Attached files
| file | filename |
|---|---|
| EX-99.4 - EXHIBIT 99.4 - Theravance Biopharma, Inc. | tm2127424d1_ex99-4.htm |
| EX-99.2 - EXHIBIT 99.2 - Theravance Biopharma, Inc. | tm2127424d1_ex99-2.htm |
| EX-99.1 - EXHIBIT 99.1 - Theravance Biopharma, Inc. | tm2127424d1_ex99-1.htm |
| 8-K - FORM 8-K - Theravance Biopharma, Inc. | tm2127424d1_8k.htm |
Exhibit 99.3

Ampreloxetine Top - line Results from Phase 3 Study (0169) in Patients with Symptomatic Neurogenic Hypotension (nOH) September 15, 2021 THERAVANCE BIOPHARMA ® , THERAVANCE ® , the Cross/Star logo and MEDICINES THAT MAKE A DIFFERENCE ® are registered trademarks of the Theravance Biopharma group of companies (in the U.S. and certain other countries). All third party trademarks used herein are the property of their respective owners. © 2021 Theravance Biopharma. All rights reserved.

Forward - looking statements 2 Under the safe harbor provisions of the U . S . Private Securities Litigation Reform Act of 1995 , the company cautions investors that any forward - looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward - looking statements or projections . Examples of forward - looking statements in this presentation may include the Company's goals, designs, strategies, plans and objectives, the potential characteristics, benefits and mechanisms of action of the Company's product and product candidates, and interpretation of the results of our clinical trials or conclusions drawn therefrom . The company’s forward - looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to additional future analysis of the data resulting from our clinical trial(s) . Other risks affecting Theravance Biopharma are in the company's Form 10 - Q filed with the SEC on August 5 , 2021 , and other periodic reports filed with the SEC .

Top - line Results: Executive Summary ‣ Primary endpoint: ▪ No statistically significant difference in OHSA #1 score at Week 4 ‣ Secondary endpoints: Ampreloxetine did not demonstrate improvement in any secondary endpoints: 1. OHSA Composite Score 2. OHDAS Composite Score 3. PGI - C 4. Falls ‣ Safety: ▪ Ampreloxetine was well - tolerated as a single daily dose administered for 4 weeks at 10 mg ▪ No safety signal (including supine hypertension) and no clinically meaningful laboratory changes ‣ PK and PD: ▪ Consistent with expectations and target engagement consistent with Phase 2 findings OHDAS, orthostatic hypotension daily activity score; OHSA, orthostatic hypotension symptom assessment; OHSA#1, orthostatic hy pot ension symptom assessment question #1; PD, pharmacodynamics; PGI - C, patient global impression of change; PK, pharmacokinetics. 3
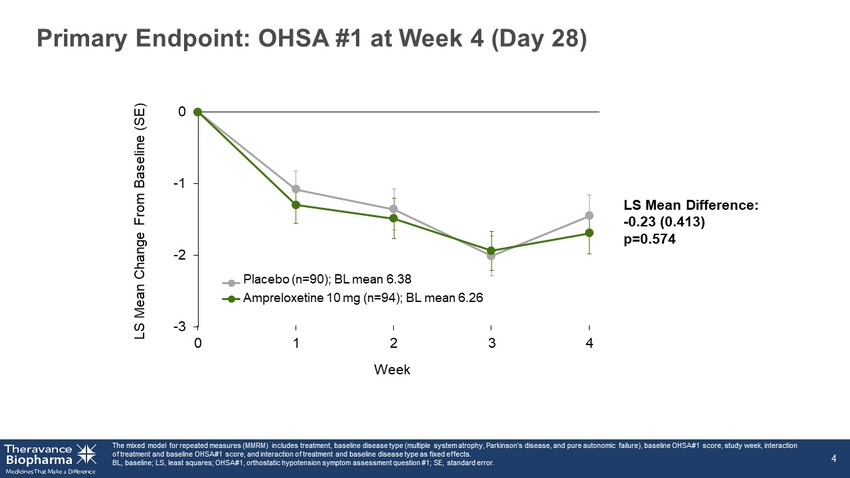
-3 -2 -1 0 0 1 2 3 4 Primary Endpoint: OHSA #1 at Week 4 (Day 28) The mixed model for repeated measures (MMRM) includes treatment, baseline disease type (multiple system atrophy, Parkinson’s dis ease, and pure autonomic failure), baseline OHSA#1 score, study week, interaction of treatment and baseline OHSA#1 score, and interaction of treatment and baseline disease type as fixed effects. BL, baseline; LS, least squares; OHSA#1, orthostatic hypotension symptom assessment question #1; SE, standard error. 4 LS Mean Change From Baseline (SE) LS Mean Difference: - 0.23 (0.413) p=0.574 Week Placebo (n=90); BL mean 6.38 Ampreloxetine 10 mg (n=94); BL mean 6.26
Safety Summary ‣ Well - tolerated as a single daily dose administered for 4 weeks at 10 mg ‣ No deaths occurred during study ‣ Serious adverse events (n=2 placebo, n=4 ampreloxetine) were not considered related to treatment ‣ Most TEAEs were mild or moderate ‣ No clinically meaningful differences in lab values, ECG parameters, or vital signs between the two groups ‣ No signal for supine hypertension, including worsening of pre - existing supine hypertension ECG, electrocardiogram; TEAE, treatment emergent adverse event. 5
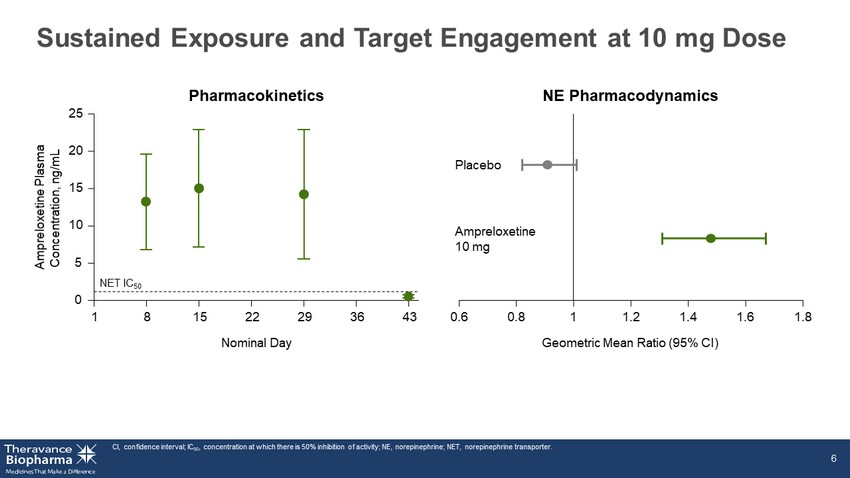
Sustained Exposure and Target Engagement at 10 mg Dose CI, confidence interval; IC 50 , concentration at which there is 50% inhibition of activity; NE, norepinephrine; NET, norepinephrine transporter. 6 NE Pharmacodynamics Pharmacokinetics Ampreloxetine Plasma Concentration, ng/mL 0 5 10 15 20 25 1 8 15 22 29 36 43 Nominal Day NET IC 50 Placebo Ampreloxetine 10 mg 0.6 0.8 1 1.2 1.4 1.6 1.8 Geometric Mean Ratio (95% CI)
