Attached files
UNITED
STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
|
☒
|
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
For the quarterly period ended March 31, 2020
OR
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
Commission File Number 001-36498
CELLULAR BIOMEDICINE GROUP, INC.
(Exact name of registrant as specified in its charter)
|
Delaware
|
|
86-1032927
|
|
State of Incorporation
|
|
IRS Employer Identification No.
|
1345 Avenue of Americas, 15th Floor
New York, New York 10105
(Address of principal
executive offices)
(347) 905 5663
(Registrant’s telephone number)
Securities
registered pursuant to Section 12(b) of the Exchange
Act:
|
Title of each class
|
Trading Symbol(s)
|
Name of each exchange on which
registered
|
|
Common Stock, par value $0.001
|
CBMG
|
The Nasdaq Global Select Market
|
Indicate
by check mark whether the registrant (1) has filed all reports
required to be filed by Section 13 or 15(d) of the
Securities Exchange Act of 1934 during the preceding 12 months (or
for such shorter period that the registrant was required to file
such reports), and (2) has been subject to such filing
requirements for the past 90 days.
Yes ☑ No ☐
Indicate
by check mark whether the registrant has submitted electronically
every Interactive Data File required to be submitted pursuant to
Rule 405 of Regulation S-T during the preceding 12 months (or
for such shorter period than the registrant was required to submit
such files). Yes ☑ No ☐
Indicate
by check mark whether the registrant is a large accelerated filer,
an accelerated filer, a non-accelerated filer, a smaller reporting
company, or an emerging growth company. See the definition of
“accelerated filer,” and “large accelerated
filer”, “smaller reporting company,” and
“emerging growth company” in Rule 12b-2 of the
Exchange Act.
|
Large
accelerated filer
|
☐
|
Accelerated
filer
|
☐
|
|
Non-accelerated
filer
|
☐
|
Smaller
reporting company
|
☒
|
|
|
|
Emerging growth
company
|
☐
|
If an
emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided
pursuant to Section 13(a) of the Exchange Act.
☐
Indicate by check
mark whether the registrant is a shell company (as defined in
Rule 12b-2 of the Exchange Act). Yes ☐ No
☒
As of
May 4, 2020, there were 19,391,343 shares of common stock, par
value $.001 per share, outstanding.
TABLE OF CONTENTS
|
PART I FINANCIAL INFORMATION
|
|
||||
|
|
|
|
|||
|
Item 1.
|
Condensed Consolidated Financial Statements
(unaudited)
|
3
|
|||
|
|
|
|
|||
|
|
Condensed Consolidated Balance Sheets (unaudited)
|
3
|
|||
|
|
|
|
|||
|
|
Condensed Consolidated Statements of Operations and Comprehensive
Loss (unaudited)
|
4
|
|||
|
|
|
|
|||
|
|
Condensed Consolidated Statements of Cash Flows
(unaudited)
|
5
|
|||
|
|
|
|
|||
|
|
Condensed
Consolidated Statements of Changes in Stockholders’ Equity
(unaudited)
|
6
|
|||
|
|
|
|
|||
|
|
Condensed Notes to Consolidated Financial Statements
(unaudited)
|
7
|
|||
|
|
|
|
|||
|
Item 2.
|
Management’s Discussion and Analysis of Financial Condition
and Results of Operations
|
21
|
|||
|
|
|
|
|||
|
Item 3.
|
Quantitative and Qualitative Disclosures About Market
Risk
|
39
|
|||
|
|
|
|
|||
|
Item 4.
|
Controls and Procedures
|
41
|
|||
|
|
|
|
|||
|
PART II OTHER
INFORMATION
|
|
||||
|
|
|
|
|||
|
Item 1.
|
Legal Proceedings
|
42
|
|||
|
|
|
|
|||
|
Item 1A.
|
Risk Factors
|
42
|
|||
|
|
|
|
|||
|
Item 2.
|
Unregistered Sales of Equity Securities and Use of
Proceeds
|
43
|
|||
|
|
|
|
|||
|
Item 3.
|
Defaults Upon Senior Securities
|
43
|
|||
|
|
|
|
|||
|
Item 4.
|
Mine Safety
Disclosures
|
43
|
|||
|
|
|
|
|||
|
Item 5.
|
Other Information
|
43
|
|||
|
|
|
|
|||
|
Item 6.
|
Exhibits
|
44
|
|||
|
|
|
|
|||
|
SIGNATURES
|
45
|
||||
2
PART I – FINANCIAL INFORMATION
Item 1. Condensed Consolidated Financial Statements
(Unaudited)
CONDENSED CONSOLIDATED BALANCE SHEETS (UNAUDITED)
AS OF MARCH 31, 2020 AND DECEMBER 31, 2019
|
|
March
31,
|
December
31,
|
|
|
2020
|
2019
|
|
Assets
|
|
|
|
Cash and cash
equivalents
|
$21,597,360
|
$15,443,649
|
|
Restricted
cash
|
-
|
17,000,000
|
|
Other
receivables
|
253,749
|
750,943
|
|
Prepaid
expenses
|
1,707,265
|
835,048
|
|
Total current
assets
|
23,558,374
|
34,029,640
|
|
|
|
|
|
Investments
|
-
|
240,000
|
|
Property, plant and
equipment, net
|
21,338,143
|
21,434,414
|
|
Right of
use
|
19,280,349
|
20,106,163
|
|
Goodwill
|
7,678,789
|
7,678,789
|
|
Intangibles,
net
|
7,035,420
|
7,376,940
|
|
Long-term prepaid
expenses and other assets
|
6,997,391
|
6,458,354
|
|
Total assets
(1)
|
$85,888,466
|
$97,324,300
|
|
|
|
|
|
Liabilities
and Stockholders' Equity
|
|
|
|
|
|
|
|
Liabilities:
|
|
|
|
Short-term
debt
|
$14,000,000
|
$14,334,398
|
|
Accounts
payable
|
1,620,314
|
2,039,686
|
|
Accrued
expenses
|
2,372,410
|
1,904,829
|
|
Taxes
payable
|
30,420
|
26,245
|
|
Other current
liabilities
|
5,509,393
|
5,367,708
|
|
Total current
liabilities
|
23,532,537
|
23,672,866
|
|
|
|
|
|
Other non-current
liabilities
|
17,204,688
|
17,933,743
|
|
Total liabilities
(1)
|
40,737,225
|
41,606,609
|
|
|
|
|
|
Commitments and
Contingencies (note 12)
|
|
|
|
|
|
|
|
Stockholders'
equity:
|
|
|
|
|
|
|
|
Preferred
stock, par value $.001, 50,000,000 shares
|
|
|
|
authorized;
none issued and outstanding as of
|
|
|
|
March
31, 2020 and December 31, 2019, respectively
|
-
|
-
|
|
|
|
|
|
Common
stock, par value $.001, 300,000,000 shares authorized;
|
|
|
|
20,427,185
and 20,359,889 issued; and 19,371,686 and 19,304,390
outstanding,
|
|
|
|
as
of March 31, 2020 and December 31, 2019, respectively
|
20,427
|
20,360
|
|
Treasury
stock at cost; 1,055,499 shares of common stock
|
(14,992,694)
|
(14,992,694)
|
|
as
of March 31, 2020 and December 31, 2019, respectively
|
|
|
|
Additional paid in
capital
|
273,535,311
|
272,117,518
|
|
Accumulated
deficit
|
(211,514,040)
|
(199,966,543)
|
|
Accumulated
other comprehensive loss
|
(1,897,763)
|
(1,460,950)
|
|
Total stockholders'
equity
|
45,151,241
|
55,717,691
|
|
|
|
|
|
Total liabilities
and stockholders' equity
|
$85,888,466
|
$97,324,300
|
_______________
|
(1)
|
The
Company’s consolidated assets as of March 31, 2020 and
December 31, 2019 included $47,512,515 and $54,668,966,
respectively, of assets of variable interest entities, or VIEs,
that can only be used to settle obligations of the VIEs. Each of
the following amounts represent the balances as of March 31, 2020
and December 31, 2019, respectively. These assets include cash and
cash equivalents of $6,166,998 and $13,424,425; other receivables
of $230,062 and $201,532; prepaid expenses of $1,518,325 and
$770,127; property, plant and equipment, net, of $20,605,081 and
$20,762,271; right of use of $12,849,397 and $13,541,518;
intangibles of $1,195,862 and $1,226,955; and long-term prepaid
expenses and other assets of $4,946,790 and $4,742,138. The
Company’s consolidated liabilities as of March 31, 2020 and
December 31, 2019 included $18,229,381 and $32,865,763,
respectively, of liabilities of the VIEs whose creditors have no
recourse to the Company. These liabilities include short-term debt
of nil and $14,334,398; accounts payable of $1,572,746 and
$1,324,792; other payables of $3,933,933 and $4,090,154; payroll
accrual of $1,423,447 and $1,208,491, which mainly includes bonus
accrual of $1,277,861 and $1,207,560; deferred income of nil and
$10,994; and other non-current liabilities of $11,299,255 and
$11,896,934. See further description in Note 3, Variable Interest
Entities.
|
The
accompanying notes are an integral part of these unaudited
condensed consolidated financial statements.
3
CELLULAR BIOMEDICINE GROUP, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE
LOSS
(UNAUDITED)
FOR THE THREE MONTHS ENDED MARCH 31, 2020 AND 2019
|
|
For the Three
Months Ended
|
|
|
|
March
31,
|
|
|
|
2020
|
2019
|
|
Net sales and
revenue
|
$-
|
$49,265
|
|
|
|
|
|
Operating
expenses:
|
|
|
|
Cost of
sales
|
-
|
8,087
|
|
General and
administrative
|
3,431,344
|
3,447,734
|
|
Selling and
marketing
|
-
|
42,260
|
|
Research and
development
|
7,759,358
|
5,968,096
|
|
Impairment of
investments
|
240,000
|
-
|
|
Total
operating expenses
|
11,430,702
|
9,466,177
|
|
Operating
loss
|
(11,430,702)
|
(9,416,912)
|
|
|
|
|
|
Other (expense)
income
|
|
|
|
Interest income,
net
|
12,772
|
97,034
|
|
Other expense,
net
|
(127,792)
|
(14,510)
|
|
Total
other (expense) income
|
(115,020)
|
82,524
|
|
Loss before
taxes
|
(11,545,722)
|
(9,334,388)
|
|
|
|
|
|
Income taxes
provision
|
(1,775)
|
(2,400)
|
|
|
|
|
|
|
|
|
|
Net
loss
|
$(11,547,497)
|
$(9,336,788)
|
|
Other comprehensive
income:
|
|
|
|
Cumulative
translation adjustment
|
(436,813)
|
396,126
|
|
Total other
comprehensive income:
|
(436,813)
|
396,126
|
|
|
|
|
|
Comprehensive
loss
|
$(11,984,310)
|
$(8,940,662)
|
|
|
|
|
|
|
|
|
|
Net loss per share
:
|
|
|
|
Basic
and diluted
|
$(0.60)
|
$(0.51)
|
|
|
|
|
|
Weighted average
common shares outstanding:
|
|
|
|
Basic
and diluted
|
19,340,982
|
18,152,429
|
The
accompanying notes are an integral part of these unaudited
condensed consolidated financial statements.
4
CELLULAR BIOMEDICINE GROUP, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
FOR THE THREE MONTHS ENDED MARCH 31, 2020 AND 2019
|
|
For the Three
Months Ended
|
|
|
|
March
31,
|
|
|
|
2020
|
2019
|
|
CASH FLOWS FROM
OPERATING ACTIVITIES:
|
|
|
|
Net
loss
|
$(11,547,497)
|
$(9,336,788)
|
|
Adjustments
to reconcile net loss to net cash
|
|
|
|
used
in operating activities:
|
|
|
|
Depreciation and
amortization
|
1,593,078
|
1,329,699
|
|
Loss on disposal of
assets
|
-
|
(23)
|
|
Stock based
compensation expense
|
936,062
|
1,124,562
|
|
Other than
temporary impairment on investments
|
240,000
|
-
|
|
Changes
in operating assets and liabilities:
|
|
|
|
Accounts
receivable
|
-
|
788
|
|
Other
receivables
|
493,853
|
(161,074)
|
|
Prepaid
expenses
|
(884,281)
|
(1,038,324)
|
|
Long-term prepaid
expenses and other assets
|
(472,222)
|
(378,024)
|
|
Accounts
payable
|
(744,090)
|
426,027
|
|
Accrued
expenses
|
486,538
|
12,704
|
|
Other current
liabilities
|
599,822
|
155,980
|
|
Taxes
payable
|
4,175
|
-
|
|
Other non-current
liabilities
|
-
|
(71,221)
|
|
Net
cash used in operating activities
|
(9,294,562)
|
(7,935,694)
|
|
|
|
|
|
CASH FLOWS FROM
INVESTING ACTIVITIES:
|
|
|
|
Proceeds
from disposal of assets
|
-
|
359
|
|
Purchases of
intangibles
|
(51,687)
|
(619,165)
|
|
Purchases of
property, plant and equipment
|
(1,582,479)
|
(3,545,355)
|
|
Net
cash used in investing activities
|
(1,634,166)
|
(4,164,161)
|
|
|
|
|
|
CASH FLOWS FROM
FINANCING ACTIVITIES:
|
|
|
|
Net proceeds from
the issuance of common stock
|
-
|
16,038,504
|
|
Proceeds from
exercise of stock options
|
481,798
|
109,261
|
|
Proceeds from
short-term debt
|
14,000,000
|
6,131,723
|
|
Repayment of
short-term debt
|
(14,315,898)
|
-
|
|
Repurchase of
treasury stock
|
-
|
(1,039,028)
|
|
Net
cash provided by financing activities
|
165,900
|
21,240,460
|
|
|
|
|
|
EFFECT OF EXCHANGE
RATE CHANGES ON CASH
|
(83,461)
|
84,032
|
|
|
|
|
|
INCREASE (DECREASE)
IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH
|
(10,846,289)
|
9,224,637
|
|
CASH, CASH
EQUIVALENTS AND RESTRICTED CASH, BEGINNING OF PERIOD
|
32,443,649
|
52,812,880
|
|
CASH, CASH
EQUIVALENTS AND RESTRICTED CASH, END OF PERIOD
|
$21,597,360
|
$62,037,517
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL
CASH FLOW INFORMATION
|
|
|
|
|
|
|
|
Income tax
refund
|
$3,200
|
$-
|
|
|
|
|
|
Cash paid for
income taxes
|
$800
|
$2,400
|
|
|
|
|
|
Interest expense
paid
|
$99,271
|
$30,506
|
|
|
|
|
|
Interest income
from pledged bank deposits received, netting off withholding
tax
|
$460,041
|
$-
|
|
|
March
31,
|
March
31,
|
|
|
2020
|
2019
|
|
Reconciliation of
cash, cash equivalents and restricted cash in condensed
consolidated statements of cash flows:
|
|
|
|
Restricted
cash
|
$-
|
$17,000,000
|
|
Cash and cash
equivalents
|
21,597,360
|
45,037,517
|
|
|
|
|
|
Cash, cash
equivalents and restricted cash
|
$21,597,360
|
$62,037,517
|
The
accompanying notes are an integral part of these unaudited
condensed consolidated financial statements.
5
CELLULAR BIOMEDICINE GROUP, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’
EQUITY
(UNAUDITED)
FOR THE THREE MONTHS ENDED MARCH 31, 2020 AND 2019
|
|
|
|
|
|
|
|
|
|
Accumulated
Other
|
|
|
|
Common
Stock
|
Preferred
Stock
|
Treasury
Stock
|
Additional
|
Accumulated
|
Comprehensive
|
|
|||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Paid in
Capital
|
Deficit
|
Income
(Loss)
|
Total
|
|
Balance at
December 31, 2019
|
20,359,889
|
$20,360
|
-
|
$-
|
1,055,499
|
$(14,992,694)
|
$272,117,518
|
$(199,966,543)
|
$(1,460,950)
|
$55,717,691
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted
stock grants
|
20,061
|
20
|
-
|
-
|
-
|
-
|
434,385
|
-
|
-
|
434,405
|
|
Accrual of
share-based compensation costs
|
-
|
-
|
-
|
-
|
-
|
-
|
501,657
|
-
|
-
|
501,657
|
|
Exercise of
stock options
|
47,235
|
47
|
-
|
-
|
-
|
-
|
481,751
|
-
|
-
|
481,798
|
|
Foreign
currency translation
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(436,813)
|
(436,813)
|
|
Net
loss
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(11,547,497)
|
-
|
(11,547,497)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
March 31, 2020
|
20,427,185
|
$20,427
|
-
|
$-
|
1,055,499
|
$(14,992,694)
|
$273,535,311
|
$(211,514,040)
|
$(1,897,763)
|
$45,151,241
|
|
|
|
|
|
|
|
|
|
|
Accumulated
Other
|
|
|
|
Common
Stock
|
Preferred
Stock
|
Treasury
Stock
|
Additional
|
Accumulated
|
Comprehensive
|
|
|||
|
|
Shares
|
Amount
|
Shares
|
Amount
|
Shares
|
Amount
|
Paid in
Capital
|
Deficit
|
Income
(Loss)
|
Total
|
|
Balance at
December 31, 2018
|
19,120,781
|
$19,121
|
-
|
$-
|
(1,001,499)
|
$(13,953,666)
|
$250,604,618
|
$(149,982,489)
|
$(1,469,192)
|
$85,218,392
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock
issued with public offering
|
1,029,412
|
1,029
|
-
|
-
|
-
|
-
|
16,037,475
|
-
|
-
|
16,038,504
|
|
Restricted
stock grants
|
20,053
|
20
|
-
|
-
|
-
|
-
|
341,919
|
-
|
-
|
341,939
|
|
Accrual of
share-based compensation costs
|
-
|
-
|
-
|
-
|
-
|
-
|
782,623
|
-
|
-
|
782,623
|
|
Exercise of
stock options
|
12,408
|
13
|
-
|
-
|
-
|
-
|
109,248
|
-
|
-
|
109,261
|
|
Treasury stock
purchase
|
-
|
-
|
-
|
-
|
(54,000)
|
(1,039,028)
|
-
|
-
|
-
|
(1,039,028)
|
|
Foreign
currency translation
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
396,126
|
396,126
|
|
Net
loss
|
-
|
-
|
-
|
-
|
-
|
-
|
-
|
(9,336,788)
|
-
|
(9,336,788)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
March 31, 2019
|
20,182,654
|
$20,183
|
-
|
$-
|
(1,055,499)
|
$(14,992,694)
|
$267,875,883
|
$(159,319,277)
|
$(1,073,066)
|
$92,511,029
|
Note:
No dividend was declared for the three months ended March 31, 2020
and 2019.
The
accompanying notes are an integral part of these unaudited
condensed consolidated financial statements.
6
CELLULAR BIOMEDICINE GROUP, INC.
FOR THE THREE MONTHS ENDED MARCH 31, 2020 AND 2019
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL
STATEMENTS
NOTE 1 – DESCRIPTION OF BUSINESS
As used
in this quarterly report, “we”, “us”,
“our”, “CBMG”, “Company” or
“our company” refers to Cellular Biomedicine Group,
Inc. and, unless the context otherwise requires, all of its
subsidiaries and variable interest entities.
Overview
We are
a clinical-stage biopharmaceutical company committed to using our
proprietary cell-based technologies to develop immunotherapies for
the treatment of cancer and stem cell therapies for the treatment
of degenerative diseases. We view ourselves as a leader in the cell
therapy industry through our diverse, multi-target, broad pipeline
ranging from immuno-oncology, featuring Chimeric antigen receptor
T-cell (CAR-T), T-cell receptor-engineered T-cell (TCR-T) and tumor
infiltrating lymphocytes (TILs) to regenerative medicine. Our focus
is to bring our potentially highly competitive products to market
while also aiming to reduce manufacturing cycle time and aggregate
cost as well as ensuring quality products of cell therapies. We
provide comprehensive and integrated research and manufacturing
services throughout the discovery, development and manufacturing
spectrum for cell-based technologies. We have two major components
to our global strategy. First, we intend on developing our own
internal pipeline, focusing on immune cell therapy, regenerative
medicine, as well as other innovative biotechnology modalities that
can leverage our infrastructure, human capital and intellectual
property. Second, we plan to partner with leading companies to
monetize our innovative technologies in markets where we do not
currently have a presence or limited resources and may
also seek to bring their technologies to markets where we have
infrastructure.
Our
end-to-end platform enables discovery, development and
manufacturing of cell-based therapies from concept to commercial
manufacturing in a cost-efficient manner. The manufacturing and
delivery of T-cell therapies involve complex, integrated processes,
comprised of isolating T-cells from patients, T-cell enrichment,
activation, viral vector transduction, expansion, harvest and
fill-finish. Our in-house cell therapy manufacturing is comprised
of a semi-automated, fully closed system and can manufacture high
quality plasmids, and serum-free reagents as well as viral vectors
for our immuno-oncology cell therapy products. Because we are
vertically integrated, we are able to reduce the aggregate cost of
cell therapies. We plan to build out our manufacturing capacity to
scale for commercial supply at an economical cost. We hone our
manufacturing process in our good manufacturing practice (GMP)
facilities in China to achieve cycle time reduction, improve
quality assurance and control and increase efficiency and early
development to understand our therapies’ efficacy. Upon
completion of our Rockville, Maryland GMP facility in late Q3, 2020
we plan to: (a) transfer protocol from our China GMP facility to
the Rockville site to support our U.S. FDA clinical trials on
anti-CD20/CD19 bi-specific CAR for NHL, and (b) initiate U.S. FDA
clinical trials on TIL for Non-Small-Cell Lung Cancer (NSCLC). Our
other objective on institutionalizing our manufacturing process is
portability and ease of tech transfer to other facilities and ease
of deployment in future locations.
In
September 2018, we executed a License and Collaboration Agreement
(hereinafter Novartis LCA) with Novartis AG (Novartis) to
manufacture and supply their U.S. FDA-approved CD19 CAR-T cell
therapy product Kymriah®
(tisagenlecleucel) in China. Pursuant to the Novartis LCA
agreement, we also granted Novartis a worldwide license to certain
of our CAR-T intellectual property for the development, manufacture
and commercialization of CAR-T products. We are entitled to an
escalating single-digit percentage royalty of Kymriah®’s net
sales in China. CBMG is responsible for the cost of bi-directional
technology transfers between the two companies. We will receive
collaboration payments equal to a single-digit escalating
percentage of net sales of Kymriah® in China,
subject to certain caps set forth under the Novartis LCA, for sales
in diffuse large B-cell lymphoma and pediatric acute lymphoblastic
leukemia indications and up to a maximum amount to be agreed upon
for sales in other indications. We are also obligated to assist
Novartis with the development of Kymriah® in China as
Novartis may request and we are responsible for a certain
percentage of the total development cost for the development of
Kymriah® in China for
indications other than diffuse large B-cell lymphoma and pediatric
acute lymphoblastic leukemia indications. As of March 31, 2020, we
have achieved several major milestones on the technology transfer
and collaboration with Novartis on commercialization of
Kymriah®, specifically:
process and analytical training, feasibility, export license for
feasibility/comparability and majority of our manufacturing
comparability run.
On
October 2, 2018, we executed a nonexclusive license agreement with
the U.S. National Cancer Institute (NCI) for ten tumor infiltrating
lymphocytes patents, pursuant to which we acquired rights to the
worldwide development, manufacture and commercialization of
autologous, tumor-reactive lymphocyte adoptive cell therapy
products, isolated from tumor infiltrating lymphocytes for the
treatment of non-small cell lung, stomach, esophagus, colorectal
and head and neck cancer(s) in humans. We plan to use our Maryland
GMP facility to launch clinical trials in the U.S. upon
institutionalizing our process development.
In
order to expedite fulfillment of patient treatment, we have been
actively developing technologies and products with strong
intellectual property protection. CBMG’s worldwide exclusive
license to the T-cell patent rights owned by Augusta University
provides an opportunity to expand the application of CBMG’s
cancer therapy-enabling technologies and to initiate clinical
trials with leading cancer hospitals. On February 14, 2019, Augusta
University granted us an exclusive, worldwide license with
sublicense rights to its patent rights to Human Alpha
Fetoprotein-Specific T-cell Receptor modified T-cells (AFP TCR-T).
We started the AFP TCR-T Investigator Initiated Trial (IIT) in
October 2019 and have commenced enrolling HCC patients in China at
the low dose since then.
Corporate History
Headquartered in
New York, the Company is a Delaware biopharmaceutical company
focused on developing treatments for cancer and orthopedic diseases
for patients in China. We also plan to develop our products
targeting certain solid tumor and other cancer indications in the
United States. The Company started its regenerative medicine
business in China in 2009 and expanded to CAR-T therapies in
2014.
7
NOTE 2 – BASIS OF PRESENTATION AND SIGNIFICANT
ACCOUNTING POLICIES
The
accompanying unaudited condensed consolidated financial statements
have been prepared in accordance with accounting principles
generally accepted in the United States of America (“U.S.
GAAP”) for interim financial information and the rules and
regulations of the Securities and Exchange Commission
(“SEC”) for reporting on Form 10-Q. Accordingly, they
do not include all the information and footnotes required by U.S.
GAAP for complete financial statements herein. The unaudited
Condensed Consolidated Financial Statements herein should be read
in conjunction with the historical consolidated financial
statements of the Company for the year ended December 31, 2019
included in our Annual Report on Form 10-K for the year ended
December 31, 2019. Operating results for the three months ended
March 31, 2020 are not necessarily indicative of the results that
may be expected for the year ending December 31, 2020.
Principles of Consolidation
Our
unaudited condensed consolidated financial statements reflect all
adjustments, which are, in the opinion of management, necessary for
a fair presentation of our financial position and results of
operations. Such adjustments are of a normal recurring nature,
unless otherwise noted. The balance sheet as of March 31, 2020 and
the results of operations for the three months ended March 31, 2020
are not necessarily indicative of the results to be expected for
any future period.
Our
unaudited condensed consolidated financial statements are prepared
in accordance with U.S. GAAP. These accounting principles require
us to make certain estimates, judgments and assumptions that affect
the reported amounts if assets and liabilities and disclosure of
contingent assets and liabilities at the date of the financial
statements and the reported amounts of revenues and expenses during
the reporting period. We believe that the estimates, judgments and
assumptions are reasonable, based on information available at the
time they are made. Actual results could differ materially from
those estimates.
Reclassification of Prior Period Presentation
Certain
reclassifications have been made to conform the prior period date
to the current presentation. These reclassifications had no
material effect on the reported results.
Liquidity and Going Concern
The
Company recorded accumulated deficit of $211,514,040, cash and cash
equivalents and restricted cash of $21,597,360 as of March 31,
2020, compared with accumulated deficit of $199,966,543, cash and
cash equivalents of $32,443,649 as of December 31, 2019. Management
believes that, based on the progress of our clinical development,
the Company can secure financial resources amid the COVID-19
pandemic to satisfy the Company’s current liabilities and the
capital expenditure needs in the next 12 months, however, there are
no guarantees that these financial resources will be secured. If
these financial resources are not secured, there is substantial
doubt about the ability of the Company to continue as a going
concern and it may be unable to realize its assets and discharge
its liabilities in the normal course of business. In order to
finance our operations, management intends to rely upon external
financing. This financing may be in the form of equity and or debt,
private placements and/or public offerings or arrangements with
private lenders. The
condensed consolidated financial statements do not include any
adjustments that might be necessary if the Company is unable to
continue as a going concern.
Recent Accounting Pronouncements
Accounting pronouncements adopted during the three months ended
March 31, 2020
In
August 2018, the FASB issued Accounting Standards Update
(“ASU”) No. 2018-13, “Fair Value Measurement (Topic
820)” which eliminates, adds and modifies certain
disclosure requirements for fair value measurements. The modified
standard eliminates the requirement to disclose changes in
unrealized gains and losses included in earnings for recurring
Level 3 fair value measurements and requires changes in unrealized
gains and losses be included in other comprehensive income for
recurring Level 3 fair value measurements of instruments. The
standard also requires the disclosure of the range and weighted
average used to develop significant unobservable inputs and how
weighted average is calculated for recurring and nonrecurring Level
3 fair value measurements. The amendment is effective for fiscal
years beginning after December 15, 2019 and interim periods within
that fiscal year with early adoption permitted. The Company adopted
Topic 820 on January 1,
2020. The adoption of the ASU 2018-13 did not have a material
impact on the Company’s consolidated financial
statements.
In
January 2017, the FASB issued ASU No. 2017-04, “Intangibles—Goodwill and Other
(Topic 350): Simplifying the Test for Goodwill
Impairment” (“ASU 2017-04”) which removes
Step 2 from the goodwill impairment test. An entity will apply a
one-step quantitative test and record the amount of goodwill
impairment as the excess of a reporting unit’s carrying
amount over its fair value, not to exceed the total amount of
goodwill allocated to the reporting unit. The new guidance does not
amend the optional qualitative assessment of goodwill impairment.
Public business entity that is a U.S. Securities and Exchange
Commission filer should adopt the amendments in this ASU for its
annual or any interim goodwill impairment test in fiscal years
beginning after December 15, 2019. Early adoption is permitted for
interim or annual goodwill impairment tests performed on testing
dates after January 1, 2017. The Company adopted ASU 2017-04 on January 1, 2020.
The adoption of the ASU 2017-04 did not have a material impact on
the Company’s consolidated financial statements.
In June
2016, the FASB issued ASU No. 2016-13, “Financial Instruments—Credit
Losses (Topic 326): Measurement of Credit Losses on Financial
Instruments.” (“ASU
2016-13”). Financial Instruments—Credit Losses (Topic
326) amends guideline on reporting credit losses for assets held at
amortized cost basis and available-for-sale debt securities. For
assets held at amortized cost basis, Topic 326 eliminates the
probable initial recognition threshold in current GAAP and,
instead, requires an entity to reflect its current estimate of all
expected credit losses. The allowance for credit losses is a
valuation account that is deducted from the amortized cost basis of
the financial assets to present the net amount expected to be
collected. For available-for-sale debt securities, credit losses
should be measured in a manner similar to current GAAP, however
Topic 326 will require that credit losses be presented as an
allowance rather than as a write-down. ASU 2016-13 affects entities
holding financial assets and net investment in leases that are not
accounted for at fair value through net income. The amendments
affect loans, debt securities, trade receivables, net investments
in leases, off balance sheet credit exposures, reinsurance
receivables and any other financial assets not excluded from the
scope that have the contractual right to receive cash. In October 2019, the FASB issued ASU
2019-10, Financial Instruments –
Credit Losses (Topic 326), Derivatives and Hedging (Topic 815) and
Leases (Topic 842), which
defers the effective date for public filers that are considered
small reporting companies as defined by the Securities and Exchange
Commission to fiscal years beginning after December 15, 2022,
including interim periods within those fiscal years. Since the
Company is a smaller reporting company, implementation is not
needed until January 1, 2023. Adoption of the standard requires
using a modified retrospective approach through a cumulative-effect
adjustment to retained earnings as of the effective date to align
existing credit loss methodology with the new standard. The Company
is evaluating the impact of this standard on its consolidated
financial statements, including accounting policies, processes, and
systems, and expects the standard will have a minor impact on its
consolidated financial statements.
8
Accounting pronouncements not yet effective to adopt
In
December 2019, the FASB issued ASU No. 2019-12, “Income
Taxes” (Topic 740): Simplifying the Accounting for Income
Taxes (“ASU 2019-12”). ASU 2019-12 will simplify the
accounting for income taxes by removing certain exceptions to the
general principles in Topic 740. The amendments also improve
consistent application of and simplify GAAP for other areas of
Topic 740 by clarifying and amending existing guidance. For public
business entities, the amendments in this Update are effective for
fiscal years, and interim periods within those fiscal years,
beginning after December 15, 2020. For all other entities, the
amendments are effective for fiscal years beginning after December
15, 2021, and interim periods within fiscal years beginning after
December 15, 2022. We do not expect that the requirements of ASU
2019-12 will have a material impact on our consolidated financial
statements.
NOTE 3 – VARIABLE INTEREST ENTITIES
VIEs
are those entities in which a company, through contractual
arrangements, bears the risk of, and enjoys the rewards normally
associated with ownership of the entity, and therefore the Company
is the primary beneficiary of the entity. Cellular Biomedicine
Group Ltd (Shanghai) (“CBMG Shanghai”) and its
subsidiaries are variable interest entities (VIEs) through which
the Company conducts stem cell and immune therapy research and
clinical trials in China. The registered shareholders of CBMG
Shanghai are Lu Junfeng and Chen Mingzhe, who together own 100% of
the equity interests in CBMG Shanghai. The initial
capitalization and operating expenses of CBMG Shanghai are funded
by our wholly foreign-owned enterprise (“WFOE”),
Cellular Biomedicine Group Ltd. (Wuxi) (“CBMG Wuxi”).
The registered capital of CBMG Shanghai is 10 million RMB and was
incorporated on October 19, 2011. Beijing Agreen Biotechnology Co.,
Ltd. (“AG”) was 100% acquired by CBMG Shanghai in
September 2014. The registered capital of AG is 5 million RMB and
was incorporated on April 27, 2011. In 2017, CBMG Shanghai
established two subsidiaries in Wuxi and Shanghai. Wuxi Cellular
Biopharmaceutical Group Ltd. was established on January 17, 2017
with registered capital of 20 million RMB and wholly owned by CBMG
Shanghai. Shanghai Cellular Biopharmaceutical Group Ltd. (“SH
SBM”) was established on January 18, 2017 with registered
capital of 100 million RMB and wholly owned by CBMG Shanghai. For
the period ended March 31, 2020 and 2019, nil and 32% of the
Company revenue is derived from VIEs respectively.
In February 2012, CBMG Wuxi provided financing to CBMG Shanghai in
the amount of $1,587,075 for working capital purposes. In
conjunction with the provided financing, exclusive option
agreements were executed granting CBMG Wuxi the irrevocable and
exclusive right to convert the unpaid portion of the provided
financing into equity interest of CBMG Shanghai at CBMG
Wuxi’s sole and absolute discretion. CBMG Wuxi and CBMG
Shanghai additionally executed a business cooperation agreement
whereby CBMG Wuxi is to provide CBMG Shanghai with technical and
business support, consulting services and other commercial
services. The shareholders of CBMG Shanghai pledged their equity
interest in CBMG Shanghai as collateral in the event CBMG Shanghai
does not perform its obligations under the business cooperation
agreement.
The Company has determined it is the primary beneficiary of CBMG
Shanghai by reference to the power and benefits criterion under ASC
Topic 810, Consolidation. This determination was reached after
considering the financing provided by CBMG Wuxi to CBMG Shanghai is
convertible into equity interest of CBMG Shanghai and the business
cooperation agreement grants the Company and its officers the power
to manage and make decisions that affect the operation of CBMG
Shanghai.
There are substantial uncertainties regarding the interpretation,
application and enforcement of PRC laws and regulations, including
but not limited to the laws and regulations governing our business
or the enforcement and performance of our contractual arrangements.
See Risk Factors below regarding “Risks Related to Our
Structure.” The Company has not provided any guarantees
related to VIEs and no creditors of VIEs have recourse to the
general credit of the Company.
As the primary beneficiary of CBMG Shanghai and its subsidiaries,
the Company consolidates in its financial statements the financial
position, results of operations and cash flows of CBMG Shanghai and
its subsidiaries, and all intercompany balances and transactions
between the Company and CBMG Shanghai and its subsidiaries are
eliminated in the consolidated financial statements.
9
The
Company has aggregated the financial information of CBMG Shanghai
and its subsidiaries in the table below. The aggregate carrying
value of assets and liabilities of CBMG Shanghai and its
subsidiaries (after elimination of intercompany transactions and
balances) in the Company’s condensed consolidated balance
sheets as of March 31, 2020 and December 31, 2019 are as
follows:
|
|
March
31,
|
December
31,
|
|
|
2020
|
2019
|
|
Assets
|
|
|
|
Cash
|
$6,166,998
|
$13,424,425
|
|
Other
receivables
|
230,062
|
201,532
|
|
Prepaid
expenses
|
1,518,325
|
770,127
|
|
Total current
assets
|
7,915,385
|
14,396,084
|
|
|
|
|
|
Property, plant and
equipment, net
|
20,605,081
|
20,762,271
|
|
Right of
use
|
12,849,397
|
13,541,518
|
|
Intangibles
|
1,195,862
|
1,226,955
|
|
Long-term prepaid
expenses and other assets
|
4,946,790
|
4,742,138
|
|
Total
assets
|
$47,512,515
|
$54,668,966
|
|
|
|
|
|
Liabilities
|
|
|
|
Short-term
debt
|
$-
|
$14,334,398
|
|
Accounts
payable
|
1,572,746
|
1,324,792
|
|
Other
payables
|
3,933,933
|
4,090,154
|
|
Accrued payroll
*
|
1,423,447
|
1,208,491
|
|
Deferred
income
|
-
|
10,994
|
|
Total current
liabilities
|
$6,930,126
|
$20,968,829
|
|
|
|
|
|
Other non-current
liabilities
|
11,299,255
|
11,896,934
|
|
Total
liabilities
|
$18,229,381
|
$32,865,763
|
* Accrued
payroll mainly includes bonus accrual of $1,277,861 and $1,207,560
as of March 31, 2020 and December 31, 2019,
respectively.
10
NOTE 4 – RESTRICTED CASH AND SHORT-TERM
DEBT
On
January 19, 2019, SH SBM, a wholly owned subsidiary of CBMG
Shanghai, entered into a credit agreement (the “Credit
Agreement”) with China Merchants Bank, Shanghai Branch (the
“Merchants Bank”). Pursuant to the Credit Agreement,
the Merchants Bank agreed to extend credit of up to 100 million RMB
(approximately $14.5 million) to SH SBM via revolving and/or
one-time credit lines. The credit period under the Credit Agreement
ran until December 30, 2019. As of December 31, 2019, all $14.3
million had been drawn down under the Credit Agreement. The Company
subsequently repaid all the bank borrowings in February
2020.
Convertible Debt
On January
28, 2020, the Board of Directors of the Company accepted the
Special Committee of the Board and its advisers’
recommendation to arrange a bridge loan (the “Bridge
Loan”) of sixteen million dollars ($16,000,000) in accordance
with a Bridge Loan Agreement entered into with Winsor Capital
Limited on January 28, 2020. TF Capital Ranok Ltd., an affiliate of
Winsor Capital Limited, is a member of the consortium that
submitted a non-binding going-private proposal to the Company on
November 11, 2019, and remained as a member of the consortium in
the schedule 13D/A filed on April 1, 2020. The Bridge Loan
Agreement is not conditioned upon the consortium bid. The Bridge
Loan was funded in three tranches. The Company received with the
first two tranches of $7 million each in January and March, 2020,
respectively, and received the last tranche of $2 million on April
2, 2020. The Company will repay all unpaid principal amount
together with the unpaid and accrued interest payable for the first
tranche on the earliest of (i) the date falling nine months from
the date of a convertible promissory note (the “Note”)
issued pursuant to the terms of the Bridge Loan Agreement, or (ii)
the occurrence of an Event of Default (as described in Section 6 of
the Note) by converting and issuing to the account holder all (but
not part) of the outstanding amount into the common stock of the
Company at a conversion price equal to the lower of (A) $19.50 per
share and (B) an amount representing a 15% discount to the volume
weighted average price over the preceding 30 trading days prior to
and including the Maturity Date (as defined in the Note). If a
consortium of investors acquires 100% of the shares of the Company
or takes the Company private by way of merger or otherwise (the
“Acquisition”), at the election of Winsor Capital
Limited, all unpaid principal amount together with the unpaid and
accrued interest payable under all tranches of the outstanding
Bridge Loan may be converted into the common stock of the Company
at a conversion price equal to the price per share payable in the
Acquisition and issued to Winsor Capital Limited and Section 3
(Repayment) of the Note shall not apply. Related interest payables
of $104,712 was recorded in other current liabilities as of March
31, 2020.
The
Company follows ASC 480-10, Distinguishing Liabilities from
Equity (“ASC 480-10”) in its evaluation of the
accounting for a hybrid instrument. A financial instrument that
embodies an unconditional obligation, or a financial
instrument other than an outstanding share that embodies a
conditional obligation, that the issuer must or may settle by
issuing a variable number of its equity shares shall be classified
as a liability (or an asset in some circumstances) if, at
inception, the monetary value of the obligation is based solely or
predominantly on any one of the following: (a) a
fixed monetary amount known at inception. (b) variations in
something other than the fair value of the issuer’s equity
shares. or (c) variations inversely related to changes in the
fair value of the issuer’s equity shares.
The
general measurement guidance in ASC 480 requires obligations that
can be settled in shares with a fixed monetary value at settlement
to be carried at fair value unless other accounting guidance
specifies another measurement attribute. The Company has determined
that ASC 835-30 is the appropriate accounting guidance for the
share-settled debt using the effective interest method over
the term of the note.
Notwithstanding the
fact that the above instruments can be settled in shares, FASB
concluded that equity classification is not appropriate
because instruments with those characteristics do not expose
the counterparty to risks and rewards similar to those of
an owner and, therefore do not create a shareholder
relationship. The Company is instead using its shares as the
currency to settle its obligation.
The
details of the short-term debt as of March 31, 2020 and December
31, 2019 are as follows:
|
|
|
|
|
As of March
31,
2020
|
As of December
31,
2019
|
|
Lender
|
Inception
date
|
Maturity
date
|
Interest
rate
|
USD
|
USD
|
|
Merchants
Bank
|
January 21, 2019 ~
January 31, 2019
|
January 21, 2020 ~
January 31, 2020
|
4.785%
|
-
|
3,496,361
|
|
Merchants
Bank
|
February 22, 2019 ~
June 24, 2019
|
February 22, 2020 ~
June 24, 2020
|
4.35%
|
-
|
10,838,037
|
|
Winsor Capital
Limited
|
January 29, 2020 ~
March 2, 2020
|
the earliest of (i)
the date falling nine months from the inception date, or (ii) the
occurrence of an event of default as defined in the loan agreement
by converting and issuing to the account holder all (but not part)
of the outstanding amount into the common stock of the
Company.
|
6%
|
14,000,000
|
-
|
|
|
|
|
|
||
|
|
|
14,000,000
|
14,334,398
|
||
11
NOTE 5 – PROPERTY, PLANT AND EQUIPMENT
As of
March 31, 2020 and December 31, 2019, property, plant and
equipment, carried at cost, consisted of the
following:
|
|
March 31,
2020
|
December 31,
2019
|
|
Office
equipment
|
$161,265
|
$160,315
|
|
Manufacturing
equipment
|
15,764,293
|
14,963,621
|
|
Computer
equipment
|
669,875
|
576,499
|
|
Leasehold
improvements
|
15,492,900
|
15,516,570
|
|
Construction
work in process
|
281,406
|
196,240
|
|
|
32,369,739
|
31,413,245
|
|
Less:
accumulated depreciation
|
(11,031,596)
|
(9,978,831)
|
|
|
$21,338,143
|
$21,434,414
|
For the three months ended March 31,
2020 and 2019, depreciation expense was $1,218,594 and $971,856,
respectively.
NOTE 6 – INVESTMENTS
The
Company’s investments represent the investment in equity
securities listed in Over-The-Counter (“OTC”) markets
of the United States of America:
|
March 31,
2020
|
Cost
|
Gross Unrealized
Gains/(losses)
|
Gross Unrealized
Losses more than 12 months
|
Gross Unrealized
Losses less than 12 months
|
Market or Fair
Value
|
|
Equity position in
Arem Pacific Corporation
|
$480,000
|
$-
|
$(240,000)
|
$(240,000)
|
$-
|
|
December 31,
2019
|
Cost
|
Gross Unrealized
Gains/(losses)
|
Gross Unrealized
Losses more than 12 months
|
Gross Unrealized
Losses less than 12 months
|
Market or Fair
Value
|
|
Equity position in
Arem Pacific Corporation
|
$480,000
|
$-
|
$(240,000)
|
$-
|
$240,000
|
There
were no sales of investments for the three months period ended
March 31, 2020 and 2019.
There
were no unrealized holding gains or losses for the investments that
were recognized in other comprehensive income for the three months
ended March 31, 2020 and 2019.
The
Company tracks each investment with an unrealized loss and
evaluates them on an individual basis for other-than-temporary
impairments, including obtaining corroborating opinions from
third-party sources, performing trend analyses and reviewing
management’s future plans. When investments have declines
determined by management to be other-than-temporary, the Company
recognizes write downs through earnings. Other-than-temporary
impairment of investments for the three month period ended March
31, 2020 and 2019 was $240,000 and nil, respectively. In March
2020, the Company contacted certain brokers to handle our Arem
Pacific Corporation (“ARPC”) restricted legend removal
from the stock certificates to convert to free-trade shares.
Because of ARPC’s non-filing status and illiquid nature of
the stock, the brokers’ compliance department summarily
rejected our request. Considering the serious doubt over the
liquidity of the ARPC stock, full impairment was made over ARPC
stock in first quarter 2020.
12
NOTE 7 – FAIR VALUE ACCOUNTING
Fair
value is defined as the price that would be received to sell an
asset or paid to transfer a liability in an orderly transaction
between market participants at the measurement date. The hierarchy
for determining that distinguishes between (1) market participant
assumptions developed based on market data obtained from
independent sources (observable inputs) and (2) an entity’s
own assumptions about market participant assumptions developed
based on the best information available in the circumstances
(unobservable inputs). The Company has adopted ASC Topic
820, Fair Value Measurement and Disclosure, which defines fair
value, establishes a framework for measuring fair value in GAAP and
expands disclosures about fair value measurements. It does not
require any new fair value measurements, but provides guidance on
how to measure fair value by providing a fair value hierarchy used
to classify the source of the information. It establishes a
three-level valuation hierarchy of valuation techniques based on
observable and unobservable inputs, which may be used to measure
fair value, and includes the following:
Level 1
– Quoted prices in active markets for identical assets or
liabilities.
Level 2
– Inputs other than Level 1 that are observable, either
directly or indirectly, such as quoted prices for similar assets or
liabilities; quoted prices in markets that are not active; or other
inputs that are observable or can be corroborated by observable
market data for substantially the full term of the assets or
liabilities.
Level 3
– Unobservable inputs that are supported by little or no
market activity and that are significant to the fair value of the
assets or liabilities.
Classification
within the hierarchy is determined based on the lowest level of
input that is significant to the fair value
measurement.
The
carrying value of financial items of the Company including cash and
cash equivalents, accounts receivable, other receivables, accounts
payable and accrued liabilities, approximate their fair values due
to their short-term nature and are classified within Level 1 of the
fair value hierarchy. As of March 31, 2020, the carrying value of
the Company’s Bridge Loan approximates fair value as the
borrowing bears interest rates that are similar to existing market
rates.
The
Company’s investments are classified within Level 2 of the
fair value hierarchy because of the insufficient volatility of the
three stocks traded in OTC market. The Company did not have any
Level 3 financial instruments as of March 31, 2020 and December 31,
2019.
Assets
measured at fair value within Level 2 on a recurring basis as of
March 31, 2020 and December 31, 2019 are summarized as
follows:
|
|
As of December 31,
2019
|
|||
|
|
Fair Value
Measurements at Reporting Date Using:
|
|||
|
|
|
Quoted Prices
in
|
Significant
Other
|
Significant
|
|
|
|
Active Markets
for
|
Observable
|
Unobservable
|
|
|
|
Identical
Assets
|
Inputs
|
Inputs
|
|
|
Total
|
(Level
1)
|
(Level
2)
|
(Level
3)
|
|
Assets:
|
|
|
|
|
|
Equity position in
ARPC
|
$240,000
|
$-
|
$240,000
|
$-
|
No
shares were acquired in the three months ended March 31, 2020 and
2019.
As of
March 31, 2020 and December 31, 2019, the Company holds 8,000,000
shares in Arem Pacific Corporation, 2,942,350 shares in Alpha Lujo,
Inc. (“ALEV”) and 2,057,131 shares in Wonder
International Education and Investment Group Corporation
(“Wonder”), respectively. Full impairment
has been provided for shares of ALEV, Wonder and ARPC as of March
31, 2020. All available-for-sale investments held by the Company at
December 31, 2019 have been valued based on level 2 inputs due to
the limited trading of these companies.
13
NOTE 8 – INTANGIBLE ASSETS
Most of
our intellectual properties are developed internally. Because we do
not capitalize our research and development expenses related to our
home-grown intellectual properties, as of March 31, 2020, the
intellectual properties acquired from the Agreen acquisition still
account for the majority of the net book value of our intangible
assets. We continue to apply the acquired Agreen intellectual
properties in our immuno-oncology research and development
activities. As such, there is no impairment on the continued use of
the acquired Agreen intellectual properties.
As of
March 31, 2020 and December 31, 2019, intangible assets, net
consisted of the following:
Patents
& knowhow & license
|
|
March 31,
2020
|
December 31,
2019
|
|
Cost
basis
|
$15,236,962
|
$15,265,211
|
|
Less:
accumulated amortization
|
(8,648,997)
|
(8,317,085)
|
|
|
|
|
|
|
$6,587,965
|
$6,948,126
|
Software
|
|
March 31,
2020
|
December 31,
2019
|
|
Cost
basis
|
$657,359
|
$612,679
|
|
Less:
accumulated amortization
|
(209,904)
|
(183,865)
|
|
|
$447,455
|
$428,814
|
|
|
|
|
|
|
|
|
|
Total intangibles,
net
|
$7,035,420
|
$7,376,940
|
All
software is provided by a third-party vendor, is not internally
developed and has an estimated useful life of five years. Patents,
knowhow and license are amortized using an estimated useful life of
five to ten years. Amortization expense for the three months
ended March 31, 2020 and 2019 was $374,484 and $357,843,
respectively.
Estimated
amortization expense for each of the ensuing years are as follows
for the twelve months ending March 31:
|
Twelve months
ending March 31,
|
Amount
|
|
2021
|
$1,495,984
|
|
2022
|
1,489,134
|
|
2023
|
1,480,299
|
|
2024
|
1,459,204
|
|
2025 and
thereafter
|
1,110,799
|
|
|
$7,035,420
|
14
NOTE 9 – LEASES
The
Company leases facilities and equipment under non-cancellable
operating lease agreements. These facilities and equipment are
located in the United States, Hong Kong and China. The Company
recognizes rental expense on a straight-line basis over the life of
the lease period.
The
Company recognized an operating liability with a corresponding ROU
asset of the same amounts based on the present value of the minimum
rental payments of such leases. Related liabilities were recorded
in other current liabilities and other non-current liabilities. We
applied the short-term lease practical expedient to all leases of
one year or less.
Quantitative
information regarding the Company’s leases is as
follows:
|
|
For the Three
Months Ended
|
|
|
|
March
31,
|
|
|
|
2020
|
2019
|
|
Lease
cost
|
|
|
|
Operating
lease cost
|
888,787
|
707,180
|
|
Short-term
lease cost
|
61,780
|
55,510
|
|
Total
lease cost
|
950,567
|
762,690
|
Supplemental
cash flow information related to leases was as
follows:
|
|
For the Three Months
Ended
|
|
|
|
March 31,
|
|
|
|
2020
|
2019
|
|
Cash paid for the amounts included in the measurement of lease
liabilities for operating leases:
|
|
|
|
Operating cashflows
|
1,228,316
|
1,268,993
|
Supplemental
balance sheet information related to leases was as
follows:
|
|
March 31,
2020
|
December 31,
2019
|
|
Operating
lease right-of-use assets
|
19,280,349
|
20,106,163
|
|
Other
current liabilities
|
2,404,520
|
2,506,413
|
|
Other
non-current liabilities
|
16,875,829
|
17,599,750
|
|
|
|
|
|
Weighted
Average Remaining Lease Term (in years): Operating
leases
|
7.7
|
7.9
|
|
|
|
|
|
Weighted
Average Discount Rate: Operating leases
|
5%
|
5%
|
15
As of
March 31, 2020, the Company has the following future minimum lease
payments due under the foregoing lease agreements:
|
Years ending March 31,
|
Amount
|
|
2021
|
$3,464,776
|
|
2022
|
3,129,891
|
|
2023
|
3,210,172
|
|
2024
|
3,143,939
|
|
2025 and
thereafter
|
11,051,249
|
|
|
|
|
|
$24,000,027
|
NOTE 10 – RELATED PARTY TRANSACTIONS
The
Company may advance petty cash to officers for business travel
purpose. As of March 31, 2020 and December 31, 2019,
other receivables due from officers for business travel purpose was
nil.
NOTE 11 – EQUITY
ASC
Topic 505 Equity paragraph 505-50-30-6
establishes that share-based payment transactions with nonemployees
shall be measured at the fair value of the consideration received
or the fair value of the equity instruments issued, whichever is
more reliably measurable.
During
the three months ended March 31, 2020 and 2019, the Company
expensed $501,657 and $782,623 associate with unvested options
awards, $434,405 and $341,939 associated with restricted common
stock, respectively.
During
the three months ended March 31, 2020 and 2019, options for 47,235
and 12,408 underlying shares were exercised, 47,235 and 12,408
shares of the Company’s common stock were issued,
respectively.
During
the three months ended March 31, 2020 and 2019, 20,061 and 20,053
shares of the Company's restricted common stock were issued to
directors, employees and advisors respectively.
On
February 21, 2020, the Special Committee of the Board of Directors
of the Company received a new preliminary non-binding proposal
letter, dated the same day, from a consortium led by Mr. Tony
(Bizuo) Liu, certain other senior management members of the
Company, Hillhouse Bio Holdings, L.P., TF Capital Ranok Ltd.,
Dangdai International Group Co., Limited, Mission Right Limited,
Maplebrook Limited, Viktor Pan, Zheng Zhou, OPEA SRL, Wealth Map
Holdings Limited and Earls Mill Limited (the “Consortium
Members”), to acquire all Shares of the Company (other than
those Shares held by the Consortium Members that may be rolled over
in connection with the transaction proposed in the Letter) for
$19.50 per Share in cash in a going-private transaction. As of
March 31, 2020, the Special Committee of the Board, with the
assistance of its advisors, has not made a decision on the
proposal.
16
NOTE 12 – COMMITMENTS AND CONTINGENCIES
Capital commitments
As of
March 31, 2020, the capital commitments of the Company are
summarized as follows:
|
|
March 31,
2020
|
|
Contracts
for acquisition of plant and equipment being or to be
executed
|
$727,979
|
|
To
be excecuted approved budget for US GMP facilities
construction
|
3,744,408
|
|
|
$4,472,387
|
NOTE 13 – STOCK BASED COMPENSATION
Our
stock-based compensation arrangements include grants of stock
options and restricted stock awards under the Stock Option Plan
(consisting of the 2009 Plan, 2011 Plan, 2013 Plan, 2014 Plan and
the 2019 Plan) and certain awards granted outside of these plans.
The compensation cost that has been charged against income related
to stock options for the three months ended March 31, 2020 and 2019
was $501,657 and $782,623, respectively. The compensation cost that
has been charged against income related to restricted stock awards
for the three months ended March 31, 2020 and 2019 was $434,405 and
$341,939, respectively.
As of
March 31, 2020, there was $1,644,172 all unrecognized compensation
cost related to an aggregate of 213,814 of non-vested stock option
awards and $2,586,983 related to an aggregate of 212,823 of
non-vested restricted stock awards. These costs are
expected to be recognized over a weighted-average period of 0.9
years for the stock options awards and 1.2 years for the restricted
stock awards.
During
the three months ended March 31, 2020 and 2019, no options of the
Company’s common stock were issued under the Stock Option
Plan.
17
The
following table summarizes stock option activity as of March 31,
2020 and December 31, 2019 and for the three months ended March 31,
2020:
|
|
Number of
Options
|
Weighted-
Average Exercise Price
|
Weighted-
Average Remaining Contractual Term (in years)
|
Aggregate
Intrinsic Value
|
|
Outstanding at
December 31, 2019
|
1,788,888
|
$12.37
|
5.4
|
$9,394,219
|
|
Grants
|
-
|
|
|
|
|
Forfeitures
|
(46,160)
|
|
|
|
|
Exercises
|
(47,235)
|
|
|
|
|
Outstanding at
March 31, 2020
|
1,695,493
|
$12.30
|
5.2
|
$8,497,235
|
|
|
|
|
|
|
|
Vested and
exercisable at March 31, 2020
|
1,481,679
|
$11.83
|
4.7
|
$8,172,473
|
|
Exercise
|
Number of
Options
|
|
|
Price
|
Outstanding
|
Exercisable
|
|
$3.00 - 4.95
|
185,547
|
185,547
|
|
$5.00 - 9.19
|
416,304
|
409,824
|
|
$9.20 - 15.00
|
487,501
|
407,828
|
|
$15.01 - 20.00
|
464,141
|
346,680
|
|
$20.10+
|
142,000
|
131,800
|
|
|
1,695,493
|
1,481,679
|
The
aggregate intrinsic value for stock options outstanding is defined
as the positive difference between the fair market value of our
common stock and the exercise price of the stock
options.
Cash
received from option exercises under all share-based payment
arrangements for the three months ended March 31, 2020 and 2019 was
$481,798 and
$109,261.
18
NOTE 14 – NET LOSS PER SHARE
Basic
and diluted net loss per common share is computed on the basis of
our weighted average number of common shares outstanding, as
determined by using the calculations outlined below:
|
|
For the Three
Months Ended
|
|
|
|
March
31,
|
|
|
|
2020
|
2019
|
|
Net
loss
|
$(11,547,497)
|
$(9,336,788)
|
|
|
|
|
|
Weighted average
shares of common stock
|
19,340,982
|
18,152,429
|
|
Dilutive effect of
stock options
|
-
|
-
|
|
Restricted stock
vested not issued
|
-
|
-
|
|
Common stock and
common stock equivalents
|
19,340,982
|
18,152,429
|
|
|
|
|
|
Net loss per basic
and diluted share
|
$(0.60)
|
$(0.51)
|
For the
three months ended March 31, 2020 and 2019, the effect of
conversion and exercise of the Company’s outstanding options
are excluded from the calculations of dilutive net loss per share
as their effects would have been anti-dilutive since the Company
had generated losses for the three months ended March 31, 2020 and
2019.
NOTE 15 – INCOME TAXES
Income
taxes are accounted for under the asset and liability method.
Deferred tax assets and liabilities are recognized for the future
tax consequences attributable to differences between the financial
statement carrying amounts of existing assets and liabilities and
their respective tax bases and operating loss and tax credit
carry-forwards. Deferred tax assets and liabilities are measured
using enacted tax rates expected to apply to taxable income in the
years in which those temporary differences are expected to be
recovered or settled. The effect of a change in tax rates on
deferred tax assets and liabilities is recognized in income in the
period during which such rates are enacted.
The
Company considers all available evidence to determine whether it is
more likely than not that some portion or all of the deferred tax
assets will be realized. The ultimate realization of deferred tax
assets is dependent upon the generation of future taxable income
during the periods in which those temporary differences become
realizable. Management considers the scheduled reversal of deferred
tax liabilities (including the impact of available carryback and
carry-forward periods), and projected taxable income in assessing
the realizability of deferred tax assets. In making such judgments,
significant weight is given to evidence that can be objectively
verified. Based on all available evidence, in particular our
three-year historical cumulative losses, recent operating losses
and U.S. pre-tax loss for the three months ended March 31, 2020, we
recorded a valuation allowance against our U.S. and China net
deferred tax assets.
In each
period since its inception, the Company has recorded a valuation
allowance for the full amount of net deferred tax assets, as the
realization of deferred tax assets is uncertain. As a result, the
Company has not recorded any federal or state income tax benefit in
the consolidated statements of operations and comprehensive income
(loss).
Pursuant to the
December 2017 Tax Cut and Jobs Act (H.R.1) (the
“TCJA”), major changes were made to the Internal
Revenue Code of 1986, as amended, including modification to the net
operating loss (“NOL”). The TCJA limits the NOL
deduction to 80% of taxable income (before the deduction). It also
generally eliminates NOL carrybacks for individuals and non-REIT
corporations, but allows indefinite NOL carryforwards. The new NOL
rules apply to losses arising in taxable years beginning in 2018.
As of March 31, 2020, the Company had net operating loss
carry-forwards of $39 million for U.S. federal income tax purposes
and $17.4 million for U.S. state income tax purposes.
The
Company’s effective tax rate differs from statutory rates of
21% for U.S. federal income tax purposes, 15% to 25% for Chinese
income tax purpose and 16.5% for Hong Kong income tax purposes due
to the effects of the valuation allowance and certain permanent
differences as it pertains to book-tax differences in the value of
client shares received for services.
Pursuant to the
Corporate Income Tax Law of the PRC, all of the Company’s PRC
subsidiaries are liable to PRC Corporate Income Taxes
(“CIT”) at a rate of 25% except for CBMG Shanghai and
Shanghai SBM.
According to
Guoshuihan 2009 No. 203, if an entity is certified as an
“advanced and new technology enterprise”, it is
entitled to a preferential income tax rate of 15%. CBMG Shanghai
obtained the certificate of “advanced and new technology
enterprise” dated October 30, 2015 with an effective period
of three years and the provision for PRC corporate income tax for
CBMG Shanghai is calculated by applying the income tax rate of 15%
from 2015. CBMG Shanghai re-applied and Shanghai SBM applied for
the certificate of “advanced and new technology
enterprise” in 2018. Both of them received approval on
November 27, 2018. On August 23, 2018, State Administration of
Taxation (“SAT”) issued a Bulletin on Enterprise Income
Tax Issues Related to the Extension of Loss Carry-forward Period
for Advanced and New Technology Enterprises and Small and
Medium-sized Technology Enterprises (“Bulletin 45”).
According to the Bulletin 45, an enterprise that obtains the two
type of qualification in 2018, is allowed to carry forward all its
prior year loss incurred between 2013 and 2017 to up to ten years
instead of five years. The same requirement applies to the
enterprise obtaining the qualification after 2018.
19
As of
March 31, 2020, all of the deferred income tax expense is offset by
changes in the valuation allowance pertaining to the
Company’s existing net operating loss carryforwards due to
the unpredictability of future profit streams prior to the
expiration of the tax losses.
NOTE 16 – SEGMENT INFORMATION
The
Company is engaged in the development of new treatments for
cancerous and degenerative diseases utilizing proprietary
cell-based technologies, which have been organized as one reporting
segment as they have substantially similar economic characteristic
since they have similar nature and economic characteristics. The
Company’s chief operating decision maker, the Chief Executive
Officer, receives and reviews the result of the operation for all
major cell platforms as a whole when making decisions about
allocating resources and assessing performance of the Company. In
accordance with FASB ASC 280-10, the Company is not required to
report the segment information.
NOTE 17 – SUBSEQUENT EVENTS
On
April 2, 2020, the Company received $2 million from the third
tranche of the Bridge Loan.
On
April 13, 2020, our Gaithersburg, Maryland site successfully
imported our Shanghai, China produced GMP grade lentiviral vector
and plasmid material designated for in vitro research and
development in the U.S.
On
April 18, 2020, the Company retained a U.S. based contract research
organization to assist with its preparation of the U.S. pre-FDA
TYPE-C meetings, Investigational New Drug (IND) gap analysis,
PRE-IND and IND application for C-CAR039 anti-CD20/CD19 bi-specific
CAR for Non-Hodgkin Lymphoma (NHL), and
Tumor Infiltrating Lymphocyte therapy (our TIL051) for
Non-Small-Cell Lung Cancer.
On
April 30, 2020, our wholly-owned subsidiary CBMG Wuxi received
approval from Nanjing Bank for a one-year line of credit of up to
CNY 30 million (approximately $4.2 million). Use of proceeds from
the credit line is limited to working capital for research and
development activities. This line of credit carries an interest
rate of not less than Loan Prime Rate (LPR) +0.5%. LPR is the
benchmark for pricing existing floating-rate loans set by
People’s Bank of China.
As of April 30, 2020, the LPR for a one-year loan is 3.85%. This
facility is only in effect while the Company remains as a publicly
traded entity. We have not drawn on this line of
credit.
20
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
The following is management’s discussion and analysis
summarizing the significant factors affecting our results of
operations, financial condition and liquidity position for the
three months ended March 31, 2020 and 2019, and should be read in
conjunction with our unaudited condensed consolidated financial
statements and related notes included elsewhere in this
filing.
This
report contains forward-looking statements. Generally, the words
“believes,” “anticipates,”
“may,” “will,” “should,”
“could,” “expect,” “plans,”
“intend,” “estimate,”
“projects,” “presidents,”
“potential,” “continue” and similar
expressions or the negative thereof or comparable terminology are
intended to identify forward-looking statements. These statements
reflect our current views with respect to future events or to our
future financial performance and involve known and unknown risks,
uncertainties and other factors which may cause our actual results,
performance or achievements to be materially different from any
future results, performance or achievements expressed or implied by
the forward-looking statements. Given these uncertainties, you
should not place undue reliance on these forward-looking
statements.
A
variety of factors, some of which are outside our control, may
cause our operating results to fluctuate significantly. They
include:
●
the severity,
magnitude, and duration of the COVID-19 pandemic, including
impacts of the pandemic and of responses to the pandemic by
governments, business and individuals on our
operations;
●
our ability to
timely complete and equip our Rockville, Maryland GMP facility amid
the COVID-19 pandemic;
●
our ability to deal
with the COVID-19 related travel challenges in connection with the
technical transfer of our institutionalized process from our
Shanghai, China facility to the new Rockville site to support
clinical trial in the U.S.;
●
our anticipated
cash needs and our estimates regarding our anticipated expenses,
capital requirements and our needs for additional
financings;
●
the success, cost
and timing of our product development activities and clinical
trials;
●
our ability and the
potential to successfully advance our technology platform to
improve the safety and effectiveness of our existing product
candidates; the potential for our identified research priorities to
advance our cancer and degenerative disease
technologies;
●
our ability to
obtain drug designation or breakthrough status for our product
candidates and any other product candidates, or to obtain and
maintain regulatory approval of our product candidates, and any
related restrictions, limitations and/or warnings in the label of
an approved product candidate;
●
the ability to
generate or license additional intellectual property relating to
our product candidates;
●
regulatory
developments in China, United States and other foreign
countries;
●
the potential of
the technologies we are developing;
●
fluctuations in the
exchange rate between the U.S. dollars and the Chinese
Yuan;
●
our plans to
continue to develop our manufacturing facilities; and
●
the additional
risks, uncertainties and other factors described under the caption
“Risk Factors” in our Annual Report on Form 10-K for
the year ended December 31, 2019.
21
We
discuss many of these risks in greater detail under the heading
“Risk Factors” included in our Annual Report on Form
10-K for the year ended December 31, 2019 filed with the Securities
and Exchange Commission on February 28, 2020.
Unless
required by law, we undertake no obligation to update or revise any
forward-looking statements to reflect new information or future
events or developments. Thus, you should not assume that our
silence over time means that actual events are bearing out as
expressed or implied in such forward-looking
statements.
For
additional information, see Item 7 of Part II,
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations — Overview” of our
2019 Annual Report on Form 10-K.
OVERVIEW
The
“Company”, “CBMG”, “we”,
“us”, “our” and similar terms refer to
Cellular Biomedicine Group, Inc. (a Delaware corporation) as a
combined entity including each of its subsidiaries and controlled
companies, unless the context otherwise requires.
Impact of COVID-19
The
COVID-19 pandemic has created new challenges for CBMG, the broader
biotech community, and society as a whole. We are prioritizing the
safety and well-being of our employees and have implemented
work-from-home policies for our U.S.- and China-based employees. In
China, employees must obtain advance permission and are only
permitted onsite as needed, and must adhere to the Company’s
COVID-19 prophylactic process and procedures. In compliance with
local rules and ordinances, our U.S. employees continue to work
remotely from home. Because of our long tradition of collaborating
with various stakeholders from different locations across different
time zones, we have not experienced major setbacks in our
operations as a result of the work-from-home policies. Commensurate
to impacts throughout the biopharma industry, we have observed some
broad-based COVID-19 supply chain related issues and are continuing
to mitigate its impact to our operations by implementing concrete
measures to prioritize the safety and physical wellbeing of our
employees. Amid the COVID-19 pandemic we are working with our
clinical studies partners in China to mitigate risk to patients
participating in our studies while taking into account regulatory,
institutional, and government guidance and policies. Because of
factors such as redirected health-care resources from partnering
hospitals, travel restrictions, and patients’ unwillingness
to go to the hospital during the outbreak we have observed delay to
our clinical studies in China. China has recently eased some of the
aforementioned restrictions and we have seen a corresponding return
to normalcy in our IIT patient enrollment. We estimate that the
COVID-19 pandemic has delayed our clinical studies schedule by
approximately one quarter. However, the actual delay cannot be
predicted and may vary by clinical study and by program depending
on a variety of currently unknown factors.
The
Company remains committed to maintaining its development plans but
acknowledges the potential impact on clinical studies amid the
rapidly evolving pandemic environment.
Recent Developments
●
On January 28,
2020, the Board of Directors of the Company accepted the Special
Committee of the Board and its advisers’ recommendation to
arrange a bridge loan (the “Bridge Loan”) of sixteen
million dollars ($16,000,000) in accordance with a Bridge Loan
Agreement entered into with Winsor Capital Limited on January 28,
2020. TF Capital Ranok Ltd., an affiliate of Winsor Capital
Limited, is a member of the consortium that submitted a non-binding
going-private proposal to the Company on November 11, 2019. The
Bridge Loan Agreement is not conditioned upon the consortium
bid.
●
On February 19,
2020, the Company commenced its collaboration with Ruijin Hospital
on a pilot clinical study on inhalation of our mesenchymal stem
cells exosomes treating severe novel coronavirus pneumonia. Other
collaborators in this pilot clinical study include the Shanghai
Public Health Clinical Center and the Wuhan Jinyintan Hospital.
Patient recruitment has slowed down amid the tapering off of the
COVID-19 outbreak in China.
●
On February 20,
2020, the Company repaid the $14.3 million short-term borrowings
from China Merchant Bank.
●
On February 20,
2020, Shanghai Cellular Biopharmaceutical Group Ltd. and Novartis
entered into a Quality Agreement for external manufacturing,
pursuant to which both parties specified the quality assurance
roles and responsibilities of Novartis AG and CBMG Shanghai with
regard to the manufacture and supply of Kymriah® to Novartis in
China.
●
On February 21,
2020, the Special Committee of the Board of Directors of the
Company received a new preliminary non-binding proposal letter,
dated the same day, from a consortium led by Mr. Tony (Bizuo) Liu,
the Chief Executive Officer of the Company, certain other senior
management members of the Company, Hillhouse Bio Holdings, L.P., TF
Capital Ranok Ltd., Dangdai International Group Co., Limited and
Mission Right Limited, Maplebrook Limited, Viktor Pan, Zheng Zhou,
OPEA SRL, Wealth Map Holdings Limited and Earls Mill Limited to
acquire all outstanding shares of common stock of the Company
(other than those shares held by members of the consortium that may
be rolled over in connection with the transaction proposed in the
letter) for $19.50 per share in cash in a going private
transaction. A consortium consisting of certain but not all of the
above consortium members submitted a preliminary non-binding
proposal to acquire the Company in a going private transaction on
November 11, 2019. The Special Committee, with the assistance of
its advisors, has been considering the proposal letter but has not
made a decision on the proposal.
●
On March 31, 2020,
the National Medical Products Administration of China (NMPA)
accepted our drug application for clinical trials in China for the
anti-BCMA CAR-T (C-CAR088) for relapsed or refractory multiple
myeloma (C-CAR088). As of April 13, 2020 we have enrolled 20
patients for the study from four hospitals. 19 patients have been
infused with C-CAR088 and 17 patients have evaluable data for
clinical efficacy. Only one grade 3 cytokine release syndrome (CRS)
has been observed. The early favorable clinical outcome warrants
continued development of C-CAR088.
●
On March 31, 2020,
encouraged by three of the four infused patients’ evaluable
data for clinical efficacy in our IIT anti-CD20/CD19 bi-specific
CAR-T for NHL, which is enabled by our bespoken fast-cycle,
economical manufacturing process, the Company decided to explore
feasibility of clinical trials in the U.S. market. The targeted NHL
indications are comprised of diffuse large B-cell lymphoma (DLBCL),
chronic lymphocytic leukemia (CLL) and follicle
center lymphoma (FCL).
22
In the
next 12 months, we aim to accomplish the following, though there
can be no assurances that we will be able to accomplish any of
these goals:
●
Bifurcate our
markets and launch clinical studies in the U.S. upon establishing
good Point of Care (POC) from the clinical studies in China and
transfer the clinical assets from Shanghai to the U.S., including
our quick cycle-time, highly differentiated, proprietary
manufacturing process comprised of short cycle-time,
semi-automation and closed system;
●
Meet with the U.S.
FDA on pre-IND filing and prepare our IND package for C-CAR039
(anti-CD20/CD19 bi-specific CAR-T) for NHL;
●
Meet with the U.S.
FDA on pre-IND filing and prepare our IND package for TIL051
for NSCLC;
●
Prepare and
submit our IND for C-CAR039 (anti-CD20/CD19 bi-specific CAR-T) for
NHL with the U.S. FDA;
●
Advance our
Rockville site’s research and development and manufacturing
to support our clinical development in the U.S.;
●
Explore the need to
bring our proprietary virus manufacturing process from Shanghai to
our Rockville site to enable U.S. clinical trials;
●
Collaborate with
Duke University on TIL process development to improve cycle time
and institutionalized scalability;
●
Explore the
feasibility of establishing a new R&D and clinical
manufacturing site in China to adapt to our rapid business
expansion and explore the addition of Contract Development, and
Manufacturing Organization (CDMO) business to support certain
specific market-oriented business strategies;
●
Evaluate our
strategy to further increase our enterprise value, and expand our
capital market strategy;
●
Pursue additional
short-term funding to shore up our balance sheet to weather the
COVID-19 pandemic;
●
Execute the
technology transfer and align the manufacturing processes with the
global CAR-T leader to support the development of the world’s
first CAR-T therapy in China;
●
Explore and
introduce a gene therapy technology platform, product development
and manufacturing for our current business to create synergy with
our cell therapy pipelines;
●
Invest more into
R&D resources and enrich our intellectual property portfolio
globally;
●
Evaluate and
implement a digital data tracking and storage technology system for
research and development, material management, GMP production and
integrated clinical data management;
●
Follow up on the
pilot clinical study on inhalation of our mesenchymal stem cells
exosomes treating severe novel coronavirus pneumonia;
●
Evaluate the
feasibility of using our mesenchymal stem cells exosomes to treat
atypical pneumonia;
●
Evaluate emerging
regenerative medicine technology platform for other indications and
review recent developments in the competitive
landscape;
●
Strengthen our
Quality Management System (QMS) centralized document control system
and electronic batch recording system for quality assurance, and
laboratory information management system (LMS) for quality
control;
●
Leverage our QMS
system and our strong scientific expertise in both the U.S. and
China;
●
Continue to field
inbound inquiries and to explore opportunities to monetize our
clinical assets;
●
Collaborate with
multinational pharmaceutical companies to co-develop cell therapy
products in China and in the U.S. by leveraging our existing
leading clinical assets or researching on new targets;
and
●
Continue to
implement International Organization for Standardization (ISO)
27001 standard to fortify our information assets
security.
23
Our
operating expenses for the three months ended March 31, 2020 were
in line with management’s plans and expectations. We had an
increase in total operating expenses of approximately $2 million
for the three-month period ended March 31, 2020, as compared to the
same period ended March 31, 2019, which was primarily attributable
to increased R&D expenses in 2020.
Corporate History
Please
refer to Note 1 of the unaudited condensed consolidated financial
statements for the corporate history.
BIOPHARMACEUTICAL BUSINESS
Our biopharmaceutical business was founded in 2009
by a team of seasoned Chinese-American executives, scientists and
doctors. In 2010, we established a facility designed and built
to comply with China’s GMP standards in Wuxi, China,
and in 2012, we established a U.S. FDA compliant
manufacturing facility in Shanghai. In November 2017, we opened our
Zhangjiang facility in Shanghai, of which 40,000 square feet, or
35% of the total facility, was designed and built to GMP standards
and dedicated to advanced cell manufacturing. We are expanding our
U.S. presence with a new 22,477 square foot Rockville, Maryland
facility scheduled to be completed in the latter part of 2020. The
Rockville site is designed to house approximately 4,500 square feet
of GMP manufacturing facility to support early stage U.S. clinical
trials. Our focus has been to serve the rapidly growing health care
market initially in China by marketing and commercializing immune
cell and stem cell therapeutics, related tools and products from
our patent-protected homegrown and acquired cell technology, as
well as by utilizing in-licensed and other acquired intellectual
properties before shifting our attention to serve the mature and
highly competitive health care market in the U.S. We continue to
explore new products and gene therapies that may require the
investment of a material amount of assets.
Our current treatment focal points are
cancer and Knee Osteoarthritis (KOA).
Cancer. We
are focusing our clinical development efforts on assets such as
C-CAR088, C-CAR039, TIL051, AFT-TCRT in China and/or U.S.
As discussed above in Item 1 –
Business, under the subheading “Overview,” we entered
into the Novartis LCA in September of 2018. With the execution of
the Novartis LCA, we have prioritized our efforts on working with
Novartis to bring Kymriah® to patients in China as soon as practicable. In
light of our collaboration with Novartis, we will no longer pursue
our own acute lymphoblastic leukemia (ALL) and diffuse large B-cell
lymphoma (DLBCL) biologics license application submission
with the NMPA. We plan to continue to leverage our cutting-edge
Chemistry, Manufacturing and Control (CMC) platform, as well as our
Quality Management System and our strong scientific expertise in
the U.S and in China, to collaborate with multinational
pharmaceutical companies to co-develop cell therapies in
China.
KOA. In 2013, we completed a Phase-I/IIa
clinical study, in China, for our KOA therapy named
ReJoin®. The trial
tested the safety and efficacy of intra-articular injections of
autologous human adipose-derived
mesenchymal progenitor cells (haMPCs) in order to reduce
inflammation and repair damaged joint cartilage. Since 2013, we
have continued clinical studies on ReJoin® and
our trial data has demonstrated
positive results on the performance of ReJoin®. Our
ReJoin® haMPC therapy
for KOA is an interventional therapy using our proprietary process,
culture and medium.
Our process is distinguishable from sole Stromal
Vascular Fraction (SVF) therapy. The immunophenotype of our haMPCs
exhibited a homogenous population expressing multiple biomarkers
such as CD73+, CD90+, CD105+, HLA-DR-, CD14-, CD34- and
CD45-. In contrast, SVF is merely a heterogeneous fraction
including preadipocytes, endothelial cells, smooth muscle cells,
pericytes, macrophages, fibroblasts and adipose-derived stem
cells.
In January 2016, we launched the
Allogeneic KOA Phase-I Trial
in China to evaluate the safety and efficacy of
AlloJoin®, an off-the-shelf haMPC therapy for the
treatment of KOA. On August 5, 2016, we completed patient treatment
for the Allogeneic KOA Phase-I trial, and on December 9, 2016, we
announced interim three-month safety data from the Allogenic KOA
Phase-I Trial in China. The interim analysis of the trial has
demonstrated a preliminary safety and tolerability profile of
AlloJoin® in the three doses tested, and no serious adverse
events (SAE) have been observed. On March 16, 2018, we announced a
positive 48-week AlloJoin® Phase-I data in China, which demonstrated good
safety and early efficacy for the slowing of cartilage
deterioration. China finalized its cell therapy regulatory pathway
in December 2017. Our AlloJoin® IND application to conduct a Phase-II clinical
trial with the NMPA was been approved in January 2019 and we
launched our Phase-II AlloJoin® clinical trial on September 12, 2019. On
September 27, 2019, we received the ReJoin® therapy application acceptance for Phase-II
clinical trials by the NMPA.
We established adult adipose-derived progenitor
cell and immuno-oncology cellular therapy platforms in
treating specific medical conditions and diseases. Our QMS have
been assessed and certified to meet the requirements of ISO 9001:
2015, and a quality manual based on GMP guidelines has been
finalized. The facilities, utilities and equipment in both
Zhangjiang and Wuxi Sites have been calibrated and/or qualified and
in compliance with requirements of local health authorities. We
installed an Enterprise Quality Management System (EQMS) in April
2019 to facilitate the quality activities. A document management
system and Laboratory Information Management System (LIMS) will be
installed and qualified in early 2020.
Our proprietary manufacturing processes and
procedures include (i) banking of allogenic cellular product and
intermediate product; (ii) manufacturing process of GMP-grade viral
vectors; (iii) manufacturing process of GMP-grade cellular
product; and (iv) analytical testing to ensure the safety,
identity, purity and potency of cellular products.
24
Recent Developments in Adoptive Immune Cell Therapy
(ACT)
The immune system plays an essential role in
cancer development and growth. In the past decade, immune
checkpoint blockade has demonstrated a major breakthrough in cancer
treatment and has currently been approved for the treatment of
multiple tumor types. ACT with TIL or gene-modified T-cells
expressing novel T-cell receptors (TCR) or chimeric antigen
receptors (CAR) is another strategy to modify the immune system to
recognize tumor cells and thus carry out an anti-tumor effector
function.
The TILs consist of tumor-resident T-cells
which are isolated and expanded ex vivo after surgical resection of
the tumor. Thereafter, the TILs are further expanded in a rapid
expansion protocol (REP). Before intravenous adoptive transfer into
the patient, the patient is treated with a lymphodepleting
conditioning regimen. TCR gene therapy and CAR gene therapy are ACT
with genetically modified peripheral blood T-cells. For both
treatment modalities, peripheral blood T-cells are isolated via
leukapheresis. These T-cells are then transduced by viral vectors
to either express a specific TCR or CAR. These treatments have
shown promising results in various tumor types.
Chimeric
antigen receptor T-cells (CAR-Ts)
According to the
U.S. National Cancer Institute’s 2013 cancer topics research
update on CAR-T-Cells, excitement is growing for
immunotherapy—therapies that harness the power of a
patient’s immune system to combat their disease, or what some
in the research community are calling the “fifth
pillar” of cancer treatment.
One
approach to immunotherapy involves engineering patients’ own
immune cells to recognize and attack their tumors. This approach is
called adoptive cell transfer. Adoptive cell transfer’s
building blocks are T-cell s, a type of immune cell collected from
the patient’s own blood. One of the well-established adoptive
cell transfer approaches is CAR-T cancer therapy. After collection,
the T-cells are genetically engineered to produce special receptors
on their surface called chimeric antigen receptors (CARs). CARs are
proteins that allow the T-cells to recognize a specific protein
(antigen) on tumor cells. These engineered CAR-T cells are then
grown until the number reaches dose level. The expanded population
of CAR-T cells is then infused into the patient. After the
infusion, if all goes as planned, the T-cells multiply in the
patient’s body and, with guidance from their engineered
receptor, recognize and kill cancer cells that harbor the antigen
on their surfaces. This process builds on a similar form of
adoptive cell transfer pioneered from NCI’s Surgery Branch
for patients with advanced melanoma. In 2013, NCI’s Pediatric
Oncology Branch commented that the CAR-T cells are much more potent
than anything they can achieve with other immuno-based treatments
being studied. Although investigators working in this field caution
that there is still much to learn about CAR T-cell therapy, the
early results from trials like these have generated considerable
optimism.
CAR-T
cell therapies, such as anti-CD19 CAR-T and anti-BCMA CAR-T, have
been tested in several hematological indications on patients that
are refractory/relapsing to chemotherapy, and many of them have
relapsed after stem cell transplantation. All of these patients had
limited treatment options prior to CAR-T therapy. CAR-T has shown
encouraging clinical efficacy in many of these patients, and some
of them have had durable clinical response for years. However, some
adverse effects, such as CRS and neurological toxicity, have been
observed in patients treated with CAR-T-cells. For example, in July
2016, Juno Therapeutics, Inc. reported the death of patients
enrolled in the U.S. Phase-II clinical trial of JCAR015 (anti-CD19
CAR-T) for the treatment of relapsed or refractory B-cell acute
lymphoblastic leukemia (B-ALL). The U.S. FDA put the trial on hold
and lifted the hold within a week after Juno provided a
satisfactory explanation and solution. Juno attributed the cause of
patient deaths to the use of Fludarabine preconditioning and they
switched to use only cyclophosphamide pre-conditioning in
subsequent enrollment.
In
August 2017, the U.S. FDA approved Novartis’
Kymriah®, a
CD19-targeted CAR-T therapy, for the treatment of patients up to 25
years old for relapsed or refractory (r/r) ALL, the most common
cancer in children. Current treatments show a rate of 80% remission
using intensive chemotherapy. However, there are almost no
conventional treatments to help patients who have relapsed or are
refractory to traditional treatment. Kymriah® has shown
results of complete and long lasting remission, and was the first
U.S. FDA-approved CAR-T therapy. In October 2017, the U.S. FDA
approved Kite Pharmaceuticals’ (Gilead) CAR-T therapy for
DLBCL the most common type of NHL in adults. The initial results of
axicabtagene ciloleucel (Yescarta), the prognosis of high-grade
chemo refractory NHL, is dismal with a medium survival time of a
few weeks. Yescarta is a therapy for patients who have not
responded to or who have relapsed after at least two other kinds of
treatment.
In May
2018, the U.S. FDA approved Novartis’ Kymriah® for intravenous
infusion for its second indication—the treatment of adult
patients with relapsed or refractory (r/r) large B-cell lymphoma
after two or more lines of systemic therapy including DLBCL not
otherwise specified, high grade B-cell lymphoma and DLBCL arising
from follicular lymphoma. Kymriah® is now the only
CAR-T cell therapy to receive U.S. FDA approval for two distinct
indications in non-Hodgkin lymphoma and B-cell ALL. On September
25, 2018, we entered into the Novartis LCA with Novartis to
manufacture and supply Kymriah® to Novartis in
China.
Besides
anti-CD19 CAR-T, anti-BCMA CAR-T has shown promising clinical
efficacy in treatment of multiple myeloma. For example, bb2121, a
CAR-T therapy targeting BCMA, has been developed by Bluebird bio,
Inc. and Celgene for previously treated patients with multiple
myeloma. Based on preliminary clinical data from the ongoing
Phase-I study CRB-401, bb2121 has been granted Breakthrough Therapy
Designation by the U.S. FDA and PRIME eligibility by the European
Medicines Agency (EMA) in November 2017. We plan to initiate our
anti-BCMA CAR-T investigator-initiated trial in the near
future.
Recent
progress in Universal Chimeric Antigen Receptor (UCAR) T-cells
showed benefits such as ease of use, availability and the drug
pricing challenge. Currently, most therapeutic UCAR products are
being developed with gene editing platforms such as CRISPR or
TALEN. For example, UCART19 is an allogeneic CAR T-cell product
candidate developed by Cellectis for treatment of CD19-expressing
hematological malignancies. UCART19 Phase-I clinical trials started
in adult and pediatric patients in Europe in June 2016 and in the
U.S. in 2017. The use of UCAR may has the potential to overcome the
limitation of the current autologous approach by providing an
allogeneic, frozen, “off-the-shelf” T-cell product for
cancer treatment.
Tumor
Infiltrating Lymphocytes (TILs)
While
CAR-T cell therapy has proven successful in treatment of several
hematological malignancies, other cell therapy approaches,
including TIL are being developed to treat solid tumors. For
example, Iovance Biotherapeutics is focused on the development of
autologous tumor-directed TILs for treatments of patients with
various solid tumor indications. Iovance is conducting several
Phase-II clinical trials to assess the efficacy and safety of
autologous TIL for treatment of patients with Metastatic Melanoma,
Squamous Cell Carcinoma of the Head and Neck, NSCLC and Cervical
Cancer in the U.S. and Europe.
25
T-Cell
Receptor-Engineered T-Cells (TCRs)
Adaptimmune is
partnering with GlaxoSmithKline to develop TCR-T therapy targeting
the NY-ESO-1 peptide, which is present across multiple cancer
types. Their NY-ESO SPEAR T-cell has been used in multiple
Phase-I/II clinical trials in patients with solid tumors and
haematological malignancies, including synovial sarcoma, myxoid
round cell liposarcoma, multiple myeloma, melanoma, NSCLC and
ovarian cancer. The initial data suggested positive clinical
responses and evidence of tumor reduction in patients. NY-ESO
SPEART T-cell has been granted breakthrough therapy designation by
the U.S. FDA and PRIME regulatory access in Europe.
Adaptimmune’s other TCR-T product, AFP SPEAR T-cell targeting
AFP peptide, is aimed at the treatment of patients with
hepatocellular carcinoma (HCC). AFP SPEAR T-cell is in a Phase-I
study and enrolling HCC patients in the U.S.
CBMG’s
Adoptive Immune Cell Therapy (ACT) Programs
In
December 2017, the Chinese government issued trial guidelines
concerning the development and testing of cell therapy products in
China. Although these trial guidelines are not yet codified as
mandatory regulation, we believe they provide a measure of clarity
and a preliminary regulatory pathway for our cell therapy
operations in an uncertain regulatory environment. On April 18 and
April 21, 2018, the Center for Drug Evaluation (CDE) posted on its
website acceptance of the IND application for CAR-T cancer
therapies in treating patients with NHL and adult ALL submitted by
the Company’s wholly-owned subsidiaries, CBMG Shanghai and
Shanghai Cellular Biopharmaceutical Group Ltd. On September 25,
2018 we entered into Novartis LCA to manufacture and supply
Kymriah® in China. As
part of the deal, Novartis took approximately a 9% equity stake in
CBMG, and CBMG is discontinuing development of its own anti-CD19
CAR-T cell therapy. This collaboration with Novartis reflects our
shared commitment to bringing the first marketed CAR-T cell
therapy, Kymriah® , a
transformative treatment option currently approved in the U.S., EU
and Canada for two difficult-to-treat cancers, to China, where the
number of patients in need remains the highest in the world.
Together with Novartis, we plan to bring the first CAR-T cell
therapy to patients in China as soon as possible. We continue to
develop CAR-T therapies other than CD 19 on our own and Novartis
has the first right of negotiation on these CAR-T developments. The
CBMG oncology pipeline includes CAR-T targeting CD20-, CD 19 and 20
and BCMA, AFP TCR-T, which could specifically eradicate AFP
positive HCC tumors and TIL technologies for solid tumors. Our
current priority is to collaborate with Novartis to bring
Kymriah® to China. At
the same time, we remain committed to developing our existing
pipeline of immunotherapy candidates for hematologic and solid
tumor cancers to help deliver potential new treatment options for
patients in China. We are striving to build a competitive research
and development function, a translational medicine unit, along with
a well-established cellular manufacturing capability and ample
capacity, to support Kymriah® in China and
our development of multiple assets in multiple indications. We
believe that these efforts will allow us to boost the
Company’s Immuno-Oncology presence. We have initiated
multiple clinical trials to evaluate C-CAR088 in MM, C-CAR039 in
NHL, anti-CD20 CAR-T in NHL for patients that have relapsed after
anti-CD19 CAR-T treatment, and AFP TCR-T in HCC.
Market for Immune Cell Therapies
Our
immune cell therapies involve the genetic engineering of T-cells to
express either chimeric antigen receptors, or CARs, or T-cell
receptors, or TCRs and TIL. These T-cells are designed to recognize
and attack cancer cells. Kymriah is a type of immune cell therapy
that is made from a patient’s own white blood cells and is a
prescription cancer treatment used in patients up to 25 years old
who have acute lymphoblastic leukemia that is either relapsing or
is refractory. It is also used in patients with non-Hodgkin
lymphoma that has relapsed or is refractory after having at least
two other kinds of treatment. On August 30, 2017, Kymriah was
approved by the U.S. FDA for the treatment of children and young
adults with ALL. By October 18, 2017, the U.S. FDA granted approval
for Yescarta for treating patients with relapsed/refractory DLBCL
and other rare large B-cell lymphomas. On May 1, 2018, the U.S.FDA
approved Kymriah for a second indication (diffuse large B-cell
lymphoma). In August 2018, Kymriah and Yescarta secured European
Union approval for the treatment of blood cancers, including B-cell
ALL and relapsed or refractory DLBCL. Health Canada approved
Kymriah as the first CAR-T therapy in Canada and the Therapeutic
Goods Administration (TGA) approved it as the first CAR-T therapy
in Australia.
In
2019, 1,762,450 new cancer cases and 606,880 cancer deaths are
projected to occur in the U.S. According to a 2018 International
Agency for Cancer publication, China, as the most populous country
in the world with an estimated population of nearly 1.42 billion,
is projected to have around 4.51 million cancer cases and 3.04
million cancer death by year 2020. A 2018 Global Cancer Statistics
Cancer Communications report states that compared (the 2018 Global
Cancer Statistics Report) to the U.S. and UK, China has a 30% and
40% higher cancer mortality among which 36.4% of the cancer-related
deaths were from the digestive tract cancers (stomach, liver and
esophagus cancer) and have relatively poorer
prognoses.
The 2018 Global
Cancer Statistics Report also reported that in 2018, lung cancer
was the most diagnosed cancer type worldwide and in China with
2,093,8761 and
733,3002 new cases
respectively. HCC is the 4th most common cancer in China and more
than 50% of new HCC cases world-wide are in China. About 466,000
new liver cancer cases each year and the mortality is around
343,7003 annually in
China. In 2018, it was estimated about 510,000 new case of NHL and
248,724 patients died from NHL worldwide4.
Multiple
myeloma accounts for 1% of all cancers and approximately 10% of all
hematological malignancies5. In 2016
there were about 138,509 incident cases worldwide. The United
States had the most cases (about 24,407) and the most deaths (about
14,212), China was the second in both measures which incident cases
were about 16,537 and deaths about 10,363. The global incidence of
multiple myeloma rose by 126% from 1990 to 2016. East Asia (China,
North Korea, and Taiwan) saw incident cases of multiple myeloma
jump by 262%, which was the largest increase among any of the 21
global regions6.
_______________
1 Chen et al. CA Cancer J Clin.
2016; 66:155-132
2 Bray F et al. CA Cancer J Clin.
2018: 68:394-424
3 Chen et al. CA Cancer J Clin.
2016; 66:155-132
4 Bray F et al. CA Cancer J Clin.
2018: 68:394-424
5 Moreau P et al., Annals of Oncol.
24 (Supplement 6): vi133–vi137, 2013)
6 Cowan AJ et al., JAMA
Oncol. 2018;4(9):1221-1227
26
Market for Stem Cell-Based Therapies
The
U.S. forecast is that shipments of treatments with stem cells, or
instruments which concentrate stem cell preparations for injection
into painful joints, will fuel an overall increase in the use of
stem cell based treatments resulting in an increase to $5.7 billion
in 2020, with key growth areas being Spinal Fusion, Sports Medicine
and Osteoarthritis of the joints. Osteoarthritis (OA) is a chronic
disease that is characterized by degeneration of the articular
cartilage, hyperosteogeny and, ultimately, joint destruction that
can affect all of the joints. According to a paper published by
Dillon CF, Rasch EK, Gu Q et al. entitled, “Prevalence of
knee osteoarthritis in the United States: Arthritis Data from the
Third National Health and Nutrition Examination Survey,” the
incidence of OA is 50% among people over age 60 and 90% among
people over age 65. KOA accounts for the majority of total OA
conditions and in adults, OA is the second leading cause of work
disability and the disability incidence rate is high (53%). The
costs of OA management has grown exponentially over recent decades,
accounting for up to 1% to 2.5% of the gross national product of
countries with aging populations, including the U.S., Canada, the
UK, France and Australia. According to the American Academy of
Orthopedic Surgeons (AAOS), the only pharmacologic therapies
recommended for OA symptom management are non-steroidal
anti-inflammatory drugs (NSAIDs) and tramadol (for patients with
symptomatic osteoarthritis). Moreover, there is no approved disease
modification therapy for OA in the world. Disease progression is a
leading cause of hospitalization and ultimately requires joint
replacement surgery. According to an article published by the
Journal of the American Medical Association, approximately 505,000
hip replacements and 723,000 knee replacements were performed in
the United States in 2014, collectively costing more than $20
billion. International regulatory guidelines on clinical
investigation of medicinal products used in the treatment of OA
were updated in 2015, and clinical benefits (or trial outcomes) of
a disease modification therapy for KOA has been well defined and
recommended. Medicinal products used in the treatment of
osteoarthritis need to provide both a symptom relief effect for at
least six months and a structure modification effect to slow
cartilage degradation by at least 12 months. Symptom relief is
generally measured by a composite questionnaire, the Western
Ontario and McMaster Universities Osteoarthritis Index (WOMAC)
score, and structure modification is measured by MRI, or
radiographic image as accepted by international communities. The
Company uses the WOMAC as the primary end point to demonstrate
symptom relief, and MRI to assess structure and regeneration
benefits as a secondary endpoint.
According
to the Foundation for the National Institutes of Health, there are
27 million Americans with OA, and symptomatic KOA occurs in 13% of
persons aged 60 and older. According to a nationwide
population-based longitudinal survey among the Chinese retired
population, approximately 8.1% of participants were found to suffer
from symptomatic knee OA. Currently no treatment exists that can
effectively preserve knee joint cartilage or slow the progression
of KOA.
According
to Alternative and Integrative Medicine, 53% of KOA patients will
degenerate to the point of disability. Conventional treatment
usually involves invasive surgery with painful recovery and
physical therapy and replacement surgeries are typically only
suggested and performed on patients in the late stage of
KOA.
Our Global Strategy
CBMG is
a drug development company focusing on developing cell therapies
first in China, to take advantage of cost efficiencies, leveraging
the expeditious IIT process in China, publish and share our data in
major conferences and scientific journals and then address the
rest-of-the-world market after safety and efficacy of those
programs are established. Our goal is to develop safe and effective
cellular therapies for indications that represent a large unmet
need in China. We intend to use our first-mover advantage in China,
against a backdrop of enhanced regulation by the central
government, to differentiate ourselves from the competition and
establish a leading position in the China cell therapeutic market.
We intend to invest and expand our clinical research capabilities
by building drug development and manufacturing infrastructure in
China and in the U.S., expanding our clinical research platform,
hiring new talent and enhancing our existing coverage. We believe
that few competitors in China are as well-equipped as we are in the
areas of clinical trial development, internationally compliant
manufacturing, quality assurance and control, as well as our
dedication to regulatory compliance and process
improvement.
The key
issues with cell therapy as modality are drug therapeutic index,
institutionalized, scalable manufacturing and an affordable price
for the patients. We believe our manufacturing platform is unique
as we utilize a semiautomatic, fully closed system, which is
expected to lead to economies of scale. Additionally, our focus on
being a fully integrated cell therapy company has enabled us to be
one of only a few companies that are able to manufacture clinical
grade viral vectors in China to cater to the increasing global
demand for cell and gene therapies.
In
China, Good Clinical Practice (GCP) only requires institutional
review board (“IRB”) approval from the hospital and
local NMPA approval for IIT, which is more expeditious than the
traditional IND route. IITs can provide early evidence of POC for
novel drugs which are more time and cost efficient than the
traditional IND approach. IITs are also good ways to identify and
develop novel platforms. Currently, we have our own drug
development pipeline in CAR-T, AFP TCR-T, TIL and KOA. Our R&D
team continues to identify additional platform cell therapy
technologies to develop internally or acquire established
technologies.
In
addition to the manufacturing of Novartis’
Kymriah®
for patients in China as contemplated by the Novartis LCA and the
Manufacture and Supply Agreement with Novartis, we are actively
developing and evaluating other therapies comprised of other CAR-T,
TCR-T and TIL therapies. We have also advanced our KOA
AlloJoin® Phase-II
clinical trial and ReJoin® Phase-II
clinical trial with the NMPA.
In
addition to our drug development efforts, we are planning on
evaluating co-development, strategic partnerships and both
in-licensing and out-licensing opportunities with high quality,
multinational partners. Such partnerships will enable us to take
advantage of the technologies of our partners while leveraging our
quality control and manufacturing infrastructure to further expand
our pipelines.
Our
proprietary and patent-protected manufacturing processes enable us
to produce, store and distribute ancillary media, viral vectors and
cellular product. Our clinical protocols include medical assessment
to qualify each patient for treatment, evaluation of each patient
before and after a specific therapy, cell infusion methodologies
including dosage, frequency and the use of adjunct therapies,
handling potential adverse effects and their proper management.
Applying our proprietary intellectual property, we plan to
customize specialize formulations to address complex diseases and
debilitating conditions.
27
Currently,
we have a total of approximately 70,000 square feet of
manufacturing space in three locations, the majority of which is in
the new Shanghai facility. We operate our manufacturing facilities
under the design of the standard GMP conditions as well ISO
standards. We employ institutionalized and proprietary process and
quality management system to optimize reproducibility and to hone
our efficiency. Our Shanghai and Wuxi facilities are designed and
built to meet GMP standards. With our integrated Plasmid, Viral
Vectors platforms, our T-cells manufacturing capacities are highly
distinguishable from other companies in the cellular therapy
industry. We are currently assessing the feasibility of expanding
manufacturing spaces in new sites in both China and the
U.S.
Most
importantly, our seasoned cell therapy team members have decades of
highly relevant experience in the U.S., China and the European
Union. We believe that these are the primary factors that make CBMG
a high-quality cell products manufacturer in China. We have been
implementing significant human resources initiatives such as stock
incentive programs, graduate school and continuing education
sponsorship and a robust health insurance plan to attract and
retain quality talent to support our rapid growth.
Our Targeted Indications and Potential Therapies
The
chart below illustrates CBMG’s pipelines:
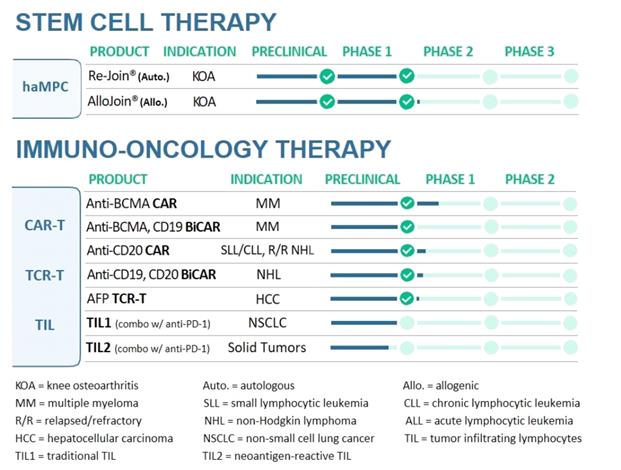
28
Immuno-oncology (I/o)
Our
CAR-T platform is built on lenti-virial vector and
second-generation CAR design, which is used by most of the current
trials and studies. We select the patient population for each asset
and indication to allow the optimal path forward for potential
regulatory approval. We integrate the state-of-the-art
translational medicine effort into each clinical study to aid in
dose selection, to investigate the mechanism of action and POC, and
to attempt to identify the optimal targeting patient population. We
plan to continue to grow our translational medicine team and engage
key opinion leaders to support our development
efforts.
We have
developed a serial of CAR-Ts to treat hematological malignancies
including CD20, CD22 and BCMA CAR-Ts, which have been proved to be
potent and effective in treating hematology tumors in the early
phase of clinical studies.
CD20 CAR
CD20 is
broadly overexpressed in a serial of B-cell malignant tumors. In
the patients relapsed after CD19 CAR-T treatment, the expression of
CD20 on target tumor cells is relatively stable. It is proven to be
an optimal target for treating CD19 CAR-T relapsing patients. We
have developed a novel CD20 CAR-Ts clinical lead product, which
demonstrated strong anti-tumor activity in both in vitro assays and
in vivo animal studies. We have filed a patent application in China
and have initiated a first in human investigator initiated trial
with CD19 CAR-T relapsed NHL patients.
CD22 CAR
CD22 is
another surface marker highly expressed in B-cell malignancies
especially in hairy cell leukemia. It also expresses in the
patients relapsed after CD19 CAR-T treatment. We have developed a
novel CD22 CARs clinical lead, which displayed effective anti-tumor
activity in in vitro cytotoxicity assays. We plan to initiate an
investigator initiated trial with CD19 CAR-T relapsing ALL patients
and hairy cell leukemia.
BCMA CAR
BCMA is
a member of the TNF receptor superfamily, universally expressed in
MM cells. It is not detectable in normal tissues except plasma and
mature B cells. It is a proven, effective and safer target for
treating refractory MM patients in several clinical trials. We have
developed unique BCMA CARs. Our BCMA CAR clinical lead exhibits
potent anti-tumor activity both in vitro and in vivo. We have filed
a patent for BCMA CAR in China and begun an investigator-initiated
trial in refractory MM patients in January 2019.
AFP TCR
We
license the AFP-TCR technology from Augusta University. We are
continuing our evaluation on the efficacy and specificity of the
AFP TCRs to identity the most appropriate candidate for a first
time in human (FTIH) study. We plan to redirect Human T-cells with
the AFP TCRs and evaluate their anti-tumor activity on in vitro
cytokine release and cytotoxicity assays; and potential
on/off-target toxicity including allo-reactivity as well as in vivo
efficacy tests in animal models.
NKG2D CAR
To
optimize our clinical development with limited resources amid the
COVID-19 pandemic environment, we have prioritized other clinical
assets over the NKG2D development.
TIL
Augmented
by the NCI technology license, CBMG is developing neoantigen
reactive TIL therapies to treat immunogenic cancers. In the early
stages of cancer, lymphocytes infiltrate into the tumor,
specifically recognizing the tumor targets and mediating anti-tumor
response. These cells are known as TIL. TIL-based therapies have
shown encouraging clinical results in early development. For
example, in Phase-II clinical studies in patients with metastatic
melanoma performed by Dr. Rosenberg, TIL therapy demonstrated
robust efficacy in patients with metastatic melanoma with objective
response rates of 56% and complete response rates of 24%. We have
started our development with NSCLC, and plan to expand into other
cancer indications.
Knee Osteoarthritis (KOA)
We are
currently pursuing two primary therapies for the treatment of KOA:
ReJoin® therapy and
AlloJoin®
therapy.
29
We
completed the Phase-I/IIa clinical trial for the treatment of KOA.
The trial tested the safety and efficacy of intra-articular
injections of autologous haMPCs in order to reduce inflammation and
repair damaged joint cartilage. The six-month follow-up clinical
data showed ReJoin® therapy to be
both safe and effective.
In the
second quarter of 2014, we completed patient enrollment for the
Phase-IIb clinical trial of ReJoin® for KOA. The
multi-center study has enrolled 53 patients to participate in a
randomized, single blind trial. We published 48 weeks’
follow-up data of Phase-I/IIa on December 5, 2014. The 48
weeks’ data indicated that patients have reported a decrease
in pain and a significant improvement in mobility and flexibility,
while the clinical data shows our ReJoin® regenerative
medicine treatment to be safe. We announced positive Phase-IIb
48-week follow-up data in January 2016, with statistically
significant evidence that ReJoin® enhanced
cartilage regeneration, which concluded the planned Phase-IIb
trial.
Osteoarthritis
is a degenerative disease of the joints. KOA is one of the most
common types of osteoarthritis. Pathological manifestation of
osteoarthritis is primarily local inflammation caused by immune
response and subsequent damage of joints. Restoration of immune
response and joint tissues are the objective of
therapies.
According
to International Journal of Rheumatic Diseases, 2011, 53% of KOA
patients will degenerate to the point of disability. Conventional
treatment usually involves invasive surgery with painful recovery
and physical therapy. As drug-based methods of management are
ineffective, the same journal estimates that some 1.5 million
patients with this disability will degenerate to the point of
requiring artificial joint replacement surgery every year. However,
only 40,000 patients will actually be able to undergo replacement
surgery, leaving the majority of patients to suffer from a
life-long disability due to lack of effective
treatment.
Adult
mesenchymal stem cells can currently be isolated from a variety of
adult human sources, such as liver, bone marrow and adipose (fat)
tissue. We believe the advantages in using adipose tissue (as
opposed to bone marrow or blood) are that it is one of the richest
sources of multipotent cells in the body, the easy and repeatable
access to fat via liposuction, and the simple cell isolation
procedures that can begin to take place even on-site with minor
equipment needs. The procedure we are testing for autologous KOA
involves extracting a very small amount of fat using a minimally
invasive extraction process which takes up to 20 minutes and leaves
no scarring. The haMPC cells are then processed and isolated on
site, and injected intraarticularly into the knee joint with
ultrasound guidance. For allogeneic KOA, we use donor haMPC
cells.
These
haMPC cells are capable of differentiating into bone, cartilage and
fat under the right conditions. As such, haMPCs are an attractive
focus for medical research and clinical development. Importantly,
we believe both allogeneic and autologously sourced haMPCs may be
used in the treatment of disease. Numerous studies have provided
preclinical data that support the safety and efficacy of allogeneic
and autologous haMPC, offering a choice for those where factors
such as donor age and health are an issue.
The
haMPCs are currently being considered as a new and effective
treatment for osteoarthritis, with a huge potential market.
Osteoarthritis is one of the ten most disabling diseases in
developed countries. Worldwide estimates are that 9.6% of men and
18.0% of women aged over 60 years have symptomatic osteoarthritis.
It is estimated that the global OA therapeutics market was worth
$4.4 billion in 2010 and is forecast to grow at a compound annual
growth rate of 3.8% to reach $5.9 billion by 2018.
In
order to bring haMPC-based KOA therapy to market, our market
strategy is to: (a) establish regional laboratories that comply
with cGMP standards in Shanghai and Beijing that meet Chinese
regulatory approval; (b) submit to the NMPA an IND package for
Allojoin™ to treat patients with donor haMPC cells; and (c)
file joint applications with Class AAA hospitals to use
ReJoin® to treat
patients with their own haMPC cells.
Our
competitors are pursuing treatments for osteoarthritis with knee
cartilage implants. However, unlike their approach, our KOA therapy
is not surgically invasive—it uses a small amount (30ml) of
adipose tissue obtained via liposuction from the patient, which is
cultured and re-injected into the patient. The injections are
designed to induce the body’s secretion of growth factors
promoting immune response and regulation, and regrowth of
cartilage. The down-regulation of the patient’s immune
response is aimed at reducing and controlling inflammation which is
a central cause of KOA.
We
believe our proprietary method, subsequent haMPC proliferation and
processing know-how will enable haMPC therapy to be a low cost and
relatively safe and effective treatment for KOA. Additionally,
banked haMPCs can continue to be stored for additional use in the
future.
Based
on current estimates, we expect to generate collaboration payment
and revenues through our sale of Kymriah® products to
Novartis within the next two years. We plan to systematically
advance our own cell therapy pipeline and timely seek BLA
opportunities to commercialize our products within the next three
years although we cannot assure you that we will be successful at
all or within the foregoing timeframe.
Competition
Many
companies operate in the cellular biopharmaceutical field. We face
competition based on several factors, including quality and breadth
of services, ability to protect our intellectual property or other
confidential information, timeliness of implementation, maintenance
of quality standards, depth of collaboration partner relationships,
price and geography. Currently there are several approved stem cell
therapies on the market including Canada’s pediatric
graft-versus-host disease and the European Commission’s
approval in March 2018 for the treatment of complex perianal
fistulas in adult Crohn’s disease. There are several public
and private cellular biopharmaceutical-focused companies outside of
China with varying phases of clinical trials addressing a variety
of diseases. We compete with these companies in bringing cellular
therapies to the market. However, our focus is to develop a core
business in the China market, with plans to expand in the U.S.
market. This difference in focus places us in a different
competitive environment from other western companies with respect
to fund raising, clinical trials, collaborative partnerships and
the markets in which we compete.
In
terms of entry barriers, the cellular biopharmaceutical business
generally requires high, upfront capital and other resources,
significant financial and time commitment in recruiting experienced
talents, a successful track record and solid reputation to build up
synergies with business partners and emphasis on cost efficiency.
Our core competitive edge is our strong capacity to cover the full
research and development process of the full life cycle of a
product, and to satisfy the increasing demand for timely
realization and localization in China of key products already
approved in foreign markets. We believe that we are able to
maintain our competitiveness by leveraging our established position
in global research and development in the cellular
biopharmaceutical market and capitalizing on the opportunities
offered by the booming pharmaceutical market in China.
30
To meet
the overall social, economic and healthcare challenges in China,
the PRC central government has a focused strategy to enable China
to compete effectively in certain designated areas of biotechnology
and the health sciences. Because of the aging population in China,
China’s Ministry of Science and Technology (MOST) has
targeted stem cell development as high priority field, and
development in this field has been intense in the agencies under
MOST. For example, the 973 Program has funded a number of stem cell
research projects such as differentiation of human embryonic stem
cells and the plasticity of adult stem cells. To the best of our
knowledge, none of the companies in China are utilizing our
proposed international manufacturing protocol and our unique
technologies in conducting what we believe will be fully compliant
NMPA-sanctioned clinical trials to commercialize cell therapies in
China. Our management believes that it is difficult for most of
these Chinese companies to turn their results into translational
stem cell science or commercially successful therapeutic products
using internationally acceptable standards.
We
compete globally with respect to the discovery and development of
new cell-based therapies, and we also compete within China to bring
new therapies to market. In the biopharmaceutical specialty
segment, namely in the areas of cell processing and manufacturing,
clinical development of cellular therapies and cell collection,
processing and storage, are characterized by rapidly evolving
technology and intense competition. Our competitors worldwide
include pharmaceutical, biopharmaceutical and biotechnology
companies, as well as numerous academic and research institutions
and government agencies engaged in drug discovery activities or
funding, in the U.S., Europe and Asia. Many of these companies are
well-established and possess technical, research and development,
financial and sales and marketing resources significantly greater
than ours. In addition, many of our smaller potential competitors
have formed strategic collaborations, partnerships and other types
of joint ventures with larger, well established industry
competitors that afford these companies potential research and
development and commercialization advantages in the technology and
therapeutic areas currently being pursued by us. Academic
institutions, governmental agencies and other public and private
research organizations are also conducting and financing research
activities which may produce products directly competitive to those
being commercialized by us. Moreover, many of these competitors may
be able to obtain patent protection, obtain government (e.g., the
U.S. FDA) and other regulatory approvals and begin commercial sales
of their products before us.
Our
primary competitors in the field of cancer immune cell therapies
include pharmaceutical, biotechnology companies such as Eureka
Therapeutics, Inc., Iovance Biotherapeutics Inc., Juno
Therapeutics, Inc. (acquired by Celgene), Kite Pharma, Inc.
(acquired by Gilead), CARSgen, Sorrento Therapeutics, Inc. and
others. Among our competitors, the ones based in and operating in
Greater China are CARsgen, Hrain Biotechnology, Nanjing Legend
Biotechnology Galaxy Biomed, Persongen, Anke Biotechnology,
Shanghai Minju Biotechnology, Unicar Therapy (Cooperated with
Terumo BCT), Wuxi Biologics, Junshi Pharma, BeiGene, Immuno China
Biotech, Chongqing Precision Biotech, Innovative Cellular
Therapeutics and China Oncology Focus Limited. Other companies in
the cancer immune cell therapies space have made inroads in China
by partnering with local companies. For example, in April, 2016,
Seattle-based Juno Therapeutics, Inc. started a new company with
WuXi AppTec in China named JW Biotechnology (Shanghai) Co., Ltd. In
January 2017, Shanghai Fosun Pharmaceutical created a joint venture
with Santa Monica-based Kite Pharma Inc. to develop, manufacture
and commercialize CAR-T and TCR products in China. The NMPA has
received IND applications for CD19 chimeric antigen receptor
T-cells cancer therapies from many companies and have granted the
initial phase of acceptance to several companies thus
far.
The
osteoarthritis industry is highly competitive and subject to rapid
and significant technological change. The large size and expanding
scope of the pain market makes it an attractive therapeutic area
for biopharmaceutical businesses. Our potential competitors include
pharmaceutical, biotechnology, medical device and specialty
pharmaceutical companies. Several of these companies have robust
drug pipelines, readily available capital and established research
and development organizations. We believe our success will be
driven by our ability to develop and commercialize treatment
options that make a meaningful difference for patients with KOA.
Our primary competitors in the field of stem cell therapy for
osteoarthritis and other indications include Mesoblast Ltd.,
Caladrius Biosciences, Inc. and others. On September 12, 2019, we
launched allogenic haMPC KOA Phase-II of the clinical trial across
six leading hospitals in China with a plan to recruit 108 patients.
We submitted our autologous adipose stem cell therapy
(ReJoin® ) KOA with IND
filing with the CDE and the application was approved by NMPA.
Additionally, in the general area of cell-based therapies for knee
osteoarthritis ailments, we potentially compete with a variety of
companies, from big pharma to specialty medical products or
biotechnology companies. Some of these companies, such as Abbvie,
Merck KGaA, Sanofi, Teva, GlaxosmithKline, Baxter, Johnson &
Johnson, Sanumed, Medtronic and Miltenyi Biotech are
well-established and have substantial technical and financial
resources compared to ours. However, as cell-based products are
only just recently emerging as viable medical therapies, many of
our more direct competitors are smaller biotechnology and specialty
medical products companies comprised of Vericel Corporation,
Regeneus Ltd., Advanced Cell Technology, Inc., Nuo Therapeutics,
Inc., ISTO technologies, Inc., Ember Therapeutics, Athersys, Inc.,
Bioheart, Inc., Mesoblast, Pluristem, Inc., Medipost Co. Ltd. and
others. There are also several non-cell-based, small molecule and
peptide clinical trials targeting knee osteoarthritis, and several
other U.S. FDA-approved treatments for knee pain.
Other
companies have OA product candidates in advanced stages of clinical
development. These product candidates include:
●
Anika Therapeutics,
Inc.’s Cingal®, which is a
mixture of Anika’s Monovisc combined with a low dose of a
commonly used immediate-release steroid. In February 2019, Anika
announced that, based on their discussions with the U.S. FDA, they
will need to conduct another Phase-III clinical trial before they
can potentially obtain approval for Cingal in the U.S.
●
Kolon TissueGene,
Inc.’s Invossa™, which is a combination of human
allogeneic chondrocytes and TGF-b1 transfected allogeneic
chondrocytes. In November 2018, Kolon TissueGene announced they
enrolled the first patient in a pivotal U.S. Phase-III trial.
According to clinicaltrials.gov, the estimated primary completion
date for the trial is April 2021.
●
Ampio
Pharmaceuticals, Inc.’s Ampion™, which is a derivative
of human serum albumin, is described as having anti-inflammatory
properties, and is formulated for immediate-release. Ampio stated
that Ampion is in Phase-III development but has not announced a
timeline for potentially submitting a Biologics License
Application, or BLA.
●
Centrexion
Therapeutics Corporation’s CNTX-4975, which is a synthetic,
ultra-pure injection of trans-capsaicin. In December 2018,
Centrexion announced completion of patient enrollment in its
Phase-III VICTORY-1 trial. Topline results are expected to be
reported in the first quarter of 2020.
●
A number of
investigational nerve growth factor antibodies are in development.
Regeneron’s fasinumab and Pfizer and Eli Lilly’s
tanezumab are both in Phase-III development. Initial results from
Phase-III clinical trials for each were announced in 2018. In
January 2019, Pfizer and Lilly announced results from a second
Phase-III study showing that the tanezumab 5 mg treatment arm met
all three co-primary endpoints at 24 weeks, however in the 2.5 mg
treatment arm, patients’ overall assessment of their OA was
not statistically different than placebo. Rapidly progressive OA
was seen in 2.1% of tanezumab-treated patients and was not observed
in the placebo arm.
●
Servier and
Galapagos NV’s S201086/GLPG1972, an ADAMTS-5 inhibitor, is
currently in Phase-II clinical development.
●
Taiwan Liposome
Company’s TLC599, which is a liposomal formulation of
dexamethasone sodium phosphate. TLC599 is currently in Phase-II
clinical development.
31
Certain
CBMG competitors also work with adipose-derived stem cells. To the
best of our knowledge, none of these companies are currently
utilizing the same technologies as ours to treat KOA, nor are we
aware of any of these companies conducting government-approved
clinical trials in China.
Some of
our targeted disease applications may compete with drugs from
traditional pharmaceutical or Traditional Chinese Medicine
companies. We do not believe that our chosen targeted disease
applications are in competition with the products and therapies
offered by traditional pharmaceutical or Traditional Chinese
Medicine companies.
We
believe we have a strategic advantage over our competitors based on
our outstanding quality management system and robust and efficient
manufacturing capability, which we believe is possessed by few, if
any, of our competitors in China, in an industry in which meeting
exacting standards and achieving extremely high purity levels is
crucial to success. In addition, in comparison to the broader range
of cellular biopharmaceutical firms, we believe we have the
advantages of cost and expediency, and a first mover advantage with
respect to commercialization of cell therapy products and
treatments in the China market.
Critical Accounting Policies and Estimates
The
discussion and analysis of our financial condition and results of
operations are based on our consolidated financial statements,
which have been prepared in accordance with accounting principles
generally accepted in the United States of America (“U.S.
GAAP”). The preparation of these financial statements
requires management to make estimates and assumptions that affect
the reported amounts of assets and liabilities and disclosure of
contingent assets and liabilities at the date of the consolidated
financial statements and the reported amounts of revenue and
expenses during the reporting period. On an ongoing basis, our
management evaluates the estimates, including those related to
revenue recognition, accounts receivable, long-lived assets,
goodwill and other intangibles, investments, stock-based
compensation, and income taxes. Of the accounting estimates we
routinely make relating to our critical accounting policies, those
estimates made in the process of determining the valuation of
accounts receivable, long-lived assets, and goodwill and other
intangibles, measuring share-based compensation expense, preparing
investment valuations, and establishing income tax valuation
allowances and liabilities are the estimates most likely to have a
material impact on our financial position and results of
operations. The Company bases its estimates on historical
experience and on various other assumptions that are believed to be
reasonable under the circumstances. However, because these
estimates inherently involve judgments and uncertainties, there can
be no assurance that actual results will not differ materially from
those estimates.
During
the three months ended March 31, 2020, we believe that there have
been no significant changes to the items that we disclosed as our
critical accounting policies and estimates in the “Critical
Accounting Policies and Estimates” section of Item 7 -
Management’s Discussion and Analysis of Financial Condition
and Results of Operations in our Annual Report on Form 10-K for the
fiscal year ended December 31, 2019.
32
Results of
Operations
Below is a discussion
of
the results of our operations for the
three months ended March 31, 2020 and 2019. These results are
not necessarily indicative of result that may be expected in any
future period. Our prospects should be considered in light of the
risks, expenses and difficulties that we may encounter. We may not
be successful in addressing these risks and
difficulties.
Comparison of Three Months Ended March 31, 2020 to Three Months
Ended March 31, 2019
The
descriptions in the results of operations below reflect our
operating results as set forth in our Condensed Consolidated
Statement of Operations filed herewith.
|
|
Three Months
Ended
March 31,
2020
|
Three Months
Ended
March 31,
2019
|
|
Net sales and
revenue
|
$-
|
$49,265
|
|
|
|
|
|
Operating
expenses:
|
|
|
|
Cost of
sales
|
-
|
8,087
|
|
General and
administrative
|
3,431,344
|
3,447,734
|
|
Selling and
marketing
|
-
|
42,260
|
|
Research and
development
|
7,759,358
|
5,968,096
|
|
Impairment of
investments
|
240,000
|
-
|
|
Total
operating expenses
|
11,430,702
|
9,466,177
|
|
Operating
loss
|
(11,430,702)
|
(9,416,912)
|
|
|
|
|
|
Other (expense)
income
|
|
|
|
Interest income,
net
|
12,772
|
97,034
|
|
Other expense,
net
|
(127,792)
|
(14,510)
|
|
Total
other (expense) income
|
(115,020)
|
82,524
|
|
Loss before
taxes
|
(11,545,722)
|
(9,334,388)
|
|
|
|
|
|
Income taxes
provision
|
(1,775)
|
(2,400)
|
|
|
|
|
|
Net
loss
|
$(11,547,497)
|
$(9,336,788)
|
|
Other comprehensive
income:
|
|
|
|
Cumulative
translation adjustment
|
(436,813)
|
396,126
|
|
Total other
comprehensive income:
|
(436,813)
|
396,126
|
|
Comprehensive
loss
|
$(11,984,310)
|
$(8,940,662)
|
|
|
|
|
|
|
|
|
|
Net loss per
share:
|
|
|
|
Basic
and diluted
|
$(0.60)
|
$(0.51)
|
|
|
|
|
|
Weighted average
common shares outstanding:
|
|
|
|
Basic
and diluted
|
19,340,982
|
18,152,429
|
* These line items include the following amounts of non-cash,
stock-based compensation expense for the periods
indicated:
|
|
Three Months
Ended
March 31,
2020
|
Three Months
Ended
March 31,
2019
|
|
General and
administrative
|
452,100
|
566,592
|
|
Selling and
marketing
|
-
|
9,816
|
|
Research and
development
|
483,962
|
548,154
|
|
|
936,062
|
1,124,562
|
33
Results of Operations
Net sales and revenue
|
|
2020
|
2019
|
Change
|
Percent
|
|
For the three
months ended March 31,
|
$-
|
$49,265
|
$(49,265)
|
(100)%
|
We are
a clinical stage company, and currently have no material revenues
or other income with similar effect.
Cost of Sales
|
|
2020
|
2019
|
Change
|
Percent
|
|
For the three
months ended March 31,
|
$-
|
$8,087
|
$(8,087)
|
(100)%
|
The
gross margin change was immaterial as currently we have no material
revenues.
General and Administrative Expenses
|
|
2020
|
2019
|
Change
|
Percent
|
|
For the three
months ended March 31,
|
$3,431,344
|
$3,447,734
|
$(16,390)
|
0%
|
No material change as compared with the period ended March
31, 2019. General and Administrative expenses primarily relate to
administrative expenses, professional fees and
depreciation.
Selling and Marketing Expenses
|
|
2020
|
2019
|
Change
|
Percent
|
|
For the three
months ended March 31,
|
$-
|
$42,260
|
$(42,260)
|
(100)%
|
There
was no sales force in 2020 and no expense incurred in first quarter
2020.
34
Research and Development Expenses
|
|
2020
|
2019
|
Change
|
Percent
|
|
For the three
months ended March 31,
|
$7,759,358
|
$5,968,096
|
$1,791,262
|
30%
|
Research
and development costs increased by approximately $1,791,000 in the
three months ended March 31, 2020 as compared to the three months
ended March 31, 2019, primarily as a result of the increase in the
staff costs of $593,000, clinical trial expenses of $620,000, and
raw material consumption of $203,000. The increase was primarily
attributed to the increased spending in the growth of our pipeline
in both liquid tumor and solid tumor development and expanding the
U.S. R&D operations at Maryland.
R&D
expenses for the three months ended March 31, 2020 and 2019 are as
follows:
|
|
For the Three
Months Ended
|
|
|
|
March
31,
|
|
|
|
2020
|
2019
|
|
Research and
pre-clinical studies
|
$2,190,626
|
$1,532,986
|
|
Development,
clinical development and studies
|
5,568,732
|
4,435,110
|
|
|
|
|
|
Total
|
$7,759,358
|
$5,968,096
|
Impairment of investments
|
|
2020
|
2019
|
Change
|
Percent
|
|
For the three
months ended March 31,
|
$240,000
|
$-
|
$240,000
|
N/A
|
The
impairment of investments for the three months ended March 31, 2020
is comprised of the recognition of other-than-temporary impairment
on the value of shares in investments of $240,000. No such expense
existed for the period ended March 31, 2019. In 2020, the Company
contacted certain brokers to handle our ARPC restricted legend
removal from the stock certificates to convert to free-trade
shares. Because of ARPC’s non-filing status and illiquid
nature of the stock, the brokers’ compliance department
summarily rejected our request. Considering the serious doubt over
the liquidity of the ARPC stock, full impairment was made over ARPC
stock in first quarter 2020.
Operating Loss
|
|
2020
|
2019
|
Change
|
Percent
|
|
For the three
months ended March 31,
|
$(11,430,702)
|
$(9,416,912)
|
$(2,013,790)
|
21%
|
The
increase in the operating loss for the three months ended March 31,
2020 as compared to the same period in 2019 is primarily due to
changes in research and development expenses and impairment of
non-current assets, each of which is described above.
Total Other (Expense) Income
|
|
2020
|
2019
|
Change
|
Percent
|
|
For the three
months ended March 31,
|
$(115,020)
|
$82,524
|
$(197,544)
|
(239)%
|
Other
expense for the three months ended March 31, 2020 was primarily
interest expense of $193,000 and foreign currency exchange loss of
$52,000, netting of subsidy income of $116,000 and interest income
of $13,000. Other income for the three months ended March 31, 2019
was primarily net interest income of $97,000, netting of foreign
currency exchange loss of $14,000.
35
Income Taxes Provision
|
|
2020
|
2019
|
Change
|
Percent
|
|
For the three
months ended March 31,
|
$(1,775)
|
$(2,400)
|
$625
|
(26)%
|
While
we have optimistic plans for our business strategy, we determined
that a valuation allowance was necessary given the current and
expected near term losses and the uncertainty with respect to our
ability to generate sufficient profits from our business model.
Therefore, we established a valuation allowance for deferred tax
assets other than the extent of the benefit from other
comprehensive income. Income tax expense for three months ended
March 31, 2020 and 2019 all represent US state tax.
Net Loss
|
|
2020
|
2019
|
Change
|
Percent
|
|
For the three
months ended March 31,
|
$(11,547,497)
|
$(9,336,788)
|
$(2,210,709)
|
24%
|
Changes
in net loss are primarily attributable to changes in operations
which are described above.
Comprehensive Loss
|
|
2020
|
2019
|
Change
|
Percent
|
|
For the three
months ended March 31,
|
$(11,984,310)
|
$(8,940,662)
|
$(3,043,648)
|
34%
|
Comprehensive
loss for the three months ended March 31, 2020 and 2019 includes a
currency translation net (loss) gain of approximately ($437,000)
and $396,000 combined with the changes in net loss,
respectively.
Liquidity and Capital Resources
We had
working capital of $25,837 as of March 31, 2020 compared to
$10,356,774 as of December 31, 2019. Our cash, cash equivalents and
restricted cash decreased to $21,597,360 at March 31, 2020 compared
to $32,443,649 at December 31, 2019, as we had an increase in cash
used in operating and investing activities partially offset by cash
inflow generated from proceeds from option exercise.
Net
cash provided by or used in operating, investing and financing
activities from continuing operations was as follows:
Net
cash used in operating activities was approximately $9,295,000 and
$7,936,000 for the three months ended March 31, 2020 and 2019,
respectively. The following table reconciles net loss to net cash
used in operating activities:
|
For the three
months ended March 31,
|
2020
|
2019
|
Change
|
|
Net
loss
|
$(11,547,497)
|
$(9,336,788)
|
$(2,210,709)
|
|
Non cash
transactions
|
2,769,140
|
2,454,238
|
314,902
|
|
Changes in
operating assets, net
|
(516,205)
|
(1,053,144)
|
536,939
|
|
Net cash used in
operating activities
|
$(9,294,562)
|
$(7,935,694)
|
$(1,358,868)
|
36
The
change in non-cash transaction was primarily due to the increase in
impairment on investment of $240,000 as well as the increase in
depreciation and amortization of $263,000 compared with same period
in 2019.
Net
cash used in investing activities was approximately $1,634,000 and
$4,164,000 in the three months ended March 31, 2020 and 2019,
respectively. The increase was primarily the result of
additional new equipment and facility improvement.
Cash
provided by financing activities was approximately $166,000 and
$21,240,000 in the three months ended March 31, 2020 and 2019,
respectively. Net cash inflow in financing activities in 2020 was
mainly attributed to the proceeds received from the exercise of
options, netting off net cash out for short-term debt. Net cash
inflow in the financing activities in 2019 was mainly attributed to
the proceeds of $16 million received from the issuance of common
stock and debt borrowings of $6 million.
Liquidity and Capital Requirements Outlook
We
anticipate that the Company will require approximately $65 million
in cash to operate as planned in the coming 12 months excluding
repayment of convertible bonds. Of this amount, approximately $52
million will be used for operations and approximately $13 million
will be used for capital expenditures, although we may revise these
plans depending on the changing circumstances of our
biopharmaceutical business. The Company’s plans can also be
adjusted by management depending on the availability of
funding.
The
Company has suffered recurring losses from operations and has a net
capital deficiency that raises substantial doubt about its ability
to continue as a going concern. In order to finance our operations,
management intends to rely upon external financing. This financing
may be in the form of equity and or debt, private placements and/or
public offerings or arrangements with private lenders.
We may
also pursue co-development of our clinical assets to defray
operating expenses. We may further explore non-dilutive financing
opportunities forging strategic partnerships with big pharma
companies. As we continue to incur losses, achieving profitability
is dependent upon the successful development of our cell therapy
business and commercialization of our technology in the research
and development phase, which is a number of years in the future.
Once that occurs, we will have to achieve a level of revenues
adequate to support our cost structure. We may never achieve
profitability, and unless and until we do, we will continue to need
to raise additional capital. Management intends to fund future
operations through additional private or public debt or equity
offerings, and may seek additional capital through arrangements
with strategic partners or from other sources.
Our
medium-to-long-term capital needs involve the further development
of our biopharmaceutical business, and may include, at
management’s discretion, new clinical trials for other
indications, strategic partnerships, joint ventures, acquisitions
of licensing rights from new or current partners and/or expansion
of our research and development programs. Furthermore, as our
therapies pass through the clinical trial process and if they gain
regulatory approval, we expect to expend significant resources on
sales and marketing of our future products, services and
therapies.
In
order to finance our medium to long-term plans, we intend to rely
upon external financing. This financing may be in the form of
equity and or debt, in private placements and/or public offerings
or arrangements with private lenders. Due to our short operating
history and our early stage of development, particularly in our
biopharmaceutical business, we may find it challenging to raise
capital on terms that are acceptable to us, or at all. Furthermore,
our negotiating position in the capital raising process may worsen
as we consume our existing resources. Investor interest in a
company such as ours is dependent on a wide array of factors,
including the state of regulation of our industry in China (e.g.
the policies of MOH and the NMPA), the U.S. and other countries,
political headwinds affecting our industry, the investment climate
for issuers involved in businesses located or conducted within
China, the risks associated with our corporate structure, risks
relating to our partners, licensed intellectual property, as well
as the condition of the global economy and financial markets in
general. Additional equity financing may be dilutive to our
stockholders; debt financing, if available, may involve significant
cash payment obligations and covenants that restrict our ability to
operate as a business; our stock price may not reach levels
necessary to induce option or warrant exercises; and asset sales
may not be possible on terms we consider acceptable. If we are
unable to raise the capital necessary to meet our medium- and
long-term liquidity needs, we may have to delay or discontinue
certain clinical trials, the licensing, acquisition and/or
development of cell therapy technologies and/or the expansion of
our biopharmaceutical business; or we may have to raise funds on
terms that we consider unfavorable. While we do not currently
expect the COVID-19 pandemic to materially impact our ability to
secure financial resources or satisfy our liquidity needs, given
the rapidly evolving global situation the actual impact cannot be
predicted and may depend on a variety of currently unknown
factors.
37
Off Balance Sheet Transactions
CBMG
does not have any off-balance sheet arrangements except the lease
and capital commitment disclosed in the unaudited condensed
consolidated financial statements.
Contractual Obligations
We have
various contractual obligations that will affect our
liquidity. The following table sets forth our contractual
obligations as of March 31, 2020.
|
|
Payments due by period
|
||||
|
|
|
Less than
|
2-3
|
4-5
|
More than
|
|
Contractual
Obligations
|
Total
|
1 year
|
years
|
years
|
5 years
|
|
Borrowings
and interest payables
|
$14,104,712
|
$14,104,712
|
$-
|
$-
|
$-
|
|
Capital
Commitment
|
4,472,387
|
4,472,387
|
-
|
-
|
-
|
|
Operating
Lease Obligations
|
24,000,027
|
3,464,776
|
6,340,063
|
6,168,124
|
8,027,064
|
|
Total
|
$42,577,126
|
$22,041,875
|
$6,340,063
|
$6,168,124
|
$8,027,064
|
38
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET
RISK
Exposure to credit,
liquidity, interest rate and currency risks arises in the normal
course of the Company’s business. The Company’s
exposure to these risks and the financial risk management policies
and practices used by the Company to manage these risks are
described below.
Credit Risk
Credit
risk is the risk that one party to a financial instrument will
cause a financial loss for the other party by failing to discharge
an obligation. The Company’s credit risk is primarily
attributable to cash at bank and receivables etc. Exposure to these
credit risks are monitored by management on an ongoing
basis.
The
Company’s cash is mainly held with well-known or state-owned
financial institutions, such as HSBC, Bank of China, China CITIC
Bank and China Merchant Bank. Management does not foresee any
significant credit risks from these deposits and does not expect
that these financial institutions may default and cause losses to
the Company.
The
maximum exposure to credit risk is represented by the carrying
amount of each financial asset in the balance sheet.
Liquidity Risk
Liquidity risk is
the risk that an enterprise may encounter deficiency of funds in
meeting obligations associated with financial liabilities. The
Company and its individual subsidiaries are responsible for their
own cash management, including short term investment of cash
surpluses and the raising of loans to cover expected cash demands.
The Company’s policy is to regularly monitor its liquidity
requirements and its compliance with lending covenants, to ensure
that it maintains sufficient reserves of cash, readily realisable
marketable investments and adequate committed lines of funding from
major financial institutions to meet its liquidity requirements in
the short and longer term.
The
following tables show the remaining contractual maturities at the
balance sheet date of the Company’s financial assets and
financial liabilities, which are based on contractual cash flows
(including interest payments computed using contractual rates or,
if floating, based on rates current at the balance sheet date) and
the earliest date the Group can be required to pay:
|
|
Contractual
undiscounted cash flow
|
|
||||
|
|
Within 1 year or
on demand
|
More than 1 year
but less than 2 years
|
More than 2 year
but less than 5 years
|
More than 5
years
|
Total
|
Carrying
amount
|
|
Financial
assets
|
|
|
|
|
|
|
|
Cash and cash
equivalents
|
21,597,360
|
-
|
-
|
-
|
21,597,360
|
21,597,360
|
|
Other
receivables
|
253,749
|
-
|
-
|
-
|
253,749
|
253,749
|
|
|
|
|
|
|
|
|
|
Sub-total
|
21,851,109
|
-
|
-
|
-
|
21,851,109
|
21,851,109
|
|
|
|
|
|
|
|
|
|
Financial
liabilities
|
|
|
|
|
|
|
|
Short-term
debt
|
14,000,000
|
-
|
-
|
-
|
14,000,000
|
14,000,000
|
|
Accounts
payable
|
1,620,314
|
-
|
-
|
-
|
1,620,314
|
1,620,314
|
|
Accrued
expenses
|
2,372,410
|
-
|
-
|
-
|
2,372,410
|
2,372,410
|
|
Other
current liabilities excluding operating lease liabilities and
deferred income
|
3,104,873
|
-
|
-
|
-
|
3,104,873
|
3,104,873
|
|
Operating
lease liabilities (lease terms over 12 months)
|
3,402,321
|
3,129,891
|
9,378,297
|
8,027,063
|
23,937,572
|
19,280,349
|
|
|
|
|
|
|
|
|
|
Sub-total
|
24,499,918
|
3,129,891
|
9,378,297
|
8,027,063
|
45,035,169
|
40,377,946
|
|
|
|
|
|
|
|
|
|
Net
amount
|
(2,648,809)
|
(3,129,891)
|
(9,378,297)
|
(8,027,063)
|
(23,184,060)
|
(18,526,837)
|
39
Interest Rate Risk
Interest-bearing
financial instruments at variable rates and at fixed rates expose
the Company to cash flow interest rate risk and fair value interest
risk, respectively. The Company’s interest rate risk arises
primarily from cash deposited at banks and short-term debt. The
Company doesn’t have any interest-bearing long-term payable/
borrowing, therefore its exposure to interest rate risk is
limited.
As at
March 31, 2020, the Company held the following interest-bearing
financial instruments:
|
|
As of March 31,
2020
|
|
|
|
Annual interest
rate
|
USD
|
|
Fixed
rate instruments
|
|
|
|
Financial
liabilities
|
|
|
|
-
Short-term debt
|
6%
|
14,000,000
|
Currency Risk
The
Company is exposed to currency risk primarily from sales and
purchases which give rise to receivables, payables that are
denominated in a foreign currency (mainly RMB). The Company has
adopted USD as its functional currency, thus the fluctuation of
exchange rates between RMB and USD exposes the Company to currency
risk.
The
following table details the Company’s exposure as of March
31, 2020 to currency risk arising from recognised assets or
liabilities denominated in a currency other than the functional
currency of the entity to which they relate. For presentation
purposes, the amounts of the exposure are shown in USD translated
using the spot rate as of March 31, 2020. Differences resulting
from the translation of the financial statements of entities into
the Company’s presentation currency are
excluded.

The
following table indicates the instantaneous change in the
Company’s net loss that would arise if foreign exchange rates
to which the Company has significant exposure at the end of the
reporting period had changed at that date, assuming all other risk
variables remained constant.

Results
of the analysis as presented in the above table represent an
aggregation of the instantaneous effects on each of the
Company’s subsidiaries’ net loss measured in the
respective functional currencies, translated into USD at the
exchange rate ruling at the end of the reporting period for
presentation purposes.
The
sensitivity analysis assumes that the change in foreign exchange
rates had been applied to re-measure those financial instruments
held by the Company which expose the Company to foreign currency
risk at the end of the reporting period, including inter-company
payables and receivables within the Company which are denominated
in a currency other than the functional currencies of the lender or
the borrower. The analysis excludes differences that would result
from the translation of the financial statements of subsidiaries
into the Company’s presentation currency.
40
ITEM 4. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Disclosure
controls and procedures include, without limitation, controls and
procedures designed to ensure that information required to be
disclosed by an issuer in the reports that it files or submits
under the Securities Exchange Act of 1934, as amended (the
“Exchange Act”) is recorded, processed, summarized and
reported within the time periods specified in the Securities and
Exchange Commission’s rules and forms. It should be
noted that the design of any system of controls is based in part
upon certain assumptions about the likelihood of future events, and
there can be no assurance that any design will succeed in achieving
its stated goals under all potential future conditions, regardless
of how remote.
We
carried out an evaluation, under the supervision and with the
participation of our management, including our Chief Executive
Officer and Chief Financial Officer, of the effectiveness of our
disclosure controls and procedures (as defined in Exchange Act
Rules 13a-15(e) and 15d-15(e)). Based upon that evaluation, our
Chief Executive Officer and Chief Financial Officer concluded that,
as of the end of the period covered in this report, our disclosure
controls and procedures were effective to ensure that information
required to be disclosed in reports filed under the Exchange Act is
recorded, processed, summarized and reported within the required
time periods and is accumulated and communicated to our management,
including our Chief Executive Officer and Chief Financial Officer,
as appropriate to allow timely decisions regarding required
disclosure.
Changes in Internal Control over Financial Reporting
During
the three months ended March 31, 2020, there was no change in our
internal control over financial reporting (as such term is defined
in Rule 13a-15(f) under the Exchange Act) that has materially
affected, or is reasonably likely to materially affect, our
internal control over financial reporting.
41
PART II – OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
We are
currently not involved in any litigation that we believe could have
a materially adverse effect on our financial condition or results
of operations.
ITEM 1A. RISK FACTORS
Investing
in our common stock involves a high degree of risk. We describe
risks associated with our business in under the caption “Risk
Factors” in our Annual Report on Form 10-K for the year ended
31, 2019. Each of the risks described in our Risk Factors may be
relevant to decisions regarding an investment in or ownership of
our stock. The occurrence of any such risks could have a
significant adverse effect on our reputation, business, financial
condition, revenue, results of operations, growth, or ability to
accomplish our strategic objectives, and could cause the trading
price of our common stock to decline. You should carefully consider
such risks and the other information contained in this report,
including our condensed consolidated financial statements and
related notes and Management’s Discussion and Analysis of
Financial Condition and Results of Operations, before making
investment decisions related to our common stock.
The
following risk factors supplement the Risk Factors contained in our
Annual Report on Form 10-K for the year ended December 31, 2019.
The following disclosures do not address all risks that may be
important to you as a stockholder.
Our
business activities for fiscal 2020 are expected to be adversely
affected by the global COVID-19
pandemic.
The
coronavirus 2019 (COVID-19) has spread globally and the World
Health Organization (WHO) has declared it a global pandemic. While
still evolving, the COVID-19 pandemic has caused significant
worldwide economic and financial turmoil, and has fueled concerns
that it will lead to a global recession. On March 13, 2020, the
United States declared a national emergency with respect to
COVID-19 and the majority of states and U.S. territories, including
the States of New York and Maryland, have since issued orders
requiring the closure of non-essential businesses and/or requiring
residents to stay at home. The New
York area and the Maryland area, including the location of the
Company’s corporate headquarter and its development facility,
is currently at the epicenter of the COVID-19 outbreak in the U.S.
The Company is following the recommendations of local health
authorities to minimize exposure risk for its team members and
visitors. The Company has required its employees in the U.S. to
work from home. The continued and prolonged implementation
of restrictions by federal, state and local authorities to slow the
spread of COVID-19 could disrupt the business, activities, and
operations of our members, as well our business and operations. The
extent to which the COVID-19 pandemic impacts our business,
operations and financial results will depend on numerous evolving
factors that we may not be able to accurately predict, including:
the duration and scope of the pandemic; governmental, business and
individuals’ actions that have been and continue to be taken
in response to the pandemic; the impact of the pandemic on economic
activity and actions taken in response; the effect on our vendors
ability to supply us with raw materials; our ability to continue
daily operations, including as a result of travel restrictions and
people working from home; and any closures of our and our business
partners’ offices and facilities. Certain business partners
(such as third-party research institution collaborators, suppliers
and other contractors) have slowed down decision-making during the
lock down in China and in U.S., or delayed progress. The COVID-19
pandemic has disrupted and delayed our in-process developments and
clinical studies for a number of our pipeline drug candidates and a
prolonged interruption to our corporate development, research or
manufacturing facilities may result in a negative impact to our
operations and further delay developments or clinical studies of
some or all of our pipeline drug candidates.
In
addition, our business is subject to risks associated with the
global spread of the COVID-19 as we operate in both China and the
U.S. Our process development of drug candidates involves key
personnel traveling between China and the U.S. on a frequent and
regular basis, which has been disrupted due to travel restrictions
and cancellation of flights. The magnitude of this negative effect
on the continuity of our business operation and supply chains
remains uncertain. The extent to which
COVID-19 or any other health epidemic may impact the
Company’s results will depend on future developments, which
are highly uncertain and cannot be predicted, including new
information which may emerge concerning the severity of COVID-19
and the actions to contain COVID-19 or treat its impact, among
others. These uncertainties impede our ability to conduct
our daily operations and could materially and adversely affect our
business, financial condition and results of
operations.
While the Company is currently implementing
solutions designed to reduce the potential impact of
COVID-19, there can be no assurance that our efforts will
adequately mitigate the risks of business disruptions and
interruptions. Further, events such as natural disasters and public
health emergencies divert our attention away from normal operations
and limited resources. Our inability to timely resume normal
operations following the pandemic disruption could adversely affect
our business, financial condition or results of operations in a
material manner.
Any
of these events could cause or exacerbate the risks and
uncertainties enumerated in the Annual Report and could materially
adversely affect our business, financial condition, results of
operations and/or stock price.
42
Our
clinical development activities for fiscal 2020 are expected to be
adversely affected by the recent COVID-19 pandemic.
Our
investigator has initiated clinical trials on our drug candidates
that have been negatively affected by the emergency quarantine
measures adopted by the Chinese government, which include holiday
extension, travel restrictions and cancellation of major events
nationwide. Disruptions or restrictions on our ability to travel or
to conduct clinical trials, as well as temporary closures of our
facilities or the facilities of our clinical trial partners and
their contract manufacturers, are expected to negatively impact our
clinical development activities in China. We have invested a
significant portion of our efforts and financial resources in the
development of clinical-stage drug candidates. We partially rely on
our third-party institution collaborators, such as hospitals for
conducting clinical trials of our drug candidates, which have been
and may continue to be affected by the emergency measures related
to the COVID-19 pandemic. The timely completion of our clinical
trials in accordance with their protocols depends, among other
things, on our ability to enroll a sufficient number of patients
who remain in the trial until its conclusion. We have experienced
difficulties in patient enrollment due to government-imposed travel
restrictions, limited access to public venues and patients’
unwillingness to visit hospitals for fear of contracting COVID-19.
Such difficulties are likely to slow enrollment significantly in,
and completion of, our clinical trials (which are mostly conducted
on-site in hospitals), as well as completion of pre-clinical
studies.
On
March 18, 2020, the U.S. FDA released guidance for conducting
clinical trials during the COVID-19 pandemic. In its guidance, the
U.S. FDA acknowledged potential impacts from the pandemic to
clinical trial conduct, for example quarantines, travel
limitations, site closures, interruptions to supply chain for
investigational products, and potential infection of site personnel
or trial participants may lead to difficulties in meeting protocol
defined procedures, including administration of investigational
product and adherence to protocol-mandated visits. As a result, the
U.S. FDA recognized that there may be unavoidable protocol
deviations, but also noted that efforts to minimize impacts on
trial integrity are important. Laboratories studying viruses and
bacteria follow a protocol known as the Biosafety Level (BSL)
standards, which are applied internationally. Protocol deviations
due to COVID-19 means trials could be interrupted due to missed
biopsies and results or data that are uninterpretable or need to be
repeated. The impact of COVID-19 on trials will vary depending on
many factors, including the nature of disease under study, the
trial design and in what region(s) the study is being conducted.
For gene and cell therapies, clinical trials may be delayed because
patients may only be treated with non-transplant standard of care
where possible with transplantation or experimental therapies
reserved for life-threatening cases (malignancies, neurocognitive
disorders) due to capacity constraint. The duration of the business
disruption, reduced patient enrollment and related operational
impact cannot be reasonably estimated at this time but are expected
to materially affect our clinical development activities. A
significant outbreak of contagious diseases in the human population
could result in a widespread health crisis that could adversely
affect the economy and financial markets of the U.S. and China,
resulting in significant clinical trial or regulatory delays, which
may also increase our development costs and could materially and
adversely affect our clinical development activities.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF
PROCEEDS
None
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not
applicable.
ITEM 5. OTHER INFORMATION
On April 30, 2020, our wholly-owned subsidiary CBMG Wuxi received
approval from Nanjing Bank for a one-year line of credit of up to
CNY 30 million (approximately $4.2 million). Use of proceeds from
the credit line is limited to working capital for research and
development activities. This line of credit carries an interest
rate of not less than Loan Prime Rate (LPR) +0.5%. LPR is the
benchmark for pricing existing floating-rate loans set by
People’s Bank of China. As of April 30, 2020, the LPR for a
one-year loan is 3.85%. This facility is only in effect while the
Company remains as a publicly traded entity. We have not drawn on
this line of credit.
43
ITEM 6. EXHIBITS
Exhibits
|
Exhibit Number
|
|
Description
|
|
|
Nanjing
Bank approval of a credit line of up to RMB 30 million to CBMG
Wuxi.
|
|
|
|
Bridge Loan Agreement, dated January 28, 2020 (incorporated by
reference to the Company’s Current Report on Form 8-K, filed
January 29, 2020)
|
|
|
|
Certification
Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 - Chief
Executive Officer and Chief Financial Officer.
|
|
|
|
Certifications
Pursuant to Section 906 of the Sarbanes-Oxley Act of
2002.
|
|
|
101.INS
|
|
XBRL
Instance Document
|
|
101.SCH
|
|
XBRL
Taxonomy Extension Schema
|
|
101.CAL
|
|
XBRL
Taxonomy Extension Calculation Linkbase
|
|
101.DEF
|
|
XBRL
Taxonomy Extension Definition Linkbase
|
|
101.LAB
|
|
XBRL
Taxonomy Extension Label Linkbase
|
|
101.PRE
|
|
XBRL
Taxonomy Extension Presentation Linkbase
|
44
SIGNATURES
In
accordance with Section 13 or 15(d) of the Securities
Exchange Act of 1934, as amended, the registrant caused this report
to be signed on its behalf by the undersigned, thereunto duly
authorized.
|
|
CELLULAR BIOMEDICINE GROUP, INC.
|
|
|
|
|
(Registrant)
|
|
|
|
|
|
|
|
|
Date:
May 6, 2020
|
By:
|
/s/
Tony (Bizuo) Liu
|
|
|
|
|
Tony
(Bizuo) Liu
|
|
|
|
|
Chief
Executive Officer and Chief Financial Officer
|
|
|
|
|
(Principal
Executive Officer and Principal Financial and Accounting
Officer)
|
|
|
|
|
|
|
45
