Attached files
| file | filename |
|---|---|
| EX-31.2 - EXHIBIT 31.2 - Aralez Pharmaceuticals Inc. | ex31_2.htm |
| EX-32.1 - EXHIBIT 32.1 - Aralez Pharmaceuticals Inc. | ex32_1.htm |
| EX-23.1 - EXHIBIT 23.1 - Aralez Pharmaceuticals Inc. | ex23_1.htm |
| EX-32.2 - EXHIBIT 32.2 - Aralez Pharmaceuticals Inc. | ex32_2.htm |
| EX-21.1 - EXHIBIT 21.1 - Aralez Pharmaceuticals Inc. | ex21_1.htm |
| EX-31.1 - EXHIBIT 31.1 - Aralez Pharmaceuticals Inc. | ex31_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2015
OR
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM TO .
Commission file number 001-37691
ARALEZ PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
|
British Columbia, Canada
|
98-1283375
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
151 Steeles Avenue East, Milton, Ontario, Canada, L9T 1Y1
(Address of registrant’s principal executive offices)
(905) 876-1118
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Name of each exchange on which registered
|
|
|
Common Shares, without par value
|
NASDAQ Global Market, Toronto Stock Exchange
|
Securities registered pursuant to Section 12(g) of the Act:
Common Shares, no par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐.
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐.
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (Check one):
|
Large accelerated filer ☐
|
Accelerated filer ☒
|
|
Non-accelerated filer ☐
|
Smaller reporting company ☐
|
Indicate by check mark whether the registrant is a shell company (as defined in 12b-2 of the Act). Yes ☐ No ☒.
The aggregate market value of the common shares held by non-affiliates of the registrant (computed by reference to the closing sale price of $10.31 for the registrant’s common shares as reported on the NASDAQ Global Market on June 30, 2015) was approximately $260,695,649. As of the close of business on March 8, 2016, there were 63,910,319 common shares issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of Aralez Pharmaceuticals Inc.’s definitive proxy statement to be filed pursuant to Regulation 14A within 120 days after the end of the registrant’s fiscal year are incorporated by reference into Part III of this Form 10-K and certain documents are incorporated by reference into Part IV.
ii
ARALEZ PHARMACEUTICALS INC.
ANNUAL REPORT ON FORM 10-K
|
PAGE
|
||
| 1 | ||
|
PART I
|
||
|
Item 1.
|
1 | |
|
Item 1A.
|
27 | |
|
Item 1B.
|
54 | |
|
Item 2.
|
54 | |
|
Item 3.
|
55 | |
|
Item 4.
|
57 | |
|
PART II
|
||
|
Item 5.
|
58 | |
|
Item 6.
|
61 | |
|
Item 7.
|
62 | |
|
Item 7A.
|
82 | |
|
Item 8.
|
82 | |
|
Item 9.
|
82 | |
|
Item 9A.
|
82 | |
|
Item 9B.
|
83 | |
|
PART III
|
||
|
Item 10.
|
84 | |
|
Item 11.
|
84 | |
|
Item 12.
|
84 | |
|
Item 13.
|
84 | |
|
Item 14.
|
84 | |
|
PART IV
|
||
|
Item 15.
|
85 | |
| 89 |
This Annual Report on Form 10-K includes “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and within the meaning of applicable securities laws in Canada. Forward-looking statements include, but are not limited to, statements about the expected benefits of the Tribute Transaction (as defined below), including growth potential, our strategies, plans, objectives, financial forecasts, goals, prospects, prospective products or product approvals, future performance or results of current and anticipated products, exposure to foreign currency exchange rate fluctuations, interest rate changes and other statements that are not historical facts, and such statements are typically identified by use of terms such as “may,” “will,” “would,” “should,” “could,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “likely,” “potential,” “continue” or the negative or similar words, variations of these words or other comparable words or phrases, although some forward-looking statements are expressed differently. You should be aware that the forward-looking statements included herein represent management’s current judgment and expectations, but our actual results, events and performance could differ materially from those in the forward-looking statements. The forward-looking statements are subject to a number of risks and uncertainties which are discussed in the section entitled “Item 1A. Risk Factors” and elsewhere in this Annual Report on Form 10-K and those described from time to time in our future reports filed with the Securities and Exchange Commission (“SEC”) and securities regulatory authorities in Canada. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee future results, events, levels of activity, performance or achievement. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, unless required by law.
All dollar amounts are expressed in U.S. dollars unless otherwise noted. All amounts are expressed on an as-converted from Canadian dollar to U.S. dollar basis and are calculated using the conversion rate as of December 31, 2015 unless otherwise noted.
Unless the context indicates otherwise, when we refer to “we,” “us,” “our,” “Aralez” or the “Company” in this Annual Report on Form 10-K, we are referring to Aralez Pharmaceuticals Inc. and its subsidiaries on a consolidated basis.
PART I
Overview
Incorporation and Registered Office
Aralez was incorporated under the British Columbia Business Corporations Act (“BCBCA”) on December 2, 2015. Our registered office is located at 666 Burrard Street, Suite 1700, Vancouver, British Columbia, V6C 2X8 and our principal executive offices are located at 151 Steeles Avenue East, Milton, Ontario, Canada, L9T 1Y1, 3 Columbus Circle, Suite 1710, New York, New York, 10019, and 56 Fitzwilliam Square, Dublin 2, Ireland.
Our Company
Aralez is a global specialty pharmaceutical company focused on delivering meaningful products to improve patients’ lives while focusing on creating shareholder value by acquiring, developing and commercializing products primarily in cardiovascular, pain and other specialty areas. Aralez’s global headquarters is located in Ontario, Canada, its U.S. headquarters is located in New York, New York and its Irish headquarters is located in Dublin, Ireland. Aralez was formed for the purpose of facilitating the business combination of POZEN Inc., a Delaware corporation (“Pozen”), and Tribute Pharmaceuticals Canada Inc., a corporation incorporated under the laws of the Province of Ontario, Canada (“Tribute”), which business combination was consummated on February 5, 2016. Aralez has had no operations as of December 31, 2015, other than business incident to the Tribute Transaction (as defined below).
Our management team has a strong track record of success in creating, leading and expanding specialty pharmaceutical companies with marketing and sales capabilities. Directed by this leadership and leveraging our competitive platform, our focus on acquiring high potential growth opportunities through aggressive business development and licensing and strategic M&A and commercializing healthcare products to provide enhanced value to a range of stakeholders is driven by the following primary strategies:
| ● | Maximize value of expanded portfolio – We plan to continue our progress toward building out our U.S. commercial organization, including growing our sales force and promoting the use of Fibricor® in the United States to grow product use moderately in the United States and which we expect will develop a relationship springboard with cardiologists ahead of the anticipated approval and commercial launch of YOSPRALA™. |
| ● | Business development through selective acquisitions – We have completed numerous transactions over the past few years to expand our portfolio offering. We will continue to pursue value-driven business development opportunities as they arise and enhance our product pipeline and expand our geographic footprint through strategically acquiring low-risk, revenue generating product candidates or approved products, particularly in the cardiovascular and pain anchor areas, but also in other specialty therapeutic areas that we anticipate are or will become revenue generating and accretive. |
| ● | Leverage platform for growth – We intend to maintain a lean, nimble and performance-oriented operating model with strong financial discipline. Our well-capitalized financial profile provides us with ample liquidity to commercialize YOSPRALA, if and when approved, and creates the opportunity for sustained long-term growth, both organically and through acquisitions, while also enabling us to have an ongoing focus on growing shareholder value. |
On February 5, 2016, pursuant to the Merger Agreement (as defined below) Aralez completed the acquisition of Tribute by way of a court approved plan of arrangement in a stock transaction with an estimated purchase price of $138 million made up of (i) $115 million related to Tribute shares, equity awards and certain warrants outstanding and (ii) $23 million in repayments of Tribute indebtedness. In connection with the transaction, Pozen and Tribute were combined under and became subsidiaries of Aralez Pharmaceuticals Inc., with Pozen treated as the acquiring company for accounting purposes (the “Tribute Transaction”). Pursuant to Rule 12g-3(a) under the Exchange Act, Aralez Pharmaceuticals Inc. is the successor issuer to Pozen. The Tribute Transaction provides the combined company with increased financial strength and product portfolio diversity and is expected to meaningfully accelerate our operating strategies.
2015 Highlights
On June 1, 2015, the Board of Directors of Pozen announced the appointment of Adrian Adams as Chief Executive Officer and Andrew I. Koven as President and Chief Business Officer, each effective as of May 31, 2015. At the same time, Pozen announced the retirement of John R. Plachetka, founder and Chief Executive Officer of Pozen.
On June 8, 2015, Pozen entered into an Agreement and Plan of Merger and Arrangement (the “Merger Agreement”), among Tribute, Aguono Limited (which was renamed Aralez Pharmaceuticals Limited and subsequently renamed Aralez Pharmaceuticals plc in connection with its re-registration as a public limited company), a limited company incorporated in Ireland (“Former Parent”), Trafwell Limited, a private limited company incorporated in Ireland, ARLZ US Acquisition Corp., a corporation incorporated under the laws of the State of Delaware and a wholly-owned subsidiary of Former Parent, and ARLZ CA Acquisition Corp., a corporation incorporated under the laws of the Province of Ontario and a wholly-owned subsidiary of Former Parent (“Can Merger Sub”) in order to effectuate the merger of Pozen and Tribute. On December 7, 2015, the Merger Agreement was amended, pursuant to which, among other things, (i) the Company replaced Former Parent as a party to the Merger Agreement, whereby, after giving effect to the merger transactions, the Company would be the ultimate parent company of the combined companies, (ii) ARLZ US Acquisition II Corp., a corporation formed under the laws of the State of Delaware, would be merged with and into Pozen, with Pozen continuing as the surviving corporation and an indirect wholly-owned subsidiary of the Company, and (iii) Can Merger Sub and Tribute would amalgamate, with the separate legal existence of Can Merger Sub ceasing and Tribute and Can Merger Sub continuing as one corporation and as a wholly-owned subsidiary of the Company.
On June 19, 2015, the Board of Directors of Pozen announced the appointment of Mark A. Glickman as Chief Commercial Officer, Eric L. Trachtenberg as Deputy General Counsel, Scott J. Charles as Senior Vice President of Finance and Jennifer L. Armstrong as Executive Vice President of Human Resources and Administration.
On September 11, 2015, the Board of Directors of Pozen announced the appointment of James P. Tursi, M.D. as Chief Medical Officer, effective as of October 1, 2015.
In the third quarter of 2015, the Company made progress toward building out its commercial organization, including preparing to recruit a 25 person high quality sales force that would begin promoting Fibricor in the United States following the consummation of the Tribute Transaction.
On December 23, 2015, the Board of Directors of Pozen announced the appointment of Scott J. Charles as Chief Financial Officer and Eric L. Trachtenberg as General Counsel, Chief Compliance Officer and Corporate Secretary, both effective as of January 1, 2016.
On December 28, 2015, Pozen announced a change in its primary aspirin active pharmaceutical ingredient (“API”) supplier in connection with the New Drug Application (“NDA”) for YOSPRALA.
On February 5, 2016, the Tribute Transaction was consummated.
On March 14, 2016, the Company resubmitted the NDA for YOSPRALA with the Food and Drug Administration (“FDA”).
Products
Primary Commercialized Products
Products Marketed in the United States
| Fibricor® and Authorized Generic |
|
In May 2015, we acquired the U.S. rights to Fibricor (fenofibric acid) and its related authorized generic. Fibricor is indicated as a complementary therapy along with diet for the treatment of severe hypertriglyceridemia and as a complementary therapy along with diet to reduce elevated low-density lipoprotein (“LDL”) cholesterol (“LDL-C”), total cholesterol (“Total-C”), triglycerides (“TG”), and apolipoprotein B (“Apo B”), and to increase high-density lipoprotein (“HDL”) cholesterol (“HDL-C”) in patients with primary hypercholesterolemia or mixed dyslipidemia. Fibricor is currently protected by four U.S. patents extending to August 20, 2027.
Fibricor is a lipid-regulating agent available as tablets for oral administration. Fibrates like Fibricor, activate peroxisome proliferator activated receptor (“PPAR”) alpha, increasing the activity of lipoprotein lipase. This typically causes a decrease in triglyceride levels. PPAR alpha activation also increases HDL production. Each tablet contains 35mg or 105mg fenofibric acid, and the 35mg tablet is the lowest dose of fenofibric acid available in the United States. Fibricor is contraindicated in patients with severe renal impairment, active liver disease, liver function abnormalities, preexisting gallbladder disease or known hypersensitivity to fenofibric acid or fenofibrate.
Hyperlipidemia Treatment Options: Hyperlipidemia is a very common chronic condition and is characterized by an excess of fatty substances called lipids, mainly cholesterol and triglycerides, in the blood. It is also called hyperlipoproteinemia because these fatty substances travel in the blood attached to proteins. This is the only way that these fatty substances can remain dissolved while in circulation. Hyperlipidemia, in general, can be divided into two subcategories: (1) hypercholesterolemia, in which there is a high level of cholesterol; and (2) hypertriglyceridemia, in which there is a high level of triglycerides, the most common form of fat. In general, lifestyle modifications such as diet, exercise and smoking cessation are the first line of treatment. If unsuccessful, pharmacologic therapy is commonly utilized for the treatment of primary and secondary dyslipidemias. In managing secondary dyslipidemia, statin therapy is commonly prescribed. For the management of major triglyceride elevations, three agents are also commonly utilized: (1) fibric acid derivatives, such as Fibricor; (2) niacin; and (3) omega-3 fatty acids. Fibricor is approved in the United States and indicated as adjunctive therapy to diet for treatment of severe hypertriglyceridemia (TG ≥ 500 mg/dL) and as adjunctive therapy to diet to reduce elevated LDL-C, Total-C, TG, and Apo B, and to increase HDL-C in patients with primary hypercholesterolemia or mixed dyslipidemia.
Competitive Analysis: Cholesterol-lowering drugs in the United States include statins, niacin, bile-acid resins, fibric acid derivatives (fibrates), cholesterol absorption inhibitors, and anti-protein convertase subtilisin-like kexin type 9 (PCSK9) inhibitors. All classes of cholesterol-lowering medicines are most effective when combined with increased exercise and a low-fat, high-fiber diet. The statin class includes some of the largest-selling prescription products in the world (e.g., Lipitor®, Zocor® and Crestor®). Statins dominate single-agent prescribing for the treatment of lipid disorders. The niacin (nicotinic acid – vitamin B3) class includes brands such as Niaspan®, which work primarily on increasing HDL cholesterol. The cholesterol absorption inhibitor class has a single product, Zetia®. The PCSK9 inhibitor are a new class of treatments that currently include Praluent® and RepathaTM. The fibrates class of cholesterol lowering treatments is composed of three competing molecules: (1) gemfibrozil (Lopid®), (2) fenofibric acid (Fibricor, Trilipix®), and (3) fenofibrate (Tricor®). The fibrate market in the United States was $2.4 billion for 2015.
Products Marketed in Canada
|
Cambia®
|
Cambia (diclofenac potassium for oral solution) is a non-steroidal anti-inflammatory drug (“NSAID”) and the only prescription NSAID available and approved in Canada for the acute treatment of migraine attacks with or without aura in adults 18 years of age or older. Cambia was licensed from Nautilus Neurosciences, Inc. (“Nautilus”) in November 2010. Cambia was approved by the FDA in June 2009 and is currently marketed by Depomed, Inc. (“Depomed”) in the United States. Cambia was approved by Health Canada in March 2012 and was commercially launched to specialists in Canada in October 2012 and broadly to all primary care physicians in February 2013. Depomed acquired Nautilus and the U.S. and Canadian rights to Cambia in December 2013.
Cambia is available as an oral solution in individual packets each designed to deliver a 50mg dose when mixed in water. Cambia is the only approved prescription NSAID available in Canada that was studied and proven to be an effective treatment for migraine according to guidelines published in September 2013 by the International Headache Society that reached statistically significant results for all four co-primary endpoints, including: (1) pain free response at two hours; (2) nausea free; (3) photophobia free (sensitivity to light); and (4) phonophobia free (sensitivity to sound). In addition, Cambia provides fast migraine pain relief within 30 minutes of dosing due in part to the significant benefits of the proprietary Dynamic Buffering Technology™ (“DBT”). DBT provides for enhanced drug absorption and bioavailability. In fasting volunteers, measurable plasma levels were observed within 5 minutes of dosing with Cambia. Peak plasma levels were achieved at approximately 15 minutes, with a range of approximately 10 to 40 minutes. NSAIDs, such as Cambia, may increase the incidence of cardiovascular adverse events such as myocardial infarction (“MI”), stroke or thrombotic events, gastrointestinal adverse events such as peptic/duodenal ulceration, perforation and gastrointestinal bleeding and are contraindicated in the third trimester of pregnancy. The risk may increase with duration of use and patients should only take this medication as prescribed by a physician.
Migraine Treatment Options in Canada: There are a number of different treatment options for migraine in Canada. Acute migraine treatment options can be broken down to three main categories: (i) triptans or 5-HT1 receptor agonists (e.g., sumatriptan, rizatriptan); (ii) ergot alkaloids (“ergots”) (e.g., ergotamine, dihydroergotamine); and (iii) NSAIDs (e.g., Cambia). Triptans may cause dizziness, nausea, weakness and chest discomfort and should not be used by patients with heart disease, uncontrolled high blood pressure, blood vessel disease or who have a history of stroke. Ergots may cause chest pain, tingling or burning sensations, nausea, vomiting, and cramps. Furthermore, ergots may reduce blood flow to the extremities (hands and feet) and may lead to tissue damage. Ergots should also not be used by anyone with heart disease, uncontrolled high blood pressure or blood vessel disease.
In September 2013, the Canadian Neurological Sciences Federation issued revised Canadian Headache Society Guidelines for Acute Drug Therapy for Migraine Headaches through the Canadian Journal of Neurological Sciences. Cambia was acknowledged as a potential first line therapy, with a fast onset of action and having a strong recommendation, high quality evidence and recommended for the acute treatment of migraine.
Migraine in Canada: Canadian studies have shown migraine prevalence rates of 23% to 26% in women, and 8% to 10% in men. Over 4,000,000 Canadians suffer from migraine in Canada and that 60% of those with migraine have one or more attacks per month while 25% of those with migraine have at least one attack per week. One Canadian study found that those with migraine lose 6.5 days of work each year resulting from their migraine and, as a result, migraine is associated with a substantial social and economic impact. A study done in 1990 calculated that 7,000,000 workdays are lost annually in Canada due to migraine. It was also found that 51% of all women suffering from migraine have never consulted a physician for their headaches.
Competitive Analysis: It is estimated that one-half of all people suffering from migraines in Canada never seek help from a physician but rather self-treat their condition with over-the-counter (“OTC”) medications such as aspirin (e.g., Bayer®), acetaminophen (e.g., Tylenol®) and OTC NSAID’s such as ibuprofen (e.g., Advil®) and naproxen sodium (e.g., Aleve®). The main prescription pharmacological agents used to treat acute migraine includes the triptan class of drugs or 5-HT1 receptor agonists as they are known and these products include sumatriptan (Imitrex®), rizatriptan (Maxalt®), zolmitriptan (Zomig®), almotriptan (Axert®), naratriptan (Amerge®), eletriptan (Relpax®) and frovatriptan (Frova®). There are also the ergot alkaloids such as ergotamine (Cafergot®) and dihydroergotamine (Migrinal®) used in some cases as are narcotics such as meperidine (e.g., Demerol®) and the combination drug of aspirin, butalbital and caffeine (e.g., Fiorinal). In spite of a number of possible treatment options for treating migraines, many of these treatments are without an authorized indication from Health Canada. The Company considers the competitive market as the triptans class, which currently sells approximately $125 million annually in Canada.
|
Fiorinal®/Fiorinal® C
|
Fiorinal (acetylsalicylic acid, caffeine and butalbital tablets and capsules) and Fiorinal C (acetylsalicylic acid, caffeine, butalbital and codeine capsules) were acquired from Novartis AG and Novartis Pharma AG in October 2014. Fiorinal and Fiorinal C were originally approved by Health Canada in 1970 for the relief of tension-type headache.
Fiorinal is a fixed dose combination drug that combines the analgesic properties of acetylsalicylic acid, with the anxiolytic and muscle relaxant properties of butalbital, and the central nervous system stimulant properties of caffeine. Fiorinal C expands on the properties of Fiorinal with the additional analgesic effect of codeine. Fiorinal and Fiorinal C are the only prescription products in Canada indicated for relief of tension type headaches. Fiorinal and Fiorinal C are currently marketed in Canada in hard gelatin capsules containing 330mg acetylsalicylic acid, 40mg caffeine, 50mg butalbital, and in the case of Fiorinal C, the addition or 15mg or 30mg of codeine. Codeine and butalbital are both habit-forming and potentially abusable. Consequently, the extended use of Fiorinal or Fiorinal C is not recommended. Fiorinal and Fiorinal C are associated with exacerbation of headache (medication overuse headaches) in susceptible patients. Repeated use of Fiorinal and Fiorinal C can lead to “rebound” headaches as each dose wears off. With repeated doses physical and psychological dependence can develop. In addition to dependence, butalbital-containing products can lead to tolerance, and at higher doses can produce withdrawal symptoms after discontinuation.
Tension-Type Headache in Canada: Tension-type headaches are the most common type of headache and are caused by muscle tightening in the back of the neck or scalp. These headaches are typically triggered by emotional stress, fatigue or depression. There are two classifications of tension-type headache: (1) episodic tension headaches, which occur randomly and less frequently; and (2) chronic tension headaches, which may occur daily or continually and the intensity of the pain may vary during a 24-hour cycle. Tension headaches differ from migraine headaches due to the lack of aura, photophobia, phonophobia and/or nausea.
Competitive Analysis: Tension-type headaches may be treated with OTC NSAIDs like Tylenol®, Advil®, Aleve®, or Aspirin®. Prescription NSAIDs may also be used, such as Naprosyn®, Anaprox®, Toradol®, as well as prescription analgesic/opiate combinations like Percocet®, Tylenol® with codeine and Fiorinal/Fiorinal C. In spite of a number of possible treatment options for treating tension-type headaches, all of these treatments, with the exception of Fiorinal and Fiorinal C, are without an authorized indication from Health Canada. The Company considers the competitive market as the prescription NSAID and prescription analgesic/opiate combination class, which has an estimated tension-type headache value of approximately $30 million annually in Canada. The OTC market for tension-type headache is estimated to be exponentially larger given the large patient population; however, the true value is extremely difficult to determine considering the broad range of indications for OTC NSAIDs.
|
Soriatane®
|
Soriatane (acitretin) is chemically known as acitretin, and is indicated for the treatment of severe psoriasis (including erythrodermic and pustular types) and other disorders of keratinization. Soriatane is a retinoid, an aromatic analog of vitamin A. Soriatane was approved in Canada in 1994 and is the first and only oral retinoid indicated for psoriasis. Soriatane is often used when milder forms of psoriasis treatments like topical steroids, emollients and topical tar-based therapies have failed.
Soriatane should be reserved for patients unresponsive to, or intolerant of, standard treatment. In addition, Soriatane should only be prescribed by physicians knowledgeable in the use of systemic retinoids. Soriatane is teratogenic (can cause birth defects) and should not be used by women who are pregnant or who are planning to become pregnant during, or within three years after stopping, treatment of Soriatane. In addition, acitretin may cause nausea, headache, itching, dry, red or flaky skin, dry or red eyes, dry or chapped lips, swollen lips, dry mouth, thirst, cystitis acne or hair loss.
Psoriasis Treatment Options: There are a number of different treatment options for psoriasis. Typically, topical agents are used for mild disease, phototherapy for moderate disease and oral systemic agents and biologicals for more severe disease. The three main traditional systemic treatments are (1) methotrexate, (2) cyclosporine and (3) retinoids. Unlike Soriatane, methotrexate and cyclosporine are immunosuppressant drugs. Methotrexate may cause a decrease in the number of blood cells made by bone marrow, may cause liver damage, lung damage, damage to the lining of the mouth, stomach or intestines and may increase the risk of developing lymphoma (cancer that begins in the cells of the immune system), among other serious side effects. Methotrexate may also cause serious or life-threatening skin reactions. Cyclosporines may cause side effects that could be very serious, such as high blood pressure and kidney and liver problems. It may also reduce the body’s ability to fight infections.
Competitive Analysis: Severe psoriasis is a condition that involves more than 10% of the body area or is physically, occupationally or psychologically disabling. Soriatane will typically be used in combination with other drugs such as topical steroids, emollients or tar-based therapies. Soriatane is most effective for treating psoriasis when it is used with phototherapy. Soriatane may be used with biologic agents, such as etanercept (Enbrel®), adalimumab (Humira®) or infliximab (Remicade®), and may also be prescribed in rotation with cyclosporine or methotrexate. Biologic therapies such as Enbrel®, Humira® and Remicade® are effective in treating severe forms of the disease, but tend to be very expensive and sometimes not reimbursed by government or other private drug plans. Cyclosporine and methotrexate are also oral agents that are often used for severe forms of psoriasis. The market for moderate to severe psoriasis in Canada, including the biologics, is estimated to be greater than $200 million for 2015.
|
Bezalip® SR
|
Bezalip SR (bezafibrate) is an established pan-peroxisome proliferator-activated receptor activator. Bezalip SR, used to treat hyperlipidemia (high cholesterol), has over 25 years of therapeutic use globally. Bezalip SR helps lower LDL-C and triglycerides while raising HDL-C levels. It also improves insulin sensitivity and reduces blood glucose levels, which in combination with the cholesterol effects may significantly lower the incidence of cardiovascular events and development of diabetes in patients with features of metabolic syndrome. Bezalip SR is contraindicated in patients with hepatic and renal impairment, pre-existing gallbladder disease, hypersensitivity to bezafibrate, or pregnancy or lactation.
Bezalip SR is currently approved in more than 40 countries worldwide, not including the United States. Bezalip SR is under license from Actavis Group PTC (“Actavis”), and we have the exclusive rights to market Bezalip SR in Canada. We also have the exclusive development and licensing rights to Bezalip SR in the United States and filed an Investigational New Drug (“IND”) that received clearance from the FDA in the United States. Clinical studies would be required prior to commercialization in the United States. The initial target indication that would be considered for pursuit in the United States is for severe hypertriglyceridemia.
Hyperlipidemia Treatment Options: Hyperlipidemia is a very common chronic condition and is characterized by an excess of fatty substances called lipids, mainly cholesterol and triglycerides, in the blood. It is also called hyperlipoproteinemia because these fatty substances travel in the blood attached to proteins. This is the only way that these fatty substances can remain dissolved while in circulation. Hyperlipidemia, in general, can be divided into two subcategories: (1) hypercholesterolemia, in which there is a high level of cholesterol; and (2) hypertriglyceridemia, in which there is a high level of triglycerides, the most common form of fat.
Competitive Analysis: Cholesterol-lowering drugs in Canada include statins, niacin, bile-acid resins, fibric acid derivatives (fibrates), and cholesterol absorption inhibitors. All classes of cholesterol-lowering medicines are most effective when combined with increased exercise and a low-fat, high-fiber diet. The statin class includes some of the largest-selling prescription products in the world (e.g., atorvastatin (Lipitor®), simvastatin (Zocor®) and rosuvastatin (Crestor®)). Statins dominate single-agent prescribing for the treatment of lipid disorders. The niacin (nicotinic acid – vitamin B3) class includes brands such as Niaspan®, which work primarily on increasing HDL cholesterol. The fibrates class of cholesterol-lowering treatments is composed of three competing molecules: (1) gemfibrozil (Lopid®), (2) bezafibrate (Bezalip SR), and (3) fenofibrate (Lipidil® in Canada or Tricor® in the United States). Clinical studies have demonstrated that bezafibrate, the active ingredient in Bezalip SR, was shown to be effective in lowering high levels of triglycerides, raising HDL cholesterol and lowering LDL cholesterol. As of the end of 2015, the annual fibrate market in Canada is estimated to be approximately $35 million.
Other Commercialized Products
In addition to the products discussed above, we also market NeoVisc® (sodium hylauronic solution - 1%), Uracyst® (sodium chondroitin sulfate - 2%), Durela® (tramadol hydrochloride), Proferrin® (heme iron polypeptide), Resultz® (isopropyl myristate), Collatamp® G (collagen-gentamycin) and a portfolio of eight products targeted in the gastroenterology and women’s health markets in Canada.
Primary Development Products
|
YOSPRALA™
|
The products in the YOSPRALA (aspirin/omeprazole delayed release tablets) portfolio, which are part of our proton pump inhibitor (“PPI”)-aspirin (“PA”) platform, are being developed with the goal of significantly reducing gastrointestinal (“GI”) ulcers and other GI complications compared to taking enteric-coated, buffered or plain aspirin alone in patients at risk of developing GI ulcers. The first candidates in the YOSPRALA product portfolio are YOSPRALA 81/40 (PA8140), which contains 81mg of enteric-coated aspirin and 40mg immediate-release omeprazole, and YOSPRALA 325/40 (PA32540), which contains 325mg of enteric-coated aspirin and 40mg immediate-release omeprazole. Both products are a coordinated-delivery tablet combining immediate-release omeprazole, a PPI, layered around a pH-sensitive enteric coating of an aspirin core. This novel, patented product is intended for oral administration once a day.
Pending FDA review and approval, YOSPRALA 81/40 and 325/40 would be indicated for patients who require aspirin (1) to reduce the combined risk of death and nonfatal stroke in patients who have had ischemic stroke or transient ischemia of the brain due to fibrin platelet emboli, (2) to reduce the combined risk of death and nonfatal MI in patients with a previous MI or unstable angina pectoris, (3) to reduce the combined risk of MI and sudden death in patients with chronic stable angina pectoris, (4) for a pre-existing condition after having undergone revascularization procedures, and (5) the omeprazole component, to decrease the risk of developing gastric ulcers in patients at risk for developing aspirin-associated gastric ulcers.
Development History and Status: YOSPRALA 81/40 and 325/40 products have completed Phase 3 clinical development testing in the United States, and we resubmitted the NDA for these products with the FDA on March 14, 2016.
We met with the FDA to discuss the overall development program requirements for YOSPRALA 81/40 and 325/40 for the secondary prevention of cardiovascular and cerebrovascular disease in patients at risk for gastric ulcers. An IND was filed in the fourth quarter of 2007. We completed a study which demonstrated that the salicylic acid component of YOSPRALA 325/40 was bioequivalent to the reference drug, enteric-coated aspirin. We filed a Special Protocol Assessment with the FDA for the design of the Phase 3 studies for the product, the primary endpoint for which is the reduction in the cumulative incidence of endoscopic gastric ulcers.
In October 2009, we began two pivotal Phase 3 and one long-term safety study for YOSPRALA 325/40. The primary endpoint of the pivotal studies, which included approximately 500 subjects per study, was a significant reduction in the cumulative incidence of gastric ulcers following administration of YOSPRALA 325/40 compared to 325mg enteric-coated aspirin over the six-month treatment period. The primary endpoint was met with statistical significance in both studies. Additionally, the studies met their key secondary endpoints, including a reduction in gastroduodenal ulceration, as well as a reduction in discontinuation due to upper gastrointestinal adverse events in subjects taking YOSPRALA 325/40 compared to 325mg enteric-coated aspirin.
In February 2012, the FDA requested an additional Phase 1 study to assess the bioequivalence of YOSPRALA 325/40 to enteric-coated aspirin 325mg with respect to acetylsalicylic acid. After the Company completed the requested bioequivalence study, the FDA made a preliminary review of the study results and the Company’s summary analyses and, based on its preliminary assessment of the information available to it at the time, the FDA did not agree that bioequivalence of YOSPRALA 325/40 to enteric-coated aspirin 325mg was demonstrated. The Company then submitted to the FDA additional information and analyses from the requested bioequivalence study, as well as other relevant pharmacokinetic, clinical pharmacology, and in vitro dissolution data as a briefing document in support of a request for a Type A meeting with the FDA. At the Type A meeting held in August 2012 (the “August 2012 Type A Meeting”), the FDA confirmed that, although it believes bioequivalence of YOSPRALA 325/40 to enteric-coated aspirin 325mg was not strictly established in our bioequivalence study according to the predetermined criteria, the results from this study, together with additional information that was submitted by the Company in the NDA, constitutes sufficient data to support the establishment of a clinical and pharmacological bridge between the product and enteric-coated aspirin 325mg. The FDA indicated that it would make a final determination during the NDA review. The FDA also indicated that a similar strategy to bridge to the reference listed drug, inclusive of a new, single pharmacokinetic study, could be utilized for a low dose version of the 325/40mg version, YOSPRALA 81/40. The Company conducted this study with the low dose version against the enteric-coated aspirin 81mg. Based on the predetermined criteria acceptable to the FDA, the study demonstrated that YOSPRALA 81/40 is bioequivalent to enteric-coated aspirin 81mg and had comparable bioavailability.
During a pre-submission meeting with respect to its NDA for YOSPRALA 325/40 in April 2012, the FDA suggested that the Company also seek approval for a lower dose formulation of the product containing 81mg of enteric-coated aspirin as part of its NDA for YOSPRALA 325/40. Absent the availability of such a lower dose formulation in the market if YOSPRALA 325/40 is approved, the FDA indicated that it might limit the indication for YOSPRALA 325/40 to use in post coronary artery bypass graft surgery with treatment duration not to exceed one year. During the August 2012 Type A Meeting, the FDA confirmed its preference to have both YOSPRALA 325/40 and a lower dose version available in the market so that physicians can have both a low and high dose option available, and agreed that, if both dosage strengths were included in the NDA and subsequently approved, the indications for both will be consistent with the full range of indications described in the current aspirin monograph.
We had generated some clinical pharmacology data and chemical, manufacturing and controls (“CMC”) data for a lower dose version of YOSPRALA 325/40 – a product that contains 81mg of enteric-coated aspirin and 40mg of immediate-release omeprazole in a single tablet known YOSPRALA 81/40. The Company filed this existing data, together with additional CMC data to be generated and evidence from the scientific literature relating to the ulcerogenic risk of 81mg of aspirin with the FDA. At this time, we do not intend to conduct Phase 3 clinical trials for YOSPRALA 81/40. We have no assurance such data will be sufficient for the FDA to approve YOSPRALA 81/40 or to allow a broader indication for YOSPRALA 325/40. The FDA will make a final determination with respect to the approvability of and indications for YOSPRALA 325/40 and 81/40 upon our re-submission of the NDA, which we resubmitted with the FDA on March 14, 2016.
The generation of additional data with respect to YOSPRALA 81/40 and the incorporation of data into the NDA for YOSPRALA 325/40 delayed submission of the NDA from the original planned submission date in the third quarter of 2012. The NDA was filed for both products in March 2013, and in May 2013, the FDA accepted the NDA for review. The FDA assigned a user fee date of January 24, 2014. As part of our continuing discussions with the FDA concerning the NDA for YOSPRALA 325/40 and 81/40 tablets, we decided to conduct an additional comparative Phase 1 pharmacokinetic study to determine the pharmacokinetic profile of the omeprazole component of YOSPRALA 81/40 tablets and compare it to that of YOSPRALA 325/40 tablets. We submitted study information and data to the FDA as it became available during the conduct of the study and FDA reviewed such information and data from the study when submitted. The final study report was submitted to the FDA in accordance with our agreed timeline. The FDA informed us that the Company’s user fee date was moved to April 25, 2014.
On April 25, 2014, we received a Complete Response Letter (“CRL”) from the FDA advising that the review of our NDA was completed and questions remained that preclude the approval of the NDA in its then current form. Specifically, an inspection of the manufacturing facility of our previously designated primary aspirin API supplier concluded with certain inspection deficiencies. There were no clinical or safety deficiencies noted with respect to either YOSPRALA 325/40 or 81/40 and no other deficiencies were noted in the CRL. On June 30, 2014, we resubmitted the NDA for YOSPRALA 325/40 and 81/40 to the FDA and the FDA notified us that the new action fee date is December 30, 2014. On May 9, 2014, the aspirin API supplier submitted a response to the FDA addressing the inspection deficiencies and subsequently submitted an update to its initial response.
On December 17, 2014, we received a second CRL from the FDA advising that the review of our NDA was completed and questions remained that preclude approval of the NDA in its then current form. In this CRL, the FDA used identical wording to that of the first CRL. There were no clinical or safety deficiencies noted with respect to either YOSPRALA 325/40 or 81/40 and no other deficiencies were noted in the CRL. FDA regulations allowed us to request a Type A meeting with the FDA to discuss the next steps required to gain approval of our NDA. The FDA granted the Type A meeting, which was held in late January 2015. At the meeting, representatives from the FDA’s Office of Compliance stated that the aspirin API supplier’s responses to the 483 inspectional observations submitted in May 2014 were still under review and the Office of Compliance would be communicating with the supplier in the coming weeks. The aspirin API supplier subsequently informed us that it received a warning letter relating to the Form 483 inspection deficiencies and submitted a plan of corrective actions to the FDA to address the matters raised in the warning letter.
On December 28, 2015, we announced that the FDA had completed re-inspection of the aspirin API supplier’s manufacturing facility and issued an additional 483 notice, citing numerous observations. The aspirin API supplier voluntarily stopped production at this facility to focus on remediating the FDA observations. We have been informed that production at this facility has resumed and it remains subject to FDA inspection.
On December 28, 2015, we also announced that significant progress had been made with respect to an alternative aspirin API supplier, which is a global leader in aspirin manufacturing, and that we have now designated this secondary supplier as our primary supplier in connection with the NDA for YOSPRALA. We are focusing our efforts toward using our previously designated secondary aspirin API supplier as our primary supplier in connection with our NDA and will include both aspirin API suppliers in the NDA package for YOSPRALA. Final agreement on the draft labeling is also pending. We resubmitted the NDA for YOSPRALA on March 14, 2016, and we believe we remain on track for potential approval and launch of YOSPRALA in 2016.
Bilastine
Bilastine is a second generation antihistamine drug for the treatment of allergic rhinoconjunctivitis and urticaria (hives). The Company has not yet chosen a trademark for bilastine. Bilastine exerts its effect as a selective histamine H1 receptor antagonist, and has an effectiveness similar to cetirizine, fexofenadine and desloratadine. It was developed in Spain by FAES Farma, S.A. The Canadian antihistamine market is currently valued at approximately $115 million per year and the leading competitors are cetirizine (Reactine®), loratadine (Claritin®), desloratadine (Aerius®) and fexofenadine (Allegra®). It has been over fifteen years since the approval of a new antihistamine in Canada.
The Company filed bilastine with Health Canada in the second quarter of 2015. Bilastine is approved in the European Union for the symptomatic treatment of allergic rhinoconjunctivitis and urticaria, but it is not approved by the FDA for any use in the United States.
The clinical efficacy of bilastine in allergic rhinitis (“AR”) and urticaria has been assessed in 10 clinical studies in which over 4,600 patients were involved. The studies on seasonal AR (“SAR”) were double-blind, placebo-controlled, parallel-group involving male and female patients over 12 years of age with symptomatic disease at the beginning of the study. In the SAR studies, the daily oral administration during 14 days of bilastine 20mg proves to have comparable efficacy to the administration of cetirizine 10mg or than the administration of desloratadine 5mg. Bilastine 20mg shows a safety and tolerability profile similar to placebo. Possible side effects of bilastine include headache and drowsiness.
The studies in urticaria were double-blind, placebo-controlled, parallel-group involving male and female patients over 18 year of age with symptomatic disease (chronic idiopathic urticaria) at the beginning of the study. In this urticaria studies the daily oral administration of 28 days of bilastine 20mg proves to have comparable efficacy to the administration of levocetirizine 5mg. Likewise, bilastine 20mg shows a safety and tolerability profile comparable to placebo.
Out-Licensed Products
|
VIMOVO®
|
VIMOVO (naproxen/esomeprazole magnesium) is the brand name for a proprietary fixed-dose combination of enteric-coated naproxen, a pain-relieving NSAID and immediate-release esomeprazole magnesium, a PPI, in a single delayed-release tablet and is a product in our PPI-NSAID (“PN”) platform. We developed VIMOVO in collaboration with AstraZeneca AB (“AstraZeneca”). On April 30, 2010, the FDA approved VIMOVO for the relief of the signs and symptoms of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, and to decrease the risk of developing gastric ulcers in patients at risk of developing NSAID-associated gastric ulcers. As of the end of December 31, 2015, VIMOVO is being sold in over 50 countries. Prescription sales of oral anti-arthritis NSAIDs in the United States in 2015 were approximately $6.3 billion.
In June 2010, we officially transferred to AstraZeneca the IND and NDA for the product. AstraZeneca is responsible for the commercialization of VIMOVO. In November 2013, AstraZeneca entered into an agreement for Horizon Pharma USA, Inc. (“Horizon”) to acquire the U.S. rights for VIMOVO. Under the terms of the agreement, we will continue to receive from Horizon a 10% royalty on net sales of VIMOVO sold in the United States, with guaranteed annual minimum royalty payments of $5 million in 2014, and $7.5 million each year thereafter, provided that the patents owned by us which cover VIMOVO are in effect and no generic forms of VIMOVO are on the market. AstraZeneca will continue to have rights to commercialize VIMOVO outside of the United States and paid us a royalty of 6% on all sales within its territory through 2015 and will pay us a royalty of 10% commencing 2016 and thereafter. See also the section entitled “Item 1. Business – Collaboration Agreements – Agreement with AstraZeneca/Horizon regarding VIMOVO®” in this Annual Report on Form 10-K.
|
Treximet®
|
Treximet (sumatriptan/naproxen sodium) is a migraine medicine that was developed by us in collaboration with Glaxo Group Limited, d/b/a GlaxoSmithKline (“GSK”). The product is formulated with our patented technology of combining a triptan, sumatriptan 85mg, with an NSAID, naproxen sodium 500mg, and GSK’s RT Technology™ in a single tablet designed for the acute treatment of migraine. On April 15, 2008, the FDA approved Treximet for the acute treatment of migraine attacks, with or without aura, in adults. Treximet is available in the United States. The market for migraine medications in the United States is valued at approximately $2.2 billion in 2015.
In May 2008, we transferred the IND and NDA for the product to GSK, which subsequently sold its rights in Treximet, including the related trademark, to Pernix Therapeutics Holdings, Inc. (“Pernix”) in August 2014. As part of GSK’s divestiture to Pernix, restrictions on our right to develop and commercialize certain additional dosage forms of sumatriptan/naproxen combinations outside of the United States had been eliminated, allowing us to seek approval for these combinations on the basis of the approved NDA. GSK was previously, and Pernix is currently, responsible for the commercialization of Treximet in the United States, while we received royalties based on net sales. In November 2011, we sold to a financial investor, CPPIB Credit Investments Inc. (“CII”), for an upfront lump-sum, our rights to future royalty and milestone payments relating to Treximet sales in the United States and certain other products containing sumatriptan/naproxen sodium developed and sold by Pernix in the United States. By virtue of the agreement, we will also be entitled to receive a 20% interest in royalties, if any, paid on net sales of Treximet and such other products in the United States to CII relating to the period commencing in the second quarter of 2018. See also the section entitled “Item 1. Business – Collaboration Agreements – Agreements with GSK, Pernix and CII regarding MT 400 (including Treximet®)” in this Annual Report on Form 10-K.
Collaboration Agreements
We have entered into and may continue to enter into collaborations with established pharmaceutical or pharmaceutical services companies to develop, commercialize and/or manufacture our products. Our existing material collaborations are described below.
Agreement with AstraZeneca/Horizon regarding VIMOVO®
In August 2006, we entered into a collaboration and license agreement, effective September 7, 2006 (the “Original AZ Agreement”), with AstraZeneca regarding the development and commercialization of proprietary fixed dose combinations of the PPI esomeprazole magnesium with the NSAID naproxen in a single tablet for the management of pain and inflammation associated with conditions such as osteoarthritis and rheumatoid arthritis in patients who are at risk for developing NSAID-associated gastric ulcers. Under the terms of the Original AZ Agreement, we granted to AstraZeneca an exclusive, fee-bearing license, in all countries of the world except Japan, under our patents and know-how relating to combinations of gastroprotective agents and NSAIDs (other than aspirin and its derivatives). We retained responsibility for the development and filing of the NDA for the product in the United States, while AstraZeneca was responsible for all development activities outside the United States, as well as for all manufacturing, marketing, sales and distribution activities worldwide. We agreed to bear all expenses related to certain specified U.S. development activities. AstraZeneca would pay all other development expenses, including all manufacturing-related expenses. The Original AZ Agreement established joint committees with representation of both AstraZeneca and us to manage the development and commercialization of the product. If consensus could not be reached between AstraZeneca and us, we generally would have the deciding vote with respect to development activities required for marketing approval of the product in the United States, and AstraZeneca generally would have the deciding vote with respect to any other matters. Pursuant to the terms of the Original AZ Agreement, we received an upfront license fee of $40.0 million from AstraZeneca.
We entered into an amendment to the Original AZ Agreement, effective as of September 6, 2007 (the “Amendment to the Original AZ Agreement”). Under the terms of the Amendment to the Original AZ Agreement, AstraZeneca agreed to pay us up to $345.0 million, in the aggregate, in milestone payments upon the achievement of certain development, regulatory and sales events. To date we have received an aggregate of $75.0 million in milestone payments, which consisted of: (1) $10.0 million payment upon execution of the Amendment to the Original AZ Agreement; (2) $20.0 million payment in recognition of the achievement of the primary endpoints and interim results for certain of the VIMOVO studies; (3) $10.0 million payment upon FDA acceptance of the VIMOVO NDA for review; (4) $20.0 million payment in May 2010 for the NDA approval of VIMOVO; and (5) $25.0 million payment in December 2010 when VIMOVO received approval (including pricing and reimbursement approval) in a major ex-U.S. market. Up to $260.0 million is payable as sales performance milestones if certain aggregate sales thresholds are achieved.
Pursuant to the Original AZ Agreement, as amended, we receive a flat, low double digit royalty rate during the royalty term on annual net sales of products made by AstraZeneca, its affiliates and sublicensees in the United States and royalties ranging from the mid-single digits to the high-teens on annual net sales of products made by AstraZeneca, its affiliates and sublicensees outside of the United States. The royalty rate may be reduced due to the loss of market share as a result of generic competition inside and outside of the United States. Our right to receive royalties from AstraZeneca for the sale of such products expires on a country-by-country basis upon the later of (a) expiration of the last-to-expire of certain patent rights relating to such products in that country, and (b) ten years after the first commercial sale of such products in such country.
Unless earlier terminated in accordance with its terms, the Original AZ Agreement, as amended, will expire upon the payment of all applicable royalties for the products commercialized under the agreement. Either party has the right to terminate by notice in writing to the other party upon or after any material breach of the agreement by the other party, if the other party has not cured the breach within 90 days after written notice to cure has been given, with certain exceptions. The parties also can terminate for cause under certain defined conditions. In addition, AstraZeneca can terminate at any time, at will, for any reason or no reason, in its entirety or with respect to countries outside the United States, upon 90 days’ notice. If terminated at will, AstraZeneca will owe us a specified termination payment or, if termination occurs after the product is launched, AstraZeneca may, at its option, under and subject to the satisfaction of conditions specified in the Original AZ Agreement, elect to transfer the product and all rights to us.
In May 2013, AstraZeneca informed us that, after a strategic business review, it had decided to cease promotion and sampling of VIMOVO by the end of the third quarter of 2013 in certain countries, including the United States and all countries in Europe, other than Spain and Portugal, which have pre-existing contractual relationships with third parties. We understand from AstraZeneca that it instead intended to focus on those countries where the product had shown growth and which AstraZeneca believed had the greatest potential for future growth.
In September 2013, we and AstraZeneca entered into a third amendment to the Original AZ Agreement which made clarifications to certain intellectual property provisions of the Original AZ Agreement to clarify that AstraZeneca’s rights under those provisions do not extend to products which contain acetylsalicylic acid. In September 2013, we and AstraZeneca also executed a letter agreement whereby we agreed that in the event that AstraZeneca divested its rights and obligations to market VIMOVO in the United States to a third-party, AstraZeneca would be relieved of its obligations under the Original AZ Agreement with respect to the United States as of the effective date of such divestiture, including its obligation under the Original AZ Agreement to guarantee the performance of such assignee and/or sublicensee.
In November 2013, AstraZeneca divested of all of its rights, title and interest to develop, commercialize and sell VIMOVO in the United States to Horizon. In connection with this divestiture, in November 2013, we and AstraZeneca entered into an Amended and Restated Collaboration and License Agreement for the United States (the “U.S. Agreement”) and an Amended and Restated License and Collaboration Agreement for outside the United States (the “ROW Agreement”), which agreements collectively amended and restated the Original AZ Agreement. With our consent pursuant to a letter agreement among us, AstraZeneca and Horizon, AstraZeneca subsequently assigned the U.S. Agreement to Horizon in connection with the divestiture. Further, the letter agreement establishes a process for AstraZeneca and Horizon to determine if sales milestones set forth in the Original AZ Agreement are achieved on a global basis and provides other clarifications and modifications required as a result of incorporating the provisions of the Original AZ Agreement into the U.S. Agreement and the ROW Agreement or as otherwise agreed by the parties.
Pursuant to an amendment of the U.S. Agreement (the “Amendment to the U.S. Agreement”) between us and Horizon, we are guaranteed an annual minimum royalty amount of $5 million in calendar year 2014 and a guaranteed annual minimum royalty amount of $7.5 million each calendar year thereafter, provided that the patents owned by us which cover VIMOVO are in effect and no generic forms of VIMOVO are in the marketplace. The Amendment to the U.S. Agreement also provides that Horizon has assumed AstraZeneca’s right to lead the on-going Paragraph IV litigation relating to VIMOVO currently pending in the United States District Court for the District of New Jersey and will assume all patent-related defense costs relating to such litigation, including reimbursement up to specified amounts of the cost of any counsel retained by us, amends certain time periods for Horizon’s delivery of quarterly sales reports to us, and provides for quarterly update calls between the parties to discuss performance of VIMOVO and Horizon’s commercialization efforts.
On December 31, 2015, we have receivables of $6.0 million related to VIMOVO royalty revenue, $4.7 million related to U.S. sales and $1.3 million related to the rest-of-the-world (“ROW”) sales.
Agreements with GSK, Pernix and CII regarding MT 400 (including Treximet®)
In June 2003, we entered into an agreement with GSK for the development and commercialization of proprietary combinations of a triptan (5-HT1B/1D agonist) and a long-acting NSAID (the “GSK Agreement”). The combinations covered by the GSK Agreement are among the combinations of MT 400 (including Treximet). Under the terms of the GSK Agreement, GSK has exclusive rights in the United States to commercialize all combinations which combine GSK’s triptans, including Imitrex® (sumatriptan succinate) or Amerge® (naratriptan hydrochloride), with a long-acting NSAID. We were responsible for development of the first combination product, while GSK provided formulation development and manufacturing.
Pursuant to the terms of the GSK Agreement, we received an initial $25.0 million payment from GSK. From May 2004 through April 2008, we received an aggregate of $55.0 million in milestone payments associated with the development and approval of Treximet. In addition, Pernix, as assignee of GSK, will pay two sales performance milestones totaling up to $80.0 million if certain sales thresholds are achieved. Pernix, as assignee of GSK, will pay royalties on all net sales of marketed products until at least the expiration of the last-to-expire issued applicable patent (October 2, 2025) based upon the scheduled expiration of currently issued patents. Pernix may reduce, but not eliminate, the royalty payable to us if generic competitors attain a pre-determined share of the market for the combination product, or if Pernix owes a royalty to one or more third parties for rights it licenses from such third parties to commercialize the product.
In November 2011, we entered into a purchase agreement with CII, pursuant to which we sold, and CII purchased, our right to receive future royalty payments arising from U.S. sales of MT 400, including Treximet. By virtue of the agreement, we will receive a 20% interest in any royalties paid on net sales of Treximet and such other products in the United States to CII relating to the period commencing in the second quarter of 2018.
In May 2014, we, GSK, CII and Pernix, entered into certain agreements in connection with GSK’s divestiture of all of its rights, title and interest to develop, commercialize and sell Treximet in the United States to Pernix. Upon the closing of the transaction in August 2014, with our consent, GSK assigned the GSK Agreement to Pernix. Immediately following the closing of the transaction, we entered into an amendment to the GSK Agreement with Pernix. This amendment, among other things, amends the royalty provisions to provide for a guaranteed quarterly minimum royalty of $4 million for the calendar quarters commencing in January 2015 and ending in March 2018 and requires that Pernix continue certain of GSK’s ongoing development activities and to undertake certain new activities, for which we will provide reasonable assistance. This amendment to the GSK Agreement also eliminates restrictions in the GSK Agreement on our right to develop and commercialize certain dosage forms of sumatriptan/naproxen combinations outside of the United States and permits us to seek approval for these combinations on the basis of the approved NDA for Treximet. Pernix also granted us a warrant to purchase 500,000 shares of Pernix common stock at an exercise price equal to $4.28 per share, which represents the closing price of Pernix common stock as reported on the NASDAQ Global Market on May 13, 2014. In the first quarter of 2015, the Company sold the warrant for $2.5 million. In July 2014, we and Pernix entered into a second amendment of the GSK Agreement, effective upon the closing of the transaction in August 2014, which will permit Pernix’s Irish affiliate (to which Pernix assigned its rights) to further assign the GSK Agreement without our prior written consent as collateral security for the benefit of certain lenders.
Sales and Marketing
The Company’s sales and marketing strategy is focused on the organic growth of existing marketed products through several key activities. First, our sales force ensures that it targets known prescribers of its medications or medications that compete with its products. We create demand by providing customers with reliable and trustworthy information from credible sources and by coordinating and facilitating continuing health education events in targeted areas. Second, we support our products by providing physicians and other healthcare practitioners with quality patient care materials. Third, we ensure that our products are accessible through all major wholesalers and distributors in Canada and plan to secure contracts with all major wholesalers and distributors in the United States, and manage our supply chain efficiently to ensure that it can meet demand.
Our current U.S. sales force is approximately 21 sales representatives, and we plan to increase this number to 25 in connection with the promotion of Fibricor. In Canada, we have 27 sales representatives. Upon FDA approval of YOSPRALA, we anticipate adding 85 additional sales representatives who will be dedicated to marketing our YOSPRALA products. The Company considers its sales force to be very experienced and well trained. All of our representatives have experience from other pharmaceutical companies including many of the largest companies in the industry. Additionally, we offer our representatives a competitive incentive plan based on the achievement of results.
Manufacturing
We currently have no manufacturing capability. We outsource the manufacturing of our proprietary products to pharmaceutical manufacturing facilities operated by third-party contractors. These facilities comply with the FDA’s current Good Manufacturing Practices (“cGMP”) regulations and applicable Health Canada regulations, including in accordance with Health Canada’s cGMP requirements. See the section entitled “Item 1. Business – Government Regulations and Other Considerations” for a further discussion regarding the regulations that pharmaceutical manufacturing facilities are subject to. We believe these facilities have sufficient excess capacity at present to meet our short and long-term objectives.
Our licensed products are manufactured by authorized, third-party, contract manufacturing organizations in various places throughout the world. Our manufacturers are all approved fabricators of pharmaceutical products according to the FDA and Health Canada, as applicable. We are responsible for secondary packaging of certain of our proprietary products at our London, Ontario facility. Our licensed products are packaged by our third-party contract manufacturers.
To date, we have entered into arrangements with third-party manufacturers for the supply of formulated and packaged clinical trial materials, active ingredients and other ingredients used in the manufacturing of our products. For example, pursuant to our agreement with Patheon Pharmaceuticals Inc. (“Patheon”), Patheon has agreed to manufacture, and we have agreed to purchase, a specified percentage of our requirements of our YOSPRALA product candidates for sale in the United States. Under our agreements with GSK, AstraZeneca and Horizon, it is the obligation of our partners to obtain commercial supplies of products developed thereunder.
Use of third-party manufacturers enables us to focus on our development and sales/commercialization activities, minimize fixed costs and capital expenditures and gain access to advanced manufacturing process capabilities and expertise. We plan to continue to rely on third-party manufacturers to manufacture our compounds and final products.
Agreements with Patheon regarding YOSPRALA™ 325/40 and YOSPRALA™ 81/40
In December 2011, we entered into a Manufacturing Services Agreement (the “Supply Agreement”) and a related Capital Expenditure and Equipment Agreement (the “Capital Agreement”), relating to the manufacture of YOSPRALA 325/40, with Patheon. The Supply Agreement and Capital Agreement were amended in July 2013 (respectively, the “Amendment to the Supply Agreement” and the “Amendment to the Capital Agreement”).
Under the terms of the Supply Agreement, as amended, Patheon has agreed to manufacture, and we have agreed to purchase, a specified percentage of the Company’s requirements of YOSPRALA 325/40 and YOSPRALA 81/40 for sale in the United States. The Amendment to the Supply Agreement expressly incorporates YOSPRALA 81/40, clarifies that the manufacturing services contemplated by the Supply Agreement include the manufacture of validation batches, but the placing of an order for such validation batches will not trigger the commencement date of the Initial Term (as defined below), updates pricing for YOSPRALA 325/40 and incorporates a new pricing schedule for YOSPRALA 81/40.
The term of the Supply Agreement extends until December 31st of the fourth year after we notify Patheon to begin manufacturing services under the Supply Agreement (the “Initial Term”), and will automatically renew thereafter for periods of two years, unless terminated by either party upon 18 months’ written notice prior to the expiration of the Initial Term or 12 months’ written notice prior to the expiration of any renewal term. In addition to usual and customary termination rights which allow each party to terminate the Supply Agreement for material, uncured breaches by the other party, we can terminate the Supply Agreement upon 30 days’ prior written notice if a governmental or regulatory authority takes any action or raises any objection that prevents us from importing, exporting, purchasing or selling YOSPRALA 325/40 or if it is determined that the formulation or sale of YOSPRALA 325/40 infringes any patent rights or other intellectual property rights of a third-party. We can also terminate the Supply Agreement upon 24 months’ prior written notice if we license, sell, assign or otherwise transfer any rights to commercialize YOSPRALA 325/40 to a third-party. The Supply Agreement contains general and customary commercial supply terms and conditions, as well as establishes pricing, subject to annual adjustments, for bulk product and different configurations of packaged product.
Under the terms of the Capital Agreement, as amended, we will be responsible for the cost of purchasing certain equipment specific to the manufacture of YOSPRALA 325/40 and YOSPRALA 81/40, the cost of which, based on current volume projections, is expected to be less than $450,000. The Amendment to the Capital Agreement provides an updated schedule, which reflects the parties’ current assumptions regarding the need for and timing of capital equipment expenditures based upon Patheon’s current and anticipated production capacity and current volume projections for YOSPRALA 325/40 and YOSPRALA 81/40. In addition, pursuant to the terms of the Amendment to the Capital Agreement, we agreed with Patheon to reduce the amount of the maximum expenditure for additional capital equipment and facility modifications to meet volume demands from $2.5 million to approximately $1.2 million in light of the revised capacity and volume assumptions.
Industry and Competition
The pharmaceutical industry is highly competitive and is characterized by rapidly changing markets, technology, emerging industry standards and frequent introduction of new products. We believe that competition in our market is based on, among other things, product safety, efficacy, convenience of dosing, reliability, availability and price. The market is dominated by a small number of highly-concentrated global competitors, many of which boast substantially greater resources than the Company. Given the size and scope of the competition, there can be no assurance that the Company will maintain or grow our current market position in its therapeutic areas, or that developments by others will not render our products or technologies non-competitive or obsolete. In addition, some of our competitors have substantially greater financial, research and development, manufacturing, marketing and human resources and greater experience than we do in product discovery, development, clinical trial management, FDA regulatory review, manufacturing and marketing, which may enable them to compete more effectively than we can.
The Company faces product competition from companies marketing competing pharmaceutical products and medical devices worldwide, particularly in the United States and Canada, and potentially on new products that could be launched in the future. See also the section entitled “Item 1. Business – Products” in this Annual Report on Form 10-K for a discussion of the other products that specifically compete with the Company’s products.
Patent and Proprietary Protection
We have obtained and intend to actively seek to obtain, when appropriate, protection for our products and proprietary technology by means of U.S., Canadian and other foreign patents, trademarks and contractual arrangements. In addition, we rely upon trade secrets and contractual agreements to protect certain of our proprietary technology and products.
While trade secret protection is an essential element of our business and we have taken security measures to protect our proprietary information and trade secrets, we cannot give assurance that our unpatented proprietary technology will afford us significant commercial protection. We seek to protect our trade secrets by entering into confidentiality agreements with third parties, employees and consultants. Our employees and consultants also sign agreements requiring that they assign to the Company their interests in intellectual property arising from their work for us. All employees sign an agreement not to engage in any conflicting employment or activity during their employment with us and not to disclose or misuse our confidential information. However, it is possible that these agreements will be breached or invalidated, and if so, there may not be an adequate corrective remedy available. Accordingly, we cannot ensure that employees, consultants or third parties will not breach the confidentiality provisions in such contracts or infringe or misappropriate our trade secrets and other proprietary rights or that the measures we are taking to protect our proprietary rights will be adequate.
We have issued U.S. and Canadian patents and pending U.S. and Canadian patent applications, as well as other pending foreign patent applications or issued foreign patents, relating to our marketed products and product candidates. We also have U.S., Canadian and other foreign patent applications pending relating to novel product concepts. There can be no assurance that our patent applications will issue as patents or, with respect to our issued patents, that they will provide us with significant protection. The following provides a general description of our patent portfolio and is not intended to represent an assessment of claim limitations or claim scope.
|
MT 400/Treximet®
|
We have four issued U.S. patents with claims relating to methods, compositions and therapeutic packages involving the use of certain NSAIDs and 5-HT receptor agonists in treating patients with migraines. Outside of the United States and Canada, we have issued patents in Australia, Europe, Hong Kong and Japan. The expected expiration date of the issued patents relating to MT 400 is in August 2017. We also have issued patents in Australia, Canada, Europe, Israel, Japan, Norway and the United States with claims relating to formulations of MT 400. We expect the patents related to formulations of MT 400 to expire in December 2023 outside the United States and in October 2025 in the United States.
|
PN (VIMOVO®)
|
We have issued patents in the United States, Australia, Canada, Europe, Eurasia, Mexico, Japan and Norway, with claims directed to certain compositions containing a combination of acid inhibitors (including PPIs) and NSAIDs. The issued patents also have claims to treatment methods involving the use of such compositions. We have pending U.S. patent applications that also have claims to compositions containing acid inhibitors and NSAIDs and to various treatment methods involving such compositions. The issued U.S. patents and related U.S. patent applications are expected to expire between May 2022 and February 2023. The European patent will expire in May 2022, but we have obtained supplementary protection certificates (“SPCs”) for VIMOVO that extend to dates between November 2025 and May 2026, depending on the country. We expect the patents outside of the United States and Europe to expire in May 2022.
We, together with AstraZeneca, have filed joint patent applications relating to VIMOVO. We have an issued U.S. patent related to the pharmacodynamics profile of VIMOVO that will expire in March 2031. Foreign counterparts, if granted, are expected to expire in September 2029. We also have a U.S. patent related to methods of treatment with VIMOVO, which will expire in October 2031. Any related patents that issue outside the U.S. are expected to expire in July 2030.
|
PA (YOSPRALA™)
|
One of the patent families covering VIMOVO also covers PA. We have issued patents in the United States, Australia, Canada, Eurasia, Europe, Israel, Japan, Mexico and Norway, with claims directed to certain compositions containing a combination of acid inhibitors (including PPIs) and NSAIDs (including aspirin). The issued patents also have claims to treatment methods involving the use of such compositions. We have pending U.S. patent applications that also have claims to compositions containing acid inhibitors and aspirin and to various treatment methods involving such compositions. The issued U.S. patents and related U.S. patent applications are expected to expire between May 2022 and February 2023. The European patent will expire in May 2022, but we expect to apply for SPCs for PA upon approval. We expect the patents outside of the United States and Europe to expire in May 2022. We have filed additional patent applications related to PA. We expect any patents that issue from these applications to expire between 2030 and 2032.
Other Patents
With respect to Cambia, we have rights to patents through our licensing agreement with Depomed, which we expect to expire in May 2017 and June 2026 in Canada. With respect to Fibricor, we have four issued patents in the United States, which we expect to expire in August 2027. In addition to the patents for the products discussed above, we also have patents or rights to patents with respect to bilastine, Durela, Moviprep, Resultz and Bedbugz.
Government Regulations and Other Considerations
The FDA in the United States, Health Canada in Canada and comparable regulatory agencies in foreign countries impose substantial requirements on the clinical development, manufacture and marketing of pharmaceutical products and product candidates. These agencies and other federal, state, provincial and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, record-keeping, approval and promotion of our product candidates. All of our product candidates will require regulatory approval before commercialization. In particular, therapeutic product candidates for human use are subject to rigorous preclinical and clinical testing and other statutory and regulatory requirements of the United States, Canada and foreign countries. Obtaining these marketing approvals and subsequently complying with ongoing statutory and regulatory requirements is costly and time-consuming. Any failure by us or our collaborators, licensors or licensees to obtain, or any delay in obtaining, regulatory approvals or in complying with other regulatory requirements could adversely affect the commercialization of our products and product candidates then being developed by us and our ability to receive product or royalty revenues.
United States Regulatory Overview
In the United States, the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act, as amended (“FFDCA”), and implements regulations. If we fail to comply with the applicable requirements at any time during the product development process, approval process or after approval, we may become subject to administrative or judicial sanctions. The steps required before a new drug product candidate may be distributed commercially in the United States generally include:
| ● | conducting appropriate preclinical laboratory evaluations of the product candidate’s chemistry, formulation and stability and preclinical studies in animals to assess the potential safety and efficacy of the product candidate; |
| ● | submitting the results of these evaluations and tests to the FDA, along with manufacturing information and analytical data, in an IND; |
| ● | initiating clinical trials under the IND and addressing any safety or regulatory concerns of the FDA; |
| ● | obtaining approval of Institutional Review Boards to introduce the drug into humans in clinical studies; |
| ● | conducting adequate and well-controlled human clinical trials that establish the safety and efficacy of the product candidate for the intended use, typically in the following three sequential, or slightly overlapping stages: |
| o | Phase 1: The product is initially introduced into human subjects or patients and tested for safety, dose tolerance, absorption, metabolism, distribution and excretion. |
| o | Phase 2: The product candidate is studied in patients to identify possible adverse effects and safety risks, to determine dosage tolerance and the optimal dosage, and to collect some efficacy data. |
| o | Phase 3: The product candidate is studied in an expanded patient population at multiple clinical study sites, to confirm efficacy and safety at the optimized dose, by measuring primary and secondary endpoints established at the outset of the study. |
| ● | submitting the results of preclinical studies and clinical trials, as well as chemistry, manufacturing and control information, on the product candidate to the FDA in an NDA; and |
| ● | obtaining FDA approval of the NDA prior to any commercial sale or shipment of the product candidate. |
The foregoing process can take a number of years and requires substantial financial resources. Each NDA must be accompanied by a user fee, pursuant to the requirements of the Prescription Drug User Fee Act (“PDUFA”) and its amendments. The FDA adjusts the PDUFA user fees on an annual basis. According to the FDA’s fee schedule, effective for the fiscal year 2016, the user fee for an application requiring clinical data, such as an NDA, is $2,374,200. The PDUFA also imposes an annual product fee for each marketed prescription drug ($114,450), and an annual establishment fee ($585,200) on facilities used to manufacture prescription drugs and biologics. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business. However, there are no waivers for product or establishment fees.
The results of preclinical studies and initial clinical trials are not necessarily predictive of the results from large-scale clinical trials, and clinical trials may be subject to additional costs, delays or modifications due to a number of factors, including the difficulty in obtaining enough patients, clinical investigators, product candidate supply and financial support.
Even after FDA approval has been obtained, further studies, including post-marketing studies, may be required. Results of post-marketing studies may limit or expand the further marketing of the products. If we propose any modifications to a product, including changes in indication, manufacturing process, manufacturing facility or labeling, a supplement to our NDA may be required to be submitted to the FDA and approved.
The FDA may also require testing and surveillance programs to monitor the effect of approved product candidates that have been commercialized, and the FDA has the power to prevent or limit further marketing of a product candidate based on the results of these post-marketing programs. Upon approval, a product candidate may be marketed only in those dosage forms and for those indications approved in the NDA.
In addition to obtaining FDA approval for each indication to be treated with each product candidate, each domestic product candidate manufacturing establishment must register with the FDA, list its product with the FDA, comply with the applicable cGMP regulations, which include requirements relating to quality control and quality assurance, as well as the corresponding maintenance of records and documentation, and permit and pass manufacturing plant inspections by the FDA. Moreover, the submission of applications for approval may require additional time to complete manufacturing stability studies. Foreign establishments manufacturing product for distribution in the United States also must list their product candidates with the FDA and comply with cGMP regulations. They are also subject to periodic inspection by the FDA or by local authorities under agreement with the FDA.
Any product candidates manufactured or distributed by us pursuant to FDA approvals are subject to extensive continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the product candidate. In addition to continued compliance with standard regulatory requirements, the FDA may also require post-marketing testing and surveillance to monitor the safety and efficacy of the marketed product. Adverse experiences and reports of adverse experiences in the medical literature with the product candidate or its components must be reported to the FDA. Product approvals may be affected and even withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product are discovered following approval.
The FFDCA also mandates that products be manufactured consistent with cGMP regulations. In complying with the cGMP regulations, manufacturers must continue to spend time, money and effort in production, record-keeping, quality control, and auditing to ensure that the marketed product meets applicable specifications and other requirements. The FDA periodically inspects manufacturing facilities to ensure compliance with cGMP regulations. Failure to comply subjects the manufacturer to possible FDA action, such as warning letters, suspension of manufacturing, seizure of the product, voluntary recall of a product or injunctive action, as well as possible civil penalties. We currently rely on, and intend to continue to rely on, third parties to manufacture our products and product candidates. These third parties will be required to comply with cGMP regulations.
Products manufactured in the United States for distribution abroad will be subject to FDA regulations regarding export, as well as to the requirements of the country to which they are shipped. These latter requirements are likely to cover the conduct of clinical trials, the submission of marketing applications, and all aspects of manufacturing and marketing. Such requirements can vary significantly from country to country.
Canadian Regulatory Overview
Health Canada is the Canadian federal authority that regulates, evaluates and monitors the safety, effectiveness, and quality of drugs, medical devices, and other therapeutic products available to Canadians. Health Canada’s regulatory process for review, approval and regulatory oversight of products is similar to the regulatory process conducted by the FDA in the United States, the European Medicines Agency in the EU, and other regulatory agencies around the world.
Prior to being given market authorization for a drug product, a manufacturer must present substantive scientific evidence of a product’s safety, efficacy and quality as required by the Food and Drugs Act (Canada) and its associated regulations, including the Food and Drug Regulations. This information is usually submitted in the form of a New Drug Submission (“NDS”) in Canada.
Health Canada performs a thorough review of the submitted information, sometimes using external consultants and advisory committees, to evaluate the potential benefits and risks of a drug. If, at the completion of the review, the conclusion is that the patient benefits outweigh the risks associated with the drug, the drug is issued a Notice of Compliance (“NOC”) and a Drug Identification Number (“DIN”), which permits the market authorization holder (i.e., the NOC and DIN holder) to market the drug in Canada.
Currently, the process for the review of a NDS typically takes approximately 1 to 2 years from the time that a manufacturer submits an NDS until Health Canada approves a drug. The time to approval varies but Health Canada target’s service standard for reviewing most NDSs is 300 days (plus an additional 45 days for screening the application). From April 1, 2014 to March 31, 2015, Health Canada’s average review time for a NDS for a new active substance was 257 days.
All establishments engaged in the fabrication, packaging/labeling, importation, distribution, and wholesale of drugs and operation of a testing laboratory relating to drugs are required to hold a Drug Establishment License to conduct one or more of the licensed activities unless expressly exempted under the Food and Drug Regulations. The basis for the issuance of a Drug Establishment License is to ensure the facility complies with cGMP as stipulated in the Food and Drug Regulations and as determined by cGMP inspection conducted by Health Canada. An importer of pharmaceutical products manufactured at foreign sites must also be able to demonstrate that the foreign sites comply with cGMP, and such foreign sites are included on the importer’s Drug Establishment License.
Regulatory obligations and oversight continue following the initial market approval of a pharmaceutical product. For example, every market authorization holder must report any new information received concerning adverse drug reactions, including timely reporting of serious adverse drug reactions that occur in Canada and any serious unexpected adverse drug reactions that occur outside of Canada. The market authorization holder must also notify Health Canada of any new safety and efficacy issues that it becomes aware of after the launch of a product.
Canadian Reimbursement Overview
After regulatory approval is received for a prescription drug, it can be sold to the public in accordance with the Food and Drugs Act and its regulations and applicable provincial pharmacy legislation and regulations. Revenues from prescription drug sales in Canada are usually generated through one of three sources:
| ● | Cash: Patients will pay “out of pocket” at their sole expense. It is estimated that 10% of all prescription dollars spent in Canada come from cash purchases. |
| ● | Private Insurance: Approximately 45% of prescription dollars spent in Canada are reimbursed via third-party private insurers, under plans generally provided by patients’ employers. Patients may be reimbursed a percentage of the cost of covered drugs minus deductibles or co-pays. The availability for reimbursement of drugs varies according to the type of reimbursement plan designed by the insurance company. There are a number of private insurers operating in Canada that provide employee plans to private and public sector employers. |
| ● | Government Drug Plans: Government drug plans cover the cost of nearly 45% of prescription dollars spent in Canada, and generally serve patients over the age of 65 or patients for whom the cost of medications represents a significant financial burden such as families receiving social assistance. Each provincial government pays the cost of drugs that are listed on their own provincial formulary, with some government drug plans requiring patients to be responsible for a co-payment. |
After regulatory approval of a drug is granted, approval for reimbursement is typically sought from provincial governments and private insurance companies. Until provincial and private reimbursement is approved, the product is sold only via cash purchases. Decisions to list drugs for reimbursement on private and government formularies vary widely depending on the drug, indications, competitive products and price.
Sales of hospital products or products dispensed in the hospital are treated differently in Canada. All medications taken while in a hospital are fully reimbursed by the provincial governments. If a patient leaves the hospital and is prescribed a drug to be taken at home, this prescription would be paid for either by cash, private insurance or public insurance plans.
Common Drug Review (“CDR”)
The CDR was implemented in 2003 to provide formulary listing recommendations for new drugs to participating publicly-funded federal, provincial and territorial drug benefit plans in Canada. The CDR is administered by the Canadian Agency for Drugs and Technologies in Health.
The CDR consists of:
| ● | a systematic review of the available clinical evidence and a review of the pharmacoeconomic data for the drug; and |
| ● | a listing recommendation made by the Canadian Expert Drug Advisory Committee. |
Based on the targeted timeframes of the CDR, a review should be completed approximately 20 to 26 weeks following receipt of a manufacturer’s submission, after which recommendations are made to participating drug plans.
At the provincial and territorial level, products are reviewed on the basis of their cost-effectiveness, comparable utility to other similar products, projected utilization and cost implications to the publicly-funded drug budget. Each submission is reviewed but there is wide variance in the formulary decisions and the time taken to make such decisions. Provinces and territories may utilize the recommendations of the CDR or perform their own analysis.
Presently, all provinces and territories except Quebec use the CDR recommendations in their assessment, but make their formulary decisions independently from the CDR. In many provinces, the formulary committee may grant “restricted or limited use approvals” for a drug as a means of regulating the size of the patient population eligible for reimbursement for the cost of the drug and by encouraging physicians to use older generation products first before prescribing newer, sometimes more costly medications. Further, if a generic drug is available, the government funded drug plans will often choose to reimburse only for the cost of the generic drug. Often, the provinces, territories and federal government may require the manufacturer to enter into a product listing agreement to have a product added to a government funded formulary. Such product listing agreements commonly contain product pricing restrictions and may contain other terms between the government agency and the manufacturer, such as volume discounts or other amounts that may be payable by the manufacturer to the government agency.
Product Pricing Regulation on Certain Patented Drug Products
Patented drug products in Canada are subject to regulation by the Patented Medicine Prices Review Board (“PMPRB”) pursuant to the Patent Act (Canada) and the Patented Medicines Regulations. Among other things, the PMPRB’s mandate is to ensure that prices of patented products in Canada are not excessive. For new patented products, the price is assessed taking into account the therapeutic improvement, if any, relative to its class and generally, the price in Canada is limited to either the cost of existing drugs sold in Canada or the median of prices for the same drug sold in other specified industrial countries. For existing patented products, prices generally cannot increase by more than maximum price increase allowed applying the PMPRB’s Consumer Price Index adjustment methodology. The PMPRB monitors compliance through a review of the average transaction price of each patented drug product as reported by the patentee over a recurring six-month reporting period (patentees of pharmaceutical products have mandatory reporting obligations to the PMPRB).
The PMPRB does not approve prices for drug products in advance of their introduction to the market. The PMPRB provides guidelines from which companies like us set their prices at the time they launch their products. All patented pharmaceutical products introduced in Canada are subject to the post-approval, post-launch scrutiny of the PMPRB. Since the PMPRB does not pre-approve prices for a patented drug product in Canada, there may be risk involved in the determination of an allowable price selected for a patented drug product at the time of introduction to the market by the Company launching such products in Canada. If the PMPRB does not agree with the pricing assumptions chosen by such company introducing a new drug product, the price chosen could be challenged by the PMPRB pursuant to the PMPRB initiating an investigation and, if it is determined, usually pursuant to an oral tribunal hearing, that the price charged is excessive, the Company may need to reduce its price of the product and a fine may be levied against the Company for any amount deemed to be in excess of the allowable price determined. Drug products that have no patents are not subject to the PMPRB’s jurisdiction.
European Union Regulatory Overview
Before a medicinal product can be supplied or marketed in the European Union (“EU”), it must first be granted a marketing authorization. There are three routes by which this may be achieved: (1) the centralized procedure whereby a single European license is granted by the European Commission permitting the supply of the product in question throughout the EU, Iceland, Norway and Lichtenstein; (2) the decentralized procedure; or (3) the mutual recognition procedure, whereby, in the case of (2) and (3), the views of one national authority (Reference Member State) are “recognized” by other authorities (Concerned Member States) when conducting their reviews. The decentralized procedure applies if the medicinal product in question has not yet received a marketing authorization in any member state at the time of the application, whereas the mutual recognition procedure applies to a currently approved medicinal product. The decentralized and mutual recognition processes lead to individual marketing authorizations in each member state for the supply of products in that country only. The centralized route is compulsory for certain products, including biotechnology products, and is optional for certain so-called “high technology” products and products containing an entirely new active substance (apart from those medicinal products containing a new active substance for treatment of specified diseases listed at paragraph 3 of the Annex to Regulation (EC) No 726/2004, which come within the compulsory centralized procedure). All products which are not authorized by the centralized route must be authorized by the decentralized or mutual recognition procedures unless the product is designed for use in a single country in which case a National Application can be made.
In making an application for a new medicinal product not governed compulsorily by the centralized procedure, typically use will be made of the decentralized procedure although the mutual recognition procedure would be used if a marketing authorization were first secured in a Reference Member State. The procedural steps for the decentralized procedure and the mutual recognition procedure are governed by Directive 2001/83/EC, as amended, and are described in the Notice to Applicants, Volume 2A Chapter 2—Mutual Recognition (updated version – February 2007). The procedures provide for set time periods for each process (decentralized – 120 days; mutual recognition - 210 days), but if consensus is not reached between all the Concerned Member States and the Reference Member State in that time, the application is referred to arbitration through the Co-ordination Group for Mutual Recognition and Decentralized Procedure (“CMD”), with subsequent referral to the Committee for Human Medicinal Products (“CHMP”). If a referral is made, the procedure is suspended, and marketing of the product would only be possible in those EU member states in which the product has been approved (prior to the conclusion of the referral procedure) by way of the mutual recognition procedure. The opinion of the CMD/CHMP, which is binding, could support or reject the objections or alternatively reach a compromise position acceptable to all EU countries concerned. The arbitration procedure may require the delivery of additional data. Once granted, any Marketing Authorization (“MA”) remains subject to pharmacovigilance and all competent authorities have the power to vary, suspend or revoke an MA on grounds of safety.
Pricing and Reimbursement
As pressures for cost containment increase, particularly in Canada, the United States and the EU, there can be no assurance that the prices we can charge for our products will be as favorable as historical pharmaceutical product prices. Reimbursement by government, private insurance organizations and other healthcare payors has become increasingly important, as has the listing of new products on large formularies, such as those of pharmaceutical benefit providers and group buying organizations. The failure of one or more products to be included on formulary lists, or to be reimbursed by government or private insurance organizations, could have a negative impact on our results of operation and financial condition.
Future Legislation or Administrative Action
The extent of U.S., Canadian and other foreign government regulation which might result from future legislation or administrative action cannot be accurately predicted. For example, in the United States, although the Food and Drug Administration Modernization Act of 1997 (“FDAMA”) modified and created requirements and standards under the FFDCA with the intent of facilitating product development and marketing, the FDA is still in the process of developing regulations implementing FDAMA and the more recent Food and Drug Administration Amendments Act of 2007 (“FDAAA”). The FDA has been actively implementing drug safety plans called Risk Evaluation and Mitigation Strategies as authorized by the FDAAA, as a condition of drug approval, or after initial marketing, if the FDA becomes aware of new safety data about the drug. These and other legislative initiatives may impose additional regulatory requirements on us and may impact approval of our drugs or our marketing plans. The actual effect of these and other developments on our business is uncertain and unpredictable.
Other Laws and Regulations
The Company’s operations are or may be subject to various federal, provincial, state and local laws, regulations and recommendations relating to the marketing of products and relationships with treating physicians, data protection, safe working conditions, laboratory and manufacturing practices, the experimental use of animals, patient safety, the export of products to certain countries and the purchase, storage, movement, use and disposal of hazardous or potentially hazardous substances. Although we believe our safety procedures comply with the standards prescribed by federal, provincial, state and local regulations, the risk of contamination, injury or other accidental harm cannot be eliminated completely. In the event of an accident, we could be held liable for any damages that result. The amount of such damages could have a materially adverse effect on our results of operations and financial condition.
Significant Customers
During the year ended December 31, 2015, on a pro forma basis, the Company had three significant pharmaceutical wholesale customers that account for approximately 51.3% (McKesson Pharmaceutical – 27.6%, Kohl & Frisch – 10.5% and Shoppers Drug Mart Inc. – 10.2%) of the Company’s sales of its commercialized products. Management believes this is normal and customary in the pharmaceutical business. These are well-known and respected customers that have a solid track record of paying all outstanding amounts owing on time and the Company does not anticipate that this will materially change in 2016.
Employees
As of March 15, 2016, the Company had a total of 107 employees, including 105 full-time and 2 part-time employees. Of these, 55 employees are in sales and marketing and the remainder are in management and administration positions, with 40 employees holding advanced degrees, including 6 with an M.D., Pharm.D. or Ph.D. degree.
Corporate Information
The Company was incorporated under the BCBCA on December 2, 2015. Our registered office is located at 666 Burrard Street, Suite 1700, Vancouver, British Columbia, V6C 2X8 and our principal executive offices are located at 151 Steeles Avenue East, Milton, Ontario, Canada, L9T 1Y1, 3 Columbus Circle, Suite 1710, New York, New York, 10019, and 56 Fitzwilliam Square, Dublin 2, Ireland. Our telephone number is (905) 876-1118. We maintain a website at www.aralez.com and will make available free of charge through this website our Annual Reports on Form 10-K, our Quarterly Reports on Form 10-Q, our Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Sections 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. We also will similarly make available, free of charge on our website, the reports filed with the SEC by our executive officers, directors and 10% stockholders pursuant to Section 16 under the Exchange Act as soon as reasonably practicable after copies of those filings are provided to us by those persons. We are not including the information contained at www.aralez.com, or at any other Internet address, as part of, or incorporating it by reference into, this Annual Report on Form 10-K. In addition, we will make available on our website (i) the charters for the committees of our Board of Directors, including the Audit Committee, Compensation Committee, Nominating/Corporate Governance Committee and Transaction Committee, and (ii) our Code of Business Conduct and Ethics governing our directors, officers and employees. We intend to disclose on our website any amendments to, or waivers from, our Code of Business Conduct and Ethics that are required to be disclosed pursuant to the rules of the SEC and the NASDAQ Global Market.
We are also required to file reports and other information with the securities commissions in all provinces in Canada, other than Quebec. You are invited to read and copy any reports, statements or other information, other than confidential filings, that we file with the provincial securities commissions (excluding the Autorité des marchés financiers). These filings are also electronically available from the Canadian System for Electronic Document Analysis and Retrieval (www.sedar.com), the Canadian equivalent of the SEC’s electronic document gathering and retrieval system.
Certain factors may have a material adverse effect on our business, financial condition and results of operations, and you should carefully consider them. Accordingly, in evaluating our business, we encourage you to consider the following discussion of risk factors in its entirety, in addition to other information contained in this Annual Report on Form 10-K, as well as our other public filings with the SEC. The risks and uncertainties described below are those we currently believe to be material, but they are not the only ones we face. If any of the following risks, or any other risks and uncertainties that we have not yet identified or that we currently consider not to be material, actually occur or become material risks, our business and financial condition could be materially and adversely affected.
Risks Related to Our Business
Our ability to generate revenues from our products is subject to attaining significant market acceptance among physicians, patients, third-party payors and the medical community.
Our current products, and other products or product candidates that we may develop, acquire or in-license, may not attain market acceptance among physicians, patients, third-party payors or the medical community. Even if a product displays a favorable efficacy and safety profile in clinical trials, market acceptance of a product will not be known until after it is launched and a product may not generate the revenues that we anticipate. The degree of market acceptance will depend upon a number of factors, including:
| ● | the acceptance by primary care doctors and other medical specialists of our products, including VIMOVO, Fibricor, our Canadian product portfolio and YOSPRALA, if and when approved, as an alternative to other therapies; |
| ● | the receipt and timing of regulatory approvals; |
| ● | the timing of market introduction of our products as well as competitive drugs; |
| ● | the availability of coverage and adequate reimbursement and pricing from government and other third-party payors; |
| ● | the price of our products, both in absolute terms and relative to alternative therapies; |
| ● | the indications for which the product is approved; |
| ● | the rate of adoption by healthcare providers; |
| ● | the rate of product acceptance by target patient populations; |
| ● | the availability of alternative therapies; |
| ● | the extent and effectiveness of marketing efforts by our collaborators, third-party distributors and agents; |
| ● | the strength of sales, marketing and distribution support; |
| ● | the existence of adverse publicity regarding our products or similar products and the pricing of pharmaceutical products generally; |
| ● | the efficacy of our products compared to alternative therapies; and |
| ● | the extent and severity of side effects as compared to alternative therapies. |
If we make strategic acquisitions, we will incur a variety of costs and may fail to realize all of the anticipated benefits of the transactions or those benefits may take longer to realize than expected. We may be unable to identify, acquire, close or integrate acquisition targets successfully.
A significant part of our business strategy includes acquiring and integrating complementary businesses, products, technologies or other assets, and forming strategic alliances and other business combinations, to help drive future growth. We may also in-license new products or compounds. Acquisitions or similar arrangements may be complex, time-consuming and expensive, and the process of negotiating the acquisition and integrating an acquired product, drug candidate, technology, business or company might result in operating difficulties and expenditures and might require significant management attention that would otherwise be available for ongoing development of our business, whether or not any such transaction is ever consummated. Moreover, we may never realize the anticipated benefits of any acquisition or forecasted sales may not materialize. For example, in 2015, we acquired the rights to manufacture, market, promote, distribute and sell Fibricor and its related authorized generic in the United States from Sun Pharmaceuticals Industries Ltd. We may not realize the anticipated benefits of, and could be subject to additional liabilities relating to, such acquisition, which could have a material adverse effect on our financial condition.
In addition, there are a number of risks and uncertainties relating to our closing transactions. If such transactions are not completed for any reason, we will be subject to several risks, including the following: (i) the market price of our common shares may reflect a market assumption that such transactions will occur, and a failure to complete such transactions could result in a negative perception by the market of us generally and a decline in the market price of our common shares; and (ii) many costs relating to the such transactions may be payable by us whether or not such transactions are completed.
If an acquisition is consummated, the integration of the acquired business, product or other assets into the Company may also be complex and time-consuming and, if such businesses, products and assets are not successfully integrated, we may not achieve the anticipated benefits, cost-savings or growth opportunities. Potential difficulties that may be encountered in the integration process include the following: integrating personnel, operations and systems, while maintaining focus on selling and promoting existing and newly-acquired products; coordinating geographically dispersed organizations; distracting management and employees from operations; retaining existing customers and attracting new customers; maintaining the business relationships the acquired company has established, including with healthcare providers, third-party payors and distributors; and managing inefficiencies associated with integrating the operations of the Company.
Furthermore, we have incurred, and may incur in the future, restructuring and integration costs and a number of non-recurring transaction costs associated with these acquisitions, combining the operations of the Company and the acquired company and achieving desired synergies. These fees and costs may be substantial. Non-recurring transaction costs include, but are not limited to, fees paid to legal, financial, regulatory, manufacturing and accounting advisors, filing fees and printing costs. Additional unanticipated costs may be incurred in the integration of the businesses of the Company and the acquired company. There can be no assurance that the elimination of certain duplicative costs, as well as the realization of other efficiencies related to the integration of the acquired business, will offset the incremental transaction-related costs over time. Therefore, any net benefit may not be achieved in the near term, the long term or at all.
Finally, these acquisitions and other arrangements, even if successfully integrated, may fail to further our business strategy as anticipated or to achieve anticipated benefits and success, expose us to increased competition or challenges with respect to our products or geographic markets, and expose us to additional liabilities associated with an acquired business, product, technology or other asset or arrangement. Any one of these challenges or risks could impair our ability to realize any benefit from our acquisition or arrangement after we have expended resources on them.
The recently consummated Tribute Transaction represents a significant acquisition for the Company and may expose us to a number of the risks identified above. We may face difficulties in connection with the integration of the Tribute business into the Company, which integration activities may be complex, time-consuming and disruptive to the operation of our business generally. In addition, the costs incurred in connection with such integration activities may be more substantial than we have anticipated and, as a result, may significantly reduce or even outweigh any benefits and efficiencies realized during our integration efforts. Finally, we may not be successful in implementing all of our plans with respect to the Tribute business and, as a result, we may not be able to achieve all of the anticipated benefits of the Tribute Transaction. Any of these factors could have a material adverse effect on our business, financial condition or results of operations or could decrease or delay the expected accretive effect of the Tribute Transaction or cause the market value of our common shares to decline.
Failure to successfully acquire, license or develop and market additional product candidates or approved products would impair our ability to grow.
As part of our growth strategy, we intend to acquire, license or develop and market additional products and product candidates. We are pursuing various therapeutic opportunities through our pipeline. The product candidates to which we allocate our resources may not end up being successful. In addition, because our internal research capabilities are limited, we may depend upon pharmaceutical, biotechnology and other researchers to sell or license products or technology to us. The success of this strategy depends partly upon our ability to identify, select, license and/or acquire promising pharmaceutical or other healthcare product candidates and products for Canada, the United States and elsewhere. Failure of this strategy would impair our ability to grow.
The process of proposing, negotiating and implementing a license or acquisition of a product candidate or approved product is lengthy and complex. Other companies, including some with substantially greater financial, marketing and sales resources, may compete with us for the license or acquisition of product candidates and approved products. We may devote resources to potential acquisitions or in-licensing opportunities that are never completed, or we may fail to realize the anticipated benefits of such efforts. We may not be able to acquire the rights to additional product candidates on terms that we find acceptable, or at all.
In addition, future acquisitions may entail numerous operational and financial risks, including:
| ● | exposure to unknown liabilities; |
| ● | disruption of our business and diversion of management’s time and attention to develop acquired products or technologies; |
| ● | incurrence of substantial debt, dilutive issuances of securities or depletion of cash to pay for acquisitions; |
| ● | higher than expected acquisition and integration costs; |
| ● | difficulty in combining the operations and personnel of any acquired businesses with our operations and personnel; |
| ● | increased amortization expenses; |
| ● | impairment of relationships with key suppliers or customers of any acquired businesses due to changes in management and ownership; and |
| ● | inability to motivate key employees of any acquired businesses. |
Further, any unapproved product candidate that we acquire may require additional development efforts prior to commercial sale, including extensive clinical testing and approval by applicable regulatory authorities. All product candidates are prone to risks of failure typical of pharmaceutical product development, including the possibility that a product candidate will not be shown to be sufficiently safe and effective for approval by applicable regulatory authorities.
We currently depend and will in the future depend on third parties to manufacture our products and product candidates. If these manufacturers fail to meet our requirements or any regulatory requirements, the product development and commercialization of our products and candidates will be delayed.
We do not have, and have no plans to develop, the internal capability to manufacture our products or product candidates. We rely upon third-party manufacturers and our partners to supply us with the commercial and developmental supplies of our products and product candidates. For example, we have a supply agreement with Patheon, pursuant to which Patheon manufactures our requirements for the sale of YOSPRALA in the United States once approved. The manufacturing facilities of our contract manufacturers must be inspected and found to be in full compliance with cGMP, quality system management requirements or similar standards before marketing approval, and we may not be able to ensure that such third parties comply with these obligations. The failure of our contract manufacturers to comply with cGMP regulations, quality system management requirements or similar regulations could result in enforcement action by the FDA or its foreign counterparts, including, but not limited to, warning letters, fines, injunctions, civil or criminal penalties, recall or seizure of products, total or partial suspension of production or importation, suspension or withdrawal of regulatory approval for approved or in-market products, refusal of the government to renew marketing applications, licenses or approve pending applications or supplements, suspension of ongoing clinical trials, imposition of new manufacturing requirements, closure of facilities and criminal prosecution. These enforcement actions could lead to a delay or suspension in production. Furthermore, the failure of our ingredient or material suppliers to comply with regulatory requirements can impact our ability to obtain approval of our products or our ability to supply the market with our products after approval. For example, in connection with the approval process for YOSPRALA, our initial primary aspirin API supplier had informed us that it received warning letters from the FDA relating to Form 483 inspection deficiencies. While the manufacturer is working to remediate these deficiencies, we have focused our efforts toward using our previously designated secondary aspirin API supplier as our primary supplier in connection with our re-submission of the YOSPRALA NDA.
There is no guarantee that manufacturers and API or other material suppliers that enter into commercial supply contracts with us will be financially viable entities going forward, or will not otherwise breach or terminate their agreements with us. If we do not have the necessary commercial supply contracts, or if Patheon is or our YOSPRALA API suppliers are, or any of our future contract manufacturers or API suppliers are, unable to satisfy our requirements or meet any regulatory requirements, and we are or will be required to find alternative sources of supply, there may be additional costs and delays in product development and commercialization of our product candidates or we may be required to comply with additional regulatory requirements.
In the event that suppliers of a product, ingredient or any materials we need to manufacture or package our products or licensed products are not available or not for sale at the time we need such ingredient or material in order to meet our required delivery schedule or on commercially reasonable terms, then we could be at risk of a product shortage or stock-out. We rely on our suppliers in many cases to ensure the adequate supply of ingredients, APIs and packaging material and for the timely delivery of orders placed by us. Should we experience a shortage in supply of a product, licensed product, or API, any material sales of such product or licensed product could be harmed or reduced and our ability to generate revenues from such product or licensed product may be impaired.
We depend heavily on the success of our unapproved product candidates, which may never be approved for commercial use. Failure to successfully commercialize our products or develop, gain approval of or commercialize our product candidates would adversely impact our financial condition and prospects.
We anticipate that for the foreseeable future our ability to achieve profitability will be dependent on the successful commercialization of our products upon regulatory approval in territories where our products are not approved, such as YOSPRALA in the United States. Before we can market and sell our products in a particular jurisdiction, we need to obtain necessary regulatory approvals (from the FDA in the United States, Health Canada in Canada and from similar foreign regulatory agencies in other jurisdictions), and in some jurisdictions, reimbursement authorization. There are no guarantees that we or our commercialization partners will obtain approval in those countries where we wish to commercialize our products. Even if we or our commercialization partners obtain additional regulatory approvals, we may never generate significant revenues from any commercial sales of our products. These approvals may not be granted on a timely basis, if at all. Nor can any assurance be given that if such approval is secured, the approved labeling will not have significant labeling limitations, including limitations on the indications for which we can market a product, or require onerous risk management programs. Further, our current or future collaboration agreements may terminate, or require us to make certain payments to our collaborators, or our collaborators may have the right to terminate their agreements with us or reduce or eliminate their payments to us under these agreements, based on our inability to obtain, or delays in obtaining, regulatory approval for our product candidates. If we fail to successfully commercialize our current and future products, we may be unable to generate sufficient revenues to sustain and grow our business, and our business, financial condition and results of operations will be adversely affected.
In addition, if our development projects are not successful or are significantly delayed, we may not recover our substantial investments in the product candidates and our failure to bring these product candidates to market on a timely basis, or at all, could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline. For example, the approval process for YOSPRALA has been delayed due to Form 483 inspection deficiencies noted by the FDA to our previously designated primary aspirin API supplier. While the manufacturer is working to remediate these deficiencies, we have focused our efforts toward using our previously designated secondary aspirin API supplier as our primary supplier in connection with our YOSPRALA NDA. We will include both aspirin API suppliers in the NDA package for YOSPRALA.
We continue to evaluate the commercial opportunities for our current products and product candidates in connection with our development of a worldwide commercialization strategy. If we are unable to develop sales and marketing capabilities on our own, or through partnerships, we will not be able to fully exploit the commercial potential of our future products and the costs of pursuing such a strategy may have a material adverse impact on our results of operations.
We continue to evaluate the commercial opportunities for our products and product candidates in connection with our development of a worldwide commercialization strategy. In June 2015, our Board appointed Adrian Adams as our new Chief Executive Officer and Andrew I. Koven as our new President and Chief Business Officer, each of whom has experience creating, leading and expanding pharmaceutical companies with marketing and sales capabilities. We have retained ownership of our PA product candidates through the clinical development and pre-commercialization stage and have developed the commercialization strategy for these products and conducted all the required pre-commercialization activities in the United States. We plan to make significant expenditures to secure commercial resources to sell YOSPRALA once approved and the products we acquired from Tribute and to expand or enhance our marketing capabilities to support our anticipated growth. Any failure or extended delay in the expansion or enhancement of our sales and marketing capabilities or inability to effectively operate in the marketplace alone or together with our partners could adversely impact our business. There can be no assurance that our sales and marketing efforts will generate significant revenues and costs of pursuing such a strategy may have a material adverse impact on our results of operations. Events or factors that may inhibit or hinder our commercialization efforts include:
| ● | building and developing our own commercial team or playing a role in the commercialization with a partner will be expensive and time-consuming and will result in high cash burn or reduced profitability; |
| ● | failure to acquire sufficient or suitable personnel to establish, oversee, or implement our commercialization strategy; |
| ● | failure to recruit, train, oversee and retain adequate numbers of effective sales and marketing personnel; |
| ● | failure to develop a commercial strategy ourselves or together with partners that can effectively reach and persuade adequate numbers of physicians to prescribe our products; |
| ● | our or our partners’ inability to secure reimbursement at a reasonable price; |
| ● | unforeseen costs and expenses associated with creating or acquiring and sustaining an independent commercial organization; |
| ● | incurrence of costs in advance of anticipated revenues and subsequent failure to generate sufficient revenue to offset additional costs; and |
| ● | ability to fund our commercialization efforts alone or together with our partners on terms acceptable to us, if at all. |
If we are unable to effectively train and equip our sales force, our ability to successfully commercialize our products will be harmed.
We are required to expend significant time and resources to train our sales force to be credible, compliant and persuasive in educating physicians to prescribe and pharmacists to dispense our products. In addition, we must train our sales force to ensure that a consistent and appropriate message about our products is being delivered to our potential customers. Our sales representatives may also experience challenges promoting multiple products when they call on physicians and their office staff. This is particularly true with respect to our products that have competing products prescribed to similar patients. If we are unable to effectively train our sales force and equip them with effective materials, including medical and sales literature to help them inform and educate potential customers about the benefits of our products and their proper administration and approved indications, our efforts to successfully commercialize our products could be put in jeopardy, which could have a material adverse effect on our financial condition, share price and operations.
Our reliance on collaborations with third parties to develop, manufacture and commercialize our development products is subject to inherent risks and may result in delays in product development and lost or reduced revenues, restricting our ability to commercialize our products and adversely affecting our profitability.
With respect to the products we have out-licensed or developed internally, we depend upon collaborations with third parties to develop and manufacture these product candidates and, in some cases, we depend substantially upon third parties to commercialize these products. As a result, our ability to develop, obtain regulatory approval of, manufacture and commercialize our existing and possibly future product candidates depends upon our ability to maintain existing, and enter into and maintain new, contractual and collaborative arrangements with others. We also engage, and may in the future to continue to engage, contract manufacturers and clinical trial investigators.
In addition, the identification of new compounds or product candidates for development has led us in the past, and may continue to require us, to enter into license or other collaborative agreements with others, including pharmaceutical companies and research institutions. Such collaborative agreements for the acquisition of new compounds or product candidates would typically require us to pay license fees, make milestone payments and/or pay royalties. For products we out-license, these agreements may result in our revenues being lower than if we developed our product candidates ourselves and in our loss of control over the development of our product candidates.
Contractors or collaborators may have the right to terminate their agreements with us after a specified notice period for any reason or upon a default by us. For example, AstraZeneca, Horizon and Pernix have the right to terminate their respective agreements with us upon a 90-day notice for any reason. Contractors or collaborators may have the right to reduce their payments to us under their agreements. For example, Pernix, AstraZeneca and Horizon have the right to reduce the royalties on net sales of products payable to us under their respective agreements if generic competitors enter the market and attain a pre-determined share of the market for products marketed under the agreements, or if they must pay a royalty to one or more third parties for rights they license from those third parties to commercialize products marketed under the agreements. Further, our current or future collaboration agreements may terminate, or our collaborators may have the right to terminate their agreements with us or reduce or eliminate their payments to us under these agreements, based on our inability to obtain, or delays in obtaining, regulatory approval for our product candidates or our contract manufacturers’ inability to manufacture our products or to supply the sufficient quantities of our products to meet market demand. If our current or future collaborators exercise termination rights they may have, or if the agreements terminate because of delays in obtaining regulatory approvals, or for other reasons, and we are not able to establish replacement or additional research and development collaborations or licensing arrangements, we may not be able to develop and/or commercialize our product candidates. Moreover, any future collaborations or license arrangements we may enter into may not be on terms favorable to us.
Collaborators may decide not to continue marketing our products in certain countries of the territory, as was the case when AstraZeneca informed us that, after a strategic business review, it had decided to cease promotion and sampling of VIMOVO by the end of the third quarter of 2013 in certain countries, including the United States and all countries in Europe, other than Spain and Portugal, which have pre-existing contractual relationships with third parties. In addition, collaborators may decide to assign their rights under our agreement to third parties. For example, we had a collaboration agreement with GSK for the development and commercialization of certain triptan combinations using our MT 400 technology, including Treximet, in the United States, and GSK subsequently divested all of its rights, title and interest to develop, commercialize and sell the licensed products in the United States to Pernix.
Other risks associated with our collaborative and contractual arrangements with others include the following:
| ● | we may not have day-to-day control over the activities of our contractors or collaborators; |
| ● | our collaborators may fail to defend or enforce patents they own on compounds or technologies that are incorporated into the products we develop with them; |
| ● | third parties may not fulfill their regulatory or other obligations; |
| ● | we may not realize the contemplated or expected benefits from collaborative or other arrangements; |
| ● | if any collaborator were to breach its agreement with us or otherwise fail to conduct collaborative activities in a timely or successful manner, the pre-clinical or clinical development or commercialization of the affected product candidate or research program would be delayed or terminated; |
| ● | our collaborators may be able to exercise control, under certain circumstances, over our ability to protect our patent rights under patents covered by the applicable collaboration agreement; and |
| ● | disagreements may arise regarding a breach of the arrangement, the interpretation of the agreement, ownership of proprietary rights, clinical results or regulatory approvals. |
These factors could lead to delays in the development of our product candidates and/or the commercialization of our products or reduction in the milestone payments we receive from our collaborators, or could result in our not being able to commercialize our products. Further, disagreements with our contractors or collaborators could require or result in litigation or arbitration, which would be time-consuming and expensive. Our ultimate success may depend upon the success and performance on the part of these third parties. If we fail to maintain these relationships or establish new relationships as required, development of our product candidates and/or the commercialization of our products will be delayed or may never be realized.
We may not be able to compete with treatments now being developed and marketed, or which may be developed and marketed in the future by other companies.
Our products and product candidates will compete with existing and new therapies and treatments. There are also likely to be numerous competitors that are engaged in the development of alternatives to our technologies and products, which could render our products, product candidates and technologies obsolete or non-competitive. For example, our primary competitors will likely include large pharmaceutical companies, biotechnology companies, universities and public and private research institutions. Some of these companies have greater research and development capabilities, experience, manufacturing, marketing, financial and managerial resources than we do. Collaborations or mergers between large pharmaceutical or biotechnology companies with competing drugs and technologies could enhance our competitors’ financial, marketing and other resources. Accordingly, our competitors may succeed in developing competing drugs or technologies, obtaining patent protection, obtaining regulatory approval for products, commercializing products or gaining market acceptance more rapidly than we can. Any delays we encounter in obtaining regulatory approvals for our product candidates, such as we experienced as a result of the CRL we received from the FDA relating to the NDA for YOSPRALA 325/40 and 81/40, increase this risk.
The competition for VIMOVO, and any other PN products that may be developed and receive regulatory approval, may come from the oral NSAID market, specifically the traditional non-selective NSAIDs (such as naproxen and diclofenac), traditional NSAID/gastroprotective agent combination products or combination product packages (such as Arthrotec® and Prevacid® NapraPAC™), combinations of NSAIDs and PPIs taken as separate pills and the only remaining COX-2 inhibitor, Celebrex®. The competition for our PA product candidates may come from aspirin itself, as well as other products used for secondary prevention.
Based upon their drug product and pipeline portfolios and the overall competitiveness of our industry, we believe that we face, and will continue to face, intense competition from other companies for securing collaborations with pharmaceutical companies, establishing relationships with academic and research institutions, and acquiring licenses to proprietary technology. Our competitors, either alone or with collaborative parties, may also succeed with technologies or products that are more effective than any of our current or future technologies or products. Many of our actual or potential competitors, either alone or together with collaborative parties, have substantially greater financial resources, and almost all of our competitors have larger numbers of scientific and administrative personnel than we do. If we cannot successfully compete with new or existing products, our marketing and sales will suffer and we may not ever receive any revenues from sales of products or may not receive sufficient revenues to achieve profitability.
For certain of our products, we depend on reimbursement from third-party payors and a failure to obtain coverage or reduction in the extent of reimbursement could reduce our product sales and revenue.
Sales of certain of our products are dependent, in part, on the availability and extent of reimbursement from government health administration authorities, private health insurers and other organizations and our continued participation in such programs. These entities may refuse to provide coverage and reimbursement, determine to provide a lower level of coverage and reimbursement than anticipated, or reduce previously approved levels of coverage and reimbursement, including in the form of higher mandatory rebates or modified pricing terms.
In certain countries, including Canada, where we sell or are seeking or may seek to commercialize our products, pricing, coverage and level of reimbursement of prescription drugs are subject to governmental control. We may be unable to timely or successfully negotiate coverage, pricing, and reimbursement on terms that are favorable to us, or such coverage, pricing, and reimbursement may differ in separate regions in the same country. A significant reduction in the amount of reimbursement or pricing for our products in one or more countries may reduce our profitability and adversely affect our financial condition. Certain countries establish pricing and reimbursement amounts by reference to the price of the same or similar products in other countries. If coverage or the level of reimbursement is limited in one or more countries, we may be unable to obtain or maintain anticipated pricing or reimbursement in current or new territories. In the United States, the EU member states, and elsewhere, there have been, and we expect there will continue to be, efforts to control and reduce healthcare costs. In the United States, for example, the price of drugs has come under intense scrutiny by the U.S. Congress. Third-party payors decide which drugs they will pay for and establish reimbursement and co-payment levels. Government and other third-party payors are increasingly challenging the prices charged for healthcare products, examining the cost effectiveness of drugs in addition to their safety and efficacy, and limiting or attempting to limit both coverage and the level of reimbursement for prescription drugs.
Changes in government regulations or private third-party payors’ reimbursement policies may reduce reimbursement for our products and adversely affect our future results. Our commercial success depends on obtaining and maintaining reimbursement at anticipated levels for our products. It may be difficult to project the impact of evolving reimbursement mechanics or the willingness of payors to cover our products. If we are unable to obtain or maintain coverage, or coverage is reduced in one or more countries, our pricing may be affected and our product sales, results of operations or financial condition could be harmed.
Failure to be included in formularies developed by managed care organizations, governments, hospitals and other organizations may negatively impact the utilization of our products, which could harm our market share and negatively impact our business, financial condition and results of operations.
Managed care organizations and other third-party payors try to negotiate the pricing of medical services and products to control their costs. Managed care organizations and pharmacy benefit managers typically develop formularies to reduce their cost for medications. Formularies can be based on the prices and therapeutic benefits of the available products. Due to their lower costs, generic products are often favored. The breadth of the products covered by formularies varies considerably from one managed care organization to another, and many formularies include alternative and competitive products for treatment of particular medical conditions. Failure to be included in such formularies or to achieve favorable formulary status may negatively impact the utilization of our products. If our products are not included within an adequate number of formularies or adequate reimbursement levels are not provided, or if those policies increasingly favor generic products, our market share and gross margins could be harmed, as could our business, financial condition, results of operations and cash flows.
For example, in July 2014, CVS Caremark and Express Scripts, Inc. removed VIMOVO from their formularies and placed it on the exclusion list. Horizon, who holds the U.S. commercialization rights for VIMOVO in exchange for royalty payments to us, estimated that approximately 20-30% of VIMOVO prescriptions in the United States could be impacted. While there was a 26% drop in VIMOVO prescriptions in the United States in the first quarter of 2015, we have seen growth in the remainder of the year such that the reported VIMOVO prescriptions by IMS Health Holdings, Inc.’s National Prescription Audit for 2015 exceed the prescriptions for 2014 by 25%. However, net sales upon which we are paid royalty only rose by 2%, indicating that managed care is having an impact on the realization of price increases through formulary control.
Generic competition of our products could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
Upon the expiration or loss of patent protection for our products, or upon the “at-risk” launch (despite pending patent infringement litigation against the generic product) by a generic competitor of a generic version of our products, we can lose a significant portion of sales of that product in a very short period, which could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
If we lose our license from any licensors, we may be unable to continue a substantial part of our business.
We have licensed certain assets, including certain intellectual property, marketing authorizations and related data, and medical commercial and technical information, used in a substantial part of our business. Such license agreements may be terminated by the licensor if we are in breach of our obligations under, or fail to perform any terms of, the agreement and fail to cure that breach. If a license agreement is terminated, then we may lose our rights to utilize the intellectual property and other assets covered by such agreement to manufacture, market, promote, distribute and sell the licensed products, which may prevent us from continuing a substantial part of our business and may result in a material and serious adverse effect on our financial condition, results of operations and any prospects for growth.
We will not be able to commercialize our product candidates if preclinical studies do not produce successful results or if clinical trials do not demonstrate safety and efficacy in humans.
We and our development partners, as applicable, conduct extensive preclinical studies and clinical trials to demonstrate the safety and efficacy in humans of our product candidates in order to obtain regulatory approval for the sale of our product candidates. Preclinical studies and clinical trials are expensive, can take many years and have uncertain outcomes. If clinical trials are unsuccessful, we will not be able to commercialize our product candidates and additional studies may be required.
We depend on key personnel and may not be able to retain these employees or recruit additional qualified personnel, which would harm our research and development and commercialization efforts as well as our ability to identify, acquire, close or integrate acquisition targets successfully.
We are highly dependent on the efforts of our key management, especially Adrian Adams, our Chief Executive Officer, and Andrew I. Koven, our President and Chief Business Officer. If we should lose the services of Mr. Adams or Mr. Koven, or are unable to replace the services of our other key personnel who may leave the Company, or if we fail to recruit other key scientific and commercial personnel, we may be unable to achieve our business objectives and growth strategies. There is intense competition for qualified scientific and commercial personnel. We may not be able to continue to attract and retain the qualified personnel necessary for developing our business. Furthermore, our future success may also depend in part on the continued service of our other key management personnel and our ability to recruit and retain additional personnel, as required by our business. Many of the other biotechnology and pharmaceutical companies with whom we compete for qualified personnel have greater financial and other resources, different risk profiles and longer histories in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high quality candidates than that which we have to offer. If we are unable to continue to attract and retain high quality personnel, the rate and success at which we can develop and commercialize products and product candidates will be limited.
Our business, financial condition and results of operations are subject to risks arising from the international scope of our operations.
Our international operations and any future international operations may expose us to risks that could negatively impact our future results. Our operations may not develop in the same way or at the same rate as might be expected in a country with an economy similar to the United States or Canada. The additional risks that we may be exposed to in these cases include, but are not limited to:
| ● | tariffs and trade barriers; |
| ● | currency fluctuations, which could decrease the Company’s revenues or increase its costs; |
| ● | regulations related to customs and import/export matters; |
| ● | tax issues, such as tax law changes and variations in tax laws; |
| ● | limited access to qualified staff; |
| ● | inadequate infrastructure; |
| ● | cultural and language differences; |
| ● | inadequate banking systems; |
| ● | different and/or more stringent environmental laws and regulations; |
| ● | restrictions on the repatriation of profits or payment of dividends; |
| ● | crime, strikes, riots, civil disturbances, terrorist attacks or wars; |
| ● | nationalization or expropriation of property; |
| ● | law enforcement authorities and courts that are weak or inexperienced in commercial matters; and |
| ● | deterioration of political relations among countries. |
Any of these factors, or any other international factors, could have a material adverse impact on our business, financial condition and results of operations and could cause the market value of our common shares to decline. Similarly, adverse economic conditions impacting our customers in these countries or uncertainty about global economic conditions could cause purchases of our products to decline, which would adversely affect our revenues and operating results. Any failure to attain our projected revenues and operating results as a result of adverse economic or market conditions could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
Due to the large portion of our business conducted in currency other than U.S. dollars, we have significant foreign currency risk.
Our consolidated financial statements are presented in accordance with U.S. generally accepted accounting principles, and we report, and will continue to report, our results in U.S. dollars. Some of our operations are conducted by subsidiaries in Canada and other countries outside of the United States. The results of operations and the financial position of these subsidiaries are recorded in the relevant foreign currencies and then translated into U.S. dollars. Any change in the value of the Canadian dollar or of the currencies in the other markets in which we operate against the U.S. dollar during a given financial reporting period would result in a foreign currency loss or gain on the translation of U.S. dollar denominated revenues and costs. The exchange rates between many of the currencies in the other markets in which we operate against the U.S. dollar have fluctuated significantly in recent years and may fluctuate significantly in the future. Consequently, our reported earnings could fluctuate materially as a result of foreign exchange translation gains or losses and may not be comparable from period to period.
We face market risks attributable to fluctuations in foreign currency exchange rates and foreign currency exposure on the translation into U.S. dollars of the financial results of our operations in Canada and Europe, including, for example, as a result of the recent strengthening of the U.S. dollar against other foreign currencies, including the Canadian dollar and the Euro. Exchange rate fluctuations could have an adverse effect on our results of operations. Both favorable and unfavorable foreign currency impacts to our foreign currency-denominated operating expenses are mitigated to a certain extent by the natural, opposite impact on our foreign currency-denominated revenue. In addition, the repurchase of principal under our U.S. dollar denominated debt may result in foreign exchange gains or losses for Canadian income tax purposes. One-half of any foreign exchange gains or losses will be included in our Canadian taxable income.
Risks related to Legislation and Regulations
As we pursue commercialization of YOSPRALA (upon approval), Fibricor, our Canadian product portfolio and other opportunities for our future products ourselves, failure to comply with the laws governing the marketing and sale of such products may result in regulatory agencies taking action against us and/or our partners, which could significantly harm our business.
As we pursue commercialization of YOSPRALA (upon approval), Fibricor, our Canadian product portfolio and other future products, we will be subject to extensive regulation by the FDA, Health Canada and the governmental authorities in other countries. In particular, there are many federal, state, provincial and local laws that we will need to comply with if we become engaged in the marketing, promoting, distribution and sale of pharmaceutical products. If we fail to comply with U.S. and Canadian regulatory requirements and those in other countries where our products are sold, we could lose our marketing approvals or be subject to civil and/or criminal penalties, injunctions, fines or other sanctions. In addition, incidents of adverse drug reactions, unintended side effects or misuse relating to our products could result in additional regulatory controls or restrictions, or even lead to withdrawal of a product from the market. The imposition of one or more of these penalties could adversely affect our revenues and our ability to conduct our business as planned. As a condition to granting marketing approval of a product, the FDA and Health Canada may require a company to conduct additional clinical trials, the results of which could result in the subsequent loss of marketing approval, changes in product labeling or new or increased concerns about side effects or efficacy of a product. Compliance with the extensive laws and regulations to which we are subject is complicated, time-consuming and expensive. We cannot assure you that we will be in compliance with all potentially applicable laws and regulations. Even minor, inadvertent irregularities can potentially give rise to claims that the law has been violated.
We are subject to various laws and regulations, including “fraud and abuse” laws, anti-bribery laws and privacy and security regulations, and a failure to comply with such laws and regulations or prevail in any litigation related to noncompliance could have a material adverse impact on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
Pharmaceutical and biotechnology companies have faced lawsuits and investigations pertaining to violations of healthcare “fraud and abuse” laws, such as the federal False Claims Act, the federal Anti-Kickback Statute, the United States Foreign Corrupt Practices Act (the “FCPA”) and other federal, state and provincial laws and regulations. We also face increasingly strict data privacy and security laws in the United States, Canada and other countries, the violation of which could result in fines and other sanctions. The United States Department of Health and Human Services Office of Inspector General recommends, and increasingly states, that pharmaceutical companies have comprehensive compliance programs and disclose certain payments made to healthcare providers or funds spent on marketing and promotion of drug products. While we have developed a corporate compliance program, we cannot assure you that we or our employees or agents are or will be in compliance with all applicable federal, state, provincial or foreign regulations and laws. If we are in violation of any of these requirements or any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines, exclusion from federal healthcare programs or other sanctions.
The FCPA, the Canadian Corruption of Foreign Public Officials Act (the “CFPOA”) and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to officials for the purpose of obtaining or retaining business. Although we require our employees to consult with our legal department prior to making any payment or gift thought to be exempt under applicable law, there is no assurance that such policies or procedures will work effectively all of the time or protect us against liability under the FCPA and/or the CFPOA for actions taken by our employees and other intermediaries with respect to our business or any businesses that we may acquire. We may operate in parts of the world that have experienced governmental corruption to some degree and, in certain circumstances, strict compliance with anti-bribery laws may conflict with local customs and practices or may require us to interact with doctors and hospitals, some of which may be state controlled, in a manner that is different from the United States and Canada. We cannot assure you that our internal control policies and procedures will protect us from reckless or criminal acts committed by our employees or agents. Violations of these laws, or allegations of such violations, could disrupt our business and result in criminal or civil penalties or remedial measures, any of which could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
We are also subject to various privacy and security regulations, including, but not limited to, the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (as amended, “HIPAA”). HIPAA mandates, among other things, the adoption of uniform standards for the electronic exchange of information in common healthcare transactions (e.g., healthcare claims information and plan eligibility, referral certification and authorization, claims status, plan enrollment, coordination of benefits and related information), as well as standards relating to the privacy and security of individually identifiable health information, which require the adoption of administrative, physical and technical safeguards to protect such information. In addition, many states have enacted comparable laws addressing the privacy and security of health information, some of which are more stringent than HIPAA. Failure to comply with these laws can result in the imposition of significant civil and criminal penalties.
Numerous other countries have, or are developing, laws governing the collection, use and transmission of personal information as well. The EU member states and other jurisdictions have adopted data protection laws and regulations, which impose significant compliance obligations. For example, the European Commission adopted the EU Data Protection Directive, as implemented into national laws by the EU member states, which imposed strict obligations and restrictions on the ability to collect, analyze, and transfer personal data, including health data from clinical trials and adverse event reporting. Data protection authorities from different EU member states have interpreted the privacy laws differently, which adds to the complexity of processing personal data in the European Union, and guidance on implementation and compliance practices are often updated or otherwise revised. Any failure to comply with the rules arising from the EU Data Protection Directive and related national laws of EU member states could lead to government enforcement actions and significant penalties against us, and adversely impact our operating results. In December 2015, a proposal for an EU Data Protection Regulation, intended to replace the current EU Data Protection Directive, was agreed between the European Parliament, the Council of the European Union and the European Commission. The EU Data Protection Regulation, which will be officially adopted in early 2016, will introduce new data protection requirements in the European Union and substantial fines for breaches of the data protection rules. The EU Data Protection Regulation, which will be applicable two years after the date of its publication in the Official Journal for the European Union, will increase our responsibility and liability in relation to personal data that we process and we may be required to put in place additional mechanisms ensuring compliance with the new EU data protection rules.
The costs of compliance with these laws and the potential liability associated with the failure to comply with these laws could adversely affect our financial condition, results of operations and cash flows.
Legislative or regulatory reform of the healthcare system may affect our ability to sell our products profitably and could adversely affect our business.
In the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory proposals to change the healthcare system in ways that could impact our ability to sell our products profitably. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (the “Health Care Reform Act”) may affect the operational results of companies in the pharmaceutical industry, including the Company and other healthcare-related industries, by imposing on them additional costs. Effective January 1, 2010, the Health Care Reform Act increased the minimum Medicaid drug rebates for pharmaceutical companies, expanded the 340B drug discount program, and made changes to affect the Medicare Part D coverage gap, or “donut hole.” The law also revised the definition of “average manufacturer price” for reporting purposes, which has the potential to affect the amount of our Medicaid drug rebates to states. Beginning in 2011, the law imposed a significant annual fee on companies that manufacture or import branded prescription drug products.
The Health Care Reform Act also added substantial new provisions affecting compliance, some of which required the entire industry to modify business practices with healthcare practitioners. Pharmaceutical manufacturers are required in 2013 to comply with the federal Physician Payments Sunshine Act, which was passed as part of the Health Care Reform Act and requires pharmaceutical companies to monitor and report payments, gifts, the provision of samples and other remuneration made to physicians and other healthcare professionals and healthcare organizations.
We are unable to predict the future course of federal or state healthcare legislation. A variety of federal and state agencies are in the process of implementing the Health Care Reform Act, including through the issuance of rules, regulations or guidance that materially affect our business. The risk of our being found in violation of these rules and regulations is increased by the fact that many of them have not been fully interpreted by applicable regulatory authorities or the courts, and their provisions are open to a variety of interpretations. The Health Care Reform Act and further changes to healthcare laws or regulatory framework that reduce our revenues or increase our compliance or other costs could also have a material adverse effect on our business, financial condition and results of operations and cash flows, and could cause the market value of our common shares to decline.
In Canada, patented drug products are subjected to regulation by the PMPRB pursuant to the Patent Act (Canada) and the Patented Medicines Regulations. The PMPRB does not approve prices for drug products in advance of their introduction to the market. The PMPRB provides guidelines from which companies like us set their prices at the time they launch their products. All patented pharmaceutical products introduced in Canada are subject to the post-approval, post launch scrutiny of the PMPRB. Since the PMPRB does not pre-approve prices for a patented drug product in Canada, there may be risk involved in the determination of an allowable price selected for a patented drug product at the time of introduction to the market by the company launching such products in Canada. If the PMPRB does not agree with the pricing assumptions chosen by such company introducing a new drug product, the price chosen could be challenged by the PMPRB pursuant to the PMPRB initiating an investigation and, if it is determined, usually pursuant to an oral tribunal hearing, that the price charged is excessive, the price of the product may be reduced and a fine may be levied against the company for any amount deemed to be in excess of the allowable price determined. Drug products that have no valid patents are not subject to the PMPRB’s jurisdiction.
Our status as a foreign corporation for U.S. federal tax purposes could be affected by IRS action or a change in U.S. tax law.
Although the Company is incorporated in Canada, the Internal Revenue Service (the “IRS”) may assert that we should be treated as a U.S. corporation (and, therefore, a U.S. tax resident) for U.S. federal income tax purposes pursuant to Section 7874 of the Internal Revenue Code of 1986, as amended (the “Code”). A corporation is generally considered a tax resident in the jurisdiction of its organization or incorporation for U.S. federal income tax purposes. As a result of the Company being an entity incorporated in the Province of British Columbia, it would generally be classified as a foreign corporation (and, therefore, a non-U.S. tax resident) under these rules. Section 7874 of the Code provides an exception pursuant to which a foreign incorporated entity may, in certain circumstances, be treated as a U.S. corporation for U.S. federal income tax purposes.
Under Section 7874 of the Code, the Company may be treated as a U.S. corporation for U.S. federal income tax purposes if former Pozen shareholders hold 80% or more of the vote or value of the Company’s shares by reason of holding stock in Pozen immediately after the Tribute Transaction and the Company’s expanded affiliated group after the Tribute Transaction does not have substantial business activities in Canada relative to its worldwide activities. As a result of the fact that the former shareholders of Pozen owned (within the meaning of Section 7874 of the Code) less than 80% (by both vote and value) of the combined entity’s stock immediately after the Tribute Transaction, we believe we qualify as a foreign corporation for U.S. federal income tax purposes following the Tribute Transaction. However, there can be no assurance that there will not exist in the future a subsequent change in the facts or in law, which might cause us to be treated as a domestic corporation for U.S. federal income tax purposes, including with retroactive effect.
Further, there can be no assurance that the IRS will agree with the position that the ownership test was satisfied. There is limited guidance regarding the application of Section 7874 of the Code, including with respect to the provisions regarding the application of the ownership test. If we were unable to be treated as a foreign corporation for U.S. federal income tax purposes, the benefits associated with enhanced global cash management, including increased liquidity resulting from access to cash generated by our non-U.S. subsidiaries, would be jeopardized.
Our tax position may be adversely affected by changes in tax law relating to multinational corporations, or increased scrutiny by tax authorities.
Under current law, we expect to be treated as a foreign corporation for U.S. federal tax purposes. However, changes to the rules in Section 7874 of the Code or the Treasury regulations promulgated thereunder could adversely affect our status as a foreign corporation for U.S. federal tax purposes, and any such changes could have prospective or retroactive application. In addition, recent legislative proposals have aimed to expand the scope of U.S. corporate tax residence, and such legislation, if passed, could have an adverse effect on us.
Moreover, the United States Congress, the Organization for Economic Co-operation and Development and other government agencies in Canada and other jurisdictions where we and our affiliates do business have had an extended focus on issues related to the taxation of multinational corporations. One example is in the area of “base erosion and profit shifting,” where payments are made between affiliates from a jurisdiction with high tax rates to a jurisdiction with lower tax rates. As a result, the tax laws in the United States, Canada and other countries in which we and our affiliates do business could change on a prospective or retroactive basis, and any such changes could adversely affect us.
Changes in tax laws and unanticipated tax liabilities could adversely affect our effective income tax rate and profitability.
We are subject to income taxes in Canada, the United States, and certain foreign jurisdictions. Our effective income tax rate in the future could be adversely affected by a number of factors including changes in the mix of earnings in countries with differing statutory tax rates, changes in the valuation of deferred tax assets and liabilities and changes in tax laws. We regularly assess all of these matters to determine the adequacy of our tax provision which is subject to discretion. If our assessments are incorrect, it could have an adverse effect on our business and financial condition.
There can be no assurance that income tax laws and administrative policies with respect to the income tax consequences generally applicable to us, to our subsidiaries, or to a U.S. or Canadian holder of common shares will not be changed in a manner which adversely affects holders of our common shares.
If we fail to comply with our reporting and payment obligations under the Medicaid Drug Rebate Program, Medicare, PMPRB obligations, governmental funded drug formularies or other governmental pricing programs, we could be subject to additional reimbursement requirements, penalties, sanctions and/or fines which could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Pricing and rebate calculations vary among products and programs. The calculations are complex and are often subject to interpretation by us, governmental or regulatory agencies and the courts. We cannot assure you that our submissions will not be found by Centers for Medicare and Medicaid Services (“CMS”) to be incomplete or incorrect. Governmental agencies may also make changes in program interpretations, requirements or conditions of participation, some of which may have implications for amounts previously estimated or paid. The Medicaid rebate amount is computed each quarter based on our submission to CMS of our current average manufacturer price and best price for the quarter. If we become aware that our reporting for a prior quarter was incorrect, or has changed as a result of recalculation of the pricing data, we are obligated to resubmit the corrected data for a period not to exceed twelve quarters from the quarter in which the data originally were due, and CMS may request or require restatements for earlier periods as well. Such restatements and recalculations increase our costs for complying with the laws and regulations governing the Medicaid Drug Rebate Program. Any corrections to our rebate calculations could result in an overage or underage in our rebate liability for past quarters, depending on the nature of the correction. Price recalculations also may affect the ceiling price at which we are required to offer our products to certain covered entities, such as safety-net providers, under the 340B drug discount program.
We are liable for errors associated with our submission of pricing data. In addition to retroactive rebates and the potential for 340B program refunds, if we are found to have knowingly submitted false average manufacturer price, average sales price (“ASP”), or best price information to the government or made a misrepresentation in the reporting of our ASP, we may be liable for civil monetary penalties. Our failure to submit monthly/quarterly average manufacturer price, ASP, and best price data on a timely basis could result in a civil monetary penalty. Such failure also could be grounds for CMS to terminate our Medicaid drug rebate agreement, pursuant to which we participate in the Medicaid program. In the event that CMS terminates our rebate agreement, federal payments may not be available under Medicaid or Medicare Part B for our covered outpatient drugs.
Federal law requires that a company must participate in the Department of Veterans Affairs Federal Supply Schedule (“FSS”) pricing program, established by Section 603 of the Veterans Health Care Act of 1992, to be eligible to have its products paid for with federal funds. If we overcharge the government in connection with our FSS contract, whether due to a misstated federal ceiling price or otherwise, we are required to refund the difference to the government. Failure to make necessary disclosures and/or to identify contract overcharges can result in allegations against us under the False Claims Act and other laws and regulations. Unexpected refunds to the government, and responding to a government investigation or enforcement action, would be expensive and time-consuming, and could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Our business involves the use of hazardous materials, and we and our third-party manufacturers must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Our third-party manufacturers’ activities involve the controlled storage, use and disposal of hazardous materials owned by us, including the components of our approved products and product candidates and other hazardous compounds. We and our manufacturers are subject to federal, state, provincial and local as well as foreign laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. Although we believe that the safety procedures utilized by our third-party manufacturers for handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental contamination or injury from these materials. In the event of an accident, federal, state, provincial or foreign authorities may curtail the use of these materials and interrupt our business operations. We do not currently maintain hazardous materials insurance coverage. If we are subject to any liability as a result of our third-party manufacturers’ activities involving hazardous materials, our business and financial condition may be adversely affected.
Risks Related to Our Financial Position and Capital Requirements
We have incurred losses since inception and we may continue to incur losses for the foreseeable future.
We have a limited operating history and even less history operating as a combined organization following the Tribute Transaction. As of December 31, 2015, Pozen had net losses of approximately $37.8 million and, on a pro forma basis combined with Tribute, $44.6 million. Our ability to receive product revenue from the sale of products is dependent on a number of factors, principally the development, regulatory approval and successful commercialization of our products and product candidates. We expect that the amount of our operating losses will fluctuate significantly from quarter to quarter principally as a result of increases and decreases in our development efforts, the timing and amount of payments that we may receive from others and the timing of our commercial expenses, including increased expenses in connection with the anticipated approval and launch of YOSPRALA. If our licensed or marketed products do not perform well in the marketplace, our revenue will be impacted and our business could be materially harmed.
We have limited product revenues and other sources of revenues. Even if we achieve profitability in the future, we cannot be certain that we will sustain profitability, which would depress the market price of our common shares and could cause our investors to lose all or a part of their investment.
Our ability to become profitable depends upon our ability to generate revenues from sales of our products. Product revenue for products which we license out is dependent upon the commercialization efforts of our partners. One of our primary sources of revenue is the royalty payments that we may receive in connection with the commercialization of VIMOVO by AstraZeneca, outside of the United States (excluding Japan), and Horizon in the United States. In the event that AstraZeneca, Horizon or any other third-party with future commercialization rights to any of our products or product candidates fails to adequately commercialize those products or product candidates because it lacks adequate financial or other resources, decides to focus on other initiatives or otherwise, our ability to successfully commercialize our products or product candidates in the applicable jurisdictions would be limited, which would adversely affect our business, financial condition, results of operations and prospects. In addition, our ability to generate future revenues depends heavily on our success in:
| ● | commercialization of our existing products and any other product candidates for which we obtain approval or that we acquire; |
| ● | obtaining FDA, and potentially Health Canada and EU, approval for YOSPRALA; |
| ● | securing Canadian approval and potentially additional foreign regulatory approvals for Treximet; and |
| ● | developing, acquiring or in-licensing and commercializing a portfolio of other product candidates in addition to our current products. |
Even if we do generate additional product sales, we may not be able to sustain profitability on a quarterly or annual basis. Our failure to become and remain profitable would depress the market price of our common shares and could impair our ability to raise capital, expand our business, diversify our product offerings or continue our operations.
We may need additional funding and may not have access to capital. If we are unable to raise capital when needed, we may need to delay, reduce or eliminate our product development or commercialization efforts.
In the future, we may need to raise additional funds to execute our evolving business strategy. We have incurred losses from operations since inception and we may continue to incur additional operating losses. Our actual capital requirements will depend upon numerous factors, including:
| ● | completing the regulatory approval process, and any further required clinical development related thereto, for YOSPRALA and other product candidates; |
| ● | our ability to commercialize or arrange for the commercialization of our products; |
| ● | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; |
| ● | the effect of competing technological and market developments; |
| ● | the timing of our receipt, if any, of milestone payments and royalties under collaborative agreements; |
| ● | the effect of changes and developments in, or termination of, our collaborative, license and other relationships; |
| ● | the terms and timing of any additional collaborative, license and other arrangements that we may establish; and |
| ● | the ability to acquire or in-license additional complementary products or products that augment our current product portfolio. |
As of December 31, 2015, we had an aggregate of $25 million in cash and cash equivalents. In connection with the closing of the Tribute Transaction, we received $75 million equity investment and $75 million convertible debt. In addition, pursuant to a Second Amended and Restated Debt Facility Agreement (the “Facility Agreement”), dated December 7, 2015, among us, Pozen, Tribute and certain lenders party thereto, we can borrow up to an additional aggregate principal amount of $200 million for acquisitions. While we believe that we will have sufficient cash reserves and cash flow to fund our operations to the point of generating continuous positive cash flow based on our current expectations of continued revenue growth, we may need to raise additional capital if we choose to expand our commercialization or development efforts more rapidly than we presently anticipate, if we develop, acquire or in-license additional products or acquire companies or if our revenues do not meet expectations. In addition, our expenses might increase beyond currently expected levels if we decide to, or any regulatory agency requires us to, conduct additional clinical trials, studies or investigations for any of our product candidates, including in connection with the FDA’s (or its foreign equivalent) consideration, or reconsideration, of our regulatory filings for our product candidates. We are planning to commercialize our PA product candidates in the United States without a commercial partner and our expenses will increase relative to prior years as we continue the transition from a development company that licenses its product candidates to other companies into a fully integrated, specialty pharmaceutical company.
We may be unable to raise additional equity funds when we desire to do so due to unfavorable market conditions in our industry, or generally, or due to other unforeseen developments in our business. Further, we cannot be certain that additional funding will be available on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our products or product candidates, or delay, cut back or abandon our plans to grow the business through acquisition or in-licensing. We also could be required to:
| ● | seek collaborators for one or more of our current or future product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available; or |
| ● | relinquish or license on unfavorable terms our rights to technologies or product candidates that we otherwise would seek to develop or commercialize ourselves. |
Any of the above events could significantly harm our business, financial condition and prospects and cause the price of our common shares to decline.
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish intellectual property rights to our product candidates.
Additional capital may be needed in the future to continue our planned operations. We may seek additional capital through a combination of private and public equity offerings, debt financings, receivables or royalty financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our existing shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect the rights of our shareholders. Debt, receivables and royalty financings may be coupled with an equity component, such as warrants to purchase shares, which could also result in dilution of our existing shareholders’ ownership. The incurrence of additional indebtedness would result in increased fixed payment obligations and could result in certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business.
Covenants imposed by the Facility Agreement restrict our business and operations in many ways and if we do not effectively manage our covenants, our financial conditions and results of operations could be adversely affected.
The Facility Agreement imposes various covenants that limit our ability and/or our subsidiaries’ ability to, among other things:
| ● | consolidate or merge with or into another person; |
| ● | enter into certain transactions with affiliates; |
| ● | pay dividends or distributions; |
| ● | create, incur or suffer liens; |
| ● | create, incur, assume guarantee or be liable with respect to indebtedness; |
| ● | acquire assets or transfer products or material assets; and |
| ● | issue equity securities senior to our common shares or convertible or exercisable for equity securities senior to our common shares. |
The covenants imposed by the Facility Agreement and our obligations to service our outstanding debt:
| ● | limit our ability to borrow additional funds for working capital, capital expenditures, acquisitions or other general business purposes; |
| ● | limit our ability to use our cash flow or obtain additional financing for future working capital, capital expenditures, acquisitions or other general business purposes; |
| ● | may require us to use a substantial portion of our cash flow from operations to make debt service payments; |
| ● | limit our flexibility to plan for, or react to, changes in our business and industry; |
| ● | place us at a competitive disadvantage compared to our less leveraged competitors; and |
| ● | increase our vulnerability to the impact of adverse economic and industry conditions. |
If we are unable to successfully manage the limitations and decreased flexibility on our business due to our debt obligations, we may not be able to capitalize on strategic opportunities or grow our business to the extent we would be able to without these limitations. Our failure to comply with any of the covenants could result in a default under the Facility Agreement, which could permit the required lenders to declare all or part of any outstanding loans to be immediately due and payable.
Risks Related to Our Intellectual Property and Product Liability
We may become involved in infringement actions which are uncertain, costly and time-consuming and could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
The pharmaceutical industry historically has generated substantial litigation concerning the manufacture, use and sale of products, and we expect this litigation activity to continue. The intellectual property rights of pharmaceutical companies, including us, are generally uncertain and involve complex legal, scientific and factual questions. In order to protect or enforce patent rights, we may initiate litigation against third parties. If we are not successful in defending an attack on our patents and maintaining exclusive rights to market one or more of our products still under patent protection, we could lose a significant portion of sales in a very short period. We may also become subject to infringement claims by third parties and may have to defend against charges that we violated patents or the proprietary rights of third parties. If we infringe the intellectual property rights of others, we could lose our right to develop or sell products, including our generic products, or could be required to pay monetary damages or royalties to license proprietary rights from third parties. The outcomes of infringement actions are uncertain and infringement actions are costly and divert technical and management personnel from their normal responsibilities.
Third parties seeking to market generic versions of branded pharmaceutical products in the United States often file Abbreviated New Drug Applications (“ANDA”) with the FDA (with a similar process in Canada and other foreign countries) containing a certification stating that the ANDA applicant believes that the patents protecting the branded pharmaceutical product are invalid, unenforceable and/or not infringed. Such certifications are commonly referred to as Paragraph IV certifications. We and Horizon are engaged in Paragraph IV litigations with several generic pharmaceutical companies with respect to our VIMOVO patents. We and AstraZeneca are also engaged in a proceeding in Canada with Mylan Pharmaceuticals ULC (“Mylan Canada”), which is seeking approval of its generic version of VIMOVO in Canada prior to the expiration of our Canadian patent. If we are unsuccessful in any of these proceedings, or once our or our licensors applicable patents expire, and the FDA or Health Canada approve a generic version of one of our marketed products, such an outcome would have a material adverse effect on sales of such product, our business and our results of operations.
If we are unable to obtain or protect intellectual property rights related to our products and product candidates, we may not be able to compete effectively in our market.
The pharmaceutical industry places considerable importance on obtaining patent and trade secret protection for new technologies, products and processes. Our success will depend, in part, on our ability, and the ability of our licensors, to obtain and to keep protection for our products and technologies under the patent laws of the United States and other countries, so that we can stop others from using our inventions. Our success also will depend on our ability to prevent others from using our trade secrets. In addition, we must operate in a way that does not infringe, or violate, the patent, trade secret and other intellectual property rights of other parties.
We cannot know how much protection, if any, our patents will provide or whether our patent applications will issue as patents. The breadth of claims that will be allowed in patent applications cannot be predicted and neither the validity nor enforceability of claims in issued patents can be assured. If, for any reason, we are unable to obtain and enforce valid claims covering our products and technology, we may be unable to prevent competitors from using the same or similar technology or to prevent competitors from marketing identical products. For example, if we are unsuccessful in protecting our patents in the litigation against several generic pharmaceutical companies who have file ANDAs for VIMOVO, such companies could market a generic version of the product prior to the expiration of our patents.
Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property or prevent others from designing around our claims. If the patent applications we hold fail to issue or if their breadth or strength of protection is threatened, it could dissuade companies from collaborating with us to develop them and threaten our ability to commercialize our products. We cannot offer any assurances about which, if any, patents will issue or whether any issued patents will be found invalid or unenforceable or will go unthreatened by third parties. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we were the first to file any patent application related to our products or any other product candidates. Furthermore, if third parties have filed such patent applications, an interference proceeding in the United States may be able to be provoked by a third-party or instituted by us to determine who was the first to invent any of the subject matter covered by the patent claims of our applications or, as in many jurisdictions, such as in Canada, the earlier filed third-party application may be cited against our patent application by a patent office in rejecting our application on the basis that the invention lacks novelty.
Our ability to obtain patents is highly uncertain because, to date, some legal principles remain unresolved, there has not been a consistent policy regarding the breadth or interpretation of claims allowed in patents in the United States and other jurisdictions, and the specific content of patents and patent applications that are necessary to support and interpret patent claims is highly uncertain due to the complex nature of the relevant legal, scientific and factual issues. Changes in either patent laws or interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property or narrow the scope of our patent protection. For example, in 2011, the Leahy-Smith America Invents Act (the “Leahy-Smith Act”) was enacted, and it included a number of significant changes to patent law in the United States. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. The United States Patent and Trademark Office has developed new and untested regulations and procedures to govern the full implementation of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective in March 2013. The Leahy-Smith Act has also introduced procedures making it easier for third parties to challenge issued patents, as well as to intervene in the prosecution of patent applications. For example, third parties have filed petitions seeking Inter Partes Review (“IPR”) of some of our VIMOVO patents and one of our Treximet patents. A number of these petitions have been denied while others are still pending or have resulted in reviews that are ongoing. Finally, the Leahy-Smith Act contains statutory provisions that require the United States Patent and Trademark Office to issue new regulations for their implementation and it may take the courts years to interpret the provisions of the new statute. Accordingly, it is too early to tell what, if any, impact the Leahy-Smith Act will have on the operation of our business and the protection and enforcement of our intellectual property. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. In addition, the Patient Protection and Affordable Care Act allows applicants seeking approval of biosimilar or interchangeable versions of biological products to initiate a process for challenging some or all of the patents covering the innovator biological product used as the reference product. This process is complicated and could result in the limitation or loss of certain patent rights. An inability to obtain, enforce and defend patents covering our proprietary technologies would materially and adversely affect our business prospects and financial condition.
Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States and Canada. As a result, we may encounter significant problems in protecting and defending our intellectual property in the United States, Canada and other countries. For example, if the issuance to us, in a given country, of a patent covering an invention is not followed by the issuance, in other countries, of patents covering the same invention, or if any judicial interpretation of the validity, enforceability, or scope of the claims in, or the written description or enablement in, a patent issued in one country is not similar to the interpretation given to the corresponding patent issued in another country, our ability to protect our intellectual property in those countries may be limited. For example, since patent protection is territorial, the teachings of a U.S. patent will generally only be protected in the United States. If we do not have a corresponding patent in another jurisdiction, the teachings of the U.S. patent may be in the public domain in such jurisdiction and free for a third-party to practice. Changes in either patent laws or in interpretations of patent laws in the United States, Canada and other countries may materially diminish the value of our intellectual property or narrow the scope of our patent protection. If we are unable to prevent material disclosure of the non-patented intellectual property related to our technologies to third parties, and there is no guarantee that we will have any such enforceable trade secret protection, we may not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, results of operations and financial condition.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our drug discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets are difficult to protect, and we cannot be certain that others will not develop the same or similar technologies on their own. We have taken steps, including entering into confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors, to protect our trade secrets and unpatented know-how. These agreements generally require that the other party keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by the Company during the course of the party’s relationship with the Company. We also typically obtain agreements from these parties, which provide that inventions conceived by the party in the course of rendering services to the Company will be our exclusive property. However, these agreements may not be honored and may not effectively assign intellectual property rights to the Company. Enforcing a claim that a party illegally obtained and is using our trade secrets or know-how is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, courts outside Canada and the United States may be less willing to protect trade secrets or know-how. The failure to obtain or maintain trade secret protection could adversely affect our competitive position.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our products.
We face an inherent business risk of exposure to significant product liability and other claims in the event that the use of our products caused, or is alleged to have caused, adverse effects. For example, we may be sued if any of our products or product candidates allegedly causes injury or is found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. The withdrawal of a product following complaints and/or incurring significant costs, including the requirement to pay substantial damages in personal injury cases or product liability cases, could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our common shares to decline.
Our product liability insurance coverage may not be sufficient to cover our claims and we may not be able to obtain sufficient coverage at a reasonable cost in the future. We will explore, on an on-going basis, expanding our insurance coverage related to the sale of our future marketed products when we obtain marketing approval for such products and commercial sales of such products begin. However, we may not be able to obtain commercially reasonable product liability insurance for any products approved for marketing. If a plaintiff brings a successful product liability claim against us in excess of our insurance coverage, if any, we may incur substantial liabilities and our business may be harmed or fail.
If our products or technologies are stolen, misappropriated or reverse engineered, others could use our products or licensed products to produce competing products or technologies.
Third parties, including our partners, contract manufacturers, contractors and others involved in our business often have access to our products, licensed products, and technologies. If our products, licensed products or technologies were stolen, misappropriated or reverse engineered, they could be used by other parties that may be able to reproduce our products, licensed products, or technologies for their own commercial gain. If this were to occur, it would be difficult for us to challenge this type of use, especially in countries with limited intellectual property protection.
Risks Related to Ownership of Our Common Shares
The price of our common shares could be volatile, which may result in significant losses to our shareholders.
The trading price of our common shares could be highly volatile and subject to wide fluctuations in response to various factors, some of which are beyond our control, including limited trading volume. In addition to the factors discussed in the “Risk Factors” of this Annual Report on Form 10-K, these factors include:
| ● | fluctuations in our operating results and revenues generated by our marketed products; |
| ● | announcements of technological innovations, acquisitions or licensing of therapeutic products or product candidates by us or our competitors; |
| ● | prolonged stock shortages from third-party manufacturers; |
| ● | published reports by securities analysts; |
| ● | positive or negative progress with our clinical trials or with regulatory approvals of our product candidates; |
| ● | commercial success of VIMOVO and our other product candidates in the marketplace once approved; |
| ● | our ability to obtain approval for YOSPRALA; |
| ● | our ability to successfully launch YOSPRALA, if and when approved; |
| ● | generic introductions of existing marketed products with no generic competition; |
| ● | governmental regulation, including reimbursement policies; |
| ● | developments in patent or other proprietary rights; |
| ● | developments in our relationships with collaborative partners or our inability to obtain consents or achieve minimum licensing terms; |
| ● | announcements by our collaborative partners regarding our products or product candidates; |
| ● | developments in new or pending litigation; |
| ● | public concern as to the safety and efficacy of our products; |
| ● | our ability to acquire or license new products or companies and the perception of the value of such transactions, and our ability to integrate and grow such products or companies; |
| ● | the sale or attempted sale of a large amount of our common shares into the market; and |
| ● | general market conditions. |
The common shares are listed on the NASDAQ Global Market and the Toronto Stock Exchange. Volatility in the market prices of our common shares may increase as a result of our common shares being listed on both the NASDAQ Global Market and the Toronto Stock Exchange because trading is split between the two markets, resulting in less liquidity on both exchanges. In addition, different liquidity levels, volume of trading, currencies and market conditions on the two exchanges may result in different prevailing trading prices.
In addition, the stock market in general, and the NASDAQ Global Market, the Toronto Stock Exchange and the stocks of biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may adversely affect the market price of our common shares, regardless of our actual operating performance.
Sales of substantial amounts of shares of our common shares in the public market could cause our share price to decline.
If our existing shareholders sell, or indicate an intention to sell, substantial amounts of our common shares in the public market, the trading price of our common shares could decline. Following the Tribute Transaction, Deerfield Private Design Fund III, L.P. (“Deerfield Private Design”) and its affiliates will beneficially own approximately 9.985% of the Company. Pursuant to the Facility Agreement, except in certain limited circumstances, Deerfield Private Design and its affiliates may not acquire a number of our common shares that would exceed 9.985% of the total number of our common shares then issued (excluding treasury shares). Any sales of substantial amounts of our common shares in the public market, including sales or distributions of shares by Deerfield Private Design, or the perception that such sales or distributions might occur, could harm the market price of our common shares and could impair our ability to raise capital through the sale of additional equity securities. Further, shareholder ownership will be diluted if we raise additional capital by issuing equity securities. In addition, our common shares that are either subject to outstanding options or reserved for future issuance under our employee benefit plans are or may become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules and Rule 144 and Rule 701 under the Securities Act. If these additional common shares are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common shares could decline.
Anti-takeover provisions in our Articles and certain provisions under the BCBCA could prevent or delay transactions that our shareholders may favor and may prevent shareholders from changing the direction of our business or management.
Provisions of our Articles and certain provisions under the BCBCA may discourage, delay or prevent a merger or acquisition that our shareholders may consider favorable, including transactions in which shareholders might otherwise receive a premium for their shares, and may also frustrate or prevent any attempt by shareholders to change the direction or management of the Company. For example, these provisions:
| ● | authorize the issuance of “blank check” preferred shares without any need for action by shareholders; |
| ● | require a 75% majority of shareholder votes cast in favor of a resolution to remove a director; |
| ● | require a 662/3% majority of shareholder votes cast in favor of a resolution to effect various amendments to our Articles; |
| ● | require that (i) in the case of shareholder action by written consent, a matter that would normally require an ordinary resolution shall require written consent by shareholders representing at least 662/3% of the votes entitled to be cast in favor of such resolution, and (ii) in the case of any other resolution of the shareholders, the written consent of shareholders representing 100% of the votes entitled to be cast in favor of such resolution; |
| ● | establish advance notice requirements for nominations for election to the board of directors; and |
| ● | require shareholder proposals for matters to be acted upon by shareholders at shareholder meetings to be submitted pursuant to, and in accordance with, the applicable provisions of the BCBCA for inclusion in the Company’s proxy materials by a date that is not later than three months prior to the anniversary date of the prior year’s shareholder meeting. |
These provisions, among others, whether alone or together, could delay or impede hostile takeovers and changes in control or changes to the composition of our board of directors or management. Any provision of our constating documents that has the effect of delaying or deterring a change in control could limit the opportunity for our shareholders to receive a premium for their common shares and could also affect the price that some investors are willing to pay for our common shares.
Provisions of Canadian law may delay, prevent or make undesirable an acquisition of all or a significant portion of our common shares or assets.
The Investment Canada Act subjects an acquisition of control of us by a non-Canadian to government review if our enterprise value as calculated pursuant to the legislation exceeds a threshold amount. A reviewable acquisition may not proceed unless the relevant Minister is satisfied that the investment is likely to be of net benefit to Canada. This could prevent or delay a change of control and may eliminate or limit strategic opportunities for shareholders to sell their common shares.
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation and these differences may make our common shares less attractive to investors.
The Company is incorporated under the laws of the Province of British Columbia, Canada, and therefore certain of the rights of holders of its shares are governed by Canadian law, including the provisions of the BCBCA, and by our Notice of Articles and Articles. These rights differ in certain respects from the rights of shareholders in typical U.S. corporations and these differences may make our common shares less attractive to investors.
An investor may be unable to bring actions or enforce judgments against us and certain of our directors.
The Company is incorporated under the laws of the Province of British Columbia. Some of our directors and officers reside principally outside of the United States and a substantial portion of our assets and a substantial portion of the assets of these persons are located outside the United States. Consequently, it may not be possible for an investor to effect service of process within the United States on us or those persons. Furthermore, it may not be possible for an investor to enforce judgments obtained in United States courts based upon the civil liability provisions of United States federal securities laws or other laws of the United States against us or those persons.
We do not expect to pay dividends for the foreseeable future, and our shareholders must rely on increases in the trading price of our common shares for returns on their investment.
Except for the $1.75 per share special cash distribution by Pozen on December 30, 2013 (representing a surplus of corporate cash and accounted for as a return of capital to stockholders), we have never paid cash dividends on our common shares and do not expect to pay dividends in the immediate future. We anticipate that the Company will retain all earnings, if any, to support our operations. Any future determination to pay dividends on our common shares will be at the sole discretion of the board of directors and will depend on, among other things, the Company’s results of operations, current and anticipated cash requirements and surplus, financial condition, contractual restrictions and financing agreement covenants, solvency tests imposed by corporate law and other factors that the board of directors may deem relevant. Holders of our common shares must rely on increases in the trading price of our shares for returns on their investment in the foreseeable future. In addition, the Facility Agreement prohibits the Company from making any cash dividend or distributing any of its assets, including its intangibles, to any of its shareholders in such capacity or its affiliates, subject to certain exceptions. The Facility Agreement also includes restrictions on the Company from incurring liens and undertaking indebtedness, subject to certain exceptions, which limitations may further impact the ability of the Company to pay any future dividends. See “– Covenants imposed by the Facility Agreement restrict our business and operations in many ways and if we do not effectively manage our covenants, our financial conditions and results of operations could be adversely affected” above.
We have incurred and will continue to incur significant increased costs as a result of operating as a public company and our management will be required to devote substantial time to compliance initiatives.
As a public company, we have incurred and will continue to incur significant legal, accounting and other expenses that we would not incur if we were a private company. In particular, the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), as well as rules subsequently implemented by the SEC, applicable securities laws in Canada, the NASDAQ Global Market and the Toronto Stock Exchange and the NASDAQ Global Market, impose significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. These rules and regulations have substantially increased our legal and financial compliance costs and have made some activities more time-consuming and costly. Further, these rules and regulations may lack specificity and are subject to varying interpretations. Their application in practice may evolve over time, as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs of compliance as a result of ongoing revisions to such corporate governance standards.
In particular, our efforts to comply with Section 404 of the Sarbanes-Oxley Act, National Instrument 52-109 – Certification of Disclosures in Issuers’ Annual and Interim Filings and the related regulations regarding our required assessment of our internal controls over financial reporting and our external auditors’ audit of that assessment requires the commitment of significant financial and managerial resources. We consistently assess the adequacy of our internal controls over financial reporting, remediate any control deficiencies that may be identified, and validate through testing that our controls are functioning as documented. While we do not anticipate any material weaknesses, the inability of management to assess our internal controls over financial reporting as effective could result in adverse consequences to us, including, but not limited to, a loss of investor confidence in the reliability of our financial statements, which could cause the market price of our stock to decline. The existence of this or one or more other material weaknesses or significant deficiencies in our internal control over financial reporting could result in errors in our financial statements, and substantial costs and resources may be required to rectify any internal control deficiencies. Although we continually review and evaluate internal control systems to allow management to report on the sufficiency of our internal controls, we cannot assure you that we will not discover weaknesses in our internal control over financial reporting. Any such weakness or failure to remediate any existing material weakness could materially adversely affect our ability to comply with applicable financial reporting requirements and the requirements of our various agreements.
We are committed to maintaining high standards of corporate governance and public disclosure, and our efforts to comply with evolving laws, regulations and standards in this regard have resulted in, and are likely to continue to result in, increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities. In addition, the laws, regulations and standards regarding corporate governance may make it more difficult, or increasingly more expensive, for us to obtain director and officer liability insurance. Further, our board members and executive officers could face an increased risk of personal liability in connection with their performance of duties. As a result, we may face difficulties attracting and retaining qualified board members and executive officers, which could harm our business. If we fail to comply with new or changed laws, regulations or standards of corporate governance, our business and reputation may be harmed.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common shares will depend in part on the research and reports that securities or industry analysts publish about us or our business. There is no guarantee that securities analysts will cover our securities, and the lack of research coverage may adversely affect our share price. If one or more of the securities analysts publish inaccurate or unfavorable research about our business, our share price would likely decline. If one or more of these securities analysts cease coverage of the Company or fail to publish reports on us regularly, demand for our common shares could decrease, which might cause our share price and trading volume to decline.
None.
The properties described below are used by the Company for general corporate purposes and, with respect to the London property, secondary packaging.
In March 2016, Tribute entered into a sublease agreement for an approximately 9,183 square foot office space located at 7100 West Credit Avenue, Mississauga, Ontario. The lease term is five years and three months, terminating on July 30, 2021. This location will serve as the global headquarters for Aralez beginning sometime in the second quarter of 2016.
In October 2015, Aralez Pharmaceuticals US Inc. (“Aralez Pharmaceuticals US”), a Delaware corporation, entered into a lease for an approximately 4,500 square foot office space located in Radnor, Pennsylvania. The lease term is five years and two months, terminating on December 31, 2020, with a five-year extension term available at Aralez Pharmaceuticals US’s option.
In September 2015, Aralez Pharmaceuticals US entered into a lease for an approximately 4,000 square foot office space located in New York, New York. The lease term is five years and two months, terminating on October 31, 2020. This office currently serves as our U.S. headquarters. We are currently seeking office space in the Princeton, New Jersey area to serve as our U.S. headquarters beginning sometime in the third quarter of 2016.
Our current global headquarters is located at 151 Steeles Avenue East, Milton, Ontario and the lease expires on August 31, 2017.
In 2004, Tribute purchased the property and building located at 544 Egerton Street in London, Ontario, Canada. Tribute’s London office’s primary function involves administrative offices and is used for secondary packaging of certain products.
VIMOVO® ANDA Litigation
Between March 14, 2011 and May 16, 2013, we received Paragraph IV Notice Letters from Dr. Reddy’s Laboratories (“DRL”), Lupin Ltd. (“Lupin”), Watson Laboratories, Inc. – Florida (“Watson”), and Mylan Pharmaceuticals Inc. (“Mylan”), stating that each had filed an ANDA with the FDA seeking regulatory approval to market a generic version of our VIMOVO product before the expiration of U.S. Patent No. 6,926,907 (the “‘907 patent”). On November 20, 2012, we received a second Notice Letter from DRL stating that DRL had filed a second ANDA with the FDA seeking regulatory approval to market a different generic formulation of the VIMOVO product before the expiration of the ‘907 patent. The ‘907 patent is assigned to POZEN and listed for the VIMOVO product in the FDA’s publication titled “Approved Drug Products with Therapeutic Equivalence Evaluations” (also known as the “Orange Book”).
On April 21, 2011, we filed suit against the first ANDA filer, DRL, in the United States District Court for the District of New Jersey (the “District Court”), asserting infringement of the ‘907 patent. We subsequently filed suit against the other three ANDA filers within 45 days of receipt of their respective Paragraph IV Notice Letters. Horizon, our current marketing partner for the VIMOVO product, is our co-plaintiff in each suit. The first suit against DRL is considered the lead case and has been consolidated with other suits for the purpose of pre-trial and discovery. On December 19, 2012, the District Court conducted a pre-trial Markman hearing to determine the proper claim construction of certain claims disputed by the parties. On May 1, 2013, the District Court issued a Markman Order construing the disputed claims. A scheduling order for the consolidated suits was issued by the District Court on June 27, 2014. Fact discovery closed in the consolidated suits on November 20, 2014, expert discovery closed on June 25, 2015, and we are currently waiting for the District Court to set a trial date (which has been delayed in part due to the retirement of the presiding judge in the case, the Honorable Joel A. Pisano).
On October 15, 2013, the United States Patent & Trademark Office (“USPTO”) issued to the Company U.S. Patent No. 8,557,285 (the “‘285 patent”). The ‘285 patent is listed in the Orange Book for the VIMOVO product and is related to the ‘907 patent. On October 23, 2013, we filed suits against DRL, Lupin, Watson and Mylan in the District Court asserting infringement of the ‘285 patent. These suits have each been consolidated with the above referenced suits involving the ‘907 patent.
On October 7, 2014, the USPTO issued to the Company U.S. Patent No. 8,852,636 (the “‘636 patent”). On October 14, 2014, the USPTO issued to the Company U.S. Patent No. 8,858,996 (the “‘996 patent”). In addition, on October 21, 2014, the USPTO issued to the Company U.S. Patent No. 8,865,190 (the “‘190 patent”). The ‘636, ‘996 and ‘190 patents are each listed in the Orange Book for the VIMOVO product and are each related to the ‘907 and ‘285 patents.
On February 3, 2015, the USPTO issued to the Company U.S. Patent No. 8,945,621 (the “‘621 patent”). The ‘621 patent is listed in the Orange Book for the VIMOVO product.
On May 13, 2015, the Company and Horizon filed suit against DRL, Lupin, Actavis (formerly known as Watson) and Mylan in the District Court asserting infringement of the ‘636 and ‘996 patents. On June 18, 2015, we filed Amended Complaints in each of the suits to assert infringement of the ‘190 patent. In its responsive pleading, Actavis filed a counterclaim alleging that its generic product does not infringe the ‘621 patent and that the ‘621 patent is invalid.
On October 20, 2015, the USPTO issued to the Company U.S. Patent No. 9,161,920 (the “‘920 patent”). On December 1, 2015, the USPTO issued to the Company U.S. Patent No. 9,198,888 (the “‘888 patent”). The ‘920 and ‘888 patents are each listed in the Orange Book for the VIMOVO product and are each related to the ‘907 and ‘285 patents.
On December 29, 2015, the USPTO issued to the Company U.S. Patent No. 9,220,698 (the “‘698 patent”). The ‘698 patent is listed in the Orange Book for the VIMOVO product.
On January 25, 2016, the Company and Horizon filed suit against Actavis in the District Court asserting infringement of the ‘920 and ‘888 patents. Actavis has not yet filed a responsive pleading. On February 10, 2015, we filed Amended Complaints against DRL, Lupin and Mylan to assert infringement of the ‘920 and ‘888 patents. In its responsive pleading, Mylan filed a counterclaim alleging that its generic product does not infringe the ‘698 patent and that the ‘698 patent is invalid. These suits are in the initial phase and a full schedule has not yet been set by the District Court.
Inter Partes Review
DRL filed a Petition for (“IPR Petition”) of the ‘285 patent with the Patent Trial and Appeal Board (“PTAB”) of the USPTO on February 24, 2015, which was denied on October 9, 2015. The Coalition for Affordable Drugs VII L.L.C. (“CFAD”) filed IPR Petitions of the ‘907 patent, the ‘996 patent and the ‘636 patent with the PTAB on May 21, 2015, June 5, 2014 and August 7, 2015, respectively, each of which was denied on December 8, 2015, December 17, 2015 and February 11, 2016, respectively.
On August 12, 2015, CFAD filed an IPR Petition of the ‘621 patent with the PTAB. On February 22, 2016 the PTAB instituted review of the claims of the ‘621 patent. The Company and Horizon have until May 23, 2016 to file a response to the petition.
On August 19, 2015, Lupin filed three separate IPR Petitions of the ‘996, ‘636 and ‘190 patents with the PTAB. On March 1, 2016 the PTAB denied Lupin’s petition for review of the ‘636 patent and instituted review of a limited number of the claims in each of the ‘996 and ‘190 patents. The Company and Horizon have until May 27, 2016 to file responses to the petitions for review of the ‘996 and ‘190 patents.
On November 12, 2015, Gray Square Pharmaceuticals, LLC (formerly known as Graybar Pharmaceuticals, LLC) filed an IPR Petition of U.S. Patent No. 7,332,183 (the “‘183 patent”) with the PTAB. The ‘183 patent is assigned to POZEN and listed with respect to Treximet in the Orange Book. The Company and our marketing partner Pernix Therapeutics Holdings, Inc. filed a Preliminary Response to Gray Square’s petition on February 16, 2016. Upon receipt of such a Preliminary Response, the PTAB has three months in which to institute or deny the IPR proceeding. If the PTAB decides to institute the IPR proceeding, Gray Square will have the opportunity to challenge the validity of the ‘183 patent in whole or in part before the PTAB via a patent validity trial.
Canada VIMOVO® Litigation
On January 20, 2015, our Canadian licensee, AstraZeneca Canada Inc. (“AstraZeneca Canada”) received a Notice of Allegation from Mylan Canada informing that Mylan Canada has filed an Abbreviated New Drug Submission in Canada for approval of its naproxen/esomeprazole magnesium tablets and alleging non-infringement of some of the claims and invalidity of Canadian Patent No. 2,449,098 (the “‘098 patent”). A Notice of Allegation is served pursuant to the Patented Medicines (Notice of Compliance) Regulations in Canada and is similar to a Paragraph IV Notice Letter in the United States, and in response, we and AstraZeneca Canada commenced a proceeding in the Federal Court of Canada (the “Canada Court”) in relation to the ‘098 patent on March 5, 2015 seeking to prohibit Health Canada from approving Mylan Canada’s generic naproxen/esomeprazole product. The Canadian proceeding is summary in nature and expected to be completed before March 5, 2017. In accordance with the schedule approved by the Canada Court, affidavit evidence of AstraZeneca Canada and the Company was served on September 11, 2015 and affidavit evidence of Mylan Canada on January 8, 2016. The parties are to complete cross-examinations on the affidavit evidence by April 29, 2016. The written records for the hearing are to be served by AstraZeneca Canada and us by July 4, 2016 and by Mylan Canada by September 2, 2016. A three-day hearing of the matter has been scheduled, commencing on November 21, 2016. The proceeding will decide whether approval for Mylan Canada’s naproxen/esomeprazole magnesium tablets will be prohibited until the expiry of the ‘098 patent because none of Mylan Canada’s allegations in respect of the ‘098 patent are justified, however the proceeding will not finally decide ‘098 patent validity or infringement. The ‘098 patent expires on May 31, 2022.
Not applicable.
PART II
ITEM 5. Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
As a result of the Tribute Transaction, all of the shares of Pozen common stock issued and outstanding immediately prior to the effective time of the Tribute Transaction were canceled and automatically converted into and became the right to receive our common shares on a one-for-one basis and Pozen became a wholly-owned subsidiary of Aralez.
Our common shares began trading on the NASDAQ Global Market under the trading symbol “ARLZ” on February 8, 2016 and on the Toronto Stock Exchange under the trading symbol “ARZ” on February 10, 2016. Previously, from October 11, 2000 until February 5, 2016, the common stock of Pozen was traded on the NASDAQ Global Market (formerly the NASDAQ National Market) under the trading symbol “POZN.” The following table sets forth the high and low sales prices per share of common stock of Pozen as reported on the NASDAQ Global Market for the periods indicated.
|
Price Range
|
||||||||
|
2015 Fiscal Year
|
High
|
Low
|
||||||
|
First Quarter
|
$
|
8.03
|
$
|
6.78
|
||||
|
Second Quarter
|
$
|
12.44
|
$
|
6.45
|
||||
|
Third Quarter
|
$
|
12.17
|
$
|
5.70
|
||||
|
Fourth Quarter
|
$
|
8.11
|
$
|
5.72
|
||||
|
Price Range
|
||||||||
|
2014 Fiscal Year
|
High
|
Low
|
||||||
|
First Quarter
|
$
|
8.99
|
$
|
7.37
|
||||
|
Second Quarter
|
$
|
9.73
|
$
|
7.56
|
||||
|
Third Quarter
|
$
|
9.59
|
$
|
5.96
|
||||
|
Fourth Quarter
|
$
|
9.71
|
$
|
7.07
|
||||
Holders of Record
The closing price of our common shares as reported on the NASDAQ Global Market and the Toronto Stock Exchange on March 8, 2016 was $5.94 and C$7.98, respectively. As of the close of business on March 8, 2016, there were approximately 108 holders of record of our common shares.
Dividends
Except for the $1.75 per share special cash distribution on December 30, 2013 (representing a surplus of corporate cash and accounted for as a return of capital to stockholders), Pozen has not declared or paid any cash dividends on shares of Pozen common stock. We have not declared or paid any cash dividends on common shares to date. We currently intend to retain all earnings to support operations and do not intend to pay cash dividends on our common shares for the foreseeable future. In addition, our ability to pay cash dividends is currently prohibited by the Facility Agreement, subject to certain exceptions. Any future determination to pay dividends on our common shares will be at the sole discretion of the board of directors and will depend on, among other things, the Company’s results of operations, current and anticipated cash requirements and surplus, financial condition, contractual restrictions and financing agreement covenants, solvency tests imposed by corporate law and other factors that the board of directors may deem relevant.
Restrictions on Share Ownership by Non-Canadians
There are no limitations under the laws of Canada or in our organizational documents on the right of foreigners to hold or vote our securities, except that the Investment Canada Act (Canada) (the “Investment Canada Act”) may require review and approval by the Minister of Innovation, Science and Economic Development (Canada) of certain acquisitions of “control” of the Company by a “non-Canadian.”
Investment Canada Act
An acquisition of control of a Canadian business by a non-Canadian is either reviewable (a “Reviewable Transaction”), in which case it is subject to both a reporting obligation and an approval process, or notifiable, in which case it is subject to only a reporting obligation. In the case of a Reviewable Transaction, the non-Canadian acquirer must submit an application for review with the prescribed information. The responsible Minister is then required to determine whether the Reviewable Transaction is likely to be of net benefit to Canada, taking into account the assessment factors specified in the Investment Canada Act and any written undertakings that may have been given by the non-Canadian acquirer.
Any investment by a non-Canadian in a Canadian business, even where control has not been acquired, can be reviewed on grounds of whether it may be injurious to national security. Where an investment is determined to be injurious to national security, Canada’s Cabinet can prohibit closing or, if closed, can order the investor to divest control. Short of a prohibition or divestment order, Canada’s Cabinet can impose terms or conditions on the investment or can require the investor to provide binding undertakings to remove the national security concern.
Competition Act
Part IX of the Competition Act (Canada) (the “Competition Act”) requires that pre-merger notification filings be submitted to the Commissioner of Competition (the “Commissioner”) in respect of certain types of transactions that exceed certain prescribed thresholds. If a proposed transaction exceeds such thresholds, subject to certain exceptions, notification filings must be submitted to the Commissioner and the statutory waiting period must expire or be terminated early or waived by the Commissioner before the transaction can be completed.
All mergers, regardless of whether they are subject to Part IX of the Competition Act, are subject to the substantive mergers provisions under Section 92 of the Competition Act. In particular, the Commissioner may challenge a transaction before the Competition Tribunal where the transaction prevents or lessens, or is likely to prevent or lessen, competition substantially in a market. The Commissioner may not make an application to the Competition Tribunal under Section 92 of the Competition Act more than one year after the transaction has been substantially completed.
Equity Compensation Plans
The following table provides information with respect to our compensation plans under which equity compensation is authorized as of December 31, 2015.
|
Plan Category
|
Number of securities to
be issued upon exercise
of outstanding options,
warrants and rights
|
Weighted-average
exercise price of
outstanding options,
warrants and rights
|
Number of securities
remaining available
for future issuance
under equity
compensation plans
|
|||||||||
|
Equity compensation plans approved by security holders(1)
|
1,909,891
|
$
|
8.29
|
2,040,688
|
||||||||
|
Equity compensation plans not approved by security holders
|
—
|
—
|
—
|
|||||||||
|
Total
|
1,909,891
|
$
|
8.29
|
2,040,688
|
||||||||
| (1) | Excludes 4,116,442 restricted stock units issued under our Equity Compensation Plans, as amended and restated. |
Stock Performance Graph
The graph below matches Pozen’s cumulative five-year total shareholder return on common stock with the cumulative total returns of the NASDAQ Composite Index, the NASDAQ Pharmaceutical Index, and the NASDAQ Biotechnology Index. The graph tracks the performance of a $100 investment in Pozen’s common stock and in each index (with the reinvestment of all dividends) from December 31, 2010 to December 31, 2015.
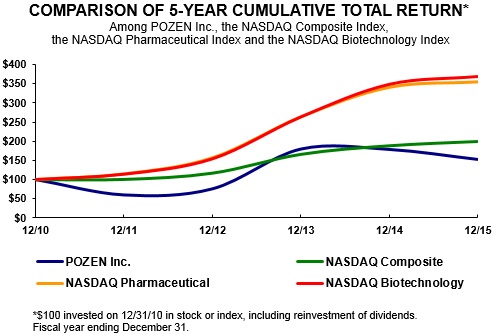
|
12/10
|
12/11
|
12/12
|
12/13
|
12/14
|
12/15
|
|||||||||||||||||||
|
POZEN Inc.
|
100.00
|
59.40
|
75.34
|
179.83
|
178.93
|
152.77
|
||||||||||||||||||
|
NASDAQ Composite
|
100.00
|
100.53
|
116.92
|
166.19
|
188.78
|
199.95
|
||||||||||||||||||
|
NASDAQ Pharmaceutical
|
100.00
|
114.48
|
156.39
|
263.04
|
340.07
|
354.40
|
||||||||||||||||||
|
NASDAQ Biotechnology
|
100.00
|
113.92
|
153.97
|
263.29
|
348.49
|
369.06
|
The stock price performance included in this graph is not necessarily indicative of future stock price performance.
Recent Sales of Unregistered Securities
There were no sales of unregistered securities during the year ended December 31, 2015.
Issuer Purchases of Equity Securities
There were no repurchases of equity securities during the fourth quarter of 2015.
The following selected financial data are derived from the audited financial statements of Pozen. The data should be read in conjunction with the financial statements, related notes and other financial information included (and incorporated by reference) herein. There is no selected financial data being presented for the Company, as it did not conduct any business as of December 31, 2015, other than business incident to the Tribute Transaction.
|
For the Years Ended December 31,
|
||||||||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
2011
|
||||||||||||||||
|
(in thousands, except per share data)
|
||||||||||||||||||||
|
Statement of Operations Data:
|
||||||||||||||||||||
|
Revenue:
|
||||||||||||||||||||
|
Royalty and licensing revenue
|
$
|
21,391
|
$
|
32,394
|
$
|
10,322
|
$
|
5,349
|
$
|
15,081
|
||||||||||
|
Sale of royalty rights, net of costs
|
—
|
—
|
—
|
—
|
71,870
|
|||||||||||||||
|
Total revenue
|
21,391
|
32,394
|
10,322
|
5,349
|
86,951
|
|||||||||||||||
|
Operating expenses:
|
||||||||||||||||||||
|
Selling, general and administrative
|
50,345
|
10,079
|
17,161
|
19,024
|
21,752
|
|||||||||||||||
|
Research and development
|
8,512
|
5,740
|
9,945
|
11,867
|
23,020
|
|||||||||||||||
|
Total operating expenses
|
58,857
|
15,819
|
27,106
|
30,891
|
44,772
|
|||||||||||||||
|
Interest and other (loss) income
|
(143
|
)
|
3,099
|
76
|
259
|
161
|
||||||||||||||
|
(Loss) income before income tax expense
|
(37,609
|
)
|
19,674
|
(16,708
|
)
|
(25,283
|
)
|
42,340
|
||||||||||||
|
Income tax expense
|
174
|
—
|
—
|
—
|
—
|
|||||||||||||||
|
Net (loss) income
|
$
|
(37,783
|
)
|
$
|
19,674
|
$
|
(16,708
|
)
|
$
|
(25,283
|
)
|
$
|
42,340
|
|||||||
|
Basic net (loss) income per common share
|
$
|
(1.16
|
)
|
$
|
0.63
|
$
|
(0.55
|
)
|
$
|
(0.84
|
)
|
$
|
1.41
|
|||||||
|
Shares used in computing basic net (loss) income per common share
|
32,590
|
31,360
|
30,450
|
30,092
|
29,925
|
|||||||||||||||
|
Diluted net (loss) income per common share
|
$
|
(1.16
|
)
|
$
|
0.60
|
$
|
(0.55
|
)
|
$
|
(0.84
|
)
|
$
|
1.40
|
|||||||
|
Shares used in computing diluted net (loss) income per common share
|
32,590
|
32,811
|
30,450
|
30,092
|
30,296
|
|||||||||||||||
|
December 31,
|
||||||||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
2011
|
||||||||||||||||
|
(in thousands)
|
||||||||||||||||||||
|
Balance Sheet Data:
|
||||||||||||||||||||
|
Cash and cash equivalents
|
$
|
24,816
|
$
|
40,582
|
$
|
32,828
|
$
|
87,314
|
$
|
119,620
|
||||||||||
|
Total assets
|
32,258
|
50,454
|
35,334
|
89,597
|
121,553
|
|||||||||||||||
|
Total liabilities
|
17,474
|
3,713
|
17,546
|
5,519
|
16,055
|
|||||||||||||||
|
Accumulated deficit
|
(134,688
|
)
|
(96,904
|
)
|
(116,579
|
)
|
(99,871
|
)
|
(74,588
|
)
|
||||||||||
|
Total stockholders’ equity
|
14,783
|
46,741
|
17,789
|
84,077
|
105,498
|
|||||||||||||||
Overview
Our Business
Aralez is a global specialty pharmaceutical company focused on delivering meaningful products to improve patients’ lives while focusing on creating shareholder value by acquiring, developing and commercializing products primarily in cardiovascular, pain and other specialty areas. Aralez’s global headquarters is located in Ontario, Canada, its U.S. headquarters is in New York, New York and its Irish headquarters is located in Dublin, Ireland. Aralez was formed for the purpose of facilitating the business combination of Pozen and Tribute, which business combination was consummated on February 5, 2016. Aralez has had no operations as of December 31, 2015, other than business incident to the Tribute Transaction.
Our management team has a strong track record of success in creating, leading and expanding specialty pharmaceutical companies with marketing and sales capabilities. Directed by this leadership and leveraging our competitive platform, our focus on acquiring high potential growth opportunities through aggressive business development and licensing and strategic M&A and commercializing healthcare products to provide enhanced value to a range of stakeholders is driven by the following primary strategies:
| ● | Maximize value of expanded portfolio – We plan to continue our progress toward building out our U.S. commercial organization, including growing our sales force and promoting the use of Fibricor in the United States to grow product use moderately in the United States and which we expect will develop a relationship springboard with cardiologists ahead of the anticipated approval and commercial launch of YOSPRALA. |
| ● | Business development through selective acquisitions – We have completed numerous transactions over the past few years to expand our portfolio offering. We will continue to pursue value-driven business development opportunities as they arise and enhance our product pipeline and expand our geographic footprint through strategically acquiring low-risk, revenue generating product candidates or approved products, particularly in the cardiovascular and pain anchor areas, but also in other specialty therapeutic areas that we anticipate are or will become revenue generating and accretive. |
| ● | Leverage platform for growth – We intend to maintain a lean, nimble and performance-oriented operating model with strong financial discipline. Our well-capitalized financial profile provides us with ample liquidity to commercialize YOSPRALA, if and when approved, and creates the opportunity for sustained long-term growth, both organically and through acquisitions, while also enabling us to have an ongoing focus on growing shareholder value. |
On February 5, 2016, pursuant to the Merger Agreement, Aralez completed the acquisition of Tribute by way of a court approved plan of arrangement in a stock transaction with an estimated purchase price of $138 million made up of (i) $115 million related to Tribute shares, equity awards and certain warrants outstanding and (ii) $23 million in repayments of Tribute indebtedness. In connection with the Tribute Transaction, Pozen and Tribute were combined under and became subsidiaries of Aralez Pharmaceuticals Inc., with Pozen treated as the acquiring company for accounting purposes. Pursuant to Rule 12g-3(a) under the Exchange Act, Aralez Pharmaceuticals Inc. is the successor issuer to Pozen. The Tribute Transaction provides the combined company with increased financial strength and product portfolio diversity and is expected to meaningfully accelerate our operating strategies.
We have incurred significant losses since our inception and have not yet generated significant revenue from product sales. As of December 31, 2015, Pozen’s accumulated deficit was approximately $134.7 million. We record revenue under the following categories: royalty revenues and licensing revenues. Our licensing revenues include upfront payments upon contract signing and additional payments if and when certain milestones in the product’s development or commercialization are reached, while the royalty payments are based on product sales. Our historical operating losses have resulted principally from our research and development activities, including clinical trial activities for our product candidates and selling, general and administrative expenses. Research and development expenses include salaries and benefits for personnel involved in our research and development activities and direct development costs, which include costs relating to the formulation and manufacturing of our product candidates, costs relating to preclinical studies, including toxicology studies, and clinical trials, and costs relating to compliance with regulatory requirements applicable to the development of our product candidates. Since inception, Pozen’s research and development expenses have represented approximately 59% of our total operating expenses. For the year ended December 31, 2015, Pozen’s research and development expenses represented approximately 14% of our total operating expenses.
Status and Expenses Related to Our Approved Products and Product Candidates
There follows a brief discussion of the status of the development of our approved products and our product candidates, as well as the costs relating to our development activities. Pozen’s direct research and development expenses were $5.4 million for the year ended December 31, 2015, $3.3 million for the year ended December 31, 2014, and $6.6 million for the year ended December 31, 2013. Our research and development expenses that are not direct development costs consist of personnel and other research and development departmental costs and are not allocated by product candidate. We generally do not maintain records that allocate our employees’ time by the projects on which they work and, therefore, are unable to identify costs related to the time that employees spend on research and development by product candidate. Total compensation and benefit costs for Pozen’s personnel involved in research and development were $2.8 million for the year ended December 31, 2015, $2.3 million for the year ended December 31, 2014, and $3.1 million for the year ended December 31, 2013. Total compensation included $0.2 million, $0.3 million, and a $0.8 million charge for non-cash stock-based compensation expense for the years ended December 31, 2015, December 31, 2014, and December 31, 2013, respectively. Other Pozen research and development department costs were $0.3 million, $0.1 million, and $0.2 million for the years ended December 31, 2015, December 31, 2014, and December 31, 2013, respectively.
Primary Commercialized Products
Products Marketed in the United States
|
Fibricor® and Authorized Generic
|
In May 2015, we acquired the U.S. rights to Fibricor (fenofibric acid) and its related authorized generic. Fibricor is indicated as a complementary therapy along with diet for the treatment of severe hypertriglyceridemia and as a complementary therapy along with diet to reduce elevated LDL-C, Total-C, TG, and Apo B, and to increase HDL-C in patients with primary hypercholesterolemia or mixed dyslipidemia. Fibricor is currently protected by four U.S. patents extending to August 20, 2027.
Fibricor is a lipid-regulating agent available as tablets for oral administration. Fibrates like Fibricor, activate PPAR alpha, increasing the activity of lipoprotein lipase. This typically causes a decrease in triglyceride levels. PPAR alpha activation also increases HDL production. Each tablet contains 35mg or 105mg fenofibric acid, and the 35mg tablet is the lowest dose of fenofibric acid available in the United States. Fibricor is contraindicated in patients with severe renal impairment, active liver disease, liver function abnormalities, preexisting gallbladder disease or known hypersensitivity to fenofibric acid or fenofibrate.
Hyperlipidemia Treatment Options: Hyperlipidemia is a very common chronic condition and is characterized by an excess of fatty substances called lipids, mainly cholesterol and triglycerides, in the blood. It is also called hyperlipoproteinemia because these fatty substances travel in the blood attached to proteins. This is the only way that these fatty substances can remain dissolved while in circulation. Hyperlipidemia, in general, can be divided into two subcategories: (1) hypercholesterolemia, in which there is a high level of cholesterol; and (2) hypertriglyceridemia, in which there is a high level of triglycerides, the most common form of fat. In general, lifestyle modifications such as diet, exercise and smoking cessation are the first line of treatment. If unsuccessful, pharmacologic therapy is commonly utilized for the treatment of primary and secondary dyslipidemias. In managing secondary dyslipidemia, statin therapy is commonly prescribed. For the management of major triglyceride elevations, three agents are also commonly utilized: (1) fibric acid derivatives, such as Fibricor; (2) niacin; and (3) omega-3 fatty acids. Fibricor is approved in the United States and indicated as adjunctive therapy to diet for treatment of severe hypertriglyceridemia (TG ≥ 500 mg/dL) and as adjunctive therapy to diet to reduce elevated LDL-C, Total-C, TG, and Apo B, and to increase HDL-C in patients with primary hypercholesterolemia or mixed dyslipidemia.
Competitive Analysis: Cholesterol-lowering drugs in the United States include statins, niacin, bile-acid resins, fibric acid derivatives (fibrates), cholesterol absorption inhibitors, and anti-protein convertase subtilisin-like kexin type 9 (PCSK9) inhibitors. All classes of cholesterol-lowering medicines are most effective when combined with increased exercise and a low-fat, high-fiber diet. The statin class includes some of the largest-selling prescription products in the world (e.g., Lipitor, Zocor and Crestor). Statins dominate single-agent prescribing for the treatment of lipid disorders. The niacin (nicotinic acid – vitamin B3) class includes brands such as Niaspan, which work primarily on increasing HDL cholesterol. The cholesterol absorption inhibitor class has a single product, Zetia. The PCSK9 inhibitor are a new class of treatments that currently include Praluent and Repatha. The fibrates class of cholesterol lowering treatments is composed of three competing molecules: (1) gemfibrozil (Lopid), (2) fenofibric acid (Fibricor, Trilipix), and (3) fenofibrate (Tricor). The fibrate market in the United States was $2.4 billion for 2015.
Products Marketed in Canada
|
Cambia®
|
Cambia (diclofenac potassium for oral solution) is an NSAID and the only prescription NSAID available and approved in Canada for the acute treatment of migraine attacks with or without aura in adults 18 years of age or older. Cambia was licensed from Nautilus in November 2010. Cambia was approved by the FDA in June 2009 and is currently marketed by Depomed in the United States. Cambia was approved by Health Canada in March 2012 and was commercially launched to specialists in Canada in October 2012 and broadly to all primary care physicians in February 2013. Depomed acquired Nautilus and the U.S. and Canadian rights to Cambia in December 2013.
Cambia is available as an oral solution in individual packets each designed to deliver a 50mg dose when mixed in water. Cambia is the only approved prescription NSAID available in Canada that was studied and proven to be an effective treatment for migraine according to guidelines published in September 2013 by the International Headache Society that reached statistically significant results for all four co-primary endpoints, including: (1) pain free response at two hours; (2) nausea free; (3) photophobia free (sensitivity to light); and (4) phonophobia free (sensitivity to sound). In addition, Cambia provides fast migraine pain relief within 30 minutes of dosing due in part to the significant benefits of the proprietary DBT. DBT provides for enhanced drug absorption and bioavailability. In fasting volunteers, measurable plasma levels were observed within 5 minutes of dosing with Cambia. Peak plasma levels were achieved at approximately 15 minutes, with a range of approximately 10 to 40 minutes. NSAIDs, such as Cambia, may increase the incidence of cardiovascular adverse events such as MI, stroke or thrombotic events, gastrointestinal adverse events such as peptic/duodenal ulceration, perforation and gastrointestinal bleeding and are contraindicated in the third trimester of pregnancy. The risk may increase with duration of use and patients should only take this medication as prescribed by a physician.
Migraine Treatment Options in Canada: There are a number of different treatment options for migraine in Canada. Acute migraine treatment options can be broken down to three main categories: (i) triptans or 5-HT1 receptor agonists (e.g., sumatriptan, rizatriptan); (ii) ergots (e.g., ergotamine, dihydroergotamine); and (iii) NSAIDs (e.g., Cambia). Triptans may cause dizziness, nausea, weakness and chest discomfort and should not be used by patients with heart disease, uncontrolled high blood pressure, blood vessel disease or who have a history of stroke. Ergots may cause chest pain, tingling or burning sensations, nausea, vomiting, and cramps. Furthermore, ergots may reduce blood flow to the extremities (hands and feet) and may lead to tissue damage. Ergots should also not be used by anyone with heart disease, uncontrolled high blood pressure or blood vessel disease.
In September 2013, the Canadian Neurological Sciences Federation issued revised Canadian Headache Society Guidelines for Acute Drug Therapy for Migraine Headaches through the Canadian Journal of Neurological Sciences. Cambia was acknowledged as a potential first line therapy, with a fast onset of action and having a strong recommendation, high quality evidence and recommended for the acute treatment of migraine.
Migraine in Canada: Canadian studies have shown migraine prevalence rates of 23% to 26% in women, and 8% to 10% in men. Over 4,000,000 Canadians suffer from migraine in Canada and that 60% of those with migraine have one or more attacks per month while 25% of those with migraine have at least one attack per week. One Canadian study found that those with migraine lose 6.5 days of work each year resulting from their migraine and, as a result, migraine is associated with a substantial social and economic impact. A study done in 1990 calculated that 7,000,000 workdays are lost annually in Canada due to migraine. It was also found that 51% of all women suffering from migraine have never consulted a physician for their headaches.
Competitive Analysis: It is estimated that one-half of all people suffering from migraines in Canada never seek help from a physician but rather self-treat their condition with OTC medications such as aspirin (e.g., Bayer), acetaminophen (e.g., Tylenol) and OTC NSAID’s such as ibuprofen (e.g., Advil) and naproxen sodium (e.g., Aleve). The main prescription pharmacological agents used to treat acute migraine includes the triptan class of drugs or 5-HT1 receptor agonists as they are known and these products include sumatriptan (Imitrex), rizatriptan (Maxalt), zolmitriptan (Zomig), almotriptan (Axert), naratriptan (Amerge), eletriptan (Relpax) and frovatriptan (Frova). There are also the ergot alkaloids such as ergotamine (Cafergot) and dihydroergotamine (Migrinal) used in some cases as are narcotics such as meperidine (e.g., Demerol) and the combination drug of aspirin, butalbital and caffeine (e.g., Fiorinal). In spite of a number of possible treatment options for treating migraines, many of these treatments are without an authorized indication from Health Canada. The Company considers the competitive market as the triptans class, which currently sells approximately $125 million annually in Canada.
|
Fiorinal®/Fiorinal® C
|
Fiorinal (acetylsalicylic acid, caffeine and butalbital tablets and capsules) and Fiorinal C (acetylsalicylic acid, caffeine, butalbital and codeine capsules) were acquired from Novartis AG and Novartis Pharma AG in October 2014. Fiorinal and Fiorinal C were originally approved by Health Canada in 1970 for the relief of tension-type headache.
Fiorinal is a fixed dose combination drug that combines the analgesic properties of acetylsalicylic acid, with the anxiolytic and muscle relaxant properties of butalbital, and the central nervous system stimulant properties of caffeine. Fiorinal C expands on the properties of Fiorinal with the additional analgesic effect of codeine. Fiorinal and Fiorinal C are the only prescription products in Canada indicated for relief of tension type headaches. Fiorinal and Fiorinal C are currently marketed in Canada in hard gelatin capsules containing 330mg acetylsalicylic acid, 40mg caffeine, 50mg butalbital, and in the case of Fiorinal C, the addition or 15mg or 30mg of codeine. Codeine and butalbital are both habit-forming and potentially abusable. Consequently, the extended use of Fiorinal or Fiorinal C is not recommended. Fiorinal and Fiorinal C are associated with exacerbation of headache (medication overuse headaches) in susceptible patients. Repeated use of Fiorinal and Fiorinal C can lead to “rebound” headaches as each dose wears off. With repeated doses physical and psychological dependence can develop. In addition to dependence, butalbital-containing products can lead to tolerance, and at higher doses can produce withdrawal symptoms after discontinuation.
Tension-Type Headache in Canada: Tension-type headaches are the most common type of headache and are caused by muscle tightening in the back of the neck or scalp. These headaches are typically triggered by emotional stress, fatigue or depression. There are two classifications of tension-type headache: (1) episodic tension headaches, which occur randomly and less frequently; and (2) chronic tension headaches, which may occur daily or continually and the intensity of the pain may vary during a 24-hour cycle. Tension headaches differ from migraine headaches due to the lack of aura, photophobia, phonophobia and/or nausea.
Competitive Analysis: Tension-type headaches may be treated with OTC NSAIDs like Tylenol, Advil, Aleve, or Aspirin. Prescription NSAIDs may also be used, such as Naprosyn, Anaprox, Toradol, as well as prescription analgesic/opiate combinations like Percocet, Tylenol with codeine and Fiorinal/Fiorinal C. In spite of a number of possible treatment options for treating tension-type headaches, all of these treatments, with the exception of Fiorinal and Fiorinal C, are without an authorized indication from Health Canada. The Company considers the competitive market as the prescription NSAID and prescription analgesic/opiate combination class, which has an estimated tension-type headache value of approximately $30 million annually in Canada. The OTC market for tension-type headache is estimated to be exponentially larger given the large patient population; however, the true value is extremely difficult to determine considering the broad range of indications for OTC NSAIDs.
|
Soriatane®
|
Soriatane (acitretin) is chemically known as acitretin, and is indicated for the treatment of severe psoriasis (including erythrodermic and pustular types) and other disorders of keratinization. Soriatane is a retinoid, an aromatic analog of vitamin A. Soriatane was approved in Canada in 1994 and is the first and only oral retinoid indicated for psoriasis. Soriatane is often used when milder forms of psoriasis treatments like topical steroids, emollients and topical tar-based therapies have failed.
Soriatane should be reserved for patients unresponsive to, or intolerant of, standard treatment. In addition, Soriatane should only be prescribed by physicians knowledgeable in the use of systemic retinoids. Soriatane is teratogenic (can cause birth defects) and should not be used by women who are pregnant or who are planning to become pregnant during, or within three years after stopping, treatment of Soriatane. In addition, acitretin may cause nausea, headache, itching, dry, red or flaky skin, dry or red eyes, dry or chapped lips, swollen lips, dry mouth, thirst, cystitis acne or hair loss.
Psoriasis Treatment Options: There are a number of different treatment options for psoriasis. Typically, topical agents are used for mild disease, phototherapy for moderate disease and oral systemic agents and biologicals for more severe disease. The three main traditional systemic treatments are (1) methotrexate, (2) cyclosporine and (3) retinoids. Unlike Soriatane, methotrexate and cyclosporine are immunosuppressant drugs. Methotrexate may cause a decrease in the number of blood cells made by bone marrow, may cause liver damage, lung damage, damage to the lining of the mouth, stomach or intestines and may increase the risk of developing lymphoma (cancer that begins in the cells of the immune system), among other serious side effects. Methotrexate may also cause serious or life-threatening skin reactions. Cyclosporines may cause side effects that could be very serious, such as high blood pressure and kidney and liver problems. It may also reduce the body’s ability to fight infections.
Competitive Analysis: Severe psoriasis is a condition that involves more than 10% of the body area or is physically, occupationally or psychologically disabling. Soriatane will typically be used in combination with other drugs such as topical steroids, emollients or tar-based therapies. Soriatane is most effective for treating psoriasis when it is used with phototherapy. Soriatane may be used with biologic agents, such as etanercept (Enbrel), adalimumab (Humira) or infliximab (Remicade), and may also be prescribed in rotation with cyclosporine or methotrexate. Biologic therapies such as Enbrel, Humira and Remicade are effective in treating severe forms of the disease, but tend to be very expensive and sometimes not reimbursed by government or other private drug plans. Cyclosporine and methotrexate are also oral agents that are often used for severe forms of psoriasis. The market for moderate to severe psoriasis in Canada, including the biologics, is estimated to be greater than $200 million for 2015.
|
Bezalip® SR
|
Bezalip SR (bezafibrate) is an established pan-peroxisome proliferator-activated receptor activator. Bezalip SR, used to treat hyperlipidemia (high cholesterol), has over 25 years of therapeutic use globally. Bezalip SR helps lower LDL-C and triglycerides while raising HDL-C levels. It also improves insulin sensitivity and reduces blood glucose levels, which in combination with the cholesterol effects may significantly lower the incidence of cardiovascular events and development of diabetes in patients with features of metabolic syndrome. Bezalip SR is contraindicated in patients with hepatic and renal impairment, pre-existing gallbladder disease, hypersensitivity to bezafibrate, or pregnancy or lactation.
Bezalip SR is currently approved in more than 40 countries worldwide, not including the United States. Bezalip SR is under license from Actavis, and we have the exclusive rights to market Bezalip SR in Canada. We also have the exclusive development and licensing rights to Bezalip SR in the United States and filed an IND that received clearance from the FDA in the United States. Clinical studies would be required prior to commercialization in the United States. The initial target indication that would be considered for pursuit in the United States is for severe hypertriglyceridemia.
Hyperlipidemia Treatment Options: Hyperlipidemia is a very common chronic condition and is characterized by an excess of fatty substances called lipids, mainly cholesterol and triglycerides, in the blood. It is also called hyperlipoproteinemia because these fatty substances travel in the blood attached to proteins. This is the only way that these fatty substances can remain dissolved while in circulation. Hyperlipidemia, in general, can be divided into two subcategories: (1) hypercholesterolemia, in which there is a high level of cholesterol; and (2) hypertriglyceridemia, in which there is a high level of triglycerides, the most common form of fat.
Competitive Analysis: Cholesterol-lowering drugs in Canada include statins, niacin, bile-acid resins, fibric acid derivatives (fibrates), and cholesterol absorption inhibitors. All classes of cholesterol-lowering medicines are most effective when combined with increased exercise and a low-fat, high-fiber diet. The statin class includes some of the largest-selling prescription products in the world (e.g., atorvastatin (Lipitor), simvastatin (Zocor) and rosuvastatin (Crestor)). Statins dominate single-agent prescribing for the treatment of lipid disorders. The niacin (nicotinic acid – vitamin B3) class includes brands such as Niaspan, which work primarily on increasing HDL cholesterol. The fibrates class of cholesterol-lowering treatments is composed of three competing molecules: (1) gemfibrozil (Lopid), (2) bezafibrate (Bezalip SR), and (3) fenofibrate (Lipidil in Canada or Tricor in the United States). Clinical studies have demonstrated that bezafibrate, the active ingredient in Bezalip SR, was shown to be effective in lowering high levels of triglycerides, raising HDL cholesterol and lowering LDL cholesterol. As of the end of 2015, the annual fibrate market in Canada is estimated to be approximately $35 million.
Other Commercialized Products
In addition to the products discussed above, we also market NeoVisc (sodium hylauronic solution - 1%), Uracyst (sodium chondroitin sulfate - 2%), Durela (tramadol hydrochloride), Proferrin (heme iron polypeptide), Resultz (isopropyl myristate), Collatamp G (collagen-gentamycin) and a portfolio of eight products targeted in the gastroenterology and women’s health markets in Canada.
Primary Development Products
|
YOSPRALA™
|
The products in the YOSPRALA (aspirin/omeprazole delayed release tablets) portfolio, which are part of our PA platform, are being developed with the goal of significantly reducing GI ulcers and other GI complications compared to taking enteric-coated, buffered or plain aspirin alone in patients at risk of developing GI ulcers. The first candidates in the YOSPRALA product portfolio are YOSPRALA 81/40 (PA8140), which contains 81mg of enteric-coated aspirin and 40mg immediate-release omeprazole, and YOSPRALA 325/40 (PA32540), which contains 325mg of enteric-coated aspirin and 40mg immediate-release omeprazole. Both products are a coordinated-delivery tablet combining immediate-release omeprazole, a PPI, layered around a pH-sensitive enteric coating of an aspirin core. This novel, patented product is intended for oral administration once a day.
Pending FDA review and approval, YOSPRALA 81/40 and 325/40 would be indicated for patients who require aspirin (1) to reduce the combined risk of death and nonfatal stroke in patients who have had ischemic stroke or transient ischemia of the brain due to fibrin platelet emboli, (2) to reduce the combined risk of death and nonfatal MI in patients with a previous MI or unstable angina pectoris, (3) to reduce the combined risk of MI and sudden death in patients with chronic stable angina pectoris, (4) for a pre-existing condition after having undergone revascularization procedures, and (5) the omeprazole component, to decrease the risk of developing gastric ulcers in patients at risk for developing aspirin-associated gastric ulcers.
Development History and Status: YOSPRALA 81/40 and 325/40 products have completed Phase3 clinical development testing in the United States, and we resubmitted the NDA for these products with the FDA on March 14, 2016.
We met with the FDA to discuss the overall development program requirements for YOSPRALA 81/40 and 325/40 for the secondary prevention of cardiovascular and cerebrovascular disease in patients at risk for gastric ulcers. An IND was filed in the fourth quarter of 2007. We completed a study which demonstrated that the salicylic acid component of YOSPRALA 325/40 was bioequivalent to the reference drug, enteric-coated aspirin. We filed a Special Protocol Assessment with the FDA for the design of the Phase 3 studies for the product, the primary endpoint for which is the reduction in the cumulative incidence of endoscopic gastric ulcers.
In October 2009, we began two pivotal Phase 3 and one long-term safety study for YOSPRALA 325/40. The primary endpoint of the pivotal studies, which included approximately 500 subjects per study, was a significant reduction in the cumulative incidence of gastric ulcers following administration of YOSPRALA 325/40 compared to 325mg enteric-coated aspirin over the six-month treatment period. The primary endpoint was met with statistical significance in both studies. Additionally, the studies met their key secondary endpoints, including a reduction in gastroduodenal ulceration, as well as a reduction in discontinuation due to upper gastrointestinal adverse events in subjects taking YOSPRALA 325/40 compared to 325mg enteric-coated aspirin.
In February 2012, the FDA requested an additional Phase 1 study to assess the bioequivalence of YOSPRALA 325/40 to enteric-coated aspirin 325mg with respect to acetylsalicylic acid. After the Company completed the requested bioequivalence study, the FDA made a preliminary review of the study results and the Company’s summary analyses and, based on its preliminary assessment of the information available to it at the time, the FDA did not agree that bioequivalence of YOSPRALA 325/40 to enteric-coated aspirin 325mg was demonstrated. The Company then submitted to the FDA additional information and analyses from the requested bioequivalence study, as well as other relevant pharmacokinetic, clinical pharmacology, and in vitro dissolution data as a briefing document in support of a request for a Type A meeting with the FDA. At the August 2012 Type A Meeting, the FDA confirmed that, although it believes bioequivalence of YOSPRALA 325/40 to enteric-coated aspirin 325mg was not strictly established in our bioequivalence study according to the predetermined criteria, the results from this study, together with additional information that was submitted by the Company in the NDA, constitutes sufficient data to support the establishment of a clinical and pharmacological bridge between the product and enteric-coated aspirin 325mg. The FDA indicated that it would make a final determination during the NDA review. The FDA also indicated that a similar strategy to bridge to the reference listed drug, inclusive of a new, single pharmacokinetic study, could be utilized for a low dose version of the 325/40mg version, YOSPRALA 81/40. The Company conducted this study with the low dose version against the enteric-coated aspirin 81mg. Based on the predetermined criteria acceptable to the FDA, the study demonstrated that YOSPRALA 81/40 is bioequivalent to enteric-coated aspirin 81mg and had comparable bioavailability.
During a pre-submission meeting with respect to its NDA for YOSPRALA 325/40 in April 2012, the FDA suggested that the Company also seek approval for a lower dose formulation of the product containing 81mg of enteric-coated aspirin as part of its NDA for YOSPRALA 325/40. Absent the availability of such a lower dose formulation in the market if YOSPRALA 325/40 is approved, the FDA indicated that it might limit the indication for YOSPRALA 325/40 to use in post coronary artery bypass graft surgery with treatment duration not to exceed one year. During the August 2012 Type A Meeting, the FDA confirmed its preference to have both YOSPRALA 325/40 and a lower dose version available in the market so that physicians can have both a low and high dose option available, and agreed that, if both dosage strengths were included in the NDA and subsequently approved, the indications for both will be consistent with the full range of indications described in the current aspirin monograph.
We had generated some clinical pharmacology data and CMC data for a lower dose version of YOSPRALA 325/40 – a product that contains 81mg of enteric-coated aspirin and 40mg of immediate-release omeprazole in a single tablet known YOSPRALA 81/40. The Company filed this existing data, together with additional CMC data to be generated and evidence from the scientific literature relating to the ulcerogenic risk of 81mg of aspirin with the FDA. At this time, we do not intend to conduct Phase 3 clinical trials for YOSPRALA 81/40. We have no assurance such data will be sufficient for the FDA to approve YOSPRALA 81/40 or to allow a broader indication for YOSPRALA 325/40. The FDA will make a final determination with respect to the approvability of and indications for YOSPRALA 325/40 and 81/40 upon our re-submission of the NDA, which we resubmitted with the FDA on March 14, 2016.
The generation of additional data with respect to YOSPRALA 81/40 and the incorporation of data into the NDA for YOSPRALA 325/40 delayed submission of the NDA from the original planned submission date in the third quarter of 2012. The NDA was filed for both products in March 2013, and in May 2013, the FDA accepted the NDA for review. The FDA assigned a user fee date of January 24, 2014. As part of our continuing discussions with the FDA concerning the NDA for YOSPRALA 325/40 and 81/40 tablets, we decided to conduct an additional comparative Phase 1 pharmacokinetic study to determine the pharmacokinetic profile of the omeprazole component of YOSPRALA 81/40 tablets and compare it to that of YOSPRALA 325/40 tablets. We submitted study information and data to the FDA as it became available during the conduct of the study and FDA reviewed such information and data from the study when submitted. The final study report was submitted to the FDA in accordance with our agreed timeline. The FDA informed us that the Company’s user fee date was moved to April 25, 2014.
On April 25, 2014, we received a CRL from the FDA advising that the review of our NDA was completed and questions remained that preclude the approval of the NDA in its then current form. Specifically, an inspection of the manufacturing facility of our previously designated primary aspirin API supplier concluded with certain inspection deficiencies. There were no clinical or safety deficiencies noted with respect to either YOSPRALA 325/40 or 81/40 and no other deficiencies were noted in the CRL. On June 30, 2014, we resubmitted the NDA for YOSPRALA 325/40 and 81/40 to the FDA and the FDA notified us that the new action fee date is December 30, 2014. On May 9, 2014, the aspirin API supplier submitted a response to the FDA addressing the inspection deficiencies and subsequently submitted an update to its initial response.
On December 17, 2014, we received a second CRL from the FDA advising that the review of our NDA was completed and questions remained that preclude approval of the NDA in its then current form. In this CRL, the FDA used identical wording to that of the first CRL. There were no clinical or safety deficiencies noted with respect to either YOSPRALA 325/40 or 81/40 and no other deficiencies were noted in the CRL. FDA regulations allowed us to request a Type A meeting with the FDA to discuss the next steps required to gain approval of our NDA. The FDA granted the Type A meeting, which was held in late January 2015. At the meeting, representatives from the FDA’s Office of Compliance stated that the aspirin API supplier’s responses to the 483 inspectional observations submitted in May 2014 were still under review and the Office of Compliance would be communicating with the supplier in the coming weeks. The aspirin API supplier subsequently informed us that it received a warning letter relating to the Form 483 inspection deficiencies and submitted a plan of corrective actions to the FDA to address the matters raised in the warning letter.
On December 28, 2015, we announced that the FDA had completed re-inspection of the aspirin API supplier’s manufacturing facility and issued an additional 483 notice, citing numerous observations. The aspirin API supplier voluntarily stopped production at this facility to focus on remediating the FDA observations. We have been informed that production at this facility has resumed and it remains subject to FDA inspection.
On December 28, 2015, we also announced that significant progress had been made with respect to an alternative aspirin API supplier, which is a global leader in aspirin manufacturing, and that we have now designated this secondary supplier as our primary supplier in connection with the NDA for YOSPRALA. We are focusing our efforts toward using our previously designated secondary aspirin API supplier as our primary supplier in connection with our NDA and will include both aspirin API suppliers in the NDA package for YOSPRALA. Final agreement on the draft labeling is also pending. We resubmitted the NDA for YOSPRALA on March 14, 2016, and we believe we remain on track for potential approval and launch of YOSPRALA in 2016.
Pozen has incurred direct development costs associated with the development of the PA program of $5.3 million during the year ended December 31, 2015. Since inception Pozen has incurred total direct development costs of $80.0 million associated with the development of the PA program. Pozen’s direct development costs do not include the cost of research and development personnel or any allocation of overhead expenses.
Bilastine
Bilastine is a second generation antihistamine drug for the treatment of allergic rhinoconjunctivitis and urticaria (hives). The Company has not yet chosen a trademark for bilastine. Bilastine exerts its effect as a selective histamine H1 receptor antagonist, and has an effectiveness similar to cetirizine, fexofenadine and desloratadine. It was developed in Spain by FAES Farma, S.A. The Canadian antihistamine market is currently valued at approximately $115 million per year and the leading competitors are cetirizine (Reactine), loratadine (Claritin), desloratadine (Aerius) and fexofenadine (Allegra). It has been over fifteen years since the approval of a new antihistamine in Canada.
The Company filed bilastine with Health Canada in the second quarter of 2015. Bilastine is approved in the European Union for the symptomatic treatment of allergic rhinoconjunctivitis and urticaria, but it is not approved by the FDA for any use in the United States.
The clinical efficacy of bilastine in AR and urticaria has been assessed in 10 clinical studies in which over 4,600 patients were involved. The studies on SAR were double-blind, placebo-controlled, parallel-group involving male and female patients over 12 years of age with symptomatic disease at the beginning of the study. In the SAR studies, the daily oral administration during 14 days of bilastine 20mg proves to have comparable efficacy to the administration of cetirizine 10mg or than the administration of desloratadine 5mg. Bilastine 20mg shows a safety and tolerability profile similar to placebo. Possible side effects of bilastine include headache and drowsiness.
The studies in urticaria were double-blind, placebo-controlled, parallel-group involving male and female patients over 18 year of age with symptomatic disease (chronic idiopathic urticaria) at the beginning of the study. In this urticaria studies the daily oral administration of 28 days of bilastine 20mg proves to have comparable efficacy to the administration of levocetirizine 5mg. Likewise, bilastine 20mg shows a safety and tolerability profile comparable to placebo.
Out-Licensed Products
|
VIMOVO®
|
VIMOVO (naproxen/esomeprazole magnesium) is the brand name for a proprietary fixed-dose combination of enteric-coated naproxen, a pain-relieving NSAID and immediate-release esomeprazole magnesium, a PPI, in a single delayed-release tablet and is a product in our PN platform. We developed VIMOVO in collaboration with AstraZeneca. On April 30, 2010, the FDA approved VIMOVO for the relief of the signs and symptoms of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis, and to decrease the risk of developing gastric ulcers in patients at risk of developing NSAID-associated gastric ulcers. As of the end of December 31, 2015, VIMOVO is being sold in over 50 countries. Prescription sales of oral anti-arthritis NSAIDs in the United States in 2015 were approximately $6.3 billion.
In June 2010, we officially transferred to AstraZeneca the IND and NDA for the product. AstraZeneca is responsible for the commercialization of VIMOVO. In November 2013, AstraZeneca entered into an agreement for Horizon to acquire the U.S. rights for VIMOVO. Under the terms of the agreement, we will continue to receive from Horizon a 10% royalty on net sales of VIMOVO sold in the United States, with guaranteed annual minimum royalty payments of $5 million in 2014, and $7.5 million each year thereafter, provided that the patents owned by us which cover VIMOVO are in effect and no generic forms of VIMOVO are on the market. AstraZeneca will continue to have rights to commercialize VIMOVO outside of the United States and paid us a royalty of 6% on all sales within its territory through 2015 and will pay us a royalty of 10% commencing 2016 and thereafter. See also the section entitled “Item 1. Business – Collaboration Agreements – Agreement with AstraZeneca/Horizon regarding VIMOVO®” in this Annual Report on Form 10-K.
Since inception Pozen has incurred total direct development costs of $96.2 million associated with the development of the PN program of which $57.1 million was funded by development revenue from AstraZeneca. Pozen’s direct development costs do not include the cost of research and development personnel or any allocation of overhead expense.
|
Treximet®
|
Treximet (sumatriptan/naproxen sodium) is a migraine medicine that was developed by us in collaboration with GSK. The product is formulated with our patented technology of combining a triptan, sumatriptan 85mg, with an NSAID, naproxen sodium 500mg, and GSK’s RT Technology™ in a single tablet designed for the acute treatment of migraine. On April 15, 2008, the FDA approved Treximet for the acute treatment of migraine attacks, with or without aura, in adults. Treximet is available in the United States. The market for migraine medications in the United States is valued at approximately $2.2 billion in 2015.
In May 2008, we transferred the IND and NDA for the product to GSK, which subsequently sold its rights in Treximet, including the related trademark, to Pernix in August 2014. As part of GSK’s divestiture to Pernix, restrictions on our right to develop and commercialize certain additional dosage forms of sumatriptan/naproxen combinations outside of the United States had been eliminated, allowing us to seek approval for these combinations on the basis of the approved NDA. GSK was previously, and Pernix is currently, responsible for the commercialization of Treximet in the United States, while we received royalties based on net sales. In November 2011, we sold to a financial investor, CII, for an upfront lump-sum, our rights to future royalty and milestone payments relating to Treximet sales in the United States and certain other products containing sumatriptan/naproxen sodium developed and sold by Pernix in the United States. By virtue of the agreement, we will also be entitled to receive a 20% interest in royalties, if any, paid on net sales of Treximet and such other products in the United States to CII relating to the period commencing in the second quarter of 2018. See also the section entitled “Item 1. Business – Collaboration Agreements – Agreements with GSK, Pernix and CII regarding MT 400 (including Treximet®)” in this Annual Report on Form 10-K.
Since inception Pozen has incurred total direct development costs of $26.6 million associated with the development of Treximet and MT 400. Pozen’s direct development costs do not include the cost of research and development personnel or any allocation of overhead expenses.
Results of Operations of Aralez Pharmaceuticals Inc.
Aralez has had no operations as of December 31, 2015, other than business incident to the Tribute Transaction.
Critical Accounting Policies and Estimates of POZEN Inc.
Management makes certain judgments and uses certain estimates and assumptions when applying accounting principles generally accepted in the United States in the preparation of our financial statements. The development and selection of the critical accounting policies, and the related disclosure about these policies, have been reviewed by the Audit Committee of our Board of Directors. We evaluate our estimates and judgments on an ongoing basis and base our estimates on historical experience and on assumptions that we believe to be reasonable under the circumstances. Our experience and assumptions form the basis for our judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may vary from what we anticipate and different assumptions or estimates about the future could change our reported results. We have critical accounting estimates in the following policy areas: revenue recognition, accrued expenses, stock-based compensation, fair value measurements and income taxes.
Revenue Recognition
Revenue for the years ended December 31, 2015, 2014, and 2013 consisted of the following:
| For the Years Ended December 31, | ||||||||||||
|
2015
|
|
2014
|
|
2013
|
||||||||
|
Royalty revenue
|
$
|
21,391,414
|
$
|
21,136,932
|
$
|
6,322,000
|
||||||
|
Other licensing revenue
|
—
|
11,257,300
|
4,000,000
|
|||||||||
|
Total royalty and licensing revenue
|
$
|
21,391,414
|
$
|
32,394,232
|
$
|
10,322,000
|
||||||
With regard to royalty revenues, royalty revenue from VIMOVO is recognized when earned, as will any other future royalty revenues with respect to the manufacture, sale or use of Pozen’s products or technology. For VIMOVO or those future arrangements where royalties are reasonably estimable, Pozen recognizes revenue based on estimates of royalties earned during the applicable period and reflects in future revenue any differences between the estimated and actual royalties. These estimates are based upon information reported to us by our collaboration partners. During the years ended December 31, 2015, 2014 and 2013, Pozen recognized $21.4 million, $21.1 million, and $6.3 million, respectively, for VIMOVO royalty revenue.
With regard to the licensing revenues, Pozen’s licensing agreements have terms that include upfront payments upon contract signing and additional payments if and when certain milestones in the product’s development or commercialization are reached. When evaluating license agreements with multiple element deliverables, Pozen considers whether the deliverables under the arrangement represent separate units of accounting. This evaluation requires subjective determinations and requires management to make judgments about the individual deliverables and whether such deliverables are separable from the other aspects of the contractual relationship. In determining the units of accounting, management evaluates certain criteria, including whether the deliverables have standalone value, based on the consideration of the relevant facts and circumstances for each arrangement. The consideration received is allocated among the separate units of accounting using the relative selling price method, and the applicable revenue recognition criteria are applied to each of the separate units.
At the inception of each agreement that includes milestone payments, Pozen evaluates whether each milestone is substantive and at risk to both parties on the basis of the contingent nature of the milestone. If the milestones are deemed substantive and the milestone payments are nonrefundable, such milestone payments are recognized upon successful accomplishment of the milestones.
Accrued Expenses, including Contracted Costs
Significant management judgments and estimates must be made and used in connection with accrued expenses, including those related to contract costs, such as costs associated with clinical trials. Specifically, Pozen must make estimates of costs incurred to date but not yet paid for or not yet invoiced in relation to contracted, external costs. Pozen analyzes the progress of product development, clinical trials and related activities, invoices received, amounts paid, and budgeted costs when evaluating the adequacy of the accrued liability for these related costs.
Pozen believes that its current assumptions and other considerations used to estimate accrued expenses for the period are appropriate. However, determining the date on which certain contract services commence, the extent of services performed on or before a given date and the cost of such, paid and unpaid, involves subjective judgments and estimates and often must be based upon information provided by third parties. In the event that management does not identify certain contract costs which have begun to be incurred or under- or over-estimates the extent of services performed or the costs of such services, management adjusts costs during the period in which the information becomes available.
Accrued costs related to product development, pre-commercialization projects and operating activities, based upon the progress of these activities covered by the related contracts, invoices received and estimated costs, totaled $6.7 million at December 31, 2015 and $0.3 million at December 31, 2014. The variance, at each of these ending periods, between the actual expenses incurred and the estimated expenses accrued was not material to the financial statements.
Stock-Based Compensation
Stock-based compensation cost is estimated at the grant date based on the fair value of the award and is recognized as expense over the requisite service period of the award. The fair value of restricted stock awards is determined by reference to the fair market value of our common stock on the date of grant. We use the Black-Scholes model to value service condition and performance condition option awards. For awards with only service conditions and graded-vesting features, we recognize compensation cost on a straight-line basis over the requisite service period. For awards with performance conditions granted we recognize compensation cost over the expected period to achieve the performance conditions, provided achievement of the performance conditions are deemed probable.
Determining the appropriate fair value model and related assumptions requires judgment, including estimating stock price volatility, forfeiture rates, and expected terms. Our expected volatility rate was estimated based on an equal weighting of the historical volatility of our common stock over a six-year period. The expected term we use was estimated based on average historical terms to exercise. The risk-free interest rate is based on seven-year U.S. Treasury securities.
Determining the appropriate amount to expense for performance-based awards based on the achievement of stated goals requires judgment, including forecasting future performance results. The estimate of expense is revised periodically based on the probability of achieving the required performance targets and adjustments are made as appropriate. The cumulative impact of any revisions is reflected in the period of change. If any applicable financial performance goals are not met, no compensation cost is recognized and any previously recognized compensation cost is reversed.
Fair Value Measurements
Financial instruments consist of cash and cash equivalents, short-term investments, accounts receivable and accounts payable. The carrying values of these amounts, other than the investment in warrants, approximate the fair value due to their short-term nature.
As part of its acquisition of Treximet from GSK, Pernix granted Pozen a warrant to purchase 500,000 shares of Pernix common stock at an exercise price of $4.28 per share. The common stock underlying the warrant was registered by Pernix with the SEC. The warrant was exercisable from August 20, 2014, the closing date of the acquisition, until February 28, 2018. The warrant was valued at $2.7 million at December 31, 2014 using the Black-Scholes valuation model discounted for the warrant’s lack of marketability and liquidity.
Short-term investment gains consisted of the investment in warrant valuation of $2.7 million on August 20, 2014, with a mark-to-market decrease of $0.1 million at December 31, 2014 and a net short-term gain of approximately $2.6 million. The warrant was sold in the first quarter of 2015 and Pozen received $2.5 million from the sale.
Pozen defines fair value (“FV”) as the price that would be received to sell an asset or paid to transfer a liability (“the exit price”) in an orderly transaction between market participants at the measurement date. The FV hierarchy for inputs maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Pozen uses the following hierarchy of inputs to measure FV:
| · | Level 1 - quoted prices in active markets for identical assets and liabilities. |
| · | Level 2 - observable inputs other than quoted prices in active markets for identical assets and liabilities, including quoted prices in active markets for instruments that are similar or quoted prices in markets that are not active for identical or similar instruments and model-derived valuations in which all significant inputs and value drivers are observable in active markets. |
| · | Level 3 - unobservable inputs that are significant to the overall valuation, for which there is little or no market data available and which require Pozen to develop its own assumptions. |
Pozen values investments using the most observable inputs available that are current as of the measurement date and classifies them according to the lowest level of inputs used. Observable inputs are inputs that market participants would use in pricing the asset or liability developed from market data obtained from independent sources. Unobservable inputs are inputs that reflect Pozen’s judgment concerning the assumptions that market participants would use in pricing the asset or liability developed from the best information available under the circumstances.
Fair value is a market-based measure considered from the perspective of a market participant who holds the asset or owes the liability rather than an entity-specific measure. Therefore, even when market assumptions are not readily available, our own assumptions are set to reflect those that market participants would use in pricing the asset or liability at the measurement date.
The financial assets for which we performed recurring measurements are cash equivalents and investments in warrants. As of December 31, 2015 and 2014, financial assets utilizing Level 1 inputs included cash equivalents. As of December 31, 2014, financial assets utilizing Level 2 inputs included investments in warrants.
Our Level 1 valuations are based on the market approach and consist primarily of quoted prices for identical items on active securities exchanges. Our Level 2 valuations may also use the market approach and are based on significant other observable inputs such as quoted prices for financial instruments not traded on a daily basis. We did not rely on Level 3 input for valuation of our securities at December 31, 2015 or 2014.
The following table sets forth our financial instruments carried at fair value within the fair value hierarchy and using the lowest level of input as of December 31, 2015 and 2014:
|
December 31, 2015
|
||||||||||||||||
|
Financial Instruments Carried at Fair Value
|
||||||||||||||||
|
Quoted
prices
in active
Markets for
identical
items
(Level 1)
|
Significant
other
observable
inputs
(Level 2)
|
Significant
unobservable
inputs
(Level 3)
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Cash and cash equivalents
|
$
|
24,815,669
|
$
|
―
|
$
|
—
|
$
|
24,815,669
|
||||||||
|
December 31, 2014
|
||||||||||||||||
|
Financial Instruments Carried at Fair Value
|
||||||||||||||||
|
Quoted prices
in active
markets for
identical items
(Level 1)
|
Significant
other
observable
inputs
(Level 2)
|
Significant
unobservable
inputs
(Level 3)
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Cash and cash equivalents
|
$
|
40,582,415
|
$
|
—
|
$
|
—
|
$
|
40,582,415
|
||||||||
|
Investment in warrants
|
—
|
2,678,773
|
—
|
2,678,773
|
||||||||||||
|
Total cash and investments
|
$
|
40,582,415
|
$
|
2,678,773
|
$
|
—
|
$
|
43,261,188
|
||||||||
Income Taxes
We account for income taxes using the liability method in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”), Topic 740, “Income Taxes” (“ASC 740”). Deferred tax assets and liabilities are determined based on temporary differences between financial reporting and tax basis assets and liabilities and are measured by applying enacted rates and laws to taxable years in which differences are expected to be recovered or settled. Further, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that the rate changes. A valuation allowance is required when it is “more likely than not” that all or a portion of deferred tax assets will not be realized. Since our inception, we have incurred substantial cumulative losses and may incur substantial and recurring losses in future periods. The utilization of the loss carryforwards to reduce future income taxes will depend on Pozen’s ability to generate sufficient taxable income prior to the expiration of the loss carryforwards. In addition, the maximum annual use of net operating loss and research credit carryforwards is limited in certain situations where changes occur in stock ownership.
We currently file income tax returns in the U.S. federal jurisdiction and the state of North Carolina and plan to file additional foreign and state returns for 2015. We are no longer subject to federal or North Carolina income tax examinations by tax authorities for years before 2012. However, the loss carryforwards generated prior to 2012 may still be subject to change if we subsequently begin utilizing these losses in a year that is open under statute and subject to federal or North Carolina income tax examinations by tax authorities.
ASC 740 prescribes a comprehensive model for how a company should recognize, measure, present and disclose in its financial statements uncertain tax positions that the company has taken or expects to take on a tax return, including a decision whether to file or not file a return in a particular jurisdiction. Our financial statements reflect expected future tax consequences of such positions presuming the taxing authorities’ full knowledge of the position and all relevant facts. We recognize any interest and penalties accrued related to unrecognized tax benefits as income tax expense. During the year ended December 31, 2015 and 2014, there were no such interest or penalties.
Historical Results of Operations of POZEN Inc.
The following table sets forth our results of operations:
|
For the Years Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
(in thousands)
|
||||||||||||
|
Revenues:
|
||||||||||||
|
Royalty revenue
|
$
|
21,391
|
$
|
21,137
|
$
|
6,322
|
||||||
|
Other licensing revenue
|
—
|
11,257
|
4,000
|
|||||||||
|
Total royalty and licensing revenue:
|
21,391
|
32,394
|
10,322
|
|||||||||
|
Operating expenses:
|
||||||||||||
|
Selling, general and administrative
|
50,345
|
10,079
|
17,161
|
|||||||||
|
Research and development
|
8,512
|
5,740
|
9,945
|
|||||||||
|
Total operating expenses
|
58,857
|
15,819
|
27,106
|
|||||||||
|
Interest and other (loss) income
|
(143
|
)
|
3,099
|
76
|
||||||||
|
(Loss) income before income tax expense
|
(37,609
|
)
|
19,674
|
(16,708
|
)
|
|||||||
|
Income tax expense
|
174
|
—
|
—
|
|||||||||
|
Net (loss) income
|
$
|
(37,783
|
)
|
$
|
19,674
|
$
|
(16,708
|
)
|
||||
Year ended December 31, 2015 compared to the year ended December 31, 2014
Revenue: We recognized total revenue of $21.4 million for the year ended December 31, 2015, as compared to total revenue of $32.4 million for the year ended December 31, 2014. The decrease in revenue was primarily due to the inclusion of $11.3 million in amortization of upfront licensing fees in 2014. Royalty revenue for the year ended December 31, 2015 consisted of $21.4 million of royalty revenue compared to $21.1 million of royalty revenue for the year ended December 31, 2014. Our licensing and collaboration agreements have terms that include upfront payments upon contract signing and additional payments if and when certain milestones in the product development or related milestones are achieved. All upfront payments have been recognized as of December 31, 2014.
Selling, general and administrative: Selling, general and administrative expenses increased by $40.2 million to $50.3 million for the year ended December 31, 2015, as compared to the same period in 2014. The increase reflects the increased activities, which included a $6.3 million expense related to our former President and Chief Executive Officer’s separation agreement, $11.5 million transaction-related expenses related to the Tribute Transaction, $2.9 million increase in employee severance and retention expense, $9.1 million of increased staffing costs, including non-cash stock-based compensation expense of $4.1 million, $10.2 million increase in pre-commercialization activities, and $0.3 million in other activities, as compared to the same period in 2014. Selling, general and administrative expenses consisted primarily of the costs of administrative personnel, facility infrastructure, business development and pre-commercialization expenses, and public company activities.
Research and development: Research and development expenses increased by $2.8 million to $8.5 million for the year ended December 31, 2015, as compared to the same period in 2014. The increase was due primarily to a $2.1 million increase in direct development costs for our PA program and other product development activities during the year ended December 31, 2015. We have included in our research and development total expenses the departmental personnel costs associated with our research and development activities and direct costs associated with pharmaceutical development, clinical trials, toxicology activities and regulatory matters.
Interest and other (loss) income: A net loss of $0.1 million, related primarily to the sale of the Pernix warrant, was incurred during the year ended December 31, 2015, compared to the year ended December 31, 2014, other income was $3.1 million and included a $2.4 million short-term investment gain related to the valuation of the Pernix warrants.
Year ended December 31, 2014 compared to the year ended December 31, 2013
Revenue: We recognized total revenue of $32.4 million for the year ended December 31, 2014, as compared to total revenue of $10.3 million for the year ended December 31, 2013. The increase in revenue was primarily due to an increase of $7.3 million in amortization of PA licensing revenue from receipt of $15.0 million upfront fee for the PA agreement with Sanofi-Aventis US LLC (“Sanofi US”) and the increase of $14.8 million in VIMOVO royalty. Revenue for the year ended December 31, 2014 consisted of $21.1 million of royalty revenue and $11.3 million of other licensing revenue, compared to $6.3 million of royalty revenue and $4.0 million of other licensing revenue for 2013.
Selling, general and administrative: Selling, general and administrative expenses decreased by $7.1 million to $10.1 million for the year ended December 31, 2014, as compared to the same period in 2013. The decrease was due primarily to lower legal costs due to partial reimbursement of litigation expenses by Horizon, decreased personnel costs, and lower market research and medical affairs costs as compared to the same period in 2013. Selling, general and administrative expenses consisted primarily of the costs of administrative personnel, facility infrastructure, business development expenses, and public company activities.
Research and development: Research and development expenses decreased by $4.2 million to $5.7 million for the year ended December 31, 2014, as compared to the same period in 2013. The decrease was due primarily to a decrease in direct development costs for our PA program including a $1.9 million FDA filing fee and departmental costs, as compared to the same period of 2013. Direct development costs for the PA program decreased by $3.3 million to $3.2 million, primarily due to the completion of the clinical trial activities and other product development activities during the year ended December 31, 2013.
Interest and other (loss) income: Interest and other income was approximately $0.1 million for each of the years ended December 31, 2014 and 2013. Other income for the year ended December 31, 2014 also included short-term investment gains consisting of the investment in warrants with an initial valuation of $2.7 million on August 20, 2014, with a mark-to-market decrease of $0.1 million at December 31, 2014 and a net of $0.4 million related to the disgorgement of short-swing profits arising from trades by a Pozen shareholder under Section 16(b) of the Exchange Act.
Income Taxes
Our effective tax rate was -0.5% for the year ended December 31, 2015 and 0.0% for the years ended December 31, 2014 and 2013. At December 31, 2015 and 2014, Pozen had federal net operating loss carryforwards of approximately $46.3 million and $53.0 million, respectively, state net economic loss carryforwards of approximately $61.1 million and $78.0 million, respectively, foreign net operating loss carryforwards of $24.3 million and $0 million, respectively, and research and development credit carryforwards of approximately $14.5 million and $14.0 million, respectively. The federal and state net operating loss carryforwards begin to expire in 2029 and 2016, respectively, and the research and development credit carryforwards begin to expire in 2018. Pozen’s federal and state net operating loss carryforwards include approximately $7.8 million of excess tax benefits resulting from stock-based compensation exercises and vestings. The tax benefit of these deductions has not been recognized in deferred tax assets. If utilized, the benefits from these deductions will be recorded as adjustments to additional paid-in capital. For financial reporting purposes, a valuation allowance has been recognized to offset the deferred tax assets related to the carryforwards based on Pozen’s assessment regarding the realizability of these deferred tax assets in future periods. Of the total increase in valuation allowance of $2.7 million, an increase of $2.7 million was allocable to current operating activities. The utilization of the loss carryforwards to reduce future income taxes will depend on Pozen’s ability to generate sufficient taxable income prior to the expiration of the loss carryforwards. In addition, the maximum annual use of net operating loss and research credit carryforwards is limited in certain situations where changes occur in stock ownership. The recognized tax benefit related to net operating loss carryforwards was approximately $2.5 million, $4.8 million, and $0 for the years ended December 31, 2015, 2014 and 2013, respectively.
Liquidity and Capital Resources
For the year ended December 31, 2015, net cash used in operating activities totaled $16.8 million compared to cash provided by operating activities of $0.4 million for the year ended December 31, 2014. The increase in cash used in operating activities primarily relates to transaction costs, severance and other merger-related expenses resulting primarily from the Tribute Transaction. For the year ended December 31, 2014 compared to the year ended December 31, 2013, cash provided by operating activities increased $1.2 million primarily related to increase in VIMOVO royalty payments received.
Net cash provided by investing activities during the year ended December 31, 2015 totaled $2.2 million, primarily due to proceeds from the sale of short-term investments. Net cash used in investing activities for December 31, 2014 was less than $0.1 million compared to the net cash provided by investing activities of $18.8 million for the year ended December 31, 2013, which related to proceeds from the sale and maturities of investments. Net cash used in financing activities during the fiscal year ended December 31, 2015 totaled $1.2 million, primarily related to payments made to net settle stock awards partially offset by proceeds from the issuance of common stock. Net cash provided by financing activities during the year ended December 31, 2014 totaled $7.4 million, primarily related to proceeds from the issuance of common stock. Net cash used in financing activities during the year ended December 31, 2013 includes a $53.7 million return of capital to stockholders.
Aralez’s current principal source of liquidity is from cash generated from royalty payments from the legacy Pozen business and the operating income of the legacy Tribute business. Our principal liquidity requirements are for working capital, operational expenses, research and development, capital expenditures and debt service payments.
Subsequent to December 31, 2015, and concurrent with Tribute Transaction, Aralez received $149.2 million of cash proceeds pursuant to the Facility Agreement ($75 million) and Amended and Restated Subscription Agreement, dated December 7, 2015 (the “Subscription Agreement”), among the Company, Pozen, Tribute and various investors ($75 million), net of certain transaction-related costs, and used part of the proceeds to pay off Tribute’s existing debt obligations including accrued interest and termination fees in the amount of $22.5 million.
The Facility Agreement includes a $75 million 6-year convertible note, which was fully drawn and bears an interest rate of 2.5% per annum and is not prepayable. Under the Facility Agreement, Aralez also has a $200 million acquisition facility until April 30, 2017. The acquisition facility is currently undrawn, can be drawn on for permitted acquisitions and is to be repaid over a 6-year period from each draw. Amounts drawn under the acquisition facility will bear an interest rate of 12.5% per annum and shall be prepayable in whole or in part at any time following the end of the sixth month after the funding date of each draw. The Facility Agreement contains affirmative and negative covenants that Aralez believes to be usual and customary. The negative covenants include, among other things, limitations on asset sales, mergers and acquisitions, indebtedness, liens and dividends.
As part of the consideration paid for the Medical Futures Inc. (“MFI”) shares, Tribute issued a one-year unsecured convertible promissory note in the aggregate amount of C$5,000,000, dated June 16, 2015, to the prior owner of MFI (“MFI Note”). The MFI Note bears an interest rate of 8% per annum and is convertible in whole or in part at the holder’s option at any time during the term into Tribute common shares at a conversion rate of C$1.777 per Tribute common share (subject to adjustment in certain events), which equals a conversion rate of C$12.213 per Aralez common shares. If the note is not converted, we will have to repay this loan along with accrued interest at its maturity date of June 16, 2016.
With our cash reserves, funds from the ongoing business and the borrowings and funding of Aralez pursuant to the Tribute Transaction and related financing transactions, we expect our cash and cash equivalents to be sufficient to cover cash needs for working capital and general corporate purposes, planned build of the Aralez sales and marketing team, payment of contractual obligations, interest payments on our indebtedness, principal repayment on the MFI Note, planned capital expenditures and any regulatory and/or sales milestones that may become due. We may need to raise additional capital if we choose to expand our commercialization or development efforts more rapidly than we presently anticipate, if we develop, acquire or in-license additional products or acquire companies or if our revenues do not meet expectations.
Contractual Obligations and Commitments
The following summarizes our contractual obligations as of December 31, 2015, and the expected timing of maturities of those contractual obligations. This table should be read in conjunction with the notes accompanying our consolidated financial statements included elsewhere in this Annual Report on Form 10-K.
|
Payments Due by Period
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than
1 Year
|
1-3
Years
|
3-5
Years
|
More
than
5 Years
|
|||||||||||||||
|
($ in thousands)
|
||||||||||||||||||||
|
Operating leases1
|
$
|
2,675
|
$
|
500
|
$
|
1,004
|
$
|
981
|
$
|
190
|
||||||||||
|
Product development agreements2
|
14,216
|
13,119
|
1,097
|
—
|
—
|
|||||||||||||||
|
Total contractual obligations
|
$
|
16,891
|
$
|
13,619
|
$
|
2,101
|
$
|
981
|
$
|
190
|
||||||||||
| 1 | These commitments are associated with operating leases. Payments due reflect fixed rent expense. |
| 2 | Amounts represent open purchase orders for ongoing commercialization and development activities for our product candidates as of December 31, 2015. These agreements may be terminated by us at any time without incurring a termination fee. |
Off-Balance Sheet Arrangements
As of December 31, 2015, we did not have any material relationships with unconsolidated entities or financial parties, such as entities often referred to as structured finance or variable interest entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes. As such, we are not exposed to any financing, liquidity, market or credit risk that could arise if we had engaged in such relationships.
New Accounting Pronouncements
Taxes
In November 2015, the FASB issued Accounting Standards Update (“ASU”) 2015-17, “Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes.” The new standard requires that deferred tax assets and liabilities be classified as noncurrent in a classified statement of financial position. The guidance is effective for financial statements issued for annual periods beginning after December 15, 2016, and interim periods within those annual periods. Earlier application is permitted for all entities as of the beginning of an interim or annual report period. The amendments in this ASU may be applied either prospectively to all deferred tax assets and liabilities or retrospectively to all periods presented. Pozen adopted this standard as of December 31, 2015 with prospective application. As a result, Pozen reclassified its deferred tax assets classified as current to noncurrent and its deferred tax liabilities classified as current to noncurrent in its December 31, 2015 consolidated balance sheet and related disclosures. Prior balance sheets were not retrospectively adjusted.
Business Combinations
In September 2015, the FASB issued ASU 2015-16, “Business Combinations (Topic 805): Simplifying the Accounting for Measurement-Period Adjustments.” The new guidance eliminates the requirements to restate prior period financial statements for measurement-period adjustments, and requires that the cumulative impact of a measurement period adjustment (including the impact on prior periods) be recognized in the reporting period in which the adjustment is identified. The new standard, which should be applied prospectively to measurement period adjustments that occur after the effective date, is effective for Aralez in the first quarter of 2016. We do not expect the adoption of the new accounting rules to have a material impact on our financial condition, results of operations or cash flows.
Debt Issuance Costs
In April 2015, the FASB issued ASU 2015-03, “Interest - Imputation of Interest (Topic 835-30): Simplifying the Presentation of Debt Issuance Costs” (“ASU 2015-03”). ASU 2015-03 requires debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the related debt liability’s carrying value, which is consistent with the presentation of debt discounts. ASU 2015-03 is effective for financial statements issued for years beginning after December 15, 2015. We do not expect the adoption of the new accounting rules to have a material impact on our financial condition, results of operations or cash flows.
Revenue from Contracts with Customers
In May 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers (Topic 606),” which requires revenue recognition based on the transfer of promised goods or services to customers in an amount that reflects consideration Aralez expects to be entitled to in exchange for goods or services. In August 2015, the FASB issued updated guidance deferring the effective date of the revenue recognition standard. The new rules supersede prior revenue recognition requirements and most industry-specific accounting guidance. The new rules will be effective for Aralez in the first quarter of 2018, with either full retrospective or modified retrospective application required. We have not yet selected a transition method and are evaluating the impact of the ASU on our financial statements.
Our cash on hand is invested in bank deposits and money market funds that invest primarily in short-term, highly-rated investments, including U.S. government securities, commercial paper and certificates of deposit guaranteed by banks and short-term corporate fixed income obligations and U.S. government and government agency obligations. Under our current policies, we do not use interest rate derivative instruments to manage our exposure to interest rate changes. Due to the short-term maturities of our investments, we do not believe that a decrease in market rates would have a significant negative impact on the value of our investment portfolio.
Aralez will face market risks attributable to fluctuations in foreign currency exchange rates and foreign currency exposure on the translation into U.S. dollars of the financial results of our operations in Canada and Europe. Both favorable and unfavorable foreign currency impacts to our foreign currency-denominated operating expenses are mitigated to a certain extent by the natural, opposite impact on our foreign currency-denominated revenue.
The financial information required by Item 8 is contained in Part IV, Item 15 of this Annual Report on Form 10-K.
None.
Evaluation of Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures designed to ensure information required to be disclosed in Company reports filed under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures are designed to provide reasonable assurance that information required to be disclosed in Company reports filed under the Exchange Act is accumulated and communicated to management, including the Company’s chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Our management, with the participation of our chief executive officer and chief financial officer, evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Annual Report on Form 10-K. Based on that evaluation, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K are functioning effectively to provide reasonable assurance that the information required to be disclosed by us in reports filed under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate to allow timely decisions regarding disclosures. A controls system, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
Management’s Annual Report on Internal Control over Financial Reporting
Our management’s report on internal control over financial reporting procedures (as defined in Rule 13a-15(f) under the Exchange Act) is included with the financial statements reflected in Part IV, Item 15 of this Annual Report on Form 10-K and is incorporated herein by reference.
Changes in Internal Control over Financial Reporting
There were no changes in the Company’s internal control over financial reporting for the quarterly period or year ended December 31, 2015 identified in connection with the evaluation required by Rules 13a-15(e) and 15d-15(e) of the Exchange Act that materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
None.
PART III
Information required under this item relating to our board of directors, executive officers and corporate governance will be included in our definitive proxy statement for the 2016 Annual Meeting of Stockholders, to be filed with the SEC within 120 days after the end of the year ended December 31, 2015 (the “2016 Proxy Statement”), and such required information is incorporated herein by reference.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics, which applies to our directors and employees (including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions). The text of our Code of Business Conduct and Ethics is posted in the “Corporate Governance” section of our website, www.aralez.com. A copy of the Code of Business Conduct and Ethics can be obtained free of charge on our website. We intend to disclose on our website any amendments to, or waivers from, our Code of Business Conduct and Ethics that are required to be disclosed pursuant to the rules of the SEC and the NASDAQ Global Market.
Information required under this item relating to executive compensation is incorporated herein by reference from information included in the 2016 Proxy Statement.
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information required under this item relating to securities authorized for issuance under equity compensation plans and to security ownership of certain beneficial owners and management is incorporated herein by reference from information included in the 2016 Proxy Statement.
Information required under this item relating to certain relationships and transactions with related parties and about director independence is incorporated herein by reference from information included in the 2016 Proxy Statement.
Information required under this item relating to the fees for professional services rendered by our independent accountants in 2015 and 2014 is incorporated herein by reference from information included in the 2016 Proxy Statement.
PART IV
(a) Financial Statements
See accompanying index to Financial Statements.
(b) Financial Statement Schedules
All schedules have been omitted because the required information is included in the financial statements or the notes thereto, or is not applicable.
(c) Index to Exhibits
The following exhibits are filed or incorporated by reference as part of this Annual Report on Form 10-K:
|
Exhibit
Number
|
Exhibit Title
|
|
|
2.1
|
Agreement and Plan of Merger and Arrangement, dated as of June 8, 2015, by and among Tribute Pharmaceuticals Canada Inc., Aguono Limited, Trafwell Limited, ARLZ US Acquisition Corp., ARLZ CA Acquisition Corp. and POZEN Inc. (incorporated by reference to Exhibit 2.1 to POZEN Inc.’s Current Report on Form 8-K filed June 11, 2015).
|
|
|
2.2
|
Amendment No. 1 to the Agreement and Plan of Merger and Arrangement, dated as of August 19, 2015, by and among Tribute Pharmaceuticals Canada Inc., Aralez Pharmaceuticals Limited (formerly Aguono Limited), Trafwell Limited, ARLZ US Acquisition Corp., ARLZ CA Acquisition Corp., ARLZ US Acquisition II Corp. and POZEN Inc. (incorporated by reference to Exhibit 2.1 to POZEN Inc.’s Current Report on Form 8-K filed December 8, 2015).
|
|
|
2.3
|
Amendment No. 2 to the Agreement and Plan of Merger and Arrangement, dated as of December 7, 2015, by and among Tribute Pharmaceuticals Canada Inc., Aralez Pharmaceuticals plc (formerly Aguono Limited), Aralez Pharmaceuticals Inc., Aralez Pharmaceuticals Holdings Limited, ARLZ US Acquisition II Corp., ARLZ CA Acquisition Corp. and POZEN Inc. (incorporated by reference to Exhibit 2.2 to POZEN Inc.’s Current Report on Form 8-K filed December 8, 2015).
|
|
|
2.4
|
Asset Purchase Agreement, dated October 2, 2014, by and among Novartis AG, Novartis Pharma AG and Tribute Pharmaceuticals Canada Inc. (incorporated by reference to Exhibit 2.1 to Tribute Pharmaceuticals’ Current Report on Form 8-K filed October 8, 2014).†
|
|
|
2.5
|
License Agreement, dated as of October 2, 2014, by and among Novartis AG, Novartis Pharma AG, Novartis Pharmaceuticals Canada Inc. and Tribute Pharmaceuticals Canada Inc. (incorporated by reference to Exhibit 2.2 to Tribute Pharmaceuticals’ Current Report on Form 8-K filed October 8, 2014).†
|
|
|
2.6
|
Share Purchase Agreement, dated as of June 16, 2015, by and among Tribute Pharmaceuticals Canada Inc. and the shareholders of Medical Futures Inc. (incorporated by reference to Exhibit 1.1 to Tribute Pharmaceuticals’ Current Report on Form 8-K filed June 22, 2015).†
|
|
|
2.7
|
Asset Purchase Agreement, dated as of May 21, 2015, by and among Tribute Pharmaceuticals Canada Inc., Mutual Pharmaceutical Company, Inc. and Sun Pharmaceutical Industries, Inc. (incorporated by reference to Exhibit 1.1 to Tribute Pharmaceuticals’ Current Report on Form 8-K filed May 28, 2015).†
|
|
3.1
|
Certificate of Incorporation of Aralez Pharmaceuticals Inc., dated as of December 2, 2015 (incorporated by reference to Exhibit 3.1 to the Registrant’s Registration Statement on Form S-4 filed December 14, 2015).
|
|
|
3.2
|
Articles of Aralez Pharmaceuticals Inc., dated as of December 11, 2015 (incorporated by reference to Exhibit 3.2 to the Registrant’s Registration Statement on Form S-4 filed December 14, 2015).
|
|
|
4.1
|
Promissory Note issued by Tribute Pharmaceuticals Canada Inc. on June 16, 2015 (incorporated by reference to Exhibit 4.1 to Tribute Pharmaceuticals’ Current Report on Form 8-K filed June 22, 2015).†
|
|
|
10.1
|
Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed February 5, 2016).+
|
|
|
10.2
|
Form of Substitute Option Agreement for U.S. Tribute Optionees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.2 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+
|
|
|
10.3
|
Form of Substitute Option Agreement for Canadian Tribute Optionees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.3 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+
|
|
|
10.4
|
Form of Nonqualified Stock Option Award Agreement for U.S. Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.4 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+
|
|
|
10.5
|
Form of Nonqualified Stock Option Award Agreement for Canadian Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.5 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+
|
|
|
10.6
|
Form of Nonqualified Stock Option Award Agreement for U.S. Directors under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.6 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+
|
|
|
10.7
|
Form of Nonqualified Stock Option Award Agreement for Canadian Directors under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.7 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+
|
|
|
10.8
|
Form Restricted Stock Unit Award Agreement for U.S. Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.8 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+
|
|
|
10.9
|
Form of Restricted Stock Unit Award Agreement for Canadian Employees under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.9 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+
|
|
|
10.10
|
Form of Restricted Stock Unit Award Agreement for U.S. Directors under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.10 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+
|
|
|
10.11
|
Form of Restricted Stock Unit Award Agreement for Canadian Directors under the Aralez Pharmaceuticals Inc. 2016 Long-Term Incentive Plan (incorporated by reference to Exhibit 99.11 to the Registrant’s Registration Statement on Form S-8 filed February 8, 2016).+
|
|
|
10.12
|
Restricted Stock Unit Agreement dated May 4, 2004 between POZEN Inc. and John R. Plachetka (incorporated by reference to Exhibit 10.4 to POZEN Inc.’s Quarterly Report on Form 10-Q filed July 30, 2004).+
|
|
10.13
|
|
First Amendment, dated September 28, 2007, to Restricted Stock Unit Agreement, dated May 4, 2004, between POZEN Inc. and John R. Plachetka (incorporated by reference to Exhibit 10.2 to POZEN Inc.’s Quarterly Report on Form 10-Q filed November 5, 2007).+
|
|
|
|
|
|
10.14
|
|
Long-Term Cash Incentive Award Agreement between POZEN Inc. and John R. Plachetka dated February 14, 2007 (incorporated by reference to Exhibit 10.4 to POZEN Inc.’s Quarterly Report on Form 10-Q filed May 3, 2007).+
|
|
|
|
|
|
10.15
|
Second Amended and Restated Facility Agreement, dated as of December 7, 2015, among Aralez Pharmaceuticals Inc., POZEN Inc., Tribute Pharmaceuticals Canada Inc., Deerfield Private Design Fund III, L.P., Deerfield International Master Fund, L.P., and Deerfield Partners, L.P. (incorporated by reference to Exhibit 10.1 to POZEN Inc.’s Current Report on Form 8-K filed December 8, 2015).
|
|
|
10.16
|
Form of Senior Secured Convertible Note issued by Aralez Pharmaceuticals Inc. (incorporated by reference to Exhibit 10.2 to the Registrant’s Registration Statement on Form S-1 filed December 31, 2015).
|
|
|
|
|
|
|
10.17
|
Second Amended and Restated Registration Rights Agreement, dated as of December 7, 2015, among Aralez Pharmaceuticals Inc., Deerfield Private Design Fund III, L.P., Deerfield International Master Fund, L.P., and Deerfield Partners, L.P. (incorporated by reference to Exhibit 10.2 to POZEN Inc.’s Current Report on Form 8-K filed December 8, 2015).
|
|
|
|
|
|
|
10.18
|
Amended and Restated Share Subscription Agreement, dated as of December 7, 2015, among Aralez Pharmaceuticals Inc., Aralez Pharmaceuticals plc, POZEN Inc., Tribute Pharmaceuticals Canada Inc., QLT Inc., Deerfield Private Design Fund III, L.P., Deerfield International Master Fund, L.P., Deerfield Partners, L.P., Broadfin Healthcare Master Fund Ltd., JW Partners, LP, JW Opportunities Fund, LLC, and JW Opportunities Master Fund, Ltd. (incorporated by reference to Exhibit 10.3 to POZEN Inc.’s Current Report on Form 8-K filed December 8, 2015).
|
|
|
|
|
|
|
10.19
|
Executive Employment Agreement between POZEN Inc. and Adrian Adams dated May 31, 2015 (incorporated by reference to Exhibit 10.3 to POZEN Inc.’s Current Report on Form 8-K filed June 3, 2015).+
|
|
|
|
|
|
|
10.20
|
Executive Employment Agreement between POZEN Inc. and Andrew I. Koven dated May 31, 2015 (incorporated by reference to Exhibit 10.4 to POZEN Inc.’s Current Report on Form 8-K filed June 3, 2015).+
|
|
|
|
|
|
|
10.21
|
Executive Employment Agreement between POZEN Inc. and Mark A. Glickman dated June 22, 2015 (incorporated by reference to Exhibit 10.7 to POZEN Inc.’s Quarterly Report on Form 10-Q filed August 10, 2015).+
|
|
|
|
|
|
|
10.22
|
Executive Employment Agreement between POZEN Inc. and Eric L. Trachtenberg dated June 22, 2015 (incorporated by reference to Exhibit 10.8 to POZEN Inc.’s Quarterly Report on Form 10-Q filed August 10, 2015).+
|
|
|
|
|
|
|
10.23
|
Executive Employment Agreement between POZEN Inc. and Jennifer L. Armstrong dated June 22, 2015 (incorporated by reference to Exhibit 10.9 to POZEN Inc.’s Quarterly Report on Form 10-Q filed August 10, 2015).+
|
|
|
|
|
|
|
10.24
|
Executive Employment Agreement between POZEN Inc. and Scott J. Charles dated July 27, 2015 (incorporated by reference to Exhibit 10.10 to POZEN Inc.’s Quarterly Report on Form 10-Q filed August 10, 2015).+
|
|
|
|
|
|
|
10.25
|
Executive Employment Agreement between POZEN Inc. and James P. Tursi, MD, dated September 11, 2015 (incorporated by reference to Exhibit 10.3 to POZEN Inc.’s Quarterly Report on Form 10-Q filed November 9, 2015).+
|
|
|
|
|
|
|
10.26
|
Form of POZEN Inc. Retention Agreement (incorporated by reference to Exhibit 10.2 to POZEN Inc.’s Current Report on Form 8-K filed June 25, 2015).+
|
|
|
|
|
|
|
10.27
|
|
Separation and General Release Agreement between POZEN Inc. and John R. Plachetka, dated May 29, 2015 (incorporated by reference to Exhibit 10.1 to POZEN Inc.’s Current Report on Form 8-K filed June 3, 2015).+
|
|
|
|
|
|
10.28
|
POZEN Inc. Employee Severance Plan and Summary Plan Description (incorporated by reference to Exhibit 10.1 to POZEN Inc.’s Current Report on Form 8-K filed June 25, 2015).+
|
|
|
|
|
|
|
10.29
|
|
Executive Employment Agreement between POZEN Inc. and William L. Hodges dated August 3, 2004 (incorporated by reference to Exhibit 10.1 to POZEN Inc.’s Quarterly Report on Form 10-Q filed October 27, 2004).+
|
|
|
|
|
|
10.30
|
First Amendment to Executive Employment Agreement between POZEN Inc. and William L. Hodges, dated September 28, 2007 (incorporated by reference to Exhibit 10.5 to POZEN Inc.’s Quarterly Report on Form 10-Q filed November 5, 2007).+
|
|
10.31
|
Manufacturing Services Agreement, dated as of December 19, 2011, by and between POZEN Inc. and Patheon Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.38 to Amendment No.1 to POZEN Inc.’s Annual Report on Form 10-K, filed June 29, 2012).†
|
|
|
10.32
|
Capital Expenditure and Equipment Agreement, dated as of December 19, 2011, by and between POZEN Inc. and Patheon Pharmaceuticals, Inc. (incorporated by reference to Exhibit 10.39 to Amendment No.1 to POZEN Inc.’s Annual Report on Form 10-K, filed June 29, 2012).
|
|
|
10.33
|
First Amendment to Manufacturing Services Agreement, between Patheon Pharmaceuticals Inc., and POZEN Inc., dated as of July 10, 2013 (incorporated by reference to Exhibit 10.2 to POZEN Inc.’s Quarterly Report on Form 10-Q, filed August 7, 2013).†
|
|
|
10.34
|
First Amendment to Capital Expenditure and Equipment Agreement, between Patheon Pharmaceuticals Inc., and POZEN Inc., dated as of July 10, 2013 (incorporated by reference to Exhibit 10.1 to POZEN Inc.’s Quarterly Report on Form 10-Q, filed August 7, 2013).†
|
|
|
10.35
|
Letter Agreement among POZEN Inc., AstraZeneca AB and Horizon Pharma U.S.A. Inc., dated as of November 18, 2013 (incorporated by reference to Exhibit 10.43 to POZEN Inc.’s Annual Report on Form 10-K, filed March 6, 2014).†
|
|
|
10.36
|
Amended and Restated Collaboration and License Agreement for the United States by and between POZEN Inc. and AstraZeneca AB, dated as of November 18, 2013 (incorporated by reference to Exhibit 10.45 to POZEN Inc.’s Annual Report on Form 10-K, filed March 6, 2014).†
|
|
|
10.37
|
Amendment No. 1 to the Amended and Restated Collaboration and License Agreement for the United States by and between POZEN Inc. and Horizon Pharma U.S.A. Inc., dated as of November 18, 2013 (incorporated by reference to Exhibit 10.44 to POZEN Inc.’s Annual Report on Form 10-K, filed March 6, 2014).†
|
|
|
10.38
|
Amended and Restated Collaboration and License Agreement for outside of the United States by and between POZEN Inc. and AstraZeneca AB, dated as of November 18, 2013 (incorporated by reference to Exhibit 10.46 to POZEN Inc.’s Annual Report on Form 10-K, filed March 6, 2014).†
|
|
|
List of subsidiaries of the Registrant (filed herewith, Exhibit 21.1).
|
||
|
Consent of Ernst & Young LLP, independent registered public accounting firm (filed herewith, Exhibit 23.1).
|
||
|
Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith, Exhibit 31.1).
|
||
|
Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 (filed herewith, Exhibit 31.2).
|
||
|
Certification of the Chief Executive Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (filed herewith, Exhibit 32.1).
|
||
|
Certification of the Chief Financial Officer pursuant to 18 U.S.C. 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (filed herewith, Exhibit 32.2).
|
||
|
101
|
The following materials from Aralez Pharmaceuticals Inc.’s Annual Report on Form 10-K for the year ended December 31, 2015, formatted in Extensible Business Reporting Language (XBRL): (i) Aralez Pharmaceuticals Inc. Balance Sheet at December 31, 2015, (ii) Aralez Pharmaceuticals Inc. Notes to Balance Sheet, (iii) POZEN Inc. Consolidated Balance Sheets at December 31, 2015 and December 31, 2014, (iv) POZEN Inc. Consolidated Statements of Comprehensive (Loss) Income for the years ended December 31, 2015, December 31, 2014 and December 31, 2013, (v) POZEN Inc. Consolidated Statements of Stockholders’ Equity at December 31, 2015, December 31, 2014 and December 31, 2013, (vi) POZEN Inc. Consolidated Statements of Cash Flows for the years ended December 31, 2015, December 31, 2014 and December 31, 2013, and (vii) POZEN Inc. Notes to Consolidated Financial Statements.
|
+Compensation Related Contract.
†Confidential treatment requested. Confidential materials omitted and filed separately with Securities and Exchange Commission.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Registrant:
|
||||
|
Aralez Pharmaceuticals Inc.
|
||||
|
Date:
|
March 15, 2016
|
By:
|
/s/ Adrian Adams
|
|
|
Adrian Adams
|
||||
|
Chief Executive Officer
|
||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
||
|
/s/ Adrian Adams
|
Chief Executive Officer (Principal Executive Officer), Director
|
March 15, 2016
|
||
|
Adrian Adams
|
||||
|
/s/ Scott J. Charles
|
Chief Financial Officer (Principal Financial Officer)
|
March 15, 2016
|
||
|
Scott J. Charles
|
||||
|
/s/ John Barnhardt
|
Principal Accounting Officer
|
March 15, 2016
|
||
|
John Barnhardt
|
||||
|
/s/ Arthur S. Kirsch
|
Director
|
March 15, 2016
|
||
|
Arthur S. Kirsch
|
||||
|
/s/ Neal F. Fowler
|
Director
|
March 15, 2016
|
||
|
Neal F. Fowler
|
||||
|
/s/ Seth A. Rudnick, M.D.
|
Director
|
March 15, 2016
|
||
|
Seth A. Rudnick, M.D.
|
||||
|
/s/ Kenneth B. Lee, Jr.
|
Director
|
March 15, 2016
|
||
|
Kenneth B. Lee, Jr.
|
||||
|
/s/ Rob Harris
|
Director
|
March 15, 2016
|
||
|
Rob Harris
|
||||
|
/s/ F. Martin Thrasher
|
Director
|
March 15, 2016
|
||
|
F. Martin Thrasher
|
||||
|
/s/ Jason M. Aryeh
|
Director
|
March 15, 2016
|
||
|
Jason M. Aryeh
|
ARALEZ PHARMACEUTICALS INC.
INDEX TO FINANCIAL STATEMENTS
|
Management’s Report on Internal Control Over Financial Reporting
|
F-2
|
|
Report of Independent Registered Public Accounting Firm
|
F-3
|
|
Report of Independent Registered Public Accounting Firm
|
F-4
|
|
Balance Sheet
|
F-5
|
|
Notes to Balance Sheet
|
F-6
|
Management’s Report on Internal Control Over Financial Reporting
Management of Aralez Pharmaceuticals Inc. (the “Company”) is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting, as defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934, is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Further, because of changes in conditions, effectiveness of internal control over financial reporting may vary over time. Management, with the participation of the Chief Executive Officer and Chief Financial Officer, evaluated the Company’s internal control over financial reporting as of December 31, 2015. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013 Framework) (COSO). As a result of this assessment and based on the criteria in the COSO framework, management has concluded that, as of December 31, 2015, the Company’s internal control over financial reporting was effective.
Ernst & Young LLP, the independent registered public accounting firm that audited the Company’s financial statement included in this Annual Report on Form 10-K, has issued an attestation report on the Company’s internal control over financial reporting, which is included herein.
|
/s/ Adrian Adams
|
/s/ Scott J. Charles
|
|||
|
Chief Executive Officer
|
Chief Financial Officer
|
|||
|
March 15, 2016
|
March 15, 2016
|
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Aralez Pharmaceuticals Inc.
We have audited the accompanying balance sheet of Aralez Pharmaceuticals Inc. as of December 31, 2015. This balance sheet is the responsibility of the Company’s management. Our responsibility is to express an opinion on this balance sheet based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the balance sheet referred to above presents fairly, in all material respects, the financial position of Aralez Pharmaceuticals Inc. at December 31, 2015 in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Aralez Pharmaceuticals Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated March 15, 2016 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
|
Raleigh, North Carolina
|
|
|
March 15, 2016
|
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Aralez Pharmaceuticals Inc.
We have audited Aralez Pharmaceuticals Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Aralez Pharmaceuticals Inc. management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Aralez Pharmaceuticals Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheet of Aralez Pharmaceuticals Inc. as of December 31, 2015 and our report dated March 15, 2016 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
|
Raleigh, North Carolina
|
|
|
March 15, 2016
|
ARALEZ PHARMACEUTICALS INC.
BALANCE SHEET
|
December 31,
|
||||
|
2015
|
||||
|
STOCKHOLDERS’ EQUITY
|
||||
|
Common shares, no par value, no maximum shares authorized; 1 share issued and outstanding at December 31, 2015
|
||||
|
Additional paid-in capital
|
$
|
1
|
||
|
Common share receivable
|
(1
|
)
|
||
|
Total shareholders’ equity
|
$
|
–
|
||
See accompanying Notes to Balance Sheet.
ARALEZ PHARMACEUTICALS INC.
NOTES TO BALANCE SHEET
| 1. | Organization |
Aralez Pharmaceuticals Inc. (“Aralez” or the “Company”) was formed on December 2, 2015, under the Business Corporations Act (British Columbia). Aralez was formed for the purpose of facilitating the business combination of POZEN Inc., a Delaware corporation (“Pozen”), and Tribute Pharmaceuticals Canada Inc., a corporation incorporated under the laws of the Province of Ontario, Canada (“Tribute”), which business combination was consummated on February 5, 2016. At inception, one common share, no par value was issued to Incatel Ltd., an entity established by Canyon CTS, a corporate and trust services company, to hold the share until completion of the proposed business combination. Prior to the consummation of the transaction, Aralez conducted no business other than business incident to such transaction.
On June 8, 2015, Pozen and Tribute agreed to a business combination under the terms of the Agreement and Plan of Merger and Arrangement, among Tribute, Aguono Limited (which was renamed Aralez Pharmaceuticals Limited and subsequently renamed Aralez Pharmaceuticals plc prior to its re-registration as a public limited company) (“Aralez Ireland”), Aralez Pharmaceuticals Holdings Limited (formerly known as Trafwell Limited) (“Holdings”), ARLZ US Acquisition Corp., ARLZ CA Acquisition Corp. (“Can Merger Sub”), and Pozen, dated as of June 8, 2015 (the “Original Merger Agreement”). On August 19, 2015, the parties amended the Original Merger Agreement pursuant to Amendment No. 1 to Agreement and Plan of Merger and Arrangement (“Amendment No. 1 to the Original Merger Agreement”), whereby ARLZ US Acquisition II Corp. was formed to replace ARLZ US Acquisition Corp. in order to optimize the corporate structure of Aralez in the future.
On December 7, 2015, the parties amended the Original Merger Agreement pursuant to Amendment No. 2 to Agreement and Plan of Merger and Arrangement (“Amendment No. 2 to the Original Merger Agreement” and, together with the Original Merger Agreement and Amendment No. 1 to the Original Merger Agreement, the “Merger Agreement”), pursuant to which, among other things, (i) the Company replaced Aralez Ireland as a party to the Merger Agreement, whereby, after giving effect to the transactions, the Company would be the ultimate parent company of the combined companies, (ii) ARLZ US Acquisition II Corp., a corporation formed under the laws of the State of Delaware, would be merged with and into Pozen, with Pozen continuing as the surviving corporation and an indirect wholly-owned subsidiary of the Company (the “Merger”), and (iii) Can Merger Sub and Tribute would amalgamate, with the separate legal existence of Can Merger Sub ceasing and Tribute and Can Merger Sub continuing as one corporation and as a wholly-owned subsidiary of the Company (the “Arrangement,” and together with the Merger, the “Tribute Transaction”).
As a result of the Merger, each share of Pozen common stock was converted into the right to receive one common share of Aralez (“Aralez Shares”), without par value. Pursuant to the Arrangement, each outstanding Tribute common share was exchanged for 0.1455 Aralez Shares.
On December 7, 2015, the Company executed the Amended and Restated Subscription Agreement (the “Subscription Agreement”) among the Company, Pozen, Tribute and various investors. Pursuant to the Subscription Agreement, immediately prior to the consummation of the Tribute Transaction, Tribute sold to the various investors up to $75 million of Tribute common shares in a private placement at a purchase price per share equal to (a) the lesser of (i) $7.20 and (ii) a 5% discount off the five-day volume weighted average price per share of Pozen common stock calculated over the five trading days immediately preceding the date of closing of the transactions, not to be less than $6.25, multiplied by (b) 0.1455 (the “Equity Price”). Based on the Equity Price of $6.25, the number of Tribute common shares sold to the various investors was 82,474,229 shares, which equaled 12,000,000 Aralez Shares based on the exchange ratio.
On December 7, 2015, the Company executed a Second Amended and Restated Facility Agreement (the “Facility Agreement”), among the Company, Pozen, Tribute and certain lenders. Pursuant to the Facility Agreement, Tribute may borrow from the lenders up to an aggregate principal amount of $275 million, of which (i) $75 million will be in the form of a 2.5% senior secured convertible promissory note due six years from issuance and convertible into Tribute shares (the “Convertible Notes”) at a conversion price equal to a 32.5% premium over the product of the Pozen Purchase Price (as defined in the Convertible Notes), multiplied by 0.1455, issued and sold by Tribute to the lenders, upon the terms and conditions of the Facility Agreement, and (ii) up to an aggregate principal amount of $200 million, which will be made available for permitted acquisitions (as defined in the Facility Agreement) and will be in the form of Secured Promissory Notes issued and sold by Aralez to the lenders (the “Acquisition Notes”), evidencing the Acquisition Loans (as defined in the First Amended and Restated Facility Agreement), upon the terms and conditions and subject to the limitations set forth in the Acquisition Notes, all subject to the terms and conditions of the Facility Agreement. In connection with the consummation of the Tribute Transaction, the Company’s obligations under the Convertible Notes were made effective, and the Convertible Notes were exchanged for convertible notes of Aralez (the “Aralez Convertible Notes”), which were convertible into Aralez Shares at a conversion price equal to a 32.5% premium over the Pozen Purchase Price (as defined in Amendment No. 2 to the Original Merger Agreement). The Aralez Convertible Notes are secured by the assets of Aralez and its subsidiaries. The Aralez Convertible Notes will thereafter be convertible into Aralez Shares.
Aralez’s registered office is located at 666 Burrard Street, Suite 1700, Vancouver, British Columbia, V6C 2X8. Aralez’s global headquarters is located in Ontario, Canada, its U.S. headquarters is in New York, New York and its Irish headquarters is located in Dublin, Ireland.
| 2. | Basis of Presentation |
The Company’s balance sheet has been prepared in accordance with U.S. generally accepted accounting principles. Separate statements of operations, cash flows, and changes in shareholders’ equity and comprehensive income have not been presented because the Company has had no operations as of December 31, 2015, other than business incident to the Tribute Transaction. The Company’s year-end is December 31st.
| 3. | Stockholders’ Equity |
The Company has no maximum authorized number of common shares issuable, and the common shares have no par value. One common share has been issued and is outstanding as of December 31, 2015.
| 4. | Subsequent Events (Unaudited) |
On February 5, 2016, Aralez announced the completion of the Tribute Transaction following the approval of the transaction by stockholders of Pozen and shareholders of Tribute. In connection with the transaction, Pozen and Tribute were combined under and became subsidiaries of Aralez, with Pozen treated as the acquiring company for accounting purposes.
The combined company will operate under Aralez, a global specialty pharmaceutical company with operations in Canada, Ireland and the United States. Under the terms of the Agreement and Plan of Merger and Arrangement, each share of Pozen common stock has been converted into the right to receive one Aralez common share and each common share of Tribute (other than dissenting shares) has been exchanged for 0.1455 Aralez common shares.
The preliminary fair value of consideration transferred as of the acquisition date of February 5, 2016 is approximately $138 million made up of (i) $115 million related to Tribute shares, equity awards and certain warrants outstanding and (ii) $23 million in repayments of Tribute indebtedness. We have not provided an allocation of the preliminary purchase price as the initial accounting for the business combination is incomplete.
Concurrent with the transaction, on February 5, 2016, the Company closed the Subscription Agreements discussed above.
POZEN INC.
INDEX TO FINANCIAL STATEMENTS
|
Management’s Report on Internal Control Over Financial Reporting
|
F-10
|
|
Report of Independent Registered Public Accounting Firm
|
F-11
|
|
Report of Independent Registered Public Accounting Firm
|
F-12
|
|
Audited Financial Statements:
|
|
|
Consolidated Balance Sheets
|
F-13
|
|
Consolidated Statements of Comprehensive (Loss) Income
|
F-14
|
|
Consolidated Statements of Stockholders’ Equity
|
F-15
|
|
Consolidated Statements of Cash Flows
|
F-16
|
|
Notes to Consolidated Financial Statements
|
F-17
|
Management’s Report on Internal Control Over Financial Reporting
Management of POZEN Inc. (Pozen) is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting, as defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934, is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of Pozen; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of Pozen are being made only in accordance with authorizations of management and directors of Pozen; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of Pozen’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Further, because of changes in conditions, effectiveness of internal control over financial reporting may vary over time. Management, with the participation of the Chief Executive Officer and Chief Financial Officer, evaluated Pozen’s internal control over financial reporting as of December 31, 2015. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated Framework (2013 Framework) (COSO). As a result of this assessment and based on the criteria in the COSO framework, management has concluded that, as of December 31, 2015, Pozen’s internal control over financial reporting was effective.
Ernst & Young LLP, the independent registered public accounting firm that audited Pozen’s financial statements included in this Annual Report on Form 10-K, has issued an attestation report on Pozen’s internal control over financial reporting, which is included herein.
|
/s/ Adrian Adams
|
/s/ Scott J. Charles
|
|||
|
Chief Executive Officer
|
Chief Financial Officer
|
|||
|
March 15, 2016
|
March 15, 2016
|
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders of Aralez Pharmaceuticals Inc.
We have audited the consolidated balance sheets of POZEN Inc. as of December 31, 2015 and 2014, and the related consolidated statements of comprehensive (loss) income, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2015. These financial statements are the responsibility of Pozen’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of POZEN Inc. at December 31, 2015 and 2014, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2015, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), POZEN Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 15, 2016 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
|
|
|
|
Raleigh, North Carolina
|
|
|
March 15, 2016
|
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of Aralez Pharmaceuticals Inc.
We have audited POZEN Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). POZEN Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on Pozen’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of Pozen; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of Pozen are being made only in accordance with authorizations of management and directors of Pozen; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of Pozen’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, POZEN Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of POZEN Inc. as of December 31, 2015 and 2014 and the related statements of comprehensive (loss) income, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2015 and our report March 15, 2016 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
|
|
|
|
Raleigh, North Carolina
|
|
|
March 15, 2016
|
POZEN INC.
CONSOLIDATED BALANCE SHEETS
|
December 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
24,815,669
|
$
|
40,582,415
|
||||
|
Investments in warrants
|
—
|
2,678,773
|
||||||
|
Accounts receivable
|
5,965,915
|
5,629,209
|
||||||
|
Prepaid expenses and other current assets
|
1,224,746
|
583,061
|
||||||
|
Property and equipment, net of accumulated depreciation
|
251,291
|
27,382
|
||||||
|
Noncurrent deferred tax asset
|
—
|
952,900
|
||||||
|
Total assets
|
$
|
32,257,621
|
$
|
50,453,740
|
||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
4,557,094
|
$
|
606,948
|
||||
|
Accrued compensation
|
5,228,853
|
1,899,935
|
||||||
|
Accrued expenses
|
6,703,016
|
253,145
|
||||||
|
Current deferred tax liability
|
—
|
952,900
|
||||||
|
Total current liabilities
|
16,488,963
|
3,712,928
|
||||||
|
Noncurrent accrued severance
|
985,415
|
—
|
||||||
|
Total liabilities
|
17,474,378
|
3,712,928
|
||||||
|
Commitments and Contingencies
|
||||||||
|
Preferred stock, $0.001 par value; 10,000,000 shares authorized, issuable in series, of which 90,000 shares are designated Series A Junior Participating Preferred Stock, none outstanding
|
—
|
—
|
||||||
|
Common stock, $0.001 par value, 90,000,000 shares authorized; 33,259,407 and 32,221,397 shares issued and outstanding at December 31, 2015 and December 31, 2014, respectively
|
33,259
|
32,221
|
||||||
|
Additional paid-in capital
|
149,437,862
|
143,613,024
|
||||||
|
Accumulated deficit
|
(134,687,878
|
)
|
(96,904,433
|
)
|
||||
|
Total stockholders’ equity
|
14,783,243
|
46,740,812
|
||||||
|
Total liabilities and stockholders’ equity
|
$
|
32,257,621
|
$
|
50,453,740
|
||||
See accompanying Notes to Consolidated Financial Statements.
POZEN INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE (LOSS) INCOME
|
For the Years Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Royalty and licensing revenue:
|
$
|
21,391,414
|
$
|
32,394,232
|
$
|
10,322,000
|
||||||
|
Operating expenses:
|
||||||||||||
|
Selling, general and administrative
|
50,344,952
|
10,078,771
|
17,160,810
|
|||||||||
|
Research and development
|
8,512,509
|
5,739,848
|
9,945,049
|
|||||||||
|
Total operating expenses
|
58,857,461
|
15,818,619
|
27,105,859
|
|||||||||
|
Interest and other (loss) income
|
(143,011
|
)
|
3,099,119
|
75,560
|
||||||||
|
(Loss) income before income tax expense
|
(37,609,058
|
)
|
19,674,732
|
(16,708,299
|
)
|
|||||||
|
Income tax expense
|
174,387
|
—
|
—
|
|||||||||
|
Net (loss) income
|
(37,783,445
|
)
|
19,674,732
|
(16,708,299
|
)
|
|||||||
|
Change in unrealized gains on marketable securities
|
―
|
―
|
3,253
|
|||||||||
|
Comprehensive (loss) income
|
$
|
(37,783,445
|
)
|
$
|
19,674,732
|
$
|
(16,705,046
|
)
|
||||
|
Basic net (loss) income per common share
|
$
|
(1.16
|
)
|
$
|
0.63
|
$
|
(0.55
|
)
|
||||
|
Shares used in computing basic net (loss) income per common share
|
32,589,795
|
31,359,867
|
30,449,721
|
|||||||||
|
Diluted net (loss) income per common share
|
$
|
(1.16
|
)
|
$
|
0.60
|
$
|
(0.55
|
)
|
||||
|
Shares used in computing diluted net (loss) income per common share
|
32,589,795
|
32,810,587
|
30,449,721
|
|||||||||
See accompanying Notes to Consolidated Financial Statements.
POZEN INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
|
Common
Stock
|
Additional
Paid-In Capital
|
Accumulated Other
Comprehensive
Income
|
Accumulated
Deficit
|
Total
Stockholders'
Equity
|
||||||||||||||||
|
Balance at December 31, 2012
|
$
|
30,322
|
$
|
183,921,159
|
$
|
(3,253
|
)
|
$
|
(99,870,866
|
)
|
$
|
84,077,362
|
||||||||
|
Exercise of common stock options
|
151
|
661,823
|
-
|
-
|
661,974
|
|||||||||||||||
|
Payments related to net settlement of stock awards
|
-
|
(522,439
|
)
|
-
|
-
|
(522,439
|
)
|
|||||||||||||
|
Issuance of common stock upon vesting of restricted stock
|
204
|
(204
|
)
|
-
|
-
|
-
|
||||||||||||||
|
Distribution to stockholders
|
-
|
(53,685,512
|
)
|
-
|
-
|
(53,685,512
|
)
|
|||||||||||||
|
Non-cash stock-based compensation
|
-
|
3,962,386
|
-
|
-
|
3,962,386
|
|||||||||||||||
|
Net loss
|
-
|
-
|
-
|
(16,708,299
|
)
|
(16,708,299
|
)
|
|||||||||||||
|
Other comprehensive income
|
-
|
-
|
3,253
|
-
|
3,253
|
|||||||||||||||
|
Balance at December 31, 2013
|
30,677
|
134,337,213
|
-
|
(116,579,165
|
)
|
17,788,725
|
||||||||||||||
|
Exercise of common stock options
|
1,484
|
7,587,445
|
-
|
-
|
7,588,929
|
|||||||||||||||
|
Payments related to net settlement of stock awards
|
-
|
(192,536
|
)
|
-
|
-
|
(192,536
|
)
|
|||||||||||||
|
Issuance of common stock upon vesting of stock awards
|
60
|
(60
|
)
|
-
|
-
|
-
|
||||||||||||||
|
Non-cash stock-based compensation
|
-
|
1,880,962
|
-
|
-
|
1,880,962
|
|||||||||||||||
|
Net income
|
-
|
-
|
-
|
19,674,732
|
19,674,732
|
|||||||||||||||
|
Balance at December 31, 2014
|
32,221
|
143,613,024
|
-
|
(96,904,433
|
)
|
46,740,812
|
||||||||||||||
|
Exercise of common stock options
|
351
|
1,734,420
|
-
|
-
|
1,734,771
|
|||||||||||||||
|
Payments related to net settlement of stock awards
|
-
|
(2,951,529
|
)
|
-
|
-
|
(2,951,529
|
)
|
|||||||||||||
|
Issuance of common stock upon vesting of stock awards
|
687
|
(687
|
)
|
-
|
-
|
-
|
||||||||||||||
|
Non-cash stock-based compensation
|
-
|
7,042,634
|
-
|
-
|
7,042,634
|
|||||||||||||||
|
Net loss
|
-
|
-
|
-
|
(37,783,445
|
)
|
(37,783,445
|
)
|
|||||||||||||
|
Balance at December 31, 2015
|
$
|
33,259
|
$
|
149,437,862
|
$
|
-
|
$
|
(134,687,878
|
)
|
$
|
14,783,243
|
|||||||||
See accompanying Notes to Consolidated Financial Statements.
POZEN INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
For the Years Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Operating Activities
|
||||||||||||
|
Net (loss) income
|
$
|
(37,783,445
|
)
|
$
|
19,674,732
|
$
|
(16,708,299
|
)
|
||||
|
Adjustments to reconcile net (loss) income to net cash (used in) provided by operating activities:
|
||||||||||||
|
Depreciation
|
16,239
|
18,933
|
29,413
|
|||||||||
|
Loss on disposal of fixed assets
|
―
|
―
|
5,205
|
|||||||||
|
Bond amortization income
|
―
|
―
|
63,389
|
|||||||||
|
Gain (loss) on investments in warrants
|
199,373
|
(2,678,773
|
)
|
―
|
||||||||
|
Non-cash stock-based compensation expense
|
7,042,634
|
1,880,962
|
3,962,386
|
|||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Accounts receivable
|
(336,706
|
)
|
(3,956,209
|
)
|
(321,000
|
)
|
||||||
|
Prepaid expenses and other current assets
|
(641,685
|
)
|
211,604
|
63,758
|
||||||||
|
Accounts payable
|
3,950,146
|
(893,723
|
)
|
268,910
|
||||||||
|
Accrued compensation and severance
|
4,314,333
|
(1,351,827
|
)
|
678,345
|
||||||||
|
Accrued expenses
|
6,449,871
|
(1,282,773
|
)
|
78,946
|
||||||||
|
Deferred revenue
|
―
|
(11,257,300
|
)
|
11,000,000
|
||||||||
|
Net cash (used in) provided by operating activities
|
(16,789,240
|
)
|
365,626
|
(878,947
|
)
|
|||||||
|
Investing activities
|
||||||||||||
|
Purchase of equipment
|
(240,148
|
)
|
(7,336
|
)
|
(1,652
|
)
|
||||||
|
Proceeds from sale of warrants
|
2,479,400
|
―
|
―
|
|||||||||
|
Sale and maturities of investments
|
―
|
―
|
18,838,000
|
|||||||||
|
Net cash provided by (used in) investing activities
|
2,239,252
|
(7,336
|
)
|
18,836,348
|
||||||||
|
Financing activities
|
||||||||||||
|
Proceeds from issuance of common stock
|
1,734,771
|
7,588,929
|
661,974
|
|||||||||
|
Distribution to shareholders
|
―
|
―
|
(53,685,512
|
)
|
||||||||
|
Payments related to net settlement of stock awards
|
(2,951,529
|
)
|
(192,536
|
)
|
(522,439
|
)
|
||||||
|
Net cash (used in) provided by financing activities
|
(1,216,758
|
)
|
7,396,393
|
(53,545,977
|
)
|
|||||||
|
Net (decrease) increase in cash and cash equivalents
|
(15,766,746
|
)
|
7,754,683
|
(35,588,576
|
)
|
|||||||
|
Cash and cash equivalents at beginning of year
|
40,582,415
|
32,827,732
|
68,416,308
|
|||||||||
|
Cash and cash equivalents at end of year
|
$
|
24,815,669
|
$
|
40,582,415
|
$
|
32,827,732
|
||||||
See accompanying Notes to Consolidated Financial Statements.
POZEN INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 1. | Significant Accounting Policies |
Organization
POZEN Inc. (together with its subsidiaries, “Pozen,” “we,” “us,” or similar pronouns) was incorporated in the State of Delaware on September 25, 1996 and is operating in a single reportable segment. On February 5, 2016, Pozen became a wholly-owned subsidiary of Aralez Pharmaceuticals Inc., a corporation incorporated under the laws of the Province of British Columbia (together with its subsidiaries, “Aralez”), which was formed for the purpose of facilitating the business combination of Pozen and Tribute Pharmaceuticals Canada Inc., a corporation incorporated under the laws of the Province of Ontario, Canada (“Tribute”) (the “Tribute Transaction”). Aralez is a global specialty pharmaceutical company focused on delivering meaningful products to improve patients’ lives while focusing on creating shareholder value by acquiring, developing and commercializing products primarily in cardiovascular, pain and other specialty areas. Aralez’s global headquarters is located in Ontario, Canada, its U.S. headquarters is in New York, New York and its Irish headquarters is located in Dublin, Ireland.
Basis of Presentation
The consolidated financial statements include the accounts of Pozen and its wholly-owned subsidiaries, Aralez Pharmaceuticals Trading DAC, Aralez Pharmaceuticals US Inc., and Aralez Pharmaceuticals R&D Inc. All intercompany transactions and balances have been eliminated.
Pozen’s consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). Such consolidated financial statements reflect all adjustments that are, in management’s opinion, necessary to present fairly, in all material respects, Pozen’s consolidated financial position, results of operations, and cash flows. There were no adjustments other than normal recurring adjustments.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts in the financial statements and accompanying notes. Actual results could differ from the estimates and assumptions used.
Revenue Recognition
Revenue for the years ended December 31, 2015, 2014 and 2013 consisted of the following royalty and other licensing revenue:
|
For the Years Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Royalty revenue
|
$
|
21,391,414
|
$
|
21,136,932
|
$
|
6,322,000
|
||||||
|
Other licensing revenue
|
―
|
11,257,300
|
4,000,000
|
|||||||||
|
Total royalty and licensing revenue
|
$
|
21,391,414
|
$
|
32,394,232
|
$
|
10,322,000
|
||||||
With regard to royalty revenue, Pozen’s licensing agreements have terms that include royalty payments based on the manufacture, sale or use of Pozen’s products or technology. VIMOVO® (naproxen and esomeprazole magnesium) royalty revenue is recognized when earned. For VIMOVO or those future arrangements where royalties are reasonably estimable, Pozen recognizes revenue based on estimates of royalties earned during the applicable period and reflects in future revenue any differences between the estimated and actual royalties. These estimates are based upon information reported to Pozen by its collaboration partners. During the years ended December 31, 2015, 2014 and 2013, Pozen recognized $21.4 million, $21.1 million, and $6.3 million, respectively, for VIMOVO royalty revenue.
Also, with regard to the licensing revenues, Pozen’s licensing agreements have had terms that include upfront payments upon contract signing and additional payments if and when certain milestones in the product’s development or commercialization are reached. Historically, the non-refundable portion of upfront payments received under Pozen’s existing agreements is deferred by Pozen upon receipt and recognized on a straight-line basis over periods ending on the anticipated date of regulatory approvals, as specified in the agreements relating to the product candidates, or the conclusion of any obligation on the part of Pozen. If regulatory approvals or other events relating to our product candidates are accelerated, delayed or not ultimately obtained, then the amortization of revenues for these products is prospectively accelerated or reduced accordingly. Milestone payments along with the refundable portions of up-front payments are recognized as licensing revenue upon the achievement of specified milestones if (i) the milestone is substantive in nature and the achievement of the milestone was not reasonably assured at the inception of the agreement; and (ii) the fees are non-refundable. Any milestone payments received prior to satisfying these revenue recognition criteria are recorded as deferred revenue. Pozen had no deferred revenue relating to milestones at December 31, 2015 or at December 31, 2014.
In March 2011, Pozen entered into a license agreement with Cilag GmbH International (“Cilag”), a division of Johnson & Johnson, for the exclusive development and commercialization of MT 400 in Brazil, Colombia, Ecuador and Peru. Cilag’s upfront payment of $0.3 million was deferred until the licensing agreement’s termination in 2014 and is included in other licensing revenue for the year ended December 31, 2014.
In September 2013, Pozen announced the signing of an exclusive license agreement with Sanofi-Aventis US LLC (“Sanofi US”) for its proton pump inhibitor-aspirin (“PA”) products, including YOSPRALA™ 81/40 and YOSPRALA 325/40, in the United States, to commercialize all PA combinations that contain 325mg or less of enteric-coated aspirin in the United States. On November 29, 2014, we executed a termination agreement with Sanofi US terminating the licensing. As of the termination date, all licenses granted to Sanofi US were terminated and all rights to the products licensed to Sanofi US under the agreement reverted to us. Pozen received an upfront payment of $15.0 million which is included within royalty and license revenue in the accompanying statements of comprehensive (loss) income. There was no revenue recognized from upfront payments for the year ended December 31, 2015. The revenue recognized from the upfront payment for the years ended December 31, 2014 and 2013 was $11.3 million and $4.0 million, respectively.
Cash, Cash Equivalents, Investments and Concentration of Credit Risk
Cash is invested in open-ended money market mutual funds, interest-bearing investment-grade debt securities and insured bank deposits. Pozen considers all highly liquid investments with maturities of 90 days or less when purchased to be cash equivalents.
Pozen invested in high-credit quality investments in accordance with its investment policy, which attempts to minimize the possibility of loss. However, cash and cash equivalents include financial instruments that potentially subject Pozen to a concentration of credit risk. Cash and cash equivalents are of a highly liquid nature and are held with high credit quality financial institutions and money market mutual fund managers. Cash held directly with U.S. financial institutions is insured up to $250,000 per account by FDIC insurance and cash held up to €100,000 in Ireland is insured by the Deposit Guarantee Scheme. Any excess amounts are uninsured. As of December 31, 2015, approximately 58% of Pozen’s cash and cash equivalents were held in fully insured bank deposits.
In connection with its acquisition of all rights, title and interest to develop, commercialize and sell Treximet® (sumatriptan/naproxen sodium) from GlaxoSmithKline (“GSK”), Pernix Therapeutics Holdings, Inc. (“Pernix”) issued Pozen a warrant to purchase 500,000 shares of Pernix common stock at an exercise price of $4.28 (the closing price of Pernix common stock as listed on the NASDAQ Global Market on May 13, 2014). The warrant was sold in the first quarter of 2015 and Pozen received $2.5 million from the sale. In connection with the sale of the warrants, Pozen recorded a loss of $0.2 million included in interest and other (loss) income in the accompanying statements of comprehensive (loss) income for the year ended December 31, 2015.
The following table sets forth our financial instruments carried at fair value as of December 31, 2015 and 2014:
|
Financial Instruments
Carried at Fair Value
|
||||||||
|
December 31,
2015
|
December 31,
2014
|
|||||||
|
Assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
24,815,669
|
$
|
40,582,415
|
||||
|
Investments in Pernix warrants
|
―
|
2,678,773
|
||||||
|
Total cash and investments
|
$
|
24,815,669
|
$
|
43,261,188
|
||||
Fair Value Measurements
Financial instruments consist of cash and cash equivalents, short-term investments, accounts receivable and accounts payable. The carrying values of these amounts, other than the investment in warrants, approximate the fair value due to their short-term nature.
As part of its acquisition of Treximet from GSK, Pernix granted Pozen a warrant to purchase 500,000 shares of Pernix common stock at an exercise price of $4.28 per share. The common stock underlying the warrant was registered by Pernix with the Securities and Exchange Commission. The warrant was exercisable from August 20, 2014, the closing date of the acquisition, until February 28, 2018. The warrant was valued at $2.7 million at December 31, 2014 using the Black-Scholes valuation model discounted for the warrant’s lack of marketability and liquidity.
Short-term investment gains consisted of the investment in warrant valuation of $2.7 million on August 20, 2014, with a mark-to-market decrease of $0.1 million at December 31, 2014 and a net short-term gain of approximately $2.6 million, which was included within interest and other (loss) income on the statements of comprehensive (loss) income for the year ended December 31, 2014. The warrant was sold in the first quarter of 2015 and Pozen received $2.5 million from the sale.
Pozen defines fair value (“FV”) as the price that would be received to sell an asset or paid to transfer a liability (“the exit price”) in an orderly transaction between market participants at the measurement date. The FV hierarchy for inputs maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Pozen uses the following hierarchy of inputs to measure FV:
|
·
|
Level 1 - quoted prices in active markets for identical assets and liabilities.
|
|
·
|
Level 2 - observable inputs other than quoted prices in active markets for identical assets and liabilities, including quoted prices in active markets for instruments that are similar or quoted prices in markets that are not active for identical or similar instruments and model-derived valuations in which all significant inputs and value drivers are observable in active markets.
|
| · | Level 3 - unobservable inputs that are significant to the overall valuation, for which there is little or no market data available and which require Pozen to develop its own assumptions. |
Pozen values investments using the most observable inputs available that are current as of the measurement date and classifies them according to the lowest level of inputs used. Observable inputs are inputs that market participants would use in pricing the asset or liability developed from market data obtained from independent sources. Unobservable inputs are inputs that reflect Pozen’s judgment concerning the assumptions that market participants would use in pricing the asset or liability developed from the best information available under the circumstances.
Fair value is a market-based measure considered from the perspective of a market participant who holds the asset or owes the liability rather than an entity-specific measure. Therefore, even when market assumptions are not readily available, our own assumptions are set to reflect those that market participants would use in pricing the asset or liability at the measurement date.
The financial assets for which we performed recurring measurements are cash equivalents and investments in warrants. As of December 31, 2015 and 2014, financial assets utilizing Level 1 inputs included cash equivalents. As of December 31, 2014, financial assets utilizing Level 2 inputs included investments in warrants.
Our Level 1 valuations are based on the market approach and consist primarily of quoted prices for identical items on active securities exchanges. Our Level 2 valuations may also use the market approach and are based on significant other observable inputs such as quoted prices for financial instruments not traded on a daily basis. We did not rely on Level 3 input for valuation of our securities at December 31, 2015 or 2014.
The following table sets forth our financial instruments carried at fair value within the fair value hierarchy and using the lowest level of input as of December 31, 2015 and 2014:
|
December 31, 2015
|
||||||||||||||||
|
Financial Instruments Carried at Fair Value
|
||||||||||||||||
|
Quoted prices in active Markets for identical items
(Level 1)
|
Significant other observable inputs
(Level 2)
|
Significant unobservable inputs
(Level 3)
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Cash and cash equivalents
|
$
|
24,815,669
|
$
|
―
|
$
|
—
|
$
|
24,815,669
|
||||||||
|
December 31, 2014
|
||||||||||||||||
|
Financial Instruments Carried at Fair Value
|
||||||||||||||||
|
Quoted prices in active markets for identical items
(Level 1)
|
Significant other observable inputs
(Level 2)
|
Significant unobservable inputs
(Level 3)
|
Total
|
|||||||||||||
|
Assets:
|
||||||||||||||||
|
Cash and cash equivalents
|
$
|
40,582,415
|
$
|
—
|
$
|
—
|
$
|
40,582,415
|
||||||||
|
Investment in warrants
|
—
|
2,678,773
|
—
|
2,678,773
|
||||||||||||
|
Total cash and investments
|
$
|
40,582,415
|
$
|
2,678,773
|
$
|
—
|
$
|
43,261,188
|
||||||||
Pozen targets investments principally in Level 1 and Level 2 cash equivalents and financial instruments and records them at FV. Pozen expects that the carrying values of cash equivalents will approximate FV because of their short maturities.
Equipment
Equipment consists primarily of computer hardware, software, furniture and fixtures and is recorded at cost. Depreciation is computed using an accelerated method over the estimated useful lives of the assets ranging from five to seven years. Accumulated depreciation as of December 31, 2015 and 2014 totaled $0.7 million.
Interest and Other (Loss) Income
Interest income was $56,400 and $43,100 for the years ended December 31, 2015 and 2014, respectively. For the year ended December 31, 2015, other (loss) income also included a loss of $199,000 related to the sale of warrants. For the year ended December 31, 2014, other (loss) income also included a short-term investments gain consisting of the investment in warrants with an initial valuation of $2.7 million and a mark-to-market decrease of $62,027, along with $377,269 related to the disgorgement of short-swing profits arising from trades by a Pozen stockholder under Section 16(b) of the Securities and Exchange Act of 1934.
Research and Development Costs, Including Clinical Trial Expenses
Research and development costs are expensed as incurred. Pozen has included in research and development expenses the personnel costs associated with research and development activities and costs associated with pharmaceutical development, clinical trials, toxicology activities, and regulatory matters.
Taxes
Income taxes are computed using the asset and liability approach, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in Pozen’s financial statements or tax returns. In estimating future tax consequences, Pozen generally considers all expected future events other than enactment of changes in tax law or rates. If it is more likely than not that some or all of a deferred tax asset will not be realized, Pozen records a valuation allowance.
Net (Loss) Income Per Share
Basic and diluted loss or net income per common share amounts have been computed using the weighted-average number of shares of common stock outstanding for the year ended December 31, 2014. During the years ended December 31, 2015 and 2013, Pozen had potential common stock equivalents related to its outstanding stock options. These potential common stock equivalents were not included, as the effect would have been antidilutive. Pozen has excluded the impact of any shares which might be issued under the Rights Plan, detailed below, from the earnings per share calculation because the Rights (as described below) are not exercisable since the specified contingent future event has not occurred.
Reconciliation of denominators for basic and diluted earnings per share computations:
|
For the Years Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Basic weighted average shares outstanding
|
32,589,795
|
31,359,867
|
30,449,721
|
|||||||||
|
Effect of dilutive employee and director awards
|
—
|
1,450,720
|
—
|
|||||||||
|
Diluted weighted-average shares outstanding
|
32,589,795
|
32,810,587
|
30,449,721
|
|||||||||
Patent Costs
Pozen expenses patent costs, including legal expenses, in the period in which they are incurred. Patent expenses are included in selling, general and administrative expenses in Pozen’s statements of comprehensive (loss) income.
Stock-Based Compensation
Stock-based compensation cost is estimated at the grant date based on the fair value of the award and is recognized as expense over the requisite service period of the award. The fair value of restricted stock awards is determined by reference to the fair market value of our common stock on the date of grant. We use the Black-Scholes model to value service condition and performance condition option awards. For awards with only service conditions and graded-vesting features, we recognize compensation cost on a straight-line basis over the requisite service period. For awards with performance conditions granted, we recognize compensation cost over the expected period to achieve the performance conditions, provided achievement of the performance conditions are deemed probable.
Accrued Expenses, including Contracted Costs
Significant management judgments and estimates must be made and used in connection with accrued expenses, including those related to contract costs, such as costs associated with clinical trials. Specifically, Pozen must make estimates of costs incurred to date but not yet paid for or not yet invoiced in relation to contracted, external costs. Pozen analyzes the progress of product development, clinical trials and related activities, invoices received, amounts paid, and budgeted costs when evaluating the adequacy of the accrued liability for these related costs.
Pozen believes that its current assumptions and other considerations used to estimate accrued expenses for the period are appropriate. However, determining the date on which certain contract services commence, the extent of services performed on or before a given date and the cost of such, paid and unpaid, involves subjective judgments and estimates and often must be based upon information provided by third parties. In the event that management does not identify certain contract costs which have begun to be incurred or under- or over-estimates the extent of services performed or the costs of such services, management adjusts costs during the period in which the information becomes available.
Accrued costs related to product development, pre-commercialization projects and operating activities, based upon the progress of these activities covered by the related contracts, invoices received and estimated costs, totaled $6.7 million at December 31, 2015 and $0.3 million at December 31, 2014. The variance, at each of these ending periods, between the actual expenses incurred and the estimated expenses accrued was not material to the financial statements.
Accrued Employee Compensation
In May 2015, we entered into a separation agreement with Pozen’s former President and Chief Executive Officer. Under the agreement he was paid specific one-time payments totaling $3.1 million, which includes special and performance bonuses, and on-going payments totaling $1.5 million, including salary continuation. The first payment was made in July 2015 and payments will continue through September 2017. While the full amount of these payments was accrued and recorded as selling, general and administrative expense for the year ended December 31, 2015, cash payments, totaling $3.4 million, were incurred for the year ended December 31, 2015.
In June 2015, we announced the adoption of an employee severance plan to provide severance benefits to eligible employees terminated involuntarily under certain circumstances. Under the plan, these employees will be paid on-going payments of approximately $4.4 million. Employees are required to render service beyond a minimum period; therefore, such benefits are being accrued over the respective service period. The first payment will be made in November 2015 and payments will continue through September 2017. For the year ended December 31, 2015, $0.7 million was recorded as R&D expense and $2.2 million was recorded as selling, general and administrative expense. Cash payments, totaling $32.0 thousand were incurred for the year ended December 31, 2015.
|
VIMOVO® ANDA Litigation
|
Between March 14, 2011 and May 16, 2013, we received Paragraph IV Notice Letters from Dr. Reddy’s Laboratories (“DRL”), Lupin Ltd. (“Lupin”), Watson Laboratories, Inc. – Florida (“Watson”), and Mylan Pharmaceuticals Inc. (“Mylan”), stating that each had filed an Abbreviated New Drug Application (“ANDA”) with the Food and Drug Administration (“FDA”) seeking regulatory approval to market a generic version of our VIMOVO product before the expiration of U.S. Patent No. 6,926,907 (the “‘907 patent”). On November 20, 2012, we received a second Notice Letter from DRL stating that DRL had filed a second ANDA with the FDA seeking regulatory approval to market a different generic formulation of the VIMOVO product before the expiration of the ‘907 patent. The ‘907 patent is assigned to POZEN and listed for the VIMOVO product in the FDA’s publication titled “Approved Drug Products with Therapeutic Equivalence Evaluations” (also known as the “Orange Book”).
On April 21, 2011, we filed suit against the first ANDA filer, DRL, in the United States District Court for the District of New Jersey (the “District Court”), asserting infringement of the ‘907 patent. We subsequently filed suit against the other three ANDA filers within 45 days of receipt of their respective Paragraph IV Notice Letters. Horizon Pharma USA, Inc. (“Horizon”), our current marketing partner for the VIMOVO product, is our co-plaintiff in each suit. The first suit against DRL is considered the lead case and has been consolidated with other suits for the purpose of pre-trial and discovery. On December 19, 2012, the District Court conducted a pre-trial Markman hearing to determine the proper claim construction of certain claims disputed by the parties. On May 1, 2013, the District Court issued a Markman Order construing the disputed claims. A scheduling order for the consolidated suits was issued by the District Court on June 27, 2014. Fact discovery closed in the consolidated suits on November 20, 2014, expert discovery closed on June 25, 2015, and we are currently waiting for the District Court to set a trial date (which has been delayed in part due to the retirement of the presiding judge in the case, the Honorable Joel A. Pisano).
On October 15, 2013, the United States Patent & Trademark Office (“USPTO”) issued to Pozen U.S. Patent No. 8,557,285 (the “‘285 patent”). The ‘285 patent is listed in the Orange Book for the VIMOVO product and is related to the ‘907 patent. On October 23, 2013, we filed suits against DRL, Lupin, Watson and Mylan in the District Court asserting infringement of the ‘285 patent. These suits have each been consolidated with the above referenced suits involving the ‘907 patent.
On October 7, 2014, the USPTO issued to Pozen U.S. Patent No. 8,852,636 (the “‘636 patent”). On October 14, 2014, the USPTO issued to Pozen U.S. Patent No. 8,858,996 (the “‘996 patent”). In addition, on October 21, 2014, the USPTO issued to Pozen U.S. Patent No. 8,865,190 (the “‘190 patent”). The ‘636, ‘996 and ‘190 patents are each listed in the Orange Book for the VIMOVO product and are each related to the ‘907 and ‘285 patents.
On February 3, 2015, the USPTO issued to Pozen U.S. Patent No. 8,945,621 (the “‘621 patent”). The ‘621 patent is listed in the Orange Book for the VIMOVO product.
On May 13, 2015, Pozen and Horizon filed suit against DRL, Lupin, Actavis (formerly known as Watson) and Mylan in the District Court asserting infringement of the ‘636 and ‘996 patents. On June 18, 2015, we filed Amended Complaints in each of the suits to assert infringement of the ‘190 patent. In its responsive pleading, Actavis filed a counterclaim alleging that its generic product does not infringe the ‘621 patent and that the ‘621 patent is invalid.
On October 20, 2015, the USPTO issued to Pozen U.S. Patent No. 9,161,920 (the “‘920 patent”). On December 1, 2015, the USPTO issued to Pozen U.S. Patent No. 9,198,888 (the “‘888 patent”). The ‘920 and ‘888 patents are each listed in the Orange Book for the VIMOVO product and are each related to the ‘907 and ‘285 patents.
On December 29, 2015, the USPTO issued to Pozen U.S. Patent No. 9,220,698 (the “‘698 patent”). The ‘698 patent is listed in the Orange Book for the VIMOVO product.
On January 25, 2016, Pozen and Horizon filed suit against Actavis in the District Court asserting infringement of the ‘920 and ‘888 patents. Actavis has not yet filed a responsive pleading. On February 10, 2015, we filed Amended Complaints against DRL, Lupin and Mylan to assert infringement of the ‘920 and ‘888 patents. In its responsive pleading, Mylan filed a counterclaim alleging that its generic product does not infringe the ‘698 patent and that the ‘698 patent is invalid. These suits are in the initial phase and a full schedule has not yet been set by the District Court.
As with any litigation proceeding, we cannot predict with certainty the patent infringement suit against DRL, Lupin, Mylan and Watson relating to a generic version of VIMOVO. Furthermore, we will have to incur additional expenses in connection with the lawsuits relating to VIMOVO, which may be substantial. In the event of an adverse outcome or outcomes, our business could be materially harmed. Moreover, responding to and defending pending litigation will result in a significant diversion of management’s attention and resources and an increase in professional fees.
Inter Partes Review
DRL filed a Petition for (“IPR Petition”) of the ‘285 patent with the Patent Trial and Appeal Board (“PTAB”) of the USPTO on February 24, 2015, which was denied on October 9, 2015. The Coalition for Affordable Drugs VII L.L.C. (“CFAD”) filed IPR Petitions of the ‘907 patent, the ‘996 patent and the ‘636 patent with the PTAB on May 21, 2015, June 5, 2014 and August 7, 2015, respectively, each of which was denied on December 8, 2015, December 17, 2015 and February 11, 2016, respectively.
On August 12, 2015, CFAD filed an IPR Petition of the ‘621 patent with the PTAB. On February 22, 2016 the PTAB instituted review of the claims of the ‘621 patent. Pozen and Horizon have until May 23, 2016 to file a response to the petition.
On August 19, 2015, Lupin filed three separate IPR Petitions of the ‘996, ‘636 and ‘190 patents with the PTAB. On March 1, 2016 the PTAB denied Lupin’s petition for review of the ‘636 patent and instituted review of a limited number of the claims in each of the ‘996 and ‘190 patents. Pozen and Horizon have until May 27, 2016 to file responses to the petitions for review of the ‘996 and ‘190 patents.
On November 12, 2015, Gray Square Pharmaceuticals, LLC (formerly known as Graybar Pharmaceuticals, LLC) filed an IPR Petition of U.S. Patent No. 7,332,183 (the “‘183 patent”) with the PTAB. The ‘183 patent is assigned to POZEN and listed with respect to Treximet in the Orange Book. Pozen and our marketing partner Pernix Therapeutics Holdings, Inc. filed a Preliminary Response to Gray Square’s petition on February 16, 2016. Upon receipt of such a Preliminary Response, the PTAB has three months in which to institute or deny the IPR proceeding. If the PTAB decides to institute the IPR proceeding, Gray Square will have the opportunity to challenge the validity of the ‘183 patent in whole or in part before the PTAB via a patent validity trial.
|
Canada VIMOVO® Litigation
|
On January 20, 2015, our Canadian licensee, AstraZeneca Canada Inc. (“AstraZeneca Canada”) received a Notice of Allegation from Mylan Pharmaceuticals ULC (“Mylan Canada”) informing that Mylan Canada has filed an Abbreviated New Drug Submission in Canada for approval of its naproxen/esomeprazole magnesium tablets and alleging non-infringement of some of the claims and invalidity of Canadian Patent No. 2,449,098 (the “‘098 patent”). A Notice of Allegation is served pursuant to the Patented Medicines (Notice of Compliance) Regulations in Canada and is similar to a Paragraph IV Notice Letter in the United States, and in response, we and AstraZeneca Canada commenced a proceeding in the Federal Court of Canada (the “Canada Court”) in relation to the ‘098 patent on March 5, 2015 seeking to prohibit Health Canada from approving Mylan Canada’s generic naproxen/esomeprazole product. The Canadian proceeding is summary in nature and expected to be completed before March 5, 2017. In accordance with the schedule approved by the Canada Court, affidavit evidence of AstraZeneca Canada and Pozen was served on September 11, 2015 and affidavit evidence of Mylan Canada on January 8, 2016. The parties are to complete cross-examinations on the affidavit evidence by April 29, 2016. The written records for the hearing are to be served by AstraZeneca Canada and us by July 4, 2016 and by Mylan Canada by September 2, 2016. A three-day hearing of the matter has been scheduled, commencing on November 21, 2016. The proceeding will decide whether approval for Mylan Canada’s naproxen/esomeprazole magnesium tablets will be prohibited until the expiry of the ‘098 patent because none of Mylan Canada’s allegations in respect of the ‘098 patent are justified, however the proceeding will not finally decide ‘098 patent validity or infringement. The ‘098 patent expires on May 31, 2022.
New Accounting Pronouncements
Taxes
In November 2015, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2015-17, “Income Taxes (Topic 740): Balance Sheet Classification of Deferred Taxes.” The new standard requires that deferred tax assets and liabilities be classified as noncurrent in a classified statement of financial position. The guidance is effective for financial statements issued for annual periods beginning after December 15, 2016, and interim periods within those annual periods. Earlier application is permitted for all entities as of the beginning of an interim or annual report period. The amendments in this ASU may be applied either prospectively to all deferred tax assets and liabilities or retrospectively to all periods presented. Pozen adopted this standard as of December 31, 2015 with prospective application. As a result, Pozen reclassified its deferred tax assets classified as current to noncurrent and its deferred tax liabilities classified as current to noncurrent in its December 31, 2015 consolidated balance sheet and related disclosures. Prior balance sheets were not retrospectively adjusted.
Business Combinations
In September 2015, the FASB issued ASU 2015-16, “Business Combinations (Topic 805): Simplifying the Accounting for Measurement-Period Adjustments.” The new guidance eliminates the requirements to restate prior period financial statements for measurement-period adjustments, and requires that the cumulative impact of a measurement period adjustment (including the impact on prior periods) be recognized in the reporting period in which the adjustment is identified. The new standard, which should be applied prospectively to measurement period adjustments that occur after the effective date, is effective for Aralez in the first quarter of 2016. Aralez does not expect the adoption of the new accounting rules to have a material impact on its financial condition, results of operations or cash flows.
Debt Issuance Costs
In April 2015, the FASB issued ASU 2015-03, “Interest - Imputation of Interest (Topic 835-30): Simplifying the Presentation of Debt Issuance Costs” (“ASU 2015-03”). ASU 2015-03 requires debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the related debt liability’s carrying value, which is consistent with the presentation of debt discounts. ASU 2015-03 is effective for financial statements issued for years beginning after December 15, 2015. Aralez does not expect the adoption of the new accounting rules to have a material impact on its financial condition, results of operations or cash flows.
Revenue from Contracts with Customers
In May 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers (Topic 606),” which requires revenue recognition based on the transfer of promised goods or services to customers in an amount that reflects consideration Aralez expects to be entitled to in exchange for goods or services. In August 2015, the FASB issued updated guidance deferring the effective date of the revenue recognition standard. The new rules supersede prior revenue recognition requirements and most industry-specific accounting guidance. The new rules will be effective for Aralez in the first quarter of 2018, with either full retrospective or modified retrospective application required. Aralez has not yet selected a transition method and is evaluating the impact of the ASU on its financial statements.
| 2. | License Agreements |
We have entered into and may continue to enter into collaborations with established pharmaceutical or pharmaceutical services companies to develop, commercialize and/or manufacture our product candidates. Our existing collaborations are described below.
Agreement with AstraZeneca/Horizon regarding VIMOVO®
In August 2006, we entered into a collaboration and license agreement, effective September 7, 2006 (the “Original AZ Agreement”), with AstraZeneca AB, a Swedish corporation (“AstraZeneca”), regarding the development and commercialization of proprietary fixed dose combinations of the proton pump inhibitor esomeprazole magnesium with the NSAID naproxen, in a single tablet for the management of pain and inflammation associated with conditions such as osteoarthritis and rheumatoid arthritis in patients who are at risk for developing NSAID-associated gastric ulcers. Under the terms of the Original AZ Agreement, we granted to AstraZeneca an exclusive, fee-bearing license, in all countries of the world except Japan, under our patents and know-how relating to combinations of gastroprotective agents and NSAIDs (other than aspirin and its derivatives). Pursuant to the terms of the Original AZ Agreement, we received an upfront license fee of $40.0 million from AstraZeneca following termination of the waiting period under the Hart-Scott-Rodino notification program.
We retained responsibility for the development and filing of the New Drug Application (“NDA”) for the product in the United States, while AstraZeneca was responsible for all development activities outside the United States, as well as for all manufacturing, marketing, sales and distribution activities worldwide. We agreed to bear all expenses related to certain specified U.S. development activities. AstraZeneca would pay all other development expenses, including all manufacturing-related expenses. The Original AZ Agreement established joint committees with representation of both AstraZeneca and us to manage the development and commercialization of the product. If consensus could not be reached between AstraZeneca and us, we generally would have the deciding vote with respect to development activities required for marketing approval of the product in the United States and AstraZeneca generally would have the deciding vote with respect to any other matters.
In September 2007, we entered into an amendment to the Original AZ Agreement, effective as of September 6, 2007. Under the terms of the amendment, AstraZeneca agreed to pay us up to $345.0 million, in the aggregate, in milestone payments upon the achievement of certain development, regulatory and sales events. In September 2007, we received a $10.0 million payment upon execution of the amendment and a $20.0 million payment in recognition of the achievement of the primary endpoints for the PN400-104 study, a study which compared acid suppression of different doses of VIMOVO (formerly PN 400), and achievement of the interim results of the PN200-301 study, a six-month comparative trial of PN 200 as compared to EC naproxen in patients requiring chronic NSAID therapy, meeting mutually agreed success criteria. In September 2009, we received a $10.0 million payment upon acceptance by the FDA of the VIMOVO NDA for review. In May 2010, we received a $20.0 million payment for the NDA approval of VIMOVO. We also received an additional $25.0 million payment in December 2010 when VIMOVO received approval (including pricing and reimbursement approval) in a major ex-U.S. market. Up to $260.0 million is payable as sales performance milestones if certain aggregate sales thresholds are achieved.
The amendment also revised the royalty rates we were to have received under the Original AZ Agreement. Prior to the effective date of the amendment, under the terms of the Original AZ Agreement, we were to receive a royalty based on annual net sales by AstraZeneca, its affiliates or sublicensees during the royalty term. The royalty rate varied based on the level of annual net sales of products made by AstraZeneca, its affiliates and sublicensees, ranging from the mid-single digits to the mid-teens. Under the amendment, we receive a flat, low double-digit royalty rate during the royalty term on annual net sales of products made by AstraZeneca, its affiliates and sublicensees, in the United States and royalties ranging from the mid-single digits to the high-teens on annual net sales of products made by AstraZeneca, its affiliates and sublicensees outside of the United States. Pursuant to the Original AZ Agreement, as amended, the royalty rate may be reduced due to the loss of market share as a result of generic competition inside and outside of the United States. Our right to receive royalties from AstraZeneca for the sale of such products expires on a country-by-country basis upon the later of (a) expiration of the last-to-expire of certain patent rights relating to such products in that country, and (b) ten years after the first commercial sale of such products in such country.
We further amended the Original AZ Agreement effective October 1, 2008 to shorten the timing of AstraZeneca’s reimbursement obligation for certain development expenses incurred by us under the Original AZ Agreement and to update the description of the target product profile studies (as defined in the Original AZ Agreement) for VIMOVO.
Unless earlier terminated in accordance with its terms, the Original AZ Agreement, as amended, will expire upon the payment of all applicable royalties for the products commercialized under the Original AZ Agreement. Either party has the right to terminate by notice in writing to the other party upon or after any material breach by the other party, if the other party has not cured the breach within 90 days after written notice to cure has been given, with certain exceptions. The parties also can terminate for cause under certain defined conditions. In addition, AstraZeneca can terminate, at any time, at will, for any reason or no reason, in its entirety or with respect to countries outside the United States, upon 90 days’ notice. If terminated at will, AstraZeneca will owe us a specified termination payment or, if termination occurs after the product is launched, AstraZeneca may, at its option, under and subject to the satisfaction of conditions specified in the Original AZ Agreement, elect to transfer the product and all rights to us.
On May 3, 2013, AstraZeneca informed us that, after a strategic business review, it had decided to cease promotion and sampling of VIMOVO by the end of the third quarter of 2013 in certain countries, including the United States and all countries in Europe, other than Spain and Portugal, which have pre-existing contractual relationships with third parties. We understand from AstraZeneca that it instead intended to focus on those countries where the product had shown growth and which AstraZeneca believed had the greatest potential for future growth.
On September 16, 2013, we and AstraZeneca entered into a third amendment to the Original AZ Agreement which made clarifications to certain intellectual property provisions of the Original AZ Agreement to clarify that AstraZeneca’s rights under those provisions do not extend to products which contain acetylsalicylic acid. On September 16, 2013, we and AstraZeneca also executed a letter agreement whereby we agreed that in the event that AstraZeneca divested its rights and obligations to market VIMOVO in the United States to a third-party, AstraZeneca would be relieved of its obligations under the Original AZ Agreement with respect to the United States as of the effective date of such divestiture, including its obligation under the Original AZ Agreement to guarantee the performance of such assignee and/or sublicensee.
On November 18, 2013, AstraZeneca divested all of its rights, title and interest to develop, commercialize and sell VIMOVO in the United States to Horizon. In connection with this divestiture, on November 18, 2013, we and AstraZeneca entered into an Amended and Restated Collaboration and License Agreement for the United States (the “U.S. Agreement”) and an Amended and Restated License and Collaboration Agreement for Outside the United States (the “ROW Agreement”), which agreements collectively amended and restated the Original AZ Agreement. With our consent pursuant to a letter agreement among us, AstraZeneca and Horizon, AstraZeneca subsequently assigned the U.S. Agreement to Horizon in connection with the divestiture. Further, the letter agreement establishes a process for AstraZeneca and Horizon to determine if sales milestones set forth in the Original AZ Agreement are achieved on a global basis and provides other clarifications and modifications required as a result of incorporating the provisions of the Original AZ Agreement into the U.S. Agreement and the ROW Agreement or as otherwise agreed by the parties.
Pursuant to an amendment of the U.S. Agreement (“Amendment to the U.S. Agreement”) between us and Horizon, we are guaranteed an annual minimum royalty amount of $5 million in calendar year 2014, and a guaranteed annual minimum royalty amount of $7.5 million each calendar year thereafter, provided that the patents owned by us which cover VIMOVO are in effect and no generic forms of VIMOVO are in the marketplace. The Amendment to the U.S. Agreement also provides that Horizon has assumed AstraZeneca’s right to lead the on-going Paragraph IV litigation relating to VIMOVO currently pending in the United States District Court for the District of New Jersey and will assume all patent-related defense costs relating to such litigation, including reimbursement up to specified amounts of the cost of any counsel retained by us, amends certain time periods for Horizon’s delivery of quarterly sales reports to us, and provides for quarterly update calls between the parties to discuss performance of VIMOVO and Horizon’s commercialization efforts.
On December 31, 2015, we accrued $6.0 million of VIMOVO royalty revenue, $4.7 million related to U.S. sales and $1.3 million related to rest-of-the-world sales.
Agreements with GSK, Pernix and CII regarding MT 400 (including Treximet®)
In June 2003, we entered into an agreement with GSK for the development and commercialization of proprietary combinations of a triptan (5-HT1B/1D agonist) and a long-acting non-steroidal anti-inflammatory drug (“NSAID”) (the “Treximet Agreement”). The combinations covered by the Treximet Agreement are among the combinations of MT 400 (including Treximet). Under the terms of the Treximet Agreement, GSK has exclusive rights in the United States to commercialize all combinations which combine GSK’s triptans, including Imitrex® (sumatriptan succinate) or Amerge® (naratriptan hydrochloride), with a long-acting NSAID. We were responsible for development of the first combination product, while GSK provided formulation development and manufacturing.
Pursuant to the terms of the Treximet Agreement, we received an initial $25.0 million payment from GSK following termination of the waiting period under the Hart-Scott-Rodino notification program and the issuance of a specified patent. In May 2004, we received a $15.0 million milestone payment as a result of our commencement of Phase 3 clinical trial activities. In October 2005, we received a $20.0 million milestone payment upon the FDA’s acceptance for review of the NDA for Treximet, the trade name for the product. On April 26, 2008, we received from GSK $20.0 million in milestone payments which were associated with the approval of, and GSK’s intent to commercialize, Treximet. In addition, Pernix, as assignee of GSK, will pay us two sales performance milestones totaling up to $80.0 million if certain sales thresholds are achieved. Pernix, as assignee of GSK, will pay us royalties on all net sales of marketed products until at least the expiration of the last-to-expire issued applicable patent (October 2, 2025) based upon the scheduled expiration of currently issued patents. Pernix may reduce, but not eliminate, the royalty payable to us if generic competitors attain a pre-determined share of the market for the combination product, or if Pernix owes a royalty to one or more third parties for rights it licenses from such third parties to commercialize the product.
The Treximet Agreement terminates on the date of expiration of all royalty obligations unless earlier terminated by either party for a material breach or by Pernix, as assignee of GSK, at any time upon 90 days’ written notice to us for any reason or no reason. Among the contract breaches that would entitle us to terminate the Treximet Agreement is Pernix’s determination not to further develop or to launch the combination product under certain circumstances. Pernix, as assignee of GSK, has the right, but not the obligation, to bring, at its own expense, an action for infringement of certain patents by third parties. If Pernix, as assignee of GSK, does not bring any such action within a certain time frame, we have the right, at our own expense, to bring the appropriate action. With regard to certain other patent infringements, we have the sole right to bring an action against the infringing third-party. Each party generally has the duty to indemnify the other Treximet Agreement damages arising from breaches of each party’s representations, warranties and obligations under the agreement, as well as for gross negligence or intentional misconduct. We also have a duty to indemnify Pernix, as assignee of GSK, for damages arising from our development and manufacture of MT 400 prior to the effective date of the Treximet Agreement, and each party must indemnify the other for damages arising from the development and manufacture of any combination product after the effective date.
On November 23, 2011, we entered into a purchase agreement with CII, pursuant to which we sold, and CII purchased, our right to receive future royalty payments arising from U.S. sales of MT 400, including Treximet. By virtue of the agreement, we will receive a 20% interest in any royalties paid on net sales of Treximet and such other products in the United States to CII relating to the period commencing in the second quarter of 2018.
On May 13, 2014, we, GSK, CII and Pernix, entered into certain agreements in connection with GSK’s divestiture of all of its rights, title and interest to develop, commercialize and sell Treximet in the United States to Pernix. Upon the closing of the transaction on August 20, 2014, with our consent, GSK assigned the Treximet Agreement to Pernix. Immediately following the closing of the transaction, we entered into an amendment to the Treximet Agreement with Pernix. The amendment, among other things, amends the royalty provisions of the Treximet Agreement to provide for a guaranteed quarterly minimum royalty of $4 million for the calendar quarters commencing on January 1, 2015 and ending on March 31, 2018 and requires that Pernix continue certain of GSK’s ongoing development activities and to undertake certain new activities, for which we will provide reasonable assistance. This amendment to the Treximet Agreement also eliminates restrictions in the Treximet Agreement on our right to develop and commercialize certain dosage forms of sumatriptan/naproxen combinations outside of the United States and permits us to seek approval for these combinations on the basis of the approved NDA for Treximet. Pernix also granted us a warrant to purchase 500,000 shares of Pernix common stock at an exercise price equal to $4.28 per share, which represents the closing price of Pernix common stock as reported on the NASDAQ Global Market on May 13, 2014. In the first quarter of 2015, Pozen sold the warrant for $2.5 million. On July 30, 2014, we and Pernix entered into a second amendment of the Treximet Agreement, effective upon the closing of the transaction on August 20, 2014, which will permit Pernix’s Irish affiliate (to which Pernix assigned its rights) to further assign the Treximet Agreement without our prior written consent as collateral security for the benefit of certain lenders.
Agreement with Sanofi-regarding PA Products
On September 3, 2013, we entered into an exclusive license and collaboration agreement with Sanofi US (the “Sanofi License Agreement”) for the commercialization of products containing a combination of immediate release omeprazole and 325mg or less of delayed release aspirin, including YOSPRALA 325/40 and YOSPRALA 81/40 in the United States. On November 29, 2014, we executed a termination agreement with Sanofi US terminating the Sanofi License Agreement. As of the termination date, all licenses granted to Sanofi US were terminated and all rights to the PA products licensed to Sanofi US under the Sanofi License Agreement reverted to us. The termination agreement further provides for the transfer of specified commercial know-how developed by Sanofi US relating to the PA products to us and allows us, and any future collaborators, to use this know-how to commercialize the products.
On March 21, 2011, we entered into a license agreement with Cilag GmbH International (“Cilag”), a division of Johnson & Johnson, for the exclusive development and commercialization of MT 400 in Brazil, Colombia, Ecuador and Peru. In December 2014, we received an executed, mutual termination from Cilag. There was no dispute between the parties regarding the license agreement and, at our request, for a period of two years after termination, Cilag has agreed to negotiate in good faith commercially reasonable terms of a supply agreement whereby Cilag would supply us or our licensees, with MT 400 for a period equal to the shorter of (i) two (2) years; or (ii) until we establish an alternative supplier. We recognized approximately $0.3 million in licensing revenue for the year ended December 31, 2014 as a result of this termination that had previously been recorded as deferred revenue.
Agreements with Patheon regarding YOSPRALA™ 325/40 and YOSPRALA™ 81/40
On December 19, 2011, we entered into a Manufacturing Services Agreement (the “Supply Agreement”) and a related Capital Expenditure and Equipment Agreement (the “Capital Agreement”), relating to the manufacture of YOSPRALA 325/40, with Patheon Pharmaceuticals Inc. (“Patheon”). The Supply Agreement and Capital Agreement were amended on July 10, 2013 (respectively, the “Amendment to the Supply Agreement” and the “Amendment to the Capital Agreement”).
Under the terms of the Supply Agreement, as amended, Patheon has agreed to manufacture, and we have agreed to purchase, a specified percentage of Pozen’s requirements of YOSPRALA 325/40 and YOSPRALA 81/40 for sale in the United States. The Amendment to the Supply Agreement expressly incorporates YOSPRALA 81/40, clarifies that the manufacturing services contemplated by the Supply Agreement include the manufacture of validation batches, but the placing of an order for such validation batches will not trigger the commencement date of the Initial Term (as defined below), updates pricing for YOSPRALA 325/40 and incorporates a new pricing schedule for YOSPRALA 81/40.
The term of the Supply Agreement extends until December 31st of the fourth year after we notify Patheon to begin manufacturing services under the Supply Agreement (the “Initial Term”), and will automatically renew thereafter for periods of two years, unless terminated by either party upon 18 months’ written notice prior to the expiration of the Initial Term or 12 months’ written notice prior to the expiration of any renewal term. In addition to usual and customary termination rights which allow each party to terminate the Supply Agreement for material, uncured breaches by the other party, we can terminate the Supply Agreement upon 30 days’ prior written notice if a governmental or regulatory authority takes any action or raises any objection that prevents us from importing, exporting, purchasing or selling YOSPRALA 325/40 or if it is determined that the formulation or sale of YOSPRALA 325/40 infringes any patent rights or other intellectual property rights of a third-party. We can also terminate the Supply Agreement upon 24 months’ prior written notice if we license, sell, assign or otherwise transfer any rights to commercialize YOSPRALA 325/40 to a third-party. The Supply Agreement contains general and customary commercial supply terms and conditions, as well as establishes pricing, subject to annual adjustments, for bulk product and different configurations of packaged product.
Under the terms of the Capital Agreement, as amended, we will be responsible for the cost of purchasing certain equipment specific to the manufacture of YOSPRALA 325/40 and YOSPRALA 81/40, the cost of which, based on current volume projections, is expected to be less than $450,000. The Amendment to the Capital Agreement provides an updated schedule, which reflects the parties’ current assumptions regarding the need for and timing of capital equipment expenditures based upon Patheon’s current and anticipated production capacity and current volume projections for YOSPRALA 325/40 and YOSPRALA 81/40. In addition, pursuant to the terms of the Amendment to the Capital Agreement, we agreed with Patheon to reduce the amount of the maximum expenditure for additional capital equipment and facility modifications to meet volume demands from $2.5 million to approximately $1.2 million in light of the revised capacity and volume assumptions.
| 3. | Stockholders’ Equity |
Shares Reserved for Future Issuance
In January 2005, Pozen approved a stockholder rights plan (the “Rights Plan”), pursuant to which Pozen entered into a Rights Agreement dated January 12, 2005 with StockTrans, Inc., as rights agent, and Pozen declared a dividend of a right to acquire one preferred share purchase right (a “Right”) for each outstanding share of Pozen’s Common Stock, $0.001 par value per share, to stockholders of record at the close of business on January 28, 2005. In connection with the Rights Plan, Pozen designated 90,000 shares of its authorized Preferred Stock as Series A Junior Participating Preferred Stock. There was no preferred stock outstanding as of December 31, 2015 or 2014. The Rights Plan has a 10-year term and contains provisions requiring a periodic review and evaluation by the Board of Directors. The Rights Plan expired on January 12, 2015.
If there is any change in the number or kind of shares of company stock outstanding or if the value of outstanding shares of company stock is substantially reduced as a result of an extraordinary dividend or distribution, Pozen’s 2010 Equity Compensation Plan (the “2010 Plan”) requires that an equitable adjustment be made to all outstanding grants to preclude dilution of rights and benefits under the plan. Therefore, as a result of the December 31, 2013 cash dividend distribution, a dividend equivalent totaling 987,000 shares was provided to all outstanding grants. The adjustments were in the form of additional restricted stock units (“RSU”) to RSU holders or an adjustment to both the outstanding number of options and their strike price, in compliance with Sections 409A and 424 of the Internal Revenue Code. In addition, the 2010 Plan provides for an adjustment to the number of common shares available for grant under the stock option plan. Therefore, as a result of the December 31, 2013 cash dividend distribution, the number of common stock available for grant was adjusted by 416,971 shares.
At December 31, 2015, shares of our common stock reserved for future issuance are as follows:
|
Common stock available for grant under stock option plans
|
2,040,688
|
|||
|
Common stock issuable pursuant to options and restricted stock units granted under equity compensations plans
|
6,026,333
|
|||
|
Total Reserved
|
8,067,021
|
| 4. | Accrued Expenses |
Accrued expenses consist of the following at:
|
December 31
|
||||||||
|
2015
|
2014
|
|||||||
|
Transaction costs
|
$
|
3,011,705
|
$
|
—
|
||||
|
Pre-commercialization costs
|
2,204,379
|
7,863
|
||||||
|
Research and development costs
|
995,547
|
55,227
|
||||||
|
Other
|
491,385
|
190,055
|
||||||
|
$
|
6,703,016
|
$
|
253,145
|
|||||
In the above table, transaction costs include $1.3 million in legal fees, $1.1 million in investment banking fairness opinion fees and $0.6 million in accounting and other professional fees; pre-commercialization costs include $0.9 in market research costs, $0.7 million in managed care and scientific messaging costs and $0.6 million in other costs.
| 5. | Income Taxes |
The provision for income taxes consists of the following:
|
($ in thousands)
|
For the Years Ended December 31,
|
|||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Current provision (benefit):
|
||||||||||||
|
Federal
|
$
|
—
|
$
|
—
|
$
|
—
|
||||||
|
State
|
174
|
—
|
—
|
|||||||||
|
Foreign
|
—
|
—
|
—
|
|||||||||
|
Total current provision
|
174
|
—
|
—
|
|||||||||
|
Deferred benefit:
|
||||||||||||
|
Federal
|
—
|
—
|
—
|
|||||||||
|
State
|
—
|
—
|
—
|
|||||||||
|
Foreign
|
—
|
—
|
—
|
|||||||||
|
Total deferred benefit
|
—
|
—
|
—
|
|||||||||
|
Total current and deferred provision
|
$
|
174
|
$
|
—
|
$
|
—
|
||||||
For the year ended December 31, 2015, loss before income tax expense of $37.6 million includes $29.1 million of foreign losses. There was no foreign income or loss for the years ended December 31, 2014 or 2013.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of Pozen’s deferred tax assets are as follows at:
|
December 31
|
||||||||
|
($ in thousands)
|
2015
|
2014
|
||||||
|
Current
|
||||||||
|
Deferred income tax assets:
|
||||||||
|
Other current assets
|
$
|
—
|
$
|
662
|
||||
|
Less valuation allowance
|
—
|
(647
|
)
|
|||||
|
Total net deferred income tax assets, current
|
$
|
—
|
$
|
15
|
||||
|
Deferred income tax liabilities:
|
||||||||
|
Investment in warrants
|
—
|
(968
|
)
|
|||||
|
Total net deferred income taxes, current
|
$
|
—
|
$
|
(953
|
)
|
|||
|
Non-current
|
||||||||
|
Deferred income tax assets (liabilities):
|
||||||||
|
Tax loss carryforwards
|
$
|
18,011
|
$
|
20,840
|
||||
|
Research and development credits
|
14,513
|
13,987
|
||||||
|
Equity compensation
|
4,375
|
6,644
|
||||||
|
Transaction costs
|
4,156
|
—
|
||||||
|
Other
|
2,833
|
39
|
||||||
|
Total gross deferred income taxes, non-current
|
43,888
|
41,510
|
||||||
|
Less valuation allowance
|
(43,888
|
)
|
(40,557
|
)
|
||||
|
Total net deferred income taxes, non-current
|
$
|
—
|
$
|
953
|
||||
|
Total net deferred income taxes
|
$
|
—
|
$
|
—
|
||||
At December 31, 2015 and 2014, Pozen had federal net operating loss carryforwards of approximately $46.3 million and $53.0 million, respectively, state net economic loss carryforwards of approximately $61.1 million and $78.0 million, respectively, foreign net operating loss carryforwards of $24.3 million and $0 million, respectively, and research and development credit carryforwards of approximately $14.5 million and $14.0 million, respectively. The federal and state net operating loss carryforwards begin to expire in 2029 and 2016, respectively, and the research and development credit carryforwards begin to expire in 2018. Pozen’s federal and state net operating loss carryforwards include approximately $7.8 million of excess tax benefits resulting from stock-based compensation exercises and vestings. The tax benefit of these deductions has not been recognized in deferred tax assets. If utilized, the benefits from these deductions will be recorded as adjustments to additional paid-in capital. A valuation allowance has been recognized to offset the deferred tax assets related to the carryforwards based on Pozen’s assessment regarding the realizability of these deferred tax assets in future periods. Of the total increase in valuation allowance of $2.7 million, an increase of $2.7 million was allocable to current operating activities. The utilization of the loss carryforwards to reduce future income taxes will depend on Pozen’s ability to generate sufficient taxable income prior to the expiration of the loss carryforwards. In addition, the maximum annual use of net operating loss and research credit carryforwards is limited in certain situations where changes occur in stock ownership. The recognized tax benefit related to net operating loss carryforwards was approximately $2.5 million, $4.8 million and $0 for the years ended December 31, 2015, 2014 and 2013, respectively.
On July 23, 2013, North Carolina enacted House Bill 998, which reduced the corporate income tax rate from 6.9% in 2013 to 6% in 2014 and 5% in 2015. On September 18, 2015, North Carolina enacted House Bill 97, which reduced the corporate income tax rate from 5% to 4% in 2016. As a result of the new enacted tax rate, Pozen adjusted its deferred tax assets in 2015 by applying the lower rate which resulted in a decrease to the deferred tax assets and a corresponding decrease to the valuation allowance of approximately $0.5 million.
The actual income tax (benefit) expense for the years ended December 31, 2015, 2014 and 2013, differed from the amounts computed by applying the U.S. federal tax rate of 35% to income (loss) before taxes as a result of the following:
|
($ in thousands)
|
|
For the Years Ended December 31,
|
|
|||||||||
|
|
|
2015
|
|
|
2014
|
|
|
2013
|
|
|||
|
(Loss) income before income tax
|
|
$
|
(37,609
|
)
|
|
$
|
19,675
|
|
|
$
|
(16,708
|
)
|
|
Federal tax rate
|
|
|
35
|
%
|
|
|
35
|
%
|
|
|
35
|
%
|
|
Federal income tax provision at statutory rate
|
|
|
(13,163
|
)
|
|
|
6,886
|
|
|
|
(5,848
|
)
|
|
State tax provision
|
|
|
(48
|
)
|
|
|
224
|
|
|
|
(215
|
)
|
|
|
|
|
(13,211
|
)
|
|
|
7,110
|
|
|
|
(6,063
|
)
|
|
Decrease (increase) in income tax benefit resulting from:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign tax rate differential
|
|
|
6,548
|
|
|
|
—
|
|
|
|
—
|
|
|
Research and development credits
|
|
|
(574
|
)
|
|
|
4
|
|
|
|
66
|
|
|
Non-deductible expenses and other
|
|
|
284
|
|
|
|
177
|
|
|
|
302
|
|
|
Change in state tax rate
|
|
|
535
|
|
|
|
35
|
|
|
|
966
|
|
|
Non-deductible executive compensation
|
|
|
1,279
|
|
|
|
—
|
|
|
|
—
|
|
|
Deferred tax asset adjustment
|
|
|
2,629
|
|
|
|
—
|
|
|
|
—
|
|
|
Change in valuation allowance
|
|
|
2,684
|
|
|
|
(7,326
|
)
|
|
|
4,729
|
|
|
Income tax expense
|
|
$
|
174
|
|
|
$
|
—
|
|
|
$
|
—
|
|
Pozen had gross unrecognized tax benefits of approximately $0.5 million as of January 1, 2015. As of December 31, 2015, the total gross unrecognized tax benefits were approximately $0.6 million and, of this total, none would reduce Pozen’s effective tax rate if recognized. Pozen does not anticipate a significant change in total unrecognized tax benefits or Pozen’s effective tax rate due to the settlement of audits or the expiration of statutes of limitations within the next 12 months.
Pozen’s policy for recording interest and penalties associated with tax audits is to record them as a component of provision for income taxes. Pozen has not recorded any interest or penalty since adoption of FASB ASC 740-10.
Pozen has analyzed its filing positions in all significant federal, state and foreign jurisdictions where it is required to file income tax returns, as well as open tax years in these jurisdictions. With few exceptions, Pozen is no longer subject to U.S. federal and state and local tax examinations by tax authorities for years before 2012, although carryforward attributes that were generated prior to 2012 may still be adjusted upon examination by the Internal Revenue Service if they either have been or will be used in a future period. No income tax returns are currently under examination by taxing authorities.
Rollforward of gross unrecognized tax positions:
|
($ in thousands)
|
|
For the Years Ended December 31,
|
|
|||||||||
|
|
|
2015
|
|
|
2014
|
|
|
2013
|
|
|||
|
Uncertain tax positions at the beginning of the year
|
|
$
|
537
|
|
|
$
|
538
|
|
|
$
|
518
|
|
|
Additions based on tax positions related to the current year
|
|
|
3
|
|
|
|
—
|
|
|
|
—
|
|
|
Additions for tax positions of prior years
|
|
|
32
|
|
|
|
—
|
|
|
|
22
|
|
|
Subtractions based on tax positions related to the current year
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Subtractions for tax positions of prior years
|
|
|
—
|
|
|
|
(1
|
)
|
|
|
(2
|
)
|
|
Balance at the end of the year
|
|
$
|
572
|
|
|
$
|
537
|
|
|
$
|
538
|
|
|
6.
|
Equity Compensation Plans
|
In June 2007, the stockholders approved the amendment and restatement of the 2000 Plan to, among other things, increase the number of shares authorized for issuance under the 2000 Plan to 6,500,000 shares and continue the various performance criteria for use in establishing specific vesting targets for certain awards. In June 2010, stockholders approved the POZEN Inc. 2010 Plan to which all grants outstanding under Pozen’s previous equity plans were issued or transferred.
The 2010 Plan provides for grants of incentive stock options, nonqualified stock options, stock awards, and other stock-based awards, such as restricted stock units and stock appreciation rights (“SARs”), to employees, non-employee directors, and consultants and advisors who perform services for us and our subsidiaries. The 2010 Plan authorizes up to 7,452,327 shares of common stock for issuance. The maximum number of shares for which any individual may receive grants in any calendar year is 1,000,000 shares. The Compensation Committee of the Board of Directors, which administers the 2010 Plan, will determine the terms and conditions of options, including when they become exercisable. Neither our Board nor the Committee can amend the 2010 Plan or options previously granted under the Plan to permit a repricing of options or SARs, without prior stockholder approval. If options granted under the 2010 Plan expire or are terminated for any reason without being exercised, or if stock awards, performance units, or other stock-based awards are forfeited or otherwise terminate, the shares of common stock underlying the grants will again be available for awards granted under the 2010 Plan.
Our statements of comprehensive (loss) income for the years ended December 31, 2015, 2014 and 2013 include the following non-cash stock-based compensation expense:
|
For the Years Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Research and development
|
$
|
172,714
|
$
|
295,631
|
$
|
765,526
|
||||||
|
Selling, general and administrative
|
6,869,920
|
1,585,331
|
3,196,860
|
|||||||||
|
Total non-cash stock-based compensation expense
|
$
|
7,042,634
|
$
|
1,880,962
|
$
|
3,962,386
|
||||||
Unrecognized stock-based compensation expense, including time-based options, performance-based options and restricted stock unit awards, expected to be recognized over an estimated weighted-average amortization period of 3.4 years, was $26.9 million at December 31, 2015.
Time-Based Stock Option Awards
No new time-based stock option awards were granted during the years ended December 31, 2015 or December 31, 2014. Previously, the fair value of each time-based award was estimated on the date of grant using the Black-Scholes option valuation model, which used the assumptions described below. Our weighted-average assumptions used in the Black-Scholes valuation model for equity awards with time-based vesting provisions granted for the year ended December 31, 2013 is shown in the following table:
|
2013
|
||||
|
Expected volatility
|
63.7%
|
|
||
|
Expected dividends
|
0%
|
|
||
|
Expected terms
|
6.0 Years
|
|||
|
Risk-free interest rate
|
1.25%
|
|
||
|
Weighted average grant date fair value
|
$
|
5.35
|
||
For the year ended December 31, 2013, the expected volatility rate was estimated based on an equal weighting of the historical volatility of Pozen common stock over approximately a six-year period and the expected term was based upon average historical terms to exercise. The risk-free interest rate was based on six-year U.S. Treasury securities. The pre-vesting forfeiture rates used of the year ended December 31, 2013 was based on historical rates. We adjust the estimated forfeiture rate based upon actual experience.
A summary of the time-based stock option awards as of December 31, 2015, and changes during the year ended December 31, 2015, are as follows:
|
Time-Based Stock Option Awards
|
Underlying
Shares
(000s)
|
Weighted-
Average
Exercise
Price
|
Average
Remaining
Contractual
Term
(years)
|
Aggregate
Intrinsic
Value
(000s)
|
||||||||||||
|
Outstanding at December 31, 2014
|
2,341
|
$
|
7.39
|
4.1
|
$
|
4,382
|
||||||||||
|
Granted
|
―
|
―
|
||||||||||||||
|
Exercised
|
(727
|
)
|
5.66
|
|||||||||||||
|
Forfeited or expired
|
(35
|
)
|
12.95
|
|||||||||||||
|
Outstanding at December 31, 2015
|
1,579
|
$
|
8.06
|
2.9
|
$
|
1,725
|
||||||||||
|
Exercisable at December 31, 2015
|
1,515
|
$
|
8.24
|
2.7
|
$
|
1,538
|
||||||||||
|
Vested or expected to vest at December 31, 2015
|
1,569
|
$
|
8.06
|
2.9
|
$
|
1,715
|
||||||||||
The aggregate intrinsic value of options outstanding represents the pretax value (the period’s closing market price, less the exercise price, times the number of in-the-money options) that would have been received by all option holders had they exercised their options at the end of the period. The exercise price of stock options granted during the years ended December 31, 2015, 2014 and 2013 was equal to the market price of the underlying common stock on the grant date. A total of 727,000 stock options were exercised during the year ended December 31, 2015 with an intrinsic value of $2.0 million, a total of 1,479,000 stock options were exercised during the year ended December 31, 2014 with an intrinsic value of $4.6 million and a total of 138,562 stock options were exercised during the year ended December 31, 2013 with an intrinsic value of $0.6 million. The fair value of shares vested during the years ended December 31, 2015, 2014 and 2013 was $1.8 million, $1.1 million and $0.6 million, respectively.
F-36
A summary of the time-based nonvested option awards as of December 31, 2015, and changes during the year ended December 31, 2015, are as follows:
|
Underlying
Shares
(000s)
|
Weighted-
Average
Exercise
Price
|
|||||||
|
Nonvested outstanding at December 31, 2014
|
477
|
$
|
3.85
|
|||||
|
Granted
|
―
|
―
|
||||||
|
Forfeited or expired
|
(3
|
)
|
3.87
|
|||||
|
Vested
|
(411
|
)
|
4.47
|
|||||
|
Nonvested outstanding at December 31, 2015
|
63
|
$
|
3.87
|
|||||
Restricted Stock and Restricted Stock Units
For the years ended December 31, 2015, 2014 and 2013, Pozen recognized $6.4 million, $1.0 million and $1.2 million, respectively, in compensation expense related to restricted stock units. Stock-based compensation expense for the year ended December 31, 2015 includes expense related to new restricted stock awards granted to certain Aralez employees and expense for the accelerated vesting of awards under the terms of the separation agreement with Pozen’s former President and Chief Executive Officer.
A summary of the restricted stock awards as of December 31, 2015, and changes during the year ended December 31, 2015, are as follows:
|
Underlying
Shares
(000s)
|
Weighted-
Average
Exercise
Price
|
|||||||
|
Restricted stock outstanding at December 31, 2014
|
1,109
|
$
|
7.14
|
|||||
|
Granted
|
3,817
|
7.80
|
||||||
|
Vested and exercised
|
(879
|
)
|
6.98
|
|||||
|
Forfeited or expired
|
(5
|
)
|
7.22
|
|||||
|
Restricted stock outstanding at December 31, 2015
|
4,042
|
$
|
7.80
|
|||||
As of December 31, 2015, there was an aggregate $26.9 million of unrecognized compensation expense related to unvested restricted stock units. Of the aggregate amount, $22.4 million unrecognized compensation expense related to unvested restricted stock units under the June 2015 award of 3,421,562 restricted stock units with a grant-date per-share fair value of $7.64. There were 4.0 million unvested restricted stock units outstanding at December 31, 2015, 627,000 unvested restricted stock units outstanding at December 31, 2014 and 523,000 unvested restricted stock units outstanding at December 31, 2013. The total fair value of restricted stock that vested and exercised during the years ended December 31, 2015, 2014 and 2013 was $6.6 million, $0.7 million and $0.9 million, respectively.
Performance-Based Stock Option Awards
In October 2011, pursuant to an incentive program (the “PA32540 incentive program”) approved by the Compensation Committee of the Board of Directors of Pozen, stock options were granted to all of Pozen’s employees, including its executive officers, to purchase an aggregate of 453,960 shares of common stock. The underlying stock options and RSUs were performance-based and focus on the successful completion of certain value-enhancing events for Pozen’s YOSPRALA product candidate. The stock options have a ten-year term and have an exercise price equal to the closing sale price of Pozen’s common stock, as reported on the NASDAQ Global Market, on the date immediately preceding the date of grant. The underlying stock options and RSUs vest in accordance with the following schedule: (a) one-third (1/3) upon the acceptance of the filing of a New Drug Application (the “NDA”) for YOSPRALA, assuming the NDA filing is made prior to December 31, 2012, (b) one-third (1/3) upon first cycle NDA approval of YOSPRALA (otherwise 16.5% upon NDA approval after first cycle), and (c) one-third (1/3) upon execution of a significant partnering transaction for YOSPRALA in a major territory as determined by the Compensation Committee of Pozen, in its sole discretion, at the time of such transaction, subject in each case to continued employment or service to Pozen.
During a pre-submission meeting with respect to its NDA for YOSPRALA in April 2012, the FDA suggested that Pozen also seek approval for a lower dose formulation of the product containing 81 mg of enteric-coated aspirin as part of its NDA for YOSPRALA. Pozen decided to include data and information relating to a lower dose formulation in its NDA. Generation of additional data with respect to lower dose formulation of YOSPRALA and incorporation of data into the NDA for YOSPRALA would delay submission of the NDA from the original planned submission date.
Therefore, in October 2012, the Compensation Committee granted performance-based incentive awards (the “PA8140 incentive program”) both to compensate the employees for the expected loss of value under the PA32540 incentive program, as well as to provide additional incentive to employees to complete the value-added activities required for submission and approval of the lower dose product. The Compensation Committee granted an aggregate of 208,740 restricted stock units to various employees of Pozen, including 105,000 restricted stock units granted to Pozen’s named executive officers at the time. The restricted stock units were performance-based and vest in accordance with the following schedule: (a) one-half (1/2) upon the acceptance by the FDA of the filing of an NDA for a lower dose YOSPRALA product candidate, and (b) one-half (1/2) upon approval by the FDA of an NDA for a lower dose YOSPRALA product candidate. In 2012, 132,883 options were forfeited in acknowledgement that certain performance goals would not be met under the PA32540 incentive program.
In April 2014, the Compensation Committee granted an aggregate of 73,000 restricted stock units to various employees of Pozen, including 65,000 restricted stock units granted to Pozen’s named executive officers at the time. The restricted stock units were performance-based and vest in accordance with the following schedule: (i) 50% upon receipt of the milestone payment by Sanofi US under the Sanofi License Agreement to be received upon approval by the FDA of the PA product candidates; and (ii) 50% upon receipt of the milestone payment by Sanofi US upon achievement of commercial readiness (as defined in the Sanofi License Agreement). The entire award was forfeited in 2014 upon the termination of the Sanofi License Agreement. In 2014, a total of 177,818 options were forfeited in acknowledgement that certain performance goals would not be met under the PA32540 incentive program and PA8140 incentive program.
During the year ended December 31, 2015, there was a compensation expense of $36,000 recorded related to performance-based awards. As of December 31, 2015, there was no unrecognized compensation expense.
A summary of the performance-based stock awards as of December 31, 2015, and changes during the year ended December 31, 2015, are as follows:
|
Underlying Shares
(000s)
|
Weighted-Average
Exercise Price
|
|||||||
|
Performance-based outstanding at December 31, 2014
|
368
|
$
|
8.12
|
|||||
|
Granted
|
154
|
8.83
|
||||||
|
Exercised
|
(40
|
)
|
4.51
|
|||||
|
Forfeited or expired
|
(76
|
)
|
8.51
|
|||||
|
Performance-based outstanding at December 31, 2015
|
406
|
$
|
8.67
|
|||||
Under the various incentive programs, there were 215,000 unvested performance-based options outstanding at December 31, 2015. No performance-based awards vested during the year ended December 31, 2015, December 31, 2014 and December 31, 2013. There were 190,000 vested performance-based options outstanding at December 31, 2015. Awards forfeited were 76,000, 199,000 and 37,000 during the years ended December 31, 2015, 2014 and 2013, respectively. Performance-based awards exercised were 40,000, 46,000 and 162,000 during the years ended December 31, 2015, 2014 and 2013, respectively. At December 31, 2015, the performance-based options had an intrinsic value of $1.5 million and a remaining weighted contractual life of 5.6 years.
| 7. | Leases |
Pozen leases its office space and certain equipment under cancellable and noncancellable operating lease agreements. Rent expense incurred by Pozen was approximately $0.4 million for each of the years ended December 31, 2015, 2014 and 2013. At December 31, 2015, noncancellable future minimum lease payments for operating leases totaled $2.7 million.
On February 16, 2009, Pozen modified certain terms to our existing lease agreement, dated November 21, 2001, relating to approximately 17,009 square feet of office space located at Exchange Office Building, Chapel Hill, North Carolina. Under the terms of the modification, the lease term was extended for an additional five years and seven months, terminating on September 30, 2015. As a result of entering into the modification, Pozen’s noncancellable future minimum lease payments for operating leases increased by approximately $2.7 million over the lease term. On July 15, 2015, Pozen signed a six-month extension to its lease, adding approximately $0.1 million to its lease commitments. This lease agreement, as amended, expires March 31, 2016.
In September 2015, Pozen’s wholly-owned subsidiary, Aralez Pharmaceuticals US Inc., entered into a lease for an approximately 4,000 square foot office space located in New York, New York. The lease term is five years and two months, terminating on October 31, 2020. In October 2015, Aralez Pharmaceuticals US Inc. entered into a lease for an approximately 4,500 square foot office space located in Radnor, Pennsylvania. The lease term is five years and two months, terminating on December 31, 2020, with a five-year extension term available at Aralez Pharmaceuticals US Inc.’s option.
Pozen had the following minimum payments under operating lease obligations that existed at December 31, 2015:
|
2016
|
$
|
500,000
|
||
|
2017
|
500,000
|
|||
|
2018
|
504,000
|
|||
|
2019
|
509,000
|
|||
|
2020
|
472,000
|
|||
|
Thereafter
|
190,000
|
|||
|
Total minimum payments
|
$
|
2,675,000
|
| 8. | Retirement Savings Plan |
Pozen has adopted a defined contribution 401(k) plan (the “Plan”) covering substantially all employees who are at least 21 years of age. Based upon management’s discretion, Pozen may elect to make contributions to the Plan. During the years ended December 31, 2015, 2014 and 2013, Pozen made contributions of $0.2 million, $0.1 million and $0.2 million, respectively, to the Plan. The Plan was amended to include all U.S. employees.
| 9. | Subsequent Events (Unaudited) |
On February 5, 2016, Aralez announced the completion of the Tribute Transaction following the approval of the transaction by stockholders of Pozen and shareholders of Tribute. In connection with the transaction, Pozen and Tribute were combined under and became subsidiaries of Aralez, with Pozen treated as the acquiring company for accounting purposes.
The combined company will operate under Aralez, a global specialty pharmaceutical company with operations in Canada, Ireland and the United States. Under the terms of the Agreement and Plan of Merger and Arrangement, each share of Pozen common stock has been converted into the right to receive one Aralez common share and each common share of Tribute (other than dissenting shares) has been exchanged for 0.1455 Aralez common shares.
The preliminary fair value of consideration transferred as of the acquisition date of February 5, 2016 is approximately $138 million made up of (i) $115 million related to Tribute shares, equity awards and certain warrants outstanding and (ii) $23 million in repayments of Tribute indebtedness. We have not provided an allocation of the preliminary purchase price as the initial accounting for the business combination is incomplete.
Concurrent with the transaction, on February 5, 2016, Tribute issued $75 million of senior secured convertible promissory notes and $75 million of Tribute shares in a private placement. In conjunction with the Tribute Transaction, the convertible promissory notes were immediately assumed by Aralez and the Tribute shares were immediately converted into Aralez shares using a conversion factor of 0.1455. Aralez also has a $200 million acquisition facility until April 30, 2017. The acquisition facility is currently undrawn, can be drawn on for permitted acquisitions and is to be repaid over a 6-year period from each draw. Amounts drawn under the acquisition facility will bear an interest rate of 12.5% per annum and shall be prepayable in whole or in part at any time following the end of the sixth month after the funding date of each draw.
| 10. | Summary of Operations by Quarters (Unaudited) |
|
2015
|
||||||||||||||||
|
1st Quarter
|
2nd Quarter
|
3rd Quarter
|
4th Quarter
|
|||||||||||||
|
Royalty and licensing revenue
|
$
|
4,404,463
|
$
|
5,200,852
|
$
|
5,820,184
|
$
|
5,965,915
|
||||||||
|
Operating expenses
|
$
|
4,245,831
|
$
|
20,495,360
|
$
|
14,013,456
|
$
|
20,102,814
|
||||||||
|
Loss before income tax expense
|
$
|
(27,358
|
)
|
$
|
(15,279,226
|
)
|
$
|
(8,176,132
|
)
|
$
|
(14,126,342
|
)
|
||||
|
Income tax expense (benefit)
|
$
|
—
|
$
|
1,001,000
|
$
|
(27,000
|
)
|
$
|
(799,613
|
)
|
||||||
|
Net loss
|
$
|
(27,358
|
)
|
$
|
(16,280,226
|
)
|
$
|
(8,149,132
|
)
|
$
|
(13,326,729
|
)
|
||||
|
Basic net loss per common share
|
$
|
0.00
|
$
|
(0.50
|
)
|
$
|
(0.25
|
)
|
$
|
(0.40
|
)
|
|||||
|
Diluted net loss per common share
|
$
|
0.00
|
$
|
(0.50
|
)
|
$
|
(0.25
|
)
|
$
|
(0.40
|
)
|
|||||
|
2014
|
||||||||||||||||
|
1st Quarter
|
2nd Quarter
|
3rd Quarter
|
4th Quarter
|
|||||||||||||
|
Royalty and licensing revenue
|
$
|
7,548,676
|
$
|
7,419,306
|
$
|
7,539,741
|
$
|
9,886,509
|
||||||||
|
Operating expenses
|
$
|
4,651,396
|
$
|
4,426,615
|
$
|
3,628,176
|
$
|
3,112,432
|
||||||||
|
Income before income tax expense
|
$
|
2,904,691
|
$
|
2,999,457
|
$
|
6,752,169
|
$
|
7,018,415
|
||||||||
|
Income tax expense
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
||||||||
|
Net income
|
$
|
2,904,691
|
$
|
2,999,457
|
$
|
6,752,169
|
$
|
7,018,415
|
||||||||
|
Basic net income per common share
|
$
|
0.09
|
$
|
0.10
|
$
|
0.21
|
$
|
0.22
|
||||||||
|
Diluted net income per common share
|
$
|
0.09
|
$
|
0.09
|
$
|
0.20
|
$
|
0.21
|
||||||||
F-41
