Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a10-13137_18k.htm |
| EX-99.2 - EX-99.2 - VIVUS INC | a10-13137_1ex99d2.htm |
| EX-99.5 - EX-99.5 - VIVUS INC | a10-13137_1ex99d5.htm |
| EX-99.3 - EX-99.3 - VIVUS INC | a10-13137_1ex99d3.htm |
| EX-99.4 - EX-99.4 - VIVUS INC | a10-13137_1ex99d4.htm |
Exhibit 99.1
Below is a graphical representation of the poster entitled “Magnitude of Weight Loss Experienced With a Low-Dose, Controlled-Release Formulation of Phentermine/Topiramate May Drive Degree of Cardiometabolic Benefit”:
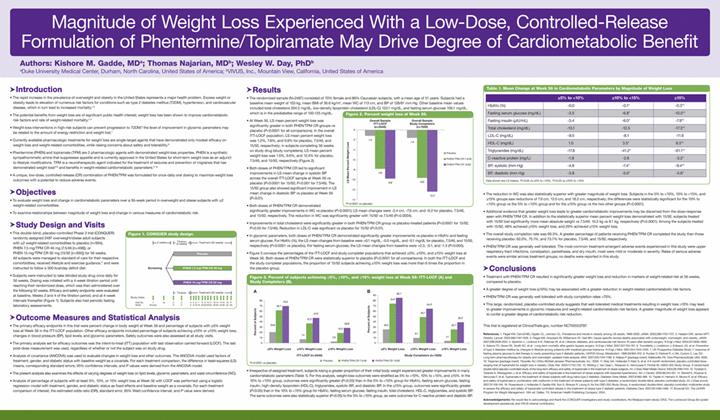
Below is a reproduction of the contents of the poster entitled “Magnitude of Weight Loss Experienced With a Low-Dose, Controlled-Release Formulation of Phentermine/Topiramate May Drive Degree of Cardiometabolic Benefit”:
Authors: Kishore M. Gadde, MD(a); Thomas Najarian, MD(b); Wesley W. Day, PhD(b)
(a)Duke University Medical Center, Durham, North Carolina, United States of America; (b)VIVUS, Inc., Mountain View, California, United States of America
· Introduction
· The rapid increase in the prevalence of overweight and obesity in the United States represents a major health problem. Excess weight or obesity leads to elevation of numerous risk factors for conditions such as type 2 diabetes mellitus (T2DM), hypertension, and cardiovascular disease, which in turn lead to increased mortality.(1)-(3)
· The potential benefits from weight loss are of significant public health interest; weight loss has been shown to improve cardiometabolic risk factors and rate of weight-related mortality.(4)-(5)
· Weight-loss interventions in high-risk subjects can prevent progression to T2DM;(6) the level of improvement in glycemic parameters may be related to the amount of energy restriction and weight lost.(7)
· Currently available pharmacologic treatments for weight loss are single-target agents that have demonstrated only modest efficacy on weight loss and weight-related comorbidities, while raising concerns about safety and tolerability.(8)
· Phentermine (PHEN) and topiramate (TPM) are 2 pharmacologic agents with demonstrated weight-loss properties. PHEN is a synthetic sympathomimetic amine that suppresses appetite and is currently approved in the United States for short-term weight loss as an adjunct to lifestyle modifications. TPM is a neurotherapeutic agent indicated for the treatment of seizures and prevention of migraines that has demonstrated weight loss(9),(10) and benefits in weight-related cardiometabolic parameters.(11)-(16)
· A unique, low-dose, controlled-release (CR) combination of PHEN/TPM was formulated for once-daily oral dosing to maximize weight loss outcomes with a potential to reduce adverse events.
· Objectives
· To evaluate weight loss and change in cardiometabolic parameters over a 56-week period in overweight and obese subjects with >2 weight-related comorbidities.
· To examine relationships between magnitude of weight loss and change in various measures of cardiometabolic risk.
· Study Design and Visits
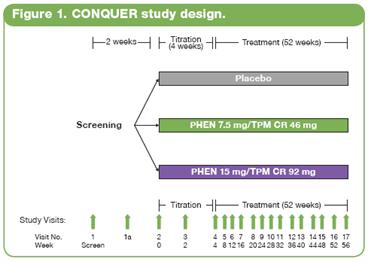
· This double-blind, placebo-controlled Phase 3 trial (CONQUER) randomly assigned 2487 overweight/obese adult subjects with >2 weight-related comorbidities to placebo (n=994), PHEN 7.5 mg/TPM CR 46 mg (7.5/46 [n=498]), or PHEN 15 mg/TPM CR 92 mg (15/92 [n=995]) for 56 weeks. All subjects were managed to standard of care for their respective comorbidities, received lifestyle and exercise guidance,(17) and were instructed to follow a 500 kcal/day deficit diet.
· Subjects were instructed to take blinded study drug once daily for 56 weeks. Dosing was initiated with a 4-week titration period until reaching their randomized dose, which was then administered over the following 52 weeks. Efficacy and safety endpoints were evaluated at baseline, Weeks 2 and 4 of the titration period, and at 4-week intervals thereafter (Figure 1). Subjects also had periodic fasting laboratory assessments.
· Outcome Measures and Statistical Analysis
· The primary efficacy endpoints in this trial were percent change in body weight at Week 56 and percentage of subjects with >5% weight loss at Week 56 in the ITT-LOCF population. Other efficacy endpoints included percentage of subjects achieving >10% or >15% weight loss; changes in blood pressure (BP), lipid levels, and glycemic parameters. Safety outcomes were also assessed at all study visits.
· The primary analysis set for efficacy outcomes was the intent-to-treat (ITT) population with last observation carried forward (LOCF). The last post-dose measurement was used, regardless of whether or not the subject was on study drug.
· Analysis of covariance (ANCOVA) was used to evaluate changes in weight loss and other outcomes. The ANCOVA model used factors of treatment, gender, and diabetic status with baseline weight as a covariate. For each treatment comparison, the difference in least-squares (LS) means, corresponding standard errors, 95% confidence intervals, and P values were derived from the ANCOVA model.
· The present analysis also examines the effects of varying degrees of weight loss on lipid levels, glycemic parameters, and waist circumference (WC).
· Analysis of percentage of subjects with at least 5%, 10%, or 15% weight loss at Week 56 with LOCF was performed using a logistic regression model with treatment, gender, and diabetic status as fixed effects and baseline weight as a covariate. For each treatment comparison of interest, the estimated odds ratio (OR), standard error, 95% Wald confidence interval, and P value were derived.
· Results
· The randomized sample (N=2487) consisted of 70% female and 86% Caucasian subjects, with a mean age of 51 years. Subjects had a baseline mean weight of 103 kg, mean BMI of 36.6 kg/m2, mean WC of 113 cm, and BP of 128/81 mm Hg. Other baseline mean values included total cholesterol 204.5 mg/dL, low-density lipoprotein cholesterol (LDL-C) 123.1 mg/dL, and fasting serum glucose 106.1 mg/dL, which is in the prediabetes range of 100-125 mg/dL.
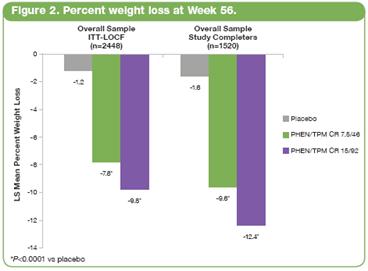
· At Week 56, LS mean percent weight loss was significantly greater in both PHEN/TPM CR groups vs placebo (P<0.0001 for all comparisons). In the overall ITT-LOCF population, LS mean percent weight loss was 1.2%, 7.8%, and 9.8% for placebo, 7.5/46, and 15/92, respectively; in subjects completing 56 weeks on study drug (study completers), LS mean percent weight loss was 1.6%, 9.6%, and 12.4% for placebo, 7.5/46, and 15/92, respectively (Figure 2).
· Both doses of PHEN/TPM CR led to significant improvements in LS mean change in systolic BP across the overall ITT-LOCF sample at Week 56 vs placebo (P<0.0001 for 15/92; P<0.001 for 7.5/46). The 15/92 group also showed significant improvement in LS mean change in diastolic BP vs placebo at Week 56 (P<0.01).
· Both doses of PHEN/TPM CR demonstrated significantly greater improvements in WC vs placebo (P<0.0001); LS mean changes were -2.4 cm, -7.6 cm, and -9.2 for placebo, 7.5/46, and 15/92, respectively. The reduction in WC was significantly greater with 15/92 vs 7.5/46 (P=0.0004).
· Improvements in total cholesterol were significantly greater in both PHEN/TPM CR group vs placebo-treated patients (P<0.0001 for 15/92, P<0.05 for 7.5/46). Reduction in LDL-C was significant vs placebo for 15/92 (P<0.01).
· In glycemic parameters, both doses of PHEN/TPM CR demonstrated significantly greater improvements vs placebo in HbA1c and fasting serum glucose. For HbA1c (%), the LS mean changes from baseline were +0.1 mg/dL, -0.0 mg/dL, and -0.1 mg/dL for placebo, 7.5/46, and 15/92, respectively (P<0.0001 vs placebo). For fasting serum glucose, the LS mean changes from baseline were +2.3, -0.1, and -1.3 (P<0.005).
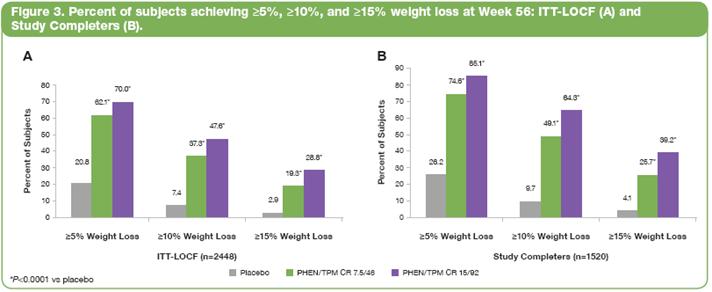
· Figure 3 conveys the percentages of the ITT-LOCF and study completer populations that achieved >5%, >10%, and >15% weight loss at Week 56. Both doses of PHEN/TPM CR were statistically superior to placebo (P<0.0001 for all comparisons). In both the ITT-LOCF and the study completer populations, the proportion of 15/92 subjects achieving >10% weight loss was more than 6 times the proportion in the placebo group.
· Irrespective of assigned treatment, subjects losing a greater proportion of their initial body weight experienced greater improvements in many cardiometabolic parameters (Table 1). For this analysis, weight-loss outcomes were stratified as 5% to <10%, 10% to <15%, and >15%. In the 10% to <15% group, outcomes were significantly greater (P<0.05) than in the 5% to <10% group for HbA1c, fasting serum glucose, fasting insulin, high-density lipoprotein (HDL-C), triglycerides, systolic BP, and diastolic BP. In the >15% group, outcomes were significantly greater (P<0.05) than in the 10% to <15% group for HbA1c, fasting serum glucose, fasting insulin, total cholesterol, HDL-C, triglycerides, and systolic BP. The same outcomes were also statistically superior (P<0.05) to the 5% to <10% group, as were outcomes for C-reactive protein and diastolic BP.
Table 1. Mean Change at Week 56 in Cardiometabolic Parameters by Magnitude of Weight Loss
|
|
|
>5% to <10% |
|
>10% to <15% |
|
>15% |
|
|
HbA1c (%) |
|
-0.0 |
|
-0.1* |
|
-0.3*† |
|
|
Fasting serum glucose (mg/dL) |
|
-3.5 |
|
-6.8* |
|
-10.0*† |
|
|
Fasting insulin (µIU/mL) |
|
-3.4 |
|
-6.0* |
|
-7.9*† |
|
|
Total cholesterol (mg/dL) |
|
-13.1 |
|
-12.5 |
|
-17.3*† |
|
|
LDL-C (mg/dL) |
|
-9.5 |
|
-8.1 |
|
-11.6 |
|
|
HDL-C (mg/dL) |
|
1.0 |
|
3.5* |
|
6.5*† |
|
|
Triglycerides (mg/dL) |
|
-17.8 |
|
-41.2* |
|
-61.1*† |
|
|
C-reactive protein (mg/L) |
|
-1.9 |
|
-2.6 |
|
-3.2* |
|
|
BP, systolic (mm Hg) |
|
-4.8 |
|
-7.4* |
|
-9.4*† |
|
|
BP, diastolic (mm Hg) |
|
-3.8 |
|
-5.0* |
|
-5.6* |
|
Data shown are LS means, *P<0.05 vs >5% to <10%, †P<0.05 vs >10% to <15%
· The reduction in WC was also statistically superior with greater magnitude of weight loss. Subjects in the 5% to <10%, 10% to <15%, and >15% groups saw reductions of 7.9 cm, 12.0 cm, and 18.2 cm, respectively; the differences were statistically significant for the 10% to <15% group vs the 5% to <10% group and for the >15% group vs the two other groups (P<0.0001).
· Additional evidence that greater weight loss leads to greater cardiometabolic improvements may be discerned from the dose-response seen with PHEN/TPM CR. In addition to the statistically superior mean percent weight loss demonstrated with 15/92, subjects treated with 15/92 lost significantly more mean absolute weight vs 7.5/46: 10.2 kg vs 8.1 kg, respectively (P<0.0001). Among the subjects treated with 15/92, 48% achieved >10% weight loss, and 29% achieved >15% weight loss.
· The overall study completion rate was 69.3%. A greater percentage of patients receiving PHEN/TPM CR completed the study than those receiving placebo: 62.0%, 75.1%, and 73.7% for placebo, 7.5/46, and 15/92, respectively.
· PHEN/TPM CR was generally well tolerated. The most common treatment-emergent adverse events experienced in this study were upper respiratory tract infections, constipation, paresthesia, and dry mouth; most were mild or moderate in severity. Rates of serious adverse events were similar across treatment groups; no deaths were reported in this study.
· Conclusions
· Treatment with PHEN/TPM CR resulted in significantly greater weight loss and reduction in markers of weight-related risk at 56 weeks, compared to placebo.
· A greater degree of weight loss (>10%) may be associated with a greater reduction in weight-related cardiometabolic risk factors.
· PHEN/TPM CR was generally well tolerated with study completion rates >70%.
· This large, randomized, placebo-controlled study suggests that well-tolerated medical treatments resulting in weight loss >10% may lead to greater improvements in glycemic measures and weight-related cardiometabolic risk factors. A greater magnitude of weight loss appears to confer a greater degree of cardiometabolic risk reduction.
This trial is registered at ClinicalTrials.gov, number NCT00553787.
References: (1) Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723-1727. (2) Haslam DW, James WPT. Obesity. Lancet. 2005;366:1197-1209. (3) Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028-2037. (4) Sjöström L, Lindroos A-K, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693. (5) Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753-761. (6) Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343-1350.(7) UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905-912. (8) Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194-1199. (9) Adipex-P [package insert]. Sellersville, PA: Teva Pharmaceuticals USA; 2005. (10) Topamax [package insert]. Titusville, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc.; 2009. (11) Bray GA, Hollander P, Klein S, et al. A 6-month randomized, placebo-controlled dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11:722-733. (12) Wilding J, Van Gaal L, Rissanen A, Vercruysse F, Fitchet M; OBES-002 Study Group. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28:1399-1410. (13) Tonstad S, Tykarski A, Weissgarten J, et al. Efficacy and safety of topiramate in the treatment of obese subjects with essential hypertension. Am J Cardiol. 2005;96:243-251. (14) Stenlof K, Rossner S, Vercrysse F, et al. Topiramate in the treatment of obese subjects with drug-naïve type 2 diabetes. Diabetes Obes Metab. 2007;9:360-368. (15) Toplak H, Hamann A, Moore R, et al. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond). 2007;31:138-146. (16) Rosenstock J, Hollander P, Gadde KM, Sun X, Strauss R, Leung A, for the OBD-205 Study Group. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30:1480-1486. (17) Brownell KD. The LEARN Program for Weight Management. 10th ed. Dallas, TX: American Health Publishing Company; 2004.
Acknowledgements: We would like to acknowledge and thank the CONQUER investigators and study coordinators, the Medpace team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.
