Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - SELLAS Life Sciences Group, Inc. | gale20170930ex321.htm |
| EX-31.1 - EXHIBIT 31.1 - SELLAS Life Sciences Group, Inc. | gale20170930ex311.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
________________________________
FORM 10-Q
________________________________
ý | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2017
OR
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ________ to ________
Commission File Number: 001-33958
Galena Biopharma, Inc.
(Exact name of registrant as specified in its charter)
________________________________
Delaware | 20-8099512 | |
(State of incorporation) | (I.R.S. Employer Identification No.) | |
2000 Crow Canyon Place, Suite 380, San Ramon, CA 94583
(855) 855-4253
(Address, including zip code, and telephone number, including
area code, of registrant's principal executive offices)
________________________________
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter time that the registrant was required to submit and post such files). Yes ý No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act (Check one):
Large accelerated filer | ¨ | Accelerated filer | ý | |||
Non-accelerated filer | ¨ | (Do not check if a smaller reporting company) | Smaller reporting company | ý | ||
Emerging growth company | ¨ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act): ¨ Yes ý No
As of November 9, 2017, Galena Biopharma, Inc. had outstanding 45,712,912 shares of common stock, $0.0001 par value per share, exclusive of treasury shares.
GALENA BIOPHARMA, INC.
FORM 10-Q - Quarterly Report
For the Quarter Ended September 30, 2017
TABLE OF CONTENTS
Part No. | Item No. | Description | Page No. | ||
I | |||||
1 | |||||
Condensed Consolidated Balance Sheets as of September 30, 2017 (unaudited) and December 31, 2016 | |||||
Condensed Consolidated Statements of Operations (unaudited) for the three and nine months ended September 30, 2017 and 2016 | |||||
Condensed Consolidated Statement of Stockholders' Equity (unaudited) for the nine months ended September 30, 2017 | |||||
Condensed Consolidated Statements of Cash Flows (unaudited) for the nine months ended September 30, 2017 and 2016 | |||||
2 | |||||
3 | |||||
4 | |||||
II | |||||
1 | Legal Proceedings | ||||
1A | Risk Factors | ||||
2 | |||||
3 | |||||
4 | |||||
5 | |||||
6 | |||||
EX-31.1 | |||||
EX-32.1 | |||||
1
NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the information contained in this quarterly report may include forward-looking statements that reflect our current views with respect to our development programs, business strategy, business plan, business combination transactions, financial performance and other future events. These statements include forward-looking statements both with respect to us, specifically, and our industry, in general. Statements that include the words “expect,” “intend,” “plan,” “believe,” “project,” “estimate,” “may,” “should,” “anticipate,” “will” and similar statements of a future or forward-looking nature identify forward-looking statements for purposes of the federal securities laws and otherwise. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. There are or will be important factors that could cause actual results to differ materially from those indicated in these statements. These factors include, but are not limited to, statements about future expectations, plans and prospects for the completion of the Proposed Merger as defined in Note 1 of the condensed consolidated financial statements below, final agreements among the U.S. Attorney's Office of the District of New Jersey, or USAO NJ, and the Department of Justice, or the DOJ, and the Company, the settlement terms among USAO NJ, DOJ and the Company, the settlement of any claims that might be made by state agencies in the future, the settlement terms with federal agencies such as U.S. Department of Defense, the Office of the Personnel Management, the Office of the Inspector General for the U.S. Department of Health and Human Services, future expectations, plans and prospects of Galena’s clinical programs, the cash projections, and other future events. These factors also include, but are not limited to, those factors set forth in the sections entitled ” Risk Factors,” “Legal Proceedings,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Quantitative and Qualitative Disclosures About Market Risk” and “Controls and Procedures” in this quarterly report, all of which you should review carefully. Please consider our forward-looking statements in light of those risks as you read this quarterly report. We undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise, except as required by law. If one or more of these or other risks or uncertainties materializes, or if our underlying assumptions prove to be incorrect, actual results may vary materially from what we anticipate. All subsequent written and oral forward-looking statements attributable to us or individuals acting on our behalf are expressly qualified in their entirety by this forward-looking statement.
2
PART I FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS
GALENA BIOPHARMA, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts in thousands, except share and per share data)
September 30, 2017 | December 31, 2016 | ||||||
(Unaudited) | |||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 12,914 | $ | 18,083 | |||
Restricted cash | 12,372 | 18,022 | |||||
Prepaid expenses and other current assets | 520 | 581 | |||||
Current assets of discontinued operations | 830 | 813 | |||||
Total current assets | 26,636 | 37,499 | |||||
Equipment and furnishings, net | 123 | 199 | |||||
In-process research and development | 9,300 | 12,864 | |||||
GALE-401 rights | 8,100 | 9,255 | |||||
Goodwill | 5,386 | 5,898 | |||||
Deposits and other assets | 50 | 96 | |||||
Total assets | $ | 49,595 | $ | 65,811 | |||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 211 | $ | 840 | |||
Accrued expenses and other current liabilities | 3,186 | 4,292 | |||||
Litigation settlement payable | 1,300 | 950 | |||||
Fair value of warrants potentially settleable in cash | 4,395 | 1,860 | |||||
Current portion of long-term debt | 12,170 | 16,397 | |||||
Current liabilities of discontinued operations | 6,759 | 6,059 | |||||
Total current liabilities | 28,021 | 30,398 | |||||
Deferred tax liability | 5,661 | 5,661 | |||||
Contingent purchase price consideration | 1,277 | 1,095 | |||||
Total liabilities | 34,959 | 37,154 | |||||
Commitments and contingencies | |||||||
Stockholders’ equity: | |||||||
Preferred stock, $0.0001 par value; 5,000,000 shares authorized; no shares issued and outstanding | — | — | |||||
Common stock, $0.0001 par value; 350,000,000 shares authorized, 41,849,725 shares issued and 41,815,975 shares outstanding at September 30, 2017; 15,224,223 shares issued and 15,190,473 shares outstanding at December 31, 2016 | 4 | 2 | |||||
Additional paid-in capital | 347,610 | 335,436 | |||||
Accumulated deficit | (329,129 | ) | (302,932 | ) | |||
Less treasury shares at cost, 33,750 shares | (3,849 | ) | (3,849 | ) | |||
Total stockholders’ equity | 14,636 | 28,657 | |||||
Total liabilities and stockholders’ equity | $ | 49,595 | $ | 65,811 | |||
See accompanying notes to condensed consolidated financial statements.
3
GALENA BIOPHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except share and per share data)
(Unaudited)
Three Months Ended September 30, | Nine Months Ended September 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | ||||||||||||
Operating expenses: | |||||||||||||||
Research and development | $ | 951 | $ | 3,624 | $ | 5,357 | $ | 15,242 | |||||||
General and administrative | 3,511 | 2,848 | 9,104 | 9,490 | |||||||||||
Total operating expenses | 4,462 | 6,472 | 14,461 | 24,732 | |||||||||||
Operating loss | (4,462 | ) | (6,472 | ) | (14,461 | ) | (24,732 | ) | |||||||
Non-operating income (expense): | |||||||||||||||
Litigation settlements | — | — | (1,300 | ) | (1,800 | ) | |||||||||
Change in fair value of warrants potentially settleable in cash | 4,115 | 3,652 | 7,822 | 14,172 | |||||||||||
Goodwill and intangible assets impairment loss | (5,231 | ) | — | (5,231 | ) | — | |||||||||
Interest expense, net | (565 | ) | (1,377 | ) | (2,225 | ) | (1,988 | ) | |||||||
Change in fair value of the contingent purchase price liability | (50 | ) | (145 | ) | (182 | ) | 5,182 | ||||||||
Total non-operating income (expense), net | (1,731 | ) | 2,130 | (1,116 | ) | 15,566 | |||||||||
Loss from continuing operations | (6,193 | ) | (4,342 | ) | (15,577 | ) | (9,166 | ) | |||||||
Income (loss) from discontinued operations | 118 | (2,587 | ) | (10,620 | ) | (8,867 | ) | ||||||||
Net loss | $ | (6,075 | ) | $ | (6,929 | ) | $ | (26,197 | ) | $ | (18,033 | ) | |||
Net loss per common share, basic and diluted: | |||||||||||||||
Basic and diluted net loss per share, continuing operations | $ | (0.15 | ) | $ | (0.41 | ) | $ | (0.45 | ) | $ | (0.97 | ) | |||
Basic and diluted net income (loss) per share, discontinued operations | $ | — | $ | (0.25 | ) | $ | (0.31 | ) | $ | (0.93 | ) | ||||
Basic and diluted net loss per share | $ | (0.15 | ) | $ | (0.66 | ) | $ | (0.76 | ) | $ | (1.90 | ) | |||
Weighted-average common shares outstanding, basic and diluted | 39,250,419 | 10,465,164 | 34,406,397 | 9,515,316 | |||||||||||
See accompanying notes to condensed consolidated financial statements.
4
GALENA BIOPHARMA, INC.
CONDENSED CONSOLIDATED STATEMENT OF STOCKHOLDERS' EQUITY
(Amounts in thousands, except share amounts)
(Unaudited)
Common Stock | Additional Paid-In Capital | Accumulated Deficit | Treasury Stock | Total | ||||||||||||||||||
Shares Issued | Amount | |||||||||||||||||||||
Balance at December 31, 2016 | 15,224,223 | $ | 2 | $ | 335,436 | $ | (302,932 | ) | $ | (3,849 | ) | $ | 28,657 | |||||||||
Issuance of common stock, net of $356 in issuance costs | 17,000,000 | 2 | 15,522 | — | — | 15,524 | ||||||||||||||||
Fair value of common stock warrants granted in connection with 2017 common stock offerings | — | — | (10,357 | ) | — | — | (10,357 | ) | ||||||||||||||
Issuance of common stock as repayment of principal and interest on long-term debt | 9,460,991 | — | 6,305 | — | — | 6,305 | ||||||||||||||||
Issuance of common stock in connection with employee stock purchase plan | 4,048 | — | 5 | — | — | 5 | ||||||||||||||||
Stock-based compensation for directors and employees | — | — | 576 | — | — | 576 | ||||||||||||||||
Fair value of common stock issued in exchange for services | 160,463 | — | 123 | — | — | 123 | ||||||||||||||||
Net loss | — | — | — | (26,197 | ) | — | (26,197 | ) | ||||||||||||||
Balance at September 30, 2017 | 41,849,725 | $ | 4 | $ | 347,610 | $ | (329,129 | ) | $ | (3,849 | ) | $ | 14,636 | |||||||||
See accompanying notes to condensed consolidated financial statements.
5
GALENA BIOPHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in thousands)
(Unaudited)
For the Nine Months Ended September 30, | ||||||||
2017 | 2016 | |||||||
Cash flows from operating activities: | ||||||||
Cash flows from continuing operating activities: | ||||||||
Net loss from continuing operations | $ | (15,577 | ) | $ | (9,166 | ) | ||
Adjustment to reconcile net loss to net cash used in operating activities: | ||||||||
Depreciation and amortization expense | 76 | 95 | ||||||
Non-cash accretion of debt issuance costs | 1,423 | 1,280 | ||||||
Non-cash GALE-401 rights impairment charge | 1,155 | — | ||||||
Non-cash In-process research and development impairment charge | 3,564 | — | ||||||
Non-cash goodwill impairment charge | 512 | — | ||||||
Issuance of common stock as repayment of interest on long-term debt | 765 | — | ||||||
Non-cash stock-based compensation | 576 | 1,830 | ||||||
Fair value of common stock issued in exchange for services | 123 | — | ||||||
Fair value of common stock issued in connection with litigation settlements | — | 2,650 | ||||||
Change in fair value of common stock warrants | (7,822 | ) | (14,172 | ) | ||||
Change in fair value of contingent consideration | 182 | (5,182 | ) | |||||
Changes in operating assets and liabilities: | ||||||||
Prepaid expenses and other assets | 107 | 381 | ||||||
Litigation settlement insurance recovery | — | 21,700 | ||||||
Litigation settlement payable | 350 | (25,000 | ) | |||||
Accounts payable | (629 | ) | (703 | ) | ||||
Accrued expenses and other current liabilities | (1,106 | ) | (1,473 | ) | ||||
Net cash used in continuing operating activities | (16,301 | ) | (27,760 | ) | ||||
Cash flows from discontinued operating activities: | ||||||||
Net loss from discontinued operations | (10,620 | ) | (8,867 | ) | ||||
Changes in operating assets and liabilities attributable to discontinued operations | 683 | (288 | ) | |||||
Net cash used in discontinued operating activities | (9,937 | ) | (9,155 | ) | ||||
Net cash used in operating activities | (26,238 | ) | (36,915 | ) | ||||
Cash flows from investing activities: | ||||||||
Cash paid for purchase of equipment and furnishings | — | (6 | ) | |||||
Net cash used in continuing investing activities | — | (6 | ) | |||||
Selling costs paid for sale of commercial assets | — | (1,050 | ) | |||||
Net cash used in discontinued investing activities | — | — | (1,050 | ) | ||||
Net cash used in investing activities | — | (1,056 | ) | |||||
2017 | 2016 | |||||||
Cash flows from financing activities: | ||||||||
Net proceeds from issuance of common stock | 15,524 | 31,804 | ||||||
Net proceeds from exercise of stock options | — | 261 | ||||||
Proceeds from exercise of warrants | — | 233 | ||||||
Proceeds from common stock issued in connection with Employee Stock Purchase Plan | 5 | 95 | ||||||
Net proceeds from issuance of long-term debt | — | 23,641 | ||||||
Change in restricted cash related to debt principal paid in common stock | 5,650 | — | ||||||
Minimum cash covenant on long-term debt | — | (18,500 | ) | |||||
Principal payments on long-term debt | (110 | ) | (4,779 | ) | ||||
Net cash provided by financing activities | 21,069 | 32,755 | ||||||
Net decrease in cash and cash equivalents | (5,169 | ) | (5,216 | ) | ||||
Cash and cash equivalents at the beginning of period | 18,083 | 29,730 | ||||||
Cash and cash equivalents at end of period | $ | 12,914 | $ | 24,514 | ||||
Supplemental disclosure of cash flow information: | ||||||||
Cash received during the periods for interest | $ | 83 | $ | 84 | ||||
Cash paid during the periods for interest | $ | 102 | $ | 636 | ||||
Supplemental disclosure of non-cash investing and financing activities: | ||||||||
Fair value of warrants issued in connection with common stock recorded as issuance cost | $ | 10,357 | $ | 9,866 | ||||
Repayment of interest and principal on long-term debt through issuance of common stock | $ | 6,305 | $ | — | ||||
Fair value of warrants issued in connection with long-term debt recorded as debt issuance costs | $ | — | $ | 1,139 | ||||
Reclassification of warrant liabilities upon exercise | $ | — | $ | 324 | ||||
6
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. Business and Basis of Presentation
Overview
Galena Biopharma, Inc. ("Company," “we,” “us,” “our,” or “Galena”) is a biopharmaceutical company developing hematology and oncology therapeutics that address unmet medical needs. The Company’s pipeline consists of multiple mid- to late-stage clinical assets, including our hematology asset, GALE-401, and our novel cancer immunotherapy programs including NeuVax™ (nelipepimut-S), GALE-301 and GALE-302. GALE-401 is a controlled release version of the approved drug anagrelide for the treatment of elevated platelets in patients with myeloproliferative neoplasms. NeuVax is currently in multiple investigator-sponsored Phase 2 clinical trials in breast cancer. GALE-301 and GALE-302 have completed early stage trials in ovarian, endometrial and breast cancers.
On January 31, 2017, the Company announced that its Board of Directors had initiated a process to explore and review a range of strategic alternatives. As a result of this process, on August 7, 2017, the Company, SELLAS Life Sciences Group Ltd, a Bermuda exempted company (“SELLAS”), Sellas Intermediate Holdings I, Inc., a Delaware corporation and a wholly owned subsidiary of Galena (“Holdings I”), Sellas Intermediate Holdings II, Inc., a Delaware corporation and a wholly owned subsidiary of Holdings I (“Holdings II”) and Galena Bermuda Merger Sub, LTD., a Bermuda corporation and a wholly owned subsidiary of the Company (“Merger Sub”), entered into an Agreement and Plan of Merger and Reorganization, as amended on November 5, 2017 (the “Merger Agreement”) pursuant to which, among other things, and subject to the satisfaction or waiver of the conditions set forth in the Merger Agreement, Merger Sub will merge with and into SELLAS, and the separate existence of Merger Sub shall cease and SELLAS will continue its corporate existence as the surviving company of the merger (the "Proposed Merger" or "Merger"). As a result of the Proposed Merger, SELLAS will become a wholly owned indirect subsidiary of Galena. See below for more information regarding the Proposed Merger.
If the Proposed Merger is completed, under the terms of the Merger Agreement, at the effective time of the Proposed Merger (the “Effective Time”), (a) each outstanding share of SELLAS (excluding shares held by Galena, Merger Sub or SELLAS, which will be canceled without conversion or payment) will be converted into the right to receive shares of Galena Common Stock based on an exchange ratio specified in the Merger Agreement and (b) each outstanding SELLAS stock option and restricted stock unit award will be assumed by Galena. No fractional shares will be issued in connection with the Proposed Merger and Galena will pay cash in lieu of any such fractional shares. Immediately following the Effective Time, (a) Galena stockholders immediately prior to the Effective Time are expected to own approximately 32.5% of the aggregate number of shares of Galena Common Stock, and (b) SELLAS shareholders immediately prior to the Effective Time are expected to own approximately 67.5% of the aggregate number of shares of Galena Common Stock, each calculated on a fully diluted basis for the combined company, except for the exclusion of 2,556,851 out-of-the money Galena warrants. Though the allocation percentage between SELLAS and Galena will remain the same, both SELLAS and Galena are subject to dilution from (i) any shares of Galena Common Stock issued in connection with a potential third party financing that SELLAS has consented to, and (ii) Galena Common Stock underlying certain Galena warrants (other than the warrants outstanding as of immediately prior to the Effective Time that were issued by Galena under the Warrant Agreement dated February 13, 2017). Upon closing of the Proposed Merger, the name of the combined company will become SELLAS Life Sciences Group, Inc. and shares of the combined company are expected to continue trading on the NASDAQ Capital Market under a new the ticker symbol, SLS.
If the Proposed Merger is completed, our three, Phase 2, investigator-sponsored clinical trials with NeuVax in breast cancer will remain ongoing. Our other development programs, GALE-401 and GALE-301/GALE-302 will be evaluated for potential internal development or strategic partnership by management of the continuing company post-Merger.
7
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
Basis of Presentation and Significant Accounting Policies
The accompanying consolidated financial statements included herein have been prepared by Galena pursuant to the generally accepted accounting principles (GAAP). Unless the context otherwise indicates, references in these notes to the "Company," “we,” “us,” “our,” or “Galena” refer (i) to Galena, our wholly owned subsidiary, Apthera, Inc., or “Apthera,” and our wholly owned subsidiary, Mills Pharmaceuticals, Inc. or "Mills."
These condensed consolidated financial statements and accompanying notes should be read in conjunction with the Company's annual consolidated financial statements and the notes hereto included in its Annual Report on Form 10-K for the year ended December 31, 2016, which was filed on March 15, 2017. The accompanying condensed financial statements at September 30, 2017 and for the three and nine months ended September 30, 2017 and 2016, respectively, are unaudited, but include all adjustments, consisting of normal recurring entries, that management believes to be necessary for a fair presentation of the periods presented. Interim results are not necessarily indicative of results for a full year. Balance sheet amounts as of December 31, 2016 have been derived from the audited financial statements as of that date.
On October 31, 2016, we announced a reverse stock split of our shares of common stock at a ratio of 1-for-20 as approved by the Board of Directors on October 26, 2016. The reverse stock split was authorized by the Company’s stockholders at the Special Meeting of Stockholders held on October 21, 2016. The reverse stock split became effective on November 11, 2016 and the Company’s common stock commenced trading on a split-adjusted basis on Wednesday, November 14, 2016. Unless otherwise stated, all shares and price per share numbers set forth in the condensed consolidated financial statements for periods prior to November 11, 2016 are presented after giving effect to the reverse stock split.
Liquidity & Management's plan - At September 30, 2017, the Company’s capital resources consisted of cash and cash equivalents of $12.9 million. On January 31, 2017, the Company announced that its Board of Directors had initiated a process to explore and review a range of strategic alternatives. As a result of this process, on August 8, 2017 we announced the Proposed Merger. In light of the strategic alternatives process, the Company limited expenditures for its operations through headcount reductions and a general deferral of programs and operational items except that we continued to support the NeuVax investigator-sponsored immunotherapy trials and we have continued to advance activities related to GALE-401 manufacturing in preparation for potential future initiation of a Phase 3 trial. The Company intends to continue to operate at these reduced levels in order to preserve liquidity while completing the Proposed Merger.
Additional funding sources that in certain circumstances may be available to the Company, include 1) approximately $12.0 million of restricted cash associated with the outstanding principal balances as of September 30, 2017 of a debenture with original principal amount of $25.5 million that we sold in May 2016 to the extent we repay the debenture through issuance of common stock in accordance with the terms of the debenture, as detailed further in Note 5; 2) a Purchase Agreement with Lincoln Park Capital, LLC (LPC); 3) At The Market Issuance Sales Agreements (collectively, the ATM) with FBR & Co. (formerly MLV & Co. LLC) and Maxim Group LLC; and 4) amendments to the outstanding warrants; and 5) private or public offerings. See Note 7 below for current restriction on our ability to use the Purchase Agreement with LPC and the ATM with FBR & Co. (formerly MLV & Co. LLC) and Maxim Group LLC. In addition, there are certain restrictions on our ability to amend the outstanding warrants and engage in a private or public offering in the Merger Agreement.
The Company cannot provide assurances that its plans for sources and uses of cash will not change or that changed circumstances will not result in the depletion of its capital resources more rapidly than it currently anticipates. Whether or not we complete the Proposed Merger, we expect to need to raise additional capital to fund our operations, whether through a sale of equity or debt securities, a strategic business transaction, the establishment of other funding facilities, licensing arrangements, asset sales or other means, in order to continue the development of the Company's product candidates and to support its other ongoing activities. However, the Company cannot be certain that it will be able to raise additional capital on favorable terms, or at all, which raises substantial doubt about the Company’s ability to continue as a going concern. The Company is currently evaluating its capital requirements in light of both pursuing the Proposed Merger and funding the development of its clinical
8
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
programs. In addition, the Company is working with SELLAS to develop a comprehensive capital program to fund all product development programs currently prioritized by SELLAS and the Company subsequent to the Proposed Merger. For example, the Merger Agreement states that subsequent to the Merger the Company will use commercially reasonable best efforts to fund the NeuVax ongoing programs in the amount of up to $3 million through the December 31, 2019.
The current unrestricted cash and cash equivalents as of the date of this filing will fund the Company's operations for at least six months from the date that the unaudited condensed consolidated financial statements as of September 30, 2017 were issued. This projection is based on our current limited operations and estimates of resolution and legal expenses associated with the ongoing government investigation and legal matters pending against the Company, and is subject to changes in our operating plans, legal matters, uncertainties inherent in our business, transaction costs incurred at closing of the Proposed Merger, that could individually or in the aggregate cause us to need to seek to replenish our existing cash and cash equivalents sooner than we project and in greater amounts that we had projected. There is no guarantee that any debt, additional equity or other funding will be available to us on acceptable terms, or at all. If we fail to obtain additional funding when needed or the Proposed Merger is not completed, we would be forced to scale back, or terminate, our operations and may not be able to consummate the Proposed Merger. The Company prepared the consolidated financial statements as of and for the three and nine months ended September 30, 2017 using the generally accepted accounting principles applicable to a going concern. These consolidate financial statements do not include any adjustments relating to the recoverability and classification of recorded assets and liabilities amounts that may be necessary should the Company be unable to continue as going concern.
Reclassifications — The prior year amounts for outstanding common stock at par and related additional paid-in capital have been reclassified to correctly present those amounts. These reclassifications had no effect on total equity, or net loss per share.
Goodwill and Intangible Assets — Goodwill and indefinite-lived intangible assets are not amortized but are tested annually for impairment at the reporting unit level, or more frequently if events and circumstances indicate impairment may have occurred. Factors the company considers important that could trigger an interim review for impairment include, but are not limited to, the following:
• | Significant changes in the manner of its use of acquired assets or the strategy for its overall business; |
• | Significant negative industry or economic trends; |
• | Significant decline in stock price for a sustained period; and |
• | Significant decline in market capitalization relative to net book value. |
Intangible assets with indefinite lives are evaluated for impairment first by a qualitative assessment to determine the likelihood of impairment. If it is determined that impairment is more likely than not, the Company will then proceed to the two step impairment test. The first step is to compare the fair value of the reporting unit to the carrying amount of the reporting unit (the “First Step”). If the carrying amount exceeds the fair value, a second step must be followed to calculate impairment (the “Second Step”). Otherwise, if the fair value exceeds the carrying amount, the intangible asset is not considered to be impaired as of the measurement date. In its review of the carrying value of the indefinite-lived intangible assets, the Company determines fair values of its indefinite-lived intangible assets using the income approach.
Intangible assets not considered indefinite-lived are reviewed for impairment when facts or circumstances suggest that the carrying value of these assets may not be recoverable. The Company’s policy is to identify and record impairment losses, if necessary, on intangible product rights when events and circumstances indicate that the assets might be impaired and the undiscounted cash flows estimated to be generated by those assets are less than the carrying amounts of those assets. Refer to Note 2 of the condensed consolidated financial statements for the Company's preliminary interim impairment analysis on intangible assets.
9
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
Goodwill is evaluated for impairment using the simplified test of goodwill impairment as defined by the FASB Accounting Standards Update No. 2017-04. Under the new guidance, goodwill impairment will be measured by the amount by which the carrying value of a reporting unit exceeds its fair value, without exceeding the carrying amount of goodwill allocated to that reporting unit. In its review of the carrying value of the goodwill for its single reporting unit and its indefinite-lived intangible assets, the Company determines fair values of its goodwill using the market approach. Refer to Note 2 of the condensed consolidated financial statements for the Company's preliminary interim impairment analysis on goodwill.
Recently Issued Accounting Pronouncements
In November 2015, the FASB issued ASU No. 2015-17, Balance Sheet Classification of Deferred Taxes or ASU 2015-17. ASU 2015-17 requires that deferred tax liabilities and assets be classified as noncurrent on the balance sheet. Previous guidance required deferred tax liabilities and assets to be separated into current and noncurrent amounts on the balance sheet. The guidance will become effective for us beginning in the first quarter of 2017 and may be applied either prospectively or retrospectively. Early adoption is permitted. At the time of adoption, we will reclassify current deferred tax amounts on our Consolidated Balance Sheets as noncurrent. The Company adopted this ASU on January 1, 2017. There was no impact to the Company’s consolidated financial statements upon adoption.
In March 2016, the FASB issued Accounting Standards Update No. 2016-09, Compensation-Stock Compensation or ASU-2016-09. ASU 2016-09 includes several areas of simplification to stock compensation including simplifications to the accounting for income taxes, classification of excess tax benefits on the Statement of Cash Flows and forfeitures. ASU 2016-09 is effective for annual reporting periods beginning after December 15, 2016. An entity that elects early adoption must adopt all of the amendments in the same period. The Company adopted this ASU on January 1, 2017. There was no impact to the Company’s consolidated financial statements upon adoption.
In January 2017, the FASB issued Accounting Standards Update No. 2017-01, Business Combinations or ASU 2017-01. ASU 2017-01 provides guidance for evaluating whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses. The guidance provides a screen to determine when an integrated set of assets and activities (a “set”) does not qualify to be a business. The screen requires that when substantially all of the fair value of the gross assets acquired (or disposed of) is concentrated in an identifiable asset or a group of similar identifiable assets, the set is not a business. If the screen is not met, the guidance requires a set to be considered a business to include, at a minimum, an input and a substantive process that together significantly contribute to the ability to create outputs and removes the evaluation as to whether a market participant could replace the missing elements. The new standard will be effective for us on January 1, 2018 and will be adopted on a prospective basis. Early adoption is permitted. The Company is currently evaluating the impact of our pending adoption of the new standard on the consolidated financial statements.
In January 2017, the FASB issued Accounting Standards Update No. 2017-04, Simplifying the Test for Goodwill Impairment. ASU 2017-04 simplifies the goodwill impairment test. Under the new guidance, goodwill impairment will be measured by the amount by which the carrying value of a reporting unit exceeds its fair value, without exceeding the carrying amount of goodwill allocated to that reporting unit. This guidance will be effective for us beginning in the first quarter of 2020 and is required to be adopted on a prospective basis. Early adoption is permitted. The Company adopted this ASU on September 30, 2017 and was used to determine the impairment loss to goodwill as of September 30, 2017.
In May 2017, the FASB issued Accounting Standard Update No. 2017-09, Compensation - Stock Compensation (Topic 718): Scope of Modification Accounting. ASU 2017-09 provides guidance about which changes to the terms or conditions of a share-based payment award require an entity to apply modification accounting in Topic 718. The new standard will be effective for us on January 1, 2018; however, early adoption is permitted. The Company is currently evaluating the impact of our pending adoption of the new standard on the consolidated financial statements.
10
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
2. Goodwill and Intangible Assets
Intangible assets include the Company’s GALE-401 rights asset and in-process research and development (IPR&D) asset which were acquired in business combinations and recorded at their respective fair values as of the acquisition date. IPR&D is related to the NeuVax asset. Intangible assets related to GALE-401 rights and IPR&D are considered indefinite-lived intangible assets and are assessed for impairment annually or more frequently if impairment indicators exist. If the associated research and development effort is abandoned, the related assets will be written-off and the Company will record a noncash impairment loss on its consolidated statements of operations. For those compounds that reach commercialization, the IPR&D assets will be amortized over their estimated useful lives.
The impairment test for indefinite-lived intangible assets is a one-step test, which compares the fair value of the intangible asset to its carrying value. If the carrying value exceeds its fair value, an impairment loss is recognized in an amount equal to the excess. Based on accounting standards, it is required that these assets be assessed at least annually for impairment unless a triggering event occurs between annual assessments which would then require an assessment in the period which a triggering event occurred.
The Company entered into the Merger Agreement on August 7, 2017 and the intangible assets were fair valued with the latest preliminary valuation as of October 26, 2017. The preliminary fair values of IPR&D and GALE-401 rights using the income approach. The preliminary interim valuation indicated that the carrying values for IPR&D and GALE-401 rights exceeded the respective carrying values and therefore, the Company recorded impairment losses were recorded to adjust the carrying values to approximate fair values. The impairment losses on GALE-401 rights and IPR&D are aggregated in the statement of operations in goodwill and intangible assets impairment loss.
The following table presents amounts presents the gross amounts of intangible assets and the impairment losses recognized to arrive at the carrying values that approximate fair values as of September 30, 2017 (in thousands):
December 31, 2016 | Impairment Loss | September 30, 2017 | |||||||||
GALE-401 rights | $ | 9,255 | $ | (1,155 | ) | $ | 8,100 | ||||
In-process research and development | 12,864 | (3,564 | ) | 9,300 | |||||||
Total other intangible assets | $ | 22,119 | $ | (4,719 | ) | $ | 17,400 | ||||
Goodwill represents the excess of purchase price over the fair value of net assets acquired by the Company in the acquisition of Apthera, Inc. in 2011 with their sole product candidate, NeuVax. Goodwill is not amortized, but assessed for impairment on an annual basis or more frequently if impairment indicators exist. The impairment model prescribes a simplified goodwill impairment test. Under the new guidance, goodwill impairment will be measured by the amount by which the carrying value of a reporting unit exceeds its fair value, without exceeding the carrying amount of goodwill allocated to that reporting unit.
During the three and nine months ended September 30, 2017, the Company experienced a significant decline in our stock price, and our stock price continued to trend lower through September 30, 2017. Following the public announcement of the entry into the Merger Agreement on August 8, 2017, our stock price declined 27%. The Company determined that this event constituted substantive changes in circumstances that would more likely than not reduce the fair value of the Company's single reporting unit below its carrying amount. Accordingly, the Company tested its goodwill for impairment as of September 30, 2017.
11
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
In determining, the fair value of the Company's sole reporting unit, the estimated fair value of our reporting unit was determined utilizing a market-based approach, as the primary input in this approach was a quoted market price in an active market. Based on our interim impairment analysis we determined that our carrying value as of September 30, 2017 exceeded the market capitalization of the Company due to the declining stock price. Based on our interim impairment analysis, we recorded a goodwill impairment charge of $0.5 million in the third quarter of 2017 to adjust goodwill to its implied fair value. After recording the goodwill impairment loss, the fair value of equity approximates the carrying value of equity. The impairment loss on goodwill was recorded to adjust the carrying value to approximate fair value. The impairment losses on GALE-401 rights and IPR&D are aggregated in the statement of operations in goodwill and intangible assets impairment loss.
The following table presents amounts presents the gross amount of goodwill and the impairment losses recognized to arrive at the carrying values that approximate fair values as of September 30, 2017 (in thousands):
December 31, 2016 | Impairment Loss | September 30, 2017 | |||||||||
Goodwill | $ | 5,898 | $ | (512 | ) | $ | 5,386 | ||||
The following table presents the impairment losses that aggregate in the statement of operations in goodwill and intangible assets impairment loss for the three and nine months ended September 30, 2017 and 2016, respectively (in thousands):
Three Months Ended September 30, | Nine months ended September 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | ||||||||||||
GALE-401 rights impairment loss | $ | (1,155 | ) | $ | — | $ | (1,155 | ) | $ | — | |||||
In-process research and development impairment loss | (3,564 | ) | — | (3,564 | ) | — | |||||||||
Goodwill impairment loss | (512 | ) | — | (512 | ) | — | |||||||||
Goodwill and intangible assets impairment loss | $ | (5,231 | ) | $ | — | $ | (5,231 | ) | $ | — | |||||
12
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
3. Fair Value Measurements
The following tables present information about our assets and liabilities measured at fair value on a recurring basis in the condensed consolidated balance sheets (in thousands):
Description | September 30, 2017 | Quoted Prices In Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Unobservable Inputs (Level 3) | |||||||||||
Assets: | |||||||||||||||
Cash equivalents | $ | 11,162 | $ | 11,162 | $ | — | $ | — | |||||||
Restricted cash equivalents | 12,093 | 12,093 | — | — | |||||||||||
Total assets measured and recorded at fair value | $ | 23,255 | $ | 23,255 | $ | — | $ | — | |||||||
Liabilities: | |||||||||||||||
Warrants potentially settleable in cash | $ | 4,395 | $ | — | $ | 4,395 | $ | — | |||||||
Contingent purchase price consideration | 1,277 | — | — | 1,277 | |||||||||||
Total liabilities measured and recorded at fair value | $ | 5,672 | $ | — | $ | 4,395 | $ | 1,277 | |||||||
Description | December 31, 2016 | Quoted Prices In Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Unobservable Inputs (Level 3) | |||||||||||
Assets: | |||||||||||||||
Cash equivalents | $ | 16,192 | $ | 16,192 | $ | — | $ | — | |||||||
Restricted cash equivalents | 17,622 | 17,622 | — | — | |||||||||||
Total assets measured and recorded at fair value | $ | 33,814 | $ | 33,814 | $ | — | $ | — | |||||||
Liabilities: | |||||||||||||||
Warrants potentially settleable in cash | $ | 1,860 | $ | — | $ | 1,860 | $ | — | |||||||
Contingent purchase price consideration | 1,095 | — | — | 1,095 | |||||||||||
Total liabilities measured and recorded at fair value | $ | 2,955 | $ | — | $ | 1,860 | $ | 1,095 | |||||||
The Company did not transfer any financial instruments into or out of Level 3 classification during the nine months ended September 30, 2017 and 2016. A reconciliation of the beginning and ending Level 3 liabilities for the nine months ended September 30, 2017 is as follows (in thousands):
Fair Value Measurements Using Significant Unobservable Inputs (Level 3) | |||
Balance, January 1, 2017 | $ | 1,095 | |
Change in the estimated fair value of the contingent purchase price consideration | 182 | ||
Balance at September 30, 2017 | $ | 1,277 | |
13
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
The fair value of the contingent purchase price consideration is measured at the end of each reporting period using Level 3 inputs in a probability-weighted, discounted cash-outflow model. The significant unobservable assumptions include the probability of achieving each milestone, the date we expect to reach the milestone, and a determination of present value factors used to discount future expected cash outflows. The decrease in the estimated fair value of the contingent purchase price consideration during the period reflects a lowering of the probability and lengthening of the timeline for the potential approval of NeuVax, as these assumptions are now based principally on our Phase 2 combination trial of trastuzumab and NeuVax with HER2 low-to-intermediate expressing patients. Previously, the valuation was based on the probability of achieving each milestone for our Phase 3 PRESENT trial, which was stopped in June 2016 and subsequently closed in the third quarter due to futility as recommended by the Independent Data Monitoring Committee ("IDMC").
See Note 8 for discussion of the Level 2 liabilities relating to warrants accounted for as liabilities.
4. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following (in thousands):
September 30, 2017 | December 31, 2016 | ||||||
Clinical trial costs | $ | 324 | $ | 3,088 | |||
Professional fees | 1,827 | 229 | |||||
Compensation and related benefits | 1,035 | 975 | |||||
Interest expense | — | — | |||||
Accrued expenses and other current liabilities | $ | 3,186 | $ | 4,292 | |||
14
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
5. Long-Term Debt
On May 10, 2016, the Company entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”), with JGB (Cayman) Newton Ltd. (the “Purchaser”) pursuant to which the Company sold to Purchaser, at a 6.375% original issue discount, a $25,530,000 Senior Secured Debenture (the “Initial Debenture”) and warrants to purchase up to 100,000 shares of the Company's common stock, $0.0001 par value per share (“Common Stock”). Net proceeds to the Company from sale of the Initial Debenture and warrants, after payment of commissions and legal fees, were approximately $23,400,000. The Initial Debenture contained no conversion features to shares of Common Stock.
The Initial Debenture carried an interest only period of six months following which the holder of the Initial Debenture had the right, at its option, to require the Company to redeem up to $1,100,000 of the outstanding principal amount of the Initial Debenture per calendar month. The Company is required to promptly, but in any event no more than three trading days after the holder delivers a redemption notice to the Company, pay the applicable redemption amount in cash or, at the Company’s election and subject to certain conditions, in shares of Common Stock. If the Company elects to pay the redemption amount in shares of Common Stock, then the shares will be delivered at the lesser of A) 7.5% discount to the average of the 3 lowest volume weighted average prices over the prior 20 trading days or B) a 7.5% discount to the prior trading day’s volume weighted average price (the “Stock Payment Price”). Pursuant to the Initial Debenture, the Company may only opt for payment in shares of Common Stock if certain equity conditions are met or waived, including, among others, that the volume weighted price of the Common Stock be at least $15.00 (the “Original Minimum Price Condition”).
The Initial Debenture was amended and restated in its entirety on August 22, 2016 (as so amended, the “Debenture”) pursuant to an Amendment Agreement, dated August 22, 2016, among the Company, the Purchaser and JGB Collateral LLC (the “Amendment Agreement”). As previously reported, interest on the Debenture is payable at the end of each month based on the outstanding principal. The Debenture matures on November 10, 2018, and accrues interest at 9% per year. In addition, on the maturity date of the Debenture (or such earlier date that the principal amount of the Debenture is paid in full by acceleration or otherwise) a fixed amount, which shall be deemed interest under the Debenture, equal to $765,900, will be due and payable to the holder of the Debenture on such date in, at the option of the Company, cash and, subject to the same conditions for the payment of interest in shares of Common Stock, shares of Common Stock or a combination of cash and Common Stock.
The Company’s obligations under the Debenture are secured under a Security Agreement by a senior lien on all of the Company’s assets, including all of the Company’s interests in its consolidated subsidiaries. Under the subsidiary guarantee agreement, each subsidiary guarantees the performance of the Company of the Purchase Agreement, Debenture and related agreements.
15
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
After giving effect to the Amendment Agreement, the Debenture contains the following modified and/or additional terms, among others:
• | With respect to interest accruing on the outstanding principal amount under the Debenture for the period prior to November 10, 2016, the Company was permitted to satisfy such interest payments in kind by adding such amount to the outstanding principal. |
• | The Purchaser can from time to time during the term of the Debenture require the Company to prepay in cash all or a portion of the outstanding principal plus accrued and unpaid interest (the “Outstanding Amount”) on written notice to the Company, provided, that such prepayment amount shall not exceed the lesser of $18,500,000 and the Outstanding Amount. If the holder elects such prepayment of the Debenture, then the number of shares subject to the warrants issued to the holder will be reduced in proportion to the percentage of principal and accrued interest required to be prepaid by the Company. In addition, the Company shall have the right to prepay in cash all (but not less than all) of the Outstanding Amount (1) at any time after November 10, 2017, or (2) upon a “change of control” (as such term is used in the Debenture), in each case with a 10% premium on the Outstanding Amount. |
• | The Purchaser shall continue to have the right, which commenced on November 10, 2016, to require the Company to redeem the Outstanding Amount, except that the maximum monthly amount of such redemptions was increased from $1,100,000 to $1,500,000; provided, that if the trading price of Common Stock is at least $8.00 per share (as may be further adjusted appropriately for stock splits, combinations or similar events) during such calendar month, then such monthly maximum redemption amount may be increased to $2,200,000 at the Purchaser’s election and if the Company has already elected to satisfy such redemptions in shares of Common Stock. In addition, notwithstanding the foregoing limitations on the monthly redemption amount, the Purchaser may elect up to three times in any 12-month period to increase the monthly maximum to $2,500,000. |
• | Among the various conditions that must be satisfied (or waived) in order for the Company to be able to elect to satisfy the monthly redemption amounts in shares of Common Stock, the Original Minimum Price Condition of $15.00 was decreased to a volume-weighted average price of $4.00 per share (the “Amended Minimum Price Condition”). |
• | Following November 10, 2016, the Purchaser may elect to convert any portion of the Outstanding Amount into shares of Common Stock at a fixed price of $12.00 per share (as adjusted appropriately for stock splits, combinations or similar events). |
• | Under the Initial Debenture, the Company was required to maintain a minimum of $24,000,000 of unencumbered cash in a restricted account as security for its obligations under the Initial Debenture. Such minimum amount has been reduced to the lesser of $18,500,000 or the Outstanding Amount. |
In addition, in accordance with the terms of the Amendment Agreement, the exercise price of the Series A Warrant was reduced from $30.20 per share to $8.60 per share (as may be further adjusted appropriately for stock splits, combinations or similar events).
16
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
On December 14, 2016, the Company and the Purchaser entered into a waiver (the “First Waiver”) pursuant to which, as contemplated by the Debenture, the Purchaser waived with respect to the calendar months of December 2016, January 2017, February 2017 and March 2017 (collectively, the “First Specified Months”) the Amended Minimum Price Condition, provided that, among other things, with respect to the First Specified Months, the volume weighted average price of the Common Stock was not less than $1.00 and the Company’s cash on hand exceeded the outstanding principal amount of the Debenture by $10 million. Furthermore, the First Waiver set out a monthly amount to be redeemed for each of the First Specified Months equal to $1,500,000 and amended the Debenture to require the Company to withdraw all cash and/or cash equivalents in excess of $18,500,000 from certain accounts and deposit such funds into an account in a form acceptable to the Purchaser, to be executed by the Company, U.S. Bank, N.A. and SVB Asset Management such that the Company requires the prior written consent of the Purchaser for certain withdrawals. The First Waiver amends the Debenture to grant the Purchaser the right to redeem any portion of the outstanding principal amount of the Debenture in Common Stock if the price per share of Common Stock on a principal trading market at any point in time of any trading day exceeds the closing price per share of the Common Stock on the immediately preceding trading day by more than 25%.
On April 1, 2017, the Company and Purchaser entered into a waiver (the “Second Waiver”) pursuant to which, as contemplated by the Debenture, the Purchaser waived with respect to the calendar months of April 2017, May 2017, June 2017, July 2017, August 2017 and September 2017 (collectively, the “Second Specified Months”) the Amended Minimum Price Condition, provided that, among other things, with respect to the Second Specified Months, the volume weighted average price of the Common Stock is not less than $0.30 and the Company’s cash on hand exceeds the outstanding principal amount of the Debenture by $10 million.
On May 1, 2017, the Purchaser, the Company and the guarantors of the Company’s obligations under the Debenture entered into an amendment agreement (the “2017 Amendment Agreement”) pursuant to which the Purchaser may, from time to time, at the Purchaser’s option waive the Amended Minimum Price Condition; provided, however, the Purchaser cannot waive the Amended Minimum Price Condition to the extent that the resulting Stock Payment Price would be less than $0.35 per share as a result of any such waiver (the “Minimum Stock Payment Price Condition”). The 2017 Amendment Agreement further provides that, in the event of any Equity Conditions Failure (as such term is defined in the Debenture) that is not, or cannot be as a result of the 2017 Amendment Agreement, waived by the Purchaser, the Company shall honor the holder redemption amounts in cash or, at the Company’s election, with the prior written consent of the Purchaser, deliver aggregate consideration in shares of Common Stock and cash in satisfaction of the applicable holder redemption amount as follows: (i) the number of shares of Common Stock equal to the quotient obtained by dividing such holder redemption amount and $0.35 (each such share having a deemed value per share at the Stock Payment Price that would have been in effect but for the Minimum Stock Payment Price Condition of $0.35 per share) and (ii) cash equal to the difference between the holder redemption amount and the aggregate deemed value of the shares of Common Stock delivered in clause (i).
As of May 1, 2017 pursuant to the 2017 Amendment Agreement, the Company estimated that the maximum number of shares of Common Stock that the Company could issue pursuant to the terms of the Debenture subsequent to May 1, 2017 was 45,000,000. As of November 9, 2017, the date of issuance of the Company's condensed consolidated financial statements for the quarter ended September 30, 2017, the Company had issued 8,102,082 shares since the 2017 Amendment Agreement.
On July 10, 2017, the Purchaser, the Company and the guarantors of the Company’s obligations under the Debenture entered into an amendment agreement (the “July 2017 Amendment Agreement”) pursuant to which the definition of “Stock Payment Price” in the Debenture was amended and restated to be the lower of (a) 80% (previously 92.5%) of the VWAP for the Trading Day immediately prior to, as the case may be, the applicable Interest Payment Date, the applicable Advance Date or, with respect to any redemption pursuant to Section 6(a) of the Debenture, the date of the applicable Holder Redemption Notice (the “Prior Day VWAP”) and (b) 80% (previously 92.5%) of the average of the three lowest VWAPs during the 20 consecutive Trading Day period immediately preceding, as the case may be, the applicable Interest Payment Date, the applicable Advance Date or, with respect to any redemption pursuant to Section 6(a) of the Debenture, the date of the applicable Holder Redemption Notice (the “Twenty Day VWAP”); provided, however, to the extent that, on any given Trading Day, the price per share of Common Stock on such Trading Day on the Principal Market equals or exceeds 115% of the Prior Day VWAP or Twenty Day VWAP, then for the such Trading Day, and such Trading Day only, each reference to eighty percent (80%) shall be deemed, for such Trading Day only, to be ninety two and one-half percent (92.5%).
17
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
The effect of the July 2017 Amendment Agreement is to increase the discount to the Prior Day VWAP and the Twenty Day VWAP granted to the Holder with respect to redemption of, or the payment of interest on, the Debenture in shares of Common Stock from 7.5% to 20%, unless the on any given Trading Day, the price per share of Common Stock on such Trading Day on the Principal Market equals or exceeds 115% of the Prior Day VWAP or Twenty Day VWAP. However, the maximum number of shares of Common Stock issuable pursuant to the Debenture was not changed by the July 2017 Amendment Agreement.
On August 7, 2017, the Company and Purchaser entered into a consent to the Debenture in which the Purchaser consented to the Company’s entry into the Merger Agreement and the Merger as well as an amendment to the Debenture in which: (a) the Company shall not prepay all or any portion of the Debenture prior to the first anniversary of the consummation of the Merger, (b) the Purchaser may increase the dollar amount of the monthly allowance up to the outstanding principal balance of the Debenture by written notice to the Company and may deliver an unlimited number of redemption notices during any calendar month, and (c) to the extent commercially reasonable under the circumstances the Purchaser shall limit the redemption amounts for any given trading day to fifteen percent (15%) of the greater of (1) the daily dollar trading volume for our common stock for such trading day and (2) the average daily dollar trading volume for our common stock for the five (5) consecutive trading days preceding such trading day.
As of September 30, 2017, the outstanding principal balance of the Debenture was $11,971,702. The current portion of long-term debt as of September 30, 2017 of $12,170,059 is net of unamortized discounts and debt issuance costs of $198,357. During the nine months ended September 30 2017, the holder of the Debenture redeemed $5,650,000 of principal, which the Company satisfied with 7,806,708 shares of our common stock. As of December 31, 2016, the outstanding principal balance of the Debenture was $17,621,702. The current portion of long-term debt of $16,397,030 as of December 31, 2016 is net of unamortized discounts and debt issuance costs of $1,224,672. Subsequent to September 30, 2017 and prior to November 9, 2017, the date of issuance of the Company's condensed consolidated financial statements for the quarter ended September 30, 2017, the holder of the Debenture redeemed an additional $1,150,000 of principal, which the Company satisfied with 3,285,711 shares of our common stock.
18
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
6. Legal Proceedings, Commitments and Contingencies
Legal Proceedings
Settled Matters
On December 16, 2015, Galena received a subpoena issued by the U.S. Attorney’s Office for the District of New Jersey, or USAO NJ, requesting the production of a broad range of documents pertaining to Galena’s marketing and promotional practices for Abstral. Through its communications with the USAO NJ and the DOJ, Galena came to understand that the investigation being undertaken by the USAO NJ and DOJ was a criminal investigation in addition to a civil investigation that could ultimately involve Galena as well as one or more former employees. Pursuant to Galena’s charter, Galena was reimbursing certain former employees’ attorney’s fees with respect to the investigation but stopped on May 1, 2017. Galena cooperated with the civil, and is continuing to cooperate with the criminal, investigations, and on September 8, 2017, DOJ announced a settlement agreement with Galena regarding the USAO NJ’s and DOJ’s investigation. The settlement agreement involves a non-criminal resolution and a civil payment in equal installments over twelve months of approximately $7.6 million, plus interest accrued since the date of reaching an agreement in principle, in return for a release of government claims in connection with the investigation. The $7.6 million civil payment is payable over four equal quarterly installments, with the first payment being made in the third quarter of 2017, and is presented in discontinued operations in the statement of operations. As set forth in that settlement agreement, for a release of all claims against Galena and its officers and directors and dismissal with prejudice of the qui tam lawsuit described below, the relator received a portion of the civil payment to the federal government. Upon payment of the settlement amount, the federal government and the relator will dismiss with prejudice their claims against Galena in the qui tam lawsuit.
In addition, there is a qui tam action pending in the U.S. District Court of the District of New Jersey related to the investigation by USAO NJ and DOJ. On September 18, 2017, the Company executed a settlement agreement with the attorneys for the relator in the qui tam action to settle their statutorily mandated attorney fees award by payment of $100,000 in cash and $200,000 in Galena Common Stock subject to court approval, which amounts were accrued during the second quarter 2017. Galena also obtained the consent of SELLAS under the terms of the Merger Agreement. However on November 7, 2017, attorneys for the qui tam relator agreed to have Galena pay the $200,000 in cash. We also obtained the consent of SELLAS under the terms of the Merger Agreement. The Company paid the $300,000 in cash for the statutorily mandated attorney fees award in the fourth quarter of 2017.
Open Matters
On October 13, 2016, Galena filed a complaint in the Circuit Court for the County of Multnomah for the State of Oregon against Aon Risk Insurance Services West, Inc. (Aon) where Galena is seeking attorney’s fees, costs and expenses incurred by Galena related to its coverage dispute with a certain insurer and for amounts Galena was required to contribute to the settlements of In re Galena Biopharma, Inc. Derivative Litigation and In re Galena Biopharma, Inc. Securities Litigation as a direct result of certain insurer’s failure to pay its full policy limits of liability and other relief. Galena and Aon are currently engaged in written discovery.
On February 13, 2017, a putative shareholder securities class action complaint was filed in the U.S. District Court for the District of New Jersey captioned, Miller v. Galena Biopharma, Inc., et al. On February 15, 2017, a putative shareholder securities class action complaint was filed in the U.S. District Court for the District of New Jersey entitled, Kattuah v Galena Biopharma, Inc., et al. The actions assert that the defendants failed to disclose that Galena's promotional practices for Abstral® (fentanyl sublingual tablets were allegedly improper and that the Company may be subject to civil and criminal liability, and that these alleged failures rendered the Company’s statements about its business misleading. Two groups of shareholders and one individual shareholder filed three motions to be appointed lead plaintiff on April 14, 2017 and April 17, 2017. Subsequently, one of the shareholders groups withdrew its motion for lead plaintiff status and the individual shareholder notified the court that he does not object to the appointment of the remaining shareholder group, GALE investor group, as lead plaintiff. On July 17, 2017, the Court approved the GALE investor group as named lead plaintiff and its counsel as lead and liaison counsel. The Court also consolidated both actions. An amended complaint was filed on October 6, 2017. It is expected that Galena and former officers and current and former employees will respond to the amended complaints through an appropriate pleading or motion.
19
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
On March 16, 2017, a complaint captioned Keller v. Ashton et al., CA No. 2:17-cv-01777 was filed in the U.S. District Court for the District of New Jersey against the Company's current directors and the Company, as a nominal defendant. The complaint purports to assert derivative claims for breach of fiduciary duty on Galena's behalf against its directors based on substantially similar facts as alleged in the putative shareholder securities class action complaints mentioned above. The Company's response to the complaint was due on June 1, 2017; however, the Court on May 21, 2017, entered a stay of the proceedings pending resolution of motions to dismiss in the securities litigations described above.
Galena also received a stockholder demand dated April 14, 2017, pursuant to 8 Del. C. Sec. 220, from a shareholder (Albert Zhang) demanding access to Galena’s books and records relating to its sales of Abstral and the U.S. Attorney’s investigation into Galena’s sale of Abstral in order for Mr. Zhang to determine, among other things, whether to file a derivative lawsuit against Galena’s management and directors. Galena has responded to the demand and Mr. Zhang has indicated that he will file a derivative complaint soon.
On April 27, 2017, a putative shareholder class action was filed in the Chancery Court of Delaware captioned Patel vs. Galena Biopharma, Inc. et. al, CA No. 2017-0325 alleging breaches of Section 225 of the Delaware General Corporate Law (DGCL) and breaches of fiduciary duties by the Galena Board of Directors regarding the voting results of authorized shares and the reverse stock split proposals in the proxy statements for the July 2016 and October 2016 stockholder meetings. On June 2, 2017, an amended verified complaint was filed along with a motion to expedite the proceedings. On June 5, 2017, Galena filed a verified petition under Section 205 of the DGCL and a motion to expedite the proceedings. On June 8, 2017, the court denied a request by the plaintiff to schedule a preliminary injunction motion and ordered a prompt trial on both the plaintiff and Galena’s claims. On June 20, 2017, the court consolidated the claims into In re Galena Biopharma, Inc., C. A. No. 2017-0423-JTL. On July 10, 2017, the court ordered that the trial of the claims be held on August 28, 30 and 31, 2017. On July 24, 2017, Galena entered into a binding settlement term sheet, which the parties will use to enter into a Stipulation of Settlement that is intended to settle the litigation currently pending in the Court of Chancery of the State of Delaware, captioned In re Galena Biopharma, Inc., C. A. No. 2017-0423-JTL. The settlement resolves the putative stockholder class action claims against Galena and/or certain of its current and former officers and directors, as well as Galena’s petition to validate certain corporate actions. The settlement will not become effective until approved by the Court. Due to the decline in the price of the Galena Common Stock, the plaintiff has demanded to renegotiate the binding settlement term sheet. On September 7, 2017, Galena moved to enforce the binding settlement term sheet. The parties have filed supporting briefs and the hearing will be held on November 30, 2017.
Under the terms of the settlement, the class will receive a settlement payment of $1.3 million, in addition to attorney fees in an amount to be approved. The settlement payment of $1.3 million consists of $50,000 in cash to be paid by the Defendants or their insurers and $1,250,000 in unrestricted shares of the Company’s common stock (“Settlement Stock”), which valuation will be based on the volume-weighted average closing price for the 20 trading days immediately preceding the day before the transfer of the Settlement Stock to the settlement fund pursuant to the terms and conditions of the settlement. The Company anticipates that the Settlement Stock will be issued, pursuant to the terms of the Stipulation of Settlement, in a transaction that is exempt from the registration requirements of the Securities Act of 1933, as amended (the “Securities Act”), pursuant to Section 3(a)(10) of the Securities Act. Any amounts awarded by the Court for attorneys’ fees will be paid in part by the settlement fund and in part by the Company’s insurance carriers. Upon the effectiveness of the proposed settlement, the Defendants will be released from the claims that were asserted or could have been asserted in the class action by class members participating in the settlement.
On July 6, 2017, a complaint captioned Jacob v. Schwartz et al., Case No. C17-01222, was filed in the Superior Court of California, County of Contra Costa against the Company's current and former directors and the Company, as a nominal defendant. The complaint purports to assert derivative claims for breach of fiduciary duty on Galena's behalf against its directors based on substantially similar facts as alleged in the derivative complaint mentioned above. The Company's response to the complaint was due on July 7, 2017; however, the court on September 5, 2017, entered a stay of the proceedings pending resolution of motions to dismiss in the securities litigations described above.
20
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
On November 7, 2017, a written demand was made on the Company by a shareholder requesting that additional financial projections and valuation analyses be made in the Company’s Form S-4 relating to the Merger, which was declared effective on November 6, 2017. The demand stated, among other thing, that, if such disclosures are not made within a reasonable period of time, the shareholder intends to file a securities class action lawsuit in federal court. The Company will review the demand letter and make the appropriate response
21
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
7. Stockholders’ Equity
Preferred Stock — The Company has authorized up to 5,000,000 shares of preferred stock, $0.0001 par value per share, for issuance. The preferred stock will have such rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, as shall be determined by the Company’s Board of Directors upon its issuance. To date, the Company has not issued any preferred shares.
Common Stock — The Company has authorized up to 350,000,000 shares of common stock, $0.0001 par value per share, for issuance.
November 2014 Purchase Agreement with Lincoln Park Capital, LLC - On November 18, 2014, the Company entered into a purchase agreement (LPC Agreement) with Lincoln Park Capital, LLC (LPC), pursuant to which the Company has the right to sell to LPC up to $50 million in shares of the Company's common stock, subject to certain limitations and conditions over the 36-month term of the LPC Agreement. Pursuant to the purchase agreement, LPC initially purchased 125,000 shares of the Company's common stock at $40.00 per share and the Company issued 31,561 shares of common stock to LPC as a commitment fee, which was recorded as a cost of capital. As a result of this initial issuance, the Company received initial net proceeds of $4.9 million, after deducting commissions and other offering expenses. The Company did not sell any shares of our common stock under the LPC Agreement during the nine months ended September 30, 2017. On February 6, 2017, the LPC Agreement was amended to decrease the total value of common stock that the Company may sell to LPC from $55,000,000 to $15,600,000. Except as noted below, the Company has $2.1 million of remaining availability under the LPC Agreement. Use of the purchase agreement with LPC is not currently available to the Company because the Company is not currently eligible to use a Form S-3 registration statement, and it does not expect to be eligible to use a Form S-3 registration statement until May 1, 2018 at the earliest.
At-The-Market Issuance Sales Agreements - On May 24, 2013, the Company entered into At-The-Market Issuance Sales Agreements (ATM) with FBR & Co. (formerly MLV & Co. LLC) and Maxim Group LLC (the Agents). From time to time during the term of the ATM, we may issue and sell through the Agents, shares of our common stock, and the Agents collect a fee equal to 3% of the gross proceeds from the sale of shares, up to a total limit of $20 million in gross proceeds. Except as noted below, the ATM is available to the Company until it is terminated by the Agents, or the Company. The Company did not sell any shares of our common stock under the ATM during the nine months ended September 30, 2017. On December 4, 2015 we replenished the ATM limit up to $20 million in gross proceeds available for future sales of our common stock. Except as noted below, the Company has $19.1 million of remaining availability under the ATM. Use of the ATM is not currently available to the Company because the Company is not currently eligible to use a Form S-3 registration statement, and it does not expect to be eligible to use a Form S-3 registration statement until May 1, 2018 at the earliest.
February 2017 Underwritten Public Offering - On February 13, 2017, the Company closed an underwritten public offering of 17,000,000 shares of common stock and warrants to purchase 17,000,000 shares of common stock priced at $1.00 per share and accompanying warrant (February 2017 Offering). The warrants are immediately exercisable with a strike price of $1.10 and will expire on the fifth anniversary of the date of issuance. The shares of common stock and the warrants were issued separately and were separately transferable immediately upon issuance. The net proceeds of the February 2017 Offering were $15.5 million, after deducting underwriting discounts and commissions and offering expenses paid by the Company. The fair value of the warrants to purchase shares of our common stock issued in connection with the February 2017 Offering was $10.4 million recorded as an issuance cost.
22
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
Shares of common stock for future issuance are reserved for as follows (in thousands):
As of September 30, 2017 | ||
Warrants outstanding | 19,557 | |
Stock options outstanding | 443 | |
Options reserved for future issuance under the Company’s 2016 Incentive Plan | 457 | |
Shares reserved for future issuance under the Employee Stock Purchase Plan | 17 | |
Total reserved for future issuance | 20,474 | |
8. Warrants
The following is a summary of warrant activity for the nine months ended September 30, 2017 (in thousands):
Warrant Issuance | Outstanding, December 31, 2016 | Granted | Exercised | Expired | Outstanding, September 30 2017 | Expiration | ||||||||||
February 2017 | — | 17,000 | — | — | 17,000 | February 2022 | ||||||||||
July 2016 | 700 | — | — | — | 700 | January 2022 | ||||||||||
January 2016 | 682 | — | — | — | 682 | January 2021 | ||||||||||
March 2015 | 700 | — | — | — | 700 | March 2020 | ||||||||||
September 2013 | 199 | — | — | — | 199 | September 2018 | ||||||||||
December 2012 | 152 | — | — | — | 152 | December 2017 | ||||||||||
April 2011 | 13 | — | — | (13 | ) | — | April 2017 | |||||||||
Other | 124 | — | — | — | 124 | November 2021 | ||||||||||
2,570 | 17,000 | — | (13 | ) | 19,557 | |||||||||||
Warrants consist of warrants potentially settleable in cash, which are liability-classified warrants, and equity-classified warrants.
23
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
Warrants classified as liabilities
Liability-classified warrants consist of warrants to purchase common stock issued in connection with equity financings in February 2017, July 2016, January 2016, March 2015, September 2013, December 2012, and April 2011. These warrants are potentially settleable in cash and were determined not to be indexed to our common stock.
Most of our warrants have a provision allowing the holders of warrants to require us to make a cash payment in the event we engage in a Fundamental Transaction. The term Fundamental Transaction is defined in each warrant agreement governing the applicable class of warrants. The cash payment is based upon a Black-Scholes analysis of the remaining value of the warrant at the time the Fundamental Transaction is effectuated. In August 2017, two holders of warrants under the warrant agreement dated February 13, 2017 asserted that the Proposed Merger constitutes a Fundamental Transaction. The Company is in process of determining the merit of this assertion.
The estimated fair value of outstanding warrants accounted for as liabilities is determined at each balance sheet date. Any decrease or increase in the estimated fair value of the warrant liability since the most recent balance sheet date is recorded in the condensed consolidated statement of operations as other income (expense). The fair value of the warrants is estimated using an appropriate pricing model with the following inputs:
As of September 30, 2017 | ||||||||||||||
Warrant Issuance | Outstanding (in thousands) | Strike price (per share) | Expected term (years) | Volatility % | Risk-free rate % | |||||||||
February 2017 | 17,000 | $ | 1.10 | 4.37 | 129.64 | % | 1.83 | % | ||||||
July 2016 | 700 | $ | 13.00 | 3.79 | 128.32 | % | 1.74 | % | ||||||
January 2016 | 682 | $ | 28.40 | 3.28 | 132.02 | % | 1.66 | % | ||||||
March 2015 | 700 | $ | 41.60 | 2.47 | 145.73 | % | 1.54 | % | ||||||
September 2013 | 199 | $ | 50.00 | 0.97 | 179.71 | % | 1.30 | % | ||||||
December 2012 | 152 | $ | 10.32 | 0.23 | 75.45 | % | 1.05 | % | ||||||
As of December 31, 2016 | ||||||||||||||
Warrant Issuance | Outstanding (in thousands) | Strike price (per share) | Expected term (years) | Volatility % | Risk-free rate % | |||||||||
July 2016 | 700 | $ | 13.00 | 4.54 | 117.82 | % | 1.82 | % | ||||||
January 2016 | 682 | $ | 28.40 | 4.03 | 120.38 | % | 1.71 | % | ||||||
March 2015 | 700 | $ | 41.60 | 3.22 | 131.46 | % | 1.52 | % | ||||||
September 2013 | 199 | $ | 50.00 | 1.72 | 164.01 | % | 1.10 | % | ||||||
December 2012 | 152 | $ | 31.60 | 0.98 | 204.55 | % | 0.84 | % | ||||||
April 2011 | 13 | $ | 13.00 | 0.31 | 103.79 | % | 0.53 | % | ||||||
The expected volatility assumptions are based on the Company's implied volatility in combination with the implied volatilities of similar publicly traded entities. The expected life assumption is based on the remaining contractual terms of the warrants. The risk-free rate is based on the zero coupon rates in effect at the time of valuation. The dividend yield used in the pricing model is zero, because the Company has no present intention to pay cash dividends.
24
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
The changes in fair value of the warrant liability for the nine months ended September 30, 2017 were as follows (in thousands):
Warrant Issuance | Warrant liability, December 31, 2016 | Fair value of warrants granted | Change in fair value of warrants | Warrant liability, September 30, 2017 | |||||||||||
February 2017 | $ | — | $ | 10,357 | $ | (6,101 | ) | $ | 4,256 | ||||||
July 2016 | 753 | — | (680 | ) | 73 | ||||||||||
January 2016 | 529 | — | (489 | ) | 40 | ||||||||||
March 2015 | 432 | — | (406 | ) | 26 | ||||||||||
September 2013 | 81 | — | (81 | ) | — | ||||||||||
December 2012 | 65 | — | (65 | ) | — | ||||||||||
April 2011 | — | — | — | — | |||||||||||
$ | 1,860 | $ | 10,357 | $ | (7,822 | ) | $ | 4,395 | |||||||
Warrants classified as equity
Equity-classified warrants consist of warrants issued in connection with consulting services provided to us. On May 8, 2013 as a part of a previous loan financing, we granted Oxford Financial LLC warrants to purchase 9,109 shares of common stock at an exercise price of $49.40 per share, which equaled the 20-day average market price of our common stock prior to the date of the grant. The warrants were valued using the Black Scholes model. The fair value assumptions for the grant included a volatility of 75.34%, expected term of seven years, risk free rate of 1.20%, and a dividend rate of 0.00%. The fair value of the warrants granted was $38.60 per share. These warrants are recorded in equity at fair value upon issuance, and not as liabilities, and are not subject to adjustment to fair value in subsequent reporting periods.
In 2016, the Company issued warrants to purchase 100,000 shares of common stock to the holder of the Debenture. The holder received 50,000 warrants upon the closing on the sale of the Debenture at an exercise price of $30.20, maturing 5 years from issuance, and in accordance with the terms of the amendment agreement, the exercise price of the warrant was reduced to $8.60 per share. The fair value assumptions for the grant included a volatility of 77.13%, expected term of 5.5 years, risk free rate of 1.26%, and a dividend rate of 0.00%. Additionally, the holder received 50,000 warrants upon the Company's public announcement of the interim analysis on June 29, 2016 at an exercise price of $8.60. The fair value assumptions for the grant included a volatility of 106.63%, expected term of 5.5 years, risk free rate of 1.35%, and a dividend rate of 0.00%.
In addition to the warrants issued to the holder of the Debenture there are 15,000 outstanding warrants issued to service providers with a weighted average exercise price of $79.40 as of September 30, 2017 and December 31, 2016. These warrants are recorded in equity at fair value upon issuance, and not as liabilities, and are not subject to adjustment to fair value in subsequent reporting periods.
25
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
9. Stock-Based Compensation
Options to Purchase Shares of Common Stock
The following table summarizes the components of stock-based compensation expense in the condensed consolidated statements of operations for the three and nine months ended September 30, 2017 and 2016, respectively (in thousands):
Three Months Ended September 30, | Nine Months Ended September 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | ||||||||||||
Research and development | $ | 34 | $ | 50 | $ | 106 | $ | 285 | |||||||
General and administrative | 118 | 483 | 470 | 1,545 | |||||||||||
Total stock-based compensation from continuing operations | $ | 152 | $ | 533 | $ | 576 | $ | 1,830 | |||||||
The Company uses the Black-Scholes option-pricing model and the following weighted-average assumptions to determine the fair value of all its stock options granted:
Three Months Ended September 30, | Nine Months Ended September 30, | ||||||||||
2017 | 2016 | 2017 | 2016 | ||||||||
Risk free interest rate | — | % | 1.23 | % | 1.87 | % | 1.25 | % | |||
Volatility | — | % | 102.77 | % | 116.41 | % | 98.91 | % | |||
Expected lives (years) | 0.00 | 5.47 | 5.92 | 5.58 | |||||||
Expected dividend yield | — | % | — | % | — | % | — | % | |||
There were no stock options granted during the three months ended September 30, 2017. The weighted-average grant date fair value of options granted during nine months ended September 30, 2017 was $1.15 per share. The weighted-average fair value of options granted during the three and nine months ended September 30, 2016 were $9.00 per share and $10.20 per share, respectively
The Company’s expected common stock price volatility assumption is based upon the Company's own implied volatility in combination with the implied volatility of a basket of comparable companies. The expected life assumptions for employee grants were based upon the simplified method provided for under ASC 718-10, which averages the contractual term of the Company’s options of ten years with the average vesting term of four years for an average of six years. The expected life assumptions for non-employees were based upon the contractual term of the option. The dividend yield assumption is zero, because the Company has never paid cash dividends and presently has no intention to do so. The risk-free interest rate used for each grant was also based upon prevailing short-term interest rates. The Company has estimated an annualized forfeiture rate of 15% for options granted to its employees, 8% for options granted to senior management and zero for non-employee directors. The Company will record additional expense if the actual forfeitures are lower than estimated and will record a recovery of prior expense if the actual forfeiture rates are higher than estimated.
As of September 30, 2017, there was $600,000 of unrecognized compensation cost related to outstanding stock options that is expected to be recognized as a component of the Company’s operating expenses over a weighted-average period of 2.19 years.
As of September 30, 2017, an aggregate of 1,325,000 shares of common stock were reserved for issuance under the Company’s 2016 Incentive Plan, including 443,000 shares subject to outstanding common stock options granted under the plan. There are 457,000 shares available for future grants based on adjustments in the 2016 Incentive Plan. The administrator of the plan determines the terms when a stock option may become exercisable. Vesting periods of stock options granted to date have not exceeded four years. The stock options will expire, unless previously exercised, no later than ten years from the grant date.
26
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
The following table summarizes stock option activity of the Company for the nine months ended September 30, 2017:
Total Number of Shares (In Thousands) | Weighted Average Exercise Price | Aggregate Intrinsic Value (In Thousands) | ||||||||
Outstanding at January 1, 2017 | 561 | $ | 41.50 | |||||||
Granted | 105 | 1.35 | ||||||||
Exercised | — | — | $ | — | ||||||
Canceled | (223 | ) | 43.01 | $ | — | |||||
Outstanding at September 30, 2017 | 443 | $ | 31.23 | $ | — | |||||
Options exercisable at September 30, 2017 | 285 | $ | 43.76 | $ | — | |||||
The aggregate intrinsic values of outstanding and exercisable stock options at September 30, 2017 were calculated based on the closing price of the Company’s common stock as reported on the NASDAQ Capital Market on September 30, 2017 of $0.35 per share. The aggregate intrinsic value equals the positive difference between the closing fair market value of the Company’s common stock and the exercise price of the underlying stock options.
10. Net Loss Per Share
The following table sets forth the potentially dilutive common shares excluded from the calculation of net loss per common share because their inclusion would be anti-dilutive (in thousands):
Three and Nine Months Ended September 30, | |||||
2017 | 2016 | ||||
Warrants to purchase common stock | 19,557 | 2,570 | |||
Options to purchase common stock | 443 | 518 | |||
Total | 20,000 | 3,088 | |||
27
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
11. Discontinued Operations
During the fourth quarter of 2015, the Company sold its rights to its commercial products Abstral® (fentanyl) Sublingual Tablets and Zuplenz® (ondansetron) Oral Soluble Film.
The following table presents amounts related to the discontinued operations in the balance sheets (in thousands):
September 30, 2017 | December 31, 2016 | ||||||
Carrying amounts of current assets of discontinued operations: | |||||||
Accounts receivable | $ | 830 | $ | 813 | |||
Total current assets of discontinued operations | 830 | 813 | |||||
Carrying amounts of current liabilities of discontinued operations: | |||||||
Accounts payable | $ | 57 | $ | 3,115 | |||
Accrued expenses and other current liabilities | 6,702 | 2,944 | |||||
Total current liabilities of discontinued operations | $ | 6,759 | $ | 6,059 | |||
The following table represents the components attributable to the commercial operations that are presented in the condensed consolidated statements of operations as discontinued operations (in thousands):
Three Months Ended September 30, | Nine months ended September 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | ||||||||||||
Additional channel obligations | (496 | ) | (520 | ) | $ | (923 | ) | $ | (2,186 | ) | |||||
Selling, general, and administrative | 614 | (2,067 | ) | (1,897 | ) | (6,681 | ) | ||||||||
Settlements associated with USAO NJ and DOJ and the qui tam action (Note 6) | — | — | (7,800 | ) | — | ||||||||||
Loss from discontinued operations | $ | 118 | $ | (2,587 | ) | $ | (10,620 | ) | $ | (8,867 | ) | ||||
Additional channel obligations included in discontinued operations is comprised of larger than anticipated returns of product expiring throughout 2016 and rebates of Abstral sales for which we are responsible through the end of the first quarter of 2016. The increase in returns and rebates was driven by larger than expected volumes through these returns and rebate channels and additional price protection provisions over which the Company has no control. During the third quarter of 2017, the Company incurred $0.5 million in additional channel obligations related to a settlement agreement and mutual release entered into with a former customer to resolve disputes over a product swap agreement of one of our former commercial products, Zuplenz.
28
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
Selling, general and administrative expense included in discontinued operations consists of all other expenses of our commercial operations that were required in order to market and sell our marketed products prior to our sales of the rights to these commercial products. These expenses include all personnel related costs, marketing, data, consulting, legal, and other outside services necessary to support the commercial operations. During the three and nine months ended September 30, 2017 and 2016, the majority of the costs incurred in selling, general, and administrative expense in discontinued operations related to legal fees from Company's external counsel and four former employees' counsel associated with the Company's cooperation with the USAO NJ and DOJ's investigation of the sales and marketing practices of Abstral. The settlement recorded in the second quarter of 2017 relates to the oral agreement with the attorneys for the relator in the qui tam action to settle the statutorily mandated attorney fees award. These legal proceedings are disclosed in Note 6. On September 7 2017, the Company entered into a written settlement agreement, with the primary D&O liability insurance carrier regarding reimbursement of these attorneys’ fees of the four former employees’ counsel associated with the USAO NJ and DOJ's investigation of the sales and marketing practices of Abstral. Under the settlement, the Company received a payment of $685,000 to reimburse the prior payments of the attorneys’ fees of the four former employees' counsel through May 1, 2017. In addition, such insurance carrier will advance the attorneys’ fees of four former employees to the extent they incur attorneys' fees as a result of the USAO NJ and DOJ investigation from May 1, 2017 forward.
12. Subsequent Events
The Company evaluated all events or transactions that occurred after September 30, 2017 up through the date these financial statements were issued. Other than as disclosed elsewhere in the notes to the condensed consolidated financial statements and below, the Company did not have any material subsequent events.
29
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
In this section, "Galena," “we,” “our,” the “Company” or “us” refer to Galena Biopharma, Inc. and its consolidated subsidiaries, Apthera, Inc., or “Apthera,” and Mills Pharmaceuticals, LLC, or "Mills."
This management’s discussion and analysis of financial condition as of September 30, 2017 and results of operations for the three and nine months ended September 30, 2017 and 2016, respectively, should be read in conjunction with management’s discussion and analysis of financial condition and results of operations included in our Annual Report on Form 10-K for the year ended December 31, 2016 which was filed with the SEC on March 15, 2017 and our other public reports filed with the SEC.
30
Overview
Galena is a biopharmaceutical company developing hematology and oncology therapeutics that address unmet medical needs. Galena’s pipeline consists of multiple mid- to late-stage clinical assets, including Galena’s hematology asset, GALE-401, and Galena’s novel cancer immunotherapy programs including NeuVax (nelipepimut-S), GALE-301 and GALE-302. NeuVax is currently in multiple investigator-sponsored Phase 2 clinical trials in breast cancer that will continue if the Merger is completed. GALE-301 and GALE-302 have completed early stage trials in ovarian, endometrial and breast cancers. GALE-401 is a controlled release version of the approved drug anagrelide for the treatment of elevated platelets in patients with myleoproliferative neoplasms, or MPNs, and Galena has completed a Phase 2 clinical trial in patients with essential thrombocythemia, or ET. If the Merger is completed, GALE-401 and GALE-301/GALE-302 will be evaluated for potential internal development or strategic partnership by management of the continuing company post-Merger.
On January 31, 2017, the Company announced that its Board of Directors had initiated a process to explore and review a range of strategic alternatives. As a result of this process, on August 7, 2017, the Company entered into the Merger Agreement further described in Note 1 to the condensed consolidated financial statements.
The chart below summarizes the current status of our clinical development pipeline but as we have noted above in Note 1 to the condensed consolidated financial statements if the Proposed Merger is completed, our three, NeuVax investigator-sponsored clinical trials will remain ongoing and our other development programs, GALE-401 and GALE-301/GALE-302 will be evaluated for potential internal development or strategic partnership by the continuing company:
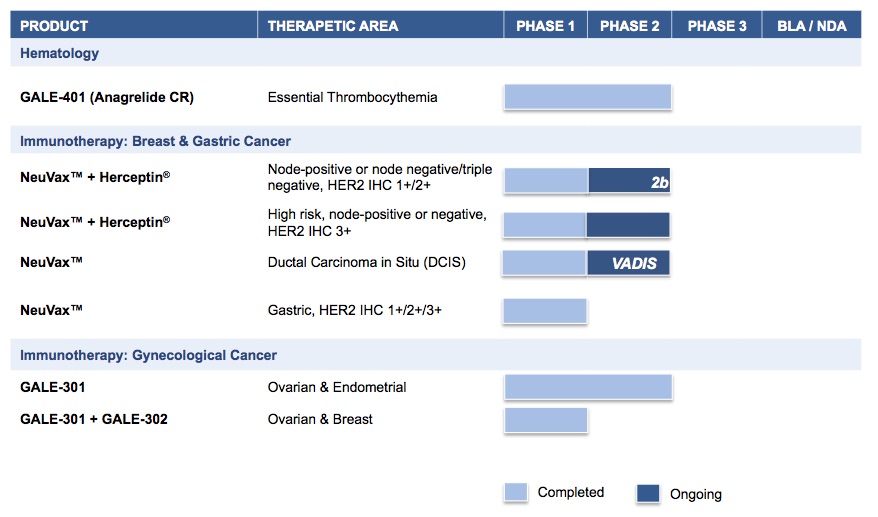
31
Hematology
GALE-401 (anagrelide controlled release (CR))
GALE-401 contains the active ingredient anagrelide, an FDA-approved product, for the treatment of patients with MPNs to lower abnormally elevated platelet levels. The currently available immediate release, or IR, version of anagrelide causes adverse reactions that are believed to be dose and plasma concentration dependent. According to the Highlights section of the FDA-approved prescribing information for AGRYLIN® (anagrelide hydrochloride) capsules, for oral use (as revised in July 2015), the most common adverse reactions (incidence ≥ 5%) are headache, palpitations, diarrhea, asthenia, edema, nausea, abdominal pain, dizziness, pain, dyspnea, cough, flatulence, vomiting, fever, peripheral edema, rash, chest pain, anorexia, tachycardia, malaise, paresthesia, back pain, pruritus and dyspepsia and may limit the use of the IR version of the drug. Therefore, reducing the maximum concentration or Cmax and increasing the half-life of the drug is hypothesized to reduce the adverse reactions, while preserving the efficacy, potentially allowing a broader use of the drug.
Multiple Phase 1 studies in 98 healthy subjects have shown GALE-401 reduces the Cmax of anagrelide and increases the half-life following oral administration, appears to be well tolerated at the doses administered, and may be capable of reducing platelet levels. The Phase 1 program provided the desired PK/PD (pharmacokinetic/pharmacodynamic) profile to enable the initiation of the Phase 2 proof-of-concept trial. The Phase 2, open label, single arm, proof-of concept trial enrolled 18 patients in the United States for the treatment of thrombocytosis, or elevated platelet counts, in patients with MPNs. Final safety and efficacy data from this Phase 2 trial were presented in December 2015. There is currently no timeline for beginning a Phase 3 trial. As discussed above, if the Merger is completed, GALE-401 will be evaluated for potential internal development or strategic partnership by management of the continuing company post-Merger.
ET is a myeloproliferative blood disorder, and is characterized by the overproduction of platelets in the bone marrow. Elevated platelets alter the normal process of blood coagulation and can lead to thromboembolic events. About a third of patients are asymptomatic at the time of diagnosis. However, many patients develop symptoms during the course of the disease that affect their quality of life.
Galena has analyzed the data from the trials and the treatment landscape for MPNs, with a current focus on ET. Subject to completion of the manufacturing of the new formulation and other internal work to prepare the Phase 3 for initiation, GALE-401 could be advanced into a Phase 3 clinical trial in ET patients who are intolerant or resistant to hydroxyurea. The Phase 3 trial is designed to compare GALE-401 (drug arm) versus best available therapy to include a sizable population of patients treated with anagrelide IR. A productive meeting with the FDA in December 2016 confirmed that the GALE-401 development program is appropriate for a new drug application, or NDA, filing using the 505(b)(2) regulatory pathway.
32
On April 27, 2017, Galena received a notice from BioVascular, Inc., which is referred to herein as BVI, the licensor of GALE-401, of an alleged material breach of Galena’s Exclusive License Agreement with BVI, dated December 20, 2013, which is referred to herein as the BVI License Agreement. On May 31, 2017, Galena received a notice of termination of the BVI License Agreement from BVI pursuant to which BVI purported to terminate the BVI License Agreement. BVI maintained that Galena had not used “Commercially Reasonable Efforts” to start the Phase 3 trial of GALE-401. On September 5, 2017, Mills Pharmaceuticals, LLC, or Mills, a wholly owned subsidiary of Galena, and BVI entered into an amendment, or the BVI License Amendment, of the BVI License Agreement, pursuant to which parties agreed to resolve their outstanding disputes over the BVI License Agreement and to modify certain terms of the BVI License Agreement, including but not limited to, (i) eliminating the 3% royalty rate on annual net sales of under $50 million and making the 4% royalty rate applicable to annual net sales of up to $100 million, (ii) making an advance payment of $350,000 for the milestone related to the initiation of the Phase 3 clinical trial payable in two tranches with the first payment of $200,000 payable on or before October 31, 2017 and the second payment of $150,000 payable thirty days after the effective time of the Merger but no later than December 31, 2017, (iii) adding a payment for a sublicense by Mills to a third party of 25% of any cash received for upfront fees or milestone payments if the sublicense is executed prior to first patient enrolled in the Phase 3 clinical trial and 17.5% of any cash received for upfront fees or milestone payments if the sublicense is executed after the first patient is enrolled in the Phase 3 clinical trial, and (iv) if the first patient is not enrolled in the Phase 3 clinical trial by December 31, 2018, BVI shall have the right to terminate the License Agreement and the advance payment shall not be repaid to Mills. Under the terms of a consent among Comerica Bank, BVI and Mills dated September 5, 2017, Comerica Bank shall receive $100,000 of the $350,000 advance payment from Mills. BVI also withdrew its notice of termination as of September 5, 2017.
Novel Cancer Immunotherapies
Our targeted cancer immunotherapy approach is currently based upon two key areas: preventing secondary recurrence of cancer, which is becoming increasingly important as the number of cancer survivors continues to grow; and, primary prevention intended to prevent ductal carcinoma in situ (DCIS) from becoming invasive breast cancer. Once a patient’s tumor becomes metastatic, the outcome is often fatal, making the prevention of recurrence a potentially critical component of overall patient care. Our secondary recurrence programs primarily target patients in the adjuvant (after-surgery) setting who have relatively healthy immune systems, but may still have residual disease. Minimal residual disease, or micrometastasis, that are undetectable by current radiographic scanning technologies, can result in disease recurrence.
Our therapies utilize an immunodominant peptide combined with the immune adjuvant, recombinant human granulocyte macrophage-colony stimulating factor (rhGM-CSF or GM-CSF), and work by harnessing the patient’s own immune system to seek out and attack any residual cancer cells. Using peptide immunogens has many potential clinical advantages, including a favorable safety profile, since these drugs may lack the toxicities typical of most cancer therapies. They also have the potential to induce immunologic memory and provide long-lasting protection with a convenient, intradermal mode of delivery.
NeuVax™ (nelipepimut-S)
NeuVax™ (nelipepimut-S) is a cancer immunotherapy targeting human epidermal growth factor receptor (HER2) expressing cancers. NeuVax is the immunodominant nonapeptide derived from the extracellular domain of the HER2 protein, a well-established and validated target for therapeutic intervention in breast and gastric carcinomas. The NeuVax vaccine is combined with GM-CSF for injection under the skin, or intradermal administration. Data has shown that an increased presence of circulating tumor cells (CTCs) may predict reduced Disease Free Survival (DFS) and Overall Survival (OS) suggesting a presence of isolated micrometastases, not detectable clinically, but, over time, can lead to recurrence of cancer, most often in distant sites. After binding to the specific HLA molecules on antigen presenting cells, the nelipepimut-S sequence stimulates specific cytotoxic T lymphocytes, or CTLs, causing significant clonal expansion. These activated CTLs recognize, neutralize and destroy, through cell lysis, HER2 expressing cancer cells, including occult cancer cells and micrometastatic foci. The nelipepimut immune response can also generate CTLs to other immunogenic peptides through inter- and intra-antigenic epitope spreading.
33
Breast Cancer: According to the National Cancer Institute (NCI), over 230,000 women in the U.S. are diagnosed with breast cancer annually. While improved diagnostics and targeted therapies have decreased breast cancer mortality in the U.S., metastatic breast cancer remains incurable. Approximately 75% to 80% of breast cancer patients have tissue test positive for some increased amount of the HER2 receptor, which is associated with disease progression and decreased survival. Only approximately 20% to 30% of all breast cancer patients-those with HER2 immunohistochemistry (IHC) 3+ disease, or IHC 2+ and fluorescence in situ hybridization (FISH) amplified-have a HER2 directed, approved treatment option available after their initial standard of care. This leaves the majority of breast cancer patients with low-to-intermediate HER2 expression (IHC 1+, 2+) ineligible for therapy and without an effective targeted treatment option to prevent cancer recurrence.
We currently have two investigator-sponsored trials (IST) ongoing with NeuVax in combination with trastuzumab (Herceptin®; Genentech/Roche). The combination of trastuzumab and NeuVax has been shown pre-clinically and in a pilot study to be synergistic. Our Phase 2b clinical trial is a randomized, multicenter, investigator-sponsored study with 300 enrolled patients HER2 1+ and 2+, HLA A2+, A3+, A24 and/or A26, node positive, and high-risk node negative patients. Eligible patients are randomized to receive NeuVax + GM-CSF + trastuzumab or trastuzumab + GM-CSF alone. Genentech/Roche is providing the trastuzumab and partial funding for this trial. Data presented in October 2016 demonstrated that this novel combination of trastuzumab and NeuVax with HER2 low-expressing patients is well tolerated and the cardiac effects of trastuzumab are not impacted by the addition of NeuVax. In February 2017, the Data Safety Monitoring Board (DSMB) reported that there were no safety concerns with the trial and the trial is not futile. The recommendation from the DSMB was to continue the trial with one revision to the statistical analysis plan regarding the timing of the pre-specified interim analysis. Given the lengthy duration of enrollment for the trial, the DSMB determined that the pre-specified interim efficacy analysis be moved up from 12 months to 6 months after the last patient is enrolled. Enrollment was completed in the third quarter of 2017; therefore, the DSMB expects to perform the interim efficacy analysis in the first quarter of 2018. The primary endpoint of the study is disease-free survival after 24-months and is expected in the fourth quarter of 2019.
Our second combination IST is a Phase 2 in HER2 3+ breast cancer patients who have completed neoadjuvant therapy with an approved regimen that includes trastuzumab and fail to achieve a pathological complete response, meaning they have microscopic evidence of residual disease and are therefore at an increased risk of disease recurrence. This multi-center, prospective, randomized, single-blinded Phase 2 trial has enrolled approximately 100 patients with a diagnosis of HER2 3+ breast cancer who are HLA A2+ or HLA A3+ and are determined to be at high-risk for recurrence. High-risk is defined as having received neoadjuvant therapy with an approved regimen that includes trastuzumab but not obtaining a pathological complete response at surgery, or those who undergo surgery as a first intervention and are found to be pathologically node-positive. These high-risk patients are known to have higher recurrence rates than other HER2 3+ breast cancer patients. Eligible patients are randomized to receive NeuVax + GM-CSF + trastuzumab or trastuzumab + GM-CSF alone. Funding for this trial was awarded through the Congressionally Directed Medical Research Program, funded through the Department of Defense, via a Breast Cancer Research Program Breakthrough Award. In February 2017, the DSMB reported that there were no safety concerns with the trial and the trial is not futile. The pre-specified interim safety analysis was completed on n=50 patients and a poster presentation in April 2017 demonstrated that the agent is well tolerated with no increased cardiotoxicity associated with giving NeuVax in combination with trastuzumab. The recommendation from the DSMB was to continue the HER2 3+ trial unmodified. Enrollment was completed in the third quarter of 2017. The primary endpoint of the study is disease-free survival after 24-months and is expected in the fourth quarter of 2019.
A Phase 2 IST with NeuVax as a single agent in patients with ductal carcinoma in situ, or DCIS, is open for enrollment. The trial is entitled, VADIS: Phase 2 trial of the nelipepimut-S peptide vaccine in women with DCIS of the breast. The trial is being run in collaboration with the National Cancer Institute, or NCI, and the University of Texas MD Anderson Cancer Center Phase I and II Chemoprevention Consortium, potentially positioning NeuVax as a treatment for earlier stage disease. The trial has an immunological endpoint evaluating NeuVax peptide-specific cytotoxic T lymphocyte (CTL; CD8+ T-cell) response in vaccinated patients. DCIS is defined by the NCI as a noninvasive condition in which abnormal cells are found in the lining of a breast duct and have not spread outside the duct to other tissues in the breast. DCIS is the most common type of breast cancer. In some cases, DCIS may become invasive cancer and spread to other tissues, and at this time, there is no way to know which lesions could become invasive. Current treatment options for DCIS include breast-conserving surgery and radiation therapy with
34
or without tamoxifen, breast-conserving surgery without radiation therapy, or total mastectomy with or without tamoxifen. According to the American Cancer Society, in 2015 there were over 60,000 diagnoses of DCIS.
A Phase 3 PRESENT (Prevention of Recurrence in Early-Stage, Node- Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment) study enrolled 758 HER2 1+/2+ patients who were node-positive and HLA A2 or A3 positive. On June 27, 2016, the Independent Data Monitoring Committee, or IDMC, recommended that the Phase 3 PRESENT clinical trial be stopped for futility. The PRESENT trial was stopped, and Galena initiated an investigation into the causes of the recommendation. Galena’s analysis of the data showed that there was a separation of the curves, albeit not statistically significant, with the control arm performing better than expected and the NeuVax arm performing consistent with Galena’s protocol assumptions for the control group. Because the study was deemed futile, Galena closed the PRESENT trial, and expects to present the data at a future medical conference.
Gastric Cancer: According to the NCI, gastric (stomach) cancer is a disease in which malignant (cancer) cells form in the lining of the stomach. Gastric cancer is often diagnosed at an advanced stage because there are no early signs or symptoms, and is the second-most common cancer among males and third-most common among females in Asia and worldwide with over 63,000 new cases a year in India. Overexpression of the HER2 receptor occurs in approximately 20% of gastric and gastro-esophageal junction adenocarcinomas, predominantly those of the intestinal type. Overall, without regard to the stage of cancer, only approximately 28% of patients with stomach cancer live at least five years following diagnosis and new adjuvant treatments are needed to prevent disease recurrence. Galena currently has an agreement with Dr. Reddy’s Laboratories to conduct a Phase 2 investigational study in gastric cancer in India, but Dr. Reddy’s has previously indicated that it would require additional data before proceeding with the Phase 2 trial.
GALE-301 and GALE-302
Our second immunotherapy franchise targets folate binding protein (FBP) receptor-alpha. FBP is a well-validated therapeutic target that is highly over-expressed in ovarian, endometrial and breast cancers, and is the source of immunogenic peptides that can stimulate cytotoxic T lymphocytes (CTLs) to recognize and destroy FBP-expressing cancer cells. Current treatments after surgery for these diseases are principally with platinum based chemotherapeutic agents. These patients suffer a high recurrence rate and most relapse with an extremely poor prognosis. GALE-301 and GALE-302 are immunogenic peptides that consist of a peptide derived from FBP combined with GM-CSF for the prevention of cancer recurrence in the adjuvant setting. GALE-301 is the E39 peptide, while GALE-302 is an attenuated version of this peptide, known as E39’. Two early stage clinical trials have been completed with our FBP peptides in ovarian, endometrial, and breast cancers. In June 2016, the U.S. Food and Drug Administration (FDA) granted two orphan-drug designations for the treatment (including prevention of recurrence) of ovarian cancer: one for GALE-301 (E39), and one for GALE-301 (E39) and GALE-302 (E39’).
Ovarian Cancer: According to the NCI Surveillance, Epidemiology, and End Results (SEER) Program, new cases of ovarian cancer occur at an annual rate of 11.9 per 100,000 women in the U.S., with an estimated 22,280 new cases and 14,240 deaths in 2016. Only 46.2% of ovarian cancer patients are expected to survive five years after diagnosis. Approximately 1.3% of women will be diagnosed with ovarian cancer at some point during their lifetime (2011 - 2013 data). The prevalence data from 2013 showed an estimated 195,767 women living with ovarian cancer in the United States. Due to the lack of specific symptoms, the majority of ovarian cancer patients are diagnosed at later stages of the disease, with an estimated 80% of women presenting with advanced-stage (III or IV) disease. These patients have their tumors routinely surgically debulked to minimal residual disease, and then are treated with platinum- and/or taxane-based chemotherapy. While many patients respond to this treatment regimen and become clinically free of disease, the majority of these patients will relapse. Depending upon their level of residual disease, the risk for recurrence after completion of primary therapy is approximately 70%. Unfortunately, for these women, once the disease recurs, treatment options are limited and the disease is most likely incurable.
35
Intellectual Property
Patents and other intellectual property rights are crucial to our success. It is our policy to protect our intellectual property rights through available means, including filing and prosecuting patent applications in the U.S. and other countries, protecting trade secrets, and utilizing regulatory protections such as data exclusivity. We also include restrictions regarding use and disclosure of our proprietary information in our contracts with third parties, and utilize customary confidentiality agreements with our employees, consultants, clinical investigators and scientific advisors to protect our confidential information and know-how. Together with our licensors, we also rely on trade secrets to protect our combined technology especially where we do not believe patent protection is appropriate or obtainable. It is our policy to operate without infringing on, or misappropriating, the proprietary rights of others. The following chart summarizes our intellectual property rights:
Drug Candidate | Jurisdiction | Indication | Claims | Status | Latest Estimated Patent Exclusivity Period |
GALE-401 | US | Vaso-occlusive | Methods of Use | 1 issued | 2020 |
GALE-401 | US, Europe, India, Japan, and UK | Platelet lowering | Anagrelide Controlled Release Formulations & Methods of Use | 4 pending and 7 issued | 2029 |
NeuVax™ (nelipepimut-S) | US, Australia, Canada, China, Europe, Hong Kong, Japan, Korea, and Mexico | Recurrence of cancers expressing low to intermediate levels of HER2/neu | Methods of Use | 6 pending and 10 issued | 2028 |
NeuVax in combination with trastuzumab | US and Australia | HER2/neu expressing cancer | Methods of Use | 2 issued | 2026 |
GALE-301/GALE-302 Combination | US, Canada, Europe and Japan | Cancers expressing Folate Binding Protein (FBP) | Compositions & Methods of Use | 1 pending and 8 issued | 2022 |
GALE-301/GALE-302 Combination | US and PCT | Cancers expressing Folate Binding Protein (FBP) | Combination Dosage Regimen | 2 pending | 2036 |
GALE-301 | US and PCT | Cancers expressing low levels of FBP (IHC 0 or 1+) | Dosage Regimen | 2 pending | 2037 |
Each of the above-referenced pending or issued patents has been licensed by Galena. To Galena’s knowledge, there are no contested proceedings or third-party claims relating to any of the above pending or issued patents.
36
Results of Operations for the Three and Nine Months Ended September 30, 2017 and 2016
(dollars in thousands) | Three Months Ended September 30, | |||||||||
2017 | 2016 | % Change | ||||||||
Operating loss | $ | (4,462 | ) | $ | (6,472 | ) | (31 | )% | ||
Non-operating income (expense) | (1,731 | ) | 2,130 | (181 | )% | |||||
Increase (loss) from discontinued operations | 118 | (2,587 | ) | (105 | )% | |||||
Net loss | $ | (6,075 | ) | $ | (6,929 | ) | (12 | )% | ||
Net loss per common share, basic and diluted: | ||||||||||
Basic and diluted net loss per share, continuing operations | $ | (0.15 | ) | $ | (0.41 | ) | (63 | )% | ||
Basic and diluted net income (loss) per share, discontinued operations | $ | — | $ | (0.25 | ) | (100 | )% | |||
Basic and diluted net loss per share | $ | (0.15 | ) | $ | (0.66 | ) | (77 | )% | ||
For the three months ended September 30, 2017, our net loss was $6.1 million compared with net loss of $6.9 million for the three months ended September 30, 2016. The decrease of $0.9 million in net loss was primarily attributable to a decrease in operating loss of $2.0 million, or 31%, and a decrease in loss from discontinued operations of $2.7 million, partially offset by an increase in non-operating expense of $3.9 million.
(dollars in thousands) | Nine Months Ended September 30, | |||||||||
2017 | 2016 | % Change | ||||||||
Operating loss | $ | (14,461 | ) | $ | (24,732 | ) | (42 | )% | ||
Non-operating income (expense) | (1,116 | ) | 15,566 | (107 | )% | |||||
Loss from discontinued operations | (10,620 | ) | (8,867 | ) | 20 | % | ||||
Net income (loss) | $ | (26,197 | ) | $ | (18,033 | ) | 45 | % | ||
Net loss per common share: | ||||||||||
Basic and diluted net loss per share, continuing operations | $ | (0.45 | ) | $ | (0.97 | ) | (54 | )% | ||
Basic and diluted net loss per share, discontinued operations | $ | (0.31 | ) | $ | (0.93 | ) | (67 | )% | ||
Basic and diluted net loss per share | $ | (0.76 | ) | $ | (1.90 | ) | (60 | )% | ||
For the nine months ended September 30, 2017, our net loss was $26.2 million compared with a net loss of $18.0 million for the nine months ended September 30, 2016. Net loss during the nine months ended September 30, 2016 was affected by two significant non-cash adjustments included in non-operating income, including a $14.2 million gain on our warrant liability due a significant decrease in our stock price and a $5.2 million gain on our contingent purchase price liability based on its revaluation following the discontinuation of our PRESENT clinical trial during the second quarter of 2016. Non-operating income totaled $15.6 million for the nine months ended September 30, 2016 compared to non-operating expense of $1.1 million for the nine months ended September 30, 2017. Operating loss decreased $10.3 million, or 42%, from the nine months ended September 30, 2016 compared to the nine months ended September 30, 2017 primarily due reducing expenses following to the discontinuation of our PRESENT clinical trial in the second quarter of 2016. The increase in loss from discontinued operations was due to the written agreement in principle with U.S. Attorney's Office of the District of New Jersey, or USAO NJ, and the Department of Justice, or DOJ, that includes a civil payment of approximately $7.6 million accrued during the first quarter of 2017, partially offset by decreased channel obligations and legal defense costs related to our former commercial products. The written agreement in principle is further discussed in Note 6 to the condensed consolidated financial statements.
Further analysis of the changes and trends in our operating results are discussed below.
37
Research and Development Expense
Research and development expenses are comprised of both (i) directs costs, which are mainly contract research organizations costs, materials and supplies, licenses and fees, consultancy fees, and milestone payments and (ii) internal or indirect costs which are mainly personnel costs, including salaries, benefits and stock-based compensation, and overhead allocations consisting of various support and facilities-related costs.
We do not track total research and development expenses by product candidate, therapeutic area or development phase, as we do not allocate internal or indirect costs by product candidate, therapeutic area or development phase. However, we manage our research and development expenses by identifying the research and development activities we anticipate will be performed during a given period and then prioritizing efforts based on scientific data, probability of successful development, market potential, available human and capital resources and other considerations. We continually review our R&D pipeline and the status of development and, as necessary, reallocate resources among the R&D portfolio that we believe will best support the future growth of our business.
Research and development expense for the three and nine months ended September 30, 2017 and 2016, respectively, was as follows (dollar amounts in thousands):
Three Months Ended September 30, | Nine Months Ended September 30, | ||||||||||||||||||||
2017 | 2016 | % Change | 2017 | 2016 | % Change | ||||||||||||||||
Research and development expense | $ | 951 | $ | 3,624 | (74 | )% | $ | 5,357 | $ | 15,242 | (65 | )% | |||||||||
The majority of our research and development expenses for the three and nine months ended September 30, 2017 relate to our three ongoing investigator sponsored studies with NeuVax and internal preparation work on our GALE-401 asset to enter a Phase 3 study. The significant decrease in research and development costs from the three and nine months ended September 30, 2017 compared to the three and nine months ended September 30, 2016 was due to the reduction in expenses following the discontinuation of our Phase 3 PRESENT trial upon the recommendation of the IDMC in the third quarter of 2016. In addition, research and development headcount has decreased from four at September 30, 2016 to two at September 30, 2017. We have reduced usage of outside service providers during 2017 in order to preserve capital and remain focused on our ongoing development programs.
Direct and unallocated indirect costs associated with research and development expense for the three and nine months ended September 30, 2017 and 2016, respectively, were as follows (dollars in thousands):
Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||||||||||
Therapeutic Area | 2017 | 2016 | % Change | 2017 | 2016 | % Change | ||||||||||||||||
Direct costs | ||||||||||||||||||||||
GALE-401 | Essential Thrombocythemia | $ | 56 | $ | 188 | (70 | )% | $ | 599 | $ | 370 | 62 | % | |||||||||
NeuVaxTM | Node-positive or node negative/triple negative HER2 IHC 1+/2+ | (238 | ) | 1,651 | (114 | )% | 339 | 8,718 | (96 | )% | ||||||||||||
NeuVaxTM + Herceptin® | Node-positive or node negative/triple negative HER2 IHC 1+/2+ | 281 | 652 | (57 | )% | 1,220 | 1,756 | (31 | )% | |||||||||||||
NeuVaxTM + Herceptin® | High risk, node-positive or negative, HER2 IHC 3+ | 80 | 119 | (33 | )% | 476 | 337 | 41 | % | |||||||||||||
GALE-301 + GALE-302 | Ovarian & breast | 172 | — | NA | 302 | 243 | 24 | % | ||||||||||||||
Indirect costs | 600 | 1,014 | (41 | )% | 2,421 | 3,818 | (37 | )% | ||||||||||||||
Total | $ | 951 | $ | 3,624 | (74 | )% | $ | 5,357 | $ | 15,242 | (65 | )% | ||||||||||
38
General and Administrative Expense
General and administrative expense includes compensation-related costs for our employees dedicated to general and administrative activities, legal fees, audit and tax fees, consultants and professional services, and general corporate expenses. General and administrative expense for the three and nine months ended September 30, 2017 and 2016, respectively, was as follows (dollars in thousands):
Three Months Ended September 30, | Nine Months Ended September 30, | ||||||||||||||||||||
2017 | 2016 | % Change | 2017 | 2016 | % Change | ||||||||||||||||
General and administrative expense | $ | 3,511 | $ | 2,848 | 23 | % | $ | 9,104 | $ | 9,490 | (4 | )% | |||||||||
The 23% increase in selling, general, and administrative expense for the three months ended September 30, 2017 compared to the three months ended September 30, 2016 was driven by a $1.3 million increase in legal expenses incurred primarily in support for the Merger Agreement and related filings, partially offset by decreases in non-cash stock based compensation of $0.4 million, $0.2 million in personnel related expenses. The 4% decrease in selling, general, and administrative expense for the nine months ended September 30, 2017 compared to the nine months ended September 30, 2016 was driven by decreases in non-cash stock based compensation of $1.1 million, director's and officer's insurance of $0.3 million, and outside services of $0.3 million, partially offset by an increase in legal expenses of $1.4 million primarily in support of the Merger Agreement.
39
Non-Operating Income (Expense), Net
Non-operating income (expense), net for the three and nine months ended September 30, 2017 and 2016, respectively, was as follows (dollars in thousands):
Three Months Ended September 30, | Nine Months Ended September 30, | ||||||||||||||||||||
2017 | 2016 | % Change | 2017 | 2016 | % Change | ||||||||||||||||
Litigation settlement | $ | — | $ | — | — | % | $ | (1,300 | ) | $ | (1,800 | ) | (28 | )% | |||||||
Change in fair value of warrants potentially settleable in cash | 4,115 | 3,652 | 13 | % | 7,822 | 14,172 | (45 | )% | |||||||||||||
Goodwill and intangible assets impairment loss | (5,231 | ) | — | NA | (5,231 | ) | — | NA | |||||||||||||
Interest expense, net | (565 | ) | (1,377 | ) | (59 | )% | (2,225 | ) | (1,988 | ) | 12 | % | |||||||||
Change in fair value of the contingent purchase price liability | (50 | ) | (145 | ) | (66 | )% | (182 | ) | 5,182 | (104 | )% | ||||||||||
Total non-operating income (expense), net | $ | (1,731 | ) | $ | 2,130 | (181 | )% | $ | (1,116 | ) | $ | 15,566 | (107 | )% | |||||||
The increases in our net non-operating expense during the three and nine months ended September 30, 2017 compared to the three and nine months ended September 30, 2016 was primarily due to significant decreases in the fair value of warrants accounted for as liabilities and the fair value of the contingent purchase price liability in 2016. The decreases in the estimated fair value of our warrant liabilities were primarily due to the decrease in our common stock price, which is one of the most impactful inputs to the pricing model we use to estimate the fair value of our warrant liabilities. In addition to the significant decrease in the fair value of warrants during the nine months ended September 30, 2016, our contingent purchase price consideration related to the approval of NeuVax also decreased. The interim analysis of the PRESENT Phase 3 clinical trial and subsequent close down of the trial triggered an intangible asset and goodwill impairment analysis of the carrying amount and the fair value was determined to exceed the carrying amount as of June 30, 2016 based on the other ongoing and planned trials with NeuVax. The Company determined the fair value of the contingent purchase price consideration at each reporting period and the lower probability and extended time line for marketing approval were updated to align with the valuation performed of NeuVax resulting in the significant decrease in the fair value which are the two largest variables impacting the liability during the three and nine months ended September 30, 2016. In addition, during the three and nine months ended September 30, 2017, the Company performed an interim impairment analysis on goodwill and intangible assets resulting in an impairment loss of $5.2 million as it was determined that the carrying values of the intangible assets exceeded their fair and the carrying value of the Company's sole reporting unit exceeded the carrying value. See Note 2 to the condensed consolidated financial statements for additional details on the goodwill and intangible assets impairment loss.
The change in fair value of warrants, goodwill and intangible assets impairment loss, and the change in contingent purchase price consideration are all non-cash in nature.
Income Taxes
For the three and nine months ended September 30, 2017 and 2016, there was no income tax benefit or expense recognized.
40
Discontinued Operations
We sold the assets of our commercial business during the fourth quarter of 2015. We believe this disposition allows us to focus our resources on our clinical development programs and maximize the value of these assets to our shareholders.
The following table represents the components attributable to the commercial operations that are presented in the condensed consolidated statements of operations as discontinued operations (in thousands):
Three Months Ended September 30, | Nine Months Ended September 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | ||||||||||||
Additional channel obligations | (496 | ) | (520 | ) | (923 | ) | (2,186 | ) | |||||||
Selling, general, and administrative | 614 | (2,067 | ) | (1,897 | ) | (6,681 | ) | ||||||||
Settlement associated with USAO NJ and DOJ and qui tam action (Note 6) | — | — | (7,800 | ) | — | ||||||||||
Income (loss) from discontinued operations | $ | 118 | $ | (2,587 | ) | $ | (10,620 | ) | $ | (8,867 | ) | ||||
Discontinued operations are comprised of net revenue, cost of revenue, and expenses attributable to our commercial operations, which were sold in the fourth quarter of 2015.
•Additional Channel Obligations included in discontinued operations in the first quarter of 2016 is comprised of larger than anticipated rebates of Abstral sales that we were responsible for through the end of the first quarter of 2016. The increase in rebates was driven by larger than expected volumes through these rebate channels and additional price protection provisions. The increase in rebates was partially offset by lower than expect patient assistance program reimbursement. The additional channel obligations for the third quarter and nine months of 2017 relate to adjusted Medicaid billings from previous quarters since the first quarter of 2014 and trailing returns from our former commercial products expiring in the fourth quarter of 2016. During the third quarter of 2017, the Company incurred $0.5 million in additional channel obligations related to a settlement agreement and mutual release entered into with a former customer to resolve disputes over a product swap agreement of one of our former commercial products, Zuplenz.
•Selling, general and administrative expense included in discontinued operations consists of all other expenses of our commercial operations that are required in order to market and sell our marketed products. These expenses include all personnel related costs, marketing, data, consulting, legal, consulting, and other outside services necessary to support the commercial operations. During the nine months ended September 30, 2017, we incurred $1.8 million related to legal expenses from the Company's external counsel and the four former employees' counsel associated with the Company's cooperation with the USAO NJ and DOJ's investigation of the sales and marketing practices of Abstral. On September 7 2017, the Company entered into a written settlement agreement, with the primary D&O liability insurance carrier regarding reimbursement of the four former employees' attorneys’ fees. Under the settlement, the Company received a payment of $685,000 to reimburse the prior payments of the four former employees' attorneys’ fees as well as such insurance carrier will advance the attorneys’ fees of four former employees to the extent they incur attorneys' fees as a result of the USAO NJ and DOJ investigation from May 1, 2017 forward. These legal proceedings are further disclosed in Part II, Item 1. These legal proceedings are disclosed in Note 6 to the condensed consolidated financial statements and Part II, Item 1.
41
Liquidity and Capital Resources
We had cash and cash equivalents of approximately $12.9 million as of September 30, 2017, compared with $18.1 million as of December 31, 2016.
During the nine months ended September 30, 2017 we raised funds of $15.5 million in net proceeds from issuance of common stock and warrants to purchase common stock and $5.7 million in redemptions of our debenture paid by the Company in shares of our common stock. Principal redemptions of our debenture paid in shares of our common stock result in the release of restricted cash to unrestricted cash. The increase was offset by $26.2 million used in operating activities.
We expect to continue to incur operating losses as we continue to advance our product candidates through the drug development and the regulatory process. In the absence of revenue, our potential sources of operational funding are proceeds from the sale of equity, funded research and development payments, debt financing arrangements, and payments received under partnership and collaborative agreements. The Company cannot provide assurances that its plans for sources and uses of cash will not change or that changed circumstances will not result in the depletion of its capital resources more rapidly than it currently anticipates. While the Company is advancing its product candidates, the Company continues to explore ways to raise additional capital, whether through a sale of equity or debt securities, a strategic business transaction, the establishment of other funding facilities, licensing arrangements, asset sales or other means. Until we complete the Proposed Merger, we may need to raise additional capital to fund our operations.
In addition to the funds raised through underwritten public offerings and the Debenture, we maintain a purchase agreement with Lincoln Park Capital LLC (LPC) and At Market Issuance Sales Agreements (collectively, the ATM) with future availability of $2.0 million and $19.1 million, respectively subject to certain terms and conditions. We cannot currently use the ATM and the purchase agreement with LPC because the Company is not currently eligible to use a Form S-3 registration statement, and it does not expect to be eligible to use a Form S-3 registration statement until May 1, 2018 at the earliest. In addition, we may be able to raise additional funds through amendments to the outstanding warrants and one or more private or public offerings. Once we become eligible to use a Form S-3 registration statement we expect to be able to use the ATM and our purchase agreement to fund our operations going forward, subject to terms of those instruments. However, even if we become eligible to use a form S-3 registration statement as of May 1, 2018, unless the market value of our common stock held by non-affiliates of the Company increases to at least $75 million, we will be limited in the amounts we may sell under Form S-3.
On January 31, 2017, the Company announced that its Board of Directors had initiated a process to explore a range of strategic alternatives focused on maximizing stockholder value. As a result of this process, on August 7, 2017, the Company entered into the Merger Agreement, pursuant to which SELLAS will become a wholly owned subsidiary of the Company, subject to the terms and conditions of the Merger Agreement. In connection with the terms of the Merger Agreement, upon consummation of the Proposed Merger, Galena securityholders immediately prior to the effective time of the Merger are expected to own approximately 32.5% of the aggregate number of shares of Galena Common Stock, and SELLAS securityholders are expected to own approximately 67.5% of the aggregate number of shares of Galena Common Stock, in each case calculated on a fully diluted basis for the continuing company, except for the exclusion of the impact of a potential third party financing consented to by SELLAS and 2,556,851 shares of Galena Common Stock issuable pursuant to out-of-the money Galena warrants.
42
The Company cannot provide assurances that its plans for sources and uses of cash will not change or that changed circumstances will not result in the depletion of its capital resources more rapidly than it currently anticipates. Even if we complete the Proposed Merger, we expect that we will need to raise additional capital to fund our operations, whether through a sale of equity or debt securities, a strategic business transaction, the establishment of other funding facilities, licensing arrangements, asset sales or other means, in order to continue the development of the Company's product candidates and to support its other ongoing activities. However, the Company cannot be certain that it will be able to raise additional capital on favorable terms, or at all, which raises substantial doubt about the Company’s ability to continue as a going concern. The Company is currently evaluating its capital requirements in light of both pursuing the Proposed Merger and funding the development of its clinical programs. In addition, the Company is working with SELLAS to develop a comprehensive capital program to fund all product development programs currently prioritized by SELLAS and the Company subsequent to the Proposed Merger. For example, the Merger Agreement states that subsequent to the Merger the Company will use commercially reasonable best efforts to fund the NeuVax ongoing programs in the amount of up to $3 million through December 31, 2019.
The current unrestricted cash and cash equivalents as of the date of this filing will fund the Company's operations for at least six months from November 9, 2017, the date that the unaudited condensed consolidated financial statements as of September 30, 2017 were issued. This projection is based on our current limited operations and estimates of legal expenses associated with the ongoing government investigation and legal matters pending against the Company, and is subject to changes in our operating plans, legal matters, uncertainties inherent in our business, transaction costs incurred at closing of the Proposed Merger, and other items that could individually or in the aggregate cause us to need to seek to replenish our existing cash and cash equivalents sooner than we project and in greater amounts that we had projected. There is no guarantee that any debt, additional equity or other funding will be available to us on acceptable terms, or at all. If we fail to obtain additional funding when needed, we would be forced to scale back, or terminate, our operations and may not be able to consummate the Proposed Merger. The Company prepared the consolidated financial statements as of and for the three and nine months ended September 30, 2017 using the generally accepted accounting principles applicable to a going concern. These consolidate financial statements do not include any adjustments relating to the recoverability and classification of recorded assets and liabilities amounts that may be necessary should the Company be unable to continue as going concern.
43
Cash Flows
The following table summarizes our cash flows from operating, investing, and financing activities for the nine months ended September 30, 2017 and 2016 (amounts in thousands):
For the Nine Months Ended September 30, | |||||||
2017 | 2016 | ||||||
Cash flows from continuing operations: | |||||||
Cash flows used in continuing operating activities | $ | (16,301 | ) | $ | (27,760 | ) | |
Cash flows used in continuing investing activities | — | (6 | ) | ||||
Cash flows provided by continuing financing activities | 21,069 | 32,755 | |||||
Total cash flows provided by continuing operating activities | 4,768 | 4,989 | |||||
Cash flows from discontinued operations: | |||||||
Cash flows used in discontinued operating activities | (9,937 | ) | (9,155 | ) | |||
Cash flows used in discontinued investing activities | — | (1,050 | ) | ||||
Total cash flows used in discontinued operations | (9,937 | ) | (10,205 | ) | |||
Total cash flows: | |||||||
Cash flows used in operating activities | (26,238 | ) | (36,915 | ) | |||
Cash flows used in investing activities | — | (1,056 | ) | ||||
Cash flows provided by financing activities | 21,069 | 32,755 | |||||
Total increase in cash and cash equivalents | $ | (5,169 | ) | $ | (5,216 | ) | |
Net Cash Flow from Operating Activities
Net cash used in operating activities decreased $10.7 million for the nine months ended September 30, 2017, compared to the nine months ended September 30, 2016. The decrease in cash used in operating activities was driven by a $11.5 million decrease in cash used in continuing operations, partially offset by an increase of $0.8 million cash used in our discontinued operating activities. The decrease in total net cash used in continuing operating activities was due to the termination of our Phase 3 PRESENT trial during the third quarter of 2016.
Net Cash Flow from Investing Activities
Net cash used in investing activities was none for the nine months ended September 30, 2017, and $1.1 million for the nine months ended September 30, 2016. The $1.1 million in 2016 related to payments of selling costs incurred from the sale of our commercial assets in the fourth quarter of 2015 that were paid in the first quarter of 2016.
Net Cash Flow from Financing Activities
Net cash provided by financing activities was $21.1 million for the nine months ended September 30, 2017, compared with $32.8 million for the nine months ended September 30, 2016. The financing activities during the nine months ended September 30, 2017 consisted of $15.5 million in net proceeds from issuance of common stock and warrants to purchase common stock and $5.7 million in redemptions of the Debenture paid by the Company in shares of our common stock. The financing activities during the nine months ended September 30, 2016 consisted of $31.8 million in net proceeds from the issuance of common stock and warrants to purchase common stock and $5.1 million in net unrestricted proceeds from the issuance of long-term debt, partially offset by $4.8 million of principal payments on long-term debt.
44
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet financing arrangements other than operating leases.
Critical Accounting Policies and Estimates
In our Annual Report on Form 10-K for the year ended December 31, 2016, we disclosed our critical accounting policies and estimates upon which our financial statements are derived. There have been no changes to these policies since December 31, 2016 that are not included in Note 1 of the accompanying condensed consolidated financial statements for the three and nine months ended September 30, 2017. Readers are encouraged to read our Annual Report on Form 10-K for the year ended December 31, 2016 in conjunction with this report.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
Not applicable, as Galena is a smaller reporting company.
45
ITEM 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this quarterly report on Form 10-Q, our principal executive officer and our principal financial officer (the “Certifying Officer”) evaluated the effectiveness of our disclosure controls and procedures. Disclosure controls and procedures are controls and procedures designed to reasonably assure that information required to be disclosed in our reports filed under the Securities Exchange Act of 1934 (the “Exchange Act”), such as this quarterly report on Form 10-Q, is recorded, processed, summarized and reported within the time periods specified in the SEC rules and forms. Disclosure controls and procedures are also designed to reasonably assure that such information is accumulated and communicated to our management, including the Certifying Officer, as appropriate to allow timely decisions regarding required disclosure. We are not currently eligible to use a short-form Form S-3 registration due to our failure to timely file multiple current reports on Form 8-K relating to our unregistered sales of equity securities under the Debenture, which we resolved by the filing of an 8-K on May 2, 2017. During the three-month period ending September 30, 2017, management implemented procedures pursuant to which a subgroup of senior managers reviews each significant Company event to determine how that event impacts the Company’s SEC reporting responsibilities, including the Company’s obligation to file current reports on Form 8-K. Based on the evaluation noted above, the Certifying Officer has concluded, that, as of three-month period ending September 30, 2017 covered by this quarterly report on Form 10-Q:
(a) | our disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed by us in the reports we file or submit under the Exchange Act was recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms; and |
(b) | our disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed by us in the reports we file or submit under the Exchange Act was accumulated and communicated to our management, including the Certifying Officer, as appropriate to allow timely decisions regarding required disclosure. |
Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting (as defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the quarter ended September 30, 2017 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our management, including the Interim CEO and CFO, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well-designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of the effectiveness of controls to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
46
PART II - OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
Please refer to Note 6 (Legal Proceedings, Commitments and Contingencies) to our condensed consolidated financial statements contained in Part I, Item 1 (Financial Statements) of this Quarterly Report on Form 10-Q, which is incorporated into this item by reference.
47
ITEM 1A. RISK FACTORS
Please refer to our note on forward-looking statements on page 2 of this Form 10-Q, which is incorporated into this item by reference.
The following description of risk factors includes any material changes to, and supersedes the description of, risk factors associated with the Company’s business previously disclosed in Part I, Item 1A of the 2016 Form 10-K and in Part II, Item 1A of the Forms 10-Q for the quarters ended March 31, 2017 and June 30, 2017, in each case under the heading “Risk Factors.” The business, financial condition and operating results of the Company can be affected by a number of factors, whether currently known or unknown, including but not limited to those described below, any one or more of which could, directly or indirectly, cause the Company’s actual financial condition and operating results to vary materially from past, or from anticipated future, financial condition and operating results. Any of these factors, in whole or in part, could materially and adversely affect the Company’s business, financial condition, operating results and stock price.
Risks Related to the Proposed Merger
The exchange ratio is not adjustable based on the market price of Galena Common Stock so the Merger consideration at the closing may have a greater or lesser value than at the time the Merger Agreement was signed.
It is currently anticipated that, at the closing of the Merger, the exchange ratio specified in the Merger Agreement, or the Exchange Ratio, would be approximately 1,268.8519 pre-split shares of Galena Common Stock for each SELLAS Share and would be within a range of approximately 126.8852 and 42.2951 post-split shares of Galena Common Stock for each SELLAS Share. These estimates are subject to adjustment prior to closing of the Merger, including adjustments to account for the issuance of any additional SELLAS Shares or Galena Common Stock, as applicable, prior to the consummation of the Merger. Issuances of Galena Common Stock involving the following securities or transactions, if any, will not impact the Exchange Ratio: (i) a potential third party financing consented to by SELLAS and (ii) any exercise of the Significantly Out-of-the-Money Galena Warrants. Any changes in the market price of Galena Common Stock before the completion of the Merger will not affect the number of shares SELLAS shareholders will be entitled to receive pursuant to the Merger Agreement. Therefore, if before the completion of the Merger the market price of Galena Common Stock declines from the market price on the date of the Merger Agreement, then SELLAS shareholders could receive Merger consideration with substantially lower value. Similarly, if before the completion of the Merger the market price of Galena Common Stock increases from the market price on the date of the Merger Agreement, then SELLAS shareholders could receive Merger consideration with substantially more value for their SELLAS Shares than the parties had negotiated for in the establishment of the Exchange Ratio. Because the Exchange Ratio does not adjust as a result of changes in the market value of Galena Common Stock, for each one percentage point that the market value of Galena Common Stock rises or declines, there is a corresponding one percentage point rise or decline, respectively, in the value of the total Merger consideration issued to SELLAS shareholders.
The market price of Galena Common Stock following the Merger may decline as a result of the Merger.
The market price of Galena Common Stock may decline as a result of the Merger for a number of reasons, including if:
• | investors react negatively to the prospects of the continuing company’s business and prospects; or |
• | the performance of the continuing company’s business or its future prospects are not consistent with the expectations of financial or industry analysts; |
• | the continuing company cannot raise the necessary capital to fund the clinical programs; or |
• | the continuing company does not achieve benefits of the Merger as rapidly or to the extent anticipated by financial or industry analysts. |
48
SELLAS and Galena securityholders will have a reduced ownership and voting interest in, and will exercise less influence over the management of, the continuing company as compared to their current ownership and voting interest in the respective companies following the completion of the Merger.
After the completion of the Merger, the current securityholders of SELLAS and Galena will own a smaller percentage of the continuing company than their ownership of their respective companies prior to the Merger. Immediately after the Merger, SELLAS securityholders will own approximately 67.5% of the Galena Common Stock and Galena securityholders, whose shares of Galena Common Stock will remain outstanding after the Merger, will own approximately 32.5% of the Galena Common Stock, in each case calculated on a fully diluted basis for the continuing company, except for the exclusion of the impact of a potential third party financing consented to by SELLAS and 2,556,851 shares of Galena Common Stock issuable pursuant to out-of-the money Galena warrants (the "Significantly Out-of-the-Money Galena Warrants"). These estimates are based on the anticipated Exchange Ratio in the Merger and are subject to adjustment. In addition, the seven-member board of directors of the continuing company, a majority of whom will be independent, will initially comprise five directors designated by SELLAS and, subject to the prior consent of SELLAS, not to be unreasonably withheld, two directors designated by Galena. Consequently, securityholders of SELLAS and Galena will be able to exercise less influence over the management and policies of the continuing company than they currently exercise over the management and policies of their respective companies.
If the conditions to the Merger are not met, the Merger may not occur.
Even if the required Merger approvals are obtained from the SELLAS shareholders and the Galena stockholders, specified conditions must be satisfied or waived to complete the Merger. Galena cannot assure you that all of the conditions will be satisfied or waived. If the conditions are not satisfied or waived, the Merger may not occur or will be delayed, and Galena and SELLAS each may lose some or all of the intended benefits of the Merger.
Failure to complete the Merger may result in Galena or SELLAS paying a termination fee to the other party and could harm the common stock price of Galena and future business and operations of each company.
If the Merger is not completed, Galena and SELLAS are subject to the following risks:
• | if the Merger Agreement is terminated under specified circumstances, Galena or SELLAS will be required to pay the other party a termination fee of $750,000 and/or up to $100,000 in expense reimbursements; |
• | the attention of Galena and SELLAS management will have been diverted to the Merger instead of being directed solely to their respective operations and the pursuit of other opportunities that may have been beneficial to Galena and/or SELLAS; |
• | the price of Galena Common Stock may decline further and remain volatile; |
• | costs related to the Merger, such as legal and accounting fees, which Galena and SELLAS estimate will total approximately $2.1 million and $2.1 million, respectively; |
• | Galena and SELLAS, if they remain stand-alone companies, may not be able to raise the necessary capital to support their respective operations; and |
• | Galena may be forced to cease its operations, dissolve and liquidate its assets. |
In addition, if the Merger Agreement is terminated and the Galena Board or the SELLAS Board determines to seek another business combination, there can be no assurance that either Galena or SELLAS will be able to find a partner willing to provide equivalent or more attractive consideration than the consideration to be provided by each party in the Merger.
During the pendency of the Merger, Galena and SELLAS may not be able to enter into a business combination with another party at a favorable price because of restrictions in the Merger Agreement, which could adversely affect their respective businesses.
Covenants in the Merger Agreement impede the ability of Galena and SELLAS to make acquisitions, subject to specified exceptions relating to fiduciary duties or complete other transactions that are not in the ordinary course of business pending completion of the Merger. As a result, if the Merger is not completed, the parties may have been placed at a disadvantage to their competitors during the period leading up to the decision to terminate the Merger. In
49
addition, while the Merger Agreement is in effect, each party is generally prohibited from soliciting, initiating, encouraging or entering into specified extraordinary transactions, such as a merger, sale of assets or other business combination, with any third party, subject to specified exceptions. Any such transactions could be favorable to such party and such party’s securityholders.
Certain provisions of the Merger Agreement may discourage third parties from submitting competing proposals, including proposals that may be superior to the arrangements contemplated by the Merger Agreement.
The terms of the Merger Agreement prohibit each of Galena and SELLAS from soliciting competing proposals or cooperating with persons making unsolicited takeover proposals, except in limited circumstances when such party’s board of directors determines in good faith, after consultation with its independent financial advisor, if any, and outside legal counsel, that an unsolicited competing proposal constitutes, or would reasonably be expected to result in, a superior competing proposal and that failure to take such action would be reasonably likely to result in a breach of the fiduciary duties of the board of directors. In addition, if Galena or SELLAS terminate the Merger Agreement under specified circumstances, including terminating because of a decision of a board of directors to recommend a superior competing proposal, Galena or SELLAS would be required to pay a termination fee of $750,000 and/or up to $100,000 in expense reimbursements to the other party. This termination fee may discourage third parties from submitting competing proposals to Galena or SELLAS or their securityholders, and may cause the respective boards of directors to be less inclined to recommend a competing proposal.
Because the lack of a public market for the SELLAS Shares makes it difficult to evaluate the fairness of the Merger, the shareholders of SELLAS may receive consideration in the Merger that is less than the fair market value of the SELLAS Shares or Galena may pay more than the fair market value of the SELLAS Shares.
The outstanding SELLAS Shares are privately held and not traded in any public market. The lack of a public market makes it extremely difficult to determine the fair market value of the SELLAS Shares. Because the percentage of Galena equity to be issued to SELLAS shareholders was determined based on negotiations between the parties, it is possible that the value of the Galena Common Stock to be received by SELLAS shareholders will be less than the fair market value of SELLAS Shares, or Galena may pay more than the fair market value of the SELLAS Shares.
Galena expects that it will need to raise additional capital prior to the completion of the Merger, which may be unavailable on acceptable terms, or at all, which may impair Galena’s ability to complete the Merger.
Galena expects that it will need to raise additional capital in order to complete the Merger, as it is unclear if it will be able meet all of its obligations at the closing of the Merger if it does not raise additional capital. Galena’s ability to raise additional capital will depend in part on conditions in the capital markets at that time, which are outside Galena’s control, on Galena’s financial prospects and the market’s perception of the Merger. Galena may be unable to raise additional capital on acceptable terms, or at all, prior to the closing of the Merger, which may impair Galena’s ability to complete the Merger.
Even if the Merger is consummated, the continuing company may fail to realize the anticipated benefits of the Merger.
The success of the Merger will depend on, among other things, the continuing company’s ability to achieve its business objectives and raise the necessary capital to fund its operations, including the successful development of its current and future product candidates. If the continuing company is not able to achieve these objectives, the anticipated benefits of the Merger may not be realized fully, may take longer to realize than expected, or may not be realized at all.
Galena and SELLAS have operated and, until the completion of the Merger, will continue to operate independently. Even if the Merger is completed, it is possible that the integration process could result in the loss of key employees, the disruption of each company’s ongoing business, an adverse impact on the value of the continuing company’s assets, or inconsistencies in standards, controls, procedures or policies that could adversely affect the continuing company’s ability to comply with reporting obligations as a public company, an inability to satisfy its obligations to third parties or to achieve the anticipated benefits of the Merger, or an inability to raise the necessary capital to fund each company’s operations. Integration efforts between the two companies will also divert management’s attention and resources. Any delays in the integration process or inability to realize the full extent of the anticipated benefits of the Merger could have an adverse effect on the continuing company’s business and the results of the continuing
50
company’s operations. Such an adverse effect may impact the value of the shares of the Galena Common Stock after the completion of the Merger.
Potential difficulties that may be encountered in the integration process include, among other things, the following:
• | raising sufficient capital to fund the current clinical programs; |
• | using the continuing company’s cash and other assets efficiently to develop the business of the continuing company; |
• | appropriately managing the liabilities of the continuing company; |
• | loss of key employees; |
• | potential unknown or currently unquantifiable liabilities associated with the Merger and the operations of the continuing company; and |
• | performance shortfalls at one or both of the companies as a result of the diversion of management’s attention caused by completing the Merger and integrating the companies’ operations. |
If the Merger is not completed, Galena may elect to liquidate its remaining assets, and there can be no assurances as to whether any cash would be available to distribute to Galena’s stockholders after paying Galena’s debts and other obligations.
If Galena does not complete the Merger, the Galena Board may elect to take the steps necessary to liquidate all of Galena’s remaining assets. The process of liquidation may be lengthy and Galena cannot make any assurances regarding the timing of completing such a process. In addition, Galena would be required to pay all of Galena’s debts and contractual obligations, and to set aside certain reserves for potential future claims. There can be no assurance as to whether any cash would be available to distribute to Galena stockholders after paying Galena’s debts and other obligations and setting aside funds for reserves, or as to the timing of any such distribution.
If Galena fails to continue to meet all applicable requirements of the Nasdaq Capital Market and NASDAQ determines to delist Galena Common Stock, the delisting could adversely affect the value and market liquidity of Galena Common Stock and harm Galena’s business and would impair Galena’s ability to complete the Merger.
It is a condition of SELLAS’ obligation to complete the Merger that Galena maintain the listing of the Galena Common Stock on the Nasdaq Capital Market where the Galena Common Stock is currently listed. In order to maintain that listing, Galena must satisfy minimum financial and other listing requirements, including the closing bid price requirement. On March 24, 2017, Galena received notice from the Listing Qualifications Department of NASDAQ that the Galena Common Stock had not met the $1.00 per share minimum bid price requirement for the previous 30 consecutive business days, and that Galena was therefore not in compliance with the requirements for continued inclusion on the Nasdaq Capital Market under NASDAQ Listing Rule 5550(a)(2). In accordance with Nasdaq Listing Rule 5810(c)(3)(A), Galena had 180 calendar days, or until September 20, 2017, to regain compliance with this minimum bid price requirement. On September 21, 2017, NASDAQ notified Galena that, while Galena had not regained compliance with the minimum bid price requirement, it was eligible for an additional 180-day grace period, or until March 19, 2018, to regain compliance with the $1.00 per share minimum bid price requirement.
NASDAQ’s determination to grant an additional 180-day grace period was based on Galena having met the continued listing requirement for market value of publicly held shares and all other applicable requirements for initial listing on the Nasdaq Capital Market, with the exception of the minimum bid price requirement, and on Galena’s written notice to NASDAQ of its intention to cure the deficiency during the second compliance period by effecting a reverse stock split, if necessary. If at any time during this second 180-day grace period the closing bid price of the Galena Common Stock is at least $1.00 per share for a minimum of ten consecutive business days, NASDAQ will provide written confirmation of compliance and this matter will be closed. Under NASDAQ Rule 5810(c)(3)(F), NASDAQ may, in its discretion require Galena to maintain a minimum bid price of at least $1.00 per share for a period in excess of ten consecutive business days, but generally no more than 20 consecutive business days, before determining Galena has demonstrated an ability to maintain long-term compliance. If Galena does not regain compliance with the minimum bid price requirement by March 19, 2018, NASDAQ will provide written notification to Galena that the Galena Common Stock will be delisted. At that time, Galena may appeal the NASDAQ staff’s determination to a Hearings Panel, which will stay the delisting process pending the decision of the Hearings Panel.
51
Galena will be required to provide a plan to regain compliance to the Hearings Panel. If the Hearings Panel decides to continue with delisting of Galena, the Hearing Panel’s decision may be appealed to the NASDAQ Listing and Hearing Review Council, but such appeal will not stay the delisting process. The closing bid price of the Galena Common Stock on the Nasdaq Capital Market was $0.30 on November 8, 2017.
While Galena intends to engage in efforts to regain compliance through, among other things, the Reverse Stock Split, and thus maintain Galena’s listing, there can be no assurance that Galena will be able to regain compliance during the applicable time periods set forth above. If Galena fails to continue to meet all applicable NASDAQ requirements in the future and NASDAQ determines to delist the Galena Common Stock, the delisting could substantially decrease trading in Galena Common Stock, adversely affect the market liquidity of Galena Common Stock, adversely affect Galena’s ability to obtain financing on acceptable terms, if at all, for the continuation of Galena’s operations and harm Galena’s business. Additionally, the market price of Galena Common Stock may decline further and Galena stockholders may lose some or all of their investment.
A failure by the continuing company to comply with the initial listing standards of NASDAQ will result in a failure of SELLAS’ obligation to complete the Merger and the Merger may not occur.
Upon the completion of the Merger, the continuing company will be required to meet the initial listing requirements to maintain the listing and continued trading of the Galena Common Stock on the Nasdaq Capital Market. These initial listing requirements are more difficult to achieve than the continued listing requirements under which Galena Common Stock is now trading. Based on information currently available to Galena, Galena anticipates that it will be unable to meet the $4.00 minimum bid price initial listing requirement at the closing of the Merger unless Galena effects the Reverse Stock Split. If Galena is unable to satisfy these requirements, a condition to SELLAS’ obligation to complete the Merger will not be satisfied and the Merger may not occur. It is also a condition of SELLAS’ obligation to complete the Merger that Galena maintain the listing of the Galena Common Stock on NASDAQ. In addition, oftentimes a reverse stock split will not result in a trading price for the affected common stock that is proportional to the ratio of the split. Galena believes that the Reverse Stock Split will be in the best interest of the continuing company and Galena stockholders. However, Galena cannot assure you that the implementation of the Reverse Stock Split will have a positive impact on the price of the Galena Common Stock.
Galena may become involved in securities class action litigation that could divert management’s attention and harm Galena’s business, and insurance coverage may not be sufficient to cover all costs and damages.
In the past, securities class action or shareholder derivative litigation often follows certain significant business transactions, such as the sale of a business division or announcement of a merger. The continuing company may become involved in this type of litigation in the future. Litigation often is expensive and diverts management’s attention and resources, which could adversely affect the continuing company’s business.
The Merger may be completed even though material adverse changes may result from the announcement of the Merger, industry-wide changes and other causes.
In general, either Galena or SELLAS can refuse to complete the Merger if there is a material adverse change affecting the other party between August 7, 2017, the date of the Merger Agreement, and the closing of the Merger. However, certain types of changes do not permit either party to refuse to complete the Merger, even if such change could be said to have a material adverse effect on Galena or SELLAS, including:
• | any effect, change, event, circumstance or development in the conditions generally affecting the industries in which SELLAS and Galena operate or the U.S. or global economy or capital markets as a whole; |
• | any natural disaster or any acts of terrorism, sabotage, military action or war or any escalation of worsening thereof; |
• | any change in accounting requirements or principles or any change in applicable laws, rules or regulations or the interpretation thereof; |
• | any effect resulting from the announcement or pendency of the Merger or any related transactions; |
• | any failure by Galena or SELLAS to meet internal projections or forecasts or third-party revenue or earnings predictions for any period ending on or after August 7, 2017; |
• | with respect to Galena, any change in the price or trading volume of Galena Common Stock; |
52
• | any rejection by a governmental body of a registration or filing by SELLAS or Galena relating to specified intellectual property rights; or |
• | with respect to SELLAS, any change in the cash position of SELLAS that results from operations in the ordinary course of business. |
If adverse changes occur and Galena and SELLAS still complete the Merger, the stock price of the continuing company may suffer. This in turn may reduce the value of the Merger to the stockholders of Galena, shareholders of SELLAS or both.
Some Galena and SELLAS executive officers and directors have interests in the Merger that are different from yours and that may influence them to support or approve the Merger without regard to your interests.
Some officers and directors of Galena and SELLAS participate in arrangements that provide them with interests in the Merger that are different from yours, including, among others, the continued service as an officer or director of the continuing company, severance and retention benefits, payment of current year incentive compensation, continued indemnification and the potential ability to sell an increased number of shares of common stock of the continuing company in accordance with Rule 144 under the Securities Act of 1933, as amended, or the Securities Act.
Immediately following the Merger, Equilibria Capital Management Limited and its affiliates will own a significant percentage of the continuing company’s outstanding common stock and will be able to exert significant control over matters subject to stockholder approval, potentially preventing new investors from influencing significant corporate decisions.
As of the effective time of the Merger, Equilibria Capital Management Limited and its affiliates are currently anticipated to own approximately 47.7% of the continuing company’s outstanding common stock. The interests of Equilibria Capital Management Limited and its affiliates may not coincide with the interests of other stockholders. Therefore, these stockholders will have the ability to influence the continuing company through their ownership positions, which may prevent or discourage unsolicited acquisition proposals or offers for the continuing company’s capital stock that you may believe are in your best interest as one of the continuing company’s securityholders.
Because the Merger will result in an ownership change under Section 382 of the Code for Galena, Galena’s pre-Merger net operating loss carryforwards and certain other tax attributes will be subject to limitation or elimination. The net operating loss carryforwards and certain other tax attributes of SELLAS and of the continuing company may also be subject to limitations as a result of ownership changes.
As of June 30, 2017, Galena had $277 million of pre-Merger federal net operating loss carryforwards. If a corporation undergoes an “ownership change” within the meaning of Section 382 of the Code, the corporation’s net operating loss carryforwards and certain other tax attributes arising before the ownership change are subject to limitations on use after the ownership change. In general, an ownership change occurs if there is a cumulative change in the corporation’s equity ownership among certain stockholders (under rules specified in Code Section 382, including, generally, stockholders holding greater than 5% of Galena’s equity and the aggregate “public group” of stockholders) that exceeds fifty percentage points by value over a rolling three-year period. Similar rules may apply under state tax laws. The Merger will result in an ownership change for Galena and, accordingly, Galena’s net operating loss carryforwards and certain other tax attributes will be subject to limitation and possibly elimination after the Merger. This limitation is based on a percentage of the value of the equity of Galena, not the amount of the net operating loss carryovers. SELLAS has not performed an analysis on whether it has experienced any ownership changes in the past. However, it is possible that SELLAS’ net operating loss carryforwards and certain other tax attributes may also be subject to limitation as a result of prior shifts in equity ownership and/or the Merger. Additional ownership changes in the future could result in additional limitations on Galena’s, SELLAS’ and the continuing company’s net operating loss carryforwards and certain other tax attributes. Consequently, even if the continuing company achieves profitability, it may not be able to utilize a material portion of Galena’s, SELLAS’ or the continuing company’s net operating loss carryforwards and certain other tax attributes, which could have a material adverse effect on cash flow and results of operations.
53
Risks Related to Galena’s Former Commercial Operations
Galena is subject to U.S. federal and state health care fraud and abuse and false claims laws and regulations, and Galena had been subpoenaed in connection with marketing and promotional practices related to Abstral (fentanyl) sublingual tablets. Prosecutions under such laws have increased in recent years and Galena may become subject to such prosecutions or related litigation under these laws. If Galena has not fully complied with such laws, Galena could face substantial penalties.
Galena’s former commercial operations and development programs are subject to various U.S. federal and state fraud and abuse laws, including, without limitation, the federal False Claims Act, federal Anti-Kickback Statute, and the federal Sunshine Act. A federal investigation led by the U.S. Attorney’s Office for the Southern District of Alabama, or the SDAL, of two of the high-prescribing physicians for Abstral (fentanyl) sublingual tablets resulted in the criminal prosecution of the two physicians for alleged violations of the federal False Claims Act and other federal statutes. On April 28, 2016, a second superseding indictment was filed in the criminal case, which added additional information about the defendant physicians and provided information regarding the facts and circumstances involving a rebate agreement between Galena and the defendant physicians’ pharmacy as well as their ownership of Galena Common Stock. The criminal trial, which began on January 4, 2017, concluded with a jury verdict on February 23, 2017 finding these physicians guilty on 19 of 20 counts. In May 2017, one physician was sentenced to twenty years in prison, and the other physician was sentenced to twenty-one years in prison. At the end of the SDAL case, SDAL dismissed count 18 of the indictment charging that the physicians conspired, through the C&R Pharmacy, to receive illegal kickbacks in exchange for prescribing Abstral. To Galena’s knowledge, Galena was not a target or subject of that investigation.
There have also been federal and state investigations of a company that has a product that competes with Abstral in the same therapeutic class, and Galena has learned that the U.S. Food and Drug Administration, or the FDA, and other governmental agencies were investigating Galena’s Abstral promotion practices. In December 2015, Galena announced it had received a subpoena from the U.S. Attorney’s Office for the District of New Jersey, or the USAO NJ, requesting the production of a broad range of documents pertaining to marketing and promotional practices related to Abstral, a product which it sold to Sentynl Therapeutics Inc. for aggregate gross consideration of $12 million in November 2015. In January 2016, Galena announced that the USAO NJ and Department of Justice, or the DOJ, were conducting a criminal and civil investigation of Galena as well as possibly one or more then-current and/or former employees. On September 8, 2017, DOJ announced a settlement agreement regarding the USAO NJ and DOJ’s investigation as to Galena. The settlement involves a non-criminal resolution agreement and a civil payment of approximately $7.6 million, plus interest accrued since the date of reaching an agreement in principle payable in equal installments over twelve months, in return for a release of government claims of Galena in connection with the investigation.
Galena may be subject to legal or administrative actions as a result of these matters, or the impact of such matters. If Galena is found to be in violation of the False Claims Act, Anti-Kickback Statute, Patient Protection and Affordable Care Act, or any other applicable state or any federal fraud and abuse laws, Galena may be subject to penalties, such as civil and criminal penalties, damages, fines, or an administrative action of exclusion from government health care reimbursement programs. Galena can make no assurances as to the time or resources that will need to be devoted to these matters or their outcome, or the impact, if any, that these matters or any resulting legal or administrative proceedings may have on Galena’s business or financial condition.
Galena is subject to many regulatory provisions that include criminal provisions. If Galena is unable to comply with these provisions in the operation of Galena’s business, Galena may become subject to civil and criminal investigations and proceedings that could have a material adverse effect on Galena’s business, financial condition and prospects.
The federal False Claims Act prohibits persons from knowingly filing, or causing to be filed, a false claim to, or the knowing use of false statements to obtain payment from, the federal government. Qui tam suits filed under the False Claims Act can be brought by any individual on behalf of the government and such individuals, commonly known as “relators” or “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. The frequency of filing qui tam actions has increased significantly in recent years, causing greater numbers of health care companies to have to defend such qui tam actions and pay substantial sums to settle such actions. A qui tam action had been filed against Galena and others as described in the settlement agreement with DOJ and USAO NJ. As set forth in that settlement agreement, for a release of all claims against Galena and its officers and directors and dismissal with prejudice of the qui tam lawsuit, the relator received a portion of approximately $7.6 million payment to the federal government. Upon payment of the settlement amount, the federal government and the relator will dismiss
54
with prejudice their claims against Galena in the qui tam lawsuit. In a separate settlement agreement, Galena paid the $300,000 in cash to the relator’s counsel for the statutory mandated attorneys fees. The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal health care program such as the Medicare and Medicaid programs. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal health care covered business, the statute has been violated. The Anti-Kickback Statute is broad, and despite a series of narrow safe harbors, prohibits many arrangements and practices that are lawful in businesses outside of the health care industry. Penalties for violations of the federal Anti-Kickback Statute include criminal penalties and civil and administrative sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal health care programs. An alleged violation of the Anti- Kickback Statute may be used as a predicate offense to establish liability pursuant to other federal laws and regulations such as the federal False Claims Act. Many states have also adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for health care items or services reimbursed by any source, not only Medicare and Medicaid programs.
The federal Patient Protection and Affordable Care Act includes provisions expanding the ability of certain relators to bring actions that would have been dismissed under prior law. When an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each separate false claim. The Deficit Reduction Act of 2005 encouraged states to enact or modify their state false claims acts to be at least as effective as the federal False Claims Act by granting states a portion of any federal Medicaid funds recovered through Medicaid-related actions. Most states have enacted state false claims laws, and many of those states included laws including qui tam provisions. The federal Patient Protection and Affordable Care Act includes provisions known as the Physician Payments Sunshine Act, which requires manufacturers of drugs, biologics, devices and medical supplies covered under Medicare and Medicaid to record any transfers of value to physicians and teaching hospitals and to report this data beginning in 2013 to the Centers for Medicare and Medicaid Services for subsequent public disclosures. Manufacturers must also disclose investment interests held by physicians and their family members. Failure to submit the required information may result in civil monetary penalties of up to $1 million per year for knowing violations and may result in liability under other federal laws or regulations. Similar reporting requirements have also been enacted on the state level in the United States, and an increasing number of countries worldwide either have adopted or are considering similar laws requiring transparency of interactions with health care professionals. In addition, some states such as Massachusetts and Vermont imposed an outright ban on certain gifts to physicians. These laws could affect Galena’s product promotional activities by limiting the kinds of interactions Galena could have with hospitals, physicians or other potential purchasers or users of Galena’s potential future commercial assets. Both the disclosure laws and gift bans also will impose administrative, cost and compliance burdens on Galena.
Galena faces product liability exposure and, if successful claims are brought against Galena, Galena may incur substantial liability if Galena’s insurance coverage for those claims is inadequate.
The commercial sale of Galena’s products after they are approved as well as the use of Galena’s product candidates in clinical trials exposes Galena to possible product liability claims. This risk exists even if a product is approved for commercial sale by the FDA and manufactured in facilities licensed and regulated by the FDA, if Galena’s products were sold to third parties, or if Galena’s product candidates are provided in clinical trials. Galena’s products and product candidates are designed to affect important bodily functions and processes. Any side effects, manufacturing defects, misuse or abuse associated with Galena’s products or product candidates could result in injury to a patient or even death. For example, because the placebo may have performed better than NeuVax in the PRESENT (Prevention of Recurrence in Early-Stage, Node-Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment) Trial, the use of NeuVax may have worsened the patient’s condition.
Product liability claims may be brought against Galena by consumers, health care providers, pharmaceutical companies or others selling or otherwise coming into contact with Galena’s products or generic versions of Galena’s products. If Galena cannot successfully defend itself against product liability claims Galena could incur substantial liabilities. Because Galena has sold Abstral and Zuplenz (ondansetron) oral soluble film and provided NeuVax as a study drug in the PRESENT Trial and other clinical trials, regardless of merit or eventual outcome, product liability claims may result in:
• | impairment of Galena’s business reputation; |
• | costs of related litigation; |
55
• | distraction of management’s attention from Galena’s primary business; or |
• | substantial monetary awards to patients or other claimants. |
Galena has obtained product liability insurance coverage for commercial product sales with a $10 million per occurrence and a $10 million annual aggregate coverage limit. Galena’s insurance coverage may not be sufficient to cover all of Galena’s product liability related expenses or losses and may not cover Galena for any expenses or losses Galena may suffer. Moreover, insurance coverage is becoming increasingly expensive and, in the future, Galena may not be able to maintain insurance coverage at a reasonable cost, in sufficient amounts or upon adequate terms to protect Galena against losses due to product liability. If Galena determines that it is prudent to increase Galena’s product liability coverage based on sales of Galena’s products, Galena may be unable to obtain this increased product liability insurance on commercially reasonable terms or at all. Large judgments have been awarded in class action or individual lawsuits based on drugs that had unanticipated side effects, including side effects that may be less severe than those of Galena’s products. A successful product liability claim or series of claims brought against Galena could cause Galena’s stock price to decline and, if judgments exceed Galena’s insurance coverage, could decrease Galena’s cash and have a material adverse effect on Galena’s business, results of operations, financial condition and prospects.
Galena’s business involves the use of hazardous materials and Galena and its third-party manufacturers and suppliers must comply with environmental laws and regulations, which can be expensive and restrict how Galena does business.
Galena’s third-party manufacturers’ and suppliers’ activities involve the controlled storage, use and disposal of hazardous materials. Galena and Galena’s manufacturers and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials even after Galena sells or otherwise disposes of the products. In some cases, these hazardous materials and various wastes resulting from their use will be stored at Galena’s contractors or manufacturers’ facilities pending use and disposal. Galena cannot completely eliminate the risk of contamination, which could cause injury to Galena’s employees and others, environmental damage resulting in costly cleanup and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although Galena expects that the safety procedures utilized by Galena’s third-party contractors and manufacturers for handling and disposing of these materials will generally comply with the standards prescribed by these laws and regulations, Galena cannot guarantee that this will be the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, Galena may be held liable for any resulting damages and such liability could exceed Galena’s resources. Galena does not currently carry biological or hazardous waste insurance coverage and Galena’s property and casualty and general liability insurance policies specifically exclude coverage for damages and fines arising from biological or hazardous waste exposure or contamination.
Galena will continue to be responsible for certain liabilities and obligations related to Abstral and Zuplenz, and if unknown liabilities were to arise it could have a material adverse effect on Galena.
Under Galena’s respective asset purchase agreements with Sentynl Therapeutics, Inc. and Midatech Pharma PLC, Galena’s future obligations under Galena’s former agreements with Orexo AB and MonoSol Rx have been assumed by Sentynl and Midatech, respectively, except that Galena will continue to be responsible for chargebacks, rebates, patient assistance and certain other product distribution channel liabilities related to Abstral and Zuplenz for a specified period of time post-closing. With respect to Abstral, Galena will continue to be responsible for returns for products sold prior to November 19, 2015, chargebacks and rebates through February 17, 2016 and patient assistance for a period subsequent to November 19, 2015 which shall terminate on the earlier of (i) February 19, 2016 or (ii) cumulative patient benefits accrued following November 19, 2015 equal to $440,000. With respect to Zuplenz, Galena will continue to be responsible for returns for a period from December 24, 2015 through December 24, 2016 for products which Galena previously sold and will be responsible from and after such period for any downstream returns from end user customers or returns from wholesalers from inventory existing as of December 24, 2015 that was sold by Galena prior to December 24, 2015. In addition, Galena will continue to be responsible for Medicaid rebates with respect to the fourth quarter of 2015 and payment claims arising from sales by customers any time prior and up to February 22, 2016. Galena is also responsible for any pre-closing liabilities and obligations related to Abstral and Zuplenz, including unknown liabilities, and have agreed in the respective asset purchase agreements to indemnify Sentynl and Midatech for any breach of Galena’s representations, warranties and covenants in the respective asset purchase agreements up to certain agreed to amounts. As presently believed by Galena, responsibilities to Sentynl and Midatech are not material, but if substantial unknown liabilities were to arise,
56
it could have a material adverse effect on Galena’s financial condition. In this regard, Galena has been advised by one of its wholesale customers that Zuplenz inventory held by that customer under an alleged agreement with Galena is approaching its expiration date and needs to be swapped with better dated Zuplenz product. That customer and Galena have settled the swap by Galena paying the customer $500,000. Midatech has advised Galena that the same Zuplenz inventory is reaching its expiration date and will be returned. Under the terms of the asset purchase agreement, Midatech maintained that the cost of the return is $1.5 million and Galena needs to pay Midatech for the return. Midatech has since withdrawn that claim. Galena believes the settlement with the customer has resolved any return issues with Midatech without additional cost to Galena. However, no assurance can be given that Galena will not face additional liabilities under these asset purchase agreements.
Risks Related to Galena’s Development Programs
Galena’s drug candidates may not receive regulatory approval or be successfully commercialized.
Before they can be marketed, Galena’s clinical trials of its product candidates are subject to inspection. The same products are subject to inspection and approval by the FDA or similar foreign governmental agencies prior to being manufactured or marketed. The inspections by the FDA could delay the completion of the clinical trial and manufacturing of the product and thus the approval of the product. The process for obtaining FDA approval is both time-consuming and costly, with no certainty of a successful outcome.
Before obtaining regulatory approval for the sale of any drug candidate, Galena must conduct extensive preclinical tests and clinical trials to demonstrate the safety and efficacy in humans of Galena’s product candidates. Although Galena’s drug candidates have exhibited no serious adverse events, or SAEs, in the Phase 1 and 1/2 clinical trial, SAEs or other unexpected side effects may arise during further testing and development, and a failure of any preclinical study or clinical trial can occur at any stage of testing. The results of preclinical and initial clinical testing of these products may not necessarily indicate the results that will be obtained from later or more extensive testing. It also is possible to suffer significant setbacks in advanced clinical trials, even after obtaining promising results in earlier trials.
Galena’s Phase 3 PRESENT clinical trial of NeuVax was stopped due to futility and, since Galena has not been able to determine the causes for the failure of the clinical trial, it may be that NeuVax is not effective as a monotherapy in the treatment of the recurrence of breast cancer, which could indicate that certain of Galena’s other product candidates may also not be effective.
On June 27, 2016, the Independent Data Monitoring Committee conducted the pre-planned interim analysis of the PRESENT Trial and recommended that Galena stop the clinical trial because of futility, as the placebo may have performed better than NeuVax in the PRESENT trial. While there may have been factors about the design or conduct of the PRESENT trial that caused its failure, it may be that NeuVax is not effective as a monotherapy in the prevention of recurrence of breast cancer. As Galena product candidates GALE-301 and GALE-302 have a similar mechanism of action to NeuVax, they may no longer be effective product candidates as a monotherapy for the prevention of the recurrence of ovarian cancer or other types of cancer.
A number of different factors could prevent Galena from obtaining regulatory approval or commercializing Galena’s product candidates on a timely basis, or at all.
Clinical trials of a drug candidate may be suspended at any time for various reasons, including if Galena or other regulatory agencies believe the subjects or patients participating in such trials are being exposed to unacceptable health risks. A suspension may come from: Galena; the FDA or other applicable regulatory authorities; an Independent Data Safety Monitoring Board, or DSMB, governing Galena’s clinical trials; or an institutional review board, or IRB, which is an independent committee registered with and overseen by the U.S. Department of Health and Human Services, or the HHS, that functions to approve, monitor and review biomedical and behavioral research involving humans. Among other reasons, adverse side effects of a drug candidate on subjects or patients in a clinical trial could result in the FDA or other regulatory authorities suspending or terminating the trial and refusing to approve a particular drug candidate for any or all indications of use.
Clinical trials of a new drug candidate require the enrollment of a sufficient number of patients, including patients who are suffering from the disease the drug candidate is intended to treat and who meet other eligibility criteria. Rates of patient enrollment are affected by many factors, and delays in patient enrollment can result in increased costs and longer development times than Galena expects at present. Patients who are enrolled at the outset of the
57
trial may eventually choose for personal reasons not to participate in the study. Galena also competes for eligible patients with other clinical trials underway, and Galena may experience delays in patient enrollment due to the dependency of other trials underway in the same patient population.
Clinical trials also require the review and oversight of IRBs, which approve and continually review clinical investigations to protect the rights and welfare of human subjects. An inability or delay in obtaining IRB approval could prevent or delay the initiation and completion of clinical trials, and the FDA may decide not to consider any data or information derived from a clinical investigation not subject to initial and continuing IRB review and approval.
In addition, cancer vaccines are a relatively new form of therapeutic treatment and a very limited number of such products have received regulatory approval. Therefore, the FDA or other regulatory authority may apply standards for approval of a new cancer vaccine that is different from past experience.
Numerous factors could affect the timing, cost or outcome of Galena’s drug development efforts, including the following:
• | difficulties or delays in enrolling patients in Galena’s planned clinical trials in conformity with required protocols or projected timelines; |
• | conditions imposed on Galena by the FDA or comparable foreign authorities regarding the scope or design of Galena’s clinical trials; |
• | difficulties or delays in arranging for third parties to conduct clinical trials of Galena’s product candidates; |
• | problems in engaging IRBs to oversee trials or problems in obtaining or maintaining IRB approval of studies; |
• | third-party contractors failing to comply with regulatory requirements or meet their contractual obligations to Galena in a timely manner; |
• | Galena’s drug candidates having very different chemical and pharmacological properties in humans than in laboratory testing and interacting with human biological systems in unforeseen, ineffective or harmful ways, and the possibility that Galena’s previous Phase 1 or Phase 2 trials will not be indicative of Galena’s drug candidates’ performance in larger patient populations; |
• | the need to suspend or terminate Galena’s clinical trials if the participants are being exposed to unacceptable health risks; |
• | insufficient or inadequate supply or quality of Galena’s drug candidates or other necessary materials necessary to conduct Galena’s clinical trials; |
• | disruption at Galena’s clinical trial sites resulting from local social or political unrest or other geopolitical factors; |
• | effects of Galena’s drug candidates not having the desired effects or including undesirable side effects or the drug candidates having other unexpected characteristics; |
• | negative or inconclusive results from Galena’s clinical trials or the clinical trials of others for drug candidates similar to Galena’s own or inability to generate statistically significant data confirming the efficacy of the product being tested; |
• | adverse results obtained by other companies developing similar drugs; |
• | modification of the drug during testing; |
• | Galena’s capital resources; and |
• | reallocation of Galena’s financial and other resources to other clinical programs. |
It is possible that none of the product candidates that Galena develops will obtain the appropriate regulatory approvals necessary for Galena to begin selling them or that any regulatory approval to market a product may be subject to limitations on the indicated uses for which Galena may market the product. The time required to obtain FDA and other approvals is unpredictable but often can take years following the commencement of clinical trials, depending upon the complexity of the drug candidate. Any analysis Galena performs of data from clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory
58
approval. Any delay or failure in obtaining required approvals could have a material adverse effect on Galena’s ability to generate revenue from the particular drug candidate.
In addition, the length of time to develop the product candidates as well as any regulatory delays in the development and regulatory approval process could cause the patent exclusivity to be unavailable or greatly reduced for each product candidate. The lack of patent exclusivity could have a material adverse effect on Galena’s ability to generate revenue from the particular drug candidate.
Galena is also subject to numerous foreign regulatory requirements governing the conduct of clinical trials, manufacturing and marketing authorization, pricing and third-party reimbursement. The foreign regulatory approval process includes all of the risks associated with the FDA approval described above as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. Approval by the FDA does not assure approval by regulatory authorities outside of the United States.
Galena is dependent upon contract manufacturers for clinical supplies of Galena’s product candidates.
Galena does not have the facilities or expertise to manufacture supplies of any of Galena’s product candidates for clinical trials. Accordingly, Galena is dependent upon contract manufacturers for these supplies. There can be no assurance that Galena will be able to secure needed supply arrangements on reasonable terms, or at all. There is also no assurance that these contract manufacturers will receive the necessary approval after any FDA inspection of their facilities to continue to manufacture the products for trials or commercial sale. Galena’s failure to secure these arrangements as needed could have a materially adverse effect on Galena’s ability to complete the development of Galena’s product candidates or, if Galena obtains regulatory approval for Galena’s product candidates, to commercialize them.
Galena’s current plans call for the manufacture of Galena’s compounds by contract manufacturers offering research grade, Good Laboratory Practices grade and Good Manufacturing Practices grade materials for preclinical studies (e.g., toxicology studies) and for clinical use. Certain of Galena’s product candidates are complex molecules requiring many synthesis steps, which may lead to challenges with purification and scale-up. These challenges could result in increased costs and delays in manufacturing. For GALE-401, Galena would change contract manufacturers for the supply of the clinical study drug and the commercial product, which may lead to delays in the initiation and completion of the Phase 3 trial and filing for regulatory approval.
In the clinical trials using NeuVax, Leukine is also administered and its availability is dependent upon a third-party manufacturer, which may or may not reliably provide Leukine, thus jeopardizing the completion of the trials.
NeuVax is administered in combination with Leukine, a recombinant human granulocyte macrophage-colony stimulating factor, which is referred to herein as rhGM-CSF or GM-CSF, available in both liquid and lyophilized forms exclusively from Genzyme Corporation, or Genzyme, a subsidiary of Sanofi-Aventis. Galena will continue to be dependent on Genzyme for Galena’s supply of Leukine in connection with the ongoing NeuVax and GALE-301/GALE-302 trials and the potential commercial manufacture of these programs. Galena has not entered into a supply agreement with Genzyme. Any temporary interruptions or discontinuation of the availability of Leukine, or any determination by Galena to change the GM-CSF used with NeuVax or GALE-301/GALE-302, may have a material adverse effect on Galena’s clinical trials and any commercialization of the assets.
Galena may not be able to establish or maintain the third-party relationships that are necessary to develop or potentially commercialize some or all of Galena’s product candidates.
Galena expects to depend on collaborators, partners, licensees, clinical research organizations and other third parties to support Galena’s discovery efforts, to formulate product candidates, to manufacture Galena’s product candidates, and to conduct clinical trials for some or all of Galena’s product candidates. Galena cannot guarantee that Galena will be able to successfully negotiate agreements for or maintain relationships with collaborators, partners, licensees, clinical investigators, vendors and other third parties on favorable terms, if at all. Galena’s ability to successfully negotiate such agreements will depend on, among other things, potential partners’ evaluation of the superiority of Galena’s technology over competing technologies and the quality of the preclinical and clinical data that Galena has generated, and the perceived risks specific to developing Galena’s product candidates. If Galena is unable to obtain or maintain these agreements, Galena may not be able to clinically develop, formulate, manufacture, obtain regulatory approvals for or commercialize Galena’s product candidates. Under certain license
59
agreements that Galena has already entered into, Galena has minimum dollar amounts per year that Galena is obligated to spend on the development of the technology Galena has licensed from Galena’s contract partners and other obligations to maintain certain licenses. If Galena fails to meet this requirement under any of Galena’s licenses that contain such requirements or any other obligations under these licenses, Galena may be in breach of Galena’s obligations under such agreement, which may result in the loss of the technology licensed. Galena cannot necessarily control the amount or timing of resources that Galena’s contract partners will devote to Galena’s research and development programs, product candidates or potential product candidates, and Galena cannot guarantee that these parties will fulfill their obligations to Galena under these arrangements in a timely fashion. Galena may not be able to readily terminate any such agreements with contract partners even if such contract partners do not fulfill its obligations to Galena.
In addition, Galena may receive notices from third parties from time to time alleging that Galena’s technology or product candidates infringe upon the intellectual property rights of those third parties. Any assertion by third parties that Galena’s activities or product candidates infringe upon its intellectual property rights may adversely affect Galena’s ability to secure strategic partners or licensees for Galena’s technology or product candidates or Galena’s ability to secure or maintain manufacturers for Galena’s compounds.
If Galena fails to meet its obligations under Galena’s license agreements, Galena may lose the ability to develop its product candidates
Galena’s business depends on Galena’s ability to license therapeutic compounds from third parties. If Galena fails to meet its obligations under Galena’s license agreements, Galena may lose the ability to develop its product candidates, which would adversely affect Galena’s business.
Galena is subject to competition and may not be able to compete successfully.
The biotechnology industry, including the cancer immunotherapy market, is intensely competitive and involves a high degree of risk. Galena competes with other companies that have far greater experience and financial, research and technical resources than Galena. Potential competitors in the United States and worldwide are numerous and include pharmaceutical and biotechnology companies, educational institutions and research foundations, many of which have substantially greater capital resources, marketing experience, research and development staffs and facilities than Galena. Some of Galena’s competitors may develop and commercialize products that compete directly with those incorporating Galena’s technology, introduce products to market earlier than Galena’s products or on a more cost effective basis. In addition, Galena’s technology may be subject to competition from other technology or methods developed using techniques other than those developed by traditional biotechnology methods. Galena’s competitors compete with Galena in recruiting and retaining qualified scientific and management personnel as well as in acquiring technologies complementary to Galena’s technology. Galena and Galena’s collaborators may face competition with respect to product efficacy and safety, ease of use and adaptability to various modes of administration, acceptance by physicians, the timing and scope of regulatory approvals, availability of resources, reimbursement coverage, price and patent position, including the potentially dominant patent positions of others. An inability to successfully complete Galena’s product development could lead to Galena having limited prospects for establishing market share or generating revenue from Galena’s technology.
GALE-401 must successfully complete a Phase 3 clinical trial and obtain regulatory approval before Galena can market the product and Galena’s competitors may obtain a successful clinical trial result and regulatory approval before Galena does.
GALE-401 contains the active ingredient, anagrelide, an FDA-approved product for the treatment of patients with MPNs, to lower abnormally elevated platelet levels. The currently available immediate release, or IR, version of anagrelide causes adverse reactions that are believed to be dose and plasma concentration dependent. According to the Highlights section of the FDA-approved prescribing information for AGRYLIN (anagrelide hydrochloride) capsules, for oral use (as revised in July 2015), the most common adverse reactions (incidence ≥ 5%) are headache, palpitations, diarrhea, asthenia, edema, nausea, abdominal pain, dizziness, pain, dyspnea, cough, flatulence, vomiting, fever, peripheral edema, rash, chest pain, anorexia, tachycardia, malaise, paresthesia, back pain, pruritus and dyspepsia. These adverse reactions may limit the use of the IR version of the drug. Therefore, reducing the maximum concentration, or Cmax, is hypothesized to reduce the adverse reactions, while preserving efficacy, potentially allowing broader use of the drug. Galena has analyzed data from multiple Phase 1 and 2 GALE-401 clinical trials and the treatment landscape for MPNs, with a current focus on ET, where Galena sees an unmet medical need in patients who are intolerant to the current standard of care. The risks include but are not
60
limited to regulatory (agreement with regulatory agency on the development plan), operational (rate of enrollment), and statistical confirmation of the safety and efficacy endpoints. In addition, pursuant to the terms of the Exclusive License Agreement with BioVascular, Inc., or BVI, dated December 20, 2013, or the BVI License Agreement, if the first patient is not enrolled in the Phase 3 clinical trial by December 31, 2018, BVI shall have the right to terminate the BVI License Agreement. Even if Galena successfully completes a Phase 3 trial, there are other potential competitors whose clinical trials may be successful and obtain regulatory approval prior to Galena’s regulatory approval.
Galena is dependent on technologies Galena licenses, and if Galena loses the right to license such technologies or fails to license new technologies in the future, Galena’s ability to develop new products would be harmed.
Galena currently is dependent on licenses from third parties for technologies relating to Galena’s product candidates. Galena’s current licenses impose, and any future licenses Galena enters into are likely to impose, various development, funding, royalty, diligence, sublicensing, insurance and other obligations on Galena. If Galena’s license with respect to any of these technologies is terminated for any reason, the development of the products contemplated by the licenses would be delayed, or suspended altogether, while Galena seeks to license similar technology or develop new non-infringing technology. The costs of obtaining new licenses are high.
Risks associated with operating in foreign countries could materially adversely affect Galena’s product development.
Galena may conduct future studies in countries outside of the United States. Consequently, Galena may be subject to risks related to operating in foreign countries. Risks associated with conducting operations in foreign countries include:
• | differing regulatory requirements for drug approvals and regulation of approved drugs in foreign countries; |
• | unexpected changes in tariffs, trade barriers and regulatory requirements; economic weakness, including inflation, or political instability in particular foreign economies and markets; compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; foreign taxes, including withholding of payroll taxes; |
• | foreign currency fluctuations, which could result in increased operating expenses or reduced revenues, and other obligations incident to doing business or operating in another country; |
• | workforce uncertainty in countries where labor unrest is more common than in the United States; |
• | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
• | business interruptions resulting from geopolitical actions, including war and terrorism. |
Risks Related to Galena’s Financial Position and Capital Requirements
Galena is conducting a review of strategic alternatives for Galena, including evaluating potential paths forward for NeuVax, GALE-301/GALE-302 and GALE-401 that could significantly impact Galena’s future operations and financial position.
In January 2017, Galena announced that the Galena Board had initiated a review of strategic alternatives that could result in changes to Galena’s business strategy and future operations, which has resulted in the Merger. If the Merger is completed, GALE-401 and GALE-301/GALE-302 will be evaluated for potential internal development or strategic partnership by management of the continuing company post-Merger. Whether or not Galena completes the Merger, if Galena determines to further develop NeuVax, GALE-301/GALE-302 and GALE-401, or all of the products, Galena will need to obtain substantial additional funding. Such funding is constrained by the disqualifications to use Regulation A and Regulation D of the SEC regulations arising from the cease and desist order issued by the SEC on April 10, 2017 in the stock promotion practices investigation. In addition, Galena’s current ineligibility to use a Form S-3 registration statement currently limits Galena’s ability to raise capital.
61
Galena may not be able to obtain sufficient financing, and may not be able to develop Galena’s product candidates.
Galena had cash and cash equivalents of approximately $12.9 million as of September 30, 2017. Galena had no revenue during the nine months ended September 30, 2017, and Galena’s cash burn from operations for the nine months ended September 30, 2017 was approximately $26.2 million. Galena believes that Galena’s existing cash and cash equivalents should be sufficient to fund Galena’s operations for at least six months from November 9, 2017 the date of issuance of Galena’s consolidated financial statements for the quarter ended September 30, 2017. This projection is based on Galena’s current limited planned operations (not taking into account certain expenses to be paid only if the Merger is completed), anticipated payments for defense costs for the governmental investigation, payments of the resolution of the SEC investigation and the Abstral governmental investigation, estimates of the defense costs to defend certain securities litigation and other matters, and Galena’s impaired ability to raise funds. The projection is also subject to changes in Galena’s operating plans, transaction costs related to the closing of the Merger, unanticipated developments and other legal matters and uncertainties inherent in Galena’s business. Galena expects that it will need to seek to replenish its existing cash and cash equivalents through dilutive and non-dilutive financings over this calendar year.
In addition, Galena had approximately $12.0 million of restricted cash associated with a debenture as of September 30, 2017. Galena cannot currently use the Purchase Agreement with Lincoln Park Capital, LLC, or LPC, and its At The Market Issuance Sales Agreements with FBR & Co. (formerly MLV & Co. LLC) and Maxim Group LLC which are referred to collectively as the ATM, because Galena is not currently eligible to use a Form S-3 registration statement and it does not expect to be eligible to use a Form S-3 registration statement until May 1, 2018. However, even if Galena becomes eligible to use a Form S-3 registration statement as of May 1, 2018, unless the market value of Galena Common Stock held by non-affiliates of Galena increases to $75 million, Galena will be limited in the amounts it may sell under Form S-3.
As discussed above, Galena received a notice of delisting from NASDAQ on March 24, 2017 because Galena’s stock price was below the required $1.00 per share for more than 30 consecutive trading days. Galena is not able to predict whether Galena’s stock price and/or the volume of shares post Reverse Stock Split will be sufficient based upon the current stock price to meet Galena’s ongoing financing requirements to maintain Galena’s operations.
If Galena fails to obtain additional future funding, Galena could be forced to scale back or terminate its operations. Galena may not be able to meet its obligations as they come due, which would raise substantial doubts as to Galena’s ability to continue as a going concern. Any such inability to continue as a going concern may result in Galena stockholders losing their entire investment. Whether or not the Merger is completed, there is no guaranty that Galena will become profitable or secure additional financing.
Galena expects to continue to incur significant operating and non-operating expenses, which may make it difficult for Galena to secure sufficient financing, and may lead to uncertainty about Galena’s ability to continue as a going concern.
Substantial funds were expended to develop Galena’s technologies and product candidates, and additional substantial funds will be required for further clinical trials of Galena’s product candidates, and to manufacture and market any products that are approved for commercial sale. Because the successful development of Galena’s products is uncertain, Galena is unable to precisely estimate the actual funds Galena will require to develop and potentially commercialize them. In addition, Galena may not be able to generate enough revenue, even if Galena is able to commercialize any of Galena’s product candidates, to become profitable.
In the event that Galena is unable to obtain additional financing as needed or if Galena incurs significant expense related to, among other things, transaction costs related to the closing of the Merger, and defense of the putative shareholder class-action and derivative complaints, Galena may not be able to meet Galena’s obligations as they come due, that in turn may raise substantial doubts as to Galena’s ability to continue as a going concern. Any such inability to continue as a going concern may result in holders of Galena Common Stock losing their entire investment. There is no assurance that Galena will secure additional financing. Galena’s financial statements contemplate that Galena will continue as a going concern and do not contain any adjustments that might result if Galena were unable to continue as a going concern. Changes in Galena’s operating plans, Galena’s existing and anticipated working capital needs, transaction costs related to the closing of the Merger, defense costs related to the recent securities and derivative lawsuits, resolution of the ongoing government investigation, increased expenses, Galena’s current inability to use a Form S-3 registration statement and even if Galena could use a Form S-3
62
registration statement limitations on the amounts that Galena could sell on a Form S-3 due to Galena’s low market capitalization, potential acquisitions, or other events may affect Galena’s ability to continue as a going concern. Future financing may be obtained through, and future development efforts may be paid for by, the issuance of debt or equity, which may have an adverse effect on Galena stockholders or may otherwise adversely affect Galena’s business.
If Galena raises funds through the issuance of debt or equity, any debt securities or preferred stock issued will have rights, preferences and privileges senior to those of holders of Galena Common Stock in the event of a liquidation. In such event, there is a possibility that once all senior claims are settled, there may be no assets remaining to pay out to the holders of Galena Common Stock. In addition, if Galena raises funds through the issuance of additional equity, whether through private placements or additional public offerings, such an issuance would dilute your ownership in Galena.
The terms of debt securities may also impose restrictions on Galena’s operations, which may include limiting Galena’s ability to incur additional indebtedness, to pay dividends on or repurchase Galena Common Stock, or to make certain acquisitions or investments. In addition, Galena may be subject to covenants requiring Galena to satisfy certain financial tests and ratios, and Galena’s ability to satisfy such covenants may be affected by events outside of Galena’s control.
You may have difficulty evaluating Galena’s business, and Galena’s historical financial information may not be representative of Galena’s future results.
Galena has closed its NeuVax PRESENT trial due to futility and plans to continue to support the NeuVax investigator-sponsored immunotherapy trials. For GALE-401, Galena has completed a Phase 2 trial of GALE-401 in patients with essential thrombocythemia, or ET. If the Merger is completed, GALE-401 will be evaluated for potential internal development or strategic partnership by management of the continuing company post-Merger. As a result, Galena will have no recurring revenues unless and until Galena is able to obtain marketing approval of one or more of Galena’s other product candidates and Galena’s historical financial information may not be representative of Galena’s future results.
Galena may be unable to comply with reporting and other requirements under federal securities laws.
As a publicly traded company, Galena is subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act. In addition, the Exchange Act requires that Galena file annual, quarterly and current reports. Galena’s failure to prepare and disclose this information in a timely manner could subject Galena to penalties under federal securities laws, expose Galena to lawsuits and restrict Galena’s ability to access financing. The Sarbanes-Oxley Act requires that Galena, among other things, establishes and maintains effective internal controls over financial reporting. From time to time Galena evaluates its existing internal controls in light of the standards adopted by the Public Company Accounting Oversight Board. It is possible that Galena, or Galena’s independent registered public accounting firm, may identify significant deficiencies or material weaknesses in Galena’s internal control over financial reporting in the future. Any failure or difficulties in implementing and maintaining these controls could cause Galena to fail to meet the periodic reporting obligations or result in material misstatements in Galena’s financial statements.
Intellectual Property Risks Related to Galena’s Business
Galena may not be able to obtain and enforce patent rights or other intellectual property rights that cover Galena’s product candidates and that are of sufficient breadth to prevent third parties from competing against Galena.
Galena’s success with respect to its product candidates will depend in part on its ability to obtain and maintain patent protection in the United States and abroad, to preserve Galena’s trade secrets, and to prevent third parties from infringing upon Galena’s proprietary rights. Galena’s patents and patent applications, however, may not be sufficient to provide protection for NeuVax or Galena’s other product candidates against commercial competition.
The active peptide found in NeuVax, the E75 peptide, has been known and studied for many years. Galena has one issued U.S. patent, US 6,514,942, covering the composition of matter of the E75 peptide, which expired in mid-2015, prior to any potential commercialization of NeuVax. Galena does not have and will not be able to obtain any composition of matter patent protection for E75. Galena also has a license from The Henry M. Jackson Foundation
63
for the Advancement of Military Medicine to issued U.S., European, Japanese, Korean, Mexican and Australian method of use patents, which expire in 2028, that are directed to a method of inducing immunity against breast cancer recurrence by administering a composition comprising the E75 peptide to patients who have both an immunohistochemistry, or IHC, rating of 1+ or 2+ for HER2/neu protein expression, as well as a fluorescence in situ hybridization, or FISH, rating of less than about 2.0 for HER2/neu gene expression. The license further includes an issued U.S. method of use patent directed to a method of inducing immunity against recurrence of any HER2/neu expressing tumors by administering the E75 peptide to patients with tumors having a FISH rating of less than about 2.0 for HER2/neu gene expression; an issued U.S. patent which includes claims to the use of E75 to reduce the risk of cancer recurrence, including bone only recurrence; and pending applications with similar claims in a number of foreign jurisdictions, all of which expire in 2028. Also included in the license is a method of use patent, which expires in 2026, that is directed to the use of NeuVax in combination with trastuzumab (Herceptin; Genentech/Roche) to treat any HER2/neu expressing cancer. Thus, Galena’s method of use patents may not prevent competitors from seeking to develop and market NeuVax for use in cancer patients who do not meet these criteria. If any such alternative uses were approved, this could lead to off-label use and price erosion for Galena’s NeuVax product. Galena may seek FDA approval for use of NeuVax to treat cancer patients who fall outside the claimed IHC and FISH ranges and for other cancers as well. Although Galena is pursuing additional patent protection for NeuVax through pending patent applications, Galena may not be able to obtain additional patent protection that would provide Galena with a significant commercial advantage.
Anagrelide hydrochloride, the sole active pharmaceutical ingredient, or API, in GALE-401, has been approved for many years and, thus, it is not possible to obtain composition of matter patents that cover anagrelide hydrochloride. As a result, competitors who obtain the requisite regulatory approval can offer products with the same API as GALE-401, so long as the competitors do not infringe any formulation patents that Galena may have or may obtain or license, if any. The only patent protection that Galena has or is likely to obtain covering GALE-401 are patents relating to specific formulations, methods using these formulations, and methods of manufacturing and packaging. Galena has an issued U.S. Patent, which expires in 2020, covering methods of using anagrelide to reduce platelet count in patients subject to veno-occlusive events. Galena has granted patents in the United States, United Kingdom and Japan, which expire in 2029, covering controlled release formulations of anagrelide and methods of use. Galena also is prosecuting pending patent applications in other territories including, but not limited to, the United States, Europe, India, and Japan, which may not issue prior to any potential commercialization of GALE-401. Galena may seek FDA approval for use of GALE-401 to treat patients with MPNs that include several hematological disorders, including ET. Although Galena is pursuing additional patent protection for GALE-401 through pending patent applications, Galena may not be able to obtain additional patent protection that would provide Galena with a significant commercial advantage.
The active peptides found in GALE-301 and GALE-302 are derived from folate binding protein, or FBP. One of the active peptides, E39, has been known and studied for many years. The other active peptide, J65, is a derivative of E39. Galena has a license from The Henry M. Jackson Foundation to issued and granted patents in the United States, Europe, Canada, and Japan, covering composition of matter for the E39 and J65 peptides alone and in combination with E39, as well as the use of these compositions for the treatment of cancer. These patents are expected to expire in 2022, prior to any potential commercialization of GALE-301. Galena also has pending U.S. and International (PCT) applications with claims to combination dosage regimens of GALE-301 and GAL-302, which, if granted, would expire in 2036. Galena also has pending U.S. and International (PCT) applications with claims to methods of inducing an immune response to tumors with an IHC rating of 0 or 1+ for folate binding protein expression which, if granted, would expire in 2037. Galena does not have and will not be able to obtain any composition of matter patent protection for the E39 peptide in any territory. The license Galena has from The Henry M. Jackson Foundation grants Galena the right to develop and market GALE-301 for any use, including methods of treating cancer. Galena’s patents may not prevent competitors from seeking to develop and market the E39 peptide alone. If any such alternative uses of compositions containing the E39 peptide were approved, this could lead to off label use and price erosion for GALE-301. Galena may seek FDA approval for use of GALE-301, alone or in combination with GALE-302, to treat cancer patients with ovarian and endometrial cancers and for other cancers as well. Although Galena is pursuing additional patent protection for GALE-301 and the combination of GALE-301 and GALE-302 through pending patent applications, Galena may not be able to obtain additional patent protection that would provide Galena with a significant commercial advantage.
Galena’s ability to obtain, maintain and enforce patents is uncertain and involves complex legal and factual questions. Accordingly, rights under any patents Galena has or may obtain or license may not provide Galena with sufficient protection for Galena’s commercial product and product candidates to afford a commercial advantage against competitive products or processes, including those from branded and generic pharmaceutical companies. In
64
addition, Galena cannot guarantee that any patents will issue from any pending or future patent applications owned by or licensed to Galena. Nor can Galena guarantee that the claims of these patents will be held valid or enforceable by the courts or will provide Galena with any significant protection against competitive products or otherwise be commercially valuable to Galena.
Changes in either the patent laws or in the interpretations of patent laws in the United States or abroad may diminish the value of Galena’s intellectual property. In addition, on September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to the U.S. patent law. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of Galena’s business. However, the Leahy-Smith Act, in particular the first-to-file provision and its implementation could increase the uncertainties and costs surrounding the prosecution of Galena’s patent applications and the enforcement of or defense of Galena’s issued patents, all of which could have a material adverse effect on Galena’s business and financial condition. Accordingly, Galena cannot predict the breadth of claims that may be allowed or enforced in Galena’s patents or in third-party patents.
While Galena intends to take actions reasonably necessary to enforce Galena’s patent rights, Galena may not be able to detect infringement of Galena’s own or in-licensed patents, which may be especially difficult for methods of manufacturing or formulation products, and Galena depends, in part, on Galena’s licensors and collaborators to protect a substantial portion of Galena’s proprietary rights. In addition, third parties may challenge Galena’s in-licensed patents and any of Galena’s own patents that Galena may obtain, which could result in the invalidation or unenforceability of some or all of the relevant patent claims. Litigation or other proceedings to enforce or defend intellectual property rights is very complex, expensive, and may divert Galena management’s attention from Galena’s core business and may result in unfavorable results that could adversely affect Galena’s ability to prevent third parties from competing with Galena.
If another party has reason to assert a substantial new question of patentability against any of Galena’s claims in Galena’s own and in-licensed patents, the third party can request that the patent claims be reexamined, which may result in a loss of scope of some claims or a loss of the entire patent. In addition to potential infringement suits and, interference and reexamination proceedings, Galena may become a party to patent opposition proceedings where either the patentability of the inventions subject of Galena’s patents are challenged, or Galena is challenging the patents of others. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful. As the medical device, biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that others may assert Galena’s commercial product and/or product candidates infringe their patent rights. If a third-party’s patents were found to cover Galena’s commercial product and product candidates, proprietary technologies or its uses, Galena or Galena’s collaborators could be enjoined by a court and required to pay damages and could be unable to continue to commercialize Galena’s products or use Galena’s proprietary technologies unless Galena obtained a license to the patent. A license may not be available to Galena or Galena’s collaborators on acceptable terms, or at all. In addition, during litigation, the patent holder could obtain a preliminary injunction or other equitable relief, which could prohibit Galena from making, using or selling Galena’s commercial product and product candidates pending a trial on the merits, which could be years away.
Proprietary trade secrets and unpatented know-how are also very important to Galena’s business. Although Galena has taken steps to protect Galena’s trade secrets and unpatented know-how, by entering into confidentiality agreements with third parties, and proprietary information and invention agreements with certain employees, consultants and advisors, third parties may still obtain this information or Galena may be unable to protect Galena’s rights. Galena also has limited control over the protection of trade secrets used by Galena’s licensors, collaborators and suppliers. There can be no assurance that binding agreements will not be breached, that Galena would have adequate remedies for any breach, or that Galena’s trade secrets and unpatented know-how will not otherwise become known or be independently discovered by Galena’s competitors. If trade secrets are independently discovered, Galena would not be able to prevent their use. Enforcing a claim that a third party illegally obtained and is using Galena’s trade secrets or unpatented know-how is expensive and time consuming, and the outcome is unpredictable.
Galena may be subject to claims that Galena’s employees, consultants or independent contractors have wrongfully used or disclosed to Galena alleged trade secrets of their other clients or former employers. As is common in the biotechnology and pharmaceutical industry, certain of Galena’s employees were formerly employed by other biotechnology or pharmaceutical companies, including Galena’s competitors or potential competitors. Moreover, Galena engages the services of consultants to assist Galena in the development of Galena’s commercial product
65
and product candidates, many of whom were previously employed at or may have previously been or are currently providing consulting services to, other biotechnology or pharmaceutical companies, including Galena’s competitors or potential competitors. Galena may be subject to claims that these employees and consultants or Galena has inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers or their former or current customers. Litigation may be necessary to defend against these types of claims. Even if Galena is successful in defending against any such claims, any such litigation would likely be protracted, expensive, a distraction to Galena’s management team, not viewed favorably by investors and other third parties, and may potentially result in an unfavorable outcome.
Galena’s product candidates may face competition sooner than expected after the expiration of Galena’s composition of matter patent protection for such products.
Galena’s composition of matter patents for many of Galena’s product candidates have expired or will expire prior to any product approval. Galena intends to seek data exclusivity or market exclusivity for Galena’s NeuVax as well as Galena’s GALE-301 and GALE-302 product candidates provided under the Federal Food, Drug and Cosmetic Act, or FDCA, and similar laws in other countries. Galena believes that these product candidates will qualify for 12 years of data exclusivity under the Biologics Price Competition and Innovation Act of 2009, or BPCIA, which was enacted as part of the Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010, which are referred to collectively as the Affordable Care Act or ACA, enacted in March 2010. Under the BPCIA, an application for a biosimilar product or biologics license application, which is referred to herein as BLA, cannot be submitted to the FDA until four years, or if approved by the FDA, until 12 years, after the original brand product identified as the reference product is approved under a BLA. The BPCIA provides an abbreviated pathway for the approval of biosimilar and interchangeable biological products. The new abbreviated regulatory pathway establishes legal authority for the FDA to review and approve biosimilars, including the possible designation of a biosimilar as “interchangeable” based on its similarity to an existing brand product. The new law is complex and is only beginning to be interpreted and implemented by the FDA. While it is uncertain when any such processes may be fully adopted by the FDA, any such processes could have a material adverse effect on the future commercial prospects for Galena’s biological product candidates. There is also a risk that the U.S. Congress could amend the BPCIA to shorten this exclusivity period, potentially creating the opportunity for biosimilar competition sooner than anticipated after the expiration of Galena’s patent protection. Moreover, the extent to which a biosimilar, once approved, will be substituted for any reference product in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
If Galena’s product candidates are not considered biologics that would qualify for exclusivity under the BPCIA, they may be eligible for market exclusivity as drugs under the FDCA. The FDCA provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to gain approval of a new drug application, or NDA, for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA, submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages, or strengths of an existing drug. This three-year exclusivity covers only the conditions associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent.
Even if, as Galena expects, NeuVax, GALE-301 and GALE-302 are considered to be reference products eligible for 12 years of exclusivity under the BPCIA or five years of exclusivity under the FDCA, another company could market competing products if the FDA approves a full BLA or full NDA for such product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of the products.
In some countries outside of the United States, peptide vaccines, such as NeuVax, GALE-301 and GALE-302, are regulated as chemical drugs rather than as biologics and may or may not be eligible for non-patent exclusivity.
66
Although Galena has received Orphan Drug designation for both GALE-301, as well as the combination of GALE-301 and GALE-302, there is no guarantee that the drugs will be successfully approved by the FDA, that they will be commercially successful in the marketplace, or that another drug will not be approved for the same indication ahead of Galena’s drugs.
Significant disruptions of information technology systems or breaches of information security could adversely affect Galena’s business.
Galena relies to a large extent upon sophisticated information technology systems to operate its business. In the ordinary course of business, Galena collects, stores and transmits large amounts of confidential information (including, but not limited to, personal information and intellectual property). Galena also has outsourced significant elements of its operations to third parties, including significant elements of Galena’s information technology infrastructure and, as a result, Galena is managing many independent vendor relationships with third parties who may or could have access to Galena’s confidential information. The size and complexity of Galena’s information technology and information security systems, and those of Galena’s third-party vendors with whom Galena contracts (and the large amounts of confidential information that is present on them), make such systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by Galena’s employees or vendors, or from malicious attacks by third parties. Such attacks are of ever-increasing levels of sophistication and are made by groups and individuals with a wide range of motives (including, but not limited to, industrial espionage and market manipulation) and expertise. While Galena has invested significantly in the protection of data and information technology, there can be no assurance that Galena’s efforts will prevent service interruptions or security breaches. Any interruption or breach in Galena’s systems could adversely affect Galena’s business operations and/or result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal, business and reputational harm to Galena or allow third parties to gain material, inside information that they use to trade in Galena’s securities.
Risks Related to Ownership of the Galena Common Stock
Galena Common Stock trades at prices less than $1.00, which is the minimum bid price requirement under NASDAQ’s continued listing standards, as such Galena Common Stock may be subject to delisting from the Nasdaq Capital Market.
As noted above, Galena is not in compliance with the NASDAQ Listing Rule 5550(a)(2), which requires the Galena Common Stock to have a minimum bid price of at least $1.00 per share. The closing bid price of the Galena Common Stock on NASDAQ was $0.30 on November 8, 2017. Galena has been granted a second 180-day grace period, or until March 19, 2018, to regain compliance with the $1.00 per share minimum bid price requirement. As discussed above, if Galena does not regain compliance with the minimum bid price requirement by March 19, 2018, NASDAQ will provide written notification to Galena that the Galena Common Stock will be delisted, subject to certain appeal rights that Galena may pursue. Galena will continue to monitor the closing bid price for its common stock and consider its available options to regain compliance with the NASDAQ minimum bid requirements, including the Reverse Stock Split and the Merger.
On January 18, 2017, Galena received an inquiry from the NASDAQ Listing Qualifications Department seeking information in connection with the subpoenas issued to Galena by the U.S. Attorney’s Office for the District of New Jersey and the U.S. Attorney’s Office for the Southern District of Alabama. Galena responded to the inquiry on February 1, 2017 and March 31, 2017. Galena has not received any follow-up inquiries from NASDAQ on this matter. If Galena is not in compliance with the listing requirements under NASDAQ Listing Rule 5502(a)(2), Galena could be delisted by NASDAQ.
On May 17, 2017, Galena received an inquiry from the NASDAQ Listing Qualifications Department seeking information in connection with the settlement with the SEC. Galena responded to the inquiry on June 13, 2017. Galena has not received any follow-up inquiries from NASDAQ on this matter. If Galena is not in compliance with the listing requirements under NASDAQ Listing Rule 5502(a)(2), Galena could be delisted by NASDAQ.
If Galena Common Stock is delisted from the Nasdaq Capital Market in the future, such securities may be traded over-the-counter on the “pink sheets.” Such alternative market, however, is generally considered to be less efficient than, and not as broad as, NASDAQ. Accordingly, delisting of Galena Common Stock from NASDAQ could have a significant negative effect on the trading volume, liquidity and market price of Galena Common Stock. In addition,
67
the delisting of Galena Common Stock could adversely affect Galena’s ability to raise capital on terms acceptable or at all and could reduce the number of investors willing to hold or acquire Galena Common Stock.
The market price and trading volume of Galena Common Stock may be volatile.
The market price of Galena Common Stock has exhibited substantial volatility recently. Between January 1, 2017 and November 8, 2017, the sale price of Galena Common Stock as reported on NASDAQ ranged from a low of $0.26 to a high of $2.41. The market price of Galena Common Stock could continue to fluctuate significantly for many reasons, including the following factors:
• | reports of the results of Galena’s clinical trials regarding the safety or efficacy of Galena’s product candidates and surrogate markers; |
• | announcements of regulatory developments or technological innovations by Galena or Galena’s competitors; |
• | announcements of business or strategic transactions or Galena’s success in finalizing such a transaction; |
• | announcements of legal or regulatory actions against Galena or any adverse outcome of any such actions; |
• | changes in Galena’s relationships with its licensors, licensees and other strategic partners; |
• | Galena’s quarterly operating results; |
• | announcements of additional potential reverse stock splits; |
• | developments in patent or other technology ownership rights; |
• | additional funds may not be available on terms that are favorable to Galena and, in the case of equity financings, may result in dilution to Galena stockholders; |
• | government regulation of drug pricing; and |
• |
• | general changes in the economy, the financial markets or the pharmaceutical or biotechnology industries. |
Factors beyond Galena’s control may also have an impact on the price of Galena Common Stock. For example, to the extent that other companies within Galena’s industry experience declines in their stock prices, the price of Galena Common Stock may decline as well.
Galena is, and in the future may be, subject to legal or administrative actions that could adversely affect Galena’s business financial condition and Galena’s business.
In early 2014, Galena and certain of its former officers and certain of its current and former directors had been the subject of shareholder derivative complaints in Oregon state court alleging, among other things, breaches of fiduciary duties and abuse of control by such officers and directors in connection with public statements purportedly issued by Galena or on its behalf and sales of the Galena Common Stock by such officers and directors, improper stock option grants, and excessive compensation of Galena’s non-employee directors. These cases were settled in June 2016.
In early 2014, Galena and certain of its former and current directors, current employee and former officers had been the subject to class action lawsuits in federal court alleging, among other things, that Galena and certain of Galena’s former and current directors, current employee and former officers violated the federal securities laws by making materially false and misleading statements and omissions in press releases and in filings with the SEC and that certain of Galena’s former and current directors and former officers sold Galena Common Stock while in possession of material non-public information. These cases were settled in June 2016.
In September 2015, a federal securities lawsuit was filed alleging, among other things, that Galena and certain of its former officers, its current and former directors and current employee violated Sections 10(b), 20(a) and 20A of the Exchange Act by launching a pump and dump scheme even though Galena and certain of its former and current directors, former officers and current employee knew the price of the Galena Common Stock would decline in the wake of the announcement that its drug NeuVax, which it claimed would prevent the recurrence of breast cancer, would not be approved by the FDA. This case was settled in July 2016.
68
On December 16, 2015, Galena received a subpoena issued by the U.S. Attorney’s Office for the District of New Jersey, or USAO NJ, requesting the production of a broad range of documents pertaining to Galena’s marketing and promotional practices for Abstral, which Galena had sold to a third party in the fourth quarter of 2015. In September 2017, the Department of Justice announced that Galena is to pay approximately $7.6 million to resolve allegations under the civil False Claims Act that it paid kickbacks to doctors to induce them to prescribe its fentanyl-based drug Abstral.
In January 2016, Galena and its former chief executive officer and director were threatened with a lawsuit by the former owners of Mills Pharmaceuticals, LLC, Galena’s wholly owned subsidiary, or Mills, alleging, among other things, breaches of the Purchase Agreement relating to the purchase of Mills by Galena and the representations and warranties contained therein, as well as certain alleged violations of Section 10(b) of the Exchange Act and Rule 10b-5 promulgated thereunder, and alleged violations of Section 20(a) of the Exchange Act. In July 2016, Galena and its former chief executive officer and director resolved the claims with the former owners of Mills.
On February 13, 2017, putative shareholder securities class action complaints were filed in federal court alleging, among other things, that Galena and certain of its former officers and directors and current employee failed to disclose that Galena’s promotional practices for Abstral (fentanyl) sublingual tablets were allegedly improper and that Galena may be subject to civil and criminal liability, and that these alleged failures rendered Galena’s statements about its business misleading. The actions were consolidated, a lead plaintiff was named by the court and an amended complaint was filed. This lawsuit is ongoing.
On March 16, 2017, a derivative complaint was filed in the federal court against Galena’s current directors and Galena, as a nominal defendant. The complaint purports to assert derivative claims for breach of fiduciary duty on Galena’s behalf against its directors based on substantially similar facts as alleged in the putative shareholder securities class action complaints filed in February 2017 mentioned above. This lawsuit is currently stayed pending resolution of the referenced securities class action lawsuits.
In April 2017, a putative shareholder class action was filed in Delaware state court alleging breaches of Section 225 of the DGCL and breaches of fiduciary duties by the Galena Board regarding the voting results of authorized share and the reverse stock split proposals in the proxy statements for the July 2016 and October 2016 Galena stockholder meetings. On July 24, 2017, Galena entered into a binding settlement term sheet involving the payment of $50,000 in cash and $1,250,000 in unrestricted shares of Galena Common Stock. The parties planned to use the term sheet to enter into a Stipulation of Settlement. The Stipulation of Settlement will not become effective until approved by the Court. Due to the decline in the price of the Galena Common Stock, the plaintiff has demanded to renegotiate the binding settlement term sheet. On September 7, 2017, Galena moved to enforce the binding settlement term sheet. The parties have filed supporting briefs and the hearing will be held on November 30, 2017.
On April 10, 2017, the SEC issued a cease and desist order against Galena and the former chief executive officer, or CEO, Mark Ahn, requiring each of them to cease and desist from any future violations of Sections 5(a), 5(b), 5(c), 17(a), and 17(b) of the Securities Act of 1933, as amended, or the Securities Act, and Section 10(b), 13(a), and 13(b)(2)(A) of the Exchange Act, and various rules thereunder, which refer to as the SEC Order. Based upon the order, Galena made a $200,000 penalty payment as well as a payment of approximately $750,000, which was the indemnification payment of Galena’s former CEO for the disgorgement and prejudgment interest payment that he was required to pay by the order. Galena made such indemnification payment after a special committee of the Galena Board determined that Galena was required under Delaware law to indemnify Galena’s former CEO for the disgorgement and prejudgment interest payment. The former CEO also made a penalty payment of $600,000. As a result of the SEC Order, Galena may not use certain exemptions from registration under the federal securities laws, including Regulation A and Regulation D. In addition, Galena is an “ineligible issuer” as the term is defined under Rule 405 promulgated under the Securities Act.
On July 2017, a complaint was filed in California state court against Galena’s current and former directors and Galena, as a nominal defendant. The complaint purports to assert derivative claims for breach of fiduciary duty on Galena’s behalf against its directors based on substantially similar facts as alleged in the derivative complaint filed in early March of 2017 mentioned above. This lawsuit is currently stayed pending resolution of the referenced securities class action lawsuits.
Galena may be subject to legal or administrative actions in the future, which could result in the payment of additional amounts and have a material adverse effect on Galena’s financial condition and results of operations. If Galena is found to be in violation of the False Claims Act, Anti-Kickback Statute, Patient Protection and Affordable Care Act,
69
federal or state securities laws, or any other applicable state or any federal fraud and abuse laws, Galena may be subject to penalties, such as civil and criminal penalties, damages, fines, or an administrative action of exclusion from government health care reimbursement programs or by the SEC. Galena can make no assurances as to the time or resources that will need to be devoted to these matters or their outcome, or the impact, if any, that these matters or any resulting legal or administrative proceedings may have on Galena’s business or financial condition but any further action in respect of any such matter by a governmental agency could have a material adverse effect on Galena’s results of operation and Galena’s business and prospects.
The settlement of these lawsuits, threatened lawsuits, investigations and administrative actions has resulted in substantial payments, some of which have not been covered by Galena’s insurance policies. Galena may continue to incur substantial unreimbursed legal fees and other expenses in connection with these or other legal and regulatory proceedings that may not qualify for coverage under, or may exceed the limits of, Galena’s applicable directors and officers liability insurance policies and could have a material adverse effect on Galena’s financial condition, liquidity, and results of operations. These matters also may distract the time and attention of Galena’s officers and directors or divert Galena’s other resources away from Galena’s ongoing development programs. An unfavorable outcome in any of these matters could damage Galena’s business and reputation or result in additional claims or proceedings against Galena.
For additional information on these legal matters, please see Note 6 (Legal Proceedings, Commitments and Contingencies) to the condensed consolidated financial statements contained in Part I, Item 1 (Financial Statements) of this Quarterly Report on Form 10-Q.
Galena has in the past and expects in the future to settle legal claims through the issuance of freely tradable Galena Common Stock, which will result in dilution to holders of Galena Common Stock and may adversely affect the market price of the Galena Common Stock.
Galena has in the past and expects in the future to settle legal claims through the issuance of freely tradable Galena Common Stock. As described in Note 6 (Legal Proceedings, Commitments and Contingencies) to the condensed consolidated financial statements contained in Part I, Item 1 (Financial Statements) of this Quarterly Report on Form 10-Q. Galena currently expects to issue $1,250,000 in unrestricted shares of Galena Common Stock valued at based on the volume-weighted average closing price for the 20 trading days immediately preceding the day before the transfer of the settlement stock to the settlement fund to settle the case captioned, Patel vs. Galena Biopharma, Inc. et. al. Payment of this amount in Galena Common Stock will cause significant dilution to Galena’s stockholders, and the amount of that dilution will vary depending on the price of the Galena Common Stock during the 20 trading days prior to such payment. In addition, the issuance of such a significant number of shares of Galena Common Stock may cause a decrease in the trading price of the Galena Common Stock.
Future sales of substantial amounts of Galena Common Stock, or the possibility that such sales could occur, could adversely affect the market price of Galena Common Stock.
Future sales in the public market of shares of Galena Common Stock, including shares referred to in the foregoing risk factors or shares issued upon exercise of Galena’s outstanding stock options or warrants, or the perception by the market that these sales could occur, could lower the market price of Galena Common Stock or make it difficult for Galena to raise additional capital.
As of November 8, 2017, Galena had reserved for issuance 443,272 shares of Galena Common Stock issuable upon the exercise of outstanding stock options at a weighted-average exercise price of $31.23 per share and 19,556,851 shares of Galena Common Stock issuable upon the exercise of outstanding warrants at a weighted-average exercise price of $4.62 per share. Upon exercise of these options and warrants, the underlying shares may be resold into the public market. In the case of outstanding options and warrants that have exercise prices that are below the market price of Galena Common Stock from time to time, Galena stockholders would experience dilution upon the exercise of these options.
Galena’s outstanding warrants may result in dilution to Galena stockholders.
Galena’s outstanding December 2012 warrants to purchase 151,565 shares of common stock as of November 8, 2017 with an exercise price as of that date of $10.32 per share contain so-called weighted-average anti-dilution provisions. These anti-dilution provisions may be triggered by the issuance of the shares being offered hereby or
70
upon any future issuance by Galena of shares of Galena Common Stock or common stock equivalents at a price per share below the then-exercise price of the warrants, subject to some exceptions.
To the extent that these anti-dilution provisions are triggered in the future, Galena would be required to reduce the exercise price of all of the warrants on either a full-ratchet or weighted-average basis, which would have a dilutive effect on Galena stockholders.
Galena may issue preferred stock in the future, and the terms of the preferred stock may reduce the value of Galena Common Stock.
Galena is authorized to issue up to five million shares of preferred stock in one or more series. The Galena Board may determine the terms of future preferred stock offerings without further action by Galena stockholders. If Galena issues preferred stock, it could affect stockholder rights or reduce the market value of Galena’s outstanding common stock. In particular, specific rights granted to future holders of preferred stock may include voting rights, preferences as to dividends and liquidation, conversion and redemption rights, sinking fund provisions, and restrictions on Galena’s ability to merge with or sell its assets to a third party.
The Debenture Galena entered into in May 2016, as subsequently amended, has resulted, and may continue to result, in significant dilution to the holders of Galena Common Stock.
On May 10, 2016, Galena entered into a Securities Purchase Agreement with JGB (Cayman) Newton Ltd, or JGB, pursuant to which Galena sold to JGB, at a 6.375% original issue discount, a $25,530,000 Senior Secured Debenture, which as subsequently amended is referred to herein as the Debenture, and warrants to purchase Galena Common Stock. As of November 9, 2017, (i) there were 45,712,912 shares of Galena Common Stock outstanding and (ii) 17,261,473 shares of Galena Common Stock had been issued by Galena pursuant to the terms of the Debenture. Assuming all the shares issuable pursuant to the terms of the Debenture subsequent to November 9, 2017 are issued at a stock payment price of $0.35, the lowest stock payment price permitted under the Debenture, Galena estimates that the maximum number of shares of common stock that Galena could issue pursuant to the terms of the Debenture subsequent to November 9, 2017 is 36,897,918.
Anti-takeover provisions of the Galena Certificate of Incorporation and the Galena Bylaws and provisions of Delaware law could delay or prevent a change of control.
Anti-takeover provisions of the Galena Certificate of Incorporation and the Galena Bylaws may discourage, delay or prevent a merger or other change of control that stockholders may consider favorable or may impede the ability of the holders of Galena Common Stock to change Galena’s management and may be constrained by other contractual agreements with third parties. These provisions of the Galena Certificate of Incorporation and the Galena Bylaws, among other things:
• | divide the Galena Board into three classes, with members of each class to be elected for staggered three-year terms; |
• | limit the right of securityholders to remove directors; |
• | prohibit stockholders from acting by written consent; |
• | regulate how stockholders may present proposals or nominate directors for election at annual meetings of stockholders; and |
• | authorize the Galena Board to issue preferred stock in one or more series, without stockholder approval. |
In addition, Section 203 of the Delaware General Corporation Law provides that, subject to limited exceptions, persons that acquire, or are affiliated with a person that acquires, more than 15% of the outstanding voting stock of a Delaware corporation such as Galena shall not engage in any business combination with that corporation, including by merger, consolidation or acquisitions of additional shares for a three-year period following the date on which that person or its affiliate crosses the 15% stock ownership threshold. Section 203 could operate to delay or prevent a change of control of Galena.
71
Galena has never declared or paid cash dividends on Galena’s capital stock and Galena does not anticipate paying cash dividends in the foreseeable future.
Galena’s business requires significant funding. Galena currently plans to invest all available funds and future earnings in the development and growth of Galena’s business and does not anticipate paying any cash dividends on Galena Common Stock in the foreseeable future, and Galena is prohibited by the terms of Galena’s outstanding indebtedness from paying dividends on any Galena Common Stock, except with the prior consent of Galena’s lenders. As a result, capital appreciation, if any, of Galena Common Stock will be Galena stockholders’ sole source of potential gain for the foreseeable future.
The terms of Galena’s outstanding indebtedness may inhibit potential acquirers.
Galena is prohibited by the terms of Galena’s outstanding indebtedness from disposing of any of Galena’s business or property, except with the consent of Galena’s lenders or if Galena were to prepay the outstanding indebtedness and related fees in accordance with the loan security agreement. Galena’s outstanding indebtedness may inhibit potential acquirers or other interested parties from seeking to acquire all or a part of Galena’s business or assets, and there is no assurance that Galena’s lenders would consent to any proposed future transaction that might be beneficial to Galena stockholders.
If the Galena Common Stock becomes subject to the penny stock rules, it may be more difficult to sell Galena Common Stock.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00 (other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system). The OTC Bulletin Board does not meet such requirements and if the price of the Galena Common Stock remains less than $5.00 and the Galena Common Stock is no longer listed on a national securities exchange such as Nasdaq, the Galena Common Stock may be deemed a penny stock. The penny stock rules require a broker-dealer, at least two business days prior to a transaction in a penny stock not otherwise exempt from those rules, to deliver to the customer a standardized risk disclosure document containing specified information and to obtain from the customer a signed and date acknowledgement of receipt of that document. In addition, the penny stock rules require that prior to effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive: (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for the Galena Common Stock, and therefore stockholders may have difficulty selling their shares.
72
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
None.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. Mine Safety Disclosures
None.
ITEM 5. Other Information
None.
73
ITEM 6. EXHIBITS
Exhibit # | Description | Form | Exhibit | Filing Date |
2.1 | S-4/A #2 | Annex A | November 6, 2017 | |
4.1 | 8-K | 4.1 | July 11, 2017 | |
10.1 | 8-K | 10.1 | August 8, 2017 | |
10.2 | 8-K | 10.2 | August 8, 2017 | |
10.3 | 8-K | 10.3 | August 8, 2017 | |
10.4 | 8-K | 10.1 | September 11, 2017 | |
31.1 | ||||
32.1 | ||||
101.INS | XBRL Instance Document.* | |||
101.SCH | XBRL Taxonomy Extension Schema.* | |||
101.CAL | XBRL Taxonomy Extension Calculation.* | |||
101.DEF | XBRL Taxonomy Extension Definition.* | |||
101.LAB | XBRL Taxonomy Extension Label.* | |||
101.PRE | XBRL Taxonomy Extension Presentation.* | |||
101.PRE | XBRL Taxonomy Extension Presentation.* | |||
* | Filed herewith. |
** | Furnished herewith. |
† | Indicates management contract or compensatory plan or arrangement. |
74
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
GALENA BIOPHARMA, INC. | |||
By: | /s/ Stephen F. Ghiglieiri | ||
Stephen F. Ghiglieri | |||
Interim Chief Executive Officer and Chief Financial Officer | |||
Date: November 9, 2017 | |||
75
