Attached files
| file | filename |
|---|---|
| EX-32.1 - EX-32.1 - HERON THERAPEUTICS, INC. /DE/ | d347497dex321.htm |
| EX-31.2 - EX-31.2 - HERON THERAPEUTICS, INC. /DE/ | d347497dex312.htm |
| EX-31.1 - EX-31.1 - HERON THERAPEUTICS, INC. /DE/ | d347497dex311.htm |
| EX-23.1 - EX-23.1 - HERON THERAPEUTICS, INC. /DE/ | d347497dex231.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
[ x ] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2016
or
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number: 001-33221
HERON THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| DELAWARE | 94-2875566 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
| 4242 CAMPUS POINT COURT, SUITE 200 SAN DIEGO, CA |
92121 | |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code:
(858) 251-4400
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class: | Name of each exchange on which registered: | |
| Common Stock, par value $0.01 per share | The NASDAQ Capital Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] No [ x ]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes [ ] No [ x ]
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [ x ] No [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [ x ] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ x ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.:
| Large accelerated filer | [ x ] | Accelerated filer | [ ] | Non-accelerated filer | [ ] | Smaller reporting company | [ ] | |||||||
| (Do not check if a smaller reporting company) | ||||||||||||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes [ ] No [ x ]
The aggregate market value of voting and non-voting common stock held by non-affiliates of the registrant as of June 30, 2016 totaled $597,117,000 based on the closing price of $18.05 as reported by The NASDAQ Capital Market. As of February 3, 2017, there were 53,574,534 shares of the Company’s common stock ($0.01 par value) outstanding.
Documents Incorporated by Reference
Portions of the registrant’s Definitive Proxy Statement related to its 2017 Annual Stockholders’ Meeting to be held on or about June 13, 2017 are incorporated by reference into Part III of this Annual Report on Form 10-K. Such Definitive Proxy Statement will be filed with the U.S. Securities and Exchange Commission within 120 days after the end of the fiscal year to which this report relates. Except as expressly incorporated by reference, the registrant’s Definitive Proxy Statement shall not be deemed to be part of this report.
Table of Contents
| TABLE OF CONTENTS | ||||
| Item 1. |
5 | |||
| Item 1A. |
22 | |||
| Item 1B. |
51 | |||
| Item 2. |
51 | |||
| Item 3. |
51 | |||
| Item 4. |
51 | |||
| Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 52 | ||
| Item 6. |
54 | |||
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
55 | ||
| Item 7A. |
67 | |||
| Item 8. |
69 | |||
| Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
97 | ||
| Item 9A. |
97 | |||
| Item 9B. |
99 | |||
| Item 10. |
100 | |||
| Item 11. |
100 | |||
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
100 | ||
| Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
101 | ||
| Item 14. |
101 | |||
| Item 15. |
102 | |||
| 103 | ||||
| 104 | ||||
2
Table of Contents
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the federal securities laws. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. You can identify forward-looking statements by the use of the words “believe,” “expect,” “anticipate,” “intend,” “estimate,” “project,” “will,” “should,” “may,” “plan,” “assume” and other expressions that predict or indicate future events and trends and which do not relate to historical matters. You should not rely on forward-looking statements, because they involve known and unknown risks, uncertainties and other factors, some of which are beyond our control. These risks, uncertainties and other factors may cause our actual results, performance or achievements to be materially different from our anticipated future results, performance or achievements expressed or implied by the forward-looking statements.
Factors that might cause these differences include the following:
• our ability to successfully commercialize, market and achieve market acceptance of SUSTOL® (granisetron) extended-release injection (“SUSTOL”) and for future product candidates, including our positioning relative to competing products;
• estimates of the outcomes of our New Drug Application (“NDA”) submission to the U.S. Food and Drug Administration (“FDA”) for CINVANTI™ (HTX-019) (“CINVANTI”) and potential regulatory approval for and commercial launch of CINVANTI;
• any limitations or unfavorable warning or cautionary language that the FDA may ultimately impose on the label for CINVANTI;
• the potential market opportunities for SUSTOL, CINVANTI and HTX-011;
• whether safety and efficacy results of our clinical trials and other required tests for approval of our product candidates provide data to warrant progression of clinical trials, potential regulatory approval or further development of any of our product candidates;
• our ability to meet the post-marketing study requirements within the FDA’s mandated timelines and to obtain favorable results and comply with standard post-marketing requirements, including U.S. federal advertising and promotion laws, federal and state anti-fraud and abuse laws, healthcare information privacy and security laws, safety surveillance, and disclosure of payments or other transfers of value to healthcare professionals and entities for SUSTOL or any of our product candidates;
• our ability to successfully develop and achieve regulatory approval for other future product candidates utilizing our proprietary Biochronomer® sustained-release drug delivery technology (“Biochronomer technology”);
• our ability to establish key collaborations and vendor relationships for our products and any other future product candidates;
• our ability to successfully develop and commercialize any technology that we may in-license or products we may acquire;
• unanticipated delays due to manufacturing difficulties, supply constraints or changes in the regulatory environment;
3
Table of Contents
• our ability to successfully operate in non-U.S. jurisdictions in which we may choose to do business, including compliance with applicable regulatory requirements and laws;
• uncertainties associated with obtaining and enforcing patents to protect our products, and our ability to successfully defend ourselves against unforeseen third-party infringement claims;
• our estimates regarding our capital requirements; and
• our ability to obtain additional financing and raise capital as necessary to fund operations or pursue business opportunities.
Any forward-looking statements in this Annual Report on Form 10-K reflect our current views with respect to future events or to our financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the section entitled “Risk Factors” in this Annual Report on Form 10-K. You should carefully review all of these factors. Given these uncertainties, you should not place undue reliance on these forward-looking statements. These forward-looking statements were based on information, plans and estimates as of the date of this Annual Report on Form 10-K, and, except as required by law, we assume no obligation to update any forward-looking statements to reflect changes in underlying assumptions or factors, new information, future events or other changes. These risk factors may be updated from time to time by our future filings under the Securities Exchange Act of 1934 (the “Exchange Act”). You should carefully review all information therein.
4
Table of Contents
In this Annual Report on Form 10-K, all references to “Heron,” the “Company,” “we,” “us,” “our” and similar terms refer to Heron Therapeutics, Inc. Heron Therapeutics®, the Heron logo, SUSTOL®, CINVANTI™ and Biochronomer® are our trademarks. All other trademarks appearing or incorporated by reference into this Annual Report on Form 10-K are the property of their respective owners.
Overview
Heron is a commercial-stage biotechnology company focused on improving the lives of patients by developing best-in-class medicines that address major unmet medical needs. We are developing novel, patient-focused solutions that apply our innovative science and technologies to already-approved pharmacological agents for patients suffering from cancer and pain.
On August 9, 2016, our first commercial product, SUSTOL, was approved by the FDA. We developed SUSTOL for the prevention of chemotherapy-induced nausea and vomiting (“CINV”). SUSTOL is indicated, in combination with other antiemetics, in adults for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy (“MEC”) or anthracycline and cyclophosphamide (“AC”) combination chemotherapy regimens. We commenced commercial sales of SUSTOL in October 2016.
We have two investigational pharmaceutical products for patients suffering from cancer or post-operative pain. CINVANTI, an intravenous formulation of the neurokinin-1 (“NK1”) receptor antagonist aprepitant, has been developed for the prevention of CINV as an adjunct to other antiemetic agents. We submitted an NDA with the FDA for CINVANTI on January 11, 2017 using the 505(b)(2) regulatory pathway. HTX-011, a long-acting formulation of the local anesthetic bupivacaine in a fixed-dose combination with the anti-inflammatory meloxicam, is being developed for the prevention of post-operative pain. HTX-011 is the subject of a broad-based Phase 2 development program designed to target the many patients undergoing a wide range of surgeries who experience significant post-operative pain.
CINV Product Portfolio
SUSTOL
SUSTOL is our first commercial product. SUSTOL was approved by the FDA on August 9, 2016, and we commenced commercial sales in the U.S. in October 2016.
SUSTOL is indicated in combination with other antiemetics in adults for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of MEC or AC combination chemotherapy regimens. SUSTOL is an extended-release, injectable 5-hydroxytryptamine type 3 (“5-HT3”) receptor antagonist that utilizes our Biochronomer technology to maintain therapeutic levels of granisetron for ³5 days. The SUSTOL global Phase 3 development program was comprised of two, large, guideline-based clinical trials that evaluated efficacy and safety in more than 2,000 patients with cancer. The efficacy of SUSTOL in preventing nausea and vomiting was evaluated in both the acute phase (day 1 following chemotherapy) and the delayed phase (days 2-5 following chemotherapy).
5
Table of Contents
SUSTOL is the first extended-release 5-HT3 receptor antagonist approved for the prevention of acute and delayed nausea and vomiting associated with both MEC and AC combination chemotherapy regimens. A standard of care in the treatment of breast cancer and other cancer types, AC regimens are among the most commonly prescribed highly emetogenic chemotherapy regimens as defined by both the National Comprehensive Cancer Network and the American Society of Clinical Oncology.
CINVANTI (HTX-019)
We submitted an NDA with the FDA for CINVANTI on January 11, 2017 using the 505(b)(2) regulatory pathway.
CINVANTI is a proprietary, intravenous formulation of aprepitant, an NK1 receptor antagonist for the prevention of CINV. NK1 receptor antagonists are typically used in combination with 5-HT3 receptor antagonists. Currently, the only injectable NK1 receptor antagonist approved in the U.S., EMEND® for Injection (fosaprepitant), contains polysorbate 80, a surfactant, which may cause hypersensitivity reactions, infusion site reactions or other adverse reactions in some patients. Our formulation for CINVANTI does not contain a surfactant and may have a lower incidence of certain types of adverse reactions than reported with the other commercially available injectable NK1 receptor antagonist.
Our NDA submission includes data demonstrating the bioequivalence of CINVANTI to EMEND for Injection, supporting its efficacy for the prevention of both acute and delayed CINV with both MEC and highly emetogenic chemotherapy (“HEC”). Results also showed CINVANTI was better tolerated than EMEND for Injection, with significantly fewer adverse events reported with CINVANTI.
Pain Management Product Portfolio
HTX-011
HTX-011, which utilizes our Biochronomer technology, is a long-acting formulation of the local anesthetic bupivacaine in a fixed-ratio combination with the anti-inflammatory meloxicam for the prevention of post-operative pain. By delivering sustained levels of both a potent anesthetic and an anti-inflammatory agent directly to the site of tissue injury, HTX-011 was designed to provide superior pain relief while potentially reducing the need for systemically administered pain medications such as opioids, which carry the risk of harmful side effects, abuse and addiction. HTX-011 is the subject of a broad-based Phase 2 development program designed to target the many patients undergoing a wide range of surgeries who experience significant post-operative pain. Following a planned End of Phase 2 meeting with the FDA, we anticipate initiating Phase 3 studies in 2017 and filing an NDA in 2018.
In the third quarter of 2016 and the first quarter of 2017, we reported positive efficacy and safety results from studies in our Phase 2 clinical development program for HTX-011. HTX-011 has been generally well tolerated in the ongoing Phase 2 program, which has involved more than 600 administrations of HTX-011. The most frequent treatment-related adverse events reported have been nausea and vomiting, which occurred at similar rates in active and control patients.
Inguinal Hernia Repair — Study 202
In September 2016, we reported interim efficacy and safety results from Study 202, a randomized, placebo-controlled, double-blind Phase 2 clinical study in patients undergoing inguinal hernia repair. The study evaluated the efficacy and safety of three
6
Table of Contents
formulations of HTX-011 and two routes of administration into the wound (injection and instillation). Instillation into the incision site is an easier and potentially safer route of administration as it avoids multiple injections around the wound (as many as 100 or more in large operations) that carry the risk of venous puncture.
The primary endpoint was the difference as compared to placebo in pain intensity as measured by the Summed Pain Intensity (SPI) score in the first 24 hours post-surgery (SPI 0-24). Important secondary endpoints included SPI for the first 48 hours post-surgery (SPI 0-48), total opioid consumption, and the percent of patients opioid-free through 96 hours post-surgery. The major findings reported in September 2016 described Part B of the study (202B), which compared our planned Phase 3 formulation of HTX-011 (HTX-011B) at 200 mg (n=30) and 400 mg (n=31) to saline placebo (n=31). The findings for the 400 mg dose of HTX-011B as compared to placebo were as follows:
• There was a 29.5% reduction in pain as measured by SPI 0-24 (p=0.0035).
• Instillation (29.9% reduction in SPI 0-24) was equally as effective as injection (29.1% reduction in SPI 0-24).
• The pain reduction was long-lasting, with a statistically significant, 25.2% reduction through 48 hours (SPI 0-48; p=0.0250).
• Mean total opioid consumption decreased by 22.4% through 96 hours post-surgery.
• The number of patients who were opioid-free through 96 hours post-surgery was substantially higher (24.1% versus 6.5%).
The frequency of treatment-related adverse events reported in Study 202B was 38.7% in the HTX-011B 200 mg group, 33.3% in the HTX-011B 400 mg group and 51.6% in the placebo group. The most frequent treatment-related adverse events reported were nausea, headache and constipation.
Bunionectomy — Study 201 and Study 208
In September 2015, we reported positive, top-line results from Study 201, a randomized, placebo-controlled, double-blind, Phase 2 clinical trial of HTX-011 in patients undergoing bunionectomy. The primary and all key secondary endpoints in this study were achieved.
In August 2016, we reported preliminary positive, top-line efficacy results from the initial portion of Study 208, a randomized, placebo- and active-controlled, double-blind Phase 2 clinical study in patients undergoing bunionectomy. This study is evaluating the efficacy and safety of two formulations of HTX-011 at 200 mg compared to the standard dose of bupivacaine solution and placebo. Bupivacaine solution is the standard-of-care agent for the management of post-operative pain. In addition, HTX-011 is being evaluated when administered via Mayo Block (nerve block via closed wound injection) or infiltration (open wound injection).
The primary endpoint was the difference as compared to placebo in pain intensity as measured by SPI 0-24. Important secondary endpoints included comparison to bupivacaine solution, the time to first use of opioid rescue medication, total opioid
7
Table of Contents
consumption and difference in pain intensity compared to placebo or bupivacaine solution when administered as a Mayo Block or infiltration. The major findings for our Phase 3 formulation of HTX-011 were as follows:
• There was a 66% reduction in pain as measured by SPI 0-24 when comparing HTX-011 administered by infiltration to placebo (p<0.0001). There was a 64% reduction in pain as measured by SPI 0-24 when comparing HTX-011 administered by infiltration to bupivacaine solution (p<0.0001).
• There was a 69% reduction in pain as measured by SPI 0-24 when comparing HTX-011 administered by nerve block to placebo (p<0.0001). There was a 71% reduction in pain as measured by SPI 0-24 when comparing HTX-011 administered by nerve block to bupivacaine solution (p<0.0001).
• Significant reductions in pain were maintained through 96 hours post-surgery (SPI 0-96) for all groups: HTX-011 by infiltration versus placebo (p=0.005), HTX-011 by infiltration versus bupivacaine solution (p=0.019), HTX-011 by nerve block versus placebo (p=0.004), and HTX-011 by nerve block versus bupivacaine solution (p=0.007).
• Mean time to first opioid rescue medication was 716% longer than for placebo (p<0.0001) and 167% longer than for bupivacaine solution (p=0.037).
• Over the first 24 hours post-surgery, patients receiving HTX-011 consumed 74% less opioids than placebo patients (p<0.0001) and 67% less than bupivacaine solution patients (p<0.0001). Over the first 96 hours post-surgery, patients receiving HTX-011 consumed 53% less opioids than placebo patients (p=0.003) and 50% less than bupivacaine solution patients (p=0.008).
In January 2017, we reported additional data from Study 208 that establishes, for the first time, the synergy between the local anesthetic bupivacaine and the anti-inflammatory meloxicam in HTX-011. Utilizing our Biochronomer technology, HTX-011 has demonstrated a statistically significant benefit over each individual component alone, providing evidence for the synergistic activity of bupivacaine and meloxicam in the HTX-011 formulation. Further data from Study 208 demonstrates that a 60 mg dose of HTX-011 produced a statistically significant reduction in both pain and opioid use compared to a 50 mg dose of bupivacaine solution, further supporting the synergy observed with the two components of HTX-011.
Abdominoplasty — Study 203
In January 2017, we reported positive, topline results from Study 203, a randomized, placebo-controlled, dose-finding, Phase 2 clinical study evaluating the efficacy and safety of locally administered HTX-011 for post-operative anesthesia following abdominoplasty surgery. HTX-011 demonstrated statistically significant reductions in both pain intensity and the use of opioid rescue medications through 96 hours following surgery.
The SPI score through 96 hours post-surgery (SPI 0-96) was significantly reduced with HTX-011 and produced a statistically significant 36.6% reduction in pain through 96 hours following surgery, as measured by SPI 0-96 (p=0.0104). Pain was consistently reduced through 96 hours with statistically significant reductions observed between 24 to 48 hours (p=0.007), 48 to 72 hours (p=0.038), and 72 to 96 hours (p=0.016) after a single administration of HTX-011.
8
Table of Contents
Additionally, HTX-011 produced significant reductions (p=0.011) in the use of opioid rescue medication through 96 hours following abdominoplasty, as compared to placebo. To date, HTX-011 continues to be generally well-tolerated in Phase 2.
Biochronomer Technology
Our proprietary Biochronomer technology is designed to deliver therapeutic levels of a wide range of otherwise short-acting pharmacological agents over a period from days to weeks with a single subcutaneous injection. Our Biochronomer technology consists of bioerodible polymers that have been the subject of comprehensive animal and human toxicology studies that have shown evidence of the safety of the polymer. When injected into subcutaneous tissue, the polymers undergo controlled hydrolysis, resulting in a controlled, sustained release of the pharmacological agent encapsulated within the Biochronomer-based composition. Furthermore, our Biochronomer technology is designed to permit more than one pharmacological agent to be incorporated, such that multimodal therapy can be delivered with a single injection.
Sales and Marketing
Our U.S.-based sales and marketing team consists of 50 employees. The sales and marketing infrastructure includes a targeted, oncology sales force to establish relationships with a focused group of oncologists and oncology nurses. The sales force is supported by sales management, internal sales support, an internal marketing group and distribution support. Additionally, the sales and marketing teams manage relationships with key accounts, such as managed care organizations, group purchasing organizations, hospital systems, oncology group networks, and government accounts.
Customers
SUSTOL is distributed in the U.S. through a limited number of specialty distributors (“Customers”) that subsequently resell SUSTOL to healthcare providers, the end users of SUSTOL.
Sales to ASD Specialty Healthcare, Inc., Cardinal Health, Inc. and McKesson Specialty Care Distribution Corporation each accounted for 10% or more of our net revenues for the year ended December 31, 2016. The loss of any of these Customers could materially and adversely affect our business, results of operations, financial condition and cash flows. Due to the relatively short lead-time required to fill orders for our products, backlog of orders is not material to our business.
We have engaged a third-party service provider to act as our exclusive distributor for commercial shipment and distribution of our products to our Customers in the U.S. In addition to distribution services, other related services, including product storage, returns, customer support, and administrative support are provided.
Competition
The biotechnology and pharmaceutical industries are extremely competitive. Our potential competitors in the field are many in number and include major and mid-sized pharmaceutical and biotechnology companies. Many of our potential competitors have significantly more financial, technical and other resources than we do, which may give them a competitive advantage. In addition, they may have substantially more experience in effecting strategic combinations, in-licensing technology, developing drugs,
9
Table of Contents
obtaining regulatory approvals and manufacturing and marketing products. We cannot give any assurances that we can compete effectively with these other biotechnology and pharmaceutical companies. Any products that we may develop or discover will compete in highly competitive markets. Our potential competitors in these markets may succeed in developing products that could render our product candidates obsolete or non-competitive.
SUSTOL faces significant competition. Currently available 5-HT3 receptor antagonists include: ALOXI® (palonosetron, marketed by Eisai, Inc. in conjunction with Helsinn Healthcare S.A.); AKYNZEO® (palonosetron combined with the NK1 receptor antagonist netupitant, marketed by Eisai); SANCUSO® (granisetron transdermal patch, marketed by ProStrakan Group Plc); and generic products including ondansetron (formerly marketed by GlaxoSmithKline plc as ZOFRAN) and granisetron (formerly marketed by Hoffman-La Roche, Inc. as KYTRIL). Generic palonosetron is also likely to be available after September 2018. Currently, ALOXI is the only 5-HT3 receptor antagonist other than SUSTOL that is approved for the prevention of delayed CINV associated with MEC regimens. No 5-HT3 receptor antagonist is approved for the prevention of delayed CINV associated with HEC regimens. According to Transparency Market Research, sales of therapeutics for the prevention of CINV approximated $620 million in 2013, and sales of such therapeutics are expected to reach $1 billion in 2020.
NK1 receptor antagonists are also administered for the prevention of CINV, in combination with 5-HT3 receptor antagonists, to augment the therapeutic effect of the 5-HT3 receptor antagonist. If CINVANTI is approved by the FDA, it will face significant competition. Currently available NK1 receptor antagonists include: AKYNZEO® (palonosetron combined with the NK1 receptor antagonist netupitant, marketed by Eisai); EMEND® (aprepitant, marketed by Merck & Co, Inc.); EMEND® for Injection (fosaprepitant, marketed by Merck & Co); VARUBI® (rolapitant, marketed by Tesaro, Inc.) and potentially other products that include an NK1 receptor antagonist that reach the market. According to FactSet Research Systems, Inc., in 2015, Merck & Co.’s EMEND and EMEND for Injection generated U.S. sales of $535 million.
If we are able to successfully develop HTX-011 for the prevention of post-operative pain, we will compete with MARCAINE (bupivacaine; marketed by Hospira, Inc.) and generic forms of bupivacaine; NAROPIN (ropivacaine; marketed by Fresenius Kabi USA, LLC) and generic forms of ropivacaine; EXPAREL® (bupivacaine liposome injectable suspension; marketed by Pacira Pharmaceuticals, Inc.) and potentially other products in development for the prevention of post-operative pain that reach the market. According to Decision Resources Group, LLC, the addressable market for HTX-011 consisted of 23 million procedures annually in the U.S. in 2012 and is expected to increase to 29 million in 2021.
Manufacturing and Clinical Supplies
We do not own or operate manufacturing facilities for the production of clinical or commercial quantities of SUSTOL or any of our product candidates. We rely on a small number of third-party manufacturers to produce our compounds and expect to continue to do so to meet the preclinical and clinical requirements of our product candidates and for all of our commercial needs. We currently have long-term commercial supply agreements with certain third-party manufacturers. Our manufacturing and processing agreements require that all third-party contract manufacturers and processors produce active pharmaceutical ingredients and finished products in accordance with the FDA’s current Good Manufacturing Practices (“cGMP”) and all other applicable laws and regulations. We maintain confidentiality agreements with potential and existing manufacturers in order to protect our proprietary rights related to SUSTOL and our drug candidates.
Some of the critical materials and components used in manufacturing SUSTOL, CINVANTI and HTX-011 are sourced from single suppliers. An interruption in the supply of a key material could significantly delay our research and development process
10
Table of Contents
or increase our expenses for commercialization or development of products. Specialized materials must often be manufactured for the first time for use in drug delivery technologies, or materials may be used in the technologies in a manner that is different from their customary commercial uses. The quality of materials can be critical to the performance of a drug delivery technology, so a reliable source that provides a consistent supply of materials is important. Materials or components needed for our drug delivery technologies may be difficult to obtain on commercially reasonable terms, particularly when relatively small quantities are required or if the materials traditionally have not been used in pharmaceutical products.
Intellectual Property
Our success will depend in large part on our ability to:
• obtain and maintain international and domestic patents and other legal protections for the proprietary technology, inventions and improvements we consider important to our business;
• prosecute and defend our patents;
• preserve our trade secrets; and
• operate without infringing the patents and proprietary rights of other parties.
We intend to continue to seek appropriate patent protection for the product candidates in our research and development programs and their uses by filing patent applications in the United States and other selected countries. We intend for these patent applications to cover, where possible, claims for composition of matter, medical uses, processes for preparation and formulations.
We have filed a number of U.S. patent applications on inventions relating to the composition of a variety of polymers, specific products, product groups and processing technology. As of December 31, 2016, we had a total of 14 issued U.S. patents and an additional 48 issued (or registered) foreign patents. The patents on the bioerodible technologies expire between January 2017 and March 2026. Currently, SUSTOL is covered by 7 patents issued in the U.S. and by 27 patents issued in foreign countries including Austria, Belgium, Canada, Denmark, the EU, Finland, France, Germany, Greece, Ireland, Italy, Luxembourg, Netherlands, Portugal, Spain, Sweden, Switzerland, Taiwan, and the United Kingdom. U.S. patents covering SUSTOL have expiration dates ranging from May 2021 to September 2024; foreign patents covering SUSTOL have expiration dates ranging from May 2021 to September 2025. Our policy is to actively seek patent protection in the United States and to pursue equivalent patent claims in selected foreign countries, thereby seeking patent coverage for novel technologies and compositions of matter that may be commercially important to the development of our business.
Although we believe that our rights under patent applications we own provide a competitive advantage, the patent positions of pharmaceutical and biotechnology companies are highly uncertain and involve complex legal and factual questions. We may not be able to develop patentable products or processes, and may not be able to obtain patents from pending applications. Even if patent claims are allowed, the claims may not issue, or in the event of issuance, may not be sufficient to protect the technology owned by or licensed to us. Any patents or patent rights that we obtain may be circumvented, challenged or invalidated by our competitors.
11
Table of Contents
We also rely on trade secrets, proprietary know-how and continuing innovation to develop and maintain our competitive position. We seek protection of these trade secrets, proprietary know-how and any continuing innovation, in part, through confidentiality and proprietary information agreements. However, these agreements may not provide meaningful protection for, or adequate remedies to protect, our technology in the event of unauthorized use or disclosure of information. Furthermore, our trade secrets may otherwise become known to, or be independently developed by, our competitors.
Government Regulation
Pharmaceutical Regulation
Pharmaceutical products that we market in the U.S. are subject to extensive government regulation. Likewise, if we seek to market and distribute any such products abroad, they would also be subject to extensive foreign government regulation.
In the United States, the FDA regulates pharmaceutical products. FDA regulations govern the testing, research and development activities, manufacturing, quality, storage, advertising, promotion, labeling, sale and distribution of pharmaceutical products. Accordingly, there is a rigorous process for the approval of new drugs and ongoing oversight of marketed products. We are also subject to foreign regulatory requirements governing clinical trials and drug products if products are tested or marketed abroad. The approval process outside the United States varies from jurisdiction to jurisdiction and the time required may be longer or shorter than that required for FDA approval.
See Item 1A. Risk Factors of this Annual Report on Form 10-K for a discussion of the factors that could adversely impact our development of commercial products and industry regulation.
Regulation in the United States
The FDA testing and approval process requires substantial time, effort and money. The FDA approval process for new drugs includes, without limitation:
• preclinical studies;
• submission in the U.S. of an Investigational New Drug application (“IND”), for clinical trials conducted in the U.S.;
• adequate and well-controlled human clinical trials to establish safety and efficacy of the product;
• review of an NDA in the U.S.; and
• inspection of the facilities used in the manufacturing of the drug to assess compliance with the FDA’s current cGMP regulations.
The FDA monitors the progress of trials conducted in the U.S. under an IND and may, at its discretion, re-evaluate, alter, suspend or terminate testing based on the data accumulated to that point and the FDA’s risk/benefit assessment with regard to the patients enrolled in the trial. The FDA may also place a hold on one or more clinical trials conducted under an IND for a drug if it deems warranted. Furthermore, even after regulatory approval of an NDA is obtained, under certain circumstances, such as later discovery of previously unknown problems, the FDA can withdraw approval or subject the drug to additional restrictions.
12
Table of Contents
Preclinical Testing
Preclinical studies include laboratory evaluation of the product and animal studies to assess the potential safety and effectiveness of the product. Most of these studies must be performed according to Good Laboratory Practices, a system of management controls for laboratories and research organizations to ensure the consistency and reliability of results.
An IND is the request for authorization from the FDA to administer an investigational new drug product to humans. The IND includes information regarding the preclinical studies, the investigational product’s chemistry and manufacturing, supporting data and literature and the investigational plan and protocol(s). Clinical trials may begin 30 days after an IND is received, unless the FDA raises concerns or questions about the conduct of the clinical trials. If concerns or questions are raised, an IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can proceed. An IND must become effective before human clinical trials begin. We have filed INDs in the U.S. and Clinical Trial Applications (“CTAs”) in the European Union (the “EU”), and we may file additional INDs and CTAs in the future. We cannot assure that submission of any additional INDs or CTAs for any of our product candidates will result in authorization to commence clinical trials.
Clinical Trials
Clinical trials involve the administration of the product candidate that is the subject of the trial to volunteers or patients under the supervision of a qualified principal investigator and in accordance with a clinical trial protocol, which sets forth details, such as the study objectives, enrollment criteria and the safety and effectiveness criteria to be evaluated. Each clinical trial must be reviewed and approved by an independent institutional review board (“IRB”) in the U.S. or ethics committee in the EU at each institution at which the study will be conducted. The IRB or ethics committee will consider, among other things, ethical factors, safety of human subjects and the possible liability of the institution arising from the conduct of the proposed clinical trial. In addition, clinical trials in the U.S. must be performed according to good clinical practices, which are enumerated in FDA regulations and guidance documents. Some studies include oversight by an independent group of experts, known as a data safety monitoring board, which authorizes whether a study may move forward based on certain data from the study and may stop the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or it may impose other conditions.
Clinical trials in the U.S. typically are conducted in sequential phases: Phases 1, 2, 3 and 4. The phases may overlap. The FDA may require that we suspend clinical trials at any time on various grounds, including if the FDA makes a finding that the subjects participating in the trial are being exposed to an unacceptable health risk.
In Phase 1 clinical trials, the investigational product is usually tested on a small number of healthy volunteers to determine safety, any adverse effects, proper dosage, absorption, metabolism, distribution, excretion and other drug effects. Follow-on Phase 1b clinical trials may also evaluate efficacy with respect to trial participants.
In Phase 2 clinical trials, the investigational product is usually tested on a limited number of patients (generally up to several hundred) to preliminarily evaluate the efficacy of the drug for specific, targeted indications, to determine dosage tolerance and
13
Table of Contents
optimal dosage, and to identify possible adverse effects and safety risks. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning Phase 3 clinical trials.
In Phase 3 clinical trials, the investigational product is administered to an expanded patient population to confirm proof of concept and efficacy claims, provide evidence of clinical efficacy and to further test for safety, generally at multiple clinical sites.
In Phase 4 clinical trials or other post-approval commitments, additional studies and patient follow-up are conducted to gain experience from the treatment of patients in the intended therapeutic indication. The FDA may require a commitment to conduct post-approval Phase 4 studies as a condition of approval. Additional studies and follow-up may be conducted to document a clinical benefit where drugs are approved under accelerated approval regulations and based on surrogate endpoints. In clinical trials, surrogate endpoints are alternative measurements of the symptoms of a disease or condition that are substituted for measurements of observable clinical symptoms. Failure to timely conduct of Phase 4 clinical trials and follow-up could result in withdrawal of approval for products approved under accelerated approval regulations.
Clinical Data Review and Approval in the U.S.
The data from the clinical trials, together with preclinical data and other supporting information that establishes a drug candidate’s safety, are submitted to the FDA in the form of an NDA, or NDA supplement (for approval of a new indication if the product candidate is already approved for another indication). Under applicable laws and FDA regulations, the FDA reviews the NDA within 60 days of receipt of the NDA to determine whether the application will be accepted for filing based on the FDA’s threshold determination that the NDA is sufficiently complete to permit substantive review. If deemed complete, the FDA will “file” the NDA, thereby triggering substantive review of the application. The FDA can refuse to file any NDA that it deems incomplete or not properly reviewable.
The FDA has established internal substantive review goals of ten months for most NDAs. The FDA has various programs, including Fast Track, priority review, and accelerated approval, which are intended to expedite or simplify the process for reviewing drugs, and/or provide for approval based on surrogate endpoints. Even if a drug qualifies for one or more of these programs, the FDA may later decide that the drug no longer meets the conditions for qualification or that the period for FDA review or approval will not be shortened. Generally, drugs that may be eligible for these programs are those for serious or life-threatening conditions, those with the potential to address unmet medical needs, and those that offer meaningful benefits over existing treatments. For example, Fast Track is a process designed to facilitate the development, and expedite the review, of drugs to treat serious diseases and fill an unmet medical need. The request may be made at the time of IND submission and generally no later than the pre-NDA meeting. The FDA will respond within 60 calendar days of receipt of the request. Priority review, which is requested at the time of an NDA submission, is designed to give drugs that offer major advances in treatment or provide a treatment where no adequate therapy exists, an initial review within six months as compared to a standard review time of ten months. Although Fast Track and priority review do not affect the standards for approval, the FDA will attempt to facilitate early and frequent meetings with a sponsor of a Fast Track designated drug and expedite review of the application for a drug designated for priority review. Accelerated approval provides an expedited approval of drugs that treat serious diseases and that fill an unmet medical need based on a surrogate endpoint. The FDA, however, is not legally required to complete its review within these periods, and these performance goals may change over time.
14
Table of Contents
If the FDA approves the NDA, it will issue an approval letter authorizing the commercial marketing of the drug with prescribing information for specific indications. As a condition of NDA approval, the FDA may require a risk evaluation and mitigation strategy (“REMS”), to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use. Additionally, the FDA will inspect the facility or the facilities at which the drug is manufactured. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing. In many cases, the outcome of the review, even if generally favorable, is not an actual approval, but a “complete response” that generally outlines the deficiencies in the submission, which may require substantial additional testing or information before the FDA will reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter.
Satisfaction of FDA requirements or similar requirements of state, local and foreign regulatory agencies typically takes several years and requires the expenditure of substantial financial resources. Information generated in this process is susceptible to varying interpretations that could delay, limit or prevent regulatory approval at any stage of the process. Accordingly, the actual time and expense required to bring a product to market may vary substantially. Data obtained from clinical activities is not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. Success in early stage clinical trials does not ensure success in later stage clinical trials. Even if a product candidate receives regulatory approval, the approval may be significantly limited to specific disease states, patient populations and dosages, or have conditions placed on it that restrict the commercial applications, advertising, promotion or distribution of these products.
Once issued, the FDA may withdraw product approval if ongoing regulatory standards are not met or if safety problems occur after the product reaches the market. In addition, the FDA may require testing and surveillance programs to monitor the safety or effectiveness of approved products which have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs. The FDA may also request or require additional Phase 4 clinical trials after a product is approved. The results of Phase 4 clinical trials can confirm the effectiveness of a product candidate and can provide important safety information to augment the FDA’s voluntary adverse drug reaction reporting system. Any products manufactured or distributed by us pursuant to FDA approvals would be subject to continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the drug. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMPs, which impose certain procedural and documentation requirements upon us and our third-party manufacturers.
In addition, both before and after approval is sought, we are required to comply with a number of FDA requirements. For example, we are required to report certain adverse reactions and production problems, if any, to the FDA, and to comply with certain limitations and other requirements concerning advertising and promotion for our products. In addition, quality control and manufacturing procedures must continue to conform to cGMP after approval, and the FDA periodically inspects manufacturing facilities to assess compliance with continuing cGMP. In addition, discovery of problems, such as safety problems, may result in changes in labeling or restrictions on a product manufacturer or NDA holder, including removal of the product from the market.
15
Table of Contents
The FDA closely regulates the marketing and promotion of drugs. Approval may be subject to post-marketing surveillance and other record-keeping and reporting obligations, and involve ongoing requirements. Product approvals may be withdrawn if compliance with regulatory standards is not maintained or if problems occur following initial marketing. A company can make only those claims relating to safety and efficacy that are approved by the FDA. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties.
Clinical Trial Conduct and Product Approval Regulation in Non-U.S. Jurisdictions
In addition to regulations in the U.S., we may be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Our clinical trials conducted in the EU must be done under an Investigational Medicinal Product Dossier, and the oversight of an ethics committee. If we market our products in foreign countries, we also will be subject to foreign regulatory requirements governing marketing approval for pharmaceutical products. The requirements governing the conduct of clinical trials, product approval, pricing and reimbursement vary widely from country to country. Whether or not FDA approval has been obtained, approval of a product by the comparable regulatory authorities of foreign countries must be obtained before manufacturing or marketing the product in those countries. The approval process varies from country to country and the time required for such approvals may differ substantially from that required for FDA approval. There is no assurance that any future FDA approval of any of our product candidates will result in similar foreign approvals or vice versa. The process for clinical trials in the EU is similar, and trials are heavily scrutinized by the designated ethics committee.
Section 505(b)(2) Applications
Some of our product candidates may be eligible for submission of applications for approval under the FDA’s Section 505(b)(2) approval process, which provides an alternate path to FDA approval for new or improved formulations or new uses of previously approved products. Section 505(b)(2) was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Act, and allows approval of NDAs that rely, at least in part, on studies that were not conducted by or for the applicant and to which the applicant has not obtained a right of reference. Such studies can be provided by published literature, or the FDA can rely on previous findings of safety and efficacy for a previously approved drug. If the 505(b)(2) applicant can establish that reliance on the FDA’s previous approval is scientifically appropriate, it may eliminate the need to conduct certain preclinical studies or clinical trials of the new product. Section 505(b)(2) applications may be submitted for drug products that represent a modification (e.g., a new indication or new dosage form) of an eligible approved drug. In such cases, the additional information in 505(b)(2) applications necessary to support the change from the previously approved drug is frequently provided by new studies submitted by the applicant. Because a Section 505(b)(2) application relies in part on previous studies or previous FDA findings of safety and effectiveness, preparing 505(b)(2) applications is generally less costly and time-consuming than preparing an NDA based entirely on new data and information from a full set of clinical trials. The FDA may approve the new product candidate for all, or some, of the label indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant. The law governing Section 505(b)(2) or FDA’s current policies may change in such a way as to adversely affect our applications for approval that seek to utilize the Section 505(b)(2) approach. Such changes could result in additional costs associated with additional studies or clinical trials and delays.
16
Table of Contents
The FDA provides that reviews and/or approvals of applications submitted under Section 505(b)(2) will be delayed in various circumstances. For example, the holder of the NDA for the listed drug may be entitled to a period of market exclusivity during which the FDA will not approve, and may not even review, a Section 505(b)(2) application from other sponsors. If the listed drug is claimed by one or more patents that the NDA holder has listed with the FDA, the Section 505(b)(2) applicant must submit a certification with respect to each such patent. If the 505(b)(2) applicant certifies that a listed patent is invalid, unenforceable or not infringed by the product that is the subject of the Section 505(b)(2) application, it must notify the patent holder and the NDA holder. If, within 45 days of providing this notice, the NDA holder sues the 505(b)(2) applicant for patent infringement, the FDA will not approve the Section 505(b)(2) application until the earlier of a court decision favorable to the Section 505(b)(2) applicant or the expiration of 30 months. The regulations governing marketing exclusivity and patent protection are complex, and it is often unclear how they will be applied in particular circumstances.
Drug Enforcement Agency Regulation
Our research and development processes involve the controlled use of hazardous materials, including chemicals. Some of these hazardous materials are considered to be controlled substances and subject to regulation by the U.S. Drug Enforcement Agency (“DEA”). Controlled substances are those drugs that appear on one of five schedules promulgated and administered by the DEA under the Controlled Substances Act (“CSA”). The CSA governs, among other things, the distribution, recordkeeping, handling, security and disposal of controlled substances. We must be registered by the DEA in order to engage in these activities, and we are subject to periodic and ongoing inspections by the DEA and similar state drug enforcement authorities to assess ongoing compliance with the DEA’s regulations. Any failure to comply with these regulations could lead to a variety of sanctions, including the revocation, or a denial of renewal, of the DEA registration, injunctions or civil or criminal penalties.
Third-Party Payor Coverage and Reimbursement
Commercial success of SUSTOL and any of our other product candidates that are approved or commercialized for any indication will depend, in part, upon the availability of coverage and reimbursement from third-party payors at the federal, state and private levels. Government payor programs, including Medicare and Medicaid, private health care insurance companies and managed care plans have attempted to control costs by limiting coverage and the amount of reimbursement for particular procedures or drug treatments. The U.S. Congress and state legislatures, from time to time, propose and adopt initiatives aimed at cost containment. Ongoing federal and state government initiatives directed at lowering the total cost of health care will likely continue to focus on health care reform, the cost of prescription pharmaceuticals and on the reform of the Medicare and Medicaid payment systems. Examples of how limits on drug coverage and reimbursement in the United States may cause reduced payments for drugs in the future include:
• changing Medicare reimbursement methodologies;
• fluctuating decisions on which drugs to include in formularies;
• revising drug rebate calculations under the Medicaid program or requiring that new or additional rebates be provided to Medicare, Medicaid and other federal or state healthcare programs; and
17
Table of Contents
• reforming drug importation laws.
Some third-party payors also require pre-approval of coverage for new drug therapies before they will reimburse health care providers that use such therapies. While we cannot predict whether any proposed cost-containment measures will be adopted or otherwise implemented in the future, the announcement or adoption of these proposals could have a material adverse effect on our ability to obtain adequate prices for our product candidates and to operate profitably.
Reimbursement systems in international markets vary significantly by country and, within some countries, by region. Reimbursement approvals must be obtained on a country-by-country basis. In many foreign markets, including markets in which we hope to sell our products, the pricing of prescription pharmaceuticals is subject to government pricing control. In these markets, once marketing approval is received, pricing negotiations could take significant additional time. As in the United States, the lack of satisfactory reimbursement or inadequate government pricing of any of our products would limit widespread use and lower potential product revenues.
Anti-Kickback, Fraud and Abuse and False Claims Regulation
We are subject to health care fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business. Healthcare providers, physicians and third-party payors play a primary role in the recommendation and prescription of SUSTOL and any other product candidates for which we obtain marketing approval. Arrangements with third-party payors and customers may expose us to applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval.
Regulations under applicable federal and state healthcare laws and regulations include the federal health care programs’ Anti-Kickback Law, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral or purchase of any good or service for which payment may be made under federal health care programs such as the Medicare and Medicaid programs. Remuneration has been broadly defined to include anything of value, including cash, improper discounts, and free or reduced price items and services. Many states have similar laws that apply to their state health care programs as well as private payors. In addition, the False Claims Act (“FCA”) imposes liability on persons who, among other things, present or cause to be presented false or fraudulent claims for payment by a federal health care program. The FCA has been used to prosecute persons submitting claims for payment that are inaccurate or fraudulent, that are for services not provided as claimed, or for services that are not medically necessary. Actions under the FCA may be brought by the Attorney General or as a qui tam action by a private individual in the name of the government. Violations of the FCA can result in significant monetary penalties and treble damages. The federal government is using the FCA, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical and biotechnology companies throughout the country, for example, in connection with the promotion of products for unapproved uses and other sales and marketing practices.
The risk of being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Moreover, recent health care reform legislation has strengthened many of these laws. For example, the Patient Protection and Affordable Care Act
18
Table of Contents
(“PPACA”), among other things, amends the intent requirement of the federal anti-kickback and criminal health care fraud statutes to clarify that a person or entity does not need to have actual knowledge of this statute or specific intent to violate it. In addition, PPACA provides that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes.
The continuing interpretation and application of these laws could have a material adverse impact on our business and our ability to compete should we commence marketing a product.
Federal and State Sunshine Laws
We must comply with federal “sunshine” laws, now known as Open Payments that require transparency regarding financial arrangements with health care providers. This would include the reporting and disclosure requirements imposed by the PPACA on drug manufacturers regarding any “payment or transfer of value” made or distributed to physicians and teaching hospitals. Failure to submit required information can result in civil monetary penalties. A number of states have laws that require the implementation of commercial compliance programs, impose restrictions on drug manufacturer marketing practices and/or require pharmaceutical companies to track and report payments, gifts and other benefits provided to physicians and other health care professionals and entities.
Foreign Corrupt Practices Act
We are subject to the Foreign Corrupt Practices Act of 1997 (“FCPA”). The FCPA and other similar anti-bribery laws in other jurisdictions, such as the U.K. Bribery Act, generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more frequent and aggressive investigations and enforcement proceedings by both the Department of Justice and the U.S. Securities and Exchange Commission (“SEC”). A determination that our operations or activities are not, or were not, in compliance with United States or foreign laws or regulations could result in the imposition of substantial fines, interruptions of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits and other legal or equitable sanctions. Other internal or government investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence.
Patient Privacy and Data Security
We are required to comply, as applicable, with numerous federal and state laws, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, and to govern the collection, use and disclosure of personal information. Other countries also have, or are developing, laws governing the collection, use and transmission of personal information. In addition, most healthcare providers who prescribe SUSTOL or who may prescribe other products we may sell in the future and from whom we may obtain patient health information are subject to privacy and security requirements under the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), as amended by the Health Information Technology and Clinical Health Act, and its implementing regulations. We are not a HIPAA covered entity, do not intend to become one, and we do not operate as a business associate to any covered entities. Therefore, these privacy and security requirements do not apply to us. However, we could be subject to civil and criminal penalties if we knowingly obtain individually identifiable health information from a covered entity in a manner that is not authorized or permitted by HIPAA
19
Table of Contents
or for aiding and abetting the violation of HIPAA. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing amount of focus on privacy and data protection issues with the potential to affect our business, including recently enacted laws in a majority of states requiring security breach notification. These laws could create liability for us or increase our cost of doing business, and any failure to comply could result in harm to our reputation, and potentially fines and penalties.
In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Environmental, Health and Safety Laws
Our operations are subject to complex and increasingly stringent environmental, health and safety laws and regulations. Further, in the future, we may open manufacturing facilities that would likely be subject to environmental and health and safety authorities in the relevant jurisdictions. These authorities typically administer laws which regulate, among other matters, the emission of pollutants into the air (including the workplace), the discharge of pollutants into bodies of water, the storage, use, handling and disposal of hazardous substances, the exposure of persons to hazardous substances, and the general health, safety and welfare of employees and members of the public. Violations of these laws could subject us to strict liability, fines or liability to third parties.
Other Laws
We are subject to a variety of financial disclosure and securities trading regulations as a public company in the U.S., including laws relating to the oversight activities of the SEC and the regulations of The NASDAQ Stock Exchange, on which our shares are traded. We are also subject to various laws, regulations and recommendations relating to safe working conditions, laboratory practices and the experimental use of animals.
Employees
As of February 3, 2017, we had 139 full-time employees; 70 are involved in research and development activities, 50 are involved in sales and marketing activities, and 19 are involved in administration, human resources, finance, legal and information technology. We believe that we are able to attract skilled and experienced personnel, but competition for personnel is intense and there can be no assurance that we will be able to attract and retain the individuals needed. None of our employees are covered by a collective bargaining agreement and management considers relations with our employees to be good.
Company Information
We were founded in February 1983 as a California corporation under the name AMCO Polymerics, Inc. (“AMCO”). AMCO changed its name to Advanced Polymer Systems, Inc. (“APS”) in 1984 and was reincorporated in the state of Delaware in 1987. APS changed its name to A.P. Pharma, Inc. (“APP”) in May 2001. In January 2014, APP changed its name to Heron Therapeutics, Inc.
We have executive offices located at 4242 Campus Point Court, Suite 200, San Diego, CA 92121. Our telephone number is (858) 251-4400. Our website address is www.herontx.com. We make our periodic and current reports available on our web-
20
Table of Contents
site, free of charge, as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. No portion of our website is incorporated by reference into this Annual Report on Form 10-K. We file our annual, quarterly and special reports, proxy statements and other information with the SEC. You may read and copy any document filed by us at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference room. Our filings with the SEC are also available to the public on the SEC’s website at http://www.sec.gov. Additional information regarding us, including our audited financial statements and descriptions of our business, is contained in the documents incorporated by reference in this Annual Report on Form 10-K. Our common stock is traded on The NASDAQ Capital Market under the symbol HRTX.
21
Table of Contents
You should carefully consider the following information about risks and uncertainties that may affect us or our business, together with the other information appearing elsewhere in this Annual Report on Form 10-K. If any of the following events, described as risks, actually occur, our business, financial condition, results of operations and future growth prospects would likely be materially and adversely affected. In these circumstances, the market price of our common stock could decline, and you may lose all or part of your investment in our securities. An investment in our securities is speculative and involves a high degree of risk. You should not invest in our securities if you cannot bear the economic risk of your investment for an indefinite period of time and cannot afford to lose your entire investment.
Risks Related to Our Business
We are substantially dependent upon the success of SUSTOL, and, if SUSTOL does not attain market acceptance by healthcare professionals and patients, our business and results of operations will suffer.
The success of our business is substantially dependent upon our ability to commercialize our recently approved product, SUSTOL. Although members of our management team have prior experience launching new drugs, SUSTOL is the first product that the Company has launched.
Further, even if our sales organization performs as expected, the revenue that we may receive from the sales of SUSTOL may be less than anticipated due to factors that are outside of our control. These factors that may affect revenue include:
• the scope of our approved product label;
• the perception of physicians and other members of the health care community of the safety and efficacy and cost-competitiveness relative to that of competing products;
• our ability to maintain successful sales, marketing and educational programs for certain physicians and other health care providers;
• our ability to raise patient and physician awareness of CINV associated with AC combination chemotherapy regimens or MEC and encourage physicians to look for incidence of CINV among patients;
• the cost-effectiveness of our product;
• acceptance by institutional formulary committees;
• patient and physician satisfaction with our product;
• the size of the potential market for our product;
• our ability to obtain adequate reimbursement from government and third-party payors;
22
Table of Contents
• unfavorable publicity concerning our product or similar products;
• the introduction, availability and acceptance of competing treatments;
• adverse event information relating to our product or similar classes of drugs;
• product liability litigation alleging injuries relating to the product or similar classes of drugs;
• our ability to maintain and defend our patents for SUSTOL;
• our ability to have SUSTOL manufactured at a commercial production level successfully and on a timely basis;
• the availability of raw materials;
• our ability to access third parties to manufacture and distribute our product on acceptable terms or at all;
• regulatory developments related to the manufacture or continued use of our product;
• any post-approval study requirements and the results thereof;
• the extent and effectiveness of sales and marketing and distribution support for our product;
• our competitors’ activities, including decisions as to the timing of competing product launches, generic entrants, pricing and discounting; and
• any other material adverse developments with respect to the potential commercialization of our product.
Our business will be adversely affected if, due to these or other factors, our commercialization of SUSTOL does not achieve the acceptance and demand necessary to sustain revenue growth. If we are unable to successfully commercialize SUSTOL, our business and results of operations will suffer.
If we are unable to develop and maintain sales, marketing and distribution capabilities or enter into agreements with third parties to sell and market SUSTOL or any other products we may develop, our product sales may be hindered.
We have established an internal sales organization for the sale, marketing and distribution of SUSTOL. In order to successfully commercialize any other product we may develop, we must increase our sales, marketing, distribution and other non-technical capabilities or make arrangements with third parties to perform these services. The development of a sales organization to market SUSTOL, or any other product we may develop, is expensive and time consuming, and we cannot be certain that we will be able to successfully develop this capacity or that this function will execute as expected. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and our business and results of operations will suffer.
23
Table of Contents
If we cannot establish satisfactory pricing of SUSTOL or any other products we may develop that is also acceptable to the U.S. government, insurance companies, managed care organizations and other payors, or arrange for favorable reimbursement policies, our product sales may be hindered and our future revenue may suffer.
The continuing efforts of the U.S. government, insurance companies, managed care organizations and other payors of health care costs to contain or reduce costs of health care may adversely affect our ability to generate adequate revenues and gross margins to make SUSTOL or any other product we may develop commercially viable. Our ability to commercialize SUSTOL or any other product we may develop successfully will depend in part on the extent to which governmental authorities, private health insurers and other organizations establish appropriate reimbursement levels for the cost of such products and related treatments and for what uses reimbursement will be provided.
Adoption of SUSTOL or any other product we may develop by the medical community may be limited if third-party payers will not offer adequate coverage. In addition, third-party payors often challenge the price and cost-effectiveness of medical products and services and such pressure may increase in the future. In many cases, uncertainty exists as to the adequate reimbursement status of newly approved healthcare products. Accordingly, SUSTOL or any other product we may develop may not be reimbursable by certain third-party payors at the time of commercial launch and potentially for an extended period of time thereafter. In addition, products may not be considered cost-effective and adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize a profit.
Legislation and regulations affecting the pricing of pharmaceuticals may change and any such changes could further limit reimbursement. Cost control initiatives may decrease coverage and payment levels for SUSTOL or any other product we may develop and, in turn, the reimbursement that we receive. We are unable to predict all changes to the coverage or reimbursement methodologies that will be applied by private or government payers to SUSTOL or any other product we may develop. If SUSTOL or any other products we develop do not receive adequate reimbursement, our revenue could be severely limited.
In the U.S., given recent federal and state government initiatives directed at lowering the total cost of health care, the U.S. Congress and state legislatures will likely continue to focus on health care reform, reducing the cost of prescription pharmaceuticals and reforming the Medicare and Medicaid systems. For example, the 2010 Patient Protection and Affordable Care Act (“PPACA”) encourages comparative effectiveness research. Any adverse findings for our products from such research may negatively impact reimbursement available for our products. Economic pressure on state budgets may result in states increasingly seeking to achieve budget savings through mechanisms that limit coverage or payment for drugs. State Medicaid programs are increasingly asking manufacturers to pay supplemental rebates and requiring prior authorization by the state program for use of any drug for which supplemental rebates are not being paid. Further, the trend toward managed health care in the U.S., which could significantly influence the purchase of health care services and products, may result in lower prices for SUSTOL or any other product we may develop for marketing. While we cannot predict whether any legislative or regulatory proposals affecting our business will be adopted, the announcement or adoption of these proposals could have a material and adverse effect on our potential revenues and gross margins.
24
Table of Contents
If we fail to comply with our reporting and payment obligations under U.S. governmental pricing and contracting programs, we could be subject to additional reimbursement requirements, penalties and fines, which could have a material adverse effect on our business, financial condition, and results of operations.
The Medicare program and certain government pricing programs, including the Medicaid drug rebate program, the Public Health Services’ 340B drug pricing program, and the pricing program under the Veterans Health Care Act of 1992 impact the reimbursement we may receive from sales of SUSTOL, or any other products that are approved for marketing. Pricing and rebate calculations vary among programs. The calculations are complex and are often subject to interpretation by manufacturers, governmental or regulatory agencies, and the courts. We are required to submit a number of different pricing calculations to government agencies on a quarterly basis. Failure to comply with our reporting and payment obligations under U.S. governmental pricing and contracting programs may result in additional payments, penalties and fines due to government agencies, which may have a material adverse effect on our business, financial condition and results of operations.
Because the results of preclinical studies and clinical trials are not necessarily predictive of future results, we can provide no assurances that HTX-011 or any other of our product candidates will have favorable results in future studies or trials or receive regulatory approval.
Positive results from preclinical studies or clinical trials should not be relied upon as evidence that later or larger-scale studies or trials will succeed. Even if our product candidates achieve positive results in early-stage preclinical studies or clinical trials, we will be required to demonstrate that these product candidates are safe and effective for use in Phase 3 clinical trials before we can seek regulatory approvals for their commercial sale. Even if our early-stage preclinical studies or clinical trials achieve the specified endpoints, the FDA may determine that these data are not sufficient to allow the commencement of Phase 3 clinical trials. There is an extremely high historical rate of failure of product candidates proceeding through clinical trials in our industry. There is no guarantee that the efficacy of any product candidate, including HTX-011, shown in early patient studies will be replicated or maintained in future studies of longer duration and/or larger patient populations. Similarly, favorable safety and tolerability data seen in short-term studies might not be replicated in studies of longer duration and/or larger patient populations. If any product candidate demonstrates insufficient safety or efficacy in any preclinical study or clinical trial, we would experience potentially significant delays in, or be required to abandon, development of that product candidate. If we delay or abandon our efforts to develop any of our product candidates, we may not be able to generate sufficient revenues to become profitable, and our reputation in the industry and in the investment community would likely be significantly damaged, each of which would cause our stock price to decrease significantly.
Our product platforms or product development efforts may not produce safe, efficacious or commercially viable products, and, if we are unable to develop new products, our business may suffer.
Our long-term viability and growth will depend upon the successful development of products through our research and development activities. Product development is very expensive and involves a high degree of risk. Only a small number of research and development programs result in the commercialization of a product. Success in preclinical work or early-stage clinical trials does not ensure that later-stage or larger-scale clinical trials will be successful. Our ability to complete our clinical trials in a timely fashion depends in large part on a number of key factors, including protocol design, regulatory and institutional review board approval, the rate of patient enrollment in clinical trials and compliance with extensive current good clinical practices (“cGCP”).
25
Table of Contents
In addition, because we fund the development of our product candidates, we may not be able to continue to fund all such development efforts to completion or to provide the support necessary to perform the clinical trials, obtain regulatory approvals, or market any approved products. If our drug delivery technologies or product development efforts fail to result in the successful development and commercialization of product candidates, or if our new products do not perform as anticipated, such events could materially adversely affect our business, financial condition, cash flows and results of operations.
We rely on third parties to conduct our preclinical testing and conduct our clinical trials, and their failure to perform their obligations in a timely and competent manner may delay development and commercialization of our product candidates and our business could be substantially harmed.
We have used contract research organizations (“CROs”) to oversee our clinical trials for SUSTOL, CINVANTI, and HTX-011, and we expect to use the same or similar organizations for our future clinical trials and pipeline programs. There can be no assurance that these CROs will perform their obligations at all times in a competent or timely fashion, and we must rigorously oversee their activities in order to be confident in their conduct of these trials on our behalf. If the CROs fail to commit resources to our product candidates, our clinical programs related to our product candidates could be delayed, terminated or unsuccessful, and we may not be able to obtain regulatory approval for, or successfully commercialize, them. Different cultural and operational issues in foreign countries could cause delays or unexpected problems with patient enrollment or with the data obtained from those locations. If we experience significant delays in the progress of our clinical trials or experience doubts with respect to the quality of data derived from our clinical trials, we could face significant delays in gaining necessary product approvals.
We also rely on third parties to assist in conducting our preclinical studies in accordance with Good Laboratory Practices and the Animal Welfare Act requirements. We, our CROs, and other third parties are required to comply with cGCP, which are regulations and guidelines enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area and comparable foreign regulatory authorities. Regulatory authorities enforce cGCP through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of our CROs fail to comply with applicable cGCP, the clinical data generated in the clinical trials may be deemed unreliable, and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot be certain that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our ongoing or future clinical trials comply with cGCP. In addition, all of our clinical trials must be conducted with product produced under cGCP. Failure to comply with these regulations may require us to repeat preclinical and clinical trials, which would delay the regulatory approval process.
Our CROs and other third parties we may engage to support our development programs are not our employees, and, except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing clinical, nonclinical and preclinical programs. Outsourcing these functions involves risk that third parties may not perform to our standards, may not produce results in a timely manner, or may fail to perform at all. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines or if the quality or accuracy of the preclinical results or clinical data they obtain is compromised due to the failure to adhere to test requirements, our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
26
Table of Contents
If our suppliers and contract manufacturers are unable to manufacture in commercially viable quantities, we could face delays in our ability to commercialize SUSTOL or any other products we may develop, our costs will increase and our product sales may be severely hindered.
If in the future any of our product candidates are approved for commercial sale, we will need to be able to consistently manufacture our products in larger quantities and be able to show equivalency to the FDA in the manufacture of our products at commercial scale as compared to development batch size. The commercial success of our products will be dependent upon the ability of our contract manufacturers to produce a product in commercial quantities at competitive costs of manufacture in a process that is validated by the FDA. We have scaled-up manufacturing for SUSTOL in order to realize important economies of scale, and these activities took time to implement, required additional capital investment, process development and validation studies and regulatory approval. We cannot guarantee that we will be successful in achieving competitive manufacturing costs through such scaled-up activities.
The manufacture of pharmaceutical products is a highly complex process in which a variety of difficulties may arise from time to time, including product loss due to material failure, equipment failure, vendor error, operator error, labor shortages, inability to obtain material, equipment or transportation, physical or electronic security breaches and natural disasters. Problems with manufacturing processes could result in product defects or manufacturing failures, which could require us to delay shipment of products or recall products previously shipped, or could impair our ability to expand into new markets or supply products in existing markets. We may not be able to resolve any such problems in a timely manner, if at all.
We expect to depend on third-party suppliers and contract manufacturers for manufacturing SUSTOL, as well as any future products that we develop; if our contract manufacturers do not perform as expected, our business could suffer.
We do not own or operate manufacturing facilities for the production of clinical or commercial quantities of any product, including SUSTOL. Our ability to successfully commercialize SUSTOL, as well as any other products or product candidates that we may develop will depend in part on our ability to arrange for other parties to manufacture our products at a competitive cost, in accordance with regulatory requirements, and in sufficient quantities for clinical testing and eventual commercialization. We currently rely on a small number of third-party manufacturers to produce compounds used in our product development activities and expect to continue to do so to meet the preclinical and clinical requirements of our potential products and for all of our commercial needs. Certain contract manufacturers are, at the present time (and are expected to be for the foreseeable future), our sole resource to manufacture certain key components of SUSTOL, as well as key components for product candidates in clinical and preclinical testing in our research and development program. Although we entered into a single-source, long-term commercial manufacturing agreement for the manufacture of SUSTOL and have a long-term agreement for the manufacture of our Biochronomer technology, we may not be able to successfully negotiate long-term agreements with any additional third parties and thereby reduce or remove our dependence on a single supplier. We may have difficulties with these manufacturer relationships, and we may not be able to find replacement contract manufacturers on satisfactory terms or on a timely basis. Also, due to regulatory and technical requirements, we may have limited ability to shift production to a different third party should the need arise. We cannot be certain that we could reach agreement on reasonable terms, if at all, with such a manufacturer. Even if we were to reach agreement, the transition of the manufacturing process to a different third party could take a significant amount of time and money, and may not be successful.
27
Table of Contents
Further, we, along with our contract manufacturers, are required to comply with FDA requirements related to product testing, quality assurance, manufacturing and documentation. Our contract manufacturers may not be able to comply with the applicable FDA regulatory requirements. They may be required to pass an FDA preapproval inspection for conformity with cGMPs before we can obtain approval to manufacture our products and will be subject to ongoing, periodic, unannounced inspection by the FDA and corresponding state agencies to ensure strict compliance with cGMP, and other applicable government regulations and corresponding foreign standards. If we and our contract manufacturers fail to achieve and maintain high manufacturing standards in compliance with cGMP, or fail to scale-up manufacturing processes in a timely manner, we may experience manufacturing errors resulting in defective products that could be harmful to patients, product recalls or withdrawals, delays or interruptions of production or failures in product testing or delivery, delay or prevention of filing or approval of marketing applications for our products, cost overruns or other problems that could seriously harm our business. Not complying with FDA requirements could result in an enforcement action, such as a product recall, or prevent commercialization of our product candidates and delay our business development activities. In addition, such failure could be the basis for the FDA to issue a warning or untitled letter or take other regulatory or legal action, including recall or seizure, total or partial suspension of production, suspension of ongoing clinical trials, refusal to approve pending applications or supplemental applications, and potentially civil and/or criminal penalties depending on the matter.
SUSTOL, CINVANTI, HTX-011 or any of our other product candidates may be in competition with other products for access to the facilities of third parties. Consequently, SUSTOL, CINVANTI, HTX-011 or any of our other product candidates may be subject to manufacturing delays if our contractors give other companies’ products greater priority than our products. For this and other reasons, our third-party contract manufacturers may not be able to manufacture SUSTOL, CINVANTI, HTX-011 or any of our other product candidates in a cost-effective or timely manner. If not manufactured in a timely manner, the clinical development of any of our product candidates or their submission for regulatory approval could be delayed, and our ability to deliver products to market on a timely basis could be impaired. This could increase our costs, cause us to lose revenue or market share and damage our reputation.
Certain of the components used in the manufacture of SUSTOL, CINVANTI, HTX-011 and our other product candidates are sourced from a single vendor.
Some of the critical materials and components used in manufacturing SUSTOL, CINVANTI, HTX-011 and our other product candidates are sourced from single suppliers. An interruption in the supply of a key material could significantly delay our research and development process or increase our expenses for commercialization or development products. Specialized materials must often be manufactured for the first time for use in drug delivery technologies, or materials may be used in the technologies in a manner different from their customary commercial uses. The quality of materials can be critical to the performance of a drug delivery technology, so a reliable source that provides a consistent supply of materials is important. Materials or components needed for our drug delivery technologies may be difficult to obtain on commercially reasonable terms, particularly when relatively small quantities are required or if the materials traditionally have not been used in pharmaceutical products.
28
Table of Contents
We face intense competition from other companies developing products for the prevention of CINV or post-operative pain.
SUSTOL faces significant competition. Currently available 5-HT3 receptor antagonists include: ALOXI® (palonosetron, marketed by Eisai, Inc. in conjunction with Helsinn Healthcare S.A.); AKYNZEO® (palonosetron combined with the NK1 receptor antagonist netupitant, marketed by Eisai); SANCUSO® (granisetron transdermal patch, marketed by ProStrakan Group Plc); and generic products including ondansetron (formerly marketed by GlaxoSmithKline plc as ZOFRAN) and granisetron (formerly marketed by Hoffman-La Roche, Inc. as KYTRIL). Generic palonosetron is also likely to be available after September 2018. Currently, ALOXI is the only 5-HT3 receptor antagonist other than SUSTOL that is approved for the prevention of delayed CINV associated with MEC regimens. No 5-HT3 receptor antagonist is approved for the prevention of delayed CINV associated with HEC regimens.
NK1 receptor antagonists are also administered for the prevention of CINV, in combination with 5-HT3 receptor antagonists, to augment the therapeutic effect of the 5-HT3 receptor antagonist. If CINVANTI is approved by the FDA, it will face significant competition. Currently available NK1 receptor antagonists include: AKYNZEO® (palonosetron combined with the NK1 receptor antagonist netupitant, marketed by Eisai); EMEND® (aprepitant, marketed by Merck & Co, Inc.); EMEND® for Injection (fosaprepitant, marketed by Merck & Co); VARUBI® (rolapitant, marketed by Tesaro, Inc.) and potentially other products that include an NK1 receptor antagonist that reach the market.
If we are able to successfully develop HTX-011 for the prevention of post-operative pain, we will compete with MARCAINE (bupivacaine; marketed by Hospira, Inc.) and generic forms of bupivacaine; NAROPIN (ropivacaine; marketed by Fresenius Kabi USA, LLC) and generic forms of ropivacaine; EXPAREL® (bupivacaine liposome injectable suspension; marketed by Pacira Pharmaceuticals, Inc.) and potentially other products in development for the prevention of post-operative pain that reach the market.
Small or early-stage companies and research institutions may also prove to be significant competitors, particularly through collaborative arrangements with large and established pharmaceutical companies. We will also face competition from these parties in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, and acquiring and in-licensing technologies and products complementary to our programs or potentially advantageous to our business. If any of our competitors succeed in obtaining approval from the FDA or other regulatory authorities for their products sooner than we do or for products that are more effective or less costly than ours, our commercial opportunity could be significantly reduced. Major technological changes can happen quickly in the biotechnology and pharmaceutical industries, and the development of technologically improved or different products or drug delivery technologies may make our product candidates or platform technologies obsolete or noncompetitive.
Our products may face competition from lower cost generic products offered by our competitors.
Pricing for therapeutics can be extremely competitive, and strict formulary guidelines enforced by payors may create significant challenges in the acceptance and profitability of branded products. The market for generic products can be very lucrative, and it is dominated by companies that may have much larger distribution capabilities than we may have in the future. It can be very difficult to predict the timing of the launch of generic products given the commonality of litigation with manufacturers over
29
Table of Contents
anticipated patent expiration. Our inability to accurately foresee and plan for generic product launches that may compete with our products may significantly impact our potential revenues from such products. Upon the expiration or loss of patent protection for a branded product, or upon the “at-risk” launch (despite pending patent infringement litigation against the generic product) by a manufacturer of a generic version of a drug that may compete with one of our products, we could quickly lose a significant portion of our sales of that product. The inability for a branded product we may sell to successfully compete against generic products could negatively impact sales of our product, reduce our ability to grow our business and significantly harm our business prospects.
If we are unable to recruit and retain skilled employees, we may not be able to achieve our objectives.
We depend on a small number of key management and personnel. Retaining our current employees and recruiting qualified personnel to perform future research and development and commercialization work will be critical to our success. Competition is always present for highly skilled and experienced personnel, and an inability to recruit or retain sufficient skilled personnel could result in delays in our business growth and development and adversely impact our research and development or commercial activities. If we lose key members of our senior management team, we may not be able to find suitable replacements and our business may be harmed as a result.
Our business strategy may include acquisitions of other businesses, products or product licenses. We may not be able to successfully manage such activities.
We may engage in strategic transactions that could cause us to incur contingent liabilities, commitments or significant expense. In the course of pursuing strategic opportunities, we may evaluate potential acquisitions or investments in strategic technologies, products or businesses. Future acquisitions or investments could subject us to a number of risks, including, but not limited to:
• our inability to appropriately evaluate and take into consideration the potential uncertainties associated with the other party to such a transaction, including, but not limited to, the prospects of that party and their existing products or product candidates and regulatory approvals;
• difficulties associated with realizing the perceived potential for commercial success with respect to any acquired technology, product or business;
• our ability to effectively integrate any new technology, product and/or business including personnel, intellectual property or business relationships into our Company;
• our inability to generate revenues from acquired technology and/or products sufficient to meet our objectives in undertaking the acquisition or even to offset the associated acquisition and maintenance costs and/or assumption of liabilities; and
• the distraction of our management from our existing product development programs and initiatives in pursuing an acquisition.
In connection with an acquisition, we must estimate the value of the transaction by making certain assumptions that may prove to be incorrect, which could cause us to fail to realize the anticipated benefits of a transaction. Any strategic transaction we may pursue may not result in the benefits we initially anticipate, may result in costs that end up outweighing the benefits and may adversely impact our financial condition and be detrimental to our future business prospects.
30
Table of Contents
Our business strategy may include entry into collaborative agreements. We may not be able to enter into collaborative agreements or may not be able to negotiate commercially acceptable terms for these agreements.
Our current business strategy may include the entry into collaborative agreements for the development and commercialization of our products and product candidates. The negotiation and consummation of these types of agreements typically involve simultaneous discussions with multiple potential collaborators and require significant time and resources from our officers, business development and research and development staff. In addition, in attracting the attention of pharmaceutical and biotechnology company collaborators, we compete with numerous other third parties with product opportunities as well as the collaborators’ own internal product opportunities. We may not be able to consummate collaborative agreements, or we may not be able to negotiate commercially acceptable terms for these agreements.
If we do enter into such arrangements, we could be dependent upon the subsequent success of these other parties in performing their respective responsibilities and the cooperation of our partners. Our collaborators may not cooperate with us or perform their obligations under our agreements with them. We cannot control the amount and timing of our collaborators’ resources that will be devoted to researching our product candidates pursuant to our collaborative agreements with them. Our collaborators may choose to pursue existing or alternative technologies in preference to those being developed in collaboration with us.
Under agreements with any collaborators we may work with in the future, we may rely significantly on them to, among other activities:
• fund research and development activities with us;
• pay us fees upon the achievement of milestones; and
• market for or with us any commercial products that result from our collaborations.
If we do not consummate collaborative agreements, we may use our financial resources more rapidly on our product development efforts, continue to defer certain development activities or forego the exploitation of certain geographic territories, any of which could have a material adverse effect on our business prospects. Further, we may not be successful in overseeing any such collaborative arrangements. If we fail to establish and maintain necessary collaborative relationships, our business prospects could suffer.
Risks Related to Our Financial Condition
We have a history of losses, we expect to generate losses in the near future, and we may never achieve or maintain profitability.
We have incurred significant operating losses and negative cash flows from operations and had an accumulated deficit of $586.0 million through December 31, 2016. We expect to continue to generate substantial losses over at least the next several years as we:
• expand product development activities with respect to our product candidates;
• conduct preclinical development and clinical trials for our product candidates;
31
Table of Contents
• pursue regulatory approvals for any current or future product candidates; and
• engage in commercialization efforts for any future approved product candidates.
In addition, the amount we spend will impact our profitability. Our spending will depend, in part, on:
• the number of product candidates we pursue;
• the progress of our research and development programs for our product candidates, including clinical trials;
• the time and expense required to pursue FDA and/or non-U.S. regulatory approvals for our product candidates, whether such approvals are obtained and the scope of any approved product label;
• the cost of possible acquisitions of technologies, compounds, product rights or companies;
• the cost of obtaining licenses to use technology owned by others for proprietary products and otherwise;
• the time and expense required to prosecute, enforce and/or challenge patent and other intellectual property rights;
• the costs of potential litigation; and
• the costs associated with recruiting and compensating a highly skilled workforce in an environment where competition for such employees may be intense.
To achieve and sustain profitability, we must, alone or in cooperation with others, successfully develop, obtain regulatory approval for, manufacture, market and sell our products, including our current work commercializing SUSTOL. We will incur substantial expenses in our efforts to develop and commercialize our products and we may never generate sufficient revenue to become profitable or to sustain profitability.
Additional capital may be needed in the future to enable us to implement our business plan, and we may be unable to raise capital, which would force us to limit or cease our operations and related product development programs.
In January 2017, we completed an underwritten public offering of our common stock for net proceeds of $163.7 million. As of December 31, 2016, we had cash, cash equivalents and short-term investments of $51.1 million, or $214.8 million in pro-forma cash, cash equivalents and short-term investments, adjusting for the January 2017 public offering. Historically, we have financed our operations, including technology and product research and development, primarily through sales of our common stock and debt financings. Our capital requirements going forward will depend on numerous factors, including but not limited to: the degree of commercial success of SUSTOL; the scope, rate of progress, results and costs of preclinical testing and clinical trials; an approval decision by the FDA with respect to CINVANTI; the timing and costs associated with the commercial launch of CINVANTI, if approved; the degree of commercial success of CINVANTI, if approved; the number and characteristics of product development programs we pursue and the pace of each program, including the timing of clinical trials; the time, cost and outcome involved in seeking other regulatory approvals; scientific progress in our research and development programs; the magnitude and scope of our research and development programs; our ability to establish and main-
32
Table of Contents
tain strategic collaborations or partnerships for research, development, clinical testing, manufacturing and marketing of our product candidates; the cost and timing of establishing sales, marketing and distribution capabilities if we commercialize products independently; the cost of establishing clinical and commercial supplies of our product candidates and any products that we may develop and general market conditions.
We may not be able to raise additional capital when needed or desired, or we may need to raise additional capital on unfavorable terms, which could result in dilution to existing stockholders.
We may not be able to raise sufficient additional capital when needed on favorable terms, or at all. If we are unable to obtain adequate funds, we may be required to curtail significantly or cease our operations.
The timing and degree of any future capital requirements will depend on many factors, including:
• our ability to successfully commercialize, market and achieve market acceptance of SUSTOL;
• the status of regulatory approval of any pending applications with the FDA, or other regulators, as the case may be, and the costs involved with pursuing regulatory approvals;
• the number and characteristics of product development programs we pursue and the pace of each program;
• the scope, rate of progress, results and costs of preclinical testing and clinical trials;
• our ability to establish and maintain strategic collaborations or partnerships for research, development, clinical testing, manufacturing and marketing of our product candidates;
• the cost and timing of establishing or enlarging sales and marketing capabilities; and
• the cost of establishing supply arrangements for clinical and commercial development of our product candidates and any products that we may develop.
If we issue additional equity securities or securities convertible into equity securities to raise funds, our stockholders will suffer dilution of their investment, and such issuance may adversely affect the market price of our common stock. Additionally, a second close of $50.0 million of our Subordinated Secured Promissory Note (the “Promissory Note”), which would provide additional capital to us if received, is subject to the achievement of a corporate milestone. There can be no assurance that we will achieve the corporate milestone required as a condition to the second close of the Promissory Note when needed, or at all.
Any new debt financing we enter into may involve covenants that restrict our operations. These restrictive covenants may include, among other things, limitations on borrowing and specific restrictions on the use of our assets, as well as prohibitions on our ability to create liens, pay dividends, redeem capital stock or make investments. Our Senior Secured Convertible Notes (“Convertible Notes”) also include restrictions on our use of cash and financial activities and both the Convertible Notes and the Promissory Note are secured by liens on substantially all of our assets. In the event that additional funds are obtained through arrangements with collaborative partners, these arrangements may require us to relinquish rights to some of our technologies,
33
Table of Contents
product candidates or products on terms that are not favorable to us or require us to enter into a collaboration arrangement that we would otherwise seek to develop and commercialize ourselves. If adequate funds are not available, we may default on our indebtedness, be required to delay, reduce the scope of, or eliminate one or more of our product development programs and reduce personnel-related and other costs, which would have a material adverse effect on our business.
Provisions contained in our debt instruments limit our ability to incur additional indebtedness.
The Convertible Notes and the Promissory Note (collectively, the “Secured Notes”) are secured by substantially all of our assets, including our bank and investment accounts, and the terms of the Secured Notes require us to seek approval from the holders of the Secured Notes before taking certain actions, including incurring certain additional indebtedness or modifying the terms of certain existing indebtedness. The Secured Notes also contain provisions that trigger events of default upon any default of our financial obligations under certain material contracts we may enter into. In addition, potential third-party lenders may be unwilling to subordinate new debt to the Secured Notes. As a result, we may not be able to raise funds through the issuance of debt in the future, which could impair our ability to finance our business obligations or pursue business expansion initiatives.
We could be exposed to significant product liability claims that could be time-consuming and costly to defend, divert management attention and adversely impact our ability to obtain and maintain insurance coverage.
The administration of drugs in humans, whether in clinical studies or commercially, carries the inherent risk of product liability claims whether or not the drugs are actually the cause of an injury. SUSTOL, our product candidates and products that we may commercially market in the future may cause, or may appear to have caused, injury or dangerous drug reactions, and we may not learn about or understand those effects until the product or product candidate has been administered to patients for a prolonged period of time.
Although we are insured against such risks up to an annual aggregate limit in connection with clinical trials and commercial sales of our products, our present product liability insurance may be inadequate and may not fully cover the costs of any claim or any ultimate damages we might be required to pay. Product liability claims or other claims related to our products, regardless of their outcome, could require us to spend significant time and money in litigation or to pay significant damages. Any successful product liability claim may prevent us from obtaining adequate product liability insurance in the future on commercially desirable or reasonable terms. In addition, product liability coverage may cease to be available in sufficient amounts or at an acceptable cost. An inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of our products. A product liability claim could also significantly harm our reputation and delay market acceptance of our products.
The investment of our cash is subject to risks, which may cause losses or adversely affect the liquidity of these investments and our results of operations, liquidity and financial condition.
Our investments of cash, cash equivalents and short-term investments are subject to general credit, liquidity, market and interest rate risks, which have been and may, in the future, be exacerbated by a U.S. and/or global financial crisis. We may realize losses in the fair value of certain of our investments or a complete loss of these investments if the credit markets tighten, which would have an adverse effect on our results of operations, liquidity and financial condition.
34
Table of Contents
Risks Related to Our Industry
Drug development involves a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
Conducting clinical trials is a lengthy, time-consuming and expensive process. For example, we incurred significant expenses in developing SUSTOL, with no guarantees that doing so would result in a commercially viable product. Before obtaining regulatory approvals for the commercial sale of any products, we, or our potential partners, must demonstrate through preclinical testing and clinical trials that our product candidates are safe and effective for their intended uses in humans. We have incurred and will continue to incur substantial expense and devote a significant amount of time to preclinical testing and clinical trials.
The outcome of clinical testing is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical studies and early clinical trials of product candidates may not be predictive of the results of later stage clinical trials. In addition, regulations are not static, and regulatory agencies, including the FDA, alter their staff, interpretations and practices and may in the future impose more stringent requirements than are currently in effect, which may adversely affect our planned drug development and/or our commercialization efforts. Satisfying the FDA’s, and other regulatory agencies’, requirements typically takes a significant number of years and can vary substantially based upon the type, complexity and novelty of the product candidate. Our business, results of operations and financial condition may be materially adversely affected by any delays in, or termination of, our clinical trials. Factors that could impede our ability to generate commercially viable products through the conduct of clinical trials include:
• insufficient funds to conduct clinical trials;
• the inability to find partners, if necessary, for support, including research, development, manufacturing or clinical needs;
• the failure of tests or studies necessary to submit an NDA, such as clinical studies, bioequivalence studies in support of a 505(b)(2) regulatory filing, or stability studies;
• the failure of clinical trials to demonstrate the safety and efficacy of our product candidates to the extent necessary to obtain regulatory approvals;
• the failure by us or third-party investigators, CROs, or other third parties involved in the research to adhere to regulatory requirements applicable to the conduct of clinical trials;
• the failure of preclinical testing and early clinical trials to predict results of later clinical trials;
• any delay in completion of clinical trials, resulting in increased costs; and
• the inability to obtain regulatory approval of our product candidates following completion of clinical trials, or delays in obtaining such approvals.
There can be no assurance that if our clinical trials are successfully initiated and completed we will be able to obtain approval by the FDA in the U.S. or similar regulatory authorities elsewhere in the world in a timely manner, if at all. If we fail to success-
35
Table of Contents
fully develop and commercialize one or more of our product candidates, we may be unable to generate sufficient revenues to attain profitability, and our reputation in the industry and in the investment community would likely be damaged, each of which would cause our stock price to decrease.
Delays in clinical testing could increase our costs and delay our ability to obtain regulatory approval and commercialize our product candidates.
Before we can receive regulatory approval for the commercial sale of our potential products, the FDA and comparable authorities in non-U.S. jurisdictions require extensive preclinical safety testing and clinical trials to demonstrate their safety and efficacy. Significant delays in preclinical and clinical testing could materially impact our product development costs and delay regulatory approval of our product candidates. Our ability to complete clinical trials in a timely manner could be impacted by, among other factors:
• delay or failure in reaching agreement with the FDA or comparable foreign regulatory authority on a trial design that we are able to execute;
• delay or failure in obtaining authorization to commence a trial or inability to comply with conditions imposed by a regulatory authority regarding the scope or design of a clinical study;
• delay or failure in reaching agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites;
• delay or failure in obtaining IRB approval or the approval of other reviewing entities, including comparable foreign entities, to conduct a clinical trial at each site;
• withdrawal of clinical trial sites from our clinical trials as a result of changing standards of care or the ineligibility of a site to participate in our clinical trials;
• delay or failure in obtaining clinical materials;
• delay or failure in recruiting and enrolling suitable subjects to participate in a trial;
• delay or failure of subjects completing a trial or returning for post-treatment follow-up;
• clinical sites and investigators deviating from trial protocol, failing to conduct the trial in accordance with regulatory requirements, or dropping out of a trial;
• inability to identify and maintain a sufficient number of trial sites, many of which may already be engaged in other clinical trial programs, including some that may be for the same indication;
• failure of our third-party clinical trial managers to satisfy their contractual duties or meet expected deadlines;
• delay or failure in adding new clinical trial sites;
36
Table of Contents
• ambiguous or negative interim results or results that are inconsistent with earlier results;
• feedback from the FDA, the IRB, data safety monitoring boards or comparable foreign entities, or results from earlier stage or concurrent preclinical and clinical studies that might require modification to the protocol;
• decisions by the FDA, the IRB, comparable foreign regulatory entities, or recommendations by a data safety monitoring board or comparable foreign regulatory entity to suspend or terminate clinical trials at any time for safety issues or for any other reason;
• unacceptable risk-benefit profiles or unforeseen safety issues or adverse side effects;
• failure to demonstrate a benefit from using a drug;
• manufacturing issues, including problems with manufacturing or obtaining from third parties sufficient quantities of a product candidate for use in clinical trials; and
• changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial.
Patient enrollment, a significant factor in the timing of clinical trials, is affected by many factors including the size and nature of the patient population, the proximity of subjects to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, the ability to obtain and maintain patient consents, whether enrolled subjects drop out before completion, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we investigate. Furthermore, we rely on CROs and clinical trial sites to ensure the proper and timely conduct of our clinical trials and while we have agreements governing their activities, we have limited influence over CROs’ actual performance.
Our failure to successfully establish, recruit for, and oversee our clinical trials could delay our product development efforts and negatively impact our business. If we experience delays in the completion of any ongoing study, the commercial prospects of HTX-011 or any of our other product candidates could be harmed, and our ability to generate product revenue will be delayed. Any delays in completing our clinical trials will increase our costs, slow our product candidates’ development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects significantly.
We may not obtain regulatory approval for our product candidates in development. Regulatory approval may also be delayed or revoked or may impose limitations on the indicated uses of a proposed product. If we are unable to obtain regulatory approval for our product candidates in development, our business will be substantially harmed.
The process for obtaining regulatory approval of a new drug is time consuming, is subject to unanticipated delays and costs and requires the commitment of substantial resources. Any product that we or our potential future collaborative partners develop must receive all necessary regulatory agency approvals or clearances before it may be marketed in the U.S. or other countries. Human pharmaceutical products are subject to rigorous preclinical and clinical testing and other requirements by the
37
Table of Contents
FDA in the U.S. and similar health authorities in foreign countries. We may not receive necessary regulatory approvals or clearances to market our product candidates currently in development in the U.S. or in other jurisdictions, as a result of changes in regulatory policies prior to approval or other events. Additionally, data obtained from preclinical and clinical activities, or from stability or bioequivalence studies, are susceptible to varying interpretations that could delay, limit or prevent regulatory agency approvals or clearances.
Our product candidates could fail to receive regulatory approval from the FDA or a comparable foreign regulatory authority for many reasons, including:
• disagreement with the design or implementation of our clinical trials;
• failure to demonstrate that the product candidate is safe and effective for its proposed indication;
• failure of clinical trial results to meet the level of statistical significance required for approval;
• the failure of third parties to manage and conduct the trials or perform necessary oversight to meet expected deadlines or to comply with regulatory requirements;
• failure to demonstrate that the product candidate’s clinical and other benefits outweigh its safety risks;
• disagreement with our interpretation of data from preclinical studies or clinical trials;
• the insufficiency of data collected from clinical trials to support the submission and filing of an NDA or other submission or to obtain regulatory approval;
• disapproval of the manufacturing processes or facilities of third-party manufacturers with whom we contract for clinical and commercial supplies; and
• changes in approval policies or regulations that render our preclinical and clinical data insufficient for approval.
The FDA or a comparable non-U.S. regulatory authority may require additional preclinical or clinical data to support approval, such as confirmatory studies and other data or studies to address questions or concerns that may arise during the FDA review process.
Even if granted, regulatory approvals may include significant limitations on the uses for which products may be marketed. Failure to comply with applicable regulatory requirements can, among other things, result in warning letters, imposition of civil penalties or other monetary payments, delay in approving or refusal to approve a product candidate, suspension or withdrawal of regulatory approval, product recall or seizure, operating restrictions, interruption of clinical trials or manufacturing, injunctions and criminal prosecution.
In addition, the marketing and manufacturing of products are subject to continuing FDA review, and later discovery of previously unknown problems with a product, its manufacture or its marketing may result in the FDA requiring further clinical research or restrictions on the product or the manufacturer, including withdrawal of the product from the market.
38
Table of Contents
Failure to obtain regulatory approval in international jurisdictions would prevent SUSTOL or any other products we may develop from being marketed abroad.
In the event we pursue the right to market and sell SUSTOL or any other products we may develop in jurisdictions other than the U.S., we would be required to obtain separate marketing approvals and comply with numerous and varying regulatory requirements in each foreign country. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the U.S. generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the U.S., it is required that the product be approved for reimbursement before the product can be approved for sale in that country. In the event we choose to pursue them, we may not obtain approvals from regulatory authorities outside the U.S. on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the U.S. does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. If we are unable in the future to obtain approval of a product candidate by regulatory authorities in non-U.S. jurisdictions, the commercial prospects of that product candidate may be significantly diminished and our business prospects could decline.
Even if our product candidates in development receive regulatory approval, they may still face future development and regulatory difficulties. If we fail to comply with continuing federal, state and foreign regulations, we could lose our approvals to market drugs, and our business would be seriously harmed.
Even if we obtain regulatory approval for our product candidates in development, they remain subject to ongoing requirements of the FDA and comparable foreign regulatory authorities, including requirements related to manufacturing, quality control, further development, labeling, packaging, storage, distribution, safety surveillance, import, export, advertising, promotion, recordkeeping, and reporting of safety and other post-market information. Following initial regulatory approval for drugs we develop, including SUSTOL or any other products we may develop, we remain subject to continuing regulatory review, including review of adverse drug experiences and clinical results that may be reported after drug products become commercially available. This would include results from any post-marketing tests or continued actions required as a condition of approval. The manufacturer and manufacturing facilities we use to make any of our drug candidates will also be subject to periodic review and inspection by the FDA. If a previously unknown problem or problems with a product or a manufacturing and laboratory facility used by us is discovered, the FDA or foreign regulatory agency may impose restrictions on that product or on the manufacturing facility, including requiring us to withdraw the product from the market. Any changes to an approved product, including the way it is manufactured or promoted, often require FDA approval before the product, as modified, can be marketed. We and our contract manufacturers will also be subject to ongoing FDA requirements for submission of safety and other post-market information. If we and our contract manufacturers fail to comply with applicable regulatory requirements, a regulatory agency may:
• issue warning letters;
• impose civil or criminal penalties;
• suspend or withdraw our regulatory approval;
• suspend or terminate any of our ongoing clinical trials;
39
Table of Contents
• refuse to approve pending applications or supplements to approved applications filed by us;
• impose restrictions on our operations;
• close the facilities of our contract manufacturers; or
• seize or detain products or require a product recall.
The occurrence of any event or penalty described above may inhibit our ability to commercialize our products and generate revenue.
Additionally, such regulatory review covers a company’s activities in the promotion of its drugs, with significant potential penalties and restrictions for promotion of drugs for an unapproved use or other inappropriate sales and marketing activities. Advertising and promotion of any product candidate that obtains approval in the U.S. will be heavily scrutinized by the FDA, the Department of Justice, and the Department of Health and Human Services’ Office of Inspector General. Violations of applicable advertising and promotion laws and regulations, including promotion of products for unapproved (or off-label) uses, are subject to enforcement letters, inquiries and investigations and civil and criminal sanctions by the FDA. We are also required to submit information on our open and completed clinical trials to public registries and databases; failure to comply with these requirements could expose us to negative publicity, fines and penalties that could harm our business. We are also required to comply with the requirements to submit to governmental authorities information on payments to physicians and certain other third parties; failure to comply with these requirements could expose us to negative publicity, fines and penalties that could harm our business.
The commercial use of our products may cause unintended side effects or adverse reactions, or incidents of misuse may occur, which could adversely affect our business.
We cannot predict whether any commercial use of our product candidates, once approved, will produce undesirable or unintended side effects that have not been evident in clinical trials conducted for such product candidates to date. Additionally, incidents of product misuse may occur. These events, including the reporting of adverse safety events, among others, could result in product recalls, product liability actions or withdrawals or additional regulatory controls (including additional regulatory scrutiny and requirements for additional labeling), all of which could have a material adverse effect on our business, financial condition, cash flows and results of operations.
If we cannot establish pricing of our product candidates acceptable to the U.S. or foreign governments, insurance companies, managed care organizations and other payors, or arrange for favorable reimbursement policies, our product sales will be severely hindered.
The continuing efforts of the U.S. and foreign governments, insurance companies, managed care organizations and other payors of health care costs to contain or reduce costs of health care may adversely affect our ability to generate adequate revenues and gross margins to make the products we develop commercially viable. Our ability to commercialize any product candidates successfully will depend in part on the extent to which governmental authorities, private health insurers and other organizations establish appropriate reimbursement levels for the cost of such products and related treatments and for what uses reimbursement will be provided.
40
Table of Contents
In the U.S., given recent federal and state government initiatives directed at lowering the total cost of health care, the U.S. Congress and state legislatures will likely continue to focus on health care reform, reducing the cost of prescription pharmaceuticals and reforming the Medicare and Medicaid systems. For example, the PPACA encourages comparative effectiveness research. Any adverse findings for our products from such research may negatively impact reimbursement available for our products. Economic pressure on state budgets may result in states increasingly seeking to achieve budget savings through mechanisms that limit coverage or payment for drugs. State Medicaid programs are increasingly asking manufacturers to pay supplemental rebates and requiring prior authorization by the state program for use of any drug for which supplemental rebates are not being paid. Further, the trend toward managed health care in the U.S., which could significantly influence the purchase of health care services and products, may result in lower prices for our products, once approved for marketing. While we cannot predict whether any legislative or regulatory proposals affecting our business will be adopted, the announcement or adoption of these proposals could have a material and adverse effect on our potential revenues and gross margins.
The pharmaceutical industry is subject to significant regulation and oversight pursuant to anti-kickback laws, false claims statutes, and anti-corruption laws, which may result in significant additional expense and limit our ability to commercialize our products. In addition, any failure to comply with these regulations could result in substantial fines or penalties.
We are subject to health care fraud and abuse regulations that are enforced by both the federal government and the states in which we conduct our business. Healthcare providers, physicians and third-party payors play a primary role in the recommendation and prescription of any product with marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products with marketing approval. Restrictions under applicable federal and state healthcare laws and regulations include, but are not limited to, the following:
• the Federal health care programs’ Anti-Kickback Law, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, lease, order or recommendation of, any good or service for which payment may be made under federal health care programs such as the Medicare and Medicaid programs;
• federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other federal health care programs that are false or fraudulent. This false claims liability may attach in the event that a company is found to have knowingly submitted false average sales price, best price or other pricing data to the government or to have unlawfully promoted its products;
• federal “sunshine” laws, now known as Open Payments, that require transparency regarding financial arrangements with health care providers, such as the reporting and disclosure requirements imposed by the PPACA on drug manufacturers regarding any “payment or transfer of value” made or distributed to physicians and teaching hospitals; and
• state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payer, including commercial insurers.
41
Table of Contents
The risk of being found in violation of these laws is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Moreover, recent health care reform legislation has strengthened many of these laws. For example, the PPACA, among other things, amends the intent requirement of the federal anti-kickback and criminal health care fraud statutes to clarify that a person or entity does not need to have actual knowledge of this statute or specific intent to violate it. In addition, PPACA provides that a claim including items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the false claims statutes. Finally, some states, such as California, Massachusetts and Vermont, mandate implementation of commercial compliance programs to ensure compliance with these laws.
In addition, a number of states have laws that require pharmaceutical companies to track and report payments, gifts and other benefits provided to physicians and other health care professionals and entities. Similarly, the federal Physician Payments Sunshine Act within PPACA requires pharmaceutical companies to report to the federal government certain payments to physicians and teaching hospitals. The Physician Payments Sunshine Act provisions require manufacturers that participate in federal health care programs to begin collecting such information after a six-month period following commercial launch of a product; however state law equivalents may require compliance beginning at commercial launch.
In addition, we may in the future be subject to the FCPA. The FCPA and similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more frequent and aggressive investigations and enforcement proceedings by both the Department of Justice and the SEC. A determination that our operations or activities are not, or were not, in compliance with U.S. or foreign laws or regulations could result in the imposition of substantial fines, interruptions of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits, and other legal or equitable sanctions. Other internal or government investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence.
Changes in laws affecting the healthcare industry could also adversely affect our revenues and profitability, including new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions related to patent protection and enforcement, healthcare availability, and product pricing and marketing. Changes in FDA regulations and regulations issued by other regulatory agencies inside and outside of the U.S., including new or different approval requirements, timelines and processes, may also delay or prevent the approval of product candidates, require additional safety monitoring, labeling changes, restrictions on product distribution or other measures that could increase our costs of doing business and adversely affect the market for our products. The enactment in the U.S. of healthcare reform, new legislation or implementation of existing statutory provisions on importation of lower-cost competing drugs from other jurisdictions and legislation on comparative effectiveness research are examples of previously enacted and possible future changes in laws that could adversely affect our business.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government-funded healthcare programs, like Medicare and Medicaid, and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our
42
Table of Contents
business and our financial results. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Any action against us for violation of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Moreover, achieving and sustaining compliance with applicable federal and state privacy, security and fraud laws may prove costly.
We may incur significant liability if it is determined that we are promoting the “off-label” use of drugs.
We are prohibited from promoting SUSTOL or any other products we may develop for “off-label” uses that are not described in its labeling and that differ from the uses approved by the FDA. Physicians may prescribe drug products for off-label uses, and such off-label uses are common across medical specialties. The FDA and other regulatory agencies do not regulate a physician’s choice of treatments. However, they do restrict pharmaceutical companies and their sales representatives’ dissemination of information concerning off-label use. The FDA and other regulatory agencies actively enforce regulations prohibiting promotion of products for off-label uses and the promotion of products for which marketing authorization has not been obtained. A company that is found to have promoted products for off-label uses may be subject to significant liability, including civil and administrative remedies as well as criminal sanctions. Notwithstanding the regulatory restrictions on off-label promotion, the FDA and other regulatory authorities allow companies to engage in truthful, non-misleading, and non-promotional scientific exchanges concerning their products.
The FDA or other regulatory authorities may conclude that we have violated applicable laws, rules or regulations, and we may therefore be subject to significant liability, including civil and administrative remedies, as well as criminal sanctions. Such enforcement actions could cause us reputational harm and divert the attention of our management from our business operations. Likewise, our distribution and contracting partners and those providing vender support services may also be the subject of regulatory investigations involving, or remedies or sanctions for, off-label promotion of SUSTOL or any other products we may develop, which may adversely impact sales of SUSTOL or any other products we may develop or trigger indemnification obligations. These consequences, could, in turn, have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
Health care reform could increase our expenses and adversely affect the commercial success of our products.
The PPACA includes numerous provisions that affect pharmaceutical companies, some of which became effective immediately upon enactment of the law, and others of which are scheduled to take effect over the next several years. For example, the PPACA seeks to expand healthcare coverage to the uninsured through private health insurance reforms and an expansion of Medicaid. The PPACA also imposes substantial costs on pharmaceutical manufacturers, such as an increase in liability for rebates paid to Medicaid, new drug discounts that must be offered to certain enrollees in the Medicare prescription drug benefit and an annual fee imposed on all manufacturers of brand prescription drugs in the U.S. The PPACA also requires increased disclosure obligations — including those required under the “sunshine” laws — and an expansion of an existing program requiring pharmaceutical discounts to certain types of hospitals and federally subsidized clinics and contains cost-containment measures that could reduce reimbursement levels for pharmaceutical products. These and other aspects of the PPACA, including the regulations that may be imposed in connection with the implementation of the PPACA, could increase our expenses and adversely affect our ability to successfully commercialize our products and product candidates.
43
Table of Contents
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with FDA regulations or similar regulations of comparable foreign regulatory authorities, provide accurate information to the FDA or comparable foreign regulatory authorities, comply with manufacturing standards we have established, comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, report financial information or data accurately, or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
We are subject to certain data privacy and security requirements, which are very complex and difficult to comply with at times. Any failure to ensure adherence to these requirements could subject us to fines, penalties and damage our reputation.
We are required to comply, as applicable, with numerous federal and state laws, including state security breach notification laws, state health information privacy laws and federal and state consumer protection laws, which govern the collection, use and disclosure of personal information. Other countries also have, or are developing, laws governing the collection, use and transmission of personal information. In addition, most healthcare providers who may prescribe products we may sell in the future and from whom we may obtain patient health information are subject to privacy and security requirements under HIPAA. We are not a HIPAA covered entity, do not intend to become one, and we do not operate as a business associate to any covered entities. Therefore, these privacy and security requirements do not apply to us. However, we could be subject to criminal penalties if we knowingly obtain individually identifiable health information from a covered entity in a manner that is not authorized or permitted by HIPAA or for aiding and abetting the violation of HIPAA. We are unable to predict whether our actions could be subject to prosecution in the event of an impermissible disclosure of health information to us. These laws could create liability for us or increase our cost of doing business, and any failure to comply could result in harm to our reputation and potentially fines and penalties.
Security breaches and other disruptions could compromise our information and expose us to liability, which would cause our business and reputation to suffer.
In the ordinary course of our business, we collect and store sensitive data, including intellectual property, our proprietary business information and that of our suppliers, as well as personally identifiable information of clinical trial participants and employ-
44
Table of Contents
ees. Similarly, our third-party providers possess certain of our sensitive data. The secure maintenance of this information is critical to our operations and business strategy. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Any such breach could compromise our networks and the information stored there could be accessed, publicly disclosed, lost or stolen. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing amount of focus on privacy and data protection issues with the potential to affect our business, including recently enacted laws in a majority of states requiring security breach notification. Thus, any access, disclosure or other loss of information, including our data being breached at our partners or third-party providers, could result in legal claims or proceedings and liability under laws that protect the privacy of personal information, disrupt our operations and damage our reputation, which could adversely affect our business.
Our use of hazardous materials could subject us to liabilities, fines and sanctions.
Our laboratory and clinical testing sometimes involve use of hazardous, radioactive or otherwise toxic materials. We are subject to federal, state and local laws and regulations governing how we use, manufacture, handle, store and dispose of these materials.
Although we believe that our safety procedures for handling and disposing of such materials comply in all material respects with all federal, state and local regulations and standards, there is always the risk of accidental contamination or injury from these materials. In the event of an accident, we could be held liable for any damages that result, and we could also be subject to fines and penalties and such liability and costs could exceed our financial resources. If we fail to comply with these regulations and standards or with the conditions attached to our operating licenses, the licenses could be revoked, and we could be subjected to criminal sanctions and substantial financial liability or be required to suspend or modify our operations. Compliance with environmental and other laws may be expensive and current or future regulations may impair our product development efforts.
Risks Related to Our Intellectual Property
If we are unable to adequately protect or enforce our intellectual property rights, we may lose valuable assets or incur costly litigation to protect our rights.
Our success will depend in part on our ability to obtain patents and maintain trade secret protection, as well as successfully defending these patents against challenges, while operating without infringing the proprietary rights of others. We have filed a number of U.S. patent applications on inventions relating to the composition of a variety of polymers, specific products, product groups and processing technology. As of December 31, 2016, we had a total of 14 issued U.S. patents and an additional 48 issued (or registered) foreign patents. The patents on the bioerodible technologies expire between January 2017 and March 2026. Currently, SUSTOL is covered by 7 patents issued in the U.S. and by 27 patents issued in foreign countries including Austria, Belgium, Canada, Denmark, the EU, Finland, France, Germany, Greece, Ireland, Italy, Luxembourg, Netherlands, Portugal, Spain, Sweden, Switzerland, Taiwan, and the United Kingdom. U.S. patents covering SUSTOL have expiration dates ranging from May 2021 to September 2024; foreign patents covering SUSTOL have expiration dates ranging from May 2021 to September 2025. Our policy is to actively seek patent protection in the United States and to pursue equivalent patent claims in selected foreign countries, thereby seeking patent coverage for novel technologies and compositions of matter that may be
45
Table of Contents
commercially important to the development of our business. Granted patents include claims covering the product composition, methods of use and methods of preparation. Our existing patents may not cover future products, additional patents may not be issued and current patents, or patents issued in the future, may not provide meaningful protection or prove to be of commercial benefit.
The patent positions of pharmaceutical companies, including ours, are uncertain and involve complex legal and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued. Consequently, our patent applications may not issue into patents, and any issued patents may not provide sufficient protection for our product candidates or provide sufficient protection to afford us a commercial advantage against competitive technologies or may be held invalid if challenged or circumvented. Patent applications in the U.S. are maintained in confidence for at least 18 months after their filing. Consequently, we cannot be certain that the patent applications we are pursuing will lead to the issuance of any patent or be free from infringement or other claims from other parties. Our competitors may also independently develop products similar to ours or design around or otherwise circumvent patents issued to us or licensed by us. In addition, the laws of some foreign countries may not protect our proprietary rights to the same extent as U.S. laws.
We may enter into collaborative agreements which may subject us to obligations that must be fulfilled and require us to manage complex relationships with third parties. In the future, if we are unable to meet our obligations or manage our relationships with our collaborators under these agreements our revenue may decrease. The loss or diminution of our intellectual property rights could result in a decision by our third-party collaborators to terminate their agreements with us. In addition, these agreements are generally complex and contain provisions that could give rise to legal disputes, including potential disputes concerning ownership of intellectual property and data under collaborations. Such disputes can lead to lengthy, expensive litigation or arbitration, requiring us to divert management time and resources to such dispute.
Because the patent positions of pharmaceutical and biotechnology companies involve complex legal and factual questions, enforceability of patents cannot be predicted with certainty. The ultimate degree of patent protection that will be afforded to products and processes, including ours, in the U.S., remains uncertain and is dependent upon the scope of protection decided upon by the patent offices, courts and lawmakers in these countries. The recently enacted America Invents Act, which reformed certain patent laws in the U.S., may create additional uncertainty. Patents, if issued, may be challenged, invalidated or circumvented. As more products are commercialized using our proprietary product platforms, or as any product achieves greater commercial success, our patents become more likely to be subject to challenge by potential competitors.
We also rely upon trade secrets, technical know-how and continuing technological innovation to develop and maintain our competitive position. We require our employees, consultants, advisors and collaborators to execute appropriate confidentiality and assignment-of-inventions agreements with us. These agreements typically provide that all materials and confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances, and that all inventions arising out of the individual’s relationship with us shall be our exclusive property. These agreements may be breached, and in some instances, we may not have an appropriate remedy available for breach of the agreements. Furthermore, our competitors may independently develop substantially equivalent proprietary information and techniques, reverse engineer our information and techniques, or otherwise gain access to our proprietary technology. We may be unable to meaningfully protect our rights in trade secrets, technical know-how and other non-patented technology. We may have to resort to litigation to protect our
46
Table of Contents
intellectual property rights, or to determine their scope, validity or enforceability. In addition, interference proceedings declared by the U.S. Patent and Trademark Office may be necessary to determine the priority of inventions with respect to our patent applications. Enforcing or defending our proprietary rights is expensive, could cause diversion of our resources and may not prove successful. In addition, courts outside the U.S. may be less willing to protect trade secrets. Costly and time-consuming litigation could be necessary to seek to enforce and determine the scope of our proprietary rights. Any failure to enforce or protect our rights could cause us to lose the ability to exclude others from using our technology to develop or sell competing products.
We may infringe on the intellectual property rights of others, and any litigation could force us to stop developing or selling potential products and could be costly, divert management attention and harm our business.
We must be able to develop products without infringing the proprietary rights of other parties. Because the markets in which we operate involve established competitors with significant patent portfolios, including patents relating to the composition of a variety of polymers, specific products, product groups and processing technology, it could be difficult for us to use our technologies or develop products without infringing the proprietary rights of others. Therefore, there is risk that third parties may make claims of infringement against our products or technologies. We may not be able to design around the patented technologies or inventions of others, and we may not be able to obtain licenses to use patented technologies on acceptable terms, or at all. If we cannot operate without infringing the proprietary rights of others, we will not be able to develop or commercialize some or all of our product candidates, and consequently will not be able to earn product revenue.
There is considerable uncertainty within the pharmaceutical industry about the validity, scope and enforceability of many issued patents in the U.S. and elsewhere in the world. We cannot currently determine the ultimate scope and validity of patents which may be granted to third parties in the future or which patents might be asserted to be infringed by any future manufacture, use or sale of our products. In part as a result of this uncertainty, there has been, and we expect that there may continue to be, significant litigation in the pharmaceutical industry regarding patents and other intellectual property rights. We may have to enforce our intellectual property rights against third parties who infringe our patents and other intellectual property or challenge our patent or trademark applications. For example, in the U.S., putative generics of innovator drug products (including products in which the innovation comprises a new drug delivery method for an existing product, such as the drug delivery market occupied by us) may file Abbreviated New Drug Applications (“ANDA”) and, in doing so, certify that their products either do not infringe the innovator’s patents or that the innovator’s patents are invalid. This often results in litigation between the innovator and the ANDA applicant. This type of litigation is commonly known as “Paragraph IV” litigation in the U.S. These litigations could result in new or additional generic competition to any of our products that may be marketed in the future and a potential reduction in product revenue.
If we are required to defend ourselves in a patent-infringement lawsuit, we could incur substantial costs, and the lawsuit could divert management attention, regardless of the lawsuit’s merit or outcome. These legal actions could seek damages and seek to enjoin testing, manufacturing and marketing of the accused product or process. In addition to potential liability for significant damages, we could be required to redesign affected products or obtain a license to continue to manufacture or market the accused product or process and any license required under any such patent may not be made available to us on acceptable terms, if at all. Competitors may sue us as a way of delaying the introduction of our products. Any litigation, including any interference or derivation proceedings to determine priority of inventions, oppositions or other post-grant review proceedings to
47
Table of Contents
patents in the U.S. or in countries outside the U.S., or litigation against our partners may be costly and time-consuming and could harm our business. We expect that litigation may be necessary in some instances to determine the validity and scope of certain of our proprietary rights. Litigation may be necessary in other instances to determine the validity, scope and/or non-infringement of certain patent rights claimed by third parties to be pertinent to the manufacture, use or sale of our products. Ultimately, the outcome of such litigation could adversely affect the validity and scope of our patent or other proprietary rights or hinder our ability to manufacture and market our products.
Periodically, we review publicly available information regarding the development efforts of others in order to determine whether these efforts may violate our proprietary rights. We may determine that litigation is necessary to enforce our proprietary rights against others. Such litigation could result in substantial expense, regardless of its outcome, and may not be resolved in our favor.
Risks Related to Our Common Stock
The price of our common stock has been and may continue to be volatile.
The stock markets, in general, and in particular with respect to biotech and life sciences companies, have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock. In addition, the limited trading volume of our stock may contribute to its volatility. Our stock price may be particularly volatile given the stage of our business.
In the past, following periods of volatility in the market price of a particular company’s securities, litigation has often been brought against that company. If litigation of this type is brought against us, it could be extremely expensive and divert management’s attention and our Company’s resources.
Our certificate of incorporation, our bylaws and Delaware law contain provisions that could discourage another company from acquiring us and may prevent attempts by our stockholders to replace or remove our current management.
Provisions of Delaware law, our certificate of incorporation and our bylaws may discourage, delay or prevent a merger or acquisition that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace or remove our board of directors. These provisions include authorizing the issuance of “blank check” preferred stock without any need for action by stockholders.
In addition, Section 203 of Delaware General Corporation Law, which is applicable to us, may discourage, delay or prevent a change in control of our Company by prohibiting stockholders owning in excess of 15% of our outstanding voting stock from merging or combining with us, unless certain approvals are obtained.
Conversion of our Convertible Notes would result in substantial dilution for our existing stockholders.
Our outstanding Convertible Notes bear interest at a rate of 6% per annum, payable quarterly in cash or in kind, at the election of the holders of the Convertible Notes. The Convertible Notes are convertible into shares of our common stock at a rate of
48
Table of Contents
1,250 shares for every $1,000 of principal and accrued interest that is being converted. In the event the holders of the Convertible Notes were to opt to convert in full the outstanding principal and accrued interest due under the Convertible Notes as of December 31, 2016, we would be required to issue an aggregate of 7,521,416 shares, representing 16% of our outstanding shares, after giving effect to such conversion. This would result in substantial dilution of our existing stockholders.
On January 18, 2017, Tang Capital Partners, LP (“TCP”) executed a waiver (the “Waiver”) pursuant to which TCP waived: (i) our obligation under the Securities Purchase Agreement, dated April 24, 2011, among us, TCP and certain other investors (the “SPA”) to maintain a sufficient number of authorized shares of our common stock to permit the conversion of TCP’s outstanding principal and interest under the Convertible Notes to our common stock; and (ii) its right to convert the Convertible Notes for the duration of the Waiver. The Waiver will remain effective until the earliest to occur of: (i) a change in the control of the Company; (ii) the Company’s stockholders approving an increase of the number of authorized shares of our common stock; and (iii) July 18, 2017. If, upon the expiration of the Waiver, TCP seeks to convert part or all of the Convertible Notes, and we do not have a sufficient number of authorized shares of our common stock to permit the conversion of the portion of the Convertible Notes being converted, then we will be obligated to make a cash payment to TCP equal to the value of the underlying shares of common stock that we are unable to deliver on conversion, based on the price of our common stock at such time.
Concentration in stockholder ownership could influence strategic actions.
Our directors, executive officers, principal stockholders and affiliated entities currently beneficially own or control a significant percentage of our outstanding common stock. Based on information set forth in a Form 4 filed with the SEC on January 23, 2017, the beneficial ownership in our common stock, as determined in accordance with Rule 13d-3 of the Exchange Act, of TCP was 8,332,907 shares, or 16% of our outstanding shares, after giving effect to the shares issued in our January 2017 equity financing. In addition, as of December 31, 2016, TCP has the right to acquire 6,017,133 shares upon conversion of the Convertible Notes.
Such a substantial concentration of common stock ownership or control could significantly influence corporate actions on various strategic matters, including, for example, receptivity to collaborations and merger or sale overtures to the extent that stockholder approval is required for such transactions. Further, covenants contained in the Secured Notes would require approval from the noteholders for any change of control transaction we might consider. Accordingly, we may only be able to pursue transactions that are supported by these large stockholders. In addition, the conversion of the Convertible Notes, the exercise of these warrants, or the sale by our current stockholders of a substantial number of shares, or the expectation that such exercises or sales may occur, could significantly reduce the market price of our common stock.
Future utilization of net operating loss carry-forwards may be impaired due to recent changes in ownership.
We believe our net operating losses and tax attributes may be subject to limitation under Section 382 of the Internal Revenue Code of 1986. As a result, our deferred tax assets, and related valuation allowance, have been reduced for the estimated impact of the net operating losses and credits that we currently estimate may expire unused. Utilization of our remaining net operating loss and research and development credit carry-forwards may still be subject to substantial annual limitations due to ownership change limitations provided by the Internal Revenue Code and similar state provisions for ownership changes after December 31, 2016, including those that may come in conjunction with future equity financings or market trades by our stockholders.
49
Table of Contents
Our business could be negatively affected as a result of the actions of activist stockholders.
Proxy contests have been waged against many companies in the biopharmaceutical industry over the last few years. If faced with a proxy contest, we may not be able to respond successfully to the contest, which would be disruptive to our business. Even if we are successful, our business could be adversely affected by a proxy contest involving us because:
• responding to proxy contests and other actions by activist stockholders can be costly and time-consuming, disrupting operations and diverting the attention of management and employees, and can lead to uncertainty;
• perceived uncertainties as to future direction may result in the loss of potential acquisitions, collaborations or in-licensing opportunities, and may make it more difficult to attract and retain qualified personnel and business partners; and
• if individuals are elected to our board of directors with a specific agenda, it may adversely affect our ability to effectively implement our strategic plan in a timely manner and create additional value for our stockholders.
These actions could cause the market price of our common stock to experience periods of volatility.
If we identify a material weakness in our internal control over financial reporting, our ability to meet our reporting obligations and the trading price of our common stock could be negatively affected.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis. Accordingly, a material weakness increases the risk that the financial information we report contains material errors.
We regularly review and update our internal controls, disclosure controls and procedures and corporate governance policies. In addition, we are required under the Sarbanes-Oxley Act of 2002 to report annually on our internal control over financial reporting. Any system of internal controls, however well designed and operated, is based in part on certain assumptions and can provide only reasonable, not absolute, assurances that the objectives of the system are met. If we, or our independent registered public accounting firm, determine that our internal controls over financial reporting are not effective, or we discover areas that need improvement in the future, these shortcomings could have an adverse effect on our business and financial results.
If we cannot conclude that we have effective internal control over our financial reporting, or if our independent registered public accounting firm is unable to provide an unqualified opinion regarding the effectiveness of our internal control over financial reporting, investors could lose confidence in the reliability of our financial statements. Failure to comply with reporting requirements could also subject us to sanctions and/or investigations by the SEC, the NASDAQ Stock Market or other regulatory authorities.
Because we do not anticipate paying any cash dividends on our common stock in the foreseeable future, capital appreciation, if any, will be the source of gain for our stockholders.
We have never declared or paid cash dividends on our common stock. We currently intend to retain all of our current and future earnings to finance the growth and development of our business. As a result, capital appreciation, if any, of our common stock will be the sole source of gain for our stockholders for the foreseeable future.
50
Table of Contents
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
We lease 28,275 square feet of laboratory and office space in San Diego, California under a lease which began on December 1, 2016 and expires on April 15, 2024. We have one 5-year option to renew this lease. We also lease 26,067 square feet of laboratory, office and warehouse space in Redwood City, California and 1,898 square feet of office space in Jersey City, New Jersey. The lease for the Redwood City space expires on May 31, 2019. The lease for the Jersey City office space expires on June 30, 2018. The annual rent expense for all properties is $2.0 million. We believe our facilities are adequate and suitable for our current needs and that we will be able to obtain new or additional leased space in the future when necessary.
We are not currently a party to any legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES.
Not applicable.
51
Table of Contents
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Information About Our Common Stock
Shares of our common stock are traded on the NASDAQ Capital Market under the symbol “HRTX”.
Set forth below are the high and low sales prices for our common stock for each full quarterly period within the two most recent fiscal years.
| Year Ended December 31, 2016 |
High | Low | ||||||
| First Quarter |
$ | 28.10 | $ | 15.22 | ||||
| Second Quarter |
$ | 25.46 | $ | 15.50 | ||||
| Third Quarter |
$ | 24.00 | $ | 15.36 | ||||
| Fourth Quarter |
$ | 20.85 | $ | 12.30 | ||||
| Year Ended December 31, 2015 |
High | Low | ||||||
| First Quarter |
$ | 16.49 | $ | 7.09 | ||||
| Second Quarter |
$ | 34.03 | $ | 10.60 | ||||
| Third Quarter |
$ | 42.25 | $ | 20.82 | ||||
| Fourth Quarter |
$ | 31.32 | $ | 20.84 | ||||
Stockholders
The number of record holders of our common stock as of February 1, 2017 was 133.
Dividend Policy
We have never paid dividends on our common stock. We currently intend to retain all available funds and any future earnings for use in the operation and expansion of our business, and we do not anticipate paying any cash dividends in the foreseeable future.
52
Table of Contents
Stock Performance Graph
The following is not deemed “filed” with the SEC and is not to be incorporated by reference into any filing we make under the Securities Act of 1933, as amended, whether made before or after the date hereof and irrespective of any general incorporation by reference language in such filing.
The following graph shows the value of an investment of $100 on December 31, 2011 in Heron Therapeutics, Inc. common stock, the NASDAQ Composite Index (U.S.) and the NASDAQ Biotechnology Index. All values assume reinvestment of the pretax value of dividends paid by companies included in these indices and are calculated as of December 31st of each year. Our common stock has traded on The NASDAQ Capital Market since January 2014. Prior to that, shares of our common stock were traded on the OTC Bulletin Board under the symbol “APPA.OB.” The comparisons shown in the graph are based upon historical data and we caution that the stock price performance shown in the graph is not indicative of, nor intended to forecast, the potential future performance of our stock.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Heron Therapeutics, Inc, the NASDAQ Composite Index
and the NASDAQ Biotechnology Index
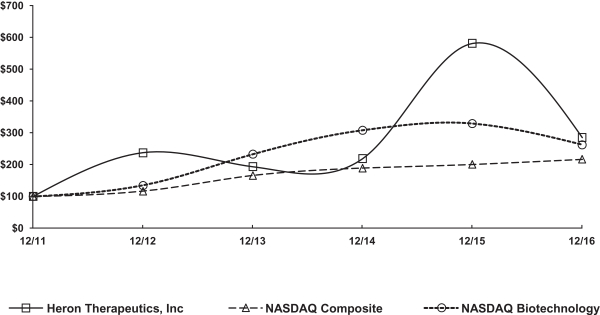
| * | $100 invested on 12/31/11 in stock or index, including reinvestment of dividends. Fiscal year ending December 31. |
|
|
12/11 | 12/12 | 12/13 | 12/14 | 12/15 | 12/16 | ||||||||||||||||||
| Heron Therapeutics, Inc. |
$ | 100.00 | $ | 236.96 | $ | 193.48 | $ | 218.70 | $ | 580.43 | $ | 284.78 | ||||||||||||
| NASDAQ Composite |
100.00 | 116.41 | 165.47 | 188.69 | 200.32 | 216.54 | ||||||||||||||||||
| NASDAQ Biotechnology |
100.00 | 134.68 | 232.37 | 307.67 | 328.76 | 262.08 | ||||||||||||||||||
53
Table of Contents
Issuer Purchases of Securities
None.
Unregistered Sales of Equity Securities and Use of Proceeds
None.
ITEM 6. SELECTED FINANCIAL DATA.
The following Selected Financial Data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in Item 7 and the Consolidated Financial Statements and Notes included in Item 8 of this Annual Report on Form 10-K.
| Years Ended December 31, | ||||||||||||||||||||
|
|
2016 | 2015 | 2014 | 2013 | 2012 | |||||||||||||||
| (In thousands, except per share amounts) | ||||||||||||||||||||
| Statements of Operations Data: |
||||||||||||||||||||
| Revenues: |
||||||||||||||||||||
| Net product sales |
$ | 1,279 | $ | — | $ | — | $ | — | $ | — | ||||||||||
| Operating expenses: |
||||||||||||||||||||
| Cost of product sales |
35 | — | — | — | — | |||||||||||||||
| Research and development |
103,125 | 61,183 | 54,833 | 32,516 | 15,174 | |||||||||||||||
| General and administrative |
21,366 | 18,395 | 11,020 | 13,032 | 6,760 | |||||||||||||||
| Sales and marketing |
47,668 | 17,347 | 8,708 | 8,909 | 1,897 | |||||||||||||||
|
|
|
|||||||||||||||||||
| Loss from operations |
(170,915) | (96,925) | (74,561) | (54,457) | (23,831) | |||||||||||||||
| Other expense, net |
(2,228) | (666) | (1,806) | (826) | (599) | |||||||||||||||
|
|
|
|||||||||||||||||||
| Loss from continuing operations |
(173,143) | (97,591) | (76,367) | (55,283) | (24,430) | |||||||||||||||
| Gain from discontinued operations |
— | — | — | — | 1,082 | |||||||||||||||
|
|
|
|||||||||||||||||||
| Net loss |
$ | (173,143) | $ | (97,591) | $ | (76,367) | $ | (55,283) | $ | (23,348) | ||||||||||
|
|
|
|||||||||||||||||||
| Basic and diluted net loss per common share — loss from continuing operations |
$ | (4.56) | $ | (2.95) | $ | (2.87) | $ | (3.42) | $ | (2.00) | ||||||||||
|
|
|
|||||||||||||||||||
| Basic and diluted net loss per common share — net loss |
$ | (4.56) | $ | (2.95) | $ | (2.87) | $ | (3.42) | $ | (1.91) | ||||||||||
|
|
|
|||||||||||||||||||
| Weighted-average common shares outstanding used to calculate basic and diluted net loss per common share |
37,925 | 33,081 | 26,569 | 16,163 | 12,223 | |||||||||||||||
|
|
|
|||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 13,414 | $ | 75,180 | $ | 72,675 | $ | 72,287 | $ | 53,506 | ||||||||||
| Short-term investments |
37,724 | 55,986 | — | — | — | |||||||||||||||
| Working capital |
23,410 | 115,016 | 60,112 | 65,933 | 49,936 | |||||||||||||||
| Total assets |
67,482 | 137,845 | 76,682 | 75,937 | 55,972 | |||||||||||||||
| Long-term liabilities |
50,000 | — | — | — | — | |||||||||||||||
| Accumulated deficit |
(585,971) | (412,828) | (315,237) | (238,870) | (183,587) | |||||||||||||||
| Total stockholders’ equity (deficit) |
(21,251) | 118,110 | 63,062 | 68,945 | 51,818 | |||||||||||||||
54
Table of Contents
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the federal securities laws. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. You can identify forward-looking statements by the use of the words “believe,” “expect,” “anticipate,” “intend,” “estimate,” “project,” “will,” “should,” “may,” “plan,” “assume” and other expressions that predict or indicate future events and trends and which do not relate to historical matters. You should not rely on forward-looking statements, because they involve known and unknown risks, uncertainties and other factors, some of which are beyond our control. These risks, uncertainties and other factors may cause our actual results, performance or achievements to be materially different from our anticipated future results, performance or achievements expressed or implied by the forward-looking statements.
Factors that might cause these differences include the following:
• our ability to successfully commercialize, market and achieve market acceptance of SUSTOL® (granisetron) extended-release injection (“SUSTOL”) and for future product candidates, including our positioning relative to competing products;
• estimates of the outcomes of our New Drug Application (“NDA”) submission to the U.S. Food and Drug Administration (“FDA”) for CINVANTI™ (HTX-019) (“CINVANTI”) and potential regulatory approval for and commercial launch of CINVANTI;
• any limitations or unfavorable warning or cautionary language that the FDA may ultimately impose on the label for CINVANTI;
• the potential market opportunities for SUSTOL, CINVANTI and HTX-011;
• whether safety and efficacy results of our clinical trials and other required tests for approval of our product candidates provide data to warrant progression of clinical trials, potential regulatory approval or further development of any of our product candidates;
• our ability to meet the post-marketing study requirements within the FDA’s mandated timelines and to obtain favorable results and comply with standard post-marketing requirements including U.S. federal advertising and promotion laws, federal and state anti-fraud and abuse laws, healthcare information privacy and security laws, safety surveillance, and disclosure of payments or other transfers of value to healthcare professionals and entities for SUSTOL or any of our product candidates;
• our ability to successfully develop and achieve regulatory approval for other future product candidates utilizing our proprietary Biochronomer® sustained-release drug delivery technology (“Biochronomer technology”);
• our ability to establish key collaborations and vendor relationships for our products and any other future product candidates;
• our ability to successfully develop and commercialize any technology that we may in-license or products we may acquire;
• unanticipated delays due to manufacturing difficulties, supply constraints or changes in the regulatory environment;
• our ability to successfully operate in non-U.S. jurisdictions in which we may choose to do business, including compliance with applicable regulatory requirements and laws;
55
Table of Contents
• uncertainties associated with obtaining and enforcing patents to protect our products, and our ability to successfully defend ourselves against unforeseen third-party infringement claims;
• our estimates regarding our capital requirements; and
• our ability to obtain additional financing and raise capital as necessary to fund operations or pursue business opportunities.
Any forward-looking statements in this Annual Report on Form 10-K reflect our current views with respect to future events or to our financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the section entitled “Risk Factors” in this Annual Report on Form 10-K. You should carefully review all of these factors. Given these uncertainties, you should not place undue reliance on these forward-looking statements. These forward-looking statements were based on information, plans and estimates as of the date of this Annual Report on Form 10-K, and except as required by law, we assume no obligation to update any forward-looking statements to reflect changes in underlying assumptions or factors, new information, future events or other changes. These risk factors may be updated from time to time by our future filings under the Securities Exchange Act of 1934. You should carefully review all information therein.
Introduction
Management’s discussion and analysis of financial condition and results of operations is provided as a supplement to the Consolidated Financial Statements and Notes, included in Item 8 of this Annual Report on Form 10-K, to help provide an understanding of our financial condition, the changes in our financial condition and our results of operations. Our discussion is organized as follows:
• Overview. This section provides a general description of our business and operating expenses.
• Critical accounting policies and estimates. This section contains a discussion of the accounting policies that we believe are important to our financial condition and results of operations and that require significant judgment and estimates on the part of management in their application. In addition, all of our significant accounting policies, including the critical accounting policies and estimates, are summarized in Note 2 to the Consolidated Financial Statements included in Item 8 of this Annual Report on Form 10-K.
• Results of operations. This section provides an analysis of our results of operations presented in the accompanying consolidated statements of comprehensive loss by comparing the results for the year ended December 31, 2016 to the results for the year ended December 31, 2015 and comparing the results for the year ended December 31, 2015 to the results for the year ended December 31, 2014.
• Liquidity and capital resources. This section provides an analysis of our cash flows and a discussion of our outstanding commitments and contingencies that existed as of December 31, 2016. Included in this discussion is our financial capacity to fund our future commitments and a discussion of other financing arrangements.
56
Table of Contents
Overview
Heron Therapeutics, Inc. is a commercial-stage biotechnology company focused on improving the lives of patients by developing best-in-class medicines that address major unmet medical needs. We are developing novel, patient-focused solutions that apply our innovative science and technologies to already-approved pharmacological agents for patients suffering from cancer and pain.
On August 9, 2016, our first commercial product, SUSTOL, was approved by the FDA. We developed SUSTOL for the prevention of chemotherapy-induced nausea and vomiting (“CINV”). SUSTOL is indicated, in combination with other antiemetics, in adults for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide combination chemotherapy regimens. We commenced commercial sales of SUSTOL in October 2016.
We have two investigational pharmaceutical products for patients suffering from cancer or post-operative pain. CINVANTI, an intravenous formulation of the neurokinin-1 receptor antagonist aprepitant, has been developed for the prevention of CINV as an adjunct to other antiemetic agents. We submitted an NDA with the FDA for CINVANTI on January 11, 2017 using the 505(b)(2) regulatory pathway. HTX-011, a long-acting formulation of the local anesthetic bupivacaine in a fixed-dose combination with the anti-inflammatory meloxicam, is being developed for the prevention of post-operative pain. HTX-011 is the subject of a broad-based Phase 2 development program designed to target the many patients undergoing a wide range of surgeries who experience significant post-operative pain.
Net Product Sales
Net product sales include revenue recognized for sales of SUSTOL to specialty distributors (“Customers”), less applicable sales allowances. See the “Critical Accounting Policies and Estimates” section of this Annual Report on Form 10-K for further details on our revenue recognition policy.
Cost of Sales
Cost of sales relates to the costs to produce, package and deliver SUSTOL to our Customers. These expenses include labor, raw materials, manufacturing and quality control overhead, and depreciation of equipment. See the “Critical Accounting Policies and Estimates” section of this Annual Report on Form 10-K for further details on our inventory policy.
Research and Development Expense
All costs of research and development are expensed in the period incurred. Research and development costs primarily consist of salaries and related expenses for personnel, stock-based compensation expense, fees paid to outside service providers and consultants, facilities costs and materials used in the clinical and preclinical trials and research and development.
At this time, due to the risks inherent in the clinical trial process, we are unable to estimate, with any certainty, the costs we will incur in the continued development of our product candidates. Other than costs for outsourced services associated with our clinical programs, we generally do not track our research and development expenses by project; rather, we track such expenses by the type of cost incurred.
57
Table of Contents
We expect our research and development expenses to increase in 2017 to support our ongoing research and development efforts for our product candidates, including costs for our ongoing HTX-011 Phase 2 program and the initiation of our HTX-011 Phase 3 program, costs for post-marketing requirements for SUSTOL, and costs associated with preclinical development efforts. The lengthy process of completing our clinical trials and seeking regulatory approval for our product candidates requires the expenditure of substantial resources. Our NDA for CINVANTI was submitted to the FDA for review, and, if accepted and approved, we expect CINVANTI to be commercially available in the first quarter of 2018.
General and Administrative Expense
General and administrative expense primarily consists of salaries, stock-based compensation expense and other related costs for personnel in executive, finance and accounting, investor relations, legal and human resource functions. Other general and administrative costs include professional fees for legal, information technology, accounting and other general corporate purposes, facility costs and insurance not otherwise included in research and development expense.
Sales and Marketing Expense
Sales and marketing expense primarily consists of salaries and related expenses, stock-based compensation expense and other related costs for sales operations, marketing and market access. Other sales and marketing costs include professional fees and commercialization costs related to SUSTOL. We expect sales and marketing expense to increase in 2017 to support the ongoing commercialization of SUSTOL, and the pre-commercialization efforts for CINVANTI. The commercial launch process requires the expenditure of substantial resources. If CINVANTI is approved, we expect it to be commercially available in the first quarter of 2018.
Other Expense, net
Other expense, net primarily consists of interest expense on the Subordinated Secured Promissory Note (the “Promissory Note”), as well as interest expense and amortization of debt discount related to our Senior Secured Convertible Notes (the “Convertible Notes”). In addition, other expense, net includes interest income earned on our cash, cash equivalents and short-term investments, impairment of fixed assets, and gains (losses) from the disposal of fixed assets.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations are based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. We evaluate our estimates on an ongoing basis, including those related to revenue recognition, inventory, accrued clinical liabilities, income taxes, and stock-based compensation. We base our estimates on historical experience and on assumptions that we believe to be reasonable under the circumstances, the results of which form the basis of making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ materially from these estimates under different assumptions or conditions.
58
Table of Contents
We believe the following critical accounting policies involve significant judgments and estimates used in the preparation of our consolidated financial statements.
Revenue Recognition
Product Sales
SUSTOL is distributed in the U.S. through a limited number of specialty distributors (“Customers”) that resell SUSTOL to healthcare providers, the end users of SUSTOL. Product sales are recorded net of sales allowances and estimated rebates, chargebacks, distributor fees, and other deductions.
Product sales are recognized as revenue when there is persuasive evidence that an arrangement exists, delivery has occurred and title has passed, the price is fixed or determinable and we are reasonably assured of collecting the resulting receivable. Product sales are recorded net of sales allowances and estimated rebates, chargebacks, distributor fees, and other deductions. Due to the recent launch of SUSTOL, we do not yet have sufficient historical data regarding the third-party payor mix and resulting rebates related to shipments of SUSTOL to our Customers. In addition, we have further determined that we do not currently have the necessary data to provide sufficient visibility into the ultimate utilization of SUSTOL, which inhibits our ability to determine a reasonable estimate of potential returns. As a result of the above, we have determined that we are not yet able to reliably make all of the estimates necessary to meet certain of the key recognition criteria to recognize product sales as revenue with respect to shipments of SUSTOL to our Customers. Accordingly, we concluded that revenue related to shipments to our Customers should be deferred, except to the extent that our Customers have resold SUSTOL to healthcare providers. As of December 31, 2016, product sales of $1.1 million to our Customers have been deferred and are recorded as deferred revenue on our consolidated balance sheet.
Product Sales Allowances
We recognize product sales allowances as a reduction of product sales in the same period the related revenue is recognized. Product sales allowances are based on amounts owed or to be claimed on the related sales. These estimates take into consideration the terms of our agreements with Customers, historical product returns, rebates or discounts taken, the shelf life of the product, and specific known market events, such as competitive pricing and new product introductions. If actual future results vary from our estimates, we may need to adjust these estimates, which could have an effect on product sales and earnings in the period of adjustment. Our product sales allowances include:
• Product Returns — We allow our Customers to return product for credit 12 months after its product expiration date. Because of the shelf life of our product and our return policy, there may be a significant period of time between the time the product is shipped and the time the credit is issued on returned product.
• Distributor Fees — We offer contractually determined discounts to our Customers. These discounts are paid on a quarterly basis within two months after the quarter in which product was shipped.
• Group Purchasing Organization (“GPO”) Discounts and Rebates — We offer cash discounts to GPO members. These discounts are taken when the GPO members purchase SUSTOL from our Customers, who then charge back to us the dis-
59
Table of Contents
count amount. Additionally, we offer volume and contract-tier rebates to GPO members. Rebates are estimated based on actual purchase levels during the quarterly or semi-annual rebate purchase period.
• GPO Administrative Fees — We pay administrative fees to GPO’s for services and access to data. These fees are based on contracted terms and are paid after the quarter in which the product was purchased by the GPO’s members.
• Medicaid Rebates — We participate in Medicaid rebate programs, which provide assistance to certain low-income patients based on each individual state’s guidelines regarding eligibility and services. Under the Medicaid rebate programs, we pay a rebate to each participating state, generally within three months after the quarter in which SUSTOL was sold.
We believe our estimated allowance for product returns requires a high degree of judgment and is subject to change based on our experience and certain quantitative and qualitative factors. We believe our estimated allowances for distributor fees, GPO discounts, rebates and administrative fees, and Medicaid rebates do not require a high degree of judgement because the amounts are settled within a relatively short period of time.
Our product sales allowances and related accruals are evaluated each reporting period and adjusted when trends or significant events indicate that a change in estimate is appropriate.
Inventory
Inventory is stated at the lower of cost or estimated net realizable value, on a first-in, first-out, or FIFO, basis. We periodically analyze our inventory levels and write down inventory that has become obsolete, inventory that has a cost basis in excess of its estimated realizable value and inventory quantities that are in excess of expected sales requirements as cost of product sales. The determination of whether inventory costs will be realizable requires estimates by management. If actual market conditions are less favorable than projected by management, additional write-downs of inventory may be required, which would be recorded as cost of product sales.
We capitalize pre-launch costs into inventory when we believe it is probable that: (i) a future economic benefit will be derived from the commercialization of the product; (ii) the FDA will approve the marketing of the product; and (iii) our process for manufacturing the product is within the specifications that we believe will be approved by the FDA for such product. In evaluating whether it is probable that we will derive future economic benefits from our pre-launch inventories and whether the pre-launch inventories are stated at the lower of cost or net realizable value, we consider, among other things, the remaining shelf life of that inventory, the current and expected market conditions, the amount of inventory on hand, and the substance of communications with the FDA during the regulatory approval process.
For the year ended December 31, 2016, we recognized cost of product sales of $35,000 for sales of SUSTOL, primarily related to shipping and distribution costs. All SUSTOL units sold in 2016 were manufactured prior to the approval of SUSTOL, and the costs to manufacture such units were recorded to research and development expense in prior periods. We expect the cost of product sales to increase in 2017, as we began capitalizing raw materials, labor and overhead related to the manufacturing of SUSTOL in the third quarter of 2016.
60
Table of Contents
Accrued Clinical Liabilities
We accrue clinical costs based on work performed, which relies on estimates of the services received from other parties and related expenses incurred. Clinical trial-related contracts vary significantly in duration, and may be for a fixed amount, based on the achievement of certain contingent events or deliverables, a variable amount based on actual costs incurred, capped at a certain limit, or a combination of these approaches. Revisions are recorded to research and development expense in the period in which the facts that give rise to the revision become known. Historically, revisions have not resulted in material changes to research and development expense, however, a modification in the protocol of a clinical trial or cancellation of a trial could result in a material charge to our results of operations.
Income Taxes
We make certain estimates and judgments in determining income tax expense for financial statement purposes. These estimates and judgments occur in the calculation of certain tax assets and liabilities, which arise from differences in the timing of recognition of revenue and expense for tax and financial statement purposes. As part of the process of preparing our consolidated financial statements, we are required to estimate our income taxes in each of the jurisdictions in which we operate. This process involves estimating our current tax exposure under the most recent tax laws and assessing temporary differences resulting from differing treatment of items for tax and financial statement purposes.
We assess the likelihood that we will be able to recover our deferred tax assets. In doing so, we consider all available evidence, both positive and negative, including our historical levels of income and losses, expectations and risks associated with estimates of future taxable income and ongoing prudent and feasible tax planning strategies in assessing the need for a valuation allowance. A valuation allowance is provided when it is more likely than not that the deferred tax assets will not be realized. At December 31, 2016, we established a valuation allowance to offset our net deferred tax assets due to the uncertainty of realizing future tax benefits from our net operating loss carryforwards and other deferred tax assets.
Additionally, we believe that our deferred tax assets may have been limited in accordance with a provision of the Internal Revenue Code of 1986, whereby net operating loss and tax credit carryforwards available for use in a given period are limited upon the occurrence of certain events, including a significant change in ownership interests. As a result, our deferred tax assets and related valuation allowance were reduced for the estimated impact of the net operating losses and credits that may expire unused.
Should there be a change in our ability to recover our deferred tax assets, we would recognize a benefit to our tax provision in the period in which we determine that it is more likely than not that we will recover our deferred tax assets.
Stock-Based Compensation
We generally grant equity-based awards under our stockholder-approved, stock-based compensation plans. We have granted, and may in the future grant, options and restricted stock awards to employees, directors, consultants and advisors under our 2007 Amended and Restated Equity Incentive Plan. In addition, all of our employees are eligible to participate in our 1997 Employee Stock Purchase Plan, which enables employees to purchase common stock at a discount through payroll deductions. Prior to our relisting on The NASDAQ Capital Market in January 2014, we issued non-plan stock option grants to
61
Table of Contents
certain employees, as set forth under Item 12 of this Annual Report on Form 10-K. These non-plan stock option grants were registered with the Securities and Exchange Commission (“SEC”) on Form S-8.
We estimate the fair value of stock options granted using the Black-Scholes option pricing model. This fair value is then amortized over the requisite service periods of the awards. The Black-Scholes option pricing model requires the input of subjective assumptions, including each option’s expected life and price volatility of the underlying stock. Expected volatility is based on our historical stock price volatility. The expected life of employee stock options represents the average of the contractual term of the options and the weighted-average vesting period, as permitted under the simplified method.
As stock-based compensation expense is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures are estimated based on historical experience. Changes in assumptions used under the Black-Scholes option pricing model could materially affect our net loss and net loss per share.
New Accounting Pronouncements
See Note 2 to the Consolidated Financial Statements included in Item 8 of this Annual Report on Form 10-K.
Results of Operations
Years Ended December 31, 2016 and 2015
Net Product Sales
For the year ended December 31, 2016, we recognized net product sales of $1.3 million for sales of SUSTOL. Due to the recent launch of SUSTOL in October 2016, we have recognized net product sales as revenue only when our Customers have sold SUSTOL to their customers, the end users. As of December 31, 2016, we have deferred $1.1 million in SUSTOL sales to our Customers. SUSTOL is our first commercial product and, as such, there was no comparable activity in 2015.
Cost of Product Sales
For the year ended December 31, 2016, we recognized cost of product sales of $35,000 for sales of SUSTOL, primarily related to shipping and distribution costs. All SUSTOL units sold in 2016 were manufactured prior to the approval of SUSTOL, and the costs to manufacture such units were recorded to research and development expense in prior periods. We expect the cost of product sales to increase in 2017, as we began capitalizing raw materials, labor and overhead related to the manufacturing of SUSTOL in the third quarter of 2016. There was no comparable activity in 2015.
62
Table of Contents
Research and Development Expense
Research and development expense consisted of the following (in thousands):
| December 31, | ||||||||
|
|
2016 | 2015 | ||||||
| SUSTOL related costs |
$ | 9,618 | $ | 24,438 | ||||
| HTX-011 related costs |
40,356 | 9,834 | ||||||
| CINVANTI related costs |
8,675 | 5,070 | ||||||
| New product development related costs |
5,358 | 3,162 | ||||||
| Personnel and related costs |
22,571 | 10,216 | ||||||
| Stock-based compensation expense |
11,316 | 4,701 | ||||||
| Facility related costs |
2,597 | 1,952 | ||||||
| Other |
2,634 | 1,810 | ||||||
|
|
|
|||||||
| Total research and development expense |
$ | 103,125 | $ | 61,183 | ||||
|
|
|
|||||||
For the year ended December 31, 2016, research and development expense increased to $103.1 million from $61.2 million for the same period in 2015. The increase in research and development expense was primarily due to an increase in clinical and manufacturing costs associated with our Phase 2 clinical program for HTX-011 of $30.5 million, an increase in personnel and related costs of $12.4 million, an increase in stock-based compensation expense of $6.6 million, an increase in CINVANTI development costs of $3.6 million, and an increase in new product development of $2.2 million. The increase in personnel and related costs was primarily due to an $8.3 million increase in headcount and related expense to support our development efforts. In addition, the increase was due to $4.1 million of costs incurred in connection with the realignment of our objectives and new development focus following the approval of SUSTOL. These increases were partially offset by a decrease of $14.8 million in SUSTOL related costs due to the completion of our Phase 3 program in 2015.
General and Administrative Expense
For the year ended December 31, 2016, general and administrative expense increased to $21.4 million from $18.4 million for the same period in 2015. The increase in general and administrative expense was primarily due to an increase in stock-based compensation expense and an increase in headcount and related expense and professional fees to support our increased development and pre-commercialization efforts.
Sales and Marketing Expense
For the year ended December 31, 2016, sales and marketing expense increased to $47.7 million from $17.3 million for the same period in 2015. The increase in sales and marketing expense was primarily due to an increase in activities in preparation for the commercial launch of SUSTOL, including the hiring and training of a sales force, as well as an increase in stock-based compensation expense.
63
Table of Contents
Other Expense, net
For the year ended December 31, 2016, other expense, net increased to $2.2 million from $0.7 million for the same period in 2015. The increase in other expense, net was primarily due to interest expense incurred in 2016 related to the Promissory Note, as well as interest expense and amortization of debt discount related to the Convertible Notes.
Years Ended December 31, 2015 and 2014
Research and Development Expense
Research and development expense consisted of the following (in thousands):
| December 31, | ||||||||
|
|
2015 | 2014 | ||||||
| SUSTOL related costs |
$ | 24,438 | $ | 33,758 | ||||
| HTX-011 related costs |
9,834 | 3,189 | ||||||
| CINVANTI related costs |
5,070 | 495 | ||||||
| New product development related costs |
3,162 | 3,077 | ||||||
| Personnel and related costs |
10,216 | 7,868 | ||||||
| Stock-based compensation expense |
4,701 | 3,329 | ||||||
| Facility related costs |
1,952 | 1,716 | ||||||
| Other |
1,810 | 1,401 | ||||||
|
|
|
|
|
|||||
| Total research and development expense |
$ | 61,183 | $ | 54,833 | ||||
|
|
|
|
|
|||||
For the year ended December 31, 2015, research and development expense increased to $61.2 million from $54.8 million for the same period in 2014. This increase was primarily a result of an increase of $6.6 million in research and development expense for clinical and manufacturing costs associated with our Phase 1 and Phase 2 clinical studies for HTX-011 and an increase of $4.6 million in costs associated with the development of CINVANTI. The increase was also due to an increase of $2.3 million in headcount and related expense for personnel and increased stock-based compensation expense of $1.4 million. These increases were partially offset by a decrease of $9.3 million in SUSTOL related costs, as our Phase 3 clinical program was completed in May 2015.
General and Administrative Expense
For the year ended December 31, 2015, general and administrative expense increased to $18.4 million compared to $11.0 million for the year ended December 31, 2014, primarily as a result of an increase in stock-based compensation expense of $3.8 million and an increase in headcount and related expense and professional fees to support our increased development and pre-commercialization efforts.
64
Table of Contents
Sales and Marketing Expense
For the year ended December 31, 2015, sales and marketing expense increased to $17.3 million compared to $8.7 million for the year ended December 31, 2014, primarily as a result of an increase of $7.5 million in costs for SUSTOL launch preparation activities and an increase in stock-based compensation expense of $1.2 million.
Other Expense, net
Other expense, net was $0.7 million for the year ended December 31, 2015, compared to $1.8 million for the year ended December 31, 2014. Other expense, net decreased in 2015 primarily due to the write-off of impaired manufacturing related equipment of $0.9 million recorded in 2014. For the year ended December 31, 2015, other expense, net consisted primarily of interest expense and amortization of debt discount related to our Convertible Notes.
Liquidity and Capital Resources
In January 2017, we completed an underwritten public offering of our common stock for net proceeds of $163.7 million. As of December 31, 2016, we had cash, cash equivalents and short-term investments of $51.1 million, or $214.8 million in pro-forma cash, cash equivalents and short-term investments, adjusting for the January 2017 public offering. This compares to $131.2 million in cash, cash equivalents and short-term investments as of December 31, 2015.
Our net cash used for operating activities for the year ended December 31, 2016 was $134.1 million compared to net cash used for operating activities of $78.5 million for the same period in 2015. Our net loss for the year ended December 31, 2016 was $173.1 million, or $4.56 per share, compared to a net loss of $97.6 million, or $2.95 per share, for the same period in 2015. The increases in net cash used for operating activities and net loss in 2016 as compared to 2015 was primarily due to costs incurred in preparation for the commercial launch of SUSTOL, as well as clinical and manufacturing costs related to our Phase 2 clinical studies for HTX-011 and costs associated with the development of CINVANTI.
Historically, we have financed our operations, including technology and product research and development, primarily through sales of our common stock and debt financings.
In August 2016, we entered into the Promissory Note with Tang Capital Partners, LP (“TCP”) whereby TCP agreed to lend us up to $100.0 million. The Promissory Note has a two-year term and bears interest of 8% per annum. The first close of $50.0 million occurred on August 5, 2016. The second close of an additional $50.0 million is subject to the achievement of a corporate milestone. There are no fees, no warrants and no equity conversion feature associated with this transaction. The Promissory Note is secured by a second-priority lien on substantially all of our assets. TCP is controlled by Tang Capital Management, LLC (“TCM”). The manager of TCM is Kevin C. Tang, who serves as the Chairman of our Board of Directors. The terms of the Promissory Note were determined by our independent directors to be no less favorable than terms that would be obtained in an arm’s length financing transaction.
In June 2015, we completed a public offering of common stock as a result of which we received $128.2 million in proceeds, net of issuance costs.
65
Table of Contents
Based on our current operating plan and projections, management believes that available cash, cash equivalents and short-term investments are sufficient to fund operations for at least one year from the date this annual report on Form 10-K is filed with the SEC. Our capital requirements going forward will depend on numerous factors, including but not limited to: the degree of commercial success of SUSTOL; the scope, rate of progress, results and costs of preclinical testing and clinical trials; an approval decision by the FDA with respect to CINVANTI; the timing and costs associated with the commercial launch of CINVANTI, if approved; the degree of commercial success of CINVANTI; the number and characteristics of product development programs we pursue and the pace of each program, including the timing of clinical trials; the time, cost and outcome involved in seeking other regulatory approvals; scientific progress in our research and development programs; the magnitude and scope of our research and development programs; our ability to establish and maintain strategic collaborations or partnerships for research, development, clinical testing, manufacturing and marketing of our product candidates; the cost and timing of establishing sales, marketing and distribution capabilities if we commercialize products independently; the cost of establishing clinical and commercial supplies of our product candidates and any products that we may develop and general market conditions.
We may not be able to raise sufficient additional capital when we need it on favorable terms, or at all. The sale of additional equity in the future may be dilutive to our stockholders. If we are unable to obtain adequate funds on reasonable terms, we may be required to curtail operations significantly or to obtain funds by entering into financing, supply or collaboration agreements on unattractive terms. There can be no assurance that our product development efforts related to any of our product candidates will be successful, that required regulatory approvals will be obtained, or that any products, including SUSTOL will be successfully marketed or achieve commercial acceptance. Until we can generate significant continuing revenues, we expect to satisfy our future cash needs through public or private equity offerings, debt financings and corporate collaboration and licensing arrangements. We cannot be certain that additional funding will be available to us on acceptable terms, or at all. Our ability to obtain new financing may be constrained by our failure to achieve significant business objectives, covenants applicable to the Convertible Notes and the Promissory Note, and numerous other factors.
The following table summarizes our contractual obligations as of December 31, 2016 (in thousands):
| Payment due by period | ||||||||||||||||||||
|
|
Total | Less than 1 year |
1-3 years |
3-5 years |
More than 5 years |
|||||||||||||||
| Operating lease obligations |
$ | 13,058 | $ | 2,259 | $ | 4,377 | $ | 2,890 | $ | 3,532 | ||||||||||
| Promissory note payable |
50,000 | — | 50,000 | — | — | |||||||||||||||
| Purchase obligations |
6,746 | 6,746 | — | — | — | |||||||||||||||
|
|
|
|||||||||||||||||||
| Total |
$ | 69,804 | $ | 9,005 | $ | 54,377 | $ | 2,890 | $ | 3,532 | ||||||||||
|
|
|
|||||||||||||||||||
On April 11, 2016, we entered into the Fourth Amendment to Lease (as amended, the “Lease Amendment”) with Metropolitan Life Insurance Company, effective as of April 11, 2016, pursuant to which we extended the lease for our Redwood City offices through May 31, 2019. Under the Lease Amendment, we also agreed to pay a basic annual rent of $1.0 million per annum through May 31, 2019 and such other amounts as set forth in the Lease Amendment.
On October 18, 2016, we entered into a lease (the “Lease”) with AP3-SD1 Campus Point LLC (the “Landlord”) pursuant to which we leased office and laboratory space located at 4242 Campus Point Court, San Diego, California for a period of seven years and four and one-half months, beginning on December 1, 2016. Pursuant to the Lease, we will pay a basic annual rent
66
Table of Contents
that rises incrementally over the term of the lease from $1.3 million for the first 12 months of the Lease to a prorated portion of $1.6 million for the last four and one-half months of the Lease, and such other amounts as set forth in the Lease. We also paid to the Landlord a security deposit of $0.1 million.
The holders of the Convertible Notes may require prepayment of such notes at any time at each holder’s option (see Notes to Consolidated Financial Statements included in this Annual Report on Form 10-K). As of December 31, 2016, $6.0 million aggregate principal amount of the Convertible Notes were outstanding.
At December 31, 2016, purchase obligations primarily consisted of commitments with third-party manufacturers in connection with the manufacturing of SUSTOL, as well as commitments with various vendors for sales and marketing support and clinical and preclinical studies. Total purchase obligations of $4.6 million were not included in our consolidated financial statements for the year ended December 31, 2016. We intend to use our current financial resources to fund our commitments under these purchase obligations.
We also enter into agreements from time to time with clinical sites and clinical research organizations for the conduct of our clinical trials. We make payments to these sites and organizations based in part upon the number of eligible patients enrolled and the length of their participation in the clinical trials. Under certain of these agreements, we may be subject to penalties in the event that we prematurely terminate these agreements. At this time, due to the variability associated with clinical site and contract research organization agreements, we are unable to estimate with certainty the future costs we will incur. We intend to use our current financial resources to fund our obligations under these commitments.
In addition, we entered into executive employment or management retention agreements with our executive officers and certain other key employees that, under certain cases, provide for a one-time severance payment and certain other benefits if these executives or employees are terminated under special circumstances. These agreements generally expire upon termination for cause or when we have met our obligations under these agreements. In connection with our realignment of goals and objectives and new development focus following the approval of SUSTOL, certain employees have or will receive a one-time severance payment upon termination, as well as other benefits as required by the executive employment or management retention agreements. We expect to fulfill our remaining obligation under these agreements by the second quarter of 2018 (see Note 6 to our Consolidated Financial Statements).
Off-Balance Sheet Arrangements
We are not involved in any “off-balance sheet arrangements” within the meaning of the rules of the SEC.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
The primary objective of our investment activities is to preserve our capital to fund operations. Our exposure to market risk for changes in interest rates relates primarily to the increase or decrease in the amount of interest income we can earn on our investment portfolio. Our risk associated with fluctuating interest income is limited to our investments in interest rate-sensitive financial instruments. Under our current policies, we do not use interest rate derivative instruments to manage this exposure to interest rate changes. We seek to ensure the safety and preservation of our invested principal by limiting default risk, market risk and reinvestment risk. We mitigate default risk by investing in short-term investment grade securities, such as treasury-
67
Table of Contents
backed money market funds, corporate debt securities and commercial paper. As a result of the generally short-term nature of our investments, a 50-basis point movement in market interest rates would not have a material impact on the fair value of our portfolio as of December 31, 2016. While changes in our interest rates may affect the fair value of our investment portfolio, any gains or losses are not recognized in our consolidated statement of comprehensive loss until the investment is sold or if a reduction in fair value is determined to be a permanent impairment. Our debt obligations on our Convertible Notes carry a fixed interest rate and, as a result, we are not exposed to interest rate risk on our convertible debt. The Promissory Note also carries a fixed interest rate. We seek to ensure the safety and preservation of our invested principal by limiting default risk, market risk and reinvestment risk. We do not have any material foreign currency obligations or other derivative financial instruments.
68
Table of Contents
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of
Heron Therapeutics, Inc.
We have audited the accompanying consolidated balance sheets of Heron Therapeutics, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of comprehensive loss, stockholders’ equity (deficit) and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements audited by us present fairly, in all material respects, the financial position of Heron Therapeutics, Inc. at December 31, 2016 and 2015, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2016, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Heron Therapeutics, Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 22, 2017 expressed an unqualified opinion thereon.
/s/ OUM & CO. LLP
San Francisco, California
February 22, 2017
69
Table of Contents
HERON THERAPEUTICS, INC.
CONSOLIDATED BALANCE SHEETS
|
|
December 31, 2016 |
December 31, 2015 |
||||||
| (In thousands, except par value amounts) |
||||||||
| ASSETS | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 13,414 | $ | 75,180 | ||||
| Short-term investments |
37,724 | 55,986 | ||||||
| Accounts receivable, net |
1,960 | — | ||||||
| Inventory |
5,340 | — | ||||||
| Prepaid expenses and other current assets |
3,705 | 3,585 | ||||||
|
|
|
|||||||
| Total current assets |
62,143 | 134,751 | ||||||
| Property and equipment, net |
5,076 | 3,049 | ||||||
| Other assets |
263 | 45 | ||||||
|
|
|
|||||||
| Total assets |
$ | 67,482 | $ | 137,845 | ||||
|
|
|
|||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 6,814 | $ | 3,300 | ||||
| Accrued clinical liabilities |
13,207 | 5,231 | ||||||
| Accrued payroll and employee liabilities |
8,414 | 4,828 | ||||||
| Other accrued liabilities |
6,288 | 4,154 | ||||||
| Deferred revenue |
1,099 | — | ||||||
| Convertible notes payable to related parties, net of discount |
2,911 | 2,222 | ||||||
|
|
|
|||||||
| Total current liabilities |
38,733 | 19,735 | ||||||
| Promissory note payable to related party |
50,000 | — | ||||||
|
|
|
|||||||
| Total liabilities |
88,733 | 19,735 | ||||||
|
|
|
|||||||
| Commitments and contingencies (see Note 5) |
||||||||
| Stockholders’ equity (deficit): |
||||||||
| Preferred stock, $0.01 par value: 2,500 shares authorized; no shares issued or outstanding at December 31, 2016 and 2015 |
— | — | ||||||
| Common stock, $0.01 par value: 75,000 shares authorized; 39,355 and 36,106 shares issued and outstanding at December 31, 2016 and 2015, respectively |
394 | 361 | ||||||
| Additional paid-in capital |
564,343 | 530,617 | ||||||
| Accumulated other comprehensive loss |
(17) | (40) | ||||||
| Accumulated deficit |
(585,971) | (412,828) | ||||||
|
|
|
|||||||
| Total stockholders’ equity (deficit) |
(21,251) | 118,110 | ||||||
|
|
|
|||||||
| Total liabilities and stockholders’ equity (deficit) |
$ | 67,482 | $ | 137,845 | ||||
|
|
|
|||||||
See accompanying Notes to Consolidated Financial Statements.
70
Table of Contents
HERON THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
| Years Ended December 31, | ||||||||||||
|
|
2016 | 2015 | 2014 | |||||||||
| (In thousands, except per share amounts) |
||||||||||||
| Revenues: |
||||||||||||
| Net product sales |
$ | 1,279 | $ | — | $ | — | ||||||
| Operating expenses: |
||||||||||||
| Cost of product sales |
35 | — | — | |||||||||
| Research and development |
103,125 | 61,183 | 54,833 | |||||||||
| General and administrative |
21,366 | 18,395 | 11,020 | |||||||||
| Sales and marketing |
47,668 | 17,347 | 8,708 | |||||||||
|
|
|
|||||||||||
| Total operating expenses |
172,194 | 96,925 | 74,561 | |||||||||
|
|
|
|||||||||||
| Loss from operations |
(170,915) | (96,925) | (74,561) | |||||||||
|
|
|
|||||||||||
| Other expense, net: |
||||||||||||
| Interest expense |
(2,664) | (958) | (887) | |||||||||
| Other income (expense) |
436 | 292 | (919) | |||||||||
|
|
|
|||||||||||
| Total other expense, net |
(2,228) | (666) | (1,806) | |||||||||
|
|
|
|||||||||||
| Net loss |
(173,143) | (97,591) | (76,367) | |||||||||
| Other comprehensive loss: |
||||||||||||
| Unrealized gains (losses) on short-term investments |
23 | (40) | — | |||||||||
|
|
|
|||||||||||
| Comprehensive loss |
$ | (173,120) | $ | (97,631) | $ | (76,367) | ||||||
|
|
|
|||||||||||
| Basic and diluted net loss per share |
$ | (4.56) | $ | (2.95) | $ | (2.87) | ||||||
|
|
|
|||||||||||
| Shares used in computing basic and diluted net loss per share |
37,925 | 33,081 | 26,569 | |||||||||
|
|
|
|||||||||||
See accompanying Notes to Consolidated Financial Statements.
71
Table of Contents
HERON THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(In thousands)
|
Common Stock |
Additional Paid-In Capital |
Accumulated Other Comprehensive Loss |
Accumulated Deficit |
Total Stockholders’ Equity (Deficit) |
||||||||||||||||||||
|
|
Shares | Amount | ||||||||||||||||||||||
| Balance, December 31, 2013 |
23,572 | $ | 237 | $ | 307,578 | $ | — | $ | (238,870) | $ | 68,945 | |||||||||||||
| Issuance of common stock and pre-funded warrants in a public offering, net |
4,751 | 47 | 58,869 | — | — | 58,916 | ||||||||||||||||||
| Conversion benefit included in Convertible Notes issued |
— | — | 309 | — | — | 309 | ||||||||||||||||||
| Issuance of common stock under Employee Stock Purchase Plan |
12 | — | 91 | — | — | 91 | ||||||||||||||||||
| Issuance of common stock upon exercise of stock options |
588 | 5 | 3,096 | — | — | 3,101 | ||||||||||||||||||
| Issuance of common stock upon exercise of warrants |
304 | 3 | (3) | — | — | — | ||||||||||||||||||
| Stock-based compensation expense |
— | — | 8,067 | — | — | 8,067 | ||||||||||||||||||
| Net loss |
— | — | — | — | (76,367) | (76,367) | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Balance, December 31, 2014 |
29,227 | 292 | 378,007 | — | (315,237) | 63,062 | ||||||||||||||||||
| Issuance of common stock in a public offering, net |
5,520 | 55 | 128,144 | — | — | 128,199 | ||||||||||||||||||
| Conversion benefit included in Convertible Notes issued |
— | — | 328 | — | — | 328 | ||||||||||||||||||
| Issuance of common stock under Employee Stock Purchase Plan |
31 | — | 257 | — | — | 257 | ||||||||||||||||||
| Issuance of common stock upon exercise of stock options |
1,042 | 11 | 9,524 | — | — | 9,535 | ||||||||||||||||||
| Issuance of common stock upon exercise of warrants |
286 | 3 | (3) | — | — | — | ||||||||||||||||||
| Stock-based compensation expense |
— | — | 14,360 | — | — | 14,360 | ||||||||||||||||||
| Net loss |
— | — | — | — | (97,591) | (97,591) | ||||||||||||||||||
| Net unrealized loss on short-term investments |
— | — | — | (40) | — | (40) | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Comprehensive loss |
— | — | — | — | — | (97,631) | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Balance, December 31, 2015 |
36,106 | 361 | 530,617 | (40) | (412,828) | 118,110 | ||||||||||||||||||
| Conversion benefit included in Convertible Notes issued |
— | — | 348 | — | — | 348 | ||||||||||||||||||
| Issuance of common stock under Employee Stock Purchase Plan |
55 | 1 | 770 | — | — | 771 | ||||||||||||||||||
| Issuance of common stock upon exercise of stock options |
798 | 8 | 6,676 | — | — | 6,684 | ||||||||||||||||||
| Issuance of common stock upon exercise of warrants |
2,396 | 24 | (24) | — | — | — | ||||||||||||||||||
| Stock-based compensation expense |
— | — | 25,956 | — | — | 25,956 | ||||||||||||||||||
| Net loss |
— | — | — | — | (173,143) | (173,143) | ||||||||||||||||||
| Net unrealized gain on short-term investments |
— | — | — | 23 | — | 23 | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Comprehensive loss |
— | — | — | — | — | (173,120) | ||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Balance, December 31, 2016 |
39,355 | $ | 394 | $ | 564,343 | $ | (17) | $ | (585,971) | $ | (21,251) | |||||||||||||
|
|
|
|||||||||||||||||||||||
See accompanying Notes to Consolidated Financial Statements.
72
Table of Contents
HERON THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | ||||||||||||
|
|
2016 | 2015 | 2014 | |||||||||
| (In thousands) | ||||||||||||
| Operating activities: |
||||||||||||
| Net loss |
$ | (173,143) | $ | (97,591) | $ | (76,367) | ||||||
| Adjustments to reconcile net loss to net cash used for operating activities: |
||||||||||||
| Stock-based compensation expense |
25,956 | 14,360 | 8,067 | |||||||||
| Depreciation and amortization |
1,099 | 734 | 578 | |||||||||
| Amortization of debt discount |
689 | 624 | 573 | |||||||||
| Impairment loss on property and equipment |
— | — | 905 | |||||||||
| Loss (gain) on disposal of property and equipment |
9 | (118) | 17 | |||||||||
| Amortization of premium on short-term investments |
274 | 89 | — | |||||||||
| Change in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
(1,960) | — | — | |||||||||
| Prepaid expenses and other current assets |
(338) | (2,443) | (419) | |||||||||
| Inventory |
(5,340) | — | — | |||||||||
| Accounts payable |
3,514 | 751 | 1,285 | |||||||||
| Accrued clinical liabilities |
7,976 | 1,420 | 2,038 | |||||||||
| Accrued payroll and employee liabilities |
3,586 | 2,097 | 165 | |||||||||
| Deferred revenue |
1,099 | — | — | |||||||||
| Other accrued liabilities |
2,482 | 1,551 | 2,876 | |||||||||
|
|
|
|||||||||||
| Net cash used for operating activities |
(134,097) | (78,526) | (60,282) | |||||||||
| Investing activities: |
||||||||||||
| Purchases of short-term investments |
(43,318) | (56,115) | — | |||||||||
| Maturities of short-term investments |
61,329 | — | — | |||||||||
| Purchases of property and equipment |
(3,135) | (1,086) | (1,438) | |||||||||
| Proceeds from the sale of property and equipment |
— | 241 | — | |||||||||
|
|
|
|||||||||||
| Net cash provided by (used for) investing activities |
14,876 | (56,960) | (1,438) | |||||||||
| Financing activities: |
||||||||||||
| Net proceeds from sale of common stock and/or pre-funded warrants |
— | 128,199 | 58,916 | |||||||||
| Proceeds from purchases under the Employee Stock Purchase Plan |
771 | 257 | 91 | |||||||||
| Proceeds from stock option exercises |
6,684 | 9,535 | 3,101 | |||||||||
| Proceeds from issuance of promissory note payable to related party |
50,000 | — | — | |||||||||
|
|
|
|||||||||||
| Net cash provided by financing activities |
57,455 | 137,991 | 62,108 | |||||||||
|
|
|
|||||||||||
| Net (decrease) increase in cash and cash equivalents |
(61,766) | 2,505 | 388 | |||||||||
| Cash and cash equivalents at beginning of year |
75,180 | 72,675 | 72,287 | |||||||||
|
|
|
|||||||||||
| Cash and cash equivalents at end of year |
$ | 13,414 | $ | 75,180 | $ | 72,675 | ||||||
|
|
|
|||||||||||
| Supplemental disclosure of cash flow information: |
||||||||||||
| Interest paid |
$ | 1,622 | $ | — | $ | — | ||||||
|
|
|
|||||||||||
See accompanying Notes to Consolidated Financial Statements.
73
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Business
Heron Therapeutics, Inc. (the “Company”, “Heron”, or “we”) is a commercial-stage biotechnology company focused on improving the lives of patients by developing best-in-class medicines that address major unmet medical needs. We are developing novel, patient-focused solutions that apply our innovative science and technologies to already-approved pharmacological agents.
On August 9, 2016, our first commercial product, SUSTOL® (granisetron) extended-release injection (“SUSTOL”), was approved by the U.S. Food and Drug Administration (“FDA”). SUSTOL is indicated, in combination with other antiemetics, in adults for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide combination chemotherapy regimens. We commenced commercial sales of SUSTOL in October 2016.
We have two investigational pharmaceutical products for patients suffering from cancer or post-operative pain. CINVANTI™ (HTX-019), an intravenous formulation of the neurokinin-1 receptor antagonist aprepitant, has been developed for the prevention of chemotherapy-induced nausea and vomiting as an adjunct to other antiemetic agents. HTX-011, a long-acting formulation of the local anesthetic bupivacaine in a fixed-dose combination with the anti-inflammatory meloxicam, is being developed for the prevention of post-operative pain.
We have incurred significant operating losses and negative cash flows from operations. As of December 31, 2016, our accumulated deficit was $586.0 million and we had $51.1 million in cash, cash equivalents and short-term investments. In January 2017, we completed an underwritten public offering of our common stock for net proceeds of $163.7 million. As of December 31, 2016, our pro-forma cash, cash equivalents and short-term investments, adjusting for the January 2017 public offering, was $214.8 million. Based on our current operating plan and projections, management believes that available cash, cash equivalents and short-term investments are sufficient to fund operations for at least one year from the date this annual report on Form 10-K is filed with the U.S. Securities and Exchange Commission (the “SEC”).
2. Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Heron Therapeutics, Inc. and its wholly owned subsidiary, Heron Therapeutics, B.V., which was organized in the Netherlands in March 2015. Heron Therapeutics, B.V. has no operations and no material assets or liabilities, and there have been no significant transactions related to Heron Therapeutics, B.V. since its inception.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the financial statements and disclosures made in the accompanying notes to the financial statements. Our critical accounting policies that involve significant judgment and estimates include revenue recognition, inventory, accrued clinical liabilities, income taxes and stock-based compensation. Actual results could differ materially from those estimates.
74
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Cash, Cash Equivalents and Short-Term Investments
Cash and cash equivalents consist of cash and highly liquid investments with original maturities from purchase date of three months or less.
Short-term investments consist of securities with maturities from purchase date of greater than three months. We have classified our short-term investments as available-for-sale securities in the accompanying consolidated financial statements. Available-for-sale securities are stated at fair market value, with unrealized gains and losses reported in other comprehensive income (loss) and realized gains and losses included in interest income (expense). The cost of securities sold is based on the specific-identification method. Interest and dividends on securities classified as available-for-sale are included in interest income.
Our bank and investment accounts have been placed under control agreements in accordance with our Senior Secured Convertible Notes (the “Convertible Notes”) and Subordinated Secured Promissory Note (the “Promissory Note”) (see Note 7).
Fair Value of Financial Instruments
Financial instruments, including cash and cash equivalents, receivables, inventory, prepaid expenses, other current assets, accounts payable and accrued expenses, are carried at cost, which is considered to be representative of their respective fair values because of the short-term maturity of these instruments. Short-term available-for-sale investments are carried at fair value (see Note 3). Our Convertible Notes and Promissory Note outstanding at December 31, 2016 do not have a readily available ascertainable market value, however, the carrying value is considered to approximate its fair value.
Concentration of Credit Risk
Cash, cash equivalents and short-term investments are financial instruments that potentially subject us to concentrations of credit risk. We deposit our cash in financial institutions. At times, such deposits may be in excess of insured limits. We may also invest our excess cash in money market funds, corporate debt securities and commercial paper. We have established guidelines relative to our diversification of our cash investments and their maturities in an effort to maintain safety and liquidity. These guidelines are periodically reviewed and modified to take advantage of trends in yields and interest rates.
Sales to ASD Specialty Healthcare, Inc., Cardinal Health, Inc. and McKesson Specialty Care Distribution Corporation each accounted for 10% or more of our net revenues for the year ended December 31, 2016. The loss of any of these customers could materially and adversely affect our business, results of operations, financial condition and cash flows.
Inventory
Inventory is stated at the lower of cost or estimated net realizable value, on a first-in, first-out, or FIFO, basis. We periodically analyze our inventory levels, and write down inventory that has become obsolete, inventory that has a cost basis in excess of its estimated realizable value and inventory quantities that are in excess of expected sales requirements as cost of product sales. The determination of whether inventory costs will be realizable requires estimates by management. If actual market conditions are less favorable than projected by management, additional write-downs of inventory may be required, which would be recorded as cost of product sales.
75
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
We capitalize pre-launch costs into inventory when we believe it is probable that: (i) a future economic benefit will be derived from the commercialization of the product; (ii) the FDA will approve the marketing of the product; and (iii) our process for manufacturing the product is within the specifications that we believe will be approved by the FDA for such product. In evaluating whether it is probable that we will derive future economic benefits from our pre-launch inventories and whether the pre-launch inventories are stated at the lower of cost or net realizable value, we consider, among other things, the remaining shelf life of that inventory, the current and expected market conditions, the amount of inventory on hand, and the substance of communications with the FDA during the regulatory approval process.
For the year ended December 31, 2016, we recognized cost of sales of $35,000 for sales of SUSTOL, primarily related to shipping and distribution costs. All SUSTOL units sold in 2016 were manufactured prior to the approval of SUSTOL, and the costs to manufacture such units were recorded to research and development expense in prior periods.
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation and amortization. Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets (primarily five years). Leasehold improvements are stated at cost and amortized on a straight-line basis over the shorter of the estimated useful life of the asset or the lease term.
Impairment of Long-Lived Assets
If indicators of impairment exist, we assess the recoverability of the affected long-lived assets by determining whether the carrying value of such assets can be recovered through undiscounted future operating cash flows. If impairment is indicated, we measure the amount of such impairment by comparing the carrying value of the asset to the fair value of the asset and record the impairment as a reduction in the carrying value of the related asset with a corresponding charge to operating expenses. Estimating the undiscounted future cash flows associated with long-lived assets requires judgment and assumptions that could differ materially from actual results.
Revenue Recognition
Product Sales
SUSTOL is distributed in the U.S. through a limited number of specialty distributors (“Customers”) that resell SUSTOL to healthcare providers, the end users of SUSTOL. Product sales are recorded net of sales allowances and estimated rebates, chargebacks, distributor fees, and other deductions.
Product sales are recognized as revenue when there is persuasive evidence that an arrangement exists, delivery has occurred and title has passed, the price is fixed or determinable and we are reasonably assured of collecting the resulting receivable. Product sales are recorded net of sales allowances and estimated rebates, chargebacks, distributor fees, and other deductions. Due to the recent launch of SUSTOL, we do not yet have sufficient historical data regarding the third-party payor mix and resulting rebates related to shipments of SUSTOL to our Customers. In addition, we have further determined that we do not currently have the necessary data to provide sufficient visibility into the ultimate utilization of SUSTOL, which inhibits our ability to determine a reasonable estimate of potential returns. As a result of the above, we have determined that we are not yet able to reliably make all of the estimates necessary to meet certain of the key recognition criteria to recognize product sales as
76
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
revenue with respect to shipments of SUSTOL to our Customers. Accordingly, we concluded that revenue related to shipments to our Customers should be deferred, except to the extent that our Customers have resold SUSTOL to healthcare providers. As of December 31, 2016, product sales of $1.1 million to our Customers have been deferred and are recorded as deferred revenue on our consolidated balance sheet.
Product Sales Allowances
We recognize product sales allowances as a reduction of product sales in the same period the related revenue is recognized. Product sales allowances are based on amounts owed or to be claimed on the related sales. These estimates take into consideration the terms of our agreements with Customers, historical product returns, rebates or discounts taken, the shelf life of the product, and specific known market events, such as competitive pricing and new product introductions. If actual future results vary from our estimates, we may need to adjust these estimates, which could have an effect on product sales and earnings in the period of adjustment. Our product sales allowances include:
• Product Returns — We allow our Customers to return product for credit 12 months after its product expiration date. Because of the shelf life of our product and our return policy, there may be a significant period of time between the time the product is shipped and the time the credit is issued on returned product.
• Distributor Fees — We offer contractually determined discounts to our Customers. These discounts are paid on a quarterly basis within two months after the quarter in which product was shipped.
• Group Purchasing Organization (“GPO”) Discounts and Rebates — We offer cash discounts to GPO members. These discounts are taken when the GPO members purchase SUSTOL from our Customers, who then charge back to us the discount amount. Additionally, we offer volume and contract-tier rebates to GPO members. Rebates are estimated based on actual purchase levels during the quarterly or semi-annual rebate purchase period.
• GPO Administrative Fees — We pay administrative fees to GPO’s for services and access to data. These fees are based on contracted terms and are paid after the quarter in which the product was purchased by the GPO’s members.
• Medicaid Rebates — We participate in Medicaid rebate programs, which provide assistance to certain low-income patients based on each individual state’s guidelines regarding eligibility and services. Under the Medicaid rebate programs, we pay a rebate to each participating state, generally within three months after the quarter in which SUSTOL was sold.
We believe our estimated allowance for product returns requires a high degree of judgment and is subject to change based on our experience and certain quantitative and qualitative factors. We believe our estimated allowances for distributor fees, GPO discounts, rebates and administrative fees, and Medicaid rebates do not require a high degree of judgement because the amounts are settled within a relatively short period of time.
Our product sales allowances and related accruals are evaluated each reporting period and adjusted when trends or significant events indicate that a change in estimate is appropriate. Changes in sales allowance estimates could materially affect our results of operations and financial position. As of December 31, 2016, our product sales allowances totaled $342,000, which included $72,000 for distributor fees, $221,000 for rebates and chargebacks and $49,000 for product returns. During the year ended December 31, 2016, we made no payments related to rebates, chargebacks or product returns.
77
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Accrued Clinical Liabilities
We accrue clinical costs based on work performed, which relies on estimates of the progress of the trials and the related expenses incurred. Clinical trial related contracts vary significantly in duration, and may be for a fixed amount, based on the achievement of certain contingent events or deliverables, a variable amount based on actual costs incurred, capped at a certain limit, or contain a combination of these elements. Revisions are recorded to research and development expense in the period in which the facts that give rise to the revision become known. Historically, revisions have not resulted in material changes to research and development expense, however, a modification in the protocol of a clinical trial or cancellation of a trial could result in a material charge to our results of operations.
Research and Development Expenses
All costs of research and development are expensed in the period incurred. Research and development costs primarily consist of salaries and related expenses for personnel, stock-based compensation expense, fees paid to outside service providers and consultants, facilities costs and materials used in the clinical and preclinical trials and research and development.
Patent Costs
We incur outside legal fees in connection with filing and maintaining our various patent applications. All patent costs are expensed as incurred and are included in general and administrative expense in the consolidated statements of comprehensive loss.
Stock-Based Compensation Expense
We estimate the fair value of stock-based payment awards using the Black-Scholes option pricing model. This fair value is then amortized using the straight-line single-option method of attributing the value of stock-based compensation to expense over the requisite service periods of the awards. The Black-Scholes option pricing model requires the input of complex and subjective assumptions, including each option’s expected life and price volatility of the underlying stock.
As stock-based compensation expense is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures are estimated based on historical data.
Warrants
We have issued warrants to purchase shares of our common stock in conjunction with certain equity financings. The terms of the warrants were evaluated to determine the appropriate classification as equity or a liability.
Income Taxes
We recognize the impact of a tax position in our consolidated financial statements if the position is more likely than not to be sustained upon examination and on the technical merits of the position. The total amount of unrecognized tax benefits, if recognized, would affect other tax accounts, primarily deferred taxes in future periods, and would not affect our effective tax rate since we maintain a full valuation allowance against its deferred tax assets (see Note 9).
78
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Comprehensive Loss
Comprehensive loss is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Unrealized gains and losses on available-for-sale securities are included in other comprehensive loss and represent the difference between our loss and comprehensive net loss for all periods presented.
Earnings per Share
Basic earnings per share (“EPS”) is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period, without consideration of common share equivalents. Diluted EPS is computed by dividing the net loss by the weighted-average number of common shares and common share equivalents outstanding for the period determined using the treasury stock method. For purposes of this calculation, stock options, warrants and common stock underlying Convertible Notes are considered to be common stock equivalents and are included in the calculation of diluted EPS only when their effect is dilutive.
Because we have incurred a net loss for all periods presented in the consolidated statements of comprehensive loss, stock options, warrants and shares of common stock underlying Convertible Notes are not included in the computation of net loss per share because their effect would be anti-dilutive.
The following table includes the number of stock options, warrants and shares of common stock underlying Convertible Notes not included in the computation as of the dates shown below (in thousands):
| As of December 31, | ||||||||||||
|
|
2016 | 2015 | 2014 | |||||||||
| Stock options outstanding |
11,845 | 8,435 | 7,918 | |||||||||
| Warrants outstanding |
600 | 3,565 | 4,108 | |||||||||
| Shares of common stock underlying Convertible Notes outstanding |
7,521 | 7,087 | 6,677 | |||||||||
Recent Accounting Pronouncements
In March 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-09, Compensation — Stock Compensation: Improvements to Employee Share-Based Payment Accounting (“ASU 2016-09”). ASU 2016-09 addresses several aspects of the accounting for share-based payment award transactions, including: (a) income tax consequences; (b) classification of awards as either equity or liabilities; and (c) classification on the statement of cash flows. ASU 2016-09 is effective for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. We plan to adopt the provisions of ASU 2016-09 in the first quarter of 2017. We do not expect the adoption of ASU 2016-09 to have a material impact on our results of operations or financial condition.
In February 2016, FASB issued ASU No. 2016-02, Leases (“ASU 2016-02”). ASU 2016-02 requires a lessee to record on the balance sheet the assets and liabilities for the rights and obligations created by leases with lease terms of more than 12 months. In addition, ASU 2016-02 requires both lessees and lessors to disclose certain key information about lease trans-
79
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
actions. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. We plan to adopt the provisions of ASU 2016-02 in the first quarter of 2019 and we are currently evaluating the impact on our results of operations and financial condition.
In July 2015, FASB issued ASU No. 2015-11, Inventory (Topic 330) (“ASU 2015-11”). ASU 2015-11 requires entities to measure inventory at the lower of cost or net realizable value. Net realizable value is the estimated selling price in the ordinary course of business, less reasonably predicable costs of completion, disposal and transportation. Subsequent measurements are unchanged for inventory measured using LIFO or the retail inventory method. ASU 2015-11 is effective for fiscal years beginning after December 15, 2016, and interim periods within fiscal years beginning after December 15, 2017. We plan to adopt the provisions of ASU 2015-11 in 2017. We do not expect the adoption of ASU 2015-11 to have a material impact on our results of operations or financial condition.
In August 2014, FASB issued ASU No. 2014-15, Presentation of Financial Statements — Going Concern (Subtopic 205-40) (“ASU 2014-15”). ASU 2014-15 requires management to assess an entity’s ability to continue as a going concern by incorporating and expanding upon certain principles that are currently in U.S. auditing standards. Specifically, the amendments (1) provide a definition of the term substantial doubt, (2) require an evaluation every reporting period, including interim periods, (3) provide principles for considering the mitigating effect of management’s plans, (4) require certain disclosures when substantial doubt is alleviated as a result of consideration of management’s plans, (5) require an express statement and other disclosures when substantial doubt is not alleviated, and (6) require an assessment for a period of one year after the date that the financial statements are issued (or available to be issued). The amendments in ASU 2014-15 are effective for the annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. We adopted the provisions of ASU 2014-15 in 2016. The adoption of ASU 2014-15 did not require any additional disclosures in our consolidated financial statements for the year ended December 31, 2016.
In May 2014, FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606) (“ASU 2014-09”). ASU 2014-09 is based on the principle that revenue should be recognized to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. ASU 2014-09 is effective for fiscal years beginning after December 15, 2017, including interim periods within those fiscal years. The provisions of ASU 2014-09 allows for either a full retrospective or a modified retrospective adoption approach. We plan to adopt the provisions of ASU 2014-09 in the first quarter of 2018 and we are currently evaluating the impact on our results of operations and financial condition.
3. Fair Value Measurements
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, is as follows:
80
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
• Level 1 — Quoted prices in active markets for identical assets or liabilities.
• Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
• Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
We measure the following financial assets at fair value on a recurring basis. The fair values of these financial assets at December 31, 2016 and 2015, respectively, were as follows (in thousands):
| Fair Value Measurements at Reporting Date Using | ||||||||||||||||
|
|
Balance at December 31, 2016 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Money market funds |
$ | 11,493 | $ | 11,493 | $ | — | $ | — | ||||||||
| United States corporate debt securities |
12,039 | — | 12,039 | — | ||||||||||||
| Foreign corporate debt securities |
14,057 | — | 14,057 | — | ||||||||||||
| United States commercial paper |
8,635 | — | 8,635 | — | ||||||||||||
| Foreign commercial paper |
2,993 | — | 2,993 | — | ||||||||||||
|
|
|
|||||||||||||||
| Total |
$ | 49,217 | $ | 11,493 | $ | 37,724 | $ | — | ||||||||
|
|
|
|||||||||||||||
| Fair Value Measurements at Reporting Date Using | ||||||||||||||||
|
|
Balance at December 31, 2015 |
Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||
| Money market funds |
$ | 67,089 | $ | 67,089 | $ | — | $ | — | ||||||||
| United States corporate debt securities |
28,715 | — | 28,715 | — | ||||||||||||
| Foreign corporate debt securities |
4,922 | — | 4,922 | — | ||||||||||||
| United States commercial paper |
7,770 | — | 7,770 | — | ||||||||||||
| Foreign commercial paper |
15,579 | — | 15,579 | — | ||||||||||||
|
|
|
|||||||||||||||
| Total |
$ | 124,075 | $ | 67,089 | $ | 56,986 | $ | — | ||||||||
|
|
|
|||||||||||||||
There were no significant transfers between level 1 and level 2 investments during the years ended December 31, 2016 and December 31, 2015.
As of December 31, 2016, short-term investments included $37.7 million of available-for-sale securities with contractual maturities of one year or less. As of December 31, 2015, we had cash equivalents consisting of $1.0 million of available-for-sale
81
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
securities with contractual maturities of less than three months and short-term investments consisting of $56.0 million of available-for-sale securities with contractual maturities of one year or less. The money market funds as of December 31, 2016 and 2015 are included in cash and cash equivalents on the consolidated balance sheet.
A company may elect to use fair value to measure accounts and loans receivable, available-for-sale and held-to-maturity securities, equity method investments, accounts payable, guarantees and issued debt. Other eligible items include firm commitments for financial instruments that otherwise would not be recognized at inception and non-cash warranty obligations where a warrantor is permitted to pay a third party to provide the warranty goods or services. If the use of fair value is elected, any upfront costs and fees related to the item such as debt issuance costs must be recognized in earnings and cannot be deferred. The fair value election is irrevocable and generally made on an instrument-by-instrument basis, even if a company has similar instruments that it elects not to measure based on fair value. Unrealized gains and losses on existing items for which fair value has been elected are reported as a cumulative adjustment to beginning retained earnings and any changes in fair value are recognized in earnings. We have elected to not apply the fair value option to our financial assets and liabilities.
We consider the carrying amount of cash and cash equivalents, receivables, inventory, prepaid expenses and other current assets, accounts payable and accrued liabilities to be representative of their respective fair values because of the short-term nature of those instruments.
Unrealized gains and losses associated with our investments are reported in accumulated other comprehensive loss. For the year ended December 31, 2016, we recorded $23,000 in net unrealized gains associated with our short-term investments. For the year ended December 31, 2015, we recorded $40,000 in net unrealized losses associated with our short-term investments.
Realized gains and losses associated with our investments, if any, are reported in the statement of comprehensive loss. There were no realized gains or losses for the years ended December 31, 2016 and 2015.
4. Balance Sheet Details
Short-Term Investments
The following is a summary of our short-term, available-for-sale securities (in thousands):
| December 31, 2016 | ||||||||||||||||
|
|
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
||||||||||||
| United States corporate debt |
$ | 12,048 | $ | — | $ | (9) | $ | 12,039 | ||||||||
| Foreign corporate debt |
14,065 | — | (8) | 14,057 | ||||||||||||
| United States commercial paper |
8,635 | — | — | 8,635 | ||||||||||||
| Foreign commercial paper |
2,993 | — | — | 2,993 | ||||||||||||
|
|
|
|||||||||||||||
| Total |
$ | 37,741 | $ | — | $ | (17) | $ | 37,724 | ||||||||
|
|
|
|||||||||||||||
82
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| December 31, 2015 | ||||||||||||||||
|
|
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
||||||||||||
| United States corporate debt |
$ | 27,750 | $ | — | $ | (35) | $ | 27,715 | ||||||||
| Foreign corporate debt |
4,927 | — | (5) | 4,922 | ||||||||||||
| United States commercial paper |
7,770 | — | — | 7,770 | ||||||||||||
| Foreign commercial paper |
15,579 | — | — | 15,579 | ||||||||||||
|
|
|
|||||||||||||||
| Total |
$ | 56,026 | $ | — | $ | (40) | $ | 55,986 | ||||||||
|
|
|
|||||||||||||||
The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. We regularly monitor and evaluate the realizable value of our marketable securities. We did not recognize any impairment losses for the years ended December 31, 2016 and 2015.
Inventory
Inventory consists of the following (in thousands):
| December 31, | ||||||||
|
|
2016 | 2015 | ||||||
| Raw materials |
$ | 1,647 | $ | — | ||||
| Work in process |
1,104 | — | ||||||
| Finished goods |
2,589 | — | ||||||
|
|
|
|||||||
| Total inventory |
$ | 5,340 | $ | — | ||||
|
|
|
|||||||
Property and Equipment
Property and equipment consists of the following (in thousands):
| December 31, | ||||||||
|
|
2016 | 2015 | ||||||
| Scientific equipment |
$ | 7,952 | $ | 5,593 | ||||
| Computer equipment and software |
1,429 | 1,272 | ||||||
| Furniture, fixtures and office equipment |
674 | 352 | ||||||
| Leasehold improvements |
1,635 | 1,384 | ||||||
|
|
|
|||||||
| Property and equipment |
11,690 | 8,601 | ||||||
| Less: accumulated depreciation and amortization |
(6,614) | (5,552) | ||||||
|
|
|
|||||||
| Property and equipment, net |
$ | 5,076 | $ | 3,049 | ||||
|
|
|
|||||||
83
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Depreciation and amortization expense for the years ended December 31, 2016, 2015 and 2014 was $1.1 million, $0.7 million and $0.6 million, respectively. As of December 31, 2016 and 2015, $1.7 million and $0.2 million of property and equipment, respectively, were in process and not depreciated during the respective years.
During the year ended December 31, 2014, we recognized $0.9 million as an impairment loss due to the write-down of a piece of manufacturing-related equipment. In 2015, we sold the equipment for cash proceeds of $0.2 million, with a net gain of $0.1 million.
Accrued Payroll and Employee Liabilities and Other Accrued Liabilities
Accrued payroll and employee liabilities consist of the following (in thousands):
| December 31, | ||||||||
|
|
2016 | 2015 | ||||||
| Accrued employee salaries and benefits |
$ | 2,236 | $ | 1,863 | ||||
| Accrued bonuses |
3,474 | 2,965 | ||||||
| Accrued expenses for realignment (see Note 6) |
2,704 | — | ||||||
|
|
|
|||||||
| Total accrued payroll and employee liabilities |
$ | 8,414 | $ | 4,828 | ||||
|
|
|
|||||||
Other accrued liabilities consist of the following (in thousands):
| December 31, | ||||||||
|
|
2016 | 2015 | ||||||
| Accrued consulting and professional fees |
$ | 4,703 | $ | 3,914 | ||||
| Accrued accounts payable |
1,039 | 36 | ||||||
| Accrued sales allowances |
342 | — | ||||||
| Deferred rent |
116 | 61 | ||||||
| Other accrued liabilities |
88 | 143 | ||||||
|
|
|
|||||||
| Total other accrued liabilities |
$ | 6,288 | $ | 4,154 | ||||
|
|
|
|||||||
5. Commitments and Contingencies
Leases
We lease 28,275 square feet of laboratory and office space in San Diego, California under a lease which began on December 1, 2016 and expires on April 15, 2024. We have one 5-year option to renew this lease. We also lease 26,067 square feet of laboratory, office and warehouse space in Redwood City, California and 1,898 square feet of office space in Jersey City, New Jersey. The lease for the Redwood City space is under a lease expiring on May 31, 2019. The lease for the Jersey City office space expires on June 30, 2018. The annual rent expense for all properties is $2.0 million. We believe our facilities are adequate and suitable for our current needs, and that we will be able to obtain new or additional leased space in the future when necessary. We also lease certain office equipment under operating lease arrangements.
84
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Annual future minimum lease payments as of December 31, 2016 are as follows (in thousands):
|
|
Operating Leases | |||
| Years ended December 31, |
||||
| 2017 |
$ | 2,259 | ||
| 2018 |
2,553 | |||
| 2019 |
1,824 | |||
| 2020 |
1,424 | |||
| 2021 |
1,466 | |||
| Thereafter |
3,532 | |||
|
|
|
|||
| Total |
$ | 13,058 | ||
|
|
|
|||
Rent expense under all operating leases totaled $2.1 million, $1.5 million and $1.4 million for the years ended December 31, 2016, 2015 and 2014, respectively.
Clinical Development Agreements
We have entered into agreements with various vendors for the research and development of product candidates, which are generally cancellable anytime at our option. Under the terms of these agreements, the vendors provide a variety of services including conducting preclinical development, research, manufacturing clinical compounds, enrolling and recruiting patients, monitoring studies, data analysis and regulatory filing assistance. Payments under these agreements typically include fees for services and reimbursement of expenses. In addition, under certain agreements, we are subject to penalties in the event we permanently discontinue performance under these agreements.
Purchase Obligations
At December 31, 2016, our purchase obligations of $6.7 million primarily consisted of commitments with third-party manufacturers in connection with the manufacturing of SUSTOL, as well as commitments with various vendors for sales and marketing support and clinical and preclinical studies. For the year ended December 31, 2016, $4.6 million of the total purchase obligations were not included in our consolidated financial statements. We intend to use our current financial resources to fund our commitments under these purchase obligations.
6. Realignment of Goals and Objectives and New Development Focus
Following the approval of SUSTOL and consistent with our transition into a commercial-stage biotechnology company, we realigned our goals and objectives and refocused our development efforts to the area of post-operative pain management. On October 18, 2016, we entered into a lease agreement for new office and laboratory space in San Diego, California (see Note 5) which became our corporate headquarters in December 2016. On September 30, 2016, the board of directors accepted the resignations of three executive officers, and these executive officers and other employees directly affected by the realignment and refocusing will be provided with one-time severance payments upon termination, continued benefits for a specified period of time and outplacement assistance.
85
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
We expect to incur total expenses of $10.4 million, $6.4 million of which is primarily for severance, and $4.0 million of which is for non-cash, stock-based compensation expense. For the year ended December 31, 2016, total expenses were $7.8 million, with $6.7 million included in research and development expense, $1.0 million in general and administrative expense and $0.1 million in sales and marketing expense. The remaining $2.6 million of total anticipated expenses relate to employees who are being retained until the fourth quarter of 2017 and are being recognized on a straight-line basis over the retention period. The Company expects to make the final payment resulting from the realignment of our goals and objectives and new development focus in the second quarter of 2018. As of December 31, 2016, we have paid $2.2 million of the total $6.4 million cash charges, and $2.7 million of the cash charges were included in accrued payroll and employee liabilities.
The expenses we expect to incur are subject to a number of assumptions, and actual results may materially differ. We may also incur other material expenses not currently contemplated due to events that may be associated with, or result from, the realignment of our goals and objectives and new development focus. We have accounted for these expenses in accordance with Accounting Standard Codification No. 420, Exit or Disposal Cost Obligations.
7. Secured Notes to Related Party
Convertible Notes
In April 2011, we entered into a securities purchase agreement for a private placement of up to $4.5 million in Convertible Notes with certain investors, including Tang Capital Partners, LP (“TCP”). TCP is controlled by Tang Capital Management, LLC (“TCM”). The manager of TCM is Kevin C. Tang, who served as a director at the time and currently serves as the Chairman of our Board of Directors. The terms of the Convertible Notes were determined by our independent directors to be no less favorable than terms that would be obtained in an arm’s length financing transaction. We received a total of $4.3 million, net of issuance costs, from the issuance of these Convertible Notes.
The Convertible Notes are secured by substantially all of our assets, including placing our bank and investment accounts under a control agreement. The Convertible Notes bear interest at 6% per annum, payable quarterly in cash or in additional principal amount of Convertible Notes, at the election of the purchasers. The Convertible Notes mature on May 2, 2021; however, the holders of the Convertible Notes may require prepayment of the Convertible Notes at any time, at each holder’s option.
The Convertible Notes are convertible into shares of our common stock at a rate of 1,250 shares for every $1,000 of outstanding principal due under the Convertible Notes. There is no right to convert the Convertible Notes to the extent that, after giving effect to such conversion, the holder would beneficially own in excess of 9.99% of our outstanding common stock. Each holder of the Convertible Notes can increase or decrease this beneficial ownership conversion limit by written notice to us, which will not be effective until 61 days after delivery of the notice.
As of December 31, 2016, we were in compliance with all covenants under the Convertible Notes. Upon the occurrence of an event of default under the Convertible Notes, the holders of the Convertible Notes have the right to require us to redeem all or a portion of their Convertible Notes.
In 2011, we filed a registration statement with the SEC to register for resale 3.5 million shares underlying the Convertible Notes. The registration statement was declared effective on July 29, 2011. The Convertible Note holders have agreed to waive
86
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
their right to require us to maintain the effectiveness of the registration statement and to register the additional shares underlying the Convertible Notes until they provide notice otherwise.
The Convertible Notes contain an embedded conversion feature that was in-the-money on the issuance dates. Based on an effective fixed conversion rate of 1,250 shares for every $1,000 of principal and accrued interest due under the Convertible Notes, the total conversion benefit at issuance exceeded the loan proceeds. Therefore, a debt discount was recorded in an amount equal to the face value of the Convertible Notes on the issuance dates and we began amortizing the resultant debt discount over the respective 10-year term of the Convertible Notes. During the year ended December 31, 2016, accrued interest of $0.3 million was paid-in-kind and rolled into the Convertible Note principal balance, which resulted in an additional debt discount of $0.3 million. For the years ended December 31, 2016, 2015 and 2014, interest expense relating to the stated rate was $0.4 million, $0.3 million, and $0.3 million, respectively, and interest expense relating to the amortization of the debt discount was $0.7 million, $0.6 million and $0.6 million, respectively.
As of December 31, 2016, the carrying value of the Convertible Notes was $2.9 million, which is comprised of the $6.0 million principal amount of the Convertible Notes outstanding, less debt discount of $3.1 million. If the $6.0 million principal amount of Convertible Notes is converted, we would issue 7.5 million shares of our common stock.
In January 2017, in connection with the public offering (see Note 12), TCP executed a waiver (the “Waiver”) pursuant to which TCP waived: (i) our obligation under the Securities Purchase Agreement, dated April 24, 2011, among us, TCP and certain other investors (the “SPA”) to maintain a sufficient number of authorized shares of our common stock to permit the conversion of TCP’s outstanding principal and interest under the Convertible Notes to our common stock; and (ii) its right to convert the Convertible Notes for the duration of the Waiver. The Waiver will remain effective until the earliest to occur of: (i) a change in the control of the Company; (ii) the Company’s stockholders approving an increase of the number of authorized shares of our common stock; and (iii) July 18, 2017. If, upon the expiration of the Waiver, TCP seeks to convert part or all of the Convertible Notes and we do not have a sufficient number of authorized shares of our common stock to permit the conversion of the portion of the Convertible Notes being converted, then we will be obligated to make a cash payment to TCP equal to the value of the underlying shares of common stock that we are unable to deliver on conversion, based on the price of our common stock at such time.
Promissory Note
In August 2016, we entered into the Promissory Note with TCP whereby TCP will lend us up to $100.0 million. The Promissory Note has a two-year term and bears interest of 8% per annum. The first close of $50.0 million occurred on August 5, 2016. The second close of an additional $50.0 million is subject to the achievement of a corporate milestone. There are no fees, no warrants and no equity conversion feature associated with this transaction. The Promissory Note is secured by a second-priority lien on substantially all of our assets. TCP is controlled by TCM. The manager of TCM is Kevin C. Tang, who serves as the Chairman of our Board of Directors. The terms of the Promissory Note were determined by our independent directors to be no less favorable than terms that would be obtained in an arm’s length financing transaction.
For the year ended December 31, 2016, interest expense was $1.6 million. As of December 31, 2016, the outstanding principal amount of the Promissory Note was $50.0 million.
87
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
8. Stockholders’ Equity
2014 Common Stock Offering
In June 2014, we sold 4.8 million shares of our common stock at a public offering price of $11.75 per share. In addition, as a component of the offering, we sold 600,000 pre-funded warrants to purchase shares of our common stock at a public offering price of $11.74 per share. The pre-funded warrants have an exercise price of $0.01 per share and expire on June 30, 2021. We received total net proceeds of $58.9 million (net of $4.0 million in issuance costs) from the sale of the common stock and the pre-funded warrants. As of December 31, 2016, all warrants from the June 2014 public offering remain outstanding.
2015 Common Stock Offering
In June 2015, we sold 5.5 million shares of our common stock at a public offering price of $24.75 per share. We received total net proceeds of $128.2 million (net of $8.4 million in issuance costs) from the sale of the common stock.
2017 Common Stock Offering
In January 2017, we sold 14.1 million shares of our common stock at a public offering price of $12.20 per share. We received total net proceeds of $163.7 million (net of $8.8 million in issuance costs) from the sale of the common stock.
Private Placement Warrants
In June 2011, we sold shares of common stock and warrants to purchase common stock in a private placement. A total of 4,000,000 warrants to purchase common stock at an exercise price of $3.60 per share were issued as part of this private placement. As of December 31, 2016, all warrants from the June 2011 Private Placement have been exercised.
During the year ended December 31, 2016, warrant holders exercised 2,965,477 warrants under the cashless exercise provision in each such holder’s warrant, which resulted in the net issuance of 2,395,700 shares of common stock and no net cash proceeds to us. During the year ended December 31, 2015, warrant holders exercised 343,813 warrants under the cashless exercise provision in each such holder’s warrant, which resulted in the net issuance of 285,713 shares of common stock and no net cash proceeds to us. During the year ended December 31, 2014, warrant holders exercised 460,706 warrants under the cashless exercise provision in the warrant agreement, which resulted in the net issuance of 303,614 shares of common stock and no net cash proceeds to us.
88
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Common Stock Reserved for Future Issuance
Shares of our common stock reserved for future issuance as of December 31, 2016 were as follows:
|
|
Number of Shares | |||
| Stock options outstanding |
11,845,376 | |||
| Stock options available for grant |
2,653,548 | |||
| Employee stock purchase plan |
144,600 | |||
| Warrants outstanding |
600,000 | |||
| Shares of common stock underlying Convertible Notes outstanding (see Note 7) |
7,521,416 | |||
|
|
|
|||
| Total shares reserved for future issuance |
22,764,940 | |||
|
|
|
|||
Employee Stock Purchase Plan
In 1997, our stockholders approved our Employee Stock Purchase Plan (the “ESPP”). In December 2007, May 2009, June 2011, May 2014, May 2015 and June 2016, our stockholders authorized increases in the number of shares reserved for issuance under the ESPP by 5,000, 10,000, 25,000, 25,000, 100,000 and 100,000 shares, respectively, for a total of 275,000 shares reserved at December 31, 2016. Under the terms of the ESPP, employees can elect to have up to a maximum of 10% of their base earnings withheld to purchase our common stock. The purchase price of the stock is 85% of the lower of the closing prices for our common stock on: (i) the first trading day in the enrollment period, as defined in the ESPP, in which the purchase is made, or (ii) the purchase date. The length of the enrollment period is six months. Enrollment dates are the first business day of May and November. In 2016, 51% of eligible employees participated in the ESPP. Under the ESPP, we issued 54,932, 30,361, and 12,028 shares in 2016, 2015 and 2014, respectively. The weighted-average exercise price per share of the purchase rights exercised during 2016, 2015 and 2014 was $14.03, $8.46 and $7.59, respectively. As of December 31, 2016, 130,400 shares of common stock have been issued under the ESPP and 144,600 shares of common stock are available for future issuance.
Stock Option Plans
We currently have one stock option plan from which we can grant options and restricted stock awards to employees, officers, directors and consultants. In December 2007, the stockholders approved our 2007 Equity Incentive Plan (the “2007 Plan”) at which time a maximum of 150,000 shares of common stock were available for grant. In May 2010, June 2011, May 2014, May 2015 and June 2016, our stockholders approved amendments to our 2007 Plan to increase the maximum number of shares of common stock available for grant by 100,000, 4,500,000, 1,750,000, 4,300,000 and 3,000,000 shares of common stock, respectively, resulting in an aggregate of 13,800,000 shares of common stock authorized for issuance as of December 31, 2016. At December 31, 2016, there were 2,653,548 shares available for future grant under the 2007 Plan. Any shares that are issuable upon exercise of options granted that expire, are cancelled, or that we receive pursuant to a net exercise of options, are available for future grant and issuance.
We also granted stock options and restricted stock awards under the 2002 Stock Incentive Plan (the “2002 Plan”) in prior years. The remaining shares available to be granted under the 2002 Plan expired in February 2012.
89
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In 2014, 2013 and 2012, we granted options to certain employees outside of our stockholder approved stock option plans. All options to purchase our common stock were granted with an exercise price that equals fair market value of the underlying common stock on the grant dates and expire no later than ten years from the date of grant. The options are exercisable in accordance with vesting schedules that generally provide for them to be fully vested and exercisable four years after the date of grant, provided, however, that we have also issued stock options awards that are subject to performance vesting requirements. All stock option grants issued outside of our stockholder approved plans have been registered on Form S-8 with the SEC.
A summary of our stock option activity and related data follows:
| Outstanding Options | ||||||||
|
|
Number of Shares |
Weighted- Average Exercise Price |
||||||
| Balance at December 31, 2013 |
6,355,681 | $ | 8.22 | |||||
| Granted |
3,181,001 | 9.40 | ||||||
| Exercised |
(756,593) | 6.34 | ||||||
| Cancelled |
(862,085) | 9.88 | ||||||
|
|
|
|||||||
| Balance at December 31, 2014 |
7,918,004 | 8.69 | ||||||
| Granted |
2,134,505 | 28.95 | ||||||
| Exercised |
(1,042,343) | 9.15 | ||||||
| Cancelled |
(575,178) | 10.42 | ||||||
|
|
|
|||||||
| Balance at December 31, 2015 |
8,434,988 | 13.64 | ||||||
| Granted |
4,920,661 | 15.98 | ||||||
| Exercised |
(798,363) | 8.37 | ||||||
| Cancelled |
(711,910) | 22.43 | ||||||
|
|
|
|||||||
| Balance at December 31, 2016 |
11,845,376 | $ | 14.44 | |||||
|
|
|
|||||||
For the year ended December 31, 2016, option holders exercised 798,363 stock options resulting in cash proceeds to us of $6.7 million.
For the year ended December 31, 2016, options cancelled (included in the above table) consisted of 707,617 options forfeited with a weighted-average exercise price of $22.35 and 4,293 options expired with a weighted-average exercise price of $35.09.
As of December 31, 2016, options exercisable have a weighted-average remaining contractual term of 5.3 years. The total intrinsic value of stock option exercises, which is the difference between the exercise price and closing price of our common stock on the date of exercise, during the years ended December 31, 2016 and 2015, was $10.4 million and $13.9 million, respectively. As of December 31, 2016 and 2015, the total intrinsic value of options outstanding and exercisable was $16.9 million and $46.6 million, respectively, which is the difference between the exercise price and closing price of our common stock.
90
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| Years Ended December 31, | ||||||||||||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||||||
|
|
Options | Weighted- Average Exercise Price |
Options | Weighted- Average Exercise Price |
Options | Weighted- Average Exercise Price |
||||||||||||||||||
| Exercisable at end of year |
4,356,665 | $ | 11.66 | 2,587,392 | $ | 8.72 | 2,237,901 | $ | 9.01 | |||||||||||||||
| Options vested or expected to vest |
11,234,529 | $ | 14.32 | 8,059,481 | $ | 13.38 | 7,841,074 | $ | 8.69 | |||||||||||||||
Exercise prices and weighted-average remaining contractual lives for the options outstanding as of December 31, 2016 were:
| Options Outstanding |
|
Range of Exercise Prices |
Weighted- Average Remaining Contractual Life (in years) |
Weighted- Average Exercise Price |
Options Exercisable |
Weighted- Average Exercise Price of Options Exercisable |
||||||||||||||||
| 581,518 |
$ | 3.80 – $ 7.00 | 1.01 | $ | 6.02 | 581,518 | $ | 6.02 | ||||||||||||||
| 1,935,473 |
$ 7.20 | 6.28 | 7.20 | 1,054,143 | 7.20 | |||||||||||||||||
| 1,793,013 |
$ | 7.61 – $ 9.05 | 7.09 | 8.93 | 1,104,899 | 8.94 | ||||||||||||||||
| 843,387 |
$ | 9.20 – 12.65 | 4.23 | 10.33 | 736,434 | 10.39 | ||||||||||||||||
| 2,271,111 |
$ 13.00 | 9.97 | 13.00 | — | — | |||||||||||||||||
| 548,025 |
$ | 13.20 – $ 16.62 | 7.38 | 15.46 | 202,650 | 14.57 | ||||||||||||||||
| 1,241,350 |
$ 16.83 | 9.71 | 16.83 | 51,320 | 16.83 | |||||||||||||||||
| 1,226,884 |
$ | 17.10 – $ 28.30 | 8.41 | 22.48 | 164,692 | 26.76 | ||||||||||||||||
| 1,203,272 |
$ | 28.73 – $ 29.86 | 8.49 | 29.44 | 395,686 | 29.44 | ||||||||||||||||
| 201,343 |
$ | 29.95 – $102.40 | 8.61 | 34.78 | 65,323 | 35.03 | ||||||||||||||||
|
|
|
|
||||||||||||||||||||
| 11,845,376 |
$ | 3.80 – $102.40 | 7.60 | 14.44 | 4,356,665 | 11.66 | ||||||||||||||||
|
|
|
|
||||||||||||||||||||
On December 31, 2016, we had reserved 11,845,376 shares of common stock for future issuance upon exercise of outstanding options granted under the 2002 Plan and the 2007 Plan, as well as the non-plan grants.
91
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Valuation and Expense Information
The following table summarizes stock-based compensation expense related to stock-based payment awards granted pursuant to all of our equity compensation arrangements for the years ended December 31, 2016, 2015 and 2014 (in thousands):
| December 31, | ||||||||||||
|
|
2016 | 2015 | 2014 | |||||||||
| Research and development |
$ | 11,316 | $ | 4,701 | $ | 3,329 | ||||||
| General and administrative |
7,402 | 6,282 | 2,532 | |||||||||
| Sales and marketing |
7,238 | 3,377 | 2,206 | |||||||||
|
|
|
|||||||||||
| Stock-based compensation expense included in operating expenses |
$ | 25,956 | $ | 14,360 | $ | 8,067 | ||||||
|
|
|
|||||||||||
As of December 31, 2016, there was $86.4 million of total unrecognized compensation cost related to non-vested, stock-based payment awards granted under all of our equity compensation plans and all non-plan option grants. Total unrecognized compensation cost will be adjusted for future changes in estimated forfeitures. We expect to recognize this compensation cost over a weighted-average period of 2.9 years.
For the years ended December 31, 2016, 2015 and 2014, we estimated the fair value of each option grant and ESPP purchase right on the date of grant using the Black-Scholes option pricing model with the following weighted-average assumptions:
Options:
| December 31, | ||||||||||||
|
|
2016 | 2015 | 2014 | |||||||||
| Risk-free interest rate |
1.7% | 1.7% | 1.9% | |||||||||
| Dividend yield |
—% | —% | —% | |||||||||
| Volatility |
89.1% | 92.7% | 99.0% | |||||||||
| Expected life (years) |
6 | 6 | 6 | |||||||||
ESPP:
| December 31, | ||||||||||||
|
|
2016 | 2015 | 2014 | |||||||||
| Risk-free interest rate |
0.4% | 0.1% | 0.1% | |||||||||
| Dividend yield |
—% | —% | —% | |||||||||
| Volatility |
68.3% | 86.7% | 53.8% | |||||||||
| Expected life (months) |
6 | 6 | 6 | |||||||||
92
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The weighted-average fair value of options granted was $11.82, $21.92 and $7.40 for the years ended December 31, 2016, 2015 and 2014, respectively.
The weighted-average fair value of shares purchased through the ESPP was $6.10, $8.23 and $3.06 for the years ended December 31, 2016, 2015 and 2014, respectively.
The risk-free interest rate assumption is based on observed interest rates on United States Treasury debt securities with maturities close to the expected term of our employee and director stock options and ESPP purchases.
The dividend yield assumption is based on our history and expectation of dividend payouts. We have never paid dividends on our common stock and we do not anticipate paying dividends in the foreseeable future.
We used our historical stock price to estimate volatility.
The expected life of employee and director stock options represents the average of the contractual term of the options and the weighted-average vesting period, as permitted under the simplified method. We have elected to use the simplified method, as we do not have enough historical exercise experience to provide a reasonable basis upon which to estimate the expected term. The expected life for the ESPP purchase rights is six months, which represents the length of each purchase period.
9. Income Taxes
For the years ended December 31, 2016, 2015 and 2014, we did not record a provision for income taxes because we have incurred operating losses for all three years.
Deferred income tax assets and liabilities arising from differences between accounting for financial statement purposes and tax purposes, less valuation allowance at year-end are as follows (in thousands):
| December 31, | ||||||||
|
|
2016 | 2015 | ||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforward |
$ | 138,727 | $ | 94,465 | ||||
| Research and development credits |
16,687 | 11,181 | ||||||
| Stock-based compensation |
10,272 | 6,726 | ||||||
| Other, net |
3,204 | 1,345 | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
168,890 | 113,717 | ||||||
|
|
|
|
|
|||||
| Valuation allowance for net deferred tax assets |
(168,890) | (113,717) | ||||||
|
|
|
|
|
|||||
| Net deferred taxes |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
93
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Taxes on income vary from the statutory federal income tax rate applied to earnings before tax on income as follows (in thousands):
| December 31, | ||||||||||||
|
|
2016 | 2015 | 2014 | |||||||||
| Statutory federal income tax rate of 34% |
$ | (58,868) | $ | (33,181) | $ | (25,965) | ||||||
| Stock-based compensation expense |
2,694 | 894 | 551 | |||||||||
| NOL not benefitted |
53,798 | 31,935 | 25,085 | |||||||||
| Non-deductible compensation |
1,864 | — | — | |||||||||
| Other, net |
512 | 352 | 329 | |||||||||
|
|
|
|
|
|
|
|||||||
| Provision for taxes |
$ | — | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
A valuation allowance is provided when it is more likely than not that the deferred tax assets will not be realized. We have established a valuation allowance to offset net deferred tax assets at December 31, 2016 and 2015 due to the uncertainty of realizing future tax benefits from our net operating loss carryforwards and other deferred tax assets. The net change in the total valuation allowance for the year ended December 31, 2016 and 2015 was an increase of $55.2 million and $40.4 million, respectively.
At December 31, 2016, we had federal and California state net operating loss (“NOL”) carryforwards of $387.6 million and $268.5 million, respectively, expiring beginning in 2018 for federal and 2017 for California state purposes. Federal NOLs of $10.0 million and state NOLs of $4.2 million relate to stock-based compensation deductions in excess of book expense, the tax effect of which would be to credit additional paid-in capital, if realized. At December 31, 2016, we had federal and California state research credit carryforwards of $11.6 million and $7.6 million, respectively, expiring beginning in 2022 for federal. The California state credits can be carried forward indefinitely.
Internal Revenue Code section 382 places a limitation on the amount of taxable income that can be offset by NOL carryforwards after a change in control (generally greater than 50% change in ownership) of a loss corporation. California has similar rules. Generally, after a change in control, a loss corporation cannot deduct NOL carryforwards in excess of the Section 382 limitation. We have performed an IRC Section 382 analysis and determined there were ownership changes in 2007, 2011, and 2013. We are currently in the process of completing the IRC Section 382 analysis for 2016 and we do not expect an ownership change for the year ended December 31, 2016. The limitation in the Federal and state carryforwards associated with the NOL and credit carryforwards reduce the deferred tax assets, which are further offset by a full valuation allowance. The limitation can result in the expiration of the NOLs and credit carryforwards available as of December 31, 2016, before utilization.
We file U.S. and state income tax returns with varying statutes of limitations. The tax years from 1997 to 2016 remain open to examination due to the carryover of unused NOL carryforwards and tax credits.
For benefits to be realized, a tax position must be more likely than not to be sustained upon examination. The amount recognized is measured as the largest amount of benefit that is greater than 50 percent likely of being realized upon settlement. It is our policy to recognize interest and penalties related to income tax matters in income tax expense. As of December 31, 2016 and 2015, we had no accrued interest and penalties related to uncertain tax positions.
94
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
As of December 31, 2016 and 2015, we had unrecognized tax benefits of $0.1 million, and the amount that would impact our effective tax rate, before the consideration of the valuation allowance, is $0.1 million. We do not expect any material changes to the estimated amount of liability associated with its uncertain tax positions within the next 12 months.
10. Employee Benefit Plan
We have a defined contribution 401(k) plan (the “Plan”) covering substantially all of our employees. In the past three calendar years, we made matching cash contributions equal to 50% of each participant’s contribution during the Plan year up to a maximum amount equal to the lesser of 3% of each participant’s annual compensation or $7,950, $7,950 and $7,800 for the years 2016, 2015 and 2014, respectively. Such amounts were recorded as expense in the corresponding years. We may also contribute additional discretionary amounts to the Plan as we may determine. For the years ended December 31, 2016, 2015 and 2014, we contributed to the Plan $0.6 million, $0.3 million and $0.2 million, respectively. No discretionary contributions have been made to the Plan since its inception.
11. Summary of Quarterly Consolidated Financial Data (unaudited)
The following is a summary of the unaudited quarterly results of operations for the years ended December 31, 2016 and 2015:
| 2016 | ||||||||||||||||
|
|
First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
||||||||||||
| (In thousands, except per share amounts) | ||||||||||||||||
| Revenues: |
||||||||||||||||
| Net product sales |
$ | — | $ | — | $ | — | $ | 1,279 | ||||||||
| Operating expenses: |
||||||||||||||||
| Cost of product sales |
— | — | — | 35 | ||||||||||||
| Research and development |
16,092 | 27,286 | 30,242 | 29,505 | ||||||||||||
| General and administrative |
5,367 | 4,774 | 5,333 | 5,892 | ||||||||||||
| Sales and marketing |
11,853 | 11,006 | 12,159 | 12,650 | ||||||||||||
|
|
|
|||||||||||||||
| Loss from operations |
(33,312) | (43,066) | (47,734) | (46,803) | ||||||||||||
| Interest income |
118 | 97 | 111 | 119 | ||||||||||||
| Interest expense |
(251) | (257) | (886) | (1,270) | ||||||||||||
| Other expense |
— | — | — | (9) | ||||||||||||
|
|
|
|||||||||||||||
| Net loss |
$ | (33,445) | $ | (43,226) | $ | (48,509) | $ | (47,963) | ||||||||
|
|
|
|||||||||||||||
| Basic and diluted net loss per share |
$ | (0.92) | $ | (1.17) | $ | (1.24) | $ | (1.22) | ||||||||
|
|
|
|||||||||||||||
95
Table of Contents
HERON THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 2015 | ||||||||||||||||
|
|
First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
||||||||||||
| (In thousands, except per share amounts) | ||||||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
$ | 14,504 | $ | 16,175 | $ | 14,241 | $ | 16,263 | ||||||||
| General and administrative |
3,587 | 4,082 | 4,127 | 6,599 | ||||||||||||
| Sales and marketing |
2,269 | 2,757 | 4,123 | 8,198 | ||||||||||||
|
|
|
|||||||||||||||
| Loss from operations |
(20,360) | (23,014) | (22,491) | (31,060) | ||||||||||||
| Interest income |
22 | 26 | 61 | 65 | ||||||||||||
| Interest expense |
(232) | (237) | (242) | (247) | ||||||||||||
| Other income |
— | 118 | — | — | ||||||||||||
|
|
|
|||||||||||||||
| Net loss |
$ | (20,570) | $ | (23,107) | $ | (22,672) | $ | (31,242) | ||||||||
|
|
|
|||||||||||||||
| Basic and diluted net loss per share |
$ | (0.70) | $ | (0.74) | $ | (0.63) | $ | (0.87) | ||||||||
|
|
|
|||||||||||||||
12. Subsequent Events
In January 2017, we sold 14.1 million shares of our common stock at a public offering price of $12.20 per share. We received total net proceeds of $163.7 million (net of $8.8 million in issuance costs) from the sale of the common stock. In connection with the public offering, TCP executed a waiver pursuant to which TCP waived its right to convert the Convertible Notes (see Note 7).
96
Table of Contents
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
(a) Disclosure Controls and Procedures; Changes in Internal Control Over Financial Reporting
Our management, with the participation of our principal executive and principal financial and accounting officers, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (the “Exchange Act”)) as of December 31, 2016. Based on this evaluation, our principal executive and principal financial and accounting officers concluded that our disclosure controls and procedures were effective as of December 31, 2016.
To address changes in our business in conjunction with the commercial launch of SUSTOL, we have deployed new processes and systems related to our inventory, cost of goods sold, revenue, cash receipts and receivables. In doing so, we have modified and enhanced our internal control over financial reporting (as such term is defined in Exchange Act Rule 13a-15(f)) during the period covered by this Annual Report on Form 10-K as a result of the implementation of these new processes and systems. Other than the above-mentioned changes, there have been no significant changes in our internal control over financial reporting that occurred during the period covered by this Annual Report on Form 10-K that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
(b) Management Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and Rule 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
• pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
• provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
• provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
97
Table of Contents
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2016. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013 Framework).
Based on our assessment, management concluded that, as of December 31, 2016, our internal control over financial reporting was effective based on those criteria.
The independent registered public accounting firm that audited the consolidated financial statements that are included in this Annual Report on Form 10-K has issued an audit report on our internal control over financial reporting. The report appears below.
(c) Report of Independent Registered Public Accounting Firm on Internal Control Over Financial Reporting
The Board of Directors and Stockholders of
Heron Therapeutics, Inc.
We have audited Heron Therapeutics, Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control–Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Heron Therapeutics, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management Report on Internal Control Over Financial Reporting included in Item 9A. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and
98
Table of Contents
expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Heron Therapeutics, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Heron Therapeutics, Inc. as of December 31, 2016 and 2015, and the related consolidated statements of comprehensive loss, stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2016, and our report dated February 22, 2017 expressed an unqualified opinion thereon.
/s/ OUM & CO. LLP
San Francisco, California
February 22, 2017
None.
99
Table of Contents
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Information required by this item will be contained in our Definitive Proxy Statement for our 2017 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A with the SEC within 120 days of December 31, 2016. Such information is incorporated herein by reference.
We have adopted a Code of Ethics that applies to our Principal Executive Officer, Principal Financial and Accounting Officer, and to all of our other officers, directors and employees. The Code of Ethics is available in the Corporate Governance section of the Investor Resources page on our website at www.herontx.com. We intend to disclose future waivers or material amendments to certain provisions of our Code of Ethics on the above-referenced website within four business days following the date of such waiver or amendment.
ITEM 11. EXECUTIVE COMPENSATION.
Information required by this item will be contained in our Definitive Proxy Statement for our 2017 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A with the SEC within 120 days of December 31, 2016. Such information is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Additional information required by this item will be contained in our Definitive Proxy Statement for our 2017 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A with the SEC within 120 days of December 31, 2016. Such information is incorporated herein by reference.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table contains information regarding our equity compensation plans as of December 31, 2016.
|
|
Number of Securities to be Issued upon Exercise of Outstanding Options |
Weighted- Average Exercise Price of Outstanding Options |
Number of Securities Remaining Available for Future Issuance under Equity Compensation Plans (Excluding Securities Reflected in the First Column) |
|||||||||
| Equity compensation plans approved by security holders |
||||||||||||
| Stock option and awards plans (1) |
8,893,666 | $ | 16.56 | 2,653,548 | ||||||||
| Employee stock purchase plan |
— | — | 144,600 | |||||||||
| Equity compensation plans not approved by security holders (2) |
2,951,710 | $ | 8.06 | — | ||||||||
|
|
|
|
|
|||||||||
| Total |
11,845,376 | $ | 14.44 | 2,798,148 | ||||||||
|
|
|
|
|
|||||||||
100
Table of Contents
| (1) | Consists of awards granted under the 2002 Plan and the 2007 Plan. |
| (2) | Consists of non-plan grants made to certain employees in 2012, 2013 and 2014. These grants were made with the same terms as stock option grants made under the 2007 Plan. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
Information required by this item will be contained in our Definitive Proxy Statement for our 2017 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A with the SEC within 120 days of December 31, 2016. Such information is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
Information required by this item will be contained in our Definitive Proxy Statement for our 2017 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A with the SEC within 120 days of December 31, 2016. Such information is incorporated herein by reference.
101
Table of Contents
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
| 1. | Consolidated Financial Statements. |
| The consolidated financial statements and supplementary data set forth in Part II of the Annual Report on Form 10-K are included herein. |
| 2. | Consolidated Financial Statement Schedules. |
| These schedules are omitted because they are not required, or are not applicable, or the required information is shown in the consolidated financial statements or notes thereto. |
| 3. | Exhibits. |
| The Exhibit Index attached to this report is incorporated by reference herein. |
102
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| HERON THERAPEUTICS, INC. | ||
| BY: |
/s/ BARRY D. QUART | |
| Barry D. Quart, Pharm.D. | ||
| Chief Executive Officer | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each individual whose signature appears below constitutes and appoints Barry D. Quart and Brian G. Drazba as his true and lawful attorney-in-fact and agent, with full power of substitution, for him and in his name, place and stead, in any and all capacities, with respect to this annual report and any and all amendments thereto, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all the said attorney-in-fact and agent or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ BARRY D. QUART Barry D. Quart, Pharm. D. |
Chief Executive Officer and Director (Principal Executive Officer) |
February 23, 2017 | ||
| /s/ BRIAN G. DRAZBA Brian G. Drazba |
Vice President, Finance and Chief Financial Officer (Principal Financial and Accounting Officer) |
February 23, 2017 | ||
| /s/ CRAIG A. JOHNSON Craig A. Johnson |
Director |
February 23, 2017 | ||
| /s/ JOHN W. POYHONEN John W. Poyhonen |
Director |
February 23, 2017 | ||
| /s/ ROBERT H. ROSEN Robert H. Rosen |
President and Director |
February 23, 2017 | ||
| /s/ KEVIN C. TANG Kevin C. Tang |
Chairman of the Board of Directors |
February 23, 2017 | ||
| /s/ CHRISTIAN WAAGE Christian Waage |
Director |
February 23, 2017 | ||
103
Table of Contents
| Exhibit | Document Description | |
| 3.1 | Certificate of Incorporation, as amended through July 29, 2009 (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2009, as Exhibit 3.1, filed on August 4, 2009) | |
| 3.2 | Amended and Restated Bylaws (incorporated by reference to our Current Report on Form 8-K, as Exhibit 3.1, filed on January 22, 2016) | |
| 3.3 | Certificate of Amendment of Certificate of Incorporation (incorporated by reference to our Current Report on Form 8-K, as Exhibit 3.1, filed on June 30, 2011) | |
| 3.4 | Certificate of Amendment to the Certificate of Incorporation (incorporated by reference to our Current Report on Form 8-K, as Exhibit 3.1, filed on January 13, 2014) | |
| 4.1 | Common Stock Certificate (incorporated by reference to our Registration on Form S-3 (Registration No. 333-162968), as Exhibit 4.1, filed on November 6, 2009) | |
| 4.2 | Form of Warrant to Purchase Shares of Common Stock (incorporated by reference to our Current Report on Form 8-K, as Exhibit 4.1, filed on June 17, 2014) | |
| 4.3 | Form of Warrant to Purchase Shares of Common Stock (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.3, filed on October 22, 2009) | |
| 4.4 | Amended and Restated Certificate of Designation, Preferences, and Rights of Series A Preferred Stock (incorporated by reference to our Current Report on Form 8-K, as Exhibit 3.C, filed on December 19, 2006) | |
| 10.1* | 1997 Employee Stock Purchase Plan, as amended to date (incorporated by reference to our Definitive Proxy Statement on Schedule 14A, as Exhibit B, filed on April 28, 2015) | |
| 10.2 | Lease Agreement between the Company and Metropolitan Life Insurance Company for lease of the Company’s offices in Redwood City dated as of November 7, 1997 (incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 1997, as Exhibit 10-E, filed on March 30, 1998) | |
| 10.3* | 2002 Equity Incentive Plan (incorporated by reference to our Registration on Form S-8 (Registration No. 333-90428), as Exhibit 99.1, filed on June 13, 2002) | |
| 10.4* | Amended and Restated 2007 Equity Incentive Plan (incorporated by reference to our Definitive Proxy Statement on Schedule 14A, as Exhibit A, filed on April 28, 2015) | |
| 10.5* | Form of 2007 Equity Incentive Plan Stock Option Agreement (incorporated by reference to our Registration on Form S-8 (Registration No. 333-148660), as Exhibit 4.3, filed on January 14, 2008) | |
| 10.6* | Form of 2007 Equity Incentive Plan Restricted Stock Unit Agreement (incorporated by reference to our Registration on Form S-8 (Registration No. 333-148660), as Exhibit 4.4, filed on January 14, 2008) | |
| 10.7* | Form of 2007 Equity Incentive Plan Restricted Stock Award Agreement (incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2007, as Exhibit 10-O, filed on March 31, 2008) | |
104
Table of Contents
| Exhibit | Document Description | |
| 10.8* | Form of Indemnification Agreement (incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2007, as Exhibit 10-S, filed on March 31, 2008) | |
| 10.9 | Registration Rights Agreement, dated as of October 22, 2009, by and among the Company and the purchasers listed therein (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.2, filed on October 22, 2009) | |
| 10.10 | Securities Purchase Agreement, dated as of April 24, 2011, by and among the Company and the purchasers listed therein (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.1, filed on April 28, 2011) | |
| 10.11 | Form of Senior Secured Convertible Note due 2021 (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.2, filed on April 28, 2011) | |
| 10.12 | Securities Agreement, dated as of April 24, 2011, by and between the Company and Tang Capital Partners, LP, as Agent for the Purchasers (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.3, filed on April 28, 2011) | |
| 10.13 | Second Amendment to Lease, dated as of April 1, 2011, by and between the Company and Metropolitan Life Insurance Company (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.4, filed on April 28, 2011) | |
| 10.14* | Management Retention Agreement, dated as of April 25, 2011, by and between the Company and Michael A. Adam (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.6, filed on April 28, 2011) | |
| 10.15 | Securities Purchase Agreement, dated June 29, 2011, by and between the Company and the purchasers listed on Schedule I thereto (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.1, filed on June 30, 2011) | |
| 10.16 | Amendment to Senior Secured Convertible Note Due 2021, dated June 29, 2011, by and between the Company and the purchasers named in the Securities Purchase Agreement, dated April 24, 2011, (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.2, filed on June 30, 2011) | |
| 10.17 | Third Amendment to Lease, dated as of July 28, 2011, by and between the Company and Metropolitan Life Insurance Company (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.1, filed on August 3, 2011) | |
| 10.18 | Registration Rights Agreement, dated July 25, 2012, by and between the Company and the purchasers named therein (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.2, filed on July 25, 2012) | |
| 10.19* | Executive Employment Agreement, dated May 1, 2013, by and between the Company and Barry D. Quart, Pharm.D. (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2013, as Exhibit 10-AI, filed on May 10, 2013) | |
| 10.20* | Executive Employment Agreement, dated May 1, 2013, by and between the Company and Robert H. Rosen (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2013, as Exhibit 10-AJ filed on May 10, 2013) | |
105
Table of Contents
| Exhibit | Document Description | |
| 10.21 | Form of Non-Qualified Stock Option Agreement (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended June 30, 2013, as Exhibit 10-AL, filed on August 8, 2013) | |
| 10.22* | Offer Letter dated October 16, 2013 between the Company and Brian G. Drazba (incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2013, as Exhibit 10-AF, filed on March 7, 2014) | |
| 10.23* | Management Retention Agreement as of October 23, 2013, by and between the Company and Brian G. Drazba (incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2013, as Exhibit 10-AG, filed on March 7, 2014) | |
| 10.24* | Executive Employment Agreement, dated November 1, 2013, by and between the Company and Paul Marshall (incorporated by reference to our Annual Report on Form 10-K for the year ended December 31, 2013, as Exhibit 10-AH, filed on March 7, 2014) | |
| 10.25* | Amendment to Executive Employment Agreement, dated May 1, 2013, as amended on April 22, 2015, by and between the Company and Dr. Barry Quart (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2015, as Exhibit 10.1, filed on May 8, 2015) | |
| 10.26* | Amendment to Executive Employment Agreement, dated May 1, 2013, as amended on April 22, 2015, by and between the Company and Robert Rosen (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2015, as Exhibit 10.2, filed on May 8, 2015) | |
| 10.27* | Amendment to Management Retention Agreement, dated October 23, 2013, as amended on April 22, 2015, by and between the Company and Brian G. Drazba (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2015, as Exhibit 10.3, filed on May 8, 2015) | |
| 10.28* | Amendment to Executive Employment Agreement, dated November 1, 2013, as amended on April 22, 2015, by and between the Company and Paul Marshall (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2015, as Exhibit 10.4, filed on May 8, 2015) | |
| 10.29+ | SUSTOL® (granisetron, extended release) Injection Commercial Manufacturing Services Agreement —Finished Final Drug Product, dated May 27, 2015, by and between the Company and Lifecore Biomedical, LLC) (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.1, filed on May 29, 2015) | |
| 10.30* | Executive Employment Agreement, dated October 12, 2015, by and between the Company and Neil Clendeninn (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2015, as Exhibit 10.2, filed on November 6, 2015) | |
| 10.31+ | Commercial Supply Agreement, dated December 8, 2015, by and between the Company and SAFC, Inc. (incorporated by reference to our Annual Report on Form 10-K/A Amendment No. 1 for the year ended December 31, 2015, as Exhibit 10.36, filed on December 23, 2016) | |
| 10.32 | Fourth Amendment to Lease, dated as of April 11, 2016, by and between the Company and Metropolitan Life Insurance Company (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.1, filed on April 15, 2016) | |
106
Table of Contents
| Exhibit | Document Description | |
| 10.33* | Executive Employment Agreement, dated January 28, 2016, by and between the Company and Kimberly Manhard (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended March 31, 2016, as Exhibit 10.1, filed on May 5, 2016) | |
| 10.34 | Security Agreement, dated as of August 5, 2016, by and among the Company, Tang Capital Partners, LP and TC Management Services, LLC (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2016, as Exhibit 10.1, filed on November 8, 2016) | |
| 10.35 | Subordinated Secured Promissory Note, dated August 5, 2016, by and between the Company and Tang Capital Partners, LP (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2016, as Exhibit 10.2, filed on November 8, 2016) | |
| 10.36 | Lease Agreement, dated October 18, 2016, by and between the Company and AP3-SD1 Campus Point LLC (incorporated by reference to our Quarterly Report on Form 10-Q for the quarter ended September 30, 2016, as Exhibit 10.3, filed on November 8, 2016) | |
| 10.37 | Waiver, dated January 18, 2017, between the Company and Tang Capital Partners, LP (incorporated by reference to our Current Report on Form 8-K, as Exhibit 10.1, filed on January 24, 2017) | |
| 23.1 | Consent of Independent Registered Public Accounting Firm (OUM & Co. LLP) | |
| 24.1 | Power of Attorney (included on the signature page hereto) | |
| 31.1 | Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 31.2 | Certification Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | |
| 32.1 | Certification Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Taxonomy Extension Schema Document | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | |
| * | Management contract or compensatory plan, contract or arrangement. |
| + | Confidential treatment has been requested with respect to certain portions of the exhibit, which portions have been omitted and filed separately with the Securities and Exchange Commission. |
107
