Attached files
U.S. SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Form 10-Q
Quarterly Report Under
the Securities Exchange Act of 1934
For Quarter Ended: March
31, 2018
Commission File Number: 000-52898
SUNSHINE BIOPHARMA INC.
(Exact name of small business issuer as specified in its
charter)
|
Colorado
|
|
20-5566275
|
|
(State
of other jurisdiction of incorporation)
|
|
(IRS
Employer ID No.)
|
6500 Trans-Canada Highway
4th Floor
Pointe-Claire, Quebec, Canada H9R 0A5
(Address of principal executive offices)
514) 426-6161
(Issuer’s Telephone
Number)
Indicate by check mark whether the registrant (1) has filed all
reports required to be filed by Section 13 or 15(d) of the
Securities Exchange Act of 1934 during the preceding 12 months (or
for such shorter period that the registrant was required to file
such reports), and (2) has been subject to such filing requirements
for the past 90 days: Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted
electronically and posted on its corporate Web site, if any, every
Interactive Data File required to be submitted and posted pursuant
to Rule 405 of Regulation S-T (§232.405 of this chapter)
during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files).
Yes ☑
No ☐
Indicate
by check mark whether the registrant is a large accelerated filer,
an accelerated filer, a non-accelerated filer, smaller reporting
company, or an emerging growth company. See the definitions of
“large accelerated filer,” “accelerated
filer”, “smaller reporting company”, and
“emerging growth company” in Rule 12b-2 of the Exchange
Act. (Check one)
|
|
Large
accelerated filer ☐
|
Accelerated
filer ☐
|
|
|
Non-accelerated
filer ☐
|
Smaller
reporting company ☒
|
|
|
Emerging
growth company ☒
|
|
If an
emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided
pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company
(as defined in Rule 12b-2 of the Exchange
Act). ☐ Yes ☑ No
The number of shares of the registrant’s only class of common
stock issued and outstanding as of May 21, 2018, was 968,512,658
shares.
TABLE OF CONTENTS
|
|
PART I.
FINANCIAL INFORMATION
|
|
Page No.
|
|
|
|
|
|
|
|
|
Item
1. Financial
Statements
|
|
3
|
||
|
|
|
|
|
|
|
Unaudited
Consolidated Balance Sheet as of March 31, 2018
|
|
3
|
||
|
|
|
|
|
|
|
Unaudited
Consolidated Statement of Operations for the Three Month Periods
Ended March 31, 2018 and 2017
|
|
4
|
||
|
|
|
|
|
|
|
Unaudited
Consolidated Statement of Cash Flows for the Three Month Periods
Ended March 31, 2018 and 2017
|
|
5
|
||
|
|
|
|
|
|
|
Notes
to Consolidated Financial Statements
|
|
6
|
||
|
|
|
|
|
|
|
Item
2. Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
|
11
|
||
|
|
|
|
|
|
|
Item
3. Quantitative and
Qualitative Disclosures About Market Risk.
|
|
16
|
||
|
|
|
|
|
|
|
Item
4. Controls and
Procedures.
|
|
16
|
||
|
|
|
|
|
|
|
|
PART II. OTHER
INFORMATION
|
|
|
|
|
|
|
|
|
|
|
Item
1. Legal
Proceedings
|
|
18
|
||
|
|
|
|
|
|
|
Item 1A.
Risk
Factors
|
|
18
|
||
|
|
|
|
|
|
|
Item
2. Unregistered
Sales of Equity Securities and Use of Proceeds
|
|
18
|
||
|
|
|
|
|
|
|
Item
3. Defaults Upon
Senior Securities
|
|
18
|
||
|
|
|
|
|
|
|
Item
4. Mine Safety
Disclosures
|
|
18
|
||
|
|
|
|
|
|
|
Item
5. Other
Information
|
|
18
|
||
|
|
|
|
|
|
|
Item
6. Exhibits
|
|
18
|
||
|
|
|
|
|
|
|
Signatures
|
|
19
|
||
2
PART I.
|
|
Unaudited
|
Audited
|
|
|
March
31,
|
December
31,
|
|
|
2018
|
2017
|
|
ASSETS
|
|
|
|
|
|
|
|
Current Assets:
|
|
|
|
Cash and cash
equivalents
|
$104,763
|
$107,532
|
|
Accounts
receivable
|
$92,390
|
$-
|
|
Prepaid
expenses
|
5,336
|
9,667
|
|
|
|
|
|
Total Current
Assets
|
202,489
|
117,199
|
|
|
|
|
|
Equipment (net of
$16,295 and $9,132 depreciation, resepctively)
|
118,444
|
59,996
|
|
Patents (net of
$58,918 amortization and $556,120 impairment)
|
-
|
-
|
|
|
|
|
|
Non-current
Assets:
|
|
|
|
Deposits
|
7,756
|
80,290
|
|
Goodwill
|
673,646
|
-
|
|
|
|
|
|
|
|
|
|
TOTAL
ASSETS
|
$1,002,335
|
$257,485
|
|
|
|
|
|
LIABILITIES AND
SHAREHOLDERS' EQUITY
|
|
|
|
|
|
|
|
Current Liabilities:
|
|
|
|
Notes
payable
|
785,137
|
516,867
|
|
Notes payable -
Related party
|
256,665
|
205,742
|
|
Bank
overdraft
|
3,915
|
-
|
|
Accounts
payable
|
113,358
|
19,314
|
|
Interest
payable
|
21,924
|
9,215
|
|
|
|
|
|
Total Current
Liabilities
|
1,180,999
|
751,138
|
|
|
|
|
|
Long-term
Liabilities:
|
|
|
|
Note
payable
|
-
|
79,710
|
|
Note payable -
Related party
|
322,021
|
-
|
|
|
|
|
|
TOTAL
LIABILITIES
|
1,503,020
|
830,848
|
|
|
|
|
|
COMMITMENTS AND
CONTINGENCIES
|
|
|
|
|
|
|
|
SHAREHOLDERS'
EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
Preferred stock,
Series A $0.10 par value per share; Authorized 850,000
Shares;
Issued
and
outstanding -0- shares.
|
-
|
-
|
|
|
|
|
|
Preferred stock,
Series B $0.10 par value per share; Authorized 500,000
Shares;
Issued
and
outstanding 500,000 shares.
|
50,000
|
50,000
|
|
|
|
|
|
Common
Stock, $0.001 par value per share; Authorized
3,000,000,000 Shares; Issued and
outstanding
949,019,532 and 918,736,498 at March 31, 2018 and
December 31, 2017, respectively
|
948,020
|
918,737
|
|
Reserved for
issuance 474,779,621 at Martch 31, 2018
|
|
|
|
|
|
|
|
Capital paid in
excess of par value
|
12,387,355
|
12,075,586
|
|
|
|
|
|
Accumulated
comprehensive income
|
(2,234)
|
504
|
|
|
|
|
|
Accumulated
(Deficit)
|
(13,883,826)
|
(13,618,190)
|
|
|
|
|
|
TOTAL SHAREHOLDERS'
EQUITY (DEFICIT)
|
(500,685)
|
(573,363)
|
|
|
|
|
|
TOTAL LIABILITIES
AND SHAREHOLDERS' EQUITY (DEFICIT)
|
$1,002,335
|
$257,485
|
See
Accompanying Notes To These Financial Statements.
3
|
|
Unaudited
|
Unaudited
|
|
|
3
Months
|
3
Months
|
|
|
Ended
|
Ended
|
|
|
March
31,
|
March
31,
|
|
|
2018
|
2017
|
|
|
|
|
|
Revenue:
|
$91,168
|
$-
|
|
|
|
|
|
General &
Administrative Expenses
|
|
|
|
|
|
|
|
Accounting
|
28,000
|
15,600
|
|
Consulting
|
4,118
|
25,937
|
|
Legal
|
27,485
|
14,904
|
|
Office
|
19,048
|
9,066
|
|
Officer &
director remuneration
|
77,787
|
42,965
|
|
Rent
|
23,059
|
-
|
|
Salaries
|
57,558
|
-
|
|
Supplies
|
14,070
|
-
|
|
Depreciation
|
6,740
|
518
|
|
|
|
|
|
Total G &
A
|
257,865
|
108,990
|
|
|
|
|
|
(Loss) from
operations
|
(166,697)
|
(108,990)
|
|
|
|
|
|
Other Income
(expense):
|
|
|
|
Foreign exchange
(loss)
|
14,868
|
(639)
|
|
Interest
expense
|
(75,467)
|
(9,144)
|
|
Loss on debt
conversions
|
(38,340)
|
(76,929)
|
|
|
|
|
|
Total Other
(Expense)
|
(98,939)
|
(86,712)
|
|
|
|
|
|
Net
(loss)
|
$(265,636)
|
$(195,702)
|
|
|
|
|
|
Basic (Loss) per
common share
|
$0.00
|
$0.00
|
|
|
|
|
|
Weighted Average
Common Shares Outstanding
|
941,606,571
|
805,604,089
|
|
|
|
|
|
Net Income
(Loss)
|
$(265,636)
|
$(195,702)
|
|
Other comprehensive
income:
|
|
|
|
Gain (Loss) from
foreign exchange translation
|
(2,738)
|
1,509
|
|
Comprehensive
(Loss)
|
(268,374)
|
(194,193)
|
|
|
|
|
|
Basic (Loss) per
common share
|
$0.00
|
$0.00
|
|
|
|
|
|
Weighted Average
Common Shares Outstanding
|
941,606,571
|
805,604,089
|
4
|
|
Unaudited
|
Unaudited
|
|
|
3
Months
|
3
Months
|
|
|
Ended
|
Ended
|
|
|
March
31,
|
March
31,
|
|
|
2018
|
2017
|
|
|
|
|
|
Cash
Flows From Operating Activities:
|
|
|
|
|
|
|
|
Net
Loss
|
$(265,636)
|
$(195,702)
|
|
Depreciation and
amortization
|
6,740
|
525
|
|
Foreign exchange
loss
|
(14,868)
|
639
|
|
Stock issued for
licenses, services, and other assets
|
-
|
-
|
|
Stock issued for
payment interest
|
1,712
|
3,022
|
|
Loss on debt
conversion
|
38,340
|
76,929
|
|
Stock issued for
payment of expenses
|
-
|
14,400
|
|
(Increase) decrease
in accounts receivable
|
(12,882)
|
-
|
|
(Increase) decrease
in prepaid expenses
|
4,331
|
161
|
|
(Increase) decrease
in deposits
|
72,534
|
-
|
|
Increase (decrease)
in Accounts Payable & accrued expenses
|
(11,435)
|
(171)
|
|
Increase (decrease)
in interest payable
|
12,709
|
(7,840)
|
|
|
|
|
|
Net
Cash Flows Used in Operations
|
(168,455)
|
(108,037)
|
|
|
|
|
|
Cash
Flows From Investing Activities:
|
|
|
|
|
|
|
|
Cash paid for
Acquisition of Subsidiary
|
(80,289)
|
|
|
Cash received from
acquisition of subsidiary
|
4,942
|
|
|
Purchase of
equipment
|
(4,783)
|
-
|
|
|
|
|
|
Net
Cash Flows Used in Investing Activities
|
(80,130)
|
-
|
|
|
|
|
|
Cash
Flows From Financing Activities:
|
|
|
|
|
|
|
|
Proceed from notes
payables
|
356,885
|
50,256
|
|
Payment of notes
payable
|
(130,908)
|
-
|
|
Advances from
related parties
|
12,240
|
|
|
Payments to related
parties
|
(1,163)
|
|
|
Note payable used
to pay expenses
|
|
13,962
|
|
Note payable used
to pay origionation fees & interest
|
11,500
|
2,000
|
|
|
|
|
|
Net
Cash Flows Provided by Financing Activities
|
248,554
|
66,218
|
|
|
|
|
|
Net Increase
(Decrease) In Cash and cash equivalents
|
(31)
|
(41,819)
|
|
Foreign currency
translation adjustment
|
(2,738)
|
1,509
|
|
Cash and cash
equivalents at beginning of period
|
107,532
|
57,453
|
|
|
|
|
|
Cash and cash
equivalents at end of period
|
$104,763
|
$17,143
|
|
|
|
|
|
Supplementary
Disclosure Of Cash Flow Information:
|
|
|
|
|
|
|
|
Stock issued for
services, licenses and other assets
|
$-
|
$14,400
|
|
Stock issued for
note conversions including interest
|
$95,052
|
$128,451
|
|
Stock issued to pay
expenses and acquisition
|
$246,000
|
$-
|
|
Cash paid for
interest
|
$9,428
|
$-
|
|
Cash paid for
income taxes
|
$-
|
$-
|
See
Accompanying Notes To These Financial Statements.
5
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three Month Interim Period Ended March 31, 2018
Note 1 – Nature of Business and Basis of
Presentation
The
Company was originally incorporated under the name Mountain West
Business Solutions, Inc. (“MWBS”) on August 31, 2006 in
the State of Colorado. Until October 2009, the Company was
operating as a business consultancy firm. Effective October 15,
2009, the Company acquired Sunshine Biopharma, Inc. in a
transaction classified as a reverse acquisition. Sunshine
Biopharma, Inc. was holding an exclusive license to a new
anticancer drug bearing the laboratory name, Adva-27a. Upon
completion of the reverse acquisition transaction, the Company
changed its name to Sunshine Biopharma, Inc. and began operating as
a pharmaceutical company focusing on the development of the
licensed Adva-27a anticancer drug.
In July
2014, the Company formed a wholly owned Canadian subsidiary,
Sunshine Biopharma Canada Inc. (“Sunshine Canada”) for
the purposes of offering generic pharmaceutical products in Canada
and elsewhere around the world. Sunshine Canada has signed
licensing agreements for four (4) generic prescription drugs for
treatment of breast cancer, prostate cancer and BPH (Benign
Prostatic Hyperplasia). Sunshine Canada is currently evaluating
several other generic products for in-licensing and is working on
securing a Drug Establishment License (“DEL”) and a
Drug Identification Number (“DIN”) per product from
Health Canada. Once the DEL and the DIN’s are secured,
Sunshine Canada will begin its generic pharmaceuticals marketing
and sales program in Canada and overseas.
On
January 1, 2018 the Company acquired all of the issued and
outstanding shares of Atlas Pharma Inc. (“Atlas”), a
Canadian privately held company. The purchase price for the shares
was Eight Hundred Forty Eight Thousand Dollars ($848,000) Canadian
($684,697 US). The purchase price included a cash payment of
$100,500 Canadian ($80,289 US), plus the issuance of 20,000,000
shares of the Company’s Common Stock valued at $246,000 or
$0.0123 per share, and a promissory note in the principal amount of
$450,000 Canadian ($358,407 US), with interest payable at the rate
of 3% per annum. Atlas is a certified company dedicated to chemical
analysis of pharmaceutical and other industrial samples.
Atlas’ operations are authorized by a Drug Establishment
License issued by Health Canada. Atlas is also registered with the
FDA.
While the agreement to acquire Atlas
Pharma Inc. was signed effective January 1, 2018, there are several
matters which are yet to be
completed. In addition, as of the date of this report, the audit of
Atlas Pharma Inc. has not been completed. As a result, various disclosures in this report
may have to be updated. The updated information may differ and
the difference may be
material.
In
March 2018, the Company formed NOX Pharmaceuticals, Inc., a wholly
owned Colorado corporation and assigned all of the Company’s
interest in the Adva27a anticancer drug to that company. NOX
Pharmaceuticals Inc.’s mission is to research, develop and
commercialize proprietary drugs including Adva-27a.
The
financial statements represent the consolidated activity of
Sunshine Biopharma, Inc., Sunshine Biopharma Canada Inc., Atlas
Pharma Inc. and NOX Pharmaceuticals, Inc. (herein collectively
referred to as the "Company").
The
Company has been and continues to work on the development of its
proprietary anticancer drug, Adva-27a. The next series of steps in
the development of Adva-27a include (i) GMP-manufacturing of a
2-kilogram quantity of the drug, (ii) completing the requisite
IND-enabling studies, and (iii) conducting Phase I clinical trials.
In the preclinical studies, Adva-27a was shown to be effective at
destroying multidrug resistant cancer cells including Pancreatic
Cancer, Breast Cancer, Lung Cancer and Uterine Sarcoma,
cells.
During
the last three month period the Company has continued to raise
money through stock sales and borrowings.
The
Company’s activities are subject to significant risks and
uncertainties, including failing to secure additional funding to
operationalize the Company’s generics business and
proprietary drug development program.
6
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three Month Interim Period Ended March 31, 2018
Basis of Presentation of Unaudited Condensed Financial
Information
The
unaudited condensed financial statements of the Company for the
three month periods ended March 31, 2018 and 2017 have been
prepared in accordance with accounting principles generally
accepted in the United States of America for interim financial
information and pursuant to the requirements for reporting on Form
10-Q and Regulation S-K. Accordingly, they do not include all the
information and footnotes required by accounting principles
generally accepted in the United States of America for complete
financial statements. However, such information reflects all
adjustments (consisting solely of normal recurring adjustments),
which are, in the opinion of management, necessary for the fair
presentation of the financial position and the results of
operations. Results shown for interim periods are not necessarily
indicative of the results to be obtained for a full fiscal year.
The balance sheet information as of March 31, 2018 was derived from
the audited financial statements included in the Company's
financial statements as of and for the year ended December 31, 2017
included in the Company’s Annual Report on Form 10-K filed
with the Securities and Exchange Commission (the “SEC”)
on April 2, 2018. These financial statements should be read in
conjunction with that report.
Between May 2014 and December
2016, the FASB issued several ASU’s on Revenue from Contracts
with Customers (Topic 606). These updates will supersede nearly all
existing revenue recognition guidance under current U.S. generally
accepted accounting principles (GAAP). The core principle is to
recognize revenues when promised goods or services are transferred
to customers in an amount that reflects the consideration to which
an entity expects to be entitled for those goods or
services.
A
five-step process has been defined to achieve this core principle,
and, in doing so, more judgment and estimates may be required
within the revenue recognition process than are required under
existing U.S. GAAP. The standards are effective for annual periods
beginning after December 15, 2017, and interim periods therein,
using either of the following transition methods: (i) a full
retrospective approach reflecting the application of the standards
in each prior reporting period with the option to elect certain
practical expedients, or (ii) a retrospective approach with the
cumulative effect of initially adopting the standards recognized at
the date of adoption (which includes additional footnote
disclosures). The Company is currently evaluating the impact of its
pending adoption of these standards on its financial statements and
has not yet determined the method by which it will adopt the
standard in 2018.
Recently Issued Accounting Pronouncements
The
amendments are effective for fiscal years beginning after December
15, 2017, and should be applied prospectively to an award modified
on or after the adoption date. Early adoption is permitted,
including adoption in an interim period. The Company does not
expect this amendment to have a material impact on its financial
statements.
7
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three Month Interim Period Ended March 31, 2018
Note 2 – Going Concern
The
accompanying financial statements have been prepared on a going
concern basis, which contemplates the realization of assets and the
satisfaction of liabilities in the normal course of business. Since
inception, the Company has had recurring operating losses and
negative operating cash flows. These factors raise substantial
doubt about the Company’s ability to continue as a going
concern.
The
Company’s continuation as a going concern is dependent on its
ability to obtain additional financing to fund operations,
implement its business model, and ultimately, attain profitable
operations. The Company will need to secure additional funds
through various means, including equity and debt financing or any
similar financing. There can be no assurance that the Company will
be able to obtain additional equity or debt financing, if and when
needed, on terms acceptable to the Company, or at all. Any
additional equity or debt financing may involve substantial
dilution to the Company’s stockholders, restrictive covenants
or high interest costs. The Company’s long-term liquidity
also depends upon its ability to generate revenues and achieve
profitability.
Note 3 – Notes Payable
During
the three months period ended March 31, 2018, the Company entered
into the following new debt arrangements:
●
On January 12,
2018, the Company received net proceeds of $100,000 in exchange for
a note payable having a face value of $102,000 and accruing
interest at the rate of 8% per annum. The note, due on October 30,
2018, is convertible after 180 days from issuance into $0.001 par
value Common Stock at a price 35% below market value. The Company
estimates that the fair value of this convertible debt approximates
the face value, so no value has been assigned to the beneficial
conversion feature.
8
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three Month Interim Period Ended March 31, 2018
●
On February 7,
2018, the Company received net proceeds of $143,000 in exchange for
a note payable having a face value of $150,000 and accruing
interest at the rate of 8% per annum. The note, due on February 7,
2019, is convertible after 180 days from issuance into $0.001 par
value Common Stock at a price 35% below market value. The Company
estimates that the fair value of this convertible debt approximates
the face value, so no value has been assigned to the beneficial
conversion feature.
●
On February 22,
2018, the Company received net proceeds of $80,000 in exchange for
a note payable having a face value of $85,000 and accruing interest
at the rate of 8% per annum. The note, due on November 30, 2018, is
convertible after 180 days from issuance into $0.001 par value
Common Stock at a price 35% below market value. The Company
estimates that the fair value of this convertible debt approximates
the face value, so no value has been assigned to the beneficial
conversion feature.
At March 31, 2018, the Company had advances from private
individuals totaling $40,651 Canadian (approximately $32,520 US).
These advances were made on an interest free basis and are
repayable on demand.
At
March 31, 2018 and December 31, 2017, accrued interest on Notes
Payable was $21,924 and $9,481, respectively.
Note 4 – Notes Payable - Related Party
On
January 1, 2018 as part of an acquisition the Company has a note
payable in the amount of $450,000 Canadian ($358,407 US) and
accruing interest at the rate of 3% per annum. The note is due on
December 31, 2023. Payments on this note are $10,000 Canadian
(approximately $8,000 US) per quarter. The outstanding principal
balance at March 31, 2018 is $353,058.
In
addition to the above, on March 31, 2018 the Company had notes
payable from related parties amounting to $256,665 and accrued
interest of $6,088.
Note 5 – Issuance of Common Stock
During
the three months ended March 31, 2018, the Company issued a total
of 29,283,034 shares of $0.001 par value Common Stock. Of these
9,283,034 shares valued at $95,052 were issued upon conversion of
outstanding notes payable, reducing the debt by $55,000 and
interest payable by $1,712 and generating a loss on conversion of
$38,340.
In
addition, 20,000,000 shares valued at $246,000 or $0.0123 per share
were issued as part of the acquisition of Atlas Pharma
Inc.
The
Company declared no dividends through March 31, 2018.
Note 6 – Commitments
The Company’s subsidiary, Atlas Pharma Inc., has entered into
long-term lease agreements for the rental of buildings which call
for minimum lease payments of $228,113 and additional lease
payments based on operating expenses. The lease expires on May 21,
2021. Minimum lease payments for the next four years are $62,213 in
2018, $62,213 in 2019, $62,213 in 2020, and $41,474 in
2021.
Note 7 – Earnings (Loss) Per Share
Earnings
(loss) per share is computed using the weighted average number of
Common Shares outstanding during the period. The Company has
adopted ASC 260 (formerly SFAS128), “Earnings per
Share”.
9
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three Month Interim Period Ended March 31, 2018
Note 8 – Goodwill
On January 1, 2018, the Company acquired all of the issued and
outstanding shares of Atlas Pharma Inc. ("Atlas"), a Canadian
privately held company. The purchase price for the shares was Eight
Hundred Forty Eight Thousand Dollars ($848,000) Canadian ($684,697
US). The book value of the fixed assets acquired was $11,051. The
remainder of the purchase price ($673,646) was applied to
Goodwill.
Note 9 – Income Taxes
Deferred
income taxes arise from the temporary differences between financial
statement and income tax recognition of net operating losses and
other items. Loss carryovers are limited under the Internal Revenue
Code should a significant change in ownership occur.
A
deferred tax asset at each date has been offset by a 100% valuation
allowance.
Note 10 – Royalties Payable
As part
of a subscription agreement entered into in 2016, the Company has
an obligation to pay a royalty of 5% of net sales on one of its
generic products (Anastrozole) for a period of three (3) years from
the date of the first sale of that product.
Note 11 – Related Party Transactions
In
addition to the related party transactions detailed in Note 5
above, during the three month period ended March 31, 2018 and 2017,
the Company paid its Officers and Directors cash compensation
totaling $77,787 and $42,965, respectively.
Note 12 - Revenue Recognition
As of January 1, 2018, we adopted ASU No. 2014-09, “Revenue
from Contracts with Customers” (ASU 2014-09). Under
the new guidance, an entity will
recognize revenue to depict the transfer of promised goods or
services to customers at an
amount that the entity expects to be entitled to in exchange for
those goods or services. Leasing revenue recognition is
specifically excluded and therefore
the new standard is only applicable to service fee and consulting
revenue. A five-step model has
been introduced for an entity to apply when recognizing revenue.
The new guidance also includes enhanced disclosure requirements. The guidance was
effective January 1, 2018 and was applied on a modified basis. The
adoption did not have an
impact on the Company's financial statements.
Note 13 - Accounts Receivable
Accounts receivable consist of trade accounts arising in the normal
course of business and are classified as current assets
and carried at original invoice
amounts less an estimate for doubtful receivables based on a review
of outstanding balances on a
monthly basis. The estimate of allowance for doubtful accounts is
based on the Company's bad debt experience, market
conditions, and aging of accounts
receivable, among other factors. If the financial condition of the
Company's customers deteriorates resulting in the customer's
inability to pay the Company's receivables as they come due,
additional allowances for doubtful accounts will be
required.
Note 14 – Subsequent Events
During
April 2018, the holder of a note payable dated September 22, 2017
elected to convert $47,000 in principal and $2,480 in accrued
interest into 13,084,511 shares of Common Stock leaving a principal
balance of -0-.
On
April 30, 2018, the holder of a note payable dated October 26, 2017
elected to convert $23,000 in principal and $917 in accrued
interest into 7,408,615 shares of Common Stock leaving a principal
balance of $92,000.
On May
3, 2018, we signed an agreement with Jitney Trade Inc. whereby the
parties agreed to extend the equity financing that was previously
announced of up to $10,000,000 Canadian (approximately $8,000,000
US) until August 31, 2018. The terms and conditions of the
financing remained unchanged at up to 400,000,000 shares of the
Company’s Common Stock at $0.025 Canadian (approximately
$0.02 US) per share.
10
ITEM 2. MANAGEMENT’S DISCUSSION AND
ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
The following discussion should be read in conjunction with our
consolidated financial statements and notes thereto included
herein. In connection with, and because we desire to take advantage
of, the “safe harbor” provisions of the Private
Securities Litigation Reform Act of 1995, we caution readers
regarding certain forward looking statements in the following
discussion and elsewhere in this report and in any other statement
made by, or on our behalf, whether or not in future filings with
the Securities and Exchange Commission. Forward looking statements
are statements not based on historical information and which relate
to future operations, strategies, financial results or other
developments. Forward looking statements are necessarily based upon
estimates and assumptions that are inherently subject to
significant business, economic and competitive uncertainties and
contingencies, many of which are beyond our control and many of
which, with respect to future business decisions, are subject to
change. These uncertainties and contingencies can affect actual
results and could cause actual results to differ materially from
those expressed in any forward looking statements made by, or on
our behalf. We disclaim any obligation to update forward looking
statements.
Overview and History
We were
incorporated in the State of Colorado on August 31, 2006 under the
name “Mountain West Business Solutions, Inc.” Until
October 2009, our business was to provide management consulting
with regard to accounting, computer and general business issues for
small and home-office based companies.
In
October 2009, we acquired Sunshine Biopharma, Inc., a Colorado
corporation holding an exclusive license to a new anticancer drug
bearing the laboratory name, Adva-27a. As a result of this
transaction we changed our name to “Sunshine Biopharma,
Inc.” and our officers and directors resigned their positions
with us and were replaced by Sunshine Biopharma, Inc.’s
management at the time, including our current CEO, Dr. Steve N.
Slilaty, and our current CFO, Camille Sebaaly each of whom remain
part of our current management. Our principal business became that
of a pharmaceutical company focusing on the development of our
licensed Adva-27a anticancer compound. In December 2015 we acquired
all issued and pending patents pertaining to our Adva-27a
technology and terminated the license.
In July
2014, we formed a wholly owned Canadian subsidiary, Sunshine
Biopharma Canada Inc. (“Sunshine Canada”), for the
purposes of offering generic pharmaceutical products in Canada and
elsewhere around the world. Sunshine Canada has signed licensing
agreements for four (4) generic prescription drugs for the
treatment of breast cancer, prostate cancer and BPH (Benign
Prostatic Hyperplasia). We have applied for and are currently
awaiting the issuance by Health Canada of a Drug Establishment
License and a Drug Identification Number for each of our four (4)
generic products in order to begin marketing of the
same.
In
January 2018, we acquired Atlas Pharma Inc. (“Atlas”),
a certified company dedicated to chemical analysis of
pharmaceutical and other industrial samples whose operations are
authorized by a Drug Establishment License issued by Health Canada.
Atlas has been generating revenues since its inception in September
2013. The revenues reported in our consolidated financial
statements for the first calendar quarter of 2018 are a result of
the Atlas operations.
In
March 2018, we formed NOX Pharmaceuticals, Inc., a Colorado
corporation, and assigned all of our interest in our Adva-27a
anticancer compound to that company. NOX Pharmaceuticals
Inc.’s mission is to research, develop and commercialize
proprietary drugs including Adva-27a.
As a
result, we are now a holding company operating through these three
wholly owned subsidiaries.
Our
principal place of business is located at 6500 Trans-Canada Highway, 4th Floor,
Pointe-Claire, Quebec, Canada H9R 0A5. Our phone number is
(514) 426-6161and our website address is
www.sunshinebiopharma.com.
We have
not been subject to any bankruptcy, receivership or similar
proceeding.
Operations
Proprietary Drug Development Operations
Since
inception, our proprietary drug development activities have been
focused on the development of a small molecule called Adva-27a for
the treatment of aggressive forms of cancer. A Topoisomerase II
inhibitor, Adva-27a has been shown to be effective at destroying
Multidrug Resistant Cancer cells including Pancreatic Cancer cells,
Breast Cancer cells, Small-Cell Lung Cancer cells and Uterine
Sarcoma cells (Published in ANTICANCER RESEARCH, Volume 32, Pages
4423-4432, October 2012). Sunshine Biopharma Inc. owns all of the
rights, as well as, all of the issued and pending worldwide patents
pertaining to Adva-27a, including U.S. Patent Number
8,236,935.
11
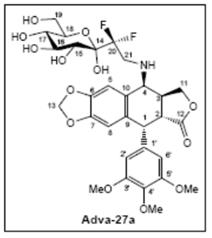
Adva-27a is a
GEM-difluorinated C-glycoside derivative of Podophyllotoxin.
Another derivative of Podophyllotoxin called Etoposide is currently
on the market and is used to treat various types of cancer
including leukemia, lymphoma, testicular cancer, lung cancer, brain
cancer, prostate cancer, bladder cancer, colon cancer, ovarian
cancer, liver cancer and several other forms of cancer. Etoposide
is one of the most widely used anticancer drugs. Adva-27a and
Etoposide are similar in that they both attack the same target in
cancer cells, namely the DNA unwinding enzyme, Topoisomerase II.
Unlike Etoposide, and other anti-tumor drugs currently in use,
Adva-27a is able to destroy Multidrug Resistant Cancer cells.
Adva-27a is the only compound known today that is capable of
destroying Multidrug Resistant Cancer. In addition, Adva-27a has
been shown to have distinct and more desirable biological and
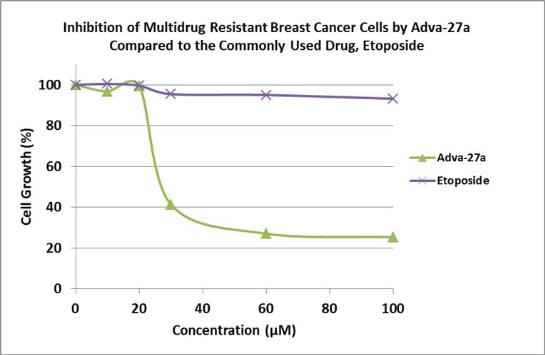
pharmacological properties compared to Etoposide. In side-by-side
studies using Multidrug Resistant Breast Cancer cells and Etoposide
as a reference, Adva-27a showed markedly improved cell killing
activity (see Figure below). Our preclinical studies to date have
shown that:
●
Adva-27a is
effective at killing different types of Multidrug Resistant cancer
cells, including Pancreatic Cancer Cells (Panc-1), Breast Cancer
Cells (MCF-7/MDR), Small-Cell Lung Cancer Cells (H69AR), and
Uterine Sarcoma Cells (MES-SA/Dx5).
●
Adva-27a is
unaffected by P-Glycoprotein, the enzyme responsible for making
cancer cells resistant to anti-tumor drugs.
●
Adva-27a has
excellent clearance time (half-life = 54 minutes) as indicated by
human microsomes stability studies and pharmacokinetics data in
rats.
●
Adva-27a clearance
is independent of Cytochrome P450, a mechanism that is less likely
to produce toxic intermediates.
●
Adva-27a is an
excellent inhibitor of Topoisomerase II with an IC50 of only 13.7
micromolar (this number has recently been reduce to 1.44 micromolar
as a result of resolving the two isomeric forms of
Adva-27a).
●
Adva-27a has shown
excellent pharmacokinetics profile as indicated by studies done in
rats.
●
Adva-27a does not
inhibit tubulin assembly.
These
and other preclinical data have been published in ANTICANCER
RESEARCH, a peer-reviewed International Journal of Cancer Research
and Treatment. The publication which is entitled “Adva-27a, a
Novel Podophyllotoxin Derivative Found to Be Effective Against
Multidrug Resistant Human Cancer Cells” [ANTICANCER RESEARCH
32: 4423-4432 (2012)] is available on our website at www.sunshinebiopharma.com.
12
We have
been delayed in our clinical development program due to lack of
funding. Our fund raising efforts are continuing and as soon as
adequate financing is in place we will continue our clinical
development program of Adva-27a by conducting the following next
sequence of steps:
●
GMP Manufacturing
of 2 kilogram for use in IND-Enabling Studies and Phase I Clinical
Trials
●
IND-Enabling
Studies
●
Regulatory Filing
(Fast-Track Status Anticipated)
●
Phase I Clinical
Trials (Pancreatic Cancer Indication)
Adva-27a’s
initial indication will be Pancreatic Cancer for which there are
currently little or no treatment options available. We are planning
to conduct our clinical trials at McGill University’s Jewish
General Hospital in Montreal, Canada. All aspects of the clinical
trials in Canada will employ FDA standards at all levels. We
estimate that the Pancreatic Cancer clinical trials will take
approximately 18 to 24 months from start to finish. Given the
terminal and limited treatment options available for the Pancreatic
Cancer, we anticipate being granted limited marketing approval
(“compassionate-use”) for our Adva-27a following a
successful Phase I clinical trial. It is likely that following such
events, we will begin to receive offers from large pharmaceutical
companies to buyout or license our drug. Alternatively, it may be
more advisable for us to continue our own development of the drug
by proceeding to Phase II clinical trials and attending to other
requirements for full marketing approval.
Our Lead Anti-Cancer Compound, Adva-27a, in 3D
Generic Pharmaceuticals Operations
In
2016, our Canadian wholly owned subsidiary, Sunshine Biopharma
Canada Inc. (“Sunshine Canada”), signed Cross
Referencing Agreements with a major pharmaceutical company for four
prescription generic drugs for the treatment of Breast Cancer,
Prostate Cancer and Enlarged Prostate. Following this acquisition
we have been working towards commencement of marketing of these
pharmaceutical products under our own Sunshine Biopharma label.
These four generic products are as follows:
●
Anastrozole (brand
name Arimidex® by AstraZeneca) for treatment of Breast
Cancer;
●
Letrozole (brand
name Femara® by Novartis) for treatment of Breast
Cancer;
●
Bicalutamide (brand
name Casodex® by AstraZeneca) for treatment of Prostate
Cancer;
●
Finasteride (brand
name Propecia® by Merck) for treatment of BPH (Benign
Prostatic Hyperplasia)
Worldwide sales of
the brand name version of these products as reported by the
respective pharmaceutical company, owner of the registered
trademark are as follows:
●
Arimidex®
$232M in 2016
●
Femara® $380M
in 2014
●
Casodex® $247M
in 2016
●
Propecia®
$183M in 2015
In June
2017, Sunshine Canada submitted an application to Health Canada for
the procurement of a Drug Establishment License
(“DEL”), a requirement for the Company’s drug
handling and pharmaceutical operations. Health Canada has assigned
the Company DEL Application No. 3002475 and File No. 17938. We are
currently awaiting Health Canada to set a date for physical
inspection of our warehouse and drug management operations. In
addition, we are currently in the process of filing applications
for a Drug Identification Number (“DIN”) for each of
its four (4) generic products, Anastrozole, Letrozole, Bicalutamide
and Finasteride. The Figure below shows our 30-Pill blister pack of
Anastrozole.
13

We
currently have twenty three (23) additional Generic Pharmaceuticals
under review for in-licensing. We believe that a larger product
portfolio will provide us with more opportunities and a greater
reach into the marketplace. Our plan is to fund our Proprietary
Drug Development Program, including Adva-27a, through the sales of
Generic Drugs. In addition to near-term revenue generation,
building the generics business infrastructure and securing the
proper permits will render us appropriately positioned for the
marketing and distribution of our own proprietary drugs as they
become available.
Analytical Chemistry Services Operations
On
January 1, 2018, we entered into an agreement (the “Atlas
Agreement”) to acquire Atlas Pharma Inc.
(“Atlas”). The purchase price was $848,000 Canadian
($684,697 US). Payment of the purchase price was comprised of (i) a
cash payment of $100,500 Canadian ($80,289 US), (ii) the issuance
of 20,000,000 shares of our Common Stock valued at $246,000, and
(iii) a promissory note in the principal amount of $450,000
Canadian ($358,407 US), with interest payable at the rate of 3% per
annum. We are required to make payments of $10,000 Canadian
(approximately $8,000 US) per calendar quarter, due and payable on
or before the end of each such calendar quarter through December
31, 2023.
Atlas
is a certified company dedicated to chemical analysis of
pharmaceutical and other industrial samples. Atlas has 9 full-time
employees and generated revenues of approximately $500,000 Canadian
(approximately $400,000 US) in 2017. Housed in a 5,250 square foot
facility, Atlas’s operations are authorized by a Drug
Establishment License (DEL) issued by Health Canada and are fully
compliant with the requirements of Good Manufacturing Practices
(GMP). Atlas is also registered with the FDA.
Atlas
is the owner of a relatively large portfolio of analytical
chemistry methodology and Standard Operating Procedure. This
intellectual property is protected as company secrets and
controlled through employee and management confidentiality
agreements.
We
intend to expand Atlas’ business operations by purchasing
additional equipment and hiring more technical and sales personnel.
Part of the expansion will include the development and addition of
new tests and new sample testing capabilities.
Results Of Operations
Comparison of Results of Operations for the three months ended
March 31, 2018 and 2017
For
the three months ended March 31, 2018, we generated $91,168 in
revenues compared to no revenues for the same three months of 2017.
All of these revenues were generated from the operations of our
newly acquired wholly owned subsidiary, Atlas Pharma Inc.
(“Atlas”), which we acquired on December 31,
2017.
General
and administrative expenses during the three month period ended
March 31, 2018 were $257,865, compared to general and
administrative expense of $108,990 incurred during the three month
period ended March 31, 2017, an increase of $148,875. This increase
is attributable to increases in the following expense
categories:
●
Accounting
Fees increased by $12,400 due to accounting expenses associated
with the acquisition of Atlas.
●
Legal
Fees increased by $12,581 as a result of legal expenses in
connection with the acquisition of Atlas.
●
Office
Expenses increased by $9,982 due the consolidation of Atlas office
expenses.
●
Rent
increased by $23,059 which is the rent for the space occupied by
Atlas.
●
Salaries increased by $57,558 which is entirely for the
full-time employees of Atlas.
●
Supplies increased by $14,070 which are largely laboratory
supplies used in the analytical testing services provided by
Atlas.
In
addition, we saw an increase of $34,822 in Officer & Director
Compensation as well as an increase of $6,222 in Depreciation, the
latter being a result of depreciation associated with the
analytical chemistry equipment of Atlas. The only expense category
that decreased was Consulting Fees by $21,819 as most of the
required work was performed by salaried Atlas
personnel.
14
We
also incurred $75,467 in interest expense during the three months
ended March 31, 2018, compared to $9,144 in interest expense during
the similar period in 2017 as a result of increased
borrowings. However, we incurred $38,340 in losses
arising from debt conversion during the three months ended March
31, 2018, compared to $76,929 in losses from debt conversion during
the similar period in 2017, a difference of $38,589 as a result of
some convertible notes having been paid off prior to
maturity.
As
a result, we incurred a net loss of $265,636 ($0.00 per share) for
the three month period ended March 31, 2018, compared to a net loss
of $195,702 ($0.00 per share) during the three month period ended
March 31, 2017.
Liquidity and Capital Resources
As
of March 31, 2018, we had cash or cash equivalents of
$104,763.
Net cash used in operating activities was $168,455
during the three month period ended March 31, 2018, compared to
$108,037 for the three month period ended March 31,
2017. We anticipate that overhead costs and other
expenses will increase in the future as we move forward with our
proprietary drug development activities and expansion of our
generic pharmaceuticals operations as well as our newly acquired
analytical chemistry services operations as discussed above.
Cash
flows from financing activities were $248,462 for the three month
periods ended March 31, 2018, compared to $66,218 during the three
months ended March 31, 2017. Cash flows used by
investing activities were $106,101 for the three month period ended
March 31, 2018 compared to $0 during the same three months period
in 2017.
During
the three months period ended March 31, 2018, we issued a total of
29,283,034 shares of our Common Stock. Of these, 9,283,034 shares
valued at $95,052 were issued upon conversion of outstanding notes
payable, reducing debt by $55,000 and interest payable by $1,712
and generating a loss on conversion of $38,340. In addition, we
issued 20,000,000 shares of our Common Stock valued at $246,000 or
$0.0123 per share as part of the acquisition of Atlas Pharma
Inc.
During
the three months ended March 31, 2018, we entered into the
following new debt arrangements:
●
On January 12,
2018, we received net proceeds of $100,000 in exchange for a note
payable having a face value of $102,000 and accruing interest at
the rate of 8% per annum. The note, due on October 30, 2018, is
convertible after 180 days from issuance into $0.001 par value
Common Stock at a price 35% below market value. We estimate that
the fair value of this convertible debt approximates the face
value, so no value has been assigned to the beneficial conversion
feature.
●
On February 7,
2018, we received net proceeds of $143,000 in exchange for a note
payable having a face value of $150,000 and accruing interest at
the rate of 8% per annum. The note, due on February 7, 2019, is
convertible after 180 days from issuance into $0.001 par value
Common Stock at a price 39% below market value. We estimate that
the fair value of this convertible debt approximates the face
value, so no value has been assigned to the beneficial conversion
feature.
●
On February 22,
2018, we received net proceeds of $80,000 in exchange for a note
payable having a face value of $85,000 and accruing interest at the
rate of 8% per annum. The note, due on November 30, 2018, is
convertible after 180 days from issuance into $0.001 par value
Common Stock at a price 39% below market value. We estimate that
the fair value of this convertible debt approximates the face
value, so no value has been assigned to the beneficial conversion
feature.
In
August 2017 we signed an agreement with Jitney Trade Inc.
(“Jitney”), a Canadian broker-dealer, to raise up to
$10 million Canadian (approximately $8 million US) in a private
offering being undertaken only in Canada (the
“Offering”) in order to provide the funding we have
estimated we need to implement our business plan. The Offering
expired on February 28, 2018 without any funds having been raised.
Since February 28, 2018, we have been engaged in negotiations with
Jitney concerning the terms for extending the Offering. On May 3,
2018, we signed an agreement with Jitney whereby the parties agreed
to extend the Offering under the same terms and conditions until
August 31, 2018.
We are not generating adequate revenues from our
operations, and our ability to implement our business plan as set
forth herein will depend on the future availability of financing.
Such financing will be required to enable us to expand our
Analytical Chemistry Services business and further develop our
Generic Pharmaceuticals operations and Proprietary Drug Development
program. We intend to raise funds through private placements of our
Common Stock and/or debt financing. We estimate that we will
require approximately $7 million ($2 million for the Analytical
Chemistry and Generic Pharmaceuticals operations and $5 million for
the Proprietary Drug Development program) to fully implement our
business plan in the future and there are no assurances that we
will be able to raise this capital. Our inability to obtain
sufficient funds from external sources when needed will have a
material adverse effect on our plan of operation, results of
operations and financial condition. Our plan is to fund our
Proprietary Drug Development Program, including Adva-27a, through
the sales of Generic Drugs if we are unable to find any additional
financing. There are also no assurances that we will generate
sufficient revenues and profits from our Proprietary Drug
Development Program to accomplish these objectives.
15
Our
cost of operations is expected to increase as we move forward with
implementation of our business plan. We do not have sufficient
funds to cover the anticipated increase in the relevant expenses.
We need to raise additional capital in order to continue our
existing operations and finance our expansion plans for the next
year. If we are successful in raising additional funds, we expect
our operations and business efforts to continue and expand. There
are no assurances this will occur.
Subsequent Events
During
April 2018, the holder of a note payable dated September 22, 2017
elected to convert $47,000 in principal and $2,480 in accrued
interest into 13,084,511 shares of Common Stock leaving a principal
balance of $0.
On
April 30, 2018, the holder of a note payable dated October 26, 2017
elected to convert $23,000 in principal and $917 in accrued
interest into 7,408,615 shares of Common Stock leaving a principal
balance of $92,000.
On May
3, 2018, we signed an agreement with Jitney Trade Inc. whereby the
parties agreed to extend the proposed equity financing that was
previously announced of up to $10,000,000 Canadian (approximately
$8,000,000 US), until August 31, 2018. The terms and conditions of
the financing remained unchanged. We intend to offer at up to
400,000,000 shares of our Common Stock at a price of $0.025
Canadian (approximately $0.02 US) per share. There are no
assurances that Jitney will sell any shares of our Common Stock in
this proposed offering.
Off Balance Sheet Arrangements
None
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET
RISK.
We
are a smaller reporting company and are not required to provide the
information under this item pursuant to Regulation
S-K.
ITEM 4. CONTROLS AND PROCEDURES.
Disclosure
Controls and Procedures – Our
management, with the participation of our Chief Executive Officer
and Chief Financial Officer, has evaluated the effectiveness of our
disclosure controls and procedures (as such term is defined in
Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of
1934, as amended (the “Exchange Act”) as of the end of
the period covered by this Report.
These
controls are designed to ensure that information required to be
disclosed in the reports we file or submit pursuant to the
Securities Exchange Act of 1934 is recorded, processed, summarized
and reported within the time periods specified in the rules and
forms of the Securities and Exchange Commission, and that such
information is accumulated and communicated to our management,
including our CEO/CFO to allow timely decisions regarding required
disclosure.
16
Based on this
evaluation, our CEO and CFO have concluded that our disclosure
controls and procedures were not effective as of March 31, 2018, at
reasonable assurance level, for the following reasons:
● Ineffective control
environment and lack of qualified full-time CFO who has SEC
experience to focus on our financial affairs;
● Lack of qualified
and sufficient personnel, and processes to adequately and timely
identify making any and all required public
disclosures;
● Deficiencies in the
period-end reporting process and accounting policies;
● Inadequate internal
controls over the application of new accounting principles or the
application of existing accounting principles to new
transactions;
● Inadequate internal
controls relating to the authorization, recognition, capture, and
review of transactions, facts, circumstances, and events that could
have a material impact on the company’s financial reporting
process;
● Deficient revenue
recognition policies;
● Inadequate internal
controls with respect to inventory transactions; and
● Improper and lack
of timely accounting for accruals such as prepaid expenses,
accounts payable and accrued liabilities.
Our
Board of Directors has assigned a priority to the short-term and
long-term improvement of our internal control over financial
reporting. We are reviewing various potential solutions to remedy
the processes that would eliminate the issues that may arise due to
the absence of separation of duties within the financial reporting
functions. Additionally, the Board of Directors will work with
management to continuously review controls and procedures to
identified deficiencies and implement remediation within our
internal controls over financial reporting and our disclosure
controls and procedures.
We
believe that our financial statements presented in this annual
report on Form 10-K fairly present, in all material respects, our
financial position, results of operations, and cash flows for all
periods presented herein.
Inherent
Limitations – Our management, including our Chief
Executive Officer and Chief Financial Officer, does not expect that
our disclosure controls and procedures will prevent all error and
all fraud. A control system, no matter how well
conceived and operated, can provide only reasonable, not absolute,
assurance that the objectives of the control system are
met. The design of any system of controls is based in
part upon certain assumptions about the likelihood of future
events, and there can be no assurance that any design will succeed
in achieving its stated goals under all potential future
conditions. Further, the design of a control system must
reflect the fact that there are resource constraints, and the
benefits of controls must be considered relative to their costs.
Because of the inherent limitations in all control systems, no
evaluation of controls can provide absolute assurance that all
control issues and instances of fraud, if any, within our company
have been detected. These inherent limitations include
the realities that judgments in decision-making can be faulty, and
that breakdown can occur because of simple error or mistake. In
particular, many of our current processes rely upon manual reviews
and processes to ensure that neither human error nor system
weakness has resulted in erroneous reporting of financial
data.
Changes in
Internal Control over Financial Reporting – There were
no changes in our internal control over financial reporting during
the three month period ended March 31, 2018, which were identified
in conjunction with management’s evaluation required by
paragraph (d) of Rules 13a-15 and 15d-15 under the Exchange Act,
that have materially affected, or are reasonably likely to
materially affect, our internal control over financial
reporting.
17
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
On
November 14, 2014, we entered into a Manufacturing Services
Agreement with Lonza Ltd. and Lonza Sales Ltd. (hereinafter jointly
referred to as “Lonza”), whereby we engaged Lonza to be
the manufacturer of our Adva-27a anticancer drug. In June 2016 we
received a sample of the pilot manufacturing run for evaluation.
Our laboratory analyses showed that, while the sample meets all of
the required chemical, physical and biological specifications, the
amount of material generated (the “Yield”) by the pilot
run was found to be significantly lower than anticipated. We are
currently working towards finding possible solutions to increase
the Yield and define a path forward. During the course of our
discussions concerning the problem of the low Yield, Lonza informed
us that they required us to pay them $687,818 prior to moving
forward with any activity pertaining to the manufacturing agreement
we have with them. We have repeatedly indicated to Lonza that a
clear path defining exactly how the extremely low Yield issue would
be addressed is imperative prior to us making any payments. We
issued a letter to them in June 2017 advising of our position. As
of the date of this Report we have not received a response to our
letter and no further action has been taken by either
party.
To the
best of our management’s knowledge and belief, there are no
other material claims that have been brought against us nor have
there been any claims threatened.
ITEM 1A. RISK FACTORS
We
are a smaller reporting company and are not required to provide the
information under this item pursuant to Regulation
S-K.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF
PROCEEDS
During
the three months period ended March 31, 2018, we issued a total of
29,283,034 shares of our Common Stock. Of these, 9,283,034 shares
valued at $95,052 were issued upon conversion of outstanding notes
payable. In addition, we issued 20,000,000 shares of our Common
Stock as part of the consideration for the acquisition of Atlas
Pharma Inc.
We
relied upon the exemption from registration provided by Section
4(2) of the Securities Act of 1933, as amended, to issue these
shares.
Other
than reduction of debt from the conversion of the outstanding
convertible notes described above, we did not receive any direct
proceeds from the issuance of these shares. The proceeds from the
notes were used for working capital.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURE
Not
Applicable.
ITEM 5. OTHER INFORMATION
None.
ITEM 6. EXHIBITS
|
Exhibit No.
|
|
Description
|
|
|
|
|
|
|
Certification
of Chief Executive Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002
|
|
|
|
|
|
|
|
Certification
of Chief Financial Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002
|
|
|
|
|
|
|
|
Certification
of Chief Executive Officer and Chief Financial Officer Pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002
|
|
101.INS
|
|
XBRL
Instance Document*
|
|
|
|
|
|
101.SCH
|
|
XBRL
Schema Document*
|
|
|
|
|
|
101.CAL
|
|
XBRL
Calculation Linkbase Document*
|
|
|
|
|
|
101.DEF
|
|
XBRL
Definition Linkbase Document*
|
|
|
|
|
|
101.LAB
|
|
XBRL
Label Linkbase Document*
|
|
|
|
|
|
101.PRE
|
|
XBRL
Presentation Linkbase Document*
|
______________________
*
Pursuant to Rule 406T of Regulation S-T, these interactive data
files are not deemed filed or part of a registration statement or
prospectus for purposes of Section 11 or 12 of the Securities Act
or Section 18 of the Securities Exchange Act and otherwise not
subject to liability.
18
SIGNATURES
Pursuant
to the requirements of Section 12 of the Securities and Exchange
Act of 1934, the Registrant has duly caused this Report to be
signed on its behalf by the undersigned, thereunto duly authorized
on May 21, 2018.
|
|
SUNSHINE BIOPHARMA, INC.
|
|
|
|
|
|
|
|
|
|
By:
|
s/ Dr.
Steve N. Slilaty
|
|
|
|
|
Dr.
Steve N. Slilaty,
|
|
|
|
|
Principal
Executive Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By:
|
s/
Camille Sebaaly
|
|
|
|
|
Camille
Sebaaly,
Principal
Financial Officer and
|
|
|
|
|
Principal
Accounting Officer
|
|
19
