Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a12-19541_18k.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a12-19541_1ex99d1.htm |
| EX-99.3 - EX-99.3 - Innoviva, Inc. | a12-19541_1ex99d3.htm |
| EX-99.5 - EX-99.5 - Innoviva, Inc. | a12-19541_1ex99d5.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a12-19541_1ex99d2.htm |
| EX-99.7 - EX-99.7 - Innoviva, Inc. | a12-19541_1ex99d7.htm |
| EX-99.6 - EX-99.6 - Innoviva, Inc. | a12-19541_1ex99d6.htm |
| EX-99.9 - EX-99.9 - Innoviva, Inc. | a12-19541_1ex99d9.htm |
| EX-99.4 - EX-99.4 - Innoviva, Inc. | a12-19541_1ex99d4.htm |
| EX-99.8 - EX-99.8 - Innoviva, Inc. | a12-19541_1ex99d8.htm |
| EX-99.10 - EX-99.10 - Innoviva, Inc. | a12-19541_1ex99d10.htm |
| EX-99.12 - EX-99.12 - Innoviva, Inc. | a12-19541_1ex99d12.htm |
| EX-99.14 - EX-99.14 - Innoviva, Inc. | a12-19541_1ex99d14.htm |
| EX-99.11 - EX-99.11 - Innoviva, Inc. | a12-19541_1ex99d11.htm |
| EX-99.15 - EX-99.15 - Innoviva, Inc. | a12-19541_1ex99d15.htm |
| EX-99.13 - EX-99.13 - Innoviva, Inc. | a12-19541_1ex99d13.htm |
Exhibit 99.16
|
ERS |
September 1 - 5, 2012 |
|
A Randomized, Crossover Study to Examine the Pharmacodynamics and Safety of a New Antimuscarinic TD-4208 in Patients with COPD
Potgieter P.D.(1), Hopkins A.(1), Liu P.(1), Quinn D.(2), Amburgey C.F.(1) and Moran E.J.(1)
(1) Theravance, Inc., South San Francisco, CA. (2) P3 Research Ltd., Wellington, New Zealand. |
|
Peter Potgieter, MD., Ph.D. Theravance, Inc. 901 Gateway Blvd. South San Francisco, CA 94080 Phone: 650-808-3726 Fax: 650-808-6441 ppotgieter@theravance.com |
ABSTRACT
Background: TD-4208 is a potent and selective inhaled muscarinic antagonist with functional lung selectivity and long duration in preclinical models of bronchoconstriction. It is currently in development for maintenance treatment of airflow obstruction in patients with COPD.
Aims: To investigate the bronchodilatory profile, safety and tolerability of nebulized TD-4208 in subjects with COPD.
Methods: Thirty-two patients aged 40-75 years of age with moderate or severe COPD were randomized in a double-blind, complete 4-way crossover study. Single doses of 350 µg or 700 µg TD-4208, active-control ipratropium bromide (500 µg) or placebo were administered using a PARI LC Plus nebulizer in each period. Baseline and serial post-dose spirometry assessments (0-25 hours) were performed. Safety evaluation included adverse events, vital signs, ECGs, and clinical laboratory results.
Results: A statistically significant improvement in peak FEV1 versus placebo of 174 mL (95% CI: 112, 235), 169 mL (95% CI: 108, 231) and 176 mL (95% CI: 114, 237) for TD-4208 350 µg, 700 µg, and ipratropium 500 µg, respectively, was observed (p<0.001 for each comparison). Similar to ipratropium, onset of action of TD-4208 was rapid. TD-4208 bronchodilation was sustained over the 25-hour monitoring period. FEV1 difference from placebo at 12 hours was 113 mL , 123 mL, and 15 mL; p <0.001, <0.001 and 0.659, and at 24 hours was 103 mL, 137 mL , and -24 mL; p 0.001, <0.001 and 0.327, for TD-4208 350 µg, 700 µg, and ipratropium 500 µg, respectively. Adverse events were generally mild and occurred with similar frequencies in all treatments, with the most common being headache and dyspnea. There were no serious adverse events.
Conclusions: TD-4208 was well tolerated and demonstrated significant peak bronchodilation with rapid onset that was sustained over 24 hours suggesting a once daily dosing regimen.
INTRODUCTION
· Muscarinic receptors mediate a variety of physiological processes including maintenance of airway tone, mucus secretion, and regulation of further ACh release.
· The expression and function of muscarinic receptors may be altered in chronic lung disease, leading to increases in airway hyper-reactivity, bronchoconstriction, and mucus hypersecretion.(1)
· Treatment with bronchodilators is central to the management of COPD, either as-needed in mild cases, or daily for patients with persistent symptoms.(2)
· Long-acting inhaled muscarinic antagonist (LAMA) bronchodilators are convenient and more effective for symptom relief than short-acting bronchodilators.(2)
· Despite increased therapeutic options, there remains a need for improved bronchodilator agents for treating COPD and asthma.
· TD-4208 is a novel, long-acting, inhaled muscarinic antagonist that is being developed as a once daily treatment of COPD and asthma.(3),(4)
AIMS
Primary Objective:
· To investigate the bronchodilatory effect of single nebulized doses of TD-4208 in male and female subjects with COPD
Key Secondary Objectives:
· To explore the duration of bronchodilatory effect of TD-4208
· To evaluate the safety and tolerability profile of TD-4208
· To evaluate single dose pharmacokinetics of TD-4208
METHODS
Key Eligibility Criteria:
· Male and female aged 40 to 75 years
· COPD meeting GOLD guidelines (2)
· FEV1/FVC ratio <0.70
· FEV1 35 to 80% predicted post-bronchodilator
· >12% (and >200 mL) response in FEV1 post ipratropium bromide
· No recent exacerbations/infections (< 6 weeks prior to screening)
· Not taking high-dose steroids (> 5 mg prednisone or >1000 µg fluticasone)
Study Design and Assessments:
· Single-dose, randomized, double-blind, active and placebo-controlled, four-period complete crossover study
· 21 day screening period followed by four in-house treatment periods with 25 hour intense monitoring (spirometry, safety and pharmacokinetics)
· Washout of 7 to 12 days between doses
· Single doses of TD-4208 350 and 700 mg, active-control agent ipratropium bromide (500 mg), and placebo, each administered using a PARI LC® Plus nebulizer with a PARI PRONEB® Ultra II Compressor
· Maintenance bronchodilators and steroids were discontinued for 12 to 72 hours prior to each dosing period depending on their duration of action
Pharmacodynamic Endpoints:
· Primary PD endpoint:
· Change in peak FEV1 relative to period baseline
· Key secondary PD endpoint:
· FEV1 value at 24 hours postdose (trough)
PATIENTS
· 32 subjects diagnosed with COPD were enrolled
· All subjects received each study treatment and completed all follow-up assessments
Table 1. Demographic and Clinical Characteristics
|
|
|
N = 32 |
|
|
Age (mean + SD) |
|
62.0 + 7.46 |
|
|
Sex M/F |
|
22/10 |
|
|
Race (W/Other) |
|
28/4 |
|
|
BMI (mean + SD) |
|
27.7 + 8.02 |
|
|
FEV1 % predicted (mean + SD) |
|
50.5 +13.74 |
|
|
FEV1 (L) (mean + SD) |
|
1.9 + 0.48 |
|
|
FEV1 % response post-ipratropium (mean + SD) |
|
23.7 + 8.75 |
|
PHARMACODYNAMIC RESULTS
Figure 1. FEV1 Change from Baseline
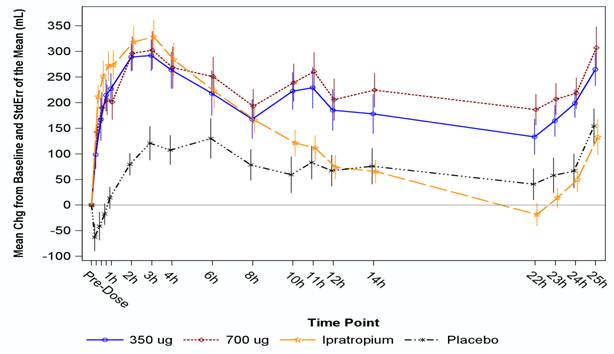
Figure 2. Mean FEV1 Peak and Trough*

PHARMACOKINETIC RESULTS
· Mean Cmax values were 0.12 and 0.25 ng/mL for the 350 and 700 µg doses
· Median Tmax values were 0.3 and 0.3 hours, respectively
· Mean half-lives (t1/2) of 3.8 and 6.7 hours, respectively
SUMMARY OF ADVERSE EVENTS
· Adverse events were generally mild
· Adverse events occurred with similar frequencies in all treatments
· Most common were headache and dyspnea
· No dry mouth reported
· No patient discontinued because of an adverse event
· There were no serious adverse events and no deaths
Table 2. Treatment-Emergent Adverse Events in >2 Patients (Safety Subjects)
|
|
|
Placebo |
|
350 mcg |
|
700 mcg |
|
Ipra. |
|
Total |
|
|
Any AEs |
|
16 (50.0%) |
|
11 (34.4%) |
|
12 (37.5%) |
|
11 (34.4%) |
|
23 (71.9%) |
|
|
Nasopharyngitis |
|
1 (3.1%) |
|
0 |
|
0 |
|
1 (3.1%) |
|
2 (6.3%) |
|
|
ECG T wave peaked |
|
1 (3.1%) |
|
2 (6.3%) |
|
1 (3.1%) |
|
1 (3.1%) |
|
2 (6.3%) |
|
|
Gout |
|
1 (3.1%) |
|
1 (3.1%) |
|
1 (3.1%) |
|
1 (3.1%) |
|
2 (6.3%) |
|
|
Headache |
|
6 (18.8%) |
|
2 (6.3%) |
|
3 (9.4%) |
|
2 (6.3%) |
|
9 (28.1%) |
|
|
Dyspnoea |
|
3 (9.4%) |
|
0 |
|
2 (6.3%) |
|
4 (12.5%) |
|
6 (18.8%) |
|
|
Nasal congestion |
|
1 (3.1%) |
|
1 (3.1%) |
|
0 |
|
0 |
|
2 (6.3%) |
|
CONCLUSIONS
· TD-4208 demonstrated significant peak bronchodilation with rapid onset
· The bronchodilation for both doses of TD-4208 was sustained for the 24 hour period
· TD-4208 was well tolerated
· TD-4208 is suitable for development as a once daily inhaled agent for COPD and asthma
REFERENCES
(1) Belmonte K.E. Proc. Am. Thorac. Soc. 2005;2(4):297-304.
(2) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Updated December 2011. Available at: http://goldcopd.com
(3) Pulido-Rios, et al., TD-4208: A Novel, Lung-Selective Muscarinic Antagonist with Sustained Bronchoprotective Activity in Preclinical Models. ATS 2009, Poster A4557.
(4) Steinfeld, et al., In vitro Characterization of TD-4208, a Lung-selective and Long-acting Muscarinic Antagonist Bronchodilator. ATS 2009, Poster A4553.
