Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - HERON THERAPEUTICS, INC. /DE/ | d240921dex991.htm |
| 8-K - FORM 8-K - HERON THERAPEUTICS, INC. /DE/ | d240921d8k.htm |
Exhibit 99.2

SUSTOL Approval August 10, 2016

Forward-Looking Statements This presentation contains “forward-looking statements” as defined by the Private Securities Litigation Reform Act of 1995. We caution investors that forward-looking statements are based on managements expectations and assumptions as of the date of this presentation, and involve substantial risks and uncertainties that could cause our clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward-looking statements. These risks and uncertainties include, but are not limited to, those associated with: the potential market opportunity for SUSTOL, the expected timing of the SUSTOL commercial launch, safety information for SUSTOL, the SUSTOL indication, and other risks and uncertainties identified in the Company’s filings with the Securities and Exchange Commission. Forward-looking statements reflect our analysis only on their stated date, and we take no obligation to update or revise these statements except as may be required by law.

SUSTOL Now approved by U.S. FDA U.S. Commercial Launch Planned For Q4 2016
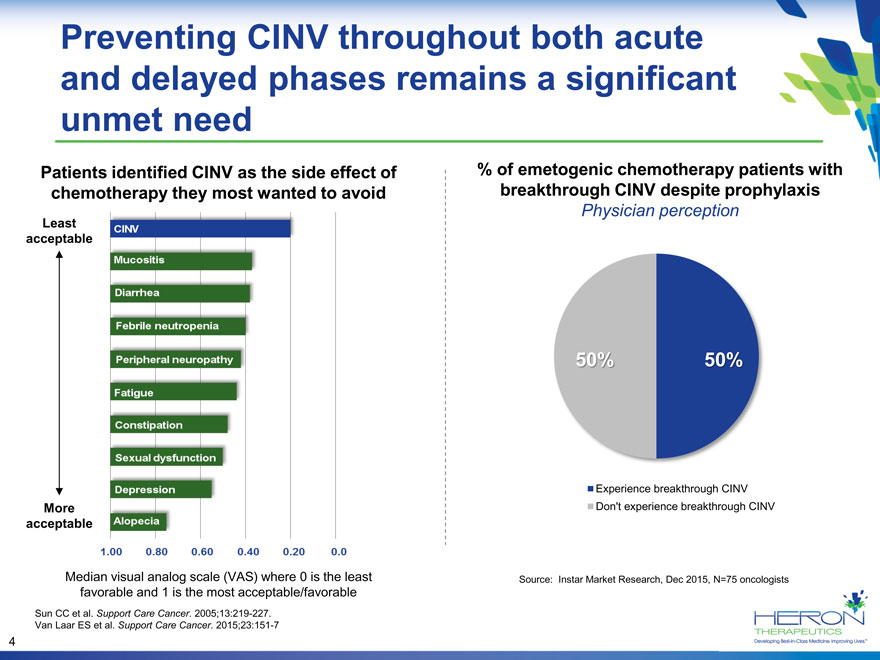
Preventing CINV throughout both acute and delayed phases remains a significant unmet need Patients identified CINV as the side effect of% of emetogenic chemotherapy patients with chemotherapy they most wanted to avoid breakthrough CINV despite prophylaxis Physician perception Least acceptable 50% 50% Experience breakthrough CINV More Don’t experience breakthrough CINV acceptable Median visual analog scale (VAS) where 0 is the least Source: Instar Market Research, Dec 2015, N=75 oncologists favorable and 1 is the most acceptable/favorable Sun CC et al. Support Care Cancer. 2005;13:219-227. Van Laar ES et al. Support Care Cancer. 2015;23:151-7 4
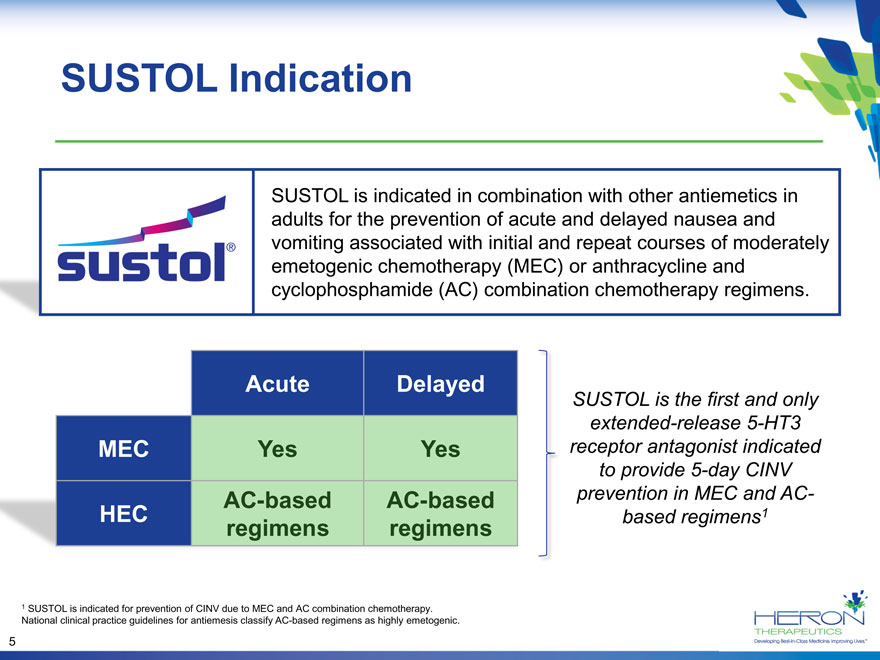
SUSTOL Indication SUSTOL is indicated in combination with other antiemetics in adults for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (MEC) or anthracycline and cyclophosphamide (AC) combination chemotherapy regimens. Acute Delayed SUSTOL is the first and only extended-release 5-HT3 MEC Yes Yes receptor antagonist indicated to provide 5-day CINV AC-based AC-based prevention in MEC and AC- HEC based regimens1 regimens regimens 1 SUSTOL is indicated for prevention of CINV due to MEC and AC combination chemotherapy. National clinical practice guidelines for antiemesis classify AC-based regimens as highly emetogenic. 5
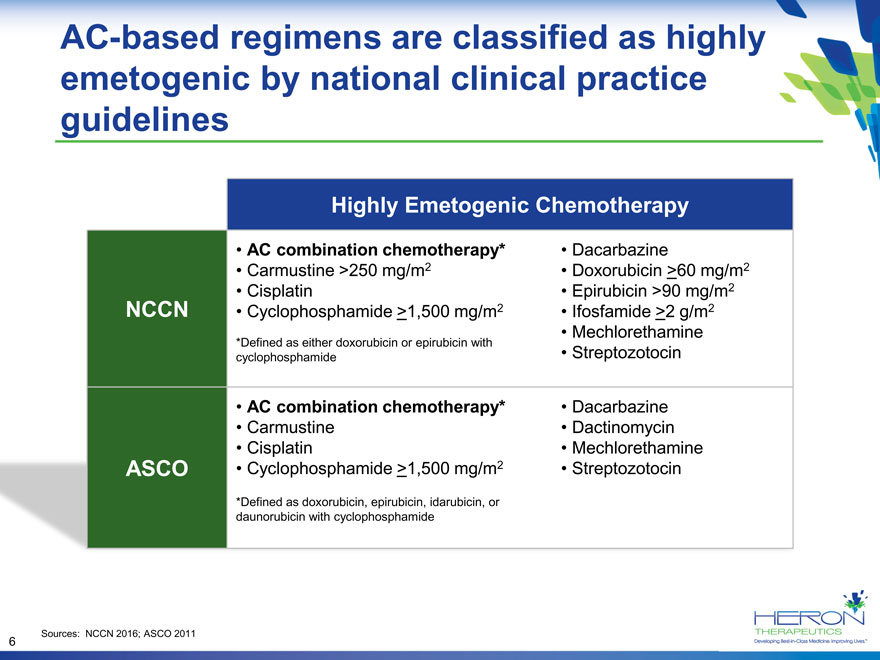
AC-based regimens are classified as highly emetogenic by national clinical practice guidelines Highly Emetogenic Chemotherapy AC combination chemotherapy* Dacarbazine Carmustine >250 mg/m2 Doxorubicin >60 mg/m2 Cisplatin Epirubicin >90 mg/m2 NCCN Cyclophosphamide >1,500 mg/m2 Ifosfamide >2 g/m2 Mechlorethamine *Defined as either doxorubicin or epirubicin with cyclophosphamide Streptozotocin AC combination chemotherapy* Dacarbazine Carmustine Dactinomycin Cisplatin Mechlorethamine ASCO Cyclophosphamide >1,500 mg/m2 Streptozotocin *Defined as doxorubicin, epirubicin, idarubicin, or daunorubicin with cyclophosphamide Sources: NCCN 2016; ASCO 2011 6
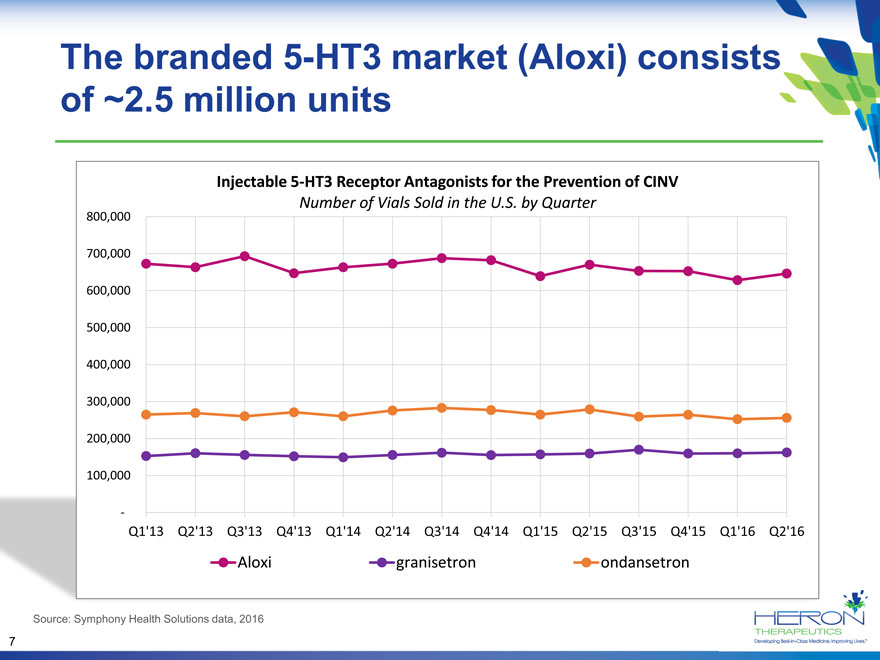
The branded 5-HT3 market (Aloxi) consists of ~2.5 million units Injectable 5-HT3 Receptor Antagonists for the Prevention of CINV Number of Vials Sold in the U.S. by Quarter 800,000 700,000 600,000 500,000 400,000 300,000 200,000 100,000 — Q1’13 Q2’13 Q3’13 Q4’13 Q1’14 Q2’14 Q3’14 Q4’14 Q1’15 Q2’15 Q3’15 Q4’15 Q1’16 Q2’16 Aloxi granisetron ondansetron Source: Symphony Health Solutions data, 2016 7
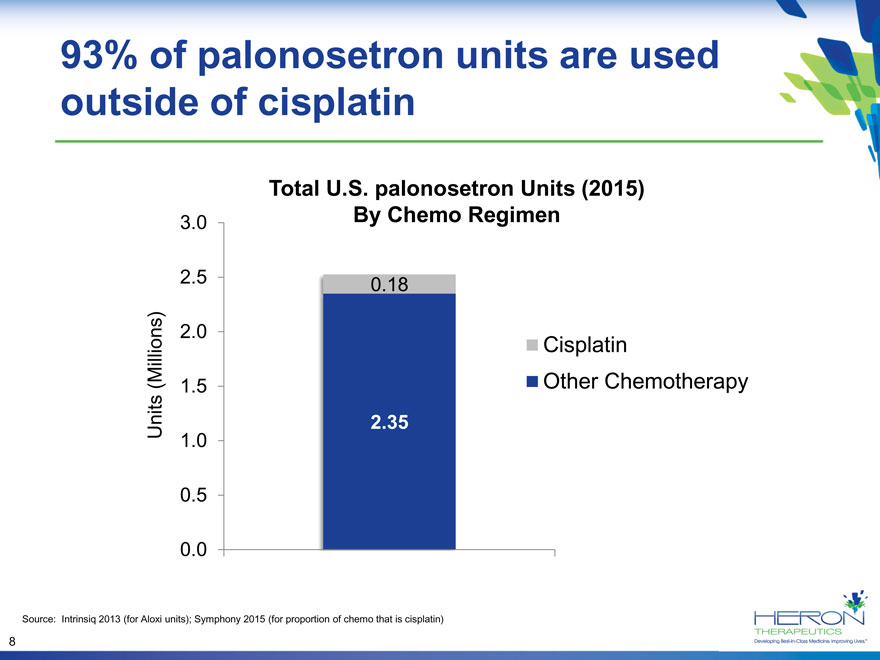
93% of palonosetron units are used outside of cisplatin Total U.S. palonosetron Units (2015) 3.0 By Chemo Regimen 2.5 0.18 (Millions) 2.0 Cisplatin 1.5 Other Chemotherapy Units 2.35 1.0 0.5 0.0 Source: Intrinsiq 2013 (for Aloxi units); Symphony 2015 (for proportion of chemo that is cisplatin) 8
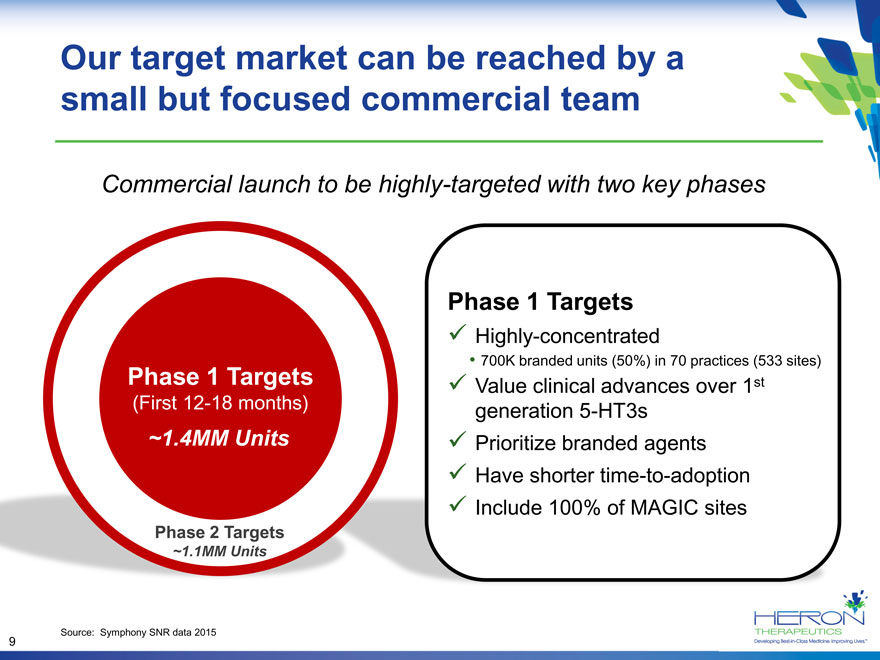
Our target market can be reached by a small but focused commercial team Commercial launch to be highly-targeted with two key phases Phase 1 Targets Highly-concentrated 700K branded units (50%) in 70 practices (533 sites) Phase 1 Targets Value clinical advances over 1st (First 12-18 months) generation 5-HT3s ~1.4MM Units Prioritize branded agents Have shorter time-to-adoption Include 100% of MAGIC sites Phase 2 Targets ~1.1MM Units Source: Symphony SNR data 2015 9
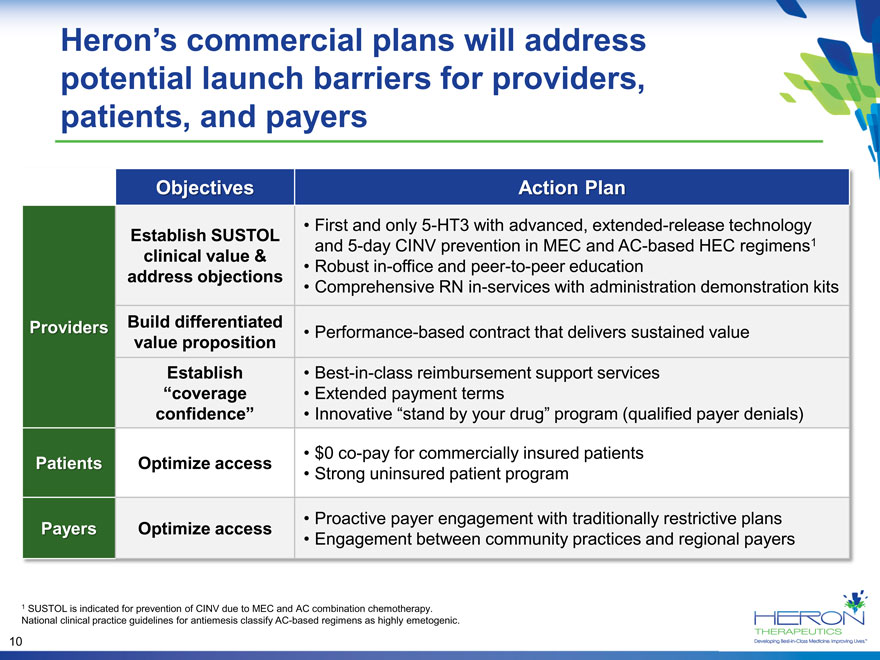
Herons commercial plans will address potential launch barriers for providers, patients, and payers Objectives Action Plan First and only 5-HT3 with advanced, extended-release technology Establish SUSTOL and 5-day CINV prevention in MEC and AC-based HEC regimens1 clinical value & address objections Robust in-office and peer-to-peer education Comprehensive RN in-services with administration demonstration kits Providers Build differentiated Performance-based contract that delivers sustained value value proposition Establish Best-in-class reimbursement support services coverage Extended payment terms confidence Innovative stand by your drug program (qualified payer denials) $0 co-pay for commercially insured patients Patients Optimize access Strong uninsured patient program Proactive payer engagement with traditionally restrictive plans Payers Optimize access Engagement between community practices and regional payers 1 SUSTOL is indicated for prevention of CINV due to MEC and AC combination chemotherapy. National clinical practice guidelines for antiemesis classify AC-based regimens as highly emetogenic. 10

U.S. commercial organization is poised for launch U.S. Commercial Organization Marketing Sales Phase 1 Targets Market Access(First 12-18 months) GPO Account Team ~1.4MM Units Payer Account Team MSLs / Nurse Educators Source: Symphony SNR data 2015 11

SUSTOL Now Approved Despite advances in the management of CINV, up to half of patients receiving chemotherapy can still experience CINV, with delayed CINV being particularly challenging to control, commented Ralph V. Boccia, MD, FACP, Medical Director, Center for Cancer and Blood Disorders. In our experience, other 5-HT3 receptor antagonists, including palonosetron, are generally effective for 48 hours or less. SUSTOL, due to its extended-release profile, represents a novel option that can protect patients from CINV for a full 5 days. “The SUSTOL clinical trial populations and results are highly representative of cancer patients in our real-world clinical practice, said Jeffrey Vacirca, MD, FACP, Chief Executive Officer and Director of Clinical Research, North Shore Hematology Oncology Associates and Vice President, Community Oncology Alliance. Use of MEC regimens is widespread, and AC-based regimens are among the most commonly prescribed highly emetogenic chemotherapy regimens. The most significant challenge for my breast cancer patients receiving AC is chemotherapy-induced nausea and vomiting. SUSTOL represents a better option to manage this devastating side effect of therapy. 12
