UNITED STATES SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C.
20549
Form 10-K
FOR ANNUAL AND TRANSITION
REPORTS PURSUANT TO SECTIONS 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
| |
|
|
|
(Mark One)
|
|
|
|
þ
|
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the fiscal year ended
December 31,
2010
|
|
or
|
|
o
|
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
|
|
|
|
For the transition period
from to
|
001-33357
(Commission file number)
PROTALIX BIOTHERAPEUTICS,
INC.
(Exact name of
registrant as specified in its charter)
| |
|
|
|
Florida
|
|
65-0643773
|
State or other jurisdiction
of
incorporation or organization
|
|
(I.R.S. Employer
Identification No.)
|
|
|
|
|
2 Snunit Street
Science Park
POB 455
Carmiel, Israel
(Address of principal
executive offices)
|
|
20100
(Zip Code)
|
Registrant’s telephone number, including area code
972-4-988-9488
Securities registered pursuant to Section 12(b) of the
Act:
| |
|
|
|
Title of Each Class
|
|
Name of Each Exchange on Which Registered
|
|
|
|
Common stock, par value $0.001 per share
|
|
NYSE AMEX
|
Securities registered pursuant to Section 12(g) of the
Act:
None
Indicate by check mark if the registrant is a well-known
seasoned issuer, as defined in Rule 405 of the Securities
Act. Yes o No þ
Indicate by check mark if the registrant is not required to file
reports pursuant to Section 13 or Section 15(d) of the
Act. Yes o No þ
Indicate by check mark whether the registrant (1) has filed
all reports required to be filed by Section 13 or 15(d) of
the Securities Exchange Act of 1934 during the preceding
12 months (or for such shorter period that the registrant
was required to file such reports), and (2) has been
subject to such filing requirements for the past
90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted
electronically and posted on its corporate Web site, if any,
every Interactive Data File required to be submitted and posted
pursuant to Rule 405 of
Regulation S-T
during the preceding 12 months (or for such shorter period
that the registrant was required to submit and post such
files). Yes o No o
Indicate by check mark if disclosure of delinquent filers
pursuant to Item 405 of
Regulation S-K
is not contained herein, and will not be contained, to the best
of registrant’s knowledge, in definitive proxy or
information statements incorporated by reference in
Part III of this
Form 10-K
or any amendment to this
Form 10-K. o
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated
filer, or a smaller reporting company. See the definitions of
“large accelerated filer,” “accelerated
filer” and “smaller reporting company” in
Rule 12b-2
of the Exchange Act. (Check one):
|
|
|
|
| Large
accelerated
filer o
|
Accelerated
filer þ
|
Non-accelerated
filer o
|
Smaller reporting
company o
|
(Do not check if a smaller
reporting company)
Indicate by check mark whether the registrant is a shell company
(as defined in
Rule 12b-2
of the Exchange
Act). Yes o No þ
The aggregate market value of the voting stock held by
non-affiliates of the Registrant, as of June 30, 2010 was
approximately $316.6 million (based upon the closing price
for shares of the Registrant’s common stock as reported by
the NYSE Amex) as of June 30, 2010 of $6.11). Shares of
common stock held by each officer, director and holder of 5% or
more of the outstanding common stock have been excluded in that
such persons may be deemed to be affiliates. This determination
of affiliate status is not necessarily a conclusive
determination for other purposes.
On February 15, 2011, approximately 81,328,699 shares
of the Registrant’s common stock, par value $0.001 per
share, were outstanding.
FORM 10-K
TABLE OF
CONTENTS
i
PART I
Except where the context otherwise requires, the terms,
“we,” “us,” “our” or “the
Company,” refer to the business of Protalix
BioTherapeutics, Inc. and its consolidated subsidiaries, and
“Protalix” or “Protalix Ltd.” refers to the
business of Protalix Ltd., our wholly-owned subsidiary and sole
operating unit.
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
The statements set forth under the captions
“Business,” “Management’s Discussion and
Analysis of Financial Condition and Results of Operations”
and “Risk Factors,” and other statements included
elsewhere in this Annual Report on
Form 10-K,
which are not historical, constitute “forward-looking
statements” within the meaning of Section 27A of the
Securities Act of 1933, as amended, and Section 21E of the
Securities Exchange Act of 1934, as amended, including
statements regarding expectations, beliefs, intentions or
strategies for the future. When used in this report, the terms
“anticipate,” “believe,”
“estimate,” “expect” and “intend”
and words or phrases of similar import, as they relate to our or
our subsidiaries or our management, are intended to identify
forward-looking statements. We intend that all forward-looking
statements be subject to the safe-harbor provisions of the
Private Securities Litigation Reform Act of 1995. These
forward-looking statements are only predictions and reflect our
views as of the date they are made with respect to future events
and financial performance, and we undertake no obligation to
update any forward-looking statement to reflect events or
circumstances after the date on which the statement is made or
to reflect the occurrence of unanticipated events, except as may
be required under applicable law. Forward-looking statements are
subject to many risks and uncertainties that could cause our
actual results to differ materially from any future results
expressed or implied by the forward-looking statements.
Examples of the risks and uncertainties include, but are not
limited to, the following:
|
|
|
| |
•
|
delays in the approval or the potential rejection of any
applications we file with the U.S. Food and Drug
Administration, or FDA, or other regulatory authorities,
including the New Drug Application (NDA) we filed with the FDA,
the marketing application we submitted to the Israeli Ministry
of Health, and the Marketing Authorization Application (MAA) we
submitted to each of the European Medicines Agency and the
National Sanitary Vigilance Agency, an agency of the Ministry of
Health of Brazil, for taliglucerase alfa;
|
| |
| |
•
|
the inherent risks and uncertainties in developing the types of
drug platforms and products we are developing;
|
| |
| |
•
|
delays in our preparation and filing of applications for
regulatory approval in the United States, the European Union,
Israel, Brazil and elsewhere;
|
| |
| |
•
|
any lack of progress of our research and development (including
the results of our clinical trials);
|
| |
| |
•
|
our ability to establish and maintain strategic license,
collaboration and distribution arrangements and to manage our
relationship with Pfizer Inc., Teva Ltd. or with any other
collaborator, distributor or partner;
|
| |
| |
•
|
obtaining on a timely basis sufficient patient enrollment in our
clinical trials;
|
| |
| |
•
|
the impact of development of competing therapies
and/or
technologies by other companies;
|
| |
| |
•
|
risks relating to biogeneric legislation
and/or
healthcare reform in the United States or elsewhere;
|
| |
| |
•
|
our ability to obtain additional financing required to fund our
research programs and the expansion of our manufacturing
capabilities;
|
| |
| |
•
|
the risk that we will not be able to develop a successful sales
and marketing organization in a timely manner, if at all;
|
| |
| |
•
|
our ability to enter into supply arrangements with the Ministry
of Health of Brazil or other parties and to supply drug product
pursuant to such arrangements;
|
|
|
|
| |
•
|
potential product liability risks, and risks of securing
adequate levels of product liability and clinical trial
insurance coverage;
|
| |
| |
•
|
the availability of reimbursement to patients from health care
payors for any of our product candidates, if approved;
|
| |
| |
•
|
the possibility of infringing a third party’s patents or
other intellectual property rights;
|
| |
| |
•
|
the uncertainty of obtaining patents covering our products and
processes and in successfully enforcing our intellectual
property rights against third parties;
|
| |
| |
•
|
the possible disruption of our operations due to terrorist
activities and armed conflict, including as a result of the
disruption of the operations of regulatory authorities, our
subsidiaries, our manufacturing facilities and our customers,
suppliers, distributors, collaborative partners, licensees and
clinical trial sites; and
|
| |
| |
•
|
other risks and uncertainties detailed in Section 1A of
this Annual Report on Form
10-K.
|
In addition, companies in the pharmaceutical and biotechnology
industries have suffered significant setbacks in advanced or
late-stage clinical trials, even after obtaining promising
earlier trial results or preliminary findings for such clinical
trials. Even if favorable testing data is generated by clinical
trials of drug products, the FDA or foreign regulatory
authorities may not accept or approve an NDA or MAA, as
applicable, filed by a pharmaceutical or biotechnology company
for such drug product. These and other risks and uncertainties
are detailed under the heading “Risk Factors” in this
Annual Report on
Form 10-K
and are described from time to time in the reports we file with
the Securities and Exchange Commission. We undertake no
obligation to update, and we do not have a policy of updating or
revising, these forward-looking statements.
We are a biopharmaceutical company focused on the development
and commercialization of recombinant therapeutic proteins based
on our proprietary
ProCellExtm
protein expression system, or ProCellEx. Using our ProCellEx
system, we are developing a pipeline of proprietary and
biosimilar or “generic” versions of recombinant
therapeutic proteins based on our plant cell-based expression
technology that target large, established pharmaceutical markets
and that rely upon known biological mechanisms of action. Our
initial commercial focus has been on complex therapeutic
proteins, including proteins for the treatment of genetic
disorders, such as Gaucher disease and Fabry disease. We believe
our ProCellEx protein expression system will enable us to
develop proprietary recombinant proteins that are
therapeutically equivalent or superior to existing recombinant
proteins currently marketed for the same indications. Because we
are primarily targeting biologically equivalent versions of
highly active, well-tolerated and commercially successful
therapeutic proteins, we believe our development process is
associated with relatively less risk compared to other
biopharmaceutical development processes for completely novel
therapeutic proteins.
Our lead product development candidate is taliglucerase alfa for
the treatment of Gaucher disease, which we are developing using
our ProCellEx protein expression system. Gaucher disease is a
rare and serious lysosomal storage disorder with severe and
debilitating symptoms. Taliglucerase alfa is our proprietary
recombinant form of glucocerebrosidase (GCD), an enzyme
naturally found in human cells that is mutated or deficient in
patients with Gaucher disease. In July 2007, we reached an
agreement with the U.S. Food and Drug Administration, or
the FDA, on the final design of our pivotal phase III
clinical trial of taliglucerase alfa through the FDA’s
special protocol assessment (SPA) process. The phase III
clinical trial was completed in September 2009 and, on
October 15, 2009, we announced positive top-line results
from the trial. On December 9, 2009, we filed our New Drug
Application (NDA) for taliglucerase alfa, and in January 2010
the FDA requested additional data regarding the Chemistry,
Manufacturing and Controls (CMC) section of our NDA. We
provided the requested data to the FDA in April 2010 and in July
2010 we received notification from the FDA that it had accepted
the filing of our NDA and assigned a Prescription Drug User Fee
Act (PDUFA) date of February 25, 2011 to taliglucerase alfa
for the treatment of Gaucher disease. In addition, in November
2010 we submitted a marketing application to the Israeli
Ministry of Health, or the Israeli MOH,
2
and a Marketing Authorization Application (MAA) to each of the
European Medicines Agency, or the EMEA, and ANVISA, the National
Sanitary Vigilance Agency, an agency of the Brazilian Ministry
of Health, or the ANVISA, for taliglucerase alfa for the
treatment of Gaucher disease.
In February 2010, the Israeli MOH completed a successful good
manufacturing practices (GMP) audit of our manufacturing
facilities in Carmiel, Israel. The audit was performed as part
of the Israeli MOH’s evaluation of our manufacturing
process for taliglucerase alfa. On February 20, 2011, we
received a letter from the FDA notifying us that the FDA had
completed its review of the Establishment Inspection Report in
connection with the FDA’s inspection of our facility in
Carmiel, Israel, and that the FDA had classified our facility as
acceptable.
In addition to our recently completed phase III clinical
trial, we initiated a double-blind, follow-on extension study as
part of the trial during the second quarter of 2008. We also
initiated a home care treatment program for patients enrolled in
the extension study and, in December 2008, we initiated a
nine-month, worldwide, multi-center, open-label, switch-over
clinical study evaluating the safety and efficacy of switching
Gaucher patients currently treated under the current standard of
care to treatment with taliglucerase alfa. The current standard
of care for Gaucher patients is enzyme replacement therapy with
Cerezymetm,
which is produced by Genzyme Corporation and, until the recent
approval of
VPRIVtm
by Shire plc in February 2010, was the only approved enzyme
replacement therapy for Gaucher disease. Enzyme replacement
therapy is a medical treatment in which recombinant enzymes are
infused into patients in whom the enzyme is lacking or
dysfunctional. Taliglucerase alfa has an amino acid, glycan and
three-dimensional structure that is very similar to Cerezyme,
which is a mammalian cell expressed version of the same protein.
We believe taliglucerase alfa may prove more cost-effective than
the currently marketed alternative due to the cost benefits of
expression through our ProCellEx protein expression system.
Under our SPA, the switch-over study is not a prerequisite for
approval of taliglucerase alfa by the FDA. In December 2009, we
filed a proposed pediatric investigation plan to the Pediatric
Committee of the EMEA which was approved during the first
quarter of 2010 and have since initiated the study. In November
2010, we announced positive preliminary data from the first
15 patients that completed the switchover clinical study of
taliglucerase alfa.
The Orphan Drug designation for taliglucerase alfa for the
treatment of Gaucher Disease provides special status to
taliglucerase alfa provided that it meets certain criteria. As a
result of the orphan designation, we are qualified for the tax
credit and marketing incentives provided under the Orphan Drug
Act of 1983. A marketing application for a prescription drug
product that has been designated as a drug for a rare disease or
condition is not subject to a prescription drug user fee unless
the application includes an indication for other than a rare
disease or condition.
On November 30, 2009, Protalix Ltd., our wholly-owned
subsidiary, and Pfizer Inc., or Pfizer, entered into an
exclusive license and supply agreement pursuant to which Pfizer
was granted an exclusive, worldwide license to develop and
commercialize taliglucerase alfa. Under the terms and conditions
of the Pfizer agreement, Protalix Ltd. retained the right to
commercialize taliglucerase alfa in Israel. In connection with
the execution of the Pfizer agreement, Pfizer made an upfront
payment to Protalix Ltd. of $60.0 million in connection
with the execution of the agreement and subsequently paid
Protalix Ltd. an additional $5.0 million upon its filing of
a proposed pediatric investigation plan to the Pediatric
Committee of the EMEA. Protalix Ltd. is also eligible to receive
potential milestone payments totaling $50.0 million for the
successful achievement of other regulatory milestones. Pfizer
and Protalix Ltd. will also share future revenues and expenses
for the development and commercialization of taliglucerase alfa
on a 60% and 40% basis, respectively, and have also agreed to a
specific allocation of the responsibilities for the continued
development efforts for taliglucerase alfa.
In July 2009, following a request by the FDA, we submitted a
treatment protocol to the FDA in order to address an expected
shortage of the current enzyme replacement therapy approved for
Gaucher disease. The treatment protocol was approved by the FDA
in August 2009. In September 2009, the FDA’s Office of
Orphan Product Development granted taliglucerase alfa Orphan
Drug Status. In January 2010, the Committee for Orphan Medicinal
Products (COMP) of the EMEA, after reviewing all relevant
clinical data, recommended that the European Commission grant
Orphan Drug designation to taliglucerase alfa for the treatment
of
3
Gaucher disease. The Orphan Drug designation in the United
States for taliglucerase alfa for the treatment of Gaucher
disease provides special status to taliglucerase alfa provided
that it meets certain criteria. As a result of the Orphan Drug
designation, we are qualified for the tax credit and marketing
incentives of the Orphan Drug Act of 1983. A marketing
application for a prescription drug product that has been
designated as a drug for a rare disease or condition is not
subject to a prescription drug user fee unless the application
includes an indication for other than a rare disease or
condition.
On July 13, 2010, we announced that the French regulatory
authority had granted an Autorisation Temporaire
d’Utilisation (ATU), or Temporary Authorization for Use,
for taliglucerase alfa for the treatment of Gaucher disease. An
ATU is the regulatory mechanism used by the French Health
Products and Safety Agency to make non-approved drugs available
to patients in France when a genuine public health need exists.
This ATU allows patients with Gaucher disease in France to
receive treatment with taliglucerase alfa before marketing
authorization for the product is granted in the European Union.
Payment for taliglucerase alfa has been secured through
government allocations to hospitals.
On August 10, 2010, Pfizer entered into a $30 million
short-term supply agreement with the Ministry of Health of
Brazil pursuant to which Protalix and Pfizer have provided
taliglucerase alfa to the Ministry of Health of Brazil for the
treatment of patients with Gaucher disease. Revenue generated
from the Ministry of Health of Brazil will be recorded by Pfizer
and we are entitled to our share of the revenue in accordance
with the terms and conditions of the Pfizer agreement. In
addition, we and the Ministry of Health of Brazil are in
discussions relating to a possible long-term supply agreement
that contemplates, among other matters, providing certain
components of our manufacturing technology to the Ministry of
Health of Brazil for implementation by it in Brazil. We are
currently unable to assess whether these discussions will result
in an agreement and we can make no assurance that we will be
able to enter into such an agreement on favorable terms, if at
all. In any event, we do not expect to enter into a long-term
supply agreement with the Ministry of Health of Brazil until we
receive marketing approval of taliglucerase alfa from the FDA or
ANVISA, if at all.
Although Gaucher disease is a relatively rare disease, it
represents a large commercial market due to the severity of the
symptoms and the chronic nature of the disease. The annual
worldwide sales of Cerezyme were approximately
$722.0 million in 2010, compared with $793.0 million
for the previous year, according to public reports by Genzyme.
According to Genzyme, it suffered a temporary interruption in
production of Cerezyme in 2009 associated with the remediation
of a contamination in one of its manufacturing facilities, and,
as a result, shipments of Cerezyme were limited. Shire reported
annual worldwide sales of VPRIV of approximately
$143.0 million in 2010.
In addition to taliglucerase alfa, we are developing an
innovative product pipeline using our ProCellEx protein
expression system. Our product pipeline currently includes,
among other candidates, (1) PRX-102, a therapeutic protein
candidate for the treatment of Fabry disease, a rare, genetic
lysosomal disorder in humans, (2) PRX-105, a plant cell
expressed pegylated recombinant acetylcholinesterase product
candidate for biodefense and other indications,
(3) pr-antiTNF, a plant cell expressed recombinant fusion
protein made from the soluble form of the human TNF receptor
(TNFR) and an antibody portion, which is being developed as a
treatment of certain immune diseases such as rheumatoid
arthritis, juvenile idiopathic arthritis, ankylosing,
spondylitis, psoriatic arthritis and plaque psoriasis,
(4) an orally administrated glucocerebrosidase enzyme for
treating Gaucher patients utilizing the oral delivery of the
recombinant enzyme produced within carrot cells and
(5) additional undisclosed therapeutic proteins, all of
which are currently being evaluated in animal studies. In March
2010, we initiated a preliminary phase I clinical trial of
PRX-105 which we completed in June 2010. We are currently
preparing for further efficacy trials of this product candidate
in larger animals. In our preclinical studies we utilized an
analogue to nerve gas. However, we anticipate that we will use
live nerve gas rather than an analogue in the proposed
additional efficacy trials in animals.
In December 2010 we held a pre-investigational new drug, or IND,
meeting with the FDA with respect to PRX-102. We expect to
submit an IND to the FDA within the next 12 months in
connection with an anticipated phase I/II study of PRX-102 and
to initiate the trial once approved, if at all.
In September 2009, we announced preliminary preclinical data
regarding pr-antiTNF. Our pr-antiTNF product candidate has an
amino acid sequence that is similar to
Enbrel®
which is one of the treatments for
4
patients of those diseases. We believe that we may be able to
reduce the development risks and time to market for our product
candidates as our product candidates are based on
well-understood proteins with known biological mechanisms of
action. Except for the rights to commercialize taliglucerase
alfa worldwide (other than Israel) which we licensed to Pfizer,
we hold the worldwide commercialization rights to our
proprietary development candidates, and we intend to establish
an internal, commercial infrastructure and targeted sales force
to market taliglucerase alfa in Israel and our other products,
if approved, in North America, the European Union and in other
significant markets, including Israel. In addition we are
continuously evaluating potential strategic marketing
partnerships.
Our ProCellEx protein expression system consists of a
comprehensive set of technologies and capabilities for the
development of recombinant proteins, including advanced genetic
engineering technology and plant cell-based protein expression
methods. Through our ProCellEx protein expression system, we can
develop highly complex recombinant therapeutic proteins all the
way to the
scale-up of
a purified product produced in compliance with current good
manufacturing practices, or cGMP. We believe that our plant
cell-based expression technology will enable us, in certain
cases, to develop and commercialize recombinant proteins without
infringing upon the method-based patents or other intellectual
property rights of third parties. The major elements of our
ProCellEx system are patent protected in most major countries.
Moreover, we expect to enjoy method-based patent protection for
the proteins we develop using our proprietary ProCellEx protein
expression technology, although there can be no assurance that
any such patents will be granted. In some cases, we may be able
to obtain patent protection for the compositions of the proteins
themselves. We have filed for United States and international
composition of matter patents for taliglucerase alfa.
Our ProCellEx protein expression system is built on flexible
custom-designed bioreactors made of polyethylene and optimized
for the development of complex proteins in plant cell cultures.
These bioreactors entail low initial capital investment, are
rapidly scalable at a low cost and require less hands-on
maintenance between cycles, compared to the highly complex,
expensive, stainless steel bioreactors typically used in
mammalian cell-based production systems. As a result, through
our ProCellEx protein expression system, we believe that we can
develop recombinant therapeutic proteins yielding substantial
cost advantages, accelerated development and other competitive
benefits as compared to mammalian cell-based protein expression
systems.
We have successfully demonstrated the feasibility of our
ProCellEx system through clinical and preclinical studies
performed by us to date including the positive efficacy and
safety data in our phase III study for taliglucerase alfa,
preclinical results in well-known models in our enzyme for Fabry
disease and pr-antiTNF, and extensive animal studies for our
acetylcholinesterase enzyme, and by expressing, on an
exploratory, research scale, many additional complex therapeutic
proteins belonging to different drug classes, such as enzymes,
hormones, monoclonal antibodies, cytokines and vaccines. The
therapeutic proteins we have expressed to date in research
models have produced the intended composition and similar
biological activity compared to their respective
human-equivalent proteins. Moreover, several of such proteins
demonstrated advantageous biological activity when compared to
the biotherapeutics currently available in the market to treat
the applicable disease or disorder. We believe that the clinical
success of taliglucerase alfa represents a strong
proof-of-concept
for our ProCellEx protein expression system and plant cell-based
protein expression technology. We also believe that the
significant benefits of our ProCellEx protein expression system,
if further substantiated in clinical trials and
commercialization of our product candidates, have the potential
to transform the industry standard for the development of
complex therapeutic proteins.
Our goal is to become a leading fully integrated
biopharmaceutical company focused on the development and
commercialization of proprietary and biosimilar or generic
versions of recombinant therapeutic proteins. To that end, we
are leveraging our ProCellEx protein expression system to
develop a pipeline of proprietary and biosimilar versions of
recombinant therapeutic proteins. In addition to the product
candidates that we are developing internally, we have entered
into agreements for additional compounds with academic
institutions, including a licensing agreement with the
technology transfer arm of Israel’s Weizmann Institute of
Science and an agreement with the technology transfer arm of the
Hebrew University of Jerusalem. In addition, we are
collaborating with other pharmaceutical companies to develop
therapeutic proteins that can benefit from the significant cost,
intellectual property and other competitive advantages of our
ProCellEx protein expression system. We entered into an
agreement with Teva Pharmaceutical Industries Ltd., or Teva, in
September 2006
5
under which we have agreed to collaborate on several matters. We
and Teva identified two proteins for research and development
activities under the agreement, but in 2009 both of the projects
were terminated for commercial reasons. Other elements of our
collaboration with Teva are currently ongoing. We also
continuously review and consider additional development and
commercialization alliances with other pharmaceutical companies
and academic institutions.
Industry
Overview
Recombinant proteins have revolutionized the treatment of a
variety of diseases and disorders. Recombinant proteins are
forms of human proteins that are produced, or expressed, using a
mammalian, plant, bacterial or yeast cell as a production
engine. In the early 1970s, a number of key scientific
breakthroughs, including, among others, the demonstration of
genetic engineering and genetic sequencing techniques, as well
as the synthesis of genes, led to the advancement of recombinant
protein technology.
As a result, the market for pharmaceutical therapeutics has
undergone a transformation as recombinant proteins and other
biologic products have become an increasingly significant
portion of the global drug market and the focus of research
worldwide. Based upon data from the Biotechnology Industry
Organization, an organization that provides information,
advocacy and business support to the biotechnology industry,
since the introduction in 1982 of recombinant human insulin, the
world’s first genetically engineered pharmaceutical
product, over 254 biotechnology drugs have been approved for
over 392 indications. According to Datamonitor, a provider of
business information to the pharmaceutical and other industries,
the overall global biologics market size is expected to grow to
$105.2 billion in 2010, from $56.1 billion in 2004,
representing a compounded annual growth rate (CAGR) of 11.1%.
Mammalian cell-based systems are the current industry standard
for expression of recombinant therapeutic glycoproteins (complex
proteins that contain sugar residues), including catalytic
enzymes and monoclonal antibodies. Mammalian cell-based systems
were first introduced in the late 1980s and are currently used
to produce many of the biotechnology industry’s largest and
most successful therapeutic proteins, including
Epogen®,
Neupogen®,
Cerezyme,
Rituxan®,
Enbrel®,
Neulasta®
and
Herceptin®.
Mammalian cell-based expression technology is based on the
introduction of a human gene encoding for a specific therapeutic
protein into the genome of a mammalian cell. The cells most
often used in connection with mammalian cell-based protein
expression are Chinese hamster ovary (CHO) cells.
Mammalian cell-based expression systems have become the dominant
system for the expression of recombinant proteins due to their
capacity for sophisticated, proper protein folding (which is
necessary for proteins to carry out their intended biological
activity), assembly and post-expression modification, such as
glycosilation (the addition of sugar residues to a protein which
is necessary to enable specific biological activity by the
protein). While bacterial and yeast cell-based expression
systems were the first protein expression systems developed by
the biotechnology industry and remain cost-effective compared to
mammalian cell-based production methodologies, proteins
expressed in bacterial and yeast cell-based systems lack the
capacity for sophisticated protein folding, assembly and
post-expression modifications, which are key factors of
mammalian cell-based systems. Accordingly, such systems cannot
be used to produce glycoproteins or other complex proteins and,
therefore, bacterial and yeast cell-based systems are limited to
the expression of the most basic, simple proteins, such as
insulin and growth hormones. Due to their significant
advantages, mammalian cell-based expression systems can produce
proteins with superior quality and efficacy compared to proteins
expressed in bacteria and yeast cell-based systems. As a result,
the majority of currently approved therapeutic proteins, as well
as those under development, are produced in mammalian cell-based
systems.
Despite the utility and widespread use of mammalian cell-based
systems, they are subject to a number of disadvantages. CHO
cells and other mammalian cells are highly sensitive and can
only be grown under near perfect conditions, requiring highly
complex, expensive, stainless steel bioreactors which tightly
regulate the required temperature, pH and oxygen levels. As a
result, such bioreactor systems are very costly and complicated
to operate. CHO cells and other mammalian cells are also
susceptible to viral infections, including human viruses, and
several cases of viral contamination have occurred recently. The
FDA and other regulatory authorities require viral inactivation
and other rigorous and detailed procedures for mammalian
cell-based
6
manufacturing processes in order to address these potential
hazards, thereby increasing the cost and time demands of such
expression systems. Furthermore, the current FDA and other
procedures only ensure screening for scientifically identified,
known viruses. Accordingly, compliance with current FDA and
other procedures does not fully guarantee that patients are
protected against transmission of unknown or new potentially
fatal viruses that may infect mammalian cells. In addition,
mammalian cell-based expression systems require large quantities
of sophisticated and expensive growth medium to accelerate the
expression process.
Several companies and research institutions have explored
alternatives to mammalian cell-based production technologies
that overcome some of these disadvantages, focusing primarily on
the expression of human proteins in genetically-modified
organisms, or GMOs, such as transgenic field-grown, whole plants
and transgenic animals. However, these alternate techniques may
be restricted by regulatory and environmental risks regarding
contamination of agricultural crops and by the difficulty in
applying cGMP standards of the pharmaceutical industry to these
expression technologies.
ProCellEx:
Our Proprietary Protein Expression System
ProCellEx is our proprietary production system that we have
developed based on our plant cell culture technology for the
development, expression and manufacture of recombinant proteins.
Our expression system consists of a comprehensive set of
capabilities and proprietary technologies, including advanced
genetic engineering and plant cell culture technology, which
enables us to produce complex, proprietary and biologically
equivalent proteins for a variety of human diseases. Our protein
expression system facilitates the creation and selection of high
expressing, genetically stable cell lines capable of expressing
recombinant proteins. The entire protein expression process,
from initial nucleotide cloning to large-scale production of the
protein product, occurs under cGMP-compliant, controlled
processes. Our plant cell culture technology uses plant cells,
such as carrot and tobacco cells, which undergo advanced genetic
engineering and are grown on an industrial scale in a flexible
bioreactor system. Cell growth, from scale up through
large-scale production, takes place in flexible, sterile,
polyethylene bioreactors which are confined to a clean-room
environment. Our bioreactors are well-suited for plant cell
growth using a simple, inexpensive, chemically-defined growth
medium as a catalyst for growth. The reactors are
custom-designed and optimized for plant cell cultures, easy to
use, entail low initial capital investment, are rapidly scalable
at a low cost and require less hands-on maintenance between
cycles. Our protein expression system does not involve mammalian
or animal components or transgenic field-grown, whole plants at
any point in the production process.
Our ProCellEx system is capable of producing proteins with an
amino acid structure practically equivalent to that of the
desired human protein, and with a very similar, although not
identical, glycan, or sugar, structure. Our internal research
and external laboratory studies have demonstrated that ProCellEx
is capable of producing recombinant proteins that exhibit a
glycan and amino acid structure similar to their
naturally-produced human counterparts. In collaboration with
Israel’s Weizmann Institute of Science, we have
demonstrated that the three-dimensional structure of a protein
expressed in our proprietary plant cell-based expression system
retains the same three-dimensional structure as exhibited by the
mammalian cell-based expressed version of the same protein. In
addition, proteins produced by our ProCellEx system maintain the
biological activity that characterize that of the
naturally-produced proteins. Based on these results, we believe
that proteins developed using our ProCellEx protein expression
system have the intended composition and correct biological
activity of their human equivalent proteins.
Competitive
Advantages of Our ProCellEx Protein Expression System
We believe that our ProCellEx protein expression system,
including our advanced genetic engineering technology and plant
cell-based protein expression methods, affords us a number of
significant advantages over mammalian, bacterial, yeast and
transgenic cell-based expression technologies, including the
following:
Ability to Penetrate Certain Patent-Protected
Markets. We seek to develop recombinant proteins
that we believe we can produce and commercialize without
infringing upon the method-based patents or other intellectual
property rights of third parties. Certain biotherapeutic
proteins available for commercial
7
sale are not protected by patents that cover the compound and
are available for use in the public domain. Rather, the process
of expressing the protein product in mammalian or bacterial cell
systems is protected by method-based patents. Using our plant
cell-based protein expression technology, we are able to express
an equivalent protein without infringing upon these method-based
patents. Moreover, we expect to enjoy method-based patent
protection for the proteins we develop using our proprietary
ProCellEx protein expression technology, although there can be
no assurance that any such patents will be granted. In some
cases, we may be able to obtain patent protection for the
compositions of the proteins themselves. We have filed for
United States and international composition of matter patents
for taliglucerase alfa.
Significantly Lower Capital and Production
Costs. Plant cells have a number of dynamic
qualities that make them well-suited for the production of
therapeutic proteins. Plant cells grow rapidly under a variety
of conditions and are not as sensitive to temperature, pH and
oxygen levels as mammalian cells. Our ProCellEx protein
expression system, therefore, requires significantly less
upfront capital expenditures as it does not use the highly
complex, expensive, stainless steel bioreactors typically used
in mammalian cell-based production systems to maintain very
specific temperature, pH and oxygen levels. Instead, we use
simple polyethylene bioreactors that are able to be maintained
at the room temperature of the clean-room in which they are
placed. This system also reduces ongoing production and
monitoring costs typically incurred by companies using mammalian
cell-based expression technologies. Furthermore, while mammalian
cell-based systems require very costly growth media at various
stages of the production process to achieve target yields of
their proteins, plant cells require only simple and much less
expensive solutions based on sugar, water and microelements at
infrequent intervals to achieve target yields. We believe that
these factors will potentially result in lower capital and
production costs for the commercial scale production of proteins
by our ProCellEx system thereby providing us with a competitive
advantage over competing protein expression technologies.
Elimination of the Risk of Viral Transmission or Infection by
Mammalian Components. By nature, plant cells do
not carry the risk of infection by human or other animal
viruses. As a result, the risk of contamination of our products
under development and the potential risk of viral transmission
from our products under development to future patients, whether
from known or unknown viruses, is eliminated. Because our
product candidates do not bear the risk of viral transmission,
we are not required by the FDA or other regulatory authorities
to perform the constant monitoring procedures for mammalian
viruses during the protein expression process that mammalian
cell-based manufacturers are required to undertake. In addition,
the production process of our ProCellEx protein expression
system is void of any mammalian components which are susceptible
to the transmission of prions, such as those related to bovine
spongiform encephalopathy (commonly known as “mad-cow
disease”). These factors further reduce the risks and
operating costs of our ProCellEx system compared to mammalian
cell-based expression systems.
More Effective and Potent End Product Relative to Mammalian
Based Systems. Our ProCellEx protein expression
system produces enzymes which have uniform glycosilation
patterns and therefore do not require the lengthy and expensive
post-expression modifications that are required for certain
proteins produced by mammalian cell-based systems, including the
proteins for the treatment of Gaucher disease. Such
post-expression modifications in mammalian cell-produced
proteins are made in order to expose the terminal mannose sugar
residues, which are structures on a protein that are key
elements in allowing the produced protein to bind to a target
cell and subsequently be taken into the target cell for
therapeutic benefit. In the production of Cerezyme, exposing
these terminal mannose sugar residues involves a multitude of
highly technical steps which add time and cost to the production
process. In addition, these steps do not guarantee the exposure
of all of the required terminal mannose sugar residues,
resulting in potentially lower effective yields and
inconsistency in potency from batch to batch. Our ProCellEx
protein expression system, by contrast, produces taliglucerase
alfa in a “ready to use” form that does not require
additional glycosilation or other modifications to make
taliglucerase alfa suitable for use in enzyme replacement
therapy for Gaucher disease. We believe this quality increases
the potency and consistency of the expressed proteins, thereby
further increasing the cost advantages of our ProCellEx protein
expression system over competing protein expression
methodologies.
8
Broad Range of Expression Capabilities. Unlike
bacterial and yeast cell-based systems, which are unable to
produce complex proteins, our ProCellEx protein expression
system is able to produce a broad array of complex glycosilated
proteins. We have successfully demonstrated the feasibility of
our ProCellEx system by producing, on an exploratory, research
scale, a variety of therapeutic proteins belonging to different
classes of recombinant drugs, such as enzymes, hormones,
monoclonal antibodies, cytokines and vaccines. We have
demonstrated that the recombinant proteins we have expressed to
date have the intended composition and correct biological
activity of their human-equivalent protein, with several of such
proteins demonstrating advantageous biological activity compared
to the currently available biotherapeutics. In specific cases,
we have been successful in expressing proteins that have not
been successfully expressed in other production systems.
Our
Strategy
Our goal is to become a leading fully integrated
biopharmaceutical company focused on the development and
commercialization of proprietary and biosimilar or generic
versions of recombinant therapeutic proteins. To achieve our
goal, we intend to:
Facilitate the successful development and commercialization
of taliglucerase alfa by Pfizer. We intend to
work with our licensee, Pfizer, to develop and commercialize
taliglucerase alfa. We have begun collaborating with Pfizer to
facilitate the transition of certain of our taliglucerase alfa
assets to Pfizer’s organization. We are cooperating with
Pfizer with respect to our Expanded Access protocol for
taliglucerase alfa in order to facilitate the participation of
additional physicians in additional sites in the protocol.
Pfizer is promoting the protocol to new clinical sites and is
recruiting additional patients. We have also begun to facilitate
relationships between Pfizer and the Gaucher community and
third-party payors. We intend to actively participate and
provide our expertise in Pfizer’s development and
commercialization efforts with respect to taligluerase alfa.
Obtain Regulatory Approval for Taliglucerase Alfa for the
Treatment of Gaucher Disease. We completed
successfully our pivotal phase III clinical trial of
taliglucerase alfa in September 2009 and announced the positive
top-line study results in October 2009 and full study results in
February 2010. We filed an NDA with the FDA in December 2009 and
in January 2010, the FDA requested additional data regarding the
Chemistry, Manufacturing and Controls (CMC) section of the
NDA. We provided the requested data to the FDA in April 2010 and
in July 2010 we received notification from the FDA that it had
accepted the filing of our NDA and assigned it a PDUFA date of
February 25, 2011. In addition, in November 2010 we
submitted a marketing application to the Israeli MOH, and with
our partner Pfizer, an MAA to each of the EMEA and to ANVISA for
taliglucerase alfa. Our phase III clinical trial was
conducted in selected leading medical centers worldwide in North
America, South America, Israel, Europe and South Africa. In the
third quarter of 2008, we initiated a double blind, follow-on
extension study as part of the phase III clinical trial in
which patients that successfully completed treatment in the
trial were given the opportunity to continue to be treated with
taliglucerase alfa at the same dose that they received in the
trial. We are compiling additional information relating to the
long term safety and efficacy of taliglucerase alfa through the
follow-on study. In addition, in the fourth quarter of 2008 we
announced the enrollment of the first patient in a worldwide,
multi-center, open-label, switch-over trial to assess the safety
and efficacy of taliglucerase alfa. The switch-over trial, which
is not a pre requisite for marketing approval from the FDA, was
designed to include 15 patients with Gaucher disease that
are currently undergoing enzyme replacement therapy with
imiglucerase (Cerezyme). Due to the shortage in 2009 of the
currently available enzyme replacement therapy for Gaucher
disease, after fully enrolling the 15 patients we extended
the trial to include up to 30 patients in total. In
December 2009, we filed a proposed pediatric investigation plan
to the Pediatric Committee of the EMEA and have since initiated
pediatric studies. We believe that taliglucerase alfa may have
cost, efficacy and potency advantages over the currently
available enzyme replacement therapy for Gaucher disease and we
intend to pursue post-marketing studies to confirm these
advantages. Although Gaucher disease is a relatively rare
disease, it represents a substantial commercial market due to
the severity of the symptoms and the chronic nature of the
disease. We believe that the approval of taliglucerase alfa as a
treatment for Gaucher disease, if at all,
9
with its potentially longer acting profile and more
cost-effective development process, may lead to an increase in
the number of patients who will be able to have access to and
afford such treatment, thereby expanding the size of the market
for Gaucher disease treatments.
Develop a Pipeline of Innovative or Biosimilar Versions of
Recombinant Therapeutic Proteins. We are
leveraging our ProCellEx protein expression system to develop a
pipeline of innovative or biosimilar versions of recombinant
proteins, with an emphasis on therapeutic treatments with large
market opportunities. We select additional therapeutic
candidates for development through in-house testing, licensing
agreements with academic institutions and collaborations with
pharmaceutical partners. We have currently identified several
product candidates that are mainly oriented towards the
specialty disease and therapeutic market segments, including
treatments for Fabry disease and an acetylcholinesterase enzyme
based therapy for biodefense and nerve gas toxicity treatments.
We have also identified several other product candidates that
are chemical equivalents of approved therapeutic products that
will no longer be patent protected within the next couple of
years, such as pr-antiTNF, our proprietary product candidate for
the treatment of certain immune diseases such as rheumatoid
arthritis. We believe our cost-effective technology will be an
important asset for the commercialization of such drug
candidates. We believe that the clinical and regulatory pathway
for many of our pipeline product programs candidates is already
established, and that this may reduce the risks and costs
associated with our clinical development programs. Furthermore,
established markets already exist for the development of most of
our current product candidates. We plan to apply the
manufacturing, clinical and regulatory experience we have gained
from the development of our lead product candidate to advance a
number of our preclinical product candidates into clinical
trials over the next few years.
Collaborate with Third Party Pharmaceutical Suppliers and
build a Targeted Sales and Marketing
Infrastructure. We have licensed to Pfizer the
right to commercialize taliglucerase worldwide, except in
Israel. We plan to establish our own, internal sales and
marketing capabilities for taliglucerase in Israel, and, for our
other product candidates, in North America, the European Union
and in other significant markets, including Israel. We believe
that the focus of our current clinical pipeline on relatively
rare genetic disorders with small patient populations and a
highly concentrated group of physicians focused on treating
patients with such disorders may facilitate our creation of a
targeted internal sales force. In addition we are continuously
evaluating potential strategic marketing partnerships with
respect to our other product candidates.
Establish Development and Commercialization Alliances with
Corporate Partners. We believe that our
technology and know-how has broad applicability to many classes
of proteins and can be used to develop and potentially enhance
numerous existing marketed protein therapeutics. We intend to
leverage our technology and know-how by pursuing development and
commercialization alliances with corporate partners for specific
products and territories in order to enable us to optimize our
resources and effectively penetrate a wider range of target
diseases and therapeutic markets. In November 2009, we entered
into a license and supply agreement with Pfizer for the
development and commercialization of taliglucerase alfa. We
entered into an agreement with Teva in September 2006 for the
development of two proteins. Although programs relating to two
proteins to be developed under the agreement were terminated for
commercial reasons in 2009, other elements of our collaboration
are still ongoing. Last, we are in various stages of discussions
with a number of multinational pharmaceutical companies
regarding additional collaboration agreements.
Acquire or In-License New Technologies, Products or
Companies. We continuously seek attractive
product candidates and innovative technologies to in-license or
acquire. We intend to focus on product candidates that would be
synergistic with our ProCellEx protein expression system and
expertise and that represent large potential market
opportunities. We believe that by pursuing selective
acquisitions of technologies in businesses that complement our
own, we will be able to enhance our competitiveness and
strengthen our market position.
Leverage Strength and Experience of Our Management Team and
Board of Directors. Our management team has
extensive experience in the biotechnology and pharmaceutical
industry. The Interim
10
Chairman of our Board of Directors, Mr. Zeev Bronfeld, is a
highly experienced Israeli health care investor. In February
2008, we appointed Professor Roger D. Kornberg, a renowned
biochemist and laureate of the Nobel Prize in Chemistry, to our
Board of Directors. We will continue to leverage their
experience and established track record as well as their
relationships across the biotechnology and pharmaceutical
industries.
Our
Pipeline Drug Candidates
Our
Lead Product Candidate, Taliglucerase Alfa
Taliglucerase alfa, our lead proprietary product candidate, is a
plant cell expressed recombinant glucocerebrosidase enzyme (GCD)
for the treatment of Gaucher disease. In July 2007, we reached
an agreement with the FDA on the final design of our pivotal
phase III clinical trial of taliglucerase alfa through the
FDA’s special protocol assessment (SPA) process. We
successfully completed our phase III pivotal clinical trial
of taliglucerase alfa in September 2009 and announced positive
top line results of the clinical trial in October 2009 and full
study results in February 2010. We submitted an NDA to the FDA
in December 2009. In January 2010, the FDA requested additional
data regarding the Chemistry, Manufacturing and Controls
(CMC) section of the NDA. We provided the requested data to
the FDA in April 2010 and in July 2010 we received notification
from the FDA that it had accepted the filing of our NDA and
assigned it a PDUFA date of February 25, 2011. In addition,
in November 2010 we submitted a marketing application to the
Israeli MOH and an MAA to each of the EMEA and ANVISA for
taliglucerase alfa for the treatment of Gaucher disease. During
the third quarter of 2008, we initiated a double blind,
follow-on extension study as part of our phase III clinical
trial of taliglucerase alfa in which patients that successfully
completed treatment in the trial were given the opportunity to
continue to be treated with taliglucerase alfa at the same dose
that they received in the trial. We are compiling additional
information relating to the long term safety and efficacy of
taliglucerase alfa through the follow-on study.
In the fourth quarter of 2008 we announced the enrollment of the
first patient in a worldwide, multi-center, open-label,
switch-over trial which has been reviewed by the FDA and is
designed to assess the safety and efficacy of taliglucerase
alfa. The switch-over trial, which is not a pre requisite for
approval, is designed to include 15 patients with Gaucher
disease that are currently undergoing enzyme replacement therapy
with imiglucerase (Cerezyme). Due to the then shortage of the
currently available enzyme replacement therapy for Gaucher
disease, after fully enrolling the 15 patients, we extended
the trial to include up to 30 patients in total. In
November 2010, we announced positive preliminary data from the
first 15 patients that completed the switchover trial. The
data indicate that patients can safely be switched to
taliglucerase alfa from Cerezyme. Patients enrolled in the trial
were switched from Cerezyme (doses ranging from
10-60 U/kg
every other week) to an equivalent dose using the same number of
units of taliglucerase alfa. The data from the first
15 patients demonstrate that maintenance of efficacy was
achieved over a nine-month period with no increased safety
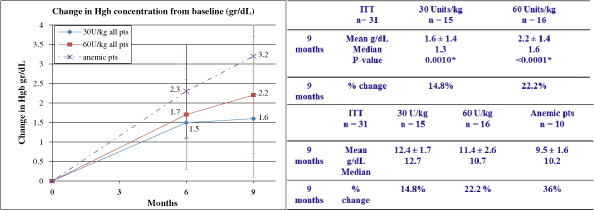
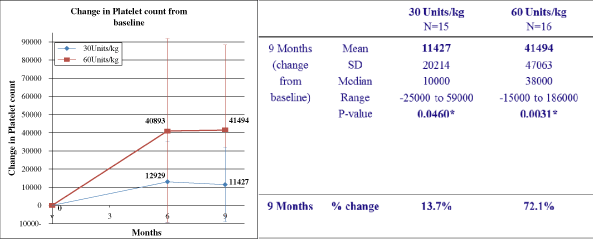
concerns. Patients’ hemoglobin and platelet counts remained
stable demonstrating hematological stability. As measured by
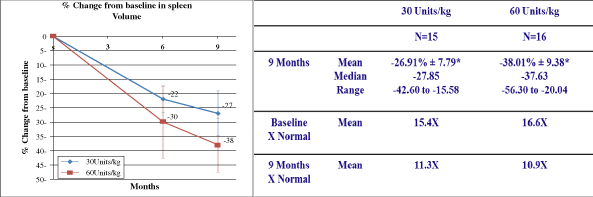
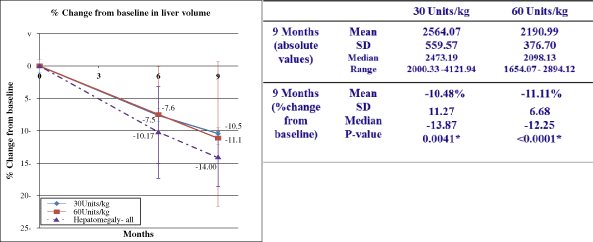
MRI, mean spleen volume and liver volume also remained stable.
There was no evidence of increased safety concerns in patients
switched from Cerezyme to taliglucerase alfa and there were no
drug related serious adverse events. Hypersensitivity reactions
were not reported in this patient group. One patient developed
non-neutralizing IgG antibodies to taliglucerase at the end of
the study.
In addition, in December 2009, we filed a proposed pediatric
investigation plan to the Pediatric Committee of the EMEA and
have since initiated pediatric studies. In clinical trials in
healthy subjects and in vivo primate studies, taliglucerase alfa
has demonstrated an increased half-life and prolonged presence
of the enzyme in the blood serum of the subjects as compared to
Cerezyme, the only enzyme replacement therapy currently marketed
to treat Gaucher disease.
We believe that taliglucerase alfa, if approved, has the
potential to offer patients and healthcare payors a more
effective and cost efficient treatment of Gaucher disease
because of the following features:
Increased Glycan Efficacy and Consistency. We
believe that our ProCellEx protein expression system produces
recombinant proteins that exhibit consistent enzymatic activity
from batch to batch. This results in a highly active product
that may achieve a desired therapeutic effect more effectively
than the
11
activity demonstrated in proteins produced through mammalian
cell-based expression systems due to its greater glycan efficacy
and consistency. This quality increases the effective
consistency in potency and further increases the cost advantages
from using our plant cell-based expression technology compared
to competing protein expression methodologies.
Longer Half-Life. The data generated in
preclinical and human clinical trials relating to the half-life
of taliglucerase alfa in the subjects’ blood serum after
infusion showed that the half-life of taliglucerase alfa is
significantly longer than that of Cerezyme when measured and
compared to publicly available data on Cerezyme.
Cost-Effective. Taliglucerase alfa is
potentially less expensive to produce as the manufacturing
process does not require the large initial
set-up
investments involved in mammalian cell-based protein production,
the extensive ongoing costs associated with growth media and
monitoring throughout the production process nor any of the
post-expression modification costs in order to modify the
glycosilation of the proteins produced through the mammalian
cell-based methodologies.
As such, we believe that taliglucerase alfa’s potential
advantages may lead taliglucerase alfa to become a highly
efficacious and cost-effective treatment alternative for Gaucher
disease patients.
In addition, we are developing a new method for delivering
active recombinant proteins systemically through oral
administration of transgenic plant cells expressing
biotherapeutic proteins. We have commenced pre-clinical studies
of an oral form of taliglucerase alfa. If proven effective, our
experimental oral taliglucerase alfa would be the first protein
to be administered orally rather than through intravenous
therapy. Our oral taliglucerase alfa is a plant cell expressed
form of GCD that is naturally encapsulated within carrot cells
genetically engineered to express the GCD enzyme. Pre-clinical
studies of oral taliglucerase alfa demonstrate the stability of
the enzyme in the cell and the capacity of the cell’s
cellulose wall to protect the enzyme against degradation in the
digestive tract in an in-vitro model of the stomach and
intestines. Additionally, rats fed with lyophilized carrot cells
expressing GCD have accumulated the active enzyme in the target
organs; the spleen and liver.
We believe that oral delivery of taliglucerase alfa presents a
number of advantages. First, the development of oral
taliglucerase alfa has the advantage of leveraging the
well-characterized mechanism of action of our
intravenously-administered taliglucerase alfa product candidate.
In addition, we believe that oral delivery of taliglucerase alfa
may dramatically change the treatment paradigm for Gaucher
patients, compared to the intravenous delivery of taliglucerase
alfa, and contribute to increased compliance and the
facilitation of treatment management. Further, oral delivery of
GCD targets the disease-specific organs without the need for
lifetime dependence on repeated intravenous infusions. Last, our
oral enzyme replacement therapy does not have the unpredictable
long term untoward effects of the inhibition of other
non-disease-specific compounds that are common in oral substrate
reduction therapy.
Our oral taliglucerase alfa product candidate is a recombinant
form of the GCD enzyme, not a small molecule. This
differentiates our oral product candidate from other early
clinical stage, experimental, small molecule, oral drugs which
are being developed for the treatment of Gaucher disease by
Genzyme and Amicus Therapeutics, Inc., or Amicus Therapeutics.
Small molecule based treatments for Gaucher disease, such as
Zavesca, have different mechanisms of action than those
associated with enzyme replacement therapy, and may be
associated with a number of side effects. We have filed patent
applications with respect to this new protein delivery mechanism
in other countries with commercially significant markets.
Currently, we are the exclusive owners of all rights to this
technology.
Gaucher
Disease Background
Gaucher disease, a hereditary, genetic disorder with severe and
debilitating symptoms, is the most prevalent lysosomal storage
disorder in humans. Lysosomal storage disorders are metabolic
disorders in which a lysosomal enzyme, a protein that degrades
cellular substrates in the lysosomes of cells, is mutated or
deficient. Lysosomes are small membrane-bound cellular
structures within cells that contain enzymes necessary for
intracellular digestion. Gaucher disease is caused by mutations
or deficiencies in the gene
12
encoding GCD, a lysosomal enzyme that catalyzes the degradation
of the fatty substrate, glucosylceramide (GlcCer). The normal
degradation products of GlcCer are glucose and ceramide, which
are easily excreted by the cells through normal biological
processes. Patients with Gaucher disease lack or otherwise have
dysfunctional GCD and, accordingly, are not able to break down
GlcCer. The absence of an active GCD enzyme leads to the
accumulation of GlcCer in lysosomes of certain white blood cells
called macrophages. Macrophages affected by the disease become
highly enlarged due to the accumulation of GlcCer and are
referred to as “Gaucher cells.” Gaucher cells
accumulate in the spleen, liver, lungs, bone marrow and brain.
Signs and symptoms of Gaucher disease may include enlarged liver
and spleen, abnormally low levels of red blood cells and
platelets and skeletal complications. In some cases, the patient
may suffer an impairment of the central nervous system.
Current
Treatments for Gaucher Disease
The standard of care for Gaucher disease is enzyme replacement
therapy using recombinant GCD to replace the mutated or
deficient natural GCD enzyme. The latest studies estimate that
there are approximately 10,000 patients suffering from
Gaucher disease worldwide. Enzyme replacement therapy is a
medical treatment in which recombinant enzymes are injected into
patients in whom the enzyme is lacking or dysfunctional.
Cerezyme, an enzyme replacement therapy commercialized by
Genzyme, and VPRIV, an enzyme replacement therapy commercialized
by Shire, are the only recombinant GCDs currently available on
the market for the treatment of Gaucher disease. According to
public reports issued by Genzyme, Cerezyme had annual sales of
approximately $722 million in 2010, compared to
$793 million in 2009. According to Genzyme it suffered a
temporary interruption in production of Cerezyme in 2009
associated with the remediation of a contamination in one of its
manufacturing facilities, and, as a result, shipments of
Cerezyme were limited during the second half of 2009. Shire has
reported sales of VPRIV equal to $143 million during 2010
after the approval of VPRIV in February 2010.
Cerezyme is produced through a mammalian cell-based protein
expression process in CHO cells. There are no known severe side
effects to the use of Cerezyme and its approved use over the
past decade suggests that it is an effective treatment of
Gaucher disease. However, Cerezyme is subject to the limitations
of most mammalian cell-based therapeutic proteins, including
lengthy and costly production processes and contamination risks.
As enzyme replacement therapy does not cure the genetic
disorder, but rather provides an external source for transfusion
of the missing or mutated enzyme, Gaucher disease patients
generally receive the treatment over their entire lifetime.
Zavesca (miglustat), which is marketed by Actelion Ltd., or
Actelion, is a small molecule drug for the treatment of Gaucher
disease. Zavesca has been approved by the FDA for use in the
United States as an oral treatment. However, it has many side
effects and the FDA has approved it only for administration to
those patients who cannot be treated through enzyme replacement
therapy, and, accordingly, have no other treatment alternative.
As a result, Zavesca’s use has been extremely limited.
Actelion has reported sales of Zavesca of approximately CHF
68.7 million (approximately $72.0 million) in 2010.
Taliglucerase
Alfa Development Program
We believe the clinical development path for taliglucerase alfa
will be similar to that followed by the existing enzyme
replacement therapy currently on the market. The primary
efficacy endpoint for our pivotal phase III study was the
reduction in size of spleen and the secondary endpoints for our
pivotal phase III study included increase in platelet and
hemoglobin counts and reduction in liver size, all of which are
generally well-established and accepted by regulatory agencies
and specifically agreed to by the FDA in the special protocol
assessment (SPA) of the final design of our pivotal
phase III clinical trial for taliglucerase alfa. The
phase III clinical trial was successfully completed in
September 2009 and we announced positive top-line results from
the trial in October 2009 and full study results in February
2010. The trial met its primary endpoint, mean reduction in
spleen volume after nine months compared with baselines, in both
60 U/kg dose and in the lower 30 U/kg dose treatment groups
(P<0.0001). In addition, the primary endpoint was observed
already after six months of treatment in both treatment groups.
Statistically significant improvements compared with baselines
were also observed in the secondary endpoints, including
increase in hemoglobin level, decrease
13
in liver size and increase in platelet count at the 60 U/kg
dose. Statistically significant improvements compared with
baselines were observed in hemoglobin level and liver size and
significant nominal elevation in platelet count in the lower
dose of 30 U/kg. However, the lower dose group did not meet the
secondary endpoint relating to platelet count. See
“— Phase III Clinical Trial.” The
primary end point for our switch-over study, which is not a
prerequisite for approval, is non deterioration in the
patient’s clinical condition as measured through
significant, well established end points such as platelet and
hemoglobin counts and spleen and liver size.
Laboratory
Testing and Preclinical Studies of Taliglucerase Alfa
We conducted several in vitro tests and in vivo preclinical
studies of taliglucerase alfa. Our preclinical rodent and
primate trials generated extensive toxicological and safety data
that demonstrated no adverse effects, even with very high doses
of taliglucerase alfa being administered via intravenous
infusions. In short term repeat dose studies in rodents and
primates and nine month repeat dose studies in primates, no
toxicity was observed at dosage levels of up to 10 times the
current dose recommended for GCD in clinical use. Furthermore,
no neutralizing antibodies were detected in any of the primates
treated in the studies. The presence of neutralizing antibodies
would have implied a likelihood of the host rejecting the
therapeutic enzyme or reacting to it in a less efficient manner.
Our laboratory and preclinical data demonstrate that
taliglucerase alfa has the potential to be an efficacious enzyme
replacement therapy for the treatment of Gaucher disease. Data
produced from these preliminary development studies show that,
relative to Cerezyme, taliglucerase alfa has:
|
|
|
| |
•
|
an equivalent to superior level of enzymatic activity;
|
| |
| |
•
|
enhanced uptake based on observed GlcCer substrate degradation;
and
|
| |
| |
•
|
a prolonged half-life.
|
Phase I
Clinical Trial
We completed a phase I clinical trial of taliglucerase alfa in
June 2006. The phase I clinical trial was a single-center,
non-randomized, open label, dose ranging study designed to
evaluate the safety and pharmacokinetics of taliglucerase alfa
in healthy subjects. The trial was conducted on healthy subjects
over a four-week period in which subjects received three single
escalating doses of taliglucerase alfa administered as
intravenous infusions.
All doses administered to subjects in the phase I clinical
trial, including the highest dose, which was the same dosage
currently suggested with respect to the treatment by Cerezyme,
demonstrated a strong safety profile. The data from our phase I
clinical trial showed that taliglucerase alfa was safe and well
tolerated at all doses.
There were no serious adverse events and no subjects withdrew
from the trial or discontinued treatment due to an adverse event.
In addition, the half-life of taliglucerase alfa was found to be
significantly longer than that of Cerezyme, based upon data
disclosed publicly by Genzyme, which was consistent with our
preclinical data.
Further, no neutralizing antibodies or adverse immunological
responses were detected in any of the subjects treated in the
phase I clinical trial. The presence of neutralizing antibodies
would imply that the human body may reject the therapeutic
enzyme.
Phase III
Clinical Trial
After the conclusion of the phase I clinical trial and
discussions with the FDA, we applied to commence a pivotal
phase III clinical trial of taliglucerase alfa without the
requirement to first complete a phase II clinical trial. In
April 2007, we received approval from the FDA to initiate a
pivotal phase III clinical trial. We submitted to the FDA a
request for a special protocol assessment (SPA) of the final
design of our pivotal
14
phase III clinical trial for taliglucerase alfa. In July
2007, we reached an agreement with the FDA on the design that we
submitted in the SPA request and in the third quarter of 2007 we
initiated enrollment and treatment of naive patients in the
phase III clinical trial. In accordance with the terms of
the SPA, the phase III clinical trial was a multi-center,
world-wide, randomized, double-blind, parallel group,
dose-ranging study to assess the safety and efficacy of
taliglucerase alfa in 31 treatment-naive patients suffering from
Gaucher disease. In the trial, patients were selected randomly
for one of two dosing arms (60 U/kg or 30 U/kg) and received
intravenous infusions of taliglucerase alfa once every two weeks
for a nine-month period. The primary endpoint of the study was a
20% mean reduction from baseline in spleen volume after nine
months, as measured by MRI. Major secondary endpoints were an
increase in hemoglobin, decrease in liver volume and increase in
platelet count. Patients enrolled in the trial were treated in
11 centers throughout Europe, Israel, North America, South
America and South Africa. We commenced enrollment and treatment
of patients in our phase III clinical trial in the third
quarter of 2007 and completed enrollment in the fourth quarter
of 2008. During the third quarter of 2008, we initiated a double
blind, follow-on extension study as part of our phase III
clinical trial in which patients that successfully completed
treatment in the trial were given the opportunity to continue to
be treated with taliglucerase alfa at the same dose that they
received in the trial. We are compiling additional information
relating to the long term safety and efficacy of taliglucerase
alfa through the follow-on study. In addition, in the fourth
quarter of 2008, we announced the enrollment of the first
patient in a worldwide, multi-center, open-label, switch-over
trial to assess the safety and efficacy of taliglucerase alfa.
The switch-over trial, which is not a pre requisite for
approval, was originally designed to include 15 patients
with Gaucher disease that are currently undergoing enzyme
replacement therapy with imiglucerase (Cerezyme). Due to the
shortage of Cerezyme in 2009, after fully enrolling the
15 patients, we extended the trial to include up to
30 patients in total. We successfully completed the
phase III clinical trial in September 2009.
In July 2009, following a request by the FDA, we submitted a
treatment protocol to the FDA in order to address an expected
shortage of the current enzyme replacement therapy approved for
Gaucher disease. The treatment protocol was approved by the FDA
in August 2009. In September 2009, the FDA’s Office of
Orphan Product Development granted taliglucerase alfa Orphan
Drug Status. In January 2010, the Committee for Orphan Medicinal
Products (COMP) of the EMEA, after reviewing all relevant
clinical data, recommended that the European Commission grant
Orphan Drug designation to taliglucerase alfa for the treatment
of Gaucher disease. The Orphan Drug designation in the United
States for taliglucerase alfa for the treatment of Gaucher
disease provides special status to taliglucerase alfa provided
that it meets certain criteria. As a result of the Orphan Drug
designation, we are qualified for the tax credit and marketing
incentives of the Orphan Drug Act of 1983. A marketing
application for a prescription drug product that has been
designated as a drug for a rare disease or condition is not
subject to a prescription drug user fee unless the application
includes an indication for other than a rare disease or
condition.
Phase III
Clinical Trial Results
We reported positive top line results of our phase III
clinical trial of taliglucerase alfa in October 2009 and full
study results in February 2010. In the clinical trial,
taliglucerase alfa significantly reduced mean spleen volume
after nine months compared with baseline in both treatment
groups. The 60 U/kg group demonstrated a statistically
significant mean reduction in spleen volume of 38.0%
(p<0.0001) and the
30 U/kg
group demonstrated a significant mean reduction in spleen volume
of 26.9% (p<0.0001). In addition, the primary endpoint was
achieved in both treatment groups after only six months of
therapy. See Figure 1 (range of spleen volume at baseline is
8X — 54X).
* P-value<0.0001.
15
Figure 1. MRI validated method-2 blinded readers,
Intra-reader variability
£
0.5%; Inter-reader variability
£
1.00%
Statistically significant improvements were also observed for
the secondary endpoints after nine months when compared to
baseline for the 60 U/kg dose. Patients demonstrated a mean
increase in hemoglobin of 2.2 g/dL or 22.2% (p<0.0001), a
mean decrease in liver volume of 11.1% (p<0.0001) and a
mean elevation in platelet count of 41,494 ml or 72.1%
(p=0.0031). For patients in the 30 U/kg dose, statistically
significant improvements after nine months compared with
baselines were observed for hemoglobin level (increased 1.6 g/dL
or 14.8%; p=0.0010) and liver size (decreased 10.48%; p=0.0041);
a nominal elevation in platelet count was also seen (11,427 ml
or 13.7%; p=0.0460). See Figures 2 through 4.
Figure 2. 1st Major Secondary endpoint:
Change in Hemoglobin (g/dL) From Baseline — ITT vs.
Anemic patients (both anemic and non anemic patients showed
an increase in Hb)
16
Figure 3. 2nd Major Secondary Endpoint: Liver
Volume % Change from Baseline, MRI validated method-2 blinded
readers; Intra-reader variability
£
0.5%; Inter-reader variability
£
1.00% (16 (52%) of the patients who presented hepatomegaly
showed a reduction of 14% on liver volume)
Figure 4. 3rd Major Secondary Endpoint:
Platelet Counts Change from Baseline
Thirty patients in the trial had Chitotriosidase measurements, a
biomarker for clinical symptoms of Gaucher disease. In these
patients, Chitotriosidase decreased from baseline in both the
30U/kg and 60U/kg groups by 47.3% and 58.4%, respectively.
The safety analysis for both treatment groups showed that
taliglucerase alfa was well tolerated and no serious or severe
adverse events were reported. Two patients in the trial
developed antibodies to taliglucerase alfa and no patients
developed neutralizing antibodies. In addition, two patients
experienced hypersensitivity reactions to taliglucerase alfa. No
anti-taliglucerase antibodies were detected in these patients
and both reactions were treated in the physicians’ clinic
and reversed.
Most adverse events were considered unrelated to taliglucerase
alfa. The most frequent mild to moderate adverse event was
headache. Other mild to moderate adverse events included
dizziness, muscle spasm, chest discomfort, nausea, skin
irritation and arthalgia.
In November 2010, we announced positive preliminary data from
the first 15 patients that completed our switchover trial
of taliglucerase alfa. The data indicate that patients can
safely be switched to taliglucerase alfa
17
from Cerezyme. Patients enrolled in the trial were switched from
Cerezyme (doses ranging from
10-60 U/kg
every other week) to an equivalent dose of taliglucerase alfa
using the same number of units of taliglucerase alfa. The data
from the interim report regarding the switchover trial
demonstrates that, on average, hemoglobin and platelet count,
spleen volume and liver volume (as measured by MRI) all remained
stable over the nine-month period, and no patients showed a
sustained clinical deterioration. The safety analysis from the
interim report (n=25, as performed at the time of data lock)
demonstrates that taliglucerase alfa was well tolerated and no
drug related severe adverse events were reported. No patients
experienced hypersensitivity reactions, one patient developed
antibodies to taliglucerase alfa and no patients developed
neutralizing antibodies. Most adverse events were considered
unrelated to taliglucerase alfa. The most frequent mild to
moderate adverse event was nasopharyngitis, a viral infection of
the upper respiratory system.
Other
Drug Candidates in Our Pipeline
We are developing other recombinant therapeutic proteins to be
expressed by our ProCellEx protein expression system, with an
emphasis on treatments for which there are large, established
pharmaceutical markets and where our proprietary protein
expression system enables us to develop and commercialize
recombinant proteins that are patent-protected and
therapeutically equivalent or superior to the existing
treatments. We select additional therapeutic candidates for
development by testing candidates in-house and through
collaborations with academic partners. We have identified
several product candidates oriented towards specialty disease
and therapeutic market segments, including treatments for Fabry
disease. We are also conducting initial research programs in the
fields of monoclonal antibodies, cytokines and vaccines. We
filed an IND with the FDA for PRX-105, our plant cell expressed
pegylated recombinant acetylcholinesterase enzyme (AChE) product
candidate, during the last quarter of 2009, and in March 2010 we
initiated a preliminary phase I clinical trial of PRX-105. We
completed the preliminary phase I clinical trial in June 2010.
We are currently preparing for further efficacy trials of this
product candidate in larger animals. In addition, we recently
held a pre-IND meeting with the FDA regarding PRX-102, our
product candidate for the treatment of Fabry disease, and we
plan to file an IND with the FDA for PRX-102 within the next
12 months. Last, we are developing a new method for
delivering active recombinant proteins systemically through oral
administration of transgenic plant cells expressing such
biotherapeutic proteins.
PRX-102
We are developing PRX-102, our proprietary plant cell expressed
modified version of the recombinant alpha-GAL-A protein, a
therapeutic enzyme for the treatment of Fabry disease. Fabry
disease is a rare, hereditary, genetic lysosomal storage
disorder in humans caused by an X-lined deficiency of the
alpha-GAL-A enzyme. Fabry disease causes harmful accumulations
of lipids in the kidneys, automatic nervous system and
cardiovascular system that result in the risk of heart attack
and stroke, and can be life-threatening. Fabry disease affects
more than 8,000 people globally. We believe that the
treatment of Fabry disease is a specialty clinical niche with
the potential for high growth. Currently there are two drugs
available on the market to treat Fabry disease. Fabrazyme, made
by Genzyme, was approved for the treatment of Fabry disease in
the European Union in 2001 and the United States in 2003.
Genzyme reported $188 million in worldwide sales of
Fabrazyme in 2010, compared to $431 million in 2009.
According to Genzyme, it suffered a temporary interruption in
production of Fabrazyme in 2009 associated with the remediation
of a contamination in one of its manufacturing facilities, and,
as a result, shipments of Fabrazyme were limited during the
second half of 2009. The other approved drug for the treatment
of Fabry disease in the European Union is Replagal, which is
sold by Shire plc. Shire reported $351 million in sales of
Replagal in 2009. According to public reports by Shire, it filed
a BLA with the FDA for Replagal in the United States in December
2009. Shire subsequently received Fast Track designation for
Replagal and withdrew its December 2009 BLA in favor of a
rolling BLA submission. Shire has reported that it withdrew that
filing in August 2010 in order to update the filing with
additional clinical information.
We are currently in the animal evaluation testing phase of the
development of PRX-102, which tests are based on a well
established mouse model for Fabry disease. In pre-clinical
studies, PRX-102 demonstrated preliminary efficacy in a Fabry
animal model. Chemical modifications made to PRX-102 improved
the
18
enzyme’s activity and stability resulting in prolonged
activity profiles and enhanced bioavailability in animals. The
modifications also have the potential to decrease the
immunogenicity of the enzyme, which is a major drawback of
currently approved therapies for Fabry disease. We held a
pre-IND meeting with the FDA in 2010 regarding PRX-102, and we
expect to file an IND with the FDA within the next
12 months. As was the case in our development of
taliglucerase alfa, our development of PRX-102 involves the
expression by our proprietary protein expression system of a
naturally occurring enzyme to be used in enzyme replacement
therapy for the treatment of Fabry disease. Based on our
experience with taliglucerase alfa, the experience of other
companies developing enzyme replacement therapies for Fabry
disease and the pre IND meeting we held with FDA in 2010, we
have reason to believe that, if favorable data is accumulated in
preclinical and phase I/II clinical trials, the FDA may allow us
to proceed directly with a pivotal phase III clinical
trial. However, there can be no assurance that we will initiate
phase I/II clinical trials and if we do, that such trials will
result in favorable data. In addition, there can be no assurance
that the FDA will allow us to proceed directly with a
phase III clinical trial after completion of a phase I/II
clinical trial.
Acetylcholinesterase
In August 2007, Protalix Ltd. licensed the rights to certain
technology under a research and license agreement with Yissum
Research and Development Company, or Yissum, and the Boyce
Thompson Institute, Inc., or Boyce Thompson. Pursuant to the
agreement, we are developing PRX-105, a proprietary plant
cell-based acetylcholinesterase (AChE) and its molecular
variants for the use in several therapeutic and prophylactic
indications, as well as in a biodefense program and an
organophosphate-based pesticide treatment program. Under the
terms of the agreement, Yissum and Boyce Thompson granted us an
exclusive, worldwide right and license to certain technology,
including patents and certain patent applications relating to
AChE for the therapeutic and prophylactic indications as well as
an exclusive license not limited to such indications with
respect to certain of those patents and patent applications. As
consideration for the license, we are obligated to pay Yissum
and Boyce Thompson, collectively, an annual, non-refundable
initial maintenance fee of $20,000, commencing on the fourth
anniversary of the execution of the agreement, which is subject
to a 12% annual increase. In addition, we are obligated to make
royalty payments equal to varying low, single-digit percentages
of net sales of products under the agreement. These royalty
rates are evaluated on a
country-by-country
basis, and are subject to reduction if a third party
commercializes a competing product or commercializes an
authorized generic version of the applicable product, subject to
certain conditions. We also have the right to grant sublicenses
relating to the licensed technology under the agreement, subject
to the payment of sublicensing fees. The fees payable in
connection with any sublicense are equal to varying percentages,
in the low-teens through the low-twenties, of the consideration
we receive in connection with the sublicense, depending on the
level of clinical development of the product at the time we
enter into the sublicense. Last, we are obligated to pay Yissum
and Boyce Thompson, collectively, milestone payments equal to
$700,000, in the aggregate, upon the achievement of certain
milestones under the license agreement.
The license agreement remains in effect until the expiration of
all obligations to Yissum and Boyce Thompson under the
agreement, determined on a
country-by-country
basis. We have the right to terminate the agreement for any
reason upon 60 days’ prior written notice to Yissum
and Boyce Thompson. Subject to certain conditions, Yissum and
Boyce Thompson may terminate the agreement immediately upon
written notice to us in connection with certain events relating
to bankruptcy, lapses in our insurance coverage, failures to
defend against third party claims or claims we may make
regarding the validity or enforceability of any licensed patent.
We or Yissum and Boyce Thompson may terminate the agreement
within 60 days after receiving written notice if the
non-terminating party passes a resolution for a voluntary wind
up, if a receiver or liquidator is appointed for the
non-terminating party, or the non-terminating party enters into
an insolvency or bankruptcy proceeding. In addition, either
party may terminate the agreement due to a material breach by
the other party if the breaching party is unable to cure the
breach within 60 days after receiving written notice of the
breach from the non-breaching party. Any termination of the
agreement will result in a loss of our rights to the licensed
technology, which will revert back to Yissum and Boyce Thompson.
To date, our in vitro experiments of PRX-105 have shown
that the acetylcholinesterase enzyme expressed in our ProCellEx
protein expression system demonstrates promising biological
activity on biochemical and
19
cellular levels. In addition, early animal studies demonstrated
that the acetylcholinesterase expressed in our ProCellEx protein
expression system was able to successfully treat animals exposed
to the nerve gas agent analogues, both when injected with our
acetylcholinesterase product candidate immediately before
exposure or when injected after exposure. In March 2010 we
initiated a preliminary phase I clinical trial of PRX-105 which
we completed in June 2010. The trial established the
pharmokinetics of PRX-105 and demonstrated that single dose
intravenous administration of PRX-105 is safe and well
tolerated. We are currently preparing for further efficacy
trials of PRX-105 in larger animals. In our preclinical studies
we utilized an analogue to nerve gas. However, we anticipate
that we will use live nerve gas in the proposed additional
efficacy trials in animals.
We are currently in discussions with different U.S. civil
and military organizations regarding certain grants for which
our acetylcholinesterase program is eligible. We anticipate
applying for specific grants during 2011 to support the further
development of our acetylcholinesterase program.
pr-antiTNF
In September 2009, we announced preliminary preclinical data
regarding an antiTNF (Tumor, Necrosis Factor) protein that we
are expressing through our proprietary ProCellEx system. We have
designed this antiTNF as pr-antiTNF. pr-antiTNF is a candidate
for the treatment of certain autoimmune diseases such as
rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing,
spondylitis, psoriatic arthritis and plaque psoriasis. Amgen
Inc. reported total sales of Enbrel of $3.5 billion in the
United States and Canada for 2010 and Pfizer has reported total
sales of Enbrel outside of the United States and Canada of
$865.0 million for 2010.
pr-antiTNF is a plant cell-expressed recombinant fusion protein
made from the soluble form of the human TNF receptor (TNFR),
fused to the Fc component of a human antibody domain. pr-antiTNF
has an identical amino acid sequence to Enbrel and our
in vitro and preclinical animal studies have demonstrated
that pr-antiTNF exhibits similar activity to Enbrel.
Specifically, pr-antiTNF binds TNF thereby inhibiting it from
binding to cellular surface TNF receptors and protects L929
cells from TNF-induced apoptosis in a dose-dependent manner. In
a
proof-of-concept
in vivo study using an established arthritis animal model,
pr-antiTNF, when injected in mice, significantly improved the
clinical arthritis parameters associated with this accepted
arthritis mouse model, including joint inflammation, swelling
and tissue degradation. We intend to conduct additional animal
studies to collect additional data to form a basis for a
discussion with the FDA to explore the regulatory pathway for
our antiTNF program. Patents for the Enbrel start to expire as
early as 2012, and we expect to use our cost effective
manufacturing platform to facilitate entry into this market upon
approval of our pr-antiTNF product, if at all. We have scheduled
a pre-IND meeting with the FDA regarding our antiTNF program in
March 2011.
Commercialization
Agreement
On November 30, 2009, Protalix Ltd. and Pfizer entered into
a license and supply agreement pursuant to which Pfizer was
granted an exclusive, worldwide license to develop and
commercialize taliglucerase alfa. Under the terms and conditions
of the Pfizer agreement, Protalix Ltd. retained the right to
commercialize taliglucerase alfa in Israel. In connection with
the execution of the Pfizer agreement, Pfizer made an upfront
payment to Protalix Ltd. of $60.0 million in connection
with the execution of the agreement and subsequently paid to
Protalix Ltd. an additional $5.0 million upon our filing of
a proposed pediatric investigation plan to the Pediatric
Committee of the EMEA. Protalix Ltd. is also eligible to receive
potential milestone payments of up to $50.0 million, in the
aggregate, for the successful achievement of other
regulatory-related milestones and to payments equal to 40% of
the net profits earned by Pfizer on sales of taliglucerase alfa.
In calculating the net profits, there are certain agreed upon
limits on the amounts that may be deducted from gross sales for
certain expenses and costs of goods sold. Protalix Ltd. retained
the manufacturing rights to taliglucerase alfa and Pfizer and
Protalix Ltd. have agreed to a specific allocation of the
responsibilities for the continued development efforts for
taliglucerase alfa. Protalix Ltd. will manufacture all of the
taliglucerase alfa needed for all purposes under the agreement
and Pfizer will purchase the taliglucerase alfa from Protalix
Ltd., subject to certain terms and conditions. The Pfizer
agreement also provides for reimbursement by Pfizer of certain
costs to be incurred by Protalix Ltd.
20
In connection with the upfront and milestone payments made under
the Pfizer agreement, Protalix Ltd. has paid a sublicense fee
equal to $1.6 million to the academic institution from who
it licensed certain technology relating to taliglucerase alfa.
Future milestone payments will be subject to a 2.5% royalty, and
all of revenues generated under the agreement will be subject to
a 0.75% royalty payable to the same institution until 2016, when
a patent related to taliglucerase alfa licensed to us will
expire. We are also required to pay a royalty equal to 3% of the
revenues of taliglucerase alfa Pizer records under the Pfizer
agreement to the OCS.
On August 10, 2010, Pfizer entered into a $30 million
short-term supply agreement with the Ministry of Health of
Brazil pursuant to which Protalix and Pfizer have provided
taliglucerase alfa to the Ministry of Health of Brazil for the
treatment of patients with Gaucher disease. In addition, we and
the Ministry of Health of Brazil are in discussions relating to
a possible long-term supply agreement that contemplates, among
other matters, providing certain components of our manufacturing
technology to the Ministry of Health of Brazil for
implementation by it in Brazil. At this time, we are unable to
assess whether these discussions will result in an agreement and
there can be no assurance that we will be able to enter into
such an agreement on favorable terms, if at all. In any event,
we do not expect to enter into a long-term supply agreement with
the Ministry of Health of Brazil until we receive marketing
approval of taliglucerase alfa from the FDA or ANVISA, if at all.
We will be subject to a withholding tax on the U.S. revenue
source portion of the payments made to us for our share of
Pfizer’s in net profits under the Pfizer agreement.
Currently, the withholding tax rate is 15%.
Strategic
Collaborations
Teva
Pharmaceutical Industries
In September 2006, we entered into a Collaboration and Licensing
Agreement with Teva for the development and manufacture of two
proteins to be identified by Teva and us using our ProCellEx
protein expression system. The agreement also identifies
additional matters for collaboration between Teva and us.
Subsequently, two proteins were identified to be researched and
developed under the agreement but in 2009, both of the projects
were terminated for commercial reasons. Pursuant to the
agreement, we have agreed to collaborate on certain additional
matters regarding proteins, including the research and
development of proteins utilizing our ProCellEx protein
expression system. See “Risk Factors — Our
strategy, in many cases, is to enter into collaboration
agreements with third parties to leverage our ProCellEx system
to develop product candidates. If we fail to enter into these
agreements or if we or the third parties do not perform under
such agreements or terminate or elect to discontinue the
collaboration, it could have a material adverse affect on our
revenues.”
Weizmann
Institute of Science
In March 2006, Protalix Ltd. entered into a research and license
agreement with the Yeda Research and Development Company
Limited, or Yeda, the technology transfer arm of the Weizmann
Institute of Science. Under the terms of the agreement, Yeda
agreed to use its technology to design a next generation of
glucoceribrosidase (GCD) for the treatment of Gaucher disease
that can be expressed using our ProCellEx protein expression
system and that may have certain benefits over the first
generation treatments used today. The technology licensed from
Yeda provides a methodology for the rational design of an
improved drug for the treatment of Gaucher disease by enzyme
replacement therapy, based on the three-dimensional crystal
structure of glucoceribrosidase (GCD) that was solved by
scientists from the Weizmann Institute of Science. Yeda has
granted us an exclusive worldwide license to use their
technology and discoveries for the development, production and
sale of enzymatically active mutations of glucoceribrosidase
(GCD) and derivatives thereof for the treatment of Gaucher
disease. Under the terms of the agreement, we are required to
take all necessary steps to develop and commercialize the
products subject to the agreement.
As consideration for the license, we agreed to pay Yeda a fixed
research budget amount, subject to certain conditions. We have
since completed the research phase of the arrangement with Yeda.
Accordingly, we are no longer making any research-related
payments to Yeda under the agreement. In addition, we are
obligated to make an annual non-refundable license fee of
$10,000 during the term of the agreement, commencing on the
fifth anniversary of the execution of the agreement until, and
including, the 19th anniversary thereof. We are
21
also obligated to make royalty payments equal to varying low,
single-digit percentages of net sales of products under the
agreement. Sublicenses relating to the licensed technology may
be granted under the agreement, subject to the payment of
sublicensing fees. The fee for any sublicense is equal to a
percentage, ranging from the low-teens through the low-twenties,
of the consideration we receive in connection with the
sublicense, depending on the level of clinical and regulatory
development of the products under the agreement at the time we
enter into the sublicense.
The license agreement remains in effect until the earlier of the
expiration of the last patent licensed under the agreement or if
there are no commercial sales of any products for a continuous
period of 20 years. Yeda may modify the exclusivity
component of the agreement by written notice to us and without
our consent. Yeda may terminate the agreement by written notice
to us if we fail to satisfy any one or more specified
milestones, and we fail to cure any such failure within a
certain time period after we receive the notice. Yeda is not
entitled to exercise this termination right if we demonstrate
that we are making all necessary efforts to achieve such
milestones, that our inability to satisfy the milestones is due
to factors beyond our control, and that the total delay with
respect to any one milestone does not exceed 12 months and
the total cumulative delay in respect of all milestones has not
exceeded 30 months. Yeda may also terminate the agreement
if we contest the validity of any of the patents included in the
agreement. We or Yeda may terminate the agreement due to a
material breach by the other party if the breach is unable to be
cured or, if curable, the breach is not cured within
21 days after the breaching party’s receipt of written
notice of the breach from the non-breaching party. In addition,
either party may terminate the agreement in connection with
certain events relating to a wind up or bankruptcy.
Intellectual
Property
We maintain a proactive intellectual property strategy which
includes patent filings in multiple jurisdictions, including the
United States and other commercially significant markets. At the
end of the first quarter of 2011, we held 20 granted patents and
84 pending patent applications with respect to various
compositions, methods of production and methods of use relating
to our ProCellEx protein expression system and our proprietary
product pipeline. At the end of the first quarter of 2011 we
also held one joint patent with a third party and held licensed
rights to six patents and seven patent applications.
Our competitive position and future success depend in part on
our ability, and that of our licensees, to obtain and leverage
the intellectual property covering our product candidates,
know-how, methods, processes and other technologies, to protect
our trade secrets, to prevent others from using our intellectual
property and to operate without infringing the intellectual
property of third parties. We seek to protect our competitive
position by filing United States, European Union, Israeli and
other foreign patent applications covering our technology,
including both new technology and improvements to existing
technology. Our patent strategy includes obtaining patents,
where possible, on methods of production, compositions of matter
and methods of use. We also rely on know-how, continuing
technological innovation, licensing and partnership
opportunities to develop and maintain our competitive position.
As of December 31, 2010, our patent portfolio consists of
several patent families (consisting of patents
and/or
patent applications) covering our technology, protein expression
methodologies and system and product candidates, as follows:
|
|
|
| |
•
|
With respect to our ProCellEx protein expression system, we have
been issued, and hold licensed rights to, patents in the United
States, the European Union, Israel, Canada, the Czech Republic,
Hungary, Japan, Poland, Mexico, Hong Kong and India, and to 10
pending patent applications. Among other things, the patents
cover the methods that we use for culturing and harvesting plant
cells and/or
tissues in consecutive cycles. The issued patents in this patent
family are expected to expire in 2017.
|
| |
| |
•
|
With respect to our ProCellEx protein expression system, we also
hold 14 patent applications relating to the large scale
production of proteins in cultured plant cells. The patents to
issue in the future based on the pending patent applications in
this patent family are expected to expire in 2028.
|
22
|
|
|
| |
•
|
We hold a patent family containing five granted patents in
India, South Africa, Russia, Australia and the Ukraine, and 36
patent applications, relating to the production of glycosylated
lysosomal proteins in our plant culture platform, particularly
proteins having a terminal mannose glycosylation, including
taliglucerase alfa. The issued patents and any patents to issue
in the future based on pending patent applications in this
patent family are expected to expire in 2024.
|
| |
| |
•
|
We hold a patent family containing one granted and three pending
patent applications relating to a system and method for
production of antibodies in a plant cell culture, and antibodies
produced in such a system. The patents to issue in the future
based on the patent applications in this patent family are
expected to expire in 2025.
|
| |
| |
•
|
We hold a patent family containing one issued patent in South
Africa and 12 pending patent applications relating to a new
method for delivering active recombinant proteins systemically
through oral administration of transgenic plant cells. The
issued patents and any patents to issue in the future based on
patent applications in this patent family are expected to expire
in 2026.
|
| |
| |
•
|
We hold a patent family containing seven pending patent
applications relating to saccharide containing protein
conjugates. The patents to issue in the future based on the
patent applications in this patent family will expire in 2028.
|
| |
| |
•
|
Our patent portfolio includes a patent that we co-own that
covers human glycoprotein hormone and chain splice variants,
including isolated nucleic acids encoding these variants. More
specifically, this patent covers a new splice variant of human
FSH. This patent was issued in the United States and is expected
to expire in 2026.
|
| |
| |
•
|
With respect to taliglucerase alfa, we have licensed the rights
to two patents from Virginia Tech Intellectual Properties, Inc.,
or Virginia Tech, that are expected to expire in 2016. We also
hold the licensed rights from Yeda to one granted and two
pending patent applications with respect to the research and
development of the glucocerebrosidase (GCD) protein.
|
| |
| |
•
|
With respect to acetylcholinesterase, we have licensed the
rights to three patents issued in the United States that are
expected to expire in 2013, 2017 and 2021, and to five patent
applications from Yissum.
|
We monitor third parties for activities that may infringe our
intellectual property, as well as the progression of third party
patent applications that may cover our product candidates or
expression methods and thus, potentially, interfere with the
development of our business. We are aware, for example, of
U.S. patents, and corresponding international counterparts
of such patents, owned by third parties that contain claims
covering methods of producing GCD. We do not believe that, if
any claim of infringement were to be asserted against us based
upon such patents, taliglucerase alfa would be found to infringe
any valid claim under such patents. However, there can be no
assurance that a court would find in our favor or that, if we
choose or are required to seek a license to any one or more of
such patents, a license would be available to us on acceptable
terms or at all.
In April 2005, Protalix Ltd. entered into a license agreement
with Icon Genetics AG, or Icon, pursuant to which we received an
exclusive worldwide license to develop, test, use and
commercialize Icon’s technology to express certain proteins
in our ProCellEx protein expression system. Under the terms of
the agreement, we are also entitled to a non-exclusive worldwide
license to make and have made other proteins expressed by using
Icon’s technology in our technology. As consideration for
the license, we are obligated to make royalty payments equal to
varying low, single-digit percentages of net sales of products
by us, our affiliates, or any sublicensees under the agreement.
In addition, we are obligated to make milestone payments equal
to $350,000, in aggregate, upon the achievement of certain
milestones.
Our license agreement with Icon remains in effect until the
earlier of the expiration of the last patent under the agreement
or, if all of the patents under the agreement expire,
20 years after the first commercial sale of any product
under the agreement. Icon may terminate the agreement upon
written notice to us that we are in material breach of our
obligations under the agreement and we are unable to remedy such
material breach within 30 days after we receive such
notice. Further, Icon may terminate the agreement in connection
23
with certain events relating to a wind up or bankruptcy, if we
make a general assignment for the benefit of our creditors, or
if we cease to conduct operations for a certain period. Icon may
also terminate the exclusivity granted to us by written notice
if we fail to reach certain milestones within a designated
period of time. Notwithstanding the termination date of the
agreement, our obligation to pay royalties under the agreement
to Icon may expire prior to the termination of this agreement,
subject to certain conditions.
In January 2005, Protalix Ltd. entered into a license agreement
with Virginia Tech, pursuant to which we received a
non-exclusive worldwide license to make, have made, use, sell,
offer for sale and import certain of Virginia Tech’s
patents. As consideration for the license, we made a one-time
license fee payment to Virginia Tech within 10 days of the
effective date of the agreement, and we are obligated to make
royalty payments equal to varying low, single-digit percentages
of net sales of licensed products by Protalix Ltd., its
subsidiaries
and/or their
affiliates. Upon commercialization of a licensed product, the
royalty payment is subject to a low, annual minimum amount. In
addition, we are obligated to make milestone payments equal to
$150,000, in aggregate, upon the achievement of certain
milestones. We have the right to grant sublicenses under the
agreement.
Our license agreement with Virginia Tech remains in effect until
the earlier of the expiration of the last patent under the
agreement or 10 years after the first commercial sale of
any licensed product. Virginia Tech may terminate the agreement
upon written notice to us that we are in material breach of our
obligations under the agreement if we are unable to remedy such
material breach within a fixed number of days after we receive
such notice, which number may be doubled if we are making good
faith efforts to achieve a cure and the extension will not
increase the damages suffered by Virginia Tech. We have the
right to terminate the agreement at any time upon prior written
notice delivered an
agreed-upon
number of days prior to the date of termination.
Manufacturing
We are obligated to manufacture all of the taliglucerase alfa
drug product needed under the Pfizer agreement, subject to
certain terms and conditions. Our drug product candidates,
including taliglucerase alfa, must be manufactured in a sterile
environment and in compliance with cGMPs set by the FDA and
other relevant foreign regulatory authorities. We use our
current facility, which has approximately 20,000 sq/ft of clean
rooms built according to industry standards, to develop, process
and manufacture taliglucerase alfa and other recombinant
proteins. We believe that the manufacturing space complies with
the good laboratory, clinical and manufacturing practices
required by the FDA and other comparable regulatory authorities
for production of pharmaceutical products on a commercial scale.
We intend to use our current manufacturing space to produce all
of the taliglucerase alfa we need in the near future, included
the taliglucerase alfa to be purchased by Pfizer. Current
capacity of our facility can serve approximately 20% of the
Gaucher disease patients that are currently under treatment. We
intend to expand our current facility in order to reach a
capacity of approximately 50% of the Gaucher disease patients
that are currently under treatment and to house the laboratory
space necessary for further development of other product
candidates in our pipeline. Total expected cost for such
expansion is currently estimated to be approximately
$25 million and the process is expected to be completed
during 2012.
We have entered into a contract with Teva pursuant to which Teva
is required to perform the final filling and freeze drying steps
for taliglucerase alfa. In addition, we have engaged a contract
manufacturer in Europe to act as an additional source of fill
and finish activities for taliglucerase alfa. According to our
agreement with Pfizer, Pfizer will be responsible for the fill
and finish activities for taliglucerase alfa. We have the right
to terminate the agreement at any time upon written notice of a
fixed number of days.
Our current facility in Israel has been granted “Approved
Enterprise” status, and we have elected to participate in
the alternative benefits program. Our facility is located in a
Zone A location, and, therefore, our income from the Approved
Enterprise will be tax exempt in Israel for a period of
10 years commencing with the year in which we first
generate taxable income from the relevant Approved Enterprise.
We currently anticipate that the benefits of this program will
be available to Protalix Ltd. until 2017 and, accordingly, we
expect to be entitled to similar tax benefits for a number of
years thereafter. To remain eligible for these tax benefits, we
must continue to meet certain conditions, and if we increase our
activities outside of Israel, for
24
example, by future acquisitions, such increased activities
generally may not be eligible for inclusion in Israeli tax
benefit programs. In addition, our technology is subject to
certain restrictions with respect to the transfer of technology
and manufacturing rights. “See Risk Factors — The
manufacture of our products is an exacting and complex process,
and if we or one of our materials suppliers encounter problems
manufacturing our products, it will have a material adverse
effect on our business and results of operations.”
Raw
Materials and Suppliers
We believe that the raw materials that we require throughout the
manufacturing process of our current and potential drug product
candidates are widely available from numerous suppliers and are
generally considered to be generic industrial biological
supplies. We rely on a single approved supplier for certain
materials relating to the current expression of our proprietary
biotherapeutic proteins through ProCellEx. We are currently in
the process of identifying additional suppliers that can provide
the same materials.
Development and regulatory approval of our pharmaceutical
products are dependent upon our ability to procure active
ingredients and certain packaging materials from sources
approved by the FDA and other regulatory authorities. Since the
FDA and other regulatory approval processes require
manufacturers to specify their proposed suppliers of active
ingredients and certain packaging materials in their
applications, FDA approval of a supplemental application to use
a new supplier in connection with any drug candidate or approved
product, if any, would be required if active ingredients or such
packaging materials were no longer available from the specified
supplier, which could result in manufacturing delays. From time
to time, we intend to continue to identify alternative
FDA-approved suppliers to ensure the continued supply of
necessary raw materials.
Competition
The biotechnology and pharmaceutical industries are
characterized by rapidly evolving technology and significant
competition. Competition from numerous existing companies and
others entering the fields in which we operate is intense and
expected to increase. Most of these companies have substantially
greater research and development, manufacturing, marketing,
financial, technological personnel and managerial resources than
we do. In addition, many specialized biotechnology companies
have formed collaborations with large, established companies to
support research, development and commercialization of products
that may be competitive with our current and future product
candidates and technologies. Acquisitions of competing companies
by large pharmaceutical or biotechnology companies could further
enhance such competitors’ financial, marketing and other
resources. Academic institutions, governmental agencies and
other public and private research organizations are also
conducting research activities and seeking patent protection and
may commercialize competitive products or technologies on their
own or through collaborations with pharmaceutical and
biotechnology companies.
We specifically face competition from companies with approved
treatments of Gaucher disease, including Genzyme, which recently
announced a proposed merger into Sanofi Aventis, and to a much
lesser extent, Actelion. In February 2010, the FDA approved
VPRIV, Shire’s enzyme replacement therapy for the treatment
of Gaucher disease and the European Commission granted marketing
authorization to VPRIV in August 2010. In addition, we are aware
of other early clinical stage, experimental, small molecule,
oral drugs which are being developed for the treatment of
Gaucher disease by Genzyme and Amicus Therapeutics, which
according to public filings by Amicus Therapeutics has been
suspended. We also face competition from companies with approved
enzyme treatments of Fabry disease, including Genzyme and Shire,
and we are aware of other early stage drugs which are being
developed for the treatment of Fabry disease, including a drug
being developed by Amicus Therapeutics.
We also face competition from companies that are developing
other platforms for the expression of recombinant therapeutic
pharmaceuticals. We are aware of companies that are developing
alternative technologies to develop and produce therapeutic
proteins in anticipation of the expiration of certain patent
claims covering marketed proteins. Competitors developing
alternative expression technologies include Crucell N.V. (which
was acquired by Johnson & Johnson during 2010), Shire
and GlycoFi, Inc. (which was acquired by
25
Merck & Co. Inc.). Other companies are developing
alternate plant-based technologies, include Biolex, Inc.,
Chlorogen, Inc., Greenovation Biotech GmbH, and Symbiosys, none
of which are cell-based. Rather, such companies base their
product development on transgenic plants or whole plants.
Several biogeneric companies are pursuing the opportunity to
develop and commercialize follow-on versions of other currently
marketed biologic products, including growth factors, hormones,
enzymes, cytokines and monoclonal antibodies, which are areas
that interest us. These companies include, among others,
Novartis AG/Sandoz Pharmaceuticals, BioGeneriX AG, Stada
Arzneimittel AG, BioPartners GmbH and Teva.
Key differentiating elements affecting the success of our
product candidates are likely to be their potency and efficacy
profiles, as well as their cost-effectiveness as compared to
other existing therapies. See “Risk Factors —
Developments by competitors may render our products or
technologies obsolete or non-competitive which would have a
material adverse effect on our business and results of
operations.”
Scientific
Advisory Board
Members of our scientific advisory board, who are experts in the
fields of plant molecular and cell biology as well as Gaucher
disease and various hematological and genetic disorders, consult
with our management within their professional areas of
expertise; exchange strategic and business development ideas
with our management; attend scientific, medical and business
meetings with our management, such as meetings with the FDA and
comparable foreign regulatory authorities, meetings with
strategic or potential strategic partners and other meetings
relevant to their areas of expertise; and attend meetings of our
scientific advisory board. We expect our scientific advisory
board to convene at least twice annually, and we frequently
consult with the individual members of our Scientific Advisory
Board. Our scientific advisory board currently includes the
following people:
| |
|
|
|
Name
|
|
Affiliation
|
|
|
|
Professor Aaron Ciechanover, M.D., D.Sc.
|
|
Laureate of the Nobel Prize in Chemistry
|
|
|
|
Distinguished research Professor at the Cancer and Vascular
Biology Research Center of the Rappaport Research Institute and
Faculty of Medicine at the Technion
|
|
|
|
American Academy of Arts and Sciences, Member
|
| |
|
Professor Gad Galili, Ph.D.
|
|
Former Chairman of the Department of Plant
|
|
|
|
Sciences, The Weizmann Institute of Science,
|
|
|
|
Rehovot, Israel
|
| |
|
Professor Ari Zimran, M.D.
|
|
Director of the Gaucher Clinic, Shaare Zedek Medical Center,
Jerusalem, Israel
|
|
|
|
Associate Professor of Medicine, Hebrew University-Hadassah
Medical School, Jerusalem, Israel
|
Government
Regulation
The testing, manufacture, distribution, advertising and
marketing of drug products are subject to extensive regulation
by federal, state and local governmental authorities in the
United States, including the FDA, and by similar authorities in
other countries. Any product that we develop must receive all
relevant regulatory approvals or clearances, as the case may be,
before it may be marketed in a particular country.
The regulatory process, which includes overseeing preclinical
studies and clinical trials of each pharmaceutical compound to
establish its safety and efficacy and confirmation by the FDA
that good laboratory, clinical and manufacturing practices were
maintained during testing and manufacturing, can take many
years, requires the expenditure of substantial resources and
gives larger companies with greater financial resources a
competitive advantage over us. Delays or terminations of
clinical trials that we undertake would likely impair
26
our development of product candidates. Delays or terminations
could result from a number of factors, including stringent
enrollment criteria, slow rate of enrollment, size of patient
population, having to compete with other clinical trials for
eligible patients, geographical considerations and others.
The FDA review process can be lengthy and unpredictable, and we
may encounter delays or rejections of our applications when
submitted. Generally, in order to gain FDA approval, we must
first conduct preclinical studies in a laboratory and in animal
models to obtain preliminary information on a compound and to
identify any potential safety problems. The results of these
studies are submitted as part of an IND application that the FDA
must review before human clinical trials of an investigational
drug can commence. Clinical trials may be terminated by the
clinical trial site, sponsor or the FDA if toxicities appear
that are either worse than expected or unexpected.
Clinical trials are normally performed in three sequential
phases and generally take two to five years, or longer, to
complete. Phase I consists of testing the drug product in a
small number of humans, normally healthy volunteers, to
determine preliminary safety and tolerable dose range.
Phase II usually involves studies in a limited patient
population to evaluate the effectiveness of the drug product in
humans having the disease or medical condition for which the
product is indicated, determine dosage tolerance and optimal
dosage and identify possible common adverse effects and safety
risks. Phase III consists of additional controlled testing
at multiple clinical sites to establish clinical safety and
effectiveness in an expanded patient population of
geographically dispersed test sites to evaluate the overall
benefit-risk relationship for administering the product and to
provide an adequate basis for product labeling. Phase IV
clinical trials may be conducted after approval to gain
additional experience from the treatment of patients in the
intended therapeutic indication.
After completion of clinical trials of a new drug product, FDA
and foreign regulatory authority marketing approval must be
obtained. Assuming that the clinical data support the
product’s safety and effectiveness for its intended use, a
New Drug Application (NDA) is submitted to the FDA for its
review. Generally, it takes one to three years to obtain
approval. If questions arise during the FDA review process,
approval may take a significantly longer period of time. The
testing and approval processes require substantial time and
effort and approval on a timely basis, if at all, or the
approval that we receive may be for a narrower indication than
we had originally sought, potentially undermining the commercial
viability of the product. Even if regulatory approvals are
obtained, approved products are subject to continual review and
holders of an approved product are required, for example, to
report certain adverse reactions and production problems, if
any, to the FDA, and to comply with certain requirements
concerning advertising and promotional labeling for the product.
Also, quality control and manufacturing procedures relating to a
product must continue to conform to cGMP after approval, and the
FDA periodically inspects manufacturing facilities to assess
compliance with cGMP. Accordingly, manufacturers must continue
to expend time, money and effort in the area of production and
quality control to comply with cGMP and other aspects of
regulatory compliance. The later discovery of previously unknown
problems or failure to comply with the applicable regulatory
requirements with respect to any product may result in
restrictions on the marketing of the product or withdrawal of
the product from the market as well as possible civil or
criminal sanctions. See also “— International
Regulation.”
Under the Orphan Drug Act of 1983, the FDA may grant orphan drug
designation to drugs and biological products intended to treat a
rare disease or condition, which is generally a disease or
condition that affects fewer than 200,000 individuals in the
United States. In September 2009, we received orphan drug
designation for taliglucerase alfa for the treatment of Gaucher
disease. The FDA grants orphan drug designation to drugs that
may provide a significant therapeutic advantage over existing
treatments and target conditions affecting 200,000 or fewer
U.S. patients per year. Orphan drug designation does not
convey any advantage in or shorten the duration of the
regulatory review and approval process. Among the other benefits
of orphan drug designation are possible funding and tax savings
to support clinical trials and for other financial incentives
and a waiver of the marketing application user fee and most
likely priority review. If a significant therapeutic advantage
over existing treatments is shown in the marketing application,
FDA may grant orphan drug approval and provide a seven-year
period of marketing exclusivity.
The FDA has a fast track program that is intended to expedite or
facilitate the process for reviewing new drugs and biological
products that meet certain criteria. Specifically, new drugs and
biological products are
27
eligible for fast track designation if they are intended to
treat a serious or life-threatening condition and demonstrate
the potential to address unmet medical needs for the condition.
Fast track designation applies to the combination of the product
and the specific indication for which it is being studied. For a
fast track product, the FDA may consider for review on a rolling
basis sections of the NDA before the complete application is
submitted, if the sponsor provides a schedule for the submission
of the sections of the NDA, the FDA agrees to accept sections of
the NDA as they become available and determines that the
schedule is acceptable, and the sponsor pays any required user
fees upon submission of the first section of the NDA. We used
the rolling submission option for our NDA for our lead product
candidate, taliglucerase alfa, which we completed in April 2010.
None of our products under development has been approved for
marketing in the United States or elsewhere. We may not be able
to obtain regulatory approval for any of our products under
development in a timely manner, if at all. Failure to obtain
requisite governmental approvals or failure to obtain approvals
of the scope requested will delay or preclude us, or our
licensees or marketing partners, from marketing our products, or
limit the commercial use of our products, and thereby would have
a material adverse effect on our business, financial condition
and results of operations. See “Risk Factors — We
may not obtain the necessary U.S. or worldwide regulatory
approvals to commercialize our drug candidates in a timely
manner, if at all, which would have a material adverse effect on
our business and results of operations.”
The United States federal government regulates healthcare
through various agencies, including but not limited to the
following: (i) the FDA, which administers the Federal Food,
Drug, and Cosmetic Act (FDCA), as well as other relevant laws;
(ii) the Center for Medicare & Medicaid Services
(CMS), which administers the Medicare and Medicaid programs;
(iii) the Office of Inspector General (OIG) which enforces
various laws aimed at curtailing fraudulent or abusive
practices, including by way of example, the Anti-Kickback Law,
the Anti-Physician Referral Law, commonly referred to as Stark,
the Anti-Inducement Law, the Civil Money Penalty Law and the
laws that authorize the OIG to exclude healthcare providers and
others from participating in federal healthcare programs; and
(iv) the Office of Civil Rights, which administers the
privacy aspects of the Health Insurance Portability and
Accountability Act of 1996 (HIPAA). All of the aforementioned
are agencies within the Department of Health and Human Services
(HHS). Healthcare is also provided or regulated, as the case may
be, by the Department of Defense through its TriCare program,
the Department of Veterans Affairs, especially through the
Veterans Health Care Act of 1992, the Public Health Service
within HHS under Public Health Service Act § 340B
(42 U.S.C. § 256b), the Department of Justice
through the Federal False Claims Act and various criminal
statutes, and state governments under the Medicaid and other
state sponsored or funded programs and their internal laws
regulating all healthcare activities. Many states also have
anti-kickback and anti-physician referral laws that are similar
to the federal laws, but may be applicable in situations where
federal laws do not apply.
Medicare is the federal healthcare program for those who are
(i) over 65 years of age, (ii) disabled,
(iii) suffering from end-stage renal disease or
(iv) suffering from Lou Gehrig’s disease. Medicare
consists of part A, which covers inpatient costs,
part B, which covers services by physicians and
laboratories, durable medical equipment and certain drugs,
primarily those administered by physicians, and part D,
which provides drug coverage for most prescription drugs other
than those covered under part B. Medicare also offers a
managed care option under part C. Medicare is administered
by CMS. In contrast, Medicaid is a state-federal healthcare
program for the poor and is administered by the states pursuant
to an agreement with the Secretary of Health and Human Services.
Most state Medicaid programs cover most outpatient prescription
drugs.
International
Regulation
We are subject to regulations and product registration
requirements in many foreign countries in which we may sell our
products, including in the areas of product standards, packaging
requirements, labeling requirements, import and export
restrictions and tariff regulations, duties and tax
requirements. The time required to obtain clearance required by
foreign countries may be longer or shorter than that required
for FDA clearance, and requirements for licensing a product in a
foreign country may differ significantly from FDA requirements.
28
Pharmaceutical products may not be imported into, or
manufactured or marketed in, the State of Israel absent drug
registration. The three basic criteria for the registration of
pharmaceuticals in Israel is quality, safety and efficacy of the
pharmaceutical product and the Israeli MOH requires
pharmaceutical companies to conform to international
developments and standards. Regulatory requirements are
constantly changing in accordance with scientific advances as
well as social and ethical values.
The relevant legislation of the European Union requires that
medicinal products, including generic versions of previously
approved products, and new strengths, dosage forms and
formulations, of previously approved products, shall have a
marketing authorization before they are placed on the market in
the European Union. Authorizations are granted after the
assessment of quality, safety and efficacy by the respective
health authorities. In order to obtain an authorization, an
application must be made to the competent authority of the
member state concerned or in a centralized procedure to the
EMEA. Besides various formal requirements, the application must
contain the results of pharmaceutical (physico-chemical,
biological or microbiological) tests, of preclinical
(toxicological and pharmacological) tests as well as of clinical
trials. All of these tests must have been conducted in
accordance with relevant European Union regulations and must
allow the reviewer to evaluate the quality, safety and efficacy
of the medicinal product. On January 2010, the Committee for
Orphan Medicinal Products (COMP) of the EMEA recommended that
the European Commission grant orphan drug designation to
taliglucerase alfa. Orphan drug designation in the European
Union is granted to medicinal products intended for the
diagnosis, prevention and treatment of life-threatening diseases
and very serious conditions that affect not more than five in
10,000 people in the European Union. Orphan drug
designation is generally given to medicinal products that treat
conditions for which no current therapy exists or are expected
to bring a significant benefit to patients over existing
therapies. If granted by the European Commission, orphan drug
designation will provide us a centralized procedure for
obtaining marketing authorization for taliglucerase alfa, with a
single marketing authorization valid throughout all EU Member
States. We may also be eligible for a number of additional
incentives including protocol assistance, reduction in
registration fees and eligibility for grants and initiatives
supporting research and development related to the orphan drug
designation.
Israeli
Government Programs
The following is a summary of the current principal Israeli tax
laws applicable to us and Protalix Ltd., and of the Israeli
Government programs from which Protalix Ltd. benefits. Some
parts of this discussion are based on new tax legislation that
has not been subject to judicial or administrative
interpretation. Therefore, the views expressed in the discussion
may not be accepted by the tax authorities in question. The
discussion should not be construed as legal or professional tax
advice and does not cover all possible tax considerations.
General
Corporate Tax Structure in Israel
Generally, Israeli companies are subject to corporate tax at the
rate of 25% on taxable income and are subject to real capital
gains tax at a rate of 25% on capital gains (other than gains
derived from the sale of listed securities that are taxed at the
prevailing corporate tax rates) derived after January 1,
2003. The corporate tax rate was reduced in June 2004, from 36%
to 35% for the 2004 tax year, 34% for the 2005 tax year, 31% for
the 2006 tax year, 29% for the 2007 tax year, 27% for the 2008
tax year, 26% for the 2009 tax year and 25% for the 2010 tax
year and thereafter. Additional, gradual corporate tax
reductions were adopted in 2008, as follows: 24% for the 2011
tax year; 23% for the 2012 tax year; 22% for the 2013 tax year;
21% for the 2014 tax year; 20% for the 2015 tax year; and 18%
thereafter. As discussed below, the corporate tax rate may be
less for income derived from an Approved Enterprise. In addition
to the corporate taxes in Israel, we are subject to a
withholding tax on the U.S. revenue source portion of the
payments made to us for our share of Pfizer’s net profits
under the Pfizer agreement. The withholding tax rate is 15%. See
“Business — Commercialization Agreement.”
Law for
the Encouragement of Capital Investments, 1959
The Law for the Encouragement of Capital Investments, 1959,
known as the Investment Law, provides certain incentives for
capital investments in a production facility (or other eligible
assets). Generally, an
29
investment program that is implemented in accordance with the
provisions of the Investment Law, referred to as an
“Approved Enterprise,” is entitled to benefits. These
benefits may include cash grants from the Israeli government and
tax benefits, based upon, among other things, the location of
the facility in which the investment is made and specific
elections made by the grantee.
The Investment Law was significantly amended effective in April
2005. Protalix Ltd. will continue to enjoy the tax benefits
under the pre-revision provisions of the Investment Law. If any
new benefits are granted to Protalix Ltd. in the future,
Protalix Ltd. will be subject to the provisions of the amended
Investment Law with respect to these new benefits. Therefore,
the following discussion is a summary of the Investment Law
prior to its amendment as well as the relevant changes contained
in the new legislation.
Under the Investment Law prior to its amendment, a company that
wished to receive benefits had to receive approval from the
“Investment Center” of the Israeli Ministry of
Industry, Trade and Labor, or the Investment Center. Each
certificate of approval for an Approved Enterprise relates to a
specific investment program in the Approved Enterprise,
delineated both by the financial scope of the investment and by
the physical characteristics of the facility or the asset, e.g.,
the equipment to be purchased and utilized pursuant to the
program.
An Approved Enterprise may elect to forego any entitlement to
the grants otherwise available under the Investment Law and,
instead, participate in an alternative benefits program under
which the undistributed income from the Approved Enterprise is
fully exempt from corporate tax for a defined period of time.
Under the alternative package of benefits, a company’s
undistributed income derived from an Approved Enterprise will be
exempt from corporate tax for a period of between two and
10 years from the first year of taxable income, depending
upon the geographic location within Israel of the Approved
Enterprise. Upon expiration of the exemption period, the
Approved Enterprise is eligible for the reduced tax rates
otherwise applicable under the Investment Law for any remainder
of the otherwise applicable benefits period (up to an aggregate
benefits period of either seven or 10 years, depending on
the location of the company or its definition as a foreign
investors’ company). If a company has more than one
Approved Enterprise program or if only a portion of its capital
investments are approved, its effective tax rate is the result
of a weighted combination of the applicable rates. The tax
benefits from any certificate of approval relate only to taxable
profits attributable to the specific Approved Enterprise. Income
from activity that is derived from different Approved
Enterprises does not enjoy these tax benefits.
A company that has an Approved Enterprise program is eligible
for further tax benefits if it qualifies as a foreign
investors’ company. A foreign investors’ company
eligible for benefits is essentially a company in which more
than 25% of the share capital (in terms of shares, rights to
profit, voting and appointment of directors) is owned (measured
by both share capital and combined share and loan capital) by
non-Israeli residents. A company that qualifies as a foreign
investors’ company and has an Approved Enterprise program
is eligible for tax benefits for a
10-year
benefit period and may enjoy a reduced corporate tax rate of 10%
to 25%, depending on the amount of the company’s shares
held by non-Israeli shareholders.
If a company that has an Approved Enterprise program is a wholly
owned subsidiary of another company, then the percentage of
foreign investments is determined based on the percentage of
foreign investment in the parent company. The tax rates and
related levels of foreign investments are set forth in the
following table:
| |
|
|
|
|
|
|
|
Rate of
|
|
|
Percent of Foreign Ownership
|
|
Reduced Tax
|
|
|
|
|
0- 49%
|
|
|
25
|
%
|
|
49- 74%
|
|
|
20
|
%
|
|
74- 90%
|
|
|
15
|
%
|
|
90-100%
|
|
|
10
|
%
|
Our original facility in Israel has been granted “Approved
Enterprise” status, and it has elected to participate in
the alternative benefits program. Under the terms of its
Approved Enterprise program, the facility is located in a top
priority location, or “Zone A,” and, therefore, the
income from that Approved Enterprise will be tax exempt in
Israel for a period of 10 years, commencing with the year
in which taxable income is
30
first generated from the relevant Approved Enterprise. The
current benefits program may not continue to be available and
Protalix Ltd. may not continue to qualify for its benefits.
A company that has elected to participate in the alternative
benefits program and that subsequently pays a dividend out of
the income derived from the Approved Enterprise during the tax
exemption period will be subject to corporate tax in respect of
the amount distributed at the rate that would have been
applicable had the company not elected the alternative benefits
program (generally 10% to 25%, depending on the extent to which
non-Israeli shareholders hold such company’s shares). If
the dividend is distributed within 12 years after the
commencement of the benefits period (or, in the case of a
foreign investor’s company, without time limitation), the
dividend recipient is taxed at the reduced withholding tax rate
of 15% applicable to dividends from approved enterprises, or at
the lower rate under an applicable tax treaty. After this
period, the withholding tax rate is 25%, or at the lower rate
under an applicable tax treaty. In the case of a company with a
foreign investment level (as defined by the Investment Law) of
25% or more, the
12-year
limitation on reduced withholding tax on dividends does not
apply. The company must withhold this tax at its source,
regardless of whether the dividend is converted into foreign
currency.
The Investment Law also provides that an Approved Enterprise is
entitled to accelerated depreciation on its property and
equipment that are included in an approved investment program.
This benefit is an incentive granted by the Israeli government
regardless of whether the alternative benefits program is
elected.
The benefits available to an Approved Enterprise are conditioned
upon terms stipulated in the Investment Law and regulations and
the criteria set forth in the applicable certificate of
approval. If Protalix Ltd. does not fulfill these conditions in
whole or in part, the benefits can be canceled and Protalix Ltd.
may be required to refund the received benefits, linked to the
Israeli consumer price index with the addition of interest or
alternatively with an additional penalty payment. We believe
that Protalix Ltd. currently operates in compliance with all
applicable conditions and criteria, but there can be no
assurance that Protalix Ltd. will continue to do so.
Furthermore, there can be no assurance that any Approved
Enterprise status granted to Protalix Ltd.’s facilities
will entitle Protalix Ltd. to the same benefits to which it is
currently entitled.
Pursuant to the March 2005 amendment to the Investment Law, the
approval of the Investment Center is required only for Approved
Enterprises that receive cash grants. Approved Enterprises that
do not receive benefits in the form of governmental cash grants,
but only tax benefits, are no longer required to obtain this
approval. Instead, these Approved Enterprises are required to
make certain investments as specified in the Investment Law.
The amended Investment Law specifies certain conditions for an
Approved Enterprise to be entitled to benefits. These conditions
include:
|
|
|
| |
•
|
the Approved Enterprise’s revenues from any single country
or a separate customs territory may not exceed 75% of the
Approved Enterprise’s total revenues; or
|
| |
| |
•
|
at least 25% of the Approved Enterprise’s revenues during
the benefits period must be derived from sales into a single
country or a separate customs territory with a population of at
least 12 million.
|
There can be no assurance that Protalix Ltd. will comply with
the above conditions in the future or that Protalix Ltd. will be
entitled to any additional benefits under the Investment Law. In
addition, it is possible that Protalix Ltd. may not be able to
operate in a way that maximizes utilization of the benefits
under the Investment Law.
From time to time, the Israeli Government has discussed reducing
the benefits available to companies under the Investment Law.
The termination or substantial reduction of any of the benefits
available under the Investment Law could materially impact the
cost of our future investments.
Encouragement
of Industrial Research and Development Law, 1984
In the past, Protalix Ltd. received grants from the Office of
the Chief Scientist of the Israeli Ministry of Industry, Trade
and Labor, the OCS, for the financing of a portion of its
research and development expenditures in Israel. As of
December 31, 2010, the OCS approved grants in respect of
Protalix Ltd.’s
31
continuing operations totaling approximately $21.5 million,
measured from inception. Protalix Ltd. is required to repay up
to 100% of grants actually received (plus interest at the LIBOR
rate applied to the grants received on or after January 1,
1999) to the OCS through payments of royalties at a rate of
3% to 6% of the revenues generated from an OCS-funded project,
depending on the period in which revenues were generated. As of
December 31, 2010, Protalix Ltd. either paid or accrued
royalties payable of $2.7 million and Protalix Ltd.’s
contingent liability to the OCS with respect to grants received
was approximately $17.2 million,
Under the Israeli Law for the Encouragement of Industrial
Research and Development, 1984 and related regulations, the
Research Law, recipients of grants from the OCS are prohibited
from manufacturing products developed using these grants outside
of the State of Israel without special approvals, although the
Research Law does enable companies to seek prior approval for
conducting manufacturing activities outside of Israel without
being subject to increased royalties. If Protalix Ltd. receives
approval to manufacture the products developed with government
grants outside of Israel, it will be required to pay an
increased total amount of royalties (possibly up to 300% of the
grant amounts plus interest), depending on the manufacturing
volume that is performed outside of Israel, as well as at a
possibly increased royalty rate.
Additionally, under the Research Law, Protalix Ltd. is
prohibited from transferring the OCS financed technologies and
related intellectual property rights outside of the State of
Israel except under limited circumstances and only with the
approval of the Research Committee of the OCS. Protalix Ltd. may
not receive the required approvals for any proposed transfer
and, if received, Protalix Ltd. may be required to pay the OCS a
portion of the consideration that it receives upon any sale of
such technology by a non-Israeli entity. The scope of the
support received, the royalties that Protalix Ltd. has already
paid to the OCS, the amount of time that has elapsed between the
date on which the know-how was transferred and the date on which
the OCS grants were received and the sale price and the form of
transaction will be taken into account in order to calculate the
amount of the payment to the OCS. Approval of the transfer of
technology to residents of the State of Israel is required, and
may be granted in specific circumstances only if the recipient
abides by the provisions of applicable laws, including the
restrictions on the transfer of know-how and the obligation to
pay royalties. No assurance can be made that approval to any
such transfer, if requested, will be granted.
In March 2005, an amendment to the Research Law was enacted. One
of the main modifications included in the amendment was an
authorization of the Research Committee to allow the transfer
outside of Israel of know-how derived from an approved program
and the related manufacturing rights. In general, the Research
Committee may approve transfer of know-how in limited
circumstances as follows:
|
|
|
| |
•
|
in the event of a sale of the know-how itself to a non
affiliated third party, provided that upon such sale the owner
of the know-how pays to the OCS an amount, in cash, as set forth
in the Research Law. In addition, the amendment provides that if
the purchaser of the know-how gives the selling Israeli company
the right to exploit the know-how by way of an exclusive,
irrevocable and unlimited license, the research committee may
approve such transfer in special cases without requiring a cash
payment.
|
| |
| |
•
|
in the event of a sale of the company which is the owner of
know-how, pursuant to which the company ceases to be an Israeli
company, provided that upon such sale, the owner of the know-how
makes a cash payment to the OCS as set forth in the Research Law.
|
| |
| |
•
|
in the event of an exchange of know-how such that in exchange
for the transfer of know-how outside of Israel, the recipient of
the know-how transfers other know-how to the company in Israel
in a manner in which the OCS is convinced that the Israeli
economy realizes a greater, overall benefit from the exchange of
know-how.
|
Another provision in the amendment concerns the transfer of
manufacturing rights. The research committee may, in special
cases, approve the transfer of manufacture or of manufacturing
rights of a product developed within the framework of the
approved program or which results therefrom, outside of Israel.
The State of Israel does not own intellectual property rights in
technology developed with OCS funding and there is no
restriction on the export of products manufactured using
technology developed with OCS funding. The technology is,
however, subject to transfer of technology and manufacturing
rights restrictions as
32
described above. For a description of such restrictions, please
see “Risk Factors — Risks Relating to Our
Operations in Israel.” OCS approval is not required for the
export of any products resulting from the research or
development or for the licensing of any technology in the
ordinary course of business.
Special
Provisions Relating to Taxation under Inflationary
Conditions
Protalix Ltd. is taxed in Israel under the Income Tax Law
(Inflationary Adjustments), 1985, generally referred to as the
Inflationary Adjustments Law. The Inflationary Adjustments Law
is highly complex, and represents an attempt to overcome the
problems presented to a traditional tax system by an economy
undergoing rapid inflation. The provisions that are material to
us are summarized below:
|
|
|
| |
•
|
Where a company’s equity, as calculated under the
Inflationary Adjustments Law, exceeds the depreciated cost of
its fixed assets (as defined in the Inflationary Adjustments
Law), a deduction from taxable income is permitted equal to this
excess multiplied by the applicable annual rate of inflation.
The maximum deduction permitted under this provision in any
single tax year is 70% of taxable income. The unused portion
linked to the Israeli consumer price index, may be carried
forward.
|
| |
| |
•
|
Where a company’s depreciated cost of fixed assets exceeds
its equity, the excess multiplied by the applicable annual rate
of inflation is added to taxable income.
|
| |
| |
•
|
Subject to specified limitations, depreciation deductions
carryforwards on fixed assets and losses are adjusted for
inflation based on the change in the consumer price index.
|
Under the Inflationary Adjustments Law, results for tax purposes
are measured in real terms, in accordance with changes in the
Israeli consumer price index. The difference between the change
in the Israeli consumer price index and the exchange rate of
Israeli currency in relation to the U.S. dollar may in
future periods cause significant differences between taxable
income and the income measured in dollars as reflected in our
consolidated financial statements.
Law for
the Encouragement of Industry (Taxes), 1969
We believe that Protalix Ltd. currently qualifies as an
“Industrial Company” within the meaning of the Law for
the Encouragement of Industry (Taxes), 1969, or the Industry
Encouragement Law. The Industry Encouragement Law defines
“Industrial Company” as a company resident in Israel
that derives 90% or more of its income in any tax year (other
than specified kinds of passive income such as capital gains,
interest and dividends) from an “Industrial
Enterprise” that it owns. An “Industrial
Enterprise” is defined as an enterprise whose major
activity in a given tax year is industrial production.
The following corporate tax benefits, among others, are
available to Industrial Companies:
|
|
|
| |
•
|
amortization of the cost of purchased know-how and patents over
an eight-year period for tax purposes;
|
| |
| |
•
|
accelerated depreciation rates on equipment and buildings;
|
| |
| |
•
|
under specified conditions, an election to file consolidated tax
returns with other related Israeli Industrial Companies; and
|
| |
| |
•
|
expenses related to a public offering are deductible in equal
amounts over three years.
|
Eligibility for the benefits under the Industry Encouragement
Law is not subject to receipt of prior approval from any
governmental authority. It is possible that Protalix Ltd. may
fail to qualify or may not continue to qualify as an
“Industrial Company” or that the benefits described
above will not be available in the future.
Tax
Benefits for Research and Development
Under specified conditions, Israeli tax laws allow a tax
deduction by a company for research and development
expenditures, including capital expenditures, for the year in
which such expenditures are incurred. These expenditures must
relate to scientific research and development projects and must
be approved by the
33
OCS. Furthermore, the research and development projects must be
for the promotion of the company and carried out by or on behalf
of the company seeking such tax deduction. However, the amount
of such deductible expenditures is reduced by the sum of any
funds received through government grants for the finance of such
scientific research and development projects. Expenditures not
so approved are deductible over a three-year period.
Employees
As of December 31, 2010, we had 240 employees, of who
35 have an M.D. or a Ph.D. in their respective scientific
fields. We believe that our relations with these employees are
good. We intend to continue to hire additional employees in
research and development, manufacturing and administration in
order to meet our operating plans. We believe that our success
will greatly depend on our ability to identify, attract and
retain capable employees. The Israeli Ministry of Labor and
Welfare is authorized to make certain industry-wide collective
bargaining agreements that apply to types of industries or
employees including ours (“Expansion Orders”). These
agreements affect matters such as cost of living adjustments to
salaries, length of working hours and week, recuperation, travel
expenses, and pension rights. Otherwise, our employees are not
represented by a labor union or represented under a collective
bargaining agreement. See “Risk Factors — We
depend upon key employees and consultants in a competitive
market for skilled personnel. If we are unable to attract and
retain key personnel, it could adversely affect our ability to
develop and market our products.”
Company
Background
Our principal business address is set forth below. Our executive
offices and our main research manufacturing facility are located
at that address. Our telephone number is +972-4-988-9488. From
May 2001 through December 31, 2006, our company had no
operations. We were originally formed as Embassy Acquisition
Corp., a Florida corporation, in November 2005 and changed our
name to Orthodontix, Inc., in April, 1992. On December 31,
2006, we acquired, through a merger with our wholly-owned
subsidiary, Protalix Acquisition Co. Ltd., all of the
outstanding shares of Protalix Ltd., in exchange for shares of
our common stock. As a result, Protalix Ltd. is now our
wholly-owned subsidiary. In connection with the merger, we
completed a
one-for-ten
reverse stock split and on February 26, 2007, we changed
our name to Protalix BioTherapeutics, Inc. Unless otherwise
indicated, all share numbers in this Annual Report on
Form 10-K
give effect to such reverse stock split.
Our wholly-owned subsidiary and sole operating unit, Protalix
Ltd., is an Israeli corporation and was originally incorporated
in Israel as Metabogal Ltd. on December 27, 1993. During
1999, Protalix Ltd. changed its focus from plant secondary
metabolites to the expression of recombinant therapeutic
proteins in plant cells, and in April 2004 changed its name to
Protalix Ltd.
ProCellExtm
is our trademark. Each of the other trademarks, trade names or
service marks appearing in this Annual Report on
Form 10-K
belongs to its respective holder.
Available
Information
Our corporate website is www.protalix.com. We make available on
our website, free of charge, our Securities and Exchange
Commission, or the Commission, filings, including our Annual
Report on
Form 10-K,
Quarterly Reports on
Form 10-Q,
Current Reports on
Form 8-K
and any amendments to these reports, as soon as reasonably
practicable after we electronically file these documents with,
or furnish them to, the Commission. Additionally, from time to
time, we provide notifications of material news including press
releases and conferences on our website. Webcasts of
presentations made by our company at certain conferences may
also be available from time to time on our website, to the
extent the webcasts are available. The content of our website is
not intended to be incorporated by reference into this report or
in any other report or document we file and any references to
these websites are intended to be inactive textual references
only.
We are also listed on the Tel Aviv Stock Exchange and,
accordingly, we submit copies of all our filings with the
Commission to the Israeli Securities Authority and the Tel Aviv
Stock Exchange. Such copies can be
34
retrieved electronically through the Tel Aviv Stock
Exchange’s internet messaging system (www.maya.tase.co.il)
and through the MAGNA distribution site of the Israeli
Securities Authority (www.magna.isa.gov.il).
Our website also includes printable versions of our Code of
Business Conduct and Ethics and the charters for each of the
Audit, Compensation and Nominating Committees of our Board of
Directors. Each of these documents is also available in print to
any shareholder who requests a copy by addressing a request to:
Protalix BioTherapeutics, Inc.
2 Snunit Street
Science Park
POB 455
Carmiel 20100, Israel
Attn: Mr. Yossi Maimon, Chief Financial Officer
You should carefully consider the risks described below
together with the other information included in this Annual
Report on
Form 10-K.
Our business, financial condition or results of operations could
be adversely affected by any of these risks. If any of these
risks occur, the value of our common stock could decline.
Risks
Related to Our Business
We
currently have no significant product revenues and will need to
raise additional capital to operate our business, which may not
be available on favorable terms, or at all, and which will have
a dilutive effect on our shareholders.
To date, we have generated no significant revenues from product
sales and only minimal revenues from research and development
services and other fees, other than the milestone payments we
received in connection with our license and supply agreement
with Pfizer. For the years ended December 31, 2010, 2009
and 2008, we had net losses of $29.0 million,
$31.4 million and $22.4 million, respectively,
primarily as a result of expenses incurred through a combination
of research and development activities and expenses supporting
those activities, which includes share-based compensation
expense. Drug development and commercialization is very capital
intensive. Until we receive approval from the FDA and other
regulatory authorities for our drug candidates, we cannot
generate significant sales from our drugs and will not have
product revenues, except for certain regulatory-related
milestone payments under the Pfizer agreement which we expect to
earn prior to any sales of taliglucerase alfa. Therefore, for
the foreseeable future, we will have to fund all of our
operations and capital expenditures from our cash on hand,
potential regulatory-related milestone payments under the Pfizer
agreement, other licensing fees and grants and the net proceeds
of any equity or debt offerings. Over the next 12 months,
we expect to spend a minimum of approximately $35.0 million
building an internal sales and marketing force for the sale of
taliglucerase alfa in Israel, expanding our manufacturing
capacity and on preclinical and clinical development for our
products candidates. Based on our current plans and capital
resources, we believe that our cash and cash equivalents
together with the regulatory milestones payments we anticipate
receiving from Pfizer will be sufficient to enable us to meet
our planned operating needs for at least 12 months.
However, changes may occur that could consume our existing
capital at a faster rate than projected, including, among
others, changes in the progress of our research and development
efforts, the cost and timing of regulatory approvals and the
costs of protecting our intellectual property rights. We may
seek additional financing to implement and fund product
development, preclinical studies and clinical trials for the
drugs in our pipeline, as well as additional drug candidates and
other research and development projects. If we are unable to
secure additional financing in the future on acceptable terms,
or at all, we may be unable to commence or complete planned
preclinical and clinical trials or obtain approval of our drug
candidates from the FDA and other regulatory authorities. In
addition, we may be forced to reduce or discontinue product
development or product licensing, reduce or forego sales and
marketing efforts and other commercialization activities or
forego attractive business opportunities in order to improve our
liquidity and to enable us to continue operations which would
have a material adverse effect on our business and results of
35
operations. Any additional sources of financing will likely
involve the issuance of our equity securities, which will have a
dilutive effect on our shareholders.
We are
not currently profitable and may never become profitable which
would have a material adverse effect on our business and results
of operations and could negatively impact the value of our
common stock.
We expect to incur substantial losses for the foreseeable future
and may never become profitable. We also expect to continue to
incur significant operating and capital expenditures, and we
anticipate that our expenses will increase substantially in the
foreseeable future as we:
|
|
|
| |
•
|
continue to undertake preclinical development and clinical
trials for our current and new drug candidates;
|
| |
| |
•
|
seek regulatory approvals for our drug candidates;
|
| |
| |
•
|
implement additional internal systems and infrastructure;
|
| |
| |
•
|
seek to license-in additional technologies to develop; and
|
| |
| |
•
|
hire additional personnel.
|
We also expect to continue to experience negative cash flow for
the foreseeable future as we fund our operating losses and
capital expenditures. As a result, we will need to generate
significant revenues in order to achieve and maintain
profitability. We may not be able to generate these revenues or
achieve profitability in the future. Any failure to achieve or
maintain profitability would have a material adverse effect on
our business and results of operations and could negatively
impact the value of our common stock.
We
have a limited operating history which may limit the ability of
investors to make an informed investment decision.
We are a clinical stage biopharmaceutical company. To date, we
have not commercialized any of our drug candidates or received
any FDA or other approval to market any drug. The successful
commercialization of our drug candidates will require us to
perform a variety of functions, including:
|
|
|
| |
•
|
continuing to undertake preclinical development and clinical
trials;
|
| |
| |
•
|
participating in regulatory approval processes;
|
| |
| |
•
|
formulating and manufacturing products; and
|
| |
| |
•
|
conducting sales and marketing activities.
|
Our operations have been limited to organizing and staffing our
company, acquiring, developing and securing our proprietary
technology and undertaking, through third parties, preclinical
trials and clinical trials of our principal drug candidates. To
date, we have commenced a phase III clinical trial in
connection with only one drug candidate, taliglucerase alfa,
which trial was completed in August 2009, and we have not
commenced the preclinical trial phase of development under Good
Laboratory Practice (GLP) standards for any of our other drug
candidates, except for our pr-antiTNF and acetylcholinesterase
product candidates. These operations provide a limited basis for
investors to assess our ability to commercialize our drug
candidates and whether to invest in us.
Our
ProCellEx protein expression system is based on our proprietary
plant cell-based expression technology which has a limited
history and any material problems with the system, which may be
unforeseen, may have a material adverse effect on our business
and results of operations.
Our ProCellEx protein expression system is based on our
proprietary plant cell-based expression technology. Our business
is dependent upon the successful development and approval of our
product candidates produced through our protein expression
system. Our ProCellEx protein expression system is novel and is
still in the early stages of development and optimization, and,
accordingly, is subject to certain risks.
36
Mammalian cell-based protein expression systems have been used
in connection with recombinant therapeutic protein expression
for more than 20 years and are the subject of a wealth of
data; in contrast, there is not a significant amount of data
generated regarding plant cell-based protein expression and,
accordingly, plant cell-based protein expression systems may be
subject to unknown risks. In addition, the protein glycosilation
pattern created by our protein expression system is not
identical to the natural human glycosilation pattern and its
long term effect on human patients is still unknown. Lastly, as
our protein expression system is a new technology, we cannot
always rely on existing equipment; rather, there is a need to
design custom-made equipment and to generate specific growth
media for the plant cells, which may not be available at
favorable prices, if at all. Any material problems with the
technology underlying our plant cell-based protein expression
system may have a material adverse effect on our business and
results of operations.
We
currently depend heavily on the success of taliglucerase alfa,
our lead product candidate. Any failure to commercialize
taliglucerase alfa, or the experience of significant delays in
doing so, will have a material adverse effect on our business,
results of operations and financial condition.
We have invested a significant portion of our efforts and
financial resources in the development of taliglucerase alfa.
Our ability to generate product revenue, depends heavily on the
successful development and commercialization of taliglucerase
alfa. In November 2009, we granted to Pfizer an exclusive
worldwide license to develop and commercialize taliglucerase
alfa except in Israel. We retained such rights in Israel. The
successful commercialization of taliglucerase alfa will depend
on several factors, including the following:
|
|
|
| |
•
|
successful completion of our ongoing studies of taliglucerase
alfa;
|
| |
| |
•
|
obtaining marketing approvals from the FDA and other foreign
regulatory authorities;
|
| |
| |
•
|
maintaining the cGMP compliance of our manufacturing facility or
establishing manufacturing arrangements with third parties;
|
| |
| |
•
|
the successful audit of our facilities by the FDA and other
foreign regulatory authorities;
|
| |
| |
•
|
Pfizer’s efforts under the Pfizer agreement;
|
| |
| |
•
|
our development of a successful sales and marketing organization
for taliglucerase in Israel;
|
| |
| |
•
|
the availability of reimbursement to patients from healthcare
payors for our drug products, if approved;
|
| |
| |
•
|
a continued acceptable safety and efficacy profile of our
product candidates following approval; and
|
| |
| |
•
|
other risks described in these Risk Factors.
|
Any failure to commercialize taliglucerase alfa or the
experience of significant delays in doing so will have a
material adverse effect on our business, results of operations
and financial condition.
Our
strategy, in many cases, is to enter into collaboration
agreements with third parties to leverage our ProCellEx system
to develop product candidates. If we fail to enter into these
agreements or if we or the third parties do not perform under
such agreements or terminate or elect to discontinue the
collaboration, it could have a material adverse affect on our
revenues.
Our strategy, in many cases, is to enter into arrangements with
pharmaceutical companies to leverage our ProCellEx system to
develop additional product candidates. Under these arrangements,
we may grant to our partners rights to license and commercialize
pharmaceutical products developed under the applicable
agreements. Our partners may control key decisions relating to
the development of the products and we may depend on our
partners’ expertise and dedication of sufficient resources
to develop and commercialize our product candidates. The rights
of our partners limit our flexibility in considering
alternatives for the commercialization of our product
candidates. To date, we have entered into a license and supply
agreement with Pfizer relating to the development and
commercialization of taliglucerase alfa and an agreement with
Teva, which relates to the development by us of two proteins,
and the licensing by Teva of such proteins in consideration for
royalties and milestone payments. Subsequently, two proteins
were identified to be researched and developed under the
agreement but in 2009, both of the projects were terminated for
37
commercial reasons. We may not identify any additional proteins
to be developed in a collaboration between us and Teva under the
agreement, which may have a material adverse effect on our
business, results of operations and financial condition. If we
or any of our partners breach or terminate the agreements that
make up such arrangements, our partners otherwise fail to
conduct their obligations under such arrangements in a timely
manner, there is a dispute about their obligations or if either
party terminates the applicable agreement or elects not to
continue the arrangement, we may not enjoy the benefits of the
agreements or receive a sufficient amount of royalty or
milestone payments from them, if any.
All of
our product candidates other than taliglucerase alfa and our
acetycholinesterase product are in preclinical or research
stages. If we are unable to develop and commercialize our
product candidates, our business will be adversely
affected.
A key element of our strategy is to develop and commercialize a
portfolio of new products in addition to taliglucerase alfa. We
are seeking to do so through our internal research programs and
strategic collaborations for the development of new products.
Research programs to identify new product candidates require
substantial technical, financial and human resources, whether or
not any product candidates are ultimately identified. Our
research programs may initially show promise in identifying
potential product candidates, yet fail to yield product
candidates for clinical development for many reasons, including
the following:
|
|
|
| |
•
|
the research methodology used may not be successful in
identifying potential product candidates;
|
| |
| |
•
|
competitors may develop alternatives that render our product
candidates obsolete;
|
| |
| |
•
|
a product candidate may on further study be shown to have
harmful side effects or other characteristics that indicate it
is unlikely to be effective or otherwise does not meet
applicable regulatory approval;
|
| |
| |
•
|
a product candidate is not capable of being produced in
commercial quantities at an acceptable cost, or at all; or
|
| |
| |
•
|
a product candidate may not be accepted by patients, the medical
community or third-party payors.
|
Any failure to develop or commercialize any of our other product
candidates may have a material adverse effect on our business,
results of operations and financial condition.
We may
not obtain the necessary U.S. or worldwide regulatory approvals
to commercialize our drug candidates in a timely manner, if at
all, which would have a material adverse effect on our business
and results of operations.
We will need FDA approval to commercialize our drug candidates
in the United States and approvals from foreign regulators to
commercialize our drug candidates elsewhere. In order to obtain
FDA approval of any of our drug candidates, we must submit to
the FDA an NDA or a Biologic License Application, a BLA,
demonstrating that the drug candidate is safe for humans and
effective for its intended use. This demonstration requires
significant research and animal tests, which are referred to as
preclinical studies, as well as human tests, which are referred
to as clinical trials. In the European Union, we must submit an
MAA to the EMEA. Satisfaction of the FDA’s and foreign
regulatory authorities’ regulatory requirements typically
takes many years, and depends upon the type, complexity and
novelty of the drug candidate and requires substantial resources
for research, development and testing. In December 2009 we
completed the filing of an NDA for taliglucerase alfa for the
treatment of Gaucher disease and received a PDUFA date of
February 25, 2011, and in November 2010, we submitted a
marketing application to the Israeli MOH and an MAA to each of
the EMEA and ANVISA for taliglucerase alfa. Our research and
clinical efforts may not result in drugs that the FDA or foreign
regulatory authorities consider safe for humans and effective
for indicated uses, which would have a material adverse effect
on our business and results of operations. After clinical trials
are completed for any drug candidate, if at all, the FDA and
foreign regulatory authorities have substantial discretion in
the drug approval process of the drug candidate in their
respective jurisdictions and may require us to conduct
additional clinical testing or to perform post-marketing studies
which would cause us to incur additional costs. Incurring such
costs could have a material adverse effect on our business and
results of operations.
38
The approval process for any drug candidate may also be delayed
by changes in government regulation, future legislation or
administrative action or changes in policy of the FDA and
comparable foreign authorities that occur prior to or during
their respective regulatory reviews of such drug candidate.
Delays in obtaining regulatory approvals with respect to any
drug candidate may:
|
|
|
| |
•
|
delay commercialization of, and our ability to derive product
revenues from, such drug candidate;
|
| |
| |
•
|
delay the regulatory-related milestone payments we anticipate
receiving from Pfizer;
|
| |
| |
•
|
require us to perform costly procedures with respect to such
drug candidate; or
|
| |
| |
•
|
otherwise diminish any competitive advantages that we may have
with respect to such drug candidate.
|
Even if we comply with all the requests of the FDA and
comparable foreign authorities, the authorities may ultimately
reject the NDA or other filing or submission we filed for
taliglucerase alfa or one or more of the NDAs or other filing or
submission we file in the future, if any, or we might not obtain
regulatory clearance in a timely manner for taliglucerase alfa
or any of our other product candidates. Companies in the
pharmaceutical and biotechnology industries have suffered
significant setbacks in advanced or late-stage clinical trials,
even after obtaining promising earlier trial results or in
preliminary findings or other comparable authorities for such
clinical trials. Further, even if favorable testing data is
generated by the clinical trials of a drug product, the FDA may
not accept or approve an NDA, MAA or other comparable submission
filed by a pharmaceutical or biotechnology company for the drug
product. Failure to obtain approval of the FDA or comparable
foreign authorities any of our drug candidates in a timely
manner, if at all, will severely undermine our business and
results of operation by reducing our potential marketable
products and our ability to generate corresponding product
revenues.
The
“fast track” designation for the development program
of taliglucerase alfa for the treatment of Gaucher disease may
not lead to a faster development or regulatory review or
approval process or increase the likelihood that taliglucerase
alfa will receive regulatory approval for the treatment of
Gaucher disease.
If a human medicine is intended for the treatment of a serious
or life-threatening condition and the medicine demonstrates the
potential to address unmet medical needs for this condition, the
sponsor of an IND may apply for FDA “fast track”
designation for a particular indication. Marketing applications
submitted by sponsors of product candidates in fast track
development may qualify for expedited FDA review under the
policies and procedures offered by the FDA, but the fast track
designation does not assure any such qualification. However,
although the FDA has granted fast track designation for
taliglucerase alfa for the treatment of Gaucher disease, we were
granted the standard review period of 10 months. The fast
track designation does not increase the likelihood that
taliglucerase alfa will receive regulatory approval for the
treatment of Gaucher disease.
Clinical
trials are very expensive, time-consuming and difficult to
design and implement and may result in unforeseen costs which
may have a material adverse effect on our business and results
of operations.
Human clinical trials are very expensive and difficult to design
and implement, in part because they are subject to rigorous
regulatory requirements. The clinical trial process is also
time-consuming. Other than taliglucerase alfa and our
acetylcholinesterase product, our drug candidates are in early
stages of preclinical studies or research stages. Other, ongoing
clinical trials of taliglucerase alfa and our
acetylcholinesterase product, and anticipated clinical trials of
our other potential drug candidates which have not yet been
initiated, will take at least several years to complete.
Preliminary and initial results from a clinical trial do not
necessarily predict final results, and failure can occur at any
stage of the trials. We may encounter problems that cause us to
abandon or repeat preclinical studies or clinical trials.
Companies in the pharmaceutical and biotechnology industries
have suffered significant setbacks in advanced clinical trials,
even after obtaining promising results in earlier trials. Data
obtained from tests are susceptible to varying interpretations
which
39
may delay, limit or prevent regulatory approval. Failure or
delay in the commencement or completion of our clinical trials
may be caused by several factors, including:
|
|
|
| |
•
|
unforeseen safety issues;
|
| |
| |
•
|
determination of dosing issues;
|
| |
| |
•
|
lack of effectiveness during clinical trials;
|
| |
| |
•
|
slower than expected rates of patient recruitment;
|
| |
| |
•
|
inability to monitor patients adequately during or after
treatment;
|
| |
| |
•
|
inability or unwillingness of medical investigators and
institutional review boards to follow our clinical
protocols; and
|
| |
| |
•
|
lack of sufficient funding to finance the clinical trials.
|
Any failure or delay in commencement or completion of any
clinical trials may have a material adverse effect on our
business and results of operations. In addition, we or the FDA
or other regulatory authorities may suspend any clinical trial
at any time if it appears that we are exposing participants in
the trial to unacceptable safety or health risks or if the FDA
or such other regulatory authorities, as applicable, find
deficiencies in our IND submissions or the conduct of the trial.
Any suspension of a clinical trial may have a material adverse
effect on our business, financial condition and results of
operations.
If the
results of our clinical trials do not support our claims
relating to any drug candidate or if serious side effects are
identified, the completion of development of such drug candidate
may be significantly delayed or we may be forced to abandon
development altogether, which will significantly impair our
ability to generate product revenues.
The results of our clinical trials with respect to any drug
candidate might not support our claims of safety or efficacy,
the effects of our drug candidates may not be the desired
effects or may include undesirable side effects or the drug
candidates may have other unexpected characteristics. Further,
success in preclinical testing and early clinical trials does
not ensure that later clinical trials will be successful, and
the results of later clinical trials may not replicate the
results of prior clinical trials and preclinical testing. The
clinical trial process may fail to demonstrate that our drug
candidates are safe for humans and effective for indicated uses.
In addition, our clinical trials may involve a specific and
small patient population. Results of early clinical trials
conducted on a small patient population may not be indicative of
future results. Adverse or inconclusive results may cause us to
abandon a drug candidate and may delay development of other drug
candidates. Any delay in, or termination of, our clinical trials
will delay the filing of NDAs with the FDA, or other filings
with other regulatory authorities, and, ultimately,
significantly impair our ability to commercialize our drug
candidates and generate product revenues which would have a
material adverse effect on our business, financial condition and
results of operations.
We may
find it difficult to enroll patients in our clinical trials,
which could cause significant delays in the completion of such
trials or may cause us to abandon one or more clinical
trials.
Most of the diseases or disorders that our product candidates
are intended to treat are relatively rare and we expect only a
subset of the patients with these diseases to be eligible for
our clinical trials. Given that each of our product candidates
other than taliglucerase alfa is in the early stages of
preclinical or research stages, we may not be able to initiate
clinical trials for each or all of our product candidates if we
are unable to locate a sufficient number of eligible subjects to
participate in the clinical trials required by the FDA
and/or other
foreign regulatory authorities. The requirements of our clinical
trials generally mandate that a patient cannot be involved in
another clinical trial for the same indication. We are aware
that our competitors have ongoing clinical trials for products
that are competitive with our product candidates and subjects
who would otherwise be eligible for our clinical trials may be
involved in such testing, rendering them unavailable for testing
of our product candidates. Our inability to enroll a sufficient
number of patients for any of our current
40
or future clinical trials would result in significant delays or
may require us to abandon one or more clinical trials
altogether, which would have a material adverse effect on our
business.
If
physicians, patients, third party payors and others in the
medical community do not accept and use our drugs, our ability
to generate revenue from sales of our products under development
will be materially impaired.
Even if the FDA or other foreign regulatory authorities approve
any of our drug candidates for commercialization, physicians and
patients, and other healthcare providers, may not accept and use
such candidates. Future acceptance and use of our products will
depend upon a number of factors including:
|
|
|
| |
•
|
perceptions by physicians, patients, third party payors and
others in the medical community, about the safety and
effectiveness of our drug candidates;
|
| |
| |
•
|
the willingness of the target patient population to try new
therapies and of physicians to prescribe these therapies;
|
| |
| |
•
|
the prevalence and severity of any side effects, including any
limitations or warnings contained in our products’ approved
labeling;
|
| |
| |
•
|
pharmacological benefit of our products relative to competing
products and products under development;
|
| |
| |
•
|
the efficacy and potential advantages relative to competing
products and products under development;
|
| |
| |
•
|
relative convenience and ease of administration;
|
| |
| |
•
|
effectiveness of education, marketing and distribution efforts
by us and our licensees and distributors, if any;
|
| |
| |
•
|
publicity concerning our products or competing products and
treatments;
|
| |
| |
•
|
reimbursement of our products by third party payors; and
|
| |
| |
•
|
the price for our products and competing products.
|
Because we expect sales of our current drug candidates, if
approved, to generate substantially all of our product revenues
for the foreseeable future, the failure of any of these drugs to
find market acceptance would have a material adverse effect on
our business and revenues from sales of our products would be
materially impaired.
Because
our clinical trials depend upon third-party researchers, the
results of our clinical trials and such research activities are
subject to delays and other risks which are, to a certain
extent, beyond our control, which could impair our clinical
development programs and our competitive position.
We depend upon independent investigators and collaborators, such
as universities and medical institutions, to conduct our
preclinical and clinical trials. These collaborators are not our
employees, and we cannot control the amount or timing of
resources that they devote to our clinical development programs.
The investigators may not assign as great a priority to our
clinical development programs or pursue them as diligently as we
would if we were undertaking such programs directly. If outside
collaborators fail to devote sufficient time and resources to
our clinical development programs, or if their performance is
substandard, the approval of our NDA, MAA and other
applications, if any, and our introduction of new drugs, if any,
may be delayed which could impair our clinical development
programs and would have a material adverse effect on our
business and results of operations. The collaborators may also
have relationships with other commercial entities, some of whom
may compete with us. If our collaborators also assist our
competitors, our competitive position could be harmed.
41
The
manufacture of our products is an exacting and complex process,
and if we or one of our materials suppliers encounter problems
manufacturing our products, it will have a material adverse
effect on our business and results of operations.
The FDA and foreign regulators require manufacturers to register
manufacturing facilities. The FDA and foreign regulators also
inspect these facilities to confirm compliance with cGMP or
similar requirements that the FDA or foreign regulators
establish. We or our materials suppliers may face manufacturing
or quality control problems causing product production and
shipment delays or a situation where we or the supplier may not
be able to maintain compliance with the FDA’s cGMP
requirements, or those of foreign regulators, necessary to
continue manufacturing our drug candidates. Any failure to
comply with cGMP requirements or other FDA or foreign regulatory
requirements could adversely affect our clinical research
activities and our ability to market and develop our products.
To date, our current facility has been audited by the FDA and
the Israeli MOH, but remains subject to audit by other foreign
regulatory authorities. There can be no assurance that we will
be able to comply with FDA or foreign regulatory manufacturing
requirements for our current facility or any facility we may
establish in the future, which would have a material adverse
effect on our business.
We
rely on third parties for final processing of taliglucerase
alfa, which exposes us to a number of risks that may delay
development, regulatory approval and commercialization of our
product candidates or result in higher product
costs.
We have no experience in the final filling and freeze drying
steps of the drug manufacturing process. According to our
license and supply agreement with Pfizer, Pfizer will be
responsible for the fill and finish activities for taliglucerase
alfa. In addition, we have engaged a European contract
manufacturer to act as an additional source of fill and finish
activities for taliglucerase alfa. We currently rely primarily
on other third-party contractors to perform the final
manufacturing steps for taliglucerase alfa on a commercial
scale. We may be unable to identify manufacturers
and/or
replacement manufacturers on acceptable terms or at all because
the number of potential manufacturers is limited and the FDA and
other regulatory authorities, as applicable, must approve any
manufacturer
and/or
replacement manufacturer, including us, and we or any such third
party manufacturer might be unable to formulate and manufacture
our drug products in the volume and of the quality required to
meet our clinical and commercial needs. If we engage any
contract manufacturers, such manufacturers may not perform as
agreed or may not remain in the contract manufacturing business
for the time required to supply our clinical or commercial
needs. In addition, contract manufacturers are subject to the
rules and regulations of the FDA and comparable foreign
regulatory authorities and face the risk that any of those
authorities may find that they are not in compliance with
applicable regulations. Each of these risks could delay our
clinical trials, the approval, if any, of taliglucerase alfa and
our other potential drug candidates by the FDA or other
regulatory authorities, or the commercialization of
taliglucerase alfa and our other drug candidates or could result
in higher product costs or otherwise deprive us of potential
product revenues.
We
have no experience selling, marketing or distributing products
and no internal capability to do so.
We currently have very limited sales, marketing or distribution
capabilities and no experience in building a sales force and
distribution capabilities. To be able to commercialize
taliglucerase alfa upon approval, if at all, in Israel, and to
commercialize any of our other product candidates, we must
either develop internal sales, marketing and distribution
capabilities, which will be expensive and time consuming, or
make arrangements with third parties to perform these services.
In November 2009, we granted to Pfizer an exclusive, worldwide
right to develop and commercialize taliglucerase alfa, but
retained such rights in Israel. If we decide to market any of
our products directly, we must commit significant financial and
managerial resources to develop a marketing and sales force with
technical expertise and with supporting distribution
capabilities. Factors that may inhibit our efforts to
commercialize our products directly and without strategic
partners include:
|
|
|
| |
•
|
our inability to recruit and retain adequate numbers of
effective sales and marketing personnel;
|
| |
| |
•
|
the inability of sales personnel to obtain access to or persuade
adequate numbers of physicians to prescribe our products;
|
42
|
|
|
| |
•
|
the lack of complementary products to be offered by sales
personnel, which may put us at a competitive disadvantage
relative to companies with more extensive product lines; and
|
| |
| |
•
|
unforeseen costs and expenses associated with creating and
sustaining an independent sales and marketing organization.
|
We may not be successful in recruiting the sales and marketing
personnel necessary to sell any of our products upon approval,
if at all, and even if we do build a sales force, it may not be
successful in marketing our products, which would have a
material adverse effect on our business, financial condition and
results of operations.
If the
market opportunities for our current product candidates are
smaller than we believe they are, our revenues may be adversely
affected and our business may suffer.
A substantial focus of our current clinical pipeline is on
relatively rare disorders with small patient populations, in
particular Gaucher disease and Fabry disease. Currently, most
reported estimates of the prevalence of these diseases are based
on studies of small subsets of the population of specific
geographic areas, which are then extrapolated to estimate the
prevalence of the diseases in the broader world population. As
new studies are performed, the estimated prevalence of these
diseases may change. There can be no assurance that the
prevalence of Gaucher disease or Fabry disease in the study
populations, particularly in these newer studies, accurately
reflect the prevalence of these diseases in the broader world
population. If the market opportunities for our current product
candidates are smaller than we believe they are, our revenues
may be adversely affected and our business may suffer.
We may
enter into distribution arrangements and marketing alliances for
certain products and any failure to successfully identify and
implement these arrangements on favorable terms, if at all, may
impair our ability to commercialize our product
candidates.
While we intend to build a sales force to market taliglucerase
alfa in Israel and other product candidates worldwide, we do not
anticipate having the resources in the foreseeable future to
develop global sales and marketing capabilities for all of the
products we develop, if any. We may pursue arrangements
regarding the sales and marketing and distribution of one or
more of our product candidates, such as our license and supply
agreement with Pfizer, and our future revenues may depend, in
part, on our ability to enter into and maintain arrangements
with other companies having sales, marketing and distribution
capabilities and the ability of such companies to successfully
market and sell any such products. Any failure to enter into
such arrangements and marketing alliances on favorable terms, if
at all, could delay or impair our ability to commercialize our
product candidates and could increase our costs of
commercialization. Any use of distribution arrangements and
marketing alliances to commercialize our product candidates will
subject us to a number of risks, including the following:
|
|
|
| |
•
|
we may be required to relinquish important rights to our
products or product candidates;
|
| |
| |
•
|
we may not be able to control the amount and timing of resources
that our distributors or collaborators may devote to the
commercialization of our product candidates;
|
| |
| |
•
|
our distributors or collaborators may experience financial
difficulties;
|
| |
| |
•
|
our distributors or collaborators may not devote sufficient time
to the marketing and sales of our products; and
|
| |
| |
•
|
business combinations or significant changes in a
collaborator’s business strategy may adversely affect a
collaborator’s willingness or ability to complete its
obligations under any arrangement.
|
We may need to enter into additional co-promotion arrangements
with third parties where our own sales force is neither well
situated nor large enough to achieve maximum penetration in the
market. We may not be successful in entering into any
co-promotion arrangements, and the terms of any co-promotion
arrangements we enter into may not be favorable to us.
43
Developments
by competitors may render our products or technologies obsolete
or non-competitive which would have a material adverse effect on
our business and results of operations.
We compete against fully integrated pharmaceutical companies and
smaller companies that are collaborating with larger
pharmaceutical companies, academic institutions, government
agencies and other public and private research organizations.
Our drug candidates will have to compete with existing therapies
and therapies under development by our competitors. In addition,
our commercial opportunities may be reduced or eliminated if our
competitors develop and market products that are less expensive,
more effective or safer than our drug products. Other companies
have drug candidates in various stages of preclinical or
clinical development to treat diseases for which we are also
seeking to develop drug products. Some of these potential
competing drugs are further advanced in development than our
drug candidates and may be commercialized earlier. Even if we
are successful in developing effective drugs, our products may
not compete successfully with products produced by our
competitors.
We specifically face competition from companies with approved
treatments of Gaucher disease, including Genzyme, Shire plc and
to a much lesser extent, Actelion. In February 2010, the FDA
approved VPRIV, Shire’s enzyme replacement therapy for the
treatment of Gaucher disease and the European Commission granted
marketing authorization to VPRIV in August 2010. In addition, we
are aware of other early stage, experimental, small molecule,
oral drugs which are being developed for the treatment of
Gaucher disease by each of Genzye and Amicus Therapeutics.
According to Amicus Therapeutics, its trial of a small molecule,
oral drug for the treatment of Gaucher disease has been
suspended. We also face competition from companies with approved
treatments of Fabry disease, including Genzyme and Shire, and we
are aware of other early stage drugs which are being developed
for the treatment of Fabry disease, including a drug being
developed by Amicus Therapeutics.
We also face competition from companies that are developing
other platforms for the expression of recombinant therapeutic
pharmaceuticals. We are aware of companies that are developing
alternative technologies to develop and produce therapeutic
proteins in anticipation of the expiration of certain patent
claims covering marketed proteins. Competitors developing
alternative expression technologies include Crucell N.V. (which
was acquired by Johnson & Johnson), Shire and GlycoFi
Inc. (which was acquired by Merck). Other companies are
developing alternate plant-based technologies, include Biolex,
Inc., Chlorogen, Inc., Greenovation Biotech GmbH and Dow
Agroscience.
Several biogeneric companies are pursuing the opportunity to
develop and commercialize follow-on versions of other currently
marketed biologic products, including growth factors, hormones,
enzymes, cytokines and monoclonal antibodies, which are areas
that interest us. These companies include, among others,
Novartis AG/Sandoz Pharmaceuticals, BioGeneriX AG, Stada
Arzneimittel AG, BioPartners GmbH and Teva.
Most of our competitors, either alone or together with their
collaborative partners, operate larger research and development
programs, staff and facilities and have substantially greater
financial resources than we do, as well as significantly greater
experience in:
|
|
|
| |
•
|
developing drugs;
|
| |
| |
•
|
undertaking preclinical testing and human clinical trials;
|
| |
| |
•
|
obtaining FDA and other regulatory approvals of drugs;
|
| |
| |
•
|
formulating and manufacturing drugs; and
|
| |
| |
•
|
launching, marketing and selling drugs.
|
These organizations also compete with us to attract qualified
personnel, acquisitions and joint ventures candidates and for
other collaborations. Activities of our competitors may impose
unanticipated costs on our business which would have a material
adverse effect on our business, financial condition and results
of operations.
44
If we
fail to adequately protect or enforce our intellectual property
rights or secure rights to third party patents, the value of our
intellectual property rights would diminish and our business,
competitive position and results of operations would
suffer.
As of December 31, 2010, we had 86 pending patent
applications and held licensed rights to seven pending patent
applications. However, the filing of a patent application does
not mean that we will be issued a patent, or that any patent
eventually issued will be as broad as requested in the patent
application or sufficient to protect our technology. Any
modification required to a current patent application may delay
the approval of such patent application which would have a
material adverse effect on our business and results of
operations. In addition, there are a number of factors that
could cause our patents, if granted, to become invalid or
unenforceable or that could cause our patent applications to not
be granted, including known or unknown prior art, deficiencies
in the patent application or the lack of originality of the
technology.
Our competitive position and future revenues will depend in part
on our ability and the ability of our licensors and
collaborators to obtain and maintain patent protection for our
products, methods, processes and other technologies, to preserve
our trade secrets, to prevent third parties from infringing on
our proprietary rights and to operate without infringing the
proprietary rights of third parties. We have filed U.S. and
international patent applications for process patents, as well
as composition of matter patents, for taliglucerase alfa.
However, we cannot predict:
|
|
|
| |
•
|
the degree and range of protection any patents will afford us
against competitors and those who infringe upon our patents,
including whether third parties will find ways to invalidate or
otherwise circumvent our licensed patents;
|
| |
| |
•
|
if and when patents will issue;
|
| |
| |
•
|
whether or not others will obtain patents claiming aspects
similar to those covered by our licensed patents and patent
applications; or
|
| |
| |
•
|
whether we will need to initiate litigation or administrative
proceedings, which may be costly, and whether we win or lose.
|
As of December 31, 2010, we hold, or have license rights
to, 26 patents. If patent rights covering our products are not
sufficiently broad, they may not provide us with sufficient
proprietary protection or competitive advantages against
competitors with similar products and technologies. Furthermore,
if the U.S. Patent and Trademark Office or foreign patent
offices issue patents to us or our licensors, others may
challenge the patents or circumvent the patents, or the patent
office or the courts may invalidate the patents. Thus, any
patents we own or license from or to third parties may not
provide any protection against our competitors and those who
infringe upon our patents.
Furthermore, the life of our patents is limited. The patents we
hold relating to our ProCellEx protein expression system will
expire in 2017. If patents issue from other currently pending
patent applications, those patents will expire between 2024 and
2028.
We
rely on confidentiality agreements that could be breached and
may be difficult to enforce which could have a material adverse
effect on our business and competitive position.
Our policy is to enter agreements relating to the non-disclosure
of confidential information with third parties, including our
contractors, consultants, advisors and research collaborators,
as well as agreements that purport to require the disclosure and
assignment to us of the rights to the ideas, developments,
discoveries and inventions of our employees and consultants
while we employ them. However, these agreements can be difficult
and costly to enforce. Moreover, to the extent that our
contractors, consultants, advisors and research collaborators
apply or independently develop intellectual property in
connection with any of our projects, disputes may arise as to
the proprietary rights to the intellectual property. If a
dispute arises, a court may determine that the right belongs to
a third party, and enforcement of our rights can be costly and
unpredictable. In addition, we rely on trade secrets and
proprietary know-how that we seek to protect in part by
45
confidentiality agreements with our employees, contractors,
consultants, advisors or others. Despite the protective measures
we employ, we still face the risk that:
|
|
|
| |
•
|
these agreements may be breached;
|
| |
| |
•
|
these agreements may not provide adequate remedies for the
applicable type of breach; or
|
| |
| |
•
|
our trade secrets or proprietary know-how will otherwise become
known.
|
Any breach of our confidentiality agreements or our failure to
effectively enforce such agreements would have a material
adverse effect on our business and competitive position.
If we
infringe the rights of third parties we could be prevented from
selling products, forced to pay damages and required to defend
against litigation which could result in substantial costs and
may have a material adverse effect on our business and results
of operations.
We have not received to date any claims of infringement by any
third parties. However, as our drug candidates progress into
clinical trials and commercialization, if at all, our public
profile and that of our drug candidates may be raised and
generate such claims. Defending against such claims, and
occurrence of a judgment adverse to us, could result in
unanticipated costs and may have a material adverse effect on
our business and competitive position. If our products, methods,
processes and other technologies infringe the proprietary rights
of other parties, we may incur substantial costs and we may have
to:
|
|
|
| |
•
|
obtain licenses, which may not be available on commercially
reasonable terms, if at all;
|
| |
| |
•
|
redesign our products or processes to avoid infringement;
|
| |
| |
•
|
stop using the subject matter claimed in the patents held by
others, which could cause us to lose the use of one or more of
our drug candidates;
|
| |
| |
•
|
defend litigation or administrative proceedings that may be
costly whether we win or lose, and which could result in a
substantial diversion of management resources; or
|
| |
| |
•
|
pay damages.
|
Any costs incurred in connection with such events or the
inability to sell our products may have a material adverse
effect on our business, financial condition and results of
operations.
If we
cannot meet requirements under our license agreements, we could
lose the rights to our products, which could have a material
adverse effect on our business.
We depend on licensing agreements with third parties to maintain
the intellectual property rights to certain of our products
under development. Presently, we have licensed rights from the
Yeda Research and Development Company Limited, the technology
transfer arm of the Weizman Institute of Science, which allow us
to use their technology and discoveries for the development,
production and sale of enzymatically active mutations of GCD and
derivatives thereof for the treatment of Gaucher disease. In
addition, pursuant to our agreement with the Yissum Research and
Development Company, or Yissum, the technology transfer arm of
the Hebrew University of Jerusalem, Israel, and the Boyce
Thompson Institute for Plant Research, at Cornell University, we
have received an exclusive worldwide right and license to
certain technology, including patents and additional patent
applications relating to acetylcholinesterase (AChE), for all
therapeutic and prophylactic indications as well as an exclusive
license not limited to such indications with respect to certain
of these patents and patent applications. Under the agreement
with Yissum, we intend to develop a proprietary plant cell-based
acetylcholinesterase (AChE) and its molecular variants for the
use in several therapeutic and prophylactic indications,
including a biodefense program. In addition, we have licensed
certain rights relating to our production of taliglucerase alfa
from Virginia Tech. Our license agreements require us to make
payments and satisfy performance obligations in order to
maintain our rights under these agreements. All of these
agreements last either throughout the life of the patents that
are the subject of the agreements, or with respect to other
licensed technology, for a number of years after the first
commercial sale of the relevant product.
46
In addition, we are responsible for the cost of filing and
prosecuting certain patent applications and maintaining certain
issued patents licensed to us. If we do not meet our obligations
under our license agreements in a timely manner, we could lose
the rights to our proprietary technology which could have a
material adverse effect on our business.
If we
in-license drug candidates, we may delay or otherwise adversely
affect the development of our existing drug candidates, which
may negatively impact our business, results of operations and
financial condition.
In addition to our own internally developed drug candidates, we
proactively seek opportunities to in-license and advance other
drug candidates that are strategic and have value-creating
potential to take advantage of our development know-how and
technology. If we in-license any additional drug candidates, our
capital requirements may increase significantly. In addition,
in-licensing additional drug candidates may place a strain on
the time of our existing personnel, which may delay or otherwise
adversely affect the development of our existing drug candidates
or cause us to re-prioritize our drug pipeline if we do not have
the necessary capital resources to develop all of our drug
candidates, which may delay the development of our drug
candidates and negatively impact our business, results of
operations and financial condition.
If we
are unable to successfully manage our growth, there could be a
material adverse impact on our business, results of operations
and financial condition.
We have grown rapidly and expect to continue to grow. We expect
to hire more employees, particularly in the areas of drug
development, manufacturing, regulatory affairs and sales and
marketing, and increase our facilities and corporate
infrastructure, further increasing the size of our organization
and related expenses. To manage our anticipated future growth,
we must continue to implement and improve our managerial,
operational and financial systems, expand our facilities and
continue to recruit and train additional qualified personnel.
Due to our limited resources, we may not be able to effectively
manage the expansion of our operations or recruit and train
additional qualified personnel. The expansion of our operations
may lead to significant costs and may divert our management and
business development resources. Any inability on the part of our
management to manage growth could delay the execution of our
business plans or disrupt our operations. If we are unable to
manage our growth effectively, we may not use our resources in
an efficient manner, which may delay the development of our drug
candidates and negatively impact our business, results of
operations and financial condition.
If we
acquire companies, products or technologies, we may face
integration risks and costs associated with those acquisitions
that could negatively impact our business, results from
operations and financial condition.
If we are presented with appropriate opportunities, we may
acquire or make investments in complementary companies, products
or technologies. We may not realize the anticipated benefit of
any acquisition or investment. If we acquire companies or
technologies, we will face risks, uncertainties and disruptions
associated with the integration process, including difficulties
in the integration of the operations of an acquired company,
integration of acquired technology with our products, diversion
of our management’s attention from other business concerns,
the potential loss of key employees or customers of the acquired
business and impairment charges if future acquisitions are not
as successful as we originally anticipate. In addition, our
operating results may suffer because of acquisition-related
costs or amortization expenses or charges relating to acquired
intangible assets. Any failure to successfully integrate other
companies, products or technologies that we may acquire may have
a material adverse effect on our business and results of
operations. Furthermore, we may have to incur debt or issue
equity securities to pay for any additional future acquisitions
or investments, the issuance of which could be dilutive to our
existing shareholders.
47
We
depend upon key employees and consultants in a competitive
market for skilled personnel. If we are unable to attract and
retain key personnel, it could adversely affect our ability to
develop and market our products.
We are highly dependent upon the principal members of our
management team, especially our President and Chief Executive
Officer, Dr. David Aviezer, Ph.D., as well as the
Interim Chairman of our Board of Directors, Zev Bronfeld, our
other directors, our scientific advisory board members,
consultants and collaborating scientists. Many of these people
have been involved with us for many years and have played
integral roles in our progress, and we believe that they will
continue to provide value to us. A loss of any of these
personnel may have a material adverse effect on aspects of our
business and clinical development and regulatory programs. We
have employment agreements with Dr. Aviezer and five other
officers that may be terminated by us or the applicable officer
at any time with varying notice periods of 60 to 90 days.
Although these employment agreements generally include
non-competition covenants and provide for severance payments
that are contingent upon the applicable employee’s
refraining from competition with us, the applicable
noncompetition provisions can be difficult and costly to monitor
and enforce. The loss of any of these persons’ services may
adversely affect our ability to develop and market our products
and obtain necessary regulatory approvals. Further, we do not
maintain key-man life insurance.
We also depend in part on the continued service of our key
scientific personnel and our ability to identify, hire and
retain additional personnel, including marketing and sales
staff. We experience intense competition for qualified
personnel, and the existence of non-competition agreements
between prospective employees and their former employers may
prevent us from hiring those individuals or subject us to suit
from their former employers. While we attempt to provide
competitive compensation packages to attract and retain key
personnel, many of our competitors are likely to have greater
resources and more experience than we have, making it difficult
for us to compete successfully for key personnel.
Our
collaborations with outside scientists and consultants may be
subject to restriction and change.
We work with chemists, biologists and other scientists at
academic and other institutions, and consultants who assist us
in our research, development, regulatory and commercial efforts,
including the members of our scientific advisory board. These
scientists and consultants have provided, and we expect that
they will continue to provide, valuable advice on our programs.
These scientists and consultants are not our employees, may have
other commitments that would limit their future availability to
us and typically will not enter into non-compete agreements with
us. If a conflict of interest arises between their work for us
and their work for another entity, we may lose their services.
In addition, we will be unable to prevent them from establishing
competing businesses or developing competing products. For
example, if a key scientist acting as a principal investigator
in any of our clinical trials identifies a potential product or
compound that is more scientifically interesting to his or her
professional interests, his or her availability to remain
involved in our clinical trials could be restricted or
eliminated.
Under
current U.S. and Israeli law, we may not be able to enforce
employees’ covenants not to compete and therefore may be
unable to prevent our competitors from benefiting from the
expertise of some of our former employees.
We have entered into non-competition agreements with all of our
employees. These agreements prohibit our employees, if they
cease working for us, from competing directly with us or working
for our competitors for a limited period. Under current
U.S. and Israeli law, we may be unable to enforce these
agreements against most of our employees and it may be difficult
for us to restrict our competitors from gaining the expertise
our former employees gained while working for us. If we cannot
enforce our employees’ non-compete agreements, we may be
unable to prevent our competitors from benefiting from the
expertise of our former employees.
48
If
product liability claims are brought against us, it may result
in reduced demand for our products or damages that exceed our
insurance coverage.
The clinical testing, marketing and use of our products exposes
us to product liability claims in the event that the use or
misuse of those products causes injury, disease or results in
adverse effects. Use of our products in clinical trials, as well
as commercial sale, could result in product liability claims. We
presently carry clinical trial liability insurance with
coverages of up to $5.0 million per occurrence and
$5.0 million in the aggregate, an amount we consider
reasonable and customary. However, this insurance coverage
includes various deductibles, limitations and exclusions from
coverage, and in any event might not fully cover any potential
claims. We may need to obtain additional clinical trial
liability coverage prior to initiating additional clinical
trials. We expect to obtain product liability insurance coverage
before commercialization of our proposed products; however, such
insurance is expensive and insurance companies may not issue
this type of insurance when we need it. We may not be able to
obtain adequate insurance in the future at an acceptable cost.
Any product liability claim, even one that was not in excess of
our insurance coverage or one that is meritless
and/or
unsuccessful, could adversely affect our cash available for
other purposes, such as research and development, which could
have a material adverse effect on our business, financial
condition and results of operations. Product liability claims
may result in reduced demand for our products, if approved,
which would have a material adverse effect on our business,
financial condition and results of operations. In addition, the
existence of a product liability claim could affect the market
price of our common stock.
Reimbursement
may not be available for our product candidates, which could
diminish our sales or affect our ability to sell our products
profitably.
Market acceptance and sales of our product candidates will
depend on worldwide reimbursement policies. Government
authorities and third-party payors, such as private health
insurers and health maintenance organizations, decide which
drugs they will pay for and establish reimbursement levels. We
cannot be sure that reimbursement will be available for any of
our product candidates, if approved for marketing and sale.
Obtaining reimbursement approval for an approved product from
every government or other third party payor is a time consuming
and costly process that could require us to provide supporting
scientific, clinical and cost-effectiveness data for the use of
our products, if and when approved, to every payor. We may not
be able to provide data sufficient to gain acceptance with
respect to reimbursement or we might need to conduct
post-marketing studies in order to demonstrate the
cost-effectiveness of any approved products, if any, to such
payors’ satisfaction. Such studies might require us to
commit a significant amount of management time and financial and
other resources. Even if a payor determines that an approved
product is eligible for reimbursement, the payor may impose
coverage limitations that preclude payment for some uses that
are approved by the FDA or other regulatory authorities. In
addition, there is a risk that full reimbursement may not be
available for high priced products. Moreover, eligibility for
coverage does not imply that any approved product will be
reimbursed in all cases or at a rate that allows us to make a
profit or even cover our costs. Also, we cannot be sure that
reimbursement amounts will not reduce the demand for, or the
price of, our product candidates. Except with respect to
taliglucerase alfa, we have not commenced efforts to have our
product candidates reimbursed by government or third-party
payors. If reimbursement is not available or is available only
to limited levels, the sales of our products, if approved may be
diminished or we may not be able to sell such products
profitably.
Reforms
in the healthcare industry and the uncertainty associated with
pharmaceutical pricing, reimbursement and related matters could
adversely affect the marketing, pricing and demand for our
products, if approved.
Increasing expenditures for healthcare have been the subject of
considerable public attention in the United States. Both private
and government entities are seeking ways to reduce or contain
healthcare costs. Numerous proposals that would effect changes
in the U.S. healthcare system have been introduced or
proposed in the U.S. Congress and in some state
legislatures within the United States, including reductions in
the pricing of prescription products and changes in the levels
at which consumers and healthcare providers are reimbursed for
purchases of pharmaceutical products. For example, the Medicare
Prescription Drug Improvement, and
49
Modernization Act of 2003 and the proposed rules thereunder
impose new requirements for the distribution and pricing of
prescription drugs that began in 2006, which could reduce
reimbursement of prescription drugs for healthcare providers and
insurers. Although we cannot predict the full effect on our
business of the implementation of this legislation, it is
possible that the new Medicare prescription drug benefit, which
will be managed by private health insurers and other managed
care organizations, will result in additional government
reimbursement for prescription drugs, which may make some
prescription drugs more affordable but may further exacerbate
industry-wide pressure to reduce prescription drug prices. We
believe that legislation that reduces reimbursement for our
product candidates could adversely impact how much or under what
circumstances healthcare providers will prescribe or administer
our products, if approved. This could materially and adversely
impact our business by reducing our ability to generate revenue,
raise capital, obtain additional collaborators and market our
products, if approved. In addition, we believe the increasing
emphasis on managed care in the United States has and will
continue to put pressure on the price and usage of
pharmaceutical products, which may adversely impact product
sales, upon approval, if at all.
We are
subject to healthcare reform measures, including legislation,
regulatory proposals and healthcare payor initiatives that may
increase our costs and adversely affect our profitability and/or
ability to obtain adequate reimbursement for our product
candidates.
The United States and some foreign jurisdictions are considering
or have enacted a number of legislative and regulatory proposals
to change the healthcare system in ways that could affect our
ability to sell our products profitably. Among policymakers and
payors in the United States and elsewhere, there is significant
interest in promoting changes in healthcare systems with the
stated goals of containing healthcare costs, improving quality
and/or
expanding access. In the United States, the pharmaceutical
industry has been a particular focus of these efforts and has
been significantly affected by major legislative initiatives.
In March 2010, the Patient Protection and Affordable Care Act,
as amended by the Health Care and Education Affordability
Reconciliation Act, or collectively, PPACA, became law in the
United States. PPACA substantially changes the way healthcare is
financed by both governmental and private insurers and
significantly affects the pharmaceutical industry. Key
provisions of PPACA specific to the pharmaceutical industry,
among others, include the following:
|
|
|
| |
•
|
An annual, nondeductible fee on any entity that manufactures or
imports certain branded prescription drugs and biologic agents
into the United States, apportioned among these entities
according to their market share in certain federal government
healthcare programs (excluding sales of any drug or biologic
product marketed for an orphan indication), beginning in 2011;
|
| |
| |
•
|
An increase in the rebates a manufacturer must pay under the
Medicaid Drug Rebate Program, retroactive to January 1,
2010, to 23.1% and 13% of the average manufacturer price for
branded and generic drugs, respectively;
|
| |
| |
•
|
A new Medicare Part D coverage gap discount program, in
which manufacturers must agree to offer 50% point-of sale
discounts off negotiated prices of applicable brand drugs to
eligible beneficiaries during their coverage gap period, as a
condition for the manufacturer’s outpatient drugs to be
covered under Medicare Part D, beginning in 2011;
|
| |
| |
•
|
Extension of manufacturers’ Medicaid rebate liability to
covered drugs dispensed to individuals who are enrolled in
Medicaid managed care organizations, effective March 23,
2010;
|
| |
| |
•
|
Expansion of eligibility criteria for Medicaid programs by,
among other things, allowing states to offer Medicaid coverage
to additional individuals beginning in April 2010 and by adding
new mandatory eligibility categories for certain individuals
with income at or below 133% of the Federal Poverty Level
beginning in 2014, thereby potentially increasing both the
volume of sales and manufacturers’ Medicaid rebate
liability;
|
| |
| |
•
|
Expansion of the entities eligible for discounts under the
Public Health Service pharmaceutical pricing program, effective
January 2010;
|
50
|
|
|
| |
•
|
New requirements to report certain financial arrangements with
physicians and others, including reporting any “transfer of
value” made or distributed to prescribers and other
healthcare providers and reporting any investment interests held
by physicians and their immediate family members during each
calendar year beginning in 2012, with reporting starting in 2013;
|
| |
| |
•
|
A new requirement to annually report drug samples that
manufacturers and distributors provide to physicians, effective
April 1, 2012;
|
| |
| |
•
|
A licensure framework for follow-on biologic products; and
|
| |
| |
•
|
A new Patient-Centered Outcomes Research Institute to oversee,
identify priorities in, and conduct comparative clinical
effectiveness research, along with funding for such research.
|
Although we cannot predict their full impact, we anticipate that
PPACA, as well as other healthcare reform measures that may be
adopted in the future, may result in more rigorous coverage and
reimbursement criteria and in additional downward pressure on
the price that we receive for any approved product, and could
adversely affect our profits and our business generally.
We may
be subject, directly or indirectly, to federal and state
healthcare fraud and abuse and false claims laws and
regulations. If we are unable to comply, or have not fully
complied, with such laws, we could face substantial
penalties.
If we obtain FDA approval for any of our product candidates and
begin commercializing those products in the United States, our
operations may be directly, or indirectly through our customers,
subject to various state and federal fraud and abuse laws,
including, without limitation, the federal Anti-Kickback Statute
and the federal False Claims Act. These laws may impact, among
other things, our proposed sales, marketing and education
programs.
The federal Anti-Kickback Statute prohibits persons from
knowingly and willfully soliciting, receiving, offering or
paying remuneration, directly or indirectly, to induce either
the referral of an individual, or the furnishing, recommending,
or arranging for a good or service, for which payment may be
made under a federal healthcare program, such as the Medicare
and Medicaid programs. The term “remuneration” is not
defined in the federal Anti-Kickback Statute and has been
broadly interpreted to include anything of value, including for
example, gifts, discounts, the furnishing of supplies or
equipment, credit arrangements, payments of cash, waivers of
payment, ownership interests and providing anything at less than
its fair market value. The reach of the Anti-Kickback Statute
was also broadened by PPACA, which, among other things, amends
the intent requirement of the federal Anti-Kickback Statute and
the applicable criminal healthcare fraud statutes contained
within 42 U.S.C.
§ 1320a-7b,
effective March 23, 2010. Pursuant to the statutory
amendment, a person or entity no longer needs to have actual
knowledge of this statute or specific intent to violate it in
order to have committed a violation. In addition, PPACA provides
that the government may assert that a claim including items or
services resulting from a violation of the federal Anti-Kickback
Statute constitutes a false or fraudulent claim for purposes of
the civil False Claims Act (discussed below) or the civil
monetary penalties statute, which imposes penalties against any
person who is determined to have presented or caused to be
presented a claim to a federal health program that the person
knows or should know is for an item or service that was not
provided as claimed or is false or fraudulent. The federal
Anti-Kickback Statute is broad, and despite a series of narrow
safe harbors, prohibits may arrangements and practices that are
lawful in businesses outside of the healthcare industry.
Penalties for violations of the federal Anti-Kickback Statute
include criminal penalties and civil sanctions such as fines,
imprisonment and possible exclusion from Medicare, Medicaid and
other healthcare programs. Many states have also adopted laws
similar to the federal Anti-Kickback Statute, some of which
apply to the referral of patients for healthcare items or
services reimbursed by any source, not only the Medicare and
Medicaid programs, and do not contain identical safe harbors.
The federal False Claims Act imposes liability on any person
who, among other things, knowingly presents, or causes to be
presented, a false or fraudulent claim for payment by a federal
healthcare program. The “qui tam” provisions of the
False Claims Act allow a private individual to bring civil
actions on behalf of the federal government alleging that the
defendant has submitted a false claim to the federal government,
and
51
to share in any monetary recovery. In addition, various states
have enacted false claims laws analogous to the False Claims
Act. Many of these state laws apply where a claim is submitted
to any third-party payer and not merely a federal healthcare
program. When an entity is determined to have violated the False
Claims Act, it may be required to pay up to three times the
actual damages sustained by the government, plus civil penalties
of $5,500 to $11,000 for each separate false claim.
Also, the Health Insurance Portability and Accountability Act of
1996, or HIPAA, created several new federal crimes, including
health care fraud, and false statements relating to health care
matters. The health care fraud statute prohibits knowingly and
willfully executing a scheme to defraud any health care benefit
program, including private third-party payers. The false
statements statute prohibits knowingly and willfully falsifying,
concealing or covering up a material fact or making any
materially false, fictitious or fraudulent statement in
connection with the delivery of or payment for health care
benefits, items or services.
We are unable to predict whether we could be subject to actions
under any of these or other fraud and abuse laws, or the impact
of such actions. Moreover, to the extent that our products will
be sold in a foreign country, we may be subject to similar
foreign laws and regulations. If we are found to be in violation
of any of the laws described above and other applicable state
and federal fraud and abuse laws, we may be subject to
penalties, including civil and criminal penalties, damages,
fines, exclusion from government healthcare reimbursement
programs and the curtailment or restructuring or our operations,
all of which could have a material adverse effect on our
business and the results of operations.
Governments
outside the United States tend to impose strict price controls
and reimbursement approval policies, which may adversely affect
our prospects for generating revenue.
In some countries, particularly European Union countries, the
pricing of prescription pharmaceuticals is subject to
governmental control. In these countries, pricing negotiations
with governmental authorities can take considerable time (six to
12 months or longer) after the receipt of marketing
approval for a product. To obtain reimbursement or pricing
approval in some countries with respect to any product candidate
that achieves regulatory approval, we may be required to conduct
a clinical trial that compares the cost-effectiveness of our
product candidate to other available therapies. If reimbursement
of our products upon approval, if at all, is unavailable or
limited in scope or amount, or if pricing is set at
unsatisfactory levels, our prospects for generating revenue, if
any, could be adversely affected which would have a material
adverse effect on our business and results of operations.
Further, if we achieve regulatory approval of any product, we
must successfully negotiate product pricing for such product in
individual countries. As a result, the pricing of our products,
if approved, in different countries may vary widely, thus
creating the potential for third-party trade in our products in
an attempt to exploit price differences between countries. This
third-party trade of our products could undermine our sales in
markets with higher prices.
Risks
Relating to Our Operations in Israel
Potential
political, economic and military instability in the State of
Israel, where the majority of our senior management and our
research and development facilities are located, may adversely
affect our results of operations.
Our executive office and operations are located in the State of
Israel. Accordingly, political, economic and military conditions
in Israel directly affect our business. Since the State of
Israel was established in 1948, a number of armed conflicts have
occurred between Israel and its Arab neighbors. Any hostilities
involving Israel or the interruption or curtailment of trade
between Israel and its present trading partners, or a
significant downturn in the economic or financial condition of
Israel, could affect adversely our operations. Since October
2000 there have been increasing occurrences of terrorist
violence. Ongoing and revived hostilities or other Israeli
political or economic factors could harm our operations and
product development and cause our revenues to decrease.
Furthermore, several countries, principally those in the Middle
East, still restrict business with Israel and Israeli companies.
These restrictive laws and policies may limit seriously our
ability to sell our products in these countries.
52
Although Israel has entered into various agreements with Egypt,
Jordan and the Palestinian Authority, there have been times
since October 2000 when Israel has experienced an increase in
unrest and terrorist activity. The establishment in 2006 of a
government in the Palestinian Authority by representatives of
the Hamas militant group has created additional unrest and
uncertainty in the region. In mid-2006, there was a war between
Israel and the Hezbollah in Lebanon, resulting in thousands of
rockets being fired from Lebanon up to 50 miles into
Israel. Our current facilities are located in northern Israel,
are in range of rockets that were fired from Lebanon into Israel
during the war and suffered minimal damages during one of the
rocket attacks. If our facilities are damaged as a result of
hostile action, our operations may be materially adversely
affected.
Our
operations may be disrupted by the obligations of our personnel
to perform military service which could have a material adverse
effect on our business.
Many of our male employees in Israel, including members of
senior management, are obligated to perform up to one month (in
some cases more) of annual military reserve duty until they
reach the age of 45 and, in the event of a military conflict,
could be called to active duty. Our operations could be
disrupted by the absence of a significant number of our
employees related to military service or the absence for
extended periods of military service of one or more of our key
employees. A disruption could have a material adverse effect on
our business.
Because
a certain portion of our expenses is incurred in New Israeli
Shekels, or NIS, our results of operations may be seriously
harmed by currency fluctuations and inflation.
We report our financial statements in U.S. dollars, our
functional currency, but we pay a meaningful portion of our
expenses in New Israeli Shekels, or NIS. As a result, we are
exposed to risk to the extent that the inflation rate in Israel
exceeds the rate of devaluation of the NIS in relation to the
U.S. dollar or if the timing of these devaluations lags
behind inflation in Israel. In that event, the U.S. dollar
cost of our operations in Israel will increase and our
U.S. dollar-measured results of operations will be
adversely affected. To the extent that the value of the NIS
increases against the dollar, our expenses on a dollar cost
basis increase. Our operations also could be adversely affected
if we are unable to guard against currency fluctuations in the
future. To date, we have not engaged in hedging transactions. In
the future, we may enter into currency hedging transactions to
decrease the risk of financial exposure from fluctuations in the
exchange rate of the U.S. dollar against the NIS. These
measures, however, may not adequately protect us from material
adverse effects.
The
tax benefits available to us require that we meet several
conditions and may be terminated or reduced in the future, which
would increase our taxes and would have a material adverse
effect on our business and results of operations.
We are able to take advantage of tax exemptions and reductions
resulting from the “Approved Enterprise” status of our
facilities in Israel. To remain eligible for these tax benefits,
we must continue to meet certain conditions, including making
specified investments in property and equipment, and financing
at least 30% of such investments with share capital. If we fail
to meet these conditions in the future, the tax benefits would
be canceled and we may be required to refund any tax benefits we
already have enjoyed. These tax benefits are subject to
investment policy by the Investment Center and may not be
continued in the future at their current levels or at any level.
In recent years the Israeli government has reduced the benefits
available and has indicated that it may further reduce or
eliminate some of these benefits in the future. The termination
or reduction of these tax benefits or our inability to qualify
for additional “Approved Enterprise” approvals may
increase our tax expenses in the future, which would reduce our
expected profits and adversely affect our business and results
of operations. Additionally, if we increase our activities
outside of Israel, for example, by future acquisitions, such
increased activities generally may not be eligible for inclusion
in Israeli tax benefit programs.
53
The
Israeli government grants we have received for certain research
and development expenditures restrict our ability to manufacture
products and transfer technologies outside of Israel and require
us to satisfy specified conditions. If we fail to satisfy these
conditions, we may be required to refund grants previously
received together with interest and penalties which could have a
material adverse effect on our business and results of
operations.
Our research and development efforts have been financed, in
part, through grants that we have received from the OCS. We,
therefore, must comply with the requirements of the Israeli Law
for the Encouragement of Industrial Research and Development,
1984, and related regulations, or the Research Law.
Under the Research Law, the discretionary approval of an OCS
committee is required for any transfer of technology developed
with OCS funding. OCS approval is not required for the export of
any products resulting from the research or development, or for
the licensing of the technology in the ordinary course of
business. We may not receive the required approvals for any
proposed transfer. Such approvals, if granted, may be subject to
the following additional restrictions:
|
|
|
| |
•
|
we may be required to pay the OCS a significant portion of the
consideration we receive upon any sale of such technology to an
entity that is not Israeli. The scope of the support received,
the royalties that were paid by us, the amount of time that
elapses between the date on which the know-how is transferred
and the date on which the grants were received, as well as the
sale price, will be taken into account in order to calculate the
amount of the payment; and
|
| |
| |
•
|
the transfer of manufacturing rights could be conditioned upon
an increase in the royalty rate and payment of increased
aggregate royalties (up to 300% of the amount of the grant plus
interest, depending on the percentage of the manufacturing that
is foreign).
|
These restrictions may impair our ability to sell our technology
assets or to outsource manufacturing outside of Israel. The
restrictions will continue to apply for a certain period of time
even after we have repaid the full amount of royalties payable
for the grants. If we fail to satisfy these conditions, we may
be required to refund grants previously received together with
interest and penalties which could have a material adverse
effect on our business and results of operations.
Investors
may have difficulties enforcing a U.S. judgment, including
judgments based upon the civil liability provisions of the U.S.
federal securities laws against us, our executive officers and
most of our directors or asserting U.S. securities laws claims
in Israel.
Most of our directors and officers are not residents of the
United States and most of their assets and our assets are
located outside the United States. Service of process upon our
non-U.S. resident
directors and officers and enforcement of judgments obtained in
the United States against us, some of our directors and
executive officers may be difficult to obtain within the United
States. We have been informed by our legal counsel in Israel
that investors may find it difficult to assert claims under
U.S. securities laws in original actions instituted in
Israel or obtain a judgment based on the civil liability
provisions of U.S. federal securities laws against us, our
officers and our directors. Israeli courts may refuse to hear a
claim based on a violation of U.S. securities laws against
us or our officers and directors because Israel is not the most
appropriate forum to bring such a claim. In addition, even if an
Israeli court agrees to hear a claim, it may determine that
Israeli law and not U.S. law is applicable to the claim. If
U.S. law is found to be applicable, the content of
applicable U.S. law must be proved as a fact which can be a
time-consuming and costly process. Certain matters of procedure
will also be governed by Israeli law. There is little binding
case law in Israel addressing the matters described above.
Israeli courts might not enforce judgments rendered outside
Israel which may make it difficult to collect on judgments
rendered against us. Subject to certain time limitations, an
Israeli court may declare a foreign civil judgment enforceable
only if it finds that:
|
|
|
| |
•
|
the judgment was rendered by a court which was, according to the
laws of the state of the court, competent to render the judgment;
|
54
|
|
|
| |
•
|
the judgment may no longer be appealed;
|
| |
| |
•
|
the obligation imposed by the judgment is enforceable according
to the rules relating to the enforceability of judgments in
Israel and the substance of the judgment is not contrary to
public policy; and
|
| |
| |
•
|
the judgment is executory in the state in which it was given.
|
Even if these conditions are satisfied, an Israeli court will
not enforce a foreign judgment if it was given in a state whose
laws do not provide for the enforcement of judgments of Israeli
courts (subject to exceptional cases) or if its enforcement is
likely to prejudice the sovereignty or security of the State of
Israel. An Israeli court also will not declare a foreign
judgment enforceable if:
|
|
|
| |
•
|
the judgment was obtained by fraud;
|
| |
| |
•
|
there is a finding of lack of due process;
|
| |
| |
•
|
the judgment was rendered by a court not competent to render it
according to the laws of private international law in Israel;
|
| |
| |
•
|
the judgment is at variance with another judgment that was given
in the same matter between the same parties and that is still
valid; or
|
| |
| |
•
|
at the time the action was brought in the foreign court, a suit
in the same matter and between the same parties was pending
before a court or tribunal in Israel.
|
Risks
Related to Investing in Our Common Stock
The
market price of our common stock may fluctuate
significantly.
The market price of our common stock may fluctuate significantly
in response to numerous factors, some of which are beyond our
control, such as:
|
|
|
| |
•
|
the announcement of new products or product enhancements by us
or our competitors;
|
| |
| |
•
|
developments concerning intellectual property rights and
regulatory approvals;
|
| |
| |
•
|
variations in our and our competitors’ results of
operations;
|
| |
| |
•
|
the results of our ongoing studies regarding our lead product
candidate taliglucerase alfa, or communications from the FDA or
other regulatory authorities regarding our NDA or MAA for
taliglucerase alfa or similar filings or submissions, including
the approval of any such filings by the FDA or other applicable
regulatory authorities, or any delay of failure by the FDA or
other applicable regulatory authorities to approve any such
filings;
|
| |
| |
•
|
changes in earnings estimates or recommendations by securities
analysts, if our common stock is covered by analysts;
|
| |
| |
•
|
developments in the biotechnology industry; and
|
| |
| |
•
|
general market conditions and other factors, including factors
unrelated to our operating performance.
|
Further, stock markets in general, and the market for
biotechnology companies in particular, have recently experienced
price and volume fluctuations. Continued market fluctuations
could result in extreme volatility in the price of our common
stock, which could cause a decline in the value of our common
stock. Price volatility of our common stock may be worse if the
trading volume of our common stock is low. We have not paid, and
do not expect to pay, any cash dividends on our common stock as
any earnings generated from future operations will be used to
finance our operations. As a result, investors will not realize
any income from an investment in our common stock until and
unless their shares are sold at a profit.
55
All
liabilities of our company have survived the merger and there
may be undisclosed liabilities that could harm our revenues,
business, prospects, financial condition and results of
operations.
Protalix Ltd. and its counsel conducted due
diligence on us that was customary and appropriate for the
reverse merger transaction consummated on December 31,
2006. However, the due diligence process may not have revealed
all our material liabilities then existing or that could be
asserted in the future against us relating to our activities
before the consummation of the merger. Any such potential
liabilities survive the merger and could harm our revenues,
business, prospects, financial condition and results of
operations.
Future
sales of our common stock could reduce our stock
price.
The market price of our common stock could drop significantly if
our existing shareholders sell a large number of shares of our
common stock or are perceived by the market as intending to sell
them. All of the shares sold in our public offering in October
2007 were freely tradable without restriction or further
registration under the federal securities laws, unless purchased
by our “affiliates” as that term is defined in
Rule 144 under the Securities Act. In addition, all of the
outstanding shares of our common stock are freely tradable
without restriction or further registration under the federal
securities laws, unless owned by our affiliates. At
December 31, 2010, there were options issued and
outstanding to purchase 7,806,671 shares of our common
stock with a weighted average exercise price of $3.73 per share.
Also at December 31, 2010, there were 3,064 shares of
common stock remaining available for future for issuance in
connection with future grants of incentives under our 2006 Stock
Incentive Plan. In addition, four of our executive officers have
entered into trading plans established under
Rule 10b5-1
under the Securities Act that allow for sales of approximately
1.3 million shares upon receipt of FDA approval of
taliglucerase alfa, if at all.
Directors,
executive officers, principal shareholders and affiliated
entities own a significant percentage of our capital stock, and
they may make decisions that an investor may not consider to be
in the best interests of our shareholders.
Our directors, executive officers, principal shareholders and
affiliated entities beneficially own, in the aggregate,
approximately 30% of our outstanding common stock. As a result,
if some or all of them acted together, they would have the
ability to exert substantial influence over the election of our
Board of Directors and the outcome of issues requiring approval
by our shareholders. This concentration of ownership may have
the effect of delaying or preventing a change in control of our
company that may be favored by other shareholders. This could
prevent the consummation of transactions favorable to other
shareholders, such as a transaction in which shareholders might
otherwise receive a premium for their shares over current market
prices.
Failure
to maintain effective internal controls in accordance with
Section 404 of the Sarbanes-Oxley Act could have a material
adverse effect on our business and operating results. In
addition, current and potential shareholders could lose
confidence in our financial reporting, which could have a
material adverse effect on the price of our common
stock.
Effective internal controls are necessary for us to provide
reliable financial reports and effectively prevent fraud. If we
cannot provide reliable financial reports or prevent fraud, our
results of operation could be harmed.
Section 404 of the Sarbanes-Oxley Act of 2002 requires
annual management assessments of the effectiveness of our
internal controls over financial reporting and a report by our
independent registered public accounting firm addressing these
assessments. We continuously monitor our existing internal
controls over financial reporting systems to confirm that they
are compliant with Section 404, and we may identify
deficiencies that we may not be able to remediate in time to
meet the deadlines imposed by the Sarbanes-Oxley Act. This
process may divert internal resources and will take a
significant amount of time and effort to complete.
If, at any time, it is determined that we are not in compliance
with Section 404, we may be required to implement new
internal control procedures and reevaluate our financial
reporting. We may experience higher
56
than anticipated operating expenses as well as increased
independent auditor fees during the implementation of these
changes and thereafter. Further, we may need to hire additional
qualified personnel. If we fail to maintain the adequacy of our
internal controls, as such standards are modified, supplemented
or amended from time to time, we may not be able to conclude on
an ongoing basis that we have effective internal controls over
financial reporting in accordance with Section 404 of the
Sarbanes-Oxley Act, which could result in our being unable to
obtain an unqualified report on internal controls from our
independent auditors. Failure to maintain an effective internal
control environment could also cause investors to lose
confidence in our reported financial information, which could
have a material adverse effect on the price of our common stock.
Compliance
with changing regulation of corporate governance and public
disclosure may result in additional expenses, divert
management’s attention from operating our business which
could have a material adverse effect on our
business.
There have been other changing laws, regulations and standards
relating to corporate governance and public disclosure in
addition to the Sarbanes-Oxley Act, as well as new regulations
promulgated by the Commission and rules promulgated by the
national securities exchanges, including the NYSE Amex and the
NASDAQ. These new or changed laws, regulations and standards are
subject to varying interpretations in many cases due to their
lack of specificity, and as a result, their application in
practice may evolve over time as new guidance is provided by
regulatory and governing bodies, which could result in
continuing uncertainty regarding compliance matters and higher
costs necessitated by ongoing revisions to disclosure and
governance practices. As a result, our efforts to comply with
evolving laws, regulations and standards are likely to continue
to result in increased general and administrative expenses and a
diversion of management time and attention from
revenue-generating activities to compliance activities. Our
board members, Chief Executive Officer and Chief Financial
Officer could face an increased risk of personal liability in
connection with the performance of their duties. As a result, we
may have difficulty attracting and retaining qualified board
members and executive officers, which could have a material
adverse effect on our business. If our efforts to comply with
new or changed laws, regulations and standards differ from the
activities intended by regulatory or governing bodies, we may
incur additional expenses to comply with standards set by
regulatory authorities or governing bodies which would have a
material adverse effect on our business and results of
operations.
We are
a holding company with no operations of our own.
We are a holding company with no operations of our own.
Accordingly, our ability to conduct our operations, service any
debt that we may incur in the future and pay dividends, if any,
is dependent upon the earnings from the business conducted by
Protalix Ltd. The distribution of those earnings or advances or
other distributions of funds by our subsidiary to us, as well as
our receipt of such funds, are contingent upon the earnings of
our subsidiary and are subject to various business
considerations and U.S. and Israeli law. If Protalix Ltd.
is unable to make sufficient distributions or advances to us, or
if there are limitations on our ability to receive such
distributions or advances, we may not have the cash resources
necessary to conduct our corporate operations which would have a
material adverse effect on our business and results of
operations.
The
issuance of preferred stock or additional shares of common stock
could adversely affect the rights of the holders of shares of
our common stock.
Our Board of Directors is authorized to issue up to
100,000,000 shares of preferred stock without any further
action on the part of our shareholders. Our Board of Directors
has the authority to fix and determine the voting rights, rights
of redemption and other rights and preferences of preferred
stock. Currently, we have no shares of preferred stock
outstanding.
Our Board of Directors may, at any time, authorize the issuance
of a series of preferred stock that would grant to holders the
preferred right to our assets upon liquidation, the right to
receive dividend payments before dividends are distributed to
the holders of common stock and the right to the redemption of
the shares, together with a premium, before the redemption of
our common stock, which may have a material adverse effect on
the rights of the holders of our common stock. In addition, our
Board of Directors, without further shareholder approval, may,
at any time, issue large blocks of preferred stock. In addition,
the ability of our
57
Board of Directors to issue shares of preferred stock without
any further action on the part of our shareholders may impede a
takeover of our company and may prevent a transaction that is
favorable to our shareholders.
Under the rules of the Tel Aviv Stock Exchange, other than
incentives under our 2006 Stock Option Plan, we may not issue
any securities of any class or series different than the common
stock that is listed on the Tel Aviv Stock Exchange for the
12-month
period immediately succeeding our initial listing, which
occurred on September 6, 2010. Subsequent to such
12-month
period, the rules of the Tel Aviv Stock Exchange allow us to
issue securities with preferential rights with respect to
dividends but such other securities may not include voting
rights. The foregoing does not limit our ability to issue and
grant options and warrants for the purchase of shares of our common
stock.
|
|
|
Item 1B.
|
Unresolved
Staff Comments
|
None.
Our manufacturing facility and executive offices are located in
Carmiel, Israel. The facilities currently contain approximately
16,000 sq/ft of manufacturing space and additional 48,000 sq/ft
of laboratory, warehouse and office space and are leased at a
rate of approximately $71,000 per month. In addition, we are
entitled to use an additional 13,000 sq/ft in the same facility,
which we intend to utilize in connection with an anticipated
expansion of our manufacturing facilities. Our facilities are
equipped with the requisite laboratory services required to
conduct our business, and we believe that the existing
facilities are adequate to meet our needs for the foreseeable
future. We have leased the facility through 2017, subject to
three options exercisable by us to extend the term for a
five-year period, for an aggregate of 15 additional years. Upon
the exercise of each option to extend the term of the lease, if
any, the then current base rent shall be increased by 10%. We
also lease an office in Ramat Gan, Israel, for approximately
$2,200 per month.
|
|
|
Item 3.
|
Legal
Proceedings
|
We are not involved in any material legal proceedings.
58
|
|
|
Item 4.
|
(Removed
and Reserved)
|
|
|
|
Item 5.
|
Market
for Registrant’s Common Equity, Related Stockholder Matters
and Issuer Purchases of Equity Securities
|
Our common stock began trading on the NYSE Amex on
March 12, 2007 under the symbol “PLX.” The
following table sets forth the quarterly high and low closing
prices for our common stock, as reported by the NYSE Amex.
| |
|
|
|
|
|
|
|
|
|
|
|
Price Range
|
|
|
|
High
|
|
Low
|
|
|
|
Fourth Quarter 2010
|
|
$
|
10.00
|
|
|
$
|
8.40
|
|
|
Third Quarter 2010
|
|
$
|
9.00
|
|
|
$
|
5.99
|
|
|
Second Quarter 2010
|
|
$
|
7.00
|
|
|
$
|
5.84
|
|
|
First Quarter 2010
|
|
$
|
7.70
|
|
|
$
|
6.56
|
|
|
Fourth Quarter 2009
|
|
$
|
12.14
|
|
|
$
|
6.62
|
|
|
Third Quarter 2009
|
|
$
|
8.31
|
|
|
$
|
4.51
|
|
|
Second Quarter 2009
|
|
$
|
5.24
|
|
|
$
|
2.10
|
|
|
First Quarter 2009
|
|
$
|
2.89
|
|
|
$
|
1.80
|
|
These quotations reflect prices between dealers and do not
include retained
mark-ups,
mark-downs and commissions and may not necessarily represent
actual transactions.
There were approximately 44 holders of record of our common
stock at February 15, 2011. A substantially greater number
of holders of our common stock are “street name” or
beneficial holders, whose shares are held of record by banks,
brokers and other financial institutions. To date, we have not
declared or paid any cash dividends on our common stock. We do
not anticipate paying any dividends on our common stock in the
foreseeable future.
59
STOCK
PERFORMANCE GRAPH
The following graph compares the cumulative total shareholder
return data for our common stock from December 31, 2005
through December 31, 2010 to the cumulative return over
such time period of (i) The AMEX Composite Index and
(ii) The AMEX Biotechnology Index. The graph assumes an
investment of $100 on December 31, 2005 in each of our
common stock, and the stocks comprising the AMEX Composite Index
and the stocks comprising the AMEX Biotechnology Index,
including dividend reinvestment, if any.
The stock price performance shown on the graph below
represents historical price performance and is not necessarily
indicative of any future stock price performance. Specifically,
during the period from December 31, 2005 through
December 31, 2006, our company did not have any operations
and our common stock was quoted on the
OTC®
Bulletin Board. The historical performance of our
common stock prior to January 2, 2007, represents the
performance of our company prior to the merger on
December 31, 2006, and, therefore, is not indicative of the
performance of our common stock after the merger or the
performance of our common stock after it was listed for trade on
the NYSE AMEX on March 12, 2007.
COMPARISON
OF 5 YEAR CUMULATIVE TOTAL
RETURN*
Among Protalix BioTherapeutics, Inc., the NYSE Amex
Composite Index
and the NYSE Arca Biotechnology Index
|
|
|
|
* |
|
$100 invested on 12/31/05 in stock or index, including
reinvestment of dividends.
Fiscal year ending December 31. |
Notwithstanding anything to the contrary set forth in any
of our previous filings under the Securities Act of 1933, as
amended, or the Exchange Act, which might incorporate future
filings made by us under those statutes, this Stock Performance
Graph will not be incorporated by reference into any of those
prior filings, nor will such report or graph be incorporated by
reference into any future filings made by us under those
Acts.
60
|
|
|
Item 6.
|
Selected
Financial Data
|
The selected consolidated financial data below should be read in
conjunction with “Management’s Discussion and Analysis
of Financial Condition and Results of Operations” and our
consolidated financial statements and the related notes included
elsewhere in this Annual Report on
Form 10-K.
The selected consolidated statements of operations data for the
years ended December 31, 2010, 2009 and 2008 and the
selected consolidated balance sheet data as of December 31,
2010 and 2009, are derived from the audited consolidated
financial statements included elsewhere in this Annual Report.
The statement of operations data for the years ended
December 31, 2006 and 2007 and the balance sheet data as of
December 31, 2006, 2007 and 2008 are derived from audited
financial statements not included in this Annual Report. The
historical results presented below are not necessarily
indicative of future results.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2006
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(In thousands, except share and per share amounts)
|
|
|
|
|
Consolidated Statement of Operations Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
388
|
|
|
$
|
11,244
|
|
|
Cost of revenues
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3,575
|
|
|
|
4,383
|
|
|
Gross profit (loss)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(3,187
|
)
|
|
|
6,861
|
|
|
Research and development expenses, net
|
|
$
|
5,246
|
|
|
$
|
13,570
|
|
|
$
|
17,401
|
|
|
$
|
21,638
|
|
|
|
29,951
|
|
|
General and administrative expenses
|
|
|
4,525
|
|
|
|
20,594
|
|
|
|
6,770
|
|
|
|
7,144
|
|
|
|
6,876
|
|
|
Finance income
|
|
|
344
|
|
|
|
2,080
|
|
|
|
1,757
|
|
|
|
529
|
|
|
|
968
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss before change in accounting principle
|
|
$
|
9,427
|
|
|
$
|
32,084
|
|
|
$
|
22,414
|
|
|
$
|
31,440
|
|
|
$
|
28,998
|
|
|
Cumulative effect of change in accounting principle
|
|
|
(37
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
9,390
|
|
|
$
|
32,084
|
|
|
$
|
22,414
|
|
|
$
|
31,440
|
|
|
$
|
28,998
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share of common stock, basic and diluted(1)
|
|
$
|
0.32
|
|
|
$
|
0.48
|
|
|
$
|
0.30
|
|
|
$
|
0.41
|
|
|
$
|
0.36
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares of common stock used in
computing net loss per share of common stock(2)
|
|
|
29,300,987
|
|
|
|
67,187,329
|
|
|
|
75,890,633
|
|
|
|
76,942,840
|
|
|
|
80,960,300
|
|
|
Consolidated Balance Sheet Data:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
15,378
|
|
|
$
|
61,813
|
|
|
$
|
42,596
|
|
|
$
|
81,266
|
|
|
$
|
35,900
|
|
|
Other assets
|
|
|
11,610
|
|
|
|
6,324
|
|
|
|
8,215
|
|
|
|
17,405
|
|
|
|
28,829
|
|
|
Total assets
|
|
|
26,988
|
|
|
|
68,137
|
|
|
|
50,811
|
|
|
|
98,671
|
|
|
|
64,729
|
|
|
Current liabilities
|
|
|
2,268
|
|
|
|
3,762
|
|
|
|
5,527
|
|
|
|
21,530
|
|
|
|
18,903
|
|
|
Total liabilities
|
|
|
2,704
|
|
|
|
4,452
|
|
|
|
6,464
|
|
|
|
82,788
|
|
|
|
76,052
|
|
|
Shareholders’ equity (net of capital deficiency)
|
|
|
24,284
|
|
|
|
63,685
|
|
|
|
44,347
|
|
|
|
15,883
|
|
|
|
(11,323
|
)
|
|
|
|
|
* |
|
Represents less than $1. |
| |
|
(1) |
|
Reflects the retroactive effects of the impact of our merger
with Protalix Ltd. in December 2006 and the resulting exchange
of shares of common stock for the ordinary shares of Protalix
Ltd. at an exchange ratio of approximately 61.08 shares of
our common stock per ordinary share of Protalix Ltd. for all
periods presented. |
| |
|
(2) |
|
In connection with the merger, we completed a
one-for-ten
reverse stock split in December 2006, therefore all share
numbers presented in this Annual Report on
Form 10-K
give retroactive effect to the reverse stock split, as
applicable. |
61
|
|
|
Item 7.
|
Management’s
Discussion and Analysis of Financial Condition and Results of
Operations
|
You should read the following discussion and analysis of our
financial condition and results of operations together with our
consolidated financial statements and the related notes included
elsewhere in this Annual Report on
Form 10-K.
Some of the information contained in this discussion and
analysis, particularly with respect to our plans and strategy
for our business and related financing, includes forward-looking
statements that involve risks and uncertainties. You should read
“Risk Factors” in Item 1A of this Annual Report
on
Form 10-K
for a discussion of important factors that could cause actual
results to differ materially from the results described in or
implied by the forward-looking statements contained in the
following discussion and analysis.
Overview
We are a biopharmaceutical company focused on the development
and commercialization of recombinant therapeutic proteins based
on our proprietary
ProCellExtm
protein expression system, or ProCellEx. Using our ProCellEx
system, we are developing a pipeline of proprietary and
biosimilar or “generic” versions of recombinant
therapeutic proteins based on our plant cell-based expression
technology that target large, established pharmaceutical markets
and that rely upon known biological mechanisms of action. Our
initial commercial focus has been on complex therapeutic
proteins, including proteins for the treatment of genetic
disorders, such as Gaucher disease and Fabry disease. We believe
our ProCellEx protein expression system will enable us to
develop proprietary recombinant proteins that are
therapeutically equivalent or superior to existing recombinant
proteins currently marketed for the same indications. Because we
are primarily targeting biologically equivalent versions of
highly active, well-tolerated and commercially successful
therapeutic proteins, we believe our development process is
associated with relatively less risk compared to other
biopharmaceutical development processes for completely novel
therapeutic proteins.
Our lead product development candidate is taliglucerase alfa for
the treatment of Gaucher disease, which we are developing using
our ProCellEx protein expression system. On December 9,
2009, we filed a New Drug Application (NDA) for taliglucerase
alfa with the FDA, and in July 2010 we received notification
from the FDA that it had accepted the filing of our NDA and
assigned taliglucerase alfa a Prescription Drug User Fee Act
(PDUFA) date of February 25, 2011. In addition, in November
2010 we submitted a marketing application to the Israeli MOH and
an MAA to each of the EMEA and ANVISA for taliglucerase alfa for
the treatment of Gaucher disease.
In addition to our recently completed phase III clinical
trial, we initiated a double-blind, follow-on extension study as
part of the trial during the second quarter of 2008. We also
initiated a home care treatment program for patients enrolled in
the extension study and, in December 2008, we initiated a
nine-month, worldwide, multi-center, open-label, switch-over
clinical study evaluating the safety and efficacy of switching
Gaucher patients currently treated under the current standard of
care to treatment with taliglucerase alfa. In December 2009, we
filed a proposed pediatric investigation plan to the Pediatric
Committee of the EMEA which was approved during the first
quarter of 2010, and have since initiated pediatric studies.
On November 30, 2009, Protalix Ltd. and Pfizer entered into
an exclusive license and supply agreement pursuant to which
Pfizer was granted an exclusive, worldwide license to develop
and commercialize taliglucerase alfa. Under the terms and
conditions of the Pfizer agreement, Protalix Ltd. retained the
right to commercialize taliglucerase alfa in Israel. In
connection with the execution of the Pfizer agreement, Pfizer
made an upfront payment to Protalix Ltd. of $60.0 million
in connection with the execution of the agreement and
subsequently paid Protalix Ltd. an additional $5.0 million
upon its filing of a proposed pediatric investigation plan to
the Pediatric Committee of the EMEA. Protalix Ltd. is also
eligible to receive potential milestone payments totaling
$50.0 million for the successful achievement of other
regulatory milestones. Pfizer and Protalix Ltd. will also share
future revenues and expenses for the development and
commercialization of taliglucerase alfa on a 60% and 40% basis,
respectively, and have also agreed to a specific allocation of
the responsibilities for the continued development efforts for
taliglucerase alfa.
On July 13, 2010, we announced that the French regulatory
authority had granted an Autorisation Temporaire
d’Utilisation (ATU), or Temporary Authorization for Use,
for taliglucerase alfa for the treatment
62
of Gaucher disease. An ATU is the regulatory mechanism used by
the French Health Products and Safety Agency to make
non-approved drugs available to patients in France when a
genuine public health need exists. This ATU allows patients with
Gaucher disease in France to receive treatment with
taliglucerase alfa before marketing authorization for the
product is granted in the European Union. Payment for
taliglucerase alfa has been secured through government
allocations to hospitals.
On August 10, 2010, Pfizer entered into a $30 million
short-term supply agreement with the Ministry of Health of
Brazil pursuant to which Protalix and Pfizer have provided
taliglucerase alfa to the Ministry of Health of Brazil for the
treatment of patients with Gaucher disease. Revenue generated
from the Ministry of Health of Brazil will be recorded by Pfizer
and we are entitled to our share of the revenue in accordance
with the terms and conditions of the Pfizer Agreement. In
addition, we and the Ministry of Health of Brazil are in
discussions relating to a possible long-term supply agreement
that contemplates, among other matters, providing certain
components of our manufacturing technology to the Ministry of
Health of Brazil for implementation by it in Brazil. At this
time, we are unable to assess whether these discussions will
result in an agreement and we can make no assurances that we
will be able to enter into such an agreement on favorable terms,
if at all. In any event, we do not expect to enter into a
long-term supply agreement with the Ministry of Health of Brazil
until we receive marketing approval of taliglucerase alfa from
the FDA or ANVISA, if at all.
In addition to taliglucerase alfa, we are developing an
innovative product pipeline using our ProCellEx protein
expression system. Our product pipeline currently includes,
among other candidates, (1) PRX-102, a therapeutic protein
candidate for the treatment of Fabry disease, a rare, genetic
lysosomal disorder in humans, (2) PRX-105, a plant cell
expressed pegylated recombinant acetylcholinesterase product
candidate for biodefense and other indications,
(3) pr-antiTNF, a plant cell expressed recombinant fusion
protein made from the soluble form of the human TNF receptor
(TNFR) and an antibody portion, which is being developed as a
treatment of certain immune diseases such as rheumatoid
arthritis, juvenile idiopathic arthritis, ankylosing,
spondylitis, psoriatic arthritis and plaque psoriasis,
(4) an orally administrated glucocerebrosidase enzyme for
treating Gaucher patients utilizing the oral delivery of the
recombinant enzyme produced within carrot cells and
(5) additional undisclosed therapeutic proteins, all of
which are currently being evaluated in animal studies. In March
2010, we initiated a preliminary phase I clinical trial of
PRX-105, our plant cell expressed pegylated recombinant
acetylcholinesterase product candidate for biodefense
indications, which we completed in June 2010. We are currently
preparing for further efficacy trials of this product candidate
in larger animals and with live nerve gas.
In December 2010 we held a pre-IND meeting with the FDA with
respect to PRX-102. We expect to submit an IND to the FDA within
the next 12 months in connection with an anticipated phase
I/II study of PRX-102 and to initiate the trial once the IND is
approved, if at all.
Our business is conducted by our wholly-owned subsidiary,
Protalix Ltd., which we acquired through a reverse merger
transaction effective December 31, 2006. The merger
transaction was treated as a recapitalization for accounting
purposes and, as such, the results of operations discussed below
are those of Protalix Ltd. Prior to the merger transaction, we
had not conducted any operations for several years. Protalix
Ltd. was originally incorporated in Israel in December 1993.
Since its inception in December 1993, Protalix Ltd. has
generated significant losses in connection with its research and
development, including the clinical development of taliglucerase
alfa. Since we do not generate significant revenue from any of
our product candidates, we expect to continue to generate losses
in connection with the continued clinical development of
taliglucerase alfa and the research and development activities
relating to our technology and other drug candidates. We except
that our expenditures in connection with the research and
development activities will continue to represent a significant
portion of our expenses for the next several years, and will
require further financial resources if the activities are
successful. As a result, we believe that our operating losses
may continue to be substantial over the next several years.
However, if taliglucerase alfa is launched successfully, we
anticipate that we will generate revenues to offset any such
losses. We may need to obtain additional funds for the
commercialization of our lead product, taliglucerase alfa, and
to further develop the research and clinical development of our
other programs.
63
Critical
Accounting Policies
Our significant accounting policies are more fully described in
Note 1 to our consolidated financial statements appearing
at the end of this Annual Report. We believe that the accounting
policies below are critical for one to fully understand and
evaluate our financial condition and results of operations.
The discussion and analysis of our financial condition and
results of operations is based on our financial statements,
which we prepared in accordance with U.S. generally
accepted accounting principles. The preparation of these
financial statements requires us to make estimates and
assumptions that affect the reported amounts of assets and
liabilities and the disclosure of contingent assets and
liabilities at the date of the financial statements, as well as
the reported revenues and expenses during the reporting periods.
On an ongoing basis, we evaluate such estimates and judgments,
including those described in greater detail below. We base our
estimates on historical experience and on various other factors
that we believe are reasonable under the circumstances, the
results of which form the basis for making judgments about the
carrying value of assets and liabilities that are not readily
apparent from other sources. Actual results may differ from
these estimates under different assumptions or conditions.
Functional
Currency
The currency of the primary economic environment in which our
operations are conducted is the dollar. As we have no
significant source of revenues, we considered the currency of
the primary economic environment to be the currency in which we
expend cash. Most of our expenses and capital expenditures are
incurred in dollars, and a significant source of our financing
has been provided in U.S. dollars.
Revenues
We recognize revenue when the earnings process is complete,
which is when revenue is realized or realizable and earned,
there is persuasive evidence a revenue arrangement exists,
delivery of goods or services has occurred, the sales price is
fixed or determinable and collectability is reasonably assured.
We recognize revenue and milestone payments received pursuant to
the Pfizer Agreement in accordance with guidance regarding
revenue recognition and accounting for revenue arrangements with
multiple deliverables. Pursuant to this guidance, we determine
whether our arrangement with Pfizer involves multiple
revenue-generating deliverables that should be accounted for as
a combined unit of accounting or separate units of accounting
for revenue recognition purposes. If we determine that there are
multiple units of accounting, the consideration from the
arrangement is allocated among the separate units based on a
relative fair value allocation. If the arrangement represents a
single unit of accounting, the revenue is recognized over the
performance obligation period. As the arrangement with Pfizer
requires our continued involvement with respect to the proposed
commercialization of taliglucerase alfa, the non-refundable,
up-front license payments we received from Pfizer are deferred
and recognized over the related performance period. We estimated
the performance period of 14 years based on the date that
the last relevant patent relating to taliglucerase alfa expires.
Under the terms and conditions of the Pfizer Agreement, we are
entitled to 40% of the profits or loss from sales of
taliglucerase alfa, and related expenses incurred, except with
respect to sales in Israel. We recognize our share of net profit
or loss under the Pfizer Agreement based on reports we receive
from Pfizer summarizing the results of the collaborative
activities under the agreement for the applicable period. Under
the terms of the Pfizer Agreement, for its subsidiaries
operating outside the United States, financial information is
included based on the fiscal year ending November 30, while
financial information for the U.S. entity is included based
on the fiscal year ending December 31.
We recognize revenues received from the sale of a product to
Pfizer upon delivery to Pfizer. The revenues represent our cost
with respect to the product sold.
64
Research
and Development Expense
We expect our research and development expense to remain our
primary expense in the near future as we continue to develop our
product candidates. Research and development expense consists of:
|
|
|
| |
•
|
internal costs associated with research and development
activities;
|
| |
| |
•
|
payments made to third party contract research organizations,
investigative sites and consultants;
|
| |
| |
•
|
manufacturing development costs;
|
| |
| |
•
|
personnel-related expenses, including salaries, benefits,
travel, and related costs for the personnel involved in research
and development;
|
| |
| |
•
|
activities relating to the advancement of product candidates
through preclinical studies and clinical trials; and
|
| |
| |
•
|
facilities and other allocated expenses, which include direct
and allocated expenses for rent and maintenance of facilities,
as well as laboratory and other supplies.
|
The following table identifies our current major research and
development projects:
| |
|
|
|
|
|
Project
|
|
Status
|
|
Expected Near Term Milestone
|
|
|
|
taliglucerase alfa for the treatment of Gaucher disease
|
|
NDA and MAA (EMEA, ANVISA and the Israeli MOH) filed
|
|
PDUFA date (February 25, 2011)
|
|
PRX 102 — alpha-GAL-A
|
|
Preclinical
|
|
File IND and initiate phase I clinical trials
|
|
Acetylcholinesterase
|
|
Phase I clinical trial
|
|
Additional phase I clinical trials
|
|
pr-antiTNF
|
|
Research
|
|
File IND and initiate phase I clinical trials
|
All of our projects, other than taliglucerase alfa and our
acetylcholinesterase product, are in the preclinical or research
phase with relatively immaterial costs. Most of our research and
development costs were incurred in connection with our
phase III clinical trial of taliglucerase alfa. Our
internal resources, employees and infrastructure are not tied to
any individual research project and are typically deployed
across all of our projects. We currently do not record and
maintain research and development costs per project.
The costs and expenses of our projects are partially funded by
grants we have received from the OCS. Each grant is deducted
from the related research and development expenses as the costs
are incurred. For additional information regarding the grant
process, see “Business — Israeli Government
Programs — Encouragement of Industrial Research and
Development Law, 1984” in Item 1 of this Annual
Report. There can be no assurance that we will continue to
receive grants from the OCS in amounts sufficient for our
operations, if at all.
At this time, due to the inherently unpredictable nature of
preclinical and clinical development processes and given the
early stage of our preclinical product development programs, we
are unable to estimate with any certainty the costs we will
incur in the continued development of the product candidates in
our pipeline for potential commercialization. Clinical
development timelines, the probability of success and
development costs can differ materially from expectations. While
we are currently focused on advancing each of our product
development programs, our future research and development
expenses will depend on the clinical success of each product
candidate, as well as ongoing assessments of each product
candidate’s commercial potential. In addition, we cannot
forecast with any degree of certainty which product candidates
may be subject to future collaborations, when such arrangements
will be secured, if at all, and to what degree such arrangements
would affect our development plans and capital requirements. See
“Risk Factors — All of our product candidates
other than taliglucerase alfa and our acetylcholinesterase
product are in pre-clinical or research stages. If we are unable
to develop and commercialize our product candidates, our
business will be adversely affected” and
“— We may not obtain the necessary U.S. or
worldwide regulatory approvals to commercialize our drug
candidates in a timely manner, if at all, which would have a
material adverse effect on our business and results of
operations.”
65
We expect our research and development expenses to continue to
be our primary expense in the future as we continue the
advancement of our clinical trials and preclinical product
development programs for our product candidates other than
taliglucerase alfa. The lengthy process of completing clinical
trials and seeking regulatory approval for our product
candidates requires expenditure of substantial resources. Any
failure or delay in completing clinical trials, or in obtaining
regulatory approvals, could cause a delay in generating product
revenue and cause our research and development expense to
increase and, in turn, have a material adverse effect on our
operations. We filed an NDA for taliglucerase alfa with the FDA
in the last quarter of 2009 and received a PDUFA date of
February 25, 2011. In addition, we submitted a marketing
application to the Israeli MOH and an MAA to each of the EMEA
and ANVISA in the fourth quarter of 2010 regarding taliglucerase
alfa. Due to the factors set forth above, we are not able to
estimate with any certainty when we would recognize any net cash
inflows from our projects. See “Risk Factors —
Clinical trials are very expensive, time-consuming and difficult
to design and implement and may result in unforeseen costs which
may have a material adverse effect on our business and results
of operations.”
Share-Based
Compensation
The discussion below regarding share-based compensation relates
to share-based compensation paid by Protalix Ltd., our
wholly-owned subsidiary.
In accordance with the guidance, we record the benefit of any
grant to a non-employee and remeasure the benefit in any future
vesting period for the unvested portion of the grants, as
applicable. In addition, we use the straight-line accounting
method for recording the benefit of the entire grant, unlike the
graded method we use to record grants made to employees.
We measure share-based compensation cost for all share-based
awards at the fair value on the grant date and recognition of
share-based compensation over the service period for awards that
we expect will vest. The fair value of stock options is
determined based on the number of shares granted and the price
of our ordinary shares, and calculated based on the
Black-Scholes valuation model. We recognize such value as
expense over the service period, net of estimated forfeitures,
using the accelerated method.
For purposes of determining the fair value of the options to
purchase common stock granted to employees and non-employees
during the fiscal year ended December 31, 2010, including
shares held by non employees that vested during such period, our
management used the fair value of our common stock which was the
closing sale price of our common stock on the NYSE Amex on the
date of calculation.
The guidance allows companies to estimate the expected term of
the option rather than simply using the contractual term of an
option. Because of lack of data on past option exercises by
employees, the expected term of the options could not be based
on historic exercise patterns. Accordingly, we adopted the
simplified method, according to which companies may calculate
the expected term as the average between the vesting date and
the expiration date, assuming the option was granted as a
“plain vanilla” option.
In performing the valuation, we assumed an expected 0% dividend
yield in the previous years and in the next years. We do not
have a dividend policy and given the lack of profitability,
dividends are not expected in the foreseeable future, if at all.
The guidance stipulates a number of factors that should be
considered when estimating the expected volatility, including
the implied volatility of traded options, historical volatility
and the period that the shares of the company are being publicly
traded. As we do not have any traded shares or options, the
expected volatility figures used in this valuation have been
calculated by using the historical volatility of traded shares
of similar companies. In addition, we examined the standard
deviation of shares of similar biotechnology companies that
engage in research and development, generally with no
significant revenues. We found that the volatility of our shares
and the shares of comparable companies was in the range of
40%-75% over periods of three to six years. The volatility used
for each grant differed based on its expected term. For the term
of each grant of our options, the historical volatility was
calculated based upon the overall trading history of the common
stock of comparable companies.
The risk-free interest rate described above has been based on
the implied yield of U.S. federal reserve zero —
coupon government bonds. The remaining term of the bonds used
for each valuation was equal to the
66
expected term of the grant. This methodology has been applied to
all grants valued by us. The guidance requires the use of a
risk — free interest rate based on the implied yield
currently available on zero — coupon government issues
of the country in whose currency the exercise price is
expressed, with a remaining term equal to the expected life of
the option being valued. This requirement has been applied for
all grants valued as part of this report.
Year
Ended December 31, 2010 Compared to the Year Ended
December 31, 2009
Revenues
We recorded revenue of $6.6 million for the year ended
December 31, 2010 compared to revenue of $388,000 for the
year ended December 31, 2009. The increase resulted
primarily from the amortization of the $65.0 million of
upfront and milestone payments received from Pfizer over the
entire fiscal year. Such payments were amortized over one month
for the year ended December 31, 2009. The payments were
recorded as deferred revenue and the amounts will be amortized
over the performance period, estimated at approximately
14 years, at a rate of approximately $1.1 million per
quarter. The amortization during the year ended
December 31, 2010 resulted in the recognition of revenue of
$4.6 million, or an increase of $4.2 million, from
$388,000 recognized during the year ended December 31,
2009. In addition, we recorded revenues in the amount of
approximately $2.0 million in connection with products
delivered to Pfizer under our license agreement. No products
were sold during 2009.
Our share
in the Collaboration Agreement
We recorded $4.6 million of income as our share in the
collaboration under the Pfizer Agreement during the year ended
December 31, 2010. This income resulted primarily from our
share of the approximately $21.8 million of revenues
generated by Pfizer’s sale of taliglucerase alfa to the
Ministry of Health of Brazil under the short term supply
agreement during Pfizer’s 2010 fiscal year. No share in the
collaboration was recorded for the year ended December 31,
2009. Under the terms and conditions of the Pfizer Agreement, we
recorded revenue equal to 40% of the profits realized from sales
of taliglucerase alfa, and related expenses incurred based on
reports we received from Pfizer summarizing the results of the
collaborative activities under the agreement for the applicable
period. Under the terms of the Pfizer Agreement, for its
subsidiaries operating outside the United States, financial
information is included based on the fiscal year ending
November 30, while financial information for the
U.S. entity is included based on the fiscal year ending
December 31.
Research
and Development Expenses
Research and development expenses were $37.7 million for
the year ended December 31, 2010, an increase of
$10.3 million, or 38%, from $27.4 million for the year
ended December 31, 2009. The increase resulted primarily
from an increase of $2.7 million in development expenses
related to salaries for personnel involved in research and
development, and $4.1 million in related subcontractors and
consultants expenses, mainly in connection with our expanded
access program for taliglucerase alfa. The increase in research
and development expenses was partially offset by the recognition
of grants from the OCS and Pfizer’s reimbursement of us,
collectively equal to $7.7 million. The offsets to our
research and development expenses for the year ended
December 31, 2010 represent an increase of approximately
$2.0 million, or 35%, compared to the recognition of grants
from the OCS equal to $5.7 million that offset our research
and development expenses for the year ended December 31,
2009.
We expect research and development expenses to continue to be
our primary expense as we enter into a more advanced stage of
preclinical trials for certain of our product candidates and
into clinical trials for PRX-102, our product candidate for the
treatment of Fabry disease, our acetylcholinesterase product
candidate and our antiTNF product candidate.
General
and Administrative Expenses
General and administrative expenses were $6.9 million for
the year ended December 31, 2010, a decrease of $268,000,
or approximately 4%, from $7.1 million for the year ended
December 31, 2009. The decrease
67
resulted primarily from a $389,000 decrease in salaries expense
during 2010. We expect that our general and administrative
expenses will increase as we add additional personnel and
continue to comply with the reporting and other obligations
applicable to public companies in the United States.
Financial
Expenses and Income
Financial income was $968,000 for the year ended
December 31, 2010, an increase of $439,000, or approximately
83%, compared to $529,000 for the year ended December 31,
2009. The increase resulted primarily from increases in the
exchange rate that resulted in income of approximately $546,000.
Year
Ended December 31, 2009 Compared to the Year Ended
December 31, 2008
Revenues
We recorded revenues of $388,000 during the year ended
December 31, 2009. The revenue represents the pro rata
amortization of the $60.0 million upfront payment and
$5.0 million milestone payment we received in connection
with our license and supply agreement with Pfizer. The payments
were recorded as deferred revenue and the amounts will be
amortized over the performance period, estimated at
approximately 14 years, at a rate of approximately
$1.1 million per quarter. No revenues were recorded during
the year ended December 31, 2008.
Research
and Development Expenses
Research and development expenses were $27.4 million for
the year ended December 31, 2009, an increase of
$5.3 million, or 24%, from $22.1 million for the year
ended December 31, 2008. The increase resulted primarily
from an increase of $1.2 million in development expenses
related to salaries for personnel involved in research and
development, and $2.2 million in related subcontractors and
consultants expenses, mainly in connection with our
phase III clinical trial of taliglucerase alfa. The
increase in research and development expenses was further
increased by the recognition of grants equal to
$5.7 million from the OCS during 2009, an increase of
approximately $1.0 million, or 22%, compared to the
recognition of grants equal to $4.7 million during 2008.
We expect research and development expenses to continue to be
our primary expense as we enter into a more advanced stage of
preclinical trials for certain of our product candidates and
into additional clinical trials for PRX-105.
General
and Administrative Expenses
General and administrative expenses were $7.1 million for
the year ended December 31, 2009, an increase of $374,000,
or approximately 6.0%, from $6.8 million for the year ended
December 31, 2008. The increase resulted primarily from a
$543,000 increase in salaries expenses during 2009.
Financial
Expenses and Income
Financial income was $529,000 for the year ended
December 31, 2009, a decrease of $1.2 million, or
approximately 70.0%, compared to $1.8 million for the year
ended December 31, 2008. The decrease resulted primarily
from a lower interest rate for deposits in 2009 which
contributed to lower financial income during 2009.
Liquidity
and Capital Resources
Sources
of Liquidity
As a result of our significant research and development
expenditures and the lack of any approved products to generate
significant product sales revenue, we have not been profitable
and have generated operating losses since our inception. To
date, we have funded our operations primarily with proceeds
equal to $31.3 million from the sale of shares of our
common stock and from sales of convertible preferred and
68
ordinary shares of Protalix Ltd., and an additional
$14.1 million in connection with the exercise of warrants
issued in connection with the sale of such ordinary shares,
through December 31, 2008. In addition, on October 25,
2007, we generated gross proceeds of $50 million in
connection with an underwritten public offering of our common
stock. Furthermore, on November 30, 2009, we entered into
an exclusive license and supply agreement with Pfizer, pursuant
to which Pfizer made an upfront payment to Protalix Ltd. of
$60.0 million in connection with the execution of the
agreement and subsequently paid to Protalix Ltd. an additional
$5.0 million upon meeting a certain milestone. Protalix
Ltd. is also eligible to receive potential milestone payments of
up to $50.0 million for the successful achievement of other
regulatory-related milestones. We are also entitled to payments
equal to 40% of the net profits earned by Pfizer on its sales of
taliglucerase alfa, if any. In calculating net profits there are
certain agreed upon limits on the amounts that may be deducted
from gross sales for certain expenses and costs of goods sold.
We believe that the funds currently available to us as are
sufficient to satisfy our capital needs for at least
12 months.
The following table summarizes our past funding sources:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
|
|
Security
|
|
Year
|
|
Shares
|
|
Amount(1)
|
|
|
|
Ordinary Shares
|
|
|
1996-2000
|
|
|
|
18,801,527
|
(2)
|
|
$
|
1,100,000
|
|
|
Series A Convertible Preferred Shares
|
|
|
2001
|
|
|
|
11,635,090
|
|
|
$
|
2,000,000
|
|
|
Series B Convertible Preferred Shares(3)
|
|
|
2004-2005
|
|
|
|
7,175,621
|
|
|
$
|
4,500,000
|
|
|
Series C Convertible Preferred Shares(4)
|
|
|
2005
|
|
|
|
5,513,422
|
|
|
$
|
7,700,000
|
|
|
Ordinary Shares(5)
|
|
|
2006
|
|
|
|
10,367,880
|
|
|
$
|
16,000,000
|
|
|
Common Stock
|
|
|
2007
|
|
|
|
10,000,000
|
|
|
$
|
50,000,000
|
|
|
|
|
|
(1) |
|
Gross proceeds; does not include proceeds from warrant exercises. |
| |
|
(2) |
|
Includes the issuance of ordinary shares to founders. |
| |
|
(3) |
|
During 2005, 1,035,569 Series B Preferred Shares were
converted on a 1:1 basis into Series C Preferred Shares for
no additional consideration. Also, in connection with such
funding, warrants to purchase 181,228 Series B Preferred
Shares were issued for no additional consideration with an
aggregate exercise price of $103,000. As of the closing date of
the merger, 168,034 of such warrants were exercised for net
proceeds equal to approximately $96,000 and 13,194 of such
warrants were forfeited. |
| |
|
(4) |
|
In connection with such funding, warrants to purchase an
additional 8,862,803 Series C Preferred Shares were granted
to the investors for no additional consideration with a total
exercise price equal to $9.0 million. As of the closing
date of the merger, 5,296,279 of such warrants were exercised
for net proceeds equal to $8.7 million, 3,384,502 were
assumed by our company and 182,022 expired. |
| |
|
(5) |
|
In connection with such funding, warrants to purchase 3,875,416
ordinary shares were issued for no additional consideration with
an aggregate exercise price equal to $5.3 million. These
warrants were exercised in full on January 31, 2007. |
Cash
Flows
Net cash used in operations was $38.5 million for the year
ended December 31, 2010. The net loss for 2010 of
$29.0 million was further increased by an increase in
accounts receivable of approximately $6.9 million and a
decrease of $4.6 million in deferred revenues. Such net
loss was partially offset by non-cash charges for share-based
compensation of $1.3 million, and depreciation of
$3.1 million. Net cash used in investing activities for
2010 was $8.0 million and consisted primarily of purchases
of property and equipment. Net cash provided from financing
activities for 2010 was approximately $501,000 due to option
exercises.
Net cash provided from operations was $44.5 million for the
year ended December 31, 2009. The net loss for 2009 of
$31.4 million was reversed by $65.0 million received
under the license and supply agreement with Pfizer. Such
reversal was partially offset by non-cash charges for
share-based compensation of $2.7 million, and depreciation
of $2.0 million. Net cash used in investing activities for
2009 was $6.2 million and consisted
69
primarily of purchases of property and equipment. Net cash
provided from financing activities for 2009 was approximately
$293,000 due to option exercise.
Future
Funding Requirements
We expect that our operating losses may continue to be
substantial over the next several years. However, we anticipate
that we will generate revenues to offset any such losses upon
the successful launch of taliglucerase alfa, if at all. We
expect to incur significant research and development expenses,
including expenses related to the hiring of personnel and the
advancement of our additional pipeline of product candidate into
the various clinical trials. We expect that general and
administrative expenses will increase as we expand our finance
and administrative staff, add infrastructure, and incur
additional costs related to our preparation for the commercial
phase for our lead product candidate, taliglucerase alfa. In
addition, we are working on the expansion of our manufacturing
facility that would meet approximately half of the market for
our lead product candidate, which would increase our capital
expenditures significantly and is estimated to cost
approximately $25 million in total.
We believe that our existing cash and cash equivalents and the
regulatory milestone payments we anticipate receiving from
Pfizer will be sufficient to enable us to fund our operating
expenses and capital expenditure requirements at least for the
next 12 months. We have based this estimate on assumptions
that are subject to change and may prove to be wrong, and we may
be required to use our available capital resources sooner than
we currently expect. Because of the numerous risks and
uncertainties associated with the development and
commercialization of our product candidates, we are unable to
estimate the amounts of increased capital outlays and operating
expenditures associated with our current and anticipated
clinical trials.
Our future capital requirements will depend on many factors,
including the progress and results of our clinical trials, costs
of commercialization activities, including product marketing,
sales and distribution and whether these efforts will be
performed internally or through some form of collaboration with
third parties, the duration and cost of discovery and
preclinical development, and laboratory testing and clinical
trials for our product candidates, the timing and outcome of
regulatory review of our product candidates, the costs involved
in preparing, filing, prosecuting, maintaining, defending and
enforcing patent claims and other intellectual property rights
and the number and development requirements of other product
candidates that we pursue.
We may need to finance our future cash needs through public or
private equity offerings, debt financings, or additional
corporate collaboration and licensing arrangements. We currently
do not have any commitments for future external funding, other
than the potential regulatory-related milestone payments from
Pfizer. We may need to raise additional funds more quickly if
one or more of our assumptions prove to be incorrect or if we
choose to expand our product development efforts more rapidly
than we presently anticipate. We may also decide to raise
additional funds even before we need them if the conditions for
raising capital are favorable. The sale of additional equity or
debt securities will likely result in dilution to our
shareholders. The incurrence of indebtedness would result in
increased fixed obligations and could also result in covenants
that would restrict our operations. Additional equity or debt
financing, grants or corporate collaboration and licensing
arrangements may not be available on acceptable terms, if at
all. If adequate funds are not available, we may be required to
delay, reduce the scope of or eliminate our research and
development programs, reduce our planned commercialization
efforts or obtain funds through arrangements with collaborators
or others that may require us to relinquish rights to certain
product candidates that we might otherwise seek to develop or
commercialize independently.
Effects
of Inflation and Currency Fluctuations
Inflation generally affects us by increasing our cost of labor
and clinical trial costs. We do not believe that inflation has
had a material effect on our results of operations during the
years ended December 31, 2008, 2009 or 2010.
Currency fluctuations could affect us by increased or decreased
costs mainly for goods and services acquired outside of Israel.
We do not believe currency fluctuations have had a material
effect on our results of operations during the years ended
December 31, 2008, 2009 or 2010.
70
Off-Balance
Sheet Arrangements
We have no off-balance sheet arrangements as of
December 31, 2009 and 2010. See Note 6 of the
consolidated financial statements for a full description of
certain contingent royalty payments.
Recently
Issued Accounting Pronouncements
In April 2010, the FASB issued ASU
No. 2010-17,
Revenue Recognition — Milestone Method (ASU
2010-017).
ASU 2010-017
provides guidance in applying the milestone method of revenue
recognition to research or development arrangements. Under this
guidance, we may recognize revenue contingent upon the
achievement of a milestone in its entirety, in the period in
which the milestone is achieved, only if the milestone meets all
the criteria within the guidance to be considered substantive.
ASU 2010-017
is effective on a prospective basis for research and development
milestones achieved in fiscal years, beginning on or after
June 15, 2010, which for our company means the year ending
December 31, 2011. Early adoption is permitted; however, we
have elected to implement ASU
2010-17
prospectively, and as a result, the effect of this guidance will
be limited to future transactions. We do not expect adoption of
this standard to have a material impact on our financial
position or results of operations as we have no material
research and development arrangements which will be accounted
for under the milestone method.
In December 2010, the FASB issued ASU
No. 2010-027,
Fees Paid to the Federal Government by Pharmaceutical
Manufacturers (ASU
2010-027).
ASU 2010-027
provides guidance concerning the recognition and classification
of the new annual fee payable by branded prescription drug
manufactures and importers on branded prescription drugs which
was mandated under the health care reform legislation enacted in
the United States in March 2010. Under this new accounting
standard, the annual fee would be presented as a component of
operating expenses and recognized over the calendar year such
fees are payable using a straight-line method of allocation
unless another method better allocates the fee over the calendar
year. ASU
2010-027 is
effective for calendar years beginning on or after
December 31, 2010, when the fee initially becomes
effective, which for our company means the year ending
December 31, 2011. As this standard relates only to
classification, the adoption of this accounting standard will
not have an impact on our financial position or results of
operations.
Contractual
Obligations
The following table summarizes our significant contractual
obligations at December 31, 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Less Than
|
|
|
|
|
|
|
|
|
More Than
|
|
|
|
|
Total
|
|
|
1 Year
|
|
|
1-3 Years
|
|
|
3-5 Years
|
|
|
5 Years
|
|
|
|
|
Operating lease obligations
|
|
$
|
5,293
|
|
|
$
|
1,360
|
|
|
$
|
2,265
|
|
|
$
|
1,668
|
|
|
|
—
|
|
|
Purchase obligations(1)
|
|
$
|
6,753
|
|
|
$
|
6,753
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Certain clinical contract and building contractor obligations
|
|
$
|
5,995
|
|
|
$
|
5,456
|
|
|
$
|
539
|
|
|
|
—
|
|
|
|
—
|
|
|
Other long term liabilities reflected
on the balance sheet under GAAP
|
|
$
|
1,663
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
$
|
1,663
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
19,704
|
|
|
$
|
13,569
|
|
|
$
|
2,804
|
|
|
$
|
1,668
|
|
|
$
|
1,663
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Represents open purchase orders issued to certain suppliers and
other vendors mainly in connection with certain improvements to
our manufacturing facility that were outstanding as of
December 31, 2010. |
71
Selected
Quarterly Financial Data (unaudited)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
|
|
|
|
2009
|
|
2010
|
|
|
|
March 31
|
|
June 30
|
|
Sept. 30
|
|
Dec. 31
|
|
March 31
|
|
June 30
|
|
Sept. 30
|
|
Dec. 31
|
|
|
|
(U.S. dollars in thousands)
|
|
|
|
Revenues
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
388
|
|
|
$
|
1,141
|
|
|
$
|
1,141
|
|
|
$
|
3,184
|
|
|
$
|
1,176
|
|
|
Net loss
|
|
$
|
5,183
|
|
|
$
|
5,427
|
|
|
$
|
5,894
|
|
|
$
|
14,936
|
|
|
$
|
7,955
|
|
|
$
|
10,041
|
|
|
$
|
2,474
|
|
|
$
|
8,528
|
|
|
Net loss per share of common stock, basic and diluted
|
|
$
|
0.07
|
|
|
$
|
0.07
|
|
|
$
|
0.08
|
|
|
$
|
0.19
|
|
|
$
|
0.10
|
|
|
$
|
0.12
|
|
|
$
|
0.03
|
|
|
$
|
0.11
|
|
|
|
|
Item 7A.
|
Quantitative
and Qualitative Disclosures About Market Risk
|
Currency
Exchange Risk
The currency of the primary economic environment in which our
operations are conducted is the dollar. We are currently have no
significant source of revenues; therefore we consider the
currency of the primary economic environment to be the currency
in which we expend cash. Approximately 50% of our expenses and
capital expenditures are incurred in dollars, and a significant
source of our financing has been provided in U.S. dollars.
Since the dollar is the functional currency, monetary items
maintained in currencies other than the dollar are remeasured
using the rate of exchange in effect at the balance sheet dates
and non-monetary items are remeasured at historical exchange
rates. Revenue and expense items are remeasured at the average
rate of exchange in effect during the period in which they
occur. Foreign currency translation gains or losses are
recognized in the statement of operations.
Approximately 35% of our costs, including salaries, expenses and
office expenses, are incurred in NIS. Inflation in Israel may
have the effect of increasing the U.S. dollar cost of our
operations in Israel. If the U.S. dollar declines in value
in relation to the NIS, it will become more expensive for us to
fund our operations in Israel. A revaluation of 1% of the NIS
will affect our income before tax by less than 1%. The exchange
rate of the U.S. dollar to the NIS, based on exchange rates
published by the Bank of Israel, was as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2008
|
|
2009
|
|
2010
|
|
|
|
Average rate for period
|
|
|
3.5878
|
|
|
|
3.933
|
|
|
|
3.733
|
|
|
Rate at year-end
|
|
|
3.8020
|
|
|
|
3.775
|
|
|
|
3.549
|
|
To date, we have not engaged in hedging transactions. In the
future, we may enter into currency hedging transactions to
decrease the risk of financial exposure from fluctuations in the
exchange rate of the U.S. dollar against the NIS. These
measures, however, may not adequately protect us from material
adverse effects due to the impact of inflation in Israel.
Interest
Rate Risk
Our exposure to market risk is confined to our cash and cash
equivalents. We consider all short term, highly liquid
investments, which include short-term deposits with original
maturities of three months or less from the date of purchase,
that are not restricted as to withdrawal or use and are readily
convertible to known amounts of cash, to be cash equivalents.
The primary objective of our investment activities is to
preserve principal while maximizing the interest income we
receive from our investments, without increasing risk. We invest
any cash balances primarily in bank deposits and investment
grade interest-bearing instruments. We are exposed to market
risks resulting from changes in interest rates. We do not use
derivative financial instruments to limit exposure to interest
rate risk. Our interest gains may decline in the future as a
result of changes in the financial markets.
|
|
|
Item 8.
|
Financial
Statements and Supplementary Data
|
See the Index to Consolidated Financial Statements on
Page F-1
attached hereto.
72
|
|
|
Item 9.
|
Changes
in and Disagreements with Accountants on Accounting and
Financial Disclosure
|
None.
|
|
|
Item 9A.
|
Controls
and Procedures
|
Evaluation
of Disclosure Controls and Procedures
We conducted an evaluation of the effectiveness of the design
and operation of our disclosure controls and procedures as of
the end of the period covered by this
Form 10-K.
The controls evaluation was conducted under the supervision and
with the participation of management, including our Chief
Executive Officer and Chief Financial Officer. Disclosure
controls and procedures are controls and procedures designed to
reasonably assure that information required to be disclosed in
our reports filed under the Exchange Act, such as this
Form 10-K,
is recorded, processed, summarized and reported within the time
periods specified in the Commission’s rules and forms.
Disclosure controls and procedures are also designed to
reasonably assure that such information is accumulated and
communicated to our management, including the Chief Executive
Officer and Chief Financial Officer, as appropriate to allow
timely decisions regarding required disclosure.
The evaluation of our disclosure controls and procedures
included a review of the controls’ objectives and design,
our implementation of the controls and their effect on the
information generated for use in this
Form 10-K.
In the course of the controls evaluation, we reviewed identified
data errors, control problems or acts of fraud, and sought to
confirm that appropriate corrective actions, including process
improvements, were being undertaken. This type of evaluation
will be performed on a quarterly basis so that the conclusions
of management, including the Chief Executive Officer and Chief
Financial Officer, concerning the effectiveness of the
disclosure controls and procedures can be reported in our
periodic reports on
Form 10-Q
and
Form 10-K.
The overall goals of these various evaluation activities are to
monitor our disclosure controls and procedures, and to modify
them as necessary. Our intent is to maintain the disclosure
controls and procedures as dynamic systems that change as
conditions warrant.
Based on the controls evaluation, our Chief Executive Officer
and Chief Financial Officer have concluded that, as of the end
of the period covered by this
Form 10-K,
our disclosure controls and procedures were effective to provide
reasonable assurance that information required to be disclosed
in our Exchange Act reports is recorded, processed, summarized
and reported within the time periods specified by the
Commission, and that material information related to our company
and our consolidated subsidiary is made known to management,
including the Chief Executive Officer and Chief Financial
Officer, particularly during the period when our periodic
reports are being prepared.
Management
Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining
adequate internal control over financial reporting to provide
reasonable assurance regarding the reliability of our financial
reporting and the preparation of financial statements for
external purposes in accordance with U.S. generally
accepted accounting principles. Internal control over financial
reporting includes those policies and procedures that:
(i) pertain to the maintenance of records that in
reasonable detail accurately and fairly reflect the transactions
and dispositions of our assets; (ii) provide reasonable
assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with
U.S. generally accepted accounting principles, and that
receipts and expenditures of our company are being made only in
accordance with authorizations of management and our directors;
and (iii) provide reasonable assurance regarding prevention
or timely detection of unauthorized acquisition, use or
disposition of our assets that could have a material effect on
our financial statements.
Management assessed our internal control over financial
reporting as of December 31, 2010, the end of our fiscal
year. Management based its assessment on criteria established in
Internal Control — Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway
Commission. Management’s assessment included evaluation of
elements such as the design and operating effectiveness of key
financial reporting controls, process documentation, accounting
policies and our overall control environment.
73
Based on our assessment, management has concluded that our
internal control over financial reporting was effective as of
the end of the fiscal year to provide reasonable assurance
regarding the reliability of financial reporting and the
preparation of financial statements for external reporting
purposes in accordance with U.S. generally accepted
accounting principles. We reviewed the results of
management’s assessment with the Audit Committee of our
Board of Directors.
Our independent registered public accounting firm has audited
management’s assessment of our internal control over
financial reporting, and issued an unqualified opinion dated
February 23, 2011 on such assessment and on our internal
control over financial reporting, which opinion is included
herein.
Inherent
Limitations on Effectiveness of Controls
Our management, including our Chief Executive Officer and Chief
Financial Officer, does not expect that our disclosure controls
and procedures or our internal control over financial reporting
will prevent or detect all error and all fraud. A control
system, no matter how well designed and operated, can provide
only reasonable, not absolute, assurance that the control
system’s objectives will be met. The design of a control
system must reflect the fact that there are resource
constraints, and the benefits of controls must be considered
relative to their costs. Further, because of the inherent
limitations in all control systems, no evaluation of controls
can provide absolute assurance that misstatements due to error
or fraud will not occur or that all control issues and instances
of fraud, if any, within a company have been detected. These
inherent limitations include the realities that judgments in
decision-making can be faulty and that breakdowns can occur
because of simple error or mistake. Controls can also be
circumvented by the individual acts of some persons, by
collusion of two or more people or by management override of the
controls. The design of any system of controls is based in part
on certain assumptions about the likelihood of future events,
and there can be no assurance that any design will succeed in
achieving its stated goals under all potential future
conditions. Projections of any evaluation of controls
effectiveness to future periods are subject to risks. Over time,
controls may become inadequate because of changes in conditions
or deterioration in the degree of compliance with policies or
procedures.
Changes
in internal controls
There were changes in our internal control over financial
reporting (as defined in
Rules 13a-15f
and 15d-15f
under the Exchange Act) that occurred during the period ended
December 31, 2010. The changes relate to the processes
associated with the evaluation of our new revenue contracts and
accounting for inventories.
|
|
|
Item 9B.
|
Other
Information
|
None.
74
PART III
|
|
|
Item 10.
|
Directors,
Executive Officers and Corporate Governance
|
Our directors and executive officers, their ages and positions
as of February 23, 2011 are as follows:
| |
|
|
|
|
|
|
|
Name
|
|
Age
|
|
Position
|
|
|
|
Directors
|
|
|
|
|
|
|
|
Zeev Bronfeld
|
|
|
59
|
|
|
Interim Chairman of the Board
|
|
David Aviezer, Ph.D., MBA
|
|
|
46
|
|
|
Director, President and Chief Executive Officer
|
|
Yoseph Shaaltiel, Ph.D.
|
|
|
57
|
|
|
Director and Executive VP, Research and Development
|
|
Alfred Akirov(1)(2)(3)
|
|
|
70
|
|
|
Director
|
|
Amos Bar Shalev(1)(2)(3)
|
|
|
58
|
|
|
Director
|
|
Yodfat Harel Gross(1)(2)(3)
|
|
|
38
|
|
|
Director
|
|
Roger D. Kornberg, Ph.D.
|
|
|
63
|
|
|
Director
|
|
Eyal Sheratzky
|
|
|
42
|
|
|
Director
|
|
Executive Officers
|
|
|
|
|
|
|
|
Einat Brill Almon, Ph.D.
|
|
|
51
|
|
|
Senior Vice President, Product Development
|
|
Yossi Maimon, CPA
|
|
|
40
|
|
|
Vice President, Chief Financial Officer, Treasurer and Secretary
|
|
Sandra L. Lauterbach
|
|
|
42
|
|
|
Vice President, Sales and Commercial Affairs
|
|
Tzvi Palash
|
|
|
54
|
|
|
Chief Operating Officer
|
|
|
|
|
(1) |
|
Member of Nominating Committee |
| |
|
(2) |
|
Member of Audit Committee |
| |
|
(3) |
|
Member of Compensation Committee |
Zeev Bronfeld. Mr. Bronfeld has served as
a director of Protalix Ltd. since 1996 and as our director since
December 31, 2006. Mr. Bronfeld brings to us vast
experience in management and value building of biotechnology
companies. Mr. Bronfeld is an experienced businessman who
is involved in a number of biotechnology companies. He is a
co-founder of Biocell Ltd. (TASE:BCEL), an Israeli publicly
traded holding company specializing in biotechnology companies
and has served as its Chief Executive Officer since 1986.
Mr. Bronfeld currently serves as a director of Biocell
Ltd., D. Medical Industries Ltd. (NASDAQ:DMED, TASE:DMDC),
Biomedix Incubator Ltd. (TASE:BMDX), Flowsense Medical Ltd.
(TASE:FLSN), E.T. View Medical Ltd. (TASE:ETVW), NextGen Biomed
Ltd. (TASE:NXGN), D.N.A. Biomedical Solutions Ltd. (TASE:DNA),
and Gefen Biomed Investments Ltd. (TASE:GEFEN), all of which are
public companies traded on the Tel Aviv Stock Exchange (except
D. Medical which is dual-listed on the Tel Aviv Stock Exchange
and the Nasdaq Stock Market LLC. Mr. Bronfeld is also a
director of each of the following privately-held companies:
Ecocycle Israel Ltd., Contipi Ltd., Spring Health Solutions
Ltd., G-Sense Ltd., Sindolor Medical Ltd., L.N. Innovative
Technologies, A.T.I Ashkelon Industries Information Technologies
Ltd., The Trendlines Group, MOFET B’Yehuda —
Industrial Research & Development in Judea Ltd.,
Incubator for Management of Technological Entrepreneurship
Misgav Ltd., A.Y.M.B. Holdings and Investments Ltd., Spring-Set
Health Solutions Ltd., Sindolor Holdings Ltd., TransBiodiesel
Ltd., Nanutra Ltd. and Entera Bio Ltd. Mr. Bronfeld
received a B.A. in Economics from the Hebrew University in 1975.
We believe Mr. Bronfeld’s qualifications to serve on
our Board of Directors include his years of experience in the
management of private and public Israeli companies, including
life science companies.
David Aviezer, Ph.D.,
MBA. Dr. Aviezer has served as Chief
Executive Officer of Protalix Ltd. since 2002 and its director
since 2005 and as our director since December 31, 2006. On
December 31, 2006, he became our President and Chief
Executive Officer. Dr. Aviezer has over 15 years of
experience in biotechnology management, advancing products from
early-stage research up to their regulatory approval and
75
commercialization. Prior to joining Protalix Ltd., from 1996 to
2002, he served as General Manager of ProChon Biotech Ltd., an
Israeli company focused on orthopedic disorders. Previously,
Dr. Aviezer was a visiting scientist at the Medical
Research Division of American Cyanamid, a subsidiary of Wyeth
which was subsequently acquired by Pfizer (NYSE:PFE), in New
York. Since 1996, Dr. Aviezer has served as an Adjunct
Lecturer at Bar Ilan University. Dr. Aviezer is the
recipient of the Clore Foundation Award and the J.F. Kennedy
Scientific Award. He holds a Ph.D. in Molecular Biology and
Biochemistry from the Weizmann Institute of Science and an MBA
from the Bar Ilan University Business School. We believe
Dr. Aviezer’s qualifications to serve on our Board of
Directors include his position as our President and Chief
Executive Officer as well as his previous experience in the
management of biotechnology companies.
Yoseph
Shaaltiel, Ph.D. Dr. Shaaltiel founded
Protalix Ltd. in 1993 and has served as a member of our Board of
Directors and as our Vice President, Research and Development
since December 31, 2006. Prior to establishing Protalix
Ltd., from 1988 to 1993, Dr. Shaaltiel was a Research
Associate at the MIGAL Technological Center. He also served as
Deputy Head of the Biology Department of the Biological and
Chemical Center of the Israeli Defense Forces and as a
Biochemist at Makor Chemicals Ltd. Dr. Shaaltiel was a
Postdoctoral Fellow at the University of California at Berkeley
and at Rutgers University in New Jersey. He has co-authored over
40 articles and abstracts on plant biochemistry and holds seven
patents. Dr. Shaaltiel received his Ph.D. in Plant
Biochemistry from the Weizmann Institute of Science, an M.Sc. in
Biochemistry from the Hebrew University and a B.Sc. in Biology
from the Ben Gurion University. We believe
Dr. Shaaltiel’s qualifications to serve on our Board
of Directors include his role in founding our company and his
continued role in the management of our company.
Alfred Akirov. Mr. Akirov has served as
our director since January 2008. Mr. Akirov is the founder,
chairman of the Board of Directors and chief executive officer
of the Alrov Group (TASE: ALRO), an Israeli publicly-traded
company listed on the Tel Aviv Stock Exchange. Mr. Akirov
founded the Alrov Group in 1978 and it is currently one of
Israel’s largest real-estate companies. The Alrov Group
holds 80% of the capital stock of Techno-Rov Holdings
(1993) Ltd., one of our shareholders. Mr. Akirov
serves in different capacities, including chairman, chief
executive officer and director, for a number of private
companies in the Alrov Group and Techno-Rov portfolios.
Mr. Akirov serves on the Executive Council and the Board of
Governors of the Tel Aviv University. We believe
Mr. Akirov’s qualifications to serve on our Board of
Directors include his years of experience in the management of
Israeli businesses.
Amos Bar Shalev. Mr. Bar Shalev has
served as our director since July 2008. Mr. Bar Shalev
served as a director of Protalix Ltd. from 2005 through
January 31, 2008, and as our director from
December 31, 2006 through January 31, 2008.
Mr. Bar Shalev was not nominated for reelection at our
annual meeting of shareholders on January 31, 2008. On
July 14, 2008, our Board of Directors appointed
Mr. Bar Shalev to serve on the board. Mr. Bar Shalev
brings to us extensive experience in managing technology
companies. Currently, Mr. Bar Shalev manages the Technorov
portfolio. Since 2011, he has served on the board of directors
of Aposense Ltd. (TASE: APOS), an Israeli publicly-traded
company listed on the Tel Aviv Stock Exchange. From 1997 through
2004, he was a Managing Director of TDA Capital Partners, a
management company of the TGF (Templeton Tadiran) Fund. From
2004 through 2007, he was the President of Win Buyer Ltd. From
2000 through 2007, Mr. Bar Shalev served the Director of
Technorov Holdings (1993) Ltd. and from 2004 through 2007
he served as a director of Golden Wings Investment Company Ltd.
He has served on the board of directors of many companies, such
as Golden Wings Investment Company Ltd., Win Buyer Ltd. and Sun
Light. He received his B.Sc. in Electrical Engineering from the
Technion, Israel in 1978 and M.B.A. from the Tel Aviv University
in 1981. He holds the highest award from the Israeli Air Force
for technological achievements. We believe Mr. Bar
Shalev’s qualifications to serve on our Board of Directors
include his years of experience in the management of Israeli
businesses.
Yodfat Harel Gross. Ms. Harel Gross has
served as our director since June 2007. Since 2006,
Ms. Harel Gross has been a Managing Director of Tamares
Capital Ltd., a private investment group with interests in real
estate, technology, manufacturing, leisure and media. At Tamares
Capital, Ms. Harel Gross serves as the Business Development
Director and the head of the Israel office. Prior to joining
Tamares Capital, from 2004 to 2006, she was the Head of the
Medical Desk of Orbotech, Ltd. (NASDAQ:ORBK), a company
providing high-tech inspection and imaging solutions for bare
printed circuit board (PCB), flat panel display (FPD) and
76
PCB assembly manufacturing worldwide. Prior to that, from 1994
to 2003, she was a Managing Director of Harel-Hertz Investment
House Ltd., a business investment company with offices in Tel
Aviv, Israel and Tokyo, Japan. In 2002, Harel-Hertz Investment
House became the Israeli representative office for ITX
Corporation, a publicly-traded company in Japan. Ms. Harel
Gross currently serves on the board of directors of Tamares
Capital, Tamares Hotels, Tamares Real Estate, Storewiz and
Halman-Aldubi Provident Funds, Ltd. Ms. Harel Gross holds a
B.A. in Communication and Political Science from Bar Ilan
University and an executive M.B.A. from Bradford University,
Great Britain. She has also completed programs in
Directors’ Studies and Advanced Advertising and Marketing
at the Israel Management Center. We believe Ms. Harel
Gross’ qualifications to serve on our Board of Directors
include her experience in the management of Israeli and other
businesses.
Roger D. Kornberg, Ph.D. Professor
Kornberg has served as our director since February 2008. He has
served as a director of Teva Pharmaceuticals (NASDAQ: TEVA,
TASE: TEVA) since 2007. Professor Kornberg is a member of the
U.S. National Academy of Sciences and the Winzer Professor
of Medicine in the Department of Structural Biology at Stanford
University, Stanford, California. He has been a member of the
faculty of Stanford University since 1972. Prior to that, he was
a professor at Harvard Medical School. Professor Kornberg is a
renowned biochemist and in 2006 he was awarded the Nobel Prize
in Chemistry in recognition for his studies of the molecular
basis of eukaryotic transcription, the process by which DNA is
copied to RNA. Professor Kornberg is also the recipient of
several awards, including the 2001 Welch Prize, the highest
award granted in the field of chemistry in the United States,
and the 2002 Leopold Mayer Prize, the highest award granted in
the field of biomedical sciences from the French Academy of
Sciences. He received his B.S. in Chemistry from Harvard
University in 1967 and his Ph.D. in Chemistry from Stanford
University in 1972. He holds honorary degrees from universities
in Europe and Israel, including the Hebrew University in
Jerusalem, where he currently is a visiting professor. We
believe Professor Kornberg’s qualifications to serve on our
Board of Directors include his expertise in chemistry and
medicine and his experience in the academic arena.
Eyal Sheratzky. Mr. Sheratzky has served
as a director of Protalix Ltd. since 2005 and as our director
since December 31, 2006. Mr. Sheratzky has served as a
director of Ituran Location & Control (NASDAQ:ITRN), a
publicly-traded company listed on the Nasdaq, since 1995 and as
a Co-Chief Executive Officer since 2003. Prior to such date, he
served as an alternate Chief Executive Officer of Ituran from
2002 through 2003 and as Vice President of Business Development
from 1999 through 2002. Mr. Sheratzky is the Chairman of
the Board of Directors of Biocell and serves as a director of
Moked Ituran Ltd. and certain of Ituran’s other
subsidiaries. Mr. Sheratzky also serves as a director of D.
Medical Industries Ltd. (NASDAQ:DMED, TASE:DMDC), as well as of
Nilimedix Ltd., its subsidiary. From 1994 to 1999 he served as
the Chief Executive Officer of Moked Services, Information and
Investments Ltd. and as legal advisor to several of
Ituran’s affiliated companies. Mr. Sheratzky holds
LL.B and LL.M degrees from Tel Aviv University School of Law and
an Executive M.B.A. degree from Kellogg University. We believe
Mr. Sheratzky’s qualifications to serve on our Board
of Directors include his years of experience in the management
of Israeli public and private businesses.
Einat Brill Almon, Ph.D. Dr. Almon
joined Protalix Ltd. in December 2004 as its Senior Vice
President, Product Development and became our Vice President,
Product Development on December 31, 2006. Dr. Almon
has many years of experience in the management of life science
projects and companies, including biotechnology and agrobiotech,
with direct experience in clinical, device and scientific
software development, as well as a strong background and work
experience in Intellectual Property. Prior to joining Protalix
Ltd., from 2001 to 2004, she served as Director of R&D and
IP of Biogenics Ltd., a company that developed an autologous
platform for tissue based protein drug delivery. Biogenics,
based in Israel, is a wholly-owned subsidiary of Medgenics Inc.
Dr. Almon has trained as a biotechnology patent agent at
leading IP firms in Israel. Dr. Almon holds a Ph.D. and an
M.Sc. in molecular biology of cancer research from the Weizmann
Institute of Science, a B.Sc. from the Hebrew University and has
carried out Post-Doctoral research at the Hebrew University in
the area of plant molecular biology.
Yossi Maimon, CPA. Mr. Maimon joined
Protalix Ltd. on October 15, 2006 as its Chief Financial
Officer and became our Vice President and Chief Financial
Officer on December 31, 2006. Prior to joining Protalix,
from 2002 to 2006, he served as the Chief Financial Officer of
Colbar LifeScience Ltd., a biomaterial
77
company focusing on aesthetics, where he led all of the
corporate finance activities, fund raisings and legal aspects of
Colbar including the sale of Colbar to Johnson and Johnson.
Mr. Maimon has a B.A. in accounting from the City
University of New York and an MBA from Tel Aviv University, and
he is a Certified Public Accountant in the United States (New
York State) and Israel.
Sandra L. Lauterbach. Ms. Lauterbach has
served as our Vice President, Sales and Commercial Affairs since
December 18, 2009. Prior to joining our company,
Ms. Lauterbach was the Vice President of Marketing,
Endocrinology of EMD Serono, Inc., from July 2008 through July
2009. Prior to that, from August 2003 through July 2008, she
served in a number of positions at Genzyme Corporation, the last
position being the Senior Director, Global Marketing of
Fabrazyme. Ms. Lauterbach holds a B.Sc. in Molecular
Biology from the University of Wisconsin and an MBA from the
University of South Florida.
Tzvi Palash. Mr. Palash has served as
Protalix Ltd.’s Chief Operating Officer since
September 6, 2010. Prior to joining Protalix Ltd., from
2006 through 2010, Mr. Palash served as a General Manager
of ColBar LifeScience Ltd., a biotechnology company specializing
in reconstructive medicine and tissue engineering that was
acquired by a division of Johnson & Johnson in 2006.
In that position, Mr. Palash served as a member of the
Global Aesthetic Management Team at the Consumer Group of
Johnson & Johnson. Prior to that, from 2001 through
2006, Mr. Palash served as the Vice President, Operations
of ColBar LifeScience. Mr. Palash holds an M.Sc. in
Biochemistry from the Hebrew University, and a B.Sc. in Biology
from the Tel Aviv University.
Section 16(a)
Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors,
executive officers and holders of more than 10% of our common
stock to file with the Commission reports regarding their
ownership and changes in ownership of our equity securities. We
believe that all Section 16 filings requirements were met
by our officers and directors during 2010. In making this
statement, we have relied solely upon examination of the copies
of Forms 3, 4 and 5, Schedule 13s and written
representations of our former and current directors, officers
and 10% shareholders.
Audit
Committee
We require that all Audit Committee members possess the required
level of financial literacy and at least one member of the Audit
Committee meet the current standard of requisite financial
management expertise as required by the NYSE Amex and applicable
rules and regulations of the SEC. Messrs. Bar Shalev and
Akirov, and Ms. Harel Gross have been appointed by the
Board of Directors to serve on the Audit Committee until their
respective successors have been duly elected.
Our Audit Committee operates under a formal charter that governs
its duties and conduct.
All members of the Audit Committee are independent from our
executive officers and management.
Our independent registered public accounting firm reports
directly to the Audit Committee.
Our Audit Committee meets with management and representatives of
our registered public accounting firm prior to the filing of
officers’ certifications with the Commission to receive
information concerning, among other things, effectiveness of the
design or operation of our internal controls over financial
reporting, as required by Section 404 of the Sarbanes-Oxley
Act of 2002.
Our Audit Committee has adopted a Policy for Reporting
Questionable Accounting and Auditing Practices and Policy
Prohibiting Retaliation against Reporting employees to enable
confidential and anonymous reporting of improper activities to
the Audit Committee.
Messrs. Bar Shalev and Akirov qualify as “audit
committee financial experts” under the applicable rules of
the SEC. In making the determination as to these
individuals’ status as audit committee financial experts,
our Board of Directors determined they have accounting and
related financial management expertise within the meaning of the
aforementioned rules, as well as the listing standards of the
NYSE Amex.
78
Code of
Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics that
includes provisions ranging from restrictions on gifts to
conflicts of interest. All of our employees and directors are
bound by this Code of Business Conduct and Ethics. Violations of
our Code of Business Conduct and Ethics may be reported to the
Audit Committee.
The Code of Business Conduct and Ethics includes provisions
applicable to all of our employees, including senior financial
officers and members of our Board of Directors and is posted on
our website (www.protalix.com). We intend to post amendments to
or waivers from any such Code of Business Conduct and Ethics.
|
|
|
Item 11.
|
Executive
Compensation
|
Compensation
Discussion and Analysis
The primary goals of the Compensation Committee of our Board of
Directors with respect to executive compensation are to attract
and retain the most talented and dedicated executives possible,
to tie annual and long-term cash and stock incentives to
achievement of specified performance objectives, and to align
executives’ incentives with shareholder value creation. To
achieve these goals, the Compensation Committee intends to
implement and maintain compensation plans that tie a portion of
executives’ overall compensation to key strategic goals
such as developments in our clinical path, the establishment of
key strategic collaborations, the
build-up of
our pipeline and the strengthening of our financial position.
The Compensation Committee evaluates individual executive
performance with a goal of setting compensation at levels the
committee believes are comparable with executives in other
companies of similar size and stage of development operating in
the biotechnology industry while taking into account our
relative performance and our own strategic goals.
Elements
of Compensation
Executive compensation consists of following elements:
Base Salary. Base salaries for our executives
are established based on the scope of their responsibilities
taking into account competitive market compensation paid by
other companies for similar positions. Generally, we believe
that executive base salaries should be targeted near the median
of the range of salaries for executives in similar positions
with similar responsibilities at comparable companies. The
companies reviewed by the Compensation Committee in making its
compensation decisions in February 2010 were as follows:
|
|
|
| |
•
|
Keryx Biopharmaceuticals, Inc.
|
| |
| |
•
|
Savient Pharmaceuticals, Inc.
|
| |
| |
•
|
Biomarin Pharmaceutical Inc.
|
| |
| |
•
|
Amicus Therapeutics, Inc.
|
| |
| |
•
|
Mannkind Corporation
|
| |
| |
•
|
Nektar Therapeutics
|
| |
| |
•
|
Theravance, Inc.
|
The Compensation Committee intends to continue reviewing and
revising the peer group periodically to ensure that it continues
to reflect companies similar to us in size and development
stage. The Compensation Committee also reviews an executive
compensation report and analysis of publicly-traded
biotechnology companies prepared by a third party for additional
data and other information regarding executive compensation for
comparative purposes.
Base salaries are usually reviewed annually, and adjusted from
time to time to realign salaries with market levels after taking
into account individual responsibilities, performance and
experience. The
79
Compensation Committee intends to perform its review for 2010
after the anticipated FDA approval of our lead product
candidate. The base salaries of our named executive officers are
set forth in “Employment Arrangements.”
Annual Bonus. The Compensation Committee has
the authority to award discretionary annual bonuses to our
executive officers. For 2010, the Compensation Committee
established a formal bonus plan for certain milestones, as
described below. The discretionary annual bonus awards were
intended to compensate officers for achieving financial,
clinical, regulatory and operational goals and for achieving
individual annual performance objectives. For any given year,
the compensation objectives vary, but relate generally to
strategic factors such as developments in our clinical path, the
execution of a license agreement for the commercialization of
product candidates, the establishment of key strategic
collaborations, the
build-up of
our pipeline and financial factors such as capital raising.
Bonuses are awarded generally based on corporate performance,
with adjustments made within a range for individual performance,
at the discretion of the Compensation Committee. The
Compensation Committee determines, on a discretionary basis, the
size of the entire bonus pool and the amount of the actual award
to each named executive officer.
The Compensation Committee selects, in its discretion, the
executive officers of our company or our subsidiary who are
eligible to receive bonuses for any given year. Any bonus
granted by the Compensation Committee will generally be paid in
the first quarter of the year, unless such bonus was, by its
terms, made payable upon the achievement of a specific
milestone. The Compensation Committee has not fixed a minimum or
maximum award for any executive officer’s annual
discretionary bonus, unless specified in the officer’s
employment agreement.
Each of our executive officers is eligible for a discretionary
annual bonus under his or her employment agreement. The
Compensation Committee determined the discretionary annual bonus
to be paid to our executive officers for performance in 2008,
2009 and 2010. The Compensation Committee has not fixed a
minimum or a maximum amount for any officer’s annual
discretionary bonus, nor is any executive officer entitled to a
minimum or maximum bonus amount under his or her employment
agreement.
On February 25, 2010, our Board of Directors, acting upon
the resolution of a majority of our independent directors,
decided to pay bonuses to our executive officers and other
employees in two tranches. The aggregate amount of all of the
bonuses awarded or reserved for award by the Board of Directors
pursuant to the resolution was approximately $2.6 million.
The first tranche of bonus payments awarded in February 2010 to
our named executive officers and other employees was for
approximately $1.1 million in the aggregate. These bonuses
were made on a discretionary basis to acknowledge and compensate
our executive officers for their contributions towards the
completion of our phase III clinical trial of our lead
product candidate, taliglucerase alfa, the upgrade of our
manufacturing facility during the years 2008 and 2009, and
specifically with the execution of the license and supply
agreement with Pfizer relating to taliglucerase alfa. The
decision to grant the awards constituting the first tranche of
bonuses in 2010 was not based on any predetermined goal set for
any named executive officer. However, in making this
compensation decision, the Compensation Committee took into
account the Board of Directors’ decision to refrain from
awarding bonuses to our executive officers and others in 2009
due to the general market conditions and our cash balance at
that time. Of the approximately $1.1 million made available
for the first tranche of the bonuses, our Board of Directors
awarded Dr. Aviezer $500,000; Dr. Shaaltiel $160,000;
Dr. Brill Almon $160,000; and Mr. Maimon $160,000.
These bonus payments were made in March 2010.
80
The remaining $1.5 million approved by our Board of
Directors in February 2010 was reserved for future payment to
our named executive officers and other employees. The second
tranche of bonus payments will generally become payable upon the
achievement of the milestones described in the following tables,
if at all:
First
shipment of taliglucerase alfa
| |
|
|
|
|
|
|
|
Anticipated Bonus
|
|
Named Executive Officer
|
|
Amount
|
|
|
|
David Aviezer, Ph.D., MBA
|
|
|
—
|
|
|
Yoseph Shaaltiel, Ph.D.
|
|
$
|
100,000
|
|
|
Einat Brill Almon, Ph.D.
|
|
$
|
20,000
|
|
|
Yossi Maimon
|
|
$
|
20,000
|
|
|
Total
|
|
$
|
140,000
|
|
Approval
of taliglucerase alfa by the FDA
| |
|
|
|
|
|
|
|
Anticipated Bonus
|
|
Named Executive Officer
|
|
Amount
|
|
|
|
David Aviezer, Ph.D., MBA
|
|
$
|
400,000
|
|
|
Yoseph Shaaltiel, Ph.D.
|
|
$
|
140,000
|
|
|
Einat Brill Almon, Ph.D.
|
|
$
|
140,000
|
|
|
Yossi Maimon
|
|
$
|
140,000
|
|
|
Total
|
|
$
|
820,000
|
|
The first shipment of taliglucerase alfa was made in 2010 and
bonuses were paid accordingly. Other than the achievement of the
corporate milestones set forth above, the anticipated amounts
allocated to each executive officer were not based upon any
predetermined goals with respect to any individual named
executive officer. Further, no individual goals have been
communicated to any individual named executive officer with
respect to the eventual payment of the bonuses. The remaining
approximately $500,000 was allocated to other employees, subject
to the same criteria.
Options. Our 2006 Stock Option Plan authorizes
us to grant options to purchase shares of common stock to our
employees, directors and consultants. Our Compensation Committee
is the administrator of the stock option plan. Stock option
grants are generally made at the commencement of employment and
following a significant change in job responsibilities or to
meet other special retention or performance objectives. The
Compensation Committee reviews and approves stock option awards
to executive officers based upon a review of competitive
compensation data, its assessment of individual performance, a
review of each executive’s existing long-term incentives,
and retention considerations. The exercise price of stock
options granted under the 2006 Stock Incentive Plan must be
equal to at least 100% of the fair market value of our common
stock on the date of grant; however, in certain circumstances,
grants may be made at a lower price to Israeli grantees who are
residents of the State of Israel. We have not awarded stock
options to any of our executive officers since February 2010,
except in connection with the hiring of our Chief Operating
Officer.
Severance and Change in Control
Benefits. Pursuant to the employment agreements
entered into with each of our executive officers based in
Israel, the executive officer is entitled to be insured by
Protalix Ltd. under a Manager’s Policy in lieu of
severance. The intention of such Manager’s Policies is to
provide the Israel-based officers with severance protection of
one month’s salary for each year of employment. In
addition, stock option agreements with each of our named
executive officers, as amended, provide that all of the
outstanding options of each Named Executive Officer are subject
to accelerated vesting immediately upon a change in control of
our company.
Other Compensation. Consistent with our
compensation philosophy, we intend to continue to maintain our
current benefits for our executive officers; however, the
Compensation Committee in its discretion may revise, amend, or
add to the officer’s executive benefits if it deems it
advisable. As an additional benefit to all of our Israel-based
Named Executive Officers and for most of our employees, we
generally contribute to
81
certain funds amounts equaling a total of approximately 15% of
their gross salaries for certain pension and other savings plans
for the benefit of the Named Executive Officers. In addition, in
accordance with customary practice in Israel, our Israel-based
executives’ agreements require us to contribute towards
their vocational studies, and to provide annual recreational
allowances, a company car and a company phone. We believe these
benefits are currently equivalent with median competitive levels
for comparable companies.
Executive Compensation. We refer to the
“Summary Compensation Table” set forth in
Section 11 of the Annual Report on
Form 10-K
for information regarding the compensation earned during the
fiscal year ended December 31, 2010 by: our President and
Chief Executive Officer, our Executive Vice President, Research
and Development, our Senior Vice President, Product Development,
our Vice President and Chief Financial Officer and our Vice
President, Sales and Commercial Affairs, who we refer to
collectively as the “Named Executive Officers.” There
are no other executive officers for 2010 whose total
compensation exceeded $100,000 during that fiscal year other
than the Named Executive Officers.
Compensation
Committee Report
The above report of the Compensation Committee does not
constitute soliciting material and shall not be deemed filed or
incorporated by reference into any other filing by us under the
Securities Act of 1933 or the Securities Exchange Act of
1934.
The Compensation Committee has reviewed and discussed the
Compensation Discussion and Analysis set forth below with our
management. Based on this review and discussion, the
Compensation Committee has recommended to our Board of Directors
that the Compensation Discussion and Analysis be included in our
Annual Report on Form 10 — K and our annual proxy
statement on Schedule 14A.
Respectfully submitted on February 23, 2011, by the members
of the Compensation Committee of the Board of Directors.
Yodfat Harel Gross
Alfred Akirov
Amos Bar Shalev
Summary
Compensation Table
The following table sets forth a summary for the fiscal years
ended December 31, 2010, 2009 and 2008, respectively, of
the cash and non-cash compensation awarded, paid or accrued by
us or Protalix Ltd. to each of our President and Chief Executive
Officer, our Executive Vice President, Research and Development,
our Senior Vice President, Product Development, our Vice
President and Chief Financial Officer and our Vice President,
Sales and Commercial Affairs, who we refer to collectively as
the “Named Executive Officers.” Our employment of Tzvi
Palash, our Chief Operating Officer, commenced in September
2010. Accordingly, he is not a Named Executive Officer for the
year ended December 31, 2010. There were no restricted
stock awards, long-term incentive plan payouts or other
compensation paid during fiscal years 2010, 2009 and 2008 by us
or Protalix Ltd. to the Named Executive Officers, except as set
forth below. All of the Named Executive Officers
82
are employees of our subsidiary, Protalix Ltd., except for
Ms. Lauterbach who is an employee of Protalix
BioTherapeutics, Inc. All currency amounts are expressed in
U.S. dollars.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nonqualified
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Equity
|
|
|
Deferred
|
|
|
|
|
|
|
|
Name and
|
|
|
|
|
|
|
|
|
|
|
Stock
|
|
|
Option
|
|
|
Incentive Plan
|
|
|
Compensation
|
|
|
All Other
|
|
|
|
|
Principal
|
|
|
|
|
Salary
|
|
|
Bonus
|
|
|
Award(s)
|
|
|
Award(s)
|
|
|
Compensation
|
|
|
Earnings
|
|
|
Compensation
|
|
|
Total
|
|
|
Position
|
|
Year
|
|
|
($)
|
|
|
($)
|
|
|
($)
|
|
|
($)
|
|
|
($)
|
|
|
($)
|
|
|
($)(1)
|
|
|
($)
|
|
|
|
|
David Aviezer, Ph.D., MBA
|
|
|
2010
|
|
|
|
508,868
|
|
|
|
—
|
|
|
|
|
|
|
|
145,537
|
|
|
|
|
|
|
|
|
|
|
|
107,972
|
|
|
|
762,377
|
|
|
President and
|
|
|
2009
|
|
|
|
427,970
|
|
|
|
500,000
|
|
|
|
|
|
|
|
529,951
|
|
|
|
|
|
|
|
|
|
|
|
62,167
|
|
|
|
1,520,088
|
|
|
Chief Executive Officer
|
|
|
2008
|
|
|
|
486,305
|
|
|
|
—
|
|
|
|
|
|
|
|
565,394
|
|
|
|
|
|
|
|
|
|
|
|
93,224
|
|
|
|
1,144,923
|
|
|
Yoseph Shaaltiel, Ph.D.
|
|
|
2010
|
|
|
|
292,095
|
|
|
|
100,000
|
|
|
|
|
|
|
|
70,286
|
|
|
|
|
|
|
|
|
|
|
|
76,491
|
|
|
|
538,872
|
|
|
Executive Vice President,
|
|
|
2009
|
|
|
|
196,271
|
|
|
|
160,000
|
|
|
|
|
|
|
|
225,336
|
|
|
|
|
|
|
|
|
|
|
|
37,981
|
|
|
|
619,588
|
|
|
Research and Development
|
|
|
2008
|
|
|
|
226,652
|
|
|
|
—
|
|
|
|
|
|
|
|
163,328
|
|
|
|
|
|
|
|
|
|
|
|
54,704
|
|
|
|
444,684
|
|
|
Einat Brill Almon, Ph.D.
|
|
|
2010
|
|
|
|
251,940
|
|
|
|
20,000
|
|
|
|
|
|
|
|
97,007
|
|
|
|
|
|
|
|
|
|
|
|
66,591
|
|
|
|
435,538
|
|
|
Senior Vice President,
|
|
|
2009
|
|
|
|
172,210
|
|
|
|
160,000
|
|
|
|
|
|
|
|
283,388
|
|
|
|
|
|
|
|
|
|
|
|
36,927
|
|
|
|
652,525
|
|
|
Product Development
|
|
|
2008
|
|
|
|
195,559
|
|
|
|
28,932
|
|
|
|
|
|
|
|
253,862
|
|
|
|
|
|
|
|
|
|
|
|
51,223
|
|
|
|
529,576
|
|
|
Yossi Maimon, CPA
|
|
|
2010
|
|
|
|
268,154
|
|
|
|
20,000
|
|
|
|
|
|
|
|
63,175
|
|
|
|
|
|
|
|
|
|
|
|
68,156
|
|
|
|
419,485
|
|
|
Vice President,
|
|
|
2009
|
|
|
|
186,478
|
|
|
|
160,000
|
|
|
|
|
|
|
|
253,030
|
|
|
|
|
|
|
|
|
|
|
|
41,051
|
|
|
|
640,559
|
|
|
Chief Financial Officer
|
|
|
2008
|
|
|
|
203,097
|
|
|
|
30,659
|
|
|
|
|
|
|
|
238,194
|
|
|
|
|
|
|
|
|
|
|
|
83,808
|
|
|
|
555,758
|
|
|
Sandra L. Lauterbach(2)
|
|
|
2010
|
|
|
|
186,410
|
|
|
|
—
|
|
|
|
|
|
|
|
405,562
|
|
|
|
|
|
|
|
|
|
|
|
62,248
|
|
|
|
654,220
|
|
|
Vice President, Sales and
|
|
|
2009
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
—
|
|
|
Commercial Affairs
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Includes employer contributions to pension and/or insurance
plans and other miscellaneous payments. |
| |
|
(2) |
|
Ms. Lauterbach joined our company in December 2009. |
The following table summarizes the grant of awards made to the
Named Executive Officers during 2010 as of December 31,
2010.
GRANTS OF
PLAN-BASED AWARDS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All
|
|
|
Option
|
|
|
|
|
|
Grant
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
|
|
|
Awards:
|
|
|
Exercise
|
|
|
Date Fair
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock
|
|
|
Number of
|
|
|
or Base
|
|
|
Value
|
|
|
|
|
|
|
|
Estimated Future Payouts Under
|
|
|
Estimated Future Payouts
|
|
|
Awards:
|
|
|
Securities
|
|
|
Price of
|
|
|
of Stock
|
|
|
|
|
|
|
|
Non-Equity Incentive Plan Awards
|
|
|
Under Equity Incentive Plan Awards
|
|
|
Number of
|
|
|
Underlying
|
|
|
Option
|
|
|
and Option
|
|
|
|
|
Grant
|
|
|
Threshold
|
|
|
Target
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares of
|
|
|
Options
|
|
|
Awards
|
|
|
Awards
|
|
Name
|
|
Date
|
|
|
($)
|
|
|
($)
|
|
|
Maximum($)
|
|
|
Threshold($)
|
|
|
Target($)
|
|
|
Maximum($)
|
|
|
Stock or Units (#)
|
|
|
(#) (1)
|
|
|
($/Sh) (2)
|
|
|
($)(3)
|
|
|
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
|
|
|
(h)
|
|
|
(i)
|
|
|
(j)
|
|
|
(k)
|
|
|
(l)
|
|
|
|
|
David Aviezer
|
|
|
Feb. 25, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
250,000
|
|
|
|
6.90
|
|
|
|
1,395,820
|
|
|
Yoseph Shaaltiel
|
|
|
Feb. 25, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
145,000
|
|
|
|
6.90
|
|
|
|
809,576
|
|
|
Einat Brill Almon
|
|
|
Feb. 25, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
130,000
|
|
|
|
6.90
|
|
|
|
725,826
|
|
|
Yossi Maimon
|
|
|
Feb. 25, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
130,000
|
|
|
|
6.90
|
|
|
|
725,826
|
|
|
Sandra L. Lauterbach
|
|
|
Feb. 7, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
160,000
|
|
|
|
6.81
|
|
|
|
739,623
|
|
|
|
|
|
(1) |
|
Represents outstanding options at December 31, 2010. |
| |
|
(2) |
|
Represents the range of the exercise price of the stock options. |
| |
|
(3) |
|
Represents the fair value as recorded on the grant date of the
stock options. |
83
Outstanding
Equity Awards at Fiscal Year-End
The following table sets forth information with respect to the
Named Executive Officers concerning equity awards as of
December 31, 2010.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Option Awards
|
|
|
Stock Awards
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Incentive
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plan
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity
|
|
|
Awards:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Incentive
|
|
|
Market or
|
|
|
|
|
|
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plan
|
|
|
Payout
|
|
|
|
|
|
|
|
|
|
|
Incentive
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Awards:
|
|
|
Value of
|
|
|
|
|
|
|
|
|
|
|
Plan
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
|
Unearned
|
|
|
|
|
|
|
|
|
|
|
Awards:
|
|
|
|
|
|
|
|
|
|
|
|
Market
|
|
|
Unearned
|
|
|
Shares,
|
|
|
|
|
Number
|
|
|
Number
|
|
|
Number
|
|
|
|
|
|
|
|
|
Number
|
|
|
Value of
|
|
|
Shares,
|
|
|
Units or
|
|
|
|
|
of Securities
|
|
|
of Securities
|
|
|
of Securities
|
|
|
|
|
|
|
|
|
of Shares or
|
|
|
Shares
|
|
|
Units or
|
|
|
Other
|
|
|
|
|
Underlying
|
|
|
Underlying
|
|
|
Underlying
|
|
|
|
|
|
|
|
|
Units
|
|
|
or Units
|
|
|
Other
|
|
|
Rights
|
|
|
|
|
Unexercised
|
|
|
Unexercised
|
|
|
Unexercised
|
|
|
Option
|
|
|
|
|
|
of Stock
|
|
|
of Stock
|
|
|
Rights
|
|
|
That
|
|
|
|
|
Options
|
|
|
Options
|
|
|
Unearned
|
|
|
Exercise
|
|
|
Option
|
|
|
That Have
|
|
|
That Have
|
|
|
That Have
|
|
|
Have Not
|
|
|
|
|
Exercisable
|
|
|
Unexercisable
|
|
|
Options
|
|
|
Price
|
|
|
Expiration
|
|
|
Not Vested
|
|
|
Not Vested
|
|
|
Not Vested
|
|
|
Vested
|
|
|
Name
|
|
(#)
|
|
|
(#)
|
|
|
(#)
|
|
|
($)
|
|
|
Date
|
|
|
(#)
|
|
|
($)
|
|
|
(#)
|
|
|
($)
|
|
|
|
|
David Aviezer
|
|
|
326,267
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0.120
|
|
|
|
8/1/2013
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
977,297
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0.972
|
|
|
|
9/10/2016
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
399,996
|
|
|
|
200,004
|
|
|
|
—
|
|
|
|
5.00
|
|
|
|
2/7/2018
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
100,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2.65
|
|
|
|
2/25/2019
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
250,000
|
|
|
|
|
|
|
|
6.90
|
|
|
|
2/25/2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yoseph Shaaltiel
|
|
|
122,162
|
(1)
|
|
|
—
|
|
|
|
—
|
|
|
|
0.001
|
|
|
|
6/30/2016
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
175,816
|
|
|
|
87,912
|
|
|
|
—
|
|
|
|
5.00
|
|
|
|
2/7/2018
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
50,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2.65
|
|
|
|
2/25/2019
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
145,000
|
|
|
|
|
|
|
|
6.90
|
|
|
|
2/25/2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Einat Brill Almon
|
|
|
116,848
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0.972
|
|
|
|
8/13/2016
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
207,520
|
|
|
|
103,752
|
|
|
|
|
|
|
|
5.00
|
|
|
|
2/7/2018
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
50,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2.65
|
|
|
|
2/25/2019
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
130,000
|
|
|
|
|
|
|
|
6.90
|
|
|
|
2/25/2020
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Yossi Maimon
|
|
|
75,964
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0.972
|
|
|
|
9/19/2016
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
116,668
|
|
|
|
58,332
|
|
|
|
|
|
|
|
5.00
|
|
|
|
2/7/2018
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
50,000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2.65
|
|
|
|
2/25/2019
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
130,000
|
|
|
|
|
|
|
|
6.90
|
|
|
|
2/25/2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sandra L. Lauterbach
|
|
|
|
|
|
|
160,000
|
|
|
|
|
|
|
|
6.81
|
|
|
|
2/7/2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Pursuant to a divorce settlement, Dr. Shaaltiel is required
to transfer 50% of these options to his former spouse. |
Option exercises during 2010 and vested stock awards for Named
Executive Officers as of December 31, 2010 were as follows:
OPTION
EXERCISES AND STOCK VESTED
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Option Awards
|
|
|
Stock Awards
|
|
|
|
|
Number of
|
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
Shares
|
|
|
Value
|
|
|
Shares
|
|
|
Value
|
|
|
|
|
Acquired on
|
|
|
Received on
|
|
|
Acquired on
|
|
|
Received on
|
|
|
|
|
Exercise
|
|
|
Exercise
|
|
|
Vesting
|
|
|
Vesting
|
|
|
Name (a)
|
|
(#)(b)
|
|
|
($)(c)
|
|
|
(#)(d)
|
|
|
($)(e)
|
|
|
|
|
David Aviezer
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Yoseph Shaaltiel
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Yossi Maimon(1)
|
|
|
140,000
|
|
|
|
68,040
|
|
|
|
—
|
|
|
|
—
|
|
|
Einat Brill Almon
|
|
|
120,000
|
|
|
|
113,919
|
|
|
|
—
|
|
|
|
—
|
|
|
Sandra L. Lauterbach
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
(1) |
|
Some of the options were exercised through net exercise with no
value received by our company in connection with the exercise. |
84
Potential
Payments upon Termination or
Change-in-Control
Each of our Named Executive Officers is entitled to be insured
by Protalix Ltd. under a Manager’s Policy in lieu of
severance upon termination. The intention of such Manager’s
Policies is to provide the Israel-based officers with severance
protection of one month’s salary for each year of
employment. We do not provide any change in control benefits to
our Named Executive Officers except that their stock option
agreements, as amended, provide that all of the outstanding
options of each Named Executive Officer are subject to
accelerated vesting immediately upon a change in control of our
company, as defined in our 2006 Stock Incentive Plan. If we had
experienced a change of control on December 31, 2010, the
value of the acceleration of the stock options held by each of
Dr. Aviezer, Dr. Shaaltiel, Dr. Brill Almon,
Mr. Maimon and Ms. Lauterbach would be
$1.8 million, $884,000, $917,000, $691,000 and $507,000,
respectively.
Employment
Arrangements
David Aviezer, Ph.D.,
MBA. Dr. Aviezer originally served as
Protalix Ltd.’s Chief Executive Officer on a consultancy
basis pursuant to a Consulting Services Agreement between
Protalix Ltd. and Agenda Biotechnology Ltd., a company
wholly-owned by Dr. Aviezer. On September 11, 2006,
Protalix Ltd. entered into an employment agreement with
Dr. Aviezer pursuant to which he agreed to be employed as
Protalix Ltd.’s President and Chief Executive Officer,
which agreement supersedes the Consultancy Services Agreement.
Dr. Aviezer currently serves as our President and Chief
Executive Officer. Dr. Aviezer’s current monthly base
salary is NIS 148,000 (approximately $40,360) and he is entitled
to an annual bonus at the Board’s discretion. The monthly
salary is subject to cost of living adjustments from time to
time. Dr. Aviezer is eligible to receive a substantial
bonus in the event of certain public offerings or acquisition
transactions, which bonus shall be at the discretion of the
Board, and certain specified bonuses in the event Protalix
achieves certain specified milestones. In connection with the
employment agreement, in addition to other options already held
by Dr. Aviezer granted to Dr. Aviezer options to
purchase 16,000 ordinary shares of Protalix Ltd. at an exercise
price equal to $59.40 per share, which we assumed as options to
purchase 977,297 shares of our common stock at $0.97 per
share. Such options vest quarterly retroactively from
June 1, 2006, over a four-year period. In addition, in 2008
we granted to Dr. Aviezer an option to purchase
600,000 shares of our common stock at an exercise price
equal to $5.00 per share. The option vests variably over a
five-year period that commenced on January 1, 2008. In
2009, we granted Dr. Aviezer an option to purchase
100,000 shares of our common stock at an exercise price
equal to $2.65 per share. As of December 31, 2009, all of
those options had fully vested. In 2010, we granted
Dr. Aviezer an option to purchase 250,000 shares of
our common stock at an exercise price equal to $6.90 per share,
which option vests quarterly over a three-year period commencing
upon FDA approval of taliglucerase alfa, if at all.
Dr. Aviezer’s employment agreement is terminable by
either party on 90 days’ written notice for any reason
and we may terminate the agreement for cause without notice.
Dr. Aviezer is entitled to be insured by Protalix Ltd.
under a Manager’s Policy in lieu of severance, company
contributions towards vocational studies, annual recreational
allowances, a company car and a company phone. Dr. Aviezer
is entitled to 24 working days of vacation. All stock options
that have not vested as of the date of termination shall be
deemed to have expired.
Yoseph
Shaaltiel, Ph.D. Dr. Shaaltiel founded
Protalix Ltd. in 1993 and currently serves as our Executive Vice
President, Research and Development. Dr. Shaaltiel entered
into an employment agreement with Protalix Ltd. on
September 1, 2001. Pursuant to the employment agreement,
his current monthly base salary is NIS 85,000 (approximately
$23,180) per month. The employment agreement is terminable by
Protalix Ltd. on 90 days’ written notice for any
reason and we may terminate the agreement for cause without
notice. In 2008 we granted to Dr. Shaaltiel an option to
purchase 263,728 shares of our common stock at an exercise
price equal to $5.00 per share. The option vests variably over a
five-year period that commenced on January 1, 2008. In
2009, we granted Dr. Shaaltiel an option to purchase
50,000 shares of our common stock at an exercise price
equal to $2.65 per share. As of December 31, 2009, all of
those options had fully vested. In 2010, we granted
Dr. Shaaltiel an option to purchase 145,000 shares of
our common stock at an exercise price equal to $6.90 per share,
which option vests quarterly over a three-year period commencing
upon FDA approval of taliglucerase alfa, if at all.
Dr. Shaaltiel is entitled to be insured by Protalix Ltd.
under a
85
Manager’s Policy in lieu of severance, company
contributions towards vocational studies, annual recreational
allowances, a company car and a company phone.
Dr. Shaaltiel is entitled to 24 working days of vacation.
Einat Brill Almon, Ph.D. Dr. Brill
Almon joined Protalix Ltd. on December 19, 2004 as its
Vice President, Product Development, pursuant to an
employment agreement effective on December 19, 2004 by and
between Protalix Ltd. and Dr. Brill Almon, and currently
serves as our Senior Vice President, Product Development.
Pursuant to the employment agreement, her current monthly base
salary is NIS 73,500 per month (approximately $20,044). She is
also entitled to certain specified bonuses in the event that
Protalix achieves certain specified clinical development
milestones within specified timelines. In connection with the
employment agreement, Protalix agreed to grant to Dr. Brill
Almon options to purchase 7,919 ordinary shares of Protalix Ltd.
at exercise prices equal to $24.36 and $59.40 per share, which
we assumed as options to purchase 483,701 shares of our
common stock at $0.40 and $0.97 per share. The options vest over
four years. In addition, in 2008 we granted to Dr. Brill
Almon an option to purchase 311,272 shares of our common
stock at an exercise price equal to $5.00 per share. The option
vests variably over a five-year period that commenced on
January 1, 2008. In 2009, we granted to Dr. Brill
Almon an option to purchase 50,000 shares of our common
stock at an exercise price equal to $2.65 per share. As of
December 31, 2009, all of those options had fully vested.
In 2010, we granted Dr. Almon an option to purchase
130,000 shares of our common stock at an exercise price
equal to $6.90 per share, which option vests quarterly over a
three-year period commencing upon FDA approval of taliglucerase
alfa, if at all. The employment agreement is terminable by
either party on 60 days’ written notice for any reason
and we may terminate the agreement for cause without notice.
Dr. Brill Almon is entitled to be insured by Protalix Ltd.
under a Manager’s Policy in lieu of severance, company
contributions towards vocational studies, annual recreational
allowances, a company car and a company phone at up to NIS 1,000
per month. Dr. Brill Almon is entitled to 22 working days
of vacation. All stock options that have not vested as of the
date of termination shall be deemed to have expired.
Yossi Maimon, CPA. Mr. Maimon joined
Protalix Ltd. as its Chief Financial Officer pursuant to an
employment agreement effective as of October 15, 2006 by
and between Protalix Ltd. and Mr. Maimon and currently
serves as our Chief Financial Officer. Pursuant to the
employment agreement, his current monthly base salary is NIS
73,500 (approximately $20,044) and Mr. Maimon is entitled
to an annual discretionary bonus and additional discretionary
bonuses in the event Protalix achieves significant financial
milestones, subject to the Board’s sole discretion. The
monthly salary is subject to cost of living adjustments from
time to time. In connection with the employment agreement,
Protalix agreed to grant to Mr. Maimon options to purchase
10,150 ordinary shares of Protalix Ltd. at an exercise price
equal to $59.40 per share, which we assumed as options to
purchase 619,972 shares of our common stock at $0.97 per
share. The first 25% of such options shall vest on the first
anniversary of the grant date and the remainder shall vest
quarterly in 12 equal increments. In addition, in 2008 we
granted to Mr. Maimon an option to purchase
175,000 shares of our common stock at an exercise price
equal to $5.00 per share. The option vests variably over a
five-year period that commenced on January 1, 2008. In
2009, we granted to Mr. Maimon an option to purchase
50,000 shares of our common stock at an exercise price
equal to $2.65 per share. As of December 31, 2009, all of
those options had fully vested. In 2010, we granted
Mr. Maimon an option to purchase 130,000 shares of our
common stock at an exercise price equal to $6.90 per share,
which option vests quarterly over a three-year period commencing
upon FDA approval of taliglucerase alfa, if at all. The
employment agreement is terminable by either party on
60 days’ written notice for any reason and we may
terminate the agreement for cause without notice.
Mr. Maimon is entitled to be insured by Protalix Ltd. under
a Manager’s Policy in lieu of severance, company
contributions towards vocational studies, annual recreational
allowances, a company car and a company phone. Mr. Maimon
is entitled to 24 working days of vacation. All stock options
that have not vested as of the date of termination shall be
deemed to have expired.
Sandra L. Lauterbach. Ms. Lauterbach
joined our company as our Vice President, Sales and Commercial
Affairs, pursuant to an employment agreement effective
December 18, 2009. Pursuant to the employment agreement,
Ms. Lauterbach’s annual base salary is $180,000 and we
may elect to pay her an annual discretionary bonus in an amount
and based upon criteria determined by either the Compensation
Committee of our Board of Directors, or the entire Board of
Directors, at their sole discretion. She is also entitled to
certain health care insurance benefits and contributions to
retirement plans, and allowances for car and cell
86
phone expenses. In connection with the employment agreement, the
Board of Directors granted to Ms. Lauterbach stock options
to purchase 160,000 shares of our common stock at an
exercise price equal to $6.81. The options vest over a four-year
period, with 25% of the options vesting upon the lapse of one
year from the date of grant and the remainder of the options
vesting on a quarterly basis in 12 equal installments,
commencing on the initial vesting date. The unvested portion of
the option will vest automatically upon a change of control of
our company. The employment agreement is terminable by either
party with 60 days’ written notice for any reason and
we may terminate the agreement for cause without notice.
2006
Stock Incentive Plan
Our Board of Directors and a majority of our shareholders
approved our 2006 Stock Incentive Plan on December 14, 2006
and cancelled our 1998 stock option plan (no options were
outstanding under the 1998 plan at that time). We have reserved
9,741,655 shares of our common stock for issuance, in the
aggregate, under the 2006 Stock Incentive Plan, subject to
adjustment for a stock split or any future stock dividend or
other similar change in our common stock or our capital
structure. As of December 31, 2010, options to acquire
3,064 shares of common stock remain available to be granted
under our 2006 Stock Incentive Plan.
Our 2006 Stock Incentive Plan provides for the grant of stock
options, restricted stock, restricted stock units, stock
appreciation rights and dividend equivalent rights, collectively
referred to as “awards.” Stock options granted under
the 2006 Stock Incentive Plan may be either incentive stock
options under the provisions of Section 422 of the Internal
Revenue Code, or non-qualified stock options. Incentive stock
options may be granted only to employees. Awards other than
incentive stock options may be granted to employees, directors
and consultants.
The 2006 Stock Incentive Plan is also designed to comply with
the provisions of the Israeli Income Tax Ordinance New Version,
1961 (including as amended pursuant to Amendment 132 thereto)
(the “tax ordinance”) and is intended to enable us to
grant awards to grantees who are Israeli residents as follows:
(i) awards to employees pursuant to Section 102 of the
tax ordinance; and (ii) awards to non-employees pursuant to
Section 3(I) of the tax ordinance. For this purpose,
“employee” refers only to employees, office holders
and directors of our company or a related entity excluding those
who are considered “Controlling Shareholders” pursuant
to, or otherwise excluded by, the tax ordinance. In accordance
with the terms and conditions imposed by the Tax Ordinance,
grantees who receive awards under the 2006 Stock Incentive Plan
may be afforded certain tax benefits in Israel as described
below.
Our Board of Directors or the Compensation Committee, referred
to as the “plan administrator,” will administer our
2006 Stock Incentive Plan, including selecting the grantees,
determining the number of shares to be subject to each award,
determining the exercise or purchase price of each award, and
determining the vesting and exercise periods of each award.
The exercise price of stock options granted under the 2006 Stock
Incentive Plan must be equal to at least 100% of the fair market
value of our common stock on the date of grant; however, in
certain circumstances, grants may be made at a lower price to
Israeli grantees who are residents of the State of Israel. If,
however, incentive stock options are granted to an employee who
owns stock possessing more than 10% of the voting power of all
classes of our stock or the stock of any parent or subsidiary of
our company, the exercise price of any incentive stock option
granted must equal at least 110% of the fair market value on the
grant date and the maximum term of these incentive stock options
must not exceed five years. The maximum term of all other awards
must not exceed 10 years (or five years in the case of an
incentive stock option granted to any participant who owns stock
representing more than 10% of the voting power of all classes of
our stock or the stock of any parent or subsidiary of our
company). The plan administrator will determine the exercise or
purchase price (if any) of all other awards granted under the
2006 Stock Incentive Plan.
Under the 2006 Stock Incentive Plan, incentive stock options and
options to Israeli grantees may not be sold, pledged, assigned,
hypothecated, transferred or disposed of in any manner other
than by will or by the laws of descent or distribution and may
be exercised during the lifetime of the participant only by the
participant. Other awards shall be transferable by will or by
the laws of descent or distribution and to the extent and in the
manner authorized by the plan administrator by gift or pursuant
to a domestic relations order
87
to members of the participant’s immediate family. The 2006
Stock Incentive Plan permits the designation of beneficiaries by
holders of awards, including incentive stock options.
If the service of a participant in the 2006 Stock Incentive Plan
is terminated for any reason other than cause, the participant
may exercise awards that were vested as of the termination date
for a period ending upon the earlier of 12 months from the
date of termination (or such shorter or longer period set forth
in the award agreement) or the expiration date of the awards
unless otherwise determined by the plan administrator. If the
service of a participant in the 2006 Stock Incentive Plan is
terminated for cause, the participant may exercise awards that
were vested as of the termination date for a period ending upon
the earlier of 14 days from the date of termination (or
such shorter or longer period set forth in the award agreement)
or the expiration date of the awards unless otherwise determined
by the plan administrator.
In the event of a corporate transaction, all awards will
terminate unless assumed by the successor corporation. Unless
otherwise provided in a participant’s award agreement, in
the event of a corporate transaction and with respect to the
portion of each award that is assumed or replaced, then such
portion will automatically become fully vested and exercisable
immediately upon termination of a participant’s service if
the participant is terminated by the successor company or us
without cause within 12 months after the corporate
transaction. With respect to the portion of each award that is
not assumed or replaced, such portion will automatically become
fully vested and exercisable immediately prior to the effective
date of the corporate transaction so long as the
participant’s service has not been terminated prior to such
date.
In the event of a change in control, except as otherwise
provided in a participant’s award agreement, following a
change in control (other than a change in control that also is a
corporate transaction) and upon the termination of a
participant’s service without cause within 12 months
after a change in control, each award of such participant that
is outstanding at such time will automatically become fully
vested and exercisable immediately upon the participant’s
termination. In addition, the stock options issued to each of
our Named Executive Officers are subject to accelerated vesting
immediately upon a change in control of our company, as defined
in our 2006 Stock Incentive Plan.
Under our 2006 Stock Incentive Plan, a corporate transaction is
generally defined as:
|
|
|
| |
•
|
a merger or consolidation in which we are not the surviving
entity, except for the principal purpose of changing our
company’s state of incorporation;
|
| |
| |
•
|
the sale, transfer or other disposition of all or substantially
all of our assets;
|
| |
| |
•
|
the complete liquidation or dissolution of our company;
|
| |
| |
•
|
any reverse merger in which we are the surviving entity but our
shares of common stock outstanding immediately prior to such
merger are converted or exchanged by virtue of the merger into
other property, whether in the form of securities, cash or
otherwise, or in which securities possessing more than forty
percent (40%) of the total combined voting power of our
outstanding securities are transferred to a person or persons
different from those who held such securities immediately prior
to such merger; or
|
| |
| |
•
|
acquisition in a single or series of related transactions by any
person or related group of persons of beneficial ownership of
securities possessing more than fifty percent (50%) of the total
combined voting power of our outstanding securities but
excluding any such transaction or series of related transactions
that the plan administrator determines not to be a corporate
transaction (provided however that the plan administrator shall
have no discretion in connection with a corporate transaction
for the purchase of all or substantially all of our shares
unless the principal purpose of such transaction is changing our
company’s state of incorporation).
|
Under our 2006 Stock Incentive Plan, a change of control is
defined as:
|
|
|
| |
•
|
the direct or indirect acquisition by any person or related
group of persons of beneficial ownership of securities
possessing more than fifty percent (50%) of the total combined
voting power of our outstanding securities pursuant to a tender
or exchange offer made directly to our shareholders and
|
88
|
|
|
| |
|
which a majority of the members of our board (who have generally
been on our board for at least 12 months) who are not
affiliates or associates of the offeror do not recommend
shareholders accept the offer; or
|
|
|
|
| |
•
|
a change in the composition of our board over a period of
12 months or less, such that a majority of our board
members ceases, by reason of one or more contested elections for
board membership, to be comprised of individuals who were
previously directors of our company.
|
Unless terminated sooner, the 2006 Stock Incentive Plan will
automatically terminate in 2016. Our Board of Directors has the
authority to amend, suspend or terminate our 2006 Stock
Incentive Plan. No amendment, suspension or termination of the
2006 Stock Incentive Plan shall adversely affect any rights
under awards already granted to a participant. To the extent
necessary to comply with applicable provisions of federal
securities laws, state corporate and securities laws, the
Internal Revenue Code, the rules of any applicable stock
exchange or national market system, and the rules of any
non-U.S. jurisdiction
applicable to awards granted to residents therein (including the
Tax Ordinance), we shall obtain shareholder approval of any such
amendment to the 2006 Stock Incentive Plan in such a manner and
to such a degree as required.
Impact
of Israeli Tax Law
The awards granted to employees pursuant to Section 102 of
the Tax Ordinance under the 2006 Stock Incentive Plan may be
designated by us as approved options under the capital gains
alternative, or as approved options under the ordinary income
tax alternative.
To qualify for these benefits, certain requirements must be met,
including registration of the options in the name of a trustee.
Each option, and any shares of common stock acquired upon the
exercise of the option, must be held by the trustee for a period
commencing on the date of grant and deposit into trust with the
trustee and ending 24 months thereafter.
Under the terms of the capital gains alternative, we may not
deduct expenses pertaining to the options for tax purposes.
Under the 2006 Stock Incentive Plan, we may also grant to
employees options pursuant to Section 102(c) of the Tax
Ordinance that are not required to be held in trust by a
trustee. This alternative, while facilitating immediate exercise
of vested options and sale of the underlying shares, will
subject the optionee to the marginal income tax rate of up to
50% as well as payments to the National Insurance Institute and
health tax on the date of the sale of the shares or options.
Under the 2006 Stock Incentive Plan, we may also grant to
non-employees options pursuant to Section 3(I) of the Tax
Ordinance. Under that section, the income tax on the benefit
arising to the optionee upon the exercise of options and the
issuance of common stock is generally due at the time of
exercise of the options.
These options shall be further subject to the terms of the tax
ruling that has been obtained by Protalix Ltd. from the Israeli
tax authorities in connection with the merger. Under the tax
ruling, the options issued by us in connection with the
assumption of Section 102 options previously issued by
Protalix Ltd. under the capital gains alternative shall be
issued to a trustee, shall be designated under the capital gains
alternative and the issuance date of the original options shall
be deemed the issuance date for the assumed options for the
calculation of the respective holding period.
89
Compensation
of Directors
The following table sets forth information with respect to
compensation of our non-employee directors during fiscal year
2010. The fees to our current directors were paid by Protalix
Ltd. Prior to January 1, 2007, Protalix Ltd. compensated
only certain of its directors, which compensation was limited to
the granting of options under its employee stock option plan.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fees
|
|
|
|
|
|
Non-Equity
|
|
Nonqualified
|
|
|
|
|
|
|
|
Earned
|
|
|
|
|
|
Incentive
|
|
Deferred
|
|
All
|
|
|
|
|
|
or Paid
|
|
Stock
|
|
Option
|
|
Plan
|
|
Compensation
|
|
Other
|
|
|
|
|
|
in Cash
|
|
Award
|
|
Awards
|
|
Compensation
|
|
Earnings
|
|
Compensation
|
|
Total
|
|
Name
|
|
($)
|
|
($)
|
|
($)
|
|
($)
|
|
($)
|
|
($)
|
|
($)
|
|
|
|
Current Directors
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Eli Hurvitz(1)
|
|
|
6,297
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6,297
|
|
|
Alfred Akirov
|
|
|
33,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
33,000
|
|
|
Amos Bar Shalev
|
|
|
50,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
50,000
|
|
|
Zeev Bronfeld
|
|
|
33,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
33,000
|
|
|
Yodfat Harel Gross
|
|
|
33,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
33,000
|
|
|
Roger D. Kornberg
|
|
|
33,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
33,000
|
|
|
Eyal Sheratzky
|
|
|
33,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
33,000
|
|
|
|
|
|
(1) |
|
Represents amounts paid to Pontifax Management Company, Ltd.
pursuant to a management consulting agreement. Mr. Hurvitz
resigned from our Board of Directors in March 2010. |
Our Board of Directors will review director compensation
annually and adjust it according to then current market
conditions and corporate governance guidelines.
Compensation
Committee Interlocks and Insider Participation
Our Compensation Committee currently consists of
Messrs. Akirov and Bar Shalev and Ms. Harel Gross, who
were appointed to the Committee during 2009. No member of our
Compensation Committee or any executive officer of our company
or of Protalix Ltd. has a relationship that would constitute an
interlocking relationship with executive officers or directors
of another entity. No Compensation Committee member is or was an
officer or employee of ours or of Protalix Ltd. Further, none of
our executive officers serves on the board of directors or
compensation committee of any entity that has one or more
executive officers serving as a member of our Board of Directors
or Compensation Committee.
|
|
|
Item 12.
|
Security
Ownership of Certain Beneficial Owners and Management and
Related Stockholder Matters
|
Security
Ownership of Certain Beneficial Owners and Management
The following table sets forth information, as of
February 15, 2011, regarding beneficial ownership of our
common stock:
|
|
|
| |
•
|
each person who is known by us to own beneficially more than 5%
of our common stock;
|
| |
| |
•
|
each director;
|
| |
| |
•
|
each of our Chief Executive Officer, our Executive Vice
President, Research and Development, our Senior Vice President, Product
Development, our Chief Financial Officer, our Vice President,
Sales and Commercial Affairs and our Chief Operating
Officer; and
|
| |
| |
•
|
all of our directors and executive officers collectively.
|
Unless otherwise noted, we believe that all persons named in the
table have sole voting and investment power with respect to all
shares of our common stock beneficially owned by them. For
purposes of these tables, a person is deemed to be the
beneficial owner of securities that can be acquired by such
person within 60 days from February 15, 2011 upon
exercise of options, warrants and convertible securities. Each
beneficial
90
owner’s percentage ownership is determined by assuming that
options, warrants and convertible securities that are held by
such person (but not those held by any other person) and that
are exercisable within such 60 days from such date have
been exercised. The information set forth below is based upon
information obtained from the beneficial owners, upon
information in our possession regarding their respective
holdings and upon information filed by the holders with the SEC.
The percentages of beneficial ownership are based on
81,328,699 shares of our common stock outstanding as of
February 15, 2011.
The address for all directors and officers is
c/o Protalix
BioTherapeutics, Inc., 2 Snunit Street, Science Park, POB 455,
Carmiel, Israel, 20100.
| |
|
|
|
|
|
|
|
|
|
|
|
Amount and Nature
|
|
|
|
|
|
of Beneficial
|
|
Percentage of
|
|
Name and Address of Beneficial Owner
|
|
Ownership
|
|
Class
|
|
|
|
Board of Directors and Executive Officers
|
|
|
|
|
|
|
|
|
|
David Aviezer, Ph.D., MBA(1)
|
|
|
1,836,895
|
|
|
|
2.3
|
%
|
|
Yoseph Shaaltiel, Ph.D.(2)
|
|
|
1,076,384
|
|
|
|
1.3
|
|
|
Alfred Akirov(3)
|
|
|
6,186,046
|
|
|
|
7.6
|
|
|
Amos Bar Shalev(4)
|
|
|
1,680
|
|
|
|
*
|
|
|
Zeev Bronfeld(5)
|
|
|
14,466,319
|
|
|
|
17.8
|
|
|
Yodfat Harel Gross
|
|
|
—
|
|
|
|
—
|
|
|
Roger D. Kornberg, Ph.D.(6)
|
|
|
37,500
|
|
|
|
*
|
|
|
Eyal Sheratzky
|
|
|
—
|
|
|
|
—
|
|
|
Einat Brill Almon, Ph.D.(7)
|
|
|
391,660
|
|
|
|
*
|
|
|
Yossi Maimon(8)
|
|
|
252,353
|
|
|
|
*
|
|
|
Sandra E. Lauterbach(9)
|
|
|
—
|
|
|
|
—
|
|
|
Tzvi Palash(10)
|
|
|
—
|
|
|
|
—
|
|
|
All executive officers and directors as a group
(12 persons)(11) 5% Holders
|
|
|
24,248,837
|
|
|
|
29.8
|
|
|
Biocell Ltd.(12)
|
|
|
14,466,319
|
|
|
|
17.8
|
|
|
Techno-Rov Holdings (1993) Ltd.(13)
|
|
|
6,186,046
|
|
|
|
7.6
|
|
|
Baillie Gifford & Co.(14)
|
|
|
5,828,161
|
|
|
|
7.2
|
|
|
|
|
|
* |
|
less than 1%. |
| |
|
(1) |
|
Consists of 1,836,895 shares of our common stock issuable
upon exercise of outstanding options within 60 days of
February 15, 2011. Does not include 416,669 shares of
common stock issuable upon exercise of outstanding options that
are not exercisable within 60 days of February 15,
2011. |
| |
|
(2) |
|
Consists of 763,754 shares of our common stock held by
Dr. Shaaltiel and 312,630 shares of our common stock
issuable upon exercise of outstanding options within
60 days of February 15, 2011. Does not include
268,260 shares of common stock issuable upon exercise of
outstanding options that are not exercisable within 60 days
of February 15, 2011. |
| |
|
(3) |
|
Consists of 6,186,046 shares of our common stock held by
Techno-Rov Holdings (1993) Ltd. Mr. Akirov is the
Chief Executive Officer of Techno-Rov Holdings and has the power
to control its investment decisions. |
| |
|
(4) |
|
Mr. Bar Shalev is the Manager of Techno-Rov Holdings. |
| |
|
(5) |
|
Consists of 14,466,319 shares of our common stock held by
Biocell Ltd. Mr. Bronfeld is a director and Chief Executive
Officer of Biocell. Mr. Bronfeld disclaims beneficial
ownership of these shares except to the extent of his pecuniary
interest therein. |
| |
|
(6) |
|
Consists of 37,500 shares of our common stock issuable upon
exercise of outstanding options within 60 days of
February 15, 2011. Does not include 12,500 shares of
common stock issuable upon exercise of outstanding options that
are not exercisable within 60 days of February 15,
2011. |
91
|
|
|
|
(7) |
|
Consists of 391,660 shares of our common stock issuable
upon exercise of outstanding options within 60 days of
February 15, 2011. Does not include 216,460 shares of
common stock issuable upon exercise of outstanding options that
are not exercisable within 60 days of February 15,
2011. |
| |
|
(8) |
|
Consists of 252,353 shares of our common stock issuable
upon exercise of outstanding options within 60 days of
February 15, 2011. Does not include 178,611 shares of
common stock issuable upon exercise of outstanding options that
are not exercisable within 60 days of February 15,
2011. |
| |
|
(9) |
|
Does not include 160,000 shares of common stock issuable
upon exercise of outstanding options that are not exercisable
within 60 days of February 15, 2011. |
| |
|
(10) |
|
Does not include 160,000 shares of common stock issuable
upon exercise of outstanding options that are not exercisable
within 60 days of February 15, 2011. |
| |
|
(11) |
|
Consists of 21,417,799 shares of our common stock and
2,831,038 shares of our common stock issuable upon exercise
of outstanding options within 60 days of February 15,
2011. Does not include 1,412,500 shares of common stock
issuable upon exercise of outstanding options that are not
exercisable within 60 days of February 15, 2011. |
| |
|
(12) |
|
The address is Moshe Aviv Tower, 7 Jabotinsky Street, Ramat Gan,
Israel. Biocell Ltd.’s investment and voting decisions are
made collectively by its board of directors. |
| |
|
(13) |
|
The address is Alrov Tower, 46 Rothschild Blvd., Tel Aviv,
Israel 66883. Mr. Akirov is the Chief Executive Officer of
Techno-Rov Holdings (1993) Ltd. and has the power to
control its investment decisions. |
| |
|
(14) |
|
Based solely on a Schedule 13G filed by Baillie
Gifford & Co. on January 25, 2011. According to
Baillie Gifford & Co., it has sole dispositive power
over 5,828,161 shares of common stock and sole voting power
over 3,369,061 shares of common stock. |
Equity
Compensation Plan Information
The following table provides information as of December 31,
2010 with respect to the shares of our common stock that may be
issued under our existing equity compensation plan.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A
|
|
B
|
|
C
|
|
|
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
Securities
|
|
|
|
|
|
|
|
Remaining
|
|
|
|
|
|
|
|
Available for
|
|
|
|
|
|
|
|
Future Issuance
|
|
|
|
Number of
|
|
Weighted
|
|
Under Equity
|
|
|
|
Securities to be
|
|
Average
|
|
Compensation
|
|
|
|
Issued
|
|
Exercise
|
|
Plans (Excluding
|
|
|
|
Upon Exercise of
|
|
Price of
|
|
Securities
|
|
|
|
Outstanding
|
|
Outstanding
|
|
Reflected in
|
|
Plan Category
|
|
Options
|
|
Options
|
|
Column A)
|
|
|
|
Equity Compensation Plans Approved by Shareholders
|
|
|
7,174,805
|
|
|
$
|
3.16
|
|
|
|
3,064
|
|
|
Equity Compensation Plans Not Approved by Shareholders
|
|
|
631,866
|
|
|
$
|
10.24
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
7,806,671
|
|
|
$
|
3.73
|
|
|
|
3,064
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Item 13.
|
Certain
Relationships and Related Transactions, and Director
Independence
|
On March 17, 2005, Protalix Ltd. entered into a Management
Services Agreement with Pontifax Management Company, Ltd. in
connection with the purchase of Protalix’s Series B
Preferred Shares by the Pontifax Funds. Pursuant to the
Management Services Agreement, Mr. Hurvitz served as a
member of our Board of Directors and later as the Chairman of
our Board of Directors until his resignation in March 2010. In
consideration for Mr. Hurvitz’s services, Protalix was
required to pay Pontifax Management Company a fee equal to
$3,000 per month plus required taxes on such payment. In
addition, in connection with the execution of the Management
Services Agreement and the later appointment of Mr. Hurvitz
as Chairman of our Board of Directors, Protalix issued to
Pontifax, in the aggregate, a number of options that, upon our
December 2006
92
merger transaction, were converted into options to purchase
3,384,502 shares of our common stock. Under the terms of
the assumed Management Services Agreement, we are obligated only
to use our best efforts to nominate Mr. Hurvitz for
election to our Board of Directors, which remains subject to the
review and approval of the Nominating Committee of the Board of
Directors and the entire Board of Directors, as applicable.
During the year 2010 through the date of Mr. Hurvitz’s
resignation, the fee payable under this agreement was increased
to $33,000 per annum, which is the same fee payable to the other
non-executive directors. No further payments were due to
Pontifax Management Company after Mr. Hurvitz’s
resignation from our Board of Directors.
On September 14, 2006, Protalix Ltd. entered into a
collaboration and licensing agreement with Teva for the
development and manufacture of two proteins using
ProCellExtm,
our proprietary protein expression system. Mr. Hurvitz, our
former Chairman, was the chairman of Teva’s board of
directors when we entered into the agreement. Phillip
Frost, M.D., a former director and a major shareholder of
our company, is the chairman of Teva’s board of directors
and Professor Kornberg, a member of our Board of Directors also
serves as a member of Teva’s board of directors. The
agreement provides that we will collaborate with Teva on the
research and development of two proteins using ProCellEx. We and
Teva identified two proteins for research and development
activities under the agreement, but in 2009 both of the projects
were terminated for commercial reasons. Other elements of our
collaboration with Teva are currently ongoing.
All related party transactions are reviewed and approved by the
Audit Committee, as required by the Audit Committee Charter.
Corporate
Governance and Independent Directors
In compliance with the listing requirements of the NYSE Amex, we
have a comprehensive plan of corporate governance for the
purpose of defining responsibilities, setting high standards of
professional and personal conduct and assuring compliance with
such responsibilities and standards. We currently regularly
monitor developments in the area of corporate governance to
ensure we are in compliance with the standards and regulations
required by the NYSE Amex. A summary of our corporate governance
measures follows.
Independent
Directors
We believe a majority of the members of our Board of Directors
are independent from management. When making determinations from
time to time regarding independence, the Board of Directors will
reference the listing standards adopted by the NYSE Amex as well
as the independence standards set forth in the Sarbanes-Oxley
Act of 2002 and the rules and regulations promulgated by the SEC
under that Act. In particular, our Audit Committee periodically
evaluates and reports to the Board of Directors on the
independence of each member of the Board. We anticipate our
audit committee will analyze whether a director is independent
by evaluating, among other factors, the following:
|
|
|
| |
•
|
Whether the member of the Board of Directors has any material
relationship with us, either directly, or as a partner,
shareholder or officer of an organization that has a
relationship with us;
|
| |
| |
•
|
Whether the member of the Board of Directors is a current
employee of our company or any of our subsidiaries, or was an
employee of our company or any of our subsidiaries within three
years preceding the date of determination;
|
| |
| |
•
|
Whether the member of the Board of Directors is, or in the three
years preceding the date of determination has been, affiliated
with or employed by (i) a present internal or external
auditor of our company or any affiliate of such auditor or
(ii) any former internal or external auditor of our company
or any affiliate of such auditor, which performed services for
us within three years preceding the date of determination;
|
| |
| |
•
|
Whether the member of the Board of Directors is, or in the three
years preceding the date of determination has been, part of an
interlocking directorate, in which any of our executive officers
serve on the Compensation Committee of another company that
concurrently employs the member as an executive officer;
|
93
|
|
|
| |
•
|
Whether the member of the Board of Directors receives any
compensation from us, other than fees or compensation for
service as a member of the Board of Directors and any committee
of the Board of Directors and reimbursement for reasonable
expenses incurred in connection with such service and for
reasonable educational expenses associated with Board of
Directors or committee membership matters;
|
| |
| |
•
|
Whether an immediate family member of the member of the Board of
Directors is a current executive officer of our company or was
an executive officer of our company within three years preceding
the date of determination;
|
| |
| |
•
|
Whether an immediate family member of the member of the Board of
Directors is, or in the three years preceding the date of
determination has been, affiliated with or employed in a
professional capacity by (i) a present internal or external
auditor of ours or any of our affiliates or (ii) any former
internal or external auditor of our company or any affiliate of
ours which performed services for us within three years
preceding the date of determination; and
|
| |
| |
•
|
Whether an immediate family member of the member of the Board of
Directors is, or in the three years preceding the date of
determination has been, part of an interlocking directorate, in
which any of our executive officers serve on the Compensation
Committee of another company that concurrently employs the
immediate family member of the member of the Board of Directors
as an executive officer.
|
The above list is not exhaustive and we anticipate that the
Audit Committee will consider all other factors which could
assist it in its determination that a director will have no
material relationship with us that could compromise that
director’s independence.
Under these standards, our Board of Directors has determined
that Messrs. Akirov and Bar Shalev and Ms. Harel Gross
are considered “independent” pursuant to the rules of
the NYSE Amex and Section 10A(m)(3) of the Securities
Exchange Act of 1934, as amended. In addition, our Board of
Directors has determined that at least two of these directors
are able to read and understand fundamental financial statements
and have substantial business experience that results in their
financial sophistication, qualifying them for membership on any
audit committee we form. Our Board of Directors has also
determined that Messrs. Akirov, Bar Shalev, Bronfeld and
Sheratzky, Ms. Harel Gross and Dr. Kornberg are
“independent” pursuant to the rules of the NYSE Amex.
The position of chairman of the board is not held by our chief
executive officer at this time. The Board of Directors does not
have a policy mandating the separation of these functions. We
believe it is in our best interest that Mr. Bronfeld serve
as the interim chairman of the board. This decision was based on
Mr. Bronfeld’s experience in the healthcare industry
in Israel and globally and his years of experience serving on
the board of directors of many public and private companies. Our
non-management directors hold formal meetings, separate from
management, at least twice per year. We have no formal policy
regarding attendance by our directors at annual shareholders
meetings, although we encourage such attendance and anticipate
most of our directors will attend these meetings.
Messrs. Bronfeld, Bar Shalev, Akirov, Aviezer and
Shaaltiel, and Ms. Harel Gross, attended our 2010 annual
meeting of shareholders. Mr. Hurvitz resigned from our
Board of Directors in March 2010.
The
Board’s Role in Risk Oversight
Our Board of Directors oversees an enterprise-wide approach to
risk management, designed to support the achievement of business
objectives, including organizational and strategic objectives,
to improve long-term organizational performance and enhance
shareholder value. The involvement of our Board of Directors in
setting our business strategy is a key part of its assessment of
management’s plans for risk management and its
determination of what constitutes an appropriate level of risk
for the company. The participation of our Board of Directors in
our risk oversight process includes receiving regular reports
from members of senior management on areas of material risk to
our company, including operational, financial, legal and
regulatory, and strategic and reputational risks. While the full
board has the ultimate oversight responsibility for the risk
management process, various committees of the board also have
responsibility for risk management. For example, financial
risks, including internal controls, are overseen by the audit
committee and risks that may be
94
implicated by our executive compensation programs are overseen
by the compensation committee. Upon identification of a risk,
the assigned board committee or our full Board of Directors
discuss or review risk management and risk mitigation
strategies. Additional review or reporting on enterprise risks
is conducted as needed or as requested by our Board of Directors
or a committee thereof.
|
|
|
Item 14.
|
Principal
Accountant Fees and Services
|
The following table sets forth fees billed to us by our
independent registered public accounting firm during the fiscal
years ended December 31, 2010 and 2009 for:
(i) services rendered for the audit of our annual financial
statements and the review of our quarterly financial statements;
(ii) services by our independent registered public
accounting firm that are reasonably related to the performance
of the audit or review of our financial statements and that are
not reported as Audit Fees; (iii) services rendered in
connection with tax compliance, tax advice and tax planning; and
(iv) all other fees for services rendered.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
|
2010
|
|
2009
|
|
|
|
|
|
Audit Fees
|
|
$
|
289,740
|
|
|
$
|
259,000
|
|
|
|
|
|
|
Audit Related Fees
|
|
$
|
28,353
|
|
|
$
|
78,039
|
|
|
|
|
|
|
Tax Fees
|
|
$
|
63,008
|
|
|
$
|
197,282
|
|
|
|
|
|
|
All Other Fees
|
|
$
|
—
|
|
|
$
|
—
|
|
|
|
|
|
Policy on
Audit Committee Pre-Approval of Audit and Permissible Non-Audit
Services of Independent Auditors
Prior to entering into the engagement letter with our
independent registered accountants, our Audit Committee approved
the 2010 audit fees. For fiscal year 2011, our Audit Committee
has approved fees for certain services to be rendered by our
independent registered accounting firm.
PART IV
|
|
|
Item 15.
|
Exhibits
and Financial Statement Schedules
|
The following documents are filed as part of this Annual Report
on
Form 10-K:
1. Financial Statements. The following Consolidated
Financial Statements of Protalix BioTherapeutics, Inc. are
included in Item 8 of this Annual Report on
Form 10-K:
| |
|
|
|
|
|
|
|
Page
|
|
|
|
|
|
|
F-2
|
|
|
|
|
|
F-3
|
|
|
|
|
|
F-4
|
|
|
|
|
|
F-5
|
|
|
|
|
|
F-6
|
|
|
|
|
|
F-7
|
|
2. Financial Statement Schedule. Financial statement
schedules have been omitted since they are either not required,
are not applicable or the required information is shown in the
consolidated financial statements or related notes.
95
3. Exhibits.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Incorporated by Reference
|
|
|
Exhibit
|
|
|
|
|
|
File
|
|
|
|
|
|
Filed
|
|
Number
|
|
Exhibit Description
|
|
Form
|
|
Number
|
|
Exhibit
|
|
Date
|
|
Herewith
|
|
|
|
|
3
|
.1
|
|
Amended and Restated Articles of Incorporation of the Company
|
|
S-4
|
|
333-48677
|
|
|
3
|
.4
|
|
March 26, 1998
|
|
|
|
|
3
|
.2
|
|
Article of Amendment to Articles of Incorporation dated
June 9, 2006
|
|
8-A
|
|
001-33357
|
|
|
3
|
.2
|
|
March 9, 2007
|
|
|
|
|
3
|
.3
|
|
Article of Amendment to Articles of Incorporation dated
December 13, 2006
|
|
8-A
|
|
001-33357
|
|
|
3
|
.3
|
|
March 9, 2007
|
|
|
|
|
3
|
.4
|
|
Article of Amendment to Articles of Incorporation dated
December 26, 2006
|
|
8-A
|
|
001-33357
|
|
|
3
|
.4
|
|
March 9, 2007
|
|
|
|
|
3
|
.5
|
|
Article of Amendment to Articles of Incorporation dated
February 26, 2007
|
|
8-A
|
|
001-33357
|
|
|
3
|
.5
|
|
March 9, 2007
|
|
|
|
|
3
|
.6
|
|
Amended and Restated Bylaws of the Company
|
|
10-Q
|
|
001-33357
|
|
|
3
|
.6
|
|
August 8, 2008
|
|
|
|
|
10
|
.1
|
|
2006 Stock Incentive Plan
|
|
10-K
|
|
001-33357
|
|
|
10
|
.1
|
|
July 13, 2007
|
|
|
|
|
10
|
.2
|
|
Employment Agreement between Protalix Ltd. and Yoseph Shaaltiel,
dated as of September 1, 2004
|
|
8-K
|
|
001-33357
|
|
|
10
|
.3
|
|
January 8, 2007
|
|
|
|
|
10
|
.3
|
|
Employment Agreement between Protalix Ltd. and Einat Almon,
dated as of December 19, 2004
|
|
8-K
|
|
001-33357
|
|
|
10
|
.3
|
|
January 8, 2007
|
|
|
|
|
10
|
.4
|
|
Employment Agreement between Protalix Ltd. and David Aviezer,
dated as of September 11, 2006
|
|
8-K
|
|
001-33357
|
|
|
10
|
.4
|
|
January 8, 2007
|
|
|
|
|
10
|
.5
|
|
Employment Agreement between Protalix Ltd. and Yossi Maimon,
dated as of October 15, 2006
|
|
8-K
|
|
001-33357
|
|
|
10
|
.5
|
|
January 8, 2007
|
|
|
|
|
10
|
.6†
|
|
License Agreement entered into as of April 12, 2005, by and
between Icon Genetics AG and Protalix Ltd.
|
|
8-K/A
|
|
001-33357
|
|
|
10
|
.6
|
|
September 20, 2007
|
|
|
|
|
10
|
.7†
|
|
Research and License Agreement between Yeda Research and
Development Company Limited and Protalix Ltd. dated as of
March 15, 2006
|
|
8-K/A
|
|
001-33357
|
|
|
10
|
.7
|
|
September 20, 2007
|
|
|
|
|
10
|
.8†
|
|
Agreement between Teva Pharmaceutical Industries Ltd. and
Protalix Ltd., dated September 14, 2006
|
|
8-K/A
|
|
001-33357
|
|
|
10
|
.8
|
|
September 20, 2007
|
|
|
|
|
10
|
.9
|
|
Lease Agreement between Protalix Ltd. and Angel Science Park
(99) Ltd., dated as of October 28, 2003 as amended on
April 18, 2005
|
|
8-K
|
|
001-33357
|
|
|
10
|
.9
|
|
January 8, 2007
|
|
|
|
|
10
|
.10
|
|
Merger Agreement and Plan of Reorganization made and entered
into as of August 21, 2006, by and among the Company,
Protalix Acquisition Co., Ltd. and Protalix Ltd.
|
|
8-K
|
|
001-33357
|
|
|
10
|
.10
|
|
January 8, 2007
|
|
|
|
|
10
|
.11
|
|
Stock Option Award Agreement grant by and between the Company
and Steven Rubin, dated as of December 31, 2006
|
|
10-K
|
|
001-33357
|
|
|
10
|
.13
|
|
March 30, 2007
|
|
|
96
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Incorporated by Reference
|
|
|
Exhibit
|
|
|
|
|
|
File
|
|
|
|
|
|
Filed
|
|
Number
|
|
Exhibit Description
|
|
Form
|
|
Number
|
|
Exhibit
|
|
Date
|
|
Herewith
|
|
|
|
|
10
|
.12
|
|
First Amendment to the December 31, 2006 Stock Option Award
Agreement by and between the Company and Steven Rubin, effective
as of February 28, 2007
|
|
10-K
|
|
001-33357
|
|
|
10
|
.16
|
|
March 30, 2007
|
|
|
|
|
10
|
.13
|
|
Scientific Advisory Board Agreement dated August 5, 2007 by
and between the Company and Aaron Ciechanover, M.D.
|
|
8-K
|
|
001-33357
|
|
|
10
|
.1
|
|
August 6, 2007
|
|
|
|
|
10
|
.14†
|
|
Research and License Agreement made on August 8, 2007, by
and between Yissum Research Development Company of Jerusalem,
the Boyce Thompson Institute and Protalix Ltd.
|
|
10-Q
|
|
001-33357
|
|
|
10
|
.1
|
|
November 14, 2007
|
|
|
|
|
10
|
.15
|
|
Unprotected Lease Agreement
|
|
10-K
|
|
001-33357
|
|
|
10
|
.21
|
|
March 17, 2008
|
|
|
|
|
10
|
.16†
|
|
Exclusive License and Supply Agreement dated as of
November 30, 2009 between Protalix Ltd. and Pfizer Inc.
|
|
10-K
|
|
001-33357
|
|
|
10
|
.16
|
|
February 26, 2010
|
|
|
|
|
10
|
.17
|
|
Employment Agreement by and between the Company and Sandra
Lauterbach dated as of December 17, 2009
|
|
8-K
|
|
001-33357
|
|
|
10
|
.1
|
|
December 27, 2009
|
|
|
|
|
10
|
.18
|
|
Employment Agreement by and between Protalix Ltd., and Tzvi
Palash dated as of August 29, 2010
|
|
8-K
|
|
001-33357
|
|
|
10
|
.1
|
|
September 7, 2010
|
|
|
|
|
10
|
.19†
|
|
License Agreement between Protalix Biotherapeutics Ltd. and
Virginia Tech Intellectual Properties, Inc.
|
|
10-Q
|
|
001-33357
|
|
|
10
|
.2
|
|
November 8, 2010
|
|
|
|
|
21
|
.1
|
|
Subsidiaries
|
|
10-K
|
|
001-33357
|
|
|
21
|
.1
|
|
February 26, 2010
|
|
|
|
|
23
|
.1
|
|
Consent of Kesselman & Kesselman, Certified Public
Accountant (Isr.), A member of PricewaterhouseCoopers
International Limited, independent registered public accounting
firm for the Registrant
|
|
|
|
|
|
|
|
|
|
|
|
X
|
|
|
31
|
.1
|
|
Certification of Chief Executive Officer pursuant to
Rule 13a-14(a)
as adopted pursuant to Section 302 of the Sarbanes-Oxley
Act of 2002
|
|
|
|
|
|
|
|
|
|
|
|
X
|
|
|
31
|
.2
|
|
Certification of Chief Financial Officer pursuant to
Rule 13a-14(a)
as adopted pursuant to Section 302 of the Sarbanes-Oxley
Act of 2002
|
|
|
|
|
|
|
|
|
|
|
|
X
|
|
|
32
|
.1
|
|
18 U.S.C. Section 1350, as adopted pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002,
Certification of Chief Executive Officer
|
|
|
|
|
|
|
|
|
|
|
|
X
|
|
|
32
|
.2
|
|
18 U.S.C. Section 1350, as adopted pursuant to
Section 906 of the Sarbanes-Oxley Act of 2002,
Certification of Chief Financial Officer
|
|
|
|
|
|
|
|
|
|
|
|
X
|
|
|
|
|
† |
|
Portions of this exhibit were omitted and have been filed
separately with the Secretary of the Securities and Exchange
Commission pursuant to the Registrant’s application
requesting confidential treatment under
Rule 24b-2
of the Exchange Act. |
97
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the
Securities Exchange Act of 1934, as amended, the registrant has
duly caused this report to be signed on its behalf by the
undersigned, thereunto duly authorized, as of
February 23, 2011.
PROTALIX BIOTHERAPEUTICS, INC.
David Aviezer, Ph.D.
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose
signature appears below constitutes and appoints David
Aviezer, Ph.D. and Yossi Maimon, and each of them, as his
true and lawful attorneys-in-fact and agents, with full power of
substitution and re-substitution, for him and in his name, place
and stead, in any and all capacities, to sign any and all
amendments to this Report on
Form 10-K,
and to file the same, with all exhibits thereto, and other
documents in connection therewith, with the Securities and
Exchange Commission, granting unto said attorneys-in-fact and
agents, and each of them, full power and authority to do and
perform each and every act and thing requisite and necessary to
be done in connection therewith, as fully to all intents and
purposes as he might or could do in person, hereby ratifying and
confirming that said attorneys-in-fact and agents, or any of
them, or their or his substitute or substitutes, may lawfully do
or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of
1934, this report has been signed below by the following persons
on behalf of the Registrant and in the capacities and on the
dates indicated.
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
/s/ David
Aviezer
David
Aviezer, Ph.D.
|
|
President, Chief Executive Officer (Principal Executive Officer)
and Director
|
|
February 23, 2011
|
|
|
|
|
|
|
|
/s/ Yossi
Maimon
Yossi
Maimon
|
|
Chief Financial Officer, Treasurer and
Secretary (Principal Financial and Accounting Officer)
|
|
February 23, 2011
|
|
|
|
|
|
|
|
/s/ Yoseph
Shaaltiel
Yoseph
Shaaltiel, Ph.D.
|
|
Executive VP, Research and Development and Director
|
|
February 23, 2011
|
|
|
|
|
|
|
|
/s/ Alfred
Akirov
Alfred
Akirov
|
|
Director
|
|
February 23, 2011
|
|
|
|
|
|
|
|
/s/ Amos
Bar Shalev
Amos
Bar Shalev
|
|
Director
|
|
February 23, 2011
|
|
|
|
|
|
|
|
/s/ Zeev
Bronfeld
Zeev
Bronfeld
|
|
Director
|
|
February 23, 2011
|
98
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
|
|
/s/ Yodfat
Harel Gross
Yodfat
Harel Gross
|
|
Director
|
|
February 23, 2011
|
|
|
|
|
|
|
|
/s/ Eyal
Sheratzky
Eyal
Sheratzky
|
|
Director
|
|
February 23, 2011
|
|
|
|
|
|
|
99
PROTALIX
BIOTHERAPEUTICS, INC.
CONSOLIDATED
FINANCIAL STATEMENTS
TABLE OF
CONTENTS
| |
|
|
|
|
|
|
|
Page
|
|
|
|
|
|
|
F-2
|
|
|
CONSOLIDATED FINANCIAL STATEMENTS
|
|
|
|
|
|
|
|
|
F-3
|
|
|
|
|
|
F-4
|
|
|
|
|
|
F-5
|
|
|
|
|
|
F-6
|
|
|
|
|
|
F-7
|
|
The dollar amounts are stated in U.S. dollars ($)
F-1
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the shareholders of
PROTALIX BIOTHERAPEUTICS, INC.
In our opinion, the consolidated balance sheets and the related
statements of operations, changes in shareholders’ equity
(capital deficiency) and cash flows present fairly, in all
material respects, the financial position of Protalix
BioTherapeutics, Inc. and its subsidiaries at December 31,
2009 and 2010, and the results of their operations and their
cash flows for each of the three years in the period ended
December 31, 2010, in conformity with accounting principles
generally accepted in the United States of America. Also, in our
opinion, the Company maintained, in all material respects,
effective internal control over financial reporting as of
December 31, 2010, based on criteria established in
Internal Control — Integrated Framework issued by the
Committee of Sponsoring Organizations of the Treadway Commission
(COSO). The Company’s management is responsible for these
financial statements, for maintaining effective internal control
over financial reporting and for its assessment of the
effectiveness of internal control over financial reporting,
included in the accompanying “Management Report on Internal
Control over Financial Reporting” appearing under
Item 9A. Our responsibility is to express opinions on these
financial statements and on the Company’s internal control
over financial reporting based on our integrated audits. We
conducted our audits in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audits to obtain
reasonable assurance about whether the financial statements are
free of material misstatement and whether effective internal
control over financial reporting was maintained in all material
respects. Our audits of the financial statements included
examining, on a test basis, evidence supporting the amounts and
disclosures in the financial statements, assessing the
accounting principles used and significant estimates made by
management, and evaluating the overall financial statement
presentation. Our audit of internal control over financial
reporting included obtaining an understanding of internal
control over financial reporting, assessing the risk that a
material weakness exists, and testing and evaluating the design
and operating effectiveness of internal control based on the
assessed risk. Our audits also included performing such other
procedures as we considered necessary in the circumstances. We
believe that our audits provide a reasonable basis for our
opinions.
A company’s internal control over financial reporting is a
process designed to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of
financial statements for external purposes in accordance with
generally accepted accounting principles. A company’s
internal control over financial reporting includes those
policies and procedures that: (i) pertain to the
maintenance of records that, in reasonable detail, accurately
and fairly reflect the transactions and dispositions of the
assets of the company; (ii) provide reasonable assurance
that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally
accepted accounting principles, and that receipts and
expenditures of the company are being made only in accordance
with authorizations of management and directors of the company;
and (iii) provide reasonable assurance regarding prevention
or timely detection of unauthorized acquisition, use or
disposition of the company’s assets that could have a
material effect on the financial statements.
Because of its inherent limitations, internal control over
financial reporting may not prevent or detect misstatements.
Also, projections of any evaluation of effectiveness to future
periods are subject to the risk that controls may become
inadequate because of changes in conditions, or that the degree
of compliance with the policies or procedures may deteriorate.
| |
|
|
|
Tel-Aviv, Israel
|
|
/s/ Kesselman
& Kesselman
|
|
February 23, 2011
|
|
Kesselman & Kesselman
|
|
|
|
Certified Public Accountant (Isr.)
|
|
|
|
A member firm of PricewaterhouseCoopers
|
|
|
|
International Limited
|
Kesselman
& Kesselman, Trade Tower, 25 Hamered Street, Tel-Aviv 68125, Israel, P.O. Box
452 Tel-Aviv 61003 Telephone: +972 -3- 7954555, Fax:+972 -3- 7954556, www.pwc.co.il
F-2
PROTALIX
BIOTHERAPEUTICS, INC.
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(U.S. dollars in thousands, except shares and per share
amounts)
|
|
|
|
|
ASSETS
|
|
CURRENT ASSETS:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
81,266
|
|
|
$
|
35,900
|
|
|
Accounts receivable:
|
|
|
|
|
|
|
|
|
|
Trade
|
|
|
|
|
|
|
7,013
|
|
|
Other
|
|
|
2,144
|
|
|
|
2,231
|
|
|
Inventories
|
|
|
|
|
|
|
1,189
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
83,410
|
|
|
|
46,333
|
|
|
|
|
|
|
|
|
|
|
|
|
FUNDS IN RESPECT OF EMPLOYEE RIGHTS UPON RETIREMENT
|
|
|
724
|
|
|
|
942
|
|
|
|
|
|
|
|
|
|
|
|
|
PROPERTY AND EQUIPMENT, NET
|
|
|
14,537
|
|
|
|
17,454
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
98,671
|
|
|
$
|
64,729
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND SHAREHOLDERS’ EQUITY (NET OF CAPITAL
DEFICIENCY)
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES:
|
|
|
|
|
|
|
|
|
|
Accounts payable and accruals:
|
|
|
|
|
|
|
|
|
|
Trade
|
|
$
|
3,406
|
|
|
$
|
6,272
|
|
|
Other
|
|
|
13,561
|
|
|
|
8,068
|
|
|
Deferred revenues
|
|
|
4,563
|
|
|
|
4,563
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
21,530
|
|
|
|
18,903
|
|
|
|
|
|
|
|
|
|
|
|
|
LONG TERM LIABILITIES:
|
|
|
|
|
|
|
|
|
|
Deferred revenues
|
|
|
60,049
|
|
|
|
55,486
|
|
|
Liability for employee rights upon retirement
|
|
|
1,209
|
|
|
|
1,663
|
|
|
|
|
|
|
|
|
|
|
|
|
Total long term liabilities
|
|
|
61,258
|
|
|
|
57,149
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
82,788
|
|
|
|
76,052
|
|
|
|
|
|
|
|
|
|
|
|
|
COMMITMENTS
|
|
|
|
|
|
|
|
|
|
SHAREHOLDERS’ EQUITY (CAPITAL DEFICIENCY):
|
|
|
|
|
|
|
|
|
|
Common Stock, $0.001 par value:
|
|
|
|
|
|
|
|
|
|
Authorized — as of December 31, 2009 and 2010,
150,000,000 shares; issued and outstanding — as
of December 31, 2009 and 2010, 80,841,237 and
81,248,472 shares, respectively
|
|
|
81
|
|
|
|
81
|
|
|
Additional paid-in capital
|
|
|
122,252
|
|
|
|
124,044
|
|
|
Accumulated deficit
|
|
|
(106,450
|
)
|
|
|
(135,448
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total shareholders’ equity (capital deficiency)
|
|
|
15,883
|
|
|
|
(11,323
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and shareholders’ equity (net of capital
deficiency)
|
|
$
|
98,671
|
|
|
$
|
64,729
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the consolidated
financial statements.
F-3
PROTALIX
BIOTHERAPEUTICS, INC.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(U.S. dollars in thousands,
|
|
|
|
|
except shares and per share amounts)
|
|
|
|
|
REVENUES
|
|
$
|
—
|
|
|
$
|
388
|
|
|
$
|
6,642
|
|
|
COMPANY’S SHARE IN COLLABORATION AGREEMENT
|
|
|
|
|
|
|
|
|
|
|
4,602
|
|
|
COST OF REVENUES
|
|
|
—
|
|
|
|
(3,575
|
)
|
|
|
(4,383
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT (LOSS)
|
|
|
—
|
|
|
|
(3,187
|
)
|
|
|
6,861
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RESEARCH AND DEVELOPMENT EXPENSES
|
|
|
(22,115
|
)
|
|
|
(27,390
|
)
|
|
|
(37,691
|
)
|
|
Less — grants and reimbursements
|
|
|
4,714
|
|
|
|
5,752
|
|
|
|
7,740
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(17,401
|
)
|
|
|
(21,638
|
)
|
|
|
(29,951
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GENERAL AND ADMINISTRATIVE EXPENSES
|
|
|
(6,770
|
)
|
|
|
(7,144
|
)
|
|
|
(6,876
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING LOSS
|
|
|
(24,171
|
)
|
|
|
(31,969
|
)
|
|
|
(29,966
|
)
|
|
FINANCIAL INCOME — NET
|
|
|
1,757
|
|
|
|
529
|
|
|
|
968
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS FOR THE YEAR
|
|
$
|
(22,414
|
)
|
|
$
|
(31,440
|
)
|
|
$
|
(28,998
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share of common stock — basic and diluted:
|
|
$
|
0.30
|
|
|
$
|
0.41
|
|
|
$
|
0.36
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares of common stock used in
computing loss per share of common stock, basic and diluted:
|
|
|
75,890,633
|
|
|
|
76,942,840
|
|
|
|
80,960,300
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the consolidated
financial statements.
F-4
PROTALIX
BIOTHERAPEUTICS, INC.
(CAPITAL
DEFICIENCY)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
|
|
|
|
|
Common
|
|
|
Common
|
|
|
Paid in
|
|
|
Accumulated
|
|
|
|
|
|
|
|
Stock
|
|
|
Stock
|
|
|
Capital
|
|
|
Deficit
|
|
|
Total
|
|
|
|
|
Number of
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
|
|
(U.S. dollars in thousands, except share data)
|
|
|
|
|
Balance at January 1, 2008
|
|
|
75,775,439
|
|
|
|
76
|
|
|
|
116,205
|
|
|
|
(52,596
|
)
|
|
|
63,685
|
|
|
Changes during 2008:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted Common Stock issued for future services(1)
|
|
|
(5,333
|
)
|
|
|
|
*
|
|
|
(3
|
)
|
|
|
—
|
|
|
|
(3
|
)
|
|
Share-based compensation
|
|
|
|
|
|
|
|
|
|
|
3,074
|
|
|
|
—
|
|
|
|
3,074
|
|
|
Exercise of options granted to employees (includes net exercise)
|
|
|
167,953
|
|
|
|
|
*
|
|
|
5
|
|
|
|
—
|
|
|
|
5
|
|
|
Net Loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(22,414
|
)
|
|
|
(22,414
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
|
75,938,059
|
|
|
|
76
|
|
|
|
119,281
|
|
|
|
(75,010
|
)
|
|
|
44,347
|
|
|
Changes during 2009:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share-based compensation
|
|
|
|
|
|
|
|
|
|
|
2,683
|
|
|
|
—
|
|
|
|
2,683
|
|
|
Exercise of options granted to employees and non-employees
(includes net exercise)
|
|
|
4,903,178
|
|
|
|
5
|
|
|
|
288
|
|
|
|
—
|
|
|
|
293
|
|
|
Net Loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(31,440
|
)
|
|
|
(31,440
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
|
80,841,237
|
|
|
|
81
|
|
|
|
122,252
|
|
|
|
(106,450
|
)
|
|
|
15,883
|
|
|
Changes during 2010:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share-based compensation
|
|
|
|
|
|
|
|
|
|
|
1,282
|
|
|
|
|
|
|
|
1,282
|
|
|
Exercise of options granted to employees and non-employees
(includes net exercise)
|
|
|
407,235
|
|
|
|
|
*
|
|
|
510
|
|
|
|
|
|
|
|
510
|
|
|
Net Loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(28,998
|
)
|
|
|
(28,998
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2010
|
|
|
81,248,472
|
|
|
|
81
|
|
|
|
124,044
|
|
|
|
(135,448
|
)
|
|
|
(11,323
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
Represents an amount less than $1. |
| |
|
(1) |
|
The Company issued a total of 8,000 shares of restricted
Common Stock in consideration for services provided by, and to
be provided by, a member of the Company’s Scientific
Advisory Board. Such services were terminated in October 2008
while the forfeiture provisions of the restricted stock were
still in effect. Accordingly, 5,333 shares of restricted
Common Stock were forfeited. |
The accompanying notes are an integral part of the consolidated
financial statements.
F-5
PROTALIX
BIOTHERAPEUTICS, INC.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
|
|
(U.S. dollars in thousands)
|
|
|
|
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Loss
|
|
$
|
(22,414
|
)
|
|
$
|
(31,440
|
)
|
|
$
|
(28,998
|
)
|
|
Adjustments required to reconcile net loss to net cash
|
|
|
|
|
|
|
|
|
|
|
|
|
|
provided by (used in) operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share based compensation
|
|
|
3,071
|
|
|
|
2,683
|
|
|
|
1,282
|
|
|
Depreciation and impairment of fixed assets
|
|
|
1,301
|
|
|
|
1,990
|
|
|
|
3,133
|
|
|
Financial income net (mainly exchange differences)
|
|
|
(270
|
)
|
|
|
(166
|
)
|
|
|
(591
|
)
|
|
Changes in accrued liability for employee rights upon retirement
|
|
|
247
|
|
|
|
265
|
|
|
|
377
|
|
|
Loss (Gain) on amounts funded in respect of employee rights upon
retirement
|
|
|
39
|
|
|
|
(81
|
)
|
|
|
(26
|
)
|
|
Loss on sale of fixed assets
|
|
|
|
|
|
|
29
|
|
|
|
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase (decrease) in deferred revenues (including non-current
portion)
|
|
|
|
|
|
|
64,612
|
|
|
|
(4,563
|
)
|
|
Decrease (increase) in accounts receivable
|
|
|
636
|
|
|
|
(1,224
|
)
|
|
|
(6,860
|
)
|
|
Increase in inventories
|
|
|
|
|
|
|
|
|
|
|
(1,189
|
)
|
|
Increase (decrease) in accounts payable and accruals
|
|
|
1,375
|
|
|
|
7,784
|
|
|
|
(1,029
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities
|
|
$
|
(16,015
|
)
|
|
$
|
44,452
|
|
|
$
|
(38,464
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment
|
|
$
|
(3,371
|
)
|
|
$
|
(6,195
|
)
|
|
$
|
(7,855
|
)
|
|
Investment in restricted deposit
|
|
|
(175
|
)
|
|
|
|
|
|
|
|
|
|
Proceeds from sale of property and equipment
|
|
|
1
|
|
|
|
73
|
|
|
|
|
|
|
Amounts funded in respect of employee rights upon retirement, net
|
|
|
(156
|
)
|
|
|
(52
|
)
|
|
|
(137
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
$
|
(3,701
|
)
|
|
$
|
(6,174
|
)
|
|
$
|
(7,992
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance cost in respect of issuance of shares
|
|
$
|
(56
|
)
|
|
|
|
|
|
|
|
|
|
Exercise of options
|
|
|
5
|
|
|
$
|
293
|
|
|
$
|
501
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) financing activities
|
|
$
|
(51
|
)
|
|
$
|
293
|
|
|
$
|
501
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EFFECT OF EXCHANGE RATE CHANGES ON CASH
|
|
|
550
|
|
|
|
99
|
|
|
|
589
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS
|
|
|
(19,217
|
)
|
|
|
38,670
|
|
|
|
(45,366
|
)
|
|
BALANCE OF CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR
|
|
|
61,813
|
|
|
|
42,596
|
|
|
|
81,266
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BALANCE OF CASH AND CASH EQUIVALENTS AT END OF YEAR
|
|
$
|
42,596
|
|
|
$
|
81,266
|
|
|
$
|
35,900
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTARY INFORMATION ON INVESTING AND FINANCING
ACTIVITIES NOT INVOLVING CASH FLOWS:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchase of property and equipment
|
|
$
|
932
|
|
|
$
|
4,525
|
|
|
$
|
2,720
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise of options granted to employees
|
|
|
|
|
|
|
|
|
|
$
|
9
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of the consolidated
financial statements.
F-6
PROTALIX
BIOTHERAPEUTICS, INC.
(U.S. dollars in thousands)
|
|
|
NOTE 1 —
|
SIGNIFICANT
ACCOUNTING POLICIES
|
Protalix BioTherapeutics, Inc. and its wholly-owned subsidiary,
Protalix Ltd. (the “Israeli Subsidiary” or
“Protalix Ltd.,” and collectively with Protalix
BioTherapeutics, Inc., the “Company”), are
biopharmaceutical companies focused on the development and
commercialization of recombinant therapeutic proteins based on
the Company’s proprietary
ProCellExtm
protein expression system (“ProCellEx”). In September
2009, the Company formed another wholly-owned subsidiary under
the laws of the Netherlands in connection with the EMEA
application process in Europe. The Company’s two
subsidiaries are referred to collectively herein as the
“Subsidiaries.” The Company’s lead product
development candidate is taliglucerase alfa for the treatment of
Gaucher disease which the Company is developing using ProCellEx.
In addition to taliglucerase alfa, the Company is developing
other certain products using ProCellEx.
In September 2009, the Company successfully completed its
phase III pivotal trial of taliglucerase alfa. In July
2010, the U.S. Food and Drug Administration
(“FDA”) notified the Company that it had accepted the
Company’s new drug application (NDA) for taliglucerase alfa
for the treatment of Gaucher disease and that it granted to
taliglucerase alfa a Prescription Drug User Fee Act (PDUFA)
action date of February 25, 2011.
In September 2009, the FDA’s Office of Orphan Product
Development granted taliglucerase alfa Orphan Drug Status. In
addition to its phase III clinical trial, the Company
initiated a clinical study in December 2008 to evaluate the
safety and efficacy of switching Gaucher disease patients
currently treated under the current standard of care to
treatment with taliglucerase alfa. This switchover-study is not
a prerequisite for the marketing approval of taliglucerase alfa.
In November 2010 the Company successfully completed the nine
month switchover trial.
On November 30, 2009, Protalix Ltd. and Pfizer Inc.
(“Pfizer”) entered into an Exclusive License and
Supply Agreement (the “Pfizer Agreement”) pursuant to
which Protalix Ltd. granted Pfizer an exclusive, worldwide
license to develop and commercialize taliglucerase alfa, except
in Israel. Under the terms and conditions of the Pfizer
Agreement, Protalix Ltd. retained the right to commercialize
taliglucerase alfa in Israel. See Note 2.
On July 13, 2010, the French regulatory authority granted
an Autorisation Temporaire d’Utilisation (“ATU”),
or Temporary Authorization for Use, for taliglucerase alfa for
the treatment of Gaucher disease. An ATU is the regulatory
mechanism used by the French Health Products and Safety Agency
to make non-approved drugs available to patients in France when
a genuine public health need exists. This ATU allows Gaucher
disease patients in France to receive treatment with
taliglucerase alfa before marketing authorization for the
product is granted in the European Union. Payment for
taliglucerase alfa has been secured through government
allocations to hospitals.
On August 10, 2010, Pfizer entered into a $30 million
short-term supply agreement with the Ministry of Health of
Brazil pursuant to which the Company and Pfizer have provided
taliglucerase alfa to the Ministry of Health of Brazil for the
treatment of Gaucher disease patients. Revenue generated from
the Ministry of Health of Brazil will be recorded by Pfizer and
the Company is entitled to its share of the revenue in
accordance with the terms and conditions of the Pfizer Agreement.
|
|
|
2.
|
Liquidity
and Financial Resources
|
Successful completion of the Company’s development programs
and its transition to normal operations is dependent upon
obtaining necessary regulatory approvals from the FDA prior to
selling its products within the United States, and foreign
regulatory approvals must be obtained to sell its products
internationally. There can
F-7
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 1 —
|
SIGNIFICANT
ACCOUNTING POLICIES (continued):
|
be no assurance that the Company will receive regulatory
approval of any of its product candidates, and a substantial
amount of time may pass before the Company achieves a level of
revenues adequate to support its operations, if at all. The
Company will also incur substantial expenditures in connection
with the regulatory approval process for each of its product
candidates during the developmental period. Obtaining marketing
approval will be directly dependent on the Company’s
ability to implement the necessary regulatory steps required to
obtain marketing approval in the United States and in other
countries. The Company cannot predict the outcome of these
activities.
Based on its current cash resources and commitments, the Company
believes it will be able to maintain its current planned
development activities and the corresponding level of
expenditures for at least the next 12 months, although no
assurance can be given that it will not need additional funds
prior to such time. If there are unexpected increases in general
and administrative expenses or research and development
expenses, the Company may need to seek additional financing
during the next 12 months.
The Company’s financial statements have been prepared in
accordance with generally accepted accounting principles in the
United States (“U.S. GAAP”).
|
|
|
c.
|
Use of
estimates in the preparation of financial
statements
|
The preparation of financial statements in conformity with
U.S. GAAP requires management to make estimates and
assumptions that affect the reported amounts of assets and
liabilities, the disclosure of contingent assets and liabilities
at the date of the financial statements and the reported amounts
of revenues and expenses during the reporting period. In
addition, the Company uses estimates and assumptions relating to
inventory reserves in its financial statements. Actual results
may differ from those estimates.
The dollar is the currency of the primary economic environment
in which the operations of the Company and its Subsidiaries are
conducted. The Company’s revenues are derived in dollars.
Most of the Company’s expenses and capital expenditures are
incurred in dollars, and the major source of the Company’s
financing has been provided in dollars.
Transactions and balances originally denominated in dollars are
presented at their original amounts. Balances in non-dollar
currencies are translated into dollars using historical and
current exchange rates for non-monetary and monetary balances,
respectively. For non-dollar transactions and other items
(stated below) reflected in the statements of operations, the
following exchange rates are used: (i) for
transactions — exchange rates at the transaction dates
or average rates; and (ii) for other items (derived from
non-monetary balance sheet items such as depreciation and
amortization, etc.) — historical exchange rates.
Currency transaction gains and losses are carried to financial
income or expenses, as appropriate.
The Company considers all short-term, highly liquid investments,
which include short-term bank deposits with original maturities
of three months or less from the date of purchase, that are not
restricted as to withdrawal or use and are readily convertible
to known amounts of cash, to be cash equivalents.
Inventories are valued at the lower of cost or market. Cost of
raw and packaging materials and purchased products is determined
using the “moving average” basis. Cost of finished
products and products in process is determined as follows: the
value of the raw and packaging materials component is determined
primarily on a
F-8
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 1 —
|
SIGNIFICANT
ACCOUNTING POLICIES (continued):
|
using the “moving average” basis; the value of the
labor and overhead component is determined on an average basis
over the production period.
Manufacturing costs for product candidates are expensed as
research and development expenses. The Company considers
regulatory approval of product candidates to be uncertain, and
product manufactured prior to regulatory approval may not be
sold unless regulatory approval is obtained. As such, the
manufacturing costs for product candidates incurred prior to
regulatory approval are not capitalized as inventory, unless
inventories for which there are orders or are probable to be
sold without the regulatory approval.
|
|
|
g.
|
Property
and equipment
|
1. Property and equipment are stated at cost, net of
accumulated depreciation and amortization.
2. The Company’s assets are depreciated by the
straight-line method on the basis of their estimated useful
lives as follows:
| |
|
|
|
|
|
Years
|
|
|
|
Laboratory equipment
|
|
5
|
|
Furniture
|
|
10-15
|
|
Computer equipment
|
|
3
|
Leasehold improvements are amortized by the straight-line method
over the expected lease term, which is shorter than the
estimated useful life of the improvements.
|
|
|
h.
|
Impairment
in value of long-lived assets:
|
The Company tests long-lived assets for impairment if an
indication of impairment exists. If the sum of expected future
cash flows of definite life of long lived assets (undiscounted
and without interest charges) is less than the carrying amount
of such assets, the Company recognizes an impairment loss, and
writes down the assets to their estimated fair values,
calculated based on expected future discounted cash flows. See
Note 3c.
Deferred taxes are determined utilizing the assets and
liabilities method based on the estimated future tax effects of
the differences between the financial accounting and tax bases
of assets and liabilities under the applicable tax laws.
Deferred tax balances are computed using the tax rates expected
to be in effect when those differences reverse. A valuation
allowance in respect of deferred tax assets is provided if,
based upon the weight of available evidence, it is more likely
than not that some or all of the deferred tax assets will not be
realized. The Company has provided a full valuation allowance
with respect to its deferred tax assets.
The guidance prohibits the recognition of deferred tax
liabilities or assets that arise from differences between the
financial reporting and tax bases of assets and liabilities that
are measured from the local currency into dollars using
historical exchange rates, and that result from changes in
exchange rates or indexing for tax purposes. Consequently, the
above mentioned differences with respect to Protalix Ltd. were
not reflected in the computation of deferred tax assets and
liabilities.
|
|
|
2.
|
Uncertainty
in income taxes
|
Tax benefits recognized in the financial statements are at least
more likely than not of being sustained, based on technical
merits. The amount of benefits recorded for these tax benefits
is measured as the largest benefit more likely than not to be
sustained.
F-9
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 1 —
|
SIGNIFICANT
ACCOUNTING POLICIES (continued):
|
The Company recognizes revenue when the earnings process is
complete, which is when revenue is realized or realizable and
earned, there is persuasive evidence a revenue arrangement
exists, delivery of goods or services has occurred, the sales
price is fixed or determinable and collectability is reasonably
assured.
|
|
|
1.
|
Revenues
from the license and supply agreement with Pfizer
|
The Company recognizes revenue and milestone payments received
pursuant to the Pfizer Agreement in accordance with guidance
regarding revenue recognition and accounting for revenue
arrangements with multiple deliverables. Pursuant to this
guidance, the Company determines whether its arrangement with
Pfizer involves multiple revenue-generating deliverables that
should be accounted for as a combined unit of accounting or
separate units of accounting for revenue recognition purposes.
If the Company determines that there are multiple units of
accounting, the consideration from the arrangement is allocated
among the separate units based on a relative fair value
allocation. If the arrangement represents a single unit of
accounting, the revenue is recognized over the performance
obligation period. As the arrangement with Pfizer requires the
Company’s continued involvement with respect to the
proposed commercialization of taliglucerase alfa, the
non-refundable, up-front license payments the Company received
from Pfizer are deferred and recognized over the related
performance period. The Company estimated the performance period
of 14 years based on the date the last relevant patent
expires. The Company adjusts the performance periods, if
appropriate, based on the applicable facts and circumstances.
|
|
|
2.
|
Revenues
from selling products to Pfizer
|
The Company recognizes revenues received from products sold to
Pfizer when the sales price is fixed or determinable and
collectability is reasonably assured.
|
|
|
3.
|
Company’s
share in the collaboration agreement
|
Under the terms and conditions of the Pfizer Agreement, the
Company is entitled to 40% of the profits or loss from sales of
taliglucerase alfa, and related expenses incurred, except with
respect to sales in Israel, where the Company retained exclusive
marketing rights. Since Pfizer bears most of the risks and
rewards relating to the agreement, the Company’s share in
the profits and loss in the agreement is recognized on a net
basis. The Company recognizes its share of net profit or loss
from the Pfizer Agreement based on reports it receives from
Pfizer summarizing the results of the collaborative activities
under the agreement for the applicable period. Under the terms
of the Pfizer Agreement, for its subsidiaries operating outside
the United States, financial information is included based on
the fiscal year ending November 30, while financial
information for the U.S. entity is included based on the
fiscal year ending December 31.
|
|
|
k.
|
Research
and development costs
|
Research and development costs are expensed as incurred and
consist primarily of personnel, subcontractors and consultants,
facilities, equipment and supplies for research and development
activities. Grants received by the Israeli Subsidiary from the
Office of the Chief Scientist of Israel’s Ministry of
Industry, Trade and Labor (the “OCS”) and other
research foundations are recognized when the grant becomes
receivable, provided there is reasonable assurance that the
Company or the Subsidiary will comply with the conditions
attached to the grant and there is reasonable assurance the
grant will be received. The grant is deducted from the research
and development expenses as the applicable costs are incurred.
Reimbursements received from Pfizer and other research
foundations are recognized when the reimbursements become
receivable, provided there is reasonable assurance that the
Company will comply with the conditions attached to the
reimbursements and there is reasonable assurance the
reimbursements will be
F-10
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 1 —
|
SIGNIFICANT
ACCOUNTING POLICIES (continued):
|
received. The reimbursements are deducted from the research and
development expenses as the applicable costs are incurred.
In connection with purchases of assets, amounts assigned to
intangible assets to be used in a particular research and
development project that have no alternative future use are
charged to research and development costs at the purchase date.
Nonrefundable advance payments for goods or services that will
be used or rendered for future research and development
activities are deferred and amortized over the period that the
goods are consumed or the related services are performed.
The Company has no other comprehensive loss components other
than net loss for the reported periods.
|
|
|
m.
|
Concentration
of credit risks and trade receivable
|
Financial instruments that potentially subject the Company to
concentration of credit risk consist principally of bank
deposits. The Company deposits these instruments with highly
rated financial institutions, mainly in Israeli banks, and, as a
matter of policy, limits the amounts of credit exposure to any
one financial institution. The Company has not experienced any
credit losses in these accounts and does not believe it is
exposed to any significant credit risk on these instruments.
The Company’s trade receivable represents amounts to be
received from Pfizer, as the Company currently receives all of
its revenues from Pfizer. The Company does not require Pfizer to
post collateral with respect to the receivables. The Company
performs periodic credit evaluations of Pfizer’s financial
condition and believes there is no significant risk with respect to
Pfizer’s payment of the receivables.
|
|
|
n.
|
Share-based
compensation
|
The Company accounts for employee’s share-based payment
awards classified as equity awards using the grant-date fair
value method. The fair value of share-based payment transactions
is recognized as an expense over the requisite service period,
net of estimated forfeitures. The Company estimates forfeitures
based on historical experience and anticipated future conditions.
The Company elected to recognize compensation cost for an award
with only service conditions that has a graded vesting schedule
using the accelerated method based on the multiple-option award
approach.
When stock options are granted as consideration for services
provided by consultants and other non-employees, the grant is
accounted for based on the fair value of the consideration
received or the fair value of the stock options issued,
whichever is more reliably measurable. The fair value of the
options granted is measured on a final basis at the end of the
related service period and is recognized over the related
service period using the straight-line method.
Basic and diluted losses per share (“LPS”) are
computed by dividing net loss by the weighted average number of
shares of Common Stock outstanding for each period.
Shares of restricted Common Stock and the shares of Common Stock
underlying outstanding options of the Company were not included
in the computation of diluted LPS because of the anti-dilutive
effect of doing so.
F-11
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 1 —
|
SIGNIFICANT
ACCOUNTING POLICIES (continued):
|
Diluted LPS does not include options and restricted shares of
Common Stock of the Company in the amount of 11,037,356,
10,660,447 and 7,761,168 shares of Common Stock for the
years 2008, 2009 and 2010, respectively.
|
|
|
p.
|
Newly
Issued Accounting Pronouncements
|
1. In October 2009, the Financial Accounting Standards
Board (“FASB”) issued an Accounting Standards Update
to ASC 605, ASU
No. 2009-13,
“Multiple Deliverable Revenue Arrangements” (“ASU
2009-13”).
ASU 2009-13
provides guidance regarding whether multiple deliverables in a
revenue arrangement exist, how the arrangement should be
separated, and how the consideration should be allocated.
Pursuant to ASU
2009-13,
when vendor specific objective evidence or third party evidence
for deliverables in an arrangement cannot be determined, a best
estimate of the selling price is required to separate
deliverables and allocate arrangement consideration, using the
relative selling price method. In addition, the residual method
of allocating arrangement consideration is no longer permitted
under ASU
2009-13. ASU
2009-13 is
effective for revenue arrangements entered into or materially
modified in fiscal years beginning on or after June 15,
2010 and will be applied to future arrangements to which the
Company becomes a party, if any.
2. In April 2010, the Financial Accounting Standards Board
issued an Accounting Standard — ASU
No. 2010-17,
Revenue Recognition — Milestone Method (ASU
2010-17).
ASU 2010-17
provides guidance in applying the milestone method of revenue
recognition to research or development arrangements. Under this
guidance management may recognize revenue contingent upon the
achievement of a milestone in its entirety, in the period in
which the milestone is achieved, only if the milestone meets all
the criteria within the guidance to be considered substantive.
ASU 2010-17
is effective on a prospective basis for research and development
milestones achieved in fiscal years beginning on or after
June 15, 2010. The Company is currently evaluating the
potential impact of ASU
2010-17 on
its consolidated financial position, results of operations and
cash flows.
|
|
|
NOTE 2 —
|
LICENSE
AND SUPPLY AGREEMENT
|
On November 30, 2009, Protalix Ltd. and Pfizer entered into
the Pfizer Agreement pursuant to which Pfizer was granted an
exclusive, worldwide license to develop and commercialize
taliglucerase alfa, except in Israel. Under the terms and
conditions of the Pfizer Agreement, Protalix Ltd. retained the
right to commercialize taliglucerase alfa in Israel. Under the
Pfizer Agreement, Pfizer made an upfront payment to Protalix
Ltd. of $60,000 in connection with the execution of the
agreement and shortly thereafter paid Protalix Ltd. an
additional $5,000 upon the Company’s filing of a proposed
pediatric investigation plan to the Pediatric Committee of the
European Medicines Agency (EMEA). Protalix Ltd. is also eligible
to receive additional potential milestone payments totaling up
to $50,000 for the successful achievement of other regulatory
milestones. Protalix Ltd. is entitled to 40% of the profits
earned on Pfizer’s sales of taliglucerase alfa. Such profit
will be calculated while, in addition to other adjustments,
taking into account Protalix Ltd.’s cost of goods sold and
Pfizer’s commercial expenses, with certain expenses capped
or borne solely by one party.
The Company has determined that the initial, non-refundable
upfront license fee payment of $60,000 together with the first
$5,000 payment will be recognized on a straight line basis as
revenue over the estimated relationship period. The Company has
estimated that the relationship period for its arrangement with
Pfizer will be approximately 14 years based on the
Company’s last material patent relating to taliglucerase
alfa to expire.
The Company’s deliverables under this collaboration include
an exclusive license to taliglucerase alfa as an enzyme
replacement therapy for the treatment of Gaucher disease,
certain research and development services as required under the
Pfizer Agreement for taliglucerase alfa, manufacturing of
taliglucerase alfa and optional participation in a joint
steering committee.
F-12
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 2 —
|
LICENSE
AND SUPPLY AGREEMENT (continued):
|
According to the terms and conditions of the Pfizer Agreement,
the Company retained manufacturing rights and sells its products
to Pfizer. In addition, the Company is entitled to reimbursement
by Pfizer of certain costs incurred by the Company in connection
with certain development expenses for taliglucerase alfa.
In connection with the payments received under the Pfizer
Agreement, Protalix Ltd. is obligated to pay certain royalties.
See Note 6a.
|
|
|
NOTE 3 —
|
PROPERTY
AND EQUIPMENT
|
a. Composition of property and equipment grouped by major
classifications is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2010
|
|
|
|
|
Laboratory equipment
|
|
$
|
10,008
|
|
|
$
|
11,478
|
|
|
Furniture and computer equipment
|
|
|
944
|
|
|
|
1,306
|
|
|
Leasehold improvements
|
|
|
5,665
|
|
|
|
11,221
|
|
|
Equipment under construction
|
|
|
2,615
|
|
|
|
1,038
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
19,232
|
|
|
$
|
25,043
|
|
|
Less — accumulated depreciation
|
|
|
|
|
|
|
|
|
|
and amortization
|
|
|
(4,695
|
)
|
|
|
(7,589
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
14,537
|
|
|
$
|
17,454
|
|
|
|
|
|
|
|
|
|
|
|
b. Depreciation and amortization in respect of property and
equipment totaled $1,301, $1,990 and $3,133 for the years ended
December 31, 2008, 2009 and 2010, respectively.
c. During the year ended December 31, 2010, the
Company recorded a total impairment of $43. The impaired long
lived assets were mainly laboratory equipment and computer
equipment.
a. Inventory at December 31, 2010 consisted of the
following:
| |
|
|
|
|
|
|
|
December 31, 2010
|
|
|
|
|
Raw materials
|
|
$
|
553
|
|
|
Finished goods
|
|
|
636
|
|
|
|
|
|
|
|
|
Total inventory
|
|
$
|
1,189
|
|
|
|
|
|
|
|
b. During the year ended December 31, 2010, the
Company recorded a $2.7 million write-down of inventory
under cost of revenues. See Note 9d.
|
|
|
NOTE 5 —
|
LIABILITY
FOR EMPLOYEE RIGHTS UPON RETIREMENT
|
The Israeli Subsidiary is required to make a severance payment
upon dismissal of an employee, or upon termination of employment
in certain circumstances. The severance pay liability to the
employees (based upon length of service and the latest monthly
salary — one month’s salary for each year
employed) is recorded on the Company’s balance sheets under
“Liability for employee rights upon retirement.” The
liability is recorded as if it were payable at each balance
sheet date on an undiscounted basis.
The liability is funded in part from the purchase of insurance
policies or by the establishment of pension funds with dedicated
deposits in the funds. The amounts used to fund these
liabilities are included in the Company’s balance sheets
under “Funds in respect of employee rights upon
retirement.” These policies are the
F-13
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 5 —
|
LIABILITY
FOR EMPLOYEE RIGHTS UPON RETIREMENT (continued):
|
Company’s assets. However, under labor agreements and
subject to certain limitations, any policy may be transferred to
the ownership of the individual employee for whose benefit the
funds were deposited. In the years ended December 31, 2008,
2009 and 2010, the Company deposited $161, $79 and $174,
respectively, with insurance companies in connection with its
severance payment obligations.
In accordance with the current employment agreements with
certain employees, the Company makes regular deposits with
certain insurance companies for accounts controlled by each
applicable employee in order to secure the employee’s
rights upon retirement. The Company is fully relieved from any
severance pay liability with respect to each such employee after
it makes the payments on behalf of the employee. The liability
accrued in respect of these employees and the amounts funded, as
of the respective agreement dates, are not reflected in the
Company’s balance sheets, as the amounts funded are not
under the control and management of the Company and the pension
or severance pay risks have been irrevocably transferred to the
applicable insurance companies (the “Contribution
Plans”).
The amounts of severance pay expenses were $563, $488 and $1,118
for the years ended December 31, 2008, 2009 and 2010,
respectively, of which $319, $335 and $531 in the years ended
December 31, 2008, 2009 and 2010, respectively, were in
respect of a Contribution Plan. Gain (loss) on amounts funded in
respect of employee rights upon retirement totaled $(39), $81
and $26 for the years ended December 31, 2008, 2009 and
2010, respectively.
The Company expects to contribute approximately $841 in the year
ending December 31, 2011 to insurance companies in
connection with its severance liabilities for its operations for
that year, $653 of which will be contributed to one or more
Contribution Plans.
During the
10-year
period following December 31, 2010, the Company expects to
pay future benefits to eight employees upon their normal
retirement age, which is anticipated to amount to $70, $26, $7,
$16 and $913 during the years 2011, 2012, 2014, 2015 and 2020,
respectively. These amounts were determined based on each such
employee’s current salary rates and the number of years of
employment that will be accumulated upon the retirement date of
each such employee. This expectation does not include additional
amounts that might be paid to employees that will cease working
for the Company before their normal retirement age.
1. The Company is obligated to pay royalties to the OCS on
proceeds from the sale of products developed from research and
development activities that were funded, partially, by grants
from the OCS. At the time the grants were received, successful
development of the related projects was not assured.
In the case of failure of a project that was partly financed as
described above, the Company is not obligated to pay any such
royalties or repay funding received from the OCS.
Under the terms of the funding arrangements with the OCS,
royalties of 3% to 6% are payable on the sale of products
developed from projects funded by the OCS, which payments shall
not exceed, in the aggregate, 100% of the amount of the grant
received (dollar linked), plus, commencing upon January 1,
2001, interest at annual rate based on LIBOR. In addition, if
the Company receives approval to manufacture products developed
with government grants outside the State of Israel, it will be
required to pay an increased total amount of royalties (possibly
up to 300% of the grant amounts plus interest), depending on the
manufacturing volume that is performed outside the State of
Israel, and, possibly, an increased royalty rate.
The Company is obligated to pay the OCS royalties in respect of
revenues of taliglucerase alfa recorded by Pfizer under the
Pfizer Agreement. Royalty expenses are included in the statement
of operations as a component of the cost revenues and were
approximately $1,950 and $769 during the year ended
December 31, 2009 and 2010, respectively.
F-14
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 6 —
|
COMMITMENTS
(continued):
|
At December 31, 2010, the maximum royalty amount payable by
the Company under these funding arrangements is approximately
$17,154 (without interest, assuming 100% of the funds are
payable).
2. The Company is a party to certain research and license
agreements. Under the agreements, the Company is obligated to
pay royalties at varying rates from its future revenues. The
aggregate royalties payable under all of the agreements is equal
to a percentages of net sales of licensed products in the teens.
As of December 31, 2009 and 2010, royalty payments in the
amount of approximately $1,625 and $115, respectively,
have become payable under the agreements due to the
execution of, and certain sales under, the Pfizer Agreement. The
royalties are included in the statement of operations as a
component of cost of revenues.
Under each agreement, the Company is also obligated to pay
milestone, licensing and other payments to the counterparties of
the agreement. The payments under the agreements are for varying
amounts and are subject to varying conditions. If all of the
contingencies with respect to milestone payments under the
research and license agreements are met, the aggregate milestone
payments payable would be approximately $950 and would be
payable, if at all, as the Company’s projects progress over
the course of a number of years. In addition, milestone payments
of $70 and $100, were paid in respect of the said agreements
during the year ended 2009 and 2010, respectively.
None of the agreements has a fixed termination date. Subject to
earlier termination for other reasons, each agreement terminates
after a certain number of years following the first commercial
sale of any licensed product under the agreement or after a
certain number of years without the initiation of commercial
sales of any product under the agreement.
|
|
|
b.
|
Subcontracting
Agreements
|
The Company has entered into
sub-contracting
agreements with several clinical providers and constructor in
Israel, the United States and certain other countries in
connection with its primary product development process and with
expenditure of the company’s manufacturing facilities. As
of December 31, 2010, total commitments under said
agreements were approximately $5,995.
The Company is a party to a number of lease agreements for its
facilities, the latest of which expires in 2017. The Company has
the option to extend certain of such agreements on three
occasions for additional five-year periods, for a total of 15
additional years. Under the leases, the aggregate monthly rental
payments are approximately $71. As of December 31, 2010,
the Company provided bank guarantees of approximately $226, in
the aggregate, to secure the fulfillment of its obligations
under the lease agreements. The future minimum lease payments
required in each of the next five years under the operating
leases for such premises are approximately as follows:
2011 — $853, 2012 — $853, 2013 —
$842, 2014 — $834 and 2015 - $834. Lease expenses
totaled $220, $780 and $891 for the years ended
December 31, 2008, 2009 and 2010, respectively.
|
|
|
d.
|
Vehicle
Lease and Maintenance Agreements
|
In July 2004, the Company entered into several three-year lease
and maintenance agreements for vehicles which are regularly
amended as new vehicles are leased. The current monthly lease
fees aggregate approximately $44. The minimum expected lease
payments for the years ending December 31, 2011, 2012 and
2013 are $514, $452 and $125, respectively.
F-15
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 6 —
|
COMMITMENTS
(continued):
|
On September 14, 2006, the Company entered into an
agreement (the “Teva Agreement”) with Teva
Pharmaceutical Industries Ltd. (“Teva”) under which
the Company agreed to collaborate on the research and
development of two proteins to be identified by Teva and the
Company, using ProCellEx. The Teva Agreement also identifies
additional matters for collaboration between Teva and the
Company. Subsequently, two proteins were identified to be
researched and developed under the agreement but in 2009, both
of the projects were terminated for commercial reasons. Other
elements of the Company’s collaboration with Teva are
currently ongoing. Eli Hurvitz, the former Chairman of the
Company’s Board of Directors, is also a former Chairman of
Teva’s Board of Directors, and Phillip Frost M.D., a former
director and an indirect shareholder of the Company, is the
current Chairman of Teva’s Board of Directors.
On August 8, 2007, the Company signed an agreement with the
Yissum Research and Development Company, the technology transfer
arm of the Hebrew University of Jerusalem, Israel, and the Boyce
Thompson Institute for Plant Research, at Cornell University,
Ithaca, New York, to develop a proprietary plant cell-based
acetylcholinesterase (AChE) and its molecular variants for the
use in several therapeutic and prophylactic indications,
including a biodefense program and organophosphate-based
pesticide treatment. Pursuant to the agreement, the Company has
received an exclusive worldwide right and license to certain
technology, including patents and additional patent applications
relating to AChE (the “Licensed Technology”), for all
therapeutic and prophylactic indications. In consideration for
the license, the Company is required to make certain milestone
payments upon its achievement of clinical milestones and
royalties from sales derived from any drugs developed by it with
the Licensed Technology. The agreement does not terminate until
either party to the agreement elect to terminate the agreement,
subject to certain terms and conditions set forth therein.
|
|
|
a.
|
Rights
of the Company’s Stock
|
The Company’s common stock is listed on the NYSE Amex and,
since September 6, 2010, on the Tel Aviv Stock Exchange.
Each share of Common Stock is entitled to one vote. The holders
of Common Stock are also entitled to receive dividends whenever
funds are legally available, when and if declared by the Board
of Directors. Since its inception, the Company has not declared
any dividends.
The preferred shares were authorized in the Company’s
Restated Articles of Incorporation on April 16, 1998. The
rights and privileges of the preferred stock may be established
by the Company’s Board of Directors. The directors have not
designated any class of preferred stock and no shares of
preferred stock have ever been issued.
|
|
|
b.
|
Stock
based compensation
|
On December 14, 2006, the Board of Directors adopted the
Protalix BioTherapeutics, Inc. 2006 Stock Incentive Plan (the
“Plan”). The grant of options to Israeli employees
under the Plan is subject to the terms stipulated by
Sections 102 and 102A of the Israeli Income Tax Ordinance.
Each option grant is subject to the track chosen by the Company,
either Section 102 or Section 102A of the Israeli
Income Tax Ordinance, and pursuant to the terms thereof, the
Company is not allowed to claim, as an expense for tax purposes,
the amounts credited to employees as a benefit, including
amounts recorded as salary benefits in the Company’s
accounts, in respect of options granted to employees under the
Plan, with the exception of the work-income
F-16
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 7 —
|
SHARE
CAPITAL (continued):
|
benefit component, if any, determined on the grant date. For
Israeli non-employees, the share option plan is subject to
Section 3(i) of the Israeli Income Tax Ordinance.
As of December 31, 2010, 3,064 shares of Common Stock
remain available for grant under the Plan.
For purposes of determining the fair value of the options and
shares of restricted Common Stock granted to employees and
non-employees, the Company’s management uses the fair value
of the Common Stock.
From January 1, 2008 through December 31, 2010, the
Company granted options to certain employees and non-employees
as follows:
|
|
|
1.
|
Options
granted to employees:
|
a) Below is a table summarizing all of the option grants to
employees for each of the three years in the period ended
December 31, 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year of
|
|
No. of Options
|
|
|
Exercise Price
|
|
|
|
|
Fair Value
|
|
|
Expiration
|
|
Grant
|
|
Granted
|
|
|
Range
|
|
|
Vesting Period
|
|
at Grant
|
|
|
Period
|
|
|
|
2008
|
|
|
2,060,000
|
|
|
$
|
2.35-$5.00
|
|
|
4 -5 years
|
|
$
|
2,914
|
|
|
10 years
|
|
2009*
|
|
|
504,000
|
|
|
$
|
2.65
|
|
|
Upon achievement of
certain milestones*
|
|
$
|
1,068
|
|
|
10 years
|
|
2009
|
|
|
120,400
|
|
|
$
|
2.65
|
|
|
4 years
|
|
$
|
212
|
|
|
10 years
|
|
2010
|
|
|
1,016,000
|
|
|
$
|
6.90
|
|
|
3 years commencing
upon achievement of a
certain milestone
|
|
$
|
5,673
|
|
|
10 years
|
|
2010
|
|
|
428,000
|
|
|
$
|
6.32-$9.66
|
|
|
4 years
|
|
$
|
2,147
|
|
|
10 years
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,128,400
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
The milestone was achieved as of December 31, 2009 and the
options vested in full. |
Set forth below are grants made by the Company to employees and
certain related parties during the three-year period ended
December 31, 2010 (such grants appear in the table above):
1. In February 2008, the Company’s Board of Directors
approved the grant of options to purchase 1,900,000 shares
of Common Stock to the Company’s Chief Executive Officer
and certain officers and employees of the Company with an
exercise price equal to $5.00 per share. The options vest
variably over a five-year period. The options expire within a
period of 10 years from the date of grant.
2. In October 2008, the Company’s Board of Directors
approved the grant of options to purchase 160,000 shares of
Common Stock to a new newly-hired officer of the Company with an
exercise price equal to $2.35 per share. The options vest over a
four-year period. The options expire within a period of
10 years from the date of grant.
3. In February 2008, the Company amended the stock option
agreements of certain executive officers. As amended, such stock
option agreements provide for the full acceleration of the
vesting period of unvested options held by such officers
immediately upon a change of control. The Company concluded that
there was no incremental increase in the value of the awards and
therefore no accounting charges need to be recorded in
connection with such modification.
F-17
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 7 —
|
SHARE
CAPITAL (continued):
|
4. In February, 2009, the Company’s Board of Directors
approved the grant of options to purchase 624,400 shares of
Common Stock to the Company’s Chief Executive Officer and
certain officers and employees of the Company with an exercise
price equal to $2.65 per share. The options vest as follows:
(i) 504,000 of the options vest immediately upon the
achievement of certain clinical and operational performance
milestones, which milestones must be achieved within one year of
the date of grant or the options will be forfeited. The options
expire within a period of 10 years from the date of grant.
The vesting conditions of these options were satisfied prior to
December 31, 2009. Accordingly, these options were fully
vested at December 31, 2009 and the Company recognized all
of the expenses for these options during 2009.
(ii) 120,400 of the options vest as follows: 25% within one
year from the date of grant, with the remainder vesting in 12
equal quarterly tranches over 36 months. The options expire
within a period of 10 years from the date of grant. The
Company’s management assumed the simplified method to
reflect the expected life regarding these options. The Company
continued to use the simplified method in the first quarter of
2009 as the Company did not have sufficient historical exercise
data to provide a reasonable basis upon which to estimate
expected term due to the limited period of time its equity
shares have been publicly traded.
5. In February 2010, the Company’s Board of Directors
approved the grant of options to purchase 1,016,000 shares
of Common Stock, in the aggregate, to the Company’s Chief
Executive Officer and certain officers and employees of the
Company with an exercise price equal to $6.90 per share. The
options vest quarterly over a three-year period commencing upon
the FDA’s approval of taliglucerase alfa, if at all. The
Company will start recording these expenses following the
FDA’s marketing approval of taliglucerase alfa, if at all.
6. In February 2010, the Company’s Board of Directors
approved the grant of options to purchase 160,000 shares of
Common Stock with an exercise price equal to $6.81 per share to
a new executive officer of the Company. The options expire
within a period of 10 years from the date of grant. The
first 25% of the options vest on the first anniversary of the
date of grant and the remaining 75% vest in 12 equal tranches on
a quarterly basis for three years thereafter.
7. In September 2010, the Company’s Board of Directors
approved the grant of options to purchase 160,000 shares of
Common Stock to a new executive officer of the Company with an
exercise price equal to $7.55 per share and options to purchase
40,000 shares of Common Stock to a new employee of the
Company with an exercise price equal to $6.32 per share. The
options vest over a four-year period, with the first 25% vesting
on the first anniversary of the applicable date of the grant and
the remaining 75% vesting in equal tranches on a quarterly basis
for a three-year period thereafter. The options expire within a
period of 10 years from the date of grant.
8. In November 2010, the Company’s Board of Directors
approved the grant of options to purchase 68,000 shares of
Common Stock to a new officer of the Company with an exercise
price equal to $9.66 per share. The options vest over a
four-year period, with the first 25% vesting on the first
anniversary of the applicable date of the grant and the
remaining 75% vesting in equal tranches on a quarterly basis for
a three-year period thereafter. The options expire within a
period of 10 years from the date of grant.
9. In September 2010, the Company’s Board of Directors
modified the terms of the options previously granted to an
executive in 2001, by extending the life of the options until
2016. At the date of modification, all of the options were fully
vested. The Company concluded that there was no incremental
increase in the value of the awards and therefore no accounting
charges need to be recorded in connection with the modifications.
F-18
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 7 —
|
SHARE
CAPITAL (continued):
|
b) The fair value of options granted during the years ended
December 31, 2008, 2009 and 2010 were $2,914, $1,280 and
$7,820, respectively. The fair value of each option granted is
estimated on the date of grant using the Black-Scholes
option-pricing model, with the following weighted average
assumptions:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
|
|
Dividend yield
|
|
|
0%
|
|
|
|
0%
|
|
|
|
0%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expected volatility
|
|
|
63%
|
|
|
|
75%
|
|
|
|
75%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Risk-free interest rate
|
|
|
2.99%
|
|
|
|
2.74%
|
|
|
|
3.23%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expected life — in years
|
|
|
6.0
|
|
|
|
9.2
|
|
|
|
8.8
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The expected volatility is based on the historical volatility of
the Common Stock and those of comparable companies. The
risk-free interest rate assumption is based on observed interest
rates appropriate for the expected term of the stock options
granted in dollar terms. The Company’s management uses the
contractual term or its expectations, based on historical
incidence of option exercises, as applicable (through 2008 and
the first quarter of 2009 — using the simplified
method), of each option as its expected life. The pre-vesting
forfeiture rate of approximately 6.3% is estimated based on
pre-vesting forfeiture experience.
The total unrecognized compensation cost of employee stock
options at December 31, 2010 is $7,317 (net of forfeiture
rate) out of which $5,656 are expected to be recognized over a
three-year period commencing upon the FDA’s approval of
taliglucerase alfa, if at all. The remaining compensation cost
of $1,661 is expected to be recognized over a weighted average
period of 1.1 years.
The total cash received from employees as a result of employee
stock option exercises for the years ended December 31,
2008, 2009 and 2010 was $5, $293 and $501, respectively. The
Company did not realize any tax benefit in connection with these
exercises.
During 2010, the Company issued 407,235 shares of Common
Stock in connection with the exercise of 429,309 options by
certain officers and employees of the Company. The Company
received cash proceeds equal to $501 in connection with such
exercises. Of such options, 98,081 were exercised on a
“net-exercise” basis.
|
|
|
2.
|
Options
and shares of restricted Common Stock granted to consultants,
directors, and other service providers:
|
a. In February 2008, the Company’s Board of Directors
approved the grant of options to purchase 50,000 shares of
Common Stock to a new director of the Company with an exercise
price equal to $3.02 per share. The options vest over a
four-year period commencing on the date of grant and expire
within a period of 10 years from the date of grant.
b. The fair value of options and shares of restricted
Common Stock granted to consultants and other non-employees
during the years ended December 31, 2008, 2009 and 2010
were $109, $0 and $0, respectively. The fair value of each
option granted is estimated on the date of grant using the
Black-Scholes option-pricing model, with the following weighted
average assumptions:
| |
|
|
|
|
|
|
|
2008
|
|
|
|
|
Dividend yield
|
|
|
0%
|
|
|
|
|
|
|
|
|
Expected volatility
|
|
|
63%
|
|
|
|
|
|
|
|
|
Risk-free interest rate
|
|
|
2.99%
|
|
|
|
|
|
|
|
|
Expected life — in years
|
|
|
10
|
|
|
|
|
|
|
|
F-19
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 7 —
|
SHARE
CAPITAL (continued):
|
The expected volatility is based on the historical volatility of
the Company’s stock and those of comparable companies. The
risk-free interest rate assumption is based on observed interest
rates appropriate for the expected term of the stock options
granted in dollar terms. The Company’s management used the
contractual terms as the expected life.
The total unrecognized compensation cost as of December 31,
2010, is $14, and it is expected to be recognized over a
weighted average period of 0.4 years.
No cash was received from consultants as a result of consultant
stock option exercises for the years ended December 31,
2008, 2009 and 2010. The Company did not realize any tax
benefits in connection with these exercises.
During the year ended December 31, 2009, the Company issued
3,853,441 shares of Common Stock in connection with the
exercise of 3,866,093 options by certain consultants, directors,
and other service providers of the Company. The Company did not
receive cash proceeds in connection with such exercises as all
of such options were exercised on a “net-exercise”
basis.
c. A summary of share option plans, and related
information, under all of the Company’s equity incentive
plans for the years ended December 31, 2008, 2009 and 2010
are as follows:
|
|
|
1.
|
Options
granted to employees:
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Weighted
|
|
|
|
|
Number
|
|
|
Average
|
|
|
Number
|
|
|
Average
|
|
|
Number
|
|
|
Average
|
|
|
|
|
of
|
|
|
Exercise
|
|
|
of
|
|
|
Exercise
|
|
|
of
|
|
|
Exercise
|
|
|
|
|
Options
|
|
|
Price
|
|
|
Options
|
|
|
Price
|
|
|
Options
|
|
|
Price
|
|
|
|
|
Outstanding at beginning of year
|
|
|
4,212,686
|
|
|
$
|
0.830
|
|
|
|
5,890,641
|
|
|
$
|
2.118
|
|
|
|
5,366,729
|
|
|
$
|
2.476
|
|
|
Changes during the year:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
2,060,000
|
|
|
|
4.794
|
|
|
|
624,400
|
|
|
|
2.650
|
|
|
|
1,444,000
|
|
|
|
7.076
|
|
|
Forfeited
|
|
|
177,237
|
|
|
|
4.405
|
|
|
|
1,400
|
|
|
|
2.650
|
|
|
|
13,441
|
|
|
|
3.551
|
|
|
Exercised(*)
|
|
|
204,808
|
|
|
|
0.565
|
|
|
|
1,146,912
|
|
|
|
0.733
|
|
|
|
429,309
|
|
|
|
1.602
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at end of year
|
|
|
5,890,641
|
|
|
$
|
2.118
|
|
|
|
5,366,729
|
|
|
$
|
2.476
|
|
|
|
6,367,979
|
|
|
$
|
3.576
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at end of year
|
|
|
3,267,607
|
|
|
$
|
0.995
|
|
|
|
3,680,382
|
|
|
$
|
1.785
|
|
|
|
4,267,850
|
|
|
$
|
2.183
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (*) |
The total intrinsic value of options exercised during the years
ended December 31, 2008, 2009 and 2010, was $450, $7,258
and $3,050, respectively.
|
F-20
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 7 —
|
SHARE
CAPITAL (continued):
|
|
|
|
2.
|
Options
granted to consultants, directors, and other service
providers:
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
Weighted
|
|
|
|
|
Number
|
|
|
Average
|
|
|
Number
|
|
|
Average
|
|
|
Number
|
|
|
Average
|
|
|
|
|
of
|
|
|
Exercise
|
|
|
of
|
|
|
Exercise
|
|
|
of
|
|
|
Exercise
|
|
|
|
|
Options
|
|
|
Price
|
|
|
Options
|
|
|
Price
|
|
|
Options
|
|
|
Price
|
|
|
|
|
Outstanding at beginning of year
|
|
|
5,254,785
|
|
|
$
|
1.250
|
|
|
|
5,304,785
|
|
|
$
|
1.285
|
|
|
|
1,438,692
|
|
|
$
|
4.697
|
|
|
Changes during the year:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
50,000
|
|
|
|
3.020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised(*)
|
|
|
|
|
|
|
|
|
|
|
3,866,093
|
|
|
|
0.016
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at end of year
|
|
|
5,304,785
|
|
|
$
|
1.285
|
|
|
|
1,438,692
|
|
|
$
|
4.697
|
|
|
|
1,438,692
|
|
|
$
|
4.697
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at end of year
|
|
|
5,133,189
|
|
|
$
|
0.902
|
|
|
|
1,407,234
|
|
|
$
|
4.674
|
|
|
|
1,421,734
|
|
|
$
|
4.653
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(*) |
|
The total intrinsic value of options exercised during the years
ended December 31, 2008, 2009 and 2010, was $0, $41,281 and
$0, respectively. |
d. The following tables summarize information concerning
outstanding and exercisable options as of December 31, 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2010
|
|
|
Options Outstanding
|
|
|
Options Exercisable
|
|
|
|
|
|
Number of
|
|
|
Weighted
|
|
|
Number of
|
|
|
Weighted
|
|
|
|
|
|
Options
|
|
|
Average
|
|
|
Options
|
|
|
Average
|
|
|
|
|
|
Outstanding
|
|
|
Remaining
|
|
|
Exercisable
|
|
|
Remaining
|
|
Exercise
|
|
|
at End of
|
|
|
Contractual
|
|
|
at End of
|
|
|
Contractual
|
|
|
Prices
|
|
|
Year
|
|
|
Life
|
|
|
Year
|
|
|
Life
|
|
|
|
|
$
|
0.001
|
|
|
|
919,207
|
|
|
|
4.42
|
|
|
|
917,875
|
|
|
|
4.42
|
|
|
$
|
0.120
|
|
|
|
1,063,653
|
|
|
|
3.30
|
|
|
|
1,063,653
|
|
|
|
3.30
|
|
|
$
|
0.399
|
|
|
|
31,673
|
|
|
|
4.35
|
|
|
|
31,673
|
|
|
|
4.35
|
|
|
$
|
0.972
|
|
|
|
1,376,047
|
|
|
|
5.48
|
|
|
|
1,376,047
|
|
|
|
5.48
|
|
|
$
|
2.350
|
|
|
|
160,000
|
|
|
|
7.82
|
|
|
|
80,000
|
|
|
|
7.82
|
|
|
$
|
2.650
|
|
|
|
612,204
|
|
|
|
8.15
|
|
|
|
553,929
|
|
|
|
8.15
|
|
|
$
|
3.020
|
|
|
|
50,000
|
|
|
|
7.10
|
|
|
|
34,375
|
|
|
|
7.10
|
|
|
$
|
5.000
|
|
|
|
1,765,345
|
|
|
|
7.10
|
|
|
|
1,244,490
|
|
|
|
7.10
|
|
|
$
|
6.320
|
|
|
|
40,000
|
|
|
|
9.66
|
|
|
|
|
|
|
|
|
|
|
$
|
6.810
|
|
|
|
160,000
|
|
|
|
9.10
|
|
|
|
|
|
|
|
|
|
|
$
|
6.900
|
|
|
|
1,013,000
|
|
|
|
9.15
|
|
|
|
|
|
|
|
|
|
|
$
|
7.550
|
|
|
|
160,000
|
|
|
|
9.66
|
|
|
|
|
|
|
|
|
|
|
$
|
9.660
|
|
|
|
68,000
|
|
|
|
9.84
|
|
|
|
|
|
|
|
|
|
|
$
|
16.700
|
|
|
|
387,542
|
|
|
|
6.00
|
|
|
|
387,542
|
|
|
|
6.00
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,806,671
|
|
|
|
|
|
|
|
5,689,584
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The aggregate intrinsic value of the total outstanding and of
total vested and exercisable options as of December 31,
2010 is $51,392 and $43,454, respectively.
F-21
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 7 —
|
SHARE
CAPITAL (continued):
|
e. The following table illustrates the effect of
share-based compensation on the statement of operations:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
|
|
Research and development expenses
|
|
$
|
1,226
|
|
|
$
|
1,489
|
|
|
$
|
630
|
|
|
General and administrative expenses
|
|
|
1,845
|
|
|
|
1,194
|
|
|
|
652
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
3,071
|
|
|
$
|
2,683
|
|
|
$
|
1,282
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Protalix BioTherapeutics, Inc. is taxed according to tax laws of
the United States. The income of the Company is, or will be,
taxed in the United States at the rate of up to 39.4%.
The Israeli Subsidiary is taxed according to Israeli tax laws:
|
|
|
1.
|
Measurement
of results for tax purposes
|
Commencing in 2008, the results of the Israeli Subsidiary are
measured for tax purposes in nominal terms. Pursuant to the
Israel Income Tax Law (Adjustments for Inflation), 1985 (the
“Adjustments Law”), the Subsidiary’s results for
tax purposes have been measured through 2007 on a real basis,
based on changes in the Israel consumer price index.
The income of the Israeli Subsidiary, other than income from
“Approved Enterprises,” is taxed in Israel at the
regular rate. See 3 below. According to the provisions of the
Law for Amending the Israel Income Tax Ordinance, 2005 of August
2005, corporate tax rates will be gradually lowered, resulting
in the corporate following tax rates for 2008 and thereafter:
2008 — 27%, 2009 — 26% and for 2010 and
thereafter — 25%.
Capital gain for assets purchased since January 1, 2003 are
subject to real capital gain tax at 25% and exempted from
inflationary capital gains tax.
On July 14, 2009, the Israel Economic Efficiency Law
(Legislation Amendments for Applying the Economic Plan for 2009
and 2010), 2009, became effective, stipulating, among other
things, an additional gradual decrease in tax rates in 2011 and
thereafter, as follows: 2011 — 24%, 2012 —
23%, 2013 — 22%, 2014 — 21%,
2015 — 20% and 2016 and thereafter — 18%.
In addition to the above decrease in corporate tax, the real
capital gain tax was reduced to be in line with corporate tax in
the year of selling the asset.
F-22
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 8 —
|
TAXES ON
INCOME (continued):
|
|
|
|
3.
|
The Law
for the Encouragement of Capital Investments, 1959 (the
“Encouragement of Capital Investments Law”)
|
Under the Encouragement of Capital Investments Law, including
Amendment No. 60 to the Encouragement of Capital
Investments Law as published in April 2005, by virtue of the
“Approved Enterprise” or “Benefited
Enterprise” status the Israeli Subsidiary is entitled to
various tax benefits as follows:
Income derived from the Approved Enterprise during a
10-year
period commencing upon the year in which the enterprise first
realizes taxable income is tax exempt, provided that the maximum
period to which it is restricted by the Encouragement of Capital
Investments Law has not elapsed.
The Israeli Subsidiary has an “Approved Enterprise”
plan since 2004 and “Benefited Enterprise” plan since
2009. The period of benefits in respect of the main enterprise
of the Company has not yet commenced. The period during which
the Company is entitled to benefits in connection with the
Approved Enterprise expires in 2017.
If the Israeli Subsidiary subsequently pays a dividend out of
income derived from the “Approved Enterprise” or
“Benefited Enterprise” during the tax exemption
period, it will be subject to a tax on the amount distributed,
including any company tax on these amounts, at the rate which
would have been applicable had such income not been exempted.
In addition to the corporate taxes in Israel, the Company might
be subject to a withholding tax on the U.S. revenue source
portion of the payments made to the Company for its share of
Pfizer’s net profits under the Pfizer Agreement. The
withholding tax rate is currently 15%.
|
|
|
b.
|
Accelerated
depreciation
|
The Israeli Subsidiary is entitled to claim accelerated
depreciation as provided by Israeli law, commencing in the first
year of operation of each asset, in respect of buildings,
machinery and equipment used by the Approved Enterprise.
|
|
|
c.
|
Conditions
for entitlement to the benefits
|
The Israeli Subsidiary’s entitlement to the benefits
described above is subject to its fulfilling the conditions
stipulated by the law, rules and regulations published
thereunder, and the instruments of approval for the specific
investment in an approved enterprise. If there is any failure by
the Israeli Subsidiary to comply with these conditions, the
benefits may be cancelled and the Subsidiary may be required to
refund the amount of the benefits, in whole or in part, with
interest. The Company received a final implementation approval
with respect to its “Approved Enterprise” from the
Investment Center.
|
|
|
d.
|
Amendment
of the Law for the Encouragement of Capital Investments,
1959
|
The Encouragement of Capital Investments Law was amended as part
of the Economic Policy Law for the years
2011-2012,
which was passed by the Israeli Knesset on December 27,
2010 (the “Capital Investments Law Amendment”).
The Capital Investments Law Amendment sets alternative benefit
tracks to those currently in effect under the provisions of the
Encouragement of Capital Investments Law.
The benefits granted to the Benefited Enterprises will be
unlimited in time, unlike the benefits granted to special
Benefited enterprises, which will be limited for a
10-year
period. The benefits shall be granted to
F-23
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 8 —
|
TAXES ON
INCOME (continued):
|
companies that will qualify under criteria set in the law; for
the most part, those criteria are similar to the criteria that
were set in the Encouragement of Capital Investments Law prior
to its amendment.
Under the transitional provisions of the Encouragement of
Capital Investments Law, the Company is entitled to take
advantage of the tax benefits available under the Encouragement
of Capital Investments Law prior to its amendment until the end
of the benefits period, as defined in the Encouragement of
Capital Investments Law. The Company will be allowed to set the
“year of election” no later than tax year 2012,
provided that the minimum qualifying investment was made not
later than the end of 2010. On each year during the benefits
period, the Company will be able to elect that the Capital
Investments Amendment apply to the Company, thereby making the
tax rates described above available to the Company. An election
to have the Capital Investments Amendment apply is
irrecoverable. The amendment was not in effect as of
December 31, 2010. Accordingly, the measurement of the
deferred income taxes, was prepared without taking the effects
of Capital Investments Amendment into consideration. See d below.
|
|
|
4.
|
The Law
for the Encouragement of Industry (Taxation), 1969:
|
The Israeli Subsidiary is an “industrial company,” as
defined under the Law for the Encouragement of Industry
(Taxation), 1969 (the “Law for the Encouragement of
Industry”). As such, the Israeli Subsidiary is entitled to
claim depreciation at increased rates for equipment used in
industrial activity, as stipulated by regulations published
under Law for the Encouragement of Industry, and has done so.
Under the provisions of the Income Tax Regulations
“Accelerated Depreciation in respect of Equipment acquired
during the Defined Period” (Temporary Orders), industrial
companies whose operations are mostly “eligible
operations” are entitled to claim accelerated depreciation
at a rate of 50% on machinery and equipment acquired from
June 1, 2008 to May 31, 2009. The accelerated
depreciation is to be claimed over two years. For the year in
which the equipment was acquired, depreciation is recorded at
the regular rate. In the second year and thereafter,
depreciation is recorded at a rate that would make the aggregate
rate 100%.
Under the regulations, the Company is entitled to accelerated
depreciation in 2010 for machines and equipment purchased in
2008 and 2009. The effect of the change in the rates of
accelerated depreciation was included in deferred taxes
described below.
|
|
|
c.
|
Tax
losses carried forward to future years
|
As of December 31, 2010, the Company had aggregate net
operating loss (NOL) carry-forwards equal to approximately
$54,000 that are available to reduce future taxable income as
follows:
The NOL carry-forward of the Company equal to approximately
$9,000 may be restricted under Section 382 of the
Internal Revenue Code (“IRC”). IRC Section 382
applies whenever a corporation with NOL experiences an ownership
change. As a result of IRC Section 382, the taxable income
for any post change year that may be offset by a pre-change NOL
may not exceed the general IRC Section 382 limitation,
which is the fair market value of the pre-change entity
multiplied by the IRC long-term tax exempt rate.
At December 31, 2010, the Israeli Subsidiary had
approximately $45,000 of NOL carry-forwards that are available
to reduce future taxable income with no limited period of use.
F-24
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 8 —
|
TAXES ON
INCOME (continued):
|
|
|
|
d.
|
Deferred
income taxes:
|
The components of the Company’s net deferred tax assets at
December 31, 2009 and 2010 were as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2010
|
|
|
|
|
In respect of:
|
|
|
|
|
|
|
|
|
|
Property and equipment
|
|
$
|
161
|
|
|
$
|
143
|
|
|
Holiday and recreation pay
|
|
|
200
|
|
|
|
296
|
|
|
Severance pay obligation
|
|
|
87
|
|
|
|
130
|
|
|
Deferred revenues
|
|
|
8,345
|
|
|
|
3,691
|
|
|
Net operating loss carry forwards
|
|
|
2,246
|
|
|
|
3,599
|
|
|
Valuation allowance
|
|
|
10,717
|
|
|
|
7,573
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
e.
|
Reconciliation
of the theoretical tax expense to actual tax
expense
|
The main reconciling items between the statutory tax rate of the
Company and the effective rate is the tax exemptions in
connections with the Approved Enterprise and the provision for
full valuation allowance in respect of tax benefits from carry
forward tax losses due to the uncertainty of the realization of
such tax benefits (see above).
In accordance with the Income Tax Ordinance, as of
December 31, 2010, all of Protalix Ltd.’s tax
assessments through tax year 2005 are considered final.
A summary of open tax years by major jurisdiction is presented
below:
| |
|
|
|
|
|
Jurisdiction:
|
|
Years:
|
|
|
|
Israel
|
|
|
2005-2010
|
|
|
United States(*)
|
|
|
2002-2010
|
|
|
Netherlands
|
|
|
2009-2010
|
|
|
|
|
|
(*) |
|
Includes federal, state and local (or similar provincial
jurisdictions) tax positions. |
F-25
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 9 —
|
SUPPLEMENTARY FINANCIAL STATEMENT INFORMATION:
|
Balance sheets:
| |
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
|
|
December 31,
|
|
|
|
|
2009
|
|
|
2010
|
|
|
|
|
a. Accounts receivable — other:
|
|
|
|
|
|
|
|
|
|
Institutions
|
|
$
|
740
|
|
|
$
|
634
|
|
|
State of Israel (see Note 6a)
|
|
|
832
|
|
|
|
878
|
|
|
Restricted deposit
|
|
|
213
|
|
|
|
226
|
|
|
Prepaid expenses
|
|
|
208
|
|
|
|
354
|
|
|
Sundry
|
|
|
151
|
|
|
|
139
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
2,144
|
|
|
$
|
2,231
|
|
|
|
|
|
|
|
|
|
|
|
|
b. Accounts payable and accruals — other:
|
|
|
|
|
|
|
|
|
|
Payroll and related expenses
|
|
$
|
2,731
|
|
|
$
|
1,199
|
|
|
Provision for vacation and recreation pay
|
|
|
799
|
|
|
|
1,235
|
|
|
Accrued expenses
|
|
|
1,931
|
|
|
|
2,030
|
|
|
Royalties payable
|
|
|
3,575
|
|
|
|
884
|
|
|
Property and equipment supplier
|
|
|
4,525
|
|
|
|
2,720
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
13,561
|
|
|
$
|
8,068
|
|
|
|
|
|
|
|
|
|
|
|
F-26
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 9 —
|
SUPPLEMENTARY
FINANCIAL STATEMENT INFORMATION (continued):
|
Statement of operations:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
|
|
c. Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred revenues from the license and supply agreement with
Pfizer
|
|
$
|
—
|
|
|
$
|
388
|
|
|
$
|
4,563
|
|
|
Revenues from selling products to Pfizer
|
|
|
|
|
|
|
|
|
|
|
2,079
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
—
|
|
|
$
|
388
|
|
|
$
|
6,642
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
d. Cost of Revenues:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of products sold
|
|
|
|
|
|
|
|
|
|
$
|
807
|
|
|
Write down of inventories
|
|
|
|
|
|
|
|
|
|
|
2,692
|
|
|
Royalties expenses
|
|
$
|
|
|
|
$
|
3,575
|
|
|
|
884
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
|
|
|
$
|
3,575
|
|
|
$
|
4,383
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
e. Research and development expenses — net:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payroll and related expenses
|
|
$
|
9,296
|
|
|
$
|
10,479
|
|
|
$
|
13,176
|
|
|
Subcontractors and consultants
|
|
|
5,289
|
|
|
|
7,469
|
|
|
|
11,550
|
|
|
Materials and consumables
|
|
|
3,799
|
|
|
|
3,852
|
|
|
|
4,947
|
|
|
Rent, insurance and maintenance
|
|
|
1,592
|
|
|
|
2,238
|
|
|
|
3,457
|
|
|
Patent registration and licensing
|
|
|
182
|
|
|
|
387
|
|
|
|
571
|
|
|
Depreciation and impairment
|
|
|
1,171
|
|
|
|
1,799
|
|
|
|
2,456
|
|
|
Other
|
|
|
786
|
|
|
|
1,166
|
|
|
|
1,534
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
22,115
|
|
|
|
27,390
|
|
|
|
37,691
|
|
|
Less — grants and reimbursements:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Development costs reimbursements from Pfizer
|
|
|
|
|
|
|
|
|
|
|
3,922
|
|
|
Grants (see Note 6a)
|
|
|
4,714
|
|
|
|
5,752
|
|
|
|
3,818
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
17,401
|
|
|
$
|
21,638
|
|
|
$
|
29,951
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
f. General and administrative expense:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payroll and related expenses
|
|
$
|
2,261
|
|
|
$
|
2,804
|
|
|
$
|
2,415
|
|
|
Management and consulting fees
|
|
|
1,335
|
|
|
|
681
|
|
|
|
221
|
|
|
Rent, insurance and maintenance
|
|
|
191
|
|
|
|
347
|
|
|
|
463
|
|
|
Professional fees
|
|
|
1,362
|
|
|
|
2,274
|
|
|
|
2,274
|
|
|
Travel
|
|
|
472
|
|
|
|
251
|
|
|
|
308
|
|
|
Depreciation
|
|
|
130
|
|
|
|
191
|
|
|
|
309
|
|
|
Other
|
|
|
1,019
|
|
|
|
596
|
|
|
|
886
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$
|
6,770
|
|
|
$
|
7,144
|
|
|
$
|
6,876
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-27
PROTALIX
BIOTHERAPEUTICS, INC.
NOTES TO
CONSOLIDATED FINANCIAL
STATEMENTS
(U.S. dollars in thousands)
|
|
|
NOTE 10 —
|
RELATED
PARTY TRANSACTIONS
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
|
|
a. Management and consulting fees to the Chairman of the
Board
|
|
$
|
33
|
|
|
$
|
33
|
|
|
$
|
6
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
b. Compensation to the non-executive directors (except
the Chairman of the Board in 2008 and 2009)
|
|
$
|
210
|
|
|
$
|
209
|
|
|
$
|
215
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
c. With respect to options granted to the Company’s Chief
Executive Officer and to a shareholder, see
Note 7(b)1.
|
|
d. In March 2005, Protalix Ltd. entered into a management services
agreement with Pontifax Management Company, Ltd. in connection
with an investment in Protalix Ltd. by affiliates of Pontifax.
The monthly management fees under the management services
agreement were $3. The management services agreement remained in
full force as long as Mr. Hurvitz served as a member of the
Company’s Board of Directors. In 2008, the annual amount
was set to $33 and in March 2010, Mr. Hurvitz resigned from
the Company’s Board of Directors. The management services
agreement is no longer in effect.
|
|
|
|
NOTE 11 —
|
SUBSEQUENT EVENTS
|
During January and February 2011, the Company issued a total of
86,065 shares of Common Stock in connection with the
exercise of options to purchase 86,065 shares of Common
Stock by certain employees of the Company. The Company received
aggregate cash proceeds equal to approximately $118 in
connection with the exercise of such options.
F-28