Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - UNIVERSAL BIOSENSORS INC | Financial_Report.xls |
| EX-32.0 - EX-32.0 - UNIVERSAL BIOSENSORS INC | d837900dex320.htm |
| EX-31.2 - EX-31.2 - UNIVERSAL BIOSENSORS INC | d837900dex312.htm |
| EX-31.1 - EX-31.1 - UNIVERSAL BIOSENSORS INC | d837900dex311.htm |
| EX-13.0 - EX-13.0 - UNIVERSAL BIOSENSORS INC | d837900dex130.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | Annual Report Pursuant To Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the fiscal year ended December 31, 2014
OR
| ¨ | Transition Report Pursuant To Section 13 or 15(d) of the Securities Exchange Act of 1934 |
Commission File Number: 000-52607
Universal Biosensors, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 98-0424072 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) |
| Universal Biosensors, Inc. 1 Corporate Avenue, Rowville, 3178, Victoria Australia |
Telephone: +61 3 9213 9000 | Not Applicable | ||
| (Address of principal executive offices) | (Registrant’s telephone number, including area code) |
(Zip Code) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Name of each exchange on which registered | |
| None | Not applicable |
Securities registered pursuant to Section 12(g) of the Act:
Title of each class
Shares of common stock, par value US$0.0001
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act:
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The approximate aggregate market value of voting and non-voting common equity held by non-affiliates of the registrant was A$26,975,748 (equivalent to US$25,411,155) as of June 30, 2014.
The number of shares outstanding of each of the registrant’s classes of common stock as of March 4, 2015:
| Title of Class |
Number of Shares | |
| Common Stock, US$.0001 par value | 175,893,533 |
Documents incorporated by reference:
Certain information contained in the registrant’s definitive Proxy Statement for the 2014 annual meetings of stockholders, to be filed not later than 120 days after the end of the fiscal year covered by this report, is incorporated by reference into Part III hereof.
Information contained on pages F-2 through F-44 of our Annual Report to Stockholders for the fiscal year ended December 31, 2014 is incorporated by reference in our response to Items 7, 7A, 8 and 9A of Part II.
Table of Contents
| Page | ||||||
| 3 | ||||||
| ITEM 1. |
4 | |||||
| ITEM 1A. |
11 | |||||
| ITEM 1B. |
21 | |||||
| ITEM 2. |
22 | |||||
| ITEM 3. |
23 | |||||
| ITEM 4. |
24 | |||||
| ITEM 5. |
25 | |||||
| ITEM 6. |
30 | |||||
| ITEM 7. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION |
31 | ||||
| ITEM 7A. |
32 | |||||
| ITEM 8. |
33 | |||||
| ITEM 9. |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
35 | ||||
| ITEM 9A. |
36 | |||||
| ITEM 9B. |
39 | |||||
| ITEM 10. |
40 | |||||
| ITEM 11. |
41 | |||||
| ITEM 12. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
42 | ||||
| ITEM 13. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
43 | ||||
| ITEM 14. |
44 | |||||
| ITEM 15. |
45 | |||||
| 49 | ||||||
Unless otherwise noted, references on this Form 10-K to “Universal Biosensors”, the “Company,” “Group,” “we,” “our” or “us” means Universal Biosensors, Inc. (“UBI”) a Delaware corporation and, when applicable, its wholly owned Australian operating subsidiary, Universal Biosensors Pty Ltd. Our principal place of business is located at 1 Corporate Avenue, Rowville, Victoria 3178, Australia. Our telephone number is +61 3 9213 9000. Unless otherwise noted, all references in this Form 10-K to “$”, “A$” or “dollars” and dollar amounts are references to Australian dollars. References to “US$” are references to United States dollars.
2
Table of Contents
This Form 10-K contains forward-looking statements that involve known and unknown risks, uncertainties and other factors that may cause our, our customers and partners’ or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements. All statements, other than statements of historical facts, are forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
| • | our business and product development strategies; |
| • | our expectations with respect to collaborative, strategic or distribution arrangements; |
| • | our expectations with respect to the timing and amounts of revenues from our customers and partners; |
| • | our expectations with respect to the services we provide to, and the development projects we undertake for, our customers and partners; |
| • | our expectations with respect to regulatory submissions, approvals, market launches of products we develop or are involved in developing; |
| • | our expectations with respect to sales of products we develop or are involved in developing and the quantities of such products to be manufactured by us; |
| • | our expectations with respect to our research and development programs, the timing of product development and our associated research and development expenses; |
| • | the ability to protect our owned or licensed intellectual property; and |
| • | our estimates regarding our capital requirements, the sufficiency of our cash resources, our debt repayment obligations and our need for additional financing. |
The words “anticipates,” believes,” “continue,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “projects,” “should,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. You should not place undue reliance on these forward-looking statements, which apply only as of the date of this Form 10-K. The forward-looking statements included in this Form 10-K do not guarantee our future performance, and actual results could differ from those contemplated by these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that we make. We undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in cautionary statements throughout this Form 10-K, particularly those set forth in section “Item 1A - Risk Factors.” However, new factors emerge from time to time and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We do not undertake to update or revise any forward-looking statements.
3
Table of Contents
The following discussion and analysis should be read in conjunction with our financial statements and related notes included elsewhere in this Form 10-K. This discussion and analysis contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth in the section entitled “Item 1A - Risk Factors” and elsewhere in this Form 10-K.
Business overview
We are a specialist medical diagnostics company focused on the research, development and manufacture of in vitro diagnostic test devices for consumer and professional point-of-care use.
We were incorporated in the State of Delaware on September 14, 2001 and our shares of common stock in the form of CHESS Depositary Interests (“CDIs”) have been quoted on the Australian Securities Exchange (“ASX”) since December 13, 2006. Our securities are not currently traded on any other public market. Our wholly owned subsidiary and primary operating vehicle, Universal Biosensors Pty Ltd (“UBS”) was incorporated as a proprietary limited company in Australia on September 21, 2001. UBS conducts our research, development and manufacturing activities in Melbourne, Australia.
Our principal place of business is 1 Corporate Avenue, Rowville, Victoria 3178, Australia. Our principal telephone number in Australia is +61 3 9213 9000. Our agent for service in the United States is Corporation Service Company of 2711 Centerville Road, Suite 400, Wilmington, DE 19808, United States. We also maintain a web site at www.universalbiosensors.com. The information contained in, or that can be accessed through, our web site is not part of this Form 10-K.
We have rights to an extensive patent portfolio, with certain patents owned by UBS and a number licensed to UBS by LifeScan, Inc. and other third party licensors. Unless otherwise noted, references to “LifeScan” in this document are references collectively or individually to LifeScan, Inc., and/or LifeScan Europe, a division of Cilag GmbH International, both affiliates of Johnson and Johnson.
We are using our electrochemical cell technology platform to develop tests for a number of different markets. Our current focus is as set out below:
| • | Coagulation testing market – We are working with Siemens Healthcare Diagnostics, Inc. (“Siemens”) in relation to a range of products for the point-of-care coagulation market, pursuant to a Collaboration Agreement with Siemens (“Collaboration Agreement”). The first such product developed with Siemens, the Xprecia StrideTM Coagulation Analyzer, received CE mark approval on December 9, 2014 and is now being released by Siemens in Europe. The CE marking is mandatory for any company prior to selling its product within the 31 countries operating in the European Economic Area. The CE marking indicates a product is fully compliant with all relevant EU legislation and can move freely within the European Economic Area. Under the terms of a supply agreement with Siemens (“Supply Agreement”), UBS acts as exclusive manufacturer of test strips for this product and two further tests still in development with Siemens. We are also developing our own PT-INR test targeted at the patient self-test market and intend to enter into distribution arrangements with respect to that test. |
| • | Blood glucose – We will provide services to LifeScan as required from time to time, pursuant to a Master Services and Supply Agreement (“Master Services and Supply Agreement”) and a development and research agreement (“Development and Research Agreement”) with LifeScan. |
| • | Other electrochemical-cell based tests – We are working on proving the broader applicability of our technology platform, including tests based on enzymatic, immunoassay and molecular diagnostic methods. We may seek to enter into collaborative arrangements, strategic alliances or distribution agreements with respect to any tests arising from this work. |
Our Strategy
We are a specialist medical diagnostics company focused on the research, development and manufacture of in vitro diagnostic test devices for consumer and professional point-of-care use. Key aspects of our strategy include:
| • | manufacturing products (test strips and meters) for our customers and partners as required; |
| • | extending our electrochemical cell technology and proving the broader applicability of our technology platform for markets with significant commercial potential. In particular, at the current time we are focusing on our own PT-INR test targeted at the patient self-test market; |
4
Table of Contents
| • | seeking to enter into collaborative, strategic or distribution arrangements with other life sciences companies or other industry participants with respect to the development and commercialization of specific tests or specific fields. We intend to enter into distribution arrangements with respect to our own PT-INR test once developed and approved for sale; |
| • | undertaking research and development work for our customers and partners; |
| • | providing post market support services to our customers and partners. |
Plan of Operations for the Remainder of the Fiscal Year Ending December 2015
Our plan of operations over the remainder of the fiscal year ending December 2015 is to:
| • | manufacture products to satisfy our customers and partners requirements; |
| • | continue to undertake research and development work for our customers and partners; |
| • | provide the necessary post-market support for our customers and partners; |
| • | prove the broader applicability of our technology platform for markets with significant commercial potential, focusing initially on enzymatic, immunoassay and molecular diagnostic point-of-care tests; |
| • | seek to enter into collaborative, strategic or distribution arrangements with other life sciences companies or other industry participants with respect to the development and commercialization of specific tests or specific fields. |
Financial information about segments
We operate in one segment. Our principal activities are the research, development and manufacture of in vitro diagnostic test devices for consumer and professional point-of-care use. Although our products are intended for sale worldwide, we operate predominantly in one geographical area, that being Australia. For details of our revenues, profit and loss and total assets for financial years ending December 31, 2014, 2013, 2012, 2011 and 2010 refer to “Item 6. Selected Financial Data”.
Description of our business
We are a specialist medical diagnostics company focused on the research, development and manufacture of in vitro diagnostic test devices for consumer and professional point-of-care use.
Industry background
We operate in the high growth, point-of-care segment of the global in vitro diagnostics (IVD) industry. A large proportion of clinical diagnostics has historically been performed by trained personnel at dedicated or centralized testing sites including hospital laboratories and commercial pathology laboratories. Significant interest has developed in techniques and technologies that allow testing to be performed “on-the-spot” (in real time at the patient’s side). Point-of-care testing can be further divided into consumer self-testing or testing of patients by one of a variety of medical or laboratory professionals in locations such as clinics, physician’s office laboratories and emergency departments. While not all tests are suited to being performed at the point-of-care, we believe our electrochemical cell technology and other technologies could be a suitable platform for adapting a number of relevant central laboratory tests to a point-of-care format.
Point-of-care tests in development and partnering strategy
We are also working to prove the broader applicability of our technology platform for markets with significant commercial potential across enzymatic, immunoassay and molecular diagnostic point-of-care tests. Our strategy is to apply the electrochemical cell technology to different fields and biomarkers and then to either enter into collaborative arrangements or strategic alliances with third parties to develop and commercialize products for those fields or, as is the case of our PT-INR test in development for the patient self-testing market, to complete the development of the products and commercialize them using distributors. To date, we have developed a blood glucose test with LifeScan and a coagulation PT-INR test with Siemens, both of which are now sold by LifeScan and Siemens, respectively. We will continue to work with Siemens to develop other test strip and reader products for the point-of-care coagulation market.
5
Table of Contents
Principal Products and Services
UBS acts as exclusive manufacturer of PT-INR coagulation test strips for Siemens’ Xprecia StrideTM Coagulation Analyzer. We will continue to work with Siemens to develop a range of other products for the point-of-care coagulation market which UBS expects to manufacture once approved for sale. UBS also conducts research and development to prove the broader applicability of our technology platform, including tests based on enzymatic, immunoassay and molecular diagnostic methods.
UBS provides LifeScan with research and development services from time to time. Between 2009 and 2013, UBS acted as a non-exclusive manufacturer of blood glucose test strips for LifeScan’s OneTouch® Verio® blood glucose testing product. While UBS no longer manufactures the OneTouch® Verio® blood glucose test strips for LifeScan, under the Master Services and Supply Agreement, UBS continues to be paid the quarterly service fee based on the number of OneTouch® Verio® strips sold, irrespective of the manufacturer of the strips in consideration of services provided. LifeScan has the option to give notice to convert the quarterly service fees, which it may only do so once it has paid cumulative quarterly service fees of US$45 million. As of December 31, 2014, LifeScan had paid cumulative quarterly service fees of US$11.8 million. In the event notice is given, LifeScan is required to the pay the quarterly service fees for the remainder of that year. In addition, LifeScan must pay a one-time lump sum fee. This one-time lump sum service fee is calculated by multiplying the sum of all quarterly service fees for the relevant year in which notice is given by a multiplier (on a sliding scale from 2.6x if notice is given in 2015 to 2x if notice is given in 2018 and beyond).
Facilities
Universal Biosensors Pty Ltd leases approximately 5,000 square meters of office, research and development and manufacturing facilities at 1 Corporate Avenue, Rowville in Melbourne, Australia. We have had ISO 13485 certification continuously at that site since May 2007. The lease for 1 Corporate Avenue expires on March 31, 2019 with an option to renew the lease for two further terms of five years each.
Raw materials
Raw materials essential to our business are purchased worldwide in the ordinary course of business from numerous suppliers. In general, these materials are available from multiple sources. Certain of our products in development may be more reliant on sole sources of supply. We seek to enter into long term contracts of supply with respect to these materials and will develop mitigation strategies, which may include development work to enable substitute materials to be used.
Distribution
With respect to certain of our products in development, including our PT-INR test, part of our strategy is to establish distribution arrangements in the future.
Regulatory clearances
In all major territories of the world, regulatory clearances are required prior to marketing diagnostic tests. The regulatory clearance requirements vary from country to country and product to product, however, regulatory clearances typically require a satisfactory “technical file”, which provides the regulatory bodies with details of the design and previous testing of the product including safety and efficacy data as well as the details of the conduct of trials which show the suitability for use of the product at the point-of-care. Regulators also require demonstration of continuing compliance with an appropriate quality management system. There is no common international regulatory body and we, or our relevant customer or partner or distributor, would be required to submit for clearance to sell in each of the major jurisdictions in which we or our relevant customers and partners seeks to market products. For example, for Europe, a “Notified Body” assesses the quality system and product technical file, whereas in the United States, the Food and Drug Administration, or “FDA”, is the regulatory body responsible for the examination of the design and performance of the device and for assessment of our quality system.
In the case of point-of-care tests, there are often additional requirements that a manufacturer must meet such as an examination of certain aspects affecting test suitability for non-professional users. In Europe, certain codified standards describe the requirements of tests whilst in the United States, tests to be used by non-laboratory professionals must gain waiver status under the United States Clinical Laboratory Improvement Amendments of 1988. Amongst other clearances, we will also require clearance for export of medical devices from the Therapeutics Goods Administration, or “TGA”, in Australia.
If we are developing a product for a customer or partner, our customers and partners are generally responsible for obtaining and maintaining all applicable regulatory approvals and determining the location and timing for submissions for regulatory clearance. We may provide a supporting role in this process. We will however be responsible for the regulatory approvals of the products which we wish to take to market through distributors.
6
Table of Contents
The importance and duration of all our patents, trademarks and licenses
We rely on a combination of patent, copyright, trademark and trade secret laws, as well as confidentiality agreements, to establish and protect our proprietary rights which in the aggregate we believe to be of material importance to us in the operation of our business. Our continued success depends to a large extent on our ability to protect and maintain our owned and licensed patents and patent applications, copyright, trademark and trade secrets.
Our point-of-care tests in development draw upon an extensive portfolio of patents and patent applications as well as know-how either owned by UBS or licensed to UBS. We patent the technology, inventions and improvements that we consider important to the development of our business.
We rely on the owned patent applications and the patents and patent applications licensed to us in the manufacture of the point-of-care diagnostic tests being developed by us and to enable us to grant rights to our customers and partners to commercialize products that we may develop.
Our owned and licensed patents extend for varying periods according to the date of patent filing or grant and the legal term of patents in the various countries where patent protection is obtained. The actual protection afforded by a patent, which can vary from country to country, depends upon the type of patent, the scope of its coverage and the availability of legal remedies in the country. Based on current product sales and our projects, the owned and licensed patents and patent applications that we consider most significant by virtue of their importance to our platform together with the last of the patents to expire within the patent family are set forth in the table below.
| Patent |
Expiration | |
| Apparatus and Method for Electrochemical Protease Sensor (this patent family relates to a sensor to detect cleavage of an electrochemical substrate for use in measuring blood or plasma coagulation in assays such as prothrombin time and thrombin potential) | Refer Note 1 | |
| Electrochemical On-Board Control Detection (this patent family relates to an on-board control system of a sensor, wherein the control system can test/verify the viability of the sensor) | Refer Note 1 | |
| Electrochemical Cell (this patent family relates to a method and an electrochemical biosensor for determining the concentration of an analyte in a carrier) | 2022 | |
| Electrochemical Method (this patent family provides an improved method and biosensor for determination of the concentration of an analyte in a carrier which provides improved accuracy, reliability and speed over prior techniques) | 2024 | |
| Electrochemical Cell (this patent family relates to an electrochemical cell for determining the concentration of an analyte in a carrier) | 2016 | |
| Electrochemical Method for Measuring Chemical Reaction Rates (this patent family relates to the measurement of the progress of a chemical reaction that generates an electroactive reaction product that is subsequently detected at an electrode amperometrically or coulometrically) | 2023 | |
| Electrochemical Cell Connector (this patent family relates to a connector to provide electrical connection between an electrochemical cell of a strip type sensor and meter circuitry) | 2026 | |
| Method and Apparatus for Rapid Electrochemical Analysis (this patent application relates to an improved method and apparatus for electrochemical analysis) | 2026 | |
| Methods and Apparatus for Analyzing a Sample in the Presence of Interferents (this patent application relates to methods and apparatus for determining analyte concentrations in a rapid and accurate manner) | 2026 | |
| System and Method for measuring an Analyte in a Sample (this patent relates to a method for measuring a temperature corrected glucose concentration over a temperature range) | 2029 | |
| Systems and Methods for Discriminating Control Solution from a Physiological Sample (this patent application relates to systems and methods for discriminating between a control solution and blood sample) | 2028 | |
| Systems and Methods of Discriminating Control Solution from a Physiological Sample (this patent application relates to systems and methods for discriminating between a control solution and a blood sample based on a summation of current values and comparing reference values to threshold values) | 2027 | |
7
Table of Contents
| 1. | The patent application is either pending, allowed, or published |
We will continue to file and prosecute patent applications when and where appropriate to attempt to protect our rights in our proprietary technologies.
Pursuant to our License Agreement with LifeScan, LifeScan is responsible for prosecution and maintenance of the patents and patent applications licensed to us by them. In the event that LifeScan elects not to proceed with the prosecution of a patent application licensed to us by them or discontinues the payment of fees, we have the right to assume and continue at our own expense the prosecution of any such patent or patent applications. We also license intellectual property from Siemens and SpeeDx Pty Ltd, who are both primarily responsible for the prosecution and maintenance of the patents and patent applications licensed to us by them.
Our ability to build and maintain our proprietary position for our technology and products will depend on our success in obtaining effective claims and those claims being enforced once granted and, with respect to intellectual property licensed to us, the licensee’s success in obtaining effective claims and those claims being enforced once granted. The patent positions of companies like ours are generally uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. Some countries in which we or our customers or partners may seek approval to sell point-of-care tests that we have been involved in developing, may fail to protect our owned and licensed intellectual property rights to the same extent as the protection that may be afforded in the United States or Australia. Some legal principles remain unresolved and there has not been a consistent policy regarding the breadth or interpretation of claims allowed in patents in the United States, the United Kingdom, the European Union, Australia or elsewhere. In addition, the specific content of patents and patent applications that are necessary to support and interpret patent claims is highly uncertain due to the complex nature of the relevant legal, scientific and factual issues. Changes in either patent laws or in interpretations of patent laws in the United States, the United Kingdom, the European Union, Australia or elsewhere may diminish the value of our intellectual property or narrow the scope of our patent protection.
Seasonality
We do not expect sales of the diagnostic tests we develop to be materially impacted by seasonality.
The practices of the registrant and the industry (respective industries) relating to working capital items.
We deal with our accounts receivables, inventory, trade payables and the supply of products in accordance with our contractual obligations to our customers, partners and suppliers.
Dependence on single customer
While we have commenced manufacturing the test strips for Siemens’ Xprecia StrideTM Coagulation Analyzer, as shown in the table below, we continue to receive a significant portion of our revenue from LifeScan. All revenue from products to December 31, 2013 was paid in connection with the manufacture of strips for LifeScan and the majority of our revenue from services is also derived from LifeScan. All revenue from products in 2014 was paid in connection with the manufacture of the test strips for Siemens’ Xprecia StrideTM Coagulation Analyzer.
| Years Ended December 31, | ||||||||||||||
| 2014 | 2013 | 2012 | ||||||||||||
| A$ | A$ | A$ | ||||||||||||
| Revenue from products |
215,486 | 10,170,804 | 19,368,745 | |||||||||||
| Revenue from services |
9,314,198 | 4,918,868 | 10,277,698 | |||||||||||
| Research and development tax incentive income |
9,935,083 | 6,279,954 | 0 | |||||||||||
| Interest income |
260,904 | 499,970 | 437,171 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| Total income |
19,725,671 | 21,869,596 | 30,083,614 | |||||||||||
|
|
|
|
|
|
|
|||||||||
| % of total income derived from |
- LifeScan | 34 | % | 62 | % | 82 | % | |||||||
| - Siemens | 14 | % | 7 | % | 16 | % | ||||||||
| - Other | 52 | % | 31 | % | 2 | % | ||||||||
8
Table of Contents
While we will no longer manufacture OneTouch® Verio® blood glucose test strips for LifeScan, we are paid a quarterly service fee based on the number of such strips sold. Our dependence on the quarterly service fees from LifeScan for a significant proportion of our revenue is likely to continue until we start to receive meaningful revenues from Siemens relating to the the Xprecia StrideTM Coagulation Analyzer and other partnering and collaborative arrangements or strategic alliances with third parties and from the sale of our own products. We started generating revenues from Siemens during 2012 and 14% of our total income in fiscal year 2014 was derived from our arrangement with Siemens.
We did not have any significant backlog orders as of December 31, 2014 and 2013.
Competitive conditions of our business
Despite Siemens having released the Xprecia StrideTM Coagulation Analyzer in Europe, our revenue is currently highly dependent on the success of the OneTouch® Verio® blood glucose product we have developed with LifeScan. OneTouch® Verio® was first launched in the Netherlands in January 2010 by LifeScan and has subsequently been launched in countries that represent over 90% of the world self-monitoring blood glucose market including North America, major European markets and Australia. LifeScan is responsible for all sales and marketing decisions and any decision to introduce the product to new territories and the timing of those decisions.
The global diabetes market place is intensely competitive and dominated by multinationals such as LifeScan, Roche, Abbott and Bayer. Changes to reimbursement of blood glucose monitoring supplies in the US market have further intensified pricing competition and margin pressures over the past 12 months. In addition, during 2013, the International Standards Organization released new guidelines that increase the accuracy and performance requirements for blood glucose monitoring systems. Although OneTouch® Verio® has been well received in the jurisdictions in which it has been launched, LifeScan controls the commercialization of the OneTouch® Verio® product and we do not know whether customers will prefer it over competitive offerings, nor the rate at which it might continue to be adopted.
Siemens is responsible for all sales and marketing decisions with respect to the products we develop for them and for any decision to introduce the products to new territories and the timing of those decisions. In December 2014, Siemens received the CE mark approval for sale of the Xprecia StrideTM Coagulation Analyzer in Europe and initiated the European limited release of its first point-of-care coagulation testing device.
The worldwide point-of-care coagulation testing market was estimated at around US$1.0 billion in 2014 and is forecast to grow by about 9% per annum to US$1.4 billion by 2018. The coagulation testing market is dominated by PT-INR testing, which represents over 65% of this market. Roche and Alere are currently the two largest players in the point-of-care professional PT-INR testing market. These companies have well established brand recognition, sales and marketing forces, and have significant resources available to support their product.
Core to our business strategy is to extend our intellectual property platform to enable other tests currently done in the central laboratory to be migrated to the point-of-care settings. Our belief is that much testing done in the central lab can more efficiently and profitably be performed at the point-of-care. With the exception of blood glucose testing, most point-of-care testing is currently conducted in professional settings. The health care professional has a choice and can request tests from a central laboratory, or services provider, or choose to have the test performed at the point-of-care. Thus we face competition not just from other companies active in the point-of-care space, but also the providers of testing who operate in centralized settings.
Our research and development expenditure during the last three fiscal years were as follows:
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| A$ | A$ | A$ | ||||||||||
| Research and development expenses |
17,136,051 | 15,483,902 | 13,482,459 | |||||||||
| Research and development tax incentive income |
(9,935,083 | ) | (6,279,954 | ) | 0 | |||||||
|
|
|
|
|
|
|
|||||||
| 7,200,968 | 9,203,948 | 13,482,459 | ||||||||||
|
|
|
|
|
|
|
|||||||
We undertake research and development for our self and on behalf of our customers and partners. Research and development activities undertaken on behalf of our customers and partners were A$9,971,035, A$10,401,575 and A$9,985,591 for the fiscal years ended 2014, 2013 and 2012, respectively.
9
Table of Contents
Employees
At March 4, 2015, we had 79 full time employees in our Melbourne facility, spanning production, engineering, quality and regulatory, research and development and administration.
Financial information about geographic areas
We operate in one segment. Our principal activities are research and development, commercial manufacture of approved medical or testing devices and the provision of services including contract research work. We operate predominantly in one geographical area, being Australia. Our total income as disclosed below is attributed to countries based on location of customer. Location has been determined generally based on contractual arrangements.
| Years Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| A$ | A$ | A$ | ||||||||||
| Home country - Australia |
10,323,745 | 6,779,924 | 437,171 | |||||||||
|
|
|
|
|
|
|
|||||||
| Foreign countries |
||||||||||||
| - Scotland |
0 | 10,170,804 | 22,454,227 | |||||||||
| - U.S.A. |
2,482,288 | 1,456,833 | 4,955,965 | |||||||||
| - Germany |
215,486 | 0 | 0 | |||||||||
| - Switzerland |
6,704,152 | 3,462,035 | 2,236,251 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total - foreign countries |
9,401,926 | 15,089,672 | 29,646,443 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total income |
19,725,671 | 21,869,596 | 30,083,614 | |||||||||
|
|
|
|
|
|
|
|||||||
Our material long-lived assets are all based in Australia.
Available Information
We file annual and quarterly reports, proxy statements and other information with the SEC. Stockholders may read and copy any reports, statements or other information that we file at the SEC’s Public Reference Room at 100 F. Street, NE, Washington, D.C 20549. Please call the Securities and Exchange Commission (the “SEC”) at 1-800-SEC-0330 for further information about the public reference rooms. Our public filings are also available from commercial document retrieval services and at the Internet Web site maintained by us at http://universalbiosensors.com and the SEC at http://www.sec.gov.
We provide without charge to each person solicited by the Proxy Statement a copy of our Annual Report on Form 10-K, including our financial statements but excluding the exhibits to Form 10-K other than Exhibit 13. The Annual Report includes a list of the exhibits that were filed with the Form 10-K, and we will furnish a copy of any such exhibit to any person who requests it upon the payment of our reasonable expenses in providing the requested exhibit. For further information, please contact our Company Secretary, Cameron Billingsley at +612 8115 9801 or write us at 1 Corporate Avenue, Rowville VIC 3178. You may also send an email to us at companysecretary@universalbiosensors.com. Our Annual Report on Form 10-K, proxy statement, and our other filings with the SEC, including the exhibits, are also available for free on our Internet site (http://universalbiosensors.com) and the SEC’s Internet site (http://www.sec.gov).
10
Table of Contents
Investing in our shares or CDIs involves a high degree of risk. Before you invest in our shares or CDIs, you should understand the high degree of risk involved. You should carefully consider the following risks and other information in this Form 10-K, including our financial statements and related notes appearing elsewhere in this Form 10-K, before you decide to invest in our shares or CDIs. If any of the events described below actually occurs, our business, financial condition and operating results could be harmed. In such an event, the market price of our CDIs would likely decline and you could lose part or all of your investment.
Our products may not be successful in the marketplace.
Our success and the success of products that we are involved in developing is ultimately dependent on the level of market acceptance and sales of those products. Market acceptance will depend on, amongst other things, the ability to provide and maintain evidence of safety, efficacy and cost effectiveness of the products, the advantages and profile over competing products, the level of support from clinicians, the relative convenience and ease of use, cost-effectiveness compared to other products, the availability of reimbursement from national health authorities, the timing of market introduction and the success of marketing and sales efforts by our customers and partners. Additionally, it is difficult to determine the market opportunity for new technologies and our estimates may not accurately reflect the actual demand in the target markets.
Our commercial opportunity will be reduced or eliminated if the size of the market opportunity is less than we expect or if our competitors develop and commercialize products that are safer, more effective, more convenient, less expensive, or reach markets sooner or are marketed better than products that we are involved in developing.
The blood glucose test strips for the One Touch® Verio® product which we developed with LifeScan were first launched in the Netherlands in January 2010 and are now available in much of the world’s self-monitored blood glucose market including North America, major European markets and Australia. While market acceptance for One Touch® Verio® has been positive to date, there is no guarantee that the product will secure and maintain adequate market share in a timely fashion.
The coagulation test strips for the Xprecia StrideTM Coagulation Analyzer which we developed with Siemens were first released in Europe in December 2014. This product represents Siemens’ first entry into this point-of-care coagulation testing market and as such, there is no track record of market acceptance for the product. As such, there can be no guarantee this product will gain market share in a timely fashion (or at all) from the market’s two largest players, Roche and Alere. These companies have well established brand recognition, sales and marketing forces, and have significant resources available to support their product.
Likewise, we cannot be sure that any other products we are involved in developing with our customers and partners, such as the other test strip and reader products for the point-of-care coagulation market that we are developing with Siemens or our own PT-INR test, will be successful in the marketplace or will secure and maintain adequate market share.
Our ability to be or maintain profitability in the future will be adversely affected if any of the products that we are involved in developing fail to achieve or maintain market acceptance or compete effectively in the market place. It would reduce or eliminate our revenues from product sales and/or manufacturing and have a material adverse effect on our business and financial position.
We are currently dependent on revenue from LifeScan.
UBI has received some initial revenue from coagulation test strip manufacturing on behalf of Siemens. Even though we expect this figure to grow significantly over time, the majority of our products and services revenue is currently derived from LifeScan. With effect from December 31, 2013, we no longer manufacture OneTouch® Verio® blood glucose test strips for LifeScan, however, we are paid a quarterly service fee based on the number of strips sold. To date, our business has been dependent on the sales of the blood glucose test strips for the OneTouch® Verio® product to fund our operations. Any changes in the level of test strip sales will directly affect the amount of the quarterly service fee paid by LifeScan and, as a result, our business.
11
Table of Contents
LifeScan has the ability to terminate the obligation to pay quarterly service fees to us in certain situations set out in the Master Services and Supply Agreement or with the agreement of Universal Biosensors. LifeScan has the option to give notice to convert the quarterly service fees, which it may only do once it has paid cumulative quarterly service fees of US$45 million. As of December 31, 2014, LifeScan has paid cumulative quarterly service fees of US$11.8 million. In the event notice is given, LifeScan is required to the pay the quarterly service fees for the remainder of that year. In addition, LifeScan must pay a one-time lump sum fee. This one-time lump sum service fee is calculated by multiplying the sum of all quarterly service fees for the relevant year in which notice is given by a multiplier (on a sliding scale from 2.6x if notice is given in 2015 to 2x if notice is given in 2018 and beyond). LifeScan may also terminate the obligation to pay quarterly service fees if certain other factors detailed in the Master Services and Supply Agreement arise, including LifeScan ceasing to sell the product, termination for breach, insolvency and bankruptcy, change of control and regulatory termination.
Even though we act as exclusive manufacturer of the coagulation test strips we developed with Siemens, the market for the Xprecia StrideTM Coagulation Analyzer is currently unknown because the product was released in December 2014. While there is no minimum commitment, if Siemens does not submit purchase orders for a certain amount of product, our manufacturing capacity may not be fully utilized. If this occurs, we will be faced with surplus capacity in our manufacturing operations and our revenues will decline. Further, Siemens may obtain the right to manufacture product or have a third party manufacture product on its behalf if certain events occur (for example, insolvency, failure to supply). The Supply Agreement with Siemens may also be terminated as a result of either party defaulting on its material obligations. If any of these circumstances arise, we would cease to have the potential to receive manufacturing revenues from the sale of product purchased by Siemens.
An important part of our strategy is to seek to enter into other collaborative arrangements or strategic alliances with respect to the development and commercialization of specific tests or in specific fields. Our dependence on LifeScan for a significant proportion of our revenue is likely to continue until we start to receive meaningful revenues from other collaborative arrangements or strategic alliances with third parties, such as our arrangement with Siemens, and/or until we receive meaningful revenues from our own products which we intend to commercialize through distributors, such as our PT-INR product that is in development.
Our current and future customers and partners may choose to utilize less of our research and development services. If the development and research work we undertake was materially reduced or ceased, we would lose an ongoing source of income which would have a material adverse effect on our business and financial position.
Inability to maintain compliance with the debt covenants.
On December 19, 2013 UBI and UBS entered into a credit agreement with Athyrium Opportunities Fund (A) LP, as administrative agent (the “Administrative Agent”) and as a lender, and Athyrium Opportunities Fund (B) LP as a lender (Athyrium A and Athyrium B together with any other lenders party thereto from time to time, the “Lenders”), which was amended on January 30, 2015, for a secured term loan of up to US$25,000,000 (the “Credit Agreement”). A first tranche loan of US$15,000,000 was drawn on December 2013 and a further two tranches each of US$5,000,000 may be drawn within 30 days of any fiscal quarter ending on or before July 31, 2015 in which UBS satisfies certain conditions. Unless the facility is otherwise terminated earlier pursuant to the terms of the Credit Agreement, UBS is required to repay the outstanding principal amount of the loans drawn down, together with all accrued and unpaid interest thereon and all other obligations on December 19, 2018 (the “Maturity Date”). The Credit Agreement is secured by substantially all of the assets of UBS and UBI, including the stock in UBS held by UBI. The obligations of UBS under the Credit Agreement are guaranteed by UBI.
UBS’ ability to maintain compliance with the covenants in our Credit Agreement is dependent upon, among other things, our ability to continue to execute our business plans and our ability to generate cash from operations. The debt facility is subject to certain specified events of default, defaults relating to non-payment, breach of covenants or inaccuracy of representations and warranties, cross-defaults to other indebtedness, bankruptcy and insolvency defaults, material judgment defaults, regulatory defaults, the occurrence of a material adverse effect, or un-remedied material breach by UBS or UBI or termination of a key contract. The occurrence of an event of default could result in the amounts owing under the Credit Agreement, including all unpaid principal and interest being due and payable, and could result in the administrative agent enforcing its security over the assets of UBS and UBI. If the loans are accelerated or commitments terminated, we could face substantial liquidity problems and may be forced to dispose of
12
Table of Contents
material assets or operations, seek to obtain equity capital, or restructure or refinance our indebtedness. Such alternative measures may not be available or successful. Also, our debt covenants may limit our ability to dispose of material assets or operations or to restructure or refinance our indebtedness. Even if we are able to restructure or refinance our indebtedness, the economic terms may not be favorable to us. In addition, an event of default under our key commercial contracts could result in a cross-default under our Credit Agreement. All of the foregoing could have serious consequences to our financial condition and results of operations and could cause us to become bankrupt or insolvent.
Deviations from expected results of operations and/or expected cash requirements could result in a default under our Credit Agreement and/or adversely affect our financial condition and results of operations.
Our principal sources of liquidity are cash flows from operations, cash and cash equivalents and availability under our debt facility. Our operating activities generally provide a proportion of cash to fund our working capital requirements and, together with borrowings under our debt facility, are expected to be sufficient to fund our operating needs and capital requirements for at least the next twelve months, based on current assumptions regarding the amount and timing of such expenditures and anticipated cash flows. Although we currently expect to remain in compliance with the Credit Agreement, based on our current expectations, any significant deviation in actual results from our expected results of operations, any significant deviation in the amounts or timing of material expenditures from current estimates, the termination of any of our key commercial contracts with LifeScan or Siemens, or other significant unanticipated expenses could result in a default under our Credit Agreement, have a material adverse effect on our financial condition and/or may result in the need for additional debt or equity financing.
Our debt covenants may affect our liquidity or limit our ability to complete acquisitions, incur debt, make investments, sell assets, merge or complete other significant transactions.
The Credit Agreement includes provisions that place limitations on a number of our activities, including but not limited to our ability to: incur additional debt; create liens on our assets or make guarantees; make certain investments or loans; pay dividends; dispose of or sell assets; or enter into acquisitions, mergers or similar transactions. These covenants could restrict our ability to pursue opportunities to expand our business operations. We are required to maintain unrestricted cash of US$2,000,000 in a specified bank account at all times.
Our business strategy and revenue relies on our ability to enter collaborative arrangements with other companies and there is a risk that we will not be able to enter into collaborative arrangements with respect to our products.
Our business strategy involves proving the broader applicability of our technology platform for a number of different products/technologies and then entering into collaborative arrangements, licensing agreements, strategic alliances or distribution arrangements for these products/technologies. We have not established any internal product sales and marketing capacity and to achieve commercial success we must enter into and maintain successful arrangements with others to sell, market and distribute products that we are involved in developing. We may not be able to enter into such collaborative arrangements, strategic alliances or distributions arrangements in a timely fashion and on acceptable terms, if at all. Our inability to enter such arrangements would be detrimental to our strategy, business and financial position. Our ability to enter into collaborative, strategic or distribution arrangements will suffer if the technologies developed by us are not perceived as being comparable or superior to established laboratory methods or other products.
If we are unable to enter collaborative or distribution arrangements with respect to certain of our products/technologies, we may have to change strategy, delay, reduce the scope of or eliminate some or all of our development programs or liquidate some or all of our assets or seek to raise additional capital. As a result, we may not be able to pursue what we consider to be worthwhile commercial opportunities and significant monies and management time invested may be rendered unproductive and worthless. Our inability to enter collaborative or strategic arrangements would thus have a material adverse effect on our business and financial position.
Entering collaborative arrangements with respect to our products will expose us to risks and uncertainties related to those collaborations and alliances.
To the extent we are able to enter into collaborative or strategic arrangements with respect to our products, we will be exposed to risks and uncertainties related to those arrangements. The customer or partner will generally make the key decisions on product choice, regulatory approvals, product launch, product manufacture and marketing and promotion. Decisions made by our partner with respect to the commercialization of the products we develop with them will significantly affect the extent and timing of revenues to
13
Table of Contents
us. For example, our partner may choose not to launch new products we develop, may choose to launch the products in a limited number of jurisdictions, may delay the launch of products, may undertake only limited sales and marketing efforts to commercialize the products, all of which would have a material adverse effect on our business and financial position. Collaborative arrangements, licensing agreements or strategic alliances will subject us to a number of risks, including the risk that:
| • | we do not control the amount and timing of resources that our strategic partners may devote to our products; |
| • | we do not control the decision to pursue a product, the timing of product launches and extent of marketing and sales activities; |
| • | our customer or partners may experience financial difficulties; |
| • | we may be required to relinquish important rights such as marketing and distribution rights; |
| • | business combinations or significant changes in a partner’s business strategy may also adversely affect a collaborator’s willingness or ability to complete its obligations under any arrangement; |
| • | a collaborator could independently move forward with a competing product developed either independently or in collaboration with others, including our competitors; and |
| • | collaborative arrangements are often terminated or allowed to expire, which would delay the development and may increase the cost of developing our products. |
Likewise, distribution arrangements will also expose us to a number of additional comparable risks, including that the distributor may not perform as required.
Allegedly defective design or the manufacture of allegedly defective products could potentially expose us to substantial costs, write-offs and reputational damage.
Allegedly defective designs or manufacture of allegedly defective products exposes us to the risk of product liability claims and product recalls, resulting in substantial costs, write-offs and potential delays in our shipment of product to customers, decreased demand for products, loss of revenue and cash flow, reputational damage, costs of related litigation, increases in our insurance premiums and increased scrutiny by regulatory agencies, claims by our customers and may trigger the dissolution of partnerships or collaborative relationships. The occurrence of certain of these events may trigger an event of default under our Credit Agreement. While we will seek to mitigate our loss by obtaining appropriate insurances and appropriate contractual protections, if we are unable to maintain our insurance at an acceptable cost or on acceptable terms with adequate coverage, or negotiate appropriate contractual protections or otherwise protect against potential product liability claims, we will be exposed to significant liabilities. Recalls would harm our business and compromise the performance of our obligations to our customers and would have a material adverse effect on our business and financial results and may result in claims by our customers or partners and may trigger the dissolution of partnerships or collaborative relationships. Any claim for damages by our customers or other claim against us could be substantial.
There are many elements to manufacturing products that can cause variability beyond acceptable limits. We may be required to discard defective products after we have incurred significant material and labor costs, resulting in manufacturing delays and delayed shipment to customers. Further, if our suppliers are unable to provide materials in conformance with specifications, we may be required to discard materials, which may also cause delays in the manufacture and shipment of products.
Reduced margins would have a material adverse effect on our business and financial position.
Our margins may be reduced and costs increased which would have a material adverse effect on our business and financial position. The two primary factors that pose this risk include increased manufacturing costs or currency fluctuations.
Increases in our costs to manufacturing products or conducting development work may decrease our margins or cause us to suffer a loss on the manufactured products. Additionally, we may suffer decreased margins due to the global reach of our business exposing us to market risk from changes in foreign currency exchange rates. While the majority of our cash reserves and expenses are in Australian dollars, we continue to deal in other currencies, particularly in the United States and Europe, which may increase costs and decrease revenues incurred in foreign currencies. Additionally, we use, from time to time, financial instruments, primarily foreign currency forward contracts to hedge certain forecasted foreign currency commitments arising from trade accounts receivables, trade accounts payable and fixed purchase obligations. These hedging activities are largely dependent upon the accuracy of our forecasts and as such, our foreign currency forward contracts may not cover our full exposure to exchange rate fluctuations. Although we believe our foreign
14
Table of Contents
exchange policies are reasonable and prudent under the circumstances, we may experience losses from un-hedged currency fluctuations, which could be significant. If our costs increase or our margins decrease, it would have an adverse effect on our business and financial position.
New product design and development and clinical testing is costly, labor intensive and the outcomes uncertain.
The design and development of different tests on our platform takes a number of years to complete, is costly and the outcomes are uncertain. Although development risk generally reduces the further a test is developed, the tests we develop have a significant degree of technical risk, and irrespective of the stage of development, design and development work and product validation, the development of the test may be unsuccessful or not warrant product commercialization. If development activities are unsuccessful, we may need to delay, reduce the scope of or eliminate some or all of our development programs and significant monies and management time invested may be rendered unproductive and worthless.
Our agreements with our customers to date have contained milestone based payments, many of which are payable upon the achievement of technical development milestones. Such milestone payments may not cover the cost of our research and development activities. In the event we are not successful in achieving the relevant development milestone, we will not receive the milestone payments associated with the milestone which would have an adverse effect on our revenue and financial position. Failure to achieve certain development milestones may have an impact on our covenants under the Credit Agreement. Furthermore, if we are unable to develop a product for a customer, it may eliminate an important revenue stream for us which may result in us not being profitable, or trigger dissolution of partnerships or collaborative relationships.
Diagnostic devices must be tested for safety and performance in laboratory and clinical trials before regulatory clearance for marketing is achieved. Such studies are costly, time consuming and unpredictable. Clinical trials may not be successful and marketing authorization may not be granted which may result in us not being profitable, or trigger dissolution of partnerships or collaborative relationships. The outcome of early clinical trials may not be predictive of the success of later clinical trials. Failed clinical trials may result in considerable investments of time and money being rendered unproductive and worthless.
Additionally, unanticipated trial costs or delays could cause substantial additional expenditure that is not reimbursed by a partner, cause us to miss milestones which trigger a financial payment or cause us or a partner to delay or modify our plans significantly. This would harm our business, financial condition and results of operations.
If we cannot maintain our intellectual property rights, our ability to make or develop point-of-care tests would be restricted or eliminated, and the value of our technology and diagnostic tests may be adversely affected.
Our ability to obtain proprietary rights, maintain trade secret protection and operate without infringing the proprietary rights of third parties is an integral part of our business.
A number of companies, universities and research institutions have or may be granted patents that cover technologies that we need to complete development of a particular product. We may choose or be required to seek licenses under third party patents which would be costly, may not be available on commercially acceptable terms, or at all. Further, we may be unaware of other third party patents or proprietary rights that are infringed by our point-of-care tests.
Much of our platform intellectual property rights are licensed to us from LifeScan. If we were to breach the License Agreement and LifeScan were to validly terminate the agreement in response, it would seriously restrict or eliminate our ability to develop and commercialize our existing and future tests which would have a material adverse effect on us as it would restrict or eliminate our existing commercialization opportunities. We also license other intellectual property from third parties as part of our other development efforts.
LifeScan and our other licensors have a considerable degree of control over the manner that the intellectual property licensed to us is maintained and protected and, as a result, we have reduced control with respect to the maintenance and protection of our licensed patent portfolio. LifeScan is responsible for the prosecution and maintenance of the intellectual property it licenses to us and we are largely dependent on them to defend proceedings or prosecute infringers. The same applies to our other licensors. Our business would be harmed if the licensed patents were infringed or misappropriated. Prosecuting third parties and defending ourselves against third-party claims would be costly, time consuming and divert management’s attention from our business, potentially leading to delays in our development or commercialization efforts. Additionally, if third parties made successful claims, we may be liable for substantial damages or license fees, be required to stop marketing the infringing product or take other actions that are adverse to our business.
15
Table of Contents
Risks associated with regulatory clearance and changes to regulation.
The products we are involved in developing are medical devices and therefore subject to extensive regulation in all major markets. The process of obtaining regulatory clearance is costly and time consuming and there can be no assurance that the required regulatory clearances will be obtained. Products cannot be commercially sold without regulatory clearance. We and our customers and partners may be unable to obtain the necessary clearances to sell or if the clearances are delayed, revoked or subject to unacceptable conditions, the product may not be able to be commercialized which would have a material adverse effect on us.
If we were required and able to change suppliers and third party contract manufacturers, applicable regulatory bodies may require new testing and compliance inspections and require that we demonstrate structural and functional comparability between the same products manufactured by different organizations, resulting in additional costs and potential delays which could be detrimental to our business.
Furthermore, regulation is ongoing and manufacturers and marketers of products are subject to continuous review and periodic inspections. Potentially costly responses may be required to be given by us and our customers including product modification, or post-marketing clinical trials as a condition of approval to further substantiate safety and efficacy or investigate issues of interest. If we or our customers fail to comply with applicable regulatory requirements it may result in fines, delays, suspensions of clearances, seizures, recalls of products, operating restrictions or criminal prosecutions and could have a material adverse effect on our operations. Any such regulatory action may also constitute an event of default under our Credit Agreement. Additionally, changes in existing regulations or the adoption of new regulations could make regulatory compliance by us more difficult in future and could hamper our ability to produce our products when we require.
Risks associated with suppliers.
Similar to most major manufacturers in our industry, we are dependent upon our suppliers for certain raw materials and components. We have preferred suppliers, making us vulnerable to supply disruption, which could harm our business and delay manufacturing operations. We seek to enter into long term contractual arrangements with certain of our suppliers, however we may not always be able to do so on acceptable terms. If our manufacturing requirements change, such long term contractual arrangements may cause us to have excess or obsolete inventory. We may not be able to guarantee the supply of certain of our materials which may in turn affect our ability to supply product to our customers. We may have difficulty locating alternative suppliers in a timely manner or on commercially acceptable terms, and switching components may require product redesign and further regulatory clearance which could significantly delay production. Likewise, our customers and partners are subject to supply risks which may delay their ability to supply customers with product which would impact our revenue and have a consequential adverse effect on our business and results of operations.
To the extent we agree to be responsible for manufacturing meters for any of our customers and partners, we anticipate that we will outsource the manufacture of these meters. There is no guarantee that we will be able to enter into any such arrangement on acceptable terms, if at all, and as a result there is a risk of lengthy and costly delays of bringing our products to market. Further, if our contract manufacturers fail to achieve and maintain required production yields or manufacturing standards, it could result in product withdrawals, delays, recalls, product liability claims and other problems that could seriously harm our business. Any meter shortages or manufacturing delays could result in delays or reduction in our revenues, with consequential adverse effect on our business and results of operations.
We face risks manufacturing product.
There are technical challenges to establishing and maintaining commercial manufacturing for products, including maintaining the consistency of our incoming raw materials, equipment design and automation, material procurement, production yields and quality control and assurance. We may fail to achieve and maintain required production yields or manufacturing standards which could result in financial loss, patient injury or death, product recalls or withdrawals, product shortages, delays or failures in product testing or delivery, breach of our agreements with any partner and other problems that could seriously harm our business.
16
Table of Contents
The success of our business is heavily dependent upon market factors such as growth of the point-of-care testing market and our ability to compete effectively within the highly competitive in vitro diagnostics market.
Our business success relies on the growth of both the existing and emerging point-of-care testing market. We cannot be sure that this market will grow as we anticipate. Such growth will require continued support and demand from payers, patients and health care professionals and the endorsement by professional bodies that influence the practice of medicine. Research and clinical data may not sufficiently support point-of-care testing, nor may the health economic benefits sufficiently support point-of-care testing as an alternative to current practice. Even if the data is compelling, significant resources may be required to educate users and change in practice may be slower and more costly than we anticipate. If point-of-care testing fails to be adopted at the rate we expect, the sector may remain unattractive to the size of partner we seek to attract and as a consequence, we may need to change our business model. This may require us to incur more cost and/or our anticipated growth will be adversely affected and our results will suffer.
We may face intense competition in development, marketing and selling point-of-care tests.
The market for in vitro diagnostics is intensely competitive, price sensitive and subject to rapid change. We and our customers and partners may be unable to accurately anticipate changes in the markets and the direction of technological innovation and the demands of end users, competitors may develop improved technologies and the market place may conclude that our products are obsolete. Our larger competitors enjoy several competitive advantages including significantly greater financial resources, greater brand recognition, greater expertise in conducting clinical trials, obtaining regulatory approvals and managing manufacturing operations, and greater experience in product sales and marketing. Early-stage companies may also prove to be significant competitors.
Competition will be faced from existing products as well as products in development. Point-of-care tests are likely to experience significant and continuing competition from traditional pathology laboratory based testing as well as other point-of-care tests. Our and our customers’ and partners’ commercial opportunity will be reduced or eliminated if competitors develop and commercialize safer, more effective, more convenient, or cheaper products, or reach the market sooner than we do. Any such developments adversely affecting the market for products developed by us may force us and our partners to reduce production or discontinue manufacturing which would cause our operating results to suffer. There can be no assurances given with respect to our or any partner’s ability to compete effectively in the competitive markets in which we operate.
Adverse economic conditions may harm our business.
Market and economic conditions have been challenging worldwide. Continuing concerns have led to increased market volatility and diminished expectations for world economies. These factors may include fluctuations in foreign exchange rates, inflation, interest rates, rate of economic growth, taxation laws, consumer spending, unemployment rates, government fiscal, monetary and regulatory policies and consumer and business sentiment. Any of these factors have the potential to cause costs to increase or revenues to decline. Continued turbulence in the US, Australian and other international markets and economies may adversely affect our ability to enter into collaborative arrangements, the behavior and financial condition of our current and any future customers and partners and the spending patterns of users of the products we are developing. This may adversely impact demand for our services and for products developed by us. In addition, economic conditions could also impact our suppliers, which may impact on their ability to provide us with materials and components which in turn may negatively impact our business.
Our operations are not currently profitable.
Our operations are not currently, and may never be profitable. To date, we have funded our operations and capital expenditures from revenue from the sale of products and provision of services and with proceeds from the sale of our securities, government grants and interest on investments. On December 19, 2013 we entered into the Credit Agreement which has provided debt financing for our business. We may, however, require additional capital to fund our business operations, which may not be available on acceptable commercial terms, or at all.
We may not be able to raise capital or secure credit if and when required.
We may not be able to raise capital or secure credit if and when required. If we are unable to raise capital or secure credit when required, we may have to delay, reduce the scope of or eliminate some or all of our development programs or commercialization efforts or liquidate some or all of our assets.
17
Table of Contents
We rely on government grants and rebates.
Our principal sources of liquidity are cash flows from operations (revenue from services and product sales) and availability under our debt facility. We have also financed our business operations through government grants and rebates, including the tax incentive income. The research and development tax incentive is one of the key elements of the Australian Government’s support for Australia’s innovation system and if eligible, provides the recipient with a tax offset for research and development activities. For the year ended December 31, 2014 we recorded research and development tax incentive income of $8.2 million. There can be no assurance that Universal Biosensors will continue to qualify and be eligible for such incentives or that the Australian Government will continue to provide incentives, offsets, grants and rebates on similar terms or at all.
The loss of a key employee or the inability to recruit and retain high caliber staff to manage future anticipated growth could have a material adverse effect on our business.
As with most growth companies, our future success is substantially dependent on our key personnel. Certain key personnel would be difficult to replace and the loss of any such key personnel may adversely impact the achievement of our objectives. Our ability to operate successfully and manage the business depends significantly on attracting and retaining additional highly qualified personnel. The loss of any key personnel may be disruptive or have a material adverse effect on the future of our business. The competition for qualified employees in scientific research and medical diagnostic industries is particularly intense and there are a limited number of persons with the necessary skills and experience.
Our primary operations are conducted at a single location. Any disruption at our facility could adversely affect our operations and increase our expenses.
Our primary operations are conducted at our Corporate Avenue facility in Melbourne, Australia. We take precautions to safeguard our facility, including security, health and safety protocols and maintain applicable insurance. However, we may be impacted by industrial action or operating equipment and facilities may not operate as intended or be unavailable as a result of unanticipated failures or other events outside of our control such as a natural disaster, fire, flood or earthquake or catastrophic breakdowns or deliberate acts of destruction. The occurrence of any of these events may restrict our ability to supply product, could cause substantial delays in our operations, damage or destroy our manufacturing equipment or inventory, and cause us to incur additional expenses. The insurance we maintain against fires, floods, earthquakes and other natural disasters may not be adequate to cover our losses in any particular case.
Investors may be subject to Australian and/or US taxation.
The receipt of dividends by Australian tax resident security holders and any subsequent disposal of our securities by any such Australian tax resident may have both United States and Australian tax consequences depending upon their individual circumstances. This may result in a security holder being subject to tax in both jurisdictions and a tax credit may or may not be available in one jurisdiction to offset the tax paid in the other jurisdiction depending upon the security holder’s individual circumstances.
The price of our shares is highly volatile and could decline significantly.
Our shares of common stock in the form of CDIs were quoted on the ASX and began trading on December 13, 2006. The price of our shares is highly volatile and could decline significantly. The market price of our shares historically has been, and we expect will continue to be, subject to significant fluctuations over short periods of time. Some of the factors that may cause the market price of our common stock to fluctuate include:
| • | the entry into, or termination of, key agreements, including key collaboration agreements and licensing agreements; |
| • | any inability to obtain additional financing on favorable terms to fund our operations and pursue our business plan if additional financing becomes necessary; |
| • | our ability to negotiate and enter into definitive agreements related to any financing transaction, if additional financing becomes necessary; |
| • | future sales of our common stock or debt or convertible debt securities or other capital-raising activities, and the terms of those issuances of securities; |
| • | future revenue streams from product sales, if any, by our collaborative partners, and the extent of demand for, and sales of, our products; |
18
Table of Contents
| • | the initiation of material developments in, or conclusion of litigation to enforce or defend any of our intellectual property rights or otherwise; |
| • | our results of operations and financial condition, including our cash reserves, cash burn and cost level; |
| • | general and industry-specific economic and regulatory conditions that may affect our ability to successfully develop and commercialize products; |
| • | the loss of key employees; |
| • | the introduction of technological innovations or other products by our competitors; |
| • | sales of a substantial number of shares of our common stock by our large stockholders; |
| • | changes in estimates or recommendations by securities analysts, if any, who cover our common stock; |
| • | issuance of shares by us, and sales in the public market of the shares issued, upon exercise of our outstanding warrants; and |
| • | period-to-period fluctuations in our financial results. |
For example, from the initial quotation of our shares in the form of CDIs on the Australian Securities Exchange on December 13, 2006 until March 4, 2015, the closing price per share of our shares ranged from a low of A$0.14 during May 2014 to a high of A$2.02 during the first quarter of the 2010 fiscal year and was A$0.33 on March 4, 2015. We may experience a material decline in the market price of our CDIs, regardless of our operating performance and therefore, a holder of our shares may not be able to sell those shares at or above the price paid by such holder for such shares. Sales by our larger shareholders may create volatility or impact how the value of our shares is perceived.
Class action litigation has been brought in the past against companies which have experienced volatility in the market price of their securities. We may become involved in this type of litigation in the future. Litigation of this type is often extremely expensive and diverts management’s attention and our resources.
Our securities are not currently traded on any United States public markets and there are currently restrictions on the ability of United States persons to acquire our securities on the ASX.
There is no public market for our shares in the United States or in any other jurisdiction other than Australia. We have not determined whether we will seek the quotation of our shares on any United States public trading market. Even if our shares are in the future listed on a United States public market, the liquidity of our shares may not improve, and the United States market price may not accurately reflect the price or prices at which purchasers or sellers would be willing to purchase or sell our common stock.
In addition, our securities are “restricted securities” as that term is defined in Rule 144 under the United States Securities Act of 1933, as amended (“Securities Act”). Restricted securities may be resold in the public market to United States persons as defined in Regulation S only if registered for resale or if they qualify for an exemption from registration under the Securities Act. We have not agreed to register any of our common stock for resale by security holders.
We may be involved in litigation.
There has been substantial litigation and other proceedings in the medical diagnostic industries. Defending against litigation and other third party claims would be costly and time consuming and would divert management’s attention from our business, which could lead to delays in our development or commercialization efforts. If third parties are successful in their claims, we might have to pay substantial damages or take other actions that are adverse to our business.
Changes in laws may adversely affect our business.
Our business and the business of our customers and partners are subject to the laws and regulations in a number of jurisdictions. Unforeseen changes in laws and government policy both in Australia, the EU, the US and elsewhere, could materially impact our’ operations, assets, contracts and profitability.
We are exposed to risks relating to evaluations of controls required by Section 404 of the Sarbanes-Oxley Act.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 (“Sarbanes-Oxley Act”) and related regulations implemented by the SEC, have substantially increased legal and financial compliance costs. We expect that our ongoing compliance with applicable laws and regulations, including the Securities Exchange
19
Table of Contents
Act of 1934 as amended (“Exchange Act”) and the Sarbanes-Oxley Act, will involve significant and potentially increasing costs. In particular, we must annually evaluate our internal controls systems to allow management to report on our internal controls. Additionally, as an “accelerated” filer with the SEC, our independent auditors must attest to our internal controls. We must perform the system and process evaluation and testing (and any necessary remediation) required to comply with the management certification and, when applicable, auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act. If we are not able to continue to satisfy the requirements of Section 404 adequately, we may be subject to sanctions or investigation by regulatory authorities, including the SEC. Any action of this type could adversely affect our financial results, investors’ confidence in our company and our ability to access capital markets, and could cause our stock price to decline.
A significant amount of our shares are controlled by individuals or voting blocks, and the interests of such individuals or voting blocks could conflict with those of the other stockholders.
Single stockholders with significant holdings or relatively small groups of stockholders have the power to influence matters requiring the approval of stockholders. Mr. Andrew Jane is one of our directors and a director of Talu Ventures Pty Ltd which holds approximately 10.1% of our shares. Control of a significant amount of our common stock by insiders could adversely affect the market price of shares. For details of our substantial stockholders and the interests of our directors, refer to “Item 12 — Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters”.
We have never paid a dividend and we do not intend to pay dividends in the foreseeable future which means that holders of shares of common stock and CDIs may not receive any return on their investment from dividends.
To date, we have not declared or paid any cash dividends on our shares or CDIs and currently intend to retain any future earnings, if any, for funding growth. We do not anticipate paying any dividends in the foreseeable future.
Our holders of CDIs are not stockholders and do not have stockholder rights.
The main difference between holding CDIs and holding our underlying shares is that a CDI holder has beneficial ownership of the equivalent number of shares instead of legal title. CDIs are exchangeable, at the option of the holder, into shares of our common stock at a ratio of 1:1. Legal title is held by CHESS Depositary Nominees Pty Ltd (“CDN”) and the shares are registered in the name of CDN and held by CDN on behalf of and for the benefit of CDI Holders. CDN is a wholly owned subsidiary of ASX Limited. CDI holders will be entitled to all the economic benefits of the shares underlying their CDIs, such as dividends (if any), bonus issues or rights issues. CDN as a stockholder of record will receive notice of stockholder meetings and be entitled to attend and vote at stockholder meetings. CDI holders will likewise be sent notices of stockholder meetings and are entitled to attend stockholder meetings but are not permitted to vote other than by giving directions on how to vote to CDN or as a proxy holder for CDN.
Our success is reliant on the accuracy, reliability and proper use of sophisticated information processing systems and management information technology and the interruption in these systems could have a material adverse effect on our business, financial condition and results of operations.
Our success is reliant on the accuracy, reliability and proper use of sophisticated information processing systems and management information technology. Our information technology systems are designed and selected in order to facilitate the entering of order entry, customer billing, to maintain customer records, to provide product traceability, to accurately track purchases, to manage accounting, finance, administration and manufacturing, generate reports and provide customer service and technical support. Any interruption in these systems could have a material adverse effect on our business, financial condition and results of operations.
Provisions in our charter documents and under Delaware law could make the possibility of our acquisition, which may be beneficial for our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove current management.
Provisions in our certificate of incorporation and our bylaws may delay or prevent an acquisition of us or a change in our management, and frustrate or prevent attempts by our stockholders to replace or remove our current management by making it more difficult to remove our current directors. Such provisions include:
| • | the division of our Board into classes whose terms expire at staggered intervals over a three year period and advance notice requirements for nominations to our Board and proposing matters that can be acted upon at shareholder meetings; |
| • | the requirement that actions by our stockholders by written consent be unanimous; |
| • | the ability of our Board to issue preferred stock. |
20
Table of Contents
Limitation on Independent Registered Public Accounting Firm’s Liability.
The Australian accounting firm we utilize for audit reports on our financial statements is subject to limitations on liability with respect to claims arising out of their audit reports, in accordance with professional standards legislation. This legislation may limit the liability of our accountant’s for damages with respect to certain civil claims arising directly or vicariously from anything done or omitted in the performance of their professional services to us, including to the lesser of (in the case of audit services) ten times the reasonable charge for the service provided and a maximum liability for audit work of A$75 million or, in relation to matters occurring prior to October 7, 2007, A$20 million. The limit does not apply to claims for breach of trust, fraud or dishonesty.
These limitations of liability may limit recovery upon the enforcement in Australian courts of any judgment under US or other foreign laws rendered against our Australian accountants based on or related to their audit report on our financial statements. Substantially all of our accountant’s assets are located in Australia. However, the professional standards legislation has not been subject to judicial consideration and therefore how the limitation will be applied by the courts and the effect of the limitation on the enforcement of foreign judgments are untested.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
21
Table of Contents
Universal Biosensors Pty Ltd leases approximately 5,000 square meters of office, research and development and manufacturing facilities at 1 Corporate Avenue, Rowville in Melbourne, Australia. The lease for the premises at 1 Corporate Avenue Rowville expires on March 31, 2019 with an option to renew the lease for two further terms of five years each.
We will manufacture our test strips using custom manufacturing equipment.
Depending on the number of strips required to be manufactured, it may become necessary in the future for us to acquire additional large scale equipment to satisfy manufacturing demand. If our existing facilities and equipment are fully utilized for the manufacture of test strips for one of our customers or our own products, we will need to secure additional or alternative facilities and establish additional large scale equipment sufficient to future manufacturing requirements.
22
Table of Contents
There are no material legal or arbitration proceedings pending against us or Universal Biosensors Pty Ltd.
23
Table of Contents
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
24
Table of Contents
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market information
Our shares of common stock are not currently traded on any established United States public trading market. We have not determined whether we will seek the quotation of our shares of common stock on any United States public trading market. We cannot assure you that we will seek to be quoted on any United States public trading market or that we would meet any applicable listing requirements.
Our shares of common stock are traded on the ASX in the form of CHESS Depositary Interests, or CDIs, under the ASX trading code “UBI”. The Clearing House Electronic Subregister System, or “CHESS”, is an electronic system which manages the settlement of transactions executed on the ASX and facilitates the paperless transfer of legal title to ASX quoted securities. CHESS cannot be used directly for the transfer of securities of U.S. domiciled companies. CDIs are used as a method of holding and transferring the legal title of these securities on the ASX which are not able to be electronically traded in CHESS. CDIs are exchangeable, at the option of the holder, into shares of our common stock at a ratio of 1:1. The main difference between holding CDIs and holding the underlying securities (in this case our shares) is that a holder of CDIs has beneficial ownership of the equivalent number of our shares instead of legal title. Legal title is held by CHESS Depositary Nominees Pty Ltd, or CDN, and the shares are registered in the name of CDN and held by CDN on behalf of and for the benefit of the holders of CDIs. CDN is a wholly owned subsidiary of ASX.
Holders of CDIs who do not wish to have their trades settled in CDIs on the ASX may request that their CDIs be converted into shares, in which case legal title to the shares of common stock are transferred to the holder of the CDIs. Likewise, stockholders who wish to be able to trade on the ASX can do so by requesting that their shares be converted into CDIs and by lodging their applicable share certificate with our share registrar and signing a share transfer form with respect to the relevant shares. Our share registrar will then transfer the shares from the stockholder to CDN and establish a CDI holding in the name of the stockholder (now a CDI holder).
High and low sale prices of our CDIs on the ASX
The sale prices of our shares traded in the form of CDIs are quoted on the ASX in Australian dollars. Our CDIs were first quoted on the ASX on December 13, 2006. Twenty minute delayed trading prices of our CDIs are available through the ASX at www.asx.com.au.
The following tables sets forth, for the periods indicated, the highest and lowest market prices in Australian dollars for our CDIs reported on the ASX:
| High A$ | Low A$ | |||||||||
| Fiscal Year 2014 |
First Quarter |
0.53 | 0.32 | |||||||
| Second Quarter |
0.41 | 0.14 | ||||||||
| Third Quarter |
0.20 | 0.15 | ||||||||
| Fourth Quarter |
0.19 | 0.14 | ||||||||
| Fiscal Year 2013 |
||||||||||
| First Quarter |
0.92 | 0.65 | ||||||||
| Second Quarter |
0.75 | 0.60 | ||||||||
| Third Quarter |
0.83 | 0.67 | ||||||||
| Fourth Quarter |
0.72 | 0.43 | ||||||||
Security details
As of March 4, 2015, there were 175,893,533 shares of our common stock issued and outstanding and 9,755,101 employee options that are exercisable for an equivalent number of shares of common stock (8,568,059 of which were exercisable or exercisable within 60 days thereafter). All of our issued and outstanding shares of common stock are fully paid.
25
Table of Contents
Under applicable U.S. securities laws all of the shares of our common stock are “restricted securities” as that term is defined in Rule 144 under the Securities Act. Restricted securities may be resold to U.S. persons as defined in Regulation S only if registered or if they qualify for an exemption from registration under the Securities Act, each as described in more detail below. We have not agreed to register any of our common stock for resale by security holders.
Rule 144(b)
Because there is no public trading market for the shares in the United States, no sales in the United States under Rule 144 other than Rule 144(b)(1)(i) are likely to occur. Under Rule 144(b)(1)(i), a person who is not deemed to have been an affiliate of ours at any time during the 90 days preceding a sale, and who has beneficially owned the shares proposed to be sold for between six months and one year may sell so long as the public information requirements of Rule 144 are satisfied, and, after one year, such person is entitled to sell the shares without having to comply with the public information or other provisions of Rule 144. A person who is deemed an affiliate during the 90 days preceding the sale who has beneficially owned the shares proposed to be sold for at least six months may sell so long as the conditions of Rule 144 are met, including the manner of sale, public information, volume limitation and notice filing provisions of Rule 144.
Holders
Currently, CDN holds the majority of our shares on behalf of and for the benefit of the holders of CDIs. The balance of the shares are held by certain of our employees generally as part of our restricted employee share scheme. Set out below is the aggregate number of our registered holders of CDIs and shares at the specific date below:
| Date | Total Number of Registered Holders |
Number of Holders that are United States Residents |
||||||
| At March 4, 2015 |
1,879 | 15 | ||||||
Dividends
To date, we have not declared or paid any cash dividends on our shares or CDIs and currently intend to retain any future earnings, if any, for funding growth. We do not anticipate paying any dividends in the foreseeable future.
Recent Sales of Unregistered Securities
| • | Warrants |
In connection with the Credit Agreement, we granted to the lenders warrants entitling the holders to purchase an aggregate total of 4,500,000 shares of our common stock at an exercise price of A$1.00 per share, exercisable at any time until December 19, 2020 (“Warrants”). The holder of a Warrant has the option to pay the exercise price in cash or by making a cashless exercise. The number of shares of common stock to be issued on exercise of the Warrants and/or the exercise price of the Warrants will be adjusted in certain circumstances including bonus issues, pro-rata issues and reorganizations of share capital.
The issue of the Warrants was made in reliance upon the exemption from registration pursuant to Regulation D, as promulgated by the Securities Act. The holders of the Warrants may not re-sell any Warrants or shares issued upon exercise of the Warrants into the U.S. or to a U.S. Person (as defined in the Securities Act) or within Australia for a period of one year after the date of issue of the relevant security unless the re-sale of the securities is registered under the Securities Act or an exemption is available.
| • | Institutional Placement |
On November 26, 2012, we placed 13,334,000 shares of common stock at A$0.90 per share to certain existing and new institutional investors based primarily in Australia, and raised an aggregate total of A$12,000,600 (before expenses of the offer) (“Placement”). Wilson HTM Corporate Finance Limited (“Wilson HTM”) acted as Lead Manager and Bookrunner for the Placement. Veritas Securities Limited acted as Co-manager to the Placement. We paid the Wilson HTM a management fee of A$180,009 and a selling fee of A$360,018 in connection with the Placement. In addition, we reimbursed Wilson HTM for certain costs and expenses
26
Table of Contents
incurred in connection with the Placement. We raised A$11,460,573 net of management and selling fees paid to the Wilson HTM in the Placement. Dr. Wilson, who was a director of the Company until August 2013, is the spouse of Mr. Steven Wilson who is a substantial stockholder and officer of the parent company of Wilson HTM.
| • | Share Purchase Plan |
On December 17, 2012 we completed a share purchase plan (“Share Purchase Plan”) offer to all holders of our securities with a registered address in Australia or New Zealand on the record date of the Share Purchase Plan and raised an aggregate total of A$1,163,442 (before expenses of the offer) through the issuance of 1,292,713 shares at a price of A$0.90 per share. Wilson HTM acted as Lead Manager for the Share Purchase Plan. We paid Wilson HTM a fee of A$17,452 in connection with managing the Share Purchase Plan. We raised A$1,145,990 net of fees paid to the Lead Manager in our Share Purchase Plan.
The offers of shares under the Placement and the Share Purchase Plan were made in offshore transactions in reliance upon the exemption from registration pursuant to Regulation S, as promulgated by the Securities Act. In order to comply with the requirements of Regulation S, investors may not re-sell any securities issued in the Placement or Share Purchase Plan into the U.S. or to a U.S. Person (as defined in the Securities Act) for a period of six months after the date of issue of the securities unless the re-sale of the securities is registered under the Securities Act or an exemption is available.
Accordingly, in order to enforce the above transfer restrictions whilst ensuring that stockholders can still trade their CDIs on ASX, certificates representing the shares will bear a restrictive legend and the CDIs will for a period of time (of not less than 6 months) have a Foreign Ownership Restriction (FOR) designation which will inform the market of the prohibition on U.S. Persons acquiring the shares or CDIs.
The proceeds from the Placement and the Share Purchase Plan have been and will be used to take advantage of opportunities for the Company’s existing point-of-care initiatives by accelerating new product development in patient self-test PT-INR, immunoassay testing and molecular diagnostic testing and to provide working capital to support new product launches and growth in manufacturing.
27
Table of Contents
Exercise of Employee Stock Options
The table below sets forth the number of employee stock options exercised and the number of shares of common stock issued within the past three financial years. We issued these shares in reliance upon exemptions from registration under Regulation S under the Securities Act of 1933, as amended.
| Period Ending |
Number of Options Exercised and Corresponding Number of Shares Issued |
Option Exercise Price |
Proceeds Received (A$) |
|||||||||
| 2012 |
||||||||||||
| February, 2012 |
6,248 | US$ | 0.26 | 1,518 | ||||||||
| June, 2012 |
55,993 | US$ | 0.22 | 12,300 | ||||||||
| August, 2012 |
38,332 | Nil | 0 | |||||||||
| October, 2012 |
8,000 | A$ | 0.70 | 5,600 | ||||||||
| November, 2012 |
6,667 | A$ | 0.70 | 4,668 | ||||||||
|
|
|
|
|
|||||||||
| 115,240 | 24,086 | |||||||||||
|
|
|
|
|
|||||||||
| 2013 |
||||||||||||
| May, 2013 |
40,000 | US$ | 0.22 | 9,020 | ||||||||
| June, 2013 |
708,640 | US$ | 0.22 | 167,051 | ||||||||
| September, 2013 |
75,993 | US$ | 0.22 | 18,690 | ||||||||
| December, 2013 |
672,392 | US$ | 0.22 | 165,837 | ||||||||
|
|
|
|
|
|||||||||
| 1,497,025 | 360,598 | |||||||||||
|
|
|
|
|
|||||||||
| 2014 |
||||||||||||
| March, 2014 |
8,333 | A$ | 0.00 | 0 | ||||||||
|
|
|
|
|
|||||||||
The funds raised have been and will be used for working capital requirements including the continued development of our existing pipeline of point-of-care tests and to identify and develop additional tests.
Restricted Employee Shares Issued to Employees
Our Employee Share Plan was adopted by the Board of Directors in 2009. The Employee Share Plan permits our Board to grant shares of our common stock to our employees and directors. The number of shares able to be granted is limited to the amount permitted to be granted at law, the ASX Listing Rules and by the limits on our authorized share capital in our certificate of incorporation. All our employees are eligible for shares under the Employee Plan. The Company currently proposes to issue A$1,000 worth of restricted shares of common stock to employees of the Company on a recurring basis, but no more frequently than annually. The restricted shares have the same terms of issue as our existing shares of common stock but are not able to be traded until the earlier of three years from the date on which the shares are issued or the date the relevant employee ceases to be an employee of the Company or any of its associated group of companies. We issue these shares in reliance upon exemptions from registration under Regulation S under the Securities Act.
The table below sets forth the restricted shares issued by the Company within the past three financial years:
| Number of Restricted Shares Issued |
Market Value of Restricted Shares Issued (A$) |
|||||||
| November, 2012 |
77,945 | 84,960 | ||||||
| May, 2013 |
917 | 1,000 | ||||||
| December, 2013 |
142,800 | 69,972 | ||||||
| June, 2014 |
2,040 | 1,000 | ||||||
28
Table of Contents
Restricted stock awards activity during the current period is as follows:
| Number of shares | Weighted average issue price A$ |
|||||||
| Balance at December 31, 2013 |
260,801 | 0.72 | ||||||
| Granted |
2,040 | 0.49 | ||||||
| Release of restricted shares |
(28,354 | ) | 0.71 | |||||
|
|
|
|
|
|||||
| Balance at December 31, 2014 |
234,487 | 0.72 | ||||||
|
|
|
|
|
|||||
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
There were no repurchases of equity securities in 2014.
Total Return Stock Performance Graph
The following line graph compares the cumulative total stockholder return on our common stock from December 31, 2009 through December 31, 2014 with the cumulative total return of a major market index and a published industry index. The graph below assumes an investment of A$100.00 on December 31, 2009 in our common stock, and compares its performance with the Standard and Poor’s/Australian Securities Exchange 200 Index and the Standard and Poor’s/Australian Securities Exchange Health Care 200 Index. We paid no dividends on our common stock during the period covered by the graph. The Indices included in the graph reflect a cumulative total return based upon the reinvestment of dividends of the stocks included in those indices. Measurement points are December 31, 2009 and the last trading day of each subsequent year end through December 31, 2014.
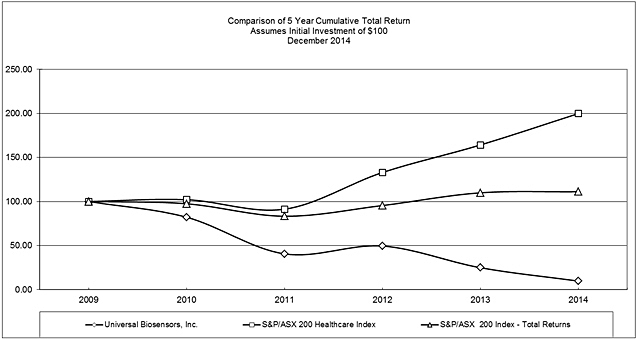
The comparisons shown in the graph above are based upon historical data. The stock price performance shown in the graph is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock. This graph shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, and will not be deemed incorporated by reference into any filing under the Securities Act, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
29
Table of Contents
ITEM 6. SELECTED FINANCIAL DATA.
The following table represents our selected financial data for the dates and periods indicated.
| Years Ended December 31, | ||||||||||||||||||||
| 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||||
| A$ | A$ | A$ | A$ | A$ | ||||||||||||||||
| Revenue |
||||||||||||||||||||
| Revenue from products |
215,486 | 10,170,804 | 19,368,745 | 12,063,582 | 11,760,009 | |||||||||||||||
| Revenue from services |
9,314,198 | 4,918,868 | 10,277,698 | 2,632,870 | 6,420,027 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenue |
9,529,684 | 15,089,672 | 29,646,443 | 14,696,452 | 18,180,036 | |||||||||||||||
| Operating costs & expenses |
||||||||||||||||||||
| Cost of goods sold |
313,374 | 10,455,567 | 17,987,049 | 12,310,302 | 10,801,062 | |||||||||||||||
| Cost of services |
242,453 | 1,187,244 | 669,042 | 708,149 | 1,481,674 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total cost of goods sold & services |
555,827 | 11,642,811 | 18,656,091 | 13,018,451 | 12,282,736 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Contribution from products & services |
8,973,857 | 3,446,861 | 10,990,352 | 1,678,001 | 5,897,300 | |||||||||||||||
| Other operating costs & expenses |
||||||||||||||||||||
| Research and development |
17,136,051 | 15,483,902 | 13,482,459 | 9,812,396 | 6,482,150 | |||||||||||||||
| General and administrative |
5,623,748 | 6,200,786 | 6,790,524 | 7,271,488 | 7,185,550 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating costs & expenses |
22,759,799 | 21,684,688 | 20,272,983 | 17,083,884 | 13,667,700 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(13,785,942 | ) | (18,237,827 | ) | (9,282,631 | ) | (15,405,883 | ) | (7,770,400 | ) | ||||||||||
| Other income/(expense) |
||||||||||||||||||||
| Interest income |
260,904 | 499,970 | 437,171 | 683,323 | 1,192,889 | |||||||||||||||
| Interest expense |
(15,905 | ) | (22,640 | ) | (29,263 | ) | 0 | 0 | ||||||||||||
| Financing costs |
(2,646,092 | ) | (797,126 | ) | 0 | 0 | 0 | |||||||||||||
| Patent fees |
(2,133,626 | ) | 0 | 0 | 0 | 0 | ||||||||||||||
| Other |
9,004,534 | 6,923,816 | (256,499 | ) | 30,443 | (33,014 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total other income/(expense) |
4,469,815 | 6,604,020 | 151,409 | 713,766 | 1,159,875 | |||||||||||||||
| Net loss before tax |
(9,316,127 | ) | (11,633,807 | ) | (9,131,222 | ) | (14,692,117 | ) | (6,610,525 | ) | ||||||||||
| Income tax benefit/(expense) |
0 | 0 | 0 | 0 | 0 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
(9,316,127 | ) | (11,633,807 | ) | (9,131,222 | ) | (14,692,117 | ) | (6,610,525 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Earnings per share |
||||||||||||||||||||
| Basic and diluted net loss per share |
(0.05 | ) | (0.07 | ) | (0.06 | ) | (0.09 | ) | (0.04 | ) | ||||||||||
| Average weighted number of shares - basic & diluted |
175,608,634 | 174,428,259 | 160,417,411 | 159,017,777 | 157,584,044 | |||||||||||||||
| Other comprehensive loss, net of tax: |
||||||||||||||||||||
| Unrealized (loss)/gain on derivative instruments |
0 | 0 | 0 | 83,339 | 0 | |||||||||||||||
| Reclassification for losses/(gains) realized in net income |
0 | 0 | (83,339 | ) | 0 | 47,412 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Other comprehensive (loss)/gain |
0 | 0 | (83,339 | ) | 83,339 | 47,412 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Comprehensive (loss)/gain |
(9,316,127 | ) | (11,633,807 | ) | (9,214,561 | ) | (14,608,778 | ) | (6,563,113 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Years Ended December 31, | ||||||||||||||||||||
| 2014 | 2013 | 2012 | 2011 | 2010 | ||||||||||||||||
| A$ | A$ | A$ | A$ | A$ | ||||||||||||||||
| Balance Sheet Data: |
|
|||||||||||||||||||
| Cash and cash equivalents |
16,329,829 | 23,742,422 | 23,649,417 | 15,089,209 | 23,271,766 | |||||||||||||||
| Total assets |
47,532,638 | 54,619,699 | 49,066,850 | 45,216,467 | 53,837,949 | |||||||||||||||
| Short term borrowings |
498,890 | 0 | 0 | 0 | 0 | |||||||||||||||
| Other liability |
2,133,626 | 0 | 0 | 0 | 0 | |||||||||||||||
| Long term secured loan |
17,499,194 | 15,857,966 | 0 | 0 | 0 | |||||||||||||||
| Total stockholders’ equity |
19,740,945 | 29,683,940 | 39,372,139 | 35,022,606 | 47,219,079 | |||||||||||||||
30
Table of Contents
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The information required by this item is incorporated by reference to our 2014 Annual Report under the caption “Management’s Discussion and Analysis of Financial Condition and Results of Operations” on pages F2 to F15.
31
Table of Contents
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The information required by this item is incorporated by reference to our 2014 Annual Report under the caption “Management’s Discussion and Analysis of Financial Condition and Results of Operations - Financial Risk Management” on pages F14 to F15.
32
Table of Contents
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
We refer you to the “Consolidated Balance Sheets”, “Consolidated Statements of Comprehensive Income”, “Consolidated Statements of Changes in Stockholders’ Equity and Comprehensive Income”, “Consolidated Statements of Cash Flows”, and “Notes to Consolidated Financial Statements”, on pages F18 through F44, and “Report of Independent Registered Public Accounting Firm” on pages F16 through F17 of our Annual Report to Stockholders for the fiscal year ended December 31, 2014, included herein.
Supplementary Financial Information
The following is a summary of the unaudited quarterly results of operations:
| Year ended December 31, 2014 | ||||||||||||||||
| Quarter Ended March 31 |
Quarter Ended June 30 |
Quarter Ended September 30 |
Quarter Ended December 31 |
|||||||||||||
| A$ | A$ | A$ | A$ | |||||||||||||
| Revenue |
||||||||||||||||
| Revenue from products |
0 | 0 | 137,294 | 78,192 | ||||||||||||
| Revenue from services |
1,351,951 | 1,513,981 | 1,949,297 | 4,498,969 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
1,351,951 | 1,513,981 | 2,086,591 | 4,577,161 | ||||||||||||
| Operating costs & expenses |
||||||||||||||||
| Cost of goods sold |
0 | 0 | 218,298 | 95,076 | ||||||||||||
| Cost of services |
16,145 | 6,777 | 127,084 | 92,447 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total cost of goods sold & services |
16,145 | 6,777 | 345,382 | 187,523 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Contribution from products & services |
1,335,806 | 1,507,204 | 1,741,209 | 4,389,638 | ||||||||||||
| Other operating costs & expenses |
||||||||||||||||
| Research and development |
4,915,187 | 4,270,368 | 4,715,444 | 3,235,052 | ||||||||||||
| General and administrative |
1,383,406 | 1,683,105 | 1,522,175 | 1,035,062 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating costs & expenses |
6,298,593 | 5,953,473 | 6,237,619 | 4,270,114 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Profit/(loss) from operations |
(4,962,787 | ) | (4,446,269 | ) | (4,496,410 | ) | 119,524 | |||||||||
| Other income/(expense) |
||||||||||||||||
| Interest income |
54,348 | 29,501 | 103,398 | 73,657 | ||||||||||||
| Interest expense |
(6,362 | ) | (4,771 | ) | (4,772 | ) | 0 | |||||||||
| Financing costs |
(657,271 | ) | (633,629 | ) | (654,095 | ) | (701,097 | ) | ||||||||
| Patent fees |
0 | 0 | 0 | (2,133,626 | ) | |||||||||||
| Other |
1,974,354 | 1,700,239 | 3,157,820 | 2,172,121 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income/(expense) |
1,365,069 | 1,091,340 | 2,602,351 | (588,945 | ) | |||||||||||
| Net loss before tax |
(3,597,718 | ) | (3,354,929 | ) | (1,894,059 | ) | (469,421 | ) | ||||||||
| Income tax benefit/(expense) |
0 | 0 | 0 | 0 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(3,597,718 | ) | (3,354,929 | ) | (1,894,059 | ) | (469,421 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Earnings per share |
||||||||||||||||
| Basic and diluted net loss per share |
(0.02 | ) | (0.02 | ) | (0.01 | ) | (0.00 | ) | ||||||||
| Other comprehensive loss, net of tax: |
||||||||||||||||
| Reclassification for gain/(loss) realized in net income |
0 | 0 | 0 | 0 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other comprehensive (loss)/gain |
0 | 0 | 0 | 0 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss |
(3,597,718 | ) | (3,354,929 | ) | (1,894,059 | ) | (469,421 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
33
Table of Contents
| Year ended December 31, 2013 | ||||||||||||||||
| Quarter Ended March 31 |
Quarter Ended June 30 |
Quarter Ended September 30 |
Quarter Ended December 31 |
|||||||||||||
| A$ | A$ | A$ | A$ | |||||||||||||
| Revenue |
||||||||||||||||
| Revenue from products |
3,745,818 | 3,455,253 | 1,396,357 | 1,573,376 | ||||||||||||
| Revenue from services |
1,063,156 | 1,315,200 | 1,117,229 | 1,423,283 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
4,808,974 | 4,770,453 | 2,513,586 | 2,996,659 | ||||||||||||
| Operating costs & expenses |
||||||||||||||||
| Cost of goods sold |
3,713,714 | 3,206,717 | 1,756,957 | 1,778,179 | ||||||||||||
| Cost of services |
77,625 | 554,388 | 236,334 | 318,897 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total cost of goods sold & services |
3,791,339 | 3,761,105 | 1,993,291 | 2,097,076 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Contribution from products & services |
1,017,635 | 1,009,348 | 520,295 | 899,583 | ||||||||||||
| Other operating costs & expenses |
||||||||||||||||
| Research and development |
4,457,929 | 3,448,202 | 3,901,823 | 3,675,948 | ||||||||||||
| General and administrative |
1,307,665 | 1,487,733 | 1,684,703 | 1,720,685 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating costs & expenses |
5,765,594 | 4,935,935 | 5,586,526 | 5,396,633 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(4,747,959 | ) | (3,926,587 | ) | (5,066,231 | ) | (4,497,050 | ) | ||||||||
| Other income/(expense) |
||||||||||||||||
| Interest income |
157,302 | 136,057 | 123,663 | 82,948 | ||||||||||||
| Interest expense |
(5,660 | ) | (5,660 | ) | (7,547 | ) | (3,773 | ) | ||||||||
| Financing costs |
0 | 0 | 0 | (797,126 | ) | |||||||||||
| Other |
(46,352 | ) | 755,415 | 4,266,790 | 1,947,963 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other income |
105,290 | 885,812 | 4,382,906 | 1,230,012 | ||||||||||||
| Net loss before tax |
(4,642,669 | ) | (3,040,775 | ) | (683,325 | ) | (3,267,038 | ) | ||||||||
| Income tax benefit/(expense) |
0 | 0 | 0 | 0 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(4,642,669 | ) | (3,040,775 | ) | (683,325 | ) | (3,267,038 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Earnings per share |
||||||||||||||||
| Basic and diluted net loss per share |
(0.03 | ) | (0.01 | ) | (0.00 | ) | (0.02 | ) | ||||||||
| Other comprehensive loss, net of tax: |
||||||||||||||||
| Unrealised gain/(loss) on derivative instruments |
12,527 | 0 | 0 | 0 | ||||||||||||
| Reclassification for (losses)/gains realised in net income |
0 | (12,527 | ) | 0 | 0 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other comprehensive (loss)/gain |
12,527 | (12,527 | ) | 0 | 0 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss |
(4,630,142 | ) | (3,053,302 | ) | (683,325 | ) | (3,267,038 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
34
Table of Contents
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
35
Table of Contents
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures. At the end of the period covered by this report, the Company evaluated the effectiveness of the design and operation of its disclosure controls and procedures. The Company’s disclosure controls and procedures are designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the Company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Paul Wright, Chief Executive Officer, and Salesh Balak, Chief Financial Officer, reviewed and participated in this evaluation. Based on this evaluation, Messrs. Wright and Balak concluded that, as of the end of the period covered by this report, the Company’s disclosure controls and procedures were effective.
Changes in Internal Control over Financial Reporting. During the fiscal quarter ended December 31, 2014, there were no changes in the Company’s internal control over financial reporting identified in connection with the evaluation referred to above in this Item 9A that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
36
Table of Contents
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| • | Pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and the dispositions of the assets of the Company; |
| • | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and the board of directors of the Company; and |
| • | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluations of effectiveness to future periods are subject to risk that controls may become inadequate because of changes in conditions or because of declines in the degree of compliance with the policies or procedures.
Our management, with the participation of the Principal Executive Officer and Principal Financial Officer, assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2014. In making this assessment, the Company’s management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control-Integrated Framework (2013).
Based on this evaluation, our management, with the participation of the Principal Executive Officer and Principal Financial Officer, concluded that, as of December 31, 2014, our internal control over financial reporting was effective.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2014 has been audited by PricewaterhouseCoopers, an independent registered public accounting firm, and PricewaterhouseCoopers has issued an attestation report on the Company’s internal control over financial reporting, which appears in the “Report of Independent Registered Public Accounting Firm” on pages F-16 to F-17 of the Annual Report, which is incorporated herein by reference and filed as Exhibit 13 to this Report on Form 10-K.
| /s/ Paul Wright |
/s/ Salesh Balak | |||
| Paul Wright | Salesh Balak | |||
| Principal Executive Officer | Principal Financial Officer | |||
March 12, 2015
37
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM ON INTERNAL CONTROL OVER FINANCIAL REPORTING
We refer you to “Report of Independent Registered Public Accounting Firm” on pages F16 to F17 of our Annual Report to Stockholders for the fiscal year ended December 31, 2014, which are included herein, for the Independent Registered Public Accounting Firm’s report with respect to the effectiveness of internal control over financial reporting.
38
Table of Contents
None.
39
Table of Contents
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
The information required by this item regarding our directors and executive officers is incorporated by reference to our Definitive Proxy Statement to be filed with the Securities and Exchange Commission in connection with our Annual Meeting of Stockholders in 2015 (the “2015 Proxy Statement”) under the caption “Management of the Company.”
The information required by this item regarding “Compliance with Section 16(a) of the Exchange Act” is incorporated by reference to the 2015 Proxy Statement under the caption “Other Matters – Section 16(a) Beneficial Ownership Reporting Compliance.”
We have adopted our Code of Ethics for Senior Financial Officers, a code of ethics that applies to our Principal Executive Officer and Principal Financial Officer. This code of ethics may be accessed and reviewed through our website at www.universalbiosensors.com. We intend to satisfy any disclosure requirement under item 5.05 of Form 8-K regarding an amendment to, or waiver from, a provision of the Code of Ethics for our Principal Executive Officer and Principal Financial Officer, by posting such information on our website at www.universalbiosensors.com
The information regarding the procedures by which security holders may recommend nominees to our Board of Directors is incorporated by reference to the 2015 Proxy Statement under the caption “Management of the Company – Board Committees – Remuneration and Nomination Committee.” There have been no material changes to the procedures by which security holders may recommend nominees to our Board of Directors.
The information required by this item regarding our Audit Committee is incorporated by reference to the 2015 Proxy Statement under the caption “Management of the Company – Board Committees – Audit and Compliance Committee.”
40
Table of Contents
ITEM 11. EXECUTIVE COMPENSATION.
The information required by this item is incorporated by reference to the 2015 Proxy Statement under the captions “Management of the Company – Compensation of Directors”, “Executive Compensation” and “Management of the Company – Board Committees – Compensation Committee Interlocks and Insider Participation.”
Discussions on the frequency of the shareholder advisory votes on executive compensation are incorporated by reference to the 2015 Proxy Statement under the caption “Executive Compensation”.
41
Table of Contents
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The information regarding the security ownership of certain beneficial owners and management is incorporated by reference to the 2015 Proxy Statement under the caption “Security Ownership of Certain Beneficial Owners and Management.”
The information regarding “Securities Authorized for Issuance under Equity Compensation Plans” is incorporated by reference to our 2015 Proxy Statement under the caption “Executive Compensation – Equity Compensation Plan Information.”
42
Table of Contents
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
The information required by this item is incorporated by reference to the 2015 Proxy Statement under the caption “Certain Relationships and Related Transactions,” and “Management of the Company.”
43
Table of Contents
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
The information required by this item is incorporated by reference to the 2015 Proxy Statement under the caption “Independent Public Accountants – Audit Fees.”
44
Table of Contents
ITEM 15. EXHIBITS, FINANCIAL STATEMENTS AND SCHEDULES.
| (a)(1) | ||||||
| The following financial statements are incorporated by reference from pages F-16 through F-44 of our Annual Report to Stockholders for the fiscal year ended December 31, 2014, as provided in Item 8 hereof: | ||||||
| F-16 | ||||||
| F-18 | ||||||
| F-19 | ||||||
| Consolidated Statements of Changes in Stockholders’ Equity and Comprehensive Income |
F-20 | |||||
| F-21 | ||||||
| F-22 | ||||||
| (a)(2) | Financial Statement Schedules – Schedule II—Valuation and Qualifying Accounts. All other schedules are omitted because of the absence of the conditions under which they are required or because the required information is included elsewhere in the financial statements. | |||||
| (a)(3) and (b) | Exhibits – Refer below. | |||||
| Exhibit Number |
Description | Location | ||
| 3.1 | Amended and restated articles of incorporation dated December 5, 2006. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 3.1. | ||
| 3.2 | Amended and restated by-laws dated December 5, 2006. | Incorporated by reference to our Amendment No. 5 to Form 10 filed on April 29, 2008 as Exhibit 3.2. | ||
| 10.1 | Amended and Restated License Agreement, between LifeScan, Inc. and Universal Biosensors Pty Ltd dated on August 29, 2011 and effective as of August 19, 2011. | Incorporated by reference to our Current Report on Form 8-K filed on August 30, 2011 as Exhibit 10.1. | ||
| 10.2 | Amended and Restated Development and Research Agreement between Cilag GmbH International and Universal Biosensors Pty Ltd dated on August 29, 2011 and effective as of August 19, 2011. | Incorporated by reference to our Current Report on Form 8-K filed on August 30, 2011 as Exhibit 10.2. | ||
| 10.3 | Form of indemnity agreement entered into with directors of us, our chief financial officer and company secretary | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.3. | ||
| 10.4 | Lease of premises 1 Corporate Avenue, Rowville, Victoria, Australia by and between Universal Biosensors Pty Ltd and Heyram Properties Pty Ltd. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.5. | ||
| 10.5 | Employee Option Plan. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.7. | ||
| 10.6 | Employment agreement between Universal Biosensors Pty Ltd and Mr. Salesh Balak effective November 27, 2006. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.8. | ||
| 10.7 | Employment agreement between Universal Biosensors Pty Ltd and Mr. Garry Chambers effective April 1, 2006. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.9. | ||
| 10.8 | Employment agreement between Universal Biosensors Pty Ltd and Dr Ronald Chatelier dated April 1, 2006. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.10. | ||
45
Table of Contents
| 10.9 | Employment agreement between Universal Biosensors Pty Ltd and Dr Alastair Hodges effective April 1, 2006. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.11. | ||
| 10.10 | Employment agreement between Universal Biosensors Pty Ltd and Mr. Adrian Oates dated August 15, 2007. | Incorporated by reference to our Form 10-K filed on March 16, 2010 as Exhibit 10.12. | ||
| 10.11 | Amended and Restated Master Services and Supply Agreement (which amends and restates the Master Services and Supply Agreement by and between Universal Biosensors Pty. Ltd., Universal Biosensors, Inc., and LifeScan, Inc. dated October 29, 2007 filed on November 14, 2007 as Exhibit 10.1 to our Quarterly Report on Form 10-Q and the First Amendment to the Master Services and Supply Agreement filed on March 30, 2009 as Exhibit 10.14 to our Annual Report on Form 10-K). | Incorporated by reference to our Quarterly Report on Form 10-Q filed on August 7, 2009 as Exhibit 10.3. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.12 | Manufacturing Initiation Payment Addendum to Master Services and Supply Agreement (which is an addendum to the Amended and Restated Master Services and Supply Agreement filed on August 7, 2009 as Exhibit 10.3 to our Quarterly Report on Form 10-Q). | Incorporated by reference to our Quarterly Report on Form 10-Q filed on August 7, 2009 as Exhibit 10.4. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.13 | Collaboration Agreement between Universal Biosensors Pty Ltd and Siemens Healthcare Diagnostics Inc. dated September 9, 2011. | Incorporated by reference to our Quarterly Report on Form 10-Q filed on November 3, 2011 as Exhibit 10.20. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.14 | Statement of Work for MAP Feasibility Project between Universal Biosensors Pty Ltd, LifeScan, Inc. and Cilag GmbH International dated October 11, 2011. | Incorporated by reference to our Quarterly Report on Form 10-Q filed on November 3, 2011 as Exhibit 10.21. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.15 | Novation Agreement and First Amendment to the Amended and Restated Master Services and Supply Agreement between Universal Biosensors, Inc., Universal Biosensors Pty Ltd, LifeScan, Inc. and Cilag GmbH International dated October 11, 2011. | Incorporated by reference to our Quarterly Report on Form 10-Q filed on November 3, 2011 as Exhibit 10.22. | ||
| 10.16 | Second Amendment to the Amended and Restated Master Services and Supply Agreement between Universal Biosensors, Inc., Universal Biosensors Pty Ltd, LifeScan, Inc. and Cilag GmbH International dated October 11, 2011. | Incorporated by reference to our Quarterly Report on Form 10-Q filed on November 3, 2011 as Exhibit 10.23. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.17 | Employment agreement between Universal Biosensors Pty Ltd and Mr. Paul Wright effective March 1, 2011. | Incorporated by reference to our Current Report on Form 8-K filed on February 25, 2011 as Exhibit 10.1. | ||
46
Table of Contents
| 10.18 | Amendment to Collaboration Agreement between Universal Biosensors Pty Ltd and Siemens Healthcare Diagnostics, Inc. dated September 20, 2012. | Incorporated by reference to our Quarterly Report on Form 10-Q/A filed on February 4, 2013 as Exhibit 10.1. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.19 | Supply Agreement between Universal Biosensors Pty Ltd and Siemens Healthcare Diagnostics, Inc. dated September 20, 2012. | Incorporated by reference to our Quarterly Report on Form 10-Q/A filed on February 4, 2013 as Exhibit 10.2. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.20 | Supplemental Agreement – Reader Product Support Obligations and Responsibilities between Universal Biosensors Pty Ltd and Siemens Healthcare Diagnostics, Inc. dated September 20, 2012. | Incorporated by reference to our Quarterly Report on Form 10-Q/A filed on February 4, 2013 as Exhibit 10.3. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.21 | Credit Agreement dated December 19, 2013 by and among Athyrium Opportunities Fund (A) LP as Administrative Agent and a Lender, Universal Biosensors Pty Ltd as borrower, Universal Biosensors, Inc. as a Guarantor, and the other Lenders and Guarantors as party thereto from time to time. | Incorporated by reference to our Current Report on Form 8-K filed on December 20, 2013 as Exhibit 10.1. | ||
| 10.22 | Third Amendment to Amended and Restated Master Services and Supply Agreement by and among Universal Biosensors, Inc., Universal Biosensors Pty Ltd, and Cilag GmbH International | Incorporated by reference to our Current Report on Form 8-K filed on December 20, 2013 as Exhibit 10.2. | ||
| 10.23 | Common Stock Purchase Warrant by and among Athyrium Opportunities Fund (A) LP and Universal Biosensors, Inc. | Incorporated by reference to our Current Report on Form 8-K filed on December 20, 2013 as Exhibit 10.3. | ||
| 10.24 | Common Stock Purchase Warrant by and among Athyrium Opportunities Fund (B) LP and Universal Biosensors, Inc. | Incorporated by reference to our Current Report on Form 8-K filed on December 20, 2013 as Exhibit 10.4. | ||
| 10.25 | Deed of Extension of Lease between Universal Biosensors Pty Ltd and Bowmayne Pty Ltd dated March 24, 2014 | Incorporated by reference to our Quarterly Report on Form 10-Q filed on April 25, 2014 as Exhibit 10.34. | ||
| 10.26 | Amendment dated January 30, 2015 to Credit Agreement by and among Athyrium Opportunities Fund (A) LP as Administrative Agent and a Lender, Universal Biosensors Pty Ltd as borrower, Universal Biosensors, Inc. as a Guarantor, and the other Lenders and Guarantors as party thereto from time to time | Incorporated by reference to our Current Report on Form 8-K filed on February 2, 2015 as Exhibit 10.1. | ||
| 13.0 | Annual Report. | Filed herewith. | ||
| 14.0 | Code of Ethics. | Incorporated by reference to our Annual Report on Form 10-K filed on March 28, 2008 as Exhibit 14.0. | ||
| 21.0 | List of Subsidiaries. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 21.0. | ||
| 24.0 | Power of Attorney. | Included on signature page. | ||
47
Table of Contents
| 31.1 | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act. | Filed herewith. | ||
| 31.2 | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act. | Filed herewith. | ||
| 32.0 | Certification of Principal Executive Officer and Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act. | Filed herewith. | ||
| 101 | The following materials from the Universal Biosensors, Inc. Annual Report on Form 10-K for the financial year ended December 31, 2014 formatted in Extensible Business Reporting Language (XBRL): (i) the Consolidated Condensed Balance Sheets, (ii) the Consolidated Condensed Statements of Comprehensive Income, (iii) the Consolidated Condensed Statements of Changes in Stockholder’s Equity, (iv) the Consolidated Condensed Statements of Cash Flows and (v) the Notes to Consolidated Condensed Financial Statements. | As provided in Rule 406T of Regulation S-T, this information is furnished herewith and not filed for purposes of Sections 11 and 12 of the Securities Act of 1933 and Section 18 of the Securities Exchange Act of 1934. | ||
48
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
| Universal Biosensors, Inc. | ||||||||
| (Registrant) | ||||||||
| Date: March 12, 2015 | ||||||||
| By: | /s/ Paul Wright | |||||||
| Paul Wright | ||||||||
| Principal Executive Officer |
POWER OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Paul Wright and Salesh Balak and each of them, his or her attorneys-in-fact, each with the power of substitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this report on Form 10-K, and to file the same, with all exhibits thereto and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them full power and authority to do and perform each and every act and all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that such attorneys in-fact and agents or any of them or his or their substitute or substitutes, may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following on behalf of the registrant and in the capacities and on the dates indicated:
| Signature |
Title |
Date | ||
| /s/ Paul Wright |
Chief Executive Officer | March 12, 2015 | ||
| Paul Wright | (Principal Executive Officer) | |||
| /s/ Salesh Balak |
Chief Financial Officer | March 12, 2015 | ||
| Salesh Balak | (Principal Financial Officer and Principal Accounting Officer) | |||
| /s/ Andrew Denver |
Director and Chairman | March 12, 2015 | ||
| Andrew Denver | ||||
| /s/ Denis Hanley |
Director | March 12, 2015 | ||
| Denis Hanley | ||||
| /s/ Andrew Jane |
Director | March 12, 2015 | ||
| Andrew Jane | ||||
| /s/ Christopher Smith |
Director | March 12, 2015 | ||
| Christopher Smith | ||||
| /s/ Marshall Heinberg |
Director | March 12, 2015 | ||
| Marshall Heinberg | ||||
49
Table of Contents
INDEX TO EXHIBITS
| Exhibit Number |
Description | Location | ||
| 3.1 | Amended and restated articles of incorporation dated December 5, 2006. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 3.1. | ||
| 3.2 | Amended and restated by-laws dated December 5, 2006. | Incorporated by reference to our Amendment No. 5 to Form 10 filed on April 29, 2008 as Exhibit 3.2. | ||
| 10.1 | Amended and Restated License Agreement, between LifeScan, Inc. and Universal Biosensors Pty Ltd dated on August 29, 2011 and effective as of August 19, 2011. | Incorporated by reference to our Current Report on Form 8-K filed on August 30, 2011 as Exhibit 10.1. | ||
| 10.2 | Amended and Restated Development and Research Agreement between Cilag GmbH International and Universal Biosensors Pty Ltd dated on August 29, 2011 and effective as of August 19, 2011. | Incorporated by reference to our Current Report on Form 8-K filed on August 30, 2011 as Exhibit 10.2. | ||
| 10.3 | Form of indemnity agreement entered into with directors of us, our chief financial officer and company secretary | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.3. | ||
| 10.4 | Lease of premises 1 Corporate Avenue, Rowville, Victoria, Australia by and between Universal Biosensors Pty Ltd and Heyram Properties Pty Ltd. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.5. | ||
| 10.5 | Employee Option Plan. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.7. | ||
| 10.6 | Employment agreement between Universal Biosensors Pty Ltd and Mr. Salesh Balak effective November 27, 2006. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.8. | ||
| 10.7 | Employment agreement between Universal Biosensors Pty Ltd and Mr. Garry Chambers effective April 1, 2006. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.9. | ||
| 10.8 | Employment agreement between Universal Biosensors Pty Ltd and Dr Ronald Chatelier dated April 1, 2006. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.10. | ||
| 10.9 | Employment agreement between Universal Biosensors Pty Ltd and Dr Alastair Hodges effective April 1, 2006. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 10.11. | ||
| 10.10 | Employment agreement between Universal Biosensors Pty Ltd and Mr. Adrian Oates dated August 15, 2007. | Incorporated by reference to our Form 10-K filed on March 16, 2010 as Exhibit 10.12. | ||
| 10.11 | Amended and Restated Master Services and Supply Agreement (which amends and restates the Master Services and Supply Agreement by and between Universal Biosensors Pty. Ltd., Universal Biosensors, Inc., and LifeScan, Inc. dated October 29, 2007 filed on November 14, 2007 as Exhibit 10.1 to our Quarterly Report on Form 10-Q and the First Amendment to the Master Services and Supply Agreement filed on March 30, 2009 as Exhibit 10.14 to our Annual Report on Form 10-K). | Incorporated by reference to our Quarterly Report on Form 10-Q filed on August 7, 2009 as Exhibit 10.3. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
50
Table of Contents
| 10.12 | Manufacturing Initiation Payment Addendum to Master Services and Supply Agreement (which is an addendum to the Amended and Restated Master Services and Supply Agreement filed on August 7, 2009 as Exhibit 10.3 to our Quarterly Report on Form 10-Q). | Incorporated by reference to our Quarterly Report on Form 10-Q filed on August 7, 2009 as Exhibit 10.4. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.13 | Collaboration Agreement between Universal Biosensors Pty Ltd and Siemens Healthcare Diagnostics Inc. dated September 9, 2011. | Incorporated by reference to our Quarterly Report on Form 10-Q filed on November 3, 2011 as Exhibit 10.20. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.14 | Statement of Work for MAP Feasibility Project between Universal Biosensors Pty Ltd, LifeScan, Inc. and Cilag GmbH International dated October 11, 2011. | Incorporated by reference to our Quarterly Report on Form 10-Q filed on November 3, 2011 as Exhibit 10.21. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.15 | Novation Agreement and First Amendment to the Amended and Restated Master Services and Supply Agreement between Universal Biosensors, Inc., Universal Biosensors Pty Ltd, LifeScan, Inc. and Cilag GmbH International dated October 11, 2011. | Incorporated by reference to our Quarterly Report on Form 10-Q filed on November 3, 2011 as Exhibit 10.22. | ||
| 10.16 | Second Amendment to the Amended and Restated Master Services and Supply Agreement between Universal Biosensors, Inc., Universal Biosensors Pty Ltd, LifeScan, Inc. and Cilag GmbH International dated October 11, 2011. | Incorporated by reference to our Quarterly Report on Form 10-Q filed on November 3, 2011 as Exhibit 10.23. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.17 | Employment agreement between Universal Biosensors Pty Ltd and Mr. Paul Wright effective March 1, 2011. | Incorporated by reference to our Current Report on Form 8-K filed on February 25, 2011 as Exhibit 10.1. | ||
| 10.18 | Amendment to Collaboration Agreement between Universal Biosensors Pty Ltd and Siemens Healthcare Diagnostics, Inc. dated September 20, 2012. | Incorporated by reference to our Quarterly Report on Form 10-Q/A filed on February 4, 2013 as Exhibit 10.1. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.19 | Supply Agreement between Universal Biosensors Pty Ltd and Siemens Healthcare Diagnostics, Inc. dated September 20, 2012. | Incorporated by reference to our Quarterly Report on Form 10-Q/A filed on February 4, 2013 as Exhibit 10.2. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.20 | Supplemental Agreement – Reader Product Support Obligations and Responsibilities between Universal Biosensors Pty Ltd and Siemens Healthcare Diagnostics, Inc. dated September 20, 2012. | Incorporated by reference to our Quarterly Report on Form 10-Q/A filed on February 4, 2013 as Exhibit 10.3. Confidentiality treatment has been granted for portions of this exhibit. These confidential portions have been omitted and were filed separately with the SEC. | ||
| 10.21 | Credit Agreement dated December 19, 2013 by and among Athyrium Opportunities Fund (A) LP as | Incorporated by reference to our Current Report on Form 8-K filed on December 20, 2013 as Exhibit 10.1. | ||
51
Table of Contents
| Administrative Agent and a Lender, Universal Biosensors Pty Ltd as borrower, Universal Biosensors, Inc. as a Guarantor, and the other Lenders and Guarantors as party thereto from time to time. | ||||
| 10.22 | Third Amendment to Amended and Restated Master Services and Supply Agreement by and among Universal Biosensors, Inc., Universal Biosensors Pty Ltd, and Cilag GmbH International | Incorporated by reference to our Current Report on Form 8-K filed on December 20, 2013 as Exhibit 10.2. | ||
| 10.23 | Common Stock Purchase Warrant by and among Athyrium Opportunities Fund (A) LP and Universal Biosensors, Inc. | Incorporated by reference to our Current Report on Form 8-K filed on December 20, 2013 as Exhibit 10.3. | ||
| 10.24 | Common Stock Purchase Warrant by and among Athyrium Opportunities Fund (B) LP and Universal Biosensors, Inc. | Incorporated by reference to our Current Report on Form 8-K filed on December 20, 2013 as Exhibit 10.4. | ||
| 10.25 | Deed of Extension of Lease between Universal Biosensors Pty Ltd and Bowmayne Pty Ltd dated March 24, 2014 | Incorporated by reference to our Quarterly Report on Form 10-Q filed on April 25, 2014 as Exhibit 10.34. | ||
| 10.26 | Amendment dated January 30, 2015 to Credit Agreement by and among Athyrium Opportunities Fund (A) LP as Administrative Agent and a Lender, Universal Biosensors Pty Ltd as borrower, Universal Biosensors, Inc. as a Guarantor, and the other Lenders and Guarantors as party thereto from time to time | Incorporated by reference to our Current Report on Form 8-K filed on February 2, 2015 as Exhibit 10.1. | ||
| 13.0 | Annual Report. | Filed herewith. | ||
| 14.0 | Code of Ethics. | Incorporated by reference to our Annual Report on Form 10-K filed on March 28, 2008 as Exhibit 14.0. | ||
| 21.0 | List of Subsidiaries. | Incorporated by reference to our General Form for Registration of Securities on Form 10 filed on April 30, 2007 as Exhibit 21.0. | ||
| 24.0 | Power of Attorney. | Included on signature page. | ||
| 31.1 | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act. | Filed herewith. | ||
| 31.2 | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act. | Filed herewith. | ||
| 32.0 | Certification of Principal Executive Officer and Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act. | Filed herewith. | ||
52
Table of Contents
| 101 | The following materials from the Universal Biosensors, Inc. Annual Report on Form 10-K for the financial year ended December 31, 2014 formatted in Extensible Business Reporting Language (XBRL): (i) the Consolidated Condensed Balance Sheets, (ii) the Consolidated Condensed Statements of Comprehensive Income, (iii) the Consolidated Condensed Statements of Changes in Stockholder’s Equity, (iv) the Consolidated Condensed Statements of Cash Flows and (v) the Notes to Consolidated Condensed Financial Statements. | As provided in Rule 406T of Regulation S-T, this information is furnished herewith and not filed for purposes of Sections 11 and 12 of the Securities Act of 1933 and Section 18 of the Securities Exchange Act of 1934. | ||
53
