Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - HUMANIGEN, INC | ex99_1.htm |
| 8-K - HUMANIGEN, INC | g832118k.htm |
Exhibit 99.2

Confidential LIVE - AIR Phase 3 Study Update: Highest Response Rates Observed in Black and African - American Patients August 2021

2 Cautionary Note Regarding Forward - Looking Statements All statements other than statements of historical facts contained in this presentation are forward - looking statements . Forward - looking statements reflect management's current knowledge, assumptions, judgment, and expectations regarding future performance or events . Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct, and you should be aware that actual events or results may differ materially from those contained in the forward - looking statements . Words such as "will," "expect," "intend," "plan," "potential," "possible," "goals," "accelerate," "continue," and similar expressions identify forward - looking statements, including, without limitation, statements regarding the effectiveness of lenzilumab in Black and African - American patients ; the potential role of lenzilumab in the treatment of COVID - 19 ; the review of our submission for emergency use authorization by the FDA ; and our other plans to explore the effectiveness of lenzilumab and other candidates in our development portfolio as therapies for other inflammation and immune - oncology indications . Forward - looking statements are subject to a number of risks and uncertainties including, but not limited to, the risks inherent in our lack of profitability and need for additional capital to grow our business ; our dependence on partners to further the development of our product candidates ; the uncertainties inherent in the development, attainment of the requisite regulatory authorizations and approvals and launch of any new pharmaceutical product ; the outcome of pending or future litigation ; and the various risks and uncertainties described in the "Risk Factors" sections of our latest annual and quarterly reports and other filings with the SEC . All forward - looking statements are expressly qualified in their entirety by this cautionary notice . You should not rely upon any forward - looking statements as predictions of future events . The Company undertakes no obligation to revise or update any forward - looking statements made in this filing to reflect events or circumstances after the date hereof or to reflect new information or the occurrence of unanticipated events, except as required by law .
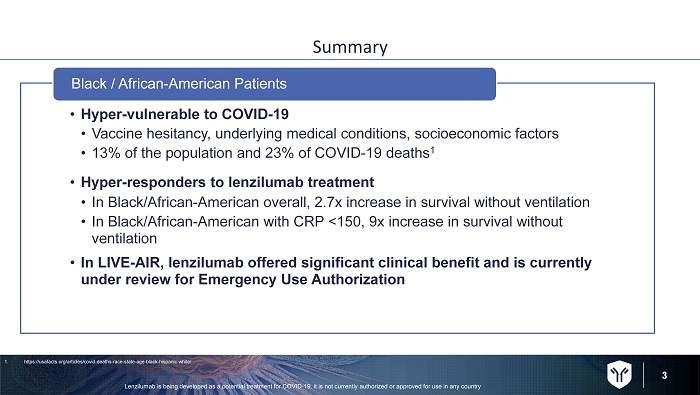
3 Summary • Hyper - vulnerable to COVID - 19 • Vaccine hesitancy, underlying medical conditions, socioeconomic factors • 13% of the population and 23% of COVID - 19 deaths 1 • Hyper - responders to lenzilumab treatment • In Black/African - American overall, 2.7x increase in survival without ventilation • In Black/African - American with CRP <150, 9x increase in survival without ventilation • In LIVE - AIR, lenzilumab offered significant clinical benefit and is currently under review for Emergency Use Authorization Black / African - American Patients Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently authorized or approved for use in any c ountry 1. https://usafacts.org/articles/covid - deaths - race - state - age - black - hispanic - white/
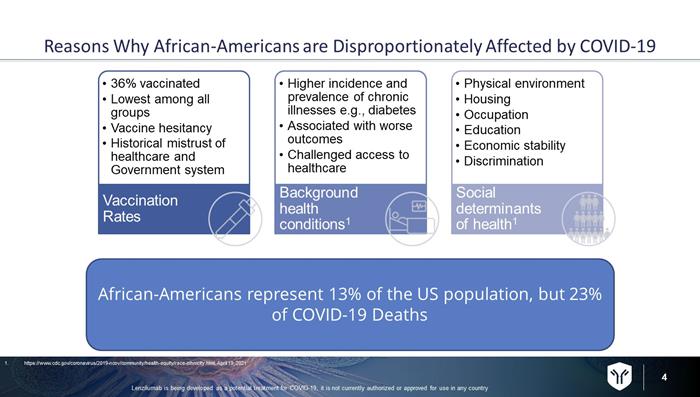
4 Reasons Why African - Americans are Disproportionately Affected by COVID - 19 • 36% vaccinated • Lowest among all groups • Vaccine hesitancy • Historical mistrust of healthcare and Government system Vaccination Rates • Higher incidence and prevalence of chronic illnesses e.g., diabetes • Associated with worse outcomes • Challenged access to healthcare Background health conditions 1 • Physical environment • Housing • Occupation • Education • Economic stability • Discrimination Social determinants of health 1 African - Americans represent 13% of the US population, but 23% of COVID - 19 Deaths 1. https://www.cdc.gov/coronavirus/2019 - ncov/community/health - equity/race - ethnicity.html, April 19, 2021 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently authorized or approved for use in any c ountry

5 Black American Vaccination Lowest by Race/Ethnicity 1 1. Kaiser Family Foundation, https://www.kff.org/coronavirus - covid - 19/issue - brief/latest - data - on - covid - 19 - vaccinations - race - ethnici ty/, July 21, 2021 Percent of total Population with at Least One COVID - 19 Vaccine Dose by Race/Ethnicity, 3 - 1 - 21 to 7 - 19 - 21

6 LIVE - AIR: Lenzilumab Phase 3 Randomized , Double - blind, Placebo - controlled Study Design Early intervention with lenzilumab, in addition to current standard of care, to prevent and treat CS and prevent progression to IMV and/or death • Randomized , double - blind, placebo - controlled, multi - center pivotal trial NCT 04351152 • All subjects received other treatment, which may have included corticosteroids and/or remdesivir Primary Endpoint: ▪ Ventilator - free survival/Survival without ventilation Key Secondary Endpoints: ▪ Ventilator - free days ▪ Duration of ICU stay ▪ Survival ▪ Time to recovery • Adults ≥ 18 years • Hospitalized • Confirmed SARS - CoV - 2 COVID - 19 pneumonia • SpO 2 ≤ 94% and pre - IMV Follow - up Assessment Day 60 Enrollment Screening Randomization Lenzilumab, 600 mg IV Q8H x3 Day 1 Placebo IV Q8H x3 Treatment Phase Follow - up Phase Daily assessments while hospitalized Endpoint Assessment Through Day 28 Evaluated through Day 28 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently authorized or approved for use in any c ountry
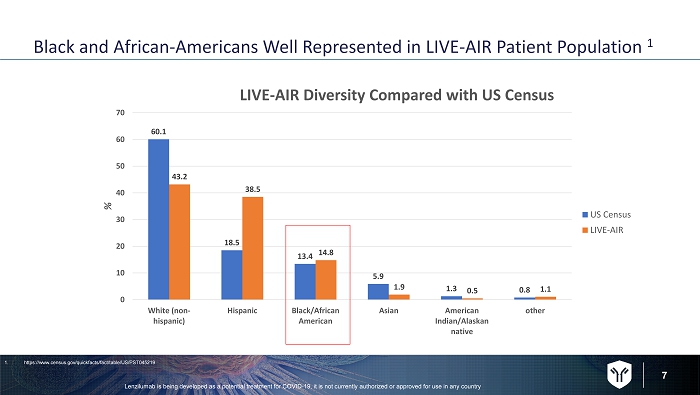
7 Black and African - Americans Well Represented in LIVE - AIR Patient Population 1 60.1 18.5 13.4 5.9 1.3 0.8 43.2 38.5 14.8 1.9 0.5 1.1 0 10 20 30 40 50 60 70 White (non- hispanic) Hispanic Black/African American Asian American Indian/Alaskan native other % LIVE - AIR Diversity Compared with US Census US Census LIVE-AIR 1. https://www.census.gov/quickfacts/fact/table/US/PST045219 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently authorized or approved for use in any c ountry

8 Primary Endpoint met: Survival without Ventilation (“SWOV”) 8 Patients who died or required ventilation K - M Estimate Population Lenzilumab Placebo Lenzilumab vs placebo hazard ratio (95% CI) P value mITT (n=479) 15.6% (11.5 – 20.9) (n=236) 22.1% (17.4 – 27.9) (n=243) 1.54 (1.02 – 2.32) 0.0403 Treatment with lenzilumab resulted in a 1.54x Increase in the likelihood of surviving and without needing to be on mechanical ventilation Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently authorized or approved for use in any c ountry
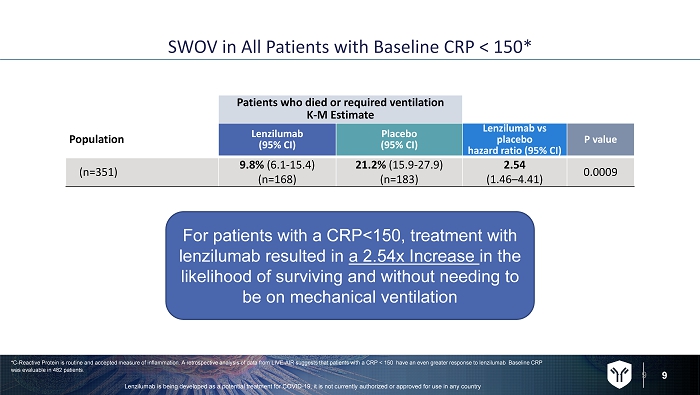
9 SWOV in All Patients with Baseline CRP < 150* 9 Patients who died or required ventilation K - M Estimate Population Lenzilumab (95% CI) Placebo (95% CI) Lenzilumab vs placebo hazard ratio (95% CI) P value (n=351) 9.8% (6.1 - 15.4) (n=168) 21.2% (15.9 - 27.9) (n=183) 2.54 (1.46 – 4.41) 0.0009 *C - Reactive Protein is routine and accepted measure of inflammation. A retrospective analysis of data from LIVE - AIR suggests tha t patients with a CRP < 150 have an even greater response to lenzilumab Baseline CRP was evaluable in 482 patients. For patients with a CRP<150, treatment with lenzilumab resulted in a 2.54x Increase in the likelihood of surviving and without needing to be on mechanical ventilation Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently authorized or approved for use in any c ountry

10 Black/African - American population with Baseline CRP < 150* 10 For Black/African - American Patients, treatment with lenzilumab resulted in a 9x Increase in the likelihood of surviving and without needing to be on mechanical ventilation Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently authorized or approved for use in any c ountry Patients who died or required ventilation K - M Estimate Population Lenzilumab Placebo Lenzilumab vs placebo hazard ratio (95% CI) P value (n=51) 4.0% (n=25) 26.9% (n=26) 8.96 (1.09 – 73.6) 0.0412 *C - Reactive Protein is routine and accepted measure of inflammation. A retrospective analysis of data from LIVE - AIR suggests tha t patients with a CRP < 150 have an even greater response to lenzilumab Baseline CRP was evaluable in 482 patients.
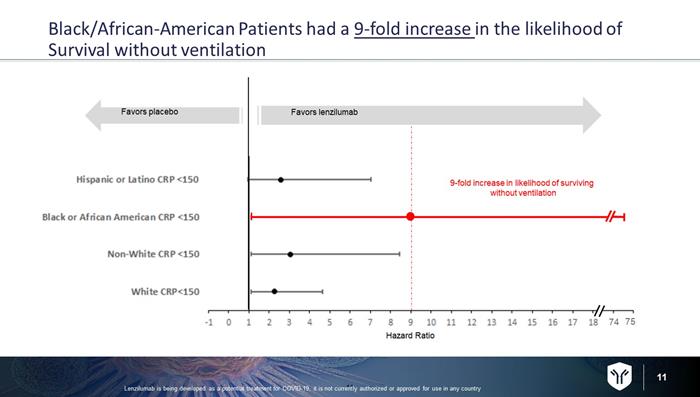
11 Black/African - American Patients had a 9 - fold increase in the likelihood of Survival without ventilation 11 Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently authorized or approved for use in any c ountry

12 Patient Reach Disease Severity Anti - viral Antibodies Outpatient Antiviral Immuno - Modulators COVID - 19 Vaccines Prophylaxis Critical patients in the ICU on mechanical ventilation and progressing rapidly 2,6,7 IMV – 2020: ~294K, 2021: ~349K, 2025: ~192K Bamlanivimab + Etesevimab Casirivimab + Imdevimab VIR - 7831 Lenzilumab; EUA Submitted Actemra (tocilizumab) Critical condition, exhibiting rapid respiratory decompensation Veklury (r emdesivir ) Narrowest Population, But Significant Broadest Population BNT162b2 mRNA - 1273 JNJ - 78436735 Newly Hospitalized Outpatient Treatment Pre/Post Exposure Prophylaxis Corticosteroids (dexamethasone) Hospitalized and hypoxic patients with confirmed diagnosis of COVID - 19 Mild - to - Moderate symptoms in high - risk non - hospitalized patients COVID - 19 negative or 90 - days post confirmed Dx Hospitalized and hypoxic patients; 12 years+, weighing at least 40kg Hospitalizations 2,6,7 – 2020: ~1.33M, 2021: ~1.58M, 2025: ~870,000 Within 3 - days of positive diagnosis; Requires presentation to infusion center; Low utilization of outpatient mAbs to date All patients 12 years+ Hospital mAbs Hospitalized - Critical Inpatient >67% of US Population At Least Partially Vaccinated 4 ~40% of COVID - 19 Patients are High - Risk 3 ~8% of Cases Require Hospitalization 5,6 ~2% of Cases Require IMV 1,5 There Remains A Significant Unmet Need For Hospitalized And Hypoxic Patients Sources: FDA Fact Sheets for Healthcare Providers for Veklury (Accessed June 29, 2021), REGEN - COV. (Accessed June 29, 2021), Bamlanivimab + Etesevimab (Accessed June 29, 2021), Actemra ( Accessed June 29, 2021), Olumiant ( Accessed August 3, 2021), Pfizer - BioNTech COVID - 19 Vaccine (Accessed June 29, 2021), Moderna COVID - 19 Vaccine (Accessed June 29, 2021). Janssen “ J&J” COVID - 19 Vaccine (Accessed June 29, 2021) using https://www.fda.gov/emergency - preparedness - and - response/mcm - legal - regulatory - and - policy - framework/emergency - use - authorization#coviddrugs 1. Based on lenzilumab Ph3 Data. 2. Humanigen Internal Forecast – Based upon 1.8% of Cases. 3. CDC: Estimated County - Level Prevalence of Selected Underlying Medical Conditions Associated with Increased Risk for Severe COVID - 19 Illness — United States, 2018. (Accessed June 29, 2021). https://www.cdc.gov/mmwr/volumes/69/wr/mm6929a1.htm?s_cid=mm6929a1_w. 4. CDC COVID Tracker: Vaccinations (Accessesed August 3 , 2 021). https://covid.cdc.gov/covid - data - tracker/#vaccinations. 5. Our World in Data / University of Oxford. (Accessed August 3, 2021). Number of US Cases YTD. https://covid.ourworldindata.org /da ta/owid - covid - data.csv. 6. CDC – COVID Data Tracker. (Accessed August 3, 2021). New Admissions of Patients with Confirmed COVID - 19, United States. https://covid.cdc.gov/covid - data - tracker/#new - hospital - admissions. 7. The COVID Tracking Pro ject – Hospitalization Increase data for dates before 7/31/21 when HHS new admissions data began (accessed June 29, 2021). https://covidtracking.com/data/download/national - history.csv JAK Inhibitor Olumiant (baricitinib) Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently authorized or approved for use in any c ountry

13 Conclusion • Higher infection rates • Higher hospitalization rates • Higher death rates Black and African Americans are disproportionately affected by COVID - 19 1 • Systemic disparities • Social determinants of health • Low vaccination rates Multiple potential reasons for this 1, 2 • 9x Increase in survival and without requiring mechanical ventilation Black/African - American patients appeared to demonstrate exceptional response rates to lenzilumab 3 Currently under review for Emergency Use Authorization by FDA 1. https://www.cdc.gov/coronavirus/2019 - ncov/covid - data/investigations - discovery/hospitalization - death - by - race - ethnicity.htm, July 16, 2021 2. Kaiser Family Foundation, https://www.kff.org/coronavirus - covid - 19/issue - brief/latest - data - on - covid - 19 - vaccinations - race - ethnici ty/, July 21, 2021 3. LIVE - AIR Phase 3 data on file at Humanigen, Inc. Lenzilumab is being developed as a potential treatment for COVID - 19, it is not currently authorized or approved for use in any c ountry
