Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a12-19541_18k.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a12-19541_1ex99d1.htm |
| EX-99.3 - EX-99.3 - Innoviva, Inc. | a12-19541_1ex99d3.htm |
| EX-99.5 - EX-99.5 - Innoviva, Inc. | a12-19541_1ex99d5.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a12-19541_1ex99d2.htm |
| EX-99.7 - EX-99.7 - Innoviva, Inc. | a12-19541_1ex99d7.htm |
| EX-99.6 - EX-99.6 - Innoviva, Inc. | a12-19541_1ex99d6.htm |
| EX-99.9 - EX-99.9 - Innoviva, Inc. | a12-19541_1ex99d9.htm |
| EX-99.4 - EX-99.4 - Innoviva, Inc. | a12-19541_1ex99d4.htm |
| EX-99.8 - EX-99.8 - Innoviva, Inc. | a12-19541_1ex99d8.htm |
| EX-99.10 - EX-99.10 - Innoviva, Inc. | a12-19541_1ex99d10.htm |
| EX-99.12 - EX-99.12 - Innoviva, Inc. | a12-19541_1ex99d12.htm |
| EX-99.14 - EX-99.14 - Innoviva, Inc. | a12-19541_1ex99d14.htm |
| EX-99.16 - EX-99.16 - Innoviva, Inc. | a12-19541_1ex99d16.htm |
| EX-99.11 - EX-99.11 - Innoviva, Inc. | a12-19541_1ex99d11.htm |
| EX-99.13 - EX-99.13 - Innoviva, Inc. | a12-19541_1ex99d13.htm |
Exhibit 99.15
POSTER P2121
Umeclidinium (GSK573719) dose response and dosing interval in COPD
Church A(1), Beerahee M(2), Brooks J(3), Mehta R(1), Shah P(3)
(1)GlaxoSmithKline, Research Triangle Park, North Carolina, USA; (2)GlaxoSmithKline, Stevenage, UK; (3)GlaxoSmithKline, Uxbridge, UK
INTRODUCTION
· Umeclidinium (UMEC; GSK573719) is a new, long-acting muscarinic antagonist in development as a COPD treatment.
· In two previous studies, no clear dose response was identified for UMEC over the dose range of 62.5mcg to 1000mcg once daily (OD).(1),(2)
OBJECTIVES
· To further characterise the dose-response of UMEC at doses of 15.6mcg to 125mcg OD, and to explore efficacy and safety at doses of 15.6 to 125mcg OD and 15.6mcg and 31.25mcg twice daily (BID) compared with tiotropium OD or placebo in subjects with COPD.
METHODS
Study design and population
· Randomised, double-blind, placebo-controlled, incomplete block, crossover study (AC4115321; NCT01372410) .
· Eligible subjects were male or female, aged 40–80 years with a history of COPD, current or former cigarette smoking of >10 pack-years, and a forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio of <0.70 and a post-salbutamol FEV1 of >35 and <70% of predicted.
Treatment
· Subjects were randomised to sequences consisting of three 7-day treatment periods, separated by 10–14 day washouts
· four OD UMEC doses (15.6, 31.25, 62.5, 125mcg) or two BID UMEC doses (15.6, 31.25mcg) were administered via dry powder inhaler
· placebo and open-label OD tiotropium (18mcg) were comparators
· each subject received 3 out of 8 possible treatments.
Endpoints
· Primary endpoint
· trough FEV1 on Day 8, defined as the mean FEV1 value obtained 23 and 24h after morning dosing on Day 7 of each treatment period
· post hoc analysis was performed without data from one investigative site due to poor compliance with good clinical practice standards.
· Secondary endpoint
· serial FEV1 over 24h on Day 7.
· Safety and pharmacokinetics were also assessed.
Model-based analysis
· OD and BID regimens were modelled jointly based on total daily dose. A population dose response model was applied and simulated based on the population variability of the parameters to provide an expected response over the dose range. A secondary efficacy analysis was performed with ANCOVA.
RESULTS
Demographics
· 244 subjects were screened, 163 were randomised and received treatment (modified intent-to-treat population), and 147 completed the study.
· Demographic characteristics of the study population included
· 85 (52%) female
· age, mean (range): 59.5 (41–80) years
· body mass index (range): 27.4 (14.7–35.3) kg/m2
· sixty-four percent of patients were current smokers with a mean smoking history of 38.2 years, and a mean smoking pack–years of 49.4
· GOLD Stage II and III with post-bronchodilator mean percent predicted FEV1 of 51.1% (35%–69%) and mean post-bronchodilator FEV1/FVC ratio of 52.3% (27%–70%)
· mean post-salbutamol FEV1 was 1.554L and FVC was 3.001L.
· Concurrent use of inhaled corticosteroids was 18% to 30% (UMEC), 20% (placebo), 25% (tiotropium).
· Mean treatment compliance was >99.3%.
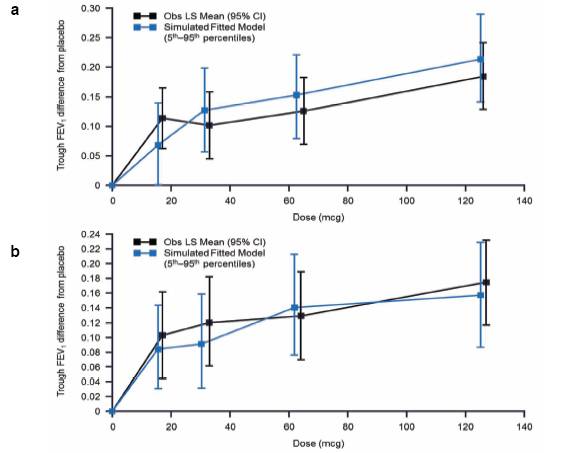
Final dose response model
· A population physiological Emax model best characterised the relationship between UMEC doses and trough FEV1 on Day 8
· a clear monotonic dose response for UMEC was observed
· a potency (ED50) estimate of 37mcg OD (95% CI: 18–57) was demonstrated with good precision (coefficient of variation [CV]: 14%); a maximum intrinsic efficacy at trough of 0.185L (95% CI: 0.153, 0.218) after OD dosing was predicted
· no difference in dose response was noted between OD and BID regimens.
· The simulated median (5th–95th percentiles) estimates of FEV1 (OD regimen) were plotted over the curve for the observed least square (LS) mean FEV1 (95% confidence [CI]) response values (Figure 1)
· simulated dose-response predictions were generally higher than the derived LS Mean data.
· Results of the post hoc analysis of the primary endpoint were similar (Figure 1b).
FIGURE 1. OBSERVED LS MEAN TROUGH FEV1 AND SIMULATED MEDIAN. (A)mITT POPULATION (B) mITT POPULATION EXCLUDING SUBJECTS FROM ONE SITE
Trough FEV1 on Day 8
· Statistically significant increases from baseline in trough FEV1 were demonstrated for all OD and BID UMEC doses and tiotropium compared with placebo (Figure 2)
· results from the post hoc analysis were similar.
· Dose ordering was observed with the largest improvements over placebo for 62.5mcg and 125mcg OD doses.
FIGURE 2. TROUGH FEV1 (L) ON Day 8. (A) mITT POPULATION (B) mITT POPULATION EXCLUDING SUBJECTS FROM ONE SITE

Serial FEV1 over 24h on Day 7
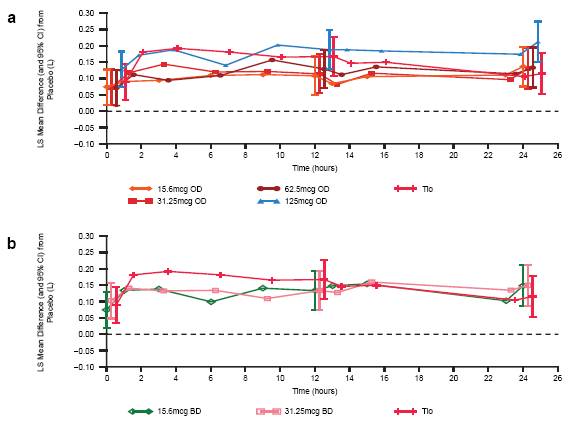
· Statistically significant improvements in FEV1 values at all time points on Day 7 were demonstrated for UMEC OD and BID doses and tiotropium (Figure 3) compared with placebo .
· The improvements in FEV1 from placebo observed at time points over the first 12h were maintained at time points over the second 12h for all OD doses of UMEC.
· UMEC 125mcg OD had more consistent increases in FEV1 from baseline across almost all time points over 24h compared with other UMEC doses or tiotropium.
· UMEC BID dosing did not offer additional benefit over OD dosing. No additional increase in FEV1 was noted after the second dose at 12h.
Safety
· Incidence of overall adverse events (AEs) was higher for UMEC 125mcg OD (18%) than for placebo (8%), tiotropium (4%), other UMEC doses (5–12%).
· The most frequent AEs were headache, nasopharyngitis, dysgeusia, or sinusitis
· headache occurred in 0%–7% of subjects and the other AEs occurred in <4% of subjects.
FIGURE 3. SERIAL FEV1 ON DAY 7. (A) UMEC OD AND TIOTROPIUM AND (B) UMEC BID AND TIOTROPIUM
· Two non-drug related, non-fatal SAEs were reported (acute respiratory failure, 15.6mcg OD; myocardial infarction, 31.25mcg OD); neither SAE was related to treatment. Both subjects were withdrawn from the study and both SAEs resolved.
· Four COPD exacerbations were reported: 1 in placebo, 2 in 15.6mcg OD, and 1 in 125mcg OD. Each of the exacerbations was treated with corticosteroids and resolved following treatment; these subjects were withdrawn from the study.
Pharmacokinetics
· UMEC Cmax occurred early (median tmax of 5–15min) followed by rapid clearance and elimination.
· Following 7 days of repeat dose treatment, there was a 1.2–1.7 -fold accumulation.
CONCLUSIONS
· Based on the population Emax dose response model of trough FEV1 data, UMEC is a potent bronchodilator with geometric mean potency of 37mcg (95% CI: 18, 57) and a predicted maximum intrinsic efficacy at trough of 0.185L (95% CI: 0.153, 0.218) after OD dosing.
· A dose ordering was demonstrated over the lower dose range of 15.6 to 125mcg OD.
· A once-daily dosing interval was confirmed.
REFERENCES
(1) Donohue J, Anzueto A, Brooks J, Crater G, Mehta R, Kalberg C. A randomized, double-blind, dose-ranging study of the novel LAMA GSK573719 in patients with COPD. Respir Med. 2012;106:970-79.
(2) Decramer M, Maltais F, Feldman G, Brooks J, Harris S, Mehta R, Crater G Sustained 24-hour bronchodilation of umeclidinium, a new long-acting muscarinic antagonist, in COPD patients. Submitted.
ACKNOWLEDGEMENTS
· This study was sponsored by GlaxoSmithKline (AC4115321; NCT01372410).
· All authors are employees of, and own stock in, GlaxoSmithKline.
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Tara N Miller, PhD, at Gardiner-Caldwell Communications and was funded by GlaxoSmithKline.

Presented at the Annual Congress of the European Respiratory Society (ERS), Vienna, Austria, September 1–5, 2012
