Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REVA Medical, Inc. | d479983d8k.htm |
Exhibit 99.1

Chairman’s Letter to Shareholders
7 February 2013
San Diego, California and Sydney, Australia (Thursday 7 February 2013, AEDT) – REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”) is pleased to provide an update on the Company’s activities in the attached letter to shareholders from its Chairman and Chief Executive Officer, Mr. Robert Stockman.
About REVA
REVA is a development stage medical device company incorporated in Delaware, USA, that is focused on the development and eventual commercialization of its proprietary bioresorbable stent products. REVA’s initial product, the ReZolve® scaffold, which is in a clinical study phase, combines REVA’s proprietary stent design with a proprietary polymer that is metabolized and cleared from the body. The ReZolve scaffold is designed to offer full x-ray visibility, clinically relevant sizing, and a controlled and safe resorption rate. In addition, by early encapsulation of the stent in the artery tissue coupled with the loss of scaffold structure over time, the ReZolve scaffold may reduce the incidence of late forming blood clots or otherwise reduce long-term disease progression, potential benefits of bioresorbable scaffolds that have yet to be proven. REVA will require clinical results and regulatory approval before it can begin selling the ReZolve scaffold.
Forward-Looking Statements
This announcement contains or may contain forward-looking statements that are based on management’s beliefs, assumptions and expectations and on information currently available to management. All statements that are not statements of historical fact, including those statements that address future operating performance and events or developments that we expect or anticipate will occur in the future, are forward-looking statements, such as those statements regarding our ability to obtain the regulatory approvals required to market our ReZolve® scaffold, our ability to timely and successfully complete our clinical trials, our ability to protect our intellectual property position, our ability to commercialize our products if and when approved, our ability to develop and commercialize new products, and our estimates regarding our capital requirements and financial performance, including profitability. You should not place undue reliance on these forward-looking statements. Although management believes these forward-looking statements are reasonable as and when made, forward-looking statements are subject to a number of risks and uncertainties that may cause our actual results to vary materially from those expressed in the forward-looking
HEAD OFFICE: 5751 Copley Drive, San Diego, CA 92111 — +1 (858) 966-3000 — +1 (858) 966-3099 (FAX) — www.revamedical.com
AUSTRALIAN OFFICE: Level 6, 175 Macquarie Street, Sydney, NSW 2000 — +61 2 9231 3322 — +61 9229 2727 (FAX) — ARBN 146 505 777
REVA Medical, Inc., is a foreign company incorporated in Delaware, USA, whose stockholders have limited liability.
| REVA Medical, Inc. – ASX Announcement | Page 2 |
statements, which risks and uncertainties are described in the “Risk Factors” section of our Annual Report on Form 10-K filed with the United States Securities and Exchange Commission (the “SEC”) on 29 February 2012. Any forward-looking statements in this announcement speak only as of the date when made. REVA does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
| United States
Investor and Media Enquiries: Cheryl Liberatore Director, Investor Relations and Marketing REVA Medical, Inc. +1 858 966-3045 |
Australia
Investor Enquiries: Kim Jacobs or Alan Taylor Inteq Limited +61 2 9231 3322
Media Enquiries: Haley Price or Rebecca Wilson Buchan Consulting +61 3 9866 4722 |
HEAD OFFICE: 5751 Copley Drive, San Diego, CA 92111 — +1 (858) 966-3000 — +1 (858) 966-3099 (FAX) — www.revamedical.com
AUSTRALIAN OFFICE: Level 6, 175 Macquarie Street, Sydney, NSW 2000 — +61 2 9231 3322 — +61 9229 2727 (FAX) — ARBN 146 505 777
REVA Medical, Inc., is a foreign company incorporated in Delaware, USA, whose stockholders have limited liability.

Letter from the Chairman & CEO
7 February 2013

7 February 2013
Dear Shareholder,
I am pleased to provide this update of our operations, clinical trial program, market developments, and upcoming events. We remain singularly focused on bringing our ReZolve®2 sirolimus-eluting bioresorbable coronary scaffold to market. This year will be transformational as we work to complete the critical milestones of analyzing and presenting the clinical data of ReZolve, enrolling patients in clinical trials of our commercial platform, ReZolve2, and laying the foundations for commercialization.
Operations Update and Clinical Program
2013 focus is to establish compelling clinical data and lay the groundwork for commercialization
During 2012 we completed enrollment in our RESTORE pilot study, which is evaluating the performance of the ReZolve scaffold that was implanted in 22 patients. We reported interim data, including data on a subset of 12 patients that had completed six-month clinical follow-up, at the TCT conference in October 2012. In those patients, there were no reported blood clots (“thrombosis”), no reported incidences of myocardial infarction (“heart attack”) and a low rate of major adverse coronary events (also known as “MACE”). Today, all 22 patients have completed six-month follow-up and we are pleased to announce that there have been no additional reported clinical events through the six-month time point.
During 2013 these patients will undergo a 12-month imaging assessment to quantitatively evaluate scaffold performance. This will be accomplished by looking inside the stented artery to evaluate the amount of residual obstruction as well as overall healing. We completed imaging assessments in two patients at an early six-month time point, and our first patient has now undergone a 12-month imaging assessment. In these three cases the stented artery is wide open (or “patent”) and it is evident the device is performing as designed (the image on the next page is from the 12-month imaging assessment). We are pleased with the results we are seeing so far and look forward to presenting more comprehensive clinical and imaging data, including any change in MACE events between six and 12 months, at the Paris Course on Revascularization (“EuroPCR”) that will be held this May in Paris, France.
HEAD OFFICE: 5751 Copley Drive, San Diego, CA 92111 — +1 (858) 966-3000 — +1 (858) 966-3099 (FAX) — www.revamedical.com
AUSTRALIAN OFFICE: Level 6, 175 Macquarie Street, Sydney, NSW 2000 — +61 2 9231 3322 — +61 9229 2727 (FAX) — ARBN 146 505 777
REVA Medical, Inc., is a foreign company incorporated in Delaware, USA, whose stockholders have limited liability.
Letter from the Chairman & CEO, 7 February 2013
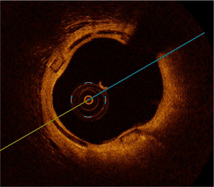
OCT image from 12-month assessment showing a patent artery
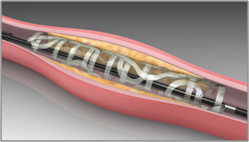
In May of last year, we announced plans to migrate from our ReZolve scaffold to a lower profile and sheathless version that will be our commercial product. This advanced product, named ReZolve2, has a reduction in profile of approximately 20%, which makes it compatible with the standard 6 French guide catheter that is customarily used to deliver stents in coronary arteries. ReZolve2 also offers a 30% increase in scaffold strength. We believe these improvements will position ReZolve2 for rapid adoption by physicians once we receive regulatory approval for commercialization.

|
|
Undeployed and deployed ReZolve2 lower-profile scaffold
We believe our early move to the ReZolve2 platform will allow REVA to deliver the most advanced and differentiated bioresorbable scaffold to patients and to the clinical community. During our most recent briefing call in November 2012, we discussed our anticipated timing for the initiation of clinical trials with ReZolve2 in up to 125 patients at approximately 30 clinical sites in Brazil, Europe, Australia, and New Zealand. This month we are shipping clinical supplies to multiple centers and will begin to screen patients for enrollment with ReZolve2.
In addition to our clinical trial milestones for 2013, which will include presenting 12-month data from the pilot study and enrolling patients with ReZolve2, REVA will continue to focus on building the manufacturing capacity and marketing infrastructure necessary to support commercial sales of ReZolve2 upon receipt of CE Marking. This will include increasing the manufacturing capacity at our San Diego facility to ensure we can meet both our clinical trial needs and market demand upon launch. We will also develop additional scaffold sizes and establish international resources to support sales of ReZolve2 in various international markets. This transition from a development-stage organization to a commercial company will occupy a large portion of REVA’s effort and resources over the next 18 months.
- 2 -
Letter from the Chairman & CEO, 7 February 2013
Bioresorbable Scaffolds Gaining Momentum
The launch of Absorb highlights the potential of bioresorbable scaffolds
The coronary stent market is substantial, with estimated 2012 worldwide revenues of $4.8 billion. However, falling prices for bare and drug-eluting metal stents have led to a decrease in revenues over the past several years. Today the market is primed for the introduction of innovative products such as bioresorbable scaffolds, which should add to revenue growth primarily due to premium pricing (the number of procedures are expected to increase modestly, at 2% to 3%, over the next few years). The market leaders for metal stents are Abbott, Boston Scientific and Medtronic. Of the three, only Abbott offers a fully bioresorbable drug-eluting scaffold option.
Abbott began selling its Absorb™ bioresorbable scaffold in limited markets in the first part of 2012 and expanded the launch in September. Currently Absorb is available in 30 countries in Europe, Asia, Latin America and the Middle East. Abbott has obtained formal reimbursement approval for Absorb in Germany with a list price of nearly €3,000, well above the current pricing of metal drug-eluting stents. Despite the fact that bioresorbable scaffolds are in their infancy, their potential to play a major role in the treatment of cardiovascular disease is being quickly recognized as conversion from metal stents to polymer scaffolds is beginning to unfold in markets where the product is available. In their January 2013 analyst report on Abbott, BMO Capital estimates the potential penetration of Absorb could be as high as 50% of cases.
At the J.P. Morgan Healthcare Conference, held last month in San Francisco, California, Abbott announced the initiation of its U.S. clinical trial program. Abbott has indicated that the ABSORB III trial will enroll 2,250 patients, and will compare Absorb against Abbott’s market-leading drug-eluting stent, Xience V. A non-inferiority finding will allow Abbott to obtain U.S. approval. Additionally, Abbott expressed plans to enroll 4,000 patients in the ABSORB IV trial, which Abbott indicated will compare target lesion failure rates between the Xience V and Absorb between one and five years. A finding of superiority against Xience V would demonstrate added clinical benefits of bioresorbable scaffolds, which may lead to differentiated product claims and potentially higher reimbursement rates in the U.S. and internationally.
REVA’s Value Proposition
We believe REVA’s potential value is greater than market activity suggests
Many of you have expressed concerns about REVA’s depressed market price. We share your frustration with the drop in share price and with the limited trading liquidity. We also appreciate the questions investors may have between news events, especially when the gaps are lengthy. We expect 2013 will be full of important events as outlined in this letter. We also believe that if Abbott continues to successfully promote the Absorb scaffold, the spotlight on REVA will increase among investors, clinicians and the broader cardiology community.
We recently announced the release of stock options and shares of common stock from mandatory escrow, including shares that I hold. I want to assure shareholders that I have no plans to sell my positions on the open market. Our board of directors and I remain excited about REVA’s prospects. As 2013 unfolds, and we successfully meet the milestones delineated previously in this letter, we believe that we will be building substantial additional value in REVA.
- 3 -
Letter from the Chairman & CEO, 7 February 2013
REVA Events
In January we presented at the J.P. Morgan Healthcare Conference in San Francisco. A copy of the J.P. Morgan presentation is available on the “Investors” section of our website at www.revamedical.com. We have been invited to present at other major U.S. investment banking conferences during 2013 and some of these are new for us, which indicates the rising awareness and interest of Wall Street in bioresorbable coronary scaffold technology. We are currently scheduled for presentations at the following banking and industry conferences:
| Cardiovascular Research Technologies (CRT) | Washington, DC | 25 February | ||
| Cowen and Company Health Care Conference | Boston, MA | 6 March | ||
| PCR Focus Group on Bioresorbable Scaffolds | Rotterdam, Netherlands | 7 – 9 March | ||
| Paris Course on Revascularization (EuroPCR) | Paris, France | 21 – 24 May | ||
| Transcatheter Cardiovascular Therapeutics (TCT) | San Francisco, CA | 27 October – 1 November |
Finally, I plan to visit Singapore, Hong Kong, and Australia between 11 March and 22 March. I look forward to having the opportunity to meet with many of you during these visits.
In Summary
The path of innovation is never without its challenges and set-backs. However, with talent, persistence, smart choices, and the support of our shareholders, challenges are overcome and the rewards can be great. REVA is in a strong position with our technology showing excellent initial clinical performance and an industry eager for innovative and differentiated new technologies. We look forward to announcing enrollment of our first ReZolve2 clinical patients shortly and continuing to update you on our progress throughout the year.
As always, we urge any investor to contact us with questions.
Kindest regards,

Robert Stockman
Chairman & Chief Executive Officer.
- 4 -
