Attached files
| file | filename |
|---|---|
| 8-K - PHARMACYCLICS INC | form8k207380_11052012.htm |
| EX-99.9 - PHARMACYCLICS INC | ex999to8k207380_11052012.htm |
| EX-99.4 - PHARMACYCLICS INC | ex994to8k207380_11052012.htm |
| EX-99.8 - PHARMACYCLICS INC | ex998to8k207380_11052012.htm |
| EX-99.6 - PHARMACYCLICS INC | ex996to8k207380_11052012.htm |
| EX-99.1 - PHARMACYCLICS INC | ex991to8k207380_11052012.htm |
| EX-99.5 - PHARMACYCLICS INC | ex995to8k207380_11052012.htm |
| EX-99.7 - PHARMACYCLICS INC | ex997to8k207380_11052012.htm |
| EX-99.3 - PHARMACYCLICS INC | ex993to8k207380_11052012.htm |
| EX-99.12 - PHARMACYCLICS INC | ex9912to8k207380_11052012.htm |
| EX-99.18 - PHARMACYCLICS INC | ex9918to8k207380_11052012.htm |
| EX-99.15 - PHARMACYCLICS INC | ex9915to8k207380_11052012.htm |
| EX-99.10 - PHARMACYCLICS INC | ex9910to8k207380_11052012.htm |
| EX-99.16 - PHARMACYCLICS INC | ex9916to8k207380_11052012.htm |
| EX-99.13 - PHARMACYCLICS INC | ex9913to8k207380_11052012.htm |
| EX-99.14 - PHARMACYCLICS INC | ex9914to8k207380_11052012.htm |
| EX-99.17 - PHARMACYCLICS INC | ex9917to8k207380_11052012.htm |
| EX-99.11 - PHARMACYCLICS INC | ex9911to8k207380_11052012.htm |
Exhibit 99.2
Chronic Lymphocytic Leukemia Oral Presentation
Title: The Btk inhibitor Ibrutinib in combination with rituximab is well tolerated and displays profound activity in high-risk Chronic Lymphocytic Leukemia (CLL) patients
Session: 642. CLL - Therapy, excluding Transplantation: New Targeted Therapies
Date/Time: Sunday, December 9, 2012 Presentation Time: 4:30 PM
Location: Georgia World Congress Center, Thomas Murphy Ballroom 4
Presenter: Jan Burger
Jan A. Burger, MD, PhD1, Michael J. Keating, MD2, William G. Wierda, MD, PhD3, Julia Hoellenriegel4*, Alessandra Ferrajoli, M.D.5, Stefan Faderl, MD6, Susan Lerner, MS7*, Gracy Zacharian2*, Xuelin Huang, PhD8*, Danelle F. James, M.D.9, Joseph J. Buggy, PhD10*, Hagop M Kantarjian2 and Susan M. O'Brien11
1Department of Leukemia, MD Anderson Cancer Center, Houston, TX
2Leukemia, MD Anderson Cancer Center, Houston, TX
3University of Texas, Houston, TX
4Department of Leukemia, The University of Texas, M.D. Anderson Cancer Center, Houston, TX
5Department of Leukemia, University of Texas, MD Anderson cancer center, Houston, TX
6Department of Leukemia, The University of Texas, M. D. Anderson Cancer Center, Houston, TX
7MD Anderson Cancer Center, Houston, TX
8MD Anderson Cancer Center, Houston
9Pharmacyclics, Inc, Sunnyvale, CA
10Pharmacyclics, Inc., Sunnyvale, CA
11Department of Leukemia, University of Texas- MD Anderson Cancer Center, Houston, TX
ABSTRACT:
CLL patients with high-risk disease features have shorter remissions and a poor outcome with conventional chemo-immunotherapy, particularly in the relapsed disease setting. Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib, thwarts B cell receptor (BCR) signaling and is a promising new targeted therapy for patients with mature B cell malignancies, particularly patients with CLL. Data from the Phase 1/2 trials demonstrated that high-risk CLL patients responded equally as well as low-risk patients to ibrutinib. Single-agent ibrutinib-treated CLL patients characteristically have delayed responses or stable disease due to persistent lymphocytosis, caused by re-distribution of tissue-resident CLL cells into the peripheral blood. To accelerate and improve responses and to expand upon the ibrutinib experience in high-risk CLL patients, we conducted a Phase 2 single-center clinical trial of ibrutinib plus rituximab, which accrued 40 patients between February and July, 2012.
Methods: Patients were treated with ibrutinib 420 mg PO daily, in combination with weekly rituximab (375 mg/m2) for weeks 1-4 (cycle 1), then daily ibrutinib plus monthly rituximab until cycle 6, followed by daily single-agent ibrutinib. Study inclusion required high-risk disease (del17p or TP53 mutation [treated or untreated], patients with PFS < 36 months after frontline chemo-immunotherapy, or relapsed CLL with del11q.
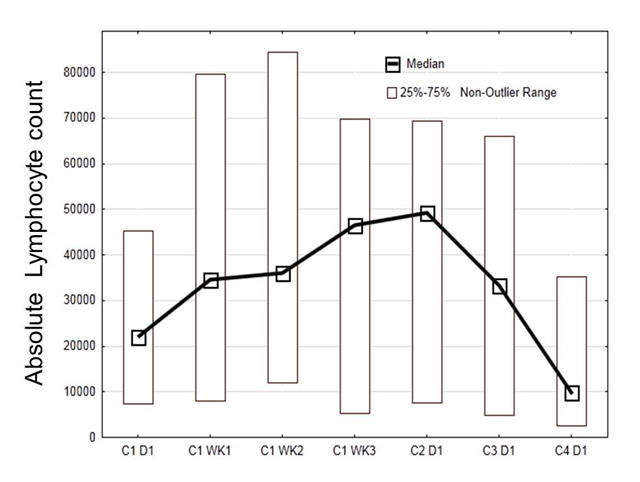
Results: Patient characteristics include a median age of 65 (range 35–82); median of 2 prior therapies, 14 female and 26 male patients. Median Rai stage was 4 (range 1–4), β2 microglobulin 4.2 mg/L (2.2 – 12.3), 31 patients had unmutated IGHV, only one patient mutated IGHV, the remaining patients had inconclusive IGHV results. 19 patients had del17p or TP53 mutation (4 without prior therapy), and 13 patients had del11q. At a median follow up of 4 months, 38 of 40 patients continue on therapy without disease progression. 1 patient died from an unrelated infectious complication, and one patient withdrew consent before starting therapy. Out of 20 patients evaluable for early response assessment at 3 months, 17 patients achieved a partial remission (PR) for an ORR of 85%, and three achieved a PR with persistent lymphocytosis. Interestingly, on this combination trial, the re-distribution lymphocytosis peaked earlier and the duration was shorter (see Figure) than with single-agent ibrutinib, presumably due to the addition of rituximab. Treatment was well tolerated, with only 13 cases of grade 3 (n=11) or grade 4 (n=2) toxicities, which were largely unrelated and transient, such as neutropenia, fatigue, pneumonia (n=1), insomnia, and bone aches. Questionnaires revealed an improved overall health and quality of life after 3 cycles of treatment in the evaluable patients (n=21).
Conclusion: Irutinib in combination with rituximab is a safe, well tolerated regimen for high-risk CLL patients, which induces very high early response rates. These encouraging data, together with the Phase 1/2 data, emphasize the need for rapid further development of ibrutinib for high-risk CLL patients, given that current alternative treatment options for this patient population are not satisfactory.
Disclosures: Burger: Pharmacyclics: Consultancy, Research Funding. Off Label Use: Ibrutinib in high-risk CLL. Faderl: Genzyme: Membership on an entity’s Board of Directors or advisory committees, Research Funding. James: Pharmacyclics: Employment, Equity Ownership. Buggy: Pharmacyclics: Employment, Equity Ownership. O'Brien: Pharmacyclics: Research Funding.
