Attached files
| file | filename |
|---|---|
| EX-21.1 - EXHIBIT 21.1 - China Health Industries Holdings, Inc. | exhibit21-1.htm |
| EX-31.2 - EXHIBIT 31.2 - China Health Industries Holdings, Inc. | exhibit31-2.htm |
| EX-32.1 - EXHIBIT 32.1 - China Health Industries Holdings, Inc. | exhibit32-1.htm |
| EX-31.1 - EXHIBIT 31.1 - China Health Industries Holdings, Inc. | exhibit31-1.htm |
| EX-10.13 - EXHIBIT 10.13 - China Health Industries Holdings, Inc. | exhibit10-13.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington,
D.C.20549
FORM 10-K
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended June 30, 2015
or
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ________________ to
________________
Commission file number: 000-51060
CHINA HEALTH INDUSTRIES HOLDINGS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 86-0827216 | |
| (State or other jurisdiction of | (I.R.S. Employer Identification No.) | |
| incorporation or organization) |
| 168 Binbei Street, Songbei District, Harbin City | ||
| Heilongjiang Province | ||
| People’s Republic of China | 150028 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: 86-451-88100688
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $0.0001 par value
Title of Class
Indicate by check mark if the registrant is a well-known
seasoned issuer, as defined in Rule 405 of the Securities Act.
[ ]
Yes [X] No
Indicate by check mark if the registrant is not required to
file reports pursuant to Section 13 or Section 15(d) of the Act.
[
] Yes [X] No
Indicate by check mark whether the registrant (1) has filed all
reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the
registrant was required to file such reports), and (2) has been subject to such
filing requirements for the past 90 days.
[X] Yes [ ] No
Indicate by check mark whether the registrant has submitted
electronically and posted on its corporate Web site, if any, every Interactive
Data File required to be submitted and posted pursuant to Rule 405 of Regulation
S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such
shorter period that the registrant was required to submit and post such files).
[X] Yes [ ] No
2
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [X]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer [ ] | Accelerated filer [ ] |
| Non-accelerated filer [ ] (Do not check if a smaller reporting company) | Smaller reporting company [X] |
Indicate by check mark whether the registrant is a shell
company (as defined in Rule 12b-2 of the Act).
[ ] Yes [X] No
The aggregate market value of the voting and non-voting common stock of the issuer held by non-affiliates as of December 31, 2014 was approximately $4,920,580.39 (44,732,549 shares of common stock held by non-affiliates) based upon the closing price of the common stock on such date.
As of September 28, 2015, there were 65,539,737 shares of common stock, par value $0.0001 issued and outstanding.
3
Table of Contents
|
Page | ||
| Part I | ||
| Item 1 | Business | 6 |
| Item 1A | Risk Factors | 27 |
| Item 1B | Unresolved Staff Comments | 27 |
| Item 2 | Properties | 27 |
| Item 3 | Legal Proceedings | 28 |
| Item 4 | Mine Safety Disclosures | 28 |
| Part II | ||
| Item 5 | Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 28 |
| Item 6 | Selected Financial Data | 31 |
| Item 7 | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 31 |
| Item 7A | Quantitative and Qualitative Disclosures About Market Risk | 35 |
4
| Item 8 | Financial Statements and Supplementary Data | 35 |
| Item 9 | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 39 |
| Item 9A | Controls and Procedures | 39 |
| Item 9B | Other Information | 41 |
| Part III | ||
| Item 10 | Directors, Executive Officers and Corporate Governance | 41 |
| Item 11 | Executive Compensation | 47 |
| Item 12 | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 48 |
| Item 13 | Certain Relationships and Related Transactions, and Director Independence | 49 |
| Item 14 | Principal Accounting Fees and Services | 49 |
| Part IV | ||
| Item 15 | Exhibits, Financial Statement Schedules | 51 |
5
PART I
| Item 1. | Business. |
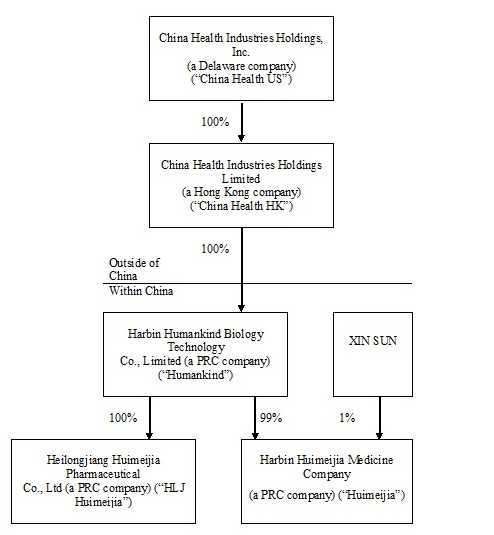
Our History and Corporate Structure
China Health Industries Holdings, Inc. (“China Health US”) was incorporated in the State of Arizona on July 11, 1996 and was the successor of the business known as Arizona Mist, Inc. which began in 1989. On May 9, 2005, it entered into a Stock Purchase Agreement and Share Exchange (effecting a reverse merger) with Edmonds 6, Inc. (“Edmonds 6”), a Delaware corporation, and changed its name to Universal Fog, Inc. Pursuant to this agreement, Universal Fog, Inc. (which has been in continuous operation since 1996) became a wholly-owned subsidiary of Edmonds 6.
China Health Industries Holdings Limited (“China Health HK”) was incorporated on July 20, 2007 in Hong Kong under the Companies Ordinance as a limited liability company. China Health HK was formed for the purpose of seeking and consummating a merger or acquisition with a business entity organized as a private corporation, partnership, or sole proprietorship as defined by FASB ACS Topic 915 (“Development Stage Entities”).
Harbin Humankind Biology Technology Co., Limited (“Humankind”) was incorporated in Harbin City, Heilongjiang Province, the People’s Republic of China (the “PRC”) on December 14, 2003, as a limited liability company under the Company Law of the PRC. Humankind is engaged in the manufacturing and sale of health products.
On August 20, 2007, the sole shareholder of China Health HK entered into a share purchase agreement (the “Share Purchase Agreement”) with the owners of Humankind. Pursuant to the Share Purchase Agreement, China Health HK purchased 100% of the ownership in Humankind for a cash consideration of $60,408 (the “Share Purchase”). Subsequent to the completion of the Share Purchase, Humankind became a wholly-owned subsidiary of China Health HK. The Share Purchase was accounted for as a “reverse merger” since the owner of Humankind owned a majority of the outstanding shares of China Health HK’s common stock immediately following the execution of the Share Purchase Agreement, it was deemed to be the acquirer in the reverse merger. Consequently, the assets and liabilities and the historical operations that have been reflected in the financial statements for periods prior to the Share Purchase are those of Humankind and have been recorded at the historical cost basis. After completion of the Share Purchase, China Health HK’s consolidated financial statements include the assets and liabilities of both China Health HK and Humankind, the historical operations of Humankind, and the operations of China Health HK and its subsidiaries from the closing date of the Share Purchase.
6
On October 14, 2008, Humankind set up a 99% owned subsidiary, Harbin Huimeijia Medicine Company (“Huimeijia”), with its primary business being manufacturing and distributing medicine. Mr. Xin Sun, the Company’s majority owner, owns 1% of Huimeijia. Huimeijia is consolidated in the consolidated financial statements of China Health HK.
On December 31, 2008, China Health HK entered into a reverse merger with Universal Fog, Inc., a U.S. publicly traded shell company (the “Transaction”). China Health HK is the acquirer in the Transaction, and the Transaction has been treated as a recapitalization of China Health US. After the Transaction and a 20:1 reverse stock split, Mr. Xin Sun owned 61,203,088 shares of common stock, representing 98.3% of the 62,234,737 total outstanding shares of common stock of China Health US. On April 7, 2009, Mr. Sun transferred 28,200,000 shares of common stock to 296 individuals, leaving him with 33,003,088 shares of common stock of China Health US, or approximately 53.03% of the total outstanding shares of common stock. Universal Fog, Inc. changed its name to China Health Industries Holdings, Inc. on February 19, 2009.
On November 22, 2013, Humankind completed the acquisition of Heilongjiang Huimeijia Pharmaceutical Co., Ltd. (“HLJ Huimeijia”) for a total purchase price of $16,339,869 (RMB100,000,000). HLJ Huimeijia was founded on October 30, 2003, and is engaged in the manufacturing and distribution of tincture, ointments, rubber paste (including hormones), topical solution, suppositories, liniment (including traditional Chinese medicine extractions), enemas and oral liquids. HLJ Huimeijia’s predecessor is Heilongjiang Xue Du Pharmaceutical Co., Ltd., which has established its brand name in the market through its supply of high quality medical products. HLJ Huimeijia is categorized as a “high and new technology” enterprise by the Science Technology Department in Heilongjiang Province. HLJ Huimeijia has 21 products which have been approved by, and have received approval numbers issued by, the China Food and Drug Administration (“CFDA”). In addition, HLJ Huimeijia is the holder of one patent for utility models, five patents for external design and three trademarks in China, including the Chinese brand name of “Xue Du” which has an established reputation among customers in northeastern China.
China Health US, China Health HK, Humankind, Huimeijia and HLJ Huimeijia are collectively referred herein to as the “Company.”
Our corporate structure as of June 30, 2015 was as below:
7
Business Overview
Humankind was incorporated in the PRC on December 14, 2003, and completed its Good Manufacturing Practice (“GMP”) certificate on April 24, 2007, which will last until February 14, 2019 and is expected to extend after that. It is in the business of the manufacture and sale of health products.
Huimeijia was incorporated in the PRC on October 14, 2008. Huimeijia received its GMP certificate on July 23, 2009, which expired as of July 22, 2014. On December 24, 2014, Humankind entered into a stock transfer agreement (the “Agreement”) with Xiuzheng Pharmaceutical Group Co., Ltd. a company incorporated under the laws of the People’s Republic of China and located in Jilin province (“Xiuzheng Pharmacy” or the “Buyer”), Mr. Xin Sun, the CEO of the Company, and Huimeijia, pursuant to which, Humankind and Mr. Xin Sun (the “Equity Holders”), shall sell their respective equity interests in Huimeijia to Xiuzheng Pharmacy. The transfer of the 100% equity interests of Huimeijia to the Buyer was for a total cash consideration of RMB 8,000,000 (approximately $1,306,186) to the Equity Holders.
8
On February 9, 2015, the four parties entered into a supplementary agreement (the “Supplementary Agreement”) to modify the terms of the Agreement, pursuant to which, the Equity Holders and Huimeijia (collectively the “Assets Transferors”) shall only sell the 19 drug approval numbers (including the tablet, capsule, powder, mixture, oral liquid, syrup and oral solution under the 19 approval numbers; licenses including the original copies of Business License, Organization Code Certificate, Tax Registration Certificate, Drug Production Permit and GMP Certificate, and other documents and original copies related to the production and operation of the 19 drugs) (the “Assets”) to Xiuzheng Pharmacy. The Equity Holders will retain the equity interests in Huimeijia, but will have the equity interests pledged to Xiuzheng Pharmacy until the Assets are transferred, at which time all the cash consideration shall be paid by the Buyer. The total cash consideration remains to be the same as under the Agreement, i.e., RMB 8,000,000 (approximately $1,306,186) to the Assets Transferors.
In the event that the Assets are failed to be transferred to the Buyer due to the fault of the Assets Transferors, the paid consideration shall be returned to the Buyer with interests accrued. If the failure of the transfer of the Assets is a result of the government policy changes or force majeure, the paid cash consideration shall be returned to the Buyer but without any interests. As of June 30, 2015, the transfer of the Assets had not been completed. The Company is striving to accelerate the process of the transfer.
HLJ Huimeijia was founded on October 30, 2003, its latest GMP certificate was effective from November 12, 2009 to December 31, 2015, and is expected to extend after that. HLJ Huimeijia engages in the manufacture and distribution of tincture, ointments, rubber paste (including hormones), topical solution, suppositories, liniment (including traditional Chinese medicine extractions), enemas and oral liquids. Its predecessor is Heilongjiang Xue Du Pharmaceutical Co., Ltd., which had established brand name in the market through its supply of high-quality drug products. HLJ Huimeijia is a “high and new technology” enterprise that provides the most comprehensive types of topical medical products in Heilongjiang Province, a northeastern province of China.
Our business is conducted through our PRC subsidiaries, Humankind and HLJ Huimeijia. Our products are primarily sold through sales agents. We plan to develop chain-stores to sell our products and to eventually sell our products online.
Products
We have, through Humankind, the license to manufacture and sell 14 health supplement products, each of which have been assigned a Guo Shi Jian Zi number, or China health food approval number (as provided below), an approval number issued by the China Food and Drug Administration (“CFDA”). A product with such a number shall include a description of the nourishment value and health function of the products on product specifications. Consumers can check the official website of CFDA to verify a health supplement product through such a number.
We have, through HLJ Huimeijia, the license to manufacture and sell 21 products. In addition, HLJ Huimeijia holds one patent for utility models, five patents for external design, and three trademarks in China, including the Chinese characters of “Xue Du,” which has a good reputation amongst customers in northeastern China.
We are licensed to sell our products, including our medical drugs, only in the PRC.
9
| (i) |
Health Supplement |
Our “QunLe” brand Sailuozhi soft capsule, which is made from frog oil, soybean isoflavone, procyanidine (made from grape seeds) and vitamin E, is for freckle removal and skin moisture supplements. The certification number issued by CFDA on September 3, 2013, is 2013B1097, with an expiration date on September 2, 2018.
On May 12, 2010, we received a patent for this product (number 200610010394.4) under the name “Run Chao” (which has since been changed to “QunLe”) with the National Bureau of Intellectual Property.
Pursuant to a technology transfer agreement dated October 12, 2007 (the “2007 Technology Transfer Agreement”), we purchased a health product known as “Kindlink” brand propolis and black ant capsule made from propolis, black ant, acanthopanax and astragalus root from Beijing Jindelikang Bio-Technology Co., Ltd (“Jindelikang”). The change of the ownership has been approved by the CFDA. This product is to boost one’s immunity. The certification number issued by the CFDA on August 20, 2004, for the license to manufacture the product is GuoShiJianZi G20040906. We have no continuing obligations under the 2007 Technology Transfer Agreement.
Pursuant to a technology transfer agreement dated January 18, 2013 (the “2013 Technology Transfer Agreement”), we purchased 12 health products from Guangzhou Aoda Biology Beauty Healthy Technology Co., Ltd, a non-affiliated party. These twelve products are the following:
- Dr. Xiao Brand Honeysuckle Pearl Capsule (Guo Shi Jian Zi G20100656), which is effective in acne removal,
- Dr. Xiao Brand Multivitamin Tablet (Guo Shi Jian Zi G20080176), which is a multivitamin and mineral supplement,
- Dr. Xiao Brand Zhengdian Capsule (Guo Shi Jian Zi 20070261), which is effective in relieving eyestrain,
- Dr. Xiao Brand Shengui Capsule (Guo Shi Jian Zi G20080297), which is effective in increasing bone density,
- Dr. Xiao Brand Multivitamin Tablet (Woman) (Guo Shi Jian Zi G20070338), which is an iron and multivitamin supplement,
- Dr. Xiao Brand Shikong Soft Capsule (Guo Shi Jian Zi 20080096), which is effective in improving memory,
- Dr. Xiao Brand Huangjingdanggui Tablet (Guo Shi Jian Zi G20080201), which is effective in improving nutritional anemia and chloasma,
- Dr. Xiao Brand Xingxing Soft Capsule (Guo Shi Jian Zi G20080080), which is effective in improving memory,
- Dr. Xiao Brand Vitamin A Fish Oil Soft Capsule (Guo Shi Jian Zi G20080406), which is effective in relieving eyestrain,
- Dr. Xiao Brand Colon Cleanser Granules (Guo Shi Jian Zi G20060061), which is effective in relaxing bowels and promoting the discharge of lead,
10
- Dr. Xiao Brand Jianli Soft Capsule (Guo Shi Jian Zi G20050710), which is effective in increasing immunity and relieving physical fatigue, and
- LB Brand Xinpin Capsule (Guo Shi Jian Zi G20050770), which is effective in dispelling chloasma.
The major suppliers of raw materials for our products who exceeded 10% of our total purchases in the fiscal years 2015 and 2014 are the following:
| Purchases | |||
| (in U.S. | % of | ||
| Name of Supplier | Dollars) | Purchases | |
| FY2015 | Shukui Wang | 4,874,929 | 73.87% |
| FY2014 | Shukui Wang | 5,151,325 | 74.32% |
For the past two fiscal years, Mr. Shukui Wang has been our biggest supplier of raw materials.
The Company typically signs monthly purchase orders with its suppliers. All purchase orders with Mr. Wang and with our other suppliers are on similar terms. We shall remit payment to a supplier’s account no later than three business days after receiving raw materials. A supplier shall deliver raw materials no later than three business days after receiving a purchase order. The cost of delivery is borne by a supplier.
| (iii) |
Medical Drugs |
Huimeijia purchased Harbin Dong Feng Medicine Company’s license to manufacture 19 medical drugs on September 16, 2008. As mentioned above, although Humankind, Mr. Xin Sun and Huimeijia has entered into the Agreement and the Supplementary Agreement with Xiuzheng Pharmacy to sell the 19 drug approval numbers to Xiuzheng Pharmacy, the transaction has not yet completed. Huimeijia remained the registered owner of the license, approved by the Heilongjiang Food and Medicine Supervising Bureau.
A description of the 19 medical drugs is as follows:
11
| Serial No. | Product | Efficacy |
| 1. | Stomach-Tonic Tablets |
Invigorating stomach and relieving pain. Used in the treatment of pain from stomach distention, eructation with fetid odor and fecal disorders caused by gasterasthenia and dyspeptic retention. |
| 2. | Pediatric Compound Sulfamethoxazole Tablets (0.125g) |
Used in the treatment of: |
| 3. | Pediatric Compound Sulfamethoxazole Tablets (0.25g) |
Same as above. |
| 4. | Pipemidic Acid Tablets |
Used to treat urinary tract infection and bacterial infection of the intestines caused by sensitive gram negative bacilli. |
| 5. | Metamizole Sodium Tablets |
Used to relieve fever caused hyperpyrexia and also for headache, migrainous headache, courbature, arthralgia, menalgia etc. The product also has strong anti-rheumatism effects and can be used for acute rheumatic arthritis, but because the product may induce severe adverse reaction, it is seldom applied in the treatment of rheumatic diseases. |
12
| 6. | Paracetamol Tablets | Used for fever caused by common cold or epidemic influenza and also for relieving light and moderate pain such as headache, arthralgia, migraines, tooth ache, courbature, neuralgia and menalgia. |
| 7. | Pediatric Paracetamol, Artificial Cow- bezoar and Chlorphenamine Maleate Tablets | Used to relieve fever, headache, aching pain in extremities, sneezing, rhinorrhea, nasal obstruction, pharyngodynia and other symptoms in children caused by common cold or epidemic influenza. |
| 8. | Compound Theophylling Hydrochloride Tablets | Used to treat bronchial asthma. |
| 9. | Powerful Loquat Syrup | Used for the treatment of coughing and reduction of sputum caused by bronchitis. |
| 10. | Purple Orange Cough Syrup | Relieving cough and eliminating sputum. Used to relieve coughing and excessive phlegm as well as expectoration. |
| 11. | Cough Syrup of Loquat Leaf | Used to clear lungs, relieve coughs and eliminate sputumand excessive phlegm. |
| 12. | Children’s Cough Syrup | Eliminating phlegm and relieving cough. Used to relieve coughs caused by the common cold in children. |
| 13. | Pentoxyverine Citrate and Ammonium Chloride Syrup | Used for cough and expectoration. |
| 14. | Schisandra Syrup | Tonifying vital energy and invigorating the kidneys. Used in the treatment for neurastheria, dizziness and insomnia |
13
| 15. | Ginseng Oral Liquid | Used to nourish renal “qi” and promote fluid production to quench thirst. Used to treat fatigue and acratia caused by deficiency of vital energy as well as poor appetite, cardiopalmus and shortness of breath, insomnia and forgetfulness. |
| 16. | Compound Fluououracil Oral Solution | Used in the therapeutic treatment of digestive tract cancer (colon carcinoma and gastric carcinoma), mammary adenocarcinoma, primary hepatic carcinoma. |
| 17. | Gossypol, Potassium Chloride and Vitamins B Capsules | Used in the treatment of uterine bleeding brought on by menopause. |
| 18. | Compound Belladonna and Aluminum Hydroxide Powder | Used for relieving stomach pain, brash (heartburn) and acid reflux caused by gastric hypersecretion. |
| 19. | Gentian and Sodium Bicarbonate Powder | Used for anorexia, gastric hypersecretion and dyspepsia. |
In addition, HLJ Huimeijia has 21 products with approval numbers issued by the CFDA as following:
| English Name | Efficacy | ||
| 1 | Enema Glycerini | Lubricating laxative. Used for constipation. | |
| 2 | Umguentum Acidi Borici Camphoratum | Dermerethistica. Used for chilblain. | |
| 3 | Ge Hong Beriberi Water | Dehumidification insecticide. Used for tinea pedis and tinea manuum caused by damp toxin brewing and binding, and other skin diseases caused by enzyme. | |
| 4 | Pelvic Inflammation Suppository | Heat-clearing and detoxifying; activating blood to promote menstruation disperse swelling and relieve pain. Used for toxin and blood stasis stagnation in the uterus, distending pain in the lower abdomen, irregular menses, algomenorrhea and leukorrhagia, as well as pelvic inflammation and annexitis with the aforementioned symptoms. |
14
| 5 | Injury and Paralysis Tincture | Warm channel and expelling cold, promoting blood circulation to arrest pain. Used to relieve pain caused by traumatic injury and sprain. | |
| 6 | Indometacin and Furazolidone Suppositories | Anti - inflammatory painkiller. Used to treat acute hemorrhoid, including internal hemorrhoids, external hemorrhoids, mixed hemorrhoids, anal fissure or archosyrinx and relieve pain; Used to ease pain after the operation of anal fissure, archosyrinx or hemorrhoids. | |
| 7 | Injury and Rheumatism Relieving Paste | Dispelling rheumatism and relieving pain. Used for headache, rheumatalgia, neuralgia, sprain and muscular soreness. | |
| 8 | Refining GouPi Cream | Relaxing tendon, invigorating the circulation of blood, dissipating cold and relieving pain. Used for arthralgia and myalgia, acute contusion, sprain, rheumatalgia, arthralgia, hypochondriac pain, muscular soreness, etc. | |
| 9 | Muskiness Pain Relieving Paste | Expelling wind and removing dampness, relaxing the tendons and unblocking collateral. Used for rheumatic arthralgia, low back cold pain, traumatic injury, etc. | |
| 10 | Muskiness Bone Strengthener Paste | Analgesia and anti-inflammatory. Used for rheumatalgia, arthralgia, backache, neuralgia, muscular soreness, sprain and contusion. | |
| 11 | Matrine Suppositories | Antibacterial and antiphlogistic drugs. Used for trichomonas and candida vaginitis, chronic cervicitis, pelvic inflammation, etc. | |
| 12 | Ethacriding Lactate Solution | Disinfectant and preservative drug. Used for disinfection of traumatic and disinfected wounds. | |
| 13 | Triamcinolone Acetonide and Neomycin Paste | Used for neurodermatitis circumscripta and chronic eczema. Also used for small-scale psoriasis. | |
| 14 | Double – Coptis Suppository | Course wind and resolving the exterior, heat-clearing and detoxifying. Used for influenza caused by affection of exogenous wind-heat, with symptoms of fever, cough and sore throat. Also used for upper respiratory tract infections and pneumonia, with symptoms of fever, cough and sore throat. | |
| 15 | Methylrosanilinium Chloride Solution | Disinfectant and preservative drug. | |
| 16 | Iodine Tincture | Disinfectant and preservative drug. | |
| 17 | Mercurochrome Solution | Disinfectant and preservative drug. | |
| 18 | Hydrogen Peroxide Solution | Disinfectant and preservative drug. | |
| 19 | Grucocorticoid. External use drug only to be used on the skin. Used for | ||
| Halcinonide Cream | dermatoneuritis and psoriasis. | ||
| 20 | Compound Fluocinonide Tincture | Grucocorticoid. Used for dermatoneuritis and psoriasis. |
15
| 21 | Policresulen Vaginal Suppository | Anti-microbial and hemostasis drug. |
Distribution
We signed a non-exclusive cooperation agreement with the Commercial Bureau of Qing’an County, Heilongjiang on September 17, 2008. Under the agreement, various affiliated companies of the Commercial Bureau provides organic food and green food products to us for distribution and sale throughout the PRC.
We order products from the Commercial Bureau and such products are delivered within 20 days of placing the order. The prices for these products fluctuate within a 3% range from its wholesale price, but we are not restricted in any way in dictating the retail prices for such products. We typically have an average profit margin of approximately 20%.
Most of our products are sold to sales agents. In fiscal year of 2014, our sales network covered 26 provinces and 4 Municipalities in China and our products were mainly sold in Beijing, Zhejiang, Jiangsu, Shanghai, Gansu, Anhui, Jilin and Liaoning provinces or cities. In fiscal year of 2015, our sales network covered 25 provinces and 4 Municipalities in China and our products were mainly sold in Beijing, Zhejiang, Jiangsu, Shanghai, Gansu, Anhui, Jilin and Shandong provinces or cities.
E-business
We are in the process of building the infrastructure to conduct our business over the internet. A B2C e-business call and sales center has been established and will become an integral part of our distribution channel in the future. We have employed graduates from Tsinghua University, Harbin Industry University and Harbin Engineering University to develop the ERP, CRM and OA software for our e-business. OA software has been used in our daily operation. The Company plans to sell its products via internet in 2016.
Our Customers
We sell most of our products to sales agents, who are our customers. The sales agents sell the products to the end users.
Our customers who contributed more than 10% of our consolidated revenues during the past two fiscal years are as following.
16
| Sales (in | ||||||
| U.S. | Percent of | |||||
| Name | Products Sold | Dollars) | Sales | |||
| FY2015 | ||||||
| Hao Liu | Waterlilies Soft Capsule, Propolis and Black Ant Capsule | 1,109,209 | 10.85% | |||
| Yufeng Shen | Waterlilies Soft Capsule, Propolis and Black Ant Capsule | 1,100,924 | 10.77% | |||
| FY2014 | ||||||
| Hao Liu | Waterlilies Soft Capsule, Propolis and Black Ant Capsule | 1,049,929 | 10.81% | |||
| Yufeng Shen | Waterlilies Soft Capsule, Propolis and Black Ant Capsule | 1,033,212 | 10.64% |
Manufacture
We manufacture our health food products on a plot of land located in Jin Xing Industrial Park, Songbei District, Harbin. On June 7, 2004, the Company entered into a Land Use Purchase Contract with the local government, pursuant to which the Company agreed to purchase the right to use a piece of land, approximately 8 acres (32,000 square meters), located in Harbin City, Heilongjiang Province for commercial purposes for a fifty-year period from June 7, 2004 through June 6, 2054, for $637,261 (RMB5,248,000). The Company has fully paid to the government the consideration for the land use right on June 13, 2004. The Department of Housing and Urban Development of Harbin City approved this transaction. The Company is in the process of applying for the title certificate from the local government. The manufacturing facility on the land is 4,000 square meters and there are five production lines which is sufficient for our purposes. We package our products in bottles, plastic containers and aluminum foil bags there.
After we acquired HLJ Huimeijia on November 22, 2013, we also manufacture our medicines and drugs using HLJ Huimeijia’s land, approximately 43,350 square meters, located in Hai-lin Economic Development Zone, Mudanjiang City. The manufacturing facilities occupy approximately 5,710 square meters. We plan to build new manufacturing facilities on the land. The expected construction cost is approximately $8,950,000 (RMB 55,000,000).
17
Our Development Strategy
We will focus on combining our products with traditional Chinese medicine, the creation of new products, and developing our B2C e-business and chain-stores. We plan to implement health management projects in our future chain-stores throughout China and establish a database of our clients’ health from data obtained from our B2C e-business and call center.
We plan to establish a one-stop shop for our customer’s health needs. From conducting a genetic profile of our customer to determine his/her susceptibility to certain types of diseases and then customizing health supplements and organic/green food to meet his/her needs, we plan to cater to our customer’s needs at all levels. With the distribution network we hope to establish through our chain stores and B2C e-businesses, we plan to eventually branch into the sale and distribution of beauty products and medical appliances.
The Future
Within the next ten years, our goals are to:
| 1. |
Increase product coverage in target markets; achieve 20%-30% coverage |
Our target market is the health industry market. Presently, we believe that our product coverage is approximately 0.2%. We plan to open distribution stores in different provinces of China to expand our coverage. We also plan to sell our products through B2C websites to our customers.
| 2. |
Enter into the medicine, health product, health industry top 500 companies in the PRC |
Currently, we are not ranked in the top 500 medicine, health product and health industry companies in the PRC. We believe that if our projected increase in revenue is achieved, we will achieve our goal of becoming one of the top 500 medicine, health product, health industry companies in China.
| 3. |
Form a diversified management group |
Currently, our management group comprises people graduated from the most prestigious universities in the PRC, such as Peking University and Renmin University of China. We plan to further diversify management group by hiring talent both in the PRC and abroad.
18
| 4. |
Create an internationally famous brand |
Currently, our products are sold under the brand names “Qunle”, “Kindlink”, “Huimeijia” and “Dr. Xiao” in the PRC. Our goal is eventually to expand our sales abroad to countries such as the United States of America, Russia, and Eastern Europe and South-east Asian countries.
| 5. |
Enter into the international market |
Currently, we sell our products only in the PRC. We plan to sell our products to Russia, East Europe, and Southeast Asia in the future.
Our Business Plan
The plans designed to meet our manufacturing, marketing and profit targets include:
Manufacturing:
| (a) |
improving the manufacturing techniques and staff training; | |
| (b) |
guaranteeing high quality material supply; | |
| (c) |
strengthening the working procedure controls; | |
| (d) |
implementing GMP to ensure a compliance standard in the food and medical industries; | |
| (e) |
ensuring that all employees have adequate training in health regulations |
Marketing:
19
Adopt an effective marketing mode to:
| (a) |
utilize direct distribution of products to chain stores nationwide; | |
| (b) |
build business alliances with well-known enterprises to create private label brands; | |
| (c) |
expand the marketing of our products beyond the traditional methods. |
Product Distribution:
| (a) |
enlarge our sales and marketing force while developing new markets; | |
| (b) |
strengthen the distribution channel by developing promotion strategies and participating in trade shows; | |
| (c) |
develop 1-3 new products to market each year; | |
| (d) |
develop new markets through innovation and research |
Our approach to manufacturing, marketing, cost control and products distribution, which is detailed above, is designed to minimize production costs and increase revenue at the same time. We feel that our procedures will enable us to reach our sales goals with an optimal manufacturing cost. The result should yield profits and a return to our investors.
Good Manufacturing Practice or “GMP” is a term that is recognized worldwide for the control and management of manufacturing and quality control testing of foods and pharmaceutical products. An important part of GMP is documentation of every aspect of the process, activities, and operations involved with drug and medical device manufacture. Additionally, GMP requires that all manufacturing and testing equipment has been qualified as suitable for use, and that all operational methodologies and procedures (such as manufacturing, cleaning, and analytical testing) utilized in the drug manufacturing process have been validated (according to predetermined specifications), to demonstrate that they can perform their purported function(s).
20
The Market for Healthcare and Beauty Products
The health product industry is one of the mainstream industries in the PRC, since it has a high level of recognition and importance. Recently there have been new policies for health products, which control quality, manufacturing, manufacturing environments and techniques. With the PRC’s large and aging population there will be a steady demand for healthcare products. It is predicted that the healthcare and beauty industry will flourish over the next 50 years.
The Healthcare Product Market in the PRC
With thousands years of history in health culture and traditional Chinese medicine, PRC currently utilizes advanced technique and production capacity to initiate a new round of health care trend, from drugs and medicines to traditional health food and nutritional supplements, and from medical devices to health management and advices. The trend demonstrates huge potential in PRC’s health products market.
In December 2012, National Development and Reform Committee and Ministry of Industry and Information issued the 12th Five –year Development Plan for Food Industry (the “Plan”), which includes the Nutrition and Health Food Manufacturing Industry in national development. According to the Plan, in the year of 2015, output value of nutrition and health food manufacturing industry in PRC will reach RMB 1,000 billion with an average annual 20% growth rate. It is believed that there will be more than 10 companies with the annual sales over RMB 10 billion. PRC will next focus on the R&D and manufacturing of high-quality protein food, dietary fiber food and health food with new functions. Undoubtedly, all these government policies will inject new power to PRC’s health industry as well as provide guaranty in terms of favorable policy and regulations.
Competition in the Healthcare Products Industry
We believe our competitors are:
Harbin DaZhong Pharmaceutical Co., Ltd.(Located in Harbin, Heilongjiang Province);
Tsinghua Unisplendour Corporation Limited (Located in Weihai City, Shandong Province);
Yeecare Company (Located in Beijing);
Heilongjiang Tianlong Pharmaceuticals Co., Ltd (Located in Heilongjiang Province); and
HPGC Renmintongtai Pharmaceuticals Co., Ltd (Located in Heilongjiang Province).
21
Our Competitive Advantages and Strategy
We believe that we have the following competitive advantages over our competitors:
| • | We have more categories of products and a diversified production line; | |
| • | We have a strong and effective research and development team; | |
| • | We are a self-owned enterprise, and have the support of the local government; | |
| • | We have a geographical advantage being located in Heilongjiang Province, the center of the healthcare industry in the PRC. |
Sales and Marketing
We plan to open more chain stores throughout the PRC. Customers who are members of our stores could enjoy discounted price of our products and services. After establishing enough stores, we plan to develop a 24-hour delivery system for our B2C e-business.
We incurred expenses of $5,640 for advertising and promotion for the fiscal year of 2015. We have budgeted approximately $500,000 for advertising and promotion for the fiscal year of 2016.
Intellectual Property
We have received a patent (200610010394.4) for our “Qunle” brand Sailuozhi soft capsule from the National Bureau of Intellectual Property. We had initially applied for and used the trade name of “RunChao” soft capsules but the trade name was changed to “Qunle”, and the change has been approved by the National Bureau of Intellectual Property.
Pursuant to a Technology Transfer Agreement dated October 12, 2007 (“Kindlink Technology Transfer Agreement”), we purchased for a total of RMB350,000 the technology, manufacturing, and trademark rights to the health product known as “Kindlink” brand propolis and black ant capsule made from propolis, black ant, acanthopanax, astragalus root from Jindelikang. The change of the ownership has been approved by the CFDA. This product is consumed to boost one’s immunity. The certification number issued by the CFDA on August 20, 2004, to permit the manufacture of the product is GuoShiJianZi G20040906. We have no continuing obligations under the Kindlink Technology Transfer Agreement.
22
We have the following 12 trademarks:
| Certificate | ||||||||
| Trademark | No. | Category | Registrant | Valid Term | ||||
| “Qunle” with an arch image | 3895929 |
No.5 : Dietetic foods adapted for medical purposes; Food preparations adapted for medical purposes; Albuminous milk; Dietetic beverages adapted for medical purposes; Milk sugar; Diabetic bread; Albuminous foodstuffs for medical purposes; Food for babies; Dietetic substances adapted for medical use; Nutritional additives for medical purposes |
Humankind | 7/7/2006 to 7/6/2016 | ||||
| “Qunle” | 3896026 |
No.5 : Food preparations adapted for medical purposes; Albuminous milk; Dietetic beverages adapted for medical purposes; Milk sugar; Diabetic bread; Albuminous foodstuffs for medical purposes; Food for babies; Dietetic substances adapted for medical use; Nutritional additives for medical purposes |
Humankind | 7/7/2006 to 7/6/2016 | ||||
| “Wangzu” | 4857905 |
No.30: Molasses for food; Honey; pollen healthy grease; tortoise tuchahoe paste; breed columbine extract; helix alga; non-medicial nutrition liquid; non-medicial nutrition powder; non-medicial nutrition capsule; sugar candy bird’s nest |
Humankind | 5/14/2008 to 5/13/2018 | ||||
| “Kindlink” | 3236981 |
No.5: Food preparations adapted for medical purposes; Dietetic substances adapted for medical use |
Humankind | 12/7/2013 to 12/06/2023 | ||||
| “Huimeijia” | 5280303 |
No.5 : Medicine for human consumption; Medical nutrition capsule; Fibres (Edible plant) [non-nutritive]; Injection; Raw material drug; Troche; suppository; Food preparations adapted for medical purposes; Dietetic foods adapted for medical purposes; Dietetic substances adapted for medical use |
Humankind | 7/21/2009 to 7/20/2019 |
23
| “Huide” | 5280304 |
No.5 : Medicines for human consumption; Medical nutrition capsule; Fibres (Edible plant) [non-nutritive]; Injection; Raw material drug; Troche; suppository; Food preparations adapted for medical purposes; Dietetic foods adapted for medical purposes; Dietetic substances adapted for medical use |
Humankind | 7/21/2009 to 7/20/2019 | ||||
|
|
||||||||
| “KDLK” | 3230404 |
No.5 : Food preparations adapted for medical purposes; Dietetic foods adapted for medical purposes; Dietetic substances adapted for medical use |
Humankind | 9/28/2013 to 9/27/2023 | ||||
|
|
||||||||
| “dr.xiao” | 5176731 |
No.5 : Disinfectant; Medicines for veterinary purposes; Insecticide; Sanitary napkin; Medicine health bag; Dental lacquer |
Humankind | 8/14/2009 to 8/13/2019 | ||||
|
|
||||||||
| “dr.xiao” | 1610828 |
No.30: non-medicial nutrition liquid; non-medicial nutrition cream; non-medicial nutrition powder; Honey; non-medicial nutrition capsule; non-medicial nutrition gum; Candy for food; Spirulina (non-medicial nutrient); Candy; Pollen healthy grease |
Humankind | 7/28/2011 to 7/27/2021 | ||||
|
|
||||||||
| “DaLeNing” | 5053772 |
No.5 : Medicine for human; Chinese patent drugs; Suppository; Tincture; Water aqua; Paste; Liniment; Medical lotion; Patch; Chemical pharmaceuticals preparations |
HLJ Huimeijia | 5/7/2009 to 5/6/2019 | ||||
|
|
||||||||
| “Xuedu” | 5053657 |
No.5 : Medicine for human; Chinese patent drugs; Suppository; Tincture; Water aqua; Paste; Liniment; Medical lotion; Patch; Chemical pharmaceuticals preparations |
HLJ Huimeijia | 5/7/2009 to 5/6/2019 | ||||
|
|
||||||||
| “Xuedu” with an image | 642099 |
No.5 : Paste |
HLJ Huimeijia | 5/21/2013 to 5/20/2023 |
24
In addition, the trademark of “LB” and its associated image under the registration number 1738881was transferred from Guangzhou Aoda Biology Beauty Healthy Technology Co., Ltd to us on March 19, 2015, from whom we acquired 12 health products in January 2013.
We have the right to use the following patents under the approval of National Bureau of Intellectual Property:
| Patent | ||||||||||
| Categories | Name | Inventor/Designer | Patent No. | Duration | Owner | |||||
| Invention Patent | Runchao Soft Capsule and Its Manufacturing Method | Xin Sun | ZL200610010394.4 | August 10, 2006- August 9, 2026 | Xin Sun | |||||
| Utility Patent | Heating System in Compression Coaster with Coating Wheels | ZhengJiang Huang | ZL201220485432.2 | September 22, 2012- September 21, 2022 | HLJ Huimeijia | |||||
| Design Patent | Packing Box for Pain- relieving Ointment | Jianjun Wang | ZL201230448116.3 | September 19, 2012- September 18, 2022 | HLJ Huimeijia | |||||
| Design Patent | Packing Box for Nasal Mucus-releiving Ointment | Jianjun Wang | ZL201230448676.9 | September 19, 2012- September 18, 2022 | HLJ Huimeijia | |||||
| Design Patent | Packing Box for Gou Pi Plaster | Jianjun Wang | ZL201230447952.X | September 19, 2012- September 18, 2022 | HLJ Huimeijia | |||||
| Design Patent | Packing Box for Tendons and Bones Strengthening Musk Ointment | Jianjun Wang | ZL201230448670.1 | September 19, 2012- September 18, 2022 | HLJ Huimeijia | |||||
| Design Patent | Packing Box for Pain- relieving Musk Ointment | Jianjun Wang | ZL201230448010.3 | September 19, 2012- September 18, 2022 | HLJ Huimeijia |
25
The laws governing our business are as follows:
| • | Pharmaceutical administration law of the PRC enacted January 12, 2001 | |
| • | Healthcare registration and administration law, enacted January 7, 2005 | |
| • | Measures for the Administration of Pharmaceutical Trade License, enacted January 4, 2004 | |
| • | Measures for the Supervision Over and Administration of Pharmaceutical Production, enacted May 8, 2004 | |
| • | Food Safety Law of the PRC, enacted June 1, 2009 | |
| • | Regulation on the Implementation of the Food Safety Law of the PRC, enacted July 20, 2009 | |
| • | Regional regulation: Heilongjiang Regional Medicinal Materials Resource Protection Bylaw, enacted January 8, 2005 | |
| • | Good Manufacturing Practice (GMP) Amendment, enacted January 17, 2011 |
In the PRC, a Good Manufacturing Practice Certification (“GMP Certification”) is required for companies that produce medical drugs and health supplements. It is also required to market our medical drugs and health supplements. According to the Administrative Rules of Drug Manufacturing and Certification issued by the CFDA of the PRC on September 7, 2005, the CFDA is responsible for the review and issuance of GMP Certification. To obtain a GMP Certification, a company shall submit its application; the CFDA will then conduct a technical review of the application materials; if such company passes the technical review, the CFDA will inspect the manufacturing site. The CFDA also conducts follow-up inspections on the manufacturing site. After the issuance of the GMP Certification, the CFDA may inspect the manufacturing site from time to time. The GMP Certifications of our wholly owned subsidiaries, Humankind, HLJ Huimeijia and Huimeijia, are valid through February 14, 2019, December 31, 2015, and July 22, 2014, respectively. Once GMP Certification is obtained, we would be able to manufacture and market our products without further governmental approval. The Company did not renew the GMP Certificate for Huimeijia due to its pending assets sale to Xiuzheng Phamacy.
26
Employees
As of June 30, 2015, we have 149 employees including 9 officers, 47 administrators, 39 sales persons and 54 workers in manufacturing. We believe that we are in compliance with local prevailing wage, contractor licensing and insurance regulations, and have good relations with our employees.
We also have 19 independent workers for packing.
| Item 1A. | Risk Factors. |
We are a smaller reporting company and therefore this item is not applicable to us.
| Item 1B. | Unresolved Staff Comments. |
Not applicable.
| Item 2. | Properties. |
All land belongs to the state in PRC. Enterprises and individuals can pay the state a fee to obtain a right to use a piece of land for commercial purpose or residential purpose for an initial period of 50 years or 70 years, respectively. The land use right can be sold, purchased, and exchanged in the market. The land use right of a successor owner will be reduced by the amount of time consumed by the predecessor owner.
We manufacture our products on a plot of land located in Jin Xing Industrial Park, Songbei District, Harbin. On June 7, 2004, the Company entered into a Land Use Purchase Contract with the local government, pursuant to which the Company agreed to purchase the right to use a piece of land, approximately 8 acres (32,000 square meters), located in Harbin County, Heilongjiang Province for commercial purposes for a fifty-year period from June 7, 2004 through June 6, 2054, for $637,261 (RMB 5,248,000). The Company has fully paid to the government the consideration for the land use right on June 13, 2004. The Department of Housing and Urban Development of Harbin City approved this transaction. The Company is in the process of applying for the title certificate from the local government. The manufacturing facility on the land is 4,000 square meters and there are five production lines which is sufficient for our operation. We package our products in bottles, plastic containers and aluminum foil bags.
After we acquired HLJ Huimeijia on November 22, 2013, we also manufacture our medicines and drugs using HLJ Huimeijia’s land, approximately 43,350 square meters, located in Hai-lin Economic Development Zone, Mudanjiang City. The manufacturing facilities occupy approximately 5,710 square meters. We plan to build new manufacturing facilities on the land. The expected construction cost is approximately $8,950,000 (RMB 55,000,000).
27
In addition, the building of HLJ Huimeijia in the book value of $1,796,166 has been mortgaged for the working capital loan in the principal amount of $1,611,967 (RMB 10,000,000).
| Item 3. | Legal Proceedings. |
We do not know of any material, active, pending or threatened proceeding against us or our subsidiaries, nor are we, or any subsidiary, involved as a plaintiff or defendant in any material proceeding or pending litigation.
| Item 4. | Mine Safety Disclosures. |
This item is not applicable to us.
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Market Information
Our common stock is traded over-the-counter on the OTC Markets QB Tier under the ticker “CHHE” and the market for the stock has been relatively inactive. The range of high and low bid quotations for the quarters of the last two years ended June 30, 2015 and 2014 for which financial statements are included is listed below. The quotations are taken from Yahoo Finance. They reflect inter-dealer prices, without retail mark-up, mark-down or commission, and may not necessarily represent actual transactions.
| Calendar Quarter | High Bid | Low Bid | ||||
| Fiscal Year ended June 30, 2014 | ||||||
| First Quarter | $ | 0.08 | $ | 0.04 | ||
| Second Quarter | $ | 1.20 | $ | 0.04 | ||
| Third Quarter | $ | 0.95 | $ | 0.21 | ||
| Fourth Quarter | $ | 0.60 | $ | 0.02 | ||
| Fiscal Year ended June 30, 2015 | ||||||
| First Quarter | $ | 0.60 | $ | 0.03 | ||
| Second Quarter | $ | 0.18 | $ | 0.11 | ||
| Third Quarter | $ | 0.24 | $ | 0.11 | ||
| Fourth Quarter | $ | 0.20 | $ | 0.15 |
28
As of September 28, 2015, we had approximately 486 shareholders of record of our common stock, such number of holders does not include street name holders who hold shares by brokerage firms. The holders of common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. Holders of the common stock have no preemptive rights and no right to convert their common stock into any other securities. There are no redemption or sinking fund provisions applicable to the common stock.
Dividends
We have not paid dividends on our common stock and do not anticipate paying such dividends in the foreseeable future. We will rely on dividends from Humankind for our funds and PRC regulations may limit the amount of funds distributed to us from Humankind, which will affect our ability to declare any dividends.
Securities Authorized for Issuance Under Equity Compensation Plans
On March 27, 2015 the Board of Directors (the “Board”) adopted the Company’s 2015 Equity Incentive Plan (the “Plan”), which became effective as of such date. The total number of authorized shares under the Plan is 6,000,000 shares of common stock. For the material features of the Plan, please see Note 17.
The following table summarizes the number of shares of our common stock authorized for issuance under our the Plan as of June 30, 2015.
Equity Compensation Plan Information
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted-average exercise price of outstanding options, warrants and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) |
| Equity compensation plans approved by security holders | - | - | - |
| Equity compensation plans not approved by security holders | 0 | - | 2,700,000(1) |
| Total |
(1) The Company granted an aggregate of 3.3 million shares of restricted shares to its CEO and an employee on March 30, 2015.
Registrar and Stock Transfer Agent
Our stock transfer agent is Interwest Transfer Company, Inc. at 1981 Murray Holladay Road, Suite 100 Salt Lake City, UT 84117. Their telephone number is (801)272-9294, and their fax number is (801) 277-3147.
Shares Eligible for Future Sale
There is no established trading market for our common stock. Future sales of substantial amounts of our common stock in the trading market could adversely affect market prices.
29
Penny Stock Regulations
Our shares of common stock are subject to the “penny stock” rules of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and various rules thereunder. In general terms, “penny stock” is defined as any equity security that has a market price less than $5.00 per share, subject to certain exceptions. The rules provide that any equity security is considered to be a penny stock unless that security is registered and traded on a national securities exchange meeting specified criteria set by the SEC, issued by a registered investment company, and excluded from the definition on the basis of price (at least $5.00 per share), or based on the issuer’s net tangible assets or revenues. In the last case, the issuer’s net tangible assets must exceed $3,000,000 if in continuous operation for at least three years or $5,000,000 if in operation for less than three years or the issuer’s average revenues for each of the past three years must exceed $6,000,000.
Trading in shares of penny stock is subject to additional sales practice requirements for broker-dealers who sell penny stocks to persons other than established customers and accredited investors. Accredited investors, in general, include individuals with assets in excess of $1,000,000 or annual income exceeding $200,000 (or $300,000 together with their spouse), and certain institutional investors. For transactions covered by these rules, broker-dealers must make a special suitability determination for the purchase of the security and must have received the purchaser’s written consent to the transaction prior to the purchase. Additionally, for any transaction involving a penny stock, the rules require the delivery, prior to the first transaction, of a risk disclosure document relating to the penny stock. A broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, and current quotations for the security. Finally, monthly statements must be sent disclosing recent price information for the penny stocks. These rules may restrict the ability of broker-dealers to trade or maintain a market in our common stock, to the extent it is penny stock, and may affect the ability of shareholders to sell their shares.
Recent Sale of Unregistered Securities
None.
Repurchase of Equity Securities
None.
30
| Item 6. | Selected Financial Data. |
Not Applicable.
| Item 7. | Management ’s Discussion and Analysis of Financial Condition and Results of Operations. |
We make certain forward-looking statements in this report. Statements concerning our future operations, prospects, strategies, financial condition, future economic performance (including growth and earnings), demand for our services, and other statements of our plans, beliefs, or expectations, including the statements contained under the captions “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business,” as well as captions elsewhere in this document, are forward-looking statements. In some cases these statements are identifiable through the use of words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “project,” “target,” “can,” “could,” “may,” “should,” “will,” “would,” and similar expressions. The forward-looking statements we make are not guarantees of future performance and are subject to various assumptions, risks, and other factors that could cause actual results to differ materially from those suggested by these forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by the forward-looking statements. Indeed, it is likely that some of our assumptions will prove to be incorrect. Our actual results and financial position will vary from those projected or implied in the forward-looking statements and the variances may be material. You are cautioned not to place undue reliance on such forward-looking statements. These risks and uncertainties, together with the other risks described from time to time in reports and documents that we file with the SEC should be considered in evaluating forward-looking statements.
The nature of our business makes predicting the future trends of our revenue, expenses, and net income difficult. Thus, our ability to predict results or the actual effect of our future plans or strategies is inherently uncertain. The risks and uncertainties involved in our business could affect the matters referred to in any forward-looking statements and it is possible that our actual results may differ materially from the anticipated results indicated in these forward-looking statements. Important factors that could cause actual results to differ from those in the forward-looking statements include, without limitation, the following:
| • | the effect of political, economic, and market conditions and geopolitical events; | |
| • | legislative and regulatory changes that affect our business; | |
| • | the availability of funds and working capital; | |
| • | the actions and initiatives of current and potential competitors; | |
| • | investor sentiment; and | |
| • | our reputation. |
We do not undertake any responsibility to publicly release any revisions to these forward-looking statements to take into account events or circumstances that occur after the date of this report. Additionally, we do not undertake any responsibility to update you on the occurrence of any unanticipated events which may cause actual results to differ from those expressed or implied by any forward-looking statements.
The following discussion and analysis should be read in conjunction with our consolidated financial statements and the related notes thereto as filed with the SEC and other financial information contained elsewhere in this report.
Except as otherwise indicated by the context, references in this report to “we,” “us,” “our,” “the Registrant,” “our Company,” or “the Company” are to China Health Industries Holdings, Inc., a Delaware corporation, China Health Industries Holdings Limited, a corporation incorporated under the laws of Hong Kong, its wholly owned subsidiary in China, Harbin Humankind Biology Technology Co. Limited (“Humankind”) and indirect 99% owned subsidiary, Harbin Huimeijia Medicine Company (“Huimeijia”) and indirect wholly owned subsidiary, Heilongjiang Huimeijia Pharmaceutical Co., Ltd. (“HLJ Huimeijia”). Unless the context otherwise requires, all references to (i) “PRC” and “China” are to the People’s Republic of China; (ii) “U.S. dollar,” “$” and “US$” are to United States dollars; (iii) “RMB” are to Renminbi Yuan of China; (iv) “Securities Act” are to the Securities Act of 1933, as amended; and (v) “Exchange Act” are to the Securities Exchange Act of 1934, as amended.
Business Overview
Our principal business operations are conducted through our wholly owned subsidiaries, Humankind and HLJ Huimeijia.
The Company owns a GMP-certified plant and facilities and has the capacity to produce 21 CFDA-approved medicines and 14 CFDA-approved health supplement products in soft capsule, hard capsule, tablet, granule and oral liquid forms. These products address the needs of key sectors, including the feminine, geriatric and children’s markets in China.
31
Our business is conducted through our sales agents and sales personnel. We sell our products directly to end customers by our own sales personnel as well as our sales agents, operating primarily in Jiangsu, Zhejiang, Gansu, Shanghai, Anhui and Beijing, where most of our revenues are generated. Our sales through agents in Beijing, Zhejiang and Jiangsu provinces accounted for 13%, 13%, and 11% of our total sales, respectively, for the year ended June 30, 2015. Although we do not currently sell our products online, we expect to do so in the future.
2016 Outlook
Overall, we anticipate our total revenues for the year ended June 30, 2016 versus the year ended June 30, 2015 to increase by 9.5% or approximately $1 million, with growth in all categories of our product sales, including the anticipated revenue from Humankind for approximately $9.6 million and from HLJ Huimeijia for approximately $1.6 million. The gross profit margin for the year ended June 30, 2016 is expected to be approximately 29%, and we estimate our overall net profit margin for the year ended June 30, 2016 to be approximately 8.9%. There is, however, no assurance that we will reach these projections.
Results of Operations
The following table summarizes the top lines of the results of our operations for the years ended June 30, 2015 and 2014, respectively:
| June 30, 2015 | June 30, 2014 | Variance | % | |||||||||
| Revenues | $ | 10,226,052 | $ | 9,709,099 | $ | 516,953 | 5.3% | |||||
| Humankind | 8,656,245 | 8,356,422 | 299,823 | 3.6% | ||||||||
| HLJ Huimeijia | 1,569,807 | 1,352,677 | 217,130 | 16.1% | ||||||||
| Cost of Goods Sold | $ | 7,290,174 | $ | 7,315,171 | $ | (24,997 | ) | -0.3% | ||||
| Humankind | 6,086,263 | 6,286,579 | (200,316 | ) | -3.2% | |||||||
| HLJ Huimeijia | 1,203,911 | 1,028,592 | 175,319 | 17.0% | ||||||||
| Gross Profit | $ | 2,935,878 | $ | 2,393,928 | $ | 541,950 | 22.6% | |||||
| Humankind | 2,569,982 | 2,069,843 | 500,139 | 24.2% | ||||||||
| HLJ Huimeijia | 365,896 | 324,085 | 41,811 | 12.9% |
Revenue
Total revenues increased by $516,953, or 5.3%, for the year ended June 30, 2015 as compared to the same period in 2014. The increase in revenues was primarily due to an increase of $299,823 or 3.6% in Humankind’s revenues and an increase of $217,130 or 16.1% in HLJ Huimeijia’s revenues for the year ended June 30, 2015 as compared to the same period in 2014. The reason for the increase of the sales revenue with a decreasing sales volume in Humankind is that from November 2014 the sales price of Humankind’s primary product, Waterlilies Soft Capsule (Sailuozhi), was adjusted. For HLJ Huimeijia, the increased revenue was mainly due to the increased sales volume of its main products. The Company strives to expand the variety of its products and has purchased 12 new products from Guangzhou Aoda Biology Beauty Healthy Technology Co. Ltd in 2013. However, the majority of the 12 products were either under the review by the CFDA or in the process of Corporate Standard Registration, a backup registration not subject to the CFDA approval but subject to the CFDA’s monitor on the production after such a registration. The Company plans to accelerate the registration process and manufacture and release four to six new products to form a series of health-care products in the near future. The Company is also considering developing new sales areas by acquiring new distributors or exploring market for medical products in order to enhance the profits.
Our total cost of sales decreased $24,997 or 0.3% for the year ended June 30, 2015 as compared to the same period in 2014. The decrease in cost of sales was primarily due to a decrease of $200,316 or 3.2% in Humankind’s cost of sales which offset an increase of $175,319 or 17% in HLJ Huimeijia’s cost of sales for the year ended June 30, 2015 as compared to the same period in 2014. This decrease was primarily due to the decrease in sales volume of Waterlilies Soft Capsule, Humankind’s primary product.
Our gross margin increased $541,950 from $2,393,928 for the year ended June 30, 2014 to $2,935,878 for the year ended June 30, 2015. This increase was attributable to the rise of the sales prices of two primary products.
Sales by Product Line
The following table summarizes a breakdown of our sales by major product line for the year ended June 30, 2015 and 2014, respectively:
32
| June 30, 2015 | June 30, 2014 | |||||||||||||||||
| % of | Quantity | % of | ||||||||||||||||
| Quantity (Unit) | Sales US$ | Sales | (Unit) | Sales US$ | Sales | |||||||||||||
| Humankind | ||||||||||||||||||
| Waterlilies Soft Capsule (Sailuozhi) | 81,459 | $ | 5,408,370 | 52.89% | 85,520 | $ | 5,265,350 | 54.2% | ||||||||||
| Propolis and Black Ant Capsule | 108,995 | 3,247,876 | 31.76% | 108,753 | 3,091,074 | 31.8% | ||||||||||||
| HLJ Huimeijia | ||||||||||||||||||
| Muskiness Bone Strengthener Paste | 1,900,489 | $ | 530,890 | 5.19% | 1,479,160 | $ | 399,882 | 4.1% | ||||||||||
| Muskiness Pain Relieving Paste | 748,259 | 224,284 | 2.19% | 566,066 | 156,573 | 1.6% | ||||||||||||
| Injury and Rheumatism relieving Paste | 730,511 | 200,400 | 1.96% | 581,949 | 136,873 | 1.4% | ||||||||||||
| Refining GouPi Cream | 968,856 | 256,856 | 2.51% | 820,742 | 192,673 | 2.0% | ||||||||||||
| Enema Glycerini | 1,546,057 | 154,746 | 1.51% | 1,721,896 | 167,589 | 1.7% | ||||||||||||
| Umguentum Acidi Borici Camphoratum | 194,747 | 62,186 | 0.61% | 336,676 | 102,402 | 1.1% | ||||||||||||
| Injury and Paralysis Tincture | 19,876 | 20,354 | 0.20% | 35,960 | 38,748 | 0.4% | ||||||||||||
| Pelvic Inflammation Suppository | 39,276 | 22,578 | 0.22% | 39,167 | 21,682 | 0.2% | ||||||||||||
| Indometacin and Furazolidone Suppositories | 101,540 | 52,359 | 0.51% | 51,739 | 24,936 | 0.3% | ||||||||||||
| Compound Fluocinonide Tincture | - | - | 0.00% | 480 | 434 | 0.0% | ||||||||||||
| Ethacriding Lactate Solution | - | - | 0.00% | 113,250 | 11,790 | 0.1% | ||||||||||||
| Ge Hong Beriberi Water | 46,597 | 14,356 | 0.14% | 155,350 | 45,009 | 0.5% | ||||||||||||
| Hydrogen Peroxide Solution | 96,710 | 10,748 | 0.11% | 240,371 | 24,554 | 0.3% | ||||||||||||
| Matrine Suppositories | - | - | 0.00% | 3,983 | 1,841 | 0.0% | ||||||||||||
| Triamcinolone Acetonide and Neomycin Paste | 292,350 | 20,049 | 0.20% | 409,952 | 27,689 | 0.3% | ||||||||||||
| Total | 29,539,748 | $ | 10,226,052 | 100.00 % | - | $ | 9,709,099 | 100.0% |
33
Operating Expenses
The following table summarizes our operating expenses for the years ended June 30, 2015 and 2014, respectively:
| June 30, 2015 | June 30, 2014 | Variance | % | |||||||||
| Operating Expenses | ||||||||||||
| Selling, general and administrative | $ | 2,123,459 | $ | 1,970,482 | $ | 152,977 | 7.8% | |||||
| Depreciation and amortization | 585,680 | 986,847 | (401,167 | ) | -40.7% | |||||||
| Research and development | - | 156,735 | (156,735 | ) | -100.0% | |||||||
| Total Operating Expenses | $ | 2,709,139 | $ | 3,114,064 | $ | (404,925 | ) | -13.0% |
Total operating expenses for the year ended June 30, 2015 decreased $404,925, or 13%, as compared to the corresponding period in 2014. The decrease in operating expenses was primarily attributable to an increase of $152,977, or 7.8% in selling, general and administrative for the year ended June 30, 2015 as compared to the same period in 2014, offset by a decrease of $401,167 or 40.7% in depreciation and amortization expense and $156,735 or 100% in research and development expense. The difference of depreciation and amortization is mainly due to the reversed adjustment of land use right amortization in 2014 caused by an estimated useful life change.
Interest Income and Interest Expense
Interest income was $97,432 for the year ended June 30, 2015, as compared to $111,810 for the year ended June 30, 2014. This decrease of $14,378, or 12.9%, was primarily due to the decrease in bank deposits. The balance of bank deposits was $21,123,027 as of June 30, 2015 and $27,232,074 as of June 30, 2014.
Interest expense was $125,608 for the year ended June 30, 2015, as compared to $111,758 for the year ended June 30, 2014. This increase of $13, 850, or 12.4% was primarily a result of the increased service charge and interest rates.
Income Taxes
Income taxes increased $234,905, from 0 for the year ended June 30, 2014 to $234,905 for the year ended June 30, 2015. The increase was due to the Company’s loss before income taxes in the amount of $658,527 for the year ended June 30, 2014, and the profit before tax for the year ended June 30, 2015,
Net Income(Loss) and Loss Per Share
Net income was $2,509 for the year ended June 30, 2015, as compared to net loss of $658,527 for the year ended June 30, 2014. The increase of $661,036, or 100.4% in net income was primarily attributable to the increase in revenues in the amount of $516,953, the decrease in cost of goods sold in the amount of $34,631, the decrease in depreciation and amortization, and research and development expenses in the amount of $401,167 and $156,735 respectively, partially offset by the increase in general and administrative expense of $214,053, and income tax in the amount of $234,905.
Income per share was $0.00004 for the years ended June 30, 2015 and loss per share was $0.01 for the same period for 2014. This decrease was caused by the above mentioned decreases in net loss.
Liquidity and Capital Resources
We believe our current working capital position, together with our expected future cash flows from operations, will be adequate to fund our operations in the ordinary course of business, anticipated capital expenditures, debt payment requirements and other contractual obligations for at least the next twelve months. However, this belief is based upon many assumptions and is subject to numerous risks, and there can be no assurance that we will not require additional funding in the future.
The following table summarizes our cash and cash equivalents position, our working capital, and our cash flow activity as of June 30, 2015 and 2014 and for each of the years then ended:
| 2015 | 2014 | |||||
| As of June 30 : | ||||||
| Cash and cash equivalents | $ | 21,123,027 | $ | 27,232,074 | ||
| Working capital | $ | 26,409,789 | $ | 25,936,346 | ||
| Inventories | $ | 841,239 | $ | 1,037,939 | ||
| For the year ended June 30 : | ||||||
| Cash provided by (used in): | ||||||
| Operating activities | $ | 1,917,140 | $ | (1,353,165 | ) | |
| Investing activities | $ | (8,682,581 | ) | $ | (131,380 | ) |
| Financing activities | $ | 628,180 | $ | 141,923 |
34
Cash and cash equivalents decreased $6,109,047, from $27,232,074 as of June 30, 2014 to $21,123,027 as of June 30, 2015, primarily attributable to net cash used in investing activities in the amount of $8,682,581, offset by net cash provided by operating activities and financing activities in the amount of $1,917,140 and $628,180 respectively.
Our working capital at June 30, 2015 was $26,409,789, compared to working capital of $25,936,346 at June 30, 2014. This increase of $473,443 or 1.8% was due to the increase of current assets of $815,668 and offset by the decrease of liabilities of $342,225.
Net cash provided in operating activities was $1,917,140 for the year ended June 30, 2015, primarily attributable to a net loss available to the Company in the amount of $222,439 and net increase in accounts payable of $103,735 and taxes payable of $226,038, partially offset by depreciation and amortization expenses of $814,941 as reconciled and net decrease in accounts receivable of $802,366, decrease of inventory of $197,703 and increase of advances from customers and other payables of $471,798.
Net cash used by investing activities was $8,682,581 for the year ended June 30, 2015, mainly due to increase in short term investment of $8,080,845. The Company entered into an investment agreement with a financial investment company in June 2015. The agreement allows the Company to invest up to RMB 50 million (approximately $8 million) for a period of one year. The rates of return on these investments were 10% if the Company does not withdraw the investment funds during the one-year period. The Company’s funds deposited with the financial investment company are not insured.
Net cash provided by financing activities was $628,180 for the year ended June 30, 2015, mainly attributable to proceeds from related party debts in the amount of $650,354 and capital contributed by non-controlling interest owner of $494,670, partially offset by payment of related party debts in the amount of $517,174. The positive effect of exchange rate changes on cash and cash equivalents in the amount of $28,214 for the year ended June 30, 2015 was mainly a result of the effect of the appreciation of the RMB to the USD on the significant amount of cash and cash equivalents held by the Company in RMB. The average exchange rates from USD to RMB were 6.1875 to 1 and 6.2036 to 1 for the years ended June 30, 2015 and 2014, respectively.
Related Party Debts
We had related party debts of $1,910,546 as of June 30, 2015, as compared to $1,776,851 as of June 30, 2014, an increase of $133,695 or 8%, which is mainly attributable to a loan in the amount of $650,543 from Mr. Xin Sun, the CEO of the Company for the construction of the factory. The loan is unsecured and non-interest bearing and has no fixed terms of repayment. There was no written agreement for the loan.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that are currently material or reasonably likely to be material to our financial position or results of operations.
Critical Accounting Policies
Management's discussion and analysis of its financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with US GAAP. Our financial statements reflect the selection and application of accounting policies which require management to make significant estimates and judgments. The discussion of our critical accounting policies contained in Note 2 to our consolidated financial statements, “Significant Accounting Policies”, is incorporated herein by reference.
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk. |
Not Applicable.
| Item 8. | Financial Statements and Supplementary Data. |
The financial statements required by this item are set forth beginning on page F-1.
35
INDEX TO CONSOLIDATED AND COMBINED STATEMENTS
Audited Consolidated and Combined Financial Statements:
Reports of Independent Registered Public Accounting Firm
Consolidated and Combined Balance Sheets as of June 30, 2015 and 2014
Consolidated and Combined Statements of Operations and Comprehensive Income For the Years Ended June 30, 2015 and 2014
Consolidated and Combined Statements of Equity For the Years Ended June 30, 2015 and 2014
Consolidated and Combined Statements of Cash Flows For the Years Ended June 30, 2015 and 2014
Notes to Consolidated and Combined Financial Statements
36
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of:
China Health
Industries Holdings, Inc.
We have audited the accompanying consolidated and combined balance sheets of China Health Industries Holdings, Inc. and Subsidiaries (the “Company”) as of June 30, 2015, and the related consolidated and combined statements of operations and comprehensive income, equity, and cash flows for the fiscal years then ended. The Company’s management is responsible for these consolidated and combined financial statements. Our responsibility is to express an opinion on these consolidated and combined financial statements based on our audits. The consolidated financial statements of the Company as of and for the year ended June 30, 2014, were audited by other auditors; whose report dated Sept. 29, 2014; express an unqualified opinion on those consolidated financial statements.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated and combined financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated and combined financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated and combined financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated and combined financial statements referred to above present fairly, in all material respects, the consolidated and combined financial positions of China Health Industries Holdings, Inc. and Subsidiaries as of June 30, 2015 and the consolidated and combined results of their operations and their cash flows for the fiscal years then ended in conformity with accounting principles generally accepted in the United States of America.
/s//CANUSWA ACCOUNTING & TAX SERVICES INC
San Jose, California
October 13, 2015
37
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of:
China Health
Industries Holdings, Inc.
We have audited the accompanying consolidated and combined balance sheets of China Health Industries Holdings, Inc. and Subsidiaries (the “Company”) as of June 30, 2014, and the related consolidated and combined statements of operations and comprehensive income, equity, and cash flows for the fiscal years then ended. The Company’s management is responsible for these consolidated and combined financial statements. Our responsibility is to express an opinion on these consolidated and combined financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated and combined financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated and combined financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated and combined financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated and combined financial statements referred to above present fairly, in all material respects, the consolidated and combined financial positions of China Health Industries Holdings, Inc. and Subsidiaries as of June 30, 2014 and the consolidated and combined results of their operations and their cash flows for the fiscal years then ended in conformity with accounting principles generally accepted in the United States of America.
/s/ KCCW Accountancy Corp.
Diamond Bar, California
September 29, 2014
38
| CHINA HEALTH INDUSTRIES HOLDINGS, INC. AND SUBSIDIARIES |
| CONSOLIDATED AND COMBINED BALANCE SHEETS |
| (Audited) |
| June 30, 2015 | June 30, 2014 | |||||
| ASSETS | ||||||
| Current assets | ||||||
| Cash and cash equivalents | $ | 21,123,027 | $ | 27,232,074 | ||
| Short term investment | 8,064,516 | - | ||||
| Notes receivable | 1,509 | 28,095 | ||||
| Accounts receivable, net | 1,431,298 | 2,230,746 | ||||
| Inventory | 841,239 | 1,037,939 | ||||
| Other receivables, net | 39,852 | 83,570 | ||||
| Advance to suppliers | 69,120 | 40,504 | ||||
| Prepaid expenses | - | 101,965 | ||||
| Total current assets | 31,570,561 | 30,754,893 | ||||
| Property, plant and equipment, net | 4,315,094 | 4,661,072 | ||||
| Intangible assets, net | 5,012,297 | 5,452,161 | ||||
| Construction in progress | 607,477 | 1,934 | ||||
| Total assets | $ | 41,505,429 | $ | 40,870,060 | ||
| LIABILITIES AND EQUITY | ||||||
| Current liabilities | ||||||
| Short-term loan | $ | 1,612,903 | $ | 1,611,967 | ||
| Accounts payable and accrued expenses | 523,946 | 627,109 | ||||
| Other payable | 29,684 | 83,798 | ||||
| Advance from customers | 770,454 | 245,308 | ||||
| Related party debts | 1,910,546 | 1,776,851 | ||||
| Wages payable | 134,615 | 69,544 | ||||
| Taxes payable | 178,625 | 403,970 | ||||
| Total current liabilities | 5,160,773 | 4,818,547 | ||||
| Equity | ||||||
| Common stock, ($0.0001 par
value, 300,000,000 shares
authorized, 65,539,737 and 62,239,737 issued and outstanding as of June 30, 2015 and 2014, respectively) |
6,404 | 6,224 | ||||
| Additional paid-in capital | 297,137 | 27,317 | ||||
| Accumulated other comprehensive income | 3,263,592 | 3,242,959 | ||||
| Statutory reserve | 38,679 | 38,679 | ||||
| Retained earnings | 32,738,642 | 32,736,081 | ||||
| Total stockholders' equity | 36,344,454 | 36,051,260 | ||||
| Non-controlling interests | 202 | 253 | ||||
| Total equity | 36,344,656 | 36,051,513 | ||||
| Total liabilities and equity | $ | 41,505,429 | $ | 40,870,060 |
The accompanying notes are an integral part of these consolidated and combined financial statements.
F-1
| CHINA HEALTH INDUSTRIES HOLDINGS, INC. AND SUBSIDIARIES |
| CONSOLIDATED AND COMBINED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME |
| (Audited) |
| For the Year Ended | ||||||
| June 30, 2015 | June 30, 2014 | |||||
| REVENUE | $ | 10,226,052 | $ | 9,709,099 | ||
| COST OF GOODS SOLD | 7,290,174 | 7,315,171 | ||||
| GROSS PROFIT | 2,935,878 | 2,393,928 | ||||
| OPERATING EXPENSES | ||||||
| Selling, general and administrative expenses | 2,123,459 | 1,970,482 | ||||
| Depreciation and amortization expenses | 585,680 | 986,847 | ||||
| Research and development expenses | - | 156,735 | ||||
| 2,709,139 | ||||||
| Total operating expenses | 3,114,064 | |||||
| INCOME(LOSS) FROM OPERATIONS | 226,739 | (720,136 | ) | |||
| OTHER INCOME/(EXPENSES) | ||||||
| Interest income | 97,432 | 111,810 | ||||
| Interest expense | (125,608 | ) | (111,758 | ) | ||
| Other income, net | 38,851 | 61,557 | ||||
| Total other income, net | 10,675 | 61,609 | ||||
| INCOME(LOSS) BEFORE INCOME TAXES | 237,414 | (658,527 | ) | |||
| Provision for income taxes | 234,905 | - | ||||
| NET INCOME (LOSS) | 2,509 | (658,527 | ) | |||
| Less: net loss attributable to non-controlling interests | (52) | (60 | ) | |||
| Net income (loss) attributable to China Health Industries Holdings | 2,561 | (658,467 | ) | |||
| Foreign currency translation gain (loss) | 20,582 | (393,356 | ) | |||
| Comprehensive income (loss) | 23,091 | (1,051,883 | ) | |||
| Less: comprehensive loss attributable to non-controlling interests | 51 | (63 | ) | |||
| COMPREHENSIVE INCOME (LOSS) ATTRIBUTABLE TO CHINA HEALTH INDUSTRIES HOLDINGS | $ | 23,040 | $ | (1,051,820 | ) | |
| Net loss attributable to China Health Industries Holdings' shareholders per share are: | ||||||
| Basic & diluted loss per share | $ | (0.00 | ) | $ | (0.01 | ) |
| Weighted average shares outstanding: | ||||||
| Basic & diluted weighted average shares outstanding | 63,044,395 | 62,239,737 | ||||
The accompanying notes are an integral part of these consolidated and combined financial statements.
F-2
| CHINA HEALTH INDUSTRIES HOLDINGS, INC. AND SUBSIDIARIES |
| CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY |
| (Audited) |
|
|
|||||||||||||||||||||||||||
|
|
Common Shares | Accumulated | |||||||||||||||||||||||||
|
|
Additional | Other | Total | Non- | |||||||||||||||||||||||
|
|
Paid-in | Retained | Statutory | Comprehensive | Stockholders' | controlling | Total | ||||||||||||||||||||
|
|
Shares | Amount | Capital | Earnings | Reserve | Income (loss) | Equity | Interest | Equity | ||||||||||||||||||
|
|
|||||||||||||||||||||||||||
|
Balance, June 30, 2013 |
62,239,737 | $ | 6,224 | $ | 27,317 | $ | 33,394,548 | $ | 38,679 | $ | 3,636,312 | $ | 37,103,080 | $ | 316 | $ | 37,103,396 | ||||||||||
|
|
|||||||||||||||||||||||||||
|
Net loss |
- | - | - | (658,467 | - | - | (658,467 | ) | (60 | ) | (658,527 | ||||||||||||||||
|
|
|||||||||||||||||||||||||||
|
Other comprehensive income |
- | - | - | - | - | (393,353 | ) | (393,353 | ) | (3 | ) | (393,356 | ) | ||||||||||||||
|
|
|||||||||||||||||||||||||||
|
Balance, June 30, 2014 |
62,239,737 | $ | 6,224 | 27,317 | $ | 32,736,081 | 38,679 | $ | 3,242,959 | $ | 36,051,260 | $ | 253 | $ | 36,051,513 | ||||||||||||
|
|
|||||||||||||||||||||||||||
|
Net loss |
- | - | - | 2,509 | - | - | 2,509 | $ | 2,509 | ||||||||||||||||||
|
|
|||||||||||||||||||||||||||
|
Accrued compensation expense
|
- | 180 | - | - | - | 180 | - | 180 | |||||||||||||||||||
|
|
|||||||||||||||||||||||||||
|
Additional Paid in capital |
- | - | 269,820 | - | - | - | 269,820 | - | 269,820 | ||||||||||||||||||
|
|
|||||||||||||||||||||||||||
|
Other comprehensive loss |
- | - | - | 52 | - | 20,633 | 20,686 | (51 | ) | 20,634 | |||||||||||||||||
|
|
|||||||||||||||||||||||||||
|
Balance, June 30, 2015 |
62,239,737 | $ | 6,404 | $ | 297,137 | $ | 32,738,642 | $ | 38,679 | $ | 3,263,592 | 36,344,454 | 202 | 36,344,656 | |||||||||||||
The accompanying notes are an integral part of these consolidated and combined financial statements.
F-3
| CHINA HEALTH INDUSTRIES HOLDINGS, INC. AND SUBSIDIARIES |
| CONSOLIDATED AND COMBINED STATEMENTS OF CASH FLOWS |
| (Audited) |
| For the Year Ended | ||||||
| June 30, 2015 | June 30, 2014 | |||||
| Cash Flows from Operating Activities | ||||||
| Net loss available to China Health Industries Holdings | $ | 2,561 | $ | (658,467 | ) | |
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: | ||||||
| Depreciation and amortization expenses | 814,941 | 1,136,042 | ||||
| Bad debt expense | - | 35,971 | ||||
| Non-controlling interests | (52 | ) | (60 | ) | ||
| Changes in operating assets and liabilities | ||||||
| Accounts receivable | 802,366 | (2,154,160 | ) | |||
| Other receivables | 43,855 | 23,555 | ||||
| Inventory | 197,703 | (44,487 | ) | |||
| Advance to suppliers, prepaid expenses and long term deposit | 73,580 | 184,324 | ||||
| Accounts payables and accrued expenses | (103,735 | ) | 91,141 | |||
| Advance from customers and other payables | 471,798 | (4,959 | ) | |||
| Wages payable | 65,161 | (114,790 | ) | |||
| Taxes payable | (226,038 | ) | 152,725 | |||
| Net cash provided by (used in) operating activities | 2,142,140 | (1,353,165 | ) | |||
| Cash Flows from Investing Activities | ||||||
| Increase in short term investment | (8,080,845 | ) | - | |||
| Decrease in notes receivable | 26,656 | 6,015 | ||||
| Purchases of property, plant and equipment | (21,624 | ) | (125,991 | ) | ||
| Purchases of intangible assets | - | (11,404 | ) | |||
| Increase in construction in progress | (606,768 | ) | - | |||
| Net cash used in investing activities | (8,682,581 | ) | (131,380 | ) | ||
| Cash Flows from Financing Activities | ||||||
| Compensation for vested shares | 180 | - | ||||
| Payment of related party debts | (517,174 | ) | (376,175 | ) | ||
| Proceeds from related party debts | 650,354 | 29,339 | ||||
| Capital contributed by non-controlling interest owner | 269,820 | - | ||||
| Proceeds from short-term loan | - | 488,759 | ||||
| Net cash provided by financing activities | 403,180 | 141,923 | ||||
| Effect of exchange rate changes on cash and cash equivalents | 28,214 | (293,837 | ) | |||
| Net decrease in cash and cash equivalents | (6,109,047 | ) | (1,636,459 | ) | ||
| Cash and cash equivalents, beginning balance | 27,232,074 | 28,868,533 | ||||
| Cash and cash equivalents, ending balance | $ | 21,123,027 | $ | 27,232,074 | ||
| Supplemental cash flow information | ||||||
| Cash paid for income taxes | $ | 336,058 | $ | 48,361 | ||
| Cash paid for interest expense | $ | 125,608 | $ | 117,623 | ||
| Non-cash activities: | ||||||
| Loan from related party for the construction of a facility | $ | 492,932 | $ | - | ||
| Reclassification from construction in progress to property, plant and equipment | - | 2,342,845 | ||||
The accompanying notes are an integral part of these consolidated and combined financial statements.
F-4
CHINA HEALTH INDUSTRIES HOLDINGS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED AND COMBINED FINANCIAL STATEMENTS
Note 1 - ORGANIZATION AND BUSINESS BACKGROUND
China Health Industries Holdings, Inc. (“China Health US”) was incorporated in the State of Arizona on July 11, 1996 and was the successor of the business known as Arizona Mist, Inc. which began in 1989. On May 9, 2005, it entered into a Stock Purchase Agreement and Share Exchange (effecting a reverse merger) with Edmonds 6, Inc. (“Edmonds 6”), a Delaware corporation, and changed its name to Universal Fog, Inc. Pursuant to this agreement, Universal Fog, Inc. (which has been in continuous operation since 1996) became a wholly-owned subsidiary of Edmonds 6.
China Health Industries Holdings Limited (“China Health HK”) was incorporated on July 20, 2007 in Hong Kong under the Companies Ordinance as a limited liability company. China Health HK was formed for the purpose of seeking and consummating a merger or acquisition with a business entity organized as a private corporation, partnership, or sole proprietorship as defined by FASB ACS Topic 915 (“Development Stage Entities”).
Harbin Humankind Biology Technology Co., Limited (“Humankind”) was incorporated in Harbin City, Heilongjiang Province, the People’s Republic of China (the “PRC”) on December 14, 2003, as a limited liability company under the Company Law of the PRC. Humankind is engaged in the manufacturing and sale of health products.
On August 20, 2007, the sole shareholder of China Health HK entered into a share purchase agreement (the “Share Purchase Agreement”) with the owners of Humankind. Pursuant to the Share Purchase Agreement, China Health HK purchased 100% of the ownership in Humankind for a cash consideration of $60,408 (the “Share Purchase”). Subsequent to the completion of the Share Purchase, Humankind became a wholly-owned subsidiary of China Health HK. The Share Purchase was accounted for as a “reverse merger” since the owner of Humankind owned a majority of the outstanding shares of China Health HK’s common stock immediately following the execution of the Share Purchase Agreement, it was deemed to be the acquirer in the reverse merger. Consequently, the assets and liabilities and the historical operations that have been reflected in the financial statements for periods prior to the Share Purchase are those of Humankind and have been recorded at the historical cost basis. After completion of the Share Purchase, China Health HK’s consolidated financial statements include the assets and liabilities of both China Health HK and Humankind, the historical operations of Humankind, and the operations of China Health HK and its subsidiaries from the closing date of the Share Purchase.
On October 14, 2008, Humankind set up a 99% owned subsidiary, Harbin Huimeijia Medicine Company (“Huimeijia”), with its primary business being manufacturing and distributing medicine. Mr. Xin Sun, the Company’s majority owner, owns 1% of Huimeijia. Huimeijia is consolidated in the consolidated financial statements of China Health HK.
On December 31, 2008, China Health HK entered into a reverse merger with Universal Fog, Inc., a U.S. publicly traded shell company (the “Transaction”). China Health HK is the acquirer in the Transaction, and the Transaction has been treated as a recapitalization of China Health US. After the Transaction and a 20:1 reverse stock split, Mr. Xin Sun owned 61,203,088 shares of common stock, representing 98.3% of the 62,234,737 total outstanding shares of common stock of China Health US. On April 7, 2009, Mr. Sun transferred 28,200,000 shares of common stock to 296 individuals, leaving him with 33,003,088 shares of common stock of China Health US, or approximately 53.03% of the total outstanding shares of common stock. Universal Fog, Inc. changed its name to China Health Industries Holdings, Inc. on February 19, 2009.
On November 22, 2013, Humankind completed the acquisition of Heilongjiang Huimeijia Pharmaceutical Co., Ltd. (“HLJ Huimeijia”) for a total purchase price of $16,339,869 (RMB100,000,000) (the “Purchase Price”). HLJ Huimeijia was founded on October 30, 2003, and is engaged in the manufacturing and distribution of tincture, ointments, rubber paste (including hormones), topical solution, suppositories, liniment (including traditional Chinese medicine extractions), enemas and oral liquids. HLJ Huimeijia’s predecessor is Heilongjiang Xue Du Pharmaceutical Co., Ltd., which has established its brand name in the market through its supply of high quality medical products. HLJ Huimeijia is categorized as a “high and new technology” enterprise by the Science Technology Department in Heilongjiang Province. HLJ Huimeijia has 21 products which have been approved by, and have received approval numbers issued by, the China State Food and Drug Administration (the “CFDA”). In addition, HLJ Huimeijia is the holder of one patent for utility models, five patents for external design and three trademarks in China, including the Chinese brand name of “Xue Du” which has an established reputation among customers in northeastern China.
China Health US, China Health HK, Humankind, Huimeijia and HLJ Huimeijia are collectively referred herein to as the “Company.”
F-5
As of June 30, 2015, the Company’s corporate structure was as follows:

Note 2 - SIGNIFICANT ACCOUNTING POLICIES
This summary of significant accounting policies of the Company is presented to assist in understanding the Company’s financial statements. The financial statements and notes are representations of the Company’s management, which is responsible for their integrity and objectivity. These accounting policies conform to generally accepted accounting principles in the United States ("US GAAP") and have been consistently applied in the preparation of the consolidated financial statements.
Principles of Consolidation
The accompanying consolidated and combined financial statements include China Health US and its four subsidiary companies, including China Health HK, Humankind, Huimeijia, and HLJ Huimeijia. All significant intercompany balances and transactions have been eliminated in consolidation and combination.
On November 22, 2013, China Health US, through its wholly owned subsidiary Humankind, completed the acquisition of HLJ Huimeijia. HLJ Huimeijia and Humankind are under the common control of Mr. Xin Sun, the CEO of the Company before and after the date of transfer. Humankind’s accounting policy adopted the guidance in ASC 805-50-05-5 for the transfer of net assets between entities under common control to apply a method similar to the pooling-of-interests method. Under this method, the financial statements of Humankind shall report results of operations for the period in which the transfer occurs as though the transfer of net assets had occurred at the beginning of the period. Results of operations for that period will thus comprise both those of the previously separate entities combined from the beginning of the period to the date the transfer is completed and those of the combined operations from that date to the end of the period. Similarly, Humankind shall present the statements of financial position and other financial information as of the beginning of the period as though the assets and liabilities had been transferred at that date. Financial statements and financial information of Humankind presented for prior years also shall be retrospectively adjusted to furnish comparative information.
F-6
Segment Reporting
ASC 280, “Segment Reporting”, established standards for reporting information about operating segments on a basis consistent with the Company's internal organizational structure as well as information about geographical areas, business segments and major customers in financial statements for details on the Company's business segments. The Company has three reportable operating segments: Humankind, HLJ Huimeijia and others. The segments are grouped based on the types of products provided.
Fair Value of Financial Instruments
The provisions of accounting guidance, FASB ASC Topic 820 that apply to the Company requires all entities to disclose the fair value of financial instruments, both assets and liabilities recognized and not recognized on the balance sheet, for which it is practicable to estimate fair value, and defines fair value of a financial instrument as the amount at which the instrument could be exchanged in a current transaction between willing parties.
Fair Value Measurements
FASB ASC Topic 820, Fair Value Measurements and Disclosures, clarifies the definition of fair value for financial reporting, establishes a framework for measuring fair value and requires additional disclosures about the use of fair value measurements.
Various inputs are considered when determining the fair value of the Company’s debt. The inputs or methodologies used for valuing securities are not necessarily an indication of the risk associated with investing in these securities. These inputs are summarized in the three broad levels listed below.
Level 1 – observable market inputs that are unadjusted quoted prices for identical assets or liabilities in active markets.
Level 2 – other significant observable inputs (including quoted prices for similar securities, interest rates, credit risk, etc.).
Level 3 – significant unobservable inputs (including the Company’s own assumptions in determining the fair value of investments).
The carrying value of financial assets and liabilities recorded at fair value is measured on a recurring or nonrecurring basis. Financial assets and liabilities measured on a non-recurring basis are those that are adjusted to fair value when a significant event occurs. The Company had no financial assets or liabilities carried and measured on a nonrecurring basis during the reporting periods. Financial assets and liabilities measured on a recurring basis are those that are adjusted to fair value each time a financial statement is prepared. The Company had no financial assets or liabilities carried and measured on a recurring basis during the reporting periods.
The availability of inputs observable in the market varies from instrument to instrument and depends on a variety of factors including the type of instrument, whether the instrument is actively traded, and other characteristics particular to the transaction. For many financial instruments, pricing inputs are readily observable in the market, the valuation methodology used is widely accepted by market participants, and the valuation does not require significant management discretion. For other financial instruments, pricing inputs are less observable in the market and may require management judgment.
Translation of Foreign Currencies
Humankind, Huimeijia and HLJ Huimeijia maintain their books and accounting records in PRC currency “Renminbi” (“RMB”), which has been determined as the functional currency. Transactions denominated in currencies other than RMB are translated into RMB at the exchange rates prevailing on the date of the transactions, as quoted by the Federal Reserve Board. Foreign currency exchange gains and losses resulting from these transactions are included in operations.
Humankind, Huimeijia and HLJ Huimeijia’s financial statements are translated into the reporting currency, the United States Dollar (“USD”). Assets and liabilities of the above entities are translated at the prevailing exchange rate at each reporting period end date. Contributed capital accounts are translated using the historical rate of exchange when capital is injected. Income and expense accounts are translated at the average rate of exchange during the reporting period. Translation adjustments resulting from the translation of these financial statements are reflected as accumulated other comprehensive income in shareholders’ equity and non-controlling interests.
F-7
Statement of Cash Flows
In accordance with Statement FASB ASC Topic 230, Statement of Cash Flows, cash flow from the Company's operations is calculated based upon the local currencies and translated to the reporting currency using an average foreign exchange rate for the reporting period. As a result, amounts related to assets and liabilities reported in the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet.
Use of Estimates and Assumptions
The preparation of financial statements in conformity with US GAAP requires management to make estimates and judgments that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities on the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company bases its estimates and judgments on historical experience and on various other assumptions and information that are believed to be reasonable under the circumstances. Estimates and assumptions of future events and their effects cannot be perceived with certainty and, accordingly, these estimates may change as new events occur, as more experience is acquired, as additional information is obtained and as the Company’s operating environment changes. Significant estimates and assumptions by management include, among others; useful lives of long-lived assets and intangible assets, valuation of inventory, accounts receivable and notes receivable, impairment analysis of long-lived assets, construction in progress, intangible assets and deferred taxes. While the Company believes that the estimates and assumptions used in the preparation of the financial statements are appropriate, actual results could differ from those estimates. Estimates and assumptions are periodically reviewed and the effects of revisions are reflected in the financial statements in the period they are determined to be necessary.
Cash and Cash Equivalents
Cash and cash equivalents include cash on hand, deposits in banks with maturities of three months or less, and all highly liquid investments which are unrestricted as to withdrawal or use, and which have original maturities of three months or less at the time of purchase.
As of June 30, 2015 and 2014, the Company’s uninsured bank balance was mainly maintained at financial institutions located in the PRC and HK, totaled $21,123,027 and $27,232,074 respectively. The Company has no insured bank balance as of June 30, 2015 and 2014, respectively.
Accounts Receivable
Accounts receivable are recorded at the invoiced amount and do not bear interest. The Company extends unsecured credit to its customers in the ordinary course of business but mitigates the associated risks by performing credit checks and actively pursuing past due accounts. An allowance for doubtful accounts is established and determined based on management’s assessment of known requirements, aging of receivables, payment and bad debt history, the customer’s current credit worthiness, changes in customer payment patterns and the economic environment. From November 1, 2013, the Company changed its credit policy by offering ninety (90) day payment terms for sales, whereas the payment terms for sales before November 1, 2013 were thirty (30) day. As a result, as of June 30, 2015 and 2014, the balances of accounts receivable were $1,431,298 and $2,230,746, respectively. The Company determines the allowance based on aging data, historical collection experience, customer specific facts and economic conditions. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company evaluated the nature of all accounts receivable then provided allowance for doubtful accounts. As of June 30, 2015 and 2014, the balances of allowance for doubtful accounts were $45,453 and $45,463, respectively.
Advance to Suppliers
The Company periodically makes advances to certain vendors for purchases of raw materials, and records these purchases as advance to suppliers. As of June 30, 2015 and 2014, advance to suppliers amounted to $69,120 and $40,504, respectively.
Prepaid Expenses
Prepaid expenses principally include prepaid R&D expenses, prepaid income taxes and amount paid to secure the use of assets or the receipt of services at a future date or continuously over one or more future periods. As of June 30, 2015 and 2014, prepaid expenses amounted to nil and $101,965, respectively.
Inventory
Inventory consists of raw materials, work in progress and finished goods of manufactured products.
F-8
Inventory is stated at lower of cost or market and consists of materials, labor and overhead. HLJ Huimeijia uses the weighted average method for inventory valuation. The other entities of the Company use the first-in, first-out (“FIFO”) method for inventory valuation. Overhead costs included in finished goods include direct labor cost and other costs directly applicable to the manufacturing process. The Company evaluates inventory for excess, slow moving, and obsolete inventory as well as inventory the volume of which is in excess of its net realizable value. This evaluation includes analysis of sales levels by product and projections of future demand. If future demand or market conditions are less favorable than the Company’s projections, a write-down of inventory may be required, and would be reflected in cost of goods sold in the period the revision is made. There was no inventory allowance provided for the year ended June 30, 2015 and 2014, respectively.
Impairment of Long-Lived Assets
The Company’s long-lived assets and other assets are reviewed for impairment in accordance with the guidance of the FASB ASC Topic 360-10, “Property, Plant, and Equipment,” and FASB ASC Topic 205, “Presentation of Financial Statements.” The Company tests for impairment losses on long-lived assets used in operations whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. Recoverability of an asset to be held and used is measured by a comparison of the carrying amount of the asset to the future undiscounted cash flows expected to be generated by the asset. If such asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value. Impairment evaluations involve management’s estimates on asset useful lives and future cash flows. Actual useful lives and cash flows could be different from those estimated by management which could have a material effect on our reporting results and financial position. Fair value is determined through various valuation techniques including discounted cash flow models, quoted market values and third-party independent appraisals, as considered necessary. As of June 30, 2015 and 2014, the Company has not experienced impairment losses on its long-lived assets. However, there can be no assurances that demand for the Company’s products or services will continue, which could result in an impairment of long-lived assets in the future.
Property, Plant and Equipment
Property, plant and equipment are carried at the lower of cost or fair value. Maintenance, repairs and minor renewals are expensed as incurred, major renewals and improvements that extend the lives or increase the capacity of plant assets are capitalized.
When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in the results of operations in the reporting period of disposition.
Depreciation is calculated on a straight-line basis over the estimated useful life of the assets. The depreciable lives applied are:
| Building, Warehouse and Improvements | 20 to 30 years |
| Office Equipment | 3 to 7 years |
| Vehicles | 5 to 15 years |
| Machinery and Equipment | 7 to 15 years |
Intangible Assets
The Company evaluates intangible assets in accordance with FASB ASC Topic 350, “Intangibles — Goodwill and Other.” Intangible assets deemed to have indefinite lives are not amortized, but are subject to annual impairment tests. If the assumptions and estimates used to allocate the purchase price are not correct, or if business conditions change, purchase price adjustments or future asset impairment charges could be required. The value of the Company’s intangible assets could be impacted by future adverse changes such as: (i) any future declines in the Company’s operating results, (ii) a decline in the valuation of technology, including the valuation of the Company’s common stock, (iii) a significant slowdown in the worldwide economy or (iv) any failure to meet the performance projections included in the Company’s forecasts of future operating results. In accordance with FASB ASC Topic 350, the Company tests intangible assets for impairment on an annual basis or more frequently if the Company believes indicators of impairment exist. Impairment evaluations involve management estimates of asset useful lives and future cash flows. Significant management judgment is required in the forecasts of future operating results that are used in the evaluations. It is possible, however, that the plans and estimates used may be incorrect. If the Company’s actual results, or the plans and estimates used in future impairment analysis, are lower than the original estimates used to assess the recoverability of these assets, we could incur additional impairment charges in a future period. Based on such evaluations, there was no impairment recorded for intangible assets for the years ended June 30, 2015 and 2014, respectively.
F-9
Construction in Progress
Construction in progress represents the costs incurred in connection with the construction of buildings or new additions to the Company’s plant facilities. Costs classified as construction in progress include all costs of obtaining the asset and bringing it to the location and condition necessary for its intended use. No depreciation is provided for construction in progress until such time as the assets are completed and are placed into service.
The Company reviews the carrying value of construction in progress for impairment whenever events and circumstances indicate that the carrying value of an asset may not be recoverable from the estimated future cash flows expected to result from its use and eventual disposition. In cases where undiscounted expected future cash flows are less than the carrying value of the assets, an impairment loss is recognized equal to an amount by which the carrying value exceeds the fair value of the assets. The factors considered by management in performing this assessment include current operating results, trends and prospects, the manner in which the property is used, and the effects of obsolescence, demand, competition and other economic factors. Based on this assessment, there was no impairment recorded for construction in progress for the years ended June 30, 2015 and 2014, respectively.
Translation of Foreign Currencies
Humankind, Huimeijia and HLJ Huimeijia maintain their books and accounting records in PRC currency “Renminbi” (“RMB”), which has been determined as the functional currency. Transactions denominated in currencies other than RMB are translated into RMB at the exchange rates prevailing on the date of the transactions, as quoted by the Federal Reserve Board. Foreign currency exchange gains and losses resulting from these transactions are included in operations.
Humankind, Huimeijia and HLJ Huimeijia’s financial statements are translated into the reporting currency, the United States Dollar (“USD”). Assets and liabilities of the above entities are translated at the prevailing exchange rate at each reporting period end date. Contributed capital accounts are translated using the historical rate of exchange when capital is injected. Income and expense accounts are translated at the average rate of exchange during the reporting period. Translation adjustments resulting from the translation of these financial statements are reflected as accumulated other comprehensive income in shareholders’ equity and non-controlling interests.
Revenue Recognition
The Company recognizes revenue when it is both earned and realized or realizable. The Company’s policy is to recognize revenue when title to the product, ownership and risk of loss have transferred to the customer, persuasive evidence of an arrangement exits and collection of the sales proceeds is reasonably assured, all of which generally occur upon shipment of goods to customers. The majority of the Company’s revenue relates to the sale of inventory to customers, and revenue is recognized when title and the risks and rewards of ownership pass to the customer. Given the nature of the Company’s business and the applicable rules guiding revenue recognition, the Company’s revenue recognition practices do not contain estimates that materially affect the results of operations. The Company records revenue at the discounted selling price and allows its customers to return products for exchange or credit subject to certain limitations. A provision for such returns is recorded based upon historical experience. There has been no provision recorded for returns based upon historical experience for the years ended June 30, 2015 and 2014, respectively.
Cost of Goods Sold
Cost of goods sold consists primarily of the costs of raw materials, freight charges, direct labor, depreciation of plants and machinery, warehousing and overhead costs associated with the manufacturing process and commission expenses.
Income Taxes
The Company adopts FASB ASC Topic 740, “Income Taxes,” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized.
In July 2006 the FASB issued FIN 48(ASC 740-10), “Accounting for Uncertainty in Income Taxes — An Interpretation of FASB Statement No. 109 (ASC 740)”, which requires income tax positions to meet a more-likely-than-not recognition threshold to be recognized in the financial statements. Under FIN 48(ASC 740-10), tax positions that previously failed to meet the more-likely-than-not threshold should be recognized in the first subsequent financial reporting period in which that threshold is met. Previously recognized tax positions that no longer meet the more-likely-than-not threshold should be derecognized in the first subsequent financial reporting period in which that threshold is no longer met.
F-10
As a result of the implementation of FIN 48 (ASC 740-10), the Company undertook a comprehensive review of its portfolio of tax positions in accordance with recognition standards established by FIN 48 (ASC 740-10). The Company recognized no material adjustments to liabilities or stockholders’ equity as a result of the implementation. The adoption of FIN 48 did not have a material impact on the Company’s financial statements.
The application of tax laws and regulations is subject to legal and factual interpretation, judgment and uncertainty. Tax laws and regulations themselves are subject to change as a result of changes in fiscal policy, changes in legislation, the evolution of regulations and court rulings. Therefore, the actual liability may be materially different from our estimates, which could result in the need to record additional tax liabilities or potentially reverse previously recorded tax liabilities or deferred tax asset valuation allowance.
Enterprise Income Tax
Under the Provisional Regulations of PRC Concerning Income Tax on Enterprises promulgated by the PRC (the “EIT Law”), income tax is payable by enterprises at a rate of 25% of their taxable income.
Value Added Tax
The Provisional Regulations of PRC Concerning Value Added Tax promulgated by the State Council came into effect on January 1, 1994. Under these regulations and the Implementing Rules of the Provisional Regulations of the PRC Concerning Value Added Tax, value added tax (“VAT”) is imposed on goods sold in, or imported into, the PRC and on processing, repair and replacement services provided within the PRC.
VAT payable in the PRC is charged on an aggregated basis at a rate of 13% or 17% (depending on the type of goods involved) on the full price collected for the goods sold or, in the case of taxable services provided, at a rate of 17% on the charges for the taxable services provided, but excluding, in respect of both goods and services, any amount paid in respect of VAT included in the price or charges, and less any deductible value added tax already paid by the taxpayer on purchases of goods and services in the same financial year. VAT for the years ended June 30, 2015 and 2014 were $88,200 and $287,441, respectively.
Sales-Related Taxes
Pursuant to the tax law and regulations of the PRC, the Company is obligated to pay 7% and 5% of the annual VAT paid as taxes on maintaining and building cities and education additional fees, both of which belong to sales-related taxes. Sales-related taxes are recorded when sales revenue is recognized. Sales-related taxes for the years ended June 30, 2015 and 2014 were $180,233 and $170,599, respectively.
Concentrations of Business and Credit Risks
All of the Company’s manufacturing is located in the PRC. There can be no assurance that the Company will be able to successfully continue to manufacture its products and failure to do so would have a material adverse effect on the Company’s financial position, results of operations and cash flows. Also, the success of the Company’s operations is subject to numerous contingencies, some of which are beyond management’s control. These contingencies include general economic conditions, prices of raw materials, competition, governmental and political conditions, and changes in regulations. Since the Company is dependent on trade in the PRC, the Company is subject to various additional political, economic and other uncertainties. Among other risks, the Company’s operations will be subject to the risks of restrictions on transfer of funds, domestic customs, changing taxation policies, foreign exchange restrictions, and political and governmental regulations.
The Company operates in China, which may give rise to significant foreign currency risks from fluctuations and the degree of volatility of foreign exchange rates between U.S. dollars and the Chinese currency RMB. The results of operations denominated in foreign currency are translated at the average rate of exchange during the reporting periods.
Earnings Per Share
Basic earnings per common share is computed by dividing net earnings applicable to common shareholders by the weighted-average number of common shares outstanding during the period. When applicable, diluted earnings per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents, consisting of shares that might be issued upon exercise of common stock options and warrants. For the years ended June 30, 2015 and 2014, the Company had no potential dilutive common stock equivalents outstanding.
F-11
Potential common shares issued are calculated using the treasury stock method, which recognizes the use of proceeds that could be obtained upon the exercise of options and warrants in computing diluted earnings per share. It assumes that any proceeds would be used to purchase common stock at the average market price of the common stock during the period.
FASB ASC Topic 260, “Earnings Per Share”, requires a reconciliation of the numerator and denominator of the basic and diluted earnings per share (EPS) computations.
Recent Accounting Pronouncements
In August 2014, The FASB has issued Accounting Standards Update (ASU) No. 2014-13, Consolidation (Topic 810): Measuring the Financial Assets and the Financial Liabilities of a Consolidated Collateralized Financing Entity. The amendments in this ASU will apply to a reporting entity that is required to consolidate a collateralized financing entity under the Variable Interest Entities guidance when: (1) the reporting entity measures all of the financial assets and the financial liabilities of that consolidated collateralized financing entity at fair value in the consolidated financial statements based on other Codification Topics; and (2) the changes in the fair values of those financial assets and financial liabilities are reflected in earnings. The amendments in this ASU are effective for public business entities for annual periods, and interim periods within those annual periods, beginning after December 15, 2015. For entities other than public business entities, the amendments are effective for annual periods ending after December 15, 2016, and interim periods beginning after December 15, 2016. Early adoption is permitted as of the beginning of an annual period. The Company does not expect the adoption to have a significant impact on its consolidated financial statements.
In August 2014, the FASB has issued Accounting Standards Update (ASU) No. 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. ASU 2014-15 is intended to define management’s responsibility to evaluate whether there is substantial doubt about an organization’s ability to continue as a going concern and to provide related footnote disclosures. The amendments are effective for annual periods ending after December 15, 2016, and interim periods within annual periods beginning after December 15, 2016. Early application is permitted for annual or interim reporting periods for which the financial statements have not previously been issued. statements. The Company does not expect the adoption to have a significant impact on its consolidated financial statements.
On November 3, 2014, the FASB issued Accounting Standards Update (“ASU”) No. 2014-16, “Derivatives and Hedging (Topic 815): Determining Whether the Host Contract in a Hybrid Financial Instrument Issued in the Form of a Share Is More Akin to Debt or to Equity (a consensus of the FASB Emerging Issues Task Force)”.The amendments permit the use of the Fed Funds Effective Swap Rate (also referred to as the Overnight Index Swap Rate, or OIS) as a benchmark interest rate for hedge accounting purposes. Public business entities are required to implement the new requirements in fiscal years (and interim periods within those fiscal years) beginning after December 15, 2015. All other types of entities are required to implement the new requirements in fiscal years beginning after December 15, 2015, and interim periods beginning after December 15, 2016. The Company does not expect the adoption to have material impact on the Company's consolidated financial statement.
On November 18, 2014, FASB issued ASU No. 2014-17, “Pushdown Accounting (a consensus of the FASB Emerging Issues Task Force)”, which allows an acquired entity to elect to apply pushdown accounting in its separate financial statements on a change-in control event. The acquired entity elects whether to apply pushdown accounting individually for each change-in-control event, and may apply pushdown accounting during the reporting period in which the change-in-control event occurs. Effective November 18, 2014, an acquired entity may apply ASU 2014-17 to future change-in-control events. The Company does not expect the adoption of ASU 2014-17 to have material impact on the Company's consolidated financial statement.
On December 23, 2014, FASB issued Accounting Standards Update (ASU) No. 2014-18, “Accounting for Identifiable Intangible Assets in a Business Combination”. The ASU contains an accounting alternative for private companies that acquire identifiable intangible assets in a business combination. Under the accounting alternative, many customer-related intangible assets and all noncompete agreements would not be recognized separately and would be subsumed into goodwill. An entity that elects this alternative is also required to adopt the alternative accounting in FASB Accounting Standards Update No. 2014-02, Accounting for Goodwill. (However, an entity that elects to adopt the goodwill alternative does not need to adopt the guidance in ASU 2014-18.) ASU 2014-18 does not require an entity to provide any incremental disclosures beyond those required by ASC 805. Once elected, the accounting alternative would be applied to all future business combinations entered into in the first annual period beginning after December 15, 2015. Early adoption would be permitted. The Company does not expect the adoption of ASU 2014-18 to have material impact on the Company's consolidated financial statement.
On January 9, 2015, FASB published ASU 2015-01, “Simplifying Income Statement Presentation by Eliminating the Concept of Extraordinary Items”. The ASU applies to all entities and is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2015. A reporting entity may apply the amendments prospectively. A reporting entity also may apply the amendments retrospectively to all prior periods presented in the financial statements. Early adoption is permitted provided that the guidance is applied from the beginning of the fiscal year of adoption. The Company does not expect the adoption of ASU 2015-01 to have material impact on the Company's consolidated financial statement.
F-12
In February 2015, FASB issued ASU No. 2015-02, Consolidation (Topic 810): Amendments to the Consolidation Analysis, which is intended to improve targeted areas of consolidation guidance for legal entities such as limited partnerships, limited liability corporations, and securitization structures. This ASU will be effective for periods beginning after December 15, 2015 for public companies. Management is evaluating the potential impact, if any, on the Company’s financial position and results of operations.
In April 2015, the FASB issued ASU No. 2015-03, Interest—Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs. The update simplifies the presentation of debt issuance costs by requiring that debt issuance costs be presented in the balance sheet as a direct deduction from the carrying amount of debt liability, consistent with debt discounts or premiums. The recognition and measurement guidance for debt issuance costs are not affected by the amendments in this update. For public companies, this update will be effective for interim and annual periods beginning after December 15, 2015, and is to be applied retrospectively. Early adoption is permitted. The Company does not expect the adoption of this guidance will have a material effect on the Company’s consolidated and combined financial statements.
In July 2015, the Financial Accounting Standards Board (FASB) issued Accounting Standard Update 2015-11: Simplifying the Measurement of Inventory. This update requires inventory to be measured at the lower of cost or net realizable value. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. Subsequent measurement is unchanged for inventory measured using LIFO or the retail inventory method. This update will be effective for the Company for all annual and interim periods beginning after December 15, 2016. The amendments in this update should be applied prospectively with earlier application permitted as of the beginning of an interim or annual reporting period. The Company does not expect this update will have a material impact on the presentation of the Company's consolidated and combined financial statements.
In August 2015, the FASB has issued Accounting Standards Update (ASU) No. 2015-15, Interest - Imputation of Interest (Subtopic 835-30): Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements - Amendments to SEC Paragraphs Pursuant to Staff Announcement at June 18, 2015 EITF Meeting. This ASU adds SEC paragraphs pursuant to the SEC Staff Announcement at the June 18, 2015, Emerging Issues Task Force meeting about the presentation and subsequent measurement of debt issuance costs associated with line-of-credit arrangements. Given the absence of authoritative guidance within ASU 2015-03 for debt issuance costs related to line-of-credit arrangements, the SEC staff would not object to an entity deferring and presenting debt issuance costs as an asset and subsequently amortizing the deferred debt issuance costs ratably over the term of the line-of-credit arrangement, regardless of whether there are any outstanding borrowings on the line-of-credit arrangement. The Company does not expect this update will have a material impact on the presentation of the Company's consolidated and combined financial statements.
NOTE 3 - ACQUISITION
On November 22, 2013, Humankind completed the acquisition of HLJ Huimeijia for a total purchase price of $16,339,869 (RMB100,000,000). HLJ Huimeijia was founded on October 30, 2003. HLJ Huimeijia is engaged in the manufacturing and distribution of tincture, ointments, rubber paste (including hormones), solution (topical), suppositories, liniment (including traditional Chinese medicine extraction), enemas and oral liquid. HLJ Huimeijia’s predecessor is Heilongjiang Xue Du Pharmaceutical Co., Ltd., which had established its brand name in the market by its medical products. HLJ Huimeijia is categorized as a “high and new technology” enterprise by the Science Technology Department in Heilongjiang Province. HLJ Huimeijia has 21 products which have been approved by, and have received approval numbers issued by, the China State Food and Drug Administration (“CFDA”). In addition, HLJ Huimeijia is a holder of one patent for utility models, five patents for external design and three trademarks in China, including the Chinese brand name of “Xue Du” that has an established reputation among customers in northeastern China.
HLJ Huimeijia and Humankind are under the common control of Mr. Xin Sun, the CEO of the Company before and after the date of the completion of the acquisition, or the transfer. Humankind’s accounting policy adopted the guidance in ASC 805-50-05-5 for the transfer of net assets between entities under common control to apply a method similar to the pooling-of-interests method. Under this method, the financial statements of Humankind shall report results of operations for the period in which the transfer occurs as though the transfer of net assets had occurred at the beginning of the period. Results of operations for that period will thus comprise those of the previously separate entities combined from the beginning of the period to the date the transfer is completed and those of the combined operations from that date to the end of the period. Similarly, Humankind shall present the statements of financial position and other financial information as of the beginning of the period as though the assets and liabilities had been transferred at that date. Financial statements and financial information of Humankind presented for prior years also shall be retrospectively adjusted to furnish comparative information.
F-13
NOTE 4 - ASSETS SALE
On December 24, 2014, Humankind entered into a stock transfer agreement (the “Agreement”) with Xiuzheng Pharmaceutical Group Co., Ltd. a company incorporated under the laws of the People’s Republic of China and located in Jilin province (“Xiuzheng Pharmacy” or the “Buyer”), Mr. Xin Sun, the CEO of the Company, and Huimeijia, pursuant to which, Humankind and Mr. Xin Sun (the “Equity Holders”), shall sell their respective equity interests in Huimeijia to Xiuzheng Pharmacy. The transfer of the 100% equity interests of Huimeijia to the Buyer was for a total cash consideration of RMB 8,000,000 (approximately $1,306,186) to the Equity Holders.
On February 9, 2015, the four parties entered into a supplementary agreement (the “Supplementary Agreement”) to modify the terms of the Agreement, pursuant to which, the Equity Holders and Huimeijia (collectively the “Assets Transferors”) shall only sell the 19 drug approval numbers (including the tablet, capsule, powder, mixture, oral liquid, syrup and oral solution under the 19 approval numbers; licenses including the original copies of Business License, Organization Code Certificate, Tax Registration Certificate, Drug Production Permit and GMP Certificate, and other documents and original copies related to the production and operation of the 19 drugs) (the “Assets”) to Xiuzheng Pharmacy. The Equity Holders will retain the equity interests in Huimeijia, but will have the equity interests pledged to Xiuzheng Pharmacy until the Assets are transferred, at which time all the cash consideration shall be paid by the Buyer. The total cash consideration remains to be the same as under the Agreement, i.e., RMB 8,000,000 (approximately $1,306,186) to the Assets Transferors. In the event that the Assets are failed to be transferred to the Buyer due to the fault of the Assets Transferors, the paid consideration shall be returned to the Buyer with interests accrued. If the failure of the transfer of the Assets is a result of the government policy changes or force majeure, the paid cash consideration shall be returned to the Buyer but without any interests.
As of June 30, 2015, the transfer of the Assets had not been completed. The Company is striving to accelerate the process of the transfer.
NOTE 5 - ACCOUNTS RECEIVABLE
The Company’s accounts receivable amounted to $1,431,298 and $2,230,746, respectively, net of allowance for doubtful accounts amounting to $45,489 and $45,463 as of June 30, 2015 and 2014, respectively.
NOTE 6 - INVENTORIES
Inventory consists of following:
| June 30, 2015 | June 30, 2014 | |||||
| Raw Materials | $ | 189,004 | $ | 524,446 | ||
| Supplies and Packing Materials | 19,565 | 10,485 | ||||
| Work-in-Progress | 261,019 | 248,304 | ||||
| Finished Goods | 371,651 | 254,704 | ||||
| Total | $ | 841,239 | $ | 1,037,939 |
For the years ended June 30, 2015 and 2014, the Company has not made provision for inventory in regards to slow moving or obsolete items.
NOTE 7 - CONSTRUCTION IN PROGRESS
Construction in progress consisted of the following:
| June 30, 2015 | June 30, 2014 | |||||
| Plant - HLJ Huimeijia | $ | 605,542 | $ | - | ||
| Warehouse - HLJ Huimeijia | - | - | ||||
| Plant and Production Lines - Huimeijia | 1,935 | 1,934 | ||||
| Interior Decoration - Humankind | - | - | ||||
| Warehouse - Humankind | - | - | ||||
| Office Building - Humankind | - | - | ||||
| Total | $ | 607,477 | $ | 1,934 |
F-14
On April 6, 2012, HLJ Huimeijia entered into an agreement with a contractor for the plant, the estimated total cost of construction was approximately $2.09 million (RMB 12,800,000), anticipated to be completed within 720 construction working days from April 20, 2012 to December 30, 2015. The construction did not start until August 16, 2014. As of June 30, 2015, 29% of construction had been completed and $605,542 (RMB 3,754,360) had been recorded as a cost of construction in progress.
NOTE 8 - PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consisted of the following:
| June 30, 2015 | June 30, 2014 | |||||
| Building, Warehouses and Improvements | $ | 4,673,771 | $ | 4,646,879 | ||
| Machinery and Equipment | 1,226,433 | 1,231,702 | ||||
| Office Equipment | 134,932 | 108,112 | ||||
| Vehicles | 232,511 | 231,570 | ||||
| Other | - | 24,180 | ||||
| Less Accumulated Depreciation | (1,952,553 | ) | (1,581,371 | ) | ||
| Total | $ | 4,315,094 | $ | 4,661,072 |
Depreciation expense was $ 364,932 and $318,761 for the year ended June 30, 2015 and 2014, respectively. Depreciation expense charged to operations was $ 141,753 and $169,565 for the year ended June 30, 2015 and 2014, respectively. Depreciation expense charged to cost of goods sold was $ 223,179 and $149,196 for the year ended June 30, 2015 and 2014, respectively.
As of June 30, 2015, the building of HLJ Huimeijia in the book value of $1,797,209 has been mortgaged for the working capital loan in the principal amount of $1,612,903 (RMB 10,000,000). As of June 30, 2014, the building of HLJ Huimeijia in the book value of $1,796,166 has been mortgaged for the working capital loan in the principal amount of $1,611,967 (RMB 10,000,000).
NOTE 9 - INTANGIBLE ASSETS
The following is a summary of intangible assets:
| June 30, 2015 | June 30, 2014 | |||||
| Land Use Rights – Humankind | $ | 1,022,255 | $ | 1,021,662 | ||
| Health Supplement Product Patents – Humankind | 4,838,709 | 4,835,901 | ||||
| Pharmaceutical Patents - HLJ Huimeijia | 144,514 | 144,430 | ||||
| Land Use Rights - HLJ Huimeijia | 699,208 | 698,802 | ||||
| Less: Accumulated Amortization | (1,692,389 | ) | (1,248,634 | ) | ||
| Total | $ | 5,012,297 | $ | 5,452,161 |
All land in the PRC belongs to the State. Enterprises and individuals can pay the State a fee to obtain the right to use a piece of land for commercial purposes or residential purposes for an initial period of 50 years or 70 years, respectively. The land use right can be sold, purchased, and exchanged in the market. The successor owner of the land use right will have the right to use the land for the time remaining on the initial period.
Amortization expense charged to operations was $443,925 and $817,281 for the year ended June 30, 2015 and 2014, respectively.
As of June 30, 2015, land use rights of HLJ Huimeijia with the book value of $699,208 had been mortgaged for a working capital loan in the principal amount of $1,612,903 (RMB 10,000,000). As of June 30, 2014, land use rights of HLJ Huimeijia with a book value of $698,802 had been mortgaged for a working capital loan in the principal amount of $1,611,967 (RMB 10,000,000).
NOTE 10 - SHORT-TERM LOAN
On November 20, 2014, HLJ Huimeijia entered into a short-term loan agreement with a bank for a working capital loan in the principal amount of RMB 10,000,000, at an interest rate of 7.8% from November 20, 2014 to November 19, 2015. The loan was secured by the land use right and the building of HLJ Huimeijia, with a maturity date of November 19, 2015. As of June 30, 2015 and 2014, the Company’s short-term loan was $1,612,903 and $1,611,967, respectively.
F-15
Interest expenses were $ $125,608 and $111,758 for the years ended June 30, 2015 and 2014, respectively.
NOTE 11 - RELATED PARTY DEBTS
Related party debts, which represent temporary short-term loans from Mr. Xin Sun, Mr. Kai Sun and Ms. Haiping Man consisted of the following:
| June 30, 2015 | June 30, 2014 | |||||
| Mr. Xin Sun | $ | 1,872,830 | $ | 1,739,157 | ||
| Mr. Kai Sun | 37,716 | 37,694 | ||||
| Total | $ | 1,910,546 | $ | 1,776,851 |
These loans are unsecured and non-interest bearing and have no fixed terms of repayment; therefore, they are deemed payable on demand. Mr. Kai Sun is a PRC citizen and family member of Xin Sun, the CEO of the Company.
NOTE 12 - INCOME TAXES
(a) Corporate income taxes
United States
China Health US was organized in the United States. China Health US had no taxable income for US income tax purposes for the years ended June 30, 2015 and 2014, respectively. As of June 30, 2014, China Health US has a net operating loss carry forward for United States income taxes. Net operating loss carry forwards are available to reduce future years’ taxable income. Management believes that the realization of the benefits from these losses appears uncertain due to the Company’s operating history and the continued losses of the US entity. Accordingly, the Company has provided a 100% valuation allowance on the deferred tax asset to reduce the asset to zero. There were no changes in the valuation allowance for the years ended June 30, 2015 and 2014. Management reviews this valuation allowance periodically and makes adjustments accordingly.
Hong Kong
China Health HK was incorporated in the Hong Kong and is subject to Hong Kong taxation on its activities conducted in Hong Kong and income arising in or derived from Hong Kong. No provision for income taxes have been made as China Health HK has no taxable income in Hong Kong.
People’s Republic of China
Under the EIT Law, the standard EIT rate is 25%. The PRC subsidiaries of the Company are subject to PRC income taxes on an entity basis on income arising in or derived from the tax jurisdiction in which they operate.
The provision for income taxes on income consists of the following for the years ended June 30, 2015 and 2014:
Provision for income taxes consisted of:
| For the Years Ended June 30, | ||||||
| 2015 | 2014 | |||||
| Current provision : | ||||||
| USA | $ | - | $ | - | ||
| China | 234,905 | - | ||||
| Total current provision | 234,905 | - | ||||
| Deferred provision: | ||||||
| USA | - | - | ||||
| China | - | - | ||||
| Total deferred provision | - | - | ||||
| Total provision for income taxes | $ | 234,905 | $ | - | ||
F-16
Significant components of deferred tax assets were as follows:
| June 30, 2015 | June 30, 2014 | |||||
| Deferred tax assets | ||||||
| Net operating loss carry forward | $ | 141,735 | $ | 301,764 | ||
| Allowance for doubtful accounts | - | 11,366 | ||||
| Valuation allowance | (141,735 | ) | (313,130 | ) | ||
| Deferred tax assets, net | $ | - | $ | - |
At June 30, 2015, based on the weight of available evidence, including cumulative losses in recent years and expectations of future taxable income, the Company determined that it was more likely than not that its deferred tax assets would be realized partly. The Company will realize its deferred tax assets according to expectations of future taxable income, which drive from the Company purchased twelve health products as “Doctor Xiao” and completed the acquisition of HLJ Huimeijia.
(b) Uncertain tax positions
There were no unrecognized tax benefits as of June 30, 2015 and 2014, respectively. Management does not anticipate any potential future adjustments in the next twelve months which would result in a material change to its tax positions. For the years ended June 30, 2015 and 2014, the Company did not incur any interest and penalties arising from its tax payments.
NOTE 13 – EARNINGS PER SHARE
Basic earnings per common share is computed by dividing net earnings applicable to common shareholders by the weighted-average number of common shares outstanding during the period. When applicable, diluted earnings per common share is determined using the weighted-average number of common shares outstanding during the period, adjusted for the dilutive effect of common stock equivalents, consisting of shares that might be issued upon exercise of common stock options and warrants.
Potential common shares issued are calculated using the treasury stock method, which recognizes the use of proceeds that could be obtained upon the exercise of options and warrants in computing diluted earnings per share. It assumes that any proceeds would be used to purchase common stock at the average market price of the common stock during the period.
FASB ASC Topic 260, Earnings Per Share, requires a reconciliation of the numerator and denominator of the basic and diluted earnings per share (EPS) computations.
For the years ended June 30, 2015 and 2014, the Company does not have potential dilutive shares.
The following table sets forth the computation of basic and diluted net income per share:
| For the Years Ended June 30, | ||||||
| 2015 | 2014 | |||||
| Net income attributable to the common stockholders | $ | (2,508 | ) | $ | (658,527 | ) |
| Basic weighted average outstanding shares of common stock | 63,044,395 | 62,239,737 | ||||
| Dilutive effect of options and warrants | - | - | ||||
| Diluted weighted average common stock and common stock equivalents | $ | 63,044,395 | $ | 62,239,737 | ||
| Earnings per share: | ||||||
| Basic | $ | (0.00 | ) | $ | (0.01 | ) |
| Diluted | $ | (0.00 | ) | $ | (0.01 | ) |
NOTE 14 - COMMITMENTS AND CONTINGENCIES
The Company’s assets are located in the PRC and revenues are derived from operations in the PRC.
F-17
In terms of industry regulations and policies, the economy of the PRC has been transitioning from a planned economy to market oriented economy. Although in recent years the Chinese government has implemented measures emphasizing the utilization of market forces for economic reforms, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial portion of productive assets in the PRC is still owned by the Chinese government. For example, all land is state owned and leased to business entities or individuals through the government’s granting of Land Use Rights. The granting process is typically based on government policies at the time of granting and can be lengthy and complex. This process may adversely affect the Company’s future manufacturing expansions. The Chinese government also exercises significant control over the PRC’s economic growth through the allocation of resources and providing preferential treatment to particular industries or companies. Uncertainties may arise with changing of governmental policies and measures.
The Company faces a number of risks and challenges not typically associated with companies in North America and Western Europe, since its assets exist solely in the PRC, and its revenues are derived from its operations therein. The PRC is a developing country with an early stage market economic system, overshadowed by the state. Its political and economic systems are very different from the more developed countries and are in a state of change. The PRC also faces many social, economic and political challenges that may produce major shocks, instabilities and even crises, in both its domestic arena and in its relationships with other countries, including the United States. Such shocks, instabilities and crises may in turn significantly and negatively affect the Company’s performance.
Since the Company terminated its rental agreement on January 9, 2013, it had no rental commitment as of June 30, 2015.
NOTE 15 - MAJOR SUPPLIERS AND CUSTOMERS
The Company had one supplier that accounted for 67% of the Company’s purchases for the year ended June 30, 2015.
The Company had three customers that in the aggregate accounted for 28% of the Company’s total sales for the year ended June 30, 2015, with each customer accounting for 12%, 10% and 6%, respectively.
The Company had one supplier that accounted for 76% of the Company’s purchases for the year ended June 30, 2014.
The Company had three customers that in the aggregate accounted for 31% of the Company’s total sales for the year ended June 30, 2014, with each customer accounting for 11%, 11% and 10%, respectively.
F-18
NOTE 16 - SEGMENT REPORTING
The Company was organized into three main business segments based on the types of products being provided to customers: HLJ Huimeijia, Humankind and others. Each of the three operating segments referenced above has separate and distinct general ledgers. The chief operating decision maker (“CODM”) receives financial information, including revenue, gross margin, operating income, and net income produced from the various general ledger systems to make decisions about allocating resources and assessing performance; however, the principal measure of segment profitability or loss used by the CODM is net income (loss) by segment.
The following tables present summary information by segment for the years ended June 30, 2015 and 2014, respectively:
| For the Year Ended June 30, 2015 | For the Year Ended June 30, 2014 | |||||||||||||||||||||||
| HLJ | HLJ | |||||||||||||||||||||||
| Huimeijia | Humankind | Others | Consolidated | Huimeijia | Humankind | Others | Consolidated | |||||||||||||||||
| Revenues | $ | 1,569,807 | $ | 8,656,245 | $ | - | $ | 10,226,052 | $ | 1,352,677 | $ | 8,356,422 | $ | - | $ | 9,709,099 | ||||||||
| Cost of revenues | 1,203,911 | 6,086,263 | - | 7,290,174 | 1,028,592 | 6,286,579 | - | 7,315,171 | ||||||||||||||||
| Gross profit | 365,896 | 2,569,982 | - | 2,935,878 | 324,085 | 2,069,843 | - | 2,393,928 | ||||||||||||||||
| Interest expense | 125,608 | - | - | 125,608 | 111,758 | - | - | 111,758 | ||||||||||||||||
| Depreciation and amortization | 72,756 | 510,987 | 1,937 | 585,680 | 175,220 | 805,630 | 5,997 | 986,847 | ||||||||||||||||
| Income tax | - | 234,905 | - | 234,905 | - | - | - | - | ||||||||||||||||
| Net loss | (426,579 | ) | 704,714 | (275,626 | ) | (2,508 | (590,555 | ) | (61,722 | ) | (6,250 | ) | (658,527 | ) | ||||||||||
| Total capital expenditures | 4,384 | 17,240 | - | 21,624 | 61,475 | 64,516 | - | 125,991 | ||||||||||||||||
| Total assets | $ | 3,358,615 | $ | 38,082,449 | $ | 64,365 | $ | 41,505,429 | $ | 3,155,438 | $ | 37,686,901 | $ | 27,721 | $ | 40,870,060 | ||||||||
F-19
NOTE 17 - STOCK BASED COMPENSATION
On March 27, 2015 the Board of Directors (the “Board”) adopted the Company’s 2015 Equity Incentive Plan (the “Plan”), which became effective as of such date. The Plan is intended to be construed as an employee benefit plan that satisfies the requirements for exemption from the restrictions of Section 16(b) of the Exchange Act. A summary of the principal provisions of the Plan is set forth below.
The aggregate number of shares of common stock that may be issued under the Plan is 6,000,000 shares. In the event that the Board determines that any dividend or other distribution, recapitalization, stock split, reverse stock split, reorganization, merger, consolidation, split-up, spin-off, combination, repurchase, or exchange of Common Stock or other securities of the Company, or other corporate transaction or event affects the common stock such that an adjustment is determined by the Board, in its sole discretion, to be necessary or appropriate in order to prevent dilution or enlargement of benefits or potential benefits intended to be made available under the Plan, the Board may, in such manner as it in good faith deems equitable, adjust any or all of (i) the number of shares of common stock or other securities of the Company (or number and kind of other securities or property) with respect to which awards may be granted, (ii) the number of shares of common stock or other securities of the Company (or number and kind of other securities or property) subject to outstanding awards, and (iii) the exercise price with respect to any stock option, or make provision for an immediate cash payment to the holder of an outstanding award in consideration for the cancellation of such award.
Individuals eligible for awards under the Plan shall consist of employees (including officers), directors and consultants, or those who will become employees (including officers), directors and consultants, of the Company and/or its subsidiaries whose performance or contribution, in the sole discretion of the Committee, benefits or will benefit the Company or any subsidiary.
The Plan was effective upon its approval by the Board and adoption by the Company. The Plan shall terminate on March 27, 2025, except with respect to awards then outstanding. After such date no further awards shall be granted under the Plan.
On March 30, 2015, the Company granted 3,000,000 and 300,000 restricted shares to Mr. Xin Sun, Chief Executive Officer and Chief Financial Officer of the Company and an employee, respectively. The vesting periods of the restricted shares under the Plan were determined based on individual stock award agreements and would be recognized as equity compensation for the fiscal years ended June 30, 2015 and 2016. The grant was pursuant to the Plan and a Restricted Stock Award Agreement, and was based on the exemption afforded by Regulation S under the Securities Act of 1933, as amended.
F-20
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. |
None.
| Item 9A. | Controls and Procedures. |
Evaluation of Disclosure Controls and Procedures
We maintain a set of disclosure controls and procedures designed to ensure that information required to be disclosed by us in our reports filed under the Securities Exchange Act, is recorded, processed, summarized and reported within the time periods specified by the SEC’s rules and forms. Disclosure controls are also designed with the objective of ensuring that this information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Based upon their evaluation as of the end of the periods covered by this report, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were not effective to satisfy the objectives, due to the material weakness in our internal control over financial reporting discussed below.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control system was designed to provide reasonable assurance to our management and our sole board member regarding the preparation and fair presentation of published financial statements.
Our management assessed the effectiveness of our internal control over financial reporting as of June 30, 2015. In making this assessment, it used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in 2013 in Internal Control—Integrated Framework. Our management has implemented and tested our internal control over financial reporting based on these criteria and did not identify any significant deficiencies and material weaknesses as of June 30, 2015. However, based on the fact that we do not have any full-time accounting personnel who have U.S. GAAP experience, our management has considered this as a material weakness and determined that as of June 30, 2015, the internal control over financial reporting was not effective.
39
In an effort to remedy this material weakness in the future, we intend to do the following:
| • |
Develop a comprehensive training and development plan, for our finance, accounting and internal audit personnel, including our Chief Financial Officer, Financial Manager, and others, in the principles and rules of U.S. GAAP, SEC reporting requirements and the application thereof. | |
|
| ||
| • |
Design and implement a program to provide ongoing company-wide training regarding the Company’s internal controls, with particular emphasis on our finance and accounting staff. | |
|
| ||
| • |
Implement an internal review process over financial reporting to review all recent accounting pronouncements and to verify that the accounting treatment identified in such report have been fully implemented and confirmed by our internal control department. In the future, we will continue to improve our ongoing review and supervision of our internal control over financial reporting. | |
|
| ||
| • |
Hire an individual that possesses the requisite U.S. GAAP experience and education. |
Despite the material weakness reported above, our management believes that our consolidated financial statements included in this report fairly present in all material respects our financial condition, results of operations and cash flows for the periods presented.
This report does not include an attestation report of our registered accounting firm regarding internal control over financial reporting. The management’s report was not subject to attestation by our registered public accounting firm pursuant to temporary rules of the SEC.
Changes in Internal Control over Financial Reporting
No changes in our internal control over financial reporting have come to management’s attention during our last fiscal year that have materially affected, or are likely to materially affect, our internal control over financial reporting.
40
Limitations on Controls
Management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all error and fraud. Any control system, no matter how well designed and operated, is based upon certain assumptions and can provide only reasonable, not absolute, assurance that its objectives will be met. Further, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the Company have been detected.
| Item 9B. | Other Information. |
None.
| Item 10. | Directors, Executive Officers and Corporate Governance. |
The following table sets forth information regarding our sole board member and executive officer. Our sole director holds office until the election and qualification of his successor.
| Name | Age | Position |
| Xin Sun | 49 | Chairman (sole director), Chief Executive Officer, Chief Financial Officer and Treasurer |
Biography
Mr. Xin Sun attended Jia Mu Si Medical College with a major in Pharmacy from 1984 to 1988. From 1988 to 1991, he was the Production Manager at Ha Yao Group Sanchine Medicine Joint-Stock Company Ltd. From 1991 to 1994, he was the District Director for the Northeast District of China for Pfizer Pharmaceuticals Limited. Thereafter, he spent one year as the Director of Marketing for Ha Yao Group Sanchine Medicine Joint-Stock Company Ltd. From 1996 to 2002, he was the Chief Executive Officer of a company he founded, Heilongjiang Bijie Chemical Industry Co., Ltd., which is no longer in existence now. He obtained his Masters of Business Administration from Renmin University of China in 2004. From 2003 to the present, he has been the President and Chief Executive Officer of Humankind. Mr. Sun is also President and General Manager of Huimeijia.
As a result of his professional experience, Mr. Xin Sun is well known in the pharmaceutical field in Harbin, PRC. While he was studying at Renmin University, Mr. Xin Sun developed many contacts in the pharmaceutical field, many of which later became district agents and other employees in Humankind’s distribution system.
41
Our sole director, Mr. Xin Sun, does not hold any directorships in other reporting companies and does not qualify as an “independent director” under the Rules of NASDAQ, Marketplace Rule 4200(a)(15).
To our knowledge, during the last ten years, none of our directors and executive officers (including those of our subsidiaries) have:
(a) had a bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time.
(b) been convicted in a criminal proceeding or been subject to a pending criminal proceeding, excluding traffic violations and other minor offenses.
(c) been subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities.
(d) been found by a court of competent jurisdiction (in a civil action), the SEC, or the Commodities Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacated.
Director Qualifications
Directors are responsible for overseeing the Company’s business consistent with their fiduciary duty to the stockholders. This significant responsibility requires highly-skilled individuals with various qualities, attributes and professional experience. Our sole director believes that there are general requirements for service on the board of directors (the “Board”) that are applicable to all directors and that there are other skills and experience that should be represented on the Board as a whole but not necessarily by each director. The existing board member considers the qualifications of director and director candidates individually and in the broader context of the Board’s overall composition and the Company’s current and future needs.
Qualifications for All Directors
In its assessment of each potential candidate, including those recommended by the stockholders, the Board will consider the nominee’s judgment, integrity, experience, independence, understanding of the Company’s business or other related industries and such other factors it determines are pertinent in light of the current needs of the Board.
42
The Board also takes into account the ability of a director to devote the time and effort necessary to fulfill his or her responsibilities to the Company.
The Board requires that each director be a recognized person of high integrity with a proven record of success in his or her field. Each director must demonstrate innovative thinking, familiarity with and respect for corporate governance requirements and practices, an appreciation of multiple cultures and a commitment to sustainability and to dealing responsibly with social issues. In addition to the qualifications required of all directors, the Board conducts interviews of potential director candidates to assess intangible qualities including the individual’s ability to ask difficult questions and, simultaneously, to work collegially. The Board does not have a specific diversity policy, but considers diversity of race, ethnicity, gender, age, cultural background and professional experiences in evaluating candidates for Board membership. Diversity is important because a variety of points of view contribute to a more effective decision-making process.
Qualifications, Attributes, Skills and Experience to be Represented on the Board as a Whole
The Board has identified particular qualifications, attributes, skills and experiences that should be represented on the Board as a whole, in light of the Company’s current needs and its business priorities. The Board believes that it should include some directors with a high level of financial literacy and some directors who possess relevant business experiences as a chief executive officer, president or similar position at a company. Marketing is the core focus of our business and the Company seeks to develop and deploy innovative and effective marketing and technology. Therefore, the Board believes that marketing and technology experience should be represented on the Board.
Presently, Mr. Xin Sun is the sole director of the Company. Mr. Xin Sun possesses many of the skills and experiences needed for our business. He has experience in the pharmaceutical industry, having previously been the District Director for the Northeast District of China for Pfizer Pharmaceuticals Limited from 1991 to 1994. He has also been the Director of Marketing for Ha Yao Group Sanchine Medicine Joint-Stock Company Ltd., where he acquired strong marketing experience. From 1996 to 2002, Mr. Xin Sun was the chief executive officer of a company he founded, Heilongjiang Bijie Chemical Industry Co., Ltd., and from 2003 to the present, he has been the president and chief executive officer of Humankind.
The Board plans to eventually increase its membership to include directors with skills and experiences complementary to Mr. Xin Sun’s background.
Board Leadership Structure and Role in Risk Oversight
Mr. Xin Sun is the Company’s Chairman and Chief Executive Officer. The Board’s role in the risk oversight of the Company includes, among other things:
| - | appointing, retaining and overseeing the work of the independent auditors, including resolving disagreements between the management and the independent auditors relating to financial reporting; |
43
| - |
approving all auditing and non-auditing services permitted to be performed by the independent auditors; | |
| - |
reviewing annually the independence and quality control procedures of the independent auditors; | |
| - |
reviewing and approving all proposed related party transactions; | |
| - |
discussing the annual audited financial statements with the management; and | |
| - |
meeting separately with the independent auditors to discuss critical accounting policies, management letters, recommendations on internal controls, the auditor’s engagement letter and independence letter and other material written communications between the independent auditors and the management. |
Compliance with Section 16(a) of Exchange Act
Section 16(a) of the Exchange Act requires our executive officers and directors and persons who own more than ten percent of a registered class of our equity securities to file with the SEC initial statements of beneficial ownership, reports of changes in ownership and annual reports concerning their ownership of our common stock and other equity securities, on Form 3, 4 and 5 respectively. Executive officers, directors and greater than ten percent shareholders are required by the SEC regulations to furnish the Company with copies of all Section 16(a) reports they file.
Based solely on our review of the copies of such reports, we believe that, with respect to the fiscal years ended June 30, 2015 our sole officer and director, and all of the persons known to us to own more than ten percent of our common stock, filed all required reports on a timely basis.
Meetings of Our Board of Directors
The Board held no meetings during the fiscal years ended June 30, 2015.
44
Board Committees
Audit Committee. We intend to establish an audit committee of the Board which will consist of soon-to-be-nominated independent directors. The audit committee’s duties will be to recommend to the Board the engagement of independent auditors to audit our financial statements and to review our accounting and auditing principles. The audit committee will review the scope, timing and fees for the annual audit and the results of audit examinations performed by the internal auditors and independent public accountants, including their recommendations to improve the system of accounting and internal controls. The audit committee will at all times be composed exclusively of directors who are, in the opinion of the Board, free from any relationship which would interfere with the exercise of independent judgment as a committee member and who possess an understanding of financial statements and generally accepted accounting principles.
Audit Committee Financial Expert. The Board currently acts as our audit committee. Since we are still a developing company, the Board is still in the process of finding an “audit committee financial expert” as defined in Regulation S-K and directors that are “independent” as that term is used in Section 10A of the Exchange Act.
Compensation Committee. We intend to establish a compensation committee of the Board. The compensation committee will review and approve our salary and benefits policies, including compensation of executive officers.
Code of Ethics
We currently do not have a Code of Ethics because we presently only have one director and one officer. We plan to adopt a Code of Ethics when the size of the Board and management increases.
Director Compensation
We did not compensate our director for the fiscal year ended June 30, 2015. Going forward, however, we intend to implement a market-based director compensation program.
Limitations on Liability
Under Delaware law, a corporation may indemnify its officers, directors, employees and agents under certain circumstances, including indemnification of such persons against liability under the Securities Act. Those circumstances include that an officer, director, employee or agent may be indemnified if the person acted in good faith and in a manner that he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was unlawful.
Article Seventh of our Articles of Incorporation provides that no director shall be personally liable to the Company or the stockholders for monetary damages for any breach of fiduciary duty by such person in his or her capacity as a director. Notwithstanding the foregoing sentence, a director shall be liable to the extent provided by applicable law, (i) for breach of his or her duty of loyalty to the Company or the stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the Delaware General Corporation law, or (iv) for any transaction from which he or she derived an improper personal benefit. No amendment to or repeal of this provision shall apply to or have any effect on the liability or alleged liability of any director of the Company for or with respect to any acts or omissions of such director occurring prior to such amendment.
45
We are also permitted to apply for insurance on behalf of any director, officer, employee or other agent for liability arising out of his or her actions, whether or not the Delaware General Corporation Law would permit indemnification.
Indemnification against Public Policy
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling an issuer pursuant to the foregoing provisions, the opinion of the SEC is that such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than by a director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The effect of indemnification may be to limit the rights of the Company and the stockholders (through stockholders’ derivative suits on behalf of the Company) to recover monetary damages and expenses against a director for breach of fiduciary duty.
Directors and Officers of Humankind
The following table sets forth certain information as of June 30, 2015 concerning the directors and executive officers of Humankind:
| Directors and Executive Officers | Position/Title | Age | ||
| Xin Sun | Chairman, Chief Financial Officer, Treasurer | 49 | ||
| Baosen Ma | President, Secretary, Director | 47 | ||
| Kai Sun | Director | 44 |
The following is a summary of the biographical information of those directors and officers of Humankind whose biographical information does not appear above:
Baosen Ma, President, Secretary and Director
Mr. Baosen Ma graduated from China University of Political Science and Law with a major in Financial Accounting. From 1987 to 1992, he was an accountant with Harbin Keluola Solar Power Co., Ltd. Thereafter, from 1992 to 1996, he was Vice General Manager and Sales Manager for Shanghai Dahua Solar Battery Co., Ltd. From 1996 to 2004, he was the East China Manager for the Ha Yao Group Sanchine Medicine Joint-Stock Ltd. In January 2004, Mr. Ma was appointed President, Secretary and Director of Humankind.
46
Kai Sun, Director
Mr. Kai Sun graduated from Mu Dan Jiang University with a major in Economics. From 1993 to 1995, he was Vice Director of Mu Dan Jiang Engine Factory. Thereafter, from 1995 to 1998, he was a Director at Mu Dan Jiang Engine Factory. From 1998 to 2004, he was an Administration Director for Mu Dan Jiang Lysine Co., Ltd. Since 2004, he has been the Administration Director at Humankind. Mr. Kai Sun is the younger brother of Mr. Xin Sun, the Company’s Chairman, Chief Financial Officer, Treasurer and sole director. In January 2004, Mr. Kai Sun was appointed a director of Humankind.
| Item 11. | Executive Compensation. |
The following Summary Compensation Table sets forth, for the years indicated, all cash compensation paid, distributed or accrued for services, including salary and bonus amounts, rendered in all capacities by our Chief Executive Officer and all other executive officers who received or are entitled to receive remuneration in excess of $100,000 during the stated periods. Mr. Xin Sun, our Chief Executive Officer and sole director, receives no additional compensation for the services he provides in his capacity as director.
SUMMARY COMPENSATION TABLE
(all figures in US Dollars)
| Non-Equity | Non-qualified | ||||||||||||||||||||||||||
| Name | Incentive | Deferred | All | ||||||||||||||||||||||||
| and | Plan | Compensation | Other | ||||||||||||||||||||||||
| Principal | Salary | Bonus | Stock | Option | Compensation | Earnings | Compensation | Total | |||||||||||||||||||
| Position | Year | ($) | ($) | Awards ($) | Awards ($) | ($) | ($) | ($) | ($) | ||||||||||||||||||
| Xin Sun, Chief Executive Officer, Chief Financial Officer | 2015 | 45,095 | 0 | 225,000 | N/A | N/A | N/A | N/A | 270,095 | ||||||||||||||||||
| 2014 | 23,330 | 0 | 0 | N/A | N/A | N/A | N/A | 23,330 |
47
Employment Agreements
We have no employment agreement with our sole principal executive officer, Mr. Xin Sun.
Equity Compensation Plan Information
The following table sets forth information regarding grants of awards to Named Executive Officer during the year ended June 30, 2015:
Grants of Plan-Based Awards
| Name | Grant date | Estimated future payouts under non-equity incentive plan awards | Estimated future payouts under equity incentive plan awards | All other stock awards: Number of shares of stock or units(#) | All other option awards: Number of securities underlying options(#) | Exercise or base price of option awards($/Sh) | Grant date fair value of stock and option awards | ||||||||||||||||||||||||||
| Threshold($) | Target($) | Maximum($) | Threshold(#) | Target(#) | Maximum(#) | ||||||||||||||||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | (k) | (l) | ||||||||||||||||||||||
| Xin Sun, Chief Executive Officer, Chief Financial Officer | - | - | - | - | - | - | - | 1,500,000(1) | - | $0.15 | $225,000 | ||||||||||||||||||||||
(1) Among 3,000,000 shares of restricted shares of common stock granted to Mr. Xin Sun, 1,500,000 shares vested on May 30, 2015, 1,500,000 shares are to vest on March 30, 2016.
Outstanding Equity Awards at Fiscal Year-End
| Option awards | Stock awards | ||||||||||||||||||||||||||
| Name | Number of securities underlying unexercised options(#) exercisable | Number of securities underlying unexercised options(#) unexercisable | Equity incentive plan awards: number of securities underlying unexercised unearned options(#) | Option exercise price($) | Option expiration date | Number of shares or units of stock that have not vested(#) | Market value of shares or units of stock that have not vested(#) | Equity incentive plan awards: number of unearned shares, units or other rights that have not vested(#) | Equity incentive plan awards: market or payout value of unearned shares, units or other rights that have not vested($) | ||||||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | ||||||||||||||||||
| Xin Sun, Chief Executive Officer, Chief Financial Officer | - | - | - | - | - | 1,500,000 | 0.20* | - | 300,000 | ||||||||||||||||||
*closing price as of June 30, 2015.
Option Exercises and Stock Vested
| Option awards | Stock awards | |||||||||||
| Name | Number of shares acquired on exercise (#) | Value realized on exercise ($) | Number of shares acquired on vesting (#) | Value realized on vesting ($) | ||||||||
| (a) | (b) | (c) | (d) | (e) | ||||||||
| Xin Sun, Chief Executive Officer, Chief Financial Officer | - | - | 1,500,000 | $225,000 | ||||||||
Directors’ and Officers’ Liability Insurance
We currently do not have insurance insuring directors and officers against liability; however, we are in the process of investigating the availability of such insurance.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
The following table sets forth certain information with respect to the beneficial ownership of our voting securities by (i) any person or group owning more than five percent of any class of voting securities, (ii) our director, (iii) our chief executive officer and president and (iv) all executive officers and directors as a group as of September 28, 2015.
48
The address of the beneficial owner listed below is Harbin Humankind Biology Technology Co. Limited, 168 Binbei Street, Songbei District, Harbin, Heilongjiang Province, PRC.
| Title of | Amount and Nature of | ||
| Class | Name | Beneficial Owner | Percent of Class (1) |
| Common Stock | Xin Sun, Chairman, Chief
Executive Officer, Chief Financial Officer and Treasurer |
20,507,188 | 31.3% |
| Common Stock | All officers and
directors as a group (1 person) |
20,507,188 | 31.3% |
(1) Based on 65,539,737 total issued and outstanding shares of the Company as of September 28, 2015.
Mr. Xin Sun has the sole power to vote and dispose of all shares of common stock listed opposite his name. Mr. Sun did not and does not own any options or convertible securities.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence. |
Our company received temporary short-term loans from its majority owner and our sole director and principal executive officer, Mr. Xin Sun, a PRC citizen. These loans are unsecured and non-interest bearing, and have no fixed terms of repayment; therefore, they are deemed payable on demand. Cash flows classified as due to majority owner are classified as cash flows from financing activities. The total borrowings from Mr. Sun were $1,872,830 and $1,739,157 as of June 30, 2015 and June 30, 2014, respectively.
Interest was imputed on the loans using the Chinese bank borrowing rate of 6.31% . There were no interested imputed on the loans for the fiscal years 2015 and 2014, respectively.
| Item 14. | Principal Accounting Fees and Services. |
We were billed by CANUSWA ACCOUNTING & TAX SERVICES INC, (“Canuswa”), our independent public accountants, for the following professional services they performed for us during the fiscal year ended June 30, 2015, and by KCCW Accountancy Corp. (“KCCW”), our former independent public accountants, for the following professional services they performed for us during the fiscal years ended June 30, 2015 and 2014, as set forth in the table below.
49
| Auditors | Audit | Audit- | Tax | Other | ||||||||||||||
| Fees | Related | Fees | Fees | |||||||||||||||
| Fees | ||||||||||||||||||
| 2015 | Canuswa | $ | 185,000 | - | - | - | ||||||||||||
| KCCW | 17,500 | |||||||||||||||||
| 2014 | KCCW | $ | 322,500 | - | - | - |
Pre-Approval Policies and Procedures
All of the services rendered to us by our independent registered public accountants were pre-approved by the Board.
50
PART IV
| Item 15. | Exhibits, Financial Statement Schedules |
| Exhibit | Filed | ||
| Index | Description of Document | Herewith | Incorporated by Reference To: |
| 3.1 | Articles of Incorporation. | Exhibit 3.1 of the Company’s Registration Statement on Form 10-SB filed with the SEC on December 1, 2004. | |
| 3.2 | By-laws. | Exhibits 3.2 of the Company’s Registration Statement on Form 10-SB filed with the SEC on December 1, 2004. | |
| 3.3 | Certificate of Amendment, dated May 11, 2005. | Exhibits 3.3of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. | |
| 3.4 | Certificate of Amendment, dated November 12, 2008. | Exhibit 3.4 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. | |
| 3.5 | Certificate of Amendment, dated February 11, 2009. | Exhibit 3.5 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. | |
| 4.1 | Specimen of Common Stock Certificate | Exhibit 4.1 of the Company’s Annual Report on Form 10-K filed with the SEC on September 29, 2014. | |
| 10.1 | English Translation of Land Use Agreement, dated June 7, 2004, between Harbin Humankind Biology Technology Co. Limited and Harbin City, Daochu District, Songbei Township, Jinxin Village. | Exhibit 10.3 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. |
51
| 10.2 | English Translation of Cooperative Agreement, dated September 17, 2008, between Harbin Humankind Biology Technology Co. Limited and the Commercial Bureau of Qing’an County. | Exhibit 10.5 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. | |
| 10.3 | English Translation of Land Purchase Agreement, dated July 7, 2009, between Harbin Humankind Biology Technology Co. Limited and Harbin Songbei District Construction and Development Management Committee. | Exhibit 10.6 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. | |
| 10.4 | English Translation of Technology Transfer Agreement, dated October 12, 2007, between Harbin Humankind Biology Technology Co. Limited and Beijing Jindelikang Bio- Technology Co., Ltd. | Exhibit 10.7 of the Company’s Annual Report on Form 10-K/A filed with the SEC on November 16, 2010. | |
| 10.5 | English Translation of Health Food Technology Transfer Agreement, dated January 18, 2013 by and between Harbin Humankind Biology Technology Co., Limited and Guangzhou Aoda Biology Beauty Healthy Technology Co., Ltd. | Exhibit 10.22 of the Company’s Annual Report on Form 10-K filed with the SEC on October 15, 2013. | |
| 10.6 | English Translation of Stock Transfer Agreement, dated April 10, 2013, by and between Stockholder of Heilongjiang Huimeijia Pharmaceuticals Co., Ltd, Liyuan Sun and Harbin Humankind Biology Technology Co., Limited. and its addendum dated June 18, 2013. | Exhibit 10.23 of the Company’s Annual Report on Form 10-K filed with the SEC on October 15, 2013. | |
| 10.7 | English Translation of Stock Transfer Agreement, dated April 10, 2013, by and between Stockholder of Heilongjiang Huimeijia Pharmaceuticals Co., Ltd, Wenbin Zhang and Harbin Humankind Biology Technology Co., Limited. and its addendum dated June 18, 2013. | Exhibit 10.24 of the Company’s Annual Report on Form 10-K filed with the SEC on October 15, 2013. |
52
| 10.8 | English Translation of Purchase Agreement, dated January 7, 2014, between Harbin Humankind Biology Technology Co. Limited and Mr. Shukui Wang. | Exhibit 10.10 of the Company’s Annual Report on Form 10-K filed with the SEC on September 29, 2014. | |
| 10.9 | English Translation of Stock Transfer Agreement dated December 24, 2014, by and among Harbin Humankind Biology Technology Co., Limited., Xin Sun, Harbin Huimeijia Medicine Company, and Xiuzheng Pharmaceutical Group Co., Ltd. | Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on December 31, 2014. | |
| 10.10 | English Translation of the Supplementary Agreement dated February 9, 2015, by and among Harbin Humankind Biology Technology Co., Limited., Xin Sun, Harbin Huimeijia Medicine Company, and Xiuzheng Pharmaceutical Group Co., Ltd. | Exhibit 10.1 of the Company’s Quarterly Report on Form 10-Q filed with the SEC on February 13, 2015. | |
| 10.11 | China Health Industries Holdings, Inc. 2015 Equity Incentive Plan, effective as of March 27, 2015 | Exhibit 10.1 of the Company’s Current Report on Form 8-K filed with the SEC on April 2, 2015. | |
| 10.12 | Form of the Restricted Stock Award Agreement | Exhibit 10.2 of the Company’s Current Report on Form 8-K filed with the SEC on April 2, 2015. | |
| 10.13 | English Translation of the Financial Management Agreement dated June 1, 2015, between Harbin Humankind Biology Technology Co., Limited and Harbin Hongxiang Investment Guarantee Co., Ltd. | x | |
| 21.1 | List of Subsidiaries. | x | |
| 31.1 | Certification of the Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | x | |
| 31.2 | Certification of the Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | x |
53
| 32.1 | Certification pursuant to U.S.C. Section 1350 as adopted pursuant to Section 906 of the Sarbanes- Oxley Act of 2002. | x | |
| 101.INS | XBRL Instance Document | ||
| 101.SCH | XBRL Taxonomy Extension Schema Document | ||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | ||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | ||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | ||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
54
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| CHINA HEALTH INDUSTRIES HOLDINGS, INC. | |
| By: /s/ Xin Sun | |
| Date: October 13, 2015 | Name: Xin Sun |
| Title: Chief Executive Officer (principal executive officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
By: /s/ Xin Sun
Name: Xin Sun
| Title: | Chief Executive Officer (principal executive officer), |
| Chief Financial Officer (principal financial officer and principal accounting officer) and sole director |
Date: October 13, 2015
55
