Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REPROS THERAPEUTICS INC. | v466666_8k.htm |
Exhibit 99.1

REPROS THERAPEUTICS CORPORATE PRESENTATION MAY 2017 Dedicated to Treating Male and Female Reproductive Disorders

SAFE HARBOR Any statements made by the Company that are not historical facts contained in these slides (or in any oral accompanying discussion) are forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are subject to various risks, uncertainties and other factors that could cause the Company’s actual results, performance or achievements to differ materially from those expressed or implied by such forward - looking statements . These statements often include words such as “may,” “will,” “expect,” “anticipate,” “continue,” “estimate,” “project,” “potential,” “intend,” “believe,” “plan,” “seek,” “could,” “can,” “should” or similar expressions . These statements are based on assumptions that the Company has made in light of the Company’s experience in the industry, as well as the Company’s perceptions of historical trends, current conditions, expected future developments and other factors the Company believes are appropriate in these circumstances . Forward - looking statements include, but are not limited to, those relating to anticipated milestones for Enclomiphene and Proellex ®, the conduct of planned clinical studies and the timing and nature of the results thereof, the markets for the Company’s products and the potential success of the Company in penetrating those markets and that the Company’s need for and use of financial resources . Such statements are based on current expectations that involve a number of known and unknown risks, uncertainties and other factors that may cause actual events to be materially different from those expressed or implied by such forward - looking statements, including the ability to raise additional needed capital on a timely basis in order for it to continue to fund development of its Enclomiphene and Proellex ® programs, the ability to have success in the clinical development of its technologies, the reliability of interim results to predict final study outcomes, and such other risks as are identified in the Company's most recent Annual Report on Form 10 - K and the subsequent quarterly report on Form 10 - Q and in the prospectus supplement and the accompanying prospectus included in the registration statement mentioned below . These documents are available on request from Repros or at www . sec . gov . Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise . In this presentation, we rely on and refer to information and statistics regarding the pharmaceutical industry . We obtained this information and these statistics from third - party sources, which we have supplemented where necessary with information from publicly available sources and our own internal estimates . Industry publications and surveys generally state that they have obtained information from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information . While we believe that each of these studies and publications is reliable, we have not independently verified such data, and we make no any representation as to the accuracy of such information . Similarly, we believe our internal research is reliable, but it has not been verified by any independent sources . 2

COMPANY OVERVIEW • Originally founded as Zonagen – an animal health company using technology from Baylor University • IPO 1993 • Key recent developments • Patrick Fourteau named as Chairman of the Board • Larry Dillaha, M.D. named President and CEO • Important patent issuance with coverage until 2027 • FDA meeting to discuss path forward for Proellex ® • European filing for Enclomiphene 3

EXPERIENCED MANAGEMENT TEAM • Larry Dillaha , M.D. – President and CEO (Feb 2017) • Sanofi, Sciele / Shionogi, Insys Therapeutics • History of clinical and regulatory success • Kathi Anderson, CPA – CFO • Joined Repros in 2002 • More than 15 years in biotech, life sciences • Joe Wernicke, M.D., Ph.D. – Chief Medical Officer • 30 years industry experience • Eli Lilly, Cyberonics • Jaye Thompson, PhD - SVP Clinical and Regulatory Affairs • Over 20 years experience in clinical research • Founded Synergos – sold to InVentiv Health 4

PROELLEX ® ( TELAPRISTONE ACETATE) FOR THE TREATMENT OF UTERINE FIBROIDS AND ENDOMETRIOSIS • Licensed from NIH, 1999 • Selective Progesterone Receptor Modulator (SPRM) • No selectivity for glucocorticoid receptor • Need for chronic intermittent dosing • Lead Indications: • Treatment of Uterine Fibroids • Treatment of Endometriosis expected to follow • Strong patent protection • NCE related thru 2021 • Off - Drug Interval thru 2027 5

SELECTIVE PROGESTERONE RECEPTOR MODULATOR (SPRM) • Evolved from Progesterone Antagonists • Mifepristone • Limited use because of high affinity for glucocorticoid receptor over progesterone receptor • Recognizing need for more selective progesterone receptor activity, SPRMs developed • Mixed agonist and antagonist activity at progesterone receptor • Relative activity is tissue specific • Minimizing activity on other steroidal receptors 6

UTERINE FIBROIDS • Benign smooth muscle tumors of uterus ( leiomyomas ) • Most common tumor of female reproductive tract • 20 - 77% of women aged 35 - 55 • Quality of Life often impacted • Heavy menstrual bleeding • Discomfort and pain • Current Therapy • Medications • NSAIDS, OCPs, GnRH agonists, Progestin releasing IUDs • Minimally Invasive Procedures • Uterine artery embolization, myomectomy, ablation • Surgery • Hysterectomy – most common reason for hysterectomy – over half of the 600,000 done annually 7

ENDOMETRIOSIS • Endometrial tissue growing outside the uterus • Infertility – Pain, including pelvic pain • Dyspareunia • Dysmenorrhea • 11% of women aged 15 - 44 • 25 - 40% of all cases of infertility • 71 - 87% of women with chronic pelvic pain • 53% teenagers with dysmenorrhea • Impact on QoL • Current Therapy • Medication • NSAIDs, opioids, OCPs, Lupron, danazol • Surgery • Laproscopic procedures – high rates of recurrence 8
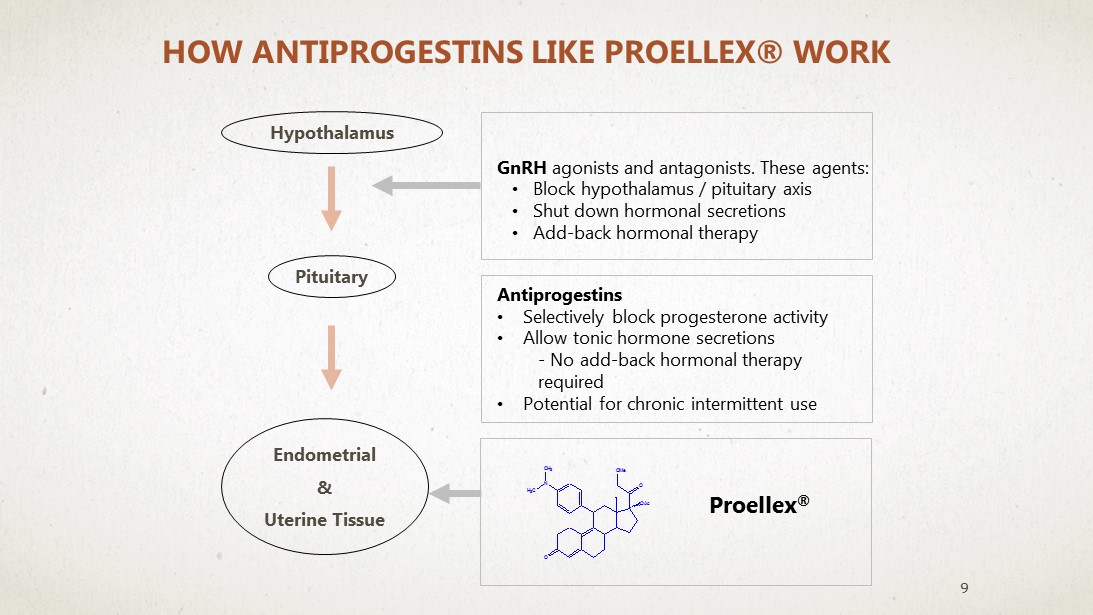
HOW ANTIPROGESTINS LIKE PROELLEX® WORK GnRH agonists and antagonists. These agents: • Block hypothalamus / pituitary axis • Shut down hormonal secretions • Add - back hormonal therapy Proellex ® Hypothalamus Pituitary Endometrial & Uterine Tissue Antiprogestins • Selectively block progesterone activity • Allow tonic hormone secretions - No add - back hormonal therapy required • Potential for chronic intermittent use 9 O O OMe N O Ac CH 3 H 3 C A1
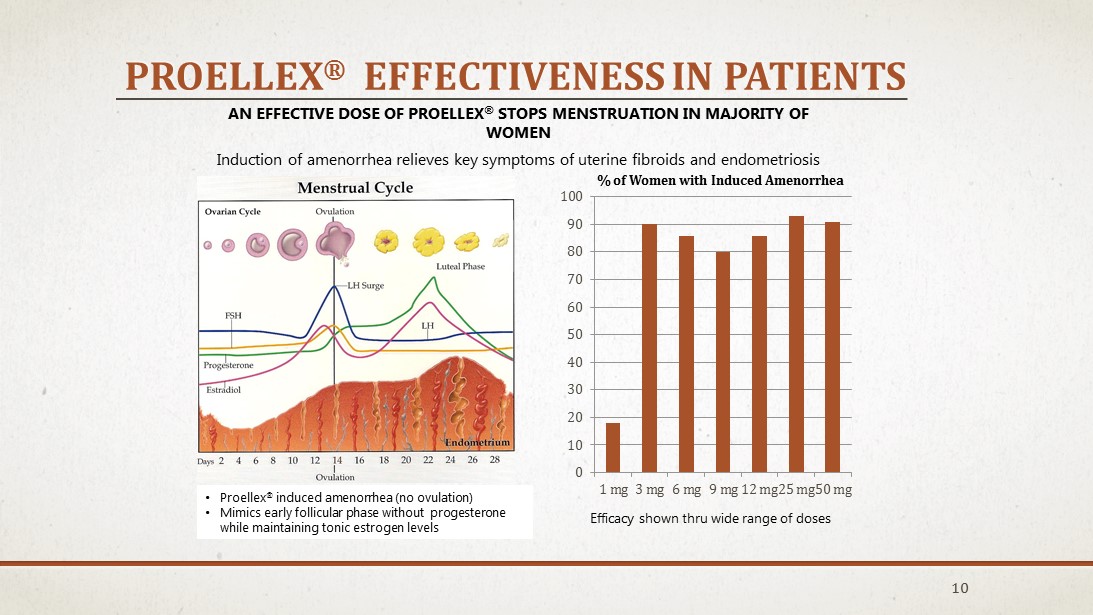
PROELLEX ® EFFECTIVENESS IN PATIENTS 10 AN EFFECTIVE DOSE OF PROELLEX ® STOPS MENSTRUATION IN MAJORITY OF WOMEN Induction of amenorrhea relieves key symptoms of uterine fibroids and endometriosis 0 10 20 30 40 50 60 70 80 90 100 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg % of Women with Induced Amenorrhea • Proellex ® induced amenorrhea (no ovulation) • Mimics early follicular phase without progesterone while maintaining tonic estrogen levels Efficacy shown thru wide range of doses
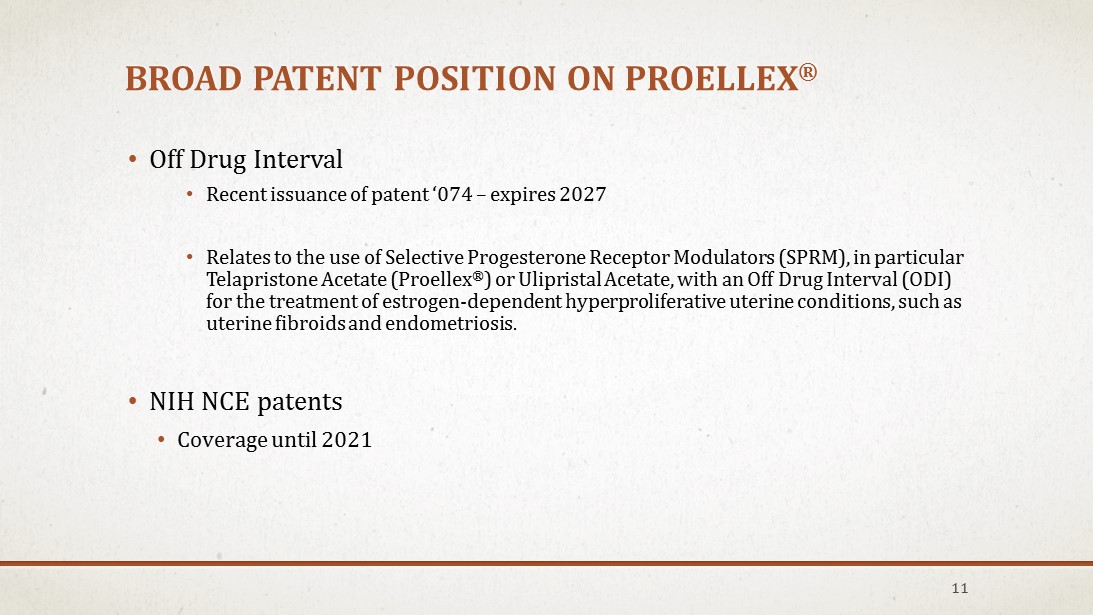
BROAD PATENT POSITION ON PROELLEX ® • Off Drug Interval • Recent issuance of patent ‘074 – expires 2027 • Relates to the use of Selective Progesterone Receptor Modulators (SPRM), in particular Telapristone Acetate (Proellex ® ) or Ulipristal Acetate, with an Off Drug Interval (ODI) for the treatment of estrogen - dependent hyperproliferative uterine conditions, such as uterine fibroids and endometriosis. • NIH NCE patents • Coverage until 2021 11
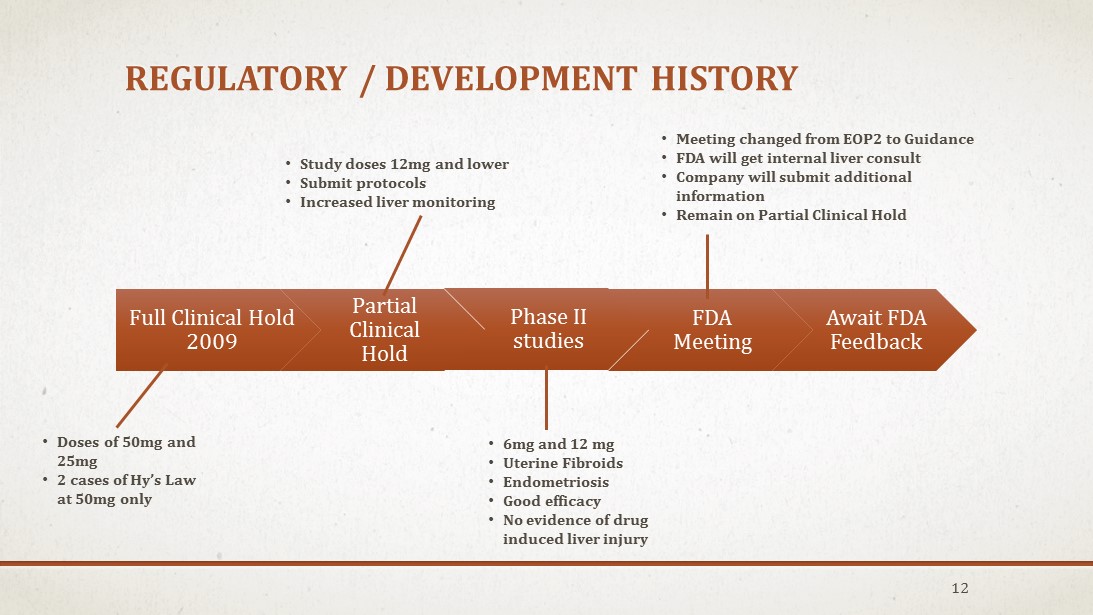
REGULATORY / DEVELOPMENT HISTORY 12 Full Clinical Hold 2009 Partial Clinical Hold Phase II studies FDA Meeting Await FDA Feedback • Study doses 12mg and lower • Submit protocols • Increased liver monitoring • Doses of 50mg and 25mg • 2 cases of Hy’s Law at 50mg only • 6mg and 12 mg • Uterine Fibroids • Endometriosis • Good efficacy • No evidence of drug induced liver injury • Meeting changed from EOP2 to Guidance • FDA will get internal liver consult • Company will submit additional information • Remain on Partial Clinical Hold

EXPECTED REGULATORY PATH FORWARD FOR PROELLEX ® • Repros has empaneled group of liver experts to review data and provide opinion • Reached agreement with division to accept additional information from Repros, including opinion from company’s liver experts, for submission to FDA internal liver panel • Submit clinical protocol along with supplemental information • FDA feedback may take 2 - 3 months 13

SIGNIFICANT MARKET OPPORTUNITY AND INVESTMENT IN THIS THERAPEUTIC AREA • Ulipristal Acetate • Gedeon Richter product Partnered with Allergan for US market • On market in EU • Esmya 5 mg for Tx of UF • Recently completed phase III • Venus II trial • Publicly stated intent to file NDA in 2H17 • $520mil by 2020 • Vilaprisan • Bayer asset V • Recently completed phase II program • Projected 1 billion euros per year • GnRH Agonists 14

ENCLOMIPHENE FOR THE TREATMENT OF SECONDARY HYPOGONADISM • NDA submitted Jan 2015 • Complete response letter received Nov 2015 • EMA filing under review present in EU market • Opinion date is anticipated in 1Q18 • Actively seeking partnering opportunities ex US 15

REPROS: NEXT STEPS AND MILESTONES • Continue discussions with FDA and obtain feedback to determine future clinical trial program for Proellex ® for Uterine Fibroids and Endometriosis • Submit Proellex ® trial design to FDA • EMA filing under review for Enclomiphene for secondary hypogonadism • New management has experience and successful track record to achieve favorable results with the FDA • Actively seeking partnering opportunities for Proellex ® in the US, global or regional markets • Actively seeking partnering opportunities for Enclomiphene outside of the US • Maximize value from patent position 16
