Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a10-13137_18k.htm |
| EX-99.1 - EX-99.1 - VIVUS INC | a10-13137_1ex99d1.htm |
| EX-99.2 - EX-99.2 - VIVUS INC | a10-13137_1ex99d2.htm |
| EX-99.5 - EX-99.5 - VIVUS INC | a10-13137_1ex99d5.htm |
| EX-99.3 - EX-99.3 - VIVUS INC | a10-13137_1ex99d3.htm |
Exhibit 99.4
Below is a graphical representation of the poster entitled “Improvements in Dyslipidemia and Other Cardiometabolic Disease Risk Factors With Low-Dose, Controlled-Release Phentermine/Topiramate”:
Below is a reproduction of the contents of the poster entitled “Improvements in Dyslipidemia and Other Cardiometabolic Disease Risk Factors With Low-Dose, Controlled-Release Phentermine/Topiramate”:
Authors: W. Timothy Garvey, MD(a); Kishore M. Gadde, MD(b); Leland Wilson(c); Craig Peterson, MS(c)
(a)University of Alabama at Birmingham, Birmingham, Alabama, United States of America; (b)Duke University, Durham, North Carolina, United States of America; (c)VIVUS, Inc., Mountain View, California, United States of America
· Introduction
· Obesity is an epidemic in the United States associated with comorbidities such as cardiovascular disease and type 2 diabetes mellitus (T2DM), as well as increased mortality.(1)-(4)
· Some of the cardiometabolic comorbidities related to obesity include dyslipidemia, hypertension, and stroke.(2) The combination of obesity, hypertension, and inflammation has been shown to cause increased atherosclerotic risk.(5)
· Weight loss, however, reduces the incidence of cardiometabolic comorbidities and improves such risk factors as triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels; it also decreases inflammation.(6),(7)
· Phentermine (PHEN) and topiramate (TPM) are 2 pharmacologic agents with demonstrated weight-loss properties. PHEN is currently approved in the United States for short-term weight loss (recommended dose is 37.5 mg/day) as an adjunct to lifestyle modifications. TPM is indicated for treatment of seizures (recommended dose is 400 mg/day) and prevention of migraines (recommended dose is 100 mg/day) and has been studied for the treatment of obesity and obesity with comorbid conditions such as T2DM and hypertension at a range of doses (64 to 384 mg/day).(8)-(13)
· A previous extended-release formulation of TPM at doses titrated up to 175 mg/day (range 50 mg/day to 175 mg/day) demonstrated weight loss in clinical trials, although side effects were significant, and prevented further development of that formulation as monotherapy.(14) Therefore, a new combination of low-dose, controlled-release (CR) PHEN/TPM for once-daily oral dosing was developed to maximize weight loss and cardiometabolic benefits, while minimizing adverse events.
· Objectives
· To evaluate weight loss over a 56-week period in overweight/obese subjects with >2 weight-related comorbidities.
· To evaluate changes in lipid parameters and inflammatory markers over a 56-week period in overweight/obese subjects with >2 weight-related comorbidities.
· Methods
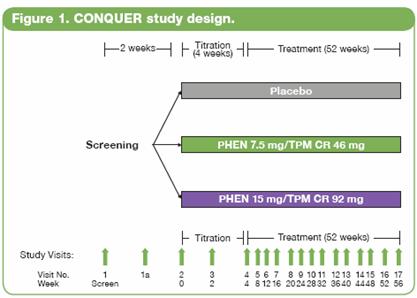
· A double-blind, placebo-controlled Phase 3 trial (CONQUER) was conducted in 2487 overweight/obese adult subjects with >2 weight-related comorbidities. Subjects were randomly assigned to placebo, PHEN 7.5 mg/TPM CR 46 mg (7.5/46), or PHEN 15 mg/TPM CR 92 mg (15/92) for 56 weeks. All subjects received lifestyle and exercise guidance.
· Subjects were instructed to take study drug once daily for 56 weeks (Figure 1). Efficacy and safety endpoints were evaluated at baseline, Weeks 2 and 4 of the titration period, and then at 4-week intervals. Subjects also had periodic fasting laboratory assessments.
· Assessments
· The primary efficacy endpoints in this trial were percent change in body weight at Week 56 and percentage of subjects with >5% weight loss at Week 56 in the intent-to-treat (ITT) population with last observation carried forward (LOCF). Other efficacy endpoints included percentage of subjects achieving >10% weight loss and changes in lipid levels (LDL-C, HDL-C, TC, ratio of TC/HDL, and TG). Safety outcomes were also assessed at all study visits.
· This analysis also examined the effects of weight loss on change in the inflammatory and metabolic markers: high-sensitivity C-reactive protein (hsCRP), fibrinogen, and adiponectin.
· Analysis of covariance (ANCOVA) was used to evaluate changes in weight loss and other outcomes. The ANCOVA model used factors of treatment, gender, and diabetic status with baseline weight as a covariate. For each treatment comparison, the difference in least-squares (LS) means, corresponding standard errors, 95% confidence intervals, and P values were derived from the ANCOVA model.
· Analysis of percentage of subjects with at least 5% or 10% weight loss at Week 56 with LOCF was performed using a logistic regression model with treatment, gender, and diabetic status as fixed effects and baseline weight as a covariate. For each treatment comparison of interest, the estimated odds ratio (OR), standard error, 95% Wald confidence interval, and P value were derived.
· Results
· The randomized population (N=2487) was 86% Caucasian and 70% female with a mean age of 51 years. Subjects had a baseline mean weight of 103 kg, mean BMI of 36.6 kg/m2, mean WC of 113 cm, and BP of 128/81 mm Hg. Other baseline mean values included TC 204.5 mg/dL and LDL-C 123.1 mg/dL.
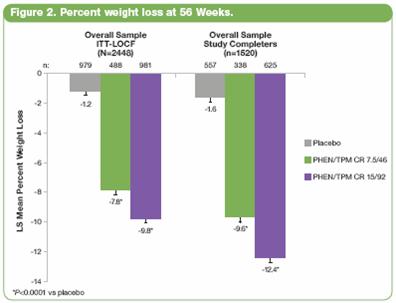
· At Week 56, LS mean percent weight loss was significantly greater in both PHEN/TPM CR groups vs placebo (P<0.0001 for all comparisons). In the overall ITT-LOCF population, LS mean percent weight loss was 1.2%, 7.8%, and 9.8% for placebo, 7.5/46, and 15/92, respectively; in subjects completing 56 weeks on study drug (study completers), LS mean percent weight loss was 1.6%, 9.6%, and 12.4% for placebo, 7.5/46, and 15/92, respectively (Figure 2).
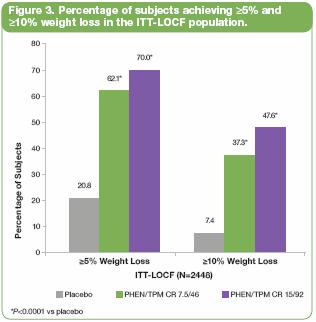
· Figure 3 displays the percentages of subjects achieving >5% weight loss and >10% weight loss in the ITT-LOCF population at week 56. Both doses of PHEN/TPM CR were statistically superior to placebo (P<0.0001 vs placebo).
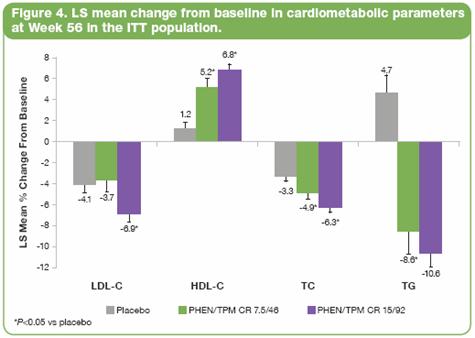
· After 56 weeks of therapy, both doses of PHEN/TPM CR resulted in improvements in atherosclerosis risk as reflected by favorable effects on lipids when compared to placebo. All doses of PHEN/TPM CR demonstrated significant improvement compared to baseline for LDL-C, HDL-C, TC, and TG (Figure 4). In addition, the TC/HDL ratio improved significantly for both treatment groups vs placebo (P<0.0001 vs placebo).
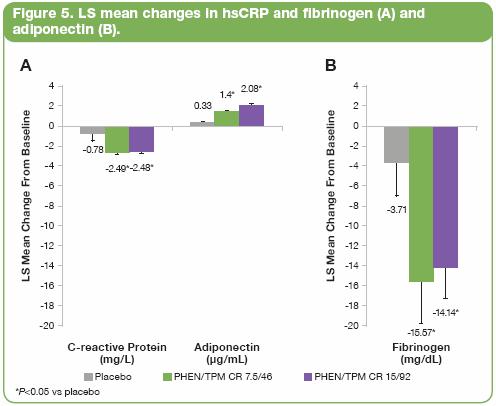
· Both PHEN/TPM CR doses were also associated with significant improvements in the inflammatory and coagulopathic markers hsCRP and fibrinogen, as well as in the adipose tissue marker adiponectin, in patients with metabolic comorbidities. The 7.5/46 dose reduced mean hsCRP levels from 7.0 mg/L at baseline to 4.2 mg/L, lowered fibrinogen from 460 mg/dL to 444 mg/dL, and increased adiponectin from 8.1 µg/mL to 9.6 µg/mL (all P<0.0005 vs baseline). The 15/92 dose led to comparable reductions in mean hsCRP (6.5 mg/L at baseline to 4.1 mg/L) and fibrinogen (454 mg/dL to 443 mg/dL), but resulted in a more pronounced change in adiponectin from 7.9 µg/mL to 10.1 µg/mL (all comparisons P<0.0001 vs baseline).
· Figure 5 shows the LS mean change in hsCRP, fibrinogen, and adiponectin in the ITT-LOCF population.
· PHEN/TPM CR was generally well tolerated; most adverse events were mild or moderate in severity. The most common treatment-emergent adverse events were respiratory tract infections, constipation, paresthesia, and dry mouth.
· Completion rates were higher in the PHEN/TPM CR groups, with discontinuation due to adverse events rates being dose related at 9%, 11.6%, and 19.3%, respectively for placebo, 7.5/46, and 15/92.
· Conclusions
· PHEN/TPM CR demonstrates significant efficacy vs placebo as a potential treatment for weight loss and was generally well tolerated, based on rates of study completion and discontinuations due to adverse events in the CONQUER trial.
· Treatment with PHEN/TPM CR improved risk factors for cardiometabolic disease, including biomarkers related to lipids, inflammation, coagulation, and adiponectin.
· These data indicate that weight loss associated with PHEN/TPM CR may reverse the cardiometabolic disease process and suggest that treatment could prevent the development or progression of cardiovascular disease and/or T2DM.
This trial is registered at ClinicalTrials.gov, number NCT00553787.
References: (1) Reaven GM. Importance of identifying the overweight patient who will benefit the most by losing weight. Ann Intern Med. 2003;138:420-423. 2. Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235-241. 3. Haslam DW, James WP. Obesity. Lancet. 2005;366:1197-1209. 4. Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763-778. 5. Rizvi AA. Hypertension, obesity, and inflammation: the complex designs of a deadly trio. Metab Syndr Relat Disord. 2010;20:1-8. 6. Van Gaal LF, Wauters MA, De Leeuw IH. The beneficial effects of modest weight loss on cardiovascular risk factors. Int J Obes Relat Metab Disord. 1997;21(suppl 1):S5-S9. 7. Kopp HP, Kopp CW, Festa A, et al. Impact of weight loss on inflammatory proteins and their association with the insulin resistance syndrome in morbidly obese patients. Arterioscler Thromb Vasc Biol. 2003;23:1042-1047. 8. Toplak H, Hamann A, Moore R, et al. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Int J Obesity. 2007;31:138-146. 9. Adipex-P [package insert]. Sellersville, PA: Teva Pharmaceuticals USA; 2005. 10. Topamax [package insert]. Titusville, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc.; 2009. 11. Bray GA, Hollander P, Klein S, et al. A 6-month randomized, placebo-controlled dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11:722-733. 12. Stenlof K, Rossner S, Vercrysse F, Kumar A, Fitchet M, Sjostrom L, for the OBDM-003 Study Group. Topiramate in the treatment of obese subjects with drug-naïve type 2 diabetes. Diabetes Obes Metab. 2006;9:360-368. 13. Tonstad S, Tykarski A, Weissgarten J, et al. Efficacy and safety of topiramate in the treatment of obese subjects with essential hypertension. Am J Cardiol. 2005;96:243-251. 14. Rosenstock J, Hollander P, Gadde KM, Sun X, Strauss R, Leung A, for the OBD-205 Study Group. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30:1480-1486.
Acknowledgements: We would like to acknowledge and thank the CONQUER investigators and study coordinators, the Medpace team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.
