Attached files
| file | filename |
|---|---|
| 8-K - 8-K - VIVUS INC | a10-13137_18k.htm |
| EX-99.1 - EX-99.1 - VIVUS INC | a10-13137_1ex99d1.htm |
| EX-99.5 - EX-99.5 - VIVUS INC | a10-13137_1ex99d5.htm |
| EX-99.3 - EX-99.3 - VIVUS INC | a10-13137_1ex99d3.htm |
| EX-99.4 - EX-99.4 - VIVUS INC | a10-13137_1ex99d4.htm |
Exhibit 99.2
Below is a graphical representation of the poster entitled “Changes in Insulin Sensitivity and Insulin Secretion in Overweight/Obese Patients Treated With Low-Dose, Controlled-Release Phentermine/Topiramate”:
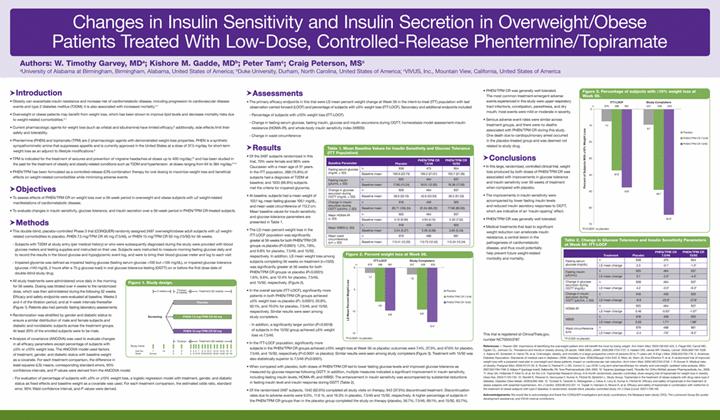
Below is a reproduction of the contents of the poster entitled “Changes in Insulin Sensitivity and Insulin Secretion in Overweight/Obese Patients Treated With Low-Dose, Controlled-Release Phentermine/Topiramate”:
Authors: W. Timothy Garvey, MD(a); Kishore M. Gadde, MD(b); Peter Tam(c); Craig Peterson, MS(c)
(a)University of Alabama at Birmingham, Birmingham, Alabama, United States of America; (b)Duke University, Durham, North Carolina, United States of America; (c)VIVUS, Inc., Mountain View, California, United States of America
· Introduction
· Obesity can exacerbate insulin resistance and increase risk of cardiometabolic disease, including progression to cardiovascular disease events and type 2 diabetes mellitus (T2DM); it is also associated with increased mortality.(1)-(4)
· Overweight or obese patients may benefit from weight loss, which has been shown to improve lipid levels and decrease mortality risks due to weight-related comorbidities.(5)-(7)
· Current pharmacologic agents for weight loss (such as orlistat and sibutramine) have limited efficacy;(8) additionally, side effects limit their safety and tolerability.
· Phentermine (PHEN) and topiramate (TPM) are 2 pharmacologic agents with demonstrated weight-loss properties. PHEN is a synthetic sympathomimetic amine that suppresses appetite and is currently approved in the United States at a dose of 37.5 mg/day for short-term weight loss as an adjunct to lifestyle modifications.(9)
· TPM is indicated for the treatment of seizures and prevention of migraine headaches at doses up to 400 mg/day;(10) and has been studied in the past for the treatment of obesity and obesity-related conditions such as T2DM and hypertension at doses ranging from 64 to 384 mg/day.(11)-(14)
· PHEN/TPM has been formulated as a controlled-release (CR) combination therapy for oral dosing to maximize weight loss and beneficial effects on weight-related comorbidities while minimizing adverse events.
· Objectives
· To assess effects of PHEN/TPM CR on weight loss over a 56-week period in overweight and obese subjects with >2 weight-related manifestations of cardiometabolic disease.
· To evaluate changes in insulin sensitivity, glucose tolerance, and insulin secretion over a 56-week period in PHEN/TPM CR-treated subjects.
· Methods
· This double-blind, placebo-controlled Phase 3 trial (CONQUER) randomly assigned 2487 overweight/obese adult subjects with >2 weight-related comorbidities to placebo, PHEN 7.5 mg/TPM CR 46 mg (7.5/46), or PHEN 15 mg/TPM CR 92 mg (15/92) for 56 weeks.
· Subjects with T2DM at study entry (per medical history) or who were subsequently diagnosed during the study were provided with blood glucose meters and testing supplies and instructed on their use. Subjects were instructed to measure morning fasting glucose daily and to record the results in the blood glucose and hypoglycemic event log, and were to bring their blood glucose meter and log to each visit
· Impaired glycemia was defined as impaired fasting glucose (fasting serum glucose >100 but <126 mg/dL), or impaired glucose tolerance (glucose >140 mg/dL 2 hours after a 75 g glucose load) in oral glucose tolerance testing (OGTT) on or before the first dose date of double-blind study drug
· All study treatments were administered once daily in the morning for 56 weeks. Dosing was titrated over 4 weeks to the randomized dose, which was then administered during the following 52 weeks. Efficacy and safety endpoints were evaluated at baseline, Weeks 2 and 4 of the titration period, and at 4-week intervals thereafter (Figure 1). Patients also had periodic fasting laboratory assessments.
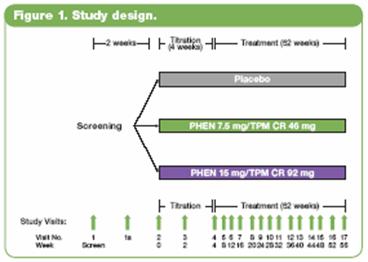
· Randomization was stratified by gender and diabetic status to ensure a similar distribution of male and female subjects and diabetic and nondiabetic subjects across the treatment groups. At least 20% of the enrolled subjects were to be male.
· Analysis of covariance (ANCOVA) was used to evaluate changes in all efficacy parameters except percentage of subjects with >5% or >10% weight loss. The ANCOVA model used factors of treatment, gender, and diabetic status with baseline weight as a covariate. For each treatment comparison, the difference in least-squares (LS) means, corresponding standard errors, 95% confidence intervals, and P values were derived from the ANCOVA model.
· For evaluation of percentage of subjects with >5% or >10% weight loss, a logistic regression model with treatment, gender, and diabetic status as fixed effects and baseline weight as a covariate was used. For each treatment comparison, the estimated odds ratio, standard error, 95% Wald confidence interval, and P values were derived.
· Assessments
· The primary efficacy endpoints in this trial were LS mean percent weight change at Week 56 in the intent-to-treat (ITT) population with last observation carried forward (LOCF) and percentage of subjects with >5% weight loss (ITT-LOCF). Secondary and additional endpoints included:
· Percentage of subjects with >10% weight loss (ITT-LOCF)
· Change in fasting serum glucose, fasting insulin, glucose and insulin excursions during OGTT, homeostasis model assessment–insulin resistance (HOMA-IR), and whole-body insulin sensitivity index (WBISI)
· Change in waist circumference
· Results
· Of the 2487 subjects randomized in this trial, 70% were female and 86% were Caucasian with a mean age of 51 years. In the ITT population, 388 (15.8%) of subjects had a diagnosis of T2DM at baseline; and 1635 (66.8%) subjects met the criteria for impaired glycemia.
· At baseline, subjects had a mean weight of 103.1 kg, mean fasting glucose 106.1 mg/dL, and mean waist circumference of 113.2 cm. Mean baseline values for insulin sensitivity and glucose tolerance parameters are presented in Table 1.
Table 1. Mean Baseline Values for Insulin Sensitivity and Glucose Tolerance (ITT Population)
|
Baseline Parameter |
|
|
|
Placebo |
|
PHEN/TPM CR |
|
PHEN/TPM CR |
|
|
Fasting serum glucose (mg/dL ± SD) |
|
n |
|
938 |
|
473 |
|
954 |
|
|
|
|
Baseline mean |
|
106.6 (23.73) |
|
106.2 (21.01) |
|
105.7 (21.36) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Fasting insulin (mU/mL ± SD) |
|
n |
|
925 |
|
464 |
|
937 |
|
|
|
|
Baseline mean |
|
17.80 (13.24) |
|
18.01 (12.92) |
|
18.38 (17.50) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in glucose excursion during OGTT (mg/dL ± SD) |
|
n |
|
928 |
|
464 |
|
937 |
|
|
|
Baseline -mean |
|
38.6 (52.10) |
|
42.6 (53.03) |
|
39.2 (51.33) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in insulin excursion during OGTT (mU/mL ± SD) |
|
n |
|
918 |
|
459 |
|
925 |
|
|
|
Baseline mean |
|
80.71 (106.24) |
|
81.04 (86.22) |
|
77.98 (80.00) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mean HOMA-IR (± SD) |
|
n |
|
925 |
|
464 |
|
937 |
|
|
|
|
Baseline mean |
|
5.15 (6.06) |
|
4.94 (4.12) |
|
5.30 (7.52) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Mean WBSI (± SD) |
|
n |
|
918 |
|
459 |
|
925 |
|
|
|
|
Baseline mean |
|
3.54 (3.27) |
|
3.36 (3.06) |
|
3.65 (3.43) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Mean waist circumference (cm ± SD) |
|
n |
|
979 |
|
488 |
|
981 |
|
|
|
|
Baseline mean |
|
113.41 (12.20) |
|
112.73 (12.42) |
|
113.24 (12.24) |
|
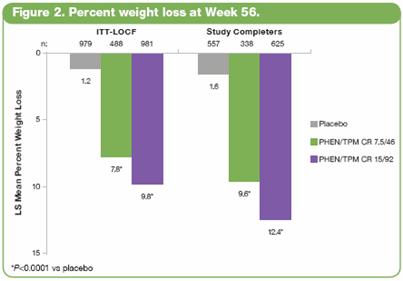
· The LS mean percent weight loss in the ITT-LOCF population was significantly greater at 56 weeks for both PHEN/TPM CR groups vs placebo (P<0.0001): 1.2%, 7.8%, and 9.8% for placebo, 7.5/46, and 15/92, respectively. In addition, LS mean weight loss among subjects completing 56 weeks on treatment (n=1520) was significantly greater at 56 weeks for both PHEN/TPM CR groups vs placebo (P<0.0001): 1.6%, 9.6%, and 12.4% for placebo, 7.5/46, and 15/92, respectively. (Figure 2).
· In the overall sample (ITT-LOCF), significantly more patients in both PHEN/TPM CR groups achieved >5% weight loss vs placebo (P< 0.0001): 20.8%, 62.1%, and 70.0% for placebo, 7.5/46, and 15/92, respectively. Similar results were seen among study completers.
· In addition, a significantly larger portion (P=0.0018) of subjects in the 15/92 group achieved >5% weight loss vs 7.5/46.
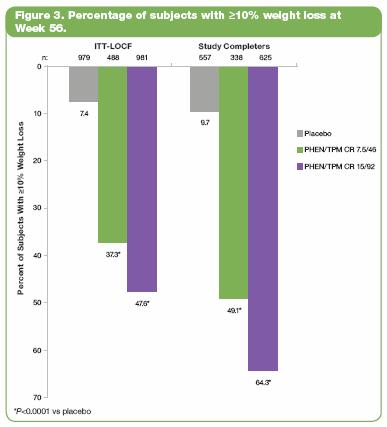
· In the ITT-LOCF population, significantly more subjects in the PHEN/TPM CR groups achieved >10% weight loss at Week 56 vs placebo; outcomes were 7.4%, 37.3%, and 47.6% for placebo, 7.5/46, and 15/92, respectively (P<0.0001 vs placebo). Similar results were seen among study completers (Figure 3). Treatment with 15/92 was also statistically superior to 7.5/46 (P<0.0001).
· When compared with placebo, both doses of PHEN/TPM CR led to lower fasting glucose levels and improved glucose tolerance as measured by glucose response following OGTT. In addition, multiple measures indicated a significant improvement in insulin sensitivity, including fasting insulin levels, HOMA-IR, and WBISI. The enhancement in insulin sensitivity was accompanied by substantial reductions in fasting insulin level and insulin response during OGTT (Table 2).
Table 2. Change in Glucose Tolerance and Insulin Sensitivity Parameters at Week 56: ITT-LOCF
|
|
|
Treatment |
|
Placebo |
|
PHEN/TPM
CR |
|
PHEN/TPM
CR |
|
|
Fasting serum glucose (mg/dL) |
|
n |
|
938 |
|
473 |
|
954 |
|
|
|
|
LS mean change |
|
2.3 |
|
-0.1* |
|
-1.3* |
|
|
|
|
|
|
|
|
|
|
|
|
|
Fasting insulin (µIU/mL) |
|
n |
|
925 |
|
464 |
|
937 |
|
|
|
|
LS mean change |
|
0.7 |
|
-3.5* |
|
-4.0* |
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in glucose excursion during OGTT (mg/dL) |
|
n |
|
928 |
|
464 |
|
937 |
|
|
|
|
LS mean change |
|
4.2 |
|
-3.5* |
|
-9.3* |
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in insulin excursion during OGTT (µIU/mL ± SD) |
|
n |
|
918 |
|
459 |
|
925 |
|
|
|
LS mean change |
|
-8.9 |
|
-22.6* |
|
-27.8* |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HOMA-IR |
|
n |
|
925 |
|
464 |
|
937 |
|
|
|
|
LS mean change |
|
0.46 |
|
-0.93* |
|
-1.07* |
|
|
|
|
|
|
|
|
|
|
|
|
|
WBISI |
|
n |
|
918 |
|
459 |
|
925 |
|
|
|
|
LS mean change |
|
0.50 |
|
1.71* |
|
1.98* |
|
|
|
|
|
|
|
|
|
|
|
|
|
Waist circumference (cm) |
|
n |
|
979 |
|
488 |
|
981 |
|
|
|
|
LS mean change |
|
-2.4 |
|
-7.6* |
|
-9.2* |
|
*P<0.005 vs placebo
· Of the randomized 2487 subjects, 1542 (62.0%) completed all study visits on therapy; 943 (37.9%) discontinued treatment. Discontinuation rates due to adverse events were 9.0%, 11.6 %, and 19.3% in placebo, 7.5/46 and 15/92, respectively. A higher percentage of subjects in the PHEN/TPM CR groups than in the placebo group completed the study on therapy (placebo, 56.7%; 7.5/46, 69.1%; and 15/92, 63.7%).
· PHEN/TPM CR was generally well tolerated. The most common treatment-emergent adverse events experienced in this study were upper respiratory tract infections, constipation, paresthesia, and dry mouth; most events were mild or moderate in severity.
· Serious adverse event rates were similar across treatment groups, and there were no deaths associated with PHEN/TPM CR during this study. One death due to cardiopulmonary arrest occurred in the placebo-treated group and was deemed not related to study drug.
· Conclusions
· In this large, randomized, controlled clinical trial, weight loss produced by both doses of PHEN/TPM CR was associated with improvements in glucose tolerance and insulin sensitivity over 56 weeks of treatment when compared with placebo.
· The improvements in insulin sensitivity were accompanied by lower fasting insulin levels and reduced insulin secretory responses to OGTT, which are indicative of an ‘insulin-sparing’ effect.
· PHEN/TPM CR was generally well tolerated.
· Medical treatments that lead to significant weight reduction can ameliorate insulin resistance, a central lesion in the pathogenesis of cardiometabolic disease, and thus could potentially help prevent future weight-related morbidity and mortality.
This trial is registered at ClinicalTrials.gov, number NCT00553787.
References: (1) Reaven GM. Importance of identifying the overweight patient who will benefit the most by losing weight. Ann Intern Med. 2003;138:420-423. (2) Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723-1727. (3) Haslam DW, James WP. Obesity. Lancet. 2005;366:1197-1209. (4) Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763-778. (5) American Diabetes Association. Standards of medical care in diabetes—2006. Diabetes Care. 2006;29(suppl 1):S4-S42. (6) Metz JA, Stern JS, Kris-Etherton P, et al. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients: impact on cardiovascular risk reduction. Arch Intern Med. 2000;160:2150-2158. (7) Pi-Sunyer X. Medical risks of obesity. Postgrad Med. 2009;121:21-33. (8) Rucker D, Padwal R, Li SK, Curioni C, Lau DCW. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194-1199. (9) Adipex-P [package insert]. Sellersville, PA: Teva Pharmaceuticals USA; 2005. (10) Topamax [package insert]. Titusville, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc.; 2009. (11) Bray GA, Hollander P, Klein S, et al. for the U.S. Topiramate Research Group. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11:722-733. (12) Stenlöf K, Rössner S, Vercruysse F, Kumar A, Fitchet M, Sjöström L, Study Group. Topiramate in the treatment of obese subjects with drug-naive type 2 diabetes. Diabetes Obes Metab. 2006;9;360-368. (13) Tonstad S, Tykarski A, Weissgarten J, Ivleva A, Levy B, Kumar A, Fitchet M. Efficacy and safety of topiramate in the treatment of obese subjects with essential hypertension. Am J Cardiol. 2005;96:243-251. (14) Toplak H, Hamann A, Moore R, et al. Efficacy and safety of topiramate in combination with metformin in the treatment of obese subjects with type 2 diabetes: A randomized, double-blind, placebo-controlled study. Int J Obes (Lond). 2007;1:138-146.
Acknowledgements: We would like to acknowledge and thank the CONQUER investigators and study coordinators, the Medpace team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.
