Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - MABVAX THERAPEUTICS HOLDINGS, INC. | mbvx8k_sep22016.htm |
Exhibit 99.1

September 2, 2016
Dear Stockholders,
MabVax Therapeutics Holdings, Inc. (NASDAQ: MBVX), has achieved several significant corporate milestones since our last shareholder letter in August 2015 that we believe help establish a framework for future progress. Perhaps even more important, we have several important upcoming milestones that should increase the value of your Company if our expectations of results prove successful.
This past year, we were able to execute on our planned clinical initiatives with our incredible team of scientists and experts who worked on and advanced our proprietary therapeutic and diagnostic cancer immunotherapy platforms. Additionally, on the capital markets side we recently completed the closing of a $9.4 million public offering and uplisted our shares onto the Nasdaq Capital Market, a step we believe will prove valuable – particularly from a higher company visibility standpoint – as we release interim data readouts on our clinical programs.
For those who have followed MabVax closely, we expect to provide updates to our progress in our two phase 1 clinical trials – our therapeutic antibody clinical program, MVT-5873 (HuMab-5B1), and our diagnostic program, MBT-2163, both in the diagnosis and treatment of pancreatic cancer. We will also be reporting in the fourth quarter 2016 on our plan to submit an investigational new drug application for a radioimmunotherapy clinical program. Together, these accomplishments and plans for the near term play an important role in bringing us to the threshold of significant corporate, scientific and medical milestones planned for the remainder of this year.
We have stated that in the third quarter we will be providing a progress report on the clinical programs and announcing interim results from the Phase 1 clinical trial for our clinical development product MVT-5873 for the treatment of pancreatic cancer before the end of the year. We are highly encouraged by the progress thus far and anticipate very meaningful results to be disclosed in this timeframe.
With these developments in place, and meaningful milestones right in front of us, we believe this is an extremely opportune time for our shareholders to be aware of our upcoming milestones.
Recent Accomplishments in Clinical and Corporate Developments
In almost every way, we have strengthened MabVax and moved our programs toward important milestones that, to date, have been on track with our past guidance. To name a few highlights:
|
·
|
We completed the preclinical development for two antibody-based products, manufactured clinical trial material, and filed two Investigational New Drug Applications (INDs) before the end of 2015.
|
|
·
|
In December of 2015 we received U.S. Food and Drug Administration (FDA) authorization to proceed with our therapeutic antibody (designated MVT-5873) in Phase 1 clinical trials.
|
|
·
|
In January 2016, we received a second authorization to proceed with our diagnostic antibody (designated MVT-2163) in a Phase 1 clinical trial.
|
|
·
|
In January 2016, we closed a $10 million non-dilutive debt financing with Oxford Finance LLC and received the first $5 million tranche.
|
|
·
|
We initiated a research collaboration with Rockefeller University with the goal of developing a next- generation HuMab-5B1 antibody under the direction of Dr. Jeffery Ravetch.
|
|
·
|
We strengthened our board of directors by adding Tom Varvaro in April 2015 and Jeffery Eisenberg in February 2016. Both new members bring significant business experience to the board.
|
|
·
|
In April 2016, Laidlaw and Company initiated equity research coverage on MabVax.
|
|
·
|
In February 2016, we initiated our MVT-5873 phase 1 trial for the treatment of pancreatic cancer at the Sara Cannon Research Institute (SCRI), and in June 2016, we expanded our patient enrollment to Memorial Sloan Kettering Cancer Center (“Memorial Sloan Kettering”).
|
|
·
|
In July 2016, we initiated patient enrollment in a Phase 1 trial for our new diagnostic imaging product, MVT-2163 for pancreatic cancer.
|
|
·
|
In August 2016, we successfully completed our underwritten offering and uplisted our common stock to the Nasdaq Capital Market.
|
One of the most significant and measurable accomplishments in 2015 was the filing of two separate INDs with the FDA. We were able to address all of the questions posed by the FDA to its satisfaction within the normal thirty-day review period, and in December 2015, we received authorization to initiate clinical trials for our lead antibody product, MVT-5873, as a therapeutic antibody product to treat pancreatic cancer. Earlier this year, we received authorization to initiate clinical trials for our antibody-based PET imaging agent, MVT-2163, to help diagnose and manage pancreatic cancer.
Our Clinical Development Pipeline
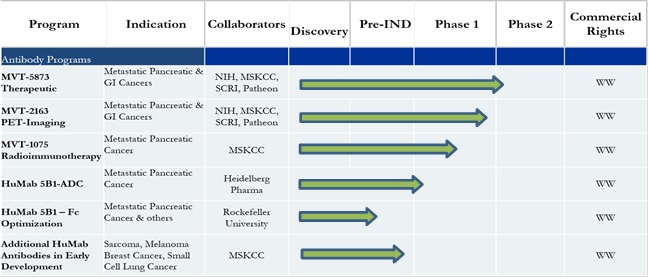
MVT-5873 – During the first quarter of 2016, we initiated the MVT-5873 therapeutic trial to evaluate the safety, tolerability, and pharmacokinetics of HuMab-5B1 as a single agent or in combination with the current standard of care chemotherapy regimen in subjects with metastatic pancreatic cancer. The first group of patients enrolled in the planned clinical trial is being entered in a traditional dose escalation regimen to assess safety and determine the recommended Phase 2 dose of the antibody. A second patient group will follow later this year to establish safety and the recommended Phase 2 dose of the antibody when administered with first-line standard of care chemotherapy. We have opened clinical trial sites at the SCRI facilities in Nashville, Tennessee, and Sarasota, Florida, as well as at Memorial Sloan Kettering.
In our clinical development program, we established a very productive relationship with SCRI for conducting our MVT-5873 clinical trial. The site in Nashville, Tennessee, has currently enrolled the majority of patients in our trial. We have opened a second SCRI site in Sarasota, Florida, in anticipation of progressing to the second part of our Phase 1 trial in which we will examine the impact of adding our antibody to first-line chemotherapy. We were able to initiate this Phase 1 trial at Memorial Sloan Kettering in the second quarter after receiving approval from Memorial Sloan Kettering’s internal review committees.
MVT-2163 – As mentioned earlier, we also initiated the MVT-2163 PET imaging trial at Memorial Sloan Kettering. This product is based on the MVT-5873 antibody with the radiolabel Zirconium 89 attached to the antibody. This new PET agent can potentially be an important new tool to aid in the diagnosis, monitoring, and assessment of pancreatic cancer patients and an attractive companion diagnostic for the MVT-5873 therapeutic product. This second Phase 1 trial is evaluating the safety, pharmacokinetics, and biodistribution of MVT-2163 in cancer patients. The trial will also determine the total dose and timing for an optimal PET scan image using the new imaging agent. Memorial Sloan Kettering is actively enrolling patients in this trial and then, after the appropriate safety assessments are complete, subsequently enrolling the patient into the MVT-5873 trial giving us a unique opportunity to both evaluate the disease and assess the impact of the therapeutic product.
MVT-1075 – We are making significant progress in expanding our product offerings using the MVT-5873 antibody platform by aggressively working to complete the preclinical work to support a radioimmunotherapy product. This product uses small doses of our MVT-5873 antibody coupled to a radioisotope to target pancreatic cancer cells and kill them. Memorial Sloan Kettering is playing a central role in the preclinical development of our new radioimmunotherapy agent. We plan to file the IND for this product late in 2016 and in the first half of 2017 enter a Phase 1 clinical trial for a product we have designated MVT-1075.
Antibody Drug Conjugate – Finally, we continue our collaboration with external partners to develop an antibody drug conjugate (ADC) using our MVT-5873 antibody linked to a toxin and again targeting pancreatic cancer. We are planning to file an IND for this product in late 2017 and initiate a Phase 1 clinical trial in 2018.
The MVT-5873 Platform – One can certainly ask why we are focused on bringing so many different versions of the MVT-5873 antibody into clinical testing. The answer is that we believe we have discovered an exceptional targeting agent in the MVT-5873 antibody. The antibody represents a highly useful directive scaffold upon which to develop more potent versions of MVT-5873. We believe our strategy will be able to improve upon current marketed products in treating pancreatic cancer. The unfortunate fact today is that the survival rates for patients diagnosed with pancreatic cancer are extremely poor, and new treatments are desperately needed. Even modest success with our new therapeutic or diagnostic agents should represent significant economic upside for us.
Emerging Pipeline of New Human Antibody Candidates – We would not be giving you a complete picture of our progress if we failed to mention the work we are doing on our emerging pipeline of new potential products. During the past year we continued to conduct preclinical work on earlier antibody discoveries to generate the necessary data to enable us to decide if they are clinical development candidates. To that end, we now have fully human antibodies that can address cancers besides pancreatic cancer, such as breast cancer, sarcoma, and small- cell lung cancer. Each of these antibodies binds to a different antigenic target on these cancers. We believe that developing additional antibody assets will continue to attract partnering interest and build value for the company.
Upcoming Report on Interim Data – We plan to report interim data on both clinical trials in the second half of this year. We believe our progress in determining the relative safety and targeting specificity of our lead antibody program will add value to the company as well as make MabVax an even more attractive partner or investment opportunity. We plan to file an IND for our radioimmunotherapy product by the end of 2016 and have preparations well under way to initiate our third Phase 1 clinical program. We also plan to report on our progress on the development of additional pipeline antibodies.
Collaborations and Partnering Discussions
Now that we have products in clinical trials, the level of activity around partnering has increased dramatically. We have ongoing discussions with multiple potential partners and we are excited that the biopharmaceutical community is beginning to take greater notice of our accomplishments. As a small company, partnering on our first programs has always been part of our strategy, and we have been engaged in discussions with several companies to that end. Our vision is to continue to build our capabilities and expertise so that we can take our follow-on product opportunities further into development in order to retain more of the economic value of each program. Below are some developments along these lines:
Memorial Sloan Kettering – We have been able to continue to strengthen our external research and development collaboration with Memorial Sloan Kettering – one of the world’s leading cancer research hospitals. Memorial Sloan Kettering serves as one of our investigative sites for our MVT-5873 therapeutic antibody Phase 1 trial. Memorial Sloan Kettering also is the key site for the MVT-2163 PET imaging trial. In fact, Memorial Sloan Kettering manufactures the MVT-2163 product for our clinical trial. We have both sponsored research and service agreements in place with Memorial Sloan Kettering to help us with the preclinical development of several products.
Rockefeller University – We have an exciting collaboration in place with Rockefeller University on development of a next-generation HuMab-5B1 aimed at improving the function of our antibody. Rockefeller University is a world-renowned center for research and graduate education in the biomedical sciences, chemistry, bioinformatics and physics and through their unique approach to science has led to some of the world’s most revolutionary contributions to biology and medicine. Our collaboration with Rockefeller is being conducted under the direction of Jeffery Ravetch, MD, PhD, a world-renowned expert on antibodies. We anticipate knowing more about the potential outcome and next steps for this effort later in 2016.
Heidelberg Pharma – We continue to work with Heidelberg Pharma, a German-based pre-clinical drug discovery and development service, on the development of our HuMab-5B1 product as an antibody drug conjugate. This has been a productive and efficient collaboration that has completed work demonstrating proof of concept for the development of our antibody as an ADC. We have demonstrated that our antibody is internalized by the cancer cell, and with the proper chemical linkage, can release the toxin payload to kill the cancer cell. The ADC approach has gained significant attention in the marketplace over the last 5 years. Several companies, including Seattle Genetics, Genentech, and ImmunoGen, have seen success with their ADC technologies. We believe that the ADC approach can improve the potency of our antibodies to treat diseases where there remains a significant unmet medical need for new treatments.
Recent Uplisting onto the Nasdaq Capital Market and Analyst Coverage
We completed our reverse stock split and successfully listed onto the Nasdaq Capital Market. We believe that this will help generate increased visibility, better liquidity, lower cost of capital, and attract a broader range of institutional investors to our story, which will be strategically advantageous as we announce interim and follow- on data from our two clinical programs in the remainder of this year. We were pleased to see earlier this year that Laidlaw & Company initiated equity research on MabVax. We believe these events, in addition to the fact that our technology platform is being recognized by the industry’s leading companies and scientists, make our story extremely compelling to our current and future shareholders.
Poised for Important Progress
We believe all of our efforts are worthwhile, as there are tens of thousands of patients suffering from pancreatic cancer who do not have adequate solutions for diagnosis and treatment of their disease. Further, these patients need new options like the products we are developing.
We can say with confidence that what we are building here we believe has all the ingredients required for long- term success and value creation for our stock. We have:
|
·
|
A proven management and board of directors and a world-class scientific team
|
|
·
|
A large and addressable market focused on an unmet medical need in the treatment of cancer
|
|
·
|
A proprietary approach to developing novel antibody-based products with significant commercial potential
|
|
·
|
A robust IP portfolio
|
|
·
|
Orphan drug designation available for our antibody products
|
|
·
|
12 months’ operating capital to complete Phase 1 milestones
|
On behalf of our board of directors and management team, we thank you for your investment in our company and your continued interest in advancing our product programs to the clinical development milestones we have outlined for 2016. Together we believe we can provide significant value accretion to our stockholders.

J. David Hansen
President and Chief Executive Officer
Forward Looking Disclaimer
This shareholder letter contains "forward-looking statements" regarding matters that are not historical facts, including statements relating to the Company's clinical trials and product development pipeline. We have no assurance that all of the product development pipeline will be fully developed by the Company. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as "anticipates," "plans," "expects," "intends," "will," "potential," "hope," and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon current expectations of the Company and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties. Detailed information regarding factors that may cause actual results to differ materially from the results expressed or implied by statements in this press release relating to the Company may be found in the Company's periodic filings with the Securities and Exchange Commission, including the factors described in the section entitled "Risk Factors" in its annual report on Form 10-K for the fiscal year ended December 31, 2015, as amended and supplemented from time to time and the Company's Quarter Reports on Form 10-Q and other filings submitted by the Company to the SEC, copies of which may be obtained from the SEC's website at www.sec.gov. The parties do not undertake any obligation to update forward-looking statements contained in this shareholder letter.
