Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - EPIRUS Biopharmaceuticals, Inc. | d8k.htm |
 Creating a Better Future April 2010 Mark Corrigan, MD, President and CEO
|
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL Forward Looking Statements 2 This presentation contains forward-looking statements within the meaning of the Private Securities
Litigation Reform Act of 1995 concerning CombinatoRx, the potential of the product Exalgo and potential royalty income to CombinatoRx, the CombinatoRx product candidates and their development and commercial potential, the CombinatoRx selective ion channel modulation platform, its combination
drug discovery technology, its collaboration agreements, and its financial condition, intellectual
property and business plans. These forward-looking statements are based on the current estimates
and assumptions of the management of CombinatoRx as of the date of this presentation and are
subject to risks, uncertainties, assumptions and other factors that may cause actual results to be
materially different from those reflected in such forward-looking statements. Important
factors that could cause actual results to differ materially from those indicated by such
forward-looking statements include: including risks related to the sale and marketing of
Exalgo by Covidien, risks related to the development and regulatory approval of CombinatoRx's
product candidates, the unproven nature of the CombinatoRx drug discovery technologies, the
ability of Covidien, Novartis and Fovea to perform their obligations under their collaboration
agreements with CombinatoRx, the ability of the Company or its collaboration partners to initiate
and successfully complete clinical trials of its product candidates, the Company's ability to
obtain additional financing or funding for its research and development and the other risks that
can be found in the "Risk Factors" section of the CombinatoRx Annual Report on Form 10-K on file with the Securities and Exchange Commission, and the other filings made by CombinatoRx
with the Securities and Exchange Commission. No forward-looking statement can be guaranteed
and actual results may differ materially from those CombinatoRx projects. CombinatoRx is providing
this information as of the date of this presentation and does not undertake any obligation to
publicly update any forward-looking statements contained in this document as a result of new
information, future events or otherwise, except as required by law.
|
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL CombinatoRx: Positioned As A Thriving Biopharmaceutical Business 3 |
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL 4 *Source: NeroInvestment April 2010 |
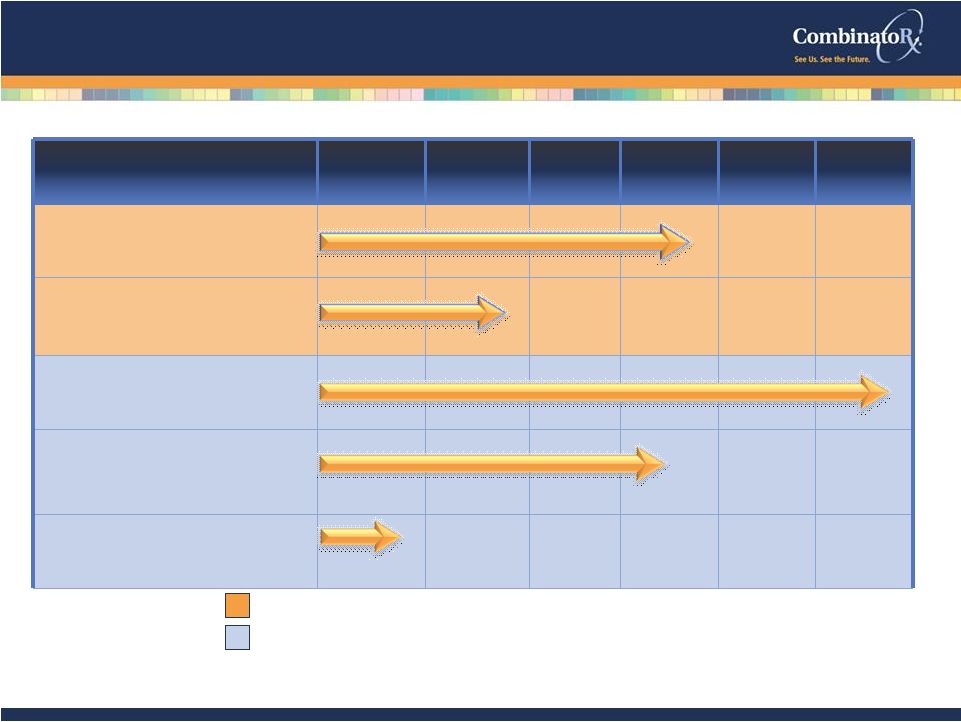 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL Pipeline: Focused on Pain and Inflammation Discovery Preclinical Phase I Phase II Phase III Market CRx-102 OA/RA Ion Channel candidates Pain Exalgo ™ (Covidien/Mallinckrodt) Pain Prednisporin™ (Sanofi-Aventis/Fovea) Ophthalmic Novartis Oncology 5 Partnered Programs Internal Programs (Synavive™) |
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL Leverage our proven pain and CNS drug development expertise • Exalgo (pain) • Tramadol ER (pain) • Actiq (pain) • Celebrex • Xanax XR (anxiety) • Imitrex, AXERT (migraine) • Aricept (Alzheimers) Underserved indications provide significant opportunities • Neuropathic pain • Osteoarthritis • Back pain • Cancer pain • Breakthrough pain >$24B WW pain market in 2007* >$33B WW pain market projected in 2017* * Datamonitor Report # DMHC2444 Pain: Opportunity to Produce Valuable Products 6 |
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL U.S. Commercial Partnership with Covidien (Mallinckrodt) – June 2009 • Received $55 million in upfront and milestone payments plus development funding • Significant tiered royalties on future sales • Launch expected in 2Q 2010 with expanded sales force Extended release Hydromorphone Exalgo ™ Indication: Moderate-to-severe pain (opioid tolerant patients requiring continuous analgesia) 7 Exalgo™ – Significant Revenue Opportunity |
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL Exalgo™ – Significant Product Opportunity US Long Acting Opioid (LAO) Market Large and Growing • 2008: 22 million Rxs - annual growth rate of 9.3% (IMS) • 2008: $4.8 billion in sales – annual growth rate of 18% (IMS) Strong performance of Opana ER- most recent LAO launched in US • Launched in US by Endo in July 2006 • 2007: 1% Rx share & 2% $ share or $83 million (IMS) • 2008: 2% Rx share & 3.4% $ share or $164 million (IMS) Strong launch performance of JURNISTA ® in Germany • Launched in Germany in 2006 by Janssen-Cilag • 2008: 3% Rx share & 5% $ share (IMS) Extended release Hydromorphone Exalgo ™ Indication: Moderate-to-severe pain (opioid tolerant patients requiring continuous analgesia) |
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL Currently no LA form of hydromorphone available in US • Only opioid of the 5 major strong opioids without a complimentary long acting formulation – key opportunity for Exalgo US Prescribers very familiar with hydromorphone IR • 2.5 million Rxs in 2008 - representing 19% share of Short Acting Opioid market with annual growth at 17% (IMS) • Provides strong foundation for potential Exalgo growth Highly concentrated prescribers in US facilitates efficient marketing and sales • 11,500 prescribers representing ~5% of total LAO prescribers (247,000) account for 50% of total LAO Rxs (WoltersKluwer Health, May 2008) Exalgo™ – Significant Product Opportunity Extended release Hydromorphone Exalgo ™ Indication: Moderate-to-severe pain (opioid tolerant patients requiring continuous analgesia) |
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL Sanofi-Aventis (Fovea) Collaboration Initiated in 2006 • $40 million in future milestones plus royalties on sales • All development fully funded by Sanofi-Aventis Potentially First Combo Therapy for Persistent Allergic Conjunctivitis • Targeting efficacy of potent steroid with improved safety profile • Concept discovered and patented by CRXX • Positive POC results in persistent allergic conjunctivitis Next Steps: • Phase 2b targeted for initiation in Q2 2010 Prednisporin™: Partnered with Sanofi-Aventis (Fovea) Prednisolone Acetate Cyclosporin A Target indications include: Persistent Allergic conjunctivitis Current Stage: Clinical *pimage is representative of delivery method Prednisporin™ * 10 |
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL Immuno-inflammatory benefits of steroids (glucocorticoids) without associated side effects • Potential NSAID/COXIB replacement in OA • Replacement of, or add-on to, some NSAIDS, steroids and DMARDs in RA. Efficacy observed in multiple Phase 2 studies • Knee OA -19.5 mm WOMAC benefit vs. placebo and 8 mm vs. prednisolone • Sustained efficacy through 1yr extension trial • Hand OA - SS AUSCAN pain improvement vs. placebo; hand pain benefit 45% vs. 23% placebo, 26% prednisolone • RA - SS ACR 20 of 63% compared to 28% placebo • Safe and well-tolerated - no
glucocorticoid-related AE’s through 1 yr extension trial
Aligned release formulation provides oral, once- daily administration Synavive™ Synavive™ 11 Dipyridamole MR Prednisolone DR Prednisolone IR Synavive™ Targeted indication: Osteoarthritis |
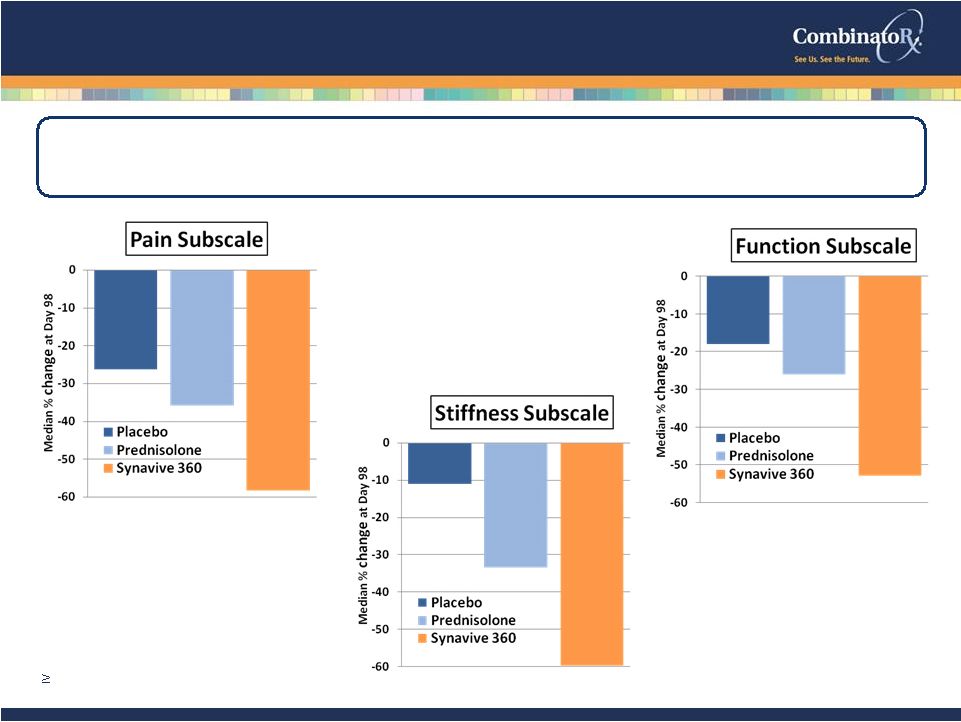 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL COMET-1: Synavive Efficacy in Knee OA Subjects with High Disease Burden • mITT LOCF, WOMAC Day 98 • Subjects with Baseline Patient Global Assessment VAS score 30 mm Placebo n=45 Pred n=40 Synavive 360 n=44 Synavive significantly improves WOMAC pain, function and stiffness measures 12 p = 0.017 p = 0.0023 p = 0.0034 |
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL COMET-1: 1 year Open Label Extension Safety Adverse Events for chronic glucocorticoid use – No worsening of glucocorticoid-related adverse events including eye changes, adrenal, glucose, and infection rate Adverse Events for chronic dipyridamole use – No bleeding or hypotension Bone mineral density (BMD) – Synavive neutral on bone effects Efficacy Sustained efficacy for subjects continued on Synavive (2.7/180) or (2.7/360) Enhanced efficacy for subjects transitioned from placebo to Synavive (2.7/180) Synavive demonstrated dissociation of efficacy and safety in a long-term extension trial 13 |
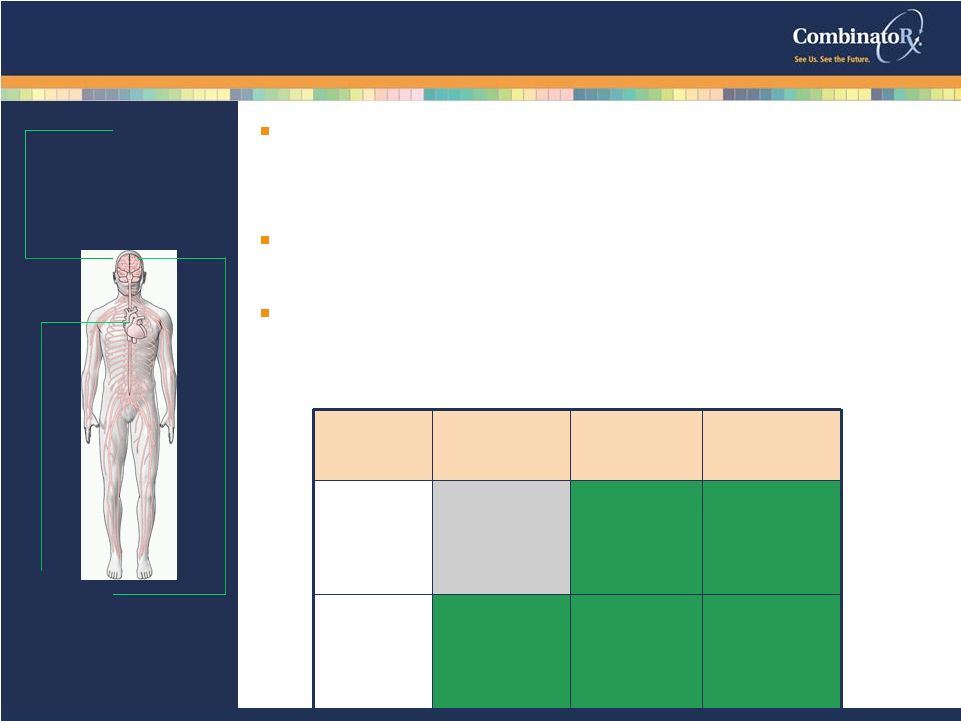 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL © Copyright Neuromed Ion Channel Blockers: Acute and Chronic Pain 14 Channel Family Acute Pain Inflammatory Pain Neuropathic Pain N-type Ca v 2.2 NO YES YES T-type Ca v 3.1-3 YES YES YES Proven Mechanism of Action • N-type directly linked to pain signal transmission • Validated target: Prialt (Ziconotide) Exclusive Patent Coverage • Covering diverse chemical series Focus on electrophysiology of calcium channel blockers • Extensive screening for off-target activity N-type NERVOUS SYSTEM chronic inflammatory pain chronic neuropathic pain T-type NERVOUS SYSTEM, HEART, VASCULAR acute and chronic pain |
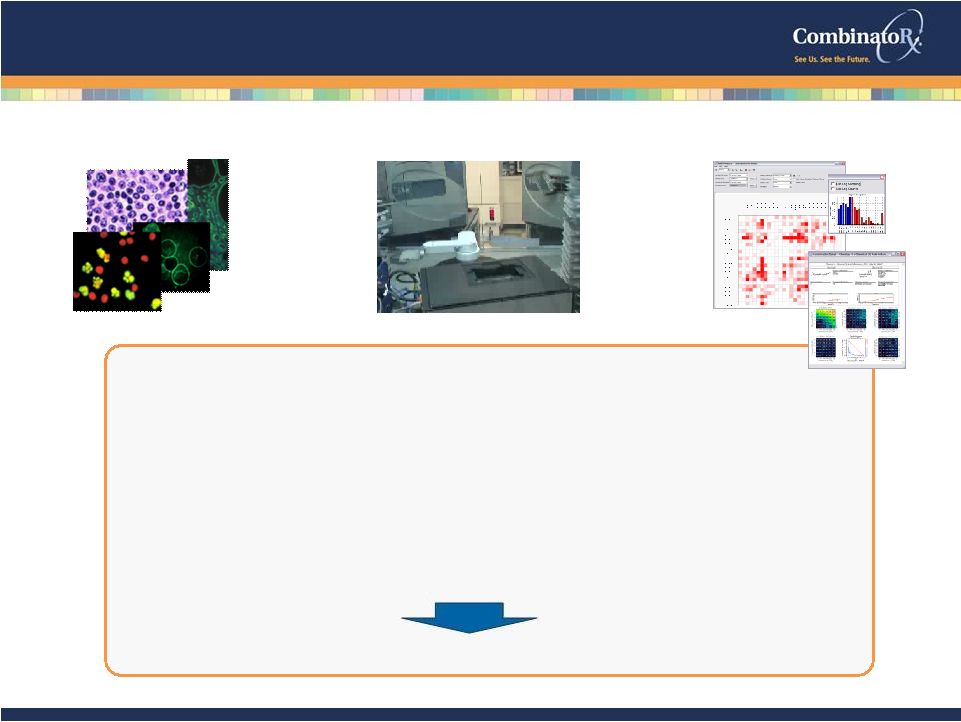 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL Diverse Library of drugs & molecular probes Cell-Based Phenotypic Assays CRXX Synergistic Drug Discovery Technology 15 cHTS Technology Platform Multi-target therapeutic discovery engine Industrialized: Systematic empirical testing of combinations >4,000 agents, >1,000 targets & mechanisms Comprehensive: High-throughput automation >250 cell lines, >20 disease-relevant tissues and normal
controls Proprietary: Software manages execution/analysis of combination activity Proven track record of discovering multi-target therapeutics
|
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL Discovery of novel anti-cancer combinations • $4m upfront; 2 year research funding • Up to $58m milestones per combination • Non-exclusive Major effort in scale and potential translation to clinic • Uniquely enabled by CRXX discovery and informatics capabilities • Broad spectrum of cell lines/cancers • Robust compound library including standards of care Valuable outputs for both partners • New development opportunities for NVS marketed and clinical compounds • New development opportunities for CRXX • New biology and intellectual property CRXX-Novartis Oncology Collaboration 16 Exploration of response by cancer type Systematic discovery of pathways and targets Exploration of response by cancer type Systematic discovery of pathways and targets cHTS/Chalice Compound library Oncology pipeline Compound library |
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL Management team with proven research/development track record • CEO: Mark Corrigan, MD • Sepracor, Pharmacia-Upjohn • EVP, R&D: Christopher Gallen, M.D., Ph.D. • Scripps, Quintiles, Premier Research, Pharmacia, Wyeth Significant resources to fund planned operations into 2014 • Ended 2009 with $25.9 million cash • $40 million milestone from Covidien received March 2010 Revenue generating collaborations (Exalgo, Prednisporin, Novartis) Pain and inflammation focused R&D Two valuable drug discovery platforms to fuel pipeline CombinatoRx Overview 17 |
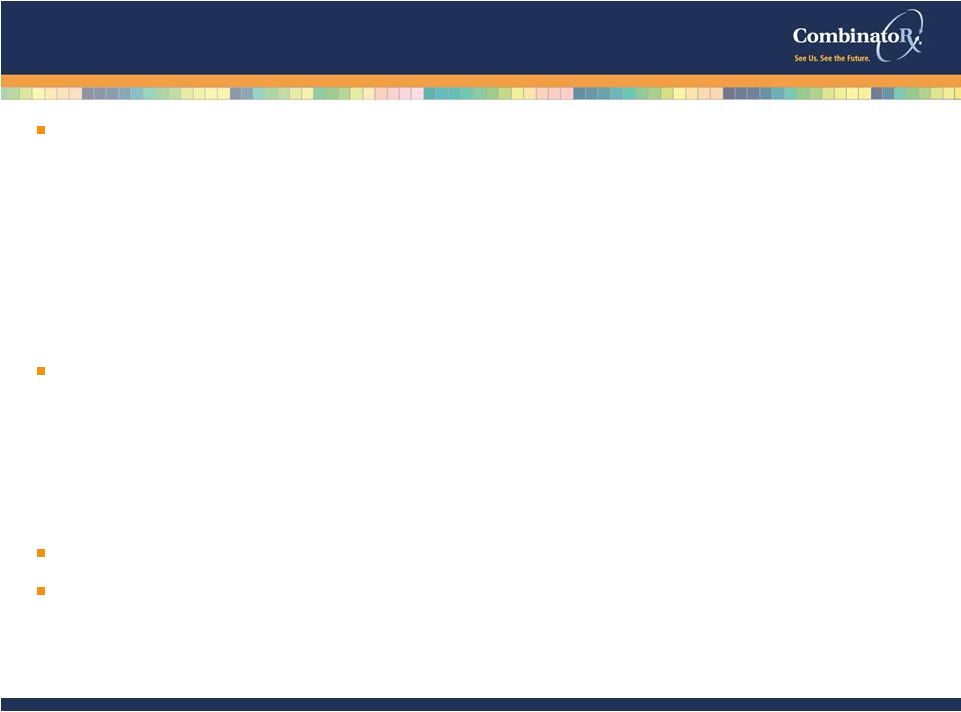
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL Product Pipeline Exalgo™ commercial partnership Exalgo™ approval Exalgo™ launch/revenue stream Synavive – advance development for the treatment of osteoarthritis Ion channel program - advance an N-type lead into development for pain Drug discovery technologies - discover new pain and inflammation product candidates for our internal portfolio Business Additional revenue-generating research and technology collaborations Maintain financial strength with sufficient resources to fund operations into 2014. Potential Near-term Value-Creating Events 18 |
 © 2009 CombinatoRx, Incorporated. All rights reserved. CONFIDENTIAL 19 Thank You |
