Attached files
| file | filename |
|---|---|
| EX-31.2 - CERTIFICATION - YBCC, Inc. | ybcc_10k-3102.htm |
| EX-32.2 - CERTIFICATION - YBCC, Inc. | ybcc_10k-3202.htm |
| EX-32.1 - CERTIFICATION - YBCC, Inc. | ybcc_10k-3201.htm |
| EX-31.1 - CERTIFICATION - YBCC, Inc. | ybcc_10k-3101.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark one)
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the fiscal year ended December 31, 2016
or
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the transition period from________________________ to _________________________
Commission file number 0-21384
YBCC, INC.
(Exact name of registrant as specified in its charter)
| Nevada | 8980 | 13-3367421 |
| (State or other jurisdiction of | (Primary Standard Industrial | (I.R.S. Employer |
| incorporation or organization) | Classification Code Number) | Identification No.) |
| 17800 CASTLETON ST. STE 386, CITY OF INDUSTRY, CA | 91748 |
| (Address of principal executive offices) | (Zip code) |
626-213-3945
Registrant’s telephone number, including area code
International Packaging and Logistics Group, Inc.
(Former name, former address and former fiscal year, if changed since last report)
| Title of each class | Name of each exchange on which registered |
| Common stock, par value $0.001 per share | None |
| Convertible Preferred stock, Series A, par value $0.0001 per share | None |
Securities registered pursuant to section 12(g) of the Act:
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒ No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes ☐ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☐ | Smaller reporting company ☒ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). ☐ Yes ☒ No
As of June 30, 2016, the aggregate market value of the shares of the Registrant’s common stock held by non-affiliates was $236,739 based upon the closing price ($0.65) of such shares as quoted on the OTC Markets as of June 30, 2016. Shares of the Registrant’s common stock held by each executive officer and director and each by each person who owns 10 percent or more of the outstanding common stock have been excluded in that such persons may be deemed to be affiliates of the Registrant. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
There were a total of 9,894,214 shares of the registrant’s common stock outstanding as of April 21, 2017.
DOCUMENTS INCORPORATED BY REFERENCE
| i |
ITEM 1. Description of Business
Overview
On August 31, 2016, YBCC, Inc. (“YBCC” or the “Company”) through its subsidiary, YibaoConfucian Co. Ltd. (“YibaoHK”) entered into a series of contractual arrangements with Shandong Confucian Biologics Co., Ltd. (“Confucian”) which is a limited liability company headquartered in, and organized under the laws of the PRC. Confucian is a manufacture and research based bio-science company. It has a large capacity to manufacture tablets, granule, oral liquid, powders, soft gels and capsules products. Confucian distributes its products through its own network and white label products. It also has access to a member-based distribution system owned by an affiliated company.
The Company through its subsidiaries possesses manufacturing permits for food product, hygienic products, sanitary products, and health products. The Company's main business includes: research and development of chondroitin and garlic oil; trading, cold storage, and pretreating of garlic, fruit, and vegetables products; trading of chemical products (excluding hazardous chemicals); import and export of goods and technology (excluding those restricted by government); and, the manufacturing and sale of health products including powder, granules, tablets, hard capsule, and soft capsule products.
History
YBCC
YBCC, formerly known as International Packaging and Logistics Group, was originally incorporated as Interactive Medical Technologies, Ltd., on June 2, 1986 in the state of Delaware. On April 17, 2008, IPLO was redomiciled from the State of Delaware to the State of Nevada.
Effective February 3, 1998, Interactive Medical Technologies, Ltd., changed its name to Kaire Holdings Incorporated, which was changed again on May 28, 2008 to International Packaging and Logistics Group, Inc.
On July 2, 2007, IPLO through its wholly-owned subsidiary, YesRx.com (“YesRx”), acquired all the outstanding shares of H&H Glass, Inc. (“H&H Glass”), in exchange for 3,915,000 shares of its common stock in a reverse triangular merger.
On May 15, 2016, the Company and Xiuhua Song (the “Purchaser”) entered into a Stock Purchase Agreement (the “Purchase Agreement”), pursuant to which IPLO (the “Seller”) sold to the Purchaser, and the Purchaser purchased from the Seller an aggregate of 3,915,000 newly issued shares of IPLO Common Stock (the “Shares”). The Shares represented 87% of the issued and outstanding shares of Common Stock. On July 1, 2016, this transaction was completed.
On July 1, 2016, Standard Resources Ltd. (“Standard”), previously IPLO’s majority stockholder, and IPLO entered into a share purchase agreement (“H&H Vend Out”) whereby Standard cancelled 3,915,000 shares of IPLO common stock held by it in exchange for all of the outstanding shares of H&H Glass. The H&H Glass Vend Out was completed on August 31, 2016.
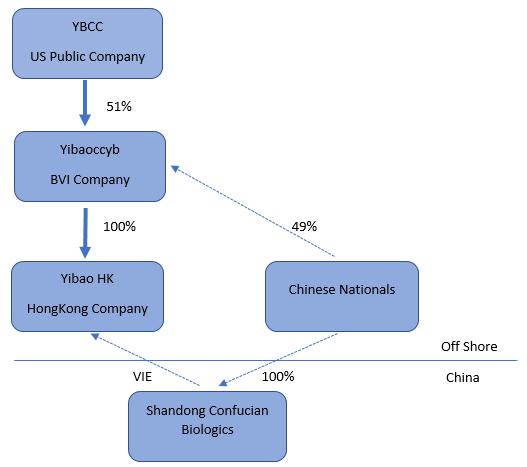
On July 1, 2016, the Company executed an Exchange Agreement with Yibaoccyb Ltd., a British Virgin Islands limited liability company (“Yibaoccyb”) and the Yibaoccyb Shareholders. On August 31, 2016, Yibaoccyb became a 51% owned subsidiary of the Company. Yibaoccyb owns 100% of YibaoConfucian Co., Ltd. (“YibaoHK”), a Hong Kong company. YibaoHK will own 100% of Shenzhen Confucian Biologics Co. Ltd. (yet to be formed, “Yibao”), which will be a wholly foreign-owned enterprise (“WFOE”) under the laws of the Peoples’ Republic of China (“PRC” or “China”). On August 31, 2016, YibaoHK entered into a series of contractual arrangements with Confucian. Our relationship with Confucian is governed by a series of contractual agreements. We do not own any equity interest in Confucian.
On December 22, 2016, the Company amended its Certificate of Incorporation (the “Amendment”). As a result of the Amendment, the Company’s corporate name changed from International Packaging and Logistics Group, Inc. to YBCC, Inc. Effective December 22, 2016, the Registrant’s ticker symbol changed from IPLO to YBAO.
| 1 |
YIBAOCCYB
Yibaoccyb is a limited liability company incorporated under the laws of the British Virgin Islands on May 30, 2016. On August 31, 2016, Yibaoccyb became a 51% owned subsidiary of IPLO. Yibaoccyb, in turn, is the sole owner of YibaoHK. Other than 100% of the issued and outstanding shares of YibaoHK, Yibaoccyb has no other assets or operations.
YIBAOHK
YibaoHK is a limited liability company incorporated under the laws of the Hong Kong on June 15, 2016. YibaoHK was formed by Yibaoccyb. YibaoHK will be the sole owner of Yibao (yet to be formed). On August 31, 2016, YibaoHK entered into a series of contractual arrangements with Confucian.
YIBAO
Yibao, a wholly foreign owned enterprise under the laws of the PRC is in the process of being established. All of the issued and outstanding shares of Yibao will be held by YibaoHK. The principal purpose of Yibao will be to manage, hold and own rights in the business of Confucian and other potential PRC businesses. Other than management contracts with the aforementioned companies and related activities, Yibao is expected to have no other separate operations of its own.
CONFUCIAN
PRC law currently has limits on foreign ownership of certain companies. To comply with these foreign ownership restrictions, we operate our businesses in China through Confucian which is a limited liability company headquartered in China and organized under the laws of China. Confucian has the licenses and approvals necessary to operate our businesses in China. We have contractual arrangements with Confucian and their respective shareholders pursuant to which we provide these companies with technology consulting and other general business operation services. Through these contractual arrangements, we also have the ability to substantially influence these companies’ daily operations and financial affairs, appoint their senior executives and approve all matters requiring shareholder approval. As a result of these contractual arrangements, which enable us to control Confucian, we are considered the primary beneficiary of Confucian. Accordingly, we consolidate the results, assets and liabilities of Confucian in our financial statements.
The following chart summarizes our organizational and ownership structure as of December 31, 2016:
Going Concern
As described in auditor’s report on our consolidated financial statements, our auditors have included a “going concern” provision in their opinion on our consolidated financial statements, expressing substantial doubt that we can continue as an ongoing business for the next twelve months.
| 2 |
CONTRACTUAL ARRANGEMENTS WITH CONFUCIAN AND THEIR SHAREHOLDERS
Our relationships with the Confucian and their shareholders are governed by a series of contractual arrangements between YibaoHK, and Confucian, which is the operating company of the Yibao Group in the PRC. Under PRC laws, each of YibaoHK and Confucian is an independent legal person and none of them is exposed to liabilities incurred by the other parties. The contractual arrangements constitute valid and binding obligations of the parties of such agreements. Each of the contractual arrangements and the rights and obligations of the parties thereto are enforceable and valid in accordance with the laws of the PRC. Other than pursuant to the contractual arrangements between YibaoHK and Confucian described below, Confucian does not transfer any other funds generated from its respective operations to any other member of the Yibao Group. On August 31, 2016, we entered into the following contractual arrangements (“VIE Agreements”) with Confucian:
Consulting Services Agreement. Pursuant to the exclusive consulting services agreements between YibaoHK and Confucian, YibaoHK has the exclusive right to provide to Confucian general business operation services, including advice and strategic planning, as well as consulting services related to the technological research and development of dietary supplements and related products (the “Services”). Under this agreement, YibaoHK owns the intellectual property rights developed or discovered through research and development, in the course of providing the Services, or derived from the provision of the Services. Confucian pays a quarterly consulting service fees in Renminbi (“RMB”) to Yibaoccyb that is equal to all of Confucian’s profits for such quarter.
Operating Agreement. Pursuant to the operating agreement among YibaoHK, Confucian and all shareholders of Confucian (collectively the “Shandong Confucian Biologics Shareholders”), YibaoHK provides guidance and instructions on Confucian’s daily operations, financial management and employment issues. Confucian Shareholders must designate the candidates recommended by YibaoHK as their representatives on the boards of directors of each of Confucian. YibaoHK has the right to appoint senior executives of Confucian. In addition, YibaoHK agrees to guarantee Confucian’ performance under any agreements or arrangements relating to Confucian’ business arrangements with any third party. Confucian, in return, agrees to pledge their accounts receivable and all of their assets to YibaoHK. Moreover, Confucian agrees that without the prior consent of YibaoHK, Confucian will not engage in any transactions that could materially affect their respective assets, liabilities, rights or operations, including, without limitation, incurrence or assumption of any indebtedness, sale or purchase of any assets or rights, incurrence of any encumbrance on any of their assets or intellectual property rights in favor of a third party or transfer of any agreements relating to their business operation to any third party. The term of this agreement is ten (10) years from August 31, 2016 and may be extended only upon YibaoHK’s written confirmation prior to the expiration of this agreement, with the extended term to be mutually agreed upon by the parties.
Equity Pledge Agreement. Under the equity pledge agreement between Confucian Shareholders and YibaoHK, Confucian Shareholders pledged all of their equity interests in Confucian to YibaoHK to guarantee Confucian’ performance of their obligations under the consulting services agreement. If Confucian or Confucian Shareholders breaches their respective contractual obligations, YibaoHK, as pledgee, will be entitled to certain rights, including the right to sell the pledged equity interests. Confucian Shareholders also agreed that upon occurrence of any event of default, YibaoHK shall be granted an exclusive, irrevocable power of attorney to take actions in the place and stead of Confucian Shareholders to carry out the security provisions of the equity pledge agreement and take any action and execute any instrument that YibaoHK may deem necessary or advisable to accomplish the purposes of the equity pledge agreement. Confucian Shareholders agreed not to dispose of the pledged equity interests or take any actions that would prejudice YibaoHK’s interest. The equity pledge agreement will expire two (2) years after Confucian’ obligations under the consulting services agreements have been fulfilled.
Option Agreement. Under the option agreement between Confucian Shareholders and YibaoHK, Confucian Shareholders irrevocably granted YibaoHK or its designated person an exclusive option to purchase, to the extent permitted under PRC law, all or part of the equity interests in Confucian for the cost of the initial contributions to the registered capital or the minimum amount of consideration permitted by applicable PRC law. YibaoHK or its designated person has sole discretion to decide when to exercise the option, whether in part or in full. The term of this agreement is ten (10) years from August 31, 2016 and may be extended prior to its expiration by written agreement of the parties.
Voting Rights Proxy Agreement. Pursuant to the proxy agreement between Confucian Shareholders and YibaoHK, Confucian Shareholders agreed to irrevocably grant a person to be designated by YibaoHK with the right to exercise Confucian Shareholders’ voting rights and their other rights, including the attendance at and the voting of Confucian Shareholders’ shares at shareholders’ meetings (or by written consent in lieu of such meetings) in accordance with applicable laws and its Articles of Association, including but not limited to the rights to sell or transfer all or any of his equity interests of Confucian, and appoint and vote for the directors and Chairman as the authorized representative of the shareholders of Confucian. The proxy agreement may be terminated by joint consent of the parties or upon 30-day written notice from YibaoHK.
| 3 |
CONFUCIAN BUSINESS
History
Confucian was founded under the laws of the People's Republic of China on October 31, 2012. Confucian is located in Food Industrial Park inside the economic development Zone of JinXiang County, Ji’ning City in the province of Shandong in China. Confucian is a limited liability company.
Overview
Confusion is a manufacture and research based bio-science company. It has a large capacity to manufacture tablets, granule, oral liquid, powders, soft gels and capsules products. Confucian distributes its products through its own network and white label products. It also has access to a member-based distribution system owned by an affiliated company.
Confusion possesses manufacturing permits for food product, hygienic products, sanitary products, and health products. Confusion's main business includes: technology study and transfer of chondroitin and garlic oil; trading, cold storage, and pretreating of garlic, fruit, and vegetables products; trading of chemical products (excluding hazardous chemicals); import and export of goods and technology (excluding those restricted by government); the manufacturing and sale of health products including powder, granules, tablets, hard capsule, and soft capsule products.
Ownership
During the phase of incorporation, Qingbao Kong accounted for 51% of the initial equity, Xiuhua Song accounted for 49%.
In 2013, Xiuhua Song transferred her 49% of equity to WenXiu Song.
In March 2016, Qingbao Kong transferred his 51% of equity to Hengchun Zhang.
As of today, the Company’s equity is owned 51% by Hengchun Zhang and 49% by Wenxiu Song
Product Overview
The Company’s main products can be divided into health food products and hygienic products
Health Food Products
| · | Phytocholesterol tableting candy, |
Phytosterol has strong anti-inflammatory effects to the human body, which can inhibit the absorption of cholesterol for human and biochemical synthesis of cholesterol. Additionally, it can promote the degradation and metabolism of cholesterol. Phytosterol can be used for prevention & therapy of coronary atherosclerosis heart disease. It has the potential for treating ulcers, skin squamous carcinoma and cervical cancer. Phytosterol may promote wound healing, muscle proliferation, enhance capillary circulation. Phytosterol may be used as blocking agent of formation of gallstones.
| · | Polydextrose tableting candy, |
Polydextrose is used for regulating blood lipid in order to reduce fat accumulation.
| · | Dunaliella Salina Haematococcus Pluvialis tableting candy, |
Dunaliella Salina Haematococcus Pluvialis is used to replenishing the body's astaxanthin, natural carotene and variety of minerals. It has an effect on antioxidant activity, protect skin, protect vision and improve immunity.
| · | Dunaliella Salina Gum Base Candy, |
Dunaliella salina is rich in antioxidant needed by the human body health, resistance to radiation and enhance human immunity of natural carotenoids and 70 kinds of minerals and trace elements.
| · | Haematococcus Pluvialis Gum Candy, |
The main component of Haematococcus Pluvialis is astaxanthin. It has six anti-aging effect protect the skin; protect eye health; helps to support the cardiovascular system; maintain a healthy joints and connective tissue; and, increase strength and endurance.
| 4 |
| · | Fish Oil Gum Candy, |
Fish Oil adjusts the blood liquid which may, prevent blood clots, cerebral thrombosis, cerebral hemorrhage and stroke. Additionally, fish oil may prevent arthritis, Alzheimer’s disease, improve the memory and vision, and control presbyopia.
| · | Earthworm Protein tableting Candy, |
Earthworm Protein is used to improve blood circulation, inhibit platelet aggregation, reduce glucose concentrations, and prevent blood clots. It may be a control efficiency for coronary heart disease, arteriosclerosis, and other hematologic disorders.
| · | Collagen Protein tableting candy, |
Collagen Protein is rich in glycine, proline hydroxyproline and other amino acid needed for human body. Collagen protein may have a beneficial effect for skin, hair, bones and muscles.
| · | Krill Oil Gum candy, |
Krill Oil is rich in EPA and DHA. This may have beneficial health effects, including cardiovascular, nerve, bones, joints, vision, and skin care.
| · | Phosphatidylserine tableting candy, |
Phosphatidylserine may improve the function of the brain by promoting the recovery of brain; and central nervous system. Phosphatidylserine can be used for auxiliary treatment dementia and age related memory loss.
| · | Milk Powder tableting candy, |
Milk powder tablet is a nutrition supplement.
Hygienic Products
The hygienic product line includes the following products:
| · | Feminine Gel products for women, |
Anti-bacteria product used as an auxiliary treatment for vaginal bacterial.
| · | Anti-bacterial skin liquid |
Anti-bacteria liquids used for skin sterilization. Inhibit the bromhidrosis and relief beriberi itch
Confucian owns a 100,000 stage purification workshops, advanced production lines and manufacturing equipment. The Company has a higher capacity for OEM processing of tablets, hard capsules, soft capsules, oral liquid, granules, and powders.
Plan
By following the Company motto of being “passionate for the health industry, bringing together the world's resources, focusing on consumer demand, and creating a win-win situation ‘, the Company is eager to develop businesses in the international health and pharmaceutical market.
The Company’s near term goal is to reach breakeven within a 6-month period time. In order to reach such goal, the Company is increasing its sales and production volume through arrangements and networking with its existing customers and its affiliated companies. Additionally, it plans to increase the size of its sales department to develop new customers.
The Company’s ultimate goal is to make the business profitable and competitive in the international health and pharmaceutical market. To achieve such goal, the Company needs to cooperate with other businesses having capital, market, technology, or products. Additionally, the Company needs to recruit sufficient workforce and talents and actively develop new technology and new products through research and development.
| 5 |
Market Overview
Domestic Markets
Through member based distribution network, the Company has access to the major markets in Jining City area and most other cities in the Shandong province.
International Market
Currently international markets show an interest in Chinese herb medicine. For instance, European and US companies in the food industry use advanced technology to extract ginkgo biloba, then add it in gum, chocolate, and other health foods. The Company focuses on product diversification and innovation. It plans to sell its produces in well-known retail stores in Europe and US, such as Walmart.
Market Opportunity
Consumers are increasingly concerned about their own health. The spending on health-related products has increased year by year, and the demand for nutrition and health food is high. According to the international standard classification, medicine and health care is one of the world's fastest growing trade sectors. Sales of health food is currently experiencing a rapid annual growth.
In China, the health products market is expanding along with the growth of economy and acceleration of aged population. People used to see health products as optional. Now they are necessities of daily life. The sixty and older group is expected to keep growing fast. The elderly group tends to draw attention to nutrition and health product, which will boost the development of the health market. In addition, young people are beginning to pay more attention to their heath, and health food and products are the new powerful impetus. Therefore, the Company believes it will be able to take advantage of this increased demand.
Competition
At present, Chinese health food manufacturers are mainly concentrated in Shandong, Jiangsu, Zhejiang, Anhui, Ningxia and a few other regions. Although in recent years, the health and production conditions of the eastern coastal areas have been improved to some extent. Overall, China’s nutrition and health food businesses are small scaled, use outdated technology, and lack brand recognition.
Intellectual Property
Confucian is actively planning research and development activities in order to patent these products.
Government Regulation
The great social demand of nutrition and health care products has led to a policy of governmental support. In December 2011, the Nutrient agency released “125 Development Plan for Food Industry”, in which nutrition and health care products manufacturing was first listed as the most important development within the industry. In addition, “the opinions of State Council on Promoting Health Development of Service Industry” published in 2013, “Notice on Promoting Health and Pension Services” published in 2014, and “Chinese Food and Nutrition Development Program” published in 2014 all had positive effects on the development of health products industry.
Employees
Confucian currently has 38 full time employees, including 2 management employees, 7 office employees handling finance and administrative functions, 4 scientific researchers and technicians; and 25 production workers.
Property
YBCC’s corporate headquarters are located at 17800 Castleton St. Ste 180, City of Industry, California 91748. YBCC rents approximately 983 square feet of office space for its headquarters. The lease began on September 1, 2016 and expires on September 30, 2018. As of December 31, 2016, the total monthly base rent is $2,802.
Confucian is located in Food Industrial Park inside the economic development Zone of JinXiang County, Jining City in the province of Shandong in China. It has three land use rights with the total cost of approximately $736,632. One land use right expires in 2063, while the other two land use rights expire in 2065. It has nearly 30,000 square meters standardized plant facilities.
| 6 |
Litigation
There are no known potential litigation matters.
You should carefully consider the risks described below together with all of the other information included in this report before making an investment decision with regard to our securities. The statements contained in or incorporated into this offering that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Industry
Our businesses are subject to fluctuations in operating results due to general economic conditions, specific economic conditions in the industries in which it operates and other external forces.
Our businesses and operations could be affected by the following, among other factors:
| - | changes in general economic conditions and specific conditions in industries in which our businesses operate that can result in the deferral or reduction of purchases by end-use customers; |
| - | the size, timing and cancellation of significant orders, which can be non-recurring; |
| - | market acceptance of new products and product enhancements; |
| - | announcements, introductions and transitions of new products by us or our competitors; |
| - | deferrals of customer orders in anticipation of new products or product enhancements introduced by us or our competitors; |
| - | changes in pricing in response to competitive pricing actions; |
| - | supply constraints; |
| - | the level of expenditures on research and development and sales and marketing programs; |
| - | our ability to achieve targeted cost reductions; |
| - | rising interest rates; and |
| - | excess facilities. |
The loss of Confucian as our operating business would have a material adverse effect on our business and the price of our common stock.
We have no equity ownership interest in Confucian. Our ability to control Confucian and consolidate its financial results is through a series of contractual agreements between it and YibaoHK. Management of Confucian is affiliates of us and the stockholders of Confucian are also our stockholders. Thus, the VIE Agreements were not entered into as a result of arms’ length negotiations because the parties to the agreement are under common control. Ms. Song, our CEO and Chairman has control over of the shares of Confucian and of our common stock. The VIE Agreements may be terminated upon the termination of the business of Confucian. Any other termination would be a breach of the agreement. While the Company believes that the VIE Agreements are legal and enforceable under PRC law, these affiliates control the parties to the VIE Agreements and it could be possible for them to cause Confucian to breach the VIE Agreements and our unaffiliated investors would have little or no recourse because of the inherent difficulties in enforcing their rights since all our assets are located in the PRC. (See, PRC laws and regulations governing Confucian' current business are sometimes vague and uncertain.) In the event that management of Confucian decides to breach the VIE Agreements, the risk of loss of the affiliated shareholders of Confucian could be lower than unaffiliated investors and the interests of the management and shareholders of Confucian would be in conflict with the interest of our other stockholders.
| 7 |
Confucian’s failure to compete effectively may adversely affect our ability to generate revenue.
Confucian competes with other companies, many of whom are developing or can be expected to develop products similar to Confucian. Confucian’s market is a large market with many competitors. Many of its competitors are more established than Confucian is, and have significantly greater financial, technical, marketing and other resources than it presently possesses. Some of Confucian’s competitors have greater name recognition and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies. We cannot assure you that Confucian will be able to compete effectively with current or future competitors or that the competitive pressures it faces will not harm it business.
We may not be able to effectively control and manage the growth of Confucian.
If Confucian’s business and markets grow and develop, it will be necessary for us to finance and manage expansion in an orderly fashion. An expansion would increase demands on its existing management, workforce and facilities. Failure to satisfy such increased demands could interrupt or adversely affect its operations and cause delay in production and delivery of its pharmaceutical prescription, over the counter and medical nutrient products as well as administrative inefficiencies.
We may require additional financing in the future and a failure to obtain such required financing will inhibit Confucian’s ability to grow.
The continued growth of Confucian’s business may require additional funding from time to time which we expect to raise in private placements of our equity or debt securities with accredited investors or by offering our securities for sale pursuant to an effective registration statement on a market where our common stock is traded. The proceeds of these funding will be forwarded to Confucian and accounted for as a loan to Confucian and eliminated during consolidation. The proceeds would be used for general corporate purposes of Confucian, which could include acquisitions, investments, repayment of debt and capital expenditures among other things. We may also use the proceeds to repurchase our capital stock or for our corporate overhead expenses. If we borrow funds we expect to be the primary obligor on any debt. Obtaining additional funding would be subject to a number of factors including market conditions, operating performance and investor sentiment, many of which are outside of our control. These factors could make the timing, amount, terms and conditions of additional funding unattractive or unavailable to us.
Our management believes that Confucian currently has sufficient funds from working capital to meet its current operating costs over the next 6 months.
The terms of any future financing may adversely affect your interest as stockholders.
If we require additional financing in the future, we may be required to incur indebtedness or issue equity securities, the terms of which may adversely affect your interests in us. For example, the issuance of additional indebtedness may be senior in right of payment to your shares upon our liquidation. In addition, indebtedness may be under terms that make the operation of Confucian's business more difficult because the lender's consent could be required before we take certain actions. Similarly, the terms of any equity securities we issue may be senior in right of payment of dividends to your common stock and may contain superior rights and other rights as compared to your common stock. Further, any such issuance of equity securities may dilute your interest in us.
We may engage in future acquisitions that could dilute the ownership interests of our stockholders, cause us to incur debt and assume contingent liabilities.
We may review acquisition and strategic investment prospects that we believe would complement the current product offerings of Confucian, augment its market coverage or enhance its technical capabilities, or otherwise offer growth opportunities. From time to time we review investments in new businesses and expect to make investments in, and to acquire, businesses, products, or technologies in the future. We expect that when we raise funds from investors for any of these purposes we will be either the issuer or the primary obligor while the proceeds will be forwarded to Confucian and accounted for as a loan to Confucian and eliminated during consolidation. In the event of any future acquisitions, we could:
| · | issue equity securities which would dilute current stockholders’ percentage ownership; |
| · | incur substantial debt; |
| · | assume contingent liabilities; or |
| · | expend significant cash. |
| 8 |
These actions could have a material adverse effect on our operating results or the price of our common stock. Moreover, even if we do obtain benefits in the form of increased sales and earnings, there may be a lag between the time when the expenses associated with an acquisition are incurred and the time when we recognize such benefits. Acquisitions and investment activities also entail numerous risks, including:
| · | difficulties in the assimilation of acquired operations, technologies and/or products; |
| · | unanticipated costs associated with the acquisition or investment transaction; |
| · | the diversion of management’s attention from other business concerns; |
| · | adverse effects on existing business relationships with suppliers and customers; |
| · | risks associated with entering markets in which Confucian has no or limited prior experience; |
| · | the potential loss of key employees of acquired organizations; and |
| · | substantial charges for the amortization of certain purchased intangible assets, deferred stock compensation or similar items. |
We cannot ensure that we will be able to successfully integrate any businesses, products, technologies, or personnel that we might acquire in the future and our failure to do so could have a material adverse effect on our and/or Confucian' business, operating results and financial condition.
We are responsible for the indemnification of our officers and directors.
Our certificate of incorporation provides for the indemnification and/or exculpation of our directors, officers, employees, agents and other entities which deal with it to the maximum extent provided, and under the terms provided, by the laws and decisions of the courts of the state of Nevada. Since we do not hold any indemnification insurance, these indemnification provisions could result in substantial expenditures, which we may be unable to recoup, which could adversely affect our business and financial conditions. Xiuhua Song, our Chairman of Board, President, Chief Executive Officer, and Chief Financial Officer are key personnel with rights to indemnification under our certificate of incorporation.
We may not have adequate internal accounting controls. While we have certain internal procedures in our budgeting, forecasting and in the management and allocation of funds, our internal controls may not be adequate.
We are constantly striving to improve our internal accounting controls. We expect to continue to improve our internal accounting control for budgeting, forecasting, managing and allocating our funds and to better account for them as we grow. There is no guarantee that such improvements will be adequate or successful or that such improvements will be carried out on a timely basis. If we do not have adequate internal accounting controls, we may not be able to appropriately budget, forecast and manage our funds, we may also be unable to prepare accurate accounts on a timely basis to meet our continuing financial reporting obligations and we may not be able to satisfy our obligations under US securities laws.
Confucian is dependent on certain key personnel and loss of these key personnel could have a material adverse effect on our business, financial condition and results of operations.
Our success is, to a certain extent, attributable to the management, sales and marketing, and manufacturing expertise of key personnel at Confucian. Xiuhua Song, our President, Chief Executive Officer and Chairman of the Board, performs key functions in the operation of our and Confucian's business. There can be no assurance that Confucian will be able to retain these officers after the term of their employment contracts expire. The loss of these officers could have a material adverse effect upon our business, financial condition, and results of operations. Confucian must attract, recruit and retain a sizeable workforce of technically competent employees. We do not carry key man life insurance for any of our key personnel or personnel at Confucian nor do we foresee purchasing such insurance to protect against a loss of key personnel and the key personnel of Confucian.
| 9 |
We and Confucian are dependent upon the services of Mrs. Song, for the continued growth and operation of our company because of her experience in the industry and her personal and business contacts in China. Although we have no reason to believe that Mrs. Song will discontinue her services with us or Confucian, the interruption or loss of her services would adversely affect our ability to effectively run Confucian's business and pursue its business strategy as well as our results of operations.
Confucian may not be able to hire and retain qualified personnel to support its growth and if it is unable to retain or hire these personnel in the future, its ability to improve its products and implement its business objectives could be adversely affected.
Competition for senior management and senior personnel in the PRC is intense, the pool of qualified candidates in the PRC is very limited, and Confucian may not be able to retain the services of its senior executives or senior personnel, or attract and retain high-quality senior executives or senior personnel in the future. This failure could materially and adversely affect our future growth and financial condition. Confucian expects to hire additional sales and plant personnel throughout fiscal year 2017 in order to accommodate its growth.
If Confucian fails to increase its brand recognition, it may face difficulty in obtaining new customers and business partners.
We believe that establishing, maintaining and enhancing Confucian’s brand in a cost-effective manner is critical to achieving widespread acceptance of Confucian’s current and future products and services and is an important element in Confucian's effort to increase its customer base and obtain new business partners. We believe that the importance of brand recognition will increase as competition in Confucian’s market develops. Some of Confucian’s potential competitors already have well-established brands in the pharmaceutical promotion and distribution industry. Successful promotion of Confucian’s brand will depend largely on its ability to maintain a sizeable and active customer base, its marketing efforts and its ability to provide reliable and useful products and services at competitive prices. Brand promotion activities may not yield increased revenue, and even if they do, any increased revenue may not offset the expenses Confucian incurs in building its brand. If Confucian fails to successfully promote and maintain its brand, or if Confucian incurs substantial expenses in an unsuccessful attempt to promote and maintain its brand, it may fail to attract enough new customers or retain its existing customers to the extent necessary to realize a sufficient return on its brand-building efforts, in which case Confucian's business, operating results and financial condition, further ours would be materially adversely affected.
Confucian's operating results may fluctuate as a result of factors beyond its control.
Confucian's operating results may fluctuate significantly in the future as a result of a variety of factors, many of which are beyond its control. These factors include:
| · | the costs of raw material and development; |
| · | the relative speed and success with which Confucian can obtain and maintain customers, merchants and vendors for its products; |
| · | capital expenditures for equipment; |
| · | marketing and promotional activities and other costs; |
| · | changes in Confucian’s pricing policies, suppliers and competitors; |
| · | the ability of Confucian’s suppliers to provide products in a timely manner to its customers; |
| · | changes in operating expenses; |
| · | increased competition in Confucian’s markets; and |
| · | other general economic and seasonal factors. |
| 10 |
Confucian faces risks related to product liability claims.
Confucian does not maintain product liability insurance. It faces the risk of loss because adverse publicity associated with product liability lawsuits, whether or not such claims are valid. It may not be able to avoid such claims. Although product liability lawsuits in the PRC are rare, and Confucian has not to date experienced significant failure of its products, there is no guarantee that it will not face such liability in the future. This liability could be substantial and the occurrence of such loss or liability may have a material adverse effect on its business, financial condition and prospects.
Confucian faces marketing risks.
Newly developed dietary supplements and technologies may not be compatible with market needs. Because markets for drugs differentiate geographically inside China, Confucian must develop and manufacture its products to accurately target specific markets to ensure product sales. If Confucian fails to invest in extensive market research to understand the health needs of consumers in different geographic areas, it may face limited market acceptance of its products, which could have material adverse effect on its sales and earnings.
We face risks relating to difficulty in defending intellectual property rights from infringement.
Our success depends on protection of the current and future technologies and products of Confucian and its ability to defend its intellectual property rights. Confucian has filed for copyright protection for the various names and brands of its products sold in the PRC. However, it is possible for its competitors to develop similar competitive products even though it has taken steps to protect its intellectual property. If we fail to protect Confucian’s intellectual property adequately, competitors may manufacture and market products similar to Confucian.
Confucian also relies on trade secrets, non-patented proprietary expertise and continuing technological innovation that it shall seek to protect, in part, by entering into confidentiality agreements with licensees, suppliers, employees and consultants. These agreements may be breached and there may not be adequate remedies in the event of a breach. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. Moreover, its trade secrets and proprietary technology may otherwise become known or be independently developed by its competitors. If patents are not issued with respect to products arising from research, Confucian may not be able to maintain the confidentiality of information relating to these products.
We face risks relating to third parties that may claim that Confucian infringes on their proprietary rights and may prevent Confucian from manufacturing and selling certain of its products.
There has been substantial litigation in the pharmaceutical industry with respect to the manufacturing, use and sale of new products. These lawsuits relate to the validity and infringement of patents or proprietary rights of third parties. We and/or Confucian may be required to commence or defend against charges relating to the infringement of patent or proprietary rights. Any such litigation could:
| · | require Confucian or us to incur substantial expense, even if covered by insurance or are successful in the litigation; |
| · | require Confucian to divert significant time and effort of its technical and management personnel; |
| · | result in the loss of Confucian’s rights to develop or make certain products; and |
| · | require Confucian or us to pay substantial monetary damages or royalties in order to license proprietary rights from third parties. |
Although patent and intellectual property disputes within our have often been settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include the long-term payment of royalties. These arrangements may be investigated by regulatory agencies and, if improper, may be invalidated. Furthermore, the required licenses may not be made available to Confucian on acceptable terms. Accordingly, an adverse determination in a judicial or administrative proceeding or a failure to obtain necessary licenses could prevent Confucian from manufacturing and selling some of its products or increase its costs to market these products.
| 11 |
In addition, when seeking regulatory approval for some of its products, Confucian is required to certify to regulatory authorities, including the SFDA that such products do not infringe upon third party patent rights. Filing a certification against a patent gives the patent holder the right to bring a patent infringement lawsuit against Confucian. Any lawsuit would delay the receipt of regulatory approvals. A claim of infringement and the resulting delay could result in substantial expenses and even prevent Confucian from manufacturing and selling certain of its products.
Confucian’s launch of a product prior to a final court decision or the expiration of a patent held by a third party may result in substantial damages to Confucian or us. If Confucian is found to infringe a patent held by a third party and become subject to such damages, these damages could have a material adverse effect on the results of its operations and financial condition.
We face risks related to research and the ability to develop new products.
Our growth and survival depends on Confucian’s ability to consistently discover, develop and commercialize new products and find new and improve on existing technologies and platforms. As such, if Confucian fails to make sufficient investments in research, be attentive to consumer needs or does not focus on the most advanced technologies, its current and future products could be surpassed by more effective or advanced products of other companies.
Risk Related To Confucian’s Industry
Confucian’s certificates, permits, and licenses related to its operations are subject to governmental control and renewal and failure to obtain renewal will cause all or part of its operations to be terminated.
Confucian is subject to various PRC laws and regulations pertaining to our industry. Confucian has attained certificates, permits, and licenses required for the operation of a dietary supplement enterprise and the manufacturing of our products in the PRC.
Confucian intends to apply for renewal of these health food production permits prior to expiration. During the renewal process, Confucian will be re-evaluated by the appropriate governmental authorities and must comply with the then prevailing standards and regulations which may change from time to time. In the event that it is not able to renew the certificates, permits and licenses, all or part of its operations may be terminated. Furthermore, if escalating compliance costs associated with governmental standards and regulations restrict or prohibit any part of its operations, it may adversely affect its operation and our profitability.
According to Drug Administration Law of the PRC and its implemental rules, SFDA approvals may be suspended or revoked prior to the expiration date under circumstances that include:
| · | producing counterfeit medicine, |
| · | producing inferior quality products, |
| · | failing to meet the drug GMP standards; |
| · | purchasing medical ingredients used in the production of products sources that do not have a Pharmaceutical Manufacturing Permit or Pharmaceutical Trade Permit; |
| · | fraudulent reporting of results or product samples in application process, |
| · | failing to meet drug labeling and direction standards, |
| · | bribing doctors or hospital personnel to entice them to use products, |
| · | producing pharmaceuticals for use or resale by companies that are not approved by the SFDA, or |
| · | the approved drug has a serious side effect. |
| 12 |
If Confucian’s products fail to receive regulatory approval or are severely limited in these products' scope of use, it may be unable to recoup considerable research and development expenditures.
Confucian’s research and development of pharmaceutical products is subject to the regulatory approval of the SFDA in China. The regulatory approval procedure for pharmaceuticals can be quite lengthy, costly, and uncertain. Depending upon the discretion of the SFDA, the approval process may be significantly delayed by additional clinical testing and require the expenditure of resources not currently available; in such an event, it may be necessary for Confucian to abandon its application. Even where approval of the product is granted, it may contain significant limitations in the form of narrow indications, warnings, precautions, or contra-indications with respect to conditions of use. If approval of Confucian’s product is denied, abandoned, or severely limited in terms of the scope of products use, it may result in the inability to recoup considerable research and development expenditures.
Price control regulations may decrease Confucian’s profitability.
The laws of the PRC provide for the government to fix and adjust prices. The prices of certain medicines Confucian distributes, including those listed in the Chinese government's catalogue of medications that are reimbursable under China's social insurance program, or the Insurance Catalogue, are subject to control by the relevant state or provincial price administration authorities. The PRC establishes price levels for products based on market conditions, average industry cost, supply and demand and social responsibility. In practice, price control with respect to these medicines sets a ceiling on their retail price. The actual price of such medicines set by manufacturers, wholesalers and retailers cannot historically exceed the price ceiling imposed by applicable government price control regulations. Although, as a general matter, government price control regulations have resulted in drug prices tending to decline over time, there has been no predictable pattern for such decreases.
None of our products are subject to price controls. It is possible that products may be subject to price control, or that price controls may be increased in the future. To the extent that Confucian’s products are subject to price control, its revenue, gross profit, gross margin and net income will be affected since the revenue we derive from Confucian’s sales will be limited and it may face no limitation on its costs. Further, if price controls affect both Confucian’s revenue and costs, its ability to be profitable and the extent of our profitability will be effectively subject to determination by the applicable regulatory authorities in the PRC.
Adverse publicity associated with Confucian’s products, ingredients or network marketing program, or those of similar companies, could harm its financial condition and operating results.
The results of Confucian’s operations may be significantly affected by the public's perception of Confucian’s product and similar companies. This perception is dependent upon opinions concerning:
| · | the safety and quality of its products and ingredients; |
| · | the safety and quality of similar products and ingredients distributed by other companies; and |
| · | its sales force. |
Adverse publicity concerning any actual or purported failure of Confucian to comply with applicable laws and regulations regarding product claims and advertising, good manufacturing practices, or other aspects of Confucian’s business, whether or not resulting in enforcement actions or the imposition of penalties, could have an adverse effect on the goodwill of Confucian and could negatively affect its sales and ability generate revenue.
In addition, Confucian’s consumers' perception of the safety and quality of its products and ingredients as well as similar products and ingredients distributed by other companies can be significantly influenced by media attention, publicized scientific research or findings, widespread product liability claims and other publicity concerning Confucian’s products or ingredients or similar products and ingredients distributed by other companies. Adverse publicity, whether or not accurate or resulting from consumers' use or misuse of Confucian’s products, that associates consumption of its products or ingredients or any similar products or ingredients with illness or other adverse effects, questions the benefits of Confucian’s or similar products or claims that any such products are ineffective, inappropriately labeled or have inaccurate instructions as to their use, could negatively impact its reputation or the market demand for Confucian’s products.
| 13 |
If Confucian fails to develop new products with high profit margins, and its high profit margin products are substituted by competitor's products, our gross and net profit margins will be adversely affected.
There is no assurance that Confucian will be able to sustain its profit margins in the future. The supplement industry is very competitive, and there may be pressure to reduce sale prices of products without a corresponding decrease in the price of raw materials. In addition, the supplement industry in China is highly competitive and new products are constantly being introduced to the market. In order to increase the sales of Confucian’s products and expand its market, it may be forced to reduce prices in the future, leading to a decrease in gross profit margin. The research and development of new products and technologies is costly and time consuming, and there are no assurances that Confucian’s research and development of new products will either be successful or completed within the anticipate timeframe, if ever at all. There is no assurance that Confucian’s competitors' new products, technologies, and processes will not render its existing products obsolete or non-competitive. To the extent that Confucian fails to develop new products with high profit margins and its high profit margin products are substituted by competitors' products, our gross profit margins will be adversely affected.
Risks Related To Doing Business In The PRC
Changes in the policies of the PRC government could have a significant impact upon the business we may be able to conduct in the PRC and the profitability of such business.
Confucian’s business operations may be adversely affected by the current and future political environment in the PRC. The PRC has operated as a socialist state since the mid-1900s and is controlled by the Communist Party of China. The Chinese government exerts substantial influence and control over the manner in which we and it must conduct our business activities. The PRC has only permitted provincial and local economic autonomy and private economic activities since 1988. The government of the PRC has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy, particularly the pharmaceutical industry, through regulation and state ownership. Our ability to operate in China may be adversely affected by changes in Chinese laws and regulations, including those relating to taxation, import and export tariffs, raw materials, environmental regulations, land use rights, property and other matters. Under current leadership, the government of the PRC has been pursuing economic reform policies that encourage private economic activity and greater economic decentralization. There is no assurance, however, that the government of the PRC will continue to pursue these policies, or that it will not significantly alter these policies from time to time without notice.
The PRC's economy is in a transition from a planned economy to a market oriented economy subject to five-year and annual plans adopted by the government that set national economic development goals. Policies of the PRC government can have significant effects on the economic conditions of the PRC. The PRC government has confirmed that economic development will follow the model of a market economy. Under this direction, we believe that the PRC will continue to strengthen its economic and trading relationships with foreign countries and business development in the PRC will follow market forces. While we believe that this trend will continue, there can be no assurance that this will be the case.
A change in policies by the PRC government could adversely affect our interests by, among other factors: changes in laws, regulations or the interpretation thereof, confiscatory taxation, restrictions on currency conversion, imports or sources of supplies, or the expropriation or nationalization of private enterprises. Although the PRC government has been pursuing economic reform policies for more than two decades, there is no assurance that the government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption, or other circumstances affecting the PRC's political, economic and social life.
The PRC laws and regulations governing Confucian’s current business operations are sometimes vague and uncertain. Any changes in such PRC laws and regulations may harm its business.
The PRC laws and regulations governing Confucian’s current business operations are sometimes vague and uncertain. The PRC’s legal system is a civil law system based on written statutes, in which system decided legal cases have little value as precedents unlike the common law system prevalent in the United States. There are substantial uncertainties regarding the interpretation and application of PRC laws and regulations, including but not limited to the laws and regulations governing its business, or the enforcement and performance of its arrangements with customers in the event of the imposition of statutory liens, death, bankruptcy and criminal proceedings. The Chinese government has been developing a comprehensive system of commercial laws, and considerable progress has been made in introducing laws and regulations dealing with economic matters such as foreign investment, corporate organization and governance, commerce, taxation and trade. However, because these laws and regulations are relatively new, and because of the limited volume of published cases and judicial interpretation and their lack of force as precedents, interpretation and enforcement of these laws and regulations involve significant uncertainties. New laws and regulations that affect existing and proposed future businesses may also be applied retroactively. We are considered a foreign persons or foreign funded enterprises under PRC laws, and as a result, we are required to comply with PRC laws and regulations. We cannot predict what effect the interpretation of existing or new PRC laws or regulations may have on its businesses. If the relevant authorities find that we are in violation of PRC laws or regulations, they would have broad discretion in dealing with such a violation, including, without limitation:
| 14 |
| · | levying fines; |
| · | revoking Confucian’s business and other licenses; |
| · | requiring that we restructure its ownership or operations; and |
| · | requiring that we discontinue any portion or all of our business. |
A slowdown, inflation or other adverse developments in the PRC economy may harm Confucian’s customers and the demand for Confucian’s services and products.
All of Confucian’s operations are conducted in the PRC and all of its revenues are generated from sales in the PRC. Although the PRC economy has grown significantly in recent years, we cannot assure you that this growth will continue. A slowdown in overall economic growth, an economic downturn, a recession or other adverse economic developments in the PRC could significantly reduce the demand for its products and harm Confucian’s business.
While the PRC economy has experienced rapid growth, such growth has been uneven among various sectors of the economy and in different geographical areas of the country. Rapid economic growth could lead to growth in the money supply and rising inflation. If prices for Confucian’s products rise at a rate that is insufficient to compensate for the rise in the costs of supplies, it may harm its profitability. In order to control inflation in the past, the PRC government has imposed controls on bank credit, limits on loans for fixed assets and restrictions on state bank lending. Such an austere policy can lead to a slowing of economic growth. In October 2004, the People's Bank of China, the PRC's central bank, raised interest rates for the first time in nearly a decade and indicated in a statement that the measure was prompted by inflationary concerns in the Chinese economy. Repeated rises in interest rates by the central bank would likely slow economic activity in China which could, in turn, materially increase its costs and also reduce demand for its products.
Governmental control of currency conversion may affect the value of your investment.
The PRC government imposes controls on the convertibility of Renminbi into foreign currencies and, in certain cases, the remittance of currency out of the PRC. We receive substantially all of our revenues in Renminbi, which is currently not a freely convertible currency. Shortages in the availability of foreign currency may restrict our ability to remit sufficient foreign currency to pay dividends, or otherwise satisfy foreign currency dominated obligations. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from the transaction, can be made in foreign currencies without prior approval from the PRC State Administration of Foreign Exchange by complying with certain procedural requirements. However, approval from appropriate governmental authorities is required where Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of bank loans denominated in foreign currencies.
The PRC government may also in the future restrict access to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currency to satisfy our currency demands, we may not be able to pay certain of our expenses as they come due.
The fluctuation of the Renminbi may harm your investment.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in the PRC's political and economic conditions. As we rely entirely on revenues earned in the PRC, any significant revaluation of the Renminbi may materially and adversely affect our cash flows, revenues and financial condition. For example, to the extent that we need to convert U.S. dollars we receive from an offering of our securities into Renminbi for Confucian’s operations, appreciation of the Renminbi against the U.S. dollar would diminish the value of the proceeds of the offering and this could harm Confucian’s business, financial condition and results of operations because it would reduce the proceeds available to us for capital investment in proportion to the appreciation of the Renminbi. In addition, the depreciation of significant RMB denominated assets could result in a charge to our income statement and a reduction in the dollar value of these assets.
| 15 |
On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the Renminbi to the U.S. dollar. Under the new policy, the Renminbi is permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. While the international reaction to the Renminbi revaluation has generally been positive, there remains significant international pressure on the PRC government to adopt an even more flexible currency policy, which could result in a further and more significant appreciation of the Renminbi against the U.S. dollar.
PRC state administration of foreign exchange ("SAFE") regulations regarding offshore financing activities by PRC residents which may increase the administrative burden we face. The failure by our shareholders who are PRC residents to make any required applications and filings pursuant to such regulations may prevent us from being able to distribute profits and could expose us and our PRC resident shareholders to liability under PRC law.
SAFE, issued a public notice ("SAFE #75") effective from November 1, 2005, which requires registration with SAFE by the PRC resident shareholders of any foreign holding company of a PRC entity. Without registration, the PRC entity cannot remit any of its profits out of the PRC as dividends or otherwise.
In October 2005, SAFE issued a public notice, the Notice on Relevant Issues in the Foreign Exchange Control over Financing and Return Investment Through Special Purpose Companies by Residents Inside China, or the SAFE notice, which requires PRC residents, including both legal persons and natural persons, to register with the competent local SAFE branch before establishing or controlling any company outside of China, referred to as an "offshore special purpose company," for the purpose of overseas equity financing involving onshore assets or equity interests held by them. In addition, any PRC resident that is the shareholder of an offshore special purpose company is required to amend its SAFE registration with the local SAFE branch with respect to that offshore special purpose company in connection with any increase or decrease of capital, transfer of shares, merger, division, equity investment or creation of any security interest over any assets located in China. Moreover, if the offshore special purpose company was established and owned the onshore assets or equity interests before the implementation date of the SAFE notice, a retroactive SAFE registration is required to have been completed before March 31, 2006. If any PRC shareholder of any offshore special purpose company fails to make the required SAFE registration and amendment, the PRC subsidiaries of that offshore special purpose company may be prohibited from distributing their profits and the proceeds from any reduction in capital, share transfer or liquidation to the offshore special purpose company. Moreover, failure to comply with the SAFE registration and amendment requirements described above could result in liability under PRC laws for evasion of applicable foreign exchange restrictions.
It is unclear whether our other PRC resident shareholders must make disclosure to SAFE. We believe that only PRC resident shareholders who receive ownership of the foreign holding company in exchange for ownership in the PRC operating company are subject to SAFE #75, there can be no assurance that SAFE will not require our other PRC resident shareholders to register and make the applicable disclosure. In addition, SAFE #75 requires that any monies remitted to PRC residents outside of the PRC be returned within 180 days; however, there is no indication of what the penalty will be for failure to comply or if shareholder non-compliance will be considered to be a violation of SAFE #75 by us or otherwise affect us.
In the event that the proper procedures are not followed under SAFE #75, we could lose the ability to remit monies outside of the PRC and would therefore be unable to pay dividends or make other distributions. Our PRC resident shareholders could be subject to fines, other sanctions and even criminal liabilities under the PRC Foreign Exchange Administrative Regulations promulgated January 29, 1996, as amended.
The PRC's legal and judicial system may not adequately protect our business and operations and the rights of foreign investors.
The PRC legal and judicial system may negatively impact foreign investors. In 1982, the National People's Congress amended the Constitution of China to authorize foreign investment and guarantee the "lawful rights and interests" of foreign investors in the PRC. However, the PRC's system of laws is not yet comprehensive. The legal and judicial systems in the PRC are still rudimentary, and enforcement of existing laws is inconsistent. Many judges in the PRC lack the depth of legal training and experience that would be expected of a judge in a more developed country. Because the PRC judiciary is relatively inexperienced in enforcing the laws that do exist, anticipation of judicial decision-making is more uncertain than would be expected in a more developed country. It may be impossible to obtain swift and equitable enforcement of laws that do exist, or to obtain enforcement of the judgment of one court by a court of another jurisdiction. The PRC's legal system is based on the civil law regime, that is, it is based on written statutes; a decision by one judge does not set a legal precedent that is required to be followed by judges in other cases. In addition, the interpretation of Chinese laws may be varied to reflect domestic political changes.
| 16 |
The promulgation of new laws, changes to existing laws and the pre-emption of local regulations by national laws may adversely affect foreign investors. However, the trend of legislation over the last 20 years has significantly enhanced the protection of foreign investment and allowed for more control by foreign parties of their investments in Chinese enterprises. There can be no assurance that a change in leadership, social or political disruption, or unforeseen circumstances affecting the PRC's political, economic or social life, will not affect the PRC government's ability to continue to support and pursue these reforms. Such a shift could have a material adverse effect on Confucian’s business and prospects.
The practical effect of the PRC legal system on Confucian’s business operations in the PRC can be viewed from two separate but intertwined considerations. First, as a matter of substantive law, the Foreign Invested Enterprise laws provide significant protection from government interference. In addition, these laws guarantee the full enjoyment of the benefits of corporate Articles and contracts to Foreign Invested Enterprise participants. These laws, however, do impose standards concerning corporate formation and governance, which are qualitatively different from the general corporation laws of the United States. Similarly, the PRC accounting laws mandate accounting practices, which are not consistent with U.S. generally accepted accounting principles. PRC's accounting laws require that an annual "statutory audit" be performed in accordance with PRC accounting standards and that the books of account of Foreign Invested Enterprises are maintained in accordance with Chinese accounting laws. Article 14 of the People's Republic of China Wholly Foreign-Owned Enterprise Law requires a wholly foreign-owned enterprise to submit certain periodic fiscal reports and statements to designated financial and tax authorities, at the risk of business license revocation. While the enforcement of substantive rights may appear less clear than United States procedures, the Foreign Invested Enterprises and Wholly Foreign-Owned Enterprises are Chinese registered companies, which enjoy the same status as other Chinese registered companies in business-to-business dispute resolution. Any award rendered by an arbitration tribunal is enforceable in accordance with the United Nations Convention on the Recognition and Enforcement of Foreign Arbitral Awards (1958). Therefore, as a practical matter, although no assurances can be given, the Chinese legal infrastructure, while different in operation from its United States counterpart, should not present any significant impediment to the operation of Foreign Invested Enterprises
Because our principal assets are located outside of the United States and all of our directors and all of our officers reside outside of the United States, it may be difficult for you to enforce your rights based on U.S. federal securities laws against us and our officers or to enforce U.S. court judgment against us or them in the PRC.
All of our directors and all of our officers reside outside of the United States. In addition, Confucian’s operating company is located in the PRC and substantially all of its assets are located outside of the United States. It may therefore be difficult for investors in the United States to enforce their legal rights based on the civil liability provisions of the U.S. Federal securities laws against us in the courts of either the U.S. or the PRC and, even if civil judgments are obtained in U.S. courts, to enforce such judgments in PRC courts. Further, it is unclear if extradition treaties now in effect between the United States and the PRC would permit effective enforcement against us or our officers and directors of criminal penalties, under the U.S. Federal securities laws or otherwise.
The relative lack of public company experience of our management team may put us at a competitive disadvantage.
Our management team lacks public company experience, which could impair our ability to comply with legal and regulatory requirements such as those imposed by Sarbanes-Oxley Act of 2002. The individuals who now constitute our senior management have never had responsibility for managing a publicly traded company. Such responsibilities include complying with federal securities laws and making required disclosures on a timely basis. Our senior management may not be able to implement programs and policies in an effective and timely manner that adequately responds to such increased legal, regulatory compliance and reporting requirements. Our failure to comply with all applicable requirements could lead to the imposition of fines and penalties and distract our management from attending to the growth of our business.
RISKS RELATED TO OUR COMMON STOCK.
Our officers and directors control us through their positions and stock ownership and their interests may differ from other stockholders.
Our officers and directors beneficially own approximately 42% of our common stock. As a result, she is able to influence the outcome of stockholder votes on various matters, including the election of directors and extraordinary corporate transactions including business combinations. Yet Mrs. Song's interests may differ from those of other stockholders. Furthermore, ownership of 42% of our common stock by our officers and directors reduces the public float and liquidity, and may affect the market price.
| 17 |
We are not likely to pay cash dividends in the foreseeable future.
We intend to retain any future earnings for use in the operation and expansion of Confucian’s business. We do not expect to pay any cash dividends in the foreseeable future but will review this policy as circumstances dictate. Should we decide in the future to do so, as a holding company, our ability to pay dividends and meet other obligations depends upon the receipt of dividends or other payments from our operating subsidiaries. In addition, our operating subsidiaries, from time to time, may be subject to restrictions on their ability to make distributions to us, including restrictions on the conversion of local currency into U.S. dollars or other hard currency and other regulatory restrictions.
Our common stock is illiquid and subject to price volatility unrelated to Confucian’s operations.
If a market for our common stock does develop, its market price could fluctuate substantially due to a variety of factors, including market perception of our ability to achieve Confucian’s planned growth, quarterly operating results of other companies in the same industry, trading volume in our common stock, changes in general conditions in the economy and the financial markets or other developments affecting Confucian or its competitors. In addition, the stock market itself is subject to extreme price and volume fluctuations. This volatility has had a significant effect on the market price of securities issued by many companies for reasons unrelated to their operating performance and could have the same effect on our common stock.
Investors may have difficulty liquidating their investment because our common stock Is subject to the "Penny Stock" rules, which require delivery of a schedule explaining the penny stock market and the associated risks before any sale.
Our common stock may be subject to regulations prescribed by the SEC relating to "penny stocks." The SEC has adopted regulations that generally define a penny stock to be any equity security that has a market price (as defined in such regulations) of less than $5 per share, subject to certain exceptions. These regulations impose additional sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors (generally institutions with assets in excess of $5,000,000 and individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 (individually) or $300,000 (jointly with their spouse). For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of these securities and have received the purchaser's prior written consent to the transaction. Additionally, for any transaction, other than exempt transactions, involving a penny stock, the rules require the delivery, prior to the transaction, of a risk disclosure document mandated by the SEC relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer's presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Legal remedies, which may be available to the investor, are as follows:
| · | If penny stocks are sold in violation of the investor's rights listed above, or other federal or state securities laws, the investor may be able to cancel his purchase and get his money back. |
| · | If the stocks are sold in a fraudulent manner, the investor may be able to sue the persons and firms that caused the fraud for damages. |
| · | If the investor has signed an arbitration agreement, however, s/he may have to pursue a claim through arbitration. |
If the person purchasing the securities is someone other than an accredited investor or an established customer of the broker-dealer, the broker-dealer must also approve the potential customer's account by obtaining information concerning the customer's financial situation, investment experience and investment objectives. The broker-dealer must also make a determination whether the transaction is suitable for the customer and whether the customer has sufficient knowledge and experience in financial matters to be reasonably expected to be capable of evaluating the risk of transactions in such securities. Accordingly, the SEC's rules may limit the number of potential purchasers of the shares of our common stock and stockholders may have difficulty selling their securities.
A large number of shares will be eligible for future sale and may depress our stock price.
We may be required, under terms of future financing arrangements, to offer a large number of common shares to the public, or to register for sale by future private investors a large number of shares sold in private sales to them.
| 18 |
Sales of substantial amounts of common stock, or a perception that such sales could occur, and the existence of options or warrants to purchase shares of common stock at prices that may be below the then-current market price of our common stock, could adversely affect the market price of our common stock and could impair our ability to raise capital through the sale of our equity securities, either of which would decrease the value of any earlier investment in our common stock.
We are authorized to issue "blank check" preferred stock, which, if issued without stockholders’ approval, may adversely affect the rights of holders of our common stock.
We are authorized to issue 50,000,000 shares of preferred stock, of which 974,730 have been issued as Series A Preferred Stock. The Series A Preferred shares are convertible into common shares on a 1:1 ratio at a fixed rate of $3 per share. Preferred shares have no voting rights, have no redemption rights and earn no dividends. Holders of Series A Convertible Preferred Stock are not permitted to convert their stock into common shares until the Company’s market capital reaches $15,000,000. Upon dissolution, liquidation or winding up of the Company, whether voluntary or involuntary, the holders of the then outstanding shares of Series A Convertible Preferred Stock shall be entitled to receive out of the assets of the Company the sum of $0.0001 per share (the “Liquidation Rate”) before any payment or distribution shall be made on any other class of capital stock of the Company ranking junior to the Series A Convertible Preferred Stock. This could dilute your ownership.
The Board of Directors is authorized under our Articles of Amendment to provide for the issuance of additional shares of preferred stock by resolution, and to fix the designation, powers, preferences and rights of the shares of each such series and the qualifications, limitations or restrictions thereof without any further vote or action by the stockholders. Any shares of preferred stock so issued are likely to have priority over the common stock with respect to dividend or liquidation rights. In the event of issuance, the preferred stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control, which could have the effect of discouraging bids for our company and thereby prevent stockholders from receiving the maximum value for their shares. We have no present intention to issue any shares of its preferred stock in order to discourage or delay a change of control. However, there can be no assurance that preferred stock will not be issued at some time in the future.
Failure to raise additional capital as needed could adversely affect the Company and its ability to grow.
The Company will need considerable amounts of capital to develop its business. It may raise funds through public or private equity offerings or debt financings. If the Company cannot raise funds on acceptable terms when needed, it may not be able to grow or maintain the business. Furthermore, such lack of funds may inhibit the Company’s ability to respond to competitive pressures or unanticipated capital needs, or may force the Company to reduce operating expenses, which could significantly harm the business and development of operations. Because the Company’s independent auditors have expressed doubt as to the Company’s ability to continue as a “going concern,” as reported in the consolidated financial statements of the Company, its ability to raise capital may be severely hampered. Similarly, the Company’s ability to borrow any such capital may be more expensive and difficult to obtain until this “going concern” issue is eliminated.
ITEM 1B. Unresolved Staff Comments
None.
ITEM 2. Description of Property
YBCC’s corporate headquarters are located at 17800 Castleton St. Ste 180, City of Industry, California 91748. YBCC rents approximately 983 square feet of office space for its headquarters. The lease began on September 1, 2016 and expires on September 30, 2018. As of December 31, 2016, total monthly base rent is $2,802.
Confucian is located in Food Industrial Park inside the economic development Zone of JinXiang County, Jining City in the province of Shandong in China. It has three land use rights with the total costs approximately $736,632. One land use right expires in 2063, while the other two land use rights expire in 2065. It has nearly 30,000 square meters standardized plant.
None.
ITEM 4. Mine Safety Disclosure
Not applicable.
| 19 |
ITEM 5. Market for Common Equity and Related Shareholder Matters
Our common stock is quoted on the Over-the Counter Market, (the “OTC Markets”), under the trading symbol “YBAO”. As of April 20, 2017, the last working day prior to the date of this report, the closing price of our securities was $0.66.
The following table set forth the quarterly high and low bid prices per share for our common stock. The bid prices reflect inter-dealer prices, without retail markup, markdown, or commission and may not represent actual transactions. The low prices are often arbitrary prices put in the system by market makers.
| High | Low | |||||||
| Year ended December 31, 2016 | ||||||||
| First quarter | $ | 0.35 | $ | 0.13 | ||||
| Second quarter | $ | 0.92 | $ | 0.13 | ||||
| Third quarter | $ | 0.68 | $ | 0.30 | ||||
| Fourth quarter | $ | 0.58 | $ | 0.30 | ||||
| Year ended December 31, 2015 | ||||||||
| First quarter | $ | 0.59 | $ | 0.15 | ||||
| Second quarter | $ | 0.22 | $ | 0.02 | ||||
| Third quarter | $ | 0.04 | $ | 0.02 | ||||
| Fourth quarter | $ | 0.12 | $ | 0.04 | ||||
Holders
As of December 31, 2016, there were approximately 833 registered shareholders of the Company’s Common Stock with 6,894,214 shares issues and outstanding.
Common Stock
Our Company’s Certificate of Incorporation, as amended, provide for authority to issue 900,000,000 shares of common stock with par value of $0.001 per share.
As of December 31, 2016, we shall have approximately 6,894,214 shares of our common stock issued and outstanding held by approximately 833 stockholders of record.
Preferred Stock
We currently have 974,730 shares of preferred stock Series A issued and outstanding. A total of 50,000,000 preferred shares with par value of $0.0001 per share are authorized.
Series A Preferred Shares
The Series A Preferred shares are convertible into common shares on a 1:1 ratio at a fixed rate of $3 per share. Preferred shares have no voting rights, have no redemption rights and earn no dividends. Holders of Series A Convertible Preferred Stock are not permitted to convert their stock into common shares until the Company’s market capital reaches $15,000,000. Upon dissolution, liquidation or winding up of the Company, whether voluntary or involuntary, the holders of the then outstanding shares of Series A Convertible Preferred Stock shall be entitled to receive out of the assets of the Company the sum of $0.0001 per share (the “Liquidation Rate”) before any payment or distribution shall be made on any other class of capital stock of the Company ranking junior to the Series A Convertible Preferred Stock.
ASC Topic 480, “Distinguishing Liabilities from Equity,” establishes standards for how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity.
| 20 |
A mandatorily redeemable financial instrument shall be classified as a liability unless the redemption is required to occur only upon the liquidation or termination of the reporting entity. A financial instrument issued in the form of shares is mandatorily redeemable if it embodies an unconditional obligation requiring the issuer to redeem the instrument by transferring its assets at a specified or determinable date (or dates) or upon an event certain to occur. A financial instrument that embodies a conditional obligation to redeem the instrument by transferring assets upon an event not certain to occur becomes mandatorily redeemable—and, therefore becomes a liability—if that event occurs, the condition is resolved, or the event becomes certain to occur.
The Company determined that the preferred shares are not mandatorily or conditionally redeemable and are properly classified as permanent equity in the accompanying unaudited condensed consolidated financial statements.
Series B Preferred Shares
Not Applicable
Dividends
We have not declared or paid any dividends on our common stock since our inception and do not anticipate paying dividends for the foreseeable future. The payment of dividends is subject to the discretion of our board of directors and depends, among other things, upon our earnings, our capital requirements, our financial condition, and other relevant factors. We intend to reinvest any earnings in the development and expansion of our business. Any cash dividends in the future to common shareholders will be payable when, as and if declared by our board of directors, based upon the board’s assessment of our financial condition and performance, earnings, need for funds, capital requirements, prior claims of preferred stock to the extent issued and outstanding, and other factors, including income tax consequences, restrictions and applicable laws. There can be no assurance, therefore, that any dividends on our common stock will ever be paid.
Transfer Agent and Registrar
The Company’s transfer agent is Jersey Stock Transfer LLC located at 1250 Sussex Turnpike, #606; Mount Freedom, NJ 07970-0606.
Securities authorized for issuance under equity compensation plans.
Not Applicable
Recent Sales of Unregistered Securities
On January 3, 2017, the Company issued 3,000,000 for $300,000. We relied upon Section 4(2) of the Securities Act of 1933, as amended for the above issuances. We believed that Section 4(2) was available because:
| · | None of these issuances involved underwriters, underwriting discounts or commissions; |
| · | We placed restrictive legends on all certificates issued; |
| · | No sales were made by general solicitation or advertising; |
| · | Sales were made only to accredited investors |
In connection with the above transactions, we provided the following to all investors:
| · | Access to all our books and records. |
| · | Access to all material contracts and documents relating to our operations. |
| · | The opportunity to obtain any additional information, to the extent we possessed such information, necessary to verify the accuracy of the information to which the investors were given access. |
| 21 |
The Company’s Board of Directors has the power to issue any or all of the authorized but unissued Common Stock without stockholder approval. The Company currently has no commitments to issue any shares of common stock.
ITEM 6. Selected Financial Data
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. In some cases, forward-looking statements are identified by terms such as “may”, “will”, “should”, “could”, “would”, “expects”, “plans”, “anticipates”, “believes”, “estimates”, “projects”, “predicts”, “potential”, and similar expressions intended to identify forward-looking statements.
These forward-looking statements are only predictions and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance, or achievements expressed or implied by such forward-looking statements. Also, these forward-looking statements represent our estimates and assumptions only as of the date of this Report. Except as otherwise required by law, we expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statement contained in this Report to reflect any change in our expectations or any change in events, conditions, or circumstances on which any of our forward-looking statements are based or to conform to actual results. We qualify all of our forward-looking statements by these cautionary statements.
Overview
The Company is a manufacture and research based bio-science company. It has large capacity to manufacture tablets, granule, oral liquid, powders, soft gels and capsules products. The Company distributes its products through its own network and white label products. It also has access to a member-based distribution system owned by an affiliated company.
The Company possesses manufacturing permits for food product, hygienic products, sanitary products, and health products. The Company's main business includes:
| · | research and development of chondroitin and garlic oil; |
| · | trading, cold storage, and pretreating of garlic, fruit, and vegetables products; |
| · | trading of chemical products (excluding hazardous chemicals); |
| · | import and export of goods and technology (excluding those restricted by government); and, |
| · | the manufacturing and sale of health products including powder, granules, tablets, hard capsule, and soft capsule products. |
Plan of Operations
By following the Company motto of being passionate for health industry, bringing together the world's resources, focusing on consumer demand, creating a win-win situation,” the Company is eager to develop businesses in the international health and pharmaceutical market.
The Company’s near term goal is to reach breakeven within a 6-month period time. In order to reach such goal, the Company is increasing its sales and production volume through arrangements and networking with its existing customers and its affiliated companies. Additionally, it plans to increase the size of its sales department to develop new customers.
The Company’s ultimate goal is to make the business profitable and competitive in the international health and pharmaceutical market. To achieve such goal, the Company needs to cooperate with other businesses having capital, market, technology, or products, recruit sufficient workforce and various talents to serve the company, and actively develop new technology and new product through research and development.
| 22 |
Results of Operations
Twelve Months Ending December 31, 2016 Compared to December 31, 2015
Revenue:
For the years ended December 31, 2016 and 2015, revenues were $1,130,743 and $273,525 respectively with an increase of $857,218 over the same period in 2015. The increase is mainly due to the production activities of the Company started in June 2015 comparing to the full year production in 2016. The increase is also due to the increase in demands from consumers for healthy products.
Cost of Goods Sold:
Cost of goods sold for the years ended December 31, 2016 and 2015 were $988,742 and $234,028 respectively, for an increase of $754,714 over the same period in 2015. This increase is mainly due to the plant had started manufacture referenced above.
Gross Profit:
Gross profits were $142,001 and $39,497 for the years ended December 31, 2016 and 2015, an increase of $102,504 over the same period in 2015. The gross profit margin as a percent of sales for the years ending December 31, 2016 and 2015 was 13% and 14% respectively with a slight decrease of 1% due to the increase in depreciation costs of newly added manufacturing related fixed assets in 2016.
Operating Expenses:
Operating expenses for the years ended December 31, 2016 and 2015 were $509,151 and $284,870 respectively for an increase of $224,281 or 79% from the same period in prior year. The major expenses for the year ended December 31, 2016 and 2015 consist of payroll, professional fee and depreciation. The increase of $224,281 was mainly due to the increase in payroll of $31,514 for increased numbers of newly recruited employees and the increase of $243,138 for additional costs related to the restructuring and corporate matters.
Other Income (Expense):
Other income (expense) consists of interest income and expenses and other non-operation related income and expenses. For the years ended December 31, 2016 and 2015, the net other expenses were $30,880 and $100,223, respectively with a decrease of $69,343. The decrease was mainly due to the reduction in interest expenses of $87,329 for an early repayment of a short term loan, offsetting by a decrease in other income of $17,986 for less governmental rewards received in 2016 comparing that in 2015.
Liquidity and Capital Resources
The Company suffered recurring losses from operations and has an accumulated deficit of $597,650 at December 31, 2016. The Company has a cash balance of $339,147 and negative working capital of $2,384,587 as of December 31, 2016. The Company has incurred losses of $398,030 and $345,596 for the years ended December 31, 2016 and 2015, respectively. The Company has not continually generated significant revenues. Unless our operations continue to generate significant revenues and cash flows from operating activities, our continued operations will depend on whether we are able to raise additional funds through various sources, such as equity and debt financing, other collaborative agreements and strategic alliances. Our management is actively engaged in seeking additional capital to fund our operations in the short to medium term. Such additional funds may not become available on acceptable terms and there can be no assurance that any additional funding that we do obtain will be sufficient to meet our needs in the long term.
Net cash provided by operating activities for the year ended December 31, 2016 amounted to $183,992, compared to $1,116,202 provided by operating activities for the year ended December 31, 2015. The decrease of $932,210 was primarily due to the increase in net loss of $52,434 and increase in net cash used in other receivable of $2.0M, offset by the increase in net cash provided by other payable of 963K.
Net cash used in investing activities for the year ended December 31, 2016 amounted to $484,504 compared to net cash used in investing activities of $754,850 in the year ended December 31, 2015, resulting in a decrease of $270,346. The decrease was mainly due to reduction in fixed assets purchases by $29K in 2016 comparing that in 2015 and intangible assets acquired in 2016 comparing $241K purchase in 2015.
| 23 |
Net cash provided by financing activities for the year ended December 31, 2016 amounted to $619,773, compared to net cash used in financing activities of $477,502 in the year ended December 31, 2015, resulting in a net increase of $1,097,275. The increase in net cash provided by financing activities was mainly due to the proceeds received from payable to related party of $1.3 million and subscription received in advance with an amount of $300K, offset by repayment of short term loan of $1.1 million.
Going Concern
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern; however, the above condition raises substantial doubt about the Company’s ability to do so. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
In order to continue as a going concern, the Company will need, among other things, additional capital resources. Management’s plans to obtain such resources for the Company include (1) obtaining capital from the sale of its equity securities, (2) sales of the Company’s products, (3) short-term and long-term borrowings from banks, and (4) short-term borrowings from stockholders or other related party(ies) when needed. However, management cannot provide any assurance that the Company will be successful in accomplishing any of its plans.
The ability of the Company to continue as a going concern is dependent upon its ability to successfully accomplish the plans described in the preceding paragraph and eventually to secure other sources of financing and attain profitable operations.
Critical Accounting Policies
The discussion and analysis of the Company’s financial condition and results of operations are based upon the Company’s consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. We continually evaluate our estimates, including those related to bad debts, allowance for obsolete inventory, and the classification of short and long-term inventory, the useful life of property and equipment and intangible assets, recovery of long-lived assets, income taxes, write-down in value of inventory, and the valuation of equity transactions. We base our estimates on historical experience and on various other assumptions that we believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Any future changes to these estimates and assumptions could cause a material change to our reported amounts of revenues, expenses, assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. We believe the following critical accounting policies affect our significant judgments and estimates used in the preparation of the financial statements.
Accounts Receivable - The Company extends credit to its customers. Accounts receivable was recorded at the contract amount after deduction of trade discounts and, allowances, if any, and do not bear interest. The allowance for doubtful accounts, when necessary, is the Company’s best estimate of the amount of probable credit losses from accounts receivable. The Company determines the allowance based on historical write-off experience, customer specific facts and economic conditions.
Inventories - Inventory is valued at the lower of cost or market. Cost is determined using first-in, first-out method. Inventory, comprised principally of finished goods, raw material and packaging material, are valued at the lower of cost or market.
Property, Plant and Equipment – Property and equipment are carried at cost and are depreciated on a straight-line basis (after taking into account their respective estimated residual value) over the estimated useful lives of the assets. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are capitalized. When assets are retired, or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the year of disposition. We examine the possibility of decreases in the value of fixed assets when events or changes in circumstances reflect the fact that their recorded value may not be recoverable. The estimated useful lives are as follows:
| Category | Estimated Life | Method |
| Manufacturing equipment | 10 | Straight Line |
| Office equipment | 5 | Straight Line |
| Buildings | 20 | Straight Line |
| 24 |
Intangible Assets - Land use rights represent the exclusive right to occupy and use a piece of land in the PRC during the contractual term of the land use right. Land use rights are carried at cost and charged to expense on a straight-line basis over the respective periods of the rights of 50 years or the remaining period of the rights upon acquisition.
Revenue Recognition - Pursuant to the guidance of ASC 605 and ASC 360, we recognize revenue when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the purchase price is fixed or determinable and collectability is reasonably assured, and no significant obligations remain. The Company has no product returns or sales discounts and allowances because goods delivered and accepted by customers are normally not returnable.
Stock-based compensation- Stock based compensation is accounted for based on the requirements of the Share-Based Payment topic of ASC 718 which requires recognition in the financial statements of the cost of employee and director services received in exchange for an award of equity instruments over the period the employee or director is required to perform the services in exchange for the award. The Accounting Standards Codification also requires measurement of the cost of employee and director services received in exchange for an award based on the grant-date fair value of the award.
Pursuant to ASC 505-50, for share-based payments to consultants and other third-parties, compensation expense is determined at the “measurement date.” The expense is recognized over the period of services or the vesting period, whichever is applicable. Until the measurement date is reached, the total amount of compensation expense remains uncertain. We record compensation expense based on the fair value of the award at the reporting date. The awards to consultants and other third-parties are then revalued, or the total compensation is recalculated based on the then current fair value, at each subsequent reporting date.
Off-Balance Sheet Arrangements
As of December 31, 2016, we did not have any off-balance sheet arrangements as defined in Item 303(a)(4)(ii) of Regulation S-K.
ITEM 7A. Quantitative and Qualitative Disclosures about Market Risk
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
The report of Independent Registered Public Accounting Firm and consolidated financial statements are set forth in this report beginning on Page F-1.
ITEM 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None
| 25 |
ITEM 9A. Controls and Procedures.
As of December 31, 2016, we conducted an evaluation under the supervision and with the participation of the Company's Chief Executive Officer and the Chief Financial Officer, management has evaluated the effectiveness of the design and operation of the Company's disclosure controls and procedures. Based on that evaluation and because of the material weaknesses in our internal control over financial reporting described below, the Chief Executive Officer and the Chief Financial Officer have concluded that the Company's disclosure controls and procedures were not effective as of December 31, 2016.
Management identified the following control deficiencies that constitute material weaknesses that are not fully remediated as of the filing date of this report:
Our size has prevented us from being able to employ sufficient resources to enable us to have an adequate level of supervision and segregation of duties within our internal control system. There is mainly one person involved in processing of transactions. Therefore, it is difficult to effectively segregate accounting duties. We have hired an additional administrative person and retained an outside professional firm to assist in mitigating the separation of duties issues on an ongoing basis. The use of the outside firm has proven successful in assisting in the separation of duties. However, additional people are not needed to do the administrative work therefore segregation of duties will continue to be an ongoing weakness.
Similarly, the Shandong Confucian Biologic Co., Ltd. operation also has a material weakness due to lack of segregation of duties. Its size has prevented us from being able to employ sufficient resources to enable us to have an adequate level of supervision and segregation of duties within our internal control system. We have retained an outside professional firm to assist in the separation of duties on an ongoing basis. The use of the outside firm has proven successful in assisting in the separation of duties.
Based on this evaluation and because of the material weaknesses, management has concluded that our internal control over financial reporting was not effective as of December 31, 2016
This annual report does not include an attestation report of the Company’s independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject attestation by the Company’s independent registered public accounting firm pursuant to rules of the SEC that permit the Company to provide only management’s report on internal control in this annual report.
Limitations on the Effectiveness of Internal Controls
Disclosure controls and procedures, no matter how well designed and implemented, can provide only reasonable assurance of achieving an entity's disclosure objectives. The likelihood of achieving such objectives is affected by limitations inherent in disclosure controls and procedures. These include the fact that human judgment in decision-making can be faulty and that breakdowns in internal control can occur because of human failures such as simple errors or mistakes or intentional circumvention of the established process.
Changes in Internal Control over Financial Reporting
There were no changes in internal control over financial reporting that occurred during the current quarter covered by this report that have materially affected, or are reasonably likely to affect, the Company's internal control over financial reporting.
None
| 26 |
ITEM 10. Directors, Executive Officers, Promoters and Control Persons; Compliance with Section 16(a) of the Exchange Act
The Company has a sole director and a sole executive officer, and the ages and positions with the Company as of December 31, 2016 are as follows:
| Name | Age | Office | Since |
| Xiuhua Song | 46 | Director, CEO and Acting CFO | August 2016 |
Mrs. Song has been the Managing Director of Shandong Yibao Biologics Co., Ltd. from May 2011 to present. Additionally, Mrs. Song has served as President of YBCC, Inc an Illinois Corporation from November 2012 to December 31, 2016. Mrs. Song received an Associate Degree in Economics and Management from HuBei University of Economics. Additionally, Mrs. Song completed the CEO training program at TsingHua University and received her Executive MBA from Peking University. Mrs. Song is an active member of the American Nutrition and Health Association. Mrs. Song plans to dedicate a minimum of 40 hours per week to the Company.
Compensation of Directors:
Mrs. Song receives salary of $120,000 per year and a guaranteed bonus of $20,000 per year starting from December 1, 2016.
Family Relationships
None.
Board of Directors, Board Meetings and Committees
Our Board is comprised of one (1) member, Xiuhua Song who is a management member of Confucian. All members of the Board serve until their terms expire or until their successors are duly elected and qualified.
Mrs. Song has been appointed as the Chairman of the Board of Directors. In this capacity, she is responsible for meeting with our Chief Financial Officer to review our financial and operating results, agendas and minutes of board and committee meetings, and presiding at the meetings of the committees of the Board.
Our Board held no formal meetings during the most recently completed fiscal year. All proceedings of the Board were conducted by resolutions, consented to in writing by all the directors and filed with the minutes of the proceedings of the directors. Such resolutions consented to in writing by the directors entitled to vote on that resolution at a meeting of the directors are, according to the corporate laws of the State of Nevada and our By-laws, as valid and effective as if they had been passed at a meeting of the directors duly called and held.
Board Committees; Director Independence
As of this date our Board has not appointed a nominating committee, audit committee or compensation committee, or committees performing similar functions nor do we have a written nominating, compensation or audit committee charter. Our Board does not believe that it is necessary to have such committees because it believes the functions of such committees can be adequately performed by our Board. Further, we are not a "listed company" under SEC rules and thus we are not required to have a compensation committee or a nominating committee. We are not currently required to have such committees. Accordingly, we do not have an “audit committee financial expert” as such term is defined in the rules promulgated under the Securities Act of 1933 and the Securities and Exchange Act of 1934. The functions ordinarily handled by these committees are currently handled by our entire Board. Our Board intends, however, to review our governance structure and institute board committees as necessary and advisable in the future, to facilitate the management of our business.
We do not believe that our incoming director is considered “independent” under Rule 4200(a)(15) of the National Association of Securities Dealers listing standards. We are not currently subject to any law, rule or regulation, however, requiring that all or any portion of our Board include "independent" directors.
| 27 |
We do not have any defined policy or procedure requirements for shareholders to submit recommendations or nominations for directors. Our Board believes that, given the early stages of our development, a specific nominating policy would be premature and of little assistance until our business operations develop to a more advanced level. We do not currently have any specific or minimum criteria for the election of nominees to our Board and we do not have any specific process or procedure for evaluating such nominees. Our Board assesses all candidates, whether submitted by management or shareholders, and makes recommendations for election or appointment.
A shareholder who wishes to communicate with our Board may do so by directing a written request addressed to our Chief Executive Officer at the address appearing on the face page of this Current Report. YBAO does not have a policy regarding the attendance of board members at the annual meeting of shareholders.
Compensation Committee Interlocks and Insider Participation
No interlocking relationship exists between our Board and the board of directors or compensation committee of any other company, nor has any interlocking relationship existed in the past.
Compliance with Section 16(a) of the Securities Exchange Act of 1934
Section 16(a) of the Securities Exchange Act of 1934 requires the Company’s directors and executive officers, and persons who own more than 10% of a registered class of the Company’s equity securities to file with the Securities and Exchange Commission initial reports of ownership and reports of changes in ownership of Common Stock and other equity securities of the Company. Officers, directors and greater than 10% shareholders are required by SEC regulations to furnish the Company with copies of all Section 16(a) forms they file.
To the Company’s knowledge, based solely on its review of the copies of such reports furnished to the Company and written representations that no other reports were required during the fiscal year ended December 31, 2016, all Section 16(a) filing requirements applicable to its officers, directors and greater than 10% beneficial owners were complied with.
Code of Ethics for the Chief Executive Officer and the Principal Financial Officer
We have not adopted a code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer, or persons performing similar functions, because of the small number of persons involved in the management of the Company.
ITEM 11. Executive Compensation
The following table sets forth certain summary information regarding compensation paid by YBCC for services rendered during the fiscal years ended December 31, 2016 and 2015, respectively, to the Company’s Chief Executive Officer and Chief Financial Officer during such period.
Summary Compensation Table
Executive Compensation:
| SUMMARY COMPENSATION TABLE | ||||||||||||||||||||||||||
| Name and principal position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||
| Xiuhua Song, CEO and CFO | 2016 | 10,000 | 1,667 | 0 | 0 | 0 | 0 | 0 | 11,667 | |||||||||||||||||
| 2015 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Owen Naccarato | 2016 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||
| 2015 | 0 | 0 | 0 | 0 | 0 | 0 | 42,000 | 42,000 | ||||||||||||||||||
| Allen Lin | 2016 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||
| 2015 | 276,000 | 0 | 0 | 0 | 0 | 0 | 50,000 | 326,000 | ||||||||||||||||||
Effective December 1, 2016, the Company entered into an Employment Agreement (the Employment Agreement) with Xiuhua Song, the Company’s CEO. Under the Employment Agreement, Ms. Song will receive a base salary of $120,000 per year and a guaranteed bonus of $20,000 per year. The term of the contract is from December 1, 2016 to December 31, 2019.
| 28 |
Outstanding Equity Awards at Fiscal Year-end
The following table sets forth certain summary information regarding outstanding equity awards as of December 31, 2016 to the Company's Chief Executive Officer and Chief Financial Officer and most highly paid executive officers during such period.
| OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END | |||||||||||||||||||||||||||
| OPTION AWARDS | STOCK AWARDS | ||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) |
Market Value of Shares or Units of Stock That Have Not Vested ($) | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested (#) | ||||||||||||||||||
| Xiuhua Song | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
Options/SAR Grants in the Last Fiscal Year:
There were no exercises of stock options, SARs or similar instruments, and no vesting of stock, including restricted stock, restricted stock units and similar instruments, during the last completed fiscal year.
Employment Agreements
Effective December 1, 2016, the Company entered into an Employment Agreement (the Employment Agreement) with Xiuhua Song, the Company’s CEO. Under the Employment Agreement, Ms. Song will receive a base salary of $120,000 per year and a guaranteed bonus of $20,000 per year. The term of the contract is from December 1, 2016 to December 31, 2019.
Employee Benefits
We currently have no plans that provide for payments or other benefits.
| 29 |
ITEM 12. Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information known to the Company with respect to the beneficial ownership of the Company’s common stock as of December 31, 2016, by (i) each person who is known by the Company to own beneficially more than 5% of the Company's common stock and (ii) the Company’s directors and executive officers, and (iii) all officers and directors of the Company as a group.
| Shares beneficially owned (1) | ||||||||
| Number of shares | Percentage of class (2) | |||||||
| Xiuhua Song | 4,020,000 | 58% | ||||||
| Eric Stoppenhagen | 350,000 | 5% | ||||||
| Officers and Directors as a group | 4,370,000 | 58% | ||||||
| (1 individual) | ||||||||
(1) As of December 31, 2016, Mrs. Song was the sole director, President, Chief Financial Officer and Secretary of the Company
(2) Percentage based on 6,894,214 shares of common stock outstanding as of December 31, 2016.
ITEM 13. Certain Relationships and Related Transaction
Xiuhua Song
During 2016, Ms Song, the Company’s Chief Executive Office and acting Chief Financial Officer, was paid compensation of $10,000.
Related Party Transactions of Confucian
Set forth below are the related party transactions since December 31, 2015, among Confucian’s shareholders, officers and/or directors, and Confucian. As a result of the share exchange transaction, we have contractual arrangements with Confucian which give us the ability to substantially influence Confucian’s daily operations and financial affairs, appoint its senior executives and approve all matters requiring shareholder approval.
| Name of related party | Relationship | |
| Wenxiu Song | Owner of Confucian | |
| Hengchun Zhang | Owner of Confucian | |
| Qinbao Kong | Xiuhua Song's spouse | |
| Xiuhua Song | CEO and Director | |
| Shandong Yibao Biologics Co., Ltd. | Affiliated company with common shareholders | |
The Company has received $34,378 deposit from Shandong Yibao Biologics Co, Ltd, which is also a customer of Confucian as of December 31, 2016. Sales to Shandong Yibao Biologics Co, Ltd for the twelve months ended December 31, 2016 and 2015 were $309,691 and $0, respectively. The purchases of raw material from Shandong Yibao Biologics Co, Ltd were $218,262 and $0 for the years of 2016 and 2015, respectively. The purchases from Shandong Yibao Biologics are mostly consisted of one raw material, and the sales to Shandong Yibao Biologics are all finished goods.
In June 2016, Shandong Yibao Biologics Co., Ltd. advanced funds to the Company $1,064,073 to pay off the short-term loan. Prior to December 2016, loan from Shandong Yibao Biologics Co., Ltd amounted to $545,545. In December 2016, Shandong Yibao Biologics assigned the loan to Xiuhua Song.
| 30 |
The Company has the following payables to related parties:
| December 31, 2016 | December 31, 2015 | |||||||
| To Xiuhua Song | $ | 2,293,434 | $ | 995,713 | ||||
| To Shandgong Yibao | – | 218,672 | ||||||
| To Hengchun Zhang | 260,169 | – | ||||||
| To Quingbao Kong | 5,873 | – | ||||||
| To Wenxiu Song | – | 138,936 | ||||||
| Total due to related parties | $ | 2,559,476 | $ | 1,353,321 | ||||
ITEM 14. Principal Accountant Fees and Services
The Company paid the following fees in each of the prior two fiscal years to its former and current independent certified public accountants, RBSM LLP, TAAD LLP and KCCW Accountancy Corp:
| For the Year Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Audit Fees | $ | 40,000 | $ | 70,040 | ||||
| Audit-Related Fees | 61,500 | 26,000 | ||||||
| Tax Fees | – | 25,000 | ||||||
| All Other Fees | – | – | ||||||
| Total Fees | $ | 101,500 | $ | 121,040 | ||||
"Audit Fees" consisted of fees billed for services rendered for the audit of the Company’s annual consolidated financial statements included in the Company’s annual reports on Form 10-K and audit related fees are for reviews of the consolidated financial statements included in the Company’s quarterly reports on Form 10-Q. Tax fees are for tax preparation and compliance services.
| 31 |
ITEM 15. Exhibits, List and Reports in Form 8-K
a) Exhibits
| 2.1 | Share Exchange Agreement among IPLO, Yibaoccyb and Xiuhua Song dated July 1, 2016 (Incorporated by reference to Exhibit 2.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on July 8, 2016.) |
| 10.1 | Stock Purchase Agreement between IPLO and Xiuhua Song dated May 15, 2016. (Incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on July 8, 2016.) |
| 10.2 | Stock Purchase Agreement among IPLO, Standard Resources Ltd., and H&H Glass Inc. dated July 1, 2016. (Incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K filed with the Securities and Exchange Commission on July 8, 2016.) |
| 10.3 | Consulting Services Agreement between YibaoHK and Shandong Confucian Biologics Co. Ltd dated August 31, 2016 (Incorporated by reference to Exhibit 99.6 to the Current Report on Form 8-K/A filed with the Securities and Exchange Commission on September 6, 2016.) |
| 10.4 | Equity Pledge Agreement between YibaoHK, Shandong Confucian Biologics Co. Ltd and the owners of Shandong Confucian Biologics Co. Ltd dated August 31, 2016 (Incorporated by reference to Exhibit 99.7 to the Current Report on Form 8-K/A filed with the Securities and Exchange Commission on September 6, 2016.) |
| 10.5 | Operating Agreement between YibaoHK, Shandong Confucian Biologics Co. Ltd and the owners of Shandong Confucian Biologics Co. Ltd dated August 31, 2016 (Incorporated by reference to Exhibit 99.8 to the Current Report on Form 8-K/A filed with the Securities and Exchange Commission on September 6, 2016.) |
| 10.6 | Voting Rights and Proxy Agreement between YibaoHK, Shandong Confucian Biologics Co. Ltd and the owners of Shandong Confucian Biologics Co. Ltd dated August 31, 2016 (Incorporated by reference to Exhibit 99.9 to the Current Report on Form 8-K/A filed with the Securities and Exchange Commission on September 6, 2016.) |
| 10.7 | Option Agreement between YibaoHK, Shandong Confucian Biologics Co. Ltd and the owners of Shandong Confucian Biologics Co. Ltd dated August 31, 2016 (Incorporated by reference to Exhibit 99.10 to the Current Report on Form 8-K/A filed with the Securities and Exchange Commission on September 6, 2016.) |
| 31.1* | Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) (Section 302 of the Sarbanes-Oxley Act of 2002) |
| 31.2* | Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) (Section 302 of the Sarbanes-Oxley Act of 2002) |
| 32.1* | Certification of the Chief Executive Officer pursuant to 18 U.S.C.ss.1350 (Section 906 of the Sarbanes-Oxley Act of 2002) |
| 32.2* | Certification of the Chief Financial Officer pursuant to 18 U.S.C.ss.1350 (Section 906 of the Sarbanes-Oxley Act of 2002) |
| 101.INS* | XBRL Instance Document |
| 101.SCH* | XBRL Schema Document |
| 101.CAL* | XBRL Calculation Linkbase Document |
| 101.DEF* | XBRL Definition Linkbase Document* |
| 101.LAB* | XBRL Label Linkbase Document |
| 101.PRE* | XBRL Presentation Linkbase Document |
* Filed herewith.
| 32 |
Signatures
In accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| YBCC, Inc. | ||
| By: | /s/ Xiuhua Song | |
| Xiuhua Song Chief Executive Officer | ||
| Dated | April 25, 2017 | |
In accordance with the Exchange Act, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| /s/ Xiuhua Song | April 25, 2017 |
| Xiuhua Song Chief Executive Officer, | |
| Principal Financial Officer and Director | |
| 33 |
YBCC, Inc.
and Subsidiaries
Consolidated Financial Statements
for the Years Ended
December 31, 2016 and 2015
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
YBCC, Inc. and Subsidiaries
We have audited the accompanying consolidated balance sheet of YBCC, Inc. and Subsidiaries (the “Company”) as of December 31, 2016, and the related consolidated statements of operations and comprehensive income, changes in equity, and cash flows for the year then ended. The Company’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2016, and the consolidated results of its operations and its cash flows for each of the year then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 2 of the consolidated financial statements, the Company has incurred significant negative cash flows from operative activities, and continuing net losses and working capital deficits. The Company’s viability is dependent upon its ability to obtain future financing and the success of its future operations. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plan in regard to these matters is also described in Note 2 to the consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ KCCW Accountancy Corp
Alhambra, California
April 21, 2017
| F-2 |

To the Stockholders and Board of Directors
Shandong Confucian Biologics Co. Ltd.
We have audited the accompanying balance sheets of Shandong Confucian Biologics Co. Ltd (the “Company”) as of December 31, 2015 and the related statement of operations, changes in stockholders’ deficit, and cash flows for the year then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatements. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Shandong Confucian Biologics Co. Ltd. as of December 31, 2015 and the results of its operations, stockholders’ deficit, and cash flows for the year ended December 31, 2015 in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company suffered a net loss from operations and has a net capital deficiency, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ TAAD, LLP
Diamond Bar, California
September 2, 2016
| F-3 |
YBCC, INC., AND SUBSIDIARIES
AS OF DECEMBER 31, 2016, AND DECEMBER 31, 2015
| December 31, 2016 | December 31, 2015 | |||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 339,147 | $ | 21,747 | ||||
| Accounts receivable - trade - net | 149,765 | 3,521 | ||||||
| Other receivable | 102,122 | 90,841 | ||||||
| Prepaid expenses | 158,869 | 195,101 | ||||||
| Inventory,net | 149,844 | 118,900 | ||||||
| Total current assets | 899,747 | 430,110 | ||||||
| Property, plant, and equipment - net | 2,518,243 | 2,404,650 | ||||||
| Intangible Assets - net | 695,109 | 760,819 | ||||||
| Other Assets | ||||||||
| Deposits | 3,215 | – | ||||||
| Total other assets | 3,215 | – | ||||||
| Total assets | $ | 4,116,314 | $ | 3,595,579 | ||||
| Liabilities and Equity | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | 69,606 | $ | 15,055 | ||||
| Accrued expenses | 71,395 | 8,389 | ||||||
| Customer deposits | 22,401 | 62,368 | ||||||
| Customer deposits-related | 34,378 | – | ||||||
| Taxes payable | 40,168 | 35,574 | ||||||
| Other payable | 486,910 | 92,661 | ||||||
| Other payable-related party | 2,559,476 | 1,353,321 | ||||||
| Short term loan | – | 1,080,614 | ||||||
| Total current liabilities | 3,284,334 | 2,647,982 | ||||||
| Total liabilities | 3,284,334 | 2,647,982 | ||||||
| Equity | ||||||||
| Convertible preferred shares: $0.0001 par value, 50,000,000 shares authorized, 974,730 Series A issued and outstanding | 98 | 98 | ||||||
| Common stock: $0.001 par value, 900,000,000 shares authorized, 6,894,214 and 4,504,214 shares issued and outstanding as of December 31,2016 and 2015, respectively | 6,894 | 4,504 | ||||||
| Additional paid in capital | 865,328 | 832,718 | ||||||
| Subscription received in advance | 300,000 | – | ||||||
| Accumulated foreign currency exchange loss | (57,173 | ) | (30,353 | ) | ||||
| Accumulated Deficit | (597,650 | ) | (323,693 | ) | ||||
| Total YBCC, Inc. stockholders' equity | 517,497 | 483,274 | ||||||
| Non-controlling interest | 314,483 | 464,323 | ||||||
| Total equity | 831,980 | 947,597 | ||||||
| Total liabilities and equity | $ | 4,116,314 | $ | 3,595,579 | ||||
The accompanying notes are integral to these consolidated financial statements.
| F-4 |
YBCC, INC., AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME
FOR THE YEARS ENDED DECEMBER 31, 2016 AND 2015
| For the Year Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Sales revenue | $ | 1,130,743 | $ | 273,525 | ||||
| Cost of goods sold | 988,742 | 234,028 | ||||||
| Gross profit | 142,001 | 39,497 | ||||||
| Operating expenses | ||||||||
| Selling expenses | 15,960 | 12,616 | ||||||
| General & administrative expenses | 493,191 | 272,254 | ||||||
| Total operating expenses | 509,151 | 284,870 | ||||||
| Loss from operation | (367,150 | ) | (245,373 | ) | ||||
| Other income (expenses) | ||||||||
| Interest income | ||||||||
| Other income | 18,925 | 36,911 | ||||||
| Interest expense | (49,805 | ) | (137,134 | ) | ||||
| Total other expenses | (30,880 | ) | (100,223 | ) | ||||
| Loss before income taxes | (398,030 | ) | (345,596 | ) | ||||
| Provision for income taxes | – | – | ||||||
| Net loss | (398,030 | ) | (345,596 | ) | ||||
| Less: net loss attributable to non controlling interest | (124,073 | ) | (169,342 | ) | ||||
| Net loss attributable to the Company | $ | (273,957 | ) | $ | (176,254 | ) | ||
| Net loss | $ | (398,030 | ) | $ | (345,596 | ) | ||
| Other comprehensive loss | ||||||||
| Foreign currency translation loss | (52,587 | ) | (46,074 | ) | ||||
| Comprehensive loss | (450,617 | ) | (391,670 | ) | ||||
| Less:comprehensive loss attributable to non-controlling interest | (25,767 | ) | (22,576 | ) | ||||
| Net comprehensive loss attributable to the Company | $ | (424,850 | ) | $ | (369,094 | ) | ||
| Loss per weighted average share of common stock - basic and diluted | $ | (0.17 | ) | $ | (0.19 | ) | ||
| Weighted average share outstanding - basic and diluted | 2,700,174 | 2,040,000 | ||||||
The accompanying notes are integral to these consolidated financial statements.
| F-5 |
YBCC, INC., AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2016 AND 2015
| Preferred
Stock Series A | Preferred
Stock Series B | Common Stock | Additional Paid-In | Subscription Received in | Accumulated Other Comprehensive | Accumulated Earnings | Non Controlling | |||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Advance | Loss | (Deficit) | Interest | Total | |||||||||||||||||||||||||
| December 31, 2014 | 974,730 | $ | 98 | 400,000 | $ | 40 | 4,961,357 | $ | 4,961 | $ | 832,221 | $ | – | $ | (6,855 | ) | $ | (147,439 | ) | $ | 656,241 | 1,339,267 | ||||||||||||||
| Divestiture of EZ Link | – | – | (400,000 | ) | (40 | ) | (457,143 | ) | (457 | ) | 497 | – | – | – | – | – | ||||||||||||||||||||
| Foreign currency translation loss | – | – | – | – | – | – | – | – | (23,498 | ) | – | (22,576 | ) | (46,074 | ) | |||||||||||||||||||||
| Net loss | – | – | – | – | – | – | – | – | – | (176,254 | ) | (169,342 | ) | (345,596 | ) | |||||||||||||||||||||
| December 31, 2015 | 974,730 | 98 | – | – | 4,504,214 | 4,504 | 832,718 | – | (30,353 | ) | (323,693 | ) | 464,323 | 947,597 | ||||||||||||||||||||||
| Effect of restructuring entities under common control | – | – | – | – | 2,040,000 | 2,040 | (2,040 | ) | – | – | – | – | – | |||||||||||||||||||||||
| Issuance of stock to legal counsel for services | – | – | – | – | 350,000 | 350 | 34,650 | – | – | – | – | 35,000 | ||||||||||||||||||||||||
| Subscription received in advance | – | – | – | – | – | – | – | 300,000 | – | – | – | 300,000 | ||||||||||||||||||||||||
| Foreign currency translation loss | – | – | – | – | – | – | – | – | (26,820 | ) | – | (25,767 | ) | (52,587 | ) | |||||||||||||||||||||
| Net loss | – | – | – | – | – | – | – | – | – | (273,957 | ) | (124,073 | ) | (398,030 | ) | |||||||||||||||||||||
| December 31, 2016 | 974,730 | $ | 98 | – | $ | – | 6,894,214 | $ | 6,894 | $ | 865,328 | $ | 300,000 | $ | (57,173 | ) | $ | (597,650 | ) | $ | 314,483 | $ | 831,980 | |||||||||||||
The accompanying notes are integral to these consolidated financial statements.
| F-6 |
YBCC, INC., AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2016 AND 2015
| For the Year Ended December 31, | ||||||||
| 2016 | 2015 | |||||||
| Cash flows from operating activities | ||||||||
| Net loss | $ | (398,030 | ) | $ | (345,596 | ) | ||
| Adjustment to reconcile net income to net cash (used in) provided by operating activities: | ||||||||
| Depreciation expense | 197,483 | 117,397 | ||||||
| Amortization | 15,405 | 11,460 | ||||||
| Legal professional fees paid by issuance of stock | 35,000 | – | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Increase in accounts receivable | (153,166 | ) | (3,629 | ) | ||||
| Decrease (increase) in other receivable | (22,557 | ) | 2,022,337 | |||||
| Decrease (increase) in prepaid expense | 25,968 | (92,926 | ) | |||||
| Increase in inventory | (40,686 | ) | (117,496 | ) | ||||
| Increase in deposits | (3,215 | ) | – | |||||
| Increase (decrease) in accounts payable and accrued expenses | 97,096 | (15,062 | ) | |||||
| Increase (decrease) in customer deposit | (37,421 | ) | 64,305 | |||||
| Increase in customer deposit-related parties | 35,947 | – | ||||||
| Increase (decrease) in tax payable | 7,296 | 14,230 | ||||||
| Increase (decrease) in other payable | 424,872 | (538,818 | ) | |||||
| Net cash provided by operating activities | 183,992 | 1,116,202 | ||||||
| Cash flow used in investing activities: | ||||||||
| Purchase of property and equipment | (484,504 | ) | (513,823 | ) | ||||
| Purchase of land use rights | – | (241,027 | ) | |||||
| Net cash used in investing activities | (484,504 | ) | (754,850 | ) | ||||
| Cash flow from financing activities: | ||||||||
| Subscription received in advance | 300,000 | – | ||||||
| Advance from (repayment to) related party | 1,373,990 | (477,502 | ) | |||||
| Repayment of loan | (1,054,217 | ) | – | |||||
| Net cash provided by (used in) financing activities | 619,773 | (477,502 | ) | |||||
| Effects of currency translation on cash | (1,861 | ) | (2,420 | ) | ||||
| Net (decrease) increase in cash and cash equivalents | 317,400 | (118,570 | ) | |||||
| Cash and cash equivalents at beginning of period | 21,747 | 140,317 | ||||||
| Cash and cash equivalents at end of period | $ | 339,147 | $ | 21,747 | ||||
| Supplementary Disclosures of Cash Flow | ||||||||
| Cash paid during the year for | ||||||||
| Interest | $ | 45,248 | $ | 137,124 | ||||
| Income tax | $ | – | $ | – | ||||
The accompanying notes are integral to these consolidated financial statements.
| F-7 |
YBCC, INC., AND SUBSIDIARIES
NOTES FOR THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016 AND 2015
NOTE 1 - ORGANIZATIONS AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Organization and Operations – YBCC, Inc., (The “Company” or “YBAO”), formerly known as International Packaging and Logistic Group, Inc., a Nevada corporation, was originally incorporated on June 2, 1986, in the state of Delaware. On April 17, 2008, the Company redomiciled form the State of Delaware to the State of Nevada.
On July 2, 2007, the Company through its wholly-owned subsidiary, YesRx.com (“YesRx”) acquired all the outstanding shares of H&H Glass, Inc. (“H&H Glass”), in exchange for 3,915,000 shares of its common stock in a reverse triangular merger.
On May 15, 2016, the Company, and Xiuhua Song (the “Purchaser”) entered into a Stock Purchase Agreement (the “Purchase Agreement”), pursuant to which IPLO (the “Seller”) would sell to the Purchaser, and the Purchaser will purchase from the Seller, an aggregate of 3,915,000 newly issued shares of IPLO Common Stock (the “Shares”), which Shares represented 87% of the issued and outstanding shares of Common Stock. On July 1, 2016, this transaction was completed.
On July 1, 2016, Standard Resources Ltd. (“Standard”) previously IPLO’s Majority Stockholder, and IPLO entered into a share purchase agreement (“H&H Vend Out”) whereby Standard would cancel 3,915,000 shares of IPLO common stock held by it in exchange for all of the outstanding shares of H&H Glass. The H&H Glass Vend Out was completed on August 31, 2016.
On July 1, 2016, the Company executed a Share Exchange Agreement (“Exchange Agreement”) by and among Yibaoccyb Limited, a British Virgin Islands limited liability company (“Yibaoccyb”), and the stockholders of 51% of Yibaoccyb’s common stock (the “Yibaoccyb Shareholders”), on the one hand, and the Company, on the other hand. Yibaoccyb owns 100% of YibaoConfucian Co., Ltd. (“YibaoHK”), a Hong Kong company. YibaoHK will own 100% of Shenzhen Confucian Biologics Co. Ltd. (yet to be formed, “Yibao”), which will be a wholly foreign-owned enterprise (“WFOE”) under the laws of the Peoples’ Republic of China (“PRC” or “China”). On August 31, 2016, YibaoHK entered into a series of contractual arrangements with Shandong Confucian Biologics Co., Ltd. (“Confucian”) which is a limited liability company headquartered in, and organized under the laws of, the PRC. The contractual arrangements are discussed below.
The Exchange Agreement was completed on August 31, 2016 concurrent with the H&H Vend Out. The Company issued 2,040,000 shares of the Registrant’s common stock (the “IPLO Shares”) to the Yibaoccyb Shareholders in exchange for 51% of the common stock of Yibaoccyb (the “Exchange Agreement”).
On December 22, 2016, the Company amended its Certificate of Incorporation (the “Amendment”). As a result of the Amendment, the Company’s corporate name was changed from International Packaging and Logistics Group, Inc. to YBCC, Inc.
Yibaoccyb Limited is a limited liability company incorporated under the laws of the British Virgin Islands on May 30, 2016. Other than all the issued and outstanding shares of Yibao Confucian Co. Ltd., Yibaoccyb has no other assets or operations.
YibaoHK is a limited liability company incorporated under the laws of the Hong Kong on June 15, 2016, which was formed by Yibaoccyb, a British Virgin Island. YibaoHK entered a series of contractual arrangements with the Confucian Co., Ltd.
Confucian was founded on October 31, 2012. Confucian is in Food Industrial Park inside the economic development Zone of JinXiang County, Jining City in the province of Shandong in China.
Confucian possesses manufacturing permits for food product, hygienic products, sanitary products, and health products. The Company's main business includes research and development of chondroitin and garlic oil; trading, cold storage, and pretreating of garlic, fruit, and vegetables products; trading of chemical products (excluding hazardous chemicals); import and export of goods and technology (excluding those restricted by China government); and, the manufacturing and sale of health products including powder, granules, tablets, hard capsule, soft capsule products.
| F-8 |
Details of the Company’s structure as of December 31, 2016, is as follow:
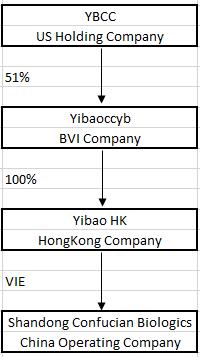
Reverse Merger Accounting – Since YBCC and Yibaoccyb were entities under Mrs. Song’s common control prior to the share exchange, the transaction was accounted for as a restructuring transaction in accordance with generally accepted accounting principles in the United States ("GAAP"). YBCC has recast prior period consolidated financial statements to reflect the conveyance of Yibaoccyb’s common shares as if the restructuring transaction had occurred as of the earliest date of the consolidated financial statements.
Basis of Accounting and Presentation - The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America.
Principles of Consolidation - The accompanying consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States of America. Confucian’s functional currency is Chinese Yuan (CNY), however, the accompanying consolidated financial statements have been re-measured and presented in United States Dollars ($).
The consolidated financial statements include the accounts of YBCC and its subsidiaries (collectively the “Company”). The Company’s subsidiaries include 51% of Yibaoccyb, YibaoHK and Confucian, of which 49% of Yibaoccyb’s consolidated operating results was shown in non-controlling interest on the consolidated balance sheets.
Intercompany accounts and transactions have been eliminated upon consolidation.
Use of Estimates - The preparation of unaudited condensed financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimate and assumptions that affect certain reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates. The most significant estimates reflected in the consolidated financial statements include allowance for doubtful accounts, allowance for inventory, depreciation, useful lives of property and equipment, deferred income taxes, useful life of intangible assets, and contingencies. Estimates and assumptions are periodically reviewed and the effects of revisions are reflected in the consolidated financial statements in the period they are determined to be necessary.
Cash and Cash Equivalents – For purpose of the statements of cash flows, the Company considers all highly liquid debt instruments purchased with a maturity of 90 days or less to be cash equivalents.
Accounts Receivable - The Company extends credit to its customers. Accounts receivable was recorded at the contract amount after deduction of trade discounts and, allowances, if any, and do not bear interest. The allowance for doubtful accounts, when necessary, is the Company’s best estimate of the amount of probable credit losses from accounts receivable. The Company determines the allowance based on historical write-off experience, customer specific facts and economic conditions.
As of December 31, 2016 and 2015, accounts receivable was $149,765 and $3,521, respectively. The Company believes that its accounts receivables are fully collectable and determined that an allowance for doubtful accounts was not necessary.
| F-9 |
Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company does not have any off-balance-sheet credit exposure related to its customers.
Inventories - Inventory is valued at the lower of cost or market. Cost is determined using first-in, first-out method.
Inventory, comprised principally of finished goods, raw material and packaging material, are valued at the lower of cost or market.
| December 31, 2016 | December 31, 2015 | |||||||
| Finished goods | $ | 7,098 | $ | 353 | ||||
| Work in progress | 106,934 | – | ||||||
| Raw materials | 20,457 | 79,282 | ||||||
| Packaging material | 15,088 | 33,859 | ||||||
| Supplies | 267 | 5,406 | ||||||
| Total | $ | 149,844 | $ | 118,900 | ||||
The Company periodically estimates an inventory allowance for estimated unmarketable inventories. Inventory amounts are reported net of such allowances, if any. There were no allowances for inventory as of December 31, 2016 and 2015.
Property, Plant and Equipment – Property, plant, and equipment are stated at cost less accumulated depreciation. The costs of a constructed asset are accumulated in the account Construction-in-Progress until the asset is placed into service. When the asset is completed and placed into service, the account Construction-in-Progress will be credited for the accumulated costs of the asset and will be debited to the appropriate Property, Plant and Equipment account. Depreciation begins after the asset has been placed into service.
Expenditures for maintenance and repairs are charged to operations; major expenditures for renewals and betterments are capitalized. Assets that are still kept in service after reaching the end of their estimated useful lives are depreciated over the estimated useful life of their residual value. Gain or loss on disposal of property, plant, and equipment is recognized as non-operating income or expenses.
Depreciation is computed by applying the following methods and estimated lives:
| Category | Estimated Life | Method |
| Manufacturing equipment | 10 | Straight Line |
| Office equipment | 5 | Straight Line |
| Buildings | 20 | Straight Line |
Intangible Assets - Land use rights represent the exclusive right to occupy and use a piece of land in the PRC during the contractual term of the land use right. Land use rights are carried at cost and charged to expense on a straight-line basis over the respective periods of the rights of 50 years or the remaining period of the rights upon acquisition.
Non-Controlling Interest – The Company accounted for its non-controlling interest of 49% in Yibaoccyb as a separate component of equity. In addition, net loss, and components of other comprehensive income are attributed to both the Company and non-controlling interest.
Revenue Recognition - The Company recognizes product revenue in accordance with ASC 605. ASC 605 requires that four basic criteria must be met before revenue can be recognized: (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred, (iii) the price paid by the customer is fixed or determinable and (iv) collection of the resulting account receivable is reasonably assured. The Company recognizes revenue for product sales upon transfer of title to the customer. Customer purchase orders and/or contracts are generally used to determine the existence of an arrangement. Shipping documents and terms and the completion of any customer acceptance requirements, when applicable, are used to verify product delivery. The Company assesses whether a price is fixed or determinable based upon the payment terms associated with the transaction and whether the sales price is subject to refund or adjustment. The Company has no product returns or sales discounts and allowances because goods delivered and accepted by customers are normally not returnable.
| F-10 |
Cost of goods sold- Cost of goods sold includes cost of inventory sold during the period, net of discounts and inventory allowances, freight and shipping costs, warranty and rework costs, and sales tax.
Impairment of Long-Live Assets – The Company applies FASB ASC 360, “Property, Plant and Equipment,” which addresses the financial accounting and reporting for the recognition and measurement of impairment losses for long-lived assets. In accordance with ASC 360, long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The Company will recognize the impairment of long-lived assets in the event the net book value of such assets exceeds the future undiscounted cash flows attributable to those assets. There are no impairment of our long-lived assets for the year ended 2016 and 2015.
Income Taxes – The Company adopts FASB ASC Topic 740, "Income Taxes,” which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the consolidated financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
In accordance with ASC Topic 740-10, “Accounting for Uncertainty in Income Taxes — An Interpretation of FASB ASC Topic 740”, which requires income tax positions to meet a more-likely-than-not recognition threshold to be recognized in the financial statements. Tax positions that previously failed to meet the more-likely-than-not threshold should be recognized in the first subsequent financial reporting period in which that threshold is met. Previously recognized tax positions that no longer meet the more-likely-than-not threshold should be derecognized in the first subsequent financial reporting period in which that threshold is no longer met. As of December 31, 2016 and 2015, management considered that the Company had no uncertain tax positions, and will continue to evaluate for uncertain positions in the future.
The application of tax laws and regulations is subject to legal and factual interpretation, judgment and uncertainty. Tax laws and regulations themselves are subject to change because of changes in fiscal policy, changes in legislation, the evolution of regulations and court rulings. Therefore, the actual liability may be materially different from our estimates, which could result in the need to record additional tax liabilities or potentially reverse previously recorded tax liabilities or deferred tax asset valuation allowance.
The Company has made a comprehensive review of its portfolio of tax positions in accordance with recognition standards established by ASC 740-10 and has not recognized any material uncertain tax positions.
In addition, companies in the PRC are required to pay an Enterprise Income Tax at 25%.
Foreign Currency Translation - The Company's functional currency is the Chinese Renminbi (RMB). The reporting currency is that of the US Dollar. Assets, liabilities and owners’ contribution are translated at the exchange rates as of the balance sheet date. Income and expenditures are translated at the average exchange rate of the year. The RMB is not freely convertible into foreign currency and all foreign currency exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US dollar at the rates used in translation.
The exchange rates used to translate amounts in RMB into USD for the purposes of preparing the financial statements were as follows:
| December 31, 2016 | |
| Balance sheet | RMB 6.94 to US $1.00 |
| Statement of operation and other comprehensive income | RMB 6.64 to US $1.00 |
| December 31, 2015 | |
| Balance sheet | RMB 6.49 to US $1.00 |
| Statement of operation and other comprehensive income | RMB 6.28 to US $1.00 |
| F-11 |
Fair Value of Financial Instruments – FASB ASC 820, “Fair Value Measurement” specifies a hierarchy of valuation techniques based upon whether the inputs to those valuation techniques reflect assumptions other market participants would use based upon market data obtained from independent sources (observable inputs). In accordance with ASC 820, the following summarizes the fair value hierarchy:
Level 1 Inputs— Unadjusted quoted market prices for identical assets and liabilities in an active market that the Company has the ability to access.
Level 2 Inputs— Inputs other than the quoted prices in active markets that are observable either directly or indirectly.
Level 3 Inputs— Inputs based on valuation techniques that are both unobservable and significant to the overall fair value measurements
ASC 820 requires the use of observable market data, when available, in making fair value measurements. When inputs used to measure, fair value fall within different levels of the hierarchy, the level within which the fair value measurement is categorized is based on the lowest level input that is significant to the fair value measurement. Valuation techniques used need to maximize the use of observable inputs and minimize the use of unobservable inputs.
The Company did not identify any assets or liabilities that are required to be presented at fair value on a recurring basis. Carrying values of non-derivative financial instruments, including cash and cash equivalents, accounts receivable, inventories, prepaid expenses, advances from customers, accounts payable, taxes payable, accrued liabilities and other payables, and loan from bank, approximated their fair values due to the short maturity of these financial instruments. There were no changes in methods or assumptions during the periods presented.
Net Earnings(loss) Per Share – Earnings/(loss) per common share is computed on the weighted average number of common shares outstanding during each year. Basic earnings per share is computed as net loss applicable to common stockholders divided by the weighted average number of common shares outstanding for the period. Diluted earnings per share reflects the potential dilution that could occur from common shares issuable through convertible preferred shares, stock options, warrants and other convertible securities when the effect would be dilutive. In this case, the Preferred Shares would not be dilutive since the conversion price is $3.00 and the quoted price is significantly lower than the conversion price. Therefore, there were no dilutive securities for the years ending December 31, 2016 and 2015, respectively.
Reclassifications – Certain classifications have been made to the prior year consolidated financial statements to conform to the current year presentation. The reclassification had no impact on previously reported net loss or accumulated deficit.
Recent Accounting Pronouncements
In February 25, 2016, FASB issued ASU-2016-02-Leases. The amendments in this Update create Topic 842, Leases, and supersede the leases requirements in Topic 840, Leases. The objective of Topic 842 is to establish the principles that lessees and lessors shall apply to report useful information to users of financial statements about the amount, timing, and uncertainty of cash flows arising from a lease. The FASB is issuing this Update to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities on the balance sheet and disclosing key information about leasing arrangements. Topic 842 affects any entity that enters into a lease (as that term is defined in this Update), with some specified scope exemptions. The guidance in this Update supersedes Topic 840, Leases. The main difference between previous GAAP and Topic 842 is the recognition of lease assets and lease liabilities by lessees for those leases classified as operating leases under previous GAAP. The core principle of Topic 842 is that a lessee should recognize the assets and liabilities that arise from leases. All leases create an asset and a liability for the lessee in accordance with FASB Concepts Statement No. 6, Elements of Financial Statements, and, therefore, recognition of those lease assets and lease liabilities represents an improvement over previous GAAP, which did not require lease assets and lease liabilities to be recognized for most leases. A lessee should recognize in the statement of financial position a liability to make lease payments (the lease liability) and a right-of-use asset representing its right to use the underlying asset for the lease term. When measuring assets and liabilities arising from a lease, a lessee (and a lessor) should include payments to be made in optional periods only if the lessee is reasonably certain to exercise an option to extend the lease or not to exercise an option to terminate the lease. Similarly, optional payments to purchase the underlying asset should be included in the measurement of lease assets and lease liabilities only if the lessee is reasonably certain to exercise that purchase option. Reasonably certain is a high threshold that is consistent with and intended to be applied in the same way as the reasonably assured threshold in the previous leases guidance. In addition, also consistent with the previous leases guidance, a lessee (and a lessor) should exclude most variable lease payments in measuring lease assets and lease liabilities, other than those that depend on an index or a rate or are in substance fixed payments. For leases with a term of 12 months or less, a lessee is permitted to make an accounting policy election by class of underlying asset not to recognize lease assets and lease liabilities. If a lessee makes this election, it should recognize lease expense for such leases generally on a straight-line basis over the lease term. The amendments in this Update are effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. The Company is still in progress of evaluating future impact of adopting this standard.
| F-12 |
In November 20, 2015, FASB issued ASU-2015-17- Income Taxes. The Board is issuing this Update as part of its initiative to reduce complexity in accounting standards (the Simplification Initiative). The objective of the Simplification Initiative is to identify, evaluate, and improve areas of generally accepted accounting principles (GAAP) for which cost and complexity can be reduced while maintaining or improving the usefulness of the information provided to users of financial statements. Current GAAP requires an entity to separate deferred income tax liabilities and assets into current and noncurrent amounts in a classified statement of financial position. To simplify the presentation of deferred income taxes, the amendments in this Update require that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. The amendments in this Update apply to all entities that present a classified statement of financial position. The current requirement that deferred tax liabilities and assets of a tax-paying component of an entity be offset and presented as a single amount is not affected by the amendments in this Update. For public business entities, the amendments in this Update are effective for financial statements issued for annual periods beginning after December 15, 2016, and interim periods within those annual periods. Earlier application is permitted for all entities as of the beginning of an interim or annual reporting period. The Company is still in progress of evaluating future impact of adopting this standard.
In March 2016, the FASB issued ASU No. 2016-09, Compensation – Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting (“ASU 2016-09”), which modifies certain aspects of the accounting for share-based payment transactions, including income taxes, classification of awards, and classification in the statement of cash flows. The Company will adopt ASU 2016-09 in its first quarter of 2018. Currently, excess tax benefits or deficiencies from the Company's equity awards are recorded as additional paid-in capital in its Consolidated Balance Sheets. Upon adoption, the Company will record any excess tax benefits or deficiencies from its equity awards in its Consolidated Statements of Operations in the reporting periods in which vesting occurs. As a result, subsequent to adoption the Company's income tax expense and associated effective tax rate will be impacted by fluctuations in stock price between the grant dates and vesting dates of equity awards. The Company is still in progress of evaluating future impact of adopting this standard.
In April 2016, the FASB issued ASU 2016-10, “Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing”. The amendments add further guidance on identifying performance obligations and also to improve the operability and understandability of the licensing implementation guidance. The amendments do not change the core principle of the guidance in Topic 606. The effective date and transition requirements for the amendments are the same as the effective date and transition requirements in Topic 606. The Company is currently in the process of evaluating the impact of the adoption on its consolidated financial statements.
In May 2016, the FASB issued ASU 2016-12, “Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients”. The amendments, among other things: (1) clarify the objective of the collectability criterion for applying paragraph 606-10-25-7; (2) permit an entity to exclude amounts collected from customers for all sales (and other similar) taxes from the transaction price; (3) specify that the measurement date for noncash consideration is contract inception; (4) provide a practical expedient that permits an entity to reflect the aggregate effect of all modifications that occur before the beginning of the earliest period presented when identifying the satisfied and unsatisfied performance obligations, determining the transaction price, and allocating the transaction price to the satisfied and unsatisfied performance obligations; (5) clarify that a completed contract for purposes of transition is a contract for which all (or substantially all) of the revenue was recognized under legacy GAAP before the date of initial application, and (6) clarify that an entity that retrospectively applies the guidance in Topic 606 to each prior reporting period is not required to disclose the effect of the accounting change for the period of adoption. The effective date of these amendments is at the same date that Topic 606 is effective. The Company is currently in the process of evaluating the impact of the adoption on its consolidated financial statements.
In October 2016, the FASB issued ASU 2016-17, “Consolidation (Topic 810): Interests Held through Related Parties That Are under Common Control”. These amendments change the evaluation of whether a reporting entity is the primary beneficiary of a variable interest entity by changing how a reporting entity that is a single decision maker of a variable interest entity treats indirect interests in the entity held through related parties that are under common control with the reporting entity. If a reporting entity satisfies the first characteristic of a primary beneficiary (such that it is the single decision maker of a variable interest entity), the amendments require that reporting entity, in determining whether it satisfies the second characteristic of a primary beneficiary, to include all of its direct variable interests in a variable interest entity and, on a proportionate basis, its indirect variable interests in a variable interest entity held through related parties, including related parties that are under common control with the reporting entity. The amendments in this ASU are effective for public business entities for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. Early adoption is permitted, including adoption in an interim period. If an entity adopts the pending content that links to this paragraph in an interim period, any adjustments should be reflected as of the beginning of the fiscal year that includes that interim period. The Company is currently in the process of evaluating the impact of the adoption on its consolidated financial statements.
In November 2016, the FASB issued ASU 2016-18, “ Statement of Cash Flows (Topic 230): Restricted Cash”. These amendments require that a statement of cash flows explain the change during the period in the total of cash, cash equivalents, and amounts generally described as restricted cash or restricted cash equivalents. As a result, amounts generally described as restricted cash and restricted cash equivalents should be included with cash and cash equivalents when reconciling the beginning-of-period and end-of-period total amounts shown on the statement of cash flows. The amendments do not provide a definition of restricted cash or restricted cash equivalents. The amendments in this ASU are effective for public business entities for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. Early adoption is permitted. The amendments should be applied using a retrospective transition method to each period presented. The Company is currently in the process of evaluating the impact of the adoption on its consolidated financial statements.
| F-13 |
In December 2016, the FASB issued ASU 2016-20, “Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers”. The amendments in ASU 2016-20 affect narrow aspects of the guidance issued in ASU 2014-09 including Loan Guarantee Fees, Contract Costs, Provisions for Losses on Construction-Type and Production-Type Contracts, Disclosure of Remaining Performance Obligations, Disclosure of Prior Period Performance Obligations, Contract Modifications, Contract Asset vs. Receivable, Refund Liabilities, Advertising Costs, Fixed Odds Wagering Contracts in the Casino Industry, and Costs Capitalized for Advisors to Private Funds and Public Funds. The effective date of these amendments are at the same date that Topic 606 is effective. Topic 606 is effective for public entities for annual reporting periods beginning after December 15, 2017, including interim reporting periods therein (i.e., January 1, 2018, for a calendar year entity). The Company is currently in the process of evaluating the impact of the adoption on its consolidated financial statements.
NOte 2 - going concern
The Company sustained operating losses of $398,030 and $345,596 during the years ended December 31, 2016 and 2015, respectively. The Company has accumulated deficit of $597,650 and $323,693 as of December 31, 2016 and 2015, respectively. The Company’s continuation as a going concern is dependent on its ability to generate sufficient cash flows from operations to meet its obligations and/or obtain additional financing, as may be required.
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern; however, the above condition raises substantial doubt about the Company’s ability to do so. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result should the Company be unable to continue as a going concern.
Management’s Plan to Continue as a Going Concern
In order to continue as a going concern, the Company will need, among other things, additional capital resources. Management’s plans to obtain such resources for the Company include (1) obtaining capital from the sale of its equity securities, (2) sales of the Company’s products, (3) short-term and long-term borrowings from banks, and (4) short-term borrowings from stockholders or other related party(ies) when needed. However, management cannot provide any assurance that the Company will be successful in accomplishing any of its plans.
The ability of the Company to continue as a going concern is dependent upon its ability to successfully accomplish the plans described in the preceding paragraph and eventually to secure other sources of financing and attain profitable operations.
NOte 3 - concentration of credit risk
We maintain our cash balance in several banks in China and United States. The consolidated cash balances as of December 31, 2016, and 2015 were $339,147 and $21,747 respectively. The cash balances in China as of December 31, 2016 and 2015 are $29,156 and $21,747 respectively. The accounts in China were not insured which we believe were exposed to credit risks on cash. Our U.S. bank account is maintained at Wells Fargo, as of December 31, 2016, and the balance of $309,991 is in excess of federal insured limit of $250,000 by $59,991.
NOTe 4 - other receivable
Total other receivable consists of balance of VAT receivable of $102,122 and $90,841 as of December 31, 2016 and 2015, respectively.
NOTe 5 - prepaid expenses
Prepaid expenses were comprised of the following:
| December 31, 2016 | December 31, 2015 | |||||||
| Prepaid property and equipment | $ | 14,792 | $ | 183,018 | ||||
| Inventory | 94,780 | 1,275 | ||||||
| Utilities | 8,420 | 9,025 | ||||||
| Other | 40,857 | 1,783 | ||||||
| Total | $ | 158,869 | $ | 195,101 | ||||
| F-14 |
Note 6 - property, plant and equipment
Property, plant and equipment as of December 31, 2016 and 2015 are summarized as following:
| December 31, 2016 | December 31, 2015 | |||||||
| Buildings | $ | 1,739,780 | $ | 1,864,722 | ||||
| Vehicles | 11,162 | 11,964 | ||||||
| Manufacturing equipment | 982,511 | 569,780 | ||||||
| Office equipment | 85,061 | 77,583 | ||||||
| Property, plant, and equipment - total | 2,818,514 | 2,524,049 | ||||||
| Less: accumulated depreciation | (300,271 | ) | (119,399 | ) | ||||
| Fixed assets, net | $ | 2,518,243 | $ | 2,404,650 | ||||
For the years ended December 31, 2016 and 2015, depreciation expense was $197,483 and $117,397, respectively.
note 7 - intangible assets -net
All land in the PRC is owned by the government and cannot be sold to any individual or entity. Instead, the government grants landholders a "land use right" after a purchase price for such "land use right" is paid to the government. The "land use right" allows the holder to use the land for 50 years and enjoys all the incidents of ownership of the land. As of December 31, 2016, and December 31, 2015, the land use rights net of accumulated amortization was $695,109 and $760,819, respectively. The use term was 50 years.
The summary of land use rights as of December 31, 2016 and 2015 are summarized as following:
| The land use right term | December 31, 2016 | December 31, 2015 | ||||||
| May, 2013 - Apr, 2063 | $ | 518,528 | $ | 555,766 | ||||
| Dec, 2015 - Sep, 2065 | 194,440 | 208,404 | ||||||
| Dec, 2015 - Sep, 2065 | 23,664 | 25,364 | ||||||
| Intangible assets- total | 736,632 | 789,534 | ||||||
| Less: accumulated amortization | (41,523 | ) | (28,715 | ) | ||||
| Intangible assets, net | $ | 695,109 | $ | 760,819 | ||||
For the year ended December 31, 2016 and 2015, amortization expense was $15,405 and $11,460, respectively.
NOTE 8 - COMMITMENTS AND CONTINGENCIES
The Company entered a lease for new office space in City of Industry, California. The lease period started October 1st, 2016 and expired on September 1st, 2018. On October 1st 2016, the Company started this lease at $2,802 per month from October 1st 2016 to September 1st 2017. From September 2nd 2017 to August 31st 2018, the monthly lease will be $2,923, resulting in the following future commitments:
| Amount | ||||
| 2017 calendar year | $ | 34,108 | ||
| 2018 calendar year | 23,384 | |||
| Total | $ | 57,492 | ||
| F-15 |
note 9 - short term loan
The Company entered into a short-term loan agreement with rural credit cooperative of Shandong. The maximum loan amount is $1,543,734 and the balances was $0 and $1,080,614 as of December 31, 2016, and December 31, 2015, respectively. The term of the loan is from August 6, 2015 and expired at August 5, 2016. The interest rate is fixed at 8.245%. Interest is calculated from the day the loan is used and due monthly. The loan was guaranteed by owner and collateralized with land use rights and 3 manufacturing buildings owned by the Company. The Company paid off the loan using funds borrowed from related party-Shandong Yibao Biologics Co., Ltd. during the period ended December 31, 2016. In December 2016, Shandong Yibao Biologics assigned the loan to Xiuhua Song.
NOTE 10 - INCOME TAX
At December 31, 2016 and 2015, based on the weight of available evidence, management determined that it was unlikely that the Company's deferred tax assets would be realized and have provided for a full valuation allowance associated with the net deferred tax assets.
The Company periodically analyzes its tax positions taken and expected to be taken and has determined that since inception there has been no need to record a liability for uncertain tax positions. The Company classifies income tax penalties and interest, if any, as part of selling, general and administrative expenses in the accompanying statements of operations. There was no accrued interest or penalties as of December 31, 2016 or 2015.
The Company is neither under examination by any taxing authority, nor has it been notified of any impending examination.
Under the Law of People’s Republic of China on Enterprise Income Tax (“EIT Law”), which was effective from January 1, 2008, domestically-owned enterprises and foreign-invested enterprises are subject to a uniform tax rate of 25%. As of December 31, 2016, the Company have net operating losses carry forward of $634,692 that begin expiring in 2018. The potential benefit of the company’s net operating losses has not been recognized in these financial statements because it is more likely-than-not the Company will not utilize the net operating losses carried forward as it does not expect to generate sufficient taxable income in future or the amount involved is not significant.
| December 31, 2016 | December 31, 2015 | |||||||
| Deferred Tax Assets and Liabilities: | ||||||||
| Net operating loss carry forwards | 597,650 | 323,693 | ||||||
| Valuation allowance | (597,650 | ) | (323,693 | ) | ||||
| Net deferred tax assets | – | – | ||||||
| F-16 |
A reconciliation between the income tax computed at the U.S. statutory rate and the Company’s provision for income tax in the PRC is as follows:
| December 31, 2016 | December 31, 2015 | |||||||
| Tax expense at statutory rate-US | 34% | 34% | ||||||
| Foreign income not recognized in the US | (34)% | (34)% | ||||||
| PRC enterprise income tax rate | 25% | 25% | ||||||
| Loss not subject to income tax | (25)% | (25)% | ||||||
| Effective income tax rates | – | – | ||||||
note 11- equity
Common stock:
On July 1, 2016, the Company issued 3,915,000 shares of common stock to Ms. Song in connection with the change of control. Subsequently, on August 31, 2016, the Company cancelled 3,915,000 shares of outstanding shares.
On August 31, 2016, the Company issued 2,040,000 shares to Yibaoccyb Shareholders in exchange for 51% of the common stock of Yibaoccyb to complete the share exchange and restructuring of entities under common control.
On December 22, 2016, the Company issued 350,000 shares to Eric Stoppenhagon in exchange for legal counsel services valued at $35,000, or $0.10 per share per agreement.
On December 23, 2016, the Company received cash payment of $300,000 in advance for issuance of 3,000,000 shares of common stock, or $0.10 per share on January 3, 2017 to seven investors, none of which is a related-party to the Company.
Series A Preferred Shares:
The Series A Preferred shares are convertible into common shares on a 1:1 ratio at a fixed rate of $3 per share. Preferred shares have no voting rights, have no redemption rights and earn no dividends. Holders of Series A Convertible Preferred Stock are not permitted to convert their stock into common shares until the Company’s market capital reaches $15,000,000. Upon dissolution, liquidation or winding up of the Company, whether voluntary or involuntary, the holders of the then outstanding shares of Series A Convertible Preferred Stock shall be entitled to receive out of the assets of the Company the sum of $0.0001 per share (the “Liquidation Rate”) before any payment or distribution shall be made on any other class of capital stock of the Company ranking junior to the Series A Convertible Preferred Stock.
ASC Topic 480, “Distinguishing Liabilities from Equity,” establishes standards for how an issuer classifies and measures certain financial instruments with characteristics of both liabilities and equity.
A mandatorily redeemable financial instrument shall be classified as a liability unless the redemption is required to occur only upon the liquidation or termination of the reporting entity. A financial instrument issued in the form of shares is mandatorily redeemable if it embodies an unconditional obligation requiring the issuer to redeem the instrument by transferring its assets at a specified or determinable date (or dates) or upon an event certain to occur. A financial instrument that embodies a conditional obligation to redeem the instrument by transferring assets upon an event not certain to occur becomes mandatorily redeemable—and, therefore becomes a liability—if that event occurs, the condition is resolved, or the event becomes certain to occur.
The Company determined that the preferred shares are not mandatorily or conditionally redeemable and are properly classified as permanent equity in the accompanying unaudited condensed consolidated financial statements.
| F-17 |
Series B Preferred Shares
During the year ended December 31, 2015, the Company received the 400,000 shares of Series B Preferred Shares being returned to the Company. As a result, there were no shares of Series B Preferred Shares issued and outstanding as of December 31, 2016 and 2015.
NOTE 12 - NON-CONTROLLING INTEREST
On July 1, 2016, the Company executed the Exchange Agreement with Yibaoccyb and the Yibaoccyb Shareholders. From and after the Closing Date, Yibaoccyb become a 51% owned subsidiary of the Company. Yibaoccyb owns 100% of YibaoHK, a Hong Kong company. YibaoHK will own 100% of Shenzhen Confucian Biologics Co. Ltd. (yet to be formed, “Yibao WFOE”), which will be wholly foreign-owned enterprise (“WFOE”) under the laws of the Peoples’ Republic of China (“PRC” or “China”). On August 31, 2016, YibaoHK entered into a series of contractual arrangements with Confucian and currently is 100% owner of Confucian.
Non-controlling interest consisted of the following:
| December 31, | December 31, | |||||||
| 2016 | 2015 | |||||||
| Beginning balance | $ | 464,323 | $ | 656,241 | ||||
| Net loss attributed to non-controlling interest | (124,073 | ) | (169,342 | ) | ||||
| Foreign currency translation loss attributable to non-controlling interest | (25,767 | ) | (22,576 | ) | ||||
| Ending balance | $ | 314,483 | $ | 464,323 | ||||
note 13 - related party transactions
The Company has received $34,378 deposit from Shandong Yibao Biologics Co, Ltd, which is also a customer of Confucian as of December 31, 2016. Sales to Shandong Yibao Biologics Co, Ltd for the twelve months ended December 31, 2016 and 2015 were $309,691 and $0, respectively. The purchases of raw material from Shandong Yibao Biologics Co, Ltd were $218,262 and $0 for the years of 2016 and 2015, respectively. The purchases from Shandong Yibao Biologics are mostly consisted of one raw material, and the sales to Shandong Yibao Biologics are all finished goods.
In June 2016, Shandong Yibao Biologics Co., Ltd. advanced funds of $1,064,073 to the Company to pay off the short-term loan. Prior to December 2016, loan from Shandong Yibao Biologics Co., Ltd amounted to $545,545. In December 2016, Shandong Yibao Biologics assigned the loan to Xiuhua Song.
The Company has the following payables to related parties:
| December 31, 2016 | December 31, 2015 | |||||||
| To Xiuhua Song | $ | 2,293,434 | $ | 995,713 | ||||
| To Shandgong Yibao | – | 218,672 | ||||||
| To Hengchun Zhang | 260,169 | – | ||||||
| To Quingbao Kong | 5,873 | – | ||||||
| To Wenxiu Song | – | 138,936 | ||||||
| Total due to related parties | $ | 2,559,476 | $ | 1,353,321 | ||||
| F-18 |
NOTE 14 - MAJOR SUPPLIERS AND CUSTOMERS
The Company purchases majority of its inventory and packaging supplies from four suppliers which accounted for 56.87% of the total purchases in year ended December 31, 2016.
The Company had two major customers for the year ended December 31, 2016: Ping Xiang Import and Export Company accounted for 46.07% of revenue and Shandong Yibao Biologics for 27.76% of revenue for the year ended December 31, 2016.
The Company purchases majority of its inventory and packaging supplies from three suppliers which accounted for 55.27% of the total purchases in year ended December 31, 2015.
The Company had one major customer for the year ended December 31, 2015: Ping Xiang Import and Export Company accounted for 64.37% of revenue for the year ended December 31, 2015.
NOTE 15 - SUBSEQUENT EVENTS
Management has evaluated subsequent events through April 21, 2017, the date which the consolidated financial statements were available to be issued. All subsequent events requiring recognition as of December 31, 2016 have been incorporated into these consolidated financial statements and there are no subsequent events that require disclosure in accordance with FASB ASC Topic 855, “Subsequent Events.”
| F-19 |
