Attached files
| file | filename |
|---|---|
| EX-32 - EX-32 - Theravance Biopharma, Inc. | a2231044zex-32.htm |
| EX-31.2 - EX-31.2 - Theravance Biopharma, Inc. | a2231044zex-31_2.htm |
| EX-31.1 - EX-31.1 - Theravance Biopharma, Inc. | a2231044zex-31_1.htm |
| EX-23.1 - EX-23.1 - Theravance Biopharma, Inc. | a2231044zex-23_1.htm |
| EX-21.1 - EX-21.1 - Theravance Biopharma, Inc. | a2231044zex-21_1.htm |
Use these links to rapidly review the document
TABLE OF CONTENTS
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
PART IV
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark One) | ||
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended December 31, 2016 |
||
OR |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to |
||
Commission File No. 001-36033
THERAVANCE BIOPHARMA, INC.
(Exact name of registrant as specified in its charter)
| Cayman Islands (State or Other Jurisdiction of Incorporation or Organization) |
98-1226628 (I.R.S. Employer Identification No.) |
|
P.O. Box 309 Ugland House, South Church Street George Town, Grand Cayman, Cayman Islands (Address of Principal Executive Offices) |
94080 (Zip Code) |
Registrant's telephone number, including area code: 650-808-6000
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
| Title of Each Class | Name of Each Exchange On Which Registered | |
|---|---|---|
| Ordinary Share $0.00001 Par Value | NASDAQ Global Market |
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT: NONE
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ý No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer. See definition of "accelerated filer and large accelerated filer" in Rule 12b-2 of the Exchange Act (Check One):
| Large accelerated filer ý | Accelerated filer o | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant based upon the closing price on the NASDAQ Global Market on June 30, 2016 was $842,602,816.
On January 31, 2017, there were 52,855,487 of the registrant's ordinary shares outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Specified portions of the registrant's definitive Proxy Statement to be issued in conjunction with the registrant's 2017 Annual Meeting of Shareholders, which is expected to be filed not later than 120 days after the registrant's fiscal year ended December 31, 2016, are incorporated by reference into Part III of this Annual Report. Except as expressly incorporated by reference, the registrant's Proxy Statement shall not be deemed to be a part of this Annual Report on Form 10-K.
THERAVANCE BIOPHARMA, INC.
2016 Form 10-K Annual Report
Table of Contents
2
Special Note regarding Forward-Looking Statements
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the "Securities Act"), and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). Such forward-looking statements involve substantial risks, uncertainties and assumptions. All statements in this Annual Report on Form 10-K, other than statements of historical facts, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans, intentions, designs, expectations and objectives could be forward-looking statements. The words "aim," "anticipate," "believe," "contemplate," "continue," "could," "designed," "developed," "drive," "estimate," "expect," "goal," "intend," "may," "mission," "opportunities," "plan," "potential," "predict," "project," "pursue," "represent," "seek," "suggest," "should," "target," "will," "would" and similar expressions (including the negatives thereof) are intended to identify forward looking statements, although not all forward looking statements contain these identifying words. These statements reflect our current views with respect to future events or our future financial performance, are based on assumptions, and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. We may not actually achieve the plans, intentions, expectations or objectives disclosed in our forward-looking statements and the assumptions underlying our forward-looking statements may prove incorrect. Therefore, you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions, expectations and objectives disclosed in the forward-looking statements that we make. Factors that we believe could cause actual results or events to differ materially from our forward-looking statements include, but are not limited to, those discussed below in "Risk Factors" in Item 1A, "Management's Discussion and Analysis of Financial Condition and Results of Operations" in Item 7 and elsewhere in this Annual Report on Form 10-K. Our forward-looking statements in this Annual Report on Form 10-K are based on current expectations and we do not assume any obligation to update any forward-looking statements for any reason, even if new information becomes available in the future. When used in this report, all references to "Theravance Biopharma", the "Company", or "we" and other similar pronouns refer to Theravance Biopharma, Inc. collectively with its subsidiaries.
3
Overview
Theravance Biopharma, Inc. ("Theravance Biopharma") is a diversified biopharmaceutical company with the core purpose of creating medicines that help improve the lives of patients suffering from serious illness.
Our pipeline of internally discovered product candidates includes potential best-in-class medicines to address the unmet needs of patients being treated for serious conditions primarily in the acute care setting. VIBATIV® (telavancin), our first commercial product, is a once-daily dual-mechanism antibiotic approved in the U.S., Europe and certain other countries for certain difficult-to-treat infections. Revefenacin (TD-4208) is a long-acting muscarinic antagonist ("LAMA") being developed as a potential once-daily, nebulized treatment for chronic obstructive pulmonary disease ("COPD"). Our neprilysin ("NEP") inhibitor program is designed to develop selective NEP inhibitors for the treatment of a range of major cardiovascular and renal diseases, including acute and chronic heart failure, hypertension and chronic kidney diseases such as diabetic nephropathy. Our research efforts are focused in the areas of inflammation and immunology, with the goal of designing medicines that provide targeted drug delivery to tissues in the lung and gastrointestinal tract in order to maximize patient benefit and minimize risk. The first program to emerge from this research is designed to develop intestinally restricted pan-Janus kinase ("JAK") inhibitors for the treatment of a range of inflammatory intestinal diseases.
In addition, we have an economic interest in future payments that may be made by Glaxo Group Limited or one of its affiliates ("GSK") pursuant to its agreements with Innoviva, Inc. ("Innoviva") (known as Theravance, Inc. prior to January 7, 2016) relating to certain drug development programs, including the combination of fluticasone furoate, umeclidinium, and vilanterol (the "Closed Triple"), currently in development for the treatment of COPD and asthma.
2016 Highlights
In 2016, we accomplished a number of key corporate goals directed towards creating medicines to help improve the lives of patients. We reported positive Phase 1 clinical results for two potentially best-in-class programs: our intestinally restricted JAK inhibitor program for inflammatory intestinal diseases and our NEP inhibitor program for cardiovascular and renal diseases and progressed candidates from pre-clinical development into early clinical development in our JAK inhibitor program. We completed enrollment in each of our three studies in the Phase 3 program for revefenacin (TD-4208) in COPD. Of these, we reported positive results from two replicate efficacy studies while the long term safety study remains ongoing. We progressed two other key programs in Phase 2 clinical development: our highly selective 5-HT4 receptor agonist velusetrag (TD-5108) in gastroparesis, for which we received Fast Track designation from the Food and Drug Administration ("FDA") for the treatment of symptoms associated with idiopathic and diabetic gastroparesis, and our norepinephrine and serotonin reuptake inhibitor (NSRI) TD-9855 in neurogenic orthostatic hypotension ("nOH"). We entered into a global license, development and commercialization agreement with Millennium Pharmaceuticals, Inc., a subsidiary of Takeda Pharmaceutical Company Limited (together, "Takeda") for TD-8954, a selective 5-HT4 receptor agonist for the treatment of enteral feeding intolerance and other gastrointestinal motility disorders. We also continued to execute our commercial strategy for VIBATIV including the progression of the Telavancin Observational Use Registry (TOURTM), a patient registry study designed to assess how VIBATIV is being used in real-world clinical settings, and the Phase 3 bacteremia study designed to expand the product's existing label. Finally, we strengthened our balance sheet through public offerings, the proceeds of which are intended for general corporate purposes including the support of key programs and objectives.
4
Our Programs
The table below summarizes the status of our approved product and our most advanced product candidates in development. Our research and development activities are concentrated primarily on four therapeutic areas—infectious disease, respiratory, gastrointestinal disease and cardiovascular and renal disease—and our commercial infrastructure is focused primarily on the acute care setting. The table also includes the status of the respiratory programs in which we have an economic interest and are being developed by GSK pursuant to agreements between Innoviva and GSK ("GSK-Partnered Respiratory Programs"). These programs consist of the Closed Triple program, the Inhaled Bifunctional Muscarinic Antagonist-Beta2 Agonist ("MABA") program and other future products that may be combined with Closed Triple or MABA. We have an economic interest in these programs through our interest in Theravance Respiratory Company, LLC ("TRC"), a limited liability company managed by Innoviva. The status of all GSK programs referenced in this Annual Report on Form 10-K solely reflects publicly available information.
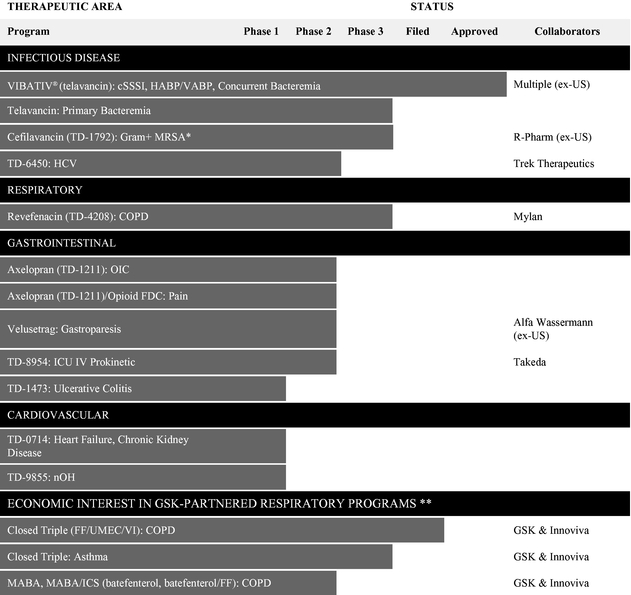
- *
- R-Pharm is conducting a Phase 3 clinical study of TD-1792 in complicated skin and soft tissues infections (cSSSI), caused by gram-positive bacteria with clinical sites in the Russian Federation and the country of Georgia. Not currently under development in the United States.
5
- **
- The information regarding the Closed Triple and the MABA programs are based solely upon publicly available information and may not reflect the most recent developments under the programs.
Glossary of Defined Terms used in Table Above:
CNS: Central Nervous System;
COPD: Chronic Obstructive Pulmonary Disease;
cSSSI: Complicated Skin and Skin Structure Infections;
FDC: Fixed Dose Combination;
FF: Fluticasone Furoate;
GI: Gastrointestinal;
HABP/VABP: Hospital-Acquired and Ventilator-Associated Bacterial Pneumonia;
HCV: Hepatitis C Virus;
ICS: Inhaled Corticosteriod;
MABA: Bifunctional Muscarinic Antagonist-Beta2 Agonist;
MRSA: Methicillin-Resistant Staphylococcus Aureus;
nOH: Neurogenic Orthostatic Hypotension;
OIC: Opioid Induced Constipation;
UMEC: Umeclidinium;
VI: Vilanterol;
Status: The most advanced stage of clinical development that has been completed or is in process;
Phase 1: initial clinical safety testing into patients or healthy human volunteers, or studies directed toward understanding the mechanisms of action of the drug;
Phase 2: further clinical safety testing and preliminary efficacy testing in a limited patient population;
Phase 3: evaluation of clinical efficacy and safety within an expanded patient population;
Filed: a marketing application has been submitted to a regulatory authority; and
Approved: approved for marketing.
Program Highlights
VIBATIV® (telavancin)
VIBATIV is a bactericidal, once-daily injectable antibiotic to treat patients with serious, life-threatening infections due to Staphylococcus aureus and other Gram-positive bacteria, including methicillin-resistant ("MRSA") strains. VIBATIV is approved in the U.S. for the treatment of adult patients with complicated skin and skin structure infections ("cSSSI") caused by susceptible Gram-positive bacteria and for the treatment of adult patients with hospital-acquired and ventilator-associated bacterial pneumonia ("HABP"/ "VABP") caused by susceptible isolates of Staphylococcus aureus when alternative treatments are not suitable. VIBATIV is indicated in the European Union ("EU") for the treatment of adults with nosocomial pneumonia, including ventilator-associated
6
pneumonia, known or suspected to be caused by MRSA when other alternatives are not suitable. VIBATIV is also indicated in Canada and Russia for cSSSI and HABP and VABP caused by Gram-positive bacteria, including MRSA.
Our focused acute care sales force currently markets VIBATIV in the U.S., and we maintain an independent sales, marketing, and medical affairs team. Outside of the U.S., our strategy is to market VIBATIV through a network of partners. To date, we have secured partners for VIBATIV in the following geographies—Canada, Middle East and North Africa, Israel, Russia, China and India. In August 2016, we and Clinigen Group ("Clinigen") reached a mutual decision for Clinigen to return commercial rights to market and distribute VIBATIV in the EU to Theravance Biopharma. On November 4, 2016, the European Commission approved the transfer of the centralized marketing authorization for VIBATIV from Clinigen to our wholly-owned Irish subsidiary, Theravance Biopharma Ireland Limited. We are in discussion with potential collaborators with the goal of establishing a new strategic commercial partnership in the EU.
Supplemental New Drug Application (sNDA) for Concurrent Staphylococcus aureus Bacteremia
In May 2016, we announced approval of our sNDA by the FDA allowing for the addition of new clinical data to the VIBATIV label concerning concurrent bacteremia in cases of HABP/VABP and cSSSI. The sNDA submission was based on the combined data from our previously conducted pivotal trials of VIBATIV in its two approved indications—cSSSI (ATLAS I and ATLAS II) and HABP/VABP (ATTAIN I and ATTAIN II). The trials were large, multi-center, multi-national, double-blind, randomized Phase 3 clinical studies enrolling and treating 3,370 adult patients, including a portion of patients with concurrent bacteremia. Importantly, these studies involved two of the largest cohorts of patients ever studied in these diseases and included one of the largest cohorts of patients with MRSA infections studied to date. Separately, we are conducting a Phase 3 registrational study in patients with Staphylococcus aureus bacteremia.
Phase 3 Registrational Study in Staphylococcus aureus Bacteremia
As part of our effort to explore additional settings in which VIBATIV may offer patients therapeutic benefit, in February 2015, we initiated a Phase 3 registrational study for the treatment of patients with Staphylococcus aureus bacteremia. The 250-patient registrational study is a multi-center, randomized, open-label study designed to evaluate the non-inferiority of telavancin in treating Staphylococcus aureus bacteremia as compared to standard therapy. Key secondary outcome measures of the study include an assessment of the duration of bacteremia post-randomization and the incidence of development of metastatic complications, as compared to standard therapy. We expect to complete the study in 2018.
Telavancin Observational Use Registry ("TOURTM") Study
Initiated in February 2015, the 1,000-patient TOURTM study is designed to assess the manner in which VIBATIV is used by healthcare practitioners to treat patients. By broadly collecting and examining data related to VIBATIV treatment patterns, as well as clinical and safety outcomes in the real world, we aim to create an expansive knowledge base to guide future development and optimal use of the drug. In February 2017, we announced that enrollment in the TOURTM study was complete.
In October 2016, we announced interim data from the TOURTM study. An initial review of data from the first 200 patients enrolled in TOUR demonstrate clinical response rates of 74% in a range of difficult-to-treat infection types including HABP/VABP, cSSSI, bone and joint infections and bacteremia. Results show 17% of the first 200 patients were considered non-evaluable with 9% deemed to have failed treatment. Clinical response was defined as cure or improvement leading to step-down oral therapy.
7
In January 2017, we announced interim data from the TOURTM study, focused on a subset of registry patients with diagnoses of bacteremia or infective endocarditis. Data demonstrated positive clinical responses in 64% of patients, with 7% of patients failing treatment and 29% considered non-evaluable. Positive clinical response was defined as cure or improvement leading to step-down oral therapy.
Long-Acting Muscarinic Antagonist—Revefenacin (TD-4208)
Revefenacin is an investigational long acting muscarinic antagonist ("LAMA") in development for the treatment of COPD. We believe that revefenacin may become a valuable addition to the COPD treatment regimen and that it represents a significant commercial opportunity. Our market research indicates there is an enduring population of COPD patients in the U.S. that either need or prefer nebulized delivery for maintenance therapy. LAMAs are a cornerstone of maintenance therapy for COPD, but existing LAMAs are only available in handheld devices that may not be suitable for every patient. Revefenacin has the potential to be a best-in-class once-daily single-agent product for COPD patients who require, or prefer, nebulized therapy. The therapeutic profile of revefenacin, together with its physical characteristics, suggest that this LAMA could serve as a foundation for combination products and for delivery in metered dose inhaler and dry powder inhaler products.
Mylan Collaboration
In January 2015, Mylan Ireland Limited ("Mylan") and we established a strategic collaboration for the development and, subject to regulatory approval, commercialization of revefenacin. Partnering with a world leader in nebulized respiratory therapies enables us to expand the breadth of our revefenacin development program and extend our commercial reach beyond the acute care setting where we currently market VIBATIV. Funding of the Phase 3 development program by Mylan strengthens our capital position and enhances our financial flexibility to advance other high-value pipeline assets alongside revefenacin.
Under the terms of the Mylan Development and Commercialization Agreement (the "Mylan Agreement"), Mylan and we are co-developing nebulized revefenacin for COPD and other respiratory diseases. We are leading the U.S. Phase 3 development program and Mylan is responsible for reimbursement of our costs related to the registrational program up until the approval of the first new drug application, after which costs will be shared. If a product developed under the collaboration is approved in the U.S., Mylan will lead commercialization and we will retain the right to co-promote the product in the U.S. under a profit-sharing arrangement (65% Mylan/35% Theravance Biopharma). Outside the U.S. (excluding China), Mylan will be responsible for development and commercialization and will pay us a tiered royalty on net sales at percentage royalty rates ranging from low double-digits to mid-teens.
Under the Mylan Agreement, Mylan paid us an initial payment of $15.0 million in cash in the second quarter of 2015. Also, pursuant to an ordinary share purchase agreement entered into on January 30, 2015, Mylan Inc., the indirect parent corporation of Mylan, made a $30.0 million equity investment in us, buying 1,585,790 ordinary shares from us in early February 2015 in a private placement transaction at a price of approximately $18.918 per share, which represented a 10% premium over the volume weighted average price per share of our ordinary shares for the five trading days ending on January 30, 2015. In February 2016, we earned a $15.0 million development milestone payment for achieving 50% enrollment in the Phase 3 twelve-month safety study. As of December 31, 2016, we are eligible to receive from Mylan additional potential development, regulatory and sales milestone payments totaling up to $205.0 million in the aggregate, with $160.0 million associated with revefenacin monotherapy and $45.0 million for future potential combination products. Of the $160.0 million associated with monotherapy, $150.0 million relates to commercialization and
8
$10.0 million relates to regulatory actions in the EU. We do not expect to earn any milestone payments from Mylan in 2017.
We retain worldwide rights to revefenacin delivered through other dosage forms, such as a metered dose inhaler or dry powder inhaler ("MDI"/"DPI"), while Mylan has certain rights of first negotiation with respect to our development and commercialization of revefenacin delivered other than via a nebulized inhalation product.
Phase 3 Study in COPD
In September 2015, we announced, with our partner Mylan, the initiation of the Phase 3 development program for revefenacin for the treatment of COPD. The Phase 3 development program, designed to support the registration of the product in the U.S., includes two replicate three-month efficacy studies and a single twelve-month safety study. The two efficacy studies examined 2 doses (88 mcg and 175 mcg) of revefenacin inhalation solution administered once-daily via nebulizer in patients with moderate to severe COPD. The Phase 3 efficacy studies were replicate, randomized, double-blind, placebo-controlled, parallel-group trials designed to provide pivotal efficacy and safety data for once-daily revefenacin over a dosing period of 12 weeks, with a primary endpoint of trough forced expiratory volume in one second (FEV1) on day 85. The Phase 3 safety study is an open-label, active comparator study of 12 months duration. In February 2016, we announced the achievement of 50% enrollment in all three of the Phase 3 clinical studies for revefenacin. The achievement of 50% enrollment in the twelve-month safety study triggered a $15.0 million milestone payment to us by Mylan.
In October 2016, we announced positive top line results from the two replicate Phase 3 efficacy studies of revefenacin in more than 1,250 moderate to very severe COPD patients. Both Phase 3 efficacy studies met their primary endpoints, demonstrating statistically significant improvements over placebo in trough forced expiratory volume in one second (FEV1) after 12 weeks of dosing for each of the revefenacin doses studied (88 mcg once daily and 175 mcg once daily). The studies also demonstrated that the 88 mcg and 175 mcg doses of revefenacin were generally well-tolerated, with comparable rates of adverse events and serious adverse events across all treatment groups (active and placebo). In addition to the two efficacy studies, the safety study has enrolled more than 1,050 patients and is expected to be completed in mid-2017. Together, the three studies enrolled approximately 2,300 patients. Should results from the safety study be supportive, we expect to file a new drug application for revefenacin with the FDA by the end of 2017.
Velusetrag (TD-5108)
Velusetrag is an oral, investigational medicine developed for gastrointestinal motility disorders. It is a highly selective agonist with high intrinsic activity at the human 5-HT4 receptor. Velusetrag is being developed in collaboration with Alfa Wassermann S.p.A. ("Alfa Wassermann") in a two-part Phase 2 program to test the efficacy, safety and tolerability of velusetrag in the treatment of patients with gastroparesis. Positive top-line results from the initial Phase 2 proof-of-concept study under this partnership, which evaluated gastric emptying, safety and tolerability of multiple doses of velusetrag, were announced in April 2014. In March 2015, we initiated a Phase 2b study of velusetrag for the treatment of patients with gastroparesis. The 200-patient study is a multi-center, double-blind, randomized, placebo-controlled, parallel-group trial which will explore the efficacy and safety of multiple doses of velusetrag in patients with diabetic or idiopathic gastroparesis. The twelve-week study will test three doses: 5, 15, and 30 mg administered once-daily. The primary endpoint will be the effect of velusetrag on symptoms in subjects with gastroparesis. The study will also evaluate the effect of velusetrag on gastric emptying, and the psychometric properties of the Gastroparesis Rating Scale, a daily patient-reported outcome measure. In February 2017, we announced the completion of enrollment in the study. We currently expect results from the Phase 2b study in mid-2017. Pursuant to our
9
agreement with Alfa Wassermann, the first Phase 2 study was, and the majority of the Phase 2b study is, funded by Alfa Wassermann.
In December 2016, the FDA granted Fast Track designation to velusetrag for the treatment of symptoms associated with idiopathic and diabetic gastroparesis. The FDA's Fast Track program was established to facilitate the development and expedite the review of drugs with the potential to treat serious conditions and address an unmet medical need.
TD-9855
TD-9855 is an investigational norepinephrine and serotonin reuptake inhibitor (NSRI). TD-9855 completed a Phase 2 study in patients with fibromyalgia, demonstrating statistically significant and clinically meaningful improvements in pain and core symptoms at the highest dose tested compared to placebo. We are assessing the potential use of TD-9855 in neurogenic orthostatic hypotension (nOH), and in May 2016, we initiated a Phase 2a study of TD-9855 in this indication. The 30 patient study is a randomized, two-part, single- and double-blind trial conducted in male and female subjects with nOH to evaluate the effect of TD-9855 in improving symptoms of nOH. The Phase 2a study is designed to evaluate postural changes in blood pressure, symptom reduction, and safety and tolerability. In February 2017, we announced our plan to amend the protocol of the Phase 2a study to allow patients who respond to continue beyond a single dose. We currently expect to complete the extended Phase 2a study by the end of 2017.
Oral Peripherally-Acting Mu Opioid Receptor Antagonist—Axelopran (TD-1211)
OIC Program
Axelopran is an investigational, once-daily, oral peripherally-active mu opioid receptor antagonist for opioid- induced constipation ("OIC"). The axelopran Phase 2 program demonstrated a clinically meaningful treatment effect in OIC patients compared to placebo. The goal for this program is to demonstrate the ability to normalize bowel function without impacting analgesia and improve a variety of GI symptoms associated with constipation, which could provide axelopran with a competitive advantage in the OIC market if demonstrated in Phase 3 studies and approved by regulatory authorities. We have developed a patient reported outcomes tool designed to measure patient symptoms which would be used in a Phase 3 registrational program and potentially generate data that could differentiate the product from the competition.
Fixed Dose Combination
In December 2014, we completed a Phase 1 study to determine the relative bioavailability of OxyContin® (oxycodone) and axelopran after oral administration as a fixed dose combination ("FDC") relative to the individual components administered together. The study examined a spray-coat application of axelopran to an opioid, OxyContin, to determine the effect of axelopran on OxyContin exposure. The study compared exposure of OxyContin alone, axelopran alone, OxyContin and axelopran administered as two separate tablets, and OxyContin spray-coated with axelopran in a FDC. Study results demonstrated that axelopran does not significantly alter systemic exposure to OxyContin when delivered as a FDC relative to when co-administered as individual tablets. A FDC of axelopran and an opioid could present an important market opportunity, as it has the potential to provide pain relief without constipation in a single abuse-deterrent pill for patients using opioids on a chronic basis.
NS5A Inhibitor—TD-6450
TD-6450 is a multivalent NS5A inhibitor. TD-6450 has successfully completed Phase 1 studies in both healthy volunteers and hepatitis C virus ("HCV") patients. In September 2015, we entered into a licensing agreement with Trek Therapeutics, PBC ("TREKtx") (the "TREKtx Agreement") granting
10
TREKtx an exclusive worldwide license for the development, manufacturing, use, marketing and sale of TD-6450 as a component in combination HCV products (the "HCV Products"). Pursuant to the TREKtx Agreement, we received an upfront payment of $8.0 million in the form of TREKtx's Series A preferred stock and will be eligible to receive future royalties based on net sales of the HCV Products. In October 2015, TREKtx initiated an open-label Phase 2a clinical trial to evaluate faldaprevir ("FDV"), an HCV protease inhibitor, combined with TD-6450 and ribavirin ("RBV") in patients infected with HCV genotype 4. In September 2016, TREKtx announced interim data from the study that showed the sustained viral response (SVR) rate four weeks after the completion of treatment (SVR4) was 100% (16 of 16) in treatment naïve patients with chronic genotype 4 HCV who received 120 mg of FDV and RBV in combination with 60 mg or 120 mg of TD-6450 for 12 weeks. In February 2017, TREKtx announced that 100% of these patients (16 of 16) had maintained SVR at twelve weeks after the completion of treatment (SVR12) as well. TREKtx is conducting a second Phase 2a study of FDV and TD-6450, with and without RBV in patients with HCV genotype 1b. In the ongoing study, TREKtx reported that 14 out of 15 patients in the study arm containing RBV achieved SVR4.
Neprilysin (NEP) Inhibitor Program (TD-0714 and TD-1439)
Neprilysin ("NEP") is an enzyme that degrades natriuretic peptides. These peptides play a protective role in controlling blood pressure and preventing cardiovascular tissue remodeling. Inhibiting NEP may result in clinical benefit for patients, including diuresis, control of blood pressure, and reversing maladaptive changes in the heart and vascular tissue in patients with congestive heart failure. Our primary objective is to develop a NEP inhibitor that could be used across a broad population of patients with cardiovascular and renal diseases, including acute and chronic heart failure and chronic kidney disease, including diabetic nephropathy. We aim to create a platform for multiple combination products with our NEP inhibitor with features that are differentiated from currently available products. Specifically, we intend to develop compounds that are non-renally cleared, dosed once-daily, dosed alone or in combination with other medicines and that may be dosed orally or intravenously.
TD-0714
Phase 1 Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD) Studies
In March 2016, we completed a Phase 1 randomized, double-blind, placebo-controlled, single ascending dose ("SAD") study in healthy volunteers of our most advanced NEP inhibitor compound, TD-0714. The study was designed to assess the safety, tolerability and pharmacokinetics of TD-0714, as well as measure biomarker evidence of target engagement and the amount of the drug that is eliminated via the kidneys. Results from the SAD study of TD-0714 demonstrate that the compound achieved maximal and sustained levels of target engagement for 24 hours after a single-dose, supporting the drug's potential for once-daily dosing. Target engagement was measured by dose-related increases in the levels of cyclic GMP (cGMP, a well-precedented biomarker of NEP engagement). TD-0714 also demonstrated very low levels of renal elimination, as evidenced by intravenous microtracer testing technology, and a favorable tolerability profile. These results met our target product profile and provide confidence for future efficacy studies of TD-0714 in a broad range of cardiovascular and renal diseases, including in patients with compromised renal function.
In October 2016, we completed a Phase 1 randomized, double-blind, placebo-controlled, multiple ascending dose ("MAD") study in healthy volunteers of TD-0714. The findings from the MAD study were consistent with the Phase 1 randomized, double-blind, placebo-controlled, SAD study in healthy volunteers we completed in March 2016, demonstrating sustained target engagement, low levels of renal elimination, and a favorable tolerability profile. Findings from the studies support clinical progression of TD-0714, which potential studies are being evaluated in the context of our overall NEPi program.
11
TD-1439
In September 2016, we progressed a second NEP inhibitor compound, TD-1439, which is structurally distinct from TD-0714, into Phase 1 randomized, double-blind, placebo-controlled, SAD and MAD studies in healthy volunteers. In February 2017, we announced favorable results from the Phase 1 SAD study. In this study, TD-1439 demonstrated characteristics consistent with our target product profile, including sustained 24-hour target engagement, low levels of renal elimination and a favorable tolerability profile. We expect to complete the Phase 1 MAD study in the first half of 2017.
We are currently evaluating next steps for the compounds in our NEPi clinical program, including compound and formulation selection, potential combinations, study population, and timing.
Intestinally Restricted Pan-Janus Kinase (JAK) Inhibitor Program (TD-1473 and TD-3504)
JAK inhibitors function by inhibiting the activity of one or more of the Janus kinase family of enzymes (JAK1, JAK2, JAK3, TYK2) that play a key role in cytokine signaling. Inhibiting these JAK enzymes interferes with the JAK/STAT signaling pathway and, in turn, modulates the activity of a wide range of pro-inflammatory cytokines. JAK inhibitors are currently approved for the treatment of rheumatoid arthritis and myelofibrosis and have demonstrated therapeutic benefit for patients with ulcerative colitis. However, these products are known to have side effects based on their systemic exposure. Our goal is to develop an orally administered, intestinally restricted pan-JAK inhibitor specifically designed to distribute adequately and predominantly to the tissues of the intestinal tract, treating inflammation in those tissues while minimizing systemic exposure. We are focused on utilizing targeted JAK inhibitors for potential treatment of a range of inflammatory intestinal diseases including ulcerative colitis.
TD-1473
Phase 1 Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD) Studies
In June 2016, we completed a Phase 1 clinical study of TD-1473, an internally-discovered JAK inhibitor that has demonstrated a high affinity for each of the JAK family of enzymes. The primary objective of the study was to evaluate the safety and tolerability of single ascending and multiple ascending doses of TD-1473 in healthy volunteers. A key secondary objective of the trial was to characterize the pharmacokinetics of TD-1473, including the determination of the amount of TD-1473 that entered systemic circulation following oral administration. Data from the study demonstrated TD-1473 to be generally well tolerated. Study results also demonstrated that systemic exposures of TD-1473 were low relative to that reported for tofacitinib, a JAK inhibitor currently in development for ulcerative colitis. At steady state, the plasma exposures of TD-1473 were significantly lower than the plasma exposure of tofacitinib.
Furthermore, subjects exhibited high stool concentrations of TD-1473, which were comparable to concentrations associated with efficacy in preclinical colitis models. Preclinical studies also demonstrated penetration of TD-1473 into the intestinal wall and membrane. The data generated from the study met our target pharmacokinetic profile and support clinical progression of the compound.
Previously announced findings from a preclinical model of colitis evaluating TD-1473 and tofacitinib demonstrated that both compounds significantly reduced disease activity scores. However, at doses providing similar preclinical efficacy, the systemic exposure of TD-1473 was much lower than that of tofacitinib and TD-1473 did not reduce systemic immune cell counts, in contrast to tofacitinib. Based on these preclinical findings, we believe that TD-1473 represents a potential breakthrough approach to treating ulcerative colitis without the risk generally associated with systemically active therapies.
12
Phase 1b Study
In October 2016, we announced dosing of the first patient in a Phase 1b clinical study of TD-1473 in patients with moderate to severe ulcerative colitis. The multi-center, randomized, double-blind, multi-dose, placebo-controlled study is designed to enroll 40 patients randomized to receive one of three doses of TD-1473 or placebo administered for 28 days in sequential fashion. The primary objectives of the study will include evaluation of the safety and tolerability of TD-1473 administered for 28 days, as well as assessment of the compound's plasma exposure following administration. A key secondary objective of the study will be the evaluation of the effect of TD-1473 on levels of a range of key ulcerative colitis biomarkers, including C-reactive protein and fecal calprotectin. Additionally, investigators are expected to evaluate a number of exploratory objectives, including changes in partial Mayo score and improvement in disease activity through endoscopic and histologic assessments. We expect data from the Phase 1b study in mid-2017. Also in October 2016, we announced that we had successfully completed the TD-1473 13-week toxicology studies, clearing the compound to progress to longer term clinical studies.
TD-3504
In September 2016, we announced plans to progress a second compound, TD-3504, from our JAK inhibitor program. TD-3504 is an innovative prodrug of tofacitinib, an investigational JAK inhibitor in development for ulcerative colitis. TD-3504 is chemically distinct from TD-1473 and is designed to release active tofacitinib into the intestinal tract. In preclinical studies, TD-3504 demonstrated rapid formation of tofacitinib in the intestinal tract, reduction in disease activity score comparable to tofacitinib, and low systemic exposure in contrast to tofacitinib. We plan to initiate a Phase 1 study of TD-3504 in healthy volunteers and ulcerative colitis patients in the first half of 2017.
Selective 5-HT4 Agonist (TD-8954)
Takeda Collaborative Arrangement
In June 2016, we entered into a License and Collaboration Agreement with Millennium Pharmaceuticals, Inc., a Delaware corporation ("Millennium") (the "Takeda Agreement"), in order to establish a collaboration for the development and commercialization of TD-8954, a selective 5-HT4 receptor agonist. Prior to the Takeda Agreement, we developed TD-8954 for potential use in the treatment of gastrointestinal motility disorders, including short-term intravenous use for enteral feeding intolerance ("EFI") to achieve early nutritional adequacy in critically ill patients at high nutritional risk, an indication for which the compound received FDA Fast Track designation. Millennium is an indirect wholly-owned subsidiary of Takeda Pharmaceutical Company Limited (TSE: 4502), a publicly-traded Japanese corporation listed on the Tokyo Stock Exchange (collectively with Millennium, "Takeda"). Under the terms of the Takeda Agreement, Takeda will be responsible for worldwide development and commercialization of TD-8954. We received an upfront cash payment of $15.0 million and will be eligible to receive success-based development, regulatory and sales milestone payments by Takeda. The first $110.0 million of potential milestones are associated with the development, regulatory and commercial launch milestones for EFI or other intravenously dosed indications. We will also be eligible to receive a tiered royalty on worldwide net sales by Takeda at percentage royalty rates ranging from low double-digits to mid-teens.
Other Programs
Economic Interest in GSK-Partnered Respiratory Programs
We are entitled to receive an 85% economic interest in any future payments that may be made by GSK (pursuant to its agreements with Innoviva) relating to certain of the respiratory programs (the "GSK-Partnered Respiratory Programs") that Innoviva partnered with GSK and assigned to Theravance
13
Respiratory Company, LLC ("TRC") in connection with Innoviva's separation of its biopharmaceutical operations into its then wholly-owned subsidiary Theravance Biopharma (the "Spin-Off"). The GSK-Partnered Respiratory Programs consist primarily of the Closed Triple program and the Inhaled Bifunctional Muscarinic Antagonist-Beta2 Agonist ("MABA") program, each of which are described in more detail below. We are entitled to this economic interest through our equity ownership in TRC. Our economic interest will not include any payments associated with RELVAR® ELLIPTA®/BREO® ELLIPTA®, ANORO® ELLIPTA® or vilanterol monotherapy. The following information regarding the Closed Triple and the MABA program is based solely upon publicly available information and may not reflect the most recent developments under the programs.
"Closed Triple" or FF/UMEC/VI (fluticasone furoate/umeclidinium bromide/vilanterol)
The Closed Triple program seeks to provide the activity of an inhaled corticosteroid (FF) plus two bronchodilators (UMEC, a LAMA, and VI, a long-acting beta2 agonist, or LABA) in a single delivery device administered once-daily. If the Closed Triple is successfully developed and commercialized, we are entitled to receive an 85% economic interest in the royalties payable by GSK to TRC on worldwide net sales, which royalties are upward-tiering from 6.5% to 10%. Previously, Innoviva and GSK announced the initiation of two global pivotal Phase 3 studies of the Closed Triple. The IMPACT study, which will enroll approximately 10,000 COPD patients, was initiated in July 2014. The IMPACT study will assess whether the Closed Triple can reduce the rate of moderate and severe exacerbations compared with two approved once-daily COPD treatments, RELVAR® ELLIPTA®/BREO® ELLIPTA® (FF/VI), an ICS/LABA combination, and ANORO® ELLIPTA® (UMEC/VI), a LAMA/LABA combination. The IMPACT study is ongoing and is expected to read out in 2017. The FULFIL study, which enrolled approximately 1,800 COPD patients was initiated in February 2015. In June 2016, GSK and Innoviva disclosed positive top-line results from the FULFIL study, in which data demonstrated superiority of the Closed Triple as compared to twice-daily SYMBICORT® TURBOHALER® (budesonide/formoterol) in improving lung function and health-related quality of life in COPD patients. In November 2016, GSK and Innoviva announced the filing of a New Drug Application ("NDA") in the U.S. for the Closed Triple for patients with COPD. In December 2016, GSK and Innoviva announced the filing of a Marketing Authorization Application ("MAA") in the EU for the Closed Triple for patients with COPD. In December 2016, GSK and Innoviva announced the initiation of the Phase 3 (CAPTAIN) study of the Closed Triple in patients with asthma. The CAPTAIN study is expected to read out in 2018.
Inhaled Bifunctional Muscarinic Antagonist-Beta2 Agonist (MABA)
GSK961081 ('081), also known as batefenterol, is an investigational, single-molecule bifunctional bronchodilator with both muscarinic antagonist and beta2 receptor agonist activity that was discovered by us when we were part of Innoviva.
If a single-agent MABA medicine containing '081 is successfully developed and commercialized, we are entitled to receive an 85% economic interest in the royalties payable by GSK to TRC on worldwide net sales, which royalties range between 10% and 20% of annual global net sales up to $3.5 billion, and 7.5% for all annual global net sales above $3.5 billion. If a MABA medicine containing '081 is commercialized only as a combination product, such as '081/FF, the royalty rate is 70% of the rate applicable to sales of the single-agent MABA medicine. If a MABA medicine containing '081 is successfully developed and commercialized in multiple regions of the world, TRC is eligible to receive contingent milestone payments from GSK. The agreements allow for total milestones of up to $125.0 million for a single-agent medicine and an incremental $125.0 million for a combination medicine. Of these amounts, $112.0 million in potential milestones remain for a single-agent medicine, and $122.0 million remain for a combination medicine. In each case, we would be entitled to receive an 85% economic interest in any such payments.
14
Theravance Respiratory Company, LLC
Prior to the June 1, 2014 separation of its biopharmaceutical operations into its then wholly-owned subsidiary Theravance Biopharma (the "Spin-Off"), Innoviva assigned to TRC its strategic alliance agreement with GSK and all of its rights and obligations under its LABA collaboration agreement with GSK other than with respect to RELVAR® ELLIPTA®/BREO® ELLIPTA®, ANORO® ELLIPTA® and vilanterol monotherapy. Our equity interest in TRC is the mechanism by which we are entitled to the 85% economic interest in any future payments made by GSK under the strategic alliance agreement and under the portion of the collaboration agreement assigned to TRC. The drug programs assigned to TRC include the Closed Triple and the MABA program, as monotherapy and in combination with other therapeutically active components, such as an inhaled corticosteroid ("ICS"), as well as any other product or combination of products that may be discovered and developed in the future under these GSK agreements.
Our Strategy
Our mission is to create value from a diverse and distinctive portfolio of assets: an approved product, a pipeline with assets at all stages of development, and a productive research platform designed for long-term growth. With our successful drug discovery and development track record, commercial infrastructure, experienced management team and efficient corporate structure, we believe that we are well positioned to create value for our shareholders and make a difference in the lives of patients.
We follow these core guiding principles in our mission to drive value creation:
- •
- Focus on insight and innovation;
- •
- Outsource non-core activities;
- •
- Create and foster an integrated environment; and
- •
- Aggressively manage uncertainty.
Our research and development activities are concentrated primarily on four therapeutic areas—infectious disease, respiratory, gastrointestinal disease and cardiovascular and renal disease—and we have established a commercial infrastructure focused primarily on the acute care setting. We manage our pipeline with the goal of optimizing program value and allocation of resources. We employ multiple strategies for commercialization of our products. Our approach may involve retaining product rights and marketing a product independently in the U.S., predominantly in the acute care setting, or we may partner a product to extend our commercial reach beyond the acute care setting, to expand our geographic reach, and/or to manage the financial risk associated with the program. Alternatively, we may monetize or divest an asset that we designate as outside our core business, where we believe the program is optimized by leveraging partner capabilities and removing or limiting our research and development costs.
Manufacturing
We rely primarily on a network of third-party manufacturers, including contract manufacturing organizations, to produce our active pharmaceutical ingredient ("API") and our drug product. We believe that we have in-house expertise to manage this network of third-party manufacturers and we believe that we will be able to continue to negotiate third-party manufacturing arrangements on commercially reasonable terms and that it will not be necessary for us to obtain internal manufacturing capacity in order to develop or commercialize our products. However, if we are unable to obtain contract manufacturing or obtain such manufacturing on commercially reasonable terms, or if
15
manufacturing is interrupted at one of our suppliers, whether due to regulatory or other reasons, we may not be able to develop or commercialize our products as planned.
We have a single source of supply of API for telavancin and another, separate single source of supply of VIBATIV drug product. If, for any reason, either single-source third-party manufacturer of telavancin API or of VIBATIV drug product is unable or unwilling to perform, or if the performance of either does not meet regulatory requirements, including maintaining current Good Manufacturing Practice ("cGMP") compliance, we may not be able to locate alternative manufacturers, enter into acceptable agreements with them or obtain sufficient quantities of API or drug product in a timely manner. Any inability to acquire sufficient quantities of API or drug product in a timely manner from current or future sources would adversely affect the commercialization of VIBATIV.
Government Regulation
The development and commercialization of VIBATIV and our product candidates by us and our collaboration partners and our ongoing research are subject to extensive regulation by governmental authorities in the United States and other countries. Before marketing in the United States, any medicine must undergo rigorous preclinical studies and clinical studies and an extensive regulatory approval process implemented by the FDA under the Federal Food, Drug, and Cosmetic Act. Outside the United States, the ability to market a product depends upon receiving a marketing authorization from the appropriate regulatory authorities. The requirements governing the conduct of clinical studies, marketing authorization, pricing and reimbursement vary widely from country to country. In any country, however, the commercialization of medicines is permitted only if the appropriate regulatory authority is satisfied that we have presented adequate evidence of the safety, quality and efficacy of our medicines.
Before commencing clinical studies in humans in the United States, we must submit to the FDA an investigational new drug application ("IND") that includes, among other things, the general investigational plan and protocols for specific human studies, and the results of preclinical studies. An IND will go into effect 30 days following its receipt by the FDA unless the FDA issues a clinical hold. Once clinical studies have begun under the IND, they are usually conducted in three phases and under FDA oversight. These phases generally include the following:
Phase 1. The product candidate is introduced into patients or healthy human volunteers and is tested for safety, dose tolerance and pharmacokinetics.
Phase 2. The product candidate is introduced into a limited patient population to assess the efficacy of the drug in specific, targeted indications, assess dosage tolerance and optimal dosage, and identify possible adverse effects and safety risks.
Phase 3. If a compound is found to be potentially effective and to have an acceptable safety profile in Phase 2 evaluations, the clinical study will be expanded to further demonstrate clinical efficacy, optimal dosage and safety within an expanded patient population.
The results of product development, preclinical studies and clinical studies must be submitted to the FDA as part of a NDA. The NDA also must contain extensive manufacturing information. The Prescription Drug User Fee Act ("PDUFA") establishes timeframes for FDA review of NDAs, with a performance goal of reviewing and acting on 90 percent of priority new molecular entity ("NME") NDA submissions within 6 months of the 60-day filing date, and to review and act on 90 percent of standard NME NDA submissions within 10 months of the 60-day filing date. The 2007 Food and Drug Administration Amendments Act gave the FDA authority to require implementation of a formal Risk Evaluation and Management Strategy to ensure that the benefits of a product outweigh its risks. At the end of the review period, the FDA communicates either approval of the NDA or a complete response listing the application's deficiencies.
16
Once approved, the FDA may withdraw the product approval if compliance with post-marketing regulatory standards is not maintained or if safety or quality issues are identified after the product reaches the marketplace. In addition, the FDA may require post-marketing studies, sometimes referred to as Phase 4 studies, to monitor the safety and effectiveness of approved products, and may limit further marketing of the product based on the results of these post-marketing studies. The FDA has broad post-market regulatory and enforcement powers, including the ability to suspend or delay issuance of approvals, seize products, withdraw approvals, enjoin violations, and initiate criminal prosecution.
If regulatory approval for a medicine is obtained, the clearance to market the product will be limited to those diseases and conditions approved by FDA and for which the medicine was shown to be effective, as demonstrated through clinical studies and specified in the medicine's labeling. Even if this regulatory approval is obtained, a marketed medicine, its manufacturer and its manufacturing facilities are subject to continual review and periodic inspections by the FDA. The FDA ensures the quality of approved medicines by carefully monitoring manufacturers' compliance with its cGMP regulations. The cGMP regulations for drugs contain minimum requirements for the methods, facilities, and controls used in manufacturing, processing, and packaging of a medicine. The regulations are intended to make sure that a medicine is safe for use, and that it has the ingredients and strength it claims to have. Discovery of previously unknown problems with a medicine, manufacturer or facility may result in restrictions on the medicine or manufacturer, including costly recalls or withdrawal of the medicine from the market.
We and our collaboration partners are also subject to various laws and regulations regarding laboratory practices, the experimental use of animals and the use and disposal of hazardous or potentially hazardous substances in connection with our research. In each of these areas, as above, the FDA and other regulatory authorities have broad regulatory and enforcement powers, including the ability to suspend or delay issuance of approvals, seize products, withdraw approvals, enjoin violations, and initiate criminal prosecution, any one or more of which could have a material adverse effect upon our business, financial condition and results of operations.
Outside the United States our ability to market our products will also depend on receiving marketing authorizations from the appropriate regulatory authorities. Risks similar to those associated with FDA approval described above exist with the regulatory approval processes in other countries.
United States Healthcare Reform
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (together the "Healthcare Reform Act"), substantially changed the way healthcare is financed by both governmental and private insurers, and impacts pricing and reimbursement with respect to our VIBATIV business, and any potential additional commercial operations. Moreover, legislative changes to the Healthcare Reform Act remain possible and appear likely in the 115th United States Congress and under the Trump Administration. We expect that the Healthcare Reform Act, as currently enacted or as it may be amended in the future, and other healthcare reform measures that may be adopted in the future, could have a material adverse effect on our industry generally and on our ability to maintain or increase sales of our existing products or to successfully commercialize our product candidates, if approved. For more information, see the risk factor under the heading "Changes in healthcare law and implementing regulations, including government restrictions on pricing and reimbursement, as well as healthcare policy and other healthcare payor cost-containment initiatives, may negatively impact our ability to generate revenues" of this Annual Report on Form 10-K.
17
Pharmaceutical Pricing and Reimbursement
We participate in and have certain price reporting obligations under the Medicaid Drug Rebate program. Our participation in the Medicaid Drug Rebate program is described in greater detail under the risk factor "If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate program or other governmental pricing programs, we could be subject to additional reimbursement requirements, penalties, sanctions and fines, which could have a material adverse effect on our business, financial condition, results of operations and growth prospects" of this Annual Report on Form 10-K.
Our ability to commercialize our products successfully, and to attract commercialization partners for our products, depends in significant part on the availability of adequate financial coverage and reimbursement from third party payors, including, in the United States, governmental payors such as the Medicare and Medicaid programs, managed care organizations, and private health insurers. The reimbursement environment is described in greater detail under the risk factor "Changes in healthcare law and implementing regulations, including government restrictions on pricing and reimbursement, as well as healthcare policy and other healthcare payor cost-containment initiatives, may negatively impact our ability to generate revenues" of this Annual Report on Form 10-K.
Fraud and Abuse Laws
Our interactions and arrangements with customers and third-party payors are subject to applicable fraud and abuse laws. These laws and the related risks are described in greater detail under the risk factor "Our relationships with customers and third-party payors are subject to applicable anti-kickback, fraud and abuse, transparency and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, exclusion, contractual damages, reputational harm and diminished profits and future earnings" of this Annual Report on Form 10-K.
Data Privacy and Protection
We are subject to laws and regulations that address privacy and data security. In the U.S., numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws (e.g., Section 5 of the FTC Act), govern the collection, use, disclosure, and protection of health-related and other personal information. These laws and related risks are described in greater detail under the risk factor "If we fail to comply with data protection laws and regulations, we could be subject to government enforcement actions (which could include civil or criminal penalties), private litigation and/or adverse publicity, which could negatively affect our operating results and business" of this Annual Report on Form 10-K.
Patents and Proprietary Rights
We will be able to protect our technology from unauthorized use by third parties only to the extent that our technology is covered by valid and enforceable patents or is effectively maintained as trade secrets. Our success in the future will depend in part on obtaining patent protection for our product candidates. Accordingly, patents and other proprietary rights are essential elements of our business. Our policy is to seek in the United States and selected foreign countries patent protection for novel technologies and compositions of matter that are commercially important to the development of our business. For proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our drug discovery process that involve proprietary know-how and technology that is not covered by patent applications, we rely on trade secret protection and confidentiality agreements to protect our interests. We require all of our employees, consultants and advisors to enter into confidentiality agreements. Where it is necessary to share our proprietary information or data with outside parties, our policy is to make available only that information and data
18
required to accomplish the desired purpose and only pursuant to a duty of confidentiality on the part of those parties.
As of December 31, 2016, we or one of our wholly-owned subsidiaries owned 434 issued United States patents and 1,681 granted foreign patents, as well as additional pending United States patent applications and foreign patent applications. The claims in these various patents and patent applications are directed to compositions of matter, including claims covering product candidates, lead compounds and key intermediates, pharmaceutical compositions, methods of use and processes for making our compounds along with methods of design, synthesis, selection and use relevant to multivalency in general and to our research and development programs in particular. In particular, our wholly-owned subsidiary Theravance Biopharma Antibiotics IP, LLC owns the following U.S. patents which are listed in the FDA Approved Drug Products with Therapeutic Equivalence Evaluations (Orange Book) for telavancin: U.S. Patent No. 6,635,618 B2, expiring on September 11, 2023; U.S. Patent No. 6,858,584 B2, expiring on August 24, 2022; U.S. Patent No. 6,872,701 B2, expiring on June 5, 2021; U.S. Patent No. 7,008,923 B2, expiring on May 6, 2021; U.S. Patent No. 7,208,471 B2, expiring on May 1, 2021; U.S. Patent No. 7,351,691 B2, expiring on May 1, 2021; U.S. Patent No. 7,531,623 B2, expiring on January 1, 2027; U.S. Patent No. 7,544,364 B2, expiring on May 1, 2021; U.S. Patent No. 7,700,550 B2, expiring on May 1, 2021; U.S. Patent No. 8,101,575 B2, expiring on May 1, 2021; and U.S. Patent No. 8,158,580 B2, expiring on May 1, 2021. Thus, the last-to-expire patent currently listed in the Orange Book for telavancin expires on January 1, 2027.
United States issued patents and foreign patents generally expire 20 years after filing. The patent rights relating to VIBATIV (telavancin) currently consist of United States patents that expire between 2019 and 2027, additional pending United States patent applications and counterpart patents and patent applications in a number of jurisdictions, including Europe. Additionally, our patent rights relating to revefenacin, velusetrag and TD-9855 currently include issued United States composition of matter patents that expire in 2025, 2025 and 2030, respectively (not including any patent term extensions that may be available under the Drug Price Competition and Patent Term Restoration Act of 1984), as well as additional issued United States patents, pending United States patent applications and counterpart patents and patent applications in a number of jurisdictions. Nevertheless, issued patents can be challenged, narrowed, invalidated or circumvented, which could limit our ability to stop competitors from marketing similar products and threaten our ability to commercialize our product candidates. Our patent position, similar to other companies in our industry, is generally uncertain and involves complex legal and factual questions. To maintain our proprietary position we will need to obtain effective claims and enforce these claims once granted. It is possible that, before any of our products can be commercialized, any related patent may expire or remain in force only for a short period following commercialization, thereby reducing any advantage of the patent. Also, we do not know whether any of our patent applications will result in any issued patents or, if issued, whether the scope of the issued claims will be sufficient to protect our proprietary position.
We are party to a license agreement with Janssen Pharmaceuticals ("Janssen") pursuant to which we have licensed rights under certain patents owned by Janssen covering an excipient used in the formulation of telavancin. Pursuant to the terms of this license agreement, we are obligated to pay royalties to Janssen based on any commercial sales of VIBATIV (telavancin). The license is terminable by us upon prior written notice to Janssen or upon an uncured breach or a liquidation event of one of the parties.
Competition
Our marketed product and our research and development programs target four therapeutic areas—infectious disease, respiratory, gastrointestinal disease and cardiovascular and renal disease—and our commercial infrastructure is focused primarily on the acute care setting. We expect that any
19
medicines that we commercialize with our collaborative partners or on our own will compete with existing and future market-leading medicines.
Many of our competitors have substantially greater financial, technical and personnel resources than we have. In addition, many of these competitors have significantly greater commercial infrastructures than we have. Our ability to compete successfully will depend largely on our ability to leverage our experience in drug discovery, development and commercialization to:
- •
- discover and develop medicines that are superior to other products in the market;
- •
- attract qualified scientific, product development and commercial personnel;
- •
- obtain patent and/or other proprietary protection for our medicines and technologies;
- •
- obtain required regulatory approvals;
- •
- commercialize approved products; and
- •
- successfully collaborate with pharmaceutical companies in the discovery, development and commercialization of new medicines.
VIBATIV (telavancin). VIBATIV competes with vancomycin, linezolid and daptomycin, generic drugs that are manufactured by a variety of companies, as well as other drugs marketed to treat complicated skin and skin structure infections and hospital acquired and ventilator associated bacterial pneumonia caused by Gram-positive bacteria. In particular, daptomycin has recently become available as a generic product and we believe the outpatient setting has been particularly impacted by its availability. Currently marketed products include but are not limited to Sivextro® (tedizolid) marketed by Merck & Co., Inc.; Teflaro® (ceftaroline) and Dalvance™ (dalbavancin) marketed by Allergan; and Orbactiv™ (oritavancin) marketed by The Medicines Company. To compete effectively with these medicines, and in particular with the relatively inexpensive generic options of vancomycin, linezolid and daptomycin, we will need to demonstrate to physicians that, based on experience, clinical data, side effect profiles and other factors, VIBATIV is a preferred injectable Staphyloccocus aureus treatment for patients not likely to respond to other Staphyloccocus aureus therapies.
Revefenacin (TD-4208) long-acting muscarinic antagonist (LAMA). If successfully developed and approved as the first once-daily nebulized LAMA, revefenacin would be expected to compete predominantly with short-acting nebulized bronchodilators used 3 to 4 times per day and has the potential to be a first line prescription or complement to single agent nebulized long-acting beta agonist (LABA) products used two times per day.
"Closed Triple" or FF/UMEC/VI (fluticasone furoate/umeclidinium bromide/vilanterol) and Inhaled Bifunctional Muscarinic Antagonist-Beta2 Agonist (MABA). If GSK successfully develops and brings to market an approved Closed Triple product, such product might compete with a number of other closed triple products that are currently under development. We believe that Chiesi Farmaceutici, AstraZeneca and Novartis all have closed triple products in late stage development for COPD and/or asthma. If GSK successfully develops and brings to market an approved MABA product, such product might compete with other MABA products that are currently under development such as AstraZeneca's AZD-8871, which is currently in Phase II studies for COPD, or dual LABA-LAMA combination products.
Research and Development
We spent $141.7 million, $129.2 million, and $168.5 million on research and development, net of reimbursements from collaboration partners, for the years ended December 31, 2016, 2015, and 2014, respectively. Additional information regarding these expenditures is included in Note 1, "Description of Operations and Summary of Significant Accounting Policies," to our consolidated financial statements in this Annual Report on Form 10-K.
20
Employees
As of December 31, 2016, we had 316 permanent employees, of which 185 were engaged in research and development activities. Of our 316 employees, 310 were located in the U.S. and six were located in Ireland. We consider our employee relations to be good.
Financial Information About Geographic Areas
Information on our total revenues attributed to geographic areas and customers who represented at least 10% of our total revenues is included in Note 3, "Segment Information," to our consolidated financial statements in this Annual Report on Form 10-K.
Corporation Information
Theravance Biopharma was incorporated in the Cayman Islands in July 2013 under the name Theravance Biopharma, Inc. Theravance Biopharma began operating as an independent, publicly-traded company on June 2, 2014 following a spin-off from Innoviva, Inc. Our corporate address in the Cayman Islands is and principal executive office is P.O. Box 309, Ugland House, Grand Cayman, KY1-1104, Cayman Islands and the address of our wholly-owned U.S. operating subsidiary Theravance Biopharma US, Inc. is 901 Gateway Boulevard, South San Francisco, California 94080. While Theravance Biopharma is incorporated under Cayman Island law, the Company became an Irish tax resident effective July 1, 2015. The address of our wholly-owned Irish operating subsidiary, Theravance Biopharma Ireland Limited, is Fitzwilliam Hall, Fitzwilliam Place, Dublin 2 Ireland.
Available Information
Our Internet address is www.theravance.com. Our investor relations website is located at http://investor.theravance.com. We make available free of charge on our investor relations website under "SEC Filings" our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, our directors' and officers' Section 16 Reports and any amendments to those reports as soon as reasonably practicable after filing or furnishing such materials to the U.S. Securities and Exchange Commission ("SEC"). The information found on our website is not part of this or any other report that we file with or furnish to the SEC. Theravance Biopharma and the Theravance Biopharma logo are registered trademarks of the Theravance Biopharma group of companies. Trademarks, tradenames or service marks of other companies appearing in this report are the property of their respective owners.
RISKS RELATING TO THE COMPANY
The risks described below and elsewhere in this Annual Report on Form 10-K and in our other public filings with the SEC are not the only risks facing the Company. Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition and/or operating results.
We anticipate that we will incur losses for the foreseeable future. We may never achieve or sustain profitability.
First as part of Innoviva, Inc. (known as Theravance, Inc. prior to January 7, 2016), and since June 2, 2014 as Theravance Biopharma, we have been engaged in discovery and development of compounds and product candidates since mid-1997. We may never generate sufficient revenue from the sale of medicines, royalties on sales by our partners or from our interest in Theravance Respiratory Company, LLC ("TRC") to achieve profitability. During the years ended December 31, 2016, 2015 and 2014, we recognized losses of $190.7 million, $182.2 million and $237.0 million, respectively, which are
21
reflected in the Shareholders' Equity on our consolidated balance sheets. We reflect cumulative net loss incurred after June 2, 2014, the effective date of the Spin-Off, as accumulated deficit on our consolidated balance sheets. We expect to continue to incur net losses at least over the next several years as we continue our drug discovery and development efforts and incur significant preclinical and clinical development costs related to our current product candidates and commercialization and development costs relating to VIBATIV® (telavancin) and, in anticipation of potential approval, revefenacin. In particular, to the extent we advance our product candidates into and through additional clinical studies without a partner, we will incur substantial expenses. We are also making additional investments in telavancin, our antibiotic that has been approved for certain difficult-to-treat infections. For example, in February 2015 we initiated a Phase 3 registrational study of telavancin for bacteremia and a patient registry study. We are incurring all of the costs and expenses associated with the commercialization of VIBATIV in the U.S., including the maintenance of an independent sales and marketing organization with appropriate technical expertise, supporting infrastructure and distribution capabilities, expanded medical affairs presence, manufacturing and third-party vendor logistics and consultant support, and post-marketing studies. We are also making additional investments in revefenacin in anticipation of potential approval. Our commitment of resources to VIBATIV, to the continued development of our existing product candidates and to our discovery programs will require significant additional funding. Our operating expenses also will increase if, among other things:
- •
- our earlier stage potential products move into later-stage clinical development, which is generally more expensive than early stage
development;
- •
- additional preclinical product candidates are selected for clinical development;
- •
- we pursue clinical development of our potential or current products in new indications;
- •
- we increase the number of patents we are prosecuting or otherwise expend additional resources on patent prosecution or defense; or
- •
- we acquire or in-license additional technologies, product candidates, products or businesses.
Other than revenues from sales of VIBATIV, our only approved medicine and potential payments under collaboration agreements, we do not expect to generate revenues from our programs for the foreseeable future. Since we or our collaborators or licensees may not successfully develop additional products, obtain required regulatory approvals, manufacture products at an acceptable cost or with appropriate quality, or successfully market and sell such products with desired margins, our expenses may continue to exceed any revenues we may receive.
In the absence of substantial licensing payments, contingent payments or other revenues from third-party collaborators, royalties on sales of products licensed under our intellectual property rights, future revenues from VIBATIV and product candidates in development that receive regulatory approval or other sources of revenues, we will continue to incur operating losses and will require additional capital to execute our business strategy. The likelihood of reaching, and the time required to reach, and then to sustain, profitability are highly uncertain. As a result, we expect to continue to incur substantial losses for the foreseeable future. We are uncertain when or if we will ever be able to achieve or sustain profitability. Failure to become and remain profitable would adversely affect the price of our securities and our ability to raise capital and continue operations.
If additional capital is not available, we may have to curtail or cease operations or we could be forced to share our rights to commercialize our product candidates with third parties on terms that may not be favorable to us.
Based on our current operating plans and financial forecasts, we believe that our cash, cash equivalents and marketable securities will be sufficient to meet our anticipated operating needs for at least the next twelve months. If our current operating plans or financial forecasts change, we may
22
require or seek additional funding sooner in the form of public or private equity or equity-linked offerings, debt financings or additional collaborations and licensing arrangements. For example, if we choose to progress any additional product candidates into later-stage development on our own, our capital needs would increase substantially. We also are making significant investments in telavancin, our approved antibiotic, which increases our operating expenses. For example, in 2015 we initiated a Phase 3 registrational study of telavancin for bacteremia and our Telavancin Observational Use Registry ("TOUR"), a patient registry study. In addition, we maintain an independent sales and marketing organization and medical affairs team focused on the acute care setting and VIBATIV. We are also making additional investments in revefenacin in anticipation of potential approval. In 2016, we increased our anticipated operating loss, primarily because of accelerated enrollment in TOUR, increased investment in our neprilysin ("NEP") inhibitor program and increased funding for the development of our intestinally restricted pan-Janus kinase ("JAK") inhibitors.
We may need to raise additional capital in the future to, among other things:
- •
- fund our discovery efforts and research and development programs;
- •
- fund our commercialization strategies for VIBATIV and any additional approved products;
- •
- progress mid-to-late stage product candidates into later-stage development, if warranted;
- •
- respond to competitive pressures; and
- •
- acquire complementary businesses or technologies.
Our future capital needs depend on many factors, including:
- •
- the scope, duration and expenditures associated with our discovery efforts and research and development programs;
- •
- continued scientific progress in these programs;
- •
- the extent to which we encounter technical obstacles in our research and development programs;
- •
- the outcome of potential licensing or partnering transactions, if any;
- •
- competing technological developments;
- •
- the extent of our proprietary patent position in telavancin and our product candidates;
- •
- our facilities expenses, which will vary depending on the time and terms of any facility lease or sublease we may enter into, and other
operating expenses;
- •
- the scope and extent of the expansion of our sales and marketing efforts;
- •
- potential litigation and other contingencies; and
- •
- the regulatory approval process for our product candidates.
We may seek to raise additional capital or obtain future funding through public or private equity offerings, debt financings or additional collaborations and licensing arrangements. We may not be able to obtain additional financing on terms favorable to us, if at all. General market conditions may make it difficult for us to seek financing from the capital markets. We may be required to relinquish rights to our technologies, product candidates or territories, or grant licenses on terms that are not favorable to us, in order to raise additional funds through collaborations or licensing arrangements. We may sequence pre-clinical and clinical studies as opposed to conducting them concomitantly in order to conserve resources, or delay, reduce or eliminate one or more of our research or development programs and reduce overall overhead expenses. If we are unable to raise additional capital or obtain future funding in sufficient amounts or on terms acceptable to us, we may have to make reductions in our workforce and may be prevented from continuing our discovery, development and
23
commercialization efforts and exploiting other corporate opportunities. This would likely harm our business, prospects and financial condition and cause the price of our securities to fall.
We may seek to obtain future financing through the issuance of debt or equity, which may have an adverse effect on our shareholders or may otherwise adversely affect our business.
If we raise funds through the issuance of additional debt, including convertible debt or equity, any debt securities or preferred shares issued will have rights, preferences and privileges senior to those of holders of our ordinary shares in the event of liquidation. The terms of our existing convertible senior notes do not restrict our ability to issue additional debt. In such event, there is a possibility that once all senior claims are settled, there may be no assets remaining to pay out to the holders of ordinary shares. In addition, if we raise funds through the issuance of additional equity, whether through private placements or public offerings, such an issuance would dilute ownership of our current shareholders that do not participate in the issuance. For example, since our Spin-Off in June 2014, we have raised an aggregate of $583.9 million through the sale of approximately 17.5 million shares and $230.0 million aggregate principal amount of 3.250% convertible senior notes due 2023 in a combination of private sale, public offerings and pursuant to our at-the-market offering program. If we are unable to obtain any needed additional funding, we may be required to reduce the scope of, delay, or eliminate some or all of, our planned research, development and commercialization activities or to license to third parties the rights to develop and/or commercialize products or technologies that we would otherwise seek to develop and/or commercialize ourselves or on terms that are less attractive than they might otherwise be, any of which could materially harm our business.
Furthermore, the terms of any additional debt securities we may issue in the future may impose restrictions on our operations, which may include limiting our ability to incur additional indebtedness, pay dividends on or repurchase our share capital, or make certain acquisitions or investments. In addition, we may be subject to covenants requiring us to satisfy certain financial tests and ratios, and our ability to satisfy such covenants may be affected by events outside of our control.
Servicing our convertible senior notes requires a significant amount of cash, and we may not have sufficient cash flow from our business to pay our debt. Additionally, holders may require us to repurchase our convertible senior notes under certain circumstances, and we may not have sufficient cash to do so.
Our ability to make interest or principal payments when due or to refinance the Notes depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate cash flow from operations sufficient to satisfy our obligations under the Notes and any future indebtedness we may incur and to make necessary capital expenditures. We may be required to adopt one or more alternatives, such as reducing or delaying investments or capital expenditures, selling assets, refinancing or obtaining additional equity capital on terms that may be onerous or highly dilutive. Our ability to refinance the Notes or future indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities on desirable terms or at all, which could result in a default on the Notes or future indebtedness.
Additionally, holders of the Notes may have the right to require us to repurchase the Notes upon the occurrence of a "fundamental change" such as a change of control of our Company or the termination of trading of our ordinary shares, as defined in the indenture, as amended, governing the Notes. We may not have sufficient funds to repurchase the Notes in cash or have the ability to arrange necessary financing on acceptable terms. Our failure to repurchase the Notes when required would result in an event of default with respect to the Notes. Any acceleration of the repayment of the Notes or future indebtedness after any applicable notice or grace periods could have a material adverse effect on our business, results of operations and financial condition.
24
If we are unable to enter into future collaboration arrangements or if any such collaborations with third parties are unsuccessful, we will be unable to fully develop and commercialize all of our product candidates and our business will be adversely affected.
We have collaborations with a number of third parties including Mylan for the development and commercialization of a nebulized formulation of revefenacin (TD-4208), our LAMA compound, Alfa Wassermann S.p.A. ("Alfa Wassermann") for velusetrag, Millennium Pharmaceuticals, Inc., an indirect wholly-owned subsidiary of Takeda Pharmaceutical Company Limited (collectively with Millennium, "Takeda") for the development and commercialization of a selective 5-HT4 receptor agonist (TD-8954) and other companies for regional development and commercialization of VIBATIV. Also, through our interest in TRC we may participate economically in Innoviva's collaborations with GSK with respect to the GSK-Partnered Respiratory Programs and we received non-marketable equity securities in connection with our September 2015 licensing agreement with Trek Therapeutics, PBC. Additional collaborations will likely be needed to fund later-stage development of certain programs that have not been licensed to a collaborator, such as our NEP inhibitor program and axelopran (TD-1211) for opioid-induced constipation and to commercialize the product candidates in our programs if approved by the necessary regulatory authorities. We may also seek collaboration arrangements with additional third parties to pursue the future commercialization of VIBATIV. Collaborations with third parties regarding our programs may require us to relinquish material rights, including revenue from commercialization of our medicines, or to assume material ongoing development obligations that we would have to fund. These collaboration arrangements are complex and time-consuming to negotiate, and if we are unable to reach agreements with third-party collaborators, we may fail to meet our business objectives and our financial condition may be adversely affected. We face significant competition in seeking third-party collaborators. We may be unable to find third parties to pursue product collaborations on a timely basis or on acceptable terms. Furthermore, for any collaboration, we may not be able to control the amount of time and resources that our partners devote to our product candidates and our partners may choose to prioritize alternative programs or otherwise be unsuccessful in their efforts with respect to our products or product candidates. Our inability to successfully collaborate with third parties would increase our development costs and may cause us to choose not to continue development of certain product candidates, would limit the likelihood of successful commercialization of some of our product candidates and could cause the price of our securities to fall.
We do not control TRC and, in particular, have no control over or access to non-public information about the GSK-Partnered Respiratory Programs.
Innoviva has assigned to TRC its strategic alliance agreement with GSK and all of its rights and obligations under its LABA collaboration agreement other than with respect to RELVAR® ELLIPTA®/BREO® ELLIPTA®, ANORO® ELLIPTA® and vilanterol monotherapy. Our equity interest in TRC entitles us to an 85% economic interest in any future payments made by GSK under the strategic alliance agreement and under the portion of the collaboration agreement assigned to TRC (the "GSK Agreements"). Our equity interest covers various drug programs including the Closed Triple combination of fluticasone furoate (FF)/umeclidinium (UMEC)/vilanterol (VI) (ICS/LAMA/LABA) and the MABA program, as monotherapy and in combination with other therapeutically active components, such as an inhaled corticosteroid ("ICS"), and any other product or combination of products that may be discovered and developed in the future under the GSK Agreements. Our economic interest does not include any payments by GSK associated with RELVAR® ELLIPTA®/BREO® ELLIPTA®, ANORO® ELLIPTA® or vilanterol monotherapy. Innoviva controls TRC and, except for certain limited consent rights, we have no right to participate in the business and affairs of TRC. Innoviva has the exclusive right to appoint TRC's manager who, among other things, is responsible for the day-to-day management of the GSK-Partnered Respiratory Programs and exercises the rights relating to the GSK-Partnered Respiratory Programs. As a result, we have no rights to participate in, or access to non-public information about, the development and commercialization of
25
the GSK-Partnered Respiratory Programs and no right to enforce rights under the GSK Agreements assigned to TRC. Moreover, we have many of the same risks with respect to our and TRC's dependence on GSK as we have with respect to our dependence on our own partners.
If the GSK-Partnered Respiratory Programs in which we have a substantial economic interest, including the Closed Triple program and MABA program, encounter delays, do not demonstrate safety and efficacy, are terminated, or if there are any adverse developments or perceived adverse developments with respect to these programs, our business will be harmed, and the price of our securities could fall.
We have no access to confidential information regarding the progress of, or plans for, the GSK-Partnered Respiratory Programs, including the Closed Triple program and the MABA program, and we have little, if any, ability to influence the progress of those programs because our interest in these programs is only through our economic interest in TRC, which is controlled by Innoviva. However, if any of the GSK-Partnered Respiratory Programs in which we have a substantial economic interest, including the Closed Triple program and MABA program, encounter delays, do not demonstrate safety and efficacy, are terminated, or if there are any adverse developments or perceived adverse developments with respect to such programs, our business will be harmed, and the price of our securities could fall. Examples of such adverse developments include, but are not limited to:
- •
- GSK deciding to delay or halt development of any of the GSK-Partnered Respiratory Programs in which we have a substantial economic interest,
including the Closed Triple or GSK961081 ('081), the lead compound in the MABA program;
- •
- the U.S. Food and Drug Administration ("FDA") and/or other regulatory authorities determining that any of the studies under these programs do
not demonstrate adequate safety or efficacy, or that additional non-clinical or clinical studies are required with respect to such programs;
- •
- safety, efficacy or other concerns arising from clinical or non-clinical studies in these programs;
- •
- any particular FDA requirements or changes in FDA policy or guidance regarding these programs; or
- •
- the emergence of new closed triple or other alternative therapies or any developments regarding these potentially competitive therapies, comparative price or efficacy of such potentially competitive therapies.
VIBATIV may not be broadly accepted by physicians, patients, third-party payors, or the medical community in general, which would have a material, adverse effect on our business.
The commercial success of VIBATIV depends upon its acceptance by physicians, patients, third-party payors and the medical community in general. VIBATIV may not be sufficiently accepted by these parties. VIBATIV competes with vancomycin (which accounts for a substantial majority of patient treatment days), linezolid and daptomycin, all relatively inexpensive generic drugs that are manufactured by a variety of companies, and a number of existing antibacterials manufactured and marketed by major pharmaceutical companies and others, and may compete against new antibacterials that are not yet on the market. If we are unable to demonstrate to physicians that, based on experience, clinical data, side effect profiles and other factors, VIBATIV is a preferred injectable treatment for treating the infections for which it is indicated, we may never generate significant revenue or profits from VIBATIV. In that case we may in the future reassess the VIBATIV business and respond in a number of ways which could include, for example, materially reducing our spending on commercialization and development efforts or other actions, any of which could cause the price of our securities to fall. Responding to ongoing challenges in the branded antibiotics market, we scaled back the size of our sales force in early 2017 and are allocating our resources with a focus on promotionally
26
sensitive territories. In addition, if we fail to meet expectations about our net sales of VIBATIV and our VIBATIV commercialization strategy, the price of our securities could fall. For example, we reduced our projected U.S. net sales target for VIBATIV for 2015 more than once.
The degree of market acceptance of VIBATIV, the rate of our VIBATIV sales and our ability to generate revenues through sales of VIBATIV depends on a number of factors, including, but not limited to:
- •
- the experiences of physicians, patients and payors with the use of VIBATIV;
- •
- the occurrence of unexpected serious adverse reactions in relation to VIBATIV;
- •
- the market price of VIBATIV relative to competing therapies, including generic therapies;
- •
- the timing, frequency and impact of price changes or changes to pricing programs;
- •
- our customer mix;
- •
- any adverse developments or perceived adverse developments with respect to Pfizer, Inc. which may adversely impact our single source of
supply for VIBATIV drug product;
- •
- any developments with, or comments by, the FDA or other regulatory agencies with respect to the manufacture, use or sale of VIBATIV;
- •
- our ability to complete our ongoing Phase 3 registrational study for use of telavancin in the treatment of patients with Staphylococcus
aureus bacteremia, the timing of any such completion, and the results of this study;
- •
- our ability to remove VIBATIV from the List of "Antineoplastic and Other Hazardous Drugs in Healthcare Settings" published by the National
Institute of Occupational Safety and Hazards (NIOSH);
- •
- the advantages and disadvantages of VIBATIV compared to alternative therapies;
- •
- our ability to find an EU commercialization partner for VIBATIV, which we have not had since August 2016 when we and Clinigen reached a mutual
decision that Clinigen will return VIBATIV EU commercial rights to us;
- •
- our ability to educate the medical community about the appropriate circumstances for use of VIBATIV;
- •
- the acceptance of VIBATIV onto formulary by hospitals and healthcare systems;
- •
- our ability to attract, train and retain appropriate numbers of sales and marketing personnel in the U.S.;
- •
- our ability to attract, train and retain medical science liaisons in the U.S. supporting physician education on the proper usage of VIBATIV;
- •
- the effectiveness of sales personnel in obtaining access to and educating adequate numbers of physicians about prescribing VIBATIV in
appropriate clinical situations;
- •
- the lack of complementary products offered by our sales personnel, which may put us at a competitive disadvantage relative to companies with
more extensive product lines; and
- •
- the reimbursement policies of government and third-party payors, including the amount of chargebacks and government rebates.
27
We market, sell and distribute VIBATIV in the U.S. without a partner and we may bear similar costs with respect to additional products in the future, which subjects us to certain risks.
We evaluate commercial strategy on a product by product basis either to engage pharmaceutical or other healthcare companies with an existing sales and marketing organization and distribution system to market, sell and distribute our products or to commercialize a product ourselves. However, we may not be able to establish these sales and distribution relationships on acceptable terms, or at all, or may encounter difficulties in commercializing a product ourselves. For any of our product candidates that receive regulatory approval in the future and are not covered by our current collaboration agreements, we will need a partner in order to commercialize such products unless we establish independent sales, marketing and distribution capabilities with appropriate technical expertise and supporting infrastructure.
VIBATIV was returned to us by Astellas Pharma Inc. ("Astellas"), our former VIBATIV collaboration partner, in January 2012, and Astellas is entitled to a ten-year, 1% royalty on future net sales of VIBATIV. On August 14, 2013, we (at the time with Innoviva) announced the reintroduction of VIBATIV to the U.S. market with the commencement of shipments into the wholesaler channel and we now maintain a VIBATIV sales force in the U.S. The risks of commercializing VIBATIV in the U.S. without a partner and commercializing any future products that we may choose to commercialize without a partner include:
- •
- costs and expenses associated with creating and maintaining an independent sales and marketing organization with appropriate technical
expertise and supporting infrastructure and distribution capability, including third- party vendor logistics and consultant support, which costs and expenses could, depending on the scope and method
of the marketing effort, exceed any product revenue from VIBATIV or any future products for several years;
- •
- our unproven ability to retain effective sales and marketing personnel and medical science liaisons in the U.S.;
- •
- the unproven ability of our sales and marketing personnel to obtain access to and educate adequate numbers of physicians about prescribing
VIBATIV, or any future products, in appropriate clinical situations;
- •
- the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with
more extensive product lines; and
- •
- bearing the full costs of further U.S. development of telavancin, the compound that is the basis of VIBATIV.
If we are not successful in maintaining an internal sales and marketing organization with appropriate experience, technical expertise, supporting infrastructure, distribution capability and the ability to obtain access to and educate adequate numbers of physicians about prescribing VIBATIV, or any future products, in appropriate clinical situations, we will have difficulty commercializing VIBATIV, or any future products, in the U.S., which would adversely affect our business and financial condition and the price of our securities could fall.
Any delay in commencing or completing clinical studies for product candidates and any adverse results from clinical or non-clinical studies or regulatory obstacles product candidates may face, would harm our business and the price of our securities could fall.
Each of our product candidates must undergo extensive non-clinical and clinical studies as a condition to regulatory approval. Non-clinical and clinical studies are expensive, take many years to complete and study results may lead to delays in further studies, new requirements for conducting future studies or decisions to terminate programs. The commencement and completion of clinical
28
studies for our product candidates may be delayed and programs may be terminated due to many factors, including, but not limited to:
- •
- lack of effectiveness of product candidates during clinical studies;
- •
- adverse events, safety issues or side effects relating to the product candidates or their formulation into medicines;
- •
- inability to raise additional capital in sufficient amounts to continue our development programs, which are very expensive;
- •
- inability to enter into partnering arrangements relating to the development and commercialization of our programs and product candidates;
- •
- the need to sequence clinical studies as opposed to conducting them concomitantly in order to conserve resources;
- •
- our inability or the inability of our collaborators or licensees to manufacture or obtain from third parties materials sufficient for use in
non-clinical and clinical studies;
- •
- governmental or regulatory delays and changes in regulatory requirements, policy and guidelines;
- •
- failure of our partners to advance our product candidates through clinical development;
- •
- delays in patient enrollment and variability in the number and types of patients available for clinical studies;
- •
- difficulty in maintaining contact with patients after treatment, resulting in incomplete data;
- •
- varying regulatory requirements or interpretations of data among the FDA and foreign regulatory authorities; and
- •
- a regional disturbance where we or our collaborative partners are enrolling patients in clinical trials, such as a pandemic, terrorist activities or war, political unrest or a natural disaster.
Our ongoing drug discovery and development efforts might not generate additional successful product candidates or approvable drugs.
Our compounds in clinical trials and our future leads for potential drug compounds are subject to the risks and failures inherent in the development of pharmaceutical products. These risks include, but are not limited to, the inherent difficulty in selecting the right drug and drug target and avoiding unwanted side effects, as well as unanticipated problems relating to product development, testing, enrollment, obtaining regulatory approvals, maintaining regulatory compliance, manufacturing, competition and costs and expenses that may exceed current estimates.
Clinical studies involving our product candidates may reveal that those candidates are ineffective, inferior to existing approved medicines, unacceptably toxic, or that they have other unacceptable side effects. In addition, the results of preclinical studies do not necessarily predict clinical success, and larger and later-stage clinical studies may not produce the same results as earlier-stage clinical studies.
Frequently, product candidates that have shown promising results in early preclinical or clinical studies have subsequently suffered significant setbacks or failed in later non-clinical or clinical studies. In some instances, there can be significant variability in safety and/or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial protocols, differences in size and type of the patient populations, varying levels of adherence to the dosing regimen and other trial protocols and the rate of dropout among clinical trial participants. Clinical and non-clinical studies of product candidates often reveal that it is not possible or practical to continue development efforts for these product candidates. In addition, the design of a clinical trial can
29
determine whether its results will support regulatory approval and flaws in the design of a clinical trial may not become apparent until the clinical trial is well underway. If our ongoing clinical studies for our current product candidates, such as the Phase 3 development program for revefenacin for the treatment of COPD and the earlier stage clinical studies for our JAK inhibitor program or our NEP inhibitor program, are substantially delayed or fail to meet their designated end points, we could fail to receive regulatory approval for one or more of these product candidates. In addition, our product candidates may have undesirable side effects or other unexpected characteristics that could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restricted label or the delay or denial of regulatory approval by regulatory authorities.
If our product candidates are not approved by regulatory authorities, including the FDA, we will be unable to commercialize them.
The FDA must approve any new medicine before it can be marketed and sold in the U.S. We will not obtain this approval for a product candidate unless and until the FDA approves a new drug application ("NDA"). We, or our collaborative partners, must provide the FDA and similar foreign regulatory authorities with data from preclinical and clinical studies that demonstrate that our product candidates are safe and effective for a defined indication before they can be approved for commercial distribution. FDA or foreign regulatory authorities may disagree with our trial design and our interpretation of data from preclinical studies and clinical trials. The processes by which regulatory approvals are obtained from the FDA and foreign regulatory authorities to market and sell a new product are complex, require a number of years, depend upon the type, complexity and novelty of the product candidate and involve the expenditure of substantial resources for research, development and testing. The FDA has substantial discretion in the drug approval process and may require us to conduct additional nonclinical and clinical testing or to perform post-marketing studies. Further, the implementation of new laws and regulations, and revisions to FDA clinical trial design guidance may lead to increased uncertainty regarding the approvability of new drugs. In addition, over the past decade, the FDA has implemented additional standards for approval of new drugs, including recommended advisory committee meetings for certain new molecular entities, and formal risk evaluation and mitigation requirements at the FDA's discretion. Even if we receive regulatory approval of a product, the approval may limit the indicated uses for which the drug may be marketed or impose significant restrictions or limitations on the use and/or distribution of such product.
In addition, in order to market our medicines in foreign jurisdictions, we, or our collaborative partners, must obtain separate regulatory approvals in each country. The approval procedure varies among countries and can involve additional testing, and the time required to obtain approval may differ from that required to obtain FDA approval. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. Conversely, failure to obtain approval in one or more jurisdictions may make approval in other jurisdictions more difficult. These laws, regulations, additional requirements and changes in interpretation could cause non-approval or further delays in the FDA's review and approval of our and our collaborative partner's product candidates, which would materially harm our business and financial condition and could cause the price of our securities to fall.
We rely on a single manufacturer for the Active Pharmaceutical Ingredient ("API") for telavancin and a separate, single manufacturer for VIBATIV drug product supply. Our business will be harmed if either of these single-source manufacturers are not able to satisfy demand and alternative sources are not available.
We have a single source of supply of API for telavancin and another, separate single source of supply of VIBATIV drug product. If, for any reason, either single-source third-party manufacturer of telavancin API or of VIBATIV drug product is unable or unwilling to perform, or if the performance
30
of either does not meet regulatory requirements, including maintaining current Good Manufacturing Practice ("cGMP") compliance, we may not be able to locate alternative manufacturers, enter into acceptable agreements with them or obtain sufficient quantities of API or drug product in a timely manner. We expect it would take approximately 24 months for an alternative manufacturer to be qualified by us and begin producing drug product for us. We currently have sufficient quantities of VIBATIV drug product on hand to meet our anticipated needs only through approximately December 2017. We have manufactured additional VIBATIV drug product supply which is currently undergoing our internal quality review prior to release. We will not know whether this supply is suitable for release until we complete our internal quality review. If we are able to release this additional supply we would have sufficient quantities of VIBATIV drug product available to meet our anticipated needs through the fall of 2018. This supply was manufactured by Pfizer, our single source manufacturer for VIBATIV, at its McPherson, Kansas facility. As recently publicly reported, Pfizer has received an FDA warning letter relating to a 2016 inspection of this facility. None of the lots cited in the warning letter are manufactured VIBATIV drug product. We also plan to have additional VIBATIV drug product manufactured for us at this facility in 2017. Given the time required to locate and qualify another acceptable drug product manufacturer, any supply delay, suspension or cessation in the manufacture and release of VIBATIV drug product would adversely affect the commercialization of VIBATIV and our obligations to our partners, as well as our Phase 3 registrational study for the treatment of patients with Staphylococcus aureus bacteremia. Similarly, any inability to acquire sufficient quantities of API in a timely manner from current or future sources would adversely affect the commercialization of VIBATIV and our ability to satisfy our obligations to our partners. If either of these were to occur, our business would be harmed.
Our previous VIBATIV commercialization partner (at the time with Innoviva) failed to maintain a reliable source of drug product supply which resulted in critical product shortages and, eventually, suspension of commercialization for well over a year. Our current agreement with Pfizer to supply VIBATIV drug product was entered into May 2012. In June 2013, the FDA approved Pfizer as a VIBATIV drug product manufacturer. On September 29, 2016, we amended our agreement with Pfizer to extend the term of the agreement to December 31, 2020. If our supply relationship with Pfizer terminates for any reason, we would need to arrange for the advance manufacture and purchase of drug product in order to manage the transition to a new supplier and such advance manufacturing and purchasing entails significant uncertainties, including the risk of purchasing excess or insufficient quantities relative to our future needs and the possible expiration of excess inventories. Any difficulties in continuing or transitioning our single source suppliers would adversely affect the commercialization of VIBATIV and our ability to satisfy our obligations to our partners and the price of our securities could fall.
We rely on a single source of supply for a number of our product candidates, and our business will be harmed if any of these single-source manufacturers are not able to satisfy demand and alternative sources are not available.
We have limited in-house production capabilities for preclinical and clinical study purposes, and depend primarily on a number of third-party API and drug product manufacturers. We may not have long-term agreements with these third parties and our agreements with these parties may be terminable at will by either party at any time. If, for any reason, these third parties are unable or unwilling to perform, or if their performance does not meet regulatory requirements, we may not be able to locate alternative manufacturers or enter into acceptable agreements with them. Any inability to acquire sufficient quantities of API and drug product in a timely manner from these third parties could delay preclinical and clinical studies and prevent us from developing our product candidates in a cost-effective manner or on a timely basis. In addition, manufacturers of our API and drug product are subject to the FDA's cGMP regulations and similar foreign standards and we do not have control over compliance with these regulations by our manufacturers.
31
Our manufacturing strategy presents the following additional risks:
- •
- because of the complex nature of many of our compounds, our manufacturers may not be able to successfully manufacture our APIs and/or drug
products in a cost effective and/or timely manner and changing manufacturers for our APIs or drug products could involve lengthy technology transfer, validation and regulatory qualification activities
for the new manufacturer;
- •
- the processes required to manufacture certain of our APIs and drug products are specialized and available only from a limited number of
third-party manufacturers;
- •
- some of the manufacturing processes for our APIs and drug products have not been scaled to quantities needed for continued clinical studies or
commercial sales, and delays in scale-up to commercial quantities could delay clinical studies, regulatory submissions and commercialization of our product candidates; and
- •
- because some of the third-party manufacturers are located outside of the U.S., there may be difficulties in importing our APIs and drug products or their components into the U.S. as a result of, among other things, FDA import inspections, incomplete or inaccurate import documentation or defective packaging.
We are subject to extensive and ongoing regulation, oversight and other requirements by the FDA with respect to VIBATIV and failure to comply with these regulations and requirements may subject us to penalties that may adversely affect our financial condition or our ability to commercialize VIBATIV.
With VIBATIV approved in certain countries, we are subject to continuing regulatory obligations, such as safety reporting requirements and additional post-marketing obligations, including regulatory oversight of promotion and marketing. Prescription drug advertising and promotion are closely scrutinized by the FDA, including substantiation of promotional claims, disclosure of risks and safety information, and the use themes and imagery in advertising and promotional materials. As with all companies selling and marketing products regulated by the FDA in the U.S., we are prohibited from promoting any uses of VIBATIV that are outside the scope of use that has been expressly approved by the FDA as safe and effective on the VIBATIV label.
The U.S. labeling for VIBATIV contains a boxed warning. Products with boxed warnings are subject to more restrictive advertising regulations than products without such warnings and FDA regulations prohibit the use of reminder advertising for VIBATIV. Further, based on its boxed warning, VIBATIV has been classified by the National Institute of Occupational Safety and Hazards (NIOSH) as a drug that represents a reproductive hazard. Beginning in mid-2018, hospitals and pharmacies that handle these classified drugs will be required to comply with a variety of procedures designed to promote patient safety, worker safety and environmental protection. We believe this classification of VIBATIV is erroneous and overstates the hazard presented to healthcare workers when handling VIBATIV, and we are working to remove VIBATIV from this list. If we fail to do so, however, the commercialization of VIBATIV could be adversely impacted as certain healthcare providers and institutions may not be in a position to comply with the additional handling requirements imposed by NIOSH.
In addition, the VIBATIV labeling for hospital-acquired and ventilator associated bacterial pneumonia ("HABP/VABP") in the U.S. and the European Union ("EU") specifies that VIBATIV should be reserved for use when alternative treatments are not suitable. These restrictions add complexity to the marketing of VIBATIV.
32
The FDA has also required that we evaluate the safety of VIBATIV use during pregnancy by developing and maintaining a prospective, observational pregnancy exposure registry study conducted in the United States. This postmarketing study remains ongoing and will continue through the end of 2019. In addition, the FDA has required that we comply with a risk evaluation and mitigation strategy ("REMS") to inform healthcare providers and patients of key risks via a communication plan. Healthcare providers periodically receive letters reminding them of the major potential risks associated with VIBATIV and patients receive a medication guide with each course of antibiotic use. The healthcare provider letter is also available on the product website. The REMS stipulates that we make assessments of the efficacy of these educational efforts and provide reports to FDA at specified intervals.
The manufacturing, labeling, packaging, adverse event reporting, advertising, promotion and recordkeeping for the approved product remain subject to extensive and ongoing regulatory requirements. If we become aware of previously unknown problems with an approved product in the U.S. or overseas or at a contract manufacturer's facilities, a regulatory authority may impose restrictions on the product, the contract manufacturers or on us, including requiring us to reformulate the product, conduct additional clinical studies, change the labeling of the product, withdraw the product from the market or require the contract manufacturer to implement changes to its facilities.
We are also subject to regulation by regional, national, state and local agencies, including the Department of Justice, the Federal Trade Commission, the Office of Inspector General of the U.S. Department of Health and Human Services ("OIG") and other regulatory bodies with respect to VIBATIV, as well as governmental authorities in those foreign countries in which any of our product candidates are approved for commercialization. The Federal Food, Drug, and Cosmetic Act, the Public Health Service Act and other federal and state statutes and regulations govern to varying degrees the research, development, manufacturing and commercial activities relating to prescription pharmaceutical products, including non-clinical and clinical testing, approval, production, labeling, sale, distribution, import, export, post-market surveillance, advertising, dissemination of information and promotion. If we or any third parties that provide these services for us are unable to comply, we may be subject to regulatory or civil actions or penalties that could significantly and adversely affect our business.
Regulatory approval for our product candidates, if any, may include similar or other limitations on the indicated uses for which we can market our medicines or the patient population that may utilize our medicines, which may limit the market for our medicines or put us at a competitive disadvantage relative to alternative therapies.
Any failure to maintain regulatory approval will limit our ability to commercialize VIBATIV or our product candidates and if we fail to comply with FDA regulations and requirements regarding VIBATIV or any of our product candidates, the FDA could potentially take a number of enforcement actions against us, including the issuance of untitled letters, warning letters, preventing the introduction or delivery of VIBATIV into interstate commerce in the United States, misbranding charges, product seizures, injunctions, and civil monetary penalties, which would materially and adversely affect our business and financial condition and may cause the price of our securities to fall.
The risks identified in this risk factor relating to regulatory actions and oversight by agencies in the U.S. and throughout the world also apply to the commercialization of any partnered products by our collaboration partners, and such regulatory actions and oversight may limit our collaboration partners' ability to commercialize such products, which could materially and adversely affect our business and financial condition, and which may cause the price of our securities to fall.
33
We may face competition from companies seeking to market generic versions of VIBATIV.
For a discussion of the risk of generic competition to VIBATIV, please see the following risk factor below "If our efforts to protect the proprietary nature of the intellectual property related to our technologies are not adequate, we may not be able to compete effectively in our current or future markets."
If our partners do not satisfy their obligations under our agreements with them, or if they terminate our partnerships with them, we may not be able to develop or commercialize our partnered product candidates as planned.
We have an exclusive development and commercialization agreement with Alfa Wassermann for velusetrag, our lead compound in the 5 HT4 program, covering the EU, Russia, China, Mexico and certain other countries. The Alfa Wassermann agreement was assigned to us in the Spin-Off and provides research and development funding for the program under license. In October 2012, we (at the time with Innoviva) also entered into a research collaboration and license agreement with Merck & Co., Inc. ("Merck") to discover, develop and commercialize novel small molecule therapeutics for the treatment of cardiovascular disease, which Merck terminated in September 2013. In January 2015, we entered into a collaboration agreement with Mylan for the development and commercialization of a nebulized formulation of our LAMA revefenacin (TD-4208). Under the terms of the agreement, we and Mylan will co-develop nebulized revefenacin for COPD and other respiratory diseases. In June 2016, we entered into a License and Collaboration Agreement with an indirect wholly-owned subsidiary of Takeda, in order to establish a collaboration for the development and commercialization of TD-8954, a selective 5-HT4 receptor agonist. Under the terms of the Agreement, Takeda will be responsible for worldwide development and commercialization of TD-8954. In connection with these agreements, these parties have certain rights regarding the use of its patents and technology with respect to the compounds in our development programs, including development and marketing rights.
We also have commercialization agreements with various partners for the commercialization of VIBATIV outside of the United States, including Canada, Middle East, North Africa, Israel, Russia, China and India. In August 2016, we and Clinigen reached a mutual decision that Clinigen will return commercial rights to market and distribute VIBATIV in the EU to Theravance Biopharma. On November 4, 2016, the European Commission authorized the transfer of the centralized marketing authorization for VIBATIV to our wholly-owned Irish subsidiary, Theravance Biopharma Ireland Limited. Therefore, we are now subject to all applicable EU regulatory obligations as the new marketing authorization holder of VIBATIV in the EU. We do not intend to commercialize VIBATIV in the EU without a partner. Therefore, if we fail to find a suitable partner to commercialize VIBATIV in the EU, we will not receive any product revenue from that region.
Our partners might not fulfill all of their obligations under these agreements, and, in certain circumstances, they or we may terminate our partnership with them as Astellas did in January 2012 with its VIBATIV agreement, as Merck did in September 2013 with the cardiovascular disease collaboration and as we and Clinigen did in August 2016 with the commercialization agreement for VIBATIV in the EU and certain other European countries. In either event, we may be unable to assume the development and commercialization responsibilities covered by the agreements or enter into alternative arrangements with a third-party to develop and commercialize such product candidates. If a partner elected to promote alternative products and product candidates such as its own products and product candidates in preference to those licensed from us, does not devote an adequate amount of time and resources to our product candidates or is otherwise unsuccessful in its efforts with respect to our products or product candidates, the development and commercialization of product candidates covered by the agreements could be delayed or terminated, and future payments to us could be delayed, reduced or eliminated and our business and financial condition could be materially and adversely affected. Accordingly, our ability to receive any revenue from the product candidates covered
34
by these agreements is dependent on the efforts of our partners. If a partner terminates or breaches its agreements with us, otherwise fails to complete its obligations in a timely manner or alleges that we have breached our contractual obligations under these agreements, the chances of successfully developing or commercializing product candidates under the collaboration could be materially and adversely affected. We could also become involved in disputes with a partner, which could lead to delays in or termination of our development and commercialization programs and time-consuming and expensive litigation or arbitration. Furthermore, termination of an agreement by a partner could have an adverse effect on the price of our ordinary shares or other securities even if not material to our business.
Because GSK is a strategic partner of Innoviva, a strategic partner of TRC and a significant shareholder of us, it may take actions that in certain cases are materially harmful to our business and to our other shareholders.
Based on our review of publicly available filings, as of December 31, 2016, GSK beneficially owned approximately 18.3% of our outstanding ordinary shares. GSK is also a strategic partner to Innoviva with rights and obligations under the strategic alliance agreement and under the collaboration agreement assigned to TRC (the "GSK-Innoviva Agreements") that may cause GSK's interests to differ from the interests of us and our other shareholders. In particular, if the Closed Triple or a MABA/ICS in either the U.S. or the EU is approved, GSK's diligent efforts obligations under the GSK-Innoviva Agreements with regard to commercialization matters will have the objective of focusing on the best interests of patients and maximizing the net value of the overall portfolio of products under the GSK-Innoviva Agreements. Following such regulatory approval, GSK's commercialization efforts will be guided by a portfolio approach across products in which we have an indirect interest through TRC and products in which we have no interest. Accordingly, GSK's commercialization efforts may have the effect of reducing the value of our interest in TRC. Furthermore, GSK has a substantial respiratory product portfolio in addition to the products covered by the GSK-Innoviva Agreements. GSK may make respiratory product portfolio decisions or statements about its portfolio which may be, or may be perceived to be, harmful to the respiratory products partnered with Innoviva and TRC. For example, GSK could promote its own respiratory products and/or delay or terminate the development or commercialization of the respiratory programs covered by the GSK-Innoviva Agreements. Also, given the potential future royalty payments GSK may be obligated to pay under the GSK-Innoviva Agreements, GSK may seek to acquire us or acquire our interests in TRC in order to effectively reduce those payment obligations and the price at which GSK might seek to acquire us may not reflect our true value. Although the actions GSK may take to acquire us are limited under our governance agreement with GSK (the "Governance Agreement"), this agreement will expire on December 31, 2017. The timing of when GSK may seek to acquire us could potentially be when it possesses information regarding the status of drug programs covered by the GSK-Innoviva Agreements that has not been publicly disclosed and is not otherwise known to us. As a result of these differing interests, GSK may take actions that it believes are in its best interest but which might not be in the best interests of either us or our other shareholders. In addition, GSK could also seek to challenge our or Innoviva's post-Spin-Off operations as violating or allowing it to terminate the GSK-Innoviva Agreements, including by violating the confidentiality provisions of those agreements or the master agreement between GSK, Innoviva and us entered into in connection with the Spin-Off, or otherwise violating its legal rights. While we believe our operations fully comply with the GSK-Innoviva Agreements, the master agreement and applicable law, there can be no assurance that we or Innoviva will prevail against any such claims by GSK. Moreover, regardless of the merit of any claims by GSK, we may incur significant cost and diversion of resources in defending them. In addition, any other action or inaction by either GSK or Innoviva that results in a material dispute, allegation of breach, litigation, arbitration, or significant disagreement between those parties may be interpreted negatively by the market or by our investors, could harm our business and cause the price of our securities to fall.
35
Examples of these kinds of issues include but are not limited to non-performance of contractual obligations and allegations of non-performance, disagreements over the relative marketing and sales efforts for Innoviva's partnered products and other GSK respiratory products, disputes over public statements, and similar matters. In general, any uncertainty about the respiratory programs partnered with GSK, the enforceability of the GSK-Innoviva Agreements or the relationship/partnership between Innoviva and GSK could result in significant reduction in the market price of our securities and other material harm to our business.
Agreements entered into with or for the benefit of GSK in connection with the Spin-Off may significantly restrict our business and affairs.
On March 3, 2014, in connection with the Spin-Off, we, Innoviva and GSK entered into a number of agreements that may significantly restrict our business and affairs. In particular, we, Innoviva and GSK entered into a three-way master agreement (the "Master Agreement") that, among other things, requires GSK's consent to make any changes to (A) the Separation and Distribution Agreement and ancillary agreements that would, individually or in the aggregate, reasonably be expected to adversely affect GSK in any material respect or (B) the TRC Limited Liability Company Agreement, which consent is not to be unreasonably withheld, conditioned or delayed, provided that GSK may withhold, condition or delay such consent in its sole discretion with respect to certain sections of the TRC Limited Liability Company Agreement and any changes to the governance structure of TRC, the confidentiality restrictions, the consent rights, and the transfer restrictions in the TRC Limited Liability Company Agreement. We and GSK also entered into (i) the Governance Agreement that, among other things, provides share purchase rights to GSK and exempts GSK from triggering our Rights Agreement until December 31, 2017, (ii) a registration rights agreement that gives GSK certain registration rights with respect to our ordinary shares held by GSK and (iii) an extension agreement that extends to us certain restrictive covenants similar to those applicable to Innoviva under the GSK-Innoviva Agreements. There can be no assurance that these restrictions will not materially harm our business, particularly given that GSK's interests may not be aligned with the interests of our business or our other shareholders.
We depend on third parties in the conduct of our clinical studies for our product candidates.
We depend on independent clinical investigators, contract research and manufacturing organizations and other third-party service providers in the conduct of our non-clinical and clinical studies for our product candidates. We rely heavily on these parties for execution of our non-clinical and clinical studies, and control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that our clinical studies are conducted in accordance with good clinical, laboratory and manufacturing practices ("GXPs") and other regulations as required by the FDA and foreign regulatory authorities, and the applicable protocol. Failure by these parties to comply with applicable regulations and practices in conducting studies of our product candidates can result in a delay in our development programs or non-approval of our product candidates by regulatory authorities.
The FDA, and equivalent authorities in other countries, enforces GXPs and other regulations through periodic inspections of trial sponsors, clinical research organizations ("CROs"), principal investigators and trial sites. If we or any of the third parties on which we have relied to conduct our clinical studies are determined to have failed to comply with GXPs (or other equivalent regulations outside the United States), the study protocol or applicable regulations, the clinical data generated in our studies may be deemed unreliable. This could result in non-approval of our product candidates by the FDA, or equivalent authorities in other countries, or we, the FDA, or equivalent authorities in other countries may decide to conduct additional audits or require additional clinical studies, which would delay our development programs, could result in significant additional costs and the price of our securities could fall.
36
We face substantial competition from companies with more resources and experience than we have, which may result in others discovering, developing, receiving approval for or commercializing products before or more successfully than we do.
Our ability to succeed in the future depends on our ability to demonstrate and maintain a competitive advantage with respect to our approach to the discovery, development and commercialization of medicines. Our objective is to discover, develop and commercialize new small molecule medicines with superior efficacy, convenience, tolerability and/or safety using our proprietary insight in chemistry, biology and multivalency, where applicable. We expect that any medicines that we commercialize with or without our collaborative partners will compete with existing or future market-leading medicines.
Many of our current and potential competitors have substantially greater financial, technical and personnel resources than we have. In addition, many of these competitors have significantly greater commercial infrastructures than we have. Our ability to compete successfully will depend largely on our ability to leverage our experience in drug discovery and development, and, more recently, commercialization, to:
- •
- discover and develop medicines that are superior to other products in the market;
- •
- attract and retain qualified personnel;
- •
- obtain patent and/or other proprietary protection for our medicines and technologies;
- •
- obtain required regulatory approvals;
- •
- develop and effectively implement commercialization strategies, with or without collaborative partners; and
- •
- successfully collaborate with pharmaceutical companies in the discovery, development and commercialization of new medicines.
Pharmaceutical companies, including companies with which we collaborate, may invest heavily to quickly discover and develop or in-license novel compounds that could make our product candidates obsolete. Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA or equivalent regulatory approval outside the United States or discovering, developing and commercializing medicines before we do. Other companies are engaged in the discovery of medicines that would compete with the product candidates that we are developing.
Any new medicine that competes with a generic or proprietary market leading medicine must demonstrate compelling advantages in efficacy, convenience, tolerability and/or safety in order to overcome severe price competition and be commercially successful. VIBATIV must demonstrate these advantages in certain circumstances, as it competes with vancomycin, linezolid and daptomycin, relatively inexpensive generic drugs that are manufactured by a number of companies, and a number of existing antibacterial drugs marketed by major and other pharmaceutical companies. If we are not able to compete effectively against our current and future competitors, our business will not grow, our financial condition and operations will suffer and the price of our securities could fall.
Certain of our directors and officers may have actual or potential conflicts of interest because of their equity ownership in Innoviva, which actual or potential conflicts may harm our business, prospects and financial condition and result in the diversion of corporate opportunities to Innoviva.
Certain of our directors and executive officers hold shares of Innoviva's common stock or rights to acquire such shares, and these holdings may be significant for some of these individuals compared to their total assets. This ownership of Innoviva common stock by our officers and most of our directors may create, or may create the appearance of, conflicts of interest when these directors and officers are
37
faced with decisions that could have different implications for Innoviva and for us. For example, potential or actual conflicts could arise relating to: our relationship with Innoviva, including Innoviva's and our respective rights and obligations under agreements entered into in connection with the Spin-Off; Innoviva's management of TRC, particularly given that we and Innoviva have different economic interests in TRC; and corporate opportunities that may be available to both companies in the future. Although we and Innoviva have implemented policies and procedures to identify and properly address such potential and actual conflicts of interest, there can be no assurance that, when such conflicts are resolved in accordance with applicable laws, such conflicts of interest will not harm our business, prospects and financial condition and result in the diversion of corporate opportunities to Innoviva.
If we lose key management or scientific personnel, or if we fail to attract and retain key employees, our ability to discover and develop our product candidates and commercialize VIBATIV and any other products that may be approved in the future will be impaired.
We are highly dependent on principal members of our management team and scientific staff, and in particular, our Chief Executive Officer, Rick E Winningham, to operate our business. Mr. Winningham has significant pharmaceutical industry experience. The loss of Mr. Winningham's services could impair our ability to discover, develop and commercialize new medicines.
If we fail to retain our qualified personnel or replace them when they leave, we may be unable to continue our discovery, development and commercialization activities, which may cause the price of our securities to fall.
In addition, our U.S. operating subsidiary's facility and most of its employees are located in northern California, headquarters to many other biotechnology and biopharmaceutical companies and many academic and research institutions. As a result, competition for certain skilled personnel in our market is intense. None of our employees have employment commitments for any fixed period of time and they all may leave our employment at will. If we fail to retain our qualified personnel or replace them when they leave, we may be unable to continue our development and commercialization activities and the price of our securities could fall.
Our business and operations would suffer in the event of significant disruptions of information technology systems or security breaches.
We rely extensively on computer systems to maintain information and manage our finances and business. In the ordinary course of business, we collect, store and transmit large amounts of confidential information (including but not limited to trade secrets or other intellectual property, proprietary business information and personal information) and it is critical that we maintain the confidentiality and integrity of such confidential information. Although we have security measures in place, our internal information technology systems and those of our CROs and other service providers, including cloud-based and hosted applications, data and services, are vulnerable to service interruptions and security breaches from inadvertent or intentional actions by our employees, service providers and/or business partners, from cyber-attacks by malicious third parties, and/or from, natural disasters, terrorism, war and telecommunication and electrical failures. Cyber-attacks are increasing in their frequency, sophistication, and intensity, and have become increasingly difficult to detect. Significant disruptions of information technology systems or security breaches could adversely affect our business operations and result in financial, legal, business and reputational harm to us, including significant liability and/or significant disruption to our business. If a disruption of information technology systems or security breach results in a loss of or damage to our data or regulatory applications, unauthorized access, use, or disclosure of, or the prevention of access to, confidential information, or other harm to our business, we could incur liability and reputational harm, we could be required to comply with federal and/or state breach notification laws and foreign law equivalents, the further development of
38
our product candidates could be delayed and the price of our securities could fall. For example, the loss of clinical trial data from completed or ongoing clinical trials of our product candidates could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Although we have security and fraud prevention measures in place, we have been subject to immaterial payment fraud activity. Moreover, there can be no assurance that such security measures will prevent service interruptions or security breaches that could adversely affect our business.
Our U.S. operating subsidiary's facility is located near known earthquake fault zones, and the occurrence of an earthquake, extremist attack or other catastrophic disaster could cause damage to our facilities and equipment, which could require us to cease or curtail operations.
Our U.S. operating subsidiary's facility is located in the San Francisco Bay Area near known earthquake fault zones and therefore will be vulnerable to damage from earthquakes. In October 1989, a major earthquake struck this area and caused significant property damage and a number of fatalities. We are also vulnerable to damage from other types of disasters, including power loss, attacks from extremist organizations, fire, floods, communications failures and similar events. If any disaster were to occur, our ability to operate our business could be seriously impaired. In addition, the unique nature of our research activities and of much of our equipment could make it difficult and costly for us to recover from this type of disaster. We may not have adequate insurance to cover our losses resulting from disasters or other similar significant business interruptions and we do not plan to purchase additional insurance to cover such losses due to the cost of obtaining such coverage. Any significant losses that are not recoverable under our insurance policies could seriously impair our business and financial condition, which could cause the price of our securities to fall.
Global health and economic, political and social conditions may harm our ability to do business, increase our costs and negatively affect our stock price.
Worldwide economic conditions remain uncertain due to the election by the United Kingdom to withdraw from the European Union (often referred to as "Brexit"), new political leadership in the United States, current economic challenges in Asia and other disruptions to global and regional economies and markets. External factors, such as potential terrorist attacks, acts of war, geopolitical and social turmoil or epidemics and other similar outbreaks in many parts of the world, could prevent or hinder our ability to do business, increase our costs and negatively affect our stock price. In addition, our operations also depend upon favorable trade relations between the U.S. and those foreign countries in which our materials suppliers have operations. A protectionist trade environment in either the U.S. or those foreign countries in which we do business, such as a change in the current tariff structures, export compliance or other trade policies, may materially and adversely affect our operations. These geopolitical, social and economic conditions could harm our business.
If we are unable to maintain effective internal controls, our business, financial position and results of operations could be adversely affected.
If we are unable to maintain effective internal controls, our business, financial position and results of operations could be adversely affected. We are subject to the reporting and other obligations under the Exchange Act, including the requirements of Section 404 of the Sarbanes-Oxley Act of 2002, which require annual management assessments of the effectiveness of our internal control over financial reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) under the Exchange Act. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States. Any failure to achieve and maintain effective internal controls could have an adverse effect on our business, financial position and results of
39
operations. In addition, since we are a "large accelerated filer" rather than an "emerging growth company" (each as defined in the Exchange Act) as of December 31, 2016 our independent registered public accounting firm will be required to attest to the effectiveness of our internal control over financial reporting annually. If our independent registered public accounting firm is unable to attest to the effectiveness of our internal control over financial reporting, investor confidence in our reported results will be harmed and the price of our securities may fall. These reporting and other obligations place significant demands on our management and administrative and operational resources, including accounting resources.
We have only been operating as a stand-alone entity since June 2, 2014 and therefore we have a limited history operating as an independent company upon which you can evaluate us.
We have only been operating as a stand-alone entity since June 2, 2014 and therefore we have a limited operating history as an independent company upon which you can evaluate us. While our biopharmaceutical business has constituted a substantial part of the historic operations of Innoviva, we did not operate as a stand-alone company.
In addition, our historical financial information prior to the Spin-Off does not necessarily reflect what our financial position, results of operations or cash flows would have been as a stand-alone company during the periods presented and is not necessarily indicative of our future financial position, future results of operations or future cash flows.
We may be treated as a U.S. corporation for U.S. federal income tax purposes.
For U.S. federal income tax purposes, a corporation generally is considered tax resident in the place of its incorporation. Theravance Biopharma is incorporated under Cayman Islands law and established tax residency in Ireland effective July 1, 2015. Therefore, it should be a non-U.S. corporation under this general rule. However, Section 7874 of the Internal Revenue Code of 1986, as amended (the "Code"), contains rules that may result in a foreign corporation being treated as a U.S. corporation for U.S. federal income tax purposes. The application of these rules is complex and there is little guidance regarding certain aspects of their application.
Under Section 7874 of the Code, a corporation created or organized outside the U.S. will be treated as a U.S. corporation for U.S. federal tax purposes if (i) the foreign corporation directly or indirectly acquires substantially all of the properties held directly or indirectly by a U.S. corporation, (ii) the former shareholders of the acquired U.S. corporation hold at least 80% of the vote or value of the shares of the foreign acquiring corporation by reason of holding stock in the U.S. acquired corporation, and (iii) the foreign corporation's "expanded affiliated group" does not have "substantial business activities" in the foreign corporation's country of incorporation relative to its expanded affiliated group's worldwide activities. For this purpose, "expanded affiliated group" generally means the foreign corporation and all subsidiaries in which the foreign corporation, directly or indirectly, owns more than 50% of the stock by vote and value, and "substantial business activities" generally means at least 25% of employees (by number and compensation), assets and gross income of our expanded affiliated group are based, located and derived, respectively, in the country of incorporation.
We do not expect to be treated as a U.S. corporation under Section 7874 of the Code, because we do not believe that the assets contributed to us by Innoviva constituted "substantially all" of the properties of Innoviva (as determined on both a gross and net fair market value basis). However, the Internal Revenue Service ("IRS") may disagree with our conclusion on this point and assert that, in its view, the assets contributed to us by Innoviva did constitute "substantially all" of the properties of Innoviva. In addition, there could be legislative proposals to expand the scope of U.S. corporate tax residence and there could be changes to Section 7874 of the Code or the Treasury Regulations
40
promulgated thereunder that could apply retroactively and could result in Theravance Biopharma being treated as a U.S. corporation.
If it were determined that we should be treated as a U.S. corporation for U.S. federal income tax purposes, we could be liable for substantial additional U.S. federal income tax on our post-Spin-Off taxable income. In addition, payments of dividends to non-U.S. holders may be subject to U.S. withholding tax.
Taxing authorities may challenge our structure and transfer pricing arrangements.
We are incorporated in the Cayman Islands, maintain subsidiaries in the Cayman Islands, the United States, the United Kingdom and Ireland, and effective July 1, 2015, we migrated our tax residency from the Cayman Islands to Ireland. Due to economic and political conditions various countries are actively considering changes to existing tax laws. We cannot predict the form or timing of potential legislative changes that could have a material adverse impact on our results of operations. In addition, significant judgment is required in determining our worldwide provision for income taxes. Various factors may have favorable or unfavorable effects on our income tax rate including, but not limited to the performance of certain functions and ownership of certain assets in tax-efficient jurisdictions such as the Cayman Islands and Ireland, together with intra-group transfer pricing agreements. Taxing authorities may challenge our structure and transfer pricing arrangements through an audit or lawsuit. Responding to or defending such a challenge could be expensive and consume time and other resources, and divert management's time and focus from operating our business. We cannot predict whether taxing authorities will conduct an audit or file a lawsuit challenging this structure, the cost involved in responding to any such audit or lawsuit, or the outcome. We may be required to pay taxes for prior periods, interest, fines or penalties, and may be obligated to pay increased taxes in the future which could result in reduced cash flows and have a material adverse effect on our business, financial condition and growth prospects.
We were a passive foreign investment company, or "PFIC," for 2014 but we believe that we are not a PFIC for 2015 and 2016, and we do not expect to be a PFIC for the foreseeable future.
For U.S. federal income tax purposes, we generally would be classified as a PFIC for any taxable year if either (i) 75% or more of our gross income (including gross income of certain 25% or more owned corporate subsidiaries) is "passive income" (as defined for such purposes) or (ii) the average percentage of our assets (including the assets of certain 25% or more owned corporate subsidiaries) that produce passive income or that are held for the production of passive income is at least 50%. In addition, whether our company will be a PFIC for any taxable year depends on our assets and income over the course of each such taxable year and, as a result, cannot be predicted with certainty until after the end of the year.
Based upon our assets and income during the course of 2014, we believe that our company and one of our company's wholly owned subsidiaries, Theravance Biopharma R&D, Inc. was a PFIC for 2014. Based upon our assets and income during the course of 2015 and 2016, we do not believe that our company is a PFIC for 2015 or 2016. We do not expect to be a PFIC for the foreseeable future based on our current business plans and current business model. For any taxable year (or portion thereof) in which our company is a PFIC that is included in the holding period of a U.S. holder, the U.S. holder is generally subject to additional U.S. federal income taxes plus an interest charge with respect to certain distributions from Theravance Biopharma or gain recognized on a sale of Theravance Biopharma shares. Similar rules would apply with respect to distributions from or gain recognized on an indirect sale of Theravance Biopharma R&D, Inc. U.S. holders of our ordinary shares may have filed an election with respect to company shares held at any time during 2014 to be treated as owning an interest in a "qualified electing fund" ("QEF") or to "mark to market" their ordinary shares to avoid the otherwise applicable interest charge consequences of PFIC treatment with respect to our
41
ordinary shares. A foreign corporation will not be treated as a QEF for any taxable year in which such foreign corporation is not treated as a PFIC. QEF and mark to market elections generally apply to the taxable year for which the election is made and all subsequent taxable years unless the election is revoked with consent of the Secretary of Treasury. U.S. holders of our ordinary shares should consult their tax advisers regarding the tax reporting implications with respect to any QEF and mark to market elections made with respect to our company and with respect to their indirect interests in Theravance Biopharma R&D, Inc.
If we are required to indemnify Innoviva, or if we are not able to collect on indemnification rights from Innoviva, our business prospects and financial condition may be harmed.
We agreed to indemnify Innoviva from and after the Spin-Off with respect to (i) all debts, liabilities and obligations transferred to us in connection with the Spin-Off (including our failure to pay, perform or otherwise promptly discharge any such debts, liabilities or obligations after the Spin-Off), (ii) any misstatement or omission of a material fact resulting in a misleading statement in our Information Statement distributed to Innoviva stockholders in connection with the Spin-Off and (iii) any breach by us of certain agreements entered into with Innoviva in connection with the Spin-Off (namely, the Separation and Distribution Agreement, the Transition Services Agreement, the Employee Matters Agreement, the Tax Matters Agreement, and the Facility Sublease Agreement). We are not aware of any existing indemnification obligations at this time, but any such indemnification obligations that may arise could be significant. Under the terms of the Separation and Distribution Agreement, Innoviva agreed to indemnify us from and after the Spin-Off with respect to (i) all debts, liabilities and obligations retained by Innoviva after the Spin-Off (including its failure to pay, perform or otherwise promptly discharge any such debts, liabilities or obligations after the Spin-Off) and (ii) any breach by Innoviva of the Separation and Distribution Agreement, the Transition Services Agreement, the Employee Matters Agreement, the Tax Matters Agreement, and the Facility Sublease Agreement. Our and Innoviva's ability to satisfy these indemnities, if called upon to do so, will depend upon our and Innoviva's future financial strength. If we are required to indemnify Innoviva, or if we are not able to collect on indemnification rights from Innoviva, our business prospects and financial condition may be harmed.
RISKS RELATED TO LEGAL AND REGULATORY UNCERTAINTY
If our efforts to protect the proprietary nature of the intellectual property related to our technologies are not adequate, we may not be able to compete effectively in our current or future markets.
We rely upon a combination of patents, patent applications, trade secret protection and confidentiality agreements to protect the intellectual property related to our technologies. Any involuntary disclosure to or misappropriation by third parties of this proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding our competitive position in our market. The status of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and is very uncertain. As of December 31, 2016, we or one of our wholly-owned subsidiaries owned 434 issued United States patents and 1,681 granted foreign patents, as well as additional pending United States and foreign patent applications. Our patent applications may be challenged or fail to result in issued patents and our existing or future patents may be invalidated or be too narrow to prevent third parties from developing or designing around these patents. If the sufficiency of the breadth or strength of protection provided by our patents with respect to a product candidate is threatened, it could dissuade companies from collaborating with us to develop product candidates and threaten our ability to commercialize products. Further, if we encounter delays in our clinical trials or in obtaining regulatory approval of our product candidates, the patent lives of the related product candidates would be reduced.
42
In addition, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, for processes for which patents are difficult to enforce and for any other elements of our drug discovery and development processes that involve proprietary know-how, information and technology that is not covered by patent applications. Although we require our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information and technology to enter into confidentiality agreements, we cannot be certain that this know-how, information and technology will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Further, the laws of some foreign countries do not protect proprietary rights to the same extent as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent material disclosure of the intellectual property related to our technologies to third parties, we will not be able to establish or, if established, maintain a competitive advantage in our market, which could materially adversely affect our business, financial condition and results of operations, which could cause the price of our securities to fall.
Under the Drug Price Competition and Patent Term Restoration Act of 1984, a company may submit an abbreviated new drug application (ANDA) under section 505(j) of the Federal Food, Drug, and Cosmetic Act to market a generic version of an approved drug. Because a generic applicant does not conduct its own clinical studies, but instead relies on the FDA's finding of safety and effectiveness for the approved drug, it is able to introduce a competing product into the market at a cost significantly below that of the original drug. Although we have multiple patents protecting VIBATIV until at least 2027 that are listed in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book, generic applicants could potentially submit "paragraph IV certifications" to FDA stating that such patents are invalid or will not be infringed by the applicant's product. We have not received any such paragraph IV notifications but if any competitors successfully challenge our patents, we would face substantial competition. If we are not able to compete effectively against such future competition, our business will not grow, our financial condition and operations will suffer and the price of our securities could fall.
Litigation or third-party claims of intellectual property infringement would require us to divert resources and may prevent or delay our drug discovery and development efforts.
Our commercial success depends in part on us and our partners not infringing the patents and proprietary rights of third parties. Third parties may assert that we or our partners are using their proprietary rights without authorization. There are third-party patents that may cover materials or methods for treatment related to our product candidates. At present, we are not aware of any patent infringement claims with merit that would adversely and materially affect our ability to develop our product candidates, but nevertheless the possibility of third-party allegations cannot be ruled out. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. Furthermore, parties making claims against us or our partners may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense against these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business.
In the event of a successful claim of infringement against us, we may have to pay substantial damages, obtain one or more licenses from third parties or pay royalties. In addition, even in the absence of litigation, we may need to obtain licenses from third parties to advance our research or allow commercialization of our product candidates, and we have done so from time to time. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize one or more of our product candidates,
43
which could harm our business significantly. In addition, in the future we could be required to initiate litigation to enforce our proprietary rights against infringement by third parties. Prosecution of these claims to enforce our rights against others would involve substantial litigation expenses and divert substantial employee resources from our business. If we fail to effectively enforce our proprietary rights against others, our business will be harmed and the price of our securities could fall.
If the efforts of our partners or future partners to protect the proprietary nature of the intellectual property related to collaboration assets are not adequate, the future commercialization of any medicines resulting from collaborations could be delayed or prevented, which would materially harm our business and could cause the price of our securities to fall.
The risks identified in the two preceding risk factors may also apply to the intellectual property protection efforts of our partners or future partners and to GSK with respect to the GSK-Partnered Respiratory Programs in which we hold an economic interest. To the extent the intellectual property protection of any partnered assets are successfully challenged or encounter problems with the United States Patent and Trademark Office or other comparable agencies throughout the world, the future commercialization of these potential medicines could be delayed or prevented. Any challenge to the intellectual property protection of a late-stage development asset, particularly those of the GSK-Partnered Respiratory Programs in which we hold an economic interest, could harm our business and cause the price of our securities to fall.
Product liability lawsuits could divert our resources, result in substantial liabilities and reduce the commercial potential of our medicines.
The risk that we may be sued on product liability claims is inherent in the development and commercialization of pharmaceutical products. Side effects of, or manufacturing defects in, products that we or our partners develop or commercialize could result in the deterioration of a patient's condition, injury or even death. The VIBATIV prescribing information describes several potential adverse effects observed during clinical trials, including increased mortality versus vancomycin in patients with HABP/VABP who had pre-existing moderate to severe renal impairment, decreased clinical response in patients with cSSSI who had pre-existing moderate/severe renal impairment, and other renal adverse events. The prescribing information includes a black box warning regarding increased mortality in patients with pre-existing moderate/severe renal impairment who were treated with VIBATIV for HABP/VABP, new onset or worsening renal impairment, use in women of childbearing potential or during pregnancy and adverse developmental outcomes observed in 3 animal species. Once a product is approved for sale and commercialized, the likelihood of product liability lawsuits tends to increase. Claims may be brought by individuals seeking relief for themselves or by individuals or groups seeking to represent a class, asserting injuries based both on potential adverse effects described in the label as well as adverse events not yet observed. Also, changes in laws outside the U.S. are expanding our potential liability for injuries that occur during clinical trials. These lawsuits may divert our management from pursuing our business strategy and may be costly to defend. In addition, if we are held liable in any of these lawsuits, we may incur substantial liabilities and may be forced to limit or forgo further commercialization of the applicable products.
Although we maintain general liability and product liability insurance, this insurance may not fully cover potential liabilities and we cannot be sure that our insurer will not disclaim coverage as to a future claim. In addition, inability to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims could prevent or inhibit the commercial production and sale of our products, which could adversely affect our business. The cost of defending any product liability litigation or other proceeding, even if resolved in our favor, could be substantial and uncertainties resulting from the initiation and continuation of product liability litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Product liability claims could also harm our reputation, which may adversely affect our and our partners' ability to commercialize our products successfully and the price of our securities could fall.
44
Changes in healthcare law and implementing regulations, including government restrictions on pricing and reimbursement, as well as healthcare policy and other healthcare payor cost-containment initiatives, may negatively impact our ability to generate revenues.
The continuing efforts of the government, insurance companies, managed care organizations and other payors of health care costs to contain or reduce costs of health care may adversely affect one or more of the following:
- •
- our or our collaborators' ability to set and collect a price we believe is reasonable for our product;
- •
- our ability to generate revenues and achieve profitability; and
- •
- the availability of capital.
The pricing and reimbursement environment for VIBATIV and any future products may change in the future and become more challenging due to, among other reasons, policies advanced by the current or any new presidential administration, federal agencies, new healthcare legislation passed by Congress or fiscal challenges faced by all levels of government health administration authorities. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and expanding access to healthcare. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. We expect to experience pricing pressures in connection with the sale of VIBATIV and other products we may bring to market, due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative proposals.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (together the "Healthcare Reform Act"), is a sweeping measure intended to expand healthcare coverage within the United States, primarily through the imposition of health insurance mandates on employers and individuals and expansion of the Medicaid program. This law substantially changes the way healthcare is financed by both governmental and private insurers, and significantly impacts the pharmaceutical industry. The Healthcare Reform Act contains a number of provisions that impact our business and operations. Changes that may affect our business include those governing enrollment in federal healthcare programs, reimbursement changes, benefits for patients within a coverage gap in the Medicare Part D prescription drug program (commonly known as the "donut hole"), rules regarding prescription drug benefits under the health insurance exchanges, changes to the Medicare Drug Rebate program, expansion of the Public Health Service's 340B drug pricing program, fraud and abuse and enforcement. These changes impact existing government healthcare programs and are resulting in the development of new programs, including Medicare payment for performance initiatives and improvements to the physician quality reporting system and feedback program.
Details of the changes to the Medicaid Drug Rebate program and the 340B program are discussed below under the risk factor "—If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate program or other governmental pricing programs, we could be subject to additional reimbursement requirements, penalties, sanctions and fines, which could have a material adverse effect on our business, financial condition, results of operations and growth prospects." In particular, on February 1, 2016, the Centers for Medicare and Medicaid Services ("CMS"), the federal agency that administers the Medicare and Medicaid programs, issued final regulations to implement the changes to the Medicaid Drug Rebate program under the Healthcare Reform Act. These regulations became effective on April 1, 2016. Congress could enact additional legislation that further increases Medicaid drug rebates or other costs and charges associated with participating in the Medicaid Drug Rebate program. The issuance of regulations and coverage expansion by various governmental agencies relating to the
45
Medicaid Drug Rebate program has and will continue to increase our costs and the complexity of compliance, has been and will be time-consuming, and could have a material adverse effect on our results of operations.
Some states have elected not to expand their Medicaid programs by raising the income limit to 133% of the federal poverty level, as is permitted under the Healthcare Reform Act. For each state that does not choose to expand its Medicaid program, there may be fewer insured patients overall, which could impact our sales, business and financial condition. Where Medicaid patients receive insurance coverage under any of the new options made available through the Healthcare Reform Act, manufacturers may be required to pay Medicaid rebates on drugs used under these circumstances, which could impact manufacturer revenues. In addition, the federal government has also announced delays in the implementation of key provisions of the Healthcare Reform Act. The implications of these delays for our sales, business and financial condition, if any, are not yet clear.
Moreover, legislative changes to the Healthcare Reform Act remain possible and appear likely in the 115th United States Congress and under the Trump Administration. We expect that the Healthcare Reform Act, as currently enacted or as it may be amended in the future, and other healthcare reform measures that may be adopted in the future, could have a material adverse effect on our industry generally and on our ability to maintain or increase sales of our existing products or to successfully commercialize our product candidates, if approved.
In addition, there have been proposals to impose federal rebates on Medicare Part D drugs, requiring federally-mandated rebates on all drugs dispensed to Medicare Part D enrollees or on only those drugs dispensed to certain groups of lower income beneficiaries. If any of these proposals are adopted they could result in Theravance owing additional rebates, which could have a negative impact on revenues from sales of our products.
Beginning on April 1, 2013, Medicare payments for all items and services under Part A and B, including drugs and biologicals, were reduced by 2% under the sequestration (i.e., automatic spending reductions) as required by federal law, which requires sequestration for most federal programs, excluding Medicaid, Social Security, and certain other programs. The law caps the cuts to Medicare payments for items and services at 2% and this will continue to 2025. As long as these cuts remain in effect, they could adversely impact payment for VIBATIV and our product candidates. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures.
If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate program or other governmental pricing programs, we could be subject to additional reimbursement requirements, penalties, sanctions and fines, which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
We participate in and have certain price reporting obligations to the Medicaid Drug Rebate program and other governmental pricing programs, and we have obligations to report average sales price under the Medicare program.
Under the Medicaid Drug Rebate program, we are required to pay a rebate to each state Medicaid program for our covered outpatient drugs that are dispensed to Medicaid beneficiaries and paid for by a state Medicaid program as a condition of having federal funds being made available to the states for our drugs under Medicaid and Medicare Part B. Those rebates are based on pricing data reported by us on a monthly and quarterly basis to CMS, the federal agency that administers the Medicaid Drug Rebate program. These data include the average manufacturer price and, in the case of innovator products, the best price for each drug which, in general, represents the lowest price available
46
from the manufacturer to any entity in the United States in any pricing structure, calculated to include all sales and associated rebates, discounts and other price concessions.
The Healthcare Reform Act made significant changes to the Medicaid Drug Rebate program, such as expanding rebate liability from fee-for-service Medicaid utilization to include the utilization of Medicaid managed care organizations as well and changing the definition of average manufacturer price. The Healthcare Reform Act also increased the minimum Medicaid rebate; changed the calculation of the rebate for certain innovator products that qualify as line extensions of existing drugs; and capped the total rebate amount at 100% of the average manufacturer price. Finally, the Healthcare Reform Act requires pharmaceutical manufacturers of branded prescription drugs to pay a branded prescription drug fee to the federal government.
On February 1, 2016, CMS issued final regulations to implement the changes to the Medicaid Drug Rebate program under the Healthcare Reform Act. These regulations became effective on April 1, 2016. The issuance of regulations and coverage expansion by various governmental agencies relating to the Medicaid Drug Rebate program has and will continue to increase our costs and the complexity of compliance, has been and will be time-consuming, and could have a material adverse effect on our results of operations.
Federal law requires that any company that participates in the Medicaid Drug Rebate program also participate in the Public Health Service's 340B drug pricing program in order for federal funds to be available for the manufacturer's drugs under Medicaid and Medicare Part B. The 340B program requires participating manufacturers to agree to charge no more than the 340B "ceiling price" for the manufacturer's covered outpatient drugs to a variety of community health clinics and other entities that receive health services grants from the Public Health Service, as well as hospitals that serve a disproportionate share of low-income patients. The Healthcare Reform Act expanded the list of covered entities to include certain free-standing cancer hospitals, critical access hospitals, rural referral centers and sole community hospitals. The 340B ceiling price is calculated using a statutory formula based on the average manufacturer price and rebate amount for the covered outpatient drug as calculated under the Medicaid Drug Rebate program. Changes to the definition of average manufacturer price and the Medicaid rebate amount under the Healthcare Reform Act and CMS's final regulations implementing those changes also could affect our 340B ceiling price calculations and negatively impact our results of operations.
The Healthcare Reform Act obligates the Secretary of the HHS to update the agreement that manufacturers must sign to participate in the 340B program to obligate a manufacturer to offer the 340B price to covered entities if the manufacturer makes the drug available to any other purchaser at any price and to report to the government the ceiling prices for its drugs. The Health Resources and Services Administration ("HRSA"), the federal agency that administers the 340B program, recently initiated the process of updating the agreement with participating manufacturers. The Healthcare Reform Act also obligates the Secretary of the HHS to create regulations and processes to improve the integrity of the 340B program. In 2015, HRSA issued proposed omnibus guidance that addresses many aspects of the 340B program, and in August 2016, HRSA issued a proposed regulation regarding an administrative dispute resolution process for the 340B program. It is unclear when or whether the guidance or regulation will be released in final form under the Trump Administration. On January 5, 2017, HRSA issued a final regulation regarding the calculation of 340B ceiling price and the imposition of civil monetary penalties on manufacturers that knowingly and intentionally overcharge covered entities. The Trump Administration has directed that this regulation, which was slated to become effective March 6, 2017, be temporarily delayed until March 21, 2017, and the regulation could be subject to further delay or other modification. Implementation of this final rule and the issuance of any other final regulations and guidance could affect our obligations under the 340B program in ways we cannot anticipate. In addition, legislation may be introduced that, if passed, would further expand the
47
340B program to additional covered entities or would require participating manufacturers to agree to provide 340B discounted pricing on drugs used in the inpatient setting.
Federal law also requires that a company that participates in the Medicaid Drug Rebate program report average sales price information each quarter to CMS for certain categories of drugs that are paid under the Medicare Part B program. Manufacturers calculate the average sales price based on a statutorily defined formula as well as regulations and interpretations of the statute by CMS. CMS uses these submissions to determine payment rates for drugs under Medicare Part B. Statutory or regulatory changes or CMS binding guidance could affect the average sales price calculations for our products and the resulting Medicare payment rate, and could negatively impact our results of operations. Also, the Medicare Part B drug payment methodology is subject to change based on potential demonstration projects undertaken by CMS or potential legislation enacted by Congress.
Pricing and rebate calculations vary across products and programs, are complex, and are often subject to interpretation by us, governmental or regulatory agencies and the courts. In the case of our Medicaid pricing data, if we become aware that our reporting for a prior quarter was incorrect, or has changed as a result of recalculation of the pricing data, we are obligated to resubmit the corrected data for up to three years after those data originally were due. Such restatements and recalculations increase our costs for complying with the laws and regulations governing the Medicaid Drug Rebate program and could result in an overage or underage in our rebate liability for past quarters. Price recalculations also may affect the ceiling price at which we are required to offer our products under the 340B drug discount program.
We are liable for errors associated with our submission of pricing data. In addition to retroactive rebates and the potential for 340B program refunds, if we are found to have knowingly submitted any false price information to the government, we may be liable for civil monetary penalties in the amount of $178,156 per item of false information. If we are found to have made a misrepresentation in the reporting of our average sales price, the Medicare statute provides for civil monetary penalties of up to $12,856 for each misrepresentation for each day in which the misrepresentation was applied. Our failure to submit the required price data on a timely basis could result in a civil monetary penalty of $17,816 per day for each day the information is late beyond the due date. Such failure also could be grounds for CMS to terminate our Medicaid drug rebate agreement, pursuant to which we participate in the Medicaid program. In the event that CMS terminates our rebate agreement, federal payments may not be available under Medicaid or Medicare Part B for our covered outpatient drugs.
CMS and the OIG have pursued manufacturers that were alleged to have failed to report these data to the government in a timely manner. Governmental agencies may also make changes in program interpretations, requirements or conditions of participation, some of which may have implications for amounts previously estimated or paid. We cannot assure you that our submissions will not be found by CMS to be incomplete or incorrect.
In order to be eligible to have our products paid for with federal funds under the Medicaid and Medicare Part B programs and purchased by the VA, Department of Defense, Public Health Service, and Coast Guard (the "Big Four agencies") and certain federal grantees, we are required to participate in the Department of Veterans Affairs ("VA") Federal Supply Schedule ("FSS") pricing program, established under Section 603 of the Veterans Health Care Act of 1992. Under this program, we are obligated to make VIBATIV available for procurement on an FSS contract and charge a price to the Big Four agencies that is no higher than the Federal Ceiling Price ("FCP"), which is a price calculated pursuant to a statutory formula. The FCP is derived from a calculated price point called the "non-federal average manufacturer price" ("Non-FAMP"), which we calculate and report to the VA on a quarterly and annual basis. Pursuant to applicable law, knowing provision of false information in connection with a Non-FAMP filing can subject a manufacturer to penalties of $178,156 for each item of false information. The FSS contract also contains extensive disclosure and certification requirements.
48
Under Section 703 of the National Defense Authorization Act for FY 2008, we are required to pay quarterly rebates on utilization of innovator products that are dispensed through the Tricare network pharmacies to Tricare beneficiaries. The rebates are calculated as the difference between the annual Non-FAMP and FCP. If we overcharge the government in connection with the FSS contract or Tricare Retail Pharmacy Rebate Program, whether due to a misstated FCP or otherwise, we are required to refund the difference to the government. Failure to make necessary disclosures and/or to identify contract overcharges can result in allegations against us under the False Claims Act and other laws and regulations. Unexpected refunds to the government, and any response to government investigation or enforcement action, would be expensive and time-consuming, and could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
If we fail to comply with data protection laws and regulations, we could be subject to government enforcement actions (which could include civil or criminal penalties), private litigation and/or adverse publicity, which could negatively affect our operating results and business.
We are subject to data protection laws and regulations (i.e., laws and regulations that address privacy and data security). In the U.S., numerous federal and state laws and regulations, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws (e.g., Section 5 of the FTC Act), govern the collection, use, disclosure, and protection of health-related and other personal information. Failure to comply with data protection laws and regulations could result in government enforcement actions and create liability for us (which could include civil and/or criminal penalties), private litigation and/or adverse publicity that could negatively affect our operating results and business. In addition, we may obtain health information from third parties (e.g., healthcare providers who prescribe our products) that are subject to privacy and security requirements under the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act ("HIPAA"). Although we are not directly subject to HIPAA—other than potentially with respect to providing certain employee benefits—we could be subject to criminal penalties if we knowingly obtain or disclose individually identifiable health information maintained by a HIPAA—covered entity in a manner that is not authorized or permitted by HIPAA. HIPAA generally requires that healthcare providers and other covered entities obtain written authorizations from patients prior to disclosing protected health information of the patient (unless an exception to the authorization requirement applies). If authorization is required and the patient fails to execute an authorization or the authorization fails to contain all required provisions, then we may not be allowed access to and use of the patient's information and our research efforts could be impaired or delayed. Furthermore, use of protected health information that is provided to us pursuant to a valid patient authorization is subject to the limits set forth in the authorization (e.g., for use in research and in submissions to regulatory authorities for product approvals). In addition, HIPAA does not replace federal, state, international or other laws that may grant individuals even greater privacy protections.
EU Member States and other jurisdictions where we operate have adopted data protection laws and regulations, which impose significant compliance obligations. For example, the EU Data Protection Directive imposes strict obligations and restrictions on the ability to collect, analyze and transfer personal data, including health data from clinical trials and adverse event reporting. Switzerland has adopted similar restrictions. Data protection authorities from the different EU Member States may interpret the applicable laws differently, and guidance on implementation and compliance practices are often updated or otherwise revised, which adds to the complexity of processing personal data in the EU. Although there are legal mechanisms to allow for the transfer of personal data from the EEA to the U.S., a decision of the European Court of Justice in the Schrems case (Case C-362/14 Maximillian Schrems v. Data Protection Commissioner) that invalidated the safe harbor framework has increased uncertainty around compliance with EU privacy law requirements. As a result of the decision, it was no longer possible to rely on the safe harbor certification as a legal basis for the transfer of personal data
49
from the EU to entities in the U.S. On February 29, 2016, however, the European Commission announced an agreement with the United States Department of Commerce (DOC) to replace the invalidated Safe Harbor framework with a new EU-U.S. "Privacy Shield." On July 12, 2016, the European Commission adopted a decision on the adequacy of the protection provided by the Privacy Shield. The Privacy Shield is intended to address the requirements set out by the European Court of Justice in its ruling by imposing more stringent obligations on companies, providing stronger monitoring and enforcement by the DOC and Federal Trade Commission, and making commitments on the part of public authorities regarding access to information. U.S. companies have been able to certify to the U.S. Department of Commerce their compliance with the privacy principles of the Privacy Shield since August 1, 2016.
On September 16, 2016, the Irish privacy advocacy group Digital Rights Ireland brought an action for annulment of the EC decision on the adequacy of the Privacy Shield before the European Court of Justice (Case T-670/16). Case T-670/16 is still pending before the Court. If, however, the European Court of Justice invalidates the Privacy Shield, it will no longer be possible to rely on the Privacy Shield certification to support transfer of personal data from the EU to entities in the US. Adherence to the Privacy Shield is not, however, mandatory. U.S.-based companies are permitted to rely either on their adherence to the Privacy Shield or on the other authorized means and procedures to transfer personal data provided by the EU Data Protection Directive. If we or our vendors fail to comply with applicable data privacy laws, or if the legal mechanisms we or our vendors rely upon to allow for the transfer of personal data from the EEA or Switzerland to the U.S. (or other countries not considered by the European Commission to provide an adequate level of data protection) are not considered adequate, we could be subject to government enforcement actions and significant penalties against us, and our business could be adversely impacted if our ability to transfer personal data outside of the EEA or Switzerland is restricted, which could adversely impact our operating results. In December 2015, a proposal for an EU General Data Protection Regulation, intended to replace the current EU Data Protection Directive, was agreed between the European Parliament, the Council of the European Union and the European Commission. The EU General Data Protection Regulation entered into force on May 24, 2016 and will apply from May 25, 2018. The Regulation will introduce new data protection requirements in the EU, as well as substantial fines for breaches of the data protection rules. The EU General Data Protection Regulation will increase our responsibility and liability in relation to personal data that we process, and we may be required to put in place additional mechanisms to ensure compliance with the new EU data protection rules.
Our relationships with customers and third-party payors are subject to applicable anti-kickback, fraud and abuse, transparency and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, exclusion, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians, distributors and third-party payors play a primary role in the distribution, recommendation and prescription of any pharmaceutical product for which we obtain marketing approval. Our arrangements with third-party payors and customers expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements through which we market, sell and distribute any products for which we have obtained or may obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations include the following:
- •
- The federal healthcare Anti-Kickback Statute prohibits any person from, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchasing, leasing, ordering or arranging for or recommending of any good or service for which payment may be made, in whole or in part, under federal and state healthcare programs such as Medicare and Medicaid. The term "remuneration" has been broadly interpreted to include
50
- •
- The federal civil False Claims Act imposes civil penalties, and provides for whistleblower or qui tam actions, against individuals or entities
for, among other things, knowingly presenting, or causing to be presented, claims for payment of government funds that are false or fraudulent, or knowingly making, or using or causing to be made or
used, a false record or statement material to a false or fraudulent claim to avoid, decrease, or conceal an obligation to pay money to the federal government. In recent years, several pharmaceutical
and other healthcare companies have faced enforcement actions under the federal False Claims Act for, among other things, allegedly submitting false or misleading pricing information to government
health care programs and providing free product to customers with the expectation that the customers would bill federal programs for the product. Federal enforcement agencies also have showed
increased interest in pharmaceutical companies' product and patient assistance programs, including reimbursement and co-pay support services, and a number of investigations into these programs have
resulted in significant civil and criminal settlements. Other companies have faced enforcement actions for causing false claims to be submitted because of the company's marketing the product for
unapproved, and thus non-reimbursable, uses. In addition, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim
for purposes of the federal civil False Claims Act. False Claims Act liability is potentially significant in the healthcare industry because the statute provides for treble damages and mandatory
penalties of $5,500 to $11,000 per false claim or statement. As a result of a recent interim final rule issued by the Department of Justice (DOJ), the penalties assessed after August 1, 2016
for violations occurring after November 2, 2015 will increase to per claim or statement penalties of $10,781 to $21,563. Because of the potential for large monetary exposure, healthcare and
pharmaceutical companies often resolve allegations without admissions of liability for significant and material amounts to avoid the uncertainty of treble damages and per claim penalties that may be
awarded in litigation proceedings. Companies may be required, however, to enter into corporate integrity agreements with the government, which may impose substantial costs on companies to ensure
compliance. Criminal prosecution is also possible for making or presenting a false or fictitious or fraudulent claim to the federal government.
- •
- HIPAA, among other things, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. HIPAA also prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document
anything of value. The Anti-Kickback Statute is subject to evolving interpretation and has been applied by government enforcement officials to a number of common business arrangements in the pharmaceutical industry. The government can establish a violation of the Anti-Kickback Statute without proving that a person or entity had actual knowledge of the statute or specific intent to violate it. There are a number of statutory exemptions and regulatory safe harbors protecting some common activities from prosecution; however, those exceptions and safe harbors are drawn narrowly. Failure to meet all of the requirements of a particular statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute, but the legality of the arrangement will be evaluated on a case-by-case basis based on the totality of the facts and circumstances. We seek to comply with the available statutory exemptions and safe harbors whenever possible, but our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability. Moreover, there are no safe harbors for many common practices, such as educational and research grants or patient assistance programs.
51
- •
- The federal Physician Payment Sunshine Act, being implemented as the Open Payments Program, imposes annual reporting requirements on certain
manufacturers of drugs, devices, or biologics for payments and other transfers of value by them, directly or indirectly, to physicians (including physician family members) and teaching hospitals, as
well as ownership and investment interests held by physicians. A manufacturer's failure to submit timely, accurately and completely the required information for all payments, transfers of value or
ownership or investment interests may result in civil monetary penalties of up to an aggregate of $150,000 per year, and up to an aggregate of $1 million per year for "knowing failures."
Manufacturers must submit reports by the 90th day of each calendar year.
- •
- Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and
claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers. Several states also require pharmaceutical companies to report expenses
relating to the marketing and promotion of pharmaceutical products in those states and to report gifts and payments to individual health care providers in those states. Some of these states also
prohibit certain marketing-related activities, including the provision of gifts, meals, or other items to certain health care providers. In addition, several states require pharmaceutical companies to
implement compliance programs or marketing codes.
- •
- Similar restrictions are imposed on the promotion and marketing of medicinal products in the EU Member States and other countries, including restrictions prohibiting the promotion of a compound prior to its approval. Laws (including those governing promotion, marketing and anti-kickback provisions), industry regulations and professional codes of conduct often are strictly enforced. Even in those countries where we may decide not to directly promote or market our products, inappropriate activity by our any international distribution partners could have implications for us.
knowing the same to contain any materially false fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services.
The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with different compliance or reporting requirements in multiple jurisdictions increase the possibility that we or our partners may fail to comply fully with one or more of these requirements. Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with applicable fraud and abuse or other healthcare laws and regulations or guidance. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we do or expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could harm our financial condition and divert resources and the attention of our management from operating our business.
52
Our business and operations, including the use of hazardous and biological materials may result in liabilities with respect to environmental, health and safety matters.
Our research and development activities involve the controlled use of potentially hazardous substances, including chemical, biological and radioactive materials. In addition, our operations produce hazardous waste products, including hazardous waste. Federal, state and local laws and regulations govern the use, manufacture, management, storage, handling and disposal of hazardous materials and wastes. We may incur significant additional costs or liabilities to comply with, or for violations of, these and other applicable laws in the future. Also, even if we are in compliance with applicable laws, we cannot completely eliminate the risk of contamination or injury resulting from hazardous materials and we may incur liability as a result of any such contamination or injury. Further, in the event of a release of or exposure to hazardous materials, including at the sites we currently or formerly operate or at sites such as landfills where we send wastes for disposal, we could be held liable for cleanup costs or damages or subject to other costs or penalties and such liability could exceed our resources. We do not have any insurance for liabilities arising from hazardous materials or under environmental laws. Compliance with or liability under applicable environmental laws and regulations or with respect to hazardous materials may be expensive, and current or future environmental regulations may impair our research, development and production efforts, which could harm our business, which could cause the price of our securities to fall.
RISKS RELATING TO OUR ORDINARY SHARES
The market price for our shares has and may continue to fluctuate widely, and may result in substantial losses for purchasers of our ordinary shares.
Our ordinary shares began trading on June 3, 2014, and the market price for our shares has and may continue to fluctuate widely, and may result in substantial losses for purchasers of our ordinary shares. To date, there is limited securities analyst coverage of our company. Limited securities analyst coverage of our company and shares is likely to reduce demand for our shares from potential investors, which likely will reduce the market price for our shares. To the extent that historically low trading volumes for our ordinary shares continues, our stock price may fluctuate significantly more than the stock market as a whole or the stock prices of similar companies. Without a larger public float of actively traded shares, our ordinary shares are likely to be more sensitive to changes in sales volumes, market fluctuations and events or perceived events with respect to our business, than the shares of common stock of companies with broader public ownership, and as a result, the trading prices for our ordinary shares may be more volatile. Among other things, trading of a relatively small volume of ordinary shares may have a greater effect on the trading price than would be the case if our public float of actively traded shares were larger. In addition, as further described below under the risk factor entitled "—Concentration of ownership will limit your ability to influence corporate matters," a number of shareholders hold large concentrations of our shares which, if sold within a relatively short timeframe, could cause the price of our shares to drop significantly.
Market prices for securities of biotechnology and biopharmaceutical companies have been highly volatile, and we expect such volatility to continue for the foreseeable future, so that investment in our ordinary shares involves substantial risk. By separating from Innoviva, there is a risk that our company may be more susceptible to market fluctuations and other adverse events than we would have been were we still a part of Innoviva. Additionally, the stock market from time to time has experienced significant price and volume fluctuations unrelated to the operating performance of particular companies.
53
The following are some of the factors that may have a significant effect on the market price of our ordinary shares:
- •
- any adverse developments or results or perceived adverse developments or results with respect to the GSK-Partnered Respiratory Programs,
including, without limitation, any delays in development in these programs, any halting of development in these programs, any difficulties or delays encountered with regard to the FDA or other
regulatory authorities in these programs, or any indication from clinical or non-clinical studies that the compounds in such programs are not safe or efficacious;
- •
- any adverse developments or results or perceived adverse developments or results with respect to our key clinical programs (for example,
revefenacin or our JAK inhibitor program), including, without limitation, any delays in development in these programs, any halting of development in these programs, any difficulties or delays
encountered with regard to the FDA or other regulatory authorities in these programs, or any indication from clinical or non-clinical studies that the compounds in such programs are not safe or
efficacious;
- •
- any further adverse developments or perceived adverse developments with respect to the commercialization of VIBATIV;
- •
- whether we achieve increased sales for VIBATIV;
- •
- any announcements of developments with, or comments by, the FDA or other regulatory authorities with respect to products we or our partners
have under development, are manufacturing or have commercialized;
- •
- any adverse developments or agreements or perceived adverse developments or agreements with respect to the relationship of Innoviva or TRC, on
the one hand, and GSK, on the other hand, including any such developments or agreements resulting from or relating to the Spin-Off;
- •
- any adverse developments or perceived adverse developments with respect to our relationship with any of our research, development or
commercialization partners, including, without limitation, disagreements that may arise between us and any of those partners;
- •
- any adverse developments or perceived adverse developments in our programs with respect to partnering efforts or otherwise;
- •
- announcements of patent issuances or denials, technological innovations or new commercial products by us or our competitors;
- •
- publicity regarding actual or potential study results or the outcome of regulatory review relating to products under development by us, our
partners or our competitors;
- •
- regulatory developments in the United States and foreign countries;
- •
- announcements with respect to governmental or private insurer reimbursement policies;
- •
- announcements of equity or debt financings;
- •
- economic and other external factors beyond our control;
- •
- loss of key personnel;
- •
- likelihood of our ordinary shares to be more sensitive to changes in sales volume, market fluctuations and events or perceived events with
respect to our business due to our small public float;
- •
- low public market trading volumes for our ordinary shares related in part to the concentration of ownership of our shares;
54
- •
- the sale of large concentrations of our shares within a relatively short timeframe;
- •
- developments or disputes as to patent or other proprietary rights;
- •
- approval or introduction of competing products and technologies;
- •
- results of clinical trials;
- •
- failures or unexpected delays in timelines for our potential products in development, including the obtaining of regulatory approvals;
- •
- delays in manufacturing adversely affecting clinical or commercial operations;
- •
- fluctuations in our operating results;
- •
- market reaction to announcements by other biotechnology or pharmaceutical companies;
- •
- initiation, termination or modification of agreements with our collaborators or disputes or disagreements with collaborators;
- •
- litigation or the threat of litigation;
- •
- public concern as to the safety of drugs developed by us; and
- •
- comments and expectations of results made by securities analysts or investors.
If any of these factors causes us to fail to meet the expectations of securities analysts or investors, or if adverse conditions prevail or are perceived to prevail with respect to our business, the price of the ordinary shares would likely drop significantly. A significant drop in the price of a company's securities often leads to the filing of securities class action litigation against the company. This type of litigation against us could result in substantial costs and a diversion of management's attention and resources.
Concentration of ownership will limit your ability to influence corporate matters.
Based on our review of publicly available filings, as of December 31, 2016 GSK beneficially owned approximately 18.3% of our outstanding ordinary shares and our directors, executive officers and investors affiliated with these individuals beneficially owned approximately 6.8% of our outstanding ordinary shares. Based on our review of publicly available filings, as of December 31, 2016 our three largest shareholders other than GSK collectively owned approximately 50% of our outstanding ordinary shares. GSK also has a right to maintain its percentage ownership in our company under the Governance Agreement, including by participating in offerings of our ordinary shares or securities convertible into our shares. These shareholders and GSK could control the outcome of actions taken by us that require shareholder approval, including a transaction in which shareholders might receive a premium over the prevailing market price for their shares.
Certain provisions in our constitutional documents may discourage our acquisition by a third-party, which could limit your opportunity to sell shares at a premium.
Our constitutional documents include provisions that could limit the ability of others to acquire control of us, modify our structure or cause us to engage in change-of-control transactions, including, among other things, provisions that:
- •
- require supermajority shareholder voting to effect certain amendments to our amended and restated memorandum and articles of association;
- •
- establish a classified board of directors;
- •
- restrict our shareholders from calling meetings or acting by written consent in lieu of a meeting;
- •
- limit the ability of our shareholders to propose actions at duly convened meetings; and
55
- •
- authorize our board of directors, without action by our shareholders, to issue preferred shares and additional ordinary shares.
These provisions could have the effect of depriving you of an opportunity to sell your ordinary shares at a premium over prevailing market prices by discouraging third parties from seeking to acquire control of us in a tender offer or similar transaction.
Our shareholders may face difficulties in protecting their interests because we are incorporated under Cayman Islands law.
Our corporate affairs are governed by our amended and restated memorandum and articles of association, by the Companies Law (2016 Revision) of the Cayman Islands and by the common law of the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under the laws of the Cayman Islands are different from those under statutes or judicial precedent in existence in jurisdictions in the U.S. Therefore, you may have more difficulty in protecting your interests than would shareholders of a corporation incorporated in a jurisdiction in the U.S., due to the different nature of Cayman Islands law in this area.
Shareholders of Cayman Islands exempted companies such as our company have no general rights under Cayman Islands law to inspect corporate records and accounts or to obtain copies of lists of shareholders. Our directors have discretion under our amended and restated memorandum and articles of association to determine whether or not, and under what conditions, our corporate records may be inspected by our shareholders, but are not obliged to make them available to our shareholders. This may make it more difficult for you to obtain the information needed to establish any facts necessary for a shareholder motion or to solicit proxies from other shareholders in connection with a proxy contest.
Our Cayman Islands counsel, Maples and Calder, is not aware of any reported class action having been brought in a Cayman Islands court. Derivative actions have been brought in the Cayman Islands courts, and the Cayman Islands courts have confirmed the availability for such actions. In most cases, the company will be the proper plaintiff in any claim based on a breach of duty owed to it, and a claim against (for example) our officers or directors usually may not be brought by a shareholder. However, based on English authorities, which would in all likelihood be of persuasive authority and be applied by a court in the Cayman Islands, exceptions to the foregoing principle apply in circumstances in which:
- •
- a company is acting, or proposing to act, illegally or beyond the scope of its authority;
- •
- the act complained of, although not beyond the scope of the authority, could be effected if duly authorized by more than the number of votes
which have actually been obtained; or
- •
- those who control the company are perpetrating a "fraud on the minority."
A shareholder may have a direct right of action against the company where the individual rights of that shareholder have been infringed or are about to be infringed.
There is uncertainty as to shareholders' ability to enforce certain foreign civil liabilities in the Cayman Islands.
We are incorporated as an exempted company limited by shares with limited liability under the laws of the Cayman Islands. A material portion of our assets are located outside of the United States. As a result, it may be difficult for our shareholders to enforce judgments against us or judgments obtained in U.S. courts predicated upon the civil liability provisions of the federal securities laws of the United States or any state of the United States.
We have been advised by our Cayman Islands legal counsel, Maples and Calder, that the courts of the Cayman Islands are unlikely (i) to recognize or enforce against Theravance Biopharma judgments of courts of the United States predicated upon the civil liability provisions of the securities laws of the
56
United States or any State; and (ii) in original actions brought in the Cayman Islands, to impose liabilities against Theravance Biopharma predicated upon the civil liability provisions of the securities laws of the United States or any State, on the grounds that such provisions are penal in nature. However, in the case of laws that are not penal in nature, although there is no statutory enforcement in the Cayman Islands of judgments obtained in the United States, the courts of the Cayman Islands will recognize and enforce a foreign money judgment of a foreign court of competent jurisdiction without retrial on the merits based on the principle that a judgment of a competent foreign court imposes upon the judgment debtor an obligation to pay the sum for which judgment has been given provided certain conditions are met. For a foreign judgment to be enforced in the Cayman Islands, such judgment must be final and conclusive and for a liquidated sum, and must not be in respect of taxes or a fine or penalty, inconsistent with a Cayman Islands' judgment in respect of the same matter, impeachable on the grounds of fraud or obtained in a manner, and or be of a kind the enforcement of which is, contrary to natural justice or the public policy of the Cayman Islands (awards of punitive or multiple damages may well be held to be contrary to public policy). A Cayman Islands court, including the Grand Court of the Cayman Islands, may stay proceedings if concurrent proceedings are being brought elsewhere, which would delay proceedings and make it more difficult for our shareholders to bring action against us.
We do not anticipate paying any cash dividends on our capital shares in the foreseeable future; as a result, capital appreciation, if any, of our ordinary shares will be your sole source of gain for the foreseeable future.
We have never declared or paid cash dividends on our capital shares. We do not anticipate paying any cash dividends on our capital shares in the foreseeable future. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business. In addition, the terms of any future debt financing arrangement may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our ordinary shares. As a result, capital appreciation, if any, of our ordinary shares will be your sole source of gain for the foreseeable future.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
Our principal physical properties in the U.S. consist of approximately 150,000 square feet of office and laboratory space leased in two buildings in South San Francisco, California. The lease expires in May 2020 and we may extend the terms for two additional five-year periods. Our Irish subsidiary operates from approximately 1,000 square feet of leased office space in Dublin, Ireland. We believe our current space is sufficient for our needs.
We are not a party to any material legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
57
ITEM 5. MARKET FOR THE REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our ordinary shares have traded on The NASDAQ Global Market under the symbol "TBPH" since June 3, 2014. Prior to this date, there was no public market for our ordinary shares. The following table sets forth the high and low closing prices of our ordinary shares on a per share basis for the periods indicated and as reported on The NASDAQ Global Market:
Calendar Quarter
|
High | Low | |||||
|---|---|---|---|---|---|---|---|
2016 |
|||||||
Fourth Quarter |
$ | 38.58 | $ | 24.57 | |||
Third Quarter |
36.97 | 22.26 | |||||
Second Quarter |
24.20 | 17.45 | |||||
First Quarter |
18.80 | 13.35 | |||||
2015 |
|||||||
Fourth Quarter |
$ | 19.51 | $ | 11.13 | |||
Third Quarter |
14.80 | 10.88 | |||||
Second Quarter |
18.63 | 12.57 | |||||
First Quarter |
21.73 | 14.70 | |||||
2014 |
|||||||
Fourth Quarter |
$ | 23.19 | $ | 13.33 | |||
Third Quarter |
33.99 | 23.05 | |||||
Second Quarter (beginning June 3) |
34.87 | 23.51 | |||||
As of January 31, 2017, there were 106 shareholders of record of our ordinary shares. As many of our ordinary shares are held by brokers and other institutions on behalf of shareholders, we are unable to estimate the total number of shareholders represented by these record holders.
Dividend Policy
We currently intend to retain any future earnings to finance our research and development efforts. We have never declared or paid cash dividends on our ordinary shares and do not intend to declare or pay cash dividends on our ordinary shares in the foreseeable future.
58
Equity Compensation Plans
The following table provides certain information with respect to all of our equity compensation plans in effect as of December 31, 2016:
Plan Category
|
Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights (a) |
Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights |
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in column (a)) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Options |
1,537,173 | $ | 26.51 | 1,080,788 | ||||||
Restricted shares |
3,609,118 | n/a | n/a | |||||||
Employee share purchase plan |
n/a | n/a | 1,064,367 | |||||||
| | | | | | | | | | | |
Equity compensation plans approved by security holders |
5,146,291 | $ | 26.51 | 2,145,155 | ||||||
Options |
693,622 |
$ |
18.06 |
23,824 |
||||||
| | | | | | | | | | | |
Equity compensation plans not approved by security holders |
693,622 | $ | 18.06 | 23,824 | ||||||
| | | | | | | | | | | |
Total |
5,839,913 | $ | 23.88 | 2,168,979 | ||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Upon the completion of the Spin-Off, we had two equity compensation plans—our 2013 Equity Incentive Plan (the "2013 EIP") and our 2013 Employee Share Purchase Plan (the "2013 ESPP"). At inception of the plans, we were authorized to issue 5,428,571 ordinary shares under the 2013 EIP and 857,142 ordinary shares under the 2013 ESPP. In October 2014, we adopted the 2014 New Employee Equity Incentive Plan (the "2014 NEEIP"). We are authorized to issue 750,000 ordinary shares under the 2014 NEEIP.
The 2013 EIP provides for the issuance of share-based awards, including restricted shares, restricted share units, options, share appreciation rights ("SARs") and other equity-based awards, to our employees, officers, directors and consultants. As of January 1 of each year, commencing on January 1, 2015 and ending on (and including) January 1, 2023, the aggregate number of ordinary shares that may be issued under the 2013 EIP shall automatically increase by a number equal to the least of 5% of the total number of ordinary shares outstanding on December 31 of the prior year, 3,428,571 ordinary shares, or a number of ordinary shares determined by our board of directors. Options may be granted with an exercise price not less than the fair market value of the ordinary shares on the grant date. Under the terms of our 2013 EIP, options granted to employees generally have a maximum term of 10 years and vest over a four-year period from the date of grant; 25% vest at the end of one year, and 75% vest monthly over the remaining three years. We may grant options with different vesting terms from time to time. Unless an employee's termination of service is due to disability or death, upon termination of service, any unexercised vested options will generally be forfeited at the end of three months or the expiration of the option, whichever is earlier.
Under the 2013 ESPP, our officers and employees may purchase ordinary shares through payroll deductions at a price equal to 85% of the lower of the fair market value of the ordinary share at the beginning of the offering period or at the end of each applicable purchase period. As of January 1 of each year, commencing on January 1, 2015 and ending on (and including) January 1, 2033, the aggregate number of ordinary shares that may be issued under the 2013 ESPP shall automatically increase by a number equal to the least of 1% of the total number of ordinary shares outstanding on December 31 of the prior year, 857,142 ordinary shares, or a number of ordinary shares determined by our board of directors. The ESPP generally provides for consecutive and overlapping offering periods of 24 months in duration, with each offering period generally composed of four consecutive six-month
59
purchase periods. The purchase periods end on either May 15 or November 15. ESPP contributions are limited to a maximum of 15% of an employee's eligible compensation.
Our 2013 ESPP also includes a feature that provides for the existing offering period to terminate and for participants in that offering period to automatically be enrolled in a new offering period when the fair market value of an ordinary share at the beginning of a subsequent offering period falls below the fair market value of an ordinary share on the first day of such offering period.
The 2014 NEEIP provides for the issuance of share-based awards, including restricted shares, restricted share units, non-qualified options and SARs, to our employees. Options may be granted with an exercise price not less than the fair market value of the ordinary shares on the grant date. Under the terms of our 2014 NEEIP, options granted to employees generally have a maximum term of 10 years and vest over a four-year period from the date of grant; 25% vest at the end of one year, and 75% vest monthly over the remaining three years. We may grant options with different vesting terms from time to time. Unless an employee's termination of service is due to disability or death, upon termination of service, any unexercised vested options will generally be forfeited at the end of three months or the expiration of the option, whichever is earlier.
Additional information regarding share-based compensation is included in Note 1, "Description of Operations and Summary of Significant Accounting Policies," and Note 8, "Share-Based Compensation," to the consolidated financial statements appearing in this Annual Report on Form 10-K.
Share Performance Graph
The graph set forth below compares the cumulative total shareholder return on our ordinary shares for the period commencing on June 3, 2014, the date on which our ordinary shares began trading on The NASDAQ Global Market, through December 31, 2016, with the cumulative total return of (i) the NASDAQ Composite Index, (ii) the NYSE Arca Pharmaceutical Index (previously labeled as the NASDAQ Pharmaceutical Index) and (iii) the NASDAQ Biotechnology Index over the same period. This graph assumes the investment of $100 on June 3, 2014 in each of (1) our ordinary shares, (2) the NASDAQ Composite Index, (3) the NYSE Arca Pharmaceutical Index and (4) the NASDAQ Biotechnology Index, and assumes the reinvestment of dividends, if any, although dividends have never been declared on our ordinary shares.
The comparisons shown in the graph below are based upon historical data. We caution that the price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our ordinary shares.
Notwithstanding anything to the contrary set forth in any of our previous or future filings under the Securities Act or the Exchange Act that might incorporate this Annual Report on Form 10-K or future filings made by us under those statutes, this Performance Graph section shall not be deemed filed with the SEC and shall not be deemed incorporated by reference into any of those prior filings or into any future filings made by us under those statutes.
60
COMPARISON OF CUMULATIVE TOTAL RETURN*
Among Theravance Biopharma, Inc., the NASDAQ Composite Index,
the NYSE Arca Pharmaceutical Index and the NASDAQ Biotechnology Index
ITEM 6. SELECTED FINANCIAL DATA
The selected consolidated summary financial data below should be read in conjunction with Part II, Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations" and Part II, Item 8, "Financial Statements and Supplementary Data", in this Annual Report on Form 10-K.
The following table sets forth certain summary historical financial information as of and for each of the years in the five-year period ended December 31, 2016, which have been derived from our (i) audited consolidated financial statements as of December 31, 2016, and 2015 and for the years ended December 31, 2016, 2015, and 2014, which are included in this Annual Report, (ii) audited combined financial statements as of December 31, 2014, 2013 and 2012 and for the years ended December 31, 2013, and 2012, which are not included in this Annual Report. In our opinion, the summary historical financial information derived from our unaudited combined financial statements is presented on a basis consistent with the information in our audited consolidated financial statements. The summary historical financial information may not be indicative of the results of operations or
61
financial position that we would have obtained if we had been an independent company during the periods presented or of our future performance as an independent company.
| |
Year Ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||
| |
(In thousands, except per share data) |
|||||||||||||||
CONSOLIDATED STATEMENT OF OPERATIONS DATA |
||||||||||||||||
Product sales |
$ | 17,603 | $ | 9,408 | $ | 4,418 | $ | — | $ | — | ||||||
Revenue from collaborative arrangements(1) |
31,045 | 32,718 | 7,270 | 226 | 130,145 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total revenue |
48,648 | 42,126 | 11,688 | 226 | 130,145 | |||||||||||
Costs and expenses: |
||||||||||||||||
Cost of goods sold(2) |
2,894 | 4,657 | 4,058 | — | — | |||||||||||
Research and development |
141,712 | 129,165 | 168,522 | 120,579 | 113,995 | |||||||||||
Selling, general and administrative |
84,509 | 90,203 | 71,647 | 35,931 | 25,725 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total costs and expenses(3) |
229,115 | 224,025 | 244,227 | 156,510 | 139,720 | |||||||||||
Loss from operations |
(180,467 | ) | (181,899 | ) | (232,539 | ) | (156,284 | ) | (9,575 | ) | ||||||
Interest expense |
(1,404 | ) | — | — | — | — | ||||||||||
Interest and other income |
1,312 | 631 | 1,865 | — | — | |||||||||||
| | | | | | | | | | | | | | | | | |
Loss before income taxes |
(180,559 | ) | (181,268 | ) | (230,674 | ) | (156,284 | ) | (9,575 | ) | ||||||
Provision for income taxes |
10,110 | 951 | 6,364 | — | — | |||||||||||
| | | | | | | | | | | | | | | | | |
Net loss |
$ | (190,669 | ) | $ | (182,219 | ) | $ | (237,038 | ) | $ | (156,284 | ) | $ | (9,575 | ) | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Basic and diluted net loss per share |
$ | (4.26 | ) | $ | (5.34 | ) | $ | (7.46 | ) | $ | (4.92 | ) | $ | (0.30 | ) | |
Shares used to compute basic and diluted net loss per share(4) |
44,711 | 34,150 | 31,755 | 31,741 | 31,741 | |||||||||||
| |
As of December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||
| |
(In thousands) |
|||||||||||||||
CONSOLIDATED BALANCE SHEETS DATA |
||||||||||||||||
Cash, cash equivalents and marketable securities(5) |
$ | 592,661 | $ | 215,294 | $ | 306,010 | $ | — | $ | — | ||||||
Working capital |
479,235 | 188,002 | 234,114 | (22,747 | ) | (11,837 | ) | |||||||||
Total assets |
639,254 | 300,116 | 337,771 | 25,177 | 20,962 | |||||||||||
Convertible senior notes, net |
222,676 | — | — | — | — | |||||||||||
Accumulated deficit |
(512,225 | ) | (321,556 | ) | (139,337 | ) | — | — | ||||||||
Parent company deficit |
— | — | — | (17,035 | ) | (6,990 | ) | |||||||||
Total shareholders' equity and parent company deficit |
350,231 | 243,065 | 289,787 | (17,035 | ) | (6,990 | ) | |||||||||
- (1)
- In
2012, there was an acceleration of deferred revenue of $125.8 million from our global collaboration agreement with Astellas Pharma Inc. ("Astellas")
for the development and commercialization of VIBATIV, which resulted from the termination of the Astellas agreement in January 2012.
- (2)
- For the year ended December 31, 2016, cost of goods sold includes charges of $0.3 million for the write-down of excess inventory primarily related to the discontinued sale of VIBATIV 250 mg vials. For the years ended December 31, 2015 and 2014, cost of goods sold includes charges of
62
$1.9 million and $2.9 million, respectively, for the write-down of VIBATIV inventory due to the dating of the product.
- (3)
- The following table discloses the allocation of share-based compensation expense included in total operating expenses:
| |
Year Ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||
| |
(In thousands) |
|||||||||||||||
Research and development |
$ | 20,202 | $ | 25,770 | $ | 21,191 | $ | 15,444 | $ | 13,192 | ||||||
Selling, general and administrative |
20,967 | 28,280 | 22,043 | 7,032 | 8,131 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total share-based compensation |
$ | 41,169 | $ | 54,050 | $ | 43,234 | $ | 22,476 | $ | 21,323 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (4)
- Prior
to the Spin-Off in June 2014, we operated as part of Innoviva and not as a separate entity. As a result, the calculation of basic and diluted net loss per
share assumes that the 32,260,105 ordinary shares issued to Innoviva stockholders in connection with the Spin-Off, less the number of ordinary shares subject to forfeiture, were outstanding from the
beginning of 2013 and 2014.
- (5)
- Cash, cash equivalents and marketable securities were not allocated to us prior to the Spin-Off.
63
ITEM 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Management's Discussion and Analysis ("MD&A") is intended to facilitate an understanding of our business and results of operations. This discussion and analysis should be read in conjunction with our consolidated financial statements and notes included in this Annual Report on Form 10-K. The information contained in this discussion and analysis or set forth elsewhere in this Annual Report on Form 10-K, including information with respect to our plans and strategy for our business, our operating expenses, and future payments under our collaboration agreements, includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 (the "Securities Act"), and Section 21E of the Securities Exchange Act of 1934 (the "Exchange Act"). Such statements are based upon current expectations that involve risks and uncertainties. You should review the section entitled "Risk Factors" in Item 1A of Part I above for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. See the section entitled "Special Note regarding Forward-Looking Statements" above for more information.
Management Overview
Theravance Biopharma, Inc. ("Theravance Biopharma") is a diversified biopharmaceutical company with the core purpose of creating medicines that help improve the lives of patients suffering from serious illness.
Our pipeline of internally discovered product candidates includes potential best-in-class medicines to address the unmet needs of patients being treated for serious conditions primarily in the acute care setting. VIBATIV® (telavancin), our first commercial product, is a once-daily dual-mechanism antibiotic approved in the U.S., Europe and certain other countries for certain difficult-to-treat infections. Revefenacin (TD-4208) is a long-acting muscarinic antagonist ("LAMA") being developed as a potential once-daily, nebulized treatment for chronic obstructive pulmonary disease ("COPD"). Our neprilysin ("NEP") inhibitor program is designed to develop selective NEP inhibitors for the treatment of a range of major cardiovascular and renal diseases, including acute and chronic heart failure, hypertension and chronic kidney diseases such as diabetic nephropathy. Our research efforts are focused in the areas of inflammation and immunology, with the goal of designing medicines that provide targeted drug delivery to tissues in the lung and gastrointestinal tract in order to maximize patient benefit and minimize risk. The first program to emerge from this research is designed to develop intestinally restricted pan-Janus kinase ("JAK") inhibitors for the treatment of a range of inflammatory intestinal diseases.
In addition, we have an economic interest in future payments that may be made by Glaxo Group Limited or one of its affiliates ("GSK") pursuant to its agreements with Innoviva, Inc. ("Innoviva") (known as Theravance, Inc. prior to January 7, 2016) relating to certain drug development programs, including the combination of fluticasone furoate, umeclidinium, and vilanterol (the "Closed Triple"), currently in development for the treatment of COPD and asthma.
In 2016, our net loss was $190.7 million, an increase of $8.5 million from $182.2 million in 2015. Our research and development expenses were $141.7 million in 2016, an increase of $12.5 million from $129.2 million in 2015, primarily driven by costs associated with the progression of our priority programs. Our selling, general and administrative expenses were $84.5 million in 2016, a decrease of $5.7 million from $90.2 million in 2015, primarily due a decrease in share-based compensation expense. Cash, cash equivalents, and marketable securities, excluding restricted cash, totaled $592.7 million on December 31, 2016.
Theravance Biopharma was incorporated in the Cayman Islands in July 2013 and became an independent, publicly-traded company on June 2, 2014 as a result of a pro rata dividend distribution by
64
Innoviva of one ordinary share of Theravance Biopharma for every three and one half shares of Innoviva common stock outstanding (the "Spin-Off"). While we are incorporated under Cayman Island law, we became an Irish tax resident effective July 1, 2015.
For the periods prior to June 2, 2014, the consolidated financial statements have been prepared using Innoviva's historical cost basis of the assets, liabilities, revenues, and expenses of the various activities that comprise the biopharmaceutical business as a component of Innoviva and reflect the results of operations, financial condition and cash flows of the biopharmaceutical business as a component of Innoviva. The statements of operations include expense allocations for general corporate overhead functions historically shared with Innoviva, including finance, legal, human resources, information technology and other administrative functions, which include the costs of salaries, benefits and other related costs, as well as consulting and other professional services. Where appropriate, these allocations were made on a specific identification basis. Otherwise, the expenses related to services provided to the biopharmaceutical business by Innoviva were allocated to Theravance Biopharma based on the relative percentages, as compared to Innoviva's other businesses, of headcount or square footage usage. The costs historically allocated to us by Innoviva for the services it has shared with us may not be indicative of the costs we have incurred or will incur for these services following the Spin-Off.
Program Highlights
VIBATIV® (telavancin)
VIBATIV is a bactericidal, once-daily injectable antibiotic to treat patients with serious, life-threatening infections due to Staphylococcus aureus and other Gram-positive bacteria, including methicillin-resistant ("MRSA") strains. VIBATIV is approved in the U.S. for the treatment of adult patients with complicated skin and skin structure infections ("cSSSI") caused by susceptible Gram-positive bacteria and for the treatment of adult patients with hospital-acquired and ventilator-associated bacterial pneumonia ("HABP"/ "VABP") caused by susceptible isolates of Staphylococcus aureus when alternative treatments are not suitable. VIBATIV is indicated in the European Union ("EU") for the treatment of adults with nosocomial pneumonia, including ventilator-associated pneumonia, known or suspected to be caused by MRSA when other alternatives are not suitable. VIBATIV is also indicated in Canada and Russia for cSSSI and HABP and VABP caused by Gram-positive bacteria, including MRSA.
Our focused acute care sales force currently markets VIBATIV in the U.S., and we maintain an independent sales, marketing, and medical affairs team. Outside of the U.S., our strategy is to market VIBATIV through a network of partners. To date, we have secured partners for VIBATIV in the following geographies—Canada, Middle East and North Africa, Israel, Russia, China and India. In August 2016, we and Clinigen Group ("Clinigen") reached a mutual decision for Clinigen to return commercial rights to market and distribute VIBATIV in the EU to Theravance Biopharma. On November 4, 2016, the European Commission approved the transfer of the centralized marketing authorization for VIBATIV from Clinigen to our wholly-owned Irish subsidiary, Theravance Biopharma Ireland Limited. We are in discussion with potential collaborators with the goal of establishing a new strategic commercial partnership in the EU.
Supplemental New Drug Application (sNDA) for Concurrent Staphylococcus aureus Bacteremia
In May 2016, we announced approval of our sNDA by the Food and Drug Administration ("FDA") allowing for the addition of new clinical data to the VIBATIV label concerning concurrent bacteremia in cases of HABP/VABP and cSSSI. The sNDA submission was based on the combined data from our previously conducted pivotal trials of VIBATIV in its two approved indications—cSSSI (ATLAS I and ATLAS II) and HABP/VABP (ATTAIN I and ATTAIN II). The trials were large, multi-center, multi-national, double-blind, randomized Phase 3 clinical studies enrolling and treating 3,370 adult patients,
65
including a portion of patients with concurrent bacteremia. Importantly, these studies involved two of the largest cohorts of patients ever studied in these diseases and included one of the largest cohorts of patients with MRSA infections studied to date. Separately, we are conducting a Phase 3 registrational study in patients with Staphylococcus aureus bacteremia.
Phase 3 Registrational Study in Staphylococcus aureus Bacteremia
As part of our effort to explore additional settings in which VIBATIV may offer patients therapeutic benefit, in February 2015, we initiated a Phase 3 registrational study for the treatment of patients with Staphylococcus aureus bacteremia. The 250-patient registrational study is a multi-center, randomized, open-label study designed to evaluate the non-inferiority of telavancin in treating Staphylococcus aureus bacteremia as compared to standard therapy. Key secondary outcome measures of the study include an assessment of the duration of bacteremia post-randomization and the incidence of development of metastatic complications, as compared to standard therapy. We expect to complete the study in 2018.
Telavancin Observational Use Registry ("TOURTM") Study
Initiated in February 2015, the 1,000-patient TOURTM study is designed to assess the manner in which VIBATIV is used by healthcare practitioners to treat patients. By broadly collecting and examining data related to VIBATIV treatment patterns, as well as clinical and safety outcomes in the real world, we aim to create an expansive knowledge base to guide future development and optimal use of the drug. In February 2017, we announced that enrollment in the TOURTM study was complete.
In October 2016, we announced interim data from the TOURTM study. An initial review of data from the first 200 patients enrolled in TOUR demonstrate clinical response rates of 74% in a range of difficult-to-treat infection types including HABP/VABP, cSSSI, bone and joint infections and bacteremia. Results show 17% of the first 200 patients were considered non-evaluable with 9% deemed to have failed treatment. Clinical response was defined as cure or improvement leading to step-down oral therapy.
In January 2017, we announced interim data from the TOURTM study, focused on a subset of registry patients with diagnoses of bacteremia or infective endocarditis. Data demonstrated positive clinical responses in 64% of patients, with 7% of patients failing treatment and 29% considered non-evaluable. Positive clinical response was defined as cure or improvement leading to step-down oral therapy.
Long-Acting Muscarinic Antagonist—Revefenacin (TD-4208)
Revefenacin is an investigational long acting muscarinic antagonist ("LAMA") in development for the treatment of COPD. We believe that revefenacin may become a valuable addition to the COPD treatment regimen and that it represents a significant commercial opportunity. Our market research indicates there is an enduring population of COPD patients in the U.S. that either need or prefer nebulized delivery for maintenance therapy. LAMAs are a cornerstone of maintenance therapy for COPD, but existing LAMAs are only available in handheld devices that may not be suitable for every patient. Revefenacin has the potential to be a best-in-class once-daily single-agent product for COPD patients who require, or prefer, nebulized therapy. The therapeutic profile of revefenacin, together with its physical characteristics, suggest that this LAMA could serve as a foundation for combination products and for delivery in metered dose inhaler and dry powder inhaler products.
Mylan Collaboration
In January 2015, Mylan Ireland Limited ("Mylan") and we established a strategic collaboration for the development and, subject to regulatory approval, commercialization of revefenacin. Partnering with
66
a world leader in nebulized respiratory therapies enables us to expand the breadth of our revefenacin development program and extend our commercial reach beyond the acute care setting where we currently market VIBATIV. Funding of the Phase 3 development program by Mylan strengthens our capital position and enhances our financial flexibility to advance other high-value pipeline assets alongside revefenacin.
Under the terms of the Mylan Development and Commercialization Agreement (the "Mylan Agreement"), Mylan and we are co-developing nebulized revefenacin for COPD and other respiratory diseases. We are leading the U.S. Phase 3 development program and Mylan is responsible for reimbursement of our costs related to the registrational program up until the approval of the first new drug application, after which costs will be shared. If a product developed under the collaboration is approved in the U.S., Mylan will lead commercialization and we will retain the right to co-promote the product in the U.S. under a profit-sharing arrangement (65% Mylan/35% Theravance Biopharma). Outside the U.S. (excluding China), Mylan will be responsible for development and commercialization and will pay us a tiered royalty on net sales at percentage royalty rates ranging from low double-digits to mid-teens.
Under the Mylan Agreement, Mylan paid us an initial payment of $15.0 million in cash in the second quarter of 2015. Also, pursuant to an ordinary share purchase agreement entered into on January 30, 2015, Mylan Inc., the indirect parent corporation of Mylan, made a $30.0 million equity investment in us, buying 1,585,790 ordinary shares from us in early February 2015 in a private placement transaction at a price of approximately $18.918 per share, which represented a 10% premium over the volume weighted average price per share of our ordinary shares for the five trading days ending on January 30, 2015. In February 2016, we earned a $15.0 million development milestone payment for achieving 50% enrollment in the Phase 3 twelve-month safety study. As of December 31, 2016, we are eligible to receive from Mylan additional potential development, regulatory and sales milestone payments totaling up to $205.0 million in the aggregate, with $160.0 million associated with revefenacin monotherapy and $45.0 million for future potential combination products. Of the $160.0 million associated with monotherapy, $150.0 million relates to commercialization and $10.0 million relates to regulatory actions in the EU. We do not expect to earn any milestone payments from Mylan in 2017.
We retain worldwide rights to revefenacin delivered through other dosage forms, such as a metered dose inhaler or dry powder inhaler ("MDI"/"DPI"), while Mylan has certain rights of first negotiation with respect to our development and commercialization of revefenacin delivered other than via a nebulized inhalation product.
Phase 3 Study in COPD
In September 2015, we announced, with our partner Mylan, the initiation of the Phase 3 development program for revefenacin for the treatment of COPD. The Phase 3 development program, designed to support the registration of the product in the U.S., includes two replicate three-month efficacy studies and a single twelve-month safety study. The two efficacy studies examined 2 doses (88 mcg and 175 mcg) of revefenacin inhalation solution administered once-daily via nebulizer in patients with moderate to severe COPD. The Phase 3 efficacy studies were replicate, randomized, double-blind, placebo-controlled, parallel-group trials designed to provide pivotal efficacy and safety data for once-daily revefenacin over a dosing period of 12 weeks, with a primary endpoint of trough forced expiratory volume in one second (FEV1) on day 85. The Phase 3 safety study is an open-label, active comparator study of 12 months duration. In February 2016, we announced the achievement of 50% enrollment in all three of the Phase 3 clinical studies for revefenacin. The achievement of 50% enrollment in the twelve-month safety study triggered a $15.0 million milestone payment to us by Mylan.
67
In October 2016, we announced positive top line results from the two replicate Phase 3 efficacy studies of revefenacin in more than 1,250 moderate to very severe COPD patients. Both Phase 3 efficacy studies met their primary endpoints, demonstrating statistically significant improvements over placebo in trough forced expiratory volume in one second (FEV1) after 12 weeks of dosing for each of the revefenacin doses studied (88 mcg once daily and 175 mcg once daily). The studies also demonstrated that the 88 mcg and 175 mcg doses of revefenacin were generally well-tolerated, with comparable rates of adverse events and serious adverse events across all treatment groups (active and placebo). In addition to the two efficacy studies, the safety study has enrolled more than 1,050 patients and is expected to be completed in mid-2017. Together, the three studies enrolled approximately 2,300 patients. Should results from the safety study be supportive, we expect to file a new drug application for revefenacin with the FDA by the end of 2017.
Velusetrag (TD-5108)
Velusetrag is an oral, investigational medicine developed for gastrointestinal motility disorders. It is a highly selective agonist with high intrinsic activity at the human 5-HT4 receptor. Velusetrag is being developed in collaboration with Alfa Wassermann S.p.A. ("Alfa Wassermann") in a two-part Phase 2 program to test the efficacy, safety and tolerability of velusetrag in the treatment of patients with gastroparesis. Positive top-line results from the initial Phase 2 proof-of-concept study under this partnership, which evaluated gastric emptying, safety and tolerability of multiple doses of velusetrag, were announced in April 2014. In March 2015, we initiated a Phase 2b study of velusetrag for the treatment of patients with gastroparesis. The 200-patient study is a multi-center, double-blind, randomized, placebo-controlled, parallel-group trial which will explore the efficacy and safety of multiple doses of velusetrag in patients with diabetic or idiopathic gastroparesis. The twelve-week study will test three doses: 5, 15, and 30 mg administered once-daily. The primary endpoint will be the effect of velusetrag on symptoms in subjects with gastroparesis. The study will also evaluate the effect of velusetrag on gastric emptying, and the psychometric properties of the Gastroparesis Rating Scale, a daily patient-reported outcome measure. In February 2017, we announced the completion of enrollment in the study. We currently expect results from the Phase 2b study in mid-2017. Pursuant to our agreement with Alfa Wassermann, the first Phase 2 study was, and the majority of the Phase 2b study is, funded by Alfa Wassermann.
In December 2016, the FDA granted Fast Track designation to velusetrag for the treatment of symptoms associated with idiopathic and diabetic gastroparesis. The FDA's Fast Track program was established to facilitate the development and expedite the review of drugs with the potential to treat serious conditions and address an unmet medical need.
TD-9855
TD-9855 is an investigational norepinephrine and serotonin reuptake inhibitor (NSRI). TD-9855 completed a Phase 2 study in patients with fibromyalgia, demonstrating statistically significant and clinically meaningful improvements in pain and core symptoms at the highest dose tested compared to placebo. We are assessing the potential use of TD-9855 in neurogenic orthostatic hypotension (nOH), and in May 2016, we initiated a Phase 2a study of TD-9855 in this indication. The 30 patient study is a randomized, two-part, single- and double-blind trial conducted in male and female subjects with nOH to evaluate the effect of TD-9855 in improving symptoms of nOH. The Phase 2a study is designed to evaluate postural changes in blood pressure, symptom reduction, and safety and tolerability. In February 2017, we announced our plan to amend the protocol of the Phase 2a study to allow patients who respond to continue beyond a single dose. We currently expect to complete the extended Phase 2a study by the end of 2017.
68
Oral Peripherally-Acting Mu Opioid Receptor Antagonist—Axelopran (TD-1211)
OIC Program
Axelopran is an investigational, once-daily, oral peripherally-active mu opioid receptor antagonist for opioid- induced constipation ("OIC"). The axelopran Phase 2 program demonstrated a clinically meaningful treatment effect in OIC patients compared to placebo. The goal for this program is to demonstrate the ability to normalize bowel function without impacting analgesia and improve a variety of GI symptoms associated with constipation, which could provide axelopran with a competitive advantage in the OIC market if demonstrated in Phase 3 studies and approved by regulatory authorities. We have developed a patient reported outcomes tool designed to measure patient symptoms which would be used in a Phase 3 registrational program and potentially generate data that could differentiate the product from the competition.
Fixed Dose Combination
In December 2014, we completed a Phase 1 study to determine the relative bioavailability of OxyContin® (oxycodone) and axelopran after oral administration as a fixed dose combination ("FDC") relative to the individual components administered together. The study examined a spray-coat application of axelopran to an opioid, OxyContin, to determine the effect of axelopran on OxyContin exposure. The study compared exposure of OxyContin alone, axelopran alone, OxyContin and axelopran administered as two separate tablets, and OxyContin spray-coated with axelopran in a FDC. Study results demonstrated that axelopran does not significantly alter systemic exposure to OxyContin when delivered as a FDC relative to when co-administered as individual tablets. A FDC of axelopran and an opioid could present an important market opportunity, as it has the potential to provide pain relief without constipation in a single abuse-deterrent pill for patients using opioids on a chronic basis.
NS5A Inhibitor—TD-6450
TD-6450 is a multivalent NS5A inhibitor. TD-6450 has successfully completed Phase 1 studies in both healthy volunteers and hepatitis C virus ("HCV") patients. In September 2015, we entered into a licensing agreement with Trek Therapeutics, PBC ("TREKtx") (the "TREKtx Agreement") granting TREKtx an exclusive worldwide license for the development, manufacturing, use, marketing and sale of TD-6450 as a component in combination HCV products (the "HCV Products"). Pursuant to the TREKtx Agreement, we received an upfront payment of $8.0 million in the form of TREKtx's Series A preferred stock and will be eligible to receive future royalties based on net sales of the HCV Products. In October 2015, TREKtx initiated an open-label Phase 2a clinical trial to evaluate faldaprevir ("FDV"), an HCV protease inhibitor, combined with TD-6450 and ribavirin ("RBV") in patients infected with HCV genotype 4. In September 2016, TREKtx announced interim data from the study that showed the sustained viral response (SVR) rate four weeks after the completion of treatment (SVR4) was 100% (16 of 16) in treatment naïve patients with chronic genotype 4 HCV who received 120 mg of FDV and RBV in combination with 60 mg or 120 mg of TD-6450 for 12 weeks. In February 2017, TREKtx announced that 100% of these patients (16 of 16) had maintained SVR at twelve weeks after the completion of treatment (SVR12) as well. TREKtx is conducting a second Phase 2a study of FDV and TD-6450, with and without RBV in patients with HCV genotype 1b. In the ongoing study, 14 out of 15 patients in the study arm containing RBV achieved SVR4.
Neprilysin (NEP) Inhibitor Program (TD-0714 and TD-1439)
Neprilysin ("NEP") is an enzyme that degrades natriuretic peptides. These peptides play a protective role in controlling blood pressure and preventing cardiovascular tissue remodeling. Inhibiting NEP may result in clinical benefit for patients, including diuresis, control of blood pressure, and reversing maladaptive changes in the heart and vascular tissue in patients with congestive heart failure.
69
Our primary objective is to develop a NEP inhibitor that could be used across a broad population of patients with cardiovascular and renal diseases, including acute and chronic heart failure and chronic kidney disease, including diabetic nephropathy. We aim to create a platform for multiple combination products with our NEP inhibitor with features that are differentiated from currently available products. Specifically, we intend to develop compounds that are non-renally cleared, dosed once-daily, dosed alone or in combination with other medicines and that may be dosed orally or intravenously.
TD-0714
Phase 1 Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD) Studies
In March 2016, we completed a Phase 1 randomized, double-blind, placebo-controlled, single ascending dose ("SAD") study in healthy volunteers of our most advanced NEP inhibitor compound, TD-0714. The study was designed to assess the safety, tolerability and pharmacokinetics of TD-0714, as well as measure biomarker evidence of target engagement and the amount of the drug that is eliminated via the kidneys. Results from the SAD study of TD-0714 demonstrate that the compound achieved maximal and sustained levels of target engagement for 24 hours after a single-dose, supporting the drug's potential for once-daily dosing. Target engagement was measured by dose-related increases in the levels of cyclic GMP (cGMP, a well-precedented biomarker of NEP engagement). TD-0714 also demonstrated very low levels of renal elimination, as evidenced by intravenous microtracer testing technology, and a favorable tolerability profile. These results met our target product profile and provide confidence for future efficacy studies of TD-0714 in a broad range of cardiovascular and renal diseases, including in patients with compromised renal function.
In October 2016, we completed a Phase 1 randomized, double-blind, placebo-controlled, multiple ascending dose ("MAD") study in healthy volunteers of TD-0714. The findings from the MAD study were consistent with the Phase 1 randomized, double-blind, placebo-controlled, SAD study in healthy volunteers we completed in March 2016, demonstrating sustained target engagement, low levels of renal elimination, and a favorable tolerability profile. Findings from the studies support clinical progression of TD-0714, which potential studies are being evaluated in the context of our overall NEPi program.
TD-1439
In September 2016, we progressed a second NEP inhibitor compound, TD-1439, which is structurally distinct from TD-0714, into Phase 1 randomized, double-blind, placebo-controlled, SAD and MAD studies in healthy volunteers. In February 2017, we announced favorable results from the Phase 1 SAD study. In this study, TD-1439 demonstrated characteristics consistent with our target product profile, including sustained 24-hour target engagement, low levels of renal elimination and a favorable tolerability profile. We expect to complete the Phase 1 MAD study in the first half of 2017.
We are currently evaluating next steps for the compounds in our NEPi clinical program, including compound and formulation selection, potential combinations, study population, and timing.
Intestinally Restricted Pan-Janus Kinase (JAK) Inhibitor Program (TD-1473 and TD-3504)
JAK inhibitors function by inhibiting the activity of one or more of the Janus kinase family of enzymes (JAK1, JAK2, JAK3, TYK2) that play a key role in cytokine signaling. Inhibiting these JAK enzymes interferes with the JAK/STAT signaling pathway and, in turn, modulates the activity of a wide range of pro-inflammatory cytokines. JAK inhibitors are currently approved for the treatment of rheumatoid arthritis and myelofibrosis and have demonstrated therapeutic benefit for patients with ulcerative colitis. However, these products are known to have side effects based on their systemic exposure. Our goal is to develop an orally administered, intestinally restricted pan-JAK inhibitor specifically designed to distribute adequately and predominantly to the tissues of the intestinal tract, treating inflammation in those tissues while minimizing systemic exposure. We are focused on utilizing
70
targeted JAK inhibitors for potential treatment of a range of inflammatory intestinal diseases including ulcerative colitis.
TD-1473
Phase 1 Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD) Studies
In June 2016, we completed a Phase 1 clinical study of TD-1473, an internally-discovered JAK inhibitor that has demonstrated a high affinity for each of the JAK family of enzymes. The primary objective of the study was to evaluate the safety and tolerability of single ascending and multiple ascending doses of TD-1473 in healthy volunteers. A key secondary objective of the trial was to characterize the pharmacokinetics of TD-1473, including the determination of the amount of TD-1473 that entered systemic circulation following oral administration. Data from the study demonstrated TD-1473 to be generally well tolerated. Study results also demonstrated that systemic exposures of TD-1473 were low relative to that reported for tofacitinib, a JAK inhibitor currently in development for ulcerative colitis. At steady state, the plasma exposures of TD-1473 were significantly lower than the plasma exposure of tofacitinib.
Furthermore, subjects exhibited high stool concentrations of TD-1473, which were comparable to concentrations associated with efficacy in preclinical colitis models. Preclinical studies also demonstrated penetration of TD-1473 into the intestinal wall and membrane. The data generated from the study met our target pharmacokinetic profile and support clinical progression of the compound.
Previously announced findings from a preclinical model of colitis evaluating TD-1473 and tofacitinib demonstrated that both compounds significantly reduced disease activity scores. However, at doses providing similar preclinical efficacy, the systemic exposure of TD-1473 was much lower than that of tofacitinib and TD-1473 did not reduce systemic immune cell counts, in contrast to tofacitinib. Based on these preclinical findings, we believe that TD-1473 represents a potential breakthrough approach to treating ulcerative colitis without the risk generally associated with systemically active therapies.
Phase 1b Study
In October 2016, we announced dosing of the first patient in a Phase 1b clinical study of TD-1473 in patients with moderate to severe ulcerative colitis. The multi-center, randomized, double-blind, multi-dose, placebo-controlled study is designed to enroll 40 patients randomized to receive one of three doses of TD-1473 or placebo administered for 28 days in sequential fashion. The primary objectives of the study will include evaluation of the safety and tolerability of TD-1473 administered for 28 days, as well as assessment of the compound's plasma exposure following administration. A key secondary objective of the study will be the evaluation of the effect of TD-1473 on levels of a range of key ulcerative colitis biomarkers, including C-reactive protein and fecal calprotectin. Additionally, investigators are expected to evaluate a number of exploratory objectives, including changes in partial Mayo score and improvement in disease activity through endoscopic and histologic assessments. We expect data from the Phase 1b study in mid-2017. Also in October 2016, we announced that we had successfully completed the TD-1473 13-week toxicology studies, clearing the compound to progress to longer term clinical studies.
TD-3504
In September 2016, we announced plans to progress a second compound, TD-3504, from our JAK inhibitor program. TD-3504 is an innovative prodrug of tofacitinib, an investigational JAK inhibitor in development for ulcerative colitis. TD-3504 is chemically distinct from TD-1473 and is designed to release active tofacitinib into the intestinal tract. In preclinical studies, TD-3504 demonstrated rapid formation of tofacitinib in the intestinal tract, reduction in disease activity score comparable to
71
tofacitinib, and low systemic exposure in contrast to tofacitinib. We plan to initiate a Phase 1 study of TD-3504 in healthy volunteers and ulcerative colitis patients in the first half of 2017.
Selective 5-HT4 Agonist (TD-8954)
Takeda Collaborative Arrangement
In June 2016, we entered into a License and Collaboration Agreement with Millennium Pharmaceuticals, Inc., a Delaware corporation ("Millennium") (the "Takeda Agreement"), in order to establish a collaboration for the development and commercialization of TD-8954, a selective 5-HT4 receptor agonist. Prior to the Takeda Agreement, we developed TD-8954 for potential use in the treatment of gastrointestinal motility disorders, including short-term intravenous use for enteral feeding intolerance ("EFI") to achieve early nutritional adequacy in critically ill patients at high nutritional risk, an indication for which the compound received FDA Fast Track designation. Millennium is an indirect wholly-owned subsidiary of Takeda Pharmaceutical Company Limited (TSE: 4502), a publicly-traded Japanese corporation listed on the Tokyo Stock Exchange (collectively with Millennium, "Takeda"). Under the terms of the Takeda Agreement, Takeda will be responsible for worldwide development and commercialization of TD-8954. We received an upfront cash payment of $15.0 million and will be eligible to receive success-based development, regulatory and sales milestone payments by Takeda. The first $110.0 million of potential milestones are associated with the development, regulatory and commercial launch milestones for EFI or other intravenously dosed indications. We will also be eligible to receive a tiered royalty on worldwide net sales by Takeda at percentage royalty rates ranging from low double-digits to mid-teens.
Other Programs
Economic Interest in GSK-Partnered Respiratory Programs
We are entitled to receive an 85% economic interest in any future payments that may be made by GSK (pursuant to its agreements with Innoviva) relating to certain of the respiratory programs (the "GSK-Partnered Respiratory Programs") that Innoviva partnered with GSK and assigned to Theravance Respiratory Company, LLC ("TRC") in connection with Innoviva's separation of its biopharmaceutical operations into its then wholly-owned subsidiary Theravance Biopharma (the "Spin-Off"). The GSK-Partnered Respiratory Programs consist primarily of the Closed Triple program and the Inhaled Bifunctional Muscarinic Antagonist-Beta2 Agonist ("MABA") program, each of which are described in more detail below. We are entitled to this economic interest through our equity ownership in TRC. Our economic interest will not include any payments associated with RELVAR® ELLIPTA®/BREO® ELLIPTA®, ANORO® ELLIPTA® or vilanterol monotherapy. The following information regarding the Closed Triple and the MABA program is based solely upon publicly available information and may not reflect the most recent developments under the programs.
"Closed Triple" or FF/UMEC/VI (fluticasone furoate/umeclidinium bromide/vilanterol)
The Closed Triple program seeks to provide the activity of an inhaled corticosteroid (FF) plus two bronchodilators (UMEC, a LAMA, and VI, a long-acting beta2 agonist, or LABA) in a single delivery device administered once-daily. If the Closed Triple is successfully developed and commercialized, we are entitled to receive an 85% economic interest in the royalties payable by GSK to TRC on worldwide net sales, which royalties are upward-tiering from 6.5% to 10%. Previously, Innoviva and GSK announced the initiation of two global pivotal Phase 3 studies of the Closed Triple. The IMPACT study, which will enroll approximately 10,000 COPD patients, was initiated in July 2014. The IMPACT study will assess whether the Closed Triple can reduce the rate of moderate and severe exacerbations compared with two approved once-daily COPD treatments, RELVAR® ELLIPTA®/BREO® ELLIPTA® (FF/VI), an ICS/LABA combination, and ANORO® ELLIPTA® (UMEC/VI), a LAMA/LABA
72
combination. The IMPACT study is ongoing and is expected to read out in 2017. The FULFIL study, which enrolled approximately 1,800 COPD patients was initiated in February 2015. In June 2016, GSK and Innoviva disclosed positive top-line results from the FULFIL study, in which data demonstrated superiority of the Closed Triple as compared to twice-daily SYMBICORT® TURBOHALER® (budesonide/formoterol) in improving lung function and health-related quality of life in COPD patients. In November 2016, GSK and Innoviva announced the filing of a New Drug Application ("NDA") in the U.S. for the Closed Triple for patients with COPD. In December 2016, GSK and Innoviva announced the filing of a Marketing Authorization Application ("MAA") in the EU for the Closed Triple for patients with COPD. In December 2016, GSK and Innoviva announced the initiation of the Phase 3 (CAPTAIN) study of the Closed Triple in patients with asthma. The CAPTAIN study is expected to read out in 2018.
Inhaled Bifunctional Muscarinic Antagonist-Beta2 Agonist (MABA)
GSK961081 ('081), also known as batefenterol, is an investigational, single-molecule bifunctional bronchodilator with both muscarinic antagonist and beta2 receptor agonist activity that was discovered by us when we were part of Innoviva.
If a single-agent MABA medicine containing '081 is successfully developed and commercialized, we are entitled to receive an 85% economic interest in the royalties payable by GSK to TRC on worldwide net sales, which royalties range between 10% and 20% of annual global net sales up to $3.5 billion, and 7.5% for all annual global net sales above $3.5 billion. If a MABA medicine containing '081 is commercialized only as a combination product, such as '081/FF, the royalty rate is 70% of the rate applicable to sales of the single-agent MABA medicine. If a MABA medicine containing '081 is successfully developed and commercialized in multiple regions of the world, TRC is eligible to receive contingent milestone payments from GSK. The agreements allow for total milestones of up to $125.0 million for a single-agent medicine and an incremental $125.0 million for a combination medicine. Of these amounts, $112.0 million in potential milestones remain for a single-agent medicine, and $122.0 million remain for a combination medicine. In each case, we would be entitled to receive an 85% economic interest in any such payments.
Theravance Respiratory Company, LLC
Prior to the June 1, 2014 separation of its biopharmaceutical operations into its then wholly-owned subsidiary Theravance Biopharma (the "Spin-Off"), Innoviva assigned to TRC its strategic alliance agreement with GSK and all of its rights and obligations under its LABA collaboration agreement with GSK other than with respect to RELVAR® ELLIPTA®/BREO® ELLIPTA®, ANORO® ELLIPTA® and vilanterol monotherapy. Our equity interest in TRC is the mechanism by which we are entitled to the 85% economic interest in any future payments made by GSK under the strategic alliance agreement and under the portion of the collaboration agreement assigned to TRC. The drug programs assigned to TRC include the Closed Triple and the MABA program, as monotherapy and in combination with other therapeutically active components, such as an inhaled corticosteroid ("ICS"), as well as any other product or combination of products that may be discovered and developed in the future under these GSK agreements.
Basis of Historical Presentation
For the periods prior to June 2, 2014, the consolidated financial statements have been prepared using Innoviva's historical cost basis of the assets, liabilities, revenues, and expenses of the various activities that comprise the biopharmaceutical business as a component of Innoviva and reflect the results of operations, financial condition and cash flows of the biopharmaceutical business as a component of Innoviva. The statements of operations include expense allocations for general corporate overhead functions historically shared with Innoviva, including finance, legal, human resources,
73
information technology and other administrative functions, which include the costs of salaries, benefits and other related costs, as well as consulting and other professional services. Where appropriate, these allocations were made on a specific identification basis. Otherwise, the expenses related to services provided to the biopharmaceutical business by Innoviva were allocated to Theravance Biopharma based on the relative percentages, as compared to Innoviva's other businesses, of headcount or square footage usage. The costs historically allocated to us by Innoviva for the services it has shared with us may not be indicative of the costs we have incurred or will incur for these services following the Spin-Off.
Critical Accounting Policies and Estimates
Our management's discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles ("GAAP"). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenue generated and expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management's judgments and estimates.
Product Sales
In the U.S., we make VIBATIV available through a limited number of distributors who sell VIBATIV to healthcare providers. Title and risk of loss transfer upon receipt by these distributors. Outside of the U.S., we make VIBATIV available through a limited number of collaborative partners who sell VIBATIV in their respective geographies.
Product sales are recorded net of estimated government-mandated rebates and chargebacks, distribution fees, estimated product returns and other deductions. We reflect such reductions in revenue as either an allowance to the related account receivable from the distributor, or as an accrued liability, depending on the nature of the sales deduction. Sales deductions are based on management's estimates that consider payor mix in target markets, industry benchmarks and experience to date. We monitor inventory levels in the distribution channel, as well as sales of VIBATIV by distributors to healthcare providers, using product-specific data provided by the distributors. We update our estimates and assumptions each quarter and if actual future results vary from our estimates, we may adjust these estimates, which could have an effect on product sales and earnings in the period of adjustment.
Sales Discounts: We offer cash discounts to certain customers in the U.S. as an incentive for prompt payment. We expect our customers to comply with the prompt payment terms to earn the cash discount. In addition, we offer contract discounts to certain direct customers. We estimate sales discounts based on contractual terms, historical utilization rates, as available, and our expectations regarding future utilization rates. We account for sales discounts by reducing accounts receivable by the full amount and recognizing the discount as a reduction of revenue in the same period the related revenue is recognized.
Chargebacks and Government Rebates: For VIBATIV sales in the U.S., we estimate reductions to product sales for qualifying federal and state government programs including discounted pricing offered to Public Health Service ("PHS") as well as government-managed Medicaid programs. Our reduction for PHS is based on actual chargebacks that distributors have claimed for reduced pricing offered to such healthcare providers and our expectation about future utilization rates. Our accrual for Medicaid
74
is based upon statutorily-defined discounts, estimated payor mix, expected sales to qualified healthcare providers, and our expectation about future utilization. The Medicaid accrual and government rebates that are invoiced directly to us are recorded in other accrued liabilities on the consolidated balance sheets. For qualified programs that can purchase our products through distributors at a lower contractual government price, the distributors charge back to us the difference between their acquisition cost and the lower contractual government price, which we record as an allowance against accounts receivable.
Distribution Fees: We have contracts with our distributors in the U.S. that include terms for distribution-related fees. We determine distribution-related fees based on a percentage of the product sales price, and we record the distribution fees as an allowance against accounts receivable.
Product Returns: We offer our distributors a right to return product purchased directly from us, which is principally based upon the product's expiration date. Our policy is to accept product returns during the six months prior to and twelve months after the product expiration date on product that had been sold to our distributors. Product return allowances are based on amounts owed or to be claimed on related sales. These estimates take into consideration the terms of our agreements with customers, historical product returns of VIBATIV experienced by Innoviva's former collaborative partner, Astellas Pharma Inc. ("Astellas"), rebates or discounts taken, estimated levels of inventory in the distribution channel, the shelf life of the product, and specific known market events, such as competitive pricing and new product introductions. We record our product return reserves as accrued other liabilities.
Allowance for Doubtful Accounts: We record allowances for potentially doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. Based on our history, we deem the risk of loss associated with these receivables to be low. As of December 31, 2016 and 2015, there was no allowance for doubtful accounts related to customer payments.
Our reserve activity for sales allowances, discounts and chargebacks is summarized as follows:
(In thousands)
|
Balance at Beginning of Period |
Charges | Deductions | Balance at End of Period |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Year ended December 31, 2015: |
|||||||||||||
Sales allowances, discounts and chargebacks |
$ | 160 | $ | 3,049 | $ | (2,451 | ) | $ | 758 | ||||
Year ended December 31, 2016: |
|||||||||||||
Sales allowances, discounts and chargebacks |
$ | 758 | $ | 6,337 | $ | (6,316 | ) | $ | 779 | ||||
There were no material changes in reserve estimates relating to the prior periods.
Inventories
Inventories consist of raw materials, work-in-process and finished goods related to the production of VIBATIV. Raw materials include VIBATIV active pharmaceutical ingredient ("API") and other raw materials. Work-in-process and finished goods include third-party manufacturing costs and labor and indirect costs we incur in the production process. Included in inventories are raw materials and work-in-process that may be used as clinical products, which are charged to research and development ("R&D") expense when consumed. In addition, under certain commercialization agreements, we may sell VIBATIV packaged in unlabeled vials that are recorded in work-in-process. Inventories are stated at the lower of cost or market value. We determine the cost of inventory using the average-cost method for each manufacturing batch.
We assess our inventory levels quarterly and write-down inventory that is expected to be at risk for expiration, that has a cost basis in excess of its expected net realizable value and inventory quantities in excess of expected requirements. This assessment requires management to utilize judgement in
75
formulating estimates and assumptions that we believe to be reasonable under the circumstances. Actual results may differ from those estimates and assumptions.
When we recognize a loss on such inventory, it establishes a new, lower cost basis for that inventory, and subsequent changes in facts and circumstances will not result in the restoration or increase in that newly established cost basis. If inventory with a lower cost basis is subsequently sold, it will result in higher gross margin for the products making up that inventory. In 2016, we recognized charges of $0.3 million for the write-down of excess inventory, and in 2015 and 2014, we recognized charges of $1.9 million and $2.9 million, respectively, to write-down inventory due to dating of the product. Finished goods is the portion of our inventory that is most at risk for product dating issues and the carrying value of our finished goods inventory was $3.5 million as of December 31, 2016. In order to realize the value of our recorded inventory, we will be dependent upon continued increases in the sales volumes of VIBATIV. Refer to Note 6, "Inventories," to the consolidated financial statements appearing in this Annual Report on Form 10-K for further information regarding the components of our inventories.
Income Taxes
The provision for income taxes in 2016 is a result of recording certain contingent tax liabilities pertaining primarily to uncertain tax positions taken with respect to transfer pricing and tax credits.
We utilize the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax basis of assets and liabilities and are measured using enacted tax rates and laws that are anticipated to be in effect when the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized. We continue to maintain a full valuation allowance against our deferred tax assets. We reassess our valuation allowance for deferred income taxes at each reporting period. If we determine that it is more likely than not that the benefit of those assets will be realized, a reversal of a portion or all of the valuation allowance would occur and result in a corresponding benefit to earnings.
We assess all material positions, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. Assessing an uncertain tax position begins with the initial determination of the position's sustainability and is measured at the largest amount of benefit that is greater than 50% likely to be realized upon ultimate settlement. The provision for income taxes, including the effective tax rates, the determination of deferred tax assets and liabilities and related valuation allowance evaluation, and the analysis of potential tax exposure items, if any, requires significant judgment. Our filings, including the positions taken therein, may be subject to audit by various taxing authorities. We may be required to pay taxes for prior periods, interest, fines or penalties, and may be obligated to pay increased taxes in the future which could result in reduced cash flows and have a material adverse effect on our business, financial condition and growth prospects.
At December 31, 2016 and 2015, we had total U.S. federal, state and foreign unrecognized tax benefits of $23.3 million and $9.2 million, respectively. Our unrecognized tax benefits would reduce our effective income tax rate if recognized. As of December 31, 2016, we do not anticipate the total amount of unrecognized income tax benefits relating to uncertain tax positions existing at December 31, 2016 to decrease in the next 12 months.
Our future income tax expense may be affected by such factors as changes in tax laws, our business, regulations, tax rates, interpretation of existing laws or regulations, the impact of accounting for share-based compensation, the impact of accounting for business combinations, our international organization, shifts in the amount of income before tax earned in the U.S. as compared with other regions in the world, and changes in overall levels of income before tax.
76
Accrued Research and Development Expenses
As part of the process of preparing financial statements, we are required to estimate and accrue expenses, the largest of which are research and development expenses. This process involves the following:
- •
- identifying services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for
the service when we have not yet been invoiced or otherwise notified of actual cost;
- •
- estimating and accruing expenses in our financial statements as of each balance sheet date based on facts and circumstances known to us at the
time; and
- •
- periodically confirming the accuracy of our estimates with selected service providers and making adjustments, if necessary.
Examples of estimated research and development expenses that we accrue include:
- •
- fees paid to clinical research organizations ("CROs") in connection with preclinical and toxicology studies and clinical studies;
- •
- fees paid to investigative sites in connection with clinical studies;
- •
- fees paid to contract manufacturing organizations ("CMOs") in connection with the production of product and clinical study materials; and
- •
- professional service fees for consulting and related services.
We base our expense accruals related to clinical studies on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical studies on our behalf. The financial terms of these agreements vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors, such as the successful enrollment of patients and the completion of clinical study milestones. Our service providers invoice us monthly in arrears for services performed. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates.
To date, we have not experienced significant changes in our estimates of accrued research and development expenses after a reporting period. However, due to the nature of estimates, there is no assurance that we will not make changes to our estimates in the future as we become aware of additional information about the status or conduct of our clinical studies and other research activities.
Non-Marketable Equity Securities
Non-marketable equity securities are recorded at cost in long-term assets, and we periodically review our non-marketable equity securities for impairment by determining whether impairment indicators are present. Common impairment indicators include a significant adverse change in the regulatory or economic environment in which the investee entity operates or cash used in operating activities and other working capital deficiencies.
If we conclude that a non-marketable equity security is impaired, we determine whether such impairment is other-than-temporary. Factors we consider to make such determination include the duration and severity of the impairment, the reason for the decline in value and the potential recovery period and our intent to sell. If any impairment is considered other-than-temporary, we will write-down the asset to its fair value and record the corresponding charge as interest and other income (loss).
77
As of December 31, 2016, we reviewed our $8.0 million investment in TREKtx for impairment and determined that no significant impairment indicators were present and no impairment charges were necessary. The realization of the value of our investment in TREKtx is dependent upon TREKtx's ability to continue to raise capital to pursue their business plan. TREKtx faces development risks and the outcome of the development is inherently uncertain, and the ultimate commercialization of their product is further dependent upon regulatory approvals, product pricing and reimbursements. As a result of these risks, it is possible that impairments of the carrying value of TREKtx can arise in future reporting periods.
Results of Operations
Product Sales and Revenue from Collaborative Arrangements
Product sales and revenues from collaborative arrangements, as compared to the prior years, were as follows:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2016 | 2015 | |||||||||||||||||||
(In thousands)
|
2016 | 2015 | 2014 | $ | % | $ | % | |||||||||||||||
Product sales |
$ | 17,603 | $ | 9,408 | $ | 4,418 | $ | 8,195 | 87 | % | $ | 4,990 | 113 | % | ||||||||
Revenue from collaborative arrangements |
31,045 | 32,718 | 7,270 | (1,673 | ) | (5 | ) | 25,448 | 350 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Total revenue |
$ | 48,648 | $ | 42,126 | $ | 11,688 | $ | 6,522 | 15 | % | $ | 30,438 | 260 | % | ||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Revenue from product sales increased to $17.6 million in 2016 compared to $9.4 million in 2015 and $4.4 million in 2014. The year-over-year increases were due to the continued growth in VIBATIV net sales. The growth was primarily due to an increase in number of customer accounts and an increase in sales volume, driven in part by the expansion of our sales infrastructure in 2015. U.S. product sales accounted for over 99% of total product sales in 2016 compared to 93% and 96% in 2015 and 2014, respectively.
Revenue from collaborative arrangements was relatively unchanged at $31.0 million in 2016 compared to $32.7 million in 2015. In 2016, collaborative arrangements revenue was primarily comprised of a $15.0 million milestone payment from Mylan for the achievement of 50% enrollment in the Phase 3 twelve-month safety study, and a $15.0 million upfront payment from the collaborative arrangement with Takeda that was entered into in June 2016.
Revenue from collaborative arrangements increased significantly in 2015 to $32.7 million compared to $7.3 million in 2014. The increase was primarily due to the recognition of $19.2 million of upfront payment from Mylan for the delivery of a license and technological know-how for revefenacin (TD-4208) and $8.0 million of upfront non-cash consideration from TREKtx for the TD-6450 licensing agreement.
Cost of Goods Sold
Cost of goods sold, as compared to the prior years, were as follows:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2016 | 2015 | |||||||||||||||||||
(In thousands)
|
2016 | 2015 | 2014 | $ | % | $ | % | |||||||||||||||
Cost of goods sold |
$ | 2,894 | $ | 4,657 | $ | 4,058 | $ | (1,763 | ) | (38 | )% | $ | 599 | 15 | % | |||||||
Cost of goods sold was $2.9 million in 2016 which included a charge of $0.3 million for the write-down of excess inventory, compared to cost of goods sold of $4.7 million in 2015 which included a
78
charge of $1.9 million for the write-down of short-dated VIBATIV inventory. Excluding the write-downs in 2016 and 2015, the cost of goods sold decreased $0.2 million from 2015 to 2016 due to the sales of VIBATIV vials that were previously written off in 2015.
The cost of goods sold of $4.1 million in 2014 included a similar inventory write-down of $2.9 million. Excluding the write-downs in 2015 and 2014, the cost of goods sold increased $1.6 million from 2014 to 2015 due to the increase in VIBATIV net sales.
Research & Development
Our research and development ("R&D") expenses consist primarily of employee-related costs, external costs, and various allocable expenses. We budget total R&D expenses on an internal department level basis, and we manage and report our R&D activities across the following four cost categories:
- 1)
- Employee-related
costs, which include salaries, wages and benefits;
- 2)
- Share-based
compensation, which includes expenses associated with our equity plans;
- 3)
- External-related
costs, which include clinical trial related expenses, other contract research fees, consulting fees, and contract manufacturing fees; and
- 4)
- Facilities and other, which include laboratory and office supplies, depreciation and other allocated expenses, which include general and administrative support functions, insurance and general supplies.
The following table summarizes our R&D expenses incurred, net of reimbursements from collaboration partners, during the periods presented:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2016 | 2015 | |||||||||||||||||||
(In thousands)
|
2016 | 2015 | 2014 | $ | % | $ | % | |||||||||||||||
Employee-related |
$ | 37,328 | $ | 38,621 | $ | 57,427 | $ | (1,293 | ) | (3 | )% | $ | (18,806 | ) | (33 | )% | ||||||
Share-based compensation |
20,202 | 25,770 | 21,191 | (5,568 | ) | (22 | ) | 4,579 | 22 | |||||||||||||
External-related |
57,576 | 38,151 | 62,975 | 19,425 | 51 | (24,824 | ) | (39 | ) | |||||||||||||
Facilities, depreciation and other allocated |
26,606 | 26,623 | 26,929 | (17 | ) | — | (306 | ) | (1 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Total research & development |
$ | 141,712 | $ | 129,165 | $ | 168,522 | $ | 12,547 | 10 | % | $ | (39,357 | ) | (23 | )% | |||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
R&D expenses increased to $141.7 million in 2016 compared to $129.2 million in 2015. The $12.5 million increase was primarily due to a $19.4 million increase in external-related costs to support our key R&D programs. The increase was partially offset by a $5.6 million decrease in share-based compensation due to lower costs associated with the long-term retention and incentive awards granted to certain employees in 2011, and a $1.3 million reduction in employee-related costs primarily due to higher expense reimbursements from the Mylan collaborative arrangement.
R&D expenses decreased $39.4 million in 2015 compared to 2014 primarily due to decreases in employee-related costs and external-related costs. The decrease in employee-related costs was primarily due to lower costs associated with the long-term retention and incentive awards granted to certain employees in 2011 and expense reimbursements from Mylan. The decrease in external-related costs was primarily due to the reimbursement of R&D costs for the revefenacin program under the Mylan collaboration agreement. Both decreases were partially offset by an increase in share-based compensation expense due primarily to new equity awards issued under our equity plans post Spin-Off.
Under certain of our collaborative arrangements we receive partial reimbursement of employee-related costs and external costs, which have been reflected as a reduction of R&D expenses of
79
$90.7 million, $55.2 million and $1.9 million for 2016, 2015 and 2014, respectively. The increase in expense reimbursements from 2014 through 2016 was primarily attributed to the continued progression of our revefenacin program that we are co-developing with Mylan.
Selling, General & Administrative
Selling, general and administrative expenses, as compared to the prior years, were as follows:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2016 | 2015 | |||||||||||||||||||
(In thousands)
|
2016 | 2015 | 2014 | $ | % | $ | % | |||||||||||||||
Selling, general and administrative |
$ | 84,509 | $ | 90,203 | $ | 71,647 | $ | (5,694 | ) | (6 | )% | $ | 18,556 | 26 | % | |||||||
Selling, general and administrative expenses decreased $5.7 million in 2016 compared to 2015. The decrease was primarily due to lower costs associated with the long-term retention and incentive awards granted to certain employees in 2011, partially offset by increased costs related to our efforts to commercialize VIBATIV.
Selling, general and administrative expenses increased $18.6 million in 2015 compared to 2014. The increase was primarily due to costs associated with the expansion of our sales and marketing organization supporting VIBATIV commercialization and due to an increase in share-based compensation expense. Share-based compensation expenses related to selling, general and administrative expenses were $28.3 million and $22.0 million in 2015 and 2014, respectively, primarily due to new equity awards issued under our equity plans post Spin-Off.
Interest Expense
Interest expense, as compared to the prior years, were as follows:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, |
|||||||||||||||||||||
| |
2016 | 2015 | ||||||||||||||||||||
(In thousands)
|
2016 | 2015 | 2014 | $ | % | $ | % | |||||||||||||||
Interest expense |
$ | 1,404 | $ | — | $ | — | $ | 1,404 | NM | $ | — | — | % | |||||||||
NM: Not Meaningful
Interest expense increased to $1.4 million in 2016 due to the November 2016 issuance of $230.0 million principal amount of 3.250% convertible senior notes due 2023. We had no interest-bearing debt in 2015 or 2014.
Interest and Other Income
Interest and other income, as compared to the prior years, were as follows:
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2016 | 2015 | |||||||||||||||||||
(In thousands)
|
2016 | 2015 | 2014 | $ | % | $ | % | |||||||||||||||
Interest and other income |
$ | 1,312 | $ | 631 | $ | 1,865 | $ | 681 | 108 | % | $ | (1,234 | ) | (66 | )% | |||||||
Interest and other income was $1.3 million in 2016 compared to $0.6 million in 2015. The $0.7 million increase was primarily due to the additional income earned from higher investment balances following our public equity and debt offerings in 2016.
80
In 2014, interest and other income of $1.9 million primarily consisted of interest income of $0.3 million and reimbursement for transition services rendered to Innoviva of $1.6 million.
Although we incurred operating losses on a consolidated basis, the provision for income taxes resulted from recording contingent tax liabilities pertaining primarily to uncertain tax positions taken with respect to transfer pricing and tax credits.
Provision for Income Taxes
| |
|
|
|
Change | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Year Ended December 31, | 2016 | 2015 | |||||||||||||||||||
(In thousands)
|
2016 | 2015 | 2014 | $ | % | $ | % | |||||||||||||||
Provision for income taxes |
$ | 10,110 | $ | 951 | $ | 6,364 | $ | 9,159 | 963 | % | $ | (5,413 | ) | (85 | )% | |||||||
In general, the provision for 2016 and 2015 resulted from recording contingent tax liabilities pertaining primarily to uncertain tax positions taken with respect to transfer pricing and tax credits. The provision for income taxes was $10.1 million, $1.0 million and $6.4 million in 2016, 2015 and 2014, respectively, although we incurred operating losses on a consolidated basis.
The provision for income taxes increased $9.2 million in 2016 compared to 2015 due to recording contingent tax liabilities pertaining primarily to uncertain tax positions taken with respect to our transfer pricing and tax credits.
As of December 31, 2016, we had $13.9 million of U.S. federal net operating loss carryforwards, as well as $5.4 million of federal research and development tax credit carryforwards which begin to expire in 2035. As of December 31, 2016, we had state operating loss carryforwards of $26.4 million which generally begin to expire in 2034, and state research and development credit carryforwards of $7.4 million to be carried forward indefinitely. We had unrecognized tax benefits of $23.2 million as of December 31, 2016. Our unrecognized tax benefits would reduce our effective income tax rate if recognized.
Liquidity and Capital Resources
We have financed our operations primarily through public offering of equity and debt securities, private placements of equity, revenue from collaboration arrangements and revenue from product sales. At December 31, 2016, we had approximately $592.7 million in cash and investments in marketable securities. Also, as of December 31, 2016, we had outstanding $230.0 million in aggregate principal amount of 3.250% convertible senior notes due 2023.
We expect to continue to incur net losses over the next several years as we continue our drug discovery efforts and incur significant preclinical and clinical development costs related to our current product candidates and commercialization and development costs relating to VIBATIV. In particular, to the extent we advance our product candidates into and through later-stage clinical studies without a partner, we will incur substantial expenses. We expect the clinical development of our key development programs will require significant investment in order to continue to advance in clinical development. In the past, we have received a number of significant payments from collaboration agreements and other significant transactions. In the future, we expect to receive revenues from product sales and potential substantial payments from future collaboration transactions if the drug candidates in our pipeline achieve positive clinical or regulatory outcomes. Our current business plan is also subject to significant uncertainties and risks as a result of, among other factors, the sales levels of VIBATIV, clinical program outcomes, whether, when and on what terms we are able to enter into new collaboration arrangements, expenses being higher than anticipated, unplanned expenses, cash receipts being lower than anticipated, and the need to satisfy contingent liabilities, including litigation matters and indemnification obligations.
81
Adequacy of cash resources to meet future needs
We expect our cash and cash equivalents and marketable securities will fund our operations for at least the next 12 months based on current operating plans and financial forecasts.
If our current operating plans or financial forecasts change, we may require additional funding sooner in the form of public or private equity offerings, debt financings or additional collaborations and licensing arrangements. However, future financing may not be available in amounts or on terms acceptable to us, if at all.
In October 2015, we entered into an Ordinary Share Purchase Agreement (the "Purchase Agreement") with funds managed by Woodford Investment Management LLP for the registered direct offering of an aggregate of 3,859,649 of our ordinary shares at a purchase price of $14.25 per share. The shares were issued pursuant to a prospectus supplement filed with the Securities and Exchange Commission ("SEC") on October 26, 2015, in connection with a takedown from our shelf registration statement on Form S-3. The closing of the transaction occurred on October 29, 2015 and the net offering proceeds were approximately $53.0 million.
On March 17, 2016, GSK purchased 1,301,015 of our unregistered ordinary shares at a price of $17.70 per share pursuant to an Ordinary Share Purchase Agreement between the Company and GSK, dated as of March 14, 2016. The aggregate gross proceeds of the purchase were approximately $23.0 million and no underwriting discounts or commissions were paid in this transaction.
Under our sales agreement with Cantor Fitzgerald & Co. ("Cantor Fitzgerald"), we may sell up to $50.0 million of our ordinary shares pursuant to an at-the-market offering program (the "ATM Agreement"). We commenced selling ordinary shares under the ATM Agreement from March 17, 2016. As of April 8, 2016, we sold approximately 770,000 of our ordinary shares at an average market price of $19.53 per share, resulting in aggregate net proceeds after offering costs of approximately $14.3 million. Since April 2016, we have ceased raising capital under the ATM agreement. If favorable financing opportunities arise, we may seek to continue to raise capital through other debt or equity offerings to fund our operations.
On May 4, 2016, we closed the sale of an aggregate of 5,479,750 of our ordinary shares at a public offering price of $21.00 per share. The shares were issued pursuant to a prospectus supplement filed with the SEC on April 29, 2016, in connection with a takedown from our shelf registration statement on Form S-3. We received net offering proceeds of approximately $107.9 million after deducting the underwriting discount and offering expenses.
On October 26, 2016, we filed a universal shelf registration statement on Form S-3 registering an indeterminate number of ordinary shares, debt and other forms of securities.
On November 2, 2016, we sold 3,850,000 ordinary shares at a price to the public of $26.00 per share (the "Shares") and $230.0 million aggregate principal amount of 3.250% convertible senior notes due 2023 (the "Notes") for net proceeds of approximately $316.2 million, after deducting underwriting discounts and commissions and other transaction expenses. On November 14, 2016, the underwriters for the Shares also exercised its option to purchase an additional 577,500 Shares for net proceeds of approximately $14.1 million, after deducting underwriting discounts and commissions, resulting in total net proceeds from both the Shares and Notes offerings of approximately $330.3 million. The Shares and the Notes (and our ordinary shares issuable upon conversion of the Notes) were offered and sold under (i) prospectus supplements dated October 27, 2016 (each, a "Prospectus Supplement" and together, the "Prospectus Supplements"), and (ii) a free writing prospectus containing the final terms of the offering of the Shares and the Notes dated October 27, 2016 and filed with the SEC.
82
Without adequate financial resources to fund our operations as presently conducted, we may be required to relinquish rights to our technologies, product candidates or territories, or grant licenses on terms that are not favorable to us, in order to raise additional funds through collaborations or licensing arrangements. We may also have to sequence pre-clinical and clinical studies as opposed to conducting them concomitantly in order to conserve resources, or delay, reduce or eliminate one or more of our research or development programs and reduce overall overhead expenses. In addition, we may have to make reductions in our workforce and may be prevented from continuing our discovery, development and commercialization efforts and exploiting other corporate opportunities.
Cash Flows
Cash flows, as compared to the prior years, were as follows:
| |
Year Ended December 31, | Change | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | 2014 | 2016 | 2015 | |||||||||||
Net cash used in operating activities |
$ | (98,989 | ) | $ | (168,857 | ) | $ | (175,155 | ) | $ | 69,868 | $ | 6,298 | |||
Net cash (used in) provided by investing activities |
(148,235 | ) | 111,039 | (106,251 | ) | (259,274 | ) | 217,290 | ||||||||
Net cash provided by financing activities |
479,226 | 81,310 | 370,621 | 397,916 | (289,311 | ) | ||||||||||
Net cash flows used in operating activities
Net cash used in operating activities was $99.0 million in 2016, consisting primarily of net loss of $190.7 million, adjusted for non-cash items such as $41.2 million for share-based compensation expense and $46.9 million of net cash inflow related to changes in operating assets and liabilities. The $46.9 million net cash inflow related to changes in operating assets and liabilities was primarily attributable to a $26.2 million net decrease in receivables from collaboration partners, principally Mylan, and $9.5 million in net tax refunds in 2016.
Net cash used in operating activities was $168.9 million in 2015, consisting primarily of net loss of $182.2 million, adjusted for non-cash items such as $54.1 million for share-based compensation expense and $8.0 million for non-cash revenue from collaborative agreements, and $37.8 million of net cash outflow related to changes in operating assets and liabilities. The $37.8 million net cash outflow related to changes in operating assets and liabilities was primarily attributable to receivables due from the Mylan collaboration agreement that was established in January 2015 and prepaid taxes in 2015.
Net cash used in operating activities was $175.2 million in 2014, consisting primarily of net loss of $237.0 million, adjusted for non-cash items such as $43.2 million for share-based compensation expense, and $12.8 million of net cash inflow related to changes in operating assets and liabilities.
Net cash flows (used in) provided by investing activities
Net cash used in investing activities was $148.2 million in 2016, consisting primarily of purchases of marketable securities of $237.6 million partially offset by maturities of marketable securities of $91.5 million.
Net cash provided by investing activities was $111.0 million in 2015, consisting primarily of maturities of marketable securities of $186.7 million partially offset by purchases of marketable securities of $73.0 million.
Net cash used in investing activities was $106.3 million in 2014, consisting primarily of purchases of marketable securities of $168.9 million partially offset by maturities of marketable securities of $65.6 million.
83
Net cash flows provided by financing activities
Net cash provided by financing activities was $479.2 million in 2016, consisting primarily of the sales of ordinary shares for total net proceeds of $253.0 million and the issuance of our convertible senior notes for a total net proceeds of $222.5 million.
Net cash provided by financing activities was $81.3 million in 2015, consisting primarily of the sales of ordinary shares to Mylan and Woodford Investment Management LLP for a total net proceeds of $79.0 million.
Net cash provided by financing activities was $370.6 million in 2014, consisting primarily of $277.5 million in cash and cash equivalents contributed from Innoviva as a result of the Spin-Off.
Commitments and Contingencies
In the first quarter of 2016, the Compensation Committee of our Board of Directors ("Compensation Committee") approved the grant of 1,575,000 performance-contingent restricted share awards ("RSAs") and 135,000 performance contingent restricted share units ("RSUs") to senior management. These grants have dual triggers of vesting based upon the achievement of certain performance conditions over a five-year timeframe from 2016 to 2020 and continued employment, both of which must be satisfied in order for the awards to vest. As of December 31, 2016, there were 1,440,000 performance-contingent RSAs and 135,000 performance-contingent RSUs outstanding.
Expense associated with these awards may be recognized during the years 2016 to 2020 depending on the probability of meeting the performance conditions. Compensation expense relating to awards subject to performance conditions is recognized if it is considered probable that the performance goals will be achieved. The probability of achievement will be reassessed at each reporting period.
In August 2016, the Compensation Committee determined not to award credit for a performance condition that occurred in the second quarter of 2016, which for accounting purposes is treated as a modification of the vesting conditions of all outstanding awards. As a result of the modification, the vesting of the first tranche of the awards changed from probable of achievement to improbable. The vesting of the second and third tranches of the awards is still considered improbable of achievement. As a result of the modification, there is a new measurement date for the second and third tranches of the awards as of the modification date. While the total number of shares under the award did not change, the remeasurement of the awards results in a higher potential compensation charge for the awards because our share price had increased since the original measurement date. The revised maximum potential expense associated with the awards could be up to $38.9 million (allocated as $16.7 million for research and development expense and $22.2 million for selling, general and administrative expense) if all of the performance conditions are achieved. For the year ended December 31, 2016, we recognized $1.8 million in share-based compensation expense related to our assessment of the probability that the performance conditions associated with the first tranche of these awards was considered to be probable of vesting. As of December 31, 2016, we determined that the remaining second and third tranches were not probable of vesting and, as a result, no compensation expense related to these tranches has been recognized in 2016.
We indemnify our officers and directors for certain events or occurrences, subject to certain limits. We believe the fair value of these indemnification agreements is minimal. Accordingly, we have not recognized any liabilities relating to these agreements as of December 31, 2016.
Off-Balance Sheet Arrangements
Our equity interest in TRC constitutes an off-balance sheet arrangement. Under the agreement governing TRC, the manager of TRC may request quarterly capital contributions from us to fund the operating costs of TRC; however, we are not obligated to make such contributions. Our equity interest
84
in TRC entitles us to an 85% economic interest in any future payments, which includes royalties and milestone payments, made by GSK under the strategic alliance agreement and under the portion of the collaboration agreement assigned to TRC by Innoviva (the "GSK Agreements"). We have determined TRC to be a variable interest entity that is not consolidated in our financial statements. See Note 11, "Spin-Off from Innoviva, Inc." in the notes to our consolidated financial statements for further information regarding our interest in TRC. The potential importance of TRC to our future financial condition and results of operations is dependent upon the progression of drug candidates covered by the GSK Agreements through development to commercialization. We rely on publicly available information about those drug candidates as we do not have access to confidential information regarding their progression or status.
Contractual Obligations and Commercial Commitments
In the table below, we set forth our enforceable and legally binding, significant obligations and future commitments, as well as obligations related to all contracts that we are likely to continue, regardless of the fact that they were cancelable as of December 31, 2016. Some of the figures that we include in this table are based on management's estimate and assumptions about these obligations, including their duration. Because these estimates and assumptions are necessarily subjective, the obligations we will actually pay in future periods may vary from those reflected in the table.
| |
Years | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
Total | Within 1 | Over 1 to 3 | Over 3 to 5 | After 5 | |||||||||||
3.250% Convertible senior notes due 2023 |
$ | 282,325 | $ | 8,700 | $ | 14,950 | $ | 14,950 | $ | 243,725 | ||||||
Facility operating leases(1) |
21,678 | 6,121 | 12,799 | 2,758 | — | |||||||||||
Purchase obligations(2) |
104,808 | 94,131 | 8,789 | 1,888 | — | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
$ | 408,811 | $ | 108,952 | $ | 36,538 | $ | 19,596 | $ | 243,725 | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- As
security for performance of certain obligations under the operating leases for our principal physical properties, we issued a letter of credit in the amount of
$0.8 million, collateralized by an equal amount of restricted cash.
- (2)
- Substantially all of this amount was subject to open purchase orders, as of December 31, 2016, that were issued under existing contracts. This amount does not represent any minimum contract termination liabilities for our existing contracts.
Recent Accounting Pronouncements
The information required by this item is included in Item 8, Note 1, "Description of Operations and Summary of Significant Accounting Policies," in our consolidated financial statements included in this Annual Report on Form 10-K.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risks in the ordinary course of our business. These risks primarily include risk related to interest rate sensitivities.
Interest Rate Sensitivity
We have invested primarily in money market funds, federal agency notes, corporate debt securities, commercial papers and U.S. treasury notes. To reduce the volatility relating to these exposures, we have put investment and risk management policies and procedures in place. The securities in our investment
85
portfolio are not leveraged and are classified as available-for-sale due to their short-term nature. We currently do not engage in hedging activities.
We performed a sensitivity analysis to determine the impact a change in interest rates would have on the value of our investment portfolio. As of December 31, 2016 and 2015, we have estimated that a hypothetical 100 basis point increase in interest rates would have resulted in a decrease in the fair market value of our investment portfolio of $2.0 million and $0.9 million, respectively. The $1.1 million change in estimated fair market value was primarily due to the increase in our investment portfolio balance at December 31, 2016 compared to December 31, 2015. Such losses would only be realized if we sold the investments prior to maturity.
We are also subject to interest rate sensitivity on our outstanding 3.250% convertible senior notes that were issued in November 2016. Increases in interest rates would result in a decrease in the fair value of our outstanding debt and decreases in interest rates would result in an increase in the fair value of our outstanding debt. These increases or decreases in the fair value of our outstanding debt would be partially offset by corresponding increases or decreases in our investment portfolio. Interest payments under the 3.250% convertible senior notes are made semi-annually, and the $230.0 million of debt principal is scheduled to be repaid in 2023.
86
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
87
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Theravance Biopharma, Inc.
We have audited the accompanying consolidated balance sheets of Theravance Biopharma, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of operations, comprehensive loss, shareholders' equity and parent company deficit and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Theravance Biopharma, Inc. at December 31, 2016 and 2015, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2016, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Theravance Biopharma, Inc.'s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 1, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
San
Jose, California
March 1, 2017
88
THERAVANCE BIOPHARMA, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except per share data)
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | |||||
Assets |
|||||||
Current assets: |
|||||||
Cash and cash equivalents |
$ | 344,709 | $ | 112,707 | |||
Short-term marketable securities |
156,387 | 59,727 | |||||
Accounts receivable, net of allowances of $779 and $758 at December 31, 2016 and 2015, respectively |
646 | 1,922 | |||||
Receivables from collaborative arrangements |
9,076 | 35,232 | |||||
Prepaid taxes |
3,060 | 12,764 | |||||
Other prepaid and current assets |
2,405 | 5,115 | |||||
Inventories |
12,220 | 10,005 | |||||
| | | | | | | | |
Total current assets |
528,503 | 237,472 | |||||
Property and equipment, net |
8,460 | 9,873 | |||||
Long-term marketable securities |
91,565 | 42,860 | |||||
Other investments |
8,000 | 8,000 | |||||
Restricted cash |
833 | 833 | |||||
Other assets |
1,893 | 1,078 | |||||
| | | | | | | | |
Total assets |
$ | 639,254 | $ | 300,116 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Liabilities and Shareholders' Equity |
|||||||
Current liabilities: |
|||||||
Accounts payable |
$ | 1,733 | $ | 18,804 | |||
Accrued personnel-related expenses |
14,021 | 10,866 | |||||
Accrued clinical and development expenses |
25,064 | 14,709 | |||||
Other accrued liabilities |
8,298 | 4,947 | |||||
Deferred revenue |
152 | 144 | |||||
| | | | | | | | |
Total current liabilities |
49,268 | 49,470 | |||||
Convertible senior notes, net |
222,676 | — | |||||
Deferred rent |
3,966 | 4,598 | |||||
Other long-term liabilities |
13,113 | 2,983 | |||||
Commitments and contingencies (Note 2, 8, and 10) |
|||||||
Shareholders' equity |
|||||||
Preferred shares, $0.00001 par value: 230 shares authorized, no shares issued or outstanding at December 31, 2016 and 2015, respectively |
— | — | |||||
Ordinary shares, $0.00001 par value: 200,000 shares authorized at December 31, 2016 and 2015; 52,833 and 37,981 shares issued and outstanding at December 31, 2016 and 2015, respectively |
1 | — | |||||
Additional paid-in capital |
862,708 | 564,691 | |||||
Accumulated other comprehensive loss |
(253 | ) | (70 | ) | |||
Accumulated deficit |
(512,225 | ) | (321,556 | ) | |||
| | | | | | | | |
Total shareholders' equity |
350,231 | 243,065 | |||||
| | | | | | | | |
Total liabilities and shareholders' equity |
$ | 639,254 | $ | 300,116 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See accompanying notes to consolidated financial statements.
89
THERAVANCE BIOPHARMA, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | |||||||
Revenue: |
||||||||||
Product sales |
$ | 17,603 | $ | 9,408 | $ | 4,418 | ||||
Revenue from collaborative arrangements |
31,045 | 32,718 | 7,270 | |||||||
| | | | | | | | | | | |
Total revenue |
48,648 | 42,126 | 11,688 | |||||||
Costs and expenses: |
||||||||||
Cost of goods sold |
2,894 | 4,657 | 4,058 | |||||||
Research and development(1) |
141,712 | 129,165 | 168,522 | |||||||
Selling, general and administrative(1) |
84,509 | 90,203 | 71,647 | |||||||
| | | | | | | | | | | |
Total costs and expenses |
229,115 | 224,025 | 244,227 | |||||||
| | | | | | | | | | | |
Loss from operations |
(180,467 | ) | (181,899 | ) | (232,539 | ) | ||||
Interest expense |
(1,404 | ) | — | — | ||||||
Interest and other income |
1,312 | 631 | 1,865 | |||||||
| | | | | | | | | | | |
Loss before income taxes |
(180,559 | ) | (181,268 | ) | (230,674 | ) | ||||
Provision for income taxes |
10,110 | 951 | 6,364 | |||||||
| | | | | | | | | | | |
Net loss |
$ | (190,669 | ) | $ | (182,219 | ) | $ | (237,038 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Net loss per share: |
||||||||||
Basic and diluted net loss per share |
$ | (4.26 | ) | $ | (5.34 | ) | $ | (7.46 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Shares used to compute basic and diluted net loss per share |
44,711 | 34,150 | 31,755 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- (1)
- Amounts include share-based compensation expense as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | 2014 | |||||||
Research and development |
$ | 20,202 | $ | 25,770 | $ | 21,191 | ||||
Selling, general and administrative |
20,967 | 28,280 | 22,043 | |||||||
| | | | | | | | | | | |
Total share-based compensation expense |
$ | 41,169 | $ | 54,050 | $ | 43,234 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
90
THERAVANCE BIOPHARMA, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | |||||||
Net loss |
$ | (190,669 | ) | $ | (182,219 | ) | $ | (237,038 | ) | |
Other comprehensive income (loss): |
||||||||||
Net unrealized gain (loss) on marketable securities |
(183 | ) | 12 | (173 | ) | |||||
| | | | | | | | | | | |
Comprehensive loss |
$ | (190,852 | ) | $ | (182,207 | ) | $ | (237,211 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
91
THERAVANCE BIOPHARMA, INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY AND PARENT COMPANY DEFICIT
(In thousands, except share data)
| |
|
|
|
|
|
|
Total Shareholders' Equity and Parent Company Deficit |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Ordinary Shares | |
Accumulated Other Comprehensive Income (Loss) |
|
|
|||||||||||||||||
| |
Additional Paid-In Capital |
Accumulated Deficit |
Parent Company Deficit |
|||||||||||||||||||
| |
Shares | Amount | ||||||||||||||||||||
Balances at December 31, 2013 (Note 1) |
— | $ | — | $ | — | $ | — | $ | — | $ | (17,035 | ) | $ | (17,035 | ) | |||||||
Contribution of net assets from Innoviva, Inc. |
32,260,105 | — | 402,787 | 91 | — | (402,878 | ) | — | ||||||||||||||
Cash contribution from Innoviva, Inc. |
— | — | — | — | — | 277,541 | 277,541 | |||||||||||||||
Net transfers from parent |
— | — | — | — | — | 222,934 | 222,934 | |||||||||||||||
Employee share-based compensation expense |
— | — | 26,315 | — | — | 17,139 | 43,454 | |||||||||||||||
Cancellation of shares distributed |
(31,285 | ) | — | — | — | — | — | — | ||||||||||||||
Repurchase of shares to satisfy tax withholding |
(7,737 | ) | — | (178 | ) | — | — | — | (178 | ) | ||||||||||||
Excess tax benefit of share-based compensation |
— | — | 282 | — | — | — | 282 | |||||||||||||||
Net unrealized loss on marketable securities |
— | — | — | (173 | ) | — | — | (173 | ) | |||||||||||||
Net loss |
— | — | — | — | (139,337 | ) | (97,701 | ) | (237,038 | ) | ||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2014 |
32,221,083 | — | 429,206 | (82 | ) | (139,337 | ) | — | 289,787 | |||||||||||||
Net proceeds from sale of ordinary shares |
5,490,013 | — | 79,017 | — | — | — | 79,017 | |||||||||||||||
Proceeds from ESPP purchases |
250,209 | — | 3,124 | — | — | — | 3,124 | |||||||||||||||
Employee share-based compensation expense |
— | — | 54,175 | — | — | — | 54,175 | |||||||||||||||
Issuance of restricted shares |
71,365 | — | — | — | — | — | ||||||||||||||||
Repurchase of shares to satisfy tax withholding |
(51,534 | ) | — | (756 | ) | — | — | — | (756 | ) | ||||||||||||
Excess tax benefit of share-based compensation |
— | — | (75 | ) | — | — | — | (75 | ) | |||||||||||||
Net unrealized gain on marketable securities |
— | — | — | 12 | — | — | 12 | |||||||||||||||
Net loss |
— | — | — | — | (182,219 | ) | — | (182,219 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2015 |
37,981,136 | — | 564,691 | (70 | ) | (321,556 | ) | — | 243,065 | |||||||||||||
Net proceeds from sale of ordinary shares |
11,978,261 | 1 | 253,027 | — | — | — | 253,028 | |||||||||||||||
Proceeds from ESPP purchases |
244,587 | — | 3,172 | — | — | — | 3,172 | |||||||||||||||
Employee share-based compensation expense |
— | — | 41,290 | — | — | — | 41,290 | |||||||||||||||
Issuance of restricted shares |
2,465,713 | — | — | — | — | — | — | |||||||||||||||
Option exercises |
197,328 | — | 4,378 | — | — | 4,378 | ||||||||||||||||
Repurchase of shares to satisfy tax withholding |
(34,182 | ) | — | (3,871 | ) | — | — | — | (3,871 | ) | ||||||||||||
Excess tax benefit of share-based compensation |
— | — | 21 | — | — | — | 21 | |||||||||||||||
Net unrealized loss on marketable securities |
— | — | — | (183 | ) | — | — | (183 | ) | |||||||||||||
Net loss |
— | — | — | — | (190,669 | ) | — | (190,669 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Balances at December 31, 2016 |
52,832,843 | $ | 1 | $ | 862,708 | $ | (253 | ) | $ | (512,225 | ) | $ | — | $ | 350,231 | |||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
92
THERAVANCE BIOPHARMA, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | |||||||
Operating activities |
||||||||||
Net loss |
$ | (190,669 | ) | $ | (182,219 | ) | $ | (237,038 | ) | |
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||
Depreciation and amortization |
3,119 | 2,989 | 3,274 | |||||||
Share-based compensation |
41,169 | 54,050 | 43,234 | |||||||
Inventory write-down |
303 | 2,096 | 2,887 | |||||||
Excess tax benefits from share-based compensation |
(21 | ) | 75 | (282 | ) | |||||
Non-cash revenue from collaboration arrangements |
— | (8,000 | ) | — | ||||||
Other |
182 | (65 | ) | — | ||||||
Changes in operating assets and liabilities: |
||||||||||
Accounts receivable |
1,276 | (1,633 | ) | (90 | ) | |||||
Receivables from collaborative arrangements |
26,156 | (33,392 | ) | (906 | ) | |||||
Receivable from Innoviva, Inc. |
— | — | 14,635 | |||||||
Prepaid taxes |
9,522 | (12,764 | ) | — | ||||||
Other prepaid and current assets |
2,710 | 963 | (2,878 | ) | ||||||
Inventories |
(3,182 | ) | 1,030 | (6,628 | ) | |||||
Other assets |
184 | (572 | ) | (211 | ) | |||||
Accounts payable |
(16,436 | ) | 8,717 | 3,917 | ||||||
Accrued personnel-related expenses, accrued clinical and development expenses, and other accrued liabilities |
17,192 | (1,039 | ) | 11,680 | ||||||
Deferred rent |
(632 | ) | (552 | ) | 376 | |||||
Income taxes payable |
— | — | — | |||||||
Deferred revenue |
448 | 295 | (7,991 | ) | ||||||
Other long-term liabilities |
9,690 | 1,164 | 866 | |||||||
| | | | | | | | | | | |
Net cash used in operating activities |
(98,989 | ) | (168,857 | ) | (175,155 | ) | ||||
| | | | | | | | | | | |
Investing activities |
||||||||||
Changes in restricted cash |
— | — | (833 | ) | ||||||
Purchases of property, equipment, and capitalized software |
(2,135 | ) | (2,647 | ) | (3,107 | ) | ||||
Purchases of marketable securities |
(237,567 | ) | (73,011 | ) | (168,893 | ) | ||||
Maturities of marketable securities |
91,467 | 186,697 | 65,564 | |||||||
Sale of short-term investments and marketable securities |
— | — | 878 | |||||||
Payments received on notes receivable, net of issuances |
— | — | 140 | |||||||
| | | | | | | | | | | |
Net cash (used in) provided by investing activities |
(148,235 | ) | 111,039 | (106,251 | ) | |||||
| | | | | | | | | | | |
Financing activities |
||||||||||
Proceeds from sale of ordinary shares, net |
253,028 | 79,017 | — | |||||||
Proceeds from issuance of 3.250% convertible senior notes, net |
222,498 | — | — | |||||||
Proceeds from ESPP purchases |
3,172 | 3,124 | — | |||||||
Proceeds from option exercises |
4,378 | — | — | |||||||
Excess tax benefits from share-based compensation |
21 | (75 | ) | 282 | ||||||
Repurchase of shares to satisfy tax withholding |
(3,871 | ) | (756 | ) | (178 | ) | ||||
Cash and cash equivalents contributed from Innoviva, Inc. (Note 1) |
— | — | 277,541 | |||||||
Transfers from Innoviva, Inc. |
— | — | 92,976 | |||||||
| | | | | | | | | | | |
Net cash provided by financing activities |
479,226 | 81,310 | 370,621 | |||||||
| | | | | | | | | | | |
Net increase in cash and cash equivalents |
232,002 | 23,492 | 89,215 | |||||||
Cash and cash equivalents at beginning of period |
112,707 | 89,215 | — | |||||||
| | | | | | | | | | | |
Cash and cash equivalents at end of period |
$ | 344,709 | $ | 112,707 | $ | 89,215 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Supplemental disclosure of cash flow information |
||||||||||
Cash paid for interest |
$ | — | $ | — | $ | — | ||||
Cash (received) paid for income taxes, net |
$ | (9,488 | ) | $ | 13,389 | $ | 4,550 | |||
Supplemental disclosure of non-cash information |
||||||||||
Contribution of net assets, excluding cash and cash equivalents, from Innoviva, Inc. (Note 11) |
$ | — | $ | — | $ | 125,337 | ||||
See accompanying notes to consolidated financial statements.
93
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Description of Operations and Summary of Significant Accounting Policies
Description of Operations
Theravance Biopharma, Inc. ("Theravance Biopharma", the "Company", or "we" and other similar pronouns) is a diversified biopharmaceutical company with the core purpose of creating medicines that help improve the lives of patients suffering from serious illness.
Our pipeline of internally discovered product candidates includes potential best-in-class medicines to address the unmet needs of patients being treated for serious conditions primarily in the acute care setting. VIBATIV® (telavancin), our first commercial product, is a once-daily dual-mechanism antibiotic approved in the U.S., Europe and certain other countries for certain difficult-to-treat infections. Revefenacin (TD-4208) is a long-acting muscarinic antagonist ("LAMA") being developed as a potential once-daily, nebulized treatment for chronic obstructive pulmonary disease ("COPD"). Our neprilysin ("NEP") inhibitor program is designed to develop selective NEP inhibitors for the treatment of a range of major cardiovascular and renal diseases, including acute and chronic heart failure, hypertension and chronic kidney diseases such as diabetic nephropathy. Our research efforts are focused in the areas of inflammation and immunology, with the goal of designing medicines that provide targeted drug delivery to tissues in the lung and gastrointestinal tract in order to maximize patient benefit and minimize risk. The first program to emerge from this research is designed to develop intestinally restricted pan-Janus kinase ("JAK") inhibitors for the treatment of a range of inflammatory intestinal diseases.
In addition, we have an economic interest in future payments that may be made by Glaxo Group Limited or one of its affiliates ("GSK") pursuant to its agreements with Innoviva, Inc. ("Innoviva") (known as Theravance, Inc. prior to January 7, 2016) relating to certain drug development programs, including the Closed Triple (the combination of fluticasone furoate, umeclidinium, and vilanterol), currently in development for the treatment of COPD and asthma.
On June 1, 2014, pursuant to a Separation and Distribution Agreement between Innoviva and Theravance Biopharma (the "Separation and Distribution Agreement"), Innoviva separated its late-stage respiratory assets partnered with GSK from its biopharmaceutical operations by transferring its discovery, development and commercialization operations (the "Biopharmaceutical Business") and contributing $393.0 million of cash, cash equivalents and marketable securities into its then wholly-owned subsidiary Theravance Biopharma. On June 2, 2014, Innoviva made a pro rata dividend distribution to its stockholders of record on May 15, 2014 of one ordinary share of Theravance Biopharma for every three and one half shares of Innoviva common stock outstanding on the record date (the "Spin-Off"). The Spin-Off resulted in Theravance Biopharma operating as an independent, publicly-traded company. Prior to June 2, 2014, Innoviva operated the Biopharmaceutical Business. While Theravance Biopharma is incorporated under Cayman Island law, the Company became an Irish tax resident effective July 1, 2015.
Basis of Presentation
For the periods prior to June 2, 2014, the consolidated financial statements have been prepared using Innoviva's historical cost basis of the assets and liabilities of the various activities that comprised the Biopharmaceutical Business of Innoviva and reflect the consolidated results of operations, financial condition and cash flows of Theravance Biopharma as a wholly-owned subsidiary of Innoviva prior to the Spin-Off. The various assets, liabilities, revenues and expenses associated with Innoviva have been allocated to the historical consolidated financial statements of Theravance Biopharma in a manner
94
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
consistent with the Separation and Distribution Agreement, discussed in Note 11, "Spin-Off from Innoviva, Inc.". Changes in parent company deficit represent Innoviva's net investment in Theravance Biopharma, after giving effect to Theravance Biopharma's net loss, parent company expense allocations, and net cash transfers to and from Innoviva.
For purposes of preparing the consolidated financial statements, the Biopharmaceutical Business was derived from Innoviva's historical consolidated financial statements, allocations of revenues, research and development ("R&D") expenses, and non-operating income and expenses to Theravance Biopharma were made on a specific identification basis. For purposes of allocating general and administrative expenses from Innoviva's historical consolidated financial statements, costs directly related to the Biopharmaceutical Business were allocated to Theravance Biopharma on a specific identification basis or based on the estimated underlying effort. Theravance Biopharma's general and administrative expenses also include allocations of Innoviva's general corporate overhead expenses, including finance, legal, human resources, information technology and other administrative functions. These allocations of general corporate overhead expenses were primarily based on the estimated underlying effort or an estimated number of full-time employees that worked with the Biopharmaceutical Business. The consolidated balance sheets of Theravance Biopharma include assets and liabilities that were allocated to Theravance Biopharma principally on a specific identification basis.
Management believes that the consolidated statements of operations and comprehensive loss include a reasonable allocation of costs incurred by Innoviva which benefited Theravance Biopharma. However, such expenses may not be indicative of the actual level of expense that would have been incurred by Theravance Biopharma if it had operated as an independent, publicly-traded company or of the costs expected to be incurred in the future. As such, the financial information herein for periods prior to the Spin-Off may not necessarily reflect the financial position, results of operations, and cash flows of Theravance Biopharma in the future or what it would have been had Theravance Biopharma been an independent, publicly-traded company during such periods.
As Theravance Biopharma was a wholly owned subsidiary of Innoviva until June 2, 2014, no separate cash accounts for the Biopharmaceutical Business were historically maintained prior to the Spin-Off and, therefore, Innoviva is presumed to have funded Theravance Biopharma's operating, investing and financing activities as necessary. For purposes of the historical consolidated financial statements prior to the Spin-Off, funding of Theravance Biopharma's expenditures is reflected in the consolidated financial statements as a component of parent company investment. In connection with the assets transfer and Spin-Off discussed above, Innoviva contributed to Theravance Biopharma cash, cash equivalents and marketable securities of $393.0 million.
We describe the Biopharmaceutical Business transferred to us by Innoviva in connection with the Spin-Off as though the Biopharmaceutical Business were our business for all historical periods described. However, Theravance Biopharma did not conduct any operations prior to the Spin-Off.
Principles of Consolidation
The consolidated financial statements include the accounts of Theravance Biopharma and its wholly owned subsidiaries, all of which are denominated in U.S. dollars. All intercompany balances and transactions have been eliminated in consolidation.
95
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Use of Management's Estimates
The preparation of consolidated financial statements in conformity with U.S. Generally Accepted Accounting Principles ("GAAP") requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ materially from those estimates. On an ongoing basis, management evaluates its significant accounting policies or estimates. We base our estimates on historical experience and other relevant assumptions that we believe to be reasonable under the circumstances. These estimates also form the basis for making judgments about the carrying values of assets and liabilities when these values are not readily apparent from other sources.
Segment Reporting
We have determined that we operate in a single segment, which is the discovery (research), development and commercialization of human therapeutics. We operate in one segment because our business offerings have similar economics and other characteristics, including the nature of products and manufacturing processes, types of customers, distribution methods and regulatory environment. We are comprehensively managed as one business segment by our Chief Executive Officer and his management team. Product sales are attributed to regions based on ship-to location and revenue from collaborative arrangements, including royalty revenue, are attributed to regions based on the location of the collaboration partner.
All capitalized property and equipment is located in the United States.
Cash and Cash Equivalents
We consider all highly liquid investments purchased with a maturity of three months or less on the date of purchase to be cash equivalents. Cash equivalents are carried at fair value.
Restricted Cash
Under certain lease agreements and letters of credit, we have pledged cash and cash equivalents as collateral. As of December 31, 2016 and 2015, restricted cash related to such agreements was $0.8 million.
Investments in Marketable Securities
We invest in marketable securities, primarily corporate notes, government, government agency, and municipal bonds. We classify our marketable securities as available-for-sale securities and report them at fair value in cash equivalents or marketable securities on the consolidated balance sheets with related unrealized gains and losses included as a component of shareholders' equity. The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity, which is included in interest income on the consolidated statements of operations. Realized gains and losses and declines in value judged to be other-than-temporary, if any, on available-for-sale securities are included in interest and other income (loss). The cost of securities sold is based on the specific identification method. Interest and dividends on securities classified as available-for-sale are included in interest income.
96
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
We regularly review all of our investments for other-than-temporary declines in estimated fair value. Our review includes the consideration of the cause of the impairment, including the creditworthiness of the security issuers, the number of securities in an unrealized loss position, the severity and duration of the unrealized losses, whether we have the intent to sell the securities and whether it is more likely than not that we will be required to sell the securities before the recovery of their amortized cost basis. When we determine that the decline in estimated fair value of an investment is below the amortized cost basis and the decline is other-than-temporary, we reduce the carrying value of the security and record a loss for the amount of such decline.
Investments in Non-Marketable Equity Securities
Non-marketable equity securities are recorded at cost in long-term assets, and we periodically review our non-marketable equity securities for impairment by determining whether impairment indicators are present. Common impairment indicators include a significant adverse change in the regulatory or economic environment in which the investee entity operates or cash used in operating activities and other working capital deficiencies.
If we conclude that any of the non-marketable equity securities are impaired, we determine whether such impairment is other-than-temporary. Factors we consider to make such determination include the duration and severity of the impairment, the reason for the decline in value and the potential recovery period and our intent to sell. If any impairment is considered other-than-temporary, we will write-down the asset to its fair value and record the corresponding charge as interest and other income (loss). We have recorded no impairment losses on our non-marketable equity securities for the periods presented.
Fair Value of Financial Instruments
We define fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
Our valuation techniques are based on observable and unobservable inputs. Observable inputs reflect readily obtainable data from independent sources, while unobservable inputs reflect our market assumptions. We classify these inputs into the following hierarchy:
Level 1—Quoted prices for identical instruments in active markets.
Level 2—Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations whose inputs are observable or whose significant value drivers are observable.
Level 3—Unobservable inputs and little, if any, market activity for the assets.
Financial instruments include cash equivalents, marketable securities, accounts receivable, receivables from Innoviva, accounts payable, and accrued liabilities. Our cash equivalents and marketable securities are carried at estimated fair value and remeasured on a recurring basis. The carrying value of accounts receivable, receivables from collaborative arrangements, accounts payable, and accrued liabilities approximate their estimated fair value due to the relatively short-term nature of these instruments.
97
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Accounts Receivable
Trade accounts receivable are recorded net of allowances for wholesaler chargebacks related to government rebate programs, cash discounts for prompt payment, distribution fees, and sales discounts. Estimates for wholesaler chargebacks for government rebates and cash discounts are based on contractual terms, historical trends and our expectations regarding the utilization rates for these programs. When appropriate, we provide for an allowance for doubtful accounts by reserving for specifically identified doubtful accounts. For the periods presented, we did not have any write-offs of accounts receivable. We perform ongoing credit evaluations of our customers and generally do not require collateral.
Concentration of Credit Risks
We invest in a variety of financial instruments and, by our policy, limit the amount of credit exposure with any one issuer, industry or geographic area for investments other than instruments backed by the U.S. federal government.
We depend on a single-source supplier of the active pharmaceutical ingredient ("API") in VIBATIV and one supplier to provide fill-finish services related to the manufacturing of VIBATIV. If any of our suppliers were to limit or terminate production or otherwise fail to meet the quality or delivery requirements needed to supply VIBATIV at levels to meet market demand, we could experience a loss of revenue, which could materially and adversely impact our results of operations.
Inventories
Inventories consist of raw materials, work-in-process and finished goods related to the production of VIBATIV. Raw materials include VIBATIV API and other raw materials. Work-in-process and finished goods include third-party manufacturing costs and labor and indirect costs we incur in the production process. Included in inventories are raw materials and work-in-process that may be used as clinical products, which are charged to research and development expense when consumed. In addition, under certain commercialization agreements, we may sell VIBATIV packaged in unlabeled vials that are recorded in work-in-process. Inventories are stated at the lower of cost or market value. We determine the cost of inventory using the average-cost method for each manufacturing batch.
We assess our inventory levels quarterly and write-down inventory that is expected to be at risk for expiration, that has a cost basis in excess of its expected net realizable value and inventory quantities in excess of expected requirements. This assessment requires management to utilize judgement in formulating estimates and assumptions that we believe to be reasonable under the circumstances. Actual results may differ from those estimates and assumptions.
When we recognize a loss on such inventory, it establishes a new, lower cost basis for that inventory, and subsequent changes in facts and circumstances will not result in the restoration or increase in that newly established cost basis. If inventory with a lower cost basis is subsequently sold, it will result in higher gross margin for the products making up that inventory. In order to realize the value of our recorded inventory, we will be dependent upon continued increases in the sales volumes of VIBATIV.
98
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Property and Equipment
Property, equipment and leasehold improvements are stated at cost, net of accumulated depreciation and depreciated using the straight-line method as follows:
Leasehold improvements |
Shorter of remaining lease terms or useful life | |
Equipment, furniture and fixtures |
5 - 7 years | |
Software and computer equipment |
3 - 5 years |
Capitalized Software
We capitalize certain costs related to direct material and service costs for software obtained for internal use. For the years ended December 31, 2016 and 2015, we capitalized costs for the replacement of our enterprise resource planning software system ("ERP System") of $0.8 million and $0.3 million, respectively. Upon being placed in service, these costs and other future capitalizable costs related to the ERP System integration will be depreciated over five years.
Impairment of Long-Lived Assets
Long-lived assets include property and equipment. The carrying value of long-lived assets is reviewed for impairment whenever events or changes in circumstances indicate that the asset may not be recoverable. An impairment loss is recognized when the total of estimated future cash flows expected to result from the use of the asset and its eventual disposition is less than its carrying amount.
Deferred Rent
Deferred rent consists of the difference between cash payments and the recognition of rent expense on a straight-line basis for the buildings we occupy. Rent expense is being recognized ratably over the life of the leases. Because our facility operating leases provide for rent increases over the terms of the leases, average annual rent expense during the initial years of the leases exceeded our actual cash rent payments. Also included in deferred rent are lease incentives of $0.9 million as of December 31, 2016, which is being recognized ratably over the life of the leases.
Revenue Recognition
Revenue is recognized when the four basic criteria of revenue recognition are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. Where the revenue recognition criteria are not met, we defer the recognition of revenue by recording deferred revenue until such time that all criteria are met.
Product Sales
We sell VIBATIV in the U.S. market by making the drug product available through a limited number of distributors, who sell VIBATIV to healthcare providers. Title and risk of loss transfer upon receipt by these distributors. We recognize VIBATIV product sales and related cost of product sales at the time title transfers to the distributors.
99
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Product sales are recorded net of estimated government-mandated rebates and chargebacks, distribution fees, estimated product returns and other deductions. We reflect such reductions in revenue as either an allowance to the related account receivable from the distributor, or as an accrued liability, depending on the nature of the sales deduction. Sales deductions are based on management's estimates that consider payor mix in target markets, industry benchmarks and experience to date. We monitor inventory levels in the distribution channel, as well as sales of VIBATIV by distributors to healthcare providers, using product-specific data provided by the distributors. Product return allowances are based on amounts owed or to be claimed on related sales. These estimates take into consideration the terms of our agreements with customers, historical product returns of VIBATIV experienced by Innoviva's former collaborative partner, Astellas Pharma Inc. ("Astellas"), rebates or discounts taken, estimated levels of inventory in the distribution channel, the shelf life of the product, and specific known market events, such as competitive pricing and new product introductions. We update our estimates and assumptions each quarter and if actual future results vary from our estimates, we may adjust these estimates, which could have an effect on product sales and earnings in the period of adjustment.
Sales Discounts: We offer cash discounts to certain customers as an incentive for prompt payment. We expect our customers to comply with the prompt payment terms to earn the cash discount. In addition, we offer contract discounts to certain direct customers. We estimate sales discounts based on contractual terms, historical utilization rates, as available, and our expectations regarding future utilization rates. We account for sales discounts by reducing accounts receivable by the full amount and recognizing the discount as a reduction of revenue in the same period the related revenue is recognized.
Chargebacks and Government Rebates: For VIBATIV sales in the U.S., we estimate reductions to product sales for qualifying federal and state government programs including discounted pricing offered to Public Health Service ("PHS") as well as government-managed Medicaid programs. Our reduction for PHS is based on actual chargebacks that distributors have claimed for reduced pricing offered to such healthcare providers and our expectation about future utilization rates. Our accrual for Medicaid is based upon statutorily-defined discounts, estimated payor mix, expected sales to qualified healthcare providers, and our expectation about future utilization. The Medicaid accrual and government rebates that are invoiced directly to us are recorded in other accrued liabilities on the consolidated balance sheets. For qualified programs that can purchase our products through distributors at a lower contractual government price, the distributors charge back to us the difference between their acquisition cost and the lower contractual government price, which we record as an allowance against accounts receivable.
Distribution Fees: We have contracts with our distributors in the U.S. that include terms for distribution-related fees. We determine distribution-related fees based on a percentage of the product sales price, and we record the distribution fees as an allowance against accounts receivable.
Product Returns: We offer our distributors a right to return product purchased directly from us, which is principally based upon the product's expiration date. Our policy is to accept product returns during the six months prior to and twelve months after the product expiration date on product that had been sold to our distributors. Product return allowances are based on amounts owed or to be claimed on related sales. These estimates take into consideration the terms of our agreements with customers, historical product returns of VIBATIV experienced by Innoviva's former collaborative partner, Astellas,
100
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
rebates or discounts taken, estimated levels of inventory in the distribution channel, the shelf life of the product, and specific known market events, such as competitive pricing and new product introductions. We record our product return reserves as accrued other liabilities.
Allowance for Doubtful Accounts: We maintain a policy to record allowances for potentially doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. As of December 31, 2016 and 2015, there was no allowance for doubtful accounts related to customer payments.
Our reserve activity for sales allowances, discounts and chargebacks is summarized as follows:
(In thousands)
|
Balance at Beginning of Period |
Charges | Deductions | Balance at End of Period |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Year ended December 31, 2015: |
|||||||||||||
Sales allowances, discounts and chargebacks |
$ | 160 | $ | 3,049 | $ | (2,451 | ) | $ | 758 | ||||
Year ended December 31, 2016: |
|||||||||||||
Sales allowances, discounts and chargebacks |
$ | 758 | $ | 6,337 | $ | (6,316 | ) | $ | 779 | ||||
There were no material changes in reserve estimates relating to the prior periods.
Collaborative Arrangements and Multiple-Element Arrangements
Revenue from non-refundable, up-front license or technology access payments under license and collaborative arrangements that are not dependent on any future performance by us is recognized when such amounts are earned. If we have continuing obligations to perform under the arrangement, such fees are recognized over the estimated period of continuing performance obligation.
We account for multiple element arrangements, such as license and development agreements in which we may provide several deliverables, in accordance with Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") Subtopic 605-25, Multiple Element Arrangements. For new or materially amended multiple element arrangements, we identify the deliverables at the inception of the arrangement and each deliverable within a multiple deliverable revenue arrangement is accounted for as a separate unit of accounting if both of the following criteria are met: (1) the delivered item or items have value to the customer on a standalone basis and (2) for an arrangement that includes a general right of return relative to the delivered item(s), delivery or performance of the undelivered item(s) is considered probable and substantially in our control. We allocate revenue to each non-contingent element based on the relative selling price of each element. When applying the relative selling price method, we determine the selling price for each deliverable using vendor-specific objective evidence ("VSOE") of selling price, if it exists, or third-party evidence ("TPE") of selling price, if it exists. If neither VSOE nor TPE of selling price exist for a deliverable, we use the best estimated selling price for that deliverable. Revenue allocated to each element is then recognized based on when the basic four revenue recognition criteria are met for each element.
Where a portion of non-refundable upfront fees or other payments received are allocated to continuing performance obligations under the terms of a collaborative arrangement, they are recorded as deferred revenue and recognized as revenue or as an accrued liability and recognized as a reduction of R&D expenses ratably over the term of our estimated performance period under the agreement. We determine the estimated performance periods, and they are periodically reviewed based on the progress
101
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
of the related program. The effect of any change made to an estimated performance period and, therefore revenue recognized, would occur on a prospective basis in the period that the change was made.
Under certain collaborative arrangements, we have been reimbursed for a portion of our R&D expenses. These reimbursements have been reflected as a reduction of R&D expense in our consolidated statements of operations, as we do not consider performing research and development services to be a part of our ongoing and central operations. Therefore, the reimbursement of research and development services and any amounts allocated to our research and development services are recorded as a reduction of R&D expense.
Amounts deferred under a collaborative arrangement in which the performance obligations are terminated will result in an immediate recognition of any remaining deferred revenue and accrued liability in the period that termination occurred, provided that there are no remaining performance obligations.
We recognize revenue from milestone payments when (i) the milestone event is substantive and its achievability was not reasonably assured at the inception of the agreement and (ii) we do not have ongoing performance obligations related to the achievement of the milestone. Milestone payments are considered substantive if all of the following conditions are met: the milestone payment (a) is commensurate with either our performance to achieve the milestone or the enhancement of the value of the delivered item or items as a result of a specific outcome resulting from our performance to achieve the milestone, (b) relates solely to past performance, and (c) is reasonable relative to all of the deliverables and payment terms (including other potential milestone consideration) within the arrangement.
Research and Development Expenses
Research and development expenses are recorded in the period that services are rendered or goods are received. Research and development expenses consist of salaries and benefits, laboratory supplies and facility costs, as well as fees paid to third parties that conduct certain research and development activities on behalf of us, net of certain external research and development expenses reimbursed under our collaborative arrangements.
As part of the process of preparing financial statements, we are required to estimate and accrue research and development expenses. This process involves the following:
- •
- identifying services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for
the service when we have not yet been invoiced or otherwise notified of actual cost;
- •
- estimating and accruing expenses in our financial statements as of each balance sheet date based on facts and circumstances known to us at the
time; and
- •
- periodically confirming the accuracy of our estimates with selected service providers and making adjustments, if necessary.
102
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Examples of estimated research and development expenses that we accrue include:
- •
- fees paid to clinical research organizations ("CROs") in connection with preclinical and toxicology studies and clinical studies;
- •
- fees paid to investigative sites in connection with clinical studies;
- •
- fees paid to contract manufacturing organizations ("CMOs") in connection with the production of product and clinical study materials; and
- •
- professional service fees for consulting and related services.
We base our expense accruals related to clinical studies on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and CROs that conduct and manage clinical studies on our behalf. The financial terms of these agreements vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors, such as the successful enrollment of patients and the completion of clinical study milestones. Our service providers invoice us monthly in arrears for services performed. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates.
Advertising Expenses
We expense the costs of advertising, including promotional expenses, as incurred. Advertising expenses were $2.5 million, $4.0 million and $1.1 million in 2016, 2015 and 2014, respectively.
Fair Value of Share-Based Compensation Awards
We use the Black-Scholes-Merton option pricing model to estimate the fair value of options granted under our equity incentive plans and rights to acquire shares granted under our employee share purchase plan ("ESPP"). The Black-Scholes-Merton option valuation model requires the use of assumptions, including the expected term of the award and the expected share price volatility. We use the "simplified" method as described in Staff Accounting Bulletin No. 107, Share-Based Payment, to estimate the expected option term.
Share-based compensation expense is calculated based on awards ultimately expected to vest and is reduced for estimated forfeitures at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Our estimated annual forfeiture rates for options are based on historical forfeiture experience.
Compensation expense for purchases under the ESPP is recognized based on the fair value of the ordinary share on the date of offering, less the purchase discount percentage provided for in the plan.
103
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Net Loss per Share
Basic net loss per share is computed by dividing net loss by the weighted-average number of shares of outstanding, less ordinary shares subject to forfeiture. Diluted net loss per share is computed by dividing net loss by the weighted-average number of shares outstanding, less ordinary shares subject to forfeiture, plus all additional ordinary shares that would have been outstanding, assuming dilutive potential common shares had been issued for other dilutive securities.
For the years ended December 31, 2016, 2015 and 2014, diluted and basic net loss per share was identical since potential common shares were excluded from the calculation, as their effect was anti-dilutive. Prior to the Spin-Off in June 2014, we operated as part of Innoviva and not as a separate entity. As a result, the calculation of basic and diluted net loss per share assumes that the 32,260,105 ordinary shares issued to Innoviva stockholders in connection with the Spin-Off, less the number of ordinary shares subject to forfeiture, were outstanding from the beginning of 2014.
Anti-dilutive Securities
The following common equivalent shares were not included in the computation of diluted net loss per share because their effect was anti-dilutive:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | 2014 | |||||||
Share issuances under equity incentive plans and ESPP |
3,709 | 4,537 | 3,475 | |||||||
Restricted shares |
33 | 202 | 424 | |||||||
Share issuances upon the conversion of convertible senior notes |
6,676 | — | — | |||||||
| | | | | | | | | | | |
|
10,418 | 4,739 | 3,899 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
In addition, there were 1,440,000 shares that are subject to performance-based vesting criteria which have been excluded from the common equivalent shares table above for the year ended December 31, 2016.
Amortization of Debt Issuance Costs from Convertible Senior Notes due 2023
On November 2, 2016, we issued $230.0 million aggregate principal amount of 3.250% convertible senior notes due 2023 (the "Notes") for net proceeds of approximately $222.5 million, after deducting underwriting discounts and commissions and other estimated transaction expenses. We incurred approximately $7.5 million in transaction costs, which will be amortized to interest expense over the estimated life of the Notes based on the effective interest method.
Income Taxes
During 2015, we adopted FASB Accounting Standards Update 2015-17, Balance Sheet Classification of Deferred Income Taxes, which requires that the Consolidated Balance Sheets reflect all deferred income tax assets and liabilities as non-current. We elected to retrospectively apply the provisions of this standard, and the adoption had no impact on our consolidated financial position or results of operations.
104
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
We utilize the asset and liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax basis of assets and liabilities and are measured using enacted tax rates and laws that are anticipated to be in effect when the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
Our unrecognized tax benefits would reduce our effective income tax rate if recognized. As of December 31, 2016, we do not anticipate the total amount of unrecognized income tax benefits relating to uncertain tax positions existing at December 31, 2016 to significantly decrease in the next twelve months.
We assess all material positions, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. Assessing an uncertain tax position begins with the initial determination of the position's sustainability and is measured at the largest amount of benefit that is greater than 50% likely to be realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed, and we will determine whether the factors underlying the sustainability assertion have changed and whether the amount of the recognized tax benefit is still appropriate.
The recognition and measurement of tax benefits requires significant judgment. Judgments concerning the recognition and measurement of a tax benefit might change as new information becomes available.
Comprehensive Loss
Comprehensive loss is comprised of net loss and changes in unrealized gains and losses on our marketable securities.
Related Parties
GSK owned 18.3% of our shares outstanding as of December 31, 2016. On March 17, 2016, GSK purchased from us 1,301,015 of our ordinary shares for an aggregate purchase price of approximately $23.0 million pursuant to a Share Purchase Agreement between GSK and us dated March 14, 2016. The Share Purchase Agreement was entered into pursuant to Section 2.1(d)(ii) of the Governance Agreement between GSK and us dated March 3, 2014 (the "Governance Agreement"), which affords GSK, on a quarterly basis, the opportunity to purchase from us ordinary shares sufficient to maintain GSK's Percentage Interest (as defined in the Governance Agreement) at the same level as prior to any exercise of share options and vesting of restricted shares that occurred during the prior quarter, and pursuant to our approval to GSK to make additional purchases, which approval was required by Section 2.1(a) of the Governance Agreement.
Robert V. Gunderson, Jr. is a member of our board of directors. We have engaged Gunderson Dettmer Stough Villeneuve Franklin & Hachigian, LLP, of which Mr. Gunderson is a partner, as our primary legal counsel. Fees incurred were $1.1 million in each of the years ended December 31, 2016, 2015 and 2014.
105
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
Recently Issued Accounting Pronouncements Not Yet Adopted
In May 2014, the FASB issued Accounting Standards Update ("ASU") 2014-09, Revenue from Contracts with Customers (Topic 606) ("ASU 2014-09"), which will replace most existing revenue recognition guidance in GAAP when it becomes effective. ASU 2014-19's core principle is that a company will recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. ASU 2014-09 defines a five-step process to achieve this core principle and, in doing so, companies may need to use more judgment and make more estimates than under the currently effective guidance. These may include identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price, and allocating the transaction price to each separate performance obligation.
ASU 2014-09 was initially to be effective for interim and annual reporting periods beginning after December 15, 2016. In August 2015, the FASB issued ASU 2015-14 which delays the effective date of ASU 2014-09 by one year and allows for early adoption as of the original effective date. ASU 2014-09 can be adopted using either of two methods: (i) retrospective application of ASU 2014-09 to each prior reporting period presented with the option to elect certain practical expedients as defined within ASU 2014-09; or (ii) retrospective application of ASU 2014-09 with the cumulative effect of initially applying ASU 2014-09 recognized at the date of initial application and providing certain additional disclosures as defined per ASU 2014-09 (the "modified retrospective method").
In March 2016, the FASB issued ASU 2016-08 which clarifies certain principal versus agent considerations under Topic 606. In April 2016, the FASB issued ASU 2016-10 which clarifies Topic 606's implementation guidance on identifying performance obligations in a contract and determining whether an entity's promise to grant a license provides a customer with either a right to use the entity's intellectual property (which is satisfied at a point in time) or a right to access the entity's intellectual property (which is satisfied over time). In May 2016, the FASB issued ASU 2016-12 which amends the guidance on transition, collectability, noncash consideration and the presentation of sales and other similar taxes. ASU 2016-12 clarifies that, for a contract to be considered completed at transition, all (or substantially all) of the revenue must have been recognized under legacy GAAP. In addition, ASU 2016-12 clarifies how an entity should evaluate the collectability threshold and when an entity can recognize nonrefundable consideration received as revenue if an arrangement does not meet the standard's contract criteria. The effective dates of ASU 2016-08, ASU 2016-10, and ASU 2016-12 are the same as the new effective date of ASU 2014-09 which is for all interim and annual reporting periods beginning after December 15, 2017, and early adoption is permitted as of the original effective date of ASU 2014-09.
We expect to adopt ASU 2014-09 in the first quarter of 2018 using the modified retrospective method. The adoption of ASU 2014-09 may have a material effect on our financial statements. Since the Spin-Off, our revenues have been derived primarily from collaboration agreements. The consideration we are eligible to receive under these agreements includes upfront payments, research and development funding, milestone payments, and royalties. Each collaboration agreement is unique and will need to be assessed separately under the five-step process under the new standard.
ASU 2014-09 differs from the current accounting standard in many respects, such as in the accounting for variable consideration, including milestone payments. Under our current accounting policy, we recognize milestone revenue using the milestone method specified in ASC 605-28, which
106
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
generally results in the recognition of the milestone payment as revenue in the period that the milestone is achieved. However, under the new accounting standard, it is possible to start to recognize milestone revenue before the milestone is achieved, subject to management's assessment of whether it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
We also recognize revenues from product sales. We have not yet completed our final review of the impact of this guidance, although we currently do not anticipate a material impact on our revenue recognition practices for product sales. We continue to review variable consideration, potential disclosures, and our method of adoption to complete our evaluation of the impact on our consolidated financial statements. In addition, we continue to monitor additional changes, modifications, clarifications or interpretations undertaken by the FASB, which may impact our current conclusions.
In February 2016, the FASB issued ASU 2016-02, Leases ("ASU 2016-02"). ASU 2016-02 is aimed at making leasing activities more transparent and comparable, and requires substantially all leases be recognized by lessees on their balance sheet as a right-of-use asset and corresponding lease liability, including leases currently accounted for as operating leases. ASU 2016-02 is effective for all interim and annual reporting periods beginning after December 15, 2018 with early adoption permitted. We are currently evaluating the impact that the adoption of ASU 2016-02 will have on our consolidated financial statements and related disclosures.
In March 2016, the FASB issued ASU 2016-09, Compensation—Stock Compensation (Topic 718) ("ASU 2016-09"). ASU 2016-09 simplifies several aspects of the accounting for share-based payment transactions, including the income tax consequences, classification of awards as equity or liabilities, an option to recognize gross share compensation expense with actual forfeitures recognized as they occur, as well as certain classifications on the statement of cash flows. ASU 2016-09 is effective for all interim and annual reporting periods beginning after December 15, 2016 with early adoption permitted. We have evaluated the potential impact of ASU 2016-09, and we do not believe that the adoption of ASU 2016-09 will have a material impact on our consolidated financial statements and related disclosures.
In May 2016, the FASB issued ASU 2016-11, Revenue Recognition (Topic 605) and Derivatives and Hedging (Topic 815) ("ASU 2016-11"). With respect to Revenue Recognition (Topic 605), ASU 2016-11 rescinds various standards codified as part of Revenue Recognition (Topic 605) in relation to the future adoption of ASU 2014-09, Revenue from Contracts with Customers (Topic 606). These rescissions include changes to topics pertaining to revenue and expense recognition for freight services in process, accounting for shipping and handling fees and costs and accounting for consideration given by a vendor to a customer. ASU 2016-11 was effective immediately upon issuance and will be adopted when we adopt ASU 2014-09. We are currently evaluating the impact that the adoption of ASU 2016-11, specific to Topic 605, will have on our consolidated financial statements and related disclosures. We do not believe ASU 2016-11, specific to Topic 815, will have a material impact on our consolidated financial statements and related disclosures.
In October 2016, the FASB issued ASU 2016-16, Income Taxes (Topic 740) ("ASU 2016-16"). ASU 2016-16 requires immediate recognition of income tax consequences of intra-company asset transfers, other than inventory transfers. Existing GAAP prohibits recognition of income tax consequences of intra-company asset transfers whereby the seller defers any net tax effect and the buyer is prohibited from recognizing a deferred tax asset on the difference between the newly created
107
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
1. Description of Operations and Summary of Significant Accounting Policies (Continued)
tax basis of the asset in its tax jurisdiction and its financial statement carrying amount as reported in the consolidated financial statements. ASU 2016-16 specifically excludes from its scope intra-company inventory transfers whereby the recognition of tax consequences will take place when the inventory is sold to third parties. Two common examples of assets included in ASU 2016-16's scope are intellectual property and property, plant and equipment. ASU 2016-16 is effective for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years with early adoption is permitted. We are currently evaluating the effect ASU 2016-16 will have on our consolidated financial statements.
2. Collaborative Arrangements
Revenues from Collaborative Arrangements
We recognized revenue from our collaborative arrangements as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | 2014 | |||||||
Mylan |
$ | 15,102 | $ | 19,175 | $ | — | ||||
Takeda Pharmaceuticals |
15,075 | — | — | |||||||
Trek Therapeutics |
— | 8,216 | — | |||||||
Various VIBATIV collaborative partners |
368 | 5,327 | 7,270 | |||||||
Other |
500 | — | — | |||||||
| | | | | | | | | | | |
Total revenue from collaborative arrangements |
$ | 31,045 | $ | 32,718 | $ | 7,270 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Mylan
Development and Commercialization Agreement
In January 2015, Mylan and we established a strategic collaboration for the development and, subject to regulatory approval, commercialization of revefenacin (TD-4208), our investigational LAMA in development for the treatment of COPD. We entered into this collaboration to expand the breadth of our revefenacin development program and extend our commercial reach beyond the acute care setting where we currently market VIBATIV.
Under the Mylan Agreement, Mylan paid us an initial payment of $15.0 million in cash in the second quarter of 2015. Also, pursuant to an ordinary share purchase agreement entered into on January 30, 2015, Mylan Inc., a subsidiary of Mylan N.V., made a $30.0 million equity investment in us, buying 1,585,790 ordinary shares from us in early February 2015 in a private placement transaction at a price of approximately $18.918 per share, which represented a 10% premium over the volume weighted average price per share of our ordinary shares for the five trading days ending on January 30, 2015.
Under the Mylan Agreement, the significant deliverables were determined to be the license, development responsibilities and committee participation. We determined that the license represents a separate unit of accounting as the license, which includes rights to our underlying technologies for revefenacin, has standalone value because the rights conveyed permit Mylan to perform all efforts necessary to use our technologies to bring the compounds through development and, upon regulatory approval, commercialization. We based the best estimate of selling price for the license using a discounted cash flow approach. We determined that development responsibilities and committee
108
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Collaborative Arrangements (Continued)
participation represent separate units of accounting as Mylan could negotiate for and/or acquire each of these services from other third parties and we based the best estimates of the respective selling prices on the nature and timing of the services to be performed.
As payments are received from Mylan, they are allocated to the three units of accounting based on the relative selling price method. Amounts allocated to the license are recognized as collaborative revenue when delivered. Amounts allocated to the development responsibilities under the Mylan Agreement are recognized proportionately with the performance of the underlying services and accounted for as reductions to R&D expense. Amounts allocated to committee participation are recognized ratably over the estimated performance periods as revenue from collaborative arrangements.
In the first quarter of 2015, upfront payments totaling $19.2 million from Mylan were allocated to the license and committee participation based on the relative selling price method. The $19.2 million consists of the initial payment of $15.0 million in cash and the $4.2 million premium related to the equity investment, which represents the difference between the closing price on January 30, 2015 and the issued price of $18.918 per share.
For the year ended December 31, 2015, we recognized $19.2 million in revenue from collaborative arrangements related primarily to the license and technological know-how delivered in the first quarter of 2015, and we recorded reductions to R&D expense of $52.6 million representing reimbursements for our development responsibilities.
For the year ended December 31, 2016, we recognized $15.1 million in revenue, primarily related to the $15.0 million milestone payment received from Mylan for the achievement of 50% enrollment in the Phase 3 twelve-month safety study, and we recorded reductions to R&D expense of $83.5 million representing reimbursements for our development responsibilities.
As of December 31, 2016, we are eligible to receive from Mylan additional potential development, regulatory and sales milestone payments totaling up to $205.0 million in the aggregate, with $160.0 million associated with revefenacin monotherapy and $45.0 million for future potential combination products. Of the $160.0 million associated with monotherapy, $150.0 million relates to commercialization and $10.0 million relates to regulatory actions in the EU. Development and regulatory milestones are deemed to be substantive milestones and will be recognized as revenue in the period upon achievement of each respective milestone. Sales milestones are considered contingent payments and are not deemed to be substantive milestones due to the fact that the achievement of the event underlying the payment predominantly relates to Mylan's performance of future commercial activities.
Takeda Pharmaceuticals
License and Collaboration Agreement
In June 2016, we entered into a License and Collaboration Agreement with Millennium Pharmaceuticals, Inc. ("Millennium") (the "Takeda Agreement"), in order to establish a collaboration for the development and commercialization of TD-8954, a selective 5-HT4 receptor agonist. Prior to the Takeda Agreement, we developed TD-8954 for potential use in the treatment of gastrointestinal motility disorders, including short-term intravenous use for enteral feeding intolerance ("EFI") to achieve early nutritional adequacy in critically ill patients at high nutritional risk, an indication for which the compound received FDA Fast Track designation. Millennium is an indirect wholly-owned
109
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Collaborative Arrangements (Continued)
subsidiary of Takeda Pharmaceutical Company Limited (TSE: 4502) (collectively with Millennium, "Takeda"). Under the terms of the Takeda Agreement, Takeda will be responsible for worldwide development and commercialization of TD-8954. We received an upfront cash payment of $15.0 million and will be eligible to receive success based development, regulatory and sales milestone payments by Takeda. The first $110.0 million of potential milestones are associated with the development, regulatory and commercial launch milestones for EFI or other intravenously dosed indications. We will also be eligible to receive a tiered royalty on worldwide net sales by Takeda at percentage royalty rates ranging from low double-digits to mid-teens.
The transactions contemplated by the Takeda Agreement closed in September 2016, following the expiration of the required waiting period under the Hart-Scott-Rodino Antitrust Improvements Act ("HSR Act"). Upon closing and the subsequent transfer of the license, technical know-how and related pharmaceutical materials, we recognized $15.1 million in revenue for the year ended December 31, 2016.
Alfa Wassermann
Development and Collaboration Agreement
In connection with the Spin-Off, we were assigned the October 2012 development and collaboration agreement between Innoviva and Alfa Wasserman for velusetrag under which the parties agreed to collaborate in the execution of a two-part Phase 2 program to test the efficacy, safety and tolerability of velusetrag in the treatment of patients with gastroparesis (a medical condition consisting of a paresis (partial paralysis) of the stomach, resulting in food remaining in the stomach for a longer time than normal). Alfa Wassermann has an exclusive option to develop and commercialize velusetrag in the European Union, Russia, China, Mexico and certain other countries, while we retain full rights to velusetrag in the United States, Canada, Japan and certain other countries. We are entitled to receive funding for the Phase 2a study and most of the Phase 2b study. If Alfa Wassermann exercises its license option at the completion of the Phase 2 program, then we are entitled to receive a $10.0 million option fee. If velusetrag is successfully developed and commercialized, we are entitled to receive potential future contingent payments totaling up to $53.5 million, and royalties on net sales by Alfa Wassermann ranging from the low teens to 20%.
Reimbursement of R&D Costs
Under certain collaborative arrangements, we are entitled to reimbursement of certain R&D costs. Our policy is to account for the reimbursement payments by our collaboration partners as reductions to R&D expense.
110
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
2. Collaborative Arrangements (Continued)
The following table summarizes the reductions to R&D expenses related to the reimbursement payments:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | 2014 | |||||||
Mylan |
$ | 83,490 | $ | 52,551 | $ | — | ||||
Alfa Wassermann |
7,113 | 2,122 | 1,764 | |||||||
Other |
134 | 483 | 120 | |||||||
| | | | | | | | | | | |
Total reduction to R&D expense |
$ | 90,737 | $ | 55,156 | $ | 1,884 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
3. Segment Information
We operate in a single segment, which is the discovery (research), development and commercialization of human therapeutics. The following table summarizes total revenue by geographic region:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | 2014 | |||||||
U.S. |
$ | 33,179 | $ | 16,981 | $ | 4,231 | ||||
Europe |
15,211 | 21,354 | 7,456 | |||||||
Asia |
254 | 2,902 | — | |||||||
Other |
4 | 889 | 1 | |||||||
| | | | | | | | | | | |
Total revenue |
$ | 48,648 | $ | 42,126 | $ | 11,688 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The following table summarizes total revenue from each of our customers or collaboration partners who individually accounted for 10% or more of our total revenue (as a percentage of total revenues) during the most recent three years:
(% of total revenue)
|
Year Ended December 31, 2016 |
|||
|---|---|---|---|---|
Mylan |
31 | % | ||
Takeda |
31 | % | ||
(% of total revenue)
|
Year Ended December 31, 2015 |
|||
|---|---|---|---|---|
Mylan |
46 | % | ||
Trek Therapeutics |
20 | % | ||
(% of total revenue)
|
Year Ended December 31, 2014 |
|||
|---|---|---|---|---|
Clinigen |
43 | % | ||
R-Pharm |
19 | % | ||
111
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Available-for-Sale Securities and Fair Value Measurements
Available-for-Sale Securities
The following table summarizes the classification of the available-for-sale securities in our consolidated balance sheets:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | |||||
Cash equivalents |
$ | 323,602 | $ | 69,126 | |||
Short-term marketable securities |
156,387 | 59,727 | |||||
Long-term marketable securities |
91,565 | 42,860 | |||||
| | | | | | | | |
Total |
$ | 571,554 | $ | 171,713 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The estimated fair value of marketable securities is based on quoted market prices for these or similar investments that were based on prices obtained from a commercial pricing service. The fair value of our marketable securities classified within Level 2 is based upon observable inputs that may include benchmark yields, reported trades, broker/dealer quotes, issuer spreads, two-sided markets, benchmark securities, bids, offers and reference data including market research publications.
Available-for-sale securities are summarized below:
| |
|
December 31, 2016 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
|
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
||||||||||
U.S. government securities |
Level 1 | $ | 69,963 | $ | 39 | $ | (47 | ) | $ | 69,955 | |||||
U.S. government agency securities |
Level 2 | 60,783 | 9 | (45 | ) | 60,747 | |||||||||
Corporate notes |
Level 2 | 98,522 | 4 | (213 | ) | 98,313 | |||||||||
Commercial paper |
Level 2 | 18,937 | — | — | 18,937 | ||||||||||
| | | | | | | | | | | | | | | | |
Marketable securities |
248,205 | 52 | (305 | ) | 247,952 | ||||||||||
Money market funds |
Level 1 | 323,602 | — | — | 323,602 | ||||||||||
| | | | | | | | | | | | | | | | |
Total |
$ | 571,807 | $ | 52 | $ | (305 | ) | $ | 571,554 | ||||||
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| |
|
December 31, 2015 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
|
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
||||||||||
U.S. government securities |
Level 1 | $ | 47,068 | $ | 4 | $ | (29 | ) | $ | 47,043 | |||||
U.S. government agency securities |
Level 2 | 31,502 | — | (37 | ) | 31,465 | |||||||||
Corporate notes |
Level 2 | 19,098 | 2 | (11 | ) | 19,089 | |||||||||
Commercial paper |
Level 2 | 4,990 | — | — | 4,990 | ||||||||||
| | | | | | | | | | | | | | | | |
Marketable securities |
102,658 | 6 | (77 | ) | 102,587 | ||||||||||
Money market funds |
Level 1 | 69,126 | — | — | 69,126 | ||||||||||
| | | | | | | | | | | | | | | | |
Total |
$ | 171,784 | $ | 6 | $ | (77 | ) | $ | 171,713 | ||||||
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
112
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. Available-for-Sale Securities and Fair Value Measurements (Continued)
At December 31, 2016, all of the available-for-sale securities had contractual maturities within two years and the weighted average maturity of marketable securities was approximately 10 months. There were no transfers between Level 1 and Level 2 during the periods presented.
We do not intend to sell the investments that are in an unrealized loss position, and it is unlikely that we will be required to sell the investments before recovery of their amortized cost basis, which may be maturity. We have determined that the gross unrealized losses on our marketable securities at December 31, 2016 were temporary in nature. All marketable securities with unrealized losses at December 31, 2016 have been in a loss position for less than twelve months or the loss is not material.
There were no sales in 2016 and 2015. During 2014, we sold available-for-sale securities totaling $0.9 million, and the related realized gains and losses were not material.
5. Long-Term Debt
In November 2016, we completed an underwritten public offering of $230.0 million of 3.250% convertible senior notes, due 2023 (the "Notes") for net proceeds of approximately $222.5 million. We incurred approximately $7.5 million in debt issuance costs, which are being amortized to interest expense over the estimated life of the Notes. The Notes bear an annual interest rate of 3.250%, payable semi-annually in arrears, on November 1 and May 1 of each year, commencing on May 1, 2017.
The Notes are our senior unsecured obligations and rank senior in right of payment to any of our indebtedness that is expressly subordinated in right of payment to the notes; equal in right of payment to any of our indebtedness that is not so subordinated; effectively junior in right of payment to any of our secured indebtedness to the extent of the value of the assets securing such indebtedness; and structurally junior to all indebtedness and other liabilities (including trade payables) of our subsidiaries.
The Notes will mature on November 1, 2023 (the "Maturity Date"), unless earlier redeemed or repurchased by us or converted. Holders may convert their notes into ordinary shares at an initial conversion rate of 29.0276 shares for each $1,000 principal amount of Notes, which is equivalent to an initial conversion price of approximately $34.45 per share, subject to adjustment, in certain circumstances (including upon the occurrence of a fundamental change), at any time prior to the close of business on the second business day immediately preceding the Maturity Date. Upon the occurrence of a fundamental change involving the Company, holders of the Notes may require the Company to repurchase all or a portion of their Notes for cash at a redemption price equal to 100% of the principal amount of the Notes to be redeemed, plus accrued and unpaid interest to, but excluding, the fundamental change repurchase date. In addition, in some circumstances, the conversion rate of the Notes will increase with a make whole premium for conversions in connection with certain fundamental changes.
The debt issuance costs related to the Notes offering were capitalized as deferred financing costs and deducted from the carrying value of the financial liability on our consolidated balance sheet at December 31, 2016.
The estimated fair value of the Notes was $266.2 million at December 31, 2016 and was based upon observable inputs that may include benchmark yields, reported trades, broker/dealer quotes, issuer spreads, two-sided markets, benchmark securities, bids, offers and reference data including market research publications (Level 2).
113
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. Inventories
Inventory consists of the following:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | |||||
Raw materials |
$ | 6,067 | $ | 6,869 | |||
Work-in-process |
2,627 | — | |||||
Finished goods |
3,526 | 3,136 | |||||
| | | | | | | | |
Total inventories |
$ | 12,220 | $ | 10,005 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
As of December 31, 2016, the $2.6 million recorded as work-in-progress was comprised of VIBATIV lots in the manufacturing process. As of December 31, 2015, there were no VIBATIV lots in the manufacturing process.
In the fourth quarter of 2016, we discontinued the sale of VIBATIV 250 mg vials which resulted in a charge of $0.3 million for the write-down of excess inventory. In 2015 and 2014, we recorded charges of $1.9 million and $2.9 million, respectively, for the write-down of VIBATIV inventory due to the dating of the product. All inventory write-downs are recorded in cost of goods sold.
7. Property and Equipment
Property and equipment consists of the following:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | |||||
Computer equipment |
$ | 1,434 | $ | 1,434 | |||
Software |
3,432 | 3,776 | |||||
Furniture and fixtures |
3,657 | 3,656 | |||||
Laboratory equipment |
26,315 | 25,603 | |||||
Leasehold improvements |
17,866 | 17,639 | |||||
| | | | | | | | |
Subtotal |
52,704 | 52,108 | |||||
Less: accumulated depreciation |
(44,244 | ) | (42,235 | ) | |||
| | | | | | | | |
Property and equipment, net |
$ | 8,460 | $ | 9,873 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
For the years ended December 31, 2016, 2015 and 2014, depreciation expense for property and equipment was $2.2 million, $2.5 million and $2.7 million, respectively.
8. Share-Based Compensation
Theravance Biopharma Equity Plans
Upon the completion of the Spin-Off, we had two equity compensation plans—our 2013 Equity Incentive Plan (the "2013 EIP") and our 2013 Employee Share Purchase Plan (the "2013 ESPP"). At inception, we were authorized to issue 5,428,571 ordinary shares under the 2013 EIP and 857,142 ordinary shares under the 2013 ESPP. In October 2014, we adopted the 2014 New Employee Equity Incentive Plan (the "2014 NEEIP"). We are authorized to issue 750,000 ordinary shares under the 2014 NEEIP.
114
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Share-Based Compensation (Continued)
The 2013 EIP provides for the issuance of share-based awards, including restricted shares, restricted share units, options, share appreciation rights ("SARs") and other equity-based awards, to our employees, officers, directors and consultants. As of January 1 of each year, commencing on January 1, 2015 and ending on (and including) January 1, 2023, the aggregate number of ordinary shares that may be issued under the 2013 EIP shall automatically increase by a number equal to the least of 5% of the total number of ordinary shares outstanding on December 31 of the prior year, 3,428,571 ordinary shares, or a number of ordinary shares determined by our board of directors. Options may be granted with an exercise price not less than the fair market value of the ordinary shares on the grant date. Under the terms of our 2013 EIP, options granted to employees generally have a maximum term of 10 years and vest over a four-year period from the date of grant; 25% vest at the end of one year, and 75% vest monthly over the remaining three years. We may grant options with different vesting terms from time to time. Unless an employee's termination of service is due to disability or death, upon termination of service, any unexercised vested options will generally be forfeited at the end of three months or the expiration of the option, whichever is earlier.
Under the 2013 ESPP, our officers and employees may purchase ordinary shares through payroll deductions at a price equal to 85% of the lower of the fair market value of the ordinary share at the beginning of the offering period or at the end of each applicable purchase period. As of January 1 of each year, commencing on January 1, 2015 and ending on (and including) January 1, 2033, the aggregate number of ordinary shares that may be issued under the 2013 ESPP shall automatically increase by a number equal to the least of 1% of the total number of ordinary shares outstanding on December 31 of the prior year, 571,428 ordinary shares or a number of ordinary shares determined by our board of directors. The ESPP generally provides for consecutive and overlapping offering periods of 24 months in duration, with each offering period generally composed of four consecutive six-month purchase periods. The purchase periods end on either May 15 or November 15. ESPP contributions are limited to a maximum of 15% of an employee's eligible compensation.
Our 2013 ESPP also includes a feature that provides for the existing offering period to terminate and for participants in that offering period to automatically be enrolled in a new offering period when the fair market value of an ordinary share at the beginning of a subsequent offering period falls below the fair market value of an ordinary share on the first day of such offering period.
The 2014 NEEIP provides for the issuance of share-based awards, including restricted shares, restricted share units, non-qualified options and SARs, to our employees. Options may be granted with an exercise price not less than the fair market value of the ordinary shares on the grant date. Under the terms of our 2014 NEEIP, options granted to employees generally have a maximum term of 10 years and vest over a four-year period from the date of grant; 25% vest at the end of one year, and 75% vest monthly over the remaining three years. We may grant options with different vesting terms from time to time. Unless an employee's termination of service is due to disability or death, upon termination of service, any unexercised vested options will generally be forfeited at the end of three months or the expiration of the option, whichever is earlier.
Innoviva's Equity Plans
Many of our employees have in the past received Innoviva stock-based compensation awards, and, therefore, the following disclosures include information regarding stock-based compensation expense allocated to Theravance Biopharma that related to Innoviva stock-based equity awards. Accordingly, the
115
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Share-Based Compensation (Continued)
amounts presented are not necessarily indicative of future performance and do not necessarily reflect the results that we would have experienced as an independent, publicly-traded company for the periods presented.
At the time of the Spin-Off, Innoviva had one active stock-based incentive plan under which it granted stock-based awards to employees, officers and consultants, the 2012 Equity Incentive Plan. All outstanding stock options and restricted stock units ("RSUs") held by (1) Innoviva employees who became our employees, and (2) members of the board of directors of Innoviva who became members of our board of directors, in connection with the Spin-Off were adjusted for the Spin-Off. Such awards, along with outstanding restricted stock awards ("RSAs") held by Innoviva employees who became our employees in connection with the Spin-Off, will continue to vest and remain outstanding based on continuing employment or service with us.
The 2012 Equity Incentive Plan provides for the grant of incentive stock options, non-statutory stock options, restricted stock awards, stock unit awards and SARs to employees, non-employee directors and consultants. Stock options were granted with an exercise price not less than the fair market value of the common stock on the grant date. Stock options granted to employees generally have a maximum term of 10 years and vest over a four year period from the date of grant; 25% vest at the end of one year, and 75% vest monthly over the remaining three years. However, Innoviva granted options with different vesting terms from time to time. Unless an employee's termination of service is due to disability or death, upon termination of service, any unexercised vested options will be forfeited at the end of three months or the expiration of the option, whichever is earlier.
On June 2, 2014, Innoviva made a pro rata dividend distribution to its stockholders of record on May 15, 2014 of one ordinary share of Theravance Biopharma for every three and one half shares of Innoviva common stock outstanding on the record date. Innoviva's outstanding stock options and RSUs, which were not entitled to the dividend distribution were adjusted for the Spin-Off. Specifically, the number of shares and exercise price for Innoviva's outstanding stock options were adjusted and the number of shares underlying Innoviva's outstanding RSUs was adjusted. All other terms of these options and RSUs remained the same; provided, however, that the vesting and expiration of these grants are based on the holder's continuing employment or service with Innoviva or us, as applicable.
Although the anti-dilution adjustments were required pursuant to the terms of each equity plan, the anti-dilution adjustments were calculated using a volume-weighted average stock price, rather than the stock price as of the date of the dividend distribution, which resulted in incremental compensation expense. The accounting impact of the adjustment to the outstanding Innoviva stock options and RSUs that occurred in connection with the Spin-Off of Theravance Biopharma was measured by comparing the fair values of the modified stock options and RSUs to our employees and directors immediately before and after the adjustment.
Innoviva Performance-Contingent Restricted Stock Awards
Over the past three years, the Compensation Committee of Innoviva's board of directors ("Innoviva's Compensation Committee") has approved grants of performance-contingent RSAs to its senior management and a non-executive officer. Generally, these awards have dual triggers of vesting based upon the achievement of certain performance goals by a pre-specified date, as well as a requirement for continued employment. When the performance goals are deemed achieved for these types of awards, time-based vesting and, as a result, recognition of stock-based compensation expense
116
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Share-Based Compensation (Continued)
commence. Included in these performance-contingent RSAs is the grant of 1,290,000 special long-term retention and incentive performance-contingent RSAs to senior management in 2011. The awards had dual triggers of vesting based upon the achievement of certain performance conditions over a six-year time frame from 2011 through December 31, 2016 and require continued employment.
In May 2014, Innoviva's Compensation Committee determined that the requisite performance conditions for the first tranche of the awards were achieved and, as a result, $7.0 million in share-based compensation expense was recognized by us during the year ended December 31, 2014.
In May 2014, Innoviva's Compensation Committee approved the modification of the remaining tranches related to these awards contingent upon the Spin-Off. The modification acknowledged the Spin-Off and permitted recognition of achievement of the original performance conditions that were met prior to the Spin-Off, triggering twelve-month service-based vesting for a portion of the equity awards. Share-based compensation expense of $6.9 million associated with this portion of the awards after the modification was fully recognized as of June 30, 2015.
During the fourth quarter of 2014, we determined that it was probable that the performance conditions associated with the vesting of the remaining RSAs outstanding under these awards would be achieved. In addition, the remaining RSAs outstanding under these awards are entitled to the pro rata dividend distribution made by Innoviva on June 2, 2014 of one ordinary share of Theravance Biopharma for every three and one half shares of Innoviva common stock. As a result, for the years ended December 31, 2016 and 2015, we recognized $1.0 million and $7.1 million, respectively, of the total share-based compensation expense of $9.5 million related to these remaining RSAs and pro rata dividends.
Employee Share Option Exchange Program
On August 28, 2015, we gave eligible option holders of the Company and its subsidiaries the opportunity to exchange some or all of their outstanding options granted under our 2013 EIP or our NEEIP before August 4, 2015, whether vested or unvested, for restricted share units (the "Exchange Program"). The Exchange Program was designed to restore the intended employee retention and incentive value of our equity awards.
In accordance with the terms of the Exchange Program, employees who held options that had an exercise price above the market price of our ordinary shares at the offer expiration date were eligible to exchange two shares subject to eligible options for one RSU granted under the terms of our 2013 EIP. The RSUs granted under the Exchange Program will vest over a three or four year service period depending on the grant date of the original option exchanged. Our executive officers and members of our board of directors were not eligible to participate in the Exchange Program.
The Exchange Program closed on September 25, 2015 and we exchanged 1,975,009 outstanding options for 987,496 RSUs with a fair value of $12.43 per share. The exchange of options for RSUs is considered a modification to the terms of the original equity award. As such, the Exchange Program resulted in an incremental share-based compensation costs of $1.4 million to be recognized, concurrently with the unamortized original compensation costs of the exchanged option awards, ratably over the new vesting period of three years. For the years ended December 31, 2016 and 2015, we recognized $0.5 million and $0.1 million, respectively, of the $1.4 million in incremental share-based compensation costs.
117
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Share-Based Compensation (Continued)
Performance-Contingent Awards
In the first quarter of 2016, the Compensation Committee of our Board of Directors ("Compensation Committee") approved the grant of 1,575,000 performance-contingent RSAs and 135,000 performance contingent RSUs to senior management. These grants have dual triggers of vesting based upon the achievement of certain performance conditions over a five-year timeframe from 2016 to 2020 and continued employment, both of which must be satisfied in order for the awards to vest. As of December 31, 2016, there were 1,440,000 performance-contingent RSAs and 135,000 performance-contingent RSUs outstanding.
Expense associated with these awards may be recognized during the years 2016 to 2020 depending on the probability of meeting the performance conditions. Compensation expense relating to awards subject to performance conditions is recognized if it is considered probable that the performance goals will be achieved. The probability of achievement will be reassessed at each reporting period.
In August 2016, the Compensation Committee determined not to award credit for a performance condition that occurred in the second quarter of 2016, which for accounting purposes is treated as a modification of the vesting conditions of all outstanding awards. As a result of the modification, the vesting of the first tranche of the awards changed from probable of achievement to improbable. The vesting of the second and third tranches of the awards is still considered improbable of achievement. As a result of the modification, there is a new measurement date for the second and third tranches of the awards as of the modification date. While the total number of shares under the award did not change, the remeasurement of the awards results in a higher potential compensation charge for the awards because our share price had increased since the original measurement date. The revised maximum potential expense associated with the awards could be up to $38.9 million (allocated as $16.7 million for research and development expense and $22.2 million for selling, general and administrative expense) if all of the performance conditions are achieved. For the year ended December 31, 2016, we recognized $1.8 million in share-based compensation expense related to our assessment of the probability that the performance conditions associated with the first tranche of these awards was considered to be probable of vesting. As of December 31, 2016, we determined that the remaining second and third tranches were not probable of vesting and, as a result, no compensation expense related to these tranches has been recognized for the year.
Share-Based Compensation Expense
The allocation of share-based compensation expense included in the consolidated statements of operations was as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | 2014 | |||||||
Research and development |
$ | 20,202 | $ | 25,770 | $ | 21,191 | ||||
Selling, general and administrative |
20,967 | 28,280 | 22,043 | |||||||
| | | | | | | | | | | |
Total share-based compensation expense |
$ | 41,169 | $ | 54,050 | $ | 43,234 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
118
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Share-Based Compensation (Continued)
Share-based compensation expense included in the consolidated statements of operations by award type was as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | 2014 | |||||||
Transferred from parent |
$ | — | $ | — | $ | 17,043 | ||||
Innoviva equity: |
||||||||||
Options |
3,973 | 5,199 | 4,378 | |||||||
RSUs |
1,547 | 3,292 | 3,169 | |||||||
RSAs |
2,597 | 7,590 | 3,796 | |||||||
Performance RSAs |
1,005 | 11,166 | 4,490 | |||||||
Theravance Biopharma equity: |
||||||||||
Options |
7,591 | 14,063 | 9,404 | |||||||
RSUs |
20,946 | 10,471 | — | |||||||
Performance RSAs and RSUs |
1,808 | — | — | |||||||
ESPP |
1,702 | 2,269 | 954 | |||||||
| | | | | | | | | | | |
Total share-based compensation expense |
$ | 41,169 | $ | 54,050 | $ | 43,234 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Total share-based compensation expense capitalized to inventory was not material for any of the periods presented.
As of December 31, 2016, the unrecognized share-based compensation cost, net of expected forfeitures, and the estimated weighted-average amortization period, using the straight-line attribution method, was as follows:
(In thousands, except amortization period)
|
Unrecognized Compensation Cost |
Weighted-Average Amortization Period (Years) |
|||||
|---|---|---|---|---|---|---|---|
Innoviva equity: |
|||||||
Options |
$ | 3,310 | 1.1 | ||||
RSUs |
215 | 0.2 | |||||
RSAs |
1,287 | 1.5 | |||||
Performance RSAs |
1 | 0.1 | |||||
Theravance Biopharma equity: |
|||||||
Options |
14,596 | 2.5 | |||||
RSUs |
48,731 | 2.9 | |||||
Performance RSAs and RSUs(1) |
4,865 | 3.5 | |||||
ESPP |
1,102 | 1.0 | |||||
| | | | | | | | |
|
$ | 74,107 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
- (1)
- Represents unrecognized share-based compensation cost associated with tranche 1 of the Theravance Biopharma performance-contingent awards described above.
119
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Share-Based Compensation (Continued)
Compensation Awards
The following table summarizes option activity under the 2013 EIP and 2014 NEEIP for the years ended December 31, 2016 and 2015:
| |
Number of Shares Subject to Outstanding Options |
Weighted-Average Exercise Price of Outstanding Options |
|||||
|---|---|---|---|---|---|---|---|
Balance at June 2, 2014 |
— | $ | — | ||||
Granted |
4,235,059 | 24.75 | |||||
Forfeited |
(272,633 | ) | 25.01 | ||||
| | | | | | | | |
Outstanding at December 31, 2014 |
3,962,426 | $ | 24.73 | ||||
Granted |
750,775 | 14.26 | |||||
Forfeited |
(2,402,037 | ) | 23.05 | ||||
| | | | | | | | |
Outstanding at December 31, 2015 |
2,311,164 | $ | 23.07 | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Granted |
474,675 | 24.06 | |||||
Exercised |
(197,328 | ) | 22.18 | ||||
Forfeited |
(357,716 | ) | 19.83 | ||||
| | | | | | | | |
Outstanding at December 31, 2016 |
2,230,795 | $ | 23.88 | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
As of December 31, 2016, the aggregate intrinsic value of the options outstanding was $18.1 million and the aggregate intrinsic value of the options exercisable was $7.6 million. As of December 31, 2015, the aggregate intrinsic value of the options outstanding was $1.4 million and the aggregate intrinsic value of the options exercisable were not material. The total estimated fair value of options vested (excluding vested options that have expired) was $7.7 million in 2016 and $10.7 million in 2015.
The following table summarizes total RSU and RSA activity (including performance RSUs and RSAs) for the years ended December 31, 2016 and 2015:
| |
Number of Shares Subject to Outstanding RSUs |
Number of Shares Outstanding Subject to Performance Conditions (RSAs) |
|||||
|---|---|---|---|---|---|---|---|
Outstanding at December 31, 2014 |
— | — | |||||
Granted |
3,399,924 | — | |||||
Forfeited |
(411,883 | ) | — | ||||
| | | | | | | | |
Outstanding at December 31, 2015 |
2,988,041 | — | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Granted |
2,344,034 | 1,575,000 | |||||
Released |
(1,185,905 | ) | — | ||||
Forfeited |
(537,052 | ) | (135,000 | ) | |||
| | | | | | | | |
Outstanding at December 31, 2016 |
3,609,118 | 1,440,000 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
As of December 31, 2016, the aggregate intrinsic value of the RSUs and RSAs outstanding was $115.1 million and $45.9 million, respectively. The total estimated fair value of RSUs vested was
120
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. Share-Based Compensation (Continued)
$21.4 million in 2016. As of December 31, 2015, the aggregate intrinsic value of the RSUs outstanding was $49.0 million and the total estimated fair value of RSUs vested was $1.6 million in 2015.
Valuation Assumptions
The range of assumptions we used to estimate the fair value of options granted and rights granted under the 2013 ESPP was as follows:
| |
Year Ended December 31, | |||||
|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | |||
Options |
||||||
Risk-free interest rate |
1.1% - 1.9% | 1.4% - 1.9% | 1.7% - 2.0% | |||
Expected term (in years) |
6 | 6 | 5 - 6 | |||
Volatility |
53% - 73% | 71% - 78% | 64% - 70% | |||
Dividend yield |
— | — | — | |||
Weighted-average estimated fair value |
$13.28 | $9.16 | $15.55 | |||
2013 ESPP |
||||||
Risk-free interest rate |
0.4% - 1.0% | 0.1% - 0.9% | 0.1% - 0.7% | |||
Expected term (in years) |
0.5 - 2.0 | 0.5 - 2.0 | 0.6 - 2.2 | |||
Volatility |
54% - 65% | 46% - 62% | 58% - 66% | |||
Dividend yield |
— | — | — | |||
Weighted-average estimated fair value |
$9.63 | $5.91 | $10.95 | |||
The range of assumptions Innoviva used to estimate the fair value of stock options granted prior to the Spin-Off was as follows:
| |
Year Ended December 31, 2014 |
|
|---|---|---|
Options |
||
Risk-free interest rate |
1.6% - 2.1% | |
Expected term (in years) |
5 - 6 | |
Volatility |
52% - 61% | |
Dividend yield |
— | |
Weighted-average estimated fair value |
$16.14 |
9. Income Taxes
Theravance Biopharma was incorporated in the Cayman Islands in July 2013 under the name Theravance Biopharma, Inc. as a wholly-owned subsidiary of Innoviva and began operations subsequent to the Spin-Off with wholly-owned subsidiaries in the Cayman Islands, U.S., United Kingdom, and Ireland. Effective July 1, 2015, Theravance Biopharma became an Irish tax resident, therefore, the loss before income taxes of Theravance Biopharma, the parent company, are included in Ireland in the tables below.
121
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. Income Taxes (Continued)
The components of the loss before income taxes were as follows:
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | 2014 | |||||||
Income (loss) before provision for income taxes: |
||||||||||
Cayman Islands |
$ | (185,099 | ) | $ | (107,074 | ) | $ | (235,306 | ) | |
United States |
(18,441 | ) | (45,960 | ) | 5,189 | |||||
Ireland |
23,323 | (27,013 | ) | — | ||||||
Other |
(342 | ) | (1,221 | ) | (557 | ) | ||||
| | | | | | | | | | | |
Total |
$ | (180,559 | ) | $ | (181,268 | ) | $ | (230,674 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The components of provision for income taxes were as follows:
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | 2014 | |||||||
Provision for income taxes: |
||||||||||
Current: |
||||||||||
Cayman Islands |
$ | — | $ | — | $ | — | ||||
United States |
9,859 | 883 | 6,223 | |||||||
Ireland |
219 | 45 | — | |||||||
Other |
32 | 23 | 141 | |||||||
| | | | | | | | | | | |
Subtotal |
10,110 | 951 | 6,364 | |||||||
| | | | | | | | | | | |
Deferred |
— | — | — | |||||||
| | | | | | | | | | | |
Total |
$ | 10,110 | $ | 951 | $ | 6,364 | ||||
| | | | | | | | | | | |
Effective tax rate |
(5.60 | )% | (0.52 | )% | (2.76 | )% | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The provision for income taxes was $10.1 million, $1.0 million and $6.4 million in 2016, 2015 and 2014, respectively, although we incurred operating losses on a consolidated basis. In general, the provision for 2016 and 2015 resulted from recording contingent tax liabilities pertaining primarily to uncertain tax positions taken with respect to transfer pricing and tax credits.
No provision for income taxes has been recognized on undistributed earnings of our foreign subsidiaries because we consider such earnings to be indefinitely reinvested. In the event of a distribution of these earnings in the form of dividends or otherwise, we may be liable for income taxes, subject to an adjustment, if any, for foreign tax credits and foreign withholdings taxes payable to certain foreign tax authorities. As of December 31, 2016, there were no undistributed earnings.
122
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. Income Taxes (Continued)
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of our deferred tax assets and liabilities are as follows:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | |||||
Deferred tax assets: |
|||||||
Net operating loss carryforwards |
$ | 2,239 | $ | 4,541 | |||
Research and development tax credit carryforwards |
3,955 | 2,405 | |||||
Fixed assets and acquired intangibles |
6,839 | 7,275 | |||||
Share-based compensation |
13,208 | 10,895 | |||||
Accruals |
2,109 | 2,285 | |||||
Other |
476 | 21 | |||||
| | | | | | | | |
Subtotal |
28,826 | 27,422 | |||||
Valuation allowance |
(28,465 | ) | (26,822 | ) | |||
| | | | | | | | |
Total deferred tax assets |
361 | 600 | |||||
| | | | | | | | |
Deferred tax liabilities: |
|||||||
Prepaid assets |
(361 | ) | (600 | ) | |||
| | | | | | | | |
Total deferred tax liabilities |
(361 | ) | (600 | ) | |||
| | | | | | | | |
Net deferred tax assets/liabilities |
$ | — | $ | — | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
In the table below, the Cayman tax rate of 0% was used in 2014. For 2016 and 2015, as a result of the Company becoming an Irish tax resident effective July 1, 2015, the tax rate reflects the Irish statutory rate of 25%. The differences between the Ireland (2016 and 2015) and Cayman Islands (2014) federal statutory income tax rate and our effective tax rates are as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2016 | 2015 | 2014 | |||||||
Provision at statutory income tax rate |
25.00 | % | 25.00 | % | 0.00 | % | ||||
Foreign rate differential |
(23.11 | ) | (14.62 | ) | (0.99 | ) | ||||
Change in valuation allowance |
(0.89 | ) | (4.42 | ) | (2.42 | ) | ||||
Share-based compensation |
(0.27 | ) | (4.15 | ) | — | |||||
Non-deductible executive compensation |
(1.07 | ) | (1.09 | ) | — | |||||
Uncertain tax positions |
(8.55 | ) | (3.88 | ) | (0.25 | ) | ||||
Research and development tax credit carryforwards |
1.93 | 2.05 | 1.00 | |||||||
Other |
1.36 | 0.59 | (0.10 | ) | ||||||
| | | | | | | | | | | |
Effective tax rate |
(5.60 | )% | (0.52 | )% | (2.76 | )% | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Realization of deferred tax assets is dependent upon future taxable income in the respective jurisdictions, if any, the timing and the amount of which are uncertain. Accordingly, the deferred tax assets have been fully offset by a valuation allowance. The valuation allowance as of December 31, 2016 increased from $26.8 million (the valuation allowance as of December 31, 2015) to $28.5 million, primarily as a result of changes to temporary differences in share-based compensation and tax credit
123
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. Income Taxes (Continued)
carryforwards. Valuation allowances require an assessment of both positive and negative evidence when determining whether it is more likely than not deferred tax assets are recoverable. Such assessment is required on a jurisdiction-by-jurisdiction basis.
As of December 31, 2016, we had $13.9 million of U.S. federal net operating losses and $5.4 million U.S. federal research and development tax credit carryforwards which begin to expire in 2035. We had state net operating losses of $26.4 million which generally begin to expire in 2034, and state research and development credit carryforwards of $7.4 million to be carried forward indefinitely.
The net operating loss deferred tax asset balances as of December 31, 2016 do not include excess tax benefits from option exercises. Shareholders' equity and parent company deficit will be credited if and when such excess tax benefits are ultimately realized.
Utilization of net operating loss and tax credit carryforwards may be subject to an annual limitation due to ownership change limitations provided by the Internal Revenue Code and similar state provisions. Annual limitations may result in expiration of net operating loss and tax credit carryforwards before some or all of such amounts have been utilized.
Our policy is to recognize interest and/or penalties related to income tax matters in income tax expense. The amount of tax expense related to interest or penalties was immaterial for the years ended December 31, 2016 and 2015.
Uncertain Tax Positions
A reconciliation of the beginning and ending balances of the total amounts of unrecognized tax benefits are as follows:
(In thousands)
|
|
|||
|---|---|---|---|---|
Unrecognized tax benefits as of December 31, 2013 |
$ | 41 | ||
Gross increase in tax positions for prior years |
— | |||
Gross increase in tax positions for current year |
1,018 | |||
| | | | | |
Unrecognized tax benefits as of December 31, 2014 |
1,059 | |||
Gross increase in tax positions for prior years |
108 | |||
Gross increase in tax positions for current year |
8,031 | |||
| | | | | |
Unrecognized tax benefits as of December 31, 2015 |
9,198 | |||
| | | | | |
Gross increase in tax positions for prior years |
157 | |||
Gross increase in tax positions for current year |
13,899 | |||
| | | | | |
Unrecognized tax benefits as of December 31, 2016 |
$ | 23,254 | ||
| | | | | |
| | | | | |
| | | | | |
The total unrecognized tax benefits of $23.3 million and $9.2 million at December 31, 2016 and 2015, respectively, if recognized, would reduce the effective tax rate in the period of recognition. As of December 31, 2016, we do not believe that it is reasonably possible that our unrecognized tax benefit will significantly decrease in the next twelve months. We currently have a full valuation allowance against our deferred tax assets, which would impact the timing of the effective tax rate benefit should any of these uncertain positions be favorably settled in the future.
124
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
9. Income Taxes (Continued)
We are subject to taxation in Ireland, the U.S., and various other jurisdictions. The tax years 2015 and forward remain open to examination in Ireland, tax years 2013 and forward remain open to examination in the U.S., and the tax years 2012 and forward remain open to examination in other jurisdictions.
Our future income tax expense may be affected by such factors as changes in tax laws, our business, regulations, tax rates, interpretation of existing laws or regulations, the impact of accounting for share-based compensation, the impact of accounting for business combinations, our international organization, shifts in the amount of income before tax earned in the U.S. as compared with other regions in the world, and changes in overall levels of income before tax.
10. Commitments and Contingencies
Operating Leases and Subleases
We lease approximately 150,000 square feet of office and laboratory space in two buildings in South San Francisco, California, under a non-cancelable operating lease that ends in May 2020. We may extend the terms of this lease for two additional five-year periods. In addition, our Irish subsidiary leases approximately 1,000 square feet of office space in Dublin, Ireland. Future minimum lease payments under the leases, exclusive of executory costs, at December 31, 2016, are as follows:
(In thousands)
|
|
|||
|---|---|---|---|---|
Years ending December 31: |
||||
2017 |
$ | 6,121 | ||
2018 |
6,305 | |||
2019 |
6,494 | |||
2020 |
2,758 | |||
Thereafter |
— | |||
| | | | | |
Total |
$ | 21,678 | ||
| | | | | |
| | | | | |
| | | | | |
Rent expenses (net of sublease income) and sublease income associated with operating leases were as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
(In thousands)
|
2016 | 2015 | 2014 | |||||||
Rent expense, net |
$ | 6,865 | $ | 6,522 | $ | 6,616 | ||||
Sublease income |
$ | 244 | $ | 186 | $ | — | ||||
Special Long-Term Retention and Incentive Cash Awards Program
In 2011, Innoviva granted special long-term retention and incentive restricted stock awards to members of senior management. The awards had dual triggers of vesting based upon the achievement of certain performance conditions over a six-year time frame from 2011 through December 31, 2016 and continued employment.
In May 2014, Innoviva's Compensation Committee approved the modification of the remaining tranches related to these awards contingent upon the Spin-Off. The modification acknowledged the
125
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. Commitments and Contingencies (Continued)
Spin-Off and permitted recognition of achievement of the original performance conditions that were met prior to the Spin-Off, triggering 12-month service-based vesting for a portion of the equity awards. The share-based compensation expense of $6.9 million associated with a portion of these awards after the modification was fully recognized as of June 30, 2015.
During the fourth quarter of 2014, we determined that it was probable that the performance conditions associated with the remaining Innoviva RSAs would be achieved. In addition, the remaining RSAs outstanding are entitled to the pro rata dividend distribution made by Innoviva on June 2, 2014 of one ordinary share of Theravance Biopharma for every three and one half shares of Innoviva common stock. As a result, for the years ended December 31, 2016 and 2015, we recognized $1.0 million and $7.1 million, respectively, of the total share-based compensation expense of $9.5 million related to these remaining RSAs and pro rata dividends.
Guarantees and Indemnifications
We indemnify our officers and directors for certain events or occurrences, subject to certain limits. We believe the fair value of these indemnification agreements is minimal. Accordingly, we have not recognized any liabilities relating to these agreements as of December 31, 2016.
11. Spin-Off from Innoviva, Inc.
On June 1, 2014, Innoviva separated its late-stage respiratory assets partnered with GSK from its biopharmaceutical operations by transferring its discovery, development and commercialization operations (the "Biopharmaceutical Business") into its then wholly-owned subsidiary Theravance Biopharma. Innoviva also contributed certain assets and liabilities from the Biopharmaceutical Business and $393.0 million of cash, cash equivalents and marketable securities to us. On June 2, 2014, Innoviva made a pro rata dividend distribution to its stockholders of record on May 15, 2014 of one ordinary share of Theravance Biopharma for every three and one half shares of Innoviva common stock outstanding on the record date. The Spin-Off resulted in Theravance Biopharma operating as an independent, publicly-traded company.
The net book value of the net assets that were transferred to us in connection with the Spin-Off was as follows:
(In thousands)
|
June 2, 2014 | |||
|---|---|---|---|---|
Cash and cash equivalents |
$ | 277,541 | ||
Marketable investment securities |
115,129 | |||
Accounts receivable |
125 | |||
Reimbursement of certain liabilities |
16,983 | |||
Prepaid and other current assets |
3,172 | |||
Inventories |
14,328 | |||
Property and equipment, net |
9,580 | |||
Accrued liabilities |
(22,342 | ) | ||
Deferred revenue |
(6,694 | ) | ||
Other liabilities |
(4,944 | ) | ||
| | | | | |
Net book value of assets transferred |
$ | 402,878 | ||
| | | | | |
| | | | | |
| | | | | |
126
THERAVANCE BIOPHARMA, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
11. Spin-Off from Innoviva, Inc. (Continued)
In connection with the Spin-Off, Innoviva and Theravance Biopharma entered into various contractual agreements to govern matters such as tax, employee benefits, and transition services. Under the Transition Services Agreement, each party pays a monthly fee to the performing party related to a variety of administrative services. For the years ended December 31, 2016 and 2015, we billed Innoviva $0.1 million and $0.4 million, respectively, and Innoviva billed us $0.1 million and $0.5 million, respectively, under the Transition Services Agreement. As of December 31, 2016, we had no material receivables due from or payables due to Innoviva.
Limited Liability Company Agreement of Theravance Respiratory Company, LLC
Prior to the Spin-Off, Innoviva assigned to Theravance Respiratory Company, LLC ("TRC"), a Delaware limited liability company formed by Innoviva, its strategic alliance agreement with GSK and all of its rights and obligations under its collaboration agreement with GSK other than with respect to RELVAR® ELLIPTA®/BREO® ELLIPTA®, ANORO® ELLIPTA® and vilanterol monotherapy. Our equity interest in TRC entitles us to an 85% economic interest in any future payments made by GSK under the strategic alliance agreement and under the portion of the collaboration agreement assigned to TRC. The drug programs assigned to TRC include the Closed Triple or FF/UMEC/VI and the MABA program, as monotherapy and in combination with other therapeutically active components, such as an inhaled corticosteroid ("ICS"), and any other product or combination of products that may be discovered and developed in the future under the GSK agreements. Our economic interest will not include any payments associated with RELVAR® ELLIPTA®/BREO® ELLIPTA®, ANORO® ELLIPTA® or vilanterol monotherapy.
On May 31, 2014, we entered into the TRC LLC Agreement with Innoviva that governs the operation of TRC. Under the TRC LLC Agreement, Innoviva is the manager of TRC, and the business and affairs of TRC are managed exclusively by the manager, including (i) day to day management of the drug programs in accordance with the existing GSK agreements, (ii) preparing an annual operating plan for TRC and (iii) taking all actions necessary to ensure that the formation, structure and operation of TRC complies with applicable law and partner agreements.
We analyzed our ownership, contractual and other interests in TRC to determine if it is a variable-interest entity ("VIE"), whether we have a variable interest in TRC and the nature and extent of that interest. We determined that TRC is a VIE. The party with the controlling financial interest, the primary beneficiary, is required to consolidate the entity determined to be a VIE. Therefore, we also assessed whether we are the primary beneficiary of TRC based on the power to direct its activities that most significantly impact its economic performance and our obligation to absorb its losses or the right to receive benefits from it that could potentially be significant to TRC, and we determined that we are not the primary beneficiary of TRC. As a result, we do not consolidate TRC in our consolidated financial statements.
127
SUPPLEMENTARY FINANCIAL DATA (UNAUDITED)
(In thousands, except per share data)
The following table presents certain unaudited consolidated quarterly financial information for the eight quarters in the periods ended December 31, 2016 and 2015. This information has been prepared on the same basis as the audited consolidated financial statements and includes all adjustments (consisting only of normal recurring adjustments) necessary to present fairly the unaudited quarterly results of operations set forth herein.
| |
For the Quarters Ended | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
March 31 | June 30 | September 30 | December 31 | |||||||||
2016 |
|||||||||||||
Total revenue |
$ | 18,410 | $ | 5,471 | $ | 19,075 | $ | 5,692 | |||||
Costs and expenses |
60,052 | 52,968 | 52,569 | 63,526 | |||||||||
Loss from operations |
(41,642 | ) | (47,497 | ) | (33,494 | ) | (57,834 | ) | |||||
Net loss |
(42,150 | ) | (47,225 | ) | (33,962 | ) | (67,332 | ) | |||||
Basic and diluted net loss per share |
$ | (1.10 | ) | $ | (1.06 | ) | $ | (0.73 | ) | $ | (1.36 | ) | |
2015 |
|||||||||||||
Total revenue |
$ | 20,401 | $ | 7,134 | $ | 10,698 | $ | 3,893 | |||||
Costs and expenses |
58,138 | 52,427 | 53,793 | 59,667 | |||||||||
Loss from operations |
(37,737 | ) | (45,293 | ) | (43,095 | ) | (55,774 | ) | |||||
Net loss(1) |
(42,474 | ) | (47,603 | ) | (47,314 | ) | (44,828 | ) | |||||
Basic and diluted net loss per share |
$ | (1.29 | ) | $ | (1.42 | ) | $ | (1.40 | ) | $ | (1.23 | ) | |
- (1)
- In the fourth quarter of 2015, we recognized a $10.8 million reduction in our 2015 provision for income taxes primary due to changes in the Company's transfer pricing.
128
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures.
We conducted an evaluation required by paragraph (d) of Rule 13a-15 of the Exchange Act as of December 31, 2016, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined under Rule 13a- 15(e) of the Exchange Act), which are controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files under the Exchange Act is recorded, processed, summarized and reported within required time periods. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Management's Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) of the Exchange Act. In connection with the preparation of this Annual Report, our management, including our Chief Executive Officer and Chief Financial Officer, assessed the effectiveness of our internal control over financial reporting as of December 31, 2016 using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in its 2013 Internal Control—Integrated Framework. Based on its assessment, our management concluded that our internal control over financial reporting was effective as of December 31, 2016.
The effectiveness of our internal control over financial reporting as of December 31, 2016 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report which is included herein.
Limitations on the Effectiveness of Controls
Our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent all error and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefit of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within Theravance Biopharma have been detected. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Control over Financial Reporting
There was no change in our internal control over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act) identified in connection with the evaluation required by paragraph (d) of Rule 13a-15 of the Exchange Act, which occurred during the fourth quarter of the year ended December 31, 2016 which has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
129
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Theravance Biopharma, Inc.
We have audited Theravance Biopharma, Inc.'s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Theravance Biopharma, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Theravance Biopharma, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Theravance Biopharma, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of operations, comprehensive loss, shareholders' equity and parent company deficit and cash flows for each of the three years in the period ended December 31, 2016 of Theravance Biopharma, Inc. and our report dated March 1, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
San
Jose, California
March 1, 2017
130
None.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
For the information required by this Item, see "Questions and Answers About Procedural Matters", "Election of Directors", "Nominees", "Audit Committee", "Meetings of the Board of Directors", "Code of Conduct", "Executive Officers" and "Section 16(a) Beneficial Ownership Reporting Compliance" in the Proxy Statement to be filed with the SEC, which sections are incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
For the information required by this Item, see "Director Compensation", "Executive Compensation" and "Compensation Committee Interlocks and Insider Participation" in the Proxy Statement to be filed with the SEC, which sections are incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
For the information required by this Item, see "Security Ownership of Certain Beneficial Owners and Management" and "Equity Compensation Plan Information" in the Proxy Statement to be filed with the SEC, which sections are incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
For the information required by this Item, see "Director Independence" and "Policies and Procedures for Related Party Transactions" in the Proxy Statement to be filed with the SEC, which sections are incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
For the information required by this Item, see "Ratification of the Appointment of Independent Registered Public Accounting Firm" and "Pre-Approval of Audit and Non-Audit Services" in the Proxy Statement to be filed with the SEC, which sections are incorporated herein by reference.
131
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
- (a)
- The
following documents are filed as part of this Annual Report on Form 10-K:
- 1.
- Financial Statements:
The following financial statements and schedules of the Registrant are contained in Part II, Item 8, "Financial Statements and Supplementary Data" of this Annual Report on Form 10-K:
- 2.
- Financial Statement Schedules:
All schedules have been omitted because of the absence of conditions under which they are required or because the required information, where material, is shown in the financial statements, financial notes or supplementary financial information.
- (b)
- Exhibits required by Item 601 of Regulation S-K
The information required by this Item is set forth on the exhibit index that follows the signature page of this report.
132
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| THERAVANCE BIOPHARMA, INC. | ||||
Date: March 1, 2017 |
By: |
/s/ RICK E WINNINGHAM Rick E Winningham Chief Executive Officer |
||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Rick E Winningham and Renee D. Gala, each of whom may act without joinder of the other, as their true and lawful attorneys-in-fact and agents, each with full power of substitution and resubstitution, for such person and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to the annual report on Form 10-K, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ RICK E WINNINGHAM Rick E Winningham |
Chairman of the Board and Chief Executive Officer (Principal Executive Officer) | March 1, 2017 | ||
/s/ RENEE D. GALA Renee D. Gala |
Senior Vice President and Chief Financial Officer (Principal Financial Officer) |
March 1, 2017 |
||
/s/ ERAN BROSHY Eran Broshy |
Director |
March 1, 2017 |
||
/s/ HENRIETTA H. FORE Henrietta H. Fore |
Director |
March 1, 2017 |
133
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ ROBERT V. GUNDERSON, JR. Robert V. Gunderson, Jr. |
Director | March 1, 2017 | ||
/s/ DONAL O'CONNOR Donal O'Connor |
Director |
March 1, 2017 |
||
/s/ BURTON G. MALKIEL, PH.D. Burton G. Malkiel, Ph.D. |
Director |
March 1, 2017 |
||
/s/ DEAN J. MITCHELL Dean J. Mitchell |
Director |
March 1, 2017 |
||
/s/ SUSAN M. MOLINEAUX, PH.D Susan M. Molineaux, Ph.D. |
Director |
March 1, 2017 |
||
/s/ PETER S. RINGROSE, PH.D. Peter S. Ringrose, Ph.D. |
Director |
March 1, 2017 |
||
/s/ GEORGE M. WHITESIDES, PH.D. George M. Whitesides, Ph.D. |
Director |
March 1, 2017 |
||
/s/ WILLIAM D. YOUNG William D. Young |
Director |
March 1, 2017 |
134
| |
|
Incorporated by Reference | |||||
|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description | Form | Filing Date/Period End Date |
||||
| 2.1 | Separation and Distribution Agreement by and between Theravance Biopharma, Inc. and Innoviva, Inc., dated June 1, 2014. | 8-K | June 3, 2014 | ||||
3.1 |
Amended and Restated Memorandum and Articles of Association |
10-12B |
April 30, 2014 |
||||
4.1 |
Specimen Share Certificate |
10-12B |
April 30, 2014 |
||||
4.2 |
Registration Rights Agreement, dated March 3, 2014 |
10-12B |
April 8, 2014 |
||||
4.3 |
Form of Rights Agreement by and between Theravance Biopharma, Inc. and Computershare Inc. |
10-12B |
April 8, 2014 |
||||
4.4 |
First Amendment to Rights Agreement by and between Theravance Biopharma, Inc. and Computershare Inc., dated November 10, 2015 |
8-K |
November 10, 2015 |
||||
4.5 |
Controlled Equity OfferingSM Sales Agreement, dated June 26, 2015, by and between Theravance Biopharma, Inc. and Cantor Fitzgerald & Co. |
S-3 |
June 26, 2015 |
||||
4.6 |
Indenture, dated as of November 2, 2016, between Theravance Biopharma, Inc. and Wells Fargo Bank, National Association, as trustee |
8-K |
November 2, 2016 |
||||
4.7 |
First Supplemental Indenture, dated as of November 2, 2016, between Theravance Biopharma, Inc. and Wells Fargo Bank, National Association, as trustee |
8-K |
November 2, 2016 |
||||
4.8 |
Form of 3.25% Convertible Senior Note due 2023 (included in Exhibit 4.7) |
8-K |
November 2, 2016 |
||||
10.1 |
Transition Services Agreement by and between Theravance Biopharma, Inc. and Innoviva, Inc., dated June 2, 2014. |
8-K |
June 3, 2014 |
||||
10.2 |
Tax Matters Agreement by and between Theravance Biopharma, Inc. and Innoviva, Inc., dated June 2, 2014. |
8-K |
June 3, 2014 |
||||
10.3 |
Employee Matters Agreement by and between Theravance Biopharma, Inc. and Innoviva, Inc., dated June 1, 2014. |
8-K |
June 3, 2014 |
||||
10.4 |
+ |
2013 Equity Incentive Plan |
S-8 |
August 18, 2014 |
|||
10.5 |
+ |
UK Addendum to the 2013 Equity Incentive Plan |
10-Q |
August 14, 2014 |
|||
10.6 |
+ |
Forms of award agreements under the 2013 Equity Incentive Plan and 2014 New Employee Equity Incentive Plan |
10-Q |
May 10, 2016 |
|||
10.7 |
+ |
2014 New Employee Equity Incentive Plan |
S-8 |
November 14, 2014 |
|||
10.8 |
+ |
2013 Employee Share Purchase Plan, as amended |
S-8 |
Aug. 18, 2014 |
|||
10.9 |
+ |
Change in Control Severance Plan |
10-12B |
April 8, 2014 |
|||
10.10 |
+ |
Cash Bonus Program |
10-12B |
November 22, 2013 |
|||
10.11 |
+ |
Form of Indemnity Agreement |
10-12B |
April 30, 2014 |
|||
| |
|
Incorporated by Reference | |||||
|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description | Form | Filing Date/Period End Date |
||||
| 10.12 | Amended and Restated Lease Agreement, 951 Gateway Boulevard, between Innoviva, Inc. and HMS Gateway Office L.P., dated January 1, 2001 | 10-12B | August 8, 2013 | ||||
10.13 |
First Amendment to Lease for 951 Gateway Boulevard effective as of June 1, 2010 between Innoviva, Inc. and ARE-901/951 Gateway Boulevard, LLC |
10-12B |
August 8, 2013 |
||||
10.14 |
Lease Agreement, 901 Gateway Boulevard, between Innoviva, Inc. and HMS Gateway Office L.P., dated January 1, 2001 |
10-12B |
August 8, 2013 |
||||
10.15 |
First Amendment to Lease for 901 Gateway Boulevard effective as of June 1, 2010 between Innoviva, Inc. and ARE-901/951 Gateway Boulevard, LLC |
10-12B |
August 8, 2013 |
||||
10.16 |
Consent to Assignment by and among ARE-901/951 Gateway Boulevard, LLC, Innoviva, Inc. and Theravance Biopharma, Inc. and Assignment and Assumption of Lease for 901 Gateway Blvd. |
10-Q |
August 14, 2014 |
||||
10.17 |
Consent to Assignment by and among ARE-901/951 Gateway Boulevard, LLC, Innoviva, Inc. and Theravance Biopharma, Inc. and Assignment and Assumption of Lease for 951 Gateway Blvd. |
10-Q |
August 14, 2014 |
||||
10.18 |
Theravance Respiratory Company, LLC Limited Liability Company Agreement, dated May 31, 2014. |
8-K |
June 3, 2014 |
||||
10.19 |
* |
Technology Transfer and Supply Agreement, dated as of May 22, 2012 between Innoviva, Inc. and Hospira Worldwide, Inc. |
10-12B |
May 7, 2014 |
|||
10.20 |
* |
First Amendment to the Technology Transfer and Supply Agreement by and between Theravance, Inc. and Hospira Worldwide, Inc., dated May 16, 2013 |
10-Q |
November 9, 2016 |
|||
10.21 |
* |
Second Amendment to the Technology Transfer and Supply Agreement by and between Theravance Biopharma Antibiotics, Inc. and Hospira Worldwide, Inc., dated October 17, 2014 |
10-Q |
November 9, 2016 |
|||
10.22 |
* |
Third Amendment to the Technology Transfer and Supply Agreement by and between Theravance Biopharma Ireland Limited and Hospira Worldwide, Inc., dated April 14, 2016 |
10-Q |
November 9, 2016 |
|||
10.23 |
* |
Fourth Amendment to the Technology Transfer and Supply Agreement by and between Theravance Biopharma Ireland Limited and Pfizer CentreOne group of Pfizer, Inc., dated September 29, 2016 |
10-Q |
November 9, 2016 |
|||
10.24 |
* |
Commercialization Agreement between Innoviva, Inc. and Clinigen Group plc, dated March 8, 2013 |
10-12B |
May 7, 2014 |
|||
| |
|
Incorporated by Reference | |||||
|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description | Form | Filing Date/Period End Date |
||||
| 10.25 | Amendment No. 1 to the License, Development, and Commercialization Agreement by and between Theravance Biopharma Ireland Limited and Clinigen Group PLC dated August 4, 2016 | 10-Q | August 9, 2016 | ||||
10.26 |
License Agreement with Janssen Pharmaceutical, dated as of May 14, 2002 |
10-Q |
August 14, 2014 |
||||
10.27 |
Collaboration Agreement between Innoviva, Inc. and Glaxo Group Limited, dated November 14, 2002(1) |
||||||
10.28 |
Strategic Alliance Agreement by and between Innoviva, Inc. and Glaxo Group Limited, dated March 30, 2004(2) |
||||||
10.29 |
Amendment to Strategic Alliance Agreement by and between Innoviva, Inc. and Glaxo Group Limited, dated October 3, 2011(3) |
||||||
10.30 |
Collaboration Agreement Amendment by and between Innoviva, Inc. and Glaxo Group Limited dated, March 3, 2014(4) |
||||||
10.31 |
Strategic Alliance Agreement Amendment by and between Innoviva, Inc. and Glaxo Group Limited dated, March 3, 2014(4) |
||||||
10.32 |
Master Agreement by and between Innoviva, Inc., Theravance Biopharma, Inc. and Glaxo Group Limited, dated March 3, 2014(4) |
||||||
10.33 |
Extension Agreement by and between the Company and Glaxo Group Limited, dated March 3, 2014 |
10-12B |
April 8, 2014 |
||||
10.34 |
Governance Agreement by and between Theravance Biopharma, Inc. and Glaxo Group Limited, dated March 3, 2014 |
10-12B |
April 8, 2014 |
||||
10.35 |
+ |
Amended Offer Letter with Rick E Winningham dated August 6, 2014 |
10-Q |
September 30, 2014 |
|||
10.36 |
+ |
Offer Letter with Frank Pasqualone May 12, 2014 |
10-Q |
August 14, 2014 |
|||
10.37 |
+ |
Offer Letter with Brett K. Haumann dated May 12, 2014 |
10-Q |
August 14, 2014 |
|||
10.38 |
+ |
Offer Letter with Renee D. Gala dated May 12, 2014 |
10-Q |
September. 30, 2014 |
|||
10.39 |
+ |
Offer Letter with Junning Lee dated August 20, 2014 |
10-Q |
September 30, 2014 |
|||
10.40 |
+ |
Offer Letter with Brad Shafer dated August 20, 2014 |
10-Q |
September 30, 2014 |
|||
10.41 |
+ |
Offer Letter with Leonard Blum dated September 15, 2014 |
10-Q |
September 30, 2014 |
|||
10.42 |
+ |
Offer Letter with Mathai Mammen dated September 15, 2014 |
10-Q |
September 30, 2014 |
|||
10.43 |
+ |
Offer Letter with Sharath Hegde May 12, 2014 |
10-Q |
May 10, 2016 |
|||
10.44 |
+ |
Offer Letter with Ken Pitzer September 15, 2014 |
10-Q |
May 10, 2016 |
|||
10.45 |
+ |
Offer Letter with Phil Worboys September 9, 2014 |
10-Q |
May 10, 2016 |
|||
| |
|
Incorporated by Reference | |||||
|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description | Form | Filing Date/Period End Date |
||||
| 10.46 | + | Forms of Equity Award Amendment | 10-12B | May 7, 2014 | |||
10.47 |
+ |
Form of TFIO Cash Award Amendment |
10-12B |
May 7, 2014 |
|||
10.48 |
+ |
Consulting Agreement with Jeff Jonker, effective as of November 14, 2014 |
10-K |
March 13, 2015 |
|||
10.49 |
* |
Development and Commercialization Agreement by and between Theravance Biopharma R&D, Inc. and Mylan Ireland Limited, dated January 30, 2015 |
8-K/A |
April 24, 2015 |
|||
10.50 |
Ordinary Share Purchase Agreement by and between Theravance Biopharma, Inc. and Mylan Inc., dated January 30, 2015 |
8-K/A |
April 24, 2015 |
||||
10.51 |
Letter Agreement by and between Theravance Biopharma, Inc. and Glaxo Group Limited, dated September 11, 2015, including the form of Ordinary Share Purchase Agreement and Schedule as attached thereto |
8-K |
September 11, 2015 |
||||
10.52 |
Ordinary Share Purchase Agreement by and between Theravance Biopharma, Inc. and Glaxo Group Limited, dated October 7, 2015 |
8-K |
October 13, 2015 |
||||
10.53 |
Ordinary Share Purchase Agreement by and among Theravance Biopharma, Inc. and the funds managed by Woodford Investment Management LLP named therein, dated as of October 26, 2015 |
8-K |
October 26, 2015 |
||||
10.54 |
Ordinary Share Purchase Agreement by and between Theravance Biopharma, Inc. and Glaxo Group Limited, dated March 14, 2016 |
10-Q |
May 10, 2016 |
||||
10.55 |
* |
License and Collaboration Agreement by and between Theravance Biopharma Ireland Limited and Millennium Pharmaceuticals, Inc. dated June 8, 2016 |
10-Q |
August 9, 2016 |
|||
10.56 |
+ |
Form of Acknowledgment for Irish Non-Employee Directors |
10-K |
March 11, 2016 |
|||
10.57 |
+ |
Irish Addendum to the 2013 Equity Incentive Plan |
10-K |
March 11, 2016 |
|||
10.58 |
+ |
Irish Addendum to the 2014 New Employee Equity Incentive Plan |
10-K |
March 11, 2016 |
|||
10.59 |
+ |
UK and Irish Addendums to the 2013 Employee Share Purchase Plan |
10-K |
March 11, 2016 |
|||
10.60 |
+ |
Theravance Biopharma, Inc. Performance Incentive Plan |
8-K |
May 6, 2016 |
|||
21.1 |
Subsidiaries of Theravance Biopharma, Inc. |
||||||
23.1 |
Consent of Independent Registered Public Accounting Firm |
||||||
24.1 |
Power of Attorney (see signature page to this Annual Report on Form 10-K) |
||||||
31.1 |
Certification of Chief Executive Officer Pursuant to Rule 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934 |
||||||
| |
|
Incorporated by Reference | |||||
|---|---|---|---|---|---|---|---|
| Exhibit Number |
Description | Form | Filing Date/Period End Date |
||||
| 31.2 | Certification of Chief Financial Officer Pursuant to Rule 13a-14(a) and 15d-14(a) under the Securities Exchange Act of 1934 | ||||||
32 |
Certifications Pursuant to 18 U.S.C. Section 1350 |
||||||
101 |
The following materials from Registrant's Annual Report on Form 10-K for the year ended December 31, 2016, formatted in Extensible Business Reporting Language (XBRL) includes: (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Operations, (iii) Consolidated Statements of Comprehensive Loss, (iv) Consolidated Statements of Shareholders' Equity and Parent Company Deficit, (v) Consolidated Statements of Cash Flows, and (vi) Notes to Consolidated Financial Statements. |
||||||
- +
- Management
contract or compensatory plan or arrangement required to be filed pursuant to Item 15(b) of Form 10-K.
- *
- Confidential
treatment has been requested for certain portions which are omitted in the copy of the exhibit electronically filed with the Securities and Exchange
Commission. The omitted information has been filed separately with the Securities and Exchange Commission pursuant to Theravance Biopharma, Inc.'s application for confidential treatment.
- (1)
- Incorporated
by reference to an exhibit filed with the quarterly report on Form 10-Q of Innoviva, Inc., filed with the Securities and Exchange
Commission on August 8, 2014.
- (2)
- Incorporated
by reference to an exhibit filed with the annual report on Form 10-K of Innoviva, Inc., filed with the Commission on March 3, 2014.
- (3)
- Incorporated
by reference to an exhibit filed with the annual report on Form 10-K of Innoviva, Inc., filed with the Commission on February 27,
2012.
- (4)
- Incorporated by reference to an exhibit filed with the current report on Form 8-K/A of Innoviva, Inc., filed with the Commission on March 6, 2014.
