Attached files
| file | filename |
|---|---|
| 8-K - HUMANIGEN, INC | p6162008k.htm |
Exhibit 99.1

0 Humanigen June 2020 “Part of the reason why some people get so severely ill... is what we call a Cytokine Storm... it’s actually the immune response that starts destroying your lungs and your tissue, and so if you can interrupt that immune response... you can avert some of the destructive effects of the virus...” - Scott Gottlieb, former FDA Commissioner Confidential

1 Disclaimer The information contained in this document has been prepared solely for informational purposes and is not an offer to sell or p urchase or a solicitation of an offer to sell or purchase any interests or securities in Humanigen , Inc. This document may not be modified, reproduced, or redistributed in whole or in part without the prior written consent of Humanigen , Inc. This document may not be distributed in any jurisdiction or to any person to which such distribution is not permitted. It is the r esp onsibility of any recipient to ensure that the documentation may be lawfully distributed to such person. Forward - Looking Statements Today’s webinar may contain forward - looking statements. Forward - looking statements reflect management's current knowledge, assum ptions, judgment and expectations regarding future performance or events. Although management believes that the expectations reflecte d i n such statements are reasonable, they give no assurance that such expectations will prove to be correct and you should be aware tha t a ctual events or results may differ materially from those contained in the forward - looking statements. Words such as "will," "expect," "intend," "plan," "potential," "possible," "goals," "accelerate," "continue," and similar expressions identify forward - looking statements, including, without l imitation, statements regarding our expectations for the Phase III study and the potential future development of lenzilumab to minimize or reduce the severity of lung dysfunction associated with severe and critical COVID - 19 infections or to be approved by FDA for such use or to help CAR - T reach its full potential or to deliver benefit in preventing GvHD. Forward - looking statements are subject to a number of risks and uncertaintie s including, but not limited to, the risks inherent in our lack of profitability and potential need for additional capital to conduct the Phase II I s tudy and grow our business; our dependence on partners to further the development of our product candidates; the uncertainties inherent in the dev elopment and launch of any new pharmaceutical product; the outcome of pending or future litigation; and the various risks and uncertaintie s d escribed in the "Risk Factors" sections and elsewhere in the Company's periodic and other filings with the Securities and Exchange Commission . All forward - looking statements made today are expressly qualified in their entirety by this cautionary notice. You should not pl ace undue reliance on any forward - looking statements, which speak only as of the date of this presentation. We undertake no obligation to revise or update any forward - looking statements made in this presentation to reflect events or circumstances after the date hereof or to reflect new information or the occurrence of unanticipated events, except as required by law.
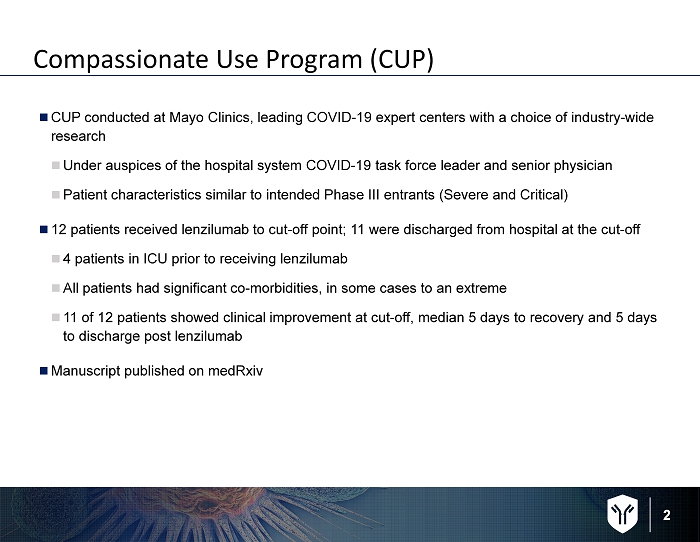
2 Compassionate Use Program (CUP) CUP conducted at Mayo Clinics, leading COVID - 19 expert centers with a choice of industry - wide research Under auspices of the hospital system COVID - 19 task force leader and senior physician Patient characteristics similar to intended Phase III entrants (Severe and Critical) 12 patients received lenzilumab to cut - off point; 11 were discharged from hospital at the cut - off 4 patients in ICU prior to receiving lenzilumab All patients had significant co - morbidities, in some cases to an extreme 11 of 12 patients showed clinical improvement at cut - off, median 5 days to recovery and 5 days to discharge post lenzilumab Manuscript published on medRxiv
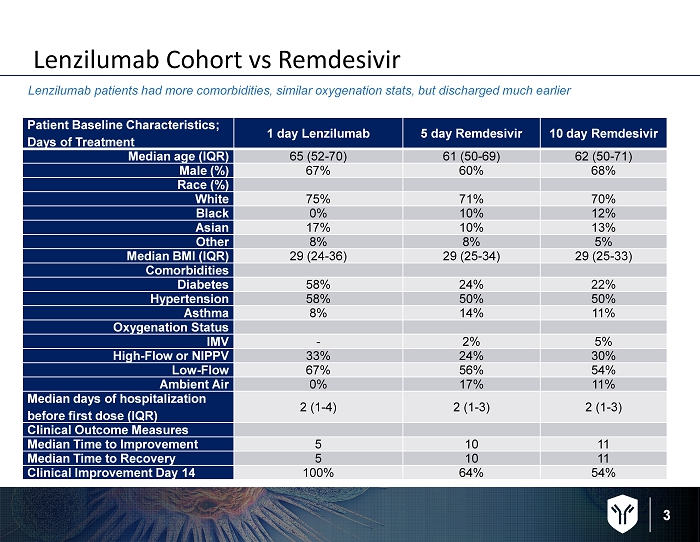
3 Lenzilumab Cohort vs Remdesivir Lenzilumab patients had more comorbidities, similar oxygenation stats, but discharged much earlier Patient Baseline Characteristics; Days of Treatment 1 day Lenzilumab 5 day Remdesivir 10 day Remdesivir Median age (IQR) 65 (52 - 70) 61 (50 - 69) 62 (50 - 71) Male (%) 67% 60% 68% Race (%) White 75% 71% 70% Black 0% 10% 12% Asian 17% 10% 13% Other 8% 8% 5% Median BMI (IQR) 29 (24 - 36) 29 (25 - 34) 29 (25 - 33) Comorbidities Diabetes 58% 24% 22% Hypertension 58% 50% 50% Asthma 8% 14% 11% Oxygenation Status IMV - 2% 5% High - Flow or NIPPV 33% 24% 30% Low - Flow 67% 56% 54% Ambient Air 0% 17% 11% Median days of hospitalization before first dose (IQR) 2 (1 - 4) 2 (1 - 3) 2 (1 - 3) Clinical Outcome Measures Median Time to Improvement 5 10 11 Median Time to Recovery 5 10 11 Clinical Improvement Day 14 100% 64% 54%
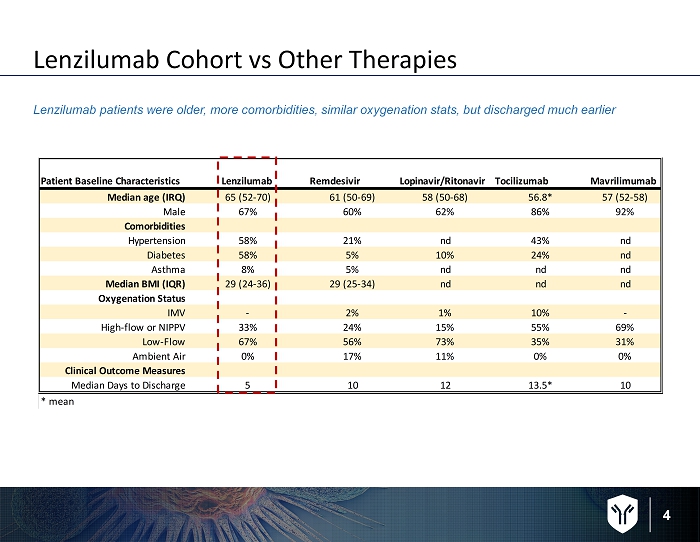
4 Lenzilumab Cohort vs Other Therapies Lenzilumab patients were older, more comorbidities, similar oxygenation stats, but discharged much earlier Patient Baseline Characteristics Lenzilumab Remdesivir Lopinavir/Ritonavir Tocilizumab Mavrilimumab Median age (IRQ) 65 (52-70) 61 (50-69) 58 (50-68) 56.8* 57 (52-58) Male 67% 60% 62% 86% 92% Comorbidities Hypertension 58% 21% nd 43% nd Diabetes 58% 5% 10% 24% nd Asthma 8% 5% nd nd nd Median BMI (IQR) 29 (24-36) 29 (25-34) nd nd nd Oxygenation Status IMV - 2% 1% 10% - High-flow or NIPPV 33% 24% 15% 55% 69% Low-Flow 67% 56% 73% 35% 31% Ambient Air 0% 17% 11% 0% 0% Clinical Outcome Measures Median Days to Discharge 5 10 12 13.5* 10 * mean
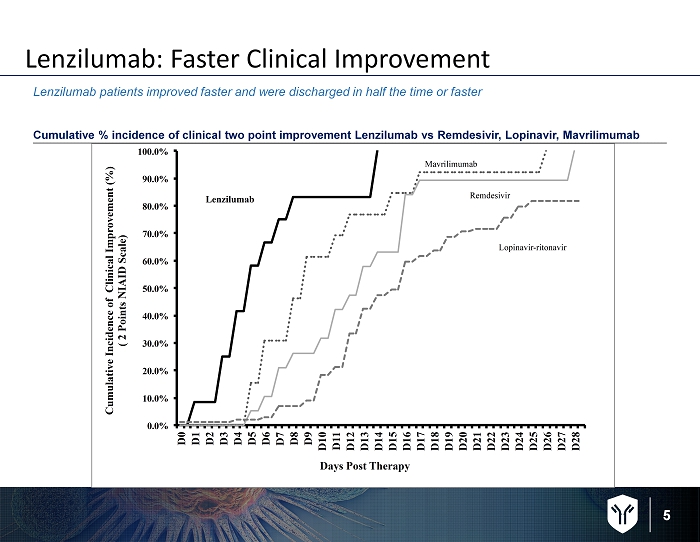
5 Lenzilumab: Faster Clinical Improvement Cumulative % incidence of clinical two point improvement Lenzilumab vs Remdesivir , Lopinavir, Mavrilimumab Lenzilumab patients improved faster and were discharged in half the time or faster 0.0% 10.0% 20.0% 30.0% 40.0% 50.0% 60.0% 70.0% 80.0% 90.0% 100.0% D 0 D 1 D 2 D 3 D 4 D 5 D 6 D 7 D 8 D 9 D 1 0 D 1 1 D 1 2 D 1 3 D 1 4 D 1 5 D 1 6 D 1 7 D 1 8 D 1 9 D 2 0 D 2 1 D 2 2 D 2 3 D 2 4 D 2 5 D 2 6 D 2 7 D 2 8 C u m u l a t i v e I n c i d e n c e o f C l i n i c a l I m p r o v e m e n t ( % ) ( 2 P o i n t s N I A I D S c a l e ) Days Post Therapy Remdesivir Lopinavir-ritonavir Mavrilimumab Lenzilumab
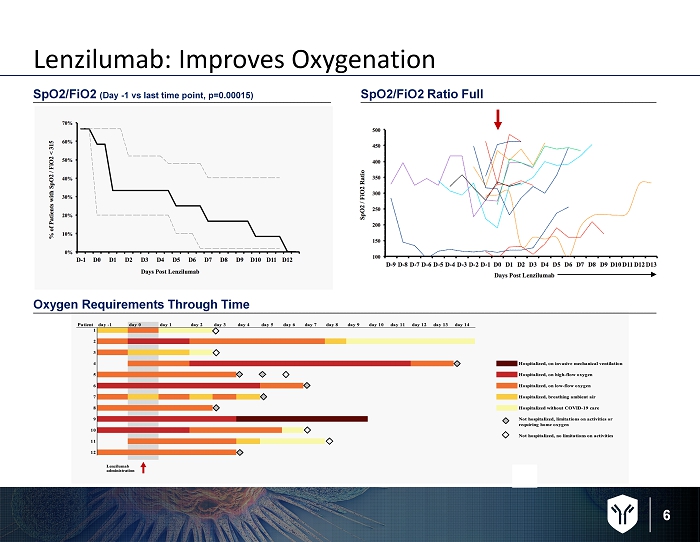
6 Oxygen Requirements Through Time SpO2/FiO2 ( Day - 1 vs last time point, p=0.00015) SpO2/FiO2 Ratio Full Lenzilumab: Improves Oxygenation
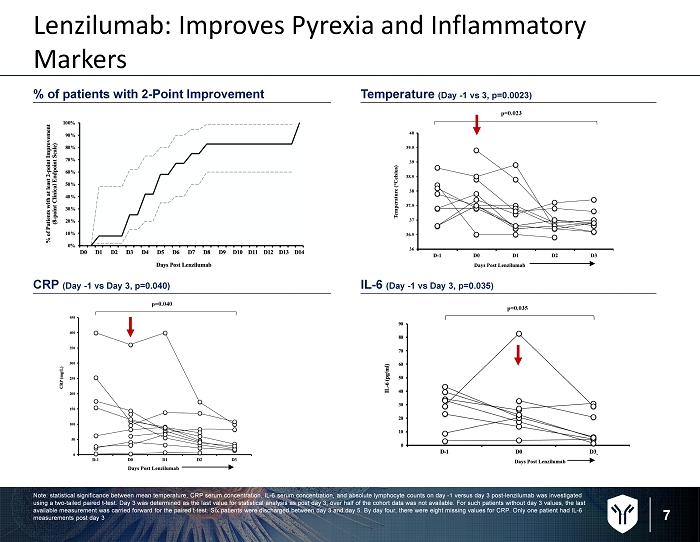
7 % of patients with 2 - Point Improvement Temperature (Day - 1 vs 3, p=0.0023) CRP ( Day - 1 vs Day 3, p=0.040) IL - 6 ( Day - 1 vs Day 3, p=0.035) Lenzilumab: Improves Pyrexia and Inflammatory Markers Note: statistical significance between mean temperature, CRP serum concentration, IL - 6 serum concentration, and absolute lymphoc yte counts on day - 1 versus day 3 post - lenzilumab was investigated using a two - tailed paired t - test. Day 3 was determined as the last value for statistical analysis as post day 3, over half of th e cohort data was not available. For such patients without day 3 values, the last available measurement was carried forward for the paired t - test. Six patients were discharged between day 3 and day 5. By day fo ur, there were eight missing values for CRP. Only one patient had IL - 6 measurements post day 3 p=0.023 p=0.040 p=0.035
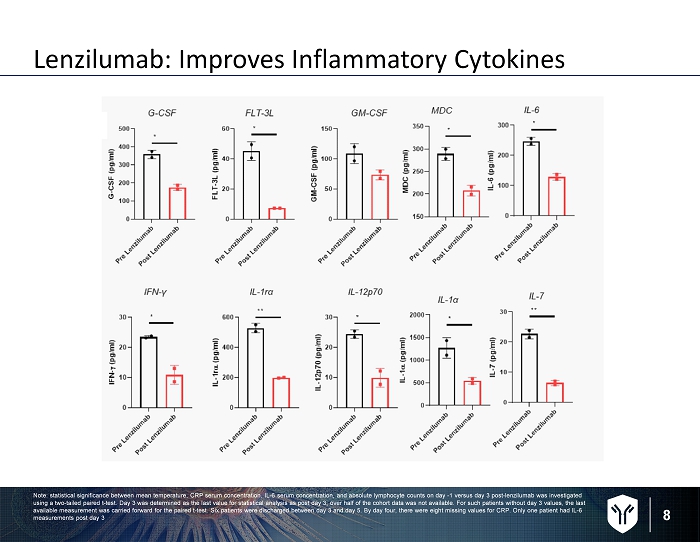
8 Lenzilumab: Improves Inflammatory Cytokines Note: statistical significance between mean temperature, CRP serum concentration, IL - 6 serum concentration, and absolute lymphoc yte counts on day - 1 versus day 3 post - lenzilumab was investigated using a two - tailed paired t - test. Day 3 was determined as the last value for statistical analysis as post day 3, over half of th e cohort data was not available. For such patients without day 3 values, the last available measurement was carried forward for the paired t - test. Six patients were discharged between day 3 and day 5. By day fo ur, there were eight missing values for CRP. Only one patient had IL - 6 measurements post day 3
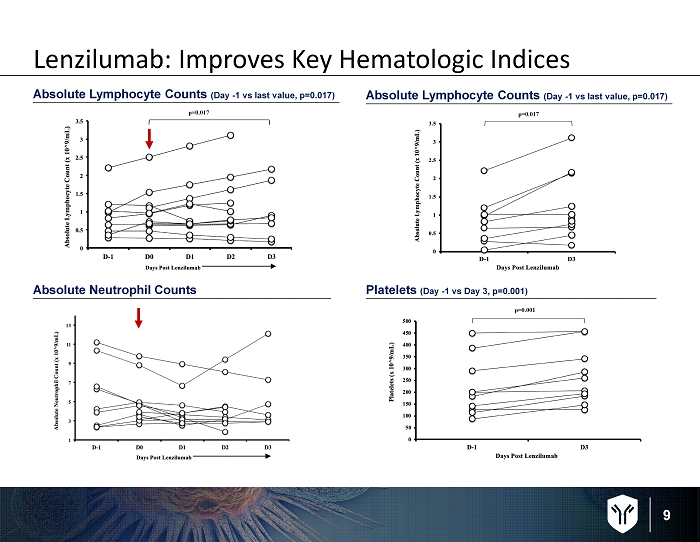
9 Absolute Lymphocyte Counts ( Day - 1 vs last value, p=0.017) Platelets ( Day - 1 vs Day 3, p=0.001) Absolute Neutrophil Counts Lenzilumab: Improves Key Hematologic Indices p=0.017 p=0.017 p=0.001 Absolute Lymphocyte Counts ( Day - 1 vs last value, p=0.017)
