Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - PSYCHEMEDICS CORP | exh_322.htm |
| EX-32.1 - EXHIBIT 32.1 - PSYCHEMEDICS CORP | exh_321.htm |
| EX-31.2 - EXHIBIT 31.2 - PSYCHEMEDICS CORP | exh_312.htm |
| EX-31.1 - EXHIBIT 31.1 - PSYCHEMEDICS CORP | exh_311.htm |
| EX-24 - EXHIBIT 24 - PSYCHEMEDICS CORP | exh_24.htm |
| EX-23.1 - EXHIBIT 23.1 - PSYCHEMEDICS CORP | exh_231.htm |
| EX-21.1 - EXHIBIT 21.1 - PSYCHEMEDICS CORP | exh_211.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended December 31, 2020
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number: 1-13738
PSYCHEMEDICS CORPORATION
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | 58-1701987 | |
| (State or Other Jurisdiction of Incorporation or Organization) |
(I.R.S. Employer Identification No.) |
| 289 Great Road Acton, Massachusetts |
01720 | |
| (Address of Principal Executive Offices) | (Zip Code) |
Registrant’s Telephone
Number Including Area Code: (978) 206-8220
Securities registered pursuant to Section 12(b) of the Act:
| Title of Class | Trading Symbol(s) | Name of each exchange on which registered |
| Common stock. $0.005 par value | PMD | The Nasdaq Stock Market, LLC. |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by a check mark if the registrant is a well-known seasoned issuer (as defined in Rule 405 of the Securities Exchange Act of 1934). Yes ☐ No ☒
Indicate by a check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934). Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files.) Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or emerging growth company. See definitions of “accelerated filer”, “large accelerated filer”, “non-accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Securities Exchange Act of 1934.
| Large Accelerated Filer | ☐ | Accelerated Filer | ☐ | Non-Accelerated Filer | ☒ |
| Smaller Reporting Company | ☒ | Emerging Growth Company | ☐ | ||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by a check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Securities and Exchange Act of 1934). Yes ☐ No ☒
As of June 30, 2020, there were 5,526,493 shares of Common Stock of the Registrant outstanding. The aggregate market value of the Common Stock of the Registrant held by non-affiliates (assuming for these purposes, but not conceding, that all executive officers, directors and 5% shareholders are “affiliates” of the Registrant) as of June 30, 2020 was approximately $22 million, computed based upon the closing price of $5.55 per share on June 30, 2020.
As of March 26, 2021, there were 5,536,493 shares of Common Stock of the Registrant outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Part III of this Annual Report on Form 10-K incorporates by reference portions of the Registrant’s definitive proxy statement, to be filed with the Securities and Exchange Commission no later than 120 days after the close of its fiscal year; provided that if such proxy statement is not filed with the Commission in such 120-day period, an amendment to this Form 10-K shall be filed no later than the end of the 120-day period.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements under “Business,” “Risk Factors,” “Legal Proceedings,” “Market for Registrant’s Common Stock and Related Stockholder Matters” and “Management Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this Annual Report on Form 10-K (this “Form 10-K”) constitute forward-looking statements under Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, including statements made with respect to future earnings, earnings per share, revenues, operating income, cash flows, competitive and strategic initiatives, potential stock repurchases, liquidity needs, dividends, future business, growth opportunities, profitability, pricing, new accounts, customer base, market share, test volume, sales volume, sales and marketing strategies, repayment under the Paycheck Protection Program (“PPP”) Loan, U.S. and foreign drug testing laws and regulations and the enforcement of such laws and regulations, required investments in plant, equipment and people, new test development, and contingencies, including litigation results. These statements involve known and unknown risks, uncertainties and other factors that may cause results, levels of activity, growth, performance, earnings per share or achievements to be materially different from any future results, levels of activity, growth, performance, earnings per share or achievements expressed or implied by such forward-looking statements.
The forward-looking statements included in this Form 10-K and referred to elsewhere are related to future events or our strategies or future financial performance. In some cases, you can identify forward-looking statements by terminology such as “may,” “should,” “believe,” “anticipate,” “future,” “potential,” “estimate,” “encourage,” “opportunity,” “growth,” “leader,” “could”, “expect,” “intend,” “plan,” “expand,” “focus,” “through,” “strategy,” “provide,” “offer,” “allow,” “commitment,” “implement,” “result,” “increase,” “establish,” “perform,” “make,” “continue,” “can,” “ongoing,” “include” or the negative of such terms or comparable terminology. All forward-looking statements included in this Form 10-K are based on information available to us as of the filing date of this report, and the Company assumes no obligation to update any such forward-looking statements. Our actual results could differ materially from the forward-looking statements.
Factors that may cause such differences include but are not limited to: (1) intense competition in the drug testing industry, particularly among companies that test utilizing hair samples; (2) risks associated with the development of markets for new products and services offered; (3) pricing policies; (4) risks associated with capacity expansion; (5) risks associated with U.S. government regulations, including, but not limited to, Food and Drug Administration (the “FDA”) regulations, (6) risks associated with our international operations, including, but not limited to, Brazilian laws, proposed laws and regulations, market development and currency risks; (7) Psychemedics' ability to maintain its reputation and brand image; (8) the ability of Psychemedics to achieve its business plans, productivity improvements, cost controls, leveraging of its global operating platform, and acceleration of the rate of innovation; (9) the direct and indirect impact of coronavirus (“COVID-19”) pandemic on our business and operations; (10) information technology system failures and data security breaches; (11) the uncertain global economy; (12) our ability to attract, develop and retain executives and other qualified employees and independent contractors, including distributors; (13) Psychemedics' ability to obtain and protect intellectual property rights; (14) litigation risks; and (15) changes in economic conditions which affect demand for our products and services.
Additional important factors that could cause actual results to differ materially from expectations reflected in our forward-looking statements include those described in Item 1A, “Risk Factors.”
| i |
| ii |
Available Information
Psychemedics Corporation (together with its wholly-owned subsidiaries, the “Company” or “Psychemedics”) maintains its principal executive office at 289 Great Road, Acton, MA 01720. Our telephone number is (978) 206-8220 and internet address is www.psychemedics.com. Our stock is traded on the NASDAQ Stock Market under the symbol “PMD”. The Company makes available, free of charge, on the Investor Information section of its website, its Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with the Securities and Exchange Commission (the “SEC”). Copies are also available, without charge, from Psychemedics Corporation, Attn: Investor Relations, 289 Great Road, Acton, MA 01720. Alternatively, reports filed with the SEC may be viewed or obtained at the SEC Public Reference Room in Washington, D.C., or the SEC’s Internet site at www.sec.gov. We do not intend for information contained in our website to be part of this Annual Report on Form 10-K.
General
Psychemedics Corporation is a Delaware corporation organized on September 24, 1986. The consolidated financial statements of the Company include the accounts and results of operations of Psychemedics Corporation and its wholly-owned subsidiary Psychemedics International, LLC (Delaware) and their jointly-owned subsidiary Psychemedics Laboratórios Ltda (Brazil). All significant inter-company balances and transactions have been eliminated in consolidation. All the Company’s assets are located within the United States. The Company provides testing services for the detection of drugs of abuse through the analysis of hair samples. The Company’s testing methods utilize a patented technology that digests the hair and releases drugs trapped in the hair without destroying the drugs. This is fundamental to the entire process because the patented method gets virtually 100% of the drug out of the hair, and if you cannot get the drug out of the hair, you cannot measure it. The Company then performs a proprietary custom-designed patented (US 10,539,580) enzyme immunoassay (“EIA”) on the liquid supernatant, with confirmation testing by mass spectrometry.
The Company’s primary application of its patented technology is as a testing service that analyzes hair samples for the presence of certain drugs of abuse. The Company’s customized proprietary EIA procedures to drug test hair samples differ from the more commonly used immunoassay procedures employed by other hair testing companies. The Company’s testing results provide quantitative information that can indicate the approximate amount of drug ingested as well as historical data, which can show a pattern of individual drug use over a longer period of time, thereby providing superior detection compared to other types of drug testing. This information is useful to employers for both applicant and employee testing, as well as treatment professionals, law enforcement agencies, school administrators, and parents concerned about their children’s drug use. The Company provides screening and confirmation by mass spectrometry using industry-accepted practices for cocaine, marijuana, PCP, amphetamines (including ecstasy, eve and Adderall®), opiates (including heroin, hydrocodone, hydromorphone, oxycodone, oxymorphone and codeine), synthetic cannabinoids (including K2, Spice, Blaze), benzodiazepines (Xanax®, Valium®, and Ativan®), nicotine and Fentanyl. In addition, in 2013, the Company launched a hair test for alcohol which also looks back on use over a 90 day period, as our hair drug tests do.
Testing services are currently performed at the Company’s Culver City, California campus located at 5832 Uplander Way and 5750 Hannum Avenue.
Background on Drug Testing with Hair
When certain chemical substances enter the bloodstream, the blood carries these substances to the hair where they become “entrapped” in the protein matrix in amounts approximately proportional to the amount ingested. The Company utilizes a patented drug extraction method followed by a unique patented EIA procedure to identify drugs in the hair. The patented drug extraction method effectively releases drugs from the hair without destroying the drugs, getting virtually 100% of the drug out of the hair. The patented method can be used with a broad range of immunoassay screen techniques and mass spectrometry methods.
The immunoassays used by the company have been patented under the name “Solid Phase Multi-Analyte Assay.” The immunoassays produced by the Psychemedics R&D team were uniquely designed specifically to meet and even exceed the standards of radioimmunoassay (“RIAH”), the original testing method created and utilized by the Company prior to 2013. Because Psychemedics is the only hair testing laboratory that manufactures its own screening assays, it has full control over all aspects of its technology, and that powerful advantage facilitated the Company's creation of its EIA assays with equivalence to its own previously FDA-cleared radioimmunoassays.
The EIA screened positive results are then confirmed by mass spectrometry. Depending upon the length of hair, the Company is able to provide historical information on drug use by the person from whom the sample was obtained. Because head hair grows approximately 1.3 centimeters per month, a 3.9 centimeter head hair sample can reflect drug ingestion over the approximate three months prior to the collection of the sample. Another option is sectional analysis of the head hair sample, in which the hair is sectioned into lengths which approximately correspond to certain time periods, thereby providing information on patterns of drug use.
| 1 |
Validation of the Company’s Proprietary Testing Methods
The process of analyzing human hair for the presence of drugs has been the subject of numerous peer-reviewed, scientific field studies. Many of the studies have been funded by the National Institute of Justice or the National Institute on Drug Abuse (“NIDA”). Several hundred research articles written by independent researchers have been published supporting the general validity and usefulness of hair analysis.
Some of the Company’s customers have also completed their own testing to validate the Company’s hair test results compared to other companies’ urine test results. These studies consistently confirmed the Company’s superior detection rate compared to urinalysis testing. When results from the Company’s hair testing methods were compared to urine results in side-by-side evaluations, 5 to 10 times as many drug abusers were accurately identified by the Company’s proprietary methods.
In 1998, the National Institute of Justice, utilizing Psychemedics’ previously utilized RIAH hair testing assay, completed a Pennsylvania Prison study where hair analysis revealed an average prison drug use level of approximately 7.9% in 1996. Comparatively, urinalysis revealed virtually no positives. After measures to curtail drug use were instituted (drug-sniffing dogs, searches and scanners), the use level fell to approximately 2% according to the results of hair analysis in 1998. Again, the urine tests showed virtually no positives. The study illustrates the usefulness of hair analysis to monitor populations and the weakness of urinalysis.
The Company has received 510k clearance from the FDA on nine EIA assays used to test head and body hair for drugs of abuse.
The Company’s decontamination wash protocol and the effects in eliminating surface contamination were analyzed in a study conducted by scientists at the Laboratory of the Federal Bureau of Investigation (the “FBI”) and published in August 2014 in the Journal of Analytical Toxicology. The FBI concluded that the use of an extended wash protocol of the type used by the Company will exclude false positive results from environmental contact with cocaine. In the study, the FBI cited Psychemedics’ studies published in 1993, 2002, 2004, and 2005, and named our Vice President of Laboratory Operations, Dr. Michael Schaffer, and our lab, in its acknowledgments. The FBI study also supported the use of metabolites known as hydroxycocaines as evidence of ingestion. These metabolites were first identified in hair by Psychemedics.
Advantages of Using the Company’s Patented Method
The Company asserts that hair testing using its patented method confers substantive advantages over detection through urinalysis. Although urinalysis testing can provide accurate drug use information, the scope of the information is short-term and is generally limited to the type of drug ingested within a few days of the test. Studies published in many scientific publications have indicated that most drugs disappear from urine within a few days.
In contrast to urinalysis testing, hair testing using the Company’s patented method can provide long-term historical drug use information resulting in a significantly wider window of detection. This window may be several months or longer depending on the length of the hair sample. The Company’s standard test offering, however, uses a 3.9 centimeter length head hair sample cut close to the scalp, which measures use for approximately three months prior to collection of the sample.
This wider window enhances the detection efficiency of hair analysis, making it particularly useful in pre-employment and random testing. Hair testing not only identifies more drug users, but it may also uncover patterns and severity of drug use (information most helpful in determining the scope of an individual’s involvement with drugs), while serving as a deterrent against drug use. Hair testing employing the Company’s patented method greatly reduces the incidence of “false negatives” associated with evasive measures typically encountered with urinalysis testing. For example, urinalysis test results are adversely impacted by excessive fluid intake prior to testing and by adulteration or substitution of the urine sample. Moreover, a drug user who abstains from use for a few days prior to urinalysis testing can usually escape detection. Hair testing is effectively free of these problems, as it cannot be thwarted by evasive measures typically encountered with urinalysis testing. Hair testing is also attractive to customers since sample collection is typically performed under close supervision yet is less intrusive and less embarrassing for test subjects.
Hair testing using the Company’s patented method (with mass spectrometry confirmation) further reduces the prospects of error in conducting drug detection tests. Urinalysis testing is more susceptible to problems such as “evidentiary false positives” resulting from passive drug exposure or poppy seeds. To combat this problem, in federally mandated testing, the opiate cutoff levels for urine testing were raised 667% (from 300 to 2,000 ng/ml) on December 1, 1998, and testing for the presence of a heroin metabolite, 6-MAM, was required. These requirements, however, effectively reduced the detection time frame for confirmed heroin use, such that 6-MAM in urine can typically only be detected for several hours post drug use. In contrast, the metabolite 6-MAM is stable in hair and can be detected for months.
In the event a positive urinalysis test result is challenged, a test on a newly collected urine sample is not a viable remedy. Unless the forewarned individual continues to use drugs prior to the date of the newly collected sample, a re-test may yield a negative result when using urinalysis testing because of temporary abstinence. In contrast, when the Company’s hair testing method is offered on a repeat hair sample, the individual suspected of drug use cannot as easily affect the results because historical drug use data remains locked in the hair fiber.
| 2 |
When compared to other hair testing methods, not only are the Company’s assays cleared by the FDA for head and body hair, the assays also employ a unique patented method of digesting hair that the Company believes allows for the most efficient release of drugs from the hair without destroying the drugs. The Company’s method of releasing drugs from hair is a key advantage and results in superior detection rates.
Disadvantages of Hair Testing
There are some disadvantages of hair testing as compared to drug detection through urinalysis. Because hair starts growing below the skin surface, drug ingestion evidence does not appear in hair above the scalp until approximately five to seven days after use. Thus, hair testing is not suitable for determining drug presence in “for cause” testing as is done in connection with an accident investigation. It does, however, provide a drug history which can complement urinalysis information in “for cause” testing.
The Company’s prices for its tests are generally somewhat higher than prices for tests using urinalysis, but the Company believes that its superior detection rates provide more value to the customer. This higher pricing policy could, however, adversely impact the growth of the Company’s sales volume and failure to obtain new business.
Hair Alcohol Testing
In 2013, the Company launched a test for alcohol using hair. This test measures average alcohol consumption over a period of approximately three months, indicates the approximate level of alcohol use during that time period, and can provide a behavioral indication of excessive use. The test measures the amount of ethyl glucuronide (EtG) in the hair – a trace metabolite of ethanol and a direct alcohol biomarker.
Intellectual Property
Certain aspects of the hair analysis method currently used by the Company are covered by US and foreign patents owned by the Company. The Company has been granted a total of twelve US patents, including a patent issued to the Company in 2011 that focuses on digesting hair and releasing drugs trapped in the hair without destroying the drugs. This patent can be used with a broad range of immunoassay screen techniques, mass spectrometry methods, and chromatographic procedures. In 2012, the Company received an additional US patent that extended the range of the patent received in 2011. More recently, two US patents related to integrity testing of hair samples issued in 2015 and 2016, and a US patent application directed to detection of multiple analytes was allowed. Additional patent applications are currently pending in the U.S. and internationally. In 2019, US Patent 10,539,580 was issued covering our Solid Phase Multi-Analyte Assay used in all our cleared EIA FDA submissions.
The Company also relies on trade secrets to protect certain aspects of its proprietary technology. The Company’s ability to protect the confidentiality of its trade secrets is dependent upon the Company’s internal safeguards and upon the laws protecting trade secrets and unfair competition.
In the event that patent protection or protection under the laws of trade secrets is not sufficient and the Company’s competitors succeed in duplicating the Company’s products, the Company’s business could be materially adversely affected.
Target Markets
Workplace
The Company focuses its primary marketing efforts on the private sector, with particular emphasis on job applicant and employee testing.
Most businesses use drug testing to screen job applicants and employees. The Hazeldon Foundation survey from 2007 indicated that 85 percent of Human Resource (“HR”) professionals believe that drug testing is an effective way to identify substance abuse. The prevalence of drug screening programs reflects a concern that drug use contributes to employee health problems and costs. As the same study found that 62 percent of HR professionals believe that absenteeism is the most significant problem caused by substance abuse and addiction, followed at 49 percent by reduced productivity, a lack of trustworthiness at 39 percent, a negative impact on the company’s external image at 32 percent, missed deadlines at 31 percent, and in certain industries, safety hazards. It has been estimated that substance abuse costs to American businesses is more than $100 billion annually.
The principal criticism of employee drug testing programs centers on the effectiveness of the testing program. Most private sector testing programs use urinalysis. Such programs are susceptible to evasive maneuvers and the inability to obtain confirmation through repeat samples in the event of a challenged result. An industry has developed over the Internet, and through direct mail, marketing a wide variety of adulterants, dilutants, clean urine and devices to assist drug users in falsifying urine test results.
Moreover, scheduled tests such as pre-employment testing and some random testing programs provide an opportunity for many drug users to simply abstain for a few days in order to escape detection by urinalysis.
The Company presents its patented hair analysis method to potential clients as a better technology well suited to employer needs. Field studies and actual client results support the accuracy and superior effectiveness of the Company’s patented technology and its ability to detect varying levels of drug use.
| 3 |
The Company performs a confirmation test of all screened positive results through mass spectrometry. The use of mass spectrometry is an industry accepted practice used to confirm a positive test result from the screening process. The Company offers its clients an expanded drug screen with mass spectrometry confirmation of cocaine, PCP, marijuana, amphetamines, opiates, synthetic cannabinoids and benzodiazepines. In addition, the Company offers a hair test for alcohol which also looks back on use over a 90 day period, as our hair drug tests do.
Professional Drivers
In 2016, Brazil started drugs of abuse testing for all professional drivers in the country using a hair test. This is a mandated program from a law passed in 2015. In the United States, a similar requirement exists for professional drivers, however, a urine test is currently required. The U.S. government is currently evaluating alternative mediums for testing of drugs of abuse for professional drivers, including hair drug tests.
Schools
The Company currently serves hundreds of schools throughout the United States and in several foreign countries. The Company offers its school clients the same five-drug screen with mass spectrometry confirmation that is used with the Company’s workplace testing service.
Parents
The Company also offers a personal drug testing service, known as “PDT-90”®, for parents concerned about drug use by their children. It allows parents to collect a small sample of hair from their child in the privacy of the home, send it to the Company’s laboratory and have it tested for drugs of abuse by the Company. The PDT-90 testing service uses the same patented method that is used with the Company’s workplace testing service.
Research
The Company is involved in the following ongoing studies involving use of drugs of abuse in various populations: In 2017, the Company partnered with an NIH-funded study titled “Adolescent Brain Cognitive Development” (“ABCD”) which expects to enroll 12,000 youths age 9-10 over a 2-2.5 year recruitment period. The objective of the ABCD consortium is to establish a national, multisite, longitudinal cohort and database by studying youth prospectively in order to examine brain and cognitive development in children and adolescents through a period (10 years) when significant development of intellectual and emotional functions occurs. Psychemedics’ role in this study is to test hair to detect use of drugs over the time period. The Company is also partnering with Olin Neuropsychiatry Research Center Institute of Living Hartford Hospital in a research study entitled, “Neurochemical and Functional Correlates of Memory in Emerging Adult Marijuana Users.” The study is aiming to better characterize the impact of heavy marijuana use on memory and is funded by a grant from NIDA.
Geographic Scope
Revenues outside the United States were 9%, 27% and 32% of consolidated revenues for years ended, 2020, 2019 and 2018, respectively.
Distribution
The Company markets its corporate drug testing services through its own sales force, distributors and webinars. The Company markets its home drug testing service, PDT-90, through the Internet.
The business in Brazil is sold through its non-exclusive distributor, Toxicologia Pardini, Ltda (“Pardini Tox”) and Sansão Holding S.A, affiliates of Instituto Hermes Pardini S.A. (“Pardini”), a provider of medical and diagnostic services in Brazil, including reference laboratory services. The agreement requires that the Psychemedics’ hair drug tests be marketed, sold, and reported in Brazil under the Psychemedics Corporation brand name, with all related materials so identified, and with actual testing services of Psychemedics’ tests to continue to be performed by Psychemedics at its laboratory in California. Either the Company or the distributor are able to cancel the distribution agreement upon 90-days’ prior written notice.
In 2016, the Company was certified as a Center of Excellence by BenchmarkPortal for its customer service function. Customer service is a key component to the sales and support function and this certification validates the efforts by the Company to support our customers.
Significant Customers and Concentration of Credit Risk
The Company had no customers that represented 10% or more of total revenue for the year ended December 31, 2020. The Company had one customer that represented 26% and 31% of total revenue for the years ended December 31, 2019 and 2018, respectively. The Company had no customers account for 10% or more of the total accounts receivable balance as of December 31, 2020. The Company had two customers that accounted for 13% and 11% of the total accounts receivable balance as of December 31, 2019.
The Company maintains its cash in bank accounts at high quality financial institutions. The individual balances, at times, may exceed federally insured limits. These deposits may be redeemed upon demand, and the Company believes that the financial institutions that hold the Company’s cash and cash equivalents are financially sound and, accordingly, minimal credit risk exists with respect to cash and cash equivalents.
| 4 |
Competition
The Company competes directly with numerous commercial laboratories that test for drugs primarily through urinalysis testing. Most of these laboratories, such as Quest Diagnostics, have substantially greater financial resources, market identity, drug testing market share, marketing organizations, facilities, and more personnel than the Company. The Company has been steadily increasing its base of corporate customers and believes that future success with new customers is dependent on the Company’s ability to communicate the advantages of implementing a drug program utilizing the Company’s patented hair analysis method.
The Company’s ability to compete is also a function of pricing. The Company’s prices for its tests are generally higher than prices for tests using urinalysis. However, the Company believes that its superior detection rates, coupled with the customer’s ability to test less frequently due to hair testing’s wider window of detection (three months versus approximately three days with urinalysis), provide more value to the customer. This pricing policy could, however, lead to slower sales growth for the Company.
The Company also competes with other hair testing laboratories. The Company distinguishes itself from hair testing competitors by emphasizing the superior results the Company obtains through use of its unique patented extraction method (getting drug out of the hair), in combination with the Company’s FDA cleared immunoassay screen.
Government Regulation
The Company is licensed as a clinical laboratory by the State of California as well as certain other states. All tests are performed according to the laboratory standards established by the Department of Health and Human Services, through the Clinical Laboratories Improvement Amendments (“CLIA”), and various state licensing statutes.
A substantial number of states regulate drug testing. The scope and nature of such regulations varies greatly from state to state and is subject to change from time to time. The Company addresses state law issues on an ongoing basis.
The Federal Food, Drug and Cosmetic Act, as amended (the “FDC Act”) requires companies engaged in the business of testing for drugs of abuse using a test (screening assay) not previously recognized by the FDA to submit their assay to the FDA for recognition prior to marketing. In addition, the laboratory performing the tests is required to be certified by a recognized agency. In 2002, the Company received 510k clearance to market all five of its assays utilizing RIAH technology.
In 2008, the Company received the first College of American Pathologists (“CAP”) certification specifically including hair testing.
In 2011, the Company received ISO/IEC 17025 International Accreditation for a broad spectrum of laboratory testing including drugs of abuse and forensics in hair and urine specimens. ISO/IEC 17025 accreditation provides formal recognition to laboratories that demonstrate technical competency and maintains this recognition through periodic evaluations to ensure continued compliance.
In 2012, the Company received 510k clearance from the FDA to market five of its assays utilizing the Company’s custom developed EIA technology.
In 2013, the Company received 510k clearance from the FDA to market two additional assays utilizing the Company’s custom developed EIA technology.
In 2015, the Brazilian government signed into law a requirement for professional drivers to take a hair drug test when obtaining or renewing their driver's license. The law also requires professional drivers to be tested when they are hired or fired. However, in March 2020 the Brazilian government ordered the closing of all driver license bureaus for a majority of 2020 and extended the license renewal period for an additional 18 months for all drivers licenses due to the COVID-19 outbreak
In 2016, the Company received accreditation from the Standards Council of Canada as an accredited testing laboratory.
In 2017, the Company received 510k clearance from the FDA to market one additional assay utilizing the Company’s custom developed EIA technology.
In 2019, the Company received 510k clearance from the FDA to market one additional assay utilizing the Company’s custom developed EIA technology.
Research and Development
The Company is continuously engaged in research and development activities. During the years ended December 31, 2020, 2019 and 2018, $1.3 million, $1.6 million and $1.6 million, respectively, were expended for research and development. The Company continues to perform research activities to develop new products and services and to improve existing products and services utilizing the Company’s proprietary technology. The Company also continues to evaluate methodologies to enhance its drug screening capabilities. Additional research using the Company’s proprietary technology is being conducted by outside research organizations through government-funded studies.
Employees
As of December 31, 2020, the Company employed 138 employees, 4 of whom were in R&D. None of the Company’s employees are subject to a collective bargaining agreement and the Company believes that overall relations with employees are good.
| 5 |
In addition to other information contained in this Form 10-K, the following risk factors should be carefully considered in evaluating Psychemedics Corporation and its business because such factors could have a significant impact on our business, operating results and financial condition. These risk factors could cause actual results to materially differ from those projected in any forward-looking statements.
The ongoing COVID-19 pandemic may continue to adversely affect our business, results of operation and financial condition.
National, state and local governments in affected regions have implemented and may continue to implement safety precautions, including but not limited to, quarantines, travel restrictions, shelter in place orders and shutdowns. These measures may disrupt normal business operations and may have significant impact on financial markets worldwide.
We continue to monitor our operations and applicable government restrictions, and we have made modifications to our normal operations because of the COVID-19 pandemic, including travel and working from home. We have also limited our in-person interactions by our customer-facing professionals. This could negatively impact our ability to market our products effectively.
The extent of the impact of COVID-19 on our operational and financial performance will depend on certain developments, including the duration and spread of the outbreak, impact on our customers, employees and vendors all of which are uncertain and cannot be predicted. A material disruption in our workplace as a result of COVID-19 could affect our ability to carry on our business operations in the ordinary course and may require additional cost and effort should employees not be able to physically on-premises.
We have incurred indebtedness under the Corona Aid, Relieve and Economic Security Act (“CARES”) Act which may be subject to audit, may not be forgivable and may eventually have to be repaid. Any repayment of such indebtedness may limit the funds available to us and may restrict our flexibility in operating business.
On May 1, 2020, we entered into a term loan with Bank of America N.A. (the “Creditor”) under the Paycheck Protection Program (“PPP”) administered by the United States Small Business Administration (“SBA”) under the CARES Act. The principal amount of the loan was $2.2 million (the “PPP Loan”), which is evidenced by a promissory note with a maturity date of May 4, 2022, two years from the commencement date. The note bears interest on the unpaid balance of one percent (1%) per annum. The PPP Loan is subject to forgiveness under the PPP upon the Company’s request to the extent that the proceeds are used to pay expenses permitted by the PPP, including payroll costs.
The U.S. Department of the Treasury has announced that it will conduct audits for PPP loans that exceed $2 million. Should we be audited by the U.S. Department of the Treasury or SBA as a result of the PPP Loan or filing an application for forgiveness, such audit or review could result in the diversion in management’s attention and resources and cause us to incur additional costs. If we were to be audited and receive an adverse outcome in such an audit, we could be required to return the full amount of the PPP Loan, in which event we may be required to finance repayment of the PPP Loan.
On November 6, 2020, the Company submitted an application for forgiveness of the entire amount due on the loan to the Creditor. The Creditor, in turn, accepted the application and issued a recommendation to the SBA on whether the Company is entitled to full, partial, or no forgiveness of the PPP Loan. While we believe we satisfied all eligibility criteria for the PPP Loan, the Company cannot provide assurance that the principal and interest amounts under the PPP Loan will be forgiven. If all or substantially all of the PPP Loan is not forgiven or it is determined that it must be repaid, we may be required to use a portion of our cash flows from operations to pay principal on the PPP Loan and interest.
Companies may develop products that compete with our products and some of these companies may be larger and better capitalized than we are.
Many of our competitors and potential competitors are larger and have greater financial resources than we do and offer a range of products broader than our products. Some of the companies with which we now compete or may compete in the future may develop more extensive research and marketing capabilities and greater technical and personnel resources than we do and may become better positioned to compete in an evolving industry. Inability to compete successfully could harm our business and prospects.
Increased competition, including price competition, could have a material impact on the Company’s net revenues and profitability.
Our business is intensely competitive, both in terms of price and service. Pricing of drug testing services is a significant factor often considered by customers in selecting a drug testing laboratory. As a result of the clinical laboratory industry undergoing significant consolidation, larger clinical laboratory providers can increase cost efficiencies afforded by large-scale automated testing. This consolidation results in greater price competition. The Company may be unable to increase cost efficiencies sufficiently, if at all, and as a result, its net earnings and cash flows could be negatively impacted by such price competition. The Company may also face increased competition from companies that do not comply with existing laws or regulations or otherwise disregard compliance standards in the industry. Additional competition, including price competition, could have a material adverse impact on the Company’s net revenues and profitability. The Company operations in Brazil are subject to price pressures with new competitors entering the market. The Company may also face changes in fee schedules, competitive bidding for laboratory services or other actions or pressures reducing payment schedules as a result of increased or additional competition.
| 6 |
Our results of operations are subject in part to variation in our customers’ hiring practices and other factors beyond our control.
Our results of operations have been and may continue to be subject to variation in our customers’ hiring practices and job creation, which in turn is dependent, to a large extent, on the general condition of the economy, especially within our major market segments. Results for a particular quarter may vary due to several factors, including but not limited to:
| • | economic conditions in our markets in general; |
| • | economic conditions affecting our customers and their particular industries; |
| • | the introduction of new products and product enhancements by us or our competitors; and |
| • | pricing and other competitive conditions. |
A failure to obtain and retain new customers, or a loss of existing customers, or a reduction in tests ordered, could impact the Company’s ability to successfully grow its business.
The Company needs to obtain and retain new customers. In addition, a reduction in tests ordered, without offsetting growth in its customer base, could impact the Company’s ability to successfully grow its business and could have a material adverse impact on the Company’s net revenues and profitability. We compete primarily based on the quality of testing, timeliness of results, reputation in the industry, the pricing of services and ability to employ qualified personnel. The Company’s failure to successfully compete on any of these factors could result in the loss of customers and a reduction in the Company’s ability to expand its customer base.
Our business could be harmed if we are unable to protect our technology.
We rely primarily on a combination of trade secrets, patents and trademark laws and confidentiality procedures to protect our technology. Despite these precautions, unauthorized third parties may infringe or copy portions of our technology. In addition, because patent applications in the United States are not publicly disclosed until either: (1) 18 months after the application filing date or (2) the publication date of an issued patent wherein applicant(s) seek only US patent protection, applications not yet disclosed may have been filed which relate to our technology. Moreover, there is a risk that foreign intellectual property laws will not protect our intellectual property rights to the same extent as United States intellectual property laws. In the absence of the foregoing protections, we may be vulnerable to competitors who attempt to copy our products, processes or technology.
Our business could be affected by IT system failures or Cybersecurity breaches.
A computer or IT system failure could affect our ability to perform tests, report test results or properly bill customers for services performed. Failures could occur as a result of the standardization of our IT systems and other system conversions, telecommunications failures, malicious human acts (such as electronic break-ins or computer viruses) or natural disasters. Sustained system failures or interruption of the Company’s systems in one or more of its operations could disrupt the Company’s ability to process and provide test results in a timely manner and/or bill the appropriate party. Failure of the Company’s information systems could adversely affect the Company’s business, profitability and financial condition.
Our technologies, systems and networks may be subject to cybersecurity breaches. Although we have experienced occasional, actual or attempted breaches of our cybersecurity, none of these breaches has had a material effect on our business, operations or reputation. If our systems for protecting against cybersecurity risks prove to be insufficient, we could be adversely affected by having our business systems compromised, our proprietary information altered, lost or stolen, or our business operations disrupted. As cyber attacks continue to evolve, we may be required to expend significant additional resources to continue to modify or enhance our protective measures or to investigate and remediate any information systems and related infrastructure security vulnerabilities.
In addition, certain third parties to whom we outsource our services and functions, or with whom we interface, store our confidential patient data or other confidential information as also subject to the same IT risks. A breach or attack affecting these outsourced third parties could negatively impact our business.
Failure to maintain confidential information could result in a significant financial impact.
The Company maintains confidential information regarding the results of drug tests and other information including credit card and payment information from our customers. The failure to protect this information could result in lawsuits, fines or penalties. Any loss of data or breach of confidentiality, such as through a computer security breach, could expose the Company to a financial liability.
Our future success will depend on the continued services of our key personnel.
Our people are a critical resource. The loss of any of our key personnel could harm our business and prospects. We may not be able to attract and retain personnel necessary for the development of our business. We do not have key personnel under contract other than 4 officers who have agreements providing for severance and non-compete covenants in the event of termination of employment following a change of control. Further, we do not have any key man life insurance for any of our officers or other key personnel.
| 7 |
There is a risk that our insurance will not be sufficient to protect us from errors and omissions liability or other claims, or that in the future errors and omissions insurance will not be available to us at a reasonable cost, if at all.
Our business involves the risk of claims of errors and omissions and other claims inherent to our business. We maintain errors and omissions and general liability insurance subject to deductibles and exclusions. There is a risk that our insurance will not be sufficient to protect us from all such possible claims. An under-insured or uninsured claim could harm our operating results or financial condition.
Our research and development capabilities may not produce viable new services or products.
In order to remain competitive, we need to continually improve our products, develop new technologies to replace older technologies that have either become obsolete or for which patent protection is has expired. It is uncertain whether we will continually be able to develop services that are more efficient, effective or that are suitable for our customers. Our ability to create viable products or services depends on many factors, including the implementation of appropriate technologies, the development of effective new research tools, the complexity of the chemistry and biology, the lack of predictability in the scientific process and the performance and decision-making capabilities of our scientists. There is no guarantee that our research and development teams will be successful in developing improvements to our technology.
Improved testing technologies, or the Company’s customers using new technologies to perform their own tests, could adversely affect the Company’s business.
Advances in technology may lead to the development of more cost-effective technologies that can be operated by third parties or customers themselves in their own offices, without requiring the services of a freestanding laboratory. Development of such technology and its use by the Company’s customers could reduce the demand for its testing services and negatively impact our revenues.
We may not be able to recruit and retain the experienced scientists and management we need to compete in our industry.
Our future success depends upon our ability to attract, retain and motivate highly skilled scientists and management. Our ability to achieve our business strategies depends on our ability to hire and retain high caliber scientists and other qualified experts. We compete with other testing companies, research companies and academic and research institutions to recruit personnel and face significant competition for qualified personnel. We may incur greater costs than anticipated, or may not be successful, in attracting new scientists or management or in retaining or motivating our existing personnel.
Our future success also depends on the personal efforts and abilities of the principal members of our senior management and scientific staff to provide strategic direction, to manage our operations and maintain a cohesive and stable environment.
Our facilities and practices may fail to comply with government regulations.
Our testing facilities and processes must be operated in conformity with current government regulations. These requirements include, among other things, quality control, quality assurance and the maintenance of records and documentation. If we fail to comply with these requirements, we may not be able to continue our services to certain customers, or we could be subject to fines and penalties, suspension of production, or withdrawal of our certifications. We operate a facility that we believe conforms to all applicable requirements. This facility and our testing practices are subject to periodic regulatory inspections to ensure compliance.
Our business could be harmed from the loss or suspension of any licenses.
The forensic laboratory testing industry is subject to significant regulation and many of these statutes and regulations are subject to change. The Company cannot assure that applicable statutes and regulations will not be interpreted or applied by a regulatory authority in a manner that would adversely affect its business. Potential sanctions for violation of these regulations could include the suspension or loss of various licenses, certificates and authorizations, which could have a material adverse effect on the Company’s business. In addition, potential delays in renewals of licenses could also harm the Company.
If our use of chemical and hazardous materials violates applicable laws or regulations or causes personal injury we may be liable for damages.
Our drug testing activities, including the analysis and synthesis of chemicals, involve the controlled use of chemicals, including flammable, combustible, and toxic materials that are potentially hazardous. Our use, storage, handling and disposal of these materials is subject to federal, state and local laws and regulations, including the Resource Conservation and Recovery Act, the Occupational Safety and Health Act and local fire codes, and regulations promulgated by the Department of Transportation, the Drug Enforcement Agency, the Department of Energy, and the California Department of Public Health and Environment. We may incur significant costs to comply with these laws and regulations in the future. In addition, we cannot completely eliminate the risk of accidental contamination or injury from these materials, which could result in material unanticipated expenses, such as substantial fines or penalties, remediation costs or damages, or the loss of a permit or other authorization to operate or engage in our business. Those expenses could exceed our net worth and limit our ability to raise additional capital.
Our operations could be interrupted by damage to our laboratory facilities.
Our operations are dependent upon the continued use of our laboratories and equipment in Culver City, California. Catastrophic events, including earthquakes, fires or explosions, could damage our laboratories, equipment, scientific data, work in progress or inventories of chemicals and may materially interrupt our business. We employ safety precautions in our laboratory activities in order to reduce the likelihood of the occurrence of certain catastrophic events; however, we cannot eliminate the chance that such events will occur. Rebuilding our facilities could be time consuming and result in substantial delays in fulfilling our agreements with our customers. We maintain business interruption insurance to cover continuing expenses and lost revenue caused by such occurrences. However, this insurance does not compensate us for the loss of opportunity and potential harm to customer relations that our inability to meet our customers’ needs in a timely manner could create.
| 8 |
Agreements we have with our employees, consultants and customers may not afford adequate protection for our trade secrets, confidential information and other proprietary information.
In addition to patent protection, we also rely on copyright and trademark protection, trade secrets, know-how, continuing technological innovation and licensing opportunities. In an effort to maintain the confidentiality and ownership of our trade secrets and proprietary information, we require our employees, consultants and advisors to execute confidentiality and proprietary information agreements. However, these agreements may not provide us with adequate protection against improper use or disclosure of confidential information and there may not be adequate remedies in the event of unauthorized use or disclosure. Furthermore, we may from time to time hire scientific personnel formerly employed by other companies involved in one or more areas similar to the activities we conduct. In some situations, our confidentiality and proprietary information agreements may conflict with, or be subject to, the rights of third parties with whom our employees, consultants or advisors have prior employment or consulting relationships. Although we require our employees and consultants to maintain the confidentiality of all proprietary information of their previous employers, these individuals, or we, may be subject to allegations of trade secret misappropriation or other similar claims as a result of their prior affiliations. Finally, others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets. Our failure or inability to protect our proprietary information and techniques may inhibit or limit our ability to compete effectively, or exclude certain competitors from the market.
We are subject to numerous political, legal, operational and other risks as a result of our international operations which could impact our business in many ways.
Although we conduct a majority of our business in the United States, a significant portion of our business is derived from Brazil. Our international operations increase our exposure to the inherent risks of doing business in international markets. Depending on the market, these risks include without limitation:
| • | changes in the local economic environment or local laws or regulations |
| • | political instability, social changes, local market practices and changes |
| • | intellectual property legal protections and remedies |
| • | trade regulations |
| • | foreign currency exchange rate fluctuations |
| • | attracting and retaining qualified employees and independent contractors including distributors |
| • | export and import and exchange controls |
| • | weak legal systems which may affect our ability to enforce contractual rights |
| • | our reliance on one distributor in Brazil |
As the Company previously disclosed, there are greater challenges and uncertainties in a new, large and developing market, such as Brazil. See Item 1. Business – Distribution, for discussion on the Company’s Brazilian distributor.
International operations also require us to devote significant management resources to implement our controls and systems in new markets, to comply with the U.S. Foreign Corrupt Practices Act and similar anti-corruption laws in non-U.S. jurisdictions and to overcome challenges based on differing languages and cultures.
International trade policies may impact demand for our products and our competitive position.
Government policies on international trade and investment such as import quotas, capital controls or tariffs, whether adopted by individual governments or addressed by regional trade blocs, can affect the demand for our services, impact the competitive position of our products or prevent us from being able to sell products in certain countries. The implementation of more restrictive trade policies, such as more detailed inspections, higher tariffs or new barriers to entry, could negatively impact our business, results of operations and financial condition. For example, a government’s adoption of “buy national” policies or retaliation by another government against such policies could have a negative impact on our results of operations.
Global operations are subject to extensive trade and anti-corruption laws and regulations.
The U.S. Foreign Corrupt Practices Act and similar foreign anti-corruption laws generally prohibit companies and their intermediaries from making improper payments or providing anything of value to improperly influence foreign government officials for the purpose of obtaining or retaining business or obtaining an unfair advantage. Recent years have seen a substantial increase in the global enforcement of anti-corruption laws. Our operations outside the United States could increase the risk of such violations. Violations of anti-corruption laws or regulations by our employees or by intermediaries acting on our behalf may result in severe criminal or civil sanctions, could disrupt our business, and result in an adverse effect on our business and results of operations or financial condition.
| 9 |
We may incur additional tax expense or become subject to additional tax exposure.
We are subject to income taxes in the United States and Brazil. Our future results of operations could be adversely affected by changes in the effective tax rate as a result of a change in the mix of earnings, changes in our method of distribution in foreign countries, changes in countries with differing statutory tax rates, changes in our Brazil-derived revenues, changes in our overall profitability, changes in tax laws or treaties or in their application or interpretation, changes in tax rates, changes in generally accepted accounting principles, changes in the valuation of deferred tax assets and liabilities, changes in the amount of earnings indefinitely reinvested offshore, the results of audits and examinations of previously filed tax returns and continuing assessments of our tax exposures. We may be subject to examination of our income tax returns by the U.S. Internal Revenue Service and other tax authorities. If our effective tax rates were to increase, or if the ultimate determination of our taxes owed is for an amount in excess of amounts previously accrued, our operating results, cash flows and financial condition could be adversely affected. For information regarding additional matters related to our taxes, please see Note 5 — "Income Taxes" to the Consolidated Financial Statements included in this Annual Report.
Currency exchange rate fluctuations affect our results of operations, as reported in our financial statements.
We currently have revenues from many countries, however, we are only subject to currency exchange risk related to the Brazilian Real. We are subject to currency exchange rate risk to the extent that our costs are denominated in currencies other than those in which we earn revenues. There can be no assurance that currency exchange rate fluctuations will not adversely affect our results of operations, financial condition and cash flows.
We also face risks arising from the imposition of exchange controls and currency devaluations. Exchange controls may limit our ability to convert foreign currencies into U.S. dollars or to remit dividends and other payments by our foreign subsidiaries or businesses located in or conducted within a country imposing controls. Currency devaluations result in a diminished value of funds denominated in the currency of the country instituting the devaluation.
Risks Related to Our Stock
Our quarterly operating results could fluctuate significantly, which could cause our stock price to decline.
Our quarterly operating results have fluctuated in the past and are likely to fluctuate in the future. Our results are impacted by the extent to which we are able to gain new customers, both domestically and internationally, competitive pricing, and on the hiring practices of our existing customers, including seasonality. Demand for drug testing can be impacted by changes in government requirements regarding testing for drugs of abuse, delays in implementation of such requirements, as well as general economic conditions. Entering into new customer contracts can involve a long lead time. Accordingly, negotiation can be lengthy and is subject to a number of significant risks, including customers’ budgetary constraints and internal reviews. Due to these and other market factors, our operating results could fluctuate significantly from quarter to quarter. In addition, we may experience significant fluctuations in quarterly operating results due to factors such as general and industry-specific economic conditions that may affect the budgets and the hiring practices of our customers.
Due to the possibility of fluctuations in our revenue and expenses, we believe that quarter-to-quarter comparisons of our operating results are not necessarily a good indication of our future performance. Our operating results in some quarters may not meet the expectations of stock market analysts and investors. If we do not meet analysts’ and/or investors’ expectations, our stock price could decline.
Our stock price could experience substantial volatility.
The market price of our common stock has historically experienced and may continue to experience extensive volatility. Our quarterly operating results, the success or failure of future development efforts, changes in general conditions in the economy or the financial markets and other developments affecting our customers, our distributors, our competitors or us could cause the market price of our common stock to fluctuate substantially. This volatility may adversely affect the price of our common stock. In the past, securities class action litigation has often been instituted following periods of volatility in the market price of a company’s securities. A securities class action suit against us could result in potential liabilities, substantial costs and the diversion of management’s attention and resources, regardless of whether we win or lose.
No assurance as to when we will resume paying dividends.
Following the first quarter 2020, in connection with, and as a result of the COVID-19 pandemic and related government programs adopted in response to the COVID-19 pandemic, we suspended our quarterly dividend and have not yet reinstated it. Because the Company has historically paid dividends, the cessation of our quarterly dividend could negatively affect our stock price. As of March 31, 2020, the Company had paid dividends on our common stock for ninety-four consecutive quarters. We currently expect to pay quarterly dividends in the future, although such payments are at the discretion of our Board of Directors, and will depend upon our financial condition, results of operations, capital requirements, government requirements and restrictions and other factors that our Board of Directors may consider at its discretion. In the absence of dividends, a return on investment in our common stock depends entirely upon future appreciation. There is no guarantee that our common stock will appreciate in value or even maintain the price at which stockholders have purchased their shares.
| 10 |
The general economic condition could deteriorate.
Our business is dependent upon new hiring and the supply of new jobs created by overall economic conditions. If the economy deteriorates, leading to a downturn in new job creation, our business and stock price could be adversely affected.
Item 1B. Unresolved Staff Comments
Not applicable.
The Company maintains its corporate offices and northeast sales offices at 289 Great Road, Acton, Massachusetts, 01720; the office consists of 6 thousand square feet and is leased through February 2024.
The Company leases two facilities for laboratory purposes in Culver City, California. The first is 14 thousand square feet of space with an additional 10 thousand square feet of storage space. This facility is leased through December 2022. The second facility of 16 thousand square feet is leased through April 2025.
The Company is involved in various suits and claims in the ordinary course of business. The Company does not believe that the disposition of any such suits or claims will have a material adverse effect on the continuing operations or financial condition of the Company.
Item 4. Mine Safety Disclosures
Not applicable.
| 11 |
Item 5. Market for Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities
The Company’s common stock is traded on the NASDAQ Stock Market under the symbol “PMD”. As of March 15, 2021, there were 165 record holders of the Company’s common stock. The number of record owners was determined from the Company’s stockholder records maintained by the Company’s transfer agent and does not include beneficial owners of the Company’s common stock whose shares are held in the names of various security holders, dealers and clearing agencies. The Company believes that the number of beneficial owners of the Company’s common stock held by others as or in nominee names exceeds 3,690.
The following table sets forth for the periods indicated the range of prices for the Company’s common stock as reported by the NASDAQ Stock Market and dividends declared by the Company.
| High | Low | Dividends | ||||||||||
| Fiscal 2020: | ||||||||||||
| First Quarter | $ | 10.69 | $ | 4.54 | $ | 0.18 | ||||||
| Second Quarter | 6.79 | 4.89 | - | |||||||||
| Third Quarter | 6.35 | 4.33 | - | |||||||||
| Fourth Quarter | 5.44 | 3.58 | - | |||||||||
| Fiscal 2019: | ||||||||||||
| First Quarter | $ | 19.64 | $ | 13.68 | $ | 0.18 | ||||||
| Second Quarter | 14.67 | 9.25 | 0.18 | |||||||||
| Third Quarter | 10.36 | 7.12 | 0.18 | |||||||||
| Fourth Quarter | 9.80 | 8.30 | 0.18 | |||||||||
Before the COVID-19 outbreak, the Company declared a dividend on February 11, 2020, which was paid on March 3, 2020, following which the quarterly dividend was suspended. Company’s current intention is to resume the payment of dividends to the extent funds are available and not required for operating purposes or capital requirements, and subject to any applicable contractual restrictions, and only then, upon approval by the Board of Directors.
Issuer Purchases of Equity Securities
During 2020, the Company did not repurchase any common shares for treasury.
Unregistered Sales of Equity Securities and Use of Proceeds
There were no unregistered sales of common stock of the Company during 2020.
| 12 |
Performance Graph
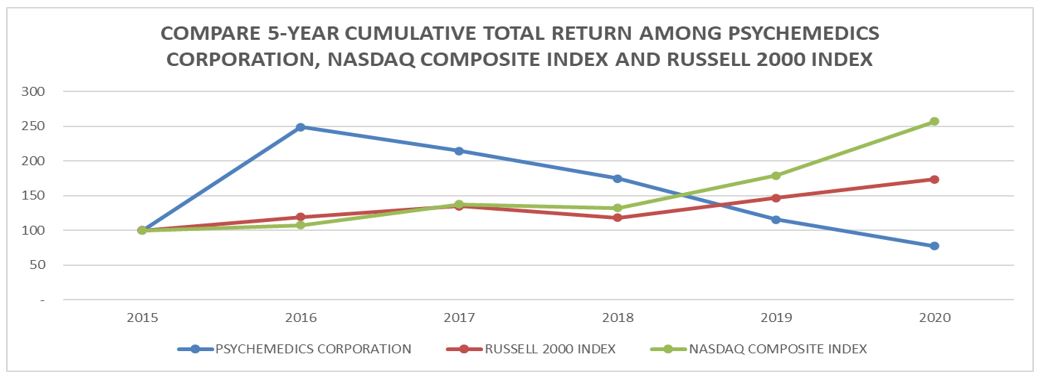
Calculated by the Company using www.yahoo.com/finance historical prices.
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |||||||||||||
| PSYCHEMEDICS CORPORATION | 100.00 | 249.31 | 214.60 | 175.15 | 115.98 | 77.71 | ||||||||||||
| RUSSELL 2000 INDEX | 100.00 | 119.48 | 135.18 | 118.72 | 146.89 | 173.86 | ||||||||||||
| NASDAQ COMPOSITE INDEX | 100.00 | 107.50 | 137.86 | 132.51 | 179.19 | 257.38 |
| (1) | The above graph assumes a $100 investment on December 31, 2015, through the end of the 5-year period ended December 31, 2020 in the Company’s Common Stock, the Russell 2000 Index and the NASDAQ Composite Index. The prices all assume the reinvestment of dividends. |
| (2) | The Russell 2000 Index is composed of the smallest 2,000 companies in the Russell 3,000 Index. The Company has been unable to identify a peer group of companies that engage in testing of drugs of abuse, except for large pharmaceutical companies where such business is insignificant to such companies’ other lines of businesses. The Company therefore uses in its proxy statements a peer index based on market capitalization. |
| (3) | The NASDAQ Composite Index includes companies whose shares are traded on the NASDAQ Stock Market. |
Item 6. Selected Financial Data
The selected financial data presented below is derived from our financial statements and should be read in connection with those statements.
| Year Ended December 31, | ||||||||||||||||||||
| 2020 | 2019 | 2018 | 2017 | 2016 | ||||||||||||||||
| (In thousands, except for per share data) | ||||||||||||||||||||
| Revenue | $ | 21,360 | $ | 37,678 | $ | 42,674 | $ | 39,701 | $ | 38,980 | ||||||||||
| Gross profit | 4,886 | 16,444 | 20,618 | 19,822 | 21,450 | |||||||||||||||
| (Loss) income from operations | (6,066 | ) | 2,998 | 7,610 | 8,157 | 10,110 | ||||||||||||||
| Net (loss) income | (3,859 | ) | 1,542 | 4,584 | 6,121 | 6,678 | ||||||||||||||
| Total assets | 24,003 | 27,531 | 24,974 | 26,508 | 25,032 | |||||||||||||||
| Working capital | 5,657 | 7,016 | 9,810 | 9,640 | 6,359 | |||||||||||||||
| Shareholders’ equity | 12,512 | 16,820 | 18,747 | 18,620 | 15,607 | |||||||||||||||
| Basic net (loss) income per share | $ | (0.70 | ) | $ | 0.28 | $ | 0.83 | $ | 1.12 | $ | 1.23 | |||||||||
| Diluted net (loss) income per share | $ | (0.70 | ) | $ | 0.28 | $ | 0.83 | $ | 1.10 | $ | 1.22 | |||||||||
| Cash dividends declared per common share | $ | 0.18 | $ | 0.72 | $ | 0.69 | $ | 0.60 | $ | 0.60 | ||||||||||
| 13 |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read together with the more detailed business information and financial statements and related notes that appear elsewhere in this annual report on Form 10-K. This annual report may contain certain “forward-looking” information within the meaning of the Private Securities Litigation Reform Act of 1995. This information involves risks and uncertainties. Actual results may differ materially from the results discussed in the forward-looking statements. Factors that might cause such a difference include, but are not limited to, those discussed in Item 1A — Risk Factors.
Overview
Psychemedics Corporation is the world’s largest provider of hair testing for drugs of abuse, utilizing a patented hair analysis method involving digestion of hair, enzyme immunoassay technology and confirmation by mass spectrometry to analyze human hair to detect abused substances. The Company’s customers include Fortune 500 companies, as well as small to mid-size corporations, schools and governmental entities, located in the United States and internationally. During the year ended December 31, 2020, the Company generated $21.4 million in revenue, while realizing a gross profit of 23% and incurring a net loss of $3.9 million and diluted net loss per share of $0.70 for the year ended December 31, 2020, versus diluted net income per share of $0.28 for fiscal year 2019, primarily due to the significant contraction in the overall economy as a result of COVID-19 resulting in a significant slow-down in job creation which substantially lowered our sales volume.
The following table sets forth, for the periods indicated, the selected statements of operations data as a percentage of total revenue:
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | 2018 | ||||||||||
| Revenues | 100.0 | % | 100.0 | % | 100.0 | % | ||||||
| Cost of revenues | 77.1 | % | 56.4 | % | 51.7 | % | ||||||
| Gross profit | 22.9 | % | 43.6 | % | 48.3 | % | ||||||
| Operating Expenses: | ||||||||||||
| General & administrative | 28.5 | % | 19.1 | % | 15.1 | % | ||||||
| Marketing & selling | 16.7 | % | 12.3 | % | 11.8 | % | ||||||
| Research & development | 6.0 | % | 4.2 | % | 3.6 | % | ||||||
| Total Operating Expenses | 51.2 | % | 35.6 | % | 30.5 | % | ||||||
| Operating (loss) income | -28.3 | % | 8.0 | % | 17.8 | % | ||||||
| Other (expense) income | -0.7 | % | 0.2 | % | 0.1 | % | ||||||
| Net (loss) income before provision for income taxes | -29.0 | % | 8.2 | % | 17.9 | % | ||||||
| (Benefit from) provision for income taxes | -11.0 | % | 4.0 | % | 7.2 | % | ||||||
| Net (loss) income | -18.0 | % | 4.2 | % | 10.7 | % | ||||||
Revenue by Geographic Region
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | 2018 | ||||||||||
| Consolidated Revenue: | ||||||||||||
| United States | $ | 19,486 | $ | 27,329 | $ | 29,189 | ||||||
| Brazil | 1,344 | 9,819 | 13,046 | |||||||||
| Other | 530 | 530 | 439 | |||||||||
| Total Revenue | $ | 21,360 | $ | 37,678 | $ | 42,674 | ||||||
| 14 |
Results for the Year Ended December 31, 2020 Compared to Results for the Year Ended December 31, 2019 (in thousands)
| 2020 | 2019 | Change | % | |||||||||||||
| Revenues | $ | 21,360 | $ | 37,678 | $ | (16,318 | ) | -43 | % | |||||||
| Cost of revenues | 16,474 | 21,234 | (4,760 | ) | -22 | % | ||||||||||
| Gross profit | 4,886 | 16,444 | (11,558 | ) | -70 | % | ||||||||||
| Operating Expenses: | ||||||||||||||||
| General & administrative | 6,095 | 7,221 | (1,126 | ) | -16 | % | ||||||||||
| Marketing & selling | 3,577 | 4,658 | (1,081 | ) | -23 | % | ||||||||||
| Research & development | 1,280 | 1,567 | (287 | ) | -18 | % | ||||||||||
| Total Operating Expenses | 10,952 | 13,446 | (2,494 | ) | -19 | % | ||||||||||
| Operating (loss) income | (6,066 | ) | 2,998 | (9,064 | ) | -302 | % | |||||||||
| Other (expense) income | (140 | ) | 58 | (198 | ) | -341 | % | |||||||||
| Net (loss) income before provision for income taxes | (6,206 | ) | 3,056 | (9,262 | ) | -303 | % | |||||||||
| (Benefit from) provision for income taxes | (2,347 | ) | 1,514 | (3,861 | ) | -255 | % | |||||||||
| Net (loss) income | $ | (3,859 | ) | $ | 1,542 | $ | (5,401 | ) | -350 | % | ||||||
Revenue: The revenue decline of 43% was primarily due to a 56% decrease in volume, offset by a 13% increase in average revenue per sample. International revenue was down 82% from 2019 to 2020, due to decline in volume from unfavorable market forces in Brazil and the COVID-19 pandemic and domestic revenue was down 29% from 2019 to 2020, also due primarily to the COVID-19 pandemic. See geographic breakdown of revenue above. The Company does not expect any change in the decline it has experienced in its Brazil driver license business as this market continues to be considerably uncertain.
Gross profit: The 70% decrease in gross profit was primarily due to lower sales volume. This lower volume was the primary factor in the gross profit percentage reduction from 44% in 2019 to 23% in 2020. In addition, gross profit was also adversely impacted by a requirement that we retain certain levels of personnel to qualify for PPP Loan forgiveness with no offsetting proportional revenue. The staffing levels we maintained did not support the volume sales noted above.
General and administrative (“G&A”) expenses: G&A expenses decreased 16% from 2019 to 2020, primarily driven by reductions in personnel after the PPP Loan covered period expired, cost-savings initiatives, including salary reductions, in response to the COVID-19 pandemic and lower international tax expense. These decreases were partially offset by higher legal expenses related to the exploration of possible strategic alternatives in an effort to enhance shareholder value.
Marketing and selling expenses: Marketing and selling expenses decreased 23% from 2019 to 2020, primarily driven by cost reduction initiatives; specifically, lower personnel related costs (including less travel and meals). In addition, lower recruiting fees and commissions from volume decline contributed to the comparative decrease.
Income Taxes: During the year ended December 31, 2020, the Company recorded a tax benefit of $2.3 million representing a tax rate of 38% compared to a tax rate of 50% in 2019. For information regarding additional matters related to our taxes, please see Note 5 — "Income Taxes" to the Consolidated Financial Statements included in this Annual Report.
| 15 |
Results for the Year Ended December 31, 2019 Compared to Results for the Year Ended December 31, 2018 (in thousands)
| 2019 | 2018 | Change | % | |||||||||||||
| Revenues | $ | 37,678 | $ | 42,674 | $ | (4,996 | ) | -12 | % | |||||||
| Cost of revenues | 21,234 | 22,056 | (822 | ) | -4 | % | ||||||||||
| Gross profit | 16,444 | 20,618 | (4,174 | ) | -20 | % | ||||||||||
| Operating Expenses: | ||||||||||||||||
| General & administrative | 7,221 | 6,430 | 791 | 12 | % | |||||||||||
| Marketing & selling | 4,658 | 5,027 | (369 | ) | -7 | % | ||||||||||
| Research & development | 1,567 | 1,551 | 16 | 1 | % | |||||||||||
| Total Operating Expenses | 13,446 | 13,008 | 438 | 3 | % | |||||||||||
| Operating income | 2,998 | 7,610 | (4,612 | ) | -61 | % | ||||||||||
| Other income | 58 | 43 | 15 | 35 | % | |||||||||||
| Net income before provision for income taxes | 3,056 | 7,653 | (4,597 | ) | -60 | % | ||||||||||
| Provision for income taxes | 1,514 | 3,069 | (1,555 | ) | -51 | % | ||||||||||
| Net income | $ | 1,542 | $ | 4,584 | $ | (3,042 | ) | -66 | % | |||||||
Revenue: Total revenue decline of 12% was primarily due to an 11% decrease in volume and a 1% decrease in average revenue per sample. International revenue was down 23% (due to decline in volume from unfavorable market forces in Brazil) and domestic revenue was down 6% from 2018 to 2019. See geographic breakdown of revenue above.
Gross profit: The decrease in gross profit was primarily due to lower sales volume. This lower volume was the primary factor in the gross margin reduction from 48% in 2018 to 44% in 2019. Gross profit was also adversely impacted by higher foreign taxes on Brazil revenue and additional costs related to the Company’s new leased facility in California.
General and administrative (“G&A”) expenses: G&A expenses included a one-time charge of $0.8 million of taxes related to the repatriation of cash from Brazil to the United States. Without this transaction, G&A expenses would have been down 1%.
Marketing and selling expenses: The decrease in marketing and selling expenses was primarily a result of lower personnel related costs in 2019, specifically lower recruiting fees and commissions.
Income Taxes: During the year ended December 31, 2019, the Company recorded a tax provision of $1.5 million representing a tax rate of 50% compared to a tax rate of 40% in 2018. Approximately 10% of the tax provision in 2019 was attributed to domestic taxes, with the other 90% attributed to Brazil. Brazil income taxes are based on sales, not pre-tax income which can cause significant changes to the effective tax rate. For information regarding additional matters related to our taxes, please see Note 5 — "Income Taxes" to the Consolidated Financial Statements included in this Annual Report.
Liquidity and Capital Resources
The Company had $2.8 million and $7.3 million of cash and cash equivalents as of December 31, 2020 and 2019, respectively. The Company’s operating activities used net cash of $4.1 million in 2020, and generated net cash of $4.3 million in 2019 and $7.9 million in 2018. Investing activities used net cash of $0.9 million in 2020, generated net cash of $2.1 million in 2019 and used net cash of $5.4 million in 2018. Financing activities provided net cash of $0.5 million in 2020 and used $3.0 million in 2019 and $5.6 million in 2018.
Operating cash used in operations of $4.1 million in 2020 primarily reflected the net loss of $3.9 million adjusted for depreciation and amortization of $2.7 million, stock compensation expense of $0.6 million, and a decrease in net deferred tax liabilities of $0.3 million. Cash used in operations was also affected by the following changes in assets and liabilities: a decrease in accounts receivable of $0.4 million, a decrease in accrued expenses of $1.8 million, and a decrease in prepaid expenses (and other current assets) of $1.6 million. The $8.4 million change in operating cash from a positive $4.3 million in 2019 to a negative $4.1 million in 2020 was primarily driven by lower net income in 2020.
Operating cash flow of $4.3 million in 2019 primarily reflected net income of $1.5 million adjusted for depreciation and amortization of $2.9 million, stock compensation expense of $0.8 million, and a decrease in net deferred tax liabilities of $0.4 million. Operating cash flow was affected by the following changes in assets and liabilities: a decrease in accounts receivable of $1.0 million, an increase in accounts payable of $0.5 million, an increase in accrued expenses of $0.7 million, and an increase in prepaid expenses (and other current assets) of $0.4 million. The operating cash flow in 2019 was $3.6 million less than in 2018 primarily due to lower net income.
| 16 |
Operating cash flow of $7.9 million in 2018 primarily reflected net income of $4.6 million adjusted for depreciation and amortization of $3.1 million, stock compensation expense of $0.6 million, and a decrease in net deferred tax liabilities of $0.3 million. Operating cash flow was affected by the following changes in assets and liabilities: an increase in accounts receivable of $0.4 million, an increase in accounts payable of $0.1 million, an increase in accrued expenses of $0.1 million, and a decrease in prepaid expenses (and other current assets) of $0.1 million. The operating cash flow in 2018 was $1.2 million less than in 2018.
Cash used in investing activities principally reflected the purchase of capital expenditures. Capital expenditures were $1.0 million, $1.7 million and $1.2 million in 2020, 2019 and 2018, respectively. In 2020, the expenditures related principally to leasehold improvements, laboratory equipment and computer software. Marketable securities transactions consisted of the sale of one certificate of deposit (“CD”) for $3.8 million in 2019 and the purchase of the same CD for $4.0 million in 2018.
Financing cash flow in 2020 principally reflected the proceeds from our PPP Loan (described further below) of approximately $2.2 million, partially offset by repayments under the Equipment Loan Arrangement. During 2020, 2019 and 2018, the Company did not repurchase any shares of common stock for treasury. The Company has authorized 750,000 shares for repurchase since June of 1998, of which 250,000 shares of common stock were authorized in March of 2008 for repurchase. Since 1998, a total of 550,684 shares have been repurchased. The Company also distributed cash dividends to its shareholders of $1.0 million in 2020, $4.0 million in 2019 and $3.8 million in 2018.
As of March 31, 2020, the Company had paid dividends over the prior ninety-four quarters. Following the first quarter of 2020, our Board of Directors suspended our quarterly dividend payment as we prioritized our liquidity and balance sheet. The Company’s intention is to reinstate the payment of dividends to the extent funds are available and not required for operating purposes or capital requirements. There can be no assurance that in the future the Company will reinstate payment of a quarterly dividend payment, or the amount of any such dividend.
At December 31, 2020, the Company’s principal sources of liquidity included approximately $2.8 million of cash on hand. Management currently believes that such funds, together with future operating profits, should be adequate to fund anticipated working capital requirements, including debt obligations, and capital expenditures for at least the next 12 months. Depending upon the Company’s results of operations, its future capital needs and available marketing opportunities, the Company may use various financing sources to raise additional funds. Such sources could include but are not limited to, issuance of common stock or debt financing, lines of credit, or equipment leasing, although there is no assurance that such financings will be available to the Company on terms it deems acceptable, if at all.
On May 4, 2020, the Company borrowed approximately $2.2 million from Bank of America, N.A., pursuant to the PPP, established under the CARES Act. The PPP Loan is subject to forgiveness under the PPP upon the Company’s request to the extent that the proceeds are used to pay expenses permitted by the PPP.
On November 6, 2020, the Company applied for forgiveness of the entire amount due on the loan. The application and recommendation from Bank of America, N.A., has been provided to the SBA. Notwithstanding our application for loan forgiveness, we are unable to predict the actual amount of loan forgiveness the SBA will approve. As of December 31, 2020, we had approximately $2.2 million outstanding under the PPP Loan and we were in full compliance with all requirements with respect to the PPP Loan. See Item 1A. Risk Factors of this Annual Report on Form 10-K.
Purchase Commitment
Operating leases consist of rent obligations for the company’s facilities and corporate office. The Company has no significant contractual obligation for supply agreements as of December 31, 2020.
Critical Accounting Policies
The Company’s significant accounting policies are described in Note 2 to the Consolidated Financial Statements included in Item 8 of this Annual Report. Management believes the most critical accounting policies are as follows:
Revenue Recognition
The Company is in the business of performing drug testing services and reporting the results thereof. The Company’s services are primarily drug and alcohol testing for its customers for an agreed-upon fee per unit tested. The revenues are recognized when the drug test is performed and reported to the customer.
The Company records revenue for the shipping of samples from the customer or independent hair collection facility to the laboratory for customers that choose to use the Company’s shipping account. The Company also records revenue for the collection of the hair sample for customers that choose to have the Company manage this process at the same time the sample test is completed and results reported to the customer. The associated costs incurred in connection with these services is recorded as costs of revenue. The Company records revenue for these services on a gross basis as it has determined it is the principal under these arrangements.
The Company also provides expert testimony, when and if necessary, to support the results of the tests, which is generally billed separately and recognized as the services are provided.
| 17 |
Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates, including bad debts, long-lived asset lives, income tax valuation, stock based compensation and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Capitalized Development Costs
We capitalize costs related to significant software projects developed or obtained for internal use in accordance with U.S. generally accepted accounting standards. Costs incurred during the preliminary project work stage or conceptual stage, such as determining the performance requirements, system requirements and data conversion, are expensed as incurred. Costs incurred in the application development phase, such as coding, testing for new software and upgrades that result in additional functionality, are capitalized and are amortized using the straight-line method over the useful life of the software for 5 years. Costs incurred during the post-implementation/operation stage, including training costs and maintenance costs, are expensed as incurred. We capitalized internally developed software costs of approximately $213 thousand, $234 thousand and $299 thousand during the years ended December 31, 2020, 2019 and 2018, respectively. The software development is for primarily for two projects. Determining whether particular costs incurred are more properly attributable to the preliminary or conceptual stage, and thus expensed, or to the application development phase, and thus capitalized and amortized, depends on subjective judgments about the nature of the development work, and our judgments in this regard may differ from those made by other companies. General and administrative costs related to developing or obtaining such software are expensed as incurred.
Allowance for Doubtful Accounts
The allowance for doubtful accounts is based on management’s assessment of the ability to collect amounts owed to it by its customers. Management reviews its accounts receivable aging for doubtful accounts and uses a methodology based on calculating the allowance using a combination of factors including the age of the receivable along with management’s judgment to identify accounts that may not be collectible. The Company routinely assesses the financial strength of its customers and, as a consequence, believes that its accounts receivable credit risk exposure is limited. The Company maintains an allowance for potential credit losses but historically has not experienced any significant losses related to individual customers or groups of customers in any particular industry or geographic area. Bad debt expense has been within management’s expectations.
Income Taxes
The Company accounts for income taxes using the liability method, which requires the Company to recognize a current tax liability or asset for current taxes payable or refundable and a net deferred tax liability for the estimated future tax effects of temporary differences between the financial statement and tax reporting bases of assets and liabilities to the extent that they are realizable. Deferred tax expense (benefit) results from the net change in deferred tax assets and liabilities during the year. A deferred tax valuation allowance is required if it is more likely than not that all or a portion of the recorded deferred tax assets will not be realized.
The Company operates within multiple taxing jurisdictions and could be subject to audit in these jurisdictions. These audits may involve complex issues, which may require an extended period of time to resolve. The Company has provided for its estimated taxes payable in the accompanying financial statements. The Company did not have any interest or penalties accrued as of December 31, 2020 or 2019. The Company does not expect the unrecognized tax benefits to change significantly over the next twelve months.
The Company’s distribution of services in Brazil subjects the Company to Brazil income taxes. These taxes are included in the total provision for income taxes reflected in the financial statements. For information regarding additional matters related to our taxes, please see Note 5 — "Income Taxes" to the Consolidated Financial Statements included in this Annual Report.
The above listing is not intended to be a comprehensive list of all of the Company’s accounting policies. In many cases, the accounting treatment of a particular transaction is specifically dictated by accounting principles generally accepted in the United States, with no need for management’s judgment in their application. There are also areas in which management’s judgment in selecting any available alternative would not produce a materially different result.
Recent Accounting Pronouncements
See Note 2 – Summary of Significant Accounting Policies in the accompanying Notes to the Consolidated Financial Statements included in this Annual Report for further detail on recent accounting pronouncements.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Not required
| 18 |
Item 8. Financial Statements and Supplementary Data
(a) Financial Statements:
| 19 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Shareholders and Board of Directors
Psychemedics Corporation
Acton, Massachusetts
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Psychemedics Corporation (the “Company”) and subsidiaries as of December 31, 2020 and 2019, the related consolidated statements of operations and comprehensive income/(loss), shareholders’ equity, and cash flows for each of the three years in the period ended December 31, 2020, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company and subsidiaries at December 31, 2020 and 2019, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2020, in conformity with accounting principles generally accepted in the United States of America.
Change in Accounting Principle
As discussed in Note 10 to the consolidated financial statements, on January 1, 2019, the Company changed its method of accounting for leases due to the adoption of ASU 2016-02, Leases (ASC 842).
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the consolidated financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ BDO USA, LLP
We have served as the Company's auditor since 2004.
Boston, Massachusetts
March 26, 2021
| 20 |
PSYCHEMEDICS CORPORATION
CONSOLIDATED BALANCE SHEETS
(in thousands, except par value)
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
| Cash and cash equivalents | $ | 2,833 | $ | 7,283 | ||||
| Accounts receivable, net of allowance for doubtful accounts of $37 and $45 at December 31, 2020 and 2019, respectively | 3,356 | 3,780 | ||||||
| Prepaid expenses and other current assets | 914 | 1,306 | ||||||
| Income tax receivable | 2,495 | 482 | ||||||
| Total Current Assets | 9,598 | 12,851 | ||||||
| Property and equipment: | ||||||||
| Computer software | 4,422 | 4,166 | ||||||
| Office furniture and equipment | 2,139 | 2,124 | ||||||
| Laboratory equipment | 15,978 | 16,195 | ||||||
| Leasehold improvements | 3,629 | 4,574 | ||||||
| 26,168 | 27,059 | |||||||
| Accumulated depreciation and amortization | (16,937 | ) | (16,197 | ) | ||||
| 9,231 | 10,862 | |||||||
| Other assets | 888 | 943 | ||||||
| Operating lease right-of-use assets | 4,286 | 2,875 | ||||||
| Total Assets | $ | 24,003 | $ | 27,531 | ||||
| LIABILITIES AND SHAREHOLDERS' EQUITY | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable | $ | 577 | $ | 617 | ||||
| Accrued expenses | 1,801 | 3,577 | ||||||
| Current portion of long-term debt | 688 | 678 | ||||||
| Current portion of operating lease liabilities | 875 | 963 | ||||||
| Total Current Liabilities | 3,941 | 5,835 | ||||||
| Long-term debt | 3,444 | 1,951 | ||||||
| Deferred tax liabilities, long-term | 211 | 550 | ||||||
| Long-term portion of operating lease liabilities | 3,895 | 2,375 | ||||||
| Total Liabilities | 11,491 | 10,711 | ||||||
| Commitments and Contingencies (Note 9) | ||||||||
| Shareholders' Equity: | ||||||||
| Preferred stock, $0.005 par value, 873 shares authorized,no shares issued or outstanding | - | - | ||||||
| Common stock, $0.005 par value; 50,000 shares authorized 6,205 shares and 6,185 shares issued at December 31, 2020 and 2019, respectively, 5,537 shares outstanding and 5,517 shares outstanding at December 31, 2020 and 2019, respectively | 31 | 31 | ||||||
| Additional paid-in capital | 32,803 | 32,249 | ||||||
| Less - Treasury stock, at cost, 668 shares | (10,082 | ) | (10,082 | ) | ||||
| Accumulated deficit | (8,606 | ) | (3,754 | ) | ||||
| Accumulated other comprehensive loss | (1,634 | ) | (1,624 | ) | ||||
| Total Shareholders' Equity | 12,512 | 16,820 | ||||||
| Total Liabilities and Shareholders' Equity | $ | 24,003 | $ | 27,531 | ||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| 21 |
PSYCHEMEDICS CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS AND
COMPREHENSIVE INCOME/(LOSS)
(in thousands, except per share amounts)
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | 2018 | ||||||||||
| Revenues | $ | 21,360 | $ | 37,678 | $ | 42,674 | ||||||
| Cost of revenues | 16,474 | 21,234 | 22,056 | |||||||||
| Gross profit | 4,886 | 16,444 | 20,618 | |||||||||
| Operating Expenses: | ||||||||||||
| General & administrative | 6,095 | 7,221 | 6,430 | |||||||||
| Marketing & selling | 3,577 | 4,658 | 5,027 | |||||||||
| Research & development | 1,280 | 1,567 | 1,551 | |||||||||
| Total Operating Expenses | 10,952 | 13,446 | 13,008 | |||||||||
| Operating (loss) income | (6,066 | ) | 2,998 | 7,610 | ||||||||
| Other (expense) income | (140 | ) | 58 | 43 | ||||||||
| Net (loss) income before provision for income taxes | (6,206 | ) | 3,056 | 7,653 | ||||||||
| (Benefit from) provision for income taxes | (2,347 | ) | 1,514 | 3,069 | ||||||||
| Net (loss) income | $ | (3,859 | ) | $ | 1,542 | $ | 4,584 | |||||
| Other Comprehensive (Loss) Income: | ||||||||||||
| Foreign currency translation, net of taxes | (10 | ) | (225 | ) | (1,161 | ) | ||||||
| Total Comprehensive (Loss) Income | $ | (3,869 | ) | $ | 1,317 | $ | 3,423 | |||||
| Basic net (loss) income per share | $ | (0.70 | ) | $ | 0.28 | $ | 0.83 | |||||
| Diluted net (loss) income per share | $ | (0.70 | ) | $ | 0.28 | $ | 0.83 | |||||
| Dividends declared per share | $ | 0.18 | $ | 0.72 | $ | 0.69 | ||||||
| Weighted average common shares outstanding: | ||||||||||||
| Basic | 5,524 | 5,514 | 5,502 | |||||||||
| Diluted | 5,524 | 5,525 | 5,547 | |||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| 22 |
PSYCHEMEDICS CORPORATION
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
(in thousands, except per share amounts)
| Common Stock | Treasury Stock | Accumulated Other | ||||||||||||||||||||||||||||||
| $0.005 | Paid-In | Accumulated | Comprehensive | |||||||||||||||||||||||||||||
| Shares | par Value | Capital | Shares | Cost | Deficit | Income (loss) | Total | |||||||||||||||||||||||||
| BALANCE, December 31, 2017 | 6,160 | $ | 31 | $ | 31,022 | 668 | $ | (10,082 | ) | $ | (2,113 | ) | $ | (238 | ) | $ | 18,620 | |||||||||||||||
| Shares issued – vested | 15 | - | - | - | - | - | - | - | ||||||||||||||||||||||||
| Tax withholding related to vested shares from employee stock plans | - | - | (93 | ) | - | - | - | - | (93 | ) | ||||||||||||||||||||||
| Stock compensation expense | - | - | 594 | - | - | - | - | 594 | ||||||||||||||||||||||||
| Cash dividends declared ($0.69 per share) | - | - | - | - | - | (3,797 | ) | - | (3,797 | ) | ||||||||||||||||||||||
| Net income | - | - | - | - | - | 4,584 | - | 4,584 | ||||||||||||||||||||||||
| Foreign currency translation, net of taxes | - | - | - | - | - | - | (1,161 | ) | (1,161 | ) | ||||||||||||||||||||||
| BALANCE, December 31, 2018 | 6,175 | 31 | 31,523 | 668 | (10,082 | ) | (1,326 | ) | (1,399 | ) | 18,747 | |||||||||||||||||||||
| Shares issued – vested | 10 | - | - | - | - | - | - | |||||||||||||||||||||||||
| Tax withholding related to vested shares from employee stock plans | - | - | (33 | ) | - | - | - | - | (33 | ) | ||||||||||||||||||||||
| Stock compensation expense | - | - | 759 | - | - | - | - | 759 | ||||||||||||||||||||||||
| Cash dividends declared ($0.72 per share) | - | - | - | - | - | (3,970 | ) | - | (3,970 | ) | ||||||||||||||||||||||
| Net income | - | - | - | - | - | 1,542 | - | 1,542 | ||||||||||||||||||||||||
| Foreign currency translation, net of taxes | - | - | - | - | - | - | (225 | ) | (225 | ) | ||||||||||||||||||||||
| BALANCE, December 31, 2019 | 6,185 | 31 | 32,249 | 668 | (10,082 | ) | (3,754 | ) | (1,624 | ) | 16,820 | |||||||||||||||||||||
| Shares issued – vested | 20 | - | - | - | - | - | - | |||||||||||||||||||||||||
| Tax withholding related to vested shares from employee stock plans | - | - | (9 | ) | - | - | - | - | (9 | ) | ||||||||||||||||||||||
| Stock compensation expense | - | - | 563 | - | - | - | - | 563 | ||||||||||||||||||||||||
| Cash dividends declared ($0.18 per share) | - | - | - | - | - | (993 | ) | - | (993 | ) | ||||||||||||||||||||||
| Net loss | - | - | - | - | - | (3,859 | ) | - | (3,859 | ) | ||||||||||||||||||||||
| Foreign currency translation, net of taxes | - | - | - | - | - | - | (10 | ) | (10 | ) | ||||||||||||||||||||||
| BALANCE, December 31, 2020 | 6,205 | $ | 31 | $ | 32,803 | 668 | $ | (10,082 | ) | $ | (8,606 | ) | $ | (1,634 | ) | $ | 12,512 | |||||||||||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| 23 |
PSYCHEMEDICS CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | 2018 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net (loss) income | $ | (3,859 | ) | $ | 1,542 | $ | 4,584 | |||||
| Adjustments to reconcile net (loss) income to net cash provided by operating activities: | ||||||||||||
| Depreciation and amortization | 2,691 | 2,914 | 3,063 | |||||||||
| ROU asset amortization | 935 | - | - | |||||||||
| Deferred income taxes | (339 | ) | (405 | ) | (288 | ) | ||||||
| Loss on sale of fixed assets | 94 | - | 6 | |||||||||
| Non-cash interest income (expense) | - | 33 | (41 | ) | ||||||||
| Stock compensation expense | 563 | 759 | 594 | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Accounts receivable | 424 | 1,049 | (355 | ) | ||||||||
| Prepaid expenses and other current assets | 392 | 64 | 145 | |||||||||
| Income tax receivable | (2,013 | ) | (482 | ) | - | |||||||
| Accounts payable | (281 | ) | (494 | ) | 77 | |||||||
| Operating lease liabilities | (914 | ) | - | - | ||||||||
| Accrued expenses | (1,776 | ) | (671 | ) | 144 | |||||||
| Net cash (used in) provided by operating activities | (4,083 | ) | 4,309 | 7,929 | ||||||||
| Cash flows from investing activities: | ||||||||||||
| Purchases of investments in short-term investments | - | - | (4,035 | ) | ||||||||
| Proceeds from sale of fixed assets | 140 | - | - | |||||||||
| Proceeds from short-term investments | - | 3,810 | - | |||||||||
| Other assets | (7 | ) | (56 | ) | (133 | ) | ||||||
| Purchases of property and equipment and capitalized software development costs | (991 | ) | (1,677 | ) | (1,191 | ) | ||||||
| Net cash (used in) provided by investing activities | (858 | ) | 2,077 | (5,359 | ) | |||||||
| Cash flows from financing activities: | ||||||||||||
| Cash dividends paid | (993 | ) | (3,970 | ) | (3,797 | ) | ||||||
| Proceeds from issuance of stock, net of tax withholding | (9 | ) | (33 | ) | (93 | ) | ||||||
| Proceeds from PPP Loan | 2,181 | - | - | |||||||||
| Proceeds from equipment financing | - | 1,416 | - | |||||||||
| Payments of equipment financing | (678 | ) | (415 | ) | (1,749 | ) | ||||||
| Net cash provided by (used in) financing activities | 501 | (3,002 | ) | (5,639 | ) | |||||||
| Effect of exchange rate changes on cash | (10 | ) | (170 | ) | (1,027 | ) | ||||||
| Net (decrease) increase in cash and cash equivalents | (4,450 | ) | 3,214 | (4,096 | ) | |||||||
| Cash and cash equivalents, beginning of year | 7,283 | 4,069 | 8,165 | |||||||||
| Cash and cash equivalents, end of year | $ | 2,833 | $ | 7,283 | $ | 4,069 | ||||||
| Supplemental disclosures of cash flow information: | ||||||||||||
| Cash paid for income taxes | $ | 249 | $ | 2,898 | $ | 3,743 | ||||||
| Cash paid for interest | $ | 75 | $ | 59 | $ | 108 | ||||||
| Cash paid for operating leases | $ | 1,038 | $ | 1,199 | $ | 994 | ||||||
| Right-of-use assets acquired through operating leases | $ | 2,346 | $ | 4,363 | $ | - | ||||||
| Non-cash investing and financing activities: | ||||||||||||
| Purchases of equipment through accounts payable and accrued liabilities | $ | 241 | $ | 1,882 | $ | 207 | ||||||
The accompanying notes are an integral part of these Consolidated Financial Statements.
| 24 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
1. Nature of Business
Company Overview
Psychemedics Corporation (the “Company”) provides hair testing for drugs of abuse, utilizing a patented hair analysis method involving digestion of hair, enzyme immunoassay and mass spectrometry to analyze hair to detect abused substances. The Company’s customers include Fortune 500 companies, as well as small to mid-size corporations, schools and governmental entities located in the United States and internationally, as well as in Brazil.
COVID-19 Pandemic
The outbreak of coronavirus (“COVID-19”) which was declared by the World Health Organization to be a pandemic, has, and is expected to continue to impact worldwide economic activity. While our domestic business has been deemed an essential business and we continue to provide services to our customers, COVID-19 has had a significant impact on our entire operations. Additionally, COVID-19’s effect on the overall economy has had an adverse impact on hiring, which is having a negative impact on our testing volume. Due to COVID-19, the Brazilian government closed all driver license bureaus and extended the renewal period for all drivers’ licenses, which has, and will continue to have a material adverse impact on expected testing volume in Brazil for the next year.
The Coronavirus Aid, Relieve and Economic Security Act (“CARES”) Act, enacted on March 27, 2020, was an emergency economic stimulus package that included spending provisions and tax cuts to strengthen the United States economy and to fund a nationwide effort to curtail the effect of COVID-19. The principal impact of the CARES Act was the adoption of the Paycheck Protection Program (“PPP”) described below. The CARES Act also provided sweeping tax changes in response to the COVID-19 pandemic, including amendments to certain provisions of the previously enacted Tax Cuts and Jobs Act (“TCJA”). The Company recognized a benefit of $2.1 million for the for the year ended December 31, 2020, as a component of income tax expense from continuing operations related to the tax provisions in the CARES Act. Based on the Company's initial assessments, the Company anticipates that the CARES Act will allow the Company to defer the employer portion of its FICA taxes to 2021 and 2022 and allow the Company to fully carryback the 2020 net operating loss, for a refund of taxes previously paid.
Liquidity and Management’s Plans
At December 31, 2020, the Company’s principal sources of liquidity included approximately $2.8 million of cash on hand. Management currently believes that such funds, together with future operating profits, should be adequate to fund anticipated working capital requirements, including debt obligations, and capital expenditures for at least the next 12 months. Depending upon the Company’s results of operations, its future capital needs and available marketing opportunities, the Company may use various financing sources to raise additional funds. Such sources could include but are not limited to, issuance of common stock or debt financing, lines of credit, or equipment leasing, although there is no assurance that such financings will be available to the Company on terms it deems acceptable, if at all.
2. Summary of Significant Accounting Policies
Risks and Uncertainties
The Company is subject to a number of risks and uncertainties similar to those of other companies, such as those associated with the continued expansion of the Company’s sales and marketing network, technological developments, intellectual property protection, development of markets for new products and services offered by the Company, the economic health of principal customers of the Company, financial and operational risks associated with expansion of testing facilities used by the Company, government regulation (including, but not limited to, Food and Drug Administration (“FDA”) regulations, Brazilian laws, proposed laws and regulations, and delays in implementation of laws and regulations), competition and general economic conditions.
Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates, including those related to bad debts, long-lived asset lives, income tax valuation and share based compensation, and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Changes in estimates are recorded in the period in which they become known.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities at the date of purchase of 90 days or less as cash equivalents. As of December 31, 2020 and 2019, there were no investments classified as cash equivalents.
| 25 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
2. Summary of Significant Accounting Policies (continued)
Property and Equipment
Property & equipment are recorded at cost. Depreciation and amortization is computed over the estimated useful lives of the assets, using the straight-line method. Repair and maintenance costs are expensed as incurred. The estimated useful lives of the assets are:
| Computer software (years) | 3 | to | 5 |
| Office furniture and equipment (years) | 3 | to | 7 |
| Laboratory equipment (years) | 5 | to | 7 |
| Leasehold improvements | Lesser of estimated useful life or lease term | ||
The Company recorded depreciation and amortization related to property and equipment and capitalized software of $2.6 million, $2.9 million, and $3.1 million in 2020, 2019 and 2018 respectively. The Company had $0.8 million of capitalized software and equipment that was not placed in service as of December 31, 2020.
Capitalized Software Development Costs
We capitalize costs related to significant software projects developed or obtained for internal use, including costs incurred in a cloud computing arrangement. Costs incurred during the preliminary project work stage or conceptual stage, such as determining the performance requirements, system requirements and data conversion, are expensed as incurred. Costs incurred in the application development phase, such as coding, testing for new software and upgrades that result in additional functionality, are capitalized and are amortized using the straight-line method over the useful life of the software for 5 years. Costs incurred during the post-implementation/operation stage, including training costs and maintenance costs, are expensed as incurred. In accordance with Company policy, during the years ended December 31, 2020 and 2019, we capitalized internally developed software costs of $213 thousand and $234 thousand, respectively. Amortization expense related to software development costs was $293 thousand, $457 thousand and $525 thousand in 2020, 2019 and 2018, respectively. Determining whether particular costs incurred are more properly attributable to the preliminary or conceptual stage, and thus expensed, or to the application development phase, and thus capitalized and amortized, depends on subjective judgments about the nature of the development work, and our judgments in this regard may differ from those made by other companies. General and administrative costs related to developing or obtaining such software is expensed as incurred.
Other Assets
Other assets primarily consist of capitalized legal costs relating to patent applications. The Company amortizes these costs over the lesser of the legal life or estimated useful life of the patent from the date of grant of the applicable patent. The typical life is twenty years. As of December 31, 2020, the Company had capitalized legal costs relating to patent applications of $1.0 million with accumulated amortization of $0.3 million, for a net balance of $0.7 million. As of December 31, 2019, the Company had capitalized legal costs relating to patent applications of $1.0 million with accumulated amortization of $0.3 million, for a net balance of $0.7 million. Amortization expense was $62 thousand, $40 thousand, and $38 thousand in 2020, 2019 and 2018, respectively. The amount of amortization related to patent applications is expected to remain below $65 thousand per year for the next five years.
Revenue Recognition
The Company is in the business of performing drug testing services and reporting the results thereof. The Company’s services are primarily drug and alcohol testing for its customers for an agreed-upon fee per unit tested. The revenues are recognized when the drug test is performed and reported to the customer.
On January 1, 2018, the Company adopted ASC 606, “Revenue from Contracts with Customers” (“ASC 606”) using the modified retrospective method. The adoption of ASC 606 did not have a material effect on the Company’s financial position or results of operations.
Revenue is recognized when control of the services is transferred to our customers, in an amount that reflects the consideration (none of which is variable) the Company expects to be entitled to in exchange for those services. The Company typically invoices customers monthly for services provided and payments are generally due within 30 to 60 days of the invoice date.
| 26 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
2. Summary of Significant Accounting Policies (continued)
The table below disaggregates our external revenue by major source (in thousands). For additional revenue detail relating to geographic breakdown of sales, see Note 14 – “Business Segment Reporting”.
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | 2018 | ||||||||||
| Consolidated Revenue: | ||||||||||||
| Testing | $ | 19,068 | $ | 34,555 | $ | 39,174 | ||||||
| Shipping / Collection (hair) | 2,174 | 2,876 | 3,159 | |||||||||
| Other | 118 | 247 | 341 | |||||||||
| Total Revenue | $ | 21,360 | $ | 37,678 | $ | 42,674 | ||||||
Testing Revenue
Drug and alcohol tests for drugs of abuse using hair, performed in the Company’s forensic laboratory in California, represents our primary service. Sales to customers are initiated through sales agreements, most of which have standard terms. Most tests are identified through a chain of custody form (“CCF”) and can therefore be uniquely tracked. Revenue is recognized when performance obligations under the terms of the contract with a customer are satisfied; generally, this occurs with the transfer of control of our service, which occurs at a specific point-in-time. The specific point-in-time is the completion of the test and availability of test results to the customer. Most tests are completed the same day that the hair specimen is received.
Substantially all tests are completed within a few days once received for processing at our laboratory in California. As the tests are performed in a forensic laboratory, the exact date and time of each test completion is available and used in the timing of recognition of revenue.
Revenue is measured as the amount of consideration the Company expects to receive in exchange for providing services. Sales taxes the Company pays concurrent with revenue-producing activities are excluded from revenue.
Shipping and Hair Collection Revenue
Shipping revenue represents the amount billed to customers related to shipping of the hair specimen and CCF (“sample”) to the Company’s laboratory. Collection revenue represents the amount billed to customers related to the collection of the hair specimen. This collection is done by third parties who have contracted with the Company. Shipping and hair collection revenue is recognized when performance obligations under the terms of the contract with a customer are satisfied; generally, this occurs with the transfer of control of the Company’s service, which occurs at a specific point-in-time. The specific point-in-time is the completion of the test (associated with the shipping or hair collection charge) and availability of test results to the customer.
Revenue is measured as the amount of consideration the Company expects to receive in exchange for providing services. As the Company controls the service before transferring to the customer, it is considered a principal in the transaction, and therefore records revenues on gross basis, with shipping and hair collection costs in costs of revenues.
Other Revenue
Other revenue represents several items including; urine testing performed by other labs, medical review officer charges, legal/testifying services, and other miscellaneous charges. The total of all of these items is less than 1% of total revenue. The amounts are generally billed to customers as services are performed, which occurs at a specific point-in-time.
Practical Expedients and Exemptions
The Company generally expenses sales commissions when incurred as they are typically not related to costs to fulfill customer contracts but relate to overall sales targets. These costs are recorded within marketing and selling expense.
Research and Development Expenses
The Company expenses all research and development costs as incurred.
| 27 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
2. Summary of Significant Accounting Policies (continued)
Income Taxes
The Company accounts for income taxes using the liability method pursuant to ASC 740, “Income Taxes”. Under this method, the Company recognizes deferred tax assets and liabilities for the expected tax consequences of temporary differences between the tax bases of assets and liabilities and their reported amounts using enacted tax rates in effect for the year the differences are expected to reverse. The Company evaluates uncertain tax positions annually and considers whether the amounts recorded for income taxes are adequate to address the Company’s tax risk profile. The Company analyzes the potential tax liabilities of specific transactions and tax positions based on management’s judgment as to the expected outcome.
Concentration of Credit Risk and Off-Balance Sheet Risk
The Company has no significant off-balance-sheet risk such as foreign exchange contracts, option contracts, or other foreign hedging arrangements. Financial instruments that potentially subject the Company to concentrations of credit risk are principally cash and accounts receivable. The Company’s policy is to place its cash in high quality financial institutions. At time, these deposits may exceed or be exempt from federally insured limits. The Company does not believe significant credit risk exists with respect to these institutions. Concentration of credit risk with respect to accounts receivable is limited to certain customers to whom the Company makes substantial sales. To reduce risk, the Company routinely assesses the financial strength of its customers and, as a consequence, believes that its accounts receivable credit risk exposure is limited. The Company maintains an allowance for potential credit losses but historically has not experienced any significant losses related to individual customers or groups of customers in any particular industry or geographic area. The Company does not require collateral.
Significant Customers
The Company had no customers that represented greater than 10% of revenue for the year ended December 31, 2020. One customer represented 26% and 31% of total revenue for the years ended December 31, 2019 and 2018, respectively. The Company had no customers that represented greater than 10% of the total accounts receivable balance as of December 31, 2020. The Company had two customers that accounted for 13% and 11% of the total accounts receivable balance as of December 31, 2019.
Stock-Based Compensation
The Company accounts for equity awards in accordance with ASC 718, “Compensation — Stock Compensation” (“ASC 718”). ASC 718 requires employee equity awards to be accounted for under the fair value method. It also requires the measurement of compensation cost at fair value on the date of grant and recognition of compensation expense over the service period for awards expected to vest. Accordingly, share-based compensation is measured at the grant date based on the fair value of the award. The Company uses the straight-line method to recognize share-based compensation over the service period of the award, which is generally equal to the vesting period. The Company uses the simplified approach to calculate the expected exercise date of options, which is one of the components used to determine the fair value of the options. This approach is used due to the small number of recipients receiving stock options not providing a reasonable basis for estimating expected term. In 2016, the Company adopted ASU 2016-09, Improvements to Employee Share-Based Payment Accounting, which simplifies several aspects of the accounting for employee share-based payment transactions including the accounting for income taxes, forfeitures, and statutory tax withholding requirements, as well as classification of related amounts within the statement of cash flows. As a result, we recognize the impact of forfeitures when they occur with no adjustment for estimated forfeitures and recognize excess tax benefits as a reduction of income tax expense regardless of whether the benefit reduces income taxes payable.
Stock compensation expense by income statement account is as follows (in thousands):
| Stock-Based Compensation | ||||||||||||
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | 2018 | ||||||||||
| Cost of revenues | $ | 50 | $ | 59 | $ | 62 | ||||||
| General & administrative | 380 | 579 | 436 | |||||||||
| Marketing & selling | 74 | 54 | 29 | |||||||||
| Research & development | 59 | 67 | 67 | |||||||||
| Total stock compensation | $ | 563 | $ | 759 | $ | 594 | ||||||
See Note 7 for additional information relating to the Company’s stock plan.
| 28 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
2. Summary of Significant Accounting Policies (continued)
Basic and Diluted Net Income per Share
Basic net income per share is computed by dividing net income available to common shareholders by the weighted average number of common shares outstanding during the period. Diluted net income per share is computed by dividing net income available to common shareholders by the weighted average number of common shares and dilutive common stock equivalents outstanding during the period. The number of dilutive common stock equivalents outstanding during the period has been determined in accordance with the treasury-stock method. Common equivalent shares consist of common stock issuable upon the exercise of outstanding options and the unvested portion of stock unit awards (“SUAs”).
Basic and diluted weighted average common shares outstanding are as follows (in thousands):
| 2020 | 2019 | 2018 | ||||||||||
| Weighted average common shares outstanding, basic | 5,524 | 5,514 | 5,502 | |||||||||
| Dilutive common equivalent shares | - | 11 | 45 | |||||||||
| Weighted average common shares outstanding, assuming dilution | 5,524 | 5,525 | 5,547 |
For the years ended December 31, 2020, 2019 and 2018, options to purchase 588 thousand, 357 thousand and 86 thousand common shares were outstanding but not included in the dilutive common equivalent share calculation as their effect would have been anti-dilutive.
Financial Instruments
Financial instruments include cash, accounts receivable and accounts payable. Estimated fair values of these financial instruments approximate carrying values due to their short-term nature. The Company has two outstanding equipment loans. One had an interest rate of the 30-day LIBOR rate + 1.75% and the other has a fixed interest rate of 3.79%. As there is a market interest rate, the carrying amount is fair value. The PPP Loan bears interest on the unpaid balance at the rate of one percent (1%) per annum.
Basis of Preparation and Consolidation
The consolidated financial statements include the financial statements of the Company and its wholly-owned subsidiaries have been prepared using accounting principles generally accepted in the United States (“U.S. GAAP”). All intercompany transactions and balances have been eliminated.
Foreign Currency Translation
To the extent sales are made through our Brazil subsidiary, such sales are transacted in Brazilian Real and translated into US dollars. Foreign currency denominated assets and liabilities are translated into U.S. dollars using the exchange rates in effect at the consolidated balance sheet date. Results of operations and cash flows are translated using the average exchange rates throughout the period. The effect of exchange rate fluctuations on translation of assets and liabilities that are in the functional currency is included as a component of shareholders’ equity in accumulated other comprehensive income (loss). The total change in foreign currency translation adjustment for the year ended December 31, 2020 was an immaterial amount and 2019 was a loss of $0.2 million. This amounted to an immaterial amount and $0.2 million after tax impact.
Segment Reporting
The Company manages its operations as one segment, drug testing services. As a result, the financial information disclosed herein materially represents all of the financial information related to the Company’s principal operating segment. See Note 14 for geographic breakdown of revenue.
| 29 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
2. Summary of Significant Accounting Policies (continued)
Recently Adopted Accounting Pronouncements
In February 2016, the Financial Accounting Standards Board (“FASB”) issued ASU 2016-02, “Leases”, which was subsequently amended by ASU 2018-10, ASU 2018-11, ASU 2018-20 and ASU 2019-01 (collectively, Topic 842). which introduced the recognition of lease assets and lease liabilities by lessees for those leases classified as operating leases under previous guidance. The new standard established a right-of-use ("ROU") model that requires a lessee to record a lease asset and liability on the balance sheet for all leases with terms longer than 12 months. The standard became effective for fiscal years beginning after December 15, 2018 and interim periods within those fiscal years. The Company adopted Topic 842 as of January 1, 2019 (see Note 10 – Operating Leases).
In August 2018, the FASB issued ASU 2018-15, “Intangibles—Goodwill and Other—Internal-Use Software: Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract”. The FASB issued ASU 2018-15 to align the requirements for capitalizing implementation costs in a cloud computing arrangement service contract with the requirements for capitalizing implementation costs incurred for an internal-use software license. ASU 2018-15 will be effective for the Company’s fiscal year 2020, with the option to early adopt prior to the effective date. The Company adopted ASU 2018-15 as of January 1, 2019 with no material impact to the Company’s consolidated financial statements and disclosures.
New Accounting Pronouncements
In December 2019, the FASB issued ASU 2019-12, “Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes”. The amendments in this update simplify the accounting for income taxes by removing certain exceptions to the general principles in ASU Topic 740. The amendments also improve consistent application of and simplify U.S. GAAP for other areas of ASU Topic 740 by clarifying and amending existing guidance. The amendments in this update are effective for interim and annual periods for the Company beginning after December 15, 2020, with early adoption permitted. The Standard may be adopted using the prospective or retrospective transition approach and could be applied to a modified retrospective basis through a cumulative-effect adjustment to retained earnings as of the beginning of the fiscal year adoption. The Company is currently evaluating the impact of this pronouncement on the Company’s consolidated financial statements and disclosures.
3. Accounts Receivable
The Company maintains an allowance for uncollectible accounts receivable based on management’s assessment of the collectability of its customer accounts by reviewing customer payment patterns and other relevant factors. The Company reviews the adequacy of the allowance for uncollectible accounts on a quarterly basis and adjusts the balance as determined necessary. Write-offs are recorded at the time a customer account is deemed uncollectable. The following is a rollforward of the Company’s allowance for doubtful accounts (in thousands):
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Balance, beginning of period | $ | 45 | $ | 67 | ||||
| Provision for doubtful accounts | 22 | 11 | ||||||
| Write-offs | (30 | ) | (33 | ) | ||||
| Balance, end of period | $ | 37 | $ | 45 | ||||
4. Accrued Expenses
Accrued expenses consist of the following (in thousands):
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Accrued compensation and employee benefits | $ | 315 | $ | 450 | ||||
| Accrued vacation expense | 379 | 399 | ||||||
| Accrued taxes | 4 | 564 | ||||||
| Accrued shipping expense | 511 | 368 | ||||||
| Accrued payables for equipment and leasehold improvements | - | 1,453 | ||||||
| Other accrued expenses | 592 | 343 | ||||||
| Total Accrued Expenses | $ | 1,801 | $ | 3,577 | ||||
| 30 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
5. Income Taxes
The income tax provision consists of the following (in thousands):
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | 2018 | ||||||||||
| Current – | ||||||||||||
| Federal | $ | (2,006 | ) | $ | 1,478 | $ | 2,117 | |||||
| State | (2 | ) | 54 | 119 | ||||||||
| Foreign | - | 348 | 1,122 | |||||||||
| Total Current Deferred – | (2,008 | ) | 1,880 | 3,358 | ||||||||
| Federal | (13 | ) | (139 | ) | (168 | ) | ||||||
| State | (326 | ) | (227 | ) | (121 | ) | ||||||
| Total Deferred | (339 | ) | (366 | ) | (289 | ) | ||||||
| Income Tax Provision | $ | (2,347 | ) | $ | 1,514 | $ | 3,069 | |||||
A reconciliation of the effective rate with the federal statutory rate is as follows:
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | 2018 | ||||||||||
| Federal statutory rate | 21.0 | % | 21.0 | % | 21.0 | % | ||||||
| State income taxes, net of federal benefit | 4.4 | % | (4.5 | %) | 0.0 | % | ||||||
| Permanent differences | 0.0 | % | (8.1 | %) | 0.2 | % | ||||||
| Stock based compensation | (0.4 | %) | 1.3 | % | 0.1 | % | ||||||
| Federal R&D Credits | 1.6 | % | (4.7 | %) | (1.7 | %) | ||||||
| Foreign taxes, net of federal benefit | (2.2 | %) | 44.5 | % | 20.5 | % | ||||||
| Difference in tax rate for carryback claim | 13.4 | % | 0.0 | % | 0.0 | % | ||||||
| Effective tax rate | 37.8 | % | 49.5 | % | 40.1 | % | ||||||
The change in effective tax rate from 2019 to 2020 was primarily driven by the Company’s carryback claim for the net loss as well as a decrease in foreign taxes. As of December 31, 2020, the Company had no federal net operating loss carryforwards since the 2020 loss will be carried back to the 2016 tax year. As of December 31, 2020, the Company had $1.7 million of state net operating loss carryforwards which expire at various dates between 2030 and 2040. As of December 31, 2020, the Company had $0.1 million of federal tax credit carryforwards that expire in 2040 and there were $1.1 million of California tax credit carryforwards relating to the years 2013 through 2020 which have an unlimited carryforward period. In 2020, the 4.4% state income tax effective rate primarily consisted of California research tax credits of 1.8%.
The components of the net deferred tax liabilities included in the accompanying balance sheets are as follows (in thousands):
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Deferred Tax Assets | ||||||||
| Allowance for doubtful accounts | $ | 9 | $ | 10 | ||||
| Accrued expenses | 112 | 87 | ||||||
| Stock-based compensation | 265 | 195 | ||||||
| R&D tax credits | 1,005 | 788 | ||||||
| Operating lease | 1,130 | 764 | ||||||
| PPP Loan expenses | 9 | - | ||||||
| NOL Carryforward | 97 | - | ||||||
| Total Deferred Tax Assets | $ | 2,627 | $ | 1,844 | ||||
| Deferred Tax Liabilities | ||||||||
| Excess of tax over book depreciation and amortization | $ | (1,775 | ) | $ | (1,696 | ) | ||
| Prepaid expenses | (48 | ) | (40 | ) | ||||
| Operating lease | (1,015 | ) | (658 | ) | ||||
| Total Deferred Tax Liabilities | (2,838 | ) | (2,394 | ) | ||||
| Net Deferred Tax Liabilities | $ | (211 | ) | $ | (550 | ) | ||
| 31 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
5. Income Taxes (continued)
Income taxes are recorded in accordance with FASB ASC Topic 740, Income Taxes (“ASC 740”), which provides for deferred taxes using an asset and liability approach. The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. A valuation allowance is provided, if, based upon the weight of available evidence, it is more likely than not that some or all of the net deferred tax assets will not be realized.
ASC 740 contains a two-step approach to recognizing and measuring uncertain tax positions (tax contingencies). The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates it is more likely than not that the position will be sustained on an audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount which is more than 50% likely of being realized upon ultimate settlement. The Company considers many factors when evaluating and estimating the Company’s tax positions and tax benefits, which may require periodic adjustments and which may not accurately forecast actual outcomes. The Company had immaterial uncertain tax positions at December 31, 2020, and no uncertain tax positions at December 31, 2019.
The Company operates within multiple taxing jurisdictions and could be subject to audit in these jurisdictions. These audits may involve complex issues, which may require an extended period of time to resolve. The Company has provided for its estimated taxes payable in the accompanying financial statements. The Company’s policy is to recognize interest and penalties related to income tax matters as a general and administrative expense, when and if incurred. Interest and penalties for the years ended December 31, 2020, 2019 or 2018 were not material. In 2019, the I.R.S. completed a standard review of the Company’s 2016 tax year. The tax years ended December 31, 2017 through December 31, 2020 remain subject to examination by all major taxing authorities.
The net (loss) income before income taxes was ($6.2) million and $3.1 million for the years ended December 31, 2020 and 2019, respectively. Net loss before income taxes in Brazil was immaterial and $1.1 million for the years ended December 31, 2020 and 2019, respectively. The pre-tax loss in Brazil in 2020 was a result of having no sales conducted through the Company’s Brazilian subsidiary and tax expense was incurred with the repatriation of cash from Brazil to the United States.
6. Preferred Stock
The Board of Directors has the authority to designate authorized preferred shares in one or more series and to fix the relative rights and preferences without vote or action by the stockholders. The Board of Directors has no present plans to designate or issue any shares of preferred stock.
7. Stock-Based Awards
The 2006 Incentive Plan initially adopted in 2006 provides for grants of options with terms of up to ten years, grants of restricted stock or stock unit awards (SUAs), issuances of stock bonuses or grants other stock-based awards plus cash based awards, to officers, directors, employees, and consultants. Such shares are issuable out of the Company’s authorized but unissued common stock. In January 2019, the 2006 Incentive Plan was amended to increase the total number of shares issuable thereunder from 850 thousand to 1.2 million. As of December 31, 2020, 45 thousand shares remained available for future grant under the 2006 Incentive Plan.
The fair value of the SUAs is determined by the closing price on the date of grant. The fair value of options is determined using a Black-Scholes model. The SUAs and options vest over a period of two to four years and are convertible or exercisable into an equivalent number of shares of the Company’s common stock provided that the employee receiving the award remains continuously employed throughout the vesting period. The Company records stock compensation expense related to the SUAs and options on a straight-line basis over the vesting term. Employees are issued shares upon vesting of SUAs, net of tax withholdings. As a result of our adoption of ASU 2016-09 in 2016, we recognize the impact of forfeitures when they occur with no adjustment for estimated forfeitures and recognize excess tax benefits as a reduction of income tax expense regardless of whether the benefit reduces income taxes payable.
| 32 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
7. Stock-Based Awards (continued)
On November 11, 2020, the Company granted SUAs covering 190 thousand shares of common stock and options to acquire up to 40 thousand shares of common stock and on December 16, 2020, the Company granted one individual SUAs covering 5 thousand shares of common stock. The SUAs vest over a period of two years for non-employee board members and four years for employees and are convertible into an equivalent number of shares of the Company’s common stock provided that the director or employee receiving the award remains employed throughout the vesting period. The stock options become exercisable over two years for non-employee board members and four years for employees and have a term of 10 years. The Company records compensation expense related to the SUAs and options on a straight-line basis over the vesting term. Employees are issued shares upon vesting, in the case of SUA’s or upon exercise of options, net of tax withholdings, unless the employee chooses to receive all shares and pay for the associated employment taxes. Upon the exercise of a stock option, the Company issues authorized but unissued shares and delivers them to the recipient. The Company does not expect to repurchase shares to satisfy stock option exercises. No other types of equity-based awards have been granted or issued under the 2006 Incentive Plan.
The following table represents all shares granted by the Company under the 2006 Incentive Plan for the last three years (shares in thousands):
| Grant Date | Type | Shares | Fair
Value Per Share(1) | |||||||
| December 16, 2020 | SUA | 5 | $ | 4.71 | ||||||
| November 11, 2020 | Options | 40 | $ | 1.13 | ||||||
| November 11, 2020 | SUA | 190 | $ | 4.07 | ||||||
| May 3, 2019 | Options | 192 | $ | 2.99 | ||||||
| May 3, 2019 | SUA | 18 | $ | 10.60 | ||||||
| July 24, 2018 | Options | 2 | $ | 5.49 | ||||||
| May 3, 2018 | SUA | 6 | $ | 21.04 | ||||||
| May 3, 2018 | Options | 117 | $ | 5.69 |
| (1) | The fair value for the SUA’s is the closing price of the Company’s stock on that date. The fair value for options represents the fair value calculated using the Black-Scholes model. Options have contractual lives of 10 years. The options granted on May 3, 2018 have a fair value of $5.69 per share based on the $21.04 grant date and exercise prices and assuming 6.25 and 5.75 year estimated terms, 38% volatility, 3.4% interest rate and a 4.2% dividend yield rate. The options granted on July 24, 2018 have a fair value of $5.49 per share based on the $19.83 grant date and exercise prices and assuming a 6.25 year estimated term, 39% volatility, 3.4% interest rate and a 4.1% dividend yield rate. The options granted on May 3, 2019 have a fair value of $2.99 per share based on the $10.60 grant date and exercise prices and assuming 6.25 and 5.75 year estimated terms, 41% volatility, 2.4% interest rate and a 3.9% dividend yield rate. The options granted on November 11, 2020 have a fair value of $1.13 per share based on the $4.07 grant date and exercise prices and assuming 6.25 and 5.75 year estimated terms, 45% volatility, 0.9% interest rate and a 4.0% dividend yield rate. For options granted during fiscal years ended December 31, 2020, 2019, and 2018, the weighted average grant date fair values were $3.47, $3.40 and $2.49, respectively. For SUAs granted during fiscal years ended December 31, 2020, 2019, and 2018, the weighted average grant date fair values were $4.89, $12.01 and $14.42, respectively. |
A summary of the Company’s stock option activity is as follows (in thousands, except price per share):
| Number of Shares | Weighted Average Exercise Price Per Share | Weighted Average Remaining Contractual Life (years) | Aggregate Intrinsic Value(2) | |||||||||||||
| Outstanding, December 31, 2019 | 584 | $ | 14.94 | 7.9 | $ | - | ||||||||||
| Granted | 40 | $ | 4.14 | |||||||||||||
| Exercised | - | - | ||||||||||||||
| Forfeited | (20 | ) | $ | 3.21 | ||||||||||||
| Outstanding, December 31, 2020 | 604 | $ | 14.31 | 7.0 | $ | - | ||||||||||
| Exercisable, December 31, 2020 | 424 | $ | 14.55 | 6.7 | $ | 35 | ||||||||||
| (2) | The aggregate intrinsic value on this table was calculated based on the amount, if any, by which the closing market price of the Company’s stock on December 31 of the applicable year exceeded the exercise price of any of the underlying options, multiplied by the number of shares subject to each such option. The closing stock price as of December 31, 2020 and 2019 was $5.09 and $9.15, respectively. |
| 33 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
7. Stock-Based Awards (continued)
A summary of the Company’s stock unit award activity is as follows (in thousands, except price per share):
| Weighted | Weighted | |||||||||||
| Number of | Average Price | Average Fair | ||||||||||
| Shares | per Share(3) | Value(3) | ||||||||||
| Outstanding & Unvested, December 31, 2019 | 24 | $ | 12.84 | $ | 311 | |||||||
| Granted | 195 | $ | 4.07 | $ | 794 | |||||||
| Converted to common stock | (20 | ) | $ | 13.00 | $ | (254 | ) | |||||
| Cancelled | (1 | ) | $ | 20.24 | $ | (20 | ) | |||||
| Forfeited | (32 | ) | $ | 5.12 | $ | (162 | ) | |||||
| Outstanding & Unvested, December 31, 2020 | 166 | $ | 4.50 | $ | 745 | |||||||
| (3) | Weighted average price per share is the weighted grant price based on the closing market price of each of the stock grants related to each transaction type. The weighted average fair value is the weighted average share price times the number of shares. |
The fair value of stock unit award vesting was $274 thousand, $223 thousand and $308 thousand for the years ended December 31, 2020, 2019 and 2018, respectively. The intrinsic value of stock unit awards converted to common stock was based on the stock price on the vesting date and amounted to $115 thousand, $144 thousand and $493 thousand for the years ended December 31, 2020, 2019 and 2018, respectively.
As of December 31, 2020, a total of 815 thousand shares of common stock were reserved for issuance under 2006 Incentive Plan. As of December 31, 2020, the unamortized fair value of outstanding options and awards was $1.1 million to be amortized over a weighted average period of approximately 3.1 years.
8. Employee Benefit Plan
The Psychemedics Corporation 401(k) Savings and Retirement Plan (the “401(k) Plan”) is a qualified defined contribution plan in accordance with Section 401(k) of the Internal Revenue Code. All employees over the age of 21 are eligible to make pre-tax contributions up to a specified percentage of their compensation. Under the 401(k) Plan, the Company may, but is not obligated to, match a portion of the employees’ contributions up to a defined maximum. Matching contributions of $198 thousand, $262 thousand and $264 thousand were made in the years ended December 31, 2020, 2019 and 2018, respectively.
9. Commitments and Contingencies
Commitments
The Company leases certain of its facilities and equipment under operating lease agreements expiring on various dates through December 2026. Total minimum lease payments, including scheduled increases, are charged to operations on the straight-line basis over the life of the respective lease. Rent expense was approximately $1.1 million, $1.2 million and $1.0 million in 2020, 2019 and 2018, respectively. See Note 10 – Operating Leases for commitments remaining under lease agreements.
Contingencies
The Company is subject legal proceedings and claims in the ordinary course of its business. The Company believes that although there can be no assurance as to the disposition of these proceedings, based upon information available to the Company as of the timing of filing of this report, the expected outcome of these matters would not have a material impact on the Company’s results of operations or financial condition.
| 34 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
10. Operating Leases
The Company has five operating leases for office and laboratory space used to conduct business. The exercise of lease renewal options is at our discretion and there are no renewals to extend the lease terms included in our Right-Of-Use (“ROU”) assets and lease liabilities as they are not reasonably certain of exercise. The Company regularly evaluates the renewal options and when they are reasonably certain of exercise. As most of the Company’s leases do not provide an implicit rate, the Company uses the incremental borrowing rate based on the information available at the lease commencement date in determining the net present value (NPV) of the lease payments.
As of December 31, 2019, the Company recognized a Right-Of-Use (“ROU”) asset of $2.9 million and an operating lease liability of $3.3 million based on the present value of the minimum rental payments as a result of adoption of ASC Topic 842. The weighted average discount rate used for leases as of December 31, 2020 is 3.9%. The weighted average lease term as of December 31, 2020 is 4.9 years. The operating lease expense for the twelve months ended December 31, 2020 and 2019, was $1.1 million and $1.2 million, respectively.
Maturities and balance sheet presentation of the Company’s lease liabilities for all operating leases as of December 31, 2020 is as follows (in thousands):
| 2021 | $ | 1,041 | ||
| 2022 | 1,028 | |||
| 2023 | 1,096 | |||
| 2024 | 1,035 | |||
| 2025 | 593 | |||
| 2026 | 458 | |||
| Total Lease Payments | 5,251 | |||
| Less Interest: | (481 | ) | ||
| Present value of lease liabilities | $ | 4,770 | ||
| Current operating lease liabilities | $ | 875 | ||
| Long-term operating lease liabilities | 3,895 | |||
| Total | $ | 4,770 |
11. Debt and Other Financing Arrangements
On March 20, 2014, the Company entered into an equipment financing arrangement with Banc of America Leasing & Capital, LLC (the “Lender”), which it amended on August 8, 2014, September 15, 2015, October 30, 2017, and December 2, 2019, including a Master Loan and Security Agreement and related documentation (collectively the “Equipment Loan Arrangement”) which provided the Company with the ability to finance, at its option, up to $16 million of new and used equipment purchases. Each such purchase financed under the Equipment Loan Arrangement is documented by the execution of an equipment note. Each note has a maturity date of 60 months from the applicable loan date. The loan on October 30, 2017 bears interest at the then current 30-day LIBOR rate + 1.75% and for the loan made on December 2, 2019 a fixed interest rate of 3.79%. Principal and interest are payable over the 60-month repayment period and principal is repayable without premium or penalty. Borrowings under the Equipment Loan Arrangement are secured by a first priority security interest in the equipment acquired with the proceeds of the equipment notes. Under the Equipment Loan Arrangement, the Company is subject to a maximum quarterly funded debt to EBITDA ratio and a minimum fixed charge coverage ratio.
On November 2, 2020, the Lender amended the Equipment Loan Arrangement in order to, among other things, waive, for the quarters ended December 31, 2020, March 31, 2021 and June 30, 2021, any minimum required funded debt to EBITDA ratio and any minimum required fixed charge coverage ratio. The Waiver and Amendment also added a requirement that the Company maintain a cash balance of at least $1,500,000 as of the end of each fiscal quarter. It also imposed a minimum required EBITDA of $1 for the fourth quarter of fiscal 2020 and $225,000 for each of the first and second quarters of fiscal 2021. It also prohibits the payment of dividends or other similar payment distributions to shareholders during the period commencing on November 1, 2020 through June 30, 2021. Thereafter such dividends and other payments may resume, provided that the funded debt to EBITDA ratio and fixed charge coverage ratio shall have been satisfied at the time of such payments. The Waiver and Amendment also waived an event of default that existed under the Equipment Loan Arrangement regarding the required funded debt to EBITDA ratio and fixed charge coverage ratio for the 12-month period ended September 30, 2020. The Company was not in compliance with all of the loan covenants under the Equipment Loan Arrangement, as amended, as of December 31, 2020.
On March 23, 2021, the Company further amended its debt arrangement to waive non-compliance and amend certain covenants through the quarter ended June 30, 2021. The Waiver and Amendment amended the Equipment Loan Arrangement in order to, among other things, waive the minimum required EBITDA of $1 for the fourth quarter of fiscal 2020. The Waiver and Amendment also amended the amount of minimum required EBITDA for the first quarter of 2021 from $225,000 to of $1. The total book value of equipment pledged as collateral for these loans as of December 31, 2020 was $3.1 million.
Under the Equipment Loan Arrangement, the Company executed notes on March 24, 2014, May 22, 2014, June 13, 2014, August 8, 2014, September 15, 2015, March 23, 2016, November 10, 2017, and December 4, 2019 in the amounts of $1.1 million, $1.9 million, $3.0 million, $1.0 million, $1.1 million, $610 thousand, $2.1 million, and $1.4 million, respectively, for total borrowings of $12.2 million, of which $0.7 million and $0.4 million was repaid in 2020 and 2019, respectively. As of December 31, 2020, only the note from November 10, 2017 and December 4, 2019 had a balance as all other notes with balances were paid off in 2018. The weighted average interest rate for these notes for the year ended December 31, 2020 was 3% and represented $75 thousand of interest expense. As of December 31, 2020, weighted average interest rate was 3%.
On May 1, 2020, the Company entered into a term loan with Bank of America N.A. under the PPP administered by the SBA under the CARES Act. The principal amount of the loan was $2,181,157, which is evidenced by a promissory note with a maturity date of May 4, 2022. The note bears interest on the unpaid balance at the rate of one percent (1%) per annum. The note contains a deferral period of six months, for which no interest or principal payments are due. The Company is in the process of applying for loan forgiveness with the SBA and expects a final approval in 2021.
The annual principal repayment requirements for debt obligations as of December 31, 2020 are as follows (in thousands):
| 2021 | $ | 688 | ||
| 2022 | 664 | |||
| 2023 | 294 | |||
| 2024 | 305 | |||
| Long-term debt from equipment financing | 1,951 | |||
| Less current portion of long-term debt from equipment financing | (688 | ) | ||
| Long-term debt from equipment financing, net of current portion | 1,263 | |||
| PPP Loan | 2,181 | |||
| Total long-term debt, net of current portion | $ | 3,444 |
| 35 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2020
12. Selected Quarterly Financial Data (Unaudited)
The following are selected quarterly financial data for the years ended December 31, 2020 and 2019 (in thousands):
| Quarter Ended - 2020 | ||||||||||||||||
| MAR 31 | JUN 30 | SEP 30 | DEC 31 | |||||||||||||
| Revenues | $ | 7,537 | $ | 3,314 | $ | 5,174 | $ | 5,335 | ||||||||
| Gross profit | 2,728 | (252 | ) | 1,133 | 1,277 | |||||||||||
| Loss from operations | (242 | ) | (3,306 | ) | (1,409 | ) | (1,109 | ) | ||||||||
| Net loss | (159 | ) | (2,050 | ) | (1,107 | ) | (543 | ) | ||||||||
| Basic net loss per share | $ | (0.03 | ) | $ | (0.37 | ) | $ | (0.20 | ) | $ | (0.10 | ) | ||||
| Diluted net loss per share | $ | (0.03 | ) | $ | (0.37 | ) | $ | (0.20 | ) | $ | (0.10 | ) | ||||
| Quarter Ended - 2019 | ||||||||||||||||
| MAR 31 | JUN 30 | SEP 30 | DEC 31 | |||||||||||||
| Revenues | $ | 9,822 | $ | 9,289 | $ | 9,852 | $ | 8,715 | ||||||||
| Gross profit | 4,408 | 4,169 | 4,382 | 3,485 | ||||||||||||
| Income (loss) from operations | 944 | 1,326 | 1,334 | (606 | ) | |||||||||||
| Net income (loss) | 627 | 768 | 677 | (530 | ) | |||||||||||
| Basic net income (loss) per share | $ | 0.11 | $ | 0.14 | $ | 0.12 | $ | (0.09 | ) | |||||||
| Diluted net income (loss) per share | $ | 0.11 | $ | 0.14 | $ | 0.12 | $ | (0.09 | ) | |||||||
| 36 |
PSYCHEMEDICS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2019
13. Other expense
Other expense consists primarily of interest expense related to the Company’s equipment financing arrangement. Interest expense for the year ended December 31, 2020, 2019 and 2018 was $75 thousand, $59 thousand and $106 thousand, respectively. There was no interest income for the year ended December 31, 2020. Interest income for the year ended December 31, 2019 and 2018 was $134 thousand $149 thousand, respectively.
14. Business Segment Reporting
The Company manages its operations as one segment, drug testing services. As a result, the financial information disclosed herein materially represents all the financial information related to the Company’s principal operating segment. All Brazil sales are though one independent distributor. The Company’s revenues by geographic region, based on the location of the customer, are as follows (in thousands):
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | 2018 | ||||||||||
| Consolidated Revenue: | ||||||||||||
| United States | $ | 19,486 | $ | 27,329 | $ | 29,189 | ||||||
| Brazil | 1,344 | 9,819 | 13,046 | |||||||||
| Other | 530 | 530 | 439 | |||||||||
| Total Revenue | $ | 21,360 | $ | 37,678 | $ | 42,674 | ||||||
All the Company’s operations are in the United States. The Company’s assets by geographic region are as follows (in thousands):
| As of December 31, | ||||||||
| Assets: | 2020 | 2019 | ||||||
| United States | $ | 24,003 | $ | 27,091 | ||||
| Brazil | - | 440 | ||||||
| Total Assets | $ | 24,003 | $ | 27,531 | ||||
| 37 |
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
| a) | Evaluation of Disclosure Controls and Procedures |
The Company carried out an evaluation as of December 31, 2020, under the supervision and with the participation of our management, including our Chief Executive Officer and Vice President, Controller as well as a third party internal control firm, of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act. Based upon that evaluation, our Chief Executive Officer and Vice President, Controller have concluded that our disclosure controls and procedures were effective as of December 31, 2020 to ensure that information required to be disclosed in the reports that the Company files or submits under the Exchange Act is (i) recorded, processed, summarized and reported, within the time periods specified in the Securities and Exchange Commission’s rules and forms and (ii) accumulated and communicated to our management, including our Chief Executive Officer and Vice President, Controller, as appropriate to allow timely decisions regarding required disclosure.
| b) | Management’s Report on Internal Control over Financial Reporting |
The Company’s management is responsible for establishing and maintaining an adequate system of internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f) and 15d-15(f). The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance, as opposed to absolute assurance, of achieving their internal control objectives.
Management conducted an assessment of the Company’s internal control over financial reporting as of December 31, 2020, based on criteria established in the 2013 Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on the assessment, management concluded that, as of December 31, 2020, the Company’s internal control over financial reporting is effective.
| c) | Changes in Internal Control over Financial Reporting |
There was no change the Company's internal control over financial reporting during the Company's most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the Company's internal control over financial reporting.
| 38 |
None.
Item 10. Directors, Executive Officers and Corporate Governance
Following is a list that sets forth as of March 26, 2021 the names, ages and positions within the Company of all of the Executive Officers of the Company and the Directors of the Company. Each such director has been nominated for reelection at the Company’s 2021 Annual Meeting, to be held on May 13, 2021 at 2:00 P.M. Due to concerns regarding the COVID-19 pandemic and to protect the safety and well-being of our stockholders, Board of Directors and employees, the Company’s 2021 Annual Meeting will be a virtual meeting conducted solely online via live webcast.
| Name | Age | Position | ||
| Raymond C. Kubacki | 76 | Chairman, Chief Executive Officer, President, Director | ||
| Charles Doucot | 55 | Executive Vice President | ||
| Andrew Limbek | 35 | Vice President, Controller | ||
| Michael I. Schaffer, Ph.D. | 76 | Vice President, Laboratory Operations | ||
| Harry Connick | 95 | Director, Audit Committee Member, Compensation Committee Member, Nominating Committee Member | ||
| Walter S. Tomenson, Jr. | 74 | Director, Audit Committee Member, Compensation Committee Member, Nominating Committee Member | ||
| Robyn C. Davis | 59 | Director, Audit Committee Member, Compensation Committee Member, Nominating Committee Member | ||
| Fred J. Weinert | 73 | Director, Audit Committee Member, Compensation Committee Member, Nominating Committee Member, Brazil Oversight Committee Member, Lead Independent Director |
All Directors hold office until the next annual meeting of stockholders or until their successors are elected. Officers serve at the discretion of the Board of Directors.
Mr. Kubacki has been the Company’s President and Chief Executive Officer since 1991. He has also served as Chairman of the Board of the Company since 2003. From March 2011 until June 2017, he served as a director of Integrated Environmental Technologies, Ltd. From 2007 until 2010, he served as a director of Protection One, Inc. and from 2004 to 2007 he served as a director of Integrated Alarm Services Group, Inc. He is also a trustee of the Center for Excellence in Education based in Washington, D.C. and holds an Executive Masters Professional Director Certification, their highest level award, from the American College of Corporate Directors, a public company director education and credentialing organization. Mr. Kubacki has been a director of the Company since 1991.
Mr. Doucot has served as Executive Vice President since January 2019. From May 2018 until January 2019, he served as Vice President Sales & Marketing. Prior to joining the Company, he served as Vice President Sales & GM of Burning Glass Technologies, a data analytics company, from January 2016 to December 2017. From April 2014 to January 2016 he served as Sr. VP and GM at Lumesse, an HR technology company, responsible for the Americas Business and starting a new business unit. From August 2009 to February 2014, he served as VP WW Sales and Marketing for Kalido, a big data and analytics company. Mr. Doucot began his career spending over 15 years at Hewlett-Packard Company with increasing levels of global responsibility.
Mr. Limbek has served as Vice President, Controller since January 2021. From March 2019 until January 2021, he served as an accounting consultant at Applied Genetic Technologies Corporation, a publicly-held clinical stage biotechnology company, where he served as interim Financial Planning & Analysis Director and reported directly to the Chief Financial Officer of the company. From June 2019 until March 2020, he served as Controller at Racepoint Global, Inc., an international independent professional services agency. From January 2018 until June 2019, he served as Assistant Controller of Racepoint Global, Inc. From March 2017 until January 2018, Mr. Limbek served as a Senior Accounting Manager at Oxford Global Resources, LLC, a temporary staffing firm. From 2014 until February 2017, he served as a Senior Manager at Bullpen Financial LLC, a financial services firm. Mr. Limbek is a Certified Public Accountant in the Commonwealth of Massachusetts.
Dr. Schaffer has served as Vice President of Laboratory Operations since 1999. Since December 2016, he has served as a member of the Drug Testing Advisory Board (DTAB) which advises the administrator of Substance Abuse and Mental Health Services Administration (SAMHSA) on drug testing activities and laboratory certification. From 1990 to 1999, he served as Director of Toxicology, Technical Manager and Responsible Person for the Leesburg, Florida laboratory of SmithKline Beecham Clinical Laboratories. From 1990 to 1999, he was also a member of the Board of Directors of the American Board of Forensic Toxicologists. Dr. Schaffer has also served as an inspector for the College of American Pathologists since 1990.
Mr. Connick served as District Attorney for Orleans Parish (New Orleans, LA) from 1974 to 2003. In 2002 Mr. Connick received from Drug Czar, John P. Walters, the Director’s Award for Distinguished Service, in recognition of exemplary accomplishment and distinguished service in the fight against illegal drugs. Mr. Connick has been a director of the Company since 2003.
| 39 |
Mr. Tomenson was a senior advisor to Integro Ltd., having retired in 2011. Mr. Tomenson was Managing Director and Chairman of Client Development of Marsh, Inc. from 1998 until 2004. From 1983 to 1998 he was Chairman of FINPRO, the financial/professional services division of Marsh, Inc. Mr. Tomenson is a Trustee of Trinity College School Fund, Inc. He also serves on the Executive Council of Inner-City Scholarship Fund. He is a board member and Vice-Chairman of the Achievement Centers for Children and Families (Delray Beach, FL). Mr. Tomenson holds an Executive Masters Professional Director Certification, their highest level award, from the American College of Corporate Directors, a public company director education and credentialing organization. Mr. Tomenson has been a director of the Company since 1999.
Ms. Davis has been managing director of Angel Healthcare Investors, LLC, an early-stage private equity investment group focused on medical devices, life sciences and specialty pharmaceutical companies since 2000. Prior to Angel Healthcare, Ms. Davis was a director of the merchant banking services practices for Barents Group, LLC, and a strategy consultant at Bain & Company. She serves as a director of Brooks Automation, Inc. (BRKS), a leading global provider of manufacturing automation solutions for the semiconductor industry, and life science sample-based services and solutions for the life sciences market, where she has served on their audit, compensation and finance committees. Ms. Davis also serves as a director of Akston Bioscience, an early-stage company developing an insulin engineering platform for multiple conditions. Ms. Davis holds an Executive Masters Professional Director Certification from the American College of Corporate Directors. Ms. Davis was elected as a director of the Company on March 16, 2021.
Mr. Weinert is an entrepreneur whose current activities are concentrated in commercial real estate, international business development and environmental consulting. He served on the Business Advisory Council for the University of Dayton from 1984 until 2005. From 1973 until 1989, Mr. Weinert held various executive positions in the Finance and Operations groups of Waste Management, Inc. and its subsidiaries, including 6 years as the President of Waste Management International, Inc. Mr. Weinert has been a director of the Company since 1991.
The information required by Item 405 of Regulation S-K will be set forth in the Proxy Statement of the Company relating to the 2021 Annual Meeting of Stockholders to be held on May 13, 2021 and is incorporated herein by reference.
The Company has a code of ethics that applies to all employees and non-employee directors. This code satisfies the requirements set forth in Item 406 of Regulation S-K and applies to all relevant persons set forth therein. The Company will mail to interested parties a copy of the Code of Ethics upon written request and without charge. Such request shall be made to our General Counsel, 289 Great Road, Acton, Massachusetts 01720.
The information required by Item 407 of Regulation S-K will be set forth in the Proxy Statement of the Company relating to the 2021 Annual Meeting of Stockholders to be held on May 13, 2021 and is incorporated herein by reference.
Item 11. Executive Compensation
The information required by this item will be set forth in the Proxy Statement of the Company relating to the 2021 Annual Meeting of Stockholders to be held on May 13, 2021 and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item will be set forth in the Proxy Statement of the Company relating to the 2021 Annual Meeting of Stockholders to be held on May 13, 2021 and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions and Director Independence
The information required by this item will be set forth in the Proxy Statement of the Company relating to the 2021 Annual Meeting of Stockholders to be held on May 13, 2021 and is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services
The information required by this item will be set forth in the Proxy Statement of the Company relating to the 2021 Annual Meeting of Stockholders to be held on May 13, 2021 and is incorporated herein by reference.
Item 15. Exhibits, Financial Statement Schedules
(a) (1) Financial Statements required by Item 15 are included and indexed in Part II, Item 8.
(a) (2) Financial Statement Schedules included in Part IV of this report. Schedule II is omitted because information is included in Notes to Financial Statements. All other schedules under the accounting regulations of the SEC are not required under the related instructions and are inapplicable and, thus have been omitted.
(a) (3) See “Exhibit Index” included elsewhere in this Report.
None.
| 40 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| PSYCHEMEDICS CORPORATION | ||
| Date: March 26, 2021 |
By:/s/ RAYMOND C. KUBACKI |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
/s/ RAYMOND C. KUBACKI Raymond C. Kubacki |
Chairman, President and Chief Executive
Officer, Director |
March 26, 2021 | ||
/s/ ANDREW LIMBEK Andrew Limbek |
Vice President, Controller |
March 26, 2021 | ||
HARRY CONNICK* Harry Connick |
Director |
|||
WALTER S. TOMENSON, JR* Walter S. Tomenson, Jr. |
Director |
|||
FRED J. WEINERT* Fred J. Weinert |
Director |
|||
ROBYN C. DAVIS* Robyn C. Davis |
Director |
|||
*By: /s/ RAYMOND C. KUBACKI |
Attorney-in-Fact |
March 26, 2021 |
| 41 |
EXHIBIT INDEX
42
