Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Protalix BioTherapeutics, Inc. | y04467e8vk.htm |
Exhibit 99.1

| Protalix BioTherapeutics January 2011 Confidential |

| Note Regarding Forward-Looking Statements This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The forward-looking statements including, among others, statements regarding expectations as to regulatory approvals, market opportunity for our product candidates, goals as to product candidate development and timing of our clinical trials, are based on Protalix's current intent, belief and expectations. These statements are not guarantees of future performance and are subject to certain risks and uncertainties that are difficult to predict. Actual results may differ materially from these forward- looking statements because of the Company's unproven business model, its dependence on new technologies, the uncertainty and timing of clinical trials, the Company's ability to develop and commercialize products, its dependence on collaborators for services and revenue, its substantial indebtedness and lease obligations, its changing requirements and costs associated with planned facilities, intense competition, the uncertainty of patent and intellectual property protection, the Company's dependence on key management and key suppliers, the uncertainty of regulation of products, the impact of future alliances or transactions and other risks described in the Company's filings with the Securities and Exchange Commission. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. Protalix undertakes no obligation to update or revise the information contained in this announcement whether as a result of new information, future events or circumstances or otherwise. 2 Confidential |

| Late Stage Biologics Program Targeting a Significant Market Best-In-Class Commercial Partner Attractive Platform Technology Promising Pipeline Lead Product taliglucerase alfa indicated for Gaucher disease Completed PIII trial and notified of NDA acceptance in July 2010 Open-label extension trial ongoing Successful Switchover trial , first stage completed Filed for regulatory approval in US (NDA submission completed 4/10), Israel (6/10) Europe (11/10) and Brazil (11/10) PDUFA date of February 25, 2011 in US Collaboration with Pfizer for commercialization of taliglucerase alfa Protalix retains rights in Israel Attractive economics with significant potential regulatory milestones Plant cell-based expression system ProCellExTM Offers significant advantages over existing expression systems including cost and safety Potentially permits an entry into certain patent protected markets PRX-105 completed preliminary Phase 1 for potential biodefense and other applications PRX-102 for Fabry's disease Oral GCD program for Gaucher disease PRX-106 for rheumatoid arthritis and other autoimmune indications 3 Confidential |
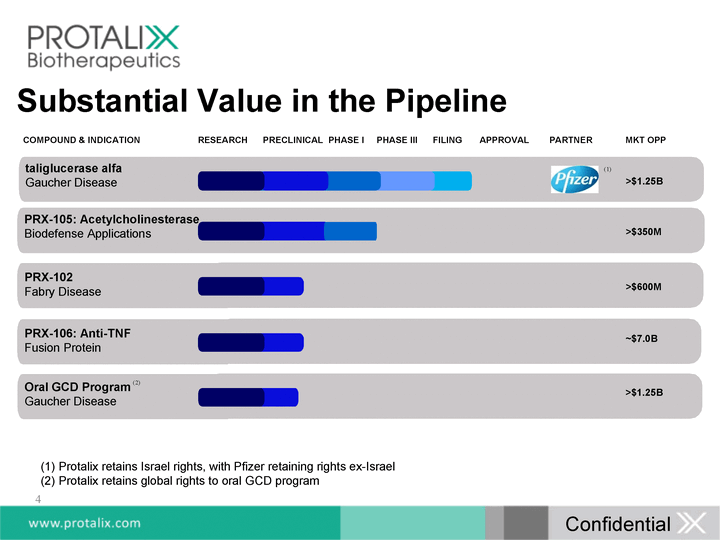
| taliglucerase alfa Gaucher Disease >$1.25B PRX-102 Fabry Disease >$600M >$350M PRX-105: Acetylcholinesterase Biodefense Applications Oral GCD Program Gaucher Disease >$1.25B COMPOUND & INDICATION RESEARCH PRECLINICAL PHASE I PHASE III FILING APPROVAL PARTNER MKT OPP PRX-106: Anti-TNF Fusion Protein ~$7.0B Substantial Value in the Pipeline 4 (1) (2) Protalix retains Israel rights, with Pfizer retaining rights ex-Israel Protalix retains global rights to oral GCD program Confidential |

| Key Advantages Cost effectiveness and scalability Flexible polyethylene bioreactors, low initial capital investment Rapid, horizontal scalability at low cost in compliance with cGMP Requires less costly "hands-on" maintenance Safety and potency No risk of mammalian viral transmission or infection to cells Hundreds of patients on drug world wide Free of any mammalian components Potentially enables penetration of certain patent protected markets May avoid infringement on method-based patents of other proteins developed with mammalian cell expression systems Proprietary Technology Platform: ProCellEx(tm) 5 Confidential |

| Key Milestones and Goals Expected for 2011 2010 Received Orphan Drug designation in Europe Completed FDA submission for taliglucerase alfa Received PDUFA date for taliglucerase alfa Completed preliminary PRX-105 Phase 1 study Granted temporary authorization for use (ATU) for taliglucerase alfa in France Preliminary top-line data from taliglucerase alfa Switchover Trial Receive US FDA approval for taliglucerase alfa Receive marketing approval for taliglucerase alfa from Israeli Ministry of Health Receive marketing approval for taliglucerase alfa in Europe and Brazil Continue enrollment of patients for taliglucerase alpha in compassionate use programs IND and Initiate clinical trials for PRX-102 IND and Initiate clinical trials for PRX-106 Advance oral delivery of GCD for Gaucher's disease towards clinical trials 6 ? ? ? ? ? ? Confidential |

| Taliglucerase alfa Confidential |
| Autosomal recessive disorder caused by mutations in the GBA gene which result in a deficiency of the lysosomal enzyme beta- glucocerebrosidase Patients accumulate large quantities of glucocerebroside in the spleen, liver, lungs and bone marrow that prevent these organs from functioning properly Enzyme replacement therapy is the current standard of treatment. If untreated, patients experience a poor quality of life and death can occur prematurely Higher prevalence among Jews from European origin and occurs at any age in life 8 Gaucher Disease Background Confidential |

| Macrophages Bone Bone Marrow Liver Lung Spleen Source: Beutler and Grabowski, The Metabolic and Molecular Bases of Inherited Disease 2001 Skeletal pathology Pulmonary hypertension/ infiltration Hepatomegaly Anemia Thrombocytopenia Splenomegaly Outcome: Death - Poor quality of life Gaucher Disease: A Genetically Inherited Disorder 9 Confidential |

| *Zavesca (r) (Actelion) is a small molecule drug approved for the treatment of Gaucher disease, however usage is extremely limited due to significant side effects Gaucher Disease: Current Market Dynamics 10 ~10,000 patients worldwide Around ~5,500 patients are being treated ~50% global market penetration Orphan disease supports premium pricing Chronic therapy Concentrated group of prescribers Annual treatment cost is ~$250,000 per year Growing market with annual $1.25 billion of enzyme sales Cerezyme (r) (Genzyme) is the major recombinant GCD on the market and is made in mammalian CHO cells Velaglucerase (Shire), a recombinant GCD produced in human cancer cells, approved in US and EU Growing Market Lucrative Market Competition* Confidential |

| Phase III Pivotal Trial Under SPA with FDA Design Multicenter, world-wide, randomized, double blind, parallel groups, dose-ranging study in 31 untreated patients with significant symptoms of Gaucher disease Patients randomized to one of two dose arms, 30 U/Kg or 60 U/kg, bi-weekly dosing for 9 months Endpoints Primary: Mean decrease in spleen volume Secondary: Decrease in liver volume, increase in hemoglobin, increase in platelet count 11 Confidential |

| Met Primary Endpoint of % Change in Spleen Volume 60 Units/kg 30 Units/kg N=16 N=15 -38.01% +- 9.38* -37.63 -56.30 to -20.04 -26.91% +- 7.79* -27.85 -42.60 to -15.58 Mean Median Range 9 Months 16.6X 15.4X Mean Baseline X Normal 10.9X 11.3X Mean 9 Months X Normal Range of Spleen volume at baseline 8X - 54X * P-value<0.0001 MRI validated method-2 blinded readers Intra-reader variability ^ 0.5%; Inter-reader variability ^ 1.00% 12 End point was achieved after 6 months in both dose groups Confidential |

| 1st Major Secondary endpoint: Change in Hemoglobin (g/dL) From Baseline - ITT vs. Anemic patients Both anemic and non anemic patients showed an increase in Hb 60 Units/kg n = 16 30 Units/kg n = 15 ITT n= 31 2.2 +- 1.4 1.6 <0.0001* 1.6 +- 1.4 1.3 0.0010* Mean g/dL Median P-value 9 months 22.2% 14.8% % change 9 months Anemic pts n = 10 60 U/kg n = 16 30 U/kg n = 15 ITT n = 31 9.5 +- 1.6 10.2 11.4 +- 2.6 10.7 12.4 +- 1.7 12.7 Mean g/dL Median 9 months 36% 22.2 % 14.8% % change 9 months 13 Confidential |

| 2nd Major Secondary Endpoint: Liver Volume % Change from Baseline 60 Units/kg 30 Units/kg 2190.99 376.70 2098.13 1654.07 - 2894.12 2564.07 559.57 2473.19 2000.33 -4121.94 Mean SD Median Range 9 Months (absolute values) -11.11% 6.68 -12.25 <0.0001* -10.48% 11.27 -13.87 0.0041* Mean SD Median P-value 9 Months (%change from baseline) MRI validated method-2 blinded readers Intra-reader variability ^ 0.5%; Inter-reader variability ^ 1.00% 16 (52%) of the patients who presented hepatomegaly showed a reduction of 14% on liver volume 14 Confidential |

| 3rd Major Secondary Endpoint: Platelet Counts Change from Baseline Baseline range 27,000/ ml - 163,000/ml 60 Units/kg 30 Units/kg N=16 41494 47063 38000 -15000 to 186000 0.0031* N=15 11427 20214 10000 -25000 to 59000 0.0460* Mean SD Median Range P-value 9 Months (change from baseline) 72.1% 13.7% % change 9 Months 15 Confidential |

| Phase III Safety Results No serious adverse events All AEs were mild or moderate in intensity and the majority of the events spontaneously resolved Headache; hypersensitivity; dizziness; muscle spasm; chest discomfort; nausea; skin irritation; arthalgia No neutralizing antibodies to taliglucerase were detected 6% of the patients developed IgG antibodies to taliglucerase alfa These patients completed the study and are being treated in the extension study 6% of the patients developed hypersensitivity reaction No anti-taliglucerase antibodies detected in the patients who experienced hypersensitivity 16 Confidential |
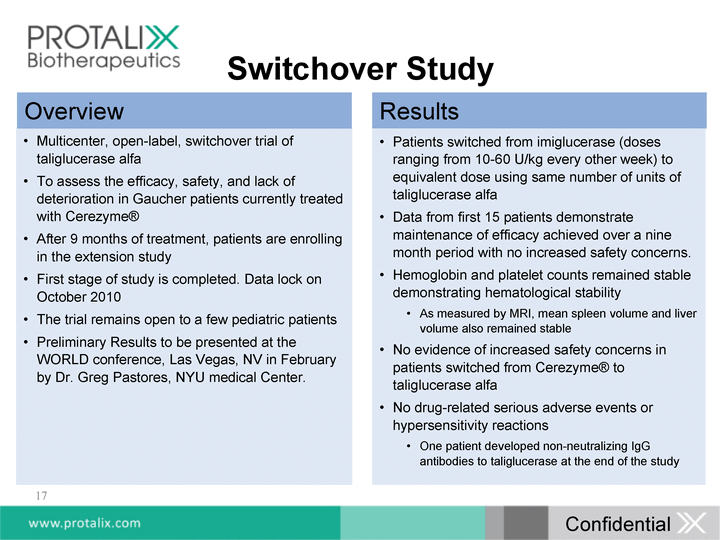
| 17 Switchover Study Multicenter, open-label, switchover trial of taliglucerase alfa To assess the efficacy, safety, and lack of deterioration in Gaucher patients currently treated with Cerezyme(r) After 9 months of treatment, patients are enrolling in the extension study First stage of study is completed. Data lock on October 2010 The trial remains open to a few pediatric patients Preliminary Results to be presented at the WORLD conference, Las Vegas, NV in February by Dr. Greg Pastores, NYU medical Center. Patients switched from imiglucerase (doses ranging from 10-60 U/kg every other week) to equivalent dose using same number of units of taliglucerase alfa Data from first 15 patients demonstrate maintenance of efficacy achieved over a nine month period with no increased safety concerns. Hemoglobin and platelet counts remained stable demonstrating hematological stability As measured by MRI, mean spleen volume and liver volume also remained stable No evidence of increased safety concerns in patients switched from Cerezyme(r) to taliglucerase alfa No drug-related serious adverse events or hypersensitivity reactions One patient developed non-neutralizing IgG antibodies to taliglucerase at the end of the study Overview Results Confidential |

| Regulatory Status Rolling NDA submission filed with FDA February 25th, 2011 PDUFA date Submission to Ministry of Health in Israel Information dossier submitted GMP audit of manufacturing facility performed Submission of MAA to EMEA in Europe November 2010 Submission to ANVISA in Brazil November 2010 Additional submissions in Rest of World in process 18 Confidential |

| FDA requested submission of a treatment protocol allowing expanded access to taliglucerase alfa in adult patients with Gaucher disease resulting from the shortage of Cerezyme Protocols approved by FDA and EMEA Patient enrollment on-going in clinical centers around the world Under a treatment protocol (US and Israel); and Compassionate use program (EU, Brazil, ROW) Patients treated in France under ATU program (temporary approval) - Paid by French Government Hundreds of patients on taliglucerase world wide Expanded Access Program Overview 19 Confidential |

| Total estimate of around 600 Gaucher patients previously treated in Brazil On 8/10/2010, Pfizer entered into a $30 million short-term supply agreement with the Ministry of Health of Brazil Protalix and Pfizer have provided taliglucerase alfa to the Ministry of Health of Brazil for the treatment of patients with Gaucher disease In addition, we and the Ministry of Health of Brazil are in discussions relating to a possible long-term supply agreement that contemplates, among other matters, providing certain components of Protalix's manufacturing technology to the Ministry of Health of Brazil for implementation by it in Brazil At this time, we are unable to assess whether these discussions will result in an agreement Brazil Program 20 Confidential |

| Terms Upfront payment of $65 million Additional near-term regulatory milestone payments of up to $50 million Pfizer and Protalix to share net profit and net loss of taliglucerase alfa on a 60% / 40 % basis to Protalix. Certain limited capped expenses Territories Pfizer retains exclusive worldwide rights outside of Israel Protalix retains exclusive commercialization rights in Israel (1) Manufacturing Protalix to manufacture taliglucerase alfa Commercialization Strategy: Collaboration 21 (1) Protalix retains global rights to oral GCD program. Confidential |

| Completed GMP Manufacturing Audits by FDA and Israel's Ministry of Health during, 2010 EMEA and ANVISA audits are prerequisite for approval Current facility can supply over 1000 patients Currently building up launch inventory Expansion of manufacturing facility in process with an estimated cost of ~$25M Manufacturing and Capacity 22 Confidential |

| Taliglucerase Milestones Over the Next 12 Months PDUFA in US (February 25, 2011) Potential approval in Israel Potential approval in Europe Potential approval in Brazil Additional data from Switchover study Filing for Approval in additional territories around the world 23 Confidential |

| Pipeline Overview Confidential |
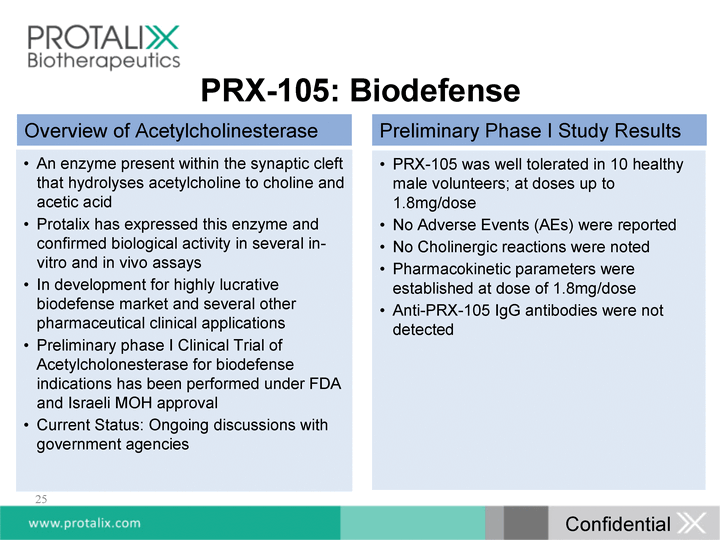
| 25 PRX-105: Biodefense An enzyme present within the synaptic cleft that hydrolyses acetylcholine to choline and acetic acid Protalix has expressed this enzyme and confirmed biological activity in several in- vitro and in vivo assays In development for highly lucrative biodefense market and several other pharmaceutical clinical applications Preliminary phase I Clinical Trial of Acetylcholonesterase for biodefense indications has been performed under FDA and Israeli MOH approval Current Status: Ongoing discussions with government agencies PRX-105 was well tolerated in 10 healthy male volunteers; at doses up to 1.8mg/dose No Adverse Events (AEs) were reported No Cholinergic reactions were noted Pharmacokinetic parameters were established at dose of 1.8mg/dose Anti-PRX-105 IgG antibodies were not detected Overview of Acetylcholinesterase Preliminary Phase I Study Results Confidential |

| 26 PRX-102: Fabry's Disease Prx-102 is a plant cell expressed, chemically modified and improved version of the human ^-Galactosidase- A enzyme Potential Advantages: Enhanced stability Longer circulatory half life Enhanced activity in target organs Reduced immunogenicity Improved kinetics 2010 Advanced R&D development including product characterization and determination of release specification Performed proof of concept in "Fabry mice" knock out model Pre-IND meeting with FDA held end of 2010 2011 Toxicological studies IND submission to FDA Q4 Initiate Phase I/II in Fabry patients Overview of Plant rh^-Galactosidase-A Development Status Confidential |

| Enbrel(tm) (etanercept) product overview: Mode of action: a competitive inhibitor of TNF Expression platform: CHO Indications: Rheumatoid Arthritis and multiple autoimmune indications Market: ~$7.0 billion PRX-106 Amino acid sequence is identical to Enbrel(tm) Current Status: Pre-IND meeting with FDA set for Q1 2011 Promising pre clinical efficacy data in arthritis (CIA) model PRX-106: Anti TNF Protein 27 Confidential |

| Oral Delivery of prGCD for Gaucher's Disease prGCD is stable in lyophilized carrot cells for months In animal studies prGCD is found in liver and spleen, Gaucher target organs when prGCD carrot cells are fed to rats Active prGCD enzyme is found in target organs following feeding Preliminary PK data demonstrates prGCD in the plasma Next Steps: Feeding experiments in large animals Pharmacokinetics and toxicology studies of oral prGCD Initiate clinical trials for oral prGCD enzyme 28 Confidential |

| Oral delivery of therapeutic proteins Long time goal for the bio pharmaceutical industry Currently only very limited success The Plant Cell Advantage The Concept: Plant cell wall (cellulose) serves as protective agent against the gastric environment and can serve as an oral administration vehicle Protein Oral Delivery Advantages 29 Confidential |

| Financial Overview Confidential |

| Financial Status 31 $ in millions Quarter Last Twelve Ending Months Ended 9/30/2010 9/30/2010 [81.2]M Shares Common Stock O/S 9/30/2010 Unaudited Unaudited Cash & Cash Equivalents $44.4 $44.4 Revenues 3.2 5.9 R & D Expenses* 3.5 28.9 G & A Expenses 1.4 7.6 Operating Cash Use (9.7) 28.7 (1) (1) Includes upfront payment from Pfizer collaboration *Less grants Confidential |

| Protalix Shareholder Perspectives 32 Top Holders Total Holdings % of Total Outstanding * Biocell 14.5mm 17.9% Techno-Rov 6.2mm 7.6% Baillie Giffold & Co. 5.8mm 7.2% Named Executive Officers 7.1mm 8.7% * Based on shares outstanding as of 9/30/10 10Q. 1.3mm shares subject to named executive officer 10b5-1 plans Triggering Event: Taliglucerase alfa FDA approval at price of either $10 or market price >5.0% Holders and Named Executive Officers Confidential |

| Late Stage Biologics Program Targeting a Significant Market Best-In-Class Commercial Partner Attractive Platform Technology Promising Pipeline Lead Product taliglucerase alfa indicated for Gaucher disease Completed PIII trial and notified of NDA acceptance in July 2010 Open-label extension trial ongoing Successful Switchover trial , first stage completed Filed for regulatory approval in US (NDA submission completed 4/10), Israel (6/10) Europe (11/10) and Brazil (11/10) PDUFA date of February 25, 2011 in US Collaboration with Pfizer for commercialization of taliglucerase alfa Protalix retains rights in Israel Attractive economics with significant potential regulatory milestones Plant cell-based expression system ProCellExTM Offers significant advantages over existing expression systems including cost and safety Potentially permits an entry into certain patent protected markets PRX-105 completed preliminary Phase 1 for potential biodefense and other applications PRX-102 for Fabry's disease Oral GCD program for Gaucher disease PRX-106 for rheumatoid arthritis and other autoimmune indications 33 Confidential |

| Q&A 34 Confidential |
