Attached files
| file | filename |
|---|---|
| EX-32.2 - EX-32.2 - Aralez Pharmaceuticals Inc. | arlz-20161231ex3227836ca.htm |
| EX-32.1 - EX-32.1 - Aralez Pharmaceuticals Inc. | arlz-20161231ex321f7090e.htm |
| EX-31.2 - EX-31.2 - Aralez Pharmaceuticals Inc. | arlz-20161231ex312901313.htm |
| EX-31.1 - EX-31.1 - Aralez Pharmaceuticals Inc. | arlz-20161231ex3111d4f51.htm |
| EX-23.1 - EX-23.1 - Aralez Pharmaceuticals Inc. | arlz-20161231ex231d3a745.htm |
| EX-21.1 - EX-21.1 - Aralez Pharmaceuticals Inc. | arlz-20161231ex211b3216f.htm |
| EX-10.6 - EX-10.6 - Aralez Pharmaceuticals Inc. | arlz-20161231ex106fcc1c9.htm |
| EX-10.3 - EX-10.3 - Aralez Pharmaceuticals Inc. | arlz-20161231ex1036550b3.htm |
| EX-10.15 - EX-10.15 - Aralez Pharmaceuticals Inc. | arlz-20161231ex1015921b6.htm |
| EX-10.14 - EX-10.14 - Aralez Pharmaceuticals Inc. | arlz-20161231ex10149a00d.htm |
| EX-10.13 - EX-10.13 - Aralez Pharmaceuticals Inc. | arlz-20161231ex1013fce4c.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
☒ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2016
OR
☐TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE TRANSITION PERIOD FROM TO .
Commission file number 001-37691
ARALEZ PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
|
British Columbia, Canada |
98-1283375 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
7100 West Credit Avenue, Suite 101, Mississauga, Ontario, Canada L5N 0E4
(Address of registrant’s principal executive offices)
(905) 876-1118
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Name of each exchange on which registered |
|
Common Shares, without par value |
NASDAQ Global Market, Toronto Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act:
Common Shares, no par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒. No ☐.
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒. No ☐.
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
|
Large accelerated filer ☐ |
Accelerated filer ☒ |
|
Non-accelerated filer ☐ |
Smaller reporting company ☐ |
Indicate by check mark whether the registrant is a shell company (as defined in 12b-2 of the Act). Yes ☐ No ☒.
The aggregate market value of the common shares held by non-affiliates of the registrant (computed by reference to the closing sale price of $4.41 for the registrant’s common shares as reported on the NASDAQ Global Market on December 30, 2016) was approximately $289,475,077. As of the close of business on March 9, 2017, there were 65,683,646 common shares issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of Aralez Pharmaceuticals Inc.’s definitive proxy statement to be filed pursuant to Regulation 14A within 120 days after the end of the registrant’s fiscal year are incorporated by reference into Part III of this Form 10-K and certain documents are incorporated by reference into Part IV.
ARALEZ PHARMACEUTICALS INC.
ANNUAL REPORT ON FORM 10-K
|
|
|
PAGE |
|
|
2 | |
|
|
|
|
|
|
3 | |
|
|
|
|
| 3 | ||
|
|
|
|
| 19 | ||
|
|
|
|
| 46 | ||
|
|
|
|
| 46 | ||
|
|
|
|
| 46 | ||
|
|
|
|
| 49 | ||
|
|
|
|
|
|
50 | |
|
|
|
|
| 50 | ||
|
|
|
|
| 53 | ||
|
|
|
|
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
54 | |
|
|
|
|
| 64 | ||
|
|
|
|
| 64 | ||
|
|
|
|
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
64 | |
|
|
|
|
| 64 | ||
|
|
|
|
| 65 | ||
|
|
|
|
|
|
66 | |
|
|
|
|
| 66 | ||
|
|
|
|
| 66 | ||
|
|
|
|
|
Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters |
66 | |
|
|
|
|
|
Certain Relationships and Related Transactions and Director Independence |
66 | |
|
|
|
|
| 66 | ||
|
|
|
|
|
|
67 | |
|
|
|
|
| 67 | ||
|
|
|
|
| 71 | ||
|
|
|
|
|
|
72 |
1
This Annual Report on Form 10-K includes “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and within the meaning of applicable securities laws in Canada. Forward-looking statements include, but are not limited to, statements about execution of our commercialization strategy with our expanded product portfolio, including Yosprala® (aspirin and omeprazole), Fibricor® (fenofibric acid) and its authorized generic, Toprol-XL® (metoprolol succinate) and its currently marketed authorized generic and Zontivity® (vorapaxar), which we expect to commence promotion of in the United States in June 2017, business development plans, our operating model and financial discipline, our objective to achieve sustained long-term growth, product launches, our strategies, plans, objectives, financial forecasts, goals, prospects, prospective products or product approvals, future performance or results of current and anticipated products, exposure to foreign currency exchange rate fluctuations, interest rate changes and other statements that are not historical facts, and such statements are typically identified by use of terms such as “may,” “will,” “would,” “should,” “could,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “likely,” “potential,” “continue” or the negative or similar words, variations of these words or other comparable words or phrases, although some forward-looking statements are expressed differently. You should be aware that the forward-looking statements included herein represent management’s current judgment and expectations, but our actual results, events and performance could differ materially from those in the forward-looking statements. The forward-looking statements are subject to a number of risks and uncertainties which are discussed in the section entitled “Item 1A. Risk Factors” and elsewhere in this Annual Report on Form 10-K and those described from time to time in our future reports filed with the Securities and Exchange Commission (“SEC”) and securities regulatory authorities in Canada. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee future results, events, levels of activity, performance or achievement. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, unless required by law.
All dollar amounts are expressed in U.S. dollars unless otherwise noted. Amounts are expressed on an as-converted from Canadian dollar to U.S. dollar basis, as applicable, and are calculated using the conversion rates as of and for the periods ended December 31, 2016 unless otherwise noted.
Unless the context indicates otherwise, when we refer to “we,” “us,” “our,” “Aralez” or the “Company” in this Annual Report on Form 10-K, we are referring to Aralez Pharmaceuticals Inc. together with its wholly-owned subsidiaries.
2
Our Company
Overview
Aralez is a global specialty pharmaceutical company focused on delivering meaningful products to improve patients’ lives while creating shareholder value by acquiring, developing and commercializing products primarily in cardiovascular, pain and other specialty areas. Our parent corporation, Aralez Pharmaceuticals Inc. (“Aralez Parent”), was incorporated under the British Columbia Business Corporations Act (“BCBCA”) on December 2, 2015. Our global headquarters is located in Mississauga, Ontario, Canada, our U.S. headquarters is located in Princeton, New Jersey, United States, and our Irish headquarters is located in Dublin, Ireland. Aralez was formed for the purpose of facilitating the business combination of POZEN Inc., a Delaware corporation (“Pozen”), and Tribute Pharmaceuticals Canada Inc., a corporation incorporated under the laws of the Province of Ontario, Canada (“Tribute”), which transaction closed on February 5, 2016.
Strategy
Our management team has a strong track record of success in creating, leading and expanding specialty pharmaceutical companies with marketing and sales capabilities. Driven by this leadership and leveraging our competitive platform, our focus on acquiring high potential growth opportunities through aggressive business development and licensing and strategic transactions, and commercializing our product portfolio to provide enhanced value to a range of stakeholders, is driven by the following primary strategies:
|
· |
Maximize value of expanded portfolio – We plan to continue our focus on execution of our commercialization strategy with respect to our broadened cardiovascular portfolio, including Yosprala® (aspirin and omeprazole) which was approved by the U.S. Food and Drug Administration (“FDA”) in September 2016, Fibricor® (fenofibric acid) and its authorized generic, Toprol-XL® (metoprolol succinate) and its currently marketed authorized generic (the “AG”), which we acquired in October 2016, and Zontivity® (vorapaxar), which we acquired in September 2016 and currently expect to commence promotion of in the United States in June 2017. |
|
· |
Business Development through selective acquisitions – We have completed several transactions in 2016 to expand our portfolio offering. We plan to continue to pursue value-driven business development opportunities as they arise in order to enhance our product pipeline through strategically acquiring Phase 3 or commercial-ready product candidates or approved revenue-generating products with growth potential, particularly in the cardiovascular and pain anchor areas. We will also continue to assess the addition of other specialty therapeutic areas through both product/portfolio acquisitions or other M&A activity with a similar focus on opportunities that we anticipate are or will become revenue generating and accretive. |
|
· |
Leverage platform for growth – We intend to maintain a lean, nimble and performance-oriented operating model with strong financial discipline. Our objective is to achieve sustained long-term growth, both organically, through products such as Yosprala, and through business development initiatives that could include M&A and/or product acquisitions, such as the recently completed purchases of Zontivity and Toprol-XL and its AG, while at all times maintaining our focus on creating shareholder value. |
2016 and More Recent Highlights
|
· |
On February 5, 2016, pursuant to an Agreement and Plan of Merger and Arrangement between Aralez Parent, Pozen, Tribute and other related parties (as amended, the “Merger Agreement”), Aralez completed the business combination of Pozen and Tribute. In connection with the transaction, Pozen and Tribute were combined under and became subsidiaries of Aralez Parent, with Pozen treated as the acquiring company for accounting purposes (the “Merger”). Pursuant to Rule 12g-3(a) under the Exchange Act, Aralez Parent is the successor issuer to Pozen. Our results of operations for the fiscal year ended December 31, 2016 include the results of operations of Tribute for the period from February 5, 2016 through December 31, 2016 and the results of operations of Pozen from January 1, 2016 to February 5, 2016. Refer to Note 2, “Business Combinations and Acquisitions,” in the |
3
accompanying notes to consolidated financial statements for additional information with respect to the acquisition of Tribute. |
|
· |
On April 20, 2016, the Company announced the promotional launch of Fibricor, comprised of both the branded product and its authorized generic, in the United States with a 25-person sales force. |
|
· |
On April 25, 2016, the Company announced Health Canada's approval of Blexten™ (bilastine 20 mg oral tablet) for the treatment of the symptoms of Seasonal Allergic Rhinitis (“SAR”) and Chronic Spontaneous Urticaria (“CSU”) (such as itchiness and hives). The approval was granted to Aralez Pharmaceuticals Trading DAC, a wholly-owned subsidiary of Aralez formed under the laws of Ireland (“Aralez Ireland”). |
|
· |
On September 6, 2016, Aralez Ireland, acquired the U.S. and Canadian rights to Zontivity pursuant to an asset purchase agreement with Schering-Plough (Ireland) Company, an Irish private unlimited company and an affiliate of Merck & Co., Inc. (“Merck”). Zontivity represents an addition to our product portfolio in cardiovascular disease and is the first and currently the only approved therapy shown to inhibit the protease-activated receptor-1 (PAR-1), the primary receptor for thrombin on the platelet, which is considered to be the most potent activator of platelets. Our results of operations for the year ended December 31, 2016 include the net revenues from sales of Zontivity from its acquisition date. |
|
· |
On September 15, 2016, the Company announced that the FDA approved Yosprala for the secondary prevention of cardiovascular and cerebrovascular events in patients at risk for aspirin-associated gastric ulcers. In connection with such approval, we expanded our U.S. sales force by 85 representatives to a total of 110 sales representatives and began commercializing Yosprala in the United States on October 3, 2016. |
|
· |
On October 31, 2016, Aralez Ireland acquired the U.S. rights to Toprol-XL and the AG pursuant to an asset purchase agreement (the “Toprol-XL Asset Purchase Agreement”) entered into between AstraZeneca AB (“AstraZeneca”), Aralez Ireland and Aralez Parent. Toprol-XL is a cardioselective beta-blocker indicated for the treatment of hypertension, alone or in combination with other antihypertensives, the long term treatment of angina pectoris and the treatment of stable, symptomatic (NYHA class II or III) heart failure of specific origins. Toprol-XL and the AG further expand our cardiovascular portfolio. Our results of operations for the year ended December 31, 2016 include the net revenues from sales of Toprol-XL and the AG from its acquisition date. |
|
· |
On December 15, 2016, the Company announced that it had entered into a rebate agreement with CaremarkPCS Health (also known as CVS Caremark), which secures formulary status for Yosprala, in the United States. |
|
· |
On December 19, 2016, the Company announced the commercial launch of Blexten for the treatment of the symptoms of SAR and CSU in Canada. Blexten is distributed in Canada by Tribute. |
|
· |
Effective on January 1, 2017, the Company entered into a rebate agreement with Express Scripts Inc., which secured formulary status for Yosprala, in the United States. |
|
· |
On January 9, 2017, the Company announced that it had submitted a Marketing Authorization Application (“MAA”) to the European Medicines Agency (“EMA”) for its investigational candidate, PA10040 (aspirin and omeprazole, which is marketed in a tablet form under the brand name Yosprala in the United States) for the secondary prevention of cardiovascular disease in patients at risk for aspirin-induced gastric ulcers. |
|
· |
On January 11, 2017, the Company announced that the United States Patent and Trademark Office (“USPTO”) had issued U.S. Patent No. 9,539,214, entitled “Compositions and Methods for Delivery of Omeprazole Plus Acetylsalicylic Acid”, which covers Yosprala. The patent is listed in FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations (also known as the “Orange Book”), bringing the total number of patents in the Orange Book for Yosprala to four. |
4
Our Products
The Company currently commercializes a number of cardiovascular products in the United States as well as products for cardiovascular, pain management, dermatological and certain other indications in Canada. In addition, the Company outlicenses certain products in exchange for royalties and/or other payments. Certain of our main products are described below.
Marketed Products – United States
Yosprala®
Yosprala is currently the only prescription fixed-dose combination of aspirin (acetylsalicylic acid), an anti-platelet agent, and omeprazole, a proton pump inhibitor (“PPI”), in the U.S. It is indicated for patients who require aspirin for secondary prevention of cardiovascular and cerebrovascular events and who are at risk of developing aspirin associated gastric ulcers. Yosprala is designed to support both cardio- and gastro-protection for at-risk patients through the proprietary Intelli-COAT™ system, which is formulated to sequentially deliver immediate-release omeprazole (40 mg) followed by a delayed-release, enteric-coated aspirin core in either 81 mg or 325 mg dose strengths. Yosprala is currently protected by four U.S. patents, the latest expiring in late 2032 with potential patent term adjustment into early 2033. We received FDA approval for Yosprala on September 14, 2016 and began commercialization in the United States on October 3, 2016, with a 110-person sales force. The competition for PPI-aspirin (“PA”) products, such as Yosprala, may come from aspirin itself, other aspirin-combination products that may be introduced, as well as other anti-platelet products used for secondary prevention of cardiovascular and cerebrovascular events.
The Company is committed to perform two post-marketing requirements related to Yosprala. One is an in-vitro study to examine the breakdown products of omeprazole at different pH levels. Pending the results of that study, the FDA has requested a pharmacokinetics study measuring the levels of these degradants in serum compared to enteric-coated omeprazole.
Toprol-XL® and its Authorized Generic
Toprol-XL is a cardioselective beta-blocker indicated for the treatment of hypertension, alone or in combination with other antihypertensives, the long term treatment of angina pectoris and treatment of stable, symptomatic (NYHA class II or III) heart failure of specific origins. Toprol-XL is an extended-release tablet that belongs to a family of high blood pressure medications known as beta-blockers. Extended-release tablets need to be taken only once a day. After swallowing Toprol-XL, the coating of the tablet dissolves, releasing a multitude of controlled release pellets filled with metoprolol succinate. Each pellet acts as a separate drug delivery unit and is designed to deliver metoprolol continuously over the dosage interval of 24 hours. We acquired the U.S. rights to Toprol-XL and the AG from AstraZeneca on October 31, 2016 in exchange for an upfront payment of $175 million, a payment for certain inventory and certain future royalties and contingent milestone payments, as described in Note 2, “Business Combinations and Acquisitions,” in the accompanying notes to consolidated financial statements in more detail. Toprol-XL and the AG compete against several generic offerings for metoprolol succinate.
Fibricor® and its Authorized Generic
Fibricor is indicated as a complementary therapy along with diet for the treatment of severe hypertriglyceridemia and as a complementary therapy along with diet to reduce elevated LDL-C, Total-C, TG, and Apo B, and to increase HDL-C in patients with primary hypercholesterolemia or mixed dyslipidemia. Fibricor is currently protected by four U.S. patents extending to August 20, 2027. In May 2015, we acquired the U.S. rights to Fibricor (fenofibric acid) and its related authorized generic. We began promoting Fibricor in the United States during the second quarter of 2016 with a 25-person U.S. sales force, which was expanded to 110 sales professionals in September 2016 in connection with the U.S. launch of Yosprala. Fibricor and its authorized generic compete against other cholesterol-lowering drugs known as fibrates. The large fibrate market is heavily genericized.
Zontivity®
Zontivity is the first and currently the only approved therapy shown to inhibit the protease-activated receptor-1 (PAR-1), the primary receptor for thrombin on the platelet, which is considered to be the most potent activator of
5
platelets. In the United States, Zontivity is indicated for the reduction of thrombotic cardiovascular events in patients with a history of heart attack (myocardial infarction) or in patients with narrowing of leg arteries, called peripheral arterial disease (PAD), and should be used in combination with daily aspirin and/or clopidogrel according to their indications or standard of care. We acquired the U.S. and Canadian rights to Zontivity from Merck on September 6, 2016 in exchange for an upfront payment of $25 million and certain future royalties and milestone payments, as described in Note 2, “Business Combinations and Acquisitions,” in the accompanying notes to consolidated financial statements in more detail.
We have commenced the commercial preparations for a relaunch of Zontivity by our U.S. sales force in 2017 and are currently assessing our plans with respect to the commercialization of Zontivity in Canada. Zontivity competes with certain products referred to as oral anti-platelets, which market is dominated by the generic offerings for clopidogrel bisulfate. There are also two newer, competitive anti-platelet offerings in this class: Effient® and Brilinta®.
Marketed Products – Canada
Cambia®
Cambia® (diclofenac potassium for oral solution) is a non-steroidal anti-inflammatory drug (“NSAID”) and currently the only prescription NSAID available and approved in Canada for the acute treatment of migraine attacks with or without aura in adults 18 years of age or older. Cambia was licensed from Nautilus Neurosciences, Inc. (“Nautilus”) in November 2010, which was acquired by Depomed, Inc. in December 2013. Cambia was approved by Health Canada in March 2012 and was commercially launched to specialists in Canada in October 2012 and broadly to all primary care physicians in February 2013.
We consider the competitive market for Cambia to be the triptan class of drugs or 5-HT1 receptor agonists as they are known, which include sumatriptan (Imitrex®), rizatriptan (Maxalt®), zolmitriptan (Zomig®), almotriptan (Axert®), naratriptan (Amerge®), eletriptan (Relpax®) and frovatriptan (Frova®).
Fiorinal®/Fiorinal® C
Fiorinal® (acetylsalicylic acid, caffeine and butalbital tablets and capsules) and Fiorinal® C (acetylsalicylic acid, caffeine, butalbital and codeine capsules) were originally approved by Health Canada in 1971 and 1970, respectively, for the relief of tension-type headaches. Fiorinal is a fixed dose combination drug that combines the analgesic properties of acetylsalicylic acid, with the anxiolytic and muscle relaxant properties of butalbital, and the central nervous system stimulant properties of caffeine. Fiorinal C expands on the properties of Fiorinal with the additional analgesic effect of codeine. Fiorinal and Fiorinal C are currently the only prescription products in Canada indicated for relief of tension type headaches. Fiorinal and Fiorinal C were acquired from Novartis AG and Novartis Pharma AG in October 2014.
We consider the competitive market for Fiorinal and Fiorinal C as the prescription NSAID class, which includes Naprosyn®, Anaprox®, Toradol®, and prescription analgesic/opiate combination class, which includes Percocet® and Tylenol® with codeine.
Soriatane®
Soriatane® (acitretin) is indicated for the treatment of severe psoriasis (including erythrodermic and pustular types) and other disorders of keratinization. Soriatane is a retinoid, an aromatic analog of vitamin A. Soriatane was approved in Canada in 1994 and is the first and currently the only oral retinoid indicated for severe psoriasis. Soriatane is often used when milder forms of psoriasis treatments like topical steroids, emollients and topical tar-based therapies have failed. Soriatane is under license from Actavis Group PTC ehf (“Actavis”), an Allergan affiliate, and we have the exclusive rights to market Soriatane in Canada.
We consider the competitive market for Soriatane to be biologic therapies such as Enbrel®, Humira® and Remicade®, and oral agents such as Cyclosporine and methotrexate.
6
Bezalip® SR
Bezalip® SR (bezafibrate) is an established pan-peroxisome proliferator-activated receptor activator. Bezalip SR, used to treat hyperlipidemia (high cholesterol), has over 25 years of therapeutic use globally. Bezalip SR helps lower LDL-C and triglycerides while raising HDL-C levels. It also improves insulin sensitivity and reduces blood glucose levels, which in combination with the cholesterol effects may significantly lower the incidence of cardiovascular events and development of diabetes in patients with features of metabolic syndrome. Bezalip SR is contraindicated in patients with hepatic and renal impairment, pre-existing gallbladder disease, hypersensitivity to bezafibrate, or pregnancy or lactation. Bezalip SR is under license from Actavis, and we have the exclusive rights to market Bezalip SR in Canada and the United States. At this time, we are only marketing Bezalip SR in Canada.
We consider the competitive market for Bezalip SR to be the fibrates class of cholesterol-lowering treatments, which is composed of three competing molecules: (1) gemfibrozil (Lopid®), (2) bezafibrate (Bezalip SR), and (3) fenofibrate (Lipidil® in Canada or Tricor® in the United States).
Proferrin®
Proferrin® (heme iron polypeptide) is an iron supplement used to prevent or treat those at risk of iron deficiency. We have the exclusive right to import and distribute Proferrin in Canada pursuant to a distribution agreement with Colorado Biolabs, Inc.
We consider the competitive market for Proferrin to be in the Heme iron class of iron supplements, which is composed of two directly competing products: (1) Hema-Fer, and (2) JAMP Heme iron, and the following indirectly competing products: (1) Polyride® and Feramax® (Polysaccharide-iron complex), and (2) Palafer® and Eurofer® (Ferrous fumarate).
BlextenTM (bilastine)
Bilastine is a second generation antihistamine drug for the symptomatic relief of allergic rhinitis and chronic spontaneous urticaria. Bilastine exerts its effect as a selective histamine H1 receptor antagonist, and has an effectiveness similar to other second generation antihistamines such as cetirizine, fexofenadine and desloratadine. It was developed in Spain by FAES Farma, S.A. In April 2016, Health Canada approved bilastine with the brand name Blexten (bilastine 20mg oral tablet) for the treatment of the symptoms of SAR and CSU (such as itchiness and hives). We began commercializing Blexten in Canada in December 2016.
We consider the competitive market for Blexten to be first generation selective histamine H1 receptor antagonists (Aerius® – desloratadine, Claritin® - loratadine, Allegra® – fexofenadine, Reactine® – cetirizine); second generation selective histamine H1 receptor antagonist (RupallTM – rupatadine; and Benadryl® – diphenhydramine and Atarax® – hydroxyzine).
Product Pipeline Updates
The Company plans to consider various avenues to commercialize Yosprala outside of the U.S., and, to this end, in January 2017, submitted a MAA to the EMA for its investigational candidate, PA10040 (aspirin and omeprazole, which is marketed in a tablet form under the brand name Yosprala in the United States), for the secondary prevention of cardiovascular disease in patients at risk for aspirin-induced gastric ulcers.
Out-Licensed Products
VIMOVO®
VIMOVO (naproxen/esomeprazole magnesium) is the brand name for a proprietary fixed-dose combination of enteric-coated naproxen, a pain-relieving NSAID and immediate-release esomeprazole magnesium, a PPI, in a single delayed-release tablet. We developed VIMOVO in collaboration with AstraZeneca. On April 30, 2010, the FDA approved VIMOVO for the relief of the signs and symptoms of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, and to decrease the risk of developing gastric ulcers in patients at risk of developing NSAID-associated gastric ulcers.
7
In 2010, we officially transferred to AstraZeneca the investigational new drug application (“IND”) and new drug application (“NDA”) for the product such that AstraZeneca became responsible for the commercialization of VIMOVO. In November 2013, AstraZeneca entered into an agreement for Horizon Pharma USA, Inc. (“Horizon”) to acquire the U.S. rights for VIMOVO. Under the terms of the agreement, we receive from Horizon a 10% royalty on net sales of VIMOVO sold in the United States, with guaranteed annual minimum royalty payments of $7.5 million. The guaranteed annual minimum royalty payments are applicable for each calendar year that certain patents which cover VIMOVO are in effect and certain types of competing products are not on the market in the United States (including competing products entering pursuant to a license to enter the market prior to expiration of the applicable patents). Horizon’s royalty payment obligation with respect to VIMOVO expires on the later of (a) the last to expire of certain patents covering VIMOVO, and (b) ten years after the first commercial sale of VIMOVO in the United States. The royalty rate may be reduced to the mid single digits in the event of a loss of market share as a result of certain competing products (including competing products entering pursuant to a license to enter the market prior to expiration of the applicable patents).
AstraZeneca will continue to have rights to commercialize VIMOVO outside of the United States and Japan and paid us a royalty of 6% on all sales within its territory through 2015, which increased to 10% commencing in the first quarter of 2016. AstraZeneca’s royalty payment obligation with respect to VIMOVO expires on a country-by country basis upon the later of (a) expiration of the last-to expire of certain patent rights related to VIMOVO in that country, and (b) ten years after the first commercial sale of VIMOVO in such country. The royalty rate may be reduced to the mid single digits in the event of a loss of market share as a result of certain competing products (including competing products entering pursuant to a license to enter the market prior to expiration of the applicable patents). As the result of an unfavorable outcome in certain patent litigation in Canada, it is expected that Mylan’s generic naproxen/esomeprazole magnesium tablets will be available in Canada shortly. See the section entitled “Item 3 – Legal Proceedings” for more information.
Treximet®
Treximet (sumatriptan/naproxen sodium) is a migraine medicine that we developed in collaboration with Glaxo Group Limited, d/b/a GlaxoSmithKline (“GSK”). The product is formulated with our patented technology of combining a triptan, sumatriptan 85mg, with an NSAID, naproxen sodium 500mg, and GSK’s RT Technology™ in a single tablet. In 2008, the FDA approved Treximet for the acute treatment of migraine attacks, with or without aura, in adults. Treximet is currently available in the United States only.
In 2008, we transferred the IND and NDA for the product to GSK, which subsequently sold its rights in Treximet, including the related trademark, to Pernix Therapeutics Holdings, Inc. (“Pernix”) in 2014. As part of GSK’s divestiture to Pernix, restrictions on our right to develop and commercialize certain additional dosage forms of sumatriptan/naproxen combinations outside of the United States had been eliminated, allowing us to seek approval for these combinations on the basis of the approved NDA. GSK was previously, and Pernix is currently, responsible for the commercialization of Treximet in the United States, while we receive royalties based on net sales. In 2011, we sold to a financial investor, CPPIB Credit Investments Inc. (“CII”), for an upfront lump-sum, our rights to future royalty and milestone payments relating to Treximet sales in the United States and certain other products containing sumatriptan/naproxen sodium developed and sold by Pernix in the United States. By virtue of the agreement, we will also be entitled to receive a 20% interest in royalties, if any, paid on net sales of Treximet and such other products in the United States to CII relating to the period commencing in the second quarter of 2018.
Sales and Marketing
The Company’s sales and marketing strategy is focused on the organic growth of existing marketed products through several key activities. First, our analytics team seeks to ensure that our sales force targets known prescribers of our medications or medications that compete with our products. We create demand by calling on and providing prescribers with reliable and trustworthy information, supported by our clinical trials and from other credible sources, and by coordinating and facilitating continuing health education events in targeted areas. Second, we support our products by providing physicians and other healthcare practitioners with quality patient care materials. Third, we endeavor to ensure that our products are accessible through all major wholesalers and distributors in the United States and Canada, and manage our supply chain efficiently to ensure that it can meet demand.
8
Our current U.S. sales force consists of approximately 110 sales representatives, including 85 sales representatives added in the third quarter of 2016 to market Yosprala, which received FDA approval in September 2016. In Canada, we have approximately 27 sales representatives. The Company considers its sales force to be very experienced and well trained. Additionally, we offer our representatives a competitive incentive plan based on the achievement of results.
Manufacturing
We currently have no manufacturing capability. We outsource the manufacturing of our proprietary products to pharmaceutical manufacturing facilities operated by third-party contractors. These facilities comply with the FDA’s current Good Manufacturing Practices (“cGMP”) regulations and applicable Health Canada regulations, including in accordance with Health Canada’s cGMP requirements. See the section entitled “Item 1. Business – Government Regulations and Other Considerations” for a further discussion regarding the regulations that pharmaceutical manufacturing facilities are subject to. We believe these facilities have sufficient excess capacity at present to meet our short and long-term objectives.
Our licensed products are manufactured by authorized, third-party, contract manufacturing organizations in various places throughout the world. Our manufacturers are all approved fabricators of pharmaceutical products according to the FDA and Health Canada, as applicable. Our proprietary and licensed products are packaged by third-party contract manufacturers.
To date, we have entered into arrangements with third-party manufacturers for the supply of formulated and packaged clinical trial materials, drug products, active ingredients and other ingredients used in the manufacturing of our products. Certain of our material manufacturing arrangements include:
|
· |
A Manufacturing Services Agreement with Patheon Pharmaceuticals Inc. (“Patheon”) pursuant to which Patheon has agreed to manufacture, and we have agreed to purchase, a specified percentage of our requirements for Yosprala for sale in the United States. |
|
· |
In connection with our acquisition of Zontivity, Merck agreed to supply Zontivity to us for a period of up to three years from closing, although we are required to transfer the packaging for the product within one year of its acquisition. |
|
· |
In connection with our acquisition of Toprol-XL and the AG, we entered into a Supply Agreement with AstraZeneca pursuant to which (except as expressly set forth therein) AstraZeneca will be our exclusive manufacturer and supplier of Toprol-XL and the AG, as described in more detail in Note 2, “Business Combinations and Acquisitions,” in the accompanying notes to consolidated financial statements. |
|
· |
Under our arrangements with GSK and Pernix for Treximet and AstraZeneca and Horizon for VIMOVO, it is the obligation of our partners to obtain commercial supplies of products developed thereunder. |
Use of third-party manufacturers enables us to focus on our development and sales/commercialization activities, minimize fixed costs and capital expenditures and gain access to advanced manufacturing process capabilities and expertise. We plan to continue to rely on third-party manufacturers to manufacture our compounds and final products.
Industry and Competition
The pharmaceutical industry is highly competitive and is characterized by rapidly changing markets, technology, emerging industry standards and frequent introduction of new products. We believe that competition in our market is based on, among other things, product safety, efficacy, convenience of dosing, reliability, availability and price. The market is dominated by a small number of highly-concentrated global competitors, many of which boast substantially greater resources than the Company. Given the size and scope of the competition, there can be no assurance that the Company will maintain or grow our current market position in its therapeutic areas, or that developments by others will not render our products or technologies non-competitive or obsolete. In addition, some of our competitors have substantially greater financial, research and development, manufacturing, marketing and human resources and greater experience than we do in product discovery, development, clinical trial management, FDA, Health Canada, and EMA regulatory review, manufacturing and marketing, which may enable them to compete more effectively than we
9
can.
The Company faces product competition from companies marketing competing pharmaceutical products and medical devices worldwide, particularly in the United States, Canada and the European Union (“EU”), and potentially on new products that could be launched in the future. See also the section entitled “Item 1. Business – Products” in this Annual Report on Form 10-K for a discussion of the other products that specifically compete with the Company’s products.
Patent and Proprietary Protection
We have obtained and intend to actively seek to obtain, when appropriate, protection for our products and proprietary technology by means of U.S., Canadian and other foreign patents, trademarks and contractual arrangements. In addition, we rely upon trade secrets and contractual agreements to protect certain of our proprietary technology and products.
While trade secret protection is an essential element of our business and we have taken security measures to protect our proprietary information and trade secrets, we cannot give assurance that our unpatented proprietary technology will afford us significant commercial protection. We seek to protect our trade secrets by entering into confidentiality agreements with third parties, employees and consultants. Our employees and consultants also sign agreements requiring that they assign to the Company their interests in intellectual property arising from their work for us. All employees sign an agreement not to engage in any conflicting employment or activity during their employment with us and not to disclose or misuse our confidential information. However, it is possible that these agreements will be breached or invalidated, and if so, there may not be an adequate corrective remedy available. Accordingly, we cannot ensure that employees, consultants or third parties will not breach the confidentiality provisions in such contracts or infringe or misappropriate our trade secrets and other proprietary rights or that the measures we are taking to protect our proprietary rights will be adequate.
We have issued U.S. and Canadian patents and pending U.S. and Canadian patent applications, as well as other pending foreign patent applications or issued foreign patents, relating to our marketed products and product candidates. We also have U.S., Canadian and other foreign patent applications pending relating to novel product concepts. There can be no assurance that our patent applications will issue as patents or, with respect to our issued patents, that they will provide us with significant protection. The following provides a general description of our patent portfolio and is not intended to represent an assessment of claim limitations or claim scope.
PN (VIMOVO)
We have issued patents in the United States, Australia, Canada, Europe, Eurasia, Israel, Mexico, Japan and Norway, with claims directed to certain compositions containing a combination of acid inhibitors (including PPIs) and NSAIDs. The issued patents also have claims to treatment methods involving the use of such compositions. We have pending U.S. patent applications that also have claims to compositions containing acid inhibitors and NSAIDs and to various treatment methods involving such compositions. The issued U.S. patents and related U.S. patent applications are expected to expire between May 2022 and February 2023. The European patent will expire in May 2022, but we have obtained supplementary protection certificates (“SPCs”) for VIMOVO that extend to dates between November 2025 and May 2026, depending on the country. We expect the patents outside of the United States and Europe to expire in May 2022.
We, together with AstraZeneca, have filed joint patent applications relating to VIMOVO. We have an issued U.S. patent and an issued Canadian patent related to the pharmacodynamics profile of VIMOVO that will expire in October of 2031 and June of 2030, respectively. Foreign counterparts, if granted, are expected to expire in September 2030. We also have two issued U.S. patents and one issued Canadian patent related to methods of treatment with VIMOVO® in patients taking low dose aspirin, which will expire as late as March of 2031 and September of 2029, respectively. Any related patents that issue outside the U.S. are expected to expire in September of 2029.
PA (YOSPRALA)
One of the patent families covering VIMOVO also covers proton pump inhibitor-aspirin (“PA”) products. We have issued patents in the United States, Australia, Canada, Eurasia, Europe, Israel, Japan, Mexico and Norway, with
10
claims directed to certain compositions containing a combination of acid inhibitors (including PPIs) and NSAIDs (including aspirin). The issued patents in Australia, Eurasia and New Zealand also have claims to treatment methods involving the use of such compositions. We have one issued patent, a pending U.S. patent application, and several non-U.S. applications that have claims directed to the use of compositions containing omeprazole and aspirin, and to various treatment methods involving such compositions.
The issued U.S. patents and related U.S. patent applications from the VIMOVO family are expected to expire between May 2022 and February 2023. The European patent will expire in May 2022, but we expect to apply for SPCs for PA upon approval. We expect the patents outside of the United States and Europe to expire in May 2022. A second family directed to PA in particular, which has issued in certain non-U.S. countries, will expire in June of 2030. A third family, also directed to PA in particular, will expire in late 2032 with potential patent term adjustment into early 2033.
MT 400 (TREXIMET)
We have four issued U.S. patents with claims relating to methods, compositions and therapeutic packages involving the use of certain NSAIDs and 5-HT receptor agonists in treating patients with migraines. Outside of the United States and Canada, we have issued patents in Australia, Europe, Hong Kong and Japan. The expected expiration date of the issued patents relating to MT 400 is in August 2017. We also have issued patents in Australia, Canada, Europe, Israel, Japan, Norway and the United States with claims relating to formulations of MT 400. We expect the patents related to formulations of MT 400 to expire in December 2023 outside the United States and in October 2025 in the United States.
Voraxapar (ZONTIVITY)
We have acquired certain patent rights from Merck relating to Zontivty. The U.S. portfolio includes two pending U.S. applications (one allowed) and fourteen issued patents. The pending cases cover voraxapar in a pharmaceutical composition or drug combination, while the issued patents cover voraxapar itself (3), intermediates (8), and synthesis (3) of voraxapar. The portfolio also includes one pending Canadian application relating to an intermediate of voraxapar, and three issued Canadian patents covering voraxapar itself, synthesis of voraxapar, and a pharmaceutical composition containing voraxapar or a drug combination.
Expiration dates for the U.S. cases range from June 2021 to July of 2028. Expiration dates for the Canadian cases range from April 2023 to June 2027. With respect to the patents covering voraxapar per se, these expire between June 2021 and May of 2024, with possible extension to 2027.
Other Patents
With respect to Cambia, we have rights to patents through our licensing agreement with Depomed, which we expect to expire in May 2017 and June 2026 in Canada. With respect to Fibricor, we have four issued patents in the United States, which we expect to expire in August 2027. In addition to the patents for the products discussed above, we also have patents or rights to patents with respect to bilastine, Durela, Moviprep, Resultz and Bedbugz.
Government Regulations and Other Considerations
The FDA in the United States, Health Canada in Canada, EMA in the EU and comparable regulatory agencies in foreign countries impose substantial requirements on the clinical development, manufacture and marketing of pharmaceutical products and product candidates. These agencies and other federal, state, provincial and local entities regulate research and development activities and the testing, manufacture, packaging, importing, distribution, quality control, safety, effectiveness, labeling, storage, record-keeping, approval and promotion of our products and product candidates. All of our product candidates will require regulatory approval before commercialization. In particular, therapeutic product candidates for human use are subject to rigorous preclinical and clinical testing and other statutory and regulatory requirements of the United States, Canada, the EU and foreign countries. Obtaining these marketing approvals and subsequently complying with ongoing statutory and regulatory requirements is costly and time-consuming. Any failure by us or our collaborators, licensors or licensees to obtain, or any delay in obtaining, regulatory approvals or in complying with other regulatory requirements could adversely affect the commercialization of our products and product candidates then being developed by us and our ability to receive product or royalty revenues.
11
United States Regulatory Overview
In the United States, the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act, as amended (“FFDCA”), and implements regulations. If we fail to comply with the applicable requirements at any time during the product development process, approval process or after approval, we may become subject to administrative or judicial sanctions. The steps required before a new drug product candidate may be distributed commercially in the United States generally include:
|
· |
conducting appropriate preclinical laboratory evaluations of the product candidate’s chemistry, formulation and stability and preclinical studies in animals to assess the potential safety and efficacy of the product candidate; |
|
· |
submitting the results of these evaluations and tests to the FDA, along with manufacturing information and analytical data, in an IND; |
|
· |
initiating clinical trials under the IND and addressing any safety or regulatory concerns of the FDA; |
|
· |
obtaining approval of Institutional Review Boards to introduce the drug into humans in clinical studies; |
|
· |
conducting adequate and well-controlled human clinical trials that establish the safety and efficacy of the product candidate for the intended use, typically in the following three sequential, or slightly overlapping stages: |
|
o |
Phase 1: The product is initially introduced into human subjects or patients and tested for safety, dose tolerance, absorption, metabolism, distribution and excretion. |
|
o |
Phase 2: The product candidate is studied in patients to identify possible adverse effects and safety risks, to determine dosage tolerance and the optimal dosage, and to collect some efficacy data. |
|
o |
Phase 3: The product candidate is studied in an expanded patient population at multiple clinical study sites, to confirm efficacy and safety at the optimized dose, by measuring primary and secondary endpoints established at the outset of the study. |
|
· |
submitting the results of preclinical studies and clinical trials, as well as chemistry, manufacturing and control information, on the product candidate to the FDA in an NDA; and |
|
· |
obtaining FDA approval of the NDA prior to any commercial sale or shipment of the product candidate. |
The foregoing process can take a number of years and requires substantial financial resources.
The results of preclinical studies and initial clinical trials are not necessarily predictive of the results from large-scale clinical trials, and clinical trials may be subject to additional costs, delays or modifications due to a number of factors, including the difficulty in obtaining enough patients, clinical investigators, product candidate supply and financial support.
Even after FDA approval has been obtained, further studies, including post-marketing studies, may be required. Results of post-marketing studies may limit or expand the further marketing of the products. If we propose any modifications to a product, including changes in indication, manufacturing process, manufacturing facility or labeling, a supplement to our NDA may be required to be submitted to the FDA and approved.
The FDA may also require testing and surveillance programs to monitor the effect of approved product candidates that have been commercialized, and the FDA has the power to prevent or limit further marketing of a product candidate based on the results of these post-marketing programs. Upon approval, a product candidate may be marketed only in those dosage forms and for those indications approved in the NDA.
12
In addition to obtaining FDA approval for each indication to be treated with each product candidate, each domestic product candidate manufacturing establishment must register with the FDA, list its product with the FDA, comply with the applicable cGMP regulations, which include requirements relating to quality control and quality assurance, as well as the corresponding maintenance of records and documentation, and permit and pass manufacturing plant inspections by the FDA. Moreover, the submission of applications for approval may require additional time to complete manufacturing stability studies. Foreign establishments manufacturing product for distribution in the United States also must list their product candidates with the FDA and comply with cGMP regulations. They are also subject to periodic inspection by the FDA or by local authorities under agreement with the FDA.
Any product candidates manufactured or distributed by us pursuant to FDA approvals are subject to extensive continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the product candidate. In addition to continued compliance with standard regulatory requirements, the FDA may also require post-marketing testing and surveillance to monitor the safety and efficacy of the marketed product. Adverse experiences and reports of adverse experiences in the medical literature with the product candidate or its components must be reported to the FDA. Product approvals may be affected and even withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product are discovered following approval.
The FFDCA also mandates that products be manufactured consistent with cGMP regulations. In complying with the cGMP regulations, manufacturers must continue to spend time, money and effort in production, record-keeping, quality control, and auditing to ensure that the marketed product meets applicable specifications and other requirements. The FDA periodically inspects manufacturing facilities to ensure compliance with cGMP regulations. Failure to comply subjects the manufacturer to possible FDA action, such as warning letters, suspension of manufacturing, seizure of the product, voluntary recall of a product or injunctive action, as well as possible civil penalties. We currently rely on, and intend to continue to rely on, third parties to manufacture our products and product candidates. These third parties will be required to comply with cGMP regulations.
Products manufactured in the United States for distribution abroad will be subject to FDA regulations regarding export, as well as to the requirements of the country to which they are shipped. These latter requirements are likely to cover the conduct of clinical trials, the submission of marketing applications, and all aspects of manufacturing and marketing. Such requirements can vary significantly from country to country.
U.S Pricing and Reimbursement Overview
In the United States, pharmaceutical products are generally paid for by private insurance, various federal or state governmental programs, “out of pocket” by the patient, or some combination of the foregoing. Recently, there has been an increased focus on drug pricing and although there are currently no direct government price controls over private sector purchases in the U.S., federal legislation requires pharmaceutical manufacturers to pay prescribed rebates on certain drugs to enable them to be eligible for reimbursement under certain public healthcare programs, such as Medicaid, and to offer brand drugs to certain federal agencies at statutorily mandated discounted prices. In addition, various states have adopted further mechanisms under Medicaid and other programs that seek to control drug prices, including by disfavoring certain higher priced drugs and by seeking supplemental rebates from manufacturers. Managed care has also become a potent force in the marketplace that increases downward pressure on the prices of pharmaceutical products.
Some recent developments on the federal level include the following:
|
· |
The Deficit Reduction Act of 2005 resulted in changes to the way average manufacturer price, or AMP, and best price are reported to the government and the formula for calculating required Medicaid rebates. In addition, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (“ACA”) increased the minimum basic Medicaid rebate for branded prescription drugs from 15.1% to 23.1% and requires pharmaceutical manufacturers to pay states rebates on prescription drugs dispensed to Medicaid managed care enrollees. The ACA also increased the additional Medicaid rebate on “line extensions” (such as extended release formulations) of solid oral dosage forms of branded products, revised the definition of AMP by changing the classes of purchasers included in the calculation, and expanded the entities eligible for discounted 340B pricing. |
|
· |
The ACA imposes a significant annual fee on companies that manufacture or import branded prescription drug |
13
products. The fee (which is not deductible for federal income tax purposes) is based on the manufacturer’s market share of sales of branded drugs and biologics (excluding orphan drugs) to, or pursuant to coverage under, specified U.S. government programs. The ACA also contains a number of provisions, including provisions governing the way that healthcare is financed by both governmental and private insurers, enrollment in federal healthcare programs, reimbursement changes, increased funding for comparative effectiveness research for use in the healthcare industry, and enhancements to fraud and abuse requirements and enforcement, that will affect existing government healthcare programs and will result in the development of new programs. |
We are unable to predict the future course of federal or state healthcare legislation and regulations, including regulations that may be issued to implement provisions of the ACA or any alternative legislation. In addition, recently, the current Presidential administration and certain legislators have made statements or proposed legislation suggesting plans to seek repeal of all or portions of the ACA and to replace the ACA with new legislation. It is unclear whether, when and how that repeal will be effectuated and what the effect on the healthcare sector will be. The ACA or any alternative legislation and further changes in the law or regulatory framework that reduce our revenues or increase our costs could also have a material adverse effect on our business, financial condition and results of operations and cash flows.
In addition, public and private healthcare payors control costs and influence drug pricing through a variety of mechanisms, including through negotiating discounts with the manufacturers and through the use of tiered formularies and other mechanisms that provide preferential access to certain drugs over others within a therapeutic class. Payors also set other criteria to govern the uses of a drug that will be deemed medically appropriate and therefore reimbursed or otherwise covered. In particular, many public and private healthcare payors limit reimbursement and coverage to the uses of a drug that are either approved by the FDA and/or appear in a recognized drug compendium. Drug compendia are publications that summarize the available medical evidence for particular drug products and identify which uses of a drug are supported or not supported by the available evidence, whether or not such uses have been approved by the FDA.
Canadian Regulatory Overview
Health Canada is the Canadian federal authority that regulates, evaluates and monitors the safety, effectiveness, and quality of drugs, medical devices, and other therapeutic products available to Canadians. Health Canada’s regulatory process for review, approval and regulatory oversight of products is similar to the regulatory process conducted by the FDA in the United States, the EMA in the EU, and other regulatory agencies around the world.
Prior to being given market authorization for a drug product, a manufacturer must present substantive scientific evidence of a product’s safety, efficacy and quality as required by the Food and Drugs Act (Canada) and its associated regulations, including the Food and Drug Regulations. This information is usually submitted in the form of a New Drug Submission (“NDS”) in Canada.
Health Canada performs a thorough review of the submitted information, sometimes using external consultants and advisory committees, to evaluate the potential benefits and risks of a drug. If, at the completion of the review, the conclusion is that the patient benefits outweigh the risks associated with the drug, the drug is issued a Notice of Compliance (“NOC”) and a Drug Identification Number (“DIN”), which permits the market authorization holder (i.e., the NOC and DIN holder) to market the drug in Canada.
Currently, the process for the review of a NDS typically takes approximately 1 to 2 years from the time that a manufacturer submits an NDS until Health Canada approves a drug. The length of time for review depends on the product being submitted and the size and quality of the submission. Health Canada’s target service standards for reviewing most NDSs is 300 days (plus an additional 45 days for screening the application). From April 1, 2015 to March 31, 2016, Health Canada’s average review time for a NDS for a new active substance was 281 days.
All establishments engaged in the fabrication, packaging/labeling, importation, distribution, and wholesale of drugs and operation of a testing laboratory relating to drugs are required to hold a Drug Establishment License to conduct one or more of the licensed activities unless expressly exempted under the Food and Drug Regulations. The basis for the issuance of a Drug Establishment License is to ensure the facility complies with cGMP as stipulated in the Food and Drug Regulations and as determined by cGMP inspection conducted by Health Canada. An importer of pharmaceutical products manufactured at foreign sites must also be able to demonstrate that the foreign sites comply with cGMP, and such foreign sites are included on the importer’s Drug Establishment License.
14
Regulatory obligations and oversight continue following the initial market approval of a pharmaceutical product. For example, every market authorization holder must report any new information received concerning adverse drug reactions, including timely reporting of serious adverse drug reactions that occur in Canada and any serious unexpected adverse drug reactions that occur outside of Canada. The market authorization holder must also notify Health Canada of any new safety and efficacy issues that it becomes aware of after the launch of a product.
Canadian Reimbursement Overview
After regulatory approval is received for a prescription drug, it can be sold to the public in accordance with the Food and Drugs Act and its regulations and applicable provincial pharmacy legislation and regulations. Revenues from prescription drug sales in Canada are usually generated through one of three sources:
|
· |
Cash: Patients will pay “out of pocket” at their sole expense. It is estimated that 10% of all prescription dollars spent in Canada come from cash purchases. |
|
· |
Private Insurance: Approximately 45% of prescription dollars spent in Canada are reimbursed via third-party private insurers, under plans generally provided by patients’ employers. Patients may be reimbursed a percentage of the cost of covered drugs minus deductibles or co-pays. The availability for reimbursement of drugs varies according to the type of reimbursement plan designed by the insurance company. There are a number of private insurers operating in Canada that provide employee plans to private and public sector employers. |
|
· |
Government Drug Plans: Government drug plans cover the cost of nearly 45% of prescription dollars spent in Canada, and generally serve patients over the age of 65 or patients for whom the cost of medications represents a significant financial burden such as families receiving social assistance. Each provincial government pays the cost of drugs that are listed on their own provincial formulary, with some government drug plans requiring patients to be responsible for a co-payment. |
After regulatory approval of a drug is granted, approval for reimbursement is typically sought from provincial governments and private insurance companies. Until provincial and private reimbursement is approved, the product is sold only via cash purchases. Decisions to list drugs for reimbursement on private and government formularies vary widely depending on the drug, indications, competitive products and price.
Sales of hospital products or products dispensed in the hospital are treated differently in Canada. All medications taken while in a hospital are fully reimbursed by the provincial governments. If a patient leaves the hospital and is prescribed a drug to be taken at home, this prescription would be paid for either by cash, private insurance or public insurance plans.
Common Drug Review (“CDR”)
The CDR was implemented in 2003 to provide formulary listing recommendations for new drugs to participating publicly-funded federal, provincial and territorial drug benefit plans in Canada. The CDR is administered by the Canadian Agency for Drugs and Technologies in Health.
The CDR consists of:
|
· |
a systematic review of the available clinical evidence and a review of the pharmacoeconomic data for the drug; and |
|
· |
a listing recommendation made by the Canadian Expert Drug Advisory Committee. |
Based on the targeted timeframes of the CDR, a review should be completed approximately 20 to 26 weeks following receipt of a manufacturer’s submission, after which recommendations are made to participating drug plans.
At the provincial and territorial level, products are reviewed on the basis of their cost-effectiveness, comparable utility to other similar products, projected utilization and cost implications to the publicly-funded drug budget. Each
15
submission is reviewed but there is wide variance in the formulary decisions and the time taken to make such decisions. Provinces and territories may utilize the recommendations of the CDR or perform their own analysis.
Presently, all provinces and territories except Quebec use the CDR recommendations in their assessment, but make their formulary decisions independently from the CDR. In many provinces, the formulary committee may grant “restricted or limited use approvals” for a drug as a means of regulating the size of the patient population eligible for reimbursement for the cost of the drug and by encouraging physicians to use older generation products first before prescribing newer, sometimes more costly medications. Further, if a generic drug is available, the government funded drug plans will often choose to reimburse only for the cost of the generic drug. Often, the provinces, territories and federal government may require the manufacturer to enter into a product listing agreement to have a product added to a government funded formulary. Such product listing agreements commonly contain product pricing restrictions and may contain other terms between the government agency and the manufacturer, such as volume discounts or other amounts that may be payable by the manufacturer to the government agency.
Product Pricing Regulation on Certain Patented Drug Products
Patented drug products in Canada are subject to regulation by the Patented Medicine Prices Review Board (“PMPRB”) pursuant to the Patent Act (Canada) and the Patented Medicines Regulations. Among other things, the PMPRB’s mandate is to ensure that prices of patented products in Canada are not excessive. For new patented products, the price is assessed taking into account the therapeutic improvement, if any, relative to its class and generally, the price in Canada is limited to either the cost of existing drugs sold in Canada or the median of prices for the same drug sold in other specified industrial countries. For existing patented products, prices generally cannot increase by more than maximum price increase allowed applying the PMPRB’s Consumer Price Index adjustment methodology. The PMPRB monitors compliance through a review of the average transaction price of each patented drug product as reported by the patentee over a recurring six-month reporting period (patentees of pharmaceutical products have mandatory reporting obligations to the PMPRB).
The PMPRB does not approve prices for drug products in advance of their introduction to the market. The PMPRB provides guidelines from which companies like us set their prices at the time they launch their products. All patented pharmaceutical products introduced in Canada are subject to the post-approval, post-launch scrutiny of the PMPRB. Since the PMPRB does not pre-approve prices for a patented drug product in Canada, there may be risk involved in the determination of an allowable price selected for a patented drug product at the time of introduction to the market by the Company launching such products in Canada. If the PMPRB does not agree with the pricing assumptions chosen by such company introducing a new drug product, the price chosen could be challenged by the PMPRB pursuant to the PMPRB initiating an investigation and, if it is determined, usually pursuant to an oral tribunal hearing, that the price charged is excessive, the Company may need to reduce its price of the product and a fine may be levied against the Company for any amount deemed to be in excess of the allowable price determined. Drug products that have no patents are not subject to the PMPRB’s jurisdiction.
European Union Regulatory Overview
Before a medicinal product can be supplied or marketed in the EU, it must first be granted a marketing authorization. There are three routes by which this may be achieved: (1) the centralized procedure whereby a single European license is granted by the European Commission permitting the supply of the product in question throughout the EU, Iceland, Norway and Lichtenstein; (2) the decentralized procedure; or (3) the mutual recognition procedure, whereby, in the case of (2) and (3), the views of one national authority (Reference Member State) are “recognized” by other authorities (Concerned Member States) when conducting their reviews. The decentralized procedure applies if the medicinal product in question has not yet received a marketing authorization in any member state at the time of the application, whereas the mutual recognition procedure applies to a currently approved medicinal product. The decentralized and mutual recognition processes lead to individual marketing authorizations in each member state for the supply of products in that country only. The centralized route is compulsory for certain products, including biotechnology products, and is optional for certain so-called “high technology” products and products containing an entirely new active substance (apart from those medicinal products containing a new active substance for treatment of specified diseases listed at paragraph 3 of the Annex to Regulation (EC) No 726/2004, which come within the compulsory centralized procedure). All products which are not authorized by the centralized route must be authorized by the decentralized or mutual recognition procedures unless the product is designed for use in a single country in which case a National Application can be made.
16
In making an application for a new medicinal product not governed compulsorily by the centralized procedure, typically use will be made of the decentralized procedure although the mutual recognition procedure would be used if a marketing authorization were first secured in a Reference Member State. The procedural steps for the decentralized procedure and the mutual recognition procedure are governed by Directive 2001/83/EC, as amended, and are described in the Notice to Applicants, Volume 2A Chapter 2—Mutual Recognition (updated version – February 2007). The procedures provide for set time periods for each process (decentralized – 120 days (if consensus is reached between all Concerned Member States, otherwise it can take longer); mutual recognition - 210 days), but if consensus is not reached between all the Concerned Member States and the Reference Member State in that time, the application is referred to arbitration through the Co-ordination Group for Mutual Recognition and Decentralized Procedure (“CMD”), with subsequent referral to the Committee for Human Medicinal Products (“CHMP”). If a referral is made, the procedure is suspended, and marketing of the product would only be possible in those EU member states in which the product has been approved (prior to the conclusion of the referral procedure) by way of the mutual recognition procedure. The opinion of the CMD/CHMP, which is binding, could support or reject the objections or alternatively reach a compromise position acceptable to all EU countries concerned. The arbitration procedure may require the delivery of additional data. Once granted, any Marketing Authorization (“MA”) remains subject to pharmacovigilance and all competent authorities have the power to vary, suspend or revoke an MA on grounds of safety.
Pricing and Reimbursement
As pressures for cost containment increase, particularly in the United States, Canada and the EU, there can be no assurance that the prices we can charge for our products will be as favorable as historical pharmaceutical product prices. Reimbursement by government, private insurance organizations and other healthcare payors has become increasingly important, as has the listing of new products on large formularies, such as those of pharmaceutical benefit providers and group buying organizations. The failure of one or more products to be included on formulary lists, or to be reimbursed by government or private insurance organizations, could have a negative impact on our results of operation and financial condition.
Future Legislation or Administrative Action
The extent of U.S., Canadian and other foreign government regulation which might result from future legislation or administrative action cannot be accurately predicted. For example, in the United States, although the Food and Drug Administration Modernization Act of 1997 (“FDAMA”) modified and created requirements and standards under the FFDCA with the intent of facilitating product development and marketing, the FDA is still in the process of developing regulations implementing FDAMA and the more recent Food and Drug Administration Amendments Act of 2007 (“FDAAA”). The FDA has been actively implementing drug safety plans called Risk Evaluation and Mitigation Strategies as authorized by the FDAAA, as a condition of drug approval, or after initial marketing, if the FDA becomes aware of new safety data about the drug. These and other legislative initiatives may impose additional regulatory requirements on us and may impact approval of our drugs or our marketing plans. The actual effect of these and other developments on our business is uncertain and unpredictable.
Other Laws and Regulations
The Company’s operations are or may be subject to various federal, provincial, state and local laws, regulations and recommendations relating to the marketing of products and relationships with treating physicians, data protection, safe working conditions, laboratory and manufacturing practices, the experimental use of animals, patient safety, the export of products to certain countries and the purchase, storage, movement, use and disposal of hazardous or potentially hazardous substances. Although we believe our safety procedures comply with the standards prescribed by federal, provincial, state and local regulations, the risk of contamination, injury or other accidental harm cannot be eliminated completely. In the event of an accident, we could be held liable for any damages that result. The amount of such damages could have a materially adverse effect on our results of operations and financial condition.
Significant Customers
For the year ended December 31, 2016, most of our product revenues were in Canada. As a result, the three significant pharmaceutical customers of the Company, which account for a significant amount of its product revenues, were McKesson Pharmaceutical – 38.5%, Shoppers Drug Mart Inc. – 19.0% and Kohl & Frisch – 14.8%. Management
17
believes this is normal and customary in the pharmaceutical business. These are well-known and respected customers that have a solid track record of paying all outstanding amounts owing on time. The profile of our customers will change prospectively as the geographic profile of our product revenues changes.
Segments and Geographic Information
We have one reporting segment. For information regarding revenue and other information regarding our results of operations, including geographic segment information, for each of our last three fiscal years, please refer to our consolidated financial statements and Note 14, “Segment Information”, in the accompanying notes to our consolidated financial statements.
Employees
As of March 9, 2017, the Company had a total of 216 employees, including 215 full-time employees and 1 part-time employee. Of these, 152 employees are in sales and marketing and the remainder are in management and administration positions. Our employees are not represented by any collective bargaining unit, and we believe our relations with our employees are good.
Available Information
We maintain a website at www.aralez.com and will make available free of charge through this website our Annual Reports on Form 10-K, our Quarterly Reports on Form 10-Q, our Current Reports on Form 8-K, our Proxy Statements on Schedule 14A, and amendments to the foregoing filed with, or furnished to, the SEC as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Except for the documents specifically incorporated by reference into this Annual Report on Form 10-K, information contained on our website or that can be accessed through our website is not incorporated by reference into this Annual Report on Form 10-K. You may also read and copy any materials we file with the SEC at the SEC’s Public Reference Room that is located at 100 F Street, N.E., Room 1580, NW, Washington, DC 20549. Information about the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330 or 1-202-551-8090.
We are also required to file reports and other information with the securities commissions in all provinces in Canada, other than Quebec. You are invited to read and copy any reports, statements or other information, other than confidential filings, that we file with the provincial securities commissions (excluding the Autorité des marchés financiers). These filings are also electronically available from the Canadian System for Electronic Document Analysis and Retrieval (www.sedar.com), the Canadian equivalent of the SEC’s electronic document gathering and retrieval system.
18
Certain factors may have a material adverse effect on our business, financial condition and results of operations, and you should carefully consider them. Accordingly, in evaluating our business, we encourage you to consider the following discussion of risk factors in its entirety, in addition to other information contained in this Annual Report on Form 10-K, as well as our other public filings with the SEC and securities regulatory authorities in Canada. The risks and uncertainties described below are those we currently believe to be material, but they are not the only ones we face. If any of the following risks, or any other risks and uncertainties that we have not yet identified or that we currently consider not to be material, actually occur or become material risks, our business and financial condition could be materially and adversely affected.
Risks Related to Our Business
Our ability to generate revenues from our products is subject to attaining significant market acceptance among physicians, patients, third-party payors and the medical community.
Our current products, and other products or product candidates that we may develop, acquire or in-license, may not attain market acceptance among physicians, patients, third-party payors or the medical community. Even if a product displays a favorable efficacy and safety profile in clinical trials, market acceptance of a product will not be known until after it is launched or relaunched and a product may not generate the revenues that we anticipate. The degree of market acceptance will depend upon a number of factors, including:
|
· |
the acceptance by doctors and other medical specialists of our products as an alternative to other therapies; |
|
· |
the receipt and timing of regulatory approvals; |
|
· |
the timing of market introduction of our products as well as competitive drugs; |
|
· |
the availability of coverage and adequate reimbursement and pricing from government and other third-party payors; |
|
· |
the price of our products, both in absolute terms and relative to alternative therapies; |
|
· |
the indications for which the product is approved; |
|
· |
the rate of adoption by healthcare providers; |
|
· |
the rate of product acceptance by target patient populations; |
|
· |
recommendations by pharmacists regarding our products relative to alternative products; |
|
· |
the availability of alternative therapies; |
|
· |
the extent and effectiveness of marketing efforts by our collaborators, third-party distributors and agents; |
|
· |
the strength of sales, marketing and distribution support; |
|
· |
the existence of adverse publicity regarding our products or similar products and the pricing of pharmaceutical products generally; |
|
· |
historical experience with a product or similar products and market perception of a product or similar products; |
19
|
· |
the efficacy of our products compared to alternative therapies; and |
|
· |
the extent and severity of side effects as compared to alternative therapies. |
Risks related to the factors above are particularly relevant to our new product acquisitions, including Fibricor, Zontivity and Toprol-XL, and our new product launches, including Yosprala (launched in the U.S. in October 2016) and Blexten (launched in Canada in December 2016). The commencement of commercialization of these products by Aralez in a short period of time will require significant efforts from us and the devotion of substantial resources as we will need to, among other things, establish the commercial infrastructure necessary to support these products. With respect to Yosprala and Blexten, our commercial organization is launching new products to the market. Prescription uptake with respect to Yosprala, for example, has thus far been slower than we expected and we may continue to face challenges relating to our launch of this product. With respect to Zontivity and Fibricor, the products were previously launched and existing market perception may make it challenging for Aralez to successfully relaunch and commercialize these products.
For our products, we depend on reimbursement from third-party payors and a failure to obtain coverage or reduction in the extent of reimbursement could reduce our product sales and revenue.
Sales of our products are dependent, in part, on the availability and extent of reimbursement from government health administration authorities, private health insurers and other organizations and our continued participation in such programs. These entities may refuse to provide coverage and reimbursement, determine to provide a lower level of coverage and reimbursement than anticipated, or reduce previously approved levels of coverage and reimbursement, including in the form of higher mandatory rebates or modified pricing terms.
In certain countries, including Canada, where we sell or are seeking or may seek to commercialize our products, pricing, coverage and level of reimbursement of prescription drugs are subject to governmental control. We may be unable to timely or successfully negotiate coverage, pricing, and reimbursement on terms that are favorable to us, or such coverage, pricing, and reimbursement may differ in separate regions in the same country. A significant reduction in the amount of reimbursement or pricing for our products in one or more countries may reduce our profitability and adversely affect our financial condition. Certain countries establish pricing and reimbursement amounts by reference to the price of the same or similar products in other countries. If coverage or the level of reimbursement is limited in one or more countries, we may be unable to obtain or maintain anticipated pricing or reimbursement in current or new territories. In the United States, the EU member states, and elsewhere, there have been, and we expect there will continue to be, efforts to control and reduce healthcare costs. In the United States, for example, the price of drugs has come under intense scrutiny by the President, U.S. Congress and other government officials and political candidates. Third-party payors decide which drugs they will pay for and establish reimbursement and co-payment levels. Government and other third-party payors are increasingly challenging the prices charged for healthcare products, examining the cost effectiveness of drugs in addition to their safety and efficacy, and limiting or attempting to limit both coverage and the level of reimbursement for prescription drugs.
Changes in government regulations or private third-party payors’ reimbursement policies may reduce reimbursement for our products and adversely affect our future results. Our commercial success depends on obtaining and maintaining reimbursement at anticipated levels for our products. It may be difficult to project the impact of evolving reimbursement mechanics or the willingness of payors to cover our products. If we are unable to obtain or maintain coverage, or coverage is reduced in one or more countries, our pricing may be affected and our product sales, results of operations or financial condition could be harmed. In addition, as the price of drugs undergoes more scrutiny, there is the possibility of retroactive price adjustments or coverage or penalties for prices that may be deemed excessive. If any such actions were applied to the Company, our business, financial condition and results of operations could be harmed.
Failure to be included in formularies, or restrictions on drugs included in formularies, developed by managed care organizations, governments, hospitals and other organizations may negatively impact the utilization of our products, which could harm our market share and negatively impact our business, financial condition and results of operations.
Managed care organizations and other third-party payors try to negotiate the pricing of medical services and products to control their costs. Managed care organizations and pharmacy benefit managers typically develop
20
formularies to reduce their cost for medications. Formularies can be based on the prices and therapeutic benefits of the available products. Due to their lower costs, generic products are often favored. The breadth of the products covered by formularies varies considerably from one managed care organization to another, and many formularies include alternative and competitive products for treatment of particular medical conditions. Failure to be included on such formularies, failure to achieve favorable formulary status, restrictions on drugs included on formularies such as prior authorizations, step edits or other limitations, or delays in implementing changes to formulary status, may negatively impact the utilization of our products. If our products are not included within an adequate number of formularies or adequate reimbursement levels are not provided, or if those policies increasingly favor generic products, our market share and gross margins could be harmed, as could our business, financial condition, results of operations and cash flows.
If we make strategic acquisitions, we will incur a variety of costs and may fail to realize all of the anticipated benefits of the transactions or those benefits may take longer to realize than expected. We may be unable to identify, acquire, close or integrate acquisition targets successfully.
A significant part of our business strategy includes acquiring and integrating complementary businesses, products, technologies or other assets, and forming strategic alliances and other business combinations, to help drive future growth. We may also in-license new products or compounds. Acquisitions or similar arrangements may be complex, time-consuming and expensive, and the process of negotiating the acquisition and integrating an acquired product, drug candidate, technology, business or company might result in operating difficulties and expenditures and might require significant management attention that would otherwise be available for ongoing development of our business, whether or not any such transaction is ever consummated. Moreover, we may never realize the anticipated benefits of any acquisition or forecasted sales may not materialize.
In addition, there are a number of risks and uncertainties relating to closing transactions. If such transactions are not completed for any reason, we will be subject to several risks, including the following: (i) the market price of our common shares may reflect a market assumption that such transactions will occur, and a failure to complete such transactions could result in a negative perception by the market of us generally and a decline in the market price of our common shares; and (ii) many costs relating to such transactions may be payable by us whether or not such transactions are completed, which costs may be significant.
If an acquisition is consummated, the integration of the acquired business, product or other assets into the Company may also be complex and time-consuming and, if such businesses, products and assets are not successfully integrated, we may not achieve the anticipated benefits, cost-savings or growth opportunities. Potential difficulties that may be encountered in the integration process include the following: integrating personnel, operations, manufacturing technology and systems, while maintaining focus on selling and promoting existing and newly-acquired products; coordinating geographically dispersed organizations; distracting management and employees from operations; retaining existing customers and attracting new customers; maintaining the business relationships of the acquired company, or the company that previously owned such product, has established, including with healthcare providers, third-party payors and distributors; and managing inefficiencies associated with integrating the operations of the Company.
Furthermore, we have incurred, and may incur in the future, restructuring and integration costs and a number of non-recurring transaction costs associated with these acquisitions, combining the operations of the Company and the acquired business and achieving desired synergies. These fees and costs may be substantial. Non-recurring transaction costs include, but are not limited to, fees paid to legal, financial, regulatory, manufacturing and accounting advisors, filing fees, transfer and other transaction-related taxes and printing costs. Additional unanticipated costs may be incurred in the integration of the businesses of the Company and the acquired business. There can be no assurance that the elimination of certain duplicative costs, as well as the realization of other efficiencies related to the integration of the acquired business, will offset the incremental transaction-related costs over time. Therefore, any net benefit may not be achieved in the near term, the long term or at all.
Finally, these acquisitions and other arrangements, even if successfully integrated, may fail to further our business strategy as anticipated or to achieve anticipated benefits and success, expose us to increased competition or challenges with respect to our products or geographic markets, and expose us to additional liabilities associated with an acquired business, product, technology or other asset or arrangement. Any one of these challenges or risks could impair our ability to realize any benefit from our acquisition or arrangement after we have expended resources on them.
21
For example, in February 2016, we completed the acquisition of Tribute, in September 2016, we completed the acquisition of the U.S. and Canadian rights to Zontivity, and in October 2016, we completed the acquisition of the U.S. rights to Toprol-XL and the AG. Such transactions represent significant acquisitions for the Company and may expose us to a number of the risks identified above. We may face difficulties in connection with the integration of such businesses with the Company, which integration activities may be complex, time-consuming and disruptive to the operation of our business generally. In particular, as part of our acquisition of the rights to Toprol-XL and the AG and Zontivity, AstraZeneca and Merck, respectively, have agreed to provide us with critical transition services, including services related to supply, technology and packaging, market access and reimbursement, sales and distribution, and certain finance and financial reporting services. We will need to work collaboratively with AstraZeneca and Merck to ensure that such services are provided in an effective and timely manner. We have limited ability to control the amount or timing of resources that AstraZeneca and Merck devote to such services. If AstraZeneca and/or Merck fail to devote sufficient time and resources to conducting such services, perform such services in a substandard manner, materially breach their obligations to conduct such services or undergo a change of control, it will delay or hinder our ability to successfully commercialize Toprol-XL and/or the AG and/or Zontivity. In addition, the costs incurred in connection with integration activities may be more substantial than we anticipated and, as a result, may significantly reduce or even outweigh any benefits and efficiencies realized during our integration efforts.
We may also face challenges transferring the assets, such as contracts, regulatory requirements and technology, to the extent applicable, associated with such acquired businesses. In addition, we may not be successful in our commercialization efforts with respect to such businesses or face increased competition or costs with respect to the acquired products and, as a result, we may not be able to achieve all of the anticipated benefits of such transactions. Any of these factors could have a material adverse effect on our business, financial condition or results of operations or could decrease or delay the expected accretive effect of such transactions or cause the market value of our common shares to decline.
Failure to successfully acquire, license or develop and market additional product candidates or approved products would impair our ability to grow.
As part of our growth strategy, we intend to acquire, license or develop and market additional products and product candidates. The product candidates to which we allocate our resources may not end up being successful. In addition, because our internal research capabilities are limited, we may depend upon pharmaceutical, biotechnology and other researchers to sell or license products or technology to us. The success of this strategy depends partly upon our ability to identify, select, license and/or acquire promising pharmaceutical or other healthcare product candidates and products for Canada, the United States and elsewhere. Failure of this strategy would impair our ability to grow.
The process of proposing, negotiating and implementing a license or acquisition of a product candidate or approved product is lengthy and complex. Other companies, including some with substantially greater financial, marketing and sales resources, may compete with us for the license or acquisition of product candidates and approved products. We may devote resources to potential acquisitions or in-licensing opportunities that are never completed, or we may fail to realize the anticipated benefits of such efforts. We may not be able to acquire the rights to additional product candidates or approved products on terms that we find acceptable, or at all.
In addition, future acquisitions may entail numerous operational and financial risks, including:
|
· |
exposure to unknown liabilities; |
|
· |
disruption of our business and diversion of management’s time and attention to develop acquired products or technologies; |
|
· |
incurrence of substantial debt, dilutive issuances of securities or depletion of cash to pay for acquisitions; |
|
· |
higher than expected acquisition and integration costs; |
|
· |
difficulty in combining the operations and personnel of any acquired businesses with our operations and personnel; |
22
|
· |
increased amortization expenses; |
|
· |
increased or unanticipated costs; |
|
· |
failure of the acquired business to achieve expected financial results; |
|
· |
increased or unexpected competition with respect to the acquired business; |
|
· |
impairment of relationships with key suppliers or customers of any acquired businesses due to changes in management and ownership; and |
|
· |
inability to motivate key employees of any acquired businesses. |
Further, any unapproved product candidate that we acquire may require additional development efforts prior to commercial sale, including extensive clinical testing and approval by applicable regulatory authorities. All product candidates are prone to risks of failure typical of pharmaceutical product development, including the possibility that a product candidate will not be shown to be sufficiently safe and effective for approval by applicable regulatory authorities and thus will never make it to market.
We currently depend and will in the future depend on third parties to manufacture our products and product candidates. If these manufacturers fail to meet our requirements or any regulatory requirements, the product development and commercialization of our products and candidates will be delayed.
We do not have, and have no plans to develop, the internal capability to manufacture our products or product candidates. We rely upon third-party manufacturers and our partners to supply us with the commercial and developmental supplies of our products and product candidates. For example, we have a supply agreement with Patheon pursuant to which Patheon manufactures our requirements for the sale of Yosprala in the United States. In connection with the acquisition of Zontivity, Merck agreed to supply the product to us for a period of up to three years post-closing (although the packaging component must be transferred within one year), after which we must establish a new manufacturer for the product. In addition, with respect to the acquisition of Toprol-XL and the AG, AstraZeneca agreed to supply such products to us for a period of at least 10 years following the closing of such acquisition. The manufacturing facilities of our third-party manufacturers may be inspected from time to time and need to be found to be in full compliance with cGMP, quality system management requirements or similar standards, and we may not be able to ensure that such third parties comply with these obligations. The failure of our contract manufacturers to comply with cGMP regulations, quality system management requirements or similar regulations could result in enforcement action by the FDA or its foreign counterparts, including, but not limited to, warning letters, fines, injunctions, civil or criminal penalties, recall or seizure of products, total or partial suspension of production or importation, suspension or withdrawal of regulatory approval for approved or in-market products, refusal of the government to renew marketing applications, licenses or approve pending applications or supplements, suspension of ongoing clinical trials, imposition of new manufacturing requirements, closure of facilities and criminal prosecution. These enforcement actions could lead to a delay or suspension in production. Furthermore, the failure of our ingredient or material suppliers to comply with regulatory requirements can impact our ability to supply the market with our products. For example, in connection with the approval process for Yosprala, our initial primary aspirin (acetylsalicylic acid) active pharmaceutical ingredient (“API”) supplier had informed us that it received warning letters from the FDA relating to Form 483 inspection deficiencies and as a result we designated our previously designated secondary aspirin API supplier as our primary supplier and approval of Yosprala was significantly delayed.
There is no guarantee that manufacturers and API or other material suppliers that enter into commercial supply contracts with us will be financially viable entities going forward, or will not otherwise breach or terminate their agreements with us. If we do not have the necessary commercial supply contracts, or if any of our current or future third party manufacturers or API suppliers are unable to satisfy our requirements or meet any regulatory requirements, and we are or will be required to find alternative sources of supply, there may be additional costs and delays in product development and commercialization of our product candidates or we may be required to comply with additional regulatory requirements.
In the event that suppliers of a product, ingredient or any materials we need to manufacture or package our products or licensed products are not available or not for sale at the time we need such ingredient or material in order to
23
meet our required delivery schedule or on commercially reasonable terms, then we could be at risk of a product shortage or stock-out. We rely on our suppliers in many cases to ensure the adequate supply of ingredients, APIs and packaging material and for the timely delivery of orders placed by us. Should we experience a shortage in supply of a product, licensed product, or API, sales of such product or licensed product could be harmed or reduced and our ability to generate revenues from such product or licensed product may be impaired.
Certain of our products may never be approved for commercial use in all desired jurisdictions. Failure to successfully commercialize our products or develop, gain approval of or commercialize our product candidates would adversely impact our financial condition and prospects.
We anticipate that an important component of our success will depend on the successful commercialization of our products upon regulatory approval in territories where our products are not approved, such as Yosprala in Europe (submitted in January 2017) and Canada. Before we can market and sell our products in a particular jurisdiction, we need to obtain necessary regulatory approvals (from the FDA in the United States, Health Canada in Canada, EMA in the EU and from similar foreign regulatory agencies in other jurisdictions), and in some jurisdictions, reimbursement authorization. There are no guarantees that we or our commercialization partners will obtain approval in those countries where we wish to commercialize our products. Even if we or our commercialization partners obtain additional regulatory approvals, we may never generate significant revenues from any commercial sales of our products. These approvals may not be granted on a timely basis, if at all. Nor can any assurance be given that if such approval is secured, the approved labeling will not have significant labeling limitations, including limitations on the indications for which we can market a product, or require onerous risk management programs. Further, our current or future collaboration agreements may terminate, or require us to make certain payments to our collaborators, or our collaborators may have the right to terminate their agreements with us or reduce or eliminate their payments to us under these agreements, based on our inability to obtain, or delays in obtaining, regulatory approval for our product candidates. If we fail to successfully commercialize our current and future products, we may be unable to generate sufficient revenues to sustain and grow our business, and our business, financial condition and results of operations will be adversely affected.
In addition, if our development projects are not successful or are significantly delayed, we may not recover our substantial investments in the product candidates and our failure to bring these product candidates to market on a timely basis, or at all, could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
Each of our products has a limited shelf life which could result in costs associated with inventory which exceeds the appropriate age limits.
Each of our products has a limited shelf life. Accordingly, product which exceeds the appropriate age limits may not be sold, may result in product returns and must be destroyed, which would have an adverse financial impact associated with the cost of writing off obsolete inventory.
We continue to evaluate the commercial opportunities for our current products and product candidates in connection with our development of a worldwide commercialization strategy. If we are unable to develop sales and marketing capabilities on our own, or through partnerships, we will not be able to fully exploit the commercial potential of our products and the costs of pursuing such a strategy may have a material adverse impact on our results of operations.
We continue to evaluate the commercial opportunities for our products and product candidates in connection with our development of a worldwide commercialization strategy. In June 2015, our Board of Directors appointed Adrian Adams as our new Chief Executive Officer and Andrew I. Koven as our new President and Chief Business Officer, each of whom has experience creating, leading and expanding pharmaceutical companies with marketing and sales capabilities. We have made significant expenditures to secure commercial resources to launch Yosprala in the United States and commercialize other existing products and anticipate that we will continue to make significant expenditures related to the commercialization of our current products or products we may acquire and to expand or enhance our marketing capabilities to support our anticipated growth. Any failure or extended delay in the expansion or enhancement of our sales and marketing capabilities or inability to effectively operate in the marketplace alone or together with our partners could adversely impact our business. There can be no assurance that our sales and marketing
24
efforts will generate significant revenues and costs of pursuing such a strategy may have a material adverse impact on our results of operations. Events or factors that may inhibit or hinder our commercialization efforts include:
|
· |
building and developing our own commercial team or playing a role in the commercialization with a partner will be expensive and time-consuming and will result in high cash burn or reduced profitability; |
|
· |
failure to acquire sufficient or suitable personnel to establish, oversee, or implement our commercialization strategy; |
|
· |
failure to recruit, train, oversee and retain adequate numbers of effective sales and marketing personnel; |
|
· |
failure to develop a commercial strategy ourselves or together with partners that can effectively reach and persuade adequate numbers of physicians to prescribe our products; |
|
· |
our or our partners’ inability to secure reimbursement at a reasonable price; |
|
· |
unforeseen costs and expenses associated with creating or acquiring and sustaining an independent commercial organization; |
|
· |
incurrence of costs in advance of anticipated revenues and subsequent failure to generate sufficient revenue to offset additional costs; and |
|
· |
ability to fund our commercialization efforts alone or together with our partners on terms acceptable to us, if at all. |
If we are unable to effectively train and equip our sales force, our ability to successfully commercialize our products will be harmed.
We are required to expend significant time and resources to train our sales force to be credible, compliant and persuasive in educating physicians to prescribe and pharmacists to dispense our products. In addition, we must train our sales force to ensure that a consistent and appropriate message about our products is being delivered to our potential customers. Our sales representatives may also experience challenges promoting multiple products when they call on physicians and their office staff. This is particularly true with respect to our products that have competing products prescribed to similar patients. If we are unable to effectively train our sales force and equip them with effective materials, including medical and sales literature to help them inform and educate potential customers about the benefits of our products and their proper administration and approved indications, our efforts to successfully commercialize our products could be put in jeopardy, which could have a material adverse effect on our financial condition, share price and operations.
Our reliance on collaborations with third parties to develop, manufacture and commercialize our products is subject to inherent risks and may result in delays in product development and lost or reduced revenues, restricting our ability to commercialize our products and adversely affecting our profitability.
We depend upon collaborations with third parties to develop, manufacture and/or supply our products and, in some cases, we depend substantially upon third parties to commercialize these products. As a result, our ability to develop, obtain regulatory approval of, manufacture and commercialize our existing and possibly future products and product candidates depends upon our ability to maintain existing, and enter into and maintain new, contractual and collaborative arrangements with others. We also engage and/or may in the future engage third party manufacturers and clinical trial investigators.
In addition, the identification of new compounds or product candidates for development has led us in the past, and may continue to require us, to enter into license or other collaborative agreements with others, including pharmaceutical companies and research institutions. Such collaborative agreements for the acquisition of new compounds or product candidates would typically require us to pay license fees, make milestone payments and/or pay royalties. For products we out-license, these agreements may result in our revenues being lower than if we developed and
25
commercialized our products or product candidates ourselves and in our loss of control over the development of our product candidates.
Contractors or collaborators may have the right to terminate their agreements with us after a specified notice period for any reason or upon a default by us. For example, AstraZeneca and Horizon, with respect to VIMOVO, and Pernix, with respect to Treximet, have the right to terminate their respective agreements with us upon a 90-day notice for any reason. Licensees may have the right to reduce their payments to us under their agreements. For example, Pernix, with respect to Treximet, and AstraZeneca and Horizon, with respect to VIMOVO, have the right to reduce the royalties on net sales of products payable to us under their respective agreements if generic competitors enter the market and attain a pre-determined share of the market for products marketed under the agreements, or if they must pay a royalty to one or more third parties for rights they license from those third parties to commercialize products marketed under the agreements. Further, our current or future collaboration agreements may terminate, or our collaborators may have the right to terminate their agreements with us or reduce or eliminate their payments to us under these agreements, based on our inability to obtain, or delays in obtaining, regulatory approval for our product candidates, certain business performance criteria or our contract manufacturers’ inability to manufacture our products or to supply the sufficient quantities of our products to meet market demand. For example, we distribute the Toprol-XL AG product through a distribution agreement with Endo Ventures Limited (“Endo”) (by assignment from Par Pharmaceuticals Inc. (“Par”) to Endo in connection with Endo International pls’s acquisition of Par), which agreement expires at the end of 2017 and may be terminated by either party under certain circumstances, including performance measures. If our current or future collaborators exercise termination rights they may have, or if the agreements terminate because of delays in obtaining regulatory approvals, business performance or for other reasons, and we are not able to establish replacement or additional research and development collaborations or licensing or commercialization arrangements, we may not be able to effectively develop and/or commercialize our products or product candidates. Moreover, any future collaborations or license arrangements we may enter into may not be on terms favorable to us.
Collaborators may decide not to continue marketing our products in certain countries, as was the case when AstraZeneca informed us that, after a strategic business review, it had decided to cease promotion and sampling of VIMOVO by the end of the third quarter of 2013 in certain countries, including the United States and all countries in Europe, other than Spain and Portugal, which have pre-existing contractual relationships with third parties. In addition, collaborators may decide to assign their rights under our agreement to third parties. For example, we had a collaboration agreement with GSK for the development and commercialization of certain triptan combinations using our MT 400 technology, including Treximet, in the United States, and GSK subsequently divested all of its rights, title and interest to develop, commercialize and sell the licensed products in the United States to Pernix.
Other risks associated with our collaborative and contractual arrangements with others include the following:
|
· |
we may not have day-to-day control over the activities of our contractors or collaborators; |
|
· |
our collaborators may fail to defend or enforce patents they own on compounds or technologies that are incorporated into the products we develop with them; |
|
· |
third parties may not fulfill their regulatory or other obligations; |
|
· |
we may not realize the contemplated or expected benefits from collaborative or other arrangements; |
|
· |
if any collaborator were to breach its agreement with us or otherwise fail to conduct collaborative activities in a timely or successful manner, the pre-clinical or clinical development or commercialization of the affected product candidate or research program would be delayed or terminated; |
|
· |
our collaborators may be able to exercise control, under certain circumstances, over our ability to protect our patent rights under patents covered by the applicable collaboration agreement; and |
|
· |
disagreements may arise regarding a breach of the arrangement, the interpretation of the agreement, ownership of proprietary rights, clinical results or regulatory approvals. |
26
These factors could lead to delays in the development of our product candidates and/or the commercialization of our products or reduction in the milestone, royalty payments or profit sharing we receive from our collaborators, or could result in our not being able to commercialize our products. Further, disagreements with our contractors or collaborators could require or result in litigation or arbitration, which would be time-consuming and expensive. Our ultimate success may depend upon the success and performance on the part of these third parties. If we fail to maintain these relationships or establish new relationships as required, development of our product candidates and/or the commercialization of our products will be delayed or may never be realized.
We are dependent upon a small number of customers for a significant portion of our revenue, and the loss of or significant reduction in sales to these customers would adversely affect our results of operations.
We sell our products in the United States and Canada to a limited number of distributors. Under this distribution model, the distributors generally take physical delivery of product and generally sell the product directly to pharmacies or patients. In addition, certain of our products may be highly dependent on a small number of customers. We expect this significant distributor/customer concentration to continue for the foreseeable future. Our ability to generate and grow sales of our products will depend, in part, on the extent to which our distributors are able to provide adequate distribution of our products on pricing terms that are favorable to us. Although we believe we can find additional or replacement distributors, if necessary, the pricing terms of such arrangements may not be as favorable to us and our revenue during any period of disruption could suffer and we might incur additional costs. In addition, these distributors/customers are responsible for a significant portion of our net trade accounts receivable balances. The loss of any large distributor/customer, a significant reduction in sales we make to them, any cancellation of orders they have made with us, or any failure to pay for the products we have shipped to them could adversely affect our results of operations.
We may not be able to compete with treatments now being developed and marketed, or which may be developed and marketed in the future by other companies.
Our products and product candidates will compete with existing and new therapies and treatments. There are also likely to be numerous competitors that are engaged in the development of alternatives to our technologies and products, which could render our products, product candidates and technologies obsolete or non-competitive. For example, our primary competitors will likely include large pharmaceutical companies, biotechnology companies, universities and public and private research institutions. Some of these companies have greater research and development capabilities, experience, and manufacturing, marketing, financial and managerial resources than we do. Collaborations or mergers between large pharmaceutical or biotechnology companies with competing drugs and technologies could enhance our competitors’ financial, marketing and other resources. Accordingly, our competitors may succeed in developing competing drugs or technologies, obtaining patent protection, obtaining regulatory approval for products, commercializing products or gaining market acceptance more rapidly than we can. Any delays we encounter in obtaining regulatory approvals for our product candidates increases this risk.
The competition for VIMOVO, and any PPI–NSAID products that may be developed and receive regulatory approval, may come from the oral NSAID market, specifically the traditional non-selective NSAIDs (such as naproxen and diclofenac), traditional NSAID/gastroprotective agent combination products or combination product packages (such as Arthrotec® and Prevacid® NapraPAC™), combinations of NSAIDs and PPIs taken as separate pills and the only remaining COX-2 inhibitor, Celebrex®. The competition for PA products, such as Yosprala, may come from aspirin itself, other aspirin-combination products that may be introduced, as well as other products used for secondary prevention of cardiovascular and cerebrovascular events. Toprol-XL and the AG compete against several generic offerings for metoprolol succinate. Zontivity competes with certain products referred to as oral anti-platelets, which market is dominated by the generic offerings for clopidogrel bisulfate. There are also two newer, competitive anti-platelet offerings in this class: Effient® and Brilinta®.
Based upon their drug product and pipeline portfolios and the overall competitiveness of our industry, we believe that we face, and will continue to face, intense competition from other companies for securing collaborations with pharmaceutical companies, establishing relationships with academic and research institutions, and acquiring licenses to proprietary technology. Our competitors, either alone or with collaborative parties, may also succeed with technologies or products that are more effective than any of our current or future technologies or products. Many of our actual or potential competitors, either alone or together with collaborative parties, have substantially greater financial resources, and almost all of our competitors have larger numbers of scientific and administrative personnel than we do. If
27
we cannot successfully compete with new or existing products, our marketing and sales will suffer and we may not ever receive any revenues from sales of products or may not receive sufficient revenues to achieve profitability.
Contractual relationships with governmental customers may impose special burdens on us and provide special benefits to those customers, including the right to change or terminate the contract in response to budgetary constraints, policy changes or competition.
A portion of our revenues come from customers that are governmental agencies or vendors to such agencies. These contracts generally contain certain rights for the benefit of the government customer, including termination for convenience, the right to place contracts out for bid before the full contract term, as well as the right to make unilateral changes in contract requirements. For example, in connection with our acquisition of the U.S. rights to Toprol-XL and the AG, we entered into a Novation Agreement with AstraZeneca and the United States of America (the “Government”) pursuant to which all of the rights and responsibilities of AstraZeneca under a VA National Contract (the “VA Contract”) between AstraZeneca and the Government were novated to a subsidiary of Aralez. The VA Contract is terminable at the convenience of the Government at any time and the Government could therefore use such right to try to renegotiate pricing terms, which could adversely affect our gross margins and results of operations.
Government contracts and subcontracts may also be subject to some or all of the following:
|
· |
termination when appropriated funding for the current fiscal year is exhausted or becomes unavailable; |
|
· |
“most-favored” pricing disclosure requirements that are designed to ensure that the government can negotiate and receive pricing akin to that offered commercially and requirements to submit proprietary cost or pricing data to ensure that government contract pricing is fair and reasonable; |
|
· |
commercial customer price tracking requirements that require contractors to monitor pricing offered to a specified class of customers and to extend price reductions offered to that class of customers to the government; |
|
· |
reporting and compliance requirements related to, among other things: equal employment opportunity, affirmative action for veterans and for workers with disabilities, and accessibility for the disabled; |
|
· |
broader audit rights than we would usually grant to non-governmental customers; and |
|
· |
specialized remedies for breach and default or failure to meet service level commitments, including setoff rights, retroactive price adjustments, and civil or criminal fraud penalties, as well as mandatory administrative dispute resolution procedures instead of state contract law remedies. |
In addition, certain violations of federal law may subject government contractors to having their contracts terminated and, under certain circumstances, suspension and/or debarment from future government contracts.
Generic competition to our products could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
Upon the expiration or loss of patent protection for our products, or upon the “at-risk” launch (despite pending patent infringement litigation against the generic product) by a generic competitor of a generic version of our products, we can lose a significant portion of sales of that product, or royalty revenue in the case of out-licensed products, in a very short period, which could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline. In addition, for products where a generic market already exists, there may be increased generic competition from current or new entrants to the generic market. For example, we currently compete with suppliers of generic versions of Toprol-XL and could face these adverse effects if additional generic competitors enter the market or if there is additional price erosion in the current market.
If we lose our license from any licensors, we may be unable to continue a substantial part of our business.
We have licensed certain assets, including certain intellectual property, marketing authorizations and related data, and medical commercial and technical information, used in a substantial part of our business. Such license
28
agreements may be terminated by the licensor if we are in breach of our obligations under, or fail to perform any terms of, the agreement and fail to cure that breach. If a license agreement is terminated, then we may lose our rights to utilize the intellectual property and other assets covered by such agreement to manufacture, market, promote, distribute and sell the licensed products, which may prevent us from continuing a substantial part of our business and may result in a material adverse effect on our financial condition, results of operations and any prospects for growth.
We will not be able to commercialize our future product candidates if preclinical studies do not produce successful results or if clinical trials do not demonstrate safety and efficacy in humans.
We and our development partners, as applicable, may conduct extensive preclinical studies and clinical trials to demonstrate the safety and efficacy in humans of our future product candidates in order to obtain regulatory approval for the sale of our future product candidates. Preclinical studies and clinical trials are expensive, can take many years and have uncertain outcomes. If clinical trials are unsuccessful, we will not be able to commercialize our future product candidates and additional studies may be required.
Developments following regulatory approval may adversely affect sales of the Company’s products.
Even after a product reaches market, certain developments following regulatory approval, including results in post-marketing requirements, Phase IV trials or other studies, may decrease demand for the Company’s products, including the following:
|
· |
the re-review of products that are already marketed; |
|
· |
new scientific information and evolution of scientific theories; |
|
· |
the recall or loss of marketing approval of products that are already marketed; |
|
· |
changing government standards or public expectations regarding safety, efficacy or labeling changes; and |
|
· |
greater scrutiny in advertising and promotion. |
Events giving rise to concerns among some prescribers and patients relating to the safety or efficacy of pharmaceutical products, whether or not scientifically justified, can lead to product recalls, withdrawals, or declining sales, as well as product liability, consumer fraud and/or other claims, including potential civil or criminal governmental actions.
For example, if the results of any post-approval studies, including the in-vitro or in-vivo post-marketing studies with Yosprala required by the FDA, demonstrated any potential/hypothetical/actual adverse effects not identified in the predecessor clinical studies, it could significantly reduce demand for the product or require the Company to take actions that could negatively affect sales, including removing the product from the market, restricting its distribution or applying for labeling changes.
We depend on key personnel and may not be able to retain these employees or recruit additional qualified personnel, which would harm our business as well as our ability to identify, acquire, close or integrate acquisition targets successfully.
We are highly dependent on the efforts of our key management, especially Adrian Adams, our Chief Executive Officer, and Andrew I. Koven, our President and Chief Business Officer. If we should lose the services of Mr. Adams or Mr. Koven, or are unable to replace the services of our other key personnel who may leave the Company, or if we fail to recruit other key scientific and commercial personnel, we may be unable to achieve our business objectives and growth strategies. There is intense competition for qualified scientific and commercial personnel. We may not be able to continue to attract and retain the qualified personnel necessary for developing our business. Furthermore, our future success may also depend in part on the continued service of our other key management personnel and our ability to recruit and retain additional personnel, as required by our business. Many of the other biotechnology and pharmaceutical companies with whom we compete for qualified personnel have greater financial and other resources, different risk profiles and longer histories in the industry than we do. They also may provide more diverse opportunities and better
29
chances for career advancement. Some of these characteristics may be more appealing to high quality candidates than that which we have to offer. If we are unable to continue to attract and retain high quality personnel, the rate and success at which we can develop and commercialize products and product candidates will be limited.
Our business, financial condition and results of operations are subject to risks arising from the international scope of our operations.
Our international operations and any future international operations may expose us to risks that could negatively impact our future results. Our operations may not develop in the same way or at the same rate as might be expected in a country with an economy similar to the United States or Canada. The additional risks that we may be exposed to in these cases include, but are not limited to:
|
· |
tariffs and trade barriers; |
|
· |
currency fluctuations, which could decrease the Company’s revenues or increase its costs; |
|
· |
regulations related to customs and import/export matters; |
|
· |
tax issues, such as tax law changes and variations in tax laws; |
|
· |
limited access to qualified staff; |
|
· |
inadequate infrastructure; |
|
· |
cultural and language differences; |
|
· |
inadequate banking systems; |
|
· |
different and/or more stringent environmental laws and regulations; |
|
· |
restrictions on the repatriation of profits or payment of dividends; |
|
· |
crime, strikes, riots, civil disturbances, terrorist attacks or wars; |
|
· |
nationalization or expropriation of property; |
|
· |
law enforcement authorities and courts that are weak or inexperienced in commercial matters; and |
|
· |
deterioration of political relations among countries. |
Any of these factors, or any other international factors, could have a material adverse impact on our business, financial condition and results of operations and could cause the market value of our common shares to decline. Similarly, adverse economic conditions impacting our customers in these countries or uncertainty about global economic conditions could cause purchases of our products to decline, which would adversely affect our revenues and operating results. Any failure to attain our projected revenues and operating results as a result of adverse economic or market conditions could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
Due to a portion of our business conducted in currency other than U.S. dollars, we have foreign currency risk.
Our consolidated financial statements are presented in accordance with U.S. generally accepted accounting principles, and we report, and will continue to report, our results in U.S. dollars. Some of our transactions are conducted in currencies other than the U.S. dollar. Any change in the value of currencies in which we transact against the U.S. dollar during a given financial reporting period would result in a foreign currency loss or gain. The exchange rates between many of the currencies in which we transact against the U.S. dollar have fluctuated significantly in recent years
30
and may fluctuate significantly in the future. Consequently, our reported earnings could fluctuate materially as a result of foreign exchange (translation) gains or losses and may not be comparable from period to period.
We face market risks attributable to fluctuations in foreign currency exchange rates and foreign currency exposure on the translation into U.S. dollars of the financial results of our operations in Canada. Exchange rate fluctuations could have an adverse effect on our results of operations. Both favorable and unfavorable foreign currency impacts to our foreign currency-denominated operating expenses are mitigated to a certain extent by the natural, opposite impact on our foreign currency-denominated revenue. In addition, the repurchase of principal under our U.S. dollar denominated debt may result in foreign exchange gains or losses for Canadian income tax purposes.
Risks related to Legislation and Regulations
As we pursue commercialization of our product portfolio and other opportunities for our future products ourselves, failure to comply with the laws governing the marketing and sale of such products may result in regulatory agencies taking action against us and/or our partners, which could significantly harm our business.
As we pursue commercialization of Yosprala, Zontivity, Toprol-XL and the AG, Fibricor and its authorized generic, our Canadian product portfolio and other future products, we will be subject to extensive regulation by the FDA, Health Canada, EMA and the governmental authorities in other countries. In particular, there are many federal, state, provincial and local laws that we will need to comply with in connection with the marketing, promoting, distribution and sale of pharmaceutical products. If we fail to comply with U.S., Canadian and European regulatory requirements and those in other countries where our products are sold, we could lose our marketing approvals or be subject to civil and/or criminal penalties, injunctions, fines or other sanctions. In addition, incidents of adverse drug reactions, unintended side effects or misuse relating to our products could result in additional regulatory controls or restrictions, or even lead to withdrawal of a product from the market. The imposition of one or more of these penalties could adversely affect our revenues and our ability to conduct our business as planned. As a condition to granting marketing approval of a product, the FDA, Health Canada, EMA or other applicable regulatory authorities may require a company to conduct additional clinical trials, the results of which could result in the subsequent loss of marketing approval, changes in product labeling or new or increased concerns about side effects or efficacy of a product. Compliance with the extensive laws and regulations to which we are subject is complicated, time-consuming and expensive. We cannot assure you that we will be in compliance with all potentially applicable laws and regulations. Even minor, inadvertent irregularities can potentially give rise to claims that the law has been violated.
We are subject to various laws and regulations, including “fraud and abuse” laws, anti-bribery laws and privacy and security regulations, and a failure to comply with such laws and regulations or prevail in any litigation related to noncompliance could have a material adverse impact on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
Pharmaceutical and biotechnology companies have faced lawsuits and investigations pertaining to violations of healthcare “fraud and abuse” laws, such as the federal False Claims Act, the federal Anti-Kickback Statute, the United States Foreign Corrupt Practices Act (the “FCPA”) and other federal, state and provincial laws and regulations. We also face increasingly strict data privacy and security laws in the United States, Canada, the EU and other countries, the violation of which could result in fines and other sanctions. The United States Department of Health and Human Services Office of Inspector General recommends, and increasingly states, that pharmaceutical companies have comprehensive compliance programs and disclose certain payments made to healthcare providers or funds spent on marketing and promotion of drug products. While we have developed a corporate compliance program, we cannot assure you that we or our employees or agents are or will be in compliance with all applicable federal, state, provincial or foreign regulations and laws. If we are in violation of any of these requirements or any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines, exclusion from federal healthcare programs or other sanctions.
The FCPA, the Canadian Corruption of Foreign Public Officials Act (the “CFPOA”) and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to officials for the purpose of obtaining or retaining business. Although we require our employees to consult with our legal department prior to making any payment or gift thought to be exempt under applicable law, there is no assurance that such policies or procedures will work effectively all of the time or protect us against liability under the FCPA and/or the CFPOA for
31
actions taken by our employees and other intermediaries with respect to our business or any businesses that we may acquire. We may operate in parts of the world that have experienced governmental corruption to some degree and, in certain circumstances, strict compliance with anti-bribery laws may conflict with local customs and practices or may require us to interact with doctors and hospitals, some of which may be state controlled, in a manner that is different from the United States and Canada. We cannot assure you that our internal control policies and procedures will protect us from reckless or criminal acts committed by our employees or agents. Violations of these laws, or allegations of such violations, could disrupt our business and result in criminal or civil penalties or remedial measures, any of which could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
We are also subject to various privacy and security regulations, including, but not limited to, the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (as amended, “HIPAA”). HIPAA mandates, among other things, the adoption of uniform standards for the electronic exchange of information in common healthcare transactions (e.g., healthcare claims information and plan eligibility, referral certification and authorization, claims status, plan enrollment, coordination of benefits and related information), as well as standards relating to the privacy and security of individually identifiable health information, which require the adoption of administrative, physical and technical safeguards to protect such information. In addition, many states have enacted comparable laws addressing the privacy and security of health information, some of which are more stringent than HIPAA. Failure to comply with these laws can result in the imposition of significant civil and criminal penalties.
Numerous other countries have, or are developing, laws governing the collection, use and transmission of personal information as well. The EU member states and other jurisdictions have adopted data protection laws and regulations, which impose significant compliance obligations. For example, the EU Data Protection Directive, as implemented into national laws by the EU member states, imposes strict obligations and restrictions on the ability to collect, analyze, and transfer personal data, including health data from clinical trials and adverse event reporting. Data protection authorities from different EU member states have interpreted the privacy laws differently, which adds to the complexity of processing personal data in the EU, and guidance on implementation and compliance practices are often updated or otherwise revised. Any failure to comply with the rules arising from the EU Data Protection Directive and related national laws of EU member states could lead to supervisory authority enforcement actions, reputational damage and significant penalties against us, adversely impacting our operating results.
In December 2015, a proposal for an EU Data Protection Regulation, intended to replace the current EU Data Protection Directive, was agreed between the European Parliament, the Council of the European Union and the European Commission. The EU General Data Protection Regulation (GDPR), which is enforceable from May 25, 2018 will expand our data protection obligations, including by imposing more stringent conditions for consent from data subjects, strengthening the rights of individuals, including the right to have personal data deleted upon request, continuing to restrict the trans-border flow of such data, requiring mandatory data breach reporting and notification, increasing penalties for non-compliance and increasing the enforcement powers of the national data protection authorities. The GDPR will increase our responsibility and liability in relation to personal data that we process and we may be required to put in place additional mechanisms to ensure compliance with the GDPR. The GDPR harmonises EU data protection laws and is intended to make it easier for multinational companies operating across the EU to comply with their data protection obligations. However, it does permit EU member states some flexibility to legislate in a number of areas, which means that inconsistencies may still arise.
The costs of compliance with these laws and the potential liability associated with the failure to comply with these laws could adversely affect our financial condition, results of operations and cash flows.
Legislative or regulatory reform of the healthcare system may affect our ability to sell our products profitably and could adversely affect our business.
In the United States and certain state and foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the healthcare system in ways that could impact our ability to sell our products profitably. The ACA may affect the operational results of companies in the pharmaceutical industry, including the Company and other healthcare-related industries, by imposing on them additional costs. Effective January 1, 2010, the ACA increased the minimum Medicaid drug rebates for pharmaceutical companies, expanded the 340B drug discount program, and made changes to affect the Medicare Part D coverage gap, or “donut hole.” The law also revised the definition of
32
“average manufacturer price” for reporting purposes, which has the potential to affect the amount of our Medicaid drug rebates to states. Beginning in 2011, the law imposed a significant annual fee on companies that manufacture or import branded (including authorized generics) prescription drug products.
The ACA also added substantial new provisions affecting compliance, some of which required the entire industry to modify business practices with healthcare practitioners. Pharmaceutical manufacturers are required in 2013 to comply with the federal Physician Payments Sunshine Act, which was passed as part of the ACA and requires pharmaceutical companies to monitor and report payments, gifts, the provision of samples and other remuneration made to physicians and other healthcare professionals and healthcare organizations.
We are unable to predict the future course of federal or state healthcare legislation. A variety of federal and state agencies are in the process of implementing the ACA, including through the issuance of rules, regulations or guidance that materially affect our business. The risk of our being found in violation of these rules and regulations is increased by the fact that many of them have not been fully interpreted by applicable regulatory authorities or the courts, and their provisions are open to a variety of interpretations. In addition, there is substantial uncertainty regarding the future of the ACA as efforts are underway to repeal and/or replace all or certain aspects of such laws. The outcome of such efforts could have a substantial impact on our business. The ACA, changes thereto or replacements thereof and further changes to healthcare laws or regulatory framework that reduce our revenues or increase our compliance or other costs could also have a material adverse effect on our business, financial condition and results of operations and cash flows, and could cause the market value of our common shares to decline.
In addition, pharmaceutical product pricing is subject to enhanced government and public scrutiny and calls for reform. Efforts by government officials or legislators to implement measures to regulate prices or payment for pharmaceutical products could adversely affect our business if implemented.
In Canada, patented drug products are subjected to regulation by the PMPRB pursuant to the Patent Act (Canada) and the Patented Medicines Regulations. The PMPRB does not approve prices for drug products in advance of their introduction to the market. The PMPRB provides guidelines from which companies like us set their prices at the time they launch their products. All patented pharmaceutical products introduced in Canada are subject to the post-approval, post-launch scrutiny of the PMPRB. Since the PMPRB does not pre-approve prices for a patented drug product in Canada, there may be risk involved in the determination of an allowable price selected for a patented drug product at the time of introduction to the market by the company launching such products in Canada. If the PMPRB does not agree with the pricing assumptions chosen by such company introducing a new drug product, the price chosen could be challenged by the PMPRB pursuant to the PMPRB initiating an investigation and, if it is determined, usually pursuant to an oral tribunal hearing, that the price charged is excessive, the price of the product may be reduced and a fine may be levied against the company for any amount deemed to be in excess of the allowable price determined. Drug products that have no valid patents are not subject to the PMPRB’s jurisdiction.
Our status as a foreign corporation for U.S. federal tax purposes could be affected by IRS action or a change in U.S. tax law.
Although the Company is incorporated in British Columbia, Canada, the Internal Revenue Service (the “IRS”) may assert that we should be treated as a U.S. corporation (and, therefore, a U.S. tax resident) for U.S. federal income tax purposes pursuant to Section 7874 of the Internal Revenue Code of 1986, as amended (the “Code”). A corporation is generally considered a tax resident in the jurisdiction of its organization or incorporation for U.S. federal income tax purposes. As a result of the Company being an entity incorporated in the Province of British Columbia, it would generally be classified as a foreign corporation (and, therefore, a non-U.S. tax resident) under these rules. Section 7874 of the Code provides an exception pursuant to which a foreign incorporated entity may, in certain circumstances, be treated as a U.S. corporation for U.S. federal income tax purposes.
Under Section 7874 of the Code, the Company may be treated as a U.S. corporation for U.S. federal income tax purposes if former Pozen shareholders hold 80% or more of the vote or value of the Company’s shares by reason of holding stock in Pozen immediately after Merger and the Company’s expanded affiliated group after the Merger does not have substantial business activities in Canada relative to its worldwide activities. As a result of the fact that the former shareholders of Pozen owned (within the meaning of Section 7874 of the Code) less than 80% (by both vote and value) of the combined entity’s stock immediately after the Merger, we believe we qualify as a foreign corporation for U.S. federal income tax purposes following the Merger. However, there can be no assurance that there will not exist in the
33
future a subsequent change in the facts or in law, which might cause us to be treated as a domestic corporation for U.S. federal income tax purposes, including with retroactive effect.
Further, there can be no assurance that the IRS will agree with the position that the ownership test was satisfied. There is limited guidance regarding the application of Section 7874 of the Code, including with respect to the provisions regarding the application of the ownership test. If we were unable to be treated as a foreign corporation for U.S. federal income tax purposes, the benefits associated with enhanced global cash management, including increased liquidity resulting from access to cash generated by our non-U.S. subsidiaries, would be jeopardized.
Our tax position may be adversely affected by changes in tax law relating to multinational corporations, or increased scrutiny by tax authorities.
Under current law, we expect to be treated as a foreign corporation for U.S. federal tax purposes. However, changes to the rules in Section 7874 of the Code or the U.S. Treasury regulations promulgated thereunder could adversely affect our status as a foreign corporation for U.S. federal tax purposes, and any such changes could have prospective or retroactive application. In addition, recent legislative proposals have aimed to expand the scope of U.S. corporate tax residence, and such legislation, if passed, could have an adverse effect on us.
Moreover, the United States Congress, the Organization for Economic Co-operation and Development and other government agencies in Canada and other jurisdictions where we and our affiliates do business have had an extended focus on issues related to the taxation of multinational corporations. One example is in the area of “base erosion and profit shifting,” where payments are made between affiliates from a jurisdiction with high tax rates to a jurisdiction with lower tax rates. As a result, the tax laws in the United States, Canada and other countries in which we and our affiliates do business could change on a prospective or retroactive basis, and any such changes could adversely affect us.
For example, in April 2016, the U.S. Treasury and IRS issued temporary regulations that expand the scope of transactions that are subject to the rules designed to eliminate the U.S. tax benefits of inversions, which regulations could limit our ability to engage in certain stock transactions in the future. Additionally, in October 2016 the U.S. Treasury and IRS issued final and temporary regulations that address whether an interest in a related corporation is debt or equity, which regulations would impact the treatment of future inter-company debt and limit the ability to deduct interest thereon.
Changes in tax laws and unanticipated tax obligations could adversely affect our effective income tax rate, other tax obligations and profitability.
We are subject to income and other taxes in Canada, the United States, and certain foreign jurisdictions. Our effective income tax rate and other tax obligations in the future could be adversely affected by a number of factors including changes in the mix of earnings in countries with differing statutory tax rates, changes in the valuation of deferred tax assets and liabilities, disagreements with taxing authorities with respect to the interpretation of tax laws and regulations and changes in tax laws. We regularly assess all of these matters to determine the adequacy of our tax provision which is subject to discretion. If our assessments are incorrect, it could have an adverse effect on our business and financial condition.
In addition, comprehensive U.S. tax reform continues to be discussed and proposed, including among other items, changes to the corporate tax rate, a border adjustment tax and changes to the U.S. taxation of foreign earnings. It is currently uncertain whether any of these changes will be enacted, and if so, the effective dates. If comprehensive tax reform occurs, our financial condition, results of operations and cash flows could be significantly impacted. However, we are unable to determine the potential impact at this time.
There can be no assurance that income and other tax laws and administrative policies with respect to the income and other tax consequences generally applicable to us, to our subsidiaries, or to a U.S. or Canadian holder of common shares will not be changed in a manner which adversely affects holders of our common shares.
34
If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate Program, Medicare, PMPRB obligations, governmental funded drug formularies or other governmental pricing programs, we could be subject to additional reimbursement requirements, penalties, sanctions and/or fines which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Pricing and rebate calculations vary among products and programs. The calculations are complex and are often subject to interpretation by us, governmental or regulatory agencies and the courts. We cannot assure you that our submissions will not be found by Centers for Medicare and Medicaid Services (“CMS”) to be incomplete or incorrect. Governmental agencies may also make changes in program interpretations, requirements or conditions of participation, some of which may have implications for amounts previously estimated or paid. The Medicaid rebate amount is computed each quarter based on our submission to CMS of our current average manufacturer price and best price for the quarter. If we become aware that our reporting for a prior quarter was incorrect, or has changed as a result of recalculation of the pricing data, we are obligated to resubmit the corrected data for a period not to exceed twelve quarters from the quarter in which the data originally were due, and CMS may request or require restatements for earlier periods as well. Such restatements and recalculations increase our costs for complying with the laws and regulations governing the Medicaid Drug Rebate Program. Any corrections to our rebate calculations could result in an overage or underage in our rebate liability for past quarters, depending on the nature of the correction. Price recalculations also may affect the ceiling price at which we are required to offer our products to certain covered entities, such as safety-net providers, under the 340B drug discount program.
We are liable for errors associated with our submission of pricing data. In addition to retroactive rebates and the potential for 340B program refunds, if we are found to have knowingly submitted false average manufacturer price, average sales price (“ASP”), or best price information to the government or made a misrepresentation in the reporting of our ASP, we may be liable for civil monetary penalties. Our failure to submit monthly/quarterly average manufacturer price, ASP, and best price data on a timely basis could result in a civil monetary penalty. Such failure also could be grounds for CMS to terminate our Medicaid drug rebate agreement, pursuant to which we participate in the Medicaid program. In the event that CMS terminates our rebate agreement, federal payments may not be available under Medicaid or Medicare Part B for our covered outpatient drugs.
Federal law requires that a company must participate in the Department of Veterans Affairs Federal Supply Schedule (“FSS”) pricing program, established by Section 603 of the Veterans Health Care Act of 1992, to be eligible to have its products paid for with federal funds. If we overcharge the government in connection with our FSS contract, whether due to a misstated federal ceiling price or otherwise, we are required to refund the difference to the government. Failure to make necessary disclosures and/or to identify contract overcharges can result in allegations against us under the False Claims Act and other laws and regulations. Unexpected refunds to the government, and responding to a government investigation or enforcement action, would be expensive and time-consuming, and could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Our business involves the use of hazardous materials, and we and our third-party manufacturers must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our third-party manufacturers’ activities involve the controlled storage, use and disposal of hazardous materials owned by us, including the components of our approved products and product candidates and other hazardous compounds. We and our manufacturers are subject to federal, state, provincial and local as well as foreign laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. Although we believe that the safety procedures utilized by our third-party manufacturers for handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental contamination or injury from these materials. In the event of an accident, federal, state, provincial or foreign authorities may curtail the use of these materials and interrupt our business operations. We do not currently maintain hazardous materials insurance coverage. If we are subject to any liability as a result of our third-party manufacturers’ activities involving hazardous materials, our business and financial condition may be adversely affected.
35
Risks Related to Our Financial Position and Capital Requirements
We have incurred losses since inception and we may continue to incur losses for the foreseeable future.
We have a limited operating history and even less history operating as a combined organization following the Merger. For the fiscal year ended December 31, 2016, we had net losses of approximately $103.0 million. Our ability to receive product revenue from the sale of products is dependent on a number of factors, principally the development, regulatory approval and successful commercialization of our products and product candidates. We expect that the amount of our operating losses will fluctuate significantly from quarter to quarter principally as a result of increases and decreases in any development efforts, the timing and amount of payments that we may receive from others and the timing of our commercial expenses, including increased expenses in connection with the commercialization of Yosprala, Zontivity, Toprol-XL, Fibricor and other current or acquired products. If our licensed or marketed products do not perform well in the marketplace, our revenue will be impacted and our business could be materially harmed.
We have had limited product revenues and other sources of revenues to date and new sources of revenue have only just been approved or acquired. Even if we achieve profitability in the future, we cannot be certain that we will sustain profitability, which would depress the market price of our common shares and could cause our investors to lose all or a part of their investment.
Our ability to become profitable depends upon our ability to generate revenues from sales of our products. New sources of product revenue have only recently been approved, in the case of Yosprala in the United States and Blexten in Canada, or acquired by the Company, in the case of Zontivity in the United States and Canada and Toprol-XL and the AG in the United States. In addition, Tribute only acquired Fibricor in May 2015. The ability of such products to generate revenues depends on a variety of factors, including the success of our commercialization efforts and competition in applicable markets. Product revenue for products which we license out is dependent upon the commercialization efforts of our partners. One of our primary sources of revenue to date is the royalty payments that we may receive in connection with the commercialization of VIMOVO by AstraZeneca, outside of the United States (excluding Japan), and Horizon in the United States. In the event that AstraZeneca, Horizon or any other third-party with future commercialization rights to any of our products or product candidates fails to adequately commercialize those products or product candidates because it lacks adequate financial or other resources, decides to focus on other initiatives or otherwise, our ability to successfully commercialize our products or product candidates in the applicable jurisdictions would be limited, which would adversely affect our business, financial condition, results of operations and prospects. In addition, our ability to generate future revenues depends in part on our success in:
|
· |
commercialization of our existing products and any other product candidates for which we obtain approval or that we acquire; |
|
· |
obtaining approval for Yosprala in Canada and the EU; |
|
· |
developing, acquiring or in-licensing and commercializing a portfolio of other products or product candidates in addition to our current products. |
Even if we do generate additional product sales, we may not be able to sustain profitability on a quarterly or annual basis. Our failure to become and remain profitable would depress the market price of our common shares and could impair our ability to raise capital, expand our business, diversify our product offerings or continue our operations.
We may need additional funding and may not have access to capital. If we are unable to raise capital when needed, we may need to delay, reduce or eliminate our product development or commercialization efforts.
In the future, we may need to raise additional funds to execute our evolving business strategy. We have incurred losses from operations since inception and we may continue to incur additional operating losses. Our actual capital requirements will depend upon numerous factors, including:
|
· |
completing the regulatory approval process, and any further required clinical development related thereto, for product candidates; |
|
· |
our ability to commercialize or arrange for the commercialization of our products; |
36
|
· |
the costs of commercialization of our products; |
|
· |
the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
|
· |
the effect of competing technological and market developments; |
|
· |
generic competition with respect to our products; |
|
· |
the timing of our payment or receipt, as applicable, of milestone payments and royalties under collaborative, license, acquisition or other agreements; |
|
· |
the effect of changes and developments in, or termination of, our collaborative, license, acquisition and other relationships; |
|
· |
the terms and timing of any additional collaborative, license, acquisition and other arrangements that we may establish; and |
|
· |
the ability to acquire or in-license additional complementary products or products that augment our current product portfolio. |
As of December 31, 2016, we had an aggregate of $64.9 million in cash and cash equivalents. In connection with the closing of the Merger, we received a $75 million equity investment and issued $75 million in convertible debt. In addition, pursuant to a Second Amended and Restated Debt Facility Agreement (the “Facility Agreement”), dated December 7, 2015, among us, Pozen, Tribute (“the Credit Parties”) and certain lenders party thereto, we had the ability to borrow up to an additional aggregate principal amount of $200 million for acquisitions. On October 31, 2016, the Company borrowed the full $200 million that had been available under the Facility Agreement for acquisitions, $175 million of which was used to fund the upfront cash closing payment for the acquisition of Toprol-XL and the AG and $25 million of which was used to replenish the Company’s cash balance for the initial upfront payment of $25 million in cash paid at the closing of the Zontivity acquisition in September 2016. In addition to this borrowing, pursuant to a consent provided by the requisite lenders under the Facility Agreement with respect to the Toprol-XL acquisition, the lenders under the Facility Agreement agreed that they and/or affiliated funds will make available additional loans to the Credit Parties in an aggregate amount of up to $250 million for the payment of the purchase price of any acquisitions permitted by the terms of the Facility Agreement (as modified by such consent) with respect to target businesses mutually approved by, and as otherwise mutually agreed upon, by the Company and the lenders, subject to the satisfaction of certain conditions set forth in the Facility Agreement. Any such loans (to the extent made available) may be borrowed in one or more advances at any time prior to April 3, 2018. We have not yet borrowed any of this additional $250 million available amount.
We may need to raise additional capital if we choose to expand our commercialization or development efforts more rapidly than we presently anticipate, if we develop, acquire or in-license additional products or acquire companies or if our revenues do not meet expectations. In addition, our expenses might increase beyond currently expected levels if we decide to, or any regulatory agency requires us to, conduct additional clinical trials, studies or investigations for any of our product candidates, including in connection with the consideration, or reconsideration, of our regulatory filings for our product candidates. We began commercializing Yosprala in the United States without a commercial partner and our expenses have increased and may continue to increase relative to prior years as we continue the transition from a development company that licenses its product candidates to other companies into a fully integrated, specialty pharmaceutical company.
We may be unable to raise additional equity funds when we desire to do so due to unfavorable market conditions in our industry, or generally, or due to other unforeseen developments in our business. Further, we cannot be certain that additional funding will be available on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the
37
development or commercialization of one or more of our products or product candidates, or delay, cut back or abandon our plans to grow the business through acquisition or in-licensing. We also could be required to:
|
· |
seek collaborators for one or more of our current or future product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available; or |
|
· |
relinquish or license on unfavorable terms our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves. |
Any of the above events could significantly harm our business, financial condition and prospects and cause the price of our common shares to decline.
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish intellectual property rights to our product candidates.
Additional capital may be needed in the future to continue our planned operations. We may seek additional capital through a combination of private and public equity offerings, debt financings, receivables or royalty financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our existing shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our shareholders. Debt, receivables and royalty financings may be coupled with an equity component, such as warrants to purchase shares, or existing debt refinanced, each of which could also result in dilution of our existing shareholders’ ownership. The incurrence of additional indebtedness would result in increased fixed payment obligations and could result in certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business.
As noted above, in connection with our acquisitions of Toprol-XL and the AG and Zontivity, we have borrowed an additional $200 million under the Facility Agreement. This substantial debt obligation could have adverse consequences, including requiring a substantial portion of cash flow from operations to be dedicated to servicing this indebtedness, thereby reducing our ability to use our cash flow to fund our operations and pursue future business opportunities and making it more difficult to satisfy our obligations with respect to indebtedness.
Covenants and financial performance thresholds imposed by the Facility Agreement restrict our business and operations in many ways and if we do not effectively manage our covenants and financial performance thresholds, our financial conditions and results of operations could be adversely affected.
The Facility Agreement imposes various covenants that limit our ability and/or our subsidiaries’ ability to, among other things:
|
· |
consolidate or merge with or into another person; |
|
· |
enter into certain transactions with affiliates; |
|
· |
pay dividends or distributions; |
|
· |
create, incur or suffer liens; |
|
· |
create, incur, assume guarantee or be liable with respect to indebtedness; |
|
· |
acquire assets or transfer products or material assets; and |
|
· |
issue equity securities senior to our common shares or convertible or exercisable for equity securities senior to our common shares. |
38
The covenants imposed by the Facility Agreement and our obligations to service our outstanding debt:
|
· |
limit our ability to borrow additional funds for working capital, capital expenditures, acquisitions or other general business purposes; |
|
· |
limit our ability to use our cash flow or obtain additional financing for future working capital, capital expenditures, acquisitions or other general business purposes; |
|
· |
may require us to use a substantial portion of our cash flow from operations to make debt service payments; |
|
· |
limit our flexibility to plan for, or react to, changes in our business and industry; |
|
· |
place us at a competitive disadvantage compared to our less leveraged competitors; and |
|
· |
increase our vulnerability to the impact of adverse economic and industry conditions. |
If we are unable to successfully manage the limitations and decreased flexibility on our business due to our debt obligations, we may not be able to capitalize on strategic opportunities or grow our business to the extent we would be able to without these limitations. Our failure to comply with any of the covenants could result in a default under the Facility Agreement, which could permit the required lenders to declare all or part of any outstanding loans to be immediately due and payable.
In addition, in connection with the Toprol-XL acquisition, the Facility Agreement was amended to include additional financial performance thresholds, including a minimum adjusted EBITDA threshold and a minimum specified revenue threshold relating to net sales of Toprol-XL and the AG received by the Company. In the event of the failure to meet both such additional financial performance thresholds, the lenders thereunder may elect to have the then outstanding principal balance of certain term loans under the Facility Agreement amortize quarterly through the maturity thereof.
To service our debt, we will be required to generate a significant amount of cash. Our ability to generate cash depends on a number of factors, some of which are beyond our control, and any failure to meet our debt service obligations would have a material adverse effect on our business, financial condition, cash flows and results of operation and could cause the market value of common shares to decline.
Our ability to satisfy our debt obligations will depend principally upon our future operating performance. As a result, prevailing economic conditions and financial, business and other factors, many of which are beyond our control, may affect our ability to make payments on our debt. If we do not generate sufficient cash flow to satisfy our debt service obligations, we may have to undertake alternative financing plans, such as refinancing or restructuring our debt, selling assets, reducing or delaying capital investments or seeking to raise additional capital. Our ability to restructure or refinance our debt will depend on the capital markets and our financial condition at such time. Any refinancing of our debt could be at higher interest rates and may require us to comply with more onerous covenants, which could further restrict our business operations. Our inability to generate sufficient cash flow to satisfy our debt service obligations or to refinance our obligations on commercially reasonable terms could have a material adverse effect on our business, financial condition, cash flows and results of operations and could cause the market value of our common shares to decline.
Risks Related to Our Intellectual Property and Product Liability
We may become involved in infringement actions which are uncertain, costly and time-consuming and could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
The pharmaceutical industry historically has generated substantial litigation concerning the manufacture, use and sale of products, and we expect this litigation activity to continue. The intellectual property rights of pharmaceutical companies, including us, are generally uncertain and involve complex legal, scientific and factual questions. In order to protect or enforce patent rights, we may initiate litigation against third parties. If we are not successful in defending an attack on our patents and maintaining exclusive rights to market one or more of our products still under patent protection,
39
we could lose a significant portion of sales in a very short period. We may also become subject to infringement claims by third parties and may have to defend against charges that we infringed patents or the proprietary rights of third parties. If we infringe the intellectual property rights of others, we could lose our right to develop or sell products, including our generic products, or could be required to pay monetary damages or royalties to license proprietary rights from third parties. The outcomes of infringement actions are uncertain and infringement actions are costly and divert technical and management personnel from their normal responsibilities.
Third parties seeking to market generic versions of branded pharmaceutical products in the United States often file Abbreviated New Drug Applications (“ANDAs”) with the FDA (with a similar process in Canada and other foreign countries) containing a certification stating that the ANDA applicant believes that the patents protecting the branded pharmaceutical product are invalid, unenforceable and/or not infringed. Such certifications are commonly referred to as Paragraph IV certifications. We and Horizon are engaged in Paragraph IV litigations with several generic pharmaceutical companies with respect to our VIMOVO patents. In addition, in December 2016, the Company received a Paragraph IV certification from Teva Pharmaceuticals USA, Inc. indicating that it had filed an ANDA for a generic version of Yosprala 81mg/40 mg and 325mg/40mg. If we are unsuccessful in any of these proceedings, or once our or our licensors’ applicable patents expire, and the FDA or Health Canada approve a generic version of one of our marketed products, such an outcome would have a material adverse effect on sales of such product, our business and our results of operations.
If we are unable to obtain or protect intellectual property rights related to our products and product candidates, we may not be able to compete effectively in our market.
The pharmaceutical industry places considerable importance on obtaining patent and trade secret protection for new technologies, products and processes. Our success will depend, in part, on our ability, and the ability of our licensors, to obtain and to keep protection for our products and technologies under the patent laws of the United States and other countries, so that we can stop others from using our inventions. Our success also will depend on our ability to prevent others from using our trade secrets. In addition, we must operate in a way that does not infringe, or violate, the patent, trade secret and other intellectual property rights of other parties.
We cannot know how much protection, if any, our patents will provide or whether our patent applications will issue as patents. The breadth of claims that will be allowed in patent applications cannot be predicted and neither the validity nor enforceability of claims in issued patents can be assured. If, for any reason, we are unable to obtain and enforce valid claims covering our products and technology, we may be unable to prevent competitors from using the same or similar technology or to prevent competitors from marketing identical products. For example, if we and our partner Horizon are unsuccessful in protecting our patents in the litigation against several generic pharmaceutical companies who have filed ANDAs for VIMOVO, such companies could market a generic version of the product prior to the expiration of our patents.
Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property or prevent others from designing around our claims. If the patent applications we hold fail to issue or if their breadth or strength of protection is threatened, it could dissuade companies from collaborating with us to develop them and threaten our ability to commercialize our products. We cannot offer any assurances about which, if any, patents will issue or whether any issued patents will be found invalid or unenforceable or will go unthreatened by third parties. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we were the first to file any patent application related to our products or any other product candidates. Furthermore, if third parties have filed such patent applications, an interference proceeding in the United States may be able to be provoked by a third-party or instituted by us to determine who was the first to invent any of the subject matter covered by the patent claims of our applications or, as in many jurisdictions, such as in Canada, the earlier filed third-party application may be cited against our patent application by a patent office in rejecting our application on the basis that the invention lacks novelty.
Our ability to obtain patents is highly uncertain because, to date, some legal principles remain unresolved, there has not been a consistent policy regarding the breadth or interpretation of claims allowed in patents in the United States and other jurisdictions, and the specific content of patents and patent applications that are necessary to support and interpret patent claims is highly uncertain due to the complex nature of the relevant legal, scientific and factual issues. Changes in either patent laws or interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property or narrow the scope of our patent protection. For example, in 2011, the Leahy-Smith
40
America Invents Act (the “Leahy-Smith Act”) was enacted, and it included a number of significant changes to patent law in the United States. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. The United States Patent and Trademark Office has developed new and untested regulations and procedures to govern the full implementation of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective in March 2013. The Leahy-Smith Act has also introduced procedures making it easier for third parties to challenge issued patents, as well as to intervene in the prosecution of patent applications. For example, third parties have filed petitions seeking Inter Partes Review (“IPR”) of some of our VIMOVO patents and one of our Treximet patents. These petitions have been denied or concluded with a final written decision upholding the challenged claims. In the IPRs concluded by a final written decision, the parties may still seek an appeal. Finally, the Leahy-Smith Act contains statutory provisions that require the United States Patent and Trademark Office to issue new regulations for their implementation and it may take the courts years to interpret the provisions of the new statute. Accordingly, it is too early to tell what, if any, impact the Leahy-Smith Act will have on the operation of our business and the protection and enforcement of our intellectual property. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. In addition, the Patient Protection and Affordable Care Act allows applicants seeking approval of biosimilar or interchangeable versions of biological products to initiate a process for challenging some or all of the patents covering the innovator biological product used as the reference product. This process is complicated and could result in the limitation or loss of certain patent rights. An inability to obtain, enforce and defend patents covering our proprietary technologies would materially and adversely affect our business prospects and financial condition.
Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States and Canada. As a result, we may encounter significant problems in protecting and defending our intellectual property in the United States, Canada and other countries. For example, if the issuance to us, in a given country, of a patent covering an invention is not followed by the issuance, in other countries, of patents covering the same invention, or if any judicial interpretation of the validity, enforceability, or scope of the claims in, or the written description or enablement in, a patent issued in one country is not similar to the interpretation given to the corresponding patent issued in another country, our ability to protect our intellectual property in those countries may be limited. For example, since patent protection is territorial, the teachings of a U.S. patent will generally only be protected in the United States. If we do not have a corresponding patent in another jurisdiction, the teachings of the U.S. patent may be in the public domain in such jurisdiction and free for a third-party to practice. Changes in either patent laws or in interpretations of patent laws in the United States, Canada and other countries may materially diminish the value of our intellectual property or narrow the scope of our patent protection. If we are unable to prevent material disclosure of the non-patented intellectual property related to our technologies to third parties, and there is no guarantee that we will have enforceable trade secret protection with respect thereto, we may not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, results of operations and financial condition.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our drug discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets are difficult to protect, and we cannot be certain that others will not develop the same or similar technologies on their own. We have taken steps, including entering into confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors, to protect our trade secrets and unpatented know-how. These agreements generally require that the other party keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by the Company during the course of the party’s relationship with the Company. We also typically obtain agreements from these parties, which provide that inventions conceived by the party in the course of rendering services to the Company will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to the Company. Enforcing a claim that a party illegally obtained and is using our trade secrets or know-how is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, courts outside Canada and the United States may be less willing to protect trade secrets or know-how. The failure to obtain or maintain trade secret protection could adversely affect our competitive position.
41
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our products.
We face an inherent business risk of exposure to significant product liability and other claims in the event that the use of our products caused, or is alleged to have caused, adverse effects. For example, we may be sued if any of our products or product candidates allegedly causes injury or is found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. The withdrawal of a product following complaints and/or incurring significant costs, including the requirement to pay substantial damages in personal injury cases or product liability cases, could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
Our product liability insurance coverage may not be sufficient to cover our claims and we may not be able to obtain sufficient coverage at a reasonable cost in the future. We will explore, on an on-going basis, expanding our insurance coverage related to the sale of our products and future marketed products when we obtain marketing approval for such products and commercial sales of such products begin. However, we may not be able to obtain commercially reasonable product liability insurance for any products approved for marketing. If a plaintiff brings a successful product liability claim against us in excess of our insurance coverage, if any, we may incur substantial liabilities and our business may be harmed or fail.
For example, if proton pump inhibitors are found, or are perceived, to create health risks, our ability to sell Yosprala could be materially adversely affected, product liability lawsuits may be brought against us, and our business could be substantially harmed.
If our products or technologies are stolen, misappropriated or reverse engineered, others could use our products or licensed products to produce competing products or technologies.
Third parties, including our partners, contract manufacturers, contractors and others involved in our business often have access to our products, licensed products, and technologies. If our products, licensed products or technologies were stolen, misappropriated or reverse engineered, they could be used by other parties that may be able to reproduce our products, licensed products, or technologies for their own commercial gain. If this were to occur, it would be difficult for us to challenge this type of use, especially in countries with limited intellectual property protection.
Risks Related to Ownership of Our Common Shares
The price of our common shares could be volatile, which may result in significant losses to our shareholders.
The trading price of our common shares could be highly volatile and subject to wide fluctuations in response to various factors, some of which are beyond our control, including limited trading volume. In addition to the factors discussed in the “Risk Factors” of this Annual Report on Form 10-K, these factors include:
|
· |
fluctuations in our operating results and revenues generated by our marketed products; |
|
· |
announcements of technological innovations, acquisitions or licensing of therapeutic products or product candidates by us or our competitors; |
|
· |
prolonged stock shortages from third-party manufacturers; |
|
· |
published reports by securities analysts; |
|
· |
positive or negative progress with our clinical trials or with regulatory approvals of our product candidates; |
|
· |
commercial success of VIMOVO, Fibricor, Yosprala, Zontivity, Toprol-XL and our other products and product candidates once approved; |
42
|
· |
generic introductions of existing marketed products with no generic competition, such as Yosprala, or additional generic competition for Toprol-XL and the AG; |
|
· |
governmental regulation, including reimbursement policies; |
|
· |
developments in patent or other proprietary rights; |
|
· |
developments in our relationships with collaborative partners or our inability to obtain consents or achieve minimum licensing terms; |
|
· |
announcements by our collaborative partners regarding our products or product candidates; |
|
· |
developments in new or pending litigation; |
|
· |
public concern as to the safety and efficacy of our products; |
|
· |
our ability to acquire or license new products or companies and the perception of the value of such transactions, and our ability to integrate and grow such products or companies; |
|
· |
the sale or attempted sale of a large amount of our common shares into the market; and |
|
· |
general market conditions. |
The common shares are listed on the NASDAQ Global Market and the Toronto Stock Exchange. Volatility in the market prices of our common shares may increase as a result of our common shares being listed on both the NASDAQ Global Market and the Toronto Stock Exchange because trading is split between the two markets, resulting in less liquidity on both exchanges. In addition, different liquidity levels, volume of trading, currencies and market conditions on the two exchanges may result in different prevailing trading prices.
In addition, the stock market in general, and the NASDAQ Global Market, the Toronto Stock Exchange and the stocks of biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may adversely affect the market price of our common shares, regardless of our actual operating performance.
Sales of substantial amounts of shares of our common shares in the public market could cause our share price to decline.
If our existing shareholders sell, or indicate an intention to sell, substantial amounts of our common shares in the public market, the trading price of our common shares could decline. Certain shareholders hold significant positions in our common shares. Any sales of substantial amounts of our common shares in the public market, including sales or distributions of shares by our large investors, or the perception that such sales or distributions might occur, could harm the market price of our common shares and could impair our ability to raise capital through the sale of additional equity securities. Further, shareholder ownership will be diluted if we raise additional capital by issuing equity securities. In addition, our common shares that are either subject to outstanding options or reserved for future issuance under our employee benefit plans are or may become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules and Rule 144 and Rule 701 under the Securities Act. If these additional common shares are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common shares could decline.
Anti-takeover provisions in our Articles and certain provisions under the BCBCA could prevent or delay transactions that our shareholders may favor and may prevent shareholders from changing the direction of our business or management.
Provisions of our Articles and certain provisions under the BCBCA may discourage, delay or prevent a merger or acquisition that our shareholders may consider favorable, including transactions in which shareholders might
43
otherwise receive a premium for their shares, and may also frustrate or prevent any attempt by shareholders to change the direction or management of the Company. For example, these provisions:
|
· |
authorize the issuance of “blank check” preferred shares without any need for action by shareholders; |
|
· |
require a 75% majority of shareholder votes cast in favor of a resolution to remove a director; |
|
· |
require a 66 2/3% majority of shareholder votes cast in favor of a resolution to effect various amendments to our Articles; |
|
· |
require that in the case of shareholder action by written consent, (i) a matter that would normally require an ordinary resolution shall require written consent by shareholders representing at least 66 2/3% of the votes entitled to be cast in favor of such resolution, and (ii) in the case of any other resolution of the shareholders, the written consent of shareholders representing 100% of the votes entitled to be cast in favor of such resolution; |
|
· |
establish advance notice requirements for nominations for election to the Board of Directors; and |
|
· |
require shareholder proposals for matters to be acted upon by shareholders at shareholder meetings to be submitted pursuant to, and in accordance with, the applicable provisions of the BCBCA for inclusion in the Company’s proxy materials by a date that is not later than three months prior to the anniversary date of the prior year’s shareholder meeting. |
These provisions, among others, whether alone or together, could delay or impede hostile takeovers and changes in control or changes to the composition of our Board of Directors or management. Any provision of our constating documents that has the effect of delaying or deterring a change in control could limit the opportunity for our shareholders to receive a premium for their common shares and could also affect the price that some investors are willing to pay for our common shares.
Provisions of Canadian law may delay, prevent or make undesirable an acquisition of all or a significant portion of our common shares or assets.
The Investment Canada Act subjects an acquisition of control of us by a non-Canadian to government review if our enterprise value as calculated pursuant to the legislation exceeds a threshold amount. A reviewable acquisition may not proceed unless the relevant Minister is satisfied that the investment is likely to be of net benefit to Canada. This could prevent or delay a change of control and may eliminate or limit strategic opportunities for shareholders to sell their common shares.
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation and these differences may make our common shares less attractive to investors.
The Company is incorporated under the laws of the Province of British Columbia, Canada, and therefore certain of the rights of holders of its shares are governed by Canadian law, including the provisions of the BCBCA, and by our Notice of Articles and Articles. These rights differ in certain respects from the rights of shareholders in typical U.S. corporations and these differences may make our common shares less attractive to investors.
An investor may be unable to bring actions or enforce judgments against us and certain of our directors.
The Company is incorporated under the laws of the Province of British Columbia. Some of our directors reside principally outside of the United States and a substantial portion of our assets and a substantial portion of the assets of these persons are located outside the United States. Consequently, it may not be possible for an investor to effect service of process within the United States on us or those persons. Furthermore, it may not be possible for an investor to enforce judgments obtained in United States courts based upon the civil liability provisions of United States federal securities laws or other laws of the United States against us or those persons.
44
We do not expect to pay dividends for the foreseeable future, and our shareholders must rely on increases in the trading price of our common shares for returns on their investment.
Except for the $1.75 per share special cash distribution by Pozen on December 30, 2013 (representing a surplus of corporate cash and accounted for as a return of capital to shareholders), we have never paid cash dividends on our common shares and do not expect to pay dividends in the immediate future. We anticipate that the Company will retain all earnings, if any, to support our operations. Any future determination to pay dividends on our common shares will be at the sole discretion of the Board of Directors and will depend on, among other things, the Company’s results of operations, current and anticipated cash requirements and surplus, financial condition, contractual restrictions and financing agreement covenants, solvency tests imposed by corporate law and other factors that the Board of Directors may deem relevant. Holders of our common shares must rely on increases in the trading price of our shares for returns on their investment in the foreseeable future. In addition, the Facility Agreement prohibits the Company from making any cash dividend or distributing any of its assets, including its intangibles, to any of its shareholders in such capacity or its affiliates, subject to certain exceptions. The Facility Agreement also includes restrictions on the Company from incurring liens and undertaking indebtedness, subject to certain exceptions, which limitations may further impact the ability of the Company to pay any future dividends. See “Covenants and financial performance thresholds imposed by the Facility Agreement restrict our business and operations in many ways and if we do not effectively manage our covenants and financial performance thresholds, our financial conditions and results of operations could be adversely affected” above.
We have incurred and will continue to incur significant increased costs as a result of operating as a public company and our management will be required to devote substantial time to compliance initiatives.
As a public company, we have incurred and will continue to incur significant legal, accounting and other expenses that we would not incur if we were a private company. In particular, the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), as well as rules subsequently implemented by the SEC, applicable securities laws in Canada, the NASDAQ Global Market and the Toronto Stock Exchange, impose significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. These rules and regulations have substantially increased our legal and financial compliance costs and have made some activities more time-consuming and costly. Further, these rules and regulations may lack specificity and are subject to varying interpretations. Their application in practice may evolve over time, as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs of compliance as a result of ongoing revisions to such corporate governance standards.
In particular, our efforts to comply with Section 404 of the Sarbanes-Oxley Act, National Instrument 52-109 - Certification of Disclosures in Issuers’ Annual and Interim Filings and the related regulations regarding our required assessment of our internal controls over financial reporting and our external auditors’ audit of that assessment requires the commitment of significant financial and managerial resources. We consistently assess the adequacy of our internal controls over financial reporting, remediate any control deficiencies that may be identified, and validate through testing that our controls are functioning as documented. While we do not anticipate any material weaknesses, the inability of management to assess our internal controls over financial reporting as effective could result in adverse consequences to us, including, but not limited to, a loss of investor confidence in the reliability of our financial statements, which could cause the market price of our stock to decline. The existence of this or one or more other material weaknesses or significant deficiencies in our internal control over financial reporting could result in errors in our financial statements, and substantial costs and resources may be required to rectify any internal control deficiencies. Although we continually review and evaluate internal control systems to allow management to report on the sufficiency of our internal controls, we cannot assure you that we will not discover weaknesses in our internal control over financial reporting. Any such weakness or failure to remediate any existing material weakness could materially adversely affect our ability to comply with applicable financial reporting requirements and the requirements of our various agreements.
We are committed to maintaining high standards of corporate governance and public disclosure, and our efforts to comply with evolving laws, regulations and standards in this regard have resulted in, and are likely to continue to result in, increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities. In addition, the laws, regulations and standards regarding corporate governance may make it more difficult, or increasingly more expensive, for us to obtain director and officer liability insurance. Further, members of the Board of Directors and executive officers could face an increased risk of personal liability in connection with their performance of duties. As a result, we may face difficulties attracting and retaining
45
qualified board members and executive officers, which could harm our business. If we fail to comply with new or changed laws, regulations or standards of corporate governance, our business and reputation may be harmed.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common shares will depend in part on the research and reports that securities or industry analysts publish about us or our business. There is no guarantee that securities analysts will cover our securities, and the lack of research coverage may adversely affect our share price. If one or more of the securities analysts publish inaccurate or unfavorable research about our business, our share price would likely decline. If one or more of these securities analysts cease coverage of the Company or fail to publish reports on us regularly, demand for our common shares could decrease, which might cause our share price and trading volume to decline.
ITEM 1B. Unresolved Staff Comments
None.
The properties described below are used by the Company for general corporate purposes.
In September 2016, Aralez Ireland entered into a lease agreement for an approximately 5,715 square foot office space located in Dublin, Ireland. The lease term is for 12 years, terminating in September 2028. This location serves as the Irish headquarters for Aralez.
In March 2016, Tribute entered into a sublease agreement for an approximately 9,183 square foot office space located at 7100 West Credit Avenue, Mississauga, Ontario. The lease term is five years and three months, terminating on July 30, 2021. This location serves as the global headquarters for Aralez. Tribute also owns a building located at 544 Egerton Street in London, Ontario, Canada, which is no longer is use by the Company and is subject to an agreement to be sold.
In March 2016, our wholly-owned subsidiary Aralez Pharmaceuticals US Inc. (“Aralez Pharmaceuticals US”), a Delaware corporation, entered into a lease for an approximately 36,602 square foot office space located in Princeton, New Jersey. The lease term is ten years and nine months, terminating in 2027. This location serves as the U.S. headquarters for Aralez.
In October 2015, Aralez Pharmaceuticals US entered into a lease for an approximately 4,500 square foot office space located in Radnor, Pennsylvania which was subsequently assigned to Aralez Pharmaceuticals Management Inc., also a wholly owned subsidiary of Aralez Pharmaceuticals Inc. The lease term is five years and two months, terminating on December 31, 2020, with a five-year extension term available at our option.
In September 2015, Aralez Pharmaceuticals US entered into a lease for an approximately 4,000 square foot office space located in New York, New York. The lease term is five years and two months, terminating on October 31, 2020.
VIMOVO ANDA Litigation
Between March 14, 2011 and May 16, 2013, Pozen, now a subsidiary of the Company, received Paragraph IV Notice Letters from Dr. Reddy’s Laboratories (“DRL”), Lupin Ltd. (“Lupin”), Watson Laboratories, Inc. – Florida (“Watson,” now “Actavis”), and Mylan Pharmaceuticals Inc. (“Mylan”), stating that each had filed an ANDA with the FDA seeking regulatory approval to market a generic version of our VIMOVO product before the expiration of U.S. Patent No. 6,926,907 (the “907 patent”). On November 20, 2012, Pozen received a second Notice Letter from DRL stating that DRL had filed a second ANDA with the FDA seeking regulatory approval to market a different generic formulation of the VIMOVO product before the expiration of the ‘907 patent. The ‘907 patent is assigned to Pozen and
46
listed for the VIMOVO product in the FDA’s publication titled “Approved Drug Products with Therapeutic Equivalence Evaluations” (also known as the “Orange Book”).
On April 21, 2011, Pozen filed suit against the first ANDA filer, DRL, in the United States District Court for the District of New Jersey (the “District Court”), asserting infringement of the ‘907 patent. Pozen subsequently filed suit against the other three ANDA filers within 45 days of receipt of their respective Paragraph IV Notice Letters. Horizon, our current marketing partner for the VIMOVO product in the U.S., is Pozen’s co-plaintiff in each suit. The first suit against DRL is considered the lead case and has been consolidated with other suits for the purpose of pre-trial and discovery. On December 19, 2012, the District Court conducted a pre-trial Markman hearing to determine the proper claim construction of certain claims disputed by the parties. On May 1, 2013, the District Court issued a Markman Order construing the disputed claims. A scheduling order for the consolidated suits was issued by the District Court on June 27, 2014.
On October 15, 2013, the United States Patent & Trademark Office (“USPTO”) issued to Pozen U.S. Patent No. 8,557,285 (the “‘285 patent”). The ‘285 patent is listed in the Orange Book for the VIMOVO product and is related to the ‘907 patent. On October 23, 2013, Pozen filed suits against DRL, Lupin, Watson and Mylan in the District Court asserting infringement of the ‘285 patent. These suits have each been consolidated with the above referenced suits involving the ‘907 patent. On May 12, 2016, the court granted DRL’s motion for summary judgment of non-infringement of the ‘907 patent with respect DRL’s second ANDA. The ruling does not apply to DRL’s first-filed ANDA, nor does it apply to the other patents asserted against DRL’s second ANDA. In January 2017, Judge Cooper conducted a six day bench trial in the lead case involving Defendants DRL and Mylan relating solely to the validity and infringement of the ‘907 and ‘285 patents. The parties are in the process of providing post-trial submissions to the District Court. It is anticipated the closing arguments will take place after post-trial submissions are complete.
On October 7, 2014, the USPTO issued to Pozen U.S. Patent No. 8,852,636 (the “‘636 patent”). On October 14, 2014, the USPTO issued to Pozen U.S. Patent No. 8,858,996 (the “‘996 patent”). In addition, on October 21, 2014, the USPTO issued to Pozen U.S. Patent No. 8,865,190 (the “190 patent”). The ‘636, ‘996 and ‘190 patents are each listed in the Orange Book for the VIMOVO product and are each related to the ‘907 and ‘285 patents.
On February 3, 2015, the USPTO issued to Pozen U.S. Patent No. 8,945,621 (the “‘621 patent”). The ‘621 patent is listed in the Orange Book for the VIMOVO product.
On May 13, 2015, Pozen and Horizon filed suit against DRL, Lupin, Actavis (formerly known as Watson) and Mylan in the District Court asserting infringement of the ‘636 and ‘996 patents. On June 18, 2015, Pozen filed Amended Complaints in each of the suits to assert infringement of the ‘190 patent.
On October 20, 2015, the USPTO issued to Pozen U.S. Patent No. 9,161,920 (the “‘920 patent”). On December 1, 2015, the USPTO issued to Pozen U.S. Patent No. 9,198,888 (the “‘888 patent”). The ‘920 and ‘888 patents are each listed in the Orange Book for the VIMOVO product and are each related to the ‘907 and ‘285 patents.
On December 29, 2015, the USPTO issued to Pozen U.S. Patent No. 9,220,698 (the “‘698 patent”). The ‘698 patent is listed in the Orange Book for the VIMOVO product.
On May 24, 2016, the USPTO issued to Pozen U.S. Patent No. 9,345,695 (the “‘695 patent”). The ‘695 patent is listed in the Orange Book for the VIMOVO product and is related to the ‘907 and ‘285 patents.
On January 25, 2016, Pozen and Horizon filed suit against Actavis in the District Court asserting infringement of the ‘920 and ‘888 patents. On March 16, 2016, the District Court consolidated this suit with the suit filed against Actavis on May 13, 2015. On February 10, 2016, Pozen filed Amended Complaints against DRL, Lupin and Mylan to assert infringement of the ‘920 and ‘888 patents. On August 10, 2016, Pozen and Horizon filed suit against DRL, Lupin, Actavis and Mylan in the District Court asserting infringement of the ’621, ’698, and ’695 patents. These suits are in the initial phase and a full schedule has not yet been set by the District Court.
On December 30, 2016, the District Court granted Actavis’ motion to enforce an alleged settlement agreement resolving all claims and counterclaims between Actavis and co-plaintiffs Pozen and Horizon in the lawsuits relating to VIMOVO. Pozen and Horizon contend that they did not agree to the settlement, and Pozen and Horizon filed notices of appeal of the District Court’s decision, on February 8, 2017 and February 9, 2017, respectively.
47
As with any litigation proceeding, we cannot predict with certainty the outcome of the patent infringement suits against DRL, Lupin, Mylan and Actavis relating to generic versions of VIMOVO. Furthermore, while Horizon is responsible for this litigation, including the costs of same, we nevertheless will have to incur additional expenses in connection with the lawsuits relating to VIMOVO, which may be substantial. Moreover, responding to and defending pending litigation results in a significant diversion of management’s attention and resources and an increase in professional fees.
Inter Partes Review
DRL filed a Petition for review (“IPR Petition”) of the ‘285 patent with the Patent Trial and Appeal Board (“PTAB”) of the USPTO on February 24, 2015, which was denied on October 9, 2015. The Coalition for Affordable Drugs VII L.L.C. (“CFAD”) filed IPR Petitions of the ‘907 patent, the ‘996 patent and the ‘636 patent with the PTAB on May 21, 2015, June 5, 2014 and August 7, 2015, respectively, each of which was denied on December 8, 2015, December 17, 2015 and February 11, 2016, respectively.
On August 12, 2015, CFAD filed an IPR Petition of the ‘621 patent with the PTAB. On February 22, 2016 the PTAB instituted review of the claims of the ‘621 patent. Pozen and Horizon filed a response on June 23, 2016. CFAD filed a reply to this response on September 22, 2016. Oral argument before the PTAB was held on November 16, 2016. On February 21, 2017, the PTAB entered a Final Written Decision in which it concluded that CFAD had not carried its burden of proving that the claims of the ‘621 patent were unpatentable.
On August 19, 2015, Lupin filed three separate IPR Petitions of the ‘996, ‘636 and ‘190 patents with the PTAB. On March 1, 2016 the PTAB denied Lupin’s petition for review of the ‘636 patent and instituted review of a limited number of the claims in each of the ‘996 and ‘190 patents. Pozen and Horizon filed responses to the petitions for review of the ‘996 and ‘190 patents on June 27, 2016. Lupin filed replies to these responses on September 16, 2016. Oral arguments before the PTAB for these matters were held on November 29, 2016. On February 28, 2017, the PTAB entered Final Written Decisions in which it concluded that Lupin had not carried its burden of proving that the claims of the ‘996 and ‘190 patents were unpatentable.
Canada VIMOVO Litigation
On January 20, 2015, our Canadian licensee, AstraZeneca Canada Inc. (“AstraZeneca Canada”) received a Notice of Allegation from Mylan Pharmaceuticals ULC (“Mylan Canada”) informing them that Mylan Canada has filed an Abbreviated New Drug Submission in Canada (“ANDS”) for approval of its naproxen/esomeprazole magnesium tablets and alleging non-infringement of some of the claims and invalidity of Pozen’s Canadian Patent No. 2,449,098 (the “‘098 patent”). A Notice of Allegation is served pursuant to the Patented Medicines (Notice of Compliance) Regulations in Canada and is similar to a Paragraph IV Notice Letter in the United States. In response, Pozen and AstraZeneca Canada commenced a proceeding in the Federal Court of Canada (the “Canada Court”) in relation to the ‘098 patent on March 5, 2015 seeking to prohibit Health Canada from approving Mylan Canada’s generic naproxen/esomeprazole product until the expiry of the ‘098 patent. The Canadian proceeding is summary in nature intended to decide only whether approval for Mylan Canada’s naproxen/esomeprazole magnesium tablets should be prohibited until the expiry of the ‘098 patent because none of Mylan Canada’s allegations in respect of the ‘098 patent are justified. The matter was heard on November 21 to 23, 2016. On February 7, 2017, the Court dismissed Pozen and AstraZeneca Canada’s request to prohibit the Minister from approving Mylan’s naproxen/esomeprazole products, deciding that certain of Mylan Canada’s allegations in respect of the ‘098 patent are justified (the “Decision”). However, this summary proceeding did not decide ‘098 patent validity or infringement. The ‘098 patent expires on May 31, 2022. Following the Decision, the Minister issued approval for Mylan’s 500/20mg strength naproxen/esomeprazole magnesium tablets on February 8, 2017
On March 23, 2016, AstraZeneca Canada received another Notice of Allegation from Mylan Canada in respect of the ‘098 patent, informing them that Mylan Canada has filed a supplemental submission for one of the strengths of its naproxen/esomeprazole magnesium tablets. This Notice of Allegation states that Mylan Canada withdrew from its ANDS the 375/20 mg strength and re-filed a supplemental submission for this strength. In this circumstance, Mylan is required to file, and has provided another Notice of Allegation in respect of the ‘098 patent. The allegations in respect of the ‘098 patent are identical to those asserted in the first Notice of Allegation. In response, Pozen and AstraZeneca Canada commenced another proceeding in the Federal Court of Canada on May 5, 2016 seeking to prohibit Health
48
Canada from approving Mylan Canada’s 375/20 mg strength naproxen/esomeprazole magnesium tablet until the expiry of the ‘098 patent. As the allegations made in respect of the ‘098 patent are identical, on the parties’ consent, the Court stayed the proceeding and the parties agreed that the outcome of the first proceeding discussed above, will determine the outcome for this new proceeding. Following the Decision, this proceeding was discontinued on February, 10, 2017. The Minister issued approval for Mylan’s 375/20 mg strength naproxen/esomeprazole magnesium tablets on February 10, 2017. It is expected that Mylan’s generic naproxen/esomeprazole magnesium tablets will be available in Canada shortly.
Yosprala Paragraph IV Certification
On November 4, 2016, the FDA website indicated that an ANDA for a generic version of Yosprala 81mg/40mg was submitted to the FDA on October 14, 2016. The Company ultimately received the related Paragraph IV Notice Letter on December 12, 2016, as described below.
On December 12, 2016, the Company received a Paragraph IV Notice Letter from Teva Pharmaceuticals USA, Inc. (“Teva”) stating that it had filed an ANDA with the FDA seeking regulatory approval to market generic versions of Yosprala 325mg/40 mg and 81mg/40mg prior to the expiration of the ‘907 patent, U.S. Patent No. 8,206,741 (the “‘741 patent”), and U.S. Patent No. 9,364,439 (the “‘439 patent”). The ‘907, ‘741, and ‘439 patents are assigned to Pozen and listed in the Orange Book for the Yosprala product.
On January 10, 2017, the USPTO issued to Pozen U.S. Patent No. 9,539,214 (the “‘214 patent”). The ‘214 patent is listed in the Orange Book for the Yosprala product. On March 13, 2017, the Company received a Paragraph IV Notice Letter regarding the ‘214 patent.
On January 23, 2017, Aralez Parent and its subsidiaries Aralez Pharmaceuticals Trading DAC, Aralez Pharmaceuticals US Inc., and Pozen Inc. filed a lawsuit in the United States District Court for the Eastern District of Texas against Teva and Teva Pharmaceutical Industries Ltd. for infringement of the ‘907, ‘741, ‘439, and ‘214 patents. The lawsuit was filed within 45 days of receipt of Teva’s Paragraph IV Notice Letter. In accordance with the Hatch-Waxman Act, as a result of having filed a timely lawsuit against Teva, a stay of approval will be imposed by the FDA on Teva’s ANDA for 30 months after the date of the Company’s receipt of Teva’s Paragraph IV Notice Letter on December 12, 2016 or until a final court decision is entered in the infringement suit in favor of Teva, whichever is earlier. The suit is in the initial phase and a full schedule has not yet been set.
As with any litigation proceeding, we cannot predict with certainty the outcome of the infringement suit relating to generic versions of Yosprala.
ITEM 4. Mine Safety Disclosures
Not applicable.
49
ITEM 5. Market for the Registrant’s Common Equity, Related Shareholder Matters and Issuer Purchases of Equity Securities
Market Information
As a result of the Merger, all of the shares of Pozen common stock issued and outstanding immediately prior to the effective time of the Merger were canceled and automatically converted into and became the right to receive our common shares on a one-for-one basis and Pozen became a wholly-owned subsidiary of Aralez.
Our common shares began trading on the NASDAQ Global Market under the trading symbol “ARLZ” on February 8, 2016 and on the Toronto Stock Exchange under the trading symbol “ARZ” on February 10, 2016. Previously, from October 11, 2000 until February 5, 2016, the common stock of Pozen was traded on the NASDAQ Global Market (formerly the NASDAQ National Market) under the trading symbol “POZN.” The following table sets forth the high and low sales prices per common share of Aralez from February 5, 2016 to December 31, 2016 and of Pozen from January 1, 2015 to February 4, 2016 as reported on the NASDAQ Global Market for the periods indicated.
|
|
|
Price Range |
|
||||
|
2016 Fiscal Year |
|
High |
|
Low |
|
||
|
First Quarter |
|
$ |
6.71 |
|
$ |
3.50 |
|
|
Second Quarter |
|
$ |
4.32 |
|
$ |
3.12 |
|
|
Third Quarter |
|
$ |
6.26 |
|
$ |
3.46 |
|
|
Fourth Quarter |
|
$ |
5.33 |
|
$ |
3.83 |
|
|
|
|
Price Range |
|
||||
|
2015 Fiscal Year |
|
High |
|
Low |
|
||
|
First Quarter |
|
$ |
8.03 |
|
$ |
6.78 |
|
|
Second Quarter |
|
$ |
12.44 |
|
$ |
6.45 |
|
|
Third Quarter |
|
$ |
12.17 |
|
$ |
5.70 |
|
|
Fourth Quarter |
|
$ |
8.11 |
|
$ |
5.72 |
|
Holders of Record
The closing price of our common shares as reported on the NASDAQ Global Market and the Toronto Stock Exchange on March 9, 2017 was $3.54 and $4.75 CAD, respectively. As of the close of business on March 9, 2017, there were approximately 1,149 holders of record of our common shares.
Dividends
We have not declared or paid any cash dividends on common shares to date. We currently intend to retain all earnings to support operations and do not intend to pay cash dividends on our common shares for the foreseeable future. In addition, our ability to pay cash dividends is currently prohibited by the Facility Agreement, subject to certain exceptions. Any future determination to pay dividends on our common shares will be made by the board of directors and will depend on, among other things, the Company’s results of operations, current and anticipated cash requirements and surplus, financial condition, contractual restrictions and financing agreement covenants, solvency tests imposed by corporate law and other factors that the board of directors may deem relevant.
Restrictions on Share Ownership by Non-Canadians
There are no limitations under the laws of Canada or in our organizational documents on the right of foreigners to hold or vote our securities, except that the Investment Canada Act (Canada) (the “Investment Canada Act”) may require review and approval by the Minister of Innovation, Science and Economic Development (Canada) of certain acquisitions of “control” of the Company by a “non-Canadian.”
50
Investment Canada Act
An acquisition of control of a Canadian business by a non-Canadian is either reviewable (a “Reviewable Transaction”), in which case it is subject to both a reporting obligation and an approval process, or notifiable, in which case it is subject to only a reporting obligation. In the case of a Reviewable Transaction, the non-Canadian acquirer must submit an application for review with the prescribed information. The responsible Minister is then required to determine whether the Reviewable Transaction is likely to be of net benefit to Canada, taking into account the assessment factors specified in the Investment Canada Act and any written undertakings that may have been given by the non-Canadian acquirer.
Any investment by a non-Canadian in a Canadian business, even where control has not been acquired, can be reviewed on grounds of whether it may be injurious to national security. Where an investment is determined to be injurious to national security, Canada’s Cabinet can prohibit closing or, if closed, can order the investor to divest control. Short of a prohibition or divestment order, Canada’s Cabinet can impose terms or conditions on the investment or can require the investor to provide binding undertakings to remove the national security concern.
Competition Act
Part IX of the Competition Act (Canada) (the “Competition Act”) requires that pre-merger notification filings be submitted to the Commissioner of Competition (the “Commissioner”) in respect of certain types of transactions that exceed certain prescribed thresholds. If a proposed transaction exceeds such thresholds, subject to certain exceptions, notification filings must be submitted to the Commissioner and the statutory waiting period must expire or be terminated early or waived by the Commissioner before the transaction can be completed.
All mergers, regardless of whether they are subject to Part IX of the Competition Act, are subject to the substantive mergers provisions under Section 92 of the Competition Act. In particular, the Commissioner may challenge a transaction before the Competition Tribunal where the transaction prevents or lessens, or is likely to prevent or lessen, competition substantially in a market. The Commissioner may not make an application to the Competition Tribunal under Section 92 of the Competition Act more than one year after the transaction has been substantially completed.
Equity Compensation Plans
See Item 12 of Part III of this Annual Report on Form 10-K.
51
Stock Performance Graph
The graph below matches Aralez’s cumulative five-year total shareholder return on common shares with the cumulative total returns of the NASDAQ Composite Index, the NASDAQ Pharmaceutical Index, and the NASDAQ Biotechnology Index. The graph tracks the performance of a $100 investment in Aralez’s common shares and in each index (with the reinvestment of all dividends) from December 31, 2011 to December 31, 2016.
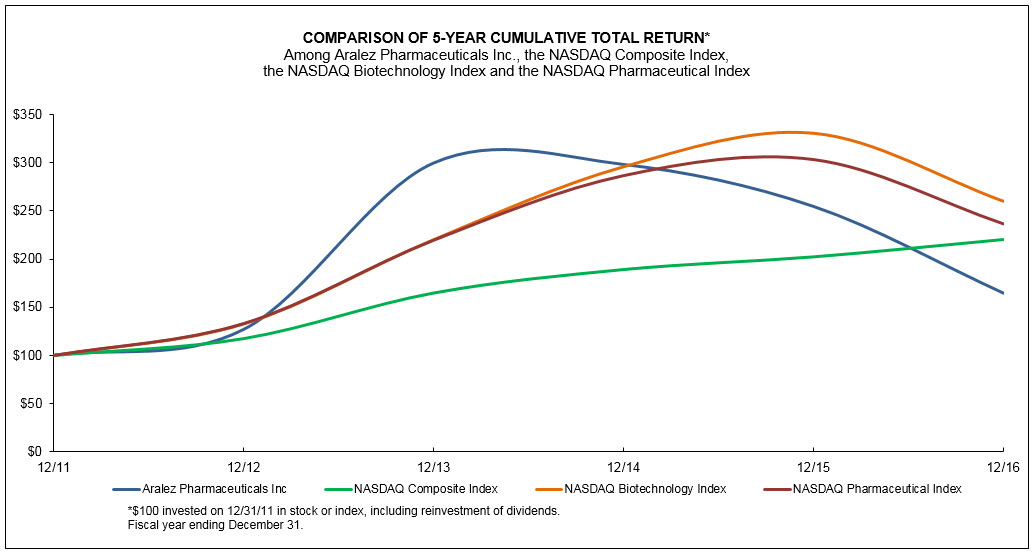
|
|
|
12/11 |
|
12/12 |
|
12/13 |
|
12/14 |
|
12/15 |
|
12/16 |
|
|
Aralez Pharmaceuticals Inc. |
|
100.00 |
|
126.84 |
|
300.11 |
|
298.61 |
|
254.94 |
|
164.61 |
|
|
NASDAQ Composite |
|
100.00 |
|
117.45 |
|
164.57 |
|
188.84 |
|
201.98 |
|
219.89 |
|
|
NASDAQ Pharmaceutical |
|
100.00 |
|
133.05 |
|
219.35 |
|
286.31 |
|
302.95 |
|
236.32 |
|
|
NASDAQ Biotechnology |
|
100.00 |
|
132.74 |
|
220.37 |
|
296.19 |
|
331.05 |
|
260.37 |
|
The stock price performance included in this graph is not necessarily indicative of future stock price performance.
Recent Sales of Unregistered Securities
There were no sales of unregistered securities during the year ended December 31, 2016.
Issuer Purchases of Equity Securities
There were no repurchases of equity securities during the fourth quarter of 2016.
52
ITEM 6. Selected Financial Data
The following selected financial data are derived from the audited financial statements of Aralez for the year ended December 31, 2016 and Pozen for the years ended December 31, 2012 through December 31, 2015. The data should be read in conjunction with the financial statements, related notes and other financial information included (and incorporated by reference) herein.
|
|
|
For the Years Ended December 31, |
|
|||||||||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
2013 |
|
2012 |
|
|||||
|
|
|
(in thousands) |
|
|||||||||||||
|
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product revenues, net |
|
$ |
25,432 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Other revenues |
|
|
28,838 |
|
|
21,391 |
|
|
32,394 |
|
|
10,322 |
|
$ |
5,349 |
|
|
Total revenues, net |
|
|
54,270 |
|
|
21,391 |
|
|
32,394 |
|
|
10,322 |
|
|
5,349 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of product revenues (exclusive of amortization shown separately below) |
|
|
11,765 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Change in fair value of contingent consideration |
|
|
750 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Amortization of intangible assets |
|
|
12,591 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Impairment of intangible assets |
|
|
4,368 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Sales, general and administrative |
|
|
118,548 |
|
|
50,345 |
|
|
10,079 |
|
|
17,161 |
|
|
19,024 |
|
|
Research and development |
|
|
8,832 |
|
|
8,512 |
|
|
5,740 |
|
|
9,945 |
|
|
11,867 |
|
|
Total costs and expenses |
|
|
156,854 |
|
|
58,857 |
|
|
15,819 |
|
|
27,106 |
|
|
30,891 |
|
|
(Loss) income from operations |
|
|
(102,584) |
|
|
(37,466) |
|
|
16,575 |
|
|
(16,784) |
|
|
(25,542) |
|
|
Interest expense |
|
|
(6,141) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Other (expense) income, net |
|
|
5,683 |
|
|
(143) |
|
|
3,099 |
|
|
76 |
|
|
259 |
|
|
(Loss) income before income tax expense |
|
|
(103,042) |
|
|
(37,609) |
|
|
19,674 |
|
|
(16,708) |
|
|
(25,283) |
|
|
Provision for (benefit from) income taxes |
|
|
(64) |
|
|
174 |
|
|
— |
|
|
— |
|
|
— |
|
|
Net (loss) income |
|
$ |
(102,978) |
|
$ |
(37,783) |
|
$ |
19,674 |
|
$ |
(16,708) |
|
$ |
(25,283) |
|
|
Basic net (loss) income per common share |
|
$ |
(1.67) |
|
$ |
(1.16) |
|
$ |
0.63 |
|
$ |
(0.55) |
|
$ |
(0.84) |
|
|
Shares used in computing basic net (loss) income per common share |
|
|
61,831 |
|
|
32,590 |
|
|
31,360 |
|
|
30,450 |
|
|
30,092 |
|
|
Diluted net (loss) income per common share |
|
$ |
(1.74) |
|
$ |
(1.16) |
|
$ |
0.60 |
|
$ |
(0.55) |
|
$ |
(0.84) |
|
|
Shares used in computing diluted net (loss) income per common share |
|
|
61,883 |
|
|
32,590 |
|
|
32,811 |
|
|
30,450 |
|
|
30,092 |
|
|
|
|
December 31, |
|
|||||||||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
2013 |
|
2012 |
|
|||||
|
|
|
(in thousands) |
|
|||||||||||||
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
64,943 |
|
$ |
24,816 |
|
$ |
40,582 |
|
$ |
32,828 |
|
$ |
87,314 |
|
|
Total assets |
|
|
517,377 |
|
|
32,258 |
|
|
50,454 |
|
|
35,334 |
|
|
89,597 |
|
|
Total liabilities |
|
|
397,891 |
|
|
17,475 |
|
|
3,713 |
|
|
17,546 |
|
|
5,519 |
|
|
Accumulated deficit |
|
|
(237,666) |
|
|
(134,688) |
|
|
(96,904) |
|
|
(116,579) |
|
|
(99,871) |
|
|
Total shareholders’ equity |
|
|
119,486 |
|
|
14,783 |
|
|
46,741 |
|
|
17,789 |
|
|
84,077 |
|
53
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Overview
Aralez is a global specialty pharmaceutical company focused on delivering meaningful products to improve patients’ lives while focusing on creating shareholder value by acquiring, developing and commercializing products primarily in cardiovascular, pain and other specialty areas. The Company currently commercializes a number of cardiovascular products in the United States as well as products for cardiovascular, pain management, dermatological and certain other indications in Canada. In addition, the Company outlicenses certain products in exchange for royalties and/or other payments.
Results of Operations
Revenues
The following table sets forth net revenues for the periods presented:
|
|
|
For the Years Ended December 31, |
|
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
|
|
(in thousands) |
|
|||||||
|
Product revenues, net |
|
$ |
25,432 |
|
$ |
— |
|
$ |
— |
|
|
Other revenues |
|
|
28,838 |
|
|
21,391 |
|
|
32,394 |
|
|
Total revenues, net |
|
$ |
54,270 |
|
$ |
21,391 |
|
$ |
32,394 |
|
Year ended December 31, 2016 compared to the year ended December 31, 2015
Product Revenues, net
Net product revenues of $25.4 million for the year ended December 31, 2016 relate to the product portfolio we acquired with the acquisition of Tribute on February 5, 2016 and primarily include revenues from sales of Bezalip, Fiorinal, Soriatane, Proferrin and Fibricor. There were no product revenues for the year ended December 31, 2015 as the acquisition of Tribute occurred in February 2016.
Other Revenues
Other revenues were $28.8 million for the year ended December 31, 2016, as compared to $21.4 million for the year ended December 31, 2015. Other revenues for the periods presented relate primarily to royalties earned on net sales of VIMOVO, Zontivity and Toprol-XL and the AG by our commercialization partners. Royalty revenues increased from $21.4 million to $28.8 million for the years ended December 31, 2015 and 2016, respectively, as a result of royalties earned on net sales of Zontivity from September 6, 2016 and Toprol-XL and the AG from October 31, 2016, as such products are sold on our behalf by Merck and AstraZeneca, respectively, for an interim period post acquisition.
Year ended December 31, 2015 compared to the year ended December 31, 2014
Other Revenues
Other revenues were $21.4 million for the year ended December 31, 2015, as compared to $32.4 million for the year ended December 31, 2014. This decrease was primarily due to the inclusion of $11.3 million in amortization of upfront licensing fees in 2014. Royalty revenue for the year ended December 31, 2015 consisted of $21.4 million of royalty revenue compared to $21.1 million of royalty revenue for the year ended December 31, 2014. Our licensing and collaboration agreements have terms that include upfront payments upon contract signing and additional payments if and when certain milestones in the product development or related milestones are achieved.
54
Costs and Expenses
The following table sets forth costs and expenses for the periods presented:
|
|
|
For the Years Ended December 31, |
|
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
|
|
(in thousands) |
|
|||||||
|
Cost of product revenues (exclusive of amortization shown separately below) |
|
$ |
11,765 |
|
$ |
— |
|
$ |
— |
|
|
Change in fair value of contingent consideration |
|
|
750 |
|
|
— |
|
|
— |
|
|
Amortization of intangible assets |
|
|
12,591 |
|
|
— |
|
|
— |
|
|
Impairment of intangible assets |
|
|
4,368 |
|
|
— |
|
|
— |
|
|
Selling, general and administrative |
|
|
118,548 |
|
|
50,345 |
|
|
10,079 |
|
|
Research and development |
|
|
8,832 |
|
|
8,512 |
|
|
5,740 |
|
|
Total costs and expenses |
|
$ |
156,854 |
|
$ |
58,857 |
|
$ |
15,819 |
|
Year ended December 31, 2016 compared to the year ended December 31, 2015
Cost of Product Revenues
Cost of product revenues were $11.8 million for the year ended December 31, 2016, which includes $1.5 million of inventory fair value step-up amortization. There were no cost of product revenues for the year ended December 31, 2015, as the acquisition of Tribute occurred in February 2016. There are no cost of revenues related to our other revenues.
Change in Fair Value of Contingent Consideration
The change in fair value of contingent consideration of $0.8 million relates to the passage of time on the contingent consideration recorded in the September 2016 Zontivity acquisition.
Amortization of Intangible Assets
Amortization of acquired intangible assets is recognized straight line over the estimated useful life of the related assets acquired in the Merger and the acquisitions of Zontivity and Toprol-XL and the AG. Amortization expense of $12.6 million for the year ended December 31, 2016 included expenses incurred from February 5, 2016, the closing date of the Merger, with respect to assets acquired in the Merger, from September 6, 2016, the closing date of the Zontivity acquisition, with respect to the Zontivity asset and from October 31, 2016, the closing date of the Toprol-XL acquisition, with respect to the Toprol-XL asset. There was no amortization of intangible assets for the year ended December 31, 2015.
Impairment of Intangible Assets
In the fourth quarter of 2016, an impairment charge of $4.4 million was recorded to write off the remaining carrying value of IPR&D recorded in the Tribute acquisition and to write down to fair value one product recorded in the Tribute acquisition, based on estimated cash flows for 2017, after which our exclusive distribution agreement is terminated.
Selling, General and Administrative Expenses
Selling, general and administrative expenses were $118.5 million and $50.3 million for the years ended December 31, 2016 and 2015, respectively. The $68.2 million increase in selling, general and administrative expenses was primarily driven by: $28.7 million of commercialization costs incurred in the United States, including (i) $13.6 million in promotional expenses, principally related to Yosprala, (ii) $9.1 million related to the build out of the U.S. sales force, and (iii) $6.0 million related to the build out of the commercial infrastructure; $12.0 million for excise tax equalization payments; $14.1 million of costs incurred to support our global corporate structure; $13.7 million of expenses related to our Canadian operation; and a $4.6 million increase in share-based compensation expense. These increases in expenses were partially offset by a decrease in severance and retention expenses of approximately $3.9
55
million compared to the year ended December 31, 2015; a decrease of $0.5 million for other expenses, including the termination of previous Pozen employees; and a $0.5 million decrease in transaction fees.
Research and Development Expenses
Research and development expenses were generally consistent at $8.8 million and $8.5 million for the years ended December 31, 2016 and 2015, respectively.
Year ended December 31, 2015 compared to the year ended December 31, 2014
Selling, General and Administrative Expenses
Selling, general and administrative expenses were $50.3 million and $10.1 million for the years ended December 31, 2015 and 2014, respectively. The $40.2 million increase reflects increased activities, which included a $6.3 million expense related to our former President and Chief Executive Officer’s separation agreement, $11.5 million transaction-related expenses related to the acquisition of Tribute, $2.9 million increase in employee severance and retention expense, $9.1 million of increased staffing costs, including non-cash stock-based compensation expense of $4.1 million, $10.2 million increase in pre-commercialization activities, and $0.2 million in other activities, as compared to the same period in 2014. Selling, general and administrative expenses consisted primarily of the costs of administrative personnel, facility infrastructure, business development and pre-commercialization expenses, and public company activities.
Research and Development Expenses
Research and development expenses were $8.5 million and $5.7 million for the years ended December 31, 2015 and 2014, respectively. The $2.8 million increase was due primarily to a $2.1 million increase in direct development costs for Yosprala and other product development activities during the year ended December 31, 2015. We have included in our research and development total expenses the departmental personnel costs associated with our research and development activities and direct costs associated with pharmaceutical development, clinical trials, toxicology activities and regulatory matters.
Interest and Other Income (Expense), net
The following table sets forth interest expense and other (expense) income, net for the periods presented:
|
|
|
For the Years Ended December 31, |
|
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
|
|
(in thousands) |
|
|||||||
|
Interest expense |
|
$ |
(6,141) |
|
$ |
— |
|
$ |
— |
|
|
Other income (expense), net |
|
|
5,683 |
|
|
(143) |
|
|
3,099 |
|
|
Total interest and other (expense) income, net |
|
|
(458) |
|
|
(143) |
|
|
3,099 |
|
Year ended December 31, 2016 compared to the year ended December 31, 2015
Interest Expense
Interest expense for the year ended December 31, 2016 was $6.1 million, primarily due to the issuance of $75.0 million aggregate principal amount of our 2.5% senior secured convertible notes in February 2016 and the October 31, 2016 borrowing of $200 million under a credit facility under the Facility Agreement with an interest rate of 12.5%. There was no interest expense for the year ended December 31, 2015.
Other Income (Expense), net
Other income, net for the year ended December 31, 2016, was $5.7 million, principally related to a $4.7 million change in the fair value of the warrants liability acquired from Tribute during the period and a $0.9 million gain on the reversal of an assumed liability in the Merger due to a contract renegotiation, partially offset by a $0.6 million loss on foreign exchange. The decrease in the fair value of the warrants liability was primarily driven by the decrease in our share price, which is an input into the Black-Scholes valuation model used to estimate the fair value of the warrants as of
56
December 31, 2016, as compared to the year ended December 31, 2015, in which other expense, net was $0.1 million, related primarily to the sale of the Pernix warrant.
Year ended December 31, 2015 compared to the year ended December 31, 2014
Other Income (Expense), net
Other expense, net for the year ended December 31, 2015 was $0.1 million, related primarily to the sale of the Pernix warrant, as compared to the year ended December 31, 2014, in which other income, net was $3.1 million and included a $2.4 million short-term investment gain related to the valuation of the Pernix warrants.
Liquidity and Capital Resources
The Company’s principal sources of liquidity are cash generated from the royalty payments received from our commercialization partners for net sales of VIMOVO, the operating income of Tribute, sales of Fibricor and its authorized generic, Yosprala, Zontivity, and Toprol-XL and the AG, and the financings executed on February 5, 2016 and October 31, 2016. Our principal liquidity requirements are for working capital; operational expenses; commercialization activities for products, including Yosprala, Zontivity, Toprol-XL and Fibricor, and product candidates; capital expenditures and debt service payments.
At December 31, 2016, we had $64.9 million of cash and cash equivalents compared to $24.8 million at December 31, 2015. We believe that we have sufficient cash and cash equivalents together with cash expected to be generated from operations, including royalty receipts, to fund our operations for at least the next twelve months, including (i) commercialization of products, (ii) relaunch of Zontivity planned for 2017, (iii) payment of contractual obligations, including any royalty payments and potential milestone payments that may become due, (iv) interest payments on our indebtedness, and (v) planned capital expenditures.
We expect to incur significant expenses in the future for the continued commercialization of our products, the relaunch of Zontivity and investments in other product opportunities and business development activities.
In connection with the Toprol-XL Asset Purchase Agreement, on October 3, 2016, the Credit Parties and the requisite lenders party to the Facility Agreement entered into a Limited Consent (the “Credit Agreement Consent”) pursuant to which such lenders consented to the Company entering into the Toprol-XL Asset Purchase Agreement and to the consummation of the transactions contemplated thereby. Pursuant to the Credit Agreement Consent, the lenders under the Facility Agreement agreed that they and/or affiliated funds will have available sufficient capital to make additional loans to Aralez in an aggregate amount of up to $250 million for the payment of the purchase price of any acquisitions permitted by the terms of the Facility Agreement (as modified by the Credit Agreement Consent) with respect to target businesses mutually approved by, and as otherwise mutually agreed upon, by the Company and the lenders under the Facility Agreement, subject to the satisfaction of certain conditions set forth in the Facility Agreement. Any such loans (to the extent made available) may be borrowed in one or more advances at any time prior to April 3, 2018.
To the extent our capital resources are insufficient to meet future operating requirements or business development activities, we may need to raise additional capital, reduce planned expenditures, or incur indebtedness. If we require additional financing in the future, we cannot assure you that it will be available to us on favorable terms, or at all, particularly if the credit and financial markets are constrained at the time we require funding.
Borrowings and Other Liabilities
At December 31, 2016, we had $75.0 million aggregate principal outstanding related to our 2.5% senior secured convertible notes due February 2022 (the “2022 Notes”) issued to certain lenders under the Facility Agreement in connection with the closing of the Merger and $200.0 million outstanding under a credit facility under the Facility Agreement, due on October 31, 2022, with an interest rate of 12.5% per annum.
See Note 9, “Debt,” in the accompanying notes to consolidated financial statements for additional information.
57
Repurchases of Common Shares
From time to time, our Board of Directors may authorize us to repurchase our common shares, subject to compliance with, among other things, the Facility Agreement. If and when our Board of Directors should determine to authorize any such action, it would be on terms and under market conditions that the Board of Directors determines are in the best interest of Aralez and its shareholders. Any such repurchases could deplete some of our cash resources.
Cash Flows
Operating Activities
Net cash used in operating activities was $83.7 million for the year ended December 31, 2016 compared to $16.8 million for the year ended December 31, 2015. The increase in cash used in operating activities was primarily due to pre-commercialization expenses incurred for the launch of Yosprala and costs related to the build out and support of the global corporate infrastructure. In addition, net cash used in operating activities included expenses related to the acquisition of Tribute, including payments of transaction and product acquisition-related expenses of approximately $15.5 million, excise tax equalization payments of $12.0 million, and severance payments of $6.6 million.
Net cash used in operating activities was $16.8 million for the year ended December 31, 2015 compared to net cash provided by operating activities of $0.4 million for the year ended December 31, 2014. The increase in cash used in operating activities primarily relates to transaction costs, severance and other merger-related expenses resulting primarily from the acquisition of Tribute.
Investing Activities
Net cash used in investing activities was $222.8 million for the year ended December 31, 2016 compared to net cash provided by investing activities of $2.2 million for the year ended December 31, 2015. Net cash used in investing activities for the year ended December 31, 2016, principally related to $175.0 million of cash consideration paid to AstraZeneca as an initial upfront payment for the Toprol-XL acquisition, $25.0 million of cash consideration paid to Merck as an initial upfront payment for the Zontivity acquisition, $17.9 million of cash consideration used to consummate the Merger, consisting of the repayment of Tribute indebtedness, net of cash acquired, $4.2 million of cash paid for the purchase of property and equipment and $0.7 million of cash payments for intangible assets. For the year ended December 31, 2015, $2.5 million was received for the sale of warrants.
Net cash used in investing activities was $2.2 million for the year ended December 31, 2015 compared to net cash used in investing activities of nil for the year ended December 31, 2014.
Financing Activities
Net cash provided by financing activities for the year ended December 31, 2016 was $346.3 million compared to net cash used in financing activities of $1.2 million for the year ended December 31, 2015. Net cash provided by financing activities for the year ended December 31, 2016 included the receipt of $200.0 million from borrowings under a credit facility under the Facility Agreement, $75.0 million from the issuance of the 2022 Notes and $75.0 million from the issuance of equity to certain investors, net of issuance costs of $0.8 million, partially offset by $3.9 million used for the repayment of a note issued to the prior owners of Medical Futures Inc., a company acquired by Tribute in 2015. See Note 9, “Debt”, in the accompanying notes to the consolidated financial statements.
Net cash used in financing activities for the year ended December 31, 2015 was $1.2 million compared to net cash provided by financing activities of $7.4 million for the year ended December 31, 2014. The net cash provided by financing activities for the year ended December 31, 2014 was primarily from the issuance of equity.
Commitments and Contingencies
Legal Proceedings
See Note 13, “Commitments and Contingencies,” in the accompanying notes to consolidated financial statements.
58
Contractual Obligations
The table below presents a summary of our contractual obligations at December 31, 2016 (in thousands):
|
|
|
Payments Due By Period |
|
|||||||||||||
|
|
|
|
|
Within |
|
|
|
|
|
More than |
|
|||||
|
Contractual Obligations (1) |
|
Total |
|
1 year |
|
1-3 Years |
|
3-5 Years |
|
5 years |
|
|||||
|
2022 Notes – principal (2) |
|
$ |
75,000 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
75,000 |
|
|
2022 Notes – interest (2) |
|
|
10,038 |
|
|
1,875 |
|
|
3,750 |
|
|
3,755 |
|
|
658 |
|
|
Credit Facility - principal (3) |
|
|
200,000 |
|
|
— |
|
|
— |
|
|
— |
|
|
200,000 |
|
|
Credit Facility - interest (3) |
|
|
150,205 |
|
|
22,945 |
|
|
50,000 |
|
|
50,068 |
|
|
27,192 |
|
|
Operating lease obligations (4) |
|
|
19,461 |
|
|
1,374 |
|
|
4,492 |
|
|
4,002 |
|
|
9,593 |
|
|
Other (5) |
|
|
4,612 |
|
|
3,873 |
|
|
211 |
|
|
211 |
|
|
317 |
|
|
Total |
|
$ |
459,316 |
|
$ |
30,067 |
|
$ |
58,453 |
|
$ |
58,036 |
|
$ |
312,760 |
|
|
(1) |
This table does not include potential future milestone payments, royalty or profit-share obligations to third parties under asset purchase, product development, license and other agreements to the extent that the timing and likelihood of such milestone payments are not known, and, in the case of royalty and profit-share obligations, if the amount of such obligations are not reasonably estimable, as discussed below. |
|
(2) |
The interest expense for the 2022 Notes includes the fixed-rate 2.5% per annum interest payable on the $75.0 million principal outstanding as of December 31, 2016. The table above assumes no conversions prior to maturity. |
|
(3) |
The interest expense on the borrowings under the Facility Agreement includes the fixed-rate 12.5% per annum interest payable on the $200.0 million currently outstanding. |
|
(4) |
Amounts represent lease obligations existing at December 31, 2016, primarily for office space. During the year ended December 31, 2016, we entered into lease agreements for our new global headquarters in Mississauga, Ontario, Canada, for our U.S. headquarters in Princeton, New Jersey, and for our Irish headquarters in Dublin, Ireland. The table above includes lease commitments for the full term of the leases under the respective agreements. Certain of such lease agreements may be terminated before the full term, including the agreement for the Princeton, New Jersey lease, which may be terminated after seven years in consideration of an early termination penalty equal to four months of rent. |
|
(5) |
Other consists of open purchase orders under agreements to purchase goods or services and non-cancelable commitments to third parties for minimum royalties payable and minimum purchase obligations under various license, distribution and manufacturing agreements. |
We have various agreements with third-parties with contingent consideration and milestone payments that are potentially payable by or to us, as more fully described in Note 2, “Business Combinations and Acquisitions” and Note 3, “Business Agreements,” in the accompanying notes to the consolidated financial statements. These payments are contingent upon achieving development, regulatory and/or sales-based milestones that may or may not ever be achieved. Therefore, our requirement to make or receive such payments in the future or at all is highly uncertain. These agreements include:
|
· |
In connection with our acquisition of Toprol-XL, we are obligated to pay certain milestone payments upon the occurrence of certain milestone events based on the annual aggregate net sales of Toprol-XL and the AG and other contingent events, which in no event will exceed $48 million in the aggregate and royalty payments of (A) 15% of total quarterly net sales of Toprol-XL and any other authorized or owned generic version of Toprol-XL that is marketed, distributed or sold by or on behalf of, or under a license or sublicense from, Aralez (other than the AG), and (B) 15% of quarterly net sales of the AG, but for purposes of royalty payments and clause (B) only, net sales do not include the supply price paid for the AG by Aralez Ireland to AstraZeneca under the supply agreement entered into between Aralez Ireland and AstraZeneca in respect of the applicable period. |
59
|
· |
In connection with our acquisition of Zontivity, we are obligated to pay certain milestone payments upon the occurrence of certain milestone events based on the annual aggregate net sales of Zontivity, any combination product containing vorapaxar sulphate and one or more other active pharmaceutical ingredients or any line extension thereof, which in no event will exceed $80 million in the aggregate and royalty payments in the low double digits based on the annual aggregate net sales of Zontivity, any combination product containing vorapaxar sulphate and one or more other active pharmaceutical ingredients or any line extension thereof. |
|
· |
Under an exclusive license and supply agreement with Faes Farma, S.A. (“Faes”), a Spanish pharmaceutical company, we have the exclusive right to sell bilastine, a product for the treatment of allergic rhinitis and chronic idiopathic urticaria (hives) in Canada, which is now named Blexten. We will owe up to $1.7 million in sales-based milestone payments to Faes if certain sales targets are met. |
|
· |
Under a license agreement with Nautilus Neurosciences, Inc. (“Nautilus”), which was acquired by Depomed, Inc. in December 2013, we have the exclusive rights to develop, register, promote, manufacture, use, market, distribute and sell Cambia in Canada. Up to $6.0 million in sales-based milestone payments may be payable over time. |
|
· |
We have a product development and profit share agreement with Actavis Group PTC ehf (“Actavis”) to develop, obtain regulatory approval of and market Bezalip SR in the United States. We may owe a milestone payment of $5.0 million to Actavis in the event that we pursue and obtain regulatory approval to market Bezalip SR in the United States. |
|
· |
In connection with our acquisition of Fibricor and its authorized generic in the United States, we may be obligated to pay up to $4.5 million in milestone payments based on annual net sales of Fibricor and its authorized generic as well as royalties ranging from the high single digits to low double digits based on annual net sales of such products. |
Off-Balance Sheet Arrangements
At December 31, 2016, we have not entered into any off-balance sheet arrangements, as contemplated by Item 303(a)(4) of Regulation S-K.
Critical Accounting Policies and Estimates
We prepare our consolidated financial statements in conformity with accounting principles generally accepted in the United States, or GAAP. The preparation of consolidated financial statements requires estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. Actual results may differ from these estimates. The accounting policies that we believe are most critical to fully understand our consolidated financial statements include those relating to: revenue recognition; intangible assets; contingent consideration; income taxes; accounting for share-based compensation; and fair value measurements.
Revenue Recognition
Principal sources of revenue are (i) product sales from the product portfolio acquired with our acquisition of Tribute, and (ii) royalty revenues from sales of VIMOVO by our commercialization partners. In all instances, revenue is recognized only when the price is fixed or determinable, persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, and collectibility of the resulting receivable is reasonably assured.
Product Sales
Revenues from the sale of products acquired in connection with our acquisition of Tribute are distributed through Canadian wholesalers to Canadian retail pharmacies and are recorded net of discounts, wholesaler fees, chargebacks, rebates, returns and allowances, and are recognized when legal title to the goods and risk of ownership has been passed to the customer, which in this case is the Canadian wholesaler. Discounts, wholesaler fees, chargebacks,
60
rebates, returns and allowances are not significant for these product sales and are not expected to be significant in the future given the Canadian marketplace.
Revenues from the sale of Yosprala in the United States are recorded on a sell through method since we do not have the historical experience to estimate returns. As such, we defer revenue and costs of inventory for all Yosprala products shipped to wholesalers in the United States until the product is sold through to the end customer. Revenue recorded since we launched Yosprala in the United States is not significant during the fiscal year 2016. Product sales from all other U.S. products (Fibricor) are recorded on a sell in method and were not significant during the fiscal year 2016.
All of our products have a returns policy that allows the customer to return pharmaceutical products within a specified period of time both prior to and subsequent to the product’s expiration date. Our estimate of the provision for returns for those products that use a sell in method is analyzed quarterly and is based upon many factors, including historical experience of actual returns and analysis of the level of inventory in the distribution channel, if any. We believe that the reserves we have established are reasonable based upon current facts and circumstances. Applying different judgments to the same facts and circumstances could result in the estimated amount for reserves to vary. If actual results vary with respect to our reserves, we may need to adjust our estimates, which could have a material effect on our results of operations in the period of adjustment.
Other Revenues
Other revenues principally include revenues from licensing arrangements with other biopharmaceutical companies (principally royalty revenues from VIMOVO), including milestones payments and royalties. Revenue from royalties is recognized when the Company has fulfilled the terms in accordance with contractual agreements and has no future obligation, and the amount of the royalty fee is determinable. Royalty revenue that is reasonably estimable and determinable is recognized based on estimates utilizing information reported to us by our commercialization partners.
Other revenues also include net revenues from sales of Zontivity, from its acquisition date, recognized net of related cost of product revenues and fees paid to Merck under a transition services agreement in effect for up to twelve months from the date of acquisition. Similarly, we also include net revenues from sales of Toprol-XL and the AG from its acquisition date, recognized net of related cost of product revenues and fees paid to AstraZeneca under a transition services agreement in effect until July 31, 2017. We record these revenues net of related cost since we are not the principal in the arrangements and expect to record this revenue similar to a royalty arrangement until we are deemed to be the principal in the sales and marketing of these products, at which point we will record net sales and costs of revenue separately.
Intangible Assets
Goodwill
Goodwill relates to amounts that arose in connection with the acquisitions of Tribute, Zontivity and Toprol-XL and the AG. Goodwill represents the excess of the purchase price over the fair value of the net assets acquired when accounted for using the acquisition method of accounting for business combinations. Goodwill is not amortized but is evaluated for impairment on an annual basis, in the fourth quarter, or more frequently if an event occurs or circumstances change that would more-likely-than-not reduce the fair value of our reporting unit below its carrying amount.
In-Process Research and Development (“IPR&D”)
IPR&D acquired in a business combination is capitalized on our consolidated balance sheets at its acquisition-date fair value. Until the underlying project is completed, these assets are accounted for as indefinite-lived intangible assets and are subject to impairment testing. Once the project is completed, the carrying value of the IPR&D is reclassified to other intangible assets, net and is amortized over the estimated useful life of the asset. Post-acquisition research and development expenses related to the IPR&D projects are expensed as incurred.
IPR&D is tested for impairment on an annual basis or more frequently if impairment indicators are present. If IPR&D becomes impaired, the carrying value of the IPR&D is written down to its revised fair value with the related impairment charge recognized in the period in which the impairment occurs. The valuation techniques utilized in
61
performing the initial valuation of IPR&D or subsequent quantitative impairment tests incorporate significant assumptions and judgments to estimate the fair value. There is no IPR&D balance as of December 31, 2016.
Other Intangible Assets, net
Other intangible assets consist of acquired technology rights. The Company amortizes its intangible assets using the straight-line method over their estimated economic lives. Costs to obtain, maintain and defend the Company's patents are expensed as incurred. We will evaluate the potential impairment of other intangible assets if events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives of these assets are no longer appropriate. Events giving rise to impairment are an inherent risk in our industry and many factors cannot be predicted. Factors that we consider in deciding when to perform an impairment review include significant changes in our forecasted projections for the asset or asset group for reasons including, but not limited to, significant under-performance of a product in relation to expectations, significant changes or planned changes in our use of the assets, significant negative industry or economic trends, and new or competing products that enter the marketplace. The impairment test is based on a comparison of the undiscounted cash flows expected to be generated from the use of the asset group and its eventual disposition to the carrying value of the asset group. If impairment is indicated, the asset is written down by the amount by which the carrying value of the asset exceeds the related fair value of the asset with the related impairment charge recognized within the statements of operations. Such impairment charges may be material to our results. The valuation techniques utilized in performing the initial valuation of other intangible assets or subsequent quantitative impairment tests incorporate significant assumptions and judgments to estimate the fair value. The use of different valuation techniques or assumptions could result in significantly different fair value estimates.
Contingent Consideration
Certain of the Company’s business acquisitions involve the potential for future payment of consideration that is contingent upon the achievement of operational and commercial milestones and royalty payments on future product sales. The fair value of contingent consideration liabilities is determined at the acquisition date using unobservable inputs. These inputs include the estimated amount and timing of projected cash flows, the probability of success (achievement of the contingent event) and the risk-adjusted discount rate used to present value the probability-weighted cash flows. Subsequent to the acquisition date, at each reporting period, the contingent consideration liability is remeasured at current fair value with changes recorded in the consolidated statements of operations. Changes in any of the inputs may result in a significantly different fair value adjustment.
Income Taxes
We account for income taxes using the liability method in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”), Topic 740, “Income Taxes” (“ASC 740”). Deferred tax assets and liabilities are determined based on temporary differences between financial reporting and tax basis assets and liabilities and are measured by applying enacted rates and laws to taxable years in which differences are expected to be recovered or settled. Further, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that the rate changes. A valuation allowance is required when it is “more-likely-than-not” that all or a portion of deferred tax assets will not be realized. Since our inception, we have incurred substantial cumulative losses and may incur substantial and recurring losses in future periods. The utilization of the loss carryforwards to reduce future income taxes will depend on our ability to generate sufficient taxable income prior to the expiration of the loss carryforwards. In addition, the maximum annual use of net operating loss and research credit carryforwards is limited in certain situations where changes occur in stock ownership.
Aralez files federal and state income tax returns, as applicable, with the tax authorities in various jurisdictions including Canada, Ireland and the United States. Pozen is no longer subject to U.S. federal or North Carolina state income tax examinations by tax authorities for years before 2013. Tribute is no longer subject to Canadian income tax examinations by tax authorities for years before 2011. However, the loss and credit carryforwards generated by Pozen and Tribute may still be subject to change to the extent these losses and credits are utilized in a year that is subject to examination by tax authorities.
ASC 740 prescribes a comprehensive model for how a company should recognize, measure, present and disclose in its financial statements uncertain tax positions that the company has taken or expects to take on a tax return, including a decision whether to file or not file a return in a particular jurisdiction. Our financial statements reflect
62
expected future tax consequences of such positions presuming the taxing authorities’ full knowledge of the position and all relevant facts. We recognize any interest and penalties accrued related to unrecognized tax benefits as income tax expense.
Share-Based Compensation
We expense the fair value of employee share-based compensation over the employees' service periods, which are generally the vesting period of the equity award. For awards with performance conditions granted, we recognize compensation cost over the expected period to achieve the performance conditions, provided achievement of the performance conditions are deemed probable. Awards with market-based conditions are expensed over the service period regardless of whether achievement of the market condition is deemed probable or is ultimately achieved. Compensation expense is measured using the fair value of the award at the grant date, adjusted for estimated forfeitures.
In order to determine the fair value of option awards on the grant date, we use the Black-Scholes option pricing model. Inherent in this model are assumptions related to expected share price volatility, estimated option life, risk-free interest rate and dividend yield. Our expected share price volatility assumption is based on the historical volatility of our stock, which is obtained from public data sources. The expected life represents the weighted average period of time that share-based awards are expected to be outstanding giving consideration to vesting schedules, historical exercise patterns and post-vesting cancellations for terminated employees that have been exhibited historically, adjusted for specific factors that may influence future exercise patterns. The risk-free interest rate is based on factual data derived from public sources. We use a dividend yield of zero as we have no intention to pay cash dividends in the foreseeable future. For performance-based awards with market conditions, the Company used a Monte Carlo simulation model to determine the fair value of awards as of the grant date.
We estimate forfeitures based on our historical experience of pre-vesting cancellations for terminated employees. Our estimated forfeiture rate is applied to all equity awards, which includes option awards and restricted stock units, including performance share units. We believe that our estimates are based on outcomes that are reasonably likely to occur. To the extent actual forfeitures differ from our estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised.
Determining the appropriate amount to expense for performance-based awards based on the achievement of stated goals requires judgment, including forecasting future performance results. The estimate of expense is revised periodically based on the probability of achieving the required performance targets and adjustments are made as appropriate. The cumulative impact of any revisions is reflected in the period of change. If any applicable financial performance goals are not met, no compensation cost is recognized and any previously recognized compensation cost is reversed.
Fair Value Measurements
The accounting standard for fair value measurements defines fair value, establishes a framework for measuring fair value in accordance with GAAP, and requires detailed disclosures about fair value measurements. Under this standard, fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The valuation techniques are based on observable and unobservable inputs. Observable inputs reflect readily obtainable data from independent sources, while unobservable inputs reflect certain market assumptions. This standard classifies these inputs into the following hierarchy:
|
· |
Level 1 Inputs — Quoted prices for identical instruments in active markets. |
|
· |
Level 2 Inputs — Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations whose inputs are observable or whose significant value drivers are observable. |
|
· |
Level 3 Inputs — Instruments with primarily unobservable value drivers. |
The fair value hierarchy level is determined by asset class based on the lowest level of significant input. In periods of market inactivity, the observability of prices and inputs may be reduced for certain instruments. This condition could cause an instrument to be reclassified between levels.
63
The carrying amount of our cash and cash equivalents approximate its fair value due to the short-term nature of these amounts. The warrants liability and contingent consideration are our only liabilities carried at fair value, and we utilized Level 3 inputs to estimate fair value. The significant unobservable inputs used in the fair value measurement of our warrants liability, which uses a Black-Scholes valuation model, include the volatility of our common shares and the expected term. The significant unobservable inputs used in the fair value measurement of our contingent consideration liability include the estimated amount and timing of projected cash flows, the probability of success (achievement of the contingent event) and the risk-adjusted discount rate used to present value the probability-weighted cash flows. The use of different inputs could result in materially different fair value estimates.
Recent Accounting Pronouncements
See Note 1, “Organization, Basis of Presentation and Accounting Policies”, in the accompanying notes to consolidated financial statements within Item 8 of this report, which is incorporated herein by reference.
ITEM 7A. Quantitative and Qualitative Disclosures about Market Risk
Our cash on hand is invested in bank deposits and money market funds that invest primarily in short-term, highly-rated investments, including U.S. government securities, commercial paper and certificates of deposit guaranteed by banks and short-term corporate fixed income obligations and U.S. government and government agency obligations. Under our current policies, we do not use interest rate derivative instruments to manage our exposure to interest rate changes. Due to the short-term maturities of our investments, we do not believe that a decrease in market rates would have a significant negative impact on the value of our investment portfolio.
Aralez will face market risks attributable to fluctuations in foreign currency exchange rates and foreign currency exposure on the translation into U.S. dollars of the financial results of our operations in Canada and Europe. Both favorable and unfavorable foreign currency impacts to our foreign currency-denominated operating expenses are mitigated to a certain extent by the natural, opposite impact on our foreign currency-denominated revenue.
ITEM 8. Financial Statements and Supplementary Data
The financial information required by Item 8 is contained in Part IV, Item 15 of this Annual Report on Form 10-K.
ITEM 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
ITEM 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures designed to ensure information required to be disclosed in Company reports filed under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures are designed to provide reasonable assurance that information required to be disclosed in Company reports filed under the Exchange Act is accumulated and communicated to management, including the Company’s chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Our management, with the participation of our chief executive officer and chief financial officer, evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Annual Report on Form 10-K. Based on that evaluation, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K are functioning effectively to provide reasonable assurance that the information required to be disclosed by us in reports filed under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate to allow timely
64
decisions regarding disclosures. A controls system, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
Management’s Annual Report on Internal Control over Financial Reporting
Our management’s report on internal control over financial reporting procedures (as defined in Rule 13a-15(f) under the Exchange Act) is included with the financial statements reflected in Part IV, Item 15 of this Annual Report on Form 10-K and is incorporated herein by reference.
Changes in Internal Control over Financial Reporting
There were no changes in the Company’s internal control over financial reporting for the quarterly period or year ended December 31, 2016 identified in connection with the evaluation required by Rules 13a-15(e) and 15d-15(e) of the Exchange Act that materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
None.
65
ITEM 10. Directors, Executive Officers and Corporate Governance
Information required under this item relating to our board of directors, executive officers and corporate governance will be included in our definitive proxy statement for the 2017 Annual and Special Meeting of Shareholders, to be filed with the SEC and with the securities regulatory authorities in Canada within 120 days after the end of the year ended December 31, 2016 (the “2017 Proxy Statement”), and such required information is incorporated herein by reference.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics, which applies to our directors and employees (including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions). The text of our Code of Business Conduct and Ethics is posted in the “Corporate Governance” section of our website, www.aralez.com and under the Company’s profile at www.sedar.com. A copy of the Code of Business Conduct and Ethics can be obtained free of charge on our website. We intend to disclose on our website any amendments to, or waivers from, our Code of Business Conduct and Ethics that are required to be disclosed pursuant to the rules of the SEC, securities regulatory authorities in Canada, and the NASDAQ Global Market and the Toronto Stock Exchange.
ITEM 11. Executive Compensation
Information required under this item relating to executive compensation is incorporated herein by reference from information included in the 2017 Proxy Statement.
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters
Information required under this item relating to securities authorized for issuance under equity compensation plans and to security ownership of certain beneficial owners and management is incorporated herein by reference from information included in the 2017 Proxy Statement.
ITEM 13. Certain Relationships and Related Transactions and Director Independence
Information required under this item relating to certain relationships and transactions with related parties and about director independence is incorporated herein by reference from information included in the 2017 Proxy Statement.
ITEM 14. Principal Accounting Fees and Services
Information required under this item relating to the fees for professional services rendered by our independent accountants in 2016 and 2015 is incorporated herein by reference from information included in the 2017 Proxy Statement.
66
ITEM 15. Exhibits, Financial Statements Schedules
(a) Financial Statements
See accompanying index to Financial Statements.
(b) Financial Statement Schedules
All schedules have been omitted because the required information is included in the financial statements or the notes thereto, or is not applicable.
(c) Index to Exhibits
The following exhibits are filed or incorporated by reference as part of this Annual Report on Form 10-K:
|
Exhibit |
|
Exhibit Title |
| 2.1 |
|
Agreement and Plan of Merger and Arrangement, dated as of June 8, 2015, by and among Tribute Pharmaceuticals Canada Inc., Aguono Limited, Trafwell Limited, ARLZ US Acquisition Corp., ARLZ CA Acquisition Corp. and POZEN Inc. (incorporated by reference to Exhibit 2.1 to POZEN Inc.’s Current Report on Form 8-K filed June 11, 2015). |
|
|
|
|
| 2.2 |
|
Amendment No. 1 to the Agreement and Plan of Merger and Arrangement, dated as of August 19, 2015, by and among Tribute Pharmaceuticals Canada Inc., Aralez Pharmaceuticals Limited (formerly Aguono Limited), Trafwell Limited, ARLZ US Acquisition Corp., ARLZ CA Acquisition Corp., ARLZ US Acquisition II Corp. and POZEN Inc. (incorporated by reference to Exhibit 2.1 to POZEN Inc.’s Current Report on Form 8-K filed December 8, 2015). |
|
|
|
|
| 2.3 |
|
Amendment No. 2 to the Agreement and Plan of Merger and Arrangement, dated as of December 7, 2015, by and among Tribute Pharmaceuticals Canada Inc., Aralez Pharmaceuticals plc (formerly Aguono Limited), Aralez Pharmaceuticals Inc., Aralez Pharmaceuticals Holdings Limited, ARLZ US Acquisition II Corp., ARLZ CA Acquisition Corp. and POZEN Inc. (incorporated by reference to Exhibit 2.2 to POZEN Inc.’s Current Report on Form 8-K filed December 8, 2015). |
|
|
|
|
| 2.4 |
|
Share Purchase Agreement, dated as of June 16, 2015, by and among Tribute Pharmaceuticals Canada Inc. and the shareholders of Medical Futures Inc. (incorporated by reference to Exhibit 1.1 to Tribute Pharmaceuticals’ Current Report on Form 8-K filed June 22, 2015).† |
|
|
|
|
| 2.5 |
|
Asset Purchase Agreement, dated as of September 6, 2016, by and between Schering-Plough (Ireland) Company, Aralez Pharmaceuticals Trading DAC and Aralez Pharmaceuticals Inc. (incorporated by reference to Exhibit 2.1 to Aralez Pharmaceutical Inc.’s (the “Registrant”) Current Report on Form 8-K/A filed December 5, 2016).† |
|
|
|
|
| 2.6 |
|
Asset Purchase Agreement, dated as of October 3, 2016, by and between AstraZeneca AB, Aralez Pharmaceuticals Trading DAC and Aralez Pharmaceuticals Inc. (incorporated by reference to Exhibit 2.1 to the Registrant’s Current Report on Form 8-K/A filed January 5, 2017).† |
|
|
|
|
| 3.1 |
|
Certificate of Incorporation of Aralez Pharmaceuticals Inc., dated as of December 2, 2015 (incorporated by reference to Exhibit 3.1 to the Registrant’s Registration Statement on Form S-4 filed December 14, 2015). |
|
|
|
|
| 3.2 |
|
Articles of Aralez Pharmaceuticals Inc., dated as of December 11, 2015 (incorporated by reference to Exhibit 3.2 to the Aralez Pharmaceuticals Inc. Registration Statement on Form S-4 filed December 14, 2015). |
|
|
|
|
67
| 10.1 |
|
Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed February 5, 2016).+ |
|
|
|
|
| 10.2 |
|
Form of Substitute Option Agreement for U.S. Tribute Optionees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.2 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+ |
|
|
|
|
| 10.3 |
|
Form of Amended and Restated Substitute Option Agreement for Canadian Tribute Optionees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (filed herewithin, Exhibit 10.3).+ |
|
|
|
|
| 10.4 |
|
Form of Nonqualified Stock Option Award Agreement for U.S. Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.4 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+ |
|
|
|
|
| 10.5 |
|
Form of Nonqualified Stock Option Award Agreement for Canadian Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.5 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+ |
|
|
|
|
| 10.6 |
|
Form of Nonqualified Stock Option Award Agreement for Irish Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (filed herewithin, Exhibit 10.6).+ |
|
|
|
|
| 10.7 |
|
Form of Nonqualified Stock Option Award Agreement for U.S. Directors under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.6 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+ |
|
|
|
|
| 10.8 |
|
Form of Nonqualified Stock Option Award Agreement for Canadian Directors under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.7 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+ |
|
|
|
|
| 10.9 |
|
Form Restricted Stock Unit Award Agreement for U.S. Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.8 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+ |
|
|
|
|
| 10.10 |
|
Form of Restricted Stock Unit Award Agreement for Canadian Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.9 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+ |
|
|
|
|
| 10.11 |
|
Form of Restricted Stock Unit Award Agreement for U.S. Directors under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.10 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+ |
|
|
|
|
| 10.12 |
|
Form of Restricted Stock Unit Award Agreement for Canadian Directors under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.11 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+ |
|
|
|
|
| 10.13 |
|
Form of Restricted Stock Unit Award Agreement for Irish Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (filed herewithin, Exhibit 10.13).+ |
|
|
|
|
| 10.14 |
|
Form Performance Share Award Agreement for U.S. Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (filed herewithin, Exhibit 10.14).+ |
|
|
|
|
| 10.15 |
|
Form Performance Share Award Agreement for Canadian Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (filed herewithin, Exhibit 10.15).+ |
|
|
|
|
68
| 10.16 |
|
Second Amended and Restated Facility Agreement, dated as of December 7, 2015, among Aralez Pharmaceuticals Inc., POZEN Inc., Tribute Pharmaceuticals Canada Inc., Deerfield Private Design Fund III, L.P., Deerfield International Master Fund, L.P., and Deerfield Partners, L.P. (incorporated by reference to Exhibit 10.1 to POZEN Inc.’s Current Report on Form 8-K filed December 8, 2015). |
|
|
|
|
| 10.17 |
|
Form of Senior Secured Convertible Note issued by Aralez Pharmaceuticals Inc. (incorporated by reference to Exhibit 10.2 to the Registrant’s Registration Statement on Form S-1 filed December 31, 2015). |
|
|
|
|
| 10.18 |
|
Second Amended and Restated Registration Rights Agreement, dated as of December 7, 2015, among Aralez Pharmaceuticals Inc., Deerfield Private Design Fund III, L.P., Deerfield International Master Fund, L.P., and Deerfield Partners, L.P. (incorporated by reference to Exhibit 10.2 to POZEN Inc.’s Current Report on Form 8-K filed December 8, 2015). |
|
|
|
|
| 10.19 |
|
Amended and Restated Share Subscription Agreement, dated as of December 7, 2015, among Aralez Pharmaceuticals Inc., Aralez Pharmaceuticals plc, POZEN Inc., Tribute Pharmaceuticals Canada Inc., QLT Inc., Deerfield Private Design Fund III, L.P., Deerfield International Master Fund, L.P., Deerfield Partners, L.P., Broadfin Healthcare Master Fund Ltd., JW Partners, LP, JW Opportunities Fund, LLC, and JW Opportunities Master Fund, Ltd. (incorporated by reference to Exhibit 10.3 to POZEN Inc.’s Current Report on Form 8-K filed December 8, 2015). |
|
|
|
|
| 10.20 |
|
Executive Employment Agreement between POZEN Inc. and Adrian Adams dated May 31, 2015 (incorporated by reference to Exhibit 10.3 to POZEN Inc.’s Current Report on Form 8-K filed June 3, 2015).+ |
|
|
|
|
| 10.21 |
|
Executive Employment Agreement between POZEN Inc. and Andrew I. Koven dated May 31, 2015 (incorporated by reference to Exhibit 10.4 to POZEN Inc.’s Current Report on Form 8-K filed June 3, 2015).+ |
|
|
|
|
| 10.22 |
|
Executive Employment Agreement between POZEN Inc. and Mark A. Glickman dated June 22, 2015 (incorporated by reference to Exhibit 10.7 to POZEN Inc.’s Quarterly Report on Form 10-Q filed August 10, 2015).+ |
|
|
|
|
| 10.23 |
|
Executive Employment Agreement between POZEN Inc. and Eric L. Trachtenberg dated June 22, 2015 (incorporated by reference to Exhibit 10.8 to POZEN Inc.’s Quarterly Report on Form 10-Q filed August 10, 2015).+ |
|
|
|
|
| 10.24 |
|
Executive Employment Agreement between POZEN Inc. and Jennifer L. Armstrong dated June 22, 2015 (incorporated by reference to Exhibit 10.9 to POZEN Inc.’s Quarterly Report on Form 10-Q filed August 10, 2015).+ |
|
|
|
|
| 10.25 |
|
Executive Employment Agreement between POZEN Inc. and Scott J. Charles dated July 25, 2015 (incorporated by reference to Exhibit 10.10 to POZEN Inc.’s Quarterly Report on Form 10-Q filed August 10, 2015).+ |
|
|
|
|
| 10.26 |
|
Executive Employment Agreement between POZEN Inc. and James P. Tursi, MD, dated September 11, 2015 (incorporated by reference to Exhibit 10.3 to POZEN Inc.’s Quarterly Report on Form 10-Q filed November 9, 2015).+ |
|
|
|
|
| 10.27 |
|
Manufacturing Services Agreement, dated as of December 19, 2011, by and between POZEN Inc. and Patheon Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.38 to Amendment No.1 to POZEN Inc.’s Annual Report on Form 10-K, filed June 29, 2012).† |
|
|
|
|
| 10.28 |
|
First Amendment to Manufacturing Services Agreement, between Patheon Pharmaceuticals Inc., and POZEN Inc., dated as of July 10, 2013 (incorporated by reference to Exhibit 10.1 to POZEN Inc.’s Quarterly Report on Form 10-Q, filed August 7, 2013).† |
|
|
|
|
69
| 10.29 |
|
Letter Agreement among POZEN Inc., AstraZeneca AB and Horizon Pharma U.S.A. Inc., dated as of November 18, 2013 (incorporated by reference to Exhibit 10.43 to POZEN Inc.’s Annual Report on Form 10-K, filed March 6, 2014).† |
|
|
|
|
| 10.30 |
|
Amended and Restated Collaboration and License Agreement for the United States by and between POZEN Inc. and AstraZeneca AB, dated as of November 18, 2013 (incorporated by reference to Exhibit 10.45 to POZEN Inc.’s Annual Report on Form 10-K, filed March 6, 2014).† |
|
|
|
|
| 10.31 |
|
Amendment No. 1 to the Amended and Restated Collaboration and License Agreement for the United States by and between POZEN Inc. and Horizon Pharma U.S.A. Inc., dated as of November 18, 2013 (incorporated by reference to Exhibit 10.44 to POZEN Inc.’s Annual Report on Form 10-K, filed March 6, 2014).† |
|
|
|
|
| 10.32 |
|
Amended and Restated Collaboration and License Agreement for outside of the United States by and between POZEN Inc. and AstraZeneca AB, dated as of November 18, 2013 (incorporated by reference to Exhibit 10.46 to POZEN Inc.’s Annual Report on Form 10-K, filed March 6, 2014).† |
|
|
|
|
| 10.33 |
|
Lease Agreement, dated as of April 18, 2016, by and between Witman Properties, L.L.C. and Alexander Road at Davanne, L.L.C. and Aralez Pharmaceuticals US Inc. (incorporated by reference to Exhibit 10.1 to Aralez Pharmaceutical Inc.’s Quarterly Report on Form 10-Q, filed May 10, 2016). |
|
|
|
|
| 10.34 |
|
Lease Guaranty dated as of April 18, 2016, by Aralez Pharmaceuticals Inc. in favor of Witman Properties, L.L.C. and Alexander Road at Davanne, L.L.C (incorporated by reference to Exhibit 10.2 to Aralez Pharmaceutical Inc.’s Quarterly Report on Form 10-Q, filed May 10, 2016). |
|
|
|
|
| 10.35 |
|
Limited Consent, dated October 3, 2016, by and among Aralez Pharmaceuticals Inc., POZEN Inc., Tribute Pharmaceuticals Canada Inc., Deerfield Private Design Fund III, L.P., Deerfield International Master Fund, L.P., and Deerfield Partners, L.P. (incorporated by reference to Exhibit 10.1 to Aralez Pharmaceutical Inc.’s Current Report on Form 8-K filed October 7, 2016). |
|
|
|
|
| 10.36 |
|
Amendment to Second Amended and Restated Facility Agreement, dated October 3, 2016, by and among Aralez Pharmaceuticals Inc., POZEN Inc., Tribute Pharmaceuticals Canada Inc., Deerfield Private Design Fund III, L.P., Deerfield International Master Fund, L.P., and Deerfield Partners, L.P. (incorporated by reference to Exhibit 10.2 to Aralez Pharmaceutical Inc.’s Current Report on Form 8-K filed October 7, 2016). |
|
|
|
|
| 10.37 |
|
Supply Agreement, dated as of October 31, 2016, by and between AstraZeneca AB and Aralez Pharmaceuticals Trading DAC (incorporated by reference to Exhibit 10.1 to Aralez Pharmaceuticals Inc.’s Current Report on Form 8-K filed November 4, 2016). † |
|
|
|
|
| 21.1 |
|
List of subsidiaries of the Registrant (filed herewith, Exhibit 21.1). |
|
|
|
|
| 23.1 |
|
Consent of Ernst & Young LLP, independent registered public accounting firm (filed herewith, Exhibit 23.1). |
|
|
|
|
| 31.1 |
|
Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith, Exhibit 31.1). |
|
|
|
|
| 31.2 |
|
Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith, Exhibit 31.2). |
|
|
|
|
| 32.1 |
|
Certification of the Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (filed herewith, Exhibit 32.1). |
|
|
|
|
| 32.2 |
|
Certification of the Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (filed herewith, Exhibit 32.2). |
|
|
|
|
70
| 101 |
|
The following materials from Aralez Pharmaceuticals Inc.’s Annual Report on Form 10-K for the year ended December 31, 2016, formatted in Extensible Business Reporting Language (XBRL): (i) Consolidated Balance Sheets at December 31, 2016 and 2015, (ii) Consolidated Statements of Operations for the years ended December 31, 2016, 2015 and 2014, (iii) Consolidated Statements of Comprehensive (Loss) Income for the years ended December 31, 2016, 2015 and 2014 (iv) Consolidated Statements of Shareholders’ Equity at December 31, 2016, 2015 and 2014, (v) Consolidated Statements of Cash Flows for the years ended December 31, 2016, 2015 and 2014, and (vi) Notes to Consolidated Financial Statements. |
+ Compensation Related Contract.
† Confidential treatment requested. Confidential materials omitted and filed separately with Securities and Exchange Commission.
Not applicable.
71
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
Registrant: |
|
|
|
|
|
|
|
|
|
Aralez Pharmaceuticals Inc. |
|
|
|
|
|
|
|
Date: March 13, 2017 |
|
By: |
/s/ Adrian Adams |
|
|
|
Adrian Adams |
|
|
|
|
Chief Executive Officer |
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
/s/ Adrian Adams |
|
Chief Executive Officer (Principal Executive Officer), Director |
|
March 13, 2017 |
|
Adrian Adams |
|
|
|
|
|
|
|
|
|
|
|
/s/ Scott J. Charles |
|
Chief Financial Officer (Principal Financial Officer, Principal Accounting Officer) |
|
March 13, 2017 |
|
Scott J. Charles |
|
|
|
|
|
|
|
|
|
|
|
/s/ Arthur S. Kirsch |
|
Director |
|
March 13, 2017 |
|
Arthur S. Kirsch |
|
|
|
|
|
|
|
|
|
|
|
/s/ Neal F. Fowler |
|
Director |
|
March 13, 2017 |
|
Neal F. Fowler |
|
|
|
|
|
|
|
|
|
|
|
/s/ Seth A. Rudnick, M.D. |
|
Director |
|
March 13, 2017 |
|
Seth A. Rudnick, M.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Kenneth B. Lee, Jr. |
|
Director |
|
March 13, 2017 |
|
Kenneth B. Lee, Jr. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Rob Harris |
|
Director |
|
March 13, 2017 |
|
Rob Harris |
|
|
|
|
|
|
|
|
|
|
|
/s/ F. Martin Thrasher |
|
Director |
|
March 13, 2017 |
|
F. Martin Thrasher |
|
|
|
|
|
|
|
|
|
|
|
/s/ Jason M. Aryeh |
|
Director |
|
March 13, 2017 |
|
Jason M. Aryeh |
|
|
|
|
72
ARALEZ PHARMACEUTICALS INC.
INDEX TO FINANCIAL STATEMENTS
|
|
|
||
|
Management’s Report on Internal Control Over Financial Reporting |
F-2 |
||
|
F-3 |
|||
|
F-4 |
|||
|
|
|
||
|
Audited Financial Statements: |
|
||
|
F-5 |
|||
|
F-7 |
|||
|
F-8 |
|||
|
F-9 |
|||
|
F-10 |
F-1
Management’s Report on Internal Control Over Financial Reporting
Management of Aralez Pharmaceuticals Inc. (Aralez) is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting, as defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934, is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of Aralez; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of Aralez are being made only in accordance with authorizations of management and directors of Aralez; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of Aralez’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Further, because of changes in conditions, effectiveness of internal control over financial reporting may vary over time. Management, with the participation of the Chief Executive Officer and Chief Financial Officer, evaluated Aralez’s internal control over financial reporting as of December 31, 2016. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013 Framework) (COSO). As a result of this assessment and based on the criteria in the COSO framework, management has concluded that, as of December 31, 2016, Aralez’s internal control over financial reporting was effective.
Ernst & Young LLP, the independent registered public accounting firm that audited Aralez’s financial statements included in this Annual Report on Form 10-K, has issued an attestation report on Aralez’s internal control over financial reporting, which is included herein.
|
|
/s/ Adrian Adams |
|
/s/ Scott J. Charles |
|
|
|
Chief Executive Officer |
|
Chief Financial Officer |
|
|
|
|
|
|
|
|
|
March 13, 2017 |
|
March 13, 2017 |
|
F-2
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Aralez Pharmaceuticals Inc.
We have audited the accompanying consolidated balance sheets of Aralez Pharmaceuticals Inc. as of December 31, 2016 and 2015, and the related consolidated statements of operations, comprehensive (loss) income, shareholders’ equity, comprehensive (loss) income, and cash flows for each of the three years in the period ended December 31, 2016. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Aralez Pharmaceuticals Inc. at December 31, 2016 and 2015, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2016, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Aralez Pharmaceuticals Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission 2013 framework and our report dated March 13, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Iselin, NJ
March 13, 2017
F-3
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Aralez Pharmaceuticals Inc.
We have audited Aralez Pharmaceuticals Inc.’s internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission 2013 framework (the COSO criteria). Aralez Pharmaceuticals Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Aralez Pharmaceuticals Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Aralez Pharmaceuticals Inc. as of December 31, 2016 and 2015, and the related consolidated statements of statements of operations, shareholders’ equity, comprehensive (loss) income, and cash flows for each of the three years in the period ended December 31, 2016 and our report dated March 13, 2017 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Iselin, NJ
March 13, 2017
F-4
ARALEZ PHARMACEUTICALS INC.
(in thousands of U.S. dollars, except share and per share data)
|
|
|
December 31, 2016 |
|
December 31, 2015 |
|
||
|
ASSETS |
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
64,943 |
|
$ |
24,816 |
|
|
Accounts receivable, net |
|
|
20,405 |
|
|
5,966 |
|
|
Inventory |
|
|
4,548 |
|
|
— |
|
|
Prepaid expenses and other current assets |
|
|
2,435 |
|
|
1,225 |
|
|
Total current assets |
|
|
92,331 |
|
|
32,007 |
|
|
Property and equipment, net |
|
|
7,316 |
|
|
251 |
|
|
Goodwill |
|
|
76,694 |
|
|
— |
|
|
Other intangible assets, net |
|
|
340,194 |
|
|
— |
|
|
Other long-term assets |
|
|
842 |
|
|
— |
|
|
Total assets |
|
$ |
517,377 |
|
$ |
32,258 |
|
|
LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
8,833 |
|
$ |
4,557 |
|
|
Accrued expenses |
|
|
32,141 |
|
|
11,932 |
|
|
Short-term contingent consideration |
|
|
10,430 |
|
|
— |
|
|
Other current liabilities |
|
|
5,870 |
|
|
— |
|
|
Total current liabilities |
|
|
57,274 |
|
|
16,489 |
|
|
Long-term debt, net |
|
|
274,441 |
|
|
— |
|
|
Deferred tax liability |
|
|
3,273 |
|
|
— |
|
|
Long-term contingent consideration |
|
|
60,685 |
|
|
— |
|
|
Other long-term liabilities |
|
|
2,218 |
|
|
986 |
|
|
Total liabilities |
|
|
397,891 |
|
|
17,475 |
|
|
Commitments and Contingencies |
|
|
|
|
|
|
|
|
Preferred shares, no par value; unlimited shares authorized, issuable in series; none outstanding |
|
|
— |
|
|
— |
|
|
Common shares, no par value, unlimited shares authorized, 65,640,607 shares issued and outstanding at December 31, 2016; common stock, $0.001 par value, 33,259,407 issued and outstanding at December 31, 2015 |
|
|
— |
|
|
33 |
|
|
Additional paid-in capital |
|
|
352,336 |
|
|
149,438 |
|
|
Accumulated other comprehensive income |
|
|
4,816 |
|
|
— |
|
|
Accumulated deficit |
|
|
(237,666) |
|
|
(134,688) |
|
|
Total shareholders’ equity |
|
|
119,486 |
|
|
14,783 |
|
|
Total liabilities and shareholders’ equity |
|
$ |
517,377 |
|
$ |
32,258 |
|
The accompanying notes are an integral part of the consolidated financial statements.
F-5
ARALEZ PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands of U.S. dollars, except share and per share data)
|
|
|
For the Years Ended December 31, |
|
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
Product revenues, net |
|
$ |
25,432 |
|
$ |
— |
|
$ |
— |
|
|
Other revenues |
|
|
28,838 |
|
|
21,391 |
|
|
32,394 |
|
|
Total revenues, net |
|
|
54,270 |
|
|
21,391 |
|
|
32,394 |
|
|
Costs and expenses: |
|
|
|
|
|
|
|
|
|
|
|
Cost of product revenues (exclusive of amortization shown separately below) |
|
|
11,765 |
|
|
— |
|
|
— |
|
|
Change in fair value of contingent consideration |
|
|
750 |
|
|
— |
|
|
— |
|
|
Amortization of intangible assets |
|
|
12,591 |
|
|
— |
|
|
— |
|
|
Impairment of intangible assets |
|
|
4,368 |
|
|
— |
|
|
— |
|
|
Selling, general and administrative |
|
|
118,548 |
|
|
50,345 |
|
|
10,078 |
|
|
Research and development |
|
|
8,832 |
|
|
8,512 |
|
|
5,740 |
|
|
Total costs and expenses |
|
|
156,854 |
|
|
58,857 |
|
|
15,818 |
|
|
(Loss) income from operations |
|
|
(102,584) |
|
|
(37,466) |
|
|
16,576 |
|
|
Interest expense |
|
|
(6,141) |
|
|
— |
|
|
— |
|
|
Other (expense) income, net |
|
|
5,683 |
|
|
(143) |
|
|
3,099 |
|
|
(Loss) income before income taxes |
|
|
(103,042) |
|
|
(37,609) |
|
|
19,675 |
|
|
(Benefit from) provision for income taxes |
|
|
(64) |
|
|
174 |
|
|
— |
|
|
Net (loss) income |
|
$ |
(102,978) |
|
$ |
(37,783) |
|
$ |
19,675 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net (loss) income per common share |
|
$ |
(1.67) |
|
$ |
(1.16) |
|
$ |
0.63 |
|
|
Diluted net (loss) income per common share |
|
$ |
(1.74) |
|
$ |
(1.16) |
|
$ |
0.60 |
|
|
Shares used in computing basic net (loss) income per common share |
|
|
61,830,967 |
|
|
32,589,795 |
|
|
31,359,867 |
|
|
Shares used in computing diluted net (loss) income per common share |
|
|
61,883,016 |
|
|
32,589,795 |
|
|
32,810,587 |
|
The accompanying notes are an integral part of the consolidated financial statements.
F-6
ARALEZ PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE (LOSS) INCOME
(in thousands of U.S. dollars)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income |
|
$ |
(102,978) |
|
$ |
(37,783) |
|
$ |
19,675 |
|
|
Other comprehensive income: |
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustments |
|
|
4,816 |
|
|
— |
|
|
— |
|
|
Other comprehensive income |
|
|
4,816 |
|
|
— |
|
|
— |
|
|
Total comprehensive (loss) income |
|
$ |
(98,162) |
|
$ |
(37,783) |
|
$ |
19,675 |
|
The accompanying notes are an integral part of the consolidated financial statements.
F-7
ARALEZ PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
(in thousands of U.S. dollars)
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other |
|
|
|
|
Total |
|
||
|
|
|
Common |
|
Additional |
|
Comprehensive |
|
Accumulated |
|
Shareholders' |
|
|||||
|
|
|
Stock |
|
Paid-In Capital |
|
Income |
|
Deficit |
|
Equity |
|
|||||
|
Balance at December 31, 2013 |
|
$ |
31 |
|
$ |
134,338 |
|
$ |
— |
|
$ |
(116,580) |
|
$ |
17,789 |
|
|
Exercise of common stock options |
|
|
1 |
|
|
7,587 |
|
|
— |
|
|
— |
|
|
7,588 |
|
|
Payments related to net settlement of stock awards |
|
|
— |
|
|
(193) |
|
|
— |
|
|
— |
|
|
(193) |
|
|
Issuance of common stock upon vesting of stock awards |
|
|
— |
|
|
|
|
|
— |
|
|
— |
|
|
- |
|
|
Non-cash share-based compensation |
|
|
— |
|
|
1,881 |
|
|
— |
|
|
— |
|
|
1,881 |
|
|
Net income |
|
|
— |
|
|
— |
|
|
— |
|
|
19,675 |
|
|
19,675 |
|
|
Balance at December 31, 2014 |
|
|
32 |
|
|
143,613 |
|
|
— |
|
|
(96,905) |
|
|
46,740 |
|
|
Exercise of common stock options |
|
|
— |
|
|
1,734 |
|
|
— |
|
|
— |
|
|
1,734 |
|
|
Payments related to net settlement of stock awards |
|
|
— |
|
|
(2,951) |
|
|
— |
|
|
— |
|
|
(2,951) |
|
|
Issuance of common stock upon vesting of stock awards |
|
|
1 |
|
|
(1) |
|
|
— |
|
|
— |
|
|
- |
|
|
Non-cash share-based compensation |
|
|
— |
|
|
7,043 |
|
|
— |
|
|
— |
|
|
7,043 |
|
|
Net loss |
|
|
— |
|
|
— |
|
|
— |
|
|
(37,783) |
|
|
(37,783) |
|
|
Balance at December 31, 2015 |
|
|
33 |
|
|
149,438 |
|
|
— |
|
|
(134,688) |
|
|
14,783 |
|
|
Issuance of common shares in connection with Merger with Tribute |
|
|
(33) |
|
|
115,169 |
|
|
— |
|
|
— |
|
|
115,136 |
|
|
Issuance of common shares to investors, net of equity issue costs |
|
|
— |
|
|
74,866 |
|
|
— |
|
|
— |
|
|
74,866 |
|
|
Warrants exercised |
|
|
— |
|
|
636 |
|
|
— |
|
|
— |
|
|
636 |
|
|
Payments related to net settlement of stock awards |
|
|
— |
|
|
362 |
|
|
— |
|
|
— |
|
|
362 |
|
|
Non-cash share-based compensation |
|
|
— |
|
|
11,865 |
|
|
— |
|
|
— |
|
|
11,865 |
|
|
Foreign currency translation adjustment |
|
|
— |
|
|
— |
|
|
4,816 |
|
|
— |
|
|
4,816 |
|
|
Net loss |
|
|
— |
|
|
— |
|
|
— |
|
|
(102,978) |
|
|
(102,978) |
|
|
Balance at December 31, 2016 |
|
$ |
— |
|
$ |
352,336 |
|
$ |
4,816 |
|
$ |
(237,666) |
|
$ |
119,486 |
|
The accompanying notes are an integral part of the consolidated financial statements.
F-8
ARALEZ PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands of U.S. dollars)
|
|
|
For the Years Ended December 31, |
|
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
Operating Activities |
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income |
|
$ |
(102,978) |
|
$ |
(37,783) |
|
$ |
19,675 |
|
|
Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities: |
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
12,968 |
|
|
16 |
|
|
19 |
|
|
Amortization of debt issuance costs |
|
|
84 |
|
|
— |
|
|
— |
|
|
Change in fair value of contingent consideration |
|
|
750 |
|
|
— |
|
|
— |
|
|
Impairment of intangible assets |
|
|
4,368 |
|
|
— |
|
|
— |
|
|
Gain (loss) on investments in warrants |
|
|
— |
|
|
199 |
|
|
(2,679) |
|
|
Unrealized foreign currency transaction gain |
|
|
(100) |
|
|
— |
|
|
— |
|
|
Loss of sale of property and equipment |
|
|
200 |
|
|
— |
|
|
— |
|
|
Change in fair value of warrants liability |
|
|
(4,744) |
|
|
— |
|
|
— |
|
|
Share-based compensation expense |
|
|
11,865 |
|
|
7,043 |
|
|
1,881 |
|
|
Benefit from deferred income taxes |
|
|
(3,952) |
|
|
— |
|
|
— |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(7,694) |
|
|
(337) |
|
|
(3,956) |
|
|
Inventory |
|
|
(819) |
|
|
— |
|
|
— |
|
|
Prepaid expenses and other current assets |
|
|
1,458 |
|
|
(642) |
|
|
212 |
|
|
Accounts payable |
|
|
(282) |
|
|
3,950 |
|
|
(894) |
|
|
Accrued expenses |
|
|
7,979 |
|
|
10,765 |
|
|
(2,635) |
|
|
Other liabilities |
|
|
(2,537) |
|
|
— |
|
|
(11,257) |
|
|
Other, net |
|
|
(297) |
|
|
— |
|
|
— |
|
|
Net cash (used in) provided by operating activities |
|
|
(83,731) |
|
|
(16,789) |
|
|
366 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investing activities |
|
|
|
|
|
|
|
|
|
|
|
Acquisitions of businesses, net of cash acquired |
|
|
(217,887) |
|
|
— |
|
|
— |
|
|
Payments for intangible assets |
|
|
(715) |
|
|
— |
|
|
— |
|
|
Purchases of property and equipment |
|
|
(4,166) |
|
|
(240) |
|
|
(7) |
|
|
Proceeds from sale of warrants |
|
|
— |
|
|
2,479 |
|
|
— |
|
|
Net cash (used in) provided by investing activities |
|
|
(222,768) |
|
|
2,239 |
|
|
(7) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing activities |
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of convertible debt |
|
|
275,000 |
|
|
— |
|
|
— |
|
|
Proceeds from issuance of common stock |
|
|
75,000 |
|
|
1,735 |
|
|
7,589 |
|
|
Payment of debt and equity issuance costs |
|
|
(778) |
|
|
— |
|
|
— |
|
|
Repayment of convertible note |
|
|
(3,922) |
|
|
— |
|
|
— |
|
|
Payment of contingent consideration |
|
|
(35) |
|
|
— |
|
|
— |
|
|
Proceeds (payments) related to settlement of stock awards |
|
|
998 |
|
|
(2,951) |
|
|
(194) |
|
|
Net cash provided by (used in) financing activities |
|
|
346,263 |
|
|
(1,216) |
|
|
7,395 |
|
|
Net increase (decrease) in cash and cash equivalents |
|
|
39,764 |
|
|
(15,766) |
|
|
7,754 |
|
|
Effect of change in foreign exchange rates on cash and cash equivalents |
|
|
363 |
|
|
— |
|
|
— |
|
|
Cash and cash equivalents at beginning of period |
|
|
24,816 |
|
|
40,582 |
|
|
32,828 |
|
|
Cash and cash equivalents at end of period |
|
$ |
64,943 |
|
$ |
24,816 |
|
$ |
40,582 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental non-cash activities: |
|
|
|
|
|
|
|
|
|
|
|
Equity issued in connection with a business acquisition (See Note 2) |
|
$ |
115,136 |
|
$ |
— |
|
$ |
— |
|
|
Non-cash additions to intangible assets (See Note 7) |
|
$ |
221 |
|
$ |
— |
|
$ |
— |
|
|
Non-cash additions to property and equipment |
|
$ |
2,828 |
|
$ |
— |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
|
|
Taxes paid |
|
$ |
3,732 |
|
$ |
— |
|
$ |
— |
|
|
Interest paid |
|
$ |
1,547 |
|
$ |
— |
|
$ |
— |
|
The accompanying notes are an integral part of the consolidated financial statements.
F-9
ARALEZ PHARMACEUTICALS INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1.ORGANIZATION, BASIS OF PRESENTATION AND ACCOUNTING POLICIES
Organization
Aralez Pharmaceuticals Inc., together with its wholly-owned subsidiaries (“Aralez” or the “Company”), is a global specialty pharmaceutical company focused on delivering meaningful products to improve patients’ lives while creating shareholder value by acquiring, developing and commercializing products primarily in cardiovascular, pain and other specialty areas. Aralez’s global headquarters is located in Mississauga, Ontario, Canada, its U.S. headquarters is located in Princeton, New Jersey, United States, and its Irish headquarters is located in Dublin, Ireland. The Company’s common shares are listed on the NASDAQ Global Market under the trading symbol “ARLZ” and on the Toronto Stock Exchange under the trading symbol “ARZ.” Aralez was formed for the purpose of facilitating the business combination of POZEN Inc., a Delaware corporation (“Pozen”), and Tribute Pharmaceuticals Canada Inc., a corporation incorporated under the laws of the Province of Ontario, Canada (“Tribute”), which closed on February 5, 2016.
On February 5, 2016, pursuant to an Agreement and Plan of Merger and Arrangement between Aralez Pharmaceuticals Inc., Pozen, Tribute and other related parties (as amended, the “Merger Agreement”), Aralez completed the acquisition of Tribute by way of a court approved plan of arrangement in a stock transaction with a purchase price of $137.6 million made up of (i) $115.1 million related to Tribute shares, equity awards and certain warrants outstanding and (ii) $22.5 million in repayments of Tribute indebtedness. In connection with this transaction, Pozen and Tribute were combined under and became wholly-owned subsidiaries of Aralez (the “Merger”). Pursuant to Rule 12g-3(a) under the Securities Exchange Act of 1934, as amended, Aralez Pharmaceuticals Inc. is the successor issuer to Pozen.
On September 6, 2016, Aralez Pharmaceuticals Trading DAC, a wholly-owned subsidiary of Aralez (“Aralez Ireland”), acquired the U.S. and Canadian rights to Zontivity® (vorapaxar), pursuant to an asset purchase agreement (the “Zontivity Asset Purchase Agreement”) with Schering-Plough (Ireland) Company, an Irish private unlimited company and an affiliate of Merck & Co., Inc. (“Merck”).
On October 31, 2016, Aralez Ireland acquired the U.S. rights to Toprol-XL® (metoprolol succinate) and its currently marketed authorized generic (the “AG”) pursuant to an asset purchase agreement (the “Toprol-XL Asset Purchase Agreement”) entered into between AstraZeneca AB (“AstraZeneca”), Aralez Ireland and Aralez Pharmaceuticals Inc.
Basis of Presentation and Consolidation
For financial reporting and accounting purposes, Pozen was the acquirer of Tribute pursuant to the Merger in a business combination. The consolidated financial statements for the years ended December 31, 2015 and 2014 reflect the results of operations and financial position of Pozen, but do not include the results of operations of Tribute because the Merger was completed on February 5, 2016. Aralez’s results of operations for the year ended December 31, 2016 include the results of Tribute from the closing date of the Merger to December 31, 2016. Aralez’s results of operations for the year ended December 31, 2016 also include the results of Zontivity and Toprol-XL from their respective acquisition dates to December 31, 2016 (See Note 2).
Aralez’s consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States. Such consolidated financial statements reflect all adjustments that are, in management’s opinion, necessary to present fairly, in all material respects, Aralez’s consolidated financial position, results of operations, and cash flows. There were no adjustments other than normal recurring adjustments. Certain reclassifications with respect to the presentation of accrued expenses were made to prior year amounts to conform with current year presentation.
The accompanying consolidated financial statements include the accounts of Aralez. All intercompany balances and transactions have been eliminated in consolidation.
F-10
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States (“GAAP”) requires the extensive use of estimates and assumptions that affect the reported amounts of assets, liabilities, revenues, expenses and related disclosures. The most significant assumptions are employed in estimates used in determining values of: inventories; long-lived assets, including goodwill, in-process research and development (“IPR&D”), and other intangible assets; accrued expenses; contingent consideration; income taxes; share-based compensation expense; as well as estimates used in accounting for contingencies and revenue recognition. Actual results could differ from these estimates.
Concentration of Risk
Financial instruments, which potentially subject the Company to concentrations of credit risk, consist principally of cash and cash equivalents, including money market funds. The Company’s investment policy places restrictions on credit ratings, maturities, and concentration by type and issuer. The Company is exposed to credit risk in the event of a default by the financial institutions holding our cash and cash equivalents to the extent recorded on the balance sheet.
The Company is also subject to credit risk from accounts receivable related to product sales and monitors its exposure within accounts receivable and records a reserve against uncollectible accounts receivable as necessary. The Company extends credit to pharmaceutical wholesale distributors and specialty pharmaceutical distribution companies, primarily in Canada and the United States, and to other international distributors. Customer creditworthiness is monitored and collateral is not required.
Cash and Cash Equivalents
Cash and cash equivalents consists of cash and short-term, interest-bearing instruments with original maturities of 90 days or less at the date of purchase.
Inventory
Inventories are stated at the lower of cost or net realizable value on a first-in, first-out basis. Cost is determined to be the purchase price for raw materials and the production cost, including materials, labor and indirect manufacturing costs, for work-in-process and finished goods. The Company analyzes its inventory levels quarterly and writes-down inventory that has become obsolete, inventory that has a cost basis in excess of its expected net realizable value, inventory in excess of expected sales requirements or inventory that fails to meet commercial sale specifications to cost of product revenues. Expired inventory is disposed of and the related costs are written off to cost of product revenues.
Property, Plant and Equipment
Fixed assets are stated at cost. Depreciation is provided using the straight-line method based on estimated useful lives or, in the case of leasehold improvements, over the lesser of the useful lives or the lease terms. Repairs and maintenance costs are expensed as incurred.
Intangible Assets
Goodwill
Goodwill relates to amounts that arose in connection with the acquisitions of Tribute, Zontivity and Toprol-XL and the AG. Goodwill represents the excess of the purchase price over the fair value of the net assets acquired when accounted for using the acquisition method of accounting for business combinations. Goodwill is not amortized but is evaluated for impairment on an annual basis, in the fourth quarter, or more frequently if an event occurs or circumstances change that would more-likely-than-not reduce the fair value of the Company's reporting unit below its carrying amount.
F-11
In-Process Research and Development (“IPR&D”)
IPR&D acquired in a business combination is capitalized on the Company's consolidated balance sheets at its acquisition-date fair value. Until the underlying project is completed, these assets are accounted for as indefinite-lived intangible assets and are subject to impairment testing. Once the project is completed, the carrying value of the IPR&D is reclassified to other intangible assets, net and is amortized over the estimated useful life of the asset. Post-acquisition research and development expenses related to the IPR&D projects are expensed as incurred. The valuation techniques utilized in performing the initial valuation of IPR&D or subsequent quantitative impairment tests incorporate significant assumptions and judgments to estimate the fair value. The Company acquired approximately $3.2 million of IPR&D assets with the acquisition of Tribute, of which $2.8 million was subsequently reclassified to other intangible assets upon receipt of regulatory approval for the related project.
IPR&D is tested for impairment on an annual basis or more frequently if impairment indicators are present. If IPR&D becomes impaired, the carrying value of the IPR&D is written down to its revised fair value with the related impairment charge recognized in the period in which the impairment occurs. In the fourth quarter of 2016, the Company recorded an impairment charge of $0.7 million for the remaining carrying value of its IPR&D. This charge is included in impairment of intangible assets on the consolidated statements of operations.
Other Intangible Assets, net
Other intangible assets consist of acquired technology rights. The Company amortizes its intangible assets using the straight-line method over their estimated economic lives. Costs to obtain, maintain and defend the Company's patents are expensed as incurred. The Company will evaluate the potential impairment of other intangible assets if events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable or that the useful lives of these assets are no longer appropriate. Events giving rise to impairment are an inherent risk in the pharmaceutical industry and many factors cannot be predicted. Factors that are considered in deciding when to perform an impairment review include significant changes in forecasted projections for the asset or asset group for reasons including, but not limited to, significant under-performance of a product in relation to expectations, significant changes or planned changes in our use of the assets, significant negative industry or economic trends, and new or competing products that enter the marketplace. The impairment test is based on a comparison of the undiscounted cash flows expected to be generated from the use of the asset group and its eventual disposition to the carrying value of the asset group. If impairment is indicated, the asset is written down by the amount by which the carrying value of the asset exceeds the related fair value of the asset with the related impairment charge recognized within the statements of operations. Such impairment charges may be material to the Company’s results. The valuation techniques utilized in performing the initial valuation of other intangible assets or subsequent quantitative impairment tests incorporate significant assumptions and judgments to estimate the fair value. The use of different valuation techniques or assumptions could result in significantly different fair value estimates. An impairment charge of $3.7 million was recorded in the fourth quarter of 2016 relating to the acquired technology rights for one product acquired in the Merger. This charge is included in impairment of intangible assets on the Company’s consolidated statements of operations (See Note 7.)
Contingent Consideration
Certain of the Company’s business acquisitions involve the potential for future payment of consideration that is contingent upon the achievement of operational and commercial milestones and royalty payments on future product sales. The fair value of contingent consideration liabilities is determined at the acquisition date using unobservable inputs. These inputs include the estimated amount and timing of projected cash flows, the probability of success (achievement of the contingent event) and the risk-adjusted discount rate used to present value the probability-weighted cash flows. Subsequent to the acquisition date, at each reporting period, the contingent consideration liability is remeasured at current fair value with changes recorded in the consolidated statements of operations. Changes in any of the inputs may result in a significantly different fair value adjustment.
Revenue Recognition
Principal sources of revenue are (i) product sales from the product portfolio acquired with our acquisition of Tribute, and (ii) royalty revenues from sales of VIMOVO by our commercialization partners. In all instances, revenue is
F-12
recognized only when the price is fixed or determinable, persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, and collectibility of the resulting receivable is reasonably assured.
Product Sales
Revenues from the sale of products acquired with our acquisition of Tribute are distributed through Canadian wholesalers to Canadian retail pharmacies and are recorded net of discounts, wholesaler fees, chargebacks, rebates, returns and allowances, and are recognized when legal title to the goods and risk of ownership has been passed to the customer which in this case is the Canadian wholesaler. Discounts, wholesaler fees, chargebacks, rebates, returns and allowances are not significant for these product sales and are not expected to be significant in the future given the Canadian marketplace.
Revenues from the sale of Yosprala® in the United States are recorded on a sell through method since we do not have the historical data to estimate returns. As such, we defer revenue and costs of inventory for all Yosprala products shipped to wholesalers in the United States until the product is sold through to the end customer. Revenue recorded since we launched Yosprala in the United States is not significant during the fiscal year 2016. Product sales from Fibricor® are recorded on a sell in method and were not significant during the fiscal year 2016.
All of our products have a returns policy that allows the customer to return pharmaceutical products within a specified period of time both prior to and subsequent to the product’s expiration date. Our estimate of the provision for returns for those products that use a sell in method is analyzed quarterly and is based upon many factors, including historical data of actual returns and analysis of the level of inventory in the distribution channel, if any. We believe that the reserves we have established are reasonable based upon current facts and circumstances. Applying different judgments to the same facts and circumstances could result in the estimated amount for reserves to vary. If actual results vary with respect to our reserves, we may need to adjust our estimates, which could have a material effect on our results of operations in the period of adjustment.
Other Revenues
Other revenues principally include revenues from licensing arrangements with other biopharmaceutical companies (principally royalty revenues from VIMOVO), including milestones payments and royalties. Revenue from royalties is recognized when the Company has fulfilled the terms in accordance with contractual agreements and has no future obligation, and the amount of the royalty fee is determinable. Royalty revenue that is reasonably estimable and determinable is recognized based on estimates utilizing information reported to us by our commercialization partners.
Other revenues also include net revenues from sales of Zontivity, from its acquisition date, recognized net of related cost of product revenues and fees paid to Merck under a transition services agreement in effect for up to twelve months from the date of acquisition. Similarly, we also include net revenues from sales of Toprol-XL and the AG from its acquisition date, recognized net of related cost of product revenues and fees paid to AstraZeneca under a transition services agreement in effect until July 31, 2017. We record these revenues net of related cost since we are not the principal in the arrangements and expect to record this revenue similar to a royalty arrangement until we are deemed to be the principal in the sales and marketing of these products, at which point we will record net sales and costs of revenue separately.
.
Income Taxes
The Company accounts for income taxes using the liability method in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”), Topic 740, “Income Taxes” (“ASC 740”). Deferred tax assets and liabilities are determined based on temporary differences between financial reporting and tax basis assets and liabilities and are measured by applying enacted rates and laws to taxable years in which differences are expected to be recovered or settled. Further, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that the rate changes. A valuation allowance is required when it is “more-likely-than-not” that all or a portion of deferred tax assets will not be realized. Since the Company’s inception, substantial cumulative losses have been incurred and substantial and recurring losses may be incurred in future periods. The utilization of the loss carryforwards to reduce future income taxes will depend on the Company’s ability to generate sufficient taxable income prior to the expiration of the loss carryforwards. In addition, the maximum annual use of net operating loss and research credit carryforwards is limited in certain situations where changes occur in stock ownership.
F-13
Aralez files federal and state income tax returns, as applicable, with the tax authorities in various jurisdictions including Canada, Ireland and the United States. Pozen is no longer subject to U.S. federal or North Carolina state income tax examinations by tax authorities for years before 2013. Tribute is no longer subject to Canadian income tax examinations by tax authorities for years before 2011. However, the loss and credit carryforwards generated by Pozen and Tribute may still be subject to change to the extent these losses and credits are utilized in a year that is subject to examination by tax authorities.
ASC 740 prescribes a comprehensive model for how a company should recognize, measure, present and disclose in its financial statements uncertain tax positions that the company has taken or expects to take on a tax return, including a decision whether to file or not file a return in a particular jurisdiction. The financial statements reflect expected future tax consequences of such positions presuming the taxing authorities’ full knowledge of the position and all relevant facts. The Company recognizes any interest and penalties accrued related to unrecognized tax benefits as income tax expense.
Share-Based Compensation
The Company expenses the fair value of employee share-based compensation over the employees' service periods, which are generally the vesting period of the equity award. For awards with performance conditions granted, the Company recognizes compensation cost over the expected period to achieve the performance conditions, provided achievement of the performance conditions are deemed probable. Awards with market-based conditions are expensed over the service period regardless of whether achievement of the market condition is deemed probable or is ultimately achieved. Compensation expense is measured using the fair value of the award at the grant date, adjusted for estimated forfeitures.
In order to determine the fair value of option awards on the grant date, the Company uses the Black-Scholes option pricing model. Inherent in this model are assumptions related to expected share price volatility, estimated option life, risk-free interest rate and dividend yield. The expected share price volatility assumption is based on the historical volatility of the Company’s stock, which is obtained from public data sources. The expected life represents the weighted average period of time that share-based awards are expected to be outstanding giving consideration to vesting schedules, historical exercise patterns and post-vesting cancellations for terminated employees that have been exhibited historically, adjusted for specific factors that may influence future exercise patterns. The risk-free interest rate is based on factual data derived from public sources. The Company uses a dividend yield of zero as it has no intention to pay cash dividends in the foreseeable future. For performance-based awards with market conditions, the Company used a Monte Carlo simulation model to determine the fair value of awards as of the grant date.
Forfeitures are estimated based on historical experience of pre-vesting cancellations for terminated employees. An estimated forfeiture rate is applied to all equity awards, which includes option awards and restricted stock units, including performance share units. The Company believes that its estimates are based on outcomes that are reasonably likely to occur. To the extent actual forfeitures differ from our estimates, such amounts will be recorded as a cumulative adjustment in the period estimates are revised.
Determining the appropriate amount to expense for awards with performance conditions based on the achievement of stated goals requires judgment, including forecasting future performance results. The estimate of expense is revised periodically based on the probability of achieving the required performance targets and adjustments are made as appropriate. The cumulative impact of any revisions is reflected in the period of change. If any applicable financial performance goals are not met, no compensation cost is recognized and any previously recognized compensation cost is reversed.
Fair Value Measurements
The accounting standard for fair value measurements defines fair value, establishes a framework for measuring fair value in accordance with GAAP, and requires detailed disclosures about fair value measurements. Under this standard, fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The valuation techniques are based on observable and unobservable inputs. Observable inputs reflect readily obtainable data from independent sources, while unobservable inputs reflect certain market assumptions. This standard classifies these inputs into the following hierarchy:
F-14
|
· |
Level 1 Inputs — Quoted prices for identical instruments in active markets. |
|
· |
Level 2 Inputs — Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations whose inputs are observable or whose significant value drivers are observable. |
|
· |
Level 3 Inputs — Instruments with primarily unobservable value drivers. |
The fair value hierarchy level is determined by asset class based on the lowest level of significant input. In periods of market inactivity, the observability of prices and inputs may be reduced for certain instruments. This condition could cause an instrument to be reclassified between levels.
The carrying amount of cash and cash equivalents approximates its fair value due to the short-term nature of these amounts. The warrants liability is carried at fair value and is included within other current liabilities on the consolidated balance sheet at December 31, 2016. The significant unobservable inputs used in the fair value measurement of our warrants liability, which uses a Black-Scholes valuation model, include the volatility of the Company’s common shares and the expected term. The contingent consideration liability is also carried at fair value, and is recorded as separate short and long-term balances on the consolidated balance sheet at December 31, 2016. The significant unobservable inputs used in the fair value measurement of the Company’s contingent consideration liability include the estimated amount and timing of projected cash flows, the probability of success (achievement of the contingent event) and the risk-adjusted discount rate used to present value the probability-weighted cash flows. The use of different inputs in the valuation of either the warrants liability or the contingent consideration liability could result in materially different fair value estimates.
Advertising Costs
The Company expenses advertising costs as incurred and is included in selling, general and administrative expense in the consolidated statements of operations. Advertising costs were approximately $12.2 million and $1.1 million for the years ended December 31, 2016 and 2015, respectively. The Company did not record any advertising costs for the year ended December 31, 2014.
Foreign Currency
The Company’s reporting currency is the U.S. dollar. The assets and liabilities of subsidiaries that have a functional currency other than the U.S. dollar, primarily the Canadian dollar, are translated into U.S. dollars at the exchange rates in effect at the balance sheet date with the results of operations of subsidiaries translated at average exchange rates for the period. The cumulative foreign currency translation adjustment is recorded as a component of accumulated other comprehensive income within shareholders’ equity.
Transactions in foreign currencies are remeasured into the functional currency of the relevant subsidiary at the exchange rate in effect at the date of the transaction. Any monetary assets and liabilities arising from these transactions are translated into the functional currency at exchange rates in effect at the balance sheet date or on settlement. Resulting gains and losses are recorded in other income (expense), net within the consolidated statements of operations.
Accumulated Other Comprehensive Income
The company is required to present, either on the face of the statement where net income is presented, in a separate statement of comprehensive income or in the notes, significant amounts reclassified out of accumulated other comprehensive income by the respective line items of net income. There were no amounts reclassified out of accumulated other comprehensive income for the years ended December 31, 2016, 2015 and 2014. Other comprehensive income for the year ended December 31, 2016 related to foreign currency translation adjustments.
Recent Accounting Pronouncements
In May 2014, the FASB issued Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (Topic 606), which requires revenue recognition based on the transfer of promised goods or services to
F-15
customers in an amount that reflects consideration Aralez expects to be entitled to in exchange for goods or services. In August 2015, the FASB issued updated guidance deferring the effective date of the revenue recognition standard. The new rules supersede prior revenue recognition requirements and most industry-specific accounting guidance. In March, April and May 2016, the FASB issued additional updated guidance, which clarifies certain aspects of the ASU and the related implementation guidance issued by the FASB-IASB Joint Transition Resource Group for Revenue Recognition. The ASU will be effective for Aralez in the first quarter of 2018, with either full retrospective or modified retrospective application required. Although the Company is still evaluating the full impact of this ASU, the Company expects to adopt it using a modified retrospective approach and that the most significant impact of the new guidance is related to the recognition of variable consideration. The new guidance requires the Company to estimate variable consideration and include in revenue amounts for which is it probable that a significant revenue reversal will not occur. This may result in revenue being recognized earlier than under the current guidance.
In January 2016, the FASB issued ASU 2016-01, Financial Instruments—Overall (Subtopic 825-10), which requires equity investments to be measured at fair value with changes in fair value recognized in net income. It allows an entity to choose to measure equity investments that do not have readily determinable fair values at cost minus impairment. It also simplifies the impairment assessment of equity investments without readily determinable fair values and eliminates the requirements to disclose the methods used to estimate fair value for instruments measured at amortized cost on the balance sheet. The amendments in the ASU are effective for Aralez in the first quarter of 2018. The Company does not expect the adoption to have a material impact on the consolidated financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), which supersedes current lease accounting guidance. The primary difference between current GAAP and the new standard is the recognition of lease assets and lease liabilities by lessees for those leases classified as operating leases under current GAAP. The standard requires a modified retrospective approach upon adoption, with practical expedients that may be available to elect. The standard is effective for Aralez in the first quarter of 2019 and early adoption is permitted. The Company is evaluating the impact of the ASU on the consolidated financial statements.
In March 2016, the FASB issued ASU 2016-09, Compensation—Stock Compensation (Topic 718), which simplifies several aspects of the accounting for share-based payment transactions, such as the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. The amendments include the requirement to recognize excess tax benefits and tax deficiencies as income tax expense or benefit, and to recognize excess tax benefits regardless of whether the benefit reduces taxes payable in the current period. It also allows an entity to make an entity-wide accounting policy election to either estimate the number of awards that are expected to vest or account for forfeitures when they occur. The amendments in the ASU are effective for Aralez in the first quarter of 2017, and early adoption is permitted. The Company does not expect the adoption to have a material impact on the consolidated financial statements.
In March 2016, the FASB issued ASU 2016-06, Derivatives and Hedging (Topic 815), which clarifies the steps required when assessing whether the economic characteristics and risks of call (put) options are clearly and closely related to the economic characteristics and risks of their debt hosts. The ASU clarifies that when a call (put) option is contingently exercisable, an entity does not have to assess whether the event that triggers the ability to exercise a call (put) is related to interest rates or credit risks. The ASU is intended to eliminate diversity in practice in assessing embedded contingent call (put) options in debt instruments. The amendments in the ASU are effective for Aralez in the first quarter of 2017, and early adoption is permitted. The Company does not expect the adoption to have a material impact on the consolidated financial statements.
In August 2016, the FASB issued ASU 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments, providing additional guidance on eight specific cash flow classification issues. The goal of the ASU is to reduce diversity in practice of classifying certain items. The amendments in the ASU are effective for Aralez in the first quarter of 2018 using a retrospective transition method, and early adoption is permitted. The Company is evaluating the impact of the ASU on the consolidated financial statements.
In January 2017, the FASB issued ASU 2017-01, Business Combinations (Topic 805): Clarifying the Definition of a Business, in an effort to clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses. The amendments of this ASU are effective for Aralez in the first quarter of 2018 on a prospective basis and early adoption is permitted. The Company is evaluating the impact of the ASU on the consolidated financial statements.
F-16
2.BUSINESS COMBINATIONS AND ACQUISITIONS
Acquisition of Tribute
On February 5, 2016, Aralez completed its acquisition of Tribute. The transaction provided Aralez with increased financial strength and product portfolio diversity with several marketed products and product candidates acquired. Pursuant to the transaction, Tribute shareholders received 0.1455 common shares of Aralez, no par value per share (the “Aralez Shares”) in exchange for each common share of Tribute, no par value per share (the “Tribute Shares”) held by such shareholders. At the effective time of the Merger, each share of Pozen common stock, $0.001 par value per share, was cancelled and automatically converted into the right to receive one Aralez Share.
Aralez valued the entire issued and to be issued share capital of Tribute at approximately $115.1 million based on Pozen’s closing share price of $5.94 on February 5, 2016 and an exchange ratio of 0.1455. Upon the close of the transaction, (a) each outstanding Tribute warrant entitled its respective holders the right to purchase 0.1455 fully-paid and non-assessable Aralez Shares for no additional consideration beyond that set out in the respective Tribute warrant; (b) each Tribute employee stock option entitled the respective holders of the option to either (i) exchange their Tribute option for a Tribute common share immediately prior to the Merger or (ii) convert into Aralez options entitling the holder to purchase that number of Aralez Shares equivalent to 0.1455 Aralez Shares for each Tribute Share originally issuable (with the exercise price of each Aralez option equal to the original exercise price adjusted for the 0.1455 conversion); and (c) each Tribute compensation option, previously granted to certain investors of Tribute in connection with private placement financings, entitled its respective holders the right to purchase 0.1455 fully-paid and non-assessable Aralez Shares, as well as 0.1455 one-half warrants for Aralez Shares, for no additional consideration beyond that set out in the respective compensation option certificate. As a result of the Merger, the warrants, employee stock options and compensation options are fully-vested and exercisable at any time prior to their respective expiration dates.
The acquisition-date fair value of the consideration transferred is as follows:
|
|
|
At |
|
|
|
|
|
February 5, 2016 |
|
|
|
|
|
(in thousands) |
|
|
|
Equity consideration |
|
$ |
115,136 |
|
|
Repayment of Tribute indebtedness |
|
|
22,488 |
|
|
Total consideration |
|
$ |
137,624 |
|
The acquisition-date fair value of total consideration transferred above excludes approximately $0.5 million related to the accelerated vesting of certain equity awards of Tribute pursuant to the Merger Agreement, which was included in share-based compensation expense during the three months ended March 31, 2016.
The transaction was accounted for as a business combination under the acquisition method of accounting. Accordingly, the tangible and identifiable intangible assets acquired and liabilities assumed were recorded at fair value as of the date of acquisition, with the remaining purchase price recorded as goodwill. The goodwill recognized is attributable primarily to strategic opportunities related to leveraging Tribute’s existing infrastructure. Goodwill is not deductible for tax purposes.
F-17
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed at the date of acquisition:
|
|
|
At |
|
|
|
|
|
February 5, 2016 |
|
|
|
|
|
(as adjusted) |
|
|
|
|
|
(in thousands) |
|
|
|
Cash |
|
$ |
4,601 |
|
|
Accounts receivable |
|
|
3,790 |
|
|
Inventory |
|
|
3,622 |
|
|
Prepaid expenses and other current assets |
|
|
1,129 |
|
|
Property, plant and equipment |
|
|
684 |
|
|
Intangible assets |
|
|
84,034 |
|
|
In-process research and development |
|
|
3,243 |
|
|
Accounts payable and accrued expenses |
|
|
(10,295) |
|
|
Note payable |
|
|
(3,604) |
|
|
Warrants liability |
|
|
(4,618) |
|
|
Other liabilities |
|
|
(7,373) |
|
|
Deferred tax liability |
|
|
(6,913) |
|
|
Total net assets acquired |
|
$ |
68,300 |
|
|
Goodwill |
|
|
69,324 |
|
|
Total consideration |
|
$ |
137,624 |
|
The fair values of intangible assets and IPR&D were determined using an income approach, including a discount rate applied to the projected net cash flows. The Company believes the assumptions are representative of those a market participant would use in estimating fair value. The fair value of intangible assets included the following:
|
|
|
Fair |
|
|
|
|
|
Value |
|
|
|
|
|
(as adjusted) |
|
|
|
|
|
(in thousands) |
|
|
|
Marketed products: |
|
|
|
|
|
Fiorinal |
|
$ |
26,954 |
|
|
Proferrin |
|
|
9,513 |
|
|
Fibricor |
|
|
10,018 |
|
|
Uracyst and Neovisc |
|
|
9,874 |
|
|
Cambia |
|
|
7,567 |
|
|
Other marketed products |
|
|
20,108 |
|
|
Total acquired technology rights |
|
$ |
84,034 |
|
The deferred tax liability of $6.9 million relates primarily to the temporary differences associated with the identifiable intangible assets, which are not deductible for tax purposes.
The operating results of Tribute for the period from February 5, 2016 to December 31, 2016, including revenues of $25.7 million, have been included in the consolidated financial statements as of and for the year ended December 31, 2016. The net loss attributable solely to Tribute is not practicably determinable for the year ended December 31, 2016 given the integration of Tribute’s operations within the combined company. The Company incurred a total of $12.9 million in transaction costs in connection with the acquisition, which were included in selling, general and administrative expenses within the consolidated statements of operations for the year ended December 31, 2016.
Acquisition of Zontivity
On September 6, 2016, Aralez Ireland acquired the U.S. and Canadian rights to Zontivity (vorapaxar), pursuant to the Zontivity Asset Purchase Agreement with Merck. Zontivity represents an addition to the Company’s product portfolio in cardiovascular disease and is the first and currently the only approved therapy shown to inhibit the protease-activated receptor-1 (PAR-1), the primary receptor for thrombin, which is considered to be the most potent activator of platelets.
F-18
The purchase price for Zontivity consists of (i) a payment of $25 million by Aralez Ireland to Merck which was made on the closing date of the acquisition, (ii) certain milestone payments to be payable by Aralez Ireland subsequent to the closing of the acquisition upon the occurrence of certain milestone events based on the annual aggregate net sales of Zontivity, any combination product containing vorapaxar sulphate and one or more other active pharmaceutical ingredients or any line extension thereof, which in no event will exceed $80 million in the aggregate, and (iii) royalty payments in the low double digits based on the annual aggregate net sales of Zontivity, any combination product containing vorapaxar sulphate and one or more other active pharmaceutical ingredients or any line extension thereof.
In connection with the Zontivity Asset Purchase Agreement, Aralez Pharmaceuticals Inc., Pozen, Tribute (collectively, the “Credit Parties”) and certain lenders party to the Second Amended and Restated Debt Facility Agreement (“Facility Agreement”) consented to Aralez Ireland entering into the Zontivity Asset Purchase Agreement and consummation of the transactions contemplated thereby. Pursuant to the terms of such consent, subject to the satisfaction of certain conditions set forth in the Facility Agreement, the Credit Parties were permitted to borrow under the credit facility until December 31, 2016, subject to an advance borrowing notice, to replenish the $25 million payment made on the closing date of the Zontivity acquisition. On October 31, 2016, the Credit Parties borrowed $25 million to replenish the Company’s cash balance for this initial upfront payment of $25 million in cash previously paid at the closing of the Zontivity acquisition. See Note 9, “Debt,” for additional information.
The acquisition-date fair value of the consideration transferred is as follows:
|
|
|
At |
|
|
|
|
|
September 6, 2016 |
|
|
|
|
|
(in thousands) |
|
|
|
Cash |
|
$ |
25,000 |
|
|
Contingent consideration |
|
|
17,600 |
|
|
Total consideration |
|
$ |
42,600 |
|
As of December 31, 2016, the fair value of the short-term and long-term contingent consideration liability in the accompanying consolidated balance sheets was $0.8 million and $17.5 million, respectively.
Pursuant to the terms of the Zontivity Asset Purchase Agreement and certain ancillary agreements entered into in connection with the acquisition, Merck has agreed to supply Zontivity to Aralez Ireland for a period of up to three years following the closing of the acquisition (although the packaging component must be transferred within one year). Merck will also provide certain transition services to Aralez Ireland following the closing of the acquisition to facilitate the transition of the supply, sale and distribution of Zontivity, including distributing Zontivity on behalf of Aralez Ireland in exchange for compensation specified in the transition services agreement. While the transition services agreement is in effect, at the end of each quarter, Merck will remit a net margin amount to Aralez Ireland, which will include a fee for its services. This net amount is included in other revenues on the consolidated statements of operations. In addition, in connection with the foregoing transactions, Merck granted Aralez Ireland, among other things, (i) an exclusive and royalty-free license to certain trademarks solely to exploit Zontivity in the U.S. and Canada and their respective territories, and (ii) an exclusive and royalty-free license to certain know-how solely in connection with the manufacture of Zontivity for exploitation in the U.S. and Canada and their respective territories.
The transaction was accounted for as a business combination under the acquisition method of accounting. Accordingly, the identifiable intangible asset acquired was recorded at fair value as of the date of acquisition, with the remaining purchase price recorded as goodwill. The goodwill recognized is attributable primarily to strategic and synergistic opportunities.
F-19
The following table summarizes the estimated preliminary fair value of the asset acquired at the date of acquisition:
|
|
|
At |
|
|
|
|
|
September 6, 2016 |
|
|
|
|
|
(in thousands) |
|
|
|
Intangible asset |
|
$ |
40,800 |
|
|
Total net asset acquired |
|
|
40,800 |
|
|
Goodwill |
|
|
1,800 |
|
|
Total consideration |
|
$ |
42,600 |
|
The purchase price allocation has been prepared on a preliminary basis and is subject to change as additional information becomes available concerning the fair value and tax basis of the asset acquired. Any adjustments to the purchase price allocation will be made as soon as practicable but no later than one year from the September 6, 2016 acquisition date.
The operating results of Zontivity for the period from September 6, 2016 to December 31, 2016, including net revenues of $1.1 million, have been included in the consolidated financial statements as of and for the year ended December 31, 2016. The Company incurred a total of $0.9 million in product acquisition-related costs in connection with the acquisition, which were included in selling, general and administrative expenses within the consolidated statements of operations for the year ended December 31, 2016.
Acquisition of Toprol-XL and the AG
On October 31, 2016, Aralez Ireland acquired the U.S. rights to Toprol-XL (metoprolol succinate) and the AG pursuant to the Toprol-XL Asset Purchase Agreement entered into between AstraZeneca, Aralez Ireland and Aralez Pharmaceuticals Inc. Toprol-XL is a cardioselective beta-blocker indicated for the treatment of hypertension, alone or in combination with other antihypertensives, the long term treatment of angina pectoris and treatment of stable, symptomatic (NYHA class II or III) heart failure of specific origins.
The purchase price payable under the Toprol-XL Asset Purchase Agreement consists of (i) a payment of $175 million by Aralez Ireland to AstraZeneca, which was made on the closing date of the transaction; (ii) certain milestone payments payable by Aralez Ireland subsequent to the closing of the transaction upon the occurrence of certain milestone events based on the annual aggregate net sales of Toprol-XL and the AG and other contingent events, which in no event will exceed $48 million in the aggregate; (iii) royalty payments of (A) 15% of total quarterly net sales of Toprol-XL and any other authorized or owned generic version of Toprol-XL that is marketed, distributed or sold by or on behalf of, or under a license or sublicense from, Aralez (other than the AG), and (B) 15% of quarterly net sales of the AG, but for purposes of royalty payments and clause (B) only, net sales do not include the supply price paid for the AG by Aralez Ireland to AstraZeneca under the supply agreement entered into between Aralez Ireland and AstraZeneca in respect of the applicable period and (iv) a payment for the value of the finished inventory of Toprol-XL and the AG at closing of the transaction, not to exceed a cap specified in the Toprol-XL Asset Purchase Agreement.
On October 31, 2016, in connection with the Toprol-XL acquisition, Aralez Ireland entered into a Supply Agreement (the “Toprol-XL Supply Agreement”) with AstraZeneca. Pursuant to the terms of the Toprol-XL Supply Agreement and except as otherwise expressly set forth therein, AstraZeneca will be the exclusive manufacturer and supplier to Aralez Ireland of Toprol-XL and the AG, each in finished bottled form for exploitation and commercialization in the United States. The initial term of the Toprol-XL Supply Agreement is 10 years (the “Toprol-XL Supply Initial Term”). The Toprol-XL Supply Agreement will continue indefinitely following the expiration of the Toprol-XL Supply Initial Term unless terminated in accordance with its terms. Except in the case of certain uncured material breaches of the Toprol-XL Supply Agreement by Aralez Ireland or certain insolvency related events affecting Aralez Ireland, AstraZeneca may not terminate the Toprol-XL Supply Agreement unless it satisfies certain conditions related to, among other things, the transfer of technology. In addition to termination rights upon certain uncured material breaches of the Toprol-XL Supply Agreement by AstraZeneca or certain insolvency related events affecting AstraZeneca, Aralez Ireland may terminate the Toprol-XL Supply Agreement at any time following the Toprol-XL Supply Initial Term upon providing 12 months prior written notice to AstraZeneca.
In connection with the Toprol-XL Asset Purchase Agreement, on October 3, 2016, the Credit Parties and certain lenders party to the Facility Agreement entered into a Limited Consent (the “Credit Agreement Consent”) pursuant to
F-20
which such lenders consented to Aralez Ireland entering into the Toprol-XL Asset Purchase Agreement and to the consummation of the transactions contemplated thereby. Pursuant to the terms of the Credit Agreement Consent, the Credit Parties were permitted to borrow under the Facility Agreement to finance the $175 million closing date payment to be made in connection with the Toprol-XL acquisition. The Credit Agreement Consent also provided that in the event the Company borrowed loans under the Facility Agreement to finance the $175 million payment (the “Toprol-XL Loans”), the Company may also elect to concurrently borrow loans under the Facility Agreement in an aggregate principal amount of $25 million to replenish the Company’s cash balance for the initial upfront payment of $25 million in cash previously paid at the closing of the Zontivity acquisition on September 6, 2016 (the “Zontivity Loans”). However, if the Company borrowed the Toprol-XL Loans at the closing of the Toprol-XL acquisition, but did not elect to concurrently borrow the Zontivity Loans, the Company would no longer be permitted to borrow, and the lenders would have no further obligation to fund, the Zontivity Loans. On October 31, 2016, the Company borrowed both the Toprol-XL Loans and the Zontivity Loans.
The acquisition date fair value of the consideration transferred is as follows:
|
|
|
At |
|
|
|
|
|
October 31, 2016 |
|
|
|
|
|
|
(in thousands) |
|
|
Cash |
|
$ |
175,000 |
|
|
Contingent consideration |
|
|
52,800 |
|
|
Cash paid for prepaid asset |
|
|
1,492 |
|
|
Total consideration |
|
$ |
229,292 |
|
As of December 31, 2016, the fair value of the short-term and long-term contingent consideration liability in the accompanying consolidated balance sheets was $9.6 million and $43.2 million, respectively.
The transaction was accounted for as a business combination under the acquisition method of accounting. Accordingly, the assets acquired were recorded at fair value as of the date of acquisition, with the remaining purchase price recorded as goodwill. The goodwill recognized is attributable primarily to strategic and synergistic opportunities.
The following table summarizes the estimated preliminary fair value of the assets acquired at the date of acquisition:
|
|
|
At |
|
|
|
|
|
October 31, 2016 |
|
|
|
|
|
(in thousands) |
|
|
|
Prepaid asset |
|
$ |
1,492 |
|
|
Intangible asset |
|
|
224,600 |
|
|
Total net assets acquired |
|
|
226,092 |
|
|
Goodwill |
|
|
3,200 |
|
|
Total consideration |
|
$ |
229,292 |
|
The purchase price allocation has been prepared on a preliminary basis and is subject to change as additional information becomes available concerning the fair value and tax basis of the assets acquired. Any adjustments to the purchase price allocation will be made as soon as practicable but no later than one year from the October 31, 2016 acquisition date.
The operating results of Toprol-XL for the period from October 31, 2016 to December 31, 2016, including net revenues of $7.7 million, have been included in the consolidated financial statements as of and for the year ended December 31, 2016. The Company incurred a total of $1.6 million in product acquisition-related costs in connection with the acquisition, which were included in selling, general and administrative expenses within the consolidated statements of operations for the year ended December 31, 2016.
F-21
Pro Forma Impact of Business Combinations
The following supplemental unaudited pro forma information presents Aralez’s financial results as if the acquisitions of Tribute, Zontivity and Toprol-XL had occurred on January 1, 2015:
|
|
|
Years Ended December 31, |
|
||||
|
|
|
2016 |
|
2015 |
|
||
|
|
|
(in thousands, except per share data) |
|
||||
|
Total revenues, net |
|
$ |
145,696 |
|
$ |
145,116 |
|
|
Net loss |
|
$ |
(67,224) |
|
$ |
(424,176) |
|
|
Diluted net loss per share |
|
$ |
(1.09) |
|
$ |
(6.70) |
|
The above unaudited pro forma information was determined based on the historical GAAP results of Aralez, Tribute, Zontivity and Toprol-XL. The historical results of Zontivity for the year ended December 31, 2015 include an intangible asset impairment charge of $289.7 million. The pro forma financial statements also include the financial results of Medical Futures Inc. (“MFI”), a company that Tribute acquired in June 2015, which included revenues of $3.8 million and net loss of $0.5 million, for the year ended December 31, 2015. The unaudited pro forma consolidated results are provided for informational purposes only and are not necessarily indicative of what Aralez’s consolidated results of operations actually would have been if the acquisition was completed on January 1, 2015 or what the consolidated results of operations will be in the future. The pro forma consolidated net loss includes pro forma adjustments relating to the following significant recurring and non-recurring items directly attributable to the business combinations, net of the pro forma tax impact utilizing applicable statutory tax rates, which were eliminated from the year ended December 31, 2016, and included in the year ended December 30, 2015, as applicable:
|
(i) |
$12.0 million of expense for excise tax equalization payments for the year ended December 31, 2016; |
|
(ii) |
$3.9 million of severance charges for the year ended December 31, 2016; |
|
(iii) |
$1.5 million of the inventory fair value step-up for the year ended December 31, 2016; |
|
(iv) |
$0.5 million of stock based compensation expense for the year ended December 31, 2016; |
|
(v) |
addition of $0.9 million and $1.9 million in cost of product sales related to the Zontivity supply agreement with Merck for the years ended December 31, 2016 and 2015, respectively; |
|
(vi) |
elimination of $15.5 million of transaction costs incurred by the combined Company for the year ended December 31, 2016, and addition of $16.3 million of transaction costs for the year ended December 31, 2015; |
|
(vii) |
elimination of $1.0 million in costs associated with the Zontivity and Toprol-XL transition services agreements for the year ended December 31, 2016, and the addition of $4.4 million in costs associated with the Zontivity and Toprol-XL transition services agreements for the year ended December 31, 2015; |
|
(viii) |
elimination of $1.8 million and $14.3 million of amortization for the years ended December 31, 2016 and 2015, respectively, and the addition of amortization of finite-lived intangible assets acquired of $22.0 million and $33.7 million for the years ended December 31, 2016 and 2015, respectively; as well as |
|
(ix) |
elimination of $0.3 million of interest expense related to the Tribute acquisition for the year ended December 31, 2016, and the addition of $20.8 million and $25.0 million in interest expense related to the financing of the Zontivity and Toprol-XL acquisitions for the years ended December 31, 2016 and 2015, respectively |
F-22
3.BUSINESS AGREEMENTS
Agreements with AstraZeneca for Toprol-XL
On October 31, 2016, Aralez Ireland acquired the U.S. rights to Toprol-XL (metoprolol succinate) and the AG. Pursuant to the Toprol-XL Asset Purchase Agreement, the purchase price consists of (i) a payment of $175 million by Aralez Ireland to AstraZeneca, which was made on the closing date of the acquisition; (ii) certain milestone payments payable by Aralez Ireland subsequent to the closing of the acquisition upon the occurrence of certain milestone events based on the annual aggregate net sales of Toprol-XL and the AG and other contingent events, which in no event will exceed $48 million in the aggregate; (iii) royalty payments of (A) 15% of total quarterly net sales of Toprol-XL and any other authorized or owned generic version of Toprol-XL that is marketed, distributed or sold by or on behalf of, or under a license or sublicense from, Aralez (other than the AG), and (B) 15% of quarterly net sales of the AG, but for purposes of royalty payments and clause (B) only, net sales do not include the supply price paid for the Toprol-XL AG by Aralez Ireland to AstraZeneca under the supply agreement entered into between Aralez Ireland and AstraZeneca in respect of the applicable period and (iv) a payment for the value of the finished inventory of Toprol-XL and the AG at closing of the transaction, not to exceed a cap specified in the Toprol-XL Asset Purchase Agreement.
Pursuant to the terms of the Toprol-XL Asset Purchase Agreement and certain ancillary agreements entered into in connection with the acquisition, AstraZeneca will be the exclusive manufacturer and supplier to Aralez Ireland of Toprol-XL and the AG, each in finished bottled form for exploitation and commercialization in the United States. The initial term of the Toprol-XL Supply Agreement is the Toprol-XL Supply Initial Term. The Toprol-XL Supply Agreement will continue indefinitely following the expiration of the Toprol-XL Supply Initial Term unless terminated in accordance with its terms. Except in the case of certain uncured material breaches of the Toprol-XL Supply Agreement by Aralez Ireland or certain insolvency related events affecting Aralez Ireland, AstraZeneca may not terminate the Toprol-XL Supply Agreement unless it satisfies certain conditions related to, among other things, the transfer of technology. In addition to termination rights upon certain uncured material breaches of the Toprol-XL Supply Agreement by AstraZeneca or certain insolvency related events affecting AstraZeneca, Aralez Ireland may terminate the Toprol-XL Supply Agreement at any time following the Toprol-XL Supply Initial Term upon providing 12 months prior written notice to AstraZeneca.
Agreements with Merck for Zontivity
On September 6, 2016, Aralez Ireland acquired the U.S. and Canadian rights to Zontivity from Merck pursuant to the Zontivity Asset Purchase Agreement. Pursuant to the Zontivity Asset Purchase Agreement, the purchase price for Zontivity consists of (i) a payment of $25 million by Aralez Ireland to Merck, which was made on the closing date of the acquisition, (ii) certain milestone payments payable by Aralez Ireland subsequent to the closing of the acquisition upon the occurrence of certain milestone events based on the annual aggregate net sales of Zontivity, any combination product containing vorapaxar sulphate and one or more other active pharmaceutical ingredients or any line extension thereof, which in no event will exceed $80 million in the aggregate, and (iii) royalty payments in the low double digits based on the annual aggregate net sales of Zontivity, any combination product containing vorapaxar sulphate and one or more other active pharmaceutical ingredients or any line extension thereof.
Pursuant to the terms of the Zontivity Asset Purchase Agreement and certain ancillary agreements entered into in connection with the acquisition, Merck has agreed to supply Zontivity to Aralez Ireland for a period of up to three years following the closing of the acquisition (although the packaging component must be transferred within one year). Merck will also provide certain transition services to Aralez Ireland following the closing of the acquisition to facilitate the transition of the supply, sale and distribution of Zontivity, including distributing Zontivity on behalf of Aralez Ireland in exchange for compensation specified in the transition services agreement. In addition, in connection with the foregoing transactions, Merck granted Aralez Ireland, among other things, (i) an exclusive and royalty-free license to certain trademarks solely to exploit Zontivity in the United States and Canada and their respective territories, and (ii) an exclusive and royalty-free license to certain know-how solely in connection with the manufacture of Zontivity for exploitation in the United States and Canada and their respective territories.
Agreement with AstraZeneca/Horizon regarding VIMOVO®
In August 2006, the Company entered into a collaboration and license agreement, effective September 7, 2006 (the “Original AZ Agreement”), with AstraZeneca regarding the development and commercialization of proprietary
F-23
fixed dose combinations of the proton pump inhibitor (“PPI”) esomeprazole magnesium with the non-steroidal anti-inflammatory drug (“NSAID”) naproxen in a single tablet for the management of pain and inflammation associated with conditions such as osteoarthritis and rheumatoid arthritis in patients who are at risk for developing NSAID-associated gastric ulcers. Under the terms of the Original AZ Agreement, the Company granted to AstraZeneca an exclusive, fee-bearing license, in all countries of the world except Japan, under our patents and know-how relating to combinations of gastroprotective agents and NSAIDs (other than aspirin and its derivatives). The Company developed VIMOVO with AstraZeneca pursuant to this collaboration arrangement, with AstraZeneca responsible for commercialization of VIMOVO.
During 2013, AstraZeneca decided to cease promotion and sampling of VIMOVO in certain countries, including the United States and all countries in Europe, other than Spain and Portugal, which have pre-existing contractual relationships with third parties. In November 2013, AstraZeneca divested of all of its rights, title and interest to develop, commercialize and sell VIMOVO in the United States to Horizon Pharma USA, Inc. (“Horizon”). In connection with this divestiture, in November 2013, the Company and AstraZeneca entered into an Amended and Restated Collaboration and License Agreement for the United States (the “U.S. Agreement”) and an Amended and Restated License and Collaboration Agreement for outside the United States and Japan (the “ROW Agreement”), which agreements collectively amended and restated the Original AZ Agreement (as amended prior to the date of the U.S. Agreement and ROW Agreement). With the Company’s consent pursuant to a letter agreement among the Company, AstraZeneca and Horizon, AstraZeneca subsequently assigned the U.S. Agreement to Horizon in connection with the divestiture. Further, the letter agreement establishes a process for AstraZeneca and Horizon to determine if certain sales milestones are achieved on a global basis and provides other clarifications and modifications required as a result of the contractual framework implemented among, or as otherwise agreed by, the parties. An additional $260.0 million is potentially payable to the Company if such sales milestones are achieved.
Under the U.S. Agreement, Horizon is obligated to pay us a 10% royalty on net sales of VIMOVO and certain other products covered thereby in the United States. Pursuant to an amendment of the U.S. Agreement (the “Amendment to the U.S. Agreement”) between the Company and Horizon, the Company is guaranteed an annual minimum royalty amount of $7.5 million each calendar year, provided that the patents owned by the Company which cover such products are in effect and certain types of competing products are not in the marketplace (including competing products entering pursuant to a license to enter the market prior to expiration of the applicable patents). The Amendment to the U.S. Agreement also provides that Horizon has assumed AstraZeneca’s right to lead the on-going Paragraph IV litigation relating to VIMOVO currently pending in the United States District Court for the District of New Jersey and will assume all patent-related defense costs relating to such litigation, including reimbursement up to specified amounts of the cost of any counsel retained by us, amends certain time periods for Horizon’s delivery of quarterly sales reports to the Company, and provides for quarterly update calls between the parties to discuss performance of VIMOVO and Horizon’s commercialization efforts.
Pursuant to the ROW Agreement, AstraZeneca will continue to have the rights to commercialize VIMOVO and certain other products covered thereby outside of the United States and Japan and paid us a royalty of 6% on net sales within the applicable territory through 2015 and started paying us a royalty of 10% of net sales commencing in the first quarter of 2016.
The royalty rates above may be reduced due to the loss of market share as a result of certain competition inside and outside of the United States, as applicable (including competing products entering pursuant to a license to enter the market prior to expiration of the applicable patents). Furthermore, our right to receive royalties from AstraZeneca or Horizon, as applicable, expires on a country-by country basis upon the later of (a) expiration of the last-to expire of certain patent rights related to the applicable product(s) in that country, and (b) ten years after the first commercial sale of such product(s) in such country. As the result of an unfavorable outcome in certain patent litigation in Canada, it is expected that Mylan’s generic naproxen/esomeprazole magnesium tablets will be available in Canada shortly. See Note 14 – Commitments and Contingencies, for more information.
Agreements with Patheon regarding Yosprala
In December 2011, the Company entered into a Manufacturing Services Agreement with Patheon Pharmaceuticals, Inc. (“Patheon”), as amended in July 2013 (as amended, the “Supply Agreement”), pursuant to which Patheon has agreed to manufacture, and the Company has agreed to purchase, a specified percentage of the Company’s requirements of Yosprala 325/40 and Yosprala 81/40 for sale in the United States. The term of the Supply Agreement
F-24
extends until December 31st of the fourth year after the date that is 60 days after the Company submits its first firm order to Patheon under the Supply Agreement (the “Initial Term”), and will automatically renew thereafter for periods of two years, unless terminated by either party upon 18 months’ written notice prior to the expiration of the Initial Term or 12 months’ written notice prior to the expiration of any renewal term. In addition to usual and customary termination rights which allow each party to terminate the Supply Agreement for material, uncured breaches by the other party, the Company can terminate the Supply Agreement upon 30 days’ prior written notice if a governmental or regulatory authority takes any action or raises any objection that prevents the Company from importing, exporting, purchasing or selling Yosprala or if it is determined that the formulation or sale of Yosprala infringes any patent rights or other intellectual property rights of a third-party. The Company can also terminate the Supply Agreement upon 24 months’ prior written notice if it licenses, sells, assigns or otherwise transfers any rights to commercialize Yosprala in the United States to a third-party. The Supply Agreement contains general and customary commercial supply terms and conditions, as well as establishes pricing, subject to annual adjustments, for bulk product and different configurations of packaged product.
Agreement to Acquire MFI
In June 2015, Tribute acquired MFI pursuant to a Share Purchase Agreement between Tribute and the former shareholders of MFI (“MFI Purchase Agreement”). The MFI acquisition diversified Tribute’s product portfolio with the addition of both marketed products, including Proferrin, and product candidates. The amounts payable pursuant to the MFI Purchase Agreement included (a) $8.5 million (CAD) in cash on closing (including a $0.2 million (CAD) deposit previously paid) to the former MFI shareholders, (b) $5.0 million (CAD) through the issuance of 3,723,008 Tribute Shares to the former MFI shareholders, (c) $5.0 million (CAD) in the form of the MFI Note, (d) retention payments of $0.5 million (CAD) to MFI employees, (e) consent payments of $3.345 million (CAD) and $2.35 million (CAD) to the former MFI shareholders payable on receipt of certain third party consents, and (f) two payments of $1.25 million (CAD) to the former MFI shareholders payable on regulatory approval of two product candidates, respectively, or change of control of Tribute. As discussed above, the MFI Note was repaid in June 2016. The $3.345 million (CAD) consent payment was made in 2015 and the $2.35 million (CAD) consent payment has not been made. The two $1.25 million (CAD) payments became payable upon the closing of the Merger. One such payment was made in full to the former shareholders of MFI and the second was paid in part with the remainder offset in settlement of certain indemnity claims by the Company against the former shareholders of MFI, in each case in 2016
Certain Other Agreements
Agreements with Sun Pharma and Frontida for Fibricor®
In May 2015, Tribute Pharmaceuticals International Inc. (“TPII”), a Barbados corporation and a wholly-owned subsidiary of Tribute, acquired the U.S. rights to Fibricor and its related authorized generic (collectively, the “Fibricor Products”) from a wholly-owned step-down subsidiary of Sun Pharmaceutical Industries Ltd. (“Sun Pharma”). Financial terms include a total payment of $10.0 million of which approximately $3.0 million was included as a liability assumed in the Merger and subsequently paid in May 2016. In addition, we may be obligated to pay up to $4.5 million in milestone payments based on annual net sales of Fibricor and its authorized generic as well as royalties ranging from the high single digits to low double digits based on annual net sales of such products. In connection with its acquisition of Fibricor, TPII also entered into a supply agreement with Sun Pharma pursuant to which Sun Pharma agreed to manufacture and supply the Fibricor Products to TPII. On June 3, 2016, Sun Pharma assigned the supply agreement to Frontida BioPharm, Inc. On June 30, 2016, TPII assigned its interest in the Fibricor Products to Aralez Ireland.
Agreements with Novartis for Fiorinal®
In 2014, Tribute entered into an asset purchase agreement (the “Asset Purchase Agreement”) with Novartis AG and Novartis Pharma AG (collectively, “Novartis”) pursuant to which Tribute acquired from Novartis the Canadian rights to manufacture, market, promote, distribute and sell Fiorinal, Fiorinal C, Visken® and Viskazide® for the relief of pain from headache and for the treatment of cardiovascular conditions (the “Novartis Products”), as well as certain other assets relating to the Novartis Products, including certain intellectual property, marketing authorizations and related data, medical, commercial and technical information, and the partial assignment of certain manufacturing and supply agreements and tenders with third parties (the “Acquired Assets”). Tribute also assumed certain liabilities arising out of the Acquired Assets and the Licensed Assets (as defined below) after the acquisition, including product liability claims or intellectual property infringement claims by third parties relating to the sale of the Novartis Products by Tribute in
F-25
Canada. In connection with the acquisition of the Acquired Assets, and pursuant to the terms of the Asset Purchase Agreement, Tribute concurrently entered into a license agreement with Novartis AG, Novartis Pharma AG and Novartis Pharmaceuticals Canada Inc., under which the Novartis entities agreed to license to Tribute certain assets relating to the Novartis Products, including certain intellectual property, marketing authorizations and related data, and medical, commercial and technical information (the “Licensed Assets”).
Agreement with Faes for BlextenTM
In 2014, Tribute entered into an exclusive license and supply agreement with Faes Farma, S.A. (“Faes”), a Spanish pharmaceutical company, for the exclusive right to sell bilastine, a product for the treatment of allergic rhinitis and chronic idiopathic urticaria (hives) in Canada, which is now named Blexten. The exclusive license is inclusive of prescription and non-prescription rights for Blexten, as well as adult and pediatric presentations in Canada. On March 31, 2016, Tribute assigned its interest in Blexten to Aralez Ireland. Regulatory approval to sell Blexten in Canada was received from Health Canada in April 2016 and the Company began commercializing Blexten in Canada in December 2016. The Company will owe sales-based milestone payments of $1.7 million to Faes if certain sales targets are met.
Agreement with Nautilus for Cambia®
In 2010, Tribute signed a license agreement with Nautilus Neurosciences, Inc. (“Nautilus”) for the exclusive rights to develop, register, promote, manufacture, use, market, distribute and sell Cambia in Canada. In 2011, Tribute and Nautilus executed the first amendment to the license agreement and in 2012 executed the second amendment to the license agreement. Nautilus was acquired by Depomed, Inc. in December 2013. Up to $6.0 million in sales-based milestone payments may be payable over time. Royalty rates are tiered and payable at rates ranging from 22.5% to 25.0% of net sales.
Agreement with Actavis for Bezalip® SR and Soriatane®
In 2008, Tribute signed a Sales, Marketing and Distribution Agreement with Actavis Group PTC ehf (“Actavis”) to perform certain sales, marketing, distribution, finance and other general management services in Canada in connection with the importation, marketing, sales and distribution of Bezalip SR and Soriatane (the “Actavis Products”). In 2010, a first amendment was signed with Actavis to grant Tribute the right and obligation to more actively market and promote the Actavis Products in Canada. In 2011, a second amendment was signed with Actavis that extended the term of the agreement, modified certain of the other terms of the agreement and increased Tribute’s responsibilities to include the day-to-day management of regulatory affairs, pharmacovigilance and medical information relating to the Actavis Products. Tribute pays Actavis a sales and distribution fee based on a percentage of the aggregate net sales of the products. In 2011, Tribute signed a Product Development and Profit Share Agreement with Actavis to develop, obtain regulatory approval of and market Bezalip SR in the United States. The Company may owe a milestone payment of $5.0 million to Actavis in the event that the Company pursues and obtains regulatory approval to market Bezalip SR in the U.S.
Agreements with GSK, Pernix and CII regarding MT 400 (including Treximet®)
In June 2003, the Company entered into an agreement with Glaxo Group Limited, d/b/a GlaxoSmithKline (“GSK”) for the development and commercialization of proprietary combinations of a triptan (5-HT1B/1D agonist) and a long-acting NSAID (the “GSK Agreement”). The combinations covered by the GSK Agreement are among the combinations of MT 400 (including Treximet®). Under the terms of the GSK Agreement, GSK had exclusive rights in the United States to commercialize all combinations which combine GSK’s triptans, including Imitrex® (sumatriptan succinate) or Amerge® (naratriptan hydrochloride), with a long-acting NSAID. The Company was responsible for development of the first combination product, while GSK provided formulation development and manufacturing.
In November 2011, the Company entered into a purchase agreement with CPPIB Credit Investments Inc. (“CII”), pursuant to which the Company sold, and CII purchased, the Company’s right to receive future royalty payments arising from U.S. sales of MT 400, including Treximet. By virtue of the agreement, the Company will receive a 20% interest in royalties, if any, paid on net sales of Treximet and such other products in the United States to CII relating to the period commencing in the second quarter of 2018.
F-26
In May 2014, the Company, GSK, CII and Pernix Therapeutics Holdings, Inc. (“Pernix”), entered into certain agreements in connection with GSK’s divestiture of all of its rights, title and interest to develop, commercialize and sell Treximet in the United States to Pernix. Upon the closing of the transaction in August 2014, with the Company’s consent, GSK assigned the GSK Agreement to Pernix. Pernix assumed the obligation to pay two sales performance milestones totaling up to $80.0 million if certain sales thresholds are achieved as well as royalties on all net sales of marketed products until at least the expiration of the last-to-expire issued applicable patent based upon the scheduled expiration of currently issued patents. Pernix may reduce, but not eliminate, the royalty payable to the Company if generic competitors attain a pre-determined share of the market for the combination product, or if Pernix owes a royalty to one or more third parties for rights it licenses from such third parties to commercialize the product. Immediately following the closing of the transaction, the Company entered into an amendment to the GSK Agreement with Pernix. This amendment, among other things, amends the royalty provisions to provide for a guaranteed quarterly minimum royalty of $4 million for the calendar quarters commencing in January 2015 and ending in March 2018 and requires that Pernix continue certain of GSK’s ongoing development activities and to undertake certain new activities, for which the Company will provide reasonable assistance. This amendment to the GSK Agreement also eliminates restrictions in the GSK Agreement on the Company’s right to develop and commercialize certain dosage forms of sumatriptan/naproxen combinations outside of the United States and permits the Company to seek approval for these combinations on the basis of the approved NDA for Treximet.
Agreement with Endo Regarding Toprol-XL AG
The Company is party to a Distribution Agreement with Endo Ventures Limited (“Endo”) pursuant to which Endo distributes the Toprol-XL AG (the “Toprol-XL AG Agreement”). The agreement was originally entered into by AstraZeneca with PAR Pharmaceutical, Inc. (“PAR”) in August 2006 and was assigned by PAR to Endo in February 2016 in connection with Endo International plc’s acquisition of PAR. AstraZeneca assigned such agreement to us in connection with our acquisition of Toprol-XL and the AG in October 2016. Pursuant to the Toprol-XL AG Agreement, Endo has the exclusive rights in the United States to promote the AG, while we retain the right to promote the branded Toprol-XL and to promote the AG to certain mail service pharmacy providers. Pursuant to the terms of the Toprol-XL AG Agreement, the Company supplies the AG product to Endo for a base purchase price, which ranges depending on dosage strength. In addition to the base purchase price, Endo pays to the Company, on a monthly basis, a deferred purchase price equal to a certain percentage of the specified profit of this business for the applicable period. The agreement expires at the end of 2017 and may be terminated by either party under certain circumstances, including performance measures.
F-27
4.FAIR VALUE
Assets and liabilities that are measured at fair value on a recurring basis
The following tables set forth the Company’s assets and liabilities that are measured at fair value on a recurring basis (in thousands) at:
|
|
|
December 31, 2016 |
|
||||||||||
|
|
|
Financial Instruments Carried at Fair Value |
|
||||||||||
|
|
|
|
|
Significant |
|
|
|
|
|
||||
|
|
|
Quoted prices in |
|
other |
|
Significant |
|
|
|
||||
|
|
|
active markets for |
|
observable |
|
unobservable |
|
|
|
||||
|
|
|
identical items |
|
inputs |
|
inputs |
|
|
|
||||
|
|
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
|
Total |
|
||||
|
Assets: |
|
|
|
|
|
|
|
|
|
||||
|
Cash and cash equivalents |
|
$ |
64,943 |
|
$ |
— |
|
$ |
— |
|
$ |
64,943 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Contingent consideration |
|
$ |
— |
|
$ |
— |
|
$ |
71,115 |
|
$ |
71,115 |
|
|
Warrants liability |
|
|
— |
|
|
— |
|
|
24 |
|
|
24 |
|
|
|
|
December 31, 2015 |
|
||||||||||
|
|
|
Financial Instruments Carried at Fair Value |
|
||||||||||
|
|
|
|
|
Significant |
|
|
|
|
|
||||
|
|
|
Quoted prices in |
|
other |
|
Significant |
|
|
|
||||
|
|
|
active markets for |
|
observable |
|
unobservable |
|
|
|
||||
|
|
|
identical items |
|
inputs |
|
inputs |
|
|
|
||||
|
|
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
|
Total |
|
||||
|
Assets: |
|
|
|
|
|
|
|
|
|
||||
|
Cash and cash equivalents |
|
$ |
24,816 |
|
$ |
― |
|
$ |
— |
|
$ |
24,816 |
|
Warrants Liability
In connection with the acquisition of Tribute, the Company assumed a liability for warrants that are treated as derivatives under accounting guidance for derivatives and hedging as they were issued with exercise prices denominated in a currency different than the Company’s reporting currency. Approximately 46,000 of the total 0.9 million common shares underlying the warrants outstanding as of December 31, 2016 are classified as liabilities. The warrants liability is valued using a Black-Scholes valuation model, which incorporates Level 3 assumptions including the volatility of the underlying share price and the expected term. The change in the fair value of the warrants liability of $4.7 million is included within other (expense) income, net in the consolidated statements of operations for the year ended December 31, 2016. A majority of the Company’s liability-classified warrants expired in the third quarter of 2016. See Note 12, “Shareholders’ Equity and Earnings Per Share,” for additional information.
Contingent Consideration
In connection with the acquisitions of Zontivity and Toprol-XL and the AG, the Company recorded short-term and long-term contingent consideration liabilities for future cash payments based on the occurrence of certain milestone events and royalty payments. The contingent consideration liability for both Zontivity and Toprol-XL and the AG is valued using a model, which incorporates Level 3 assumptions, including the estimated amount and timing of projected cash flows, the probability of success (achievement of the contingent event) and the risk-adjusted discount rate used to present value the probability-weighted cash flows. The Zontivity contingent consideration liability decreased by $1.9 million from its acquisition date, while there was no change in the fair value of the Toprol-XL contingent consideration liability between its acquisition date and December 31, 2016. See Note 2, “Business Combinations and Acquisitions,” for additional information.
F-28
Level 3 Disclosures
The following table provides quantitative information associated with the fair value measurement of the Company’s Level 3 inputs at December 31, 2016:
|
|
|
|
|
|
|
|
|
|
Range of |
|
|
|
|
(in thousands) |
|
Valuation technique |
|
Unobservable Inputs |
|
Inputs Utilized |
|
|
|
Warrants liability |
|
$ |
24 |
|
Black-Scholes |
|
Volatility |
|
57% |
|
|
|
|
|
|
|
|
|
Expected term in years |
|
0.4 |
|
|
Contingent consideration |
|
|
71,115 |
|
Monte Carlo |
|
Volatility |
|
33% - 68% |
|
|
|
|
|
|
|
|
|
Discount rate |
|
13% |
|
The table below provides a roll-forward of the warrants liability fair value balances that used Level 3 inputs (in thousands):
|
Balance at December 31, 2015 |
|
$ |
— |
|
Warrants liability assumed in Merger |
|
|
4,618 |
|
Change in fair value during the period |
|
|
(4,744) |
|
Impact of foreign exchange |
|
|
150 |
|
Balance at December 31, 2016 |
|
$ |
24 |
The table below provides a roll-forward of the contingent consideration liability fair value balances that used Level 3 inputs (in thousands):
|
Balance at December 31, 2015 |
|
$ |
— |
|
|
Contingent consideration recorded in ZONTIVITY acquisition |
|
|
17,600 |
|
|
Contingent consideration recorded in Toprol-XL acquisition |
|
|
52,800 |
|
|
Cash payments |
|
|
(35) |
|
|
Change in fair value during the period |
|
|
750 |
|
|
Balance at December 31, 2016 |
|
$ |
71,115 |
|
For the year ended December 31, 2016, the change in fair value of contingent consideration was primarily due to the passage of time.
Assets and liabilities measured at fair value on a non-recurring basis
The following table sets forth the Company’s assets that were measured at fair value on a non-recurring basis at December 31, 2016 (in thousands):
|
|
|
December 31, 2016 |
|
||||||||||
|
|
|
Fair value measurement at reporting date |
|
||||||||||
|
|
|
|
|
Significant |
|
|
|
|
|
||||
|
|
|
Quoted prices in |
|
other |
|
Significant |
|
Total impairment |
|
||||
|
|
|
active markets for |
|
observable |
|
unobservable |
|
charge recorded |
|
||||
|
|
|
identical items |
|
inputs |
|
inputs |
|
for the year ended |
|
||||
|
|
|
(Level 1) |
|
(Level 2) |
|
(Level 3) |
|
December 31, 2016 |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets: |
|
|
|
|
|
|
|
|
|
||||
|
IPR&D (Note 1) |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
695 |
|
|
Acquired technology rights (Note 7) |
|
|
— |
|
|
— |
|
|
— |
|
|
3,673 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-29
5.INVENTORY
Inventory consisted of the following at:
|
|
|
December 31, 2016 |
|
December 31, 2015 |
|
||
|
|
|
(in thousands) |
|
||||
|
Raw materials |
|
$ |
1,129 |
|
$ |
— |
|
|
Work-in-process |
|
|
189 |
|
|
— |
|
|
Finished goods |
|
|
3,230 |
|
|
— |
|
|
Total Inventory |
|
$ |
4,548 |
|
$ |
— |
|
6.PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consisted of the following at:
|
|
|
|
|
|
|
Estimated |
|
||
|
|
|
December 31, 2016 |
|
December 31, 2015 |
|
Life |
|
||
|
|
|
(in thousands) |
|
(in years) |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
Furniture and fixtures |
|
$ |
486 |
|
$ |
223 |
|
5 - 7 |
|
|
Equipment |
|
|
460 |
|
|
568 |
|
5 - 7 |
|
|
Leasehold improvements |
|
|
895 |
|
|
188 |
|
5 - 10 |
|
|
Land, buildings and improvements |
|
|
275 |
|
|
— |
|
25 - 40 |
|
|
Construction in progress |
|
|
5,437 |
|
|
— |
|
|
|
|
|
|
|
7,553 |
|
|
979 |
|
|
|
|
Less: Accumulated depreciation |
|
|
(237) |
|
|
(728) |
|
|
|
|
|
|
$ |
7,316 |
|
$ |
251 |
|
|
|
Depreciation expense was approximately $0.4 million, $0.02 million and $0.02 million for the years ended December 31, 2016, 2015 and 2014, respectively.
7.GOODWILL AND OTHER INTANGIBLE ASSETS, NET
Goodwill
The table below provides a roll-forward of the goodwill balance (as adjusted, in thousands):
|
Goodwill balance at December 31, 2015 |
|
$ |
— |
|
|
Goodwill from acquisition of Tribute |
|
|
69,324 |
|
|
Goodwill from acquisition of ZONTIVITY |
|
|
1,800 |
|
|
Goodwill from acquisition of Toprol-XL |
|
|
3,200 |
|
|
Impact of foreign exchange |
|
|
2,370 |
|
|
Goodwill balance at December 31, 2016 |
|
$ |
76,694 |
|
There were no accumulated impairment losses to goodwill at December 31, 2016.
F-30
Other Intangible Assets, Net
Other intangible assets, net consisted of the following at:
|
|
|
December 31, 2016 |
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
Weighted |
|
|||
|
|
|
Gross Carrying |
|
Accumulated |
|
Net Carrying |
|
Average |
|
|||
|
|
|
Amount |
|
Amortization |
|
Amount |
|
Life |
|
|||
|
|
|
(in thousands) |
|
(in years) |
|
|||||||
|
Toprol-XL |
|
$ |
224,600 |
|
$ |
(1,275) |
|
$ |
223,325 |
|
10 |
|
|
ZONTIVITY |
|
|
40,800 |
|
|
(3,757) |
|
|
37,043 |
|
11 |
|
|
Tribute Merger and other |
|
|
87,268 |
|
|
(7,442) |
|
|
79,826 |
|
11 |
|
|
Acquired technology rights |
|
$ |
352,668 |
|
$ |
(12,474) |
|
$ |
340,194 |
|
|
|
The gross carrying amount of acquired technology rights from the Merger increased $3.2 million between the Merger closing date and December 31, 2016 due to (i) the addition of $2.8 million reclassified from acquired IPR&D for Blexten, which was approved in April 2016, (ii) approximately $1.0 million in regulatory milestones due to Faes as a result of the approval of Blexten, and (iii) $3.1 million from the impact of foreign currency translation adjustments between the Canadian and U.S. dollars. Partially offsetting these increases was an impairment charge of $3.7 million recorded in the fourth quarter of 2016 relating to the acquired technology rights for one product acquired in the Merger. This charge is included in Impairment of intangible assets on the Company’s statements of operations. The carrying value for this product was written down to fair value based on its estimated cash flows for 2017, after which the Company’s exclusive distribution agreement is terminated. The remaining carrying value of $0.5 million at December 31, 2016 will be amortized over the next twelve months.
Amortization expense was $12.6 million for the year ended December 31, 2016. There was no amortization expense for the years ended December 31, 2015 and 2014.
The estimated aggregate amortization of intangible assets as of December 31, 2016, for each of the five succeeding years and thereafter is as follows:
|
|
|
Estimated |
|
|
|
|
|
Amortization |
|
|
|
For the Years Ending December 31, |
|
Expense |
|
|
|
|
|
(in thousands) |
|
|
|
2017 |
|
$ |
34,083 |
|
|
2018 |
|
|
34,083 |
|
|
2019 |
|
|
34,083 |
|
|
2020 |
|
|
34,083 |
|
|
2021 |
|
|
34,083 |
|
|
Thereafter |
|
|
169,779 |
|
|
Total amortization expense |
|
$ |
340,194 |
|
8.ACCRUED EXPENSES
Accrued expenses consisted of the following at:
|
|
|
December 31, 2016 |
|
December 31, 2015 |
|
||
|
|
|
(in thousands) |
|
||||
|
Accrued professional fees |
|
$ |
6,258 |
|
$ |
3,012 |
|
|
Accrued marketing fees |
|
|
4,852 |
|
|
— |
|
|
Accrued revenue reserves |
|
|
3,783 |
|
|
— |
|
|
Accrued royalties |
|
|
2,996 |
|
|
— |
|
|
Accrued employee-related expenses |
|
|
9,153 |
|
|
5,229 |
|
|
Other accrued liabilities |
|
|
5,099 |
|
|
3,691 |
|
|
Total accrued expenses |
|
$ |
32,141 |
|
$ |
11,932 |
|
F-31
Exit and Disposal Activities
In connection with the Merger, the Company incurred certain exit costs, primarily severance benefits to former Pozen and Tribute employees. The Company incurred severance expense of $2.4 million during the year ended December 31, 2016, which is primarily included within selling, general and administrative expenses in the consolidated statements of operations.
The following table summarizes the exit activity within accrued expenses and other long-term liabilities in the consolidated balance sheets (in thousands):
|
Accrued severance balance at December 31, 2015 |
|
$ |
3,986 |
|
Accrued severance liability assumed in the Merger |
|
|
2,484 |
|
Severance expense |
|
|
2,375 |
|
Cash payments |
|
|
(6,577) |
|
Impact of foreign exchange |
|
|
32 |
|
Accrued severance balance at December 31, 2016 |
|
$ |
2,300 |
The Company expects to pay the remaining $2.3 million in 2017.
9.DEBT
Convertible Notes
On February 5, 2016, Aralez issued $75.0 million aggregate principal of 2.5% senior secured convertible notes due February 2022 (“2022 Notes”) resulting in net proceeds to Aralez, after debt issuance costs, of $74.5 million in connection with the Facility Agreement, which was executed in December 2015 among the Credit Parties and certain lenders. The 2022 Notes are convertible into common shares of Aralez at an initial conversion premium of 32.5%, subject to adjustment upon certain events, which is equivalent to an initial conversion price of approximately $8.28 per common share. Holders of the 2022 Notes may convert the 2022 Notes at any time and the 2022 Notes are not pre-payable by Aralez. Interest is payable to the note holders quarterly in arrears on the first business day of each January, April, July and October. Interest expense for the year ended December 31, 2016 was $1.8 million, which includes the amortization of debt issuance costs. The Company estimated the fair value of the $75.0 million aggregate principal amount of the outstanding 2022 Notes to be approximately $60.5 million as of December 31, 2016, using a bond plus call option model that utilizes Level 3 fair value inputs. The carrying amount of the 2022 Notes was $74.5 million as of December 31, 2016, which is the principal amount outstanding, net of $0.5 million of unamortized debt issuance costs to be amortized over the remaining term of the 2022 Notes.
Credit Facility
Under the terms of the Facility Agreement, Aralez also had the ability to borrow from the lenders up to $200 million under a credit facility until April 30, 2017. The credit facility can be drawn upon for permitted acquisitions and is to be repaid on the sixth anniversary from each draw. Amounts drawn under the credit facility will bear an interest rate of 12.5% per annum and shall be prepayable in whole or in part at any time following the end of the sixth month after the funding date of each draw. The Facility Agreement contains various representations and warranties, and affirmative and negative covenants, customary for financings of this type, including, among other things, limitations on asset sales, mergers and acquisitions, indebtedness, liens and dividends.
On October 31, 2016, Aralez drew down $25 million under the credit facility to replenish the Company’s cash balance for the initial upfront payment of the $25 million in cash previously paid at the closing of the Zontivity acquisition in September 2016 and drew down an additional $175 million to finance the upfront cash payment for the acquisition of Toprol-XL and the AG.
Interest is payable to the note holders quarterly in arrears on the first business day of each January, April, July and October. Interest expense for the year ended December 31, 2016 was $4.2 million, which includes the amortization of debt issuance costs. The Company estimated the fair value of the $200.0 million aggregate principal amount of the outstanding borrowings under the credit facility under the Facility Agreement to be approximately $204.8 million as of December 31, 2016, using a bond model that utilizes Level 3 fair value inputs. The carrying amount of the borrowings
F-32
under the credit facility was $199.9 million as of December 31, 2016, which is the principal amount outstanding, net of $0.1 million of unamortized debt issuance costs to be amortized over the remaining term of the credit facility.
In addition, pursuant to a consent to the Facility Agreement entered into in connection with the acquisition of Toprol-XL and the AG, the lenders under the Facility Agreement agreed that they and/or affiliated funds will have available sufficient capital to make additional loans to Aralez in an aggregate amount of up to $250 million for the payment of the purchase price of any acquisitions permitted by the terms of the Facility Agreement (as modified by such consent) with respect to target businesses mutually approved by, and as otherwise mutually agreed upon, by Aralez and the lenders, subject to the satisfaction of certain conditions set forth in the Facility Agreement. At the time of such consent, the Facility Agreement was amended to include additional financial performance thresholds, including a minimum adjusted EBITDA threshold and a minimum specified revenue threshold relating to net sales of Toprol-XL and the AG received by the Company. The performance thresholds are not applicable to the Company’s performance as of December 31, 2016.
MFI Note
On June 16, 2015, Tribute acquired MFI. As part of the consideration paid, Tribute issued a one-year unsecured convertible promissory note in the aggregate amount of C$5.0 million ($3.9 million) to the prior owner of MFI (“MFI Note”). The MFI Note had an interest rate of 8% per annum and was convertible in whole or in part at the holder’s option during the term into Aralez common shares at a conversion rate of approximately C$12.21 per Aralez common share. The MFI Note was repaid in full along with accrued interest at its maturity date of June 16, 2016, for a total payment of approximately $4.2 million.
10.INCOME TAXES
Income (loss) before income taxes, classified by source of income (loss), is as follows:
|
|
|
For the Years Ended December 31, |
|
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
|
|
(in thousands) |
|
|||||||
|
Canadian |
|
$ |
(25,424) |
|
$ |
— |
|
$ |
— |
|
|
U.S. |
|
|
(6,582) |
|
|
(8,508) |
|
|
19,675 |
|
|
Irish |
|
|
(85,294) |
|
|
(29,101) |
|
|
— |
|
|
Other Foreign |
|
|
14,258 |
|
|
— |
|
|
— |
|
|
Income (loss) before income taxes |
|
$ |
(103,042) |
|
$ |
(37,609) |
|
$ |
19,675 |
|
The provision for income taxes consists of the following:
|
|
|
For the Years Ended December 31, |
|
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
Current provision: |
|
(in thousands) |
|
|||||||
|
Canadian |
|
$ |
45 |
|
$ |
— |
|
$ |
— |
|
|
U.S. Federal |
|
|
2,182 |
|
|
— |
|
|
— |
|
|
U.S. State |
|
|
1,629 |
|
|
174 |
|
|
— |
|
|
Irish |
|
|
— |
|
|
— |
|
|
— |
|
|
Other Foreign |
|
|
32 |
|
|
— |
|
|
— |
|
|
Total current provision |
|
|
3,888 |
|
|
174 |
|
|
— |
|
|
Deferred benefit: |
|
|
|
|
|
|
|
|
|
|
|
Canadian |
|
|
(3,147) |
|
|
— |
|
|
— |
|
|
U.S. Federal |
|
|
(614) |
|
|
— |
|
|
— |
|
|
U.S. State |
|
|
(182) |
|
|
— |
|
|
— |
|
|
Irish |
|
|
— |
|
|
— |
|
|
— |
|
|
Other Foreign |
|
|
(9) |
|
|
— |
|
|
— |
|
|
Total deferred benefit |
|
|
(3,952) |
|
|
— |
|
|
— |
|
|
Total current and deferred provision (benefit) |
|
$ |
(64) |
|
$ |
174 |
|
$ |
— |
|
F-33
The actual income tax (benefit) expense for the years ended December 31, 2016, 2015 and 2014, differed from the amounts computed by applying the Canadian federal tax rate in 2016 of 26.5% resulting from the Merger and the U.S. federal tax rate of 35% in 2015 and 2014 to income (loss) before taxes as a result of the following:
|
|
|
For the Years Ended December 31, |
|
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
|
|
(in thousands) |
|
|||||||
|
(Loss) income before income tax |
|
$ |
(103,042) |
|
$ |
(37,609) |
|
$ |
19,675 |
|
|
Statutory tax rate |
|
|
26.5 |
% |
|
35 |
% |
|
35 |
% |
|
Income tax provision at statutory rate |
|
|
(27,306) |
|
|
(13,163) |
|
|
6,886 |
|
|
U.S. State tax provision |
|
|
1,140 |
|
|
(48) |
|
|
224 |
|
|
|
|
|
(26,166) |
|
|
(13,211) |
|
|
7,110 |
|
|
Decrease (increase) in income tax benefit resulting from: |
|
|
|
|
|
|
|
|
|
|
|
Foreign tax rate differential |
|
|
12,594 |
|
|
6,548 |
|
|
— |
|
|
Research and development credits |
|
|
(296) |
|
|
(574) |
|
|
4 |
|
|
Non-deductible expenses and other |
|
|
171 |
|
|
819 |
|
|
212 |
|
|
Non-deductible executive compensation |
|
|
3,965 |
|
|
1,279 |
|
|
— |
|
|
Non-deductible transaction costs |
|
|
3,272 |
|
|
|
|
|
|
|
|
Non-deductible excise tax |
|
|
2,160 |
|
|
— |
|
|
— |
|
|
Notional interest deduction |
|
|
(4,115) |
|
|
— |
|
|
— |
|
|
Deferred tax asset adjustment |
|
|
1,533 |
|
|
2,629 |
|
|
— |
|
|
Change in valuation allowance |
|
|
6,818 |
|
|
2,684 |
|
|
(7,326) |
|
|
Income tax expense |
|
$ |
(64) |
|
$ |
174 |
|
$ |
— |
|
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of Aralez’s deferred tax assets are as follows:
|
|
|
December 31 |
|
||||
|
|
|
2016 |
|
2015 |
|
||
|
Non-current |
|
|
|
|
|
|
|
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
Tax loss carryforwards |
|
$ |
35,430 |
|
$ |
18,011 |
|
|
Research and development credits |
|
|
15,064 |
|
|
14,513 |
|
|
Equity compensation |
|
|
6,258 |
|
|
4,375 |
|
|
Transaction costs |
|
|
308 |
|
|
4,156 |
|
|
Other |
|
|
2,268 |
|
|
2,833 |
|
|
Total deferred tax assets |
|
|
59,328 |
|
|
43,888 |
|
|
Less valuation allowance |
|
|
(50,706) |
|
|
(43,888) |
|
|
Net deferred tax assets |
|
$ |
8,622 |
|
$ |
— |
|
|
Deferred tax liabilities: |
|
|
|
|
|
|
|
|
Intangible assets |
|
|
(11,066) |
|
|
— |
|
|
Total deferred tax liabilities |
|
|
(11,066) |
|
|
— |
|
|
Net deferred tax liability |
|
$ |
(2,444) |
|
$ |
— |
|
The net deferred tax liability of $2.44 million consists of the deferred tax liability of $3.27 million offset by a deferred tax asset of $0.83 million included within other long-term assets on the balance sheet.
At December 31, 2016 and 2015, Aralez had Canadian net operating loss carryforwards of approximately $34.6 million and $0 million, respectively, U.S. federal net operating loss carryforwards of approximately $41.6 million and $46.3 million, respectively, U.S. state net operating loss carryforwards of approximately $41.7 million and $61.1 million, respectively, Irish net operating loss carryforwards of $110.6 million and $24.3 million, respectively, and U.S. research and development credit carryforwards of approximately $14.8 million and $14.5 million, respectively. The Canadian, U.S. federal and U.S. state net operating loss carryforwards begin to expire in 2026, 2030 and 2017, respectively, and the U.S. research and development credit carryforwards begin to expire in 2019. Aralez’s U.S. federal and U.S. state net operating loss carryforwards include approximately $7.8 million of excess tax benefits related to tax deductions from stock-based compensation. The tax benefit of these deductions has not been recognized in deferred tax assets. If utilized,
F-34
the benefits from these deductions will be recorded as adjustments to additional paid-in capital. Based upon the accumulation of historical losses in material jurisdictions, a valuation allowance has been recognized to offset a significant portion of the deferred tax assets due to the uncertainty surrounding Aralez’s ability to realize these deferred tax assets in future periods. Certain deferred tax assets in the U.S. and Canada are considered to be realizable due to the existence of taxable income in a carryback period or reversing deferred tax liabilities.
The utilization of the loss carryforwards to reduce future income taxes will depend on Aralez’s ability to generate sufficient taxable income prior to the expiration of the loss carryforwards. In addition, the maximum annual use of net operating loss and research credit carryforwards is limited in certain situations where changes occur in stock ownership, including the change in ownership resulting from the Merger. The cash tax benefit related to net operating loss carryforwards was approximately $3.2 million, $2.5 million and $4.8 million for the years ended December 31, 2016, 2015 and 2014, respectively.
Aralez had gross unrecognized tax benefits of approximately $0.6 million as of both December 31, 2016 and December 31, 2015 and, of this total, none would reduce Aralez’s effective tax rate if recognized. Aralez does not anticipate a significant change in total unrecognized tax benefits or Aralez’s effective tax rate due to the settlement of audits or the expiration of statutes of limitations within the next 12 months.
The following table summarizes the activity related to the Company’s unrecognized tax benefits (in thousands):
|
|
|
For the Years Ended December 31, |
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|||
|
|
|
(in thousands) |
|||||||
|
Beginning balance |
|
$ |
572 |
|
$ |
537 |
|
$ |
538 |
|
(Decreases) increases related to prior year tax positions |
|
|
16 |
|
|
32 |
|
|
(1) |
|
Increases related to current year tax positions |
|
|
— |
|
|
3 |
|
|
— |
|
Ending balance |
|
$ |
588 |
|
$ |
572 |
|
$ |
537 |
Aralez’s policy for recording interest and penalties associated with tax audits is to record them as a component of provision for income taxes. Aralez has not recorded any interest or penalty since adoption of FASB ASC 740-10.
Aralez files federal and state income tax returns, as applicable, with the tax authorities in various jurisdictions including Canada, Ireland and the United States. Pozen is no longer subject to U.S. federal or North Carolina state income tax examinations by tax authorities for years before 2013. Tribute is no longer subject to Canadian income tax examinations by tax authorities for years before 2011. However, the loss and credit carryforwards generated by Pozen and Tribute may still be subject to change to the extent these losses and credits are utilized in a year that is subject to examination by tax authorities.
The Company has not provided for taxes as it relates to permanently reinvested foreign earnings. While it is not practicable to estimate the potential income taxes the Company does not believe the distribution of existing foreign earnings would result in a material tax cost.
11.EARNINGS PER SHARE
Basic and Diluted Net Loss Per Common Share
Basic net loss per common share has been computed by dividing net loss by the weighted average number of shares outstanding during the period. Except where the result would be antidilutive to income from continuing operations, diluted net loss per common share is computed assuming the conversion of convertible obligations and the elimination of the interest expense related to the 2022 Notes, the exercise of options to purchase common shares, the exercise of warrants, and the vesting of restricted stock units (“RSUs”), as well as their related income tax effects. Diluted net loss per common share differs from basic net loss per common share for the year ended December 31, 2016
F-35
given potential common shares underlying the warrants liability are dilutive when considering the unrealized gain recognized for the change in the fair value of the warrants during the period.
|
|
|
|
For the Year Ended December 31, |
|
||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
|
|
|
(in thousands, except share and per share data) |
|
||||||
|
Net (loss) income, basic |
|
$ |
(102,978) |
|
$ |
(37,783) |
|
$ |
19,675 |
|
|
Effect of dilutive securities: |
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of warrants liability |
|
|
(4,744) |
|
|
— |
|
|
— |
|
|
Net loss, diluted |
|
$ |
(107,722) |
|
$ |
(37,783) |
|
$ |
19,675 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares used in calculating basic net loss per common share |
|
|
61,830,967 |
|
|
32,589,795 |
|
|
31,359,867 |
|
|
Effect of dilutive securities: |
|
|
|
|
|
|
|
|
|
|
|
Effect of dilutive stock options |
|
|
— |
|
|
— |
|
|
1,450,720 |
|
|
Warrants to purchase common shares - liability-classified |
|
|
52,049 |
|
|
— |
|
|
— |
|
|
Shares used in calculating diluted net loss per common share |
|
|
61,883,016 |
|
|
32,589,795 |
|
|
32,810,587 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income per common share, basic |
|
$ |
(1.67) |
|
$ |
(1.16) |
|
$ |
0.63 |
|
|
Net (loss) income per common share, diluted |
|
$ |
(1.74) |
|
$ |
(1.16) |
|
$ |
0.60 |
|
Potential common shares excluded from the calculation of diluted net loss per common share as their inclusion would have been antidilutive were:
|
|
|
|
|
||||
|
|
|
For the Year Ended December 31, |
|
||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|
|
|
|
|
|
|
|
|
|
Options to purchase common shares, RSUs and PSUs |
|
7,388,888 |
|
— |
|
— |
|
|
Warrants to purchase common shares - equity-classified |
|
92,223 |
|
— |
|
— |
|
|
2022 Notes convertible into common shares |
|
8,190,534 |
|
— |
|
— |
|
The Company assumed outstanding warrants in connection with the acquisition of Tribute. The warrants are classified either as a liability, if the exercise price is denominated in Canadian dollars, or as equity if the exercise price is denominated in U.S. dollars. The following is a summary of warrants outstanding and exercisable as of December 31, 2016, and grouped in accordance with their respective expiration dates, with Canadian dollar exercise prices translated to U.S. dollars at the foreign exchange rate in effect at December 31, 2016:
|
|
|
No. of Warrants |
|
Weighted-Average |
|
|
Quarterly period of expiration |
|
Outstanding |
|
Exercise Price |
|
|
Q2 2017 |
|
46,497 |
|
$ |
4.71 |
|
Q1 2018 |
|
599,278 |
|
|
4.12 |
|
Q3 2018 |
|
15,815 |
|
|
3.78 |
|
Q4 2019 |
|
107,670 |
|
|
4.81 |
|
Q3 2020 |
|
109,968 |
|
|
4.09 |
|
Q1 2021 |
|
50,521 |
|
|
2.91 |
|
|
|
929,749 |
|
$ |
4.16 |
12.SHARE-BASED COMPENSATION
Summary of Share-Based Compensation Plans
In December 2015, the Company’s Board of Directors adopted the Aralez Pharmaceuticals 2016 Long-Term Incentive Plan (the “2016 Plan”), which became effective on February 5, 2016, upon consummation of the Merger. The 2016 Plan is the only existing plan in which the Company is authorized to grant equity-based awards. The 2016 Plan provides for grants of stock options, stock appreciation rights, stock awards, stock units, performance shares, performance units, and other stock-based awards to employees, directors, and consultants. Under the 2016 Plan, the Company initially reserved 2,300,000 common shares for grant plus (i) the number of shares available for issuance under
F-36
both the Pozen Inc. 2010 Equity Compensation Plan and the Amended and Restated Option Plan of Tribute Pharmaceuticals Canada Inc. that were not subject to outstanding awards upon the effective date and (ii) the number of shares required to cover each stock option granted in substitution of stock options held by employees of Tribute, as required to consummate the Merger. At December 31, 2016, there were 1,922,805 common shares remaining available for grant under the 2016 Plan.
Summary of Share-Based Compensation Expense
Share-based compensation expense recorded in the consolidated statements of operations for the years ended December 31, 2016, 2015 and 2014, was as follows:
|
|
|
Years Ended December 31, |
|
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
|
|
(in thousands) |
|
|||||||
|
Selling, general and administrative |
|
$ |
11,537 |
|
$ |
6,870 |
|
$ |
1,585 |
|
|
Research and development |
|
|
328 |
|
|
173 |
|
|
296 |
|
|
Total non-cash share-based compensation expense |
|
$ |
11,865 |
|
$ |
7,043 |
|
$ |
1,881 |
|
Included in the table above is approximately $0.5 million of share-based compensation expense related to the accelerated vesting of certain Tribute equity awards upon consummation of the Merger, which was recorded as selling, general and administrative expense for the year ended December 31, 2016.
Options to Purchase Common Shares
A summary of option activity for the year ended December 31, 2016 is as follows:
|
|
|
|
|
Weighted- |
|
|
Weighted- |
|
|
|
||
|
|
|
Underlying |
|
Average |
|
|
Average |
|
|
|
||
|
|
|
Shares |
|
Exercise |
|
|
Remaining |
|
Intrinsic |
|
||
|
Stock Option Awards |
|
(in thousands) |
|
Price |
|
|
Contractual Term |
|
Value |
|
||
|
Outstanding at December 31, 2015 |
|
1,985 |
|
$ |
8.18 |
|
|
2.5 years |
|
|
|
|
|
Granted |
|
2,467 |
|
|
3.77 |
|
|
|
|
|
|
|
|
Exercised |
|
(682) |
|
|
3.15 |
|
|
|
|
|
|
|
|
Forfeited or expired |
|
(705) |
|
|
8.40 |
|
|
|
|
|
|
|
|
Outstanding at December 31, 2016 |
|
3,065 |
|
$ |
5.85 |
|
|
4.8 years |
|
$ |
1,335,972 |
|
|
Vested and expected to vest at December 31, 2016 |
|
2,988 |
|
$ |
5.89 |
|
|
4.7 years |
|
$ |
1,308,370 |
|
|
Exercisable at December 31, 2016 |
|
2,082 |
|
$ |
6.59 |
|
|
1.6 years |
|
$ |
856,113 |
|
The weighted average grant date fair value for option awards granted during the year ended December 31, 2016 was $2.54 per option. No option awards were granted during the years ended December 31, 2015 and 2014.
A total of 681,683 stock options were exercised during the year ended December 31, 2016 with an intrinsic value of $0.8 million, a total of 727,000 stock options were exercised during the year ended December 31, 2015 with an intrinsic value of $2.0 million and a total of 1,479,000 stock options were exercised during the year ended December 31, 2014 with an intrinsic value of $4.6 million. The fair value of shares vested during the years ended December 31, 2016, 2015 and 2014 was $3.6 million, $1.8 million and $1.1 million, respectively.
Unrecognized stock-based compensation expense related to stock options, expected to be recognized over an estimated weighted-average amortization period of 2.0 years, was $2.6 million as of December 31, 2016.
The fair value of each option award was estimated on the date of grant using the Black-Scholes option valuation model. The weighted-average assumptions used in the Black-Scholes option valuation model for the year ended December 31, 2016 are shown in the following table:
F-37
|
|
|
2016 |
|
|
|
Expected volatility |
|
|
50.7 |
% |
|
Expected dividends |
|
|
— |
|
|
Expected term |
|
|
4 |
Years |
|
Risk-free interest rate |
|
|
0.79 |
% |
For the year ended December 31, 2016, the expected volatility rate was estimated based on an equal weighting of the historical volatility of the Company’s common shares over a period matching the expected term and the expected term was based upon average historical terms to exercise. The risk-free interest rate was based on U.S. Treasury securities with a maturity matching the expected term. The pre-vesting forfeiture rates used for the year ended December 31, 2016 was based on historical rates. The estimated forfeiture rate is adjusted based upon actual experience.
RSUs and PSUs
A summary of RSU, including performance restricted stock units (“PSUs”), activity for the year ended December 31, 2016, is as follows:
|
|
|
|
|
Weighted- |
|
|
|
|
|
Underlying |
|
Average |
|
|
|
|
|
Shares |
|
Grant Date |
|
|
|
Restricted Stock Units, including PSUs |
|
(in thousands) |
|
Fair Value |
|
|
|
Nonvested restricted stock units at December 31, 2015 |
|
4,042 |
|
$ |
7.80 |
|
|
Granted |
|
1,636 |
|
|
4.48 |
|
|
Vested |
|
(1,306) |
|
|
7.64 |
|
|
Forfeited or expired |
|
(48) |
|
|
5.00 |
|
|
Nonvested restricted stock units at December 31, 2016 |
|
4,324 |
|
$ |
6.62 |
|
During the year ended December 31, 2016, 654,737 PSUs with both market-based and service conditions were granted with an aggregate grant-date fair value of $2.8 million. The PSUs granted in 2016 were tied to a three-year relative total shareholder return (“TSR”) as the performance goal (measured against companies in the NASDAQ biotechnology index with annual revenue between $50 million and $500 million). The actual number of shares awarded is adjusted to between 50% and 200% of the target award amount based upon achievement of the pre-determined goals. TSR relative to peers is considered a market condition under applicable authoritative guidance and the Company used a Monte Carlo simulation model to determine the fair value of these awards as of the grant date.
Unrecognized stock-based compensation expense related to RSUs, expected to be recognized over an estimated weighted-average amortization period of 2.4 years, was $22.9 million at December 31, 2016.
13.COMMITMENTS AND CONTINGENCIES
Operating Leases
The Company leases office space and certain equipment under cancellable and non-cancelable operating lease agreements. Rent expense was approximately $0.8 million, $0.4 million, $0.4 million for the years ended December 31, 2016, 2015 and 2014 respectively. Future minimum payments under our non-cancelable lease agreements at December 31, 2016 were as follows (in thousands):
|
2017 |
|
$ |
1,374 |
|
|
2018 |
|
|
2,235 |
|
|
2019 |
|
|
2,257 |
|
|
2020 |
|
|
2,239 |
|
|
2021 |
|
|
1,763 |
|
|
Thereafter |
|
|
9,593 |
|
|
Total minimum payments |
|
$ |
19,461 |
|
F-38
On March 31, 2016, the lease relating to approximately 17,009 square feet of office space located at Exchange Office Building, Chapel Hill, North Carolina, expired in accordance with its terms, as amended. In April 2016, the Company entered into an agreement to lease approximately 36,602 square feet of office space for its U.S. headquarters in Princeton, New Jersey. Pursuant to the lease agreement, the Company issued a letter of credit in the amount of $0.3 million to the property owner as a security deposit, which is classified as restricted cash and included within other current assets on the consolidated balance sheet as of December 31, 2016.
Supply Agreements
The Company has various supply, license, distribution and manufacturing agreements with third parties that include purchase minimums or minimum royalties. Pursuant to these agreements, the Company has minimum future obligations of approximately $2.4 million as of December 31, 2016.
Legal Proceedings
The Company is currently party to legal proceedings arising in the normal course of business, principally patent litigation matters. The Company has assessed such legal proceedings and does not believe that it is probable that a liability has been incurred or that the amount of any potential liability or range of losses can be reasonably estimated. As a result, the Company has not recorded any loss contingencies for any of these matters as of December 31, 2016. While it is not possible to determine the outcome of these matters, in the event of an adverse outcome or outcomes, the Company’s business could be materially harmed. The Company intends to vigorously defend its intellectual property rights.
VIMOVO® ANDA Litigation
Between March 14, 2011 and May 16, 2013, Pozen, now a subsidiary of the Company, received Paragraph IV Notice Letters from Dr. Reddy’s Laboratories (“DRL”), Lupin Ltd. (“Lupin”), Watson Laboratories, Inc. – Florida (“Watson,” now “Actavis”), and Mylan Pharmaceuticals Inc. (“Mylan”), stating that each had filed an Abbreviated New Drug Application (“ANDA”) with the U.S. Food and Drug Administration (“FDA”) seeking regulatory approval to market a generic version of our VIMOVO product before the expiration of U.S. Patent No. 6,926,907 (the “907 patent”). On November 20, 2012, Pozen received a second Notice Letter from DRL stating that DRL had filed a second ANDA with the FDA seeking regulatory approval to market a different generic formulation of the VIMOVO product before the expiration of the ‘907 patent. The ‘907 patent is assigned to Pozen and listed for the VIMOVO product in the FDA’s publication titled “Approved Drug Products with Therapeutic Equivalence Evaluations” (also known as the “Orange Book”).
On April 21, 2011, Pozen filed suit against the first ANDA filer, DRL, in the United States District Court for the District of New Jersey (the “District Court”), asserting infringement of the ‘907 patent. Pozen subsequently filed suit against the other three ANDA filers within 45 days of receipt of their respective Paragraph IV Notice Letters. Horizon, our current marketing partner for the VIMOVO product in the U.S., is Pozen’s co-plaintiff in each suit. The first suit against DRL is considered the lead case and has been consolidated with other suits for the purpose of pre-trial and discovery. On December 19, 2012, the District Court conducted a pre-trial Markman hearing to determine the proper claim construction of certain claims disputed by the parties. On May 1, 2013, the District Court issued a Markman Order construing the disputed claims. A scheduling order for the consolidated suits was issued by the District Court on June 27, 2014.
On October 15, 2013, the United States Patent & Trademark Office (“USPTO”) issued to Pozen U.S. Patent No. 8,557,285 (the “‘285 patent”). The ‘285 patent is listed in the Orange Book for the VIMOVO product and is related to the ‘907 patent. On October 23, 2013, Pozen filed suits against DRL, Lupin, Watson and Mylan in the District Court asserting infringement of the ‘285 patent. These suits have each been consolidated with the above referenced suits involving the ‘907 patent. On May 12, 2016, the court granted DRL’s motion for summary judgment of non-infringement of the ‘907 patent with respect DRL’s second ANDA. The ruling does not apply to DRL’s first-filed ANDA, nor does it apply to the other patents asserted against DRL’s second ANDA. In January 2017, Judge Cooper conducted a six day bench trial in the lead case involving Defendants DRL and Mylan relating solely to the validity and infringement of the ‘907 and ‘285 patents. The parties are in the process of providing post-trial submissions to the District Court. It is anticipated the closing arguments will take place after post-trial submissions are complete.
F-39
On October 7, 2014, the USPTO issued to Pozen U.S. Patent No. 8,852,636 (the “‘636 patent”). On October 14, 2014, the USPTO issued to Pozen U.S. Patent No. 8,858,996 (the “‘996 patent”). In addition, on October 21, 2014, the USPTO issued to Pozen U.S. Patent No. 8,865,190 (the “190 patent”). The ‘636, ‘996 and ‘190 patents are each listed in the Orange Book for the VIMOVO product and are each related to the ‘907 and ‘285 patents.
On February 3, 2015, the USPTO issued to Pozen U.S. Patent No. 8,945,621 (the “‘621 patent”). The ‘621 patent is listed in the Orange Book for the VIMOVO product.
On May 13, 2015, Pozen and Horizon filed suit against DRL, Lupin, Actavis (formerly known as Watson) and Mylan in the District Court asserting infringement of the ‘636 and ‘996 patents. On June 18, 2015, Pozen filed Amended Complaints in each of the suits to assert infringement of the ‘190 patent.
On October 20, 2015, the USPTO issued to Pozen U.S. Patent No. 9,161,920 (the “‘920 patent”). On December 1, 2015, the USPTO issued to Pozen U.S. Patent No. 9,198,888 (the “‘888 patent”). The ‘920 and ‘888 patents are each listed in the Orange Book for the VIMOVO product and are each related to the ‘907 and ‘285 patents.
On December 29, 2015, the USPTO issued to Pozen U.S. Patent No. 9,220,698 (the “‘698 patent”). The ‘698 patent is listed in the Orange Book for the VIMOVO product.
On May 24, 2016, the USPTO issued to Pozen U.S. Patent No. 9,345,695 (the “‘695 patent”). The ‘695 patent is listed in the Orange Book for the VIMOVO product and is related to the ‘907 and ‘285 patents.
On January 25, 2016, Pozen and Horizon filed suit against Actavis in the District Court asserting infringement of the ‘920 and ‘888 patents. On March 16, 2016, the District Court consolidated this suit with the suit filed against Actavis on May 13, 2015. On February 10, 2016, Pozen filed Amended Complaints against DRL, Lupin and Mylan to assert infringement of the ‘920 and ‘888 patents. On August 10, 2016, Pozen and Horizon filed suit against DRL, Lupin, Actavis and Mylan in the District Court asserting infringement of the ’621, ’698, and ’695 patents. These suits are in the initial phase and a full schedule has not yet been set by the District Court.
On December 30, 2016, the District Court granted Actavis’ motion to enforce an alleged settlement agreement resolving all claims and counterclaims between Actavis and co-plaintiffs Pozen and Horizon in the lawsuits relating to VIMOVO. Pozen and Horizon contend that they did not agree to the settlement, and Pozen and Horizon filed notices of appeal of the District Court’s decision, on February 8, 2017 and February 9, 2017, respectively.
As with any litigation proceeding, we cannot predict with certainty the outcome of the patent infringement suits against DRL, Lupin, Mylan and Actavis relating to generic versions of VIMOVO. Furthermore, while Horizon is responsible for this litigation, including the costs of same, we nevertheless will have to incur additional expenses in connection with the lawsuits relating to VIMOVO, which may be substantial. Moreover, responding to and defending pending litigation results in a significant diversion of management’s attention and resources and an increase in professional fees.
Inter Partes Review
DRL filed a Petition for review (“IPR Petition”) of the ‘285 patent with the Patent Trial and Appeal Board (“PTAB”) of the USPTO on February 24, 2015, which was denied on October 9, 2015. The Coalition for Affordable Drugs VII L.L.C. (“CFAD”) filed IPR Petitions of the ‘907 patent, the ‘996 patent and the ‘636 patent with the PTAB on May 21, 2015, June 5, 2014 and August 7, 2015, respectively, each of which was denied on December 8, 2015, December 17, 2015 and February 11, 2016, respectively.
On August 12, 2015, CFAD filed an IPR Petition of the ‘621 patent with the PTAB. On February 22, 2016 the PTAB instituted review of the claims of the ‘621 patent. Pozen and Horizon filed a response on June 23, 2016. CFAD filed a reply to this response on September 22, 2016. Oral argument before the PTAB was held on November 16, 2016. On February 21, 2017, the PTAB entered a Final Written Decision in which it concluded that CFAD had not carried its burden of proving that the claims of the ‘621 patent were unpatentable.
On August 19, 2015, Lupin filed three separate IPR Petitions of the ‘996, ‘636 and ‘190 patents with the PTAB. On March 1, 2016 the PTAB denied Lupin’s petition for review of the ‘636 patent and instituted review of a limited
F-40
number of the claims in each of the ‘996 and ‘190 patents. Pozen and Horizon filed responses to the petitions for review of the ‘996 and ‘190 patents on June 27, 2016. Lupin filed replies to these responses on September 16, 2016. Oral arguments before the PTAB for these matters were held on November 29, 2016. On February 28, 2017, the PTAB entered Final Written Decisions in which it concluded that Lupin had not carried its burden of proving that the claims of the ‘996 and ‘190 patents were unpatentable.
Canada VIMOVO® Litigation
On January 20, 2015, our Canadian licensee, AstraZeneca Canada Inc. (“AstraZeneca Canada”) received a Notice of Allegation from Mylan Pharmaceuticals ULC (“Mylan Canada”) informing them that Mylan Canada has filed an Abbreviated New Drug Submission in Canada (“ANDS”) for approval of its naproxen/esomeprazole magnesium tablets and alleging non-infringement of some of the claims and invalidity of Pozen’s Canadian Patent No. 2,449,098 (the “‘098 patent”). A Notice of Allegation is served pursuant to the Patented Medicines (Notice of Compliance) Regulations in Canada and is similar to a Paragraph IV Notice Letter in the United States. In response, Pozen and AstraZeneca Canada commenced a proceeding in the Federal Court of Canada (the “Canada Court”) in relation to the ‘098 patent on March 5, 2015 seeking to prohibit Health Canada from approving Mylan Canada’s generic naproxen/esomeprazole product until the expiry of the ‘098 patent. The Canadian proceeding is summary in nature intended to decide only whether approval for Mylan Canada’s naproxen/esomeprazole magnesium tablets should be prohibited until the expiry of the ‘098 patent because none of Mylan Canada’s allegations in respect of the ‘098 patent are justified. The matter was heard on November 21 to 23, 2016. On February 7, 2017, the Court dismissed Pozen and AstraZeneca Canada’s request to prohibit the Minister from approving Mylan’s naproxen/esomeprazole products, deciding that certain of Mylan Canada’s allegations in respect of the ‘098 patent are justified (the “Decision”). However, this summary proceeding did not decide ‘098 patent validity or infringement. The ‘098 patent expires on May 31, 2022. Following the Decision, the Minister issued approval for Mylan’s 500/20mg strength naproxen/esomeprazole magnesium tablets on February 8, 2017
On March 23, 2016, AstraZeneca Canada received another Notice of Allegation from Mylan Canada in respect of the ‘098 patent, informing them that Mylan Canada has filed a supplemental submission for one of the strengths of its naproxen/esomeprazole magnesium tablets. This Notice of Allegation states that Mylan Canada withdrew from its ANDS the 375/20 mg strength and re-filed a supplemental submission for this strength. In this circumstance, Mylan is required to file, and has provided another Notice of Allegation in respect of the ‘098 patent. The allegations in respect of the ‘098 patent are identical to those asserted in the first Notice of Allegation. In response, Pozen and AstraZeneca Canada commenced another proceeding in the Federal Court of Canada on May 5, 2016 seeking to prohibit Health Canada from approving Mylan Canada’s 375/20 mg strength naproxen/esomeprazole magnesium tablet until the expiry of the ‘098 patent. As the allegations made in respect of the ‘098 patent are identical, on the parties’ consent, the Court stayed the proceeding and the parties agreed that the outcome of the first proceeding discussed above, will determine the outcome for this new proceeding. Following the Decision, this proceeding was discontinued on February, 10, 2017. The Minister issued approval for Mylan’s 375/20 mg strength naproxen/esomeprazole magnesium tablets on February 10, 2017. It is expected that Mylan’s generic naproxen/esomeprazole magnesium tablets will be available in Canada shortly.
Yosprala Paragraph IV Certification
On November 4, 2016, the FDA website indicated that an ANDA for a generic version of Yosprala 81mg/40mg was submitted to the FDA on October 14, 2016. The Company ultimately received the related Paragraph IV Notice Letter on December 12, 2016, as described below.
On December 12, 2016, the Company received a Paragraph IV Notice Letter from Teva Pharmaceuticals USA, Inc. (“Teva”) stating that it had filed an ANDA with the FDA seeking regulatory approval to market generic versions of Yosprala 325mg/40 mg and 81mg/40mg prior to the expiration of the ‘907 patent, U.S. Patent No. 8,206,741 (the “‘741 patent”), and U.S. Patent No. 9,364,439 (the “‘439 patent”). The ‘907, ‘741, and ‘439 patents are assigned to Pozen and listed in the Orange Book for the Yosprala product.
On January 10, 2017, the USPTO issued to Pozen U.S. Patent No. 9,539,214 (the “‘214 patent”). The ‘214 patent is listed in the Orange Book for the Yosprala product. On March 13, 2017, the Company received a Paragraph IV Notice Letter regarding the ‘214 patent.
F-41
On January 23, 2017, Aralez Parent and its subsidiaries Aralez Pharmaceuticals Trading DAC, Aralez Pharmaceuticals US Inc., and Pozen Inc. filed a lawsuit in the United States District Court for the Eastern District of Texas against Teva and Teva Pharmaceutical Industries Ltd. for infringement of the ‘907, ‘741, ‘439, and ‘214 patents. The lawsuit was filed within 45 days of receipt of Teva’s Paragraph IV Notice Letter. In accordance with the Hatch-Waxman Act, as a result of having filed a timely lawsuit against Teva, a stay of approval will be imposed by the FDA on Teva’s ANDA for 30 months after the date of the Company’s receipt of Teva’s Paragraph IV Notice Letter on December 12, 2016 or until a final court decision is entered in the infringement suit in favor of Teva, whichever is earlier. The suit is in the initial phase and a full schedule has not yet been set.
As with any litigation proceeding, we cannot predict with certainty the outcome of the infringement suit relating to generic versions of Yosprala.
14.SEGMENT INFORMATION
Aralez has one operating segment, the acquisition, development and commercialization of products primarily in cardiovascular, pain and other specialty areas for the purpose of delivering meaningful products to improve patients’ lives while focusing on creating shareholder value. The Company’s entire business is managed by a single management team, which reports to the Chief Executive Officer.
The geographic segment information provided below is classified based on the major geographic regions in which the Company operates. Revenues in the U.S. consist primarily of Vimovo royalties, and for the year ended December 31, 2016, also include Toprol-XL and Zontivity net revenues from their respective acquisition dates.
|
|
|
For the Years Ended December 31, |
|
|||||||
|
|
|
2016 |
|
2015 |
|
2014 |
|
|||
|
|
|
(in thousands) |
|
|||||||
|
Net revenues: |
|
|
|
|
|
|
|
|
|
|
|
United States |
|
$ |
30,077 |
|
$ |
21,391 |
|
$ |
32,394 |
|
|
Canada |
|
|
24,193 |
|
|
— |
|
|
— |
|
|
Total revenues, net |
|
|
54,270 |
|
|
21,391 |
|
|
32,394 |
|
|
|
|
December 31, 2016 |
|
December 31, 2015 |
|
||
|
|
|
(in thousands) |
|
||||
|
Long-lived assets: |
|
|
|
|
|
|
|
|
United States |
|
$ |
281,399 |
|
$ |
251 |
|
|
Canada |
|
|
143,647 |
|
|
— |
|
|
Total long-lived assets |
|
$ |
425,046 |
|
$ |
251 |
|
15. RETIREMENT SAVINGS PLAN
The Company has an employee savings and retirement plan which is qualified under Section 401(k) of the Internal Revenue Code. The Company made matching contributions for the years ended December 31, 2016, 2015 and 2014 of $0.5 million, $0.2 million and $0.1 million, respectively.
F-42
16. SELECTED QUARTERLY FINANCIAL DATA (UNAUDITED)
The following table presents selected quarterly financial data for the years ended December 31, 2016 and 2015.
|
|
|
Three Months Ended |
|
||||||||||
|
|
|
|
Mar. 31, 2016 |
|
|
June 30, 2016 |
|
|
Sept. 30, 2016 |
|
|
Dec. 31, 2016 |
|
|
|
|
(In thousands, except per share data) |
|||||||||||
|
Total revenues, net |
|
$ |
8,057 |
|
$ |
12,578 |
|
$ |
13,628 |
|
$ |
20,007 |
|
|
Cost of product revenues |
|
|
2,538 |
|
|
3,360 |
|
|
3,362 |
|
|
2,505 |
|
|
Other operating costs |
|
|
43,143 |
|
|
26,339 |
|
|
29,900 |
|
|
45,707 |
|
|
Net loss |
|
$ |
(33,788) |
|
$ |
(17,475) |
|
$ |
(20,599) |
|
$ |
(31,116) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net loss per common share |
|
$ |
(0.65) |
|
$ |
(0.27) |
|
$ |
(0.32) |
|
$ |
(0.48) |
|
|
Diluted net loss per common share |
|
$ |
(0.73) |
|
$ |
(0.27) |
|
$ |
(0.32) |
|
$ |
(0.48) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended |
|
||||||||||
|
|
|
|
Mar. 31, 2015 |
|
|
June 30, 2015 |
|
|
Sept. 30, 2015 |
|
|
Dec. 31, 2015 |
|
|
|
|
(In thousands, except per share data) |
|||||||||||
|
Total revenues, net |
|
$ |
4,404 |
|
$ |
5,201 |
|
$ |
5,820 |
|
$ |
5,966 |
|
|
Other operating costs |
|
|
4,246 |
|
|
20,495 |
|
|
14,013 |
|
|
20,103 |
|
|
Net loss |
|
$ |
(27) |
|
$ |
(16,280) |
|
$ |
(8,149) |
|
$ |
(13,327) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net loss per common share |
|
$ |
— |
|
$ |
(0.50) |
|
$ |
(0.25) |
|
$ |
(0.40) |
|
|
Diluted net loss per common share |
|
$ |
— |
|
$ |
(0.50) |
|
$ |
(0.25) |
|
$ |
(0.40) |
|
17. SUBSEQUENT EVENTS
On February 23, 2017, Aralez Pharmaceuticals US Inc. (“Aralez US”), a Delaware company and a wholly-owned, indirect subsidiary of Aralez Pharmaceuticals Inc., entered into a Novation Agreement with AstraZeneca Pharmaceuticals LP (“AstraZeneca LP”) and the United States of America (the “Government”) pursuant to which all of the rights and responsibilities of AstraZeneca LP under that certain VA National Contract signed February 11, 2016 and effective April 29, 2016 between AstraZeneca LP and the Government (the “VA Contract”) were novated to Aralez US (the “Novation Agreement”). The Novation Agreement was entered into pursuant to the Toprol-XL Asset Purchase Agreement.
Under the VA Contract, Aralez US provides all requirements of certain pharmaceutical products containing metroprolol succinate as the active pharmaceutical ingredient at fixed prices for the U.S. Department of Veterans Affairs and certain other United States federal government agencies. The VA Contract has a one-year term, renewable at the option of the Government for four successive additional one year terms. The VA Contract is terminable at the convenience of the Government at any time.
F-43
