Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a12-19541_18k.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a12-19541_1ex99d1.htm |
| EX-99.3 - EX-99.3 - Innoviva, Inc. | a12-19541_1ex99d3.htm |
| EX-99.5 - EX-99.5 - Innoviva, Inc. | a12-19541_1ex99d5.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a12-19541_1ex99d2.htm |
| EX-99.6 - EX-99.6 - Innoviva, Inc. | a12-19541_1ex99d6.htm |
| EX-99.9 - EX-99.9 - Innoviva, Inc. | a12-19541_1ex99d9.htm |
| EX-99.4 - EX-99.4 - Innoviva, Inc. | a12-19541_1ex99d4.htm |
| EX-99.8 - EX-99.8 - Innoviva, Inc. | a12-19541_1ex99d8.htm |
| EX-99.10 - EX-99.10 - Innoviva, Inc. | a12-19541_1ex99d10.htm |
| EX-99.12 - EX-99.12 - Innoviva, Inc. | a12-19541_1ex99d12.htm |
| EX-99.14 - EX-99.14 - Innoviva, Inc. | a12-19541_1ex99d14.htm |
| EX-99.16 - EX-99.16 - Innoviva, Inc. | a12-19541_1ex99d16.htm |
| EX-99.11 - EX-99.11 - Innoviva, Inc. | a12-19541_1ex99d11.htm |
| EX-99.15 - EX-99.15 - Innoviva, Inc. | a12-19541_1ex99d15.htm |
| EX-99.13 - EX-99.13 - Innoviva, Inc. | a12-19541_1ex99d13.htm |
Exhibit 99.7
POSTER P1795
Efficacy and safety of fluticasone furoate (FF)/vilanterol (VI) compared with fluticasone propionate/salmeterol combination (FP/SAL) in adults and adolescents with persistent asthma
Woodcock A(1), Bleecker ER(2), Lötvall J(3), O’Byrne PM(4), Bateman ED(5), Medley H(6), Ellsworth A(7), Jacques L(6), Busse WW(8)
(1)University of Manchester, Manchester, UK; (2)Wake Forest University Health Sciences Winston-Salem, NC, USA; (3)University of Gothenburg, Sweden; (4)Michael G DeGroote School of Medicine, Hamilton, Ontario, Canada; (5)University of Cape Town, Cape Town, South Africa; (6)Respiratory Medicines Development Centre, GlaxoSmithKline, London, UK; (7)Quantitative Sciences Division, GlaxoSmithKline, Research Triangle Park, NC, USA; (8)University of Wisconsin, Madison, WI, USA
INTRODUCTION
· For asthma patients symptomatic despite ICS therapy, a long-acting beta2 agonist (LABA) may be added.(1),(2)
· Current ICS/LABA combinations for asthma such as FP/SAL are administered twice daily; once-daily dosing of ICS/LABA may improve symptom control by improving patient adherence.
· The combination of FF (an ICS) and VI (a LABA) is in development as a once-daily therapy for asthma.
OBJECTIVES
· To compare the efficacy of FF/VI 100/25mcg administered once daily in the evening with FP/SAL 250/50mcg administered twice daily (morning and evening) over a 24-week treatment period in patients >12 years of age with persistent asthma.
METHODS
· Phase III, multi-centre, randomised, double-blind, double-dummy, parallel group study.
· Eligible patients: aged >12 years; >12% and >200mL reversibility of FEV1 with salbutamol; evening pre-bronchodilator FEV1 40–85% pred; documented use of ICS for >12 weeks with stable ICS dose (FP 250mcg twice daily or equivalent) for >4 weeks.
· After 4-week run-in on FP 250mcg twice daily, patients were randomised to 24 weeks’ treatment with
· FF/VI 100/25mcg once daily (evening dosing) via novel dry power inhaler
· FP/SAL 250/50mcg twice daily via DISKUSTM/ACCUHALERTM.
RESULTS
Study population and demographics (Table 1)
· Of 1564 patients screened, 806 were randomised (intent-to-treat [ITT] population) and 715 completed the study.
Efficacy
· Clinically important improvements from baseline in 0–24h serial weighted mean (wm) FEV1 after 24 weeks (primary endpoint) were seen with both FF/VI (341mL) and FP/SAL (377mL)
· the adjusted mean treatment difference was not statistically significant (–37mL [95% CI: –88, 15], p=0.162; Fig. 1).
Table 1. Patient demographics and baseline lung function (ITT population)
|
|
|
FF/VI |
|
FP/SAL |
|
|
|
|
100/25mcg |
|
250/50mcg |
|
|
|
|
once daily |
|
twice daily |
|
|
|
|
(N=403) |
|
(N=403) |
|
|
Mean age, years (range) |
|
43.8 (12–79) |
|
41.9 (12–80) |
|
|
Female, n (%) |
|
244 (61) |
|
245 (61) |
|
|
Race, n (%) |
|
|
|
|
|
|
White |
|
242 (60) |
|
232 (58) |
|
|
Asian |
|
124 (31) |
|
125 (31) |
|
|
African American/African heritage |
|
36 (9) |
|
43 (11) |
|
|
Other(a) |
|
1 (<1) |
|
3 (<1) |
|
|
%reversibility of FEV1(b), L, mean (SD) |
|
26.4 (14.44) |
|
29.0 (18.04) |
|
|
Pre-dose FEV1 (L), mean (SD) |
|
2.011 (0.6389) |
|
2.048 (0.6246) |
|
|
%predicted FEV1 , mean (SD) |
|
68.0 (11.68) |
|
68.8 (11.01) |
|
(a)Native Hawaiian or other Pacific islander (n=1 [<1%] FF/VI; n=1 [<1%] FP/SAL); African American/African heritage and White (n=0 FF/VI; n= 2 [<1%] FP/SAL)
(b)Screening values
Figure 1. Adjusted means for 0–24h wmFEV1 at Week 24 (ITT population)

LS=least squares; CI=confidence interval
· No statistically significant differences were reported between FF/VI and FP/SAL for serial wmFEV1 (0–4h) and clinic visit trough FEV1 (secondary endpoints).
· A greater number of patients receiving FF/VI vs. FP/SAL had an improvement of >0.5 points (minimally important difference)(3) from baseline in their Total Asthma Quality of Life Questionnaire (+12) score (‘other’ endpoint; post-hoc analysis) at Week 24 (46% vs. 38%).
Safety
· Incidences of on-treatment adverse events (AEs), treatment-related AEs and serious AEs (SAEs) were similar between treatments (Table 2)
· no SAEs were considered treatment-related
· no deaths were reported during the study.
· No clinically relevant differences between FF/VI and FP/SAL were reported for 24-h urinary cortisol (UC) excretion (adjusted treatment ratio 0.85 [95% CI: 0.72, 1.02], p=0.075) (Fig. 2) or vital signs.
Figure 2. Adjusted ratios to baseline for 24-h UC excretion at Week 24 (UC population)
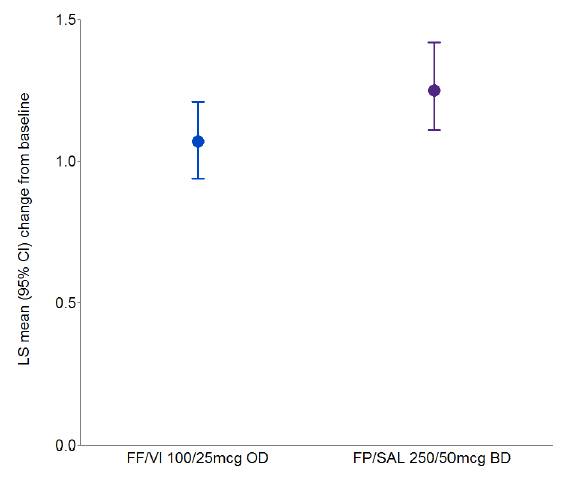
LS=least squares; CI=confidence interval
Table 2. Summary of AEs and SAEs (ITT population)
|
|
|
|
|
FP/SAL |
|
|
|
|
FF/VI 100/25mcg |
|
250/50mcg |
|
|
|
|
once daily |
|
twice daily |
|
|
n (%) |
|
(N=403) |
|
(N=403) |
|
|
On-treatment AEs |
|
213 (53) |
|
198 (49) |
|
|
Nasopharyngitis |
|
46 (11) |
|
46 (11) |
|
|
Headache |
|
34 (8) |
|
41 (10) |
|
|
Upper respiratory tract infection |
|
26 (6) |
|
16 (4) |
|
|
Treatment-related AEs |
|
19 (5) |
|
15 (4) |
|
|
SAEs |
|
4 (<1) |
|
5 (1) |
|
AEs occurring in >5% of patients in any treatment group are presented
CONCLUSIONS
· There was no difference between once-daily FF/VI and twice-daily FP/SAL in improving lung function in patients with persistent asthma.
· FF/VI improved quality of life (post-hoc analysis) compared with FP/SAL.
· No safety issues were identified with either treatment.
REFERENCES
(1) National Institutes of Health. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/index.htm. Last accessed 6 August 2012.
(2) GINA 2011. Available at: http://www.ginasthma.org/uploads/users/files/ GINA_Report_2011.pdf. Last accessed 6 August 2012.
(3) Juniper EF, et al. J Clin Epidemiol 1994;47:81–7.
ACKNOWLEDGEMENTS
· The presenting author, L Jacques, is an employee of and holds stocks/shares in GlaxoSmithKline.
· This study was funded by GlaxoSmithKline; GSK Study Code HZA113091, Clinicaltrials.gov NCT01147848.
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Lisa Moore at Gardiner-Caldwell Communications and was funded by GlaxoSmithKline.

Presented at the European Respiratory Society Annual Congress 2012 Vienna, Austria, 1–5 September, 2012
