Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Unilife Corp | d723828d8k.htm |
| EX-99.1 - EX-99.1 - Unilife Corp | d723828dex991.htm |
 Fiscal
Year
2014
3
rd
Quarter
Earnings
Call
May
12,
2014
Exhibit 99.2 |
 2
This
presentation
contains
forward
looking
statements
under
the
safe
harbor
provisions
of
the
US
securities
laws.
These
forward-looking
statements
are
based
on
management’s
beliefs
and
assumptions
and
on
information
currently
available
to
our
management.
Our
management
believes
that
these
forward-looking
statements
are
reasonable
as
and
when
made.
However
you
should
not
place
undue
reliance
on
any
such
forward
looking
statements
as
these
are
subject
to
risks
and
uncertainties.
Please
refer
to
our
press
releases
and
our
SEC
filings
for
more
information
regarding
the
use
of
forward
looking
statements.
Cautionary
Note
Regarding
Forward-Looking
Statements |
 Opening Comments
3
$22.6MM in cash receipts from customers during the first nine
months of FY 2014, with $10.9MM during Current Quarter
$60MM debt financing with OrbiMed
Generating cash receipts from ten ongoing customer programs,
including several wearable injector programs
Clear pathway to profitability via existing supply agreements
secured to-date
Scale-up of manufacturing in preparation for commercial sales
One of the products from the Unifill platform commencing commercial sales
in the first quarter of FY 2015 (Quarter ending September 30, 2014)
Two other products from the Unifill platform commencing commercial sales
in the second quarter of FY 2015 (Quarter ending December 31, 2014)
|
 4
Participating in Large, Growing Markets
* Based on internal estimates and independent market research data
Market Segment
Market Size*
Market Trends / Unmet Needs
Position of Unilife
Prefilled Syringes
(ready-to-fill syringes)
•
$2B market
•
13.5% CAGR
•
$5B market by 2024
•
Needlestick safety compliance.
•
High-performance biologics
•
Modular design, multiple application
•
Differentiation from competitors
•
Broad, customizable platform for all
prefilled drugs, biologics and vaccines
•
Long-term contracts signed to-date
include Sanofi and Hikma
Wearable Injectors
(Wearable, disposable
systems)
•
New, emerging market
•
20-40% CAGR
•
$8.8B market by 2024
•
Self-injection of viscous biologics
•
Customizable, platform-based systems
for large drug portfolios
•
Easy and ready to use for patients
•
Establishing
leadership
position
for
large,
rapid-growth
market
sector
•
Several customer programs underway
including MedImmune
Drug Reconstitution
Delivery Systems
(dual-chamber syringes)
•
New, emerging market
•
20% CAGR or more
•
$500MM-$1B by 2024
•
Reconstitute or mixing liquid or dry
drug combination products
•
Ventless, orientation-free mixing
•
More intuitive steps of use
•
Extensive, customizable platform inc.
EZMix, EZMix Prodigy & AutoMix
•
Emerging leader. Low competition
•
Lifecycle management opportunities
Auto-Injectors
(Disposable / smart
reusable systems)
•
$200MM market
•
20-30% CAGR
•
$1B market by 2024
•
Biologics for patient self-injection
•
True end-of-dose indicators
•
Compact, convenient, user-centric
•
Shift to smart reusable systems
•
Only company with both disposable and
smart reusable auto-injectors
•
Customizable. True end-of-dose
Ocular Delivery
(Accurate, precise drug
delivery to the eye)
•
New, emerging market
•
20-30% CAGR
•
$250MM market 2024
•
Localized or targeted drug delivery
•
Small doses measured in uL
•
Proliferation of drug depots
•
Trend from surgery to clinic
•
Establishing leadership position
•
Technology enables quantum shift in
dose accuracy and precision
Novel Delivery
(ie. novel devices for
target organ delivery)
•
New, emerging market
•
20-30% CAGR
•
$250MM market 2024
•
Enable
novel
drug
commercialization
•
New routes of administration
•
Long-term contracts with high ASP
•
Leader for novel delivery devices
•
Novartis
contract
now
in
2
stage with
long-term commercial potential
nd |
 Unilife is Much Like an Annuity Business Model
5
Differentiated devices become part of the regulatory drug label
Long-term supply contracts that ensure continuity of supply
Each supply contract provides recurring annual revenue with
intrinsic growth year over year
New customers increase revenue and rate of revenue growth
B2B model enables minimal selling costs and encourages
attractive operating margins
Strong, growing IP portfolio creates a barrier for new competition
|
 Scaling up for Existing and New Programs
6
Ten ongoing customer programs as of March 31, 2014
Expanding operational infrastructure and cleanrooms
Unifill
®
platform of prefilled syringes
Capacity for existing line largely sold or reserved for customers
Additional lines on order for other Unifill products
Q1 2015 –
Commence commercial sales of one of our Unifill products
Q2 2015 –
Commence commercial sales of two other Unifill products
Commercial sales to accelerate through FY 2015 and FY 2016
Manufacturing scale-up occurring across other platforms
|
 OrbiMed Debt Financing
7
$60MM debt financing with OrbiMed, the world’s largest
dedicated healthcare investor
$40MM received by Unilife to-date
Unilife has the option to receive two extra tranches of $10MM
each in December 2014 and June 2015*
Interest only payments of approximately $1MM per quarter
Cash flow savings of $400,000 per quarter compared to previous debt
service payments
Option to repay loan prior to Maturity Date in 2020
Provides capital to drive growth as several large contracts with
existing customers proceed to commercial rollout
Provided the Company is in compliance with the terms of the agreement
|
 Cash
Receipts from Customers 8 |
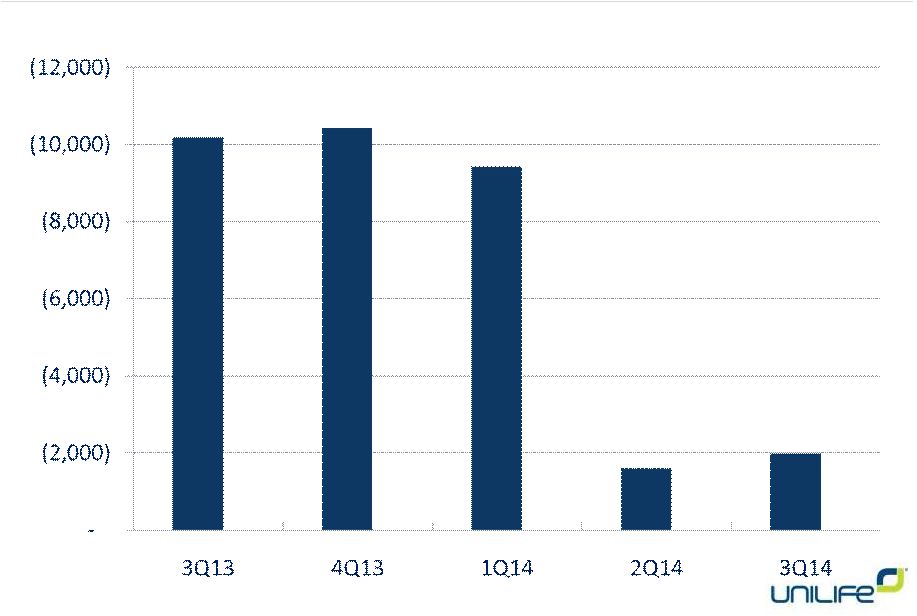 Net
Operating Cash Flow Loss 9 |
 Financial Results for Third Quarter of Fiscal 2014
10
Revenue of $1.4MM*
Deferred revenue increased by $8.3MM to $18.4MM
$39.7MM of total cash and cash equivalents including restricted
cash as of March 31, 2014
Three Months Ended
March 31,
Nine Months Ended
March 31,
2014
2013
2014
2013
Revenues
$1.4MM
$0.7MM
$8.1MM
$2.1MM
Research & development
$8.0MM
$5.5MM
$22.2MM
$15.2MM
Selling, general & administrative
$6.6MM
$7.3MM
$19.9MM
$22.2MM
Net loss
$15.1MM
$14.1MM
$42.6MM
$41.2MM
Net loss per share –
diluted
$0.15
$0.17
$0.44
$0.52
Adjusted net loss**
$11.7MM
$9.4MM
$27.1MM
$28.2MM
Adjusted net loss per share -
diluted
$0.12
$0.12
$0.28
$0.36
* Revenue of $2.7 million anticipated to be recognized during the Q3 has instead already been
recognized in Q4 of Fiscal Year 2014 based on the timing of the receipt of certain
documentation. This will be incremental to revenue from additional sources . ** Adjusted
net loss excludes non-cash share-based compensation expense, depreciation and amortization and interest expense. |
 Fiscal Year 2014 Full Year Guidance
11
Expected recognized revenue for FY 2014 to be in range of
$12MM -
$15MM
Commercial sales to begin comprising larger share of revenue
from FY 2015 onwards |
 Closing Statements
12
Increasing cash position via cash receipts and OrbiMed deal
Increasing base of pharmaceutical and biotech customers
Increasing number of customer programs with ten now
ongoing and activity underway across all product platforms
Increasing revenue with $12 -
$15MM target for FY 2014
while continuing to narrow cash losses and invest in R&D
Increasing manufacturing capacities and ordering new lines in
preparation for commercial sales in FY 2015 |
 Questions
|
