Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - SELLAS Life Sciences Group, Inc. | gale-20160310ex991.htm |
| 8-K - 8-K - SELLAS Life Sciences Group, Inc. | gale-201603108xk.htm |

Q4 & YEAR END, 2015 Earnings Report & Corporate Update

FORWARD LOOKING STATEMENT This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements about our 2015 revenue from the sale of Abstral®, our launch of Zuplenz®, the divestiture of the commercial operations including the two commercial products, the issuance and exclusivity of patents, and the progress of development of Galena’s product candidates, including patient enrollment in our clinical trials, time to complete the trials, and expected time periods for results. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those identified under “Risk Factors” in Galena’s Annual Report on Form 10-K for the year ended December 31, 2015 and most recent Quarterly Reports on Form 10-Q filed with the SEC. Actual results may differ materially from those contemplated by these forward- looking statements. Galena does not undertake to update any of these forward- looking statements to reflect a change in its views or events or circumstances that occur after the date of this press release. 2

EARNINGS CALL PARTICIPANTS Presenters Mark W. Schwartz, Ph.D. President & Chief Executive Officer Bijan Nejadnik, M.D. Executive Vice President, Chief Medical Officer John T. Burns, CPA Controller & Principal Accounting Officer Other Participants Remy Bernarda, MBA SVP, Investor Relations & Corporate Communications Tom Knapp, Esq Interim General Counsel 3

OVERVIEW Mark W. Schwartz, Ph.D. President & Chief Executive Officer

2015 MILESTONES NeuVax™ Enroll N=700 into PRESENT trial Complete enrollment in Phase 3 PRESENT trial GALE-301 GALE- 302 Report Top-Line Phase 2a clinical data Report 1-Year Phase 2a analysis Report GALE-301 + GALE-302 Phase 1b data GALE-401 (anagrelide CR) Report Top-Line efficacy and safety data Report Final Phase 2 data 5
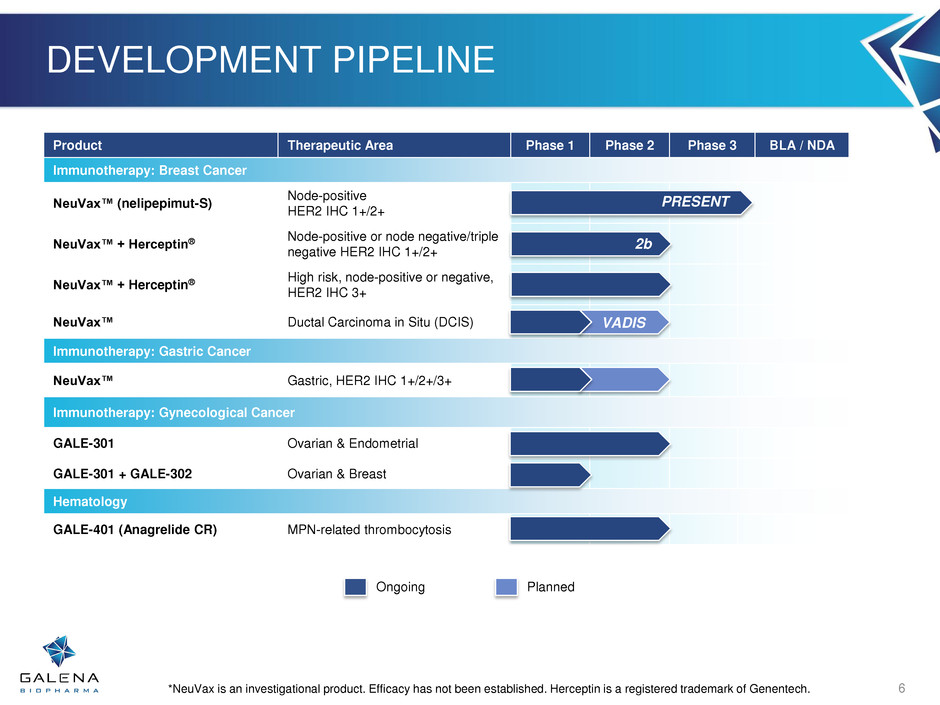
DEVELOPMENT PIPELINE Product Therapeutic Area Phase 1 Phase 2 Phase 3 BLA / NDA Immunotherapy: Breast Cancer NeuVax™ (nelipepimut-S) Node-positive HER2 IHC 1+/2+ NeuVax™ + Herceptin® Node-positive or node negative/triple negative HER2 IHC 1+/2+ NeuVax™ + Herceptin® High risk, node-positive or negative, HER2 IHC 3+ NeuVax™ Ductal Carcinoma in Situ (DCIS) Immunotherapy: Gastric Cancer NeuVax™ Gastric, HER2 IHC 1+/2+/3+ Immunotherapy: Gynecological Cancer GALE-301 Ovarian & Endometrial GALE-301 + GALE-302 Ovarian & Breast Hematology GALE-401 (Anagrelide CR) MPN-related thrombocytosis PRESENT *NeuVax is an investigational product. Efficacy has not been established. Herceptin is a registered trademark of Genentech. Ongoing Planned VADIS 6 2b

CLINICAL DEVELOPMENT Bijan Nejadnik, M.D. Executive Vice President, Chief Medical Officer 7

PHASE 3 PRESENT TRIAL PER SPA 1 2 3 4 Interim analysis by IDMC at n=70 events Endpoint DFS at n=141 events /36 months Dosing by Month + 1 booster dose every 6 months thereafter 5 6 Adjuvant breast cancer patients, randomized 1:1 Double blind Node positive HLA A2/A3+ HER2 IHC 1+/2+ Stratified by stage, type of surgery, hormone receptor, and menopausal status Enrollment complete: n=758 Patients Study Population + GM-CSF Placebo + GM-CSF 8 Prevention of Recurrence in Early-Stage, Node Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment

PRESENT TRIAL DEFINITIONS Qualifying Event: • A recurrence of the primary cancer, either locally (breast), regionally (lymph nodes), or distantly (metastatic) • Occurrence of another cancer • Death from any cause Central Imaging: Independent radiology group to evaluate radiographic images • Team includes 3 Radiologists blinded to the treatment assignment Endpoint Adjudication Committee (EAC): an independent team of physicians expert in breast cancer diagnosis that evaluate every potential event • Team includes 2 Oncologists & 1 Radiologist blinded to the treatment assignment Interim Analysis: a pre-specified futility and safety analysis on the first 70 adjudicated events which evaluates the probability of the study to achieve its pre- specified objectives Independent Data Monitoring Committee (IDMC): an independent team of physicians monitoring the overall conduct, status, and safety data of the trial and will perform the interim analysis and provide the recommendation • Team includes two medical oncologists, one cardiologist, and one statistician 9

ENDPOINT ADJUDICATION COMMITTEE (EAC) Patient is Symptomatic •Physician identifies a potential event •Submits the case for evaluation Result Sent to Central Imaging for Assessment •Independent readers (3 radiologists) evaluate the patient scans Independent Medical Group Collects & Reviews •Receives the data and reviews the case •Prepares a detailed patient case review for the EAC EAC Evaluates •Makes final determination if case qualifies as an event Qualifying Event Adjudicated •Affirmed or denied 10

PRESENT INTERIM ANALYSIS 70 Events Confirmed by the EAC Galena Compiles Data • Prepares a detailed review on 70 patients with events and overall safety data set (n=758) • Submits to IDMC IDMC Evaluates • Evaluates 70 patients with events and overall safety data set (n=758) •Makes recommendation on futility and continuation of trial Interim Analysis Results •Estimated timing: End of Q2 11

NEUVAX: DEVELOPMENT COLLABORATIONS Phase Treatment HER2 Status Indication Trial Status Protocol Defined # of Patients Collaborations 3 Single agent PRESENT Study 1+, 2+ BREAST Node Positive HLA A2+, A3+ Enrolled 13 countries ~140 centers 700 (enrolled 758) 2b Combination with trastuzumab 1+, 2+ BREAST Node Positive or High Risk Node Negative HLA A2+, A3+, A24+, A26+ Enrolling U.S. only 33 centers 300 2 Combination with trastuzumab 3+ high risk BREAST Node Positive HLA A2+, A3+ Enrolling U.S. only 28 centers 100 2 Single agent VADIS Study 1+, 2+,3+ BREAST Ductal Carcinoma in Situ (DCIS) HLA A2+ Planned U.S. only 4 centers 48 2 Single agent 1+, 2+,3+ GASTRIC HLA A2+, A3+ Planned India Only 50 12

DUCTAL CARCINOMA IN SITU (DCIS) Cancer that starts inside the milk ducts The most common type of breast cancer • 60,000 new cases a year 13 Sources: American Cancer Society; National Cancer Institute

NEUVAX: VADIS TRIAL (DCIS) 14 Program Goal: Broaden footprint of NeuVax development to include primary prevention. Should the study results be positive, NeuVax may potentially be evaluated in a large randomized trial for primary prevention of invasive breast cancer. • Study Objectives: Primary • Evaluate for nelipepimut-S specific cytotoxic T-cells Secondary • Toxicity profile • Immune response to other tumor antigens • Polyfunctional cytokine responses • Presence of DCIS at resection

GALE-301 & GALE-302: CURRENT CLINICAL DEVELOPMENT 15 Phase Treatment Cancer Type Target Indication Current Status # of Enrolled Patients 1/2a GALE-301 Ovarian, Endometrial HLA A2+ Ovarian Enrolled 51 1b GALE-301 & GALE-302 Ovarian, Breast HLA A2+ Ovarian / Breast Enrolled 39

GALE-401 ANAGRELIDE CONTROLLED RELEASE (CR) Anagrelide •Active ingredient •Reduces the elevated platelet count and the risk of thrombosis in patients with myeloproliferative neoplasms (MPNs) •MPNs are hematological malignancies in which the bone marrow cells develop and function abnormally Immediate Release •Approved for the treatment of patients with thrombocythemia, secondary to MPNs • IR formulation can cause unacceptable side effects believed to be Cmax-related and has largely limited the use due to early treatment withdrawal GALE-401 •Controlled Release (CR) formulation may decrease the frequency or severity of side effects •Phase 2, Proof-of-Concept Trial Results •Well tolerated with primarily Grade 1 and 2 toxicities •Efficacy compares favorably to historical anagrelide IR 16

CLINICAL DEVELOPMENT Bijan Nejadnik, M.D. Executive Vice President, Chief Medical Officer 17

FINANCE John T. Burns, CPA Controller & Principal Accounting Officer 18
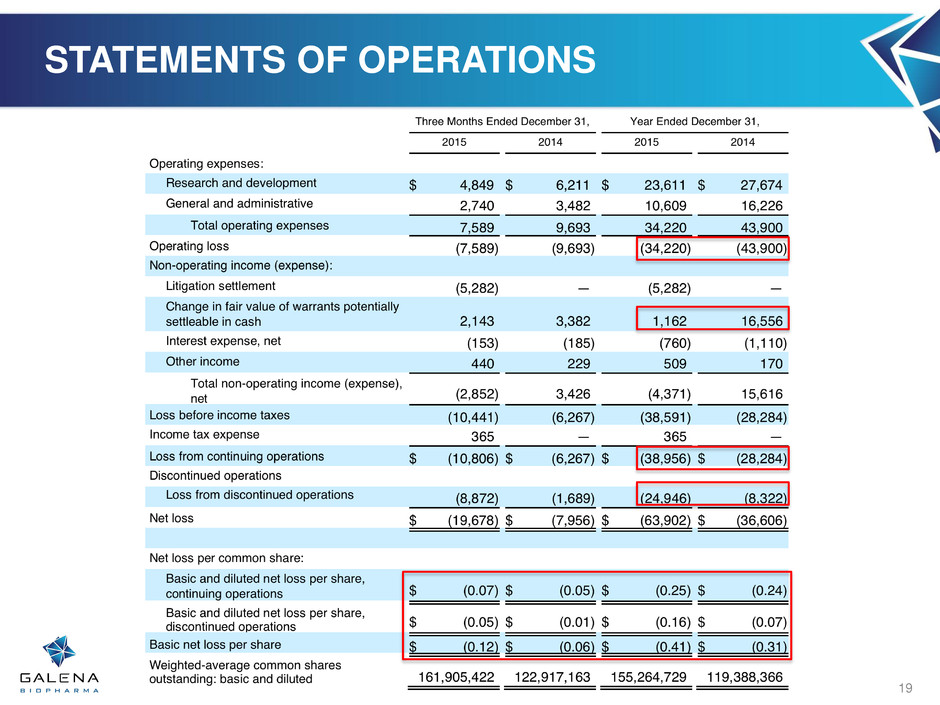
STATEMENTS OF OPERATIONS 19
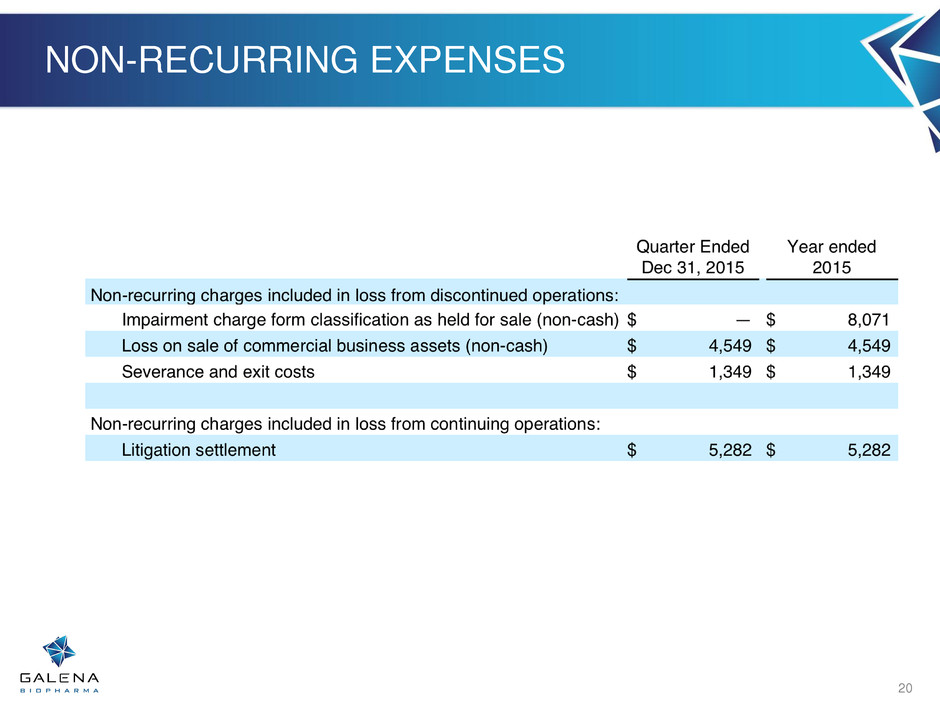
NON-RECURRING EXPENSES 20

CASH & CASH EQUIVALENTS 21

FINANCIAL OVERVIEW Cash Position (as of 2/29/15) $38.2 million Q1 Remaining Burn $2.5-$3.5 million Q2 Projected Burn $13-$15 million Includes legal settlement & fees ~$4-$5 million Debt (as of 12/31/15) $4.7 million Shares Outstanding 182 million Market Cap (9 March 16) ~$170 million 22

MILESTONES & CLOSING REMARKS Mark W. Schwartz, Ph.D. President and Chief Executive Officer 23

Adds ~10k patients >$3B Combo: High risk, HER2 3+ NEUVAX: SIGNIFICANT U.S. COMMERCIAL OPPORTUNITY 24 adds ~10k patients >$2.5B Combo Node Pos or Neg HER2 1+, 2+ HLA-A2, A3, A24, A26 PRESENT 50-60k patients >$2B Source: Global Data 2015/Medtrack. Pricing estimates based on a 20% premium to the current average annual price of Herceptin® (U.S. Dollars).

2016 MILESTONES 25 PROGRAM MILESTONE PROJECTED DATE NeuVax™ (nelipepimut-S) Initiate DCIS trial March/April PRESENT: Reach 70 events March/April PRESENT: Interim analysis Q2 Combo H&N 1+/2+ Interim safety data Q4 Combo H&N 1+/2+ A24/A26 data Q4 GALE-301 GALE-302 Present 301/302 booster data Q2 Present GALE-301 Phase 2a two year data Q4 GALE-401 (anagrelide CR) Confirmation of 505(b)2 pathway 2H Publish final Phase 2 report Q4

1st IN CLASS PROGRAMS WITH EXPANSION OPPORTUNITIES Mid-stage clinical trials have proven T-cell generation • NeuVax™ (nelipepimut-S) Phase 2 trial demonstrated 2% of the patient’s T-Cells become CD8+, HER2 directed • GALE-301 Phase 1/2: Two year DFS estimate in optimal dose group is 85.7% vaccine vs. 33.6% control (p<.02) Targeting “high value” settings: Prevention of recurrence in breast and ovarian cancer are areas of clear unmet medical needs • No approved drugs for these women with limited late stage competition Multiple trials ongoing as stand-alone therapies and in-combination with other agents Breast & Ovarian are just a start – HER2 and Folate Binding Protein expressed in numerous cancer types HER2 Breast Gastric Prostate Non-Small Cell Lung Bladder Colorectal Ovarian Head & Neck Folate Binding Protein Ovarian Endometrial Breast 26

WHY WE’RE HERE Source: E75 vaccine's final tests start in S.A. By Don Finley, January 22, 2012; Photo credit: Kin Man Hui/San Antonio Express-News/ZUMAPress “I've had several friends who've had (breast cancer) and then…it came back and they had to go through treatment again. So this would be wonderful, not to have to come back.” – First NeuVax Phase 3 patient 27
