Attached files
| file | filename |
|---|---|
| 8-K - HUMANIGEN, INC | s6162108k.htm |
Exhibit 99.1

Humanigen 2021 Annual Stockholder Meeting June 17

2 Cautionary Note Regarding Forward - Looking Statements All statements other than statements of historical facts contained in this presentation are forward - looking statements . Forward - looking statements reflect management's current knowledge, assumptions, judgment, and expectations regarding future performance or events . Although management believes that the expectations reflected in such statements are reasonable, they give no assurance that such expectations will prove to be correct, and you should be aware that actual events or results may differ materially from those contained in the forward - looking statements . Words such as "will," "expect," "intend," "plan," "potential," "possible," "goals," "accelerate," "continue," and similar expressions identify forward - looking statements, including, without limitation, statements regarding our request for and receipt of an Emergency Use Authorization from FDA for lenzilumab in COVID - 19 ; our request and receipt of Marketing Authorization or Conditional Marketing Authorization for lenzilumab in COVID - 19 by the MHRA ; and our other plans relating to lenzilumab as a result of the release of the topline results . Forward - looking statements are subject to a number of risks and uncertainties including, but not limited to, the risks inherent in our lack of profitability and need for additional capital to grow our business ; our dependence on partners to further the development of our product candidates ; the uncertainties inherent in the development, attainment of the requisite regulatory authorizations and approvals and launch of any new pharmaceutical product ; the outcome of pending or future litigation ; and the various risks and uncertainties described in the "Risk Factors" sections of our latest annual and quarterly reports and other filings with the SEC . All forward - looking statements are expressly qualified in their entirety by this cautionary notice . You should not rely upon any forward - looking statements as predictions of future events . We undertake no obligation to revise or update any forward - looking statements made in this presentation to reflect events or circumstances after the date hereof, to reflect new information or the occurrence of unanticipated events, to update the reasons why actual results could differ materially from those anticipated in the forward - looking statements, in each case, except as required by law .
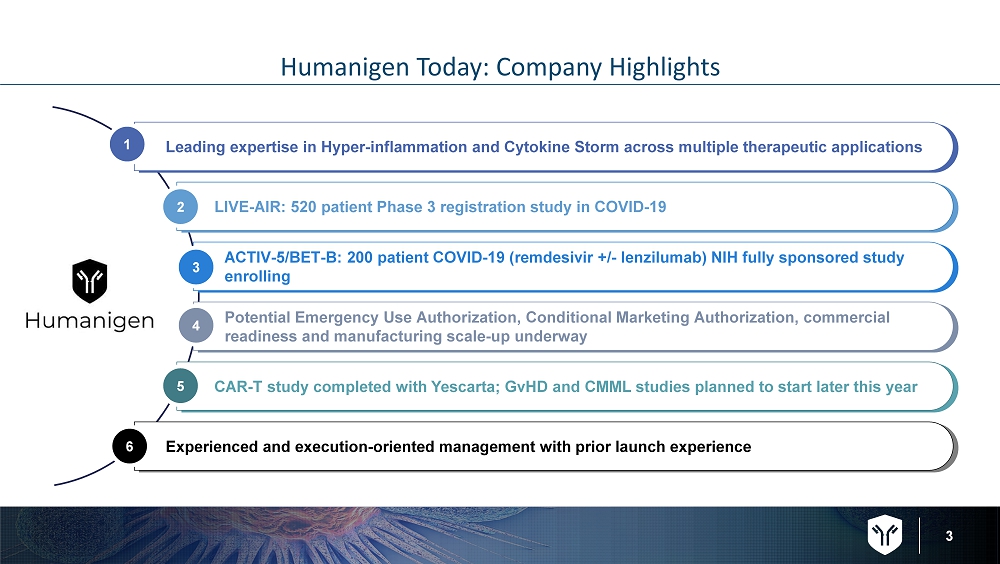
3 Humanigen Today: Company Highlights Leading expertise in Hyper - inflammation and Cytokine Storm across multiple therapeutic applications ACTIV - 5/BET - B: 200 patient COVID - 19 (remdesivir +/ - lenzilumab ) NIH fully sponsored study enrolling LIVE - AIR: 520 patient Phase 3 registration study in COVID - 19 CAR - T study completed with Yescarta ; GvHD and CMML studies planned to start later this year Experienced and execution - oriented management with prior launch experience Potential Emergency Use Authorization, Conditional Marketing Authorization, commercial readiness and manufacturing scale - up underway 1 2 6 3 4 5

4 Experienced and Execution - Oriented Management Tim Morris, CPA – Chief Operating and Chief Financial Officer Retired CFO Iovance ; raised > $2.2Bn over 29 years Extensive deal experience with >90 transactions, combined value $4.8B 5 NDA/MAA and associated commercial prep and launch experience Cameron Durrant, MD, MBA – Chief Executive Officer Serial biotech experience as Exec Chair, CEO, CFO Led previous deals with Gilead while at J&J Launched 5 blockbusters Adrian Kilcoyne, MD, MBA – Chief Medical Officer 15 years of biopharma experience, including COVID - 19 and CAR - T Medical Affairs, Evidence Generation and R&D Directed numerous projects spanning oncology and lymphoma programs Omar Ahmed, PharmD – SVP, Clinical, Medical and Scientific Affairs 20 years biopharma experience, led multiple blockbuster launches Led development of Janssen’s immunology portfolio strategy Bob Atwill, MBA – Head of Asia - Pacific Region 30 years of biopharma experience, including cell therapy Extensive network in Asia - Pacific, BD+L, fundraising and clinical development Ed Jordan, MBA – Chief Commercial Officer Senior commercial roles across the healthcare industry Extensive product launch experience including immunology, oncology, hematology Dale Chappell, MD, MBA – Chief Scientific Officer Worked under Dr. Steven Rosenberg, ‘father’ of cancer immunology Studied tumor immunology at National Cancer Institute where he published in field of T - cell therapy, immunology pathways, and GM - CSF Founder of Black Horse Capital Advisors

5 Highly Credentialed and Engaged Board Rainer Boehm, MD, MBA Former interim CEO Novartis Pharma; Chief Commercial and Chief Medical Affairs Officer, Novartis Pharma; EVP, Novartis Oncology Board member Cellectis Ron Barliant , JD Of Counsel with Goldberg Kohn US bankruptcy judge for N. Illinois for 14 years, extensive legal and bankruptcy experience Cheryl Buxton Vice Chair, Global Pharmaceuticals, Korn Ferry Former HR Director, J&J Pharmaceuticals Cameron Durrant, MD, MBA – Chief Executive Officer Serial biotech experience as Exec Chair, CEO, CFO Led previous deals with Gilead while at J&J Launched 5 blockbusters Dale Chappell, MD, MBA – Chief Scientific Officer Worked under Dr. Steven Rosenberg, ‘father’ of cancer immunology Studied tumor immunology at National Cancer Institute where he published in field of T - cell therapy, immunology pathways, and GM - CSF Founder of Black Horse Capital Advisors
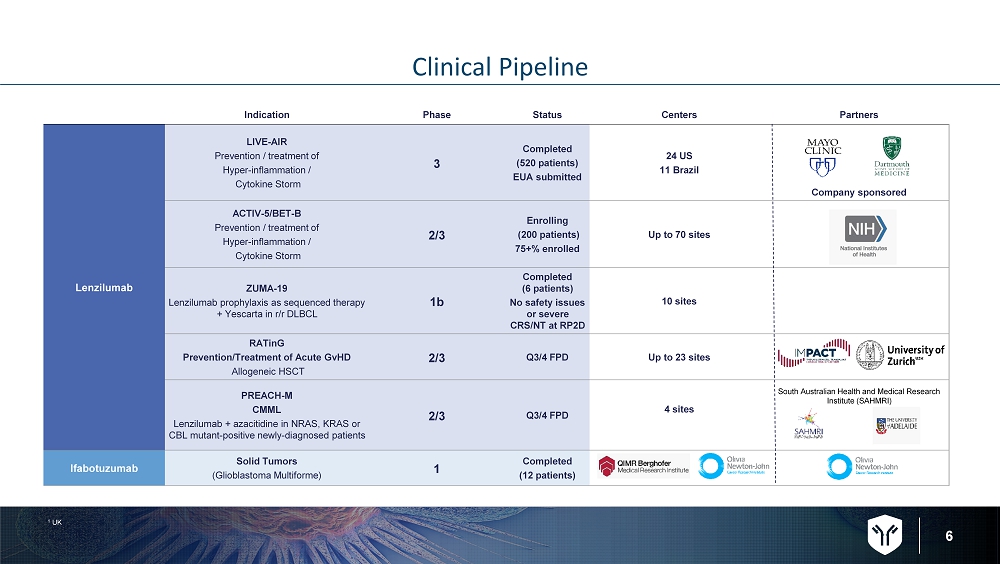
6 Clinical Pipeline Indication Phase Status Centers Partners Lenzilumab LIVE - AIR Prevention / treatment of Hyper - inflammation / Cytokine Storm 3 Completed (520 patients) EUA submitted 24 US 11 Brazil Company sponsored ACTIV - 5/BET - B Prevention / treatment of Hyper - inflammation / Cytokine Storm 2/3 Enrolling (200 patients) 75+% enrolled Up to 70 sites ZUMA - 19 Lenzilumab prophylaxis as sequenced therapy + Yescarta in r/r DLBCL 1b Completed (6 patients) No safety issues or severe CRS/NT at RP2D 10 sites RATinG Prevention/Treatment of Acute GvHD Allogeneic HSCT 2/3 Q3/4 FPD Up to 23 sites PREACH - M CMML Lenzilumab + azacitidine in NRAS, KRAS or CBL mutant - positive newly - diagnosed patients 2/3 Q3/4 FPD 4 sites South Australian Health and Medical Research Institute (SAHMRI) Ifabotuzumab Solid Tumors (Glioblastoma Multiforme) 1 Completed (12 patients) 1 UK
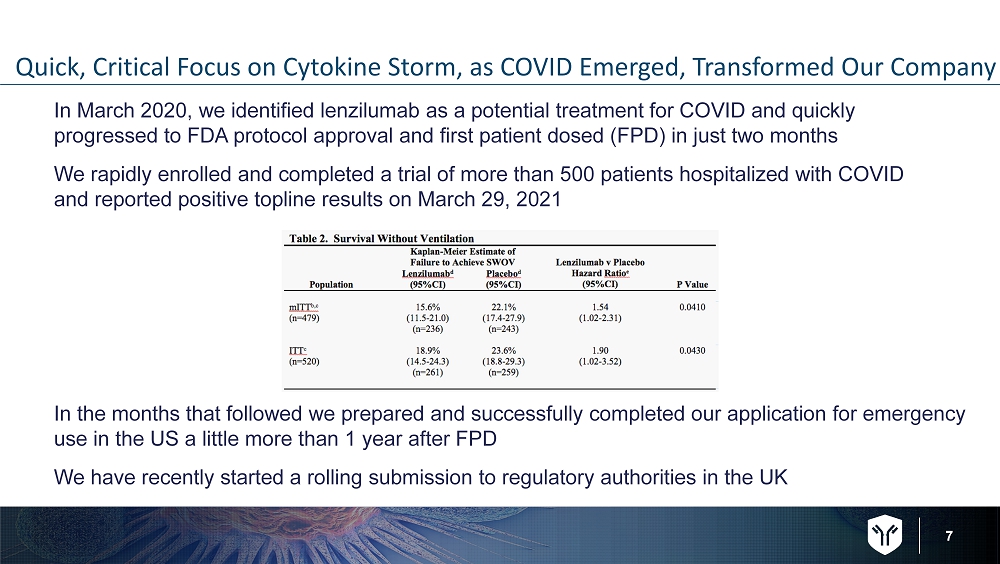
7 Quick, Critical Focus on Cytokine Storm, as COVID Emerged, Transformed Our Company In March 2020, we identified lenzilumab as a potential treatment for COVID and quickly progressed to FDA protocol approval and first patient dosed (FPD) in just two months We rapidly enrolled and completed a trial of more than 500 patients hospitalized with COVID and reported positive topline results on March 29, 2021 In the months that followed we prepared and successfully completed our application for emergency use in the US a little more than 1 year after FPD We have recently started a rolling submission to regulatory authorities in the UK
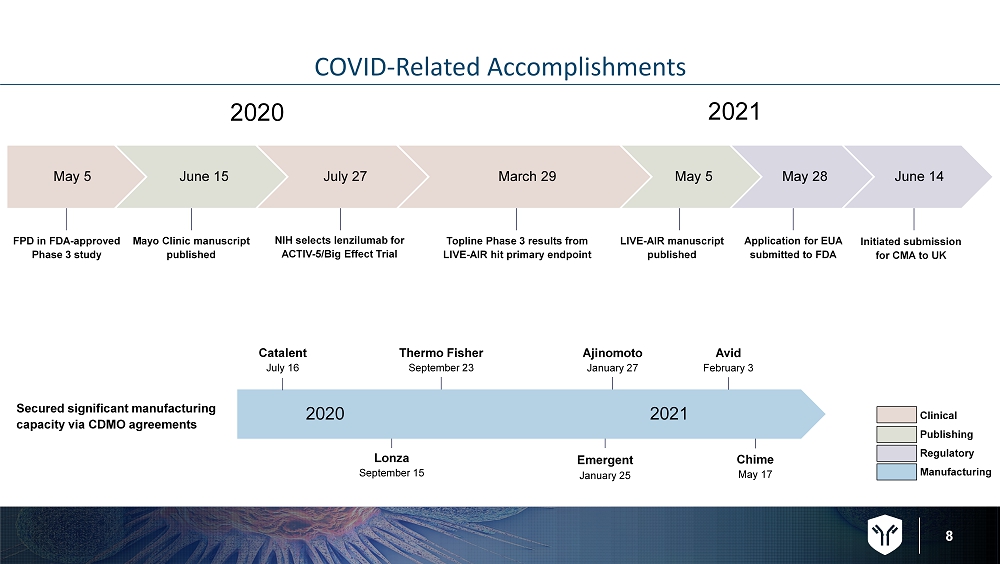
8 COVID - Related Accomplishments May 5 June 15 July 27 March 29 May 5 May 28 June 14 2020 2021 FPD in FDA - approved Phase 3 study Mayo Clinic manuscript published NIH selects lenzilumab for ACTIV - 5/Big Effect Trial Topline Phase 3 results from LIVE - AIR hit primary endpoint LIVE - AIR manuscript published Application for EUA submitted to FDA Initiated submission for CMA to UK Clinical Publishing Regulatory Manufacturing Catalent July 16 Lonza September 15 Thermo Fisher September 23 Emergent January 25 Ajinomoto January 27 Chime May 17 2020 2021 Secured significant manufacturing capacity via CDMO agreements Avid February 3
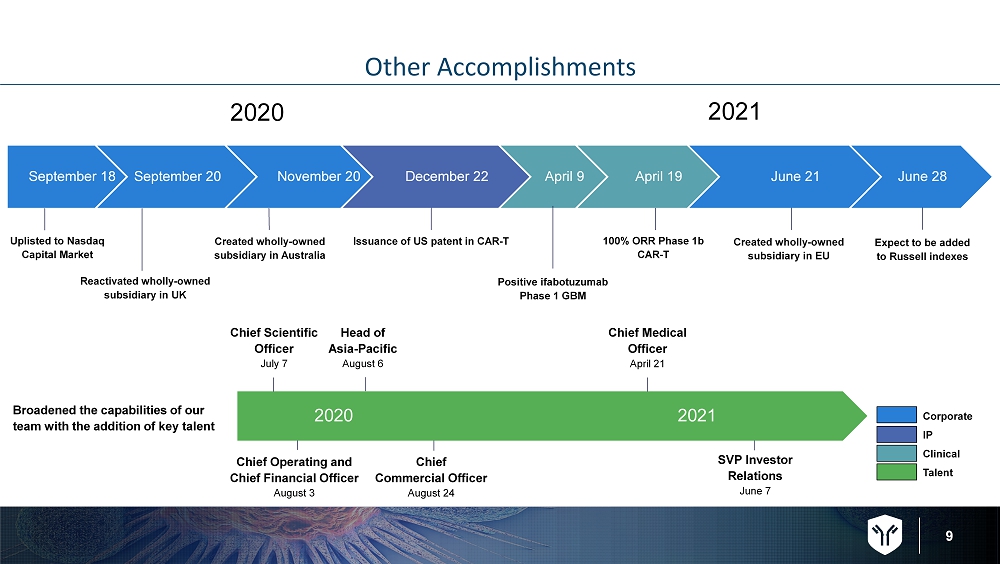
9 Other Accomplishments September 18 September 20 November 20 December 22 April 9 April 19 June 21 June 28 Uplisted to Nasdaq Capital Market Reactivated wholly - owned subsidiary in UK Issuance of US patent in CAR - T Positive ifabotuzumab Phase 1 GBM 100% ORR Phase 1b CAR - T Created wholly - owned subsidiary in EU Expect to be added to Russell indexes Corporate IP Clinical Talent Broadened the capabilities of our team with the addition of key talent Chief Scientific Officer July 7 Chief Medical Officer April 21 SVP Investor Relations June 7 2020 2021 Chief Operating and Chief Financial Officer August 3 Head of Asia - Pacific August 6 Chief Commercial Officer August 24 2020 2021 Created wholly - owned subsidiary in Australia

10 Clinical Accomplishments Enabled Access to Capital Our ability to successfully identify and demonstrate the potential clinical benefit of lenzilumab in COVID - 19 significantly improved our access to capital ▪ Private placement @ $4.35/share (reverse - split adjusted) raised ~$72MM gross ▪ Public offering @ $8.50/share raised ~$78MM gross ▪ Public offering @ $18.50/share raised ~$100MM gross ▪ Entered Loan and Security Agreement with Hercules Capital, drew initial $25MM, ability to draw a second tranche of either $25MM or $35MM and a third tranche of $20MM, subject to certain conditions In total we have raised >$250MM and secured access for up to an additional $55MM

11 Financial Overview Current Balance (MM) • Proforma post - Q1 cash equivalents $187 • Via Hercules, up to an additional $ 55 • Shares outstanding 59

12 Others are Following, But We Plan to Advance Our Lead By Continuing to Invest in . . . □ Manufacturing with global leaders □ Preparing for commercialization □ Advancing clinical development and data in CAR - T, GvHD, CMML, COVID and non - COVID respiratory and other conditions □ Expanding our IP as a result of non - obvious findings from our programs

13 Goals: Next 12 Months □ Commercial scale manufacture of lenzilumab □ Commence commercialization in countries where lenzilumab use authorized/approved □ Announce data from ACTIV - 5/BET - B □ Submit lenzilumab Biologics License Application to FDA □ Initiate Phase 2 potential registrational CAR - T study □ Initiate Phase 2/3 acute GvHD study □ Initiate Phase 2/3 CMML study □ Evaluate potential studies of lenzilumab in non - COVID respiratory and other conditions
