Attached files
U.S. SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Form 10-Q
Quarterly Report Under
the Securities Exchange Act of 1934
For Quarter Ended: September 30, 2010
Commission File Number: 000-52898
SUNSHINE BIOPHARMA INC.
(Exact name of small business issuer as specified in its charter)
|
Colorado
|
20-5566275
|
|
|
(State of other jurisdiction
of incorporation)
|
(IRS Employer ID No.)
|
2015 Peel Street
5th Floor
Montreal, Quebec, Canada H3A 1T8
(Address of principal executive offices)
6100 Royalmount Ave.
Montreal, Quebec, Canada H4P 2R2
(Former Address)
(514) 764-9698
(Issuer’s Telephone Number)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days: Yes þ No o.
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes o No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o
|
Accelerated filer o
|
|
Non-accelerated filer o (Do not check if a
smaller reporting company)
|
Smaller reporting company þ
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). o Yes þ No
The number of shares of the registrant’s only class of common stock issued and outstanding as of November 11, 2010, was 30,613,007 shares.
1
TABLE OF CONTENTS
PART I.
FINANCIAL INFORMATION
|
Page No.
|
||
|
Item 1.
|
Financial Statements
|
4
|
|
Consolidated Balance Sheet as of September 30, 2010 (unaudited)
|
4
|
|
|
Unaudited Statement of Operations for the Three Month Period
Ended September 30, 2010
|
5
|
|
|
Unaudited Consolidated Statements of Operations for the Nine Month Period Ended
September 30, 2010 and for the Period beginning August 17, 2009 through
September 30, 2010
|
6
|
|
|
Unaudited Statement of Shareholders Equity
|
7
|
|
|
Unaudited Consolidated Statement of Cash Flows for the for the Nine Month
Period Ended September 30, 2010 and for the Period
beginning August 17, 2009 (Inception) through September 30, 2010
|
8
|
|
|
Notes to Consolidated Financial Statements
|
9
|
|
|
Item 2.
|
Management’s Discussion and Analysis of Financial Condition and
Results of Operations/Plan of Operation.
|
10
|
|
Item 3.
|
Quantitative and Qualitative Disclosures About Market Risk.
|
15
|
|
Item 4.
|
Controls and Procedures.
|
15
|
|
PART II
|
||
|
OTHER INFORMATION
|
||
|
Item 1.
|
Legal Proceedings.
|
15
|
|
Item 1A.
|
Risk Factors
|
15
|
|
Item 2.
|
Unregistered Sales of Equity Securities and Use of Proceeds.
|
16
|
|
Item 3.
|
Defaults Upon Senior Securities.
|
16
|
|
Item 4.
|
[Removed and Reserved]
|
16
|
|
Item 5.
|
Other Information.
|
16
|
|
Item 6.
|
Exhibits.
|
16
|
|
Signatures
|
17
|
2
PART I.
ITEM 1. FINANCIAL STATEMENTS
SUNSHINE BIOPHARMA, INC.
(f/k/a Mountain West Business Solutions, Inc.)
Consolidated Balance Sheet
(A Development Stage Company)
|
Unaudited
September
30, 2010
|
Audited
December
31, 2009
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 108,148 | $ | 112,116 | ||||
|
Total Current Assets
|
108,148 | 112,116 | ||||||
|
TOTAL ASSETS
|
$ | 108,148 | $ | 112,116 | ||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
||||||||
|
Current Liabilities:
|
||||||||
|
Accounts payable
|
$ | 23,781 | $ | 14,314 | ||||
|
TOTAL LIABILITIES
|
$ | 23,781 | $ | 14,314 | ||||
|
SHAREHOLDERS' EQUITY
|
||||||||
|
Preferred stock, $.10 par value per share; Authorized
1,000,000 Shares; Issued and outstanding 850,000 shares.
|
$ | 73,000 | $ | 73,000 | ||||
|
Common Stock, $.001 par value per share; Authorized
200,000,000 Shares; Issued and outstanding 29,876,674
shares and 29,660,007 shares
|
29,877 | 29,660 | ||||||
|
Capital paid in excess of par value
|
1,343,055 | 1,196,272 | ||||||
|
Accumulated other comprehensive (Loss)
|
- | - | ||||||
|
(Deficit) accumulated during the development stage
|
(1,361,565 | ) | (1,201,130 | ) | ||||
|
TOTAL SHAREHOLDERS' EQUITY
|
$ | 84,367 | $ | 97,802 | ||||
|
TOTAL LIABILITIES AND SHAREHOLDERS' EQUITY
|
$ | 108,148 | $ | 112,116 | ||||
See Accompanying Notes To These Financial Statements.
3
SUNSHINE BIOPHARMA, INC.
(f/k/a Mountain West Business Solutions, Inc.)
Unaudited Statement of Operations
(A Development Stage Company)
|
Unaudited
3 Months
Ended
September
30, 2010
|
||||
|
Revenue:
|
$ | - | ||
|
General & Administrative Expenses
|
||||
|
Accounting
|
2,000 | |||
|
Office
|
143 | |||
|
Legal
|
12,858 | |||
|
Professional fees
|
(1,527,500 | ) | ||
|
Stock Transfer fees
|
225 | |||
|
Total General & Administrative Expenses
|
(1,512,274 | ) | ||
|
Net (Loss)
|
$ | 1,512,274 | ||
|
Basic (Loss) per common share
|
$ | 0.05 | ||
|
Weighted Average Common Shares Outstanding
|
29,690,563 | |||
See Accompanying Notes To These Financial Statements.
4
SUNSHINE BIOPHARMA, INC.
(f/k/a Mountain West Business Solutions, Inc.)
Unaudited Consolidated Statement of Operations
(A Development Stage Company)
|
9 Months
Ended
September
30, 2010
|
August 17,
2009 (inception)
through
September
30, 2009
|
August 17,
2009 (inception)
through
September
30, 2010
|
||||||||||
|
Revenue:
|
$ | - | $ | - | $ | - | ||||||
|
General & Administrative Expenses
|
||||||||||||
|
Accounting
|
9,795 | 2,500 | 17,395 | |||||||||
|
Office
|
283 | 905 | 2,338 | |||||||||
|
Incorporation Costs
|
- | - | 3,000 | |||||||||
|
Legal
|
41,166 | 25,600 | 86,909 | |||||||||
|
Merger costs
|
- | 155,150 | 155,150 | |||||||||
|
Misc. Expenses
|
- | - | 708 | |||||||||
|
Professional fees
|
47,000 | - | 47,000 | |||||||||
|
Public Relations
|
8,366 | 3,000 | 49,264 | |||||||||
|
Stock Transfer Fees
|
3,825 | - | 3,825 | |||||||||
|
Writedown of intangible assets
|
50,000 | 945,974 | 995,976 | |||||||||
|
Total General & Administrative Expenses
|
160,435 | 1,133,129 | 1,361,565 | |||||||||
|
Net (Loss)
|
$ | (160,435 | ) | $ | (1,133,129 | ) | $ | (1,361,565 | ) | |||
|
Basic (Loss) per common share
|
$ | (0.01 | ) | $ | (0.12 | ) | ||||||
|
Weighted Average Common Shares Outstanding
|
29,690,563 | 9,724,171 | ||||||||||
See Accompanying Notes To These Financial Statements.
5
SUNSHINE BIOPHARMA, INC.
(f/k/a Mountain West Business Solutions, Inc.)
Unaudited Statement of Shareholders’ Equity
(A Development Stage Company)
|
Number of
Common
Shares Issued
|
Common
Stock
|
Capital Paid
In Excess
of Par Value
|
Number of
Preferred
Shares Issued
|
Preferred
Stock
|
Deficit
Accumulated
During the
Development
Stage
|
Total
|
||||||||||||||||||||||
|
Balance at August 17, 2009 (Inception)
|
- | $ | - | $ | - | - | $ | - | $ | - | $ | - | ||||||||||||||||
|
August 17, 2009 issued 703,118
shares of par value $.001 common
stock for services valued at or
$.004 per share
|
703,118 | 703 | 2,297 | - | - | - | 3,000 | |||||||||||||||||||||
|
August 19, 2009 issued 218,388
shares of par value $.001 common
stock for services valued at or
$.004 per share
|
218,388 | 218 | 714 | - | - | - | 932 | |||||||||||||||||||||
|
August 20, 2009 issued 17,109,194
shares of par value $.001 common
stock and 730,000 shares of par
value $0.10 preferred stock for
license Agreement Advanomics: Common
valued at or $.004 per share and preferred
valued at or $.086 per share
|
17,109,194 | 17,109 | 55,891 | 850,000 | 73,000 | - | 146,000 | |||||||||||||||||||||
|
September-October 2009; Private
Placement – The Company sold
2,220,552 shares of par value $.001
common stock for cash of $649,000 or
$.2922 per share
|
2,220,552 | 2,221 | 646,779 | - | - | - | 649,000 | |||||||||||||||||||||
|
September 30, 2009 issued 1,710,748
shares of par value $.001 common
stock for asset purchase from Sunshine
Bio Investment valued at or $.2922 per
share
|
1,710,748 | 1,711 | 498,289 | - | - | - | 500,000 | |||||||||||||||||||||
|
October 31, 2009 Outstanding stock of
MWBS counted as issued for MWBS
net deficit
|
888,000 | 888 | (30,353 | ) | - | - | - | (29,465 | ) | |||||||||||||||||||
|
November 16, 2009 Note conversions,
several, Principal of $26,500 and interest
of $2,965
|
6,810,000 | 6,810 | 22,655 | - | - | - | 29,465 | |||||||||||||||||||||
|
Fractional Shares
|
7 | - | - | - | - | - | - | |||||||||||||||||||||
|
Net (Loss)
|
- | - | - | - | - | (1,201,130 | ) | (1,201,130 | ) | |||||||||||||||||||
|
Balance at December 31, 2009
|
29,660,007 | 29,660 | 1,196,272 | 850,000 | 73,000 | (1,201,130 | ) | 97,802 | ||||||||||||||||||||
|
June 2, 2010 issued 1,675,000 shares of
par value $.001 common stock for
services valued at or $.94 per share
|
1,675,000 | 1,675 | 1,572,825 | - | - | - | 1,574,500 | |||||||||||||||||||||
|
September 30, 2010 reversed issuance of
1,625,000 shares of par value $.001
common stock for services valued at or
$.94 per share
|
(1,625,000 | ) | (1,625 | ) | (1,525,875 | ) | - | - | - | (1,527,500 | ) | |||||||||||||||||
|
September 30, 2010 issued 166,667
shares of par value $.001 common stock
for cash at or $.60 per share
|
166,667 | 167 | 99,833 | - | - | - | 100,000 | |||||||||||||||||||||
|
Net (Loss)
|
- | - | - | - | - | (160,435 | ) | (160,435 | ) | |||||||||||||||||||
|
Balance at September 30, 2010 (Unaudited)
|
29,876,67 4 | $ | 29,877 | $ | 1,343,055 | 850,000 | $ | 73,000 | $ | (1,361,565 | ) | $ | 84,367 | |||||||||||||||
See Accompanying Notes To These Financial Statements.
6
SUNSHINE BIOPHARMA, INC.
(f/k/a Mountain West Business Solutions, Inc.)
Unaudited Consolidated Statement of Cash Flows
(A Development Stage Company)
|
9 Months
Ended
September
30, 2010
|
August 17,
2009 (inception)
through
September
30, 2009
|
August 17,
2009 (inception)
through
September
30, 2010
|
||||||||||
|
Cash Flows From Operating Activities:
|
||||||||||||
|
Net (Loss)
|
$ | (160,435 | ) | $ | (1,133,129 | ) | $ | (1,361,565 | ) | |||
|
Adjustments to reconcile net loss to net cash used in
operating activities:
|
||||||||||||
|
Stock issued for licenses, services, and other assets
|
47,000 | 663,974 | 696,932 | |||||||||
|
Increase in Accounts Payable
|
9,467 | 0 | 23,781 | |||||||||
|
Net Cash Flows (used) in operations
|
(103,968 | ) | (469,155 | ) | (640,852 | ) | ||||||
|
Cash Flows From Investing Activities:
|
||||||||||||
|
Net Cash Flows (used) in Investing activities
|
- | - | - | |||||||||
|
Cash Flows From Financing Activities:
|
||||||||||||
|
Issuance of common stock
|
100,000 | 649,000 | 749,000 | |||||||||
|
Net Cash Flows provided by financing activities
|
100,000 | 649,000 | 749,000 | |||||||||
|
Net Increase (Decrease) In Cash and cash equivalents
|
(3,968 | ) | 179,845 | 108,148 | ||||||||
|
Cash and cash equivalents at beginning of period
|
112,116 | - | - | |||||||||
|
Cash and cash equivalents at end of period
|
$ | 108,148 | $ | 179,845 | $ | 108,148 | ||||||
|
Supplementary Disclosure Of Cash Flow Information:
|
||||||||||||
|
Stock issued for services, licenses and other assets
|
$ | 47,000 | $ | 663,974 | $ | 649,932 | ||||||
|
Stock issued for note conversions
|
$ | - | $ | - | $ | 29,465 | ||||||
|
Stock issued for net deficit of MWBS
|
$ | - | $ | - | $ | (29,465 | ) | |||||
|
Cash paid for interest
|
$ | - | $ | - | $ | - | ||||||
|
Cash paid for income taxes
|
$ | - | $ | - | $ | - | ||||||
See Accompanying Notes To These Financial Statements.
7
Sunshine Biopharma, Inc
Notes To Unaudited Financial Statements
For The Three Month and Nine Month Interim Period Ended September 30, 2010
Note 1 - Unaudited Financial Information
The unaudited financial information included for the three month and nine month interim period ended September 30, 2010 was taken from the books and records without audit. However, such information reflects all adjustments, consisting only of normal recurring adjustments, which in the opinion of management are necessary to reflect properly the results of the interim periods presented. The results of operations for the three month interim period ended September 30, 2010 are not necessarily indicative of the results expected for the fiscal year ended December 31, 2010.
Note 2 - Financial Statements
For a complete set of footnotes, reference is made to the Company’s Report on Form 10-K for the year ended December 31, 2009 as filed with the Securities and Exchange Commission and the audited financial statements included therein.
Note 3 – Stock Issuance
On September 30, 2010 the Company commenced a private offering of its Common Stock. It is offering up to 6,000,000 shares of Common Stock at an offering price of $0.60 per share. As of September 30, 2010, the Company had sold 166,667 shares in this Offering and received gross proceeds of $100,000 therefrom.
Also in September 2010 the Company reversed the issuance of 1,625,000 shares of stock that it originally issued in exchange for services valued at $1,527,500.
8
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
The following discussion should be read in conjunction with our consolidated financial statements and notes thereto included herein. In connection with, and because we desire to take advantage of, the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, we caution readers regarding certain forward looking statements in the following discussion and elsewhere in this report and in any other statement made by, or on our behalf, whether or not in future filings with the Securities and Exchange Commission. Forward looking statements are statements not based on historical information and which relate to future operations, strategies, financial results or other developments. Forward looking statements are necessarily based upon estimates and assumptions that are inherently subject to significant business, economic and competitive uncertainties and contingencies, many of which are beyond our control and many of which, with respect to future business decisions, are subject to change. These uncertainties and contingencies can affect actual results and could cause actual results to differ materially from those expressed in any forward looking statements made by, or on our behalf. We disclaim any obligation to update forward looking statements.
Overview and History
We were incorporated in the State of Colorado on August 31, 2006 under the name “Mountain West Business Solutions, Inc.” During our fiscal year ended July 31, 2009 our business was to provide management consulting with regard to accounting, computer and general business issues for small and home-office based companies. Effective October 15, 2009, we executed an agreement to acquire Sunshine Biopharma, Inc., a Colorado corporation (“SBI”), in exchange for the issuance of 21,962,000 shares of our Common Stock and 850,000 shares of Convertible Preferred Stock, each convertible into twenty (20) shares of our Common Stock (the “Agreement”). As a result of this transaction our officers and directors resigned their positions with us and were replaced by our current management. The effectiveness of the Agreement was conditional upon various conditions being satisfied, including the filing of our Form 10-K for our fiscal year ended July 31, 2009 and SBI changing its name to Sunshine Etopo, Inc. These conditions were satisfied and Sunshine Etopo (formerly SBI) is now a wholly owned subsidiary of our Company. Also as a result of this transaction we have changed our name to “Sunshine Biopharma, Inc.”
We entered into this transaction because of our former management’s belief that by doing so we will significantly increase our shareholders’ future opportunity to enhance the value of their respective ownership in our Company. In addition, our former Board of Directors approved a “spin-off” of our wholly owned subsidiary company Mountain West Beverage, Inc. The terms of this “spin-off” provide for a dividend to be issued to our shareholders of one share of common stock for every share that our shareholders owned as of October 14, 2009, the record date of the dividend.
In January 2010, our Board of Directors adopted a resolution changing our fiscal year from July 31 to December 31, effective December 31, 2009. Article VIII, Section 2 of our Bylaws provides the authority for our Board of Directors to establish our fiscal year on a date in their sole discretion. Our Board undertook this resolution in order to have the fiscal year coincide with the fiscal year end for our wholly owned operating subsidiary company.
As a result of the transaction described above we have revised our current business plan to that of a pharmaceutical company focused on the research, development and commercialization of drugs for the treatment of various forms of cancer.
We have moved our principal place of business to 2015 Peel Street, 5th Floor, Montreal, Quebec, Canada H3A 1T8. From October 2009 through September 30, 2010, our principal place of business was located at 6100 Royalmount Ave., Montreal, Quebec, Canada H4P 2R2.
We have not been subject to any bankruptcy, receivership or similar proceeding.
Results Of Operations
Comparison of Results of Operations for the nine months ended September 30, 2010 and 2009
9
For the nine months ended September 30, 2010 and from inception (August 17, 2009) through September 30, 2010, we did not generate any revenues.
General and administrative expenses during the nine month period ended September 30, 2010 were $160,435, compared to general and administrative expense of $1,133,129 incurred from August 17, 2009 (inception) through September 30, 2009, a decrease of $972,694. During the aforesaid period in 2009, our general and administrative expense included $945,974 in write downs of intangible assets, compared to a write down of $50,000 undertaken during the nine month period ended September 30, 2010. In addition, the aforesaid 2009 period included $155,150 in costs associated with our merger. Our legal and accounting costs increased during the three month period ended September 30, 2010, from $28,100 during the period from inception through September 30, 2010, to $50,961, primarily as a result of our status as a public company. We also incurred costs of $47,000 paid by the issuance of shares of our Common Stock to various consultants, which we did not incur in 2009. As a result, we incurred a net loss of ($160,435) (approximately $0.01 per share) for the nine month period ended September 30, 2010.
Because we did not generate any revenues during the prior fiscal year, following is our plan of operation.
Plan of Operation
As a result of the transaction described above we have revised our current business plan to that of a pharmaceutical company focused on the research, development and commercialization of drugs for the treatment of various forms of cancer. Our lead compound, Adva-27a (difluoro-etoposide), a multi-purpose anti-tumor compound, is expected to enter Phase I clinical trials in 2011. We have licensed our technology on an exclusive basis from Advanomics Corporation, a privately held Canadian company (“Advanomics”), and we are planning to initiate our own R&D program as soon as practicable, once financing is in place. There are no assurances that we will obtain the financing necessary to allow us to implement this aspect of our business plan, or to enter clinical trials.
Carbon-Difluoride Technology
Many therapeutically important compounds contain diester bonds that link different parts of the molecule together. Diester bonds are naturally unstable often leading to suboptimal performance when the molecule is administered to patients. Diester bonds have specific three-dimensional, as well as electrostatic properties that cannot be easily mimicked by other bonds. Bonds that do not mimic the diester bond correctly invariably render the compound inactive. In collaboration with L’Institut National des Sciences Appliquées de Rouen in France (“INSA”), Advanomics has developed a way to replace the diester bond with a Carbon-Difluoride bond which acts as a diester isostere. An isostere is a different chemical structure that mimics the properties of the original. In the body, Carbon-Difluoride compounds are resistant to metabolic degradation but recognized similarly to the diester compounds (See Figure 1).
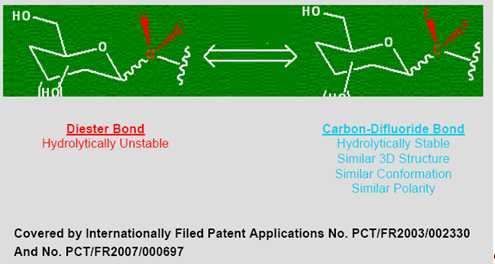
Figure 1
10
While no assurances can be provided, we are planning to expand our product line through acquisitions and/or in-licensing as well as in-house research & development.
Our Lead Compound, Adva-27a
Our initial drug candidate is Adva-27a (difluoro-etoposide), a Carbon-Difluoride derivative of Etoposide, targeted for various forms of cancer. If sufficient funding can be obtained, Adva-27a is expected to enter Phase I clinical trials in Canada during 2011. Etoposide is currently on the market and has been for over 20 years. It is sold under different brand names by various drug companies including, VePesid, VP-16, Etopophos and Vumon or Teniposide (Bristol-Myers Squibb, the original developer), Toposar (Sicor/Pfizer), Lastet (Nippon Kayaku Ltd) and Etoposide (TEVA, Bedford Laboratories, Supergen, American Pharmaceutical Partners, Watson Pharmaceuticals, and Genpharm). Etoposide is an effective anti-tumor compound and is currently in use to treat various types of cancer including leukemia, lymphoma, testicular cancer, breast cancer, lung cancer, brain cancer, prostate cancer, bladder cancer, colon cancer, ovarian cancer, liver cancer and several other forms of cancer. It is also being tested in clinical trials against other types of cancer, such as Kaposi's sarcoma. Etoposide is administered both intravenously and orally as liquid capsules.
Etoposide suffers from molecular instability leading to reduced efficacy and high toxicity. Using its Carbon-Difluoride platform technology (see Figure 1), Advanomics has constructed several Difluoro derivatives of Etoposide by replacing the labile diester bond between the sugar and the toxin moieties of the existing Etoposide molecule with a Carbon-Difluoride bond (Figure 1). All Difluoro substituted constructs were found to be completely stable. Advanomics subsequently tested these constructs for their ability to kill cancer cells in vitro by conducting side-by-side experiments against the standard Etoposide compound. The results of these studies, which have been published in our patent application PCT/FR2007/000697, are summarized in Table 1. One of the constructs, Adva-27a, showed enhanced cancer cell killing activity over the existing Etoposide molecule (see Table 1).
This new compound, which we call Difluoro-Etoposidetm is entering Phase I clinical trials in Canada in 2011. Subject to receipt of financing, we anticipate the Phase I clinical trials to be completed by late 2011 at which time we will apply for limited marketing approval (see “Clinical Trials,” below).

Table 1
11
Clinical Trials
We are entering Phase I clinical trials for our lead compound, Adva-27a in Canada in 2011. The planned clinical trials for small-cell lung cancer and/or multi-drug resistant breast cancer will be conducted at the McGill University Hospital Centers in Montreal (Canada). All aspects of the planned clinical trials in Canada will employ FDA standards at all levels. We anticipate the clinical trials to begin in early 2011 and be completed within twelve (12) months from the date of commencement, at which time we together with our licensor (Advanomics) will file for limited marketing approval with the regulatory authorities in Canada and the FDA in the U.S. (see Marketing below).
Marketing
According to the American Cancer Society, nearly 1.5 million new cases of cancer are diagnosed in the U.S. each year. Given the terminal and limited treatment options available for the indications we are planning to study, we anticipate being granted limited marketing approval (“compassionate-use”) for our Adva-27a following receipt of funding and a successful Phase I. There are no assurances that either will occur. Such limited approval will allow us to make the drug available to various hospitals and health care centers for experimental therapy and/or “compassionate-use, thereby generating some revenues. As with the existing Etoposide, our Adva-27a is anticipated to be usable to treat virtually all forms of cancer and supervising physicians are at liberty to prescribe it once it is available on the market. Similarly to the existing Etoposide, our Adva-27a product will be a single-treatment blister-pack comprised of 20 gel-caps each containing 50 milligrams of difluoro-etoposide for a total of 1 gram per pack.
Intellectual Property
We are the exclusive licensee for the US territory of Advanomics’ Adva-27a (difluoro-etoposide) which is covered by international patent applications filed on April 25, 2006 (PCT/FR2007/000697). This patent, which is issued in France and still pending elsewhere, is owned by L’Institut National des Sciences Appliquées de Rouen (France) and is licensed exclusively on a worldwide basis to Advanomics Corporation, who has subsequently issued to us an exclusive license for the US.
Our Lead Anti-Cancer Compound, Adva-27a, in 3D
Liquidity and Capital Resources
As of September 30, 2010, we had cash and cash equivalents of $108,148.
Net cash used in operating activities was $103,980 during the nine months ended September 30, 2010. We anticipate that overhead costs in current operations will increase in the future upon commencement of clinical trials.
12
Cash flows provided or used in investing activities were $-0- in the nine months ended September 30, 2010. Cash flows provided or used by financing activities were $100,000 during this period.
We are not generating revenue from our operations, and our ability to implement our new business plan for the future will depend on the future availability of financing. Such financing will be required to enable us to further develop our testing, research and development capabilities and continue operations. We intend to raise funds through private placements of our common stock and possibly through short-term borrowing. Relevant thereto, on September 30, 2010, we commenced a private offering of our Common Stock whereby we are offering up to 6,000,000 shares of our Common Stock at an offering price of $.60 per share and hope to raise up to $3,600,000 in gross proceeds. As of September 30, 2010, the date of this Report, we have accepted subscriptions of $100,000.00. We intend to utilize the proceeds derived from this offering to allow us to commence Phase I clinical trials for our lead compound, Adva-27a, a multi-purpose anti-tumor compound, manufacturing of this drug for purposes of Phase I, animal toxicology studies, regulatory filings and data management and working capital.
We estimate that we will require approximately $5 million in debt and/or equity capital to fully implement our business plan in the near future and there are no assurances that we will be able to raise this capital. While we have engaged in discussions with various investment banking firms, venture capitalists and private investors to provide us these funds, as of the date of this report we have not reached any agreement with any party that has agreed to provide us with the capital necessary to effectuate our new business plan or otherwise enter into a strategic alliance to provide such funding. Our inability to obtain sufficient funds from external sources when needed will have a material adverse affect on our plan of operation, results of operations and financial condition.
Our cost to continue operations as they are now conducted is nominal, but these are expected to increase once we commence Phase I clinical trials. We do not have sufficient funds to cover the anticipated increase in these expenses and our current cash position is critical. We need to raise additional funds in order to continue our existing operations, to initiate research and development activities, and to finance our plans to expand our operations for the next year. If we are successful in raising additional funds, our research and development efforts will continue and expand.
Critical Accounting Estimates
The discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, we evaluate our estimates based on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. The following represents a summary of our critical accounting policies, defined as those policies that we believe are the most important to the portrayal of our financial condition and results of operations and that require management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effects of matters that are inherently uncertain.
Inflation
Although our operations are influenced by general economic conditions, we do not believe that inflation had a material effect on our results of operations during the nine month period ended September 30, 2010.
13
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
We are a smaller reporting company and are not required to provide the information under this item pursuant to Regulation S-K.
ITEM 4. CONTROLS AND PROCEDURES.
Disclosure Controls and Procedures - Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) as of the end of the period covered by this report.
These controls are designed to ensure that information required to be disclosed in the reports we file or submit pursuant to the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission, and that such information is accumulated and communicated to our management, including our CEO and CFO, as appropriate, to allow timely decisions regarding required disclosure.
Based on this evaluation, our CEO and CFO concluded that our disclosure controls and procedures were effective as of September 30, 2010, at the reasonable assurance level. We believe that our consolidated financial statements presented in this Form 10-Q fairly present, in all material respects, our financial position, results of operations, and cash flows for all periods presented herein.
Inherent Limitations - Our management, including our Chief Executive Officer and Chief Financial Officer, do not expect that our disclosure controls and procedures will prevent all error and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. The design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdown can occur because of simple error or mistake. In particular, many of our current processes rely upon manual reviews and processes to ensure that neither human error nor system weakness has resulted in erroneous reporting of financial data.
Changes in Internal Control over Financial Reporting - There were no changes in our internal control over financial reporting during the nine month period ended September 30, 2010, which were identified in conjunction with management’s evaluation required by paragraph (d) of Rules 13a-15 and 15d-15 under the Exchange Act, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
None
ITEM 1A. RISK FACTORS
We are a smaller reporting company and are not required to provide the information under this item pursuant to Regulation S-K.
14
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF
PROCEEDS
During the three month period ended September 30, 2010, we issued 100,000 shares of our Common Stock as part of a private offering we commenced whereby we are offering up to 6,000,000 shares of our Common Stock at a price of $0.60 per share. We relied upon the exemption from registration provided by Regulation D and S as promulgated under the Securities Act of 1933, as amended, to issue these shares.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None
ITEM 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS.
[Removed and reserved.]
ITEM 5. OTHER INFORMATION
None
ITEM 6. EXHIBITS
|
Exhibit No.
|
Description
|
|
31.1
|
Certification of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
31.2
|
Certification of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002
|
|
32
|
Certification of Chief Executive Officer and Chief Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
15
SIGNATURES
Pursuant to the requirements of Section 12 of the Securities and Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized on November 12, 2010.
|
SUNSHINE BIOPHARMA, INC.
By: s/Dr. Steve N. Slilaty
Dr. Steve N. Slilaty, Principal Executive Officer
|
|
|
By: s/Camille Sebaaly
Camille Sebaaly, Principal Financial Officer
and Principal Accounting Officer
|
