Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Cellular Biomedicine Group, Inc. | ebig_8k.htm |
EXHIBIT 99.1

February 19, 2013
1
(OTCQB: EBIGD)

Statements made in this presentation relating to plans, strategies, economic
performance and trends, projections of results of specific activities or investments, and
other statements that are not descriptions of historical facts may be forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995,
Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange
Act of 1934. Forward-looking information is inherently subject to risks and
uncertainties, and actual results could differ materially from those currently
anticipated due to a number of factors, which include, but are not limited to, risk
factors inherent in doing business. Forward-looking statements may be identified by
terms such as "may," "will," "should," "could," "expects," "plans," "intends,"
"anticipates," "believes," "estimates," "predicts," "forecasts," "potential," or
"continue," or similar terms or the negative of these terms. Although we believe that
the expectations reflected in the forward-looking statements are reasonable, we
cannot guarantee future results, levels of activity, performance or achievements. The
Company has no obligation to update these forward-looking statements.
performance and trends, projections of results of specific activities or investments, and
other statements that are not descriptions of historical facts may be forward-looking
statements within the meaning of the Private Securities Litigation Reform Act of 1995,
Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange
Act of 1934. Forward-looking information is inherently subject to risks and
uncertainties, and actual results could differ materially from those currently
anticipated due to a number of factors, which include, but are not limited to, risk
factors inherent in doing business. Forward-looking statements may be identified by
terms such as "may," "will," "should," "could," "expects," "plans," "intends,"
"anticipates," "believes," "estimates," "predicts," "forecasts," "potential," or
"continue," or similar terms or the negative of these terms. Although we believe that
the expectations reflected in the forward-looking statements are reasonable, we
cannot guarantee future results, levels of activity, performance or achievements. The
Company has no obligation to update these forward-looking statements.
This presentation is strictly intended to provide general information about our
company and business. This presentation does not, nor does any part hereof,
company and business. This presentation does not, nor does any part hereof,
constitute an offer of securities.
Safe Harbour

Our Vision
CBMG applies advancements in biotechnology
to establish pipelines of cell-based therapeutic
products leading to fulfillment of disease
treatment in China.
to establish pipelines of cell-based therapeutic
products leading to fulfillment of disease
treatment in China.
3
4
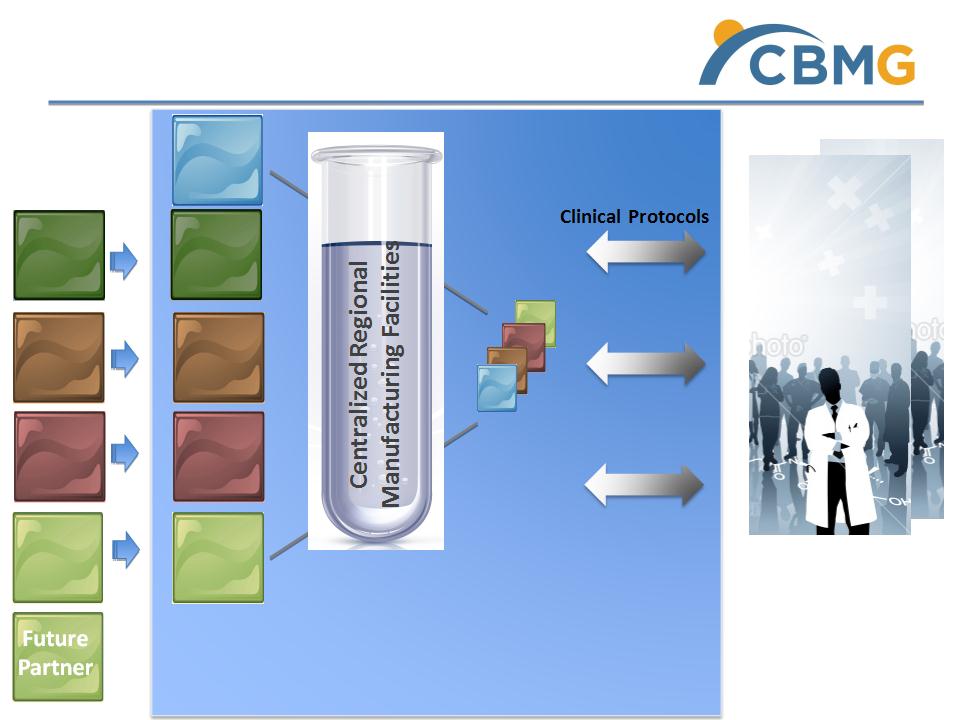
Business Model: Cellular Medicine
Translational Platform in China
• Principal Investigators
• Expert Group
• Ethic Committee
• Hospital Authorities
• MOH, SFDA
• Regulators
• Pricing Bureau
• Insurance Co
• CROs
• External Labs
Clinical Trials
• Quality Standard
• Regional GMP fabs
• Safety
• Cost
• Suppliers
• Auditors
R & D
Lab
Lab
Partner
A
A
JV A
Cell Lab
Cell Lab
Partner
C
C
Licensed
Cell Line
Partner
B
B
JV B
Cell Lab
Cell Lab
Partner
D
D
Licensed
Cell Line
Regional
Hospitals &
Patients
Hospitals &
Patients
Cell Products
& Therapies
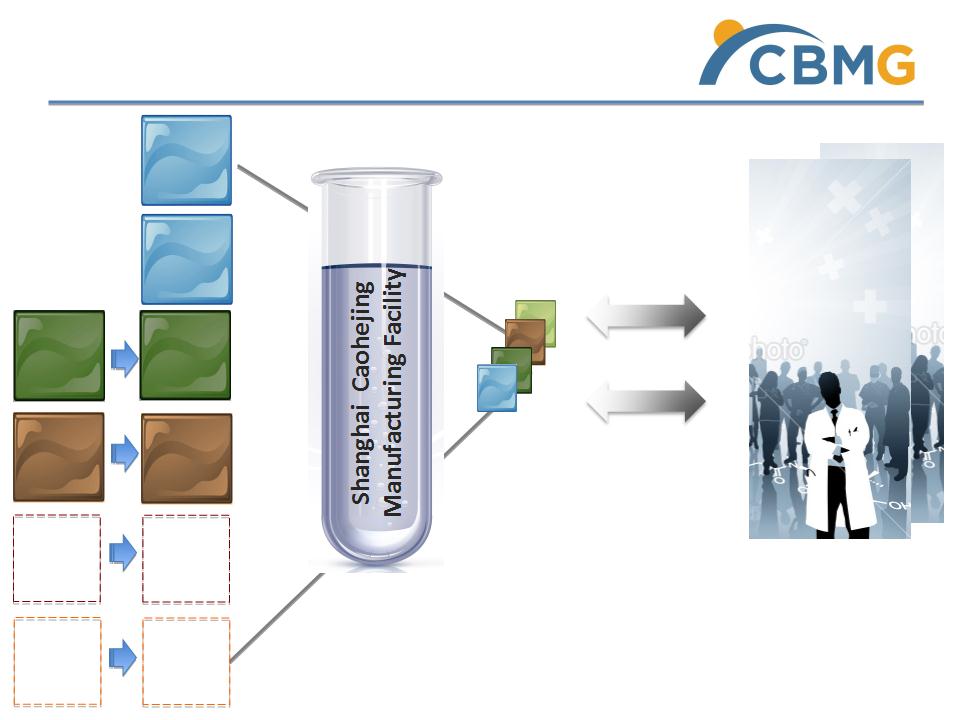
Cellular Therapies Platform - Current Status
Clinical Protocols
Clinical Trials
haMPC
Cell Lab
Partner
A
A
Partner
B
B
Licensed
Motor
Neuron
Cell Line
Partner
A
A
JV
TC-DC
Cell Lab
Partner
C
C
Parkinson
Cell Line
Cell Line
4 Indications
Knee Osteoarthritis
Lupus
Liver Cancer
Spinal muscular atrophy
Shanghai
Renji Hospital
and
85 Hospital
huMPC
Cell Lab
Cancer
Cell Line
Cell Line
Business Model
|
CBMG has built the first stage of a comprehensive platform that can support
multiple cell lines with multiple partners. |
||
|
Therapies developed fit
China’s patients profile |
Highly regulated by
Government regulation. Very difficult for foreign company to establish a cellular facility in China. |
We in-house develop, in-
license technologies. We establish JVs with
partners. We own trials and
clinical protocols. |
|
Autologous cell
therapies are regulated as medical technology |
Build high quality
manufacturing facilities in large strategic cities |
Emerging industry, no
industry standard available yet |
|
Multiple inflection points exist for each platform as therapies enter into clinical
use. |
||
Winning Strategies
|
First mover advantage in a vast Chinese market - be the first to pursue national
compliance and set standards for two clinical pathways of cell therapies - autologous cells applied as medical technologies and allogeneic cells for drug. |
|
Shortened clinical pathways exist in China for autologous cell-based therapies,
thus greatly reducing the risk of time-to-money. |
|
Demonstrated very high capital efficiency in operations - a fraction of the cost
and 3 times translation speed in comparison to similar trials in US, enabling multiple trials with limited capital. |
|
Led by a team of US trained executives with extensive connections in both US
and China and experienced in large business creation, management believes CBMG is poised to be the best translational medicine platform in the world.
|

Tony Liu
§Chairman of Audit Committee
§Corporate VP of Alibaba Group; CFO of HiChina
§Former GM, Corporate Strategy of Microsoft
§Former Corporate Accounting Director of Microsoft
Corporate Governance -
Independent Directors
8
Dai Jianping, MD (pending board’s confirmation)
§Professor, Beijing Neurosurgical Institute and Beijing Tiantan
Hospital, Capital University of Medical Sciences
Hospital, Capital University of Medical Sciences
§Vice Chairman of Chinese Association of Medical Doctors
§Vice Chairman of Chinese Association of Hospitals
§Foreign Associate, Institute of Medicine (IOM)
Lu Daopei, MD (pending board’s confirmation)
§Professor of Hematology, Beijing Medical University
§Member of Chinese Academy of Engineering
§Nationally renowned hematologist in stem cells (bone marrow)
transplant for the treatment of leukemia
transplant for the treatment of leukemia

Management Team
Wen Tao (Steve) Liu, PhD
Chairman and CEO
§PhD, Physical Chemistry, Rensselaer Polytechnic Institute, New York
§Former President & CEO, Seeo Inc.
§Former President & CEO, Shanghai Hua Hong NEC Electronics Company
Wei (William) Cao, PhD, BM
President, COO and Director
§PhD, Pharmacology from Medical College of Virginia
§Former Research Fellow in Pathology, Harvard Medical School
§Former Fellow in Cardiothoracic Surgery, Stanford Univ Medical School
§Former General Manager, Affymetrix Greater China
Andy Chan, JD, MBA
Chief Financial Officer, Board Secretary
§Jurisprudence Doctorate (JD), South Texas College of Law
§Master in Business Administration from Nova University
§Former VP, BD & China Operations, Jazz Semiconductor
§Former Senior Director, Strategic Business Development, AlliedSignal
Aerospace
Aerospace
9

Helen Zhang, BM
Vice President, Technology and Manufacturing
§Former Associate Director, Harvard Gene Therapy Initiative, Harvard
Medical School
Medical School
§Former Max-Delbruck Research Fellow, National Research Center for
Molecular Medicine, Berlin, Germany
Molecular Medicine, Berlin, Germany
Zhou Mingyuan, PhD
Chief Scientist
§Post Doctoral Associate and Instructor, University of Illinois at Chicago
§Former Senior Scientist & Associate Director, Gene Therapy Center,
University of Pennsylvania
§Former Senior Scientist, Wistar Cancer Institute
Management Team
10
Chase Dai, MD, PhD
Vice President and GM of Autologous Products Business Unit
§Former CEO of Meijin Genetic Biotechnology
§Former Deputy Chief Physician of Cardiology Department, Beijing 301
PLA Hospital

Auditor, Tax, Accountant , Legal Advisor
11
|
Auditor | BDO
Ranked # 8 in Top 100 Audit Firms in 2012
|
|
International Tax Advisor | Ernest & Young
Tax expertise for CBMG’s international structure
|
|
Investment Banker | Maxim
With renowned, authoritative biomedicine analyst Jason
Kolbert |
|
Law Firm | Richardson Patel
Corporate and Securities Law Advisor
|
|
IRPR | ProActive Capital Group, LLC
Investor relations and public relations
|
Knee Osteoarthritis (KOA)
Prognosis
12
Market size
>20 Million
Current therapies
Pain killers and steroids,
even amputation
even amputation
53% of KOA patients will
become disabled in later
years.
become disabled in later
years.
In China, 40,000 patients
go through knee
replacement surgery
go through knee
replacement surgery
each year.
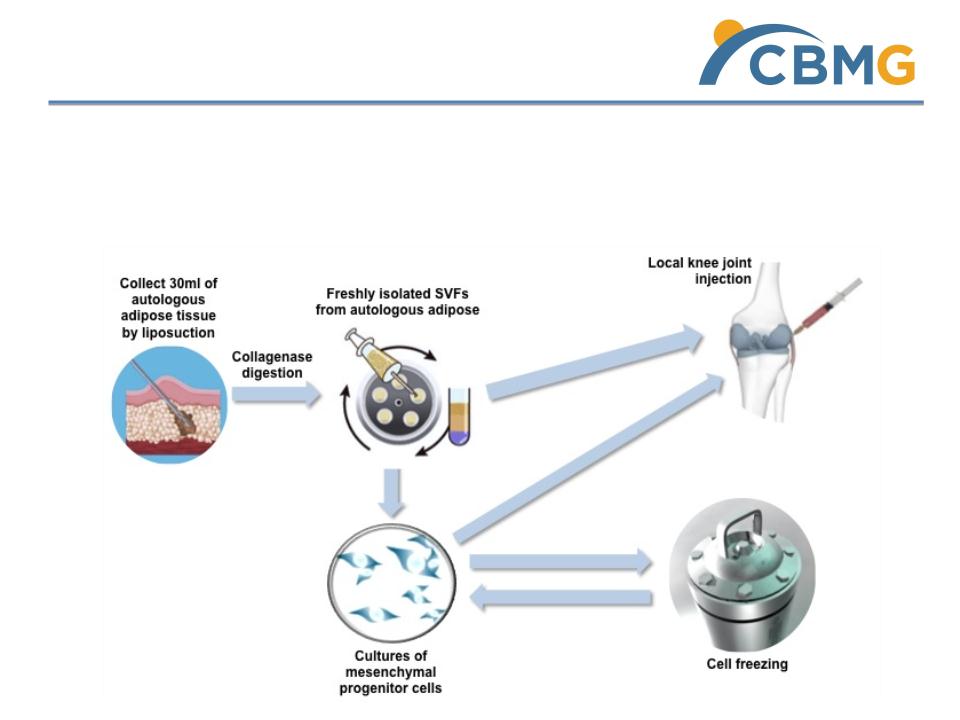
haMPC Therapy for KOA (Phase I Trial)
Our Treatment: Intra- articular injections of haMPC
Mechanism of action:
ü MPC has anti-inflammatory effect by secreting chemicals to down regulate immune response
ü MPC can also regenerate damaged joint tissues
13
2013-2-11
Hepatic Cellular Carcinoma (HCC)
Prognosis
Market size
45% worldwide HCC patients
are in China
are in China
>300,000 new patients per
year in China
year in China
90% of liver cancers are HCC
Current Therapy
Surgical removal of
tumor, plus local
chemotherapy
tumor, plus local
chemotherapy
Post surgery and standard
treatment, 2-years recurrence
rate is 51%
treatment, 2-years recurrence
rate is 51%
Medium survival time is 13
months
months
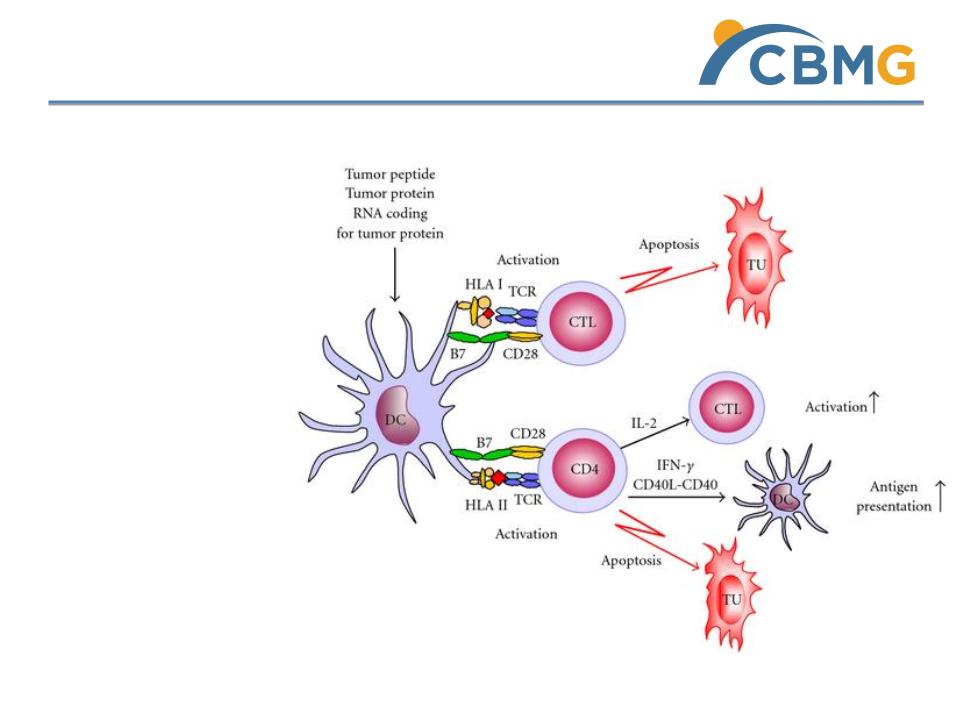
TC-DC Therapy for HCC (Phase I Trial)
TC - DC Therapy for HCC
Multiple subcutaneous
injections of patients dendritic
cells that are loaded with
patient’s own liver cancer stem
cells
injections of patients dendritic
cells that are loaded with
patient’s own liver cancer stem
cells
Mechanism of action
Patient’s own dendritic cells are
educated to efficiently
presenting cancer stem cells so
as to trigger effective cascade
immune response against
cancer stem cells
educated to efficiently
presenting cancer stem cells so
as to trigger effective cascade
immune response against
cancer stem cells
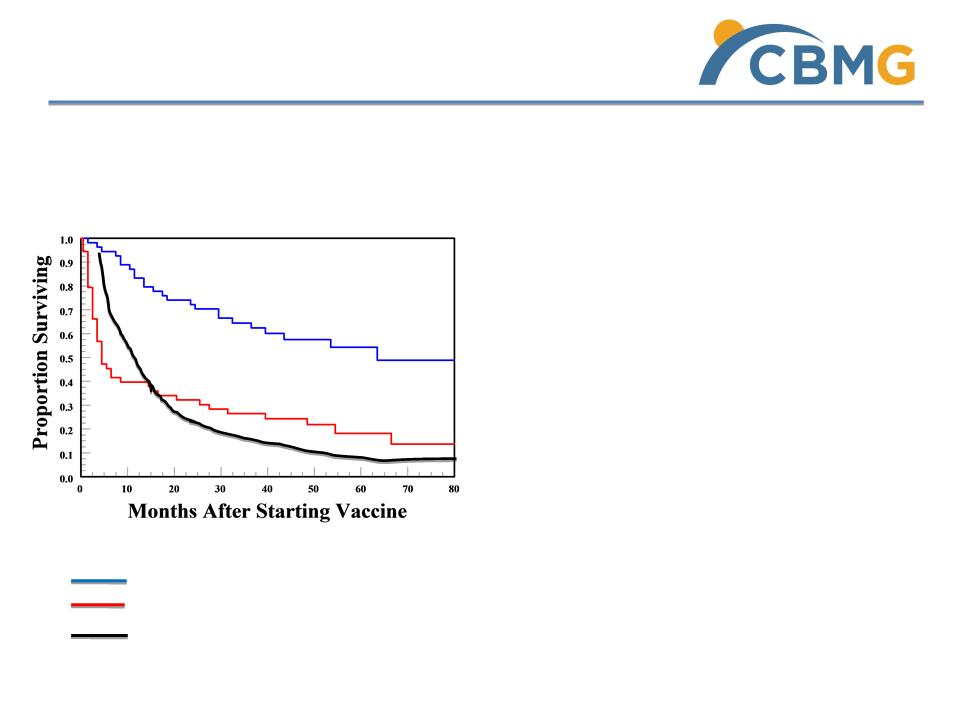
ü Strong safety profile
ü Autologous cells, no rejection
ü Extraordinary US phase II melanoma
trial result: 5 year overall survival 54%
in TC-DC group, vs. 10% in control
group
trial result: 5 year overall survival 54%
in TC-DC group, vs. 10% in control
group
ü TC-DC group medium survival time
4.5 years, vs. 1.3 years for control
group with standard therapies
4.5 years, vs. 1.3 years for control
group with standard therapies
Median OS = NR at 54 months 5-year OS =54%;
Historical control group with standard therapies
Metastatic melanoma Phase II trial results
TC- DC therapy group
Tumor cells only as control group
TC - DC, a Proven Therapy by US Trials
|
|
Preclinical
|
IRB
|
Ph I
|
Ph II
|
|
haMPC
- Knee Osteoarthritis
|
|
|
|
|
|
TC - DC
- Hepatic carcinoma
|
|
|
|
|
Pipeline of Autologous Cell Therapies
Leverage U.S. skin cancer trialed data
Work in Progress
• haMPC is autologous cells and is classified as Medical Technology
• TC - DC is also classified as Medical Technology
Completed
|
|
Preclinical
|
IRB
|
Ph I
|
Ph II
|
Ph III
|
|
huMPC
- Systemic lupus
ethrytematosus - Glaucoma
|
|
|
|
|
|
|
MNP
- Spinal muscular atrophy
|
|
|
|
|
|
Pipeline of Allogeneic Cell Therapies
Accomplished in U.S. or other laboratories
* Assumed allogeneic stem cell programs will follow the drug approval path, which include 3 phases trials
Work in Progress

|
Technology
|
Indication
|
2013
|
2014
|
2015
|
2016
|
2017
|
2018
|
|
haMPC
|
Knee Osteoarthritis
|
|
|
|
|
|
|
|
huMPC
|
Systemic Lupus
ethrytematosus |
|
|
|
|
|
|
|
TC - DC
|
Hepatocellular
Carcinoma (Liver cancer)* |
|
|
|
|
|
|
|
MNP
|
Spinal muscular
atrophy type I** |
|
|
|
|
|
|
Estimated Completion Timeline for
Active Trials
* Compassionate use is approved prior to completion of clinical trial
** Apply for orphan drug approval, i.e., two phases of trial are required for approval
19
Autologous cell therapy
Allogeneic cell therapy

Facility and Credentials
ü CBMG Shanghai facility is awarded ISO-14644 cleanroom
certification by ENV Inc. , a service provider of GMP
certification certified by FDA
certification by ENV Inc. , a service provider of GMP
certification certified by FDA
ü Shanghai and Wuxi facilities accredited the ISO
(International Standards Organization) 9001:2008
certification by internationally recognized quality
management system SGS
(International Standards Organization) 9001:2008
certification by internationally recognized quality
management system SGS
ü Both CBMG Shanghai and Wuxi facility are GMP certified by
SFDA certified institute
SFDA certified institute
ü CBMG Shanghai received Provisional Accreditation for its haMPC production and clinical application by
International l Cellular Medicine Society of AABB
International l Cellular Medicine Society of AABB
ü Wuxi Government authorized CBMG Wuxi as “Wuxi Stem Cell Technology Translation Center”
ü CBMG President William Cao is an elected member of Expert Committee, The 3rd Medical Technology
Approval Office, Chinese Medical Doctor Association, Ministry of Health, PRC
Approval Office, Chinese Medical Doctor Association, Ministry of Health, PRC
ü Dr Cao is appointed member of the Committee of International Cellular Medicine Society and American
Association of Blood Banks for the Development of the Standards for the practice of Cell-Based
Medicine
Association of Blood Banks for the Development of the Standards for the practice of Cell-Based
Medicine
20

1.Potential emerging leader in the cellular biomedicine
industry in China
industry in China
2.A platform supporting multiple therapeutic pipelines for
maximized valuation creation
maximized valuation creation
3.Two stage one trials are in progress: knee osteoarthritis
(KOA) and hepatocellular carcinoma (liver cancer)
(KOA) and hepatocellular carcinoma (liver cancer)
4.Clear advantages operating in China: large patient base and
reduced time-to-market for products and treatments
reduced time-to-market for products and treatments
5.A solid management team with principled corporate
governance
governance
6.Intent to up list to Nasdaq as soon as practically possible
Summary

Thank You
22
Contact:
Jeff Ramson
Investor Relations
ProActive Capital Group
646 -863 - 6341
Sarah Kelly
Director Of Corporate Communications,
CBMG
sarah.kelly@cellbiomedgroup.com
(OTCQB: EBIGD)

23
TC - DC - tumor cell specific dendritic cell therapy
haMPC - adipose derived mesenchymal progenitor cells
huMPC - umbilical cord derived mesenchymal progenitor cells
MNP - embryo derived motor neuron precursor cells
Neuronal SC - embryo derived neuronal cells
Glossary
