Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Aralez Pharmaceuticals Inc. | a16-7860_18k.htm |
Exhibit 99.1
“Corporate Overview & Strategic Vision” Dundee Investor Meetings April 4-6, 2016

This presentation includes certain statements that constitute “forward-looking statements” within the meaning of applicable securities laws. Forward-looking statements include, but are not limited to, statements regarding our 2016 financial guidance, the anticipated April launch of Fibricor, including related promotional and sales initiatives, and growing Fibricor use in the U.S., the anticipated YOSPRALA launch in 4Q 2016, pending FDA approval, YOSPRALA having the ability to achieve mid single digit market share in the secondary prevention patient population, our plan to expand to up to 300 sales professionals, our market research indicating that HCPs intend to use YOSPRALA in 14-26% of their aspirin+PPI/H2 antagonist patients, YOSPRALA capturing a quarter to a third of secondary prevention patients, the successful integration of Tribute, opportunistic and transformative M&A that are near-term revenue generating and accretive, our plan to submit YOSPRALA in Europe in 3Q 2016, YOSPRALA in Canada in 2017 and MT400 (Treximet) in Canada by 3Q 2016, and other statements that are not historical facts, and such statements are typically identified by use of terms such as “may,” “will,” “would,” “should,” “could,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “likely,” “potential,” “continue” or the negative or similar words, variations of these words or other comparable words or phrases, although some forward-looking statements are expressed differently. You should be aware that the forward-looking statements included herein represent management’s current judgment and expectations, and are based on current estimates and assumptions made by management in light of its experience and perception of historical trends, current conditions and expected future developments, as well as other factors that it believes are appropriate and reasonable under the circumstances, but there can be no assurance that such estimates and assumptions will prove to be correct and, as a result, the forward-looking statements based on those assumptions could prove to be incorrect. Accordingly, actual results, level of activity, performance or achievements or future events or developments could differ materially from those expressed or implied in the forward-looking statements. Material factors or assumptions that were applied in providing financial guidance for the year ending December 31, 2016, including with respect to the statements that Aralez’s net revenues are expected to be in the range of $48 million to $58 million, non-GAAP SG&A expenses are expected to be in the range of $85 million to $100 million and non-GAAP R&D expenses are expected to be in the range of $8 million to $12 million, include, but are not limited to, the material factors and assumptions outlined in this presentation and under the caption “Cautionary Note Regarding Forward-Looking Statements” in the Company’s press release dated March 15, 2016 announcing results for the fourth quarter and full-year ended December 31, 2015, and the Company’s 2016 financial guidance. Readers are cautioned that actual future operating results and economic performance of the Company, including with respect to our net revenues, non-GAAP SG&A expenses and non-GAAP R&D expense for the year ending December 31, 2016, are subject to a number of risks and uncertainties, including, among other things described below, our inability to build, acquire or contract with a sales force of sufficient scale for the commercialization of YOSPRALA™ in a timely and cost-effective manner, our failure to successfully commercialize our product candidates; costs and delays in the development and/or FDA approval of our product candidates (including YOSPRALA), including as a result of the need to conduct additional studies or due to issues with third-party API or finished product manufacturers, or the failure to obtain such approval of our product candidates for all expected indications, including as a result of changes in regulatory standards or the regulatory environment during the development period of any of its product candidates; the inability to maintain or enter into, and the risks resulting from our dependence upon, collaboration or contractual arrangements necessary for the development, manufacture, commercialization, marketing, sales and distribution of any products, including our dependence on AstraZeneca and Horizon for the sales and marketing of VIMOVO, our dependence on Patheon for the manufacture of YOSPRALA™ 81/40 and YOSPRALA™ 325/40; our ability to protect our intellectual property and defend our patents; regulatory obligations and oversight; failure to make, integrate and maintain new acquisitions, such as the integration of Tribute; fluctuations in the value of certain foreign currencies, including the Canadian dollar, in relation to the U.S. dollar, and other world currencies; changes in government regulations, including tax laws and unanticipated tax liabilities; and general adverse economic, market and business conditions, and could differ materially from what is currently expected as set out in this presentation. Our operations involve risks and uncertainties, many of which are outside of our control, and any one or any combination of these risks and uncertainties could also affect whether the forward-looking statements ultimately prove to be correct and could cause our actual results, level of activity, performance or achievements or future events or developments to differ materially from those expressed or implied by the forward-looking statements. These risks and uncertainties include those risks detailed from time-to-time under the caption “Risk Factors” and elsewhere in the Company’s SEC filings and reports and Canadian securities law filings, including in our Annual Report on Form 10-K for the year ended December 31, 2015 which are available on EDGAR at www.sec.gov, on SEDAR at www.sedar.com, and on the Company’s website at www.aralez.com, and those described from time to time in our future reports filed with the Securities and Exchange Commission and applicable securities regulatory authorities in Canada. You should not place undue importance on forward-looking statements and should not rely upon this information as of any other date. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, unless required by law. Disclaimer

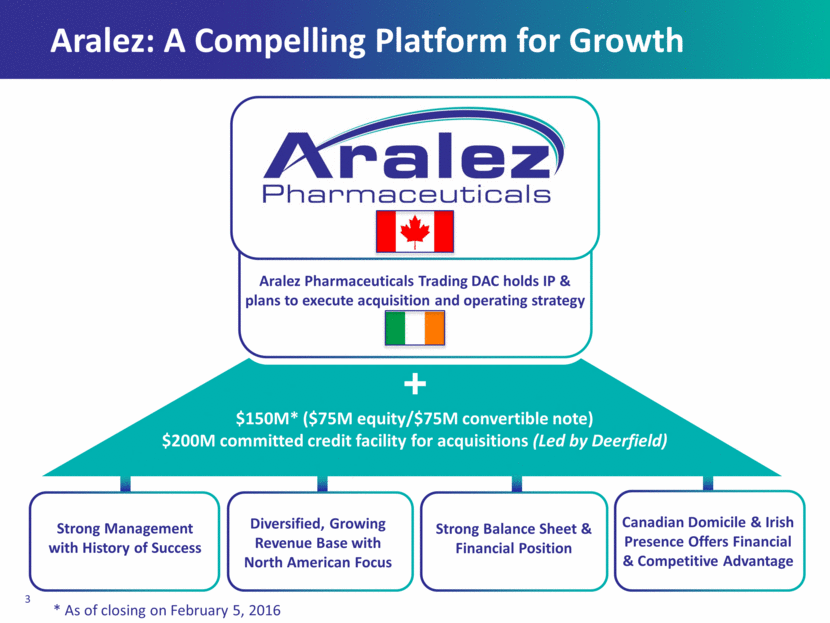
Aralez: A Compelling Platform for Growth Strong Management with History of Success Diversified, Growing Revenue Base with North American Focus Strong Balance Sheet & Financial Position Canadian Domicile & Irish Presence Offers Financial & Competitive Advantage $150M* ($75M equity/$75M convertible note) $200M committed credit facility for acquisitions (Led by Deerfield) Aralez Pharmaceuticals Trading DAC holds IP & plans to execute acquisition and operating strategy * As of closing on February 5, 2016
The Aralez Investment Thesis Aralez is a new global specialty pharmaceutical company designed to: Maximize the value of expanded portfolio, growing revenue base and geographic footprint across North America and Europe Build on our anchor positions in cardiovascular disease and pain management and opportunistically expanding into other specialty therapeutic areas Maintain a lean, nimble and performance-oriented operating model Build value organically and leverage competitive platform to accelerate transformation and execute growth strategy Access high potential growth opportunities through aggressive BD&L and strategic M&A Align with shareholder interests with a strong focus on creating shareholder value

Focus on Cardiovascular & Pain Therapies YOSPRALA™: Combination of delayed-release aspirin and immediate-release omeprazole for the secondary prevention of heart attack and stroke in patients at risk for developing aspirin-associated gastric ulcers FDA PDUFA goal date of September 14, 2016; targeted U.S. launch in early 4Q 2016 Patent protection to 2023, with potential for extension to 2032 VIMOVO®: (partnered): Combination of esomeprazole and naproxen for osteoarthritis in patients at risk of developing NSAID associated gastric ulcers Horizon Pharma (US) – 10% on US sales; annual minimum of $7.5M; Patented until 2031 AstraZeneca (ex-US) – 50+ countries; 10% on all territory sales Royalties of $21.4M in 2015 TREXIMET®/MT400: (US partnered; ex-US Aralez): Combination of sumatriptan and naproxen sodium for acute migraine in patients 12 years of age and older Pernix (US) – Royalty sold to CPPIB; receive 20% of CPPIB receipts starting April 2018 Treximet® – Adolescent Dose recently FDA approved FIBRICOR®: A fenofibric acid formulation that is a lipid regulating agent indicated as adjunctive therapy to diet for treatment of severe hypertriglyceridemia (> 500 mg/dL), primary hypercholesterolemia or mixed dyslipidemia
Aralez: Regulatory Status U.S.: PA32540/PA8140 NDA filing (YOSPRALA) 505b(2) application Proposed label for secondary prevention of CVD per aspirin monograph (excluding acute MI) Resubmitted NDA March 14, 2016 using our new primary aspirin supplier and an alternative supplier FDA PDUFA goal date of September 14, 2016; targeting launch in early 4Q 2016 CANADA: MT400 Interacting with Health Canada Targeting new drug submission (NDS) in Canada in Q3 2016 Bilastine Undergoing regulatory review in Canada YOSPRALA Targeting new drug submission in 2017 EUROPE: PA10040 MAA filing (YOSPRALA) US NDA package will form core of submission CMC, bioequivalence and pharmacodynamics studies, supporting clinical rationale for PA10040 will complete the dossier Scientific Advice from MEB, no further development required for submission Planning to file MAA in EU in 3Q 2016
Fibricor: Opportunity in Perspective Fibricor is a fenofibric acid formulation that is a lipid regulating agent indicated as adjunctive therapy to diet for treatment of severe hypertriglyceridemia (> 500 mg/dL), primary hypercholesterolemia or mixed dyslipidemia Fibricor and its Authorized Generic version launched in October 2009 but have not been promoted since that time Fibricor, a small product, competes in the large triglyceride lowering medication market Focus will be to maximize distribution for the short-term Personally selected a 25 person high quality sales force to promote Fibricor to key cardiologists to commence in April 2016 Develop “relationship springboard” ahead of and supporting potential YOSPRALA launch Promote alongside YOSPRALA, pending FDA approval, to grow Fibricor use in the U.S. Product Profile Market & Product Opportunity Focused Commercialization Strategy

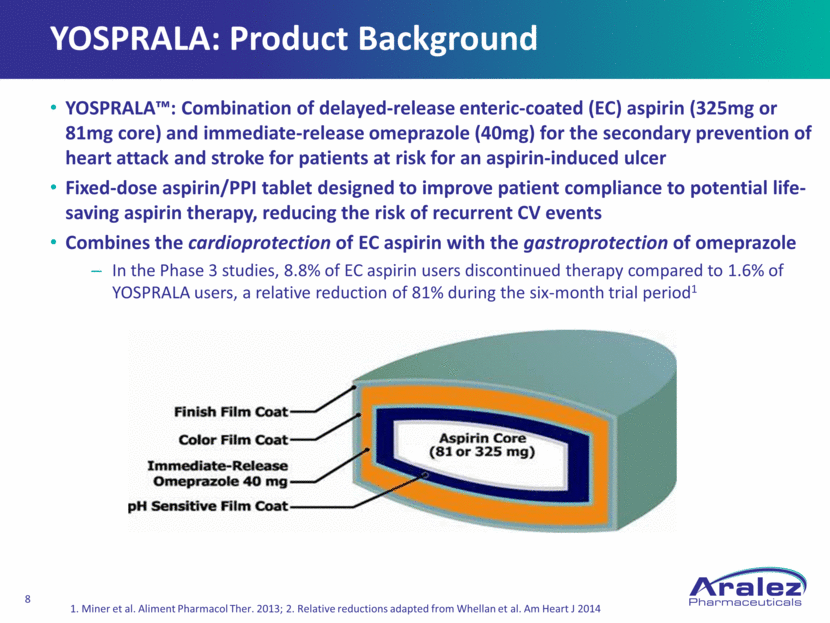
YOSPRALA: Product Background YOSPRALA™: Combination of delayed-release enteric-coated (EC) aspirin (325mg or 81mg core) and immediate-release omeprazole (40mg) for the secondary prevention of heart attack and stroke for patients at risk for an aspirin-induced ulcer Fixed-dose aspirin/PPI tablet designed to improve patient compliance to potential life-saving aspirin therapy, reducing the risk of recurrent CV events Combines the cardioprotection of EC aspirin with the gastroprotection of omeprazole In the Phase 3 studies, 8.8% of EC aspirin users discontinued therapy compared to 1.6% of YOSPRALA users, a relative reduction of 81% during the six-month trial period1 1. Miner et al. Aliment Pharmacol Ther. 2013; 2. Relative reductions adapted from Whellan et al. Am Heart J 2014
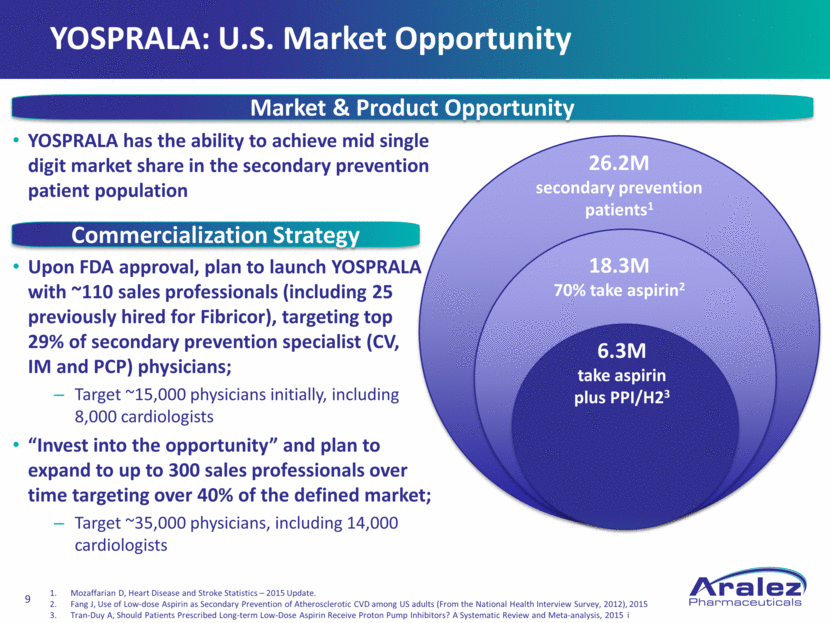
YOSPRALA: U.S. Market Opportunity YOSPRALA has the ability to achieve mid single digit market share in the secondary prevention patient population Upon FDA approval, plan to launch YOSPRALA with ~110 sales professionals (including 25 previously hired for Fibricor), targeting top 29% of secondary prevention specialist (CV, IM and PCP) physicians; Target ~15,000 physicians initially, including 8,000 cardiologists “Invest into the opportunity” and plan to expand to up to 300 sales professionals over time targeting over 40% of the defined market; Target ~35,000 physicians, including 14,000 cardiologists Market & Product Opportunity Commercialization Strategy 26.2M secondary prevention patients1 18.3M 70% take aspirin2 6.3M take aspirin plus PPI/H23 Mozaffarian D, Heart Disease and Stroke Statistics – 2015 Update. Fang J, Use of Low-dose Aspirin as Secondary Prevention of Atherosclerotic CVD among US adults (From the National Health Interview Survey, 2012), 2015 Tran-Duy A, Should Patients Prescribed Long-term Low-Dose Aspirin Receive Proton Pump Inhibitors? A Systematic Review and Meta-analysis, 2015 i
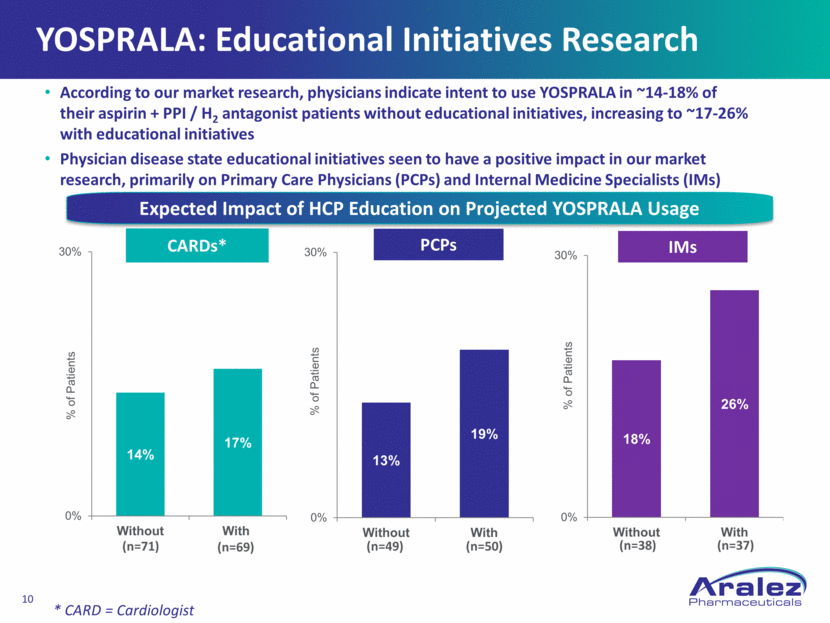
YOSPRALA: Educational Initiatives Research CARDs* IMs PCPs (n=71) (n=69) (n=49) (n=50) (n=38) (n=37) According to our market research, physicians indicate intent to use YOSPRALA in ~14-18% of their aspirin + PPI / H2 antagonist patients without educational initiatives, increasing to ~17-26% with educational initiatives Physician disease state educational initiatives seen to have a positive impact in our market research, primarily on Primary Care Physicians (PCPs) and Internal Medicine Specialists (IMs) Expected Impact of HCP Education on Projected YOSPRALA Usage * CARD = Cardiologist 13% 19% 0% 30% Without With % of Patients 18% 26% 0% 30% Without With % of Patients 14% 17% 0% 30% Without With % of Patients
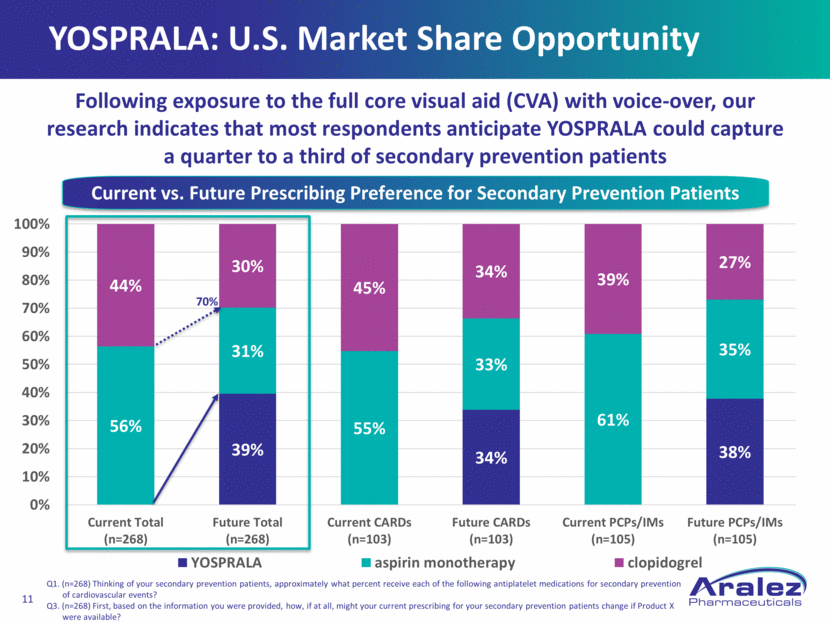
YOSPRALA: U.S. Market Share Opportunity Q1. (n=268) Thinking of your secondary prevention patients, approximately what percent receive each of the following antiplatelet medications for secondary prevention of cardiovascular events? Q3. (n=268) First, based on the information you were provided, how, if at all, might your current prescribing for your secondary prevention patients change if Product X were available? Following exposure to the full core visual aid (CVA) with voice-over, our research indicates that most respondents anticipate YOSPRALA could capture a quarter to a third of secondary prevention patients Current vs. Future Prescribing Preference for Secondary Prevention Patients
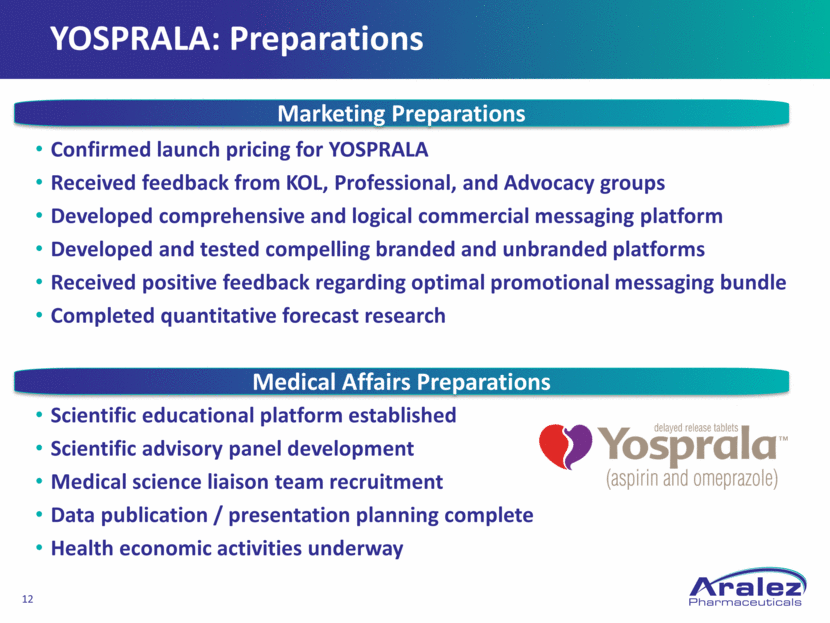
YOSPRALA: Preparations Confirmed launch pricing for YOSPRALA Received feedback from KOL, Professional, and Advocacy groups Developed comprehensive and logical commercial messaging platform Developed and tested compelling branded and unbranded platforms Received positive feedback regarding optimal promotional messaging bundle Completed quantitative forecast research Scientific educational platform established Scientific advisory panel development Medical science liaison team recruitment Data publication / presentation planning complete Health economic activities underway Marketing Preparations Medical Affairs Preparations
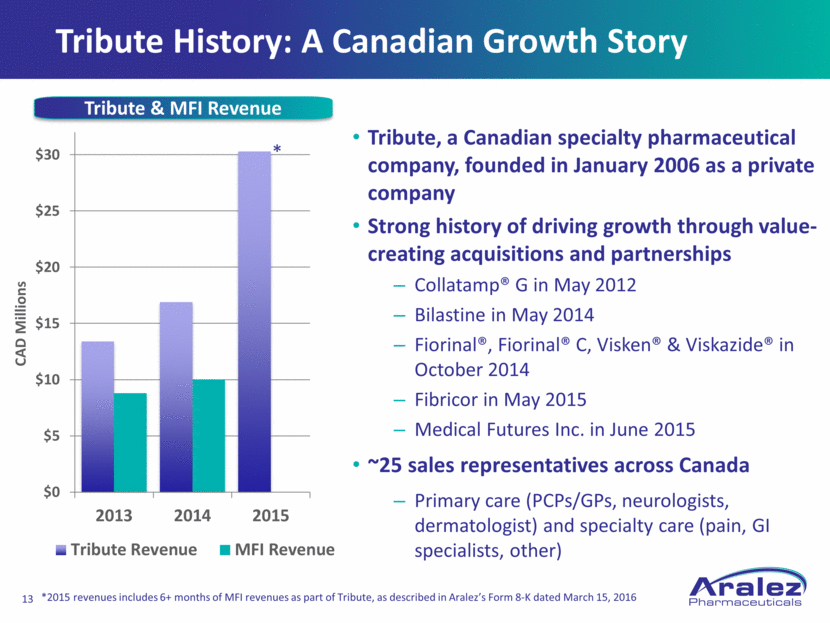
Tribute History: A Canadian Growth Story Tribute, a Canadian specialty pharmaceutical company, founded in January 2006 as a private company Strong history of driving growth through value-creating acquisitions and partnerships Collatamp® G in May 2012 Bilastine in May 2014 Fiorinal®, Fiorinal® C, Visken® & Viskazide® in October 2014 Fibricor in May 2015 Medical Futures Inc. in June 2015 ~25 sales representatives across Canada Primary care (PCPs/GPs, neurologists, dermatologist) and specialty care (pain, GI specialists, other) CAD Millions Tribute & MFI Revenue * *2015 revenues includes 6+ months of MFI revenues as part of Tribute, as described in Aralez’s Form 8-K dated March 15, 2016 $0 $5 $10 $15 $20 $25 $30 2013 2014 2015 Tribute Revenue MFI Revenue
Aralez: Canadian Portfolio Growth Drivers Cambia® Exclusive license in Canada from Depomed, Inc. Cambia (diclofenac) was approved by Health Canada in 2009 for the acute treatment of migraine attached with or without aura in adults 18 years of age or older Durela® Exclusive license in Canada from Cipher Pharmaceuticals Inc. Durela (tramadol) was approved by Health Canada in 2011 for the management of moderate to moderately severe pain in adults who require continuous treatment for several days or more Soriatane® Soriatane (acitretin) was approved by Health Canada in 1994 for the treatment for severe psoriasis (includes erythrodermic and pustular types) and other disorders of keratinization The first and only oral retinoid indicated for severe psoriasis
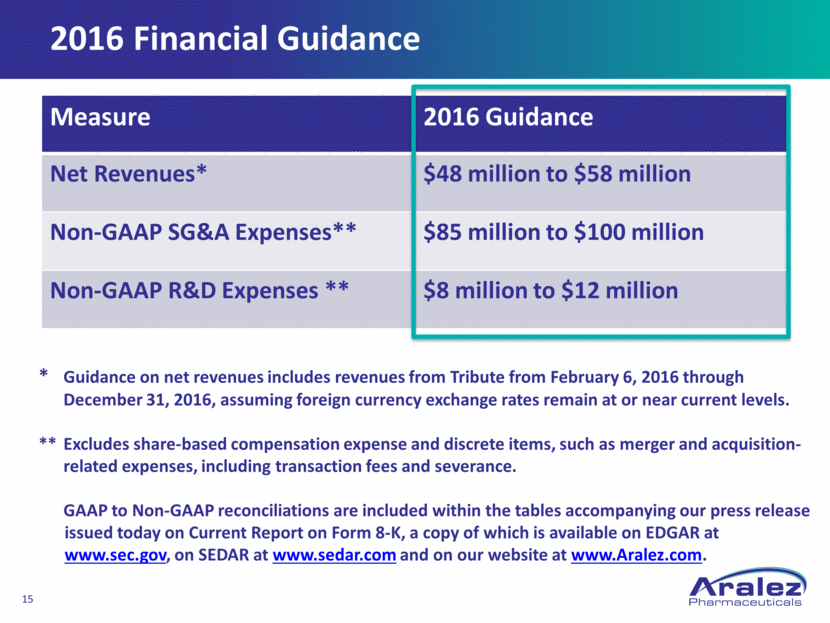
2016 Financial Guidance Measure 2016 Guidance Net Revenues* $48 million to $58 million Non-GAAP SG&A Expenses** $85 million to $100 million Non-GAAP R&D Expenses ** $8 million to $12 million * Guidance on net revenues includes revenues from Tribute from February 6, 2016 through December 31, 2016, assuming foreign currency exchange rates remain at or near current levels. ** Excludes share-based compensation expense and discrete items, such as merger and acquisition- related expenses, including transaction fees and severance. GAAP to Non-GAAP reconciliations are included within the tables accompanying our press release issued today on Current Report on Form 8-K, a copy of which is available on EDGAR at www.sec.gov, on SEDAR at www.sedar.com and on our website at www.Aralez.com.
Business Development: Framework and Focus Near-term EBITDA accretive* and revenue generating products Approved products in Cardiovascular and Pain anchor areas Opportunistic approach to other specialty therapeutic areas meeting specified criteria US, Canada and ex-North American geographies Aligned, opportunistic and transformative M&A Focus & Priorities Business Development Value Creation Strategy Corporate Structure with Strong Financial Position Enhances Competitiveness * Excluding discrete items, including merger and acquisition-related expenses.
Our Near Term Priorities Complete Integration of Tribute Pharmaceuticals Canada Inc. Continued financially disciplined build up of organization Prepare for sales force deployment in April 2016 to promote Fibricor Prepare for anticipated YOSPRALA launch in 4Q 2016, pending FDA approval, and execute a targeted, invest into the opportunity, commercialization strategy Plan to submit YOSPRALA in Europe in 3Q 2016 and MT400 (Treximet) in Canada by year-end Bilastine undergoing regulatory review in Canada Actively assess and execute Business Development and M&A opportunities that are near-term revenue generating and accretive Shareholder Value Creation

“Corporate Overview & Strategic Vision” Dundee Investor Meetings April 4-6, 2016

