Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☐ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2015
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from___________ to __________
Commission file number 001-36646
Asterias Biotherapeutics, Inc.
(Exact name of registrant as specified in its charter)
|
Delaware
|
46-1047971
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
6300 Dumbarton Circle
Fremont, California 94555
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code
(510) 456-3800
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Name of exchange on which registered
|
|
|
Series A Common Stock, $0.0001 par value
|
NYSE MKT
|
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐
|
Accelerated filer ☐
|
|
Non-accelerated filer ☒ (Do not check if a smaller reporting company)
|
Smaller reporting company☐
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act):
Yes o No x
The aggregate market value of shares of voting and non-voting common equity held by non-affiliates of the registrant on June 30, 2015 (based on the closing price for shares of the registrant’s common stock as reported on the NYSE MKT under the symbol AST on that date) was approximately $70,005,592.
As of March 23, 2016, there were outstanding 38,352,150 shares of Series A Common Stock, par value $0.0001 per share.
DOCUMENTS INCORPORATED BY REFERENCE
2
Asterias Biotherapeutics, Inc.
|
Page
Number
|
|||
|
Part I.
|
Financial Information
|
||
|
Item 1 -
|
5
|
||
|
Item 1A
|
21
|
||
|
Item 1B
|
38
|
||
|
Item 2 -
|
38
|
||
|
Item 3 -
|
39
|
||
|
Item 4 -
|
39
|
||
|
Part II.
|
Other Information
|
||
|
Item 5 -
|
40
|
||
|
Item 6 -
|
42
|
||
|
Item 7 -
|
43
|
||
|
Item 7A
|
50
|
||
|
Item 8 -
|
55
|
||
|
Item 9 -
|
80
|
||
|
Item 9A-
|
80
|
||
|
Item 9B
|
81
|
||
|
Part III.
|
Item 10 -
|
82
|
|
|
Item 11 -
|
86
|
||
|
Item 12 -
|
92
|
||
|
Item 13 -
|
95
|
||
|
Item 14 -
|
98
|
||
|
Part IV
|
Item 15 -
|
101
|
|
|
105
|
|||
PART I
Certain statements contained herein are forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for Asterias, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management constitute forward-looking statements. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements and as such should be evaluated together with the many uncertainties that affect the businesses of Asterias, particularly those mentioned in the cautionary statements found in Asterias’ filings with the Securities and Exchange Commission. Asterias disclaims any intent or obligation to update these forward-looking statements
References to “Asterias,” “our” or “us” means Asterias Biotherapeutics, Inc.
The description or discussion, in this Form 10-K, of any contract or agreement is a summary only and is qualified in all respects by reference to the full text of the applicable contract or agreement.
Preliminary Note Regarding Ownership of Our Common Stock
As of March 23, 2016, we had 530 shareholders of record and there were 38,352,150 shares of our Series A Common Stock (“Series A Shares”) outstanding, of which 21,747,569 Series A Shares were held by our parent BioTime, Inc. ("BioTime"). These Series A Shares held by BioTime account for 56.7% of our Series A Shares outstanding as a whole. Accordingly, we are a consolidated subsidiary of BioTime.
Overview
Asterias is a clinical-stage biotechnology company focused on developing and commercializing novel therapies in the emerging fields of cell therapy and regenerative medicine. We have two core technology platforms. The first is an immunotherapy platform to teach cancer patients’ immune systems to attack their tumors. The second is pluripotent stem cell platform. Pluripotent cells are a type of stem cell capable of becoming all of the cell types in the human body. We are focused on developing therapies to treat conditions with high unmet medical needs and inadequate available therapies, with an initial focus on the therapeutic areas of oncology and neurology.
In October of 2013, we acquired intellectual property, cell lines, preclinical and clinical data, and other assets from Geron Corporation ("Geron") and also acquired rights to use certain human embryonic stem ("hES") cell lines and to practice certain patents from our parent company, BioTime, Inc. ("BioTime"). From the assets acquired in these transactions, we have prioritized the development of our two core technology platforms. From our immunotherapy platform, we are developing two programs. AST-VAC1 (telomerase loaded, autologous dendritic cells) which could teach a patient's own cells to recognize and fight cancer cells in acute myelogenous leukemia(AML). Together with our collaboration partner, Cancer Research United Kingdom (“CRUK”) we are also developing AST-VAC2 (telomerase loaded, -allogeneic dendritic cells), -derived from pluripotent stem cells which could provide 'off the shelf' cells that will teach a patient's immune system to recognize and fight cancer cells, in non-small cell lung cancer. We believe that our immunotherapy programs have potential for application in additional cancer indications. From our pluripotent stem cell platform we are developing AST-OPC1, oligodendrocyte progenitor cells, in an initial clinical indication of spinal cord injury, with potential for later expansion into other neurodegenerative diseases such as stroke and multiple sclerosis.
Products Under Development
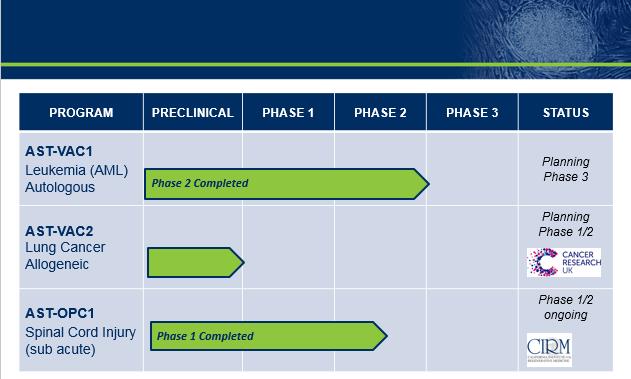
Product Candidates
AST-VAC1 and AST-VAC2, Cancer Vaccine Candidates Targeting Telomerase
We are developing two experimental immunotherapeutic programs, AST-VAC1 and AST-VAC2, each designed to attack cancer cells by targeting the cancer cell’s expression of telomerase. Both product candidates use an immune cell type known as dendritic cells to stimulate immune responses to telomerase. Dendritic cells are antigen processing and presenting cells which are potent initiators of a cellular and antibody-mediated immune response Telomerase is a ubiquitous cancer antigen, expressed at high levels in nearly all human cancers, but at very low levels or not at all in normal human cells. The premise underlying these vaccines is to “teach” the patient’s own immune system to attack cancer cells while sparing other normal healthy cells.
AST-VAC1: Autologous Telomerase-loaded, Dendritic Cells
AST-VAC1 is an autologous product candidate, or a product that is derived from cells that come from the treated patient. AST-VAC1 consists of mature antigen-presenting dendritic cells pulsed with RNA for the protein component of human telomerase (“hTERT”) and a portion of a lysosomal targeting signal ("LAMP"). LAMP directs the telomerase RNA to the lysosome, the subcellular organelle that directs the RNA to a particular part of the cell membrane. AST-VAC1 is injected into the patient’s skin, with the objective of the dendritic cells to travel to the lymph nodes and instruct cytotoxic T-cells to kill tumor cells that express telomerase on their surface.
A Phase 2 clinical trial of AST-VAC1 was conducted at six U.S. medical centers in patients with acute myeloid leukemia (“AML”) in complete clinical remission. The trial examined the safety and feasibility of a prime-boost vaccination regimen (an initial injection ("prime") followed by multiple additional injections ("boost") to generate and extend the duration of telomerase immunity. This trial completed patient enrollment in December 2009. Thirty three patients with AML entered the study in their first or second complete remission. Prior to or shortly after completing consolidation chemotherapy, patients underwent leukapheresis, a process of collecting of white blood cells directly from the patient. AST-VAC1 was produced at a centralized manufacturing facility from the patient-specific white blood cells. Patient blood cells were differentiated to dendritic cells in culture, modified to express telomerase linked to the LAMP targeting signal, aliquoted and cryopreserved. AST-VAC1 was released for patient dosing contingent on several product specifications that included identity of mature dendritic cells, confirmation of telomerase expression, number of viable cells per dose after thawing, and product sterility.
AST-VAC1 was successfully manufactured and released in 24 out of the 33 patients enrolled in the study. Three patients progressed prior to vaccination, therefore only 21 of the 24 patients for whom AST-VAC1 was successfully manufactured and released received vaccine. The 21 patients were vaccinated weekly for six weeks, with AST-VAC1 administered intra-dermally (injection into the skin), followed by a non-treatment period of four weeks, and then subsequent boost injections every other week for 12 weeks. Monthly extended boost injections were then administered until the vaccine product supply was depleted or the patient relapsed.
Twenty-one patients received AST-VAC1 in the study, including 19 in clinical remission and two in early relapse. AST-VAC1 was found to have a favorable safety and tolerability profile in this study over multiple vaccinations, with up to 32 serial vaccinations administered (median = 17). Idiopathic thrombocytopenic purpura (bleeding into the skin caused by low platelets in blood) (grade 3-4) was reported in one patient. Other toxicities (grade 1-2) included rash or headache. These data from the trial were presented at the December 2010 American Society of Hematology annual meeting.
Patient immune response to telomerase after vaccination with AST-VAC1 was evaluated using a test called the enzyme-linked immunosorbent spot (“ELISPOT”) assay to measure the presence of activated T-cells specific to hTERT. Positive immune responses were detected in 55% of patients.
We have performed follow-up data collection on the 19 patients treated in complete remission to determine the long term effects of the AST-VAC1 administration on remission duration and disease-free survival. The results of this data collection were reported in an oral presentation at the American Society of Clinical Oncology annual meeting in May 2015. Eleven of 19 patients (58%) remained in complete remission at a median follow-up of 52 months. These results compare to historical data suggesting that between 20-40% of patients would be expected to be relapse free at 3-4 years. Additionally, of the 7 patients in the higher risk over 60 year old group, 4 (57%) remained relapse free at a median follow up of 54 months. Historically, relapse free survival rates in this population have been 10-20% at 3-4 years. We are in the process of preparing a manuscript describing our findings, and performing certain process development, clinical and regulatory activities.
We have conducted an End of Phase 2 meeting with the FDA with the goal of reviewing the proposed clinical development plan for AST-VAC1. In February 2016, we announced that the FDA indicated general agreement with Asterias' proposed development plan for registration of AST-VAC1 through a single Phase 3 trial to support an accelerated development pathway and BLA filing. In this study, Asterias will assess the impact of AST-VAC1 compared to placebo on the duration of relapse-free-survival as the primary endpoint, and on overall survival as the secondary endpoint in patients who have achieved complete remission using standard therapies. The proposed trial will include AML patients 60 years and older, along with younger individuals who are at high risk for relapse and are not candidates for allogeneic bone marrow transplantation. Pending positive results, this trial could be the basis for accelerated approval of AST-VAC1. We currently plan to submit a request for a Special Protocol Assessment (SPA) to the FDA to confirm the primary endpoint and other design elements of this pivotal Phase 3 trial.
AST-VAC2: hES Cell-Derived Allogeneic Dendritic Cells
AST-VAC2 is an allogeneic, or non-patient specific, cancer vaccine candidate designed to stimulate patient immune responses to telomerase. AST-VAC2 is produced from hES cells and can be modified with any antigen. We believe that the use of hES, as opposed to collecting and using the patient’s own blood, as the starting material for AST-VAC2 provides a scalable system for the production of a large number of vaccine doses in a single lot. Allogeneic vaccine production has the potential to have lower manufacturing costs, “off-the-shelf” availability and broader patient availability, and ensure product consistency. In addition, we believe that this approach has the potential to stimulate a more robust immune response through an adjuvant effect of the immune mismatch between the genetic makeup of AST-VAC2 and patients. Further, we believe AST-VAC2 may be synergistic with immune checkpoint inhibitors currently in development for many cancer indications. This is because immune checkpoint inhibitors function by relieving suppressive mechanisms exerted on T-cells by the tumor, whereas AST-VAC2 is designed to specifically target the T-cells to attack the telomerase expressing tumor cells.
Product Development Strategy for AST-VAC2
During September 2014, we entered into a Clinical Trial and Option Agreement with Cancer Research UK (“CRUK”) with CRUK and Cancer Research Technology Limited, (“CRT”), a wholly-owned subsidiary of CRUK the “CRUK Agreement”. Under the CRUK Agreement, CRUK has agreed to fund Phase 1/2 clinical development of our AST-VAC2 product candidate loaded with the same LAMP-telomerase construct we have used in AST-VAC1. Under the terms of the CRUK Agreement, we are responsible, at our own cost, for completing process development and manufacturing scale-up of the AST-VAC2 manufacturing process and transferring the resulting cGMP-compatible process to CRUK. CRUK is responsible, at its own cost, for manufacturing clinical grade AST-VAC2 and for carrying out the Phase 1/2 clinical trial of AST-VAC2.
In January 2016 we announced that we had completed the technology transfer of the AST-VAC2 manufacturing process to CRUK. CRUK is now verifying and scaling up the production of AST-VAC2 in their facility in preparation for pilot and full cGMP campaigns. Upon successful completion of AST-VAC2 production campaigns, Cancer Research UK’s Centre for Drug Development (“CDD”) will submit a Clinical Trial Authorization application to the UK regulatory authorities for the Phase 1/2 clinical trial in non-small cell lung cancer, which will be sponsored, managed and funded by CDD. The clinical trial will examine the safety, immunogenicity and activity of AST-VAC2 and position the immunotherapy to be tested for numerous clinical indications. We will continue to serve in a collaborative and advisory role with CRUK throughout this process.
Upon completion of the Phase 1/2 study, we will have an exclusive first option to acquire the data generated in the trial. If we exercise that option we will be obligated to make payments upon the execution of the license agreement, upon the achievement of various milestones, and then royalties on sales of products. In connection with the CRUK Agreement, we sublicensed to CRUK for use in the clinical trials and product manufacturing process certain patents that have been licensed or sublicensed to us by third parties. We would also be obligated to make payments to those licensors and sublicensors upon the achievement of various milestones, and then royalties on sales of products if AST-VAC2 is successfully developed and commercialized.
AST-OPC1 Glial Progenitor Cells
Our AST-OPC1 product candidate is comprised of oligodendrocyte progenitor cells, which are cells that become oligodendrocytes after injection, derived from a cGMP master cell bank of undifferentiated hES cells that has been fully qualified for human use. These cells, which are stored frozen until ready for use, are produced under cGMP conditions and screened for adventitious agents.
Oligodendrocytes are nature’s neuronal insulating cells. Like the insulation covering an electrical wire, oligodendrocytes enable the conduction of electrical impulses along nerve fibers throughout the central and peripheral nervous system. They are also known to promote neural growth, as well as induce blood vessel formation around nerve axons. AST-OPC1 cells reproduce all of the natural functions of oligodendrocytes in animal models, including: producing myelin that wraps around nerve fibers; producing neurotrophic factors which encourage neuro-regeneration and sprouting of new nerve endings, and inducing new blood vessels which provide nutrients and remove waste matter from neural tissue as it functions in the body.
The pathology of spinal cord injury involves extensive loss of the myelin sheath produced by oligodendrocytes at the site of injury.
There are currently no drugs approved by the United States Food and Drug Administration (“FDA”) specifically for the treatment of spinal cord injury, although methylprednisolone, a corticosteroid generally used as an anti-inflammatory drug, is sometimes prescribed on an off-label basis to reduce acute inflammation in the injured spinal cord immediately after injury. It is believed that in order to effect substantial benefit in treating this complex injury, multiple mechanisms of action are required, such as re-myelination of the demyelinated axons, generation of new blood vessels to repair the ischemic damage from injury, and the presence of biologics that cause neuro-sprouting or new nerve growth to enable the severed axons to repair. In studies to date, AS-OPC1 cells have been shown to exhibit all three effects.
Multiple studies in a validated rat model of spinal cord injury have been performed using AST-OPC1. These studies have shown that a single injection of AST-OPC1 cells at the site of injury produces durable re-myelination, new blood vessel formation, and new neuronal sprouting, all of which result in sustained and significant improvement in the animal’s locomotion within several months after injection. These data provided the rationale to initiate testing of AST-OPC1 to treat acute spinal cord injury in humans.
Phase I Safety Trial
After FDA authorization, AST-OPC1 was tested in patients with acute spinal cord injury beginning in October 2010. The trial was an open label design conducted at seven U.S. neuro-trauma sites. Five subjects were treated in the trial, each of whom had a sub-acute functional complete thoracic (chest) spinal cord lesion. Patients enrolled in the study received a single dose of 2 x 10 6 cells at the injury site between seven and 14 days after injury. All subjects received temporary low dose immune suppression treatment for 60 days. The primary endpoint of the study was safety, with secondary endpoints of neurologic function assessed by five different validated measures of sensory and motor function. Each subject received a screening MRI, and if treated and entered into the treatment protocol, received eight follow-up MRIs in the first year and multiple physical exams and laboratory testing. The patients then entered a separate protocol after the first year which will follow them intermittently over a period of 15 years.
As of March 23, 2016, the first patient has completed all five years of their follow-up data set and the remaining 4 patients have completed their four year follow-up data set. No surgical complications during or post-surgery have been observed, and there have been no significant adverse events to date in any patient attributable to the AST-OPC1 product, the surgery to deliver the cells, or the immunosuppressive regimen. There have been five minor adverse events possibly related to AST-OPC1 such as transient fever and nerve pain. There have been no unexpected neurological changes to date, nor has there been evidence of adverse changes or cavitation on multiple MRIs. MRI results in four of the five subjects are consistent with prevention of lesion cavity formation. Immune monitoring, conducted in some of the patients, has not detected any evidence of immune responses to AST-OPC1 at time periods of up to one year post-transplant.
Phase I/IIa Dose Escalation Study: Subjects with Neurologically Complete Cervical Spinal Cord Injuries
Based on the results of the completed Phase I trial of AST-OPC1 in thoracic Spinal Cord Injury (“SCI”), we obtained permission from the FDA in August 2014 to initiate a Phase I/IIa dose escalation trial in patients with neurologically complete cervical spinal cord injuries. Individuals with neurologically complete cervical SCI have an enormous unmet medical need due to the loss of function in all four limbs as well as multiple additional impairments such as impaired bowel and bladder function, reduced sensation, spasticity, sudden changes in blood pressure, deep vein thrombosis, sexual dysfunction, increased infections, skin pressure sores, and chronic pain. These individuals frequently require significant assistance for their care and activities of daily living. One recent published study estimated the lifetime costs of care for a person who suffers a cervical SCI at age 25 to be $4.2 million (Y. C. Cao and M. J. DeVivo (2009)).
Scientifically, the injured cervical spinal cord is a much better location than the upper or middle thoracic spinal cord to test the safety and potential activity of AST-OPC1. This is partly due to the fact that damaged and demyelinated nerve axons in thoracic injuries need to regrow over several spinal segments in order to restore neural function. In contrast, damaged and demyelinated nerve axons in cervical injuries only need to regrow a short distance to restore neural function.
We initiated enrollment of the Phase I/IIa dose escalation trial of AST-OPC1 in patients with complete cervical injuries in March 2015. The trial is designed to assess safety and activity of three escalating doses of AST-OPC1 in complete cervical SCI, the first targeted indication for AST-OPC1. The trial is an open-label, single-arm study in patients with sub-acute, C-5 to C-7, neurologically complete cervical SCI. These individuals have lost all sensation and movement below their injury site with severe paralysis of the upper and lower limbs. AST-OPC1 will be administered 14 to 30 days post-injury. Patients will be followed by neurological exams and imaging methods to assess the safety and activity of the product. We completed enrollment in the first (2 million cells) dose cohort in August 2015. No serious adverse events related to AST-OPC1, the administration procedure, or the immunosuppressive regimen have been observed to date. We are currently open for enrollment in the second (10 million cells) cohort. Following collection of initial safety data from this second dose cohort, we plan to seek FDA concurrence to increase the robustness of the proof of concept in the Phase I/IIa clinical trial by expanding enrollment. Asterias has received a Strategic Partnerships Award grant from the California Institute for Regenerative Medicine, which provides for up to $14.3 million of non-dilutive funding for the Phase I/IIa clinical trial and other product development activities for AST-OPC1, subject to achieving certain milestones.
Additionally, in February 2016, we announced that the FDA had granted our application for Orphan Drug Designation of AST-OPC1 for the treatment of acute spinal cord injury.
AST-OPC1 CIRM Grant
The California Institute for Regenerative Medicine, or CIRM, provided us a Strategic Partnerships Award grant that provides for up to $14.3 million of non-dilutive funding for the Phase I/IIa clinical trial and other product development activities for AST-OPC1, subject to achieving certain milestones. The grant will provide partial funding for the SCIStar study and for product development efforts to refine and scale manufacturing methods to support commercialization. Under our amended agreement effective March 2, 2016, CIRM will disburse the grant funds to us contingent on our achievement of certain specific progress milestones. As the distributions of the CIRM grant are subject to meeting certain milestones, there can be no assurance that we will receive the entire amount granted. In addition, pursuant to the Award, we agreed to notify and report to CIRM information relating to serious adverse events, studies, press releases clinical trial information and routine communications in accordance with an agreed schedule.
As of March 28, 2016, we have received $7.8 million of payments from CIRM, and recent progress on the Phase l/2a dose escalation trial for OPC-1 is expected to result in a further $2.5 million payment under the terms of the existing CIRM award grant.
Failure to timely achieve milestones or otherwise satisfy CIRM regarding any delay could lead CIRM to suspend payments. The foregoing description of our arrangement with CIRM is a summary only and is qualified by reference to the Notice of Grant Award, dated as of October 16, 2014, the Amendment to Notice of Grant Award, dated as of November 26, 2014, and Amendment No. 2 to the Notice of Grant Award, dated as of March 2, 2016 between us and CIRM.
We will need to raise additional capital in order to conduct the Phase I/IIa clinical trial and any subsequent clinical trial and product development work. We intend to apply for a supplementary CIRM grant to provide funding for the clinical trial expansion.
Manufacturing and Process Development Technologies
We have sufficient existing clinical grade lots of AST-OPC1 for the ongoing Phase 1/2 trial, and cGMP master and working cell banks of undifferentiated human embryonic stem (hES) cells of the H1 and H7 cell lines. Both the H1 and H7 hES cell lines have been routinely expanded under either cGMP (H1) or pilot (H7) conditions. No limit to the expandability of hES cell lines has been observed. The cGMP cell banks of undifferentiated hES cells have been qualified for human biologics production per FDA guidelines. They exhibit normal chromosomal structure and are considered suitable for the production of biologics for human clinical use.
Additionally we have completed construction of and are currently validating a cGMP manufacturing facility at our Fremont, California headquarters. This facility is intended for use to produce additional cGMP cell banks and AST-OPC1 to supply Phase 3 clinical development and early commercial drug supply.
Intellectual Property
Our policy is to seek to protect our proprietary position by, among other methods, filing United States and foreign patent applications related to our proprietary technologies and any improvements that we consider important to the development and implementation of our business and strategy. In addition to relying on patents, we rely on trade secrets, know-how, and continuing technological advancement to maintain our competitive position. We will enter into intellectual property, invention, and non-disclosure agreements with our employees, and it will be our practice to enter into confidentiality agreements with our consultants. There can be no assurance, however, that these measures will prevent the unauthorized disclosure or use of our trade secrets and know-how, or that others may not independently develop similar trade secrets and know-how or obtain access to our trade secrets, know-how, or proprietary technology.
Our success depends, in part, upon our ability to obtain and maintain patent and other intellectual property protection for our product candidates including compositions-of-matter, dosages, and formulations, manufacturing methods, and novel applications, uses and technological innovations related to our product candidates and core technologies. We also rely on trade secrets, know-how and continuing technological innovation to further develop and maintain our competitive position. Our business would be negatively impacted if we are not successful in developing additional proprietary technologies that are patentable.
We cannot ensure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications that may be filed by us in the future, nor can we ensure that any of our existing or subsequently granted patents will be useful in protecting our drug candidates, technological innovations, and processes. The claims of any patents that are issued may not provide meaningful protection, may not provide a basis for commercially viable products or may not provide us with any competitive advantages. Because of the extensive time required for clinical development and regulatory review of a product candidate, it is possible that any patent related to our product candidates may expire before any of our product candidates can be commercialized, or may remain in force for only a short period of time following commercialization, thereby reducing the advantage afforded by any such patent. In addition, others may independently develop similar or alternative technologies, duplicate any of our technologies and, if patents are licensed or issued to us, design around the patented technologies licensed to or developed by us. Therefore, our competitors may be able to commercialize similar products, or may be able to duplicate our business strategy, without infringing our patents or otherwise using our intellectual property.
The protection afforded by any particular patent depends upon many factors, including the type of patent, scope of coverage encompassed by the granted claims, availability of extensions of patent term, availability of legal interpretation of patent laws in the United States and other countries that could diminish our ability to protect our inventions and to enforce our intellectual property rights. Furthermore, others may have patents that relate to our technology or business that may prevent us from marketing our product candidates unless we are able to obtain a license to those patents. Accordingly, while our ability to maintain and solidify our proprietary position for our products and core technologies will depend, in part, on our success in obtaining and enforcing valid patent claims, we cannot predict with certainty the enforceability of any granted patent claims or of any claims that may be granted from our patent applications.
The biotechnology and pharmaceutical industries are characterized by extensive litigation and other challenges regarding patents and other intellectual property rights that involve complex legal and factual questions making our patent position generally uncertain. Any existing or subsequently granted patents may be challenged, invalidated, found unenforceable, circumvented or infringed. We have been in the past and are currently involved in administrative proceedings with respect to our patents and patent applications and may, as a result of our extensive portfolio, be involved in such proceedings in the future. Additionally, in the future, we may claim that a third party infringes our intellectual property or a third party may claim that we infringe its intellectual property. In any of the administrative proceedings or in litigation, we may incur significant expenses, damages, attorneys’ fees, costs of proceedings and experts’ fees, and management and employees may be required to spend significant time in connection with these actions.
A patent interference proceeding may be instituted with the United States Patent and Trademark Office (“USPTO”) when more than one person files a patent application covering the same technology, or if someone wishes to challenge the validity of an issued patent on patents and applications filed before March 16, 2013. At the completion of the interference proceeding, the USPTO will determine which competing applicant is entitled to the patent, or whether an issued patent is valid. Patent interference proceedings are complex, highly contested legal proceedings, and the USPTO’s decision is subject to appeal. This means that if an interference proceeding arises with respect to any of our patent applications, we may experience significant expenses and delay in obtaining a patent, and if the outcome of the proceeding is unfavorable to us, the patent could be issued to a competitor rather than to us. For patents and applications filed after March 16, 2013 a derivation proceeding may be initiated where the USPTO may determine if one patent was derived from the work of an inventor on another patent. Inventorship may also be challenged in litigation.
In addition to interference proceedings, the USPTO can reevaluate issued patents at the request of a third party seeking to have the patent invalidated. There are proceedings at the USPTO (ex parte reexamination, post grant review, or inter partes review proceeding), which allow third parties to challenge the validity of an issued patent where there is a reasonable likelihood of invalidity. As with the USPTO interference proceedings, these USPTO proceedings will be very expensive to contest and can result in the cancelation of a patent. This means that patents owned or licensed by us may be subject to further administrative challenges and may be lost if the outcome of the challenge is unfavorable to us.
There are also challenges to obtaining patents in countries outside of the United States. In particular, under European patent law and the patent laws of certain other countries, oppositions to the issuance of patents may be filed. These foreign proceedings can be very expensive to contest and can result in significant delays in obtaining a patent or can result in a denial of a patent application. Also in Europe, there is uncertainty about the eligibility of hES cell subject matter for a patent. The European Patent Convention prohibits the granting of European patents for inventions that concern "uses of human embryos for industrial or commercial purposes". A recent decision at the Court of Justice of the European Union interpreted parthenogeneticly produced hES cells as patentable subject matter. Consequently, the European Patent Office now recognizes that human pluripotent stem cells (including human ES cells) can be created without a destructive use of human embryos as of June 5, 2003, and patent applications relating to hES cell subject matter with a filing and priority date after this date are no longer automatically excluded from patentability under Article 53 (a) EPC and Rule 28(c) EPC.
We may benefit from a variety of regulatory frameworks in the United States, Europe, China and other territories that provide periods of non-patent-based exclusivity for qualifying drug products. See “FDA and Foreign Regulation.”
The patent portfolio that we acquired pursuant to the Asset Contribution Agreement with Geron, dated January 4, 2013 (the "Asset Contribution Agreement"), includes over 400 patents and patent applications previously owned or licensed to Geron that are directed to pluripotent stem cell-, human hES cell-, and dendritic cell-based product opportunities. The portfolio encompasses a number of cell types that can be made from hES cells, including hepatocytes (liver cells), cardiomyocytes (heart muscle cells), neural cells (nerve cells, including dopaminergic neurons and oligodendrocytes), chondrocytes (cartilage cells), pancreatic islet β cells, osteoblasts (bone cells), hematopoietic cells (blood-forming cells) and dendritic cells. Also included in the patent portfolio are technologies for growing hES cells without the need for cell feeder layers or conditioned media, and novel synthetic growth surfaces.
In addition, in February 2016, we executed a broad, non-exclusive cross-license with BioTime and its subsidiary ES Cell International Pte Ltd. Under this license, we have received: (i) non-exclusive worldwide rights in a range of therapeutic fields of use to a further 35 patents and applications relating to hES cells, and (ii) non-exclusive worldwide rights for therapeutic applications of pluripotent stem cell-derived neural and cardiac cells to 22 patents and applications relating to hydrogel formulations.
The patent positions for our two most advanced programs are summarized below.
Dendritic cells: The patent rights relevant to dendritic cells include rights licensed from third parties and various patent families directed to the growth of hES cells and their differentiation into dendritic cells. This portfolio is related to our AST-VAC1 and AST-VAC2 products. There are issued patents in the United States, Australia, Europe, Canada, China, Hong Kong, and Japan. The expiration dates of these patents range from 2019 to 2025. The commercial success of our AST-VAC1 and AST-VAC2 products depends, in part, upon our ability to exclude competition in these products with this patent portfolio, regulatory exclusivity, or a combination of both.
Neural cells: The patent rights relevant to neural cells, such as oligodendrocyte progenitor cells, include various patent families directed to the growth of hES cells and their differentiation into neural cells. These patent rights also include rights licensed from the University of California. There are issued patents in the United States, Australia, Canada, United Kingdom, Japan, China, Hong Kong, India, Korea, Singapore and Israel. The expiration dates of these patents will be within 2020 to 2030. The commercial success of our AST-OPC1 product depends, in part, upon our ability to exclude competition in this product with this patent portfolio, regulatory exclusivity, or a combination of both.
In addition, Asterias has patent protection in the Unites States and various other jurisdictions for producing cardiomyocytes, pancreatic islet cells, hepatocytes, chondrocytes, hematopoietic cells, and osteoblasts. The expiration dates of these patents range from 2020 to 2032. Should a competitor not be able to market a product covered by these patents or if Asterias cannot license these patents before their expiration, the benefits for procurement and maintenance of these rights would not be fully realized and the associated costs would not be fully reimbursed.
Licensed Stem Cell Technology and Stem Cell Product Development Agreements
Telomerase Sublicense
We received the Telomerase Sublicense from Geron in connection with our acquisition of Geron’s stem cell assets. The Telomerase Sublicense grants us an exclusive sublicense under certain patents owned by the University of Colorado’s University License Equity Holdings, Inc. relating to telomerase and entitles us to use the technology covered by the patents in the development of AST-VAC1 and AST-VAC2 as immunological treatments for cancer. Under the Telomerase Sublicense, we paid Geron a one-time upfront license fee of $65,000, and we will pay Geron an annual license maintenance fee of $10,000 due on each anniversary of the effective date of the agreement, and a 1% royalty on sales of any products that we may develop and commercialize that are covered by the sublicensed patents. The Telomerase Sublicense will expire concurrently with the expiration of Geron’s license. That license will terminate during April 2017 when the licensed patents expire. The Telomerase Sublicense may also be terminated by us by giving Geron 90 days written notice, by us or by Geron if the other party breaches its obligations under the sublicense agreement and fails to cure their breach within the prescribed time period, or by us or by Geron upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings, or upon an assignment of a substantial portion of the assets for the benefit of creditors by the other party.
We are obligated to indemnify Geron, Geron’s licensor, and certain other parties for certain liabilities, including those for personal injury, product liability, or property damage relating to or arising from the manufacture, use, promotion or sale of a product, or the use by any person of a product made, created, sold or otherwise transferred by us or our sublicensees that is covered by the patents sublicensed under this agreement.
License Agreement with University of California
Geron assigned to us its Exclusive License Agreement with The Regents of the University of California for patents covering a method for directing the differentiation of multipotential hES cells to glial-restricted progenitor cells that generate pure populations of oligodendrocytes for remyelination and treatment of spinal cord injury. Pursuant to this agreement, we have an exclusive worldwide license under such patents, including the right to grant sublicenses, to create products for biological research, drug screening, and human therapy using the licensed patents.
Under the license agreement, we will be obligated to pay the university a royalty of 1% from sales of products that are covered by the licensed patent rights, and a minimum annual royalty of $5,000 starting in the year in which the first sale of a product covered by any licensed patent rights occurs, and continuing for the life of the applicable patent right under the agreement. The royalty payments due are subject to reduction, but not by more than 50%, to the extent of any payments that we may be obligated to pay to a third party for the use of patents or other intellectual property licensed from the third party in order to make, have made, use, sell, or import products or otherwise exercise our rights under the Exclusive License Agreement. We will be obligated to pay the university 7.5% of any proceeds, excluding debt financing and equity investments, and certain reimbursements, that we receive from sublicensees, other than our affiliates and joint ventures relating to the development, manufacture, purchase, and sale of products, processes, and services covered by the licensed patent.
The license agreement will terminate on the expiration of the last-to-expire of the university's issued licensed patents. If no further patents covered by the license agreement are issued, the license agreement would terminate in 2024. The university may terminate the agreement in the event of our breach of the agreement. We can terminate the agreement upon 60 days' notice.
World-Wide Non Exclusive WARF License
On October 7, 2013, we entered into a Non-Exclusive License Agreement with the Wisconsin Alumni Research Foundation (“WARF”) under which we were granted a worldwide non-exclusive license under certain WARF patents and WARF-owned embryonic stem cell lines to develop and commercialize therapeutic, diagnostic and research products. The licensed patents include patents covering methods for growth and differentiation of primate embryonic stem cells. The licensed stem cell lines include the H1, H7, H9, H13 and H14 hES cell lines.
In consideration of the rights licensed to us, we have agreed to pay WARF an upfront license fee and have agreed to pay, payments upon the attainment of specified clinical development milestones, royalties on sales of commercialized products, and, subject to certain exclusions, a percentage of any payments that we may receive from any sublicenses that we may grant to use the licensed patents or stem cell lines.
The license agreement will terminate with respect to licensed patents upon the expiration of the last licensed patent to expire. We may terminate the license agreement at any time by giving WARF prior written notice. WARF may terminate the license agreement if payments of earned royalties, once begun, cease for a specified period of time or if we and any third parties collaborating or cooperating with us in the development of products using the licensed patents or stem cell lines fail to spend a specified minimum amount on research and development of products relating to the licensed patents or stem cell lines for a specified period of time.
WARF also has the right to terminate the license agreement if we breach the license agreement or become bankrupt or insolvent or if any of the licensed patents or stem cell lines are offered to creditors.
We will indemnify WARF and certain other designated affiliated entities from liability arising out of or relating to the death or injury of any person or damage to property due to the sale, marketing, use, or manufacture of products that are covered by the licensed patents, or licensed stem cells, or inventions or materials developed or derived from the licensed patents or stem cell lines.
ViaCyte Cross-License
As part of a settlement agreement in May 2014 related to litigation in the United States District Court for the Northern District of California (Civil Action No. C12-04813), ViaCyte, Inc. (“ViaCyte”) and Asterias granted to each other a royalty free, fully paid license to each other’s technology relating to endoderm lineage cells including definitive endoderm and gut endoderm cells, only to the extent necessary to allow the licensee to make, use, sell, offer for sale, or import endodermal lineage cells. The Asterias patents that were licensed to ViaCyte in the settlement include those based on U.S. Patent Application No. 11/262,633. The ViaCyte patents that were licensed to us in the settlement included those based on U.S. Patent Application Nos. 11/021,618, 12/093,590, 10/584,338, 11/165,305, 11/317,387, and 11/860,494.
Royalty Agreement with Geron
In connection with our acquisition of Geron’s stem cell assets, we entered into a Royalty Agreement with Geron pursuant to which we agreed to pay Geron a 4% royalty on net sales (as defined in the Royalty Agreement), by us or any of our affiliates or sales agents, of any products that we develop and commercialize that are covered by the patents Geron contributed to us. In the case of sales of such products by a person other than us or one of our affiliates or sales agents, we will be required to pay Geron 50% of all royalties and cash payments received by us or by our affiliate in respect of a product sale. Royalty payments will be subject to proration in the event that a product covered by a patent acquired from Geron is sold in combination with another product that is not covered by a patent acquired from Geron. The Royalty Agreement will terminate at the expiration or termination date of the last issued patent contributed by Geron under the Royalty Agreement. We estimate that the latest patent expiration date will be 2032.
Clinical Trial and Option Agreement with Cancer Research United Kingdom
During September 2014, we entered into a Clinical Trial and Option Agreement (the “CRUK Agreement”) with Cancer Research UK (“CRUK”) and Cancer Research Technology Limited, (“CRT”), a wholly-owned subsidiary of CRUK, pursuant to which CRUK has agreed to fund Phase I/IIa clinical development of our AST-VAC2 product candidate. We will, at our own cost, complete process development and manufacturing scale-up of the AST-VAC2 manufacturing process and will transfer the resulting cGMP-compatible process to CRUK. CRUK will, at its own cost, manufacture clinical grade AST-VAC2 and will carry out the Phase I/IIa clinical trial of AST-VAC2 in cancer patients in both resected early-stage and advanced forms of lung cancer. We will have an exclusive first option to obtain a license to use the data from the clinical trial. If we exercise that option we will be obligated to make payments upon the execution of the License Agreement, upon the achievement of various milestones, and then royalties on sales of products, and, if we sublicense product development or commercialization rights to a third party, we would pay CRT a share of any sublicense revenues we receive from the third party, with CRT’s share varying from a high of 40% in the case of a sublicense entered into prior to commencement of a Phase II clinical trial, to substantially lower rates in the case of a sublicense entered into at various later stages of clinical development but prior to completion of a Phase III clinical trial, and as low as 7.5% in the case of a sublicense entered into after completion of a Phase III clinical trial. In connection with the CRUK Agreement, we sublicensed to CRUK for use in the clinical trials and product manufacturing process certain patents that have been licensed or sublicensed to us by third parties. We would also be obligated to make payments to those licensors and sublicensors upon the achievement of various milestones, and then royalties on sales of products if AST-VAC2 is successfully developed and commercialized.
If we decline to exercise our option, CRT will then have an option to obtain a license to use our intellectual property relating to AST-VAC2 to continue the development and commercialization of AST-VAC2 and related products for which we will be entitled to receive a share of the revenue relating to development and partnering proceeds. The CRT’s option will be exercisable by CRT for four months from when our option expires.
The CRUK Agreement will expire upon the earliest of (i) the date we obtain a license to use the clinical data pursuant to an exercise of our option, (ii) the date CRT obtains a license to continue the development and commercialization of AST-VAC2 pursuant to an exercise of our option and (iii) the expiration of both our option and the CRT’s option. Notwithstanding the foregoing, any party may terminate the CRUK Agreement prior to its expiration for events including (i) a party materially breaches the agreement and such breach is not cured within 60 days after the non-breaching party delivers written notice, (ii) any party is insolvent or liquidated or (iii) if regulatory approval of the clinical trial is not obtained within two years after the parties complete the technology transfer phase of the agreement, or if regulatory approval is revoked, withdrawn or otherwise terminated, or if a regulatory authority orders a halt or hold on the clinical trial for more than 18 months. In addition, CRUK will have the right to terminate the CRUK Agreement under certain other circumstances.
The CRUK Agreement contains customary representations, warranties and covenants from us and CRUK, as well as customary provisions relating to indemnity, confidentiality and other matters.
Services Agreement with Cell Therapy Catapult Services Limited
We entered into a Services Agreement (the “Services Agreement”) with Cell Therapy Catapult Services Limited (“Catapult”), a research organization specializing in the development of technologies which speed the growth of the cell and gene therapy industry. Under the Services Agreement, Catapult will license to us, certain background intellectual property and will develop a scalable manufacturing and differentiation process for our human embryonic stem cell derived AST-VAC2 allogeneic (non-patient specific) dendritic cancer vaccine development program. In consideration for the license and Catapult’s performance of services, we agreed to make aggregate payments of up to GBP £4,350,000 over the next five years. At our option of, up to GBP £3,600,000 of such payments may be settled in shares of our Series A Common Stock.
The Services Agreement may be terminated by we for any reason upon 60 days prior written notice. Catapult may terminate the Services Agreement on 60 days prior written notice if it encounters a technical issue that would prevent it from completing the services at all or without obtaining additional resources, or if the estimated time and cost of completing the services will be exceeded and both parties do not reach agreement on revised time and cost terms. Catapult may terminate the Services Agreement in the event we fail to pay any amount due under the Services Agreement 30 days after Catapult makes a written demand for payment. In addition, a non-breaching party may terminate the Services Agreement upon the occurrence a material breach that is not remedied within 30 days. Either party may terminate the Services Agreement in the event the other party becomes subject to insolvency, receivership, liquidation, or a similar event.
Manufacturing
We entered into a new lease for a 44,000 square foot facility in Fremont, California at which we constructed a cGMP compliant facility for the production of our product candidates that cost approximately $4.9 million of which we used $4.4 million in a tenant improvement allowance from the landlord. Construction began at the Fremont facility during the first quarter of 2015 and all facilities were moved in during the fourth quarter of 2015. The lease on our Menlo Park facility expired in January 2016.
Marketing
Because our planned products are still in the research and development stage, we will not initially need to have our own marketing personnel. If we are successful in developing marketable products, we will need to build our own marketing and distribution capability for our products, which would require the investment of significant financial and management resources, or we will need to find collaborative marketing partners, independent sales representatives, or wholesale distributors for the commercial sale of those products.
If we market products through arrangements with third parties, we may pay sales commissions to sales representatives or we may sell or consign products to distributors at wholesale prices. This means that our gross profit from product sales may be less than would be the case if we were to sell our products directly to end users at retail prices through our own sales force. On the other hand, selling to distributors or through independent sales representatives would allow us to avoid the cost of hiring and training our own sales employees. There can be no assurance we will be able to negotiate distribution or sales agreements with third parties on favorable terms to justify our investment in our products or achieve sufficient revenues to support our operations.
Competition
We face substantial competition in our business, and that competition is likely to intensify further as new products and technologies reach the market. Superior new products are likely to sell for higher prices and generate higher profit margins once acceptance by the medical community is achieved. Those companies that are successful in introducing new products and technologies to the market first may gain significant economic advantages over their competitors in the establishment of a customer base and track record for the performance of their products and technologies. Such companies will also benefit from revenues from sales that could be used to strengthen their research and development, production, and marketing resources. All companies engaged in the medical products industry face the risk of obsolescence of their products and technologies as more advanced or cost effective products and technologies are developed by their competitors. As the industry matures, companies will compete based upon the performance and cost effectiveness of their products.
The stem cell industry is characterized by rapidly evolving technology and intense competition. Our competitors include major multinational pharmaceutical companies, specialty biotechnology companies, and chemical and medical products companies operating in the fields of regenerative medicine, cell therapy, tissue engineering, and tissue regeneration. Many of these companies are well-established and possess technical, research and development, financial, and sales and marketing resources significantly greater than ours. In addition, certain smaller biotech companies have formed strategic collaborations, partnerships, and other types of joint ventures with larger, well established industry competitors that afford these companies’ potential research and development and commercialization advantages. Academic institutions, governmental agencies, and other public and private research organizations are also conducting and financing research activities which may produce products directly competitive to those we are developing.
We believe that some of our competitors are trying to develop hES cell, iPS cell, and mesenchymal stem cell-based technologies and products that may compete with our potential stem cell products based on efficacy, safety, cost, and intellectual property positions.
We may also face competition from companies that have filed patent applications relating to the cloning or differentiation of stem cells. We may be required to seek licenses from these competitors in order to commercialize certain of our proposed products, and such licenses may not be granted.
Government Regulation
Government authorities at the federal, state and local level, and in other countries, extensively regulate among other things, the development, testing, manufacture, quality, approval, distribution, labeling, packaging, storage, record keeping, marketing, import/export and promotion of drugs, biologics, and medical devices. Authorities also heavily regulate many of these activities for human cells, tissues and cellular and tissue-based products or HCT/Ps.
FDA and Foreign Regulation
We believe that the FDA will regulate most of our proposed products as biologicals. In the United States, the FDA regulates drugs and biologicals under the Federal Food, Drug and Cosmetic Act or FDCA, the Public Health Service Act, or PHSA, and implementing regulations. In addition, establishments that manufacture human cells, tissues, and cellular and tissue-based products are subject to additional registration and listing requirements, including current good tissue practice regulations. Many of our proposed products will be reviewed by the FDA staff in its Center for Biologics Evaluation and Research (“CBER”) Office of Cellular, Tissue and Gene Therapies.
Clinical Development
Our domestic biological products will be subject to rigorous FDA review and approval procedures. After testing in animals to evaluate the potential efficacy and safety of the product candidate, an IND must be submitted to the FDA to obtain authorization for human testing. Extensive clinical testing, which is generally done in three phases, must then be undertaken at one or more hospitals or medical centers to demonstrate optimal use, safety, and efficacy of each product in humans. Each clinical trial will also be subject to review by an independent Institutional Review Board (“IRB”) at each institution at which the trial will occur. The IRB will consider, among other things, ethical factors, the safety of human subjects, and the possible liability of the institution.
Clinical trials are generally conducted in three “phases.” Phase 1 clinical trials are conducted in a small number of healthy volunteers or volunteers with the target disease or condition to assess safety. Phase 2 clinical trials are conducted with groups of patients afflicted with the target disease or condition in order to determine preliminary efficacy, optimal dosages and expanded evidence of safety. In some cases, an initial trial is conducted in diseased patients to assess both preliminary efficacy and preliminary safety, in which case it is referred to as a Phase 1/2 trial. Phase 3 trials are large-scale, multi-center, comparative trials and are conducted with patients afflicted with the target disease or condition in order to provide enough data to demonstrate the efficacy and safety required by the FDA. The FDA closely monitors the progress of each of the three phases of clinical testing and may, at its discretion, re-evaluate, alter, suspend, or terminate the clinical trial based upon the data which have been accumulated to that point and its assessment of the risk/benefit ratio to the intended patient population. All adverse events must be reported to the FDA. Monitoring of all aspects of the study to minimize risks is a continuing process. The time and expense required to perform this clinical testing can far exceed the time and expense of the research and development initially required to create the product.
Applications for Marketing Approval
No action can be taken to market any therapeutic product in the United States until an appropriate application, which in the case of a cell therapy or vaccine product will be a Biologics License Application (“BLA”), has been approved by the FDA. Submission of the application is no guarantee that the FDA will find it complete and accept it for filing. If an application is accepted for filing, following the FDA’s review, the FDA may grant marketing approval, request additional information or deny the application if it determines that the application does not provide an adequate basis for approval. FDA regulations also restrict the export of therapeutic products for clinical use prior to BLA approval. To date, the FDA has not granted marketing approval to any hES-based therapeutic products and it is possible that the FDA or foreign regulatory agencies may subject our product candidates to additional or more stringent review than drugs or biologicals derived from other technologies.
The FDA offers several programs to expedite development of products that treat serious or life-threatening illnesses and that provide meaningful therapeutic benefits to patients over existing treatments. A product may be eligible for breakthrough therapy designation if it treats a serious or life-threatening disease or condition and preliminary clinical evidence indicates it may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Features of breakthrough therapy designation include intensive guidance from FDA on an efficient development program, intensive involvement of FDA staff in a proactive, collaborative review process, and rolling review of marketing applications. Under its accelerated approval regulations, the FDA may approve a product based on a surrogate endpoint that is reasonably likely to predict clinical benefits or based on an effect on a clinical endpoint other than survival or irreversible morbidity. The applicant will then be required to conduct additional, post-approval confirmatory trials to verify and describe clinical benefit, and the product may have certain post-marketing restrictions as necessary to assure safe use. The FDA may withdraw approval granted under the traditional route or under an accelerated approval, if it is warranted. The FDA may also consider ways to use the accelerated approval pathway for rare or very rare diseases, and a new review designation has been created to help foster the innovation of promising new therapies with the potential to shorten the timeframe for conducting pivotal trials and speed up patient access to the approved product. There is no assurance that the FDA will grant breakthrough therapy or accelerated approval status to any of our product candidates.
Combination Products
If we develop any products that are used with medical devices, they may be considered combination products, which are defined by the FDA to include products comprised of two or more regulated components or parts such as a biologic and a device. When regulated independently, biologics and devices each have their own regulatory requirements. However, the regulatory requirements for a combination product comprised of a biologic administered with a delivery device can be more complex, because in addition to the individual regulatory requirements for each component, additional combination product regulatory requirements may apply. There is an Office of Combination Products at the FDA that coordinates the review of such products and determines the primary mode of action of a combination product. The definition and regulatory requirements for combination products may differ significantly among other countries in which we may seek approval of our product candidates.
Post-Approval Matters
Even after initial FDA approval has been obtained, further studies may be required to provide additional data on safety or to gain approval for the use of a product as a treatment for clinical indications other than those initially targeted. Use of a product during testing and after marketing could reveal side effects that could delay, impede, or prevent FDA marketing approval, result in an FDA-ordered product recall, or in FDA-imposed limitations on permissible uses or in withdrawal of approval. For example, if the FDA becomes aware of new safety information after approval of a product, it may require us to conduct further clinical trials to assess a known or potential serious risk and to assure that the benefit of the product outweigh the risks. If we are required to conduct such a post-approval study, periodic status reports must be submitted to the FDA. Failure to conduct such post-approval studies in a timely manner may result in substantial civil or criminal penalties. Data resulting from these clinical trials may result in expansions or restrictions to the labeled indications for which a product has already been approved.
FDA Regulation of Manufacturing
The FDA regulates the manufacturing process of pharmaceutical products, and human tissue and cell products, requiring that they be produced in compliance with cGMP. See “Manufacturing.” The FDA regulates and inspects equipment, facilities, laboratories and processes used in the manufacturing and testing of products prior to providing approval to market products. If after receiving approval from the FDA, a material change is made to manufacturing equipment or to the location or manufacturing process, additional regulatory review may be required. The FDA also conducts regular, periodic visits to re-inspect the equipment, facilities, laboratories and processes of manufacturers following an initial approval. If, as a result of those inspections, the FDA determines that that equipment, facilities, laboratories or processes do not comply with applicable FDA regulations and conditions of product approval, the FDA may seek civil, criminal or administrative sanctions and/or remedies against the manufacturer, including suspension of manufacturing operations. Issues pertaining to manufacturing equipment, facilities or processes may also delay the approval of new products undergoing FDA review.
FDA Regulation of Advertising and Product Promotion
The FDA also regulates the content of advertisements used to market pharmaceutical and biological products. Claims made in advertisements concerning the safety and efficacy of a product, or any advantages of a product over another product, must be supported by clinical data filed as part of a BLA or an amendment to a BLA, and must be consistent with the FDA approved labeling and dosage information for that product.
Foreign Regulation
Sales of pharmaceutical and biological products outside the United States are subject to foreign regulatory requirements that vary widely from country to country. Even if FDA approval has been obtained, approval of a product by comparable regulatory authorities of foreign countries must be obtained prior to the commencement of marketing the product in those countries. The time required to obtain such approval may be longer or shorter than that required for FDA approval.
California State Regulations
The state of California has adopted legislation and regulations that require institutions that conduct stem cell research to notify, and in certain cases obtain approval from, a Stem Cell Research Oversight Committee (“SCRO Committee”) before conducting the research. Advance notice, but not approval by the SCRO Committee, is required in the case of in vitro research that does not derive new stem cell lines. Research that derives new stem cell lines, or that involves fertilized human oocytes or blastocysts, or that involves clinical trials or the introduction of stem cells into humans, or that involves introducing stem cells into animals, requires advanced approval by the SCRO Committee. Clinical trials may also entail approvals from an IRB at the medical center at which the study is conducted, and animal studies may require approval by an Institutional Animal Care and Use Committee.
All hES cell lines that will be used in our research must be acceptably derived. To be acceptably derived, the pluripotent stem cell line must have either:
| · | Been listed on the National Institutes of Health Human Embryonic Stem Cell Registry, or |
| · | Been deposited in the United Kingdom Stem Cell Bank, or |
| · | Been derived by, or approved for use by, a licensee of the United Kingdom Human Fertilisation and Embryology Authority, or |
| · | Been derived in accordance with the Canadian Institutes of Health Research Guidelines for Human Stem Cell Research under an application approved by the National Stem Cell Oversight Committee, or |
| · | Been derived in accordance with the Japanese Guidelines for Derivation; or |
| · | Been approved by CIRM in accordance with California Code of Regulations Title 17, Section 100081; or |
| · | Been derived under the following conditions: |
| (a) | Donors of gametes, embryos, somatic cells, or human tissue gave voluntary and informed consent; |
| (b) | Donors of gametes, embryos, somatic cells, or human tissue did not receive valuable consideration. This provision does not prohibit reimbursement for permissible expenses as determined by an IRB; |
| (c) | Donation of gametes, embryos, somatic cells, or human tissue was overseen by an IRB (or, in the case of foreign sources, an IRB-equivalent); and |
| (d) | Individuals who consented to donate stored gametes, embryos, somatic cells, or human tissue were not reimbursed for the cost of storage prior to the decision to donate. |
Other hES lines may be deemed acceptably derived if they were derived in accordance with (a), (b), and (d) above and the hES line was derived prior to the publication of the National Academy of Sciences guidelines on April 26, 2005 and a SCRO Committee has determined that the investigator has provided sufficient scientific rationale for the need for use of the line, which should include establishing that the proposed research cannot reasonably be carried out with covered lines that did have IRB approval.
California regulations also require that certain records be maintained with respect to stem cell research and the materials used, including:
| · | A registry of all human stem cell research conducted, and the source(s) of funding for this research. |
| · | A registry of human pluripotent stem cell lines derived or imported, to include, but not necessarily limited to: |
| (a) | The methods utilized to characterize and screen the materials for safety; |
| (b) | The conditions under which the materials have been maintained and stored; |
| (c) | A record of every gamete donation, somatic cell donation, embryo donation, or product of somatic cell nuclear transfer that has been donated, created, or used; |
| (d) | A record of each review and approval conducted by the SCRO Committee. |
California Proposition 71
During November 2004, California State Proposition 71 (“Prop. 71”), the California Stem Cell Research and Cures Initiative, was adopted by state-wide referendum. Prop. 71 provides for a state-sponsored program designed to encourage stem cell research in the State of California, and to finance such research with State funds totaling approximately $295,000,000 annually for 10 years beginning in 2005. This initiative created CIRM, which will provide grants, primarily but not exclusively, to academic institutions to advance both hES cell research and adult stem cell research.
Medicare, Medicaid, and Similar Reimbursement Programs
Sales of our products will depend, in part, on the extent to which the costs of our products will be covered by third-party payors, such as government health programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly challenging the prices charged for medical products and services. Additionally, the containment of healthcare costs has become a priority of federal and state governments and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. If these third-party payors do not consider our products to be cost-effective compared to other therapies, they may not cover our products after approved as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010, collectively referred to as the ACA, enacted in March 2010, is expected to have a significant impact on the health care industry. ACA is expected to expand coverage for the uninsured while at the same time containing overall healthcare costs. With regard to pharmaceutical products, among other things, ACA is expected to expand and increase industry rebates for drugs covered under Medicaid programs and make changes to the coverage requirements under the Medicare Part D program. We cannot predict the impact of ACA on pharmaceutical companies, as many of the ACA reforms require the promulgation of detailed regulations implementing the statutory provisions which has not yet occurred. In addition, although the United States Supreme Court upheld the constitutionality of most of the ACA, some states have indicated that they intend to not implement certain sections of the ACA, and some members of the U.S. Congress are still working to repeal parts of the ACA. These challenges add to the uncertainty of the legislative changes enacted as part of ACA.
In addition, in some non-U.S. jurisdictions, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of us placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the United States and generally tend to be significantly lower.
Additional Information
We were incorporated in September 2012 under the name BioTime Acquisition Corporation in the state of Delaware. We changed our name to Asterias Biotherapeutics, Inc. in March 2013. Our principal executive offices are located at 6300 Dumbarton Circle, Fremont, California 94555. Our telephone number is 510-456-3800. We currently maintain an Internet website at www.asteriasbiotherapeutics.com.
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012. We will remain an "emerging growth company" until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of our first sale of common equity securities, such as our common stock, pursuant to an effective registration statement under the Securities Act; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission (the “SEC”). We refer to the Jumpstart Our Business Startups Act of 2012 herein as the "JOBS Act," and references herein to "emerging growth company" shall have the meaning associated with it in the JOBS Act.
Employees
As of March 16, 2016 we employed 41 persons on a full time basis. Seventeen of our employees hold Ph.D. degrees in one or more fields of science. None of our employees are subject to a collective bargaining agreement. All of our employees are based in the United States.
Our business is subject to various risks, including those described below. You should consider the following risk factors, together with all of the other information included in this report, which could materially adversely affect our proposed operations, our business prospects, and financial condition, and the value of an investment in our business. There may be other factors that are not mentioned here or of which we are not presently aware that could also affect our business operations and prospects.
Risks Related to Our Business Operations
We have a history of operating losses and negative cash flows
Since our inception in September 2012, we have incurred operating losses and negative cash flow, and we expect to continue to incur losses and negative cash flow in the future. Our net losses for the fiscal years ended December 31, 2015, 2014, and 2013 were $15.0 million, $10.1 million, and $22.4 million respectively, and we had an accumulated deficit of $48.2 million and $33.2 million as of December 31, 2015 and 2014, respectively. Our net loss for the year ended December 31, 2013 and our accumulated deficit as of that date include $17.5 million charged as in-process research and development expenses (“IPR&D”) in accordance with Accounting Standards Codification (“ASC”) 805-50 on account of our acquisition of certain assets from Geron. See Notes 2 and 3 to the Financial Statements. BioTime previously funded our formation and operating costs but we do not expect BioTime to continue to do so in the future. We have limited cash resources and will depend upon future equity financings, research grants, financings through collaborations with third parties, and sales of BioTime common shares that we have as a source of funding for our operations. There is no assurance that we will be able to obtain the financing we need from any of those sources, or that any such financing that may become available will be on terms that are favorable to us and our shareholders.
Failure to attract and retain skilled personnel and key relationships could impair our research and development efforts
Our operations are still in the start-up stage and we had only 38 employees as of December 31, 2015. We will need to recruit and hire additional qualified research scientists, laboratory technicians, clinical development, and management personnel. Competition for these types of personnel is intense and we may experience delays in hiring the qualified people that we need. The inability to attract and retain sufficient qualified management, scientific, or technical personnel may significantly delay or prevent the achievement of our product development and other business objectives and could have a material adverse effect on our business, operating results and financial condition. We partially rely on BioTime to provide financial accounting management and personnel, human resources, and intellectual property management. We will also rely on consultants and advisors who are either self-employed or employed by other organizations, and they may have conflicts of interest or other commitments, such as consulting or advisory contracts with other organizations, that may affect their ability to perform services for us.
We will spend a substantial amount of our capital on research and development but we might not succeed in developing products and technologies that are useful in medicine
| · | The product development work we plan to do is costly, time consuming and uncertain as to its results. |
| · | We will attempt to develop new medical products and technologies that might not prove to be safe and efficacious in human medical applications. Many of the products and technologies that we will seek to develop have not been applied in human medicine and have only been used in laboratory studies in vitro or in animals. Only two of the product candidates that we have acquired have been used in clinical trials, and those were early stage trials involving only a small number of patients. |
| · | If we are successful in developing a new technology or product, refinement of the new technology or product and definition of the practical applications and limitations of the technology or product may take years and require the expenditure of large sums of money. |
The amount and pace of research and development work that we can do or sponsor, and our ability to commence and complete clinical trials required to obtain FDA and foreign regulatory approval of our products, depends upon the amount of money available to us
| · | We may have to limit our laboratory research and development work based on the amount of our cash resources. |
| · | We plan to seek research and development grants from government agencies and to enter into collaborative product development agreements through which third parties will provide funding or otherwise bear the cost of research and development or clinical trials of our product candidates. There is no assurance that the amount of any grants that we may receive will be adequate for our needs. The agreements we entered into to date with CIRM and CRUK are subject to termination if certain milestones are not achieved. Hence, there is no assurance that we will receive the full value of the agreement with either entity. |
| · | Unless we are able to generate sufficient revenue or raise additional funds when needed, it is likely that we will be unable to continue our planned activities, even if we make progress in our research and development projects. |
We will need to issue additional equity or debt securities in order to raise additional capital needed to pay our operating expenses
| · | We plan to incur substantial research and product development expenses, and we will need to raise additional capital to pay operating expenses until we are able to generate sufficient revenues from product sales, royalties, and license fees. |
| · | It is likely that additional sales of equity or debt securities will be required in the near future to meet our short-term capital needs, unless we receive substantial research grants and revenues from the sale of any products that receive regulatory approval or we are successful in licensing or sublicensing our technology and we receive substantial licensing fees and royalties. |
| · | Sales of additional equity securities could result in the dilution of the interests of present shareholders. |
The condition of certain cells, cell lines and other biological materials that we acquired from Geron could impact the time and cost of commencing our research and product development programs
The cells, cell lines and other biological materials that we acquired are being stored under cryopreservation protocols intended to preserve their functionality. We have successfully completed the verification of the viability of three lots of AST-OPC1 cells that we intend to use in clinical trials. However, the functional condition of the other materials cannot be certified until they are tested in an appropriate laboratory setting by qualified scientific personnel using validated equipment. We intend to perform that testing on the cells that we intend to use in our research and development programs as the need arises.
To the extent that the cells we plan to use are not sufficiently functional for our purposes, we would need to incur the time and expense of regenerating cell lines from cell banks, or regenerating cell banks from cell stocks, which could delay and increase the cost of our research and development work using those cells.
Sales of any products we may develop may be adversely impacted by the availability of competing products
| · | In order to compete with other products, particularly those that sell at lower prices, our products will have to provide medically significant advantages. |
| · | Physicians and hospitals may be reluctant to try a new product due to the high degree of risk associated with the application of new technologies and products in the field of human medicine. |
| · | There also is a risk that our competitors may succeed at developing safer or more effective products that could render our products and technologies obsolete or noncompetitive. |
Any products that receive regulatory approval may be difficult and expensive to manufacture on a commercial scale
| · | hES derived therapeutic cells have only been produced on a small scale and not in quantities and at levels of purity and viability that will be needed for wide scale commercialization. If we are successful in developing products that consist of hES cells or other cells or products derived from hES or other cells, we will need to develop, alone or in collaboration with one or more pharmaceutical companies or contract manufacturers, technology for the commercial production of those products. |
| · | Our hES cell or other cell-based products are likely to be more expensive to manufacture on a commercial scale than most other drugs on the market today. The high cost of manufacturing a product will require that we charge our customers a high price for the product in order to cover our costs and earn a profit. If the price of our products is too high, hospitals and physicians may be reluctant to purchase our products, especially if lower priced alternative products are available, and we may not be able to sell our products in sufficient volumes to recover our costs of development and manufacture or to earn a profit. |
We do not have our own marketing, distribution, and sales resources for the commercialization of any products that we might successfully develop
| · | If we are successful in developing marketable products, we will need to build our own marketing, distribution, and sales capability for our products, which would require the investment of significant financial and management resources, or we will need to find collaborative marketing partners, independent sales representatives, or wholesale distributors for the commercial sale of our products. |
| · | If we market products through arrangements with third parties, we may pay sales commissions to sales representatives or we may sell or consign products to distributors at wholesale prices. As a result, our gross profit from product sales may be lower than it would be if we were to sell our products directly to end users at retail prices through our own sales force. |
| · | There can be no assurance that we will able to negotiate distribution or sales agreements with third parties on favorable terms to justify our investment in our products or achieve sufficient revenues to support our operations. |
We do not have the ability to independently conduct clinical trials required to obtain regulatory approvals for our therapeutic product candidates
We will need to rely on third parties, such as CRUK, contract research organizations, data management companies, contract clinical research associates, medical institutions, clinical investigators and contract laboratories to conduct any clinical trials that we may undertake for our products. We may also rely on third parties to assist with our preclinical development of therapeutic product candidates. If we outsource clinical trials, we may be unable to directly control the timing, conduct and expense of our clinical trials. If we enlist third parties to conduct clinical trials and they fail to successfully carry out their contractual duties or regulatory obligations or fail to meet expected deadlines, if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to clinical protocols or regulatory requirements or for other reasons, our preclinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our therapeutic product candidates.
We will have certain obligations and may incur liabilities arising from clinical trials, and we do not yet know the scope of any resulting expenses that might arise
We face the risk of incurring liabilities to patients who participate in clinical trials of our product candidates if they incur any injuries as a result of their participation. We will also be obligated to obtain information and prepare reports about the health of the clinical trial patients. In addition, we have assumed Geron’s obligations to obtain information and prepare reports about the health of patients, and we have assumed any liabilities to those patients that might arise from any injuries they may have incurred, as a result of their participation in the clinical trials of Geron’s GRN-OPC1 cell replacement therapy for spinal cord damage and its GRN-VAC1 immunological therapy for certain cancers. We are not aware of any claims by patients alleging injuries suffered as a result of any of those clinical trials, but if any claims are made and if liability can be established, the amount of any liability that we may incur, depending upon the nature and extent of any provable injuries, could exceed our insurance coverage, and the amount of the liability could be material to our financial condition.
Our business could be adversely affected if we lose the services of the key personnel upon whom we depend
Our stem cell research program will be directed primarily by our President of Research and Development, Dr. Jane S. Lebkowski, our Chief Operating Officer, Dr. Katharine E. Spink, and our Chief Medical Officer, Dr. Edward D. Wirth. The loss of their services could have a material adverse effect on us.
Our business and operations could suffer in the event of system failures
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruption of our operations. For example, the loss of data for our product candidates could result in delays in our regulatory filings and development efforts and significantly increase our costs. To the extent that any disruption or security breach was to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our product candidates could be delayed.
Failure of our internal control over financial reporting could harm our business and financial results
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting for external purposes in accordance with accounting principles generally accepted in the U.S. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our financial statements; providing reasonable assurance that receipts and expenditures of our assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected. Our growth and entry into new products, technologies and markets will place significant additional pressure on our system of internal control over financial reporting. Any failure to maintain an effective system of internal control over financial reporting could limit our ability to report our financial results accurately and timely or to detect and prevent fraud.
We will initially rely in part on financial systems maintained by BioTime and upon services provided by BioTime personnel. BioTime will allocate certain expenses among itself, us, and BioTime’s other subsidiaries, which creates a risk that the allocations may not accurately reflect the benefit of an expenditure or use of financial or other resources by us, BioTime as our parent company, and the BioTime subsidiaries among which the allocations are made.
Risks Related to Our Industry
We will face certain risks arising from regulatory, legal, and economic factors that affect our business and the business of other pharmaceutical and biological product development companies. Because we are a small company with limited revenues and limited capital resources, we may be less able to bear the financial impact of these risks than larger companies that have substantial income and available capital.
If we do not receive FDA and other regulatory approvals we will not be permitted to sell our products
The cell-based products that we are developing cannot be sold until the FDA and corresponding foreign regulatory authorities approve the products for medical use. To date, long-term safety and efficacy has not been demonstrated in clinical trials for any of our therapeutic product candidates. The need to obtain regulatory approval to market a new product means that:
| · | we will have to conduct expensive and time consuming clinical trials of new products. The full cost of conducting and completing clinical trials necessary to obtain FDA and foreign regulatory approval of a new product cannot be presently determined, but could exceed our current financial resources; |
| · | clinical trials and the regulatory approval process for a cell-based product can take several years to complete. As a result, we will incur the expense and delay inherent in seeking FDA and foreign regulatory approval of new products, even if the results of clinical trials are favorable; |
| · | data obtained from preclinical and clinical studies is susceptible to varying interpretations that could delay, limit, or prevent regulatory agency approvals. Delays in the regulatory approval process or rejections of an application for approval of a new drug or cell-based product may be encountered as a result of changes in regulatory agency policy; |
| · | because the therapeutic products we plan to develop with hES and iPS technology involve the application of new technologies and approaches to medicine, the FDA or foreign regulatory agencies may subject those products to additional or more stringent review than drugs or biologics derived from other technologies. No therapeutic product based on hES or iPS technology has been approved by the FDA to date. |
| · | a product that is approved may be subject to restrictions on use; |
| · | the FDA can limit or withdraw approval of a product if problems arise; and |
| · | we will face similar regulatory issues in foreign countries. |
Clinical trial failures can occur at any stage of the testing and we may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent commercialization of our current or future therapeutic product candidates
All of our product candidates are either at early stages of clinical development or at the preclinical or research stages of development. Clinical trial failures or delays can occur at any stage of the trials, and may be directly or indirectly caused by a variety of factors, including but not limited to:
| · | delays in securing clinical investigators or trial sites for our clinical trials; |
| · | delays in obtaining Institutional Review Board (“IRB”) and other regulatory approvals to commence a clinical trial; |
| · | slower than anticipated rates of patient recruitment and enrollment, or failing to reach the targeted number of patients due to competition for patients from other trials; |
| · | limited or no availability of coverage, reimbursement and adequate payment from health maintenance organizations and other third party payors for the use of agents used in our clinical trials; |
| · | negative or inconclusive results from clinical trials; |
| · | unforeseen side effects interrupting, delaying, or halting clinical trials of our therapeutic product candidates, and possibly resulting in the FDA or other regulatory authorities denying approval of our therapeutic product candidates; |
| · | unforeseen safety issues; |
| · | uncertain dosing issues; |
| · | approval and introduction of new therapies or changes in standards of practice or regulatory guidance that render our clinical trial endpoints or the targeting of our proposed indications obsolete; |
| · | inability to monitor patients adequately during or after treatment or problems with investigator or patient compliance with the trial protocols; |
| · | inability to replicate in large controlled studies safety and efficacy data obtained from a limited number of patients in uncontrolled trials; |
| · | inability or unwillingness of medical investigators to follow our clinical protocols; and |
| · | unavailability of clinical trial supplies. |
Government imposed bans or restrictions, and religious, moral and ethical concerns on the use of hES cells could prevent us from developing and successfully marketing stem cell products
| · | Government imposed bans or restrictions on the use of embryos or hES cells research and development in the United States and abroad could generally constrain stem cell research, thereby limiting the market and demand for any of our products that receive regulatory approval. In March 2009, President Obama lifted certain restrictions on federal funding of research involving the use of hES cells, and in accordance with President Obama’s executive order, the National Institutes of Health has adopted new guidelines for determining the eligibility of hES cell lines for use in federally funded research. The central focus of the proposed guidelines is to assure that hES cells used in federally funded research were derived from human embryos that were created for reproductive purposes, were no longer needed for this purpose, and were voluntarily donated for research purposes with the informed written consent of the donors. hES cells that were derived from embryos created for research purposes rather than reproductive purposes, and other hES cells that were not derived in compliance with the guidelines, are not eligible for use in federally funded research. |
| · | California law requires that stem cell research be conducted under the oversight of a stem cell research oversight (“SCRO”) committee. Many kinds of stem cell research, including the derivation of new hES cell lines, may only be conducted in California with the prior written approval of the SCRO. A SCRO could prohibit or impose restrictions on the research we plan to do. |
| · | The use of hES cells gives rise to religious, moral and ethical issues regarding the appropriate means of obtaining the cells and the appropriate use and disposal of the cells. These considerations could lead to more restrictive government regulations or could generally constrain stem cell research thereby limiting the market and demand for any of our products that receive regulatory approval. |
Risks Related to Our Intellectual Property
If our efforts to protect our proprietary technologies are not adequate, we may not be able to compete effectively in our market.
We rely upon a combination of patents, trade secret protection and contractual arrangements to protect the intellectual property related to our technologies. We will only be able to protect our products and proprietary information and technology by preventing unauthorized use by third parties to the extent that our patents, trade secrets, and contractual position allow us to do so. Any disclosure to or misappropriation by third parties of our trade secrets or confidential information could compromise our competitive position. Moreover, we are involved in, have in the past been involved in, and may in the future be involved in legal or administrative proceedings involving our intellectual property and initiated by third parties, which proceedings can result in significant costs and commitment of management time and attention. As our product candidates continue in development, third parties may attempt to challenge the validity and enforceability of our patents and proprietary information and technologies.
We also are involved in, have in the past been involved in, and may in the future be involved in initiating legal or administrative proceedings involving the product candidates and intellectual property of our competitors. These proceedings can result in significant costs and commitment of management time and attention, and there can be no assurance that our efforts would be successful in preventing or limiting the ability of our competitors to market competing products. Composition-of-matter patents relating to the active pharmaceutical ingredient are generally considered to be the strongest form of intellectual property protection for pharmaceutical products, as such patents provide protection not limited to any one method of use. Method-of-use patents protect the use of a product for the specified method(s), and do not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method. We rely on a combination of these and other types of patents to protect our product candidates, and there can be no assurance that our intellectual property will create and sustain the competitive position of our product candidates.
Biotechnology and pharmaceutical product patents involve highly complex legal and scientific questions and can be uncertain. Any patent applications that we own or license may fail to result in issued patents. Even if patents do successfully issue from our applications, third parties may challenge their validity or enforceability, which may result in such patents being narrowed, invalidated, or held unenforceable. Even if our patents and patent applications are not challenged by third parties, those patents and patent applications may not prevent others from designing around our claims and may not otherwise adequately protect our product candidates. If the breadth or strength of protection provided by the patents and patent applications we hold with respect to our product candidates is threatened, competitors with significantly greater resources could threaten our ability to commercialize our product candidates. Discoveries are generally published in the scientific literature well after their actual development, and patent applications in the United States and other countries are typically not published until 18 months after filing, and in some cases are never published. Therefore, we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned and licensed patents or patent applications, or that we or our licensors were the first to file for patent protection covering such inventions. Subject to meeting other requirements for patentability, for United States patent applications filed prior to March 16, 2013, the first to invent the claimed invention is entitled to receive patent protection for that invention while, outside the United States, the first to file a patent application encompassing the invention is entitled to patent protection for the invention. The United States moved to a “first to file” system under the Leahy-Smith America Invents Act, or AIA, effective March 16, 2013. The effects of this change and other elements of the AIA are currently unclear, as the United States Patent and Trademark Office, or USPTO, is still implementing associated regulations, and the applicability of the AIA and associated regulations to our patents and patent applications have not been fully determined. This new system also includes new procedures for challenging issued patents and pending patent applications, which creates additional uncertainty. We may become involved in opposition or interference proceedings challenging our patents and patent applications or the patents and patent applications of others, and the outcome of any such proceedings are highly uncertain. An unfavorable outcome in any such proceedings could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology and compete directly with us, or result in our inability to manufacture, develop or commercialize our product candidates without infringing the patent rights of others.
In addition to the protection afforded by patents, we seek to rely on trade secret protection and confidentiality agreements to protect proprietary know-how, information, or technology that is not covered by our patents. Although our agreements require all of our employees to assign their inventions to us, and we require all of our employees, consultants, advisors and any third parties who have access to our trade secrets, proprietary know-how and other confidential information and technology to enter into appropriate confidentiality agreements, we cannot be certain that our trade secrets, proprietary know-how and other confidential information and technology will not be subject to unauthorized disclosure or that our competitors will not otherwise gain access to or independently develop substantially equivalent trade secrets, proprietary know-how and other information and technology. Furthermore, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property globally. If we are unable to prevent unauthorized disclosure of our intellectual property related to our product candidates and technology to third parties, we may not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business and operations.
Intellectual property disputes with third parties and competitors may be costly and time consuming, and may negatively affect our competitive position.
Our commercial success may depend on our avoiding infringement of the patents and other proprietary rights of third parties as well as on enforcing our patents and other proprietary rights against third parties. Pharmaceutical and biotechnology intellectual property disputes are characterized by complex, lengthy and expensive litigation over patents and other intellectual property rights. We may initiate or become a party to, or be threatened with, future litigation or other proceedings regarding intellectual property rights with respect to our product candidates and competing products.
As our product candidates progress toward commercialization, we or our collaboration partners may be subject to patent infringement claims from third parties. We attempt to ensure that our product candidates do not infringe third party patents and other proprietary rights. However, the patent landscape in competitive product areas is highly complex, and there may be patents of third parties of which we are unaware that may result in claims of infringement. Accordingly, there can be no assurance that our product candidates do not infringe proprietary rights of third parties, and parties making claims against us may seek and obtain injunctive or other equitable relief, which could potentially block further efforts to develop and commercialize our product candidates. Any litigation involving defense against claims of infringement, regardless of the merit of such claims, would involve substantial litigation expense and would be a substantial diversion of management time.
We intend, if necessary, to vigorously enforce our intellectual property in order to protect the proprietary position of our product candidates. Active efforts to enforce our patents may include litigation, administrative proceedings, or both, depending on the potential benefits that might be available from those actions and the costs associated with undertaking those efforts against third parties. We carefully review and monitor publicly available information regarding products that may be competitive with our product candidates and assert our intellectual property rights where appropriate.
We may consider administrative proceedings and other means for challenging third party patents and patent applications. Third parties may also challenge our patents and patent applications, through interference, reexamination, inter partes review, and post-grant review proceedings before the USPTO or through other comparable proceedings, such as oppositions or invalidation proceedings, before foreign patent offices. An unfavorable outcome in any such challenge could require us to cease using the related technology and to attempt to license rights to it from the prevailing third party, which may not be available on commercially reasonable terms, if at all, in which case our business could be harmed. Even if we are successful, participation in administrative proceedings before the USPTO or a foreign patent office may result in substantial costs and time on the part of our management and other employees.
Furthermore, there is a risk that any public announcements concerning the status or outcomes of intellectual property litigation or administrative proceedings may adversely affect the price of our stock. If securities analysts or our investors interpret such status or outcomes as negative or otherwise creating uncertainty, our common stock price may be adversely affected.
Our reliance on third parties and agreements with collaboration partners requires us to share our trade secrets, which increases the possibility that a competitor may discover them or that our trade secrets will be misappropriated or disclosed.
Our reliance on third party contractors to develop and manufacture our product candidates is based upon agreements that limit the rights of the third parties to use or disclose our confidential information, including our trade secrets and know-how. Despite the contractual provisions, the need to share trade secrets and other confidential information increases the risk that such trade secrets and information are disclosed or used, even if unintentionally, in violation of these agreements. In the highly competitive markets in which our product candidates are expected to compete, protecting our trade secrets, including our strategies for addressing competing products, is imperative, and any unauthorized use or disclosure could impair our competitive position and may have a material adverse effect on our business and operations.
In addition, our collaboration partners are larger, more complex organizations than ours, and the risk of inadvertent disclosure of our proprietary information may be increased despite their internal procedures and contractual obligations in place with our collaboration partners. Despite our efforts to protect our trade secrets and other confidential information, a competitor’s discovery of such trade secrets and information could impair our competitive position and have an adverse impact on our business.
We have an extensive worldwide patent portfolio. The cost of maintaining our patent protection is high and maintaining our patent protection requires continuous review and compliance in order to maintain worldwide patent protection. We may not be able to effectively maintain our intellectual property position throughout the major markets of the world.
The USPTO and foreign patent authorities require maintenance fees and payments as well as continued compliance with a number of procedural and documentary requirements. Noncompliance may result in abandonment or lapse of the subject patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance may result in reduced royalty payments for lack of patent coverage in a particular jurisdiction from our collaboration partners or may result in competition, either of which could have a material adverse effect on our business.
We have made, and will continue to make, certain strategic decisions in balancing costs and the potential protection afforded by the patent laws of certain countries. As a result, we may not be able to prevent third parties from practicing our inventions in all countries throughout the world, or from selling or importing products made using our inventions in and into the United States or other countries. Third parties may use our technologies in territories in which we have not obtained patent protection to develop their own products and, further, may infringe our patents in territories which provide inadequate enforcement mechanisms, even if we have patent protection. Such third party products may compete with our product candidates, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
The laws of some foreign countries do not protect proprietary rights to the same extent as do the laws of the United States, and we may encounter significant problems in securing and defending our intellectual property rights outside the United States.
Many companies have encountered significant problems in protecting and defending intellectual property rights in certain countries. The legal systems of certain countries do not always favor the enforcement of patents, trade secrets, and other intellectual property rights, particularly those relating to pharmaceutical and biotechnology products, which could make it difficult for us to stop infringement of our patents, misappropriation of our trade secrets, or marketing of competing products in violation of our proprietary rights. Proceedings to enforce our intellectual property rights in foreign countries could result in substantial costs and divert our efforts and attention from other aspects of our business, and could put our patents in these territories at risk of being invalidated or interpreted narrowly, or our patent applications at risk of not granting, and could provoke third parties to assert claims against us. We may not prevail in all legal or other proceedings that we may initiate and, if we were to prevail, the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Intellectual property rights do not address all potential threats to any competitive advantage we may have.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and intellectual property rights may not adequately protect our business or permit us to maintain our competitive advantage. The following examples are illustrative:
| · | Others may be able to make cellular treatments that are the same as or similar to our current or future product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed. |
| · | We or any of our licensors or strategic partners might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed. |
| · | We or any of our licensors or strategic partners might not have been the first to file patent applications covering certain of our inventions. |
| · | Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights. |
| · | The prosecution of our pending patent applications may not result in granted patents. |
| · | Granted patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors. |
| · | Patent protection on our product candidates may expire before we are able to develop and commercialize the product, or before we are able to recover our investment in the product. |
| · | Our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for such activities, as well as in countries in which we do not have patent rights, and may then use the information learned from such activities to develop competitive products for sale in markets where we intend to market our product candidates. |
If we fail to meet our obligations under license agreements, we may lose our rights to key technologies on which our business depends
Our business will depend in part on several technologies that are based in part on technology licensed from third parties, including the University of Colorado, the University of California, and the Wisconsin Alumni Research Foundation. Those third-party license agreements impose obligations on us, including payment obligations and obligations to pursue development of commercial products under the licensed patents or technology. If a licensor believes that we have failed to meet our obligations under a license agreement, the licensor could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation our ability to carry out the development and commercialization of potential products, and our ability to raise capital, could be significantly and negatively affected. If our license rights were restricted or ultimately lost, we would not be able to continue to use the licensed technology in our business.
The price and sale of any of our products that receive regulatory approval may be limited by health insurance coverage and government regulation
Success in selling any of our products that receive regulatory approval may depend in part on the extent to which health insurance companies, HMOs, and government health administration authorities such as Medicare and Medicaid will pay for the cost of the products and related treatment. Until we actually introduce a new product into the medical market place we will not know with certainty whether adequate health insurance, HMO, and government coverage will be available to permit the product to be sold at a price high enough for us to generate a profit. In some foreign countries, pricing or profitability of health care products is subject to government control which may result in low prices for our products. In the United States, there have been a number of federal and state proposals to implement similar government controls, and new proposals are likely to be made in the future.
The implementation of the ACA in the United States may adversely affect our business.
As a result of the March 2010 adoption of the ACA in the United States, substantial changes are being made to the current system for paying for healthcare in the United States, including programs to extend medical benefits to millions of individuals who currently lack insurance coverage. The changes contemplated by the ACA are subject to rule-making and implementation timelines that extend for several years, as well as initiatives in Congress to amend or repeal the law, and this uncertainty limits our ability to forecast changes that may occur in the future. However, implementation of the ACA has already begun with respect to certain significant cost-saving measures, including changes to several government healthcare programs that may cover the cost of our future products, including Medicaid, Medicare Parts B and D, and these efforts could have a materially adverse impact on our future financial prospects and performance. For example, with respect to Medicaid, in order for a manufacturer’s products to be reimbursed by federal funding under Medicaid, the manufacturer must enter into a Medicaid rebate agreement with the Secretary of the United States Department of Health and Human Services, and must pay certain rebates to the states based on utilization data provided by each state to the manufacturer and to CMS, and based on pricing data provided by the manufacturer to the federal government. The states share this savings with the federal government, and sometimes implement their own additional supplemental rebate programs. Under the Medicaid drug rebate program, the rebate amount for most branded drug products was previously equal to a minimum of 15.1% of the Average Manufacturer Price, or AMP, or the AMP less Best Price, whichever is greater. Effective January 1, 2010, the ACA generally increases the size of the Medicaid rebates paid by manufacturers for single source and innovator multiple source (brand name) drug product from a minimum of 15.1% to a minimum of 23.1% of the AMP, subject to certain exceptions, for example, for certain clotting factors, the increase is limited to a minimum of 17.1% of the AMP. For non-innovator multiple source (generic) products, the rebate percentage is increased from a minimum of 11.0% to a minimum of 13.0% of AMP. In 2010, the ACA also newly extended this rebate obligation to prescription drugs covered by Medicaid managed care organizations. These increases in required rebates may adversely affect our future financial prospects and performance. The ACA also creates new rebate obligations for products under Medicare Part D, a partial, voluntary prescription drug benefit created by the United States federal government primarily for persons 65 years old and over. The Part D drug program is administered through private insurers that contract with CMS. Beginning in 2011, the healthcare reform law generally requires that in order for a drug manufacturer’s products to be reimbursed under Medicare Part D, the manufacturer must enter into a Medicare Coverage Gap Discount Program agreement with the Secretary of the United States Department of Health and Human Services, and reimburse each Medicare Part D plan sponsor an amount equal to 50% savings for the manufacturer’s brand name drugs and biologics which the Part D plan sponsor has provided to its Medicare Part D beneficiaries who are in the “donut hole” (or a gap in Medicare Part D coverage for beneficiaries who have expended certain amounts for drugs). The Part D plan sponsor is responsible for calculating and providing the discount directly to its beneficiaries and for reporting these amounts paid to CMS’s contractor, which notifies drug manufacturers of the rebate amounts it must pay to each Part D plan sponsor. The rebate requirement could adversely affect our future financial performance, particularly if contracts with Part D plans cannot be favorably renegotiated or the Part D plan sponsors fail to accurately calculate payments due in a manner that overstates our rebate obligation. The ACA also introduced a biosimilar pathway that will permit companies to obtain FDA approval of generic versions of existing biologics based upon reduced documentation and data requirements deemed sufficient to demonstrate safety and efficacy than are required for the pioneer biologics. The new law provides that a biosimilar application may be submitted as soon as four years after the reference product is first licensed, and that the FDA may not make approval of an application effective until 12 years after the reference product was first licensed. With the likely introduction of biosimilars in the United States, we expect in the future to face greater competition from biosimilar products, including a possible increase in patent challenges. The FDA has reported meeting with sponsors who are interested in developing biosimilar products, and is developing regulations to implement the abbreviated regulatory review pathway. Regarding access to our products, the ACA established and provided significant funding for a Patient-Centered Outcomes Research Institute to coordinate and fund Comparative Effectiveness Research, or CER. While the stated intent of CER is to develop information to guide providers to the most efficacious therapies, outcomes of CER could influence the reimbursement or coverage for therapies that are determined to be less cost-effective than others. Should any of our products be determined to be less cost effective than alternative therapies, the levels of reimbursement for these products, or the willingness to reimburse at all, could be impacted, which could materially impact our future financial prospects and results.
Risks Related to Our Relationship With BioTime
We are a subsidiary of BioTime, and accordingly our business is substantially controlled by BioTime.
As of March 23, 2016, BioTime owns approximately 56.7% of our issued and outstanding Series A Shares. Because BioTime owns a majority of the outstanding Series A Shares, it has the voting power to elect our entire Board of Directors. Presently, four of the eight members of our Board of Directors are also directors or officers of BioTime, and another director is an employee of Broadwood Capital, Inc., which is the general partner of Broadwood Partners, L.P., the partnership that is the largest shareholder of BioTime. Some of our directors also serve on the Boards of Directors of one or more of BioTime’s other subsidiaries. The relationship of our directors with BioTime means that we will not have a Board of Directors making business decisions on our behalf independent from BioTime. Even those of our directors who do not serve on the BioTime Board of Directors will be elected to our Board of Directors by BioTime, and they may be removed from our Board by BioTime, as the majority shareholder.
BioTime could cause corporate actions to be taken even if the interests of BioTime conflict with the interests of our other shareholders. This concentration of voting power could have the effect of deterring or preventing a change in control that might be beneficial to our other shareholders.
As the majority shareholder, BioTime will have the voting power to approve or disapprove any matter or corporate transaction presented to our shareholders for approval, including but not limited to:
| · | any amendment of our certificate of incorporation or bylaws; |
| · | any merger or consolidation of us with another company; |
| · | any recapitalization or reorganization of our capital stock; |
| · | any sale of assets or purchase of assets; or |
| · | a corporate dissolution or a plan of liquidation of our business. |
We partially rely upon BioTime for certain services and resources
Although we have our own research facilities, scientific personnel, and some management personnel, we partially rely on BioTime to provide certain management and administrative services, including patent prosecution, certain legal services, accounting, financial management, and controls over financial accounting and reporting. We have entered into a Shared Facilities and Services Agreement with BioTime under which we have agreed to bear costs allocated to us by BioTime for the use of BioTime human resources and for services and materials provided for our benefit by BioTime. We pay BioTime 105% of its costs of providing personnel and services to us, and for any use of its facilities by us, including an allocation of general overhead based on that use. We may also share the services of some research personnel with BioTime.
If BioTime’s human resources and facilities are not sufficient to serve both BioTime’s needs and ours, we will have to hire additional personnel of our own, either on a full-time or part-time basis, as employees or as consultants, and the cost of doing so could be greater than the costs that would be allocated to us by BioTime. Also, any new personnel that we may need to hire may not be as familiar with our business or operations as BioTime’s personnel, which means that we would incur the expense and inefficiencies related to training new employees or consultants.
Conflicts of interest may arise from our relationship with BioTime
Our relationship with BioTime could give rise to certain conflicts of interest that could have an impact on our research and development programs, business opportunities, and operations generally.
| · | We and BioTime or any of its other subsidiaries may determine to engage in research and development of the same or similar products or technologies, or products that would otherwise compete in the market place. Even if we utilize different technologies than BioTime or its other subsidiaries, we could find ourselves in competition with them for research scientists, financing and other resources, licensing, manufacturing, and distribution |
| · | Because we are a subsidiary of BioTime, BioTime could prevent us from engaging in research and development programs, investments, business ventures, or agreements to develop, license, or acquire products or technologies that would or might compete with those owned, licensed, or under development by BioTime or any of its other subsidiaries. |
| · | BioTime may determine that some of our patents or technology would be useful in its business or that of another BioTime subsidiary, and BioTime or another BioTime subsidiary may hold patents or technology that we may determine would be useful in our business. In such cases we may enter into license or sublicense agreements with BioTime or another BioTime subsidiary for the use of such patents or technology. Conflicts of interest will arise in determining the scope and financial terms of any such licenses or sublicenses, including the fields of use permitted, licensing fees, and royalties, if any, and other matters. |
| · | BioTime and its other subsidiaries will engage for their own accounts in research and product development programs, investments, and business ventures, and we will not be entitled to participate or to receive an interest in those programs, investments, or business ventures. BioTime and its other subsidiaries will not be obligated to present any particular research and development, investment, or business opportunity to us, even if the opportunity would be within the scope of our research and development plans or programs, business objectives, or investment policies. These opportunities may include, for example, opportunities to acquire businesses or assets, including but not limited to patents and other intellectual property that could be used by us or by BioTime or by any of BioTime’s other subsidiaries. Our respective boards of directors will have to determine which company should pursue those opportunities, taking into account relevant facts and circumstances at the time, such as the financial and other resources of the companies available to acquire and utilize the opportunity, and the best “fit” between the opportunity and the business and research and development programs of the companies. However, since BioTime will have the ultimate power to elect the members of our Board of Directors, BioTime may have the ultimate say in decision making with respect to the allocation of opportunities |
| · | If we enter into any patent or technology license or sublicense, or any other agreement with BioTime or with another BioTime subsidiary, the BioTime companies that are parties to the agreement may have a conflict of interest in determining how and when they should enforce their rights under the agreement if the other BioTime company that is a party were to default or otherwise fail to perform any of its obligations under the agreement. |
| · | One of our significant assets is 3,852,880 BioTime common shares that we held as of December 31, 2015. Through the Asset Contribution Agreement we acquired 8,902,077 BioTime common shares from BioTime in October 2013 and we sold a portion of those shares during June 2014. We expect to sell the remaining BioTime common shares from time to time, or to pledge those shares as collateral for loans, to raise capital to finance our operations. Because a sale of those shares could have a depressing effect on the market value of BioTime common shares, BioTime will have a continuing interest in the number of shares we sell, the prices at which we sell the shares, and time and manner in which the shares are sold. Further, we may need or find it desirable to sell BioTime common shares at the same time as BioTime, or other BioTime subsidiaries that hold BioTime common shares, also desire to sell some of their BioTime common shares. Concurrent sales of BioTime common shares by us, BioTime, or other BioTime subsidiaries could have a depressing effect on the market price of the BioTime common shares, lowering the price at which we and they are able to sell BioTime common shares and resulting in lower net proceeds from the sales. We plan to coordinate any future sales of our BioTime common shares with BioTime and its other subsidiaries in order to provide an orderly and controlled process for raising capital through the sale of BioTime shares. This will include an agreement as to the number of shares to be sold, the time period or “market window” for selling shares, the use of a common securities broker-dealer, and a fair allocation of net sales based on average sales prices during any trading day on which we and they sell BioTime shares. |
| · | Each conflict of interest will be resolved by our respective boards of directors in keeping with their fiduciary duties and such policies as they may implement from time to time. However, the terms and conditions of patent and technology licenses and other agreements between us and BioTime or other BioTime subsidiaries will not be negotiated on an arm’s-length basis due to BioTime’s ownership of a controlling interest in us and due to the commonality of directors serving on our respective boards of directors. |
Risks Related to Our Dependence on Third Parties
We could lose our CIRM grant if we fail to meet the clinical trial milestones that are a condition to CIRM’s obligation to provide funding
We are depending upon our grant from CIRM as a source of financing for the costs of conducting our Phase 1/2a clinical trial and process development of AST-OPC1. Under the terms of the CIRM grant, as amended effective March 2, 2016, we must meet certain progress milestones pertaining to the clinical trial in order to receive additional payments. If we fail to meet the milestones, payments will be delayed. Additionally, under the agreement CIRM has the right to suspend payment upon the occurrence of certain Suspension Events, which could force us to postpone, delay, or discontinue the clinical trial and development work for the product.
Establishing and maintaining strategic alliances is a key component of our business strategy. If we are unable to establish and maintain strategic alliances for our therapeutic product candidates, we may have to reduce or delay our product development or increase our expenditures
A key component of our current strategy for developing, manufacturing and commercializing our therapeutic product candidates will be entering into strategic alliances with pharmaceutical companies or other industry participants to advance our programs and enable us to maintain our financial and operational capacity. We will face significant competition in seeking appropriate alliances. We may not be able to negotiate alliances on acceptable terms, if at all. If our strategic alliances do not result in the successful development and commercialization of our product candidates or if one or more of our collaborators terminates its agreement with us, we may not receive any future research and development funding or milestone or royalty payments under the collaboration. If we do not receive the funding we expect under these agreements, our continued development of our product candidates could be delayed and we may need to obtain additional funding, which may be unavailable or available only on unfavorable terms.
If we are able to enter into product development and marketing arrangements with pharmaceutical companies, we may license product development, manufacturing, and marketing rights to the pharmaceutical company or to a joint venture company formed with the pharmaceutical company. Under such arrangements we might receive only a royalty on sales of the products developed or an equity interest in a joint venture company that develops the product. As a result, our revenues from the sale of those products may be substantially less than the amount of revenues and gross profits that we might receive if we were to develop, manufacture, and market the products ourselves.
We may become dependent on possible future collaborations to develop and commercialize many of our product candidates and to provide the manufacturing, regulatory compliance, sales, marketing and distribution capabilities required for the success of our business
We may enter into various kinds of collaborative research and development, manufacturing, and product marketing agreements to develop and commercialize our products. Any future milestone payments and cost reimbursements from collaboration agreements could provide an important source of financing for our research and development programs, thereby facilitating the application of our technology to the development and commercialization of our products, but there are risks associated with entering into collaboration arrangements.
There is a risk that we could become dependent upon one or more collaborative arrangements for product development or manufacturing or as a source of revenues from the sale of any products that may be developed by us alone or through one of the collaborative arrangements. A collaborative arrangement upon which we might depend might be terminated by our collaboration partner or they might determine not to actively pursue the development or commercialization of our products. A collaboration partner also may not be precluded from independently pursuing competing products and drug delivery approaches or technologies.
There is a risk that a collaboration partner might fail to perform its obligations under the collaborative arrangements or may be slow in performing its obligations. In addition, a collaboration partner may experience financial difficulties at any time that could prevent it from having available funds to contribute to the collaboration. If a collaboration partner fails to conduct its product development, manufacturing, commercialization, regulatory compliance, sales and marketing or distribution activities successfully and in a timely manner, or if it terminates or materially modifies its agreements with us, the development and commercialization of one or more product candidates could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue product development, manufacturing, and commercialization on our own.
Industry and other market data used in this Annual Report, including those undertaken by us or our engaged consultants, may prove to be unrepresentative of current and future market conditions or future results
This Annual Report includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties, and surveys and studies we commissioned, regarding the market potential for our product candidates. Although we believe that such information has been obtained from sources believed to be reliable, neither the sources of such data, nor we, can guarantee the accuracy or completeness of such information. While we believe these industry publications and third party research, surveys and studies are reliable, we have not independently verified such data. With respect to the information from third party consultants, the results of that study represent the independent consultants’ own methodologies, assumptions, research, analysis, projections, estimations, composition of respondent pool, presentation of data, and adjustments, each of which may ultimately prove to be incorrect, and cause actual results and market viability to differ materially from those presented in such report. Readers should not place undue reliance on this information.
Risks Pertaining to Our Common Stock
Ownership of our common stock will entail certain risks associated with the volatility of prices for our shares and the fact that we do not pay dividends on our common stock.
The price of our common stock may rise and fall rapidly
The market price of our common stock like that of the shares of many biotechnology companies, may be highly volatile. The price of our common stock may rise or fall rapidly as a result of a number of factors, including:
| · | sales or potential sales of substantial amounts of our common stock; |
| · | results of preclinical testing or clinical trials of our product candidates or those of our competitors; |
| · | announcements about us or about our competitors, including clinical trial results, regulatory approvals, new product introductions and commercial results; |
| · | the cost of our development programs; |
| · | the success of competitive products or technologies; |
| · | litigation and other developments relating to our issued patents or patent applications or other proprietary rights or those of our competitors; |
| · | conditions in the pharmaceutical or biotechnology industries; |
| · | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
| · | variations in our financial results or those of companies that are perceived to be similar to us, including the failure of our earnings to meet analysts’ expectations; and |
| · | general economic, industry and market conditions. |
Many of these factors are beyond our control. The stock markets in general, and the market for pharmaceutical and biotechnological companies in particular, have been experiencing extreme price and volume fluctuations which have affected the market price of the equity securities without regard to the operating performance of the issuing companies. Broad market fluctuations, as well as industry factors and general economic and political conditions, may adversely affect the market price of our common stock.
The recently enacted JOBS Act allows us to postpone the date by which we must comply with certain laws and regulations intended to protect investors and to reduce the amount of information we provide in our reports filed with the Commission, which could undermine investor confidence in our company and adversely affect the market price of our securities.
The recently enacted JOBS Act is intended to reduce the regulatory burden on “emerging growth companies.” As defined in the JOBS Act, a public company whose initial public offering of common equity securities occurred after December 8, 2011 and whose annual gross revenues are less than $1.0 billion will, in general, qualify as an emerging growth company until the earliest of:
| · | the last day of its fiscal year following the fifth anniversary of the date of its initial public offering of common equity securities; |
| · | the last day of its fiscal year in which it has annual gross revenue of $1.0 billion or more; |
| · | the date on which it has, during the previous three-year period, issued more than $1.0 billion in non-convertible debt; and |
| · | the date on which it is deemed to be a “large accelerated filer,” which will occur at such time as we (a) have an aggregate worldwide market value of common equity securities held by non-affiliates of $700 million or more as of the last business day of its most recently completed second fiscal quarter, (b) have been required to file annual and quarterly reports under the Securities Exchange Act of 1934 for a period of at least 12 months, and (c) have filed at least one annual report pursuant to the Securities Exchange Act of 1934. |
Under this definition, we are an emerging growth company and could remain an emerging growth company until as late as December 31, 2019.
The JOBS Act provides that, so long as we qualify as an emerging growth company, we will, among other things:
| · | be exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting; |
| · | be exempt from the “say on pay” provisions (requiring a non-binding stockholder vote to approve compensation of certain executive officers) and the “say on golden parachute” provisions (requiring a non-binding stockholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations) of the Dodd-Frank Act and certain disclosure requirements of the Dodd-Frank Act relating to compensation of our chief executive officer; |
| · | be permitted to omit the detailed compensation discussion and analysis from proxy statements and reports filed under the Securities Exchange Act of 1934 and instead provide a reduced level of disclosure concerning executive compensation; and |
| · | be exempt from any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements. |
Although we are still evaluating the JOBS Act, we currently take advantage of the reduced regulatory and reporting requirements that are available to us so long as we qualify as an “emerging growth company,” except that we have irrevocably elected not to take advantage of the extension of time to comply with new or revised financial accounting standards available under Section 102(b) of the JOBS Act. Among other things, this means that our independent registered public accounting firm will not be required to provide an attestation report on the effectiveness of our internal control over financial reporting so long as we qualify as an emerging growth company, which may increase the risk that weaknesses or deficiencies in our internal control over financial reporting go undetected. Likewise, so long as we qualify as an emerging growth company, we may elect not to provide you with certain information, including certain financial information and certain information regarding compensation of our executive officers, that we would otherwise have been required to provide in filings we make with the Commission, which may make it more difficult for investors and securities analysts to evaluate our company. As a result, investor confidence in our company and the market price of our securities may be materially and adversely affected.
Our stock price could decline due to the large number of outstanding shares of our common stock eligible for future sale
Sales of substantial amounts of our common stock in the public market, or the perception that those sales could occur, could cause the market price of our common stock to decline. Sales of substantial amounts of common stock could also make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem appropriate.
We do not currently intend to pay dividends on any of our classes of securities and, consequently, your ability to achieve a return on your investment will depend on the appreciation in the price of our securities.
We have never declared or paid any cash dividends on any class of our securities. We currently intend to retain any future earnings to fund our future growth and do not expect to declare or pay any dividend on any class of our securities in the foreseeable future. As a result, you may only realize a gain on your investment in our securities if the market price of our securities appreciates and you sell your securities at a price above your cost after accounting for any taxes. The price of our securities may not appreciate in value or ever exceed the price that you paid for our securities.
The price of our common stock, and the value of our assets, will be affected by changes in the value of the BioTime common shares that we own
As of December 31, 2015, we held 3,852,880 of the 8,902,077 BioTime common shares we received from BioTime under the Asset Contribution Agreement. The value of our common stock will reflect, in part, the value of the BioTime common shares that we hold. The value of the BioTime common shares we hold will vary with the price at which BioTime common shares trade in the public market. The market price of BioTime common shares will be impacted by a number of factors, including the results of BioTime’s operations.
If securities analysts do not publish research or reports about our business or if they downgrade our stock, the price of our securities could decline.
The current trading market for our common stock relies in part on the research and reports that industry or financial analysts publish about us, our business, our markets and our competitors. We do not control these analysts. If securities analysts do not cover us, the lack of research coverage may adversely affect the market price of our common stock. Furthermore, if one or more of the analysts who do cover us downgrade our stock or if those analysts issue other unfavorable commentary about us or our business, our stock price would likely decline. If one or more of these analysts cease coverage of us or fails to regularly publish reports on us, we could lose visibility in the market and interest in our stock could decrease, which in turn could cause our stock price or trading volume to decline.
You may experience dilution of your ownership interests because of the future issuance of additional shares of our common stock and our preferred stock
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present shareholders. We are currently authorized to issue an aggregate of 150,000,000 shares of common stock, consisting of 75,000,000 Series A Shares and 75,000,000 of our Series B Shares. We are also authorized to issue 5,000,000 shares of “blank check” preferred stock. As of March 23, 2016, we had issued and outstanding 38,352,150 Series A Shares. We have also reserved 350,000 Series A Shares for issuance upon the exercise of outstanding warrants, and 8,000,000 Series A Shares for issuance under a stock option and stock purchase plan.
We may issue additional shares of common stock or other securities in order to raise additional capital, or in connection with hiring or retaining employees or consultants, or in connection with future acquisitions of licenses to technology or rights to acquire products, in connection with future business acquisitions, or for other business purposes. The future issuance of any such additional shares of common stock or other securities may create downward pressure on the trading price of our common stock.
We may also issue 5,000,000 shares of preferred stock having rights, preferences, and privileges senior to the rights of our common stock with respect to dividends, rights to share in distributions of our assets if we liquidate our company, or voting rights. Any preferred stock may also be convertible into Series A Shares on terms that would be dilutive to holders of common stock.
In addition, as previously announced, we intend to distribute warrants to our shareholders on a pro-rata basis (except for BioTime), and the exercise of such warrants, if any, could result in dilution.
None
We occupied an office and research facility located at 230 Constitution Drive, Menlo Park, California under a sublease from BioTime through January 2016. In November 2015, we moved to our new office and research facility located at 6300 Dumbarton Circle, Fremont, California as discussed below.
On December 30, 2013, we entered into a lease for this office and research facility located in Fremont, California, consisting of an existing building with approximately 44,000 square feet of space. The building will be used by us primarily as a laboratory and production facility that can be used to produce human embryonic stem cells and related products under current good manufacturing procedures (cGMP). We completed construction of certain tenant improvements for our use, which cost approximately $4.9 million, of which a maximum of $4.4 million was paid to us by the landlord.
The lease is for a term of 96 months commencing on October 1, 2014, with two available five-year options to extend the term, upon one year written notice from us. During the first 15 months of the lease term, from October 1, 2014 through December 31, 2015, we paid monthly base rent of $51,000 representing 22,000 square feet rather than 44,000 square feet. Beginning on January 1, 2016, base rent will increase to $105,000 per month and increase by approximately 3% annually on every October 1 thereafter.
In addition to monthly base rent, we pay all real estate taxes, insurance and the cost of maintenance, repair and replacement of the leased premises. During the first 15 months of the lease term, we will pay only 50% of the real estate taxes assessed on the premises provided that we are not in default in performing its obligations under the lease beyond any notice and cure periods. However, if any improvements or alterations to the premises that we construct or add are assessed for real property tax purposes at a valuation higher than the valuation of the improvements on the premises on the date it signed the lease, we will pay 100% of the taxes levied on the excess assessed valuation.
We also currently pays $3,300 per month for the use of approximately 120 square feet of the office space in New York City that is used to conduct meetings and other business affairs. The lease originally for one year commencing July 1, 2014 was extended through June 30, 2016 and is expected to terminate on this date.
From time to time, we may be involved in routine litigation incidental to the conduct of our business. We are not presently involved in any material litigation or proceedings, and to our knowledge no such litigation or proceedings are contemplated.
Not applicable
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities |
Our Series A Shares have been traded on the NYSE MKT under the symbol “AST” since October 7, 2014. From July 2014 through October 7, 2014 our Series A Shares traded on the OTC Bulletin Board under the ticker symbol “ASTYV.” The following table sets forth the range of high and low closing prices for our Series A Shares during 2015 and the third and fourth quarters of 2014 as report by the NYSE MKT and the OTC BB.
|
Quarter Ended
|
High
|
Low
|
||||||
|
September 30, 2014
|
$
|
7.35
|
$
|
2.07
|
||||
|
December 31, 2014
|
$
|
6.25
|
$
|
3.07
|
||||
|
March 31, 2015
|
$
|
8.65
|
$
|
3.30
|
||||
|
June 30, 2015
|
$
|
14.77
|
$
|
3.70
|
||||
|
September 30, 2015
|
$
|
5.92
|
$
|
3.48
|
||||
|
December 31, 2015
|
$
|
5.45
|
$
|
3.78
|
||||
Over-the-counter market quotations on the OTC Bulletin Board may reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
As of March 23, 2016, we had 530 holders of record of our Series A Shares.
The following table shows certain information concerning the options outstanding and available for issuance under all of our compensation plans and agreements as of December 31, 2015:
|
Plan Category
|
Number of Shares
to
be Issued upon
Exercise of
Outstanding
Options, Warrants,
and Rights(1)
|
Weighted Average
Exercise Price of
the Outstanding
Options, Warrants,
and Rights
|
Number of Shares
Remaining Available
for Future Issuance
under Equity
Compensation Plans
|
|||||||||
|
Asterias Equity Compensation Plan Approved by Shareholders (1)
|
5,178,411
|
$
|
3.17
|
2,067,000
|
||||||||
| (1) | Includes 48,306 restricted stock units granted under the Asterias Equity Incentive Plan. |
Additional information concerning our Equity Incentive Plan, the stock options and restricted stock units may be found in Note 7 to the Financial Statements.
Dividend Policy
We have never paid cash dividends on our capital stock and we do not anticipate paying cash dividends in the foreseeable future, but intend to retain our capital resources for reinvestment in our business. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon our financial condition, results of operations, capital requirements and other factors as our Board of Directors deems relevant.
Performance Measurement Comparison (1)
The following graph compares total stockholder returns of Asterias Biotherapeutics, Inc. for the last six months beginning July 31, 2015 to two indices: the NYSE Amex Market Value – U.S. Companies (Amex Market Value) and the NYSE Arca Biotechnology Index (NYSE Arca Biotechnology Index). The total return for our stock and for each index assumes the reinvestment of dividends, although we have never declared dividends on Asterias Biotherapeutics, Inc., and is based on the returns of the component companies weighted according to their capitalizations as of the end of each quarterly period. The NYSE Amex Market Value tracks the aggregate price performance of equity securities of U.S. companies listed therein. The NYSE Arca Biotechnology Index represents biotechnology companies, trading on NYSE MKT under the Standard Industrial Classification (SIC) Code Nos. 283 (Drugs) and 382 (Laboratory Apparatus and Analytical, Optical) main categories (2834: Pharmaceutical Preparations; 2835: Diagnostic Substances; 2836: Biological Products; 3826: Laboratory Analytical Instruments; and 3829: Measuring & Controlling Devices). Asterias Biotherapeutics, Inc. common stock trades on the NYSE MKT and is a component of the NYSE Amex Market Value – US Companies.
Comparison of 18 Month Cumulative Quarterly Total Return on Investment
|
7/18/2014
|
9/30/2014
|
12/31/2014
|
3/31/2015
|
6/30/2015
|
9/30/2015
|
12/31/2015
|
|||||||||||||||||||||||
|
Asterias Biotherapeutics, Inc.
|
Return %
|
69.75
|
-16.92
|
69.35
|
-59.00
|
-18.35
|
-18.13
|
||||||||||||||||||||||
|
Cum $
|
100.00
|
168.33
|
90.00
|
187.22
|
127.78
|
107.50
|
109.17
|
||||||||||||||||||||||
|
AMEX Market Value (US Companies)
|
Return %
|
-5.27
|
2.68
|
-0.38
|
-5.79
|
-8.13
|
-7.67
|
||||||||||||||||||||||
| Cum $ |
100.00
|
97.37
|
90.99
|
94.53
|
88.08
|
74.46
|
70.76
|
||||||||||||||||||||||
|
NYSE Arca Biotechnology Index
|
Return %
|
-1.22
|
0.12
|
2.76
|
0.74
|
-9.84
|
0.66
|
||||||||||||||||||||||
| Cum $ |
100.00
|
113.60
|
126.42
|
146.72
|
154.09
|
126.21
|
140.82
|
||||||||||||||||||||||
Asterias Biotherapeutics, Inc., the Amex Market Value and Amex Biotechnology Index(2)
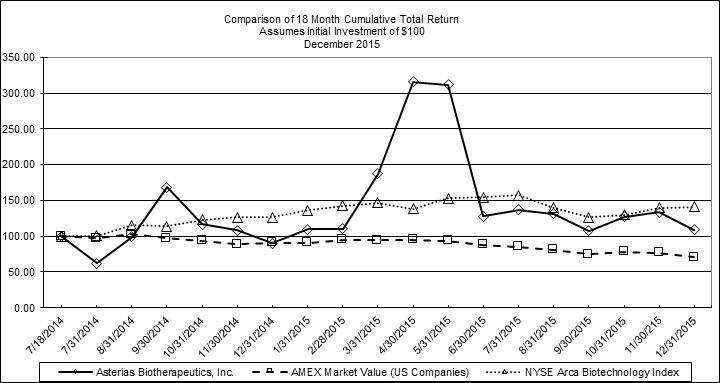
| (1) | This Section is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference in any filing of BioTime under the Securities Act of 1933, or the Securities Exchange Act of 1934, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. |
| (2) | Shows the cumulative total return on investment assuming an investment of $100 in each of Asterias Biotherapeutics, Inc., the Amex Market Value and NYSE Arca Biotechnology Index on July 18, 2014. The cumulative total return on Asterias stock has been computed based on a price of $3.60 per share, the price at which Asterias’ shares closed on July 18, 2014. |
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
REVENUES:
|
||||||||||||
|
Royalties from product sales
|
$
|
535
|
$
|
189
|
$
|
-
|
||||||
|
Sale of cell lines
|
40
|
-
|
-
|
|||||||||
|
Grant income
|
3,007
|
1,035
|
-
|
|||||||||
|
Total revenues
|
3,582
|
1,224
|
-
|
|||||||||
|
Cost of sales
|
(268
|
)
|
(95
|
)
|
-
|
|||||||
|
Gross profit
|
3,314
|
1,129
|
-
|
|||||||||
|
OPERATING EXPENSE:
|
||||||||||||
|
Research and development
|
(17,321
|
)
|
(13,310
|
)
|
(4,319
|
)
|
||||||
|
Acquired in-process research and development(1)
|
-
|
-
|
(17,459
|
)
|
||||||||
|
General and administrative
|
(7,901
|
)
|
(5,280
|
)
|
(3,883
|
)
|
||||||
|
Total operating expenses
|
(25,222
|
)
|
(18,590
|
)
|
(25,661
|
)
|
||||||
|
Loss from operations
|
(21,908
|
)
|
(17,461
|
)
|
(25,661
|
)
|
||||||
|
OTHER INCOME/(EXPENSE):
|
||||||||||||
|
Interest expense, net
|
(341
|
)
|
(10
|
)
|
(2
|
)
|
||||||
|
Other income (expense), net
|
(6
|
)
|
(2
|
)
|
2
|
|||||||
|
Total other expense, net
|
(347
|
)
|
(12
|
)
|
-
|
|||||||
|
LOSS BEFORE INCOME TAX BENEFIT
|
(22,255
|
)
|
(17,473
|
)
|
(25,661
|
)
|
||||||
|
Deferred income tax benefit
|
7,252
|
7,376
|
3,281
|
|||||||||
|
NET LOSS
|
$ |
(15,003
|
)
|
$ |
(10,097
|
)
|
$ |
(22,380
|
)
|
|||
|
BASIC AND DILUTED NET LOSS PER SHARE
|
$
|
(0.42
|
)
|
$
|
(0.33
|
)
|
$
|
(2.90
|
)
|
|||
|
WEIGHTED AVERAGE SHARES OUTSTANDING: BASIC AND DILUTED
|
35,443
|
30,720
|
7,726
|
|||||||||
| (1) | Represents the value of incomplete research and development projects acquired from Geron which Asterias intends to continue. |
|
December 31,
2015 |
December 31,
2014 |
|||||||
|
Balance Sheet Data:
|
||||||||
|
Cash and cash equivalents
|
$
|
11,183
|
$
|
3,076
|
||||
|
Total assets
|
66,978
|
44,114
|
||||||
|
Total liabilities
|
21,879
|
11,483
|
||||||
|
Accumulated deficit
|
(48,239
|
)
|
(33,236
|
)
|
||||
|
Total stockholders' equity
|
45,099
|
32,631
|
||||||
The following Management's Discussion and Analysis of Financial Condition and Results of Operations is intended to provide information necessary to understand Asterias’ audited financial statements for the years ended December 31, 2015, 2014 and 2013, and highlight certain other information which, in the opinion of management, will enhance a reader's understanding of Asterias’ financial condition, changes in financial condition and results of operations. In particular, the discussion is intended to provide an analysis of significant trends and material changes in our financial position and the operating results of our business during the year ended December 31, 2015 as compared to 2014. This discussion should be read in conjunction with our financial statements for the period ended December 31, 2015 and related notes included elsewhere in this Annual Report on Form 10-K. These historical financial statements may not be indicative of Asterias’ future performance. This Management's Discussion and Analysis of Financial Condition and Results of Operations contains a number of forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risks described throughout this filing, particularly in "Item 1A. Risk Factors.”
Critical Accounting Policies
Revenue recognition – We comply with ASC 605-10 and record revenue when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable, and collectability is reasonably assured. Grant income is recognized as revenue when earned. Royalty revenues consist of royalty payments on sales of products under license agreements. We recognize revenue in the quarter in which the royalty reports are received rather than the quarter in which the sales took place. When we are entitled to receive up-front nonrefundable licensing or similar fees pursuant to agreements under which we have no continuing performance obligations, the fees are recognized as revenues when collection is reasonably assured. When we receive up-front nonrefundable licensing or similar fees pursuant to agreements under which we do have continuing performance obligations, the fees are deferred and amortized ratably over the performance period. If the performance period cannot be reasonably estimated, we amortize nonrefundable fees over the life of the contract until such time that the performance period can be more reasonably estimated. Milestone payments, if any, related to scientific or technical achievements are recognized in income when the milestone is accomplished if (a) substantive effort was required to achieve the milestone, (b) the amount of the milestone payment appears reasonably commensurate with the effort expended, and (c) collection of the payment is reasonably assured.
Patent costs – Costs associated with obtaining patents on products or technology developed are expensed as general and administrative expenses when incurred.
Available for sale securities, at fair value – Marketable equity securities and debt securities not classified as held-to-maturity are classified as available-for-sale. Available-for-sale securities, including the OncoCyte common stock we hold (see Note 2 to our financial statements included elsewhere in this report), are carried at fair value, with the unrealized gains and losses, net of tax, reported in other comprehensive income. Realized gains and losses, and declines in value judged to be other-than-temporary related to equity securities, are included in other income and expense, net.
Property, plant and equipment – Property, plant and equipment includes equipment, fixtures and leasehold improvements stated at cost and depreciated using the straight-line method over a period of 36 to 120 months.
Intangible assets – Intangible assets with finite useful lives are amortized over estimated useful lives and intangible assets with indefinite lives are not amortized but rather are tested at least annually for impairment. Acquired in-process research and development intangible assets are accounted depending on whether they were acquired as part of an acquisition of a business, or assets that do not constitute a business. When acquired in conjunction with the acquisition of a business, these assets are considered to be indefinite-lived until the completion or abandonment of the associated research and development efforts and are capitalized as an asset. If and when development is complete, the associated assets would be deemed finite-lived and would then be amortized based on their respective estimated useful lives at that point in time. However, when acquired in conjunction with an acquisition of assets that do not constitute a business (such as our acquisition of assets from Geron), in accordance with the accounting rules in ASC 805-50, such intangible assets related to in process research and development (“IPR&D”) are expensed upon acquisition.
Impairment of long-lived assets – Our long-lived assets, including tangible assets, will be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be fully recoverable. If an impairment indicator is present, we will evaluate recoverability by a comparison of the carrying amount of the assets to future undiscounted net cash flows expected to be generated by the assets. If the assets are impaired, the impairment will be recognized and measured by the amount by which the carrying amount exceeds the estimated fair value of the assets.
Warrants to purchase common stock – We generally account for warrants issued in connection with equity financings as a component of equity. None of the warrants issued by us as of December 31, 2015 include a conditional obligation to issue a variable number of shares; nor was there a deemed possibility that we may need to settle the warrants in cash. If we were to issue warrants with a conditional obligation to issue a variable number of shares or with the deemed possibility of a cash settlement, we would record the fair value of the warrants as a liability at each balance sheet date and records changes in fair value in other income and expense in our statements of operations.
Accounting for BioTime Shares – We account for the BioTime shares we hold as available-for-sale equity securities in accordance with ASC 320-10-25, Investments-Debt and Equity Securities, as the shares have a readily determinable fair value quoted on the NYSE MKT and are held principally for future working capital purposes, as necessary. These shares are measured at fair value and reported as current assets on the balance sheet based on the closing trading price of the security as of the date being presented. Unrealized holding gains and losses are excluded from the statements of operations and reported in equity as part of other comprehensive income or loss until realized. Realized gains and losses are reclassified out of other comprehensive income or loss and included in equity, as an increase or decrease in additional paid-in capital consistent with, and pursuant to, ASC 805-50, transactions between entities under common control.
Research and development – Research and development expenses consist of costs incurred for company-sponsored, collaborative and contracted research and development activities. These costs include direct and research-related overhead expenses including salaries, payroll taxes, consulting fees, research and laboratory fees, rent of research facilities, amortization of intangible assets, and license fees paid to third parties to acquire patents or licenses to use patents and other technology. Asterias expenses research and development costs as such costs are incurred. Research and development expenses incurred and reimbursed under grants approximate the grant income recognized in the statements of operations.
Income taxes – Beginning October 1, 2013, we began filing our own separate U.S. federal tax return. For California purposes our activity will continue to be included in BioTime’s California combined tax return. Our operations through September 30, 2013 were included in BioTime’s consolidated U.S. federal income tax return. For California, our activity beginning with the 2013 calendar year has been included in BioTime’s combined tax return. The provision for income taxes was previously determined as if we had filed separate tax returns for the periods presented. Accordingly, the effective tax rate for us in periods subsequent to December 31, 2013 could vary from its historical effective tax rates depending on our future legal structure and related tax elections. We account for income taxes in accordance with the accounting principles generally accepted in the United States, which prescribe the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. Generally, we are subject to income tax examinations by major taxing authorities for all years since inception. We will recognize accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of December 31, 2015 and 2014. Management is currently unaware of any tax issues under review.
Stock-based compensation – We adopted accounting standards governing share-based payments, which require the measurement and recognition of compensation expense for all share-based payment awards made to directors and employees, including employee stock options, based on estimated fair values. Consistent with those guidelines, we utilize the Black-Scholes-Merton option pricing model. Our determination of fair value of share-based payment awards on the date of grant using that option-pricing model is affected by our stock price as well as by assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, our expected stock price volatility over the term of the awards, and actual and projected employee stock option exercise behaviors. Because our common stock has only 18 months of trading history as of December 31, 2015, for the years ended December 31, 2015 and 2014, we estimated the expected volatility of the awards from the historical volatility of selected public companies within the biotechnology industry with comparable characteristics to us, including similarity in size, lines of business, market capitalization, revenue and financial leverage. We determined the expected volatility assumption using the frequency of daily historical prices of comparable public company’s common stock for a period equal to the expected term of the options. The expected term of options granted is based upon the “simplified method” provided under Staff Accounting Bulletin, Topic 14, or SAB Topic 14. The risk-free rate is based on the U.S. Treasury rates in effect during the corresponding period of grant. Although the fair value of employee stock options is determined in accordance with FASB guidance, the key inputs and assumptions may change as we develop our own company estimates, experience and key inputs including our expected term and stock price volatility based on the trading history of our stock on the NYSE:MKT. Changes in these subjective assumptions can materially affect the estimated value of equity grants and the stock-based compensation that we record in our financial statements.
Asterias also, at times, issues restricted stock or restricted stock units (RSUs) to its executive officers, employees, and members of its Board of Directors (the Board), which are restricted and unvested common shares issued at fair market value on the date of grant. Restricted stock and restricted stock unit compensation expense is recognized on a straight-line basis over the requisite service period of generally four years, based on the grant-date fair value of the stock. Restricted stock is considered legally issued and outstanding on the grant date. Once the RSUs are vested, equivalent common shares will be issued or issuable to the grantee and therefore the RSUs are not included in total common shares issued and outstanding until vested.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718 and FASB ASC 505-50, Equity-Based Payments to Non-Employees (“ASC 505-50”). Stock option awards issued to non-employees, principally consultants and employees of BioTime or employees of BioTime subsidiaries who perform services for Asterias, are accounted for at fair value using the Black-Scholes option pricing model. Management believes that the fair value of the stock options is more reliably measured than the fair value of services received. Asterias records compensation expense based on the then-current fair values of the stock options at each financial reporting date. Compensation recorded during the service period is adjusted in subsequent periods for changes in the fair value of the stock options until the earlier of the date at which the non-employee’s performance is complete or a performance commitment is reached, which is generally when the stock option award vests. Compensation expense for non-employee grants is recorded on a straight-line basis in the statements of operations.
Fair value of financial instruments – ASC 820, Fair Value Measurements, clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability.
ASC 820 requires that the valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 establishes a three tier value hierarchy, which prioritizes inputs that may be used to measure fair value as follows:
| · | Level 1 – Observable inputs that reflect quoted prices for identical assets or liabilities in active market |
| · | Level 2 – Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| · | Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The carrying amounts of current assets and current liabilities approximate their fair value because of the relatively short period until they mature or are required to be settled, except for the investment in BioTime and OncoCyte shares, which are carried at fair value based on Level 1 inputs.
Results of Operations
Comparison of Years Ended December 31, 2015 and 2014
For the years ended December 31, 2015 and 2014 we recorded net losses of $15.0 million and $10.1 million, respectively.
Revenues
The following table shows certain information about our revenues for the years ended December 31, 2015 and 2014 (in thousands, except for percentages):
|
Year Ended
December 31,
|
$
Increase/
|
%
Increase/
|
||||||||||||||
|
2015
|
2014
|
Decrease
|
Decrease
|
|||||||||||||
|
Royalties from product sales
|
$
|
535
|
$
|
189
|
$
|
+ 346
|
+ 183
|
%
|
||||||||
|
Sale of cell lines
|
40
|
-
|
+ 40
|
+ 100
|
%
|
|||||||||||
|
Grant income
|
3,007
|
1,035
|
+ 1,972
|
+ 191
|
%
|
|||||||||||
|
Total revenues
|
3,582
|
1,224
|
+ 2,358
|
+ 193
|
%
|
|||||||||||
|
Cost of sales
|
(268
|
)
|
(95
|
)
|
+ 173
|
+ 185
|
%
|
|||||||||
|
Total gross profit
|
$
|
3,314
|
$
|
1,129
|
$
|
+ 2,185
|
+ 194
|
%
|
||||||||
Our royalty revenues from product sales is entirely from non-exclusive license agreements with Stem Cell Technologies, Inc., Corning Life Science, Life Tech, and Millipore which we assumed as part of consideration received from Geron under the Asset Contribution Agreement.
Grant income in 2015 is entirely from CIRM which awarded us a $14.3 million grant for clinical development of AST-OPC1. We received our first payment from CIRM in the amount of $917,000 during October 2014. We received the next four payments totaling $5.6 million in 2015. We received our sixth payment of $1.2 million during January 2016. Under our Amendment 2 to the Notice of Grant Award, effective March 2, 2016, the remaining $6.5 million will be dispersed to us upon our achievement of certain clinical milestones.
Expenses
The following table shows our operating expenses for the years ended December 31, 2015 and 2014 (in thousands, except for percentages):
|
Year Ended
December 31,
|
$
Increase/
|
%
Increase/
|
||||||||||||||
|
2015
|
2014
|
Decrease
|
Decrease
|
|||||||||||||
|
Research and development expenses
|
$
|
17,321
|
$
|
13,310
|
$
|
+ 4,011
|
+ 30
|
%
|
||||||||
|
General and administrative expenses
|
7,901
|
5,280
|
+ 2,621
|
+ 50
|
%
|
|||||||||||
Research and development expenses – Research and development expenses increased by approximately $4 million to $17.3 million for the year ended December 31, 2015 compared to $13.3 million for the year ended December 31, 2014. The increases in research and development expenses, during 2015 are primarily attributed to an increase of $1.5 million in clinical trial expenses, an increase of $1.6 million in salaries and related benefits, an increase of $1.1 million stock based compensation, an increase of $554,000 outside services, an increase of $260,000 in legal expense, an increase of $213,000 in consulting expense, and increase of $200,000 in utilities, an increase of $177,000 in recruiting expense, an increase of $143,000 in travel related expenses, an increase of $96,000 in building maintenance and repairs, an increase of $73,000 in lab supplies, and an increase of $35,000 in allocation related expenses. The increases are offset by a decrease of $2.1 million in amortization of intangible assets.
General and administrative expenses – General and administrative expenses increased by approximately $2.6 million to $7.9 million for the year ended December 31, 2015 compared to $5.3 million for the year ended December 31, 2014. The increase in general and administrative expenses is primarily comprised of the following increases in expenses incurred: $1.0 million in stock based compensation related to general consulting, $501,000 in legal expense, $415,000 in consulting expense, $276,000 in audit and tax related services, $272,000 in investor and public relations, $267,000 in stock based compensation for outside directors, $209,000 in recruiting fees, $101,000 in travel related expenses, and $91,000 in professional development. Those increases were in part offset by a decrease of $566,000 in stock based compensation for employees and a decrease of $255,000 in salaries related expense which was mostly due to a decrease in severance pay.
Comparison of Years Ended December 31, 2014 and 2013
For the years ended December 31, 2014 and 2013 we recorded net losses of $10.1 million and $22.4 million, respectively.
Revenues
The following table shows certain information about our revenues for the years ended December 31, 2014 and 2013 (in thousands, except for percentages).
|
Year Ended
December 31,
|
$
Increase/
|
%
Increase/
|
|||||||||||||||
|
2014
|
2013
|
Decrease
|
Decrease
|
||||||||||||||
|
Royalties from product sales
|
$
|
189
|
$
|
-
|
$
|
+ 189
|
+ 100
|
%
|
|||||||||
|
Grant income
|
1,035
|
-
|
+ 1,035
|
+ 100
|
%
|
||||||||||||
|
Total revenues
|
1,224
|
-
|
+ 1,224
|
+ 100
|
%
|
||||||||||||
|
Cost of sales
|
(95
|
)
|
-
|
+ 95
|
+ 100
|
%
|
|||||||||||
|
Total gross profit
|
$
|
1,129
|
$
|
-
|
$
|
+ 1,129
|
+ 100
|
%
|
|||||||||
Our royalty revenues from product sales is entirely from non-exclusive license agreements with Stem Cell Technologies, Inc., Corning Life Science, Life Tech, and Millipore which Asterias assumed as part of consideration received from Geron under the Asset Contribution Agreement.
Grant revenue in 2014 is entirely from CIRM which awarded us a $14.3 million grant for clinical development of AST-OPC1. CIRM will disburse the grant funds to us over four years in accordance with a quarterly disbursement schedule, subject to our attainment of certain progress and safety milestones. We received the first payment from CIRM in the amount of $917,000 during October 2014.The next four payments totaling $5.6 million were received in 2015. The sixth payment of $1.2 million was received during January 2016.
Expenses
The following table shows our operating expenses for the years ended December 31, 2014 and 2013 (in thousands, except for percentages).
|
|
Year Ended
December 31,
|
|
$
Increase/
|
|
%
Increase/
|
||||||||||||
|
|
2014
|
|
2013
|
|
Decrease
|
|
Decrease
|
||||||||||
|
Research and development expenses
|
$
|
13,310
|
$
|
4,319
|
$
|
+8,991
|
+208
|
%
|
|||||||||
|
Acquired in-process research and development expenses
|
-
|
17,459
|
-17,459
|
-100
|
%
|
||||||||||||
|
General and administrative expenses
|
5,280
|
3,883
|
+ 1,397
|
+36
|
%
|
||||||||||||
Research and development expenses – Research and development expenses increased by approximately $9.0 million to $13.3 million for the year ended December 31, 2014 compared to $4.3 million for the year ended December 31, 2013. In addition, during 2013 we recognized $17.5 million of IPR&D in connection with the consummation of our acquisition of assets from Geron. The increases in research and development expenses, excluding IPR&D, during 2014 were primarily attributed to an increase of $4.1 million of amortization of intangible assets acquired under Asset Contribution Agreement on October 1, 2013, and increases in expenses reflecting the ramp up of our AST-OPC1 and AST-VAC2 programs: $2.4 million of salaries, payroll related expenses and employee stock-based compensation allocated to research and development expenses, $546,000 of license and patent related expenses, $377,000 of rent and facilities maintenance related expenses allocated to research and development expenses due to a new lease in Fremont which commenced on October 1, 2014, $362,000 of laboratory expense and supplies expenses, $328,000 of outside research and services allocated to research and development expenses, $277,000 of depreciation expenses allocated to research and development expenses, $252,183 of clinical trial related expenses, $166,000 of contract manufacturing related expenses allocated to research and development expenses, $84,000 of insurance expense allocated to research and development expenses, and $54,000 of travel, lodging and meals allocated to research and development expenses.
Research and development expenses during 2013 included $17.5 million of IPR&D expense related to the assets we acquired in 2013 under the Asset Contribution Agreement. Other research and development expenses incurred during 2014 and 2013 included laboratory study expenses, patent and technology license fees, employee compensation, rent, insurance, and science-related consultants’ fees and amortization allocated to research and development expenses.
General and administrative expenses – General and administrative expenses increased by approximately $1.4 million to $5.3 million for the year ended December 31, 2014 compared to $3.9 million for the year ended December 31, 2013. The increase in general and administrative expenses was primarily comprised of the following increases in expenses incurred: $1.2 million of salaries, payroll related expenses and employee stock-based compensation allocated to general and administrative expenses, $196,000 of investor and public relations expenses, $184,000 in state corporation and franchise taxes, $115,000 of accounting, audit, and tax services, $98,000 of rent and facilities maintenance related expenses allocated to general and administrative expenses, $69,000 of overhead allocated from BioTime generally for accounting and corporate secretarial related services and general liability insurance allocated to general and administrative expenses, and $46,000 of cash compensation to directors. Those increases were in part offset by a decrease of $419,000 in legal expenses reflecting non-recurring expenses that we incurred in 2013 related to the Asset Contribution Agreement transactions, including preparing registration statements for filing with the SEC and a decrease of $102,000 in general office and computer supplies expenses.
Liquidity and Capital Resources
At December 31, 2015, we had $11.2 million of cash and cash equivalents on hand, held 3,852,880 BioTime common shares and 192,644 shares of OncoCyte common stock, with a market value of $15.8 million and $1.2 million, respectively. We may raise capital from time to time through the sale of our Series A Shares or other securities, and our BioTime common shares. We may sell our Series A Shares or other securities in public offerings registered under the Securities Act of 1933, as amended (the “Securities Act”), or in private placements to select investors. We may sell our BioTime common shares, from time to time, by any method that is deemed to be an “at-the-market” equity offering as defined in Rule 415 promulgated under the Securities Act, including sales made directly on or through the NYSE MKT or any other existing trading market for the common shares in the U.S. or to or through a market maker, at prices related to the prevailing market price, or through block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction, or through one more of the foregoing transactions. We may sell the BioTime common shares through Cantor Fitzgerald & Co. or such other broker-dealer as BioTime may designate. We may also sell some or all of our BioTime common shares and OncoCyte common shares by any other method permitted by law, including in privately negotiated transactions. We will bear all broker-dealer commissions payable in connection with the sale of our Series A Shares or other securities and our BioTime common shares. Broker-dealers may receive commissions or discounts from us (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The prices at which we may issue and sell our Series A Shares or other securities and our BioTime common shares in the future are not presently determinable and will depend upon many factors, including prevailing prices for those securities in the public market.
As of March 23, 2016, we have outstanding warrants to purchase 350,000 shares of our common stock at an exercise price of $5.00 per share that will expire on September 30, 2016. We will receive $1.8 million if all of the warrants are exercised. There can be no assurance that the warrants will be exercised. In addition, as previously announced, we intend to distribute warrants to our shareholders (except for BioTime) on a pro-rata basis, and therefore to the extent such warrants are exercised, we will receive proceeds from the exercise of such warrants.
We plan to use the cash we have available to develop certain of our product candidates and technology, to acquire new stem cell products and technology through licenses or similar agreements from other companies, and to defray overhead expenses and to pay general and administrative expenses. We may also use available funds for any clinical trials of products that we may conduct. We expect to continue to incur operating losses and negative cash flows. BioTime contributed to the funding of our business activities from inception through December 31, 2015 but there can be no assurance they will continue to do so in the future.
We have been awarded a $14.3 million Strategic Partnership III grant by CIRM to help fund our clinical development of AST-OPC1. The grant provides funding for us to reinitiate clinical development of AST-OPC1 in subjects with spinal cord injury, to expand clinical testing of escalating doses in the target population intended for future pivotal trials, and for product development efforts to refine and scale manufacturing methods to support eventual commercialization. CIRM has and will continue to disburse the grant funds to us over four years in accordance with a quarterly disbursement schedule, subject to our attainment of certain progress and safety milestones. We received the first payment from CIRM in the amount of $917,000 during October 2014. The next four payments totaling $5.6 million were received in 2015. The sixth payment of $1.2 million was received during January 2016. As the distributions of the CIRM grant are subject to meeting certain progress and go/no-go milestones, there can be no assurance that we will receive the entire amount granted.
Pursuant to the CRUK Agreement, CRUK has agreed to fund Phase 1/2 clinical development of our AST-VAC2 product candidate. We will, at our own cost, complete process development and manufacturing scale-up of the AST-VAC2 manufacturing process and will transfer the resulting cGMP-compatible process to CRUK. CRUK will, at its own cost, manufacture clinical grade AST-VAC2 and will carry out the Phase 1/2 clinical trial of AST-VAC2 in cancer patients both resected early-stage and advanced forms of lung cancer.
Since inception, we have incurred net losses and has funded its operations primarily through the support from BioTime, issuance of equity securities, payments from research grants, and royalties from product sales. At December 31, 2015, we had an accumulated deficit of $48.2 million, working capital of $19.5 million and stockholders’ equity of $45.1 million. We have evaluated our projected cash flows and believe that our cash and cash equivalents of $11.2 million as of December 31, 2015, and our available for sale securities of $17.0 million based on BioTime closing stock price of $4.10 as of December 31, 2015 will be sufficient to fund our operations at least through 2016. However, clinical trials being conducted by us will be funded in part with funds from the $14.3 million grant awarded in 2014 by the California Institute of Regenerative Medicine (“CIRM”) and not from cash on hand. If we were to lose our grant funding or if the value of our available for sale securities decreases,we may be required to delay, postpone, or cancel our clinical trials or limit the number of clinical trial sites, or otherwise reduce or curtail our operations unless we are able to obtain from another source of adequate financing that could be used for our clinical trials.
The unavailability or inadequacy of financing or revenues to meet future capital needs could force us to modify, curtail, delay, or suspend some or all aspects of our planned operations. Sales of additional equity securities could result in the dilution of the interests of our current shareholders.
Cash Flows
Cash used in operations
Since our inception, we have incurred losses from operations and negative cash flows from our operations. During the year ended December 31, 2015, our total research and development expenditures were $17.3 million and our general and administrative expenditures were $7.9 million. Net loss for the year ended December 31, 2015 amounted to $15.0 million. Our sources of cash during 2015 primarily consisted of $535,000 from royalty revenues on product sales by licensees and a research grant payment of $5.6 million from CIRM. As of December 31, 2015 and 2014, we had a working capital surplus of $19.5 million and $11.9 million, respectively, and an accumulated deficit of $48.2 million and $33.2 million, respectively, based on our operating losses and the expensed IPR&D.
Net cash used in operating activities during the year ended December 31, 2015 amounted to $12.4 million. The difference between the net loss and net cash used in operating activities during the year ended December 31, 2015 was primarily attributable to increases in stock-based compensation paid to employees of $3.6 million, amortization of intangible assets of $2.7 million, deferred grant income of $2.5 million, accrued expenses of $584,000, depreciation expense of $564,000, shares issued to a vendor for services of $486,000, amortization of prepaid rent with BioTime stock of $85,000, and deferred rent liabilities of $85,000. The difference was offset by our deferred income tax benefit of $7.2 million, decreases in prepaid expenses and other current assets of $680,000, long term assets of $95,000, $85,000 of our expenses paid by BioTime which is reflected in “amount due to BioTime” on our December 31, 2015 Statements of Cash flows, and $24,000 in accounts payable.
Investing and financing activities
During the year ended December 31, 2015, we used $4.3 million in cash to pay for construction in progress for our Fremont facility and $313,000 to purchase equipment.
In May 2015, we received $11.7 million from the exercise of 5,000,000 warrants to purchase our common stock which were issued to Broadwood Partners, L.P and a trust previously established by George Karfunkel as part of their purchase of BioTime shares from us in June 2014.
In February 2015, we raised approximately $5.5 million in aggregate gross proceeds from the sale of 1,410,255 shares of our common stock at a price of $3.90 per share through an underwritten public offering and a private placement. Broadwood Partners, L.P., British & American Investment Trust PLC and Pedro Lichtinger purchased an aggregate of 1,025,640 of the shares. Broadwood Partners, L.P. is BioTime’s largest shareholder and one of its directors, Neal C. Bradsher, is President, and one of BioTime’s directors, Richard T. LeBuhn, is Senior Vice President, of Broadwood Capital, Inc., the investment manager of Broadwood Partners, L.P. Pedro Lichtinger was our former President and Chief Executive Officer and a member of our Board of Directors. British & American Investment Trust PLC is an affiliate of a stockholder of us and BioTime.
During May, June, November, and December of 2015, we raised approximately $4.8 million in gross proceeds from the sale of 685,465 shares of our common stock at a weighted average price of $7.01 per share in “at-the-market” transactions.
During year ended December 31, 2015, we incurred financing costs of $665,000, received $29,000 in cash from the exercise of our stock options by two employees at an exercise price of $2.34 per share. We also received $3.8 million from our landlord on reimbursable construction in progress.
Contractual Obligations
As of December 31, 2015 our contractual obligations for the next five years and thereafter were as follows (in thousands):
|
Principal Payments Due by Period
|
||||||||||||||||||||
|
Contractual Obligations (1)
|
Total
|
Less Than
1 Year
|
1-3 Years
|
4-5 Years
|
After
5 Years
|
|||||||||||||||
|
Operating leases (2)
|
$
|
9,405
|
1,328
|
2,656
|
2,817
|
2,604
|
||||||||||||||
|
Capital lease (3)
|
$
|
39
|
8
|
16
|
15
|
-
|
||||||||||||||
| (1) | This table does not include payments to key employees that could arise if they were involuntary terminated or if their employment terminated following a change in control. |
| (2) | Includes the lease of our principal office and laboratory facilities in Fremont, California, in Menlo Park, California, and our New York office. |
| (3) | Includes one capital lease for phone equipment. |
Off-Balance Sheet Arrangements
As of December 31, 2015 and 2014, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of SEC Regulation S-K.
Foreign Currency Exchange Risk
We are not presently exposed in a significant degree to foreign exchange currency risks because we are not otherwise conducting international business at this time, and we do not engage in foreign currency hedging activities. If we engage in international transactions, we will need to translate foreign currencies into U.S. dollars for reporting purposes, and currency fluctuations could have a greater impact on our financial results.
Credit Risk
We place some of our cash in U.S. banks and invest most of our cash in money market funds. Deposits with banks may temporarily exceed the amount of insurance provided on such deposits. We will monitor the cash balances in the accounts and adjust the cash balances as appropriate, but if the amount of a deposit at any time exceeds the federally insured amount at a bank, the uninsured portion of the deposit could be lost, in whole or in part, if the bank were to fail. Our investments in money market funds are not insured or guaranteed by the United States government or any of its agencies.
Interest Rate Risk
We invest most of our cash in money market funds. The primary objective of our investments will be to preserve principal and liquidity while earning a return on our invested capital, without incurring significant risks. Our future investment income is not guaranteed and may fall short of expectations due to changes in prevailing interest rates, or we may suffer losses in principal if the net asset value of a money market fund falls below $1 per share.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Asterias Biotherapeutics, Inc.
We have audited Asterias Biotherapeutics, Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO criteria”). Asterias Biotherapeutics, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Item 9A, Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Asterias Biotherapeutics, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Asterias Biotherapeutics, Inc. as of December 31, 2015 and 2014, and the related statements of operations, comprehensive loss, stockholders’ equity, and cash flows for the years then ended and our report dated March 29, 2016 expressed an unqualified opinion thereon.
/s/ OUM & CO. LLP
San Francisco, California
March 29, 2016
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Asterias Biotherapeutics, Inc.
We have audited the accompanying balance sheets of Asterias Biotherapeutics, Inc. as of December 31, 2015 and 2014, and the related statements of operations, comprehensive loss, stockholders’ equity, and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Asterias Biotherapeutics, Inc. at December 31, 2015 and 2014, and the results of its operations and its cash flows for the years then ended.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Asterias Biotherapeutics, Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 29, 2016 expressed an unqualified opinion thereon.
/s/ OUM & CO. LLP
San Francisco, California
March 29, 2016
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Asterias Biotherapeutics, Inc.
We have audited the accompanying statements of operations, comprehensive loss, stockholders’ equity, and cash flows of Asterias Biotherapeutics, Inc. (the “Company”) for the year ended December 31, 2013. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. Our audit of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audit provide a reasonable basis for our opinions.
In our opinion, the financial statements of Asterias Biotherapeutics, Inc. referred to above present fairly, in all material respects, the results of their operations and their cash flows for the year ended December 31, 2013, in conformity with accounting principles generally accepted in the United States of America.
/s/ Rothstein Kass
New York, New York
March 17, 2014
ASTERIAS BIOTHERAPEUTICS, INC.
BALANCE SHEET
(IN THOUSANDS EXCEPT PAR VALUE AMOUNTS)
|
December 31,
2015
|
December 31,
2014
|
|||||||
|
ASSETS
|
||||||||
|
CURRENT ASSETS
|
||||||||
|
Cash and cash equivalents
|
$
|
11,183
|
$
|
3,076
|
||||
|
Available-for-sale securities, at fair value
|
17,006
|
14,374
|
||||||
|
Grant receivable
|
-
|
118
|
||||||
|
Landlord receivable
|
567
|
378
|
||||||
|
Prepaid expenses and other current assets
|
1,033
|
438
|
||||||
|
Total current assets
|
29,789
|
18,384
|
||||||
|
NONCURRENT ASSETS
|
||||||||
|
Intangible assets, net
|
20,816
|
23,502
|
||||||
|
Property, plant and equipment, net
|
5,756
|
1,045
|
||||||
|
Construction in progress
|
-
|
406
|
||||||
|
Investment in affiliates
|
416
|
416
|
||||||
|
Deferred tax asset
|
9,744
|
-
|
||||||
|
Other assets
|
457
|
361
|
||||||
|
TOTAL ASSETS
|
$
|
66,978
|
$
|
44,114
|
||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES
|
||||||||
|
Amount due to parent, BioTime, Inc.
|
$
|
530
|
$
|
615
|
||||
|
Accounts payable
|
747
|
737
|
||||||
|
Accrued expenses and other current liabilities
|
1,183
|
430
|
||||||
|
Capital lease liability
|
7
|
-
|
||||||
|
Deferred rent liability
|
-
|
2
|
||||||
|
Deferred grant income
|
2,513
|
-
|
||||||
|
Deferred tax liabilities, current portion
|
5,274
|
4,713
|
||||||
|
Total current liabilities
|
10,254
|
6,497
|
||||||
|
LONG-TERM LIABILITIES
|
||||||||
|
Capital lease liability
|
26
|
-
|
||||||
|
Deferred tax liabilities, net of current portion
|
7,020
|
4,514
|
||||||
|
Deferred rent liability, net of current portion
|
179
|
94
|
||||||
|
Lease liability
|
4,400
|
378
|
||||||
|
TOTAL LIABILITIES
|
21,879
|
11,483
|
||||||
|
Commitments and contingencies (see Note 8)
|
||||||||
|
STOCKHOLDERS’ EQUITY
|
||||||||
|
Preferred Stock, $0.0001 par value, authorized 5,000,000 shares; none issued and outstanding
|
-
|
-
|
||||||
|
Common Stock, $0.0001 par value, authorized 75,000 Series A Common Stock and 75,000 Series B Common Stock; 38,228 and 30,902 shares Series A Common Stock issued and outstanding at December 31, 2015 and 2014, respectively; no Series B Common Stock issued and outstanding at December 31, 2015 and 2014
|
4
|
3
|
||||||
|
Additional paid-in capital
|
92,900
|
66,367
|
||||||
|
Accumulated other comprehensive gain (loss) on available-for-sale investments
|
434
|
(503
|
)
|
|||||
|
Accumulated deficit
|
(48,239
|
)
|
(33,236
|
)
|
||||
|
Total stockholders’ equity
|
45,099
|
32,631
|
||||||
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY
|
$
|
66,978
|
$
|
44,114
|
||||
The accompanying notes are an integral part of these financial statements.
ASTERIAS BIOTHERAPEUTICS, INC.
STATEMENTS OF OPERATIONS
(IN THOUSANDS, EXCEPT PER SHARE DATA)
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
REVENUES:
|
||||||||||||
|
Royalties from product sales
|
$
|
535
|
$
|
189
|
$
|
-
|
||||||
|
Sale of cell lines
|
40
|
-
|
||||||||||
|
Grant income
|
3,007
|
1,035
|
-
|
|||||||||
|
Total revenues
|
3,582
|
1,224
|
-
|
|||||||||
|
Cost of sales
|
(268
|
)
|
(95
|
)
|
-
|
|||||||
|
Gross profit
|
3,314
|
1,129
|
-
|
|||||||||
|
OPERATING EXPENSE:
|
||||||||||||
|
Research and development
|
(17,321
|
)
|
(13,310
|
)
|
(4,319
|
)
|
||||||
|
Acquired in-process research and development
|
-
|
-
|
(17,459
|
)
|
||||||||
|
General and administrative
|
(7,901
|
)
|
(5,280
|
)
|
(3,883
|
)
|
||||||
|
Total operating expenses
|
(25,222
|
)
|
(18,590
|
)
|
(25,661
|
)
|
||||||
|
Loss from operations
|
(21,908
|
)
|
(17,461
|
)
|
(25,661
|
)
|
||||||
|
OTHER INCOME/(EXPENSE):
|
||||||||||||
|
Interest expense, net
|
(341
|
)
|
(10
|
)
|
(2
|
)
|
||||||
|
Other income (expense), net
|
(6
|
)
|
(2
|
)
|
2
|
|||||||
|
Total other expenses, net
|
(347
|
)
|
(12
|
)
|
-
|
|||||||
|
LOSS BEFORE INCOME TAX BENEFIT
|
(22,255
|
)
|
(17,473
|
)
|
(25,661
|
)
|
||||||
|
Deferred income tax benefit
|
7,252
|
7,376
|
3,281
|
|||||||||
|
NET LOSS
|
$ |
(15,003
|
)
|
$ |
(10,097
|
)
|
$ |
(22,380
|
)
|
|||
|
BASIC AND DILUTED NET LOSS PER SHARE
|
$
|
(0.42
|
)
|
$
|
(0.33
|
)
|
$
|
(2.90
|
)
|
|||
|
WEIGHTED AVERAGE SHARES OUTSTANDING: BASIC AND DILUTED
|
35,443
|
30,720
|
7,726
|
|||||||||
The accompanying notes are an integral part of the financial statements
ASTERIAS BIOTHERAPEUTICS, INC.
STATEMENTS OF COMPREHENSIVE LOSS
(IN THOUSANDS)
|
Years ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
NET LOSS
|
$
|
(15,003
|
)
|
$
|
(10,097
|
)
|
$
|
(22,380
|
)
|
|||
|
Other comprehensive loss, net of tax:
|
||||||||||||
|
Unrealized gain/(loss) on available for sale securities, net of taxes
|
937
|
2,432
|
(2,935
|
)
|
||||||||
|
COMPREHENSIVE LOSS
|
$
|
(14,066
|
)
|
$
|
(7,665
|
)
|
$
|
(25,315
|
)
|
|||
The accompanying notes are an integral part of the financial statements
ASTERIAS BIOTHERAPEUTICS, INC.
STATEMENT OF STOCKHOLDERS’ EQUITY
(IN THOUSANDS)
|
Common Stock
|
Additional
|
Accumulated
Other
Comprehensive
|
|
|||||||||||||||||||||||||||||||||
|
Series A
|
Series B
|
Paid-In
|
Income
|
Accumulated |
Subscription
|
Stockholders’
|
||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
Capital
|
(Loss)
|
Deficit
|
Receivable
|
Equity
|
||||||||||||||||||||||||||||
|
Balance as of January 1, 2013
|
—
|
$
|
—
|
52
|
$
|
-
|
$
|
52
|
$
|
—
|
$
|
(759
|
)
|
$
|
(50
|
)
|
$
|
(757
|
)
|
|||||||||||||||||
|
Common stock, at $2.40 per share, issued to Geron in connection with acquisition of various assets on October 1, 2013, net of issuance costs of $541,800
|
6,538
|
1
|
—
|
—
|
15,120
|
—
|
—
|
—
|
15,121
|
|||||||||||||||||||||||||||
|
Common stock, at $2.40 per share, and common stock warrants issued to BioTime in connection with transfer of various assets on October 1, 2013
|
—
|
—
|
21,773
|
2
|
58,975
|
—
|
—
|
—
|
58,977
|
|||||||||||||||||||||||||||
|
Common stock, at $2.40 per share, and common stock warrants issued to an investor for cash
|
—
|
—
|
2,136
|
-
|
5,000
|
—
|
—
|
—
|
5,000
|
|||||||||||||||||||||||||||
|
Reduction of subscription receivable
|
—
|
—
|
—
|
—
|
—
|
—
|
—
|
50
|
50
|
|||||||||||||||||||||||||||
|
Unrealized loss on available-for-sale securities, net of taxes
|
—
|
—
|
—
|
—
|
—
|
(2,935
|
)
|
—
|
—
|
(2,935
|
)
|
|||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
—
|
—
|
704
|
—
|
—
|
—
|
704
|
|||||||||||||||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
—
|
—
|
(22,380
|
)
|
—
|
(22,380
|
)
|
|||||||||||||||||||||||||
|
Balance as of December 31, 2013
|
6,538
|
1
|
23,961
|
2
|
79,851
|
(2,935
|
)
|
(23,139
|
)
|
—
|
53,780
|
|||||||||||||||||||||||||
|
Common stock issued to officer at $2.34 per share
|
—
|
—
|
200
|
-
|
468
|
—
|
—
|
—
|
468
|
|||||||||||||||||||||||||||
|
Sale of BioTime shares
|
—
|
—
|
—
|
—
|
(10,366
|
)
|
—
|
—
|
—
|
(10,366
|
)
|
|||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
—
|
—
|
1,580
|
—
|
—
|
—
|
1,580
|
|||||||||||||||||||||||||||
|
Restricted stock granted for compensation at $2.34 per share
|
—
|
—
|
200
|
-
|
234
|
—
|
—
|
—
|
234
|
|||||||||||||||||||||||||||
|
Warrants issued to outside investors as part of the sale of 5,000,000 BioTime shares
|
—
|
—
|
—
|
—
|
3,184
|
—
|
—
|
—
|
3,184
|
|||||||||||||||||||||||||||
|
Series B converted to Series A on October 1, 2014
|
24,361
|
2
|
(24,361
|
)
|
(2
|
)
|
—
|
—
|
—
|
—
|
||||||||||||||||||||||||||
|
Exercise of options
|
3
|
—
|
—
|
—
|
8
|
—
|
—
|
—
|
8
|
|||||||||||||||||||||||||||
|
Deferred tax liability adjustment on BioTime shares
|
—
|
—
|
—
|
—
|
(8,592
|
)
|
—
|
—
|
—
|
(8,592
|
)
|
|||||||||||||||||||||||||
|
Unrealized gain on available-for-sale securities, net of taxes
|
—
|
—
|
—
|
—
|
—
|
2,432
|
—
|
—
|
2,432
|
|||||||||||||||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
—
|
—
|
(10,097
|
)
|
—
|
(10,097
|
)
|
|||||||||||||||||||||||||
|
Balance as of December 31, 2014
|
30,902
|
3
|
—
|
|
—
|
|
66,367
|
|
(503
|
)
|
(33,236
|
)
|
|
—
|
|
32,631
|
||||||||||||||||||||
|
Stock-based compensation
|
145
|
—
|
—
|
—
|
3,625
|
—
|
—
|
—
|
3,625
|
|||||||||||||||||||||||||||
|
Shares retired to pay employee taxes
|
(24
|
)
|
—
|
—
|
—
|
(98
|
)
|
—
|
—
|
—
|
(98
|
)
|
||||||||||||||||||||||||
|
Unrealized gain on available-for-sale securities, net of deferred tax liability of $488.
|
—
|
—
|
—
|
—
|
—
|
937
|
—
|
—
|
937
|
|||||||||||||||||||||||||||
|
Sale of common stock under at-the- market transactions
|
686
|
—
|
—
|
—
|
4,839
|
—
|
—
|
4,839
|
||||||||||||||||||||||||||||
|
Financing costs to issue common stock
|
—
|
—
|
—
|
—
|
(665
|
)
|
—
|
—
|
—
|
(665
|
)
|
|||||||||||||||||||||||||
|
Exercise of warrants
|
5,000
|
1
|
—
|
—
|
11,700
|
—
|
—
|
—
|
11,701
|
|||||||||||||||||||||||||||
|
Common stock issued in Private Placement
|
1,026
|
—
|
—
|
—
|
4,000
|
—
|
—
|
—
|
4,000
|
|||||||||||||||||||||||||||
|
Common stock issued in public offering
|
385
|
—
|
—
|
—
|
1,500
|
—
|
—
|
—
|
1,500
|
|||||||||||||||||||||||||||
|
Exercise of stock options
|
12
|
—
|
—
|
—
|
29
|
—
|
—
|
—
|
29
|
|||||||||||||||||||||||||||
|
OncoCyte common stock received as a dividend in kind from BioTime, net of taxes
|
—
|
—
|
—
|
—
|
1,117
|
—
|
—
|
—
|
1,117
|
|||||||||||||||||||||||||||
|
Common stock issued for services
|
96
|
—
|
—
|
—
|
486
|
—
|
—
|
—
|
486
|
|||||||||||||||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
—
|
—
|
(15,003
|
)
|
—
|
(15,003
|
)
|
|||||||||||||||||||||||||
|
Balance as of December 31, 2015
|
38,228
|
$
|
4
|
—
|
$
|
—
|
$
|
92,900
|
$
|
434
|
$
|
(48,239
|
)
|
$
|
—
|
$
|
45,099
|
|||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
ASTERIAS BIOTHERAPEUTICS, INC.
STATEMENTS OF CASH FLOWS
(IN THOUSANDS)
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||||||
|
Net loss
|
$
|
(15,003
|
)
|
$
|
(10,097
|
)
|
$
|
(22,380
|
)
|
|||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Acquired in-process research and development
|
-
|
-
|
17,459
|
|||||||||
|
Depreciation expense
|
564
|
530
|
221
|
|||||||||
|
Stock-based compensation
|
3,625
|
1,814
|
704
|
|||||||||
|
Common stock issued to a supplier for services
|
486
|
-
|
-
|
|||||||||
|
Amortization of intangible assets
|
2,686
|
4,789
|
725
|
|||||||||
|
Amortization of prepaid rent
|
85
|
85
|
85
|
|||||||||
|
Gain on sale of equipment, net
|
-
|
-
|
(2
|
)
|
||||||||
|
Deferred income tax benefit
|
(7,252
|
)
|
(7,376
|
)
|
(3,281
|
)
|
||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Grant receivable
|
118
|
(118
|
)
|
-
|
||||||||
|
Prepaid expenses and other current assets
|
(680
|
)
|
(182
|
)
|
(423
|
)
|
||||||
|
Other long term assets
|
(95
|
)
|
-
|
-
|
||||||||
|
Accounts payable
|
(24
|
)
|
120
|
567
|
||||||||
|
Accrued expenses and other current liabilities
|
584
|
199
|
96
|
|||||||||
|
Deferred rent liability
|
85
|
94
|
-
|
|||||||||
|
Deferred grant income
|
2,513
|
-
|
-
|
|||||||||
|
Amount due to parent, BioTime
|
(85
|
)
|
(1,449
|
)
|
4,902
|
|||||||
|
Net cash used in operating activities
|
(12,393
|
)
|
(11,591
|
)
|
(1,327
|
)
|
||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||||||
|
Purchase of equipment and furniture
|
(313
|
)
|
(115
|
)
|
(1,247
|
)
|
||||||
|
Payments on construction in progress
|
(4,279
|
)
|
(219
|
)
|
-
|
|||||||
|
Proceeds from sale of equipment and furniture
|
-
|
-
|
28
|
|||||||||
|
Proceeds from the sale of available-for-sale investments
|
-
|
12,661
|
-
|
|||||||||
|
Payment of security deposits
|
(1
|
)
|
(307
|
)
|
(55
|
)
|
||||||
|
Net cash provided by/(used in) investing activities
|
(4,593
|
)
|
12,020
|
(1,274
|
)
|
|||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||||||
|
Proceeds from sales of common shares
|
10,339
|
468
|
-
|
|||||||||
|
Proceeds from sales of common shares and warrants
|
-
|
-
|
5,000
|
|||||||||
|
Proceeds from exercises of stock options
|
29
|
8
|
-
|
|||||||||
|
Proceeds from exercise of warrants
|
11,700
|
-
|
-
|
|||||||||
|
Payment of financing costs
|
(665
|
)
|
-
|
-
|
||||||||
|
Repayment of capital lease obligation
|
(1
|
)
|
-
|
-
|
||||||||
|
Reimbursement from landlord on construction in progress
|
3,789
|
-
|
-
|
|||||||||
|
Shares retired to pay for employees’ taxes
|
(98
|
)
|
-
|
-
|
||||||||
|
Payment to Geron in connection with acquisition of assets on October 1, 2013
|
-
|
-
|
(228
|
)
|
||||||||
|
Net cash provided by financing activities
|
25,093
|
476
|
4,772
|
|||||||||
|
NET INCREASE IN CASH AND CASH EQUIVALENTS
|
8,107
|
905
|
2,171
|
|||||||||
|
At beginning of period
|
3,076
|
2,171
|
-
|
|||||||||
|
At end of period
|
$
|
11,183
|
3,076
|
$
|
2,171
|
|||||||
|
SUPPLEMENTAL SCHEDULE OF NON-CASH FINANCING AND INVESTING ACTIVITIES:
|
||||||||||||
|
OncoCyte common stock received as a dividend in kind from BioTime, net of taxes
|
$
|
1,117
|
$
|
-
|
$
|
-
|
||||||
|
Purchase of equipment and furniture, contributed by BioTime
|
$
|
-
|
$
|
-
|
$
|
(459
|
)
|
|||||
|
Construction in progress in accounts payable and accrued expenses
|
$
|
-
|
$
|
186
|
$
|
-
|
||||||
|
Landlord receivable
|
$
|
(189
|
) |
$
|
(378
|
)
|
$
|
-
|
||||
|
Lease liability
|
$
|
189
|
$
|
378
|
$
|
-
|
||||||
|
Available-for-sale BioTime securities contributed by BioTime
|
$
|
-
|
$
|
-
|
$
|
34,985
|
||||||
|
Cancellation of indebtedness to BioTime
|
$
|
-
|
$
|
-
|
$
|
5,000
|
||||||
|
Transaction costs paid by BioTime, on behalf of Asterias
|
$
|
-
|
$
|
-
|
$
|
300
|
||||||
|
Intangible assets acquired from Geron
|
$
|
-
|
$
|
-
|
$
|
29,017
|
||||||
|
Adjustment to deferred tax liability arising from difference in book versus tax basis on Geron intangible assets acquired
|
$
|
-
|
$
|
-
|
$
|
11,558
|
||||||
|
Investment in affiliates, contributed by BioTime
|
$
|
-
|
$
|
-
|
$
|
416
|
||||||
|
Common stock and common stock warrants issued to BioTime and Geron in connection with acquisition and transfer of assets
|
$
|
-
|
$
|
-
|
$
|
74,098
|
||||||
|
Reduction of subscription receivable
|
$
|
-
|
$
|
-
|
$
|
(50
|
)
|
|||||
The accompanying notes are an integral part of these financial statements.
ASTERIAS BIOTHERAPEUTICS, INC.
NOTES TO FINANCIAL STATEMENTS
1. Organization, Basis of Presentation and Liquidity
Asterias Biotherapeutics, Inc. (“Asterias”) was incorporated in Delaware on September 24, 2012. Asterias is a majority-owned and controlled subsidiary of BioTime, Inc. (“BioTime”).
Asterias is a biotechnology company focused on the emerging fields of cell therapy and regenerative medicine. We have two core technology platforms. The first is a type of stem cell capable of becoming all of the cell types in the human body, a property called pluripotency. The second is the use of a cell type called “dendritic cells” to teach cancer patients’ immune systems to attack their tumors. We are focused on developing therapies to treat conditions with high unmet medical need and inadequate available therapies, with an initial focus on the therapeutic areas of neurology and oncology
The financial statements presented herein, and discussed below, have been prepared on a stand-alone basis. The financial statements are presented in accordance with accounting principles generally accepted in the U.S. (“GAAP”) and with the accounting and reporting requirements to Form 10-K and Article 10 of Regulation S-X of the Securities and Exchange Commission (“SEC”).
BioTime has consolidated the results of Asterias into BioTime’s consolidated results based on BioTime’s ability to control Asterias’ operating and financial decisions and policies through the ownership of Asterias common stock throughout the periods presented. BioTime owned 57.1% and 70.6% of the outstanding Asterias common stock at December 31, 2015 and 2014, respectively.
BioTime allocates expenses such as salaries and payroll related expenses incurred and paid on behalf of Asterias based on the amount of time that particular employees devote to Asterias affairs. Other expenses such as legal, accounting, travel, and entertainment expenses are allocated to Asterias to the extent that those expenses are incurred by or on behalf of Asterias. BioTime also allocates certain overhead expenses such as insurance, internet, and telephone expenses based on a percentage determined by management. These allocations are made based upon activity-based allocation drivers such as time spent, percentage of square feet of office or laboratory space used, and percentage of personnel devoted to Asterias operations or management. Management evaluates the appropriateness of the percentage allocations on a quarterly basis and believes that this basis for allocation is reasonable.
In connection with the services performed by employees of BioTime, or employees of other BioTime commonly controlled and consolidated subsidiaries within the BioTime group of affiliated entities, Asterias grants stock options to those employees performing services for Asterias and records stock-based compensation expense in the accompanying statements of operations for these services performed in the periods presented.
Liquidity – Since inception, Asterias has incurred operating losses and has funded its operations primarily through the support from BioTime, issuance of equity securities, payments from research grants, and royalties from product sales. At December 31, 2015, Asterias had an accumulated deficit of $48.2 million, working capital of $19.5 million and stockholders’ equity of $45.1 million. Asterias has evaluated its projected cash flows and believes that its cash and cash equivalents of $11.2 million as of December 31, 2015, available for sale securities and potential financial support from BioTime, if needed, will be sufficient to fund Asterias’ operations at least through 2016. However, clinical trials being conducted by Asterias will be funded in part with funds from the $14.3 million grant awarded in 2014 by CIRM and not from cash on hand. If Asterias was to lose its grant funding it may be required to delay, postpone, or cancel its clinical trials or limit the number of clinical trial sites, or otherwise reduce or curtail its operations unless it is able to obtain from another source of adequate financing that could be used for its clinical trials. Such financing, if necessary, may not be available to Asterias at acceptable terms, or if at all. Sales of additional equity securities would result in the dilution of interests of current shareholders.
2. Summary of Significant Accounting Policies
Revenue recognition – Asterias complies with ASC 605-10 and records revenue when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable, and collectability is reasonably assured. Grant income is recognized as revenue when earned; the costs associated with these activities are reflected as a component of research and development expenses in the statements of operations and the revenues recognized from such activities approximate these costs. Royalty revenues consist of royalty payments on sales of products under license agreements. Asterias recognizes revenue in the quarter in which the royalty reports are received rather than the quarter in which the sales took place. When Asterias is entitled to receive up-front nonrefundable licensing or similar fees pursuant to agreements under which Asterias has no continuing performance obligations, the fees are recognized as revenues when collection is reasonably assured. When Asterias receives up-front nonrefundable licensing or similar fees pursuant to agreements under which Asterias does have continuing performance obligations, the fees are deferred and amortized ratably over the performance period. If the performance period cannot be reasonably estimated, Asterias amortizes nonrefundable fees over the life of the contract until such time that the performance period can be more reasonably estimated. Milestone payments, if any, related to scientific or technical achievements are recognized in income when the milestone is accomplished if (a) substantive effort was required to achieve the milestone, (b) the amount of the milestone payment appears reasonably commensurate with the effort expended, and (c) collection of the payment is reasonably assured.
Use of estimates – The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period with consideration given to materiality. Significant estimates and assumptions used include allowances for uncollectible accounts receivables, deferred income taxes, and tax reserves, including valuation allowances for deferred tax assets, evaluation of asset impairment, the useful life of depreciable and definite-lived intangible assets, and the variables and assumptions used to calculate and record stock-based compensation. Actual results could differ materially from those estimates.
Cash and cash equivalents – Asterias considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. Cash and cash equivalents consist of money market funds of $11.2 million and $3.1 million at December 31, 2015 and December 31, 2014, respectively.
Available-for-sale securities, at fair value – Marketable equity and debt securities not classified as held-to-maturity are classified as available-for-sale. Available-for-sale securities are carried at fair value, with the unrealized gains and losses, net of tax, reported in other comprehensive income. Realized gains and losses, and declines in value judged to be other-than-temporary related to equity securities, are included in other income/(expense), net.
As further discussed in Note 4, on December 31, 2015, Asterias received shares of common stock in another subsidiary of BioTime, OncoCyte, accounted for as an available-for-sale security, at fair value.
Property, plant and equipment – Property, plant and equipment includes equipment, fixtures and leasehold improvements stated at cost and depreciated using the straight-line method over a period of their estimated useful lives ranging from 36 to 120 months.
Construction in progress – Construction in progress is stated at cost and is not depreciated until the underlying asset is placed into service (see Note 3).
Intangible assets – Intangible assets with finite lives are amortized over their estimated useful lives, and intangible assets with indefinite lives are not amortized but rather are tested at least annually for impairment. Acquired in-process research and development intangible assets are accounted for depending on whether they were acquired as part of an acquisition of a business, or as assets that do not constitute a business. When acquired in conjunction with the acquisition of a business, these assets are considered to be indefinite-lived until the completion or abandonment of the associated research and development efforts and are capitalized as an asset. If and when development is complete, the associated assets would be deemed finite-lived and would then be amortized based on their respective estimated useful lives at that point in time. However, when acquired in conjunction with the acquisition of assets that do not constitute a business, such intangible assets related to in-process research and development are expensed upon acquisition.
Impairment of long-lived assets – Asterias’ long-lived assets, including intangible assets, will be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be fully recoverable. If an impairment indicator is present, Asterias will evaluate recoverability by a comparison of the carrying amount of the assets to future undiscounted net cash flows expected to be generated by the assets. If the assets are impaired, the impairment will be recognized and measured by the amount by which the carrying amount exceeds the estimated fair value of the assets.
Warrants to purchase common stock – Asterias generally accounts for warrants issued in connection with equity financings as a component of equity. None of the warrants issued by Asterias as of December 31, 2015 and 2014 include a conditional obligation to issue a variable number of shares; nor was there a deemed possibility that Asterias may need to settle the warrants in cash.
Accounting for BioTime Shares – Asterias accounts for the BioTime shares of common stock it holds as available-for-sale equity securities in accordance with ASC 320-10-25, Investments-Debt and Equity Securities, as the shares have a readily determinable fair value quoted on the NYSE MKT and are held principally for future working capital purposes, as necessary. These shares are measured at fair value and reported as current assets on the balance sheet based on the closing trading price of the security as of the date being presented. Unrealized holding gains and losses are excluded from the statements of operations and reported in equity as part of other comprehensive income or loss until realized. Realized gains and losses are reclassified out of other comprehensive income or loss and included in equity, as an increase or decrease in additional paid-in capital consistent with, and pursuant to, ASC 805-50, transactions between entities under common control.
Patent costs – Costs associated with obtaining patents on products or technology developed are expensed as general and administrative expenses when incurred.
Reclassification– Certain prior year amounts in the statement of cash flows have been reclassified to conform to the current year presentation.
Research and development – Research and development expenses consist of costs incurred for company-sponsored, collaborative and contracted research and development activities. These costs include direct and research-related overhead expenses including salaries, payroll taxes, consulting fees, research and laboratory fees, rent of research facilities, amortization of intangible assets, and license fees paid to third parties to acquire patents or licenses to use patents and other technology. Asterias expenses research and development costs as such costs are incurred. Research and development expenses incurred and reimbursed under grants approximate the grant income recognized in the statements of operations.
Income taxes – As of October 1, 2013, Asterias has filed its own U.S. federal tax returns. Operations prior to that period were included in BioTime’s consolidated U.S. federal tax return. For California purposes Asterias’ activity will continue to be included in BioTime’s California combined tax return. Asterias accounts for income taxes in accordance with ASC 740, Income Taxes, which prescribe the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. Generally, Asterias is subject to income tax examinations by major taxing authorities for all years since inception. Asterias will recognize accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of December 31, 2015, and 2014.
Stock-based compensation – Asterias has adopted accounting standards governing share-based payments, which require the measurement and recognition of compensation expense for all share-based payment awards made to directors and employees, including employee stock options, based on estimated fair values less estimated forfeitures. Consistent with those guidelines, Asterias utilizes the Black-Scholes Merton option pricing model. Asterias’ determination of fair value of share-based payment awards on the date of grant using that option-pricing model is affected by Asterias’ stock price as well as by assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, Asterias’ expected stock price volatility over the term of the awards; the expected term of options granted, derived from using the simplified method under SEC Staff Accounting Bulletin Topic 14; and a risk-free rate based on the U.S. Treasury rates in effect during the corresponding expected term of the grant.
Asterias also, at times, issues restricted stock or restricted stock units (RSUs) to its executive officers, employees, and members of its Board of Directors (the Board), which are restricted and unvested common shares issued at fair market value on the date of grant. Restricted stock and restricted stock unit compensation expense is recognized on a straight-line basis over the requisite service period of generally four years, based on the grant-date fair value of the stock. Restricted stock is considered legally issued and outstanding on the grant date. Once the RSUs are vested, equivalent common shares will be issued or issuable to the grantee and therefore the RSUs are not included in total common shares issued and outstanding until vested.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718 and FASB ASC 505-50, Equity-Based Payments to Non-Employees (“ASC 505-50”). Stock option awards issued to non-employees, principally consultants and employees of BioTime or employees of BioTime subsidiaries who perform services for Asterias, are accounted for at fair value using the Black-Scholes option pricing model. Management believes that the fair value of the stock options is more reliably measured than the fair value of services received. Asterias records compensation expense based on the then-current fair values of the stock options at each financial reporting date. Compensation recorded during the service period is adjusted in subsequent periods for changes in the fair value of the stock options until the earlier of the date at which the non-employee’s performance is complete or a performance commitment is reached, which is generally when the stock option award vests. Compensation expense for non-employee grants is recorded on a straight-line basis in the statements of operations.
Fair value of financial instruments – ASC 820, Fair Value Measurements, clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability.
ASC 820 requires that the valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 establishes a three tier value hierarchy, which prioritizes inputs that may be used to measure fair value as follows:
|
●
|
Level 1– Observable inputs that reflect quoted prices for identical assets or liabilities in active markets.
|
| ● | Level 2– Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| ● | Level 3– Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The carrying amounts of current assets and current liabilities approximate their fair value because of the relatively short period until they mature or are required to be settled, except for the investment in BioTime and OncoCyte shares, which are carried at fair value based on Level 1 inputs.
Comprehensive income/loss – ASC 220, Comprehensive Income, requires that an entity’s change in equity or net assets during a period from transactions and other events from non-owner sources be reported. Asterias reports unrealized gains and losses on its available-for-sale securities as other comprehensive income/(loss).
Basic and diluted net loss per share – Basic net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding for the period. Diluted net loss per share reflects the weighted-average number of shares of common stock outstanding plus the potential effect of dilutive securities or contracts which are convertible to common stock, such as options and warrants (using the treasury stock method) and shares issuable in future periods, such as restricted stock or RSUs, except in cases where the effect would be anti-dilutive.
The computations of basic and diluted net loss per share are as follows (in thousands, except per share data):
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Net loss
|
$
|
(15,003
|
)
|
$
|
(10,097
|
)
|
$
|
(22,380
|
)
|
|||
|
Weighted average common shares outstanding – basic and diluted
|
|
35,443
|
|
30,720
|
|
7,726
|
||||||
|
Net loss per share – basic and diluted
|
$
|
(0.42
|
)
|
$
|
(0.33
|
)
|
$
|
(2.90
|
)
|
|||
The following common stock equivalents were excluded from the computation of diluted net loss per share of common stock for the periods presented because including them would have been antidilutive (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Stock options and restricted stock units
|
|
5,178
|
|
3,347
|
|
2,840
|
||||||
|
Warrants
|
|
3,500
|
|
8,500
|
|
3,500
|
||||||
Recently Issued Accounting Pronouncements – The following accounting standards, which are not yet effective, are presently being evaluated by Asterias to determine the impact that they might have on its financial statements.
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2016-02, “Leases (Topic 842)”, which requires lessees recognize assets and liabilities for leases with lease terms greater than twelve months in the statement of financial position. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. ASU 2016-02 also requires improved disclosures to help users of financial statements better understand the amount, timing and uncertainty of cash flows arising from leases. The update is effective for fiscal years beginning after December 15, 2018, including interim reporting periods within that reporting period. Early adoption is permitted. Asterias is currently evaluating the impact the adoption of ASU 2016-02 will have on its financial statements.
In November 2015, the FASB issued ASU 2015-17, “Income Taxes (Topic 740)”: Balance Sheet Classification of Deferred Taxes”, which changes how deferred taxes are classified on company’s balance sheets. The ASU eliminates the current requirement to present deferred tax liabilities and assets as current and noncurrent on the balance sheet. Instead, companies will be required to classify all deferred tax assets and liabilities as noncurrent. The amendments are effective for annual financial statements beginning after December 15, 2016, and interim periods within those annual periods. Asterias is currently evaluating the impact the adoption of ASU 2015-17 will have on its financial statements.
In May 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers (Topic 606)”, which supersedes nearly all existing revenue recognition guidance under GAAP. The core principle of ASU 2014-09 is to recognize revenues when promised goods or services are transferred to customers in an amount that reflects the consideration to which an entity expects to be entitled for those goods or services. ASU 2014-09 defines a five-step process to achieve this core principle and, in doing so, more judgment and estimates may be required within the revenue recognition process than are required under existing GAAP.
The revised revenue standard is effective for public entities for annual periods beginning after December 15, 2017, and interim periods therein, using either of the following transition methods: (i) a full retrospective approach reflecting the application of the standard in each prior reporting period with the option to elect certain practical expedients, or (ii) a retrospective approach with the cumulative effect of initially adopting ASU 2014-09 recognized at the date of adoption (which includes additional footnote disclosures). Asterias is currently evaluating the impact of Asterias’ pending adoption of ASU 2014-09 on its financial statements and has not yet determined the method by which it will adopt the standard in fiscal 2018.
In August 2014, the FASB issued ASU No. 2014-15 “Presentation of Financial Statements – Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern” requiring management to evaluate on a regular basis whether any conditions or events have arisen that could raise substantial doubt about the entity’s ability to continue as a going concern. The guidance 1) provides a definition for the term “substantial doubt,” 2) requires an evaluation every reporting period, interim periods included, 3) provides principles for considering the mitigating effect of management’s plans to alleviate the substantial doubt, 4) requires certain disclosures if the substantial doubt is alleviated as a result of management’s plans, 5) requires an express statement, as well as other disclosures, if the substantial doubt is not alleviated, and 6) requires an assessment period of one year from the date the financial statements are issued. This standard is effective for the annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. Early application is permitted. Asterias is currently evaluating the impact, if any, the adoption of ASU 2014-15 will have on its financial statements.
3. Balance Sheet Components
Property, plant and equipment, net
As of December 31, 2015 and 2014, property, plant and equipment were comprised of the following (in thousands):
|
|
|
Years Ended December 31,
|
||||||
|
|
2015 | 2014 | ||||||
|
Property, plant and equipment
|
$
|
7,072
|
$
|
1,796
|
||||
|
Accumulated depreciation
|
(1,316
|
)
|
(751
|
)
|
||||
|
Property, plant and equipment, net
|
$
|
5,756
|
$
|
1,045
|
||||
Equipment includes $33,800 financed by capital lease borrowings in October 2015 for phone equipment for the new facility in Fremont. Depreciation expense amounted to $564,000, $530,000, and $221,000 for the years ended December 31, 2015, 2014 and 2013, respectively.
Construction in progress
There is no construction in progress as of December 31, 2015 and $406,000 as of December 31, 2014, relates entirely to the improvements for Asterias’ Fremont, California, facility.
Under the terms of the lease agreement, the landlord provided Asterias with a tenant improvement allowance of $4.4 million, which Asterias used to construct a laboratory and production facility that will be used to produce human embryonic stem cell and related products under current good manufacturing procedures (“cGMP”). Of the $4.9 million total cost, $4.4 million qualifies for reimbursement under the tenant improvement allowance. As of December 31, 2015, Asterias received $3.8 million from the landlord and has a remaining $0.6 million receivable from the landlord. . Reimbursable amounts due to Asterias but not yet paid by the landlord as of period end are recorded by Asterias as a landlord receivable with a corresponding increase to lease liability since Asterias has contractually earned the right to that cash
The facility was completed and placed into service in November 2015 (see Note 8).
4. Investment in BioTime and in BioTime Subsidiaries
Investment in BioTime
At December 31, 2015 and 2014, Asterias held 3,852,880 of the 8,902,077 BioTime common shares that Asterias received under an Asset Contribution Agreement, and which are included at fair value in current assets in its balance sheet as the shares are available for use and could be sold at fair value for liquidity purposes at any time. The investment is classified as “available for sale.” Available-for-sale securities are carried at fair value, with the unrealized gains and losses, net of tax, reported in other comprehensive income until realized. Realized gains and losses are reclassified out of other comprehensive income or loss and included in equity, as an increase or decrease in additional paid-in capital. (See Note 2). “Accounting for BioTime shares.”
Asterias reviews various factors in determining whether it should recognize an other-than-temporary impairment charge for its marketable securities, including its intent and ability to hold the investment for a period of time sufficient for any anticipated recovery in market value, and the length of time and extent to which the fair value has been less than its cost basis. Based on consideration of these factors, as of December 31, 2015 and 2014, no other-than-temporary impairment was recognized.
Investment in OncoCyte
On December 31, 2015, in connection with BioTime’s distribution of OncoCyte common stock to BioTime shareholders, on a pro rata basis, Asterias received 192,644 shares of OncoCyte common stock from BioTime as a dividend in kind. On this date, BioTime shareholders, including Asterias, received one share of OncoCyte common stock for every twenty shares of BioTime common stock held. Asterias recorded the fair value of the OncoCyte common stock as contributed capital from BioTime. The OncoCyte common stock distribution resulted in a taxable gain to Asterias of $819,000 (see Note 10).
The OncoCyte shares are included in available-for-sale securities at fair value in current assets in Asterias’ balance sheet as the shares are traded on NYSE: MKT (symbol “OCX”) and available for working capital purposes. As of December 31, 2015, the OncoCyte shares are valued at $1.2 million based on the OncoCyte closing price on that date.
The OncoCyte common stock investment is classified as “available for sale.” Available-for-sale securities are carried at fair value, with the unrealized gains and losses, net of tax, reported in other comprehensive income or loss until realized. Realized gains and losses, if any, are included in other income and expense, net, in the statements of operations (See Note 2).
Investments in Affiliates
Asterias’ investments in the OrthoCyte and Cell Cure Neurosciences stock received from BioTime were recorded at BioTime’s historical cost. The investment is carried using the cost method of accounting (see Note 15).
5. Intangible assets
As of December 31, 2015 and, 2014, Asterias had capitalized intangible assets acquired from Geron Corporation, primarily related to patents and other intellectual property rights related to hES cells. These assets are being amortized over the estimated economic lives of the patents on a straight-line basis, which approximates the pattern of consumption over their estimated useful lives of 10 years.
Intangible assets net of accumulated amortization at December 31, 2015 and, 2014 are shown in the following table (in thousands):
|
Years Ended December 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Intangible assets
|
$
|
26,860
|
$
|
26,860
|
||||
|
Accumulated amortization
|
(6,044
|
)
|
(3,358
|
)
|
||||
|
Intangible assets, net
|
$
|
20,816
|
$
|
23,502
|
||||
Asterias recognized $2.7 million, $4.8 million, and 725,000 in amortization expense of intangible assets for the years ended December 31, 2015, 2014 and 2013, respectively.
Amortization of intangible assets for periods subsequent to December 31, 2015 is as follows (in thousands):
|
Year Ending
December 31,
|
Amortization
Expense
|
|||
|
2016
|
$
|
2,686
|
||
|
2017
|
2,686
|
|||
|
2018
|
2,686
|
|||
|
2019
|
2,686
|
|||
|
2020
|
2,686
|
|||
|
Thereafter
|
7,386
|
|||
|
Total
|
$
|
20,816
|
||
6. Common Stock and Warrants
At December 31, 2015, Asterias had outstanding 38,228,120 Series A Shares and no Series B Shares. At December 31, 2014, Asterias had outstanding 30,902,152 Series A Shares and no Series B Shares. All outstanding Series B Shares were converted into Series A Shares on October 3, 2014.
Common Stock Issuance
During the year ended December 31, 2015, Asterias raised approximately $5.5 million in aggregate gross proceeds from the sale of 1,410,255 shares of common stock at a price of $3.90 per share through an underwritten public offering and a private placement. Broadwood Partners, L.P., British & American Investment Trust PLC, and Pedro Lichtinger purchased an aggregate of 1,025,640 of the shares. Broadwood Partners, L.P. is BioTime’s largest shareholder and one of its directors, Neal C. Bradsher, is President, and one of Asterias’ directors, Richard T. LeBuhn, is Senior Vice President, of Broadwood Capital, Inc., the investment manager of Broadwood Partners, L.P. Pedro Lichtinger was Asterias’ former President and Chief Executive Officer and a former member of its Board of Directors (see Note 15). British & American Investment Trust PLC is an affiliate of an investor that owns more than 5% of the outstanding Asterias Series A Shares.
On April 10, 2015, Asterias entered into an at-the-market issuance sales agreement (the “Sales Agreement”) with MLV & Co. LLC (“MLV”), under which MLV, as the sales agent, at Asterias’ discretion and at such times that Asterias may determine from time to time, may sell up to a maximum of $20.0 million of Asterias Series A Shares, subject to certain limitations, including the number of shares registered and available under Asterias’ previously filed and currently effective shelf registration statement on Form S-3 (File No. 333-200745). Asterias is not required to sell any shares at any time during the term of the Sales Agreement. Asterias has agreed to pay MLV a commission of up to 3% of the gross proceeds of the sale of any Series A Shares sold through MLV as agent under the Sales Agreement.
During May, June, November, and December of 2015, Asterias raised approximately $4.8 million in gross proceeds from the sale of 685,465 shares of its common stock at a weighted average price of $7.01 per share in “at-the-market” transactions.
Asterias issued 5,000,000 shares of common stock pursuant to the exercise of 5,000,000 warrants on May 21, 2015, as described under “Warrants” below.
In December 2015, pursuant to a services agreement with Cell Therapy Catapult Services Limited, Asterias issued 96,479 shares of Asterias Series A common stock with a fair value of $486,000 (see Note 13).
During the year ended December 31, 2014, Asterias sold 200,000 Series B Shares at $2.34 per share for $468,000 to Pedro Lichtinger, its former President and Chief Executive Officer.
Asterias has issued warrants to purchase its common shares. Activity related to warrants in 2015 and 2014, is presented in the table below (in thousands, except per share and weighted average exercise prices):
|
Number of
Warrants
|
Per share
exercise
price
|
Weighted
Average
Exercise
Price
|
||||||||||
|
Outstanding, January 1, 2013
|
-
|
$
|
-
|
$
|
-
|
|||||||
|
Issued in 2013
|
3,500
|
5.00
|
5.00
|
|||||||||
|
Outstanding, December 31, 2013
|
3,500
|
5.00
|
5.00
|
|||||||||
|
Issued in 2014
|
5,000
|
2.34
|
2.34
|
|||||||||
|
Outstanding, December 31, 2014
|
8,500
|
$
|
2.34-5.00
|
$
|
3.44
|
|||||||
|
Exercised in 2015
|
(5,000
|
)
|
2.34
|
2.34
|
||||||||
|
Outstanding, December 31, 2015
|
3,500
|
$
|
5.00
|
$
|
5.00
|
|||||||
At December 31, 2015, 3,500,000 warrants to purchase common shares with an exercise price of $5.00 and a weighted average remaining contractual life of 0.75 years were outstanding (see Note 15). At December 31, 2014, 8,500,000 warrants to purchase common shares with a weighted average exercise price of $3.44 and a weighted average remaining contractual life of 0.99 years were outstanding.
The warrants to purchase 3,500,000 shares of its common stock will expire on September 30, 2016. In February 2016, of the warrants to purchase 3,500,000 shares, 3,150,000 were returned to Asterias by BioTime as part of an asset transfer between Asterias and BioTime. As of March 23, 2016, the warrants to purchase 3,150,000 shares were retired by Asterias. To the extent we distribute new warrants to our shareholders that are substantially identical as the warrants surrendered by BioTime, BioTime has agreed to waive its right to receive such new warrants. (See Note 15). Asterias will receive $1.8 million if all of the remaining 350,000 warrants are exercised at $5.00 per share.
7. Equity Incentive Plan
During March 2013, Asterias’ Board of Directors approved an Equity Incentive Plan (the “Plan”) under which Asterias has reserved 4,500,000 shares of common stock for the grant of stock options or the sale of restricted stock. Initially, Asterias issued Series B Shares under the Plan. Since the date on which all of the outstanding Series B Shares were converted into Series A Shares, Asterias has issued Series A Shares under the Plan. The Plan also permits Asterias to issue such other securities as its Board of Directors or the Compensation Committee administering the Plan may determine. Asterias’ stockholders approved the Plan in September 2013.
During May 2015, Asterias’ Board of Directors approved an amendment that would increase the number shares authorized for issuance under the Plan by 3,500,000 shares. This amendment was approved by the shareholders at the 2015 annual meeting of shareholders held on July 9, 2015.
No options may be granted under the Plan more than ten years after the date upon which the Plan was adopted by the Board of Directors, and no options granted under the Plan may be exercised after the expiration of ten years from the date of grant. Under the Plan, options to purchase common stock may be granted to employees, directors and certain consultants at prices not less than the fair market value at date of grant, subject to certain limited exceptions for options granted in substitution of other options. Options may be fully exercisable immediately, or may be exercisable according to a schedule or conditions specified by the Board of Directors or the Compensation Committee. The Plan also permits Asterias to award restricted stock for services rendered or to sell common stock to employees subject to vesting provisions under restricted stock agreements that provide for forfeiture of unvested shares upon the occurrence of specified events under a restricted stock award agreement. Asterias may permit employees or consultants, but not officers or directors, who purchase stock under restricted stock purchase agreements, to pay for their shares by delivering a promissory note that is secured by a pledge of their shares.
Asterias may also grant stock appreciation rights (“SARs”) and hypothetical units issued with reference to Asterias common stock (“Restricted Stock Units”, or RSUs) under the Plan. An SAR is the right to receive, upon exercise, an amount payable in cash or shares or a combination of shares and cash, as determined by the Board of Directors or the Compensation Committee, equal to the number of shares subject to the SAR that is being exercised multiplied by the excess of (a) the fair market value of a share of Asterias common stock on the date the SAR is exercised, over (b) the exercise price specified in the SAR Award agreement.
The terms and conditions of a grant of Restricted Stock Units is determined by the Board of Directors or Compensation Committee. No shares of stock will be issued at the time a Restricted Stock Unit is granted, and Asterias will not be required to set aside a fund for the payment of any such award. A recipient of Restricted Stock Units will have no voting rights with respect to the Restricted Stock Units. Upon the expiration of the restrictions applicable to a Restricted Stock Unit, Asterias will either issue to the recipient, without charge, one share of common stock per Restricted Stock Unit or cash in an amount equal to the fair market value of one share of common stock.
Stock Options and Restricted Stock Units
As of December 31, 2015, Asterias had outstanding to certain officers, employees, and directors, options to purchase a total of 5,130,105 shares of common stock at a weighted average exercise price of $3.16 per share and 48,306 restricted stock units.
A summary of Asterias’ Plan activity and related information follows (in thousands, except per share amounts):
|
Shares
Available
for Grant
|
Number of
Options and
Restricted
Stock Units
Outstanding
|
Weighted
Average
Exercise
Price
|
||||||||||
|
January 1, 2014
|
1,660
|
2,840
|
$
|
2.34
|
||||||||
|
Options granted
|
(1,590
|
)
|
1,590
|
2.50
|
||||||||
|
Restricted stock units issued
|
(400
|
)
|
200
|
-
|
||||||||
|
Options exercised
|
(3
|
)
|
2.34
|
|||||||||
|
Options expired/forfeited
|
1,280
|
(1,280
|
)
|
2.34
|
||||||||
|
December 31, 2014
|
950
|
3,347
|
2.42
|
|||||||||
|
Increase in option pool
|
3,500
|
-
|
-
|
|||||||||
|
Options granted
|
(2,005
|
)
|
2,005
|
3.81
|
||||||||
|
Options exercised
|
-
|
(12
|
)
|
2.34
|
||||||||
|
Options forfeited/cancelled
|
9
|
(9
|
)
|
3.22
|
||||||||
|
Restricted stock units vested
|
(200
|
)
|
-
|
|||||||||
|
Restricted stock units issued
|
(388
|
)
|
194
|
3.90
|
||||||||
|
Restricted stock units vested
|
-
|
(145
|
)
|
-
|
||||||||
|
Restricted stock units forfeited
|
1
|
-
|
3.90
|
|||||||||
|
December 31, 2015
|
2,067
|
5,178
|
$
|
3.17
|
||||||||
Stock-Based Compensation Expense
The weighted-average estimated fair value of stock options granted during the years ended December 31, 2015 and 2014 was $2.92 and $1.50 per share respectively, using the Black-Scholes Merton model with the following weighted-average assumptions:
|
Years Ended
December 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Expected life (in years)
|
6.10
|
$
|
3.98
|
|||||
|
Risk-free interest rates
|
1.74
|
%
|
1.31
|
%
|
||||
|
Volatility
|
77.78
|
%
|
83.49
|
%
|
||||
|
Dividend yield
|
0
|
%
|
0
|
%
|
||||
The risk-free rate is based on the rates in effect at the time of grant for zero coupon U.S. Treasury notes with maturities approximately equal to each grant’s expected life. A dividend yield of zero is applied since Asterias has not historically paid dividends and does not expect to pay dividends in the foreseeable future. The expected volatility is based upon the volatility of Asterias’ own trading stock and of a group of publicly traded industry peer companies. The expected term of options granted is derived from using the simplified method under SEC Staff Accounting Bulletin Topic 14.
Stock-based compensation expense is recognized based on awards that are ultimately expected to vest, and as a result, the amount has been reduced by estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures are estimated based on Asterias’ historical experience and future expectations.
The determination of stock-based compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If Asterias had made different assumptions, its stock-based compensation expense, and net loss for years ended December 31, 2015 and 2014, may have been significantly different.
Asterias does not recognize deferred income taxes for incentive stock option compensation expense, and records a tax deduction only when a disqualified disposition has occurred.
Operating expenses include stock-based compensation expense as follows (in thousands):
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
Research and development
|
$
|
1,604
|
$
|
478
|
$
|
195
|
||||||
|
General and administrative
|
2,021
|
1,336
|
509
|
|||||||||
|
Total stock-based compensation expense
|
$
|
3,625
|
$
|
1,814
|
$
|
704
|
||||||
At December 31, 2015 and 2014, Asterias had $6.7 million and $3.3 million of total unrecognized compensation expense, net of estimated forfeitures, related to the Plan that will be recognized over a weighted-average period of approximately 2.4 and 3.97 years, respectively.
In November 2015, Asterias expensed approximately $589,000 in non-cash stock-based compensation for modifications to, and accelerated vesting of, stock options held by a former executive on the termination date of his employment.
8. Commitments and Contingencies
Leases
Total rent expense for all rented facilities for the years ended December 31, 2015, 2014, and 2013 was $1.0 million, $831,000 and $456,000, respectively.
At December 31, 2015, Asterias had commitments consisting of an operating lab equipment lease, a sublease of its current office and research facility, a lease of its satellite office in New York, a lease for its future office and research facility in Fremont, California and a capital lease for phone equipment.
Asterias subleased from BioTime an office and research facility located in Menlo Park, California. The lease was for a term of three years commencing January 7, 2013. Base rent is $32,000 per month, plus real estate taxes and certain costs of maintaining the leased premises. The lease ended on January 31, 2016. Effective November 2015, Asterias began occupancy at an office and research facility located at 6300 Dumbarton Circle, Fremont, California as discussed below.
On December 30, 2013, Asterias entered into a lease for an office and research facility located in Fremont, California, consisting of an existing building with approximately 44,000 square feet of space. The building will be used by Asterias primarily as a laboratory and production facility that can be used to produce human embryonic stem cells and related products under current good manufacturing procedures. As of December 31, 2015 Asterias completed the tenant improvements, which cost approximately $4.9 million, of which the maximum of $4.4 million was paid to Asterias by the landlord. The landlord’s obligation to fund the tenant improvements expired on December 31, 2015.
In January 2014, Asterias paid the landlord a $300,000 security deposit and the landlord allowed access and use of the premises beginning in March 2014 to allow for the construction of the tenant improvements. The lease is for a term of 96 months, commencing on October 1, 2014, with two available five-year options to extend the term, upon one year written notice by Asterias. During the first 15 months of the lease term, from October 1, 2014 through December 31, 2015, Asterias will pay monthly base rent of $51,000 representing 22,000 square feet rather than 44,000 square feet provided that Asterias is not in default in performing its obligations under the lease beyond any notice and cure periods. Beginning on January 1, 2016, base rent will increase to $105,000 per month and increase by approximately 3% annually on every October 1 thereafter.
In addition to monthly base rent, Asterias will pay all real estate taxes, insurance and the cost of maintenance, repair and replacement of the leased premises. During the first 15 months of the lease term, Asterias will pay only 50% of the real estate taxes assessed on the premises provided that Asterias is not in default in performing its obligations under the lease beyond any notice and cure periods. However, if any improvements or alterations to the premises that Asterias constructs or adds are assessed for real property tax purposes at a valuation higher than the valuation of the improvements on the premises on the date it signed the lease, Asterias will pay 100% of the taxes levied on the excess assessed valuation.
Asterias is considered the owner of the asset under cooperating construction under ASC 840-40-55 as Asterias, among other things, has the primary obligation to pay for construction costs and Asterias will retain exclusive use of the building for its office and research facility requirements after construction is completed. In addition, the lease does not qualify for sale-leaseback accounting under ASC 840-40-25, Accounting for Leases, Sale-Leaseback Transactions, due to Asterias' significant continuing involvement with the facility that Asterias considers to be other than a normal leaseback as defined by ASC 840-40-25. In accordance with this guidance, amounts previously expended by Asterias for construction would continue to be reported as construction in progress in Asterias’ financial statements, and the landlord reimbursement proceeds received, including amounts earned by Asterias but not yet paid by the landlord at period end, are reported as a lease liability. The property was placed in service in November 2015 and Asterias will depreciate the property and the lease payments allocated to the landlord liability which is accounted for as debt service payments on that liability using the finance method of accounting per ASC 840-40-55. As of December 31, 2015 and 2014, Asterias had incurred $4.9 million and $406,000, respectively, of construction costs included in construction in progress, of which $4.4 million and $378,000 is the lease liability included in long term liabilities at December 31, 2015 and 2014, respectively.
Asterias was provided access and rights to use the property beginning in March 2014 with “free-rent” until the lease payments commenced on October 1, 2014, as described above. Asterias commenced expensing rent beginning in March 2014 in accordance with ASC 840-20-25-10 and 11, Rent Expense During Construction. Accordingly, during the years ended December 31, 2015 and 2014, respectively, Asterias has expensed approximately $715,400 and $153,000 included in the statements of operations and a deferred rent balance of approximately 179,000 and $94,000 included in long-term liabilities as of December 31, 2015 and 2014, respectively.
Asterias also currently pays $4,000 per month for the use of approximately 120 square feet of the office space in New York City that is used to conduct meetings and other business affairs. The lease originally for one year commencing July 1, 2014 was extended through June 30, 2016 and will terminate on this date.
Remaining minimum annual lease payments under the various operating leases for the years ending after December 31, 2015 are as follows (in thousands):
|
Year Ending
December 31,
|
Minimum Lease
Payments
|
|||
|
2016
|
$
|
1,328
|
||
|
2017
|
1,309
|
|||
|
2018
|
1,347
|
|||
|
2019
|
1,387
|
|||
|
2020
|
1,430
|
|||
|
Thereafter
|
2,604
|
|||
|
Total
|
$
|
9,405
|
||
Litigation – General
Asterias will be subject to various claims and contingencies in the ordinary course of its business, including those related to litigation, business transactions, employee-related matters, and others. When Asterias is aware of a claim or potential claim, it assesses the likelihood of any loss or exposure. If it is probable that a loss will result and the amount of the loss can be reasonably estimated, Asterias will record a liability for the loss. If the loss is not probable or the amount of the loss cannot be reasonably estimated, Asterias discloses the claim if the likelihood of a potential loss is reasonably possible and the amount involved could be material. Asterias is not aware of any claims likely to have a material adverse effect on its financial condition or results of operations.
Employment Contracts
Asterias has entered into employment contracts with certain executive officers. Under the provisions of the contracts, Asterias may be required to incur severance obligations for matters relating to changes in control, as defined, and involuntary terminations. At December 31, 2015, total potential severance obligations in connection with the termination of employment contracts approximated $816,000 for termination without cause and $1.5 million for termination due to a change in control. (See Note 15).
Indemnification
In the normal course of business, Asterias may provide indemnifications of varying scope under Asterias’ agreements with other companies or consultants, typically Asterias’ clinical research organizations, investigators, clinical sites, suppliers and others. Pursuant to these agreements, Asterias will generally agree to indemnify, hold harmless, and reimburse the indemnified parties for losses and expenses suffered or incurred by the indemnified parties arising from claims of third parties in connection with the use or testing of Asterias’ products and services. Indemnification provisions could also cover third party infringement claims with respect to patent rights, copyrights, or other intellectual property pertaining to Asterias products and services. The term of these indemnification agreements will generally continue in effect after the termination or expiration of the particular research, development, services, or license agreement to which they relate. The potential future payments Asterias could be required to make under these indemnification agreements will generally not be subject to any specified maximum amount. Historically, Asterias have not been subject to any claims or demands for indemnification. Asterias also maintains various liability insurance policies that limit Asterias’ exposure. As a result, Asterias believes the fair value of these indemnification agreements is minimal. Accordingly, Asterias has not recorded any liabilities for these agreements as of December 31, 2015 and 2014.
9. Shared Facilities and Service Agreement
On April 1, 2013, Asterias and BioTime executed a Shared Facilities and Services Agreement (“Shared Facilities Agreement”). Under the terms of the Shared Facilities Agreement, BioTime will allow Asterias to use its premises and equipment located at Alameda, California for the sole purpose of conducting business. BioTime will provide basic accounting, billing, bookkeeping, payroll, treasury, collection of accounts receivable (excluding the institution of legal proceedings or taking of any other action to collect accounts receivable), payment of accounts payable, and other similar administrative services to Asterias. BioTime may also provide the services of attorneys, accountants, and other professionals who may also provide professional services to BioTime and its other subsidiaries. BioTime will also provide Asterias with the services of its laboratory and research personnel, including BioTime employees and contractors, for the performance of research and development work for Asterias at the premise.
BioTime will charge Asterias a fee for the services and usage of facilities, equipment, and supplies aforementioned. For each billing period, BioTime will equitably prorate and allocate its employee costs, equipment costs, insurance costs, lease costs, professional costs, software costs, supply costs, and utilities costs, between BioTime and Asterias based upon actual documented use and cost by or for Asterias or upon proportionate usage by BioTime and Asterias, as reasonably estimated by BioTime. Asterias shall pay 105% of the allocated costs (the “Use Fee”). The allocated cost of BioTime employees and contractors who provide services will be based upon records maintained of the number of hours of such personnel devoted to the performance of services.
The Use Fee will be determined and invoiced to Asterias on a quarterly basis for each calendar quarter of each calendar year. If the Shared Facilities Agreement terminates prior to the last day of a billing period, the Use Fee will be determined for the number of days in the billing period elapsed prior to the termination of the Shared Facilities Agreement. Each invoice will be payable in full by Asterias within 30 days after receipt. Any invoice or portion thereof not paid in full when due will bear interest at the rate of 15% per annum until paid, unless the failure to make a payment is due to any inaction or delay in making a payment by BioTime employees from Asterias funds available for such purpose, rather than from the unavailability of sufficient funds legally available for payment or from an act, omission, or delay by any employee or agent of Asterias.
In addition to the Use Fees, Asterias will reimburse BioTime for any out of pocket costs incurred by BioTime for the purchase of office supplies, laboratory supplies, and other goods and materials and services for the account or use of Asterias, provided that invoices documenting such costs are delivered to Asterias with each invoice for the Use Fee. Furthermore, BioTime will have no obligation to purchase or acquire any office supplies or other goods and materials or any services for Asterias, and if any such supplies, goods, materials or services are obtained for Asterias, BioTime may arrange for the suppliers thereof to invoice Asterias directly.
Asterias in turn may charge BioTime or any Other Subsidiary for similar services provided by Asterias at the same rate and terms as aforementioned. “Other Subsidiary” means a subsidiary of BioTime other than Asterias and other than a subsidiary of Asterias.
The Shared Facilities Agreement terminates on December 31, 2016, provided that, unless otherwise terminated under another provision of the Shared Facilities Agreement, the term of the Shared Facilities Agreement will automatically be renewed and the termination date will be extended for an additional year each year after December 31, 2016, unless either party gives the other party written notice stating that the Shared Facilities Agreement will terminate on December 31 of that year.
BioTime allocated $282,000, $201,000, and $102,000 of general overhead expenses to Asterias during the years ended December 31, 2015, 2014 and 2013, respectively.
10. Income Taxes
The primary components of the deferred tax assets and liabilities at December 31, 2015 and 2014 were as follows (in thousands):
|
Deferred tax assets:
|
2015
|
2014
|
||||||
|
Net operating loss carryforwards
|
$
|
9,939
|
$
|
2,243
|
||||
|
Research and development credits
|
1,898
|
884
|
||||||
|
Stock based compensation and other
|
801
|
484
|
||||||
|
Valuation allowance
|
(2,894
|
)
|
-
|
|||||
|
Total deferred tax assets
|
9,744
|
3,611
|
||||||
|
Deferred tax liabilities:
|
||||||||
|
Patents and licenses
|
(7,020
|
)
|
(8,125
|
)
|
||||
|
Securities held as available for sale
|
(5,274
|
)
|
(4,713
|
)
|
||||
|
Total deferred tax liabilities
|
(12,294
|
)
|
(12,838
|
)
|
||||
|
Net deferred tax liabilities
|
$
|
(2,550
|
)
|
$
|
(9,227
|
)
|
||
Income taxes differed from the amounts computed by applying the U.S. federal income tax of 34% to pretax losses from operations as a result of the following:
|
Years Ended December
31,
|
|||||||||
|
2015
|
2014
|
||||||||
|
Computed tax benefit at federal statutory rate
|
34
|
%
|
34
|
%
|
|||||
|
Permanent differences
|
(3
|
%)
|
(3
|
%)
|
|||||
|
State tax benefit, net of effect on federal income taxes
|
13
|
%
|
8
|
%
|
|||||
|
Change in valuation allowance
|
(13
|
%)
|
-
|
||||||
|
Research and development credits
|
2
|
%
|
2
|
%
|
|||||
|
Other
|
-
|
1
|
%
|
||||||
|
33
|
%
|
42
|
%
|
||||||
As of December 31, 2015, Asterias has net operating loss carryforwards of approximately $23.4 million and $22.5 million respectively for federal and California tax purposes, which expire between 2032 and 2035. In addition, as of December 31, 2015, Asterias has federal and California research tax credit carry forwards of $925,000 and $973,000, respectively. The federal credits expire between 2032 and 2035, while the state tax credits have no expiration date.
A deferred income tax benefit of approximately $7.3 million was recorded for the year ended December 31, 2015, of which approximately $7.4 million was related to the federal tax benefit and $0.1 million was related to state tax expense. A deferred income tax benefit of approximately $7.4 million was recorded for the year ended December 31, 2014, of which approximately $5.2 million was related to federal taxes and $2.2 million was related to state taxes. Asterias established deferred tax liabilities primarily related to its acquisition of certain intellectual property and available for sale securities held in BioTime and OncoCyte common stock. It is more likely than not that the deferred tax assets are fully realizable since these income tax benefits are expected to be available to offset such deferred tax liabilities, except for the California deferred tax assets for which Asterias has established a valuation allowance as of December 31, 2015.
In June 2014, Asterias sold 5,000,000 BioTime shares that resulted in a taxable gain of approximately $10.3 million. Asterias received the BioTime shares from BioTime as part of the consideration for the Asterias common stock and warrants issued to BioTime under an Asset Contribution Agreement among BioTime, Asterias, and Geron Corporation, in a tax free transaction. This taxable gain was offset by available net operating losses, resulting in no income taxes due from the sale. The transaction was treated as a deemed distribution by Asterias and recorded as charge to equity.
On December 31, 2015, BioTime distributed 4.7 million shares of OncoCyte common stock to its shareholders, including Asterias, on a pro rata basis as a dividend in kind. As part of the distribution of OncoCyte common stock, Asterias, as it also holds BioTime common stock, received 192,644 shares of OncoCyte common stock as contributed capital from BioTime resulting in a taxable gain to Asterias of $819,000. Asterias has sufficient current year losses from operations to offset the entire taxable gain resulting in no income taxes due. As the distribution was treated as a dividend in kind for financial reporting purposes, Asterias recorded the tax effect of this gain through equity consistent with BioTime’s treatment of the distribution in accordance with ASC 740-20-45-11(g). As disclosed in Note 4, Asterias holds the OncoCyte common stock as an available for sale security on its balance sheet.
As of December 31, 2015 and 2014, Asterias recorded a $5.3 million and $4.7 million deferred tax liability, respectively, for the temporary taxable differences in the basis of the investments still held by Asterias in BioTime and OncoCyte common stock.
Internal Revenue Code Section 382 places a limitation (“Section 382 Limitation”) on the amount of taxable income that can be offset by net operating loss (“NOL”) carryforwards after a change in control (generally greater than 50% change in ownership within a three-year period) of a loss corporation. California has similar rules. Generally, after a control change, a loss corporation cannot deduct NOL carryforwards in excess of the Section 382 Limitation. Due to these “change in ownership” provisions, utilization of the NOL and tax credit carryforwards may be subject to an annual limitation regarding their utilization against taxable income in future periods.
Asterias will file an income tax return in the U.S. federal jurisdiction, and may file income tax returns in various U.S. states and foreign jurisdictions.
Asterias may be subject to potential examination by U.S. federal, U.S. states or foreign jurisdiction authorities in the areas of income taxes. These potential examinations may include questioning the timing and amount of deductions, the nexus of income among various tax jurisdictions and compliance with U.S. federal, U.S. state and foreign tax laws. Asterias' management does not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months.
11. Segment Information
Operating segments are defined as components of an enterprise that engage in business activities for which separate financial information is available and evaluated by the chief operating decision maker in deciding how to allocate resources and assess performance. Asterias’ executive management team represents its chief operating decision maker. The executive management team reviews financial information presented on a consolidated basis for purposes of allocating resources and evaluating financial performance and there are no managers who are held accountable for levels or components below the consolidated unit level. To date, management has viewed Asterias’ operations as one segment.
12. Selected Quarterly Financial Information (unaudited) (in thousands)
Asterias has derived this data from the unaudited interim financial statements that, in Asterias’ opinion, have been prepared on substantially the same basis as the audited financial statements contained in this report and include all normal recurring adjustments necessary for a fair presentation of the financial information for the periods presented. These unaudited quarterly results should be read in conjunction with the financial statements and notes thereto included in this report. The operating results in any quarter are not necessarily indicative of the results that may be expected for any future period.
|
Year Ended December 31, 2015
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
||||||||||||
|
Total gross profit
|
$
|
728
|
$
|
734
|
$
|
1,247
|
$
|
605
|
||||||||
|
Operating expenses
|
5,265
|
5,541
|
6,183
|
8,233
|
||||||||||||
|
Loss from operations before deferred tax benefits
|
(4,557
|
)
|
(4,859
|
)
|
(5,068
|
)
|
(7,771
|
)
|
||||||||
|
Basic and diluted net loss per share
|
(0.09
|
)
|
(0.10
|
)
|
(0.09
|
)
|
(0.13
|
)
|
||||||||
|
Year Ended December 31, 2014
|
||||||||||||||||
|
Total gross profit
|
$
|
62
|
$
|
(21
|
)
|
$
|
42
|
$
|
1,045
|
|||||||
|
Operating expenses
|
3,694
|
4,287
|
4,037
|
6,572
|
||||||||||||
|
Loss from operations before deferred tax benefits
|
(3,636
|
)
|
(4,315
|
)
|
(3,995
|
)
|
(5,527
|
)
|
||||||||
|
Basic and diluted net loss per share
|
(0.07
|
)
|
(0.09
|
)
|
(0.05
|
)
|
(0.11
|
)
|
||||||||
13. License and Royalty Obligations
Services Agreement with Cell Therapy Catapult Services Limited
In October 2015, Asterias entered into a Services Agreement (the “Services Agreement”) with Cell Therapy Catapult Services Limited (“Catapult”), a research organization specializing in the development of technologies which speed the growth of the cell and gene therapy industry. Under the Services Agreement, Catapult will license to Asterias, certain background intellectual property and will develop a scalable manufacturing and differentiation process for Asterias’ human embryonic stem cell derived dendritic cell cancer vaccine development program. In consideration for the license and Catapult’s performance of services, Asterias agreed to make aggregate payments of up to GBP £4,350,000 over the next five years (approximately $6.4 million based on the foreign currency exchange rate on December 31, 2015). At the option of Asterias, up to GBP £3,600,000 (approximately $5.3 million based on the foreign currency exchange rate on December 31, 2015) of such payments may be settled in shares of Asterias Series A Common Stock instead of cash. If Asterias elects to pay for the services in stock and Catapult is unable to sell the stock in the market within 60 days of issuance, after reasonable and diligent efforts through its broker, Catapult may request that the unsold portion of the stock payment, if any, be paid by Asterias in cash at a value equal to approximately 91% of the total amount that was issued in stock. This right by Catapult to put the unsold shares back to Asterias for cash expires the earlier to occur of the sale of the stock in the market or after 60 days of issuance.
The Services Agreement may be terminated by Asterias for any reason upon 60 days prior written notice. Catapult may terminate the Services Agreement on 60 days prior written notice if it encounters a technical issue that would prevent it from completing the services at all or without obtaining additional resources, or if the estimated time and cost of completing the services will be exceeded and Catapult and Asterias do not reach agreement on revised time and cost terms. Catapult may terminate the Services Agreement in the event Asterias fails to pay any amount due under the Services Agreement 30 days after Catapult makes a written demand for payment. In addition, a non-breaching party may terminate the Services Agreement upon the occurrence a material breach that is not remedied within 30 days. Either party may terminate the Services Agreement in the event the other party becomes subject to insolvency, receivership, liquidation, or a similar event.
Advance payments for research and development services to be performed by Catapult are deferred and recognized as research and development expense ratably as the services are performed. Advance payments related to licenses will be expensed when paid due to the experimental nature of the project. Pursuant to the Services Agreement, if there are any issued, but unsold Asterias stock, to Catapult for payment of services and the 60-day put right has not expired as of the period end being reported on, Asterias will present that amount as “temporary” equity in accordance with ASC 480-10-S99. Once the put right expires or the shares are sold by Catapult, the temporary equity amount will be reclassified by Asterias to permanent equity without adjustment to the carrying value of the stock.
In December 2015, pursuant to the Services Agreement, Asterias issued 96,479 shares of Asterias Series A Common Stock with a fair market value of $486,000 at the time of issuance which Asterias reclassified into permanent equity since all of these shares were sold in the market by Catapult as of December 31, 2015.
Asterias License from WARF
Asterias has entered into a Non-Exclusive License Agreement with WARF under which Asterias was granted a worldwide non-exclusive license under certain WARF patents and WARF-owned embryonic stem cell lines to develop and commercialize therapeutic, diagnostic and research products. The licensed patents include patents covering methods for growth and differentiation of primate embryonic stem cells. The licensed stem cell lines include the H1, H7, H9, H13 and H14 hES cell lines.
In consideration of the rights licensed, Asterias has agreed to pay WARF an upfront license fee, payments upon the attainment of specified clinical development milestones, royalties on sales of commercialized products, and, subject to certain exclusions, a percentage of any payments that Asterias may receive from any sublicenses that it may grant to use the licensed patents or stem cell lines.
The license agreement will terminate with respect to licensed patents upon the expiration of the last licensed patent to expire. Asterias may terminate the license agreement at any time by giving WARF prior written notice. WARF may terminate the license agreement if payments of earned royalties, once begun, cease for a specified period of time or if Asterias and any third parties collaborating or cooperating with Asterias in the development of products using the licensed patents or stem cell lines fail to spend a specified minimum amount on research and development of products relating to the licensed patents or stem cell lines for a specified period of time. WARF also has the right to terminate the license agreement if Asterias breaches the license agreement or becomes bankrupt or insolvent or if any of the licensed patents or stem cell lines are offered to creditors
Asterias License from the University of California
Geron assigned to Asterias its Exclusive License Agreement with The Regents of the University of California for patents covering a method for directing the differentiation of multipotential hES cells to glial-restricted progenitor cells that generate pure populations of oligodendrocytes for remyelination and treatment of spinal cord injury. Pursuant to this agreement, Asterias has an exclusive worldwide license under such patents, including the right to grant sublicenses, to create products for biological research, drug screening, and human therapy using the licensed patents. Under the license agreement, Asterias will be obligated to pay the university a royalty of 1% from sales of products that are covered by the licensed patent rights, and a minimum annual royalty of $5,000 starting in the year in which the first sale of a product covered by any licensed patent rights occurs, and continuing for the life of the applicable patent right under the agreement. The royalty payments due are subject to reduction, but not by more than 50%, to the extent of any payments that Asterias may be obligated to pay to a third party for the use of patents or other intellectual property licensed from the third party in order to make, have made, use, sell, or import products or otherwise exercise its rights under the Exclusive License Agreement. Asterias will be obligated to pay the university 7.5% of any proceeds, excluding debt financing and equity investments, and certain reimbursements, that its receives from sublicensees, other than Asterias’ affiliates and joint ventures relating to the development, manufacture, purchase, and sale of products, processes, and services covered by the licensed patent. The license agreement will terminate on the expiration of the last-to-expire of the university's issued licensed patents. If no further patents covered by the license agreement are issued, the license agreement would terminate in 2024. The university may terminate the agreement in the event of Asterias’ breach of the agreement. Asterias can terminate the agreement upon 60 days' notice.
Asterias Sublicense from Geron
Asterias has received from Geron an exclusive sublicense under certain patents owned by the University of Colorado’s University License Equity Holdings, Inc. relating to telomerase (the “Telomerase Sublicense”). The Telomerase Sublicense entitles Asterias to use the technology covered by the patents in the development of AST-VAC1 and AST-VAC2 as immunological treatments for cancer. Under the Telomerase Sublicense, Asterias paid Geron a one-time upfront license fee of $65,000, and will pay Geron an annual license maintenance fee of $10,000 due on each anniversary of the effective date of the Telomerase Sublicense, and a 1% royalty on sales of any products that Asterias may develop and commercialize that are covered by the sublicensed patents. The Telomerase Sublicense will expire concurrently with the expiration of Geron’s license. That license will terminate during April 2017 when the licensed patents expire. The Telomerase Sublicense may also be terminated by Asterias by giving Geron 90 days written notice, by Asterias or by Geron if the other party breaches its obligations under the sublicense agreement and fails to cure their breach within the prescribed time period, or by Asterias or by Geron upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings, or upon an assignment of a substantial portion of the assets for the benefit of creditors by the other party.
14. Clinical Trial and Option Agreement and CIRM Grant Award
During September 2014, Asterias entered into a Clinical Trial and Option Agreement (the “CRUK Agreement”) with Cancer Research UK (“CRUK”) and Cancer Research Technology Limited, (“CRT”), a wholly-owned subsidiary of CRUK, pursuant to which CRUK has agreed to fund Phase 1/2 clinical development of Asterias’ human embryonic stem cell derived AST-VAC2 allogeneic (non-patient specific) dendritic cancer vaccine product candidate. Asterias will, at its own cost, complete process development and manufacturing scale-up of the AST-VAC2 manufacturing process and will transfer the resulting cGMP-compatible process to CRUK. CRUK will, at its own cost, manufacture clinical grade AST-VAC2 and will carry out the Phase 1/2 clinical trial of AST-VAC2 in cancer patients both resected early-stage and advanced forms of lung cancer. Asterias will have an exclusive first option to obtain a license to use the data from the clinical trial. If Asterias exercises that option, then Asterias will be obligated to make payments upon the execution of the License Agreement, upon the achievement of various milestones, and royalties on sales of products. In connection with the CRUK Agreement, Asterias sublicensed to CRUK for use in the clinical trials and product manufacturing process certain patents that have been licensed or sublicensed to us by third parties. Asterias would also be obligated to make payments to those licensors and sublicensors upon the achievement of various milestones, and then royalties on sales of products if AST-VAC2 is successfully developed and commercialized.
On October 16, 2014 Asterias signed a Notice of Grant Award (“NGA”) with CIRM, effective October 1, 2014, with respect to a $14.3 million grant award for clinical development of Asterias’ product, AST-OPC1. The NGA includes the terms under which CIRM will release grant funds to Asterias. CIRM will disburse the grant funds to Asterias through July 1, 2018 in accordance with a quarterly disbursement schedule, subject to Asterias’ attainment of certain progress and safety milestones. Asterias received the first payment from CIRM in the amount of $917,000 in October 2014, and the second, third, fourth, and fifth payments totaling $5.6 million in 2015 as of December 31, 2015.
15. Subsequent Events
On February 16, 2016, Asterias entered into a Cross-License Agreement (the “Cross-License”) with its majority parent, BioTime, Inc. ("BioTime") and BioTime's wholly owned subsidiary ES Cell International Pte Ltd (“ESI”). Under the terms of a Cross-License, Asterias received a fully-paid, non-royalty-bearing, world-wide, non-exclusive, sub-licensable license under certain BioTime patents and related patent rights and ESI patents and related patent rights specified in the Cross-License, for all purposes in the Asterias Licensed Field during the term of the license. The Asterias Licensed Field includes all therapeutic applications of use for certain BioTime patents and ESI patents except all therapeutic applications of use involving pluripotent stem cell-derived cells of the following lineages: (a) bone and orthopedic soft tissues, including but not limited to ligament, tendon, meniscus, cartilage, and intervertebral disc; (b) vascular endothelium and perivascular cells including vascular smooth muscle and vascular pericytes; (c) adipose tissue; and (d) retinal pigment epithelium. The Asterias Licensed Field also includes all applications of use for certain other BioTime patents involving live human pluripotent stem cell-derived cell therapies directed to the neural spinal cord (excluding cartilage and bone of the spine) and the myocardium; and also live human pluripotent stem cell-derived glial cell therapies directed to the central nervous system.
Under the terms of the Cross-License, BioTime and ESI received a fully-paid, non-royalty-bearing, world-wide, non-exclusive, sub-licensable license in, to, and under the certain Asterias patents and related patent rights for all purposes in the BioTime/ESI Licensed Field during the term of the license. The BioTime/ESI Licensed Field includes all fields of use except any and all applications (a) to treat disorders of the nervous system, and (b) utilizing the immune system to prevent, treat, or cure cancer, and (c) involving the use of cells comprising, derived from, or manufactured using, human embryonic stem cells or human induced pluripotent stem cells for in vitro assay applications, including but not limited to drug discovery and development, drug monitoring, drug toxicology testing, and consumer products testing.
Asterias also entered into a Share Transfer Agreement with BioTime and ESI pursuant to which (a) Asterias transferred to BioTime 2,100,000 shares of common stock of OrthoCyte Corporation (“OrthoCyte) and 21,925 ordinary shares of Cell Cure Neurosciences Ltd (“Cell Cure”), each a majority-owned subsidiary of BioTime and (b) BioTime transferred to Asterias 75,771 shares of Series A Common Stock of Asterias and warrants to purchase 3,150,000 Series A common stock of Asterias at an exercise price of $5.00 per share, expiring September 30, 2016 (“Warrants”), as additional consideration for the license of patents and patent rights from Asterias under the Cross License. On March 20, 2016, the warrants to purchase 3,150,000 shares of Series A common stock were retired by Asterias.
On February 29, 2016, Asterias appointed Stephen L. Cartt as Chief Executive Officer and as a member of the Board of Directors of Asterias (the "Board"). Asterias and the Mr. Cartt entered into an employment agreement (the "Employment Agreement"), which provides for an annual base salary of $452,400, a grant of stock options to purchase 1,000,000 shares Asterias common stock at an exercise price of $3.64 per share, and a grant of 200,000 restricted shares of Asterias common stock. Subject to the Mr. Cartt’s continued employment with Asterias, the stock options vest in equal monthly installments over 48 months commencing on March 31, 2016, and 50% of the restricted stock vests on August 31, 2016 and February 28, 2017. In addition, Mr. Cartt is eligible for an annual Bonus Opportunity up to 50% of his base salary (the "Bonus Opportunity"). Asterias’ Board, or its Compensation Committee, has absolute discretion in determining whether and to what extent any payment under the Bonus Opportunity are to be made based on performance criteria that the Board, or its Compensation Committee, may determine from time to time.
Mr. Cartt’s employment agreement contains provisions entitling him to severance benefits under certain circumstances. If Asterias terminates Mr. Cartt’s employment without "Cause" or if he resigns for "Good Reason" otherwise than within 12 months following a "Change of Control") as those terms are defined in the Employment Agreement, he will be entitled to severance benefits. If Mr. Cartt has been employed by Asterias for one year or less, his severance benefits will be include payment of six months base salary and 50% of Mr. Cartt’s Bonus Opportunity. If Mr. Cartt is employed with Asterias for more than one year, his severance benefits will include payment of 12 months base salary, 100% of his Bonus Opportunity, accelerated vesting of all unvested restricted stock previously granted, and accelerated vesting of 50% of unvested stock options previously granted. If Mr. Cartt’s employment is terminated without "Cause" or if he resigns for "Good Reason" within twelve months within a Change of Control, his severance benefits will be include payment of 100% of his base salary, 100% of the Bonus Opportunity, and accelerated vesting of all unvested restricted stock and stock options previously granted.
On March 10, 2016, Asterias entered into a Separation Agreement (the "Separation Agreement") with its former President and Chief Executive Officer. Under the terms of the Separation Agreement, and consistent with the obligations under his employment agreement, Asterias agreed to pay the former executive severance in the amount of $475,000 plus accrued and unpaid wages and unused vacation, and accelerated vesting of any unvested restricted stock and 50% of unvested stock options that were previously granted to the former executive.
On March 21, 2016, Asterias entered into a Separation Agreement (the "Separation Agreement") with its former Chief Financial Officer. Under the terms of the Separation Agreement, and consistent with the obligations under her employment agreement, Asterias agreed to pay the former executive an aggregate of $128,000 comprising of severance in the amount of $82,500, accrued and unpaid wages and unused vacation in the amount of $18,000, and potential bonus opportunity in the amount of $27,500.
As of March 28, 2016, Asterias received $7.8 million of payments from CIRM, and recent progess on the Phase 1/2a dose escalation trial for OPC-1 is expected to result in a further $2.5 million payment under the terms of the existing CIRM award grant.
Not applicable.
Evaluation of Disclosure Controls and Procedures
It is management’s responsibility to establish and maintain adequate internal control over all financial reporting pursuant to Rule 13a-15 under the Securities Exchange Act of 1934 (“Exchange Act”). Our management, including our principal executive officer, our principal operations officer, and our principal financial officer, have reviewed and evaluated the effectiveness of our disclosure controls and procedures as of a date within ninety (90) days of the filing date of this Annual Report on Form 10-K. Following this review and evaluation, management collectively determined that our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act (i) is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms; and (ii) is accumulated and communicated to management, including our chief executive officer, our chief operations officer, and our chief financial officer, as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the period covered by this Annual Report on Form 10-K that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f), is a process designed by, or under the supervision of, our principal executive officer, our principal operations officer, and our principal financial officer, and effected by our Board of Directors, management, and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| ● | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
| ● | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| ● | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2015, based on criteria established in the 2013 Internal Control - Integrated Framework issued by COSO. Based on this assessment, management believes that, as of that date, our internal control over financial reporting was effective.
This annual report includes an attestation report of our registered public accounting firm regarding internal control over financial reporting for the year ended December 31, 2015. The attestation is included with the accounting firm's report on our audited financial statements.
None
PART III
Our directors are:
Don M. Bailey, 70, has been a director and our Chairman since February 2016. Mr. Bailey served as President and Chief Executive Officer of Questcor Pharmaceuticals, Inc. from November 2007 until Questcor was acquired by Mallinckrodt plc in August 2014. He was also on the Board of Directors of Mallinckrodt plc from August 2014 to February 2016. He initially joined the Questcor board in 2006 as an independent director in 2006. Mr. Bailey was the non-executive Chairman of STAAR Surgical Company from April 2005 until January 2014 and on its board until June 2014. STAAR Surgical Company is a leader in the development, manufacture, and marketing of minimally invasive ophthalmic products employing proprietary technologies. Mr. Bailey was the Chairman of the board of directors of Comarco, Inc., a defense services company transformed into a wireless communication products company, from 1998 until 2007, where he served as its Chief Executive Officer from 1991 until 2000. Mr. Bailey holds a B.S. degree in mechanical engineering from the Drexel Institute of Technology, an M.S. degree in operations research from the University of Southern California, and an M.B.A. from Pepperdine University. Mr. Bailey also serves on two boards of Chapman University.
Mr. Bailey’s qualifications to serve on our Board include his extensive knowledge of the pharmaceuticals industry and his significant experience as an executive and/or board member of publicly traded and private pharmaceutical companies.
Stephen L. Cartt, 53, has been our President and Chief Executive Officer and a director since February 2016. Mr. Cartt served as Chief Operating Officer of Questcor Pharmaceuticals, Inc., from February 2012 until August 2014, when the company was acquired by Mallinckrodt plc. Mr. Cartt served as Chief Operating Officer of the Autoimmune and Rare Diseases Business Unit of Mallinckrodt plc on an interim basis following the merger with Questcor from August 2014 until October 2014. Mr. Cartt joined Questcor as Executive Vice President, Corporate Development, during March 2005. He was later appointed Chief Business Officer before being appointed Chief Operating Officer. Mr. Cartt was a private consultant from August 2002 until March 2005. From March 2000 through August 2002, Mr. Cartt was the Senior Director of Strategic Marketing for Elan Pharmaceuticals. Prior to that, Mr. Cartt held a variety of R&D and Commercial positions at ALZA Corporation during the period July 1985 to March 2000. Mr. Cartt holds a B.S. degree from the University of California at Davis in Biochemistry, and an M.B.A. from Santa Clara University.
Mr. Cartt brings to our Board his many years of experience in the pharmaceutical industry, including experience in senior management of a Questcor, a rapidly growing biopharmaceutical company prior to its acquisition by Mallinckrodt plc.
Andrew Arno, 56, has 30 years of experience working with emerging growth companies. He is currently Vice Chairman of “The Special Equities Group” at Chardan Capital Markets, LLC, a privately held investment banking firm, and from June 2013 until July 2015 served as Managing Director of Emerging Growth Equities, an investment bank, and Vice President of Sabr, Inc., a family investment group. He was previously President of LOMUSA Limited, an investment banking firm. From 2009 to 2012, Mr. Arno served as Vice Chairman and Chief Marketing Officer of Unterberg Capital, LLC, an investment advisory firm that he co-founded. He was also Vice Chairman and Head of Equity Capital Markets of Merriman Capital LLC, an investment banking firm, and served on the board of the parent company, Merriman Holdings, Inc. Mr. Arno currently serves on the board of Smith Micro Software, Inc.
Mr. Arno brings over 30 years’ experience handling a wide range of corporate and financial matters and his background as an investment banker and strategic advisor to emerging growth companies qualifies him to serve on our board of directors.
Alfred D. Kingsley, 73, joined our Board of Directors and became Chairman of the Board during September 2012 and Executive Chairman during March 2014. Mr. Kingsley is Chairman of the Board of BioTime, Inc. ("BioTime"). Mr. Kingsley has been general partner of Greenway Partners, L.P., a private investment firm, and President of Greenbelt Corp., a business consulting firm, since 1993. Greenbelt Corp. served as BioTime’s financial advisor from 1998 until June 30, 2009. Mr. Kingsley was Senior Vice-President of Icahn and Company and its affiliated entities for more than 25 years. Mr. Kingsley holds a BS degree in economics from the Wharton School of the University of Pennsylvania, and a J.D. degree and LLM in taxation from New York University Law School.
Mr. Kingsley’s long career in corporate finance and mergers and acquisitions includes substantial experience in helping companies to improve their management and corporate governance, and to restructure their operations in order to add value for shareholders. Mr. Kingsley has been instrumental in structuring our initial equity financings, and in negotiating the Asset Contribution Agreement with Geron through which we acquired most of our stem cell assets. Mr. Kingsley, along with entities that he controls, is currently one of BioTime’s largest shareholders.
Richard T. LeBuhn, 51, has served since June 2006 as Senior Vice President of Broadwood Capital, Inc., the investment manager of Broadwood Partners, L.P. Previously, Mr. LeBuhn was Principal of Broadfield Capital Management, LLC, a private investment firm, from 2005 to 2006, and Vice President of Derchin Management, a private investment firm, from July 2002 to May 2005. Earlier in his career, Mr. LeBuhn founded and was Managing Member of Triple Eight Capital, LLC, an investment analysis and financial advisory firm, was Managing Director of Craig Drill Capital, Inc., a private investment firm, and served as an operating business manager for Chubb and Son, Inc., the property and casualty insurance division of The Chubb Corporation. Mr. LeBuhn received a MBA in Finance with Distinction from Columbia University Graduate School of Business in 1996. He received a BA in Economics from St. Lawrence University in 1988. Mr. LeBuhn currently serves as a director of Comarco, Inc.
Mr. LeBuhn’s qualifications to serve on our Board include, among others, his extensive experience as an investor in public companies, his extensive financial analyst background, his financial and management expertise, and his ability to provide advice on various matters, including matters pertaining to accounting and corporate governance matters.
Robert W. Peabody, 61, has been our Chief Financial Officer since June 2013 and is Senior Vice-President, Chief Operating Officer, and Chief Financial Officer of BioTime. Mr. Peabody joined BioTime in October 2007 as its Senior Vice-President and Chief Operating Officer also served on an interim basis as Chief Financial Officer from September 2010 until October 2011. Mr. Peabody reassumed the role as Chief Financial Officer in May 2013. Prior to joining BioTime, Mr. Peabody served as a Vice-President of Advanced Cell Technology, Inc., and also served on their board of directors from 1998 to 2006. Prior to joining ACT, Mr. Peabody spent 14 years as a Regional Controller for Ecolab, Inc., a Fortune 500 specialty chemical manufacturer and service company. He has also been an audit manager for Ernst and Young where he was a Certified Public Accountant on the audit staff serving the firm’s clients whose shares are publicly traded. Mr. Peabody received a Bachelor Degree in Business Administration from the University of Michigan.
Mr. Peabody brings to our Board many years of experience as a senior executive and in accounting and financial reporting, including in the stem cell and biotechnology industry as Chief Financial Officer of our parent company BioTime and as Vice President and director of Advance Cell Technology.
Natale S. Ricciardi, 67, spent his entire 39-year biopharmaceutical career at Pfizer Inc, retiring in 2011 as a member of the Pfizer Executive Leadership Team after holding the positions of President, Pfizer Global Manufacturing and Senior Vice President of Pfizer Inc. In addition to his corporate leadership role, he was directly responsible for all of Pfizer’s internal and external supply organization, a global enterprise that grew to more than 100 manufacturing facilities supplying small and large molecule pharmaceuticals, vaccines, consumer, nutrition and animal health products. Mr. Ricciardi maintained responsibility for global manufacturing activities from 2004 through 2011. Previously, from 1999 to 2004, he had oversight for Pfizer’s US manufacturing operations and from 1995 to 1999 was Vice President of Manufacturing for Pfizer’s Animal Health Group. Mr. Ricciardi received a B.E. degree in Chemical Engineering from The City College of New York (CCNY) and an MBA in Finance and International Business from Fordham University. He currently serves on the Advisory Board of HealthCare Royalty Partners and is a Director of Dynavax Technologies Corporation. He also serves as a member of the CCNY 21st Century Foundation.
Mr. Ricciardi’s long career at Pfizer Inc. in a corporate leadership role and his extensive experience leading Pfizer's global manufacturing and supply operations qualifies him to serve on our board of directors.
Judith Segall, 62, has been our Corporate Secretary since September 2012 and has served as a Vice President and as Secretary of BioTime since 1990. Ms. Segall also serves on the Board of Directors of BioTime. Ms. Segall received a B.S. in Nutrition and Clinical Dietetics from the University of California at Berkeley in 1989.
Ms. Segall brings to our Board more than 25 years of experience as an executive and director of BioTime. During that time, she has developed a wealth of knowledge of business operations and management.
Michael D. West, Ph.D., 63, Dr. West has been a director of Asterias since 2012, and was our Vice President from September 2012 to December 2015, except for an interim period from April 2014 to June 2014 when he served as our President and Chief Executive Officer Dr. West has been Chief Executive Officer of BioTime from October 2007 to October 2015, has been Co-Chief Executive Officer since October 2015, and has served on BioTime’s Board of Directors since 2002. Prior to becoming BioTime’s Chief Executive Officer, Dr. West served as Chief Executive Officer, President, and Chief Scientific Officer of Advanced Cell Technology, Inc., a company engaged in developing human stem cell technology for use in regenerative medicine. Dr. West also founded Geron Corporation, and from 1990 to 1998 he was a director and Vice-President of Geron, where he initiated and managed programs in telomerase diagnostics, oligonucleotide-based telomerase inhibition as anti-tumor therapy, and the cloning and use of telomerase in telomerase-mediated therapy wherein telomerase is utilized to immortalize human cells. From 1995 to 1998 he organized and managed the research between Geron and its academic collaborators, James Thomson and John Gearhart, which led to the first isolation of human embryonic stem and human embryonic germ cells. Dr. West received a B.S. Degree from Rensselaer Polytechnic Institute in 1976, an M.S. Degree in Biology from Andrews University in 1982, and a Ph.D. from Baylor College of Medicine in 1989 concentrating on the biology of cellular aging.
Dr. West is an internationally renowned pioneer and expert in stem cell research, and has extensive academic and business experience in age-related degenerative diseases, telomerase molecular biology, and human embryonic stem cell research and development. Dr. West brings to our Board the proven ability to conceive of and manage innovative research and development programs that have made scientifically significant discoveries in the field of human embryonic stem cells, and the ability to build companies focused on the great potential of regenerative medicine.
Audit Committee
The audit committee currently consists of Mr. LeBuhn (chair), Mr. Arno and Mr. Ricciardi. Our board of directors has determined that each member of the audit committee is an independent director under Section 803(A)(2) of the NYSE MKT Company Guide and meets the applicable additional eligibility standards for audit committee service under Section 803(B)(2) of the NYSE MKT Company Guide. In addition, our board of directors has determined that Mr. LeBuhn qualifies as an “audit committee financial expert” as defined in Item 407(d)(5) of Regulation S-K under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Mr. LeBuhn’s expertise is based on his experience and qualifications described under “Directors” above.
The primary purpose of the audit committee is to oversee our accounting and financial reporting processes, including our internal controls system, and audits of our financial statements. The responsibilities of the audit committee include appointing and providing for the compensation of an independent registered public accounting firm to conduct an annual audit of our financial statements, overseeing the work and evaluating the performance of the independent auditor, reviewing and evaluating our accounting principles and practices, approving all professional services to be provided to us by our independent registered public accounting firm, reviewing and overseeing any related party transactions, overseeing implementation and enforcement of our insider trading policy and reviewing and evaluating any significant financial risk exposures facing our company and the steps our management has taken to control and monitor such exposures. The audit committee is governed by a written charter approved by our board of directors. A copy of the Audit Committee charter has been posted on our internet website and may be found at www.asteriasbiotherapeutics.com.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics (“Code of Ethics”) that applies to our principal executive officers, our principal financial officer and accounting officer, our other executive officers, and our directors. The purpose of the Code of Ethics is to promote (i) honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; (ii) full, fair, accurate, timely, and understandable disclosure in reports and documents that we file with or submit to the SEC and in our other public communications; (iii) compliance with applicable governmental rules and regulations; (iv) prompt internal reporting of violations of the Code of Ethics to an appropriate person or persons identified in the Code of Ethics; and (v) accountability for adherence to the Code of Ethics. A copy of our Code of Ethics has been posted on our internet website and can be found at www.asteriasbiotherapeutics.com. We intend to disclose any future amendments to certain provisions of our Code of Ethics, and any waivers of those provisions granted to our principal executive officer, principal financial officer, principal accounting officer or controller or persons performing similar functions, by posting the information on our website within four business days following the date of the amendment or waiver.
Family Relationships
There are no family relationships among any of our officers or directors.
Process for Communicating with Board Members
Interested parties may communicate with any and all members our Board by transmitting correspondence addressed to one or more Directors by name at the following address: Asterias Biotherapeutics, Inc., 6300 Dumbarton Circle, Fremont, California 94555, c/o Corporate Secretary. Communications from our stockholders to one or more Directors will be collected and organized by our Corporate Secretary and will be forwarded to the Chairman of the Board or to the identified Director(s) as soon as practicable. If multiple communications are received on a similar topic, the Corporate Secretary may, in his or her discretion, forward only representative correspondence.
The Chairman of the Board will determine whether any communication addressed to the entire Board should be properly addressed by the entire Board or a committee thereof. If a communication is sent to the Board or a Committee, the Chairman of the Board or the Chairman of that committee, as the case may be, will determine whether a response to the communication is warranted.
Executive Officers
Our executive officers are Stephen L. Cartt, President and Chief Executive Officer, Russell L. Skibsted, Chief Financial Officer, Jane S. Lebkowski, Ph.D., President of Research and Development, Katharine E. Spink, Ph.D., Vice President and Chief Operating Officer, and Edward D. Wirth III, Chief Medical Officer. The biographies of Mr. Cartt are included above under “Directors.”
Russell L. Skibsted, 57, became our Chief Financial Officer in March 2016, and is also the Chief Financial Officer of BioTime, Inc. ("BioTime"), which is the majority-owner of Asterias. Prior to joining BioTime in November 2015, Mr. Skibsted was Chief Financial Officer of Proove Biosciences, Inc. since 2014. From 2013 to 2014, Mr. Skibsted was Managing Director and Chief Financial Officer of RSL Ventures, where he provided financial consulting services to public and private companies in the life sciences sector. Mr. Skibsted served as Senior Vice President, Chief Financial Officer and Secretary of Aeolus Pharmaceuticals, a publicly traded biopharma company, from 2010 to 2013, and was Senior Vice President and Chief Business Officer of Spectrum Pharmaceuticals, a publicly traded biopharmaceutical company, from 2006 to 2009. From 2004 to 2006, Mr. Skibsted served as Chief Financial Officer of Hana Biosciences, and from 2000 to 2004 he served as Chief Financial Officer and Portfolio Management Partner of Asset Management Company, a venture capital firm. Mr. Skibsted holds a B.A. in economics from Claremont McKenna College and an MBA from the Stanford Graduate School of Business.
Jane S. Lebkowski, Ph.D., 59, became our President of Research and Development during March 2013 after a 13-year career at Geron where she served as Senior Vice President, Cell Therapies from 2004 to 2011, and also as Chief Scientific Officer from 2009 to 2011. From August 1999 until January 2004, Dr. Lebkowski served as Vice President of Cell Therapies, and from April 1998 until August 1999, she served as Senior Director, Cell and Gene Therapies at Geron. Dr. Lebkowski managed research and development of Geron’s immunotherapy products for cancer treatment and its human embryonic stem cell based products for regenerative medicine. Prior to joining Geron, she spent more than ten years at Applied Immune Sciences and then at Rhone Poulenc Rorer, which acquired Applied Immune Sciences in 1995, advancing from research scientist to Vice President of Research and Development. Dr. Lebkowski has co-authored numerous scientific publications. Dr. Lebkowski holds a B.S. in Chemistry and Biology from Syracuse University, and a Ph.D. from Princeton University.
Katharine E. Spink, Ph.D., 41, became our Vice President and Chief Operating Officer during March 2013, after an eight year career at Geron where she served as Senior Vice President of Alliance Management and Cell Therapy Product Development, and of Operations, Cell Therapies during 2011; Vice President of Operations, Regenerative Medicine Programs from 2009 to 2011; and Senior Director of Program Operations, Regenerative Medicine from 2008 to 2009. From January 2007 until January 2008, Dr. Spink served as Program Director for Cardiovascular Disease, and was Assistant Director, and then Associate Director of Corporate Development during 2003 to 2006. Dr. Spink holds a B.A. in Biochemistry from Rice University, and a Ph.D. in Cancer Biology from Stanford University School of Medicine.
Edward D. Wirth, III, M.D., Ph.D., 50, became our Chief Translational Officer during March 2013, and has been our Chief Medical Officer since May 2015. Prior to that Mr. Wirth served as Chief Science Officer at InVivo Therapeutics Corporation from 2011 to 2012. From 2004 to 2011, Dr. Wirth served as Medical Director for Regenerative Medicine at Geron, where he led the world’s first clinical trial of a human embryonic stem cell derived product, GRNOPC1 in patients with subacute spinal cord injuries. Dr. Wirth held academic appointments at Rush-Presbyterian St. Luke’s Medical Center and at the University of Chicago from 2002 to 2004, and was a member of the faculty of the University of Florida from 1996 to 2002. Dr. Wirth received his Ph.D. and M.D. and from the University of Florida in 1992 and 1994, respectively.
Other Key Employees
Casey C. Case, Ph.D., 60, became our Senior Vice President of Research and Nonclinical Development in March 2014. Before joining Asterias, Dr. Case served as Executive Vice President of Research at SanBio Inc. from 2006 to 2014, developing cell therapies for stroke and neurodegenerative conditions. Previously, Dr. Case was Vice President of Research and Operations at Sangamo Biosciences Inc. from 1997to 2005, Director of Cell Biology at Tularik Inc. from 1993 to 1997, and Director of Transcription Research at OSI Pharmaceuticals Inc. from 1989 to 1993. Dr. Case received his Ph.D. in Biochemistry from the University of California, Davis in 1985, and did postdoctoral research at UCLA from 1985 to 1989. Dr. Case and has over 40 issued patents.
Compensation Committee Interlocks and Insider Participation in Compensation Decisions
The compensation committee currently consists of Mr. Ricciardi (chair), Mr. Arno and Mr. LeBuhn. Our Board of Directors has determined that each member of the Compensation Committee is an independent director under, as defined in the NYSE MKT Company Guide. We did not have a Compensation Committee prior to June 24, 2013, and compensation of our executive officers during the last fiscal year prior to that date was determined by our Board of Directors. Executive officers who also served as directors did not vote on matters pertaining to their own personal compensation. None of the directors who served on the Compensation Committee during the last fiscal year was a current or former officer or employee of Asterias, or had any relationship with Asterias requiring disclosure in this report under Item 404 of SEC Regulation S-K. During last fiscal year, none of our executive officers served as (a) a member of the compensation committee (or other board committee performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers served on our Board of Directors, (b) a director of another entity, one of whose executive officers served on our Board of Directors, or (c) a member of the compensation committee (or other board committee performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers served on our Board of Directors, except Michael D. West, and Robert W. Peabody, served on the board of directors our parent company BioTime.
Summary Compensation
The following table provides information regarding the compensation earned during the fiscal years ended December 31, 2015 and December 31, 2014 by our former President and Chief Executive Officer, and the two next most highly compensated executive officers (collectively, our “Named Executive Officers”).
SUMMARY COMPENSATION TABLE
|
Name
|
Principal Position
|
Year
|
Salary
|
Bonus
|
Option
Awards (1)
|
Stock
Awards
|
All other
Compensation (2)
|
Total
|
|||||||||||||||||||||||
|
Pedro Lichtinger (8)
|
President and Chief Executive Officer
|
2015
|
$
|
407,000
|
-
|
$
|
524,263
|
(3)
|
$
|
73,841
|
(3)
|
$
|
9,000
|
$
|
1,014,104
|
||||||||||||||||
|
2014
|
$
|
224,359
|
-
|
$
|
1,409,456
|
(4)
|
$
|
468,000
|
(4)
|
$
|
8,333
|
$
|
2,110,148
|
||||||||||||||||||
|
Katharine E. Spink
|
Vice President and Chief Operating Officer
|
2015
|
$
|
267,500
|
-
|
$
|
622,848
|
(5)(6)
|
$
|
87,432
|
(7)
|
$
|
13,250
|
$
|
991,030
|
||||||||||||||||
|
2014
|
$
|
250,000
|
-
|
-
|
-
|
$
|
12,500
|
$
|
262,500
|
||||||||||||||||||||||
|
2013
|
$
|
157,500
|
-
|
293,898
|
(6)
|
-
|
$
|
5,156
|
$
|
456,554
|
|||||||||||||||||||||
|
Georgia Erbez (9)
|
Vice President and Chief Financial Officer
|
2015
|
$
|
47,596
|
-
|
$
|
1,304,979
|
(9)
|
-
|
-
|
$
|
1,352,575
|
|||||||||||||||||||
| (1) | Option awards are valued at the aggregate grant date fair value, as if all options were fully vested and exercisable at the date of grant. Values are computed in accordance with FASB Accounting Standards Codification (ASC) Topic 718. We used the Black-Sholes-Merton Pricing Model to compute option fair values based on applicable exercise and stock prices, an expected option term ranging from 4.52 to 6.08 years, volatility ranging from 75.03% to 78.38%, and bond equivalent yield discount rates ranging from 0.66% to 1.905% depending on the date of grant. |
| (2) | Other compensation consists entirely of employer contributions to employee accounts under BioTime’s 401(k) plan in which employees of BioTime subsidiaries, including Asterias, are entitled to participate. |
| (3) | Mr. Lichtinger was granted options to purchase 200,000 Series A Shares under the 2013 Equity Incentive Plan. In addition, Mr. Lichtinger received 17,949 RSUs. These RSUs and options were granted under Asterias’ 2013 Equity Incentive Plan. The options are subject to time-based vesting whereby one quarter of the options shall vest upon completion of 12 full months of continuous employment of Mr. Lichtinger by Asterias measured from the grant date, and the balance of the options shall vest in 36 equal monthly installments commencing on the first anniversary of the date of grant, based upon the completion of each month of continuous employment of Mr. Lichtinger by Asterias. Each RSU represents a contingent right to receive one share of Asterias' Series A Shares. The RSUs are subject to time-based vesting in four equal quarterly payments beginning on June 30, 2015. In connection with the separation agreement entered into with Mr. Lichtinger on March 10, 2016, half of the unvested options granted to Mr. Lichtinger were cancelled following his departure from Asterias on February 29, 2016. |
| (4) | Mr. Lichtinger was granted was granted options to purchase 1,000,000 Series A Shares and 200,000 shares of restricted stock at $2.34 per share under the 2013 Equity Incentive Plan in 2014. The restricted stock was subject to restrictions on transfer and to forfeiture until the shares vested. The restricted stock vested at the rate of 16,667 shares per month while Mr. Lichtinger remained employed by Asterias. The options are subject to time-based vesting in equal monthly installments over a four-year period beginning on the one month anniversary from the date of the grant, provided that Mr. Lichtinger remains employed with Asterias through the vesting dates. All of the unvested options granted to Mr. Lichtinger were cancelled and all of the unexercised vested options were forfeited following his departure from Asterias on February 29, 2016. |
| (5) | Dr. Spink was granted options to purchase 100,000 Series A Shares under the 2013 Equity Incentive Plan in 2015. The options are exercisable at an exercise price of $5.45 per share. The options are subject to time-based vesting in equal calendar quarterly installments over a four-year period beginning December 1, 2015, provided that Dr. Spink remains employed with Asterias through the vesting dates. |
| (6) | Dr. Spink was granted options to purchase 100,000 Series A Shares and 200,000 Series A Shares under the 2013 Equity Incentive Plan in 2015 and 2013 respectively. The options are exercisable at an exercise price of $3.90 per share and $2.34 per share for 2015 and 2013, respectively. The 2015 options are subject to time-based vesting whereby one quarter of the options shall vest upon completion of 12 full months of continuous employment of Dr. Spink by Asterias measured from the grant date, and the remaining balance of the options shall vest in 36 equal monthly installments commencing on the first anniversary of the date of grant, based upon the completion of each month of continuous employment of Dr. Spink by Asterias The 2013 options are subject to time-based vesting in equal monthly installments over a four-year period beginning on the one month anniversary from the date of the grant, provided that Dr. Spink remains employed with Asterias through the vesting dates. |
| (7) | Dr. Spink received 21,153 RSUs in 2015. These RSUs and options were granted under Asterias’ 2013 Equity Incentive Plan. Each RSU represents a contingent right to receive one share of Asterias’ Series A Shares. The RSUs are subject to time-based vesting in four equal quarterly payments beginning on June 30, 2015. Each RSU represents a contingent right to receive one share of Asterias’ Series A Shares. |
| (8) | Half of the unvested options granted to Mr. Lichtinger were cancelled following his departure from Asterias on February 29, 2016. |
| (9) | Ms. Erbez was appointed as our Chief Financial Officer on November 9, 2015. As a part of Ms. Erbez’s initial compensation package, she was granted options to purchase 380,000 Series A Shares under the 2013 Equity Incentive Plan. The options are exercisable at an exercise price of $5.16 per share. The options are subject to time-based vesting whereby one quarter of the options shall vest upon completion of 12 full months of continuous employment of Ms. Erbez by Asterias measured from the grant date, and the balance of the options shall vest in 36 equal monthly installments commencing on the first anniversary of the date of grant, based upon the completion of each month of continuous employment of Ms. Erbez by Asterias. All of the unvested options granted to Ms. Erbez were cancelled upon her departure from Asterias as of March 2016. |
Stock Options Outstanding at Year End
The following table summarizes certain information concerning Asterias stock options held by our Named Executive Officers as of December 31, 2015.
|
Name
|
Number of
Securities
Underlying
Unexercised
Options
Exercisable
|
Number of
Securities
Underlying
Unexercised
Options
Unexercisable
|
Option
Exercise
Price
|
Option
Expiration
Date
(1)
|
Number of
Shares or
Units of
Stock that
have not
Vested
(2)
|
Market
Value of
Shares or
Units of
Stock
that have
not
Vested
|
|||||||||||||||
|
437,500
|
562,500
|
(1)
|
$
|
2.34
|
June 8, 2021
|
||||||||||||||||
|
Pedro Lichtinger(5)
|
|||||||||||||||||||||
| - |
200,000
|
(3)
|
$
|
3.90
|
February 12, 2025
|
||||||||||||||||
|
5,983
|
$
|
23,513
|
|||||||||||||||||||
|
133,333
|
66,667
|
(1)
|
$
|
2.34
|
March 9, 2020
|
||||||||||||||||
|
Katharine E. Spink
|
|||||||||||||||||||||
| - |
100,000
|
(3)
|
$
|
3.90
|
February 12, 2025
|
||||||||||||||||
|
12,500
|
87,500
|
(4)
|
$
|
5.45
|
November 3, 2025
|
||||||||||||||||
|
7,052
|
$
|
27,714
|
|||||||||||||||||||
|
Georgia Erbez(6)
|
-
|
380,000
|
(3)
|
$
|
5.16
|
November 8, 2025
|
-
|
-
|
|||||||||||||
| (1) | These options are exercisable for Asterias Series A Shares and become exercisable in 48 equal monthly installments during the term of employment by Asterias. |
| (2) | These RSUs are subject to time-based vesting and will vest entirely on March 31, 2016. |
| (3) | These options are subject to time-based vesting whereby one quarter of the options shall vest upon completion of 12 full months of continuous employment by Asterias measured from the grant date, and the balance of the options shall vest in 36 equal monthly installments commencing on the first anniversary of the date of grant, based upon the completion of each month of continuous employment by Asterias. |
| (4) | These options are subject to time-based vesting in equal quarterly installments over a four-year period beginning December 1, 2015, provided that Dr. Spink remains employed with Asterias through the vesting dates. |
| (5) | Half of the unvested options granted to Mr. Lichtinger were cancelled following his departure from Asterias on February 29, 2016. |
| (6) | All of the options granted to Ms. Erbez were unvested and cancelled upon her departure from Asterias as of March 2016. |
Other Compensation Plans
We do not have any pension plans, defined benefit plans, or non-qualified deferred compensation plans. We do make contributions to 401(k) plans for participating executive officers and other employees.
Risk Considerations and Recoupment Policies
The Compensation Committee considers, in establishing and reviewing the executive compensation program, whether the program encourages unnecessary or excessive risk taking. Our executive compensation arrangements include a fixed salary that provides a steady income so that executives do not feel pressured to focus exclusively on stock price performance or short term financial targets to the detriment of our long-term operational and strategic objectives. We supplement fixed salaries with discretionary bonus awards based on the executive’s performance as well as the performance of Asterias, and bonus awards based on Astrerias’ receipt of research grant funding. The stock options that we have granted to our executive officers under the Equity Incentive Plan vest over four years, assuring that the executives take a long-term perspective in viewing their equity ownership.
Because Asterias has not adopted compensation plans, or made incentive awards, based on quantified financial performance measures, we have not adopted specific policies regarding the adjustment or recovery of awards or payments if the relevant performance measures are restated or otherwise adjusted in a manner that would reduce the size of an award or payment. We may adopt such policies, however, if we adopt incentive compensation plans or grant incentive bonuses based on financial performance measures.
Tax Considerations
Section 162(m) of the Internal Revenue Code places a $1 million limit on the amount of compensation that a company can deduct in any one year for compensation paid to its chief executive officer and the three most highly-compensated executive officers employed by the company at the end of the year, other than the company’s chief financial officer. The $1 million deduction limit does not apply to compensation that is performance-based and provided under a shareholder-approved plan. The Compensation Committee has never awarded cash compensation, in the form of salary and bonuses, in excess of the $1 million limit. Asterias’ stock option awards are designed to qualify for tax deductibility. Notwithstanding the foregoing, we may elect to pay compensation to executive officers that may not be fully deductible if we believe that is necessary to attract, retain and reward high-performing executives.
Compensation of Directors
Directors and members of committees of the Board of Directors who are our salaried employees or officers are entitled to receive compensation as employees or officers but are not compensated for serving as directors or attending meetings of the Board or committees of the Board. All directors are entitled to reimbursements for their out-of-pocket expenses incurred in attending meetings of the Board or committees of the Board.
For 2014, each director who is not an Asterias officer or employee, other than the Chairman of the Board of Directors, received an annual fee of $15,000 in cash, plus a fee of $1,000 for regular or special meeting of the Board attended in person and $500 for regular or special meeting of the Board attended by telephone conference, and options to purchase 20,000 Series A Shares under our 2013 Equity Incentive Plan. For 2014, the Chairman of the Board of Directors received an annual fee of $50,000 in cash, plus $1,000 for each regular or special meeting of the Board attended in person and $500 for regular or special meeting of the Board attended by telephone conference, and options to purchase 75,000 Series A Shares under our 2013 Equity Incentive Plan.
The annual fee of cash will be paid, and the stock options granted will vest and become exercisable, in four equal quarterly installments, provided that the director remains a director on the last day of the applicable quarter. The options will expire if not exercised five years from the date of grant.
Directors who serve on the Audit Committee and the Compensation Committee shall receive, in addition to other fees payable to them as directors, the following annual fees:
| · | Audit Committee Chairman: $10,000 |
| · | Audit Committee Member other than Chairman: $7,000 |
| · | Compensation Committee Chairman: $7,500 |
| · | Compensation Committee Member other than Chairman: $5,000 |
| · | Nominating and Corporate Governance Committee Chairman: $7,500 |
| · | Nominating and Corporate Governance Committee Member other than Chairman: $5,000 |
Members of the Audit Committee and Compensation Committee receive a fee of $1,000 for meetings attended in person and $500 for meetings attended by telephone conference.
Because we are a subsidiary of BioTime, directors are also eligible to receive stock options or to purchase restricted stock under the BioTime’s 2012 Equity Incentive Plan. An award to any of our directors under BioTime’s 2012 Equity Incentive Plan may be made only if approved by the BioTime Board of Directors or by its compensation committee.
The following table summarizes certain information concerning the compensation paid during the past fiscal year to each of the current members of the persons who served as directors during the year ended December 31, 2015 and who were not employees of Asterias or BioTime on the date the compensation was earned.
|
Name
|
Fees
Earned or
Paid in
Cash
|
Option
Awards (1)
|
Total
|
|||||||||
|
Alfred D. Kingsley(2)
|
$
|
57,500
|
$
|
143,991
|
$
|
201,491
|
||||||
|
Andrew Arno
|
$
|
48,000
|
$
|
63,002
|
$
|
111,002
|
||||||
|
Richard LeBuhn
|
$
|
51,500
|
$
|
63,002
|
$
|
114,502
|
||||||
|
Natale S. Ricciardi
|
$
|
51,500
|
$
|
63,002
|
$
|
114,502
|
||||||
|
Robert W. Peabody(3)
|
$
|
4,750
|
$ | - |
$
|
4,750
|
||||||
| (1) | Those of our directors who were serving on the Board of Directors on April 1, 2015 and who are not salaried employees of Asterias each received an award of stock options entitling them to purchase 20,000 Series A Shares as partial compensation for serving on the Board of Directors for a period of one year, except Mr. Kingsley. The options will vest and become exercisable in equal quarterly installments over a one-year period, but must be reported here at the aggregate grant date fair value, as if all options were fully vested and exercisable at the date of grant. Values are computed in accordance with FASB Accounting Standards Codification (ASC) Topic 718. We used the Black-Sholes-Merton Pricing Model to compute option fair values. The assumptions underlying the valuation of the Asterias options are as follows: stock price of $6.22, exercise price of $6.22, expected term of 2.72 years, volatility of 81.91%, and a bond equivalent yield discount rate of 0.86%. |
| (2) | Mr. Kingsley received 75,000 stock options as partial compensation for serving in his capacity as Chairman of the Board. The assumptions underlying the valuation of the Asterias options are as follows: stock price of $3.85, exercise price of $3.90, expected term of 2.72 years, volatility of 80.82%, and a bond equivalent yield discount rate of 1.03%. |
| (3) | Payment of director fees to Mr. Peabody began following his resignation as Chief Financial Officer of BioTime and Astrerias in November 2015. |
Employment Agreements; Change in Control Payments
Pedro Lichtinger
Mr. Lichtinger was appointed as our President and Chief Executive Officer on June 9, 2014 and we entered into an employment agreement with Mr. Lichtinger, whereby Mr. Lichtinger was paid an annual base salary of $400,000 per year. In February 2015, Mr. Lichtinger received a $7,000 increase to his annual salary, which was pro-rated based on his June 9, 2014 hiring date. Mr. Lichtinger was granted options under our Equity Incentive Plan to purchase 1,000,000 shares of our Series A Shares. The options granted have an exercise price of $2.34 per share, which is based on the fair market value of our Series A Shares as determined by our Board of Directors, and will vest, and thereby become exercisable, in 48 equal monthly installments based upon Mr. Lichtinger’s continued employment, and will expire if not exercised in seven years from the date of grant. In addition, Mr. Lichtinger received 200,000 shares of “Restricted Stock” under our Equity Incentive Plan which are subject to restrictions on transfer and to forfeiture until the shares vest. The Restricted Stock will vest at the rate of 16,667 shares per month while Mr. Lichtinger remains employed by us. Mr. Lichtinger may receive salary increases, bonuses, and additional awards of stock options and Restricted Stock approved by the Board of Directors, implementing a compensation philosophy which targets the 50th percentile of a group of comparator companies selected by the Board of Directors or a compensation committee of the Board of Directors, and in the case of bonuses, based on the attainment of goals or milestones set by the Board of Directors or a compensation committee of the Board of Directors.
Mr. Lichtinger’s employment agreement contains provisions entitling him to severance benefits under certain circumstances. If we terminate Mr. Lichtinger’s employment without “cause” or if he resigns for “good reason” as those terms are defined in his employment agreement, he will be entitled to severance benefits. If Mr. Lichtinger has been employed by Asterias for one year or less, his severance benefits will be payment of three months base salary. If he has been employed by us for more than one year, his severance benefits will be payment of six months base salary and 50% of his then unvested Asterias stock options will vest. The cash severance compensation may be paid in a lump sum or, at our election, in installments consistent with the payment of the executive’s salary while employed by us. If Mr. Lichtinger’s employment is terminated without “cause” or if he resigns for “good reason” within twelve months following a “Change of Control,” he will be entitled to twelve months base salary, payable in a lump sum, and 100% of his then unvested Asterias options will vest and the restrictions on 100% of his Restricted Stock will expire.
Mr. Lichtinger served as our President and Chief Executive Officer through February 29, 2016. On March 10, 2016, we entered into a separation agreement with Mr. Lichtinger. Under the terms of the separation agreement, and consistent with the terms of his employment agreement we agreed to pay Mr. Lichtinger severance in the amount of $475,000 plus accrued and unpaid wages and unused vacation. In addition, the severance agreement provides for accelerated vesting of any unvested restricted stock and 50% of unvested stock options that were previously granted to Mr. Lichtinger.
Georgia Erbez
We entered into an employment agreement with Georgia Erbez, whereby Ms. Erbez was paid an annual base salary of $330,000 per year. In addition to Ms. Erbez’s annual salary, in November 2015, she received a grant of options to purchase 380,000 Series A shares (145,223 ISO and 234,777 NQSO). The options granted have an exercise price of $5.16 and are subject to time-based vesting whereby one quarter of the options shall vest upon completion of 12 full months of continuous employment of Ms. Erbez by Asterias measured from the grant date, and the remaining balance of the options shall vest in 36 equal monthly installments commencing on the first anniversary of the date of grant, based upon the completion of each month of continuous employment of Ms. Erbez by Asterias.
Ms. Erbez’s employment agreement contains provisions entitling her to severance benefits under certain circumstances. If Ms. Erbez has been employed by Asterias for one year or less, her severance benefits will be payment of three months base salary. If she has been employed by us for more than one year, her severance benefits will be payment of six months base salary and 50% of her then unvested Asterias stock options will vest. The cash severance compensation may be paid in a lump sum or, at our election, in installments consistent with the payment of the executive’s salary while employed by us. If Ms. Erbez’s employment is terminated without “cause” within twelve months following a “Change of Control,” she will be entitled to twelve months base salary, payable in a lump sum, and 100% of her then unvested Asterias options will vest. In order to receive the severance benefits, Ms. Erbez must execute a general release of all claims against us and must return all our property in her possession.
Ms. Erbez served as our Chief Financial Officer through March 11, 2016. On March 21, 2016, we entered into a separation agreement with Mr. Erbez. Under the terms of the separation agreement, and consistent with the obligations under her employment agreement, we agreed to pay Ms. Erbez severance in the amount of $110,000 plus accrued and unpaid wages and unused vacation.
Dr. Katharine E. Spink
We entered into an employment agreement with Dr. Spink, whereby Dr. Spink is paid an annual base salary of $255,000 per year, and in July 2015, Dr. Spink received a $25,000 increase in her annual salary. In addition to Dr. Spink’s annual salary, during 2015 Dr. Spink was granted options to purchase 100,000 Series A Shares under the 2013 Equity Incentive Plan in February and in November. The options granted in February are exercisable at an exercise price of $3.90 per share and are subject to time-based vesting whereby one quarter of the options shall vest upon completion of 12 full months of continuous employment of Dr. Spink by Asterias measured from the grant date, and the remaining balance of the options shall vest in 36 equal monthly installments commencing on the first anniversary of the date of grant, based upon the completion of each month of continuous employment of Dr. Spink by Asterias. The options granted in November are exercisable at an exercise price of $5.45 per share and are subject to time-based vesting in equal calendar quarterly installments over a four-year period beginning December 1, 2015, provided that Dr. Spink remains employed with Asterias through the vesting dates.
Dr. Spink’s employment agreement contains provisions entitling her to severance benefits under certain circumstances. If we terminate Dr. Spink’s employment without “cause” as those terms are defined in her employment agreement, she will be entitled to severance benefits. If Dr. Spink has been employed by Asterias for one year or less, her severance benefits will be payment of three months base salary. If she has been employed by us for more than one year, her severance benefits will be payment of six months base salary and 50% of her then unvested Asterias stock options will vest. The cash severance compensation may be paid in a lump sum or, at our election, in installments consistent with the payment of the executive’s salary while employed by us. If Dr. Spink's employment is terminated without “cause” within twelve months following a “Change of Control,” she will be entitled to twelve months base salary, payable in a lump sum, and 100% of her then unvested Asterias options will vest and the restrictions on 100% of her Restricted Stock will expire.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management, and Related Stockholder Matters |
Equity Compensation Plan Information
SECURITIES AUTHORIZED FOR ISSUANCE UNDER 2013 EQUITY INCENTIVE PLAN
The following table provides information as of December 31, 2015 about our Series A Shares that may be issued upon the exercise of options, warrants and rights under our existing equity compensation plan (in thousands).
|
Plan category
|
Number of
securities
to be issued upon
exercise of
outstanding
options,
warrants and
rights
|
Weighted-average
exercise price of
outstanding options,
warrants and rights
|
Number of
securities
remaining
available
for future issuance
under equity
compensation
plans
|
|||||||||
|
Equity compensation plans approved by security holders
|
5,178
|
$
|
3.17
|
2,067
|
||||||||
|
Equity compensation plans not approved by security holders
|
—
|
—
|
—
|
|||||||||
|
Total
|
5,178
|
$
|
3.17
|
2,067
|
||||||||
Security Ownership of Certain Beneficial Owners and Management
The following table and accompanying footnotes present information about the beneficial ownership of our Series A Shares, as of March 23, 2016 by each shareholder known by us to be the beneficial owner of 5% or more of our common stock. Information concerning certain beneficial owners of more than 5% of the common stock is based upon information disclosed by such owners in their reports on Schedule 13D or Schedule 13G. Under Rule 13d-3, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights. As a result, the percentage of outstanding shares of any person as shown in this table does not necessarily reflect the person’s actual ownership or voting power with respect to the number of shares of common stock actually outstanding as of the date of this Annual Report. As of the date of this Annual Report, there were 38,352,150 Series A Shares issued and outstanding.
Except as indicated in footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them, based on information provided to us by such stockholders.
Security Ownership of Certain Beneficial Owners
|
Number of Shares
|
Percent
of Total
|
|||||||
|
BioTime, Inc.
1301 Harbor Bay Parkway, Suite 100
Alameda, CA 94502
|
21,747,569
|
56.70
|
%
|
|||||
|
Romulus Films, Ltd(1)
Wessex House
1 Chesham Street
London SW1X 8ND
United Kingdom
|
2,913,430
|
7.53
|
%
|
|||||
| (1) | Includes 2,563,430 Series A Shares held by Romulus Films and affiliates and 350,000 Series A Shares that Romulus Films may acquire upon the exercise of warrants at $5.00 per share expiring on September 30, 2016. |
The following table and accompanying footnotes present information about the beneficial ownership of our Series A Shares, as of March 23, 2016 by each of our Named Executive Officers, directors, and all our executive officers and directors as a group.
Security Ownership of Management
|
Number of Shares
|
Percent of Total
|
|||||||
|
Stephen L. Cartt(1)
|
41,666
|
*
|
||||||
|
Russell L. Skibsted
|
-
|
*
|
||||||
|
Pedro Lichtinger(2)
|
1,367,521
|
3.49
|
%
|
|||||
|
Katharine E. Spink(3)
|
207,590
|
*
|
||||||
|
Georgia Erbez(4)
|
-
|
*
|
|
|||||
|
Robert W. Peabody(5)
|
112,772
|
*
|
||||||
|
Michael D. West(6)
|
86,377
|
*
|
||||||
|
Judith Segall(7)
|
13,125
|
*
|
||||||
|
Alfred D. Kingsley(8)
|
230,000
|
*
|
||||||
|
Richard LeBuhn(9)
|
45,000
|
*
|
||||||
|
Andrew Arno(9)
|
45,000
|
*
|
||||||
|
Natale S. Ricciardi(9)
|
45,000
|
*
|
||||||
|
Don Bailey(10)
|
18,750
|
*
|
||||||
|
All executive officers and directors as a group (13 persons)(11)
|
1,334,102
|
3.36
|
%
|
|||||
* Less than 1%
| (1) | Includes 41,666 Series A Shares that may be acquired upon the exercise of certain stock options that will become exercisable within 60 days. Excludes 958,334 Series A Shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. Excludes 200,000 shares of Restricted Stock as to which the restrictions on transfer have not expired or will not expire within 60 days. |
| (2) | Includes 328,205 Series A Shares owned by Mr. Lichtinger, 200,000 shares of Restricted Stock as to which the restrictions on transfer have expired, 833,333 Series A Shares that may be acquired upon the exercise of certain stock options, and 5,983 Restricted Stock Units (“RSUs”) granted in February 2015 under Asterias’ 2013 Equity Incentive Plan. Mr. Lichtinger is no longer our President and Chief Executive Officer and has resigned from our Board of Directors. His beneficial ownership information is provided herewith because he is a Named Executive Officer during 2015. |
| (3) | Includes 177,726 Series A Shares that may be acquired upon the exercise of certain stock options, 22,812 Series A Shares that may be acquired upon the exercise of stock options that will become exercisable within 60 days and 7,052 RSUs granted in February 2015 under Asterias’ 2013 Equity Incentive Plan that will vest within 60 days. Each RSU represents a contingent right to receive one share of Asterias’ Series A Shares. The RSUs are subject to time-based vesting in four equal quarterly payments beginning on June 30, 2015. Excludes 364,464 Series A Shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
| (4) |
Ms. Erbez is no longer the Chief Financial Officer of the Company, and her beneficial ownership information is provided herewith because she was a Named Executive Officer during 2015.
|
| (5) | Includes 41 Series A Shares owned by Mr. Peabody, 99,478 Series A Shares that may be acquired upon the exercise of certain stock options, 12,292 Series A Shares that may be acquired upon the exercise of stock options that will become exercisable within 60 days and 961 RSUs granted in February 2015 under Asterias’ 2013 Equity Incentive Plan that will vest within 60 days. Each RSU represents a contingent right to receive one share of Asterias’ Series A Shares. The RSUs are subject to time-based vesting in four equal quarterly payments beginning on June 30, 2015. Excludes 83,230 Series A Shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
| (6) | Includes 80,416 Series A Shares that may be acquired upon the exercise of certain stock options, 5,000 Series A Shares that may be acquired upon the exercise of stock options that will become exercisable within 60 days and 961 RSUs granted in February 2015 under the Asterias’ 2013 Equity Incentive Plan that will vest within 60 days. Each RSU represents a contingent right to receive one share of Asterias’ Series A Shares. The RSUs are subject to time-based vesting in four equal quarterly payments beginning on June 30, 2015. Excludes 34,584 Series A Shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
| (7) | Includes 11,874 Series A Shares that may be acquired upon the exercise of certain stock options and 1,251 Series A Shares that may be acquired upon the exercise of stock options that will become exercisable within 60 days. Excludes 16,875 Series A Shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
| (8) | Includes 225,000 Series A Shares that may be acquired upon the exercise of certain stock options and 5,000 Series A Shares that may be acquired upon the exercise of stock options that will become exercisable within 60 days. Excludes 15,000 Series A Shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
| (9) | Includes 35,000 Series A Shares that may be acquired upon the exercise of certain stock and 10,000 Series A Shares that may be acquired upon the exercise of stock options that will become exercisable within 60 days. Excludes 15,000 Series A Shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
| (10) | Includes 18,750 Series A Shares that may be acquired upon the exercise of stock options that will become exercisable within 60 days. Excludes 56,250 Series A Shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
| (11) | Includes 476 Series A Shares owned by Dr. Lebkowski, 41 Series A Shares owned by Mr. Peabody, 1,144,284 Series A Shares that may be acquired upon the exercise of certain stock options, 168,022 Series A Shares that may be acquired upon the exercise of certain stock options that will become exercisable within 60 days, and 21,279 RSUs granted in February 2015 under Asterias’ 2013 Equity Incentive Plan that will vest within 60 day. Excludes 1,847,694 Series A Shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days, 200,000 shares of Restricted Stock as to which the restrictions have not expired and will not expire within 60 days and beneficial ownership of Pedro Lichtinger and Georgia Erbez, who are not an executive officer or a director as of the date of this report. |
The following is a description of transactions, during the 2015 fiscal year, to which we have been a party, in which the amount involved exceeded or will exceed $120,000, and in which any related person had a direct or indirect material interest.
2015 Private Placement
In February 2015, Asterias raised approximately $5.5 million in aggregate gross proceeds from the sale of 1,410,255 Series A Shares at a price of $3.90 per share through an underwritten public offering and a private placement. Broadwood Partners, L.P., British & American Investment Trust PLC and Pedro Lichtinger purchased an aggregate of 1,025,640 of the shares. Broadwood Partners, L.P. is BioTime’s largest shareholder, of its directors, Neal C. Bradsher, is President of Broadwood Capital, Inc., the investment manager of Broadwood Partners, L.P., and one of Asterias’ directors, Richard T. LeBuhn, is Senior Vice President of Broadwood Capital, Inc., Pedro Lichtinger is Asterias’ Chief Executive Officer and a member of its Board of Directors. British & American Investment Trust PLC is an affiliate of a stockholder of Asterias and BioTime.
Cross License Agreements and Related Share Transfer Agreement
On February 16, 2016, we entered into a Cross-License Agreement (the “Cross-License”) with our majority parent, BioTime, Inc. ("BioTime") and BioTime's wholly owned subsidiary ES Cell International Pte Ltd (“ESI”). Under the terms of a Cross-License, we received a fully-paid, non-royalty-bearing, world-wide, non-exclusive, sub-licensable license under certain BioTime patents and related patent rights and ESI patents and related patent rights specified in the Cross-License, for all purposes in the Asterias Licensed Field during the term of the license. The Asterias Licensed Field includes all therapeutic applications of use for certain BioTime patents and ESI patents except all therapeutic applications of use involving pluripotent stem cell-derived cells of the following lineages: (a) bone and orthopedic soft tissues, including but not limited to ligament, tendon, meniscus, cartilage, and intervertebral disc; (b) vascular endothelium and perivascular cells including vascular smooth muscle and vascular pericytes; (c) adipose tissue; and (d) retinal pigment epithelium. The Asterias Licensed Field also includes all applications of use for certain other BioTime patents involving live human pluripotent stem cell-derived cell therapies directed to the neural spinal cord (excluding cartilage and bone of the spine) and the myocardium; and also live human pluripotent stem cell-derived glial cell therapies directed to the central nervous system.
Under the terms of the Cross-License, BioTime and ESI received a fully-paid, non-royalty-bearing, world-wide, non-exclusive, sub-licensable license in, to, and under the certain Asterias patents and related patent rights for all purposes in the BioTime/ESI Licensed Field during the term of the license. The BioTime/ESI Licensed Field includes all fields of use except any and all applications (a) to treat disorders of the nervous system, and (b) utilizing the immune system to prevent, treat, or cure cancer, and (c) involving the use of cells comprising, derived from, or manufactured using, human embryonic stem cells or human induced pluripotent stem cells for in vitro assay applications, including but not limited to drug discovery and development, drug monitoring, drug toxicology testing, and consumer products testing.
We also entered into a Share Transfer Agreement with BioTime and ESI pursuant to which (a) Asterias transferred to BioTime 2,100,000 shares of common stock of OrthoCyte Corporation (“OrthoCyte) and 21,925 ordinary shares of Cell Cure Neurosciences Ltd (“Cell Cure”), each a majority-owned subsidiary of BioTime and (b) BioTime transferred to Asterias 75,771 shares of Series A Common Stock of Asterias and warrants to purchase 3,150,000 Series A common stock of Asterias at an exercise price of $5.00 per share, expiring September 30, 2016 (“Warrants”), as additional consideration for the license of patents and patent rights from Asterias under the Cross License.
The value of the consideration given and received by Asterias and BioTime was approximately $4.0 million. Asterias engaged Lake Street Capital as its independent valuation firm in the transaction. Lake Street Capital provided an valuation opinion to us Asterias in connection with the transaction.
Approval by the Board of Directors
All of the transactions described above were reviewed directly by the Board, and the Board determined whether to approve or withhold approval of each transaction. The Board applied such criteria as it determined to be appropriate in connection with its evaluation of each proposed transaction on a transaction by transaction basis, and did not have any written guidelines, other than BioTime’s Code of Ethics, governing the Board’s exercise of its discretion. The directors considered such factors as they deemed relevant to the particular transaction.
During March 2013, we adopted a Related Person Transaction Policy that will apply to transactions exceeding $120,000 in which any of our officers, directors, beneficial owners of more than 5% of our common stock, or any member of their immediate family, has a direct or indirect material interest, determined in accordance with the policy (a “Related Party Transaction”). However, the Related Party Transaction will not include transactions between us and BioTime or any subsidiary of BioTime.
A Related Person Transaction must be reported to our outside legal counsel, and will be subject to review and approval by our “Disinterested Directors,” prior to effectiveness or consummation, to the extent practical. In addition, any Related Person Transaction that is ongoing in nature will be reviewed by our Disinterested Directors annually to ensure that the transaction has been conducted in accordance with any previous approval and that all required disclosures regarding the transaction are made. A “Disinterested Director” is defined in our Related Person Transaction Policy as a member of our Board of Directors who (a) does not have a financial interest (directly or through any trust or beneficial ownership of more than 5% of any class of voting securities of any business entity) in the Related Person Transaction, and (b) does not have an Immediate Family Member with a financial interest (directly or through any trust or beneficial ownership of more than 5% of any class of voting securities of any business entity) in the Related Person Transaction.
As appropriate for the circumstances, the Disinterested Directors will review and consider:
| · | the interest of the officer, director, beneficial owner of more than 5% of any class of our voting securities, or any member of their immediate family (“Related Person”) in the Related Person Transaction; |
| · | the approximate dollar value of the amount involved in the Related Person Transaction; |
| · | the approximate dollar value of the amount of the Related Person’s interest in the transaction without regard to the amount of any profit or loss; |
| · | whether the transaction was undertaken in the ordinary course of our business; |
| · | whether the transaction with the Related Person is proposed to be, or was, entered into on terms no less favorable to us than terms that could have been reached with an unrelated third party; |
| · | the purpose of, and the potential benefits to the transaction to us; and |
| · | any other information regarding the Related Person Transaction or the Related Person in the context of the proposed transaction that would be material to investors in light of the circumstances of the particular transaction. |
Our Disinterested Directors will review all relevant information available to it about a Related Person Transaction. Our Disinterested Directors may approve or ratify the Related Person Transaction only if they determine that, under all of the circumstances, the transaction is in, or is not in conflict with, our best interests. Our Disinterested Directors may, in their sole discretion, impose such conditions as they deem appropriate on us or the Related Person in connection with approval of the Related Person Transaction.
Following the creation of our Audit Committee in June 2013, the Audit Committee makes all determinations under the Related Person Transaction Policy instead of our Disinterested Directors.
The information required by Item 407(a) of Regulation S-K on Director Independence is incorporated herein by reference to "Corporate Governance" in this Form 10-K/A.
Director Independence
Asterias Biotherapeutics is a “controlled company” under the Corporate Governance Rules of the NYSE MKT, which means that a majority of our issued and outstanding voting stock is controlled by a single person or related group of persons (namely BioTime), and that Asterias has elected controlled company status.
A controlled company is exempted from certain rules otherwise applicable to companies whose securities are listed on NYSE MKT, including (i) the requirement that a company have a majority of independent directors, (ii) the requirement that nominations to Asterias’ Board be either selected or recommended by a nominating committee consisting solely of independent directors, and (iii) the requirement that officers’ compensation be either determined or recommended by a compensation committee consisting solely of independent directors.
Pursuant to the NYSE MKT Company Guide, no director qualifies as independent until the Board makes an affirmative determination to that effect. In making this determination, the Board must affirmatively conclude that the director does not have a material relationship with us that would interfere with the exercise of his or her independent judgment in carrying out the responsibilities of a director. The Board considers, among other factors, the director’s current and historic relationships with us and our competitors, suppliers, customers and auditors, including compensation directly or indirectly paid to the director; the director’s professional and family relationships with management and other directors; the relationships that the director’s current and former employers may have with us; and the relationships between us and other companies of which the director may be a director or executive officer.
We have four independent directors on our Board. After considering the factors described in the previous paragraph, the Board has determined that Richard T. LeBuhn, Andrew Arno, Natale S. Ricciardi and Don Bailey are independent. The NYSE MKT Company Guide requires that the independent directors meet on a regular basis as often as necessary to fulfill their responsibilities, including at least annually in executive session. In addition to their meetings as members of the Audit and Compensation Committees, of which they are the sole members, Mr. LeBuhn, Mr. Arno and Mr. Ricciardi meet at least annually without the presence of non–independent directors and management in person in fulfillment of this NYSE MKT requirement during fiscal year 2014, in executive session each time.
Michael D. West and Judith Segall do not qualify as “independent” because they are full-time employees of our parent company, BioTime. Alfred D. Kingsley does not qualify as “independent” because he served for a period of time as our Executive Chairman and also because he received compensation for serving in an executive role as Chairman of certain of BioTime’s subsidiaries. Robert W. Peabody does not qualify as "independent" because he was our Chief Financial Officer within the past three years. Stephen L. Cartt does not qualify as "independent" because he is currently our President and Chief Executive Officer.
Compliance with Section 16(A) of the Securities Exchange Act of 1934
Section 16(a) of Exchange Act, requires our directors and executive officers and persons who own more than ten percent (10%) of a registered class of our equity securities to file with the SEC initial reports of ownership and reports of changes in ownership of common shares and other Asterias equity securities. Officers, directors and greater than ten percent beneficial owners are required by SEC regulations to furnish us with copies of all reports they file under Section 16(a).
To our knowledge, based solely on our review of the copies of such reports furnished to us, all Section 16(a) filing requirements applicable to our officers, directors, and greater than ten percent beneficial owners were complied with during the fiscal year ended December 31, 2014.
General
OUM & Co., LLP (“OUM”) served as our independent registered public accountants and audited our annual financial statements for the fiscal years ended December 31, 2015 and 2014.
On June 30, 2014, KPMG LLP acquired certain assets of ROTHSTEIN-KASS, P.A. (d/b/a Rothstein Kass & Company, P.C.) and certain of its affiliates (“Rothstein Kass”), our independent registered public accounting firm. As a result of this transaction, on June 30, 2014, Rothstein Kass resigned as our independent registered public accounting firm.
Rothstein Kass had audited our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2013, and for the periods from inception (September 24, 2012) to December 31, 2012 and December 31, 2013, as set forth in their report. Following their resignation, our Board of Directors approved the engagement of OUM as our new independent registered public accounting firm.
The audit reports of Rothstein Kass on Asterias’ financial statements for the years ended December 31, 2013 and December 31, 2012 did not contain an adverse opinion or a disclaimer of opinion, and were not qualified or modified as to uncertainty, audit scope or accounting principles.
During the fiscal years ended December 31, 2013 and December 31, 2012, and through the subsequent interim period preceding Rothstein Kass’ resignation, there were no disagreements between us and Rothstein Kass on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedures, which disagreements, if not resolved to the satisfaction of Rothstein Kass would have caused them to make reference thereto in their reports on our financial statements for such years.
During the fiscal years ended December 31, 2013 and to December 31, 2012, and through the subsequent interim period preceding Rothstein Kass’ resignation, there were no reportable events within the meaning set forth in Item 304(a)(1)(v) of Regulation S-K.
During the fiscal years ended December 31, 2013 and December 31, 2012, and through the subsequent interim period preceding OUM’s engagement, we did not consult with OUM on either (1) the application of accounting principles to a specified transaction, either completed or proposed; or the type of audit opinion that may be rendered on our financial statements, and OUM did not provide either a written report or oral advise to us that OUM concluded was an important factor considered by us in reaching a decision as to the accounting, auditing or financial reporting issue; or (2) any matter that was either the subject of a disagreement, as defined in Item 304(a)(1)(iv) of Regulation S-K, or a reportable event, as defined in Item 304(a)(1)(v) of Regulation S-K.
The following table presents the aggregate fees billed for professional services rendered by Rothstein Kass and OUM for the year ended December 31, 2014 and December 31, 2013.
Audit Fees, Audit Related Fees, Tax Fees and Other Fees
The following table sets forth the aggregate fees billed to Asterias during the fiscal years ended December 31, 2015 and 2014 by Rothstein Kass, OUM, and Bolar, Hirsh, and Jennings:
|
Fiscal
2015 ($)
|
Fiscal
2014 ($)
|
|||||||
|
Audit Fees (1)
|
$
|
309,000
|
$
|
372,000
|
||||
|
Audit Related Fees (2)
|
68,200
|
62,800
|
||||||
|
Tax Fees (3)
|
20,500
|
60,300
|
||||||
|
Total Fees
|
$
|
397,700
|
$
|
495,100
|
||||
| (1) | Audit Fees consist of fees billed for professional services rendered for the audit of Asterias’ consolidated annual financial statements included in our Annual Report on Form 10-K and review of the interim consolidated financial statements included in our Quarterly Reports on Form 10-Q and services that are normally provided by our independent registered public accountants in connection with statutory and regulatory filings or engagements. For the fiscal years ended December 31, 2015 and 2014, aggregate fees for professional services billed by Rothstein Kass were $0 and $125,000, respectively and by OUM were $309,000 and $247,000, respectively. |
| (2) | Audit-Related Fees consist of fees billed for assurance and related services that are reasonably related to the performance of the audit or review of Asterias’ consolidated financial statements and are not reported under “Audit Fees.” This category includes fees related to non-routine SEC filings. For the fiscal years ended December 31, 2015 and 2014, aggregate fees for professional services billed by Rothstein Kass were $62,200 and $55,800, respectively and by OUM were $6,000 and $7,000, respectively. |
| (3) | Tax Fees were billed for services including assistance with tax compliance and the preparation of tax returns, tax consultation services, assistance in connection with tax audits and tax advice related to mergers, acquisitions and dispositions. For the fiscal years ended December 31, 2015 and 2014, fees for professional services billed by Rothstein Kass were $0 and $10,000, respectively, by Bolar, Hirsh, and Jennings were $20,500 and 50,300, respectively, and none by OUM. |
Pre-Approval of Audit and Permissible Non-Audit Services
Our Audit Committee has considered whether the provisions of services described in the table above are compatible with maintaining auditor independence. Our Audit Committee requires pre-approval of all audit and non-audit services in one of two methods, and each of the permitted non-auditing services described above has been pre-approved by the Audit Committee. Under the first method, the engagement to render the services would be entered into pursuant to pre-approval policies and procedures established by the Audit Committee, provided (i) the policies and procedures are detailed as to the services to be performed, (ii) the Audit Committee is informed of each service, and (iii) such policies and procedures do not include delegation of the Audit Committee’s responsibilities under the Exchange Act to Asterias’ management. Under the second method, the engagement to render the services would be presented to and pre-approved by the Audit Committee (subject to the de minimis exceptions for non-audit services described in Section 10A(i)(1)(B) of the Exchange Act that are approved by the Audit Committee prior to the completion of the audit). The Chairman of the Audit Committee has the authority to grant pre-approvals of audit and permissible non-audit services by the independent registered public accounting firm, provided that all pre-approvals by the Chairman must be presented to the full Audit Committee at its next scheduled meeting.
The prior approval of the Board of Directors is required for the engagement of our auditors to perform any non-audit services for us. Other than de minimis services incidental to audit services, non-audit services shall generally be limited to tax services such as advice and planning and financial due diligence services. All fees for such non-audit services must be approved by the Board of Directors, except to the extent otherwise permitted by applicable SEC regulations.
PART IV
| (a-1) | Financial Statements. |
The following financial statements of Asterias Biotherapeutics, Inc. are filed in the Form 10-K:
Balance sheets
Statements of operations
Statements of comprehensive loss
Statements of stockholders' equity/(deficit)
Statements of cash flows
Notes to Financial Statements
| (a-2) | Financial Statement Schedules |
All schedules are omitted because the required information is inapplicable or the information is presented in the financial statements or the notes thereto.
| (a-3) | Exhibits. |
|
Exhibit Numbers
|
Description
|
|
|
2.1
|
Asset Contribution Agreement, dated January 4, 2013, by and among BioTime, Inc., BioTime Acquisition Corporation, and Geron Corporation. (1) Schedules to the Asset Contribution Agreement have been omitted. Asterias agrees to furnish supplementally a copy of the omitted schedules to the Commission upon request
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation (2)
|
|
|
3.2
|
Bylaws (2)
|
|
|
4.1
|
Specimen of Series A Common Stock Certificate (3)
|
|
|
4.2
|
Warrant Agreement, dated October 1, 2013, by Asterias Biotherapeutics, Inc. for the benefit of BioTime, Inc. (4)
|
|
|
4.3
|
Form of Warrant (Included in Exhibit 4.2) (4)
|
|
|
4.4
|
Warrant Agreement, dated October 1, 2013, by Asterias Biotherapeutics, Inc. for the benefit of Romulus Films Ltd. (4)
|
|
|
4.5
|
Form of Warrant (Included in Exhibit 4.4) (4)
|
|
|
10.1
|
Stock and Warrant Purchase Agreement, dated January 4, 2013, between BioTime Acquisition Corporation and Romulus Films Ltd. (2)
|
|
|
10.2
|
Sublease dated April 1, 2013 between BioTime, Inc. and BioTime Acquisition Corporation (2)
|
|
|
10.3
|
Shared Facilities and Services Agreement, dated April 1, 2013, between Asterias Biotherapeutics, Inc. and BioTime, Inc. (2)
|
|
10.4
|
Promissory Note, dated April 1, 2013, payable to BioTime, Inc. (2)
|
|
|
10.5
|
2013 Equity Incentive Plan (5)
|
|
|
10.6
|
Form of Employee Incentive Stock Option Agreement (6)
|
|
|
10.7
|
Form of Non-employee Director Stock Option Agreement (6)
|
|
|
10.8
|
Employment Agreement, dated as June 24, 2013, between Thomas Okarma and Asterias Biotherapeutics, Inc. (6)
|
|
|
10.9
|
Employment Agreement, dated as of June 24, 2013, between Katharine E. Spink and Asterias Biotherapeutics, Inc. (6)
|
|
10.10
|
Employment Agreement, dated as of June 24, 2013, between Jane S. Lebkowski and Asterias Biotherapeutics, Inc. (6)
|
|
|
10.11
|
Share Exchange Agreement, dated September 25, 2012, between Thomas Okarma and BioTime Acquisition Corporation (6)
|
|
|
10.12
|
Royalty Agreement, dated October 1, 2013 between Asterias Biotherapeutics, Inc. and Geron Corporation (7)
|
|
|
10.13
|
Exclusive Sublicense Agreement between Geron Corporation and Asterias Biotherapeutics, Inc. (7)
|
|
|
10.14
|
Sublicense Agreement between BioTime, Inc. and Asterias Biotherapeutics, Inc. (7)
|
|
|
10.15
|
Exclusive License Agreement, dated February 20, 2003, and First Amendment thereto dated September 7, 2004, between The Regents of the University of California and Geron Corporation (7)
|
|
|
10.16
|
Non-exclusive License Agreement, dated October 7, 2013, between the Wisconsin Alumni Research Foundation and Asterias Biotherapeutics, Inc. (Portions of this exhibit have been omitted pursuant to a request for confidential treatment) (7)
|
|
|
10.17
|
Lease, dated December 30, 2013, by and between BMR 6300 Dumbarton Circle, LP, and Asterias Biotherapeutics, Inc. (8)
|
|
|
10.18
|
Warrant Agreement, dated June 16, 2014, by Asterias Biotherapeutics, Inc. for the benefit of certain warrant holders (9)
|
|
|
10.19
|
Form of Warrant (included in Exhibit 10.18) (9)
|
|
|
10.20
|
Employment Agreement, dated as of June 9, 2014, between Pedro Lichtinger and Asterias Biotherapeutics, Inc. (9)
|
|
|
10.21
|
Employment Agreement, dated as of June 12, 2014, between Pedro Lichtinger and Asterias Biotherapeutics, Inc. (9)
|
|
|
10.22
|
Stock Purchase Agreement, dated as of June 12, 2014, between Pedro Lichtinger and Asterias Biotherapeutics, Inc. (9)
|
|
|
10.22
|
Purchase Agreement, dated as of June 13, 2014, between Broadwood Partners, L.P. and Asterias Biotherapeutics, Inc. (9)
|
|
10.22
|
Purchase Agreement, dated as of June 13, 2014, between The George Karfunkel 2007 Grantor Trust #1 and Asterias Biotherapeutics, Inc. (9)
|
|
|
10.23
|
Registration Rights Agreement, dated June 16, 2014, between The George Karfunkel 2007 Grantor Trust #1, Broadwood Partners, L.P., and Asterias Biotherapeutics, Inc. (9)
|
|
|
10.28
|
At the Market Issuance Sales Agreement, dated April 10, 2015, by and between the Registrant and MLV & Co. LLC. (12)
|
|
|
10.29
|
Employment Agreement with Edward D. Wirth III, dated June 16, 2013. (13)
|
|
|
10.30
|
Services Agreement, dated October 8, 2015 by Aserias and Catapult. (14)
|
|
|
Employment Agreement dated November 2, 2015 between Georgia Erbez and Asterias Biotherapeutics, Inc. *
|
||
|
Employment Agreement dated February 28, 2016 between Stephen L. Cartt and Asterias Biotherapeutics, Inc. *
|
||
|
Separation Agreement, as of March 10, 2016, between Pedro Lichtinger and Asterias Biotherapeutics, Inc. *
|
||
| 10.34 | Amendment to the Notice of Award from the California Institute of Regenerative Medicine dated March 2, 2016 * + | |
|
Separation Agreement, as of March 21, 2016, between Georgia Erbez and Asterias Biotherapeutics, Inc. *
|
||
|
Consent of OUM & Co. LLP *
|
||
|
Consent of Rothstein Kass*
|
||
|
Rule 13a-14(a)/15d-14(a) Certification.*
|
||
|
Section 1350 Certification.*
|
||
|
101
|
Interactive Data File
|
|
101.INS
|
XBRL Instance Document *
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema *
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase *
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase *
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase *
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Document *
|
| + | Portions of this exhibit have been omitted pursuant to a confidential treatment order from the Securities and Exchange Commission. |
| ++ | Portions of this exhibit have been omitted pursuant to a request for confidential treatment and have been separately filed with the Securities and Exchange Commission. |
| (1) | Incorporated by reference to Asterias’ Current Report on Form 8-K filed by BioTime, Inc. with the Securities and Exchange Commission on January 8, 2013. |
| (2) | Incorporated by reference to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on April 3, 2013. |
| (3) | Incorporated by reference to Amendment No. 3 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on September 3, 2013 |
| (4) | Incorporated by reference to Current Report on Form 8-K filed with the Securities and Exchange Commission on October 1, 2013. |
| (5) | Incorporated by reference to Amendment No. 1 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on June 26, 2013. |
| (6) | Incorporated by reference to Amendment No. 2 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on August 13, 2013. |
| (7) | Incorporated by reference to Asterias’ Quarterly Report on Form 10-Q for the quarter ended September 30, 2013. |
| (8) | Incorporated by reference to Asterias’ Annual Report on Form 10-K for the year ended December 31, 2013. |
| (9) | Incorporated by reference to Post-Effective Amendment No. 4 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on June 19, 2014. |
| (10) | Incorporated by reference to Asterias’ Quarterly Report on Form 10-Q/A-1 for the quarter ended September 30, 2014 |
| (11) | Incorporated by reference to Asterias' Annual Report on Form 10-K, for the year ended December 31, 2014 |
| (12) | Incorporated by reference to Asterias' Current Report on Form 8-K, filed April 10, 2015 |
| (13) | Incorporated by reference to Asterias' Quarterly Report on Form 10-Q, for the period ended June 30, 2015 |
| (14) | Incorporated by reference to Asterias' Current Report on Form 8-K, filed August 10, 2015 |
* Filed herewith.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized on the 29th day of March, 2016.
|
ASTERIAS BIOTHERAPEUTICS, INC.
|
||
|
By:
|
/s/ Stephen L. Cartt
|
|
|
Stephen L. Cartt,
|
||
|
President and Chief Executive Officer
|
||
|
Signature
|
Title
|
Date
|
||
|
/s/ Stephen L. Cartt
|
Chief Executive Officer and
|
March 29, 2016
|
||
|
STEPHEN L. CARTT
|
Director (Principal Executive Officer)
|
|||
|
/s/ Russell L. Skibsted
|
Chief Financial Officer (Principal
|
|||
|
RUSSELL L. SKIBSTED
|
Financial and Accounting Officer)
|
March 29, 2016
|
||
|
/s/ Andrew Arno
|
Director
|
March 29, 2016
|
||
|
ANDREW ARNO
|
||||
|
/s/ Don M. Bailey
|
Director
|
March 29, 2016
|
||
|
DON M. BAILEY
|
||||
|
/s/ Alfred D. Kingsley
|
Director
|
March 29, 2016
|
||
|
ALFRED D. KINGSLEY
|
||||
|
/s/ Richard LeBuhn
|
Director
|
March 29, 2016
|
||
|
RICHARD LEBUHN
|
||||
|
/s/ Robert W. Peabody
|
Director
|
March 29, 2016
|
||
|
ROBERT W. PEABODY
|
||||
|
/s/ Natale Ricciardi
|
Director
|
March 29, 2016
|
||
|
NATALE RICCIARDI
|
||||
|
/s/ Judith Segall
|
Director
|
March 29, 2016
|
||
|
JUDITH SEGALL
|
||||
|
/s/ Michael D. West
|
Director
|
March 29, 2016
|
||
|
MICHAEL D. WEST
|
105
