Attached files
U.S. SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
Form 10-Q
Quarterly Report Under
the Securities Exchange Act of 1934
For Quarter Ended: September
30, 2016
Commission File Number: 000-52898
SUNSHINE BIOPHARMA INC.
(Exact name of small business issuer as specified in its
charter)
|
Colorado
|
|
20-5566275
|
|
(State
of other jurisdiction of incorporation)
|
|
(IRS
Employer ID No.)
|
469 Jean-Talon West
3rd Floor
Montreal, Quebec, Canada H3N 1R4
(Address of principal executive offices)
(514) 764-9698
(Issuer’s Telephone Number)
Indicate by check mark whether the registrant (1) has filed all
reports required to be filed by Section 13 or 15(d) of the
Securities Exchange Act of 1934 during the preceding 12 months (or
for such shorter period that the registrant was required to file
such reports), and (2) has been subject to such filing requirements
for the past 90 days: Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted
electronically and posted on its corporate Web site, if any, every
Interactive Data File required to be submitted and posted pursuant
to Rule 405 of Regulation S-T (§232.405 of this chapter)
during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files).
Yes ☑ No ☐
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated filer,
or a smaller reporting company. See the definitions of “large
accelerated filer,” “accelerated filer” and
“smaller reporting company” in Rule 12b-2 of the
Exchange Act.
|
Large
accelerated filer
|
☐
|
Accelerated
filer
|
☐
|
|
|
|
|
|
|
Non-accelerated
filer
|
☐
|
Smaller
reporting company
|
☑
|
|
(Do not
check if a smaller reporting company)
|
|
|
|
Indicate by check mark whether the registrant is a shell company
(as defined in Rule 12b-2 of the Exchange
Act). ☐ Yes ☑ No
The number of shares of the registrant’s only class of common
stock issued and outstanding as of November 14, 2016, was
686,399,858 shares.
TABLE OF CONTENTS
PART I.
FINANCIAL INFORMATION
|
|
|
Page
No.
|
|
|
|
|
|
Item
1.
|
Financial
Statements
|
3
|
|
|
Unaudited
Consolidated Balance Sheet as of September 30, 2016
|
3
|
|
|
Unaudited
Consolidated Statement of Operations for the three and nine month
Period Ended September 30, 2016
|
4
|
|
|
Unaudited
Consolidated Statement of Cash Flows for the nine month Period
Ended September 30, 2016 and 2015
|
5
|
|
|
Notes
to Unaudited Consolidated Financial Statements
|
6
|
|
Item
2.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations/Plan of Operation.
|
14
|
|
Item
3.
|
Quantitative
and Qualitative Disclosures About Market Risk.
|
20
|
|
Item
4.
|
Controls
and Procedures.
|
21
|
|
|
|
|
|
|
PART II
|
|
|
|
OTHER INFORMATION
|
|
|
|
|
|
|
Item
|
|
|
|
|
|
|
|
Item
1.
|
Legal
Proceedings
|
22
|
|
Item 1A.
|
Risk
Factors
|
22
|
|
Item
2.
|
Unregistered
Sales of Equity Securities and Use of Proceeds
|
22
|
|
Item
3.
|
Defaults
Upon Senior Securities
|
22
|
|
Item
4.
|
Mine
Safety Disclosures
|
22
|
|
Item
5.
|
Other
Information
|
22
|
|
Item
6.
|
Exhibits
|
23
|
|
|
Signatures
|
24
|
2
|
Sunshine
Biopharma, Inc.
|
|
|
|
Consolidated
Balance Sheet
|
|
|
|
|
|
|
|
|
Unaudited
|
Audited
|
|
|
September 30,
|
December 31,
|
|
|
2016
|
2015
|
|
ASSETS
|
|
|
|
|
|
|
|
Current
Assets:
|
|
|
|
|
|
|
|
Cash
and cash equivalents
|
$21,789
|
$50,798
|
|
Receivables
and prepaid expenses
|
1,006
|
3,111
|
|
|
|
|
|
Total
Current Assets
|
22,795
|
53,909
|
|
|
|
|
|
Equipment
(net of $1,360and $479 depreciation resepctively)
|
5,777
|
4,314
|
|
Patents
(net of $48,060 and $3,772 amortization respectively)
|
570,750
|
615,038
|
|
|
|
|
|
TOTAL
ASSETS
|
$599,322
|
$673,261
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES
AND SHAREHOLDERS' EQUITY
|
|
|
|
|
|
|
|
Current
liabilities:
|
|
|
|
|
|
|
|
Notes
payable
|
248,994
|
305,178
|
|
Notes
payable - related party
|
-
|
835,394
|
|
Accounts
payable
|
19,280
|
46,591
|
|
Accounts
payable - related party
|
-
|
80,487
|
|
Accrued
expenses
|
-
|
-
|
|
Interest
payable
|
8,406
|
2,656
|
|
|
|
|
|
Total
current liabilities
|
276,680
|
1,270,306
|
|
|
|
|
|
TOTAL
LIABILITIES
|
276,680
|
1,270,306
|
|
|
|
|
|
COMMITMENTS
AND CONTINGENCIES
|
|
|
|
|
|
|
|
SHAREHOLDERS'
EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
Preferred
stock, Series A $0.10 par value per share;
|
|
|
|
Authorized
850,000 Shares; Issued
|
|
|
|
and
outstanding -0- shares.
|
-
|
-
|
|
|
|
|
|
Preferred
stock, Series B $0.10 par value per share;
|
|
|
|
Authorized
500,000 Shares; Issued
|
|
|
|
and
outstanding 500,000 shares.
|
50,000
|
50,000
|
|
|
|
|
|
Common
Stock, $0.001 per share;
|
|
|
|
Authorized
3,000,000,000 Shares; Issued
|
|
|
|
and
outstanding 636,025,383 and 198,265,118 at
|
|
|
|
September
30, 2016 and December 31, 2015 respectively
|
636,025
|
198,265
|
|
|
|
|
|
Capital
paid in excess of par value
|
11,088,339
|
8,235,217
|
|
|
|
|
|
Accumulated
other comprehensive income
|
1,769
|
740
|
|
|
|
|
|
Accumulated
(Deficit)
|
(11,453,491)
|
(9,081,267)
|
|
|
|
|
|
TOTAL
SHAREHOLDERS' EQUITY (DEFICIT)
|
322,642
|
(597,045)
|
|
|
|
|
|
TOTAL
LIABILITIES AND SHAREHOLDERS' EQUITY (DEFICIT)
|
$599,322
|
$673,261
|
See
Accompanying Notes To These Financial Statements.
3
|
Sunshine
Biopharma, Inc.
|
|
|
|
|
|
Unaudited
Consolidated Statement Of Operations and Comprehensive
Loss
|
|
|
||
|
|
|
|
|
|
|
|
Unaudited
|
Unaudited
|
Unaudited
|
Unaudited
|
|
|
3 Months
|
3 Months
|
9 Months
|
9 Months
|
|
|
Ended
|
Ended
|
Ended
|
Ended
|
|
|
September 30,
|
September 30,
|
September 30,
|
September 30,
|
|
|
2016
|
2015
|
2016
|
2015
|
|
|
|
|
|
|
|
Revenue:
|
$-
|
$-
|
$-
|
$1,708
|
|
|
|
|
|
|
|
General
& Administrative Expenses
|
|
|
|
|
|
|
|
|
|
|
|
Accounting
|
46,311
|
2,700
|
59,280
|
60,260
|
|
Amortization
& depreciation
|
14,963
|
-
|
45,169
|
-
|
|
Consulting
|
24,054
|
6,011
|
156,477
|
107,432
|
|
Generic
drug license fees
|
11,442
|
-
|
19,203
|
-
|
|
Legal
|
9,483
|
26,104
|
53,272
|
103,346
|
|
Licenses
|
-
|
64,986
|
-
|
259,094
|
|
Office
|
4,832
|
3,187
|
16,764
|
8,911
|
|
Officer
& director renumeration
|
376
|
-
|
255,719
|
50,000
|
|
Research
& development
|
-
|
8,657
|
32,793
|
8,657
|
|
Stock
Transfer Fee
|
1,548
|
1,408
|
6,997
|
10,043
|
|
Travel
|
3,007
|
-
|
11,337
|
-
|
|
|
|
|
|
|
|
Total
G & A
|
116,016
|
113,053
|
657,011
|
607,743
|
|
|
|
|
|
|
|
(Loss)
from operations
|
(116,016)
|
(113,053)
|
(657,011)
|
(606,035)
|
|
|
|
|
|
|
|
Other
Income (expense):
|
-
|
|
|
|
|
Forign
exchange gain
|
-
|
204
|
|
204
|
|
Interest
expense
|
(12,129)
|
(9,755)
|
(29,080)
|
(49,186)
|
|
Gain
on interest forgiveness
|
381
|
|
381
|
9,465
|
|
Litigation
settlement proceeds
|
-
|
|
25,000
|
-
|
|
Debt
release
|
7,790
|
|
7,790
|
|
|
Loss
on debt conversions
|
(1,465,646)
|
(80,211)
|
(1,719,304)
|
(359,677)
|
|
|
|
|
|
|
|
Total
Other (Expense)
|
(1,469,604)
|
(89,762)
|
(1,715,213)
|
(399,194)
|
|
|
|
|
|
|
|
Net
(loss)
|
$(1,585,620)
|
$(202,815)
|
$(2,372,224)
|
$(1,005,229)
|
|
|
|
|
|
|
|
Basic
(Loss) per common share
|
$0.00
|
$0.00
|
$(0.01)
|
$(0.01)
|
|
|
|
|
|
|
|
Weighted
Average Common Shares Outstanding
|
581,464,440
|
130,069,136
|
353,977,067
|
109,827,383
|
|
|
|
|
|
|
|
Net
Income (Loss)
|
$(1,585,620)
|
$(202,815)
|
$(2,372,224)
|
$(1,005,229)
|
|
Other
comprehensive income:
|
|
|
|
|
|
Gain
from foreign exchange translation
|
(305)
|
(3,234)
|
1,029
|
1,123
|
|
Comprehensive
(Loss)
|
(1,585,925)
|
(206,049)
|
(2,371,195)
|
(1,004,106)
|
|
|
|
|
|
|
|
Basic
(Loss) per common share
|
$0.00
|
$0.00
|
$(0.01)
|
$(0.01)
|
|
|
|
|
|
|
|
Weighted
Average Common Shares Outstanding
|
581,464,440
|
130,069,136
|
353,977,067
|
109,827,383
|
See
Accompanying Notes To These Financial
Statements.
4
|
Sunshine
Biopharma, Inc.
|
|
|
|
Unaudited
Consolidated Statement Of Cash Flows
|
|
|
|
|
|
|
|
|
Unaudited
|
Unaudited
|
|
|
9 Months
|
9 Months
|
|
|
Ended
|
Ended
|
|
|
September 30,
|
September 30,
|
|
|
2016
|
2015
|
|
Cash
Flows From Operating Activities:
|
|
|
|
|
|
|
|
|
|
|
|
Net
Gain (Loss)
|
$(2,372,224)
|
$(1,005,229)
|
|
|
|
|
|
Adjustments
to reconcile net loss to net cash used in
|
|
|
|
operating
activities:
|
|
|
|
|
|
|
|
Depreciation
and amortization (Equipment and Patents)
|
45,169
|
-
|
|
Stock
issued for licenses, services, and other assets
|
419,500
|
116,500
|
|
Stock
issued for payment interest
|
6,520
|
6,406
|
|
Debt
forgiveness
|
(1,313)
|
|
|
Loss
on debt conversion
|
1,719,304
|
359,677
|
|
Stock
issued for payment of expenses
|
-
|
9,160
|
|
(Increase)
In accounts receivable
|
-
|
-
|
|
(Increase)
decrease in prepaid expenses
|
2,105
|
(11,411)
|
|
Increase
(decrease) in Accounts Payable & accrued expenses
|
(107,799)
|
(129)
|
|
Increase
in interest payable
|
5,750
|
175
|
|
|
|
|
|
Net
Cash Flows (used) in operations
|
(282,988)
|
(524,851)
|
|
|
|
|
|
Cash
Flows From Investing Activities:
|
|
|
|
|
|
|
|
|
|
|
|
Purchase
of equipment
|
(2,343)
|
-
|
|
|
|
|
|
Net
Cash Flows (used) in Investing activities
|
(2,343)
|
-
|
|
|
|
|
|
Cash
Flows From Financing Activities:
|
|
|
|
|
|
|
|
Proceed
from note payable
|
131,150
|
232,840
|
|
Note
payable used to pay expenses
|
|
-
|
|
Note
payable used to pay origionation fees & interest
|
20,015
|
36,140
|
|
Sale
of common stock
|
104,128
|
236,550
|
|
|
|
|
|
Net
Cash Flows provided by financing activities
|
255,293
|
505,530
|
|
|
|
|
|
|
|
|
|
Net
Increase (Decrease) In Cash and cash equivalents
|
(30,038)
|
(19,321)
|
|
Foreign
currency translation adjustment
|
1,029
|
1,123
|
|
Cash
and cash equivalents at beginning of period
|
50,798
|
143,423
|
|
|
|
|
|
Cash
and cash equivalents at end of period
|
$21,789
|
$125,225
|
|
|
|
|
|
Supplementary
Disclosure Of Cash Flow Information:
|
|
|
|
|
|
|
|
Stock
issued for services, licenses and other assets
|
$419,500
|
$116,500
|
|
Stock
issued for note conversions including interest
|
$2,767,254
|
$576,735
|
|
Cash
paid for interest
|
$-
|
$-
|
|
Cash
paid for income taxes
|
$-
|
$-
|
See
Accompanying Notes To These Financial
Statements.
5
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three and Nine Month Interim Periods Ended September 30,
2016
Note 1 – Description of Business
Mountain
West Business Solutions, Inc. (“MWBS”) was incorporated
on August 31, 2006 in the State of Colorado. Sunshine Etopo, Inc.
(formerly Sunshine Biopharma, Inc.) was incorporated in the State
of Colorado on August 17, 2009. Effective October 15, 2009, MWBS
was acquired by Sunshine Etopo, Inc. in a transaction classified as
a reverse acquisition. MWBS concurrently changed its name to
Sunshine Biopharma, Inc. Sunshine Etopo, Inc. has been inactive and
was recently dissolved.
In July
2014, the Company formed a wholly owned Canadian subsidiary,
Sunshine Biopharma Canada Inc. The financial statements represent
the consolidated activity of Sunshine Biopharma, Inc. and Sunshine
Biopharma Canada Inc. (hereinafter together referred to as the
"Company"). The Company was formed for the purposes of conducting
research, development and commercialization of drugs for the
treatment of various forms of cancer. The Company may also engage
in any other business that is permitted by law, as designated by
the Board of Directors of the Company.
The
Company’s wholly owned Canadian subsidiary, Sunshine
Biopharma Canada Inc. (“Sunshine Canada”) was formed
for the purposes of offering generic pharmaceutical products in
Canada and elsewhere around the globe. Sunshine Canada has recently
signed licensing agreements for four (4) generic prescription drugs
for treatment of cancer and BPH (Benign Prostatic Hyperplasia). In
addition, Sunshine Canada is currently evaluating several other
generic products for in-licensing and is working on securing a Drug
Establishment License (“DEL”) and a Drug Identification
Number (“DIN”) per product from Health Canada. Once the
DEL and the DIN’s are secured, Sunshine Canada will begin its
generic pharmaceuticals marketing and sales program in Canada and
overseas.
During
the last three month period the Company has continued to raise
money through stock sales and borrowings.
The
Company’s activities are subject to significant risks and
uncertainties, including failing to secure additional funding to
operationalize the Company’s generics business and
proprietary drug development program.
Note 2 – Summary of Significant Accounting
Policies
This
summary of significant accounting policies is presented to assist
the reader in understanding the Company's financial statements. The
consolidated financial statements and notes are representations of
the Company's management, which is responsible for their integrity
and objectivity. These accounting policies conform to generally
accepted accounting principles and have been consistently applied
in the preparation of the financial statements.
PRINCIPLES OF CONSOLIDATION
The
accompanying consolidated financial statements include the accounts
of the Company and its wholly owned subsidiary. All intercompany
accounts and transactions have been eliminated in
consolidation.
6
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three and Nine Month Interim Periods Ended September 30,
2016
USE OF ESTIMATES
The
preparation of financial statements in conformity with U.S.
generally accepted accounting principles requires management to
make estimates and assumptions that affect the reported amounts of
assets and liabilities and disclosure of contingent assets and
liabilities at the date of the financial statements and the
reported amounts of revenues and expenses during the reporting
period. The more significant estimates and assumptions made by
management are valuation of equity instruments, depreciation of
property and equipment, and deferred tax asset valuation. Actual
results could differ from those estimates as the current economic
environment has increased the degree of uncertainty inherent in
these estimates and assumptions.
CASH AND CASH EQUIVALENTS
For the
Balance Sheets and Statement of Cash Flows, all highly liquid
investments with maturity of 90 days or less are considered to be
cash equivalents. The Company had a cash balance of $21,789 and
$50,798 as of September 30, 2016 and December 31, 2015,
respectively. At times such cash balances may be in excess of the
FDIC limit of $250,000.
EARNINGS PER SHARE
The
Company has adopted the FASB ASC Topic 260 regarding earnings /
loss per share, which provides for calculation of
“basic” and “diluted” earnings / loss per
share. Basic earnings / loss per share includes no dilution and is
computed by dividing net income / loss available to common
shareholders by the weighted average common shares outstanding for
the period. Diluted earnings / loss per share reflect the potential
dilution of securities that could share in the earnings of an
entity similar to fully diluted earnings / loss per
share.
Other
than the Notes Payable specified under Note 5 below, there were no
potentially dilutive instruments outstanding during the interim
period ended September 30, 2016 or the year ended December 31,
2015.
INCOME TAXES
The
Company follows the asset and liability method of accounting for
deferred income taxes. The asset and liability method requires the
recognition of deferred tax assets and liabilities for the expected
future tax consequences of temporary differences between financial
accounting and tax bases of assets and liabilities. The Company
accounts for income taxes pursuant to ASC 740. There was no
increase in liabilities for unrecognized tax benefits as a result
of this implementation. The Company recognizes accrued interest
related to unrecognized tax benefits in interest expense and
penalties in general and administrative expense.
7
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three and Nine Month Interim Periods Ended September 30,
2016
FOREIGN CURRENCY
The
Company has a wholly owned subsidiary in Canada and the functional
currency for that subsidiary is Canadian dollars. However, the
reporting currency for the Company is in U.S. dollars. To come to
this conclusion the Company considered the direction of ASC section
830-10-55.
Selling
Price and Market – As an office is located in Canada; the
Company is performing consulting services to Canadian based
customers on a limited basis. The Company has not had any product
sales but anticipates the majority of its customers will pay in
U.S. dollars. This indicates the functional currency is U.S.
dollars.
Financing
– The Company’s financing has been generated largely in
U.S. dollars from the United States. This indicates the functional
currency is U.S. dollars.
Expenses
– The majority of expense are paid in U.S. dollars. The
expenses generated in PRC are paid by a monthly or weekly cash
transfer from the U.S. when the expenses are due, resulting in very
little foreign currency exposure. This indicates the functional
currency is U.S. dollars.
Intercompany
Transactions – The Company has a few transactions each month
between the U.S. and Canadian office. This indicates the functional
currency is U.S. dollars.
REVENUE RECOGNITION
Since
inception, the Company has been focused on the research,
development and commercialization of drugs for the treatment of
various forms of cancer. Following its recent entry into the
generic pharmaceuticals business, the Company has become a fully
integrated pharmaceutical company offering generic and proprietary
drugs for the treatment of cancer and other acute and chronic
indications. The Company intends to recognize revenues from the
sales of generic pharmaceuticals, if or when they occur, at the
times the funds from such sales are received. In the event the
Company provides consulting services in the future, revenues from
such services will be recognized when the services are rendered and
invoiced.
AMORTIZATION AND DEPRECIATION
Equipment
is stated at historical cost less accumulated depreciation and
accumulated impairment losses. Depreciation is calculated on a
declining balance method over the estimated useful lives. The
Company’s equipment consists of computer, furniture and
laboratory equipment.
Patents
are stated at cost of acquisition and amortized over the shorter of
the term of the patent life, 20 years, or the remaining life of the
underlying patents.
8
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three and Nine Month Interim Periods Ended September 30,
2016
GOING CONCERN
The
accompanying financial statements have been prepared on a going
concern basis, which contemplates the realization of assets and the
satisfaction of liabilities in the normal course of business. Since
inception, the Company has had recurring operating losses and
negative operating cash flows. These factors raise substantial
doubt about the Company’s ability to continue as a going
concern.
The
Company’s continuation as a going concern is dependent on its
ability to obtain additional financing to fund operations,
implement its business model, and ultimately, to attain profitable
operations. The Company will need to secure additional funds
through various means, including equity and debt financing or any
similar financing. There can be no assurance that the Company will
be able to obtain additional debt or equity financing, if and when
needed, on terms acceptable to the Company, or at all. Any
additional equity or debt financing may involve substantial
dilution to the Company’s stockholders, restrictive covenants
or high interest costs. The Company’s long-term liquidity
also depends upon its ability to generate revenues and achieve
profitability.
The
accompanying financial statements do not include any adjustments
relating to the recoverability and classification of recorded asset
amounts or the amounts and classification of liabilities that might
be necessary should the Company be unable to continue as a going
concern.
See the
Notes in the 2015 Form 10-K audited consolidated financial
statements for a complete summary of the Company’s
significant accounting policies.
Note 3 – Unaudited Financial Information
The
unaudited financial information included for the three and nine
month interim periods ended September 30, 2016 was taken from the
books and records of the Company without audit. However, such
information reflects all adjustments, consisting only of normal
recurring adjustments, which in the opinion of management are
necessary to reflect properly the results of the interim periods
presented. The results of operations for the three and nine month
interim periods ended September 30, 2016 are not necessarily
indicative of the results expected for the fiscal year ending
December 31, 2016.
Note 4 – Notes Payable
A Note
Payable having a face value of $19,142 and a maturity date of
December 31, 2016 was entered into on December 31, 2015. This Note
accrues interest at a rate of 12% per annum and became convertible
after December 31, 2015 into $0.001 par value Common Stock at a
price 35% below market value.
A Note
payable having a principal balance of $83,000 as of December 31,
2015 was fully converted, together with $3,320 of accrued interest
thereon, into $0.001 par value Common Stock during the three month
period ended March 31, 2016. In connection therewith, 9,906,049
shares of $0.001 par value Common Stock valued at $146,658 were
issued generating a loss of $63,658 on conversion.
9
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three and Nine Month Interim Periods Ended September 30,
2016
A Note
payable having a face value of $203,036 with interest of 12% was
due June 30, 2016. This Note was convertible after December 31,
2015 into $0.001 par value Common Stock at a price 35% below market
value. During the six month period ended June 30, 2016, a total of
$38,036 in principal was converted into $0.001 par value Common
Stock, leaving a principal balance of $165,000. In connection with
this conversion, 7,705,186 shares of $0.001 par value Common Stock
valued at $231,156 were issued, generating a loss of $193,120 on
conversion. On July 1, 2016, the Company executed a new note
payable having a face value of $174,852 comprised of the principal
amount of this Note ($165,000.00) plus all accrued interest accrued
thereon ($9,852.00). The new note is due on March 31, 2017. It also
accrues interest at 12% and is convertible at a price 35% below
market value.
A Note
payable having a face value of $85,000 executed on February 18,
2016, was fully converted, together with $3,400 of accrued interest
thereon, into $0.001 par value Common Stock during the nine month
period ended September 30, 2016. In connection therewith,
27,538,058 shares of $0.001 par value Common Stock valued at
$172,436 were issued generating a loss of $84,033 on
conversion.
On June
30, 2016, the Company received net proceeds of $49,150 in exchange
for a note payable executed on July 1, 2016 having a face value of
$55,000 and accruing interest at the rate of 10% per annum. The
Note, due on April 1, 2017, is convertible after 180 days from
issuance into $0.001 par value Common Stock at a price 40% below
market value. The Company estimates that the fair value of this
convertible debt approximates the face value, so no value has been
assigned to the beneficial conversion feature.
At
September 30, 2016 and December 31, 2015, accrued interest on Notes
Payable was $8,406 and $2,656, respectively.
Note 5 – Notes Payable Related Entity
On
October 8, 2015, the Company acquired U.S. Patent Number 8,236,935
(the “Patent”) for the anticancer compound, Adva-27a,
which includes all rights to this intellectual property within the
United States in exchange for an interest-free note payable for
$4,320,000 with annual payments of $360,000 due and payable on or
before December 31 commencing in 2016 and continuing until paid in
full. The note was collateralized by the Patent. Pursuant to an
amended agreement effective December 28, 2015, this note was
cancelled and replaced with a new note having a face value of
$210,519, comprised of $155,940 in principal amount which is the
seller’s (Advanomics Corporation, a related party, book value
of the Patent plus $54,579 as an adjustment for the currency
exchange difference. See Note 12 below. This new, interest-free
note was automatically convertible into 80,968,965 shares of the
Company’s $0.001 par value Common Stock upon the Company
completing an increase in its authorized capital such that a
sufficient number of Common Shares is available for issuance. On
July 6, 2016, the Company amended its Articles of Incorporation,
increasing its authorized capital to 3,000,000,000 shares of $0.001
par value Common Stock and on July 8, 2016 the Company issued
80,968,965 shares of $0.001 par value Common Stock to Advanomics
Corporation in exchange for cancellation of this note.
10
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three and Nine Month Interim Periods Ended September 30,
2016
On
December 28, 2015 the Company acquired the worldwide issued and
pending patents under PCT/FR2007/000697 and PCT/CA2014/000029 (the
“Patents”) for the anticancer compound, Adva-27a, which
include all worldwide rights to this intellectual property in
exchange for a note payable for $12,822,499, with interest accruing
at 2% per year beginning January 1, 2016 and quarterly payments of
$70,000 plus interest commencing the end of March 2016 and
continuing until December 2020 when the entire principal balance
and all accrued interest will be due. The note was collateralized
by the Patents. Pursuant to an amended agreement, effective
December 28, 2015, this note was cancelled and replaced with a new
convertible note having a face value of $624,875, comprised of
$462,870 in principal amount which is the seller’s
(Advanomics Corporation, a related party, book value of the
Patents, plus a $162,005 amount as an adjustment for the currency
exchange difference. See Note 12 below. This new, interest-free
note is automatically convertible into 240,336,451 shares of $0.001
par value Common Stock upon the Company completing an increase in
its authorized capital such that a sufficient number of Common
Shares are available for issuance. On July 6, 2016, the Company
amended its Articles of Incorporation, increasing its authorized
capital to 3,000,000,000 shares of $0.001 par value Common Stock
and on July 8, 2016 the Company issued 240,336,451 shares of $0.001
par value Common Stock to Advanomics Corporation in exchange for
cancellation of this note.
During
the period ended September 30, 2016, a total of $835,394 in related
party principal amount of debt was converted into 321,305,416
shares of $0.001 par value Common Stock leaving a principal balance
of $-0-. In connection with this conversion, the Common Stock
issued was valued at $2,217,007 generating a loss of $1,381,613 on
conversion.
Note 6 – Issuance of Common Stock
During
the nine months ended September 30, 2016, the Company issued a
total of 437,760,265 shares of $0.001 par value Common Stock. Of
these 366,454,709 shares valued at $2,767,254 were issued upon
conversion of outstanding notes payable, reducing the debt by
$1,041,430 and interest payable by $6,520 and generating a loss on
conversion of $1,719,304.
In
addition, the Company issued 7,000,000 shares of $0.001 par value
Common Stock for $105,000 Canadian (approximately $79,128 US) and
5,555,556 shares of $0.001 par value Common Stock for
$25,000.
The
Company also issued 10,000,000 shares of $0.001 par value Common
Stock to an unrelated party for services valued at $100,000 or
$0.01 per share, the market value of the shares at the time of
issuance. These services are for a two year period but were fully
expensed during the period ended September 30, 2016.
In
addition, the Company issued 5,750,000 shares of $0.001 par value
Common Stock to an unrelated party for services valued at $43,700
or $0.0076 per share, the market value of the shares at the time of
issuance.
The
Company also issued 7,000,000 shares of $0.001 par value Common
Stock to an unrelated party for services valued at $23,800 or
$0.0034 per share, the market value of the shares at the time of
issuance.
11
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three and Nine Month Interim Periods Ended September 30,
2016
The
Company declared no dividends through September 30,
2016.
Note 7 – Issuance of Series “B” Preferred
Stock
During
the year ended December 31, 2015, the Company authorized 500,000
shares of $0.10 par value Series “B” Preferred Stock.
The Series “B” Preferred Stock is non-convertible,
non-redeemable and non-retractable. It has superior liquidation
rights to the Common Stock at $0.10 per share and gives the holder
the right to 1,000 votes per share. All 500,000 shares of Series
“B” Preferred Stock were issued to the CEO of the
Company in exchange for services valued at $50,000.
Note 8 – Earnings (Loss) Per Share
Earnings
(loss) per share is computed using the weighted average number of
Common Shares outstanding during the period. The Company has
adopted ASC 260 (formerly SFAS128), “Earnings per
Share”.
Note 9 -- Generic Drugs Licenses
During
the nine month period ended September 30, 2016, the Company entered
into Generic Drugs License Agreements for the following four
Generic Drugs:
●
Anastrozole
(brand name Arimidex® by AstraZenica) for treatment of Breast
Cancer
●
Letrozole
(brand name Femara® by Novartis) for treatment of Breast
Cancer
●
Bicalutamide
(brand name Casodex® by AstraZenica) for treatment of Prostate
Cancer
●
Finasteride
(brand name Propecia® by Merck) for treatment of Benign
Prostatic Hyperplasia
The cost of these Licenses has been fully expensed. The Company
determined it was appropriate to fully expense these costs when
incurred.
12
Sunshine
Biopharma, Inc.
Notes
To Unaudited Consolidated Financial Statements
For The
Three and Nine Month Interim Periods Ended September 30,
2016
For a
complete set of footnotes, reference is made to the Company’s
Report on Form 10-K for the year ended December 31, 2015, as filed
with the Securities and Exchange Commission and the audited
consolidated financial statements and notes included
therein.
Note 11 – Income Taxes
Deferred
income taxes arise from the temporary differences between financial
statement and income tax recognition of net operating losses and
other items. Loss carryovers are limited under the Internal Revenue
Code should a significant change in ownership occur.
At the
year ended December 31, 2015 the Company had approximately
$11,453,491 in unused federal net operating loss carryforwards,
which begin to expire principally in the year 2026. A deferred tax
asset at each date of approximately $4,421,048 resulting from the
loss carry forwards has been offset by a 100% valuation allowance.
The change in the valuation allowance for the periods ended
September 30, 2016 and December 31, 2015 was approximately $912,955
and $843,206, respectively.
Note 12 – Related Party Transactions
Certain
members of the Company’s management, including Dr. Steve N.
Slilaty, the Company’s President, CEO and a
Director/shareholder and Camille Sebaaly, the Company’s CFO,
Secretary and a Director/shareholder, hold similar positions with
Advanomics Corporation, the seller of the Adva-27a patents recently
acquired by the Company (see Note 5).
Note 13 – Litigation Settlement Proceeds
In
February 2015 the Company filed an action in the Circuit Court of
the 11th Judicial Circuit for Miami-Date County, Florida related to
a convertible note that we issued to the defendant. This matter was
settled during the first calendar quarter of 2016. The Company
received a one-time payment of $25,000 as part of the terms of
settlement.
Note 14 – Subsequent Events
On
October 14, 2016, the Company issued 10,000,000 shares of $0.001
par value Common Stock to an unrelated party for financial
consulting services valued at $41,000 or $0.0041 per
share.
On
October 18, 2016, the holder of the convertible note having a face
value of $174,852 elected to convert $74,852 in principal amount
into 40,374,475 shares of $0.001 par value Common Stock, leaving a
principal balance of $100,000.
13
PART I.
ITEM 2. MANAGEMENT’S DISCUSSION AND
ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
The following discussion should be read in conjunction with our
consolidated financial statements and notes thereto included
herein. In connection with, and because we desire to take advantage
of, the “safe harbor” provisions of the Private
Securities Litigation Reform Act of 1995, we caution readers
regarding certain forward looking statements in the following
discussion and elsewhere in this report and in any other statement
made by, or on our behalf, whether or not in future filings with
the Securities and Exchange Commission. Forward looking statements
are statements not based on historical information and which relate
to future operations, strategies, financial results or other
developments. Forward looking statements are necessarily based upon
estimates and assumptions that are inherently subject to
significant business, economic and competitive uncertainties and
contingencies, many of which are beyond our control and many of
which, with respect to future business decisions, are subject to
change. These uncertainties and contingencies can affect actual
results and could cause actual results to differ materially from
those expressed in any forward looking statements made by, or on
our behalf. We disclaim any obligation to update forward looking
statements.
Overview and History
We
were incorporated in the State of Colorado on August 31, 2006 under
the name “Mountain West Business Solutions,
Inc.” Until October 2009, our business was to
provide management consulting with regard to accounting, computer
and general business issues for small and home-office based
companies. Effective October 15, 2009, we executed an agreement to
acquire Sunshine Biopharma, Inc., a Colorado corporation, in
exchange for the issuance of 21,962,000 shares of our Common Stock
and 850,000 shares of Convertible Preferred Stock, each convertible
into twenty (20) shares of our Common Stock. As a result of this
transaction we changed our name to “Sunshine Biopharma, Inc.
and our officers and directors resigned their positions with us and
were replaced by our current management. The majority of the Common
Shares and all of the Convertible Preferred Shares we issued for
this transaction were issued to Advanomics Corporation, a privately
held Canadian company (“Advanomics”). As a result of
the issuance of this stock, Advanomics became a related party. On
December 21, 2011, Advanomics exercised its right to convert the
850,000 shares of Series “A” Preferred Stock it held in
our Company into 17,000,000 shares of Common Stock.
We operated under a technology license agreement
with Advanomics until December 2015, at which time we acquired all
of the worldwide right to the technology and became direct owner of
all issued and pending patents pertaining to our technology.
See “Plan of Operation – Intellectual Property”
below.
Our principal place of business is located at 469
Jean-Talon West, 3rd
Floor, Montreal, Quebec, Canada H3N
1R4. Our phone number is (514) 764-9698 and our website
address is www.sunshinebiopharma.com.
We
have not been subject to any bankruptcy, receivership or similar
proceeding.
Results Of Operations
Comparison of Results of Operations for the nine months ended
September 30, 2016 and 2015
For
the nine months ended September 30, 2016 and 2015, we did not
generate any significant revenue.
General
and administrative (“G&A”) expenses during the nine
month period ended September 30, 2016 were $657,011, compared to
G&A expense of $607,743 incurred during the nine month period
ended September 30, 2015, an increase of $49,268. While this
overall increase is not very large, some of the individual items in
our G&A expenses saw a significant increase or decrease during
the nine month period ended September 30, 2016. Specifically, we
saw an increase in our executive compensation of $205,719. This
increase was a result of stock issued to each of our officers and
directors during the second quarter of 2016. All of the executive
compensation paid during both 2016 and 2015 arose from the issuance
of shares of our Common or Preferred Stock. We also incurred
$49,045 in additional consulting fees as a result of stock based
payments to consultants we engaged to provide services related to
our Generic Pharmaceuticals Business. In addition, we saw a $24,136
increase in research and development expense during the nine months
ended September 30, 2016, compared to no R&D expenditure during
the similar period in 2015. We also incurred $19,190 in additional
office and travel expenses during the nine months ended September
30, 2016. In addition, we incurred $45,169 in amortization and
depreciation during the nine months ended September 30, 2016 which
we did not incur during the same period in 2015. This amount of
amortization and depreciation is largely related to the patents we
purchased in 2015. Finally, we saw an increase of $19,203 in
generic drugs licenses expenses which we did not have in the same
period in 2015. This expense is the result of new generic drugs
license agreements we signed during the period ended September 30,
2016. See Note 9 of the Financial Statements. These increases were
offset by our no longer being required to pay a license fee, which
saved $259,094 because we acquired the patent rights which were
previously the subject of the license fee. See “Plan of
Operation – Intellectual Property” below, as well as a
decrease of $50,074 in legal fees as the litigation we were
involved in was settled in exchange for a $25,000 payment made to
us during the nine month period ended September 30,
2016.
14
We
also incurred $29,080 in interest expense during the nine months
ended September 30, 2016, compared to $49,186 in interest expense
during the similar period in 2015 as a result of decreased
borrowings. However, we incurred $1,719,304 in losses
arising from debt conversion during the nine months ended September
30, 2016, compared to $359,677 on losses from debt conversion
during the nine months ended September 30, 2015, a difference of
$1,359,627. Although we retired over $1,000,000 in convertible debt
(including the related party debt in the amount of $835,394 for the
patents purchased in 2015) during the nine month period ended
September 30, 2016, we do not anticipate that we will fund our
operations using convertible debt financing moving forward. We also
negotiated the release of $7,790 of outstanding debt during the
nine months ended September 30, 2016
As
a result, we incurred a net loss of $2,372,224 (approximately $0.01
per share) for the nine month period ended September 30, 2016,
compared to a net loss of $1,005,229 (approximately $0.01 per
share) during the nine month period ended September 30,
2015.
Comparison of Results of Operations for the three months ended
September 30, 2016 and 2015
General
and administrative expenses during the three month period ended
September 30, 2016 were $116,016, compared to general and
administrative expense of $113,053 incurred during the three month
period ended September 30, 2015, an increase of $2,963. While this
increase was not overly significant, the various components of our
general and administrative expense varied significantly from prior
results of operations.
Specifically,
licensing fees decreased from $64,986 to nil during the three
months ended September 30, 2016 compared to the similar period in
2015 because we acquired the patent rights which were previously
the subject of the license fee. See “Plan of Operation
– Intellectual Property” below. Research and
development costs also decreased by $8,657, as our management
concentrated on developing our newly launched Generics
Pharmaceuticals Business. See “Plan of Operation”
below. Legal fees decreased by $16,621 because we were
no longer involved in any litigation. Offsetting these decreases
were increases in generic drugs licensing fees, accounting fees,
consulting fees and amortization and depreciation expenses. We
incurred $11,442 in generic drugs licenses expenses which we did
not incur in the same period in 2015. This expense is the result of
new generic drugs license agreements we signed during the period
ended September 30, 2016. See Note 9 of the Financial Statements.
We also incurred $43,611 in increased accounting fees during the
three months ended September 30, 2016, compared to the similar
period in 2015 because we used stock based compensation to pay for
all of 2016 bookkeeping and tax related services. The stock issued
in connection with these services was expensed at market value
during the three month period ended September 30, 2016. Similarly,
consulting fees increased by $18,043 during the three months ended
September 30, 2016 compared to the similar period in 2015 for
services related to financing and development of the Generic
Pharmaceuticals Business. Finally, we incurred $14,963 in
amortization and depreciation expenses during the three months
ended September 30, 2016 which we did not incur during the same
period in 2015, largely related to the patents we acquired in
2015.
We
also incurred $12,129 in interest expense during the three months
ended September 30, 2016, compared to $9,755 in interest expense
during the similar period in 2015 as a result of decreased
borrowings. However, we incurred $1,465,646 in losses
arising from debt conversion during the three months ended
September 30, 2016, compared to $80,211 during the corresponding
period in 2015. We also negotiated the release of $7,790 of
outstanding debt during the three months ended September 30,
2016.
As
a result, we incurred a net loss of $1,585,620 (approximately $0.00
per share) during the three month period ended September 30, 2016,
compared to a net loss of $202,815 (approximately $0.00 per share)
during the three month period ended September 30,
2015.
Because
we did not generate any significant revenues during our prior two
fiscal years, following is our Plan of Operation.
Plan of Operation
Until
recently, we have been operating as a pharmaceutical company
focused on the research, development and commercialization of drugs
for the treatment of various forms of cancer. Following
our recent entry and expansion into the generic pharmaceuticals
business, we have become a fully integrated pharmaceutical company
offering generic and proprietary drugs for the treatment of cancer
and other acute and chronic indications. In what follows, we
describe our generic pharmaceuticals operations followed by our
proprietary drug development activities.
15
Generic Pharmaceuticals Operations
On July
25, 2014, we formed Sunshine Biopharma Canada Inc. (“Sunshine
Canada”), a Canadian wholly owned subsidiary for the purposes
of conducting generic pharmaceuticals business in Canada and
elsewhere around the globe. Sunshine Canada recently signed Cross
Referencing Agreements with a major pharmaceutical company for four
prescription generic drugs for the treatment of Breast Cancer,
Prostate Cancer and Enlarged Prostate. We will market and sell
these new pharmaceutical products under our own Sunshine Biopharma
label. These four generic products are as follows:
●
Anastrozole (brand
name Arimidex® by AstraZenica) for treatment of Breast
Cancer
●
Letrozole (brand
name Femara® by Novartis) for treatment of Breast
Cancer
●
Bicalutamide (brand
name Casodex® by AstraZenica) for treatment of Prostate
Cancer
●
Finasteride (brand
name Propecia® by Merck) for treatment of BPH (Benign
Prostatic Hyperplasia)
Worldwide sales of
the brand name version of these products as reported by the
respective owner of the registered trademark are as
follows:
●
Arimidex®
$250M in 2015
●
Femara® $380M
in 2014
●
Casodex® $267M
in 2015
●
Propecia®
$183M in 2015
Sunshine Canada is
currently in the process of securing a Drug Identification Number
(“DIN”) for each of these products from Health Canada.
The Company is also working on finding an appropriate facility and
obtaining a Drug Establishment License (“DEL”) from
Health Canada. Upon receipt of the DEL and DIN’s, we will be
able to accept orders for our own label SBI-Anastrozole,
SBI-Letrozole, SBI-Bicalutamide and SBI-Finasteride. While no
assurances can be provided, we hope to build a much larger
portfolio of “SBI” label Generic Pharmaceuticals over
time.
While
no assurances can be provided and subject to the availability of
adequate financing, of which there is no assurance, we anticipate
that profits from the sales of Generic Products will be used to
finance our proprietary drug development program, including
Adva-27a, our flagship anticancer compound. In addition to
near-term revenue generation, building the generics business
infrastructure and securing the proper permits will render Sunshine
Canada appropriately positioned for the marketing and distribution
of our proprietary Adva-27a drug candidate, provided that Adva-27a
is approved for such marketing and distribution, of which there can
be no assurance.
Proprietary Drug Development Operations
Since
inception, our proprietary drug development activities have been
focused on the development of a small molecule called Adva-27a for
the treatment of aggressive cancers. A Topoisomerase II inhibitor,
Adva-27a has been shown to be effective at destroying Multidrug
Resistant cancer cells including Pancreatic Cancer cells, Breast
Cancer cells, Small-Cell Lung Cancer cells and Uterine Sarcoma
cells (Published in ANTICANCER RESEARCH, Volume 32, Pages
4423-4432, October 2012). Sunshine Biopharma is direct owner of all
issued and pending worldwide patents pertaining to Adva-27a
including U.S. Patent Number 8,236,935 (See “Intellectual
Property” below).
Adva-27a Preclinical Studies
Adva-27a is a
GEM-difluorinated C-glycoside derivative of Podophyllotoxin,
targeted for various forms of cancer. If we are
successful in our current financing efforts, Adva-27a is expected
to enter Phase I clinical trials for pancreatic cancer and, in
parallel, multidrug resistant breast cancer in late 2017. See
“Clinical Development Path” and “Clinical
Trials” below. Another derivative of
Podophyllotoxin called Etoposide is currently on the market and is
used to treat various types of cancer including leukemia, lymphoma,
testicular cancer, lung cancer, brain cancer, prostate cancer,
bladder cancer, colon cancer, ovarian cancer, liver cancer and
several other forms of cancer. Like Etoposide, Adva-27a
is a Topoisomerase II inhibitor; however, unlike Etoposide and
other anti-tumor drugs currently in use, Adva-27a is able to
destroy Multidrug Resistant cancer cells. Adva-27a is a
new chemical entity and has been shown to have distinct and more
desirable biological properties compared to
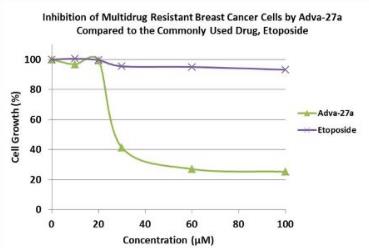
Etoposide. Most notably, Adva-27a is very effective
against Multidrug Resistant breast cancer cells while Etoposide has
no activity against this aggressive form of cancer (see Figure
1). In other side-by-side studies against Etoposide as a
reference, Adva-27a showed markedly improved cell killing activity
in various other cancer types. Our preclinical studies to date have
shown that:
●
Adva-27a is
effective at killing different types of Multidrug Resistant cancer
cells, including:
°
Breast
Cancer Cells (MCF-7/MDR)
°
Small-Cell Lung
Cancer Cells (H69AR)
°
Uterine Sarcoma
Cells (MES-SA/Dx5)
°
Pancreatic Cancer
Cells (Panc-1)
16
●
Adva-27a is
unaffected by P-Glycoprotein, the enzyme responsible for making
cancer cells resistant to anti-tumor drugs.
●
Adva-27a has
excellent clearance time (half-life = 54 minutes) as indicated by
human microsomes stability studies and pharmacokinetics data in
rats.
●
Adva-27a clearance
is independent of Cytochrome P450, a mechanism that is less likely
to produce toxic intermediates.
●
Adva-27a is an
excellent inhibitor of Topoisomerase II with an IC50 of only 13.7
micromolar (this number has recently been reduce to 1.44 micromolar
as a result of resolving the two isomeric forms of
Adva-27a).
●
Adva-27a has shown
excellent pharmacokinetics profile as indicated by studies done in
rats.
●
Adva-27a does not
inhibit tubulin assembly.
These
and other preclinical data have been published in ANTICANCER
RESEARCH, a peer-reviewed International Journal of Cancer Research
and Treatment. The manuscript entitled “Adva-27a,
a Novel Podophyllotoxin Derivative Found to Be Effective Against
Multidrug Resistant Human Cancer Cells” appeared in print in
the October 2012 issue of the journal [ANTICANCER
RESEARCH 32: 4423-4432
(2012)]. A copy of the full manuscript as it appeared in
the journal is available on our website at www.sunshinebiopharma.com.
Clinical Development Path
The
early stage preclinical studies for our lead compound, Adva-27a,
were successfully completed and the results have been published in
ANTICANCER RESEARCH 32:
4423-4432 (2012). We have been delayed in our
implementation of our clinical development program due to lack of
funding. Our fund raising efforts are continuing and as
soon as adequate financing is in place we will continue our
clinical development program of Adva-27a by conducting the next
sequence of steps comprised of the following. There are
no assurances we will be successful in our fund raising
efforts:
●
GMP
Manufacturing of 2 kilogram for use in IND-Enabling Studies and
Phase I Clinical Trials
●
IND-Enabling
Studies
●
Regulatory Filing
(Fast-Track Status Anticipated)
●
Phase
I Clinical Trials (Pancreatic Cancer and Multidrug Resistant Breast
Cancer)
17
GMP Manufacturing
On
November 14, 2014, we entered into a Manufacturing Services
Agreement with Lonza Ltd. and Lonza Sales Ltd. (hereinafter jointly
referred to as “Lonza”), whereby we engaged Lonza to be
the manufacturer of our Adva-27a anticancer drug (the “Lonza
Agreement”). Lonza is one of the world’s
leading and most-trusted manufacturers of pharmaceutical
ingredients. Headquartered in Basel, Switzerland, Lonza
has more than 40 major manufacturing facilities
worldwide. The Lonza Agreement was effective
November 10, 2014, has a term of 5 years, and may be extended or
terminated earlier as provided in the Lonza Agreement.
In June
2015 we received a sample of the scale-up manufacturing process for
evaluation and confirmation of adherence to
specifications. Based upon our laboratory analyses, the
sample meets all of the required chemical, physical and biological
specifications. The amount of material (the
“Yield”) generated by this pilot run was found to be
significantly lower than anticipated and we are currently working
towards finding possible solutions to increase the Yield and define
a path forward. During the course of our discussions concerning the
problem of the low Yield, Lonza informed us that they required us
to pay them $687,818 prior to moving forward with any activity
pertaining to the agreement we have with them for the manufacturing
of our compound. We have repeatedly indicated to Lonza that a clear
path defining exactly how the extremely low yield issue would be
addressed is imperative prior to us making any payments. As of the
date of this report, neither party has changed its
position.
Clinical Trials
Adva-27a’s
initial indication will be pancreatic cancer and multidrug
resistant breast cancer for which there are currently little or no
treatment options available. In June 2011 we concluded
an agreement with McGill University’s Jewish General Hospital
in Montreal, Canada to conduct Phase I clinical trials for these
two indications. All aspects of the planned clinical
trials in Canada will employ FDA standards at all
levels. Subject to obtaining the necessary financing, we
now anticipate that Phase I clinical trials will commence in mid to
late 2017 and we estimate that it will take 18 months to complete,
at which time we expect to receive limited marketing approval for
“compassionate-use” under the FDA and similar
guidelines in Canada. See “Potential Future
Opportunities” below.
Potential Future Opportunities
According to the
American Cancer Society, nearly 1.5 million new cases of cancer are
diagnosed in the U.S. each year. Given the terminal and
limited treatment options available for the pancreatic cancer and
multidrug resistant breast cancer indications we are planning to
study, we anticipate being granted limited marketing approval
(“compassionate-use”) for our Adva-27a following
receipt of funding and a successful Phase I clinical
trial. There are no assurances that either will
occur. Such limited approval will allow us to make the
drug available to various hospitals and health care centers for
experimental therapy and/or “compassionate-use”,
thereby generating some revenues in the near-term.
We
believe that upon successful completion of Phase I Clinical Trials
we may receive one or more offers from large pharmaceutical
companies to buyout or license our drug. However, there
are no assurances that our Phase I Trials will be successful, or if
successful, that any pharmaceutical companies will make an
acceptable offer to us. In the event we do not
consummate such a transaction, we will require significant capital
in order to manufacture and market our new drug.
Intellectual Property
Effective October
8, 2015, we executed a Patent Purchase Agreement (the
“October Purchase Agreement”), with Advanomics, a
related party, pursuant to which we acquired all of the right,
title and interest in and to U.S. Patent Number 8,236,935 (the
“US Patent”) for our anticancer compound,
Adva-27a. On December 28, 2015, we executed a second
Patent Purchase Agreement (the “December Purchase
Agreement”), with Advanomics, pursuant to which we acquired
all of the right, title and interest in and to all of the remaining
worldwide rights covered by issued and pending patents under
PCT/FR2007/000697 and PCT/CA2014/000029 (the “Worldwide
Patents”) for our anticancer compound, Adva-27a.
Effective December
28, 2015, we entered into amendments (the “Amendments”)
of these Purchase Agreements pursuant to which the total purchase
price was reduced from $17,142,499 to $618,810, the book value of
this intellectual property on the financial statements of
Advanomics. Further, the Amendments provided for
automatic conversion of the promissory notes representing the new
purchase price into an aggregate of 321,305,416 shares of our
Common Stock once we increase our authorized capital such that
these shares can be issued. The October and December
Purchase Agreements and Amendments thereof provide us with direct
ownership of all worldwide patents and rights pertaining to
Adva-27a.
18
Our Lead Anti-Cancer Compound, Adva-27a, in 3D
Liquidity and Capital Resources
As
of September 30, 2016, we had cash or cash equivalents of
$21,789.
Net
cash used in operating activities was $282,988 during the nine
month period ended September 30, 2016, compared to $524,851 for the
nine month period ended September 30, 2015. We
anticipate that overhead costs in current operations will increase
in the future as we move forward with our Generic Pharmaceuticals
and Proprietary Drug Developments operations discussed
above.
Cash
flows from financing activities were $255,293 for the nine month
periods ended September 30, 2016, compared to $505,530 during the
nine months ended September 30, 2015. Cash flows used by
investing activities were $2,343 for the nine months ended
September 30, 2016, compared to $0 for the nine month periods ended
September 30, 2015.
At
September 30, 2016, we had issued and outstanding 636,025,383
shares of our Common Stock. During the nine months ended September
30, 2016, we issued a total of 437,760,265 shares of our Common
Stock. Of these, 366,454,709 shares valued at $2,767,254 were
issued pursuant to conversion of outstanding convertible notes
payable, reducing debt by $1,041,430 and interest payable by $6,520
and generating a loss on conversion of $1,719,304 for the
period.
On
July 1, 2016, we issued 5,750,000 shares of our $0.001 par value
Common Stock in exchange for services.
On
July 1, 2016, we executed a Convertible Promissory Note payable
having a face value of $55,000.00. The proceeds from this Note were
received by us on September 30, 2016 and were recorded in our
Financial Statements included in this report as a current
liability.
On
July 1, 2016, we executed a Convertible Promissory Note payable
having a face value of $174,852, comprised of the principal amount
($165,000.00) and all accrued interest ($9,852.00) on the
Convertible Promissory Note payable that matured on September 30,
2016. See Note 4 to Financial Statements.
On
July 6, 2016, we amended our Articles of Incorporation, increasing
our authorized capital to 3,000,000,000 shares of Common Stock,
$0.001 par value per share, and 30,000,000 shares of Preferred
Stock, $0.10 par value per share.
On
July 8, 2016, we issued 321,305,416 shares of our $0.001 par value
Common Stock to Advanomics Corporation (“Advanomics”),
a related party, in exchange for cancellation of the two notes
payable in the aggregate principal amount of $835,394 applicable to
our acquisition of the patent rights discussed above and in Note 5
to our Financial Statements.
We also
issued 7,000,000 shares of Common Stock for $105,000 Canadian
(approximately $79,128 US) to finance our Generic Pharmaceuticals
operations and 5,555,556 shares of Common Stock for $25,000 to pay
for our filing expenses.
19
In
addition, we issued, 10,000,000 shares of $0.001 par value Common
Stock to an unrelated party for services valued at $100,000 or
$0.01 per share. These services are for a two year period but were
fully expensed during the period ended June 30, 2016.
We also
issued 36,000,000 shares of our Common Stock for services rendered
to us by our current directors, valued at $252,000 or $0.007 per
share.
We
are generating only nominal revenue from our operations, and our
ability to implement our business plan for the future will depend
on the future availability of financing. Such financing will be
required to enable us to continue our Generic Pharmaceuticals
business and Proprietary Drug Development operations. We intend to
raise funds through private placements of our Common Stock and
through short-term borrowing. We estimate that we will require
approximately $5 million in debt and/or equity capital to fully
implement our business plan in the future and there are no
assurances that we will be able to raise this capital. While we
have engaged in discussions with various investment banking firms
and venture capitalists to provide us these funds, as of the date
of this report we have not reached any agreement with any party
that has agreed to provide us with the capital necessary to
effectuate our business plan. Our inability to obtain sufficient
funds from external sources when needed will have a material
adverse effect on our plan of operation, results of operations and
financial condition.
Our
cost to continue operations as they are now conducted is nominal,
but these are expected to increase as we advance our Generic
Pharmaceuticals business and we commence Phase I clinical trials.
We do not have sufficient funds to cover the anticipated increase
in these expenses. We need to raise additional funds in order to
continue our existing operations and advance our plans to expand
our operations for the next year. If we are successful in raising
additional funds, our Generic Pharmaceuticals business and
Proprietary Drug Development efforts will continue and
expand.
Subsequent Events
On
October 14, 2016, we issued 10,000,000 shares of $0.001 par value
Common Stock to an unrelated party for financial consulting
services.
On
October 18, 2016, the holder of the convertible note having a face
value of $174,852 elected to convert $74,852 in principal amount
into 40,374,475 shares of $0.001 par value Common Stock, leaving a
principal balance of $100,000.
Inflation
Although
our operations are influenced by general economic conditions, we do
not believe that inflation had a material effect on our results of
operations during the nine month period ended September 30,
2016.
Critical Accounting Estimates
The
discussion and analysis of our financial condition and results of
operations are based upon our consolidated financial statements,
which have been prepared in accordance with accounting principles
generally accepted in the United States. The preparation of these
consolidated financial statements requires us to make estimates and
judgments that affect the amounts of assets, liabilities, revenues
and expenses, and related disclosure of contingent assets and
liabilities. On an on-going basis, we evaluate our estimates based
on historical experience and on various other assumptions that are
believed to be reasonable under the circumstances, the results of
which form the basis for making judgments about the carrying values
of assets and liabilities that are not readily apparent from other
sources. Actual results may differ from these estimates under
different assumptions or conditions. The following represents a
summary of our critical accounting policies, defined as those
policies that we believe are the most important to the portrayal of
our financial condition and results of operations and that require
management’s most difficult, subjective or complex judgments,
often as a result of the need to make estimates about the effects
of matters that are inherently uncertain.
We
are a smaller reporting company and are not required to provide the
information under this item pursuant to Regulation
S-K.
20
ITEM 4. CONTROLS AND PROCEDURES.
Disclosure Controls
and Procedures - Our
management, with the participation of our Chief Executive Officer
and Chief Financial Officer, has evaluated the effectiveness of our
disclosure controls and procedures (as such term is defined in
Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of
1934, as amended (the “Exchange Act”)) as of the end of
the period covered by this report.
These
controls are designed to ensure that information required to be
disclosed in the reports we file or submit pursuant to the
Securities Exchange Act of 1934 is recorded, processed, summarized
and reported within the time periods specified in the rules and
forms of the Securities and Exchange Commission, and that such
information is accumulated and communicated to our management,
including our CEO and CFO, as appropriate, to allow timely
decisions regarding required disclosure.
Based
on this evaluation, our CEO and CFO concluded that our disclosure
controls and procedures were effective as of September 30, 2016, at
the reasonable assurance level. We believe that our consolidated
financial statements presented in this Form 10-Q fairly present, in
all material respects, our financial position, results of
operations, and cash flows for all periods presented
herein.
Inherent
Limitations - Our
management, including our Chief Executive Officer and Chief
Financial Officer, do not expect that our disclosure controls and
procedures will prevent all error and all fraud. A control system,
no matter how well conceived and operated, can provide only
reasonable, not absolute, assurance that the objectives of the
control system are met. The design of any system of controls is
based in part upon certain assumptions about the likelihood of
future events, and there can be no assurance that any design will
succeed in achieving its stated goals under all potential future
conditions. Further, the design of a control system must reflect
the fact that there are resource constraints, and the benefits of
controls must be considered relative to their costs. Because of the
inherent limitations in all control systems, no evaluation of
controls can provide absolute assurance that all control issues and
instances of fraud, if any, within our company have been detected.
These inherent limitations include the realities that judgments in
decision-making can be faulty, and that breakdown can occur because
of simple error or mistake. In particular, many of our current
processes rely upon manual reviews and processes to ensure that
neither human error nor system weakness has resulted in erroneous
reporting of financial data.
Changes in Internal
Control over Financial Reporting - There were no changes in our internal
control over financial reporting during the nine month period ended
September 30, 2016, which were identified in conjunction with
management’s evaluation required by paragraph (d) of Rules
13a-15 and 15d-15 under the Exchange Act, that have materially
affected, or are reasonably likely to materially affect, our
internal control over financial reporting.
21
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
In June
2015 we received a sample of the scale-up manufacturing process for
evaluation and confirmation of adherence to
specifications. Based upon our laboratory analyses, the
sample meets all of the required chemical, physical and biological
specifications. The amount of material (the
“Yield”) generated by this pilot run was found to be
significantly lower than anticipated and we are currently working
towards finding possible solutions to increase the Yield and define
a path forward. During the course of our discussions concerning the
problem of the low Yield, Lonza informed us that they required us
to pay them $687,818 prior to moving forward with any activity
pertaining to the agreement we have with them for the manufacturing
of our compound. We have repeatedly indicated to Lonza that a clear
path defining exactly how the extremely low yield issue would be
addressed is imperative prior to us making any payments. As of the
date of this report, neither party has changed its
position.
Other
than the above, we are not party to any material legal proceedings,
nor have any such actions been threatened against us.
ITEM 1A. RISK FACTORS
We
are a smaller reporting company and are not required to provide the
information under this item pursuant to Regulation
S-K.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF
PROCEEDS
During
the nine months ended September 30, 2016, we issued a total of
437,760,265 shares of $0.001 par value Common Stock. Of these,
366,454,709 shares valued at $2,767,254 were issued upon conversion
of outstanding notes payable, reducing debt by $1,041,430 and
interest payable by $6,520 and generating a loss on conversion of
$1,719,304. We relied upon the exemption from registration provided
by Regulation D promulgated under the Securities Act of 1933, as
amended, to issue these shares. The proceeds of these loans were
used to acquire patents and for working capital.
In
addition, we issued 7,000,000 shares of $0.001 par value Common
Stock for $105,000 Canadian (approximately $79,128 US) and
5,555,556 shares of $0.001 par value Common Stock for $25,000. We
used these funds for working capital.
We also
issued 10,000,000 shares of $0.001 par value Common Stock to an
unrelated party for services valued at $100,000 or $0.01 per share,
the market value of the shares at the time of issuance. These
services are for a two year period but were fully expensed during
the period ended September 30, 2016.
In
addition, we issued 5,750,000 shares of $0.001 par value Common
Stock to an unrelated party for services valued at $43,700 or
$0.0076 per share, the market value of the shares at the time of
issuance.
Wey
also issued 7,000,000 shares of $0.001 par value Common Stock to an
unrelated party for services valued at $23,800 or $0.0034 per
share, the market value of the shares at the time of
issuance.
In
addition, we issued 36,000,000 shares of $0.001 par value Common
Stock for services rendered to the Company by our Directors valued
at $252,000 or $0.007 per share, the market value of the shares at
the time of issuance.
Except
as otherwise indicated, herein, we relied upon the exemption from
registration provided by Section 4(a)(2) promulgated under the
Securities Act of 1933, as amended, to issue the aforesaid
shares.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None
ITEM 4. MINE SAFETY DISCLOSURE
Not
Applicable
ITEM 5. OTHER INFORMATION
None
22
ITEM 6. EXHIBITS
|
Exhibit No.
|
|
Description
|
|
|
|
|
|
31.1
|
|
Certification
of Chief Executive Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002
|
|
|
|
|
|
31.2
|
|
Certification
of Chief Financial Officer Pursuant to Section 302 of the
Sarbanes-Oxley Act of 2002
|
|
|
|
|
|
32
|
|
Certification
of Chief Executive Officer and Chief Financial Officer Pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002
|
|
101.INS
|
|
XBRL
Instance Document*
|
|
101.SCH
|
|
XBRL
Schema Document*
|
|
101.CAL
|
|
XBRL
Calculation Linkbase Document*
|
|
101.DEF
|
|
XBRL
Definition Linkbase Document*
|
|
101.LAB
|
|
XBRL
Label Linkbase Document*
|
|
101.PRE
|
|
XBRL
Presentation Linkbase Document*
|
|
|
|
|
______________________
*
Pursuant to Rule 406T of Regulation S-T, these interactive data
files are not deemed filed or part of a registration statement or
prospectus for purposes of Section 11 or 12 of the Securities Act
or Section 18 of the Securities Exchange Act and otherwise not
subject to liability.
23
SIGNATURES
Pursuant
to the requirements of Section 12 of the Securities and Exchange
Act of 1934, the Registrant has duly caused this Report to be
signed on its behalf by the undersigned, thereunto duly authorized
on November 14, 2016.
|
|
SUNSHINE
BIOPHARMA, INC.
|
|
|
|
|
|
|
|
|
|
By:
|
s/ Dr. Steve N.
Slilaty
|
|
|
|
|
Dr. Steve N.
Slilaty,
|
|
|
|
|
Principal Executive
Officer
|
|
|
|
|
|
|
|
|
|
|
|
|
|
By:
|
s/ Camille
Sebaaly
|
|
|
|
|
Camille
Sebaaly,
Principal Financial
Officer and
|
|
|
|
|
Principal
Accounting Officer
|
|
24
