Attached files
| file | filename |
|---|---|
| EX-10.56 - EX-10.56 - Unilife Corp | unis-ex1056_1136.htm |
| EX-32.2 - EX-32.2 - Unilife Corp | unis-ex322_687.htm |
| EX-32.1 - EX-32.1 - Unilife Corp | unis-ex321_688.htm |
| EX-31.2 - EX-31.2 - Unilife Corp | unis-ex312_690.htm |
| EX-31.1 - EX-31.1 - Unilife Corp | unis-ex311_689.htm |
| EX-23.1 - EX-23.1 - Unilife Corp | unis-ex231_1882.htm |
| EX-21 - EX-21 - Unilife Corp | unis-ex21_13.htm |
| EX-12.1 - EX-12.1 - Unilife Corp | unis-ex121_14.htm |
| EX-10.85 - EX-10.85 - Unilife Corp | unis-ex1085_2159.htm |
| EX-10.84 - EX-10.84 - Unilife Corp | unis-ex1084_316.htm |
| EX-10.83 - EX-10.83 - Unilife Corp | unis-ex1083_2158.htm |
| EX-10.82 - EX-10.82 - Unilife Corp | unis-ex1082_1800.htm |
| EX-10.81 - EX-10.81 - Unilife Corp | unis-ex1081_318.htm |
| EX-10.80 - EX-10.80 - Unilife Corp | unis-ex1080_1799.htm |
| EX-10.79 - EX-10.79 - Unilife Corp | unis-ex1079_686.htm |
| EX-10.55 - EX-10.55 - Unilife Corp | unis-ex1055_315.htm |
| EX-10.37 - EX-10.37 - Unilife Corp | unis-ex1037_282.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended June 30, 2016
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 001-34540
UNILIFE CORPORATION
(Exact name of registrant as specified in its charter)
|
Delaware |
|
27-1049354 |
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
|
|
|
|
|
250 Cross Farm Lane, York, Pennsylvania |
|
17406 |
|
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code (717) 384-3400
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
|
Name of Each Exchange on Which Registered |
|
Common Stock, par value $0.01 per share |
|
The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or Section 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☒ |
|
|
|
|
|
|||
|
Non-accelerated filer |
|
☐ (Do not check if a smaller reporting company) |
|
Smaller reporting company |
|
☐ |
Indicated by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the common equity held by non-affiliates of the registrant as of December 31, 2015, the last business day of the registrant’s most recently completed second fiscal quarter was $69.8 million, computed by reference to the closing sale price of our common stock. For purposes of the foregoing calculation only, the registrant has assumed that all officers and directors of the registrant are affiliates.
As of October 17, 2016, there were 17,342,043 shares of our common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Definitive Proxy Statement to be filed with the Commission pursuant to Regulation 14A in connection with the registrant’s 2016 Annual Meeting of Stockholders, to be filed subsequent to the date hereof, are incorporated by reference into Part III of this Report. Such Definitive Proxy Statement will be filed with the Securities and Exchange Commission not later than 120 days after the conclusion of the registrant’s fiscal year ended June 30, 2016.
Explanatory Note
Unilife Corporation (the “Company,” “we,” “our” or “us”) is filing this Annual Report on Form 10-K (this “Annual Report on Form 10-K” or this “2016 10-K”) for the fiscal year ended June 30, 2016.
On May 8, 2016, the Company announced an investigation into violations of the Company’s policies and procedures and possible violations of law and regulation by the Company’s former Chief Executive Officer, Alan Shortall, whose employment with the Company ceased on March 11, 2016, and its former Chairman, Jim Bosnjak, who resigned from the Company’s Board of Directors (the “Board”) on August 24, 2015 (the “Investigation”).
The Investigation identified certain related party and other transactions which the Company had not previously publicly disclosed or recorded in its financial statements. As a result of the Investigation, the Company was not able to timely file the 2016 10-K. Also, as the result of Investigation, the Company was not able to timely file its Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2016 (the “March 2016 10-Q”).Therefore, the Company is filing the 2016 10-K and is concurrently filing the March 2016 10-Q along with (i) an amendment to the Company’s Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2015 (the “September 2015 10-Q Amendment”); (ii) an amendment to the Company’s Quarterly Report on Form 10-Q for the fiscal quarter ended December 31, 2015 (the “December 2015 10-Q Amendment”); and (iii) an amendment to the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2015 (the “2015 10-K Amendment”) These amendments are being made to correct immaterial errors in the previously reported financial statements and to disclose certain material weaknesses in the Company’s internal control over financial reporting and disclosure controls and procedures. See “Explanatory Note – Summary of Amendments” below for a summary of the specific amendments reflected in the September 2015 10-Q Amendment, the December 2015 10-Q Amendment and the 2015 10-K Amendment.
Common Stock Reverse Stock Split
On May 13, 2016, pursuant to prior stockholder authorization, the Company effected a reverse split of the Company’s common stock, pursuant to which every ten (10) shares of common stock outstanding before the reverse split were converted into one (1) share of common stock after the reverse split. All share and per share amounts, and exercise and conversion prices for all periods presented herein have been adjusted to reflect the reverse split as if it had occurred at the beginning of the first period presented.
Background and Results of the Investigation
The Company announced the Investigation on May 8, 2016. The Board established a Special Committee to oversee the Investigation. Independent counsel conducted the Investigation with the assistance of an advisory firm with forensic accounting expertise. The Investigation was completed on October 7, 2016.
Set forth below is a summary of the final results of the Investigation, all of which have been previously disclosed.
Bosnjak Mortgage Correspondence
In 2015, Mr. Shortall and Mr. Bosnjak, without authorization from or knowledge of the Company or its Board, caused to be transmitted to a mortgage broker for Mr. Shortall correspondence from Mr. Bosnjak that contained inaccurate statements about the Company’s financial support for Mr. Shortall’s purchase of and relocation to a new home. The investigation into the matters described in this paragraph did not identify any financial loss to the Company and the Company has corrected the inaccurate statements to the mortgage broker.
Shortall Fund Transfers
Mr. Shortall deposited $2,264,475 (the “Shortall Funds”) of his own funds into the Company’s bank account on June 29, 2015 and then caused the Company to disburse from the Shortall Funds $1,351,553 to third parties to complete Mr. Shortall’s purchase of his new home on July 23, 2015, and the remainder back to himself on July 28, 2015.
In addition to the Shortall Funds, during fiscal years 2013 through 2016, under Mr. Shortall’s direction, the Company accepted checks and wires from Mr. Shortall in the aggregate amount of approximately $340,000 and disbursed the same amount of funds to Mr. Shortall or his designees but did not deposit such checks or receive such wires from Mr. Shortall until five days to thirty-six days after the Company’s disbursement of the funds. The Company believes such transactions constituted loans from the Company to Mr. Shortall. In addition, Mr. Shortall wired funds and provided personal checks to the Company in the aggregate amount of approximately $253,000, not including the Shortall Funds, which wires and checks the Company received and deposited, as
applicable, prior to or within a day of the Company disbursing the same amounts to Mr. Shortall. The transfers noted in this paragraph are referred to collectively herein as, the “Shortall Fund Transfers”.
The investigation into the matters described in this section entitled “Shortall Fund Transfers” did not identify any financial loss to the Company.
Bosnjak Payments to Mr. Shortall
Mr. Shortall and Mr. Bosnjak failed to disclose to the Company $170,000 in personal payments during 2011 from Mr. Bosnjak to Mr. Shortall which payments did not involve Company funds, and also failed to disclose that, during the period from 2010 to Mr. Bosnjak’s resignation, Mr. Shortall, for reasons that the Company has been unable to determine, expected to be paid or loaned substantial amounts of money by Mr. Bosnjak. The investigation into the matters described in this paragraph did not involve Company funds and did not identify any financial loss to the Company.
Bosnjak Loan Payments and Unreimbursed Personal Expenses
Between July 2014 and July 2015, Mr. Shortall caused approximately $62,000 in Company funds to be transmitted to a third party, which fund transmittals the Company believes were made for the purpose of satisfying certain of Mr. Bosnjak’s commitments to pay interest to such third party on a loan secured by some of Mr. Bosnjak’s shares of Company stock (the “Bosnjak Loan Payments”). The Company believes that the Bosnjak Loan Payments constituted loans from the Company to Mr. Bosnjak, and the Company is evaluating potential actions to recover these funds.
From fiscal 2013 through fiscal 2016, Mr. Shortall caused the Company to pay for personal expenses, approximately $88,000 of which was not repaid to the Company (the “Unreimbursed Personal Expenses”). The Company believes the Unreimbursed Personal Expenses constituted loans from the Company to Mr. Shortall, and the Company has demanded repayment of the Unreimbursed Personal Expenses.
Other than as described in this section entitled “Bosnjak Loan Payments and Unreimbursed Personal Expenses,” the Investigation did not identify any financial loss to the Company. The Company is evaluating claims it may have arising from matters identified by the Investigation as well as any additional actions it may determine to pursue with respect to these claims. With respect to the Bosnjak Loan Payments and Unreimbursed Personal Expenses, because collection of such amounts is uncertain, the Company recorded these amounts in the applicable periods as Selling, General and Administrative Expense.
Advanced Withholding Payments
In March 2016, July 2015 and December 2014, in connection with the vesting of restricted shares of the Company’s common stock, the Company paid associated withholding taxes on behalf of three executive officers, its Vice President of Quality and Regulatory Affairs and Chief Compliance Officer, its Senior Vice President and Chief Commercial Officer, and its former President and Chief Operating Officer, in an aggregate amount of approximately $240,000 prior to being reimbursed by such executive officers. With the exception of one $400 underpayment, which the Company collected in July, 2016, such executive officers repaid the Company in full within a range of 18 to 120 days from the date of the withholding payment. The Company believes such advances constituted loans.
Founder Transactions
The Company investigated (the “Founders’ Shares Investigation”) certain issues related to the November 2009 issuance (the “UMSL Share Issuance”) of shares by Unilife Medical Solutions Limited (“UMSL”) to one of the Company’s founding shareholders, Roger Williamson, and whether Mr. Shortall may have been a beneficial owner of the UMSL shares or the CHESS Depositary Interests (“CDIs”) issued by the Company (the “Founder CDIs”) in exchange for the UMSL shares in connection with UMSL’s redomiciliation from Australia to Delaware in January 2010.
In connection with the Founders’ Shares Investigation, the Company determined that the UMSL Share Issuance was a valid corporate action. While the Company believes as a result of the Investigation that Mr. Shortall had business relationships unrelated to the Company with Mr. Williamson, the Company did not find sufficient evidence to conclude that Mr. Shortall was the beneficial owner of the Founder CDIs.
The Company initially disclosed the UMSL Share Issuance in its Registration Statement on Form 10, which became effective on February 11, 2010 (the “Form 10”). In connection with the Founders’ Shares Investigation, the Company discovered that, as of the effective date of the Form 10, Mr. Williamson was the beneficial owner of 21,782,241 CDIs, representing approximately 6.75% of the
Company’s common stock, but lacks access to sufficient information to determine whether Mr. Williamson was the beneficial owner of additional Company securities. The Form 10 did not disclose Mr. Williamson’s beneficial ownership of Company securities.
The Founders’ Shares Investigation did not identify any financial loss to the Company.
The Company has reported information regarding the Investigation to the SEC.
Controls and Procedures Considerations
Management, under the supervision of the Company’s new CEO and the Company’s CFO, and oversight of the Board, conducted an evaluation of the effectiveness of the Company’s internal control over financial reporting as of June 30, 2016 based on the COSO 2013 Framework. Based on this evaluation, management has determined that under the oversight of the Board and the leadership of Mr. Shortall, the Company did not have an effective control environment, risk assessment process, information and communication process and monitoring activities. Additionally, because of the Company’s findings from the Investigation, the Company is unable to rely on certain personnel, processes and internal controls, and as such, various material weaknesses existed at June 30, 2016. In light of such material weaknesses, management has concluded that the Company’s internal control over financial reporting was ineffective as of June 30, 2016. In addition, the Company has concluded that, as of such dates, there were material weaknesses in the Company’s disclosure controls and procedures (together with the material internal control weaknesses, the “Material Weaknesses”) as a result of the material internal control weaknesses.
The Company is committed to remediating the Material Weaknesses as promptly as possible and the implementation of the Company’s remediation plans has commenced. See Part II, Item 9A. Controls and Procedures below for additional information regarding the Material Weaknesses and such remediation process.
Summary of Amendments
As noted above, (i) the Company’s receipt and disbursement of the Shortall Funds were not reflected in the Company’s relevant financial statements originally filed with the SEC; (ii) none of the Company’s receipt and disbursement of the Shortall Funds, the Shortall Fund Transfers, the Bosnjak Loan Payments, the Unreimbursed Personal Expenses, or the Advanced Withholding Payments (collectively, the “Related Party Transactions”) was reflected in the Company’s relevant related party disclosures originally filed with the SEC; and (iii) as a result of the Investigation, management has determined that the Material Weaknesses existed as of June 30, 2015, as of the end of each of the first three quarters of fiscal 2016, and as of June 30, 2016.
As a result of the foregoing, the Company is filing the 2015 10-K Amendment, the September 2015 10-Q Amendment and the December 2015 10-Q Amendment. These amendments are being made to correct immaterial errors in the previously reported financial statements and to disclose the Material Weaknesses. In addition, the Material Weaknesses that existed as of March 31, 2016 and June 30, 2016 and the relevant Related Party Transactions are disclosed where appropriate in the March 2016 10-Q and this 2016 10-K. The amended documents and the March 2016 10-Q and the 2016 10-K have all been filed concurrently.
The specific amendments reflected in the 2015 10-K Amendment, the September 2015 10-Q Amendment and the December 2015 10-Q Amendment are summarized below.
Amendments Reflected in the 2015 10-K Amendment
The 2015 10-K Amendment is being filed by the Company to amend the following sections of the Company’s Annual Report on Form 10-K for the year ended June 30, 2015:
|
• |
The Section entitled “Cautionary Note Regarding Forward-Looking Information”: to add disclosure regarding certain risks; |
|
• |
Part I, Item 1A. Risk Factors: to add disclosure regarding certain risks; |
|
• |
Part II, Item 6. Selected Financial Data: to record the related party restricted cash balance equal to the Shortall Funds ($2,264,475); |
|
• |
Part II, Item 8. Financial Statements and Supplementary Data: to correct the immaterial errors discovered as a result of the Investigation to: |
|
|
o |
record the related party restricted cash balance equal to the Shortall Funds ($2,264,475) and the same amount as due to a related party on the consolidated balance sheet and to record the receipt of the Shortall funds and the corresponding amount due to a related party within the operating section of the Company’s consolidated statement of cash flows; |
|
|
o |
identify each of the Related Party Transactions as related party transactions and to add disclosure regarding the same; and |
|
|
o |
amend the reports of the Company’s independent registered public accounting firm, KPMG LLP, regarding the Company’s internal control over financial reporting and financial statements; |
|
• |
Part II, Item 9A. Controls and Procedures: to amend management’s evaluation of disclosure controls and procedures and its assessment of internal control over financial reporting to state that such controls were not effective as of June 30, 2015, disclose the Material Weaknesses at June 30, 2015, and discuss the Company’s remediation plan; |
|
• |
Part III, Item 13. Certain Relationships and Related Transactions, and Director Independence: to identify each of the Related Party Transactions as related party transactions and to add disclosure regarding the same; and |
|
• |
Part IV, Item 15. Exhibits, Financial Statement Schedules: to file a new consent of KPMG LLP and, as required by Rule 12b-15 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), file new certifications of our principal executive officer and principal financial officer as exhibits to the 2015 10-K Amendment. |
Amendments Reflected in the September 2015 10-Q Amendment
The following sections of the Company’s Quarterly Report on Form 10-Q for period ended September 30, 2015 are being amended pursuant to the September 2015 10-Q Amendment:
|
• |
Part I, Item 1 – Financial Statements to correct the immaterial errors discovered as a result of the Investigation: |
|
|
o |
to amend the presentation of information for the fiscal year ended June 30, 2015 in the consolidated balance sheet to record the related party restricted cash balance equal to the Shortall Funds ($2,264,475) and the same amount as due to a related party; |
|
|
o |
to amend the consolidated statement of cash flows to reflect the Company’s disbursement of the Shortall Funds and the corresponding reduction of restricted cash all within the operating section of the consolidated statement of cash flows for the three months ended September 30, 2015; and |
|
|
o |
to identify certain of the Related Party Transactions as related party transactions and to add disclosure regarding the same. |
|
• |
Part I, Item 4 – Controls and Procedures: to amend management’s evaluation of disclosure controls and procedures and its assessment of internal control over financial reporting to state that such controls were not effective as of September 30, 2015, disclose the Material Weaknesses at September 30, 2015, and discuss the Company’s remediation plan; and |
|
• |
Part II, Item 6 – Exhibits and Financial Statement Schedule: to, as required by Rule 12b-15 under the Exchange Act, file new certifications of our principal executive officer and principal financial officer as exhibits to the Company’s Quarterly Report on Form 10-Q for the period ended September 30, 2015. |
Amendments Reflected in the December 2015 10-Q Amendment
The following sections of the Company’s Quarterly Report on Form 10-Q for period ended December 31, 2015 are being amended pursuant to the December 2015 10-Q Amendment:
|
• |
Part I, Item 1 – Financial Statements to correct the immaterial errors discovered as a result of the Investigation: |
|
|
o |
to amend the presentation of information for the fiscal year ended June 30, 2015 in the consolidated balance sheet to record the related party restricted cash balance equal to the Shortall Funds ($2,264,475) and the same amount as due to a related party; |
|
|
o |
to amend the consolidated statement of cash flows to reflect the Company’s disbursement of the Shortall Funds and the corresponding reduction of restricted cash all within the operating section of the consolidated statement of cash flows for the six months ended December 31, 2015; |
|
|
o |
to identify certain of the Related Party Transactions as related party transactions and to add disclosure regarding the same; and |
|
|
o |
to amend the interim review “Report of Independent Registered Public Accounting Firm” therein to include a revised report of KPMG LLP. |
|
• |
Part I, Item 4 – Controls and Procedures: to amend management’s evaluation of disclosure controls and procedures and its assessment of internal control over financial reporting to state that such controls were not effective as of December 31, 2015, disclose the Material Weaknesses at December 31, 2015, and discuss the Company’s remediation plan; and |
Management and Board Changes
The Company no longer has any business relationship with Mr. Shortall or Mr. Bosnjak. As of March 11, 2016, (i) Mr. Shortall’s employment as Chief Executive Officer of the Company ceased and Mr. Shortall resigned from his position as Chairman of the Board, (ii) the Board appointed Mary Kate Wold to serve as its new Chair, and (iii) the employment of Ramin Mojdeh, Ph.D. as the Company’s President and Chief Operating Officer ceased.
Effective March 14, 2016, the Board appointed John Ryan as the Company’s Interim President and Chief Executive Officer of the Company. The Board subsequently appointed Mr. Ryan as the Company’s President and Chief Executive Officer and also appointed Mr. Ryan to serve as a member of the Board, in each case, effective July 28, 2016.
On July 25, 2016, the Company’s employment of Mark Iampietro as the Company’s Vice President of Quality and Regulatory Affairs and Chief Compliance Officer was ended by the Company without cause.
On July 28, 2016:
|
|
• |
the Board appointed Michael E. Kamarck to serve as a member of the Board; |
|
|
• |
the Board appointed Ian Hanson as the Company’s Chief Operating Officer in addition to his roles as the Company’s Senior Vice President; |
|
|
• |
due to results of the Investigation, Dennis P. Pyers was removed from his position as the Company’s Senior Vice President, Controller, Treasurer and Chief Accounting Officer and was appointed as the Company’s Senior Advisor, Special Projects; the Board appointed David Hastings as the Company’s Chief Accounting Officer and Treasurer in addition to his roles as the Company’s Senior Vice President and Chief Financial Officer; |
|
|
• |
the Board appointed Stephanie Walters as Senior Vice President, General Counsel and Secretary; and |
|
|
• |
the Board appointed Molly Weaver, Ph.D., as Vice President of Quality and Regulatory Affairs and Chief Compliance Officer. |
Investigation and Litigation Related to this Matter
The Company has reported the final results of the Investigation to the SEC and to The NASDAQ Stock Market LLC (“NASDAQ”), and the Company continues to cooperate fully with the SEC with respect to the SEC’s ongoing investigation. The SEC or other external parties could request further documents and information from the Company. The Company and certain of its current and former directors and officers have also been named as defendants in a number of lawsuits filed in connection with the matters set forth in this Explanatory Note. For information concerning the SEC’s ongoing investigation and such lawsuits, see Part I, Item 3. “Legal Proceedings” of this 2016 10-K.
Matters Relating to NASDAQ and Our Common Stock and ASX and our CDIs
The filing of this 2016 10-K and the March 2016 10-Q were delayed as a result of the Investigation. As a result of such delay, on May 17, 2016, the Company received a notice from the Listing Qualifications department of NASDAQ stating that, because the Company had not yet filed the March 2016 10-Q, the Company was no longer in compliance with NASDAQ Listing Rule 5250(c)(1), which requires listed companies to timely file all required periodic financial reports with the SEC. The notice received from NASDAQ stated that the Company had 60 calendar days from the date of the notice to submit a plan to regain compliance with NASDAQ’s continued listing requirements.
On July 18, 2016, the Company timely submitted a plan to NASDAQ as to how it planned to regain compliance with NASDAQ’s continued listing requirements. The staff at NASDAQ subsequently granted the Company an exception to file the March 2016 10-Q and any other delinquent SEC filings on or before November 7, 2016 in order to enable the Company to regain compliance with the listing rules.
On September 19, 2016, the Company received a notice from the Listing Qualifications department of NASDAQ stating that, because the Company had not yet filed this 2016 10-K, the Company is not in compliance with NASDAQ Listing Rule 5250(c)(1). The Company timely submitted to NASDAQ an updated compliance plan on October 4, 2016.
As noted above, the Company is concurrently filing with the SEC the March 2016 10-Q and the 2016 10-K with the 2015 10-K Amendment, the September 2015 10-Q Amendment and the December 2015 10-Q Amendment. Consequently, the Company currently believes that it has adequately remedied its non-compliance with NASDAQ Listing Rule 5250(c)(1) within NASDAQ’s terms of exception. However, there can be no assurance that NASDAQ will concur that we have remedied such non-compliance.
On October 17, 2016, the Company received a notice from the Listing Qualifications department of NASDAQ stating that, because the Company did not maintain a minimum Market Value of Listed Securities (“MVLS”) of $50,000,000 for the last 30 consecutive business days, the Company was no longer in compliance with NASDAQ Listing Rule 5450(b)(2)(A).
The Company has been provided a period of 180 calendar days, or until April 17, 2017, to regain compliance with the minimum MVLS listing requirement. If at any time on or before April 17, 2017, the MVLS of the Company’s common stock closes at $50,000,000 or more for a minimum of 10 consecutive business days, NASDAQ will provide the Company with written confirmation that the Company has achieved compliance with the minimum MVLS listing requirement and the matter will be closed.
In the event that the Company does not regain compliance with the minimum MVLS listing requirement on or before April 17, 2017, NASDAQ will provide the Company with written notification that its securities are subject to delisting. At that time, the Company would be permitted to appeal the delisting determination to a NASDAQ Hearings Panel or apply to transfer its common stock to The NASDAQ Capital Market (provided that it satisfied the requirement for continued listing on that market). (See Part I, Item 1A Risk Factors of this 2016 10-K – “We are not in compliance with the requirements of NASDAQ for continued listing, and if NASDAQ does not concur that we have adequately remedied our non-compliance with NASDAQ Listing Rule 5250(c)(1) or we do not adequately remedy our non-compliance with NASDAQ Listing Rule 5450(b)(2)(A), our common stock may be delisted from trading on NASDAQ, which could have a material adverse effect on us and our stockholders” below).
The Company was also required to file audited financial statements with the Australian Securities Exchange (the “ASX”) no later than September 30, 2016 (the “ASX Deadline”). The Company was not able to file such audited financial statements by the ASX Deadline. As a result, pursuant to ASX rules, trading in the Company’s CDIs on the ASX was to be suspended prior to the opening of trading on the ASX on October 3, 2016, however, the ASX accepted the Company’s request for an immediate voluntary suspension of trading and as such, ASX halted trading of the Company’s CDIs on the ASX prior to the opening of trading on September 30, 2016 in Australia. Such trading in Australia will not resume until after the Company files the audited financial statements included in this 2016 10-K with the ASX, which the Company is doing concurrently with filing this 2016 10-K with the SEC.
As noted above, the Company is concurrently filing with the SEC the March 2016 10-Q and the 2016 10-K with the 2015 10-K Amendment, the September 2015 10-Q Amendment and the December 2015 10-Q Amendment. Consequently, the Company currently believes that it has adequately remedied its non-compliance with NASDAQ’s listing rules within NASDAQ’s terms of exception. However, there can be no assurance that NASDAQ will concur that we have remedied our current non-compliance (see Part I, Item 1A Risk Factors of this 2016 10-K – “We have not been in compliance with the requirements of NASDAQ for continued listing, and if NASDAQ does not concur that we have adequately remedied our non-compliance, our common stock may be delisted from trading on NASDAQ, which could have a material adverse effect on us and our stockholders” below).
FORM 10-K
FOR THE FISCAL YEAR ENDED JUNE 30, 2015
TABLE OF CONTENTS
|
|
|
|
|
|
|
|
Item 1. |
|
|
11 |
|
|
|
Item 1A. |
|
|
20 |
|
|
|
Item 1B. |
|
|
35 |
|
|
|
Item 2. |
|
|
35 |
|
|
|
Item 3. |
|
|
35 |
|
|
|
Item 4. |
|
|
37 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
Item 5. |
|
|
38 |
|
|
|
Item 6. |
|
|
39 |
|
|
|
Item 7. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
40 |
|
|
Item 7A. |
|
|
53 |
|
|
|
Item 8. |
|
|
55 |
|
|
|
Item 9. |
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
|
100 |
|
|
Item 9A. |
|
|
100 |
|
|
|
Item 9B. |
|
|
102 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
Item 10. |
|
|
105 |
|
|
|
Item 11. |
|
|
105 |
|
|
|
Item 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
105 |
|
|
Item 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
114 |
|
|
Item 14. |
|
|
115 |
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
Item 15. |
|
|
116 |
|
|
References to the “Company”, “we”, “our” or “us” include Unilife Corporation and its consolidated subsidiaries, including Unilife Medical Solutions Limited, or UMSL, unless the context otherwise requires. References to “Unilife” are references solely to Unilife Corporation.
Trademarks, Trade Names and Service Marks
UNILIFE ® , UNIFILL ® , UNITRACT ® , UNIFILL FINESSE ® , RITA ® , PRECISION-THERAPY ® , FLEX-THERAPY ® , EZMIX ® , OCU-JECT ® , DEPOT-JECT ® , MICRO-JECT ® , and OCU-MIX ® are registered trademarks of the Company in the United States and in many international jurisdictions. UNIFILL ALLURE™, UNIFILL NEXUS™, UNIFILL SELECT™, EZMIX GENESIS™, EZMIX PRODIGY™, EZMIX ENGAGE™, IMPERIUM™, LISA™, AUTOMIX PRESTO™, READYTOGO™, and FLEXWEAR™ are common law trademarks of the Company in the United States and in many international jurisdictions. All trademarks, trade names or service marks referred to in this Annual Report on Form 10-K are the property of the Company unless otherwise indicated.
Cautionary Note Regarding Forward-Looking Information
This Annual Report on Form 10-K contains forward-looking statements. All statements that address operating performance, events or developments that we expect or anticipate will occur in the future are forward-looking statements. The forward-looking statements are contained principally in the sections entitled “Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions intended to identify forward-looking statements. These forward-looking statements include statements about the following:
|
|
• |
our ability to obtain additional funding to continue our operations and pay our expenses; |
|
|
• |
our exposure to litigation, regulatory proceedings and government enforcement actions as a result of the findings of the Investigation; |
|
|
• |
our ability to successfully execute our new refocused business strategy; |
|
|
• |
our ability to remediate material weaknesses in our disclosure controls and procedures and internal control over financial reporting; |
|
|
• |
our ability to comply with the requirements of NASDAQ for continued listing; |
|
|
• |
our ability to develop and achieve substantial sales of our products to our customers; |
|
|
• |
legal and regulatory requirements and developments in the U.S. and foreign countries; |
|
|
• |
the clinical development, therapeutic efficacy and market acceptance of our customers’ product candidates; |
|
|
• |
the ability to satisfy our debt obligations and comply with our restrictive covenants; |
|
|
• |
existing, recently enacted and future legislation and reimbursement practices regarding the healthcare system; |
|
|
• |
our financial performance; |
|
|
• |
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
|
|
• |
our ability to continue as a going concern for the next 12 months; |
|
|
• |
the success of competing products that are or become available; |
|
|
• |
obtaining and maintaining intellectual property protection for our technology and products; |
|
|
• |
our ability to maintain and perform under our customer agreements; |
|
|
• |
our exposure to manufacturing and other business disruptions; |
|
|
• |
our ability to limit our exposure to product liability lawsuits; |
|
|
• |
our exposure to scrutiny and increased expenses as a result of being a public company; |
|
|
• |
the impact on the cost and availability of raw materials and certain components due to potential supply changes; |
|
|
• |
our ability to maintain and protect our information technology systems; |
|
|
• |
our failure to recruit or retain key personnel or to retain our executive officers. |
These forward-looking statements are based on management’s beliefs and assumptions and on information currently available to our management. However, you should not place undue reliance on any such forward-looking statements because such statements speak only as of the date when made. We do not undertake any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. In addition, forward-looking statements are subject to certain risks and uncertainties that could cause actual results, events and developments to differ materially from our historical experience and our present expectations or projections. These risks and uncertainties include, but are not limited to, those described in “Item 1A. Risk Factors” and elsewhere in this Annual Report on Form 10-K and those that may be described from time to time in our future reports that we file with the Securities and Exchange Commission including, without limitation, the Company’s ability to become and remain current on all of its required periodic filings with the SEC in accordance with the Securities Exchange Act of 1934, as amended (the “Exchange Act”); expenditures that may be incurred by the Company in connection with any reduction in force; the definitive findings of the Investigation; negative reactions from the Company’s creditors, stockholders, strategic partners or customers to the definitive findings of the Investigation; that the Company’s common stock will be delisted from trading on NASDAQ; the Company’s ability to comply with or obtain waivers under the Company’s debt instruments; the potential that the Company will be required to amend its previous public filings with the SEC and/or restate its previously issued financial statements and the impact and result of any such amendments and/or restatements; the existence of material weaknesses in internal control over financial reporting and the timing and expense of any necessary remediation of control deficiencies; the impact (including costs) and results of any litigation or regulatory inquiries or investigations related to the findings of the Investigation; and the financial impact to the Company as a result of the foregoing. You should read completely this Annual Report on Form 10-K, the documents that we have filed as exhibits to this Annual Report on Form 10-K and the documents that we reference in this Annual Report on Form 10-K. The forward-looking statements contained in this Annual Report on Form 10-K are subject to the safe-harbor protection provided by the Private Securities Litigation Reform Act of 1995 and Section 21E of the Exchange Act.
On May 13, 2016, pursuant to prior stockholder authorization, the Company effected a reverse split of the Company’s common stock, pursuant to which every ten (10) shares of common stock outstanding before the reverse split were converted into one (1) share of common stock after the reverse split. All share and per share amounts, and exercise and conversion prices for all periods presented in this 2016 10-K have been adjusted to reflect the reverse split as if it had occurred at the beginning of the first period presented.
General
Unilife Corporation was incorporated under the laws of the State of Delaware in 2009 and is based in the Commonwealth of Pennsylvania. The Company began operations in Australia in 2002.
We are a designer, manufacturer, and supplier of innovative injectable drug delivery systems that can enhance and differentiate the injectable products of our pharmaceutical and biotechnology customers. We believe our products are differentiated from conventional products, with innovative features and functionality designed to optimize the safe, simple, and convenient administration of injectable therapies. The majority of our products are designed for sale directly to pharmaceutical and biotechnology companies who are expected to supply them as drug-device combination products, pre-filled and ready for administration by patients or health-care providers. We are able to customize products within each of our platforms to address specific customer, therapy, patient and/or commercial requirements.
Although we have a broad portfolio of injectable drug delivery systems, we are primarily focused on our portfolio of wearable injector products. We expect that by focusing on active and new customer programs in our portfolio of wearable injector systems, we can improve our operating efficiencies and better position the Company to take advantage of commercial opportunities within the fast-growing market for wearable injectors. Our wearable injector customers include Amgen Inc., MedImmune LLC (“MedImmune”), and Sanofi S.A. (“Sanofi”).
In addition to the filling, assembly and/or packaging of our products with injectable therapies, our customers are also, with respect to most of our products, responsible for the regulatory approval, sale and marketing of their final drug-device combination products. While at this point our products have not been sold to end users with our customers’ injectable therapies, we can generate revenue from customization programs, upfront fees, device and development materials and exclusivity fees.
Our focus is to operate our business with financial discipline, rigor, and efficiency, and to reduce our annual operating expenses. In parallel with targeted investment in research and development to support current and new customer programs relating to our portfolio of wearable injectors, we continue to evaluate opportunities to reduce our costs and improve operating efficiencies. During fiscal year 2016 we implemented several cost reduction and business realignment initiatives pursuant to which we reduced our headcount by approximately 90 employees. Such reduction is expected to reduce annual operating costs by approximately $7.9 million. Subsequent to that, the Company eliminated 10 additional positions in July 2016. The Company’s workforce was reduced to approximately 140 employees as of July 28, 2016, a reduction of more than 40% since January 2016 and a reduction of approximately 50% since July 1, 2015. A portion of this reduction is due to the Company’s determination not to backfill certain open positions. The Company has recorded a charge from severance and related costs from these cost reduction initiatives of approximately $0.7 million in the aggregate. The Company does not believe that these cost reduction initiatives will negatively impact its ability to serve its customers.
Given that our primary focus is now on wearable injector products, we performed an evaluation of our current contracts relating to other product platforms and have determined that continued investment in other product platforms is not beneficial to the Company at this time. We are currently in various stages of negotiation with the customers for such products to wind down our activities under those customer contracts. We do not expect that these negotiations will impact our wearable injector products, and the outcome of these negotiations is still uncertain. Regardless of the result of such negotiations, we intend to continue to prosecute and maintain the intellectual property related to the majority of our non-wearable injector products in the event it becomes financially attractive for the Company to further develop, customize, license or sell those products in the future. As part of this evaluation the Company recorded in its third fiscal quarter ended March 31, 2016 operating results a non-cash impairment charge of $26.6 million primarily related to certain prefilled syringe capital equipment located in its York facility as well certain capital equipment that was under construction held at various suppliers.
11
We build long-term relationships with pharmaceutical and biotechnology companies in which we design, develop, manufacture, and supply them with innovative injectable drug systems that can be used to enhance and differentiate their injectable therapies. An injectable drug delivery system is a product that forms a part of a drug-device combination product that is utilized by a pharmaceutical or biotechnology company to facilitate the administration of a dose of an injectable therapy by patients or healthcare providers.
We believe our portfolio of injectable drug delivery systems, most notably our wearable injectors, is the most extensive and customer-centric in the industry and can accommodate the needs of a wide range of injectable therapies. While our proprietary products are designed with innovative features and functionality, they utilize standard materials to support drug compatibility and can be supplied to customers for seamless integration with standard filling processes and equipment.
Our products are designed to be produced as sub-assemblies, which are ready for filling with a measured dose of an injectable therapy, and the final assembly or packaging is conducted after filling. Once our products have been filled, assembled and/or packaged with an injectable therapy, they become classified for regulatory purposes as drug-device combination products. While we have yet to do so, we expect to submit most of our products to the U.S. Food and Drug Administration, or the FDA, and other pertinent foreign regulatory agencies, when our customers are seeking approval for their specific drug-device combination products. We will also file with international regulators, or support our partners in their international filings. In such instances, our customers will ultimately be responsible for seeking and obtaining regulatory approval of the drug-device combination product.
Each of our supply agreements that are currently in effect reflect such business-to-business partnerships under which we will sell our products to pharmaceutical customers who are ultimately responsible for the regulatory approval, marketing and sale of the drug-device combination product. At the present time, none of our current product platforms has been evaluated by the FDA. Although we have multiple programs with large pharmaceutical companies under contract, none of our devices has been used by patients with a prescribed therapy to date.
Our modern U.S. production facility in York, Pennsylvania, advanced operational capabilities, quality and regulatory processes, and industry relationships allow us to support the injectable drug delivery requirements of our customers during the clinical development, regulatory approval process and lifecycle management of their injectable therapies.
Long-Term Customer Collaborations
Our proprietary injectable drug delivery systems can become part of the regulatory label for our customers’ drug-device combination products. In some cases, our products may be an important factor in the regulatory approval, clinical use and marketing of our customers’ drug-device combination products. In light of the length of regulatory processes, clinical requirements, investment in product development, uniqueness of our proprietary technology and/or other commercial factors that may be involved with switching to an alternative supplier of an injectable drug delivery system, our customers often seek to secure long-term continuity of supply for our products by signing supply contracts with us that can span periods of up to 15 years.
Prior to the supply of a product to our customers, we may receive payments from our customers in the form of upfront or exclusivity fees or fees for the customization of our product platforms to address specific customer, therapy, patient or commercial requirements. Some customers seek to have a customization program completed prior to the signing of a commercial supply contract. Others may sign customization and supply contracts that encompass all stages of a long-term collaboration, including commercial sales.
We believe that if and when our customers begin selling our products commercially, our long-term supply contracts will provide predictable, recurring revenue and intrinsic growth that can be generated over periods of up to 15 years. We also expect to generate attractive blended operating margins as products from our wearable injector product platforms reach peak sales volumes.
Diversified Revenue Sources
In addition to commercial product sales, there are multiple other revenue streams that could be derived from various customer agreements, including pre-commercial product sales for customer activities such as clinical drug trials, license fees, milestone-based customization program payments, upfront fees, exclusivity payments and royalties from sales. The combination of revenue streams generated from each customer varies, based upon specific contract terms, timing, the scope of customization requirements, the commercial opportunity for the target therapy, as well as other competitive market factors.
A significant proportion of our revenue to date has been derived from customer payments relating to the customization of products from one or more of our platforms to address specific customer, therapy, patient or commercial requirements prior to the
12
commercial launch of the drug-device combination product. Customers have also paid us upfront or exclusivity fees prior to the commercial launch of their drug-device combination products utilizing one or more of our product platforms. As our customers scale-up to, and begin, commercial launch of such drug-device combination products utilizing our products, we believe that product sales will progressively become a greater proportion of our total revenue.
Use of Our Products by Our Customers
Our products are designed for supply to pharmaceutical and biotechnology customers, or their appointed contract manufacturing organizations, in a sterile format where they are ready to be filled, assembled and/or packaged with a measured dose of an injectable therapy. We utilize standard materials within our products to support drug compatibility requirements. Components and related materials such as glass and elastomers are purchased by us from a network of established suppliers. These components and related materials are designed to be shipped either to our production facility in York, Pennsylvania, or to the designated facilities of production and supply partners to be manufactured into sub-assemblies. The sub-assemblies are shipped ready to be filled and assembled into a final combination product.
In addition to being responsible for the filling, assembly and/or packaging of the drug-device combination product, our customers are responsible for the regulatory approval of their drug-device combination product by agencies such as the FDA, the European Medicines Agency, or EMA, or other foreign regulatory authorities, as well as the sale and marketing of their final drug-device combination product.
Product Platforms
As part of our commitment to identify and address unmet or emerging market needs for the delivery of injectable therapies, we pursue a platform-based approach to product design that encompasses product development capabilities and state-of-the-art manufacturing technology. The base technology underlying each platform is fully developed and protected by a comprehensive intellectual property portfolio. Our approach to modular platform design allows for efficient customization to meet the specific customer, therapy, patient or commercial requirements.
Our customer-centric business structure enables us to respond quickly and efficiently to customer requirements. We have established cross-functional teams that possess deep scientific expertise and strong technical capabilities. This strong level of industry and product expertise and customer-centric business structure represents a key competitive advantage allowing us to develop and supply innovative, customized products with accelerated timelines.
We have developed a broad portfolio of innovative, differentiated injectable drug delivery systems that are designed for use with a range of injectable therapies. While our primary focus is our portfolio of wearable injectors, existing and prospective customers may also seek to utilize additional proprietary products from a series of platform-based technologies across device segments including pre-filled syringes, disposable and reusable auto-injectors, drug reconstitution delivery systems, ocular delivery systems, and other systems for the targeted delivery of injectable therapies. Multiple product configurations and proprietary features are available within each platform, and we are able to further customize each product to specific customer, therapy, patient, or commercial requirements.
Average selling prices for our products can vary significantly across product platforms. Pricing within each platform can also vary, depending upon the level of product customization required, the number of units to be purchased by the customer, as well as other market dynamics. Customization, industrialization, development, and licensing fees accounted for substantially all of our consolidated revenue during the fiscal years ended June, 2016, 2015, and 2014.
Wearable Injectors
Our wearable injector portfolio is designed to support growing pharmaceutical demand for disposable drug delivery systems that can be worn on the body of a patient over pre-configured periods such as several minutes or several hours during the subcutaneous injection of a measured dose of an injectable therapy. All of our wearable injector products are designed to allow pharmaceutical and biotechnology companies to supply their therapies to end users in a pre-filled, pre-assembled and ready-to inject format for simple and convenient self-injection to minimize disruption to normal daily life during the period of therapy administration.
This portfolio includes the Precision-Therapy™ platform of bolus injection devices and the Flex-Therapy™ platform of basal infusion devices that are designed to contain and deliver injectable therapies with a measured dose volume between 2mL and 10mL. We have also created smaller volume wearable injector platforms which are designed to enhance the containment, delivery and use of biologics with dose volumes less than 2mL that might otherwise be administered in prefilled syringes or disposable auto injectors. Our Imperium™ platform combines the therapeutic advantages of an insulin drug pump with the simplicity and low cost of an insulin pen, and is designed to deliver concentrated insulins up to U-500 to help patients to gain better control over their blood glucose levels.
13
Each of our scalable, flexible platforms utilize standard materials such as glass cartridges and elastomers, do not require sterilization in their final form (no terminal sterilization required), and are designed ready for integration with standard filling processes and equipment. Products can be pre-configured to control the duration, rate and volume of dose delivery for an injectable therapy to the target patient. Our wearable injector products require only three device-related simple user steps to commence the therapy which can be summarized as (1) peel off the adhesive liner, (2) stick the product onto the body and (3) click the button to commence the injection.
Our wearable injectors can be configured to include a soft catheter that is automatically inserted into the body with the needle, and the catheter remaining in the body following retraction of the needle, enabling drug delivery and supporting patient comfort during drug delivery without an exposed needle. Another feature is a user interface with visual, audible, and/or tactile indication that can inform the user during each stage of use.
With many pharmaceutical and biotechnology companies seeking to select one wearable injector technology for use with multiple target injectable therapies or indications, we have developed our platforms so that each product can be efficiently customized to address specific therapy, patient or commercial requirements. Multiple customization options are available, including: dose volume ranging from 0.5mL up to 10mL; adaptability for drug dose viscosity; pre-configurable delivery duration periods from seconds to hours; bolus, basal, or variable dose delivery rates; and wireless data connectivity.
Other Drug Delivery Systems
We have developed a number of product platforms, including our Unifill® platform of pre-filled syringes, our LISA™ smart reusable and RITA™ disposable auto-injectors, our EZMix® platform of drug reconstitution delivery systems, and our Ocu-ject®, Ocu-mix®, and Depot-ject® ocular delivery systems.
We also have a strong background in the development of novel drug delivery systems that can enable and enhance the commercialization and administration of specialized injectable` device technologies. For example, our Micro-Ject™ delivery system is designed to optimize the accurate and precise delivery of therapies with microliter doses that are unsuited to conventional device technologies, including to the eye or other organs.
Research and Development
While we have invested extensively to develop a suite of products for the delivery of most injectable therapies, we expect that our primary research and development focus moving forward will relate to the customization of existing and next-generation wearable injector technologies that enable us to support new and existing customer programs. We will need to continue to invest in research and development and innovate with respect to such products in order to compete successfully.
We incurred research and development expenses of $43.2 million, $52.5 million, and $34.1 million during fiscal years 2016, 2015 and 2014, respectively. As part of our ongoing cost reduction efforts, we expect research and development expense to decrease substantially in fiscal year 2017 relative to fiscal year 2016 and prior years.
Sales and Marketing
Our existing and prospective customers are pharmaceutical and biotechnology companies that develop and market a range of injectable therapies to treat a large number of acute, chronic and rare diseases. The majority of these existing and prospective customers are large, multinational businesses based in the U.S., Europe and Japan. Our customers typically have a number of injectable therapies, such as biologics including monoclonal antibodies, that are either approved or in clinical development and can be combined with our delivery systems to create a drug-device combination product. Customers are responsible for the regulatory submission of a drug-device combination product, and assuming approval is obtained, for the marketing and sale of such drug-device combination product as well.
We use a variety of direct channels to secure business with existing and prospective customers. In particular, we believe that our ability to secure long-term customer agreements is enhanced by having commercial development personnel with deep industry expertise and a strong network of relationships with pharmaceutical and biotechnology companies. This personnel is regularly in discussion with existing and prospective customers to identify and address their immediate or emerging requirements.
14
We are at various stages in the establishment and expansion of commercial relationships with pharmaceutical and biotechnology companies. It can take between one and three years or longer to execute a commercial supply agreement with a customer once a product request or business opportunity has entered our commercial pipeline. During this period, we and our prospective customer may enter into one or more initial agreements that may include materials transfer agreements or feasibility agreements whereby we or the customer may undertake a technical review process covering matters such as product development, quality and regulatory affairs, marketing, clinical affairs, manufacturing, material selection, formulation science and human factor studies. We may receive revenue from the prospective customer during this evaluation period for services we perform or evaluation units of our products we supply.
Once we are selected by a customer to customize or supply injectable drug delivery systems for use with one or more of their injectable therapies, we typically begin to generate revenue through sources including customization programs, product and product material sales, exclusivity fees, upfront fees or royalties. Certain of our customer contracts include exclusivity rights subject to, for example, annual minimum volume commitments, therapeutic markets and/or product configurations.
As of June 30, 2016, we were generating payments from multiple wearable injector programs involving a variety of our customers’ approved or under clinical development brand-name and generic products. We are not always aware of the products or product candidates for which our customers intend to use our products, and we are required under the terms of our customer contracts to keep what information we know about our customers’ programs confidential to protect our customers’ confidential commercial information.
Principal Customers
We are primarily focusing on active and new customer programs in our portfolio of wearable injectors. At the present time, although some of our customers have approved therapies, none of them has received or applied for regulatory clearance or approval from the FDA or from a foreign regulatory authority for the commercial sale of a drug-device combination product that incorporates our products.
Throughout the remainder of fiscal year 2017, we expect that our products may be utilized by our customers for a range of activities including, for example, compatibility studies, filling and packaging line validation, human clinical trials and human factor studies. Therefore, our revenue has been historically and is currently predominantly tied to the achievement of product development and other events that occur during the various stages in the establishment and advancement of commercial relationships with our customers. As a result, the customers that constitute a larger proportion of our revenue may change from year to year, or quarter to quarter, as different milestones are achieved and other events occur with different customers. A significant portion of our business is generated by a small number of major customers. As our industry, market demand and our customer base changes, our major customers may also change. Our top three customers generated over 91.0% of our total revenue in fiscal year 2016.
Amgen Inc.
In February 2016, we announced a strategic collaboration with Amgen Inc. for injectable drug delivery systems. This collaboration, which includes licensing, investment, development, and supply agreement components, is centered upon the use of Unilife’s portfolio of prefilled, customizable wearable injectors for medicines to enhance the patient experience. Subsequent to June 30, 2016, we have commenced wearable injector development programs under this collaboration.
Sanofi for Precision-Therapy®
In September 2014, we signed a worldwide master services and commercial supply agreement with Sanofi to be the sole provider of cartridge-based wearable injectors for all of Sanofi’s applicable large dose volume drugs, excluding insulins, for a minimum of 15 years. Additionally, the agreement allows Sanofi to make our wearable injectors available to Sanofi’s partners for use with applicable molecules under joint collaborations. In addition to an upfront payment and product sales, we anticipate we will continue to receive payments for customization programs relating to Sanofi molecules and indications as well as from customization programs conducted under joint collaborations with Sanofi partners.
MedImmune
In November 2013, we signed an agreement with MedImmune, the global biologics arm of AstraZeneca, to customize and supply products from our portfolio of wearable injectors for use with several target product candidates from MedImmune’s portfolio. We continue to receive payments for customization services from MedImmune under the agreement.
15
We have previously announced contracts with other customers covering products from across our portfolio of injectable drug delivery systems. Our primary focus is now on active and new customer programs in our wearable injector portfolio. We expect this primary focus to enhance operating efficiencies and better position Unilife to take advantage of commercial opportunities within the fast-growing wearable injector market, where we have industry-leading technology and a strong customer base.
Manufacturing
We manufacture sub-assemblies of our products at our manufacturing facility in York, Pennsylvania, which is registered with the FDA for the manufacture of medical devices. As discussed below under the header “Component Supply,” we outsource the production of many components and other raw materials used in the assembly of our products to third-party suppliers. We supply our products ready to be filled, assembled and/or packaged with a customer’s injectable therapy utilizing standard filling processes and equipment. The filling, assembly and/or packaging of our customers’ drug-device combination products incorporating our products is conducted either by our customers, or a contract manufacturing organization engaged by our customers.
Our manufacturing operations consist of a 165,000 square foot facility located on a 38 acre site in York, Pennsylvania that was opened in December 2010. In addition to multiple Class 7 and Class 8 clean rooms, the facility includes administrative offices, research and development laboratories, prototyping and automation fabrication facilities, a warehouse and expansion space. Activities we may undertake at our York facility include product design and development, production of sub-assemblies, packaging, quality assurance and supply chain management. All manufacturing and manufacturing related activities we undertake are guided by advanced business systems, such as SAP ERP that complement those systems commonly used by our customers.
Our quality management system, or QMS, is regularly audited by regulatory authorities for compliance with the requirements of, and certified to, ISO 13485, Medical Devices – Quality Management Systems – Requirements, for regulatory purposes, which is equivalent to Quality System Regulations, or QSR, for FDA compliance. As an FDA registered medical device manufacturer, we are periodically audited by the FDA pursuant to the QSRs and Current Good Manufacturing Practice regulations, or cGMP, with the last audit occurring in 2013 where there were no formal findings. In addition, our QMS is regularly audited by existing and prospective customers.
Existing clean room space is used for the manufacture of components and related materials into sub-assembly components by using assembly lines. Although we commenced the process of expanding the size of our cleanroom space in calendar year 2015, we have not completed such expansion and we have not expanded our building footprint. Given the Company’s current capital resources, the Company does not intend to commit additional resources to complete this expansion at this time.
The manufacturing systems we use vary depending on the type of product, its stage of commercialization and the unit production volume requirements of our customers. We utilize a modular platform-based design for our products to allow for an efficient, scalable approach to manufacturing. We believe that the long-term, forward-looking nature of the supply with our customers, gives us sufficient time to scale-up production capacities in line with demand.
In addition to our own production capacity, we work with a number of industry partners to whom we outsource various aspects of the production process, including the supply of elastomers, glass forming, injection molding, sterilization, and fill-finish. This broad supply chain enables the use of preferred materials from familiar component suppliers and helps to ensure that our products meet our customers’ rigorous quality standards.
Additionally, in November 2014 we announced the signing of a global strategic alliance agreement with Flextronics (now known as Flex), a leading end-to-end supply chain solutions company, to further expand the production capacity and scale-up capability of our product portfolio. Our agreement with Flex supports commercial demand from pharmaceutical customers seeking to leverage our products and services to enhance and differentiate their injectable therapies. In addition to using our own manufacturing facilities in York, Pennsylvania, Flex provides a secondary source of supply to us and our pharmaceutical customers for the manufacture and sourcing of components of our products. Flex is a leading and trusted provider of supply chain solutions for the pharmaceutical industry, and has long-term relationships with many of our current and prospective customers. Flex and their global manufacturing capabilities further enhance our supply chain network and generate attractive manufacturing and supply chain efficiencies and robustness.
Component Supply
We source many components and other materials utilized in the production of our products from a variety of qualified suppliers, with significant expertise in the medical device and pharmaceutical and biotechnology sectors. Some proprietary components are
16
manufactured by appointed vendors utilizing molding capital equipment that Unilife owns. All of our suppliers are regularly audited by us to ensure compliance with applicable regulatory and quality requirements.
Intellectual Property
Patents, trademarks and other proprietary rights are very important to our business. We generate our own intellectual property and hold numerous patents and patent applications covering our existing and future products and technologies. We also rely on a combination of trade secrets and manufacturing know-how to protect our intellectual property. We enter into non-disclosure agreements with our vendors, suppliers and our customers to ensure the confidentiality of our technology. In addition, we require that all our employees sign confidentiality, non-compete and intellectual property assignment agreements. Our focus is to safeguard the intellectual property surrounding our products, manufacturing processes, and other related technologies to protect not only our position and growth opportunities, but also that of our strategic partners and customers.
Our patent portfolio covers current and anticipated product offerings, as well as process improvements and other value-added innovations which streamline our customers’ filling processes. Additionally, since our product architecture is focused, at least in part, on component modularity, as new products are configured to meet customer needs, the modular components of such products are generally covered by existing patents or pending applications and new applications are pursued to cover the specific aggregation or configuration of the modular components. This means that each of our products is protected by virtue of one or more patents or pending applications relating to the product’s constituent components. As new products are innovated to meet specific customer, therapy, patient or commercial requirements, such new products will have preliminary patent protection at least to the extent that they leverage one or more of the existing protected components. Similarly, since our product platforms offer broad customizability for our customers, such customized products will be preliminarily protected by our existing patent portfolio which will be strengthened by further patent applications directed to the specific product configurations. We believe that this approach provides broad and readily-available patent protection to Unilife, even at the earliest phase of product development or customization.
As of July 2016, we had approximately 280 issued patents relating to our product platforms across more than 24 worldwide jurisdictions, including the U.S., the European Union, Australia, Japan, Israel, and China. These broad jurisdictions are strategically selected to reflect our customers’ markets, competitor spaces, and growth regions. Though granted patents expire at varying dates based on the filing date of the related application, the life of patents relating to our wearable injectors products expire between calendar years 2032 and 2036, with some patents for other products expiring earlier. Pending and/or future filed patent applications covering relevant advancements in these technologies could extend well beyond 2036 once granted.
We also have over 300 additional patent applications at various stages of examination with the United States Patent and Trademark Office and other international patent organizations, which may be granted as patents over the next two to five years. Additional patent applications are expected to be filed with national and international patent organizations during fiscal year 2017. Our patent filings broadly cover all of our existing product platforms, and pending applications address product improvements and next generation technologies.
Additionally, we have a broad and growing trademark portfolio. We have secured trademark registrations for the marks UNILIFE ® , UNIFILL ® , UNITRACT ® , UNIFILL FINESSE ® , RITA ® , PRECISION-THERAPY ® , FLEX-THERAPY ® , EZMIX ® , OCU-JECT ® , DEPOT-JECT ® , MICRO-JECT ® , and OCU-MIX ® in the U.S. and, for some such marks, in other key countries. We have pending trademark applications for several of these marks in other jurisdictions. UNIFILL ALLURE™, UNIFILL NEXUS™, UNIFILL SELECT™, EZMIX GENESIS™, EZMIX PRODIGY™, EZMIX ENGAGE™, IMPERIUM™, LISA™, AUTOMIX PRESTO™, READYTOGO™, and FLEXWEAR™ are our common law trademarks in the United States and in many international jurisdictions. We also have trademark registrations and pending applications for our other proprietary product platforms in a number of key countries, including the U.S.
Competition
The market for injectable drug delivery systems is highly competitive. As compared to products such as disposable (hypodermic) syringes, the production and supply of our injectable drug delivery systems is more specialized, with a need for us to conform to high quality expectations and specific requirements. Significant capital investment is required to establish the operational capabilities, quality and regulatory systems and industry expertise to develop, manufacture and supply injectable drug delivery systems such as our product platforms, which can limit the number of new entrants into our market.
We compete against many companies, both public and private, that range in size from small, highly focused businesses to large diversified multinational manufacturers of medical devices and healthcare equipment. Most of these competitors are based in the U.S. and Europe. Some of the large and established companies include Becton, Dickinson and Company, or BD, SCHOTT forma vitrum AG, Gerresheimer Bünde GmbH, West Pharmaceutical Services, Inc., Medtronic MiniMed, Inc., Insulet Corporation, and Ypsomed
17
Group. Collectively, these companies represent the majority of revenue for our market, with BD and Medtronic MiniMed, Inc. having the largest share. Some of these competitors are also current or potential suppliers of components or materials to us for use with our products.
A core business focus for many of our competitors is the production of commodity components such as glass barrels or elastomers and therefore these competitors are less likely to offer the diversity of suppliers that Unilife offers. Also, unlike Unilife, there are few established competitors that take responsibility for the entire injectable drug delivery system. Consequently, many pharmaceutical and biotechnology companies commonly purchase glass-based components from one supplier, and elastomer components from another supplier and are responsible for the delivery system.
Regulations
Our products are designed to be produced as sub-assemblies, which are ready for filling with a measured dose of an injectable therapy, and the final assembly or packaging is conducted after filling. Once our products have been filled, assembled and/or packaged with an injectable therapy, they become classified for regulatory purposes as drug-device combination products. While we have yet to do so, we expect to submit most of our products to the U.S. Food and Drug Administration, or the FDA, when our customers are seeking FDA approval for their specific drug-device combination products. We will also file with international regulators, or support our partners in their international filings. In such instances, our customers will ultimately be responsible for seeking and obtaining regulatory approval of the drug-device combination product.
Each of our supply agreements that are currently in effect reflect such business-to-business partnerships under which we will sell our products to pharmaceutical customers who are ultimately responsible for the regulatory approval, marketing and sale of the drug-device combination product. Although we may also submit some of these devices to the FDA as a 510(k) filing for clearance or premarket approval, as appropriate for the class of device (or other foreign regulatory processes), our customers would remain responsible for regulatory approval of the drug-device combination product. At the present time, none of our current product platforms has been evaluated by the FDA.
In order to be eligible to market and sell a drug-device combination product, a customer will need to submit and receive approval of a New Drug Application, or NDA, biologics license application, or BLA, abbreviated new drug application, or ANDA, the appropriate prior approval supplement to an existing NDA, BLA or ANDA, or the foreign equivalent of any of the foregoing, which may include documentation of summative human factor studies, records of completion of all required clinical trials, and compliance with cGMP across the development, production and delivery of the drug-device combination product, including process validation of the commercial-scale manufacturing and assembly processes that will be used to create the components for filling, assembly and/or packaging of the drug-device combination product. For a drug-device combination product, it is possible that our customers will be required to submit marketing applications through both the drug or biologic and medical device pathways. The process of obtaining FDA marketing clearance or approval can be lengthy, expensive, and uncertain. We cannot be sure how the FDA, EMA or other foreign regulatory authority will regulate our customers’ drug-device combination products, in which case the path to regulatory approval would be different and could be more lengthy and costly. As an FDA-registered medical device manufacturer and a component supplier to our customers, we are required to comply with cGMP for any products we supply to our customers for clinical or commercial use.
We accordingly maintain our facility certifications and operate our QMS in compliance with FDA, European and other relevant international standards. Doing so minimizes regulatory risk to our customers by demonstrating that we have the ability to supply cGMP-compliant components for their drug-device combination products. For instance, our customers must be able to demonstrate to the FDA or a foreign regulatory authority that the manufacturing and assembly processes for their drug-device combination products are manufactured under cGMP before those processes can be used for clinical or commercial production for the U.S. market.
Commercial and Regulatory Status
Our products include our wearable injectors, disposable and reusable auto-injectors, drug reconstitution delivery systems, pre-filled syringes, ocular delivery systems and other systems for the targeted delivery of injectable therapies. It is the responsibility of our customers to apply for and obtain final regulatory approval from the FDA or foreign regulatory authorities of each drug-device combination product that incorporates any of our products before the drug-device combination product can be sold commercially to end users. This is the commercial and regulatory process contemplated in each of our current customer supply agreements.
At the present time, although some of our customers have approved therapies, none of our customers has received regulatory clearance or approval from the FDA or a foreign regulatory authority for the commercial sale of a drug-device combination product that incorporates any of our products. It is also our understanding that none of our customers has applied for regulatory approval of such a drug-device combination product. At the present time, none of our products has been sold by our customers with their
18
injectable therapies. Once any of our customers apply for regulatory approval to sell a drug-device combination product, it will be the responsibility of the customer to obtain final product approvals from the FDA or a foreign regulatory authority.
We anticipate that, under the terms of certain customer agreements, we will assist our customers in the preparation and submission of their regulatory approval application.
Other Regulations
Pervasive and continuing regulation
Our customers are subject to pervasive and continuing government regulation by the FDA and various other federal and state agencies and are also subject to similar regulation by foreign governmental agencies. To varying degrees, such regulatory agencies require compliance by our customers with laws and regulations governing the development, testing, manufacturing, labeling, advertising, marketing, distribution, and post-market surveillance of our customers’ drug-device combination products that utilize our products.
Manufacturing and operations
As an FDA-registered medical device manufacturer and a component supplier to our customers, we are required to comply with cGMP for any products we supply to our customers for clinical or commercial use. Our QMS is regularly audited by regulatory bodies, such as the FDA. In addition, our QMS is also regularly audited by existing and prospective customers. Our QMS is certified to ISO 13485 and operates in compliance with cGMP. If we are unable to manufacture our products in the quantities and at the prices set forth in our customer agreements, satisfy our customers’ manufacturing scale-up needs, comply with cGMP or otherwise meet the specific terms of our customer agreements, such failures could have a material adverse impact on our business, prospects, results of operations, cash flows and financial condition.
We are also subject to various federal, state and local laws and regulations, both in the U.S. and other international territories where we conduct business, relating to such matters as safe working conditions, laboratory and manufacturing practices and the use, handling and disposal of hazardous or potentially hazardous substances used in connection with our research and development and manufacturing operations. Although we believe we are in compliance with these laws and regulations in all material respects, we cannot provide assurance that we will not be required to incur significant costs to comply with such laws or regulations in the future.
Employees
As of June 30, 2016, we had approximately 160 employees, of whom 138 were engaged in operations, including research and development, quality assurance and manufacturing activities, 2 were engaged in sales and marketing activities and 20 were engaged in finance, legal, and other administrative functions.
Most of our employees are located at either our York, Pennsylvania or King of Prussia, Pennsylvania facilities. None of our employees are represented by a labor union or covered by a collective bargaining agreement. We consider our relations with our employees to be good.
Corporate History
Unilife was incorporated in Delaware on July 2, 2009 as a wholly-owned subsidiary of UMSL. On January 27, 2010, Unilife became the parent company of UMSL upon completion of the redomiciliation under Australian law and UMSL’s stockholders and option holders exchanged their interests in UMSL for equivalent interests in Unilife. On February 16, 2010, Unilife’s common stock began trading on The NASDAQ Stock Market LLC under the symbol “UNIS.” Our principal executive offices are located at 250 Cross Farm Lane, York, Pennsylvania 17406. Our telephone number is (717) 384 3400.
Financial Information about Geographical Areas
See Note 4 to our consolidated financial statements for information regarding our revenue by geographic area.
Available Information
We maintain an internet website at www.unilife.com. Our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports are available free of charge through the “Investor Relations” portion of our
19
website, as soon as reasonably practicable after they are filed with the Securities and Exchange Commission. The information posted on our website is not incorporated into this Annual Report on Form 10-K.
Our business faces many risks. We believe the risks described below are the material risks that we face. However, the risks described below may not be the only risks we face. Additional unknown risks or risks that we currently consider immaterial may also impair our business operations. If any of the events or circumstances described below actually occurs, our business, financial condition or results of operations could suffer, and the trading price of our shares of common stock could decline significantly. Investors should consider the specific risk factors discussed below, and the other information contained or incorporated by reference herein and the other documents that we file from time to time with the Securities and Exchange Commission.
Risks Relating to Our Business
Matters relating to or arising from the Investigation, including regulatory proceedings, litigation and potential additional expenses, may adversely affect our business and results of operations.
On May 8, 2016, we announced an investigation into violations of the Company’s policies and procedures and possible violations of law and regulation by the Company’s former Chief Executive Officer, Alan Shortall, and its former Chairman, Jim Bosnjak (the “Investigation”) (see the “Explanatory Note” above for a summary of the final results of the Investigation).
To date, we have incurred significant expenses related to legal, accounting, and other professional services in connection with the Investigation.
The Company and certain of its current and former directors and officers have been named as defendants in a number of lawsuits filed in connection with the Investigation (see Part I, Item 3. Legal Proceedings in this Annual Report on Form 10-K for additional information regarding such lawsuits). We will continue to incur legal fees in connection with these pending cases and, pursuant to applicable law and the Company’s Bylaws, will incur expenses for the reimbursement of legal fees of present and former officers and directors under indemnification obligations. The expense of continuing to defend such litigation may be significant.
The Company is unable at this time to predict the timing or outcome of these lawsuits or of similar lawsuits that may be filed in relation to the findings of the Investigation or to predict what action regulatory authorities may take, if any, in relation to such findings, or the impact (including costs) of such lawsuits and/or actions by regulatory authorities. If any of the lawsuits related to the Investigation are adversely decided, we may be liable for significant damages directly or under our indemnification obligations, which could adversely affect our business, operations, cash flows and/or financial condition and damage our reputation. Further, the amount of time that will be required to resolve these lawsuits is unpredictable and these actions may divert management’s attention from the day-to-day operations of our business, which could adversely affect our business, operations, cash flows and/or financial condition.
The expenses incurred and expected to be incurred in connection with the Investigation, the impact of the findings of the Investigation, including on the confidence of our investors, employees and customers, the remediation efforts required as a result of the Investigation and the diversion of the attention of our management team that has occurred and is expected to continue to occur as a result of the Investigation, could adversely affect, our business, operations, cash flows and/or financial condition.
The findings of the Investigation could also subject the Company (or persons it may be required to indemnify) to possible criminal, civil or administrative sanctions, penalties, or investigations, in addition to potential private securities and other litigation. In this regard, regulatory authorities may consider that the loans described in the “Explanatory Note” above under the headings “Shortall Fund Transfers,” “Bosnjak Loan Payments and Unreimbursed Personal Expenses” and “Advanced Withholding Payments” to constitute prohibited Company loans to executive officers and directors in violation of Section 402 of the Sarbanes-Oxley Act of 2002, which prohibits personal loans to a director or executive officer of a public company.
As a result of the matters reported above, we are exposed to greater risks associated with litigation, regulatory proceedings and government enforcement actions. Such future investigations or additional lawsuits may adversely affect our business, operations, cash flows and/or financial condition.
In addition to the above, the findings of the Investigation may limit or even prevent our ability to raise capital, may negatively impact employee morale and may result in attrition among our workforce.
20
We have identified material weaknesses in our disclosure controls and procedures and internal control over financial reporting. If not remediated, our failure to establish and maintain effective disclosure controls and procedures and internal control over financial reporting could result in material misstatements in our financial statements and a failure to meet our reporting and financial obligations, each of which could have a material adverse effect on our financial condition and the trading price of our common stock.
Management, under the supervision of the Company’s new CEO and the Company’s CFO, and oversight of the Board, conducted an assessment of the effectiveness of the Company’s internal control over financial reporting as of June 30, 2016 based on the COSO 2013 Framework. Based on this evaluation, management has determined that under the oversight of the Board and the leadership of Mr. Shortall, the Company did not have an effective control environment, risk assessment process, information and communication process and monitoring activities. Additionally, because of the Company’s findings from the Investigation, the Company is unable to rely on certain personnel, processes and internal controls, and as such, various material weaknesses existed at June 30, 2016. In light of such material weaknesses, management has concluded that the Company’s internal control over financial reporting was ineffective as of June 30, 2016. In addition, the Company has concluded that, as of such dates, there were material weaknesses in the Company’s disclosure controls and procedures (together with the material internal control weaknesses, the “Material Weaknesses”) as a result of the material internal control weaknesses.
Maintaining effective disclosure controls and procedures and effective internal control over financial reporting are necessary for us to produce reliable financial statements and the Company is committed to remediating its material weaknesses in such controls as promptly as possible. The implementation of the Company’s remediation plans has commenced (see Part II, Item 9A. Controls and Procedures in this Annual Report on Form 10-K for additional information regarding such material weaknesses and such remediation process). However, there can be no assurance as to when such material weaknesses will be remediated or that additional material weaknesses will not arise in the future. Any failure to remediate such material weaknesses, or the development of new material weaknesses in our disclosure controls and procedures or internal control over financial reporting, could result in material misstatements in our financial statements and cause us to fail to meet our reporting and financial obligations, which in turn could have a material adverse effect on our business, financial condition and the trading price of our common stock.
We need additional funding to continue our operations and meet our research and development, capital and general and administrative expenses. Such funding may not be available on favorable terms, if at all, and may be dilutive to our existing stockholders. Without modifications to our existing payment obligations or receipt of additional funding, our existing cash and other sources of liquidity may not be sufficient to fund our operations for the next twelve months. If additional capital is not available, we may have to further curtail our operations, or take other actions that could adversely impact our stockholders.
Our business does not generate the cash necessary to finance our operations and has consumed substantial amounts of cash to date. We incurred net losses during fiscal years 2016, 2015 and 2014 of $100.8 million, $90.8 million and $57.9 million, respectively. We expect to continue to incur losses and use cash during fiscal year 2017 and through at least fiscal year 2018. Without modifications to our existing payment obligations or receipt of additional funding, we will require additional capital to continue our operations for the next twelve months. As of June 30, 2016, cash and cash equivalents were $18.7 million, restricted cash was $2.4 million and the book value of our long-term debt was $105.1 million.. In addition, at September 30, 2016, cash and cash equivalents were $6.0 million and restricted cash was $2.1 million.
If we are unable to obtain additional financing on acceptable terms and when needed, we may default under one or more of our debt obligations. A breach of any of the covenants related to our debt instruments could result in a higher rate of interest to be paid, the lenders could elect to declare all amounts outstanding under the applicable debt instruments to be immediately due and payable. If the lenders were to make such a demand for repayment, we would be unable to pay the obligations as we do not have existing facilities or sufficient cash on hand to satisfy these obligations and lenders could foreclose on their security interest in our intellectual property and other assets. These factors continue to raise substantial doubt about our ability to continue as a going concern.
On February 22, 2016, the Company and certain of its subsidiaries entered into a Securities Purchase Agreement (the “Counterparty SPA”) with Amgen Inc. (the “Counterparty”), pursuant to which Counterparty agreed to purchase from the Company a new series of 6% Senior Secured Convertible Notes Due 2023 in the aggregate original principal amount of up to $55.0 million (the “Notes”). The Notes may be issued in up to three separate closings. The Company issued to Counterparty the first Note in the aggregate original principal amount of $30.0 million on February 22, 2016 (the “2016 Convertible Note”) and Counterparty paid to the Company $30.0 million in exchange therefor. Counterparty may purchase up to an additional $25.0 million in Notes over the next two years, $15.0 million of which may be purchased in January 2017 (the “2017 Convertible Note”) and $10.0 million of which may be purchased in January 2018 (the “2018 Convertible Note”). There can be no assurance that Counterparty will elect to purchase the 2017 Convertible Note and/or the 2018 Convertible Note.
Further, we are not currently eligible to register the offer and sale of our securities using a registration statement on Form S-3 and we will not become eligible until we have timely filed certain periodic reports required under the Exchange Act for 12 consecutive
21
calendar months. As a result, the Company will not be able obtain financing under the Controlled Equity Offering Sales Agreement between the Company and Cantor Fitzgerald & Co. or the equity purchase agreement between the Company and Lincoln Park Capital Fund, LLC at least until the Company is eligible to register the offer and sale of our securities using a registration statement on Form S-3.
Our near-term capital needs depend on many factors, including:
|
|
• |
our ability to carefully manage our costs and expenses; |
|
|
• |
the amount and timing of amounts paid under our customer contracts; and |
|
|
• |
our ability to successfully utilize currently existing equity facilities. |
The Company has been implementing cost reduction measures as it focuses operations on the programs of key strategic customers. If we are unable to obtain adequate financing on acceptable terms when needed, we will be required to implement further cost reduction strategies. These reductions could significantly impact activities related to the commercialization and sale of our products, and could result in significant harm to our business, financial condition and results of operations. In addition, these reductions could cause us to further curtail our operations, or take other actions that would adversely impact our stockholders. If we are unable to secure additional financing on terms acceptable to us and on a timely basis, we may seek stockholder approval to downsize or wind down our operations through liquidation, bankruptcy or a sale of our assets.
If we raise additional funds through the issuance of equity securities, the percentage ownership of our existing stockholders will be reduced, our stockholders may experience additional dilution in net book value, and such equity securities may have rights, preferences or privileges senior to those of our existing stockholders.
We need additional funding to meet our capital needs and our debt obligations include covenants which may limit our ability to raise capital.
At June 30, 2016, the book value of our long-term debt was $105.1 million. The Amended OrbiMed Credit Agreement and the Metro Bank Loan (each defined below) contain certain restrictive covenants. The Amended OrbiMed Credit Agreement contains certain customary covenants, including among other things, covenants relating to financial performance and liquidity targets. The Amended OrbiMed Credit Agreement also contains covenants that, among other things, require us to obtain consent from the Lender prior to incurring certain indebtedness or assuming or guaranteeing the indebtedness of another entity or individual. Moreover, the Metro Bank Loan is secured by a mortgage lien and a continuing security interest in the personal property of Unilife Cross Farm LLC, a wholly owned subsidiary of the Company (“Cross Farm”), and contains certain customary covenants, including the maintenance of $2.4 million of restricted cash that must remain in cash deposits (on which FNB has a first priority security interest). In addition, we are required to maintain a cash balance of $3.0 million inclusive of the $2.4 million of restricted cash. Furthermore, the issuance of equity securities in excess of 40% of our outstanding shares of common stock to a person or group (within the meaning of Rule 13d-5 of the Exchange Act) constitutes an event of default under the Amended OrbiMed Credit Agreement. These foregoing restrictive covenants may limit or even prevent our ability to raise capital. If we are unable comply with such covenants or obtain a waiver from our lenders, then it may be more difficult for us to operate our business or we may default on our obligations to our lenders.
As a result of the delayed filing of our Quarterly Report on Form 10-Q for the fiscal period ended March 31, 2016 (the “Third Quarter 10-Q”) and this Annual Report on Form 10-K with the SEC, we are not currently eligible to use a registration statement on Form S-3 to register the offer and sale of securities, which may adversely affect our ability to raise future capital or complete acquisitions.
We are not currently eligible to register the offer and sale of our securities using a registration statement on Form S-3 and we will not become eligible until we have timely filed certain periodic reports required under the Exchange Act for 12 consecutive calendar months. There can be no assurance when we will meet this requirement, which depends in part upon our ability to file our periodic reports on a timely basis in the future. Should we wish to register the offer and sale of our securities to the public before we are eligible to do so on Form S-3, our transaction costs and the amount of time required to complete the transaction could increase, making it more difficult to execute any such transaction successfully and potentially having an adverse effect on our financial condition.
We may not be able to successfully execute our new refocused business strategy.
In July 2016, we announced that the Company will focus primarily on active and new customer programs in its portfolio of wearable injector systems. This primary focus on wearable injectors is expected to enhance operating efficiencies and better position the Company to take advantage of commercial opportunities within the fast-growing market for wearable injectors. There can be no
22
assurances that our new strategic direction will result in the commercialization and sale of our products and we cannot provide any assurances that our focus on active and new customer programs in our portfolio of wearable injector systems will be successful.
We have forecasted cost savings from our plan to refocus our business strategy based on a number of assumptions and expectations which, if achieved, would improve our cash flows from operating activities. However, there can be no assurance that the expected results will be achieved. The estimated costs and benefits associated with the plan are preliminary and may vary based on various factors including: the timing of execution of the plan, outcome of negotiations with third parties, and changes in management’s assumptions and projections. As a result, delays and unexpected costs may occur, which could result in our not realizing all, or any, of the anticipated benefits associated with the plan.
In connection with this new focus, the Company has been evaluating the prospects of its non-wearable injector customer and supplier programs. The Company is attempting to negotiate terminations of certain non-wearable injector customer and supplier contracts. There can be no assurance that the Company will be able to successfully negotiate the termination of these contracts and the Company may incur material expenses in exiting customer and/or supplier contracts. Certain of these customer contracts contain material cancellation penalties which the Company is in the process of negotiating. While the Company believes it may be able to eliminate or significantly reduce these penalties, there can be no assurance that the Company will be successful in doing so.
Further, our new business strategy can potentially present risks that may otherwise harm our business, including failure to meet customer or regulatory requirements due to the loss of employees or inadequate transfer of knowledge and negative impact on employee morale and increasing attrition among our workforce.
We are subject to litigation that could adversely affect our business.
We are or have been involved in a number of lawsuits. See “Part I, Item 3. Legal Proceedings” in this Annual Report on Form 10-K for a more detailed description of these lawsuits. We intend to contest all of these cases vigorously and will explore all options, as appropriate, in the best interests of the Company. However, as with all litigation, there is a possibility that we will suffer adverse decisions or verdicts of substantial amounts, or that we will enter into monetary settlements in one or more of these cases, which may or may not be covered by insurance. The time and cost of such litigation, as well as the ultimate outcome of such litigation, whether or not we are successful, could divert management’s attention from our business and could adversely affect our business, operations, cash flows and/or financial condition.
Recent changes in our management team may be disruptive to, or cause uncertainty in, our business, results of operations, financial condition, and the market price of our common stock.
During the second half of fiscal year 2016, the Company implemented significant changes in its Board and management team composition (see the “Explanatory Note – Management and Board Changes” to this Annual Report on Form 10-K for additional information regarding such changes to our Board and management team composition). These changes may be disruptive to or cause uncertainty in our business. The failure to ensure a smooth transition and effective transfer of knowledge involving senior employees could also negatively affect our business. These matters could have a material adverse impact on our results of operations, financial condition, and the market price of our common stock.
We are not in compliance with the requirements of NASDAQ for continued listing, and if NASDAQ does not concur that we have adequately remedied our non-compliance with NASDAQ Listing Rule 5250(c)(1) or we do not adequately remedy our non-compliance with NASDAQ Listing Rule 5450(b)(2)(A), our common stock may be delisted from trading on NASDAQ, which could have a material adverse effect on us and our stockholders.
We were delinquent in the filing of the Third Quarter 10-Q and this Annual Report on Form 10-K for the fiscal year ended June 30, 2016. As a result, we have not been in compliance with the listing rules of NASDAQ. On July 18, 2016, and subsequently on October 4, 2016, we submitted a compliance plan and an updated compliance plan, respectively, to NASDAQ and were granted an exception to file our Third Quarter 10-Q and any other delinquent periodic financial reports on or before November 7, 2016 in order to enable us to regain compliance with the listing rules. We filed our Third Quarter 10-Q concurrently with the filing of this Annual Report on Form 10-K. As a result, we currently believe that we have adequately remedied our non-compliance with NASDAQ’s Listing Rule 5250(c)(1) within NASDAQ’s terms of exception. However, because the Company did not maintain a minimum Market Value of Listed Securities (“MVLS”) of $50,000,000 for the last 30 consecutive business days, the Company is no longer in compliance with NASDAQ Listing Rule 5450(b)(2)(A). The Company has been provided a period of 180 calendar days, or until April 17, 2017, to regain compliance with the minimum MVLS listing requirement. If at any time on or before April 17, 2017, the MVLS of the Company’s common stock closes at $50,000,000 or more for a minimum of 10 consecutive business days, NASDAQ will provide the Company with written confirmation that the Company has achieved compliance with the minimum MVLS listing requirement and the matter will be closed. There can be no assurance that NASDAQ will concur that we have remedied our non-compliance with NASDAQ Listing Rule 5250(c)(1) or that we will be able to adequately remedy our non-compliance with NASDAQ
23
Listing Rule 5450(b)(2)(A), in which case our common stock could remain subject to delisting by NASDAQ. If our common stock were delisted, there can be no assurance whether or when it would again be listed for trading on NASDAQ or any other securities exchange. In addition, the market price of our shares might decline and become more volatile, and our stockholders might find that their investment in our shares has limited liquidity. Furthermore, institutions whose charters do not allow them to hold securities in unlisted companies might sell our shares, which could have a further adverse effect on the price of our stock.
Our fiscal year 2016 consolidated financial statements state that our recurring losses from operations and limited cash resources raise substantial doubt about our ability to continue as a going concern.
Our continuation as a going concern is dependent upon our attaining and maintaining profitable operations, generating continued cash payments from customers under new or existing contracts and/or raising additional capital. In addition, our consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Our liquidity is highly dependent on our ability to improve our financial condition. Our failure to obtain new or additional financing could impair our ability to both serve our existing customer base and develop prospective customers and could result in our failure to continue to operate as a going concern.
The uncertainty regarding our ability to continue as a going concern may have an adverse effect on our customer and supplier relationships.
Our relationships with our existing and prospective customers and suppliers are predicated on the belief that we will continue to operate as a going concern. Certain of our existing customers may terminate their agreements with us and certain of our prospective customers may not enter into agreements with us if there is uncertainty regarding our ability to continue as a going concern. This may have an adverse effect on our ability to grow our revenue, which is a key component of our plan to continue as a going concern. Current and future suppliers may be less likely to grant us credit, resulting in a negative impact on our working capital and cash flows.
We do not expect to be profitable until we either achieve commercial scale production and sales of our proprietary injectable drug delivery systems or receive sufficient upfront fees and other payments from customers to offset our total operating expenditures.
At the present time, none of our customers has received regulatory clearance or approval from the U.S. Food and Drug Administration, or the FDA, or a foreign regulatory authority for the commercial sale of a drug-device combination product that incorporates any of our products. It is also our understanding that, at the present time, none of our customers has applied for regulatory approval of a drug-device combination product using our technology. None of our products are currently being sold by our customers with their injectable therapies. To date, we have derived substantially all of our revenue from upfront payments, exclusivity fees, device and development materials and milestone-based fees from customers. Many of our customers’ injectable therapies that are planned to be used in combination with our products are in various stages of clinical development. We have a broad portfolio of proprietary product platforms, including wearable injectors, insulin delivery systems, disposable and reusable auto-injectors, pre-filled syringes, drug reconstitution delivery systems, ocular delivery systems and other systems for the targeted delivery of injectable therapies. We have incurred, and will continue to incur, research and development expenses related to the development and manufacturing scale-up of products for our customers. During fiscal year 2016, we invested $43.2 million to address our research and development requirements, including employee costs, equipment, materials, tooling, prototypes and outside contract services. We expect to continue investing in certain capital equipment to support increasing customer demand for our products and services. We will also incur significant general and administrative expenses such as assisting our customers with seeking regulatory approvals and complying with the requirements related to being a public company in both the United States and Australia. We do not expect to be profitable until we generate sufficient revenue from product sales, upfront fees, milestone-based payments or royalty fees to offset our total operating expenditures.
If we experience problems or delays in securing additional agreements to supply our products and services to customers, our business, including our ability to generate additional revenue, will be materially and adversely affected.
To date, we have signed commercial supply contracts and other agreements with several pharmaceutical and biotechnology companies. However, our ability to generate additional revenue will also depend on our ability to successfully negotiate additional agreements for our proprietary product platforms with new or existing customers and, notwithstanding potential upfront or milestone-based payments, to begin supplying substantial commercial quantities of such products under such agreements. Given the substantial size, complexity and long-term duration of many of these prospective agreements, they can take significant time to negotiate and finalize. We cannot provide assurance of whether we will be able to enter into additional agreements for our products or what the terms of any such agreements will be. If we are unable to secure additional supply agreements for our products and services in a timely manner or obtain favorable terms under such agreements, our ability to generate additional revenue aside from our existing contracts will be materially and adversely affected.
24
Our devices will be subject to regulatory oversight and approvals either as combination products or as devices. We cannot be sure how the FDA, European Medicines Agency (“EMA”) or other foreign regulatory authority will regulate our customers’ drug-device combination products or our devices and/or whether we or our customers will be successful in obtaining and/or maintaining regulatory approval.
Our products, outside of our reusable auto-injector and certain systems for targeted drug delivery, are designed to be supplied to customers as sub-assembly components. Such sub-assembly components are ready for filling with a measured dose of an injectable therapy, and the final assembly or packaging is to be conducted by our customers or a third party. Once our products are filled, assembled and/or packaged by our customers or a third party with an injectable therapy, they will become classified for regulatory purposes as drug-device combination products. In such instances, our customers will ultimately be responsible for seeking and obtaining regulatory approval of the drug-device combination product. Each of our supply agreements that are currently in effect reflect such business-to-business partnerships under which we will sell our products to pharmaceutical customers who are ultimately responsible for the regulatory approval, marketing and sale of the drug-device combination product.
In order to be eligible to market and sell a drug-device combination, a customer will need to submit and receive approval of an NDA, BLA, ANDA, sBLA or the foreign equivalent of any of the foregoing.
For a drug-device combination product, it is possible that our customers will be required to submit marketing applications through both the drug or biologic and medical device pathways. The process of obtaining FDA marketing clearance or approval is lengthy, expensive, and uncertain. We cannot be sure how the FDA, EMA or other foreign regulatory authority will regulate our customers’ drug-device combination products, in which case the path to regulatory approval would be different and could be more lengthy and costly. We also cannot be sure that our customers’ drug-device combination products will be cleared or approved in a timely fashion, or at all, which could impact our customers’ ability to market their drug-device combination products that utilize our products. If the FDA does not approve or clear the drug-device combination products in a timely fashion or at all, or if there are ongoing issues with obtaining approval of drug-device combination products involving our proprietary product platforms and our customers’ injectable therapies, our business and financial condition will be adversely affected.
Moreover, the approval process may also require changes to our customers’ drug-device combination products or result in limitations on the indicated uses of our customers’ drug-device combination products, which could negatively affect us. As a result, our customers’ expectations with respect to marketing approval or clearance may prove to be inaccurate, and our customers may not be able to obtain marketing approval or clearance in a timely manner or at all. We could face similar issues if we submit any devices to the FDA or a foreign regulatory authority for approval, as appropriate for the class of device (or other foreign regulatory processes). We may be required to make changes to our device or we may not be able to obtain marketing approval or clearance in a timely manner or at all.
In addition, regulatory requirements in the United States and outside the United States can, at any time, require prompt action to maintain compliance, particularly, when product modifications are required. Following the introduction of a drug-device combination product or a device, these agencies will also periodically review our or our customers’ manufacturing processes and the performance of our customers’ drug-device combination products or our devices.
Our or our customers’ failure to comply with cGMP, failure to comply with adverse event reporting, clinical trials and other requirements of these agencies could delay or prevent the production, marketing or sale of our customers’ drug-device combination products or our devices and result in fines, delays, suspensions or prevention of regulatory clearances, closure of manufacturing sites, seizures or recalls of products and damage to our and our customers’ reputations.
We and our customers are subject to extensive regulation by governments around the world, and if these regulations are not complied with, existing and future operations may be curtailed, and we could be subject to liability.
Our devices and our customers’ drug-device combination products that utilize our products are subject to extensive regulation by governmental authorities in the United States, Europe and other countries, including the FDA. Not only do these regulations present challenges during the regulatory approval process as discussed above, but after our devices or our customers’ drug-device combination products that utilize our products are approved and placed in the market, numerous regulatory requirements will apply. These include, but are not limited to QSR, labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or “off label” uses, medical device reporting regulations and post-market surveillance regulations, and laws and regulations that govern the development, testing, manufacturing, advertising, marketing and distribution of medical devices, including our devices and our customers’ drug-device combination products that utilize our products. The FDA has broad post-market and regulatory enforcement powers.
25
As a registered device manufacturer and supplier of the drug delivery device component of a combination product, we are subject to unannounced and preapproval inspections by the FDA of our manufacturing facility to determine our compliance with QSR and cGMP.
Failure to comply with applicable regulatory requirements can result in an enforcement action by the FDA or other regulatory authority, which may include any or all of the following sanctions: fines, injunctions, consent decrees and civil penalties, recall or seizure of our products or our customers’ drug-device combination products, operating restrictions, partial suspensions or total shutdown of production, refusing our customers’ requests for regulatory approvals of their drug-device combination products or new intended uses, as applicable, refusing our requests for regulatory approval of our devices, withdrawing our customers’ or our regulatory approvals that may be granted and criminal prosecution.
The therapeutic efficacy of certain of our customers’ therapies is either unproven in humans or has only been proven in limited circumstances, and we may not be able to successfully develop and sell our products in combination with our customers’ therapies.
While some of our customers will be using our products with established, approved therapies, in certain instances, the benefits of our customers’ therapies as injectable therapies is either unproven or has only been proven in limited circumstances. Our ability to generate revenue from our products will depend heavily on the successful development, commercialization and sales of our customers’ therapies, which is subject to many potential risks. For example, data developed in clinical trials or following the commercialization of our customers’ therapies may show that such therapies do not prove to be effective treatments for the targets they are being designed to act against (or as effective as other treatments available). In clinical trials or following commercialization, it may be shown that our customers’ therapies interact with human biological systems in unforeseen, ineffective or harmful ways. If our customers’ therapies are associated with undesirable side effects or have characteristics that are unexpected, our customers may need to abandon clinical development or discontinue commercial sales or limit clinical development or sales to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. As a result of these and other risks described herein that are inherent in the development and sale of therapeutic agents, our customers may never successfully develop or successfully commercialize their therapies or the commercialization of our customers’ therapies may be abandoned or severely limited, which may limit our profitability with respect to such customers, and we may not be successful in achieving commercial scale production and sales of our injectable drug delivery systems in combination with our customers’ therapies.
Certain of the injectable therapies being targeted for use with our products are not approved, but are in various phases of clinical development. These injectable therapies may be independently terminated by our customers prior to submission of a regulatory filing or even after our customers receive regulatory approval, resulting in the cessation of any revenue associated with that contract or program.
We work with pharmaceutical and biotechnology companies who are targeting the use of our products with a variety of injectable therapies. While some of our customers have approved therapies, certain of our customers’ injectable therapies are not approved, and are in various phases of clinical development. None of our customers with approved therapies has received regulatory clearance or approval from the FDA or a foreign regulatory authority for the commercial sale of a drug-device combination product that incorporates its approved therapy and one of our products. The clinical development of these pipeline therapies can be terminated by our customers at any stage. Furthermore, our customers could obtain regulatory approval for their injectable therapies and their drug-device combination products that include our product, and decide for business reasons not to market and sell their drug-device combination product. Prior investments we have made in manufacturing capacity or research and development will then not result in the generation of revenue that would have previously been anticipated through our customers’ regulatory approval, launch and post-market sales of the drug-device combination product within target domestic and international markets.
Our customers may terminate their contracts or cease doing business with us in the event of a breach by us, for strategic reasons or, in some cases, for no reason.
While the term of our customer contracts may be for up to 15 years, our customers may decide to terminate their contracts or cease doing business with us in the event of a breach by us, for strategic reasons or, in some cases, for no reason. In such event, we may not receive associated payments or revenue from such customer or under the relevant contract and we may not be able to recoup investments we have made in manufacturing capacity or research and development in connection with such customer or customer contract. Accordingly, if our customers terminate their contracts with us, it could have a material adverse impact on our business, prospects, results of operations, cash flows and financial condition.
26
Our commercial success depends upon the attainment of significant market acceptance of our customers’ product candidates, if approved, among physicians, patients, healthcare payers or the medical community.
Even if our customers obtain regulatory approval for their product candidates, their product candidates may not gain sufficient levels of market acceptance among physicians, healthcare payers, patients or the medical community to make them commercially feasible. Market acceptance of our customers’ product candidates, if they receive approval, depends on a number of factors, including the:
|
|
• |
efficacy and safety of our customers’ product candidates; |
|
|
• |
clinical indications for which their product candidates are approved; |
|
|
• |
acceptance by physicians, patients and the medical community of their product candidates as a safe and effective treatment; |
|
|
• |
potential and perceived advantages of their product candidates over alternative treatments; |
|
|
• |
safety of their product candidates seen in a broader patient group; |
|
|
• |
prevalence and severity of any side effects; |
|
|
• |
product labeling or product insert requirements of the FDA or other regulatory authorities; |
|
|
• |
timing of market introduction of their product candidates as well as competitive products; |
|
|
• |
cost of treatment in relation to alternative treatments; |
|
|
• |
availability of coverage and adequate reimbursement and pricing by third party payers and government authorities; |
|
|
• |
relative convenience and ease of administration; and |
|
|
• |
effectiveness of their sales and marketing efforts. |
If our customers’ product candidates are approved but fail to achieve market acceptance among physicians, patients or healthcare payers, we may not be able to generate anticipated revenue. This may limit our ability to generate anticipated revenue from our prior investment in assembly lines and other resources. Moreover, even if we achieve commercial scale production and sales of our injectable drug delivery systems in combination with our customers’ injectable therapies, such customers may face indirect competition from companies who develop and market other brand name, biosimilar or generic injectable therapies as well as alternative treatments and delivery methods that compete with our customers’ drug-device combination products that utilize our products, which may have a material adverse effect on our results of operations, our financial condition and/or cash flows.
Most brand name injectable therapies will face future competition from generic or biosimilar therapies, which could significantly reduce their commercial viability.
Brand name injectable therapies will usually become exposed to competition from generic or biosimilar rivals at some time after their regulatory approval and commercial launch. The average selling price and market share of brand name injectable therapies can be significantly diminished following the introduction of generic or biosimilar competition. These factors may result in our customers using our products with their brand name injectable therapies seeking to withdraw such injectable therapy from the market, or change market tactics in a way that makes the use of our products cost prohibitive. This may result in the termination of supply contracts and the significant loss of revenue.
Our debt obligations include covenants restricting our business which may adversely affect us.
On March 12, 2014, Unilife Medical Solutions, Inc., a wholly owned subsidiary of the Company, or the Borrower, entered into a Credit Agreement with ROS Acquisition Offshore LP, or the Lender or ROS, as amended by the First Amendment to the Credit Agreement, dated September 30, 2014, the Second Amendment to the Credit Agreement, dated June 30, 2015, the Third Amendment to the Credit Agreement, dated October 13, 2015, the Fourth Amendment to the Credit Agreement, dated December 31, 2015, the Fifth Amendment to the Credit Agreement, dated January 31, 2016, the Sixth Amendment to the Credit Agreement, dated February 9, 2016, the Seventh Amendment to the Credit Agreement, dated February 16, 2016, and the Eighth Amendment to the Credit Agreement, dated February 22, 2016 (as so amended, the “Amended OrbiMed Credit Agreement”). Pursuant to and subject to the terms of the Amended OrbiMed Credit Agreement, the Lender agreed to provide term loans to the Borrower in the aggregate principal amount of up to $70 million. The Borrower has received the full $70 million under the Amended OrbiMed Credit Agreement. The interest rate is 9.25% plus the greater of the three month LIBO Rate (as defined in the Amended OrbiMed Credit Agreement) or 1.0%.
27
Unless the loan facility is otherwise terminated earlier pursuant to the terms of the Amended OrbiMed Credit Agreement, the Borrower is required to repay in full the unpaid principal amount of the loans drawn down, together with all accrued and unpaid interest thereon plus a 10.0% repayment premium on March 12, 2020. The Borrower can make voluntary repayments at any time of any unpaid principal amount of the loans, plus a 10.0% repayment premium. The Borrower must make mandatory prepayments in certain prescribed circumstances, including, without limitation, certain dispositions of assets and certain casualty events. In such events, the Borrower must prepay to Lender 100% of the net cash proceeds received.
The obligations of the Borrower under the Amended OrbiMed Credit Agreement are: (i) secured by substantially all assets of the Borrower, subject to the security interest in certain assets granted to Counterparty on February 22, 2016, and (ii) guaranteed by the Company and each of its subsidiaries. Such guarantees are secured by substantially all assets of the guarantors. The security interests granted by Borrower, the Company, Cross Farm, UMSL and Unitract Syringe Pty Limited, or Unitract Syringe, are evidenced by, among other things, a Pledge and Security Agreement, a Mortgage and Security Agreement and a General Security Deed.
The Amended OrbiMed Credit Agreement contains certain customary covenants, including among other things, covenants relating to financial performance and liquidity targets. The Amended OrbiMed Credit Agreement also contains covenants that, among other things, require us to obtain consent from the Lender prior to paying dividends, making certain investments, incurring debt or liens (with certain exceptions), changing the nature of our business, assuming or guaranteeing the indebtedness of another entity or individual, selling or otherwise disposing of a substantial portion of our assets, merging or consolidating with another entity, or issuing equity securities in excess of 40% of our outstanding shares of common stock to a person or group (within the meaning of Rule 13d-5 of the Exchange Act).
A breach of any of the covenants in the Amended OrbiMed Credit Agreement could result in a default under that agreement. Upon the occurrence of an event of default, a default interest rate of 14.25% per annum plus the greater of three-month LIBO Rate or 1.0% shall apply during the existence of a default. There is also a risk that the Lender could obtain rights to the secured assets in the event we default on our obligations under the Amended OrbiMed Credit Agreement. Additionally, the Lender could elect to declare all amounts outstanding under the Amended OrbiMed Credit Agreement to be immediately due and payable, and terminate all commitments to extend further credit.
In addition, on October 20, 2010, Cross Farm entered into a Loan Agreement with First National Bank (“FNB”), formerly Metro Bank, as amended in connection with the Amended OrbiMed Credit Agreement on March 12, 2014, or the Metro Bank Loan. The Metro Bank Loan is secured by a mortgage lien and a continuing security interest in Cross Farm’s personal property, and contains certain customary covenants, including the maintenance of $2.4 million of restricted cash that must remain in cash deposits (on which FNB has a first priority security interest). In addition, we are required to maintain a cash balance of $3.0 million inclusive of the $2.4 million of restricted cash. Upon the occurrence of an event of default under the agreement evidencing the Metro Bank Loan, there is a risk that FNB could obtain rights to the secured assets. Additionally, FNB could elect to declare all amounts outstanding under the Metro Bank Loan to be immediately due and payable.
Furthermore, on or about December 17, 2010, Keystone Redevelopment Group, LLC made a loan to Cross Farm in the original principal amount of $2.25 million which was secured by a mortgage lien. Keystone Redevelopment Group, LLC assigned the loan and mortgage, or the Keystone/CFA Loan, to Commonwealth Financing Authority. Upon the occurrence of an event of default under the agreements evidencing the Keystone/CFA Loan, there is a risk that Commonwealth Financing Authority could obtain rights to the mortgaged property.
On February 22, 2016, the Company and Counterparty entered into the Counterparty SPA, pursuant to which the 2016 Convertible Note was issued. Upon an occurrence of an event of default under the 2016 Convertible Note, Counterparty could elect to declare all amounts outstanding under the 2016 Convertible Note to be immediately due and payable.
There are cross-default provisions in the Amended OrbiMed Credit Agreement, Metro Bank Loan, Keystone/CFA Loan and the 2016 Convertible Note, so that a default under one debt instrument could trigger a default under the others. As noted above, events of default under these debt instruments could result in the applicable counterparties demanding repayment of our debt. If such counterparties were to make such a demand for repayment, we would be unable to pay the obligations as we do not have existing facilities or sufficient cash on hand to satisfy these obligations. These factors continue to raise substantial doubt about our ability to continue as a going concern.
We may face significant uncertainty in the industry due to government healthcare reform.
The healthcare industry in the United States is subject to fundamental changes due to the ongoing healthcare reform and the political, economic and regulatory influences. In March 2010, comprehensive healthcare reform legislation was signed into law in the United States through the passage of the Patient Protection and Affordable Health Care Act and the Health Care and Education Reconciliation Act. Among other initiatives, the legislation provides for a 2.3% annual excise tax on the sales of certain medical
28
devices in the United States, commencing in January 2013. A manufacturer of a taxable medical device may, in certain circumstances, sell a taxable medical device tax-free for use by the purchaser for further manufacture (or for resale by the purchaser to a second purchaser for further manufacture), or for export (or for resale for export). We believe that the 2.3% annual excise tax is not applicable to us because our products are sold to our customers as components for further assembly by our customers. However, we cannot give assurances that the U.S. Internal Revenue Service will treat the sale of our products to our customers for use in their drug-device combination products as tax-free sales. Accordingly, this enacted excise tax may adversely affect our operating expenses and results of operations. In addition, various healthcare reform proposals have also emerged at the state level. We cannot predict with certainty what healthcare initiatives, if any, will be implemented at the state level, or what the ultimate effect of federal healthcare reform or any future legislation or regulation may have on us or on our customers’ purchasing decisions regarding our products and services.
Some companies we may utilize for the supply of components are also competitors, and they could elect to cease supply relationships with us in the future for competitive reasons.
Some companies we may utilize for the supply of components for our proprietary products also develop and market their own products that compete with ours. These companies may elect to cease supply relationships with us in the future for competitive reasons. This may disrupt our supply chain, cause difficulties in the qualification of new sources of supply and impair our ability to supply customer orders. Such events may have a material adverse effect on our results of operations, our financial condition and/or cash flows.
The injectable drug delivery systems industry is very competitive.
The market for injectable drug delivery systems is highly competitive. We compete against many companies, both public and private, that range in size from small, highly focused businesses to large diversified multinational manufacturers of medical devices and healthcare equipment. Our larger competitors have greater financial and human resources, distribution channels and sales and marketing capabilities than we do which may provide them a competitive advantage in acquiring new customers.
Additionally, while our customizable products and multi-source strategy for components and raw materials provides us significant differentiation in the medical device markets, it also makes supplier relationships a key aspect of our business model. Many of our competitors are vertically integrated and may source their own raw materials and/or produce their own commodity components such as plastics, glass or elastomers. As such, these competitors have greater control over the supply of these components and are therefore not subject to the same supply chain risks to which we are subject. Furthermore, because we do not produce these commodity components ourselves, we may need to purchase certain components from commodity component suppliers who are also our device competitors. Such supplier-competitors may have a disincentive to meet our supply chain needs on terms that are favorable to us.
We are also subject to competition from our customers. In particular, our customers can decide to develop their own injectable drug delivery systems internally. Moreover, we may face indirect competition from companies who develop and market alternative treatments and delivery methods that compete with our customers’ drug-device combination products that utilize our products.
Our ability to compete effectively depends upon our ability to distinguish our company and our products from our competitors and their products. Factors affecting our competitive position include, for example, product design and performance, pricing of our services and products, product safety, sales, marketing and distribution capabilities, success and timing of new product development and introductions, intellectual property protection and our financial wherewithal and perception thereof.
We may be unable to protect our intellectual property rights or may infringe on the intellectual property rights of others.
Our success depends in part on our ability to obtain and maintain protection in the United States and other countries of the intellectual property relating to or incorporated into our technology and products. Granted patents expire at varying dates based on the filing date of the related application. Patents relating to our wearable injectors expire between calendar years 2032 and 2036, with some patents for other products expiring earlier. Pending and/or future filed patent applications covering relevant advancements in our technologies could extend well beyond 2036 once granted. Our pending and future patent applications may not issue as patents or, if issued, may not issue in a form that will provide us with any competitive advantage or adequate protection. In particular, the injectable drug delivery systems which we are developing and for which we have filed patent applications are relatively new inventions, and we cannot be sure that we will be able to obtain patents on these inventions. Our issued and future patents may be challenged, narrowed, invalidated or circumvented, which could limit our ability to stop competitors from marketing similar technology or products or limit the length of terms of patent protection we may have for our technology or products. Changes in patent laws or their interpretation in the United States and other countries could also diminish the value of our intellectual property or narrow the scope of our patent protection. In addition, the legal systems of certain countries do not favor the aggressive enforcement of patents, and the laws of foreign countries may not protect our rights to the same extent as the laws of the United States. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing technology or products similar or identical to ours. In
29
order to preserve and enforce our patent and other intellectual property rights, we may need to make claims or file lawsuits against third parties. This can entail significant costs to us and divert our management’s attention from developing and commercializing our technology or products.
There also can be no assurance that third parties will not assert that our technology or products infringe their patent or other intellectual property rights. Any claims, with or without merit, could be time-consuming, result in costly litigation, divert the efforts of our technical and management personnel or require us to pay substantial damages. If we are unsuccessful in defending ourselves against these types of claims, we may be required to do one or more of the following:
|
|
• |
stop, delay or abandon our ongoing or planned commercialization of the product that is the subject of the suit; |
|
|
• |
attempt to obtain a license to sell or use the relevant technology or substitute technology, which license may not be available on reasonable terms or at all; |
|
|
• |
redesign the product that uses the relevant technology; or |
|
|
• |
pay damages which could adversely impact our financial condition and ability to execute our business plan and operations. |
If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected.
In addition to patented technology, we rely on our unpatented proprietary technology, trade secrets, processes and know-how. We generally seek to protect this information by confidentiality agreements with our employees, consultants, scientific advisors, contractors and third parties. These agreements may be breached, and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known or be independently developed by competitors. To the extent that our employees, consultants, scientific advisors or contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
We, as an FDA-registered medical device manufacturer and component supplier to our customers, are required to comply with cGMP for any products we supply to our customers for clinical or commercial use. If we fail to comply with cGMP for our manufacturing facility, our business and our results of operations would be harmed.
Our QMS is regularly audited by regulatory authorities for compliance with the requirements of, and certified to, ISO 13485, Medical Devices – Quality Management Systems – Requirements, for regulatory purposes, which is equivalent to QSR for FDA compliance. As an FDA registered medical device manufacturer, we are periodically audited by the FDA pursuant to the QSRs and cGMP with the last audit occurring in 2013 where there were no formal findings. In addition, our QMS is regularly audited by existing and prospective customers.
If we do not continue to have our QMS in compliance with ISO certifications or receive major non-conformances by the FDA or other foreign regulatory authorities during audits of our QSRs and cGMP, we may experience regulatory related delays as a result. If we do not comply with cGMP when supplying any products to our customers for clinical or commercial use, our failure could have a material adverse impact on our business, prospects, results of operations, cash flows and financial condition.
If we or our suppliers experience interruptions in manufacturing operations, our business will suffer.
We currently manufacture our products at our York, Pennsylvania facility, with no alternate facilities available. We also use a number of suppliers to supply components for our products. If we or our suppliers were to experience a manufacturing disruption as a result of damage or destruction of the building, equipment failure, acts of God or other force majeure events, our ability to satisfy our obligations to our customers would be adversely affected, which would harm our business and our results of operations.
The sale of any of our proprietary products could be stopped, delayed or made less profitable if our manufacturing facility fails to provide us with sufficient quantities of our proprietary products or fails to do so at acceptable quality levels or prices and in a timely manner.
To manufacture our proprietary products in the quantities and at the prices that we believe would be required to meet anticipated market demand of future customers, or in the event of increased orders from our current customers, we would need to increase manufacturing capacity, which would involve typical scale-up challenges. In addition, any expansion to our existing commercial-scale manufacturing capabilities may require us to either outsource manufacturing capabilities to third parties and/or to invest substantial funds and hire and retain technical personnel who have the necessary manufacturing experience. We may not successfully complete any manufacturing scale-up activities required to increase existing manufacturing capabilities in a timely manner, or at all.
30
Product defects could adversely affect the results of our operations.
The design, manufacture and marketing of our products involve certain inherent risks. Manufacturing or design defects, unanticipated use of our products, or inadequate disclosure of risks relating to the use of the product can lead to injury or other adverse events. These events could lead to recalls or safety alerts relating to our products (either voluntary or required by the FDA or similar governmental authorities in other countries), and could result, in certain cases, in the removal of a product from the market. Any recall could result in significant costs, the loss of a customer, negative publicity and damage to our reputation that could reduce demand for our products. Personal injuries relating to the use of our products can also result in product liability claims being brought against us. In some circumstances, such adverse events could also cause delays in the launch of new products.
Because our proprietary products, in connection with our customers’ injectable therapies, will be drug-device combination products, we also face additional risks of recalls that could be caused by our customers’ therapies. Any such recall could similarly result in significant costs, negative publicity and damage to our reputation, even if caused by a customer’s products. We may also realize significant costs and delay in finding a substitute customer to sell into the affected market, if one can be found at all.
We may be sued for product liability claims, which could adversely affect our business.
The design, manufacture and marketing of our products carry a significant risk of product liability claims. We may be held liable if any product we develop and sell to our customers causes injury or is found otherwise unsuitable during product testing, manufacturing, marketing, sale or consumer use. In addition, since our products will be used in our customers’ drug-device combination products, we may be sued for product liability even if the claimed injury is caused by our customers’ injectable therapies and not our products. We carry product liability insurance in the amount of $10 million. However, if there were to be product liability claims against us, our insurance may be insufficient to cover the expense of defending against such claims, or may be insufficient to pay or settle such claims. Furthermore, we may be unable to obtain adequate product liability insurance coverage for sales of any of our products or our customers’ drug-device combination products that utilize our products. If such insurance is insufficient to protect us, our results of operations will suffer. If any product liability claim is made against us, our reputation and future sales will be damaged, even if we have adequate insurance coverage. We also intend to seek product liability insurance for any products that we may develop or acquire and any of our products that are used in combination with our customers’ injectable therapies in the future. There is no guarantee that such coverage will be available when we seek it or at a reasonable cost to us.
Our relationships with customers will be subject to applicable state, federal and healthcare laws and regulations, which could result in criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third party payers will play a primary role in the recommendation and prescribing of any drug-device combination products for which our customers obtain marketing approval. Our future arrangements with our customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may affect the business or financial arrangements and relationships through which we would market, sell and distribute our products. Although we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third party payers, federal and state healthcare laws and regulations may be applicable to our business.
Efforts to ensure that our business arrangements with third parties are compliant with applicable healthcare laws and regulations will involve the expenditure of appropriate, and possibly significant, resources. Nonetheless, it is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment and the curtailment or restructuring of our operations.
We cannot give any assurance that we will be able to complete the development and sale of new or customized injectable drug delivery systems in response to the emerging needs of our pharmaceutical and biotechnology customers.
Our injectable drug delivery systems, including customized products, are a response to changes in medical technologies, industry standards and the emerging needs of our pharmaceutical and biotechnology customers. Our success will depend on our ability to continue developing and selling new injectable drug delivery systems and customizing our existing products to meet the changing needs of our customers. The development and customization of these injectable drug delivery systems requires significant research and development and expenditures of capital. There can be no assurance that our customization and development efforts will result in successful products that meet the changing needs of our customers.
31
We will continue to incur significant costs as a result of being a public company in both the United States and Australia.
We are subject to the periodic reporting requirements of the Exchange Act. Being a public reporting company in the United States entails significant expense, including costs required for us to comply with the Sarbanes-Oxley Act of 2002. In addition, because our shares of common stock are also listed on the ASX, in the form of CHESS Depositary Interests (“CDIs”), we are also required to file financial information and make certain other filings with the ASX. Our status as a reporting company in both the United States and Australia makes some activities more time-consuming and costly and causes us to incur legal, accounting and other expenses that are higher than those that are typically incurred by companies that are subject to reporting requirements in only one jurisdiction. Being subject to multiple and sometimes competing reporting frameworks also subjects us to greater risk of non-compliance.
The costs of raw materials have a significant impact on the level of expenses that we incur. If the prices of raw materials and related factors such as energy prices increase, and if we cannot pass those price increases on to our customers, our results of operations and financial condition would suffer.
We use a number of raw materials including polymer plastics. The prices of many of these raw materials, such as those sourced from petroleum-based raw materials, are cyclical and volatile. While we would generally attempt to pass along increased costs to our customers in the form of sales price increases, we might not be able to do so for competitive or contract-related reasons or otherwise. If we cannot set or adjust our prices to reflect the costs of our raw materials, our results of operations and our financial condition will suffer.
Disruptions in the supply of key raw materials and difficulties in the supplier qualification process could adversely impact our operations.
We employ a supply chain management strategy which seeks to source components and materials from a number of established third party companies. Where possible, we seek to establish more than one contract for the supply of a particular component, material or service. However, there is a risk that our supply lines may be interrupted in the event of a supplier production problem, material recall or financial difficulties. If one of our suppliers is unable to supply materials required for production of our products or our strategies for managing these risks are unsuccessful, we may be unable to complete the production of sufficient quantities of product to fulfill customer orders, or complete the qualification of new replacement materials for some programs in time to meet future production requirements. Prolonged disruptions in the supply of any of our key raw materials or components, and difficulty in completing qualification of new sources of supply or in implementing the use of replacement materials or new sources of supply could have a material adverse effect on our results of operations, our financial condition or cash flows.
We are subject to regulations related to conflict minerals, which could adversely impact our operations.
Pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act, the SEC issued final rules regarding disclosure of the use of tantalum, tin, and tungsten (or their ores) and gold, or conflict minerals, which are mined from the Democratic Republic of the Congo and adjoining countries. We are now required to report on the source of any conflict minerals used in our products, as well as the process we use to determine the source of such materials. We have and will continue to incur expenses as we work with our suppliers to remain in compliance with these requirements and compliance with these requirements could adversely affect the sourcing, supply, and pricing of our raw materials. We may face reputational challenges if we determine that certain of our products contain minerals not determined to be conflict free or if we are unable to sufficiently verify the origins for all conflict minerals used in our products. In addition, we may encounter challenges satisfying customers who require that all of the components of our products be certified as conflict free. If we are unable to comply with these requirements, it could have a material adverse effect on our results of operations, our financial condition or cash flows.
Impairment of our goodwill, which represents a significant portion of our total assets, would adversely affect our operating results and we may never realize the full value of our goodwill.
As of June 30, 2016, we had $9.4 million of goodwill on our balance sheet, which represented 10.7% of our total assets. We recorded this goodwill primarily from our historical acquisition activities. Goodwill is subject to, at a minimum, an annual impairment assessment of its carrying value. Any material impairment of our goodwill would likely have a material adverse impact on our results of operations.
Global economic conditions and risks could adversely affect our business and operations.
In recent years, the commercial and financial markets have been faced with very challenging global economic conditions, particularly in the United States and Europe. Certain of our customers and potential customers are international pharmaceutical and biotechnology companies based in the United States or in Europe. Deterioration in the global economic environment, particularly in those regions, may result in decreased demand for our products and services, downward pressure on the prices for our products, longer
32
sales cycles, and slower adoption of new technologies. A weakening of macroeconomic conditions may also adversely affect our suppliers based in the United States or Europe, which could result in interruptions in supply in the future. Similarly, this international exposure of our business may subject us to less intellectual property protection in some countries outside the U.S., non-U.S. regulatory requirements, trade protection measures and import or export licensing requirements. There can be no assurance that a deterioration of economic conditions in international markets will not adversely affect our future results. Moreover, changes in foreign currency exchange rates can affect the value of our assets and liabilities, and the amount of our revenue and expenses. We do not currently try to mitigate our exposure to currency exchange rate risks by using hedging transactions. We cannot predict future changes in foreign currency exchange rates, and as a result, we may suffer losses as a result of future fluctuations.
Changes in reimbursement practices of third party payers could affect the demand for some of our products and the prices at which they are sold and adversely affect our financial condition and results of operations.
Sales of our products, if any, may depend, in part, on the extent to which healthcare providers and facilities are reimbursed by government authorities, private insurers and other third party payers for the costs of our products. The coverage policies and reimbursement levels of third party payers, which can vary among public and private sources and by country, may affect which products customers purchase and the prices they are willing to pay for those products in a particular jurisdiction. Reimbursement rates can also affect the acceptance rate of new technologies and products. Legislative or administrative reforms to reimbursement systems in the United States or abroad, or changes in reimbursement rates by private payers, could adversely affect customer demand or the price customers are willing to pay for our products, which could in turn adversely impact our financial condition and results of operations.
Failure to successfully implement, manage and/or integrate critical information systems, disruption of these systems or material breaches of the security of systems involved in our operations may adversely affect our business and customer relationships.
We rely on our information technology systems, or ITS, to process, transmit, and store electronic information in our day-to-day operations. Some of the information stored is commercially sensitive, such as information regarding our customers and our intellectual property. We actively update our ITS to ensure protection and to prevent redundancy. However, electronic attacks, system crashes, destruction from unexpected tragedies and unauthorized access is a common risk to all ITS in today’s business world. There can be no assurances that our protective measures will prevent the events mentioned or other similar instances which could have a significant impact on our business and customer relationships.
We depend on our executive officers and key personnel and the loss of them could adversely affect our business.
Our success depends upon the efforts and abilities of our executive officers and other key personnel, particularly Mr. John Ryan, our President and Chief Executive Officer and Ian Hanson, our Chief Operating Officer and Senior Vice President, to provide strategic direction, manage our operations and maintain a cohesive and stable environment. Although we have employment agreements with Messrs. Ryan and Hanson, as well as incentive compensation plans that provide various economic incentives for them to remain with us, these agreements and incentives may not be sufficient to retain them. Our ability to operate successfully and manage our potential future growth also depends significantly upon our ability to attract, retain and motivate highly skilled and qualified research, technical, clinical, regulatory, sales, marketing, managerial and financial personnel. We face intense competition for such personnel, and we may not be able to attract, retain and motivate these individuals. The loss of our executive officers or other key personnel for any reason or our inability to hire, retain and motivate additional qualified personnel in the future could prevent us from sustaining or growing our business.
Failure to effectively execute on our cost reduction measures could result in total costs that are greater than expected or revenues that are less than anticipated.
The Company has been implementing cost reduction measures as it focuses operations on the programs of key strategic customers. During fiscal year 2016, the Company implemented several cost reduction and business realignment initiatives pursuant to which the Company reduced its headcount by approximately 90 employees. Such reduction is expected to reduce annual operating costs by approximately $7.9 million. Subsequent to that, the Company eliminated 10 additional positions in July 2016. The Company’s workforce was reduced to approximately 140 employees as of July 28, 2016, a reduction of more than 40% since January 2016 and a reduction of approximately 50% since July 1, 2015. A portion of this reduction is due to the Company’s determination not to backfill certain open positions. The Company has recorded a charge from severance and related costs from these cost reduction initiatives of approximately $0.7 million in the aggregate. The Company does not believe that these cost reduction initiatives will negatively impact its ability to serve its customers. We may have further cost reduction initiatives in the future. Risks associated with such measures include adverse effects on employee morale and the failure to meet operational targets due to the loss of employees, any of which may impair our ability to achieve anticipated cost reductions or may otherwise harm our business.
33
Risk Factors Related to Our Shares of Common Stock
The trading price of our shares of common stock may fluctuate significantly.
The price of our shares of common stock may be volatile, which means that it could decline substantially within a short period of time. The trading price of the shares may fluctuate, and investors may experience a decrease in the value of the shares that they hold, sometimes regardless of our operating performance or prospects. The trading price of our common stock could fluctuate significantly for many reasons, including the following:
|
|
• |
future announcements concerning our business and that of our competitors including in particular, the progress of the commercialization of our products and sale of our injectable drug delivery systems; |
|
|
• |
unsuccessful capital raising activities; |
|
|
• |
employee departures; |
|
|
• |
regulatory developments; |
|
|
• |
the impact (including costs) and results of any litigation or regulatory inquiries or investigations related to the findings of the Investigation; |
|
|
• |
quarterly variations in operating results; |
|
|
• |
negative reporting about us in the press; |
|
|
• |
introduction of new products or changes in product pricing policies by us or our competitors; |
|
|
• |
acquisition or loss of significant customers, distributors or suppliers; |
|
|
• |
business acquisitions or divestitures; |
|
|
• |
fluctuations of investor interest in the injectable drug delivery systems industry; and |
|
|
• |
fluctuations in the economy, world political events or general market conditions. |
If there are substantial sales of our shares of common stock, our share price could decline.
As of October 17, 2016, we had 17,342,043 shares of common stock outstanding. All of those shares of common stock other than 386,425 shares held by our affiliates are freely tradable under the Securities Act. Shares held by our affiliates are eligible for resale pursuant to Rule 144. If our stockholders sell a large number of shares of common stock, or the short interest position increases significantly, the market may perceive that our stockholders might sell a large number of shares, which could cause the price of our common stock to decline significantly.
In addition, as of October 17, 2016, 1,887,847 shares of our common stock are subject to outstanding stock options and warrants. We have registered the shares issuable upon the exercise of options granted under our Amended and Restated 2009 Stock Incentive Plan. If these options and warrants are exercised and the holders sell their shares, such sales could have an adverse effect on the market price of our common stock.
We do not intend to pay cash dividends in the foreseeable future.
For the foreseeable future, we do not intend to declare or pay any dividends on our common stock. We intend to retain our earnings, if any, to finance the development of our business and products. Any future decision to declare or pay dividends will be made by our Board and will depend upon a number of factors including our financial condition and results of operations. In addition, under our current bank financing agreements, we are not permitted to pay cash dividends without the prior written consent of the lender.
We may be subject to arbitrage risks.
Investors may seek to profit by exploiting the difference, if any, in the price of our shares of common stock on the NASDAQ and our CDIs on the ASX. Such arbitrage activities could cause our stock price in the market with the higher value to decrease to the price set by the market with the lower value.
34
Our certificate of incorporation, bylaws, and the Delaware General Corporation Law may delay or deter a change of control transaction.
Certain provisions of our certificate of incorporation and bylaws may have the effect of deterring takeovers, such as those provisions authorizing our Board to issue, from time to time, any series of preferred stock and fix the designation, powers, preferences and rights of the shares of such series of preferred stock; prohibiting stockholders from acting by written consent in lieu of a meeting; requiring advance notice of stockholder intention to put forth director nominees or bring up other business at a stockholders’ meeting; prohibiting stockholders from calling a special meeting of stockholders; requiring a 66 2/3% majority stockholder approval in order for stockholders to amend our bylaws or adopt new bylaws; and providing that, subject to the rights of preferred shares, the number of directors is to be fixed exclusively by our Board. Section 203 of the Delaware General Corporation Law, from which we did not elect to opt out, provides that if a holder acquires 15% or more of our stock without prior approval of our Board, that holder will be subject to certain restrictions on its ability to acquire us within three years. These provisions may delay or deter a change of control of us, and could limit the price that investors might be willing to pay in the future for shares of our common stock.
None.
Our 165,000 square foot global headquarters and manufacturing facility is located on 38 acres of land in York, Pennsylvania. The facility includes 110,000 square feet of production space and 54,000 square feet of office space. The property is subject to a mortgage held by Metro Bank.
We also lease 52,000 square feet of office space in King of Prussia, Pennsylvania (“King of Prussia Facility”) to support our research and development activities. The initial term of the King of Prussia Facility lease expires on June 30, 2022. Our annual occupancy expense under this lease is $1.2 million. As previously disclosed in a Current Report on Form 8-K dated July 28, 2016, on June 20, 2016, we subleased a portion (the “Subleased Portion”) of the King of Prussia Facility. During the term of the sublease, which commenced on October 1, 2016 and will end on March 31, 2019, the Company will receive an aggregate of approximately $1.3 million in rent with respect to the Subleased Portion. During the same time period, the Company will be obligated under the Company’s lease agreement relating to the King of Prussia Facility to pay an aggregate of approximately $1.9 million in rent with respect to the Subleased Portion. Assuming the sublessor exercises its renewal option, the Company will receive approximately an additional $1.9 million over the renewal term of April 1, 2019 through June 30, 2022 and the Company will be obligated under the Company’s lease agreement relating to the King of Prussia Facility to pay an aggregate of approximately $2.5 million over the same time period. The Company ceased using the Subleased Portion as of July 20, 2016.
Talbot Smith and SEC Investigation
A former employee, Talbot Smith, who was terminated for cause by Unilife, filed a civil complaint in the United States District Court of the Eastern District of Pennsylvania on August 30, 2013, and an amended complaint on March 5, 2014, alleging that he was wrongly terminated in retaliation for making allegations about the Company’s compliance practices. Following the discovery process, Mr. Smith dismissed his claims against the Company with prejudice. In connection with the resolution and dismissal of the action, Mr. Smith agreed to make a payment to the Company to settle counter claims the Company had brought against him. Mr. Smith received no payment as part of the resolution and dismissal of his claims against the Company, his attorney received a reduced portion of her fees from the Company’s insurer, and the matter is now concluded.
As previously disclosed, subsequent to the filing of an OSHA complaint by Mr. Smith, we received a subpoena from the staff of the U.S. Securities and Exchange Commission (the “Staff”) requesting the Company to provide certain information to the Staff, which is generally consistent with the meritless allegations made by Mr. Smith in his OSHA complaint. In his complaint filed in the United States District Court for the Eastern District of Pennsylvania, Mr. Smith stated that he provided the Staff with information about his allegations in July and August 2012. The Company responded to that subpoena and has received additional subpoenas and requests for information from the Staff, requesting additional information consistent with the first subpoena. The Staff has also requested information about public statements made by the Company’s former Chief Executive Officer. The Company has provided the requested information.
The Company has also reported to the SEC on the Company’s findings from the Investigation, has responded to questions from the Staff regarding the Investigation and is cooperating fully with the Staff. At this time, the SEC has not indicated whether any fines or penalties will be assessed against the Company in relation to these matters.
35
On May 8, 2016, the Company announced an investigation into violations of the Company’s policies and procedures and possible violations of laws and regulations by the Company’s former Chief Executive Officer, Alan Shortall, whose employment with the Company ceased on March 11, 2016, and its former Chairman, Jim Bosnjak, who resigned from the Company’s Board of Directors (the “Board”) on August 24, 2015 (the “Investigation”). The Investigation was completed on October 7, 2016, and the Company has reported to the SEC on the Company’s findings from the Investigation, has responded to questions from the Staff regarding the findings, and is cooperating fully with the Staff. To date, the SEC has not indicated whether any fines or penalties will be assessed against the Company in relation to these matters. The Company is unable to predict what action the SEC or other regulatory authority may take, if any, in relation to these matters or the impact, if any, of any such action on the Company’s business, operations, cash flows and/or financial condition. If any fines or penalties are assessed against the Company they may be material.
Cambridge Retirement System
As previously disclosed, on January 8, 2014, the Company was served with a derivative complaint filed in the Delaware Chancery Court (the “Court”) by Cambridge Retirement System (“Cambridge”), a purported stockholder of the Company, against its then-current Board of Directors to recover allegedly “excessive and wasteful” compensation paid to the non-executive directors since 2010. In June, 2014, pursuant to the Company’s motion to dismiss the complaint, the Court dismissed Cambridge’s complaint with respect to the directors’ equity grants, but denied the motion with respect to their cash compensation. The Company filed an answer to the remaining claims in July 2014 and, in June 2015, the Company and Cambridge entered into a Memorandum of Understanding (“MOU”) agreeing to the basic terms of a non-monetary settlement of the action.
On March 18, 2016, Cambridge agreed to voluntarily dismiss its derivative complaint. No compensation in any form was provided to either Cambridge or its counsel in exchange for its agreement to voluntarily dismiss the lawsuit. Because Cambridge agreed to voluntarily dismiss the lawsuit, the MOU has become null and void and of no further legal effect. On March 18, 2016, the Court entered a stipulated order regarding notice of the proposed dismissal of all claims in the derivative action (the “Proposed Dismissal Order”). On April 18, 2016, the Court entered that stipulation as an order, dismissing the case with prejudice.
Biodel Inc.
On September 14, 2015, Unilife Medical Solutions Inc., a subsidiary of the Company (“UMSI”),was served with a complaint filed in the Superior Court of the State of Connecticut by Biodel, Inc. (“Biodel”) seeking (1) to temporarily enjoin UMSI from entering into a transaction that would jeopardize the Company’s ability to perform its obligations under the Customization and Commercial Supply Agreement effective April 8, 2013 (as amended, the “First Biodel Agreement”) between Biodel and UMSI; and (2) damages under the Connecticut Unfair Trade Practices Act. Biodel alleged that UMSI had engaged in unfair and deceptive trade practices by purportedly misrepresenting its ability and willingness to satisfy its obligations under the First Biodel Agreement and requesting additional payments from Biodel to satisfy the Company’s obligations. Additionally, Biodel filed a demand for arbitration with the American Arbitration Association (AAA) asserting that UMSI had breached its obligations relating to the timing and scope of its performance under the First Biodel Agreement. The Company filed counterclaims in the arbitration for commercial disparagement and breach of the confidentiality provisions of the agreement.
On September 2, 2016, Biodel, the Company and UMSI entered into an Asset Purchase and License Agreement (the “Second Biodel Agreement”) which provides: (a) for the termination of the First Biodel Agreement; (b) for the grant of an exclusive license for a six-month term to the intellectual property rights related to the Unilife mixing device; (c) a six-month term during which Biodel can exercise an option (the “Option”) to purchase certain assets associated with the First Biodel Agreement for $1.5 million (the “Potential Asset Sale”) and extend Biodel’s license, for fees based on intellectual prosecution and maintenance costs determined on an annual basis; (c) dismissal, with prejudice, of all active proceedings in connection with the litigation and arbitration proceedings pending between Biodel and UMSI. Under the Second Biodel Agreement, each party also releases the other party of all liability, waives all claims with prejudice, and forever holds the other party harmless from any damages arising out of relating to the First Biodel Agreement. Biodel and UMSI each paid their respective attorneys’ fees and UMSI paid no monetary amount to Biodel in connection with this resolution.
Edward Fine
On March 24, 2016, Edward Fine filed a complaint against the Company and Unilife Medical Solutions Limited (“UMSL”) in the Superior Court of New Jersey. The complaint alleges that the Company and UMSL are in breach of contract and have been unjustly enriched as a result of UMSL’s failure to pay certain required payments under a consultancy agreement between Mr. Fine and UMSL. Pursuant to the complaint, Mr. Fine is seeking monetary damages in the amount of $0.3 million in the aggregate. The Company believes that Mr. Fine’s claims and demands for relief are wholly without merit and the Company is vigorously defending the action. On August 15, 2016, we filed an Answer, Affirmative Defenses and Counterclaims, wherein we asserted counterclaims against Mr. Fine for fraud, civil conspiracy, unjust enrichment, breach of contract, and breach of the implied covenant of good faith and fair dealing arising out of Mr. Fine’s role in certain previously disclosed transactions involving Mr. Fine and Jim Bosnjak, the
36
Company’s former Chairman of the Board. For additional information regarding such transactions, see “Explanatory Note - Bosnjak Loan Payments and Unrepaid Personal Expenses” above. This action is currently in a 450-day discovery period which commenced on July 29, 2016.
Class Action Litigation
On May 26 and 27, 2016, two putative class actions were filed in the United District Court for the Southern District of New York alleging that in violation of Rule 10b-5 and Section 20(a) of the Securities Exchange Act of 1934, the Company and four individual defendants made false and/or misleading statements and/or failed to disclose: (1) that the Company’s former CEO and former Chairman of the Board of Directors had violated the Company’s policies and procedures and had engaged in violations of law and regulations; (2) that the Company lacked adequate internal controls over accounting and financial reporting; (3) that, as a result, the Company would be unable to file its Quarterly Report on Form 10-Q for the period ended March 31, 2016 by the prescribed filing deadline; and (4) that, as a result of the foregoing, the Company’s financial statements, as well as its statements about the Company’s business, operations, and prospects, were false and misleading and/or lacked a reasonable basis. The putative class actions were brought on behalf of purchasers of the Company’s securities between February 3, 2014 and May 23, 2016. On August 24, 2016, the Court consolidated the two actions, appointed lead plaintiffs and lead counsel, and set a deadline of October 24, 2016 for Plaintiffs to file an amended complaint. The Company intends to vigorously contest this lawsuit.
Derivative Litigation
On July 11, July 28, and August 1, 2016, respectively, derivative complaints were filed in the Court of Common Pleas in York County, Pennsylvania against 11 current or former directors and/or officers, alleging (i) breach of their fiduciary duties, (ii) unjust enrichment, (iii) abuse of control, (iv) gross mismanagement, and (v) corporate waste. The complaints allege, among other things, that the individual defendants breached the fiduciary duties they owed to the Company by (1) grossly mismanaging the Company and perpetuating a variety of self-serving schemes to benefit themselves and other interested parties and (2) making and/or causing the Company to make false/misleading statements or omissions of fact in its public disclosures. The complaints further allege that as a result of this alleged conduct, the Company will lose and expend millions of dollars. The Company intends to vigorously contest these lawsuits.
Kahle Automation, S.r.l.
On August 17, 2016, Kahle Automation, S.r.l. (“Kahle”) filed a complaint against UMSI in the United States District Court for the District of New Jersey. The complaint alleges that UMSI breached contracts with Kahle for Kahle’s supply of automation systems for UMSI’s Nexus and Finesse product lines. Kahle seeks monetary damages of $4.2 million which includes alleged damages that we believe are not recoverable, such as $0.9 million for bank fees, and $0.8 million for lost profits. Kahle also seeks injunctive relief enjoining UMSI’s from using the Nexus System and requiring UMSI to take delivery of work in process related to the Finesse System. UMSI disputes Kahle’s allegations that UMSI terminated its agreement with Kahle for the Finesse System. We intend to defend ourselves vigorously against these claims.
The Company believes that depending on the outcome, certain of these matters may have a material impact to the Company or its business. See Part I, Item 1A Risk Factors – “Matters relating to or arising from the Investigation, including regulatory proceedings, litigation and potential additional expenses, may adversely affect our business and results of operations” of this 2016 10-K.
Not applicable.
37
|
Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Market Information
Commencing February 16, 2010, our shares of common stock began trading on the Global Market of The NASDAQ Stock Market LLC, or the NASDAQ Global Market, under the symbol “UNIS”. Our shares of common stock have also traded in the form of CDIs representing one-sixtieth of a share of our common stock, on the ASX under the symbol “UNS” since January 18, 2010. Prior to that date, the ordinary shares of our predecessor UMSL were traded on the ASX under the symbol “UNI”.
The following table sets forth, for the periods indicated, the high and low prices for our common stock on the NASDAQ Global Market:
|
Period |
|
High |
|
|
Low |
|
||
|
Fiscal Year 2016: |
|
|
|
|
|
|
|
|
|
First Quarter |
|
|
23.50 |
|
|
|
8.60 |
|
|
Second Quarter |
|
|
11.40 |
|
|
|
4.20 |
|
|
Third Quarter |
|
|
12.90 |
|
|
|
6.00 |
|
|
Fourth Quarter |
|
|
7.30 |
|
|
|
2.31 |
|
|
Fiscal Year 2015: |
|
|
|
|
|
|
|
|
|
First Quarter |
|
|
33.10 |
|
|
|
22.00 |
|
|
Second Quarter |
|
|
41.00 |
|
|
|
20.00 |
|
|
Third Quarter |
|
|
49.00 |
|
|
|
32.30 |
|
|
Fourth Quarter |
|
|
41.30 |
|
|
|
20.00 |
|
Holders
As of October 17, 2016, we had 17,342,043 shares of common stock outstanding, and there were 359 holders of record of our common stock, including CHESS Depositary Nominees which held shares of our common stock on behalf of 7,972 CDI holders. The closing sales price for our common stock on October 17, 2016 was $1.63 as reported by the NASDAQ Global Market.
Dividends
We currently intend to retain any earnings to finance research and development and the operation and expansion of our business and do not anticipate paying any cash dividends for the foreseeable future. The declaration and payment of any dividends in the future by us will be subject to the sole discretion of our board of directors and will depend upon many factors, including our financial condition, earnings, capital requirements of our operating subsidiaries, covenants associated with certain of our debt obligations, legal requirements, regulatory constraints and other factors deemed relevant by our board of directors. Moreover, if we determine to pay any dividend in the future, there can be no assurance that we will continue to pay such dividends. In addition, under our bank financing agreements, we are not permitted to pay cash dividends without the prior written consent of the lender.
Issuer Purchases of Equity Securities
We did not purchase any shares of our common stock during the year ended June 30, 2016.
38
The performance graph shown below compares the change in cumulative total stockholder return on shares of common stock with the NASDAQ Stock Market Index (US) and the Nasdaq Health Care Index (US) from fiscal year 2012 through fiscal year 2016. The graph sets the beginning value of shares of common stock and the indices at $100, and assumes that all quarterly dividends were reinvested at the time of payment. This graph does not forecast future performance of shares of common stock.
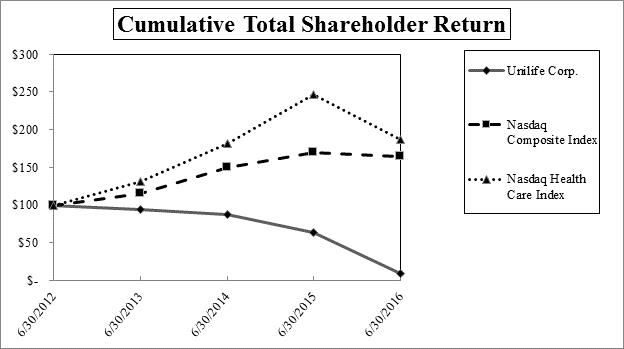
|
|
|
6/30/2012 |
|
|
6/28/2013 |
|
|
6/30/2014 |
|
|
6/30/2015 |
|
|
6/30/2016 |
|
|||||
|
Unilife Corp. |
|
|
100.00 |
|
|
|
93.79 |
|
|
|
87.57 |
|
|
|
63.61 |
|
|
|
9.73 |
|
|
Nasdaq Composite Index |
|
|
100.00 |
|
|
|
115.95 |
|
|
|
150.19 |
|
|
|
169.91 |
|
|
|
164.99 |
|
|
Nasdaq Health Care Index |
|
|
100.00 |
|
|
|
131.58 |
|
|
|
181.64 |
|
|
|
247.05 |
|
|
|
187.69 |
|
The following table presents our selected consolidated financial data as of and for each of the fiscal years in the five-year period ended June 30, 2016. The statements of operations data for the fiscal years 2016, 2015 and 2014 and the balance sheet data as of June 30, 2016 and 2015 have been derived from our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K. All such data should be read in conjunction with the information under “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our consolidated financial statements and the related notes thereto included elsewhere in this report. The statements of operations data for the fiscal years ended June 30, 2013 and 2012 and the balance sheet data as of June 30, 2014, 2013 and 2012 have been derived from our audited consolidated financial statements not included in this Annual Report on Form 10-K. Historical results are not necessarily indicative of the results to be expected in the future.
|
|
|
Fiscal Years Ended June 30, |
|
|||||||||||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|
2012 |
|
|||||
|
|
|
(In thousands, except share data) |
|
|||||||||||||||||
|
Statements of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue(a) |
|
$ |
14,841 |
|
|
$ |
13,158 |
|
|
$ |
14,689 |
|
|
$ |
2,743 |
|
|
$ |
5,519 |
|
|
Net loss |
|
|
(100,783 |
) |
|
|
(90,849 |
) |
|
|
(57,899 |
) |
|
|
(63,198 |
) |
|
|
(52,302 |
) |
|
Basic and diluted net loss per share |
|
|
(7.04 |
) |
|
|
(8.10 |
) |
|
|
(5.90 |
) |
|
|
(7.79 |
) |
|
|
(7.75 |
) |
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
|
$ |
87,661 |
|
|
$ |
95,413 |
|
|
$ |
80,665 |
|
|
$ |
68,076 |
|
|
$ |
81,956 |
|
|
Long-term debt, including current portion |
|
|
105,114 |
|
|
|
79,455 |
|
|
|
54,345 |
|
|
|
23,546 |
|
|
|
28,413 |
|
|
(a) |
Includes $2.3 million, $2.6 million, and $4.1 million in connection with our former exclusive licensing agreement and our industrialization agreement with Sanofi in the fiscal years ended June 30, 2014, 2013, and 2012, respectively. |
39
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes appearing elsewhere in this Annual Report on Form 10-K. This discussion and analysis includes certain forward-looking statements that involve risks, uncertainties and assumptions. You should review the “Risk Factors” section of this Annual Report on Form 10-K for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by such forward-looking statements. See “Cautionary Note Regarding Forward-Looking Information” at the beginning of this report. References to our fiscal year refer to the fiscal year ending June 30.
Overview
We are a designer, manufacturer, and supplier of innovative injectable drug delivery systems that can enhance and differentiate the injectable products of our pharmaceutical and biotechnology customers. We believe our products are differentiated from conventional products, with innovative features and functionality designed to optimize the safe, simple, and convenient administration of injectable therapies. The majority of our products are designed for sale directly to pharmaceutical and biotechnology companies who are expected to supply them as drug-device combination products, pre-filled and ready for administration by end-users, such as patients or health-care providers. We customize products within each of our platforms to address specific customer, therapy, patient and/or commercial requirements.
Although we have a broad portfolio of proprietary product platforms, we have focused our business on our wearable injector products. We expect that by focusing primarily on active and new customer programs in our portfolio of wearable injector systems, we will improve our operating efficiencies and better position the Company to take advantage of commercial opportunities within the fast-growing market for wearable injectors. Our wearable injector customers include Amgen Inc., MedImmune LLC (“MedImmune”), and Sanofi S.A. (“Sanofi”).
Common Stock Reverse Stock Split
On May 13, 2016, pursuant to prior stockholder authorization, the Company effected a reverse split of the Company’s common stock, pursuant to which every ten (10) shares of common stock outstanding before the reverse split were converted into one (1) share of common stock after the reverse split. All share and per share amounts, and exercise and conversion prices for all periods presented herein have been adjusted to reflect the reverse split as if it had occurred at the beginning of the first period presented.
Investigation and Amendments to Financial Statements
On May 8, 2016, the Company announced an investigation into violations of the Company’s policies and procedures and possible violations of law and regulation by the Company’s former Chief Executive Officer, Alan Shortall, whose employment with the Company ceased on March 11, 2016, and its former Chairman, Jim Bosnjak, who resigned from the Company’s Board of Directors (the “Board”) on August 24, 2015 (the “Investigation”).
The Investigation identified certain related party and other transactions which the Company had not previously publicly disclosed or recorded in its financial statements. Also, as the result of Investigation, the Company was not able to timely file its Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2016 (the “March 2016 10-Q”) or this Annual Report on Form 10-K (the “2016 10-K”). As a result, the Company is filing this 2016 10-K and is concurrently filing the March 2016 10-Q along with (i) an amendment to the Company’s Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2015 (the “September 2015 10-Q Amendment”); (ii) an amendment to the Company’s Quarterly Report on Form 10-Q for the fiscal quarter ended December 31, 2015 (the “December 2015 10-Q Amendment”); and (iii) an amendment to the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2015 (the “2015 10-K Amendment”). These amendments are being made to correct immaterial errors in the previously reported financial statements and to disclose certain material weaknesses in the Company’s internal control over financial reporting and disclosure controls and procedures. See “Explanatory Note” above for a summary of the results of the Investigation and of the specific amendments reflected in the September 2015 10-Q Amendment, the December 2015 10-Q Amendment and the 2015 10-K Amendment.
Management and Board Changes
The Company no longer has any business relationship with Mr. Shortall or Mr. Bosnjak. As of March 11, 2016, (i) Mr. Shortall’s employment as Chief Executive Officer of the Company ceased and Mr. Shortall resigned from his position as Chairman of the Board, (ii) the Board appointed Mary Kate Wold to serve as its new Chair, and (iii) the employment of Ramin Mojdeh, Ph.D. as the Company’s President and Chief Operating Officer ceased.
40
Effective March 14, 2016, the Board appointed John Ryan as the Company’s Interim President and Chief Executive Officer of the Company. The Board subsequently appointed Mr. Ryan as the Company’s President and Chief Executive Officer and also appointed Mr. Ryan to serve as a member of the Board, in each case, effective July 28, 2016.
On July 25, 2016, the Company’s employment of Mark Iampietro as the Company’s Vice President of Quality and Regulatory Affairs and Chief Compliance Officer was ended by the Company without cause.
On July 28, 2016:
|
|
• |
the Board appointed Michael E. Kamarck to serve as a member of the Board; |
|
|
• |
the Board appointed Ian Hanson as the Company’s Chief Operating Officer in addition to his roles as the Company’s Senior Vice President; |
|
|
• |
due to results of the Investigation, Dennis P. Pyers was removed from his position as the Company’s Senior Vice President, Controller, Treasurer and Chief Accounting Officer and was appointed as the Company’s Senior Advisor, Special Projects; |
|
|
• |
the Board appointed David Hastings as the Company’s Chief Accounting Officer and Treasurer in addition to his roles as the Company’s Senior Vice President and Chief Financial Officer; |
|
|
• |
the Board appointed Stephanie Walters as Senior Vice President, General Counsel and Secretary; and |
|
|
• |
the Board appointed Molly Weaver, Ph.D., as Vice President of Quality and Regulatory Affairs and Chief Compliance Officer. |
Investigation and Litigation Related to this Matter
The Company has reported the final results of the Investigation to the SEC and to The NASDAQ Stock Market LLC (“NASDAQ”), and the Company continues to cooperate fully with the SEC with respect to the SEC’s ongoing investigation. The SEC or other external parties could request further documents and information from the Company. The Company and certain of its current and former directors and officers have also been named as defendants in a number of lawsuits filed in connection with the matters set forth in this Explanatory Note. For information concerning the SEC’s ongoing investigation and such lawsuits, see Part I, Item 3. “Legal Proceedings” of this 2016 10-K.
Matters Relating to NASDAQ and Our Common Stock and ASX and our CDIs
The filing of this 2016 10-K and the March 2016 10-Q were delayed as a result of the Investigation. As a result of such delay, on May 17, 2016, the Company received a notice from the Listing Qualifications department of NASDAQ stating that, because the Company had not yet filed the March 2016 10-Q, the Company was no longer in compliance with NASDAQ Listing Rule 5250(c)(1), which requires listed companies to timely file all required periodic financial reports with the SEC. The notice received from NASDAQ stated that the Company had 60 calendar days from the date of the notice to submit a plan to regain compliance with NASDAQ’s continued listing requirements.
On July 18, 2016, the Company timely submitted a plan to NASDAQ as to how it planned to regain compliance with NASDAQ’s continued listing requirements. The staff at NASDAQ subsequently granted the Company an exception to file the March 2016 10-Q and any other delinquent SEC filings on or before November 7, 2016 in order to enable the Company to regain compliance with the listing rules.
On September 19, 2016, the Company received a notice from the Listing Qualifications department of NASDAQ stating that, because the Company had not yet filed this 2016 10-K, the Company is not in compliance with NASDAQ Listing Rule 5250(c)(1). The Company timely submitted to NASDAQ an updated compliance plan on October 4, 2016.
As noted above, the Company is concurrently filing with the SEC the March 2016 10-Q and the 2016 10-K with the 2015 10-K Amendment, the September 2015 10-Q Amendment and the December 2015 10-Q Amendment. Consequently, the Company currently believes that it has adequately remedied its non-compliance with NASDAQ Listing Rule 5250(c)(1) within NASDAQ’s terms of exception. However, there can be no assurance that NASDAQ will concur that we have remedied such non-compliance.
On October 17, 2016, the Company received a notice from the Listing Qualifications department of NASDAQ stating that, because the Company did not maintain a minimum Market Value of Listed Securities (“MVLS”) of $50,000,000 for the last 30 consecutive business days, the Company was no longer in compliance with NASDAQ Listing Rule 5450(b)(2)(A).
41
The Company has been provided a period of 180 calendar days, or until April 17, 2017, to regain compliance with the minimum MVLS listing requirement. If at any time on or before April 17, 2017, the MVLS of the Company’s common stock closes at $50,000,000 or more for a minimum of 10 consecutive business days, NASDAQ will provide the Company with written confirmation that the Company has achieved compliance with the minimum MVLS listing requirement and the matter will be closed.
In the event that the Company does not regain compliance with the minimum MVLS listing requirement on or before April 17, 2017, NASDAQ will provide the Company with written notification that its securities are subject to delisting. At that time, the Company would be permitted to appeal the delisting determination to a NASDAQ Hearings Panel or apply to transfer its common stock to The NASDAQ Capital Market (provided that it satisfied the requirement for continued listing on that market). (See Part I, Item 1A Risk Factors of this 2016 10-K – “We are not in compliance with the requirements of NASDAQ for continued listing, and if NASDAQ does not concur that we have adequately remedied our non-compliance with NASDAQ Listing Rule 5250(c)(1) or we do not adequately remedy our non-compliance with NASDAQ Listing Rule 5450(b)(2)(A), our common stock may be delisted from trading on NASDAQ, which could have a material adverse effect on us and our stockholders” below).
The Company was also required to file audited financial statements with the Australian Securities Exchange (the “ASX”) no later than September 30, 2016 (the “ASX Deadline”). The Company was not able to file such audited financial statements by the ASX Deadline. As a result, pursuant to ASX rules, trading in the Company’s CDIs on the ASX was to be suspended prior to the opening of trading on the ASX on October 3, 2016, however, the ASX accepted the Company’s request for an immediate voluntary suspension of trading and as such, ASX halted trading of the Company’s CDIs on the ASX prior to the opening of trading on September 30, 2016 in Australia. Such trading in Australia will not resume until after the Company files the audited financial statements included in this 2016 10-K with the ASX, which the Company is doing concurrently with filing this 2016 10-K with the SEC.
Key Factors Affecting Performance and Financial Condition
We are party to several agreements with our customers, including customers with whom we have entered into customization, development and/or supply agreements. The customization, industrialization and development fees and other payments received from customers in connection with these agreements and development programs accounted for the majority of our revenue during the fiscal year ended June 30, 2016.
Longer customer development timelines and increases in capital expenses and headcount have impacted us from a liquidity standpoint. Historically, we have funded our operations primarily from a combination of term loans, equity issuances, borrowings under our bank mortgages, and payments from various customers. See “Liquidity and Capital Resources Discussion” below.
Revenue
Our revenue is currently generated from customization, industrialization and development fees (many of which are recognized on the milestone basis of accounting). Customization, industrialization and development fees accounted for substantially all of our consolidated revenue for the fiscal year ended June 30, 2016. We expect that the Company’s revenue will continue to fluctuate on a quarter to quarter basis. A significant portion of our business is generated by a small number of major customers. Our top three customers generated approximately 91% of our total revenue in fiscal 2016. As our industry, market demand and our customer base changes, our major customers may also change. However, we expect that a significant portion of our future annual revenue will be concentrated among a few customers.
Operating Expenses
Our operating expenses had been increasing primarily as a result of increased research and development efforts in response to increasing demand from our customers for our products and services and anticipated market demand. The increase in research and development costs also related to the costs of products and components supplied to existing and prospective customers to support evaluation processes and user studies that are typically undertaken prior to the anticipated signing of customer agreements. However, during fiscal year 2016 we implemented several cost reduction and business realignment initiatives that were previously disclosed, pursuant to which we reduced our headcount by approximately 90 employees. Such headcount reductions are expected to reduce annual operating costs by approximately $7.9 million. We expect to further reduce our annual operating costs by not backfilling certain open positions. We do not believe that these cost reduction initiatives will negatively impact our ability to serve our customers.
Also as a result of these initiatives, our research and development expense decreased from approximately $17.0 million in the fourth quarter of fiscal year 2015 to approximately $7.4 million in the fourth quarter of fiscal year 2016, or approximately 56%. Selling, general and administrative expense decreased from approximately $9.4 million in the fourth quarter of fiscal year 2015, to approximately $7.6 million in the fourth quarter of fiscal year 2016, or approximately 20%.
42
Significant Developments in the Industry
We believe that our existing wearable injector contracts could provide significant revenue growth in relation to prior periods. Known trends in the industry that we believe will have a material favorable impact on our revenue include a shift in the focus of large pharmaceutical and biotechnology companies’ product development activities to biologic therapies, an emphasis within health-care providers to patient self-administration and a growing demand for passive safety for injectable drug delivery. There has been a marked shift in the product development activities of large customers toward biologic therapies, and the majority of therapies in the pipeline of large pharmaceutical and biotechnology companies are complex biologic therapies. The characteristics of many of these therapies (including, for example, large dose volumes and increased viscosity) necessitates administration by injection using innovative injectable drug delivery systems such as our products. We believe that we are well-positioned to meet what we expect to be a growing demand for innovative injectable drug delivery systems in light of the focus on biologic therapies. Concurrently with the shift toward biologic therapies is an emphasis towards patient self-administration. Patient self-administration is viewed as a growing trend in order to reduce demand pressure on the health-care system as well as reducing costs, especially for treatment of chronic illnesses. Devices suitable for self-administration of injectable therapies need to be safe and intuitive to use. We believe that our products are well suited for safe and intuitive patient self-administration of injectable therapies.
Critical Accounting Policies and Estimates
We prepare our audited consolidated financial statements in accordance with accounting principles generally accepted in the U.S., or U.S. GAAP. This requires management to make certain estimates, judgments and assumptions that could affect the amounts reported in the audited consolidated financial statements and accompanying notes. We believe there are several accounting policies that are critical to understanding our historical and future performance, as these policies affect the reported amounts of revenue and other significant areas that involve management’s judgments, estimates and assumptions. These critical accounting policies and estimates have been discussed with our audit committee.
The preparation of our audited consolidated financial statements requires us to make judgments, estimates and assumptions that affect the amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, we evaluate these judgments, estimates and assumptions. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable at such time, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other independent sources. Actual results may ultimately differ from these estimates.
While there are a number of accounting policies, methods and estimates affecting our consolidated financial statements as addressed in Note 4 to our audited consolidated financial statements, areas that are particularly significant and critical include:
Revenue Recognition
We recognize revenue from industrialization and development fees, licensing fees and product sales. We recognize revenue from sales of products at the time of shipment when title passes to the customer. We recognize up front, non-refundable fees ratably over the expected life of the related agreement. Revenue from industrialization and development fees is recognized as services are rendered, under the completed contract method, under the proportional performance method or upon achievement of the “at risk” substantive milestone events, which represent the culmination of the earnings process related to such events. Substantive milestones can include specific deliverables such as product design, prototype availability, user tests, manufacturing proof of principle and the various steps to complete the industrialization of the product. The terms of these contracts provide for customer payments to be made as services are rendered or substantive milestones are achieved. We consider whether a milestone is substantive at the inception of the agreement. The consideration earned from the achievement of a milestone must meet all of the following criteria to be considered substantive:
|
|
• |
It is commensurate with either our performance to achieve the milestone, or the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from our performance to achieve the milestone; |
|
|
• |
It relates solely to past performance; and |
|
|
• |
It is reasonable relative to all of the deliverables and payment terms (including other potential milestone consideration) within the arrangement. |
Payment terms are considered to be standard commercial terms. Revenue is recognized when each substantive milestone has been achieved and we have no future performance obligations related to the substantive milestone. Fees for completed substantive milestones which are dependent upon customer acceptance for non-refundable payment or, if paid, are refundable pending customer acceptance are recognized upon customer acceptance and the termination of refund rights.
43
We recognized $14.8 million, $13.2 million and $14.7 million of revenue during fiscal years 2016, 2015 and 2014, respectively, as follows:
During the fiscal year 2016, we recognized $4.5 million in revenue related to substantive milestones that were completed during the year pursuant to customer agreements to provide customization and development services, clinical support services, collaborative research activities and testing support services. Milestones completed during the year included various customization activities, device design, devices developed for use in customer evaluation testing, compatibility testing, user studies, and verification activities.
During fiscal year 2016, we recognized $5.3 million in revenue related to services rendered on a time and materials basis, proportional performance method and/or straight-line basis over the requisite service period pursuant to customer agreements to provide various customization and development services. During the fiscal year ended June 30, 2016, we recognized $5.0 million in revenue related to an agreement with a customer to provide exclusivity for a defined period of time to evaluate certain drug delivery alternatives as the requisite exclusivity period expired during the fiscal year.
Goodwill
Goodwill is the excess of purchase price over the fair value of net assets acquired in business acquisitions. Goodwill is subject to, at a minimum, an annual impairment assessment of its carrying value at the reporting unit level. Additional impairment assessments would be performed if events and circumstances warranted such additional assessments during the year. The Company has one reporting unit which includes its product lines, the base technology which it obtained as part of its November 2002 acquisition of Unitract Syringe Pty Limited, and the manufacturing capability which it obtained in its January 2007 acquisition of Integrated BioSciences, Inc.
As required under generally accepted accounting principles, the Company is required to evaluate goodwill for impairment at least annually and more frequently if certain trigger events occur. The Company determined that the culmination of the Strategic Process and the decision to focus primarily on the wearable injector business constituted trigger events that occurred in the quarter ended March 31, 2016 requiring an evaluation of the carrying value of the Company’s goodwill. Potential impairment of goodwill is identified by comparing the fair value with its carrying value.
The Company performed a goodwill valuation as of March 31, 2016 using a combination of the market approach and a discounted cash flow method under the income approach. The Company determined an overall business enterprise value (determined by the fair of equity plus the fair value of debt) by taking a weighted average form the results of the discounted cash flow under the income approach (40%) and the market approach based on the Company’s market capitalization (60%). Under the income approach, the Company calculates the fair value of its reporting unit based on the present value of estimated future cash flows. Considerable management judgment is necessary to evaluate the impact of operating and external market changes and to estimate the future cash flows used to measure fair value. The Company’s estimate of cash flows consider past performance, current and anticipated market conditions, and internal projections and operating plans which incorporate estimates for sales growth, profitability and capital spending. Additional assumptions include forecasted growth rates and estimated discount rates which are risk adjusted for current market conditions. The Company believes such assumptions reflect current and anticipated market conditions and are consistent with those that would be used by other marketplace participants for similar purposes but are subject to change due to changing conditions. The weighting of the results of each method to assess the overall business enterprise value was considered reasonable as greater weight was given to the market capitalization based on the quoted stock price as this is considered more reliable information as it is publicly available and not subject to management judgment or estimate, whereas, discounted cash flow under the income approach is subject to management estimates and judgments.
Although based on the results of the business enterprise value calculation there was no indication of impairment of goodwill, the Company determined as a result of the continuing losses incurred by the Company and its negative equity, and other indicators of impairment including, the significant long-lived asset impairment recorded as of March 31, 2016 and the drop in the Company’s stock price, that an additional step 2 analysis was warranted under generally accepted accounting principles.
The Company performed a Step 2 valuation of goodwill by valuing certain intangible assets (primarily patents, trademarks and tradenames). The Company considered the impact of all assets and liabilities in allocating the fair value of the Company to determine the implied fair value of goodwill and whether any impairment is indicated. The Company believes these intangible assets would generate the most significant difference between fair value and book value as part of completing a Step 2 purchase price allocation for the purpose of determining the value of goodwill for impairment. The valuation of intangible assets was performed using the relief from royalty method under the income approach. This method utilizes the Company’s revenue projections and assigns values based on determination of appropriate royalty rates considering relevant industry information. The Company then utilized the fair value of its intangible assets along with the implied fair value of its other assets and liabilities to determine the residual fair value compared to the
44
carrying value of goodwill. The residual fair value based on the results of the Step 2 valuation indicated there was no impairment of goodwill.
The Company considered that the results of the valuation of goodwill indicated there was no impairment of goodwill and also indicated significant cushion between the implied fair value of goodwill and its carrying value and, therefore, the Company has concluded that no goodwill impairment existed as of March 31, 2016.
The Company completed its annual goodwill valuation as of June 30, 2016. The Company performed a goodwill valuation as of June 30, 2016 using a combination of the market approach and a discounted cash flow method under the income approach to determine the overall business enterprise value and then performed a Step 2 valuation of the fair value of its assets and liabilities to determine the residual fair value of goodwill. The Step 2 valuation also valued the Company’s intangible assets using the relief from royalty method under the income approach. Based on the results of the goodwill valuation as of June 30, 2016, there was no impairment of goodwill. The Company considered that the results of the valuation of goodwill also indicated significant cushion between the implied fair value of goodwill and its carrying value and, therefore, the Company has concluded that no goodwill impairment existed as of June 30, 2016.
The Company did not record any goodwill impairments during fiscal years 2016, 2015 or 2014.
Definite-lived intangible assets include patents which are amortized on a straight-line method over their estimated useful lives of 15 years and are included in other assets. The Company reviews intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When factors indicate a possible impairment, if the sum of the estimated undiscounted future cash flows expected to result from the use and eventual disposition of an asset is less than the carrying amount of the asset, an impairment may be recognized. Measurement of an impairment loss is based on the excess of the carrying value of the asset over its fair value. Definite-lived intangible assets were less than $0.1 million as of June 30, 2016 and 2015 and are recorded in other assets on the consolidated balance sheet. There were no impairments recorded on intangible assets during the years ended June 30, 2016, 2015 or 2014.
Research and Development Expense
Research and development expense consist primarily of payroll and related personnel expenses (including share-based compensation expense), fees paid to external service providers, costs of materials, components and supplies, costs for facilities, tooling and equipment and costs related to customization and development service arrangements and developing prototype products and samples used for various evaluation, testing and related activities for existing and potential customers.
Share-Based Compensation
We grant equity awards to our employees, directors, consultants and service providers. Certain employee and director awards vest over stated vesting periods and others also require achievement of specific performance or market conditions. We expense the grant-date fair value of awards to employees and directors over their respective vesting periods. To the extent that employee and director awards vest only upon the achievement of a specific performance condition, expense is recognized over the period from the date management determines that the performance condition is probable of achievement through the date they are expected to be met. Awards granted to consultants and service providers are sometimes granted for past services, in which case their fair value is expensed on their grant date, while other awards require future service, or the achievement of performance or market conditions. Timing of expense recognition for consultant awards is similar to that of employee and director awards; however, aggregate expense is re-measured each quarter-end based on the then fair value of the award through the vesting date of the award. We estimate the fair value of stock options using the Black-Scholes option-pricing model, with the exception of market-based grants, which are valued based on the Monte Carlo option pricing model. Option pricing methods require the input of highly subjective assumptions, including the expected stock price volatility.
Property, plant and equipment
We evaluate the recoverability of the recorded value of long-lived assets periodically to determine if facts and circumstances exist that would indicate that the assets might be impaired or that the useful lives should be modified. As part of this valuation, we develop projections of undiscounted future cash flows of the asset or the asset group. Our projections of undiscounted cash flows include a combination of revenue from customization and development activities, capital expenses that may be necessary to support projected product sales and commercial product sales. As customization and development activities are completed, commercial product sales are expected to scale-up. Expectations of future commercial product sales included in the projections used for our impairment analysis are based on customer-specific information as well as market estimates relating to the anticipated therapies being targeted for use with our products. These projections also include assumptions of future sales growth and profitability based on
45
contracts entered into with customers as well as future contracts to be entered into based on the current discussions and negotiations with existing and prospective customers.
Sales projections are based on assumptions including a transition in the market toward patient self-administration of injectable therapies as well as transitions in the market toward biologic therapies in the pharmaceutical industry development pipeline. Our future sales could also be impacted by factors such as our ability to obtain new and retain existing customers, the timing and extent of the customers’ product development activities as well as the regulatory approval process, drug efficacy and industry acceptance of injectable therapies. If our future sales or projections of future sales are impacted by any one or more of the preceding factors, we will reassess the recorded value of the long-lived assets. If impairment is indicated, an adjustment will be made to reduce the carrying amount of these assets to their fair value.
Under ASC 360 Property, Plant, and Equipment, the Company is required to evaluate the recoverability of the carrying amount of its long-lived assets whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. In February 2016, the Company completed its review of strategic alternatives and announced the formation of a strategic collaboration centered upon the use of the Company’s portfolio of prefilled, customizable wearable injectors. In connection with this focus, the Company has been evaluating the prospects of its non-wearable injector customer and supplier programs. The Company’s evaluation of those programs and potential disposition of certain assets, the Company determined that certain of its long lived-assets are impaired. The Company recorded a $26.6 million non-cash asset impairment charge in the third quarter of fiscal 2016 to machinery and equipment and construction in progress. The impairment was based on the future expected use of certain equipment in the production of its product. The equipment is customized for the production of medical devices and as a result of the decision not to continue to produce certain products the related equipment was written-off as any value was determined to be nominal, if any.
Fair value measurements
In accordance with Accounting Standards Codification (“ASC”) 820, Fair Value Measurements and Disclosures, we measure fair value based on a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that observable inputs be used when available. The fair value hierarchy is broken down into three levels based on the source of inputs.
The carrying value of financial instruments such as accounts receivable, accounts payable and accrued expenses are reasonable estimates of their fair value because of the short maturity of these items. We believe that the current carrying amount of our long-term debt approximates fair value based on the adjustment of carrying value of our term loan to its fair value as of June 30, 2016. With respect to the consideration provided to ROS (as defined below) in the form of warrants and the terms of the Eighth Amendment to the Credit Agreement and the Sixth Amendment to the Royalty Agreement, the Company evaluated whether the debt was modified or extinguished pursuant to ASC 470-50, Debt – Modifications and Extinguishments. The Company determined that the previous debt was extinguished and recorded the modified debt at fair value ($51.3 million). The Company recorded a gain on debt extinguishment during fiscal 2016 of $2.9 million which consisted of the remeasurement of the debt at fair value offset by the value of the warrants as of the Counterparty Effective Date and the deferred financing costs previously associated with the term loan
We have elected to measure our royalty liability at fair value in accordance with ASC 825, Financial Instruments. The fair value of the royalty liability is based on significant inputs not observable in the market, which require it to be reported as a Level 3 liability within the fair value hierarchy. The valuation uses a methodology and assumptions that we believe would be made by a market participant. In particular, the valuation analysis uses a discounted cash flow methodology under the income approach based on the present value sum of payments to be made in the future. The fair value of the royalty liability is estimated by applying a risk adjusted discount rate to the adjusted royalty revenue stream. These fair value estimates are most sensitive to changes in the payment stream.
We have elected to measure our derivative financial instruments in accordance with ASC 815-40, Derivative and Hedging — Contracts in Entity’s Own Equity. Instruments which do not have fixed settlement provisions are deemed to be derivative instruments.
The fair value of the warrant liability is based on a Black-Scholes valuation. The fair value estimates are most sensitive to changes in our share price.
The fair value of the derivative liability is based on the average of a Monte Carlo model and a lattice model. The fair value estimates are most sensitive to changes in our share price.
Interest expense
We recognize interest expense in the consolidated statements of operations and comprehensive loss for all debt instruments using the effective interest method. The effective interest method is a method of calculating the amortized cost of a financial liability and of allocating the interest expense over the relevant period. The effective interest rate is the rate that exactly discounts the estimated
46
future cash payments through the expected life of the financial instrument to the net carrying amount of the financial liability. The application of the method has the effect of recognizing expense payable on the instrument evenly in proportion to the amount outstanding over the period to maturity or repayment. In calculating the effective interest rate, we estimate cash flows considering all contractual terms of the financial instrument, including fees for early redemption and all other premiums and discounts.
Recently Issued Accounting Pronouncements
See note 4 “Recently Issued Accounting Pronouncements” to our consolidated financial statements included in this Annual Report on Form 10-K.
Basis of Presentation
Revenue
We derive revenue primarily from industrialization and development programs with our customers and licensing agreements. The agreements with our customers generally provide for fees to be paid to us for providing specific products or services. Certain of these agreements provide for fees to be paid upon completion of certain agreed-upon milestones. In instances where these milestones are substantive, we recognize revenue when these agreed-upon substantive milestones have been completed and there is no further performance obligation related to the substantive milestone. Certain of our agreements provide for fees to be paid for specific services to be rendered or the provision of certain deliverables, and we recognize revenue upon completion of the related service or deliverable. Certain of our agreements provide for fees to be paid on an ongoing basis over the life of the agreement for agreed-upon services, and we recognize revenue ratably over the requisite service period. We also recognize revenue on certain agreements under the proportional performance method.
Operating expenses
Operating expenses primarily include costs related to research and development, selling, general and administrative expenses, as well as depreciation and amortization expense.
Research and development costs
Research and development costs consist primarily of payroll and related personnel expenses (including share-based compensation expense), fees paid to external service providers, costs of materials, components and supplies, costs for facilities, tooling and equipment and costs related to customization and development service arrangements and developing prototype products and samples used for various evaluation, testing and related activities for existing and potential customers.
Selling, general and administrative costs
Selling, general and administrative costs include corporate payroll and related benefit costs (including share-based compensation expense), marketing and commercial development costs, quality assurance and regulatory costs, accounting and financial related costs, information and technology costs, legal and professional fees, and corporate facility costs.
Depreciation
Depreciation is calculated on a straight-line basis over the estimated useful lives of the related assets, which range from 40 years for our York, Pennsylvania facility to 2 to 15 years for machinery, equipment, furniture and software and the lesser of the lease term or estimated useful life for leasehold improvements. Intangible assets are being amortized using the straight-line method over their estimated useful lives of 15 years.
Interest expense
Interest expense includes the cash and non-cash interest cost for all debt instruments. Interest expense is recognized under the effective interest method such that non-cash interest includes the additional expense recognized over and above the cash interest paid during a period as a result of the application of the effective interest method.
Change in fair value of financial instruments
Change in fair value of financial instruments includes the change in the Amended Royalty Agreement (defined below) liability, the Warrant liability, the Derivative liability, and the Preferred stock conversion liability, which are marked to fair value on a quarterly basis.
47
Net loss includes the results from revenue recognized during the period after deducting all operating and non-operating expenses.
Results of Operations
The following table summarizes our results of operations for fiscal years 2016, 2015 and 2014:
|
|
|
Fiscal Years Ended June 30, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
|
(in thousands, except per share data) |
|
|||||||||
|
Revenue |
|
$ |
14,841 |
|
|
$ |
13,158 |
|
|
$ |
14,689 |
|
|
Research and development |
|
|
43,191 |
|
|
|
52,487 |
|
|
|
34,111 |
|
|
Selling, general and administrative |
|
|
43,196 |
|
|
|
36,176 |
|
|
|
27,894 |
|
|
Asset impairment charge |
|
|
26,580 |
|
|
|
— |
|
|
|
— |
|
|
Depreciation and amortization |
|
|
5,491 |
|
|
|
4,923 |
|
|
|
4,079 |
|
|
Total operating expenses |
|
|
118,458 |
|
|
|
93,586 |
|
|
|
66,084 |
|
|
Operating loss |
|
|
(103,617 |
) |
|
|
(80,428 |
) |
|
|
(51,395 |
) |
|
Interest expense |
|
|
10,187 |
|
|
|
6,368 |
|
|
|
7,332 |
|
|
Change in fair value of financial instruments |
|
|
(9,899 |
) |
|
|
4,279 |
|
|
|
(600 |
) |
|
Gain on debt extinguishment |
|
|
(2,881 |
) |
|
|
— |
|
|
|
— |
|
|
Other (income) expense, net |
|
|
(241 |
) |
|
|
(226 |
) |
|
|
(228 |
) |
|
Net loss |
|
$ |
(100,783 |
) |
|
$ |
(90,849 |
) |
|
$ |
(57,899 |
) |
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share |
|
$ |
(7.04 |
) |
|
$ |
(8.10 |
) |
|
$ |
(5.90 |
) |
Fiscal Year 2016 Compared to Fiscal Year 2015
Revenue. Revenue increased by $1.7 million, or 12.8%, during fiscal year 2016 compared to fiscal year 2015 due to more revenue being recognized related to development activities for various customers. During fiscal year 2016, we recognized $4.5 million in revenue related to substantive milestones that were completed during the year pursuant to customer agreements to provide customization and development services, clinical support services, collaborative research activities and testing support services. During fiscal year 2016, we recognized $5.3 million in revenue related to services rendered on a time and materials basis, proportional performance method, completed contract method, and/or straight-line basis over the requisite service period pursuant to customer agreements to provide various customization and development services. During fiscal 2016, we recognized $5.0 million in revenue related to an agreement with a customer to provide exclusivity for a defined period of time to evaluate certain drug delivery alternatives as the requisite exclusivity period expired during the fiscal year. During fiscal year 2015, we recognized $5.1 million in revenue related to substantive milestones that were completed during the year pursuant to customer agreements to provide customization and development services, clinical support services, collaborative research activities and testing support services. During fiscal year 2015, we recognized $8.1 million in revenue related to services rendered on a time and materials basis pursuant to customer agreements to provide various customization and development services.
Research and development expenses. Research and development expenses decreased in fiscal year 2016 by $9.3 million, or 17.7%, compared to fiscal year 2015 primarily due to decreased tooling, prototype, and material costs of $5.8 million, decreased third-party contracting costs of $1.2 million, decreased payroll and related costs of $1.0 million, decreased travel costs of $1.0 million and decrease in other costs of $1.2 million offset by increased share-based compensation expense of $0.9 million.
Selling, general and administrative expenses. Selling, general and administrative expenses increased in fiscal year 2016 by $7.0 million, or 19.4%, compared to fiscal year 2015, primarily due to increased transaction costs of $5.0 million in connection with the Strategic Process and increased legal fees of $1.2 million offset by decreased travel costs of $0.9 million and decreased other costs of $0.2 million. Share-based compensation increased by $1.2 million and payroll and related costs increased by $0.7 million primarily in connection with the departure of our former CEO and COO in March 2016 which were partially offset by cost reductions as a result of business realignment initiatives.
Asset impairment charge. The Company incurred $26.6 million of non-cash asset impairments during the year ended June 30, 2016 related to non-wearable injector machinery and equipment.
48
Depreciation and amortization expense. Depreciation and amortization expense increased in fiscal year 2016 by $0.6 million, or 11.5%, compared to fiscal year 2015, primarily as a result of additional equipment previously placed in service.
Interest expense. Interest expense increased in fiscal year 2016 by $3.8 million, or 60.0%, compared to fiscal year 2015, primarily attributable to interest on the OrbiMed Financing ($3.2 million) and the 2016 Convertible Note ($0.7 million) offset by a decrease in capitalized interest ($0.1 million).
Change in fair value of financial instruments. Change in fair value of financial instruments decreased by $14.2 million in fiscal year 2016 compared to fiscal year 2015. A decrease of $7.9 million is related to the change in the fair value of the Royalty liability in connection with the OrbiMed Financing which is revalued each quarter. A decrease of $9.2 million is due to revaluation of the warrant liability due to a decrease in the Company’s share price. A decrease of $1.3 million is due to revaluation of the derivative liability due to a decrease in the Company’s share price. An increase of $4.2 million is related to the revaluation of the Preferred Stock Conversion liability.
Net loss and net loss per share. Net loss during fiscal years 2016 and 2015 was $100.8 million and $90.8 million, respectively. Basic and diluted net loss per share was $7.04 and $8.10 during fiscal years 2016 and 2015, respectively, on weighted average shares outstanding of 14,467,510 and 11,219,490 during fiscal years 2016 and 2015, respectively. The increase in the weighted average shares outstanding was primarily due to the New Sales Agreement and Purchase Agreement (each as defined below) as well as conversions of preferred shares under the Preferred Stock Purchase Agreement.
Fiscal Year 2015 Compared to Fiscal Year 2014
Revenue. Revenue decreased by $1.5 million, or 10.4%, during fiscal year 2015 compared to fiscal year 2014 due to less revenue being recognized related to development activities for various customers. During fiscal year 2015, we recognized $5.1 million in revenue related to substantive milestones that were completed during the year pursuant to customer agreements to provide customization and development services, clinical support services, collaborative research activities and testing support services. During fiscal year 2015, we recognized $8.1 million in revenue related to services rendered on a time and materials basis, proportional performance method and/or straight-line basis over the requisite service period pursuant to customer agreements to provide various customization and development services. During fiscal year 2014, we recognized $8.1 million in revenue related to substantive milestones that were completed during the year pursuant to customer agreements to provide customization and development services, clinical support services, collaborative research activities and testing support services. During fiscal year 2014, we recognized $4.3 million in revenue related to services rendered on a time and materials basis pursuant to customer agreements to provide various customization and development services. Also, during fiscal year 2014, we recognized $2.3 million of revenue related to our development agreement with Sanofi which was superseded by a supply agreement with Sanofi.
Research and development expenses. Research and development expenses increased in fiscal year 2015 by $18.4 million, or 53.9%, compared to fiscal year 2014 primarily due to increased payroll and related costs of $7.5 million and increased share-based compensation expense of $0.7 million related to increased headcount to support ongoing and future customer programs, as well as increased material and tooling costs of $8.5 million, and increased other costs of $1.7 million related to customer programs. The increased investment in research and development during fiscal year 2015 related to the supply of products and components to existing customers including for customization, industrialization and development programs and prospective customers to support evaluation processes and user studies that are typically undertaken prior to the anticipated signing of contracts.
Selling, general and administrative expenses. Selling, general and administrative expenses increased in fiscal year 2015 by $8.3 million, or 29.7%, compared to fiscal year 2014, primarily due to increased share-based compensation expense of $2.8 million, increased legal and professional fees of $2.3 million, increased payroll and related costs of $2.2 million and increased other administrative costs of $1.0 million.
Depreciation and amortization expense. Depreciation and amortization expense increased in fiscal year 2015 by $0.8 million, or 20.7%, compared to fiscal year 2014, primarily as a result of machinery and equipment placed in service during the fiscal year 2015.
Interest expense. Interest expense decreased in fiscal year 2015 by $1.0 million, or 13.1%, compared to fiscal year 2014, as a result of lower interest expense of $4.7 million due to debt that was refinanced with a term loan with the Lender in the prior year and interest of $2.2 million that was capitalized as part of construction in progress during the year partially offset by higher interest during the year of $5.9 million related to the term loan with the Lender.
Change in fair value of financial instruments. Change in fair value of financial instruments increased by $4.9 million in fiscal year 2015 compared to fiscal year 2014, primarily due to higher royalty rates as a result of the Amendment to the Royalty Agreement we entered into with the Lender on September 30, 2014.
49
Net loss and net loss per share. Net loss during fiscal years 2015 and 2014 was $90.8 million and $57.9 million, respectively. Basic and diluted net loss per share was $8.10 and $5.90 during fiscal years 2015 and 2014, respectively, on weighted average shares outstanding of 11,219,490 and 9,806,266 during fiscal years 2015 and 2014, respectively. The increase in the weighted average shares outstanding was primarily due to the issuance of common stock in connection with our February 2015 equity financing as well as shares issued under the Sales Agreement during fiscal year 2015.
Liquidity and Capital Resources
As of September 30, 2016, the Company’s unaudited cash balance was approximately $8.1 million, including $2.1 million of restricted cash. Under the Company’s debt facilities, the Company was required to have a cash and restricted cash balance of $5.1 million at September 30, 2016 and $5.0 million at October 31, 2016. As of June 30, 2016, cash and cash equivalents were $18.7 million, restricted cash was $2.4 million and the book value of our long-term debt was $105.1 million.
The Company incurred recurring losses from operations as well as negative cash flows from operating activities during fiscal years 2016, 2015, and 2014, and anticipates incurring additional losses and negative cash flows until such time that it can generate sufficient revenue from the sale, customization, or exclusive use and licensing of its wearable injectors to pharmaceutical and biotechnology customers. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The Company has taken or intends to take the steps delineated below to address its cash requirements, the success of which is largely beyond the Company’s control.
Amgen Inc.
On December 31, 2015, the Company entered into an exclusivity agreement (the “Exclusivity Agreement”) with Amgen Inc. (the “Counterparty”). The Exclusivity Agreement was entered into in connection with the previously announced review of strategic alternatives to maximize shareholder value (the “Strategic Process”). Pursuant to the Exclusivity Agreement, the Company agreed to negotiate a potential transaction exclusively with the Counterparty and the Counterparty paid to the Company a non-refundable $15.0 million deposit as consideration for non-exclusive and exclusive rights and licenses provided for in the Exclusivity Agreement.
On February 22, 2016 (the “Counterparty Closing Date”), the Company and the Counterparty announced the formation of a strategic collaboration for the Counterparty’s use of the Company’s injectable drug delivery systems with the Counterparty’s product portfolio. In connection with the strategic collaboration, the Company entered into a Securities Purchase Agreement (the “Counterparty SPA”) with the Counterparty, pursuant to which the Counterparty agreed to purchase from the Company a new series of 6% Senior Secured Convertible Notes Due 2023 in the aggregate original principal amount of up to $55.0 million (each, a “Note” and, collectively, the “Notes”). The Notes are convertible into Common Stock, and mature on February 22, 2023 (the “Counterparty Maturity Date”) in accordance with the terms and conditions of the Notes, as more fully described in note 10 – “Long-term Debt” to the consolidated financial statements.
Pursuant to the Counterparty SPA, the Notes may be issued in up to three separate closings. The Company issued to the Counterparty the first Note in the aggregate original principal amount of $30.0 million on the Counterparty Closing Date (the “2016 Convertible Note”) and the Counterparty paid to the Company $30.0 million in exchange therefor. The 2016 Convertible Note is secured by certain inventory and intellectual property assets related to a specific device licensed to the Counterparty (the “Collateral”).
In connection with the transactions contemplated by the Counterparty SPA, the Company granted the Counterparty exclusive rights to the Company’s wearable injectors within select drug classes for use with certain Counterparty assets, while preserving rights the Company previously granted to other customers. The Company has also granted the Counterparty non-exclusive rights to all of the Company’s proprietary delivery systems within the therapeutic areas of oncology, inflammation, bone health, nephrology, cardiovascular and neuroscience. On the Counterparty Closing Date, the Counterparty paid to the Company a non-refundable $20.0 million fee (the “License Fee”) in consideration for such licenses.
OrbiMed
On February 22, 2016, in connection with the formation of the strategic collaboration with the Counterparty, Unilife Medical Solutions, Inc., a subsidiary of the Company (the “Borrower”), entered into an Eighth Amendment (the “Eighth Amendment to the Credit Agreement”) to the Credit Agreement, dated March 12, 2014, by and between ROS Acquisition Offshore LP (the “Lender”), an affiliate of OrbiMed Advisors (“OrbiMed”), and the Borrower (the “Credit Agreement,” and, as amended the “Amended Credit Agreement” or the “OrbiMed Financing”). Pursuant to and subject to the terms of the Eighth Amendment to the Credit Agreement, the Lender agreed to: (i) defer all obligations of the Borrower to pay interest to the Lender for the period from January 1, 2016 through February 22, 2018 at the rate specified in the Amended Credit Agreement, which interest will be added to the outstanding principal amount of the loan on the last day of each interest period; (ii) enable the Counterparty to take a security interest in certain inventory and intellectual property assets related to a specific device licensed to the Counterparty; and (iii) remove the minimum cash receipts
50
covenant for all future periods. In addition, on February 22, 2016, the Borrower entered into the Sixth Amendment to the Royalty Agreement (the “Royalty Agreement,”) with Royalty Opportunities S.A.R.L. (“ROS”). Pursuant to and subject to the terms of the Sixth Amendment to the Royalty Agreement, ROS agreed to waive any rights to royalty payments otherwise payable as a result of the License Fee and the proceeds of the Notes with the Counterparty, and to defer royalty payments payable on revenues received by the Company from the Counterparty until after the end of the first fiscal quarter in which the Company sells a commercial quantity of devices developed for the Counterparty. In connection with entering into the Eighth Amendment to the Credit Agreement and the Sixth Amendment to the Royalty Agreement, the Company issued to ROS a warrant to purchase, at any time until February 22, 2026, 1,673,981 shares of Common Stock, at an exercise price of $12.50 per share, subject to adjustment for certain events. See note 10 “Long Term Debt” for accounting considerations relating to such warrant.
Cost Reduction Initiatives
As previously disclosed, during fiscal 2016 we implemented several cost reduction and business realignment initiatives pursuant to which we reduced our headcount by approximately 90 employees which initiatives are expected to reduce annual operating costs by approximately $7.9 million. Subsequent to that, the Company eliminated 10 additional positions in July 2016. In part, as a result of these headcount reductions, the Company’s workforce was reduced to approximately 140 employees as of July 28, 2016, a reduction of more than 40% since January 2016 and a reduction of approximately 50% since July 1, 2015. A portion of this reduction is due to the Company’s determination not to backfill certain open positions. The Company has recorded a charge from severance and related costs from these cost reduction initiatives of approximately $0.7 million in the aggregate. The Company does not believe that these cost reduction initiatives will negatively impact its ability to serve its customers.
In addition, the Company, on June 20, 2016, subleased a portion (the “Subleased Portion”) of its King of Prussia, Pennsylvania facility (the “Facility”). During the term of the sublease, which commenced on October 1, 2016 and will end on March 31, 2019, the Company will be entitled to receive an aggregate of approximately $1.3 million in rent with respect to the Subleased Portion. During the same time period, the Company will be obligated under the Company’s lease agreement relating to the Facility to pay an aggregate of approximately $1.9 million in rent with respect to the Subleased Portion. Assuming the sublessor exercises its renewal option, the Company will be entitled to receive approximately an additional $1.9 million over the renewal term of April 1, 2019 through June 30, 2022 and the Company will be obligated under the Company’s lease agreement relating to the Facility to pay an aggregate of approximately $2.5 million over the same time period. The Company ceased using the Subleased Portion as of July 20, 2016. The Company will record a non-cash charge, the amount of which the Company has not yet determined as of the date of this Annual Report on Form 10-K, which amount will be based on the discounted fair value of the difference between the amounts received from the sublessor and the amounts paid to the landlord.
Cash Receipts
The Company expects to generate cash receipts from wearable injector customers during fiscal 2017 and the Company continues to have business development discussions with current and prospective wearable injector customers. The Company is, however, unable to predict the amount, if any, or the timing of such receipts or any proceeds from these business development discussions.
Fundraising Efforts
The Company’s ability to raise capital will be limited and there can be no assurance that financing will be available when needed. The Company will not be able to obtain financing through offerings of its securities registered under the Securities Act of 1933, as amended, for the near future and until the Company can prepare, file with the SEC, and cause to become effective a registration statement on Form S-1. We are not currently eligible to register the offer and sale of our securities using a registration statement on Form S-3, cannot use our existing Form S-3 and will not become eligible to use Form S-3 until we have timely filed certain periodic reports required under the Exchange Act for 12 consecutive calendar months. As a result, the Company will not be able to obtain financing under the Controlled Equity Offering Sales Agreement that the Company entered into with Cantor Fitzgerald & Co.on July 29, 2015 (the “New Sales Agreement”) or the equity purchase agreement that the Company entered into with Lincoln Park Capital Fund, LLC (“LPC”) on July 29, 2015 (the “LPC Purchase Agreement”) at least until the Company is eligible to register the offer and sale of our securities using a registration statement on Form S-3.
Pursuant to the Counterparty SPA, the Counterparty may purchase up to an additional $15.0 million in Notes in January 2017 (the “2017 Convertible Note”), and up to an additional $10.0 million in Notes in January 2018 (the “2018 Convertible Note”). See note 10 “Long-Term Debt – Senior Secured Convertible Note” for more information regarding the Notes. There can be no assurance as to when or if the Counterparty will purchase all or any part of the 2017 Convertible Note or the 2018 Convertible Note. The Counterparty’s willingness to purchase the 2017 Convertible Note or the 2018 Convertible Note may be impacted by any financing the Company receives prior to January 2017 or January 2018, respectively.
51
The Company is engaged in advanced discussions to secure up to $10 million of funding (the “Potential Transaction”) contingent upon the completion of due diligence, the execution of a definitive agreement with respect to the Potential Transaction and the filing of (i) this 2016 10-K; (ii) the March 2016 10-Q; (iii) the December 2015 10-Q Amendment; (iv) the September 2015 10-Q Amendment; and (v) the 2015 10-K Amendment. There can be no assurance that the Company will be able to enter into a definitive agreement with respect to the Potential Transaction and complete the closing of the Potential Transaction. Consummation of the Potential Transaction would require the consent of the Lender, which cannot be assured.
The Company has also engaged a financial advisory firm to further assist with fundraising efforts. There is no assurance that the financial advisory firm will be successful in these efforts.
The Company believes that potential proceeds from business development discussions, the 2017 Convertible Note, the Potential Transaction and fundraising efforts along with potential customer cash receipts, will provide the Company with enough liquidity to fund its operations for the next twelve months. However, there can be no assurance that any cash from such business development discussions, issuance of the 2017 Convertible Note, the Potential Transaction, fundraising efforts, or customer receipts will be available when needed, as such sources of liquidity largely are beyond the Company’s control. If we are unable to obtain financing when needed, we may be in default under one or more of our debt obligations unless we are able to obtain waivers from our lenders. A breach of any of the covenants related to our debt instruments could result in a higher rate of interest to be paid or the lenders could elect to declare all amounts outstanding under the applicable agreements to be immediately due and payable. If the lenders were to make such a demand for repayment, we would be unable to pay the obligations as we do not have existing facilities or sufficient cash on hand to satisfy these obligations. Under the circumstances, we also would be unable to pay our other obligations as they come due, which could prompt our creditors to pursue other remedies. These factors continue to raise substantial doubt about our ability to continue as a going concern. The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of this uncertainty.
The following table summarizes our cash flows during the fiscal years 2016, 2015 and 2014:
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Net cash (used in) provided by: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
(28,432 |
) |
|
$ |
(55,201 |
) |
|
$ |
(36,601 |
) |
|
Investing activities |
|
|
(17,322 |
) |
|
|
(16,633 |
) |
|
|
(12,149 |
) |
|
Financing activities |
|
|
52,179 |
|
|
|
75,750 |
|
|
|
51,287 |
|
Fiscal Year 2016 Compared to Fiscal Year 2015
Net Cash Used in Operating Activities
Net cash used in operating activities during fiscal year 2016 was $28.4 million compared to $55.2 million during fiscal year 2015. The decrease in net cash used in operating activities was primarily due to increased cash receipts from customers recorded as deferred revenue and a decrease in research and development expense offset by an increase in selling, general, and administrative expense.
Net Cash Used in Investing Activities
Net cash used in investing activities during fiscal year 2016 and fiscal year 2015 was $17.3 million and $16.6 million, respectively. This increase was primarily as a result of costs incurred in connection with the purchase of machinery and related equipment, facility expansion and cleanroom reconfiguration.
Net Cash Provided by Financing Activities
Net cash provided by financing activities during fiscal year 2016 was $52.2 million compared to $75.8 million during fiscal year 2015. During fiscal year 2016, we received $30.0 million in net proceeds from issuance of our convertible note in February 2016, $10.0 million in aggregate proceeds from the additional tranche under the Amended Credit Agreement with the Lender, $9.4 million of proceeds in connection with our public offering of common stock under the New Sales Agreement and the LPC Purchase Agreement and $7.2 million in net proceeds from the issuance of preferred stock which was partially offset by $0.6 million in principal debt repayments, $1.2 million in royalty liability payments, $1.6 million in shares forfeited in lieu of payroll taxes, $0.6
52
million in financing costs and $0.3 million in preferred stock dividends. During fiscal year 2015, we received $44.7 million in net proceeds from our public offering of common stock in February 2015, $20.0 million in aggregate proceeds from the two additional tranches under the Amended Credit Agreement with the Lender and $12.4 million of proceeds in connection with our public offering of common stock under the old sales agreement which was partially offset by $0.6 million in principal debt repayments and $0.7 million in royalty liability payments.
Fiscal Year 2015 Compared to Fiscal Year 2014
Net Cash Used in Operating Activities
Net cash used in operating activities during fiscal year 2015 was $55.2 million compared to $36.6 million during fiscal year 2014. The increase in net cash used in operating activities was primarily due to increased research and development costs of $17.7 million (exclusive of noncash expenses), and net other items $0.9 million.
Net Cash Used in Investing Activities
Net cash used in investing activities during fiscal year 2015 and fiscal year 2014 was $16.6 million and $12.1 million, respectively. This increase was primarily as a result of costs incurred in connection with the purchase of machinery and related equipment, facility expansion and cleanroom reconfiguration.
Net Cash Provided by Financing Activities
Net cash provided by financing activities during fiscal year 2015 was $75.8 million compared to $51.3 million during fiscal year 2014. During fiscal year 2015, we received $44.7 million in net proceeds from our public offering of common stock in February 2015, $20.0 million in aggregate proceeds from the two additional tranches under the Amended Credit Agreement with the Lender and $12.4 million of proceeds in connection with our public offering of common stock under the old sales agreement which was partially offset by $0.6 million in principal debt repayments and $0.7 million in royalty liability payments. During fiscal year 2014, we received $40.0 million from our March 2014 term loan, $16.9 million in connection with our public offering of common stock under the old sales agreement and $2.5 million upon the exercise of stock options. These amounts were partially offset by principal debt payments and financing costs of $8.1 million.
Contractual Obligations and Commitments
The following table provides information regarding our contractual obligations as of June 30, 2016:
|
|
|
Payments Due by Period |
|
|||||||||||||||||
|
|
|
Total |
|
|
Less Than 1 Year |
|
|
1-3 Years |
|
|
3-5 Years |
|
|
More Than 5 Years |
|
|||||
|
|
|
(In thousands) |
|
|||||||||||||||||
|
Long-term debt and related interest |
|
$ |
184,644 |
|
|
$ |
1,495 |
|
|
$ |
15,349 |
|
|
$ |
106,318 |
|
|
$ |
61,482 |
|
|
Operating leases |
|
|
6,374 |
|
|
|
891 |
|
|
|
1,561 |
|
|
|
2,592 |
|
|
|
1,330 |
|
|
Purchase obligations |
|
|
440 |
|
|
|
440 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total contractual obligations |
|
$ |
191,458 |
|
|
$ |
2,826 |
|
|
$ |
16,910 |
|
|
$ |
108,910 |
|
|
$ |
62,812 |
|
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements as such term is defined in the SEC rules.
We are exposed to market risk from changes in interest rates and foreign currency exchange rates. Changes in these factors could cause fluctuations in our results of operations and cash flows.
Interest Rate Risk
We face exposure to changes in interest rates primarily relating to our variable rate long-term debt pursuant to the OrbiMed Amended Credit Agreement. As of June 30, 2016, we had $70.0 million outstanding principal under the Amended Credit Agreement. The loans bear interest at 9.25% per annum plus LIBOR or 1.0% (whichever is greater), payable in cash quarterly, and as otherwise described in the Amended Credit Agreement. On June 30, 2016, the three-month LIBOR rate was 0.65%. Accordingly, we used a 10.25% interest rate. As of June 30, 2016, a ten basis point adverse change in LIBOR would not impact our total monthly interest
53
expense related to the OrbiMed Credit Agreement as our interest expense would not be tied to LIBOR unless or until LIBOR was greater than 1.0%.
We also face exposure to interest rate risk relating to our cash and cash equivalents that are invested in money market funds with highly liquid short term investments. We currently do not utilize derivative instruments to mitigate changes in interest rates.
As of June 30, 2016, a ten basis point adverse change in interest rates relating to our cash and cash equivalents that are invested in money market funds with highly liquid short term investments would have decreased our aggregate reported cash and cash equivalents by less than 1.0%.
Foreign Currency Exchange Rate Fluctuations
Certain of our revenues are derived from payments under our exclusive agreements received in euros while we incur most of our expenses in U.S. dollars and Australian dollars. In addition, a portion of our cash and cash equivalents and investments are held at Australian banking institutions and are denominated in Australian dollars. We are exposed to foreign currency exchange rate risks on these amounts. We currently do not utilize options or forward contracts to mitigate changes in foreign currency exchange rates. For U.S. reporting purposes, we translate all assets and liabilities of our non-U.S. entities into U.S. dollars using the exchange rate as of the end of the related period and we translate all revenues and expenses of our non-U.S. entities using the average exchange rate during the applicable period.
As of June 30, 2016, a ten percent adverse change in foreign exchange rates versus the U.S. dollar would have decreased our aggregate reported cash and cash equivalents by less than 1.0%.
54
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
|
|
Page |
|
|
|
|
|
|
|
|
|
|
|
56 |
|
|
|
|
|
59 |
|
|
|
|
|
60 |
|
|
|
|
|
61 |
|
|
|
Consolidated Statements of Cash Flows for the fiscal years ended June 30, 2016, 2015 and 2014 |
|
|
62 |
|
|
|
|
63 |
|
|
|
Management’s Report on Internal Control Over Financial Reporting |
|
|
100 |
|
55
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Unilife Corporation:
We have audited Unilife Corporation and subsidiaries (the Company) internal control over financial reporting as of June 30, 2016, based on criteria established in Internal Control Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weaknesses have been identified and included in management’s assessment:
|
• |
Ineffective tone at the top and design and operation of controls to monitor, investigate and communicate non-compliance with the Company’s Code of Conduct; |
|
• |
Insufficient number of trained resources with responsibility and accountability for financial reporting processes and controls; |
|
• |
Ineffective continuous risk assessment process; |
|
• |
Ineffective information and communication processes and monitoring activities regarding related party transactions; |
|
• |
Ineffective operation of certain process level controls due to management override of controls, including related party transactions and loans and advances to executives and a former Board member; |
|
• |
Ineffective design and implementation and documentation of management review controls; and |
|
• |
Ineffective program change and access general information technology controls resulting in ineffective process level automated controls, and ineffective compensating manual controls. |
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Unilife Corporation and subsidiaries as of June 30, 2016 and 2015, and the related consolidated statements of operations and comprehensive loss, stockholders’ (deficit) equity, and cash flows for each of the years in the three-year period ended June 30, 2016. These material weaknesses were considered in determining the nature, timing, and extent of audit tests applied in our audit of the 2016 consolidated financial statements, and this report does not affect our report dated October 21, 2016, which expressed an unqualified opinion on those consolidated financial statements. Our report on the consolidated financial statements dated October 21, 2016 contains an explanatory paragraph that states there is substantial doubt about the Company’s ability
56
to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
In our opinion, because of the effect of the aforementioned material weaknesses on the achievement of the objectives of the control criteria, the Company has not maintained effective internal control over financial reporting as of June 30, 2016, based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
/s/ KPMG LLP
Harrisburg, Pennsylvania
October 21, 2016
57
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Unilife Corporation:
We have audited the accompanying consolidated balance sheets of Unilife Corporation and subsidiaries (the Company) as of June 30, 2016 and 2015, and the related consolidated statements of operations and comprehensive loss, stockholders’ (deficit) equity, and cash flows for each of the years in the three-year period ended June 30, 2016. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Unilife Corporation and subsidiaries as of June 30, 2016 and 2015, and the results of their operations and their cash flows for each of the years in the three-year period ended June 30, 2016, in conformity with U.S. generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in note 3 to the consolidated financial statements, the Company has incurred recurring losses from operations and has limited cash resources, which raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in note 3. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of June 30, 2016, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated October 21, 2016, expressed an adverse opinion on the effectiveness of the Company’s internal control over financial reporting.
/s/ KPMG LLP
Harrisburg, Pennsylvania
October 21, 2016
58
UNILIFE CORPORATION AND SUBSIDIARIES
|
|
|
June 30, 2016 |
|
|
June 30, 2015 |
|
||
|
|
|
(in thousands, except share data) |
|
|||||
|
Assets |
|
|
|
|
|
|
|
|
|
Current Assets: |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
18,702 |
|
|
$ |
12,303 |
|
|
Restricted cash |
|
|
2,400 |
|
|
|
2,400 |
|
|
Restricted cash - related party |
|
|
— |
|
|
|
2,264 |
|
|
Accounts receivable |
|
|
374 |
|
|
|
1,530 |
|
|
Inventories |
|
|
89 |
|
|
|
151 |
|
|
Prepaid expenses and other current assets |
|
|
1,645 |
|
|
|
656 |
|
|
Total current assets |
|
|
23,210 |
|
|
|
19,304 |
|
|
Property, plant and equipment, net |
|
|
54,773 |
|
|
|
66,148 |
|
|
Goodwill |
|
|
9,423 |
|
|
|
9,685 |
|
|
Other assets |
|
|
255 |
|
|
|
276 |
|
|
Total assets |
|
$ |
87,661 |
|
|
$ |
95,413 |
|
|
Liabilities and Stockholders’ Deficit |
|
|
|
|
|
|
|
|
|
Current Liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
2,662 |
|
|
$ |
4,042 |
|
|
Due to related party |
|
|
— |
|
|
|
2,264 |
|
|
Accrued expenses |
|
|
13,710 |
|
|
|
5,074 |
|
|
Current portion of long-term debt |
|
|
669 |
|
|
|
775 |
|
|
Deferred revenue |
|
|
1,660 |
|
|
|
4,942 |
|
|
Total current liabilities |
|
|
18,701 |
|
|
|
17,097 |
|
|
Long-term debt, less current portion |
|
|
104,445 |
|
|
|
78,680 |
|
|
Warrant liability |
|
|
3,351 |
|
|
|
— |
|
|
Derivative liability |
|
|
347 |
|
|
|
— |
|
|
Deferred revenue |
|
|
47,550 |
|
|
|
17,550 |
|
|
Total liabilities |
|
|
174,394 |
|
|
|
113,327 |
|
|
Contingencies (Note 9) |
|
|
|
|
|
|
|
|
|
Stockholders’ Deficit: |
|
|
|
|
|
|
|
|
|
Redeemable convertible preferred stock, Series A — subject to redemption, $0.01 par value, 790 and 0 shares authorized, 0 and 0 shares issued, and 0 and 0 shares outstanding as of June 30, 2016 and June 30, 2015, respectively |
|
|
— |
|
|
|
— |
|
|
Preferred stock, $0.01 par value, 50,000,000 shares authorized as of June 30, 2016; none issued and outstanding as of June 30, 2016 and June 30, 2015 |
|
|
— |
|
|
|
— |
|
|
Common stock, $0.01 par value, 350,000,000 shares authorized as of June 30, 2016; 17,488,032 and 13,197,616 shares issued, and 17,411,651 and 13,194,749 shares outstanding as of June 30, 2016 and June 30, 2015, respectively |
|
|
175 |
|
|
|
132 |
|
|
Additional paid-in-capital |
|
|
398,862 |
|
|
|
366,005 |
|
|
Accumulated deficit |
|
|
(485,363 |
) |
|
|
(384,580 |
) |
|
Accumulated other comprehensive income |
|
|
380 |
|
|
|
669 |
|
|
Treasury stock, at cost, 76,381 shares as of June 30, 2016 and 2,867 shares at June 30, 2015 |
|
|
(787 |
) |
|
|
(140 |
) |
|
Total stockholders’ deficit |
|
|
(86,733 |
) |
|
|
(17,914 |
) |
|
Total liabilities and stockholders’ deficit |
|
$ |
87,661 |
|
|
$ |
95,413 |
|
See accompanying notes to the consolidated financial statements.
59
UNILIFE CORPORATION AND SUBSIDIARIES
Consolidated Statements of Operations and Comprehensive Loss
|
|
|
12 months ended June 30, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
|
(in thousands, except per share data) |
|
|||||||||
|
Revenue |
|
$ |
14,841 |
|
|
$ |
13,158 |
|
|
$ |
14,689 |
|
|
Research and development |
|
|
43,191 |
|
|
|
52,487 |
|
|
|
34,111 |
|
|
Selling, general and administrative |
|
|
43,196 |
|
|
|
36,176 |
|
|
|
27,894 |
|
|
Asset impairment charge |
|
|
26,580 |
|
|
|
— |
|
|
|
— |
|
|
Depreciation and amortization |
|
|
5,491 |
|
|
|
4,923 |
|
|
|
4,079 |
|
|
Total operating expenses |
|
|
118,458 |
|
|
|
93,586 |
|
|
|
66,084 |
|
|
Operating loss |
|
|
(103,617 |
) |
|
|
(80,428 |
) |
|
|
(51,395 |
) |
|
Interest expense |
|
|
10,187 |
|
|
|
6,368 |
|
|
|
7,332 |
|
|
Change in fair value of financial instruments |
|
|
(9,899 |
) |
|
|
4,279 |
|
|
|
(600 |
) |
|
Gain on debt extinguishment |
|
|
(2,881 |
) |
|
|
— |
|
|
|
— |
|
|
Other expense (income), net |
|
|
(241 |
) |
|
|
(226 |
) |
|
|
(228 |
) |
|
Net loss |
|
|
(100,783 |
) |
|
|
(90,849 |
) |
|
|
(57,899 |
) |
|
Other comprehensive (income) loss, net: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation |
|
|
289 |
|
|
|
2,128 |
|
|
|
(340 |
) |
|
Comprehensive loss |
|
$ |
(101,072 |
) |
|
$ |
(92,977 |
) |
|
$ |
(57,559 |
) |
|
Net loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share |
|
$ |
(7.04 |
) |
|
$ |
(8.10 |
) |
|
$ |
(5.90 |
) |
See accompanying notes to the consolidated financial statements.
60
UNILIFE CORPORATION AND SUBSIDIARIES
Consolidated Statements of Stockholders’ (Deficit) Equity
|
|
|
Common Stock |
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
||||||
|
|
|
Shares |
|
|
Amount |
|
|
Additional Paid-In Capital |
|
|
Accumulated Deficit |
|
|
Other Comprehensive Income |
|
|
Treasury Stock |
|
|
Total |
|
|||||||
|
|
|
(In thousands, except share data) |
|
|||||||||||||||||||||||||
|
Balance as of June 30, 2013 |
|
|
9,560,256 |
|
|
$ |
96 |
|
|
$ |
269,017 |
|
|
$ |
(235,832 |
) |
|
$ |
2,457 |
|
|
$ |
(140 |
) |
|
$ |
35,598 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(57,899 |
) |
|
|
— |
|
|
|
— |
|
|
|
(57,899 |
) |
|
Foreign currency translation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
340 |
|
|
|
— |
|
|
|
340 |
|
|
Share-based compensation expense |
|
|
159,310 |
|
|
|
2 |
|
|
|
8,314 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
8,316 |
|
|
Issuance of common stock from public offerings, net of issuance costs |
|
|
501,215 |
|
|
|
5 |
|
|
|
16,851 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
16,856 |
|
|
Issuance of common stock upon exercise of stock options |
|
|
140,947 |
|
|
|
1 |
|
|
|
2,919 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,920 |
|
|
Balance as of June 30, 2014 |
|
|
10,361,728 |
|
|
$ |
104 |
|
|
$ |
297,101 |
|
|
$ |
(293,731 |
) |
|
$ |
2,797 |
|
|
$ |
(140 |
) |
|
$ |
6,131 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(90,849 |
) |
|
|
— |
|
|
|
— |
|
|
|
(90,849 |
) |
|
Foreign currency translation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,128 |
) |
|
|
— |
|
|
|
(2,128 |
) |
|
Share-based compensation expense |
|
|
989,008 |
|
|
|
10 |
|
|
|
11,765 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
11,775 |
|
|
Issuance of common stock from public offerings, net of issuance costs |
|
|
1,845,880 |
|
|
|
18 |
|
|
|
57,116 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
57,134 |
|
|
Issuance of common stock upon exercise of stock options |
|
|
1,000 |
|
|
|
— |
|
|
|
23 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
23 |
|
|
Balance as of June 30, 2015 |
|
|
13,197,616 |
|
|
$ |
132 |
|
|
$ |
366,005 |
|
|
$ |
(384,580 |
) |
|
$ |
669 |
|
|
$ |
(140 |
) |
|
$ |
(17,914 |
) |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(100,783 |
) |
|
|
— |
|
|
|
— |
|
|
|
(100,783 |
) |
|
Foreign currency translation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(289 |
) |
|
|
— |
|
|
|
(289 |
) |
|
Conversion of redeemable convertible preferred stock |
|
|
2,784,693 |
|
|
|
28 |
|
|
|
11,623 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
11,651 |
|
|
Remeasurement of redeemable convertible preferred stock |
|
|
— |
|
|
|
— |
|
|
|
(1,047 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(1,047 |
) |
|
Share-based compensation expense |
|
|
912,615 |
|
|
|
9 |
|
|
|
13,880 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
13,889 |
|
|
Shares forfeited in lieu of payroll taxes |
|
|
(111,368 |
) |
|
|
(1 |
) |
|
|
(979 |
) |
|
|
— |
|
|
|
— |
|
|
|
(647 |
) |
|
|
(1,627 |
) |
|
Issuance of common stock from public offerings, net of issuance costs |
|
|
704,476 |
|
|
|
7 |
|
|
|
9,380 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
9,387 |
|
|
Balance as of June 30, 2016 |
|
|
17,488,032 |
|
|
$ |
175 |
|
|
$ |
398,862 |
|
|
$ |
(485,363 |
) |
|
$ |
380 |
|
|
$ |
(787 |
) |
|
$ |
(86,733 |
) |
See accompanying notes to the consolidated financial statements.
61
UNILIFE CORPORATION AND SUBSIDIARIES
Consolidated Statements of Cash Flows
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
|
(in thousands) |
|
|||||||||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(100,783 |
) |
|
$ |
(90,849 |
) |
|
$ |
(57,899 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
5,491 |
|
|
|
4,923 |
|
|
|
4,079 |
|
|
Asset impairment charge |
|
|
26,580 |
|
|
|
— |
|
|
|
— |
|
|
Share-based compensation expense |
|
|
13,889 |
|
|
|
11,775 |
|
|
|
8,316 |
|
|
Recognition of deferred revenue |
|
|
(8,875 |
) |
|
|
(125 |
) |
|
|
(3,187 |
) |
|
Non-cash interest expense |
|
|
8,279 |
|
|
|
1,896 |
|
|
|
457 |
|
|
Change in fair value of financial instruments |
|
|
(9,899 |
) |
|
|
4,279 |
|
|
|
(600 |
) |
|
Gain on debt extinguishment |
|
|
(2,881 |
) |
|
|
— |
|
|
|
— |
|
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted cash - related party |
|
|
2,264 |
|
|
|
(2,264 |
) |
|
|
— |
|
|
Accounts receivable |
|
|
757 |
|
|
|
330 |
|
|
|
(266 |
) |
|
Inventories |
|
|
62 |
|
|
|
(9 |
) |
|
|
(71 |
) |
|
Prepaid expenses and other current assets |
|
|
(989 |
) |
|
|
452 |
|
|
|
(704 |
) |
|
Other assets |
|
|
16 |
|
|
|
87 |
|
|
|
(427 |
) |
|
Accounts payable |
|
|
(1,249 |
) |
|
|
431 |
|
|
|
1,062 |
|
|
Due to related party |
|
|
(2,264 |
) |
|
|
2,264 |
|
|
|
— |
|
|
Accrued expenses |
|
|
5,177 |
|
|
|
2,259 |
|
|
|
139 |
|
|
Deferred revenue |
|
|
35,993 |
|
|
|
9,350 |
|
|
|
12,500 |
|
|
Net cash used in operating activities |
|
|
(28,432 |
) |
|
|
(55,201 |
) |
|
|
(36,601 |
) |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment |
|
|
(17,322 |
) |
|
|
(16,633 |
) |
|
|
(12,149 |
) |
|
Net cash used in investing activities |
|
|
(17,322 |
) |
|
|
(16,633 |
) |
|
|
(12,149 |
) |
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Principal payments on long-term debt and capital lease obligations |
|
|
(639 |
) |
|
|
(606 |
) |
|
|
(7,616 |
) |
|
Payment of former CEO loan |
|
|
(600 |
) |
|
|
— |
|
|
|
— |
|
|
Payment of royalty liability |
|
|
(1,227 |
) |
|
|
(749 |
) |
|
|
— |
|
|
Proceeds from former CEO loan |
|
|
600 |
|
|
|
— |
|
|
|
— |
|
|
Proceeds from issuance of long-term debt |
|
|
10,000 |
|
|
|
20,000 |
|
|
|
40,000 |
|
|
Proceeds from the issuance of common stock, net of issuance costs |
|
|
9,387 |
|
|
|
57,134 |
|
|
|
16,856 |
|
|
Proceeds from the issuance of convertible debt |
|
|
30,000 |
|
|
|
— |
|
|
|
— |
|
|
Proceeds from the issuance of preferred stock, net of issuance costs |
|
|
7,172 |
|
|
|
— |
|
|
|
— |
|
|
Proceeds from the exercise of options to purchase common stock |
|
|
— |
|
|
|
23 |
|
|
|
2,534 |
|
|
Payment of financing costs |
|
|
(607 |
) |
|
|
(52 |
) |
|
|
(487 |
) |
|
Dividend payment on redeemable convertible preferred stock |
|
|
(280 |
) |
|
|
— |
|
|
|
— |
|
|
Shares forfeited in lieu of payroll taxes |
|
|
(1,627 |
) |
|
|
— |
|
|
|
— |
|
|
Net cash provided by financing activities |
|
|
52,179 |
|
|
|
75,750 |
|
|
|
51,287 |
|
|
Effect of exchange rate changes on cash |
|
|
(26 |
) |
|
|
19 |
|
|
|
95 |
|
|
Net increase in cash and cash equivalents |
|
|
6,399 |
|
|
|
3,935 |
|
|
|
2,632 |
|
|
Cash and cash equivalents at beginning of period |
|
|
12,303 |
|
|
|
8,368 |
|
|
|
5,736 |
|
|
Cash and cash equivalents at end of period |
|
$ |
18,702 |
|
|
$ |
12,303 |
|
|
$ |
8,368 |
|
|
Supplemental disclosure of cash flow information |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid for interest |
|
$ |
4,179 |
|
|
$ |
6,467 |
|
|
$ |
3,222 |
|
|
Supplemental disclosure of non-cash activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment in accounts payable and accrued expenses |
|
$ |
2,674 |
|
|
$ |
497 |
|
|
$ |
991 |
|
|
Purchases of property, plant and equipment pursuant to capital lease agreements |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
125 |
|
See accompanying notes to the consolidated financial statements.
62
UNILIFE CORPORATION AND SUBSIDIARIES
Notes to Consolidated Financial Statements
1. Description of Business
Unilife Corporation and subsidiaries (the “Company”) was incorporated under the laws of the State of Delaware in 2009 and is based in the Commonwealth of Pennsylvania. The Company began operations in Australia in 2002.
The Company is a designer, manufacturer, and supplier of innovative injectable drug delivery systems that can enhance and differentiate the injectable products of our pharmaceutical and biotechnology customers. While the Company has a broad portfolio of proprietary product platforms, the Company focused the business on the Company’s wearable injector products. The Company believes its products are differentiated from conventional products, with innovative features and functionality designed to optimize the safe, simple, and convenient administration of injectable therapies. The majority of the Company’s products are designed for sale directly to pharmaceutical and biotechnology companies who are expected to supply them as drug-device combination products, pre-filled and ready for administration by end-users, such as patients or health-care providers. The Company customizes products within each of our platforms to address specific customer, therapy, patient and/or commercial requirements.
The Company is focusing primarily on active and new customer programs in its portfolio of wearable injector systems, which the Company expects will improve our operating efficiencies and better position the Company to take advantage of commercial opportunities within the fast-growing market for wearable injectors. The Company’s wearable injector customers include Amgen Inc., MedImmune LLC (“MedImmune”), and Sanofi S.A. (“Sanofi”).
In addition to the filling, assembly and/or packaging of the Company’s products with injectable therapies, the Company’s customers are also, with respect to most of our products, responsible for the regulatory approval, sale and marketing of their final drug-device combination products. While at this point the Company’s products have not been sold to end users with our customers’ injectable therapies, the Company can generate revenue from customization programs, upfront fees, device and development materials, and exclusivity fees.
The Company believes that continual investment in research and development is important to the development and sale of the Company’s innovative, proprietary products and related services. However, the Company continues to implement cost reduction measures as the Company focuses operations on the industrialization and development of programs with key strategic wearable injector customers. During fiscal year 2016, the Company implemented several cost reduction and business realignment initiatives pursuant to which the Company reduced its headcount by approximately 90 employees. Subsequent to that, the Company eliminated 10 additional positions in July 2016. The Company’s workforce was reduced to approximately 140 employees as of July 28, 2016, a reduction of more than 40% since January 2016 and a reduction of approximately 50% since July 1, 2015. A portion of this reduction is due to the Company’s determination not to backfill certain open positions. The Company has recorded a charge from severance and related costs from these cost reduction initiatives of approximately $0.7 million in the aggregate.
With the Company’s primary focus now on its wearable injector products, the Company performed an evaluation of its current contracts for its other products and determined that continued investment in those products is ultimately not beneficial to the Company at this time. The Company is currently in various stages of negotiation with the customers for such products to wind down Unilife’s activities under those customer contracts. The Company does not expect that these negotiations will impact its wearable injector products and the outcome of these negotiations is still uncertain. Regardless of the result of such negotiations, the Company intends to continue to prosecute and maintain the intellectual property related to the majority of its non-wearable injector products in the event it becomes financially attractive for the Company to further develop and customize those products in the future. As part of this evaluation, the Company has recorded in its third quarter operating results an impairment charge of $26.6 million primarily related to certain prefilled syringe capital equipment located in its York facility as well certain capital equipment that was under construction held at various suppliers.
2. Internal Investigation
The Company announced an investigation into violations of the Company’s policies and procedures and possible violations of laws and regulations by the Company’s former Chief Executive Officer, Alan Shortall, whose employment with the Company ceased on March 11, 2016, and its former Chairman, Jim Bosnjak, who resigned from the Company’s Board of Directors (the “Board”) on August 24, 2015 (the “Investigation”). The Board established a Special Committee to oversee the Investigation. Independent counsel conducted the Investigation with the assistance of an advisory firm with forensic accounting expertise. The Investigation was completed on October 7, 2016 and no material financial loss was identified.
63
The filing of this Annual Report on Form 10-K and the Company’s Form 10-Q for the quarter ended March 31, 2016 were delayed as a result of the Investigation. In addition, the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2015 and the Company’s Form 10-Q’s for the first and second quarters of fiscal year 2016 were amended to correct immaterial misstatements to the financial statements and omissions of related party disclosures and to disclose certain material weaknesses in the Company’s internal control over financial reporting and disclosure controls and procedures. As a result of such delay, on May 17, 2016, the Company received a notice from the Listing Qualifications department of NASDAQ stating that, because the Company had not yet filed the March 2016 10-Q, the Company was no longer in compliance with NASDAQ Listing Rule 5250(c)(1), which requires listed companies to timely file all required periodic financial reports with the SEC. The notice received from NASDAQ stated that the Company had 60 calendar days from the date of the notice to submit a plan to regain compliance with NASDAQ’s continued listing requirements.
On July 18, 2016, the Company timely submitted a plan to NASDAQ as to how it planned to regain compliance with NASDAQ’s continued listing requirements. The staff at NASDAQ subsequently granted the Company an exception to file the March 2016 10-Q and any other delinquent SEC filings on or before November 7, 2016 in order to enable the Company to regain compliance with the listing rules.
On September 19, 2016, the Company received a notice from the Listing Qualifications department of NASDAQ stating that, because the Company had not yet filed this 2016 10-K, the Company is not in compliance with NASDAQ Listing Rule 5250(c)(1). The Company timely submitted to NASDAQ an updated compliance plan on October 4, 2016.
3. Liquidity
As of June 30, 2016, the Company’s cash and cash equivalents were $18.7 million, restricted cash was $2.4 million and the book value of our long-term debt was $105.1 million. Under the Company’s debt facilities, the Company was required to have a cash and restricted cash balance of $5.4 million at June 30, 2016.
The Company incurred recurring losses from operations as well as negative cash flows from operating activities during fiscal years 2016, 2015, and 2014, and anticipates incurring additional losses and negative cash flows until such time that it can generate sufficient revenue from the sale, customization, or exclusive use and licensing of its wearable injectors to pharmaceutical and biotechnology customers. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The Company has taken or intends to take the steps delineated below to address its cash requirements, the success of which are largely beyond the Company’s control.
The Company expects to generate cash receipts from wearable injector customers during fiscal 2017 and the Company continues to have business development discussions with current and prospective wearable injector customers. The Company is, however, unable to predict the amount, if any, or the timing of such receipts or any proceeds from these business development discussions.
The Company has engaged a financial advisory firm to assist with fundraising efforts. There is no assurance that the financial advisory firm will be successful in these efforts.
In February 2016, the Company and certain of its subsidiaries entered into a Securities Purchase Agreement with the Counterparty (“Counterparty SPA”), pursuant to which the Counterparty agreed to purchase from the Company a new series of 6% Senior Secured Convertible Notes Due 2023 in the aggregate original principal amount of up to $55.0 million (the “Notes”). The Company issued to the Counterparty the first Note in the aggregate original principal amount of $30.0 million in February 2016 and the Counterparty paid to the Company $30.0 million in exchange therefor. Pursuant to the Counterparty SPA, the Counterparty may purchase up to an additional $25.0 million in Notes over the next two years, $15.0 million of which may be purchased in January 2017 (the “2017 Convertible Note”) and $10.0 million of which may be purchased in January 2018 (the “2018 Convertible Note”). There can be no assurance as to when or if the Counterparty will purchase all or any part of the 2017 Convertible Note and/or the 2018 Convertible Note.
The Company’s ability to raise capital will be limited and there can be no assurance that financing will be available when needed. The Company will not be able to obtain financing through offerings of its securities registered under the Securities Act of 1933, as amended, for the near future and until the Company can prepare, file with the SEC, and cause to become effective a registration on Form S-1. Further, we are not currently eligible to register the offer and sale of our securities using a registration statement on Form S-3, cannot use our existing Form S-3 and will not become eligible to use Form S-3 until we have timely filed certain periodic reports required under the Exchange Act for 12 consecutive calendar months.
64
The Company believes that potential proceeds from business development discussions, the 2017 Convertible Note and fundraising efforts along with potential customer cash receipts, will provide the Company with enough liquidity to fund its operations for the next twelve months. However, there can be no assurance that any cash from such business development discussions, issuance of the 2017 Convertible Note, fundraising efforts, or customer receipts will be available when needed, as such sources of liquidity largely are beyond the Company’s control. If we are unable to obtain financing when needed, we may be in default under one or more of our debt obligations unless we are able to obtain waivers from our lenders. A breach of any of the covenants related to our debt instruments could result in a higher rate of interest to be paid or the lenders could elect to declare all amounts outstanding under the applicable agreements to be immediately due and payable. If the lenders were to make such a demand for repayment, we would be unable to pay the obligations as we do not have existing facilities or sufficient cash on hand to satisfy these obligations. Under the circumstances, we also would be unable to pay our other obligations as they come due, which could prompt our creditors to pursue other remedies. These factors continue to raise substantial doubt about our ability to continue as a going concern. The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of this uncertainty.
4. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of Unilife Corporation and its wholly-owned subsidiaries. The consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). All significant intercompany accounts and transactions have been eliminated in consolidation.
On January 27, 2010, Unilife became the parent company of UMSL upon completion of the redomiciliation under Australian law and UMSL’s stockholders and option holders exchanged their interests in UMSL for equivalent interests in Unilife.
References to the “Company” include Unilife Corporation and its consolidated subsidiaries, including UMSL, unless the context otherwise requires. References to “Unilife” are references solely to Unilife Corporation.
References to A$ mean the lawful currency of the Commonwealth of Australia. References to € or euros are to the lawful currency of the European Union.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. The estimates are principally in the areas of revenue recognition, royalty liability valuation, warrant liability valuation, derivative liability valuation and share-based compensation expense. Management bases its estimates on historical experience and various assumptions that are believed to be reasonable under the circumstances. Actual results could differ from those estimates.
Cash and Cash Equivalents
Cash and cash equivalents consist primarily of cash on hand, deposits at banks and other short-term highly liquid investments with original maturities of three months or less. Cash equivalents are stated at cost, which approximates fair value.
Accounts Receivable
Accounts receivable are stated at amounts due from customers, which also represents the net realizable amount. The Company evaluates the collectability of its accounts receivable on a periodic basis and has historically not recorded an allowance for doubtful accounts. In instances in which management becomes aware of circumstances that may impair a particular customer’s ability to meet its obligation, the related receivable would be written off.
Inventories
Inventories consist primarily of raw materials. Inventories are stated at the lower of cost or market, with cost determined using the first in, first out method. The Company routinely reviews its inventory for obsolete, slow moving or otherwise impaired inventory and records estimated impairments in the periods in which they occur.
65
Property, plant and equipment, including significant improvements, are recorded at cost, net of accumulated depreciation and amortization. Repairs and maintenance are expensed as incurred.
Depreciation and amortization expense is recorded on a straight-line method over the estimated useful life of the asset as listed below:
|
Asset Category |
|
Useful Lives |
|
Building |
|
40 years |
|
Machinery and equipment |
|
2 to 15 years |
|
Computer software |
|
3 to 7 years |
|
Furniture and fixtures |
|
3 to 7 years |
|
Leasehold improvements |
|
Shorter of leasehold improvement life or remaining term of lease |
Interest cost incurred in connection with the development and construction of significant new machinery and equipment, as well as facility related costs have been capitalized as one of the elements of cost and are being amortized over the asset’s respective useful life. Interest capitalized during the years ended June 30, 2016 and June 30, 2015 was $0.4 million and $2.2 million, respectively. There was no capitalized interest during the year ended June 30, 2014.
The Company evaluates the recoverability of the recorded value of long-lived assets periodically to determine if facts and circumstances exist that would indicate that the assets might be impaired or that the useful lives should be modified. As part of this valuation, the Company develops projections of undiscounted future cash flows of the asset or asset group. The projections of undiscounted cash flows include a combination of revenue from customization and development activities, capital expenses that may be necessary to support projected product sales and commercial product sales. As customization and development activities are completed, commercial product sales are expected to scale-up. Expectations of future commercial product sales included in the projections used for the impairment analysis are based on customer-specific information as well as market estimates relating to the anticipated drugs and therapies being targeted for use with the Company’s products. These projections also include assumptions of future sales growth and profitability based on contracts entered into with customers as well as future contracts to be entered into based on the current discussions and negotiations with existing and prospective customers.
Sales projections are based on assumptions including a transition in the market toward patient self-administration of injectable therapies as well as transitions in the market toward biological-based drugs in the pharmaceutical industry development pipeline. The Company’s future sales could also be impacted by factors such as its ability to obtain new and retain existing customers, the timing and extent of the customers’ drug development activities as well as the regulatory approval process, drug efficacy and industry acceptance of injectable therapies. If the Company’s future sales or its projections of future sales are impacted by any one or more of the preceding factors, it will reassess the recorded value of the long-lived assets. If impairment is indicated, an adjustment will be made to reduce the carrying amount of these assets to their fair value.
In connection with the Company’s focus on wearable injector products, the Company has been evaluating the prospects of its non-wearable injector customer and supplier programs. As a result of negotiations related to those supplier and customer programs and the Company’s evaluation of those programs and potential disposition of certain assets, the Company determined that certain of its long lived-assets are impaired. The Company incurred $26.6 million of non-cash asset impairments primarily related to machinery and equipment and construction in process in the third quarter of fiscal 2016.
Goodwill and Intangible Assets
Goodwill is the excess of purchase price over the fair value of net assets acquired in business acquisitions. Goodwill is subject to, at a minimum, an annual impairment assessment of its carrying value at the reporting unit level. Additional impairment assessments would be performed if events and circumstances warranted such additional assessments during the year. The Company has one reporting unit which includes its product lines, the base technology which it obtained as part of its November 2002 acquisition of Unitract Syringe Pty Limited, and the manufacturing capability which it obtained in its January 2007 acquisition of Integrated BioSciences, Inc.
As required under generally accepted accounting principles, the Company is required to evaluate goodwill for impairment at least annually and more frequently if certain trigger events occur. The Company determined that the culmination of the Strategic Process and the decision to focus primarily on the wearable injector business constitute trigger events that occurred in the quarter ended March 31, 2016 requiring an evaluation of the carrying value of the Company’s goodwill. Potential impairment of goodwill is identified by comparing the fair value with its carrying value.
66
The Company performed a goodwill valuation as of March 31, 2016 using a combination of the market approach and a discounted cash flow method under the income approach. The Company determined an overall business enterprise value (determined by the fair of equity plus the fair value of debt) by taking a weighted average from the results of the discounted cash flow under the income approach (40%) and the market approach based on the Company’s market capitalization (60%). Under the income approach, the Company calculates the fair value of its reporting unit based on the present value of estimated future cash flows. Management judgment is necessary to evaluate the impact of operating and external market changes and to estimate the future cash flows used to measure fair value. The Company’s estimate of cash flows considers past performance, current and anticipated market conditions, and internal projections and operating plans which incorporate estimates for sales growth, profitability and capital spending. Additional assumptions include forecasted growth rates and estimated discount rates which are risk adjusted for current market conditions. The Company believes such assumptions reflect current and anticipated market conditions and are consistent with those that would be used by other marketplace participants for similar purposes but are subject to change due to changing conditions. The weighting of the results of each method to assess the overall business enterprise value was considered reasonable as greater weight was given to the market capitalization based on the quoted stock price as this is considered more reliable information as it is publicly available and not subject to management judgment or estimate, whereas, discounted cash flow under the income approach is subject to management estimates and judgments.
Although based on the results of the business enterprise value calculation there was no indication of impairment of goodwill, the Company determined as a result of the continuing losses incurred by the Company and its negative equity, and other indicators of impairment including, the significant long-lived asset impairment recorded as of March 31, 2016 and the drop in the Company’s stock price, that an additional step 2 analysis was warranted under generally accepted accounting principles.
The Company performed a Step 2 valuation of goodwill by valuing certain intangible assets (primarily patents, trademarks and tradenames). The Company considered the impact of all assets and liabilities in allocating the fair value of the Company to determine the implied fair value of goodwill and whether any impairment is indicated. The Company believes these intangible assets would generate the most significant difference between fair value and book value as part of completing a Step 2 purchase price allocation for the purpose of determining the value of goodwill for impairment. The valuation of intangible assets was performed using the relief from royalty method under the income approach. This method utilizes the Company’s revenue projections and assigns values based on determination of appropriate royalty rates considering relevant industry information. The Company then utilized the fair value of its intangible assets along with the implied fair value of its other assets and liabilities to determine the residual fair value compared to the carrying value of goodwill.
The residual fair value based on the results of the Step 2 valuation indicated there was no impairment of goodwill. The Company considered that the results of the valuation of goodwill indicated there was no impairment of goodwill and also indicated significant cushion between the estimated fair value of goodwill and its carrying value and, therefore, the Company has concluded that no goodwill impairment existed as of March 31, 2016.
The Company completed its annual goodwill valuation as of June 30, 2016. The Company performed a goodwill valuation as of June 30, 2016 using a combination of the market approach and a discounted cash flow method under the income approach to determine the overall business enterprise value and then performed a Step 2 valuation of the fair value of its assets and liabilities to determine the residual fair value of goodwill. The Step 2 valuation also valued the Company’s intangible assets using the relief from royalty method under the income approach. Based on the results of the goodwill valuation as of June 30, 2016, there was no impairment of goodwill. The Company considered that the results of the valuation of goodwill also indicated significant cushion between the implied fair value of goodwill and its carrying value and, therefore, the Company has concluded that no goodwill impairment existed as of June 30, 2016.
The Company did not record any goodwill impairments during fiscal years 2016, 2015 or 2014.
Definite-lived intangible assets include patents which are amortized on a straight-line method over their estimated useful lives of 15 years and are included in other assets. The Company reviews intangible assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. When factors indicate a possible impairment, if the sum of the estimated undiscounted future cash flows expected to result from the use and eventual disposition of an asset is less than the carrying amount of the asset, an impairment may be recognized. Measurement of an impairment loss is based on the excess of the carrying value of the asset over its fair value. Definite-lived intangible assets were less than $0.1 million as of June 30, 2016 and 2015 and are recorded in other assets on the consolidated balance sheet. There were no impairments recorded on intangible assets during the years ended June 30, 2016, 2015 or 2014.
67
Deferred financing costs are included as a direct deduction from the carrying amount of that debt liability on the consolidated balance sheets and consist of costs incurred in connection with debt financings. These costs are amortized and included in interest expense over the term of the related debt using the effective interest rate method.
Income Taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Deferred tax assets are recorded to the extent the Company believes they will more likely than not be realized. In making such determinations, the Company considers all available positive and negative evidence, including future reversals of existing temporary differences, projected future taxable income, tax planning strategies and recent financial operations. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected more likely than not to be realized.
The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company’s policy is to include interest and penalties related to uncertain tax positions within the provision (benefit) for income taxes within the Company’s consolidated statements of operations and comprehensive loss.
Fair Value of Financial Instruments
In accordance with Accounting Standards Codification (“ASC”) 820, Fair Value Measurements and Disclosures, the Company measures fair value based on a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that observable inputs be used when available. The fair value hierarchy is broken down into three levels based on the source of inputs.
The carrying value of financial instruments such as accounts receivable, accounts payable and accrued expenses are reasonable estimates of their fair value because of the short maturity of these items. The Company believes that the current carrying amount of its long-term debt approximates fair value based on the adjustment of the carrying value of the term loan to its fair value as of June 30, 2016.
The Company has elected to measure its royalty liability at fair value in accordance with ASC 825, Financial Instruments. The fair value of the royalty liability is based on significant inputs not observable in the market, which require it to be reported as a Level 3 liability within the fair value hierarchy. The valuation uses a methodology and assumptions that the Company believes would be made by a market participant. In particular, the valuation analysis uses a discounted cash flow methodology under the income approach based on the present value sum of payments to be made in the future. The fair value of the royalty liability is estimated by applying a risk adjusted discount rate to the adjusted royalty revenue stream. These fair value estimates are most sensitive to changes in the payment stream.
The Company accounts for derivative financial instruments in accordance with ASC 815, Derivative and Hedging — Contracts in Entity’s Own Equity. Instruments which do not have fixed settlement provisions are deemed to be derivative instruments and are valued based on an average of a Monte Carlo and lattice model. The Preferred Stock Conversion valuation analysis used the estimated dividend rate based on the volume-weighted average price of the Company’s common stock at the date the Preferred Stock Conversion is measured. The warrant liability is valued using a Black-Scholes option-pricing model
Share-Based Compensation
The Company grants equity awards to its employees, directors, consultants and service providers. Certain employee and director awards vest over stated vesting periods and others also require achievement of specific performance or market conditions. The Company expenses the grant-date fair value of awards to employees and directors over their respective vesting periods. To the extent that employee and director awards vest only upon the achievement of a specific performance condition, expense is recognized over the period from the date management determines that the performance condition is probable of achievement through the date they are expected to be met. Awards granted to consultants and service providers are sometimes granted for past services, in which case their fair value is expensed on their grant date, while other awards require future service, or the achievement of performance or market conditions. Timing of expense recognition for consultant awards is similar to that of employee and director awards; however,
68
aggregate expense is re-measured each quarter-end based on the then fair value of the award through the vesting date of the award. The Company estimates the fair value of stock options using the Black-Scholes option-pricing model, with the exception of market-based grants, which are valued based on the Monte Carlo option pricing model. Option pricing methods require the input of highly subjective assumptions, including the expected stock price volatility.
Foreign Currency Translation
The Australian dollar is the functional currency for the Company’s Australian operations. Assets and liabilities denominated in foreign currencies are translated into U.S. dollars at the rate of exchange existing at the end of the period. Revenues and expenses are translated at the average exchange rates during the applicable period. Adjustments resulting from these translations are recorded in accumulated other comprehensive income within the Company’s consolidated balance sheets and will be included in income upon sale or liquidation of the foreign investment. Gains and losses from foreign currency transactions, denominated in a currency other than the functional currency, are recorded in other income within the Company’s consolidated statements of operations and comprehensive loss and aggregated less than $0.1 million for each of the fiscal years ended June 30, 2016, 2015 and 2014.
Comprehensive Loss
Comprehensive loss includes net loss and other comprehensive loss (income). The Company’s other comprehensive loss (income) consists only of foreign currency translation adjustments.
Revenue Recognition
The Company recognizes revenue from industrialization and development fees, licensing fees and product sales. The Company recognizes revenue from sales of products at the time of shipment when title passes to the customer. The Company recognizes up front, non-refundable fees ratably over the expected life of the related agreement. Revenue from industrialization and development fees is recognized as services are rendered, under the completed contract method, under the proportional performance method or upon achievement of the “at risk” substantive milestone events, which represent the culmination of the earnings process related to such events. Substantive milestones can include specific deliverables such as product design, prototype availability, user tests, manufacturing proof of principle and the various steps to complete the industrialization of the product. The terms of these contracts provide for customer payments to be made as services are rendered or substantive milestones are achieved. The Company considers whether a milestone is substantive at the inception of the agreement. The consideration earned from the achievement of a milestone must meet all of the following criteria to be considered substantive:
|
|
• |
It is commensurate with either of the Company’s performance to achieve the milestone, or the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the Company’s performance to achieve the milestone; |
|
|
• |
It relates solely to past performance; and |
|
|
• |
It is reasonable relative to all of the deliverables and payment terms (including other potential milestone consideration) within the arrangement. |
Payment terms are considered to be standard commercial terms. Revenue is recognized when each substantive milestone has been achieved and the Company has no future performance obligations related to the substantive milestone. Fees for completed, substantive milestones, which are dependent upon customer acceptance for non-refundable payment or, if paid, are refundable pending customer acceptance are recognized upon customer acceptance or the termination of refund rights.
Advertising Costs
Advertising costs are expensed in the period incurred. The Company incurred total advertising costs of $0.2 million for each of the fiscal years ended June 30, 2016, 2015 and 2014.
Research and Development Costs
Research and development expenses consist primarily of payroll and related personnel expenses (including share-based compensation expense), fees paid to external service providers, costs of materials, components and supplies, costs for facilities, tooling and equipment and costs related to developing prototype products and samples used for various evaluation, testing and related activities for existing and potential customers. Research and development expenses are included in operating expenses when incurred. Research and development expenses include costs related to the ongoing development and expansion of the Company’s broad portfolio of injectable drug delivery systems as well as costs incurred in relation to customization, industrialization and development
69
agreements with its customers. These costs are not segregated from the overall research and development costs as they are not readily distinguishable from the rest of the Company’s ongoing research and development expenses.
Interest Expense
The Company recognizes interest expense in the consolidated statements of operations and comprehensive loss for all debt instruments using the effective interest method. The effective interest method is a method of calculating the amortized cost of a financial liability and of allocating the interest expense over the relevant period. The effective interest rate is the rate that exactly discounts the estimated future cash payments through the expected life of the financial instrument to the net carrying amount of the financial liability. The application of the method has the effect of recognizing expense payable on the instrument evenly in proportion to the amount outstanding over the period to maturity or repayment. In calculating the effective interest rate, the Company estimates cash flows considering all contractual terms of the financial instrument, including fees for early redemption and all other premiums and discounts.
Net Loss Per Share
Basic net loss per share is computed as net loss divided by the weighted average number of shares outstanding during the period. Diluted net earnings per share reflect the potential dilution that could occur from common stock issued through common stock equivalents. The dilutive effect of potential common stock, consisting of non-participating restricted stock and outstanding options to purchase common stock, is calculated using the treasury stock method.
Unvested share-based payment awards that contain nonforfeitable rights to dividends or dividend equivalents, whether paid or unpaid, are considered participating securities and are included in the computation of net loss per share according to the two class method if the impact is dilutive. Shares of the Company’s unvested restricted stock are considered participating securities. However, in the event of a net loss, participating securities are excluded from the calculation of both basic and diluted net loss per share.
Business Segments
The Company operates in one reportable segment, which includes the design, development and manufacture of injectable drug delivery systems. Revenues by geographic location based on location of customer are as follows:
|
|
|
Years Ended June 30, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
|
(In thousands) |
|
|||||||||
|
Domestic |
|
$ |
11,680 |
|
|
$ |
9,834 |
|
|
$ |
5,702 |
|
|
International |
|
|
3,161 |
|
|
|
3,324 |
|
|
|
8,987 |
|
|
|
|
$ |
14,841 |
|
|
$ |
13,158 |
|
|
$ |
14,689 |
|
Reclassifications
Certain prior year amounts in the consolidated financial statements have been reclassified to conform to the current year presentation.
On December 2, 2015, the Company received a written notice from the Listing Qualifications Department of The NASDAQ Stock Market LLC indicating that, for the 30 consecutive business days ended December 1, 2015, the bid price for the Company’s common stock had closed below the $1.00 per share minimum bid price requirement for continued listing on The Nasdaq Global Market under Nasdaq Listing Rule 5450(a)(2). In response to the preceding notice, on May 13, 2016, pursuant to prior stockholder authorization, the Company effected a reverse split of the Company’s common stock, pursuant to which every ten (10) shares of common stock outstanding before the reverse split were converted into one (1) share of common stock after the reverse split. All share and per share amounts, and exercise and conversion prices for all periods presented herein have been adjusted to reflect the reverse split as if it had occurred at the beginning of the first period presented.
The retrospective adoption of ASU 2015-03 “Simplifying the Presentation for Debt Issuance Costs” decreased other assets and long-term debt by $1.0 million in the accompanying consolidated balance sheet at June 30, 2015.
Recently Issued Accounting Pronouncements
In May 2014, FASB issued ASU 2014-09 “Revenue from Contracts with Customers” and amended by ASU 2016-08 “Principle versus Agent Considerations”, ASU 2016-10 “Identifying Performance Obligations and Licensing”, and ASU 2016-12 “Narrow-
70
Scope Improvements and Practical Expedients”. The guidance requires an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to a customer. The ASU will replace most existing revenue recognition guidance in U.S. GAAP when it becomes effective. In August 2015, the FASB issued ASU 2015-14 “Revenue from Contracts with Customers” which deferred the effective date of ASU 2014-09 for all entities by one year to annual reporting periods beginning after December 15, 2017, including interim reporting periods within that reporting period. Early application is permitted only as of annual periods beginning after December 15, 2016, including interim reporting periods within that reporting period. With the deferral, the new standard is effective for the Company, on July 1, 2018, with early adoption permitted one year prior. The standard permits the use of either the retrospective or cumulative effect transition method. The Company has not selected a transition method nor has it determined the effect of the standard on its ongoing financial reporting.
In June 2014, FASB issued ASU 2014-12 “Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period” which is part of ASC 718: Compensation-Stock Compensation. The guidance requires that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition and not be reflected in the estimate of the grant-date fair value of the award. The guidance is effective for annual periods beginning after December 15, 2015. The guidance can be applied prospectively for all awards granted or modified after the effective date or retrospectively to all awards with performance targets outstanding as of the beginning of the earliest annual period presented in the financial statements and to all new or modified awards thereafter. The Company does not expect a material impact on its financial condition, results of operations or cash flows from the adoption of this guidance.
In August 2014, FASB issued ASU 2014-15 “Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern”. The guidance requires an entity to perform a going concern assessment by evaluating its ability to meet its obligations for a look-forward period of one year from the financial statement issuance date. Disclosures are required if it is probable an entity will be unable to meet its obligations within the look-forward period. Incremental substantial doubt disclosure is required if the probability is not mitigated by management’s plans. The guidance is effective for all entities for the first annual period ending after December 15, 2016 and interim periods thereafter. Early application is permitted. The Company is currently evaluating the impact this guidance will have on its financial disclosures; however, as the guidance only impacts disclosure, the adoption of this guidance is not expected to have any impact on the Company’s financial condition, results of operations and cash flows.
In April 2015, FASB issued ASU 2015-03 “Simplifying the Presentation for Debt Issuance Costs”. The guidance requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The existing recognition and measurement guidance for debt issue costs is not affected by the new guidance. In August 2015, the FASB issued a clarification that debt issue costs related to line-of-credit arrangements were not within the scope of the new guidance and therefore should continue to be accounted for as deferred assets in the balance sheet, consistent with existing GAAP. The guidance is effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. The Company has adopted this guidance and applied the provisions of this guidance retrospectively to all reporting periods. The adoption of this guidance did not have a material impact on the Company’s results of operations, cash flows or financial position.
In November 2015, the FASB issued new guidance simplifying the balance sheet classification of deferred taxes. The new guidance requires that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. The current requirement that deferred tax liabilities and assets of a tax-paying component of an entity be offset and presented as a single amount is not affected by the new guidance. The new guidance is effective for the Company on July 1, 2017, with early adoption permitted as of the beginning of an interim or annual reporting period. The new guidance may be applied either prospectively to all deferred tax liabilities and assets or retrospectively to all periods presented. The Company is evaluating the impact that the new guidance will have on its consolidated financial statements and related disclosures; however, at the present time the Company has recorded a valuation allowance against its deferred tax assets based on the history of losses incurred.
In January 2016, the FASB issued new guidance related to the recognition and measurement of financial assets and liabilities. The new guidance makes targeted improvements to GAAP impacting equity investments (other than those accounted for under the equity method or consolidated), financial liabilities accounted for under the fair value election, and presentation and disclosure requirements for financial instruments, among other changes. The new guidance is effective for the Company on July 1, 2018, with early adoption prohibited other than for certain provisions. The Company is evaluating the impact that the new guidance will have on its consolidated financial statements and related disclosures.
In February 2016, the FASB issued guidance that will change the requirements for accounting for leases. The principal change under the new accounting guidance is that lessees under leases classified as operating leases will recognize a right-of-use asset and a lease liability. Current lease accounting does not require lessees to recognize assets and liabilities arising under operating leases on the balance sheet. Under the new guidance, lessees (including lessees under leases classified as finance leases and operating leases) will recognize a right-to-use asset and a lease liability on the balance sheet, initially measured as the present value of lease payments under the lease. Expense recognition and cash flow presentation guidance will be based upon whether the lease is classified as an operating
71
lease or a finance lease (the classification criteria for distinguishing between finance leases and operating leases is substantially similar to the classification criteria for distinguishing between capital leases and operating leases under current guidance). The standard is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. Early adoption is permitted. The new standard must be adopted using a modified retrospective transition approach for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements; the guidance provides certain practical expedients. The Company is currently evaluating this guidance to determine its impact on the Company’s results of operations, cash flows and financial position.
In March 2016, the FASB issued new guidance designed to simplify several aspects of the accounting for share-based payment transactions, including guidance relating to accounting for income taxes with respect to share-based payment awards; providing generally that excess tax benefits related to share-based awards should be recorded as a reduction to income tax expense (currently, excess tax benefits generally are recorded to additional-paid-in-capital); providing generally that excess tax benefits related to share-based awards should be classified along with other income tax cash flows as an operating activity (currently, excess tax benefits generally are separated from other income tax cash flows and classified as a financing activity); providing that an entity may make an accounting policy election either to base compensation cost accruals on the number of awards expected to vest (as required by current guidance) or to account for forfeitures when they occur; modifying the current exception to liability classification such that partial cash settlement of an award for tax withholding purposes would not result, by itself, in liability classification of the award if the amount withheld does not exceed the maximum statutory tax rate in the employees' applicable jurisdictions (currently, an award cannot qualify for equity classification, rather than liability classification, if the amount withheld exceeds the minimum statutory withholding requirements); and providing that cash paid by an employer when directly withholding shares for tax withholding purposes should be classified as a financing activity on the statement of cash flows (currently there is no authoritative guidance addressing this classification issue). The guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2016. Early adoption is permitted (if early adoption occurs in an interim period, any adjustments will be reflected as of the beginning of the fiscal year that includes the interim period). Depending on the particular issue addressed by the guidance, application of the guidance will be made prospectively, retrospectively or subject to a retrospective transition method. The Company is currently evaluating the potential impact of adopting this guidance on the Company's results of operations, cash flows and financial position.
5. Equity Transactions and Share-Based Compensation
During the fiscal year ended June 30, 2013, the Company issued 446,097 shares of common stock for net proceeds of $9.6 million, net of issuance costs, pursuant to a securities purchase agreement. In connection with the securities purchase agreement, the Company issued two warrants to purchase an aggregate of 158,699 shares of common stock. The warrants are exercisable at $30.00 per share and will expire five years from the date of grant. The warrants contain exchange features whereby the warrant holders can exchange the warrants for cash or common stock equal to the value of the warrants at the time of exchange, which value is based upon a contractual formula. Based on the terms of the agreements, the Company has determined that the warrants should be classified as a liability. As of March 31, 2013, the Company recorded a liability of $3.0 million related to the negotiated value of the warrants. In April 2013, the exchange feature was exercised for one of the warrants and a total of 142,422 shares of common stock were issued in settlement of a warrant to purchase 148,699 shares of common stock and the related warrant liability of $2.8 million was reclassified to equity. As of June 30, 2013, one warrant to purchase 10,000 shares of common stock remained outstanding. During the fiscal year ended June 30, 2014, the warrant was exercised in exchange for 1,948 shares of common stock and the related warrant liability of $0.4 million was reclassified to equity.
During the fiscal year ended June 30, 2014, the Company granted certain directors 22,750 shares of common stock which may not be sold or transferred until such time as the director leaves the board of directors for any reason, including a change in control, or other permitted circumstances. The weighted average grant date fair value of the shares was $39.50 per share.
During the fiscal year ended June 30, 2015, the Company granted certain directors 17,500 shares of common stock which may not be sold or transferred until such time as the director leaves the board of directors for any reason, including a change in control, or other permitted circumstances. The weighted average grant date fair value of the shares was $28.30 per share.
During the fiscal year ended June 30, 2015, the Company issued 1,265,000 shares of common stock and raised $44.7 million, net of issuance costs, through an underwritten registered public offering. The Company intends to use the proceeds from the public offering for investments in its plant, equipment, systems and personnel to further develop its manufacturing and operational capabilities to satisfy current and future customer orders and for working capital and other general corporate purposes.
During the fiscal year ended June 30, 2016, the Company granted certain directors 17,500 shares of common stock which may not be sold or transferred until such time as the director leaves the board of directors for any reason, including a change in control, or other permitted circumstances. The weighted average grant date fair value of the shares was $8.80 per share.
72
On July 29, 2015, the Company entered into a Controlled Equity Offering Sales Agreement (the “New Sales Agreement”) with Cantor Fitzgerald & Co., pursuant to which the Company may, from time to time, issue and sell shares of common stock, having an aggregate offering price of up to $25.0 million. During fiscal year 2016, the Company has issued 380,011 shares for net proceeds of $4.6 million under the New Sales Agreement. The Company used the proceeds for working capital needs and other general corporate purposes.
On July 29, 2015, the Company entered into an equity purchase agreement (the “LPC Purchase Agreement”) with Lincoln Park Capital Fund, LLC (“LPC”), pursuant to which the Company may sell, from time to time, to LPC up to $45.0 million in shares of the Company’s common stock through July 2017, subject to certain limitations and conditions set forth in the LPC Purchase Agreement. During fiscal year 2016, the Company issued 324,465 shares of common stock to LPC and received net proceeds of approximately $4.8 million after expenses. The Company used the proceeds for working capital needs and other general corporate purposes.
The Company recognized share-based compensation expense related to equity awards to employees, directors, consultants and service providers of $13.9 million, $11.8 million and $8.3 million during the fiscal years ended June 30, 2016, 2015 and 2014, respectively.
As of June 30, 2016, the total compensation cost related to all non-vested awards not yet recognized was $8.8 million. This amount is expected to be recognized over a remaining weighted average period of 1.65 years.
Stock Options and Warrants
The Company has granted stock options to certain employees and directors under the Employee Share Option Plan (the “Plan”). The Plan is designed to assist in the motivation and retention of employees and directors and to recognize the importance of employees and directors to the long-term performance and success of the Company. The Company has also granted stock options to certain service providers outside of the Plan. The majority of the options to purchase common stock vest on the anniversary of the date of grant, which ranges from one to three years. Additionally, certain stock options vest upon the closing price of the Company’s common stock reaching certain minimum levels, as defined in the agreements. Share-based compensation expense related to options granted to employees and directors is recognized on a straight-line method over the related vesting term. Share-based compensation expense related to options granted to service providers is recognized ratably over each vesting tranche of the options.
During the fiscal year ended June 30, 2010, the Company adopted the 2009 Stock Incentive Plan (the “Stock Incentive Plan”). The Stock Incentive Plan initially provided for a maximum of 600,000 shares of common stock to be reserved for the issuance of stock options and other stock-based awards. Commencing on January 1, 2012, and on each January 1st thereafter, through January 1, 2014, the share reserve automatically adjusted so that it was equal to 17.5% of the weighted average number of shares of common stock outstanding reduced by the sum of any shares of common stock issued under the Stock Incentive Plan and any shares of common stock subject to outstanding awards under the Stock Incentive Plan.
During the fiscal year ended June 30, 2015, the Stock Incentive Plan was amended and restated (the “Amended and Restated 2009 Stock Incentive Plan” or “Amended Stock Plan”) to change how the number of shares of common stock that may be issued under the Amended Stock Plan is calculated, to increase the number of shares of common stock available for issuance under the Amended Stock Plan by 1.0 million and to reapprove the Amended Stock Plan for purposes of refreshing the stockholder approval requirement.
Under the terms of the LPC Purchase Agreement, the Company was required to obtain the consent of LPC prior to completing the Preferred Stock Purchase Agreement. The Company obtained such consent on November 9, 2015 and contemporaneously issued a five-year warrant to purchase 90,000 shares of Common Stock to LPC at an exercise price of $10.00 per share. The Company performed a Black-Scholes valuation on the warrant and valued the warrant at $5.40 per share of Common Stock. Accordingly, the Company recorded $0.5 million during fiscal year 2016 associated with the issuance of the warrant as a component of redeemable convertible preferred stock issuance cost. LPC has not exercised any warrants as of June 30, 2016. On June 30, 2016, the Company performed a Black-Scholes valuation on the warrant liability to revalue the warrant and valued the warrant at $1.15 per share of Common Stock. The warrant liability was revalued to $0.1 million at June 30, 2016.
On February 22, 2016, in connection with entering into the Eighth Amendment to the Credit Agreement and the Sixth Amendment to the Royalty Agreement, the Company issued to ROS warrants to purchase 1,673,981 shares of Common Stock, with an exercise price of $12.50 per share, subject to adjustment for certain events, which may be exercised at any time and from time to time until February 22, 2026. Upon issuance, the Company performed a Black-Scholes valuation on the warrant and valued the warrant at $7.20 per share of Common Stock. Accordingly, the Company recorded a $12.1 million warrant liability during fiscal year 2016 associated with the issuance of the warrant. ROS has not exercised any warrants as of June 30, 2016. On June 30, 2016, the Company performed a Black-Scholes valuation on the warrant liability to revalue the warrant and valued the warrant at $1.94 per share of Common Stock. The warrant liability was revalued to $3.2 million at June 30, 2016.
73
The following is a summary of activity related to stock options held by employees and directors during the fiscal year ended June 30, 2016:
|
|
|
Number of Options |
|
|
Weighted Average Exercise Price |
|
|
Weighted Average Remaining Contractual Life |
|
|
Aggregate Intrinsic Value |
|
||||
|
|
|
|
|
|
|
|
|
|
|
(in years) |
|
|
(in thousands) |
|
||
|
Outstanding as of July 1, 2015 |
|
|
250,817 |
|
|
$ |
37.82 |
|
|
|
|
|
|
|
|
|
|
Cancelled |
|
|
(140,265 |
) |
|
|
34.79 |
|
|
|
|
|
|
|
|
|
|
Expired |
|
|
(8,000 |
) |
|
|
61.90 |
|
|
|
|
|
|
|
|
|
|
Outstanding as of June 30, 2016 |
|
|
102,552 |
|
|
|
40.10 |
|
|
|
5.9 |
|
|
$ |
— |
|
|
Exercisable as of June 30, 2016 |
|
|
85,552 |
|
|
$ |
40.60 |
|
|
|
5.7 |
|
|
$ |
— |
|
The following is a summary of activity related to stock options and warrants held by persons other than employees and directors during the fiscal year ended June 30, 2016:
|
|
|
Number of Options & Warrants |
|
|
Weighted Average Exercise Price |
|
|
Weighted Average Remaining Contractual Life |
|
|
Aggregate Intrinsic Value |
|
||||
|
|
|
|
|
|
|
|
|
|
|
(in years) |
|
|
(in thousands) |
|
||
|
Outstanding as of July 1, 2015 |
|
|
105,000 |
|
|
$ |
42.01 |
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
1,763,981 |
|
|
|
12.37 |
|
|
|
|
|
|
|
|
|
|
Expired |
|
|
(75,000 |
) |
|
|
46.40 |
|
|
|
|
|
|
|
|
|
|
Outstanding as of June 30, 2016 |
|
|
1,793,981 |
|
|
$ |
12.68 |
|
|
|
9.3 |
|
|
$ |
— |
|
|
Exercisable as of June 30, 2016 |
|
|
1,793,981 |
|
|
$ |
12.68 |
|
|
|
9.3 |
|
|
$ |
— |
|
The aggregate intrinsic value is defined as the difference between the market value of the Company’s common stock as of the end of the period and the exercise price of the in-the-money stock options. The total intrinsic value of stock options exercised during the fiscal years ended June 30, 2016, 2015 and 2014 was $0.0 million, $0.1 million and $1.8 million, respectively.
The Company currently uses authorized and unissued shares to satisfy stock option exercises.
The weighted average fair value of stock options granted during the fiscal years ended June 30, 2016 and 2014 was $12.37 and $16.48 per share, respectively. There were no stock options granted during the fiscal year ended June 30, 2015.
The Company used the following weighted average assumptions in calculating the fair value of options and warrants granted during the fiscal year ended June 30, 2016 and 2014:
|
|
|
2016 |
|
2014 |
|
||
|
Number of warrants and stock options granted |
|
|
1,763,981 |
|
|
50,000 |
|
|
Expected dividend yield |
|
|
0.00 |
% |
|
0.00 |
% |
|
Risk-free interest rate |
|
|
1.96 |
% |
|
1.52 |
% |
|
Expected volatility |
|
|
71.90 |
% |
|
55.00 |
% |
|
Expected life (in years) |
|
|
9.74 |
|
|
5.50 |
|
The fair value of each stock option and warrant was estimated at the grant date using the Black-Scholes option pricing model. The Company has not historically paid dividends to its stockholders and, as a result, assumed a dividend yield of 0%. The risk free interest rate is based upon the rates of U.S. Treasury bonds with a term equal to the expected term of the option. The expected volatility used to value options granted after January 27, 2010 is based upon a blended rate of the historical share price of the Company’s stock on the Australian Securities Exchange and the volatility of peer companies traded on U.S. exchanges operating in the same industry as the Company. The expected term of the options to purchase common stock issued to employees and directors is based upon the simplified method, which is the mid-point between the vesting date of the option and its contractual term unless a reasonable alternate term is estimated by management. The expected term of the options to purchase common stock issued to consultants and service providers is based on the contractual term of the awards.
74
Restricted Stock Awards and Units
The Company has granted shares of restricted stock to certain employees, directors and consultants under the Amended Stock Incentive Plan. During the period prior to vesting, the holder of the non-vested restricted stock will have the right to vote and the right to receive all dividends and other distributions declared. All non-vested shares of restricted stock are reflected as outstanding; however, they have been excluded from the calculation of basic earnings per share.
For employees, the fair value of restricted stock is measured on the date of grant using the price of the Company’s common stock on that date. Share-based compensation expense for restricted stock issued to employees is recognized on a straight-line basis over the requisite service period, which is generally the longest vesting period. For restricted stock granted to consultants, the fair value of the awards will be re-valued on a quarterly basis and marked to market until vested. Share-based compensation expense for restricted stock issued to consultants is recognized ratably over each vesting tranche.
During the fiscal year ended June 30, 2015, the shareholders approved the issuance of 400,000 shares of restricted stock to the Company’s then Chairman and Chief Executive Officer, Alan Shortall. In connection with Mr. Shortall’s separation from the Company, the Company and Mr. Shortall entered into a General Release effective as of March 14, 2016 (the “Shortall Agreement”). Under the Shortall Agreement, such 400,000 unvested restricted shares became fully vested. The Company recorded $3.5 million in share-based compensation expense relating to this vesting and reversed $3.3 million in expense which related to the original grant.
The following is a summary of activity related to restricted stock awards and units during the fiscal year ended June 30, 2016:
|
|
|
Number of Restricted Stock Awards |
|
|
Weighted Average Grant Date Fair Value |
|
||
|
Unvested as of July 1, 2015 |
|
|
1,073,185 |
|
|
$ |
28.83 |
|
|
Granted |
|
|
623,973 |
|
|
|
4.48 |
|
|
Vested |
|
|
(712,687 |
) |
|
|
23.17 |
|
|
Cancelled |
|
|
(101,523 |
) |
|
|
35.66 |
|
|
Unvested as of June 30, 2016 |
|
|
882,948 |
|
|
$ |
15.41 |
|
The total fair value of shares vested during the fiscal years ended June 30, 2016, 2015 and 2014 was $16.5 million, $3.4 million and $6.0 million, respectively.
Preferred Stock Purchase Agreement
On November 9, 2015, the Company entered into and closed a Preferred Stock Purchase Agreement (the “Preferred Stock Purchase Agreement”) with a Cayman Islands exempted mutual fund (the “Fund”). Pursuant to the Preferred Stock Purchase Agreement, the Company issued and sold to the Fund 790 shares of the Company’s newly designated Series A Redeemable Convertible Preferred Stock of the Company, par value $0.01 per share (the “Series A Preferred Stock”), at a 5% original issue discount and at a purchase price of $10,000 per share for total gross proceeds to the Company of $7.5 million. Prior to the full conversion of the Series A Preferred stock (as more fully discussed below), the Series A Preferred Stock was convertible into shares of Common Stock at a fixed conversion price of $10.00 per share (the “Conversion Price”). The shares of Series A Preferred Stock were offered and sold in a registered direct offering (the “Offering”) pursuant to the Company’s shelf registration statement (File No. 333-197122), which was declared effective by the SEC on October 3, 2014.
From the date of issuance, each share of Series A Preferred Stock accrued dividends at a rate of 8.0% per annum (the “Dividend Rate”), subject to adjustment as discussed below, on its face value of $10,000 (the “Face Value”), payable upon conversion or redemption of such share and when, as and if otherwise declared by the Board. Dividends were paid either in cash or in shares of Common Stock at the Company’s sole discretion and were valued at (i) if there was no Trigger Event (as defined below), (A) 95.0% of the average of the 5 lowest individual daily volume weighted average prices of the Common Stock on the Trading Market during the applicable Measurement Period, which may be non-consecutive, less $0.50 per share of Common Stock, not to exceed (B) 100% of the lowest sales price on the last day of such Measurement Period less $0.50 per share of Common Stock or (ii) following any Trigger Event, (A) 80.0% of the lowest daily volume weighted average price during any Measurement Period for any conversion by Holder, less $1.00 per share of Common Stock, not to exceed (B) 80.0% of the lowest sales price on the last day of any Measurement Period, less $1.00 per share of Common Stock. “Trigger Event” is defined as including, among other events, our breach of the Certificate of Designations and any transaction documents, the occurrence of certain defaults under our material agreements, the suspension of our NASDAQ listing, bankruptcy, the appointment of a receiver, our failure to timely file any report under the Securities Exchange Act of 1934, as amended, or the unenforceability of any material provision of the Certificate of Designations. “Trading Market” is defined as the principal trading exchange or market for the Common Stock. “Measurement Period” is defined as the period beginning on the date of issuance of any such shares of Series A Preferred Stock and ending, if no Trigger Event has
75
occurred 3 trading days, and if a Trigger Event has occurred 30 trading days, after the number of shares have been delivered with respect to a conversion notice.
The Dividend Rate was adjusted (i) downward by an amount equal to 100 basis points for each amount, if any, equal to $0.50 per share of Common Stock that the volume weighted average price of our Common Stock on any trading day rose above $15.00, down to a minimum of 0.0%; and (ii) upward by an amount equal to 150 basis points for each amount, if any, equal to $0.50 per share of Common Stock that volume weighted average price of our Common Stock on any trading day fell below $7.00, up to a maximum of 15.0%. In addition, the Dividend Rate was adjusted upward by 10.0% upon any Trigger Event.
Each share of Series A Preferred Stock was convertible into such number of shares of Common Stock equal to the Face Value divided by the Conversion Price. Upon any conversion, the Company issued Common Stock at the Conversion Price and paid the dividend and conversion premium (“Dividend”) (in one instance in cash and the remaining instances in stock at the Company’s discretion). The Company was prohibited from issuing shares of Common Stock upon conversion of the Series A Preferred Stock if, as a result of the conversion, the holder, together with its affiliates, would beneficially own more than 4.99% of the total number of shares of the Company’s Common Stock then issued and outstanding, subject to adjustment up to 9.99% upon 61 days’ notice from the investor, which is referred to herein as the “Beneficial Ownership Limitation”. The Preferred Stock Purchase Agreement also contains representations, warranties and covenants customary for transactions of this type.
In November 2015 and December 2015, the Fund delivered to the Company notices of conversion totaling an aggregate of 300 shares of Series A Preferred Stock (the “Initial Conversion Notices”) and the Company issued an aggregate of 1,025,499 shares of Common Stock and paid $0.3 million in cash to satisfy the Initial Conversion Notices. Calculations in the Initial Conversion Notices were based upon the occurrence of a Trigger Event.
As described above, the amount of any Dividend varied based on the Company’s share price during the applicable Measurement Period. If the Company’s share price declined during the Measurement Period with respect to a conversion notice, the number of shares owed to the Fund pursuant to such conversion notice would have changed and the Company was then required to issue the additional shares owed. During December 2015, the Company issued an additional 518,784 shares of Common Stock as additional Dividend with respect to the Conversion Notices as a result of a decline in the share price during the applicable Measurement Periods.
On January 4, 2016, the Fund delivered to the Company a notice of conversion for 40 shares of Series A Preferred Stock (the “January 4th Conversion Notice” and together with the Initial Conversion Notices, the “Conversion Notices”) and the Company issued the Fund 246,036 shares of Common Stock. During January 2016, the Company issued an additional 162,706 shares of Common Stock as additional Dividend with respect to the Conversion Notices as a result of a decline in the share price during the applicable Measurement Periods.
On February 3, 2016, Company entered into the First Amendment to the Preferred Stock Purchase Agreement with the Fund. Pursuant to the First Amendment to the Preferred Stock Purchase Agreement, the Company acknowledged that the Fund had at all times fully and completely complied with all of its obligations under the Preferred Stock Purchase Agreement. The Fund has converted all of the Preferred Shares, and the parties entered into the First Amendment to the Preferred Stock Purchase Agreement to resolve the final and total of number shares of the Company’s Common Stock to be delivered by the Company to the Fund as a result of the conversion.
Pursuant to the First Amendment to the Purchase Agreement, in full accord and satisfaction of all obligations under the Purchase Agreement and the remaining transaction documents (as defined in the Preferred Stock Purchase Agreement), the Company agreed to issue to the Fund an additional 831,668 shares (collectively, the “Shares”) of Common Stock, the approximate amount that may be issued under Nasdaq Listing Rule 5635(d) without shareholder approval which the Company did not obtain. On February 3, 2016, the Company issued and delivered to the Fund 725,000 of the Shares. On February 11, 2016, the Company issued and delivered to the Fund the remaining 106,668 Shares.
Pursuant to the First Amendment to the Purchase Agreement, the Company has no further obligations to the Fund with respect to any of the Series A Preferred Stock, Conversion Notices (as defined in the Company’s Certificate of Designations of Preferences, Rights and Limitations of Series A Preferred Stock) or any of the transaction documents. The Company issued 2,784,693 shares of Common Stock to the Fund in connection with the Preferred Stock Purchase Agreement, as amended by the First Amendment to the Preferred Stock Purchase Agreement. The Fund is no longer the holder of any Series A Preferred Stock.
The First Amendment to the Preferred Stock Purchase Agreement contained a mutual release of claims between the Company and the Fund and contained customary representations and warranties made by such parties. The Company also agreed to provide the Fund with indemnification for breaches of the First Amendment to the Preferred Stock Purchase Agreement and for certain third-party claims, and the Fund agreed to continue the same activity restrictions provided for in the Preferred Stock Purchase Agreement.
76
The Company accounted for the Series A Preferred Stock and the related Dividend as two separate units, i.e. Series A Preferred Stock and Preferred Stock Conversion. The Company determined that the Series A Preferred Stock should be classified as temporary equity based on the requirement to provide registered shares of the Company’s Common Stock upon conversion and the related Dividend should be classified as a liability at fair value. Accordingly, the proceeds recorded as temporary equity for the Series A Preferred Stock represented the proceeds from the issuance less initial fair value of Preferred Stock Conversion and related issuance costs. As a result, on November 9, 2015, the Company recorded the net proceeds of $7.2 million between the Series A Preferred Stock ($2.8 million) and the initial Preferred Stock Conversion at its fair value ($4.4 million). After accounting for all Conversion Notices and First Amendment to the Preferred Stock Purchase Agreement, the Redeemable Convertible Preferred Stock, Series A, was reclassed from temporary equity to permanent equity and is valued at $0.0 million at June 30, 2016. The Preferred Stock Conversion was remeasured periodically and the Company recorded a charge of $4.2 million during fiscal year 2016 through change in fair value of financial instruments . At June 30, 2016, the Preferred Stock Conversion was valued at $0.0.
6. Property, Plant and Equipment
Property, plant and equipment consist of the following:
|
|
|
June 30, 2016 |
|
|
June 30, 2015 |
|
||
|
|
|
(in thousands) |
|
|||||
|
Building |
|
$ |
32,362 |
|
|
$ |
32,359 |
|
|
Machinery and equipment |
|
|
19,537 |
|
|
|
27,530 |
|
|
Computer software |
|
|
2,986 |
|
|
|
2,910 |
|
|
Furniture and fixtures |
|
|
1,386 |
|
|
|
1,345 |
|
|
Construction in progress |
|
|
13,870 |
|
|
|
17,601 |
|
|
Land |
|
|
2,036 |
|
|
|
2,036 |
|
|
Leasehold improvements |
|
|
437 |
|
|
|
270 |
|
|
|
|
|
72,614 |
|
|
|
84,051 |
|
|
Less: accumulated depreciation and amortization |
|
|
(17,841 |
) |
|
|
(17,903 |
) |
|
Property, plant and equipment, net |
|
$ |
54,773 |
|
|
$ |
66,148 |
|
Under ASC 360 Property, Plant, and Equipment, the Company is required to evaluate the recoverability of the carrying amount of its long-lived assets whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. In February 2016, the Company completed its review of strategic alternatives and announced the formation of a strategic collaboration centered upon the use of the Company’s portfolio of prefilled, customizable wearable injectors. In connection with this focus, the Company has been evaluating the prospects of its non-wearable injector customer and supplier programs. As a result of negotiations related to those supplier and customer programs through September 1, 2016 and the Company’s evaluation of those programs and potential disposition of certain assets, the Company determined that certain of its long lived-assets are impaired. The Company incurred $26.6 million of non-cash asset impairments to machinery and equipment and construction in progress during the fiscal year ended June 30, 2016. The impairment was based on the future expected use of certain equipment in the production of its product. The equipment is customized for the production of medical devices and as a result of the decision not to continue to produce certain products the related equipment was written-off as any residual salvage value was determined to be nominal, if any.
Construction in progress as of June 30, 2016 and 2015 consisted of amounts incurred in connection with machinery and equipment and facility related costs, including capitalized interest. Interest capitalized during the fiscal years ended June 30, 2016 and 2015 was $0.4 million and $2.2 million, respectively. There was no capitalized interest during the fiscal year ended June 30, 2014.
7. Goodwill
The changes in the carrying amount of goodwill during the fiscal years ended June 30, 2016 and 2015 are as follows:
|
|
|
(in thousands) |
|
|
|
Balance as of July 1, 2014 |
|
$ |
11,830 |
|
|
Foreign currency translation |
|
|
(2,145 |
) |
|
Balance as of June 30, 2015 |
|
|
9,685 |
|
|
Foreign currency translation |
|
|
(262 |
) |
|
Balance as of June 30, 2016 |
|
$ |
9,423 |
|
77
Accrued expenses consist of the following:
|
|
|
June 30, 2016 |
|
|
June 30, 2015 |
|
||
|
|
|
(In thousands) |
|
|||||
|
Accrued payroll and other employee related expenses |
|
$ |
2,755 |
|
|
$ |
2,781 |
|
|
Accrued cost related to construction in process |
|
|
2,623 |
|
|
|
314 |
|
|
Accrued transaction costs |
|
|
5,000 |
|
|
|
— |
|
|
Accrued other |
|
|
3,332 |
|
|
|
1,979 |
|
|
Total accrued expenses |
|
$ |
13,710 |
|
|
$ |
5,074 |
|
The Company recorded $5.0 million in transaction costs during fiscal year 2016 relating to the Strategic Process.
9. Commitments and Contingencies
The Company leases certain facilities, office equipment and automobiles under non-cancellable operating leases that expire on various dates through June 2022. The future minimum lease payments related to the Company’s non-cancellable operating lease commitments net of subleased income related to our King of Prussia offices, that have initial or remaining non-cancellable lease terms in excess of one year as of June 30, 2016 were as follows:
|
For the Year Ending June 30, |
|
(In thousands) |
|
|
|
2017 |
|
$ |
891 |
|
|
2018 |
|
|
716 |
|
|
2019 |
|
|
845 |
|
|
2020 |
|
|
1,283 |
|
|
2021 |
|
|
1,309 |
|
|
Thereafter |
|
|
1,330 |
|
|
|
|
$ |
6,374 |
|
In June 2016, we subleased a portion (the “Subleased Portion”) of our King of Prussia offices. During the term of the sublease, which commenced on October 1, 2016 and will end on March 31, 2019, the Company will receive an aggregate of approximately $1.3 million in rent with respect to the Subleased Portion. During the same time period, the Company will be obligated under the Company’s lease agreement relating to our King of Prussia offices to pay an aggregate of approximately $1.9 million in rent with respect to the Subleased Portion. Assuming the sublessor exercises its renewal option, the Company will receive approximately an additional $1.9 million over the renewal term of April 1, 2019 through June 30, 2022 and the Company will be obligated under the Company’s lease agreement relating to the King of Prussia Facility to pay an aggregate of approximately $2.5 million over the same time period. The Company ceased using the Subleased Portion as of July 20, 2016.
Rental expenses under operating leases during the fiscal years ended June 30, 2016, 2015 and 2014 were $1.2 million, $0.9 million and $0.6 million, respectively.
From time to time, the Company is involved in various legal proceedings, claims, suits and complaints arising out of the normal course of business. Based on the facts currently available to the Company, management believes that these claims, suits and complaints are adequately provided for, covered by insurance, without merit or that it is not probable that an unfavorable outcome will result.
In addition, the Company is or was involved in the following legal proceedings. A former employee, Talbot Smith, who was terminated for cause by Unilife, filed a civil complaint in the United States District Court of the Eastern District of Pennsylvania on August 30, 2013, and an amended complaint on March 5, 2014, alleging that he was wrongly terminated in retaliation for making allegations about the Company’s compliance practices. Following the discovery process, Mr. Smith dismissed his claims against the Company with prejudice. In connection with the resolution and dismissal of the action, Mr. Smith agreed to make a payment to the Company to settle counter claims the Company had brought against him. Mr. Smith received no payment as part of the resolution and dismissal of his claims against the Company, his attorney received a reduced portion of her fees from the Company’s insurer, and the matter is now concluded.
As previously disclosed, subsequent to the filing of an OSHA complaint by Mr. Smith, we received a subpoena from the staff of the U.S. Securities and Exchange Commission (the “Staff”) requesting the Company to provide certain information to the Staff, which
78
is generally consistent with the meritless allegations made by Mr. Smith in his OSHA complaint. In his complaint filed in the United States District Court for the Eastern District of Pennsylvania, Mr. Smith stated that he provided the Staff with information about his allegations in July and August 2012. The Company responded to that subpoena and has received additional subpoenas and requests for information from the Staff, requesting additional information consistent with the first subpoena. The Staff has also requested information about public statements made by the Company’s former Chief Executive Officer. The Company has provided the requested information.
On May 8, 2016, the Company announced an investigation into violations of the Company’s policies and procedures and possible violations of laws and regulations by the Company’s former Chief Executive Officer, Alan Shortall, whose employment with the Company ceased on March 11, 2016, and its former Chairman, Jim Bosnjak, who resigned from the Company’s Board of Directors (the “Board”) on August 24, 2015 (the “Investigation”). The Investigation was completed on October 7, 2016, and the Company has reported to the SEC on the Company’s findings from the Investigation, has responded to questions from the Staff regarding the findings, and is cooperating fully with the Staff. To date, the SEC has not indicated whether any fines or penalties will be assessed against the Company in relation to these matters. The Company is unable to predict what action the SEC or other regulatory authority may take, if any, in relation to these matters or the impact, if any, of any such action on the Company’s business, operations, cash flows and/or financial condition. If any fines or penalties are assessed against the Company they may be material.
As previously disclosed, on January 8, 2014, the Company was served with a derivative complaint filed in the Delaware Chancery Court (the “Court”) by Cambridge Retirement System (“Cambridge”), a purported stockholder of the Company, against its then-current Board of Directors to recover allegedly “excessive and wasteful” compensation paid to the non-executive directors since 2010. In June, 2014, pursuant to the Company’s motion to dismiss the complaint, the Court dismissed Cambridge’s complaint with respect to the directors’ equity grants, but denied the motion with respect to their cash compensation. The Company filed an answer to the remaining claims in July 2014 and, in June 2015, the Company and Cambridge entered into a Memorandum of Understanding (“MOU”) agreeing to the basic terms of a non-monetary settlement of the action.
On March 18, 2016, Cambridge agreed to voluntarily dismiss its derivative complaint. No compensation in any form was provided to either Cambridge or its counsel in exchange for its agreement to voluntarily dismiss the lawsuit. Because Cambridge agreed to voluntarily dismiss the lawsuit, the MOU has become null and void and of no further legal effect. On March 18, 2016, the Court entered a stipulated order regarding notice of the proposed dismissal of all claims in the derivative action (the “Proposed Dismissal Order”). On April 18, 2016, the Court entered that stipulation as an order, dismissing the case with prejudice.
On September 14, 2015, UMSI was served with a complaint filed in the Superior Court of the State of Connecticut by Biodel, Inc. (“Biodel”) seeking (1) to temporarily enjoin UMSI from entering into a transaction that would jeopardize the Company’s ability to perform its obligations under the Customization and Commercial Supply Agreement effective April 8, 2013 (as amended, the “First Biodel Agreement”) between Biodel and UMSI; and (2) damages under the Connecticut Unfair Trade Practices Act. Biodel alleged that UMSI had engaged in unfair and deceptive trade practices by purportedly misrepresenting its ability and willingness to satisfy its obligations under the First Biodel Agreement and requesting additional payments from Biodel to satisfy the Company’s obligations.Additionally, Biodel filed a demand for arbitration with the American Arbitration Association (AAA) asserting that UMSI had breached its obligations relating to the timing and scope of its performance under the First Biodel Agreement. The Company filed counterclaims in the arbitration for commercial disparagement and breach of the confidentiality provisions of the agreement.
On September 2, 2016, Biodel, the Company and UMSI entered into an Asset Purchase and License Agreement (the “Second Biodel Agreement”) which provides: (a) for the termination of the First Biodel Agreement; (b) for the grant of an exclusive license for a six-month term to the intellectual property rights related to the Unilife mixing device; (c) a six-month term during which Biodel can exercise an option (the “Option”) to purchase certain assets associated with the First Biodel Agreement for $1.5 million (the “Potential Asset Sale”) and extend Biodel’s license, for fees based on intellectual prosecution and maintenance costs determined on an annual basis; (c) dismissal, with prejudice, of all active proceedings in connection with the litigation and arbitration proceedings pending between Biodel and UMSI. Under the Second Biodel Agreement, each party also releases the other party of all liability, waives all claims with prejudice, and forever holds the other party harmless from any damages arising out of relating to the First Biodel Agreement. Biodel and UMSI each paid their respective attorneys’ fees and UMSI paid no monetary amount to Biodel in connection with this resolution.
On March 24, 2016, Edward Fine filed a complaint against the Company and Unilife Medical Solutions Limited (“UMSL”) in the Superior Court of New Jersey. The complaint alleges that the Company and UMSL are in breach of contract and have been unjustly enriched as a result of UMSL’s failure to pay certain required payments under a consultancy agreement between Mr. Fine and UMSL. Pursuant to the complaint, Mr. Fine is seeking monetary damages in the amount of $288,000 in the aggregate. The Company believes that Mr. Fine’s claims and demands for relief are wholly without merit and the Company is vigorously defending the action. On August 15, 2016, we filed an Answer, Affirmative Defenses and Counterclaims, wherein we asserted counterclaims against Mr. Fine for fraud, civil conspiracy, unjust enrichment, breach of contract, and breach of the implied covenant of good faith and fair
79
dealing arising out of Mr. Fine’s role in certain previously disclosed transactions involving Mr. Fine and Jim Bosnjak, the Company’s former Chairman of the Board. This action is currently in a 450-day discovery period which commenced on July 29, 2016.
On May 26 and 27, 2016, two putative class actions were filed in the United States District Court for the Southern District of New York alleging that in violation of Rule 10b-5 and Section 20(a) of the Securities Exchange Act of 1934, the Company and four individual defendants made false and/or misleading statements and/or failed to disclose: (1) that the Company’s former CEO and former Chairman of the Board of Directors had violated the Company’s policies and procedures and had engaged in violations of law and regulations; (2) that the Company lacked adequate internal controls over accounting and financial reporting; (3) that, as a result, the Company would be unable to file its Quarterly Report on Form 10-Q for the period ended March 31, 2016 by the prescribed filing deadline; and (4) that, as a result of the foregoing, the Company’s financial statements, as well as its statements about the Company’s business, operations, and prospects, were false and misleading and/or lacked a reasonable basis. The putative class actions were brought on behalf of purchasers of the Company’s securities between February 3, 2014 and May 23, 2016. On August 24, 2016, the Court consolidated the two actions, appointed lead plaintiffs and lead counsel, and set a deadline of October 24, 2016 for Plaintiffs to file an amended complaint. The Company intends to vigorously contest this lawsuit.
On July 11, July 28, and August 1, 2016, respectively, derivative complaints were filed in the Court of Common Pleas in York County, Pennsylvania against 11 current or former directors and/or officers, alleging (i) breach of their fiduciary duties, (ii) unjust enrichment, (iii) abuse of control, (iv) gross mismanagement, and (v) corporate waste. The complaints allege, among other things, that the individual defendants breached the fiduciary duties they owed to the Company by (1) grossly mismanaging the Company and perpetuating a variety of self-serving schemes to benefit themselves and other interested parties and (2) making and/or causing the Company to make false/misleading statements or omissions of fact in its public disclosures. The complaints further allege that as a result of this alleged conduct, the Company will lose and expend millions of dollars. The Company intends to vigorously contest these lawsuits.
On August 17, 2016, Kahle Automation, S.r.l. (“Kahle”) filed a complaint against Unilife Medical Solutions, Inc. (“UMS”) in the United States District Court for the District of New Jersey. The complaint alleges that UMS breached contracts with Kahle for Kahle’s supply of automation systems for UMS’s Nexus and Finesse product lines. Kahle seeks monetary damages of $4.2 million which includes alleged damages that we believe are not recoverable, such as $0.9 million for bank fees, and $0.8 million for lost profits. Kahle also seeks injunctive relief enjoining UMS from using the Nexus System and requiring UMS to take delivery of work in process related to the Finesse System. UMS disputes Kahle’s allegations that UMS terminated its agreement with Kahle for the Finesse System. We intend to defend ourselves vigorously against these claims.
The Company believes that depending on the outcome, certain of these matters may have a material impact to the Company or its business.
10. Long-Term Debt
Long-term debt consists of the following:
|
|
|
June 30, 2016 |
|
|
June 30, 2015 |
|
||
|
|
|
(In thousands) |
|
|||||
|
10.25% Term loan, due March 2020 |
|
$ |
57,227 |
|
|
$ |
55,518 |
|
|
Royalty agreement liability |
|
|
5,120 |
|
|
|
9,930 |
|
|
6.00% Senior secured convertible note, due February 2023 |
|
|
29,066 |
|
|
|
— |
|
|
6.00% Mortgage loan, due December 2031 |
|
|
12,370 |
|
|
|
12,812 |
|
|
5.00% Commonwealth of Pennsylvania financing authority loan, due January 2021 |
|
|
1,977 |
|
|
|
2,033 |
|
|
Other |
|
|
— |
|
|
|
142 |
|
|
|
|
|
105,760 |
|
|
|
80,435 |
|
|
Less: unamortized debt issue costs |
|
|
(646 |
) |
|
|
(980 |
) |
|
|
|
|
105,114 |
|
|
|
79,455 |
|
|
Less: current portion of long-term debt |
|
|
(669 |
) |
|
|
(775 |
) |
|
Total long-term debt |
|
$ |
104,445 |
|
|
$ |
78,680 |
|
Term Loan
On March 12, 2014, (the “Closing Date”), Unilife Medical Solutions, Inc., a wholly owned subsidiary of the Company (the “Borrower”), entered into a Credit Agreement with ROS Acquisition Offshore LP (the “Lender”), an affiliate of OrbiMed Advisors
80
(“OrbiMed”) (the “Credit Agreement,” and, as amended the “Amended Credit Agreement” or the “OrbiMed Financing”). Pursuant to and subject to the terms of the Credit Agreement, the Lender agreed to provide term loans to the Borrower in the aggregate principal amount of up to $60.0 million (the “Loans”). A first tranche loan of $40.0 million was drawn on the Closing Date and a further two tranches each of $10.0 million were committed by the Lender and were to be funded on each of December 15, 2014 and June 15, 2015, subject to and in accordance with the terms of the Credit Agreement. On September 30, 2014, the Borrower entered into a First Amendment to the Credit Agreement to accelerate the funding of the two additional tranches pursuant to which it received the proceeds from the first $10.0 million tranche on October 1, 2014 and the proceeds from the second $10.0 million tranche on November 10, 2014.
On October 13, 2015, the Company entered into the Third Amendment to the Credit Agreement, pursuant to which the Lender agreed to provide Borrower under the Amended Credit Agreement, up to an aggregate additional principal amount of $10.0 million, less fees and expenses incurred in connection with the Third Amendment to the Credit Agreement and the Second Amendment to the Royalty Agreement (as defined below). During the fiscal quarter ended December 31, 2015, the Company received the full amount of additional proceeds under the Amended Credit Agreement in the amount of $10.0 million. The Third Amendment to the Credit Agreement also modified the Borrower’s liquidity covenant whereby, under the Amended Credit Agreement, the Borrower is now required to maintain a cash balance of $3.0 million as of October 13, 2015, rather than $5.0 million.
The Loan bears interest at 9.25% per annum plus the greater of three-month LIBOR or 1.0%, payable in cash quarterly and as otherwise described in the Amended Credit Agreement. A default interest rate of 14.25% per annum plus the greater of three-month LIBOR or 1.0% shall apply during the existence of a default under the Amended Credit Agreement. The Loans are interest-only until March 12, 2020 (the “Maturity Date”).
Unless the loan facility is otherwise terminated earlier pursuant to the terms of the Amended Credit Agreement, the Borrower is required to repay in full the unpaid principal amount of the Loans drawn down, together with all accrued and unpaid interest thereon plus a 10.0% repayment premium on the Maturity Date. The Borrower can make voluntary repayments at any time of any unpaid principal amount of the Loans, plus a 10.0% repayment premium. The Borrower must make mandatory prepayments in certain prescribed circumstances, including, without limitation, certain dispositions of assets and certain casualty events. In such events, the Borrower must prepay to Lender 100% of the net cash proceeds received.
The obligations of the Borrower under the Amended Credit Agreement are guaranteed by the Company and each of its subsidiaries and the Amended Credit Agreement is secured by the assets of the Company and its subsidiaries. The security interests granted by Borrower, the Company, Unilife Cross Farm LLC (“Cross Farm”), Unilife Medical Solutions Limited (“UMSL”) and Unitract Syringe Pty Limited (“Unitract Syringe”) are evidenced by, among other things, the Pledge and Security Agreement, dated as of March 14, 2014, by the Borrower, the Company, Cross Farm, UMSL, and Unitract Syringe in favor of Lender, for itself and as agent for Royalty Opportunities S.A.R.L. (“ROS”), the Mortgage and Security Agreement, dated March 12, 2014, by and between Cross Farm and Lender, for itself and as agent of ROS, and the General Security Deed, dated as of March 12, 2014, by Unitract Syringe, UMSL, and the Company in favor of the Lender, for itself and as agent of ROS.
The Amended Credit Agreement also contains certain customary covenants, as well as covenants relating to achieving minimum cash revenue targets at the end of each calendar year which has been eliminated as discussed below, maintaining a minimum liquidity target of $3.0 million, and the execution of certain customer and employment agreements in form and substance satisfactory to lender. In the event of default, Borrower must prepay to Lender any unpaid principal amount of the loans drawn down, together with all accrued and unpaid interest thereon plus a 10.0% repayment premium. An event of default could also result in the Lender enforcing its security over the assets of Borrower, the Company, Cross Farm, UMSL and Unitract Syringe in accordance with the terms of the Amended Credit Agreement and the related security agreements. On June 30, 2015, the Company entered into a Second Amendment to the Credit Agreement to remove the minimum cash revenue target for the six month period ended June 30, 2015. On November 6, 2015, the Borrower received a waiver from the Lender of the minimum cash revenue target for the calendar year ending December 31, 2015.
In connection with entering into the Credit Agreement, the Borrower entered into a Royalty Agreement with ROS which entitles ROS to receive royalty payments.
On October 13, 2015, the Borrower entered into the Second Amendment to the Royalty Agreement (the “Amended Royalty Agreement”) with ROS. Pursuant to and subject to the terms of the Second Amendment to the Royalty Agreement, Borrower has agreed to pay ROS 4.52% on the first $50.0 million of net sales in each fiscal year, plus 1.75% of net sales in excess of $50.0 million and up to and including $100.0 million in each fiscal year, plus 0.438% of net sales in excess of $100.0 million in each fiscal year, up from 3.875%, 1.50% and 0.375%, respectively. Borrower continues to have the right to buy out the Amended Royalty Agreement at any time on or before March 12, 2018 at a reduced amount; however, under the Amended Royalty Agreement, the buy-out amounts have increased. On March 13, 2017 and on March 13, 2018, the buy-out amount increases up to a maximum of approximately $37.2 million under the Second Amendment to the Royalty Agreement, as compared to approximately $26.3 million under the First
81
Amendment to the Credit Agreement. The buy-out amount varies based on when the buy-out option is exercised and would, in each case, be reduced by amounts previously paid by Borrower to ROS pursuant to the Amended Royalty Agreement. In the event of default under the Amended Credit Agreement, OrbiMed will have a put option that will make the royalty amounts due immediately. The Amended Royalty Agreement has a term commencing on March 12, 2014 and ending on the earlier of (i) March 12, 2024 and (ii) the date of payment of the purchase price pursuant to the exercise of a put option by the Lender or the exercise of a buy-out option by the Borrower. As the Company has elected to value the Amended Royalty Agreement at fair value, the put option feature does not meet the criterion of ASC 815-15-25-1b and thus is not separated from the host contract and accounted for as a derivative instrument.
On December 31, 2015, the Borrower entered into a Fourth Amendment to the Credit Agreement with the Lender. Pursuant to and subject to the terms of the Fourth Amendment to the Credit Agreement, the Lender agreed to defer the due date for the December 31, 2015 interest payment (in the amount of $1.7 million) (the “Interest Payment) to February 5, 2016. Additionally, the Borrower agreed to pay interest on such deferred amount from December 31, 2015 at the rate set forth in the Amended Credit Agreement and to pay all fees and expenses incurred by the Lender in connection with the Fourth Amendment to the Credit Agreement.
On January 31, 2016, the Borrower entered into the Fifth Amendment to the Credit Agreement with the Lender. Pursuant to and subject to the terms of the Fifth Amendment to the Credit Agreement, the Lender agreed to further defer the due date for the Interest Payment to February 9, 2016. Additionally, the Borrower agreed to pay interest on such deferred amount from December 31, 2015 at the rate set forth in the Amended Credit Agreement and to pay all fees and expenses incurred by the Lender in connection with the Fifth Amendment to the Credit Agreement.
On January 31, 2016, the Borrower entered into the Third Amendment to the Royalty Agreement with ROS. The Third Amendment to the Royalty Agreement became effective as of January 29, 2016. Pursuant to and subject to the terms of the Third Amendment to the Royalty Agreement, ROS agreed to defer the due date for (i) $0.1 million of the January 30, 2016 royalty payment to February 1, 2016, and (ii) $0.7 million of the January 30, 2016 royalty payment to February 9, 2016.
On February 9, 2016, the Borrower entered into the Sixth Amendment to the Credit Agreement with the Lender. Pursuant to and subject to the terms of the Sixth Amendment to the Credit Agreement, the Lender agreed to further defer the due date for the Interest Payment to February 16, 2016. Additionally, the Borrower agreed to pay interest on such deferred amount from December 31, 2015 at the rate set forth in the Amended Credit Agreement and to pay all fees and expenses incurred by the Lender in connection with the Sixth Amendment to the Credit Agreement.
On February 9, 2016, the Borrower entered into the Fourth Amendment to the Royalty Agreement with ROS. Pursuant to and subject to the terms of the Fourth Amendment to the Royalty Agreement, ROS agreed to defer the due date for $0.7 million of the January 30, 2016 royalty payment to February 16, 2016.
On February 16, 2016, the Borrower entered into the Seventh Amendment to the Credit Agreement with the Lender. Pursuant to and subject to the terms of the Seventh Amendment to the Credit Agreement, the Lender agreed to further defer the due date for the Interest Payment to February 26, 2016. Additionally, the Borrower agreed to pay interest on such deferred amount from December 31, 2015 at the rate set forth in the Amended Credit Agreement and to pay all fees and expenses incurred by the Lender in connection with the Seventh Amendment to the Credit Agreement.
On February 16, 2016, the Borrower entered into the Fifth Amendment to the Royalty Agreement with ROS. Pursuant to and subject to the terms of the Fifth Amendment to the Royalty Agreement, ROS agreed to defer the due date for $0.7 million of the January 30, 2016 royalty payment to February 26, 2016.
On February 22, 2016, the Borrow entered into the Eighth Amendment to the Credit Agreement with the Lender. Pursuant to and subject to the terms of the Eighth Amendment to the Credit Agreement, the Lender agreed to, among others, (i) defer all obligations of the Borrower to pay interest to the Lender for the period from January 1, 2016 through the two year anniversary of the Counterparty Closing Date at the rate specified in the Amended Credit Agreement, which interest will be added to the outstanding principal amount of the loan on the last day of each interest period; (ii) enable the Counterparty to take a security interest in certain inventory and intellectual property assets related to a specific device licensed to the Counterparty (the “Collateral”); and (iii) remove the minimum cash receipts covenant for all future periods.
On February 22, 2016, the Borrower entered into the Sixth Amendment to the Royalty Agreement with ROS. Pursuant to and subject to the terms of the Sixth Amendment to the Royalty Agreement, ROS agreed to waive any rights to royalty payments otherwise payable as a result of the License Fee and the proceeds of the Notes, and to defer royalty payments payable on revenues received by the Company from the Counterparty until after the end of the first fiscal quarter in which the Company sells a commercial quantity of devices developed for the Counterparty.
82
In connection with entering into the Eighth Amendment to the Credit Agreement and the Sixth Amendment to the Royalty Agreement, the Company issued to ROS warrants to purchase 1,673,981 share of Common Stock, with an exercise price of $12.50 per share, subject to adjustment for certain events, which may be exercised at any time and from time to time until February 22, 2026. In respect to the consideration provided to ROS in the form of warrants and the terms of the Eighth Amendment to the Credit Agreement and the Sixth Amendment to the Royalty Agreement, the Company evaluated whether the debt was modified or extinguished pursuant to ASC 470-50, Debt – Modifications and Extinguishments. The Company determined that the previous debt was extinguished and recorded the modified debt at fair value ($51.3 million). The Company recorded a gain on debt extinguishment for the fiscal year ended June 30, 2016 of $2.9 million which consisted of the remeasurement of the debt at fair value offset by the value of the warrants as of the Counterparty Effective Date and the deferred financing costs previously associated with the term loan.
Except for the requirement to timely file the March 2016 10-Q, the failure to maintain an effective registration statement, and the failure to timely file documents, which non-compliance was subsequently waived, the Company was in compliance with all the loan covenants set forth in the Amended Credit Agreement as of June 30, 2016.
On September 30, 2016, the Borrower entered into a letter agreement (the “OrbiMed Letter Agreement”) with the Lender pursuant to which the Lender agreed to waive (a) the requirements in Sections 7.1(c) and 7.1(d) of the Amended Credit Agreement for the Borrower to provide audited financial statements of the Company together with certain other information within 90 days after the end of the Company’s fiscal year ended June 30, 2016, provided that the Borrower furnishes such information by the earlier of (i) November 7, 2016, and (ii) five business days of when the Company files the 2016 10-K with the SEC, and (b) any “Event of Default” that has occurred or would occur under Section 9.1(c) of the Amended Credit Agreement, solely as a result of thereof.
Pursuant to the OrbiMed Letter Agreement, the Lender also agreed to waive any “Event of Default”, if any, through September 30, 2016 under Section 9.1(c) of the Amended Credit Agreement as a result of any failure to furnish the Lender notice of any new Material Agreement (as defined in the Amended Credit Agreement) or amendments or terminations of Material Agreements within the timeframe set forth in Section 7.1(m) of the Amended Credit Agreement, but only to the extent that that Borrower provided any such notices to the Lender prior to September 30, 2016.
The Borrower made certain representations and warranties to the Lender in the OrbiMed Letter Agreement with respect to the content of the Amgen Letter Agreement. In reliance on such representations and warranties, the Lender waived any Event of Default under Section 9.1(f) of the Amended Credit Agreement that may have occurred as a result of any breach of Section 6.3 of the Counterparty SPA, solely due to the Company’s delay in timely filing the Securities Filings with the SEC. There were no other changes to the terms of the Amended Credit Agreement in connection with the OrbiMed Letter Agreement.
The Borrower and ROS entered into a letter agreement dated October 20, 2016 (the “ROS Letter Agreement”), pursuant to which ROS agreed, until 11:59 p.m. New York City time on July 1, 2017, to waive all rights under the Amended Credit Agreement and the other Loan Documents (as defined in the Amended Credit Agreement) to declare an “Event of Default” or other breach under such documents as a result of the Company’s or the Borrower’s failure to maintain an effective registration statement, as required by the warrant issued by the Company to ROS, dated February 22, 2016, to purchase 1,673,981 shares of Common Stock. Pursuant to the ROS Letter Agreement, ROS acknowledged and agreed that the Borrower’s receipt of notice of the effectiveness of a registration statement would cure any such breach.
In accordance with the ROS Letter Agreement, ROS also agreed to waive all rights under the Amended Credit Agreement and the other Loan Documents to declare an “Event of Default” or other breach under such documents as a result of the Company’s or the Borrower’s failure to timely file documents (the “Delayed Filings”) with the SEC or ASX since February 22, 2016. Pursuant to the ROS Letter Agreement, ROS acknowledged and agreed that the filing by the Borrower of the Delayed Filings with the SEC and the ASX will cure any such breach so long as the Borrower files such Delayed Filings by November 7, 2016.
The Company determined that the Amended Credit Agreement and the Amended Royalty Agreement should be accounted for as two separate units. Accordingly, the Company allocated the proceeds from the Loans on a residual basis between the two units based on their relative fair values. As a result, the royalty liability was determined to have a fair value of $7.0 million and the initial $40.0 million provided under the Credit Agreement was allocated to the remaining proceeds of $33.0 million. The $20.0 million from the two additional tranches that were funded during fiscal year 2015 and the $10.0 million received during fiscal year 2016 were reflected as incremental debt. The carrying value of the debt will be accreted to the face value over the loan term based on the effective interest rate. The royalty liability will be adjusted to fair value on a quarterly basis. As of June 30, 2016, the fair value of the royalty liability was $5.1 million.
There are cross-default provisions in the Amended Credit Agreement, Metro Bank loan (as described below) and Keystone/CFA Loan (as described below), so that a default under one agreement could trigger a default under the others. Metro Bank, the Lender under the Amended Credit Agreement, Keystone Redevelopment Group, LLC and Commonwealth Financing Authority are parties to an intercreditor agreement.
83
Senior Secured Convertible Note
On February 22, 2016, the Company and certain of its subsidiaries entered into a Securities Purchase Agreement (the “Counterparty SPA”) with Amgen Inc. (the “Counterparty”), pursuant to which Counterparty agreed to purchase from the Company a new series of 6% Senior Secured Convertible Notes Due 2023 in the aggregate original principal amount of up to $55.0 million (the “Notes”). The Notes may be issued in up to three separate closings. The Company issued to Counterparty the first Note in the aggregate original principal amount of $30.0 million on February 22, 2016 (the “2016 Convertible Note”) and Counterparty paid to the Company $30.0 million in exchange therefor. Counterparty may purchase up to an additional $25.0 million in Notes over the next two years, $15.0 million of which may be purchased in January 2017 (the “2017 Convertible Note”) and $10.0 million of which may be purchased in January 2018 (the “2018 Convertible Note”). There can be no assurance that Counterparty will elect to purchase the 2017 Convertible Note and/or the 2018 Convertible Note.
Interest under the 2016 Convertible Note accrues at a rate of 6% per year and will be paid quarterly in arrears through the addition of the amount of such interest to the then outstanding principal amount. All or part of the principal and accrued interest will be repaid through (i) discounted pricing on purchases by the Counterparty of the Company’s products, (ii) credits taken by the Counterparty against development and customization fees for devices, and (iii) credits against per-unit royalties otherwise payable to the Company for the manufacture and sale of the Company’s products. In addition, the Company has the right to prepay in cash all or part of the principal and accrued interest at any time upon 15 business days’ prior notice, subject to the Counterparty’s conversion right with respect to the contemplated prepayment amount. The Company is required to pay in cash any amounts of principal and accrued interest outstanding at the Counterparty Maturity Date.
The 2016 Convertible Note is convertible at the Counterparty’s election into shares of Common Stock at any time after February 22, 2016 and prior to the Counterparty Maturity Date, at a price per share that is 90% of the volume weighted average price of such shares during the twenty (20) trading days preceding the applicable conversion date (the “Discounted Sale Price”), subject to a floor price of $12.50 per share (the “Conversion Rate Floor Price”). The Conversion Rate Floor Price is subject to customary adjustments for certain capital events.
The Counterparty may cause the redemption of the 2016 Convertible Note upon any event of default by the Company. Events of default under the 2016 Convertible Note include, among others, a failure by the Company to convert the 2016 Convertible Note upon proper notice by the Counterparty or pay principal and interest on the 2016 Convertible Note when due; an acceleration of any other indebtedness under the Amended Credit Agreement or other indebtedness of the Company in excess of $1.0 million; a bankruptcy of the Company; a judgment against the Company in excess of $1.0 million; a representation or warranty made in the Transaction Documents is materially false or misleading when made; a material breach by the Company of a covenant or other term or condition in the Transaction Documents; the Transaction Documents cease to be effective; the termination or amendment of the Eighth Amendment to the Credit Agreement or the Sixth Amendment to the Royalty Agreement; and the incurrence of a lien on collateral that is not a permitted lien. The Company is required to redeem for cash the 2016 Convertible Note upon a change of control of the Company in an amount equal to 101% of the aggregate principal and accrued interest outstanding as of the change of control.
The 2016 Convertible Note also provides the Counterparty with certain rights to acquire additional shares of Common Stock or other securities or assets of the Company, as applicable, in the event: (i) the Company grants, issues or sells any options, convertible securities or rights to purchase stock, warrants, securities or other property pro rata to the holders of Common Stock; or (ii) the Company makes certain other distributions to Company stockholders such that, in the case of (i) or (ii), the Counterparty receives, in addition to the shares of Common Stock otherwise issuable upon conversion of the 2016 Convertible Note, the shares of Common Stock or other securities or assets, as applicable, that the Counterparty would have been entitled to receive if the Counterparty had converted the 2016 Convertible Note into Common Stock immediately prior to such event.
The 2016 Convertible Note is secured by the Collateral. The Counterparty has agreed to preserve license rights granted to other customers for any license rights granted prior to a foreclosure. The terms and conditions of the 2017 Convertible Note and the 2018 Convertible Note, if purchased by the Counterparty, are substantially the same as the terms and conditions of the 2016 Convertible Note, except that the “Conversion Rate Floor Price” will be the greater of (x) $12.50, (y) the closing sale price of the Common Stock on the trading day preceding the issuance date, and (z) the book value per share of Common Stock on the trading day immediately preceding the issuance date.
The Company determined that the conversion feature should be accounted for as a stock put option and would be bifurcated from the value of the 2016 Convertible Note and treated as a derivative liability. The initial fair value of the derivative liability was determined to be $1.7 million. The fair value of this liability will be adjusted to fair market value on a recurring basis. As of June 30, 2016, the fair market value was determined to be $0.3 million.
The Counterparty may purchase up to an additional $15.0 million in Notes in January 2017, and up to an additional $10.0 million in Notes in January 2018.
84
On September 29, 2016, the Company and the Counterparty entered into a letter agreement (the “Counterparty Letter Agreement”). Pursuant to the Counterparty Letter Agreement, Amgen agreed (i) until 11:59 p.m. New York City time on July 1, 2017, to waive any and all rights whatsoever that the Counterparty has or may have under the Securities Purchase Agreement that the Company entered into with Counterparty in February 2016 (the “Counterparty SPA”) and certain related transaction documents to declare an “Event of Default” under the 2016 Convertible Note as a result of the Company’s failure to timely file the March 2016 10-Q or the 2016 10-K (together, the “Securities Filings”); and (ii) that the filing by the Company of the Securities Filings with the SEC will cure any breach of Section 6.3 of the Counterparty SPA as a result of the Company’s failure to timely file the Securities Filings with the SEC.
Under section 6.3 of the Counterparty SPA, the Company is required to, until the date on which the Counterparty has sold all the shares of the Company’s common stock into which the Notes are convertible (the “Conversion Shares”) and none of the Notes are outstanding, (i) timely file all reports required to be filed with the SEC pursuant to the Securities Exchange Act of 1934 (the “Exchange Act”) or the rules and regulations thereunder and (ii) not take any action or file any document (whether or not permitted by the Securities Act of 1933 (the “Securities Act”) or the rules promulgated thereunder) to terminate or suspend the Company’s reporting and filing obligations under the Exchange Act or Securities Act, (iii) take all actions necessary to maintain the Company’s eligibility to register the Conversion Shares for resale by the Counterparty on Form S-3, and (iv) use its commercially reasonable efforts to take all action as may be required as a condition to the availability of Rule 144 under the Securities Act with respect to the Company’s common stock.
Mortgage Loans
In October 2010, Cross Farm entered into the Loan Agreement with First National Bank (formerly known as Metro Bank), pursuant to which First National Bank provided Cross Farm with two mortgage loans in the amounts of $14.25 million (“First Mortgage”) and $3.75 million (“Second Mortgage”). The proceeds received were used to finance the purchase of land and construction of the Company’s corporate headquarters and manufacturing facility in York, Pennsylvania. In connection with the credit agreement, the Company entered into the Metro Bank Amendment pursuant to which the Second Mortgage due October 2020 was repaid. Cross Farm is paying principal and interest on the First Mortgage, with interest at a fixed rate of 6.00%.
The original First National Bank loan documents contain certain customary covenants, including the maintenance of a debt service reserve account in the amount of $2.4 million, classified as restricted cash on the consolidated balance sheets, which will remain in place until Cross Farm and First National Bank agree on the financial covenants. In addition the Company is required to maintain a cash balance of $5.0 million inclusive of the $2.4 million reserve account. The terms of the original First National Bank loan documents allow the Company to use the debt service reserve account to pay monthly debt service on the mortgage loans, so long as the balance in the account is at least $1.6 million and is replenished to $2.4 million every six months. The Company was in compliance with its debt covenants as of June 30, 2016. However, there can be no assurance that the Company will be able to maintain the debt service reserve account balance for a period of 12 months from March 31, 2016. Cross Farm may prepay the loan without penalty. The U.S. Department of Agriculture has guaranteed $8.0 million of the mortgage loan due December 2031. In connection with the First Mortgage, the Company has given First National Bank a lien on the building and real estate and the debt service reserve account.
Secured Lending Facility
In August 2011, the Company entered into a Master Lease Agreement (the “Lease Agreement”) with Varilease Finance, Inc. (“Varilease”) for up to $10.0 million of secured financing for production equipment for its Unifill syringe. Based on the Company’s continuing involvement throughout the term of the agreement and the integral nature of the production equipment, the transaction was being accounted for as a financing. Over the term of the Lease Agreement, the Company made 27 monthly installments based upon the amount drawn. This facility had an effective interest rate of 14.00%. The secured lending facility contained covenants and provisions for events of default customarily found in lease agreements.
As previously disclosed on September 30, 2013, Varilease and CCA Financial LLC (collectively, the “Lessors”) filed an action in the State of Michigan in the Circuit Court for the County of Oakland, Case No. 2013-136458-CK seeking a judgment confirming the terms of the lease. The Company removed the action to the U.S. District Court for the Eastern District of Michigan, Case No. 2:13-CV-14238-SFC-LJM, on October 4, 2013. Under the Lease Agreement, Lessors and the Company were to negotiate a buyout rate at the end of the two-year lease term, which Lessors represented to the Company during the lease negotiations would be 15% of the amount financed. When the Company notified Lessors that it wanted to exercise the buyout of the equipment, Lessors claimed a buyout rate significantly higher than 15%. Under the terms of the lease, if the parties were unable to agree on a buyout rate by the end of the lease term, the lease would automatically renew for an additional 12-month period and the Company would be responsible for another year of lease payments. Lessor’s action in Michigan state court asked the court to confirm that the parties were unable to agree on a buyout rate and therefore under the terms of the lease the lease was automatically extended for one year.
85
As previously disclosed, the Company also filed suit on September 30, 2013 against Lessors in the U.S. District Court for the Eastern District of Michigan, Case No. 2:13-cv-14174-SFC-LJM alleging, among other things, that Lessors fraudulently induced the Company into entering the lease by making misrepresentations about the buyout rate. The Company sought, among other things, to have the federal court enforce a 15% buyout rate and to enjoin Lessors from declaring a default under the lease and taking possession of the equipment for which the Company would have to impair the carrying value of assets. On October 17, 2013, in a stipulated order, the U.S. District Court ordered that the Company continue to make the same monthly payments under the lease, which as long as the Company made timely payments, Lessors shall not declare a default, and that Lessors were required to provide advance notice of a default.
As previously disclosed, the Company entered into a Confidential Mutual Release and Settlement Agreement (the “Definitive Settlement Agreement”), effective December 30, 2013, with the Lessors. The Definitive Settlement Agreement provided that it will obtain title to all equipment under the equipment lease upon the payment to the Lessors of approximately $4.8 million over the next twelve months. In addition, under the Definitive Settlement Agreement the Company and the Lessors released each other from any and all claims related to the companion lawsuits, as well as dismissed such lawsuits. In connection with the Definitive Settlement Agreement, during the year ended June 30, 2014, the Company recognized $3.6 million of interest expense representing the difference between the carrying value of the debt and the present value of the settlement amount.
During the year ended June 30, 2014, the Company paid $4.7 million (including $3.5 million with proceeds from the March 12, 2014 Credit Agreement) to the Lessors in satisfaction of the Company’s remaining obligations under the Definitive Settlement Agreement. Effective March 12, 2014 the Lessors released all liens and security interest in all of the Company’s assets subject to the Lease Agreement.
Commonwealth of Pennsylvania Financing Authority Loan
In December 2010, Cross Farm received a $2.25 million loan from the Commonwealth of Pennsylvania for land and the construction of its current manufacturing facility. The loan bears interest at a rate of 5.00% per annum, matures in January 2021 and is secured by a third mortgage on the facility. In connection with the loan agreement, Cross Farm entered into an intercreditor agreement by which the Commonwealth of Pennsylvania agreed that it would not exercise its rights in the event of a default by Cross Farm without the consent of Metro Bank, which holds the first mortgage on the facility.
Loan from our Former CEO
On September 30, 2015, the Company obtained a loan in the amount of $0.6 million from Alan Shortall, the Company’s former Chairman and Chief Executive Officer. During February 2016, the loan was repaid in full including payment of interest to Mr. Shortall at the minimum applicable federal rate, which interest was less than $0.1 million.
As of June 30, 2016, aggregate maturities of long-term obligations are as follows:
|
For the Year Ending June 30, |
|
(In thousands) |
|
|
|
2017 |
|
$ |
669 |
|
|
2018 |
|
|
780 |
|
|
2019 |
|
|
958 |
|
|
2020 |
|
|
58,679 |
|
|
2021 |
|
|
3,270 |
|
|
Thereafter |
|
|
41,404 |
|
|
|
|
$ |
105,760 |
|
86
The Company’s net loss per share is as follows:
|
|
|
Year Ended June 30, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
|
(In thousands, except share and per share data) |
|
|||||||||
|
Numerator |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(100,783 |
) |
|
$ |
(90,849 |
) |
|
$ |
(57,899 |
) |
|
Deemed dividend on Series A Preferred Stock |
|
|
(1,047 |
) |
|
|
— |
|
|
|
— |
|
|
Net loss attributable to common stockholders |
|
$ |
(101,830 |
) |
|
$ |
(90,849 |
) |
|
$ |
(57,899 |
) |
|
Denominator |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares used to compute basic net loss per share |
|
|
14,467,510 |
|
|
|
11,219,490 |
|
|
|
9,806,267 |
|
|
Effect of dilutive options to purchase common stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Weighted average number of shares used to compute diluted net loss per share |
|
|
14,467,510 |
|
|
|
11,219,490 |
|
|
|
9,806,267 |
|
|
Basic and diluted net loss per share |
|
$ |
(7.04 |
) |
|
$ |
(8.10 |
) |
|
$ |
(5.90 |
) |
Due to the Company’s net losses, unvested shares of restricted stock (participating securities) totaling 850,989, 699,021 and 268,778 were excluded from the calculation of basic and diluted net loss per share during the fiscal years ended June 30, 2016, 2015 and 2014, respectively.
In addition, stock options (non-participating securities) totaling 900,566, 350,060, and 464,273 during the fiscal years ended June 30, 2016, 2015 and 2014, respectively, were excluded from the calculation of diluted net loss per share as their effect would have been anti-dilutive. Certain of these stock options were excluded solely due to the Company’s net loss position. Had the Company reported net income during the fiscal years ended June 30, 2016, 2015 and 2014, these shares would have had an effect of 0, 14,574, and 32,385 diluted shares, respectively, for purposes of calculating diluted net loss per share. The impact of the potential conversion of 2016 Convertible Note of 2,452,237 diluted shares was also excluded from the calculation of diluted net loss per share for the fiscal year ended June 30, 2016 as their effect would have been anti-dilutive.
12. Income Taxes
For the fiscal years ended June 30, 2016, 2015 and 2014, income (loss) before income taxes consists of the following:
|
|
|
Years Ended June 30, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
|
|
(In thousands) |
|
|||||||||
|
Domestic |
|
$ |
(100,011 |
) |
|
$ |
(89,617 |
) |
|
$ |
(58,784 |
) |
|
International |
|
|
(772 |
) |
|
|
(1,232 |
) |
|
|
885 |
|
|
|
|
$ |
(100,783 |
) |
|
$ |
(90,849 |
) |
|
$ |
(57,899 |
) |
Tax Rate Reconciliation
Income tax expense (benefit) is as follows:
|
|
|
Years Ended June 30, |
|
|||||||||||||||||||||||||||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||||||||||||||||||||||||||
|
|
|
Current |
|
|
Deferred |
|
|
Total |
|
|
Current |
|
|
Deferred |
|
|
Total |
|
|
Current |
|
|
Deferred |
|
|
Total |
|
|||||||||
|
|
|
(In thousands) |
|
|||||||||||||||||||||||||||||||||
|
U.S. Federal |
|
$ |
— |
|
|
$ |
(31,335 |
) |
|
$ |
(31,335 |
) |
|
$ |
— |
|
|
$ |
(27,385 |
) |
|
$ |
(27,385 |
) |
|
$ |
— |
|
|
$ |
(18,415 |
) |
|
$ |
(18,415 |
) |
|
State |
|
|
— |
|
|
|
(9,936 |
) |
|
|
(9,936 |
) |
|
|
— |
|
|
|
(8,684 |
) |
|
|
(8,684 |
) |
|
|
— |
|
|
|
(5,839 |
) |
|
|
(5,839 |
) |
|
International |
|
|
— |
|
|
|
(232 |
) |
|
|
(232 |
) |
|
|
— |
|
|
|
(369 |
) |
|
|
(369 |
) |
|
|
— |
|
|
|
266 |
|
|
|
266 |
|
|
Changes in valuation allowance |
|
|
— |
|
|
|
41,503 |
|
|
|
41,503 |
|
|
|
— |
|
|
|
36,438 |
|
|
|
36,438 |
|
|
|
— |
|
|
|
23,988 |
|
|
|
23,988 |
|
|
Income tax provision |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
87
Income tax expense (benefit) was $0 for the fiscal years ended June 30, 2016, 2015 and 2014 and differed from the amounts computed by applying the U.S. federal income tax rate to pretax income (loss) as a result of the following:
|
|
|
Years Ended June 30, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
2014 |
|
|||
|
Tax at U.S. statutory rate |
|
|
(35 |
)% |
|
|
(35 |
)% |
|
|
(35 |
)% |
|
State taxes, net of federal benefit |
|
|
(6 |
)% |
|
|
(6 |
)% |
|
|
(6 |
)% |
|
Non-deductible and non-taxable items |
|
|
— |
|
|
|
1 |
% |
|
|
— |
|
|
Change in valuation allowance |
|
|
41 |
% |
|
|
40 |
% |
|
|
41 |
% |
|
|
|
|
0 |
% |
|
|
0 |
% |
|
|
0 |
% |
Significant Components of Deferred Taxes
The tax effects of temporary differences and net operating losses that give rise to significant portions of deferred tax assets (liabilities) at June 30, 2016 and 2015 are presented below:
|
|
|
June 30, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
|
|
(In thousands) |
|
|||||
|
Net operating loss carryforwards |
|
$ |
150,868 |
|
|
$ |
110,876 |
|
|
Share-based compensation expense |
|
|
27,548 |
|
|
|
20,548 |
|
|
Deferred revenue |
|
|
5,622 |
|
|
|
6,951 |
|
|
Property, plant and equipment |
|
|
5,395 |
|
|
|
4,324 |
|
|
Debt extinguishment |
|
|
(6,196 |
) |
|
|
— |
|
|
Inventory reserves |
|
|
3,596 |
|
|
|
3,416 |
|
|
Accruals/Reserves |
|
|
154 |
|
|
|
101 |
|
|
Valuation allowance |
|
|
(186,987 |
) |
|
|
(146,216 |
) |
|
Net deferred taxes |
|
$ |
— |
|
|
$ |
— |
|
The valuation allowance for deferred tax assets as of June 30, 2016 and 2015 was $187.0 million and $146.2 million, respectively. The net change in the total valuation allowance was an increase of $40.8 million. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible or prior to the expiration of the net operating loss carryforwards. Management considers the scheduled reversal of deferred tax liabilities (including the impact of available carryback and carryforward periods), projected future taxable income, and tax-planning strategies in making the assessment as to the realizability of deferred tax assets. Based upon the level of historical taxable income and uncertainty regarding projections for future taxable income over the periods in which the deferred tax assets are deductible or can be utilized, management does not believe it is more likely than not that the Company will realize the benefits of these net operating losses and deductible temporary differences, as of June 30, 2016 and 2015. Therefore, a full valuation allowance has been provided as of June 30, 2016 and 2015. The amount of the net deferred tax assets considered realizable; however, could change if estimates of future taxable income during the carryforward period are increased.
As of June 30, 2016, the Company had net operating loss carryforwards for U.S federal, state and Australian income tax purposes of approximately $347.0 million, $347.0 million and $23.0 million, respectively, which are available to offset future taxable income. The U.S. federal and state net operating loss carryforwards begin to expire in 2023. The Australian net operating losses do not expire.
The Australian net operating loss carryforwards of approximately $23.0 million as of June 30, 2016 are subject to either the continuity of ownership or same business test (as defined under Australian tax law) that could limit or substantially eliminate the Company’s ability to use these carryforwards. If there have been or will be changes in the Company’s ownership or Australian business operations before these net operating loss carryforwards are utilized, they may be unavailable to reduce taxable income in the future. Further, under provision of the Internal Revenue Code, the utilization of a U.S corporation’s federal and state net operating loss carryforwards may be significantly limited following a change in ownership of greater than 50% within a three-year period. The Company’s federal and state net operating loss carryforwards may, therefore, be subject to an annual limitation. In addition, state net operating loss carryforwards may be further limited in Pennsylvania, which has a limitation equal to the greater of 20% of taxable income after modifications and apportionment, or $5.0 million on state net operating losses utilized in any one year.
Management has evaluated the tax positions taken and has concluded that no liability for unrecognized tax benefits was required to be recorded for the fiscal years ended June 30, 2016, 2015 and 2014.
88
The Company files Australian, U.S. federal and state income tax returns. The Company is not subject to examination in any jurisdiction at this time. As a result of the net operating losses in prior years, the statute of limitations will remain open for a period following any utilization of net operating loss carryforwards and as such these periods remain subject to examination.
13. Employee Benefit Plan
The Company has a retirement savings 401(k) plan covering all U.S. employees (the “Plan”). Participating employees may contribute up to 100% of their pre-tax earnings, subject to the statutory limits. Effective January 1, 2012, the Company began a discretionary match to participant contributions into the Plan. The Company contributes fifty cents for each dollar a participant contributes, with a maximum of 3% of a participant’s eligible earnings. The contributions made by the Company vest 50% upon two years of service and 100% upon three years of service. During the fiscal years ended June 30, 2016, 2015 and 2014, the Company paid $0.4 million, $0.5 million and $0.3 million, respectively, to match employee contributions.
Additionally, during the fiscal year ended June 30, 2015, the Company made a discretionary contribution of 1% of compensation, as defined, to all eligible employees, which amounted to $0.1 million. During the fiscal year ended June 30, 2016 and June 30, 2014, the Company did not make any discretionary contributions.
14. Revenue
The Company recognized $14.8 million, $13.2 million and $14.7 million of revenue during the fiscal years ended June 30, 2016, 2015 and 2014, respectively.
During the fiscal year ended June 30, 2016, three customers accounted for 48%, 22% and 21% of consolidated revenue, respectively. During the fiscal year ended June 30, 2015, three customers accounted for 35%, 32% and 24% of consolidated revenue, respectively. During the fiscal year ended June 30, 2014, three customers accounted for 34%, 23% and 15% of consolidated revenue, respectively.
During the fiscal year ended June 30, 2016, revenue attributed to France accounted for 21% of consolidated revenue. During the fiscal year ended June 30, 2015, revenue attributed to France accounted for 24% of consolidated revenue. During the fiscal year ended June 30, 2014, revenue attributed to Jordan and France accounted for 34% and 15% of consolidated revenue, respectively. All other revenue is attributed to the United States.
During the fiscal year ended June 30, 2016, the Company recognized $4.5 million of revenue, respectively, related to substantive milestones, as follows:
The Company recognized $1.7 million of revenue during the fiscal year ended June 30, 2016 pursuant to a feasibility agreement with a customer related to substantive milestones that were completed and accepted. This agreement provides for certain customization and development activities for a drug delivery system to be performed for the customer and provides for payments to be made upon the completion of agreed-upon substantive milestones. An initial up-front payment of $0.1 million was determined to be non-substantive and was recognized on a straight line basis over the expected term of the agreement. The remaining milestones were determined to be substantive at the time the agreement was entered into. Substantive milestones that were achieved during the fiscal year ended June 30, 2016 are as follows:
|
|
• |
$0.5 million for development and delivery of additional human factor stimuli and a report on updated product requirements; and |
|
|
• |
$1.2 million for development and delivery of semi-functional prototypes and related feasibility, product requirement, and risk management reports. |
There are no remaining substantive milestones under this agreement.
89
The Company recognized $2.1 million of revenue during the fiscal year ended June 30, 2016 pursuant to a master services and supply agreement with a customer related to substantive milestones that were completed and accepted. This agreement provides for certain customization and development activities for a drug delivery system to be performed for the customer and provides for payments to be made upon the completion of agreed-upon substantive milestones. An initial up-front payment of $1.1 million was determined to be non-substantive and is being recognized on a straight-line basis over the expected term of the agreement. The remaining milestones were determined to be substantive at the time the agreement was entered into. Substantive milestones that were achieved during the fiscal year ended June 30, 2016 are as follows:
|
|
• |
$0.6 million for development and delivery of a complete system layout; |
|
|
• |
$0.3 million for development and delivery of components for a human factor study; |
|
|
• |
$0.6 million for development and delivery of feasibility devices for testing; and |
|
|
• |
$0.6 million for development and delivery of a clinical production process. |
The remaining substantive milestones as of June 30, 2016 are as follows:
|
|
• |
$0.4 million for development and delivery of components for a human factor study; |
|
|
• |
$0.4 million for completion of testing of assembly equipment; |
|
|
• |
$0.3 million for completion of filling process of clinical devices; |
|
|
• |
$0.4 million for delivery of containers for the filling process; and |
|
|
• |
$0.3 million for delivery of devices for clinical studies. |
The Company recognized $0.3 million of revenue during the fiscal year ended June 30, 2016 pursuant to a feasibility agreement with a customer related to substantive milestones that were completed and accepted. This agreement provides for certain customization and development activities for a drug delivery system to be performed for the customer and provides for payments to be made upon the completion of agreed-upon milestones. An initial up-front payment of $0.5 million was determined to be non-substantive and was recognized on a straight-line basis over the expected term of the agreement. The remaining milestones were determined to be substantive at the time the agreement was entered into. Substantive milestones that were achieved during the fiscal year ended June 30, 2016 are as follows:
|
|
• |
$0.3 million for development and delivery of a summary report related to testing and documentation activities. |
There are no remaining substantive milestones under this agreement.
The Company recognized $0.4 million of revenue during the fiscal year ended June 30, 2016 pursuant to a master services and supply agreement with a customer related to substantive milestones that were completed and accepted. This agreement provides for certain customization and development activities for a drug delivery system to be performed for the customer and provides for payments to be made upon the completion of agreed-upon substantive milestones. An initial up-front payment of $1.0 million was determined to be non-substantive and was recognized on a straight-line basis over the expected term of the agreement. The remaining milestones were determined to be substantive at the time the agreement was entered into. Substantive milestones that were achieved during the fiscal year ended June 30, 2016 are as follows:
|
|
• |
$0.4 million for development and delivery of feasibility devices for testing; |
The remaining substantive milestones as of June 30, 2016 are as follows:
|
|
• |
$0.6 million for delivery of design transfer for the device and the related filling equipment and fixtures; and |
|
|
• |
$0.3 million for commissioning of the pilot line. |
During the fiscal year ended June 30, 2016, the Company recognized $5.3 million in revenue related to services rendered on a time and materials basis, proportional performance method, the completed contract method and/or straight line basis over the requisite service period pursuant to customer agreements to provide various customization and development services. During the fiscal year ended June 30, 2016, the Company recognized $5.0 million in revenue related to an agreement with a customer to provide exclusivity for a defined period of time to evaluate certain drug delivery alternatives as the requisite exclusivity period expired during the fiscal year.
On December 31, 2015, the Company entered into an exclusivity agreement (the “Exclusivity Agreement”) with Amgen Inc. (the “Counterparty”). Pursuant to the Agreement, the Counterparty paid to the Company a non-refundable $15.0 million license fee
90
(the “First License Fee”). Furthermore on February 22, 2016, the Company granted the Counterparty exclusive rights to the Company’s wearable injectors within select drug classes for use with certain assets, while preserving rights the Company previously granted to other customers. The Company has also granted to the Counterparty non-exclusive rights to all of the Company’s proprietary delivery systems within the therapeutic areas of oncology, inflammation, bone health, nephrology, cardiovascular and neuroscience. The Counterparty paid to the Company an additional non-refundable $20.0 million fee (the “Second License Fee”) in consideration for such licenses. Both the First License Fee and the Second License Fee were recorded in long-term deferred revenue as of June 30, 2016 and were paid as consideration for the following non-exclusive and exclusive rights and licenses:
|
|
• |
The Company granted to the Counterparty a perpetual, worldwide non-exclusive license under the patents, know-how and technology of the Company for the Company to develop, manufacture and supply wearable injector devices existing as of the closing (including any improvements or modified versions) for use with certain large volume drug products of the Counterparty. |
|
|
• |
In addition, the Company granted to the Counterparty a perpetual, worldwide exclusive license under the patents, know-how and technology of the Company for the Company to develop, manufacture and supply the Company’s 1mL wearable injector existing as of the closing (including any improvements or modified version to the same) for use with certain small volume drug products. Except as discussed below, the wearable injector devices will be developed and manufactured by the Company. The Counterparty will be required to pay the Company an amount for each device manufactured by the Company, based on annual volumes and device features. |
In addition to the Exclusivity Agreement, the Company also entered into a Master Development and Supply Agreement with the Counterparty on the Counterparty Closing Date that captures key terms for the development, production and supply of the Company’s delivery systems. The Company has a pre-existing Master Feasibility and Customization Agreement with the Counterparty entered into in the ordinary course of our business on December 2, 2015.
During the fiscal year ended June 30, 2015, the Company recognized $5.1 million of revenue related to substantive milestones, as follows:
The Company recognized $2.7 million of revenue during the fiscal year ended June 30, 2015 pursuant to a feasibility agreement with a customer related to substantive milestones that were completed and accepted during the year. This agreement provides for certain customization and development activities for a drug delivery system to be performed for the customer and provides for payments to be made upon the completion of agreed-upon substantive milestones. An initial up-front payment of $0.1 million was determined to be non-substantive and is being recognized on a straight-line basis over the expected term of the agreement. The remaining milestones were determined to be substantive at the time the agreement was entered into. Substantive milestones that were achieved during the fiscal year ended June 30, 2015 were as follows:
|
|
• |
$0.4 million for development and delivery of a detailed project plan and a failure mode and effects analysis report; |
|
|
• |
$0.4 million for development and delivery of a report on preliminary product requirements and a risk management plan; |
|
|
• |
$1.5 million for development and delivery of human factor stimuli and related supporting documents; and |
|
|
• |
$0.4 million for development and delivery of additional human factor stimuli. |
The remaining substantive milestones as of June 30, 2015 are as follows:
|
|
• |
$0.5 million for development and delivery of additional human factor stimuli and a report on updated product requirements; and |
|
|
• |
$1.2 million for development and delivery of semi-functional prototypes and related feasibility, product requirement, and risk management reports. |
The Company recognized $0.9 million of revenue during the fiscal year ended June 30, 2015 pursuant to a feasibility agreement with a customer related to substantive milestones that were completed and accepted during the year. This agreement provides for certain customization and development activities for a drug delivery system to be performed for the customer and provides for payments to be made upon the completion of agreed-upon milestones. An initial up-front payment of $0.5 million was determined to be non-substantive and is being recognized on a straight-line basis over the expected term of the agreement. The remaining milestones were determined to be substantive at the time the agreement was entered into. Substantive milestones that were achieved during the fiscal year ended June 30, 2015 were as follows:
|
|
• |
$0.5 million for development and delivery of a report on device design options as well as potential manufacturing and assembly processes; and |
|
|
• |
$0.4 million for development and delivery of product samples and related supporting documents. |
91
The remaining substantive milestone as of June 30, 2015 is as follows:
|
|
• |
$0.2 million for development and delivery of a summary report related to testing and documentation activities. |
The Company recognized $0.3 million of revenue during the fiscal year ended June 30, 2015 pursuant to a feasibility agreement with a customer related to substantive milestones that were completed and accepted during the year. This agreement provides for certain customization and development activities for a drug delivery system to be performed for the customer and provides for payments to be made upon the completion of agreed-upon milestones. The milestones were determined to be substantive at the time the agreement was entered into. Substantive milestones that were achieved during the fiscal year ended June 30, 2015 were as follows:
|
|
• |
$0.1 million for development and delivery of a report related to human factor studies and quality requirements; |
|
|
• |
$0.1 million for development and delivery of devices for compatibility and stability functional testing and related reporting; and |
|
|
• |
$0.1 million for development and delivery of devices for human factor study and related reporting. |
There are no remaining substantive milestones under this agreement.
The Company recognized $0.2 million of revenue during the fiscal year ended June 30, 2015 pursuant to a feasibility agreement with a customer related to substantive milestones that were completed and accepted during the year. This agreement provides for certain customization and development activities for a drug delivery system to be performed for the customer and provides for payments to be made upon the completion of agreed-upon milestones. The milestones were determined to be substantive at the time the agreement was entered into. Substantive milestones that were achieved during the fiscal year ended June 30, 2015 were as follows:
|
|
• |
$0.1 million for development of customized devices for testing; and |
|
|
• |
$0.1 million for development and delivery of testing activities and related reporting. |
There are no remaining substantive milestones under this agreement.
The Company recognized $1.0 million of revenue during the fiscal year ended June 30, 2015 pursuant to a master services and supply agreement with a customer related to substantive milestones that were completed and accepted during the year. This agreement provides for certain customization and development activities for a drug delivery system to be performed for the customer and provides for payments to be made upon the completion of agreed-upon substantive milestones. An initial up-front payment of $1.1 million was determined to be non-substantive and is being recognized on a straight-line basis over the expected term of the agreement. The remaining milestones were determined to be substantive at the time the agreement was entered into. Substantive milestones that were achieved during the fiscal year ended June 30, 2015 were as follows:
|
|
• |
$0.2 million for development and delivery of a report defining device requirements; |
|
|
• |
$0.2 million for development and delivery of a report defining system requirements; |
|
|
• |
$0.2 million for development and delivery of devices for testing; |
|
|
• |
$0.2 million for development and delivery of a report defining production requirements; and |
|
|
• |
$0.2 million for development and delivery of components for a human factor study. |
The remaining substantive milestones as of June 30, 2015 are as follows:
|
|
• |
$0.6 million for development and delivery of a complete system layout; |
|
|
• |
$0.3 million for development and delivery of components for a human factor study; |
|
|
• |
$0.6 million for development and delivery of feasibility devices for testing; |
|
|
• |
$0.6 million for development and delivery of a clinical production process; |
|
|
• |
$0.4 million for development and delivery of components for a human factor study; |
|
|
• |
$0.4 million for completion of testing of assembly equipment; |
|
|
• |
$0.3 million for completion of filling process of clinical devices; |
|
|
• |
$0.4 million for delivery of containers for the filling process; and |
|
|
• |
$0.3 million for delivery of devices for clinical studies. |
92
During the fiscal year ended June 30, 2015, the Company recognized $8.1 million of revenue related to services rendered on a time and materials basis, proportional performance method and/or straight-line basis over the requisite service period pursuant to customer agreements to provide various customization and development services.
During the fiscal year ended June 30, 2014, the Company recognized $8.1 million of revenue related to substantive milestones, as follows:
The Company recognized $1.3 million of revenue during the fiscal year ended June 30, 2014 pursuant to a clinical supply agreement with a customer related to substantive milestones that were completed and accepted during the year. This agreement provides for the customization and development activities for a drug delivery system for a customer and provides for payments to be made upon the completion of agreed-upon milestones. The milestones were determined to be substantive at the time the agreement was entered into. Substantive milestones that were achieved during the fiscal year ended June 30, 2014 were as follows:
|
|
• |
$0.4 million for development and delivery of devices to be used by the customer for compatibility testing; |
|
|
• |
$0.1 million for delivery of development and testing activities; |
|
|
• |
$0.6 million for delivery of development, testing and verification activities; |
|
|
• |
$0.1 million for development and delivery of testing materials; and |
|
|
• |
$0.1 million for certain support and testing activities. |
There are no remaining substantive milestones under this agreement.
The Company recognized $0.8 million of revenue during the fiscal year ended June 30, 2014 pursuant to a customization and commercial supply agreement with a customer related to a substantive milestone that was completed during the year. This agreement provides for the development of customized component parts for the customer to use in a drug-device combination product and provides for payments to be made upon the completion of agreed-upon milestones. The milestones were determined to be substantive at the time the agreement was entered into. The substantive milestone achieved during the fiscal year ended June 30, 2014 were as follows:
|
|
• |
$0.8 million for customization and delivery of devices for compatibility and initial evaluation testing. |
The remaining substantive milestones were as follows:
|
|
• |
$0.8 million for delivery of devices for regulatory filings; and |
|
|
• |
$0.2 million for certain delivery of services supporting the customer’s regulatory approval process. |
The Company recognized $0.7 million of revenue during the fiscal year ended June 30, 2014 pursuant to a materials transfer agreement with a customer related to substantive milestones that were completed during the year. This agreement provides for certain materials and related services to the customer and provides for payments to be made upon the completion of agreed-upon milestones. The milestones were determined to be substantive at the time the agreement was entered into. The substantive milestones achieved during the fiscal year ended June 30, 2014 were as follows:
|
|
• |
$0.4 million for delivery of testing materials; |
|
|
• |
$0.1 million for delivery of device design requirements report; and |
|
|
• |
$0.2 million for delivery of customization activities. |
There were no remaining substantive milestones under this agreement.
The Company recognized $0.3 million of revenue during the fiscal year ended June 30, 2014 pursuant to a collaborative research agreement with a customer related to substantive milestones that were completed during the year. This agreement provides for certain materials and related services to the customer and provides for payments to be made upon the completion of agreed-upon milestones. The milestones were determined to be substantive at the time the agreement was entered into. The substantive milestones achieved during the fiscal year ended June 30, 2014 were as follows:
|
|
• |
$0.1 million for customization and delivery of devices for evaluation and user study purposes; and |
|
|
• |
$0.2 million for customization and delivery of devices for evaluation activities. |
There were no remaining substantive milestones under this agreement.
93
The Company recognized $5.0 million of revenue during the fiscal year ended June 30, 2014 pursuant to a binding license, development and supply agreement with a customer related to a substantive milestone that was completed during the year. This agreement provides for the development of customized devices and drug delivery systems for the customer to use in a drug-device combination product and provides for payments to be made upon the completion of agreed-upon milestones. The milestones were determined to be substantive at the time the agreement was entered into. The substantive milestone achieved during the fiscal year ended June 30, 2014 was as follows:
|
|
• |
$5.0 million for customization, design and production of a prototype device for incorporation into a drug delivery system. |
The remaining substantive milestones were as follows:
|
|
• |
$5.0 million for delivery and acceptance of application for regulatory approval; and |
|
|
• |
$1.0 million each upon regulatory approval for up to 20 drug-delivery device combination products. |
During the fiscal year ended June 30, 2014, the Company recognized $4.3 million in revenue related to services rendered on a time and materials basis during the period pursuant to customer agreements to provide various customization and development services. During the fiscal year ended June 30, 2014, the Company recognized the final $2.3 million of revenue related to its licensing agreement with Sanofi.
15. Departure of Officers; Appointment of Officers
As of March 11, 2016, Alan D. Shortall’s employment as Chief Executive Officer of the Company ceased and Mr. Shortall resigned from his positions as Chairman of the Board and as a member of the Board. In addition, as of March 11, 2016 the employment of Ramin Mojdeh, Ph.D. as the Company’s President and Chief Operating Officer ceased.
Effective March 14, 2016, the Board appointed John Ryan as the Interim President and Chief Executive Officer of the Company. On July 28, 2016, the Board appointed Mr. Ryan as the Company’s President and Chief Executive Officer and also appointed Mr. Ryan to serve as a member of the Board.
The Company and Mr. Shortall entered into a General Release effective as of March 14, 2016 (the “Shortall Agreement”) pursuant to which the Company agreed to pay to Mr. Shortall the following lump sum cash payments: (i) $0.4 million, which amount represented twelve months of severance pay at Mr. Shortall’s base salary as of March 11, 2016; (ii) $0.4 million in full satisfaction of amounts otherwise owed pursuant to the Employment Agreement, effective as of October 1, 2011, between the Company and Mr. Shortall, as amended (the “Shortall Employment Agreement”); (iii) $0.4 million in full satisfaction of any bonus to which Mr. Shortall may have been entitled to for 2015; (iv) $57 thousand in respect of Mr. Shortall’s unused vacation time; (v) $20 thousand as reimbursement for the reasonable and appropriate relocation expenses that Mr. Shortall previously incurred in connection with his relocation to the King of Prussia, Pennsylvania area; and (vi) $0.1 million as reimbursement for all reasonable relocation expenses incurred by Mr. Shortall and his family for repatriation to Australia. In addition, the Company agreed to pay $20 thousand of the legal fees incurred by Mr. Shortall in connection with the Shortall Agreement and to pay for the cost of Mr. Shortall’s group health coverage under any Company benefit plan for twelve months in accordance with the Consolidated Omnibus Budget Reconciliation Act of 1985 (“COBRA”). The cash payments set forth in (i)-(iv) and (vi) above were not paid to Mr. Shortall as they were offset in full by withholding obligations of the Company as a result of the vesting of restricted shares described below.
Under the Shortall Agreement, 400,000 unvested restricted shares that were granted to Mr. Shortall by the Company pursuant to the Restricted Stock Agreement, dated November 14, 2014, became fully vested. The Company recorded $3.5 million in share-based compensation expense relating to this vesting and reversed $3.3 million in expense which related to the original grant.
Pursuant to the Shortall Agreement, the Company and Mr. Shortall executed a mutual release of claims. In addition, Mr. Shortall agreed to assign all inventions and works created by Mr. Shortall during his employment with the Company, to the extent that any such inventions and works were not previously assigned to the Company. Mr. Shortall also agreed to continue to comply with the confidentiality, non-solicitation and non-compete obligations under the Shortall Employment Agreement and to certain trading restrictions with respect to the shares of Common Stock which he holds.
The Shortall Agreement contains customary representations and warranties on the part of Mr. Shortall.
On March 11, 2016, the Company and Mr. Shortall entered into a Consulting Agreement (the “Shortall Consulting Agreement”), pursuant to which Mr. Shortall agreed to provide to the Company consulting, organizational and strategic services until March 11, 2018, as directed or authorized from time to time by the Board or the Company’s Chief Executive Officer (collectively, the “Shortall Services”). On July 28, 2016, the Shortall Consulting Agreement was terminated as a result of Mr. Shortall’s failure to fulfill his duties under the Shortall Consulting Agreement pursuant to Paragraph 6 thereof.
94
Pursuant to the Shortall Consulting Agreement, Mr. Shortall agreed to provide the Shortall Services exclusively to the Company and to not render any type of services to a competitor of the Company’s business from March 11, 2016 until March 11, 2018.
In consideration of the Shortall Services, the Company had agreed to (i) pay to Mr. Shortall an amount equal to twelve thousand five hundred dollars per calendar month from March 11, 2016 until March 11, 2018; (ii) issue to Mr. Shortall 1,000 shares of Common Stock per calendar month from March 11, 2016 until March 11, 2018; and (iii) if prior to March 11, 2021 the per share closing price of the Common Stock exceeded certain thresholds, issue to Mr. Shortall a number of shares of Common Stock (based on the stock price milestone that is achieved), up to a maximum of 100,000 shares of Common Stock. As Mr. Shortall was considered to be a “related party” of the Company under the Listing Rules of the Australian Securities Exchange (“ASX”), the Company determined that the above-described issuances of shares under the Consulting Agreement would be subject to shareholder approval under ASX Listing Rule 10.11. As a result of the findings from the Investigation, the Company has determined not to issue these shares and therefore will not seek shareholder approval for the issuance.
The Company and Dr. Mojdeh entered into a General Release effective as of March 14, 2016 (the “Mojdeh Agreement”) pursuant to which the Company agreed to pay to Dr. Mojdeh the following cash payments: (i) $0.4 million, which amount represents twelve months of severance pay at Dr. Mojdeh’s base salary as of March 11, 2016, to be paid in equal installments over a twelve-month period; and (ii) $32 thousand in respect of Dr. Mojdeh’s unused vacation time, to be paid in a lump sum. The Company also agreed to pay to Dr. Mojdeh an amount equal to $0.3 million, to be paid in equal installments over a twelve-month period, which amount represents Dr. Mojdeh’s target bonus pursuant to the Employment Agreement, effective as of July 1, 2012, between the Company and Dr. Mojdeh, as amended (the “Mojdeh Employment Agreement”). In addition, the Company agreed to pay up to $20 thousand of the legal fees incurred by Dr. Mojdeh in connection with the Mojdeh Agreement and to pay for the cost of Dr. Mojdeh’s group health coverage under any Company benefit plan for twelve months in accordance with COBRA. The cash payments described in this paragraph were not paid to Dr. Mojdeh as such amounts (other than the $20 thousand payment for the legal fees incurred by Dr. Mojdeh in connection with the Mojdeh Agreement) were offset in full by withholding obligations of the Company as a result of such share issuance to Dr. Mojdeh and the vesting of restricted shares described below.
Pursuant to the Mojdeh Agreement, the Company granted to Dr. Mojdeh on March 14, 2016 380,000 fully vested and transferable shares of Common Stock. The Company recorded $3.3 million in share-based compensation expense related to this issuance. Under the Mojdeh Agreement, 16,750 unvested restricted shares that were granted to Dr. Mojdeh by the Company pursuant to the Restricted Stock Agreements, dated May 28, 2013 and May 15, 2014, became fully vested.
Pursuant to the Mojdeh Agreement, the Company and Dr. Mojdeh executed a mutual release of claims. In addition, Dr. Mojdeh agreed to assign all inventions and works created by Dr. Mojdeh during his employment with the Company. Dr. Mojdeh also agreed to continue to comply with the confidentiality, non-solicitation and non-compete obligations under the Mojdeh Employment Agreement and to certain trading restrictions with respect to the shares of Common Stock which he holds.
The Mojdeh Agreement contains customary representations and warranties on the part of Dr. Mojdeh.
On July 25, 2016, the Company’s employment of Mark Iampietro as the Company’s Vice President of Quality and Regulatory Affairs and Chief Compliance Officer was ended by the Company without cause. Pursuant to the Employment Agreement, dated November 6, 2014, by and between the Company and Mr. Iampietro, Mr. Iampietro is entitled to (i) receive $252,000 over a period of 12 months following the termination, which amount represents Mr. Iampietro’s base salary as of the termination, (ii) continue to receive group health benefits for a period of 12 months following the termination, and (iii) receive $88,200 over a period of 12 months following the termination, which amount represents the amount of the bonus earned by and paid to Mr. Iampietro in 2015 as well as the target bonus for which Mr. Iampietro was eligible to earn in 2016. In addition, all of Mr. Iampietro’s outstanding and unvested options and other stock-based awards immediately vested.
On July 28, 2016:
|
|
• |
the Board appointed Michael E. Kamarck to serve as a member of the Board; |
|
|
• |
the Board appointed Ian Hanson as the Company’s Chief Operating Officer in addition to his roles as the Company’s Senior Vice President; |
|
|
• |
due to results of the Investigation, Dennis P. Pyers was removed from his position as the Company’s Senior Vice President, Controller, Treasurer and Chief Accounting Officer and was appointed as the Company’s Senior Advisor, Special Projects; and |
|
|
• |
the Board appointed David Hastings as the Company’s Chief Accounting Officer and Treasurer in addition to his roles as the Company’s Senior Vice President and Chief Financial Officer; |
|
|
• |
the Board appointed Stephanie Walters as Senior Vice President, General Counsel and Secretary; and |
95
|
|
• |
the Board appointed Molly Weaver, Ph.D., as Vice President of Quality and Regulatory Affairs and Chief Compliance Officer. |
16. Fair Value Measurements
The Company categorizes its assets and liabilities measured at fair value into a fair value hierarchy that prioritizes the inputs used in pricing the asset or liability. The three levels of the fair value hierarchy are as follows:
Level 1 — Quoted prices in active markets for identical assets or liabilities.
Level 2 — Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data.
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. This includes certain pricing models, discounted cash flow methodologies and similar techniques that use significant unobservable inputs.
The levels in the fair value hierarchy within which a fair value measurement in its entirety falls is based on the lowest level input that is significant to the fair value measurement in its entirety.
The following table presents the Company’s liabilities that are measured at fair value on a recurring basis for the periods presented:
|
|
|
|
|
|
|
Basis of Fair Value Measurement |
|
|||||||||
|
|
|
Total Fair Value Measurements |
|
|
Quoted Market Prices in Active Markets for Identical Assets (Level 1) |
|
|
Significant Other Observable Inputs (Level 2) |
|
|
Significant Unobservable Inputs (Level 3) |
|
||||
|
|
|
(In thousands) |
|
|||||||||||||
|
June 30, 2016: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Royalty agreement liability |
|
$ |
5,120 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
5,120 |
|
|
Warrant liability |
|
|
3,351 |
|
|
|
— |
|
|
|
— |
|
|
|
3,351 |
|
|
Derivative liability |
|
|
347 |
|
|
|
— |
|
|
|
— |
|
|
|
347 |
|
|
June 30, 2015: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Royalty agreement liability |
|
$ |
9,930 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
9,930 |
|
The following table presents the changes in the fair value of the level 3 financial instruments for the fiscal years ended June 30, 2016 and 2015.
|
|
|
Royalty Agreement liability |
|
|
Preferred Stock Conversion Liability |
|
|
Warrant Liability |
|
|
Derivative Liability |
|
||||
|
June 30, 2014 |
|
$ |
6,400 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
Royalty payments |
|
|
(749 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Increase in royalty liability |
|
|
4,279 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
June 30, 2015 |
|
$ |
9,930 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
Initial measurement |
|
|
— |
|
|
|
4,424 |
|
|
|
12,539 |
|
|
|
1,672 |
|
|
Cash payments |
|
|
(1,227 |
) |
|
|
(280 |
) |
|
|
— |
|
|
|
— |
|
|
Non-cash conversions |
|
|
— |
|
|
|
(8,341 |
) |
|
|
— |
|
|
|
— |
|
|
Increase (decrease) in liability |
|
|
(3,583 |
) |
|
|
4,197 |
|
|
|
(9,188 |
) |
|
|
(1,325 |
) |
|
June 30, 2016 |
|
$ |
5,120 |
|
|
$ |
— |
|
|
$ |
3,351 |
|
|
$ |
347 |
|
Following is a description of the valuation methodologies used to measure the royalty agreement liability, the warrant liability, and the derivative liability. There have been no changes in the methodology used during the fiscal year ended June 30, 2016.
The fair value of the royalty agreement liability is based on a discounted cash flow methodology under the income approach based on the present value sum of payments expected to be made in the future. The fair value is estimated by applying a risk adjusted discount rate to the expected royalty payment stream. These fair value estimates are most sensitive to changes in the payment stream and royalty rates.
96
The fair value of the warrant liability is based on a Black-Scholes valuation. The fair value estimates are most sensitive to changes in the Company’s share price.
The fair value of the derivative liability is based on the average of a Monte Carlo model and a lattice model. The fair value estimates are most sensitive to changes in the Company’s share price.
Other Financial Instruments
The carrying amount of the Company’s cash equivalents, which includes certificates of deposit, accounts receivable, accounts payable and accrued expenses approximate fair value due to the short term maturities of these items. The estimated fair value of the Company’s debt approximates its carrying value based upon the rates that the Company would currently be able to receive for similar instruments of comparable maturity.
17. Related Party Transactions
Administrative and Consulting Fees
The Company has an agreement with a consulting firm, of which a member of the Company’s board of directors is the principal. Under the terms of the agreement, the Company pays a fee for finance, accounting and secretarial consulting services within Australia. Amounts paid to the consulting entity during the fiscal years ending June 30, 2016, 2015 and 2014 were $0.2 million, $0.2 million and $0.2 million, respectively.
Loan from Mr. Shortall
On September 30, 2015, the Company obtained a loan in the amount of $600,000 from Alan Shortall, the Company’s former Chairman and Chief Executive Officer. During February 2016, the loan was repaid in full including payment of interest to Mr. Shortall at the minimum applicable federal rate, which interest was less than $2,000.
Bosnjak Mortgage Correspondence
In 2015, Mr. Shortall and Mr. Bosnjak, without authorization from or knowledge of the Company or its Board, caused to be transmitted to a mortgage broker for Mr. Shortall from Mr. Bosnjak correspondence that contained inaccurate statements about the Company’s financial support for Mr. Shortall’s purchase of and relocation to a new home. The investigation into the matters described in this paragraph did not identify any financial loss to the Company and the Company has corrected the inaccurate statements to the mortgage broker.
Shortall Fund Transfers
Mr. Shortall deposited $2,264,475 (the “Shortall Funds”) of his own funds into the Company’s bank account on June 29, 2015 and then caused the Company to disburse from the Shortall Funds $1,351,553 to third parties to complete Mr. Shortall’s purchase of his new home on July 23, 2015, and the remainder back to himself on July 28, 2015.
In addition to the Shortall Funds, during fiscal years 2014 through 2016, under Mr. Shortall’s direction, the Company accepted checks and wires from Mr. Shortall in the aggregate amount of approximately $300,000 and disbursed the same amount of funds to Mr. Shortall or his designees but did not deposit such checks or receive such wires from Mr. Shortall until eight days to thirty-six days after the Company’s disbursement of the funds. The Company believes such transactions constituted loans from the Company to Mr. Shortall. The amount of such loans were approximately $6,000, $224,000, and $70,000 in fiscal years 2016, 2015 and 2014, respectively. In addition, Mr. Shortall wired funds and provided personal checks to the Company in the aggregate amount of approximately $91,000, not including the Shortall Funds, which wires and checks the Company received and deposited, as applicable, prior to or within a day of the Company disbursing the same amounts to Mr. Shortall. The amount of such transfers were approximately $0, $28,000 and $63,000 in fiscal years 2016, 2015 and 2014, respectively.
The investigation into the matters described in this section entitled “Shortall Fund Transfers” did not identify any financial loss to the Company.
Bosnjak Loan Payments and Unreimbursed Personal Expenses
Between July 2014 and July 2015, Mr. Shortall caused approximately $62,000 in Company funds to be transmitted to a third party, which fund transmittals the Company believes were made for the purpose of satisfying certain of Mr. Bosnjak’s commitments to pay interest to such third party on a loan secured by some of Mr. Bosnjak’s shares of Company stock (the “Bosnjak Loan
97
Payments”). The Company believes that the Bosnjak Loan Payments constituted loans from the Company to Mr. Bosnjak, and the Company is evaluating potential actions to recover these funds. The collection of such amounts is uncertain and the Company has recorded approximately $12,000 and $50,000 as Selling, General and Administrative Expense in fiscal year 2016 and 2015, respectively.
From fiscal 2014 through fiscal 2016, Mr. Shortall caused the Company to pay for personal expenses, approximately $88,000 of which was not repaid to the Company (the “Unreimbursed Personal Expenses”). The Company believes the Unreimbursed Personal Expenses constituted loans from the Company to Mr. Shortall, and the Company has demanded repayment of the Unreimbursed Personal Expenses. The collection of such amounts is uncertain and the Company has recorded approximately $0, $60,000 and $28,000 as Selling, General and Administrative Expense in fiscal year 2016, 2015 and 2014, respectively.
Advanced Withholding Payments
In March 2016, July 2015 and December 2014, in connection with the vesting of restricted shares of the Company’s common stock, the Company paid associated withholding taxes on behalf of three executive officers, its Vice President of Quality and Regulatory Affairs and Chief Compliance Officer, its Senior Vice President and Chief Commercial Officer, and its former President and Chief Operating Officer, in an aggregate amount of approximately $240,000 prior to being reimbursed by such executive officers. With the exception of one $400 underpayment, which the Company collected in July, 2016, such executive officers repaid the Company in full within a range of 18 to 120 days from the date of the withholding payment. The Company believes such advances constituted loans. The amount of such advances were approximately $146,000, $94,000 and $0 in fiscal years 2016, 2015 and 2014, respectively. The March 2016 delayed repayments are appropriately reflected as a receivable in the Company’s financial statements as of June 30, 2016 and were reimbursed to the Company during July 2016.
18. Quarterly Results (unaudited)
|
|
|
Quarter Ended September 30, 2015 |
|
|
Quarter Ended December 31, 2015 |
|
|
Quarter Ended March 31, 2016 |
|
|
Quarter Ended June 30, 2016 |
|
||||
|
|
|
(In thousands, except per share data) |
|
|||||||||||||
|
Year Ended June 30, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
3,187 |
|
|
$ |
4,499 |
|
|
$ |
822 |
|
|
$ |
6,333 |
|
|
Net loss |
|
|
(25,864 |
) |
|
|
(25,423 |
) |
|
|
(41,779 |
) |
|
|
(7,717 |
) |
|
Basic and diluted loss per share |
|
$ |
(2.08 |
) |
|
$ |
(1.98 |
) |
|
$ |
(2.68 |
) |
|
$ |
(0.47 |
) |
|
|
|
Quarter Ended September 30, 2014 |
|
|
Quarter Ended December 31, 2014 |
|
|
Quarter Ended March 31, 2015 |
|
|
Quarter Ended June 30, 2015 |
|
||||
|
|
|
(In thousands, except per share data) |
|
|||||||||||||
|
Year Ended June 30, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues |
|
$ |
1,380 |
|
|
$ |
5,403 |
|
|
$ |
2,921 |
|
|
$ |
3,454 |
|
|
Net loss |
|
|
(22,262 |
) |
|
|
(19,387 |
) |
|
|
(23,105 |
) |
|
|
(26,095 |
) |
|
Basic and diluted loss per share |
|
$ |
(2.12 |
) |
|
$ |
(1.80 |
) |
|
$ |
(1.99 |
) |
|
$ |
(2.16 |
) |
Per share amounts for the quarters may not add to the annual amount due to differences in the weighted average common shares outstanding during the period.
19. Subsequent Events
On October 17, 2016, the Company received a notice from the Listing Qualifications department of NASDAQ stating that, because the Company did not maintain a minimum Market Value of Listed Securities (“MVLS”) of $50,000,000 for the last 30 consecutive business days, the Company was no longer in compliance with NASDAQ Listing Rule 5450(b)(2)(A).
The Company has been provided a period of 180 calendar days, or until April 17, 2017, to regain compliance with the minimum MVLS listing requirement. If at any time on or before April 17, 2017, the MVLS of the Company’s common stock closes at $50,000,000 or more for a minimum of 10 consecutive business days, NASDAQ will provide the Company with written confirmation that the Company has achieved compliance with the minimum MVLS listing requirement and the matter will be closed.
98
In the event that the Company does not regain compliance with the minimum MVLS listing requirement on or before April 17, 2017, NASDAQ will provide the Company with written notification that its securities are subject to delisting. At that time, the Company would be permitted to appeal the delisting determination to a NASDAQ Hearings Panel or apply to transfer its common stock to The NASDAQ Capital Market (provided that it satisfied the requirement for continued listing on that market).
99
None.
Disclosure Controls and Procedures
Disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (“Exchange Act”)) are designed to provide reasonable assurance that information required to be disclosed in reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC and that such information is accumulated and communicated to our management, including the Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosures.
All internal control systems, no matter how well designed and tested, have inherent limitations, including, among other things, the possibility of human error, circumvention or disregard. Therefore, even those systems of internal control that have been determined to be effective can provide only reasonable assurance that the objectives of the control system are met and may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As outlined in the Explanatory Note to this 2016 10-K, the Company’s current management discovered violations of Company policies and procedures and possible violations of laws and regulations by Alan Shortall, the Company's former Chief Executive Officer, and Jim Bosnjak, the Company’s former Chairman and member of the Board of Directors (“Board”). Mr. Shortall’s employment with the Company ceased on March 11, 2016, and Mr. Bosnjak resigned from the Board on August 24, 2015. The Board established a Special Committee to oversee an independent investigation. External counsel conducted the investigation with the assistance of an advisory firm with forensic accounting expertise (the “Investigation”). The Investigation did not identify any material financial loss to the Company.
In connection with the Investigation, the Company carried out an evaluation, of the effectiveness of the design and operation of its disclosure controls and procedures as of June 30, 2016. Due to the material weaknesses in internal control over financial reporting as described in “Management’s Report on Internal Control over Financial Reporting” below, our CEO and CFO have concluded that our disclosure controls and procedures were not effective, and were not operating at a reasonable assurance level as of June 30, 2016.
Management’s Report on Internal Control over Financial Reporting
The Company’s management is responsible for establishing and maintaining effective internal control over financial reporting and for assessing the effectiveness of internal control over financial reporting, as defined in Rule 13a-15(f) under the Exchange Act. Internal control over financial reporting refers to a process designed by, or under the supervision of, our Chief Executive Officer and our Chief Financial Officer and effected by our Board, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
|
|
• |
pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
|
|
• |
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and members of our Board; and |
|
|
• |
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on our financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness (as defined in Rule 12b-2 under the Exchange Act) is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of a company’s annual or interim financial statements will not be prevented or detected on a timely basis.
Management, under the supervision of the Company’s new CEO and the Company’s CFO, and oversight of the Board, conducted an assessment of the effectiveness of internal control over financial reporting. Management used the criteria set forth by
100
the Committee of Sponsoring Organizations of the Treadway Commission’s 2013 Framework (the “COSO 2013 Framework”). Management has determined the following material weaknesses existed at June 30, 2016:
The Company, under the oversight of the Board and the leadership of Mr. Shortall, did not have an effective control environment, risk assessment process, information and communication process and monitoring activities; specifically:
|
|
• |
The Company failed to establish a tone at the top that demonstrated its commitment to integrity and ethical values. Mr. Shortall created instances where certain personnel participated in override of the Company’s policies and procedures and internal controls without exercising the appropriate professional skepticism and failed to communicate the override of controls to others. |
|
|
• |
The Company did not have an effective annual process in place to ensure that all employees, including management, confirmed their compliance with the Company’s Business Conduct Policy and that deviations from the expected standards of conduct were identified and remedied in a timely manner. |
|
|
• |
The Company did not have an effective, documented and continuous risk assessment process to identify and analyze risks of financial misstatement due to error and/or fraud, including management override of controls, and determine an appropriate action to manage the financial reporting risks. |
|
|
• |
The Company did not have effective information and communication and monitoring controls to ensure the timely identification and communication of related party transactions to financial reporting personnel, management, and the Board, to enable appropriate financial reporting and disclosure of such transactions. |
As a consequence of the inappropriate tone at the top and the above-mentioned entity level deficiencies, the following process level control deficiencies were identified:
|
|
• |
Ineffective operation of certain process level controls due to management override of controls resulting from the dominant influence of the former CEO, including ineffective process-level controls over the accounting for related party transactions and the evaluation of transactions with senior executives and a former Board member that represented loans and advances. In addition, the Company did not involve those employees with the appropriate knowledge and expertise to evaluate the business purpose of the transactions and compliance with laws and regulations. |
|
|
• |
Ineffective design and implementation and documentation of management review controls, specifically, the management review controls did not adequately address or document management's expectations, criteria for investigation, the level of precision used in the performance of the review control, and how outliers were identified, investigated and resolved. |
Certain of these control deficiencies resulted in immaterial misstatements in the preliminary consolidated financial statements as at and for the year ended June 30, 2016 which were corrected in the Company’s consolidated financial statements included in the 2016 10-K. Other deficiencies resulted in no misstatements in the consolidated financial statements. However, these control deficiencies create a reasonable possibility that a material misstatement to the consolidated financial statements will not be prevented or detected on a timely basis. Accordingly, we concluded that the control deficiencies represent material weaknesses in the Company’s internal control over financial reporting and our internal control over financial reporting was not effective as of June 30, 2016.
The independent registered public accounting firm, KPMG LLP, has expressed an adverse report on the operating effectiveness of our internal control over financial reporting as of June 30, 2016. KPMG LLP’s report appears on page 56 of this 2016 10-K.
Remediation of Material Weaknesses
The Company continues to work, to strengthen our internal control over financial reporting. We are committed to ensuring that such controls are designed and operating effectively. Our Board and management take internal controls over financial reporting and the integrity of the Company's financial statements seriously and believe that the remediation steps described below, including with respect to personnel changes, were and are essential steps to establishing and maintaining strong and effective internal controls over
101
financial reporting and addressing the tone at the top concerns that contributed to the material weaknesses identified. The following actions and plans will be or have been implemented:
|
|
• |
The Board replaced Mr. Shortall effective March 2016 with our then interim and now current CEO, John Ryan, effective March 2016. Mr. Bosnjak resigned in August 2015. Mary Kate Wold, President and CEO of the Church Pension Group, a former finance executive at Wyeth and previously Unilife's Vice Chair and Lead Independent Director, assumed the role of Board Chair. In addition, the Controller is no longer serving as Chief Accounting Officer, Controller or Treasurer. The Company appointed David Hastings as the Company’s Chief Accounting Officer and Treasurer along with Mr. Hastings’ current role as Chief Financial Officer. The Company has also appointed a new independent Board member. |
|
|
• |
Management has evaluated and revised the assignment of authorities and financial reporting responsibilities and roles and has made staffing changes including, without limitation, those noted above; and the Company will increase technical training to those employees involved in the financial reporting process. |
|
|
• |
The Company has increased communication and will increase training to employees and the Board regarding the ethical values of the Company and the requirement to comply with laws, rules, regulations, and Company policies, including the Business Conduct Policy and Insider Trading Policy, and the importance of accurate and transparent financial reporting. In addition, the Company will revise its process to ensure that all employees annually confirm compliance with the Company’s Business Conduct Policy and that deviations are identified and timely remediated. |
|
|
• |
Under the supervision of the Board, the Company will emphasize to key leadership the importance of setting appropriate tone at the top and of appropriate behavior with respect to accurate financial reporting and adherence to the Company’s internal control over financial reporting framework and accounting policies. |
|
|
• |
The Board will work with the Company to implement an internal audit function and develop a risk based plan that will monitor the Company’s adherence to its policies and procedures including, without limitation, those policies and procedures related to the identification and disclosure of related party transactions, and to review any areas of concern or emphasis that the Board has identified as part of its oversight. |
|
|
• |
The Company will update its policies and procedures to require the identification of related party transactions, transactions with senior executives, and to enhance the review and approval for these types of transactions and ensure their disclosure; and will train all employees on such updated policies. |
|
|
• |
Management review controls will be reassessed to determine the appropriate level of precision required to mitigate the potential for a material misstatement. In addition, the Company will enhance its design and implementation and supporting documentation over management review controls to make clear: (i) management’s expectations related to transactions that are subject to such controls; (ii) the level of precision and criteria used for investigation; and (iii) evidence that all outliers or exceptions that should have been identified are investigated. |
Changes in Internal Control over Financial Reporting
Except for the management review controls deficiency and the Company’s ineffective adoption of COSO 2013 disclosed above, there were no changes in our internal controls over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the fourth quarter ended June 30, 2016 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
As noted above, the Company began the process of enhancing existing controls and designing and implementing additional controls and procedures in response to the material weaknesses.
Letter Agreement
The Borrower and ROS entered into a letter agreement dated October 20, 2016 (the “ROS Letter Agreement”), pursuant to which ROS agreed, until 11:59 p.m. New York City time on July 1, 2017, to waive all rights under the Amended OrbiMed Credit Agreement and the other Loan Documents (as defined in the Amended OrbiMed Credit Agreement) to declare an “Event of Default” or other breach under such documents as a result of the Company’s or the Borrower’s failure to maintain an effective registration
102
statement, as required by the warrant issued by the Company to ROS, dated February 22, 2016, to purchase 1,673,981 shares of Common Stock. Pursuant to the ROS Letter Agreement, ROS acknowledged and agreed that the Borrower’s receipt of notice of the effectiveness of a registration statement would cure any such breach.
In accordance with the ROS Letter Agreement, ROS also agreed to waive all rights under the Amended OrbiMed Credit Agreement and the other Loan Documents to declare an “Event of Default” or other breach under such documents as a result of the Company’s or the Borrower’s failure to timely file documents (the “Delayed Filings”) with the Securities and Exchange Commission or ASX since February 22, 2016. Pursuant to the ROS Letter Agreement, ROS acknowledged and agreed that the filing by the Borrower of the Delayed Filings with the Securities and Exchange Commission and the ASX will cure any such breach so long as the Borrower files such Delayed Filings by November 7, 2016.The foregoing description of the ROS Letter Agreement does not purport to be complete and is qualified in its entirety by reference to the full text of the ROS Letter Agreement, which is attached as Exhibit 10.85 to this Annual Report on Form 10-K and is incorporated into this Annual Report on Form 10-K by reference.
Hanson Employment Agreement
On October 21, 2016, the Company entered into an Employment Agreement with Ian Hanson (the “Hanson Employment Agreement”), pursuant to which Mr. Hanson will serve as the Company’s Senior Vice President and Chief Operating Officer and as a member of the Company’s Executive Leadership Team.
The term of the Hanson Employment Agreement commenced on October 21, 2016 and will expire on December 31, 2018; provided that the Hanson Employment Agreement will automatically renew for one-year periods annually thereafter unless either party gives the other party thirty days’ written notice prior to the relevant expiration date of its intention not to renew the agreement.
Pursuant to the Hanson Employment Agreement, Mr. Hanson’s annual base salary is $350,000. In addition, Mr. Hanson is eligible to earn an annual cash bonus in amounts and percentages as determined by the Compensation Committee of the Board (the “Compensation Committee”). The target cash bonus opportunity for each year will be no lower than forty percent of Mr. Hanson’s base salary. Mr. Hanson’s cash bonuses are subject to his achievement of such goals and objectives as the Compensation Committee may determine.
Pursuant to the Hanson Employment Agreement, Mr. Hanson is eligible to participate in those of the Company’s benefits programs (including any equity incentive plan of the Company or its affiliates) and other benefits provided to similarly situated employees of the Company. Mr. Hanson is also entitled to receive four weeks of paid vacation per calendar year.
Any stock options and other stock-based awards that Mr. Hanson may receive from the Company will be governed by the applicable underlying award agreement and the terms of the Company’s 2009 Stock Incentive Plan or any successor plan under which the award is granted.
Under the Hanson Employment Agreement, the Company will reimburse Mr. Hanson for all reasonable and necessary expenses incurred by him in carrying out his duties under the agreement in accordance with the Company’s business expense policies.
During and following Mr. Hanson’s employment, the Company will provide Mr. Hanson with indemnification for acts performed in his capacity as an employee and/or officer of the Company equivalent to the indemnification and directors’ and officers’ insurance coverage applicable to the then-current officers of the Company.
Pursuant to the Hanson Employment Agreement, if Mr. Hanson’s employment with the Company is terminated (i) by the Company without “Cause” (as defined in the Hanson Employment Agreement), (ii) as a result of Mr. Hanson’s resignation for “Good Reason” (as defined in the Hanson Employment Agreement), or (iii) as a result of the Company’s non-renewal of the Hanson Employment Agreement, then, subject to Mr. Hanson’s execution of a general release, Mr. Hanson will be entitled to: (A) the continued payment by the Company of Mr. Hanson’s base salary and health insurance premiums for a period of twelve months (which will be increased to 18 months if the termination event occurs within one year of a “Change in Control” (as defined in the Hanson Employment Agreement) of the Company), and (B) a payment by the Company of an amount equal to the greater of the amount of the annual incentive bonus, if any, earned by Mr. Hanson for the last completed bonus year prior to the year in which his employment was terminated and 40% of Mr. Hanson’s base salary as in effect immediately prior to such termination, which amount will be payable over twelve months. In addition, in the event of such a termination of the Hanson Employment Agreement, all of Mr. Hanson’s then unvested time-vested equity awards will be accelerated.
The Hanson Employment Agreement does not alter Mr. Hanson’s previously executed Confidentiality, Non-Competition and Intellectual Property Agreement, which provides that Mr. Hanson will be subject to certain non-competition and non-solicitation covenants during the term of Mr. Hanson’s employment with the Company and for a period of two years thereafter.
103
The foregoing description of the Hanson Employment Agreement does not purport to be complete and is qualified in its entirety by reference to the full text of the Hanson Employment Agreement, which is attached as Exhibit 10.83 to this Annual Report on Form 10-K and is incorporated into this Annual Report on Form 10-K by reference.
104
Directors and Corporate Governance
The information required by this Item 10 regarding directors and corporate governance is incorporated by reference to our definitive proxy statement for our 2016 Annual Meeting of Stockholders, or the 2016 Proxy Statement, under the headings “Proposal No. 1 – Election of Directors” and “Proposal No. 1 – Information on Our Board of Directors and Corporate Governance.”
Executive Officers
The information required by this Item 10 regarding executive officers is incorporated by reference to our 2016 Proxy Statement under the heading “Executive Officers.”
Compliance with Section 16(a) of the Exchange Act
The information concerning Compliance with Section 16(a) of the Exchange Act is incorporated by reference to our 2016 Proxy Statement under the heading “Section 16(a) Beneficial Ownership Reporting Compliance.”
Code of Ethics
The information concerning our Code of Business Conduct and Ethics is incorporated by reference to our 2016 Proxy Statement under the heading “Proposal No. 1– Information on Our Board of Directors and Corporate Governance – Meetings and Committees of the Board – Code of Business Conduct and Ethics.”
The information required by this Item 11 is incorporated by reference to the 2016 Proxy Statement under the headings of “Executive Compensation” and “Director Compensation”.
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
Except as set forth below, the information required by Item 12 is incorporated by reference to the 2016 Proxy Statement under the heading “Security Ownership of Certain Beneficial Owners and Management,” and “Equity Compensation Plan Information.”
ASX-Required Disclosure
Corporations Act 2001 (Cth)
We are not subject to Chapters 6, 6A, 6B and 6C of the Corporations Act dealing with the acquisition of our shares (in particular, relating to substantial shareholdings and takeovers).
Under the Delaware General Corporation Law, we are generally permitted to purchase or redeem our outstanding shares out of funds legally available for that purpose without obtaining stockholder approval, provided that (i) our capital is not impaired; (ii) such purchase or redemption would not cause our capital to become impaired; (iii) the purchase price does not exceed the price at which the shares are redeemable at our option and (iv) immediately following any such redemption, we shall have outstanding one or more shares of one or more classes or series of stock, which shares shall have full voting powers. Our certificate of incorporation does not create any further limitation on our purchase or redemption of our shares.
Australian Disclosure Requirements
In addition to our primary listing on the NASDAQ Global Market, our shares of common stock are also quoted in the form CDIs on the Australian Securities Exchange (ASX) and trade under the symbol “UNS”. As part of our ASX listing, we are required to comply with various disclosure requirements as set out under the ASX Listing Rules. The following information is intended to comply with the ASX Listing Rules and is not intended to fulfill information required by this Annual Report on Form 10-K.
105
The information required herein is incorporated by reference to the 2016 Proxy Statement under the headings “Security Ownership of Certain Beneficial Owners and Management.”
Distribution of Common Stock and CDI Holders as of September 30, 2016
|
|
|
CDIs |
|
|||||
|
|
|
Number of Holders |
|
|
Number of CDIs |
|
||
|
1 — 1,000 |
|
|
1,349 |
|
|
|
659,657 |
|
|
1,001 — 5,000 |
|
|
2,157 |
|
|
|
6,213,102 |
|
|
5,001 — 10,000 |
|
|
1,066 |
|
|
|
8,535,118 |
|
|
10,001 — 100,000 |
|
|
2,717 |
|
|
|
96,388,589 |
|
|
100,001 — and over |
|
|
683 |
|
|
|
280,246,654 |
|
|
|
|
|
7,972 |
|
|
|
392,043,120 |
|
The number of stockholders holding less than a marketable parcel of shares of common stock was 4,196 based on the closing market price as of September 30, 2016.
There is no current on-market buy-back of our securities.
Twenty Largest CDI Holders as of September 30, 2016
|
Rank |
|
Name |
|
Number of CDIs Held |
|
|
% of CDIs Outstanding |
|
||
|
1. |
|
Citicorp Nominees Pty Limited |
|
|
6,906,818 |
|
|
|
1.76 |
|
|
2. |
|
Mr Dennis Banks + Mrs Janine Banks <Banks Super Fund A/C> |
|
|
5,961,935 |
|
|
|
1.52 |
|
|
3. |
|
Mr Simon Charles McCreed |
|
|
5,750,000 |
|
|
|
1.47 |
|
|
4. |
|
Mr Evan Philip Clucas + Ms Leanne Jane Weston <Kuranga Nursery Super A/C> |
|
|
5,475,988 |
|
|
|
1.40 |
|
|
5. |
|
Mrs Joyce Maria Cootes |
|
|
5,150,000 |
|
|
|
1.31 |
|
|
6. |
|
Bond Street Custodians Limited <Grays - D07122 A/C> |
|
|
5,000,000 |
|
|
|
1.28 |
|
|
7. |
|
Hertogs Investments Pty Limited |
|
|
5,000,000 |
|
|
|
1.28 |
|
|
8. |
|
Billnted Pty Ltd <Excellent Adventure A/C> |
|
|
4,800,000 |
|
|
|
1.22 |
|
|
9. |
|
J & N Kaal Pty Limited <Kaal Super Fund A/C> |
|
|
4,427,470 |
|
|
|
1.13 |
|
|
10. |
|
Admark Investments Pty Ltd <The Pinto Family A/C> |
|
|
4,296,078 |
|
|
|
1.10 |
|
|
11. |
|
Bond Street Custodians Limited <Grays - D07026 A/C> |
|
|
4,000,000 |
|
|
|
1.02 |
|
|
12. |
|
Brispot Nominees Pty Ltd <House Head Nominee No 1 A/C> |
|
|
3,363,915 |
|
|
|
0.86 |
|
|
13. |
|
Mr Bradley Gavin Downes |
|
|
3,034,169 |
|
|
|
0.77 |
|
|
14. |
|
Bond Street Custodians Limited <Grays - D07331 A/C> |
|
|
2,500,000 |
|
|
|
0.64 |
|
|
15. |
|
Lucky Pom Pty Ltd <Grayson A/C> |
|
|
2,500,000 |
|
|
|
0.64 |
|
|
16. |
|
Mrs Cherie Ann Lauder + Mr John William Lauder <J&C Lauder Family S/F A/C> |
|
|
2,433,932 |
|
|
|
0.62 |
|
|
17. |
|
Mr Ashley James Griffiths |
|
|
2,200,000 |
|
|
|
0.56 |
|
|
18. |
|
Regnal Superannuation Pty Ltd <Regnal Super Fund No 2 A/C> |
|
|
2,094,000 |
|
|
|
0.53 |
|
|
19. |
|
Mr Joseph Zanca + Mrs Szerenke Zanca <Zanacorp Super Fund A/C> |
|
|
2,048,520 |
|
|
|
0.52 |
|
|
20. |
|
Mr Brett Anthony Wall |
|
|
1,990,998 |
|
|
|
0.51 |
|
|
Total |
|
|
|
|
78,933,823 |
|
|
|
20.13 |
|
General Information
The name of our Secretary is Ms. Stephanie Walters.
The complete mailing address, including zip code, of our principal executive offices is 250 Cross Farm Lane, York, Pennsylvania 17406.
106
The address of the principal registered office in Australia is Suite 1A, Level 2, 802 Pacific Hwy, Gordon NSW 2082, Australia and our telephone number there is +61 2 8346 6500. The ASX Liaison Officer is Mr. Jeff Carter.
Registers of securities are held as follows:
|
|
(a) |
For CDIs in Australia at Computershare Investor Services Pty Limited, Level 2, 45 St Georges Terrace Perth WA 6000 Australia, Investor Enquiries +61 8 9323 2000 (within Australia) +61 3 9415 4677 (outside Australia); and |
|
|
(b) |
For Common Stock in the United States at Computershare Investor Services, 250 Royall Street, Canton, MA 02021 USA, Tel: 800 662 7232. |
Voting Rights
Unilife’s by-laws provide that each stockholder has one vote for every share of common stock entitled to vote held of record by such stockholder and a proportionate vote for each fractional share of stock entitled to vote so held, unless otherwise provided by Delaware General Corporation Law or in the certificate of incorporation. Holders of restricted stock awards have the same voting rights as holders of shares of common stock.
If holders of CDIs wish to attend Unilife’s general meetings, they will be able to do so. Under the ASX Listing Rules, Unilife, as an issuer of CDIs, must allow CDI holders to attend any meeting of the holders of the underlying securities unless relevant U.S. law at the time of the meeting prevents CDI holders from attending those meetings.
In order to vote at such meetings, CDI holders have the following options:
(a) instructing CDN, as the legal owner, to vote the Unilife common stock underlying their CDIs in a particular manner. The instruction form must be completed and returned to Unilife’s share registry prior to the meeting;
(b) informing Unilife that they wish to nominate themselves or another person to be appointed as CDN’s proxy for the purposes of attending and voting at the general meeting;
(c) converting their CDIs into a holding of Unilife common stock prior to the record date for the meeting and voting these at the meeting (however, if thereafter the former CDI holder wishes to sell their investment on ASX, it would be necessary to convert Unilife common stock back to CDIs).
As holders of CDIs will not appear on Unilife’s share register as the legal holders of Unilife common stock, they will not be entitled to vote at Unilife stockholder meetings unless one of the above steps is undertaken.
CDI Voting Instruction Forms and details of these alternatives will be included in each notice of meeting sent to CDI holders by Unilife.
Holders of options and phantom stock units are not entitled to vote.
Australian Corporate Governance Statement
The board of directors and employees of Unilife Corporation (“Unilife” or the “Company”) are committed to developing, promoting and maintaining a strong culture of good corporate governance and ethical conduct.
The board of directors confirms that our corporate governance framework is generally consistent with the Australian Securities Exchange’s (“ASX”) Corporate Governance Council’s “Corporate Governance Principles and Recommendations (3rd Edition)” (“ASX Governance Recommendations”), other than as set out below. To this end, we provide below a review of its governance framework using the same numbering as adopted for the principles as set out in the ASX Governance Recommendations.
This Corporate Governance Statement is current as of October 21, 2016 and has been approved by our board of directors.
Copies of our charters, codes and policies may be downloaded from the corporate governance section of our website ( www.unilife.com ).
We redomiciled to the United States in January 2010 and listed on The NASDAQ Global Market in February 2010. As a result and to meet the NASDAQ listing requirements, the policies and practices that we adopted are predominantly “US-focused”.
107
Principle 1 — Lay solid foundations for management and oversight
Recommendation 1.1 — Establish the functions reserved to the board and those delegated to senior executives and disclose those functions
The primary responsibility of:
(a) the board of directors is to exercise their business judgment to act in what they reasonably believe to be in the best interests of our stockholders’; and
(b) the Chief Executive Officer is to oversee the day-to-day performance of Unilife (pursuant to powers delegated to the board of directors).
The board of directors’ responsibilities are recognized and documented on an aggregated basis by the Charter of the board of directors, which is available on the corporate governance section of our website.
While day-to-day management has been delegated to the Chief Executive Officer, it is noted that the following matters are specifically reserved for the attention of the board of directors:
(a) providing input into and final approval of management’s development of corporate strategy and performance objectives;
(b) reviewing, ratifying and monitoring systems of risk management and internal control, codes of conduct, and legal compliance;
(c) ensuring appropriate resources are available to senior executives;
(d) approving and monitoring the progress of major capital expenditure, capital management and acquisitions and divestments; and
(e) approving and monitoring financial and other reporting.
Recommendation 1.2 — Disclose the process for submitting a director to shareholders as a candidate for election as a director and provide information regarding the director’s candidacy
The Nominating and Corporate Governance Committee is responsible for evaluating nominees for election as director, determining the criteria for selecting new directors, and selecting or recommending to the board director nominees, although this may instead be handled by our Board of Directors. We undertake appropriate checks before appointing a person, or putting forward to stockholders a candidate for election as a director. Each year, we provide information to shareholders regarding director nominees to enable them to make an informed decision on whether or not to re-elect a director, including the director’s relevant qualifications and experience and the skills they bring to the board of directors; details of any other material directorships currently held by the candidate, the term of office already served by the director, whether the director is considered to be independent, and a recommendation by the board of directors with respect to the re-election of the director. We will provide similar information regarding any candidate standing for election as a director for the first time.
Recommendation 1.3 — Enter into written agreements with directors and senior executives
Other than for one recently appointed executive officer, we maintain written employment agreements with each of our named executive officers and maintain letters of appointment for all of our non-executive directors. Director obligations are set forth in the Charter of the board of directors and in the Charters of each of the committees of our board of directors, all of which are available on the corporate governance section of our website. For the fiscal year ended June 30, 2016, there were no material variations to any of the Company's employment agreements with then executive officers except as previously disclosed.
Recommendation 1.4 — Accountability of company Secretary to the board through the chair, on all matters to do with the proper functioning of the board
Our Secretary, Ms. Stephanie Walters, is directly accountable to the board of directors through the Chairman of the board of directors for monitoring our compliance in respect of all corporate governance matters and compliance with all disclosure obligations. Each director is able to communicate directly with Ms. Walters.
108
Recommendation 1.5 — Establish a policy concerning diversity and disclose it. The policy should include requirements for the board of directors to establish measurable objectives for achieving gender diversity and for the board of directors to assess annually both the objectives and progress in achieving them
The board of directors has adopted a Diversity Policy which includes the responsibility to establish appropriate and measurable diversity objectives and for the board of directors to assess regularly the overall effectiveness of the objectives and annually review the progress in achieving the diversity objectives.
Unilife is committed to driving diversity across all levels of the Company. Diversity at Unilife signifies not only a blend of races, genders, ages, ethnicities, religions, cultural backgrounds, languages, social backgrounds and military service but also a range of experiences, perspectives, skill sets, capabilities and thought. We seek to hire the most qualified person for each position while also prioritizing diversity. In accordance with the Company's Diversity Policy, the following measurable objectives have been adopted by the Nominating and Corporate Governance Committee:
|
|
• |
Recruit new employees and directors from a broad pool of talent, including targeting sources of diverse candidates, which will increase the diversity of Unilife over time; |
|
|
• |
Include at least one woman on every interview team, particularly from the target function (i.e., at least one female engineer to interview engineer candidates); |
|
|
• |
Hold focus group meetings with diverse employees annually on how to recruit diverse candidates and to encourage them to participate actively in recruitment; and |
|
|
• |
Review job postings for potential impact on diversity recruiting and confirm the posting will not dissuade diverse candidates from applying. |
As of May, 2016, women represented approximately 30% (57 of 192) of the total employee base, 27% (4 of 15) of the executive management and 20% (1 out of 5) of the board of directors.
Recommendation 1.6 — Disclose the process for evaluating the performance of the board of directors, its committees and individual directors
In accordance with the Nominating and Corporate Governance Committee Charter, the Nominating and Corporate Governance Committee periodically undertakes a formal review of the performance of the board of directors, its committees and individual directors.
A performance evaluation of the board of directors, its committees and individual directors was commenced during fiscal year 2016 in accordance with the Nominating and Corporate Governance Committee Charter and completed in August 2016.
Recommendation 1.7 — Disclose the process for evaluating the performance of its senior executives
The Compensation Committee periodically undertakes a formal review of its senior executives against agreed goals and objectives.
A performance evaluation of senior executives was undertaken during fiscal year 2016.
Principle 2 — Structure the board of directors to add value
Recommendation 2.1 — The board of directors should establish a nomination committee
We have established a Nominating and Corporate Governance Committee which consists of three members, all of whom are independent directors (including the Chairman of the Nominating and Corporate Governance Committee). The members of the Nominating and Corporate Governance Committee are Mr. Galle (Chair), Mr. Lund and Ms. Wold. The Nominating and Corporate Governance Committee is governed by the Nominating and Corporate Governance Committee Charter. The Nominating and Corporate Governance Committee Charter includes the process and criteria for selecting new directors, a copy of which is available on the corporate governance section of the Company’s website.
Recommendation 2.2 — Companies should have a board skills matrix
The Nominating and Corporate Governance Committee seeks to recruit and retain directors with a variety of skills and backgrounds, which may change as our Company grows and develops. The Nominating and Corporate Governance Committee has not established a board skills matrix, given that our company is changing rapidly and therefore our needs at the board of directors level
109
are evolving. The process and considerations involved in appointing a director are included in our annual Proxy Statement under “Information on our Board of Directors and Corporate Governance” and the skills of our directors are set forth in our annual Proxy Statement under “Proposal No. 1 — Election of Directors.” The Nominating and Corporate Governance Committee has considered and is satisfied that the composition of the Board reflects an appropriate range of skills and experience for Unilife to effectively discharge its responsibilities.
Recommendation 2.3 — Disclose the names of the directors considered by the board to be independent directors
Recommendation 2.4 — A majority of the board of directors should be independent directors
The Company considers that a director is an independent director where that director is free from any relationship that, in the opinion of the Board, would interfere with, or could be reasonably be perceived to materially interfere with, the exercise of independent judgment in carrying out the responsibilities of a director, and otherwise meets the requirements of independence set forth in the rules of NASDAQ and the ASX, any regulatory agency (including the Securities and Exchange Commission) and any additional Board guidelines.
The board of directors is currently comprised of seven directors. The seven directors include six non-executive directors (including the Chairman) and one executive director (being the Chief Executive Officer). Five of the six non-executive directors are considered “independent” in accordance with the NASDAQ listing rules and the ASX listing rules. These independent directors are Ms. Wold and Messrs. Lund, Galle, Kamarck and Hamill. Ms. Wold is the Chairman of the board of directors.
The length of service of each director on the Board is as follows:
Ms. Wold (since May 2010), Mr. Lund (since November 2009), Mr. Galle (since June 2008), Mr. Hamill (since August 2015), Mr. Carter (since April 2006), Mr. Ryan (since July 2016) and Mr. Kamarck (since July 2016).
Recommendation 2.5 — The Chairman should be an independent director and the roles of Chairman and Chief Executive Officer should not be exercised by the same individual
The board of directors elects its Chairman of the board of directors and appoints the Chief Executive Officer according to its view of what is best for the Company at any given time. Ms. Wold is Chairman and Mr. Ryan is the CEO of the Company.
In compliance with the ASX Governance Recommendations, the Chairman of the board of directors is an independent director and the roles of the Chairman and the Chief Executive Officer of the Company are not currently exercised by the same individual. However, the board of directors does not believe there should be a fixed rule as to whether the offices of Chairman of the board of directors and the Chief Executive Officer should be vested in the same person or two different people, or whether the Chairman of the board of directors should be an employee of the Company or should be elected from among the non-employee directors. Our needs and the individuals available to serve in these roles may dictate different outcomes at different times, and the board of directors believes that retaining flexibility in these decisions is in our best interest and our stockholders’. In the event that the offices of the Chairman of the board of directors and the Chief Executive Officer are vested in the same person, our board of directors will, in accordance with governance best practices, appoint a lead independent director.
Recommendation 2.6 — Disclosure of a program for director induction
The Nominating and Corporate Governance Committee Charter requires such committee to develop orientation materials for new directors and corporate governance-related continuing education for all directors. Indeed our board of directors does periodically receive continuing education to develop and maintain the skills and knowledge needed to perform their roles as directors effectively.
Furthermore, at our expense, the board of directors collectively or directors (acting as individuals) are entitled to seek advice from independent external advisers in relation to any matter which is considered necessary to fulfill their relevant duties and responsibilities. Individual directors seeking such advice must obtain the approval of the Chairman. Any advice so obtained will be made available to the board of directors.
Principle 3 — Act ethically and responsibly
Recommendation 3.1 — Establish a Code of Conduct and disclose it
The Company has adopted a Code of Business Conduct and Ethics, as well as an Insider Trading Policy. Each of these has been prepared in accordance with Nasdaq rules and the ASX Governance Recommendations. They are both available on the corporate governance section of our website.
110
Principle 4 — Safeguard integrity in corporate reporting
Recommendation 4.1 — The board of directors should establish an Audit Committee. The Audit Committee should: (a) consist of non-executive directors only; (b) consist of a majority of independent directors; (c) be chaired by an independent chair who is not chair of the board of directors; and (d) have at least three members
The Company has established an Audit Committee which consists of three members, all of whom are non-executive and independent directors (including the Chairman of the Audit Committee). The members of the Audit Committee are Mr. Lund (Chair), Ms. Wold, and Mr. Hamill. The Audit Committee is governed by the Audit Committee Charter, a copy of which is available on our website.
Information regarding the skills, experience and expertise of directors, including Audit Committee members in accordance with U.S. disclosure requirements, is available on our website.
We refer shareholders to the “Additional ASX Required Disclosures” section further below for information relating to the number of times each committee has met in the financial year ending 30 June 2016 and the individual attendances of the members at those meetings.
Recommendation 4.2 — The board should, before it approves the entity’s financial statements for a financial period, receive from its CEO and CFO a declaration that, in their opinion, the financial records of the entity have been properly maintained and that the financial statements comply with the appropriate accounting standards and give a true and fair view of the financial position and performance of the entity and that the opinion has been formed on the basis of a sound system of risk management and internal control which is operating effectively
We refer shareholders to Item 9A of this Annual Report on Form 10-K. As stated above, Item 9A of this Annual Report on Form 10-K discloses information regarding the Company’s controls and procedures, including management’s evaluation of the effectiveness of our disclosure controls and procedures and management’s evaluation of the effectiveness of our internal control over financial reporting. Please also see the “Explanatory Note” above which discusses the results of the Investigation and amendments to certain of the Company’s prior filings with the SEC as a result of findings from the Investigation.
Furthermore, consistent with the Sarbanes-Oxley Act of 2002, our Chief Executive Officer and Chief Financial Officer provide certifications at the end of each Annual Report on Form-10K that we file. These certifications confirm the opinions of these executives that, among other things: the financial statements, and other financial information included in the report, fairly present in all material respects our financial condition, results of operations and cash flows as of, and for, the periods presented in the report.
Recommendation 4.3 — The external auditor should attend the annual general meeting of shareholders and be available to answer questions
The Company’s external auditor, KPMG, attends the Company’s annual general meeting of shareholders and is available to answer questions from shareholders directly following that meeting.
Principle 5 — Make timely and balanced disclosure
Recommendation 5.1 — Establish a written policy for complying with continuous disclosure obligations under the ASX listing rules and disclose that policy
Unilife is committed to providing timely and balanced disclosure to the market and, in consequence, to meeting its continuous disclosure requirements. We established a Disclosure Committee for the purpose of ensuring significant matters requiring public disclosure are communicated to management and disclosed in a timely manner.
In accordance with our commitment to fully comply with our continuous disclosure requirements, we have adopted a Disclosure Policy, together with other internal mechanisms and reporting requirements. A copy of the Company's Disclosure Policy is available on the corporate governance section of the Company's website.
We refer shareholders to Item 9A of this Annual Report on Form 10-K. As stated above, Item 9A of this Annual Report on Form 10-K discloses information regarding the Company’s controls and procedures, including management’s evaluation of the effectiveness of our disclosure controls and procedures. Please also see the “Explanatory Note” above which discusses the results of the Investigation and amendments to certain of the Company’s prior filings with the SEC as a result of findings from the Investigation.
111
Principle 6 — Respect the rights of stockholders
Recommendation 6.1 — Provide information about the company and its governance to investors via its website
Recommendation 6.2 — Design and implement an investor relations program to facilitate effective two-way communication with investors
Recommendation 6.3 — Disclose the policies and processes in place to facilitate and encourage participation at meetings of stockholders
Recommendation 6.4 — Provide stockholders the option to receive communications from, and send communications to, the entity and its security registry electronically.
We communicate information, including our annual reports, SEC filings, investor call details, copies of our corporate governance charters, background information on directors and executive officers, board committee assignments, media releases, and product information and demonstrations, to stockholders through our website. We provide advanced notice of group briefings, including earning calls, stockholder meetings and investor events, and to the extent practicable, provide webcast links on our website. Key financial information and stock performance are also available on our website. Stockholders can raise questions to us by contacting us by telephone, facsimile, post or email, with relevant contact details being available on our website.
All stockholders are invited to attend our Annual Meeting of Stockholders, either in person or by proxy. The board of directors regards the Annual Meeting as an excellent forum in which to discuss issues relevant to the Company and thereby encourages full participation by stockholders. Stockholders have an opportunity to submit questions to the board of directors and our auditors. The meeting is also webcast to provide access to those stockholders who are unable to attend the Annual Meeting.
We seek to use multiple modes of communication, including electronic communication, to ensure that our communication with stockholders is frequent and done with ease. Stockholders (including holders of CHESS Depositary Interests) may communicate with the board of directors electronically by submitting an email to stephanie.walters@unilife.com. All incoming communications are screened by our Company Secretary and transmitted to the intended recipient absent safety or security issues. Stockholders may also elect to receive email alerts from the Company by registering on our website and by following @unilife on Twitter.
Principle 7 — Recognize and manage risk
Recommendation 7.1 — (a) Establish a committee(s) to oversee risk which has at least three members, a majority of whom are independent directors, and is chaired by an independent director; and disclose the charter of the committee, the members of the committee, and the number of times the committee and the individual attendance of committee members; or (b) if there is no such committee, disclose the processes it employs for overseeing the entity’s risk management framework
We do not have a board committee that is tasked solely with overseeing Company risk. The board of directors is responsible for reviewing, ratifying and monitoring systems of risk management and internal control, codes of conduct, and legal compliance. In addition, the Audit Committee is responsible for (i) discussing guidelines and policies to govern the process by which risk assessment and management is undertaken and handled; and (ii) reviewing the effectiveness and adequacy of our internal control structure and considering with management, the internal auditors and the independent auditor whether any changes to the internal control structure are appropriate.
We have implemented an enterprise-risk management program, which is designed to ensure that risks including, amongst others, technology risks, economic risks, financial risks, regulatory risks and other operational risks are identified, evaluated and mitigated to enable the achievement of our goals.
We refer shareholders to the “Explanatory Note” above which discusses the results of the Investigation and to Item 9A of this Annual Report on Form 10-K which discusses Material Internal Control Weaknesses and the Company’s remediation plans related thereto.
Recommendation 7.2 — Review the company’s risk management framework at least annually so that it continues to be sound and disclose whether such a review has taken place
Management provides the board of directors with frequent updates on the state of our business, including the risks that we face from time-to-time. These updates include up-to-date financial information, operational activity, clinical status and competitor updates. Management provides the board of directors with an annual review of management’s enterprise risk management assessment, which identifies particular events or circumstances relevant to our objectives (risks and opportunities), assessing them in terms of likelihood and magnitude of impact, determining a response strategy, and monitoring progress. These updates are founded on internal communications that are fostered internally through management meetings and other internal communications. These processes operate in addition to our Quality System, complaint handling processes, employee policies and standard operating procedures. The
112
board of directors conducted its last annual review of management’s enterprise risk management assessment in May 2015 and will conduct its next review in December 2016.
As a U.S. public company, we are required to comply with the provisions of the Sarbanes-Oxley Act of 2002, which requires us to establish policies and internal controls over financial reporting to reduce certain risks to the business, including potential fraud. Our internal controls are audited annually by KPMG, its auditors, and by Protiviti, an independent consultant, to ensure compliance, and the results of their audits are reported to the audit committee. The Chief Executive Officer and Chief Financial Officer are required to evaluate our internal controls on an annual basis and to certify to our auditors and the audit committee the following:
|
|
• |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting, which are reasonably likely to adversely affect our ability to record, process, summarize and report financial information; and |
|
|
• |
Any fraud, whether or not material, that involves management or other employees who have a significant role in our internal control over financial reporting. |
In addition, the board of directors holds regular meetings for the purposes of discussing and reviewing operational developments.
We refer shareholders to the “Explanatory Note” above which discusses the results of the Investigation and to Item 9A of this Annual Report on Form 10-K which discusses Material Internal Control Weaknesses and the Company’s remediation plans related thereto.
Recommendation 7.3 — Disclose if the Company has an internal audit function, how the function is structured and what role it performs. If the Company does not have an internal audit function, disclose that fact and the processes it employs for evaluating and continually improving the effectiveness of its risk management and internal control processes
Due to our current size and business circumstances, we have not had an internal audit function.
As required under the Sarbanes-Oxley Act of 2002, we are required to evaluate and test our internal controls over financial reporting and we engage the services of an outside consultant to assist in carrying out these duties. As the outside consultant is independent of management, it provides for ongoing evaluation and opportunities to improve our risk management and internal processes.
We refer shareholders to the “Explanatory Note” above which discusses the results of the Investigation and to Item 9A of this Annual Report on Form 10-K which discusses Material Internal Control Weaknesses and the Company’s remediation plans related thereto, including that we intend to implement an internal audit function and develop a risk based plan that will monitor our adherence to its policies and procedures.
Recommendation 7.4 — Disclose any material exposure to economic, environmental and social sustainability risks and, if it does, how it manages those risks
We discuss material risks we face in Item 1A of this Annual Report on Form 10-K, including economic, environmental and social sustainability risks. We manage risk in accordance with the processes and procedures discussed in response to Recommendations 7.1 through 7.3.
Principle 8 — Remunerate fairly and responsibly
Recommendation 8.1 — Establish a Remuneration Committee
We have established a Compensation Committee to review and assess executive and director compensation. A copy of the Compensation Committee Charter is available on the corporate governance section of our website.
Our Compensation Committee consists of three members, all of whom are independent directors (including the Chairman of the Compensation Committee). The members of the Compensation Committee are Mr. Lund (Chair), Mr. Galle and Mr. Hamill.
Recommendation 8.2 — Disclose policies and practices of the company regarding the remuneration of non-executive and remuneration of executive directors and other senior executives
Our Compensation Committee is responsible for making recommendations to the Board in relation to the Company’s policies and practices regarding the remuneration of non-executive directors and the remuneration of executive directors and other senior
113
executives, separately. Further details regarding the compensation policies and practices for executive and non-executive directors and other senior executives will be set out in our Annual Proxy statement.
A copy of the Compensation Committee Charter is available on our website.
Recommendation 8.3 — Establish a policy on whether participants in an equity-based compensation plan are permitted to enter into transactions which limit the economic risk of participating in the plan and disclose that policy or summary of it
Our Insider Trading Policy, which is available on the corporate governance section of our website, restricts participants in our Amended and Restated 2009 Stock Incentive Plan from entering into transactions which limit the economic risk of participating in such plan.
Additional ASX Required Disclosures
|
|
(a) |
There were 41 board meetings and the audit, compensation, and nominating and corporate governance committees held 6, 13 and 2 meetings, respectively, during the reporting period. Director attendance at Board and Committee meetings is set out below. |
|
|
|
Meetings of the Board |
|
|
Audit Committee |
|
|
Compensation Committee |
|
|
Nominating and Governance Committee |
|
|
||||
|
Alan Shortall* |
|
|
25 |
|
|
|
2 |
|
** |
|
8 |
|
** |
|
1 |
|
** |
|
Slavko James Joseph (Jim) Bosnjak* |
|
|
2 |
|
|
|
— |
|
|
|
1 |
|
|
|
— |
|
|
|
William Galle |
|
|
35 |
|
|
|
2 |
|
** |
|
13 |
|
|
|
2 |
|
|
|
John Lund |
|
|
38 |
|
|
|
6 |
|
|
|
12 |
|
|
|
2 |
|
|
|
Mary Kate Wold |
|
|
40 |
|
|
|
6 |
|
|
|
9 |
|
** |
|
2 |
|
|
|
Jeff Carter |
|
|
34 |
|
|
|
2 |
|
** |
|
5 |
|
** |
|
1 |
|
** |
|
Harry Hamill* |
|
|
38 |
|
|
|
5 |
|
|
|
12 |
|
|
|
2 |
|
** |
|
* |
Only served on the Board for a portion of the reporting period. |
|
** |
Did not serve on the Committee during the reporting period. |
Two of our current directors, John Ryan and Michael Kamarck, are not included in the above table as they were appointed to the Board after the conclusion of the reporting period.
|
|
(b) |
The names of Unilife’s substantial holders, and the number of equity securities in which each substantial holder and their associates have a relevant interest, as disclosed in substantial holding notices given to Unilife. If a substantial holding notice discloses that related bodies corporate have the same relevant interest in the same number of equity securities, the annual report need only include the name of the holding company. |
The names of our substantial holders and their respective equity holdings are set forth in our annual Proxy Statement under “Security Ownership of Certain Beneficial Owners and Management.”
|
|
(c) |
If during the reporting period any securities were purchased on-market: (i) under or for the purposes of an employee incentive plan; or (ii) to satisfy the entitlements of the holders of options or other rights to acquire securities granted under an employee incentive plan, then disclose (i) the total number of securities purchased during the reporting period and (ii) the average price per security at which the securities were purchased during the reporting period. |
We did not purchase any securities on market: (i) under or for the purposes of an employee incentive plan; or (ii) to satisfy the entitlements of the holders of options or other rights to acquire securities granted under an employee incentive plan.
The information required by this Item 13 is incorporated by reference to the 2016 Proxy Statement under the headings “– Information on Our Board of Directors and Corporate Governance” and “Certain Relationships and Related Party Transactions.”
114
The information required by this Item 14 is incorporated into this report by reference to the 2016 Proxy Statement under the heading “Proposal No. 2 – Ratification of Appointment of the Independent Registered Public Accounting Firm.”
115
(a) Documents filed as part of this report:
(1) Financial Statements
The financial statements required by this Item 15 are set forth in Part II, Item 8 of this report.
(b) Exhibits. The following Exhibits are filed as a part of this report:
|
Incorporated by Reference Herein |
|
|
|
|
|
|||||
|
Exhibit |
|
Description of Exhibit |
|
Included |
|
Form |
|
Exhibit |
|
Filing Date |
|
3.1 |
|
Certificate of Incorporation of Unilife Corporation |
|
|
|
10 |
|
3.1 |
|
November 12, 2009 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.2 |
|
Certificate of Designations of Series A Redeemable Convertible Preferred Stock of Unilife Corporation, filed with the Secretary of State of the State of Delaware on November 6, 2015 |
|
|
|
8-K |
|
3.1 |
|
November 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.3 |
|
Certificate of Amendment to Certificate of Incorporation of Unilife Corporation |
|
|
|
8-K |
|
3.1 |
|
November 17, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.4 |
|
Certificate of Amendment to Certificate of Incorporation of Unilife Corporation |
|
|
|
8-K |
|
3.1 |
|
May 12, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
3.5 |
|
Amended and Restated Bylaws of Unilife Corporation |
|
|
|
8-K |
|
3.1 |
|
August 17, 2010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
4.1 |
|
Form of Common Stock Certificate |
|
|
|
10 |
|
4.1 |
|
November 12, 2009 |
|
|
|
|
|
|
|
|
|
|
|
|
|
4.2 |
|
Form of Indenture |
|
|
|
S-3 |
|
4.4 |
|
June 30, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1+ |
|
Consultancy Agreement, dated as of January 22, 2009 between Unilife Medical Solutions Limited and Joblak Pty Ltd |
|
|
|
10 |
|
10.15 |
|
November 12, 2009 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.2+ |
|
Unilife Corporation Amended and Restated 2009 Stock Incentive Plan |
|
|
|
DEF 14A |
|
Annex A |
|
October 2, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.3+ |
|
Unilife Medical Solutions Limited Exempt Employee Share Plan |
|
|
|
10 |
|
10.19 |
|
November 12, 2009 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.4 |
|
Amended and Restated Operating Agreement dated December 14, 2009 of Unilife Cross Farm LLC |
|
|
|
10/A |
|
10.26 |
|
January 6, 2010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.5+ |
|
Form of Unilife Corporation Nonstatutory Stock Option Notice |
|
|
|
10-Q |
|
10.2 |
|
March 24, 2010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.6+ |
|
Employment Agreement, dated as of July 27, 2010 between Unilife Corporation and Dennis P. Pyers |
|
|
|
10-K |
|
10.46 |
|
September 28, 2010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.7 |
|
Loan Agreement between Metro Bank and Unilife Cross Farm LLC dated as of October 20, 2010 |
|
|
|
8-K |
|
10.1 |
|
October 26, 2010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.8 |
|
Term Note in the principal amount of $14,250,000 dated as of October 20, 2010 |
|
|
|
8-K |
|
10.2 |
|
October 26, 2010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.9 |
|
Guaranty and Suretyship Agreement dated October 20, 2010 (Unilife Corporation) |
|
|
|
8-K |
|
10.4 |
|
October 26, 2010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.10 |
|
Guaranty and Suretyship Agreement dated October 20, 2010 (Unilife Medical Solutions, Inc.) |
|
|
|
8-K |
|
10.5 |
|
October 26, 2010 |
|
|
|
|
|
|
|
|
|
|
|
|
116
|
Incorporated by Reference Herein |
|
|
|
|
|
|||||
|
Exhibit |
|
Description of Exhibit |
|
Included |
|
Form |
|
Exhibit |
|
Filing Date |
|
|
Form of Warrant issued to Keystone Redevelopment Group, LLC and L2 Architecture on December 2, 2010 |
|
|
|
POS AM |
|
10.58 |
|
December 10, 2010 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.12+ |
|
Employment Agreement, effective October 1, 2011 between Unilife Corporation and Alan D. Shortall |
|
|
|
10-Q |
|
10.4 |
|
November 9, 2011 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.13+ |
|
Employment Agreement, effective July 1, 2012 between Unilife Corporation and Ramin Mojdeh, Ph.D. |
|
|
|
8-K |
|
10.1 |
|
June 15, 2012 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.14+ |
|
Letter Agreement, dated May 14, 2013, between Unilife Corporation and Ramin Mojdeh, Ph.D. |
|
|
|
10-K |
|
10.75 |
|
September 13, 2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.15+ |
|
Amendment to Employment Agreement, effective September 12, 2013 between Unilife Corporation and Ramin Mojdeh, Ph.D. |
|
|
|
10-K |
|
10.76 |
|
September 13, 2013 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.16* |
|
Credit Agreement, dated as of March 12, 2014, by and between Unilife Medical Solutions, Inc. and ROS Acquisition Offshore LP |
|
|
|
10-Q/A |
|
10.1 |
|
September 29, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.17 |
|
Royalty Agreement, dated as of March 12, 2014, by and between Royalty Opportunities S.A.R.L. and Unilife Medical Solutions, Inc. |
|
|
|
10-Q/A |
|
10.2 |
|
September 29, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.18 |
|
General Security Deed, dated as of March 12, 2014, by Unitract Syringe Pty Limited, Unilife Medical Solutions Limited and Unilife Corporation in favor of ROS Acquisition Offshore LP |
|
|
|
10-Q |
|
10.3 |
|
May 12, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.19 |
|
Omnibus Waiver and Amendment, dated as of March 12, 2014, by and among Unilife Cross Farm LLC, Unilife Medical Solutions, Inc., Unilife Corporation and Metro Bank |
|
|
|
10-Q |
|
10.4 |
|
May 12, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.20 |
|
Guarantee, dated as of March 12, 2014, by Unilife Corporation, Unilife Cross Farms LLC, Unilife Medical Solutions Limited and Unitract Syringe Pty Limited in favor of ROS Acquisition Offshore LP and Royalty Opportunities S.A.R.L. |
|
|
|
10-Q |
|
10.7 |
|
May 12, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.21 |
|
Pledge and Security Agreement, dated as of March 12, 2014, by Unilife Medical Solutions, Inc., Unilife Cross Farms LLC, Unilife Medical Solutions Limited and Unitract Syringe Pty Limited in favor of ROS Acquisition Offshore LP |
|
|
|
10-Q/A |
|
10.3 |
|
September 29, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.22 |
|
Open-End Commercial Mortgage and Security Agreement, dated as of March 12, 2014, by and between Unilife Cross Farms LLC and ROS Acquisition Offshore LP, for itself and as agent for Royalty Opportunities S.A.R.L. |
|
|
|
10-Q |
|
10.9 |
|
May 12, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.23+ |
|
Employment Agreement, dated September 15, 2014, between Unilife Corporation and John C. Ryan, Esq. |
|
|
|
8-K |
|
10.1 |
|
September 19, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.24+ |
|
Amendment to Employment Agreement, dated September 15, 2014, between Unilife Corporation and Alan D. Shortall |
|
|
|
8-K |
|
10.2 |
|
September 19, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
117
|
Incorporated by Reference Herein |
|
|
|
|
|
|||||
|
Exhibit |
|
Description of Exhibit |
|
Included |
|
Form |
|
Exhibit |
|
Filing Date |
|
|
Amendment to Employment Agreement, dated September 17, 2014, between Unilife Corporation and Alan D. Shortall |
|
|
|
8-K |
|
10.3 |
|
September 19, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.26+ |
|
Amendment to Employment Agreement, dated September 15, 2014, between Unilife Corporation and Ramin Mojdeh, Ph.D. |
|
|
|
8-K |
|
10.4 |
|
September 19, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.27+ |
|
Amendment to Employment Agreement, dated September 17, 2014, between Unilife Corporation and Ramin Mojdeh, Ph.D. |
|
|
|
8-K |
|
10.5 |
|
September 19, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.28 |
|
First Amendment to the Credit Agreement, dated September 30, 2014, between Unilife Medical Solutions, Inc. and ROS Acquisition Offshore LP |
|
|
|
10-Q |
|
10.1 |
|
November 12, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.29 |
|
First Amendment to the Royalty Agreement, dated September 30, 2014, between Unilife Medical Solutions, Inc. and Royalty Opportunities S.A.R.L. |
|
|
|
10-Q |
|
10.2 |
|
November 12, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.30+ |
|
Employment Agreement, dated November 6, 2014, between Unilife Corporation and Mark V. Iampietro |
|
|
|
10-Q |
|
10.3 |
|
November 12, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.31+ |
|
Employment Agreement, dated January 9, 2015, between Unilife Corporation and David C. Hastings |
|
|
|
8-K |
|
10.1 |
|
January 14, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.32+ |
|
Amendment to Employment Agreement, dated January 9, 2015, between Unilife Corporation and Ramin Mojdeh, Ph.D. |
|
|
|
8-K |
|
10.3 |
|
January 14, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.33+ |
|
Amendment to Employment Agreement, dated January 9, 2015, between Unilife Corporation and John C. Ryan |
|
|
|
8-K |
|
10.4 |
|
January 14, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.34+ |
|
Form of Restricted Stock Agreement under the Unilife Corporation 2009 Stock Incentive Plan, dated November 14, 2014, between Unilife Corporation and Alan D. Shortall (4,000,000 shares of common stock) |
|
|
|
S-8 |
|
4.2 |
|
November 14, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.35+ |
|
Form of Restricted Stock Agreement under the Unilife Corporation Amended and Restated 2009 Stock Incentive Plan |
|
|
|
10-Q |
|
10.1 |
|
February 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.36+ |
|
Form of Restricted Stock Units Notice under the Unilife Corporation Amended and Restated 2009 Stock Incentive Plan (US Directors) |
|
|
|
10-Q |
|
10.2 |
|
February 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.37+ |
|
Form of Restricted Stock Units Notice under the Unilife Corporation Amended and Restated 2009 Stock Incentive Plan (Employees) |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.38+ |
|
Form of Restricted Stock Agreement under the Unilife Corporation Amended and Restated 2009 Stock Incentive Plan (Australian Directors) |
|
|
|
10-Q |
|
10.3 |
|
February 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.39 |
|
Purchase Agreement, dated as of July 29, 2015, between Unilife Corporation and Lincoln Park Capital Fund, LLC |
|
|
|
8-K (Film No. 151014075) |
|
10.1 |
|
July 30, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
118
|
Incorporated by Reference Herein |
|
|
|
|
|
|||||
|
Exhibit |
|
Description of Exhibit |
|
Included |
|
Form |
|
Exhibit |
|
Filing Date |
|
|
Controlled Equity OfferingSM Sales Agreement, dated July 29, 2015, between Unilife Corporation and Cantor Fitzgerald & Co. |
|
|
|
8-K (Film No. 151014081) |
|
10.1 |
|
July 30, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.41 |
|
Second Amendment to the Credit Agreement, dated June 30, 2015, between Unilife Medical Solutions, Inc. and ROS Acquisition Offshore LP |
|
|
|
10-K |
|
10.43 |
|
September 14, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.42+ |
|
Employment Agreement, dated September 10, 2015 between Unilife Corporation and Dennis P. Pyers |
|
|
|
10-K |
|
10.44 |
|
September 14, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.43 |
|
Third Amendment to Credit Agreement, dated October 13, 2015 by and among Unilife Medical Solutions, Inc. and ROS Acquisition Offshore LP. |
|
|
|
8-K |
|
10.1 |
|
October 16, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.44 |
|
Second Amendment to Royalty Agreement, dated October 13, 2015 by and among Unilife Medical Solutions, Inc. and Royal Opportunities S.À R.L. |
|
|
|
8-K |
|
10.2 |
|
October 16, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.45 |
|
Amended and Restated Promissory Note, dated as of October 13, 2015, for up to $70,000,000 by Unilife Medical Solutions, Inc. in favor of ROS Acquisition Offshore LP |
|
|
|
8-K |
|
10.3 |
|
October 16, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.46 |
|
Waiver to Credit Agreement, dated November 6, 2015 by and among Unilife Medical Solutions, Inc. and ROS Acquisition Offshore |
|
|
|
10-Q |
|
10.4 |
|
November 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.47 |
|
Promissory Note, dated September 30, 2015, for $600,000 by Unilife Corporation in favor of Alan D. Shortall |
|
|
|
10-Q |
|
10.5 |
|
November 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.48+ |
|
Fourth Amendment to Employment Agreement, dated October 13, 2015, by and between Unilife Corporation and Alan D. Shortall |
|
|
|
10-Q |
|
10.6 |
|
November 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.49+ |
|
Fifth Amendment to Employment Agreement, dated October 13, 2015, by and between Unilife Corporation and Ramin Mojdeh, Ph.D. |
|
|
|
10-Q |
|
10.7 |
|
November 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.50+ |
|
First Amendment to Employment Agreement, dated October 13, 2015, by and between Unilife Corporation and David C. Hastings |
|
|
|
10-Q |
|
10.8 |
|
November 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.51+ |
|
First Amendment to Employment Agreement, dated October 13, 2015, by and between Unilife Corporation and Dennis P. Pyers |
|
|
|
10-Q |
|
10.9 |
|
November 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.52+ |
|
Second Amendment to Employment Agreement, dated October 13, 2015, by and between Unilife Corporation and John C. Ryan |
|
|
|
10-Q |
|
10.10 |
|
November 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.53 |
|
Stock Purchase Agreement, dated November 9, 2015, by and between the Company and an institutional investor |
|
|
|
8-K |
|
10.1 |
|
November 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.54 |
|
Waiver and Consent Agreement, dated November 9, 2015, by and between the Company and Lincoln Park Capital Fund, LLC |
|
|
|
8-K |
|
10.2 |
|
November 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
119
|
Incorporated by Reference Herein |
|
|
|
|
|
|||||
|
Exhibit |
|
Description of Exhibit |
|
Included |
|
Form |
|
Exhibit |
|
Filing Date |
|
|
Letter Agreement, dated September 29, 2016, between Unilife Corporation, Unilife Medical Solutions, Inc. and Amgen Inc. |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.56 |
|
Letter Agreement, dated September 30, 2016, between Unilife Medical Solutions, Inc. and ROS Acquisition Offshore LP |
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10.57 |
|
Warrant to Purchase Common Stock, dated November 9, 2015, issued by the Company to Lincoln Park Capital Fund, LLC |
|
|
|
8-K |
|
10.3 |
|
November 9, 2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.58 |
|
Fourth Amendment to the Credit Agreement, dated December 31, 2015, by and among Unilife Medical Solutions, Inc., ROS Acquisition Offshore LP and the other Creditor Obligors party thereto |
|
|
|
8-K |
|
10.1 |
|
January 7, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.59* |
|
Exclusivity Agreement, dated December 31, 2015, by and between the Company and Amgen Inc. |
|
|
|
10-Q/A |
|
10.14 |
|
August 9, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.60 |
|
Fifth Amendment to the Credit Agreement, dated January 31, 2016, by and among Unilife Medical Solutions, Inc., ROS Acquisition Offshore LP and the other Creditor Obligors party thereto |
|
|
|
10-Q |
|
10.1 |
|
October 24, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.61 |
|
Third Amendment to Royalty Agreement, dated January 31, 2016, by and among Unilife Medical Solutions, Inc. and Royal Opportunities S.À R.L. |
|
|
|
10-Q |
|
10.2 |
|
October 24, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.62 |
|
First Amendment to Exclusivity Agreement, dated January 31, 2016, by and between Unilife Corporation and Amgen Inc. |
|
|
|
10-Q |
|
10.3 |
|
October 24, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.63 |
|
First Amendment to Stock Purchase Agreement, dated February 3, 2016, by and between Unilife Corporation and an institutional investor |
|
|
|
8-K |
|
10.1 |
|
February 3, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.64 |
|
Second Amendment to Exclusivity Agreement, dated February 5, 2016, by and between Unilife Corporation and Amgen Inc. |
|
|
|
10-Q |
|
10.5 |
|
October 24, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.65 |
|
Sixth Amendment to the Credit Agreement, dated February 9, 2016, by and among Unilife Medical Solutions, Inc., ROS Acquisition Offshore LP and the other Creditor Obligors party thereto |
|
|
|
10-Q |
|
10.6 |
|
October 24, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.66 |
|
Fourth Amendment to Royalty Agreement, dated February 9, 2016, by and among Unilife Medical Solutions, Inc. and Royal Opportunities S.À R.L. |
|
|
|
10-Q |
|
10.7 |
|
October 24, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.67 |
|
Third Amendment to Exclusivity Agreement, dated February 15, 2016, by and between Unilife Corporation and Amgen Inc. |
|
|
|
10-Q |
|
10.8 |
|
October 24, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.68 |
|
Seventh Amendment to the Credit Agreement, dated February 16, 2016, by and among Unilife Medical Solutions, Inc., ROS Acquisition Offshore LP and the other Creditor Obligors party thereto |
|
|
|
10-Q |
|
10.9 |
|
October 24, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.69 |
|
Fifth Amendment to Royalty Agreement, dated February 16, 2016, by and among Unilife Medical Solutions, Inc. and Royal Opportunities S.À R.L. |
|
|
|
10-Q |
|
10.10 |
|
October 24, 2016 |
|
|
|
|
|
|
|
|
|
|
|
|
120
121
|
+ |
Indicates a management contract or compensatory plan. |
|
* |
Confidential treatment has been requested for certain provisions of this Exhibit pursuant to Rule 24b-2 under the Securities Exchange Act of 1934, as amended. |
|
# |
This certification is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended. or otherwise subject to the liability of that section, nor shall it be deemed incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended. |
122
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
UNILIFE CORPORATION |
||
|
|
|
|
|
By: |
|
/s/ John Ryan |
|
Name: |
|
John Ryan |
|
Title: |
|
President and Chief Executive Officer |
|
|
|
|
|
Date: |
|
October 21, 2016 |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
|
Name |
|
Title |
|
Date |
|
|
|
|
||
|
/s/ John Ryan |
|
President and Chief Executive Officer |
|
October 21, 2016 |
|
John Ryan |
|
(Principal Executive Officer) |
|
|
|
|
|
|
||
|
/s/ David C. Hastings |
|
Senior Vice President, Chief Financial Officer, Chief Accounting Officer and Treasurer |
|
October 21, 2016 |
|
David C. Hastings |
|
(Principal Financial and Accounting Officer) |
|
|
|
|
|
|
||
|
/s/ Mary Katherine Wold |
|
Chair |
|
October 21, 2016 |
|
Mary Katherine Wold |
|
|
|
|
|
|
|
|
||
|
/s/ John Lund |
|
Director |
|
October 21, 2016 |
|
John Lund |
|
|
|
|
|
|
|
|
||
|
/s/ Harry A. Hamill |
|
Director |
|
October 21, 2016 |
|
Harry A. Hamill |
|
|
|
|
|
|
|
|
||
|
/s/ Jeff Carter |
|
Director |
|
October 21, 2016 |
|
Jeff Carter |
|
|
|
|
|
|
|
|
||
|
/s/ William Galle |
|
Director |
|
October 21, 2016 |
|
William Galle |
|
|
|
|
|
|
|
|
||
|
/s/ Michael E. Kamarck |
|
Director |
|
October 21, 2016 |
|
Michael E. Kamarck |
|
|
|
|
|
|
|
|
||
|
/s/ John Ryan |
|
Director |
|
October 21, 2016 |
|
John Ryan |
|
|
|
|
123
