Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a12-19541_18k.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a12-19541_1ex99d1.htm |
| EX-99.3 - EX-99.3 - Innoviva, Inc. | a12-19541_1ex99d3.htm |
| EX-99.5 - EX-99.5 - Innoviva, Inc. | a12-19541_1ex99d5.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a12-19541_1ex99d2.htm |
| EX-99.7 - EX-99.7 - Innoviva, Inc. | a12-19541_1ex99d7.htm |
| EX-99.9 - EX-99.9 - Innoviva, Inc. | a12-19541_1ex99d9.htm |
| EX-99.4 - EX-99.4 - Innoviva, Inc. | a12-19541_1ex99d4.htm |
| EX-99.8 - EX-99.8 - Innoviva, Inc. | a12-19541_1ex99d8.htm |
| EX-99.10 - EX-99.10 - Innoviva, Inc. | a12-19541_1ex99d10.htm |
| EX-99.12 - EX-99.12 - Innoviva, Inc. | a12-19541_1ex99d12.htm |
| EX-99.14 - EX-99.14 - Innoviva, Inc. | a12-19541_1ex99d14.htm |
| EX-99.16 - EX-99.16 - Innoviva, Inc. | a12-19541_1ex99d16.htm |
| EX-99.11 - EX-99.11 - Innoviva, Inc. | a12-19541_1ex99d11.htm |
| EX-99.15 - EX-99.15 - Innoviva, Inc. | a12-19541_1ex99d15.htm |
| EX-99.13 - EX-99.13 - Innoviva, Inc. | a12-19541_1ex99d13.htm |
Exhibit 99.6
POSTER P1794
Efficacy and safety of fluticasone furoate/vilanterol (FF/VI) once-daily for 24 weeks in persistent asthma
O’Byrne PM(1), Bleecker ER(2), Bateman ED(3), Busse WW(4), Woodcock A(5), Forth R(6), Toler T(7), Jacques L(8), Lötvall J(9)
(1)Michael G DeGroote School of Medicine, Hamilton, Ontario, Canada; (2)Center for Genomics and Personalized Medicine, Wake Forest University Health Sciences Winston-Salem, NC, USA; (3)Department of Medicine, University of Cape Town, Cape Town, South Africa; (4)Department of Medicine, University of Wisconsin, Madison, WI, USA; (5)School of Translational Medicine, University of Manchester, Manchester, UK; (6)Quantitative Sciences Division, GlaxoSmithKline, RTP, NC, USA; (7)Respiratory Medicines Development Center, GlaxoSmithKline, RTP, NC, USA; (8)Respiratory Medicines Development Centre, GlaxoSmithKline, London, UK; (9)Krefting Research Centre, University of Gothenburg, Sweden
INTRODUCTION
· Inhaled corticosteroids (ICS) are considered the most effective anti-inflammatory treatments for all severities of persistent asthma.(1)
· Adding a long-acting beta2 agonist (LABA) to an ICS improves asthma control and reduces exacerbation frequency.(2)
· FF and VI are, respectively, a novel ICS and LABA in development as a combined (FF/VI) once-daily therapy for asthma and COPD.
OBJECTIVES
· To compare the efficacy and safety of once-daily FF/VI with once-daily FF and twice-daily fluticasone propionate (FP) in patients >12 years of age with moderate-to-severe persistent asthma.
· To demonstrate non-inferiority of once-daily FF 200mcg vs. twice-daily FP 500mcg in trough FEV1, using a non-inferiority margin of 125mL.
METHODS
· Phase III, randomised, multi-centre, double-blind, double-dummy, parallel-group study.
· Patients: asthma for >12 weeks; documented use of ICS (with/without LABA) for >12 weeks with stable ICS dose (FP 500mcg twice daily (or equivalent) or mid-dose ICS/LABA) for >4 weeks; pre-bronchodilator FEV1 40–90% predicted.
· Following a 4-week run-in, patients were randomised (1:1:1) to receive one of the following for 24 weeks
· FF/VI 200/25mcg once daily (evening dosing) via novel dry powder inhaler (nDPI)
· FF 200mcg once daily (evening dosing) via nDPI
· FP 500mcg twice daily (morning/evening dosing) via DISKUSTM/ACCUHALERTM.
RESULTS
Study population and demographics (Table 1)
· Of 1206 patients screened, 586 were randomised and dosed (intent-to-treat [ITT] population) and 476 completed the study.
Table 1. Patient demographics and baseline characteristics (ITT population)
|
|
|
FF/VI |
|
|
|
|
|
|
|
|
|
|
200/25mcg |
|
FF 200mcg |
|
FP 500mcg |
|
Total |
|
|
|
|
OD (N=197) |
|
OD (N=194) |
|
BD (N=195) |
|
(N=586) |
|
|
Age, years |
|
46.6 (15.05) |
|
44.6 (14.33) |
|
47.3 (14.06) |
|
46.2 (14.51) |
|
|
Female gender, n (%) |
|
116 (59) |
|
113 (58) |
|
116 (59) |
|
345 (59) |
|
|
Screening pre- |
|
2.017 |
|
2.072 |
|
2.017 |
|
2.035 |
|
|
bronchodilator FEV1 , L |
|
(0.6226) |
|
(0.6432) |
|
(0.6659) |
|
(0.6435) |
|
|
Screening % predicted |
|
62.99 |
|
63.27 |
|
63.59 |
|
63.28 |
|
|
FEV1 |
|
(12.335) |
|
(12.575) |
|
(12.412) |
|
(12.421) |
|
|
Screening % |
|
29.58 |
|
29.17 |
|
29.56 |
|
29.44 |
|
|
reversibility FEV1 |
|
(19.828) |
|
(17.035) |
|
(16.375) |
|
(17.790) |
|
Values are mean (SD) unless otherwise stated
Efficacy
· FF/VI demonstrated benefit over FF and FP in mean change from baseline in trough (pre-bronchodilator, pre-dose) FEV1 at Week 24 (co-primary endpoint; ANCOVA analysis; last observation carried forward): 394mL FF/VI; 201mL FF; 183mL FP
· treatment differences (95% CI): FF/VI vs. FF 193mL (108, 277); FF/VI vs. FP 210mL (127, 294) (both p<0.001)
· FF 200mcg was non-inferior to FP 500mcg: treatment difference 18mL (–66mL, 102mL)
· repeated measures analysis was consistent (Fig. 1).
· FF/VI demonstrated benefit over FF and FP in weighted mean 0–24h post-dose serial FEV1 after 24 weeks (co-primary endpoint; ANCOVA analysis): 2.668L FF/VI; 2.532L FF; 2.462L FP
· treatment differences (95% CI): FF/VI vs. FF 136mL (1, 270; p=0.048); FF/VI vs. FP 206mL (73, 339; p=0.003)
· individual serial FEV1 assessments are shown in Fig. 2.
· There were significantly more % rescue-free 24-h periods (powered secondary endpoint) (11.7 [p<0.001], equivalent to 0.8 per week) and % symptom-free 24-h periods (secondary endpoint) (8.4 [p=0.01], 0.6 per week) compared with baseline for FF/VI vs. FF.
· No statistical difference between FF/VI and FF in Total Asthma Quality of Life Questionnaire score at either Weeks 12 or 24 (secondary endpoint).
Figure 1. Repeated measures analysis of change from baseline in trough FEV1 over 24 weeks (ITT population)

LS = least squares; CI = confidence interval
Figure 2. Adjusted mean change from baseline of individual serial FEV1 assessments at Week 24 (ITT population)
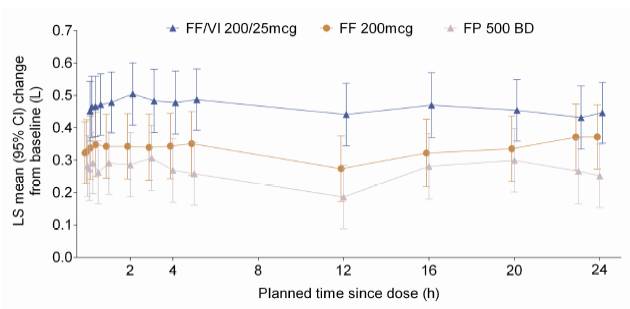
LS = least squares; CI = confidence interval
Safety
· Incidences of on-treatment adverse events (AEs) and treatment-related AEs are summarised in Table 2.
· Incidence of serious AEs (SAEs): FF/VI 3%, FF <1%, FP 1%
· two SAEs were considered treatment related (atrial fibrillation in FF/VI group, haemoptysis in FP group); both resolved.
· Ratio to baseline for 24-h urinary cortisol excretion at Week 24: 0.84 FP, 0.91 FF, 0.98 FF/VI.
· There were no clinically meaningful differences in systolic and diastolic blood pressure, pulse rate or ECG at Week 24 across treatment groups.
Table 2. Summary of AEs by treatment group (ITT population)
|
|
|
FF/VI |
|
|
|
|
|
|
|
|
200/25mcg |
|
FF 200mcg OD |
|
FP 500mcg BD |
|
|
n (%) |
|
OD (N=197) |
|
(N=194) |
|
(N=195) |
|
|
On-treatment AEs |
|
92 (47) |
|
90 (46) |
|
97 (50) |
|
|
Treatment-related AEs |
|
17 (9) |
|
8 (4) |
|
16 (8) |
|
|
AEs leading to discontinuation of study drug or withdrawal from study |
|
7 (4) |
|
3 (2) |
|
2 (1) |
|
|
Most frequent on-treatment AEs |
|
|
|
|
|
|
|
|
Nasopharyngitis |
|
25 (13) |
|
27 (14) |
|
39 (20) |
|
|
Headache |
|
11 (6) |
|
13 (7) |
|
15 (8) |
|
|
Cough |
|
3 (2) |
|
6 (3) |
|
13 (7) |
|
AEs occurring in >5% of patients in any treatment group are presented
CONCLUSIONS
· Treatment with once-daily FF/VI 200/25mcg over 24 weeks was associated with significantly greater improvements in lung function and asthma stability versus once-daily FF 200mcg and was generally well tolerated in this asthma population.
· Once-daily FF 200mcg was not inferior to twice-daily FP 500mcg, as assessed by trough FEV1.
REFERENCES
(1) GINA 2011. Available at: http://www.ginasthma.org/uploads/users/ files/GINA_Report_2011.pdf. Last accessed 6 August 2012.
(2) O’Byrne PM, et al. Chest 2008;134:1192–9.
ACKNOWLEDGEMENTS
· The presenting author, PM O’Byrne, declares the following real or perceived conflicts of interest during the last 3 years in relation to this presentation: consulting for and research funding from GlaxoSmithKline.
· This study was funded by GlaxoSmithKline; GSK Study Code HZA106829, Clinicaltrials.gov NCT01134042.
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Tom Gallagher at Gardiner-Caldwell Communications and was funded by GlaxoSmithKline.

Presented at the European Respiratory Society Annual Congress 2012 Vienna, Austria, 1–5 September, 2012
