Attached files
| file | filename |
|---|---|
| EX-31.2 - EX-31.2 - REVA Medical, Inc. | a59113exv31w2.htm |
| EX-31.1 - EX-31.1 - REVA Medical, Inc. | a59113exv31w1.htm |
| EX-32.1 - EX-32.1 - REVA Medical, Inc. | a59113exv32w1.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2010
or
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 000-54192
REVA MEDICAL, INC.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
33-0810505 (I.R.S. Employer Identification No.) |
| 5751 Copley Drive, Suite B, San Diego, CA (Address of principal executive offices) |
92111 (Zip Code) |
Registrant’s telephone number, including area code:
(858) 966-3000
(858) 966-3000
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Name of Each Exchange on Which Registered | |
| Common Stock, $0.0001 par value per share | Australian Securities Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by
Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for
such shorter period that the registrant was required to file such reports), and (2) has been
subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its
corporate Web site, if any, every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months
(or for such shorter period that the registrant was required to submit and post such files).
Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K
(§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of
registrant’s knowledge, in definitive proxy or information statements incorporated by reference in
Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a
non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated
filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
(Check one):
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer þ | Smaller reporting company o | |||
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the
Act). Yes o No þ
The
registrant did not have a public float on the last business day of its most recently completed second fiscal
quarter because there was no market for the registrant’s common equity as of such date.
As of March 15, 2011, there were 18,735,853 shares of the registrant’s Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
| Document Description | 10-K Part | |
Portions of the registrant’s notice of annual meeting of
stockholders and proxy statement to be filed pursuant to
Regulation 14A within 120 days after registrant’s fiscal year
end of December 31, 2010 are incorporated by reference into
Part III of this report
|
III |
REVA MEDICAL, INC.
FORM 10-K –– ANNUAL REPORT
For the Fiscal Year Ended December 31, 2010
For the Fiscal Year Ended December 31, 2010
Table of Contents
i
Table of Contents
PART I
Forward-Looking Statements
This Annual Report on Form 10-K contains forward-looking statements concerning our business,
operations, and financial performance and condition as well as our plans, objectives, and
expectations for business operations and financial performance and condition. Any statements
contained herein that are not of historical facts may be deemed to be forward-looking statements.
You can identify these statements by words such as “aim,” “anticipate,” “assume,” “believe,”
“could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,”
“potential,” “positioned,” “should,” “target,” “will,” “would,” and other similar expressions that
are predictions of or indicate future events and future trends. These forward-looking statements
are based on current expectations, estimates, forecasts, and projections about our business and the
industry in which we operate and management’s beliefs and assumptions and are not guarantees of
future performance or development and involve known and unknown risks, uncertainties, and other
factors that are in some cases beyond our control. As a result, any or all of our forward-looking
statements in this Form 10-K may turn out to be inaccurate. Factors that may cause such differences
include, but are not limited to, the risks described under “Risk Factors,” including:
| • | our history of net losses and our expectation of significant operating losses for the foreseeable future; |
| • | inability to obtain regulatory clearance or approval for any of our products; |
| • | increases in our projected expenditures on research and development and administrative activities; |
| • | failure of our ReZolvetm stent to meet our required clinical specifications; |
| • | failure of our products to gain market acceptance domestically or internationally; |
| • | less than anticipated growth in the market for bioresorbable stents generally; |
| • | changes in the regulatory environment which may adversely impact the commercialization of our products and result in significant additional capital expenditures; |
| • | inability to attract or retain skilled personnel for our product development and commercialization efforts; |
| • | inability to protect our intellectual property and operate our business without infringing upon the intellectual rights of others, which could result in litigation and significant expenditures; and |
| • | refusal of third-party payors to reimburse our customers for use of products. |
Potential investors and other readers are urged to consider these factors carefully in
evaluating the forward- looking statements and are cautioned not to place undue reliance on the
forward-looking statements. These forward- looking statements speak only as of the date of this
Form 10-K. Unless required by law, we do not intend to publicly update or revise any
forward-looking statements to reflect new information or future events or otherwise. You should,
however, review the factors and risks we describe in the reports we will file from time to time
with the Securities and Exchange Commission, or SEC, after the date of this Form 10-K.
Currency
Unless indicated otherwise in this Annual Report on Form 10-K, all references to “$” or
“dollars” refer to United States dollars, the lawful currency of the United States of America.
References to “A$” refer to Australian dollars, the lawful currency of the Commonwealth of
Australia.
Corporate Information
We were incorporated in Delaware in October, 2010. Our principal executive offices are located
at 5751 Copley Drive, Suite B, San Diego, CA 92111, and our telephone number is (858) 966-3000. Our
website address is www.revamedical.com. The information on, or accessible through, our
website is not part of this report. Unless the context implies otherwise, references in this report
and the information incorporated herein by reference to “REVA Medical,” “REVA,” the “Company,”
“we,” “us,” and “our” refer to REVA Medical, Inc.
1
Table of Contents
We have applied for trademark registrations for the trademark ReZolvetm in
the United States, European Union, Brazil, and Australia. All other trademarks, trade names, and
service marks appearing in this report are the property of their respective owners. Use or display
by us of other parties’ trademarks, trade dress, or products is not intended to and does not imply
a relationship with, or endorsement or sponsorship of, us by the trademark or trade dress owner.
Item 1. Business
Overview
We are a development stage medical device company focused on the development and eventual
commercialization of our proprietary bioresorbable stent products. Stents are minimally invasive,
implantable medical devices which are used by interventional cardiologists for the treatment of
coronary artery disease. Stents help stabilize diseased arteries by propping them open and
restoring blood flow. Our stent products have not yet been approved by regulatory authorities and
will require extensive clinical testing and regulatory approval before they can be sold and
generate any revenue. As a result, our efforts to generate revenue from our stent products will
take several years, even if our clinical results are favorable. Our stent products are designed to
provide the same benefits as traditional metal stents, including inhibiting restenosis, or the
renarrowing of the previously treated artery, with the additional benefit of being dissolved by the
body over time. Our lead product, the ReZolvetm stent, combines our proprietary
stent design with a proprietary polymer that is metabolized and cleared from the body over time. We
believe that, due to a number of risks associated with commercially available metal stents,
bioresorbable stents will be the next major advance in coronary stent technology, and, if approved
for commercialization by the relevant regulatory authorities, we believe the
ReZolvetm stent will enable us to compete effectively in the stent market which
was approximately $5.3 billion worldwide in 2009.
Over the last ten years, we have continued to advance our technology in both its design and
polymer composition and have undertaken significant laboratory and preclinical testing which has
shown that our technology and the ReZolvetm stent are safe and effective across
various animal models. We have funded much of our research and development to date with investments
from health care venture capital funds, along with investments from global medical device
manufacturers Medtronic, Inc., or Medtronic, and Boston Scientific Corporation, or BSC. The design
of our ReZolvetm stent is finalized, and we intend to initiate a pilot human
clinical trial in the second quarter of 2011.
We believe that if the ReZolvetm stent is approved by regulatory
authorities, the technology has the potential to provide patients with better therapeutic outcomes
and physicians with more effective clinical tools. The ReZolvetm stent is
designed to offer full x-ray visibility, clinically relevant sizing, and a controlled and safe
resorption rate. In addition, by early encapsulation of the stent in the artery tissue coupled with
the loss of stent structure over time, the ReZolvetm stent may reduce the
incidence of late forming blood clots, or thrombosis, a rare but serious problem associated with
drug-eluting metal stents currently on the market.
Market Opportunity
Coronary Artery Disease
Cardiovascular disease, or CVD, is a term used to describe all diseases and conditions that
relate to the heart and blood vessels. Coronary arteries, which supply blood to heart muscle, are
susceptible to the build up of plaque, which can block or inhibit blood flow, a condition known as
coronary artery disease. If the coronary arteries become too narrow as a result of this plaque
build up, cardiac tissue may become starved of nutrients and oxygen, and the result is severe chest
pain, known as angina. As artery narrowing becomes more severe, death of cardiac muscle downstream
from the blockage can occur due to a lack of oxygen. The sudden death of cardiac muscle can result
in a life threatening condition that is commonly known as a heart attack, or myocardial infarction.
In many developed countries, coronary artery disease is the leading cause of death in both
males and females. The World Health Organization, or WHO, has reported that, since 1990, more
people have died from coronary artery disease than from any other cause. In a January 2011 report
published by the WHO CVD was the number one cause of death globally, with an estimated 17.1 million
people dying from CVD in 2004, representing 29% of all global deaths. Of these deaths, an estimated
7.2 million deaths were due to coronary artery disease, the largest single contributor to CVD
deaths.
2
Table of Contents
The American Heart Association reported that in 2006, CVD was the leading cause of death in
the United States, or U.S., claiming 831,272 lives, or 34.3% of all deaths. Of this amount,
coronary artery disease is the greatest contributor and accounted for 425,425 deaths in the U.S. in
2006, or approximately one in every six deaths, and is estimated to cost the U.S. government,
directly and indirectly, an estimated $177.1 billion in 2010. According to the American Heart
Association, more than 17 million people in the U.S. have a history of heart attack or angina
pectoris (chest pain due to coronary disease) or both, and approximately 1.2 million Americans will
have a new or recurrent coronary attack annually.
The European Heart Network has reported that each year CVD accounts for approximately
4.3 million deaths, or 48% of all deaths, in Europe and over 2 million deaths, or 42% of all
deaths, in the European Union, or EU. Of this amount, the European Heart Network estimated that
coronary artery disease is the most common single cause of death in Europe and the EU, accounting
for approximately 1.92 million deaths per year in Europe, or approximately 21% of all male deaths
and 22% of all female deaths in Europe, and accounting for approximately 741,000 deaths per year in
the EU, or approximately 15% of all female deaths and 16% of all male deaths in the EU. In
addition, the Australia Institute of Health and Welfare has also reported that CVD was the primary
cause of death in 2007 in Australia, accounting for 46,623 deaths, or around one-third of all
deaths in 2007, and coronary artery disease kills more Australians that any other disease,
accounting for 22,727 deaths in 2007, or 16.5% of all deaths in Australia.
There have been a number of studies undertaken to determine the major risk factors that
predispose individuals to an increased risk of coronary artery disease which include those set out
below:
The WHO has reported that 80% to 90% of people dying from coronary artery disease have one or
more of the major risk factors that are influenced by lifestyle. One of the primary lifestyle based
risk factors for coronary artery disease is obesity, which has been increasing in developed
nations. According to the American Heart Association, over 144 million adults in the U.S., or 66%
of adults age 20 and older, are overweight or obese. Australia and other developed nations are
following similar trends. The WHO has also reported that cardiovascular disease is shifting to
developing nations. An increase in obesity in developing nations could increase the prevalence of
coronary artery disease in the future.
Current Interventional Treatments for Coronary Artery Disease
The treatment options available to patients with coronary artery disease vary between invasive
and non-invasive techniques and within these groups there are a variety of interventions that have
varying degrees of benefits and side effects. Due to the lifestyle risk factors associated with
coronary artery disease, interventions that can reverse these factors, such as living a healthy and
active lifestyle, are used for the prevention and treatment of coronary artery disease. Lifestyle
changes include regular exercise, smoking cessation, and healthy diet and nutrition. The evidence
to date shows that the healthy lifestyle alternative is not being well adopted in developed or
developing societies, likely due to technological advancements which are leading to more inactive
lifestyles.
Medication therapy using cholesterol lowering medications, beta blockers, diuretics, aspirin,
nitroglycerin, calcium channel blockers, and angiotensin-converting enzyme inhibitors aim to reduce
blood pressure and blood cholesterol levels and aid in the treatment of coronary artery disease.
Although drug therapy for coronary artery disease can improve quality of life and also prolong
survival, many of these current therapies must be combined with stenting to achieve satisfactory
long-term solutions for a large number of patients.
3
Table of Contents
When lifestyle changes and medications fail to prevent the development of coronary artery
disease, open heart surgery and less invasive interventional therapies are usually required to
restore blood flow to heart muscle to maintain adequate functioning of the heart. A number of
surgical procedures and interventional therapies have been developed over the past four decades to
treat coronary artery disease, each with the goal of quickly and safely restoring blood flow. This
goal is accomplished by surgically rerouting the flow of blood around the lesion or using
interventional techniques to reopen the artery. The treatment of coronary artery disease has
experienced significant innovation and has evolved from invasive surgical approaches to
minimally-invasive catheter-based therapies. This innovation has generally resulted in less severe
procedure-related complications, as well as reduced costs due to shorter procedure and recovery
times. Physicians have rapidly adopted these new therapies because of these benefits. The main
treatment options typically prescribed by treating physicians and available to patients are:
Coronary Artery Bypass Surgery. Bypass surgery, also called coronary artery bypass grafting,
or CABG, creates a detour around a blocked coronary artery with a new blood vessel, or graft. This
is an extremely invasive surgical technique whereby open heart surgery is required to route blood
flow around a blockage or narrowing of a coronary artery. During CABG, a surgeon takes a vein or an
artery from the patient’s chest, leg, or another part of the patient’s body and connects, or
grafts, it to the blocked artery. The grafted artery bypasses the blockage. This allows oxygen-rich
blood to reach the heart muscle. Surgeons can bypass multiple blocked coronary arteries during one
surgery.
Balloon Angioplasty. In the late 1970s, a significant advancement in the treatment of coronary
artery disease was made, providing physicians with a minimally-invasive therapy called balloon
angioplasty, in which a physician inserts a slender balloon-tipped catheter into the femoral artery
in the patient’s groin to a trouble spot in an artery of the heart. At the site of the blockage,
the balloon is inflated, compressing the plaque or widening the narrow coronary artery so that
blood can flow more easily. This therapy was rapidly adopted by physicians because it was minimally
invasive and resulted in shorter hospital and recovery times when compared to bypass surgery.
However, while providing advantages over bypass surgery, the long-term effectiveness of balloon
angioplasty is limited by restenosis. Restenosis following balloon angioplasty includes the
components of elastic recoil of the artery wall and the formation of scar tissue within the artery,
and typically requires a repeat of the balloon angioplasty procedure or bypass surgery to overcome
the artery renarrowing. While angioplasty in isolation was successful in initially restoring blood
flow, according to a study published in 1994 by The New England Journal of Medicine, restenosis
occurred within six months in about 40% of cases. In addition, some patients experienced abrupt
vessel closure after angioplasty, which led to major complications including death, heart attack,
and emergency bypass surgery.
Bare-Metal Stents. To address the issues of abrupt vessel closure and high rates of restenosis
following angioplasty, coronary stents were developed to improve clinical outcomes after
angioplasty. Stents are small tube-like devices used by interventional cardiologists to stabilize
an artery by propping it open and restoring blood flow after balloon angioplasty. The introduction
of stents in the early 1990s led to the reduction of restenosis and minimized abrupt closure of
blood vessels. The stents currently being used in clinical practice are flexible metal wire mesh
tubes that are permanently placed in the coronary artery. Coronary stents are typically mounted on
a balloon and expanded, stretching the open stent to the desired diameter during implantation. In
coronary stenting, we believe the key clinical measures of success or failure of the therapy are:
| • | Target Lesion Revascularization, or TLR, which measures the incidence of restenting or bypass surgery required, due to failure of the initial coronary angioplasty and stenting; and |
| • | Material Adverse Coronary Events, or MACE, being events of death, ischemia, or heart attack, where the target is to have as low a rate of MACE as possible. |
While the development of the bare-metal stent minimized the issues and complications of the
acute collapse of the artery wall, restenosis continued to be a significant problem following the
introduction of bare-metal stents, with as many as 30% of patients continuing to have restenosis
following the coronary stent placement, according to a study published in 1994 by The New England
Journal of Medicine.
Drug-Eluting Metal Stents. After coronary stents were introduced, physicians determined that
the cause of restenosis was not necessarily the recurrence of coronary artery disease but the
body’s inflammatory response to the trauma caused by the angioplasty procedure and the coronary
stent. This led to a number of methods designed to overcome restenosis, the most common being the
use of pharmacological agents to treat restenosis at the site. The desire to introduce drug therapy
at the site of the lesion resulted in the development of a combination device known as a
drug-eluting stent. These metal stents combine a thin polymer coating and therapeutic drug that
minimize the
4
Table of Contents
build up of scar tissue during the wound healing process after angioplasty and stent delivery. The
drug-eluting metal stents currently on the market contain a variety of drugs that are intended to
prevent restenosis which range from cytotoxic drugs (paclitaxel) to immunosuppressants (sirolimus,
zotarolimus, and everolimus). Delivery of drugs locally to the artery wall, using lower dosages
than would be required for systemic applications, has been shown to inhibit the events which might
lead to restenosis. Patients usually also undergo treatment with aspirin and anti-clotting or
antiplatelet drugs, such as clopidogrel (Plavix) or ticlopidine (Ticlid) after stenting, to reduce
the incidence of blood clots, or thrombosis. Early indications were that drug-eluting metal stents
succeeded in reducing the issue of restenosis with some clinical trials with drug-eluting metal
stents demonstrating a restenosis rate of under 10%, according to a study published in 2006 by The
New England Journal of Medicine.
Bioresorbable Stents. While studies showed drug-eluting metal stents have succeeded in
lowering the rates of restenosis, safety concerns were raised when studies suggested risks
associated with late-stent thrombosis and failure to restore natural movement of the artery. When
coronary stents were originally conceived, work was undertaken to develop them from biodegradable
polymers so they would dissolve or resorb over time, unlike metal stents that remain in place
permanently. This work was directed towards the use of stents as a temporary device to treat the
temporary issue of the coronary artery healing after the balloon angioplasty in order to prevent
renarrowing of arteries. More recently, the focus has been on producing a coronary stent that is
safe and overcomes the potential for late-stent thrombosis. The first bioresorbable stent was
developed by researchers at Duke University in the early 1980s. While there have been a number of
researchers developing bioresorbable stents intended to be resorbed by the body over time, there
are many technical challenges, and to date, no coronary bioresorbable stents are available for sale
in Europe and the United States.
Coronary Stent Market
The global market for coronary stents can be broken down into three main sub-markets: the
U.S., Europe, and Asia, which is principally comprised of Japan and includes Australia. Europe
represents a large market and will be the first market targeted by REVA, assuming we achieve the CE
Mark approval required to authorize the sale of our ReZolvetm stent in the
European Union.
In 2009, total annual revenues from coronary stent sales were approximately $5.3 billion, of
which drug-eluting stents accounted for approximately $4.4 billion of this market. In 2009, annual
revenues for coronary stent sales were:
| • | approximately $2.2 billion in the U.S. with sales of approximately 1.4 million stent implants, with these stents constituting approximately 90% of all interventional procedures in the U.S.; |
| • | approximately $2.3 billion in Europe with sales of approximately 1.8 million stent implants, with these stents constituting approximately 90% of all interventional procedures in Europe; and |
| • | approximately $0.7 billion in Japan with sales of approximately 0.3 million stent implants, with these stents constituting approximately 88% of all interventional procedures in Japan. |
Drug-eluting stents account for approximately 70% of stent usage worldwide. From 2006 to 2007,
there was a reduction in market size and sales of drug-eluting stents by over $1 billion which we
believe was due in part to the concerns regarding increased risk of late-stent thrombosis. We
believe there are four companies with significant market share which have received both FDA and CE
Mark approval for five drug-eluting stents.
Our Products
ReZolveTM Stent
The ReZolvetm stent is a fully bioresorbable polymer stent. After
implantation, the stent is fully captured within the artery wall. As the vessel remodels and heals,
the stent gradually degrades and is benignly cleared from the body to overcome a number of the
issues caused by permanent metal stents. As the stent degrades and is resorbed, there is an
integration of artery tissue into the space previously occupied by the stent.
We developed an early version of the stent that was not drug-coated. After extensive
preclinical testing of the early version stent, we performed a small human clinical trial in 2007
with 25 patients in Brazil and Germany. We were successful in deploying the stent and demonstrated
the stent’s ability to dilate and hold open the artery as
5
Table of Contents
anticipated and consistent with the results of our preclinical data. However, at approximately four
months, we saw adverse device performance resulting in a higher than anticipated number of patients
requiring retreatment with another stent. At approximately the three-year time point, we performed
follow-up imaging on a subset of patients that were both retreated and not retreated. This imaging
shows stability of the artery, which we believe supports our claims as to the safety of the polymer
as it degrades.
After extensive investigative analysis, we concluded that embrittlement of the polymer was one
of the primary underlying causes for the retreatments in the first study. In addition to planned
changes to the stent’s design, we modified the polymer formulation, developed more predictive bench
testing, and incorporated the results of our analysis into the design of the revised
ReZolvetm stent.
We plan to implant the ReZolvetm stent using a balloon mounted angioplasty
catheter. We currently pre-mount the ReZolvetm stent onto the balloon as part
of the manufacturing process prior to the final sterilization. While the handling and storage
requirements of the ReZolvetm stent are not expected to vary from those
commonly used in clinical practice with metal stents, we currently plan to include a sheath over
the ReZolvetm stent to protect the device until it is finally positioned in the
coronary artery. Other than the use of the sheath, we believe that the
ReZolvetm stent will be able to be implanted in humans using standard clinical
procedures, which we believe will be important to rapid adoption of the product by physicians
following receipt of regulatory approval required for commercialization. It is possible that future
device iterations will not utilize a sheathed delivery system. The intended key features of our
ReZolvetm stent include:
| Intended Key Features | Details | |
Deliverable for intended use
|
Able to be implanted with minimally invasive techniques. Resorbs leaving no device. | |
Efficacy
|
Restores natural movement to artery following resorption. | |
Minimal recoil
|
Limited stent recoil, which we believe decreases the risk of restenosis. | |
Expansion range
|
Clinically-relevant expansion range due to ratchet mechanism, which allows our stents to be ratcheted open to achieve various sizes during the implant procedure. | |
Treats 2.75 mm diameter
arteries and above
|
Meets standard size requirements and is able to treat a variety of patients. | |
Standard deployment
|
Catheter mounted with handling and storage the same as current clinical practice. | |
Radiopaque
|
Visible by the cardiologist seeking to check the placement in the artery during and post-implant. |
To address clinical requirements, we will develop several sizes of the
ReZolvetm stent and also several lengths, to address the most common lesions
being treated in coronary arteries.
Our ReZolvetm stent, which consists of our “slide & lock” design and
licensed polymer technology, has passed the following preclinical tests:
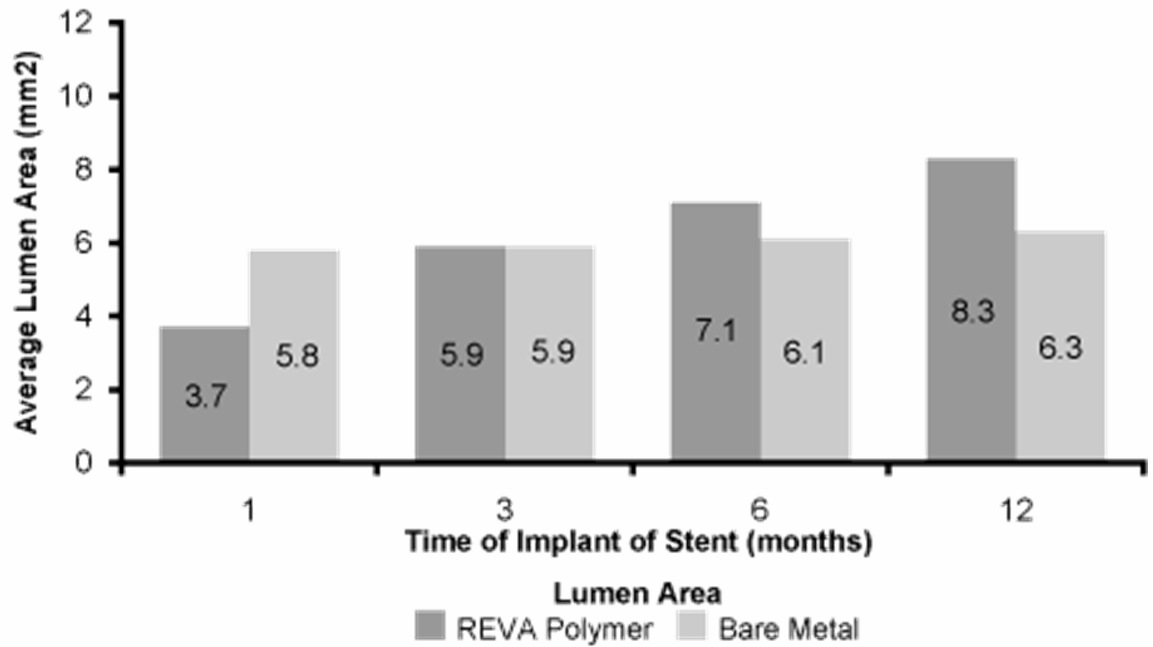
| • | Comparative Testing. Animal implants have been undertaken to assess our stent technology functionality as compared to commercially available metal stents. We have undertaken significant laboratory and preclinical testing on the development of our stent technology over the last ten years which has shown that our technology and the ReZolvetm stent is safe and effective in animals, with more than 1,000 stents tested across various animal models. In the 90 days following implant, our preclinical tests have shown that the ReZolvetm stent maintains the opening of the artery. Studies of our technology show that as the polymer degrades, the artery heals and becomes more like an unstented artery. Following stent implant, the lumen size, or the inside area of the artery, increased after our stent started to and continued to resorb over time, leaving a more normal lumen area. The lumen size of arteries supported by metal stents implanted as a control in these animal studies was almost unchanged. The following chart summarizes some of our animal studies. |
6
Table of Contents
Below: Animal testing of change in lumen size from time of implant
| • | Strength, Embrittlement, and Fatigue Testing. We have conducted engineering and life cycle testing with machines that are designed to replicate both the physiological conditions in the coronary artery as well as measure the maximum stress levels that our technology can withstand. To date, these preclinical tests have demonstrated satisfactory design and polymer strength, low levels of embrittlement of the polymer, and resistance to fatigue prior to significant degradation of the stent. |
| • | Biocompatibility Testing. The biological response to our stent technology has been evaluated by assessing healing in the animal coronary arteries using standard microscopy for stented arteries. To date, these studies have demonstrated the polymer is safe and no adverse response occurs in the artery even while the polymer degrades. |
| • | Rate of Degradation Testing. We have tested degradation of the polymer, as the stent needs to maintain its structural integrity for approximately a 90-day period following stenting to allow sufficient time for the artery to heal. To date, these studies have demonstrated that our technology maintains its structural integrity and strength during the critical 90-day period. By design, at 12 months, the stent no longer has significant mechanical strength, and the polymer begins to resorb and be eliminated from the animal’s body which continues for approximately four years, after which only tiny particles of the original polymer remain. A study of the byproducts resulting from the resorption of our stent showed no accumulation in key organs or tissues of the animal’s body and a substantial portion of the byproducts were cleared from the body. |
| • | Toxicity Testing. Our polymer material has been tested for toxicity and has been shown to date to be safe. As required by ISO-10993-1 regulations, our technology has undergone laboratory testing for genotoxicity. To date, our studies have shown that there is no change to the DNA or chromosomes of cells tested and no other genetic effect showing our polymer is genotoxic. In addition, we have also conducted tests for several other types of toxicity which have demonstrated to date that the polymer is safe. In addition to these laboratory tests, we have also conducted follow-up tests on the patients who were implanted with an early generation of our stents in 2007, and after three years of exposure to the polymer and breakdown of products in these patients, the vessels remain open and no long-term adverse clinical events related to the stent have been reported. |
| • | Testing of Drug Coating. The act of placement of the stent in the artery can injure the diseased vessel and the body’s wound-healing process can cause excessive scar tissue to form inside the stent, or in-stent restenosis. The drug sirolimus has been shown to minimize the overgrowth of tissue thereby minimizing the incidence of in-stent restenosis. In animal studies, we have tested the effects of the drug sirolimus, which is applied to the stent surface of the ReZolvetm stent as a coating. This drug is already used for other drug-eluting stents due to a recognized safety profile and efficacy at reducing restenosis. Our studies demonstrated no major drug toxicity. |
7
Table of Contents
Potential Advantages of the ReZolvetm Stent Design
We have designed our ReZolvetm stent to overcome many of the limitations
associated with currently marketed bare-metal and drug-eluting stents. We are currently working to
prove out our design features, and although we do not have human clinical data to support our
assertions, we have performed extensive bench and preclinical testing, including animal models,
that provides data and results that indicate our stent could include the following benefits:
| • | Restoration of the Vessel Movement and Decreased Risk of Adverse Effect. We believe adverse long-term reactions will be reduced due to the ability of our stent to be resorbed by the body over time. As our ReZolvetm stent dissolves, and the lesion has healed, the expansion and contraction of the vessel is restored without the restrictions of a permanent metal structure. We believe our stent has the potential to minimize disease progression downstream as the artery and blood flow are restored. |
| • | Minimization of Thrombosis Risk and Reduction of Long-Term Drug Therapy. We believe the potential for late-stent thrombosis is reduced because the stent becomes fully encapsulated into the artery where it safely dissolves over time. We believe these characteristics will help in reducing the incidence of blood clots, potentially decreasing the need for prolonged anti-platelet drug therapy. |
| • | Enhanced Applications for Future Medical Treatment. We believe as our ReZolvetm stent dissolves, the potential complications to subsequent medical treatments are reduced. A patient can likely undergo restenting, receive treatment more easily for lesions which are located downstream from the original stent, and undergo surgical procedures to the arteries without metal obstruction. In addition, we believe a significant potential application of our products is for their use as delivery vehicles for agents such as drugs and genes in coronary arteries for the treatment of a number of different lesions, including the treatment and reduction of vulnerable plaque. As a result, we believe our ReZolvetm stent will be able to treat a broader range of lesions more safely than today’s stent alternatives. The ReZolvetm stent also will not inhibit the use of MRI, thereby allowing physicians to non-invasively study coronary flow. As the stent loses radiopacity following implant, we believe the same will be true with CT imaging. |
We believe that due to risks associated with the commercially available bare-metal and
drug-eluting stents, bioresorbable stents will be the next major advance in coronary stent
development. Bioresorbable stents can potentially provide interventional cardiologists with more
treatment options to better address a broader range of coronary lesions which is not possible with
metal stents today. Our ReZolvetm stent is designed with the following features
to overcome a number of the limitations of other bioresorbable stents which are currently under
development:
| • | Proprietary Stent Design and Strong and Resilient Polymer. Our proprietary “slide & lock” design enables the ReZolvetm stent to be expanded with minimal deformation of the polymer; therefore, maintaining the strength of the material. Our proprietary polymer is also less prone to breaking than other polymers that have been tested for this application, and we believe the strength is maintained during the critical 90-day healing period following stent placement. We believe our ability to customize our polymer formulation will allow us to create products for additional applications. For example, we believe our polymer and stent technology could eventually be used to develop stents for use in peripheral arteries of the leg where stents are prone to crushing and fractures, such as in femoral arteries. |
| • | No Change to Clinical Practice. Our stent with its sheath can be deployed using a standard balloon catheter and does not require any change to storage, handling, or delivery of the stent. The stent can be stored at controlled room temperature conditions without the necessity of heating or refrigeration prior to use. |
| • | Controlled Resorption Rate. The polymer we use is designed to degrade from the body in a predictable and safe manner. The polymer degrades and can then be cleared from the body. The degradation profile of future polymer formulations may be adjusted to maximize the benefit for patient outcomes. |
| • | Biocompatible and Safe. While we do not have any human clinical data with respect to the current version of our polymer, we previously performed human clinical trials with an earlier version of our polymer and this version did not show any adverse biological reactions at 34 months post-stenting. In developing our current polymer, we modified our original polymer to enhance mechanical properties and address structural issues identified in our human clinical trials. The current polymer is similar in composition and contains approximately 85% of the same material as the original version of our polymer. The previous and the current |
8
Table of Contents
| polymers have demonstrated equal biocompatibility in preclinical testing. Our animal studies to date have shown that at 12 months post-stenting in pigs and rabbits that current version of our polymer has not shown any indications of adverse biological reactions while the stent material is degrading, consistent with the original version of our polymer. We believe the current version of our polymer addresses the structural issues identified in our human clinical trials without adversely affecting biocompatibility. |
| • | Visible Using Standard Imaging Techniques. Our ReZolvetm stent is visible under x-ray, thereby allowing physicians the ability to see the stent during placement and at early patient evaluations. It is also compatible with MRI and CT imaging, both of which imaging technologies may become more widely used in the diagnosis and treatment of coronary artery disease. |
The disadvantage of our ReZolvetm stent is that, initially, our
ReZolvetm stent is not designed to address smaller diameter vessel applications
or highly calcified, or hard and complex, lesions. As a result, the ReZolvetm
stent will not initially be able to address the needs of all patients requiring a coronary stent.
Our Strategy
Our goal is to become a world leader in the development and commercialization of bioresorbable
stent products used in the coronary and peripheral arteries of the human body. To achieve this
goal, we are pursuing the following business strategies:
| • | Demonstrate the Clinical Safety and Efficacy and Gain Regulatory Approval of our ReZolveTM Stent. We intend to demonstrate the clinical safety and efficacy of our ReZolvetm stent through carefully structured clinical studies with pilot human clinical trials of our ReZolvetm stent expected to begin in the second quarter of 2011. We plan to use these pilot studies to support our application to start the pivotal human clinical trial required to obtain CE Mark approval in the EU which will allow for commercial sales of the ReZolvetm stent in the EU. We have developed a clinical and regulatory plan designed to achieve CE Mark approval and are targeting first commercial product sales in the EU by the end of 2013. We intend to use the data from the CE Mark human trials to support applications for an Investigational Device Exemption, or IDE, for U.S. clinical trials which we expect to commence following the CE Mark human trials. |
| • | Commercialize and Drive Adoption of our ReZolveTMStent. Following regulatory approvals, we plan to commercialize our products. Once the clinical trials designed to achieve CE Marking are underway, our strategy will be to advance our commercialization plan in anticipation of the approval to allow for commercial sales of our products in the EU and related markets such as Australia. In order to meet commercial demand for our products, we intend to invest in the expansion of our manufacturing capabilities to required levels. Based on the early clinical data from our human clinical trials in the EU, we will then plan the commencement of our larger and more costly U.S. Food and Drug Administration, or FDA, clinical trial in order to seek to achieve PMA for commercial sales of our products in the U.S. We have granted BSC an option for a worldwide, exclusive right to sell, market and distribute our products, subject to certain requirements. See “— Distribution and License Agreements” for additional information. |
| • | Build Awareness and Support Among Leading Physicians. Our clinical development strategy is to closely collaborate with key opinion leaders in the field of interventional cardiology. We believe these key opinion leaders can be valuable advocates of our technology and be important in the market adoption of our products once our products are approved and commercialized. In addition, we intend to look to these physicians to generate and publish scientific data that further supports the benefits of our stent technology. |
| • | Leverage Our Technology Platform into Other Therapeutic Areas. We believe that our technology is applicable in other therapeutic areas outside of coronary artery disease. For example, we intend to pursue the use of our technology for the treatment of peripheral artery disease. Currently, we believe treatments of peripheral artery disease, particularly in the femoral artery, have demonstrated only marginal benefit. We believe, however, the application of our technology to the development of a bioresorbable peripheral stent could be significant in this expanding market. |
| • | Explore Licensing Opportunities. We intend to explore opportunities to leverage our intellectual property portfolio through licensing our technology to third parties or through the establishment of partnerships. For example, we are currently seeking a partner interested in licensing our side-chain crystalizable polymer for use as a flowable cement for orthopedic applications. |
9
Table of Contents
| • | Expand and Strengthen Our Intellectual Property Portfolio. We plan to continue to expand our current intellectual property portfolio. While we believe that our current portfolio will allow us to effectively market our products for the treatment of coronary artery disease, we plan to originate, license, and acquire additional intellectual property to enhance our existing position and enable us to more effectively protect our technology. |
| • | Provide the Highest Quality Products for Our Customers and Patients. We have assembled a team of experienced professionals in the medical device industry who are focused on patient safety and product quality. We incorporate these principles in every aspect of our business including product development, manufacturing, quality assurance, and clinical research. We intend to build on this foundation by offering only the highest quality products to patients and physician customers. |
Our Technology
“Slide & Lock” Stent Design
Our patent protected “slide & lock” mechanism is based on a ratchet system where as the stent
expands on a catheter mounted balloon, the “teeth” on the sliding parts pass through brackets in
the stent, preventing them from passing back, and locks in the stent diameter. The current version
of the “slide & lock” design is designed to be uniform throughout. The design is implemented with
two sets of components: backbones and ‘U’-shaped struts.
We believe our “slide & lock” design offers the following advantages as compared to
commercially available metal stents and drug-eluting stents, and bioresorbable stents in
development:
| • | Non-Deformable Design. All metal stents currently commercially available use deforming technology where the stent is crimped down onto the catheter mounted balloon and as the balloon expands, so does the device, until it reaches the desired size. Stents that bend open in this manner are called “deformable” stents. When polymers are stretched, they can lose strength and become prone to breakage. We believe our non-deformable ratcheting design is a key component to developing a strong bioresorbable stent. |
| • | Spiral Design which Maximizes Strength while Minimizing Bulk. In metal stents, the ultimate strength of the material prevents excessive recoil of the device post expansion. Polymer stents do not have the strength of metal and often break, recoil to a smaller diameter, or collapse entirely. We believe our spiral design offers an appropriate level of radial strength to overcome these issues while minimizing bulk. |
| • | Large Expansion Range One potential drawback of utilizing existing polymer stent technology is the lack of expansion range which can be achieved during implantation. The “slide & lock” mechanism allows our stents to be ratcheted open to achieve various sizes, similar to commercially available metal stents, and overcomes this drawback. Existing technology generally requires that treating physicians more accurately assess the correct size of the coronary artery based on angiography, since further expansion of the device post implant, a common technique to improve the position of the stent in relation to the artery wall, is limited due to potential fracture and recoil. With a limited expansion range, additional stent sizes may be required to accommodate existing clinical practice. We believe that with our “slide & lock” technology, the physician will be able to further expand the stent, as currently done in clinical practice, without the need for excessive stent sizes. |
Polymer Composition
Our patent protected polymer is an iodinated, tyrosine-derived polycarbonate.
In January 2004, we entered into an exclusive license for a polymer material invented at
Rutgers University in New Jersey for use in stents, stent coating, and embolics. We have continued
to develop and enhance the polymer in collaboration with Rutgers University. In July 2010, we
entered into a new license agreement with Rutgers University that broadens our exclusive rights to
the original polymer family and all new polymer compositions developed from this family to cover
all vascular applications. See “— Distribution and License Agreements” for additional information.
We believe the polymer we use offers the following advantages as compared to other
polymer-based stents:
| • | Strength. Strength and structural integrity is important during the critical 90 day healing period following stenting. We have developed our polymer such that, in conjunction with our design, it maintains the strength and structural integrity necessary to support an artery during the critical 90 day healing period. We believe our specific polymer formulation is less prone to cracking and breakage than other polymers. |
10
Table of Contents
| • | Biocompatibility. While we do not have any human clinical data with respect to the current version of our polymer, we previously performed human clinical trials with an earlier version of our polymer and this version did not show any adverse biological reactions at 34 months post-stenting. In developing our current polymer, we modified our original polymer to enhance mechanical properties and address structural issues identified in our human clinical trials. The current polymer is similar in composition and contains approximately 85% of the same material as the original version of our polymer. The previous and the current polymers have demonstrated equal biocompatibility in preclinical testing. Our animal studies to date have shown that at 12 months post-stenting in pigs and rabbits, the current version of our polymer has not shown any indications of adverse biological reactions while the stent material is degrading, consistent with the original version of our polymer. We believe the current version of our polymer addresses the structural issues identified in our human clinical trials without adversely affecting biocompatibility or safety. |
| • | Predictable Degradation and Resorption. Our polymer degrades into metabolites (being three monomers and carbon dioxide) and can then be cleared from the body. The degradation profile of future polymer formulations may be adjusted for specific applications. We believe future generations of our bioresorbable stent may employ formulations that will allow a more rapid degradation process to occur which will facilitate, for example, the short-term treatment of vulnerable plaque with drugs. |
| • | Visibility. The use of iodine in the polymer enables our stent to be visible under x-ray as well as standard fluoroscopy. This visibility is similar to commercially available metal stents (made of stainless steel), and we believe this differs from other products currently in development where only the end markers on the device are visible. Improved visibility allows interventional cardiologists to more accurately assess the implant quality and position. |
Drug Coating
Our ReZolveTM stent can be configured as a drug-eluting stent coated with a
therapeutic agent that is designed to inhibit restenosis of the artery in the same location. We
intend to use the drug, sirolimus, an anti-restenotic drug used in other drug-eluting stents. This
drug is commercially available from a number of different sources and is FDA approved.
A target dose of 80 μg of sirolimus is coated onto the outside surface of the
ReZolvetm stent using a polymer solution containing the drug. The polymer used
in the coating solution is the same polymer used for the stent structure. In laboratory and animal
studies to date, we have demonstrated that there is a controlled release of the drug over 30 days,
with most of the drug released from the polymer within 90 days. We believe this early and slow
release characteristic optimizes the efficacy of the drug, while more complete delivery at 90 days
may decrease the impact of the drug on the healing process.
Preclinical Testing
We have undertaken significant laboratory and preclinical testing on the development of our
stent technology over the last ten years which has shown that our technology and the
ReZolvetm stent is safe and effective in animals, with more than 1,000 stents
tested across various animal models.
We have completed our preparatory work including formal animal trials with the
ReZolvetm
stent for submission to the Brazilian and German regulatory bodies.
Upon receipt of regulatory approvals, we plan to initiate our next series of human trials,
commencing with a pilot study and then a randomized clinical trial to support our CE Mark
application.
Clinical Development Program
We have developed a clinical and regulatory plan which is designed to achieve CE Mark approval
by the end of 2013. We intend to use the data from the CE Mark human trials in order to support
future applications for an IDE for U.S. clinical trials.
11
Table of Contents
Assuming we obtain CE Mark approval for our ReZolvetm stent, we
believe we will be in a position to generate commercial sales in the EU. If we are successful in
generating such sales, we anticipate using the revenue to fund the U.S. human clinical trials, as
well as our other development activities. Commercial sales in the
United States for our
ReZolvetm
stent can only occur
after completion of a U.S. FDA human clinical trial and Premarket Approval, or PMA, from the FDA.
Besides the experience of our management team, we are also engaging consultants who specialize
in interventional cardiology to support our regulatory submissions and clinical trial efforts. We
recently hired Jeffrey Anderson to fill the role of Vice President of Clinical/Regulatory Affairs
and oversee strategy and execution of our clinical program.
Human Clinical Trial — Pilot Study
Our immediate clinical focus is to commence a 50 patient pilot clinical trial of the
ReZolvetm
stent in Brazil and sites in Germany. We have applied for our initial
regulatory approvals in Brazil and Germany. This pilot study will be a non-randomized
trial that will utilize industry standard measures of safety and efficacy in evaluating the
performance of the device. Enrolled patients will be monitored on a regular basis including at one,
six, and 12 month intervals after implant of the device, and annually thereafter, for a period of
up to five years. Based on the results of the pilot study, we, in consultation with our Scientific
Advisory Board, will make a decision on the commencement of our CE Mark trials.
Upon receiving the necessary approvals, we plan to commence the pilot study in the second
quarter of 2011 and expect that the clinical follow-up data from the patients in the pilot study
will provide further indication as to whether the device will be ready to commence the pivotal
human clinical trial. If the results are successful, we may initiate broader enrollment in other
countries to support CE Mark Application. This pilot study is not designed to enable scientific
conclusions to be drawn or regulatory approvals to be received.
Pivotal Human Clinical Trial — CE Mark
European Union Regulations
In the EU, the European Medical Devices Directive, or MDD, 93/42/EEC sets out the general
requirements for clinical trials and other essential requirements to support CE Marking and there
are numerous directives and standards regulating the design, manufacture, clinical trials, and
labeling for medical devices. For the ReZolvetm stent to bear the CE Mark and
be sold commercially throughout the EU, we will need to complete a human clinical trial of the
ReZolvetm stent, as well as complete supporting work to comply with the
requirements of MDD.
Australian Regulations
In Australia, the Therapeutic Goods Administration, or TGA, is responsible for administering
the Therapeutic Goods Act with the ReZolvetm stent falling under the category
of an Implantable Medical Device. The TGA maintains the Australian Register of Therapeutic Goods.
Unless exempt, all therapeutic goods for human use, including medical devices, must be included on
the register before they may be imported, supplied in, or exported from Australia. Any use of an
unapproved medical device in humans in Australia, even in pilot trials, requires an exemption from
the requirement for inclusion on the register. We plan to conduct part of the CE Mark human
clinical trial in two or three well-recognized Australian centers. In addition to agreeing to trial
protocols and obtaining ethical approvals at these centers, we will seek an exemption from the
Australian Register of Therapeutic Goods for the CE Mark human clinical trial in Australia.
CE Mark Trial Structure
We currently plan to conduct our pivotal human clinical trial for CE Mark approval based on:
| • | the enrollment of up to 350 patients; | ||
| • | Non-inferiority trial of the ReZolvetm stent against a commercially available drug-eluting metal stent with implants being on a randomized 2:1 basis with two of our stents implanted for every control stent; | ||
| • | engaging approximately 25 centers in the trial across the EU, Brazil, Australia, and New Zealand; |
12
Table of Contents
| • | Primary endpoint will be late loss (reduction of internal artery diameter) and comparable major adverse coronary events or MACE (death, ischemia, heart attack); | ||
| • | Clinical follow-up on all patients on a regular basis including at one, six, 12 months, and annually thereafter for a period of up to five years, after implant of the device; and | ||
| • | Interventional follow-up at 9 months or 12 months on a subset of patients in order to visualize the healing process. |
We plan to commence enrollment of patients for the CE Mark trial following successful results
from our pilot study. We plan to complete enrollment of all patients in the CE Mark trial in the
third quarter of 2012 and be in a position to apply for CE Mark in 2013. However, no
guarantee can be given that we will achieve our expected results at the clinical trials or that CE
Mark will be attained in a timely fashion or at all.
Follow-on Human Clinical Trial — U.S. FDA Clinical Trial
U.S. Regulations
In the United States, medical devices are subject to review and approval by the FDA, which
regulates the clinical testing, manufacture, labeling, storage, record keeping, distribution, and
promotion of medical devices, primarily pursuant to the requirements of the Food, Drug, and
Cosmetic Act and other regulatory requirements. Medical devices are classified as Class I, II, or
III according to risk. Devices classified as Class III, such as the ReZolveTM stent,
require FDA approval of a PMA application prior to commercialization.
To obtain FDA approval to market our products, the FDA requires proof of safety and efficacy
in human clinical trials performed under an Investigational Device
Exemption, or IDE. An IDE application must contain preclinical test
data supporting the safety of the product for human investigational use, information on
manufacturing processes and procedures, proposed clinical protocols, and other information. If the
IDE application is accepted, human clinical trials may begin.
The IDE application is generally approved by the FDA for a specified number of patients and
investigational sites. Clinical trials may begin once the FDA approves the IDE and the
Institutional Review Board at each participating clinical site approves the trial protocol.
U.S. Human Trials and FDA Approval
Based on the outcome of the pivotal CE Mark human trial, we plan to conduct human clinical
trials in the United States. The trial is expected to be a randomized trial of at least 2,000
patients.
Pursuant to our clinical and regulatory strategy, the timing of the commencement of the U.S.
FDA clinical trial will be determined after consideration of the CE Mark results, our capacity to
manage multiple trials concurrently and the availability of future funding.
Manufacturing
Our operations are based at our ISO 13485-2003 certified facility in San Diego, an
approximately 17,000 square foot facility dedicated to the development and manufacture of our
products. The facility includes laboratories for chemistry, engineering, and product assembly,
including clean rooms and quality assurance laboratories. We believe that the San Diego facility
will have the capacity to produce the quantities of stents required for our planned clinical
trials. In the future, assuming we receive the necessary regulatory approvals for our products, we
expect to expand our manufacturing capacity in line with demand for our products. Our lease expires
in August 2011.
Certain portions of the stent manufacturing process currently are completed by external
parties in ISO-certified facilities. We have not entered into any material agreements with any
third parties regarding our manufacturing process. Our suppliers have no contractual obligation to
supply, and we are not obligated to
purchase from them, any components used in our ReZolvetm stent which may
result in supply interruptions. The strategy of outsourcing selected manufacturing processes is
intended to minimize capital and operating costs while at the same time maintaining required
quality standards.
13
Table of Contents
Our process of manufacture for our stent technology, including the
ReZolvetm stent, involves six main steps, some of which currently involve a
degree of manual intervention. We plan to make this process semi-automated during 2012 as we
anticipate ramping up our manufacturing capabilities to further improve capacity and yields. These
six steps are as follows:
| • | Polymer Manufacture. Currently outsourced to SurModics, Inc. | ||
| • | Sheet Preparation (Film Pressing). Performed at our facility. | ||
| • | Lasing (Cutting the Stent Pieces from the Polymer Sheet). Currently outsourced to Resonetics. | ||
| • | Drug Coating. Performed at our facility. | ||
| • | Final Assembly, Mounting on the Catheter, Quality Assurance, and Packaging. Performed at our facility. | ||
| • | Sterilization. Currently outsourced to Sterigenics. |
Currently, our polymer manufacturer, SurModics; our catheter supplier, Bavaria Medizin
Technologies GMBH; and our lasing process carried out by Resonetics are single-sourced. While
certain other products and components come from single source suppliers, we believe alternative
suppliers are readily available, though in many cases we have not qualified these suppliers. If
necessary, we could locate second source suppliers; however, any interruption or delay in obtaining
products from third-party suppliers, or our inability to obtain products from alternate sources at
acceptable prices in a timely manner, could impair our ability to meet the demand for our planned
clinical trials. Most of our suppliers have no contractual obligation to supply us with, and we are
not contractually obligated to purchase from them, any of the components used in our products. Any
supply interruption would limit our ability to manufacture our products, which could delay
completion of clinical trials or commercialization of our products.
We have implemented a quality management system which is designed to comply with FDA
regulations and ISO standards governing our medical device products. These regulations carefully
control the design, manufacture, testing, and release of our products and product components, as
well as raw material receipt and control. We also have controlled methods for the consistent
manufacturing of our products and product components at our facilities. All key outsourcing
partners are generally ISO-certified to help assure a continual supply of high quality components.
Competition
The coronary stent industry is highly competitive. Many of our competitors have significantly
greater financial resources, human resources, and expertise in research and development,
manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals, and
marketing approved products than we do. Many of these competitors also have more established
reputations with our target customers and developed worldwide distribution channels. These
competitors include Abbott Vascular, Boston Scientific Corporation,
or BSC, Johnson & Johnson, and Medtronic. Smaller or early-stage
companies may also prove to be significant competitors, particularly if they enter into
collaborative arrangements with large and established companies. These third parties compete with
us in recruiting and retaining qualified scientific and management personnel, establishing clinical
trial sites, and patient registration for clinical trials, as well as in acquiring technologies and
technology licenses complementary to our programs or advantageous to our business. As a result, we
cannot provide assurances that we will be able to compete effectively against these competitors or
their products.
Although the field of interventional cardiology is extremely competitive with high performance
requirements for products, we believe interventional cardiologists have historically been rapid
adopters of new technology. While physicians may recommend alternative treatments such as drug
therapy, bypass surgery, angioplasty, or bare-metal stenting, we expect the primary competition for
our products will be drug-eluting stents and other bioresorbable stents. There have been a number
of companies working to develop bioresorbable or polymer stents. Abbott Vascular is developing its
Bioresorbable Vascular Scaffold, called Absorb, which received CE Mark approval in January 2011.
Biotronik, a private, European
company, is developing its second generation Dreams magnesium-based resorbable stent.
Biotronik has announced that clinical trials of this device commenced in August 2010.
14
Table of Contents
Because of the size of the market opportunity for coronary artery disease, competitors have
historically dedicated and, we expect, will continue to dedicate significant resources to
aggressively promote their products. New product developments that could compete with us more
effectively are likely because the coronary artery disease treatment market is characterized by
extensive research efforts and technological progress. Accordingly, competitors may develop
technologies and products that are safer, more effective, easier to use, or less expensive than our
ReZolvetm stent.
We believe our success is likely to be driven by, and depends on, our ability to innovate,
manufacture in commercial quantities, obtain regulatory approvals and reimbursement, and
successfully market and sell our ReZolvetm stent. We expect to encounter
potential customers who, due to existing relationships with our competitors, are committed to or
prefer the products offered by these competitors. To compete effectively, we must demonstrate that
our products are attractive alternatives to other devices and treatments by differentiating our
products on the basis of safety, efficacy, performance, ease of use, brand and name recognition,
reputation, service, and cost-effectiveness.
Research and Development
Since inception, we have devoted a significant amount of resources to develop our
ReZolvetm stent. Our research and development expenses were $11.4 million in
2008, $10.3 million in 2009, and $6.8 million in 2010. After the 2010 decrease, we expect our
research and development expenditures to increase as we devote significant resources to developing
our products, in particular, completing the clinical trials necessary to support regulatory
approval.
Sales and Marketing
As a development stage company, we do not have a sales and marketing organization and have no
experience in the sale, marketing and distribution of stents. To achieve commercial success for any
approved product we must further develop a sales and marketing organization or enter into
arrangements with others to market and sell our products.
In most countries throughout the world, a significant portion of a patient’s medical expenses
is covered by third-party payors. In the United States, hospitals and physicians generally rely on
third-party payors, such as Medicare, private health insurance plans, and health maintenance
organizations to reimburse all or part of the cost of medical devices and the related surgical
procedures. Reimbursement in the EU varies from country-to-country and often hospital-to-hospital.
We believe that numerous hospitals have established budgets to purchase coronary stents and the
purchase decision is often driven by the interventional cardiologists.
Currently, coronary stents are sold through distribution channels in the United States and
around the developed world, primarily targeting interventional cardiologists who treat patients
likely to require stenting. We believe the costs and barriers to develop a distribution channel
focused around one group of products are large. We may therefore consider partnering with a
distribution or sales channel. In addition, we have entered into a Distribution Option agreement
with BSC relating to the sale and distribution of our stent technology in markets in which the
technology is approved for sale. The terms of this agreement are described under “— Distribution
and License Agreements.”
Our sales strategy will depend on our product roll-out which is dependent upon our products
receiving the necessary regulatory approvals and clearances:
| • | The EU will be the initial target commercial market because CE Marking is our first clinical objective; | ||
| • | Australia will be the second target commercial market because we believe regulatory approvals in Australia will closely follow CE Marking, and Australia can serve as a base for the Asian market; and | ||
| • | The United States will be the third target commercial market upon completion of U.S. FDA trials and our product receiving PMA. |
Intellectual Property
We rely on a combination of patents, trade secrets, and copyright, together with
non-disclosure and confidentiality agreements, to establish and protect our proprietary rights in
our technologies. Our patents and patent applications covering the fundamental technology
underlying our “slide & lock” design have been developed internally, while the polymer has been
either licensed or developed by us.
15
Table of Contents
As of March 15, 2011, our patent portfolio comprises, on a worldwide basis, approximately 268
issued U.S. and foreign patents that we own directly or for which we are the exclusive licensee. We
have been issued 25 U.S. patents and have 27 U.S. patent applications which are pending in the
United States Patent and Trademark Office. The last to expire of our
issued or allowed patents expires in
2029. For these 52 technology patents, we have sought intellectual property protection outside of
the United States and have been issued 134 foreign patents and have 82 pending foreign
applications. We do not know if any of our patent applications will be issued, nor do we know
whether our patents, if issued, will cover our technology or will be able to be successfully
enforced. Even if valid and enforceable, our patents may not be sufficiently broad to prevent
others from inventing a stent like ours, despite our patent rights. We have received no
communications from third parties concerning the patentability, validity, or enforceability of our
patents or patent applications. We believe that the remaining time on our patents provides adequate
time to generate revenues from commercialization, subject to timing of the regulatory and clinical
pathway.
We actively monitor our intellectual property position, with new developments periodically
reviewed to identify prudent extensions to our patent portfolio to ensure that we lockup key
technology, as well as to maximize our defensive strategy through the coverage of similar
technology developments. We have an in-house patent counsel and also employ external patent
attorneys to assist us in managing our intellectual property portfolio.
The industry we operate in has been subject to a large number of patent filings and patent
infringement litigation. Whether we would, upon commercialization, infringe any patent claim will
not be known with certainty unless and until a court interprets the patent claim in the context of
litigation. If an infringement allegation is made against us, we may seek to invalidate the
asserted patent claim and may allege non-infringement of the asserted patent claim. In order for us
to invalidate a U.S. patent claim, we would need to rebut the presumption of validity afforded to
issued patents in the United States with clear and convincing evidence of invalidity, which is a
high burden of proof. To date, none of our patents or patent applications have been subject to
reexamination, interference, or other legal challenge.
We require all employees to sign confidentiality and invention assignment agreements under
which they are bound to assign to us inventions made during the term of their employment. These
agreements prohibit our employees from using, disclosing, or bringing onto the premises any
proprietary information belonging to a third party. In addition, our consultants are required to
sign agreements under which they must assign to us any inventions that relate to our business.
These agreements also prohibit our consultants from incorporating into any inventions the
proprietary rights of third parties without informing us. It is our policy to require all employees
to document potential inventions and other intellectual property in laboratory notebooks and to
disclose inventions to patent counsel in written form.
We also rely on confidentiality restrictions and trade secret protection to protect our
technology. We generally require our consultants and other parties who may be exposed to our
proprietary technology to sign non-disclosure agreements which prohibit such parties from
disclosing or using our proprietary information except as may be authorized by us.
Distribution and License Agreements
Boston Scientific Distribution Agreement
On December 7, 2007, we entered into a Distribution Option Agreement with Boston Scientific,
or BSC, pursuant to which we granted BSC an option for a worldwide, exclusive right to sell,
market, and distribute our products, provided BSC’s exclusive rights shall become non-exclusive
with respect to any product that BSC directly competes with in any country or territory where such
competitive activity occurred. The Distribution Option Agreement requires us to negotiate the terms
of a distribution agreement with BSC upon BSC’s exercise of the option. However, there is no
guarantee that we will be able to agree on terms for the distribution agreement. If BSC exercises
its option, we shall negotiate in good faith with BSC to enter into a mutually acceptable
definitive distribution agreement, which definitive distribution agreement shall include the
following provisions: (i) the term of such distribution agreement shall not be less than five
(5) years; (ii) the transfer price for our products shall be equal to 50% of BSC’s average
selling price for such products; (iii) BSC shall not be required to make any payments, other than
the transfer price for products, with respect to the sale, marketing, or distribution of such
products, (iv) we shall meet all legal and regulatory requirements as well as BSC quality
standards, with respect to the design, development, and manufacturing of all products; (v) BSC
shall have sole discretion over all marketing and sales decisions relating to the products; and
(vi) BSC shall be the exclusive distributor of such products during the term of such distribution
agreement so long as BSC does not commence the selling, marketing, or distribution of a directly
competitive stent product, with any distribution agreement becoming non-exclusive with respect to
jurisdiction and
16
Table of Contents
the product at such time as BSC first sells, markets, or distributes directly
competitive stent products in the same jurisdiction. If we are unable to agree on the terms of a
definitive distribution agreement with BSC through good faith negotiations within 90 days of BSC’s
exercise of its option, or if BSC delivers at any time written notice to us that it is electing not
to exercise its option, then we shall be permitted to sell, market, and distribute our products
pertaining to such option to a third party; provided, however, the terms of an offer to any third
party and the definitive agreement establishing such third party’s right to sell, market, and
distribute such products, shall not be on terms more favorable than the terms offered by us to BSC.
BSC’s distribution option, to the extent not previously exercised, terminates 90 days
following our achievement of all of the following clinical milestones: (i) our delivery of clinical
data to BSC concerning imaging, death, acute myocardial infarction, stent thrombosis, and target
lesion revascularization relating to the one year follow-up of at least 200 of our implanted
resorbable drug coated stents from human clinical trials, or Implanted Stents, (ii) our delivery of
clinical data to BSC concerning core lab acute gain, late loss, and binary angiographic restenosis
relating to the eight to nine month angiographic follow-up of at least 100 Implanted Stents, and
(iii) our delivery of clinical data to BSC relating to the eight to nine month intravascular
ultrasounds of at least 40 Implanted Stents.
Under the Distribution Option Agreement, we have also agreed not to take certain actions which
would prevent BSC from exercising its distribution option, provided that we may market, sell, or
distribute any product on a non-exclusive basis in any country or territory where BSC directly
competes with such product. In addition, if we receive regulatory approval for any product in any
country or territory outside of the U.S. prior to submission to the FDA, and (i) BSC does not
exercise its distribution option within 90 days following written notice from us of the approval,
or (ii) if BSC exercises it distribution option but is unable to agree with us on the terms of the
distribution arrangement within 90 days of BSC’s exercise of its option, then we may sell, market,
and distribute such product in any foreign country or territory where the product has received
approval, directly or through any third party that is not a direct competitor of BSC, provided
however, that any such arrangement must be terminable without cost to BSC on no more than 90 days’
written notice.
License Agreements
Effective July 1, 2010, we entered into an Exclusive License Agreement, or the Rutgers
License, with Rutgers, the State University of New Jersey, or Rutgers, which superseded our
existing Exclusive License Agreement with Rutgers, dated January 21, 2004. Under the new Rutgers
License, Rutgers granted us an exclusive, worldwide license, with the right to sublicense, under
certain patent and other intellectual property rights to develop and commercialize products that
utilize certain polymers in the vascular field. In the event that Rutgers sublicenses its patent
rights to intellectual property that is owned by Rutgers or was jointly invented by us and Rutgers,
to a third party, such patent-related costs shall be shared pro rata with either Rutgers or the
applicable sublicensee. If Rutgers sublicenses inventions and improvements solely owned by us,
Rutgers shall pay us a significant percentage of all income and consideration Rutgers receives from
such sublicenses. Under the Rutgers License, we will be required to pay annual license maintenance
fees until a product is commercially sold in a major market. We will also be required to pay
royalties on the sale of the products, with annual minimum royalties owing for different classes of
products. We will also be required to make milestone payments upon the achievement of certain
development, regulatory, commercialization, and change of control milestones. In order to maintain
our rights under the Rutgers License, we have to satisfy certain development and commercialization
obligations specified in the agreement. The term of the Rutgers License continues until the
expiration of the last to expire of the patents licensed to us under
the agreement, or currently 2030.
We entered into a Royalty and License Agreement, or the Integra License, with Integra
LifeSciences Corporation, or Integra, dated January 30, 2004, pursuant to which Integra granted us
an exclusive license, with the right to sublicense, under certain patent and other intellectual
property rights to develop and commercialize products that are covered by such patent rights in the
field of blood vessels. Under the Integra License, we are required to pay to Integra a per-unit
royalty on stent products that incorporate certain polycarbonates. The term of the Integra License
continues until the later of (i) expiration of the last to expire of the patents licensed to us
under the Integra License, or 2023 or (ii) the expiration of certain patent rights licensed by
Rutgers to us.
Terms of the Rutgers License and Integra License include provisions for royalty payments on
any future sales of products, if any, utilizing this technology, with provisions for minimum
royalties once product sales begin. The amount of royalties varies depending upon type of product,
use of product, stage of product, location of sale, and ultimate sales volume and price, and ranges
from a minimum of approximately $70 per product sale to a maximum of approximately $100 per product
sale, with license provisions for escalating minimum royalties that could be as high as $2.2
million per year. Additionally, in the event we receive certain milestone payments related to this
technology, the licenses require that 20% of the milestone amount be paid to the licensors.
17
Table of Contents
Additional terms of the Rutgers License and Integra License include annual licensing payments
of $150,000 in 2008 and $175,000 in 2009 and annually thereafter until the underlying technology
has been commercialized. Terms of the licenses also include other payments to occur during
commercialization that could total $950,000, payment of $350,000 upon a change in control of
ownership, and payment of patent filing, maintenance, and defense fees. The license terms remain in
effect until the last patent expires or ten years after commercialization.
Third-Party Reimbursement
In most countries throughout the world, a significant portion of a patient’s medical expenses
is covered by third-party reimbursement. In many countries, including the United States,
third-party payors consist of both government funded insurance programs and private insurance
programs. While each payor develops and maintains its own coverage and reimbursement policies, the
vast majority of payors have established policies for stents. We believe that our products
generally will fall within the existing reimbursement guidelines, although some refinement in
policies may be needed for our products. Before we can obtain reimbursement for our
ReZolvetm stent in the United States, FDA approval will be required.
In the United States, the Center for Medicare and Medicaid Services, or CMS, is the government
entity responsible for administering the Medicare program. CMS establishes Medicare coverage and
reimbursement policies for medical products and procedures and such policies are periodically
reviewed and updated. While private payors vary in their coverage and payment policies, the
Medicare program is viewed as a benchmark. Both CMS and commercial payors have established coverage
and reimbursement policies for the stents that are currently on the market. There are also
established reimbursement codes describing current products and procedures using those existing
products. However, there are no assurances that existing policies or reimbursement codes would be
used for the resorbable stents that we are currently developing. There are also no assurances that
existing payment rates for such reimbursement codes will continue to be at the same levels.
Outside of the United States, there are many reimbursement programs through private payors as
well as government programs. In some countries, government reimbursement is the predominant program
available to patients and hospitals. While the vast majority of countries have existing
reimbursement for stents, a small number of countries may require us to gather additional clinical
data before recognizing coverage and reimbursement for our products. We intend to complete the
requisite clinical studies and obtain coverage and reimbursement approval in countries where it
makes economic sense to do so.
In certain regions, such as Europe, innovative pricing and reimbursement agreements are being
used to balance the interests and objectives of medical technology manufacturers, payers, parties
assessing health technology, clinicians, and patients. Manufacturers and health technology
assessors/assessments, or HTAs, are increasingly using risk sharing
and value-based schemes as a way
to obtain HTA approval to finance the technology or device from a limited public health budget.
HTAs typically have two elements, clinical effectiveness and cost effectiveness. Some countries in
Europe have national HTA, for example, France, Germany, and Sweden, and others have regional ones,
such as, Italy, Spain, and the United Kingdom. Some manufacturers who proactively propose such
schemes to HTAs may gain competitive advantage. Each country within Europe has its own system of
pricing and reimbursement for medical devices and products.
In Australia, the Department of Health and Ageing is the government department and Medicare is
the government entity responsible for administering the Medicare Benefits Scheme and the Medicare
Benefits Schedule, or MBS. Medicare establishes coverage and reimbursement policies for medical
products and procedures and such policies are periodically reviewed and updated. Medicare and MBS
have established coverage and reimbursement policies for the stents that are currently on the
market. There are also established reimbursement codes describing current products and procedures
using those products. However, similar to the United States, there are no assurances that existing
policies or reimbursement codes will be used for the resorbable stents that we are currently
developing. There are also no assurances that existing payment rates for such reimbursement codes
will continue to be at the same level.
18
Table of Contents
In addition, in the United States, governmental and private sector payors have instituted
initiatives to limit the growth of health care costs, using, for example, price regulation or
controls and competitive pricing programs. Some third-party payors also require pre-approval of
coverage for new or innovative devices or therapies before they will reimburse health care
providers who use such devices or therapies. Providers also have sought ways to manage costs, such
as through the use of group purchasing organizations. We believe that the economic benefits
provided by the ReZolvetm stent to physicians and hospitals through shorter
procedure times and lower overall procedure costs will be viewed by providers and third-party
payors as cost-effective. However, there remains uncertainty as to whether our products will be
viewed as sufficiently cost-effective to warrant adequate coverage and reimbursement levels.
Government Regulation
United States
Our products are combination products because they are comprised of two or more regulated
components, a drug and a device, that are physically combined and produced as a single product. In
the United States, a combination product is assigned by the FDA to one of the Agency’s centers,
such as the Center for Drug Evaluation and Research, or CDER, or the Center for Devices and
Radiological Health, or CDRH. The center to which the product is assigned will have primary
jurisdiction over the PMA of the combination product. The FDA identifies the center with primary
authority over a combination product based on an assessment of the combination product’s “primary
mode of action.” Because the primary mode of action for our products is that of a medical device,
we anticipate that our products will be regulated as devices by the FDA under the Federal Food,
Drug, and Cosmetic Act, and CDRH will have primary jurisdiction over our PMA application. However,
it is possible the FDA may assign our products to be regulated by CDER. We believe that the drug
component of our products will be reviewed by CDER, which will consult with and assist CDRH in its
review of our PMA application. The drug will not require separate FDA approval. If the FDA does
assign our products to be regulated by CDER, the drug component of the product will in all
likelihood not require separate CDER approval.
FDA regulations govern the following activities that we and our suppliers, licensors, and
partners perform and will continue to perform to ensure that the products we distribute
domestically or export internationally are safe and effective for their intended uses:
| • | product design and development; | ||
| • | product testing; | ||
| • | product manufacturing; | ||
| • | product safety; | ||
| • | product labeling; | ||
| • | product storage; | ||
| • | record keeping; | ||
| • | premarket approval; | ||
| • | advertising and promotion; | ||
| • | production; and | ||
| • | product sales and distribution. |
FDA’s Premarket Clearance and Approval Requirements. The FDA classifies medical devices into
one of three classes. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining,
life-supporting, or implantable devices or devices deemed not substantially equivalent to a
previously cleared
510(k) device, are placed in Class III, requiring PMA. All of our current products in
development are Class III devices and will require FDA approval after submission and review of our
PMA application. A PMA must be supported by extensive data, including but not limited to,
technical, preclinical, clinical trials, manufacturing, and labeling to demonstrate to the FDA’s
satisfaction the safety and efficacy of the device. A PMA must also contain a full description of
the device and its components and a full description of the methods, facilities, and controls used
for manufacturing of the device.
19
Table of Contents
Product Modifications. New PMAs or PMA supplements are required for all significant
modifications to the manufacturing process, labeling, use, and design of a device that is approved
through the PMA process. PMA supplements often require submission of the same type of information
as an initial application for PMA, except that the supplement is limited to information needed to
support any changes from the device covered by the original PMA application. Certain modifications
may not require as extensive clinical data or the convening of an advisory panel.
Clinical Trials. A clinical trial is almost always required to support a PMA application.
Clinical trials for our product candidates require the submission of an application for an IDE to
the FDA, which is supported by appropriate data. The IDE application must be supported by
appropriate data, such as animal and laboratory testing results, showing that it is safe to test
the device in humans and that the testing protocol is scientifically sound. The IDE must be
approved in advance by the FDA for a specified number of patients. Clinical trials may begin once
the application is reviewed and cleared by the FDA, as well as the appropriate institutional review
boards at the clinical trial sites. Clinical trials must be conducted in accordance with applicable
regulations and policies and are subject to extensive record keeping and reporting requirements.
Our clinical trials must be conducted under the oversight of an institutional review board at the
relevant clinical trial site and in accordance with applicable regulations and policies including,
but not limited to, the FDA’s good clinical practice regulations. We, the FDA, or the institutional
review board at each site at which a clinical trial is being performed may suspend a clinical trial
at any time for various reasons, including a belief that the risks to the subjects of the clinical
trial outweighs the anticipated benefits.
Pervasive and Continuing Regulation. After a device is placed on the market, numerous
regulatory requirements apply. These include:
| • | Good Manufacturing Practices, or GMP, and the Quality System Regulation, or QSR, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation, and other quality assurance procedures during all aspects of the manufacturing process; | ||
| • | labeling regulations and FDA prohibitions against the promotion of products for unapproved or “off-label” uses; | ||
| • | medical device reporting regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; and | ||
| • | post-market surveillance regulations, which will apply when necessary to protect the public health or to provide additional safety and efficacy data for the device. |
The FDA has broad post-market and regulatory enforcement powers. We will be subject to
unannounced inspections by the FDA and the Food and Drug Branch of the California Department of
Health Services, or CDHS, to determine our compliance with the QSR and other regulations. These
inspections may also include an inspection of the manufacturing facilities of our subcontractors.
In addition, the supplier and manufacturers of the drug and drug coating used by us will be subject
to inspections by the FDA and other regulatory authorities to determine their compliance with the
strictly enforced GMP regulations.
In addition, discovery of previously unknown problems with a medical device, manufacturer, or
facility may result in restrictions on the marketing or manufacturing of an approved device,
including costly recalls or withdrawal of the device from the market. For instance, BSC and Johnson
& Johnson have experienced safety and manufacturing problems with their drug eluting stent
products, and have conducted significant and costly recalls in response to these issues. Failure to
comply with applicable regulatory requirements may result in enforcement action being taken by the
FDA, which may include any of the following sanctions:
| • | fines, injunctions, consent decrees, and civil penalties; | ||
| • | recall or seizure of our products; | ||
| • | operating restrictions, partial suspension, or total shutdown of production; | ||
| • | refusing our requests for PMA or new intended uses; | ||
| • | withdrawing PMA that are already granted; and | ||
| • | criminal prosecution. |
20
Table of Contents
The FDA also has the authority to require us to repair, replace, or refund the cost of any
medical device that we have manufactured or distributed. If any of these events were to occur, they
could have a material adverse effect on our business.
We are also subject to a wide range of federal, state, and local laws and regulations,
including those related to the environment, health and safety, and land use.
Fraud and Abuse. Our operations will be directly, or indirectly through our customers, subject
to various state and federal fraud and abuse laws, including, without limitation, the federal
Anti-Kickback Statute and False Claims Act. These laws may impact, among other things, our proposed
sales and marketing programs.
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting,
offering, receiving, or providing remuneration, directly or indirectly, in exchange for or to
induce either the referral of an individual, or the furnishing or arranging for a good or service,
for which payment may be made under a federal health care program such as the Medicare and Medicaid
programs.
Several courts have interpreted the statute’s intent requirement to mean that if any one
purpose of an arrangement involving remuneration is to induce referrals of federal health care
covered business, the statute has been violated. The Anti-Kickback Statute is broad and prohibits
many arrangements and practices that are lawful in businesses outside of the health care industry.
Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or
beneficial arrangements, Congress authorized the Department of Health and Human Services, Office of
Inspector General, or OIG, to issue a series of regulations, known as the “safe harbors.” These
safe harbors set forth provisions that, if all their applicable requirements are met, will assure
health care providers and other parties that they will not be prosecuted under the Anti-Kickback
Statute. The failure of a transaction or arrangement to fit precisely within one or more safe
harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However,
conduct and business arrangements that do not fully satisfy each applicable safe harbor may result
in increased scrutiny by government enforcement authorities such as the OIG. Penalties for
violations of the federal Anti-Kickback Statute include criminal penalties and civil sanctions such
as fines, imprisonment, and possible exclusion from Medicare, Medicaid, and other federal health
care programs. Many states have also adopted laws similar to the federal Anti-Kickback Statute,
some of which apply to the referral of patients for health care items or services reimbursed by any
source, not only the Medicare and Medicaid programs.
The federal False Claims Act prohibits persons from knowingly filing or causing to be filed a
false claim to, or the knowing use of false statements to obtain payment from, the federal
government. Suits filed under the False Claims Act, known as “qui tam” actions, can be brought by
any individual on behalf of the government and such individuals, sometimes known as “relators” or,
more commonly, as “whistleblowers”, may share in any amounts paid by the entity to the government
in fines or settlement. The frequency of filing of qui tam actions has increased significantly in
recent years, causing greater numbers of health care companies to have to defend a False Claim
action. When an entity is determined to have violated the federal False Claims Act, it may be
required to pay up to three times the actual damages sustained by the government, plus civil
penalties ranging from $5,500 to $11,000 for each separate false claim. Various states have also
enacted laws modeled after the federal False Claims Act.
In addition to the laws described above, the Health Insurance Portability and Accountability
Act of 1996 created two new federal crimes: health care fraud and false statements relating to
health care matters. The health care fraud statute prohibits knowingly and willfully executing a
scheme to defraud any health care benefit program, including private payors. A violation of this
statute is a felony and may result in fines, imprisonment, or exclusion from government sponsored
programs. The false statements statute prohibits knowingly and willfully falsifying, concealing, or
covering up a material fact or making any materially false, fictitious, or fraudulent statement in
connection with the delivery of or payment for health care benefits, items, or services. A
violation of this statute is a felony and may result in fines or imprisonment.
If our operations are found to be in violation of any of the laws described above and other
applicable state and federal fraud and abuse laws, we may be subject to penalties, including civil
and criminal penalties,
damages, fines, exclusion from government health care programs, and the curtailment or
restructuring of our operations.
21
Table of Contents
Patient Protection and Affordable Care Act. Our operations will also be impacted by the federal
Patient Protection and Affordable Care Act of 2010, as modified by the Health Care and Education
Reconciliation Act of 2010, which we refer to as the Health Care Act. The Health Care Act imposes a
2.3 percent excise tax on sales of medical devices by manufacturers. Taxable devices include any
medical device defined in section 201(h) of the FDCA and intended for use by humans, with limited
exclusions for devices purchased by the general public at retail for individual use. There is no
exemption for small companies, and we expect to begin paying the tax in 2013. The Health Care Act
also requires manufacturers to report to the Department of Health and Human Services detailed
information about financial arrangements with physicians and teaching hospitals. These reporting
provisions preempt state laws that require reporting of the same information, but not those that
require reports of different or additional information. Failure to comply subjects the manufacturer
to significant civil monetary penalties. We expect compliance with the Health Care Act to impose
significant administrative and financial burdens on us.
International
International sales of medical devices are subject to relevant foreign governmental
regulations, which vary substantially from country to country. The time required to obtain
clearance or approval by a particular country may be longer or shorter than that required for FDA
clearance or approval, and the requirements may be different.
The primary regulatory environment in Europe is that of the EU, which consists of 27 countries
encompassing most of the major countries in Europe. Three member states of the European Free Trade
Association, Iceland, Norway, and Liechtenstein have voluntarily adopted laws and regulations that
mirror those of the EU with respect to medical devices. Other countries, such as Switzerland, have
entered into Mutual Recognition Agreements and allow the marketing of medical devices that meet EU
requirements. The EU has adopted three core directives concerning medical devices: Medical Devices
Directive, In-Vitro Diagnostic Medical Devices Directive, and Active Implantable Medical Devices
Directive and the European Committees for Standardization, or CEN, have promulgated voluntary
standards regulating the design, manufacture, clinical trials, labeling, and adverse event
reporting for medical devices. A manufacturer may only place a medical device on the market or put
into service a device in the EU and European Free Trade Association when the device has undergone
the relevant conformity assessment process set out in the relevant medical devices directives. This
conformity assessment process is a process which verifies the device conforms to the essential
requirements of the directives. Devices that comply with the requirements of relevant directives
will be entitled to bear CE Marking, indicating that the device conforms with the essential
requirements of the applicable directives and, accordingly, can be commercially distributed
throughout the member states of the EU, the member states of the European Free Trade Association,
and countries which have entered into a Mutual Recognition Agreement. The method of assessing
conformity varies depending on the type and class of the product, but normally involves a
combination of self-assessment by the manufacturer and a third-party assessment by a designated
Notified Body, an independent and neutral institution appointed in one of the countries in the EU
to conduct the conformity assessment. This assessment is conducted by the designated Notified Body
in one member state of the EU, the European Free Trade Association, or one country which has
entered into a Mutual Recognition Agreement and is required for most of the medical devices in
order for a manufacturer to obtain CE Marking and to commercially distribute the product throughout
these countries. This assessment may also consist of an audit of the manufacturer’s quality system
and specific testing of the manufacturer’s device so as to ensure compliance with ISO 13485
certification, which are voluntary harmonized standards. Compliance with these ISO certifications
establishes that some of the general requirements of the directives are presumed to be fulfilled.
Each member state country of the EU has implemented the Medical Device Directives into national
laws and these laws are enforced by competent authorities in each member state. For example, in the
United Kingdom the authority is the Medicines and Healthcare Products Regulatory Agency.
Before any medical device can be supplied within Australia, it must be included on the
Australian Register of Therapeutic Goods and comply with the provisions of the Australian
Therapeutic Goods Act. Compliance generally requires, among other things:
| • | Full technical documentation demonstrating compliance to all relevant standards and regulations. | ||
| • | Full quality assurance certification to the key international standard. | ||
| • | The ability of the manufacturer to undertake post market surveillance processes. |
22
Table of Contents
However, much of the documentation produced for obtaining the CE Marking in Europe can be used
to obtain registration in Australia and the regulatory requirements with respect to the approval of
medical devices are similar to European regulations.
Employees
As of December 31, 2010, we had 43 employees, including 41 full-time employees and two
part-time employees, of which 35 were in research and development and 8 were in general and
administrative functions. We have never had a work stoppage, and none of our employees are covered
by collective bargaining agreements or represented by a labor union. We believe our employee
relations are good.
Executive Officers
Our executive officers and their ages and backgrounds as of December 31, 2010, are as follows:
Robert B. Stockman, age 57, our co-founder, has served as our Chairman of the Board and
director since 1999 and our Chief Executive Officer since August 2010. He has also served as a
director of HeartWare Limited, and subsequently HeartWare International, Inc., an ASX and NASDAQ
listed medical device company, since December 2006. Since 1999, Mr. Stockman has been the President
and Chief Executive Officer of Group Outcome LLC, a U.S.-based merchant banking firm which deploys
its capital and that of its financial partners in private equity and venture capital investments in
medical technology companies. Mr. Stockman also co-founded Centrimed, Inc., an internet-based
software company, that was acquired by the Global Healthcare Exchange, LLC, and led the buyouts of
Ioptex Research, an intraocular lens manufacturer, and two Johnson & Johnson divestitures, “A” Company
Orthodontics, Inc. and Critikon Company, LLC, each of which was subsequently acquired. Prior to
establishing Group Outcome LLC, Mr. Stockman spent 18 years with Johnston Associates, Inc. and
Narragansett Capital Corporation, where he focused on venture capital investments and merger
advisory work in health care. Mr. Stockman holds a Bachelors Degree from Harvard College and a
Master in Business Administration from The Tuck School at Dartmouth College.
Robert K. Schultz, Ph.D., age 54, has served as our President and Chief Operating Officer
since 2003. His background comprises more than 30 years in pharmaceutical, medical device and
combination products. Prior to joining REVA Medical, Dr. Schultz held positions of Vice President
of Research and Development and Vice President of Technology Strategy and Licensing for Dura
Pharmaceuticals, a specialty respiratory pharmaceutical and pulmonary drug delivery company, and
Research Specialist for 3M Pharmaceuticals, a diversified international technology company. He
obtained his Ph.D. in Pharmaceutics and his B.S. degree in Pharmacy from the University of
Minnesota.
Katrina Thompson, age 52, has served our Chief Financial Officer since 2003. Prior to joining
REVA Medical in 2003, Ms. Thompson held senior positions in the telecom, real estate development,
commercial nursery and high tech industries and spent the early part of her career with Price
Waterhouse, a provider of tax, audit and advisory services. Ms. Thompson received her B.S. in
Business Administration from San Diego State University.
Donald Brandom, Ph.D., age 51, is our Vice President of Product Development and has directed
our biomaterials activities since 2003. During his 18 years of industry experience, he has held
management and product development positions in the aerospace materials, microelectronics materials
and medical device industries. Dr. Brandom earned his Ph.D. in Materials Engineering Science at
Virginia Tech and has a B.S. in Chemistry from the University of California, Davis.
Eric Schmid, age 38, is our Vice President of Advanced Technologies. He served as a Vice
President since September 2007. From November 2005 through September 2007, Mr. Schmid served as our
Program Director and Principal Engineer, and from January 2003 through November 2005, he served as
our Principal Engineer and Manager of Stent Development and Design. Mr. Schmid has more than 15
years of experience in medical device design and development. Before joining REVA Medical, he
developed novel products and technologies for health care and medical device companies including
Abbott Laboratories, Guidant Corporation, Medtronic and BSC. Mr. Schmid completed graduate studies
in Chemical
Engineering at the University of California, San Diego, has a B.S. in Engineering from Harvey
Mudd College and holds multiple patents relating to medical devices and technologies.
23
Table of Contents
Joan Zeltinger, Ph.D., age 48, has served as our Vice President of Scientific Affairs since
June 2004 and has directed our biological and preclinical activities since 2000. Dr. Zeltinger has
18 years of industry research and business experience that includes numerous publications and
patents. Dr. Zeltinger previously directed the bioresorbable coronary graft and tissue engineered
heart valve programs at Advanced Tissue Sciences, a tissue engineering company, and chaired the
American Society for Testing and Materials, or ASTM, standard development for combination medical
products. She received a Ph.D. in Biology from the University of South Carolina with post-doctoral
work conducted at the University of Washington, School of Medicine, and has a B.S. in Biology from
the University of North Dakota.
General Information
The address of our principal place of business is 5751 Copley Drive, Suite B, San Diego, CA
92111. We also maintain a website at www.revamedical.com. We make available on our website,
free of charge, our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on
Form 8-K and any amendments to those reports, as soon as reasonably practicable after we
electronically file such material with, or furnish it to, the SEC. Our SEC reports can be accessed
through the Investor Relations section of our internet website. The information found on our
internet website is not part of this or any other report we file with or furnish to the SEC.
The public may read and copy any materials that we file with the SEC at the SEC’s Public
Reference Room located at 100 F Street, N.E., Washington, D.C. 20549. The public may obtain
information on the operation of the Public Reference Room by calling the SEC at (202) 551-8090. The
SEC also maintains electronic versions of our reports on its website at www.sec.gov.
Item 1A. Risk Factors
Investing in our CDIs or common stock involves a high degree of risk. You should carefully
consider the risks described below and all of the other information set forth in this Form 10-K,
including our consolidated financial statements and the related notes and “Management’s Discussion
and Analysis of Financial Condition and Results of Operations,” before deciding to invest in our
common stock. If any of the events or developments described below occurs, our business, financial
condition, or results of operations could be negatively affected. In that case, the market price of
our CDIs or common stock could decline, and you could lose all or part of your investment.
Risks Related to Our Business
We have a history of net losses and we may never achieve or maintain profitability.
We are a development stage medical device company. We have incurred net losses since our
inception, including net losses of approximately $12.7 million, $13.8 million, and $23.5 million
for the fiscal years ended December 31, 2008, 2009, and 2010 respectively. As of December 31, 2010,
our accumulated deficit was approximately $128.9 million. Currently, we have no products approved
for sale in any jurisdiction. We expect to continue to incur significant operating losses for the
foreseeable future as we incur costs associated with:
| • | designing and conducting the human pilot studies and human clinical trials required to obtain regulatory approval for our ReZolvetm stent; | ||
| • | seeking regulatory approvals in the EU, Australia, and the United States for our ReZolvetm stent; | ||
| • | further product research and development efforts; | ||
| • | growing, maintaining, and protecting our intellectual property; | ||
| • | expanding our manufacturing, sales, and marketing capabilities; | ||
| • | broadening our infrastructure and systems in order to meet the needs of our operations; and | ||
| • | complying with the requirements related to being a public company in the United States and a company listed on the Australian Securities Exchange, or ASX. |
24
Table of Contents
We cannot predict the extent of our future operating losses and accumulated deficit, and we
may never generate sufficient revenues to achieve or sustain profitability. To become and remain
profitable, we must succeed in developing and obtaining required regulatory approvals and
commercializing products with significant market potential. This will require us to succeed in a
range of challenging activities, including all of the activities listed above. We may never succeed
in these activities, and we may never obtain regulatory approvals in the markets in which we expect
to operate or otherwise generate revenues sufficient to achieve profitability. If we do achieve
profitability, we may not be able to sustain it.
Our ability to generate revenue depends upon the successful clinical development, regulatory
approval, and commercialization of our ReZolvetm stent.
Our ReZolvetm stent and any other products that we develop in the future
will require extensive clinical testing, regulatory approval, and significant marketing efforts
before they can be sold and generate any revenue. Our efforts to generate revenue may not succeed
for a number of reasons including:
| • | we may experience delays in the development program for our ReZolvetm stent, including the initiation and successful completion of our planned human pilot and clinical trials; | ||
| • | our ReZolvetm stent may not demonstrate safety and efficacy in our clinical trials; | ||
| • | we may not be able to obtain regulatory approvals for our ReZolvetm stent in the markets in which we expect to operate, or the approved indications for our ReZolvetm stent may be narrower than we currently anticipate; | ||
| • | our ReZolvetm stent may not be accepted in the marketplace by physicians and patients; | ||
| • | physicians may not receive adequate coverage and reimbursement for procedures using our ReZolvetm stent; | ||
| • | new product introductions by our competitors or any rapid technological change may make our technology and product candidates, including the ReZolvetm stent, obsolete; | ||
| • | we may not be able to manufacture our ReZolvetm stent in commercial quantities or at an acceptable cost; | ||
| • | we are wholly dependent on the efforts undertaken by suppliers of critical components for our ReZolvetm stent, including the stent polymer and the process of lasing the stent components, and we may be significantly impacted by any regulatory delays or barriers that our suppliers may encounter; and | ||
| • | we may be sued for infringement of intellectual property rights and could be prevented from manufacturing or selling the ReZolvetm stent or our future product candidates. |
We cannot market the ReZolvetm stent in the EU until we receive a CE Mark
or in the United States until we receive a PMA. We cannot guarantee that we will receive regulatory
approval for our ReZolvetm stent on a timely basis, or at all. Our operating
plan is based in part on our expectations regarding the timing for receipt of the required
regulatory approvals for our ReZolvetm stent. If we experience significant
delays in the regulatory approval process, we may be unable to reduce our expenditures in a timely
manner to compensate for such delays and we may not have adequate financial or other resources to
complete the regulatory approval process. Accordingly, a significant delay in the regulatory
approval process for our ReZolvetm stent would have a material adverse effect
on our business and financial condition. In addition, we may be required to raise additional
financing to fund our operations through various means, including equity or debt financing, which
could be dilutive to existing stockholders or require us to relinquish important rights to our
technology or products.
We will depend heavily on the success of our lead product candidate, our
ReZolvetm stent. Any factors that negatively impact sales of this product will
adversely affect our business, financial condition, and results of operations.
Assuming we can obtain the required regulatory approvals, we expect to derive substantially
all of our revenues from sales of our first product candidate, the ReZolvetm
stent. Accordingly, our ability to generate revenues in the future is reliant on our ability to
market and sell this device. The degree of market acceptance for our ReZolvetm
stent will depend on a number of factors, including:
25
Table of Contents
| • | the perceived advantages and disadvantages of the ReZolvetm stent over existing products and competitive treatments and technologies; | ||
| • | the safety and efficacy of the ReZolvetm stent and prevalence and severity of any adverse events or side effects especially as it relates to survival, quality of life, and bleeding; | ||
| • | the ease of use of the ReZolvetm stent compared to existing products and competitive treatments and technologies; | ||
| • | our ability to provide additional clinical data regarding the potential long-term benefits provided by the ReZolvetm stent; | ||
| • | the strength of our sales and marketing initiatives; and | ||
| • | the price of the ReZolvetm stent and the third-party coverage and reimbursement for procedures using our ReZolvetm stent. |
If the ReZolvetm stent does not achieve an adequate level of acceptance by
physicians, patients, and health care payors, we may not generate or maintain positive gross
margins and we may not become profitable or be able to sustain profitability. Even if the
ReZolvetm stent does achieve market acceptance, we may not be able to sustain
it or otherwise achieve it to a degree which would support the ongoing viability of our operations.
Physicians may not widely adopt our ReZolvetm stent unless they determine,
based on experience, long-term clinical data, and published peer reviewed journal articles, that
the use of our ReZolvetm stent provides a safe and effective alternative to
other existing treatments for coronary artery disease.
We believe that physicians will not widely adopt our ReZolvetm stent
unless they determine, based on experience, long-term clinical data, and published peer reviewed
journal articles, that the use of our ReZolvetm stent provides a safe and
effective alternative to other existing treatments for coronary artery disease. We cannot provide
any assurance that the data collected from our current and planned clinical trials will be
sufficient to demonstrate that the ReZolvetm stent is an attractive alternative
to other stent procedures. If we fail to demonstrate safety and efficacy that is at least
comparable to other stents that have received regulatory approval and that are available on the
market, our ability to successfully market the ReZolvetm stent will be
significantly limited. Even if the data collected from clinical studies or clinical experience
indicate positive results, each physician’s actual experience with the
ReZolvetm stent will vary. We also believe that published peer-reviewed journal
articles and recommendations and support by influential physicians regarding the
ReZolvetm stent will be important for market acceptance and adoption, and we
cannot assure you that we will receive these recommendations and support, or that supportive
articles will be published.
We may need substantial additional funding and may be unable to raise capital when needed, which
would force us to delay, reduce, or eliminate our product development programs or commercialization
efforts.
Our capital requirements will depend on many factors, including achievement of regulatory
approval of our products and the growth of revenue, the amount of expenditures on intellectual
property and technologies, the number of clinical trials which we conduct, and the extent of new
product development. To the extent that our existing capital is insufficient to meet these
requirements and cover any losses, we will need to raise additional funds through financings or
borrowings and our commercialization efforts would be delayed or reduced or may cease entirely. Any
equity or debt financing, if available at all, may be on terms that are not favorable to us. Equity
financings could result in dilution to our existing security holders, and the securities issued in
future financings may have rights, preferences, and privileges that are senior to those of our
existing security holders. If our need for capital arises because of significant losses, the
occurrence of these losses may make it more difficult for us to raise the necessary capital.
26
Table of Contents
We compete against companies that have longer operating histories, more established or approved
products, and greater resources than we do, which may prevent us from achieving further market
penetration or improving operating results.
Competition in the medical device industry is intense. Our products will compete against
products offered by substantial, global, public companies, such as Johnson & Johnson, Medtronic,
Abbott Laboratories, and BSC, as well as several private companies, such as Biotronik SE & Co. KG.
The four global medical device competitors have significantly greater technical, regulatory,
financial, manufacturing, and human resources than we do and have established reputations and
approved metal stent products and/or significantly greater name recognition, as well as
distribution channels and sales and marketing capabilities that are significantly larger and more
established than ours. For example, Johnson & Johnson, Medtronic, Abbott Laboratories, and BSC
constituted over 95% of the global $5.3 billion of stent sales in 2009.
Additional competitors, including those with a bioresorbable stent technology, may enter the
market, and we are likely to compete with companies offering new technologies in the future. We
also face competition from other medical therapies which may focus on our target market as well as
competition from manufacturers of pharmaceutical and other devices that have not yet been
developed. Competition from these companies could adversely affect our business.
Our ability to compete effectively depends upon our ability to distinguish our company and our
products from our competitors and their products. We believe the factors affecting our competitive
position include:
| • | name and brand recognition; | ||
| • | relationships with physicians and patients; | ||
| • | the availability of other products and procedures, including bundled product offerings; | ||
| • | product performance and design; | ||
| • | product safety and the availability of supporting clinical data; | ||
| • | sales, marketing and distribution capabilities; | ||
| • | success and timing of new product development and introductions; and | ||
| • | intellectual property protection. |
The industry in which we operate has also undergone, and is expected to continue to undergo,
rapid and significant technological change, and we expect competition to intensify as technical
advances are made. Our competitors may develop and commercialize stents or other medical device or
pharmaceutical products that are safer or more effective, have fewer side effects, or are less
expensive than any products that we
may develop. For example, we are aware of companies that are developing various other
less-invasive technologies for treating cardiovascular disease, which could limit the market
potential for our stents. We also compete with our competitors in recruiting and retaining
qualified scientific and management personnel, establishing clinical trial sites, and patient
registration for clinical trials, as well as in acquiring technologies and technology licenses
complementary to our programs or advantageous to our business. For all the foregoing reasons, we
may not be able to compete successfully against our current and future competitors.
Product liability claims could damage our reputation or adversely affect our business.
The design, manufacture, and sale of human medical devices, particularly implantable
life-sustaining medical devices, carries an inherent risk of product liability claims and other
damage claims. Such liability claims may be expensive to defend and may result in large judgments
against us. A product liability or other damages claim, product recall, or product misuse,
regardless of the ultimate outcome, could require us to spend significant time and money in
litigation or to pay significant damages and could seriously harm our business. We maintain
clinical trial insurance and limited product liability insurance. We cannot be certain that such
insurance will be sufficient to cover all claims that may be made against us. Our insurance
policies generally must be renewed on an annual basis. We may not be able to maintain or increase
such insurance on acceptable terms or at reasonable costs. A successful claim brought against us in
excess, or outside, of our insurance coverage could seriously harm our financial condition and
results of operations. Such claims against us, regardless of their merit, could result in
significant awards against us that could materially adversely harm our business, financial
condition, results of operations, and prospects. A product liability or other damages claim,
product recall, or product misuse involving any type of coronary stent, but especially involving
one of ours, could also materially and adversely damage our reputation and affect our ability to
attract and retain customers, irrespective of whether or not the claim or recall was meritorious.
27
Table of Contents
We have limited capabilities and manufacturing personnel, and if our manufacturing facilities are
unable to provide an adequate supply of our ReZolvetm stent to support our
clinical trials our regulatory approval timeline may be delayed.
We currently manufacture our ReZolvetm stent at our facilities in San
Diego, California. If there was a disruption to our existing manufacturing facility or the
surrounding area, for example, due to a natural disaster, we would have no other means of
manufacturing our ReZolvetm stent until we were able to restore the
manufacturing capability at our facility or develop alternative manufacturing facilities. If we are
unable to produce sufficient quantities of our ReZolvetm stent for use in our
current and planned clinical trials, or if our manufacturing process yields substandard product,
our regulatory approval process may be delayed.
Further, assuming we get approval for our ReZolvetm stent, we currently
have limited resources and facilities and no prior history of commercially manufacturing products.
In addition, we will need to obtain the necessary regulatory approvals to manufacture our
ReZolvetm stent for commercialization. In order to produce commercial
quantities of the ReZolvetm stent, we will need to increase substantially the
production processes and efficiency of our manufacturing operations. There are significant
technical and regulatory challenges to increasing manufacturing capacity and efficiency, and
developing commercial-scale manufacturing facilities will require the investment of additional
funds and hiring and retaining additional management and technical personnel who have the necessary
manufacturing experience. We may not successfully complete any required increase in a timely or
economically viable manner or at all. In addition, we may not be able to receive the necessary
regulatory approvals for our manufacturing facilities on a timely basis or at all. If we are unable
to manufacture a sufficient or consistent supply of the ReZolvetm stent or any
other product we are developing, or if we cannot do so efficiently, our revenues, business, and
financial prospects would be adversely affected.
We rely on specialized suppliers for certain components and processes in the manufacture of our
ReZolvetm stent.
We rely on suppliers for several critical components used in our ReZolvetm
stent, including the stent polymer and the process of lasing our stent components. We also
outsource the sterilization of the finished product. Our reliance on third-party suppliers subjects
us to risks that could harm our business, including:
| • | we are not a major customer of many of our suppliers, and these suppliers may therefore give other customers’ needs higher priority than ours; | ||
| • | our suppliers have no contractual obligation to supply, and we are not obligated to purchase from them, any components used in our ReZolvetm stent which may result in supply interruptions; | ||
| • | our polymer is complex and must be manufactured to extremely tight tolerances and specifications with the result that our suppliers, especially new suppliers, may make errors in manufacturing that could negatively affect the efficacy or safety of the ReZolvetm stent or cause our components not to be delivered on time or at all or to be delivered outside of specifications; | ||
| • | the availability of second-source suppliers may be extremely limited or their implementation as a supplier may be lengthy due to the tight tolerances and specifications which we require for the ReZolvetm stent; and | ||
| • | switching suppliers or changes to our service providers may require product redesign and submission to the regulatory authorities to whom we are seeking approval for our ReZolvetm stent. |
Additionally, we may experience problems or delays in our own manufacturing and assembly
process. Our current product development plan is predicated on maintaining strong relationships and
supply with several external parties to manufacture components of our ReZolvetm
stent. If we are unsuccessful in this regard or are unable to secure or maintain agreements with
these manufacturers on favorable terms or at all, our ability to obtain regulatory approval for our
products will be harmed.
28
Table of Contents
If we are unable to retain or hire key personnel, we may not be able to sustain or grow our
business.
Our ability to operate successfully and manage our potential future growth depends
significantly upon our ability to attract, retain, and motivate highly skilled and qualified
research, technical, clinical, regulatory, sales, marketing, managerial, and financial personnel.
We compete for talent with numerous companies, as well as universities and non-profit research
organizations. Our future success also depends on the personal efforts and abilities of the
principal members of our senior management and scientific staff to provide strategic direction,
manage our operations, and maintain a cohesive and stable environment. Except with respect to our
agreements with Robert B. Stockman, our Chief Executive Officer, Robert Schultz, our President and
Chief Operating Officer, and Katrina Thompson, our Chief Financial Officer, we have not entered
into any employment agreements with our executive officers, nor do we maintain key man life
insurance on the lives of any of the members of our senior management. Although we have a stock
option plan pursuant to which we provide our executive officers with various economic incentives to
remain employed with us, these incentives may not be sufficient to retain them. The loss of key
personnel for any reason or our inability to hire, retain, and motivate additional qualified
personnel in the future could prevent us from sustaining or growing our business.
BSC has an option to distribute our ReZolvetm stent, which may limit our
ability to negotiate more favorable terms with other potential distributors.
In December 2007, we entered into a Distribution Option Agreement with BSC under which we
granted BSC an option to negotiate the right to be the worldwide, exclusive distributor of our
ReZolvetm stent. If BSC exercises its option, we are required to negotiate in
good faith with BSC to enter into a mutually acceptable definitive distribution agreement. If we
are unable to agree on the terms of a definitive distribution agreement with BSC, the restrictions
in the Distribution Option Agreement may limit our ability to negotiate more favorable terms with
other potential distribution partners.
If we do not enter into a distribution arrangement with BSC, we will need to identify another
distribution partner for the sale of our product or develop our own sales network. Any delay or
problems associated with a distribution partner or our own sales network could have a serious
impact on our sales and our financial performance.
We do not have any experience in marketing, selling, or distributing products. Our current
strategy is to select a distribution partner to assist in the marketing and sale of our product in
jurisdictions where it is approved for commercial sale. There is no guarantee that BSC will
exercise its option to distribute our products under the Distribution Option Agreement, or that we
will be able to reach a definitive distribution agreement with BSC. If we do not enter into a
distribution arrangement with BSC, we will need to identify another distribution partner for the
sale of our product or develop our own sales and marketing network. There can be no assurance that
we will be able to identify and enter into a distribution arrangement with a third party
distributor on acceptable terms or at all. In the event that we decide to develop our own sales,
distribution, and marketing capabilities, we will have to invest significant amounts of financial
and management resources. In developing these sales, marketing, and distribution functions
ourselves, we will face a number of risks, including:
| • | the inability to attract and build a significant, successful, or qualified marketing or sales force; | ||
| • | the cost of establishing, training, and providing regulatory oversight for a marketing or sales force may be substantial; and | ||
| • | the significant legal and regulatory risks in medical device marketing and sales, and any failure to comply with all legal and regulatory requirements for sales, marketing, and distribution could result in enforcement action by the FDA or other authorities and could jeopardize our ability to market the product or could subject us to substantial liability. |
Any delay or problems associated with a distribution partner or our own sales network could
have a serious impact on our sales and our financial performance.
Based on our current operating plan, we may be subject to the risks associated with operating in
multiple foreign markets.
Our operations are primarily located in the United States. In addition to seeking a PMA in the
United States, we currently intend to seek regulatory approvals for our
ReZolvetm stent in the EU and Australia. If we expand into these and additional
foreign markets outside the United States, we will be subject to new business risks, including:
| • | failure to fulfill foreign regulatory requirements on a timely basis or at all to market the ReZolvetm stent or other future products; |
29
Table of Contents
| • | availability of, and changes in, reimbursement within prevailing foreign health care payment systems; | ||
| • | adapting to the differing laws and regulations, business and clinical practices, and patient preferences in foreign countries; | ||
| • | difficulties in managing foreign relationships and operations, including any relationships that we establish with foreign partners, distributors, or sales or marketing agents; | ||
| • | limited protection for intellectual property rights in some countries; | ||
| • | difficulty in collecting accounts receivable and longer collection periods; | ||
| • | costs of enforcing contractual obligations in foreign jurisdictions; | ||
| • | recessions in relevant foreign countries; | ||
| • | political instability and unexpected changes in diplomatic and trade relationships; | ||
| • | currency exchange rate fluctuations; and | ||
| • | potentially adverse tax consequences. |
If we are successful in introducing our ReZolvetm stent or future products
into foreign markets, we will be affected by these additional business risks, which may adversely
impact our business, financial condition, and results of operations. In addition, expansion into
additional foreign markets imposes additional burdens on our executive and administrative
personnel, research and sales departments, and general managerial resources. Our efforts to
introduce our current or future products into foreign markets may not be successful, in which case
we may have expended significant resources without realizing the expected benefit. Ultimately, the
investment required for expansion into foreign markets could exceed the results of operations
generated from this expansion.
Risk Factors Related to Regulation
In order to commence human clinical trials we will need to obtain regulatory and other approvals.
If we are unable to achieve or are delayed in achieving such approvals, this could have a
significant effect on our timeline and ability to commercialize our technology.
To date, we have performed a series of preclinical trials in animals which will be used to
support an application to commence the pilot human clinical trial and the pivotal CE Mark human
clinical trial. There is no guarantee that we will obtain the necessary regulatory approvals to
commence human clinical trials, and there is no guarantee that additional work and preclinical
testing will not be required by the regulatory bodies before they allow us to commence human
clinical trials. Before we can commence our human clinical trials, we require approvals from:
| • | the relevant Ethics Committees (Investigational Review Boards) in each of our chosen clinical trial centers; and | ||
| • | the relevant regulatory bodies as required in the applicable countries, such as Brazil and Germany. |
In the United States, prior to conducting human clinical trials, we will need to obtain
approval of an IDE application from the FDA. Before we can sell our products in the United States,
PMA is required from the FDA, which is a lengthy and uncertain process. The procedure for
submitting an application for PMA is lengthy, expensive, and typically requires extensive
preclinical and clinical trial data as well as considerable technical data. Submitted data will
need to be obtained in accordance with FDA QSR.
We are planning to use the clinical trial data obtained in Australia and the EU in order to
facilitate a more expedient U.S. approval process. There is a risk that the FDA may not allow those
results to be used in the PMA application which would result in a delay and increase in costs of
U.S. approvals.
30
Table of Contents
We cannot predict the outcome of the human clinical trials. If the ReZolvetm
stent does not meet our required clinical specifications or causes adverse or unexpected events,
then we may need to further modify the design or technology used in the
ReZolvetm stent. There is no guarantee that we will be able to address any
issues arising from the clinical trials which could be catastrophic for our future prospects.
The outcome of human clinical trials cannot be predicted, even when preclinical results are
favorable. If our ReZolvetm stent causes adverse issues in human clinical
trials, such as restenosis, stroke, thrombosis, and/or death, then it is likely the human clinical
trial will need to be halted. In such case, we may need to modify our technology to address these
issues while also meeting the market requirements for stent products. Our clinical trials may also
be suspended or terminated at any time by EU regulatory authorities, the U.S. Data Safety and
Monitoring Board or by us including during the closing stages of enrollment of the trial and the
subsequent patient follow-up period lasting up to 12 months in the event that, for example, there
should be a series of adverse clinical events such as heart attack or stroke. There is no guarantee
that if there are adverse results arising in the human clinical trials that the issues will be able
to be successfully addressed and overcome. If we are unable to address these issues, we will not be
able to commercialize our technology, and it will likely have a nominal value.
We performed a small first human clinical trial in 2007 with 25 patients in Brazil and Germany
on an early version of our stent. We achieved deployment success, demonstrating the stent’s ability
to dilate and hold the lesion as anticipated and consistent with the results of our preclinical
data. However, at approximately four months, we saw adverse device performance resulting in a
higher than anticipated number of patients requiring retreatment with another stent. These issues
were primarily associated with the brittle nature of the polymer that resulted in fractured
supporting elements of the stent. We addressed these issues by modifying the design and the
composition of the polymer used in our ReZolvetm stent. These modification
activities have been our primary focus for the past three years, during which time we used cash for
operating activities of nearly $33.9 million, to the exclusion of other development activities and
opportunities.
The completion of our clinical trial program could also be substantially delayed or prevented
by several factors, including:
| • | delays in receiving the necessary regulatory approvals to commence the CE Mark Trial; | ||
| • | slower than expected rates of patient recruitment and enrollment, including as a result of our competitors undertaking similar clinical trials or having functionally comparable products that have received approval for sale; | ||
| • | failure of patients to complete the clinical trial; | ||
| • | patients preferring to use approved devices or other experimental treatments or devices rather than our ReZolvetm stent; | ||
| • | unforeseen safety issues; | ||
| • | perceived lack of product efficacy during clinical trials; | ||
| • | inability or unwillingness of patients or medical investigators to follow our clinical trial protocols; | ||
| • | inability to monitor patients adequately during or after treatment; | ||
| • | risks associated with trial design, which may result in a failure of the trial to show statistically significant results even if the product is effective; | ||
| • | governmental and regulatory delays or changes in regulatory requirements, policies, or guidelines; | ||
| • | varying interpretation of data by regulatory agencies; and | ||
| • | perceived lack of product efficacy during clinical trials. |
The process of obtaining marketing approval or clearance from regulatory authorities for our
ReZolvetm stent, or any future products or enhancements or modifications to any
products, could:
| • | take a significant period of time; |
31
Table of Contents
| • | require the expenditure of substantial resources; | ||
| • | involve rigorous preclinical and clinical testing; | ||
| • | require changes to our products; and | ||
| • | result in limitations on the indicated uses of the products. |
There can be no assurance that we will receive the required approvals from the regulatory
authorities or, if we do receive the required approvals, that we will receive them on a timely
basis or that we will otherwise be able to satisfy the conditions of such approval, if any. The
failure to receive product approval clearance by the regulatory authorities will have a material
adverse effect on our business, financial condition or results of operations.
We do not have long-term data regarding the safety and efficacy of our
ReZolvetm stent. Any long-term data that is generated may not be consistent
with our limited short-term data, which could affect the regulatory approval of our products or the
rate at which our products are adopted.
An important factor in our clinical trials, upon which the safety and efficacy of our
ReZolvetm stent may be measured, is the rate of restenosis, or the renarrowing
of the treated artery over time, and the rate of reintervention, or retreatment following the
procedures using our ReZolvetm stent. We believe that physicians and regulators
will compare the rates of long-term restenosis and reintervention for our
ReZolvetm stent against other bioresorbable, drug-eluting, or bare-metal stent
procedures and other alternative procedures.
If, in our planned pivotal clinical trial, we fail to demonstrate restenosis and
reintervention rates, as well as other clinical trial endpoints and performance, comparable to
other stents that have been approved by the FDA and other regulatory authorities, our ability to
successfully market our ReZolvetm stent may be significantly limited. If the
long-term rates of restenosis and reintervention do not meet regulators’ or physicians’
expectations, our ReZolvetm stent may not receive regulatory approval or, if
approved, may not become widely adopted and physicians may recommend that patients receive
alternative treatments. Another important factor upon which the safety and efficacy of our
ReZolvetm stent will be measured is the incidence of late-stent thrombosis
following procedures using our ReZolvetm stent. We cannot assure you that our
long-term data, once obtained, will prove a lower incidence of late-stent thrombosis as compared to
drug-eluting metal stents. If the results obtained from our clinical trials indicate that our
products are not as safe or effective as other treatment options or as effective as current
short-term data would suggest, our products may not be approved, adoption of our products may
suffer and our business would be harmed.
We plan to operate in multiple regulatory environments that require costly and time consuming
approvals.
We will need to obtain regulatory approval in each jurisdiction in which we intend to
commercialize our ReZolvetm stent. The required regulatory requirements will
vary from country to country. In addition, the laws and regulations regarding the manufacture and
sale of our products will be subject to future changes, as are administrative interpretations and
policies of regulatory agencies. If we fail to comply with applicable laws or regulations, we could
be subject to enforcement actions. Enforcement actions could include product seizures, recalls,
withdrawal of clearances or approvals, and civil and criminal penalties, which in each case would
harm our business.
Our planned manufacturing facilities and the manufacturing facilities of our suppliers must comply
with applicable regulatory requirements. If we fail to achieve regulatory approval for these
manufacturing facilities, our business and our results of operations would be harmed.
Completion of our clinical trials and commercialization of our products require access to, or the
development of, manufacturing facilities that meet applicable regulatory standards to manufacture a
sufficient supply of our products. Approvals are required to achieve CE Marking in Europe, and
similar approvals must be obtained from the FDA for facilities that manufacture our products for
U.S. commercial purposes. Suppliers of components and products used to manufacture our products
must also comply with applicable regulatory requirements, which often require significant time,
money, resources, and record-
keeping and quality assurance efforts and subject us and our suppliers to potential regulatory
inspections and stoppages. If we or our suppliers fail to comply with the regulatory requirements
for our manufacturing operations our commercialization efforts could be delayed, which would harm
our business and our results of operations.
32
Table of Contents
We may not meet regulatory quality standards applicable to our manufacturing and quality
processes, which could have an adverse effect on our business, financial condition, or results of
operations.
Even after products have received marketing approval or clearance, product approvals and
clearances by the regulatory bodies can be withdrawn due to failure to comply with regulatory
standards or the occurrence of problems following initial approval. As a device manufacturer, we
will be required to demonstrate and maintain compliance with a variety of regulatory requirements,
including the FDA’s QSR. The QSR is a complex regulatory scheme that covers the methods and
documentation of the design, testing, control, manufacturing, labeling, quality assurance,
packaging, storage, and shipping of our products. The FDA enforces the QSR through periodic
unannounced site inspections.
In addition, the U.S. federal medical device reporting regulations require us to provide
information to the FDA whenever there is evidence that reasonably suggests that a device may have
caused or contributed to a death or serious injury or, if a malfunction were to occur, could cause
or contribute to a death or serious injury. Compliance with applicable regulatory requirements is
subject to continual review and is rigorously monitored through periodic inspections by the FDA. If
we fail to comply with the QSR or to take satisfactory corrective action in response to an adverse
QSR inspection, this could result in enforcement actions, including a public warning letter, a
shutdown of or restrictions on our manufacturing operations, delays in approving or clearing a
product, refusal to permit the import or export of our products, a recall or seizure of our
products, fines, injunctions, civil or criminal penalties, or other sanctions, any of which could
cause our business and operating results to materially suffer.
In the EU, we are required to maintain certain ISO certifications in order to sell our
products and must undergo periodic inspections by notified bodies to obtain and maintain these
certifications. We have received a Certificate of Registration certifying that our Quality
Management System complies with the requirements of ISO 13485:2003. If in the future we fail to
continue to comply with ISO regulations, the FDA or EU regulatory authorities may withdraw
clearance to market, require a product recall, or take other enforcement action.
Our operations involve hazardous materials, and we must comply with environmental laws and
regulations, which can be expensive.
Our research and development activities involve the controlled use of hazardous chemicals. Our
operations also produce hazardous waste products. We are subject to a variety of federal, state,
and local regulations relating to the use, handling, storage, and disposal of these materials. We
generally contract with third parties for the disposal of such substances. We cannot eliminate the
risk of accidental contamination or injury from these materials. We may be required to incur
substantial costs to comply with current or future environmental and safety regulations. If an
accident or contamination occurred, we would likely incur significant costs associated with civil
penalties or criminal fines. Current or future environmental regulation may impair our research,
development, or production efforts.
If we fail to obtain and maintain adequate level of reimbursement for our products by third-party
payors, there may be no commercially viable markets for our products or the markets may be much
smaller than expected.
The availability and levels of reimbursement by governmental and other third-party payors
affect the market for our products. Reimbursement and health care payment systems vary
significantly by country, and include both government sponsored health care and private insurance.
Payors may attempt to limit coverage and the level of reimbursement of new therapeutic products.
Government and other third-party payors also continually attempt to contain or reduce the costs of
health care by challenging prices charged for health care products and services.
To obtain reimbursement or pricing approval in some countries, we may be required to produce
clinical data, which may involve one or more clinical trials, that compares the cost-effectiveness
of our products to other available therapies. In addition, the efficacy, safety, performance, and
cost-effectiveness of our products in comparison to any competing products may determine the
availability and level of reimbursement for our products.
We believe that future reimbursement may be subject to increased restrictions both in the
United States and in international markets. Future legislation, regulation, or reimbursement
policies of third-party payors may adversely affect the demand for our products currently under
development and limit our ability to sell our products on a profitable basis. We cannot predict how
pending or future legislative and regulatory proposals would influence the manner in which medical
devices, including ours, are purchased or covered and reimbursed. For example, the American
Recovery and Reinvestment Act of 2009 includes funding to study the comparative effectiveness of
health
care treatments and strategies. This funding will be used, among other things, to conduct,
support, or synthesize research that compares and evaluates the risks and benefits, clinical
outcomes, effectiveness, and appropriateness of medical products. Although Congress has indicated
that this funding is intended to improve the quality of health care, it remains unclear how the
research will impact coverage, reimbursement, or other third-party payor policies.
33
Table of Contents
If reimbursement for our products is unavailable or limited in scope or amount or if pricing
is set at unsatisfactory levels, market acceptance of our products would be impaired and our future
revenues would be materially adversely affected.
Health care reform legislation could adversely affect our future revenue and financial condition.
In recent years, there have been numerous initiatives on the federal and state levels for
comprehensive reforms affecting the payment for, the availability of and reimbursement for health
care services in the United States. These initiatives have ranged from proposals to fundamentally
change federal and state health care reimbursement programs, including providing comprehensive
health care coverage to the public under governmental funded programs, to minor modifications to
existing programs. Recently, President Obama and members of Congress passed and continue to propose
significant reforms to the U.S. health care system. Both the U.S. Senate and House of
Representatives have conducted hearings about U.S. health care reform and a number of bills have
been proposed in Congress.
In addition, recent legislation and many of these proposed bills include funding to assess the
comparative effectiveness of medical devices. It is unclear what impact the comparative
effectiveness analysis would have on our products or our financial results. The ultimate content or
timing of any future health care reform legislation, and its impact on medical device companies
such as us, is impossible to predict. If significant reforms are made to the health care system in
the United States, or in other jurisdictions, those reforms may have a material adverse effect on
our financial condition and results of operations.
In March 2010, Congress enacted comprehensive health care reform legislation known as the
Patient Protection and Affordable Care Act of 2010, or the PPACA. While the PPACA involves
expanding coverage to more individuals, it includes new regulatory mandates and other measures
designed to constrain medical costs. The PPACA also imposes significant new taxes on medical device
manufacturers that are expected to cost the medical device industry up to $20 billion over the next
decade. There are also stringent new reporting requirements of financial relationships between
device manufacturers and physicians and teaching hospitals. Complying with PPACA could
significantly increase our costs and adversely affect our business and financial condition.
Our operations will also be impacted by the PPACA, as modified by the Health Care and
Education Reconciliation Act of 2010, which we refer to as the Health Care Act. The Health Care Act
imposes a 2.3 percent excise tax on sales of medical devices by manufacturers. We expect our stent
products to fall within the scope of this tax. There is no exemption for small companies, and we
expect to begin paying the tax in 2013. The Health Care Act also requires manufacturers to report
to the Department of Health and Human Services detailed information about financial arrangements
with physicians and teaching hospitals. These reporting provisions preempt state laws that require
reporting of the same information, but not those that require reports of different or additional
information. Failure to comply subjects the manufacturer to significant civil monetary penalties.
We expect compliance with the Health Care Act to impose significant administrative and financial
burdens on us.
We are subject to various federal and state laws pertaining to health care fraud and abuse,
including anti-kickback, self-referral, false claims, and fraud laws, and any violations by us of
such laws could result in fines or other penalties.
Our commercial, research, and other financial relationships with health care providers and
institutions are subject to various federal and state laws intended to prevent health care fraud
and abuse. The federal anti-kickback statute prohibits the knowing offer, receipt, or payment of
remuneration in exchange for or to induce the referral of patients or the use of products or
services that would be paid for in whole or part by Medicare, Medicaid, or other federal health
care programs. Remuneration has been broadly defined to include anything of value, including cash,
improper discounts, and free or reduced price items and services. Many states have similar laws
that apply to their state health care programs as well as private payors. Violations of the
anti-kickback laws can result in exclusion from federal health care programs and substantial civil
and criminal penalties.
34
Table of Contents
The federal False Claims Act, or FCA, imposes liability on persons who, among other things,
present or cause to be presented false or fraudulent claims for payment by a federal health care
program. The FCA has been used to prosecute persons submitting claims for payment that are
inaccurate or fraudulent, that are for services not provided as claimed, or for services that are
not medically necessary. The FCA includes a whistleblower provision that allows individuals to
bring actions on behalf of the federal government and share a portion of the recovery of successful
claims. If our marketing or other arrangements were determined to violate anti-kickback or related
laws, including the FCA, then our revenues could be adversely affected, which would likely have a
material adverse effect on our business, financial conditions, and results of operations.
State and federal authorities have aggressively targeted medical device companies for alleged
violations of these anti-fraud statutes, based on improper research or consulting contracts with
doctors, certain marketing arrangements that rely on volume-based pricing, off-label marketing
schemes, and other improper promotional practices. Companies targeted in such prosecutions have
paid substantial fines in the hundreds of millions of dollars or more, have been forced to
implement extensive corrective action plans, and have often become subject to consent decrees
severely restricting the manner in which they conduct their business. If we become the target of
such an investigation or prosecution based on our contractual relationships with providers or
institutions, or our marketing and promotional practices, we could face similar sanctions which
would materially negatively affect our business.
If we are found to have violated laws protecting the confidentiality of patient health information,
we could be subject to civil or criminal penalties, which could increase our liabilities and harm
our reputation or our business.
There are a number of federal and state laws in the United States protecting the
confidentiality of certain patient health information, including patient records, and restricting
the use and disclosure of that protected information. In particular, the U.S. Department of Health
and Human Services promulgated patient privacy rules under the Health Insurance Portability and
Accountability Act of 1996, or HIPAA. These privacy rules protect medical records and other
personal health information by limiting their use and disclosure, giving individuals the right to
access, amend, and seek accounting of their own health information, and limiting most use and
disclosures of health information to the minimum amount reasonably necessary to accomplish the
intended purpose. If we are found to be in violation of the privacy rules under HIPAA, we could be
subject to civil or criminal penalties, which could increase our liabilities, harm our reputation,
and have a material adverse effect on our business, financial condition, and results of operations.
Risk Factors Related to Intellectual Property
We rely on certain licenses for patents and other technology related to our products. The
termination of these agreements could delay or prevent us from being able to commercialize our
products.
We depend on licenses to certain patents and other technology used in our
ReZolvetm stent and stent components. For example, we rely on certain licensed
patents from Rutgers University for the polymer we use in our ReZolvetmstent.
In order to maintain our rights under the Rutgers License Agreement, we must satisfy certain
development and commercialization obligations. If we fail to satisfy these obligations, and
licenses to these patents were provided to one or more of our competitors, our ability to compete
may be diminished. Furthermore, if we fail to comply with our material obligations under this
license agreement, the license may be terminated and we could lose license rights that are
important to our business. In addition, the license agreement expires on the expiration of last to
expire patents under this agreement which is approximately 2030, and there is no guarantee we will
be able to renew the license agreement on commercially reasonable terms.
In addition, we expect that we will need to license other technology or patents to
commercialize future products. These licenses may not be available to us on commercially reasonable
terms, or at all, which could adversely affect our results of operations and growth prospects.
35
Table of Contents
If we are unable to obtain, maintain, and enforce intellectual property protection covering our
products, others may be able to make, use, or sell products substantially the same as ours, which
could adversely affect our ability to compete in the market.
Our commercial success is dependent in part on obtaining, maintaining, and enforcing
intellectual property rights, including patents, covering our ReZolvetm stent
and future product candidates. If we are unable to obtain, maintain, and enforce intellectual
property protection covering our products, others may be able to make, use, or sell products
that are substantially the same as ours without incurring the sizeable development and
licensing costs that we have incurred, which would adversely affect our ability to compete in the
market. Currently, our patent portfolio is comprised, on a worldwide basis, of close to 270 issued
U.S. and foreign patents which we own directly or for which we are the exclusive licensee and that
expire as late as 2030. Pending patent applications could further extend our patent portfolio life.
However, patents may not be issued based on any pending or future patent applications owned by or
licensed to us and, moreover, issued patents owned or licensed to us now or in the future may be
found by a court to be invalid or otherwise unenforceable. Also, even if our patents are determined
by a court to be valid and enforceable, they may not be sufficiently broad to prevent others from
marketing products similar to ours or designing around our patents, despite our patent rights, nor
do they provide us with freedom to operate unimpeded by the patent rights of others.
We have also licensed certain intellectual property from third parties related to our
products, and we rely on them to file and prosecute patent applications and maintain patents and
otherwise protect the licensed intellectual property. We cannot be certain that such activities by
third parties have been or will be conducted in compliance with applicable laws and regulations or
will result in valid and enforceable patents and other intellectual property rights. In addition,
we cannot be certain that our licensors will allocate sufficient resources or prioritize their or
our enforcement of such patents or defense of such claims to protect our interests in the licensed
patents.
The patent positions of medical device companies can be highly uncertain and involve complex
legal and factual questions for which important legal principles remain unresolved. No consistent
policy regarding the breadth of claims allowed in patents in these fields has emerged to date in
the United States or in many foreign jurisdictions. Both the U.S. Supreme Court and the Court of
Appeals for the Federal Circuit have made, and will likely continue to make, changes in how the
patent laws of the U.S. are interpreted. In addition, Congress is currently considering legislation
that would change provisions of the patent law. We cannot predict future changes in the
interpretation of patent laws or changes to patent laws which might be enacted into law. Those
changes may materially affect our patents, our ability to obtain patents, or the patents and
applications of our collaborators and licensors. The patent situation in the medical device and
disease diagnostic fields outside the United States is even more uncertain.
We have a number of foreign patents and applications. However, the laws of some foreign
jurisdictions do not protect intellectual property rights to the same extent as laws in the United
States, and many companies have encountered significant difficulties in obtaining, protecting, and
defending such rights in foreign jurisdictions. If we encounter such difficulties or we are
otherwise precluded from effectively protecting our intellectual property rights in foreign
jurisdictions, our business prospects could be substantially harmed.
We also rely on trade-secret protection to protect our interests in proprietary know-how and
for processes for which patents are difficult to obtain or enforce. We may not be able to protect
our trade secrets adequately. We have limited control over the protection of trade secrets used by
our licensors, collaborators, and suppliers. Although we use reasonable efforts to protect our
trade secrets, our employees, consultants, contractors, outside scientific collaborators, and other
advisors may unintentionally or willfully disclose our information to competitors. Enforcing a
claim that a third party illegally obtained and is using any of our trade secrets is expensive and
time consuming, and the outcome is unpredictable. In addition, courts outside the United States are
sometimes less willing to protect trade secrets. We rely, in part, on non-disclosure and
confidentiality agreements with our employees, consultants, and other parties to protect our trade
secrets and other proprietary technology. These agreements may be breached and we may not have
adequate remedies for any breach. Moreover, others may independently develop equivalent proprietary
information, and third parties may otherwise gain access to our trade secrets and proprietary
knowledge. Any disclosure of confidential data into the public domain or to third parties could
allow our competitors to learn our trade secrets and use the information in competition against us.
Claims that our current or future products infringe or misappropriate the proprietary rights of
others could adversely affect our ability to sell those products and cause us to incur additional
costs.
Intellectual property rights, including in particular patent rights, play a critical role in
the stent and stent delivery systems in the medical device industry, and therefore in our business.
We face significant risks relating to patents, both as to our own patent position as well as to
patents held by third parties. If any third-party intellectual property claim against us is
successful, we could be prevented from commercializing our ReZolvetm stent or
other products.
There are numerous U.S. and foreign issued patents and pending patent applications owned by
third parties with patent claims in areas that are the focus of our product development efforts. We
are aware of patents owned by third parties, to which we do not have licenses, that relate to,
among other things:
36
Table of Contents
| • | stent structures and materials; | ||
| • | catheters used to deliver stents; and | ||
| • | stent manufacturing and coating processes. |
Moreover, because patent applications can take many years to issue, there may be currently
pending applications, unknown to us, which may later result in issued patents that pose a material
risk to us.
We expect that we could be increasingly subject to third-party infringement claims as our
revenues increase, we are faced with more competitors, and the functionality of products and
technology in different industry segments overlaps. Third parties may currently have, or may
eventually be issued, patents on which our current or future products or technologies may infringe.
For example, we are aware of certain patents and patent applications owned by third parties that
cover different aspects of stent designs, polymer composition, and related technologies. Any of
these third parties might make a claim of infringement against us.
All of the major companies in the stent and related markets, including BSC, Abbott
Laboratories, Johnson & Johnson, and Medtronic have been involved in patent litigation relating to
stents since at least 1997. The stent and related markets have experienced rapid technological
change and obsolescence in the past, and our competitors have strong incentives to stop or delay
the introduction of new products and technologies. We may pose a competitive threat to many of the
companies in the stent and related markets. Accordingly, many of these companies will have a strong
incentive to take steps, through patent litigation or otherwise, to prevent us from commercializing
our products.
Any litigation, regardless of its outcome, would likely result in the expenditure of
significant financial resources and the diversion of management’s time and resources. In addition,
litigation in which we are accused of infringement may cause negative publicity, adversely impact
prospective customers, cause product shipment delays, prohibit us from manufacturing, marketing, or
selling our current or future products, require us to develop non-infringing technology, make
substantial payments to third parties, or enter into royalty or license agreements, which may not
be available on acceptable terms or at all. If a successful claim of infringement were made against
us and we could not develop non-infringing technology or license the infringed or similar
technology on a timely and cost-effective basis, our revenues may decrease substantially and we
could be exposed to significant liability. A court could enter orders that temporarily,
preliminarily, or permanently prevent us or our customers from making, using, selling, offering to
sell, or importing our current or future products, or could enter an order mandating that we
undertake certain remedial activities. Claims that we have misappropriated the confidential
information or trade secrets of third parties can have a similar negative impact on our reputation,
business, financial condition, or results of operations.
We may need to initiate lawsuits to protect or enforce our patents and other intellectual property
rights, which could be expensive and, if we lose, could cause us to lose some of our intellectual
property rights, which would harm our ability to compete in the market.
We rely on patents to protect a portion of our intellectual property and our competitive
position. Patent law relating to the scope of claims in the technology fields in which we operate
is still evolving and, consequently, patent positions in the medical device industry are generally
uncertain. In order to protect or enforce our patent rights, we may initiate patent litigation
against third parties, such as infringement suits or interference proceedings. Litigation may be
necessary to:
| • | assert claims of infringement; | ||
| • | enforce our patents; | ||
| • | protect our trade secrets or know-how; or | ||
| • | determine the enforceability, scope, and validity of the proprietary rights of others. |
Any lawsuits that we initiate could be expensive, take significant time, and divert
management’s attention from other business concerns. Litigation also puts our patents at risk of
being invalidated or interpreted narrowly and our patent applications at risk of not issuing.
Additionally, we may provoke third parties to assert claims against us. We may not prevail in any
lawsuits that we initiate and the damages or other remedies awarded, if any, may not be
commercially valuable. The occurrence of any of these events may have a material adverse
effect on our business, financial condition and results of operations.
37
Table of Contents
Risks Related to Our CDIs and Common Stock
The market price of our CDIs and common stock may be volatile and fluctuate significantly, which
could result in substantial losses for investors.
Our CDIs and common stock had not been publicly traded prior to our initial public offering,
which was completed in December 2010. Our securities are listed for sale only on the ASX. Since our
listing is recent, we have not yet established a trading history and are unable to predict how
active or liquid the market for our CDIs will become. As a result, the market price of our CDIs may
be volatile and fluctuate significantly. Among the factors that may cause the market price of our
CDIs to fluctuate are the risks described in this “Risk Factors” section and other factors,
including:
| • | announcements regarding the regulatory status of our ReZolvetm stent and future product candidates; | ||
| • | any reported adverse effects in our human clinical trials for our ReZolvetm stent; | ||
| • | announcements of technological innovations or new products by us or our competitors; | ||
| • | announcements of contracts, acquisitions, or strategic alliances by us or our competitors; | ||
| • | changes in the estimates of the future size and growth rate of our markets; | ||
| • | changes in market valuations or earnings of our competitors; | ||
| • | changes in legislation or regulatory policies, practices, or actions; | ||
| • | the commencement or outcome of litigation involving our company, our general industry or both; | ||
| • | recruitment or departure of one or more members our executive management team; | ||
| • | changes in our capital structure, such as future issuances of securities or the incurrence of additional debt; | ||
| • | actual or expected sales of our CDIs or common stock by existing holders; | ||
| • | the trading volume of our CDIs; and | ||
| • | changes in general economic, industry, and market conditions. |
The stock markets in general, and the markets for medical technology companies in particular,
have experienced volatility that has often been unrelated to the operating performance of
particular companies. These broad market and industry factors may materially harm the market price
of our CDIs. Litigation has often been brought against companies whose securities have experienced
volatility in market price. Class-action litigation, even if unsuccessful, could be costly to
defend and divert management’s attention and resources, which could further materially harm our
financial condition and results of operations.
Investors may experience difficulties in selling our CDIs due to the relatively limited liquidity
of shares traded on ASX
REVA’s listing on ASX should not be taken as implying that there is a ready liquid market for
our CDIs, particularly as we have a limited number of stockholders. Some ASX listed companies do
not develop active trading markets and therefore an active trading market for our CDIs may not
fully develop or be sustained. It may be more difficult for an investor to realize his or her
investment on ASX than it would be to realize an investment in a company whose shares or other
securities are quoted on the New York Stock Exchange or the NASDAQ Stock Market.
38
Table of Contents
There is no guarantee that we will maintain our listing on ASX, or qualify for listing on a
securities exchange in the U.S.
We cannot assure investors that we will always retain a listing on ASX. If we fail to retain
such a listing, certain investors may decide to sell their securities, which could have an adverse
impact on the share price. In addition, our common stock is not listed for trading on any U.S.
national securities exchange. There is no assurance that we can qualify in the future for listing
any of our securities on the New York Stock Exchange or the NASDAQ Stock Market.
Some of our existing stockholders can exert control over us and may not make decisions that are in
the best interests of all stockholders.
As of December 31, 2010, officers, directors, and stockholders holding more than five percent
of our outstanding shares collectively controlled approximately 65% of our outstanding common stock
based on their respective beneficial ownership of our common stock (assuming conversion of CDIs).
As a result, these stockholders, if they act together, would be able to exert a significant degree
of influence over our management and affairs and over matters requiring stockholder approval,
including the election of directors and approval of significant corporate transactions.
Accordingly, this concentration of ownership may harm the market price of our shares by delaying or
preventing a change in control, even if a change is in the best interests of our other
stockholders. In addition, the interests of this concentration of ownership may not always coincide
with the interests of other stockholders and, accordingly, they could cause us to enter into
transactions or agreements that we would not otherwise consider.
Future sales of our common stock may depress our share price.
Sales of a substantial number of CDIs in the public market, or the perception that these sales
may occur, could cause the market price of our CDIs to decline. The holders of an aggregate of
21,446,971 shares of our outstanding common stock have certain rights to cause us to file a
registration statement on their behalf and to include their shares in registration statements that
we may file on behalf of other stockholders.
We intend to file a registration statement covering common stock issued or reserved for such
issuance under our stock incentive plans. In addition, our 2010 Equity Incentive Plan provides for
annual increases in the number of shares available for issuance under the plan. Once we register
these shares, they can be freely sold under the federal securities laws and may be tradable under
state securities laws upon the holder’s satisfaction of such laws or an exemption therefrom,
subject to vesting provisions or other contractual arrangements. We may sell additional common stock
in subsequent public offerings, which may adversely affect the market price for our CDIs and common
stock.
We have broad discretion in the use of our assets, including the net proceeds from our initial
public offering, and our investment of these proceeds may not yield a favorable return which could
harm our business and depress the price of our securities.
Our management has discretion in the application of our assets, including the net proceeds
from our initial public offering, and may use them for a broad range of purposes. Accordingly, you
will have to rely upon the judgment of our management with respect to the use of the Company’s
assets. Our management may spend a portion or all of the Company’s assets, including the net
proceeds from our initial public offering, in ways that holders of our securities may not desire or
that may not yield a significant return or any return at all. The failure by our management to
apply these funds effectively could harm our business and depress the price of our securities.
Pending their use, we may also invest the net proceeds from our initial public offering in a manner
that does not produce income or that loses value.
We do not currently intend to pay dividends on our CDIs or common stock and, consequently, your
ability to achieve a return on your investment will depend on appreciation in the price of our
CDIs.
We currently intend to invest our future earnings, if any, to fund the development and growth
of our business. The payment of dividends will be at the discretion of our board of directors and
will depend on our results of operations, capital requirements, financial condition, future
prospects, contractual arrangements, restrictions imposed by applicable law, any limitations on
payments of dividends present in any debt agreements we may enter into, and other factors our board
of directors may deem relevant. If we do not pay dividends, your ability to achieve a return on
your investment in our company will depend on any future appreciation in the market price of our
CDIs. There is no guarantee that our CDIs will appreciate in value or even maintain the price at
which you have purchased your CDIs.
39
Table of Contents
We incur exchange rate risks relating to listing on ASX.
Our shares of common stock in the form of CDIs are listed on ASX and priced in Australian
Dollars. However, our reporting currency is U.S. Dollars. As a result, movements in foreign
exchange rates may cause the price of our securities to fluctuate for reasons unrelated to our
financial condition or performance and may result in a discrepancy between our actual results of
operations and investors’ expectations of returns on our securities expressed in Australian
Dollars.
We expend substantial costs and management resources to comply with the laws and regulations
affecting public companies in the U.S. as well as listing requirements on ASX, which may adversely
affect our operating results, and failure to achieve and maintain effective internal control over
financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could cause investors
to lose confidence in our operating results and in the accuracy of our financial reports and could
have a material adverse effect on our business and on the price of our common stock.
As a public company in the U.S. with equity securities listed on ASX, we are required,
pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, to furnish a report by
management on, among other things, the effectiveness of our internal control over financial
reporting. Our first report on compliance with Section 404 is expected to be in connection with our
financial statements for the year ending December 31, 2011. The controls and other procedures are
designed to ensure that information required to be disclosed by us in the reports that we file with
the SEC are disclosed accurately and are recorded, processed, summarized, and reported within the
time periods specified in SEC rules and forms. We are in the process of conforming our internal
control procedures to the requirements of Section 404 and we may not be able to complete our
evaluation, testing, and any required remediation needed to comply with Section 404 in a timely
fashion. Our independent registered public accounting firm was not engaged to perform an audit of
our internal control over financial reporting. Our independent registered public accounting firm’s
audit included consideration of internal control over financial reporting as a basis for designing
audit procedures that are appropriate in the circumstances, but not for the purpose of expressing
an opinion on the effectiveness of our internal control over financial reporting. Accordingly, no
such opinion was expressed. Even if we develop effective controls, these new controls may become
inadequate because of changes in conditions or the degree of compliance with these policies or
procedures may deteriorate. Even after we develop these new procedures, additional weaknesses in
our internal control over financial reporting may be discovered. In order to fully comply with
Section 404, we will need to retain additional employees to supplement our current finance staff,
and we may not be able to do so in a timely manner, or at all. In addition, in the process of
evaluating our internal control over financial reporting we expect that certain of our internal
control practices will need to be updated to comply with the requirements of Section 404 and the
regulations promulgated thereunder, and we may not be able to do so on a timely basis, or at all.
In the event that we are not able to demonstrate compliance with Section 404 in a timely manner, or
are unable to produce timely or accurate financial statements, we may be subject to sanctions or
investigations by regulatory authorities such as the SEC, and investors may lose confidence in our
operating results and the price of our CDIs could decline. Furthermore, if we or our auditors are
unable to certify that our internal control over financial reporting is effective and in compliance
with Section 404, we may be subject to sanctions or investigations by regulatory authorities such
as the SEC and we could lose investor confidence in the accuracy and completeness of our financial
reports, which would have a material adverse effect on our business and on the price of our CDIs
and our ability to access the capital markets.
Furthermore, as a U.S. public company with equity securities listed on ASX, we incur
additional legal, accounting, and other expenses. In addition, changing laws, regulations, and
standards relating to corporate governance and public disclosure, including regulations implemented
by the SEC, may increase legal and financial
compliance costs and make some activities more time consuming. Our shares of our common stock
are publicly traded on ASX in the form of CDIs. As a result, we must comply with the ASX Listing
Rules. We have policies and procedures that we believe are designed to provide reasonable assurance
of our compliance with the ASX Listing Rules. If, however, we do not follow those procedures and
policies, or they are not sufficient to prevent non-compliance, we could be subject to liability,
fines, and lawsuits. These laws, regulations, and standards are subject to varying interpretations
and, as a result, their application in practice may evolve over time as new guidance is provided by
regulatory and governing bodies. We intend to invest resources to comply with evolving laws,
regulations, and standards, and this investment may result in increased general and administrative
expenses and a diversion of management’s time and attention from revenue-generating activities to
compliance activities. If, notwithstanding our efforts to comply with new laws, regulations, and
standards, we fail to comply, regulatory authorities may initiate legal proceedings against us and
our business may be harmed.
40
Table of Contents
Failure to comply with these rules might also make it more difficult for us to obtain certain
types of insurance, including director and officer liability insurance, and we might be forced to
accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or
similar coverage. The impact of these events could also make it more difficult for us to attract
and retain qualified persons to serve on our board of directors, on committees of our board of
directors or as members of senior management.
Provisions of our certificate of incorporation, our bylaws, and Delaware law could make an
acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent
attempts by our stockholders to replace or remove the current members of our board and management.
Certain provisions of our certificate of incorporation and our bylaws could discourage, delay,
or prevent a merger, acquisition, or other change of control that stockholders may consider
favorable, including transactions in which you might otherwise receive a premium for your CDIs.
Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or
remove members of our board of directors. These provisions also could limit the price that
investors might be willing to pay in the future for our CDIs, thereby depressing the market price
of our CDIs. Stockholders who wish to participate in these transactions may not have the
opportunity to do so. These provisions:
| • | allow the authorized number of directors to be changed only by resolution of our board of directors; | ||
| • | provide that our stockholders may only remove our directors for cause; | ||
| • | establish a classified board of directors, such that not all members of the board of directors may be elected at one time; | ||
| • | authorize our board of directors to issue without stockholder approval up to 100,000,000 shares of common stock, that, if issued, would dilute our ownership and could operate as a “poison pill” to dilute the ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our board of directors; | ||
| • | authorize our board of directors to issue without stockholder approval up to 5,000,000 shares of preferred stock, the rights of which will be determined at the discretion of the board of directors that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our board of directors; | ||
| • | require that stockholder actions must be effected at a duly called stockholder meeting or by unanimous written consent; | ||
| • | establish advance notice requirements for stockholder nominations to our board of directors or for stockholder proposals that can be acted on at stockholder meetings; | ||
| • | limit who may call stockholder meetings; and | ||
| • | require the approval of the holders of 80% of the outstanding shares of our capital stock entitled to vote in order to amend certain provisions of our amended and restated certificate of incorporation and amended and restated bylaws. |
In addition, we are governed by the provisions of Section 203 of the Delaware General
Corporation Law, which may, unless certain criteria are met, prohibit large stockholders, in
particular those owning 15% or more of the voting rights on our common stock, from merging or
combining with us for a prescribed period of time.
Item 1B. Unresolved Staff Comments
We do not have any unresolved staff comments relating to our periodic or current reports.
41
Table of Contents
Item 2. Properties
Our primary facility is located at 5751 Copley Drive, Suite B, San Diego, California, where we
occupy approximately 17,000 square feet of research and office space. The lease on this facility
expires in August 2011. We lease a second lab facility that is located at 3030 Bunker Hill Street,
San Diego, California, where we occupy approximately 2,500 square feet of research space. The lease
on this facility expires in October 2011.
We do not own any real property. We believe that our leased facilities are adequate to meet
our needs for the foreseeable future.
Item 3. Legal Proceedings
We may from time to time become subject to various claims and legal actions during the
ordinary course of our business. We are not party to any legal proceedings at the date of filing
of this Annual Report on Form 10-K.
Item 4. REMOVED AND RESERVED
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of
Equity Securities
Market Information
Commencing December 23, 2010, our shares of common stock began trading in the form of CHESS
Depositary Interests (“CDIs”), each CDI representing one-tenth of a share of our common stock, on
the Australian Securities Exchange (“ASX”) under the symbol “RVA.” Prior to such time, there was no
public market for our securities. Between December 23, 2010 and December 31, 2010, the closing
price of our CDIs ranged from a low closing price of A$1.20 to a high closing price of A$1.25, or a
low closing price per share of common stock of $12.13 and a high closing price of $12.55 after
giving effect to the ten-for-one CDI-to-common stock exchange ratio and after converting to U.S.
dollars using the closing exchange rate applicable on the relevant date as reported by the Reserve
Bank of Australia.
As of March 15, 2011 we had 32,765,503 shares of common stock issued and outstanding with
approximately 670 holders of record. The holders included CHESS Depositary Nominees Pty Limited,
which held 8,098,609 shares of our common stock in the form of CDIs on behalf of the CDI holders;
there were approximately 603 registered owners of our CDIs on March 15, 2011.
Stock Price Performance Graph
The
following graph compares our total common stock return, after giving
effect to the ten-for-one CDI-to-common stock exchange ratio and after converting to U.S.
dollars using the closing exchange rate
applicable on the relevant date as reported by the Reserve Bank of Australia, with the total return for (i) the NASDAQ Composite Index and (ii)
the NASDAQ Biotechnology Index for the period from December 23, 2010 (the date our common stock
commenced trading on the Australian Securities Exchange) through December 31, 2010. The figures
represented below assume an investment of $100 in our common stock at the closing price of $12.52
per share of common stock on December 23, 2010, and in the NASDAQ Composite Index and the NASDAQ
Biotechnology Index on December 23, 2010, and the reinvestment of dividends into shares of common
stock. The comparisons in the table are required by the SEC and are not intended to forecast or be
indicative of possible future performance of our common stock. This graph shall not be deemed
“soliciting material” or to be “filed” for purposes of Section 18 of the Exchange Act or otherwise
subject to the
liabilities under that Section, and shall not be deemed to be incorporated by reference into
any of our filings under
the Securities Act or the Exchange Act.
42
Table of Contents
Dividend Policy
We have never declared or paid any cash dividends on our common stock. We currently intend to
retain all available funds and any future earnings to support operations and finance the growth and
development of our business and do not intend to pay cash dividends on our common stock or CDIs for
the foreseeable future. Any future determination related to our dividend policy will be made at the
discretion of our board of directors.
Use of Proceeds from Public Offering of Common Stock
On December 16, 2010, we closed our initial public offering, in which we sold 77,272,730 CDIs,
representing 7,727,273 shares of our common stock, at a price to the public of A$1.10 per CDI or
A$11.00 per share. The aggregate offering price for CDIs sold in the offering was A$85.0 million
(which equated to approximately US$84.3 million). The offer and sale of all of the CDIs in the
initial public offering were registered under the Securities Act pursuant to a registration
statement on Form S-1 (File No. 333-168852), which was declared effective by the SEC on November
15, 2010. We also lodged a Prospectus with the Australian Securities and Investments Commission
prior to the allotment and issuance of CDIs under the offering. The offering commenced as of
November 29, 2010 and did not terminate before all of the securities registered in the registration
statement were sold. There was no underwriter and no selling stockholders in the offering. Inteq
Limited, or Inteq, acted as our placement agent in connection with the offering of our CDIs outside
of the United States to non-U.S. residents. We raised approximately $75.8 million in net
proceeds after deducting placement agent fees and other offering expenses. No payments were made by
us to directors, officers or persons owning ten percent or more of our common stock or to their
associates, or to our affiliates, other than payments in the ordinary course of business to
officers for salaries and to non-employee directors as compensation for board or board committee
service.
Of the net proceeds received in the initial public offering, we expect to use approximately:
| • | $40.0 million for research and development activities, including continuing development of our ReZolvetm stent; | ||
| • | $10.0 million for clinical trials; | ||
| • | $4.0 million for building commercial infrastructure, including manufacturing capacity expansion; and | ||
| • | the balance for working capital and other general corporate purposes. |
The foregoing expected use of the net proceeds represents our current intentions based upon
our present plans and business condition. The amounts and timing of our actual expenditures may
vary significantly and will depend upon
numerous factors, including the timing and success of our development efforts and clinical
trials. We plan to commence clinical trials in the United States after we receive acceptable data
from the European clinical trials. Due to the regulatory requirements in the United States that
require a study with a large number of patients, we anticipate needing additional funding in order
to carry out the U.S. clinical trials.
Pending the use of net proceeds, we have invested the offering proceeds in short-term,
interest-bearing obligations, investment grade instruments, certificates of deposit, or guaranteed
obligations of the U.S. government.
43
Table of Contents
Item 6. Selected Financial Data
We have derived our statements of operations data for the years ended December 31, 2006 and
2007 and our balance sheet data as of December 31, 2006, 2007, and 2008 from our audited financial
statements which are not included in this Form 10-K. We have derived our statements of operations
data for the years ended December 31, 2008, 2009, and 2010 and our balance sheet data as of
December 31, 2009 and 2010 from our audited financial statements appearing elsewhere in this Form
10-K. Our audited financial information is prepared and presented in accordance with generally
accepted accounting principles in the U.S., or U.S. GAAP. Our selected consolidated financial data
should be read together with “Management’s Discussion and Analysis of Financial Condition and
Results of Operations” and our consolidated financial statements and related notes included
elsewhere in this Form 10-K.
| Year Ended December 31, | ||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | ||||||||||||||||
| (In thousands, except per share data) | ||||||||||||||||||||
Statements of Operations Data: |
||||||||||||||||||||
Operating Expense: |
||||||||||||||||||||
Research and development |
$ | 6,605 | $ | 8,536 | $ | 11,378 | $ | 10,272 | $ | 6,826 | ||||||||||
General and administrative |
1,520 | 2,247 | 2,205 | 2,241 | 3,292 | |||||||||||||||
Loss from operations |
(8,125 | ) | (10,783 | ) | (13,583 | ) | (12,513 | ) | (10,118 | ) | ||||||||||
Other Income (Expense): |
||||||||||||||||||||
Interest income |
194 | 94 | 124 | 26 | 116 | |||||||||||||||
Interest expense (1) |
(1,474 | ) | (2,745 | ) | (1,874 | ) | (1,579 | ) | (1,549 | ) | ||||||||||
Interest from amortization |
— | — | — | — | 2,283 | |||||||||||||||
Gain (loss) on change in fair value of
preferred stock rights and warrant
liabilities |
— | (47 | ) | 2,617 | 215 | (990 | ) | |||||||||||||
Loss on extinguishment of notes payable |
— | — | — | — | (13,285 | ) | ||||||||||||||
Other income |
23 | 2 | 2 | 7 | 36 | |||||||||||||||
Net Loss |
(9,382 | ) | (13,479 | ) | (12,714 | ) | (13,844 | ) | (23,507 | ) | ||||||||||
Cumulative dividends and deemed
dividends on Series H convertible
preferred stock |
— | (63 | ) | (1,074 | ) | (2,358 | ) | (7,200 | ) | |||||||||||
Net Loss Attributable to Common
Stockholders |
$ | (9,382 | ) | $ | (13,542 | ) | $ | (13,788 | ) | $ | (16,202 | ) | $ | (30,707 | ) | |||||
Net Loss Per Share (2): |
||||||||||||||||||||
Net loss per share, basic and diluted |
$ | (3.56 | ) | $ | (5.02 | ) | $ | (5.06 | ) | $ | (5.91 | ) | $ | (7.72 | ) | |||||
Shares used to compute net loss per
share, basic and diluted |
2,637,843 | 2,695,245 | 2,727,191 | 2,739,229 | 3,975,144 | |||||||||||||||
| (1) | Includes amounts pertaining to related parties of $1,456, $2,619, $1,794, $1,532, and $1,510 for the years ended December 31, 2006, 2007, 2008, 2009, and 2010, respectively. | |
| (2) | See Note 3 to our consolidated financial statements for an explanation of the method used to compute the net loss per share and the number of shares used in the computation of the per share amounts. |
44
Table of Contents
| As of December 31, | ||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
Balance Sheet Data: |
||||||||||||||||||||
Cash and cash equivalents |
$ | 2,585 | $ | 8,648 | $ | 8,036 | $ | 7,233 | $ | 81,747 | ||||||||||
Working capital |
2,708 | 7,244 | 13,621 | 6,085 | 80,984 | |||||||||||||||
Total assets |
4,310 | 9,436 | 16,524 | 8,442 | 83,475 | |||||||||||||||
Notes payable (1) |
20,550 | 19,822 | 19,883 | 20,304 | — | |||||||||||||||
Accrued interest on notes payable (2) |
2,539 | 4,375 | 5,813 | 6,971 | — | |||||||||||||||
Repayment premium on notes payable (3) |
11,100 | 11,100 | 11,100 | 11,100 | — | |||||||||||||||
Preferred stock warrant liability |
— | 800 | 995 | 780 | — | |||||||||||||||
Total liabilities |
34,987 | 41,654 | 39,867 | 40,402 | 1,528 | |||||||||||||||
Convertible preferred stock |
28,982 | 39,994 | 61,913 | 69,071 | — | |||||||||||||||
Deficit accumulated during the development stage |
(60,694 | ) | (74,172 | ) | (86,887 | ) | (101,033 | ) | (128,903 | ) | ||||||||||
Total stockholders’ equity (deficit) |
(59,659 | ) | (72,212 | ) | (85,256 | ) | (101,031 | ) | 81,947 | |||||||||||
| (1) | Includes $20,275, $19,547, $19,636, and $20,029 as of December 31, 2006, 2007, 2008, and 2009, respectively, held by related parties. | |
| (2) | Includes $2,482, $4,298, $5,718, and $6,857 due to related parties as of December 31, 2006, 2007, 2008, and 2009, respectively. | |
| (3) | Includes $10,550 due to related parties as of each date presented except for December 31, 2010. |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
You should read the following discussion and analysis of our financial condition and results
of operations in conjunction with the “Selected Consolidated Financial Data” and our consolidated
financial statements and the related notes thereto that appear elsewhere in this Annual Report on
Form 10-K. In addition to historical information, the following discussion and analysis includes
forward-looking information that involves risks, uncertainties, and assumptions. Actual results and
the timing of events could differ materially from those anticipated by these forward-looking
statements as a result of many factors, including those discussed under “Risk Factors” elsewhere in
this Annual Report on Form 10-K. See also “Forward-Looking Statements” included elsewhere in this
Annual Report on Form 10-K.
Overview
We are a development stage medical device company working toward commercialization of our
proprietary technologies to provide minimally invasive medical devices for treatment of conditions
in the human body. Since the inception of our company in 1998, our efforts have been concentrated
on the development of a stent for use in coronary applications. We currently are in the later
stages of developing a bioresorbable drug-eluting coronary stent that we have named the
ReZolvetm stent. In a clinical use, this stent is implanted by an
interventional cardiologist during a minimally invasive surgery to a coronary artery location with
a delivery catheter system. The stent combines our proprietary stent design with a proprietary
polymer that is metabolized and cleared from the body over time, leaving the body free of a
permanently implanted device. We have invested significant time and funds in the development of the
ReZolvetm stent and have performed significant scientific research, engineering
development, and testing of the stent in laboratory and preclinical studies. We believe the results
of this testing have shown the technology to be safe and effective and that it is suitable for
final development and human clinical studies.
We believe that due to the risks and limitations associated with commercially available metal
stents, bioresorbable stents will be the next major advance in coronary stent technology. Because
we have designed our stent to provide the same benefits as traditional metal stents, but with the
additional benefit of eliminating the need for a permanently implanted device, we believe that if
we are able to complete development and clinical testing of the stent, if we are able to
successfully implement manufacturing processes and procedures, and if it is approved for sale by
the relevant
regulatory authorities, our stent will enable us to compete effectively in the worldwide stent
market. Worldwide revenues from coronary stent sales approximated $5.3 billion in 2009.
45
Table of Contents
Our development of the ReZolvetm stent has progressed to the point where
we have tested and selected the polymer formulation, tested and selected the anti-restenotic drug
and coating process, iterated and are in the process of finalizing the design, and identified and
implemented preliminary methods to produce the stent. As part of this process, in 2007 we enrolled
patients in a small clinical study that proved the viability of our technology while confirming the
areas needing further development and we have been advancing the product design and features since.
We intend to initiate a pilot human clinical trial of the stent to commence in the second quarter
of 2011. Assuming acceptable results from this pilot clinical trial, we will initiate a larger
scale human clinical trial that will provide the data needed to apply for CE Mark approval in
Europe. If and when we receive CE Mark approval, we plan to sell our stent in Europe. In order to
produce quantities of the stent large enough to accommodate the clinical trials and commercial
needs, when that time arrives, we will need to scale-up our manufacturing processes and expand our
capabilities to allow for such things as additional stent dimensions. We have begun preliminary
development of the methods and processes for the manufacturing scale-up and we plan to work on the
dimensional and other aspects in 2011.
During our development of the ReZolvetm stent, we have invented,
co-invented, and licensed a portfolio of proprietary technologies. Our design-related technologies
have been invented by our employees and consultants and our materials-related technologies have
been either invented by our employees or licensed from, or co-invented with, Rutgers, The State
University of New Jersey. We consider our patent portfolio to be significant and have invested
considerable time and funds to develop and maintain it. Our goal is to perform feasibility tests on
additional technologies in our patent portfolio at the same time we are finalizing our
ReZolvetm stent and, if feasibility is proven, determine a course of
development for potential products and, thus, provide a follow-on product pipeline.
During our development efforts, we have also pursued, tested, and abandoned development
programs that we determined would not lead to feasible products or for which a product could not be
developed in a timeframe that would allow for reasonable commercialization. The largest of these
abandoned programs centered on development of a thin metal stent technology for use in small blood
vessels. Although abandoned in 2002 after approximately $13 million had been invested and used,
this technology became the basis for the “slide & lock” mechanism we are currently using.
Additionally, the company licensed a potential anti-restenotic drug in 2001 with the intent to
develop it for use as a stand-alone drug or as a complement to our stent product. Although the
drug’s development was abandoned in 2004 after we had invested approximately $6 million, the
knowledge we gained from that program was used in our development of the drug coating for the
ReZolvetm stent. We also formed a wholly owned subsidiary in Germany in 2007 to
facilitate our clinical trials and our planned commercialization of products; we have not used this
subsidiary yet for any operating activities.
We have performed all of our research and development activities from one location in San
Diego, California. As of December 31, 2010, we had 43 employees, a majority of which are degreed
professionals and four of whom are PhDs. We leverage our internal expertise with contract research
and preclinical laboratories, outside polymer and catheter manufacturing, and other outside
services as needed. We have two clean rooms and multiple engineering and chemistry labs at our
facility, in addition to our corporate and administrative office. We are ISO certified to the
medical device standard 13485:2003 and intend to maintain the certification to support our
commercialization plans.
We have not yet developed a product to a saleable stage and we have not, therefore, generated
any product or other revenues. Our development efforts have been funded with a variety of capital
received from angel investors, venture capitalists, strategic partners, hedge funds and
individuals. Since our inception, we have received approximately $154 million in equity proceeds
and $29 million from issuance of notes payable (such notes payable were converted to common stock
upon consummation of our initial public offering in December 2010). As of December 31, 2010, we had
approximately $82 million in cash available for operations. We have incurred substantial losses
since our inception. As of December 31, 2010, we had accumulated a deficit of approximately $129
million. We expect our losses to continue for the next several years as we continue our development
work and, if these efforts are successful and we are able to obtain approval to sell our products,
we expect to commence commercial sales thereafter.
Our company was founded in California in June 1998 and named MD3, Inc. We changed our name to
REVA Medical, Inc. in March 2002. In October 2010, we reincorporated from the State of California
to the State of Delaware. As a result, the rights of our stockholders are now governed by the
Delaware General Corporation Law.
46
Table of Contents
Key Components of our Results of Operations
Since we are still in a pre-revenue stage and our activities are focused on further developing
and testing our bioresorbable coronary stent with the goal of commercially selling it, as well as
performing minimal research and tests to determine the feasibility of other product possibilities,
our operating results primarily consist of research and development expenses, general and
administrative expenses, and other expenses that are primarily the carrying costs of the debt and
equity securities we have issued to fund our development efforts.
Research and Development Expenses: Our research and development expenses arise from a
combination of internal and external costs. Our internal costs primarily consist of employee
salaries and benefits, facility and other overhead expenses, and engineering and other supplies
that we use in our labs for prototyping, testing, and producing our stents and other product
possibilities. Our external costs primarily consist of contract research, engineering consulting,
polymer production costs, polymer lasing costs, catheter system and restenotic drug purchases,
preclinical and clinical study expenses, and license fees paid for the technology underlying our
polymer materials. All research and development costs are expensed when incurred. Through December
31, 2010, we have incurred approximately $74.3 million in research and development expenses since
our inception, which represents approximately 79% of our cumulative operating expenses. We
anticipate that we will continue our research and development activities at their current levels,
but that we will have a significant increase in our clinical trial costs once we begin our pilot
clinical trial in the second quarter of 2011 and, if successful, our larger follow-on clinical
trial that will provide the data for our CE Mark application.
General and Administrative Expenses: Our general and administrative expenses consist primarily
of salaries and benefits for our executive officers and administrative staff, corporate office and
other overhead expenses, legal expenses including patent filing and maintenance costs, audit and
tax fees, and travel expenses. Although our patent portfolio is one of our most valuable assets, we
record legal costs related to patent development, filing, and maintenance as expenses when the
costs are incurred since the underlying technology associated with these assets is purchased or
incurred in connection with our research and development efforts and the future realizable value
cannot be determined. Through December 31, 2010, we have incurred approximately $20.2 million in
general and administrative expenses since our inception, which represents approximately 21% of our
cumulative operating expenses. We anticipate that we will continue to invest in patents at similar
levels as we have in the past. We also anticipate that we will continue to expand our corporate
infrastructure, including the addition of personnel and reporting systems, to support the needs of
being a public company and to prepare for commercial sales of our products, which will increase our
general and administrative expenses significantly.
Other Expense and Income: A majority of our non-operating expenses consist of interest expense
that arose from our notes payable. The notes were issued to individuals and investment funds that
also provided equity capital to us. All the notes, along with the accumulated accrued interest,
converted into common stock upon our initial public offering in December 2010. Although the notes
were issued between 2003 and 2006, the terms of the notes allowed us to accrue and record the
interest due on them, but defer payment of both the principal balance and the interest. Interest
had accrued at rates between 4.25 percent and 9.25 percent annually. A portion of the notes
payable, in the amount of $5.5 million, had been required to be repaid at three times their
principal balance, if repaid, but not if converted to stock. Through December 31, 2010, we recorded
approximately $9.6 million of interest and $11.1 million in repayment premiums on our notes
payable. Additionally, in October 2010, we modified the conversion features of the notes payable,
but did not repay or change any repayment terms of the notes. In accordance with applicable
accounting requirements, the conversion feature modifications resulted in our recording $13.3
million of loss on extinguishment of notes payable and $2.3 million in interest income from
amortization of note premium during the year ended December 31, 2010.
In conjunction with issuing our notes payable, we issued warrants to purchase preferred stock;
these warrants were exercised for cash and on a net issuance basis upon consummation of our initial
public offering in December 2010 and none remained outstanding at December 31, 2010. We recorded
non-cash interest expense for the initial value of the warrants and recorded gains and losses for
subsequent changes in fair value of the warrants. Through December 31, 2010, a total of $1.8
million in net expense has been recorded for these warrants.
Concurrent
with the completion of our initial public offering, or our IPO, all of our outstanding convertible preferred stock,
non-voting common stock, notes payable, and accrued interest on notes payable converted to common
stock. Additionally, all
outstanding warrants were exercised for common stock, either through a cash payment to us or
on a net exercise basis. We also issued common stock for cumulative dividends on our Series H
convertible preferred stock. A total of 22,419,771 shares of common stock were issued from these
conversions, exercises, and dividends.
47
Table of Contents
Since our inception, when we have had excess cash on hand we have invested in short-term
high-quality marketable securities such as certificates of deposit and U.S. Treasury Bills.
Earnings from these investments are recorded as interest income; through December 31, 2010, we have
recorded a total of approximately $1.1 million in such interest income.
Critical Accounting Policies and Significant Estimates
Our consolidated financial statements are prepared in accordance with accounting principles
generally accepted in the U.S. The preparation of our financial statements and related disclosures
requires us to make estimates, assumptions, and judgments that affect the reported amounts of
assets, liabilities, preferred stock, stockholders’ equity, expenses, and the presentation and
disclosures related to those items. We base our estimates and assumptions on historical experience
and other factors that we believe to be reasonable under the circumstances. We evaluate our
estimates and assumptions on an ongoing basis; changes in our estimates and assumptions are
reasonably likely to occur from period to period. Additionally, actual results could differ
significantly from the estimates we make. To the extent there are material changes in our estimates
or material differences between our estimates and our actual results, our future financial
statement presentation, financial condition, results of operations, and cash flows will be
affected.
While our significant accounting policies are described in more detail in Note 3 to our
consolidated financial statements included elsewhere in this Annual Report on Form 10-K, we believe
the following accounting policies involve a greater degree of judgment and complexity than our
other accounting policies and, therefore, are the most critical to understanding and evaluating our
consolidated financial condition and results of operations.
Research and Development Costs: We expense research and development costs as incurred. These
costs primarily consist of employee salaries and benefits, facility and overhead expenses,
engineering and lab supplies, contract research, engineering consulting, polymer production costs,
polymer lasing costs, catheter system and restenotic drug purchases, preclinical and clinical study
expenses, and technology license fees. Our preclinical and clinical expenses are incurred on a
contract basis and generally span a period from a few months to longer than a year. We record costs
incurred under these contracts as the work occurs and make payments according to contractual terms.
Until a contract is completed, we estimate the amount of work performed and accrue for estimated
costs that have been incurred but not paid. As actual costs become known, we adjust our accruals.
We expect our clinical expense accruals to increase as we enroll patients in our pilot trial, which
we expect to initiate during the second quarter of 2011, and, if successful, our follow-on larger
clinical trial. We expect to make estimates as to the work performed throughout the term of these
trials, which is expected to be five years. As a public company, we are required to make these
estimates in shorter time frames and with less actual data than we have in the past, which may
result in our estimates being less accurate and subject to possible material changes in our
accruals, which could also materially affect our results of operations within any fiscal period. To
date, there have been no material changes in our research and development expense estimates.
Notes Payable: We record our notes payable at their face values, accrue interest on the notes
at their stated interest rates, and amortize or accrete any related discounts or premiums over the
original term of a note using the effective interest method. When we amend a note, such as
extending its maturity date, we perform an analysis based on applicable accounting guidelines to
determine if the amendment results in an accounting impact. We first consider whether the amendment
would qualify as a troubled debt restructuring. If the amendment is not considered a troubled debt
restructuring, we consider whether the amendment should be accounted for as an extinguishment or a
modification of debt. If the amendment is determined to be an extinguishment of a note, we remove
the carrying value of the note, recording an extinguishment gain or loss to non-operating expense
in our statement of operations, and record the note at its fair value as determined using the
amended terms. We then amortize or accrete the difference between the fair value and the face value
of the note over the amended term of the note using the effective interest method. If the note has
an embedded conversion feature and the amendment is determined to be a modification of the note, as
defined by accounting standards, then any increase in the fair value of the conversion feature
resulting from the amendment is accounted for as a reduction in the carrying amount of the note (as
an additional discount or reduction in premium) with a corresponding increase in additional paid-in
capital. All amounts amortized or accreted over the term of a note are recorded as interest expense
or interest income in our statement of operations. All notes payable and related accrued interest
were converted to common stock upon our IPO
48
Table of Contents
Preferred Stock Warrant Liability: Periodically, we have issued warrants to purchase preferred
stock in conjunction with issuing notes payable. When we issue a warrant to purchase preferred
stock, we record the fair value of the warrant as a liability, as required by accounting standards,
with the related expense amortized to interest in our statement of operations over the term of the
note payable. The warrant liability is adjusted to its current fair value at each reporting date
until the earlier of its exercise or the end of its contractual life. Our warrants have lives
ranging from five to ten years. To determine the fair value of the warrant liability, we utilize
the Black-Scholes option-pricing model, which requires use of subjective assumptions. The
assumptions used represent our best estimates, but these estimates involve inherent uncertainties.
We use an estimate of the value of the underlying preferred stock, a life equal to the warrant’s
contractual life, risk-free interest rates that correspond to the warrant’s remaining life, and an
estimate of volatility based on the market trading prices of comparative peer companies. As a
result of our use of estimates, if factors change and we use different assumptions, the amount of
our warrant liability could be materially different in the future. All preferred stock warrants
were exercised upon our IPO.
Common Stock Warrants: We have issued warrants to purchase common stock only in conjunction
with issuances of convertible preferred stock; these warrants had five-year lives. When we issue a
warrant to purchase common stock, we record the fair value of the warrant on the date of issuance
as a component of stockholders’ equity and reduce the recorded proceeds of the related preferred
stock by an equal amount. To determine the fair value of a common warrant, we utilize the same
approach as we use to value warrants issued to purchase preferred stock. All of our common stock
warrants were exercised upon our IPO.
Income Taxes: We are currently subject to taxation in U.S. and California jurisdictions, but
because we have not yet reached the stage of generating revenue and we have accumulated significant
tax losses, we have not been required to pay income taxes. To account for the tax effects of our
accumulated losses and other tax-related items, we use the asset and liability method, as defined
by accounting standards, and we adopted the guidance related to accounting for uncertainties in
income taxes as of January 1, 2009. Under this guidance, we determine our deferred tax assets and
liabilities based on the estimated future tax consequences of current temporary differences between
the financial statement carrying amounts of the assets and liabilities and their respective tax
bases, using tax rates that we expect to be in effect when the temporary difference will be
realized. After we have quantified our deferred tax assets and liabilities, we evaluate their
ability to be realized. If a deferred tax asset or liability is determined to have less than a 50%
likelihood of being upheld upon an examination by taxing authorities, or if we are unable to make
such a determination, we do not recognize the tax asset or liability. We also establish a valuation
allowance for a deferred tax asset or liability when we determine that it is likely to not be
realized. In determining our tax accounting, we use estimates and make assumptions that we believe
are reasonable; however, the ultimate tax determination involves significant judgment that is
subject to audit by tax authorities and actual tax outcomes could vary significantly from our
estimates. Additionally, as a result of our using estimates, if factors change and we use different
estimates, the amounts we have determined related to our tax positions could be materially
different in the future.
As of December 31, 2010, we had aggregate federal and California state net operating loss
carryforwards of approximately $98.0 million and $95.0 million, respectively, which may be
available to offset future taxable income for income tax purposes; the federal carryforwards begin
to expire in 2019 and the California carryforwards begin to expire in 2011. At December 31, 2010,
we also had federal and California research tax credit carryforwards of approximately $3.2 million
and $3.0 million, respectively; the federal carryforwards begin to expire in 2020 and the
California carryforwards have no expiration. In accordance with provisions of Internal Revenue Code
Sections 382 and 383, our net operating loss and research tax credit carryforwards may not be fully
usable to offset future taxable income because of cumulative changes in the ownership of our
company. We have not completed an analysis to see if our carryforwards would be limited; until we
complete this analysis we have removed the deferred tax assets for net operating losses and the
research credits from our deferred tax asset schedule. We do not expect these tax estimates and
positions to change by December 31, 2011. In each period since our inception, we have recorded a
valuations allowance for the full amount of our scheduled net deferred tax asset, as the
realization of the deferred tax asset is uncertain and, as a result, we have not recorded any
federal or California income tax benefit in our statement of operations. Upon the completion of our
initial public offering, we became subject to certain Australian tax regulations in addition to
those in U.S. jurisdictions.
Stock-Based Compensation: We have granted stock options to employees and consultants for the
purchase of common stock. These options generally have a ten-year life during which the option
holder can exercise at any time, they generally vest over a four- or five-year service period, and
their exercise price equals the fair market value of our common stock on the date they are granted.
Prior to 2006, we accounted for the options granted to employees in accordance with accounting
standards that required us to record compensation expense only if an option’s exercise
price was lower than the fair market price of our common stock on the date of grant or if we
changed any terms to the option after it was granted. Since January 1, 2006, when we adopted the
required accounting standard on a modified prospective basis, we have been recording compensation
expense for options granted to employees. Since inception, we have accounted for options granted to
consultants in accordance with accounting guidance that requires us to record compensation expense.
49
Table of Contents
For options granted to employees, we determine the amount of compensation expense by
estimating the fair value of each option on its date of grant and then we amortize that fair value
on a straight-line basis over the period the employee provides service, which generally is the
five-year expected life, and record the expense in our statement of operations as either research
and development expense or general and administrative expense based on the employee’s work
classification. We estimate the fair value by using the Black-Scholes option pricing model, which
is more fully described above. For the model inputs, we use the estimated value of the underlying
common stock, a risk-free interest rate that corresponds to the vesting period of the option, an
expected life of the option equal to 6.5 years, and an estimate of volatility based on the market
trading prices of comparative peer companies. Additionally, we reduce the amount of recorded
compensation expense to allow for potential forfeitures of the options; the forfeiture rate is
based on our actual historical forfeitures and has ranged from approximately 2.5 percent to 5.3
percent. For options granted to consultants, we estimate the fair value at the date of grant and at
each subsequent accounting date and record compensation expense in our statement of operations
based on the fair value during the service period of the consultant, which is generally the
five-year vesting period. We estimate the fair value by using the Black-Scholes option pricing
model with the same approach to inputs and assumptions as we use to estimate the fair value of
options granted to employees. As a result of our use of estimates, if factors change and we use
different assumptions, the amount of our stock-based compensation expense could be materially
different in the future.
During the past five years, we have made very few grants of options. We made one grant of
options to purchase a total of 1,467,500 shares to three employees and five directors during 2010.
We made one grant of options to purchase a total of 50,000 shares to three consultants in 2009. We
made one grant of options to purchase a total of 643,500 shares to 40 employees in 2008. We expect
to increase our frequency of granting options beginning in 2011 and, combined with the effects of
the 2010 option grants, expect our stock-based compensation to increase.
Fair Value of Stock: Because our stock was not publicly traded prior to our initial public
offering in December 2010, its fair value was determined by our board of directors on various
dates, including the dates we granted options to purchase common stock. Our board, which includes
members who are experienced in valuing the securities of early-stage companies, considered a number
of subjective and objective factors in their determination, including:
| • | The prices of our convertible preferred stock sold to outside investors in arm’s-length transactions, and the rights, preferences, and privileges of each series of stock; | ||
| • | Our results of operations, our financial position, the status of our research and development efforts, including preclinical trial results, and the length of time until occurrence of clinical trials and a commercial product; | ||
| • | The market values of medical device companies that are in a stage of development or industry similar to us; | ||
| • | The lack of liquidity of both our preferred and common stock as a private company; | ||
| • | Contemporaneous valuations performed by an unrelated valuation specialist in accordance with methodologies outlined in the AICPA Practice Aid Valuation of Privately-Held-Company Equity Securities Issued as Compensation; | ||
| • | The likelihood and timing of achieving a liquidity event, such as an initial public offering, given prevailing market conditions; and | ||
| • | The material risks related to our business. |
We believe our historical fair value estimates of our common and preferred stock were
reasonable and consistent with the AICPA valuation guidance for private companies. In connection
with preparing our financial statements for our IPO, we reassessed the estimated fair values of our
stock for financial reporting purposes for the period from January 1, 2009 through the date of our
initial public offering and incorporated our conclusions into our contemporaneous valuations during
that period. We reviewed the valuation models and the related inputs we were
using and, due to the proximity of the initial public offering, determined that a probability
weighted expected return
50
Table of Contents
model (“PWERM”) was more appropriate and would provide a better estimate
of the value of our stock than the option pricing method we had used previously. Accordingly, we
applied the PWERM model to reassess our common stock fair values for 2009 and to calculate the
values for 2010. The type and timing of each potential liquidity event used for the 2010 valuations
were heavily influenced by the commencement of our initial public offering process while the
December 31, 2009 valuation was based on our best estimate at the time of the type and timing of a
liquidity event for the Company. Since we had no corporate milestones during 2009 or 2010 that
would significantly affect the valuation of our stock, we ratably increased the values during 2009
and 2010. We used these reassessed fair values as inputs in our valuations of options to purchase
common stock and warrants to purchase common and preferred stock for the years ended December 31,
2009 and 2010 and in our deemed dividend calculations during 2010.
Results of Operations
Comparison of the Years Ended December 31, 2009 and 2010
| Year Ended | ||||||||||||
| December 31, | % | |||||||||||
| 2009 | 2010 | Change | ||||||||||
| (In thousands) | ||||||||||||
Research and development expense |
$ | 10,272 | $ | 6,826 | (34 | %) | ||||||
General and administrative expense |
$ | 2,241 | $ | 3,292 | 47 | % | ||||||
Interest income |
$ | 26 | $ | 116 | >100 | % | ||||||
Interest expense |
$ | 1,579 | $ | 1,549 | (2 | %) | ||||||
Interest income from amortization of notes payable premium |
$ | — | $ | 2,283 | >100 | % | ||||||
Loss on extinguishment of notes payable |
$ | — | $ | 13,285 | >100 | % | ||||||
Gain (loss) on change in fair value of preferred stock warrant liability |
$ | 215 | $ | (990 | ) | >100 | % | |||||
Other income |
$ | 7 | $ | 36 | >100 | % | ||||||
Cumulative and deemed dividends on Series H convertible preferred stock |
$ | 2,358 | $ | 7,200 | >100 | % | ||||||
Research and development expense decreased $3.4 million for the year ended December 31, 2010
compared to the year ended December 31, 2009. The decrease was primarily due to development
progress on our ReZolvetm stent program. During 2009, we produced and tested
polymer formulations. After selecting a formulation in mid-2009, we reduced the number of batches
we were producing, which resulted in a $1.5 million decrease in polymer costs. Additionally, work
performed in 2009 on our delivery system was not repeated in 2010, which resulted in a decrease of
$0.6 million. Due to the stage of development, our need for contracted research and engineering
services decreased, resulting in a $0.3 million decrease in related expense. Additionally, during
2010 we were awarded a non-recurring U.S. federal government grant of $0.7 million for
reimbursement of research and development expenses. The remainder of the change between years is
due to decreases in expenses related to compensation, engineering tools, lab supplies, and
preclinical and clinical study costs.
General and administrative expense increased $1.1 million for the year ended December 31, 2010
compared to the year ended December 31, 2009, primarily due to
an increase of $0.8 million in
stock-based compensation as a result of options granted to executives and board members in the
fourth quarter of 2010.
Interest income increased $90,000 for the year ended December 31, 2010 compared to the year
ended December 31, 2009, primarily as a result of interest earned on proceeds from our initial
public offering during December 2010. Absent the IPO proceeds, our interest earning would have
decreased due to lower rates at which we earned interest due to general economic conditions.
Interest expense remained relatively unchanged for the year ended December 31, 2010 compared
to the year ended December 31, 2009 since the balances of our notes payable and the rates of
interest on those notes remained consistent between periods.
Interest income from amortization of notes payable premium and the loss on extinguishment of
notes payable are non-recurring items that arose during the fourth quarter of 2010 when we modified
the conversion features of our notes payable. Although we did not repay the notes or modify any
repayment terms and although the notes converted into common stock upon the IPO, our evaluation of
the modifications to the notes required that we record these non-recurring items in accordance with
generally accepted accounting principles.
51
Table of Contents
The fair value change in our preferred stock warrant liability resulted in a loss of $1.0
million for the year ended December 31, 2010 compared to a gain of $0.2 million for the year ended
December 31, 2009, primarily as a result of increases in the estimated value of the underlying
preferred stock. These increases in estimated values resulted from a change in our valuation
assumptions due to our initial public offering that was completed in December 2010.
Other income remained relatively immaterial for the year ended December 31, 2010 and did not
change significantly compared to the same period in 2009.
The $4.8 million increase in cumulative and deemed dividends recorded for the year ended
December 31, 2010 compared to the same period in 2009 relates to the issuance of Series H
convertible preferred stock in the third quarter of 2009 and the second quarter of 2010. Terms of
the Series H stock provided for a six percent cumulative dividend, compounded quarterly, from the
date of issuance. The deemed dividends, totaling $4.4 million in 2010, were non-recurring and
non-cash items that arose when we issued Series H stock under previously agreed terms at a price
per share that was lower than the estimated fair value of our common stock on the dates of
issuance. The cumulative dividends were paid in common stock and the underlying preferred stock was
converted to common stock upon consummation of our initial public offering in December 2010. As a
result, there will be no continuing dividends or related financial effects.
Comparison of the Years Ended December 31, 2008 and 2009
| Year Ended | ||||||||||||
| December 31, | % | |||||||||||
| 2008 | 2009 | Change | ||||||||||
| (In thousands) | ||||||||||||
Research and development expense |
$ | 11,378 | $ | 10,272 | (10 | %) | ||||||
General and administrative expense |
$ | 2,205 | $ | 2,241 | 2 | % | ||||||
Interest income |
$ | 124 | $ | 26 | (79 | %) | ||||||
Interest expense |
$ | 1,874 | $ | 1,579 | (16 | %) | ||||||
Gain (loss) on change in fair value of preferred stock
rights and warrant liabilities |
$ | 2,617 | $ | 215 | (92 | %) | ||||||
Other income |
$ | 2 | $ | 7 | >100 | % | ||||||
Cumulative dividends on Series H convertible preferred stock |
$ | 1,074 | $ | 2,358 | >100 | % | ||||||
Research and development expense decreased $1.1 million for the year ended December 31, 2009
compared to the year ended December 31, 2008. The decrease was primarily due to $1.4 million in
preclinical testing; the timing and magnitude of testing is variable depending upon the types of
tests and data needed to support development. Additional decreases included $273,000 of
non-recurring engineering consulting services, $195,000 in polymer costs due to timing of
production runs, and other numerous individually insignificant items. The decreases were offset by
increases of $353,000 in payroll and benefits due to added personnel, $135,000 in engineering and
lab supplies, and $147,000 in depreciation from new equipment and cleanroom improvements.
General and administrative expense increased $36,000 for the year ended December 31, 2009
compared to the year ended December 31, 2008. This increase primarily resulted from branding and
website update expenses of $77,000 in 2009. The lack of change in general and administrative
expense reflects stability in our headcount and related compensation and stable spending as our
administrative infrastructure and legal and consulting activities have remained consistent.
Interest income decreased $98,000 for the year ended December 31, 2009 compared to the year
ended December 31, 2008. Although our average cash balances were higher in 2009 than 2008, the rate
at which we earned interest was significantly lower due to general economic conditions.
Interest expense decreased $295,000 for the year ended December 31, 2009 compared to the year
ended December 31, 2008. This decrease was due primarily to decreases in interest rates for our
debt that is indexed to the prime rate of interest and a decrease in the amount of debt discount
amortization from notes payable.
The fair value change in our preferred stock rights and warrant liabilities was $2.4 million
for the year ended December 31, 2009 compared to the year ended December 31, 2008. The change is a
combination of a decrease of
$2.8 million from our preferred stock rights due to remeasuring the rights and an increase of
$0.4 million from our preferred stock warrant liability due to an increase in the value of the
underlying preferred stock values.
52
Table of Contents
Other income for the years ended December 31, 2008 and 2009 consisted of only individually
insignificant non-operating transactions.
The $1.3 million increase in dividends recorded in 2009 compared to 2008 relates to the
issuance of new Series H convertible preferred stock in September 2009 and a full year accrual of
dividends on Series H convertible preferred stock issued in September and December 2008.
Liquidity and Capital Resources
Sources of Liquidity
We are considered a “development stage” enterprise, as we have not yet generated revenues from
the sale of products. Although we have been researching and developing new technologies and product
applications and have initiated the first human clinical trial of our bioresorbable stent, we do
not anticipate having a product available for sale for at least the next several years. Until
revenue is generated from a saleable product, we expect to continue to incur substantial operating
losses and experience significant net cash outflows. We have incurred losses since our inception in
June 1998 and as of December 31, 2010, we had an accumulated deficit of $128.9 million.
In December 2010 we completed an initial public offering of our common stock (in the form of
CDIs) primarily to investors in Australia, Hong Kong, London and the United States and to retail
investors in Australia. We issued 7,727,273 shares of common stock at $10.91 per share for gross
proceeds of $84.3 million. We incurred $8.5 million in issuance costs in connection with our
initial public offering. Concurrent with the completion of our initial public offering, all of our
outstanding convertible preferred stock, non-voting common stock, notes payable, and accrued
interest on notes payable converted to common stock. Additionally, all outstanding warrants were
exercised for common stock, either through a cash payment to us or on a net exercise basis. We also
issued common stock for cumulative dividends on our Series H convertible preferred stock. A total
of 22,419,771 shares of common stock were issued from these conversions, exercises, and dividends.
Prior to our initial public offering, we funded our operations from a combination of private
placements of our equity securities, for which we received aggregate net proceeds of $68.9 million,
and issuances of notes payable, for which we received $28.5 million in net proceeds. Our receipts
from financing activities have total $173.5 million since our inception.
Based on our current operating plans, we believe that the proceeds from our initial public
offering will be sufficient to meet our capital and operating needs for at least the next three
years and that our cash balances at December 31, 2010 will be sufficient to satisfy our liquidity
requirements and provide sufficient working capital to carry out our business objectives.
Cash Flows
Below is a summary of our cash flows from operating activities, investing activities, and
financing activities for the periods indicated.
| Year Ended December 31, | ||||||||||||
| 2008 | 2009 | 2010 | ||||||||||
| (In thousands) | ||||||||||||
Net cash used for operating activities |
$ | (12,598 | ) | $ | (12,569 | ) | $ | (8,719 | ) | |||
Net cash provided by (used for) investing activities |
(8,013 | ) | 6,766 | (300 | ) | |||||||
Net cash provided by financing activities |
20,000 | 5,000 | 83,535 | |||||||||
Effect of foreign exchange rates |
(1 | ) | — | (2 | ) | |||||||
Net increase (decrease) in cash and cash equivalents |
$ | (612 | ) | $ | (803 | ) | $ | 74,514 | ||||
Net Cash Flow from Operating Activities
Net cash used for operating activities during 2008 primarily reflects the net loss of $12.7
million and $2.8 million related to the change in fair value of the preferred stock right. These
items were offset by $501,000 of net cash
provided by changes in operating assets and liabilities, $1.8 million of non-cash interest in
our notes payable, $257,000 of depreciation and amortization, $195,000 related to the change in the
fair value of the preferred stock warrant liability and $138,000 of stock-based compensation and
other expense.
53
Table of Contents
Net cash used for operating activities during 2009 primarily reflects the net loss of $13.8
million. We also used cash of $790,000 for the net changes in operating assets and liabilities,
including payments of certain long-term preclinical study costs, and $215,000 related to the change
in the fair value of the preferred stock warrant liability. These items were offset by $1.6 million
of non-cash interest on notes payable, $466,000 of depreciation and amortization, including
additional amortization related to the clean room and lab space we added in the beginning of 2009,
and $235,000 of stock-based compensation and other expense.
Net cash used for operating activities during 2010 primarily reflects the net loss of $23.5
million, cash used of $372,000 for changes in operating assets and liabilities, and non-cash
interest income of $734,000. These items were offset by non-cash expenses of $13.3 million for
extinguishment of notes payable, $990,000 from the change in the fair value of the preferred stock
warrant liability, $471,000 of depreciation and amortization, and $1.1 million of stock-based
compensation and other expense.
Net Cash Flow from Investing Activities
Net cash used in investing activities during the years ended December 31, 2008, 2009, and 2010
consisted primarily of the net purchases of short-term investment securities of $7.5 million in
2008 that matured during the first six months of 2009. Additionally, purchases of property and
equipment accounted for $516,000 in 2008, $733,000 in 2009, and $300,000 in 2010, with the majority
of the 2008 and 2009 purchases relating to the additions of a cleanroom and lab space and equipment
for those additions and furniture and equipment for our administrative offices.
Net Cash Flow from Financing Activities
Net cash provided by financing activities during the years ended December 31, 2008, 2009, and
2010 consisted of net proceeds of $20.0 million, $5.0 million, and $7.5 million respectively, from
the sale of our Series H convertible preferred stock, net of repurchases. Additionally, during
2010, we received $84.3 million in cash proceeds from issuance of common stock upon our initial
public offering, $263,000 in cash proceeds from exercises of warrants, and we paid $8.5 million in
costs related to our initial public offering.
Operating Capital and Capital Expenditure Requirements
To date, we have not commercialized any products. We do not anticipate generating any revenue
unless and until we successfully obtain CE Mark or FDA marketing approval for, and begin selling,
the ReZolvetm stent or one of our other product possibilities. We anticipate
that we will continue to incur substantial net losses for the next several years as we continue our
development work, conduct and complete preclinical and clinical trials, expand our corporate
infrastructure, and prepare for the potential commercial launch of our products.
We believe that our existing cash and cash equivalents will be sufficient to meet our
anticipated cash requirements for at least the next three years. If our available cash and cash
equivalents are insufficient to satisfy our liquidity requirements, or if we develop additional
products or pursue additional applications for our products, we may seek to sell additional equity
or debt securities, or obtain a credit facility. The sale of additional equity and debt securities
may result in additional dilution to our stockholders. If we raise additional funds through the
issuance of debt securities, these securities could have rights senior to those of our common stock
and could contain covenants that would restrict our operations. We may require additional capital
beyond our currently forecasted amounts. For example, we will need to raise additional funds in
order to build our sales force and commercialize our products. Any such required additional capital
may not be available on reasonable terms, if at all. If we are unable to obtain additional
financing, we may be required to reduce the scope of, delay, or eliminate some or all of our
planned clinical trials, research, development, and commercialization activities, which could
materially harm our business.
Our forecasts for the period of time through which our financial resources will be adequate to
support our operations and the costs to complete development of products are forward-looking
statements and involve risks and uncertainties, and actual results could vary materially and
negatively as a result of a number of factors, including the factors discussed in the “Risk
Factors” section of this prospectus. We have based these estimates on assumptions that may prove to
be wrong, and we could utilize our available capital resources sooner than we currently expect.
54
Table of Contents
Because of the numerous risks and uncertainties associated with the development of medical
devices, such as our ReZolvetm stent, we are unable to estimate the exact
amounts of capital outlays and operating expenditures necessary to complete development, continue
ongoing preclinical studies, conduct human clinical trials, and successfully deliver a commercial
product to market. Our future funding requirements will depend on many factors, including, but not
limited to:
| • | the time and effort it will take to successfully complete the development and testing of the ReZolvetm stent; | ||
| • | the time and effort it will take to identify, develop, and scale-up manufacturing processes; | ||
| • | the scope, enrollment rate, and costs of our human clinical trials; | ||
| • | the scope of research and development for any of our other product opportunities; | ||
| • | the cost of filing and prosecuting patentable technologies and defending and enforcing our patent and other intellectual property rights; | ||
| • | the terms and timing of any collaborative, licensing, and other arrangements that we may establish; | ||
| • | the requirements, cost, and timing of regulatory approvals; | ||
| • | the cost and timing of establishing sales, marketing, and distribution capabilities; | ||
| • | the cost of establishing clinical and commercial supplies of our products and any products that we may develop; | ||
| • | the effect of competing technological and market developments; and | ||
| • | the cost and ability to license technologies for future development. |
Future capital requirements will also depend on the extent to which we acquire or invest in
businesses, products and technologies, although we currently have no commitments or agreements
relating to any of these types of transactions.
Contractual Obligations, Commitments, and Contingencies
The following table summarizes our outstanding contractual obligations as of December 31,
2010:
| Payments Due by Period | ||||||||||||
| Total | Under 1 Year | Over 1 Year | ||||||||||
Operating lease obligations |
289 | 289 | — | |||||||||
Purchase obligations |
196 | 196 | — | |||||||||
Total contractual obligations |
$ | 485 | $ | 485 | $ | — | ||||||
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Recent Accounting Pronouncements
In January 2010, the Financial Accounting Standards Board, or FASB, issued guidance that
expands the interim and annual disclosure requirements of fair value measurements, including the
information about movement of assets between Level 1 and 2 of the three-tier fair value hierarchy
established under its fair value measurement guidance. This guidance also requires separate
disclosure for purchases, sales, issuances, and settlements in the reconciliation for fair value
measurements using Level 3 methodologies. Except for the detailed disclosure in the Level 3
reconciliation, which is effective for the fiscal years beginning after December 15, 2010, all the
other disclosures
under this guidance became effective during our year ended December 31, 2010. We adopted the
relevant provisions of this guidance on January 1, 2010 and it had no financial impact on our
financial statements.
55
Table of Contents
In February 2010, the FASB issued an update to the accounting standard regarding subsequent
events. The update requires evaluation of subsequent events through the date financial statements
are issued for SEC filers, amends the definition of SEC filer, and changes required disclosures.
The new accounting guidance was effective on February 24, 2010 and did not have a material
financial impact on our financial statements upon adoption.
Other accounting standards that have been issued or proposed by the FASB or other
standards-setting bodies that do not require adoption until a future date are not expected to have
a material impact on our financial statements upon adoption.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Sensitivity
Our cash and cash equivalents of $81.7 million at December 31, 2010 consisted of cash and
money market funds that will be used for working capital purposes. We do not enter into investments
for trading or speculative purposes. Our primary exposure to market risk is interest income
sensitivity, which is affected by changes in the general level of interest rates in the U.S.
Because of the short-term nature of our cash and cash equivalents, we do not believe that we have
any material exposure to changes in their fair values as a result of changes in interest rates.
Foreign Currency Risk
To date, our purchases from foreign suppliers and consultants have been minimal and have been
denominated primarily in the currencies of Australia and the EU. Accordingly, we have had and will
continue to have exposure to foreign currency exchange rate fluctuations. We do not enter into
foreign currency hedging transactions. Although our foreign subsidiary is non-operational, its
functional currency is the Euro; accordingly, the effects of exchange rate fluctuations on the net
assets of the subsidiary are accounted for as translation gains or losses in accumulated other
comprehensive income within stockholders’ equity. A change of 10% or more in foreign currency
exchange rates of the Australian dollar or the Euro would have a material impact on our financial
position and results of operations if we continue or increase our purchases denominated in
currencies other than the U.S. dollar.
Related Party Transactions
Our related parties include the members of our Board of Directors and investors with five
percent or more of our outstanding securities. As of December 31, 2010, our related parties
collectively represented approximately 65 percent of our outstanding stock. Transactions with our
related parties historically consisted of notes payable issued to members of our board of
directors, or firms they represented, or to the investors that held in excess of five percent of
our securities. All of our notes payable together with accrued interest converted into common stock
upon consummation of our initial public offering in December 2010.
Item 8. Financial Statements and Supplementary Data
See the list of financial statement filed with the report under Part IV — Item 15 below.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
56
Table of Contents
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, including our Chief Executive Officer and Chief Financial Officer, evaluated
the effectiveness of our disclosure controls and procedures as of December 31, 2010. The term
“disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the
Exchange Act, means controls and other procedures of a company that are designed to ensure that
information required to be disclosed by us in the reports that we file or submit under the Exchange
Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s
rules and forms. Disclosure controls and procedures include, without limitation, controls and
procedures designed to ensure that information required to be disclosed by us in the reports that
we file or submit under the Exchange Act is accumulated and communicated to our management,
including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely
decisions regarding required disclosure. In designing and evaluating the disclosure controls and
procedures, management recognizes that any controls and procedures, no matter how well designed and
operated, can provide only reasonable, and not absolute, assurance of achieving the desired
objectives. In reaching a reasonable level of assurance, management necessarily applies its
judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on
this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our
disclosure controls and procedures were effective as of December 31, 2010 at the reasonable
assurance level.
Exemption from Management’s Report on Internal Control Over Financial Reporting for the Fiscal Year
Ended December 31, 2010
This annual report does not include a report of management’s assessment regarding internal
control over financial reporting or an attestation report of the Company’s registered public
accounting firm due to a transition period established by rules of the SEC for newly public
companies.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting (as defined in Rules
13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the quarterly period ended
December 31, 2010 that has materially affected, or is reasonably likely to materially affect, our
internal control over financial reporting.
Item 9B. Other Information
None.
PART III
Certain information required by Part III is omitted from this report because the Company will
file a definitive proxy statement within 120 days after the end of its fiscal year pursuant to
Regulation 14A (the “Proxy Statement”) for its annual meeting of stockholders to be held on May 23,
2011, and certain information included in the Proxy Statement is incorporated herein by reference.
Item 10. Directors, Executive Officers, and Corporate Governance
(a) The information required by this item will be set forth in the Proxy Statement and is
incorporated in this report by reference.
(b) Identification of Executive Officers. Information concerning our executive officers is set
forth under “Executive Officers” in Part I of this Annual Report on Form 10-K and is incorporated
herein by reference.
(c) The information required by this item will be set forth in the Proxy Statement and is
incorporated in this report by reference.
(d) The information required by this item will be set forth in the Proxy Statement and is
incorporated in this report by reference.
57
Table of Contents
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics that applies to all of our officers,
directors and employees. We have posted a copy of our Code of Business Conduct and Ethics, and
intend to post amendments to this code, or any waivers of its requirements, on our website at
www.revamedical.com.
Australian Disclosure Requirements
As part of our ASX listing, we are required to comply with various disclosure requirements as
set out under the ASX Listing Rules. The following information is intended to comply with the ASX
Listing Rules and is not intended to fulfill information required by Part III of this Annual Report
on Form 10-K.
Substantial Shareholders
The number of equivalent CHESS Depository Interests, or CDIs, held by our substantial
shareholders (those being the equity holders, together with their associates, who have a relevant
interest in at least 5 percent of our voting shares), assuming conversion of common stock into CDIs
(ten CDIs are equivalent to one share of common stock) as of March 15, 2011 are as follows:
| % of Total | ||||||||
| Equivalent | Equivalent | |||||||
| Number of | Outstanding | |||||||
| CDIs Held | CDIs | |||||||
Domain Partners |
53,869,050 | 16.4 | % | |||||
Saints Capital Everest, L.P. |
45,774,651 | 14.0 | % | |||||
Group Outcome Investors/Robert B. Stockman |
30,144,755 | 9.2 | % | |||||
Brookside Capital Partners |
29,650,222 | 9.0 | % | |||||
Cerberus and affiliates |
28,844,260 | 8.8 | % | |||||
Medtronic, Inc. |
22,558,280 | 6.9 | % | |||||
Distribution of Equity Security Holders as of March 15, 2011
As of March 15, 2011, we had a total of 32,765,503 shares of common stock issued and
outstanding, which equates to 327,655,030 CDIs. A portion of the outstanding securities was held as
CDIs. The following table presents the number of shares of common stock and CDIs held, as well as
the number of shares underlying outstanding stock options and restricted stock issuances.
| Restricted Common Stock | ||||||||||||||||||||||||||||||||
| Common Stock | CDIs | Options (unlisted) | (unlisted) | |||||||||||||||||||||||||||||
| Number of | Number of | Number of | Number of | Number of | Number of | Number of | Number of | |||||||||||||||||||||||||
| Holders | Shares | Holders | CDIs | Holders | Shares | Holders | Shares | |||||||||||||||||||||||||
1 — 1,000 |
9 | 3,414 | 51 | 15,415 | 8 | 7,000 | — | — | ||||||||||||||||||||||||
1,001 — 5,000 |
6 | 20,500 | 138 | 443,648 | 12 | 38,500 | 1 | 5,000 | ||||||||||||||||||||||||
5,001 — 10,000 |
5 | 38,963 | 98 | 862,354 | 5 | 40,500 | — | — | ||||||||||||||||||||||||
10,001 — 100,000 |
22 | 866,639 | 273 | 8,727,046 | 17 | 741,500 | — | — | ||||||||||||||||||||||||
100,001 and over |
24 | 23,737,378 | 43 | 70,937,627 | 8 | 2,590,000 | — | — | ||||||||||||||||||||||||
| 66 | 24,666,894 | 603 | 80,986,090 | 50 | 3,417,500 | 1 | 5,000 | |||||||||||||||||||||||||
The number of shareholders holding less than a marketable parcel of CDIs (being a parcel of
securities not less than A$500) as of March 15, 2011 was 32.
58
Table of Contents
Top 20 Equity Security Holders
The table below shows the top 20 holders of our CDIs as of March 15, 2011:
| Number of | % of CDIs | |||||||||||
| CDIs Held | Outstanding | |||||||||||
| 1 | Citicorp Nominees Pty Limited |
13,499,252 | 16.7 | % | ||||||||
| 2 | HSBC Custody Nominees (Australia) Limited — GSCO ECA |
9,818,182 | 12.1 | % | ||||||||
| 3 | Medtronic Inc. |
9,570,000 | 11.8 | % | ||||||||
| 4 | HSBC Custody Nominees (Australia) Limited |
9,302,369 | 11.5 | % | ||||||||
| 5 | CS Fourth Nominees Pty Ltd |
9,201,300 | 11.4 | % | ||||||||
| 6 | UBS Nominees Pty Ltd |
2,637,182 | 3.3 | % | ||||||||
| 7 | Kenneth Rainin Charitable Lead Annuity Trust No. 3 dtd 3/26/90 |
2,454,545 | 3.0 | % | ||||||||
| 8 | UBS Nominees Pty Ltd <PB SEG A/C> |
1,818,182 | 2.3 | % | ||||||||
| 9 | Pennsylvania State University |
1,284,840 | 1.6 | % | ||||||||
| 10 | RBC Dexia Investor Services Australia Nominees Pty Ltd <BkCust A/C> |
1,136,363 | 1.4 | % | ||||||||
| 11 | JP Morgan Nominees Australia Limited <Cash Income A/C> |
1,054,747 | 1.3 | % | ||||||||
| 12 | Saints Capital Everest LP |
909,091 | 1.1 | % | ||||||||
| 13 | Asia Union Investments Pty Ltd |
909,000 | 1.1 | % | ||||||||
| 14 | Mr. Robert Schneider |
625,000 | 0.8 | % | ||||||||
| 15 | HSBC Custody Nominees (Australia) Limited <No 2 A/C> |
513,688 | 0.6 | % | ||||||||
| 16 | Moore Family Nominee Pty Ltd <Moore Family Super Fund A/C> |
500,000 | 0.6 | % | ||||||||
| 17 | Jarek Kanikula |
447,760 | 0.6 | % | ||||||||
| 18 | OC Funds Management Limited |
364,100 | 0.4 | % | ||||||||
| 19 | Warman Investments Pty Ltd |
363,800 | 0.4 | % | ||||||||
| 20 | National Nominees Limited |
341,900 | 0.4 | % | ||||||||
Total CDIs held by top 20 shareholders |
66,751,301 | 82.4 | % | |||||||||
Total CDIs held by all other shareholders |
14,234,789 | 17.6 | % | |||||||||
The table below provides a list of the top 20 holders of our securities at March 15, 2011
taking into account securities held both in the form of common stock and CDIs and prepared on the
assumption that all common stock in REVA Medical, Inc. is held as CDIs.
| Number of | % of CDIs | |||||||||||
| CDIs Held | Outstanding | |||||||||||
| 1 | Domain Partners V, L.P. |
52,625,830 | 16.1 | % | ||||||||
| 2 | Saints Capital Everest LP |
45,774,651 | 14.0 | % | ||||||||
| 3 | Brookside Capital Partners Fund |
29,650,222 | 9.0 | % | ||||||||
| 4 | Medtronic, Inc. |
22,558,280 | 6.9 | % | ||||||||
| 5 | Citicorp Nominees Pty Limited |
13,499,252 | 4.1 | % | ||||||||
| 6 | Kenneth Rainin Charitable Lead Annuity Trust No. 3 dtd 3/26/90 |
13,470,695 | 4.1 | % | ||||||||
| 7 | Group Outcome Investors I, LLC |
13,411,750 | 4.1 | % | ||||||||
| 8 | Cerberus Series Four Holdings, LLC |
10,464,860 | 3.2 | % | ||||||||
| 9 | Cerberus International, LTD. |
9,955,530 | 3.0 | % | ||||||||
| 10 | HSBC Custody Nominees (Australia) Limited — GSCO ECA |
9,818,182 | 3.0 | % | ||||||||
| 11 | HSBC Custody Nominees (Australia) Limited |
9,302,369 | 2.8 | % | ||||||||
| 12 | CS Fourth Nominees Pty Ltd |
9,201,300 | 2.8 | % | ||||||||
| 13 | Gordon E. Nye |
8,235,310 | 2.5 | % | ||||||||
| 14 | Thomas Andrew Steinke |
7,383,880 | 2.3 | % | ||||||||
| 15 | Colleen Diane O’Connell |
7,383,870 | 2.3 | % | ||||||||
| 16 | Cerberus Partners, L.P. |
5,206,410 | 1.6 | % | ||||||||
| 17 | C. Raymond Larkin, Jr. |
3,517,490 | 1.1 | % | ||||||||
| 18 | Frederich H. Moll, M.D. |
3,345,610 | 1.0 | % | ||||||||
| 19 | Gabriel Assets, LLC |
2,955,790 | 0.9 | % | ||||||||
| 20 | Timothy J. Barberich |
2,702,680 | 0.8 | % | ||||||||
Total CDIs held by top 20 shareholders |
280,463,961 | 85.6 | % | |||||||||
Total CDIs held by all other shareholders |
47,191,069 | 14.4 | % | |||||||||
59
Table of Contents
Options Unlisted
As of March 15, 2011, we had 3,417,500 options to purchase shares of common stock on issue
under the 2010 Equity Incentive Plan and the 2001 Stock Option/Stock Issuance Plan. These options
are held by 50 individuals.
Restricted Securities
The following shareholders are subject to the escrow periods outlined below commencing from
the date of quotation of our CDIs on ASX, being 23 December 2010
(Australian Eastern time).
| Number of shares of | ||||||||
| common stock/options | Expiry of escrow | |||||||
| Escrowed party | Type | subject to escrow | period (Australian time) | |||||
Robert Stockman |
ASX imposed | 813,221 | 23 December 2012 | |||||
Group Outcome Investors I, LLC |
ASX imposed | 1,356,248 | 23 December 2012 | |||||
Lisa Stockman |
ASX imposed | 226,423 | 23 December 2012 | |||||
Gordon Nye |
ASX imposed | 885,792 | 23 December 2012 | |||||
Brian Dovey |
ASX imposed | 62,500 | 23 December 2012 | |||||
Robert Thomas |
ASX imposed | 62,500 | 23 December 2012 | |||||
Anne Keating |
ASX imposed | 62,500 | 23 December 2012 | |||||
James Schiro |
ASX imposed | 62,500 | 23 December 2012 | |||||
Daniel Frank |
ASX imposed | 61,750 | 23 December 2012 | |||||
Donald Brandom |
Executive team — voluntary | 175,000 | 23 June 2011 | |||||
Robert Schultz |
Executive team — voluntary | 715,000 | 23 June 2011 | |||||
Eric Schmid |
Executive team — voluntary | 285,000 | 23 June 2011 | |||||
Katrina Thompson |
Executive team — voluntary | 355,000 | 23 June 2011 | |||||
Joan Zeltinger |
Executive team — voluntary | 120,000 | 23 June 2011 | |||||
Beaver Creek Fund Ltd |
Shareholder — voluntary | 184,493 | 23 June 2011 | |||||
Medtronic, Inc. |
Shareholder — voluntary | 1,291,467 | 23 June 2011 | |||||
Saints Capital Everest, LP |
Shareholder — voluntary | 4,483,668 | 23 June 2011 | |||||
Domain Partners1 |
Shareholder — voluntary | 5,375,028 | 23 June 2011 | |||||
Cerberus2 |
Shareholder — voluntary | 2,868,052 | 23 June 2011 | |||||
Brookside Capital Partners Fund, LP |
Shareholder — voluntary | 2,767,430 | 23 June 2011 | |||||
Steinke Family, LLC |
Shareholder — voluntary | 1,476,775 | 23 June 2011 | |||||
Blue Group Trust |
Shareholder — voluntary | 205,738 | 23 June 2011 | |||||
Hoskins Family Trust |
Shareholder — voluntary | 169,898 | 23 June 2011 | |||||
Timothy Barberich |
Shareholder — voluntary | 270,186 | 23 June 2011 | |||||
Raymond Larkin |
Shareholder — voluntary | 351,488 | 23 June 2011 | |||||
Frederic Moll |
Shareholder — voluntary | 333,986 | 23 June 2011 | |||||
Kenneth Rainin Trust |
Shareholder — voluntary | 1,101,122 | 23 June 2011 | |||||
| 1. | Domain Partners means Domain Partners V LP and DP V Associates, LP | |
| 2. | Cerberus means Cerberus America Series Two Holdings, LLC, Cerberus International Ltd, Cerberus Partners, LP, Cerberus Series Four Holdings, LLC and Gabriel Assets, LLC |
Voting Rights
Our amended and restated certificate of incorporation and by-laws provide that each
stockholder has one vote for every share of common stock entitled to vote held of record by such
stockholder. In addition, although holders of restricted stock are subject to restrictions on
transfer until vesting, restricted stockholders have the same voting rights as holders of shares of
common stock (including prior to vesting).
If holders of CDIs wish to attend our general meetings, they will be able to do so. Under the
ASX Listing Rules, REVA Medical, Inc., as an issuer of CDIs, must allow CDI holders to attend any
meeting of the holders of the underlying securities unless relevant U.S. law at the time of the
meeting prevents CDI holders from attending those meetings.
60
Table of Contents
In order to vote at such meetings, CDI holders have the following options:
| (a) | instructing CHESS Depository Nominee, as the legal owner, to vote the shares of REVA Medical common stock underlying their CDIs in a particular manner. The instruction form must be completed and returned to our share registry prior to the meeting; | |
| (b) | informing REVA Medical that they wish to nominate themselves or another person to be appointed as CDN’s proxy for the purposes of attending and voting at the general meeting; | |
| (c) | converting their CDIs into a holding of shares of REVA Medical common stock and voting these at the meeting (however, if thereafter the former CDI holder wishes to sell their investment on ASX, it would be necessary to convert shares of common stock back to CDIs). This must be done prior to the record date for the meeting. |
As holders of CDIs will not appear on REVA Medical’s share register as the legal holders of
the shares of common stock, they will not be entitled to vote at our shareholder meetings unless
one of the above steps is undertaken.
Proxy forms and details of these alternatives will be included in each notice of meeting sent
to CDI holders by REVA Medical.
Holders of options are not entitled to vote.
Required Statements
REVA Medical makes the following disclosures:
| (a) | There is no current on-market buy-back of the Company’s securities. | |
| (b) | REVA Medical, Inc. was incorporated in the state of Delaware in the United States of America. | |
| (c) | REVA Medical, Inc. is not subject to Chapters 6, 6A, 6B or 6C of the Corporations Act dealing with the acquisitions of shares (including substantial shareholdings and takeovers). | |
| (d) | Under the Delaware General Corporation Law, shares are generally freely transferable subject to restrictions imposed by U.S. federal or state securities laws, by our certificate of incorporation or by-laws or by an agreement signed with the holders of the shares at issue. Our amended and restated certificate of incorporation and by-laws do not impose any specific restrictions on transfer. | |
| Delaware General Corporation Law prohibits a publicly held Delaware Corporation from engaging in a “business combination” with an “interested shareholder” for a period of three years following the time the person became an interested shareholder, unless the business combination or acquisition of shares that resulted in a shareholder becoming an interested shareholder is approved in a prescribed manner. A “business combination” can include a merger, asset or share sale or other transaction resulting in a financial benefit to an interested shareholder. Generally, an interested shareholder is a person who, together with its affiliates and associates, owns (or within three years prior to the determination of interested shareholder status did own) 15% or more of a corporation’s voting shares. The existence of this provision would be expected to have an anti-takeover effect with respect to transactions not approved in advance by the board of directors, or the Board, including discouraging attempts that might result in a premium over the market price for the shares of common stock held by shareholders. | ||
| (e) REVA Medical, Inc. has used the cash (and assets in a form readily convertible to cash) that it had at the time of admission to ASX in a manner consistent with its stated business objectives (as described in the Australian prospectus and supplementary prospectus lodged with the Australian Securities and Investments Commission with respect to our IPO) from the time of our admission to ASX to December 31, 2010. |
General Information
The name of the Company Secretary is Katrina Thompson.
61
Table of Contents
The address of our registered office in Australia is c/o Inteq Limited, Level 6, 175 Macquarie
Street, Sydney NSW 2000, telephone +61 2 9231 3322.
Registers of securities are held at Computershare Investor Services Pty. Limited, Level 3, 60
Carrington Street, Sydney NSW 2000, Investor Enquiries: 1300 855 080.
Quotation has been granted for CDIs on the ASX Limited.
Australian Corporate Governance Statement
The Board is committed to promoting and strengthening good corporate governance practices and
procedures throughout the Company. The Board has evaluated the Company’s corporate governance
policies and practices in light of the ASX Corporate Governance Council’s Corporate Governance
Principles and Recommendations (“ASX Corporate Governance Principles”) in force from the date of
its listing on ASX until the end of the Company’s financial year ended December 31, 2010. A summary
of the approach adopted by the Company during that period is set out below. Recent amendments to
the ASX Corporate Governance Principles will apply to the Company with respect to its financial
year ending December 31, 2011. Where appropriate, the Company has addressed its transition to the
amended ASX Corporate Governance Principles below.
Principle 1 — Lay solid foundations for management and oversight
Recommendation 1.1 — Establish the functions reserved to the Board and those delegated to senior
executives and disclose those functions
The Board’s responsibilities are defined by the Corporate Governance Guidelines, a copy of
which is available on the Company’s website at www.revamedical.com, and there is a clear
delineation between the Chairman of the Board’s responsibility for the Company’s strategy and
activities, and the day-to-day management of operations conferred upon the Company’s officers.
Recommendation 1.2 — Disclose the process for evaluating the performance of senior executives
In accordance with its charter, the Compensation Committee reviews and approves corporate and
personal performance goals and objectives relevant to the compensation of all executive officers,
evaluates the performance of each executive officer in light of those goals and objectives, and
sets each executive officer’s compensation, including but not limited to salary, bonus, incentive
compensation, and equity awards based on such an evaluation. In addition, the Compensation
Committee is responsible for regularly reviewing the Company’s compensation, recruitment,
retention, and termination policies for senior executives.
Recommendation 1.3 — Provide the information required to be disclosed under Principle 1
Reporting Requirement
The Company fully complied with Recommendations 1.1, 1.2, and 1.3 from the date of its listing
on ASX to the end of its financial year ended December 31, 2010.
In setting the compensation for our executive officers, our Board placed significant emphasis
on the recommendation of our Chief Executive Officer (other than with respect to determining his
own compensation), considering our overall performance during the prior fiscal year, the
executive’s individual contributions during the prior fiscal year, the individual’s annual
performance reviews based on achievement of annual goals, and relevant
market data. With respect to new hires, our Board considered an executive’s background and
historical compensation in lieu of prior year performance. For 2010 and 2011, our Board utilized
research and informal benchmarking based on its personal knowledge of companies in the medical
device industry. Our Board used this market data as one component of determining executive
compensation. We expect to retain an independent compensation consultant to assist us with our
benchmarking process going forward. Further information regarding executive compensation for the
year ended December 31, 2010, as required by Item 11, is incorporated by reference to the
applicable information in our Definitive Proxy Statement for our 2011 Annual Meeting of
Stockholders, to be filed pursuant to Registration 14A with the Securities and Exchange Commission
within 120 days of December 31, 2010. Such information is incorporated herein by reference.
62
Table of Contents
Principle 2 — Structure the Board to add value
Recommendation 2.1 — A majority of the Board should be independent directors
Recommendation 2.2 — The Chair should be an independent director
Recommendation 2.3 — The roles of Chair and Chief Executive Officer should not be exercised by the
same individual
The Company has complied with Recommendation 2.1, but is not compliant with Recommendations
2.2 and 2.3. While the majority of the Board is comprised of independent directors for ASX
purposes, the Chairman is not an independent director and he also serves as the Company’s Chief
Executive Officer, contrary to ASX recommendation. The Board believes that Mr. Stockman is not able
to exert undue influence on the decision-making process or the governance functions of the Board,
despite Mr. Stockman not being independent. In addition, while the Chairman and Chief Executive
Officer roles have not been separated, the Company has also appointed Dr. Schultz as President and
Chief Operating Officer with responsibility for the Company’s day-to-day operations. Dr. Schultz
attends board meetings by invitation but not as a Director. The Board believes that this creates a
collaborative management style approach between the Chairman and President with appropriate checks
and balances.
Recommendation 2.4 — The Board should establish a Nomination Committee
In connection with our IPO, the Board established a Nominating and Corporate Governance
Committee which consists only of non-executive, independent directors to oversee the selection and
appointment practices of the Company. The members of the Nominating and Corporate Governance
Committee are Anne Keating (Chair), Gordon Nye, and James Schiro. All members are considered
independent directors for both ASX and SEC purposes. A copy of the Nominating and Corporate
Governance Committee Charter is available on the Company’s website at www.revamedical.com.
Recommendation 2.5 — Disclose the process for evaluating the performance of the Board, its
committees, and individual directors
As the Company is still in an early stage of development, it has not yet undertaken a formal
review of the Board’s performance. However, the Company’s Corporate Governance Guidelines provide
for an annual self-assessment of the Board’s performance to be provided to the Nominating and
Corporate Governance Committee.
Recommendation 2.6 — Provide the information required to be disclosed under Principle 2
Reporting Requirement
Except as disclosed above, the Company fully complied with Recommendations 2.1 to 2.6 from the
date of its listing on ASX to the end of its financial year ended December 31, 2010.
Identification and Evaluation of Nominees for Directors
Our nominating and governance committee uses a variety of methods for identifying and
evaluating nominees for director. Our nominating and governance committee regularly assesses the
appropriate size and composition of the Board, the needs of the Board and the respective committees
of the Board, and the qualifications of candidates in
light of these needs. Candidates may come to the attention of the nominating and governance
committee through stockholders, management, current members of the Board, or search firms. The
evaluation of these candidates may be based solely upon information provided to the nominating and
governance committee or may also include discussions with persons familiar with the candidate, an
interview of the candidate or other actions the nominating and governance committee deems
appropriate, including the use of third parties to review candidates.
In accordance with the amendments to the ASX Corporate Governance Principles, the Company’s
next Annual Report for the financial year ending December 31, 2011 will include disclosure as to
the mix of skills and diversity for which the Board is looking to achieve in its membership. As
outlined below with respect to Principle 3, the Company intends to evaluate and formalize its
policy in relation to diversity over the next six months.
63
Table of Contents
Director Biographies and Qualifications
Brian Dovey has served as our director since June 2001. Mr. Dovey has been a Partner of
Domain Associates, L.L.C., a private venture capital management firm focused on life sciences,
since 1988. Since joining Domain, he has served on the board of
directors of over 35 private and
public companies and has been Chairman of five. Prior to joining Domain, Mr. Dovey spent six years
at Rorer Group, Inc., a New York Stock Exchange listed pharmaceutical and medical device company
(now part of Sanofi-Aventis), including as president from 1986 to 1988. Previously, Mr. Dovey was
president of Survival Technology, Inc., a start-up medical products company. He also held
management positions with Howmedica, Inc., Howmet Corporation, and New York Telephone Company. Mr.
Dovey has served as both president and chairman of the National Venture Capital Association. He is
Chair of the Wistar Institute, a non-profit preclinical biomedical research company. Mr. Dovey
serves on the board of directors and is also Chairman at the Center for Venture Education (Kauffman
Fellows Program). He was also a former board member of the industry association representing the
medical device industry, as well as the association representing consumer pharmaceuticals. Mr.
Dovey currently sits on the board of directors of Orexigen Therapeutics, Inc., a biopharmaceutical
company focused on the development of pharmaceutical product candidates for the treatment of
obesity. Mr. Dovey has also served as a member of the board of directors for the following
publicly traded companies over the past five years: Align Technology, Inc., Cardiac Science, Inc.
and Neose Technologies, Inc. Mr. Dovey received his B.A. from Colgate University and an M.B.A.
from the Harvard Business School.
Qualifications: We believe Mr. Dovey is qualified to sit on our Board due to his strong
financial expertise, his previous service as a director on over 30 private and public companies,
his executive experience at a medical device company and his extensive experience at a health care
venture capital firm.
Anne Keating has served as a director of the Company since October 2010. Ms. Keating is
currently a director of a number of ASX listed companies in a range of different industries,
including Ardent Leisure Management Limited (formerly Macquarie Leisure Management
Limited), a general management service provider, Goodman Group Limited (formerly Macquarie
Goodman), a property development and management company, and Clearview Wealth Limited, a life insurance and financial services provider. She is also a member of the Advisory
Council of RBS Australia. Ms. Keating is also a Director for the Garvan Institute of Medical
Research and an Inaugural Governor for the Cerebral Palsy Foundation. From 1993 to 2001, Ms.
Keating held the position of General Manager, Australia for United Airlines, and from 1994 to 1998,
she was a Governor for the American Chamber of Commerce. She was also a Delegate to the
Australian/American Leadership Dialogue for 14 years. Ms. Keating was an inaugural board
member of the Victor Chang Cardiac Research Institute for ten years and also served on the board of
NRMA/Insurance/IAG for nine years. She has also held former directorships with Spencer Street
Station Redevelopment Holdings Limited, Easy FM China Pty Ltd, Radio 2CH Pty Ltd and Workcover
Authority of New South Wales.
Qualifications: We believe Ms. Keating is qualified to sit on our Board due to her extensive
business and governance experience, including her positions on a number of boards of ASX listed
companies. Ms. Keating also brings Australian medical research experience from her years of
service with the Garvan Institute of Medical Research and the Victor Chang Cardiac Research
Institute.
Gordon Nye has served as our director since 1999. He is a Managing Director of Group Outcome
LLC, a U.S.-based merchant banking firm which deploys its capital and that of its financial
partners in private equity and venture capital investments in medical technology companies. He is
currently the Chief Executive Officer of Zeltiq Aesthetics, Inc., a medical device company, a
position he has held since September 2009. From August 2003 to July 2009, Mr. Nye served as
general partner of Prism Venture Partners, a venture capital firm, where he was a
member of the life sciences investment team. Prior to that time, he served as our Chief
Executive Officer from 2001 to 2003 and President and Chief Executive Officer of two former Johnson
& Johnson divisions — “A” Company Orthodontics, Inc. and Critikon Company, LLC — after they were
acquired in management buyouts. He has also held a variety of marketing, sales and general
management roles for L.A. Gear, Inc., Olin Ski Company, Inc., Reebok, Ltd. and The Gillette
Company. Mr. Nye has also served on the board of directors of Insulet, Inc., a medical device
company, from 2004 to 2008. Mr. Nye received his MBA from the Amos Tuck School of Business at
Dartmouth College where he also received his undergraduate degree.
Qualifications: We believe Mr. Nye’s qualifications to sit on our Board include his
knowledge of the medical device business, his in-depth operating experience as a senior executive
of Zeltiq Aesthetics, Inc. and two former Johnson & Johnson divisions, and his service on other
company boards.
64
Table of Contents
Robert Thomas has served as our director since July 2010. He has also been a director and
non-executive Chairman of the Board of HeartWare Limited, and subsequently HeartWare International,
Inc., a U.S. medical device company listed on ASX and NASDAQ, since November 2004. He is
currently a director of a number of Australian public companies, including Virgin Blue Holdings
Limited and Tower Australia Limited. Between October 2004 and September 2008, Mr. Thomas was a
consultant to Citigroup Corporate and Investment Bank and was Chairman of Global Corporate and
Investment Bank, Australia and New Zealand of Citigroup Global Markets Australia Pty Limited
between March 2003 and September 2004. Prior to that time, Mr. Thomas was Chief Executive Officer
of Citigroup’s (formerly known as Salomon Smith Barney) Corporate and Investment Bank, Australia
and New Zealand from October 1999 until February 2003. Mr. Thomas is a director of O’Connell
Street Associates and Grahger Capital Securities as well as being President of the State Library
Council of New South Wales in Australia. Mr. Thomas holds a Bachelor of Economics from Monash
University, Australia. He is currently Chairman of the Stockbrokers Association of Australia and
is a Master Stockbroker. Mr. Thomas is also a Fellow of the Financial Services Institute of
Australia and the Australian Securities Institute of Company Directors.
Qualifications: We believe Mr. Thomas is qualified to sit on our Board due to his extensive
investment banking experience, including his leadership of finance and strategic transactions, and
his experience in governance and risk management across a wide range of industries. Mr. Thomas
also brings capital market and economics expertise to the Board from his years of service as a
securities analyst and experience as a director of ASX listed companies.
Robert Stockman, our co-founder, has served as our Chairman of the Board and director since
1999 and our Chief Executive Officer since August 2010. He has also served as a director of
HeartWare Limited, and subsequently HeartWare International, Inc., an ASX and NASDAQ listed medical
device company, since December 2006. Since 1999, Mr. Stockman has been the President and Chief
Executive Officer of Group Outcome LLC, a U.S.-based merchant banking firm which deploys its
capital and that of its financial partners in private equity and venture capital investments in
medical technology companies. Mr. Stockman also co-founded Centrimed, Inc., an internet-based
software company, that was acquired by the Global Healthcare Exchange, LLC, and led the buyouts of
Iopter, an intraocular lens manufacturer, and two Johnson & Johnson divestitures, “A” Company
Orthodontics, Inc. and Critikon Company, LLC, each of which was subsequently acquired. Prior to
establishing Group Outcome LLC, Mr. Stockman spent 18 years with Johnston Associates, Inc. and
Narragansett Capital Corporation, where he focused on venture capital investments and merger
advisory work in health care. Mr. Stockman holds a Bachelor’s Degree from Harvard College and a
Master in Business Administration from The Tuck School at Dartmouth College.
Qualifications: We believe Mr. Stockman is qualified to sit on our Board due to his extensive
experience as an entrepreneur driving the growth of five medical products companies, his experience
as an executive of several medical device companies and his experience as an executive in the
investment banking industry, particularly in private equity and venture capital investments in
medical technology. Mr. Stockman’s qualifications also include his strong financial background,
including his work early in his career at Price Waterhouse, a provider of tax, audit and advisory
services, and his ability to provide financial expertise to the board, including an understanding
of financial statements, corporate finance, accounting and capital markets.
James Schiro has served as our director since November 2010. Mr. Schiro was Chief Executive
Officer of Zurich Financial Services from May 2002 to December 2009, after serving as Chief
Operating Officer — Group Finance since March 2002. He joined Price Waterhouse in 1967, where he
held various management positions. In 1994, he was elected Chairman and senior partner of Price
Waterhouse, and in 1998 became Chief Executive Officer of PricewaterhouseCoopers, after the merger
of Price Waterhouse and Coopers & Lybrand. Mr. Schiro is also a Director of PepsiCo, Royal Philips
Electronics and Goldman Sachs. In addition, Mr. Schiro is a director with various non-profit organizations, including St.
John’s University, a trustee of the Institute for Advanced Study
and an advisory board member of the Tsinghua University School of Economics and Management.
Qualifications: We believe Mr. Schiro is qualified to sit on our Board due to his extensive
executive experience and his service on other public company boards.
Director Independence
In accordance with our corporate governance principles, the majority of our Board members are
independent directors. Our Board considers that a director is independent when the director is not
an officer or employee of the Company or its subsidiaries, does not have any relationship which
would, or could reasonably appear to, materially interfere with independent judgment, and otherwise
meets the independence requirements under the rules of NASDAQ, ASX and the SEC. Our Board has
reviewed the materiality of any relationship that each of our directors has with us, either
directly or indirectly. Based on this review, our Board has determined that:
65
Table of Contents
| • | Robert B. Stockman is not considered to be an independent director under the rules of NASDAQ, ASX or the SEC; | ||
| • | Anne Keating, Gordon E. Nye, James Schiro and Robert Thomas are considered to be independent directors under the rules of NASDAQ, ASX and the SEC; and | ||
| • | Brian Dovey is considered to be an independent director under the rules of NASDAQ and the SEC, but is not considered to be independent under ASX standards. |
There are no family relationships among our officers and directors, nor are there any
arrangements or understandings between any of our directors or officers or any other person
pursuant to which any officer or director was, or is, to be selected as an officer or director.
Membership of Board Committees
During 2010, all directors attended 75% or more of the meetings of the Board and the Board
committees on which he or she served during 2010. Directors serve on the following committees:
| Nominating | ||||||||||||
| and Corporate | ||||||||||||
| Audit | Compensation | Governance | ||||||||||
| Committee | Committee | Committee | ||||||||||
Mr. Stockman (Chairman) |
— | — | — | |||||||||
Mr. Dovey* |
X | X | — | |||||||||
Ms. Keating** |
— | — | Chair | |||||||||
Mr. Nye** |
— | Chair | X | |||||||||
Mr. Schiro** |
X | — | X | |||||||||
Mr. Thomas** |
Chair | X | — | |||||||||
| * | Independent Director under the rules of NASDAQ and the SEC, but not considered independent under the ASX | |
| ** | Independent Director under the rules of NASDAQ, ASX, and the SEC |
Nominating Committee
During the fiscal year ended December 31, 2010, our nominating and corporate governance
committee held no meetings. The members of our nominating and corporate governance committee are
Ms. Keating and Messrs. Nye and Schiro.
Professional Advice
From time to time, the Board may seek independent professional advice at the expense of the
Company. There is no formal policy in place with respect to retention of such independent
consultants.
Annual Board Review
The nominating and corporate governance committee conducts annual evaluations of the Board.
The first evaluation will occur in 2011.
Further information regarding Directors, as required by Item 10, will be contained in our
Definitive Proxy Statement for our 2011 Annual Meeting of Stockholders, to be filed pursuant to
Registration 14A with the Securities and Exchange Commission within 120 days of December 31, 2010.
Such information is incorporated herein by reference.
Principle 3 — Promote ethical and responsible decision-making
Recommendation 3.1 — Establish a Code of Conduct and disclose it
66
Table of Contents
Recommendation 3.2 — Establish a policy concerning trading in company securities by directors,
senior executives, and employees, and disclose the policy or a summary of that policy
The Company has adopted a Code of Business Conduct and Ethics, an Insider Trading Policy, and
a Related Party Transaction Policy. A copy of each policy is available on the Company’s website at
www.revamedical.com.
Recommendation 3.3 — Provide the information required to be disclosed under Principle 3
Reporting Requirement
The Company fully complied with Recommendations 3.1 and 3.2 from the date of its listing on
ASX to the end of its financial year ended December 31, 2010.
The recommendations under Principle 3 have been amended with effect from the Company’s
financial year which began on January 1, 2011 to provide that companies should establish a policy
concerning diversity which should include requirements for the Board to establish measurable
objectives for achieving gender diversity and for the Board to assess annually both the objectives
and progress in achieving them. The Company’s next Annual Report will include disclosure regarding
its measurable objectives with respect to gender diversity and in relation to the proportion of
women at various levels throughout the organization. The Company is in the process of evaluating
its policy on diversity with a view to adopting a formal diversity policy within the next six
months.
Principle 4 — Safeguard integrity in financial reporting
Recommendation 4.1 — The Board should establish an Audit Committee
Recommendation 4.2 — The Audit Committee should be structured so that it: (a) consists only of
non-executive directors; (b) consists of a majority of independent directors; (c) is chaired by an
independent Chair, who is not Chair of the Board; and (d) has at least three members
In connection with our IPO, the Board established an Audit Committee which consists only of
non-executive directors and a majority of independent directors to oversee the management of
financial and internal risks. The members of the Audit Committee are Robert Thomas (Chair), James
Schiro, and Brian Dovey. Robert Thomas and James Schiro are both considered independent directors
for ASX purposes; however, Brian Dovey is not considered to be independent for ASX purposes but is
considered to be independent under SEC rules.
Recommendation 4.3 — The Audit Committee should have a formal charter
The Audit Committee has adopted a formal charter, a copy of which is available on the
Company’s website at www.revamedical.com.
Recommendation 4.4 — Provide the information required to be disclosed under Principle 4
During the fiscal year ended December 31, 2010, our audit committee held one meeting. The
members of our audit committee are Messrs. Dovey, Schiro and Thomas, each of whom attended the
committee meeting held in
2010.
The Company fully complied with Recommendations 4.1, 4.2, 4.3, and 4.4 from the date of its
listing on ASX to the end of its financial year ended December 31, 2010.
Further information regarding the audit committee, as required by Item 10, will be contained
in our Definitive Proxy Statement for our 2011 Annual Meeting of Stockholders, to be filed pursuant
to Registration 14A with the Securities and Exchange Commission within 120 days of December 31,
2010. Such information is incorporated herein by reference.
Principle 5 — Make timely and balanced disclosure
Recommendation 5.1 — Establish written policies designed to ensure compliance with ASX Listing
Rule disclosure requirements and to ensure accountability at a senior executive level for that
compliance and disclose those policies or a summary of those policies
67
Table of Contents
The Company is committed to providing timely and balanced disclosure to the market in
accordance with its continuous disclosure obligations. A copy of the Company’s Continuous
Disclosure Policy is available on its website at www.revamedical.com.
Recommendation 5.2 — Provide the information required to be disclosed under Principle 5
Reporting Requirement
The Company fully complied with Recommendations 5.1 and 5.2 from the date of its listing on
ASX to the end of the financial year ended December 31, 2010.
Principle 6 — Respect the rights of shareholders
Recommendation 6.1 — Design a communications policy for promoting effective communication with
shareholders and encouraging their participation at general meetings and disclose that policy or a
summary of that policy
The Company has adopted a Shareholder Communications Policy for shareholders wishing to
communicate with the Board, which is included in the Company’s Corporate Governance Guidelines, a
copy which is available on the Company’s website at www.revamedical.com. The Company seeks
to utilize numerous modes of communication, including electronic communication, to ensure that its
communication with Shareholders is frequent, clear, and accessible.
All shareholders are invited to attend the Company’s annual meeting either in person or by
proxy. The Board regards the annual meeting as an excellent forum in which to discuss issues
relevant to the Company and accordingly encourages full participation by shareholders. Shareholders
have an opportunity to submit questions to the Board and auditors. The meeting will also be webcast
to provide access to those shareholders who are unable to attend the annual general meeting.
Recommendation 6.2 — Provide the information required to be disclosed under Principle 6
Reporting Requirement
The Company has fully complied with Recommendations 6.1 and 6.2 from the date of its listing
on ASX to the end of its financial year ended December 31, 2010.
The Company did not hold an annual meeting during the period from the date of its listing on
ASX to the end of its financial year ended December 31, 2010. Accordingly, the Company did not
comply with the equivalent of section 250RA of the Corporations Act during the reporting period.
Principle 7 — Recognize and manage risk
Recommendation 7.1 — Establish policies for the oversight and management of material business
risks and disclose a summary of those policies
Recommendation 7.2 — The Board should require management to design and implement the risk
management and internal control system to manage the Company’s material business risk and report to
it on whether those risks are being managed effectively. The Board should disclose that management
has reported to it as to the effectiveness of the Company’s management of its material business
risks
The Board’s Role in Risk Oversight
Our Board’s role in risk oversight includes receiving reports from members of management on a
regular basis regarding material risks faced by the Company and applicable mitigation strategies
and activities, at least on a quarterly basis. The reports cover the critical areas of operations,
sales and marketing, development, regulatory and quality affairs, intellectual property, clinical
development, and legal and financial affairs. Our Board and its committees consider these reports;
discuss matters with management and identify and evaluate any potential strategic or operational
risks, and appropriate activity to address those risks.
68
Table of Contents
We have adopted a risk management policy which sets out how we identify, assess and manage
risk in business operations, a copy of which is available in the “Investors — Corporate Governance”
section of our website www.revamedical.com.
Recommendation 7.3 — The Board should disclose whether it has received assurance from the Chief
Executive Officer (or equivalent) and the Chief Financial Officer (or equivalent) that the
declaration provided in accordance with section 295A of the Corporations Act is founded on a sound
system of risk and internal control and that system is operating effectively in all material
respects in relation to financial reporting risks
As the Company prepares and files its financial statements under U.S. accounting practices and
laws, management is required to provide representations to the Board on a wide range of issues,
including the effectiveness of the Company’s disclosure controls and procedures as well as the
design or operation of internal control over financial reporting. However, as the Company is
incorporated in the United States and is not bound by the financial reporting provisions under the
Australian Corporations Act 2001 (Cth), no declaration is required under section 295A of the
Corporations Act. To this end, shareholders’ attention is drawn to Item 9A of this Annual Report on
Form 10-K and the certifications provided by the Chief Executive Officer and the Chief Financial
Officer at the end of the Form 10-K. As stated above, Item 9A of this Annual Report on Form 10-K
discloses information regarding the Company’s controls and procedures and management’s evaluation
of the effectiveness of our internal control over financial reporting.
Recommendation 7.4 — Provide the information required to be disclosed under Principle 7
Reporting Requirement
Except as disclosed above, the Company fully complied with Recommendations 7.1, 7.2, 7.3, and
7.4 from the date of its listing on ASX to the end of its financial year ended December 31, 2010.
Principle 8 — Remunerate fairly and responsibly
Recommendation 8.1 — The Board should establish a remuneration committee
In connection with our IPO, the Board established a Compensation Committee which consists of a
majority of independent directors. The members of the Compensation Committee are Gordon Nye
(Chair), Brian Dovey, and Robert Thomas. Gordon Nye and Robert Thomas are both considered to be
independent for ASX purposes; however, Brian Dovey is not considered to be independent for ASX
purposes but is considered to be independent under the SEC rules. A copy of the Compensation
Committee charter is available on the Company’s website at www.revamedical.com.
Recommendation 8.2 — Clearly distinguish the structure of non-executive directors’ remuneration
from that of executive directors and senior executives
In accordance with its charter, the Compensation Committee is responsible for ensuring that
the structure of non-executive and executive directors’ compensation is clearly distinguished. The
Company has adopted a non-executive director compensation policy pursuant to which non-executive
directors are compensated for their services to the Board. Non-executive director compensation
comprises a base salary as well as the ability to receive annual grants of options at the Board’s
discretion. The Company has adopted a separate executive compensation program which extends to its
Chairman and consists of a base salary, equity-based incentives, and severance benefits as well as
other benefits such as health insurance. In addition, the Company’s Chairman is entitled to receive
a cash bonus of up to 30% of his salary for each year.
Recommendation 8.3 — Provide the information required to be disclosed under Principle 8
During the fiscal year ended December 31, 2010, our compensation committee held no meetings.
The members of our compensation committee are Messrs. Dovey, Nye and Thomas.
None of the Company’s non-executive directors will be entitled to any retirement benefits.
Further information regarding the compensation committee, as required by Item 10, will be
contained in our Definitive Proxy Statement for our 2011 Annual Meeting of Stockholders, to be
filed pursuant to Registration 14A with the Securities and Exchange Commission within 120 days of
December 31, 2010. Such information is incorporated herein by reference.
69
Table of Contents
Reporting Requirement
The Company fully complied with Recommendations 8.1, 8.2, and 8.3 from the date of its listing
on ASX to the end of its financial year ended December 31, 2010.
Item 11. Executive Compensation
Information required by this item will be contained in our Definitive Proxy Statement for our
2011 Annual Meeting of Stockholders, to be filed pursuant to Registration 14A with the Securities
and Exchange Commission within 120 days of December 31, 2010. Such information is incorporated
herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information required by this item will be contained in our Definitive Proxy Statement for our
2011 Annual Meeting of Stockholders, to be filed pursuant to Registration 14A with the Securities
and Exchange Commission within 120 days of December 31, 2010. Such information is incorporated
herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information required by this item will be contained in our Definitive Proxy Statement for our
2011 Annual Meeting of Stockholders, to be filed pursuant to Registration 14A with the Securities
and Exchange Commission within 120 days of December 31, 2010. Such information is incorporated
herein by reference.
Item 14. Principal Accountant Fees and Services
Information required by this item will be contained in our Definitive Proxy Statement for our
2011 Annual Meeting of Stockholders, to be filed pursuant to Registration 14A with the Securities
and Exchange Commission within 120 days of December 31, 2010. Such information is incorporated
herein by reference.
PART IV
Item 15. Exhibits and Financial Statement Schedules
The following documents are filed as part of this Annual Report on Form 10-K:
| 1. | Financial Statements — The following financial statements are included in this report: |
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets
Consolidated Statements of Operations
Consolidated Statements of Cash Flows
Consolidated Statements of Preferred Convertible Stock and Stockholders’ Equity (Deficit)
Notes to Consolidated Financial Statements
Consolidated Balance Sheets
Consolidated Statements of Operations
Consolidated Statements of Cash Flows
Consolidated Statements of Preferred Convertible Stock and Stockholders’ Equity (Deficit)
Notes to Consolidated Financial Statements
| 2. | List of Financial Statement Schedules — All schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto. | |
| 3. | Exhibits — The exhibits listed in the accompanying Index to Exhibits are filed or incorporated by reference as part of this Annual Report on Form 10-K. |
70
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934,
the Registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the
undersigned, thereunto duly authorized.
| REVA Medical, Inc. |
||||
| Dated: March 30, 2011 | By: | /s/ Robert B. Stockman | ||
| Name: | Robert B. Stockman | |||
| Title: | Chairman of the Board and Chief Executive Officer |
|||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been
signed below by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
/s/ Robert B. Stockman
|
Chairman of the Board and | March 30, 2011 | ||
| Chief Executive Officer (Principal Executive Officer) |
||||
/s/ Katrina Thompson
|
Chief Financial Officer | March 30, 2011 | ||
| (Principal Financial Officer and Principal Accounting Officer) |
||||
/s/ Robert K. Schultz, Ph.D.
|
President and | March 30, 2011 | ||
| Chief Operating Officer | ||||
/s/ Brian Dovey
|
Director | March 30, 2011 | ||
/s/ Anne Keating
|
Director | March 30, 2011 | ||
/s/ Gordon E. Nye
|
Director | March 30, 2011 | ||
/s/ James J. Schiro
|
Director | March 30, 2011 | ||
/s/ Robert Thomas
|
Director | March 30, 2011 |
71
Table of Contents
INDEX TO FINANCIAL STATEMENTS
| F-2 | ||||
| F-3 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-10 |
F - 1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
REVA Medical, Inc.
REVA Medical, Inc.
We have audited the accompanying consolidated balance sheets of REVA Medical, Inc. (a development
stage company) (the Company) as of December 31, 2009 and 2010, and the related consolidated
statements of operations, convertible preferred stock and stockholders’ equity (deficit), and cash
flows for each of the three years in the period ended December 31, 2010, and for the period from
June 3, 1998 (inception) to December 31, 2010. These consolidated financial statements are the
responsibility of the Company’s management. Our responsibility is to express an opinion on the
consolidated financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight
Board (United States). Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement. We
were not engaged to perform an audit of the Company’s internal control over financial reporting.
Our audits included consideration of internal control over financial reporting as a basis for
designing audit procedures that are appropriate in the circumstances, but not for the purpose of
expressing an opinion on the effectiveness of the Company’s internal control over financial
reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test
basis, evidence supporting the amounts and disclosures in the financial statements, assessing the
accounting principles used and significant estimates made by management, and evaluating the overall
financial statement presentation. We believe that our audits provide a reasonable basis for our
opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all
material respects, the consolidated financial position of the Company at December 31, 2009 and
2010, and the consolidated results of its operations and its cash flows for each of the three years
in the period ended December 31, 2010, and for the period from June 3, 1998 (inception) to December
31, 2010, in conformity with U.S. generally accepted accounting principles.
| /s/ Ernst & Young LLP | ||||
San Diego, California
March 30, 2011
March 30, 2011
F - 2
Table of Contents
REVA Medical, Inc.
(a development stage company)
(a development stage company)
Consolidated Balance Sheets
(in thousands)
(in thousands)
(page 1 of 2)
| December 31, | ||||||||
| 2009 | 2010 | |||||||
Assets |
||||||||
Current Assets: |
||||||||
Cash and cash equivalents |
$ | 7,233 | $ | 81,747 | ||||
Prepaid expenses and other current assets |
68 | 765 | ||||||
Total current assets |
7,301 | 82,512 | ||||||
Property and equipment, net |
1,134 | 963 | ||||||
Other non-current assets |
7 | — | ||||||
Total Assets |
$ | 8,442 | $ | 83,475 | ||||
Liabilities, Convertible Preferred Stock, and Stockholders’ Equity (Deficit) |
||||||||
Current Liabilities: |
||||||||
Accounts payable |
$ | 817 | $ | 937 | ||||
Accrued expenses and other current liabilities |
399 | 591 | ||||||
Total current liabilities |
1,216 | 1,528 | ||||||
Long-term notes payable, net ($20,029 held by related parties at December 31, 2009; none
held at December 31, 2010) |
20,304 | — | ||||||
Accrued interest on long-term notes payable ($6,857 to related parties at December 31, 2009;
none at December 31, 2010) |
6,971 | — | ||||||
Repayment premium on long-term notes payable ($10,550 to related parties at December
31, 2009; none at December 31, 2010) |
11,100 | — | ||||||
Preferred stock warrant liability |
780 | — | ||||||
Other long-term liabilities |
31 | — | ||||||
Total long-term liabilities |
39,186 | — | ||||||
Total Liabilities |
40,402 | 1,528 | ||||||
Commitments and contingencies (Note 10) |
||||||||
See Convertible Preferred Stock and Stockholders’ Equity (Deficit) on next page
The accompanying notes are an integral part of these financial statements.
F - 3
Table of Contents
REVA Medical, Inc.
(a development stage company)
Consolidated Balance Sheets
(in thousands, except share and per share amounts)
(a development stage company)
Consolidated Balance Sheets
(in thousands, except share and per share amounts)
(page 2 of 2)
| December 31, | ||||||||
| 2009 | 2010 | |||||||
Liabilities, Convertible Preferred Stock, and Stockholders’ Equity (Deficit) — continued |
||||||||
Convertible Preferred Stock ($0.0001 par value; 20,676,918 shares authorized at December 31,
2009 and none at December 31, 2010): |
||||||||
Series A — 1,814,558 shares authorized, issued, and outstanding at December 31, 2009 and none at
December 31, 2010; liquidation preference of $1,803 at December 31, 2009 and none at December 31, 2010 |
382 | — | ||||||
Series B — 833,333 shares authorized, issued, and outstanding at December 31, 2009 and none at
December 31, 2010; liquidation preference of $987 at December 31, 2009 and none at December 31, 2010 |
1,000 | — | ||||||
Series C — 558,374 shares authorized, issued, and outstanding at December 31, 2009 and none at
December 31, 2010; liquidation preference of $1,085 at December 31, 2009 and none at December 31, 2010 |
1,100 | — | ||||||
Series D — 819,673 shares authorized, issued, and outstanding at December 31, 2009 and none at
December 31, 2010; liquidation preference of $1,973 at December 31, 2009 and none at December 31, 2010 |
2,000 | — | ||||||
Series E — 2,550,980 shares authorized and 2,450,980 shares issued and outstanding at December 31, 2009
and none at December 31, 2010; liquidation preference of $29,600 at December 31, 2009 and none at
December 31, 2010 |
15,000 | — | ||||||
Series F — 1,000,000 shares authorized at December 31, 2009 and none at December 31, 2010; no shares
issued or outstanding |
— | — | ||||||
Series G-1 — 3,500,000 shares authorized and 1,014,199 shares issued and outstanding at December 31, 2009
and none at December 31, 2010; liquidation preference of $10,000 at December 31, 2009 and none at
December 31, 2010 |
9,500 | — | ||||||
Series G-2 — 600,000 shares authorized at December 31, 2009 and none at December 31, 2010;
no shares issued or outstanding |
— | — | ||||||
Series H — 9,000,000 shares authorized and 6,172,784 shares issued and outstanding at December 31, 2009
and none at December 31, 2010; liquidation preference of $47,164 at December 31, 2009 and none at
December 31, 2010 |
40,089 | — | ||||||
Total Convertible Preferred Stock |
69,071 | — | ||||||
Stockholders’ Equity (Deficit): |
||||||||
Common stock — $0.0001 par value; 30,000,000 shares authorized at December 31, 2009 and
100,000,000 shares authorized at December 31, 2010; 2,610,745 shares issued and
outstanding at December 31, 2009 and 32,760,503 shares issued and outstanding
at December 31, 2010 |
— | 3 | ||||||
Non-voting common stock — $0.0001 par value; 130,000 shares authorized and 128,484
shares issued and outstanding at December 31, 2009 and none at December 31, 2010 |
— | — | ||||||
Class B common stock — $0.0001 par value; no shares authorized at December 31, 2009
and 25,000,000 shares authorized at December 31, 2010; no shares issued or outstanding |
— | — | ||||||
Undesignated preferred stock — $0.0001 par value; no shares authorized at December 31, 2009
and 5,000,000 shares authorized at December 31, 2010; no shares issued or outstanding |
— | — | ||||||
Additional paid-in capital |
— | 210,847 | ||||||
Accumulated other comprehensive income |
2 | — | ||||||
Deficit accumulated during the development stage |
(101,033 | ) | (128,903 | ) | ||||
Total Stockholders’ Equity (Deficit) |
(101,031 | ) | 81,947 | |||||
Total Liabilities, Convertible Preferred Stock, and Stockholders’ Equity (Deficit) |
$ | 8,442 | $ | 83,475 | ||||
The accompanying notes are an integral part of these financial statements.
F - 4
Table of Contents
REVA Medical, Inc.
(a development stage company)
(a development stage company)
Consolidated Statements of Operations
(in thousands, except share and per share amounts)
(in thousands, except share and per share amounts)
| Period from | ||||||||||||||||
| June 3, 1998 | ||||||||||||||||
| (inception) to | ||||||||||||||||
| Year Ended December 31, | December 31, | |||||||||||||||
| 2008 | 2009 | 2010 | 2010 | |||||||||||||
Operating Expense: |
||||||||||||||||
Research and development |
$ | 11,378 | $ | 10,272 | $ | 6,826 | $ | 74,321 | ||||||||
General and administrative |
2,205 | 2,241 | 3,292 | 20,192 | ||||||||||||
Loss from operations |
(13,583 | ) | (12,513 | ) | (10,118 | ) | (94,513 | ) | ||||||||
Other Income (Expense): |
||||||||||||||||
Interest income |
124 | 26 | 116 | 1,095 | ||||||||||||
Related party interest expense |
(1,794 | ) | (1,532 | ) | (1,510 | ) | (21,113 | ) | ||||||||
Interest expense |
(80 | ) | (47 | ) | (39 | ) | (952 | ) | ||||||||
Interest from amortization of notes payable premium |
— | — | 2,283 | 2,283 | ||||||||||||
Gain (loss) on change in fair value of preferred
stock rights and warrant liabilities |
2,617 | 215 | (990 | ) | 1,795 | |||||||||||
Loss on extinguishment of notes payable |
— | — | (13,285 | ) | (13,285 | ) | ||||||||||
Other income (expense) |
2 | 7 | 36 | (40 | ) | |||||||||||
Net Loss |
(12,714 | ) | (13,844 | ) | (23,507 | ) | (124,730 | ) | ||||||||
Cumulative dividends and deemed dividends on
Series H convertible preferred stock |
(1,074 | ) | (2,358 | ) | (7,200 | ) | (10,695 | ) | ||||||||
Net Loss Attributable to Common Stockholders |
$ | (13,788 | ) | $ | (16,202 | ) | $ | (30,707 | ) | $ | (135,425 | ) | ||||
Net Loss Per Common Share: |
||||||||||||||||
Net loss per share, basic and diluted |
$ | (5.06 | ) | $ | (5.91 | ) | $ | (7.72 | ) | |||||||
Shares used to compute net loss per share,
basic and diluted |
2,727,191 | 2,739,229 | 3,975,144 | |||||||||||||
The accompanying notes are an integral part of these financial statements.
F - 5
Table of Contents
REVA Medical, Inc.
(a development stage company)
(a development stage company)
Consolidated Statements of Cash Flows
(in thousands)
(in thousands)
| Period from | ||||||||||||||||
| June 3, 1998 | ||||||||||||||||
| (inception) to | ||||||||||||||||
| Year Ended December 31, | December 31, | |||||||||||||||
| 2008 | 2009 | 2010 | 2010 | |||||||||||||
Cash Flows from Operating Activities: |
||||||||||||||||
Net loss |
$ | (12,714 | ) | $ | (13,844 | ) | $ | (23,507 | ) | $ | (124,730 | ) | ||||
Non-cash adjustments to reconcile net loss to net cash used for
operating activities: |
||||||||||||||||
Depreciation and amortization |
257 | 466 | 471 | 2,841 | ||||||||||||
Loss (gain) on property and equipment disposal and impairment |
(2 | ) | — | — | 585 | |||||||||||
Stock-based compensation |
112 | 227 | 1,185 | 2,028 | ||||||||||||
Interest on notes payable |
1,837 | 1,579 | (734 | ) | 8,562 | |||||||||||
Repayment premium on notes payable |
— | — | — | 11,100 | ||||||||||||
Loss (gain) on change in fair value of preferred stock warrant
liability |
195 | (215 | ) | 990 | 970 | |||||||||||
Gain on change in fair value of preferred stock rights liability |
(2,812 | ) | — | — | (2,765 | ) | ||||||||||
Loss on extinguishment of notes payable |
— | — | 13,285 | 13,285 | ||||||||||||
Other non-cash expenses (income) |
28 | 8 | (37 | ) | 43 | |||||||||||
Changes in operating assets and liabilities: |
||||||||||||||||
Prepaid expenses and other current assets |
65 | 47 | (697 | ) | (765 | ) | ||||||||||
Other non-current assets |
(7 | ) | — | 7 | — | |||||||||||
Accounts payable |
396 | (426 | ) | 120 | 937 | |||||||||||
Accrued expenses and other current liabilities |
47 | (411 | ) | 198 | 560 | |||||||||||
Net cash used for operating activities |
(12,598 | ) | (12,569 | ) | (8,719 | ) | (87,349 | ) | ||||||||
Cash Flows from Investing Activities: |
||||||||||||||||
Purchases of property and equipment |
(516 | ) | (733 | ) | (300 | ) | (4,550 | ) | ||||||||
Sales of property and equipment |
2 | — | — | 161 | ||||||||||||
Purchases of short-term investments |
(8,789 | ) | — | — | (17,886 | ) | ||||||||||
Maturities of short-term investments |
1,290 | 7,499 | — | 17,886 | ||||||||||||
Net cash provided by (used for) investing activities |
(8,013 | ) | 6,766 | (300 | ) | (4,389 | ) | |||||||||
Cash Flows from Financing Activities: |
||||||||||||||||
Proceeds from issuances of convertible preferred stock, net of costs |
20,000 | 5,000 | 8,034 | 68,917 | ||||||||||||
Proceeds from issuances of common stock |
— | — | 84,278 | 84,933 | ||||||||||||
Initial public offering costs |
— | — | (8,490 | ) | (8,490 | ) | ||||||||||
Proceeds from exercises of warrants |
— | — | 263 | 263 | ||||||||||||
Repurchases of stock |
— | — | (550 | ) | (638 | ) | ||||||||||
Proceeds from issuances of notes payable |
— | — | — | 28,600 | ||||||||||||
Repayments of notes payable |
— | — | — | (100 | ) | |||||||||||
Net cash provided by financing activities |
20,000 | 5,000 | 83,535 | 173,485 | ||||||||||||
Net increase (decrease) in cash and cash equivalents |
(611 | ) | (803 | ) | 74,516 | 81,747 | ||||||||||
Effect of foreign exchange rates |
(1 | ) | — | (2 | ) | — | ||||||||||
Cash and cash equivalents at beginning of period |
8,648 | 8,036 | 7,233 | — | ||||||||||||
Cash and cash equivalents at end of period |
$ | 8,036 | $ | 7,233 | $ | 81,747 | $ | 81,747 | ||||||||
Supplemental Cash and Non-Cash Information (also see Consolidated
Statements of Stockholders’ Equity for non-cash transactions that arose upon
our IPO in December 2010): |
||||||||||||||||
Cash paid for interest |
$ | 37 | $ | — | $ | — | $ | 126 | ||||||||
Preferred stock issued upon conversion of notes payable |
$ | — | $ | — | $ | — | $ | 7,950 | ||||||||
The accompanying notes are an integral part of these financial statements.
F - 6
Table of Contents
REVA Medical, Inc.
(a development stage company)
(a development stage company)
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
Period from June 3, 1998 (inception) to December 31, 2010
(in thousands, except share and per share amounts)
Period from June 3, 1998 (inception) to December 31, 2010
(in thousands, except share and per share amounts)
(page 1 of 3)
| Accumulated | Deficit | Total | |||||||||||||||||||||||||||||||||||||||
| Other | Accumulated | Stock- | |||||||||||||||||||||||||||||||||||||||
| Convertible | Common Stock | Additional | Compre- | During the | holders’ | ||||||||||||||||||||||||||||||||||||
| Preferred Stock | Voting | Non-Voting | Paid-In | hensive | Development | Equity | |||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Income(Loss) | Stage | (Deficit) | ||||||||||||||||||||||||||||||||
Common stock issued June 1998 to July 1999
for cash at $0.0001 to $0.67 per share |
— | $ | — | 2,452,088 | $ | — | — | $ | — | $ | 278 | $ | — | $ | — | $ | 278 | ||||||||||||||||||||||||
Net loss June 3, 1998 (inception) to November
30, 1999 |
— | — | — | — | — | — | — | — | (492 | ) | (492 | ) | |||||||||||||||||||||||||||||
Recapitalization of Company December 1999 |
— | — | — | — | — | — | (492 | ) | — | 492 | — | ||||||||||||||||||||||||||||||
Series A preferred stock issued December 1999
in exchange for common stock on a 1-for-1
basis upon recapitalization of Company |
1,618,058 | 185 | (1,618,058 | ) | — | — | — | (185 | ) | — | — | (185 | ) | ||||||||||||||||||||||||||||
Series A and Series B preferred stock issued
December 1999 for cash at $1.007 and $1.20
per share, respectively |
1,029,833 | 1,197 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Series C preferred stock issued July 2000 for
cash at $1.97 per share |
558,374 | 1,100 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Series D preferred stock issued February 2001
for cash at $2.44 per share |
819,673 | 2,000 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Series E preferred stock issued June 2001 to
February 2002 for cash at $6.12 per share |
2,450,980 | 15,000 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Series G-1 preferred stock issued October 2004
for cash at $9.86 per share |
709,939 | 7,000 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Issuance costs on Series G-1 preferred stock |
— | (500 | ) | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
Series G-1 preferred stock issued October 2004
upon conversion of notes payable and
accrued interest at $9.86 per share |
304,260 | 3,000 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Series H preferred stock issued December 2007
for cash at $6.5066 per share |
1,536,901 | 10,000 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Issuance costs on Series H preferred stock |
— | (100 | ) | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
Series H preferred stock issued December 2007
upon conversion of notes payable and
accrued interest at $6.5066 per share |
793,629 | 5,164 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Value of rights in 2007 of possible future
issuances of Series H preferred stock |
— | (3,905 | ) | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
Cumulative dividends in 2007 on Series H preferred
stock at $0.3995 per share per year |
— | 63 | — | — | — | — | (63 | ) | — | — | (63 | ) | |||||||||||||||||||||||||||||
Value of beneficial conversion feature on
convertible notes payable |
— | — | — | — | — | — | 365 | — | — | 365 | |||||||||||||||||||||||||||||||
Fair value of warrants to purchase Series E
and Series F preferred stock reclassified to
long-term liability upon adoption of
accounting pronouncement |
— | — | — | — | — | — | (435 | ) | — | — | (435 | ) | |||||||||||||||||||||||||||||
Change in fair value during 2007 of embedded
conversion features of notes payable |
— | — | — | — | — | — | 744 | — | — | 744 | |||||||||||||||||||||||||||||||
Fair value of warrants issued September 2003 in
connection with notes payable to purchase
82,805 shares of Series E preferred stock |
— | — | — | — | — | — | 315 | — | — | 315 | |||||||||||||||||||||||||||||||
Fair value of warrants issued April 2004 in
connection with notes payable to purchase
53,354 shares of Series F preferred stock |
— | — | — | — | — | — | 230 | — | — | 230 | |||||||||||||||||||||||||||||||
Fair Value of warrants issued December 2007 in
connection with Series H preferred stock to
purchase 466,108 shares of common stock |
— | (210 | ) | — | — | — | — | 210 | — | — | 210 | ||||||||||||||||||||||||||||||
Common stock issued December 1999 to October
2000 for cash at $0.10 to $0.20 per share |
— | — | 910,500 | — | — | — | 106 | — | — | 106 | |||||||||||||||||||||||||||||||
(continued on page 2 of 3) |
|||||||||||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F - 7
Table of Contents
REVA Medical, Inc.
(a development stage company)
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
Period from June 3, 1998 (inception) to December 31, 2010
(in thousands, except share and per share amounts)
(a development stage company)
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
Period from June 3, 1998 (inception) to December 31, 2010
(in thousands, except share and per share amounts)
(page 2 of 3)
| Accumulated | Deficit | Total | |||||||||||||||||||||||||||||||||||||||
| Other | Accumulated | Stock- | |||||||||||||||||||||||||||||||||||||||
| Convertible | Common Stock | Additional | Compre- | During the | holders’ | ||||||||||||||||||||||||||||||||||||
| Preferred Stock | Voting | Non-Voting | Paid-In | hensive | Development | Equity | |||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Income(Loss) | Stage | (Deficit) | ||||||||||||||||||||||||||||||||
(continued from page 1 of 3) |
|||||||||||||||||||||||||||||||||||||||||
Common stock issued February 2001 to October
2006 upon exercise of stock options for cash
at $0.10 to $1.00 per share |
— | — | 1,055,715 | — | — | — | 456 | — | — | 456 | |||||||||||||||||||||||||||||||
Common stock repurchased August 2000 for
cash at $0.0001 per share |
— | — | (189,500 | ) | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
Non-voting common stock issued May 2001 for
technology license valued at $0.25 per share |
— | — | — | — | 481,813 | — | 13 | — | — | 13 | |||||||||||||||||||||||||||||||
Non-voting common stock repurchased August
2004 for cash at $0.25 per share |
— | — | — | — | (353,329 | ) | — | (88 | ) | — | — | (88 | ) | ||||||||||||||||||||||||||||
Non-voting common stock vested July 2005 |
— | — | — | — | — | — | 60 | — | — | 60 | |||||||||||||||||||||||||||||||
Non-cash distribution of assets to stockholders
July 2002 |
— | — | — | — | — | — | (60 | ) | — | — | (60 | ) | |||||||||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 504 | — | — | 504 | |||||||||||||||||||||||||||||||
Translation adjustment |
— | — | — | — | — | — | — | 3 | — | 3 | |||||||||||||||||||||||||||||||
Net loss December 1, 1999 (recapitalization) to
December 31, 2007 |
— | — | — | — | — | — | — | — | (74,173 | ) | (74,173 | ) | |||||||||||||||||||||||||||||
Balance at December 31, 2007 |
9,821,647 | $ | 39,994 | 2,610,745 | $ | — | 128,484 | $ | — | $ | 1,958 | $ | 3 | $ | (74,173 | ) | $ | (72,212 | ) | ||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (12,714 | ) | (12,714 | ) | |||||||||||||||||||||||||||||
Translation adjustment |
— | — | — | — | — | — | — | (1 | ) | — | (1 | ) | |||||||||||||||||||||||||||||
Comprehensive loss |
(12,715 | ) | |||||||||||||||||||||||||||||||||||||||
Series H preferred stock issued September and
December for cash at $6.5066 per share |
3,073,800 | 20,000 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Realized value of rights to possible future
issuances of Series H preferred stock |
— | 1,140 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Fair value of warrants to purchase 614,760 shares
of common stock issued in connection with
Series H preferred stock issuance |
— | (295 | ) | — | — | — | — | 295 | — | — | 295 | ||||||||||||||||||||||||||||||
Cumulative dividends on Series H preferred
stock at $0.3995 per share per year |
— | 1,074 | — | — | — | — | (1,074 | ) | — | — | (1,074 | ) | |||||||||||||||||||||||||||||
Change in fair value of embedded conversion
feature |
— | — | — | — | — | — | 338 | — | — | 338 | |||||||||||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 112 | — | — | 112 | |||||||||||||||||||||||||||||||
Balance at December 31, 2008 |
12,895,447 | $ | 61,913 | 2,610,745 | $ | — | 128,484 | $ | — | $ | 1,629 | $ | 2 | $ | (86,887 | ) | $ | (85,256 | ) | ||||||||||||||||||||||
Net loss and comprehensive loss |
— | — | — | — | — | — | — | — | (13,844 | ) | (13,844 | ) | |||||||||||||||||||||||||||||
Series H preferred stock issued September for
cash at $6.5066 per share |
768,454 | 5,000 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Fair value of warrants to purchase 153,692 shares
of common stock issued in connection with
Series H preferred stock issuance |
— | (200 | ) | — | — | — | — | 200 | — | — | 200 | ||||||||||||||||||||||||||||||
Cumulative dividends on Series H preferred
stock at $0.3995 per share per year |
— | 2,358 | — | — | — | — | (2,056 | ) | — | (302 | ) | (2,358 | ) | ||||||||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 227 | — | — | 227 | |||||||||||||||||||||||||||||||
Balance at December 31, 2009 |
13,663,901 | $ | 69,071 | 2,610,745 | $ | — | 128,484 | $ | — | $ | — | $ | 2 | $ | (101,033 | ) | $ | (101,031 | ) | ||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F - 8
Table of Contents
REVA Medical, Inc.
(a development stage company)
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
Period from June 3, 1998 (inception) to December 31, 2010
(in thousands, except share and per share amounts)
(a development stage company)
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
Period from June 3, 1998 (inception) to December 31, 2010
(in thousands, except share and per share amounts)
(page 3 of 3)
| Accumulated | Deficit | Total | |||||||||||||||||||||||||||||||||||||||
| Other | Accumulated | Stock- | |||||||||||||||||||||||||||||||||||||||
| Convertible | Common Stock | Additional | Compre- | During the | holders’ | ||||||||||||||||||||||||||||||||||||
| Preferred Stock | Voting | Non-Voting | Paid-In | hensive | Development | Equity | |||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Income(Loss) | Stage | (Deficit) | ||||||||||||||||||||||||||||||||
Balance at December 31, 2009 |
13,663,901 | $ | 69,071 | 2,610,745 | $ | — | 128,484 | $ | — | $ | — | $ | 2 | $ | (101,033 | ) | $ | (101,031 | ) | ||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | (23,507 | ) | (23,507 | ) | |||||||||||||||||||||||||||||
Translation adjustment |
— | — | — | — | — | — | — | (2 | ) | — | (2 | ) | |||||||||||||||||||||||||||||
Comprehensive loss |
(23,509 | ) | |||||||||||||||||||||||||||||||||||||||
Series H preferred stock issued June for cash at
$6.5066 per share |
1,075,831 | 7,000 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Proceeds in June from Series H preferred stock
escrow fund |
— | 484 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Fair value of warrants to purchase 215,165 shares
of common stock issued in connection with
Series H preferred stock issuance |
— | (840 | ) | — | — | — | — | 840 | — | — | 840 | ||||||||||||||||||||||||||||||
Purchase for reissuance in March of Series H
preferred stock and warrants to purchase 92,214
shares of common stock for cash at $0.99 per share |
(461,071 | ) | (550 | ) | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||
Reissuance in May of Series H preferred stock and
warrants to purchase 92,214 shares of common
stock for cash at $0.99 per share |
461,071 | 550 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Deemed dividends on Series H preferred stock |
— | — | — | — | — | — | 4,363 | — | (4,363 | ) | — | ||||||||||||||||||||||||||||||
Cumulative dividends on Series H preferred
stock at $0.3995 per share per year |
— | 2,837 | — | — | — | — | (2,837 | ) | — | — | (2,837 | ) | |||||||||||||||||||||||||||||
Change in fair value of embedded conversion feature |
— | — | — | — | — | — | 11,161 | — | — | 11,161 | |||||||||||||||||||||||||||||||
Common stock issued February upon exercise of
stock options for cash at $0.10 to $1.40 per share |
— | — | 2,714 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Common stock issued December upon conversion
of preferred convertible stock |
(14,739,732 | ) | (78,552 | ) | 14,929,713 | 1 | — | — | 78,551 | — | — | 78,552 | |||||||||||||||||||||||||||||
Common stock issued December upon conversion
of non-voting common stock |
— | — | 128,484 | — | (128,484 | ) | — | — | — | — | — | ||||||||||||||||||||||||||||||
Common stock issued December upon conversion
of long-term notes payable and accrued interest |
— | — | 5,638,778 | 1 | — | — | 28,664 | — | — | 28,665 | |||||||||||||||||||||||||||||||
Transfer of repayment premium on long-term notes
payable in December upon conversion of notes |
— | — | — | — | — | — | 11,100 | — | — | 11,100 | |||||||||||||||||||||||||||||||
Common stock issued December upon exercise of
warrants for cash at $3.28 to $6.5066 per share |
— | — | 49,535 | — | — | — | 263 | — | — | 263 | |||||||||||||||||||||||||||||||
Common stock issued December upon net exercise
of warrants at $3.28 to $6.5066 per share |
— | — | 700,034 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Transfer of preferred stock warrant liability in
December upon exercise of warrants |
— | — | — | — | — | — | 1,770 | — | — | 1,770 | |||||||||||||||||||||||||||||||
Common stock issued December upon initial public
offering at $10.9065 per share |
— | — | 7,727,273 | 1 | — | — | 84,277 | — | — | 84,278 | |||||||||||||||||||||||||||||||
Issuance costs of initial public offering |
— | — | — | — | — | — | (8,490 | ) | — | — | (8,490 | ) | |||||||||||||||||||||||||||||
Common stock issued December for cumulative
dividends on Series H convertible preferred stock |
— | — | 973,227 | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | — | 1,185 | — | — | 1,185 | |||||||||||||||||||||||||||||||
Balance at December 31, 2010 |
— | $ | — | 32,760,503 | $ | 3 | — | $ | — | $ | 210,847 | $ | — | $ | (128,903 | ) | $ | 81,947 | |||||||||||||||||||||||
F - 9
Table of Contents
REVA Medical, Inc.
(a development stage company)
(a development stage company)
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
1. Description of Business
REVA Medical, Inc. (“REVA”) was incorporated in California in 1998 under the name MD3, Inc. In
March 2002, we changed our name to REVA Medical, Inc. In October 2010, we reincorporated in
Delaware. We established a non-operating wholly owned subsidiary, REVA Germany GmbH, in 2007. In
these notes the terms “us,” “we,” or “our” refer to REVA and our consolidated subsidiary unless
context dictates otherwise.
In December 2010, we completed an initial public offering of our common stock, as more fully
described in Note 2 below.
We are currently developing proprietary designs and biomaterial technologies that will be used
primarily for a bioresorbable stent to treat vascular disease in humans. We initiated the first
human clinical trial of our bioresorbable stent during 2007 and plan to initiate a pilot clinical
trial in the second quarter of 2011.
2. Stage of Company, Capital Resources, and Basis of Presentation
Development Stage and Capital Resources: We are considered a “development stage” enterprise, as we
have not yet generated revenues from the sale of products. Although we have been researching and
developing new technologies and product applications and have initiated the first human clinical
trial of our bioresorbable stent, we do not anticipate having a product available for sale for at
least the next several years. Until revenue is generated from a saleable product, we expect to
continue to incur substantial operating losses and experience significant net cash outflows. As
discussed below, we completed an initial public offering of our common stock in December 2010. We
believe that we have sufficient capital to fund our operations at least through December 31, 2011.
Initial Public Offering: In December 2010 we completed an initial public offering (the “IPO”) of
our common stock in Australia. We issued 7,727,273 shares of common stock at $10.9065 per share for
gross proceeds of $84,278. We incurred $8,490 in issuance costs in connection with the IPO. Our
stock is traded in the form of CHESS Depository Interests (“CDIs”) on the Australian Securities
Exchange; each share of our common stock is equivalent to ten CDIs. Our trading symbol is “RVA.”
Concurrent with the completion of our IPO, all of our outstanding convertible preferred stock,
non-voting common stock, notes payable, and accrued interest on notes payable converted to common
stock. Additionally, all outstanding warrants were exercised for common stock, either through a
cash payment to us or on a net exercise basis. We also issued common stock for cumulative dividends
on our Series H convertible preferred stock. A total of 22,419,771 shares of common stock were
issued from these conversions, exercises, and dividends.
Consolidation: Our consolidated financial statements, including these notes, include the accounts
of REVA and of our subsidiary, REVA Germany GmbH. All intercompany transactions and balances, if
any, have been eliminated in consolidation.
Use of Estimates: In order to prepare our financial statements in conformity with accounting
principles generally accepted in the United States, we are required to make estimates and
assumptions that affect the reported amounts in the financial statements and accompanying notes.
Our most significant estimates relate to, or have related to, expense accruals and fair market
value determinations of notes payable and embedded conversion features, common and preferred stock
warrants, preferred stock rights liability, and stock-based compensation. Actual results could
differ from our estimates.
F - 10
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
3. Significant Accounting Policies (continued)
Cash and Cash Equivalents: All highly liquid investments with original maturities of three months
or less are classified as cash equivalents.
Short-Term Investments: Excess cash is invested in high-quality marketable securities, primarily
issuances of agencies of the United States government, with maturities of less than one year.
Short-term investments are stated at cost, adjusted for accretion of issuance discounts, which
approximates fair value due to their short-term nature and are classified as held-to-maturity. We
held no short-term investments at December 31, 2009 and December 31, 2010.
Property and Equipment: Property and equipment is stated at cost, less accumulated depreciation
and amortization. Depreciation is determined using the straight-line method over the estimated
useful lives of the related assets, generally five years. Amortization of leasehold improvements is
determined using the straight-line method over the lesser of the useful life of the asset or the
term of the underlying lease. Upon disposition or retirement of an asset, its cost and related
accumulated depreciation or amortization is removed from the accounts and any gain or loss is
recognized in the consolidated statements of operations.
Patents: Costs related to patent development, filing, and maintenance are expensed as incurred
since the underlying technology associated with these assets is purchased or incurred in connection
with our research and development efforts and the future realizable value cannot be determined.
Impairment of Long-Lived Assets: We review our long-lived assets for impairment whenever events or
changes in circumstances indicate that the carrying amount of an asset may not be recoverable and
exceeds its undiscounted future cash flows. The amount of impairment, if any, is determined by
comparing an asset’s estimated fair value to the asset’s respective carrying amount. During the
years ended December 31, 2008, 2009, and 2010 we determined there were no indications of asset
impairment. During the period from June 3, 1998 (inception) through December 31, 2010 we recorded
$502 in losses from impairment of long-lived assets.
Concentrations of Credit Risk: Our cash, cash equivalents, and short-term investments are subject
to concentrations of credit risk to the extent the balances exceed limits that are insured by the
Federal Deposit Insurance Corporation. Cash and cash equivalents are maintained in a bank account,
the balance of which generally exceeds the insured limits. Short-term investments are held in
custody by a large financial asset manager. We maintain our cash balances and investments in
accordance with our investment policy to limit exposure to concentrations of credit risk and
changes in market conditions. We have not experienced any losses in our investments and believe we
are not exposed to significant credit risk related to our cash and cash equivalents.
Preferred Stock Warrant Liability: We record the value of warrants issued for the purchase of
preferred stock as a liability when the warrants provide for issuance of shares that would be
contingently redeemable and, therefore, might require a future transfer of assets. Until the time
the warrants are exercised or expire, the fair value is assessed at each reporting date utilizing
the Black-Scholes model and any change in value is recorded as a component of other income
(expense). The fair value of the preferred stock warrants was estimated to be $780 as of December
31, 2009; there were no warrants outstanding as of December 31, 2010. The increase and decrease in
the fair value was $195, $(215), and $990 for the years ended December 31, 2008, 2009, and 2010,
respectively, and was $1,225 for the period from June 3, 1998 (inception) to December 31, 2010. The
following valuation assumptions were used for these reporting dates:
| Year Ended December 31, | ||||||||||||
| 2008 | 2009 | 2010 | ||||||||||
Assumed risk-free interest rate |
2.3 | % | 1.7 to 2.7 | % | 0.7 to 1.1 | % | ||||||
Assumed volatility |
88.8 | % | 78.4 | % | 63.9 | % | ||||||
Expected life (in years) |
4.0 to 5.3 | 3.0 to 4.3 | 2.0 to 3.3 | |||||||||
Expected dividend yield |
0.0 to 6.1 | % | 0.0 to 6.1 | % | 0.0 to 6.1 | % | ||||||
F - 11
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
3. Significant Accounting Policies (continued)
Preferred Stock Warrant Liability (continued): The preferred stock warrant liability fair value of
$435 along with a corresponding reduction in additional paid-in capital was first recorded in 2007
upon the adoption of new accounting guidance. Upon the exercises of preferred stock warrants in
connection with our IPO (see Note 2) the preferred stock warrant liability was reclassified to
additional paid-in capital.
Research and Development: Research and development costs are expensed as incurred. These costs
include salaries, employee benefits, laboratory supplies, consulting services, manufacturing
products and services, preclinical and clinical costs, technology license fees, laboratory
equipment depreciation, facility costs, and certain indirect costs.
Segment Information: We operate in one business segment, which is the development and
commercialization of medical devices.
Income Taxes: Income taxes are accounted for using the asset and liability method, under which the
current income tax expense or benefit is the amount of income tax expected to be payable or
refundable in the current year. Deferred tax assets and liabilities are recorded for the estimated
future tax consequences of temporary differences between the financial statement carrying amounts
of assets and liabilities and their respective tax bases, and for operating loss and tax credit
carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to
apply to taxable income in the year in which the temporary differences are expected to be recovered
or settled. We evaluate the realizability of our deferred tax assets and establish a valuation
allowance when it is more likely than not that all or a portion of our deferred tax assets will not
be realized.
On January 1, 2009 we adopted new accounting guidance to account for uncertainty in income taxes.
The guidance prescribes a recognition threshold and measurement attribute criteria for financial
statement recognition and measurement of tax positions taken or expected to be taken in a tax
return. To recognize a benefit, a tax position must be more-likely-than-not to be sustained upon
examination by taxing authorities. We do not recognize tax benefits that have a less than 50
percent likelihood of being sustained. We are subject to taxation in U.S. and California
jurisdictions. Our policy is to recognize interest and tax penalties in income tax expense; no
interest or tax penalties have been recorded through December 31, 2010. As of December 31, 2010,
our tax years beginning January 1, 2000 remain subject to examination by taxing authorities.
Stock-Based Compensation: We account for stock-based compensation by measuring and recognizing
expense for all stock-based payments made to employees and directors based on estimated grant date
fair values. We use the straight-line method to allocate compensation expense to reporting periods
over each optionee’s requisite service period, which is generally the vesting period, and estimate
the fair value of stock-based awards to employees and directors using the Black-Scholes option
valuation model. The Black-Scholes model requires the input of subjective assumptions, including
volatility, the expected term, and the fair value of the underlying common stock on the date of
grant, among other inputs. We record the option value to compensation expense based on the
financial statement category for which an optionee’s services are rendered and cash compensation is
recorded. We adjust stock-based compensation expense for estimated option forfeitures based on our
five-year historical average of actual forfeitures.
We account for stock options issued to consultants as expense at their fair value over the related
service period, as determined in accordance with authoritative guidance. We periodically revalue
the stock options as they vest.
Foreign Currency: The functional currency of our subsidiary REVA Germany GmbH is the Euro. Balance
sheet accounts of our subsidiary are translated into United States dollars using the exchange rate
in effect at the balance sheet date while expenses are translated using the average exchange rate
in effect during the period. Gains and losses arising
from translation of our subsidiary’s financial statements are recorded to accumulated other
comprehensive income (loss). These gains and losses, in the aggregate, were insignificant through
December 31, 2010.
F - 12
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
3. Significant Accounting Policies (continued)
Preferred Stock Warrant Liability (continued): The preferred stock warrant liability fair value of
$435 along with a corresponding reduction in additional paid-in capital was first recorded in 2007
upon the adoption of new accounting guidance. Upon the exercises of preferred stock warrants in
connection with our IPO (see Note 2) the preferred stock warrant liability was reclassified to
additional paid-in capital.
Research and Development: Research and development costs are expensed as incurred. These costs
include salaries, employee benefits, laboratory supplies, consulting services, manufacturing
products and services, preclinical and clinical costs, technology license fees, laboratory
equipment depreciation, facility costs, and certain indirect costs.
Segment Information: We operate in one business segment, which is the development and
commercialization of medical devices.
Income Taxes: Income taxes are accounted for using the asset and liability method, under which the
current income tax expense or benefit is the amount of income tax expected to be payable or
refundable in the current year. Deferred tax assets and liabilities are recorded for the estimated
future tax consequences of temporary differences between the financial statement carrying amounts
of assets and liabilities and their respective tax bases, and for operating loss and tax credit
carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to
apply to taxable income in the year in which the temporary differences are expected to be recovered
or settled. We evaluate the realizability of our deferred tax assets and establish a valuation
allowance when it is more likely than not that all or a portion of our deferred tax assets will not
be realized.
On January 1, 2009 we adopted new accounting guidance to account for uncertainty in income taxes.
The guidance prescribes a recognition threshold and measurement attribute criteria for financial
statement recognition and measurement of tax positions taken or expected to be taken in a tax
return. To recognize a benefit, a tax position must be more-likely-than-not to be sustained upon
examination by taxing authorities. We do not recognize tax benefits that have a less than 50
percent likelihood of being sustained. We are subject to taxation in U.S. and California
jurisdictions. Our policy is to recognize interest and tax penalties in income tax expense; no
interest or tax penalties have been recorded through December 31, 2010. As of December 31, 2010,
our tax years beginning January 1, 2000 remain subject to examination by taxing authorities.
Stock-Based Compensation: We account for stock-based compensation by measuring and recognizing
expense for all stock-based payments made to employees and directors based on estimated grant date
fair values. We use the straight-line method to allocate compensation expense to reporting periods
over each optionee’s requisite service period, which is generally the vesting period, and estimate
the fair value of stock-based awards to employees and directors using the Black-Scholes option
valuation model. The Black-Scholes model requires the input of subjective assumptions, including
volatility, the expected term, and the fair value of the underlying common stock on the date of
grant, among other inputs. We record the option value to compensation expense based on the
financial statement category for which an optionee’s services are rendered and cash compensation is
recorded. We adjust stock-based compensation expense for estimated option forfeitures based on our
five-year historical average of actual forfeitures.
We account for stock options issued to consultants as expense at their fair value over the related
service period, as determined in accordance with authoritative guidance. We periodically revalue
the stock options as they vest.
Foreign Currency: The functional currency of our subsidiary REVA Germany GmbH is the Euro. Balance
sheet accounts of our subsidiary are translated into United States dollars using the exchange rate
in effect at the balance sheet
date while expenses are translated using the average exchange rate in effect during the period.
Gains and losses arising from translation of our subsidiary’s financial statements are recorded to
accumulated other comprehensive income (loss). These gains and losses, in the aggregate, were
insignificant through December 31, 2010.
F - 13
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
3. Significant Accounting Policies (continued)
Comprehensive Loss: Comprehensive loss is the change in equity during a period from transactions
and other events and circumstances from non-owner sources. Net loss and other comprehensive loss,
including unrealized gains and losses from foreign currency translations, are reported net of their
related tax effect to arrive at comprehensive loss.
Net Loss Per Common Share: Basic net loss per common share is calculated by dividing the net loss
attributable to common stockholders by the weighted average number of common shares outstanding for
the period, without consideration for common stock equivalents. Diluted net loss per share is
computed by dividing the net loss attributable to common stockholders by the weighted average
number of common share equivalents outstanding for the period determined using the treasury-stock
method and the if-converted method, as applicable. For purpose of this calculation, convertible
preferred stock, common stock options, preferred and common stock warrants, and convertible notes
payable are considered to be common stock equivalents and are included in the calculation of
diluted net loss per share only when their effect is dilutive. The calculation excludes any impact
related to accrued but undeclared dividends.
The following table presents the potential common shares outstanding or common share equivalents
that were excluded from the computation of diluted net loss per share because including them would
have been antidilutive:
| Year Ended December 31, | ||||||||||||
| 2008 | 2009 | 2010 | ||||||||||
Convertible preferred stock |
13,085,428 | 13,853,882 | — | |||||||||
Common stock options |
1,623,000 | 1,563,214 | 3,026,800 | |||||||||
Convertible notes payable and accrued interest |
2,991,461 | 3,124,041 | — | |||||||||
Convertible preferred stock warrants |
289,851 | 289,851 | — | |||||||||
Common stock warrants |
1,080,868 | 1,234,560 | — | |||||||||
| 19,070,608 | 20,065,548 | 3,026,800 | ||||||||||
Fair Value Measurements: On January 1, 2008, we adopted new accounting guidance which defines fair
value, establishes a framework for measuring fair value, and expands disclosures about fair value
measurements. Fair value is defined as the exchange price that would be received for an asset or
paid to transfer a liability (an exit price) in the principal or most advantageous market for the
asset or liability in an orderly transaction between market participants on the measurement date.
Valuation techniques used to measure fair value must maximize the use of observable inputs and
minimize the use of unobservable inputs. We use a fair value hierarchy that is based on three
levels of inputs, of which the first two are considered observable and the last unobservable. Our
assets and liabilities are classified as Level 1, 2, or 3 within the following fair value
hierarchy:
Level 1 — Quoted prices in active markets that we have the ability to access for identical assets
or liabilities;
Level 2 — Inputs other than Level 1 that are directly or indirectly observable, such as quoted
prices for identical or similar assets and liabilities; quoted prices in markets that are not
active; or other inputs that are observable or can be corroborated by observable market data for
substantially the full term of the assets or liabilities such as interest rates, yield curves, and
foreign currency spot rates; and
Level 3 — Unobservable inputs that are supported by little or no market activity and that are
significant to the fair value of the assets and liabilities
F - 14
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
3. Significant Accounting Policies (continued)
Fair Value Measurements (continued): On January 1, 2009, we implemented the new accounting
guidance for nonfinancial assets and liabilities that are remeasured at fair value on a
non-recurring basis. We have not elected to measure any nonfinancial assets or liabilities at fair
value that were not previously required to be remeasured at fair value.
In January 2010, the Financial Accounting Standards Board (“FASB”) issued guidance that expands the
interim and annual disclosure requirements of fair value measurements, including the information
about movement of assets between Level 1 and 2 of the three-tier fair value hierarchy established
under its fair value measurement guidance. This guidance also requires separate disclosure for
purchases, sales, issuances, and settlements in the reconciliation for fair value measurements
using Level 3 methodologies. Except for the detailed disclosure in the Level 3 reconciliation,
which is effective for the fiscal years beginning after December 15, 2010, all the other
disclosures under this guidance became effective during the year ended December 31, 2010. We
adopted the relevant provisions of this guidance effective January 1, 2010.
The adoption of the various elements of the fair value guidance in 2008, 2009, and 2010 did not
result in a material impact to our financial statements in any period.
Assets and Liabilities Measured at Fair Value on a Recurring Basis: As of December 31, 2009, we
classified the $780 in preferred stock warrant liability as a Level 3 within the fair value
hierarchy. We had no assets at December 31, 2009 or 2010 and no liabilities at December 31, 2010
that required measurement at fair value.
Our Level 3 financial liabilities have consisted of long-term liabilities related to warrants
issued for the purchase of preferred stock. Measurement of fair value for the warrants is made
utilizing the Black-Scholes model. Changes in the fair values of the preferred stock warrant
liability is recorded as a component of other income (expense). The Level 3 activity is as follows:
| Level 3 | ||||
Balance at December 31, 2007 |
$ | — | ||
Transfer in upon adoption of fair value guidance: |
||||
Preferred stock rights liability |
3,952 | |||
Preferred stock warrant liability |
800 | |||
Transfer to preferred stock upon settlement |
(1,140 | ) | ||
Change in fair value of preferred stock rights liability |
(2,812 | ) | ||
Change in fair value of preferred stock warrant liability |
195 | |||
Balance at December 31, 2008 |
995 | |||
Change in fair value of preferred stock warrant liability |
(215 | ) | ||
Balance at December 31, 2009 |
780 | |||
Change in fair value of preferred stock warrant liability |
990 | |||
Transfer to additional paid-in capital upon exercise of warrants |
(1,770) | |||
Balance at December 31, 2010 |
$ | — | ||
Fair Value of Financial Instruments not Measured at Fair Value on a Recurring Basis: The carrying
value of certain of our financial instruments are not adjusted to fair value on a recurring basis.
These items include cash, cash equivalents, accounts payable, and accrued expenses and are
considered to be reasonable estimates of their respective fair values due to their short-term
nature.
F - 15
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
3. Significant Accounting Policies (continued)
Recent Accounting Pronouncements: In February 2010, the FASB issued an update to the accounting
standard regarding subsequent events. This update amends the authoritative guidance for subsequent
events that was previously issued and exempts Securities and Exchange Commission registrants from
the requirement to disclose the date through which it has evaluated subsequent events for either
original or restated financial statements. This standard does not apply to subsequent events or
transactions that are within the scope of other applicable generally acceptable accounting
principles that provides different guidance on the accounting treatment for subsequent events or
transactions. Our adoption of this standard did not have a material impact on our financial
statements.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting
bodies that do not require adoption until a future date are not expected to have a material impact
on our financial statements upon adoption.
4. Notes Payable
We had no outstanding notes payable as of December 31, 2010 since all of the notes payable, along
with all accrued interest on the notes, converted to common stock upon our IPO in December 2010
(see Note 2). Our notes payables as of December 31, 2009 were as follows:
| December 31, | ||||
| 2009 | ||||
6.25% Unsecured notes |
$ | 3,800 | ||
Repayment premium on 6.25% notes |
7,600 | |||
6.75% Unsecured convertible notes |
1,750 | |||
Repayment premium on 6.75% notes |
3,500 | |||
2005 Unsecured convertible notes |
10,000 | |||
2006 Unsecured convertible note |
5,000 | |||
Unamortized debt discount |
(246 | ) | ||
| $ | 31,404 | |||
The 6.25 percent unsecured notes were issued between June 2003 and September 2003. The notes
contained provisions for a repayment premium of two times face value and we recorded the premium as
interest expense in 2003 with a corresponding increase in the carrying value of the debt during the
initial term of the notes. Interest on the notes was due at maturity and was not subject to the
repayment premium. For the years ended December 31, 2008 and 2009, interest expense of $238 was
recorded each year. For the year ended December 31, 2010 and the period from June 3, 1998
(inception) to December 31, 2010, interest expense was $228 and $1,767, respectively. The notes
originally matured in 2003; their maturities were extended to December 31, 2011 through a series of
amendments between 2003 and 2010. During the year ended December 31, 2010, we amended the notes to
require conversion into common stock at a rate of $3.28 per share if our IPO successfully
completed; the notes and accrued interest converted into 1,697,297 shares of common stock in
December 2010. We reclassified the repayment premium to additional paid-in capital upon conversion
of the notes. In connection with issuing these notes, we issued warrants to the note holders to
purchase up to 82,805 shares of Series E preferred stock at $6.0383 per share. The warrants were
exercisable immediately and expired in 2013. The value of the warrants was calculated to be $315,
which was recorded as interest expense in 2003.
During December 2010, the warrants were exercised on a net issuance basis and we issued 37,160
shares of common stock to the holders.
F - 16
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
4. Notes Payable (continued)
The 6.75 percent unsecured convertible notes were issued between November 2003 and April 2004 and
were convertible into Series F preferred stock at the option of the note holders at a $3.28 per
share conversion rate. These notes also contained provisions for repayment premiums of two times
face value; we recorded the premium as interest expense during 2003 and 2004 with a corresponding
increase in the carrying value of the debt. Interest on the notes was subject to conversion at the
option of the note holders, and was not subject to the repayment premium. For the years ended
December 31, 2008 and 2009, interest expense of $118 was recorded each year. For the year ended
December 31, 2010 and the period from June 3, 1998 (inception) to December 31, 2010, respectively,
interest expense of $113 and $819 was recorded. The notes’ original maturity date of May 2004 was
extended to December 31, 2011 through a series of amendments between 2004 and 2010. During the year
ended December 31, 2010, we amended the notes to require conversion into common stock if our IPO
successfully completed; the notes and accrued interest converted into 783,317 shares of common
stock in December 2010. We reclassified the repayment premium to additional paid-in capital upon
conversion of the notes. In connection with issuing these notes, we had issued warrants to the note
holders to purchase up to 53,354 shares of Series F preferred stock at $3.28 per share. The
warrants were exercisable and expired in 2014. The value of the warrants was calculated to be $230,
which was recorded as interest expense in 2004. During December 2010, the warrants were exercised
for cash and on a net issuance basis and we issued 42,859 shares of common stock to the holders.
Between June 2005 and July 2006, we issued $15,000 in unsecured convertible notes payable to a
single holder. The notes accrued interest at the prime rate plus one percent, compounded annually,
and payment of the interest was deferred until December 31, 2011 or later. The notes were
convertible in certain circumstances at any time at the holder’s option into our Series G-1
preferred stock at $9.86 per share. For the years ended December 31, 2008, 2009, and 2010 and for
the period from June 3, 1998 (inception) to December 31, 2010, respectively, interest expense of
$1,082, $802, $803, and $5,528 was recorded. The notes’ original maturity dates were extended to
December 31, 2011 or later through a series of amendments between 2007 and 2010. During the year
ended December 31, 2010, we amended the conversion rate of the notes to $6.50 per share and added a
requirement for conversion into common stock if our IPO successfully completed; the notes and
accrued interest converted into 3,158,164 shares of common stock in December 2010.
Since the dates of origination of the various convertible and non-convertible notes payable
discussed above, there have been a number of amendments to the underlying terms of the notes,
primarily to extend the notes’ maturity dates and change certain conversion provisions. At each
amendment date, we performed an analysis based on the applicable accounting guidelines to determine
if the amendment resulted in an accounting impact. We first considered whether the amendment would
qualify as a troubled debt restructuring. If the amendment was not considered a troubled debt
restructuring, we considered whether the amendment should be accounted for as an extinguishment or
a modification of debt. If the note had an embedded conversion feature and the amendment was
considered a modification, rather than an extinguishment, then any increase in the fair value of
the conversion feature as a result of the amendment was accounted for as a reduction in the
carrying amount of the note, as an additional discount, with a corresponding increase in additional
paid-in capital. If the amendment was considered to cause an extinguishment, we record a loss on
extinguishment to other income and expense for the carrying value of the note payable at the time
of modification and also determine a new fair value for the note and record such fair value as a
liability with a corresponding decrease in additional paid-in capital. We amortize or accrete any
resulting premium or discount over the term of the note using the effective interest method as
interest expense in our statement of operations. The impact of the accounting for the various
amendments to the notes payable resulted in a loss on extinguishment of notes payable of $13,285
for the year ended December 31, 2010 and an increase or decrease in interest expense for the years
ended December 31, 2008, 2009, and 2010 and for the period from June 3, 1998 (inception) to
December 31, 2010 of $399, $421, $(1,878), and $(1,042), respectively. The balance of the
unamortized debt discount related to the notes payable was $246 as of
December 31, 2009. Since all the notes converted to common stock upon our IPO in December 2010, no
related discounts or premiums remained at December 31, 2010.
F - 17
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
5. Balance Sheet Details
| December 31, | ||||||||
| 2009 | 2010 | |||||||
Property and equipment: |
||||||||
Furniture and office equipment |
$ | 332 | $ | 351 | ||||
Laboratory equipment |
2,131 | 2,400 | ||||||
Leasehold improvements |
559 | 559 | ||||||
| 3,022 | 3,310 | |||||||
Accumulated depreciation and amortization |
(1,888 | ) | (2,347 | ) | ||||
| $ | 1,134 | $ | 963 | |||||
Accrued expenses and other current liabilities: |
||||||||
Accrued salaries and other employee costs |
$ | 199 | $ | 353 | ||||
Accrued operating expenses |
112 | 192 | ||||||
Accrued use taxes |
51 | 15 | ||||||
Deferred rent |
37 | 31 | ||||||
| $ | 399 | $ | 591 | |||||
6. Income Taxes
We have reported net losses for all periods through December 31, 2010; therefore, no provision for
income taxes has been recorded.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying
amount of assets and liabilities for financial reporting purposes and the amounts used for income
tax purposes. Significant components of our deferred tax assets and liabilities at December 31,
2009 and 2010 are as follows:
| December 31, | ||||||||
| 2009 | 2010 | |||||||
Deferred Tax Assets: |
||||||||
Interest on notes payable |
$ | 2,776 | $ | — | ||||
Investment write-off loss carryforward |
1,034 | 1,034 | ||||||
Depreciation |
46 | 154 | ||||||
Accrued operating expenses |
39 | 38 | ||||||
Other |
62 | 64 | ||||||
| 3,957 | 1,290 | |||||||
Less: valuation allowance |
(3,957 | ) | (1,290 | ) | ||||
Net deferred tax assets |
$ | — | $ | — | ||||
At December 31, 2010 we had aggregate federal and California state net operating loss carryforwards
of approximately $98,000 and $95,000, respectively, which may be available to offset future taxable
income for income tax purposes. The federal net operating loss carryforwards begin to expire in
2019 and the California carryforwards begin to expire in 2011. At December 31, 2010, we also had
federal and California state research tax credit carryforwards of
approximately $3,250 and $2,980, respectively. The federal carryforwards begin to expire in 2020
and the California carryforwards have no expiration. Additionally, at December 31, 2010, we had
California manufacturers’ investment tax credit carryforwards of approximately $56 that begin to
expire in 2011.
F - 18
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
6. Income Taxes (continued)
Under the Internal Revenue Code (“IRC”) Sections 382 and 383, annual use of our net operating loss
and research tax credit carryforwards to offset taxable income may be limited based on cumulative
changes in ownership. We have not completed an IRC Section 382/383 analysis regarding the
limitations on carryforwards. Until this analysis is completed, we have removed the deferred tax
assets for net operating losses and research credits from our deferred tax asset schedule and
recorded a corresponding decrease in the valuation allowance. At December 31, 2009 and 2010, our unrecognized income tax benefits and uncertain tax positions were not material and would not, if recognized, affect the effective tax rate. We do not expect these tax positions
to change within 12 months after December 31, 2010 and, as a result, do not expect that the
unrecognized tax benefits will change by December 31, 2011. We recognize interest and/or penalties related to uncertain tax positions as a component of income tax expense. For the years ended December 31, 2009 and 2010, we did not recognize any interest or penalties.
We have established a valuation allowance against our net deferred tax assets due to the
uncertainty surrounding the realization of those assets. We periodically evaluate the
recoverability of the deferred tax assets and, when it is determined that it is
more-likely-than-not that the deferred tax assets are realizable, the valuation allowance will be
reduced. Due to the existence of the valuation allowance, future changes in our unrecognized tax
benefits will not impact our effective tax rate.
The following table provides reconciliation between income taxes computed at the federal statutory
rate and our provision for income taxes:
| Year Ended December 31, | ||||||||||||
| 2008 | 2009 | 2010 | ||||||||||
Federal income taxes at 34% |
$ | (4,323 | ) | $ | (4,707 | ) | $ | (7,993 | ) | |||
State income taxes, net of federal benefit |
(741 | ) | (807 | ) | (1,370 | ) | ||||||
Research and development credits |
(809 | ) | (760 | ) | (682 | ) | ||||||
Stock-based compensation expense |
45 | 90 | 472 | |||||||||
Increase in valuation allowance |
6,631 | 6,061 | 310 | |||||||||
Loss on extinguishment of notes payable |
— | — | 5,291 | |||||||||
Loan premium payoff |
— | — | 4,421 | |||||||||
Change in fair value of preferred warrants |
78 | (86 | ) | 394 | ||||||||
Change in fair value of preferred stock
purchase rights |
(1,120 | ) | — | — | ||||||||
Other |
239 | 209 | (843 | ) | ||||||||
| $ | — | $ | — | $ | — | |||||||
7. Convertible Preferred Stock
All of our outstanding convertible preferred stock converted into 14,929,713 shares of common stock
upon our IPO in December 2010 and we have not subsequently issued any new preferred stock.
Dividends: The holders of Series H convertible preferred stock were entitled to receive cumulative
dividends in preference to any declaration or payment of dividends on common stock at the rate of
six percent per annum, compounded quarterly, which equated to $0.3995 per share. We recorded the
liquidation value of $6,332 for these dividends through the date of our IPO by increasing the
carrying value of the Series H convertible preferred stock and reducing additional paid-in capital,
or in the case where we had no remaining additional paid-in capital, we increased our deficit
accumulated in the development stage. These dividends became payable upon our IPO in December 2010;
as a result, we issued 973,227 shares of common stock for the Series H cumulative dividends. No
other series of preferred stock was entitled to receive dividends.
F - 19
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
7. Convertible Preferred Stock (continued)
Series H Convertible Preferred Stock: During 2007, we issued certain rights to purchase Series H
preferred stock for $6.5066 per share, with such purchases to occur when technology milestones were
achieved in 2008 and 2009. The fair value of the purchase rights was estimated to be $3,905 upon
their issuance in 2007 and was recorded as a reduction to the carrying value of Series H preferred
stock and as a long-term liability that would convert to preferred stock as purchases occurred
under the purchase rights. At each subsequent reporting date, the fair value of any remaining
purchase rights was recalculated and any change in fair value was recorded as a component of other
income and expense. As of December 31, 2008, the fair value of the remaining purchase rights was
determined to be nil, as future milestones were deemed to be unachievable within scheduled
timeframes. As such, we reclassified the remaining fair value of $1,140 to increase the carrying
value of the Series H preferred stock. For the year ended December 31, 2008, the change in fair
value of the purchase rights liability resulted in $2,812 of income. The fair value of the
remaining purchase rights liability as of December 31, 2009 was also determined to be nil. All
purchases under the purchase rights were completed in June 2010.
During June 2010, we completed the sale of 1,075,831 shares of Series H convertible preferred stock
for net proceeds of approximately $7,484, including $484 in proceeds from the related escrow fund.
This Series H convertible preferred stock was sold at a price per share below the estimated fair
value of our common stock. Accordingly, we recorded a deemed dividend on the Series H convertible
preferred stock of $693, which is equal to the number of shares of Series H convertible preferred
stock sold multiplied by the difference between the estimated fair value of the underlying common
stock and the Series H conversion price per share. The deemed dividend was recognized as an
adjustment to the net loss attributable to common stockholders since the preferred stock was
convertible, but not mandatorily redeemable.
Limitations on Ability to Declare Dividends and Certain Other Transactions: Under the terms of
certain agreements, including our facility lease, we are subject to limitations on our ability to
pay dividends, incur liens or additional debt, redeem stock, and engage in merger, consolidation,
or asset sale transactions, among other restrictions.
Preferred Stock Warrants: In conjunction with the issuance of certain of our notes payable, we
issued warrants to purchase shares of Series E and Series F convertible preferred stock; see Note 4
regarding the terms of those warrants.
In conjunction with the issuance of certain notes payable that were converted into Series H
preferred stock during 2007, we issued warrants to purchase 153,692 shares of Series H convertible
preferred stock with an exercise price of $6.5066 per share. These warrants were exercisable and
had an expiration date of December 7, 2012. During December 2010, the warrants were exercised for
cash and on a net issuance basis and we issued 71,531 shares of common stock to the holders.
Common Stock Warrants: Holders of Series H convertible preferred stock received warrants to
purchase our common stock at the rate of one common warrant for every five Series H shares
purchased. As a result, we issued warrants to purchase 1,449,725 shares of common stock between
December 2007 and June 2010. The values of these warrants were calculated to be $210, $295, $200,
and $840 at their respective issuance dates and were recorded as additional paid-in capital and a
reduction to the carrying value of the Series H convertible preferred stock. The warrants were
exercisable at $6.5066 per share and had an expiration date of December 7, 2012. During December
2010, the warrants were exercised for cash and on a net issuance basis and we issued 598,019 shares
of common stock to the holders.
F - 20
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
8. Stock-Based Compensation
Our 2010 Equity Incentive Award Plan was adopted in October 2010; this plan was a follow-on to our
2001 Stock Option/Stock Issuance Plan, which would expire in 2011. The two plans are collectively
referred to herein as the “Plan.” The Plan provides for awards of incentive and non-qualified stock
options to purchase up to 4,179,338 shares of our common stock at an option price per share of at
least 85 percent of the fair market value of our common stock on the date of grant. The Plan
provides for an annual increase to the number of shares reserved for issuance under the Plan equal
to three percent of the outstanding shares of the Company. The term of the options granted under
the Plan may not exceed ten years. The Plan also provides for restricted stock awards. Option and
award vesting is determined by the Board of Directors upon each grant and is generally over a four-
or five-year period. All options are immediately exercisable upon grant and are subject to
repurchase by us at the exercise price in the event an employee terminates service prior to being
vested.
Activity under the Plan is as follows:
| Weighted | ||||||||||||||||
| Weighted | Average | |||||||||||||||
| Average | Remaining | Aggregate | ||||||||||||||
| Options | Exercise | Contractual | Intrinsic | |||||||||||||
| Outstanding | Price | Term (years) | Value | |||||||||||||
Balance at December 31, 2007 |
988,000 | $ | 1.03 | |||||||||||||
Granted |
643,500 | $ | 1.40 | |||||||||||||
Cancelled |
(21,000 | ) | $ | 1.14 | ||||||||||||
Balance at December 31, 2008 |
1,610,500 | $ | 1.17 | |||||||||||||
Granted |
50,000 | $ | 1.80 | |||||||||||||
Cancelled |
(109,786 | ) | $ | 1.24 | ||||||||||||
Balance at December 31, 2009 |
1,550,714 | $ | 1.19 | |||||||||||||
Granted |
1,467,500 | $ | 11.00 | |||||||||||||
Cancelled |
(1,200 | ) | $ | 1.40 | ||||||||||||
Exercised |
(214 | ) | $ | 1.40 | ||||||||||||
Balance at December 31, 2010 |
3,016,800 | $ | 5.96 | 7.62 | $ | 19,428 | ||||||||||
Vested at December 31, 2010 |
1,163,600 | $ | 1.11 | 4.74 | $ | 13,137 | ||||||||||
All options outstanding under the Plan as of December 31, 2010 were exercisable and no options have
expired since inception of the Plan. At December 31, 2010, we had approximately $8,737 of total
unrecognized compensation costs related to unvested employee options that are expected to be
recognized over a weighted average period of 3.62 years.
Stock-based compensation included in research and development expense totaled $62, $133, and $320
for the years ended December 31, 2008, 2009, and 2010, respectively. Stock-based compensation
included in general and administrative expense totaled $50, $94, and $865 for the years ended
December 31, 2008, 2009, and 2010, respectively.
No tax benefits arising from stock-based compensation have been recognized in the consolidated
statements of operations through December 31, 2010.
The fair value of options vested during the years ended December 31, 2008, 2009, and 2010 was $111,
$198, and $946 respectively.
F - 21
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
8. Stock-Based Compensation (continued)
The fair value of grants during the year ended December 31, 2010 was determined to be $6.26 per
share based on the following value assumptions: assumed risk-free
interest rate of 1.85%;
assumed volatility of 63.9%; expected option life of 6.5 years; and, expected dividend yield of
zero percent. No grants to employees were made in 2009. During the year ended December 31, 2008, we
made one grant of options for which the fair market value was estimated to $1.07 per share based in
the following value assumptions: assumed risk-free interest rate of
2.82%; assumed
volatility of 88.8%; expected option life of 6.5 years; and, expected dividend yield of zero
percent.
The assumed risk-free interest rate was based on the implied yield on a U.S. Treasury zero-coupon
issue with a remaining term equal to the expected life of the option. The assumed volatility was
calculated from the historical market prices of a selected group of publicly traded companies
considered to be our peers. We used peer group data due to the fact that we have no historical
trading data. The expected option life was calculated using the simplified method under the
accounting standard for stock compensation and a ten-year option expiration. The simplified method
is used since we believe our option activity as a public company will differ from that of our own
historical experience. The expected dividend yield of zero reflects that we have not paid cash
dividends since inception and do not intend to pay cash dividends in the foreseeable future.
During 2009, consultants were granted options to purchase 50,000 shares of common stock. During
2008 and for the year ended December 31, 2010 we did not grant options to consultants. Grants to
consultants resulted in stock compensation expense of $2, $30, $239, and $383 during the years
ended December 31 2008, 2009, and 2010 and for the period from June 3, 1998 (inception) to December
31, 2010, respectively. The fair value of these awards was determined using the Black-Scholes model
with the following assumptions: Assumed risk-free interest rate of 1.3% to 3.4%; assumed
volatility of 64% to 80%; expected option life of 5.5 to 8.7 years; and, expected dividend yield of
zero percent.
Prior to establishment of the Plan, we had issued non-qualified options to purchase common stock to
certain employees and consultants under similar terms to those issued under the Plan. Options to
purchase a total of 577,500 shares were issued. Through December 31, 2010, a total of 492,000
shares have been purchased at a weighted average purchase price of $0.15 per share, a total of
75,500 shares have been forfeited, and a total of 10,000 shares with a weighted average exercise
price of $0.25 per share and a weighted average remaining life of 0.2 years remain outstanding.
9. Retirement Plan
During 2003 we adopted a qualified 401(k) profit sharing plan (the “401(k) Plan”) for the benefit
of our employees. Employees are eligible to participate in the 401(k) Plan the month following hire
and may defer up to 25 percent of their total compensation, up to the maximum allowed under IRS
regulations, on an annual basis. We are required to match 25 percent of an employee’s deferral
amount, up to a maximum of four percent of the employee’s compensation. We may, at our discretion,
make additional contributions. Employees are immediately vested in the employer matching
contributions. Our contributions to the 401(k) Plan were $27, $28, $24, and $171 for the years
ended December 31, 2008, 2009, and 2010 and for the period from June 3, 1998 through December 31,
2010, respectively.
F - 22
Table of Contents
Notes to Consolidated Financial Statements
(in thousands, except share, per share, and per unit amounts)
(in thousands, except share, per share, and per unit amounts)
10. Commitments and Contingencies
We have licensed certain patents and other intellectual property rights related to the composition
and coating of our bioresorbable stent and our other biomaterial products. Terms of these licenses
include provisions for royalty payments on any future sales of products, if any, utilizing this
technology, with provisions for minimum royalties once product sales begin. The amount of royalties
varies depending upon type of product, use of product, stage of product, location of sale, and
ultimate sales volume, and ranges from a minimum of approximately $70.00 per unit to a maximum of
approximately $100.00 per unit sold, with license provisions for escalating minimum royalties that
could be as high as $2,200 per year. Additionally, in the event we sublicense the technology and
receive certain milestone payments, the licenses require that 20 percent of the milestone amount be
paid to the licensors.
Additional terms of the technology licenses included annual licensing payments of $150 in 2008 and
$175 in 2009 and 2010 and annually thereafter until the underlying technology has been
commercialized. Terms of the licenses also include other payments to occur during commercialization
that could total $950, payment of $350 upon a change in control of ownership, and payment of patent
filing, maintenance, and defense fees. The license terms remain in effect until the last patent
expires.
In connection with our development activities, we periodically enter into contracts with
consultants and vendors. These contracts are generally cancelable with 30 days’ written notice. As
of December 31, 2010 the minimum future payments on these contracts totaled approximately $196.
We currently lease our office facilities under non-cancelable operating leases that expire in
August 2011 and October 2011. We recorded rent expense of $320, $394, $436, and $2,942 for the
years ended December 31, 2008, 2009, and 2010 and for the period from June 3, 1998 (inception)
through December 31, 2010, respectively. Future minimum payments under the leases as of December
31, 2010 total $289.
11. Related Parties
Our related parties include the members of our Board of Directors and investors with five percent
or more of our outstanding securities. As of December 31, 2010, our related parties collectively
represented approximately 65 percent of our outstanding voting stock. We had issued long-term debt
to certain of these related parties; these related party transactions are disclosed in our
consolidated balance sheets and statements of operations.
12. Selected Quarterly Financial Information (unaudited)
The following table presents our unaudited quarterly results of operations for 2009 and 2010. The
sum of the quarterly per share amounts may not equal the amounts presented for the full year due to
differences in the weighted average number of shares outstanding as calculated quarterly compared
to annually.
| Quarter | ||||||||||||||||||||
| First | Second | Third | Fourth | Year | ||||||||||||||||
2009: |
||||||||||||||||||||
Loss from operations |
$ | (3,068 | ) | $ | (3,311 | ) | $ | (3,049 | ) | $ | (3,085 | ) | $ | (12,513 | ) | |||||
Net loss |
(3,383 | ) | (3,645 | ) | (3,391 | ) | (3,425 | ) | (13,844 | ) | ||||||||||
Net loss attributable to common stockholders |
(3,928 | ) | (4,203 | ) | (3,987 | ) | (4,084 | ) | (16,202 | ) | ||||||||||
Net loss per common share, basic and diluted |
$ | (1.43 | ) | $ | (1.53 | ) | $ | (1.46 | ) | $ | (1.49 | ) | $ | (5.91 | ) | |||||
2010: |
||||||||||||||||||||
Loss from operations |
$ | (2,231 | ) | $ | (2,618 | ) | $ | (2,491 | ) | $ | (2,778 | ) | $ | (10,118 | ) | |||||
Net loss |
(2,976 | ) | (3,387 | ) | (3,073 | ) | (14,071 | ) | (23,507 | ) | ||||||||||
Net loss attributable to common stockholders |
(3,631 | ) | (8,456 | ) | (3,870 | ) | (14,750 | ) | (30,707 | ) | ||||||||||
Net loss per common share, basic and diluted |
$ | (1.33 | ) | $ | (3.08 | ) | $ | (1.41 | ) | $ | (1.93 | ) | $ | (7.72 | ) | |||||
F - 23
Table of Contents
INDEX TO EXHIBITS
| Exhibit | ||
| Number | Description of Exhibits | |
|
3.1
|
Amended and Restated Certificate of Incorporation.* | |
|
3.2
|
Amended and Restated Bylaws to be effective upon completion of this offering.* | |
|
4.1
|
Form of Stock Certificate.* | |
4.2
|
Form of Amended and Restated Investors’ Rights Agreement, by and among REVA Medical, Inc. and the holders of our preferred stock set forth therein.* | |
10.1
|
Telecom Business Center Business Lease between FSP Telecom Business Center Limited Partnership and REVA Medical, Inc. dated December 18, 2001.* | |
10.2
|
First Amendment to Telecom Business Center Business Lease between FSP Telecom Business Center Limited Partnership and REVA Medical, Inc. dated January 3, 2005.* | |
10.3
|
Second Amendment to Telecom Business Center Business Lease between ARI Commercial Properties, Inc. and REVA Medical, Inc. dated February 18, 2006.* | |
10.4
|
Third Amendment to Telecom Business Center Business Lease between ARI Commercial Properties, Inc. and REVA Medical, Inc. dated December 14, 2006.* | |
10.5
|
Fourth Amendment to Telecom Business Center Business Lease between ARI Commercial Properties, Inc. and REVA Medical, Inc. dated May 7, 2008.* | |
10.6
|
Agreement and Plan of Merger, dated October 13, 2004, by and among REVA Medical, Inc., Boston Scientific Corporation, RMI Acquisition Corp. and certain stockholder representatives set forth therein.* | |
10.7
|
Amendment No. 1 to the Agreement and Plan of Merger, dated December 7, 2007, by and among REVA Medical, Inc., Boston Scientific Corporation, RMI Acquisition Corp. and certain stockholder representatives set forth therein.* | |
10.8
|
Securities Purchase Agreement between Boston Scientific Corporation and REVA Medical, Inc. dated October 13, 2004.* | |
10.9
|
Amendment No. 1 to Securities Purchase Agreement between Boston Scientific Corporation and REVA Medical, Inc. dated December 7, 2007.* | |
10.10
|
Distribution Option Agreement, dated December 7, 2007, by and between REVA Medical, Inc. and Boston Scientific Corporation.* | |
10.11
|
Exclusive License Agreement Number between Rutgers, The State University of New Jersey and REVA Medical, Inc. dated July 1, 2010.++* | |
10.12
|
Royalty and License Agreement between Integra/LifeSciences Corporation and REVA Medical, Inc. dated February 2, 2004.++* | |
10.13
|
2001 Stock Option/Stock Issuance Plan.+* | |
10.14
|
Form of Stock Option Agreement.+* | |
10.15
|
Form of Addendum to Stock Option Agreement.+* | |
10.16
|
2010 Equity Incentive Plan.+* | |
10.17
|
Form of Stock Option Agreement.+* | |
10.18
|
Form of Stock Option Agreement entered into with Robert Thomas and Anne Keating.+* | |
10.19
|
Form of Director and Officer Indemnification Agreement.+* | |
10.20
|
Employment Agreement, dated July 1, 2010, by and between REVA Medical, Inc. and Robert B. Stockman.+* | |
10.21
|
Employment Agreement, dated October 21, 2010, by and between REVA Medical, Inc. and Robert Schultz.+* | |
10.22
|
Employment Agreement, dated October 21, 2010, by and between REVA Medical, Inc. and Katrina Thompson.+* | |
10.23
|
Form of Offer Management Agreement between REVA Medical, Inc. and Inteq Limited.* | |
10.24
|
Form of CDI Subscription Application for non U.S. investors.* | |
10.25
|
Form of CDI Subscription Application for U.S. investors.* | |
10.26
|
Form of Escrow Deed between REVA Medical, Inc. and Computershare Investor Services Pty Limited.* | |
21.1
|
List of Subsidiaries.* |
Table of Contents
| Exhibit | ||
| Number | Description of Exhibits | |
31.1
|
Certification of Principal Executive Officer Required Under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended. | |
31.2
|
Certification of Principal Financial Officer Required Under Rule 13a-14(a) and 15d-14(a) of the Securities Exchange Act of 1934, as amended. | |
32.1
|
Certification of Principal Executive Officer and Principal Financial Officer Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. §1350. | |
99.1
|
Section 13 of the ASX Settlement Rules.* |
| + | Management Compensation Plan | |
| ++ | Confidential Treatment Request | |
| * | Filed as exhibits to the registrant’s Registration Statement on Form S-1 (File No. 333-168852), effective November 15, 2010, and incorporated herein by reference. |
