Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - SELLAS Life Sciences Group, Inc. | gale20160630ex321.htm |
| EX-31.2 - EXHIBIT 31.2 - SELLAS Life Sciences Group, Inc. | gale20160630ex312.htm |
| EX-31.1 - EXHIBIT 31.1 - SELLAS Life Sciences Group, Inc. | gale20160630ex311.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
________________________________
FORM 10-Q
________________________________
ý | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended June 30, 2016
OR
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |

Commission File Number: 001-33958
Galena Biopharma, Inc.
(Exact name of registrant as specified in its charter)
________________________________
Delaware | 20-8099512 | |
(State of incorporation) | (I.R.S. Employer Identification No.) | |
2000 Crow Canyon Place, Suite 380, San Ramon, CA 94583
(855) 855-4253
(Address, including zip code, and telephone number, including
area code, of registrant's principal executive offices)
________________________________
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter time that the registrant was required to submit and post such files). Yes ý No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
Large accelerated filer | ¨ | Accelerated filer | ý | |||
Non-accelerated filer | ¨ | (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | ||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act): ¨ Yes ý No
As of July 31, 2016, Galena Biopharma, Inc. had outstanding 213,968,953 shares of common stock, $0.0001 par value per share, exclusive of treasury shares.
GALENA BIOPHARMA, INC.
FORM 10-Q - Quarterly Report
For the Quarter Ended June 30, 2016
TABLE OF CONTENTS
Part No. | Item No. | Description | Page No. | ||
I | |||||
1 | |||||
Condensed Consolidated Balance Sheets as of June 30, 2016 (unaudited) and December 31, 2015 | |||||
Condensed Consolidated Statements of Operations (unaudited) for the three and six months ended June 30, 2016 and 2015 | |||||
Condensed Consolidated Statement of Stockholders' Equity (unaudited) for the six months ended June 30, 2016 | |||||
Condensed Consolidated Statements of Cash Flows (unaudited) for the six months ended June 30, 2016 and 2015 | |||||
2 | |||||
3 | |||||
4 | |||||
II | |||||
1 | Legal Proceedings | ||||
1A | Risk Factors | ||||
6 | |||||
EX-31.1 | |||||
EX-31.2 | |||||
EX-32.1 | |||||

1
PART I FINANCIAL INFORMATION
ITEM 1. FINANCIAL STATEMENTS
GALENA BIOPHARMA, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts in thousands, except share and per share data)
June 30, 2016 | December 31, 2015 | ||||||
(Unaudited) | |||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 19,590 | $ | 29,730 | |||
Restricted cash | 24,401 | 401 | |||||
Litigation settlement insurance recovery | — | 21,700 | |||||
Prepaid expenses and other current assets | 1,210 | 1,398 | |||||
Current assets of discontinued operations, net | 83 | 392 | |||||
Total current assets | 45,284 | 53,621 | |||||
Equipment and furnishings, net | 259 | 335 | |||||
In-process research and development | 12,864 | 12,864 | |||||
GALE-401 rights | 9,255 | 9,255 | |||||
Goodwill | 5,898 | 5,898 | |||||
Deposits and other assets | 218 | 171 | |||||
Total assets | $ | 73,778 | $ | 82,144 | |||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 1,273 | $ | 1,597 | |||
Accrued expenses and other current liabilities | 4,703 | 5,292 | |||||
Litigation settlement payable | 5,100 | 25,000 | |||||
Fair value of warrants potentially settleable in cash | 9,264 | 14,518 | |||||
Current portion of long-term debt | 23,157 | 4,739 | |||||
Current liabilities of discontinued operations | 3,727 | 5,925 | |||||
Total current liabilities | 47,224 | 57,071 | |||||
Deferred tax liability | 5,418 | 5,418 | |||||
Contingent purchase price consideration | 815 | 6,142 | |||||
Total liabilities | 53,457 | 68,631 | |||||
Commitments and contingencies | |||||||
Stockholders’ equity: | |||||||
Preferred stock, $0.0001 par value; 5,000,000 shares authorized; no shares issued and outstanding | — | — | |||||
Common stock, $0.0001 par value; 275,000,000 shares authorized, 182,996,385 shares issued and 182,321,385 shares outstanding at June 30, 2016; 162,581,753 shares issued and 161,906,753 shares outstanding at December 31, 2015 | 18 | 15 | |||||
Additional paid-in capital | 314,639 | 296,730 | |||||
Accumulated deficit | (290,487 | ) | (279,383 | ) | |||
Less treasury shares at cost, 675,000 shares | (3,849 | ) | (3,849 | ) | |||
Total stockholders’ equity | 20,321 | 13,513 | |||||
Total liabilities and stockholders’ equity | $ | 73,778 | $ | 82,144 | |||
See accompanying notes to condensed consolidated financial statements.
2
GALENA BIOPHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except share and per share data)
(Unaudited)
Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||
2016 | 2015 | 2016 | 2015 | ||||||||||||
Operating expenses: | |||||||||||||||
Research and development | $ | 6,175 | $ | 7,197 | $ | 11,618 | $ | 13,022 | |||||||
General and administrative | 3,117 | 1,886 | 6,642 | 4,973 | |||||||||||
Total operating expenses | 9,292 | 9,083 | 18,260 | 17,995 | |||||||||||
Operating loss | (9,292 | ) | (9,083 | ) | (18,260 | ) | (17,995 | ) | |||||||
Non-operating income (expense): | |||||||||||||||
Litigation settlements | (1,800 | ) | — | (1,800 | ) | — | |||||||||
Change in fair value of warrants potentially settleable in cash | 14,392 | (4,267 | ) | 10,520 | (3,115 | ) | |||||||||
Interest expense, net | (519 | ) | (207 | ) | (611 | ) | (432 | ) | |||||||
Change in fair value of the contingent purchase price liability | 5,497 | 83 | 5,327 | (238 | ) | ||||||||||
Total non-operating income (expense), net | 17,570 | (4,391 | ) | 13,436 | (3,785 | ) | |||||||||
Income (loss) from continuing operations | 8,278 | (13,474 | ) | (4,824 | ) | (21,780 | ) | ||||||||
Loss from discontinued operations | (2,889 | ) | (2,186 | ) | (6,280 | ) | (4,417 | ) | |||||||
Net income (loss) | $ | 5,389 | $ | (15,660 | ) | $ | (11,104 | ) | $ | (26,197 | ) | ||||
Net income (loss) per common share: | |||||||||||||||
Basic and diluted net income (loss) per share, continuing operations | $ | 0.05 | $ | (0.08 | ) | $ | (0.03 | ) | $ | (0.15 | ) | ||||
Basic and diluted net loss per share, discontinued operations | $ | (0.02 | ) | $ | (0.02 | ) | $ | (0.03 | ) | $ | (0.03 | ) | |||
Basic and diluted net income (loss) per share | $ | 0.03 | $ | (0.10 | ) | $ | (0.06 | ) | $ | (0.18 | ) | ||||
Weighted-average common shares outstanding: basic | 182,034,593 | 161,383,398 | 180,703,456 | 148,647,581 | |||||||||||
Weighted-average common shares outstanding: diluted | 185,477,330 | 161,383,398 | 180,703,456 | 148,647,581 | |||||||||||
See accompanying notes to condensed consolidated financial statements.
3
GALENA BIOPHARMA, INC.
CONDENSED CONSOLIDATED STATEMENT OF STOCKHOLDERS' EQUITY
(Amounts in thousands, except share amounts)
(Unaudited)
Common Stock | Additional Paid-In Capital | Accumulated Deficit | Treasury Stock | Total | ||||||||||||||||||
Shares Issued | Amount | |||||||||||||||||||||
Balance at December 31, 2015 | 162,581,753 | $ | 15 | $ | 296,730 | $ | (279,383 | ) | $ | (3,849 | ) | $ | 13,513 | |||||||||
Issuance of common stock | 19,772,727 | 3 | 20,186 | — | — | 20,189 | ||||||||||||||||
Common stock warrants issued in connection with January 2016 common stock offering | — | — | (5,590 | ) | — | — | (5,590 | ) | ||||||||||||||
Common stock warrants issued in connection with debt financing | — | — | 1,139 | — | — | 1,139 | ||||||||||||||||
Issuance of common stock upon exercise of warrants | 408,058 | — | 557 | — | — | 557 | ||||||||||||||||
Issuance of common stock in connection with employee stock purchase plan | 67,017 | — | 78 | — | — | 78 | ||||||||||||||||
Stock-based compensation for directors and employees | — | — | 1,278 | — | — | 1,278 | ||||||||||||||||
Exercise of stock options | 166,830 | — | 261 | — | — | 261 | ||||||||||||||||
Net loss | — | — | — | (11,104 | ) | — | (11,104 | ) | ||||||||||||||
Balance at June 30, 2016 | 182,996,385 | $ | 18 | $ | 314,639 | $ | (290,487 | ) | $ | (3,849 | ) | $ | 20,321 | |||||||||
See accompanying notes to condensed consolidated financial statements.
4
GALENA BIOPHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in thousands)
(Unaudited)
For the Six Months Ended June 30, | ||||||||
2016 | 2015 | |||||||
Cash flows from operating activities: | ||||||||
Cash flows from continuing operating activities: | ||||||||
Net loss from continuing operations | $ | (4,824 | ) | $ | (21,780 | ) | ||
Adjustment to reconcile net loss to net cash used in operating activities: | ||||||||
Depreciation and amortization expense | 777 | 181 | ||||||
Non-cash stock-based compensation | 1,278 | 759 | ||||||
Change in fair value of common stock warrants | (10,520 | ) | 3,115 | |||||
Change in fair value of contingent consideration | (5,327 | ) | 238 | |||||
Changes in operating assets and liabilities: | ||||||||
Prepaid expenses and other assets | 141 | 256 | ||||||
Litigation settlement insurance recovery | 21,700 | — | ||||||
Litigation settlement payable | (19,900 | ) | — | |||||
Accounts payable | (324 | ) | (825 | ) | ||||
Accrued expenses and other current liabilities | (589 | ) | (1,380 | ) | ||||
Net cash used in continuing operating activities | (17,588 | ) | (19,436 | ) | ||||
Cash flows from discontinued operating activities: | ||||||||
Net loss from discontinued operations | (6,280 | ) | (4,417 | ) | ||||
Changes in operating assets and liabilities attributable to discontinued operations | (839 | ) | 437 | |||||
Net cash used in discontinued operating activities | (7,119 | ) | (3,980 | ) | ||||
Net cash used in operating activities | (24,707 | ) | (23,416 | ) | ||||
Cash flows from investing activities: | ||||||||
Cash paid for purchase of equipment and furnishings | (6 | ) | (34 | ) | ||||
Net cash used in continuing investing activities | (6 | ) | (34 | ) | ||||
Selling costs paid for sale of commercial assets | (1,050 | ) | — | |||||
Cash paid for commercial assets | — | (534 | ) | |||||
Net cash used in discontinued investing activities | (1,050 | ) | — | (534 | ) | |||
Net cash used in investing activities | (1,056 | ) | (568 | ) | ||||
Cash flows from financing activities: | ||||||||
Net proceeds from issuance of common stock | 20,189 | 47,416 | ||||||
Net proceeds from exercise of stock options | 261 | 1 | ||||||
Proceeds from exercise of warrants | 233 | — | ||||||
Proceeds from common stock issued in connection with ESPP | 78 | 110 | ||||||
Net proceeds from issuance of long-term debt | 23,641 | — | ||||||
Minimum cash covenant on long-term debt | (24,000 | ) | — | |||||
Principal payments on long-term debt | (4,779 | ) | (1,914 | ) | ||||
Net cash provided by financing activities | 15,623 | 45,613 | ||||||
Net (decrease) increase in cash and cash equivalents | (10,140 | ) | 21,629 | |||||
Cash and cash equivalents at the beginning of period | 29,730 | 23,650 | ||||||
Cash and cash equivalents at end of period | $ | 19,590 | $ | 45,279 | ||||
Supplemental disclosure of cash flow information: | ||||||||
Cash received during the periods for interest | $ | 49 | $ | 2 | ||||
Cash paid during the periods for interest | $ | 606 | $ | 312 | ||||
Supplemental disclosure of non-cash investing and financing activities: | ||||||||
Fair value of warrants issued in connection with common stock recorded as cost of equity | $ | 5,590 | $ | 10,296 | ||||
Reclassification of warrant liabilities upon exercise | $ | 324 | $ | — | ||||
5
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. Business and Basis of Presentation
Overview
Galena Biopharma, Inc. (“we,” “us,” “our,” “Galena” or the “Company”) is a biopharmaceutical company committed to the development and commercialization of hematology and oncology therapeutics that address unmet medical needs. The Company’s pipeline consists of multiple mid- to late-stage clinical assets, including our hematology asset, GALE-401, our novel cancer immunotherapy programs including NeuVax™ (nelipepimut-S), GALE-301 and GALE-302. GALE-401 is a controlled release version of the approved drug anagrelide for the treatment of elevated platelets in patients with myeloproliferative neoplasms. GALE-401 has completed a Phase 2 trial and we are advancing the asset into a pivotal trial. NeuVax is currently in multiple Phase 2 trials. GALE-301 is in a Phase 2a clinical trial in ovarian and endometrial cancers and in a Phase 1b clinical trial given sequentially with GALE-302.
We are seeking to build value for shareholders through pursuit of the following objectives:
• | Developing hematology and oncology assets through clinical development, targeting areas of unmet medical need. Our hematology asset is targeting the treatment of patients with essential thrombocythemia (ET) to reduce elevated platelet counts. Our immunotherapy programs are currently targeting two key areas: secondary prevention intended to significantly decrease the risk of disease recurrence in breast, gastric, and ovarian cancers; and primary prevention intended to cease or delay ductal carcinoma in situ (DCIS) from becoming invasive breast cancer. |
• | Expand our development pipeline by enhancing the clinical and geographic footprint of our technologies. We intend to accomplish this through the initiation of new clinical trials and potentially through the acquisition of additional development programs. |
• | Leverage partnerships and collaborations, as well as investigator-sponsored trial arrangements, to maximize the scope of potential clinical opportunities in a cost effective and efficient manner. |
6
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
Basis of Presentation and Significant Accounting Policies
The accompanying consolidated financial statements included herein have been prepared by Galena pursuant to the generally accepted accounting principles (GAAP). Unless the context otherwise indicates, references in these notes to the “Company,” “we,” “us” or “our” refer (i) to Galena, our wholly owned subsidiaries, Apthera, Inc., or “Apthera,” and our wholly owned subsidiary, Mills Pharmaceuticals, LLC or "Mills."
At June 30, 2016, the Company’s capital resources consisted of cash and cash equivalents of $19.6 million not including $24.4 million of restricted cash. The Company will need to continue to incur significant expenses to advance our development portfolio and will need to raise additional capital to finance such activities.
On July 13, 2016, we closed the sale to certain institutional investors of 28,000,000 shares of common stock at a purchase price per share of $0.45 in a registered direct offering, and warrants to purchase up to 14,000,000 shares of common stock with an exercise price of $0.65 per share in a concurrent private placement. The warrants are initially exercisable six months and one day following issuance and have a term of five years from the date of issuance. The net proceeds to Galena after deducting placement agent fees and estimated offering expenses were approximately $11.7 million. The Company intends to use the net proceeds from this offering to fund its clinical trials of its product candidates, to augment its working capital, and for general corporate purposes. The current unrestricted cash and cash equivalents as of the date of this filing will fund the Company's operations for at least six months.
Additional funding was received July 13, 2016 upon the closing of an underwritten registered direct offering of our common stock and a private placement of warrants for net proceeds of $11.7 million to the Company. Additional funding sources that are, or in certain circumstances may be, available to the Company, include 1) approximately $24 million of restricted cash associated with our $25.5 million sale of Debentures by amending the current agreement with the holder to reduce the minimum cash covenant as detailed in Note 4; 2) a Purchase Agreement with Lincoln Park Capital, LLC; and 3) At Market Issuance Sales Agreements (ATM) with FBR & Co. (formerly MLV & Co. LLC) and Maxim Group LLC. The Purchase Agreement and ATM are unavailable to the Company until 75 days after the closing of our July 2016 financing. The Company cannot provide assurances that its plans will not change or that changed circumstances will not result in the depletion of its capital resources more rapidly than it currently anticipates. The Company is seeking and will need to raise additional capital, whether through a sale of equity or debt securities, a strategic business, the establishment of other funding facilities, licensing arrangements, asset sales or other means, in order to continue the development of the Company's product candidates and to support its other ongoing activities. However, the Company cannot be certain that it will be able to raise additional capital on favorable terms, or at all, which raises substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Discontinued Operations — As described in Note 11, during the quarter ended September 30, 2015 the Company met the relevant criteria for reporting the commercial operations as held for sale and in discontinued operations, pursuant to FASB Topic 205-20, Presentation of Financial Statements - Discontinued Operations, and FASB Topic 360, Property, Plant, and Equipment. During the quarter ended December 31, 2015, the Company completed the sale of the commercial products and the related assets.
Uses of Estimates in Preparation of Financial Statements — The preparation of these consolidated financial statements in accordance with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ materially from those estimates.
Principles of Consolidation — The consolidated financial statements include the accounts of Galena and its wholly owned subsidiaries. All material intercompany accounts have been eliminated in consolidation.
7
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
Reclassifications — Certain prior year amounts have been reclassified to conform to current year presentation. These reclassifications had no effect on net loss per share. The Company has reclassified the financial information for the three and six months ended June 30, 2015 to present the Company's commercial business as discontinued operations in the accompanying financial statements as the commercial business was divested in the fourth quarter of 2015.
Cash and Cash Equivalents — The Company considers all highly liquid debt instruments with an original maturity of 90 days or less to be cash equivalents. Cash equivalents consist primarily of amounts invested in money market accounts and demand deposits.
Restricted Cash — Restricted cash consists of certificates of deposit on hand with the Company’s financial institutions as collateral for its corporate credit cards and the minimum cash covenant per the Company's outstanding Debentures as described in Note 4.
Fair Value of Financial Instruments — The carrying amounts reported in the balance sheet for cash equivalents, accounts receivable, accounts payable, and capital leases approximate their fair values due to their short-term nature and market rates of interest.
Equipment and Furnishings — Equipment and furnishings are stated at cost and depreciated using the straight-line method based on the estimated useful lives (generally three to five years) of the related assets.
Goodwill and Intangible Assets — Goodwill and indefinite-lived intangible assets are not amortized but are tested annually for impairment at the reporting unit level, or more frequently if events and circumstances indicate impairment may have occurred. Factors the Company considers important that could trigger an interim review for impairment include, but are not limited to, the following:
• | Significant changes in the manner of its use of acquired assets or the strategy for its overall business; |
•Significant negative industry or economic trends;
•Significant decline in stock price for a sustained period; and
•Significant decline in market capitalization relative to net book value.
Goodwill and other intangible assets with indefinite lives are evaluated for impairment first by a qualitative assessment to determine the likelihood of impairment. If it is determined that impairment is more likely than not, the Company will then proceed to the two step impairment test. The first step is to compare the fair value of the reporting unit to the carrying amount of the reporting unit. If the carrying amount exceeds the fair value, a second step must be followed to calculate impairment. Otherwise, if the fair value of the reporting unit exceeds the carrying amount, the goodwill is not considered to be impaired as of the measurement date. In its review of the carrying value of the goodwill for its single reporting unit and its indefinite-lived intangible assets, the Company determines fair values of its goodwill using the market approach, and its indefinite-lived intangible assets using the income approach.
Intangible assets not considered indefinite-lived are reviewed for impairment when facts or circumstances suggest that the carrying value of these assets may not be recoverable. The Company’s policy is to identify and record impairment losses, if necessary, on intangible assets when events and circumstances indicate that the assets might be impaired and the undiscounted cash flows estimated to be generated by those assets are less than the carrying amounts.
In connection with the interim analysis of the PRESENT Phase 3 clinical trial and subsequent close down of the trial, the Company performed an impairment analysis of the intangible asset and goodwill. The fair value was determined to exceed to the carrying amount as of June 30, 2016 based on the other ongoing and and planned trials with NeuVax. As a result, no impairment was deemed necessary to these assets as of June 30, 2016.
8
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
Acquisitions and In-Licensing — For all in-licensed products and technologies, we perform an analysis to determine whether we hold a variable interest or a controlling financial interest in a variable interest entity. On the basis of our interpretations and conclusions, we determine whether the acquisition falls under the purview of variable interest entity accounting and if so, consider the necessity to consolidate the acquisition. As of June 30, 2016, we determined there were no variable interest entities required to be consolidated.
We also perform an analysis to determine if the assets and liabilities acquired in an acquisition qualify as a "business." The excess of the purchase price over the fair value of the net assets acquired can only be recognized as goodwill in a business combination. The Company completes its valuation analysis no later than twelve months from the date of the acquisition.
Contingent Purchase Price Consideration — Contingent consideration in business combinations is recorded at the estimated fair value as of the acquisition date. The fair value of the contingent consideration is re-measured at each reporting period with any adjustments in fair value included in our consolidated statement of comprehensive loss.
Patents and Patent Application Costs — Although the Company believes that its patents and underlying technology have continuing value, the amount of future benefits to be derived from the patents is uncertain. Patent costs are, therefore, expensed as incurred.
Litigation Settlement Payable and Insurance Recoveries — There can be a significant time lag between the time that legal fees are incurred and the insurance reimbursement available to offset the related costs. The legal costs are recorded in the period they are incurred, and the insurance recoveries for those costs are recorded in the period when the insurance reimbursement is deemed probable.
Share-based Compensation — The Company follows the provisions of the FASB ASC Topic 718, Compensation — Stock Compensation (“ASC 718”), which requires the measurement and recognition of compensation expense for all stock-based payment awards made to employees, non-employee directors, and consultants, including stock options and warrants. Stock compensation expense based on the grant date fair value estimated in accordance with the provisions of ASC 718 is recognized as an expense over the requisite service period.
For stock options and warrants granted as consideration for services rendered by non-employees, the Company recognizes compensation expense in accordance with the requirements of FASB ASC Topic 505-50 (“ASC 505-50”), Equity Based Payments to Non- Employees. Non-employee option and warrant grants that do not vest immediately upon grant are recorded as an expense over the vesting period. At the end of each financial reporting period prior to vesting, the value of these options and warrants, as calculated using the Black-Scholes option-pricing model, is re-measured using the fair value of the Company’s common stock and the non-cash compensation recognized during the period is adjusted accordingly. Since the fair market value of options and warrants granted to non-employees is subject to change in the future, the amount of the future compensation expense will include fair value re-measurements until the stock options are fully vested.
Research and Development Expenses — Research and development costs are expensed as incurred. Included in research and development costs are wages, benefits and other operating costs, facilities, supplies, external services and overhead related to our research and development departments, and clinical trial expenses.
Clinical trial expenses include direct costs associated with contract research organizations (CROs), as well as patient-related costs at sites at which our trials are being conducted. Direct costs associated with our CROs are generally payable on a time and materials basis, or when certain enrollment and monitoring milestones are achieved. Expense related to a milestone is recognized in the period in which the milestone is achieved or in which we determine that it is more likely than not that it will be achieved.
9
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
The invoicing from clinical trial sites can lag several months. We accrue these site costs based on our estimate of upfront set-up costs upon the screening of the first patient at each site, and the patient related costs based on our knowledge of patient enrollment status at each site.
Income Taxes — The Company recognizes liabilities or assets for the deferred tax consequences of temporary differences between the tax basis of assets or liabilities and their reported amounts in the financial statements in accordance with FASB ASC 740-10, Accounting for Income Taxes (“ASC 740-10”). These temporary differences will result in taxable or deductible amounts in future years when the reported amounts of the assets or liabilities are recovered or settled. ASC 740-10 requires that a valuation allowance be established when management determines that it is more likely than not that all or a portion of a deferred asset will not be realized. The Company evaluates the realizability of its net deferred income tax assets and valuation allowances as necessary, at least on an annual basis. During this evaluation, the Company reviews its forecasts of income in conjunction with other positive and negative evidence surrounding the realizability of its deferred income tax assets to determine if a valuation allowance is required. Adjustments to the valuation allowance will increase or decrease the Company’s income tax provision or benefit. The recognition and measurement of benefits related to the Company’s tax positions requires significant judgment, as uncertainties often exist with respect to new laws, new interpretations of existing laws, and rulings by taxing authorities. Differences between actual results and the Company’s assumptions or changes in the company’s assumptions in future periods are recorded in the period they become known.
There was no income tax expense or benefit for the three and six month periods ended June 30, 2016 and 2015. We continue to maintain a full valuation allowance against our net deferred tax assets.
Concentrations of Credit Risk — Financial instruments that potentially subject the Company to significant concentrations of credit risk consist principally of cash and cash equivalents. The Company maintains cash balances in several accounts with two banks, which at times are in excess of federally insured limits. As of June 30, 2016, the Company’s cash equivalents were invested in money market mutual funds. The Company’s investment policy does not allow investment in any debt securities rated less than “investment grade” by national ratings services. The Company has not experienced any losses on its deposits of cash and cash equivalents. The Company maintains significant cash and cash equivalents at two financial institutions that are in excess of federally insured limits.
Comprehensive Loss — Comprehensive loss consists of our net loss, with no other comprehensive income items for the periods presented.
Effect of Recent Accounting Pronouncements
In February 2016, the FASB issued Accounting Standards Update No. 2016-02, Leases ("ASU 2016-02"). ASU 2016-02 provides accounting guidance for both lessee and lessor accounting models. Among other things, lessees will recognize a right-of-use asset and a lease liability for leases with a duration of greater than one year. For income statement purposes, ASU 2016-02 will require leases to be classified as either operating or finance. Operating leases will result in straight-line expense while finance leases will result in a front-loaded expense pattern. The new standard will be effective for us on January 1, 2019 and will be adopted using a modified retrospective approach which will require application of the new guidance at the beginning of the earliest comparative period presented. We are currently evaluating the effect that the updated standard will have on our consolidated financial statements and related disclosures, however, we anticipate recognition of additional assets and corresponding liabilities related to leases on our balance sheet upon adoption.
In March 2016, the FASB issued Accounting Standards Update No. 2016-09, Compensation-Stock Compensation ("ASU 2016-09"). ASU 2016-09 changes several aspects of the accounting for share-based payment transactions including the income tax consequences, classification of awards as either equity or liabilities, employee tax withholding, calculation of shares for use in diluted earnings per share, and classification on the statement of cash flows. The new standard will be effective for us on January 1, 2017. Early adoption is available. We are currently evaluating the effect that the updated standard will have on our consolidated financial statements and related disclosures.
10
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
2. Fair Value Measurements
The Company follows ASC 820, Fair Value Measurements and Disclosures, (“ASC 820”) for the Company’s financial assets and liabilities that are re-measured and reported at fair value at each reporting period, and are re-measured and reported at fair value at least annually using a fair value hierarchy that is broken down into three levels. Level inputs are defined as follows:
Level 1 — quoted prices in active markets for identical assets or liabilities.
Level 2 — other significant observable inputs for the assets or liabilities through corroboration with market data at the measurement date.
Level 3 — significant unobservable inputs that reflect management’s best estimate of what market participants would use to price the assets or liabilities at the measurement date.
The Company categorized its cash equivalents as Level 1. The valuations for Level 1 were determined based on a “market approach” using quoted prices in active markets for identical assets. Valuation of these assets does not require a significant degree of judgment. The Company categorized its warrants potentially settleable in cash as Level 2 inputs. The warrants are measured at market value on a recurring basis and are being marked to market each quarter-end until they are completely settled. The warrants are valued using an appropriate pricing model, using assumptions consistent with our application of ASC 718. The contingent purchase price consideration is categorized as Level 3 inputs and is measured at its estimated fair value on a recurring basis and is adjusted at each quarter-end until it is completely settled. The contingent purchase price consideration is valued based on the expected timing of milestones, the expected probability of success for each milestone and discount rates based on a corporate debt interest rate index publicly issued.
The following tables present information about our assets and liabilities measured at fair value on a recurring basis in the condensed consolidated balance sheets (in thousands):
Description | June 30, 2016 | Quoted Prices In Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Unobservable Inputs (Level 3) | |||||||||||
Assets: | |||||||||||||||
Cash equivalents | $ | 18,169 | $ | 18,169 | $ | — | $ | — | |||||||
Total assets measured and recorded at fair value | $ | 18,169 | $ | 18,169 | $ | — | $ | — | |||||||
Liabilities: | |||||||||||||||
Warrants potentially settleable in cash | $ | 9,264 | $ | — | $ | 9,264 | $ | — | |||||||
Contingent purchase price consideration | 815 | — | — | 815 | |||||||||||
Total liabilities measured and recorded at fair value | $ | 10,079 | $ | — | $ | 9,264 | $ | 815 | |||||||
Description | December 31, 2015 | Quoted Prices In Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Unobservable Inputs (Level 3) | |||||||||||
Assets: | |||||||||||||||
Cash equivalents | $ | 29,171 | $ | 29,171 | $ | — | $ | — | |||||||
Total assets measured and recorded at fair value | $ | 29,171 | $ | 29,171 | $ | — | $ | — | |||||||
Liabilities: | |||||||||||||||
Warrants potentially settleable in cash | $ | 14,518 | $ | — | $ | 14,518 | $ | — | |||||||
Contingent purchase price consideration | 6,142 | — | — | 6,142 | |||||||||||
Total liabilities measured and recorded at fair value | $ | 20,660 | $ | — | $ | 14,518 | $ | 6,142 | |||||||
11
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
The Company did not transfer any financial instruments into or out of Level 3 classification during the six months ended June 30, 2016 and 2015. A reconciliation of the beginning and ending Level 3 liabilities for the six months ended June 30, 2016 is as follows (in thousands):
Fair Value Measurements Using Significant Unobservable Inputs (Level 3) | |||
Balance, January 1, 2016 | $ | 6,142 | |
Change in the estimated fair value of the contingent purchase price consideration | (5,327 | ) | |
Balance at June 30, 2016 | $ | 815 | |
The fair value of the contingent purchase price consideration is measured at the end of each reporting period using Level 3 inputs in a probability-weighted, discounted cash-outflow model. The significant unobservable assumptions include the probability of achieving each milestone, the date we expect to reach the milestone, and a determination of present value factors used to discount future expected cash outflows. The change in the estimated fair value of the contingent purchase price consideration during the quarter ended June 30, 2016 reflects an adjusted probability and time line for the potential approval of NeuVax associated with the Phase 2 combination trial with trastuzumab. Previously, the valuation was measured using the probability and time line of the Phase 3 PRESENT trial, which was stopped in June 2016 due to futility as recommended by the Independent Data Monitoring Committee ("IDMC").
See Note 7 for discussion of the Level 2 liabilities relating to warrants accounted for as liabilities.
3. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following (in thousands):
June 30, 2016 | December 31, 2015 | ||||||
Clinical trial costs | $ | 3,208 | $ | 3,294 | |||
Professional fees | 560 | 435 | |||||
Compensation and related benefits | 935 | 1,535 | |||||
Interest expense | — | 28 | |||||
Accrued expenses and other current liabilities | $ | 4,703 | $ | 5,292 | |||
12
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
4. Long-term Debt
On May 8, 2013, we entered into a loan and security agreement with Oxford Finance LLC, as collateral agent, and related lenders under which we borrowed the first tranche of $10 million (the "Loan"). The Loan payment terms include 12 months of interest-only payments at the fixed coupon rate of 8.45%, followed by 30 months of amortization of principal and interest until maturity in November 2016. In connection with the Loan, we paid the lender a 1% cash facility fee and a 5.5% cash final payment and granted to the lenders seven-year warrants to purchase up to 182,186 shares of our common stock at an exercise price of $2.47, which equaled a 20-day average market price of our common stock prior to the date of the grant. On May 10, 2016, the Company prepaid the outstanding principal amount and cash final payment.
On May 10, 2016, the Company entered into a Securities Purchase Agreement, with certain purchasers pursuant to which the Company sold, at a 6.375% original issue discount, a total of $25,530,000 Senior Secured Debentures (the “Debentures”) and warrants to purchase up to 2.0 million shares of the Company's common stock. Net proceeds to the Company from sale of the Debentures, after payment of commissions and legal fees, were approximately $23,400,000. The Debentures mature November 10, 2018, accrue interest at 9% per year, and do not contain any conversion features into shares of our common stock. The Company intends to use the net proceeds from this offering to fund the costs associated with the close down of the Phase 3 PRESENT study of NeuVax and other clinical trials of our product candidates and to augment its working capital and for general corporate purposes.
The Debentures carry an interest only period of six months following which the holder shall have the rights, at its option, to require the Company to redeem up to $1,100,000 per month of the outstanding principal amount of these Debentures. Interest is payable at the end of each month based on the outstanding principal.
The Company is required to promptly, but in any event no more than three trading days after the holder delivers a redemption notice to the Company, pay the applicable redemption amount in cash or, at the Company’s election and subject to certain conditions, in shares of the Company's common stock. If the Company elects to pay the redemption amount in shares of its common stock, then the shares will be delivered at the lesser of A) 7.5% discount to the average of the 3 lowest volume weighted average prices over the prior 20 trading days or B) a 7.5% discount to the prior trading day’s volume weighted average price. The Company may only opt for payment in shares of common stock if certain equity conditions are met. The Company, at its option, may also force the holder to redeem up to double the monthly redemption principal amount of the Debentures but not less than the monthly payment.
Based on the recommendation of the IDMC to stop the PRESENT Trial, the holder has the right to require the Company to prepay in cash all, or any portion, of the outstanding principal amount of this Debenture funded in cash by the holder on the closing date, plus all accrued and unpaid interest. If the holder elects such prepayment of the Debentures, then the number of shares subject to the warrants issued to the holder will be reduced in proportion to the percentage of principal required and accrued interest to be prepaid by the Company. The Purchaser received 1 million warrants upon the closing on the sale of the Debentures at an exercise price of $1.51, maturing 5 years from issuance. Additionally, the Purchasers received 1 million warrants upon the Company's public company announcement of the interim analysis on June 29, 2016 at an exercise price of $0.43. As of June 30, 2016 and the date of this filing, the holder had not exercised its right to require the Company to prepay in cash all, or any portion, of the outstanding principal amount of the Debentures. The holder's right to require the Company to prepay the outstanding principal amount expires 30 trading days after June 29, 2016, the announcement date of the recommendation of the IDMC. Therefore as of June 30, 2016 the Debentures, net of unamortized discounts, are presented as current liabilities on the condensed consolidated balance sheet.
The Company’s obligations under the Debenture can be accelerated in the event the Company undergoes a change in control and other customary events of default. In the event of default and acceleration of the Company’s obligations, the Company would be required to pay all amounts of principal and interest then outstanding under the Debenture in cash. The Company’s obligations under the Debentures are secured under a Security Agreement by a senior lien on all of the Company’s assets, including all of the Company’s interests in its consolidated subsidiaries. Until the holder exercises its right to require the Company to prepay some or all of the loan, the Company must also maintain a minimum of $24.0 million in cash, which is included in restricted cash as of June 30, 2016.
13
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
Armentum Partners, LLC (the “Placement Agent”) acted as the placement agent in the offering of the Debentures and the Company agreed to pay the Placement Agent a fee equal to 2% of the funds received from the sale of the Debentures. The Company paid half of the placement fee upon funding with the remaining payable at such time as the IDMC recommended in favor of the continuation of the PRESENT Trial or the holder waives the Company’s obligation to prepay the Debenture as a result of the IDMC’s recommendation.
5. Legal Proceedings, Commitments and Contingencies
Legal Proceedings
On December 3, 2015, we agreed in principle to resolve and settle the consolidated shareholder derivative action, In re Galena Biopharma, Inc. Derivative Litigation, Civil Action No. 3:14-cv-00382-SI, pending in the United States District Court for the District of Oregon against us and certain of our current and former officers and directors. On April 21, 2016, the District Court of Oregon held the final approval hearing of the settlement with the derivative plaintiffs after which the District Court continued the final approval hearing until June 23, 2016 and requested the parties submit additional briefing by June 9, 2016 on the fee request by the derivative plaintiffs’ attorneys. Following the hearing on June 23, 2016, on June 24, 2016, the U.S. District Court for the District of Oregon entered a final order and judgment in In re Galena Biopharma, Inc. Derivative Litigation, granting final approval to the settlement.
On the same day, the Court also issued an opinion and order awarding attorney’s fees of $4.5 million plus costs, which will be paid by our insurance carriers. The settlement includes a payment of $15 million in cash by our insurance carriers, which we used to fund a portion of the class action settlement, and cancellation of 1,200,000 outstanding director stock options. The settlement also requires that we adopt and implement certain corporate governance measures. The settlement does not include any admission of wrongdoing or liability on the part of us or the individual defendants and includes a full release of us and the current and former officers and directors in connection with the allegations made in the consolidated federal derivative actions and state court derivative actions.
On December 3, 2015, we also agreed in principal to resolve and settle the securities putative class action lawsuit, In re Galena Biopharma, Inc. Securities Litigation, Civil Action No. 3:14-cv-00367-SI, pending against us, certain of our current and former officers and directors and other defendants in the United States District Court for the District of Oregon. Following the hearing on June 23, 2016, on June 24, 2016, the U.S. District Court for the District of Oregon entered a final order and partial judgment in In re Galena Biopharma, Inc. Securities Litigation, granting final approval of the settlement. On the same day, the Court also issued an opinion and order awarding attorney’s fees of $4.5 million plus costs, which is paid out of the settlement funds. The settlement agreement provides for a payment of $20 million to the class and the dismissal of all claims against us and our current and former officers and directors in connection with the consolidated federal securities class actions. Of the $20 million settlement payment to the class, $16.7 million was paid by our insurance carriers and $2.3 million in cash was paid by us on July 1, 2016, along with $1 million in shares of our common stock (480,053 shares) paid by us on July 6, 2016. We will be responsible for defense costs and any settlements or judgments incurred for any related opt-out lawsuits. As of June 30, 2016 our insurance carriers paid $21.7 million. The Company paid $2.3 million in cash and $1 million in common stock on July 1, 2016.
In July 2016, we have resolved claims brought by shareholders that relate to the securities litigation mentioned above in one case for $150,000 plus $150,000 in shares (291,262) of our common stock, and in another case for $1.5 million in shares of our common stock (3,366,750 shares). The shares issued in connection with such settlements are included in the secondary offering filed on July 25, 2016. The settlements do not include any admission of wrongdoing or liability on the part of us or any of the current or former directors and officers and includes a full release of us and the current and former directors and officers in connection with the allegations made. We are not aware of any other claims made by shareholders who have opted out of the securities litigation.
14
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
The litigation settlements are summarized as follow as of June 30, 2016 (in thousands)
Amount | |||
Class action settlement | $ | 20,000 | |
Derivative settlement | 5,000 | ||
Shareholders securities litigation settlements | $ | 1,800 | |
Total settlements | $ | 26,800 | |
Paid by the insurance carriers | $ | 21,700 | |
Payable by the company in cash (paid in July 2016) | 2,450 | ||
Payable by the company in common stock (paid in July 2016) | 2,650 | ||
Total settlements | $ | 26,800 | |
We are aware that the SEC is investigating certain matters relating to the use of certain outside investor-relations professionals by us and other public companies. We have been in contact with the SEC staff through our counsel and are cooperating with the investigation and in discussions with the SEC staff to resolve the investigation.
A federal investigation of two of the high-prescribing physicians for Abstral has resulted in the criminal prosecution of the two physicians for alleged violations of the federal False Claims Act and other federal statutes. The criminal trial is set for October 2016. We have received a trial subpoena for documents in connection with that investigation and we have been in contact with the U.S. Attorney’s Office for the Southern District of Alabama, which is handling the criminal trial, and are cooperating in the production of documents. On April 28, 2016, a second superseding indictment was filed in the criminal case, which added additional information about the defendant physicians and provided information regarding the facts and circumstances involving a rebate agreement between the Company and the defendant physicians’ pharmacy as well as their ownership of our stock. Certain former employees have received trial subpoenas to appear at the trial and provide oral testimony. We have agreed to reimburse those former employees’ attorney’s fees. To our knowledge, we are not a target or subject of that investigation.
There also have been federal and state investigations of a company that has a product that competes with Abstral in the same therapeutic class, and we have learned that the FDA and other governmental agencies are investigating our Abstral promotion practices. On December 16, 2015, we received a subpoena issued by the U.S. Attorney’s Office in District of New Jersey requesting the production of a broad range of documents pertaining to our marketing and promotional practices for Abstral. We have been in contact with the U.S. Attorney’s Office for the District of New Jersey and are cooperating in the production of the requested documents. We are unable to predict whether we could become subject to legal or administrative actions as a result of these matters, or the impact of such matters. If we are found to be in violation of the False Claims Act, Anti-Kickback Statute, Patient Protection and Affordable Care Act, or any other applicable state or any federal fraud and abuse laws, we may be subject to penalties, such as civil and criminal penalties, damages, fines, or an administrative action of exclusion from government health care reimbursement programs. We can make no assurances as to the time or resources that will need to be devoted to these matters or their outcome, or the impact, if any, that these matters or any resulting legal or administrative proceedings may have on our business or financial condition.
15
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
6. Stockholders’ Equity
Preferred Stock — The Company has authorized up to 5,000,000 shares of preferred stock, $0.0001 par value per share, for issuance. The preferred stock will have such rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, as shall be determined by the Company’s Board of Directors upon its issuance. To date, the Company has not issued any preferred shares.
Common Stock — The Company has authorized up to 275,000,000 shares of common stock, $0.0001 par value per share, for issuance. On July 14, 2016, shareholders approved a 75,000,000 share increase to the Company's authorized shares of common stock up to 350,000,000 shares.
November 2014 Purchase Agreement with Lincoln Park Capital, LLC - On November 18, 2014, the Company entered into a purchase agreement with Lincoln Park Capital, LLC (LPC), pursuant to which the Company has the right to sell to LPC up to $50 million in shares of the Company's common stock, subject to certain limitations and conditions over the 36 month term of the purchase agreement. Pursuant to the purchase agreement, LPC initially purchased 2.5 million shares of the Company's common stock at $2.00 per share and the Company issued 631,221 shares of common stock to LPC as a commitment fee, which was recorded as a cost of capital. As a result of this initial issuance, the Company received initial net proceeds of $4.9 million, after deducting commissions and other offering expenses. In addition to LPC’s initial purchase of our common stock under the purchase agreement, during the first quarter of 2015, we received net proceeds of $4.4 million from LPC’s subsequent purchases of a total of 2.7 million shares of our common stock, excluding the commitment fee shares. There were no sales of our common stock under the LPC purchase agreement during the six months ended June 30, 2016.
At Market Issuance Sales Agreements - On May 24, 2013 the Company entered into At Market Issuance Sales Agreements (ATM) with FBR & Co. (formerly MLV & Co. LLC) and Maxim Group LLC (the Agents). From time to time during the term of the ATM, we may issue and sell through the Agents, shares of our common stock, and the Agents collect a fee equal to 3% of the gross proceeds from the sale of shares, up to a total limit of $20 million in gross proceeds. The ATM is available to the Company until it is terminated by the Agents or the Company. During the first quarter of 2015, we received $2.3 million in net proceeds from the sale of 1.4 million shares of our common stock through the ATM. There were no sales of our common stock under the ATM during the six months ended June 30, 2016.
March 2015 Underwritten Public Offering - On March 18, 2015 the Company closed an underwritten public offering of 24,358,974 units at a price to the public of $1.56 per unit for gross proceeds of $38 million (the "March 2015 Offering"). Each unit consists of one share of common stock, and a warrant to purchase 0.50 of a share of common stock at an exercise price of $2.08 per share. The March 2015 Offering included an over-allotment option for the underwriters to purchase an additional 3,653,846 shares of common stock and/or warrants to purchase up to 1,826,923 shares of common stock. On March 18, 2015, the underwriters exercised their over-allotment option to purchase warrants to purchase an aggregate of 1,826,923 shares of common stock. On April 10, 2015, the underwriters exercised their over-allotment option to purchase 3,653,846 shares of common stock for additional net proceeds of $5.4 million. The total net proceeds of the March 2015 Offering, including the exercise of the over-allotment option to purchase the warrants, were $40.8 million, after deducting underwriting discounts and commissions and offering expenses payable by the Company.
January 2016 Underwritten Public Offering - On January 12, 2016 the Company closed an underwritten public offering of 19,772,727 units at a price to the public of $1.10 per unit for gross proceeds of $21.8 million (the "January 2016 Offering"). Each unit consists of one share of common stock, and a warrant to purchase 0.60 of a share of common stock at an exercise price of $1.42 per share. The January 2016 Offering included an over-allotment option for the underwriters to purchase an additional 2,965,909 shares of common stock and/or warrants to purchase up to 1,779,545 shares of common stock. On January 12, 2016, the underwriters exercised their over-allotment option to purchase warrants to purchase an aggregate of 1,779,545 shares of common stock. The underwriters did not exercise their over-allotment option to purchase 2,965,909 shares of our common stock. The total net proceeds of the January 2016 Offering, including the exercise of the over-allotment option to purchase the warrants, were $20.2 million, after deducting underwriting discounts and commissions and offering expense paid by the Company.
16
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
Shares of common stock for future issuance are reserved for as follows (in thousands):
As of June 30, 2016 | ||
Warrants outstanding | 37,418 | |
Stock options outstanding | 10,309 | |
Options reserved for future issuance under the Company’s 2007 Incentive Plan | 10,923 | |
Shares reserved for future issuance under the Employee Stock Purchase Plan | 461 | |
Total reserved for future issuance | 59,111 | |
7. Warrants
The following is a summary of warrant activity for the six months ended June 30, 2016 (in thousands):
January 2016 Warrants | March 2015 Warrants | September 2013 Warrants | December 2012 Warrants | Other Equity Financing Warrants | Warrants issued to Consultants and Debtors | Total | ||||||||||||||
Outstanding, January 1, 2016 | — | 14,006 | 3,973 | 3,031 | 816 | 482 | 22,308 | |||||||||||||
Issued | 13,643 | — | — | — | — | 2,000 | 15,643 | |||||||||||||
Exercised | — | — | — | — | (502 | ) | — | (502 | ) | |||||||||||
Expired | — | — | — | — | (31 | ) | — | (31 | ) | |||||||||||
Outstanding, June 30, 2016 | 13,643 | 14,006 | 3,973 | 3,031 | 283 | 2,482 | 37,418 | |||||||||||||
Expiration | January 2021 | March 2020 | September 2018 | December 2017 | Varies 2016-2017 | Varies 2014-2021 | ||||||||||||||
Warrants consist of warrants potentially settleable in cash, which are liability-classified warrants, and equity-classified warrants.
Warrants classified as liabilities
Liability-classified warrants consist of warrants to purchase common stock issued in connection with equity financings in January 2016, March 2015, September 2013, December 2012, April 2011, March 2011, and March 2010. These warrants are potentially settleable in cash and were determined not to be indexed to our common stock.
The estimated fair value of outstanding warrants accounted for as liabilities is determined at each balance sheet date. Any decrease or increase in the estimated fair value of the warrant liability since the most recent balance sheet date is recorded in the condensed consolidated statement of operations as other income (expense). The fair value of the warrants is estimated using an appropriate pricing model with the following inputs:
As of June 30, 2016 | |||||||||||||||||||||||
January 2016 Warrants | March 2015 Warrants | September 2013 Warrants | December 2012 Warrants | April 2011 Warrants | March 2010 Warrants | ||||||||||||||||||
Strike price | $ | 1.42 | $ | 2.08 | $ | 2.50 | $ | 1.75 | $ | 0.65 | $ | 1.92 | |||||||||||
Expected term (years) | 4.52 | 3.72 | 2.22 | 1.48 | 0.81 | 0.25 | |||||||||||||||||
Volatility % | 113.70 | % | 122.36 | % | 139.26 | % | 167.41 | % | 218.93 | % | 218.93 | % | |||||||||||
Risk-free rate % | 0.94 | % | 0.82 | % | 0.61 | % | 0.51 | % | 0.42 | % | 0.19 | % | |||||||||||
17
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
As of December 31, 2015 | |||||||||||||||||||||||
March 2015 Warrants | September 2013 Warrants | December 2012 Warrants | April 2011 Warrants | March 2011 Warrants* | March 2010 Warrants | ||||||||||||||||||
Strike price | $ | 2.08 | $ | 2.50 | $ | 1.83 | $ | 0.65 | $ | 0.65 | $ | 2.02 | |||||||||||
Expected term (years) | 4.22 | 2.72 | 1.98 | 1.31 | 0.18 | 1.00 | |||||||||||||||||
Volatility % | 75.85 | % | 74.70 | % | 76.37 | % | 65.60 | % | 47.98 | % | 71.41 | % | |||||||||||
Risk-free rate % | 1.58 | % | 1.24 | % | 1.05 | % | 0.77 | % | — | % | — | % | |||||||||||
*The March 2011 warrants expired in March 2016. The March 2010 warrants do not expire until September 2016.
The expected volatility assumptions are based on the Company's implied volatility in combination with the implied volatilities of similar publicly traded entities. The expected life assumption is based on the remaining contractual terms of the warrants. The risk-free rate is based on the zero coupon rates in effect at the time of valuation. The dividend yield used in the pricing model is zero, because the Company has no present intention to pay cash dividends.
The changes in fair value of the warrant liability for the six months ended June 30, 2016 were as follows (in thousands):
January 2016 Warrants | March 2015 Warrants | September 2013 Warrants | December 2012 Warrants | April 2011 Warrants | Other Equity Financing Warrants | Total | |||||||||||||||||||||
Warrant liability, January 1, 2016 | $ | — | $ | 10,337 | $ | 1,933 | $ | 1,565 | $ | 537 | $ | 146 | $ | 14,518 | |||||||||||||
Fair value of warrants issued | 5,590 | — | — | — | — | — | 5,590 | ||||||||||||||||||||
Fair value of warrants exercised | — | — | — | — | (278 | ) | (46 | ) | (324 | ) | |||||||||||||||||
Change in fair value of warrants | (1,515 | ) | (6,668 | ) | (1,148 | ) | (909 | ) | (184 | ) | (96 | ) | (10,520 | ) | |||||||||||||
Warrant liability, June 30, 2016 | $ | 4,075 | $ | 3,669 | $ | 785 | $ | 656 | $ | 75 | $ | 4 | $ | 9,264 | |||||||||||||
Warrants classified as equity
Equity-classified warrants consist of warrants issued in connection with consulting services provided to us and warrants issued in connection with debt financings. On May 10, 2016 upon closing on the sale of Debentures, we granted the holder warrants to purchase up to 1,000,000 shares of common stock at an exercise price of $1.51. The warrants were valued using an appropriate pricing model. The fair value assumptions for the grant included a volatility of 77.13%, expected term of five and five tenths years, risk-free rate of 1.26%, and a dividend rate of 0.00%. The fair value of the warrants granted was $0.87 per share. These warrants are recorded in equity at fair value upon issuance. Additionally, on June 29, 2016 upon closing on the public announcement of the interim analysis of the PRESENT trial, we granted the holder warrants to purchase up to 1,000,000 shares of common stock at an exercise price of $0.43.The warrants were valued using an appropriate pricing model. The fair value assumptions for the grant included a volatility of 106.63%, expected term of 5.5 years, risk-free rate of 1.35%, and a dividend rate of 0.00%. The fair value of the warrants granted was $0.27 per share. These warrants are recorded in equity at fair value upon issuance.
18
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
8. Stock-Based Compensation
Options to Purchase Shares of Common Stock — The Company follows the provisions ASC 718, which requires the measurement and recognition of compensation expense for all share-based payment awards made to employees, non-employee directors, including employee stock options. Stock compensation expense based on the grant date fair value estimated in accordance with the provisions of ASC 718 is recognized as an expense over the requisite service period.
For stock options and warrants granted in consideration for services rendered by non-employees, the Company recognizes compensation expense in accordance with the requirements of ASC Topic 505-50. Non-employee option and warrant grants that do not vest immediately upon grant are recorded as an expense over the vesting period. At the end of each financial reporting period prior to vesting, the value of these options and warrants, as calculated using the Black-Scholes option-pricing model, is re-measured using the fair value of the Company’s common stock and the non-cash compensation recognized during the period is adjusted accordingly. Since the fair market value of options and warrants granted to non-employees is subject to change in the future, the amount of the future compensation expense will include fair value re-measurements until the stock options and warrants are fully vested.
The following table summarizes the components of stock-based compensation expense in the condensed consolidated statements of comprehensive loss for the three and six months ended June 30, 2016 and 2015, respectively (in thousands):
Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||
2016 | 2015 | 2016 | 2015 | ||||||||||||
Research and development | $ | 108 | $ | 93 | $ | 235 | $ | 170 | |||||||
General and administrative | 514 | 296 | 1,043 | 590 | |||||||||||
Total stock-based compensation from continuing operations | $ | 622 | $ | 389 | $ | 1,278 | $ | 760 | |||||||
The Company uses the Black-Scholes option-pricing model and the following weighted-average assumptions to determine the fair value of all its stock options granted:
Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||
2016 | 2015 | 2016 | 2015 | ||||||||
Risk free interest rate | 1.41 | % | 1.69 | % | 1.41 | % | 1.50 | % | |||
Volatility | 76.30 | % | 73.32 | % | 75.63 | % | 74.20 | % | |||
Expected lives (years) | 6.25 | 5.76 | 6.25 | 6.09 | |||||||
Expected dividend yield | — | % | — | % | — | % | — | % | |||
The weighted-average fair value of options granted during the three and six months ended June 30, 2016 were $1.36 per share and $0.86 per share, respectively.
19
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
The Company’s expected common stock price volatility assumption is based upon the Company's own implied volatility in combination with the implied volatility of a basket of comparable companies. The expected life assumptions for employee grants were based upon the simplified method provided for under ASC 718-10, which averages the contractual term of the Company’s options of ten years with the average vesting term of four years for an average of six years. The expected life assumptions for non-employees were based upon the contractual term of the option. The dividend yield assumption is zero, because the Company has never paid cash dividends and presently has no intention to do so. The risk-free interest rate used for each grant was also based upon prevailing short-term interest rates. The Company has estimated an annualized forfeiture rate of 15% for options granted to its employees, 8% for options granted to senior management and zero for non-employee directors. The Company will record additional expense if the actual forfeitures are lower than estimated and will record a recovery of prior expense if the actual forfeiture rates are higher than estimated.
As of June 30, 2016, there was $3,755,000 of unrecognized compensation cost related to outstanding options that is expected to be recognized as a component of the Company’s operating expenses over a weighted-average period of 2.79 years.
As of June 30, 2016, an aggregate of 26,500,000 shares of common stock were reserved for issuance under the Company’s 2007 Incentive Plan, including 10,309,000 shares subject to outstanding common stock options granted under the plan. On July 14, 2016, the shareholders approved the 2016 Incentive Plan into which the available shares in the 2007 Incentive Plan were transferred. There are 10,923,000 shares available for future grants based on adjustments in the 2016 Incentive Plan. The administrator of the plan determines the terms when an option may become exercisable. Vesting periods of options granted to date have not exceeded four years. The options will expire, unless previously exercised, no later than ten years from the grant date.
The following table summarizes option activity of the Company:
Total Number of Shares (In Thousands) | Weighted Average Exercise Price | Aggregate Intrinsic Value (In Thousands) | ||||||||
Outstanding at January 1, 2016 | 13,262 | $ | 2.58 | |||||||
Granted | 166 | 1.27 | ||||||||
Exercised | (167 | ) | 1.57 | $ | 56 | |||||
Canceled | (2,952 | ) | 3.07 | $ | — | |||||
Outstanding at June 30, 2016 | 10,309 | $ | 2.44 | $ | — | |||||
Options exercisable at June 30, 2016 | 5,970 | $ | 2.97 | $ | — | |||||
The aggregate intrinsic values of outstanding and exercisable options at June 30, 2016 were calculated based on the closing price of the Company’s common stock as reported on The NASDAQ Capital Market on June 30, 2016 of $0.47 per share. The aggregate intrinsic value equals the positive difference between the closing fair market value of the Company’s common stock and the exercise price of the underlying options.
20
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
9. Net Income (Loss) Per Share
Basic and diluted earnings per share are calculated as follows:
Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||
2016 | 2015 | 2016 | 2015 | ||||||||||||
Numerator: | |||||||||||||||
Net income (loss) (in thousands) | $ | 5,389 | $ | (15,660 | ) | $ | (11,104 | ) | $ | (26,197 | ) | ||||
Denominator: | |||||||||||||||
Weighted average number of common shares outstanding | 182,034,593 | 161,383,398 | 180,703,456 | 148,647,581 | |||||||||||
Effect of dilutive securities | |||||||||||||||
Stock options | 677,279 | — | — | — | |||||||||||
Warrants | 2,765,458 | — | — | — | |||||||||||
Dilutive potential common shares | 3,442,737 | — | — | — | |||||||||||
Shares used in calculating diluted earnings per share | 185,477,330 | 161,383,398 | 180,703,456 | 148,647,581 | |||||||||||
Basic net income (loss) per share | $ | 0.03 | $ | (0.10 | ) | $ | (0.06 | ) | $ | (0.18 | ) | ||||
Diluted net income (loss) per share | $ | 0.03 | $ | (0.10 | ) | $ | (0.06 | ) | $ | (0.18 | ) | ||||
The following table sets forth the potentially dilutive common shares excluded from the calculation of net income (loss) per common share because their inclusion would be anti-dilutive (in thousands):
Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||
2016 | 2015 | 2016 | 2015 | ||||||||
Warrants to purchase common stock | 21,517 | 22,308 | 37,418 | 22,308 | |||||||
Options to purchase common stock | 6,442 | 11,411 | 10,309 | 11,411 | |||||||
Total | 27,959 | 33,719 | 47,727 | 33,719 | |||||||
21
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
10. License Agreements
As part of its business, the Company enters into licensing agreements with third parties that often require milestone and royalty payments based on the progress of the licensed assets through development and commercial stages. Milestone payments may be required, for example, upon approval of the product for marketing by a regulatory agency, and the Company may be required to make royalty payments based upon a percentage of net sales of the product. The expenditures required under these arrangements in any period may be material and are likely to fluctuate from period to period.
These arrangements sometimes permit the Company to unilaterally terminate development of the product and thereby avoid future contingent payments; however, the Company is unlikely to cease development if the compound successfully achieves clinical testing objectives.
On January 12, 2014, we acquired worldwide rights to anagrelide controlled release (CR) formulation, which we renamed GALE-401, through our acquisition of Mills Pharmaceuticals, LLC ("Mills") and Mills became a wholly owned subsidiary. GALE-401 contains the active ingredient anagrelide, an FDA-approved product that has been in use since the late 1990s for the treatment of essential thrombocythemia (ET). Mills holds an exclusive license to develop and commercialize anagrelide CR formulation, pursuant to a license agreement with BioVascular, Inc. Under the terms of the license agreement, Mills has agreed to pay BioVascular, Inc. a mid-to-low single digit royalty on net revenue from the sale of licensed products as well as future cash milestone payments based on the achievement of specified regulatory milestones. Mills is also responsible for patent prosecution and maintenance. BioVascular has advised the Company of an alleged breach of the BioVascular agreement which the Company has denied. Both parties are discussing the dispute and it is anticipated that the dispute will be resolved.
On November 19, 2015, the Company and Sentynl Therapeutics Inc., a Delaware corporation (“Sentynl”), entered into and closed upon an Asset Purchase Agreement (the “Purchase Agreement”), pursuant to which the Company agreed to sell to Sentynl and Sentynl agreed to purchase from the Company, certain assets of the Company related to and including its Abstral® (fentanyl) sublingual tablets product (“Abstral”). The assets sold and assigned to Sentynl pursuant to the Purchase Agreement included all of the Company’s rights and interests in the Asset Purchase Agreement by and between the Company and Orexo AB (“Orexo”) dated March 15, 2013, and the License Agreement by and between the Company and Orexo dated March 18, 2013 (collectively, the “Orexo Agreements”). The Company’s future obligations under the Orexo Agreements were assumed by Sentynl pursuant to such assignment. In connection with such assignment, Orexo released the Company from any future obligations under the Orexo Agreements. The Purchase Agreement further provides that the Company will continue to be responsible for any pre-closing liabilities and obligations related to Abstral, as well for certain channel liabilities and rebates related to Abstral for a period of time post-closing.
The total potential consideration payable to the Company under the Purchase Agreement is $12 million, comprised of an $8 million upfront payment and up to an aggregate of $4 million, consisting of two one-time payments based on Sentynl's achievement of "net sales" of Abstral in amounts ranging from $25 million to $35 million.
On December 17, 2015, the Company and Midatech Pharma PLC, a public limited company organized under the laws of England and Wales (“Midatech”), entered into an Asset Purchase Agreement (the “Purchase Agreement”), pursuant to which the Company agreed to sell to Midatech and Midatech agreed to purchase from the Company, certain assets of the Company related to and including its Zuplenz® (ondansetron) Oral Soluble Film (“Zuplenz”). The assets to be sold and assigned to Midatech pursuant to the Purchase Agreement include all of the Company’s rights and interests in the License and Supply Agreement by and between the Company and MonoSol Rx, LLC (“MonoSol”) dated July 17, 2014 (the “MonoSol License”). The Company’s future obligations under the MonoSol agreement will be assumed by Midatech pursuant to such assignment. The Purchase Agreement further provides that the Company will continue to be responsible for any pre-closing liabilities and obligations related to Zuplenz, as well for certain rebates and channel liabilities related to Zuplenz for a period of time post-closing. The transaction was completed on December 24, 2015.
22
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
The total potential consideration payable to the Company under the Purchase Agreement is $29.75 million, comprised of an $3.75 million upfront payment upon the closing and up to an aggregate of $26 million, consisting of four one-time payments based on Midatech's achievement of "net sales" of Zuplenz in amounts ranging from $12 million to $70 million.
Through a separate agreement with MonoSol entered into on December 16, 2015 (the “MonoSol License Amendment”), (i) the Company and MonoSol agreed to amend the MonoSol License in order to reduce the number of field representatives that the Company is required to maintain with respect to Zuplenz, and (ii) the Company paid MonoSol $900,000 of the upfront fee payable to the Company under the Purchase Agreement and 20% of any future milestone payments received by the Company under the Purchase Agreement.
On December 24, 2015, the Company and Midatech closed upon the Purchase Agreement. In connection with the closing of the transactions contemplated by the Purchase Agreement, the Company assigned to Midatech all of its rights to and interests in the Company’s License and Supply Agreement, dated July 17, 2014 (the “MonoSol License”). As a result of such assignment, Midatech assumed all of the Company’s obligations under the MonoSol License.
11. Discontinued Operations, Assets Held for Sale
During the fourth quarter of 2015, The Company sold its rights to its commercial products Abstral® (fentanyl) Sublingual Tablets and Zuplenz® (ondansetron) Oral Soluble Film.
The Company entered into an agreement with a third party firm to assist the company with the divestiture of its commercial operations including identifying potential acquirers. Pursuant to the terms of the agreement, the Company paid a success fee to the third party firm in an amount of $900,000 and agreed to pay 5% of realized future revenue and payment streams.
The Company entered into compensatory arrangements related to the divestiture of our commercial business with certain members of commercial management. Under the terms of these arrangements, the Company paid a retention fee to the three employees in a combined total amount equal to $352,000 or 3% of cash consideration received as upfront payment in the transactions. These employees also received severance payments equal to one month’s salary for between four and seven months. In addition to these compensatory agreements loss from discontinued operations includes one-time termination benefits provided to employees who were part of the commercial business and did not accept employment opportunities at the companies that purchased Abstral and Zuplenz.
The following table describes the net proceeds from the sale and the assets and liabilities sold, net of selling costs (in thousands):
Sale of Abstral and related assets on November 19, 2015 | Sale of Zuplenz and related assets on December 24, 2015 | ||||||
Net proceeds from sales | |||||||
Total consideration | $ | 8,348 | $ | 3,750 | |||
Less selling costs* | (815 | ) | (1,050 | ) | |||
Proceeds from sale, net of selling costs | $ | 7,533 | $ | 2,700 | |||
*Selling costs related to the sale of Zuplenz and related assets were included in accrued liabilities as of December 31, 2015 and were paid in the first quarter of 2016. All other amounts were received or paid in the fourth quarter of 2015.
23
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
In addition to the upfront proceeds received from the sale of Abstral and Zuplenz and their related assets, the Company is eligible to receive up to $30 million in future milestone payments based on future net revenue of the products. The additional consideration will be recognized in the period that the net revenue milestones are achieved.
The following table presents amounts related to the discontinued operations in the balance sheets (in thousands):
June 30, 2016 | December 31, 2015 | ||||||
Carrying amounts of assets included as part of discontinued operations: | |||||||
Accounts receivable, net | $ | 83 | $ | 392 | |||
Total current assets of discontinued operations, net | 83 | 392 | |||||
Carrying amounts of liabilities included as part of discontinued operations: | |||||||
Accounts payable | $ | 1,299 | $ | 1,491 | |||
Accrued expenses and other current liabilities | 2,428 | 4,434 | |||||
Total current liabilities of discontinued operations | $ | 3,727 | $ | 5,925 | |||
The following table represents the components attributable to the commercial operations that are presented in the condensed consolidated statements of operations as discontinued operations (in thousands):
Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||
2016 | 2015 | 2016 | 2015 | ||||||||||||
Net revenue | $ | — | $ | 3,382 | $ | — | $ | 6,132 | |||||||
Additional channel obligations | (656 | ) | — | (1,666 | ) | — | |||||||||
Cost of revenue | — | (468 | ) | — | (861 | ) | |||||||||
Amortization of certain acquired intangible assets | — | (442 | ) | — | (588 | ) | |||||||||
Research and development | — | (94 | ) | — | (179 | ) | |||||||||
Selling, general, and administrative | (2,233 | ) | (4,581 | ) | (4,614 | ) | (8,921 | ) | |||||||
Non-operating income (expense) | — | 17 | — | — | |||||||||||
Impairment charge from classification as assets held for sale | — | — | — | — | |||||||||||
Loss from discontinued operations | $ | (2,889 | ) | $ | (2,186 | ) | $ | (6,280 | ) | $ | (4,417 | ) | |||
Additional channel obligations included in discontinued operations in the first half of 2016 is comprised of larger than anticipated rebates of Abstral sales for which we are responsible for through the end of the first quarter of 2016. The increase in rebates was driven by larger than expected volumes through these rebate channels and additional price protection provisions over which the Company has no control. The increase in rebates was partially offset by lower than expect patient assistance program reimbursement.
Selling, general and administrative expense included in discontinued operations consists of all other expenses of our commercial operations that are required in order to market and sell our marketed products. These expenses include all personnel related costs, marketing, data, consulting, legal, and other outside services necessary to support the commercial operations. During the three and months ended June 30,2016 we incurred $2.2 million and $4.6 million respectively, in selling, general, and administrative expense in discontinued operations. of which $2.2 million and $4.4 million, for the three and six months ended June 30, 2016, respectively, related to legal fees from external counsel associated with document production for the subpoenas related to the sales and marketing practices of Abstral. These legal proceedings are further disclosed in Part II., Item 1.
24
GALENA BIOPHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS - Continued
(Unaudited)
The following table presents significant operating non-cash items and capital expenditures related to discontinued operations (in thousands):
June 30, 2016 | June 30, 2015 | ||||||
Depreciation and amortization | $ | — | $ | 20 | |||
Stock-based compensation | $ | — | $ | 512 | |||
Purchases of property and equipment | $ | — | $ | (34 | ) | ||
Cash paid for acquisition of Zuplenz rights | $ | — | $ | (500 | ) | ||
12. Subsequent Events
The Company evaluated all events or transactions that occurred after June 30, 2016 up through the date these financial statements were issued. Other than as disclosed elsewhere in the notes to the condensed consolidated financial statements and below, the Company did not have any material recognizable or unrecognizable subsequent events.
On July 13, 2016, we closed the sale to certain institutional investors of 28,000,000 shares of common stock at a purchase price per share of $0.45 in a registered direct offering, and warrants to purchase up to 14,000,000 shares of common stock with an exercise price of $0.65 per share in a concurrent private placement. The warrants are initially exercisable six months and one day following issuance and have a term of five years from the date of issuance. The net proceeds to Galena after deducting placement agent fees and estimated offering expenses were approximately $11.7 million. The Company intends to use the net proceeds from this offering to fund its clinical trials of its product candidates, to augment its working capital, and for general corporate purposes.
On August 8, 2016, we amended the November 2014 Purchase Agreement with Lincoln Park Capital, LLC to increase the number of shares of our common stock that we can direct LPC to purchase from time to time, at its sole discretion and subject to certain conditions from 400,000 to 500,000 shares, which number of shares may increase up to 750,000 provided that the closing stock price of our common stock is not below $0.40 on the purchase date, and further may be increased to up to 1,000,000 shares, provided that the closing stock price of our common stock is not below $0.75 on the purchase date; provided, however, that the LPC’s committed obligation under any single regular purchase shall not exceed $2,000,000. In addition, we have removed the “floor price” at which the purchase price of shares of our common stock may be sold to LPC on any given day. The Company continues to control the timing and amount of any sales of our common stock to LPC. In addition, the Company may direct LPC to purchase additional amounts as accelerated purchases if on the date of a regular purchase the closing sale price of the Common Stock is not below $0.50 on the purchase date. Last, LPC will not purchase an aggregate number of shares that would result in the beneficial ownership by LPC and its affiliates of more than 4.99% of the then issued and outstanding shares of our common stock. All other terms of the purchase agreement remain the same.
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
In this section, "Galena," “we,” “our,” “ours” and “us” refer to Galena Biopharma, Inc. and its consolidated subsidiaries, Apthera, Inc., or “Apthera,” and Mills Pharmaceuticals, LLC, or "Mills."
This management’s discussion and analysis of financial condition as of June 30, 2016 and results of operations for the three and six months ended June 30, 2016 and 2015, respectively, should be read in conjunction with management’s discussion and analysis of financial condition and results of operations included in our Amended Annual Report on Form 10-K for the year ended December 31, 2015 which was filed with the SEC on March 11, 2016.
The discussion and analysis below includes certain forward-looking statements related to the development of our products in the U.S., our future financial condition and results of operations and potential for profitability, the sufficiency of our cash resources, our ability to obtain additional equity or debt financing, possible partnering or other strategic opportunities for the development of our products, as well as other statements related to the progress and timing of our product commercialization and development activities, present or future licensing, collaborative or financing arrangements or that otherwise relate to future periods, which are all forward-looking statements as defined by the Private Securities Litigation Reform Act of 1995. These statements represent, among other things, the expectations, beliefs, plans and objectives of management and/or assumptions underlying or judgments concerning the future financial performance and other matters discussed in this document. The words “may,” “will,” “should,” “plan,” “believe,” “estimate,” “intend,” “anticipate,” “project,” and “expect” and similar expressions are intended to connote forward-looking statements. All forward-looking statements involve certain risks, including the uncertainties and other factors described in our Amended Annual Report on Form 10-K for the year ended December 31, 2015 that could cause our actual development activities, financial condition and results of operations, and business prospects and opportunities to differ materially from these expressed in, or implied by, those forward-looking statements. We caution investors not to place significant reliance on the forward-looking statements contained in this report. These statements, like all statements in this report, speak only as of the date of this report (unless another date is indicated) and we undertake no obligation to update or revise forward-looking statements.
25
Overview
Galena Biopharma, Inc. (“we,” “us,” “our,” “Galena” or the “Company”) is a biopharmaceutical company committed to the development and commercialization of hematology and oncology therapeutics that address unmet medical needs. The Company’s pipeline consists of multiple mid- to late-stage clinical assets, including our hematology asset, GALE-401, our novel cancer immunotherapy programs including NeuVax™ (nelipepimut-S), GALE-301 and GALE-302. GALE-401 is a controlled release version of the approved drug anagrelide for the treatment of elevated platelets in patients with myeloproliferative neoplasms. GALE-401 has completed a Phase 2 trial and we are advancing the asset into a pivotal trial. NeuVax is currently in multiple Phase 2 trials. GALE-301 is in a Phase 2a clinical trial in ovarian and endometrial cancers and in a Phase 1b clinical trial given sequentially with GALE-302.
We are seeking to build value for shareholders through pursuit of the following objectives:
• | Developing hematology and oncology assets through clinical development, targeting areas of unmet medical need. Our hematology asset is targeting the treatment of patients with essential thrombocythemia (ET) to reduce elevated platelet counts. Our immunotherapy programs are currently targeting two key areas: secondary prevention intended to significantly decrease the risk of disease recurrence in breast, gastric, and ovarian cancers; and primary prevention intended to cease or delay ductal carcinoma in situ (DCIS) from becoming invasive breast cancer. |
• | Expand our development pipeline by enhancing the clinical and geographic footprint of our technologies. We intend to accomplish this through the initiation of new clinical trials and potentially through the acquisition of additional development programs. |
• | Leverage partnerships and collaborations, as well as investigator-sponsored trial arrangements, to maximize the scope of potential clinical opportunities in a cost effective and efficient manner. |
The chart below summarizes the current status of our clinical development pipeline:
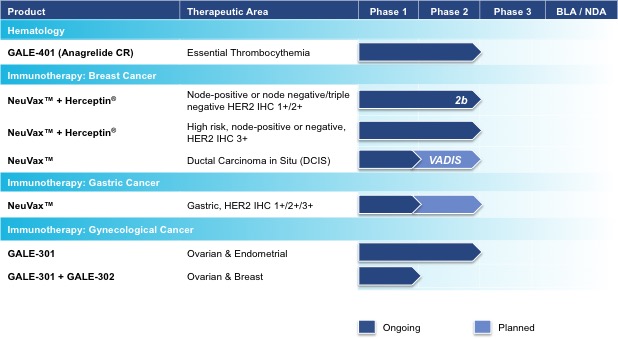
26
Hematology
GALE-401 (anagrelide controlled release (CR))
GALE-401 contains the active ingredient anagrelide, an FDA-approved product, for the treatment of patients with myeloproliferative neoplasms (MPNs) to lower abnormally elevated platelet levels. The currently available immediate release (IR) version of anagrelide causes adverse events that are believed to be dose and plasma concentration dependent. These adverse events may limit the use of the IR version of the drug. Therefore, reducing the maximum concentration (Cmax) is hypothesized to reduce the side effects, but preserve efficacy, potentially allowing a broader use of the drug.
Multiple Phase 1 studies in 98 healthy subjects have shown GALE-401 reduces the Cmax of anagrelide following oral administration, appears to be well tolerated at the doses administered, and to be capable of reducing platelet levels. The Phase 1 program provided the desired PK/PD (pharmacokinetic/pharmacodynamic) profile to enable the initiation of the ongoing Phase 2 proof-of-concept trial. The Phase 2, open label, single arm, proof-of-concept trial enrolled 18 patients in the United States for the treatment of thrombocytosis, or elevated platelet counts, in patients with MPNs. Final safety and efficacy data from this pilot Phase 2 trial were presented in December 2015 and demonstrated a prolonged clinical benefit with a potentially improved safety profile. We plan to submit a final Phase 2 manuscript at the end of 2016.
We have analyzed our data and the treatment landscape for MPNs, with a current focus on Essential Thrombocythemia (ET) where we see an unmet medical need in patients who are intolerant to the current standard of care. We are advancing GALE-401 into a pivotal trial and we plan to meet with the FDA by the end of the year to discuss our Phase 2/3 clinical trial design, development opportunities in ET patients, and confirmation of the 505(b)2 regulatory pathway for approval.
Thrombocythemia is a myeloproliferative blood disorder. It is characterized by the production of too many platelets in the bone marrow. Too many platelets make normal clotting of blood difficult. It can be either reactive or primary (also termed essential and caused by a myeloproliferative disease). Although often symptomless (particularly when it is a secondary reaction), it can predispose to thrombosis in some patients. Primary Thrombocytosis (essential thrombocythemia or ET) is due to a failure to regulate the production of platelets (autonomous production) and is a feature of a number of myeloproliferative disorders. About a third of patients are asymptomatic at the time of diagnosis.
Novel Cancer Immunotherapies
Our targeted cancer immunotherapy approach is currently based upon two key areas: preventing secondary recurrence of cancer, which is becoming increasingly important as the number of cancer survivors continues to grow; and, primary prevention intended to treat breast cancer earlier in the treatment spectrum. Once a patient’s tumor becomes metastatic, the outcome is often fatal, making the prevention of recurrence a potentially critical component of overall patient care. Our programs primarily target patients in the adjuvant (after-surgery) setting who have relatively healthy immune systems, but may still have residual disease. Minimal residual disease, or single cancer cells (occult cancer cells) or micrometastasis, that are undetectable by current radiographic scanning technologies, can result in disease recurrence.
Our therapies utilize an immunodominant peptide combined with the immune adjuvant, recombinant human granulocyte macrophage-colony stimulating factor (rhGM-CSF), and work by harnessing the patient’s own immune system to seek out and attack any residual cancer cells. Using peptide immunogens has many potential clinical advantages, including a favorable safety profile, since these drugs may lack the toxicities typical of most cancer therapies. They also have the potential to evoke long-lasting protection through activation of the immune system and a convenient, intradermal mode of delivery. We are currently engaged in multiple clinical trials with NeuVax™ (nelipepimut-S), GALE-301, and GALE-302, targeting the prevention of recurrence in breast, gastric, ovarian and endometrial cancers.
27
NeuVax™ (nelipepimut-S)
NeuVax™ (nelipepimut-S) is a cancer immunotherapy targeting human epidermal growth factor receptor (HER2) expressing cancers. NeuVax is the immunodominant nonapeptide derived from the extracellular domain of the HER2 protein, a well-established and validated target for therapeutic intervention in breast and gastric carcinomas. The NeuVax vaccine is combined with GM-CSF for injection under the skin, or intradermal administration. Data has shown that an increased presence of circulating tumor cells (CTCs) may predict Disease Free Survival (DFS) and Overall Survival (OS) suggesting a presence of isolated micrometastases, not detectable clinically, but, over time, can lead to recurrence, most often in distant sites. After binding to the specific HLA molecules on antigen presenting cells, the nelipepimut-S sequence stimulates specific cytotoxic T lymphocytes, or CTLs, causing significant clonal expansion. These activated CTLs recognize, neutralize and destroy, through cell lysis, HER2 expressing cancer cells, including occult cancer cells and micrometastatic foci. The nelipepimut immune response can also generate CTLs to other immunogenic peptides through inter- and intra-antigenic epitope spreading.
Breast Cancer: According to the National Cancer Institute (NCI), over 230,000 women in the U.S. are diagnosed with breast cancer annually. While improved diagnostics and targeted therapies have decreased breast cancer mortality in the U.S., metastatic breast cancer remains incurable. Approximately 75% to 80% of breast cancer patients have tissue test positive for some increased amount of the HER2 receptor, which is associated with disease progression and decreased survival. Only approximately 20% to 30% of all breast cancer patients-those with HER2 immunohistochemistry (IHC) 3+ disease, or IHC 2+ and fluorescence in situ hybridization (FISH) amplified-have a HER2 directed, approved treatment option available after their initial standard of care. This leaves the majority of breast cancer patients with low-to-intermediate HER2 expression (IHC 1+/2+) ineligible for therapy and without an effective targeted treatment option to prevent cancer recurrence.
We currently have two investigator-sponsored trials ongoing with NeuVax in combination with trastuzumab (Herceptin®; Genentech/Roche). The combination of trastuzumab and NeuVax has been shown pre-clinically and in a pilot study to be synergistic. Our Phase 2b clinical trial is a randomized, multicenter, investigator-sponsored, 300 patient study enrolling HER2 1+ and 2+, HLA A2+, A3+, A24 and/or A26, node positive, and high-risk node negative patients. The endpoint is disease free survival and preliminary safety data will be presented in the fourth quarter of this year. Eligible patients will be randomized to receive NeuVax + GM-CSF + trastuzumab or trastuzumab + GM-CSF alone. The primary endpoint of the study is disease-free survival. Genentech/Roche is providing the trastuzumab and partial funding for this trial.
Our second combination trial is a Phase 2 in HER2 3+ breast cancer patients who have completed neoadjuvant therapy with an approved regimen that includes trastuzumab and fail to achieve a pathological complete response, meaning they have microscopic evidence of residual disease and are therefore at an increased risk of disease recurrence. This multi-center, prospective, randomized, single-blinded Phase 2 trial is enrolling approximately 100 patients with a diagnosis of HER2 3+ breast cancer who are HLA A2+ or HLA A3+ and are determined to be at high-risk for recurrence. High-risk is defined as having received neoadjuvant therapy with an approved regimen that includes trastuzumab but not obtaining a pathological complete response at surgery, or those who undergo surgery as a first intervention and are found to be pathologically node-positive. These high-risk patients are known to have higher recurrence rates than other HER2 3+ breast cancer patients. Eligible patients will be randomized to receive NeuVax + GM-CSF (granulocyte macrophage-colony stimulating factor) + trastuzumab or trastuzumab + GM-CSF alone. The primary endpoint of the study is disease-free survival. Funding for this trial was awarded through the Congressionally Directed Medical Research Program (CDMRP), funded through the Department of Defense (DoD), via annual Congressional legislation known as the Defense Appropriations Act. The grant was a Breast Cancer Research Program (BCRP) Breakthrough Award.
28
We have also announced our intent to initiate a Phase 2 trial with NeuVax as a single agent in patients with ductal carcinoma in situ, or DCIS, in collaboration with the NCI, potentially positioning NeuVax as a treatment for earlier stage disease. The trial will have an immunological endpoint evaluating NeuVax peptide-specific cytotoxic T lymphocyte (CTL; CD8+ T-cell) response in vaccinated patients. The trial is currently suspended pending discussion around the outcome of the PRESENT clinical trial investigation. DCIS is defined by the NCI as a noninvasive condition in which abnormal cells are found in the lining of a breast duct, and is the most common type of breast cancer. The abnormal cells have not spread outside the duct to other tissues in the breast. In some cases, DCIS may become invasive cancer and spread to other tissues, and at this time, there is no way to know which lesions could become invasive. Current treatment options for DCIS include breast-conserving surgery and radiation therapy with or without tamoxifen, breast-conserving surgery without radiation therapy, or total mastectomy with or without tamoxifen. According to the American Cancer Society, in 2015 there were over 60,000 diagnoses of DCIS.
On June 24, 2016, the assembled Independent Data Monitoring Committee, or IDMC, met to conduct a pre-planned safety and futility analysis of the Phase 3 PRESENT (Prevention of Recurrence in Early-Stage, Node- Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment) clinical trial. On June 27, 2016, the IDMC recommended that the Phase 3 trial “be stopped for futility unless it is determined that there has been a systematic reversal in the study drug treatments in the two arms, in which case the IDMC should reevaluate the clinical evidence.” We immediately stopped the PRESENT trial, and initiated an investigation into the causes of the recommendation. Our analysis of the data shows that there was a separation of the curves, albeit not statistically significant, with the control arm performing better than expected and the NeuVax arm performing consistent with our protocol assumptions for the control group. Because the study is deemed futile, we are officially closing the PRESENT trial, and we expect to present the data at a future medical conference.
Gastric Cancer: According to the NCI, gastric (stomach) cancer is a disease in which malignant (cancer) cells form in the lining of the stomach. Almost all gastric cancers are adenocarcinomas (cancers that begin in cells that make and release mucus and other fluids). Other types of gastric cancer are gastrointestinal carcinoid tumors, gastrointestinal stromal tumors, and lymphomas. Infection with bacteria called Helicobacter pylori (H. pylori) is a common cause of gastric cancer and age, diet, and stomach disease can affect the risk of developing gastric cancer. Gastric cancer is often diagnosed at an advanced stage because there are no early signs or symptoms. Gastric, or stomach cancer, is the second-most common cancer among males and third-most common among females in Asia and worldwide with over 63,000 new cases a year in India, where an initial clinical trial of NeuVax will be run. Overexpression of the HER2 receptor occurs in approximately 20% of gastric and gastro-esophageal junction adenocarcinomas, predominantly those of the intestinal type. Overall, without regard to the stage of cancer, only approximately 28% of patients with stomach cancer live at least five years following diagnosis and new adjuvant treatments are needed to prevent disease recurrence.
We currently have an agreement with Dr. Reddy’s Laboratories to conduct a Phase 2 investigational study in gastric cancer in India.
GALE-301 and GALE-302
Our second immunotherapy franchise targets folate binding protein (FBP) receptor-alpha. FBP is a well-validated therapeutic target that is highly over-expressed in ovarian, endometrial and breast cancers, and is the source of immunogenic peptides that can stimulate cytotoxic T lymphocytes (CTLs) to recognize and destroy FBP-expressing cancer cells. Current treatments after surgery for these diseases are principally with platinum based chemotherapeutic agents. These patients suffer a high recurrence rate and most relapse with an extremely poor prognosis. GALE-301 and GALE-302 are immunogenic peptides that consist of a peptide derived from FBP combined with GM-CSF for the prevention of cancer recurrence in the adjuvant setting. GALE-301 is the E39 peptide, while GALE-302 is an attenuated version of this peptide, known as E39’. Two trials are ongoing with our FBP peptides: the GALE-301 Phase 2a portion of the Phase 1/2a clinical trial is ongoing in ovarian and endometrial adenocarcinomas, and the GALE-301 plus GALE-302 Phase 1b clinical trial is ongoing in breast and ovarian cancers.
29
Ovarian Cancer: According to the NCI Surveillance, Epidemiology, and End Results (SEER) Program, new cases of ovarian cancer occur at an annual rate of 11.9 per 100,000 women in the United States, with an estimated 22,280 cases for 2016. Although ovarian cancer represents about 1.3% of all cancers, it represents about 2.4% of all cancer deaths, or an estimated 14,180 deaths in 2015. Approximately 1.3% of women will be diagnosed with ovarian cancer at some point during their lifetime (2010 - 2012 data). The prevalence of ovarian cancer in the U.S. is about 192,000 women, and the five-year survivorship for women with ovarian cancer is 45.6%. Due to the lack of specific symptoms, the majority of ovarian cancer patients are diagnosed at later stages of the disease, with an estimated 80% of women presenting with advanced-stage (III or IV) disease. These patients have their tumors routinely surgically debulked to minimal residual disease, and then are treated with platinum- and/or taxane-based chemotherapy. While many patients respond to this treatment regimen and become clinically free-of-disease, the majority of these patients will relapse. Depending upon their level of residual disease, the risk for recurrence after completion of primary therapy is approximately 70%. Unfortunately for these women, once the disease recurs, treatment options are limited and the disease is most likely incurable.
According to the NCI SEER Program, new cases of endometrial cancer occur at an annual rate of 25.1 per 100,000 women in the U.S., with an estimated 54,870 cases for 2015. Although endometrial cancer represents about 3.3% of all cancers, it represents about 1.7% of all cancer deaths, or an estimated 10,170 deaths in 2015. Approximately 2.8% of women will be diagnosed with endometrial cancer at some point during their lifetime (2010 - 2012 data). The prevalence of endometrial cancer in the U.S. is about 620,000 women, and the five-year survivorship for women with endometrial cancer is 81.7%.
Intellectual Property
Patents and other intellectual property rights are crucial to our success. It is our policy to protect our intellectual property rights through available means, including filing and prosecuting patent applications in the U.S. and other countries, protecting trade secrets, and utilizing regulatory protections such as data exclusivity. We also include restrictions regarding use and disclosure of our proprietary information in our contracts with third parties, and utilize customary confidentiality agreements with our employees, consultants, clinical investigators and scientific advisors to protect our confidential information and know-how. Together with our licensors, we also rely on trade secrets to protect our combined technology especially where we do not believe patent protection is appropriate or obtainable. It is our policy to operate without infringing on, or misappropriating, the proprietary rights of others. The following chart summarizes our intellectual property rights:
Drug Candidate | Indication | Scope | Estimated Exclusivity Period |
GALE-401 (Anagrelide Controlled Release) | Platelet Lowering | Pending and/or issued | 2029 |
NeuVax™ (nelipepimut-S) | Breast cancer recurrence | Pending and/or issued | 2028 |
NeuVax™ (nelipepimut-S) | Gastric | Pending and/or issued | 2028 |
NeuVax™ (nelipepimut-S) | DCIS | Pending and/or issued | 2028 |
NeuVax™ in combination with trastuzumab | Breast cancer | Pending and/or issued | 2026 |
NeuVax™ in combination with other compounds | Breast cancer | Pending and/or issued | 2037 |
GALE-301 & GALE-302 | Breast, ovarian and endometrial cancer | Pending and/or issued | 2035 |
30
Recent Operational Developments
Discontinued NeuVax™ (nelipepimut-S) Phase 3, PRESENT Interim Analysis based on Independent Data Monitoring Committee Recommendation
On June 24, 2016, the assembled IDMC met to conduct a pre-planned safety and futility analysis of the Phase 3 PRESENT (Prevention of Recurrence in Early-Stage, Node- Positive Breast Cancer with Low to Intermediate HER2 Expression with NeuVax Treatment) Trial. On June 27, 2016, the IDMC recommended that the study be stopped for futility unless it is determined that there has been a systematic reversal in the study drug treatments in the two arms, in which case the IDMC should reevaluate the clinical evidence. We immediately stopped the PRESENT trial, and initiated an investigation into the causes of the recommendation. Our analysis of the data shows that there was a separation of the curves, albeit not statistically significant, with the control arm performing better than expected and the NeuVax arm performing consistent with our protocol assumptions for the control group. Because the study is deemed futile, we are officially closing the PRESENT trial, and we expect to present the data at a future medical conference.
Derivative and Securities Litigation - The U.S. District Court for the District of Oregon granted final approval of the settlements previously reported.
On December 3, 2015, we reached an agreement in principle to settle the consolidated shareholder derivative action, In re Galena Biopharma, Inc. Derivative Litigation, Civil Action No. 3:14-cv-00382-SI pending against us and certain of our current and former officers and directors. Following the hearing on June 23, 2016, on June 24, 2016, the U.S. District Court for the District of Oregon entered a final order and judgment in In re Galena Biopharma, Inc. Derivative Litigation, granting final approval to the settlement. On the same day, the Court also issued an opinion and order awarding attorney’s fees of $4.5 million plus costs, which will be paid by our insurance carriers. The settlement includes a payment of $15 million in cash by our insurance carriers, which we will use to fund a portion of the class action settlement, and cancellation of 1,200,000 outstanding director stock options. The settlement also requires that we adopt and implement certain corporate governance measures. The settlement does not include any admission of wrongdoing or liability on the part of us or the individual defendants and includes a full release of us and the current and former officers and directors in connection with the allegations made in the consolidated federal derivative actions and state court derivative actions.
On December 3, 2015, we also agreed in principal to resolve and settle the securities putative class action lawsuit, In re Galena Biopharma, Inc. Securities Litigation, Civil Action No. 3:14-cv-00367-SI, pending against us, certain of our current and former officers and directors and other defendants in the United States District Court for the District of Oregon. Following the hearing on June 23, 2016, on June 24, 2016, the U.S. District Court for the District of Oregon entered a final order and partial judgment in In re Galena Biopharma, Inc. Securities Litigation, granting final approval of the settlement. On the same day, the Court also issued an opinion and order awarding attorney’s fees of $4.5 million plus costs, which is paid out of the settlement funds. The agreement provides for a settlement payment of $20 million to the class and the dismissal of all claims against us and the other defendants in connection with the consolidated federal securities class actions. Of the $20 million settlement payment to the class, $16.7 million was paid by our insurance carriers and $2.3 million in cash was paid by us on July 1, 2016, along with $1 million in shares of our common stock (480,053 shares) paid by us on July 6, 2016. We will be responsible for defense costs and any settlements or judgments incurred for any related opt-out lawsuits.
Securities Litigation Opt Out Claims
We have resolved claims brought by shareholders and others that relate to the securities litigation mentioned above in one case for $150,000 plus $150,000 in shares (291,262) of our common stock, and in another case for $1.5 million in shares of our common stock (3,138,951 shares). The 3,430,213 shares issued in connection with such settlements are included in the offering covered by this prospectus. The settlements do not include any admission of wrongdoing or liability on the part of us or any of the current or former directors and officers and includes a full release of us and the current and former directors and officers in connection with the allegations made. We are not aware of any other claims made by shareholders who have opted out of the securities litigation.
31
Presented GALE-401 Combined Safety Data
On June 13, 2016 we presented combined safety data from our GALE-401 clinical trials at the European Hematology Association 21stCongress. A total of six trials have been run with GALE-401, five Phase 1 trials in healthy volunteers (N=98), and one Phase 2 single arm, open label pilot study in MPN patients (N=18). The poster, entitled, “Anagrelide Controlled Release (GALE-401) Safety Profile Consistently Well Tolerated in Myeloproliferative Neoplasms Patients and Healthy Volunteers” was designed to characterize the safety profile of GALE-401 in all subjects treated to date. The results demonstrated that GALE-401 is well tolerated in MPN patients as well as in healthy volunteers and we observed predominantly mild to moderate toxicities that did not reveal any unexpected AEs.
Received Two Orphan Drug Designations for GALE-301 and GALE-301/GALE-302
On June 10, 2016, we announced that the U.S. Food and Drug Administration, or FDA, granted two orphan-drug designations for Galena’s two cancer immunotherapy peptides derived from Folate Binding Protein (FBP) for the treatment (including prevention of recurrence) of ovarian cancer: one for GALE-301 (E39), and one for GALE-301 (E39) and GALE-302 (E39’).
Presented GALE-301 Phase 1/2a Primary Analysis
On June 6, 2016, we presented the primary analysis from the Company’s GALE-301 Phase 1/2a clinical trial at the American Society of Clinical Oncology Annual Meeting 2016. The poster, entitled, “The primary analysis of a phase I/IIa dose finding trial of a folate binding protein vaccine, E39 + GM-CSF in ovarian and endometrial cancer patients to prevent recurrence,” demonstrated that the vaccine is well tolerated and immunogenic. In the optimal dose group, the results demonstrate potential clinical benefit for GALE-301 to prevent recurrence in these patients, and that boosters may sustain this effect.
Received Fast Track Designation for NeuVax™ (nelipepimut-S) PRESENT Clinical Trial
On June 1, 2016, we announced that the FDA has designated NeuVax™ (nelipepimut-S), combined GM-CSF, as a Fast Track development program for the treatment of patients with early stage, node positive breast cancer with low to intermediate HER2 expression, otherwise known as HER2 1+ or 2+, following standard of care.
Appointment of Mary Ann Gray, Ph.D. to Board of Directors
Effective April 25, 2016, the Board increased the number of directors from eight to nine directors and appointed Mary Ann Gray, Ph.D. as a Class III director. Dr. Gray will be listed in Galena’s 2016 Proxy Statement as a nominee for election at our 2016 Annual Meeting of Stockholders. Dr. Gray is President of Gray Strategic Advisors, LLC, which provides strategic advice to both public and private biotechnology companies. Previously, she spent three and a half years with the Federated Kaufmann Fund focusing on both public and private healthcare investments. Prior, Dr. Gray was a sell-side biotechnology analyst for nine years. Earlier in her career, Dr. Gray held scientific positions at Schering Plough and NeoRx, managed pre-clinical toxicology studies for the National Cancer Institute through Battelle Memorial Institute, and worked in a hospital laboratory. Dr. Gray currently serves on the board of directors of several publicly traded biotechnology companies: TetraLogic, Inc., Juniper Pharmaceuticals, Senomyx, Inc. Previously, Dr. Gray also served on the boards of Acadia Pharmaceuticals, Dyax Corp., GTC Biotherapeutics, Inc., Telik, and Apthera, Inc. (private). Dr. Gray has a Ph.D. in Pharmacology from the University of Vermont where she focused on novel chemotherapeutic agents for the treatment of cancer, and she received her B.S. in biology from the University of South Carolina. She completed her postdoctoral work at Northwestern University Medical School and Yale University School of Medicine.
32
Presented GALE-301/GALE-302 Clinical Booster Data
On April 19, 2016 data from the booster phase of the Company’s GALE-301/GALE-302 Phase 1/2a clinical trial was presented at the American Association for Cancer Research (AACR) Annual Meeting. The poster, entitled, “Comparing an attenuated booster (E39’) vs. E39 booster to potentiate the clinical benefit of the folate binding protein (FBP)-derived vaccine (E39 + GM-CSF) in a phase I/IIa trial to prevent recurrence in endometrial (EC) and ovarian cancer (OC) patients,” randomized patients to two different boosters: E39 (GALE-301), versus E39’ (GALE-302). The purpose of the study was to evaluate the immune responses and determine which booster, if either, would provide a sustained immune response and potentially longer disease free survival (DFS) rates. The use of the wildtype peptide (GALE-301/E39) demonstrated the same tolerable safety profile as the attenuated peptide (GALE-302/E39’) with only Grade 1 local reactions and minimal Grade 2 toxicities. Importantly, the percentage of patients who received two booster inoculations and remained disease free was significantly better in the drug treatment arm, versus the control arm (p=0.02), regardless of which booster was used.
Announced a Notice of Allowance of a U.S. Patent for NeuVax
On February 8, 2016, we announced the United States Patent Office issued a Notice of Allowance for an additional U.S. patent application covering multiple uses of NeuVax™ (nelipepimut-S): inducing and maintaining an immune response to HER2 expressing tumor cells in patients in clinical remission with a tumor having a fluorescence in situ hybridization (FISH) rating of less than about 2.0 (FISH <2.0); inducing and sustaining a cytotoxic T-lymphocyte (CTL) response to HER2 in patients in clinical remission from a tumor with a FISH rating of less than about 2.0 (FISH < 2.0); reducing risk of cancer recurrence in patients in clinical remission from a tumor with a FISH rating of less than about 2.0 (FISH < 2.0); and preventing bone only recurrence of a HER2 expressing cancer. This patent will expand both the protection and the potential population of cancer patients NeuVax may address. Once issued, the patent will expire in 2028, not including any patent term extensions.
Presented Observational Study Data in Gastric Cancer Patients at the ASCO 2016 Gastrointestinal Cancers Symposium
On January 21, 2016, we presented data from an observational study in gastric cancer patients at the American Society of Clinical Oncology (ASCO) 2016 Gastrointestinal Cancers Symposium. The study was conducted by our partner, Dr. Reddy’s Laboratories Ltd, who will conduct a Phase 2 clinical trial of NeuVax in gastric cancer patients in India. The poster, entitled, “An observational study evaluating the expression of HER2 (1+, 2+, and 3+) with HLA A2+/A3+ in gastric adenocarcinoma patients” showed that approximately 25% of the patients met the projected clinical protocol population of all levels of expression of HER2 and HLA A2+ and/or A3+ as defined for the planned NeuVax Phase 2 clinical trial. Results indicate an acceptable potential for enrollment rate, given the high incidence of gastric cancer in this population, and will inform the screen failure rate in the planned Phase 2 clinical study.
Closed Public Offering and Debt Financing
On January 12, 2016, we closed the previously announced underwritten public offering of common stock and warrants. The net proceeds to us were approximately $20.2 million.
On May 10, 2016, we closed on a Securities Purchase Agreement, with certain purchasers pursuant to which the Company sold, at a 6.375% original issue discount, a total of $25.5 million Senior Secured Debentures. The net proceeds to us were approximately $23.4 million.
On July 13, 2016, we closed the previously announced underwritten registered direct offering of common stock and warrants. The net proceeds to us were approximately $11.7 million.
33
Results of Operations for the Three and Six Months Ended June 30, 2016 and 2015
For the three months ended June 30, 2016, our net income was $5.4 million compared with a net loss of $15.7 million for the three months ended June 30, 2015. Income from continuing operations for the three months ended June 30, 2016 was $8.3 million compared with a loss from continuing operations of $13.5 million for the three months ended June 30, 2015, which was primarily driven by a non-cash net change in our warrant liability of $18.7 million from a $4.3 million loss in the first quarter of 2015 to a $14.4 million gain in the first quarter of 2016. In addition, to the non-cash gain on our warrant liability, the revaluation of our contingent purchase price consideration as of June 30, 2016 resulted in an additional $5.5 million non-cash gain for the three months then ended compared to a $0.1 million non-cash gain for the three months ended June 30, 2015.
For the six months ended June 30, 2016, our net loss was $11.1 million compared with a net loss of $26.2 million for the six months ended June 30, 2015. Loss from continuing operations for the six months ended June 30, 2016 was $4.8 million compared with a loss from continuing operations of $21.8 million for the six months ended June 30, 2015, which was primarily driven by a non-cash net change in our warrant liability of $13.6 million from a $3.1 million loss in the first half of 2015 to a $10.5 million gain in the first half of 2016. In addition, to the non-cash gain on our warrant liability, the revaluation of our contingent purchase price consideration as of June 30, 2016 resulted in an additional $5.3 million non-cash gain for the three months then ended compared to a $0.2 million non-cash loss for the three months ended June 30, 2015.
During the third quarter of 2015, the Company completed its strategic review and concluded to solely focus its resources on its clinical development pipeline and our management and Board of Directors committed to pursue a plan to sell or otherwise divest the Company’s commercial business. These actions caused the Company to meet the relevant criteria for reporting the Company’s commercial business as discontinued operations. Discontinued operations for the three and six months ended June 30, 2016 and 2015 is comprised of the revenue, expenses, gains and losses of our commercial business. Our loss from discontinued operations for the three months ended June 30, 2016 was $2.9 million compared with a loss from discontinued operations of $2.2 million for the three months ended June 30, 2015. Loss from discontinued operations for the six months ended June 30, 2016 was $6.3 million compared with a loss from discontinued operations of $4.4 million for the six months ended June 30, 2015.
Further analysis of the changes and trends in our operating results are discussed below.
(dollars in thousands) | Three Months Ended June 30, | |||||||||
2016 | 2015 | % Change | ||||||||
Operating loss | $ | (9,292 | ) | $ | (9,083 | ) | (2 | )% | ||
Non-operating income (expense) | 17,570 | (4,391 | ) | (500 | )% | |||||
Loss from discontinued operations | (2,889 | ) | (2,186 | ) | (32 | )% | ||||
Net income (loss) | $ | 5,389 | $ | (15,660 | ) | 134 | % | |||
Net income (loss) per common share: | ||||||||||
Basic and diluted net income (loss) per share, continuing operations | $ | 0.05 | $ | (0.08 | ) | 163 | % | |||
Basic and diluted net income (loss) per share, discontinued operations | $ | (0.02 | ) | $ | (0.02 | ) | — | % | ||
Basic and diluted net income (loss) per share | $ | 0.03 | $ | (0.10 | ) | 130 | % | |||
34
(dollars in thousands) | Six Months Ended June 30, | |||||||||
2016 | 2015 | % Change | ||||||||
Operating loss | $ | (18,260 | ) | $ | (17,995 | ) | (1 | )% | ||
Non-operating income (expense) | 13,436 | (3,785 | ) | (455 | )% | |||||
Loss from discontinued operations | (6,280 | ) | (4,417 | ) | (42 | )% | ||||
Net loss | $ | (11,104 | ) | $ | (26,197 | ) | 58 | % | ||
Net loss per common share: | ||||||||||
Basic and diluted net loss per share, continuing operations | $ | (0.03 | ) | $ | (0.15 | ) | 80 | % | ||
Basic and diluted net loss per share, discontinued operations | $ | (0.03 | ) | $ | (0.03 | ) | — | % | ||
Basic and diluted net loss per share | $ | (0.06 | ) | $ | (0.18 | ) | 67 | % | ||
Research and Development Expense
Research and development expense consists primarily of clinical trial expenses, compensation-related costs for our employees dedicated to research and development activities, and licensing fees and patent prosecution costs. Research and development expense for the three and six months ended June 30, 2016 and 2015, respectively, was as follows (dollars in thousands):
Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||
2016 | 2015 | % Change | 2016 | 2015 | % Change | ||||||||||||||||
Research and development expense | $ | 6,175 | $ | 7,197 | (14 | )% | $ | 11,618 | $ | 13,022 | (11 | )% | |||||||||
The majority of our research and development expenses to date relate to our Phase 3 PRESENT clinical trial using NeuVax as a HER2 directed cancer immunotherapy under evaluation to prevent breast cancer recurrence after standard of care treatment. The trial costs are more significant during the recruitment and enrollment phase. We established more than 140 sites in 13 counties and screened over 3,300 patients in order to enroll qualifying patients who currently have no available treatment options to maintain their disease-free status after their standard of care. Once the patient is enrolled, they enter the monitoring phase, which lasts the later of three years of treatment or an event (recurrence or death). On April 14, 2015 we announced the completion of over-enrollment in the PRESENT trial of 758 patients, which was 7.7% higher than called for under our FDA-approved Special Protocol Assessment.
The decrease of 14% for the three months ended June 30, 2016 compared to the three months ended June 30, 2015 in research and development expense was primarily due to the decrease in enrollment efforts surrounding our Phase 3 PRESENT clinical trial, partially offset by additional consulting expenses incurred preparing for the interim analysis of PRESENT on June 24, 2016. The decrease of 11% for the six months ended June 30, 2016 compared to the six months ended June 30, 2015 in research and development expense was primarily due to the decrease in enrollment efforts surrounding our Phase 3 PRESENT clinical trial, partially offset by additional consulting expenses incurred preparing for the interim analysis of PRESENT on June 24, 2016.
The completion of over-enrollment in April 2015 reduced expenses related to the trial as we entered the monitoring phase and continued toward our interim analysis on June 24, 2016, which resulted in the stop and eventual termination of the Phase 3 PRESENT trial in the third quarter of 2016. The decrease in recruitment and enrollment expenses related to the Phase 3 PRESENT clinical trial were partially offset by recruitment, enrollment, and monitoring expenses in our other ongoing or planned clinical trials. We expect the second half 2016 research and development expenses to decrease as we close down the Phase 3 PRESENT trial upon the recommendation of the IDMC, will reduce headcount, and will focus on our other ongoing and planned clinical trials. The close out of the Phase 3 PRESENT trial is expected to cost $2.5 - $3.5 million over the second half of 2016.
35
General and Administrative Expense
General and administrative expense includes compensation-related costs for our employees dedicated to general and administrative activities, legal fees, audit and tax fees, consultants and professional services, and general corporate expenses. General and administrative expense for the three and six months ended June 30, 2016 and 2015, respectively, was as follows (dollars in thousands):
Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||
2016 | 2015 | % Change | 2016 | 2015 | % Change | ||||||||||||||||
General and administrative expense | $ | 3,117 | $ | 1,886 | 65 | % | $ | 6,642 | $ | 4,973 | 34 | % | |||||||||
The 65% increase in selling, general, and administrative expense for the three months ended June 30, 2016 compared to the three months ended June 30, 2015 was driven by an increase of $0.8 million in legal expenses incurred with final approval of the settlement of our class action and derivative litigation on June 24, 2016 and the ongoing SEC investigation and other governmental investigations, as well as $0.2 million in stock based compensation. The 34% increase in selling, general, and administrative expense for the six months ended June 30, 2016 compared to the six months ended June 30, 2015 was driven by an increase of $0.4 million in legal expenses incurred with final approval of the settlement of our class action derivative litigation on June 24, 2016 and the ongoing SEC investigation and other governmental investigations as well as $0.4 million in stock based compensation. We also incurred additional personnel expenses including recruitment fees for two new directors for our Board and replacing our chief financial officer.
Non-Operating Income (Expense)
Non-operating income (expense) for the three and six months ended June 30, 2016 and 2015, respectively, was as follows (dollars in thousands):
Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||
2016 | 2015 | % Change | 2016 | 2015 | % Change | ||||||||||||||||
Change in fair value of warrants potentially settleable in cash | $ | 14,392 | $ | (4,267 | ) | (437 | )% | $ | 10,520 | $ | (3,115 | ) | (438 | )% | |||||||
Interest expense, net | (519 | ) | (207 | ) | 151 | % | (611 | ) | (432 | ) | 41 | % | |||||||||
Change in fair value of the contingent purchase price liability | 5,497 | 83 | 6,523 | % | 5,327 | (238 | ) | (2,338 | )% | ||||||||||||
Total non-operating income (expense), net | $ | 19,370 | $ | (4,391 | ) | (541 | )% | $ | 15,236 | $ | (3,785 | ) | (503 | )% | |||||||
The increase in our net non-operating income (expense) during the three and six months ended June 30, 2016 compared to the three and six months ended June 30, 2015 was primarily due to a significant decrease in the change in fair value of warrants accounted for as liabilities. This decrease in the estimated fair value of our warrant liabilities was primarily due to the decrease in our common stock price, which is one of the most impactful inputs into the pricing model we use to estimate the fair value of our warrant liabilities. The stock price as of June 30, 2016 was $0.47, a decrease of 65% and 68% for the three and six months ended June 30, 2016, respectively. In addition to the decrease in the fair value of warrants, our contingent purchase price consideration related to the approval of NeuVax also decreased. The interim analysis of the PRESENT Phase 3 clinical trial and subsequent close down of the trial triggered an intangible asset and goodwill impairment analysis of the carrying amount and the fair value was determined to exceed the carrying amount as of June 30, 2016 based on the other ongoing and planned trials with NeuVax. The contingent purchase price consideration is fair valued at each reporting period and the lower probability and extended time line for approval were updated to align with the valuation performed of NeuVax and significantly decreased the fair value which are the two largest variables impacting the liability.
The change in fair value of warrants and the change in contingent purchase price consideration are both non-cash. Management believes these adjustments may not always accurately reflect the operating, economic activities, or obligations undertaken by the Company.
36
Income Taxes
For the three and six months ended June 30, 2016 and 2015, there was no income tax benefit or expense recognized.
Discontinued Operations
During the quarter ended September 30, 2015, we completed a strategic review of our commercial business and operations, and as a result of that review we sold the assets of our commercial business during the fourth quarter of 2015. We believe this disposition allows us to focus our resources on our valuable and expanding clinical development programs and maximize the value of these assets to our shareholders.
The following table represents the components attributable to the commercial operations that are presented in the condensed consolidated statements of operations as discontinued operations (in thousands):
Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||
2016 | 2015 | 2016 | 2015 | ||||||||||||
Net revenue | $ | — | $ | 3,382 | $ | — | $ | 6,132 | |||||||
Additional channel obligations | (656 | ) | — | $ | (1,666 | ) | — | ||||||||
Cost of revenue | — | (468 | ) | — | (861 | ) | |||||||||
Amortization of certain acquired intangible assets | — | (442 | ) | — | (588 | ) | |||||||||
Research and development | — | (94 | ) | — | (179 | ) | |||||||||
Selling, general, and administrative | (2,233 | ) | (4,581 | ) | (4,614 | ) | (8,921 | ) | |||||||
Non-operating income (loss) | — | 17 | — | — | |||||||||||
Impairment charge from classification of assets held for sale | — | — | — | — | |||||||||||
Loss from discontinued operations | $ | (2,889 | ) | $ | (2,186 | ) | $ | (6,280 | ) | $ | (4,417 | ) | |||
Discontinued operations are comprised of net revenue, cost of revenue, and expenses attributable to our commercial operations, which were sold in the fourth quarter of 2015.
•Net Revenue included in discontinued operations comprises revenue from the sale of Abstral, which was provided by our commercial operations.
•Additional Channel Obligations included in discontinued operations in the first quarter of 2016 is comprised of larger than anticipated rebates of Abstral sales that we are responsible for through the end of the first quarter of 2016. The increase in rebates was driven by larger than expected volumes through these rebate channels and additional price protection provisions. The increase in rebates was partially offset by lower than expect patient assistance program reimbursement. The additional channel obligations for the three months ended June 30, 2016 relate to adjusted Medicaid billings from previous quarters since the first quarter of 2014.
•Cost of revenue included in discontinued operations consists of direct products costs and related overhead, Abstral royalties based on net revenue, inventory obsolescence, and other direct costs.
•Research and development expense included in discontinued operations consists of expenses related to our Abstral RELIEF trial and other product stability costs.
•Selling, general and administrative expense included in discontinued operations consists of all other expenses of our commercial operations that are required in order to market and sell our marketed products. These expenses include all personnel related costs, marketing, data, consulting, legal, consulting, and other outsider services necessary to support the commercial operations. During the three and six months ended June 30,2016 we incurred $2.2 million and $4.6 million respectively, in selling, general, and administrative expense in discontinued operations, of which $2.2 million and $4.4 million for the three and six months ended June 30, 2016, respectively, related to legal expenses from external counsel associated with document production for the subpoenas related to the sales and marketing practices of Abstral. We expect the third quarter of 2016 to incur similar legal expenses and wind down during the fourth quarter of the year. These legal proceedings are further disclosed in Part II, Item 1.
37
Liquidity and Capital Resources
We had cash and cash equivalents of approximately $19.6 million as of June 30, 2016, compared with $29.7 million as of December 31, 2015.
The decrease of approximately $10.1 million in our cash and cash equivalents from December 31, 2015 to June 30, 2016 was attributable primarily to $24.7 million used in operating activities, $1.1 million in selling expenses related to the sale of our commercial products in the fourth quarter of 2015, and $4.7 million in payments on long-term debt. The decrease was partially offset by $20.2 million in net proceeds from issuance of common stock and warrants to purchase common stock.
On May 10, 2016, we entered into a Securities Purchase Agreement, with certain purchasers pursuant to which the Company sold, at a 6.375% original issue discount, a total of $25.5 million Senior Secured Debentures (the “Debenture”) and warrants to purchase up to 2.0 million shares of the our common stock. Net proceeds to the Company from sale of the Debentures, after payment of commissions and legal fees, were approximately $23.4 million. The Debentures mature November 10, 2018, and accrue interest at 9% per year and contain no conversion features to shares of our common stock. The Debentures carry an interest only period of six months following which the holder shall have the rights, at its option, to require the Company to redeem up to $1,100,000 of the outstanding principal amount of the Debentures. Interest is payable at the end of each month based on the outstanding principal. We can pay the applicable redemption amount in cash or, at the Company’s election and subject to certain conditions, in shares of the Company's common stock. The Company, at its option, may also force the holder to redeem up to double the monthly redemption principal amount of the Debentures but not less than the monthly payment.
The Company’s obligations under the Debenture can be accelerated in the event the Company undergoes a change in control and other customary events of default. In the event of default and acceleration of the Company’s obligations, the Company would be required to pay all amounts of principal and interest then outstanding under the Debentures in cash. The Company’s obligations under the Debentures are secured under a Security Agreement by a senior lien on all of the Company’s assets, including all of the Company’s interests in its consolidated subsidiaries. The Company must also maintain a minimum of $24.0 million in cash based on the public announcement of the interim analysis of the PRESENT Trial, which is included in restricted cash as of June 30, 2016. The Company is in discussions with the holder in the event that the holder does not require the Company to prepay the outstanding principal to potentially amend terms of the Debenture. Their right to require the Company to prepay the outstanding principal amount expires 30 trading days after June 29, 2016, the announcement date of the interim analysis. Therefore as of June 30, 2016 the Debentures, net of unamortized discounts, are presented as current liabilities on the condensed consolidated balance sheet.
On July 13, 2016, we closed the sale to certain institutional investors of 28,000,000 shares of common stock at a purchase price per share of $0.45 in a registered direct offering, and warrants to purchase up to 14,000,000 shares of common stock with an exercise price of$0.65 per share in a concurrent private placement. The warrants are initially exercisable six months and one day following issuance and have a term of five years from the date of issuance. The net proceeds to Galena after deducting placement agent fees and estimated offering expenses are expected to be approximately $11.7 million. The Company intends to use the net proceeds from this offering to fund its clinical trials of its product candidates, to augment its working capital, and for general corporate purposes. The current unrestricted cash and cash equivalents as of the date of this filing will fund the Company's operations for at least six months.
The Company will need to continue to incur significant expenses to advance our development portfolio and will need to raise additional capital to finance such activities. The Company cannot be certain that it will be able to raise additional capital on favorable terms, or at all, which raises substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
38
In addition to the sale of Debentures and July 2016 registered direct equity offering, the Company has a Purchase Agreement with Lincoln Park Capital, LLC and At Market Issuance Sales Agreements (ATM) with FBR & Co. (formerly MLV & Co. LLC) and Maxim Group LLC. The Purchase Agreement and ATM are unavailable to the Company until 75 days post closing of our July 2016 financing. The Company cannot provide assurances that its plans will not change or that changed circumstances will not result in the depletion of its capital resources more rapidly than it currently anticipates. The Company is seeking and will need to raise additional capital, whether through a sale of equity or debt securities, a strategic business collaboration with a pharmaceutical company, the establishment of other funding facilities, licensing arrangements, asset sales or other means, in order to continue the development of the Company's product candidates and to support its other ongoing activities. However, the Company cannot provide assurances that such additional capital will be available on acceptable terms or at all.
Cash Flows
The following table summarizes our cash flows from operating, investing, and financing activities for the six months ended June 30, 2016 and 2015 ($ in thousands):
For the Six Months Ended June 30, | |||||||
2016 | 2015 | ||||||
Cash flows from continuing operations: | |||||||
Cash flows used in continuing operating activities | $ | (17,588 | ) | $ | (19,436 | ) | |
Cash flows used in continuing investing activities | (6 | ) | (34 | ) | |||
Cash flows provided by continuing financing activities | 15,623 | 45,613 | |||||
Total cash flows provided by (used in) continuing operating activities | (1,971 | ) | 26,143 | ||||
Cash flows from discontinued operations: | |||||||
Cash flows used in discontinued operating activities | (7,119 | ) | (3,980 | ) | |||
Cash flows used in discontinued investing activities | (1,050 | ) | (534 | ) | |||
Total cash flows used in discontinued operating activities | (8,169 | ) | (4,514 | ) | |||
Total cash flows: | |||||||
Cash flows used in operating activities | (24,707 | ) | (23,416 | ) | |||
Cash flows used in investing activities | (1,056 | ) | (568 | ) | |||
Cash flows provided by financing activities | 15,623 | 45,613 | |||||
Total increase (decrease) in cash and cash equivalents | $ | (10,140 | ) | $ | 21,629 | ||
Net Cash Flow from Operating Activities
Net cash used in operating activities increased $1.3 million for the six months ended June 30, 2016, compared to the six months ended June 30, 2015. The increase in cash used in operating activities was driven by an increase of $3.1 million cash used in our discontinued operating activities, partially offset by a reduction of $1.8 million for the continuing operating activities. The increase in total net cash used in continuing operating activities was due to payments made for severance and exit costs related to our sales and marketing team and payments on channel liabilities for Abstral and Zuplenz.
39
Net Cash Flow from Investing Activities
Net cash used in investing activities was $1.1 million for the six months ended June 30, 2016, compared with $0.6 million for the six months ended June 30, 2015. The increase was due to $1.1 million of payments for selling costs paid in the first quarter of 2016 that were incurred from the sale of commercial assets in the fourth quarter of 2015 compared to the $0.5 million milestone payment for U.S. rights to Zuplenz during the first quarter of 2015.
Net Cash Flow from Financing Activities
Net cash provided by financing activities was $15.6 million for the six months ended June 30, 2016, compared with $45.6 million for the six months ended June 30, 2015. The decrease was primarily attributable to $47.4 million in net proceeds from the issuance of common stock and warrants to purchase common stock during the six months ended June 30, 2015 compared to $20.2 million in net proceeds from the issuance of common stock and warrants to purchase common stock during the six months ended June 30, 2016. The cash flows provided by financing activities were partially offset by principal payments on long-term debt of $4.8 million and $1.9 million for the six month periods ended June 30, 2016 and 2015, respectively.
40
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet financing arrangements other than operating leases.
Critical Accounting Policies and Estimates
In our Annual Report on Form 10-K for the year ended December 31, 2015, we disclosed our critical accounting policies and estimates upon which our financial statements are derived. There have been no changes to these policies since December 31, 2015 that are not included in Note 1 of the accompanying condensed consolidated financial statements for the three and six months ended June 30, 2016. Readers are encouraged to read our Annual Report on Form 10-K in conjunction with this report.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
The primary objective of our investment activities is to preserve capital. We do not utilize hedging contracts or similar instruments.
We are exposed to certain market risks relating primarily to interest rate risk on our cash and cash equivalents and risks relating to the financial viability of the institutions which hold our capital and through which we have invested our funds. We manage the latter risks by investing primarily in money market mutual funds.
In addition, we are exposed to foreign currency exchange rate fluctuations relating to payments we make to certain vendors and suppliers and license partners using foreign currencies. We do not hedge against foreign currency risks. Consequently, changes in exchange rates could adversely affect our operating results and stock price. Such losses have not been significant to date.
41
ITEM 4. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
As of the end of the period covered by this quarterly report on Form 10-Q, our principal executive officer and our principal financial officer (the “Certifying Officers”), evaluated the effectiveness of our disclosure controls and procedures. Disclosure controls and procedures are controls and procedures designed to reasonably assure that information required to be disclosed in our reports filed under the Securities Exchange Act of 1934 (the “Exchange Act”), such as this quarterly report on Form 10-Q, is recorded, processed, summarized and reported within the time periods specified in the SEC rules and forms. Disclosure controls and procedures are also designed to reasonably assure that such information is accumulated and communicated to our management, including the Certifying Officers, as appropriate to allow timely decisions regarding required disclosure. Based on these evaluations, the Certifying Officers have concluded, that, as of the end of the period covered by this quarterly report on Form 10-Q:
(a) | our disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed by us in the reports we file or submit under the Exchange Act was recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms; and |
(b) | our disclosure controls and procedures were effective to provide reasonable assurance that material information required to be disclosed by us in the reports we file or submit under the Exchange Act was accumulated and communicated to our management, including the Certifying Officers, as appropriate to allow timely decisions regarding required disclosure. |
Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting that occurred during the three and six months ended June 30, 2016 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
42
PART II - OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
On December 3, 2015, we agreed in principle to resolve and settle the consolidated shareholder derivative action, In re Galena Biopharma, Inc. Derivative Litigation, Civil Action No. 3:14-cv-00382-SI, pending in the United States District Court for the District of Oregon against us and certain of our current and former officers and directors. On April 21, 2016, the District Court of Oregon held the final approval hearing of the settlement with the derivative plaintiffs after which the District Court continued the final approval hearing until June 23, 2016 and requested the parties submit additional briefing by June 9, 2016 on the fee request by the derivative plaintiffs’ attorneys. Following the hearing on June 23, 2016, on June 24, 2016, the U.S. District Court for the District of Oregon entered a final order and judgment in In re Galena Biopharma, Inc. Derivative Litigation, granting final approval to the settlement.
On the same day, the Court also issued an opinion and order awarding attorney’s fees of $4.5 million plus costs, which will be paid by our insurance carriers. The settlement includes a payment of $15 million in cash by our insurance carriers, which we used to fund a portion of the class action settlement, and cancellation of 1,200,000 outstanding director stock options. The settlement also requires that we adopt and implement certain corporate governance measures. The settlement does not include any admission of wrongdoing or liability on the part of us or the individual defendants and includes a full release of us and the current and former officers and directors in connection with the allegations made in the consolidated federal derivative actions and state court derivative actions.
On December 3, 2015, we also agreed in principal to resolve and settle the securities putative class action lawsuit, In re Galena Biopharma, Inc. Securities Litigation, Civil Action No. 3:14-cv-00367-SI, pending against us, certain of our current and former officers and directors and other defendants in the United States District Court for the District of Oregon. The District Court has set the final approval hearing of such settlement for June 23, 2016. Following the hearing on June 23, 2016, on June 24, 2016, the U.S. District Court for the District of Oregon entered a final order and partial judgment in In re Galena Biopharma, Inc. Securities Litigation, granting final approval of the settlement. On the same day, the Court also issued an opinion and order awarding attorney’s fees of $4.5 million plus costs, which is paid out of the settlement funds. The agreement provides for a settlement payment of $20 million to the class and the dismissal of all claims against us and the other defendants in connection with the consolidated federal securities class actions. Of the $20 million settlement payment to the class, $16.7 million was paid by our insurance carriers and $2.3 million in cash was paid by us on July 1, 2016, along with $1 million in shares of our common stock (480,053 shares) paid by us on July 6, 2016. We will be responsible for defense costs and any settlements or judgments incurred for any related opt-out lawsuits.
We have resolved claims brought by shareholders that relate to the securities litigation mentioned above in one case for $150,000 plus $150,000 in shares (291,262) of our common stock, and in another case for $1.5 million in shares of our common stock (3,366,750 shares). The shares issued in connection with such settlements are included in the secondary offering filed on July 25, 2016. The settlements do not include any admission of wrongdoing or liability on the part of us or any of the current or former directors and officers and includes a full release of us and the current and former directors and officers in connection with the allegations made. We are not aware of any other claims made by shareholders who have opted out of the securities litigation.
We are aware that the SEC is investigating certain matters relating to the use of certain outside investor-relations professionals by us and other public companies. We have been in contact with the SEC staff through our counsel and are cooperating with the investigation and in discussions with the SEC staff to resolve the investigation.
A federal investigation of two of the high-prescribing physicians for Abstral has resulted in the criminal prosecution of the two physicians for alleged violations of the federal False Claims Act and other federal statutes. The criminal trial is set for October 2016. We have received a trial subpoena for documents in connection with that investigation and we have been in contact with the U.S. Attorney’s Office for the Southern District of Alabama, which is handling the criminal trial, and are cooperating in the production of documents. On April 28, 2016, a second superseding indictment was filed in the criminal case, which added additional information about the defendant physicians and provided information regarding the facts and circumstances involving a rebate agreement between the Company and the defendant physicians’ pharmacy as well as their ownership of our stock. Certain former employees have received trial subpoenas to appear at the trial and provide oral testimony. We have agreed to reimburse those former employees’ attorney’s fees. To our knowledge, we are not a target or subject of that
43
investigation.
There also have been federal and state investigations of a company that has a product that competes with Abstral in the same therapeutic class, and we have learned that the FDA and other governmental agencies are investigating our Abstral promotion practices. On December 16, 2015, we received a subpoena issued by the U.S. Attorney’s Office in District of New Jersey requesting the production of a broad range of documents pertaining to our marketing and promotional practices for Abstral. We have been in contact with the U.S. Attorney’s Office for the District of New Jersey and are cooperating in the production of the requested documents. We are unable to predict whether we could become subject to legal or administrative actions as a result of these matters, or the impact of such matters. If we are found to be in violation of the False Claims Act, Anti-Kickback Statute, Patient Protection and Affordable Care Act, or any other applicable state or any federal fraud and abuse laws, we may be subject to penalties, such as civil and criminal penalties, damages, fines, or an administrative action of exclusion from government health care reimbursement programs. We can make no assurances as to the time or resources that will need to be devoted to these matters or their outcome, or the impact, if any, that these matters or any resulting legal or administrative proceedings may have on our business or financial condition.
44
ITEM 1A. RISK FACTORS
In addition the risk factors included in our Annual Report on Form 10-K for the year ended December 31, 2015 filed and our most recent Quarterly Report on Form 10-Q for the three months ended March 31, 2016 with the SEC, you should consider the following new or updated risk factors:
Our common stock is currently trading at prices less than $1.00, which is the minimum bid price requirement under NASDAQ’s continued listing standards. If our common stock continues to trade at such prices, our common stock may be subject to delisting from the NASDAQ Capital Market.
The continued listing requirements of the NASDAQ Capital Market require that the closing bid price of our common stock not be less than $1.00. Following our announcement on June 29, 2016, that we had stopped our PRESENT trial, the closing bid price of our common stock has been less than $1.00. If the closing bid price of our common stock remains under $1.00 for a period of at least 30 consecutive trading days, we expect to receive a letter from NASDAQ advising us that we are not in compliance with its continued listing requirements under NASDAQ Listing Rule 5502(a)(2). In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we would then have 180 calendar days to regain compliance with the $1.00 minimum bid price requirement. We can regain compliance with the $1.00 minimum bid listing requirement if the closing bid price of our is at least $1.00 per share for a minimum of ten consecutive trading days during this initial 180-day compliance period. If compliance is not achieved within the 180-day period, NASDAQ would provide written notification to us that our common stock is subject to delisting.
In the event that we fail to regain compliance with NASDAQ continued listing standards by the expiration of the applicable cure period or any extension period, NASDAQ will commence suspension and delisting procedures with respect to our common stock, which could impair the value of your investment. If our common stock is delisted from NASDAQ Capital Market in the future, such securities may be traded over-the-counter on the “pink sheets.” Such alternative market, however, is generally considered to be less efficient than, and not as broad as, NASDAQ. Accordingly, delisting of our common stock from NASDAQ could have a significant negative effect on the trading volume, liquidity and market price of our common stock. In addition, the delisting of our common stock could adversely affect our ability to raise capital on terms acceptable to us or at all and could reduce the number of investors willing to hold or acquire our common stock.
We are, and in the future may be, subject to legal or administrative actions that could adversely affect our financial condition and our business.
We are aware that the SEC is investigating certain matters relating to the use of certain outside investor-relations professionals by us and other public companies. We have been in contact with the SEC staff through our counsel and are cooperating with the investigation and in discussions with the SEC staff.
A federal investigation of two of the high-prescribing physicians for Abstral has resulted in the criminal prosecution of the two physicians for alleged violations of the federal False Claims Act and other federal statutes. The criminal trial is set for October 2016. We have received a trial subpoena for documents in connection with that investigation and we have been in contact with the U.S. Attorney’s Office for the Southern District of Alabama, which is handling the criminal trial, and are cooperating in the production of documents. On April 28, 2016, a second superseding indictment was filed in the criminal case, which added additional information about the defendant physicians and provided information regarding the facts and circumstances involving a rebate agreement between the Company and the defendant physicians’ pharmacy as well as their ownership of our stock. Certain former employees have received trial subpoenas to appear at the trial and provide oral testimony. We have agreed to reimburse those former employees’ attorney’s fees. To our knowledge, we are not a target or subject of that investigation.
There also have been federal and state investigations of a company that has a product that competes with Abstral in the same therapeutic class, and we have learned that the FDA and other governmental agencies are investigating our Abstral promotion practices. On December 16, 2015, we received a subpoena issued by the U.S. Attorney’s Office in District of New Jersey requesting the production of a broad range of documents pertaining to our marketing and promotional practices for Abstral. We have been in contact with the U.S. Attorney’s Office for the District of New Jersey and are cooperating in the production of the requested documents. We are unable to predict whether we could become subject to legal or administrative actions as a result of these matters, or the impact of such matters.
If we are found to be in violation of the False Claims Act, Anti-Kickback Statute, Patient Protection and Affordable
45
Care Act, or any other applicable state or any federal fraud and abuse laws, we may be subject to penalties, such as civil and criminal penalties, damages, fines, or an administrative action of exclusion from government health care reimbursement programs. We can make no assurances as to the time or resources that will need to be devoted to these matters or their outcome, or the impact, if any, that these matters or any resulting legal or administrative proceedings may have on our business or financial condition.
Litigation is inherently uncertain. We have incurred and may continue to incur substantial unreimbursed legal fees and other expenses in connection with these or other legal and regulatory proceedings that may not qualify for coverage under, or may exceed the limits of, our applicable directors and officers liability insurance policies and could have a material adverse effect on our financial condition, liquidity, and results of operations. These matters also may distract the time and attention of our officers and directors or divert our other resources away from our ongoing development programs. An unfavorable outcome in any of these matters could damage our business and reputation or result in additional claims or proceedings against us.
The terms of our outstanding indebtedness may inhibit potential acquirers.
We are prohibited by the terms of our outstanding indebtedness from disposing of any of our business or property, except with the consent of our lenders or if we were to prepay the outstanding indebtedness and related fees in accordance with the loan security agreement. Our outstanding indebtedness may inhibit potential acquirers or other interested parties from seeking to acquire all or a part of our business or assets, and there is no assurance that our lenders would consent to any proposed future transaction that might be beneficial to our stockholders.
You may have difficulty evaluating our business, and our historical financial information may not be representative of our future results.
We recently announced the recommendation by the IDMC to stop the PRESENT Trial for futility and based upon our investigation we closed down the trial. As a result, we will focus our resources on our pipeline of other product candidates. Thus, we will have no recurring revenues unless and until we are able to obtain marketing approval of one or more of our other product candidates and our historical financial information may not be representative of our future results.
Risks Relating to Our Financial Position and Capital Requirements
We may not be able to obtain sufficient financing, and may not be able to develop our product candidates.
We had cash and cash equivalents of approximately $19.6 million as of June 30, 2016. In addition, we have approximately $24 million of restricted cash that is reserved for a lender who has the option to redeem all, or part, of such amount within 30 trading days of our public announcement on June 29, 2016, of the discontinuation of the Phase 3 PRESENT Trial upon the IDMC’s recommendation on June 27, 2016. As of the date of this prospectus, the lender has not redeemed the approximately $24 million in full or in part.
We had no revenue for the quarter ending June 30, 2016, and our cash burn from operations for the quarter ending June 30, 2016 was approximately $11.5 million. In addition, we paid off our loan with Oxford Finance LLC for a total of $3.1 million. The Company has stopped the PRESENT Trial and has completed the investigation. based upon the results of the investigation, the estimated cost to close out the PRESENT Trial will be between $2.5 million to $3.5 million. On July 1, 2016, we paid $2.3 million for the securities class action settlement. We believe that our existing cash and cash equivalents together with the net proceeds we received on July 13, 2016 from selling 28,000,000 shares of common stock and 14,000,000 warrants (the “July Financing”) should be sufficient to fund our operations for at least six months. This projection is based on our current planned operations, stopping the PRESENT Trial and investigation of the causes of the failure of such clinical trial, anticipated payments for defense costs for the cooperation and discussions with the staff in the SEC investigation and other governmental investigations, resolution of the SEC investigation and is subject to changes in our plans and uncertainties inherent in our business. We will need to seek to replenish our existing cash and cash equivalents prior to the end of 2016. We also have funding available under our amended purchase agreement with Lincoln Park Capital Fund, LLC and sales agreements with MLV & Co. and Maxim Group LLC described in the previously filed prospectuses, but there is no guarantee that such funding will be available to us on favorable terms or will be sufficient to meet all of our future funding needs. Additionally, in connection with the July Financing, we have agreed not to issue any shares of our common stock (including under our purchase agreement with Lincoln Park Capital Fund, LLC and under our sales agreements with MLV & Co. and Maxim Group LLC) for a period of 75 days from the date of the closing of the July Financing.
46
At our annual meeting of stockholders adjourned on July 15, 2016, our stockholders approved an increase in our authorized common stock from 275,000,000 to 350,000,000. We are not able to predict whether these additional shares will be sufficient based upon our current stock price to meet the Company’s ongoing financing requirements to maintain the Company’s operations. If we fail to obtain additional future funding when needed, we could be forced to scale back or terminate our operations, or to seek to merge with or to be acquired by another company. We may not be able to meet our obligations as they come due, raising substantial doubts as to our ability to continue as a going concern. Any such inability to continue as a going concern may result in our common stock holders losing their entire investment. There is no guaranty that we will become profitable or secure additional financing.
We expect to continue to incur significant research and development expenses, which may make it difficult for us to attain profitability, and may lead to uncertainty about our ability to continue as a going concern.
Substantial funds were expended to develop our technologies and product candidates, and additional substantial funds will be required for further preclinical testing and clinical trials of our product candidates, and to manufacture and market any products that are approved for commercial sale. Because the successful development of our products is uncertain, we are unable to precisely estimate the actual funds we will require to develop and potentially commercialize them. In addition, we may not be able to generate enough revenue, even if we are able to commercialize any of our product candidates, to become profitable.
In the event that we are unable to achieve or sustain profitability or to secure additional financing, we may not be able to meet our obligations as they come due, raising substantial doubts as to our ability to continue as a going concern. Any such inability to continue as a going concern may result in our common stock holders losing their entire investment. There is no guaranty that we will become profitable or secure additional financing. Our financial statements contemplate that we will continue as a going concern and do not contain any adjustments that might result if we were unable to continue as a going concern. Changes in our operating plans, our existing and anticipated working capital needs, the acceleration or modification of our expansion plans, increased expenses, potential acquisitions or other events will all affect our ability to continue as a going concern. Future financing may be obtained through, and future development efforts may be paid for by, the issuance of debt or equity, which may have an adverse effect on our security holders or may otherwise adversely affect our business.
If we raise funds through the issuance of debt or equity, any debt securities or preferred stock issued will have rights, preferences and privileges senior to those of holders of our common stock in the event of a liquidation. In such event, there is a possibility that once all senior claims are settled, there may be no assets remaining to pay out to the holders of common stock. In addition, if we raise funds through the issuance of additional equity, whether through private placements or additional public offerings, such an issuance would dilute your ownership in us.
The terms of debt securities may also impose restrictions on our operations, which may include limiting our ability to incur additional indebtedness, to pay dividends on or repurchase our capital stock, or to make certain acquisitions or investments. In addition, we may be subject to covenants requiring us to satisfy certain financial tests and ratios, and our ability to satisfy such covenants may be affected by events outside of our control.
Risks Relating to Our Development Programs
Our Phase 3 PRESENT clinical trial has been stopped due to futility and though we are conducting an investigation of the causes for the failure of the clinical trial, we are not certain that the investigation will result in the reason(s) for the failure.
On June 27, 2016, the Independent Data Monitoring Committee conducting the pre-planned interim analysis of the
PRESENT Trial recommended that we stop the clinical trial because of futility. We have conducted an investigation of the causes of the failure of the trial, which has resulted in the trial being closed down. The investigation has not yet provided the reasons for the failure of the clinical trial. The investigation has caused us to unblind the data of the clinical trial. The PRESENT Trial may no longer likely be considered a registrational clinical trial. Even if we determine the causes of the failure, NeuVax may never be approved for the treatment of patients with Node positive HER2 negative breast cancer.
47
ITEM 6. EXHIBITS
Exhibit Number | Description | ||
4.1 | 9% Original Issues Discount Senior Secured Debenture of Galena Biopharma, Inc. ** | ||
4.2 | Series A Common Stock Purchase Warrant assigned to JGB (Cayman) Newton Ltd.** | ||
4.3 | Series B Common Stock Purchase Warrant assigned to JGB (Cayman) Newton Ltd.** | ||
10.1 | Securities Purchase Agreement dated May 10, 2016 between Galena Biopharma, Inc. and Purchasers.** | ||
10.2 | Subsidiary Guarantee dated May 10, 2016 between Galena Biopharma, Inc. and JGB Collateral LLC. ** | ||
10.3 | Registration Rights Agreement dated May 10, 2016 between Galena Biopharma, Inc. and Purchasers.** ** | ||
10.4 | Security Agreement dated May 10, 2016 between Galena Biopharma, Inc. and JGB Collateral LLC.** | ||
31.1 | Sarbanes-Oxley Act Section 302 Certification of Mark W. Schwartz, Ph.D.* | ||
31.2 | Sarbanes-Oxley Act Section 302 Certification of John T. Burns* | ||
32.1 | Sarbanes-Oxley Act Section 906 Certification of Mark W. Schwartz, Ph.D., and John T. Burns* | ||
101.INS | XBRL Instance Document.* | ||
101.SCH | XBRL Taxonomy Extension Schema.* | ||
101.CAL | XBRL Taxonomy Extension Calculation.* | ||
101.DEF | XBRL Taxonomy Extension Definition.* | ||
101.LAB | XBRL Taxonomy Extension Label.* | ||
101.PRE | XBRL Taxonomy Extension Presentation.* | ||
101.PRE | XBRL Taxonomy Extension Presentation.* | ||
* | Filed herewith. |
** | Previously filed as an Exhibit to the Company’s Form 10-Q filed on May 10, 2016 (File No. 001-33958) and incorporated herein by reference. |
48
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
GALENA BIOPHARMA, INC. | |||
By: | /s/ Mark W. Schwartz | ||
Mark W. Schwartz, Ph.D. | |||
President and Chief Executive Officer | |||
Date: August 9, 2016 | |||
By: | /s/ John T. Burns | ||
John T. Burns | |||
Vice President, Finance and Corporate Controller (Principal Accounting Officer) | |||
Date: August 9, 2016 | |||
49
