Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - Vitamin Shoppe, Inc. | vsi-ex322x123116.htm |
| EX-32.1 - EXHIBIT 32.1 - Vitamin Shoppe, Inc. | vsi-ex321x123116.htm |
| EX-31.2 - EXHIBIT 31.2 - Vitamin Shoppe, Inc. | vsi-ex312x123116.htm |
| EX-31.1 - EXHIBIT 31.1 - Vitamin Shoppe, Inc. | vsi-ex311x123116.htm |
| EX-23.1 - EXHIBIT 23.1 - Vitamin Shoppe, Inc. | vsi-ex231x123116.htm |
| EX-21.1 - EXHIBIT 21.1 - Vitamin Shoppe, Inc. | vsi-ex211x123116.htm |
| EX-10.65 - EXHIBIT 10.65 - Vitamin Shoppe, Inc. | vsi-ex1065x123116.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
or
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
for the transition period from to .
Commission file number: 001-34507
VITAMIN SHOPPE, INC.
(Exact name of registrant as specified in its charter)
Delaware | 11-3664322 | |
(State or Other Jurisdiction of Incorporation or Organization) | (IRS Employer Identification No.) | |
300 Harmon Meadow Blvd.
Secaucus, New Jersey 07094
(Addresses of Principal Executive Offices, including Zip Code)
(201) 868-5959
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Class | Name of the exchange on which registered | |
Common Stock, $0.01 par value per share | New York Stock Exchange | |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ¨ Yes x No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ¨ Yes x No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. x Yes ¨ No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). x Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer | x | Accelerated filer | ¨ | ||
Non-accelerated filer | ¨ | (Do not check if a smaller reporting company) | Smaller reporting company | ¨ | |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ¨ Yes x No
The aggregate market value of the registrant’s voting and non-voting common stock held by non-affiliates of the registrant was approximately $717,583,499 as of June 25, 2016, the last business day of the registrant’s most recently completed second fiscal quarter, based on the closing price of the common stock on the New York Stock Exchange.
As of January 28, 2017, Vitamin Shoppe, Inc. had 23,424,956 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The information required by Part III of this report, to the extent not set forth herein, is incorporated by reference from the Registrant’s definitive Proxy Statement to be filed for the 2017 Annual Meeting of the Stockholders.
TABLE OF CONTENTS
Page | ||
Item 1. | ||
Item 1A. | ||
Item 1B. | ||
Item 2. | ||
Item 3. | ||
Item 4. | ||
Item 5. | ||
Item 6. | ||
Item 7. | ||
Item 7A. | ||
Item 8. | ||
Item 9. | ||
Item 9A. | ||
Item 9B. | ||
Item 10. | ||
Item 11. | ||
Item 12. | ||
Item 13. | ||
Item 14. | ||
Item 15. | ||
2
EX 10.65 |
EX 21.1 |
EX 23.1 |
EX 31.1 |
EX 31.2 |
EX 32.1 |
EX 32.2 |
EX-101 INSTANCE DOCUMENT |
EX-101 SCHEMA DOCUMENT |
EX-101 CALCULATION LINKBASE DOCUMENT |
EX-101 DEFINITION LINKBASE DOCUMENT |
EX-101 LABELS LINKBASE DOCUMENT |
EX-101 PRESENTATION LINKBASE DOCUMENT |
3
Forward-Looking Statements
This Annual Report on Form 10-K contains “forward-looking” statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, as amended, including, without limitation, statements regarding future financial results and performance, future business prospects, implementation of omni-channel retailing, revenue, stores, our ability to implement strategic initiatives, realize improved financial results and meet market expectations, our ability to identify efficiencies and cost reduction opportunities and realize the benefits of potential savings, share and debt repurchases, product offerings, contract manufacturing, supply chain network utilization, intellectual property, integration of acquisitions, store transformation costs, future inventory charges, potential charges related to Nutri-Force, estimated costs to open a new distribution center, working capital, liquidity, capital expenditures, interest costs, industry based factors, including the level of competition in the vitamin, mineral and supplement industry, continued demand from the primary markets Vitamin Shoppe, Inc. (the “Company” or “we”) serves, consumer perception of our products, the availability of raw materials, as well as economic conditions generally and factors more specific to the Company such as compliance with manufacturing, healthcare, environmental and other regulations, changes in accounting standards, certifications and practices and restrictions imposed by the Company’s Convertible Notes and our Revolving Credit Facility (as defined below), including financial covenants and limitations on the Company’s ability to incur additional indebtedness and the Company’s future capital requirements, and other risks, uncertainties and factors set forth under Item 1A., entitled “Risk Factors” in this Annual Report on Form 10-K. You can identify these forward-looking statements by the use of words such as “outlook”, “believes”, “expects”, “potential”, “continues”, “may”, “will”, “should”, “seeks”, “predicts”, “intends”, “plans”, “estimates”, “anticipates”, “target”, “could” or the negative version of these words or other comparable words. These statements are subject to various risks and uncertainties, many of which are outside our control, including, among others, product liability claims and recalls, the availability of insurance, the strength of the economy, changes in the overall level of consumer spending, the performance of the Company’s products within the prevailing retail environment, trade restrictions, changes in tax policy, regulatory restrictions, political environment, international operations, availability of suitable store locations at appropriate terms, new credit card technology, e-commerce relationships, disruptions of manufacturing, warehouse or distribution facilities or information systems, and other specific factors discussed herein and in other Securities and Exchange Commission (the “SEC”) filings by us (including our reports on Forms 10-K and 10-Q filed with the SEC).
We believe that all forward-looking statements are based on reasonable assumptions when made; however, we caution that it is impossible to predict actual results or outcomes or the effects of risks, uncertainties or other factors on anticipated results or outcomes with certainty and that, accordingly, one should not place undue reliance on these statements. Forward-looking statements speak only as of the date when made and we undertake no obligation to update these statements in light of subsequent events or developments. Actual results may differ materially from anticipated results or outcomes discussed in any forward-looking statement.
Electronic Access to Company Reports
Our investor website can be accessed at www.vitaminshoppe.com under “Investor Relations”. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed with or furnished to the SEC pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934, as amended, are available free of charge on our investor website under the caption “SEC Filings” promptly after we electronically file those materials with, or furnish those materials to, the SEC. No information contained on any of our websites is intended to be included as part of, or incorporated by reference into, this Annual Report on Form 10-K. Information relating to corporate governance at our Company, including our Corporate Governance Guidelines, our Standards of Business Conduct for all directors, officers, and employees, and information concerning our directors, Committees of the Board, including Committee charters, and transactions in Company securities by directors and executive officers, is available at our investor website under the captions “Corporate Governance” and “SEC Filings”. Paper copies of these filings and corporate governance documents are available to stockholders free of charge by written request to Investor Relations, Vitamin Shoppe, Inc., 300 Harmon Meadow Blvd., Secaucus, New Jersey 07094. Documents filed with the SEC are also available on the SEC’s website at www.sec.gov.
4
PART I
Unless the context requires otherwise, references in this Annual Report on Form 10-K to “VSI”, the “Company”, “we”, “us” and “our” collectively refer to Vitamin Shoppe, Inc., its wholly owned subsidiary, Vitamin Shoppe Industries Inc. (“VS Industries”) and the wholly owned subsidiaries of VS Industries. References to “Fiscal” or “Fiscal Year” mean the fifty-three weeks ended December 31, 2016 and the fifty-two weeks ended December 26, 2015 and December 27, 2014 for Fiscal Year 2016, Fiscal Year 2015 and Fiscal Year 2014, respectively, and references to “Fiscal” and “Fiscal Year” for other years are similarly based on a fifty-two week or fifty-three week fiscal year, as applicable.
Item 1. Business
Overview of our Company
We are a multi-channel specialty retailer and contract manufacturer of vitamins, minerals, herbs, specialty supplements, sports nutrition and other health and wellness products. We market approximately 900 nationally recognized brands as well as our own brands, which include The Vitamin Shoppe®, BodyTech®, True Athlete®, Mytrition®, plnt®, ProBioCare®, Next Step® and Betancourt Nutrition®. We believe we offer one of the largest varieties of products among vitamin, mineral and supplement (“VMS”) retailers and continue to refine our assortment with approximately 7,000 stock keeping units (“SKUs”) offered in our typical store and approximately 10,000 additional SKUs available through e-commerce. Our broad product offering enables us to provide our customers with a depth of selection of products that may not be readily available at other specialty retailers or mass merchants, such as discount stores, supermarkets, drugstores and wholesale clubs. We believe our product offering and emphasis on product knowledge and customer service helps us meet the needs of our target customer and serves as a foundation for enhancing customer loyalty.
We sell our products through three operating segments: retail, direct and manufacturing. In our retail segment, which includes Vitamin Shoppe and Super Supplements retail store formats, we have leveraged our store economic model by opening a total of 137 stores from the beginning of Fiscal Year 2014 through Fiscal Year 2016. As of December 31, 2016, we operated 775 stores located in 45 states, the District of Columbia and Puerto Rico, primarily located in retail centers and stand alone locations. In our direct segment, we sell our products directly to consumers through the internet, primarily at www.vitaminshoppe.com. Our e-commerce sites complement our in-store experience by extending our retail product offerings and enable us to access customers outside our retail markets and those who prefer to shop online. Our manufacturing segment provides custom manufacturing and private labeling of VMS products, and develops and markets our own branded products for both sales to third parties and for the VSI product assortment.
During Fiscal 2015, the Company began development of a strategic plan focused on upgrading our customers’ experience across our retail and e-commerce channels, the “reinvention strategy”. The Company worked with outside consultants to analyze qualitative and quantitative information relevant to our customers’ experience. The reinvention strategy is focused on upgrading the customer experience to inspire our target customers with changes to curate our product assortment, opportunities to increase private brands penetration, enhancements to the in-store and digital experience, store layout, as well as changes to improve the effectiveness of our loyalty program. The Company incurred approximately $9.0 million of selling, general and administrative costs during Fiscal 2016 in connection with the reinvention, of which approximately $6.0 million of such costs are expected to be on-going. These costs include additional internal resources, improvements to store network connectivity, and outside consultants. The Company is in the process of testing several initiatives and expects to realize improved financial results from the reinvention beginning in Fiscal 2017. In addition, we have engaged a consulting firm in Fiscal 2016 to identify other efficiencies and cost reduction opportunities focusing on product sourcing, store operations, pricing and promotions, and corporate expenses. During Fiscal 2016, we incurred $3.8 million of costs in connection with identifying other efficiencies and cost reduction opportunities. As a result of this cost reduction project, we have identified savings potential with an estimated value of at least $24.0 million on an annualized basis.
In Fiscal 2015, we also performed a review of certain business operations. As part of this review, the Company implemented changes to more closely align Super Supplements stores with current processes and assortments in the Vitamin Shoppe retail stores. As a result, net costs of $1.8 million and $1.0 million were incurred during Fiscal 2015 and Fiscal 2016, respectively. Annual cost savings resulting from these actions are estimated to be $1 million to $2 million. In addition, the Company decided to cease operations in Canada, and as a result net costs of $0.9 million and $1.9 million were incurred in Fiscal 2015 and Fiscal 2016, respectively. The annual cost savings related to ceasing operations in Canada are estimated to be approximately $1.0 million. Costs for these two initiatives include lease liabilities, markdown charges on inventory and employee severance.
5
Segment Information
We operate through three business segments: retail, which includes Vitamin Shoppe and Super Supplements retail store formats, direct, which consists of our e-commerce formats, and manufacturing, which consists of the Nutri-Force manufacturing operations. For additional information, refer to Note 15, “Segment and Product Data” to our consolidated financial statements included in this Annual Report on Form 10-K.
Retail. Through our retail store formats, we believe we differentiate ourselves in the VMS industry. What makes us unique is our broad selection of VMS products and our stores are staffed with trained and knowledgeable employees, who we refer to as Health Enthusiasts®, and who are able to inform our customers about product features and assist in product selection.
Since the beginning of Fiscal 2014 through Fiscal 2016, we have opened 137 stores, expanding our presence in our existing markets as well as entering new markets. In Fiscal 2017, the rate of new store growth and remodeling of existing stores is being further evaluated as part of the reinvention. In addition, our new stores since the beginning of Fiscal 2013 are approximately 2,900 square feet compared to the average of our total store portfolio of approximately 3,500 square feet.
Direct. We sell our products directly to consumers through the internet, primarily at www.vitaminshoppe.com. Our e-commerce sites complement our in-store experience by extending our retail product offerings with approximately 10,000 additional SKUs that are not available in our stores and enable us to access customers outside our retail markets and those who prefer to shop online.
Manufacturing. Through Nutri-Force, we provide custom manufacturing and private labeling of VMS products and develop and market our own branded products for both sales to third parties and for the VSI product assortment.
In conjunction with the Company’s reinvention, we are increasing our focus on customer centric initiatives toward becoming an omni-channel based retailer. As recently launched initiatives, including buy online pickup in store and subscription sales, continue to develop, the interrelationship among the ways customers can purchase products from VSI results in sales that are generated and fulfilled across multiple channels. We believe the historical structure of separate segments for retail stores and e-commerce will no longer be representative of our operating performance. As a result, beginning in Fiscal 2017, the Company will be updating its segment reporting to better align with its omni-channel strategy. These changes include combining the current retail and direct operating segments into one retail operating segment. In addition, certain costs currently classified as corporate costs, such as retail and direct management costs, will be allocated to the retail operating segment. VSI has revised its internal management structure to align with our omni-channel strategy.
Industry
The VMS industry is large, estimated to be approximately $41 billion in 2016 according to the Nutrition Business Journal (“NBJ”), we believe is fragmented, and continued growth is expected as health and wellness trends continue. According to the NBJ, the VMS industry is expected to register a CAGR of 6.2% from 2016 to 2020. Sports supplements and meal replacement categories are projected to post the highest CAGRs at 8.3% and 7.3%, respectively.
Increased focus on healthy diet and nutrition, along with growing fitness and wellness program participation, serves as a positive trend for the nutritional supplements industry. Retailers of VMS products primarily include specialty retailers and mass merchants, such as discount stores, supermarkets, drugstores and wholesale clubs. The specialty retailers typically cater to the more sophisticated VMS customer by focusing on selection and customer service, while the mass merchants generally offer a limited assortment comprised of more mainstream products with less customer service. NBJ anticipates that the specialty retail channel will grow at an average rate of 6.5% through 2020. Additionally, NBJ forecasts the internet channel to grow at an average rate of 10.2% from 2016 to 2020.
Although long-term prospects noted above suggest continued growth, recent trends have created volatility in the near term and we expect continued volatility. Recent industry trends have been mixed, driven in part by the prospects of more federal and state involvement in the industry. A lack of clarity on regulation appears to be dissuading manufacturers from investing in and developing new ingredients/products. Additionally, negative publicity about the nutritional supplement industry has increased over the past few years and adds further uncertainty to the fundamental outlook. With product innovation remaining slower than in past years, and negative headlines/media at heightened levels, VMS industry headwinds appear poised to persist over the near term.
6
Over recent years, there has been a shift of market share from specialty retailers to other channels such as mass market retailers, club chains, drugstore chains and e-commerce companies. This broader competitive channel availability of VMS products represents a challenge for VSI to keep pace with industry growth rates.
Industry and market data contained or incorporated by reference in this Form 10-K were obtained through company research, surveys and studies conducted by third parties and industry and general publications or based on our experience in the industry. We have not independently verified market and industry data from third-party sources.
Competitive Strengths
We believe there is an opportunity to capitalize on the VMS industry dynamics, and we plan to further develop the following competitive strengths as part of the foundation of our reinvention strategy:
Value-Added Customer Service. We believe we offer a high degree of customer service. We place a strong emphasis on employee training and customer service, and view our Health Enthusiasts as a source for health and wellness information while assisting our customers with their product selections.
Product Selection, Including a Strong Assortment of Private Brands. We believe we have a broad merchandise assortment. We complement our assortment with our private brands merchandise which accounted for approximately 21% of our net sales in Fiscal 2016.
Attractive Customer Base. We have a large base of customers who proactively manage their health and wellness through the use of vitamins and supplements. In Fiscal 2016, 86% of our net sales (excluding Nutri-Force net sales) were attributable to our Healthy Awards customers. Our no-fee Healthy Awards Program promotes brand loyalty among our customers and allows our customers to earn points redeemable for future purchases, approximately 66% of which were redeemed in Fiscal 2016. We also utilize our Healthy Awards Program database to track customer purchasing patterns across our retail and direct business segments, analyze market and industry trends and create targeted merchandising and marketing strategies. In Fiscal 2016, we announced enhancements to this program, including the issuance of certificates on a quarterly basis.
Highly Refined Real Estate Strategy. We apply demanding criteria to our retail site selection. We locate our stores primarily in attractive stand-alone locations or endcap (corner) positions in retail centers. We believe that the location and visibility of our real estate is an important component of our customer acquisition strategies.
Multi-Channel Retailer. We are a multi-channel retailer, distributing products through our retail stores and our e-commerce sites, enabling us to access customers outside our retail markets and those who prefer to shop online. This business model affords us multiple touch points of interaction with our customers, which allows us to gather data and communicate with them in person, through our call center and via the internet.
Experienced Management Team with Proven Track Record. We have assembled a management team across a broad range of disciplines with extensive experience in building leading national specialty retailers.
Business Strategy
We intend to pursue the following key strategies in order to execute our reinvention strategy:
• | Upgrading our Customers’ Shopping Experience – We are focusing on enhancing both our in-store and digital experience. This will include improvements to our store layout, the remodeling of stores, changes to our product assortment, increasing the penetration of private brands and continuing to enhance the features and functionality of our e-commerce sites and providing our customers with a more personalized shopping experience; |
• | Building Relationships – To enhance relationships with our customers including improving the utilization of our Health Enthusiasts, customer relationship management program, website and mobile functionality, in order to personalize our relationship and gain a greater share of their discretionary spending; |
• | Store and Comparable Sales Growth – To increase sales and profitability of our existing store base as well as continue opening new stores in the future. We are further evaluating changes to our store format in order to enhance our customers’ shopping experience; |
• | Cost Reduction Initiatives – To improve operating performance through initiatives focused on reducing costs in areas such as product sourcing, promotions and various other reductions to selling, general and administrative expenses; |
• | Private Brands – We are focused on the development of new private brands and the extension of existing private brands to establish a point of differentiation for our customers; and |
• | Vertical integration – The acquisition of the manufacturing operations of Nutri-Force allows us to better control the production and timing of new product introductions and should enhance profitability. We intend to focus on increasing |
7
the portion of the VSI private brands assortment manufactured by Nutri-Force in order to leverage capacity, and implement a series of initiatives designed to improve the financial performance of Nutri-Force.
Store Counts and Locations
We plan to open approximately 15 new stores in Fiscal 2017 and the rate of new store growth and remodeling of existing stores is being further evaluated as part of the reinvention strategy. The following table shows the change in our network of stores for the Fiscal Years 2012 through 2016:
Fiscal Year | ||||||||||||||
2016 | 2015 | 2014 | 2013 | 2012 | ||||||||||
Store Data: | ||||||||||||||
Stores open at beginning of year | 758 | 717 | 659 | 579 | 528 | |||||||||
Stores opened | 26 | 50 | 61 | 52 | 54 | |||||||||
Stores acquired | — | — | — | 31 | — | |||||||||
Stores closed | (9 | ) | (9 | ) | (3 | ) | (3 | ) | (3 | ) | ||||
Stores open at end of year | 775 | 758 | 717 | 659 | 579 | |||||||||
8
New stores have typically required approximately four to five years to mature, generating lower store level sales in the initial years than our mature stores. As a result, new stores generally have a negative impact on our overall operating margin. In addition, our new stores since the beginning of Fiscal 2013 are approximately 2,900 square feet compared to the average of our total store portfolio of approximately 3,500 square feet. As these stores mature, we expect them to contribute positively to our operating results. The following table reflects our store count by state, as well as the District of Columbia and Puerto Rico at December 31, 2016:
Stores Open at December 31, 2016 | Stores Open at December 31, 2016 | |||||
Alabama | 5 | Nebraska | 2 | |||
Arizona | 12 | Nevada | 8 | |||
Arkansas | 2 | New Hampshire | 6 | |||
California | 90 | New Jersey | 33 | |||
Colorado | 8 | New Mexico | 3 | |||
Connecticut | 11 | New York | 73 | |||
Delaware | 3 | North Carolina | 26 | |||
District of Columbia | 1 | Ohio | 25 | |||
Florida | 77 | Oklahoma | 3 | |||
Georgia | 24 | Oregon | 9 | |||
Hawaii | 7 | Pennsylvania | 30 | |||
Idaho | 2 | Rhode Island | 2 | |||
Illinois | 41 | South Carolina | 16 | |||
Indiana | 13 | South Dakota | 1 | |||
Iowa | 3 | Tennessee | 13 | |||
Kansas | 3 | Texas | 54 | |||
Kentucky | 5 | Utah | 3 | |||
Louisiana | 8 | Vermont | 1 | |||
Maine | 2 | Virginia | 26 | |||
Maryland | 22 | Washington | 34 | |||
Massachusetts | 21 | Wisconsin | 6 | |||
Michigan | 19 | |||||
Minnesota | 10 | |||||
Missouri | 8 | |||||
Mississippi | 1 | Puerto Rico | 3 | |||
Total | 775 | |||||
As of December 31, 2016, we leased the property for all of our 775 stores. Our typical lease terms are ten years, with one or two five-year renewal options. We do not believe that any individual store property is material to our financial condition or results of operations. Of the leases for our stores, 30 expire in Fiscal 2017, 95 expire in Fiscal 2018, 107 expire in Fiscal 2019, 95 expire in Fiscal 2020, 94 expire in Fiscal 2021 and the balance expire in Fiscal 2022 or thereafter. For the majority of our leases, renewal options remain available.
Products
We organize our products by category enabling comparisons between different brands within each product sub-category. In addition, our stores are staffed with experienced and knowledgeable Health Enthusiasts, many of whom are regular and informed VMS consumers. Our Health Enthusiasts are equipped with tablets to inform our customers about product features and assist our customers in product selection. To further inform our customers, our stores are equipped with Aisle 7®, an independent source of health and wellness information.
We offer a comprehensive selection of vitamins, minerals, herbs, homeopathic remedies, specialty supplements such as fish oil, probiotics, glucosamine and Co Q10, sports nutrition, weight management, as well as natural bath and beauty products. Our offering includes approximately 17,000 SKUs from approximately 900 brands, including our own brands such as The Vitamin Shoppe®, BodyTech®, True Athlete®, Mytrition®, plnt®, ProBioCare®, Next Step® and Betancourt Nutrition®
9
brands which include products such as Ultimate Man, Ultimate Woman, Ultimate 10 Probiotic, Whey Tech and Whey Tech Pro 24 Proteins. We also offer a comprehensive assortment from leading national brands such as Optimum Nutrition®, Cellucor®, Garden of Life®, Quest Nutrition®, Solaray®, Solgar® and Nature’s Way®. This extensive assortment is designed to provide our customers with a unique selection of available products to help them achieve their health and wellness goals. Sales of our branded products accounted for approximately 21% of our net sales in Fiscal 2016.
Key Product Categories
Below is a comparison of our net merchandise sales by major product category and the respective percentage of our total net merchandise sales for the periods shown (dollars in thousands).
Fiscal 2016 (a) | Fiscal 2015 (b) | Fiscal 2014 (b) | ||||||||||||||||||
Product Category | Dollars | % | Dollars | % | Dollars | % | ||||||||||||||
Vitamins, Minerals, Herbs and Homeopathy | $ | 339,597 | 26.4 | % | $ | 320,872 | 25.4 | % | $ | 311,863 | 25.8 | % | ||||||||
Sports Nutrition | 408,288 | 31.7 | % | 421,293 | 33.3 | % | 419,804 | 34.7 | % | |||||||||||
Specialty Supplements | 308,945 | 24.0 | % | 289,938 | 22.9 | % | 288,045 | 23.8 | % | |||||||||||
Other | 230,252 | 17.9 | % | 232,399 | 18.4 | % | 190,285 | 15.7 | % | |||||||||||
Total | 1,287,082 | 100.0 | % | 1,264,502 | 100.0 | % | 1,209,997 | 100.0 | % | |||||||||||
Delivery Revenue | 2,161 | 2,047 | 3,049 | |||||||||||||||||
$ | 1,289,243 | $ | 1,266,549 | $ | 1,213,046 | |||||||||||||||
(a) | Fiscal 2016 includes a 53rd week. |
(b) | Fiscal 2015 and Fiscal 2014 figures have been restated to conform with changes to Fiscal 2016 product category classifications. |
Vitamins, Minerals, Herbs and Homeopathy
Vitamins and minerals are recommended to maintain health, proactively to improve health and in support of specific health conditions. These products help prevent nutrient deficiencies that can occur when diet alone does not provide all the necessary vitamins and minerals our bodies need. The vitamin and mineral product category includes multi-vitamins, which many consider to be a foundation of a healthy regimen, lettered vitamins, such as Vitamins A, C, D, E, and B-complex, along with major and trace minerals such as calcium, magnesium, chromium and zinc.
Herbs offer a natural remedy to address specific conditions. Certain herbs help support specific body systems, including ginkgo to support brain function and milk thistle to help support liver function, as well as other less common herbs such as black cohosh for menopause support. Herbal products include whole herbs, standardized extracts, herb combination formulas and teas. Homeopathic remedies offer our customers the ability to address health concerns while providing the safety of having no known drug interactions or side effects.
With approximately 6,500 SKUs in this product category, a wide range of potency levels and multiple delivery systems, our customers have many choices to fit their individual needs.
Sports Nutrition
Our sports nutrition consumers are looking for products to help maintain or supplement a healthy lifestyle. These products are used in conjunction with cardiovascular conditioning, weight training and sports activities. Major categories in sports nutrition include protein and weight gain powders, meal replacements, weight management, and pre and post-workout supplements to either support energy production or enhance recovery after exercise. Our sports nutrition products are offered in many convenient forms, such as powders, tablets, capsules, soft gels and liquids. Our sports nutrition consumers include the sports enthusiast, weekend warrior, endurance athlete, marathoner, serious bodybuilder, as well as those seeking to maintain a healthy fitness level. We offer approximately 2,000 SKUs in sports nutrition.
Specialty Supplements
Specialty supplements help supply higher levels of nutrients than diet alone can provide, help individuals stay healthy, and support specific conditions and life stages such as childhood, pregnancy, menopause and aging. Categories of specialty supplements include omega fatty acids, probiotics and condition specific formulas. Certain specialty supplements, such as
10
organic greens, psyllium fiber and soy proteins, provide added support during various life stages. Folic acid is specifically useful during pregnancy. Super antioxidants, such as coenzyme Q-10, grapeseed extract and pycnogenol, address specific conditions. High ORAC (oxygen radical absorptive capacity) fruit concentrates such as; gogi, mangosteen, pomegranate and blueberry help prevent oxygen radical damage. Other specialty supplement formulas are targeted to support specific organs, biosystems and body functions. We offer approximately 4,500 SKUs of specialty supplements.
Other
Our “Other” category represents all other product classifications we stock that are not included within the previously described categories. These products include items such as on the go bars, drinks and snacks, natural beauty and personal care. Our on the go bars, drinks and snacks offer our customers access to an offering of protein, low carb and natural bars, protein, energy and functional beverages and natural snacks. Natural beauty and personal care products offer an alternative to traditional products that often contain synthetic and/or other ingredients that our customers find objectionable. Our customers choose these products over more traditional products because they contain organic and natural ingredients, are free of pesticides or not tested on animals and/or are more closely aligned with the health and wellness goals of our customers. We offer approximately 4,000 SKUs for our Other category. In Fiscal 2016, Fiscal 2015 and Fiscal 2014, our Other product category includes net merchandise sales to third parties of Nutri-Force of $50.0 million, $56.6 million and $40.3 million, respectively.
Delivery Revenue
Delivery revenue represents amounts billed to customers for shipping fees.
Access to New Products
One of the many components of customer satisfaction is the introduction of new products. We identify customer and market trends by listening to our customers, Health Enthusiasts, vendors, contract manufacturers and market influencers. We maintain active partnerships with our vendors to stay on top of their product offerings and to bring new products to our customers quickly. In addition, we have a knowledgeable team in-house who focuses on bringing new Vitamin Shoppe branded products to our offering. Each year we launch many new products under our own brands, including the launch in Fiscal 2016 of approximately 60 new products. These include new product expansions into sustained release proteins and Isotech 42 ready to drink clear proteins in our BodyTech® brand, and the expansion of our plnt® brand with the addition of key items such as protein based meal replacement powders, mushroom immune formula and adaptogenic herb formulas.
Manufacturing
Through Nutri-Force, we provide custom manufacturing and private labeling of VMS products and develop and market our own branded products for both sales to third parties and for the VSI product assortment. Our manufacturing operations, which are located in Miami Lakes, Florida, produce tablets, capsules, soft-gels and powders. By operating our own manufacturing facilities, we believe we have the ability to better control the production and timing of new product introductions, and maintain high standards of product quality.
Suppliers and Inventory
The Company had one supplier from whom we purchased at least 5% of our merchandise during Fiscal 2016, two suppliers from whom we purchased at least 5% of our merchandise during Fiscal 2015 and one supplier from whom we purchased at least 5% of our merchandise during Fiscal 2014. We purchased approximately 11% of our total merchandise from these suppliers during Fiscal 2016 and approximately 17% during Fiscal 2015 and 12% during Fiscal 2014.
We consider numerous factors in supplier selection, including, but not limited to, quality, price, credit terms, and product offerings. As is customary in our industry, we generally do not have long-term contracts with any supplier and most suppliers may discontinue selling to us at any time.
We strive to maintain sufficient inventory to enable us to provide a high level of service to our customers. Inventory, accounts receivable and accounts payable levels, payment terms and return policies are in accordance with standard business procedures. We maintain a distribution network which we use in conjunction with a just-in-time inventory ordering system that we use to replenish our stores based upon customer demand of a given product or products. Our working capital requirements for merchandise inventory will continue to increase as we continue to open additional stores and expand our distribution network. Currently, our practice is to establish an inventory level of approximately $145,000 at cost for each of our stores, the cost of which is partially offset by vendor incentive and allowance programs. Additionally, 30 day payment terms have been extended to us by some of our suppliers allowing us to effectively manage our inventory and working capital. We believe that our buying power enables us to receive favorable pricing terms and enhances our ability to obtain high demand merchandise.
11
Warehouse and Distribution
We operate our supply chain primarily from two Company operated distribution center facilities. The Company operates distribution centers in North Bergen, New Jersey and Ashland, Virginia. By operating our own facilities we gain greater control over operations and costs. Our products manufactured by Nutri-Force are warehoused and distributed through its Miami Lakes, Florida facilities.
In the fourth quarter of 2016, the Company entered into an agreement to lease a warehousing and distribution facility located in Avondale, Arizona, which we expect to open before the end of Fiscal 2017. We previously utilized a third-party logistics provider to service the west coast, and we believe operating our own facility will provide improved service levels and network efficiencies.
Regulatory and Quality Control
The Food and Drug Administration (“FDA”) is the regulatory authority charged with overseeing the products we offer and the Federal Trade Commission (“FTC”) regulates the advertising of those products.
Our Scientific and Regulatory Affairs (“S&RA”) and Legal departments review all aspects of our Company’s FDA and FTC regulatory processes, ensuring compliance with regulations. We have established processes to review the underlying safety and efficacy of our branded products, including The Vitamin Shoppe®, BodyTech®, True Athlete®, Mytrition®, plnt®, ProBioCare®, Next Step® and Betancourt Nutrition®. These processes include review of the ingredients’ safety information, product formulation, product form, product labeling, the efficacy and claim support for the product and any marketing materials. All consumer communications that deal with product and health issues must be approved by S&RA prior to being disseminated to the public.
We have standard procedures whereby all potential Vitamin Shoppe contract manufacturers are reviewed and approved before they can supply any of our branded products. In addition, all potential new products are evaluated and approved prior to being accepted into our branded product lines.
Our relationships with manufacturers require that all of our branded products not be adulterated or misbranded under any provisions of the Federal Food, Drug, and Cosmetic Act (“FDCA”) and the regulations promulgated thereunder. This includes, but is not limited to, compliance with applicable Current Good Manufacturing Practices (“cGMP”). This means that ingredients in our products must be tested for identity, purity, quality, strength, and composition before being incorporated into our branded products, and that our final branded products must again be tested for identity, purity, quality, strength, and composition prior to being released. All of these products require a certificate of analysis, which includes certification to 100% of label claim.
We have established a standard quality control operating procedure that calls for on-site audits of our contract manufacturers’ facilities and processes, and have established an internal team that will audit each of these facilities and work with our contract manufacturers to resolve any noncompliance with dietary supplement cGMP regulations. We require that our manufacturers have certificates of analysis (such as for microbial testing and label testing).
Third party vendors, are also subject to a standard review, must comply with our vendor purchase agreement, including guaranteeing that all third-party products are lawful and manufactured in compliance with applicable cGMPs, and are required to carry adequate insurance policies to satisfy our standards. Each new product proposed to be carried by us is reviewed by our S&RA department. They reject those products that they believe may present undue risk or be unsafe.
Healthy Awards Program
Our Healthy Awards Program, which we established over 15 years ago, encourages our customers to make repeat purchases and enables us to enhance customer loyalty. The program is free of charge to join, and members earn one point for every dollar they spend, and points are accumulated toward a credit certificate which can be applied to a future purchase. Beginning in Fiscal 2016, the Company implemented enhancements to the program, including the issuance of credit certificates on a quarterly basis compared with annual issuances under the previous program. We enrolled approximately 2.1 million new members in Fiscal 2016. The number of active members between retail and online shoppers was approximately 6.3 million as of December 31, 2016. An active member is a customer that has purchased an item within the last twelve months.
We utilize our Healthy Awards Program database to track customer purchasing patterns across our retail and direct business segments, analyze market and industry trends and create targeted merchandising and marketing strategies. In addition, it provides us with customer and demographic data we use to assist us in the selection of future store locations.
12
Marketing
We believe our high quality real estate is one of our primary marketing tools, as we locate our stores in high-visibility areas. We also conduct targeted marketing efforts by sending promotional offers to members of our Healthy Awards Program, and we continue to develop our digital marketing and social media strategies. We advertise in national magazines, and engage in local advertising via direct mail, radio and television for certain new stores.
We promote our own branded products, including The Vitamin Shoppe®, BodyTech®, True Athlete®, Mytrition®, plnt®, ProBioCare®, Next Step® and Betancourt Nutrition® through our retail channel by placing the products in strategic and highly visible locations in our stores.
Competition
The U.S. nutritional supplements retail industry is highly competitive and fragmented. Competition is based primarily on quality, product assortment, price, customer service, convenience, marketing support and availability of new products. We compete with publicly and privately owned companies with broad geographical market coverage and product categories. We compete with other specialty and mass market retailers, including GNC®, Whole Foods®, Natural Grocers®, Sprouts Farmers Market®, Vitamin World®, Costco® and other club chains, Wal-Mart®, drugstore chains including Rite-Aid®, CVS® and Walgreens®, internet and mail order companies, including Amazon.com®, Puritan’s Pride®, Vitacost.com®, Bodybuilding.com®, Doctors Trust®, Swanson® and iHerb®, in addition to a variety of independent health and vitamin stores and e-commerce outlets.
Insurance and Risk Management
We purchase insurance to partially offset standard risks in our industry, including policies to cover products liability, travel liability, auto liability and other casualty and property risks. We are self-insured and utilize high deductible programs for certain losses related to our employee medical benefits, workers’ compensation and general liability, although we maintain stop-loss coverage with third-party insurers to limit our liability exposure. Our insurance rates are based on our safety record, claims experience and trends in the insurance industry.
We face an inherent risk of exposure to product liability claims if, among other things, the use of our products results in injury. With respect to product liability coverage, we carry insurance coverage typical of our industry and product lines. Our coverage involves self-insured retentions with primary and excess liability coverage above the retention amount. We have the ability to refer certain claims to our contract manufacturers, third-party vendors and their respective insurers to pay the costs associated with any claims arising from those contract manufacturers’ or third-party vendors’ products. Our insurance covers claims that are not adequately covered by a contract manufacturer’s or third-party vendor’s insurance and provides for excess secondary coverage above the limits provided by our contract manufacturers or third-party vendors. We believe we have obtained a prudent amount of insurance for the insurable risks associated with our business. Our experience is that our insurance costs have increased in the past, and may increase in the future.
Tradenames and Other Intellectual Property
We believe trademark protection is particularly important to the maintenance of the recognized proprietary brand names under which we market our products. We own trademarks or trade names that we use in conjunction with the sale of our products, including The Vitamin Shoppe®, BodyTech®, True Athlete®, Mytrition®, plnt®, ProBioCare®, Next Step® and Betancourt Nutrition® brand names. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position. We protect our intellectual property rights through a variety of methods including trademark and trade secret laws, as well as confidentiality agreements and proprietary information agreements with vendors, employees, consultants and others who have access to our proprietary information. Protection of our intellectual property often affords us the opportunity to enhance our position in the marketplace by precluding our competitors from using or otherwise exploiting our technology and brands. The carrying value of our trademarks and brands, which are primarily indefinite lived intangible assets, was $78.9 million at December 31, 2016 and $79.5 million at December 26, 2015.
Sales from International Sources
For each of the last three years, less than 1.0% of our sales have been derived from international sources.
Employees
As of December 31, 2016, we had a total of 3,887 full-time and 1,616 part-time employees, of whom 4,334 were employed in our retail channel and 1,169 were employed in corporate, manufacturing, distribution and direct channel support
13
functions. None of our employees belong to a union or are a party to any collective bargaining or similar agreement except for certain employees at one of our Seattle based stores, who are members of the United Food & Commercial Workers Local No. 367. We consider our relationships with our employees to be good.
Environmental
We are subject to numerous federal, state, local and foreign laws and regulations governing our operations, including the handling, transportation and disposal of our products and our non-hazardous and hazardous substances and wastes, as well as emissions and discharges into the environment, including discharges to air, surface water and groundwater. Failure to comply with those laws and regulations could result in costs for corrective action, penalties or the imposition of other liabilities. Changes in environmental laws or the interpretation thereof or the development of new facts could also cause us to incur additional capital and operational expenditures to maintain compliance with environmental laws and regulations. We also are subject to laws and regulations that impose liability and cleanup responsibility for releases of hazardous substances into the environment without regard to fault or knowledge about the condition or action causing the liability. Under certain of these laws and regulations, such liabilities can be imposed for cleanup of previously owned or operated properties. The presence of contamination from those substances or wastes could also adversely affect our ability to utilize our leased properties. Compliance with environmental laws and regulations has not had a material effect upon our earnings or financial position; however, if we violate any environmental obligation, it could have a material adverse effect on our business or financial performance.
Government Regulation
The formulation, manufacturing, processing, labeling, packaging, advertising and distribution of our products are subject to regulation by various federal agencies, including the FDA, the FTC, the Consumer Product Safety Commission, the U.S. Department of Agriculture (“USDA”) and the Environmental Protection Agency (“EPA”). These activities are also regulated by various agencies of the states and localities in which our products are sold. The FDA, under the Federal Food, Drug, and Cosmetic Act (“FDCA”) regulates the processing, formulation, safety, manufacture, packaging, labeling and distribution of dietary supplements (including vitamins, minerals, and herbs) and cosmetics. The FTC regulates the advertising of these products.
The Dietary Supplement Health and Education Act of 1994 (“DSHEA”) amended the FDCA to establish a new framework governing the composition, safety, labeling and marketing of dietary supplements. “Dietary supplements” are defined as vitamins, minerals, herbs, other botanicals, amino acids and other dietary substances for human use to supplement the diet, as well as concentrates, metabolites, constituents, extracts or combinations of such dietary ingredients. Generally, under DSHEA, dietary ingredients that were on the market prior to October 15, 1994 may be used in dietary supplements without notifying the FDA. New dietary ingredients (i.e., not marketed in the U.S. prior to October 15, 1994) must be the subject of a new dietary ingredient notification submitted to the FDA unless the ingredient has been “present in the food supply as an article used for food” without being “chemically altered.” A new dietary ingredient notification must provide the FDA with evidence of a “history of use or other evidence of safety” establishing that use of the dietary ingredient “will reasonably be expected to be safe.” A new dietary ingredient notification must be submitted to the FDA at least 75 days before the initial marketing of the new dietary ingredient. There can be no assurance that the FDA will accept the evidence of safety for any new dietary ingredients that we may want to market, and the FDA’s refusal to accept such evidence could prevent the marketing of such dietary ingredients. In 2011, the FDA issued draft guidance regarding new dietary ingredient notifications, including the scope of the notification requirement and the content of such notifications, and in August 2016, the FDA issued revised draft guidance. While the revised draft guidance is not enforceable, it may be deemed to represent the FDA’s current point of view. Should the FDA enforce the draft guidance as currently written, it would have a negative effect on the innovation and continued marketing of dietary supplements. There is no certainty that the FDA will accept any particular evidence of safety for any new dietary ingredient. The FDA’s refusal to accept such evidence could prevent the marketing of those dietary ingredients.
DSHEA permits “statements of nutritional support” to be included in labeling for dietary supplements without premarket FDA approval. Such statements must be submitted to the FDA within 30 days of first use in marketing and must be accompanied by a label disclosure that “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.” Such statements may describe how a particular dietary ingredient affects the structure, function or general well-being of the body, or the mechanism of action by which a dietary ingredient may affect body structure, function or well-being, but may not expressly or implicitly represent that a dietary supplement will diagnose, cure, mitigate, treat, or prevent a disease. Any statement of nutritional support we make in labeling must possess scientific evidence substantiating that the statement is truthful and not misleading. If the FDA were to determine that a particular statement of nutritional support was an unacceptable drug claim or an unauthorized version of a health claim about disease risk reduction for a food product, or if the FDA were to determine that a particular claim was not adequately supported by existing scientific data or was false or misleading, we would be prevented from using that claim. In addition, the
14
FDA deems internet materials as labeling; therefore, our internet materials must comply with FDA requirements and could be the subject of regulatory action by the FDA, or by the FTC if that agency, reviewing the materials as advertising, considers the materials false and misleading.
DSHEA provides that so-called “third-party literature,” such as a reprint of a peer-reviewed scientific publication linking a particular dietary ingredient with health benefits, may be used “in connection with the sale of a dietary supplement to consumers” without the literature being subject to regulation as labeling. Such literature must not be false or misleading; the literature may not “promote” a particular manufacturer or brand of dietary supplement; and a balanced view of the available scientific information on the subject matter must be presented. If the literature fails to satisfy each of these requirements, we may be prevented from disseminating such literature with our products, and any dissemination could subject our product to regulatory action as an illegal drug.
In June 2007, the FDA published current Good Manufacturing Practice (“cGMP”) regulations that govern the manufacturing, packing and holding of dietary ingredients and dietary supplements. cGMP regulations require dietary supplements to be prepared, packaged and held in compliance with strict rules, and require quality control provisions similar to those in the cGMP regulations for drugs. The FDA could inspect one of our facilities or those of one of our contract manufacturers and determine that the facility was not in compliance with these regulations, and cause affected products made or held in the facility to be subject to FDA enforcement actions. We believe our manufacturing and distribution facilities and practices comply with these rules. In addition, as is common practice in the industry, we rely on our third-party contract manufacturers to ensure that the products they manufacture and sell to us comply with all applicable regulatory requirements and seek representations and warranties in our agreements with these contract manufacturers confirming such compliance.
The FDA has broad authority to enforce the provisions of the FDCA applicable to foods, dietary supplements, and cosmetics, including powers to issue a public warning letter to a company, to publicize information about illegal products, to request a recall of illegal products from the market, and to request the United States Department of Justice to initiate a seizure action, an injunction action, or a criminal prosecution in the U. S. courts.
The FTC exercises jurisdiction over the advertising of foods, dietary supplements and cosmetics. In recent years, the FTC has instituted numerous enforcement actions against dietary supplement companies for failure to have adequate substantiation for claims made in advertising or for the use of false or misleading advertising claims. As a result of our efforts to comply with applicable statutes and regulations, we have from time to time reformulated, eliminated or relabeled certain of our products and revised certain provisions of our sales and marketing program. The FTC has broad authority to enforce its laws and regulations applicable to foods, dietary supplements and cosmetics, including the ability to institute enforcement actions which often result in consent decrees, injunctions, and the payment of civil penalties by the companies involved. Failure to comply with the FTC’s laws and regulations could impair our ability to market our products.
We are also subject to regulation under various state and local laws that include provisions governing, among other things, the registration, formulation, manufacturing, packaging, labeling, advertising and distribution of foods, dietary supplements and cosmetics. In addition, in the future, we may become subject to additional laws or regulations administered by the FDA or by other federal, state, local or foreign regulatory authorities, to the repeal of laws or regulations that we consider favorable, such as DSHEA, or to more stringent interpretations of current laws or regulations. In the future, we believe the dietary supplement industry will likely face increased scrutiny from federal and state regulatory authorities. It is difficult to predict the effect future laws, regulations, repeals or interpretations will have on our business. However, such changes in the regulatory landscape could require the reformulation of certain products, recalls or discontinuance of certain products, additional administrative requirements, revised or additional labeling, increased scientific substantiation or other new requirements. Any such changes could have a material adverse effect on our business or financial performance.
Corporate Information
We were incorporated in Delaware on September 27, 2002. Our principal executive offices are located at 300 Harmon Meadow Blvd., Secaucus, New Jersey 07094.
Item 1A. Risk Factors
You should carefully consider the following factors, in addition to other information in this Annual Report on Form 10-K, in evaluating our Company and our business.
15
Risks Related to Our Business and Industry
We operate in a highly competitive industry and our failure to compete effectively could materially and adversely affect our sales and growth prospects.
The U.S. nutritional supplements retail industry is a large and highly fragmented industry. We compete primarily against other specialty retailers, supermarkets, drugstores, mass merchants, multi-level marketing organizations and e-commerce companies. This market is highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market. As certain products become more mainstream, with broader distribution, we experience increased competition for those products. For example, as the trend in favor of low carb products developed, we experienced increased competition for our low carb products from supermarkets, drug stores, mass merchants and other food companies. Increased competition from companies that distribute through retail, e-commerce or wholesale channels could have a material adverse effect on our financial condition and results of operations. Certain of our competitors may have significantly greater financial, technical and marketing resources than we do. In addition, our competitors may be more effective and efficient in introducing new products. Furthermore, if we fail to increase the utilization of our supply chain network, fail to maximize the efficiency of our ship direct to customers strategy, or fail to provide our customers with an attractive omni-channel experience, our business and results of operations could be materially and adversely affected. We may not be able to compete effectively, and any of the factors listed above may cause price reductions, reduced margins and losses of our market share.
Unfavorable publicity or consumer perception of our products and any similar products distributed by other companies could have a material adverse effect on our reputation, which could result in decreased sales and significant fluctuations in our business, financial condition and results of operations.
We depend significantly on consumer perception regarding the safety and quality of our products, as well as similar products distributed by other companies. Consumer perception of products can be significantly influenced by adverse publicity in the form of published scientific research, national media attention or other publicity, whether or not accurate, that associates consumption of our products or any other similar products with illness or other adverse effects, or questions the benefits of our or similar products or that claims that any such products are ineffective. A new product may initially be received favorably, resulting in high sales of that product, but that sales level may not be sustainable as consumer preferences change. Future scientific research or publicity could be unfavorable to our industry or any of our particular products and may not be consistent with earlier favorable research or publicity. Unfavorable research or publicity could have a material adverse effect on our ability to generate sales.
Our failure to appropriately and timely respond to changing consumer preferences and demand for new products and services could significantly harm our customer relationships and our business, financial condition and results of operations.
Our business is subject to changing consumer trends and preferences. Our failure to accurately predict or react to these trends could negatively impact consumer opinion of us as a source for the latest products, which in turn could harm our customer relationships and cause us to lose market share. The success of our product offerings depends upon a number of factors, including our ability to:
• | anticipate customer needs; |
• | innovate and develop new products; |
• | successfully introduce new products in a timely manner; |
• | price our products competitively with retail and online competitors; |
• | deliver our products in sufficient volumes and in a timely manner; and |
• | differentiate our product offerings from those of our competitors. |
If we do not introduce new products or make enhancements to meet the changing needs of our customers in a timely manner, some of our products could be rendered obsolete, which could have a material adverse effect on our sales and other operating results.
We continue to explore new strategic initiatives, including our reinvention strategy, but we may not be able to successfully execute on, or realize the expected benefits from the implementation of, our strategic initiatives, and our pursuit of new strategic initiatives may pose significant costs and risks.
In Fiscal 2015, we began development of our reinvention strategy to refocus our business on market-based opportunities for stronger growth. As part of our reinvention strategy, we are comprehensively reviewing our customer experience. Our reinvention strategy may include initiatives to optimize product assortment, integration of technology and e-
16
commerce, changes to the layout and design of our retail stores, and improvements in service levels provided by our Health Enthusiasts. Our strategic initiatives are also focused on, among other things, developing a presence in new international markets through franchise, wholesale and retail distribution opportunities, developing new products, and evaluating acquisitions and joint ventures. Our future operating results are dependent, in part, on our management’s success in implementing the reinvention strategy and other strategic initiatives, and as a result could divert management’s attention from our existing business as management focuses on developing the initiative and related operations. Also, our short-term operating results could be unfavorably impacted by the opportunity and financial costs associated with the implementation of our strategic plans, such as consulting fees incurred in connection with the reinvention strategy, and we might not realize the benefits from such strategies. In addition, we may not be successful in achieving the intended objectives of the reinvention strategy and other strategic initiatives in a timely manner or at all.
We may experience product recalls, withdrawals or seizures, which could materially and adversely affect our business, financial condition and results of operations.
We may be subject to product recalls, withdrawals or seizures if any of the products we sell or the products that we manufacture for third parties is believed to cause injury or illness or if we are alleged to have violated governmental regulations in the manufacturing, labeling, promotion, sale or distribution of those products. A significant recall, withdrawal or seizure of any of the products we manufacture or sell may require significant management attention, would likely result in substantial and unexpected costs and may materially and adversely affect our business, financial condition or results of operations. Furthermore, a recall, withdrawal or seizure of any of our products may adversely affect consumer confidence in our brands and thus decrease consumer demand for our products. As is common in the VMS industry, except with respect to the products that we manufacture at our manufacturing facility, we rely on our contract manufacturers and suppliers to ensure that the products they manufacture and sell to us comply with all applicable regulatory and legislative requirements. In general, we seek representations and warranties, indemnification and/or insurance from our contract manufacturers and suppliers. However, even with adequate insurance and indemnification, any claims of non-compliance could significantly damage our reputation and consumer confidence in our products. In addition, the failure of those products to comply with applicable regulatory and legislative requirements could prevent us from marketing the products or require us to recall or remove such products from the market, which in certain cases could materially and adversely affect our business, financial condition and results of operations.
Disruptions at our or our contract manufacturers’ manufacturing facilities or loss of our or their manufacturing certifications could materially and adversely affect our business, financial condition, results of operations and customer relationships.
Our private brands merchandise accounted for approximately 21% of our net sales in Fiscal 2016. Any significant disruption in a contract manufacturers’ manufacturing facilities for any reason, including regulatory requirements, an FDA determination that the facility is not in compliance with the cGMP regulations, the loss of certifications, power interruptions, destruction of or damage to facilities, terrorist attacks, civil unrest, war or the perceived threat thereof, fires, hurricanes and other natural disasters could disrupt our contract manufacturers’ ability to manufacture products for the Vitamin Shoppe assortment as well as disrupt our ability to manufacture products for our contract manufacturing customers and our own branded products. Any such disruption could have a material adverse effect on our business, financial condition and results of operations. While we do not believe it would be difficult to source our products from other contract manufacturers, a transition period would be required in order to source our own branded products from other contract manufacturers.
Nutri-Force has incurred operating losses, which are expected to continue in Fiscal 2017. We plan on implementing initiatives to turnaround the performance of Nutri-Force which may not be successful in achieving the expected improvements, or may require more time and resources than expected to implement.
The success of the turnaround of our manufacturing operation will depend in large part on our ability to implement a series of initiatives designed to reduce complexity, such as reduce the manufacture of unprofitable product and focus on core customers, improve efficiencies, establish core processes and reduce costs and expenses. If the turnaround plan does not achieve its intended results, the anticipated improvements to our operating results may not be realized fully or at all, or may take longer to realize than expected, and the costs to implement this plan could be significantly higher than expected. We expect the turnaround plan to be time consuming, and require substantial resources and effort. There can be no assurance that the plan will be successful and as a result, may adversely affect our business, financial results and operations.
Our customers for whom we contract manufacture may significantly influence our business, financial condition and results of operations.
Our contract manufacturing business is dependent on demand for the products we manufacture for our customers and we have no control or influence over the market demand for those products. Demand for our customers’ products can be
17
adversely affected by, among other things, regulatory issues, the loss of patent or other intellectual property rights protection, the emergence of competing products, competition from other contract manufacturers, negative public or consumer perception of those products or our industry and changes in the marketing strategies for such products.
If production volumes of products that we manufacture for third parties and related revenues are not maintained, it may have a material adverse effect on our business, financial condition and results of operations. Additionally, any changes in product mix due to our customers’ products may adversely affect our results of operations.
Increases in the price or shortages of supply of key raw materials could materially and adversely affect our business, financial condition and results of operations.
Our products and the products we manufacture for third parties are composed of certain key raw materials. If the prices of these raw materials were to increase significantly, it could result in a significant increase to us in the prices charged to us for our own branded products and third-party products. Raw material prices may increase in the future and we may not be able to pass on those increases to customers who purchase our products. A significant increase in the price of raw materials that cannot be passed on to customers could have a material adverse effect on our business, financial condition and results of operations.
We are reliant upon the supply of raw materials that meet our specifications and the specifications of third parties for which we manufacture. If any raw material is adulterated and does not meet our specifications or third parties’ specifications, it could significantly impact our ability to manufacture products and could materially and adversely affect our business, financial condition and results of operations.
In addition, if we are no longer able to obtain products from one or more of our suppliers on terms reasonable to us or at all, our ability to perform under contracts with third parties for whom we manufacture products and our customer relationships could be materially and adversely affected. Events such as terrorist attacks, civil unrest or war, or the perceived threat thereof, may also have a significant adverse effect on raw material availability essential to the manufacturing of our products which could have a material adverse effect on our business, financial condition and results of operations.
The cost of construction materials we use to build and remodel our stores is also subject to significant price volatility based on market and economic conditions. Higher construction material prices would increase the capital expenditures needed to construct a new store or remodel an existing store and could increase the rent payable by the Company under its leases.
We currently rely primarily on two warehouse and distribution facilities to distribute most of the products we sell. Disruptions to these warehouse and distribution facilities could adversely affect our business.
Our primary warehouse and distribution operations are currently concentrated in two locations; in North Bergen, New Jersey and in Ashland, Virginia. Any significant disruption to our two primary distribution centers operations for any reason, such as a flood, fire or hurricane, could adversely affect our product distributions and sales until we are able to secure an alternative distribution method. Unexpected delays in deliveries or increases in transportation costs (including through increased fuel costs) could significantly decrease our sales and operating results. In addition, labor shortages in the transportation industry or long-term disruptions to the national and international transportation infrastructure that lead to delays or interruptions of deliveries could negatively affect our business.
In addition, during Fiscal 2017, we plan on opening a warehousing and distribution facility in Avondale, Arizona. If we fail to successfully open and effectively utilize this new distribution center, it could have an adverse affect on our financial results.
Failure to increase the utilization of our supply chain network could have a material adverse effect on our business.
If we fail to increase the utilization of our supply chain network and expand functionality of our information technology systems, we could experience increased costs associated with diminished productivity and operating inefficiencies related to the flow of goods through our supply chain, which could have a material adverse effect on our financial results.
Our existing stores, or any stores we open in the future, may not achieve sales and operating levels consistent with historical results. In addition, our growth strategy includes the addition of a number of new stores each year. We may not be able to successfully implement this strategy on a timely basis or at all, and our business could be materially and adversely affected if we are unable to successfully negotiate favorable lease terms.
Since the beginning of Fiscal 2014, we have opened 137 stores, expanding our presence in our existing markets as well as entering new markets. Historically, our new stores have reached sales that are consistent with our mature stores over the
18
course of approximately four to five years. As part of our reinvention strategy, we have reduced the number of planned new store openings in Fiscal 2017, as we assess the performance of recently remodeled stores. Our new stores opened since the beginning of Fiscal 2013 average approximately 2,900 square feet compared to the average of our total store portfolio of approximately 3,500 square feet. Existing stores, or any new stores we open in the future, may not achieve sales and operating levels consistent with our historical results. In addition, customer migration from retail stores to e-commerce may also reduce store potential. The failure of our existing stores and new stores to achieve sales and operating levels consistent with our historical results could have a material adverse effect on our financial condition and operating results. As of December 31, 2016, we leased 775 stores along with our corporate headquarters, additional office space and manufacturing and distribution facilities. The store leases are generally for a term of ten years and we have options to extend most leases for a minimum of five years. Our business, financial condition, and operating results could be materially and adversely affected if we are unable to continue to negotiate acceptable lease and renewal terms.
In addition, our growth continues to depend, in part, on our ability to open and operate new stores successfully. The success of this strategy depends upon, among other things, the identification of suitable sites for store locations, the negotiation of acceptable lease terms, the hiring, training and retention of competent sales personnel, and the effective management of inventory to meet the needs of new and existing stores on a timely basis. Our continued expansion will also place increased demands on our operational, managerial and administrative resources. These increased demands could cause us to operate our business less effectively, which in turn could cause deterioration in the financial performance of our existing stores. Further, our new store openings may result in reduced net sales volumes in the direct channel, as well as in our existing stores in those markets. We expect to fund our expansion through cash flow from operations and, if necessary, by borrowings under our revolving credit facility. If we experience a decline in performance, we may slow or discontinue store openings. If we fail to successfully implement these strategies, our financial condition and operating results may be materially and adversely affected.
Some of our new stores may be located in areas where we have little or no presence or brand awareness. Those markets may have different competitive conditions, market conditions, consumer tastes and discretionary spending patterns than our existing markets, which may cause our new stores to be less successful than stores in our existing markets. Alternatively, many of our new stores will be located in areas where we have existing stores. Although we have experience in these markets, increasing the number of locations in these markets may result in inadvertent over-saturation of markets and temporarily or permanently divert customers and sales from our existing stores, thereby adversely affecting our overall financial performance.
The loss of key management could negatively affect our business.
Our success largely depends on the efforts and abilities of our senior executive group and key personnel. The loss of the services of one or more of our key executives or personnel, or the increased demands placed on our key executives and personnel by our continued growth could adversely affect our financial performance and our ability to execute our strategies. Our continued success also depends on our ability to attract and retain qualified team members to meet our future growth needs. We may not be able to attract and retain necessary team members to operate our business.
Our inability to attract, train and retain highly qualified Health Enthusiasts could adversely impact our business, financial condition and results of operations.
Our success depends on the continued contributions of our Health Enthusiasts, and the loss of these contributions could have a material adverse effect on our business. We must attract, train and retain a large and growing number of qualified Health Enthusiasts, while controlling related labor costs and maintaining our core values. Our ability to control labor and benefit costs is subject to numerous external factors, including regulatory changes, prevailing wage rates, and healthcare and other insurance costs. We compete with other retail and non-retail businesses for these Health Enthusiasts and invest significant resources in training and motivating them. There is no assurance that we will be able to attract or retain qualified Health Enthusiasts in the future, which could have a material adverse effect on our business, financial condition and results of operations.
If we fail to protect our brand names, competitors may adopt tradenames that dilute the value of our brand names.
We may be unable or unwilling to strictly enforce our tradenames in each jurisdiction in which we do business. In addition, because of the differences in foreign trademark laws concerning proprietary rights, our trademarks may not receive the same degree of protection in foreign countries as they do in the U.S. Also, we may not always be able to successfully enforce our trademarks against competitors or against challenges by others. Our failure to successfully protect our trademarks could diminish the value and efficacy of our past and future marketing efforts, and could cause customer confusion, which could, in turn, materially and adversely affect our sales and profitability.
19
Disruptions in our information systems could damage our reputation, be expensive to remedy and have a material adverse effect on our business and results of operations.
We rely extensively on information systems for point-of-sale processing in our stores, our e-commerce business, supply chain, manufacturing operations, financial reporting, human resources and various other processes and transactions. Our information systems, including those provided and maintained by third-party service providers, are subject to damage or interruption from power outages or other types of damage, including those due to computer and telecommunications failures, natural events including hurricanes, fires, floods, earthquakes, tornadoes, high winds and other severe weather, and from events caused by humans, including computer viruses, physical or electronic break-ins and acts of war or terrorism. Any of these events could cause system interruptions, delays and loss of critical data, and could prevent us from accepting and fulfilling customer orders, process and receive shipments of products, process financial and credit card transactions and providing services, which could make our product offerings less attractive and subject us to liability as well as result in lost customer confidence. Additionally, changes in technology could cause our information systems to become obsolete and it may be necessary to incur additional costs to upgrade such systems, and if our information systems prove inadequate to handle our growth, we could lose customers, which could have a material adverse effect on our business, financial condition and results of operations. Our systems are not fully redundant and our disaster recovery planning may not be sufficient. In addition, we may have inadequate insurance coverage to compensate for any related losses. Any of these events could damage our reputation, be expensive to remedy and have a material adverse effect on our business and results of operations.
If we fail to protect the integrity and security of customer-related and other confidential information, we could be exposed to litigation, increased costs and reputational damage, and our business, results of operations and financial condition could be materially and adversely affected.
The use of individually identifiable data by us, our customers, our Health Enthusiasts and others is regulated at the state, federal and international levels. Privacy and information security laws and regulations change from time to time, and increasing costs of compliance with those laws and regulations and related technology investments could materially and adversely affect our business and results of operations. Additionally, the success of our e-commerce operations depends upon the secure transmission of confidential information over public networks, including the use of cashless payments, and we use computers in substantially all other aspects of our business operations, including for point-of-sale processing in our stores. Such uses give rise to cybersecurity risks, including security breach, espionage, system disruption, theft and inadvertent release of information. While we have taken significant steps to protect customers’ personal information, consumer preferences and credit card information, and other confidential information including our employees’ private information and financial and strategic data about the Company and our business partners, the intentional or negligent actions of Health Enthusiasts, our suppliers or others may undermine our security measures. As a result, unauthorized parties may obtain access to our data systems and misappropriate confidential data. Furthermore, because the methods used to obtain unauthorized access change frequently and may not be immediately detected, we may be unable to anticipate these methods or implement preventative measures, and our incident response efforts may not be entirely effective. Any preventative measures we implement may have the potential to negatively affect our relations with our customers or decrease activity on our websites by making them less user-friendly. If our data security is compromised, it could have a material adverse effect on our reputation, results of operations and financial condition, materially increase the costs we incur to protect against those events in the future and subject us to additional legal risk and a competitive disadvantage. In addition, our customers could lose confidence in our ability to protect their personal information, which could cause them to stop shopping at our stores or online. The loss of confidence from a data security breach involving Health Enthusiasts could hurt our, and their, reputation and as a result cause Health Enthusiast recruiting and retention challenges.
Natural disasters and unusually adverse weather conditions could cause permanent or temporary damage to our distribution centers or stores, impair our ability to purchase, receive or replenish inventory or cause customer traffic to decline, all of which could result in lost sales and otherwise materially and adversely affect our results of operations.
The occurrence of one or more natural disasters, such as hurricanes, fires, floods, earthquakes, tornadoes, high winds and other severe weather, could materially and adversely affect our operations and results of operations. To the extent these events result in the closure of our distribution centers, our corporate headquarters, or a significant number of our stores, or to the extent they adversely affect one or more of our key suppliers, our operations and results of operations could be materially and adversely affected through an inability to make deliveries to our stores and through lost sales. In addition, these events could result in increases in fuel (or other energy) prices or a fuel shortage, delays in opening new stores, the temporary lack of an adequate work force in a market, the temporary or long-term disruption in the supply of products from suppliers, delay in the delivery of goods to our distribution centers or stores, the temporary reduction in the availability of products in our stores and disruption to our information systems, as noted above. These events also could have indirect consequences, such as increases in the cost of insurance, if they were to result in significant loss of property or other insurable damage.
20
Our e-commerce business is dependent on certain third parties. Changes in business practices or terms by such third parties could have a material adverse effect on our results of operations.
Our e-commerce business has several third-party relationships that contribute to our ability to generate revenue from a variety of online sources. These relationships may be dependent upon third-party tools, such as search engines, or established business terms negotiated by the Company, or utilization of third party marketplaces. If the economics of these relationships or the use of the third-party tools used to drive revenue change materially, this could affect our decision to maintain these relationships, and could result in lost sales and otherwise materially and adversely affect our financial performance.
If we do not successfully develop and maintain a relevant omni-channel experience for our customers, our business and results of operations could be materially and adversely affected.
Omni-channel retailing is rapidly evolving, and we must keep pace with changing customer expectations and new developments by our competitors. Our customers are increasingly using computers, tablets, mobile phones, and other devices to shop online. As part of our omni-channel strategy, we are making technology investments. If we are unable to make, improve, or develop relevant customer-facing technology in a timely manner, our ability to compete and our business and results of operations could be materially and adversely affected. In addition, if our e-commerce businesses or our other customer-facing technology systems do not function as designed, we may experience a loss of customer confidence, lost sales, or data security breaches, any of which could materially and adversely affect our business and results of operations.
We have significant lease obligations, which may require us to continue paying rent for store locations that we no longer operate.
Our stores are leased. We are subject to risks associated with our current and future real estate leases. Our costs could increase because of changes in the real estate markets and supply or demand for real estate sites. We generally cannot cancel our leases, so if we decide to close or relocate a location, we may nonetheless be committed to perform our obligations under the applicable lease including paying the base rent for the remaining lease term. As each lease expires, we may fail to negotiate renewals, either on commercially acceptable terms or any terms at all and may not be able to find replacement locations that will provide for the same success as current store locations. Of the current leases for our stores, 30 expire in Fiscal 2017, 95 expire in Fiscal 2018, 107 expire in Fiscal 2019, 95 expire in Fiscal 2020, 94 expire in Fiscal 2021 and the balance expire in Fiscal 2022 or thereafter.
Our international operations may result in additional market risks, which may harm our business.
As of December 31, 2016, we had 7 international franchise stores in Panama, 5 in Guatemala, 3 in Costa Rica and 2 in Paraguay, and also distribute products to other countries and manufacture products for third parties in other countries. In addition, if the opportunity arises, we may expand our operations into new and high-growth international markets. However, we are subject to risks associated with international operations, including but not limited to: (i) fluctuations in currency exchange rates; (ii) changes in international staffing and employment issues; (iii) tariff and other trade barriers; (iv) greater difficulty in using and enforcing our intellectual property rights; (v) failure to understand the local culture and market; (vi) inconsistent product regulation or sudden policy changes by foreign agencies or governments; (vii) compliance with U.S. laws applicable to international operations, including the Foreign Corrupt Practices Act and regulations promulgated by the Office of Foreign Asset Control; (viii) compliance with foreign laws, including tax laws and financial accounting standards; and (ix) political and economic instability and developments. Any of these risks could have a material adverse effect on our international operations and our growth strategy.
In addition, there is no assurance that we will expand our operations in new international markets. To expand our operations into new international markets, we may enter into business combination transactions, make acquisitions or enter into strategic partnerships, joint ventures or alliances, any of which may be material. We may enter into these transactions to acquire other businesses or products to expand our products or take advantage of new developments and potential changes in the industry. Our lack of experience operating in new international markets and our lack of familiarity with local economic, political and regulatory systems could prevent us from achieving the results that we expect on our anticipated time frame or at all. If we are unsuccessful in expanding into new or high-growth international markets, it could adversely affect our operating results and financial condition.
Legal and Regulatory Risks
We may incur material product liability claims, which could increase our costs and adversely affect our reputation with our customers, which in turn could materially adversely affect our business, financial condition and results of operations.
21
As a retailer, direct marketer and manufacturer of products designed for human consumption, we are subject to product liability claims if the use of our products or the products that we manufacture for third parties is alleged to have resulted in injury or to include inadequate instructions for use or inadequate warnings concerning possible side effects and interactions with other substances. Most of our products and the products that we manufacture for third parties are vitamins, minerals, herbs and other ingredients that are classified as foods or dietary supplements and are not subject to pre-market regulatory approval in the U. S. Our products or the products that we manufacture for third parties could contain contaminated substances, and some of our products and the products that we manufacture for third parties contain ingredients that do not have long histories of human consumption. Previously unknown adverse reactions resulting from human consumption of these ingredients could occur. In addition, third-party manufacturers produce many of the products we sell. We rely on these manufacturers to ensure the integrity of their ingredients and formulations. As a distributor of products manufactured by third parties, we may also be liable for various product liability claims for products we do not manufacture. While we attempt to manage these risks by obtaining indemnification agreements from the manufacturers of products that we sell (other than our own branded products) and insurance, third parties may not satisfy their indemnification obligations to us and/or our insurance policies may not be sufficient or available. A product liability claim against us, whether with respect to products of a third party that we sell, our branded products or products that we manufacture for third parties, could result in increased costs and could adversely affect our reputation with our customers, which in turn could materially adversely affect our business, financial condition and results of operations.
We may not be able to obtain insurance coverage in the future at current rates, or we may experience unfavorable claims.
While we believe we will be able to obtain liability insurance in the future, because of increased selectivity by insurance providers we may only be able to obtain such insurance at increased rates and/or with reduced coverage levels. Additionally, we may experience unfavorable claims. Changes in insurance rates, reduced coverage levels, or unfavorable claims could reduce our income from operations.
Compliance with governmental regulations could increase our costs significantly and adversely affect our operating income.
The processing, formulation, manufacturing, packaging, labeling, advertising and distribution of our products and the products that we manufacture for third parties are subject to federal laws and regulation by one or more federal agencies, including the FDA, the FTC, the USDA and the EPA. These activities are also regulated by various state, local and international laws and agencies of the states and localities in which our products and the products that we manufacture for third parties are sold. Regulations may prevent or delay the introduction, or require the reformulation, of our products or the products that we manufacture for third parties, which could result in lost sales and increased costs to us. A regulatory agency may not accept the evidence of safety for any new ingredients that we may want to market, may determine that a particular ingredient is not a legal dietary ingredient under DSHEA, may determine that a particular product or product ingredient presents an unacceptable health risk, may determine that a particular statement of nutritional support on our products or that parties use on the products we manufacture for them, or that we want to use on our products or that third parties want to use on the products we manufacture for them, is an unacceptable drug claim or an unauthorized version of a food “health claim.” A regulatory agency may determine that particular claims are not adequately supported by available scientific evidence. Any such regulatory determination would prevent us or third parties, as applicable, from marketing particular products or using certain statements on those products, or force us to recall a particular product, which could adversely affect our sales of those products
We are subject to environmental, health and safety laws and regulations, which could subject us to liabilities, increase our costs or restrict our operations in the future.
Our operations are subject to a variety of environmental, health and safety laws and regulations in each of the jurisdictions in which we operate. These laws and regulations govern, among other things, air emissions, wastewater discharges, the handling and disposal of hazardous substances and wastes, soil and groundwater contamination and employee health and safety. We are also subject to laws and regulations governing the handling and disposal of raw materials, non-compliant products and waste, the handling of regulated material that is included in our products or products that we manufacture for third parties and the disposal of products at the end of their useful life. These laws and regulations have increasingly become more stringent, and we may incur additional expenses to ensure compliance with existing or new requirements in the future. Any failure by us to comply with environmental, health and safety requirements could result in the limitation or suspension of our operations, including operations at our manufacturing facility. We also could incur monetary fines, civil or criminal sanctions, third-party claims or cleanup or other costs as a result of violations of or liabilities under such requirements. In addition, compliance with environmental, health and safety requirements could restrict our ability to expand our facilities or require us to acquire costly pollution control equipment, incur other significant expenses or modify our manufacturing processes.
22
Our manufacturing facilities use, store and dispose of hazardous substances in connection with the manufacturing processes. It is possible that these facilities may expose us to environmental liabilities associated with historical site conditions that have not yet been discovered. Some environmental laws impose liability for contamination on current and former owners and operators of affected sites, regardless of fault. If remediation costs or potential claims for personal injury or property or natural resource damages resulting from contamination arise, they may be material and may not be recoverable under any contractual indemnity or otherwise from prior owners or operators or any insurance policy. Additionally, we may not be able to successfully enforce any such indemnity or insurance policy in the future. In the event that new or previously unknown contamination is discovered or new cleanup obligations are otherwise imposed at any of our currently or previously owned or operated facilities, we may be required to take additional, unplanned remedial measures and record charges for which no reserves have been recorded.
Congress and/or regulatory agencies may impose additional laws or regulations or change current laws or regulations, and state attorneys general may increase enforcement of existing or new laws, and compliance with new or changed governmental regulations, or any state attorney proceeding, could increase our costs significantly and materially and adversely affect our business, financial condition and results of operations.
From time to time, Congress, the FDA, the FTC, or other federal, state, local or foreign legislative and regulatory authorities may impose additional laws or regulations that apply to us, repeal laws or regulations that we consider favorable to us or impose more stringent interpretations of current laws or regulations. We are not able to predict the nature of such future laws, regulations, repeals or interpretations or to predict the effect that additional governmental regulation, when and if it occurs, would have on our business in the future. Those developments could require reformulation of certain products to meet new standards, recalls or discontinuance of certain products (including products that we sell and products that we manufacture for third parties) not able to be reformulated, additional record-keeping requirements, increased documentation of the properties of certain products, additional or different labeling, additional scientific substantiation, adverse event reporting or other new requirements. Any developments of this nature could increase our costs significantly and could have a material adverse effect on our business, financial condition and results of operations.
In July 2011, the FDA issued draft guidance governing the notification of new dietary ingredients (“NDIs”) and in August 2016, the FDA issued revised draft guidance. We believe that the draft guidance, if implemented as proposed, would have a material impact on our operations. FDA enforcement of the NDI guidance as written could require us to incur additional expenses, which could be significant, and negatively affect our business in several ways, including, but not limited to, the detention and refusal of admission of imported products, the injunction of manufacturing of any dietary ingredients or dietary supplements until the FDA determines that those ingredients or products are in compliance, and the potential imposition of penalties for non-compliance.
Our failure to comply with FTC regulations could result in substantial monetary penalties and could adversely affect our operating results.
The FTC exercises jurisdiction over the advertising of dietary supplements and has instituted numerous enforcement actions against dietary supplement companies, including us, for failure to have adequate substantiation for claims made in advertising or for the use of false or misleading advertising claims. Failure by us to comply with applicable regulations could result in substantial monetary penalties, which could have a material adverse effect on our financial condition or results of operations.
We may be subject to intellectual property litigation and infringement claims by others.
We may be subject to intellectual property litigation and infringement claims initiated by others, other competitors or entities may assert rights in, or ownership of, our trademarks and other intellectual property rights or in marks that are similar to ours, and we may not be able to successfully resolve these types of conflicts to our satisfaction. Claims and litigation of this nature could cause us to incur significant expenses or prevent us from manufacturing, selling or using some of our products or the products that we manufacture for third parties, which could, in turn, adversely affect our sales and profitability.
Changes in accounting standards and estimates could have a material adverse effect on our results of operations and financial position.
Generally accepted accounting principles and the related authoritative guidance for many aspects of our business, including revenue recognition, inventories, goodwill and intangible assets, leases, income taxes and stock-based compensation, are complex and involve subjective judgments. Changes in these rules or changes in the underlying estimates, assumptions or judgments by our management could have a material adverse effect on our results of operations. For example, recently issued authoritative guidance for lease accounting will result in a significant increase to long-term assets and liabilities given we have a significant number of leases.
23
The accounting method for our convertible debt securities that may be settled in cash could have a material effect on our reported financial results.
In May 2008, the Financial Accounting Standards Board, which we refer to as FASB, issued FASB Staff Position No. APB 14-1, Accounting for Convertible Debt Instruments That May Be Settled in Cash Upon Conversion (Including Partial Cash Settlement), which has subsequently been codified as Accounting Standards Codification 470-20, Debt with Conversion and Other Options, which we refer to as ASC 470-20.
Under ASC 470-20, an entity must separately account for the liability and equity components of the convertible debt instruments (including our Convertible Notes) that may be settled entirely or partially in cash upon conversion in a manner that reflects the Company’s economic interest cost. The effect of ASC 470-20 on the accounting for the Convertible Notes is that the equity component is required to be included in the additional paid-in capital section of stockholders’ equity on our consolidated balance sheet, and the value of the equity component would be treated as original issue discount for purposes of accounting for the debt component of the Convertible Notes. As a result, we are required to record a greater amount of non-cash interest expense in current periods presented as a result of the amortization of the discounted carrying value of the Convertible Notes to their face amount over the term of the Convertible Notes. We report lower net income in our financial results because ASC 470-20 requires interest to include both the current period’s amortization of the debt discount and the instrument’s coupon interest, which could adversely affect our reported or future financial results and the trading price of our common stock.
In addition, under certain circumstances, convertible debt instruments (including the Convertible Notes) that may be settled entirely or partly in cash are currently accounted for utilizing the treasury stock method, the effect of which is that the shares issuable upon conversion of the Convertible Notes are not included in the calculation of diluted earnings per share except to the extent that the conversion value of the Convertible Notes exceeds their principal amount. Under the treasury stock method, for diluted earnings per share purposes, the transaction is accounted for as if the number of shares of common stock that would be necessary to settle such excess, if we elected to settle such excess in shares, are issued. We cannot be sure that the accounting standards in the future will continue to permit the use of the treasury stock method. If we are unable to use the treasury stock method in accounting for the shares issuable upon conversion of the Convertible Notes, then our diluted earnings per share would be adversely affected.
Risks Related to our Capital Structure
Our debt, and potential future additional indebtedness, could adversely affect our results of operations and financial condition and otherwise adversely impact our operating income and growth prospects.
As of December 31, 2016, our total consolidated indebtedness was $133.4 million, consisting of borrowings under our Convertible Senior Notes, our Revolving Credit Facility and our capital lease liabilities.
Our current and potential future debt financing could:
• | increase our vulnerability to general adverse economic, industry and competitive conditions; |
• | require us to dedicate a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow to fund working capital, new store growth and other capital expenditures, research and development efforts and other general corporate purposes; |
• | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
• | place us at a competitive disadvantage compared to our competitors that have less debt; and |
• | limit our ability to borrow additional funds. |
Restrictions in the agreements governing our existing and future indebtedness may prevent us from taking actions that we believe would be in the best interest of our business.
The agreements governing our existing indebtedness contain, and the agreements governing our future indebtedness will likely contain, customary restrictions on us or our subsidiaries, including covenants that restrict us or our subsidiaries, as the case may be, from incurring additional indebtedness, granting liens on our assets, making investments, consolidating or merging with another business, selling or otherwise disposing of our assets, paying dividends and entering into transactions with our affiliates.
Our ability to comply with these covenants and other provisions of our Revolving Credit Facility may be affected by changes in our operating and financial performance, changes in general business and economic conditions, adverse regulatory developments or other events beyond our control. The breach of any of these covenants could result in a default under our debt, which could cause those and other obligations to become immediately due and payable. In addition, these restrictions may
24
prevent us from taking actions that we believe would be in the best interest of our business and may make it difficult for us to successfully execute our business strategy or effectively compete with companies that are not similarly restricted.
Our ability to continue to access credit on the terms previously obtained for the funding of our operations and capital projects may be limited due to changes in credit markets.
In the past, the credit markets and the financial services industry have experienced disruption characterized by the bankruptcy, failure, collapse or sale of various financial institutions, increased volatility in securities prices, diminished liquidity and credit availability and intervention from the U.S. and other governments. Continued concerns about the systemic impact of potential long-term or widespread downturn, energy costs, geopolitical issues, the availability and cost of credit, the global commercial and residential real estate markets and related mortgage markets and reduced consumer confidence have contributed to increased market volatility. The cost and availability of credit has been and may continue to be adversely affected by these conditions. We cannot be certain that funding for our capital needs will be available from our existing financial institutions and the credit markets if needed, and if available, to the extent required and on acceptable terms. The Revolving Credit Facility matures in 2018, and the Convertible Notes mature in 2020. If we cannot renew or refinance this facility and our notes upon their maturities or, more generally, obtain funding when needed, in each case on acceptable terms, we may be unable to continue our current rate of growth, which may have an adverse effect on our revenues and results of operations.
Servicing our debt requires a significant amount of cash, and we may not have sufficient cash flow from our business to pay our substantial debt.
Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our indebtedness, including the Convertible Notes, depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not continue to generate cash flow from operations in the future sufficient to service our debt and make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional equity capital on terms that may be onerous or highly dilutive. Our ability to refinance our indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
Despite our current debt levels, we may still incur substantially more debt or take other actions which would intensify the risks discussed above.
Despite our current consolidated debt levels, we and our subsidiaries may be able to incur substantial additional debt in the future, subject to the restrictions contained in our Revolving Credit Facility. We will not be restricted under the terms of the indenture governing the Convertible Notes from incurring additional debt, securing existing or future debt, recapitalizing our debt or taking a number of other actions that are not limited by the terms of the indenture governing the Convertible Notes. Our Revolving Credit Facility restricts our ability to incur additional indebtedness, including secured indebtedness, but if the facility matures or is repaid, we may not be subject to such restrictions under the terms of any subsequent indebtedness.
In December 2015, we issued $143.8 million of 2.25% Convertible Senior Notes due 2020, which could dilute our existing stockholders’ equity and lower our reported earnings per share.
We issued $143.8 million of indebtedness in December 2015 in the form of 2.25% Convertible Senior Notes due 2020. The issuance of the Convertible Notes substantially increased our principal payment obligations. The holders of the Convertible Notes are entitled to convert the Convertible Notes into shares of our common stock under certain circumstances which would dilute our existing stockholders and lower our reported per share earnings.
In addition, in the event the conditional conversion feature of the Convertible Notes is triggered, holders of Convertible Notes will be entitled to convert the Convertible Notes at any time during specified periods at their option. If one or more holders elect to convert their Convertible Notes, unless we elect to satisfy our conversion obligation by delivering solely shares of our common stock (other than paying cash in lieu of delivering any fractional share), we would be required to settle a portion or all of our conversion obligation through the payment of cash, which could adversely affect our liquidity. In addition, even if holders do not elect to convert their Convertible Notes, we could be required under applicable accounting rules to reclassify all or a portion of the outstanding principal of the Convertible Notes as a current rather than long-term liability, which would result in a material reduction of our net working capital.
25
The convertible notes hedge and warrant transactions we entered into in connection with the issuance of the Convertible Notes may affect the value of the Convertible Notes and our common stock.
In connection with the pricing of the Convertible Notes, we entered into convertible note hedge transactions with the option counterparties. The convertible note hedge transactions are expected generally to reduce the potential dilution upon conversion of the Convertible Notes and/or offset any cash payments we are required to make in excess of the principal amount of converted Convertible Notes, as the case may be. We also entered into warrant transactions with the option counterparties. However, the warrant transactions could separately have a dilutive effect on our common stock to the extent that the market price per share of our common stock exceeds the applicable strike price of the warrants.
In addition, the option counterparties or their respective affiliates may modify their hedge positions by entering into or unwinding various derivatives with respect to our common stock and/or purchasing or selling our common stock or other securities of ours in secondary market transactions prior to the maturity of the Convertible Notes (and are likely to do so during any observation period related to a conversion of the Convertible Notes). This activity could also cause or avoid an increase or a decrease in the market price of our common stock or the Convertible Notes, which could affect the noteholders’ ability to convert the Convertible Notes and, to the extent the activity occurs during any observation period related to a conversion of the Convertible Notes, it could affect the number of shares and value of the consideration that the holders will receive upon conversion of the Convertible Notes.
In addition, if any such convertible note hedge and warrant transactions fail to become effective, the option counterparties may unwind their hedge positions with respect to our common stock, which could adversely affect the value of our common stock and the value of the Convertible Notes.
Hedging instruments often involve counterparty risks.
We will be subject to risk with respect to our counterparties to the convertible notes hedge transactions. Counterparty risk is the risk that the other party in a derivative transaction will not fulfill its contractual obligation. Changes in the credit quality of our counterparties with respect to their derivative transactions may affect the value of those instruments. By entering into derivatives, we assume the risk that these counterparties could experience financial hardships that could call into question their continued ability to perform their obligations.
If a counterparty becomes bankrupt or otherwise fails to perform its obligations under a derivative contract due to financial difficulties, it is likely to result in a default under such derivative contract, unless such default is cured. Default by a party with whom we enter into a hedging transaction may result in the loss of unrealized profits, leaving us with unsecured exposure and force us to cover our resale commitments, if any, at the then current market price. It may not always be possible to dispose of or close out a hedging position without the consent of the hedging counterparty, and we may not be able to enter into an offsetting contract in order to cover our risk. We cannot assure our shareholders that a liquid secondary market will exist for hedging instruments purchased or sold, and we may be required to maintain a position until exercise or expiration, which could result in losses.
Furthermore, upon the bankruptcy of a counterparty, we may experience significant delays in obtaining any recovery under the derivative contract in a dissolution, assignment for the benefit of creditors, liquidation, winding-up, bankruptcy, or other analogous proceeding. In addition, in the event of the insolvency of a counterparty to a derivative transaction, the derivative transaction would typically be terminated at its fair market value. If we are owed this fair market value in the termination of the derivative transaction and these claims are unsecured, we will be treated as general creditors of such counterparty, and will not have any claim with respect to the underlying security. We may obtain only a limited recovery or may obtain no recovery in such circumstances and the enforceability of agreements for hedging transactions may depend on compliance with applicable statutory and other regulatory requirements and, depending on the identity of the counterparty, applicable international requirements.
Our failure to meet market expectations could adversely affect the market price and volatility of our stock.
We believe that the price of our stock generally reflects market expectations for our future operating results. Any failure to meet, or delay in meeting, these expectations, including our comparable store sales growth rates, gross margin, earnings and earnings per share or new store openings, could cause the market price of our stock to decline, as could changes in our stock repurchase policies.
Item 1B. Unresolved Staff Comments
None.
26
Item 2. Properties
As of December 31, 2016, there were 775 Vitamin Shoppe and Super Supplements retail stores open in the United States and Puerto Rico. See “Item 1—Business—Store Counts and Locations” for additional information on the growth in our network of stores for Fiscal 2012 through 2016 and the location of our stores as of December 31, 2016. As of December 31, 2016, we leased the property for all of our stores. We do not believe that any individual store property is material to our financial condition or results of operation, however, more highly populated geographic areas may have a higher concentration of store locations. Of the leases for our stores as of December 31, 2016, 30 expire in Fiscal 2017, 95 expire in Fiscal 2018, 107 expire in Fiscal 2019, 95 expire in Fiscal 2020, 94 expire in 2021 and the balance expire in Fiscal 2022 or thereafter. We have options to extend most of these leases for a minimum of five years.
Our leased properties also include the following:
Location | Description | Square Footage | Lease Termination Year | Renewal Options | |||||
North Bergen, New Jersey | Warehouse, Distribution Center and Corporate Offices | 230,000 | 2018 | One Five-Year Renewal Option | |||||
Ashland, Virginia | Warehousing and Distribution Center | 312,000 | 2028 | Three Five-Year Renewal Options | |||||
Avondale, Arizona | Warehousing and Distribution Center | 187,000 | 2029 | Three Five-Year Renewal Options | |||||
Secaucus, New Jersey | Corporate Headquarters | 71,000 | 2029 | Two Five-Year Renewal Options | |||||
Miami Lakes, Florida | Manufacturing Facilities | 212,000 | 2018 and 2021 | Two Five-Year Renewal Options and Three Five-Year Renewal Options | |||||
The Company closed its three stores in Ontario, Canada and the Seattle, Washington distribution center in Fiscal 2016.
We believe that all of our current facilities are in good condition.
Item 3. Legal Proceedings
The Company is party to various lawsuits arising from time to time in the normal course of business, many of which are covered by insurance. As of December 31, 2016, the Company was not party to any material legal proceedings. Although the impact of the final resolution of these matters on the Company’s financial condition, results of operations or cash flows is not known, management does not believe that the resolution of these lawsuits will have a material adverse effect on the financial condition, results of operations or liquidity of the Company.
Item 4. Mine Safety Disclosures
Not applicable.
27
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity
Securities
Market Information
Since October 28, 2009, our common stock has been traded on the New York Stock Exchange (“NYSE”) under the trading symbol “VSI”. At December 31, 2016, there were 23,424,055 common shares outstanding, and the closing sale price of our common stock was $23.75. Also as of that date, we had approximately 189 common shareholders of record. The table below sets forth the high and low sale prices of our common stock for the periods indicated:
Fiscal period | High | Low | |||||
2016 Quarter ended: | |||||||
March | $ | 33.67 | $ | 26.02 | |||
June | 31.66 | 27.13 | |||||
September | 32.31 | 26.23 | |||||
December | 28.41 | 21.90 | |||||
2015 Quarter ended: | |||||||
March | $ | 48.85 | $ | 39.64 | |||
June | 44.54 | 37.57 | |||||
September | 38.87 | 32.73 | |||||
December | 34.41 | 26.57 | |||||
Issuer Purchases of Equity Securities
The following table summarizes the Company’s purchases of shares of common stock during the quarter ended December 31, 2016 :
Period | Total Number of Shares (or Units) Purchased (1) | Average Price Paid per Share (or Unit) | Total Number of Shares (or Units) Purchased as Part of Publicly Announced Plans or Programs (2) | Maximum Number (or Approximate Dollar Value) of Shares (or Units) that May Yet Be Purchased Under the Plans or Programs (in thousands) (2) | |||||||||
September 25, 2016 through October 22, 2016 | — | $ | — | — | $ | 40,066 | |||||||
October 23, 2016 through November 19, 2016 | — | $ | — | — | $ | 40,066 | |||||||
November 20, 2016 through December 31, 2016 | 399,546 | $ | 25.10 | 398,371 | $ | 30,066 | |||||||
Totals | 399,546 | 398,371 | |||||||||||
(1) | Includes 1,175 shares withheld to cover required tax payments on behalf of employees as their restricted shares vest. |
(2) | On August 5, 2014, May 6, 2015 and November 23, 2015, the Company’s board of directors approved share repurchase programs that enable the Company to purchase up to an aggregate of $300 million of its shares of common stock from time to time over three year periods ending on August 4, 2017, May 5, 2018 and November 22, 2018, respectively. |
Stock Performance Graph
The line graph below compares the cumulative total stockholder return on the Company’s common stock with the Russell 2000 Index (RUT), S&P Retail Index (SPXRT) and the NYSE Composite Index (NYA) for the five year period from December 31, 2011 through December 31, 2016. The graph assumes an investment of $100 made at the closing of trading on December 30, 2011, in (i) the Company’s common stock, (ii) the stocks comprising the RUT, (iii) the stocks comprising the SPXRT and (iv) the stocks comprising the NYA. All values assume reinvestment of the full amount of all dividends, if any, into
28
additional shares of the same class of equity securities at the frequency with which dividends are paid on those securities during the applicable time period.
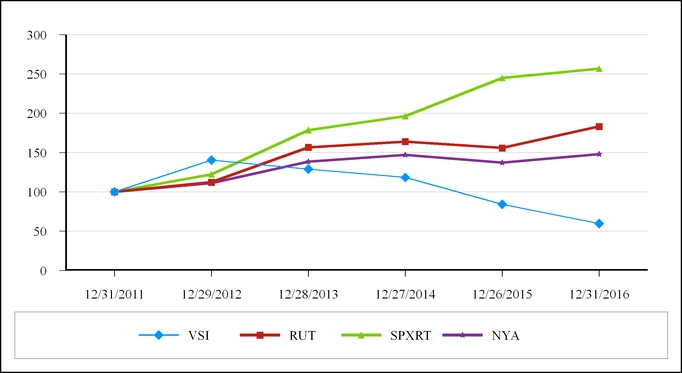
12/31/2011 | 12/29/2012 | 12/28/2013 | 12/27/2014 | 12/26/2015 | 12/31/2016 | ||||||||||||
Vitamin Shoppe, Inc. | 100.00 | 140.37 | 128.99 | 118.28 | 84.03 | 59.55 | |||||||||||
Russell 2000 Index | 100.00 | 112.31 | 156.71 | 164.01 | 155.85 | 183.17 | |||||||||||
S&P Retail Index | 100.00 | 122.23 | 178.55 | 196.28 | 244.93 | 256.69 | |||||||||||
NYSE Composite Index | 100.00 | 111.22 | 138.47 | 146.92 | 137.20 | 147.88 | |||||||||||
This graph and the accompanying table are not “soliciting material”, are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference in any filing by us under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
Share Repurchase Programs
On August 5, 2014, May 6, 2015 and November 23, 2015, the Company’s board of directors approved share repurchase programs that enable the Company to purchase up to an aggregate of $300 million of its shares of common stock from time to time over three year periods ending on August 4, 2017, May 5, 2018 and November 22, 2018, respectively. As of December 31, 2016, 8,064,325 shares have been repurchased for a total of $269.9 million. The shares were retired upon repurchase. For additional information, refer to Note 11., “Share Repurchase Program”, to our consolidated financial statements included in this Annual Report on Form 10-K.
Dividends
We have not paid cash dividends on our common stock and we do not anticipate paying any cash dividends in the foreseeable future.
Item 6. Selected Financial Data
We have derived the selected financial data presented below from our consolidated financial statements for the Fiscal Years ended December 31, 2016, December 26, 2015, December 27, 2014, December 28, 2013, and December 29, 2012.
29
Financial results for all fiscal years presented are based on a 52-week period, with the exception of financial results for the Fiscal Year ended December 31, 2016 which are based on a 53-week period, unless otherwise stated. The selected financial data for the Fiscal Years ended December 31, 2016, December 26, 2015, and December 27, 2014 presented below, should be read in conjunction with such consolidated financial statements and notes included herein and in conjunction with Item 7., “Management’s Discussion and Analysis of Financial Condition and Results of Operations”.
Fiscal Year Ended | |||||||||||||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | December 28, 2013 | December 29, 2012 | |||||||||||||||
(data presented in thousands, except for share, per share data, number of stores and average store square footage) | |||||||||||||||||||
Statement of Operations Data: | |||||||||||||||||||
Net sales | $ | 1,289,243 | $ | 1,266,549 | $ | 1,213,046 | $ | 1,087,469 | $ | 950,902 | |||||||||
Cost of goods sold | 862,887 | 847,634 | 808,787 | 709,823 | 617,920 | ||||||||||||||
Gross profit | 426,356 | 418,915 | 404,259 | 377,646 | 332,982 | ||||||||||||||
Selling, general and administrative expenses (1) | 380,779 | 329,922 | 301,603 | 267,354 | 233,610 | ||||||||||||||
Income from operations | 45,577 | 88,993 | 102,656 | 110,292 | 99,372 | ||||||||||||||
Interest expense, net | 9,523 | 1,105 | 495 | 495 | 659 | ||||||||||||||
Income before provision for income taxes | 36,054 | 87,888 | 102,161 | 109,797 | 98,713 | ||||||||||||||
Provision for income taxes | 11,090 | 34,717 | 40,920 | 43,251 | 37,888 | ||||||||||||||
Net income | $ | 24,964 | $ | 53,171 | $ | 61,241 | $ | 66,546 | $ | 60,825 | |||||||||
Weighted average shares outstanding: | |||||||||||||||||||
Basic | 23,875,540 | 28,954,804 | 30,239,183 | 29,992,620 | 29,473,711 | ||||||||||||||
Diluted | 24,067,686 | 29,203,429 | 30,664,105 | 30,541,057 | 30,110,237 | ||||||||||||||
Net income per share: | |||||||||||||||||||
Basic | $ | 1.05 | $ | 1.84 | $ | 2.03 | $ | 2.22 | $ | 2.06 | |||||||||
Diluted | $ | 1.04 | $ | 1.82 | $ | 2.00 | $ | 2.18 | $ | 2.02 | |||||||||
Other Financial Data: | |||||||||||||||||||
Depreciation and amortization of fixed and intangible assets | $ | 38,780 | $ | 38,495 | $ | 34,219 | $ | 28,026 | $ | 23,076 | |||||||||
Acquisition and integration related costs (2) | $ | — | $ | 1,874 | $ | 10,242 | $ | 4,336 | $ | 1,281 | |||||||||
Operating Data: | |||||||||||||||||||
Number of stores at end of period | 775 | 758 | 717 | 659 | 579 | ||||||||||||||
Total retail square feet at end of period | 2,709 | 2,662 | 2,568 | 2,390 | 2,130 | ||||||||||||||
Average store square footage at end of period | 3,495 | 3,511 | 3,582 | 3,627 | 3,679 | ||||||||||||||
Net sales per store (3) | $ | 1,431 | $ | 1,426 | $ | 1,453 | $ | 1,471 | $ | 1,468 | |||||||||
Comparable store sales (4) | (1.5 | )% | 0.1 | % | 2.8 | % | 3.5 | % | 8.2 | % | |||||||||
E-commerce comparable sales (5) | 3.8 | % | (0.6 | )% | 11.2 | % | 14.4 | % | na | ||||||||||
Balance Sheet Data: | |||||||||||||||||||
Working capital | $ | 151,548 | $ | 157,089 | $ | 125,382 | $ | 172,341 | $ | 153,453 | |||||||||
Total assets | 734,184 | 748,691 | 722,391 | 682,064 | 586,285 | ||||||||||||||
Total debt, including capital lease obligations | 133,371 | 123,525 | 8,195 | 347 | 168 | ||||||||||||||
Stockholders’ equity | 439,996 | 475,301 | 551,934 | 528,340 | 447,418 | ||||||||||||||
30
(1) | Fiscal 2016 includes impairment charges of $32.6 million on goodwill and $6.6 million on the customer relationships intangible asset of Nutri-Force. |
(2) | For Fiscal 2015, these amounts represent costs incurred related to the integration of Nutri-Force. In Fiscal 2014, these amounts related to acquisition costs of $3.4 million and integration costs of $1.4 million ($0.6 million for Nutri-Force and $0.8 million for Super Supplements), charges to cost of goods sold for the inventory valuation step-up of $4.5 million and the contingent consideration adjustment for the Nutri-Force acquisition of $1.0 million. In Fiscal 2013 and 2012, these amounts represent costs incurred related to the acquisition and integration of Super Supplements. |
(3) | Net sales per store are calculated by dividing retail net sales by the number of stores open at the end of the period. |
(4) | A new retail store is included in comparable store sales after 410 days of operation, and acquired retail stores from the Super Supplements acquisition are included in comparable store sales after 365 days. For Fiscal 2016, comparable store sales growth is based on a 52-week period. |
(5) | For Fiscal 2016, e-commerce comparable sales is based on a 52-week period. |
For additional information on certain costs included in our operating results, refer to Note 17., “Selected Quarterly Financial Information (unaudited)” to our consolidated financial statements included in this Annual Report on Form 10-K.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with the consolidated financial statements and notes thereto included as part of this Annual Report on Form 10-K. The discussion in this section contains forward-looking statements that are based upon current information and expectations. We sometimes identify forward-looking statements with such words as “may”, “expect”, “intend”, “anticipate”, “plan”, “believe”, “seek”, “should”, “estimate”, “outlook”, “trends”, “future benefits”, “strategies”, “goals” and similar words. The forward-looking statements contained herein, include, without limitation, statements concerning future revenue sources and concentration, gross profit margins, selling and marketing expenses, general and administrative expenses, capital resources, liquidity, capital expenditures, new stores, integration of acquisitions, retail inflation, additional financings or borrowings and are subject to risks and uncertainties including, but not limited to, those discussed below and elsewhere in this Annual Report on Form 10-K that could cause actual results to differ materially from the results contemplated by these forward-looking statements. We also urge you to carefully review the risk factors set forth in Item 1A. – “Risk Factors”. See also “Forward-Looking Statements” for additional information regarding forward-looking statements.
References to “Fiscal” or “Fiscal Year” mean the fifty-three weeks ended December 31, 2016 and the fifty-two weeks ended December 26, 2015 and December 27, 2014 for Fiscal Year 2016, Fiscal Year 2015 and Fiscal Year 2014, respectively.
Overview
We are a multi-channel specialty retailer and contract manufacturer of vitamins, minerals, herbs, specialty supplements, sports nutrition and other health and wellness products. We market approximately 900 nationally recognized brands as well as our own brands, which include The Vitamin Shoppe®, BodyTech®, True Athlete®, Mytrition®, plnt®, ProBioCare®, Next Step® and Betancourt Nutrition®. We believe we offer one of the largest varieties of products among VMS retailers and continue to refine our assortment with approximately 7,000 SKUs offered in our typical store and approximately 10,000 additional SKUs available through e-commerce. Our broad product offering enables us to provide our customers with a depth of selection of products that may not be readily available at other specialty retailers or mass merchants, such as discount stores, supermarkets, drugstores and wholesale clubs. We believe our product offering and emphasis on product knowledge and customer service helps us meet the needs of our target customer and serves as a foundation for enhancing strong customer loyalty.
During Fiscal 2015, the Company began development of a strategic plan focused on upgrading our customers’ experience across our retail and e-commerce channels, the “reinvention strategy”. The Company worked with outside consultants to analyze qualitative and quantitative information relevant to our customers’ experience. The reinvention strategy is focused on upgrading the customer experience to inspire our target customers with changes to curate our product assortment, opportunities to increase private brands penetration, enhancements to the in-store and digital experience, store layout, as well as changes to improve the effectiveness of our loyalty program. The Company incurred approximately $9.0 million of selling, general and administrative costs during Fiscal 2016 in connection with the reinvention, of which approximately $6.0 million of such costs are expected to be on-going. These costs include additional internal resources, improvements to store network connectivity, and outside consultants. The Company is in the process of testing several initiatives and expects to realize improved financial results from the reinvention beginning in Fiscal 2017. In addition, we have engaged a consulting firm in Fiscal 2016 to identify other efficiencies and cost reduction opportunities focusing on product sourcing, store operations, pricing and promotions, and corporate expenses. During Fiscal 2016, we incurred $3.8 million of costs in connection with
31
identifying other efficiencies and cost reduction opportunities. As a result of this cost reduction project, we have identified savings potential with an estimated value of at least $24.0 million on an annualized basis.
In Fiscal 2015, we also performed a review of certain business operations. As part of this review, the Company implemented changes to more closely align Super Supplements stores with current processes and assortments in the Vitamin Shoppe retail stores. As a result, net costs of $1.8 million and $1.0 million were incurred during Fiscal 2015 and Fiscal 2016, respectively. Annual cost savings resulting from these actions are estimated to be $1 million to $2 million. In addition, the Company decided to cease operations in Canada, and as a result, net costs of $0.9 million and $1.9 million were incurred in Fiscal 2015 and Fiscal 2016, respectively. In Fiscal 2016, the Company realized a $3.0 million tax benefit resulting from the write-off of the Canada investment. The annual cost savings related to ceasing operations in Canada are estimated to be approximately $1.0 million. Costs for these two initiatives include lease liabilities, markdown charges on inventory and employee severance.
On December 9, 2015, the Company issued $143.8 million of its 2.25% Convertible Senior Notes due 2020 (the “Convertible Notes”). The Convertible Notes are senior unsecured obligations of VSI. Interest is payable on the Notes on June 1 and December 1 of each year, commencing on June 1, 2016 until their maturity date of December 1, 2020. In connection with the issuance of the Convertible Notes, the Company entered into convertible note hedge transactions for which it paid an aggregate $26.4 million. In addition, the Company sold warrants for which it received aggregate proceeds of $13.0 million. The net proceeds from the Convertible Notes of $125.7 million, net of commissions and offering costs of $4.6 million, was used to repurchase shares of our common stock under the Company’s share repurchase programs. For additional information, refer to Note 8., “Credit Arrangements” and Note 11., “Share Repurchase Programs” to our consolidated financial statements included in this Annual Report on Form 10-K.
The Company’s board of directors approved share repurchase programs that enable the Company to purchase up to an aggregate of $300 million of its shares of common stock from time to time over three year periods ending on August 4, 2017, May 5, 2018 and November 22, 2018, respectively. As of December 31, 2016, 8,064,325 shares have been repurchased for a total of $269.9 million. The shares were retired upon repurchase. For additional information, refer to Note 11., “Share Repurchase Program”, to our consolidated financial statements included in this Annual Report on Form 10-K.
On June 6, 2014, the Company acquired all of the outstanding equity interests of Nutri-Force, a company which provides custom manufacturing and private labeling of vitamins, dietary supplements, nutraceuticals and nutritional supplements, as well as, develops and markets its own branded products. The total purchase price was $86.1 million in cash. For additional information, refer to Note 3., “Acquisitions”, to our consolidated financial statements included in this Annual Report on Form 10-K. During Fiscal 2015, we incurred $1.9 million of integration costs primarily related to professional fees. In addition, we incurred a $1.4 million charge in Fiscal 2015 to increase the allowance for doubtful accounts for Nutri-Force, related to one wholesale customer that abruptly ceased operations.
Since the acquisition in Fiscal 2014, Nutri-Force, our manufacturing reporting unit, has experienced disruption in its ability to optimize production capacity, volatility in sales performance, loss of third party customers, and correspondingly has experienced lower service levels to customers. In the fiscal fourth quarter, the Company performed valuation analyses, including our annual quantitative analysis of the manufacturing reporting unit as of October 22, 2016, based on the operating plan for Fiscal 2017 and then a subsequent updated long range projection due to further deterioration in operating results, which indicated that the carrying value of Nutri-Force exceeded its fair value. The Company proceeded to step two of the impairment analysis. Based on the results of these analyses, the Company recorded impairment charges of $32.6 million on goodwill and $6.6 million on the customer relationships intangible asset of Nutri-Force, which are included in selling, general and administrative expenses in the consolidated statement of income. For additional information, refer to Note 5., “Goodwill and Intangible Assets”, to our consolidated financial statements included in this Annual Report on Form 10-K.
The Company has engaged outside consultants to perform an assessment of the operations of Nutri-Force to determine the actions and resources required to turnaround this business unit. As a result, the Company will likely incur expenses, charges and capital expenditures during Fiscal 2017 related to the turnaround of Nutri-Force.
Trends and Other Factors Affecting Our Business
Our performance is affected by industry trends including, among others, demographic, health and lifestyle preferences, as well as other factors, such as industry media coverage and governmental actions. For example, our industry is subject to potential regulatory activity and other legal matters that could affect the credibility of a given product or category of products. Consumer trends, the overall impact on consumer spending, which may be affected heavily by current economic conditions, and limited product innovation and introductions in the VMS industry can dramatically affect purchasing patterns. Even though our business model allows us to respond to changing industry trends by introducing new products and adjusting our product mix and sales incentives, such actions may not offset adverse trends.
32
Additionally, our performance is affected by competitive trends such as the entry and expansion of competitors, changes in pricing and promotional strategies or expansion of product assortment by various competitors. Over recent years, there has been a shift of market share from specialty retailers to other channels such as mass market retailers, club chains, drugstore chains and e-commerce companies. This broader competitive channel availability of VMS products represents a challenge for VSI to keep pace with industry growth rates. We also have observed more competition in our assortment pricing and promotional strategy to increase our market share.
Our historical results have also been significantly influenced by our new store openings. Since the beginning of Fiscal 2014, we have opened 137 stores and as of December 31, 2016 operate 775 stores located in 45 states, the District of Columbia and Puerto Rico. As part of our reinvention strategy, we have reduced the number of planned new store openings in Fiscal 2017 as we assess the performance of recent remodeled stores.
New stores have typically required approximately four to five years to mature, generating lower store level sales in the initial years than our mature stores. As a result, new stores generally have a negative impact on our overall operating margin. In addition, our new stores since the beginning of Fiscal 2013 are approximately 2,900 square feet compared to the average of our total store portfolio of approximately 3,500 square feet. Additionally, stores opened in new markets have lower brand awareness compared to stores in existing markets, and as a result initially experience a lower sales volume than stores opened in existing markets. As these stores mature, we expect them to contribute positively to our operating results.
In the fourth quarter of 2016, the Company entered into an agreement to lease a warehousing and distribution facility in Avondale, Arizona, which we expect to open before the end of Fiscal 2017. We expect to incur approximately $16.0 million to $17.0 million of capital expenditures related to the opening of this facility. We previously utilized a third-party logistics provider to service the west coast. We believe operating our own facility will provide improved service levels and network efficiencies.
Critical Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting period. Critical accounting policies are those that are the most important portrayal of our financial condition and results of operations, and require our most difficult, subjective and complex judgments as a result of the need to make estimates about the effect of matters that are inherently uncertain. While our significant accounting policies are described in more detail in the notes to our consolidated financial statements, our most critical accounting policies, discussed below, pertain to revenue recognition, inventories, impairment of long-lived assets, and goodwill and other intangible assets. In applying such policies, we must use some amounts that are based upon our informed judgments and best estimates. Estimates, by their nature, are based on judgments and available information. The estimates that we make are based upon historical factors, current circumstances and the experience and judgment of management. We evaluate our assumptions and estimates on an ongoing basis.
Revenue Recognition. We recognize revenue upon sale of our products when merchandise is sold “at point of sale” in retail stores or upon delivery to a direct customer. Wholesale revenue is recognized when risk of loss, title and insurable risks have transferred to the customer. All revenue is recognized net of sales returns. In addition, we classify amounts billed to customers that represent shipping fees as sales. To arrive at net sales, gross sales are reduced by deferred sales, customer discounts, actual customer returns, and a provision for estimated future customer returns, which is based on management’s review of historical and current customer returns. Sales taxes collected from customers are presented on a net basis and as such are excluded from revenue.
Inventories. Inventories are stated at the lower of cost or market value. Cost is determined using the weighted average method. As applied to inventories, cost means in principle the sum of the applicable expenditures and charges directly or indirectly incurred in bringing the product to its existing condition and location. Finished goods inventory includes costs on freight on internally transferred merchandise, and costs associated with our buying department, distribution facilities, and manufacturing overhead, which are capitalized into inventory and then expensed as merchandise is sold. In addition, the cost of inventory is reduced by purchase discounts and other allowances received from certain of our vendors. We adjust our inventory to reflect situations in which the cost of inventory is not expected to be recovered. We regularly review our inventory, including when a product is close to expiration and not expected to be sold, when a product has reached its expiration date, or when a product is not expected to be saleable. In determining the reserves for these products we consider factors such as the amount of inventory on hand and its remaining shelf life, and current and expected market conditions, including management forecasts and levels of competition. In addition, we have established a reserve for estimated inventory shrinkage between physical inventories. Physical inventories and cycle counts are taken on a regular basis, and inventory is adjusted accordingly. For each
33
reporting period, we estimate inventory shrinkage based on a historical trend analysis. We have evaluated the current level of inventory considering historical trends and other factors, and based on our evaluation, have recorded adjustments to reflect inventory at net realizable value. These adjustments are estimates, which could vary significantly from actual results if future economic conditions, customer demand or competition differ from expectations. These estimates require us to make assessments about the future demand for our products in order to identify such inventory items as slow moving, expiring, obsolete or in excess of need. These future estimates are subject to the ongoing accuracy of management’s forecasts of market conditions, industry trends and competition. We are also subject to volatile changes in specific product demand as a result of unfavorable publicity, government regulation and rapid changes in demand for new and improved products or services. Inventory reserves were $8.6 million and $7.3 million at December 31, 2016 and December 26, 2015, respectively.
Long-Lived Assets. We evaluate long-lived assets, including fixed assets and intangible assets with finite useful lives, periodically for impairment whenever events or changes in circumstances indicate that the carrying amount of any such asset may not be recoverable. If the sum of our estimated undiscounted future cash flows is less than the asset’s carrying value, we recognize an impairment loss, measured as the amount by which the carrying value exceeds the fair value of the asset. These estimates of cash flow require significant management judgment and certain assumptions about future sales and expense growth rates, devaluation and inflation. As such, these estimates may differ from actual cash flows. The Company recognized impairment charges of $0.8 million during Fiscal 2016 on fixed assets related to five of its underperforming retail locations still in use in the Company's operations. The Company recognized impairment charges of $1.2 million during Fiscal 2015 on fixed assets related to five of its underperforming retail locations, three of which are still in use in the Company’s operations, and three retail locations in Ontario, Canada which the Company closed during Fiscal 2016. The Company recognized impairment charges of $0.4 million during Fiscal 2014 on fixed assets related to three of its underperforming retail locations, two of which are still in use in the Company’s operations. Impairment charges are included in selling, general and administrative expenses in the consolidated statements of income.
Goodwill and Other Intangible Assets. On an annual basis, or whenever impairment indicators exist, we perform an evaluation of goodwill and indefinite-lived intangible assets. In the absence of any impairment indicators, goodwill and other indefinite-lived intangible assets are tested in the fourth quarter of each fiscal year. With regards to goodwill, our evaluations are based on our three reporting units. The evaluations of goodwill and indefinite-lived intangible assets may first consider qualitative factors to determine whether the existence of events or circumstances leads to a determination that it is more likely than not that the fair value of a reporting unit or indefinite-lived intangible asset is less than its carrying value. A quantitative evaluation is performed if the qualitative evaluation results in a more likely than not determination or if a qualitative evaluation is not performed. Our quantitative evaluation for goodwill utilizes the discounted cash flow method, based on operating projections, as well as the market multiples method. For indefinite-lived tradenames, we utilize the royalty relief method in our quantitative evaluations. For those intangible assets which have definite lives, we amortize their cost on a straight-line basis over their estimated useful lives, the periods of which vary based on their particular contractual terms.
Our annual impairment review requires extensive use of accounting judgment and financial estimates. Judgments regarding the existence of impairment indicators are based on market conditions and operational performance of the business. Future events could cause us to conclude that impairment indicators exist, and therefore that goodwill and other intangible assets may be impaired. The valuation of goodwill and indefinite-lived intangible assets is affected by, among other things, our business plan for the future and estimated results of future operations. Changes in the business plan, operating results, or application of alternative assumptions that are different than the estimates used to develop the valuation of the assets may materially impact their valuation.
In Fiscal 2016, the Company performed a quantitative analysis of its retail and direct reporting units and determined that the fair value of these reporting units was greater that their respective carrying values. As a result, the Company believes the fair values of each of these reporting units and indefinite-lived tradenames substantially exceeds their respective carrying values.
Since the acquisition in Fiscal 2014, Nutri-Force, our manufacturing reporting unit, has experienced disruption in its ability to optimize production capacity, volatility in sales performance, loss of third party customers, and correspondingly has experienced lower service levels to customers. Based upon the operating results of Nutri-Force during the three months ended June 25, 2016, we concluded that an impairment trigger occurred for the manufacturing reporting unit and therefore an impairment test was performed. A discounted cash flow model was prepared using the internal forecast, including an estimate of long-term future growth rates and a discount rate determined by management to be commensurate with the risk inherent in this forecast. The results of this analysis determined the fair value of the manufacturing reporting unit exceeded its carrying value, and as a result, we concluded the goodwill assigned to the reporting unit was not impaired. However, the fair value of the manufacturing reporting unit exceeded its carrying value by approximately 5%, which was not considered to be a substantial excess over the carrying value. While financial results during the three months ended September 24, 2016 did not improve, we continued to closely monitor the financial performance of Nutri-Force. During the fiscal fourth quarter, the performance of
34
Nutri-Force declined and expectations of future years were reduced significantly due to on-going churn in third party customers and its inability to reduce costs. In the fiscal fourth quarter, the Company performed valuation analyses, including our annual quantitative analysis of the manufacturing reporting unit as of October 22, 2016, based on the operating plan for Fiscal 2017 and then a subsequent updated long range projection due to further deterioration in operating results, which indicated that the carrying value of Nutri-Force exceeded its fair value. The Company proceeded to step two of the impairment analysis. Based on the results of these analyses, the Company recorded impairment charges of $32.6 million on goodwill and $6.6 million on the customer relationships intangible asset of Nutri-Force, which are included in selling, general and administrative expenses in the consolidated statement of income.
The Company has engaged outside consultants to perform an assessment of the operations of Nutri-Force to determine the actions and resources required to turnaround this business unit. As a result, the Company will likely incur expenses, charges and capital expenditures during Fiscal 2017 related to the turnaround of Nutri-Force.
General Definitions for Operating Results
Net Sales consist of sales, net of sales returns, deferred sales, customer incentives and a provision for estimated future returns. Total comparable net sales include sales generated by retail stores and e-commerce sales in both reporting periods. Sales generated by retail stores after 410 days of operation and sales generated by acquired retail stores from the Super Supplements acquisition after 365 days are included in comparable net sales. Sales to third parties of manufactured products generated by Nutri-Force are considered non-comparable sales.
Cost of goods sold includes the cost of inventory sold, costs of warehousing, distribution, manufacturing and store occupancy costs and excludes depreciation and amortization related to the retail and direct segments that is included within selling, general and administrative expenses. Warehousing, distribution and manufacturing costs, which are capitalized into inventory and then expensed as merchandise is sold, include freight to transfer merchandise, costs associated with our buying department, distribution facilities and manufacturing overhead. Store occupancy costs include rent, common area maintenance, real estate taxes and utilities.
Gross profit is net sales minus cost of goods sold.
Selling, general and administrative expenses consist of depreciation and amortization of fixed and intangible assets, operating payroll and related benefits, advertising and promotion expense, and other selling, general and administrative expenses.
Income from operations consists of gross profit minus selling, general and administrative expenses.
Interest expense, net includes interest on our Convertible Notes and Revolving Credit Facility, letters of credit fees, interest on our capital leases, as well as amortization of financing costs, reduced by interest income earned from highly liquid investments (investments purchased with an original maturity of three months or less).
Key Performance Indicators and Statistics
We use a number of key indicators of financial condition and operating results to evaluate the performance of our business, including the following (in thousands):
Fiscal Year Ended | |||||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | |||||||||
Net sales | $ | 1,289,243 | $ | 1,266,549 | $ | 1,213,046 | |||||
Increase (Decrease) in total comparable net sales (1) | (0.9 | )% | — | % | 3.7 | % | |||||
Increase (Decrease) in comparable store net sales | (1.5 | )% | 0.1 | % | 2.8 | % | |||||
Increase (Decrease) in e-commerce comparable net sales | 3.8 | % | (0.6 | )% | 11.2 | % | |||||
Gross profit as a percent of net sales | 33.1 | % | 33.1 | % | 33.3 | % | |||||
Income from operations | $ | 45,577 | $ | 88,993 | $ | 102,656 | |||||
(1) | Total comparable net sales are comprised of comparable retail store sales and e-commerce sales. |
35
The following table shows the growth in our network of stores for Fiscal 2016, 2015 and 2014:
Fiscal Year | ||||||||
2016 | 2015 | 2014 | ||||||
Stores open at beginning of year | 758 | 717 | 659 | |||||
Stores opened | 26 | 50 | 61 | |||||
Stores closed | (9 | ) | (9 | ) | (3 | ) | ||
Stores open at end of year | 775 | 758 | 717 | |||||
Results of Operations
The information presented below is for the Fiscal years ended December 31, 2016, December 26, 2015 and December 27, 2014 and was derived from our audited consolidated financial statements, which, in the opinion of management, includes all adjustments necessary for a fair presentation of our financial position and operating results for such periods and as of such dates. The following table summarizes our results of operations for the Fiscal years ended December 31, 2016, December 26, 2015 and December 27, 2014 as a percentage of net sales:
Fiscal Year Ended | ||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | ||||||
Net sales | 100.0 | % | 100.0 | % | 100.0 | % | ||
Cost of goods sold | 66.9 | % | 66.9 | % | 66.7 | % | ||
Gross profit | 33.1 | % | 33.1 | % | 33.3 | % | ||
Selling, general and administrative expenses | 29.5 | % | 26.0 | % | 24.9 | % | ||
Income from operations | 3.5 | % | 7.0 | % | 8.5 | % | ||
Interest expense, net | 0.7 | % | 0.1 | % | — | % | ||
Income before provision for income taxes | 2.8 | % | 6.9 | % | 8.4 | % | ||
Provision for income taxes | 0.9 | % | 2.7 | % | 3.4 | % | ||
Net income | 1.9 | % | 4.2 | % | 5.0 | % | ||
Figures may not sum due to rounding.
The results of operations presented for the Fiscal year ended December 31, 2016 are based on a 53-week period ("Fiscal 2016"). The results of operations presented for the Fiscal years ended December 26, 2015 and December 27, 2014 are each based on a 52-week period ("Fiscal 2015" and "Fiscal 2014").
Fiscal 2016 Compared to Fiscal 2015
2016 Financial and Operating Highlights:
• | Net sales increased 1.8% |
• | Total comparable net sales decreased 0.9% |
• | Opened 26 retail stores |
• | Transformed 4 stores into new reinvented stores |
• | Launched new responsive website |
• | Repurchased 2.6 million common shares for a total of $66.0 million |
• | Impairment charges of $39.2 million on Nutri-Force goodwill and intangible asset |
• | Fully diluted earnings per share of $1.04 |
Outlook for 2017, management expects:
• | Total comparable net sales growth of flat to low single digit negative |
• | To open approximately 15 new stores |
• | To remodel 10 to 15 stores into new format |
• | Fully diluted earnings per share in the range of $1.95 to $2.20. This excludes any potential charges associated with the implementation of strategic initiatives to improve performance at Nutri-Force. |
• | Capital expenditures of approximately $45 million |
36
The following tables summarize our results of operations for Fiscal 2016 and Fiscal 2015 (in thousands):
Fiscal Years Ended | ||||||||||||||
December 31, 2016 | December 26, 2015 | $ Change | % Change | |||||||||||
Net sales | $ | 1,289,243 | $ | 1,266,549 | $ | 22,694 | 1.8 | % | ||||||
Cost of goods sold | 862,887 | 847,634 | 15,253 | 1.8 | % | |||||||||
Cost of goods sold as % of net sales | 66.9 | % | 66.9 | % | ||||||||||
Gross profit | 426,356 | 418,915 | 7,441 | 1.8 | % | |||||||||
Gross profit as % of net sales | 33.1 | % | 33.1 | % | ||||||||||
Selling, general and administrative expenses | 380,779 | 329,922 | 50,857 | 15.4 | % | |||||||||
SG&A expenses as % of net sales | 29.5 | % | 26.0 | % | ||||||||||
Income from operations | 45,577 | 88,993 | (43,416 | ) | (48.8 | )% | ||||||||
Income from operations as % of net sales | 3.5 | % | 7.0 | % | ||||||||||
Interest expense, net | 9,523 | 1,105 | 8,418 | 761.8 | % | |||||||||
Income before provision for income taxes | 36,054 | 87,888 | (51,834 | ) | (59.0 | )% | ||||||||
Provision for income taxes | 11,090 | 34,717 | (23,627 | ) | (68.1 | )% | ||||||||
Net income | $ | 24,964 | $ | 53,171 | $ | (28,207 | ) | (53.0 | )% | |||||
Net Sales
The increase in net sales was primarily the result of our 53rd week sales of $20.2 million. On a 52 week basis, increases in specialty supplements product categories of $13.9 million and in vitamins, minerals, herbs and homeopathy product categories of $12.9 million were offset by decreases in sports nutrition product categories of $19.1 million and Nutri-Force net merchandise sales to third parties of $7.2 million.
Net sales for our three business segments, as well as a discussion of the changes in each segment’s net sales from the comparable prior year period, are provided below (in thousands):
Fiscal Years Ended | ||||||||||||||
December 31, 2016 | December 26, 2015 | $ Change | % Change | |||||||||||
Net Sales: | ||||||||||||||
Retail (a) | $ | 1,109,202 | $ | 1,081,123 | $ | 28,079 | 2.6 | % | ||||||
Direct (b) | 130,024 | 128,825 | 1,199 | 0.9 | % | |||||||||
Manufacturing (c) | 87,684 | 91,159 | (3,475 | ) | (3.8 | )% | ||||||||
Segment net sales | 1,326,910 | 1,301,107 | 25,803 | 2.0 | % | |||||||||
Elimination of intersegment revenues | (37,667 | ) | (34,558 | ) | (3,109 | ) | 9.0 | % | ||||||
Total net sales | $ | 1,289,243 | $ | 1,266,549 | $ | 22,694 | 1.8 | % | ||||||
(a) | The change in retail sales resulted from an increase in non-comparable store sales of $25.4 million and retail sales in the 53rd week of $18.2 million partially offset by a decrease in comparable store sales of $15.5 million, or 1.5%. The decrease in comparable store sales was driven by a decline in average transaction value and lower customer traffic. |
(b) | Direct sales increased primarily due to an increase in comparable e-commerce sales of $4.7 million, or 3.8%, and direct sales in the 53rd week of $1.4 million, offset by a decrease in non-comparable e-commerce sales and lower catalog sales totaling $5.0 million. The increase in comparable e-commerce sales was primarily due to effective customer acquisition and promotional activities. The decrease in non-comparable e-commerce sales was primarily due to the discontinuation of the Super Supplements website and catalog. |
(c) | Manufacturing sales reflect a decrease in product manufactured for third parties of $6.6 million partially offset by an increase of $3.1 million in product manufactured for the Vitamin Shoppe assortment. Manufacturing sales in the 53rd week were $1.2 million of which $0.6 million was product manufactured for the Vitamin Shoppe assortment and $0.6 million was product manufactured for third parties. |
37
Cost of Goods Sold
The dollar increase of cost of goods sold was primarily due to the increase in sales resulting from the 53rd week. Cost of goods sold as a percentage of net sales was flat. Improvement in product margin of 0.5% was offset by 0.3% related to Nutri-Force and 0.2% of deleverage of retail occupancy costs. Cost of goods sold for Fiscal 2016 includes $0.4 million related to Super Supplements conversion costs and Canada stores closing costs and for Fiscal 2015 includes a $1.3 million charge for the write-off of USPlabs® products which the Company ceased selling.
Selling, General and Administrative Expenses
Fiscal Years Ended | ||||||||||||||
December 31, 2016 | December 26, 2015 | $ Change | % Change | |||||||||||
SG&A Expenses (in thousands): | ||||||||||||||
Store Payroll and Benefits (a) | $ | 135,722 | $ | 128,217 | $ | 7,505 | 5.9 | % | ||||||
Store Payroll & benefit as % of net sales | 10.5 | % | 10.1 | % | ||||||||||
Advertising and Promotion (b) | 21,897 | 21,621 | 276 | 1.3 | % | |||||||||
Advertising & promotion as % of net sales | 1.7 | % | 1.7 | % | ||||||||||
Other SG&A (c) | 223,160 | 180,084 | 43,076 | 23.9 | % | |||||||||
Other SG&A as % of net sales | 17.3 | % | 14.2 | % | ||||||||||
Total SG&A Expenses | $ | 380,779 | $ | 329,922 | $ | 50,857 | 15.4 | % | ||||||
(a) | Store payroll and benefits increased primarily due to the increase in head count added to operate new stores and an increase in the average wage rates. |
(b) | Advertising and promotion as a percentage of net sales was flat. Higher retail expenditures and digital advertising was substantially offset by lower expenditures related to Nutri-Force. |
(c) | Other selling, general and administrative expenses increased primarily due to impairment charges of $32.6 million on goodwill and $6.6 million on the customer relationships intangible asset of Nutri-Force. Excluding these impairment charges, other selling, general and administrative expenses increased by $3.8 million. |
Income from Operations
Operating income (loss) for our three business segments are provided below (in thousands):
Fiscal Years Ended | ||||||||||||||
December 31, 2016 | December 26, 2015 | $ Change | % Change | |||||||||||
Income (Loss) from operations: | ||||||||||||||
Retail (a) | $ | 197,450 | $ | 192,598 | $ | 4,852 | 2.5 | % | ||||||
% of net sales | 17.8 | % | 17.8 | % | ||||||||||
Direct (b) | 18,737 | 20,904 | (2,167 | ) | (10.4 | )% | ||||||||
% of net sales | 14.4 | % | 16.2 | % | ||||||||||
Manufacturing (c) | (44,223 | ) | (1,977 | ) | (42,246 | ) | 2,136.9 | % | ||||||
% of net sales | (50.4 | )% | (2.2 | )% | ||||||||||
Corporate costs (d) | (126,387 | ) | (122,532 | ) | (3,855 | ) | 3.1 | % | ||||||
% of net sales | (9.8 | )% | (9.7 | )% | ||||||||||
Income from operations | $ | 45,577 | $ | 88,993 | $ | (43,416 | ) | (48.8 | )% | |||||
(a) | Retail income from operations as a percentage of net sales was flat. A 0.8% improvement in product margin was offset by 0.4% from store payroll and benefits costs, 0.2% related to occupancy costs and 0.2% from supply chain costs. |
(b) | The decrease in direct income from operations as a percentage of net sales is primarily due to a reduction in operating margin generated by on-line marketplaces and from an increase in promotional pricing and delivery expense. |
(c) | The year ended December 31, 2016 includes impairment charges of $32.6 million on goodwill and $6.6 million on the customer relationships intangible asset of Nutri-Force. In addition, the manufacturing segment recognized an increase in |
38
costs as compared to the prior year due to operational inefficiencies. The year ended December 26, 2015 includes a $1.4 million charge for accounts receivable for one wholesale customer which were deemed uncollectible.
(d) | The increase in corporate costs is primarily due to costs incurred in connection with the reinvention, including outside consultants. |
Provision for Income Taxes
The effective tax rate for Fiscal 2016 was 30.8%, compared to 39.5% for Fiscal 2015. The effective tax rate decreased primarily due to a $3.0 million tax benefit resulting from the write-off of the Canada investment.
Fiscal 2015 Compared To Fiscal 2014
The following tables summarize our results of operations for Fiscal 2015 and Fiscal 2014 (in thousands):
Fiscal Years Ended | ||||||||||||||
December 26, 2015 | December 27, 2014 | $ Change | % Change | |||||||||||
Net sales | $ | 1,266,549 | $ | 1,213,046 | $ | 53,503 | 4.4 | % | ||||||
Cost of goods sold | 847,634 | 808,787 | 38,847 | 4.8 | % | |||||||||
Cost of goods sold as % of net sales | 66.9 | % | 66.7 | % | ||||||||||
Gross profit | 418,915 | 404,259 | 14,656 | 3.6 | % | |||||||||
Gross profit as % of net sales | 33.1 | % | 33.3 | % | ||||||||||
Selling, general and administrative expenses | 329,922 | 301,603 | 28,319 | 9.4 | % | |||||||||
SG&A expenses as % of net sales | 26.0 | % | 24.9 | % | ||||||||||
Income from operations | 88,993 | 102,656 | (13,663 | ) | (13.3 | )% | ||||||||
Income from operations as % of net sales | 7.0 | % | 8.5 | % | ||||||||||
Interest expense, net | 1,105 | 495 | 610 | 123.2 | % | |||||||||
Income before provision for income taxes | 87,888 | 102,161 | (14,273 | ) | (14.0 | )% | ||||||||
Provision for income taxes | 34,717 | 40,920 | (6,203 | ) | (15.2 | )% | ||||||||
Net income | $ | 53,171 | $ | 61,241 | $ | (8,070 | ) | (13.2 | )% | |||||
The results of Nutri-Force, included in the Company’s results of operations, reflect a full year for Fiscal 2015 and the period from June 6, 2014 through December 27, 2014 for Fiscal 2014.
Net Sales
The increase in net sales was the result of an increase in our total non-comparable net sales of $53.8 million, which includes an increase in Nutri-Force net sales of $16.3 million to third parties. Sales increased $24.0 million in the Other
product category (which includes on the go bars, drinks and snacks, as well as natural beauty and personal care products).
Sales in the Sports Nutrition category (which includes sports and performance nutrition and weight management products)
were relatively flat with the increase in sales of sports and performance nutrition products substantially offset by the decrease
in sales of weight management products. In addition, the growth rate in sales of sports and performance nutrition products is
below historical trends.
39
Net sales for our three business segments, as well as a discussion of the changes in each segment’s net sales from the comparable prior year period, are provided below (in thousands):
Fiscal Years Ended | ||||||||||||||
December 26, 2015 | December 27, 2014 | $ Change | % Change | |||||||||||
Net Sales: | ||||||||||||||
Retail (a) | $ | 1,081,123 | $ | 1,042,054 | $ | 39,069 | 3.7 | % | ||||||
Direct (b) | 128,825 | 130,644 | (1,819 | ) | (1.4 | )% | ||||||||
Manufacturing (c) | 91,159 | 48,102 | 43,057 | 89.5 | % | |||||||||
Segment net sales | 1,301,107 | 1,220,800 | 80,307 | 6.6 | % | |||||||||
Elimination of intersegment revenues | (34,558 | ) | (7,754 | ) | (26,804 | ) | 345.7 | % | ||||||
Total net sales | $ | 1,266,549 | $ | 1,213,046 | $ | 53,503 | 4.4 | % | ||||||
(a) | The change in retail sales resulted from an increase in non-comparable store sales of $38.5 million and in comparable store sales of $0.6 million, or 0.1%. The increase in comparable store sales was driven by average transaction value substantially offset by a decrease in customer traffic. |
(b) | Direct sales declined due to a decrease in catalog sales of $1.0 million and a decrease in e-commerce sales of $0.8 million, or 0.6%. |
(c) | Manufacturing sales reflect an increase of $26.8 million in product manufactured for the Vitamin Shoppe assortment and an increase of $16.3 million in product manufactured for third parties. |
Cost of Goods Sold
The dollar increase of cost of goods sold was primarily due to an increase in sales. The increase of cost of goods
sold as a percentage of net sales was primarily due to 0.5% of de-leverage of retail occupancy costs partially offset by 0.2%
related to Nutri-Force. Cost of goods sold for Fiscal 2015 includes a $1.3 million charge for the write-off of USPlabs® products which the Company ceased selling and for Fiscal 2014 includes a $4.5 million charge from adjusting Nutri-Force inventory to fair value as part of purchase accounting.
Selling, General and Administrative Expenses
Fiscal Years Ended | ||||||||||||||
December 26, 2015 | December 27, 2014 | $ Change | % Change | |||||||||||
SG&A Expenses (in thousands): | ||||||||||||||
Store Payroll and Benefits (a) | $ | 128,217 | $ | 119,499 | $ | 8,718 | 7.3 | % | ||||||
Store Payroll & benefit as % of net sales | 10.1 | % | 9.9 | % | ||||||||||
Advertising and Promotion (b) | 21,621 | 19,290 | 2,331 | 12.1 | % | |||||||||
Advertising & promotion as % of net sales | 1.7 | % | 1.6 | % | ||||||||||
Other SG&A (c) | 180,084 | 162,814 | 17,270 | 10.6 | % | |||||||||
Other SG&A as % of net sales | 14.2 | % | 13.4 | % | ||||||||||
Total SG&A Expenses | $ | 329,922 | $ | 301,603 | $ | 28,319 | 9.4 | % | ||||||
(a) | Store payroll and benefits increased primarily due to the increase in head count added to operate new stores and higher medical benefits costs. |
(b) | Advertising and promotion increased with the addition of Nutri-Force of $1.7 million and an increase in digital advertising of $0.8 million partially offset by lower retail expenditures of $0.2 million. |
(c) | Other SG&A expenses include an increase in costs related to Nutri-Force of $5.8 million and increased depreciation and amortization expenses of $4.0 million. In addition, other SG&A increased as a result of management realignment charges of $3.4 million, reinvention costs of $2.7 million, a charge to increase the allowance for doubtful accounts for Nutri-Force of $1.4 million and a net reduction in acquisition related costs of $2.9 million. |
40
Income from Operations
Operating income (loss) for our three business segments are provided below (in thousands):
Fiscal Years Ended | ||||||||||||||
December 26, 2015 | December 27, 2014 | $ Change | % Change | |||||||||||
Income (Loss) from operations: | ||||||||||||||
Retail (a) | $ | 192,598 | $ | 194,864 | $ | (2,266 | ) | (1.2 | )% | |||||
% of net sales | 17.8 | % | 18.7 | % | ||||||||||
Direct (b) | 20,904 | 22,755 | (1,851 | ) | (8.1 | )% | ||||||||
% of net sales | 16.2 | % | 17.4 | % | ||||||||||
Manufacturing (c) | (1,977 | ) | (1,830 | ) | (147 | ) | 8.0 | % | ||||||
% of net sales | (2.2 | )% | (3.8 | )% | ||||||||||
Corporate costs (d) | (122,532 | ) | (113,133 | ) | (9,399 | ) | 8.3 | % | ||||||
% of net sales | (9.7 | )% | (9.3 | )% | ||||||||||
Income from operations | $ | 88,993 | $ | 102,656 | $ | (13,663 | ) | (13.3 | )% | |||||
(a) | Decrease in retail income from operations as a percentage of net sales is due to 0.5% related to occupancy costs and 0.4% from payroll and benefits costs. |
(b) | Decrease in direct income from operations as a percentage of net sales is due to 0.7% related to advertising and promotion expenses and 0.4% related to product margin. |
(c) | During the period ended December 26, 2015, the manufacturing segment recognized an increase in costs as compared to the prior year due to operational inefficiencies, and includes a $1.4 million charge for accounts receivable for one wholesale customer which were deemed uncollectible. The period ended December 27, 2014 includes a $4.5 million charge from adjusting Nutri-Force inventory to fair value as part of purchase accounting. |
(d) | The increase in corporate costs includes an increase in depreciation and amortization expenses of $4.0 million. In addition, corporate costs increased as a result of management realignment charges of $3.4 million, reinvention costs of $2.7 million and a net reduction in acquisition related costs of $2.9 million. |
In addition to the items noted above, income from operations in Fiscal 2015 includes $1.8 million of Super Supplements conversion costs and $0.9 million of closing costs for the stores in Canada.
Provision for Income Taxes
The effective tax rate for Fiscal 2015 was 39.5%, compared to 40.1% for Fiscal 2014. The effective tax rate
decreased primarily due to a decrease in permanent non-deductible items during Fiscal 2015 as compared to Fiscal 2014.
Key Indicators of Liquidity and Capital Resources
The following table provides key indicators of our liquidity and capital resources (in thousands):
December 31, 2016 | December 26, 2015 | ||||||
Balance Sheet Data: | |||||||
Cash and cash equivalents | $ | 2,833 | $ | 15,104 | |||
Working capital (a) | 151,548 | 157,089 | |||||
Total assets | 734,184 | 748,691 | |||||
Total debt (b) | 133,371 | 123,525 | |||||
(a) Working capital is total current assets minus total current liabilities.
(b) Total debt includes the outstanding balance on the Company's Revolving Credit Facility, the net balance of its Convertible Notes and its capital lease obligations.
41
Fiscal Year Ended | |||||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | |||||||||
Other Information: | |||||||||||
Depreciation and amortization of fixed and intangible assets | $ | 38,780 | $ | 38,495 | $ | 34,219 | |||||
Cash Flows Provided By (Used In): | |||||||||||
Operating activities | $ | 93,373 | $ | 60,667 | $ | 100,147 | |||||
Investing activities | (40,359 | ) | (39,430 | ) | (125,184 | ) | |||||
Financing activities | (65,304 | ) | (18,428 | ) | (36,877 | ) | |||||
Effect of exchange rate changes on cash and cash equivalents | 19 | 129 | 44 | ||||||||
Net (decrease) increase in cash and cash equivalents | $ | (12,271 | ) | $ | 2,938 | $ | (61,870 | ) | |||
Liquidity and Capital Resources
Our primary uses of cash have been to fund working capital, operating expenses and capital expenditures related primarily to the build-out of new stores, the remodeling of existing stores and information technology investments as well as to repurchase shares of our common stock. Historically, we have financed our requirements predominately through internally generated cash flow, supplemented with short-term financing. In Fiscal 2015, we issued $143.8 million of Convertible Notes to fund the repurchase of shares of our common stock. Refer to Note 8., “Credit Arrangements”, to our consolidated financial statements included in this Annual Report on Form 10-K for additional information. We believe that the cash generated by operations and cash and cash equivalents, together with the borrowing availability under our Revolving Credit Facility, will be sufficient to meet our working capital needs for the next twelve months, our store growth plans, costs and investments related to our reinvention strategy, systems development, store improvements, the opening of our new distribution center and interest payments on the Convertible Notes, as well as the repurchase of shares of our common stock and our Convertible Notes from time to time in negotiated or open market transactions subject to market conditions.
We purchased $66.0 million of common stock under our $300.0 million share repurchase programs during Fiscal 2016. Refer to Note 11., “Share Repurchase Programs”, to our consolidated financial statements included in this Annual Report on Form 10-K for additional information. We invested $40.1 million in capital expenditures during Fiscal 2016, including costs for building new stores, remodeling existing stores, information technology and investments resulting from our reinvention strategy. During Fiscal 2017 we plan to spend approximately $45 million in capital expenditures, including costs for building new stores, remodeling existing stores, information technology, the opening of our new distribution center and investments resulting from our reinvention strategy. We opened 26 new stores and closed 9 stores during Fiscal 2016. We plan to open approximately 15 new stores in Fiscal 2017. Our working capital requirements for merchandise inventory will continue to increase as we continue to open additional stores. Currently, our practice is to establish an inventory level of approximately $145,000 at cost for each of our stores, the cost of which is partially offset by vendor incentive and allowance programs. Additionally, 30 day payment terms have been extended to us by some of our suppliers allowing us to effectively manage our inventory and working capital.
The Company is subject to concentrations of credit risk associated with cash and cash equivalents, and at times holds cash balances in excess of Federal Deposit Insurance Corporation limits. Currently, the Company’s cash management practice is to hold cash balances in quality institutions and invest in highly liquid and secure investments.
We were in compliance with all covenants relating to our Revolving Credit Facility and Convertible Notes as of December 31, 2016. We expect to be in compliance with these same covenants during Fiscal 2017 as well.
Cash Provided by Operating Activities
Net cash provided by operating activities was $93.4 million and $60.7 million during Fiscal 2016 and Fiscal 2015, respectively. The $32.7 million increase in net cash flows from operating activities is primarily due to the timing of accounts payable disbursements and an increase in inventory purchases in Fiscal 2015 related primarily to the transition of Vitamin Shoppe production of private brands to Nutri-Force and the opening of new stores.
Net cash provided by operating activities was $60.7 million and $100.1 million during Fiscal 2015 and Fiscal 2014, respectively. The $39.5 million decrease in net cash flows from operating activities is primarily due to an increase in inventory purchases to support activities including the transition to a new third-party warehouse and the transition of products to our manufacturing facility.
42
Cash Used in Investing Activities
Net cash used in investing activities was $40.4 million during Fiscal 2016 as compared to $39.4 million during Fiscal 2015. Capital expenditures during Fiscal 2016 and 2015 were used primarily for the build-out of new stores, the remodeling of existing stores and information technology investments.
Net cash used in investing activities was $39.4 million during Fiscal 2015 as compared to $125.2 million during Fiscal 2014. The $85.8 million decrease in cash used in investing activities is primarily due to the $81.5 million for the acquisition of Nutri-Force in Fiscal 2014.
Cash Used in Financing Activities
Net cash used in financing activities was $65.3 million in Fiscal 2016 as compared to $18.4 million in Fiscal 2015. The $46.9 million increase in cash used in financing activities was primarily due to purchases of common stock under the Company’s share repurchase programs of $66.0 million in Fiscal 2016 and $146.1 million in Fiscal 2015 partially offset by the net proceeds from the issuance of Convertible Notes of $125.7 million in Fiscal 2015.
Net cash used in financing activities was $18.4 million in Fiscal 2015 as compared to $36.9 million in Fiscal 2014. The $18.4 million decrease in cash used in financing activities was primarily due to purchases of common stock under the
Company’s share repurchase programs of $146.1 million in Fiscal 2015 and $57.8 million in Fiscal 2014 partially offset by
the net proceeds from the issuance of Convertible Notes of $125.7 million in Fiscal 2015 and by net borrowings under the
Company’s Revolving Credit Facility of $8.0 million in Fiscal 2014. In addition, proceeds from exercises of stock options
decreased $8.0 million in Fiscal 2015 as compared to Fiscal 2014.
Revolving Credit Facility
The terms of our Revolving Credit Facility extend through October 11, 2018, and allow the Company to borrow up to $90.0 million, subject to the terms of the facility, with a Company option to increase the facility up to a total of $150.0 million. For information regarding the terms of our Revolving Credit Facility, refer to Note 8., “Credit Arrangements”, to our consolidated financial statements included in this Annual Report on Form 10-K. As of December 31, 2016, the Company had $11.0 million of borrowings outstanding on its Revolving Credit Facility. The largest amount borrowed at any given point during Fiscal 2016 was $27.0 million. The unused available line of credit under the Revolving Credit Facility at December 31, 2016 was $76.1 million.
Convertible Notes
On December 9, 2015, the Company issued $143.8 million of its 2.25% Convertible Notes. The Convertible Notes are senior unsecured obligations of VSI. Interest is payable on the Convertible Notes on June 1 and December 1 of each year, commencing on June 1, 2016 until their maturity date of December 1, 2020. For additional information regarding the terms of our Convertible Notes, refer to Note 8., “Credit Arrangements”, to our consolidated financial statements included in this Annual Report on Form 10-K.
Contractual Obligations and Commercial Commitments
As of December 31, 2016, our lease commitments and contractual obligations were as follows (in thousands):
Fiscal year ending | Total | Operating Leases Real Estate (1) | Convertible Notes | Interest on Convertible Notes | Operating Leases Equipment | Capital Lease Obligations | ||||||||||||||||||
2017 | $ | 126,087 | $ | 122,219 | $ | — | $ | 3,234 | $ | 254 | $ | 380 | ||||||||||||
2018 | 116,261 | 112,672 | — | 3,234 | — | 355 | ||||||||||||||||||
2019 | 97,475 | 93,886 | — | 3,234 | — | 355 | ||||||||||||||||||
2020 | 225,403 | 78,064 | 143,750 | 3,234 | — | 355 | ||||||||||||||||||
2021 | 65,841 | 65,630 | — | — | — | 211 | ||||||||||||||||||
Thereafter | 175,018 | 175,018 | — | — | — | — | ||||||||||||||||||
$ | 806,085 | $ | 647,489 | $ | 143,750 | $ | 12,936 | $ | 254 | $ | 1,656 | |||||||||||||
43
(1) | Store operating leases included in the above table do not include contingent rent based upon sales volume. Operating leases do not include common area maintenance costs or real estate taxes that are paid to the landlord during the year, which combined represented approximately 18.8% of our minimum lease obligations for Fiscal 2016. |
We are not party to any long-term purchase commitments. Our typical merchandise purchase orders are generally performed upon within a four to six week period. However, as of December 31, 2016, we have an obligation, excluded from the above commitments, of approximately $12.8 million to purchase an agreed upon supply of our own branded merchandise and raw materials during Fiscal 2017 which has been produced by, and resides with, the applicable vendors.
In addition to the contractual obligations set forth in the table above, we have employment agreements with certain of our executives and an executive severance policy for all our officers that provide for compensation and certain other benefits. Under certain circumstances, these agreements and the policy provide for severance or other payments.
Off-Balance Sheet Arrangements
We have not created, and are not party to, any special-purpose or off-balance sheet entities for the purpose of raising capital, incurring debt or operating our business. We do not have any off-balance sheet arrangements or relationships with entities that are not consolidated into our financial statements that have or are reasonably likely to have a material current or future effect on our financial condition, changes in financial condition, revenues, expenses, results of operations, liquidity, capital expenditures or capital resources. The Company has commitments for its operating leases, primarily related to its stores, distribution centers, as well as its manufacturing and corporate facilities, which are not reflected on our balance sheet.
Effects of Inflation
We do not believe that our sales or operating results have been materially affected by inflation during the periods presented in our financial statements. During Fiscal 2016, retail price inflation was less than 1%. During Fiscal 2017, we anticipate market driven cost inflation to be in the range of 0% to 2%. Additionally, we may experience increased cost pressure from our suppliers which could have an adverse effect on our gross profit results in the future.
Recent Accounting Pronouncements
Except as discussed in Note 2., “Summary of Significant Accounting Policies”, to our consolidated financial statements included in this Annual Report on Form 10-K, we have considered all new accounting pronouncements and have concluded that there are no new pronouncements that may have a material impact on our results of operations, financial condition, or cash flows, based on current information.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk
The Company’s market risks relate primarily to changes in interest rates. Market risk represents the risk of changes in the value of market risk sensitive instruments caused by fluctuations in interest rates and commodity prices. Changes in these factors could cause fluctuations in the results of our operations and cash flows.
Our Revolving Credit Facility carries a floating interest rate and, therefore, our statements of income and our cash flows are exposed to changes in interest rates. As of December 31, 2016, there was $11.0 million of borrowings outstanding on our Revolving Credit Facility. At December 31, 2016, a hypothetical 10% change in the floating interest rate would have a de minimis impact on our consolidated financial statements.
Our Convertible Notes carry a fixed interest rate and, therefore, have no market risk.
Item 8. Financial Statements and Supplementary Data
The response to this item is incorporated herein by reference to the financial statements and supplementary financial data in Item 15. “Exhibits and Financial Statement Schedules” appearing at the end of this Annual Report on Form 10-K.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
44
Item 9A. Controls and Procedures
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, who are our principal executive officer and principal financial officer, respectively, of the design and operation of our disclosure controls and procedures as such term is defined in Rules 13a-15(e) and 15d—15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) as of December 31, 2016, pursuant to Exchange Act Rules 13a-15 and 15d-15. Based on such evaluation, the Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at a reasonable assurance level as of December 31, 2016.
Management’s Report on Internal Control Over Financial Reporting
See Item 15. “Exhibits and Financial Statement Schedules” appearing at the end of this Annual Report on Form 10-K for Management’s Report on Internal Control Over Financial Reporting.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting during the quarter ended December 31, 2016, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our management, including the Chief Executive Officer and Chief Financial Officer, do not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the Company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Item 9B. Other Information
None.
45
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Information with respect to this Item will be included in the Company’s Proxy Statement to be filed in April 2017, which is incorporated herein by reference under the captions “Proposal One – Election of Directors”, “Corporate Governance”, “Executive Officers” and “Section 16(a) Beneficial Ownership Reporting Compliance”.
Item 11. Executive Compensation
Information with respect to this Item will be included in the Company’s Proxy Statement to be filed in April 2017, which is incorporated herein by reference under the captions, “Director Compensation”, “Compensation Discussion and Analysis” and “Executive Compensation”.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information with respect to this Item will be included in the Company’s Proxy Statement to be filed in April 2017, which is incorporated herein by reference under the captions “Security Ownership” and “Equity Compensation Plan Information”.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information with respect to this Item will be included in the Company’s Proxy Statement to be filed in April 2017, which is incorporated herein by reference under the captions “Corporate Governance – Director Independence”, “Corporate Governance – Policies with Respect to Transactions with Related Persons” and “Certain Relationships and Related Party Transactions, and Director Independence”.
Item 14. Principal Accounting Fees and Services
Information with respect to this Item will be included in the Company’s Proxy Statement to be filed in April 2017, which is incorporated herein by reference under the caption “Principal Accountant Fees and Services”.
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a) | The following documents are filed as part of this annual report on Form 10-K: |
1. | The following consolidated financial statements listed below are filed as a separate section of this annual report on Form 10-K: |
Management’s Reports and Reports of Independent Registered Public Accounting Firm—Deloitte & Touche LLP.
Consolidated Balance Sheets as of December 31, 2016 and December 26, 2015.
Consolidated Statements of Income for the Fiscal years ended December 31, 2016, December 26, 2015 and December 27, 2014.
Consolidated Statements of Comprehensive Income for the Fiscal years ended December 31, 2016, December 26, 2015 and December 27, 2014.
Consolidated Statements of Stockholders’ Equity for the Fiscal years ended December 31, 2016, December 26, 2015 and December 27, 2014.
Consolidated Statements of Cash Flows for the Fiscal years ended December 31, 2016, December 26, 2015 and December 27, 2014.
Notes to Consolidated Financial Statements for the Fiscal years ended December 31, 2016, December 26, 2015 and December 27, 2014.
46
2. | Exhibits: |
Exhibit No. | Description | ||
2.1 | Asset Purchase Agreement, dated as of December 17, 2012, by and among Super Supplements, Inc., John Wurts, Vitamin Shoppe Mariner, Inc. and, solely for certain specified provisions thereof, Vitamin Shoppe, Inc. (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on December 18, 2012 (File No. 001-34507)) | ||
2.2 | Amendment No. 1 to Asset Purchase Agreement, dated as of December 30, 2012, by and among Super Supplements, Inc., John Wurts, Vitamin Shoppe Mariner, Inc. and Vitamin Shoppe, Inc. (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on January 2, 2013 (File No. 001-34507)) | ||
2.3 | LLC Interest Purchase Agreement, dated as of June 6, 2014, by and among VS Hercules LLC, FDC Vitamins, LLC, MBF/FDC Acquisition, LLC, FDC Management, LLC, FDC Limited II, LLC, Nutri-Force Nutrition, Inc., the individuals listed therein and, solely for certain specified provisions thereof, Vitamin Shoppe, Inc. (Incorporated by reference to Exhibit 2.1 in our Current report on Form 8-K filed on June 9, 2014 (File No. 001-34507)) | ||
3.1 | Amended and Restated Certificate of Incorporation of Vitamin Shoppe, Inc. (Incorporated by reference to Exhibit 3.1 in our Current Report on Form 8-K filed on June 10, 2016 (File No. 001-34507)) | ||
3.2 | Fourth Amended and Restated By-laws of Vitamin Shoppe Inc. (Incorporated by reference to Exhibit 3.2 in our Annual Report on Form 10-K filed on February 23, 2016 (File No. 001-34507)) | ||
4.1 | Specimen certificate for shares of common stock, $0.01 par value, of Vitamin Shoppe, Inc. (Incorporated by reference to Exhibit 4.4 in Amendment No. 4 to our Registration Statement No. 333-160756 on Form S-1 filed on October 14, 2009 (File No. 333-160756)) | ||
4.2 | Indenture, dated as of December 9, 2015, by and between Vitamin Shoppe, Inc. and Wilmington Trust, National Association. (Incorporated by reference to Exhibit 4.1 in our Current Report on Form 8-K filed on December 10, 2015 (File No. 001-34507)) | ||
10.1 | Base Convertible Bond Hedge Confirmation, dated as of December 3, 2015, by and between Vitamin Shoppe, Inc. and Bank of America, N.A. (Incorporated by reference to Exhibit 10.2 in our Current Report on Form 8-K filed on December 10, 2015 (File No. 001-34507)) | ||
10.2 | Base Convertible Bond Hedge Confirmation, dated as of December 3, 2015, by and between Vitamin Shoppe, Inc. and J.P. Morgan Chase Bank, National Association, London Branch. (Incorporated by reference to Exhibit 10.3 in our Current Report on Form 8-K filed on December 10, 2015 (File No. 001-34507)) | ||
10.3 | Base Warrant Confirmation, dated as of December 3, 2015, by and between Vitamin Shoppe, Inc. and Bank of America, N.A. (Incorporated by reference to Exhibit 10.4 in our Current Report on Form 8-K filed on December 10, 2015 (File No. 001-34507)) | ||
10.4 | Base Warrant Confirmation, dated as of December 3, 2015, by and between Vitamin Shoppe, Inc. and J.P. Morgan Chase Bank, National Association, London Branch. (Incorporated by reference to Exhibit 10.5 in our Current Report on Form 8-K filed on December 10, 2015 (File No. 001-34507)) | ||
10.5 | Additional Convertible Bond Hedge Confirmation, dated as of December 8, 2015, by and between Vitamin Shoppe, Inc. and Bank of America, N.A. (Incorporated by reference to Exhibit 10.6 in our Current Report on Form 8-K filed on December 10, 2015 (File No. 001-34507)) | ||
10.6 | Additional Convertible Bond Hedge Confirmation, dated as of December 8, 2015, by and between Vitamin Shoppe, Inc. and J.P. Morgan Chase Bank, National Association, London Branch. (Incorporated by reference to Exhibit 10.7 in our Current Report on Form 8-K filed on December 10, 2015 (File No. 001-34507)) | ||
47
10.7 | Additional Warrant Confirmation, dated as of December 8, 2015, by and between Vitamin Shoppe, Inc. and Bank of America, N.A. (Incorporated by reference to Exhibit 10.8 in our Current Report on Form 8-K filed on December 10, 2015 (File No. 001-34507)) | ||
10.8 | Additional Warrant Confirmation, dated as of December 8, 2015, by and between Vitamin Shoppe, Inc. and J.P. Morgan Chase Bank, National Association, London Branch. (Incorporated by reference to Exhibit 10.9 in our Current Report on Form 8-K filed on December 10, 2015 (File No. 001-34507)) | ||
10.9 | Amended and Restated Loan and Security Agreement, dated as of January 20, 2011, by and among Vitamin Shoppe Industries Inc. and VS Direct Inc., as Borrowers, Vitamin Shoppe, Inc., as Guarantor, the Lenders and Issuing Bank from time to time party thereto, and JPMorgan Chase Bank, N.A. as Administrative Agent. (Incorporated by reference to Exhibit 10.2 in our Annual Report on Form 10-K filed on March 9, 2011 (File No. 001-34507)) | ||
10.10 | First Amendment to Amended and Restated Loan and Security Agreement, dated as of January 10, 2013, by and among Vitamin Shoppe Industries Inc., VS Direct Inc. and Vitamin Shoppe Mariner, Inc., as Borrowers, each guarantor party thereto, the lenders party thereto, and JPMorgan Chase Bank, N.A., as Agent, the Issuing Bank and a Lender. (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on October 16, 2013 (File No. 001-34507)) | ||
10.11 | Second Amendment to Amended and Restated Loan and Security Agreement and First Amendment to Existing Guarantees, dated as of October 11, 2013, by and among Vitamin Shoppe Industries Inc., VS Direct Inc., Vitamin Shoppe Mariner, Inc., and Vitamin Shoppe Global, Inc., as Borrowers, each guarantor party thereto, and JPMorgan Chase Bank, N.A., as Agent. (Incorporated by reference to Exhibit 10.2 in our Current Report on Form 8-K filed on October 16, 2013 (File No. 001-34507)) | ||
10.12 | Third Amendment to Amended and Restated Loan and Security Agreement, dated as of December 2, 2015, by and among Vitamin Shoppe Industries Inc., VS Direct Inc., Vitamin Shoppe Mariner, Inc., and Vitamin Shoppe Global, Inc., VS Hercules LLC, FDC Vitamins LLC, Betancourt Sports Nutrition, LLC, Vitamin Shoppe Procurement Services, Inc., as Borrowers, the guarantors parties thereto, the lenders parties thereto, and JPMorgan Chase Bank, N.A., as Agent. (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on December 10, 2015 (File No. 001-34507)) | ||
10.13 | Fourth Amendment to Amended and Restated Loan and Security Agreement, dated as of January 29, 2016, by and among Vitamin Shoppe Industries Inc., VS Direct Inc., Vitamin Shoppe Mariner, Inc., and Vitamin Shoppe Global, Inc., VS Hercules LLC, FDC Vitamins LLC, Betancourt Sports Nutrition, LLC, Vitamin Shoppe Procurement Services, Inc., as Borrowers, the guarantors parties thereto, the lenders parties thereto, and JPMorgan Chase Bank, N.A., as Agent. (Incorporated by reference to Exhibit 10.13 in our Annual Report on Form 10-K filed on February 23, 2016 (File No. 001-34507)) | ||
10.14 | Intellectual Property Security Agreement, dated as of September 25, 2009, by and among Vitamin Shoppe Industries Inc., VS Direct Inc. and Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) and JPMorgan Chase Bank, N.A., as Administrative Agent. (Incorporated by reference to Exhibit 99.5 in our Current Report on Form 8-K filed on September 30, 2009 (File No. 333-134983-02)) | ||
10.15 | Second Amended and Restated Intellectual Property Security Agreement, dated as of October 6, 2014, by and between Vitamin Shoppe Industries Inc., as Grantor and JPMorgan Chase Bank, N.A., as Administrative Agent. (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on October 10, 2014 (File No. 001-34507)) | ||
10.16 | Stock Pledge Agreement, dated as of September 25, 2009, by and between Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.), as Pledgor, and JPMorgan Chase Bank, N.A., as Pledgee. (Incorporated by reference to Exhibit 99.6 in our Current Report on Form 8-K filed on September 30, 2009 (File No. 333-134983-02)) | ||
10.17 | Amended and Restated Stock Pledge Agreement, dated as of October 11, 2013, by and between Vitamin Shoppe Industries Inc., as Pledgor, and JPMorgan Chase Bank, N.A., as Pledgee. (Incorporated by reference to Exhibit 10.3 in our Current Report on Form 8-K filed on October 16, 2013 (File No. 001-34507)) | ||
10.18 | Stock Pledge Agreement, dated as of August 21, 2014, by and between Vitamin Shoppe Global, Inc., as Pledgor, and JPMorgan Chase Bank, N.A., as Pledgee. (Incorporated by reference to Exhibit 10.3 in our Current Report on Form 8-K filed on August 27, 2014 (File No. 001-34507)) | ||
48
10.19 | Stock Pledge Agreement, dated as of August 21, 2014, by and between VS Hercules LLC, as Pledgor, and JPMorgan Chase Bank, N.A., as Pledgee. (Incorporated by reference to Exhibit 10.4 in our Current Report on Form 8-K filed on August 27, 2014 (File No. 001-34507)) | ||
10.20 | Stock Pledge Agreement, dated as of August 21, 2014, by and between FDC Vitamins, LLC, as Pledgor, and JPMorgan Chase Bank, N.A., as Pledgee. (Incorporated by reference to Exhibit 10.5 in our Current Report on Form 8-K filed on August 27, 2014 (File No. 001-34507)) | ||
10.21 | Guarantee of Vitamin Shoppe Industries Inc. and Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.), dated as of September 25, 2009, of obligations of VS Direct Inc. under the Amended and Restated Loan and Security Agreement, as amended. (Incorporated by reference to Exhibit 99.8 in our Current Report on Form 8-K filed on September 30, 2009 (File No. 333-134983-02)) | ||
10.22 | Guarantee of VS Direct Inc. and Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.), dated as of September 25, 2009, of obligations of Vitamin Shoppe Industries Inc. under the Amended and Restated Loan and Security Agreement, as amended. (Incorporated by reference to Exhibit 99.9 in our Current Report on Form 8-K filed on September 30, 2009 (File No. 333-134983-02)) | ||
10.23 | Guarantee of Vitamin Shoppe, Inc., Vitamin Shoppe Industries Inc. and VS Direct Inc., dated as of January 10, 2013, of obligations of Vitamin Shoppe Mariner, Inc. under the Amended and Restated Loan Agreement, as amended. (Incorporated by reference to Exhibit 10.5 in our Current Report on Form 8-K filed on October 16, 2013 (File No. 001-34507)) | ||
10.24 | Guarantee of Vitamin Shoppe, Inc., Vitamin Shoppe Industries Inc., VS Direct Inc. and Vitamin Shoppe Mariner, Inc., dated as of October 11, 2013, of the obligations of Vitamin Shoppe Global, Inc. under the Amended and Restated Loan Agreement, as amended. (Incorporated by reference to Exhibit 10.7 in our Current Report on Form 8-K filed on October 16, 2013 (File No. 001-34507)) | ||
10.25 | Guarantee, dated as of August 21, 2014, by Vitamin Shoppe, Inc., Vitamin Shoppe Industries Inc., VS Direct Inc., Vitamin Shoppe Mariner, Inc., Vitamin Shoppe Global, Inc., VS Hercules LLC, FDC Vitamins, LLC and Betancourt Sports Nutrition, LLC, of the obligations of one another under the Amended and Restated Loan Agreement, as amended. (Incorporated by reference to Exhibit 10.2 in our Current Report on Form 8-K filed on August 27, 2014 (File No. 001-34507)) | ||
10.26 | Joinder Agreement, dated as of January 10, 2013, by and between Vitamin Shoppe Mariner, Inc., and JPMorgan Chase Bank, N.A. (Incorporated by reference to Exhibit 10.4 in our Current Report on Form 8-K filed on October 16, 2013 (File No. 001-34507)) | ||
10.27 | Joinder Agreement, dated as of October 11, 2013, by and between Vitamin Shoppe Global, Inc., and JPMorgan Chase Bank, N.A. (Incorporated by reference to Exhibit 10.6 in our Current Report on Form 8-K filed on October 16, 2013 (File No. 001-34507)) | ||
10.28 | Joinder Agreement, dated as of August 21, 2014, by and between VS Hercules LLC, FDC Vitamins, LLC, Betancourt Sports Nutrition, LLC, and JPMorgan Chase Bank, N.A. (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on August 27, 2014 (File No. 001-34507)) | ||
10.29 | Joinder Agreement, dated as of March 20, 2015 by and between Vitamin Shoppe Procurement Services and JPMorgan Chase Bank, N.A. (Incorporated by reference to Exhibit 10.29 in our Annual Report on Form 10-K filed on February 23, 2016 (File No. 001-34507)) | ||
10.30 | Form of Indemnification Agreement by and among executive officer, Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.29 in Amendment No. 4 to our Registration Statement No. 333-160756 on Form S-1 filed on October 14, 2009 (File No. 333-160756)) | ||
10.31 | Form of Indemnification Agreement by and among director, Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.30 in Amendment No. 4 to our Registration Statement No. 333-160756 on Form S-1 filed on October 14, 2009 (File No. 333-160756)) | ||
49
10.32 | VS Parent, Inc. 2006 Stock Option Plan. * (Incorporated by reference to Exhibit 10.27 in Amendment No. 5 to our Registration Statement No. 333-160756 on Form S-1 filed on October 22, 2009 (File No. 333-160756)) | ||
10.33 | 2009 Vitamin Shoppe Equity Incentive Plan. * (Incorporated by reference to Exhibit 10.27 in Amendment No. 2 to our Registration Statement No. 333-160756 on Form S-1 filed on September 22, 2009 (File No. 333-160756)) | ||
10.34 | Vitamin Shoppe 2009 Equity Incentive Plan Amended and Restated Through April 6, 2012 * (Incorporated by reference to Annex A of the Definitive Proxy Statement of Vitamin Shoppe, Inc. filed on April 12, 2012 (File No. 001-34507)) | ||
10.35 | Vitamin Shoppe 2010 Employee Stock Purchase Plan. * (Incorporated by reference to Exhibit 10.16 in our Annual Report on Form 10-K filed on March 17, 2010 (File No. 001-34507)) | ||
10.36 | Vitamin Shoppe, Inc. Executive Severance Pay Policy, amended and restated effective as of October 29, 2014. (Incorporated by reference to Exhibit 10.35 in our Annual Report on Form 10-K filed on February 23, 2016 (File No. 001-34507)) | ||
10.37 | Director Compensation Plan and Stock Ownership Guidelines.* (Incorporated by reference to Exhibit 10.2 in our Current Report on Form 8-K filed on January 4, 2016 (File No. 001-34507)) | ||
10.38 | Vitamin Shoppe, Inc. Executive Severance Pay Policy, amended and restated effective as of March 4, 2016 (Incorporated by reference to Exhibit 10.01 in our Quarterly Report on Form 10-Q filed on May 4, 2016 (File No. 001-34507)) | ||
10.39 | Employment and Non-Competition Agreement, dated as of September 9, 2009, among Richard Markee, VS Parent, Inc., VS Direct, Inc. Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.26 in Amendment No. 2 to our Registration Statement No. 333-160756 on Form S-1 filed on September 22, 2009 (File No. 333-160756)) | ||
10.40 | Amendment No. 1 to Employment and Non-Competition Agreement, dated as of February 28, 2011, by and among Richard Markee, Vitamin Shoppe, Inc. and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.32 in our Annual Report on Form 10-K filed on March 9, 2011 (File No. 001-34507)) | ||
10.41 | Amendment No. 2 to Employment and Non-Competition Agreement, dated as of March 29, 2012, by and among Richard Markee, Vitamin Shoppe, Inc. and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on April 2, 2012 (File No. 001-34507)) | ||
10.42 | Employment Agreement, effective January 1, 2015, by and between Vitamin Shoppe, Inc. and Vitamin Shoppe Industries Inc. and Richard Markee. * (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on January 7, 2015 (File No. 001-34507)) | ||
10.43 | Letter Agreement, dated as of December 31, 2015, among Vitamin Shoppe, Inc., Vitamin Shoppe Industries Inc. and Richard Markee. * (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on January 4, 2016 (File No. 001-34507)) | ||
10.44 | Employment and Non-Competition Agreement, dated as of March 3, 2015, among Colin Watts and Vitamin Shoppe, Inc., Vitamin Shoppe Industries Inc. and all of their subsidiaries and affiliates. * (Incorporated by reference to Exhibit 99.2 in our Current Report on Form 8-K filed on March 4, 2015 (File No. 001-34507)) | ||
10.45 | Amended and Restated Employment and Non-Competition Agreement, dated as of June 12, 2006, by and among Anthony Truesdale, VS Parent, Inc., Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.17 in Amendment No. 1 to our Registration Statement No. 333-134983 on Form S-4 filed on June 14, 2006 (File No. 333-134983-02)) | ||
50
10.46 | Amendment to Amended and Restated Employment and Non-Competition Agreement, dated as of December 28, 2007, by and among Anthony Truesdale, VS Parent, Inc., Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.36 in our Annual Report on Form 10-K filed on March 28, 2008 (File No. 333-134983-02)) | ||
10.47 | Amendment No. 2 to Employment and Non-Competition Agreement, dated as of September 25, 2009 by and among Anthony Truesdale, VS Parent, Inc., Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 99.2 in our Current Report on Form 8-K filed on September 30, 2009 (File No. 333-134983-02)) | ||
10.48 | Amendment No. 3 to Employment and Non-Competition Agreement, dated as of February 28, 2011, by and among Anthony Truesdale, Vitamin Shoppe, Inc. and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.31 in our Annual Report on Form 10-K filed on March 9, 2011 (File No. 001-34507)) | ||
10.49 | Amendment No. 4 to Employment and Non-Competition Agreement, dated as of March 29, 2012, by and among Anthony Truesdale, Vitamin Shoppe, Inc. and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.2 in our Current Report on Form 8-K filed on April 2, 2012 (File No. 001-34507)) | ||
10.50 | Letter Agreement, dated as of March 31, 2015, by and between Vitamin Shoppe, Inc. and Anthony Truesdale. * (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on April 7, 2015 (File No. 001-34507)) | ||
10.51 | Employment and Non-Competition Agreement, dated as of January 15, 2007, by and among Louis H. Weiss, VS Parent, Inc., VS Direct, Inc., Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) and Vitamin Shoppe Industries, Inc. * (Incorporated by reference to Exhibit 10.29 in our Current Report on Form 8-K filed on January 16, 2007 (File No. 333-134983-02)) | ||
10.52 | Amendment to Employment and Non-Competition Agreement, dated as of December 28, 2007, by and among Louis H. Weiss, VS Parent, Inc., VS Direct, Inc., Vitamin Shoppe, Inc. (f/k/a VS Holdings, Inc.) and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.33 in our Annual Report on Form 10-K filed on March 28, 2008 (File No. 333-134983-02)) | ||
10.53 | Amendment No. 2 to Employment and Non-Competition Agreement, dated as of March 29, 2012, by and among Louis H. Weiss, Vitamin Shoppe, Inc. and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.4 in our Current Report on Form 8-K filed on April 2, 2012 (File No. 001-34507)) | ||
10.54 | Amendment No. 3 to Employment and Non-Competition Agreement, dated as of March 27, 2015, by and among Louis H. Weiss, Vitamin Shoppe, Inc. and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.2 in our Current Report on Form 8-K filed on March 30, 2015 (File No. 001-34507)) | ||
10.55 | Letter Agreement, dated as of January 29, 2016, among Vitamin Shoppe, Inc., Vitamin Shoppe Industries Inc. and Louis H. Weiss (Incorporated by reference to Exhibit 10.53 in our Annual Report on Form 10-K filed on February 23, 2016 (File No. 001-34507)) | ||
10.56 | Letter Agreement, dated as of February 10, 2011, by and between Brenda Galgano and Vitamin Shoppe Industries, Inc. * (Incorporated by reference to Exhibit 10.29 in our Annual Report on Form 10-K filed on March 9, 2011 (File No. 001-34507)) | ||
10.57 | Employment and Non-Competition Agreement, dated as of March 29, 2012, by and among Brenda Galgano, Vitamin Shoppe, Inc. and Vitamin Shoppe Industries Inc. * (Incorporated by reference to Exhibit 10.5 in our Current Report on Form 8-K filed on April 2, 2012 (File No. 001-34507)) | ||
10.58 | Amendment to Employment and Non-Competition Agreement, dated as of March 27, 2015, by and among Vitamin Shoppe, Inc., Vitamin Shoppe Industries Inc. and Brenda Galgano. * (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on March 30, 2015 (File No. 001-34507)) | ||
51
10.59 | Lease Agreement, dated as of May 2, 2002, by and between Hartz Mountain Industries, Inc. and Vitamin Shoppe Industries Inc. (Incorporated by reference to Exhibit 10.22 in our Registration Statement No. 333-134983 on Form S-4 filed on June 13, 2006 (File No. 333-134983-02)) | ||
10.60 | Lease Agreement, dated as of August 27, 2012, by and between CLF Ashland, LLC and Vitamin Shoppe Industries Inc. (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on August 31, 2012 (File No. 001-34507)) | ||
10.61 | Offer Letter, dated as of March 24, 2016 among Vitamin Shoppe, Inc., Vitamin Shoppe Industries Inc. and Brenda Galgano * (Incorporated by reference to Exhibit 10.03 in our Quarterly Report on Form 10-Q filed on May 4, 2016 (File No. 001-34507)) | ||
10.62 | Master Confirmation - Capped Accelerated Share Repurchase, dated as of November 3, 2014, by and among Vitamin Shoppe, Inc. and JP Morgan Securities LLC, as Agent for JPMorgan Chase Bank, National Association, London Branch. (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on November 6, 2014 (File No. 001-34507)) | ||
10.63 | Master Confirmation - Capped Accelerated Share Repurchase, dated as of December 7, 2015, by and among Vitamin Shoppe, Inc. and JP Morgan Securities LLC, as Agent for JPMorgan Chase Bank, National Association, London Branch. (Incorporated by reference to Exhibit 10.10 in our Current Report on Form 8-K filed on December 10, 2015 (File No. 001-34507)) | ||
10.64 | Agreement, dated as of January 12, 2016, by and between the Company and Carlson Capital. (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on January 12, 2016 (File No. 001-34507)) | ||
10.65 | Lease Agreement dated as of December 21, 2016, by and between Vitamin Shoppe Procurement Services, Inc. and Coldwater Industrial Associates 3, LLC (Filed herewith) | ||
10.66 | Offer Letter, dated as of June 6, 2016, among Vitamin Shoppe, Inc., Vitamin Shoppe Industries Inc. and Jason Reiser * (Incorporated by reference to Exhibit 10.1 in our Current Report on Form 8-K filed on June 16, 2016 (File No. 001-34507)) | ||
10.67 | Form of Performance Stock Unit Award Agreement (Incorporated by reference to Exhibit 10.06 in our Quarterly Report on Form 10-Q filed on May 4, 2016 (File No. 001-34507)) | ||
10.68 | Form of Non-Qualified Stock Option Agreement (Incorporated by reference to Exhibit 10.07 in our Quarterly Report on Form 10-Q filed on May 4, 2016 (File No. 001-34507)) | ||
10.69 | Form of Restricted Stock Award Agreement (Incorporated by reference to Exhibit 10.08 in our Quarterly Report on Form 10-Q filed on May 4, 2016 (File No. 001-34507)) | ||
21.1 | Subsidiaries of the Registrant. (Filed herewith) | ||
23.1 | Consent of Independent Registered Public Accounting Firm. (Filed herewith) | ||
31.1 | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. (Filed herewith) | ||
31.2 | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. (Filed herewith) | ||
32.1 | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 – Chief Executive Officer. (Filed herewith ) | ||
32.2 | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 – Chief Financial Officer. (Filed herewith ) | ||
52
101 | The following financial information from the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2016, formatted in eXtensible Business Reporting Language (XBRL): (a) Consolidated Balance Sheets as of December 31, 2016 and December 26, 2015; (b) Consolidated Statements of Income for the fiscal years ended December 31, 2016, December 26, 2015, and December 27, 2014; (c) Consolidated Statements of Comprehensive Income for the fiscal years ended December 31, 2016, December 26, 2015, and December 27, 2014; (d) Consolidated Statements of Stockholders’ Equity for the fiscal years ended December 31, 2016, December 26, 2015, and December 27, 2014; (e) Consolidated Statements of Cash Flows for the fiscal years ended December 31, 2016, December 26, 2015, and December 27, 2014; and (f) Notes to Consolidated Financial Statements for the fiscal years ended December 31, 2016, December 26, 2015, and December 27, 2014. | ||
* Management contract or compensation plan or arrangement.
53
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on March 1, 2017.
VITAMIN SHOPPE, INC. | |||
By: | /s/ Colin Watts | ||
Colin Watts Chief Executive Officer (Principal Executive Officer) | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Name | Title | Date | ||||
By: | /s/ John Bowlin | Non-Executive Chairman, Director | March 1, 2017 | |||
John Bowlin | ||||||
By: | /s/ Colin Watts | Chief Executive Officer, Director (Principal Executive Officer) | March 1, 2017 | |||
Colin Watts | ||||||
By: | /s/ Brenda Galgano | EVP, Chief Financial Officer (Principal Financial Officer) | March 1, 2017 | |||
Brenda Galgano | ||||||
By: | /s/ Daniel Lamadrid | SVP, Chief Accounting Officer (Principal Accounting Officer) | March 1, 2017 | |||
Daniel Lamadrid | ||||||
By: | /s/ B. Michael Becker | Director | March 1, 2017 | |||
B. Michael Becker | ||||||
By: | /s/ Catherine Buggeln | Director | March 1, 2017 | |||
Catherine Buggeln | ||||||
By: | /s/ Deborah M. Derby | Director | March 1, 2017 | |||
Deborah M. Derby | ||||||
By: | /s/ David H. Edwab | Director | March 1, 2017 | |||
David H. Edwab | ||||||
By: | /s/ Richard L. Markee | Director | March 1, 2017 | |||
Richard L. Markee | ||||||
By: | /s/ Guillermo Marmol | Director | March 1, 2017 | |||
Guillermo Marmol | ||||||
54
By: | /s/ Beth M. Pritchard | Director | March 1, 2017 | |||
Beth M. Pritchard | ||||||
By: | /s/ Timothy J. Theriault | Director | March 1, 2017 | |||
Timothy J. Theriault | ||||||
55
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined under the Exchange Act) to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America (“GAAP”). Such internal control includes those policies and procedures that (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets; and (ii) provide reasonable assurance (A) that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors; and (B) regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2016. In making this assessment, it used the criteria set forth in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) published in 2013. Based on this assessment, management has determined that, as of December 31, 2016, our internal control over financial reporting is effective based on those criteria.
The Company’s internal control over financial reporting as of December 31, 2016 has been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their attestation report which appears herein.
March 1, 2017
/s/ Colin Watts | /s/ Brenda Galgano | |
Colin Watts | Brenda Galgano | |
Chief Executive Officer | EVP and Chief Financial Officer | |
MANAGEMENT’S RESPONSIBILITY FOR FINANCIAL STATEMENTS
The management of Vitamin Shoppe, Inc. is responsible for the preparation, objectivity and integrity of the consolidated financial statements and other information contained in this Annual Report on Form 10-K. The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America and include some amounts that are based on management’s informed judgments and best estimates.
Deloitte & Touche LLP, an independent registered public accounting firm, has audited these consolidated financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States) and has expressed herein their unqualified opinion on those financial statements.
The Audit Committee of the Board of Directors, which oversees all of the Company’s financial reporting process on behalf of the Board of Directors, consists solely of independent directors, meets with the independent registered public accounting firm, internal auditors and management periodically to review their respective activities and the discharge of their respective responsibilities. Both the independent registered public accounting firm and the internal auditors have unrestricted access to the Audit Committee, with or without management, to discuss the scope and results of their audits and any recommendations regarding the system of internal controls.
March 1, 2017
/s/ Colin Watts | /s/ Brenda Galgano | |
Colin Watts | Brenda Galgano | |
Chief Executive Officer | EVP and Chief Financial Officer | |
56
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Vitamin Shoppe, Inc.
Secaucus, New Jersey
We have audited the internal control over financial reporting of Vitamin Shoppe, Inc. and Subsidiary (the “Company”) as of December 31, 2016, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements as of and for the fiscal year ended December 31, 2016 of the Company and our report dated March 1, 2017 expressed an unqualified opinion on those financial statements.
/s/ Deloitte & Touche LLP
Parsippany, New Jersey
March 1, 2017
57
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Vitamin Shoppe, Inc.
Secaucus, New Jersey
We have audited the accompanying consolidated balance sheets of Vitamin Shoppe, Inc. and Subsidiary (the “Company”) as of December 31, 2016 and December 26, 2015, and the related consolidated statements of income, comprehensive income, stockholders’ equity, and cash flows for each of the three fiscal years in the period ended December 31, 2016. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2016 and December 26, 2015, and the results of their operations and their cash flows for each of the three fiscal years in the period ended December 31, 2016, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 1, 2017 expressed an unqualified opinion on the Company’s internal control over financial reporting.
/s/ Deloitte & Touche LLP
Parsippany, New Jersey
March 1, 2017
58
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share data)
December 31, 2016 | December 26, 2015 | ||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 2,833 | $ | 15,104 | |||
Accounts receivable, net of allowance of $1,061 and $897 in 2016 and 2015, respectively | 7,367 | 7,437 | |||||
Inventories | 241,736 | 226,830 | |||||
Prepaid expenses and other current assets | 33,717 | 25,194 | |||||
Total current assets | 285,653 | 274,565 | |||||
Property and equipment, net | 139,132 | 140,158 | |||||
Goodwill | 210,633 | 243,269 | |||||
Other intangibles, net | 79,489 | 87,270 | |||||
Other long-term assets | 19,277 | 3,429 | |||||
Total assets | $ | 734,184 | $ | 748,691 | |||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
Current liabilities: | |||||||
Revolving credit facility | $ | 11,000 | $ | 8,000 | |||
Accounts payable | 65,606 | 41,217 | |||||
Deferred sales | 5,209 | 20,483 | |||||
Accrued expenses and other current liabilities | 52,290 | 47,776 | |||||
Total current liabilities | 134,105 | 117,476 | |||||
Convertible notes, net | 120,874 | 115,410 | |||||
Deferred rent | 37,489 | 39,889 | |||||
Other long-term liabilities | 1,720 | 615 | |||||
Commitments and contingencies | |||||||
Stockholders’ equity: | |||||||
Preferred stock, $0.01 par value; 250,000,000 shares authorized and no shares issued and outstanding at December 31, 2016 and December 26, 2015 | — | — | |||||
Common stock, $0.01 par value; 400,000,000 shares authorized, 23,585,240 shares issued and 23,424,055 shares outstanding at December 31, 2016, and 25,993,715 shares issued and 25,873,581 shares outstanding at December 26, 2015 | 236 | 260 | |||||
Additional paid-in capital | 80,727 | 139,827 | |||||
Treasury stock, at cost; 161,185 shares at December 31, 2016 and 120,134 shares at December 26, 2015 | (6,430 | ) | (5,225 | ) | |||
Accumulated other comprehensive loss | — | (60 | ) | ||||
Retained earnings | 365,463 | 340,499 | |||||
Total stockholders’ equity | 439,996 | 475,301 | |||||
Total liabilities and stockholders’ equity | $ | 734,184 | $ | 748,691 | |||
See accompanying notes to consolidated financial statements.
59
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF INCOME
(In thousands, except share and per share data)
Fiscal Year Ended | |||||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | |||||||||
Net sales | $ | 1,289,243 | $ | 1,266,549 | $ | 1,213,046 | |||||
Cost of goods sold | 862,887 | 847,634 | 808,787 | ||||||||
Gross profit | 426,356 | 418,915 | 404,259 | ||||||||
Selling, general and administrative expenses | 380,779 | 329,922 | 301,603 | ||||||||
Income from operations | 45,577 | 88,993 | 102,656 | ||||||||
Interest expense, net | 9,523 | 1,105 | 495 | ||||||||
Income before provision for income taxes | 36,054 | 87,888 | 102,161 | ||||||||
Provision for income taxes | 11,090 | 34,717 | 40,920 | ||||||||
Net income | $ | 24,964 | $ | 53,171 | $ | 61,241 | |||||
Weighted average common shares outstanding | |||||||||||
Basic | 23,875,540 | 28,954,804 | 30,239,183 | ||||||||
Diluted | 24,067,686 | 29,203,429 | 30,664,105 | ||||||||
Net income per common share | |||||||||||
Basic | $ | 1.05 | $ | 1.84 | $ | 2.03 | |||||
Diluted | $ | 1.04 | $ | 1.82 | $ | 2.00 | |||||
See accompanying notes to consolidated financial statements.
60
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(In thousands)
Fiscal Year Ended | |||||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | |||||||||
Net income | $ | 24,964 | $ | 53,171 | $ | 61,241 | |||||
Other comprehensive income: | |||||||||||
Foreign currency translation adjustments | 60 | 23 | 3 | ||||||||
Other comprehensive income | 60 | 23 | 3 | ||||||||
Comprehensive income | $ | 25,024 | $ | 53,194 | $ | 61,244 | |||||
See accompanying notes to consolidated financial statements.
61
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands, except share data)
Common Stock | Treasury Stock | Additional Paid-In Capital | Accumulated Other Comprehensive (Loss) Income | Retained Earnings | |||||||||||||||||||||||||
Shares | Amounts | Shares | Amounts | Total | |||||||||||||||||||||||||
Balance at December 28, 2013 | 30,531,550 | $ | 305 | (6,316 | ) | $ | (280 | ) | $ | 302,314 | $ | (86 | ) | $ | 226,087 | $ | 528,340 | ||||||||||||
Comprehensive income | — | — | — | — | — | 3 | 61,241 | 61,244 | |||||||||||||||||||||
Equity compensation | — | — | — | — | 6,901 | — | — | 6,901 | |||||||||||||||||||||
Issuance of restricted shares | 194,929 | 2 | — | — | (2 | ) | — | — | — | ||||||||||||||||||||
Purchases of treasury stock | — | — | (51,140 | ) | (2,415 | ) | — | — | — | (2,415 | ) | ||||||||||||||||||
Purchases of shares under Share Repurchase Programs | (1,183,714 | ) | (12 | ) | — | — | 57,803 | — | — | (57,815 | ) | ||||||||||||||||||
Cancellation of restricted shares | (14,691 | ) | — | — | — | — | — | — | — | ||||||||||||||||||||
Issuance of shares under employee stock purchase plan | 24,289 | — | — | — | 923 | — | — | 923 | |||||||||||||||||||||
Exercises of stock options | 553,974 | 6 | — | — | 9,387 | — | — | 9,393 | |||||||||||||||||||||
Tax benefits on exercise of equity awards | — | — | — | — | 5,363 | — | — | 5,363 | |||||||||||||||||||||
Balance at December 27, 2014 | 30,106,337 | 301 | (57,456 | ) | (2,695 | ) | 267,083 | (83 | ) | 287,328 | 551,934 | ||||||||||||||||||
Comprehensive income | — | — | — | — | — | 23 | 53,171 | 53,194 | |||||||||||||||||||||
Equity compensation | — | — | — | — | 5,402 | — | — | 5,402 | |||||||||||||||||||||
Issuance of restricted shares | 271,716 | 3 | — | — | (3 | ) | — | — | — | ||||||||||||||||||||
Issuance of shares | 5,184 | — | — | — | 167 | — | — | 167 | |||||||||||||||||||||
Purchases of treasury stock | — | — | (62,678 | ) | (2,530 | ) | — | — | — | (2,530 | ) | ||||||||||||||||||
Purchases of shares under Share Repurchase Programs | (4,328,055 | ) | (43 | ) | — | — | (146,065 | ) | — | — | (146,108 | ) | |||||||||||||||||
Cancellation of restricted shares | (145,117 | ) | (2 | ) | — | — | 2 | — | — | — | |||||||||||||||||||
Issuance of shares under employee stock purchase plan | 27,187 | — | — | — | 892 | — | — | 892 | |||||||||||||||||||||
Exercises of stock options | 56,463 | 1 | — | — | 1,351 | — | — | 1,352 | |||||||||||||||||||||
Equity portion of convertible notes, net | — | — | — | — | 24,948 | — | — | 24,948 | |||||||||||||||||||||
Bond hedge purchase | — | — | — | — | (26,407 | ) | — | — | (26,407 | ) | |||||||||||||||||||
Warrant sale | — | — | — | — | 12,966 | — | — | 12,966 | |||||||||||||||||||||
Tax benefits on exercise of equity awards | — | — | — | — | (509 | ) | — | — | (509 | ) | |||||||||||||||||||
Balance at December 26, 2015 | 25,993,715 | 260 | (120,134 | ) | (5,225 | ) | 139,827 | (60 | ) | 340,499 | 475,301 | ||||||||||||||||||
62
Comprehensive income | — | — | — | — | — | 60 | 24,964 | 25,024 | |||||||||||||||||||||
Equity compensation | — | — | — | — | 6,380 | — | — | 6,380 | |||||||||||||||||||||
Issuance of restricted shares | 196,777 | 2 | — | — | (2 | ) | — | — | — | ||||||||||||||||||||
Issuance of shares | 11,942 | — | — | — | 333 | — | — | 333 | |||||||||||||||||||||
Purchases of treasury stock | — | — | (41,051 | ) | (1,205 | ) | — | — | — | (1,205 | ) | ||||||||||||||||||
Purchases of shares under Share Repurchase Programs | (2,552,556 | ) | (26 | ) | — | — | (65,985 | ) | — | — | (66,011 | ) | |||||||||||||||||
Cancellation of restricted shares | (103,362 | ) | (1 | ) | — | — | 1 | — | — | — | |||||||||||||||||||
Issuance of shares under employee stock purchase plan | 33,442 | 1 | — | — | 822 | — | — | 823 | |||||||||||||||||||||
Exercises of stock options | 5,282 | — | — | — | 90 | — | — | 90 | |||||||||||||||||||||
Tax benefits on exercise of equity awards | — | — | — | — | (739 | ) | — | — | (739 | ) | |||||||||||||||||||
Balance at December 31, 2016 | 23,585,240 | $ | 236 | (161,185 | ) | $ | (6,430 | ) | $ | 80,727 | $ | — | $ | 365,463 | $ | 439,996 | |||||||||||||
See accompanying notes to consolidated financial statements.
63
VITAMIN SHOPPE, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
Fiscal Year Ended | |||||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | |||||||||
Cash flows from operating activities: | |||||||||||
Net income | $ | 24,964 | $ | 53,171 | $ | 61,241 | |||||
Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||
Depreciation and amortization of fixed and intangible assets | 38,780 | 38,495 | 34,219 | ||||||||
Impairment charge on goodwill | 32,636 | — | — | ||||||||
Impairment charge on intangible asset | 6,594 | — | — | ||||||||
Impairment charges on fixed assets | 797 | 1,177 | 419 | ||||||||
Contingent consideration for acquisition of FDC Vitamins, LLC | — | (959 | ) | 959 | |||||||
Amortization of deferred financing fees | 957 | 237 | 164 | ||||||||
Amortization of debt discount on convertible notes | 4,690 | 223 | — | ||||||||
Deferred income taxes | (13,683 | ) | (1,364 | ) | (3,950 | ) | |||||
Deferred rent | (3,226 | ) | (2,294 | ) | (503 | ) | |||||
Equity compensation expense | 6,292 | 5,491 | 6,901 | ||||||||
Issuance of shares for services rendered | 333 | 167 | — | ||||||||
Tax benefits on exercises of equity awards | 739 | 509 | (5,363 | ) | |||||||
Changes in operating assets and liabilities: | |||||||||||
Accounts receivable | 70 | 2,939 | 1,499 | ||||||||
Inventories | (13,078 | ) | (38,284 | ) | (2,458 | ) | |||||
Prepaid expenses and other current assets | (8,521 | ) | 3,889 | 3,782 | |||||||
Other long-term assets | 116 | (139 | ) | 2,441 | |||||||
Accounts payable | 26,522 | (3,709 | ) | (9,869 | ) | ||||||
Deferred sales | (15,277 | ) | (2,011 | ) | 787 | ||||||
Accrued expenses and other current liabilities | 2,921 | 394 | 8,483 | ||||||||
Other long-term liabilities | 747 | 2,735 | 1,395 | ||||||||
Net cash provided by operating activities | 93,373 | 60,667 | 100,147 | ||||||||
Cash flows from investing activities: | |||||||||||
Capital expenditures | (40,068 | ) | (39,403 | ) | (42,957 | ) | |||||
Acquisition of FDC Vitamins, LLC | — | 487 | (81,538 | ) | |||||||
Trademarks and other intangible assets | (291 | ) | (514 | ) | (689 | ) | |||||
Net cash used in investing activities | (40,359 | ) | (39,430 | ) | (125,184 | ) | |||||
Cash flows from financing activities: | |||||||||||
Borrowings under revolving credit agreement | 82,000 | 47,000 | 15,000 | ||||||||
Repayments of borrowings under revolving credit agreement | (79,000 | ) | (47,000 | ) | (7,000 | ) | |||||
Proceeds from issuance of convertible notes | — | 143,750 | — | ||||||||
Debt issuance costs on convertible notes | (2 | ) | (4,593 | ) | — | ||||||
Bond hedge purchase | — | (26,407 | ) | — | |||||||
Proceeds from sale of warrants | — | 12,966 | — | ||||||||
Contingent consideration payment for acquisition of FDC Vitamins, LLC | — | (4,041 | ) | — | |||||||
Bank overdraft | (1,041 | ) | 6,973 | — | |||||||
64
Payments of capital lease obligations | (207 | ) | (80 | ) | (152 | ) | |||||
Proceeds from exercises of common stock options | 90 | 1,352 | 9,393 | ||||||||
Issuance of shares under employee stock purchase plan | 823 | 892 | 923 | ||||||||
Purchases of treasury stock | (1,205 | ) | (2,530 | ) | (2,415 | ) | |||||
Purchases of shares under Share Repurchase Programs | (66,011 | ) | (146,108 | ) | (57,815 | ) | |||||
Tax benefits on exercises of equity awards | (739 | ) | (509 | ) | 5,363 | ||||||
Deferred financing fees and other | (12 | ) | (93 | ) | (174 | ) | |||||
Net cash used in financing activities | (65,304 | ) | (18,428 | ) | (36,877 | ) | |||||
Effect of exchange rate changes on cash and cash equivalents | 19 | 129 | 44 | ||||||||
Net increase (decrease) in cash and cash equivalents | (12,271 | ) | 2,938 | (61,870 | ) | ||||||
Cash and cash equivalents beginning of year | 15,104 | 12,166 | 74,036 | ||||||||
Cash and cash equivalents end of year | $ | 2,833 | $ | 15,104 | $ | 12,166 | |||||
Supplemental disclosures of cash flow information: | |||||||||||
Interest paid | $ | 3,715 | $ | 440 | $ | 249 | |||||
Income taxes paid | $ | 33,655 | $ | 33,659 | $ | 37,652 | |||||
Supplemental disclosures of non-cash investing activities: | |||||||||||
Liability for purchases of property and equipment | $ | 4,630 | $ | 7,497 | $ | 8,379 | |||||
Assets acquired under capital lease | $ | 1,589 | $ | — | $ | — | |||||
See accompanying notes to consolidated financial statements.
65
VITAMIN SHOPPE, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Basis of Presentation
Vitamin Shoppe, Inc. (“VSI”), is incorporated in the State of Delaware, and through its wholly-owned subsidiary, Vitamin Shoppe Industries Inc. (“Subsidiary” or “Industries” together with VSI, the “Company”), is a multi-channel specialty retailer and contract manufacturer of nutritional products. Sales of both national brands and our own brands of vitamins, minerals, herbs, specialty supplements, sports nutrition and other health and wellness products (“VMS products”) are made through VSI-operated retail stores and the internet to customers located primarily in the United States. The Company manufactures products for both sales to third parties as well as for the VSI product assortment.
The consolidated financial statements for the fiscal years ended December 31, 2016, December 26, 2015 and December 27, 2014 include the accounts of VSI and Subsidiary. All intercompany transactions and balances have been eliminated in consolidation.
The Company’s fiscal year ends on the last Saturday in December. As used herein, the term “Fiscal Year” or “Fiscal” refers to a 52-week or 53-week period, ending on the last Saturday in December. Fiscal 2016 is a 53-week fiscal year.
On June 6, 2014, the Company acquired all of the outstanding equity interests of FDC Vitamins, LLC d/b/a Nutri-Force Nutrition (“Nutri-Force”), a company which provides custom manufacturing and private labeling of vitamins, dietary supplements, nutraceuticals and nutritional supplements, as well as, develops and markets its own branded products. The total purchase price was $86.1 million in cash. Refer to Note 3. Acquisitions for additional information.
2. Summary of Significant Accounting Policies
Use of Estimates—The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosures of contingent assets and liabilities at the date of the financial statements, and revenue and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents—Cash and cash equivalents include all highly liquid investments with original maturities of ninety days or less. The Company reclassifies cash overdrafts to accounts payable.
Accounts Receivable—Through Nutri-Force, the Company sells product to third-party wholesale customers. The Company monitors the financial condition of its third-party wholesale customers and establishes an allowance for doubtful accounts for balances estimated to be uncollectible. In addition, customer allowances including promotional discounts and allowances are provided to wholesale customers based on various contract terms and are recorded as a reduction to revenue.
The following table details the activity and balances for the Company’s customer allowances for the years ended December 31, 2016, December 26, 2015 and December 27, 2014 (in thousands):
Balance at Beginning of Fiscal Year | Additions | Deductions | Balance at End of Fiscal Year | ||||||||||||
Period Ended December 31, 2016 | $ | 897 | $ | 3,097 | $ | (2,933 | ) | $ | 1,061 | ||||||
Period Ended December 26, 2015 | $ | 1,883 | $ | 2,752 | $ | (3,738 | ) | $ | 897 | ||||||
Period Ended December 27, 2014 | $ | — | $ | 3,194 | $ | (1,311 | ) | $ | 1,883 | ||||||
Inventories—Inventories are stated at the lower of cost or market value. Cost is determined using the weighted average method. Finished goods inventory includes costs of freight on internally transferred merchandise, and costs associated with our buying department and distribution facilities, as well as manufacturing overhead which are capitalized into inventory and then expensed as merchandise is sold. In addition, the cost of inventory is reduced by purchase discounts and other allowances received from certain of our vendors. The Company estimates losses for expiring inventory and the net realizable value of inventory based on when a product is close to expiration and not expected to be sold, when a product has reached its expiration date, or when a product is not expected to be saleable. In determining the reserves for these products, consideration is given to such factors as the amount of inventory on hand, the remaining shelf life, current and expected market conditions, historical trends and the likelihood of recovering the inventory costs based on anticipated demand. The following table details the activity and balances for the Company’s reserve for inventory for the years ended December 31, 2016, December 26, 2015 and December 27, 2014 (in thousands):
66
Balance at Beginning of Fiscal Year | Amounts Charged to Cost of Goods Sold | Write-Offs Against Reserves | Balance at End of Fiscal Year | ||||||||||||
Fiscal Year Ended December 31, 2016 | $ | 7,253 | $ | 11,067 | $ | (9,707 | ) | $ | 8,613 | ||||||
Fiscal Year Ended December 26, 2015 (1) | $ | 5,797 | $ | 11,088 | $ | (9,632 | ) | $ | 7,253 | ||||||
Fiscal Year Ended December 27, 2014 (1) | $ | 2,640 | $ | 8,764 | $ | (5,607 | ) | $ | 5,797 | ||||||
(1) Fiscal 2015 and Fiscal 2014 figures have been restated to include the reserve for inventory of Nutri-Force.
Property and Equipment, Net—Property and equipment, net is stated at cost less accumulated depreciation and amortization. Depreciation and amortization are provided for on a straight-line basis over the estimated useful lives of the related assets. Furniture, fixtures and equipment are generally depreciated over seven years. Leasehold improvements are amortized generally over the shorter of their useful lives or related lease terms. The direct internal and external costs associated with the development of the features and functionality of the Company’s website, transaction processing systems, telecommunications infrastructure and network operations, are capitalized and are amortized on a straight line basis over the estimated useful lives of generally five years. Capitalization of costs begins when the preliminary project stage is completed and management authorizes and commits to funding the computer software project and that it is probable that the project will be completed and the software will be used to perform the function intended. Depreciation of the assets commences when they are put into use. Expenditures for repairs and maintenance are expensed as incurred and expenditures for major renovations and improvements are capitalized. Upon retirement or disposition of property and equipment, the applicable cost and accumulated depreciation are removed from the accounts and any resulting gains or losses are included in the results of operations.
Impairment of Long-Lived Assets—The Company reviews its long-lived assets for impairment whenever events or changes in circumstances, including store closures, indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets held and used is measured by a comparison of the carrying amount of an asset to undiscounted pre-tax future net cash flows expected to be generated by that asset. If the undiscounted future cash flows are not adequate to recover the carrying value of the asset, an impairment loss is recognized for the amount by which the carrying amount of the assets exceeds the fair value of the assets.
Goodwill and Other Intangibles—Goodwill and other indefinite-lived intangibles are not amortized. Evaluations for impairment are performed at least annually, in the fourth quarter of each year, or whenever impairment indicators exist. Goodwill is evaluated for impairment at the reporting unit level (the Company’s operating segments). The evaluation of goodwill and other indefinite-lived intangibles may first consider qualitative factors to determine whether the existence of events or circumstances leads to a determination that it is more likely than not that the fair value is less than its carrying value. A quantitative evaluation is performed if the qualitative evaluation results in a more likely than not determination or if a qualitative evaluation is not performed. The Company’s quantitative impairment tests involve calculating the fair value of each reporting unit using the discounted cash flow analysis method along with the market multiples method which is used for additional validation of the fair value calculated. These valuation methods require certain assumptions and estimates be made by the Company regarding certain industry trends and future profitability. It is the Company’s policy to conduct goodwill impairment testing from information based on current business projections, which include projected future revenues and cash flows. The cash flows utilized in the discounted cash flow analysis are based on five-year financial forecasts developed internally by management. Cash flows for each reporting unit are discounted using an internally derived weighted average cost of capital which reflects the costs of borrowing for the funding of each unit as well as the risk associated with the units themselves. If the carrying amount of a reporting unit exceeds its fair value, the Company would compare the implied fair value of the reporting unit goodwill with its carrying value. To compute the implied fair value of goodwill, the Company would assign the fair value of the reporting unit to all of the assets and liabilities of that unit (including any unrecognized intangible assets) as if the reporting unit had been acquired in a business combination. The excess of the fair value of a reporting unit over the amounts assigned to its assets and liabilities is the implied fair value of goodwill. Also as part of the quantitative test, the Company conducts the test using a 10% decrease in its revenue projections as an additional sensitivity test to ensure the reporting unit’s fair value is greater than its carrying value should events in the future be less favorable than anticipated. For indefinite-lived tradenames, we utilize the royalty relief method in our quantitative evaluations. Under the royalty relief method, a royalty rate is determined based on comparable licensing arrangements which is applied to the revenue projections for the applicable indefinite-lived tradename and the fair value is calculated using a discounted cash flow analysis. To the extent that the implied fair value associated with the goodwill and indefinite-lived intangible assets is less than the recorded value, this would result in a write down of the carrying value of the asset. Impairment tests between annual tests may be
67
undertaken if an event occurs or circumstances change that could reduce the fair value of a reporting unit below its carrying value. The valuation of the goodwill and indefinite-lived intangible assets is affected by, among other things, the Company’s projections for the future and estimated results of future operations. Changes in the business plan or operating results that are different than the estimates used to develop the valuation of the assets may impact these valuations. For those intangible assets which have definite lives, the Company amortizes their cost on a straight-line basis over their estimated useful lives, the periods of which vary based on their particular contractual terms.
In Fiscal 2016, the Company performed a quantitative analysis of its retail and direct reporting units and determined that the fair value of these reporting units was greater that their respective carrying values. As a result, the Company believes the fair values of each of these reporting units and indefinite-lived tradenames substantially exceeds their respective carrying values.
During Fiscal 2016, the Company also performed quantitative analyses of its manufacturing reporting unit and determined the carrying value of the manufacturing reporting unit exceeded its fair value, which resulted in the write-off of the corresponding goodwill of $32.6 million and the customer relationship intangible asset of $6.6 million. Refer to Note 5. Goodwill and Intangible Assets for additional information.
There have been no impairment charges related to goodwill or other intangibles during Fiscal 2015 and Fiscal 2014.
Rent Expenses, Deferred Rent and Landlord Construction Allowances—Rent expense and rent incentives, including landlord construction allowances, are recognized on a straight-line basis over the lease term. The Company records rent expense for stores, distribution centers and manufacturing facilities as a component of cost of goods sold. The Company accounts for landlord construction allowances as lease incentives and records them as a component of deferred rent, which is recognized in cost of goods sold over the lease term.
Revenue Recognition—The Company recognizes revenue when merchandise is sold “at point of sale” in retail stores or upon delivery to a direct customer. In addition, shipping fees billed to customers are classified as sales. Amount recognized as shipping revenue during Fiscal 2016, Fiscal 2015 and Fiscal 2014, were $2.2 million, $2.0 million and $3.0 million respectively. Nutri-Force sells product primarily to third-party customers and to our retail and direct segments. Wholesale revenue is recognized when risk of loss, title and insurable risks have transferred to the customer, net of estimated returns and allowances. To arrive at net sales, gross sales are reduced by deferred sales, customer discounts, actual customer returns and a provision for estimated future customer returns, which is based on management’s review of historical and current customer returns. Sales taxes collected from customers are presented on a net basis and as such are excluded from revenue.
Cost of Goods Sold—The Company includes the cost of inventory sold, costs of warehousing, distribution, manufacturing and store occupancy costs in cost of goods sold and excludes depreciation and amortization related to the retail and direct segments, which is included within selling, general and administrative expenses. Warehousing, distribution and manufacturing costs, which are capitalized into inventory and then expensed as merchandise is sold, include freight on internally transferred merchandise as well as for shipments to direct and wholesale customers and costs associated with our buying department and distribution facilities, as well as manufacturing overhead. Store occupancy costs include rent, common area maintenance, real estate taxes and utilities.
Vendor Allowances—Vendor allowances include discounts, allowances and rebates received from vendors and are based on various contract terms. Vendor allowances are recognized as either purchase discounts which represent a reduction of product cost, funding which is capitalized into inventory and recognized in the statement of income as the merchandise is sold, or direct offset which represents funding subject to immediate recognition in the statement of income, depending on the nature of the allowance.
Frequent Buyer Program—The Company has a frequent buyer program (“Healthy Awards Program”), whereby customers earn points toward free merchandise based on the dollar volume of purchases. Beginning in Fiscal 2016, points are earned each calendar quarter and must be redeemed within the subsequent calendar quarter or they expire. In previous years, points were earned each calendar year and must be redeemed within the first three months of the following year or they expire. Sales are deferred at the time points are earned based on the value of points that are projected to be redeemed, which are based on historical redemption data. The Company records a liability in the period points are earned with a corresponding reduction of sales.
Store Pre-opening Costs—Costs associated with the opening of new retail stores and start up activities are expensed as incurred.
68
Advertising Costs—The costs of advertising for online marketing arrangements, magazines, direct mail and radio are expensed as incurred, or the first time the advertising takes place. Advertising expense was $21.9 million, $21.6 million and $19.3 million for Fiscal 2016, Fiscal 2015 and Fiscal 2014, respectively.
Online Marketing Arrangements—The Company has entered into online marketing arrangements with various online companies. These agreements are established for periods of 24 months, 12 months or, in some cases, a lesser period and generally provide for compensation based on revenue sharing upon the attainment of stipulated revenue amounts, a percentage of the media expenditure managed by the online partner, or based on the number of visitors that the online company refers to the Company. The Company had no fixed payment commitments during Fiscal 2016, Fiscal 2015 and Fiscal 2014.
Income Taxes—Deferred income tax assets and liabilities are recorded in accordance with the liability method. Deferred income taxes have been provided for temporary differences between the tax bases and financial reporting bases of the Company’s assets and liabilities using the tax rates and laws in effect for the periods in which the differences are expected to reverse.
The Company accounts for tax positions based on the provisions of the accounting literature related to accounting for uncertainty in income tax positions. Such literature provides guidance for the recognition threshold and measurement attribute for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. For tax positions that are not more likely than not sustainable upon audit, the Company recognizes the largest amount of the benefit that is more likely than not to be sustained. The Company makes estimates of the potential liability based on our assessment of all potential tax exposures. In addition, the Company uses factors such as applicable tax laws and regulations, current information and past experience with similar issues to make these assessments. The tax positions are analyzed regularly and adjustments are made as events occur that warrant adjustments for those positions. The Company records interest expense and penalties payable to relevant tax authorities as income tax expense.
Concentrations of Credit Risk—Financial instruments, which potentially subject the Company to concentrations of credit risk, include accounts receivable from wholesale customers as well as debit and credit card processors of retail transactions. As of December 31, 2016 and December 26, 2015, five customers represented approximately 58% and 53%, respectively, of the accounts receivable from wholesale customers. Accounts receivable from debit and credit card processors, included in prepaid expenses and other current assets on the consolidated balance sheets, totaled $10.6 million at December 31, 2016 and $10.2 million at December 26, 2015.
The Company had one supplier from whom we purchased at least 5% of our merchandise during Fiscal 2016, two suppliers from whom we purchased at least 5% of our merchandise during Fiscal 2015 and one supplier from whom we purchased at least 5% of our merchandise during Fiscal 2014. We purchased approximately 11% of our total merchandise from these suppliers during Fiscal 2016 and approximately 17% during Fiscal 2015 and 12% during Fiscal 2014.
The Company is subject to concentrations of credit risk associated with cash and cash equivalents, and at times holds cash balances in excess of Federal Deposit Insurance Corporation limits.
Stock-Based Compensation—Stock-based compensation cost is measured at the grant date based on the fair value of awards and is recognized as expense on a straight-line basis over the requisite service period for each separately vesting portion of the award, net of anticipated forfeitures. With the exception of restricted shares, performance share units and restricted share units, determining the fair value of stock-based awards at the grant date requires considerable judgment, including estimating expected volatility, expected term and risk-free rate. Compensation expense resulting from the granting of restricted shares, performance share units and restricted share units is based on the grant date fair value of those common shares and is recognized generally over the two to three year vesting period for restricted shares, the approximately three year vesting period for performance share units and over the quarterly or one year vesting periods for restricted share units. For accounting purposes, the expense for performance based stock options, performance based restricted shares and performance share units is calculated and recorded, based on the determination that the achievement of the pre-established performance targets are probable, over the relevant service period. The vesting requirements for performance based stock options and performance based restricted shares permit a catch-up of vesting at the end of the vesting period.
Expense related to shares purchased under the Company’s Employee Stock Purchase Plan (“ESPP”) is accounted for based on fair value recognition requirements similar to stock options. ESPP participation occurs each calendar quarter (the “Participation Period”) and the expense of which is subject to employee participation in the plan. Under the ESPP, participating employees are allowed to purchase shares at 85% of the lower of the market price of the Company’s common stock at either the first or last trading day of the Participation Period. Compensation expense related to the ESPP is based on the estimated fair value of the discount and purchase price offered on the estimated shares to be purchased under the ESPP. Expense is calculated quarterly, based on the employee contributions made over the applicable three-month Participation Period, using volatility and risk free rates applicable to that three-month period.
69
Net Income Per Share—The Company’s basic net income per share excludes the dilutive effect of stock options, unvested restricted shares, unvested performance share units and unvested restricted share units. It is based upon the weighted average number of common shares outstanding during the period divided into net income.
Diluted net income per share reflects the potential dilution that would occur if securities or other contracts to issue common stock were exercised or converted into common stock. Stock options, unvested restricted shares, unvested performance share units, warrants and unvested restricted share units are included as potential dilutive securities for the periods applicable, using the treasury stock method to the extent dilutive.
The components of the calculation of basic net income per common share and diluted net income per common share are as follows (in thousands except share and per share data):
Fiscal Year Ended | |||||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | |||||||||
Numerator: | |||||||||||
Net income | $ | 24,964 | $ | 53,171 | $ | 61,241 | |||||
Denominator: | |||||||||||
Basic weighted average common shares outstanding | 23,875,540 | 28,954,804 | 30,239,183 | ||||||||
Effect of dilutive securities: | |||||||||||
Stock options | 68,272 | 97,114 | 235,057 | ||||||||
Restricted shares | 115,287 | 150,353 | 184,995 | ||||||||
Performance share units | 7,173 | — | — | ||||||||
Restricted share units | 1,414 | 1,158 | 4,870 | ||||||||
Diluted weighted average common shares outstanding | 24,067,686 | 29,203,429 | 30,664,105 | ||||||||
Basic net income per common share | $ | 1.05 | $ | 1.84 | $ | 2.03 | |||||
Diluted net income per common share | $ | 1.04 | $ | 1.82 | $ | 2.00 | |||||
Stock options, restricted shares and performance share units for the fiscal years ended December 31, 2016, December 26, 2015 and December 27, 2014 for 24,140, 48,538 and 18,089 shares, respectively, have been excluded from the above calculation as they were anti-dilutive.
The Company has the intent and ability to settle the principal portion of its Convertible Notes in cash, and as such, has applied the treasury stock method, which has resulted in the underlying convertible shares being anti-dilutive in Fiscal 2016 and 2015 as the Company’s average stock price from the issuance of the Convertible Notes through December 31, 2016 was less than the conversion price. Refer to Note 8. Credit Arrangements for additional information on the Convertible Notes.
Recent Accounting Pronouncements— Except as noted below, the Company has considered all new accounting pronouncements and has concluded that there are no new pronouncements that may have a material impact on its results of operations, financial condition, or cash flows, based on current information.
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2014-09 (“ASU 2014-09”), Revenue from Contracts with Customers (Topic 606). Under ASU 2014-09, an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. In July 2015, the FASB deferred the effective date of ASU 2014-09 by one year. ASU 2014-09 will be effective for annual reporting periods beginning after December 15, 2017 for public companies and early adoption of ASU 2014-09 is permitted for public companies for annual reporting periods beginning after December 15, 2016. The Company currently expects this guidance will not have a material impact on the Company's consolidated financial statements. However, the Company is still evaluating ASU 2014-09 including the determination of the transition approach it will utilize.
In February 2016, the FASB issued Accounting Standards Update No. 2016-02 (“ASU 2016-02”), Leases (Topic 842). ASU 2016-02 was issued by the FASB to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities on the balance sheet and disclosing key information about leasing arrangements. The main difference between previous GAAP and Topic 842 is the recognition of lease assets and lease liabilities by lessees for those leases classified as operating leases under previous GAAP. ASU 2016-02 will require modified retrospective application at the beginning of our first quarter of Fiscal 2019, but permits adoption in an earlier period. The Company currently expects this guidance will not have a material impact on the Company's results of operations. However, the Company is still evaluating
70
ASU 2016-02 in order to determine the impact of this guidance on the Company’s balance sheet and anticipates this guidance will result in a significant increase to long-term assets and liabilities given we have a significant number of leases.
In March 2016, the FASB issued Accounting Standards Update No. 2016-09 (“ASU 2016-09”), Compensation-Stock Compensation (Topic 718) Improvements to Employee Share-Based Payment Accounting. ASU 2016-09 addresses simplification of several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. ASU 2016-09 is effective for public companies for annual reporting periods beginning after December 15, 2016, and interim periods within those fiscal years. Early adoption of ASU 2016-09 is permitted. The Company has evaluated ASU 2016-09 and does not expect the impact of this guidance to have a material impact on the Company's consolidated financial statements. However, under certain circumstances, this guidance could have an impact on the Company’s effective tax rate as changes between tax and book treatment of equity compensation will be recognized in the provision for income taxes beginning in Fiscal 2017. The Company currently estimates the adoption of this guidance will increase the effective tax rate by 1.1 percentage points in Fiscal 2017.
3. Acquisitions
Nutri-Force
On June 6, 2014, the Company acquired all of the outstanding equity interests of Nutri-Force. The total purchase price was $86.1 million in cash, which includes $5.0 million of contingent consideration which was paid in Fiscal 2015. See Note 15. Segment and Product Data for additional information. The acquisition was funded by cash on hand. The results of operations of the acquired business are included in the Company’s results from the acquisition date.
The Company has recorded its accounting for this acquisition in accordance with accounting guidance on business combinations. The acquisition resulted in goodwill primarily related to the expected benefits resulting from vertical integration as well as growth opportunities. The Company recorded $1.9 million and $4.0 million of acquisition and integration related costs during Fiscal 2015 and Fiscal 2014, respectively, which are included in the consolidated statement of income within selling, general and administrative expenses.
The purchase price of the acquisition has been allocated to the net tangible and intangible assets acquired, with the remainder recorded as goodwill on the basis of estimated fair values. The goodwill was allocated to the Company’s manufacturing segment. The allocation is as follows (in thousands):
Consideration transferred | $ | 81,538 | ||
Working capital adjustment | (487 | ) | ||
Estimated contingent consideration | 4,041 | (a) | ||
Total consideration | $ | 85,092 | ||
Less: net identifiable assets acquired | ||||
Current assets | 33,798 | |||
Non-current assets | 10,008 | |||
Intangible assets | 18,800 | |||
Current liabilities | (10,150 | ) | ||
Total net identifiable assets acquired | $ | 52,456 | ||
Goodwill | $ | 32,636 | ||
(a) | In the fourth quarter of Fiscal 2014, the Company recorded approximately $1.0 million of additional contingent consideration, which is included in the consolidated statement of income within selling, general and administrative expenses. |
As a result of fair value accounting for the acquisition, current assets includes an inventory valuation step-up of $4.5 million, which was charged to cost of goods sold during Fiscal 2014. Intangible assets consist of brands totaling $10.0 million, customer relationships of $7.5 million and internally-developed software of $1.3 million which are being amortized over their estimated useful lives of 18 years, 20 years and 5 years, respectively. The goodwill of $32.6 million is being amortized for tax purposes.
71
From June 6, 2014 through December 27, 2014, the acquired business generated net sales to third parties of $40.3 million and a pre-tax net loss of $1.8 million, excluding acquisition and integration costs. The pre-tax net loss includes the $4.5 million of charges related to the inventory valuation step-up noted above. The results represent the manufacturing segment. Pro forma results are not presented as the acquisition was not significant to the operating results for Fiscal 2016, Fiscal 2015 or Fiscal 2014.
4. Inventories
The components of inventories are as follows (in thousands):
December 31, 2016 | December 26, 2015 | ||||||
Finished goods | $ | 222,046 | $ | 211,879 | |||
Work-in-process | 7,566 | 6,180 | |||||
Raw materials | 12,124 | 8,771 | |||||
$ | 241,736 | $ | 226,830 | ||||
5. Goodwill and Intangible Assets
Goodwill is allocated between the Company’s segments (reporting units), retail, direct and manufacturing. The following table discloses the carrying value of all intangible assets (in thousands):
December 31, 2016 | December 26, 2015 | ||||||||||||||||||||||||||
Gross Carrying Amount | Accumulated Amortization | Impairment Charges | Net | Gross Carrying Amount | Accumulated Amortization | Net | |||||||||||||||||||||
Intangible assets: | |||||||||||||||||||||||||||
Goodwill | $ | 243,269 | $ | — | $ | 32,636 | $ | 210,633 | $ | 243,269 | $ | — | $ | 243,269 | |||||||||||||
Tradenames - Indefinite-lived | 68,405 | — | — | 68,405 | 68,405 | — | 68,405 | ||||||||||||||||||||
Brands | 10,000 | 1,435 | — | 8,565 | 10,000 | 880 | 9,120 | ||||||||||||||||||||
Customer relationships | 7,500 | 906 | 6,594 | — | 7,500 | 594 | 6,906 | ||||||||||||||||||||
Tradenames - Definite-lived | 4,964 | 3,073 | — | 1,891 | 4,673 | 2,722 | 1,951 | ||||||||||||||||||||
Software | 1,300 | 672 | — | 628 | 1,300 | 412 | 888 | ||||||||||||||||||||
$ | 335,438 | $ | 6,086 | 39,230 | $ | 290,122 | $ | 335,147 | $ | 4,608 | $ | 330,539 | |||||||||||||||
Intangible amortization expense for Fiscal 2016, Fiscal 2015 and Fiscal 2014 was $1.5 million, $2.3 million and $1.7 million, respectively. The annual impairment tests for goodwill and tradenames were performed during the fourth quarter of Fiscal 2016.
In Fiscal 2016, the Company performed a quantitative analysis of its retail and direct reporting units and determined that the fair value of these reporting units was greater that their respective carrying values. As a result, the Company believes the fair values of each of these reporting units and indefinite-lived tradenames substantially exceeds their respective carrying values.
Since the acquisition in Fiscal 2014, Nutri-Force, our manufacturing reporting unit, has experienced disruption in its ability to optimize production capacity, volatility in sales performance, loss of third party customers, and correspondingly has experienced lower service levels to customers. Based upon the operating results of Nutri-Force during the three months ended June 25, 2016, we concluded that an impairment trigger occurred for the manufacturing reporting unit and therefore an impairment test was performed. A discounted cash flow model was prepared using the internal forecast, including an estimate of long-term future growth rates and a discount rate determined by management to be commensurate with the risk inherent in this forecast. The results of this analysis determined the fair value of the manufacturing reporting unit exceeded its carrying value, and as a result, we concluded the goodwill assigned to the reporting unit was not impaired. However, the fair value of the manufacturing reporting unit exceeded its carrying value by approximately 5%, which was not considered to be a substantial
72
excess over the carrying value. While financial results during the three months ended September 24, 2016 did not improve, we continued to closely monitor the financial performance of Nutri-Force. During the fiscal fourth quarter, the performance of Nutri-Force declined and expectations of future years were reduced significantly due to on-going churn in third party customers and its inability to reduce costs. In the fiscal fourth quarter, the Company performed its annual quantitative analysis of the manufacturing reporting unit as of October 22, 2016, based on the operating plan for Fiscal 2017, and then a subsequent analysis based on an updated long range projection due to further deterioration in operating results, which indicated that the carrying value of Nutri-Force exceeded its fair value. The Company proceeded to step two of the impairment analysis. Based on the results of these analyses, the Company recorded impairment charges of $32.6 million on goodwill and $6.6 million on the customer relationships intangible asset of Nutri-Force, which are included in selling, general and administrative expenses in the consolidated statement of income.
There have been no impairment charges related to goodwill or other intangibles during Fiscal 2015 and Fiscal 2014.
The useful lives of the Company’s definite-lived intangible assets are between 3 to 18 years. The expected amortization expense on definite-lived intangible assets on the Company’s consolidated balance sheet at December 31, 2016, is as follows (in thousands):
Fiscal 2017 | $ | 1,073 | |
Fiscal 2018 | 1,067 | ||
Fiscal 2019 | 915 | ||
Fiscal 2020 | 807 | ||
Fiscal 2021 | 807 | ||
Thereafter | 6,415 | ||
$ | 11,084 | ||
6. Property and Equipment
Property and equipment consists of the following (in thousands):
December 31, 2016 | December 26, 2015 | ||||||
Leasehold improvements | $ | 173,216 | $ | 168,830 | |||
Furniture, fixtures and equipment | 184,786 | 170,391 | |||||
Software | 78,089 | 59,049 | |||||
436,091 | 398,270 | ||||||
Less: accumulated depreciation and amortization | (305,777 | ) | (274,222 | ) | |||
Subtotal | 130,314 | 124,048 | |||||
Construction in progress | 8,818 | 16,110 | |||||
$ | 139,132 | $ | 140,158 | ||||
Depreciation and amortization expense on property and equipment for the fiscal years ended December 31, 2016, December 26, 2015 and December 27, 2014 was approximately $37.3 million, $36.1 million and $32.5 million, respectively. The Company recognized impairment charges of $0.8 million during Fiscal 2016 on fixed assets related to five of its underperforming retail locations still in use in the Company’s operations. The Company recognized impairment charges of $1.2 million during Fiscal 2015 on fixed assets related to five of its underperforming retail locations, three of which are still in use in the Company’s operations, and three retail locations in Ontario, Canada which the Company closed during Fiscal 2016. The Company recognized impairment charges of $0.4 million during Fiscal 2014 on fixed assets related to three of its underperforming retail locations, two of which are still in use in the Company's operations.
Depreciation and amortization expense on property and equipment for the Company’s retail and direct segments is recorded in selling, general and administrative expenses on the consolidated statements of income. Depreciation on property and equipment used in the manufacturing process is recorded in cost of goods sold on the consolidated statements of income. All other depreciation and amortization for the manufacturing segment is recorded in selling, general and administrative expenses on the consolidated statements of income.
73
7. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following (in thousands):
December 31, 2016 | December 26, 2015 | ||||||
Accrued salaries and related expenses | $ | 13,861 | $ | 10,115 | |||
Sales tax payable and related expenses | 7,669 | 6,975 | |||||
Other accrued expenses | 30,760 | 30,686 | |||||
$ | 52,290 | $ | 47,776 | ||||
The Company is involved in ongoing examinations with various taxing authorities regarding non-income based tax matters for Fiscal 2016 and prior. The final obligation to these authorities may be subject to either an increase or decrease to the initial estimates recorded. As of December 31, 2016, the Company believes the reserves for these matters are adequately provided for in its consolidated financial statements, the reserves of which are reflected in “Sales tax payable and related expenses” in the table above.
8. Credit Arrangements
Convertible Senior Notes due 2020
On December 9, 2015, the Company issued $143.8 million of its 2.25% Convertible Senior Notes due 2020 (the “Convertible Notes”). The Convertible Notes are senior unsecured obligations of VSI. Interest on the Convertible Notes is payable on June 1 and December 1 of each year, commencing on June 1, 2016 until their maturity date of December 1, 2020. The Company may not redeem the Convertible Notes prior to the maturity date.
Prior to July 1, 2020, the Convertible Notes will be convertible only under the following circumstances: (1) during any calendar quarter commencing after the calendar quarter ending on March 31, 2016, if the last reported sale price of the common stock for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending on the last trading day of the immediately preceding calendar quarter is greater than or equal to 130% of the conversion price on each applicable trading day; (2) during the five business day period after any ten consecutive trading day period in which the trading price per $1,000 principal amount of Convertible Notes for such trading day was less than 98% of the product of the last reported sale price of our common stock and the conversion rate on each such trading day; or (3) upon the occurrence of specified corporate events. On or after July 1, 2020 until the close of business on the second scheduled trading day immediately preceding the maturity date, holders may convert all or any portion of their notes, in multiples of $1,000 principal amount, at the option of the holder regardless of the foregoing circumstances.
The Convertible Notes will be convertible at an initial conversion rate of 25.1625 shares of the Company’s common stock per $1,000 principal amount of the Convertible Notes, which is equivalent to an initial conversion price of approximately $39.74. The conversion rate will be subject to adjustment in some events but will not be adjusted for any accrued and unpaid interest. In addition, following certain corporate events that occur prior to the maturity date, the Company is required to increase, in certain circumstances, the conversion rate for a holder who elects to convert its Convertible Notes in connection with such a corporate event including customary conversion rate adjustments in connection with a “make-whole fundamental change” as defined. Upon conversion, the Company may satisfy its conversion obligation by paying or delivering, as applicable, cash, shares of its common stock or a combination of cash and shares of its common stock, at its election.
The Company allocated the principal amount of the Convertible Notes between its liability and equity components (see table below). The carrying amount of the liability component was determined by measuring the fair value of a similar debt instrument of similar credit quality and maturity that did not have the conversion feature. The carrying amount of the equity component, representing the embedded conversion option, was determined by deducting the fair value of the liability component from the principal amount of the Convertible Notes as a whole. The equity component was recorded to additional paid-in capital and is not remeasured as long as it continues to meet the conditions for equity classification. The excess of the principal amount of the Convertible Notes over the carrying amount of the liability component was recorded as a debt discount, and is being amortized to interest expense using an effective interest rate of 3.8% over the term of the Convertible Notes. The Company allocated the total amount of transaction costs incurred to the liability and equity components using the same proportions as the proceeds from the Convertible Notes. Transaction costs attributable to the liability component were recorded as a direct deduction from the liability component of the Convertible Notes, and are being amortized to interest expense using
74
the effective interest method through the maturity date. Transaction costs attributable to the equity component were netted with the equity component of the Convertible Notes in additional paid-in capital.
The Convertible Notes consist of the following components (in thousands):
December 31, 2016 | December 26, 2015 | ||||||
Liability component: | |||||||
Principal | $ | 143,750 | $ | 143,750 | |||
Conversion feature | (24,800 | ) | (24,800 | ) | |||
Liability portion of debt issuance costs | (3,802 | ) | (3,800 | ) | |||
Amortization | 5,726 | 260 | |||||
Net carrying amount | $ | 120,874 | $ | 115,410 | |||
Equity component: | |||||||
Conversion feature | $ | 24,800 | $ | 24,800 | |||
Equity portion of debt issuance costs | (793 | ) | (793 | ) | |||
Deferred taxes | 941 | 941 | |||||
Net carrying amount | $ | 24,948 | $ | 24,948 | |||
In connection with the issuance of the Convertible Notes, the Company entered into convertible note hedge transactions for which it paid an aggregate $26.4 million. In addition, the Company sold warrants for which it received aggregate proceeds of $13.0 million. The convertible note hedge transactions are expected generally to reduce potential dilution of the Company’s common stock upon any conversion of notes and/or offset any cash payments the Company is required to make in excess of the principal amount of converted notes. However, the warrant transaction could separately have a dilutive effect to the extent that the market value per share of the Company’s common stock exceeds the applicable strike price of the warrant transactions, which is approximately $52.99 at inception. As these transactions meet certain accounting criteria, the convertible note hedge and warrant transactions are recorded in stockholders’ equity, are not accounted for as derivatives and are not remeasured each reporting period.
The net proceeds from the Convertible Notes and related transactions of $125.7 million, net of commissions and offering costs of $4.6 million, are being used to repurchase shares of the Company’s common stock under the Company’s share repurchase programs. Refer to Note 11. Share Repurchase Programs for additional information.
Revolving Credit Facility
As of December 31, 2016 and December 26, 2015, the Company had $11.0 million and $8.0 million, respectively, of borrowings outstanding on its Revolving Credit Facility.
Subject to the terms of the Revolving Credit Facility, which has a maturity date of October 11, 2018, the Company may borrow up to $90.0 million, with a Company option to increase the facility up to a total of $150.0 million. The availability under the Revolving Credit Facility is subject to a borrowing base calculated on the value of certain accounts receivable as well as certain inventory of the Company. The obligations thereunder are secured by a security interest in substantially all of the assets of the Company. Under the Revolving Credit Facility, VSI has guaranteed the Company’s obligations, and Industries and its wholly-owned subsidiaries have each guaranteed the obligations of the other respective entities. The Revolving Credit Facility provides for affirmative and negative covenants affecting the Company. The Revolving Credit Facility restricts, among other things, the Company’s ability to incur indebtedness, create or permit liens on the Company’s assets, declare or pay dividends and make certain other restricted payments, consolidate, merge or recapitalize, sell assets, make certain investments, loans or other advances, enter into transactions with affiliates, change our line of business, and restricts the types of hedging activities the Company can enter into. The largest amount borrowed at any given point during Fiscal 2016 was $27.0 million. The unused available line of credit under the Revolving Credit Facility at December 31, 2016 was $76.1 million.
Borrowings under the Revolving Credit Facility accrue interest, at the Company’s option, at the rate per annum based on an “alternative base rate” plus 0.25% or 0.50% or the adjusted Eurodollar rate plus 1.25% or 1.50%, in each case with the higher spread applicable in the event that the aggregate amount of the borrowings under the Revolving Credit Facility exceeds 50% of the borrowing base availability under the Revolving Credit Facility. The weighted average interest rate for the Revolving Credit facility for Fiscal 2016 was 1.78%. The commitment fee on the undrawn portion of the $90.0 million Revolving Credit Facility was 0.25% as of December 31, 2016 and December 26, 2015.
75
Interest expense, net for Fiscal 2016, 2015 and 2014 consists of the following (in thousands):
Fiscal Year Ended | |||||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | |||||||||
Amortization of debt discount on Convertible Notes | $ | 4,690 | $ | 223 | $ | — | |||||
Interest on Convertible Notes | 3,335 | 159 | — | ||||||||
Amortization of deferred financing fees | 957 | 237 | 164 | ||||||||
Interest / fees on the Revolving Credit Facility and other interest | 541 | 487 | 344 | ||||||||
Interest income | — | (1 | ) | (13 | ) | ||||||
Interest expense, net | $ | 9,523 | $ | 1,105 | $ | 495 | |||||
9. Income Taxes
The provision for income taxes for Fiscal 2016, Fiscal 2015 and Fiscal 2014 consists of the following (in thousands):
Fiscal Year Ended | |||||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | |||||||||
Current: | |||||||||||
Federal | $ | 20,923 | $ | 30,696 | $ | 38,432 | |||||
State | 3,850 | 5,385 | 6,438 | ||||||||
Total current | 24,773 | 36,081 | 44,870 | ||||||||
Deferred: | |||||||||||
Federal | (11,655 | ) | (1,283 | ) | (3,497 | ) | |||||
State | (2,028 | ) | (81 | ) | (453 | ) | |||||
Total deferred | (13,683 | ) | (1,364 | ) | (3,950 | ) | |||||
Provision for income taxes | $ | 11,090 | $ | 34,717 | $ | 40,920 | |||||
A reconciliation of the statutory Federal income tax rate and effective rate of the provision for income taxes is as follows:
Fiscal Year Ended | ||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | ||||||
Federal statutory rate | 35.0 | % | 35.0 | % | 35.0 | % | ||
State income taxes, net of Federal income tax benefit | 2.5 | % | 3.4 | % | 4.2 | % | ||
Write-off of Canada investment | (8.3 | )% | — | — | ||||
Other | 1.6 | % | 1.1 | % | 0.9 | % | ||
Effective tax rate | 30.8 | % | 39.5 | % | 40.1 | % | ||
76
Deferred income taxes reflect the net tax effects of temporary differences between the carrying value of assets and liabilities for financial reporting purposes and amounts used for income tax purposes. The temporary differences and carryforwards that give rise to deferred tax assets and liabilities at December 31, 2016 and December 26, 2015 are as follows (in thousands):
December 31, 2016 | December 26, 2015 | ||||||
Deferred tax assets: | |||||||
Net operating loss carryforward | $ | 2,535 | $ | 1,806 | |||
Deferred rent | 10,775 | 11,389 | |||||
Tenant allowance | 3,938 | 4,215 | |||||
Deferred sales | 1,019 | 4,011 | |||||
General accrued liabilities | 7,132 | 6,790 | |||||
Deferred wages and compensation | 863 | 569 | |||||
Inventory | 7,443 | 7,205 | |||||
Equity compensation expense | 3,815 | 3,400 | |||||
Debt | 995 | 1,002 | |||||
Other | 2,735 | 3,299 | |||||
41,250 | 43,686 | ||||||
Valuation allowance | (2,535 | ) | (1,806 | ) | |||
Deferred tax assets | 38,715 | 41,880 | |||||
Deferred tax liabilities: | |||||||
Trade name and goodwill | (15,590 | ) | (29,777 | ) | |||
Accumulated depreciation | (4,589 | ) | (9,488 | ) | |||
Prepaid expenses | (1,689 | ) | (2,012 | ) | |||
Deferred tax liabilities | (21,868 | ) | (41,277 | ) | |||
Net deferred tax asset | $ | 16,847 | $ | 603 | |||
The net deferred tax assets are included in other long-term assets on the consolidated balance sheets.
Management periodically assesses whether the Company is more likely than not to realize some or all of its deferred tax assets. As of December 31, 2016, with the exception of $2.5 million of deferred tax assets arising from a foreign and state net operating loss carryforward against which there is a valuation allowance (see above table), management determined that the Company is more likely than not to realize the deferred tax assets detailed above. Realization of deferred tax assets associated with the state net operating loss carryforwards is dependent upon generating sufficient taxable income prior to their expiration by tax jurisdiction.
The Company and its subsidiaries file income tax returns in the U.S. federal jurisdiction, various state jurisdictions, Puerto Rico and Canada. The Company recognizes interest related to uncertain tax positions in income tax expense. The Company is no longer subject to U.S. federal examinations by tax authorities for years before 2014 and for state examinations before 2010. However, the tax authorities still have the ability to review the relevance of net operating loss carryforwards created in closed years if such tax attributes are utilized in open years (subsequent to 2010).
The Company has domestic (U.S. state) and foreign net operating losses of approximately $8.2 million and $8.3 million at December 31, 2016, against which a full valuation allowance is recorded. Domestic net operating losses generated will continue to expire annually through Fiscal 2032. The Company’s foreign net operating loss is generated through operations in Canada, and will expire in Fiscal 2035.
10. Stock Based Compensation
Equity Incentive Plans - The Company has two equity incentive plans that provide stock based compensation to certain directors, officers, consultants and employees of the Company; the 2006 Stock Option Plan (the “2006 Plan”) and the Vitamin Shoppe 2009 Equity Incentive Plan amended and restated through April 2012 (the “2009 Plan”), under which the Company has granted stock options (includes non-qualified as well as performance based stock options), restricted shares (includes time based as well as performance based restricted shares), performance share units and restricted share units. The issuance of up to 7,453,678 shares of common stock is authorized under these plans. As of December 31, 2016, there were
77
2,065,074 shares available to grant under both plans, which includes 161,185 shares currently held by the Company as treasury stock. Restricted shares, performance share units and restricted share units are issued at a value not less than the fair market value of the common shares on the date of the grant and stock options are exercisable at no less than the fair market value of the underlying shares on the date of grant. Equity awards of restricted shares generally shall become vested between two and three years subsequent to the date on which such equity grants were awarded. Performance share units shall become vested approximately three years subsequent to the date on which such equity grants were awarded. Stock options awarded shall become vested in three or four equal increments on each of the anniversaries of the date on which such equity grants were awarded and generally have a maximum term of 10 years. However, regarding performance based restricted shares, performance share units and performance based stock options, vesting is dependent not only on the passage of time, but also on the attainment of certain internal performance metrics. The vesting requirements for performance based restricted shares and performance based stock options permit a catch-up of vesting at the end of the vesting period. For accounting purposes, the expense for performance based stock options, performance based restricted shares and performance share units is calculated and recorded, based on the determination that the achievement of the pre-established performance targets are probable, over the relevant service period. Restricted share units generally shall become vested quarterly, or one year, subsequent to the date on which such equity grants were awarded.
The following table summarizes restricted shares for the 2009 Plan as of December 31, 2016 and changes during Fiscal 2016:
Number of Unvested Restricted Shares | Weighted Average Grant Date Fair Value | |||||
Unvested at December 26, 2015 | 398,562 | $ | 42.65 | |||
Granted | 181,712 | $ | 29.45 | |||
Vested | (104,095 | ) | $ | 46.87 | ||
Canceled/forfeited | (103,362 | ) | $ | 42.06 | ||
Unvested at December 31, 2016 | 372,817 | $ | 35.20 | |||
The total intrinsic value of restricted shares vested during Fiscal 2016, Fiscal 2015 and Fiscal 2014 was $2.5 million, $6.3 million and $5.7 million, respectively.
The following table summarizes stock options for the 2006 and 2009 Plans as of December 31, 2016 and changes during Fiscal 2016:
Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (years) | Aggregate Intrinsic Value (in thousands) | |||||||||
Outstanding at December 26, 2015 | 284,838 | $ | 22.65 | |||||||||
Granted | 264,072 | $ | 29.21 | |||||||||
Exercised | (5,282 | ) | $ | 17.11 | ||||||||
Canceled/forfeited | (40,831 | ) | $ | 33.19 | ||||||||
Outstanding at December 31, 2016 | 502,797 | $ | 25.30 | 5.39 | $ | 1,443 | ||||||
Vested or expected to vest at December 31, 2016 | 484,921 | $ | 25.30 | 5.39 | — | |||||||
Vested and exercisable at December 31, 2016 | 256,797 | $ | 21.62 | 1.65 | $ | 1,422 | ||||||
The total intrinsic value of options exercised during Fiscal 2016, Fiscal 2015 and Fiscal 2014 was $0.1 million, $1.0 million and $16.0 million, respectively. The cash received from options exercised during Fiscal 2016, Fiscal 2015 and Fiscal 2014 was $0.1 million, $1.4 million and $9.4 million, respectively.
78
The following table summarizes performance share units for the 2009 Plan as of December 31, 2016 and changes during Fiscal 2016:
Number of Unvested Performance Share Units | Weighted Average Grant Date Fair Value | |||||
Unvested at December 26, 2015 | — | — | ||||
Granted | 134,927 | $ | 30.42 | |||
Vested | — | — | ||||
Canceled/forfeited | (9,912 | ) | $ | 30.26 | ||
Unvested at December 31, 2016 | 125,015 | $ | 30.43 | |||
Performance share units granted during Fiscal 2016 shall become vested on December 29, 2018 if the performance criteria are achieved. Performance share units can vest at a range of 25% to 150% based on the achievement of pre-established performance targets.
The following table summarizes restricted share units for the 2009 Plan as of December 31, 2016 and changes during Fiscal 2016:
Number of Unvested Restricted Share Units | Weighted Average Grant Date Fair Value | |||||
Unvested at December 26, 2015 | 11,280 | $ | 37.25 | |||
Granted | 21,451 | $ | 30.68 | |||
Vested | (17,341 | ) | $ | 34.92 | ||
Canceled/forfeited | — | $ | — | |||
Unvested at December 31, 2016 | 15,390 | $ | 30.71 | |||
The total intrinsic value of restricted share units vested during Fiscal 2016, Fiscal 2015 and Fiscal 2014 was $0.4 million, $0.6 million and $0.3 million, respectively.
Compensation expense attributable to stock-based compensation for Fiscal 2016 was $6.3 million, for Fiscal 2015 was $5.5 million and for Fiscal 2014 was $6.9 million. As of December 31, 2016, the remaining unrecognized stock based compensation expense for non-vested stock options, restricted shares, performance share units and restricted share units to be expensed in future periods is $7.6 million, and the related weighted average period over which it is expected to be recognized is 1.7 years. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. The Company estimates forfeitures based on its historical forfeiture rate since the inception of granting stock based awards. The estimated value of future forfeitures for stock options, restricted shares, performance share units and restricted share units as of December 31, 2016 is approximately $0.6 million.
The weighted average grant date fair value of stock options was $7.96 and $18.99 for Fiscal 2016 and Fiscal 2014, respectively. For Fiscal 2014, this valuation represents the fair value of subsequent annual tranches of performance based stock option grants. No stock options were granted in Fiscal 2015. The fair value of each option grant was estimated on the date of grant using the Black-Scholes option-pricing model with the following assumptions:
December 31, 2016 | December 27, 2014 | ||||
Expected dividend yield | — | % | — | % | |
Weighted average expected volatility | 32.4 | % | 35.3 | % | |
Weighted average risk-free interest rate | 1.2 | % | 1.4 | % | |
Expected holding period(s) | 4.00 years | 4.00 - 4.43 years | |||
Treasury Stock — As part of the Company’s equity incentive plans, the Company makes required tax payments on behalf of employees as their restricted shares vest. The Company withholds the number of vested shares having a value on the date of vesting equal to the minimum statutory tax obligation. The shares withheld are recorded as treasury shares. During Fiscal 2016, the Company purchased 41,051 shares in settlement of employees’ tax obligations for a total of $1.2 million. The
79
Company accounts for treasury stock using the cost method. These shares are available to grant under the Company’s equity incentive plans.
11. Share Repurchase Programs
The Company’s board of directors approved share repurchase programs that enable the Company to purchase up to an aggregate of $300 million of its shares of common stock from time to time over three year periods ending on August 4, 2017, May 5, 2018 and November 22, 2018, respectively. As of December 31, 2016, 8,064,325 shares have been repurchased for a total of $269.9 million. The repurchase program does not obligate the Company to acquire any specific number of shares of its common stock and may be suspended, terminated or modified at any time for any reason, including market conditions, the cost of repurchasing such shares, the availability of alternative investment opportunities, liquidity, and other factors deemed appropriate. These factors may also affect the timing and amount of share repurchases.
During Fiscal 2016 and Fiscal 2015, the Company repurchased 1,670,837 and 1,182,990 shares, respectively, of its common stock in the open market. The shares were retired upon repurchase. Open market share repurchases were $47.0 million in Fiscal 2016 and $44.2 million in Fiscal 2015 with average repurchase prices per share of $28.13 and $37.38, respectively. In Fiscal 2016, the Company also repurchased 646,666 shares of its common stock for $19.0 million, or $29.40 per share, under a 10b5-1 program which the Company entered into to purchase shares under predetermined criteria.
Additionally, the Company has entered into accelerated share repurchase (“ASR”) arrangements with financial institutions. In exchange for an up-front payment, the financial institutions initially deliver shares of the Company’s common stock. The total number of shares ultimately delivered, and therefore the average repurchase price paid per share, is determined at the end of the purchase period of each ASR based on the volume weighted-average price of the Company’s common stock during that period. The shares are retired in the periods they are delivered, and each up-front payment is accounted for as a reduction to stockholders’ equity in the Company’s Consolidated Balance Sheet in the period the payment was made. The Company reflects each ASR as a repurchase of common stock in the period delivered for purposes of calculating earnings per share and as a forward contract indexed to its own common stock. The ASRs met all of the applicable criteria for equity classification, and therefore, were not accounted for as derivative instruments.
The following table summarizes the Company’s ASR arrangements:
Beginning of ASR Period | Up-front Payment (in millions) | Initial Share Deliveries | End of ASR Period | Final Shares Delivered | Average Repurchase Price | ||||||||||
November, 2014 | $ | 50.0 | 982,714 | January, 2015 | 88,325 | $ | 46.68 | ||||||||
December, 2015 | $ | 50.0 | 1,391,940 | February, 2016 | 235,053 | $ | 30.73 | ||||||||
In December 2015, the Company also repurchased 1,664,800 shares of its common stock for $51.9 million, or $31.17 per share, from purchasers of the Convertible Notes in privately negotiated transactions.
12. Benefit Plans
The Company sponsors the Vitamin Shoppe Industries, Inc. 401(k) Plan (“401k Plan”). Employees who have completed one month of service are eligible to participate in the 401k Plan. The 401k Plan provides for participant contributions of 1% to 100% of participant compensation into deferred savings, subject to IRS limitations. The 401k Plan provides for Company contributions upon the participant meeting the eligibility requirements. Participants are 100% vested in the Company matching contribution upon receipt. The Company matching contribution is 100% of the first 3% of participant compensation contributed to the 401k Plan and 50% of the next 2% of participant compensation contributed to the 401k Plan. The Company may make discretionary contributions for each 401k Plan year.
The Company recognized expenses for the 401k Plan of $2.2 million in Fiscal 2016, $1.9 million in Fiscal 2015 and $1.6 million in Fiscal 2014.
80
13. Lease Commitments
The Company has non-cancelable real estate operating leases, which expire through 2036. These leases generally contain renewal options for periods ranging from 1 to 10 years and require the Company to pay costs such as real estate taxes and common area maintenance. Contingent rentals are paid based on a percentage of gross sales as defined by lease agreements. The following table provides the net rental expense for all real estate operating leases (in thousands):
Fiscal Year Ended | |||||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | |||||||||
Minimum rentals | $ | 122,039 | $ | 117,578 | $ | 107,456 | |||||
Contingent rentals | 88 | 154 | 103 | ||||||||
122,127 | 117,732 | 107,559 | |||||||||
Less: Sublease rentals | (274 | ) | (273 | ) | (245 | ) | |||||
Net rental expense | $ | 121,853 | $ | 117,459 | $ | 107,314 | |||||
As of December 31, 2016, the Company’s real estate lease commitments are as follows (in thousands):
Fiscal year | Total Operating Leases (1) | ||
2017 | $ | 122,219 | |
2018 | 112,672 | ||
2019 | 93,886 | ||
2020 | 78,064 | ||
2021 | 65,630 | ||
Thereafter | 175,018 | ||
$ | 647,489 | ||
(1) | Store operating leases included in the above table do not include contingent rent based upon sales volume. Operating leases do not include common area maintenance costs or real estate taxes that are paid to the landlord during the year, which combined represented approximately 18.8% of our minimum lease obligations for Fiscal 2016. |
14. Legal Proceedings
The Company is party to various lawsuits arising from time to time in the normal course of business, some of which are covered by insurance. As of December 31, 2016, the Company was not party to any material legal proceedings. Although the impact of the final resolution of these matters on the Company’s financial condition, results of operations or cash flows is not known, management does not believe that the resolution of these lawsuits will have a material adverse effect on the financial condition, results of operations or liquidity of the Company.
15. Segment and Product Data
The Company currently operates three business segments, retail, direct and manufacturing. The operating segments are segments of the Company for which separate financial information is available and for which operating results are evaluated regularly by executive management in deciding how to allocate resources and in assessing performance. The Company’s management evaluates segment operating results based on several indicators. The primary key performance indicators are sales and operating income for each segment. The table below represents key financial information for each of the Company’s business segments as well as corporate costs. The retail segment primarily includes the Company’s retail stores. The retail segment generates revenue primarily through the sale of VMS products through Vitamin Shoppe and Super Supplements retail stores in the United States and Puerto Rico. The direct segment generates revenue through the sale of VMS products primarily through the Company’s websites. The Company’s websites offer customers online access to a full assortment of approximately 17,000 SKUs. The manufacturing segment supplies the retail and direct segments, along with various thirds parties, with finished products for sale. Corporate costs represent all other expenses not allocated to the retail, direct or manufacturing segments which include, but are not limited to: human resources, legal, retail management, direct management, finance, information technology, depreciation (primarily related to assets utilized by the retail and direct business segments as well as corporate assets) and amortization, and various other corporate level activity related expenses. Intercompany sales transactions are eliminated in consolidation.
81
The Company’s segments are designed to allocate resources internally and provide a framework to determine management responsibility. The Company has allocated $165.3 million and $45.3 million of its recorded goodwill to the retail and direct segments, respectively. The Company does not have identifiable assets separated by segment, with the exception of the identifiable assets of the manufacturing segment, which were $62.3 million and $88.4 million as of December 31, 2016 and December 26, 2015, respectively. Capital expenditures for the manufacturing segment during Fiscal 2016 were $2.5 million, during Fiscal 2015 were $3.5 million and from the acquisition date of June 6, 2014 through December 27, 2014 were approximately $0.5 million. At December 31, 2016 and December 26, 2015, long lived assets of the manufacturing segment were $20.1 million and $60.4 million, respectively. Depreciation and amortization expense, included in selling, general and administrative expenses, for the manufacturing segment during Fiscal 2016 was $1.7 million, during Fiscal 2015 was $1.5 million and from the acquisition date of June 6, 2014 through December 27, 2014 was $0.9 million.
The following table contains key financial information of the Company’s business segments (in thousands):
Fiscal Year Ended | |||||||||||
December 31, 2016 (1) | December 26, 2015 | December 27, 2014 | |||||||||
Net sales: | |||||||||||
Retail | $ | 1,109,202 | $ | 1,081,123 | $ | 1,042,054 | |||||
Direct | 130,024 | 128,825 | 130,644 | ||||||||
Manufacturing | 87,684 | 91,159 | 48,102 | ||||||||
Segment net sales | 1,326,910 | 1,301,107 | 1,220,800 | ||||||||
Elimination of intersegment revenues | (37,667 | ) | (34,558 | ) | (7,754 | ) | |||||
Net sales | 1,289,243 | 1,266,549 | 1,213,046 | ||||||||
Income (loss) from operations: | |||||||||||
Retail | 197,450 | 192,598 | 194,864 | ||||||||
Direct | 18,737 | 20,904 | 22,755 | ||||||||
Manufacturing (2) | (44,223 | ) | (1,977 | ) | (1,830 | ) | |||||
Corporate costs (3) | (126,387 | ) | (122,532 | ) | (113,133 | ) | |||||
Income from operations | $ | 45,577 | $ | 88,993 | $ | 102,656 | |||||
(1) | Fiscal 2016 includes a 53rd week. Net sales for the 53rd week were $20.2 million and income from operations for the 53rd week was $3.3 million. |
(2) | In Fiscal 2016, loss from operations for the manufacturing segment includes impairment charges of $32.6 million on goodwill and $6.6 million on the customer relationships intangible asset of Nutri-Force. In Fiscal 2015, loss from operations for the manufacturing segment includes a $1.4 million charge for accounts receivable for one wholesale customer which were deemed uncollectible, and in Fiscal 2014 includes $4.5 million in charges related to the inventory valuation step up for inventory sold subsequent to the acquisition of Nutri-Force. |
(3) | Corporate costs include (in thousands): |
Fiscal Year Ended | |||||||||||
December 31, 2016 | December 26, 2015 | December 27, 2014 | |||||||||
Depreciation and amortization expenses | $ | 37,103 | $ | 37,004 | $ | 32,968 | |||||
Cost reduction project (a) | 3,761 | — | — | ||||||||
Canada stores closing costs (b) | 2,057 | — | — | ||||||||
Super Supplements conversion costs (c) | 1,275 | — | — | ||||||||
Reinvention strategy costs (d) | 541 | 2,723 | — | ||||||||
Management realignment charges (e) | — | 3,396 | — | ||||||||
Acquisition and integration costs (f) | — | 1,874 | 4,777 | ||||||||
Contingent consideration for Nutri-Force acquisition | — | — | 959 | ||||||||
(a) | Outside consulting costs relating to a project to identify and implement cost reduction opportunities. |
(b) | Charges primarily related to lease terminations. |
(c) | Costs primarily related to the closure of the Seattle distribution center. |
(d) | The costs represent outside consultants fees in connection with the Company's "reinvention strategy". |
82
(e) | Management realignment charges primarily consist of severance, sign-on bonuses, recruiting and relocation costs. |
(f) | For Fiscal 2015, represents integration costs related to the acquisition of Nutri-Force, consisting primarily of professional fees. In Fiscal 2014, represents acquisition costs of $3.4 million and integration costs of $1.4 million ($0.6 million for Nutri-Force and $0.8 million for Super Supplements). |
The following table represents net merchandise sales by major product category (in thousands):
Fiscal Year Ended | |||||||||||
Product Category | December 31, 2016 (a) | December 26, 2015 (b) | December 27, 2014 (b) | ||||||||
Vitamins, Minerals, Herbs and Homeopathy | $ | 339,597 | $ | 320,872 | $ | 311,863 | |||||
Sports Nutrition | 408,288 | 421,293 | 419,804 | ||||||||
Specialty Supplements | 308,945 | 289,938 | 288,045 | ||||||||
Other | 230,252 | 232,399 | 190,285 | ||||||||
Total | 1,287,082 | 1,264,502 | 1,209,997 | ||||||||
Delivery Revenue | 2,161 | 2,047 | 3,049 | ||||||||
$ | 1,289,243 | $ | 1,266,549 | $ | 1,213,046 | ||||||
(a) | Fiscal 2016 includes a 53rd week. |
(b) | Fiscal 2015 and Fiscal 2014 figures have been restated to conform with changes to Fiscal 2016 product category classifications. |
For each of the last three years, less than 1.0% of our sales have been derived from international sources.
16. Fair Value of Financial Instruments
The fair value hierarchy requires the categorization of assets and liabilities into three levels based upon the assumptions (inputs) used to price the assets or liabilities. Level 1 provides the most reliable measure of fair value, while Level 3 generally requires significant management judgment. The three levels are defined as follows:
• | Level 1: Unadjusted quoted prices in active markets for identical assets and liabilities. |
• | Level 2: Observable inputs other than those included in Level 1. For example, quoted prices for similar assets or liabilities in active markets or quoted prices for identical assets or liabilities in inactive markets. |
• | Level 3: Unobservable inputs reflecting management’s own assumptions about the inputs used in pricing the asset or liability. |
The Company’s financial instruments include cash, accounts receivable, accounts payable and its Revolving Credit Facility. The Company believes that the recorded values of these financial instruments approximate their fair values due to their nature and respective durations.
The Company's financial instruments also include its Convertible Notes (in thousands):
December 31, 2016 | December 26, 2015 | ||||||
Fair Value | $ | 111,563 | $ | 119,784 | |||
Carrying Value | 120,874 | 115,410 | |||||
The fair value of the Convertible Notes was determined based on inputs that are observable in the market or that could be derived from, or corroborated with, observable market data, including the trading price of the Company’s Convertible Notes, when available, the Company’s stock price and interest rates based on similar debt issued by parties with credit ratings similar to the Company (Level 1 or 2).
Certain assets are measured at fair value on a non-recurring basis, that is, the assets are subject to fair value adjustments in certain circumstances such as when there is evidence of impairment. These measures of fair value, and related inputs, are considered Level 2 or 3 measures under the fair value hierarchy.
83
17. Selected Quarterly Financial Information (unaudited)
The following table summarizes the Fiscal 2016 and Fiscal 2015 quarterly results (in thousands, except for share data):
Fiscal Quarter Ended | |||||||||||||||
March | June | September | December (1) | ||||||||||||
Fiscal Year Ended December 31, 2016 | |||||||||||||||
Net sales | $ | 336,774 | $ | 332,717 | $ | 314,887 | $ | 304,865 | |||||||
Gross profit | 116,247 | 107,824 | 102,125 | 100,160 | |||||||||||
Income (loss) from operations | 27,262 | 20,724 | 20,273 | (22,682 | ) | ||||||||||
Net income (loss) | 14,782 | 10,433 | 11,363 | (11,614 | ) | ||||||||||
Net income (loss) per common share: | |||||||||||||||
Basic | $ | 0.60 | $ | 0.44 | $ | 0.48 | $ | (0.50 | ) | ||||||
Diluted | $ | 0.59 | $ | 0.44 | $ | 0.48 | $ | (0.49 | ) | ||||||
Fiscal Year Ended December 26, 2015 | |||||||||||||||
Net sales | $ | 336,835 | $ | 322,338 | $ | 313,886 | $ | 293,490 | |||||||
Gross profit | 114,649 | 108,260 | 104,709 | 91,297 | |||||||||||
Income from operations | 30,955 | 23,564 | 23,357 | 11,117 | |||||||||||
Net income | 18,700 | 14,241 | 14,098 | 6,132 | |||||||||||
Net income per common share: | |||||||||||||||
Basic | $ | 0.63 | $ | 0.49 | $ | 0.49 | $ | 0.22 | |||||||
Diluted | $ | 0.63 | $ | 0.48 | $ | 0.48 | $ | 0.22 | |||||||
(1) Net loss for the fiscal quarter ended December 2016 reflects a $3.0 million tax benefit resulting from the write-off of the Canada investment.
The following table summarizes certain items for Fiscal 2016 and Fiscal 2015 which impacted quarterly results on a pre-tax basis (in thousands):
Fiscal Quarter Ended | |||||||||||||||
March | June | September | December | ||||||||||||
Fiscal Year Ended December 31, 2016 | |||||||||||||||
Super Supplements conversion costs (1) | $ | 1,046 | $ | — | $ | — | $ | — | |||||||
Canada stores closing costs (2) | 931 | 1,864 | (906 | ) | — | ||||||||||
Reinvention strategy costs (3) | 541 | — | — | — | |||||||||||
Cost reduction project (4) | — | 1,492 | 2,269 | — | |||||||||||
Impairment charges on goodwill and intangible asset (5) | — | — | — | 39,230 | |||||||||||
Fiscal Year Ended December 26, 2015 | |||||||||||||||
Integration costs (6) | $ | 360 | $ | 410 | $ | 617 | $ | 487 | |||||||
Management realignment charges (7) | — | 2,174 | — | 1,222 | |||||||||||
Accounts receivable bad debt reserve charge (8) | — | 1,370 | — | — | |||||||||||
Reinvention strategy costs (3) | — | — | 1,026 | 1,697 | |||||||||||
Super Supplements conversion costs (1) | — | — | — | 1,766 | |||||||||||
Product write-off (9) | — | — | — | 1,330 | |||||||||||
Canada stores closing costs (2) | — | — | — | 885 | |||||||||||
(1) | In Fiscal 2016, costs primarily related to the closure of the Seattle distribution center. In Fiscal 2015, conversion costs primarily include inventory reserve charges, product markdowns and accelerated depreciation. |
(2) | In Fiscal 2016, charges primarily related to lease terminations. The credit in the fiscal quarter ended September 2016 relates to a reversal of lease liabilities previously accrued. In Fiscal 2015, costs include inventory reserve charges, impairment charges to fixed assets and severance charges. |
(3) | The costs represent outside consultants fees in connection with the Company’s “reinvention strategy”. |
(4) | Outside consulting costs relating to a project to identify and implement cost reduction opportunities. |
84
(5) | Impairment charges of $32.6 million on goodwill and $6.6 million on the customer relationships intangible asset of Nutri-Force. |
(6) | Represents integration costs related to the acquisition of Nutri-Force, consisting primarily of professional fees. |
(7) | Management realignment charges primarily consist of severance, sign-on bonuses, recruiting and relocation costs. |
(8) | Represents a charge to increase the allowance for doubtful accounts for Nutri-Force, related to one wholesale customer that abruptly ceased operations. |
(9) | Represents a charge to inventory reserves for the write-off of USPlabs® products which the Company ceased selling. |
85
