Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☐ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2014
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from___________ to __________
Commission file number 000-55046
Asterias Biotherapeutics, Inc.
(Exact name of registrant as specified in its charter)
|
Delaware
|
46-1047971
|
|
|
(State or other jurisdiction of incorporation or organization)
|
(I.R.S. Employer Identification No.)
|
230 Constitution Drive
Menlo Park, California 94025
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code
(650) 433-2900
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Name of exchange on which registered
|
|
|
Series A Common Stock, $0.0001 par value
|
NYSE MKT
|
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ☐
|
Accelerated filer ☐
|
|
Non-accelerated filer ☒ (Do not check if a smaller reporting company)
|
Smaller reporting company☐
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act):
Yes o No x
The Registrant's Series A Common Stock did not have a market price as of the last day of the Registrant's second fiscal quarter, therefore the aggregate market value of the outstanding shares of Series A Common Stock as of such date cannot be calculated.
As of March 9, 2015, there were outstanding 32,312,407 shares of Series A Common Stock, par value $0.0001 per share.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement for its Annual Meeting of Stockholders or Annual Report on Form 10-K/A, to be filed on or before April 30, 2015, are incorporated by reference into Part III of this Report.
Asterias Biotherapeutics, Inc.
|
Page
Number
|
|||
|
Part I.
|
Financial Information
|
||
|
Item 1 -
|
5
|
||
|
Item 1A
|
24
|
||
|
Item 1B
|
38
|
||
|
Item 2 -
|
38
|
||
|
Item 3 -
|
39
|
||
|
Item 4 -
|
39
|
||
|
Part II.
|
Other Information
|
||
|
Item 5 -
|
39
|
||
|
Item 6 -
|
41
|
||
|
Item 7 -
|
42
|
||
|
Item 7A -
|
47
|
||
|
Item 8 -
|
52
|
||
|
Item 9 -
|
75
|
||
|
Item 9A-
|
75
|
||
|
Item 9B
|
75
|
||
|
Part III.
|
Item 10 -
|
76
|
|
|
Item 11 -
|
76
|
||
|
Item 12 -
|
76
|
||
|
Item 13 -
|
76
|
||
|
Item 14 -
|
76
|
||
|
|
|||
|
Part IV
|
Item 15 -
|
77
|
|
|
80
|
|||
PART I
Certain statements contained herein are forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for Asterias, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management constitute forward-looking statements. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects,” “estimates”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. Actual results may differ materially from the results anticipated in these forward-looking statements and as such should be evaluated together with the many uncertainties that affect the businesses of Asterias, particularly those mentioned in the cautionary statements found in Asterias’ filings with the Securities and Exchange Commission. Asterias disclaims any intent or obligation to update these forward-looking statements
References to “Asterias,” “our” or “us” means Asterias Biotherapeutics, Inc.
The description or discussion, in this Form 10-K, of any contract or agreement is a summary only and is qualified in all respects by reference to the full text of the applicable contract or agreement.
Preliminary Note Regarding Ownership of Our Common Stock
As of February 26, 2015, we had 542 shareholders of record and there were 32,312,407 shares of our Series A Common Stock (“Series A Shares”) outstanding as of March 9, 2015, of which 21,823,340 Series A Shares were held by our parent BioTime, Inc. ("BioTime"). These Series A Shares held by BioTime account for 67.5% of our Series A Shares outstanding as a whole. Accordingly, we are a consolidated subsidiary of BioTime.
Overview
We are a biotechnology company focused on the emerging field of regenerative medicine. Our core technologies center on stem cells capable of becoming all of the cell types in the human body, a property called pluripotency. We plan to develop therapeutic products from “pluripotent” stem cells to treat diseases or injuries in a variety of medical fields, with an initial focus on the therapeutic areas of neurology and oncology. We have three clinical stage product candidates: AST-OPC1 for spinal cord injuries; AST-VAC2 for lung cancer, and AST-VAC1 for prostate cancer and acute myelogenous leukemia. Our product development efforts are presently focused primarily on AST-OPC1 and AST-VAC2.
“Regenerative medicine” refers to an emerging field of therapeutic product development that may allow all human cell and tissue types to be manufactured on an industrial scale. This new technology is made possible by the isolation of human embryonic stem ("hES") cells, and by the development of induced pluripotent stem ("iPS") cells which are created from regular cells of the human body using technology that allows adult cells to be “reprogrammed” into cells with pluripotency much like hES cells. Pluripotent hES and iPS cells have the unique property of being able to branch out into each and every kind of cell in the human body, including the cell types that make up the brain, the blood, the heart, the lungs, the liver, and other tissues. Unlike adult-derived stem cells that have limited potential to become different cell types, pluripotent stem cells may have vast potential to supply an array of new regenerative therapeutic products, especially those targeting the large and growing markets associated with age-related degenerative disease. Unlike pharmaceuticals that require a molecular target, therapeutic strategies in regenerative medicine are generally aimed at regenerating affected cells and tissues, and therefore may have broader applicability. We believe that regenerative medicine represents a revolution in the field of biotechnology with the promise of providing therapies for diseases previously considered incurable.
Additional Information
We were incorporated in September 2012 under the name BioTime Acquisition Corporation in the state of Delaware. We changed our name to Asterias Biotherapeutics, Inc. in March 2013. Our principal executive offices are located at 230 Constitution Drive, Menlo Park, California 94025. Our telephone number is 650-433-2900. We currently maintain an Internet website at www.asteriasbiotherapeutics.com.
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012. We will remain an "emerging growth company" until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of our first sale of common equity securities, such as our common stock, pursuant to an effective registration statement under the Securities Act; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission (the “SEC”). We refer to the Jumpstart Our Business Startups Act of 2012 herein as the "JOBS Act," and references herein to "emerging growth company" shall have the meaning associated with it in the JOBS Act.
Business Strategy
In October of 2013, we acquired intellectual property, cell lines and other human embryonic stem cell related assets from Geron Corporation ("Geron") and also acquired rights to use certain human embryonic stem ("hES") cell lines and to practice certain patents from our parent company, BioTime, Inc. ("BioTime"). From the assets acquired in these transactions, we have prioritized the development of two technology platforms: human embryonic stem cells and allogeneic dendritic cell vaccines. We have selected one program from each platform as our initial development focus. We are developing AST-OPC1, glial cells derived from our human embryonic stem cell platform, in an initial clinical indication of spinal cord injury, with potential for later expansion into other neurodegenerative diseases such as stroke and multiple sclerosis. With our collaboration partner, Cancer Research United Kingdom (“CRUK”) we are developing AST-VAC2, allogeneic dendritic cells derived from human embryonic stem cells and loaded with the telomerase antigen, in non-small cell lung cancer in collaboration with CRUK. We believe AST-VAC2 also has potential for application in additional cancer indications.
Products Under Development
Our products candidates are summarized in the following table:
|
Product Candidate
Description
|
Target Market
|
Estimated Number of
Potential
Patients(1)
|
Status
|
|
|
AST-OPC1 – Glial Cells
|
Current development focus:
Spinal Cord Injury ("SCI")
|
12,000 new cases per year in U.S.
|
Phase I Trial in thoracic SCI completed in U.S. Five Patients treated – no serious adverse events related to the AST-OPC1 drug product to date. Phase I/IIa dose escalation trial underway in cervical SCI. Obtained a grant for up to $14.3 million grant obtained from California Institute of Regenerative Medicine (CIRM) to provide matching funds for clinical trial and process development.
|
|
|
Additional potential markets:
|
||||
|
Multiple Sclerosis (“MS”)
|
180,000 new cases per year in U.S.
|
Proof of principle achieved in animal models.
|
||
|
Stroke
|
800,000 new cases per year in U.S.
|
Pre-clinical research.
|
||
| AST-VAC2 – | ||||
|
Allogeneic Dendritic Cells loaded with Telomerase antigen
|
Current development focus:
|
|||
|
Non-small Cell Lung Cancer
|
166,000 new cases per year in U.S.
|
Cells derived and characterization studies performed (parameters analyzed showed normal cell functions in vitro(2)).
Proof of concept established in multiple human in vitro(2) systems. Manufacturing process being transferred to partner (Cancer Research UK) for conduct of Phase I/IIa trial.
|
||
|
Additional potential markets:
Multiple cancer types, antigens and infectious diseases
|
| (1) | The estimates of the numbers of potential patients shown in the table are based on data for the United States only and do not include potential patients in other countries. |
| (2) | In vitro means in tissue culture dishes. |
Additional product candidates that we may determine to develop from various cell types that we acquired from Geron are summarized in the following table:
|
Product Candidate
Description
|
Target Market
|
Estimated Number of Potential
Patients (1)
|
Status
|
|
|
AST-VAC1 – Autologous Monocyte – Derived Dendritic Cells (infused cells derived from the treated patient)
|
Cancer
|
Prostate: 240,000 new cases per year in U.S.
|
Phase I study in metastatic prostate cancer completed (Journal of Immunology, 2005, 174: 3798-3807).
|
|
|
Acute myelogenous leukemia: more than 12,000 new cases per year in U.S.
|
Phase I/II study in acute myelogenous leukemia completed. Manuscript in preparation.
|
|||
| (1) | The estimates of the numbers of potential patients shown in the table are based on data for the United States only and do not include potential patients in other countries. |
Patents and Patent Applications
The patent portfolio that we acquired pursuant to the Asset Contribution Agreement with Geron, dated January 4, 2013 (the "Asset Contribution Agreement") includes over 450 patents and patent applications relating to hES cell-based product opportunities. This portfolio consists primarily of patents and patent applications previously owned by Geron, and also includes patent families licensed to Geron by third parties. The patents and patent applications cover a number of cell types that can be made from hES cells, including hepatocytes (liver cells), cardiomyocytes (heart muscle cells), neural cells (nerve cells, including dopaminergic neurons and oligodendrocytes), chondrocytes (cartilage cells), pancreatic islet β cells, osteoblasts (bone cells), hematopoietic cells (blood-forming cells) and dendritic cells. Also included in the patent portfolio are technologies for growing hES cells without the need for cell feeder layers, and novel synthetic growth surfaces.
We believe that this is one of the largest and broadest portfolios of patents related to hES and iPS technology owned by any company or institution. In addition, as a subsidiary of BioTime, we expect to have opportunities to acquire licenses to use patents, patent applications and know-how in the hES and iPS fields owned by or licensed to BioTime and its other subsidiaries. BioTime and its subsidiaries own or have licensed rights to more than 350 patents in the hES and iPS fields. Except for licenses described in this report, the specific patents that we may license or sublicense from BioTime and its other subsidiaries, and the financial and other terms and conditions of those licenses and sublicenses, have not yet been determined.
Trademark Notice
Asterias Biotherapeutics, the Asterias logo and other trademarks of Asterias appearing in this report are the property of Asterias. All other trademarks, service marks and trade names in this report are the property of their respective owners. We have omitted the ® and ™ designations, as applicable, for the trademarks used in this report.
Product Candidates
AST-OPC1 Glial Progenitor Cells
Our AST-OPC1 product is comprised of glial progenitor cells, which are cells that become glial cells after injection, derived from a cGMP master cell bank of undifferentiated hES cells that has been fully qualified for human use. These cells, which are stored frozen until ready for use, are produced under cGMP conditions and screened for adventitious agents. These glial progenitor cells were the first hES cell-derived cellular therapy to enter human clinical testing when Geron initiated a Phase 1 trial of these cells in spinal cord injury in 2010.
Glial cells are nature’s neuronal insulating cells. Like the insulation covering an electrical wire, glial cells enable the conduction of electrical impulses along nerve fibers throughout the central and peripheral nervous system. They are also known to promote neural growth, as well as induce blood vessel formation around nerve axons. AST-OPC1 cells reproduce all of the natural functions of glial cells in animal models, including: producing myelin that wraps around nerve fibers; producing neurotrophic factors which encourage neuro-regeneration and sprouting of new nerve endings, and inducing new blood vessels which provide nutrients and remove waste matter from neural tissue as it functions in the body.
The pathology of spinal cord injury involves extensive loss of the myelin sheath (insulation) produced by glial cells at the site of injury. Although neurons are lost, the prime pathology of spinal cord injury is loss of glial insulation which prevents transmission of nerve impulses above or below the point of injury.
There are currently no drugs approved by the United States Food and Drug Administration (“FDA”) specifically for the treatment of spinal cord injury, although methylprednisolone, a corticosteroid generally used as an anti-inflammatory drug, is sometimes prescribed on an off-label basis to reduce acute inflammation in the injured spinal cord immediately after injury. It is believed that in order to effect substantial benefit in treating this complex injury, multiple mechanisms of action are required, such as re-myelination of the demyelinated axons, generation of new blood vessels to repair the ischemic damage from injury, and the presence of biologics that cause neuro-sprouting or new nerve growth to enable the severed axons to repair. In studies to date, AST-OPC1 cells have been shown to exhibit all three effects, and therefore we believe they have potential to effectively treat acute spinal cord injury.
Geron performed multiple studies in a validated rat model of spinal cord injury showing that a single injection of AST-OPC1 cells at the site of injury produces durable re-myelination, new blood vessel formation, and new neuronal sprouting, all of which result in sustained and significant improvement in the animal’s locomotion within several months after injection. These data provided the rationale to initiate the world’s first clinical trial using hES cell-derived glial cells (AST-OPC1) to treat acute spinal cord injury in humans. A large body of evidence derived from this research showed the following observations:
| · | AST-OPC1 survives for at least 12 months in the spinal cord after injection into animal models of spinal cord injury. |
| · | The injected cells result in sustained and significant improvement in locomotor activity in the spinal cord injured animals in models of both thoracic and cervical injuries. |
| · | The growth of the AST-OPC1 cells after injection reduces cavities that normally form after injury in both animal models and human spinal cord injury. |
| · | AST-OPC1 cells migrate up to Five centimeters in both directions from the site of injection in rodent models of spinal cord injury. No toxicity was seen in the animals after injection – no systemic toxicity, nerve pain, benign growths (known as teratomas), or toxicity of any kind other than rare observations of benign cyst-like structures at the point of injection. Extensive in vitro immune assays demonstrated the absence of direct immune recognition of AST-OPC1 by human immune cells. |
| · | The cyst-like structures that appeared in certain rat model studies were microscopic in size, had very few dividing cells, did not grow, and were found exclusively in the spinal cord injury site where the AST-OPC1 cells were injected. Because of the discovery of the cyst-like structures in early animal models, the FDA placed Geron’s planned clinical trial on hold. The presence of cyst-like structures was investigated in additional animal studies. In four separate animal studies using the clinical grade AST-OPC1 product, cyst-like structures were found in the frequencies shown in the following table: |
|
Number of Animals
|
Number of
|
|
|
Developing Cyst-Like Structures
|
Animals Studied
|
|
|
5
|
128
|
|
|
0
|
62
|
|
|
1
|
68
|
|
|
1
|
108
|
After discussions that Geron had with the FDA, the clinical trial investigators, and the data monitoring safety board, the unanimous opinion was that these cyst-like structures were of low risk to subjects and the clinical trial was permitted to proceed. Nevertheless a plan was developed to monitor subjects in clinical trials for the development of such cyst-like structures. In the completed Phase I safety study in which Five patients received AST-OPC1 cells in their injured spinal cords, no cyst-like structures were detected in multiple magnetic resonance imaging exams (“MRIs”) during a one year follow-up, or in annual long term follow-up MRIs out to 3-4 years in all subjects.
Phase I Safety Trial
After FDA authorization, Geron began the world’s first hES cell trial in patients with acute spinal cord injury in October 2010. The trial was an open label design conducted at seven U.S. neuro-trauma sites. Five subjects were treated in the trial, each of whom had a sub-acute functional complete thoracic (chest) spinal cord lesion. Patients enrolled in the study received a single dose of 2 x 106 cells at the injury site between seven and 14 days after injury. All subjects received temporary low dose immune suppression treatment for 60 days. The primary endpoint of the study was safety, with secondary endpoints of neurologic function assessed by five different validated measures of sensory and motor function. Each subject received a screening MRI, and if treated and entered into the treatment protocol, received eight follow-up MRIs in the first year and multiple physical exams and laboratory testing. The patients then entered a separate protocol after the first year which will follow them intermittently over a period of 15 years.
As of March 2015, all five patients have completed their three year follow-up data set. No surgical complications during or post-surgery have been observed, and there have been no significant adverse events to date in any patient attributable to the AST-OPC1 product, the surgery to deliver the cells, or the immunosuppressive regimen. There have been five minor adverse events possibly related to AST-OPC1 such as transient fever and nerve pain. There have been no unexpected neurological changes to date, nor has there been evidence of adverse changes or cavitation on multiple MRIs. MRI results in four of the five subjects are consistent with prevention of lesion cavity formation. Immune monitoring, conducted in some of the patients, has not detected any evidence of immune responses to AST-OPC1 at time periods of up to one year post-transplant, an important clinical finding that was predicted by extensive in vitro immune testing of AST-OPC1 prior to initiating the trial.
Phase I/IIa Dose Escalation Study: Subjects with Neurologically Complete Cervical Spinal Cord Injuries
Based on the results of the completed Phase I trial of AST-OPC1 in thoracic Spinal Cord Injury (“SCI”), we obtained permission from the FDA in August 2014 to initiate a Phase I/IIa dose escalation trial in patients with neurologically complete cervical spinal cord injuries. We believe that there are both medical and scientific rationales for the transition to subjects with cervical SCI. Individuals with neurologically complete cervical SCI have an enormous unmet medical need due to the loss of function in all four limbs as well as multiple additional impairments such as impaired bowel and bladder function, reduced sensation, spasticity, sudden changes in blood pressure, deep vein thrombosis, sexual dysfunction, increased infections, skin pressure sores, and chronic pain. These individuals frequently require significant assistance for their care and activities of daily living. One recent published study estimated the lifetime costs of care for a person who suffers a cervical SCI at age 25 to be $4.2 million (Y. C. Cao and M. J. DeVivo (2009)).
Scientifically, the injured cervical spinal cord is a much better location than the upper or middle thoracic spinal cord to test the safety and potential activity of AST-OPC1. This is partly due to the fact that damaged and demyelinated nerve axons in thoracic injuries need to regrow over several spinal segments in order to restore neural function. In contrast, damaged and demyelinated nerve axons in cervical injuries only need to regrow a short distance to restore neural function. Therefore, in cervical injuries, regeneration and/or repair of damaged axons mediated by AST-OPC1 could result in substantial re-innervation of cervical segments and thereby have a significant impact on upper extremity motor and/or sensory function.
The advantages of conducting clinical trials in patients with neurologically complete cervical SCI was recently further demonstrated by published studies done by J. D. Steeves and others together with the Spinal Cord Outcomes Partnership Endeavor (“SCOPE”) (Steeves et al, Top Spinal Cord Inj Rehabil 2012). These studies analyzed several large SCI databases and found that only 21% and 26% of people living with C4-C7 cervical sensorimotor complete spinal cord injury recovered two or more upper extremity motor levels at 24 and 48 weeks after injury, respectively. If this rate could be increased by 20 percentage points (i.e. to 41-46% of subjects recovering two or more upper extremity motor levels), the authors calculated that a trial could achieve sufficient statistical power with approximately 200 subjects enrolled. Those studies further showed that an improvement of two or more motor levels led to a statistically significant increase in the Spinal Cord Independence Measure (“SCIM”) self-care subscore, suggestive of a measurable association between improvement in neurological function and a clinically meaningful functional outcome.
Near-Term Product Development Strategy for AST-OPC1
We initiated enrollment of the Phase I/IIa dose escalation trial of AST-OPC1 in patients with complete cervical injuries in March 2015. The trial is designed to assess safety and activity of escalating doses of AST-OPC1 in complete cervical SCI, the first targeted indication for AST-OPC1. The trial is an open-label, single-arm study in patients with sub-acute, C-5 to C-7, neurologically complete cervical SCI. These individuals have lost all sensation and movement below their injury site with severe paralysis of the upper and lower limbs. AST-OPC1 will be administered 14 to 30 days post-injury. Patients will be followed by neurological exams and imaging methods to assess the safety and activity of the product. We are implementing an initiative to accelerate the current timelines for the AST-OPC1 clinical program by approximately six months in order to obtain safety and efficacy readouts more rapidly, and plan to seek FDA concurrence to increase the robustness of the proof of concept in the Phase I/IIa clinical trial by expanding enrollment from 13 patients to up to 40 patients. The company believes these changes will increase the statistical confidence of the safety and efficacy readouts and position the product for potential accelerated regulatory approvals. Asterias has received a Strategic Partnerships Award grant from the California Institute for Regenerative Medicine, which provides for up to $14.3 million of non-dilutive funding for the Phase I/IIa clinical trial and other product development activities for AST-OPC1, subject to achieving certain milestones.
In addition to the ongoing Phase I/IIa study, we are conducting additional research and planning for subsequent trials and for other possible indications for the use of AST-OPC1. Ongoing and intended near term activities in the AST-OPC1 program encompass five main categories of activities: Regulatory; Clinical; Product Development; Quality; and Research, briefly summarized below.
Regulatory: These activities include, but are not limited to, preparing regulatory packages for each clinical site for submission to the FDA, preparing a briefing package to discuss ongoing product development activities with FDA staff, and amending the existing IND to enable expansion of the Phase I/IIa study from 13 to up to 40 patients.
Clinical: The activities include, but are not limited to (i) selecting and engaging clinical sites and vendors for the conduct of the clinical trial, (ii) training and qualifying neurosurgeons and clinical sites to identify and enroll subjects, to prepare the AST-OPC1 drug product at the clinical sites, and to conduct the trial according to the IND protocol, (iii) monitoring conduct of the study to ensure it is performed in accordance with the IND protocol, and (iv) consulting with investigators and key opinion leaders to further define clinical development plans for subsequent trials. We must also ensure an adequate supply chain of (1) the investigational drug product (AST-OPC1), (2) syringe positioning devices used to inject the AST-OPC1 cells, and (3) dose preparation kits used to thaw and prepare the AST-OPC1 cells for injection.
Product Development: Activities in this category will include work to improve the scale and efficiency of the manufacturing process for AST-OPC1 in preparation for larger future trials if the Phase I/IIa trial is successful.
Quality: We will be required to test the purity, stability and potency of the AST-OPC1 drug product used in the proposed trial and also to improve the assays currently used to define the product attributes as we progress to later stage clinical studies using the AST-OPC1 drug product.
Research: We plan to try to develop novel methods to measure purity and potency of the AST-OPC1 drug product. We plan to investigate possible improvements to the current methods used to manufacture AST-OPC1 and also to test AST-OPC1 in animal models of other neurodegenerative diseases to identify other potential clinical applications for the product, such as sub-cortical stroke.
We will need to raise additional capital in order conduct the Phase I/IIa clinical trial and any subsequent clinical trial and product development work. We intend to apply for a supplementary CIRM grant to provide funding for the clinical trial expansion from 13 to up to 40 subjects, and we may sell some of our BioTime common shares or additional shares of our capital stock to finance the clinical trials, including later Phase trials.
AST-OPC1 CIRM Grant
We have been awarded a $14.3 million Strategic Partnership III grant by CIRM to help fund our clinical development of AST-OPC1. The grant will provide funding for the Phase I/IIa dose escalation study of AST-OPC1 in subjects with complete cervical spinal cord injury, and for product development efforts to refine and scale manufacturing methods to support eventual commercialization. CIRM will disburse the grant funds to us over four years in accordance with a quarterly disbursement schedule, subject to our attainment of certain specific progress and safety milestones. As the distributions of the CIRM grant are subject to meeting certain progress and go/no-go milestones, there can be no assurance that we will receive the entire amount granted. In addition, pursuant to the Award, we agreed to notify and report to CIRM information relating to serious adverse events, studies, press releases clinical trial information and routine communications in accordance with an agreed schedule.
As of March 9, 2015 we have received $3,186,069 of payments from CIRM and have achieved the first three clinical trial milestones: executing contracts with our clinical vendors and service providers in the fourth quarter in 2014, completing release of sufficient drug product to supply the trial in the fourth quarter in 2014, and opening our first clinical site in the first quarter of 2015. Future clinical and regulatory milestones that must be timely achieved include enrollment of our first AST-OPC1 patient in the first quarter of 2015, executing contracts with clinical trial sites, initiating and completing enrollment with our remaining intended clinical sites by the end of the third quarter of 2015, and receiving data monitoring committee approval to advance patients to enroll in the second dosing cohort for AST-OPC1 before the end of 2015. We are also required to achieve additional clinical trial milestones through the third quarter of 2017, as well as process development milestones relating to FDA, cGMP, and other manufacturing and technology transfer issues through the third quarter of 2018. Failure to timely achieve milestones or otherwise satisfy CIRM regarding any delay could lead CIRM to suspend payments. The foregoing description of our arrangement with CIRM is a summary only and is qualified by reference to the Notice of Grant Award, dated as of October 16, 2014, and the Amendment to Notice of Grant Award, dated as of November 26, 2014, between us and CIRM.
AST-OPC1 for the treatment of multiple sclerosis and other diseases
In addition or as an alternative to spinal cord injury, we may test the AST-OPC1 cells in other alternative indications, including multiple sclerosis (“MS”) and stroke. AST-OPC1 may also be useful in the treatment of MS focal lesions, especially those in the spinal cord. Because of its functional properties, AST-OPC1 is a candidate for the repair of central nervous system lesions found in subjects with MS. In these lesions, axons are “demyelinated,” meaning that they have lost the sheaths that provide insulation for nerve conduction. In many cases, lesions located in the spinal cord of patients with MS are responsible for progressive clinical deterioration and a loss of ambulatory function. AST-OPC1 may have the potential to repair such spinal cord lesions and to reverse clinical deterioration associated with the lesions. Preclinical studies were conducted by Dr. Jeffrey Kocsis at Yale University. In these studies, Dr. Kocsis’ group created lesions resembling those seen in MS. AST-OPC1 was implanted seven days after induction of the lesion. Progressive remyelination of the lesion was observed which was durable for at least one year and was not observed in control animals that did not receive AST-OPC1. We believe that research provides support for potential clinical testing of local delivery of AST-OPC1 in the spinal cord of patients with progressive MS, and we are exploring potential development paths to assess the safety and utility of AST-OPC1 in treating MS spinal cord lesions.
Additionally, a growing body of evidence supports the use of AST-OPC1 as a treatment for white matter stroke. Based upon the three documented mechanisms of action of AST-OPC1 – re-myelination, vascularization, and neurotrophin release – we are collaborating with Dr. Thomas Carmichael at the University of California Los Angeles (“UCLA”) to study AST-OPC1 in animal models of white matter stroke in an attempt to generate proof of concept data for the application of AST-OPC1 in this large, unmet medical need.
AST-VAC2 and AST-VAC1, Technology For Potential New Cancer Vaccines
We acquired from Geron two experimental therapeutic cancer vaccines designed to target cancer cells by targeting the cancer cell’s expression of telomerase. Telomerase is a ubiquitous cancer target, expressed at high levels in all human cancers but at very low levels or not at all, in normal human cells. The premise underlying these vaccines is to “teach” the patient’s own immune system to attack cancer cells while sparing other cells. This may be possible by repeatedly exposing the immune system to a substance (an antigen) that is either specifically expressed or over-expressed by cancer cells in a way that subsequently induces an immune response to any cells that express that antigen on their surface. We believe that the characteristics of telomerase make it an ideal antigen for cancer vaccines.
AST-VAC2: hES Cell-Derived Dendritic Cells
Dendritic cells can be likened to the quarterback of the immune system. They are antigen processing and presenting cells which are potent initiators of a cellular and humoral (antibody) immune response. Immature dendritic cells initiate an antigen specific suppressive response, such as would be required to terminate an abnormal autoimmune reaction as occurs in diseases like rheumatoid arthritis, and systemic lupus erythematosis. Mature dendritic cells, on the other hand, initiate active cellular and humoral immunity such as is required for immune targeting of cancer and infectious disease. AST-VAC2 is a mature dendritic cell population that is produced from hES cells that can be modified with any antigen. There is a significant amount of global clinical literature that describes the use of mature dendritic cells isolated from peripheral blood samples and used to stimulate immune responses to a target antigen in various vaccination schemes, especially in various cancers (see our discussion of AST-VAC1, below). Although effective in generating an antigen specific immune response, and in several cases showing a significant clinical impact, the drawbacks of autologous peripheral blood-derived dendritic cell vaccination schemes such as AST-VAC1 are the limited supply of cells, the high cost of production, the long production time, and high patient to patient variability. As a second generation dendritic cell technology, AST-VAC2 is designed to specifically obviate theses drawbacks. AST-VAC2 can be produced in large quantities, similar to the other hES cell-based therapeutic cells. Additionally, because AST-VAC2 is an allogeneic cell, it is believed to be potentially more potent than an autologous dendritic cell, by means of partial antigen mismatch in the HLA system (Human Leukocyte Antigen - markers of immune system types, akin to blood types) which may have a broad immunostimulatory effect similar to the adjuvants used in many vaccines.
Quality control can be standardized and the product can be shown to have uniform potency. Cost of goods is dramatically lower than autologous approaches, and the multi-dose batch production and cryo-preservation enables “on-demand” availability. It is generally agreed that partial HLA matching between dendritic cell and patient will be required to optimize efficacy and reduce side effects. The H1 hES cell line, qualified for human use by Geron, can provide a single HLA match on HLA-A2 (a specific HLA type) for approximately 47% of North American Caucasians. Addition of dendritic cells manufactured from one additional hES cell line will capture approximately 70% of North American Caucasians. The feasibility of AST-VAC2 differentiation from multiple hES cell lines has been demonstrated.
The differentiation process for AST-VAC2 has been optimized, the protocol is patent protected and clinically compliant (suitable for use in humans), and no serum or animal feeder cells are used. The production protocol is robust, achieving fully matured dendritic cells within 34 days. Four growth factors are used to drive hES cell differentiation to dendritic cells, and they are serially removed during the process: VEGF, SCF, BMP-4 and GMCSF. The hES cell-derived dendritic cells can be irradiated, which may shorten the animal studies required for IND submission, because irradiation prevents cell division of the injected AST-VAC2 dendritic cells, potentially eliminating concerns of growth of non-dendritic cells in the product. Lastly, cryo-preservation in low concentration of DMSO (Dimethyl Sulfoxide – a chemical used to stabilize cells during freezing) is feasible, thereby potentially enabling direct thaw and injection in the clinic.
AST-VAC2 cells have been extensively characterized in vitro and have high migratory and antigen presenting functionality with limited phagocytic activity (ability to engulf other cells – not a characteristic of dendritic cells), as would be expected for mature dendritic cells. They express high levels of all the appropriate surface markers defining them as mature human dendritic cells. AST-VAC2 cells are phenotypically similar to dendritic cells derived from peripheral blood mononuclear cells, further enabling them to be potentially used in lieu of peripheral blood derived dendritic cell vaccination protocols. AST-VAC2 and peripheral blood monocyte derived dendritic cells produce similar cytokine profiles (patterns of biologically active proteins) before and after antigen stimulation. AST-VAC2 has been shown to demonstrate functionality in chemotactic responses (cells are specifically attracted by certain molecules) and T-cell stimulation. AST-VAC2 in-vitro stimulates a TH-1 type cytokine production (T-helper 1 – a subtype of T cells) from lymphocytes in a mixed lymphocyte reaction in vitro (a test in which lymphocytes from two different individuals are mixed together to determine whether one individual "recognizes" the other's lymphocyte type) resulting in highly activated antigen restricted T-cell populations (lymphocytes that recognizes only one specific substance). In vitro studies have demonstrated that a single HLA match between AST-VAC2 cells and responding lymphocytes is required to stimulate antigen specific T-cell responses. AST-VAC2 has been shown to retain antigen presentation functionally (ability to "present" antigen on its surface to induce an immune response in another cell) after cryo-preservation. Irradiation of AST-VAC2 after introduction of antigen eliminates the proliferative capacity of the dendritic cells and removes any safety concerns due to the presence of any residual undifferentiated embryonic stem cells in the preparation. Irradiated and cryo-preserved AST-VAC2 cells are fully capable of presenting antigen to T-cells, resulting in antigen specific T-cell activation.
A clinical protocol for the potential first-in-man safety study of AST-VAC2 has been outlined for non-small cell lung cancer (“NSCLC”) in collaboration with CRUK, which has agreed to conduct a Phase I/IIa clinical trial. Telomerase, a ubiquitous tumor antigen, would be the first antigen to be used with AST-VAC2. The intended first-in-man trial design includes treatment of both early stage (resected) and advanced stage NSCLC patients. Patients would initially be restricted to HLA-A 2.1. Three cohorts of patients would receive six vaccinations each; patients with resected disease would be treated at two different doses (1 x 106 and 1 x 107 cells per vaccination), advanced disease patients would be tested at the 1 x 107 cells per vaccination dose.
The route of the administration would be intradermal. The primary endpoint would be to investigate the safety and toxicity of AST-VAC2, with secondary endpoints of immune response to the telomerase antigen introduced into AST-VAC2. The clinical and immunological monitoring would be achieved with a standard immune test such as ELISPOT and tetramer analysis (a biochemical assay to measure a specific antigen). Clinical responses would be monitored by rates of relapse in the resected disease setting, and by progression-free survival, and overall survival in the advanced disease patients.
In summary, AST-VAC2, a second generation dendritic cell technology, has been demonstrated to exhibit a mature dendritic cell phenotype of reproducibly characterized cellular composition. The cells activate allogenic T-cells and migrate in response to chemokine stimulation. AST-VAC2 stimulates a TH-1 type cytokine production and can present antigen delivered to the cells in either mRNA, or protein form. AST-VAC2 can stimulate Class 1 and Class 2 antigen specific T-cells (two types of antigens - type 1 is within a cell, type 2 is outside the cell) and has been shown to prime and stimulate naive antigen restricted T-cells even with only a single HLA-antigen match. Lastly, the feasibility of cryo-presentation and irradiation without alteration of AST-VAC2 function has been demonstrated. These attributes will potentially allow for a greater margin of safety in clinical studies utilizing AST-VAC2 and reduce the number of additional preclinical studies required for an IND submission. Specifically, long-term cell survival and engraftment studies may not be required for an AST-VAC2 IND submission. The first clinical trials of AST-VAC2 using telomerase as the antigen are planned and if the outcome of those trials demonstrate safety and activity, Asterias may examine the potential of AST-VAC2 with telomerase in other cancer indications, or the use of AST-VAC2 to deliver other antigens.
Near-term Product Development Strategy for AST-VAC2
During September 2014, we entered into a Clinical Trial and Option Agreement (the “CRUK Agreement”) with CRUK and Cancer Research Technology Limited, (“CRT”), a wholly-owned subsidiary of CRUK, pursuant to which CRUK has agreed to fund Phase I/IIa clinical development of our AST-VAC2 product candidate. We will, at our own cost, complete process development and manufacturing scale-up of the AST-VAC2 manufacturing process and will transfer the resulting cGMP-compatible process to CRUK. CRUK will, at its own cost, manufacture clinical grade AST-VAC2 and will carry out the Phase I/IIa clinical trial of AST-VAC2 in cancer patients both resected early-stage and advanced forms of lung cancer. We will have an exclusive first option to obtain a license to use the data from the clinical trial. If we exercise that option we will be obligated to make payments upon the execution of the license agreement, upon the achievement of various milestones, and then royalties on sales of products. In connection with the CRUK Agreement, we sublicensed to CRUK for use in the clinical trials and product manufacturing process certain patents that have been licensed or sublicensed to us by third parties. We would also be obligated to make payments to those licensors and sublicensors upon the achievement of various milestones, and then royalties on sales of products if AST-VAC2 is successfully developed and commercialized.
We are completing scale up and technology transfer of the manufacturing process for the AST-VAC2 drug product to CRUK. Following completion of the technology transfer, CRUK will initiate cGMP production to support the first-in-man clinical study of AST-VAC2 cancer immunotherapy in NSCLC. We are also developing and transferring to CRUK the quality, purity and potency assays needed for release testing of clinical grade AST-VAC2, and the immunological monitoring assays that will be used to measure patient immune responses in the clinical trial. CRUK will bear primary responsibility for performing the cGMP manufacturing of clinical grade AST-VAC2, filing the regulatory dossier, and for conducting the Phase I/IIa study of AST-VAC2 in NSCLC. We will continue to serve in a collaborative and advisory role with CRUK throughout this process. Upon completion of the Phase I/IIa study, we will have an exclusive first option to acquire the data generated in the trial.
Telomerase Therapeutic Vaccine (AST-VAC1)
We acquired from Geron rights to its immunological cancer therapy product AST-VAC1, including the IND for clinical trials conducted by Geron and the related drug master files. AST-VAC1 is an autologous product (using cells that come from the treated patient) consisting of mature antigen-presenting dendritic cells pulsed with RNA for the protein component of human telomerase (“hTERT”) and a portion of a lysosomal targeting signal ("LAMP"). LAMP directs the telomerase RNA to the lysosome, the subcellular organelle that directs the RNA to a particular part of the cell membrane. AST-VAC1 is injected into the patient’s skin; and from there the dendritic cells travel to the lymph nodes and instruct cytotoxic T-cells (T-cells that "kill" other cells) to kill tumor cells that express telomerase on their surface.
A Geron-sponsored Phase I/II clinical trial of AST-VAC1 was conducted at six U.S. medical centers in patients with acute myelogenous leukemia (“AML”) in complete clinical remission. The trial examined the safety and feasibility of a prime-boost vaccination regimen (an initial injection ("prime") followed by multiple additional injections ("boost")) to generate and extend the duration of telomerase immunity. Geron evaluated the immune response to AST-VAC1 and explored the effects of vaccination on minimal residual disease and relapse rates. This trial completed patient enrollment in December 2009.
In the Phase I/II clinical trial, patients with AML entered the study in their first or second complete remission. Prior to or shortly after completing consolidation chemotherapy, patients underwent leukapheresis (collection of white blood cells) to harvest normal peripheral blood mononuclear (white blood) cells for vaccine manufacture. AST-VAC1 was produced at a centralized manufacturing facility from the patient-specific leukapheresis harvests. Patient mononuclear cells were differentiated in culture to immature dendritic cells, which were transfected with messenger RNA encoding hTERT and LAMP. Transfected dendritic cells were matured, aliquoted and cryopreserved. AST-VAC1 was released for patient dosing contingent on several product specifications that included identity of mature dendritic cells, confirmation of positive transfection with hTERT, number of viable cells per dose after thawing, and product sterility.
AST-VAC1 was successfully manufactured and released in 24 out of the 33 patients enrolled in the study. These results reflect the variability of patient derived starting material that is often associated with an autologous, patient-specific product.
Three patients progressed prior to vaccination, therefore only 21 of the 24 patients for whom AST-VAC1 was successfully manufactured and released received vaccine. The 21 patients were vaccinated weekly for six weeks with AST-VAC1 administered intra-dermally, followed by a non-treatment period of four weeks, and then subsequent boost injections every other week for 12 weeks. Monthly extended boost injections were then administered until the vaccine product supply was depleted or the patient relapsed.
Twenty-one patients received AST-VAC1 in the study, including 19 in clinical remission and two in early relapse. Of the 19 patients in clinical remission, eight were considered at intermediate risk for relapse and 11 were at high risk for relapse as predicted by their cytogenetics (gene expression pattern in the AML cells), FAB type (French-American-British classification of AML into eight subtypes), or because they were in second clinical remission. Thirteen out of 21 patients in the trial remained in clinical remission at a median duration of follow-up from first vaccination of 13.2 months. At 12 months after vaccination with AST-VAC1, estimated disease-free survival was 81% for patients at high-risk of relapse (95% CI: 42-95%). The confidence interval (“CI”) of 95% means that the true value is between 42 and 95 with a probability of 95%. Previously published data on this patient population suggests that approximately 45% of patients would normally remain free from relapse at this stage. AST-VAC1 was found to have a favorable safety and tolerability profile in this study over multiple vaccinations, with up to 32 serial vaccinations administered (median = 17). Idiopathic thrombocytopenic purpura (bleeding into the skin caused by low platelets in blood) (grade 3-4) was reported in one patient. Other toxicities (grade 1-2) included rash or headache. These data from the Phase I/II trial were presented at the December 2010 American Society of Hematology annual meeting.
Expression of WT-1, a marker of minimal residual disease, was sequentially analyzed by qPCR (quantitative polymerase chain reaction – a method to identify DNA modules) in 21 patients. The 13 patients who remain in clinical remission remain negative for WT-1, while six of seven with clinical relapse were WT-1 positive. One patient was positive for WT-1 prior to vaccination with AST-VAC1 and became WT-1 negative during the course of vaccination. This patient relapsed after 30 months.
Patient immune response to telomerase after vaccination with AST-VAC1 was evaluated using a test called the enzyme-linked immunosorbent spot (“ELISPOT”) assay to measure the presence of activated T-cells specific to hTERT. Positive immune responses were detected in 55% of patients.
We have performed follow-up data collection on the 21 patients treated in the study at the six participating U.S. medical centers to determine the long term effects, if any, of the AST-VAC1 administration on remission duration and disease-free survival. We are in the process of preparing a manuscript describing our findings, and intend to submit an abstract for presentation at the American Society of Clinical Oncology meeting in June 2015.
Early Study of AST-VAC1 in Prostate Cancer
A prior clinical study using AST-VAC1 in metastatic hormone refectory prostate cancer was published in the Journal of Immunology in 2005. Telomerase loaded autologous monocyte-derived dendritic cells were administered to 20 patients with metastatic prostate cancer. Treatment was well tolerated with no significant adverse reactions. In 19 of 20 subjects, telomerase specific T lymphocytes were generated in the peripheral blood after vaccination. Vaccination was associated with a reduction of prostate-specific antigen velocity (a measure of disease progression), although no clinical responses were observed in this preliminary study. This study provided the rationale for the Phase I/II trial in AML described above.
Manufacturing and Process Development Technologies
The cGMP banks of undifferentiated hES cells that we acquired from Geron have been well characterized and validated, although they will need to be tested using validated equipment in order to verify their functionality after being stored under cryopreservation protocols. Both the H1 and H7 hES cell lines were routinely expanded under either cGMP (H1) or pilot (H7) conditions at Geron’s manufacturing facility. No limit to the expandability of hES cell lines has been observed. Geron’s cGMP cell banks of undifferentiated hES cells have been qualified for human biologics production per FDA guidelines. They are free of a long list of potential contaminants or adventitious agents of human or animal origin. They exhibit normal G-banding karyotype (chromosomal structure) and are considered suitable for the production of biologics for human clinical use. All of the therapeutic cells are manufactured according to a shared and standardized three stage procedure. Stage 1 is the expansion of the undifferentiated hES cells, currently performed in standard cell culture vessels coated with extracellular matrix. Stage 2 is the product specific differentiation step in which various factors are added sequentially to drive the differentiation of the hES cell down a desired and specific differentiation lineage. Stage 3 is the harvest, formulation, fill and finish stage in which the differentiated cells are aliquoted and stored frozen in the vapor phase of liquid nitrogen tanks indefinitely. Sensitive assays have been developed to detect the presence of contaminating undifferentiated hES cells in the various product formulations.
hES Cell Differentiation Patents Sublicensed by BioTime
We acquired from BioTime a non-exclusive, world-wide, royalty-free sublicense to use certain hES differentiation patents owned by BioTime’s subsidiary, ES Cell International Pte Ltd. (“ESI”). The ESI patents cover methods of modulating the differentiation of hES cells by inhibiting the spontaneous differentiation of hES cells in culture. The technology will be useful in maintaining and expanding populations of undifferentiated hES cells in culture which can then be used in directed differentiation protocols to obtain cells for potential therapeutic use.
cGMP hES Cell Lines Provided by BioTime
BioTime has developed research and clinical grade hES cell lines that it markets for both basic research and therapeutic product development. BioTime will provide us with ampules of five hES cell lines produced by ESI under cGMP. These hES cell lines are among the best characterized and documented cell lines available today, including documented full genomic sequencing. These hES cell lines are included in the Stem Cell Registry of the National Institutes of Health (“NIH”), making them eligible for use in federally funded research. These lines may be used as alternative starting material for producing some of the hES derived cell types acquired from Geron.
Patents and Trade Secrets
The patent portfolio that we acquired from Geron includes over 450 patents and patent applications owned or licensed to Geron relating to human hES cell-based product opportunities. This portfolio consists primarily of patents and patent applications owned by Geron, and also include patent families exclusively licensed to Geron by the third parties, and assigned to us.
The patent portfolio includes patents and patent applications covering a number of cell types that can be made from hES cells, including hepatocytes (liver cells), cardiomyocytes (heart muscle cells), neural cells (nerve cells, including dopaminergic neurons and oligodendrocytes), chondrocytes (cartilage cells), pancreatic islet β cells, osteoblasts (bone cells), hematopoietic cells (blood-forming cells) and dendritic cells. Also included in the patent portfolio are technologies for growing hES cells without the need for cell feeder layers, and novel synthetic growth surfaces.
Patents and Expiration Dates
The patents we acquired from Geron and that have been licensed to us by assignment of third party licenses have been issued in certain key countries and will expire at various times.
Oligodendrocyte progenitor cells: The patent rights relevant to oligodendrocyte progenitor cells include rights licensed from the University of California and various developed patent families covering the growth of hES cells and their differentiation into neural cells. There are issued patents in the United States, Australia, Canada, China, United Kingdom, Japan, Singapore and Israel. The expiration dates of these patents range from 2023 to 2029.
Cardiomyocytes: The patent rights relevant to cardiomyocytes include various patent families covering the growth of hES cells and their differentiation into cardiomyocytes. There are issued patents in the United States, Australia, Canada, China, United Kingdom, Hong Kong, Korea, Japan, India, Singapore and Israel. The expiration dates of these patents range from 2022 to 2029.
Pancreatic islet cells: The patent rights relevant to pancreatic islet cells include various patent families covering the growth of hES cells and their differentiation into pancreatic islet cells. There are issued patents in the United States, Australia, Canada, United Kingdom, Hong Kong, Korea, Japan, China, Singapore and Israel. The expiration dates of these patents are in 2022 to 2028.
Hepatocytes: The patent rights relevant to hepatocytes include various patent families covering the growth of hES cells and their differentiation into hepatocytes. There are issued patents in the United States, Australia, Canada, United Kingdom, Korea, India, Singapore and Israel. The expiration dates of these patents are in 2020 to 2029.
Neural cells: The patent rights relevant to neural cells include various patent families covering the growth of hES cells and their differentiation into neural cells. There are issued patents in the United States, Australia, Canada, United Kingdom, Japan, China, Hong Kong, India, Korea, Singapore and Israel. The expiration dates of these patents are in 2020 to 2023.
Hematopoietic cells: The patent rights relevant to hematopoietic cells include rights licensed from certain third parties and various patent families covering the growth of hES cells and their differentiation into hematopoietic cells. There are issued patents in the United States, Australia, United Kingdom, Singapore and Israel. The expiration dates of these patents are in 2022 to 2029.
Osteoblasts: The patent rights relevant to osteoblasts include various patent families covering the growth of hES cells and their differentiation into osteoblasts. There are issued patents in the Australia, United Kingdom, India, Singapore and Israel. The expiration dates of these patents are in 2022.
Chondrocytes: The patent rights relevant to chondrocytes include various patent families covering the growth of hES cells and their differentiation into chondrocytes. There are issued patents in the United States, Australia, Canada, Korea, Singapore and Israel. The expiration dates of these patents are in 2022 to 2023.
Dendritic cells: The patent rights relevant to dendritic cells include rights licensed from third parties and various patent families covering the growth of hES cells and their differentiation into dendritic cells. There are issued patents in the United States, Australia, Europe, Canada, China, Hong Kong, and Japan. The expiration dates of these patents range from 2019 to 2025.
Platform patents: The platform patent rights include various patent families covering the growth of hES cells. There are issued patents in the United States, Australia, Canada, United Kingdom, Hong Kong, China, India, Japan, Singapore and Israel. The expiration dates of these patents range from 2018 to 2030.
ViaCyte Patent Interference Proceedings
During May 2014, we entered into a settlement agreement with ViaCyte, Inc. (“ViaCyte”) concerning certain litigation in the United States District Court for the Northern District of California (Civil Action No. C12-04813) seeking the reversal of two adverse determinations by the United States Patent and Trademark Office with respect to two patent applications in U.S. Patent Interference 105,734, involving U.S. patent 7,510,876 (ViaCyte) and U.S. patent application 11/960,477 (Geron), and U.S. Patent Interference 105,827 involving U.S. patent 7,510,876 (ViaCyte) and U.S. patent application 12/543,875 (Geron), along with four Opposition Proceedings pending before the Australian Patent Office pertaining to priority rights and the validity of each party’s patents relating to endodermal precursor cells. Under the terms of the settlement agreement, the parties granted to each other a royalty free, fully paid license to each other’s technology relating to endoderm lineage cells including definitive endoderm and gut endoderm cells, only to the extent necessary to allow the licensee to make, use, sell, offer for sale, or import endodermal lineage cells. The Asterias patents that were licensed to ViaCyte in the settlement include US Patent Application No 11/161,633. The ViaCyte patents that were licensed to us in the settlement included US Patent Application Nos. 11/021,618, 11/093,590, 10/584,338, 11/165,305, 11/317,387, and 11/860,494.
General Risks Related to Obtaining and Enforcing Patent Protection
Our patents and patent applications are directed to compositions of matter, formulations, methods of use and/or methods of manufacturing, as appropriate. The patent positions of pharmaceutical and biotechnology companies, including ours, are generally uncertain and involve complex legal and factual questions. Our business could be negatively impacted by any of the following:
| · | The claims of any patents that are issued may not provide meaningful protection, may not provide a basis for commercially viable products or may not provide us with any competitive advantages; |
| · | Our patents may be challenged by third parties; |
| · | Others may have patents that relate to our technology or business that may prevent us from marketing our product candidates unless we are able to obtain a license to those patents; |
| · | The pending patent applications to which we have rights may not result in issued patents; |
| · | Our patents may expire; and |
| · | We may not be successful in developing additional proprietary technologies that are patentable |
In addition, others may independently develop similar or alternative technologies, duplicate any of our technologies and, if patents are licensed or issued to us, design around the patented technologies licensed to or developed by us. Moreover, we could incur substantial costs in litigation if we have to defend ourselves in patent lawsuits brought by third parties or if we initiate such lawsuits
In Europe, the European Patent Convention prohibits the granting of European patents for inventions that concern "uses of human embryos for industrial or commercial purposes." The European Patent Office is presently interpreting this prohibition broadly, and is applying it to reject patent claims that pertain to hES cells. However, this broad interpretation is being challenged through the European Patent Office appeals system. As a result, we do not yet know whether or to what extent we will be able to obtain patent protection for our hES cell technologies in Europe.
There is a risk that any patent applications that we file and any patents that we hold or later obtain could be challenged by third parties and be declared invalid or infringing on third party claims. A patent interference proceeding may be instituted with the United States Patent and Trademark Officer (“USPTO”) when more than one person files a patent application covering the same technology, or if someone wishes to challenge the validity of an issued patent on patents and applications filed before March 16, 2013. At the completion of the interference proceeding, the USPTO will determine which competing applicant is entitled to the patent, or whether an issued patent is valid. Patent interference proceedings are complex, highly contested legal proceedings, and the USPTO’s decision is subject to appeal. This means that if an interference proceeding arises with respect to any of our patent applications, we may experience significant expenses and delay in obtaining a patent, and if the outcome of the proceeding is unfavorable to us, the patent could be issued to a competitor rather than to us. For patents and applications filed after March 16, 2013 a derivation proceeding may be initiated where the USPTO may determine if one patent was derived from the work of an inventor on another patent. In addition to interference proceedings, the USPTO can re-examine issued patents at the request of a third party seeking to have the patent invalidated. After March 16, 2013 an inter partes review proceeding will allow third parties to challenge the validity of an issued patent where there is a reasonable likelihood of invalidity. This means that patents owned or licensed by us may be subject to re-examination and may be lost if the outcome of the re-examination is unfavorable to us. This means that patents owned or licensed by us may be subject to re-examination and may be lost if the outcome of the re-examination is unfavorable to us.
Post Grant Review under the new America Invents Act now makes available opposition-like proceedings in the United States. As with the USPTO interference proceedings, Post Grant Review proceedings will be very expensive to contest and can result in significant delays in obtaining patent protection or can result in a denial of a patent application. Also, a derivation proceeding may be instituted by the USPTO or an inventor alleging that a patent or application was derived from the work of another inventor.
Oppositions to the issuance of patents may be filed under European patent law and the patent laws of certain other countries. As with the USPTO interference proceedings, these foreign proceedings can be very expensive to contest and can result in significant delays in obtaining a patent or can result in a denial of a patent application.
The enforcement of patent rights often requires litigation against third-party infringers, and such litigation can be costly to pursue. Even if we succeed in having new patents issued or in defending any challenge to issued patents, there is no assurance that our patents will be comprehensive enough to provide us with meaningful patent protection against our competitors.
In addition to relying on patents, we rely on trade secrets, know-how, and continuing technological advancement to maintain our competitive position. We will enter into intellectual property, invention, and non-disclosure agreements with our employees, and it will be our practice to enter into confidentiality agreements with our consultants. There can be no assurance, however, that these measures will prevent the unauthorized disclosure or use of our trade secrets and know-how, or that others may not independently develop similar trade secrets and know-how or obtain access to our trade secrets, know-how, or proprietary technology.
Licensed Stem Cell Technology and Stem Cell Product Development Agreements
Telomerase Sublicense
We received the Telomerase Sublicense from Geron in connection with our acquisition of Geron’s stem cell assets. The Telomerase Sublicense grants us an exclusive sublicense under certain patents owned by the University of Colorado’s University License Equity Holdings, Inc. relating to telomerase and entitles us to use the technology covered by the patents in the development of AST-VAC1 and AST-VAC2 as immunological treatments for cancer. Under the Telomerase Sublicense, we paid Geron a one-time upfront license fee of $65,000, and we will pay Geron an annual license maintenance fee of $10,000 due on each anniversary of the effective date of the agreement, and a 1% royalty on sales of any products that we may develop and commercialize that are covered by the sublicensed patents. The Telomerase Sublicense will expire concurrently with the expiration of Geron’s license. That license will terminate during April 2017 when the licensed patents expire. The Telomerase Sublicense may also be terminated by us by giving Geron 90 days written notice, by us or by Geron if the other party breaches its obligations under the sublicense agreement and fails to cure their breach within the prescribed time period, or by us or by Geron upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings, or upon an assignment of a substantial portion of the assets for the benefit of creditors by the other party.
We are obligated to indemnify Geron, Geron’s licensor, and certain other parties for certain liabilities, including those for personal injury, product liability, or property damage relating to or arising from the manufacture, use, promotion or sale of a product, or the use by any person of a product made, created, sold or otherwise transferred by us or our sublicensees that is covered by the patents sublicensed under this agreement.
License Agreement with University of California
Geron assigned to us its Exclusive License Agreement with The Regents of the University of California for patents covering a method for directing the differentiation of multipotential hES cells to glial-restricted progenitor cells that generate pure populations of oligodendrocytes for remyelination and treatment of spinal cord injury. Pursuant to this agreement, we have an exclusive worldwide license under such patents, including the right to grant sublicenses, to create products for biological research, drug screening, and human therapy using the licensed patents.
Under the license agreement, we will be obligated to pay the university a royalty of 1% from sales of products that are covered by the licensed patent rights, and a minimum annual royalty of $5,000 starting in the year in which the first sale of a product covered by any licensed patent rights occurs, and continuing for the life of the applicable patent right under the agreement. The royalty payments due are subject to reduction, but not by more than 50%, to the extent of any payments that we may be obligated to pay to a third party for the use of patents or other intellectual property licensed from the third party in order to make, have made, use, sell, or import products or otherwise exercise our rights under the Exclusive License Agreement. We will be obligated to pay the university 7.5% of any proceeds, excluding debt financing and equity investments, and certain reimbursements, that we receive from sublicensees, other than our affiliates and joint ventures relating to the development, manufacture, purchase, and sale of products, processes, and services covered by the licensed patent.
The license agreement will terminate on the expiration of the last-to-expire of the university's issued licensed patents. If no further patents covered by the license agreement are issued, the license agreement would terminate in 2024. The university may terminate the agreement in the event of our breach of the agreement. We can terminate the agreement upon 60 days' notice.
World-Wide Non Exclusive WARF License
On October 7, 2013, we entered into a Non-Exclusive License Agreement with the Wisconsin Alumni Research Foundation (“WARF”) under which we were granted a worldwide non-exclusive license under certain WARF patents and WARF-owned embryonic stem cell lines to develop and commercialize therapeutic, diagnostic and research products. The licensed patents include patents covering primate embryonic stem cells as compositions of matter, as well as methods for growth and differentiation of primate embryonic stem cells. The licensed stem cell lines include the H1, H7, H9, H13 and H14 hES cell lines.
In consideration of the rights licensed to us, we have agreed to pay WARF an upfront license fee and have agreed to pay, payments upon the attainment of specified clinical development milestones, royalties on sales of commercialized products, and, subject to certain exclusions, a percentage of any payments that we may receive from any sublicenses that we may grant to use the licensed patents or stem cell lines.
The license agreement will terminate with respect to licensed patents upon the expiration of the last licensed patent to expire. We may terminate the license agreement at any time by giving WARF prior written notice. WARF may terminate the license agreement if payments of earned royalties, once begun, cease for a specified period of time or if we and any third parties collaborating or cooperating with us in the development of products using the licensed patents or stem cell lines fail to spend a specified minimum amount on research and development of products relating to the licensed patents or stem cell lines for a specified period of time.
WARF also has the right to terminate the license agreement if we breach the license agreement or become bankrupt or insolvent or if any of the licensed patents or stem cell lines are offered to creditors.
We will indemnify WARF and certain other designated affiliated entities from liability arising out of or relating to the death or injury of any person or damage to property due to the sale, marketing, use, or manufacture of products that are covered by the licensed patents, or licensed stem cells, or inventions or materials developed or derived from the licensed patents or stem cell lines.
Royalty Agreement with Geron
In connection with our acquisition of Geron’s stem cell assets, we entered into a Royalty Agreement with Geron pursuant to which we agreed to pay Geron a 4% royalty on net sales (as defined in the Royalty Agreement), by us or any of our affiliates or sales agents, of any products that we develop and commercialize that are covered by the patents Geron contributed to us. In the case of sales of such products by a person other than us or one of our affiliates or sales agents, we will be required to pay Geron 50% of all royalties and cash payments received by us or by our affiliate in respect of a product sale. Royalty payments will be subject to proration in the event that a product covered by a patent acquired from Geron is sold in combination with another product that is not covered by a patent acquired from Geron. The Royalty Agreement will terminate at the expiration or termination date of the last issued patent contributed by Geron under the Royalty Agreement. We estimate that the latest patent expiration date will be 2029.
Clinical Trial and Option Agreement with Cancer Research United Kingdom
During September 2014, we entered into a Clinical Trial and Option Agreement (the “CRUK Agreement”) with Cancer Research UK (“CRUK”) and Cancer Research Technology Limited, (“CRT”), a wholly-owned subsidiary of CRUK, pursuant to which CRUK has agreed to fund Phase I/IIa clinical development of our AST-VAC2 product candidate. We will, at our own cost, complete process development and manufacturing scale-up of the AST-VAC2 manufacturing process and will transfer the resulting cGMP-compatible process to CRUK. CRUK will, at its own cost, manufacture clinical grade AST-VAC2 and will carry out the Phase I/IIa clinical trial of AST-VAC2 in cancer patients in both resected early-stage and advanced forms of lung cancer. We will have an exclusive first option to obtain a license to use the data from the clinical trial. If we exercise that option we will be obligated to make payments upon the execution of the License Agreement, upon the achievement of various milestones, and then royalties on sales of products, and, if we sublicense product development or commercialization rights to a third party, we would pay CRT a share of any sublicense revenues we receive from the third party, with CRT’s share varying from a high of 40% in the case of a sublicense entered into prior to commencement of a Phase II clinical trial, to substantially lower rates in the case of a sublicense entered into at various later stages of clinical development but prior to completion of a Phase III clinical trial, and as low as 7.5% in the case of a sublicense entered into after completion of a Phase III clinical trial. In connection with the CRUK Agreement, we sublicensed to CRUK for use in the clinical trials and product manufacturing process certain patents that have been licensed or sublicensed to us by third parties. We would also be obligated to make payments to those licensors and sublicensors upon the achievement of various milestones, and then royalties on sales of products if AST-VAC2 is successfully developed and commercialized.
If we decline to exercise our option, CRT will then have an option to obtain a license to use our intellectual property relating to AST-VAC2 to continue the development and commercialization of AST-VAC2 and related products for which we will be entitled to receive a share of the revenue relating to development and partnering proceeds. The CRT’s option will be exercisable by CRT for four months from when our option expires.
The CRUK Agreement will expire upon the earliest of (i) the date we obtain a license to use the clinical data pursuant to an exercise of our option, (ii) the date CRT obtains a license to continue the development and commercialization of AST-VAC2 pursuant to an exercise of our option and (iii) the expiration of both our option and the CRT’s option. Notwithstanding the foregoing, any party may terminate the CRUK Agreement prior to its expiration for events including (i) a party materially breaches the agreement and such breach is not cured within 60 days after the non-breaching party delivers written notice, (ii) any party is insolvent or liquidated or (iii) if regulatory approval of the clinical trial is not obtained within two years after the parties complete the technology transfer phase of the agreement, or if regulatory approval is revoked, withdrawn or otherwise terminated, or if a regulatory authority orders a halt or hold on the clinical trial for more than 18 months. In addition, CRUK will have the right to terminate the CRUK Agreement under certain other circumstances.
The CRUK Agreement contains customary representations, warranties and covenants from us and CRUK, as well as customary provisions relating to indemnity, confidentiality and other matters.
Share Ownership in OrthoCyte and Cell Cure Neurosciences
Under the Asset Contribution Agreement, BioTime transferred to us a portion of their share ownership in two subsidiaries, OrthoCyte Corporation (“OrthoCyte”) and Cell Cure Neurosciences Ltd (“Cell Cure Neurosciences”). We received 10% of the shares of OrthoCyte and 6% of the ordinary shares of Cell Cure Neurosciences outstanding as of January 4, 2013.
OrthoCyte
OrthoCyte was organized in October 2010 and is developing cellular therapies to treat orthopedic disorders, diseases and injuries. Its lead products are human embryonic progenitor cell (“hEPC”) lines for cartilage repair. OrthoCyte has identified several progenitor cell lines that display chondrogenic (cartilage-producing) potential. These lines are currently in the pre-clinical testing phase to optimize effective cartilage repair.
As the population ages, osteoarthritis and spinal disc degeneration have a significant impact on mobility and health and current non-surgical treatments tend to target the reduction of pain and inflammation as opposed to repairing tissue deficits. To date, the development of cell-based therapeutics to treat damaged cartilage has met with mixed success. Autologous chondrocytes have been tested as a means to provide cartilage-producing cells but this approach is hampered by a multi-step process that first requires harvesting of chondrocytes from donor tissues, followed by in vitro culture expansion of the harvested cells. Primary chondrocytes have very limited capacity for in vitro expansion and will typically lose their biological characteristics within a short period of in vitro culture. Mesenchymal stem cells have been tested extensively as a source of cellular therapeutics for cartilage treatment but have met with very limited success, possibly as a result of their propensity to differentiate further into bone.
In a recently published study, OrthoCyte scientists, working in collaboration with scientists from BioTime’s subsidiary LifeMap Sciences, Inc., demonstrated that certain hEPC lines, derived using BioTime’s PureStem™ technology, are progenitors to diverse skeletal tissues of the human body. These cell lines bear diverse molecular markers that distinguish them from each other and from mesenchymal stem cells. The molecular markers of these cell lines suggest the lines may therefore be applicable to the repair of different types of bone, cartilage, and tendon for the treatment of degenerative diseases afflicting these tissue types such as non-healing bone fractures, osteoarthritis and degeneration of intervertebral discs, and tendon tears (tendinosis).
Cell Cure Neurosciences
Cell Cure Neurosciences is an Israel-based biotechnology company focused on developing stem cell-based therapies for retinal and neurological disorders, including the development of retinal pigment epithelial cells for the treatment of macular degeneration, and treatments for multiple sclerosis. Cell Cure Neurosciences’ lead product is OpRegen®, a proprietary formulation of embryonic stem cell-derived retinal pigmented epithelial (“RPE”) cells developed to address the high, unmet medical needs of people suffering from age-related macular degeneration (“dry AMD”).
On February 16, 2015, the first clinical trial of OpRegen® opened at Hadassah University Medical Center in Jerusalem. The clinical trial is entitled “Phase I/IIa Dose Escalation Safety and Efficacy Study of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium Cells Transplanted Subretinally in Patients with Advanced Dry-Form Age-Related Macular Degeneration with Geographic Atrophy”. Patient enrollment is expected to begin shortly. The Phase I/IIa clinical trial will evaluate three different dose regimens of OpRegen®. Following transplantation, the patients will be followed for 12 months at specified intervals, to evaluate the safety and tolerability of OpRegen®. Following the initial 12 month period, patients will continue to be monitored at longer intervals for an additional period of time. A secondary objective of the clinical trial will be to examine the ability of transplanted OpRegen® to engraft, survive, and moderate disease progression in the patients. In addition to thorough characterization of visual function, a battery of ophthalmic imaging modalities will be used to quantify structural changes and rate of geographic atrophy expansion.
AMD is the leading cause of blindness and visual impairment in the aging population, and 30% of Americans aged 75 and older have some form of AMD. The U.S. Centers for Disease Control and Prevention estimate that about 1.8 million people in the United States have advanced stage AMD and another 7.3 million have an earlier stage and are at risk of vision impairment from the disease. Most people are afflicted with the dry form of AMD, for which there is currently no effective treatment.
Manufacturing
We are subleasing from BioTime a 24,000 square-foot building in Menlo Park, California that was previously used by Geron for research and development and manufacturing for its hES programs.
We have also entered into a new lease for a 44,000 square foot facility in Fremont, California at which we plan to construct a cGMP compliant facility for the production of our product candidates, using a $4,400,000 tenant improvement allowance from the landlord. We anticipate initiating construction at the Fremont facility during the first quarter of 2015.
Marketing
Because our planned products are still in the research and development stage, we will not initially need to have our own marketing personnel. If we are successful in developing marketable products we will need to build our own marketing and distribution capability for our products, which would require the investment of significant financial and management resources, or we will need to find collaborative marketing partners, independent sales representatives, or wholesale distributors for the commercial sale of those products.
If we market products through arrangements with third parties, we may pay sales commissions to sales representatives or we may sell or consign products to distributors at wholesale prices. This means that our gross profit from product sales may be less than would be the case if we were to sell our products directly to end users at retail prices through our own sales force. On the other hand, selling to distributors or through independent sales representatives would allow us to avoid the cost of hiring and training our own sales employees. There can be no assurance we will be able to negotiate distribution or sales agreements with third parties on favorable terms to justify our investment in our products or achieve sufficient revenues to support our operations.
Competition
We face substantial competition in our business, and that competition is likely to intensify further as new products and technologies reach the market. Superior new products are likely to sell for higher prices and generate higher profit margins once acceptance by the medical community is achieved. Those companies that are successful in introducing new products and technologies to the market first may gain significant economic advantages over their competitors in the establishment of a customer base and track record for the performance of their products and technologies. Such companies will also benefit from revenues from sales that could be used to strengthen their research and development, production, and marketing resources. All companies engaged in the medical products industry face the risk of obsolescence of their products and technologies as more advanced or cost effective products and technologies are developed by their competitors. As the industry matures, companies will compete based upon the performance and cost effectiveness of their products.
The stem cell industry is characterized by rapidly evolving technology and intense competition. Our competitors include major multinational pharmaceutical companies, specialty biotechnology companies, and chemical and medical products companies operating in the fields of regenerative medicine, cell therapy, tissue engineering, and tissue regeneration. Many of these companies are well-established and possess technical, research and development, financial, and sales and marketing resources significantly greater than ours. In addition, certain smaller biotech companies have formed strategic collaborations, partnerships, and other types of joint ventures with larger, well established industry competitors that afford these companies’ potential research and development and commercialization advantages. Academic institutions, governmental agencies, and other public and private research organizations are also conducting and financing research activities which may produce products directly competitive to those we are developing.
We believe that some of our competitors are trying to develop hES cell, iPS cell, and mesenchymal stem cell-based technologies and products that may compete with our potential stem cell products based on efficacy, safety, cost, and intellectual property positions.
We may also face competition from companies that have filed patent applications relating to the cloning or differentiation of stem cells. We may be required to seek licenses from these competitors in order to commercialize certain of our proposed products, and such licenses may not be granted.
Government Regulation
Government authorities at the federal, state and local level, and in other countries, extensively regulate among other things, the development, testing, manufacture, quality, approval, distribution, labeling, packaging, storage, record keeping, marketing, import/export and promotion of drugs, biologics, and medical devices. Authorities also heavily regulate many of these activities for human cells, tissues and cellular and tissue-based products or HCT/Ps.
FDA and Foreign Regulation
We believe that the FDA will regulate most of our proposed products as biologicals. In the United States, the FDA regulates drugs and biologicals under the Federal Food, Drug and Cosmetic Act or FDCA, the Public Health Service Act, or PHSA, and implementing regulations. In addition, establishments that manufacture human cells, tissues, and cellular and tissue-based products are subject to additional registration and listing requirements, including current good tissue practice regulations. Many of our proposed products will be reviewed by the FDA staff in its Center for Biologics Evaluation and Research (“CBER”) Office of Cellular, Tissue and Gene Therapies.
Clinical Development
Our domestic biological products will be subject to rigorous FDA review and approval procedures. After testing in animals to evaluate the potential efficacy and safety of the product candidate, an IND must be submitted to the FDA to obtain authorization for human testing. Extensive clinical testing, which is generally done in three phases, must then be undertaken at one or more hospitals or medical centers to demonstrate optimal use, safety, and efficacy of each product in humans. Each clinical trial will also be subject to review by an independent Institutional Review Board (“IRB”) at each institution at which the trial will occur. The IRB will consider, among other things, ethical factors, the safety of human subjects, and the possible liability of the institution.
Clinical trials are generally conducted in three “phases.” Phase I clinical trials are conducted in a small number of healthy volunteers or volunteers with the target disease or condition to assess safety. Phase II clinical trials are conducted with groups of patients afflicted with the target disease or condition in order to determine preliminary efficacy, optimal dosages and expanded evidence of safety. In some cases, an initial trial is conducted in diseased patients to assess both preliminary efficacy and preliminary safety, in which case it is referred to as a Phase I/II trial. Phase III trials are large-scale, multi-center, comparative trials and are conducted with patients afflicted with the target disease or condition in order to provide enough data to demonstrate the efficacy and safety required by the FDA. The FDA closely monitors the progress of each of the three phases of clinical testing and may, at its discretion, re-evaluate, alter, suspend, or terminate the clinical trial based upon the data which have been accumulated to that point and its assessment of the risk/benefit ratio to the intended patient population. All adverse events must be reported to the FDA. Monitoring of all aspects of the study to minimize risks is a continuing process. The time and expense required to perform this clinical testing can far exceed the time and expense of the research and development initially required to create the product.
Applications for Marketing Approval
No action can be taken to market any therapeutic product in the United States until an appropriate application, which in the case of a cell therapy or vaccine product will be a Biologics License Application (“BLA”), has been approved by the FDA. Submission of the application is no guarantee that the FDA will find it complete and accept it for filing. If an application is accepted for filing, following the FDA’s review, the FDA may grant marketing approval, request additional information or deny the application if it determines that the application does not provide an adequate basis for approval. FDA regulations also restrict the export of therapeutic products for clinical use prior to BLA approval. To date, the FDA has not granted marketing approval to any hES-based therapeutic products and it is possible that the FDA or foreign regulatory agencies may subject our product candidates to additional or more stringent review than drugs or biologicals derived from other technologies.
The FDA offers several programs to expedite development of products that treat serious or life-threatening illnesses and that provide meaningful therapeutic benefits to patients over existing treatments. A product may be eligible for breakthrough therapy designation if it treats a serious or life-threatening disease or condition and preliminary clinical evidence indicates it may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Features of breakthrough therapy designation include intensive guidance from FDA on an efficient development program, intensive involvement of FDA staff in a proactive, collaborative review process, and rolling review of marketing applications. Under its accelerated approval regulations, the FDA may approve a product based on a surrogate endpoint that is reasonably likely to predict clinical benefits or based on an effect on a clinical endpoint other than survival or irreversible morbidity. The applicant will then be required to conduct additional, post-approval confirmatory trials to verify and describe clinical benefit, and the product may have certain post-marketing restrictions as necessary to assure safe use. The FDA may withdraw approval granted under the traditional route or under an accelerated approval, if it is warranted. The FDA may also consider ways to use the accelerated approval pathway for rare or very rare diseases, and a new review designation has been created to help foster the innovation of promising new therapies with the potential to shorten the timeframe for conducting pivotal trials and speed up patient access to the approved product. There is no assurance that the FDA will grant breakthrough therapy or accelerated approval status to any of our product candidates.
Combination Products
If we develop any products that are used with medical devices, they may be considered combination products, which are defined by the FDA to include products comprised of two or more regulated components or parts such as a biologic and a device. For example, we may seek a license from BioTime to use certain of their hydrogel products to administer one or more of our hES cell-based therapy products. When regulated independently, biologics and devices each have their own regulatory requirements. However, the regulatory requirements for a combination product comprised of a biologic administered with a delivery device can be more complex, because in addition to the individual regulatory requirements for each component, additional combination product regulatory requirements may apply. There is an Office of Combination Products at the FDA that coordinates the review of such products and determines the primary mode of action of a combination product. The definition and regulatory requirements for combination products may differ significantly among other countries in which we may seek approval of our product candidates.
Post-Approval Matters
Even after initial FDA approval has been obtained, further studies may be required to provide additional data on safety or to gain approval for the use of a product as a treatment for clinical indications other than those initially targeted. Use of a product during testing and after marketing could reveal side effects that could delay, impede, or prevent FDA marketing approval, result in an FDA-ordered product recall, or in FDA-imposed limitations on permissible uses or in withdrawal of approval. For example, if the FDA becomes aware of new safety information after approval of a product, it may require us to conduct further clinical trials to assess a known or potential serious risk and to assure that the benefit of the product outweigh the risks. If we are required to conduct such a post-approval study, periodic status reports must be submitted to the FDA. Failure to conduct such post-approval studies in a timely manner may result in substantial civil or criminal penalties. Data resulting from these clinical trials may result in expansions or restrictions to the labeled indications for which a product has already been approved.
FDA Regulation of Manufacturing
The FDA regulates the manufacturing process of pharmaceutical products, and human tissue and cell products, requiring that they be produced in compliance with cGMP. See “Manufacturing.” The FDA regulates and inspects equipment, facilities, laboratories and processes used in the manufacturing and testing of products prior to providing approval to market products. If after receiving approval from the FDA, a material change is made to manufacturing equipment or to the location or manufacturing process, additional regulatory review may be required. The FDA also conducts regular, periodic visits to re-inspect the equipment, facilities, laboratories and processes of manufacturers following an initial approval. If, as a result of those inspections, the FDA determines that that equipment, facilities, laboratories or processes do not comply with applicable FDA regulations and conditions of product approval, the FDA may seek civil, criminal or administrative sanctions and/or remedies against the manufacturer, including suspension of manufacturing operations. Issues pertaining to manufacturing equipment, facilities or processes may also delay the approval of new products undergoing FDA review.
FDA Regulation of Advertising and Product Promotion
The FDA also regulates the content of advertisements used to market pharmaceutical and biological products. Claims made in advertisements concerning the safety and efficacy of a product, or any advantages of a product over another product, must be supported by clinical data filed as part of a BLA or an amendment to a BLA, and must be consistent with the FDA approved labeling and dosage information for that product.
Foreign Regulation
Sales of pharmaceutical and biological products outside the United States are subject to foreign regulatory requirements that vary widely from country to country. Even if FDA approval has been obtained, approval of a product by comparable regulatory authorities of foreign countries must be obtained prior to the commencement of marketing the product in those countries. The time required to obtain such approval may be longer or shorter than that required for FDA approval.
Federal Funding of Research
The United States government and its agencies have until recently been prevented from funding research which involves the use of human embryonic tissue. President Bush issued Executive Orders on August 9, 2001 and June 20, 2007 that permitted federal funding of research on hES cells using only the limited number of hES cell lines that had already been created as of August 9, 2001. On March 9, 2009, President Obama issued an Executive Order rescinding President Bush’s August 9, 2001 and June 20, 2007 Executive Orders. President Obama’s Executive Order also instructed the National Institutes of Health to review existing guidance on human stem cell research and to issue new guidance on the use of hES cells in federally funded research, consistent with President’s new Executive Order and existing law. The NIH has adopted new guidelines that went into effect July 7, 2009. The central focus of the new guidelines is to assure that hES cells used in federally funded research were derived from human embryos that were created for reproductive purposes, were no longer needed for this purpose, and were voluntarily donated for research purposes with the informed written consent of the donors. Those hES cells that were derived from embryos created for research purposes rather than reproductive purposes, and other hES cells that were not derived in compliance with the guidelines, are not eligible for use in federally funded research.
California State Regulations
The state of California has adopted legislation and regulations that require institutions that conduct stem cell research to notify, and in certain cases obtain approval from, a Stem Cell Research Oversight Committee (“SCRO Committee”) before conducting the research. Advance notice, but not approval by the SCRO Committee, is required in the case of in vitro research that does not derive new stem cell lines. Research that derives new stem cell lines, or that involves fertilized human oocytes or blastocysts, or that involves clinical trials or the introduction of stem cells into humans, or that involves introducing stem cells into animals, requires advanced approval by the SCRO Committee. Clinical trials may also entail approvals from an IRB at the medical center at which the study is conducted, and animal studies may require approval by an Institutional Animal Care and Use Committee.
All hES cell lines that will be used in our research must be acceptably derived. To be acceptably derived, the pluripotent stem cell line must have either:
| · | Been listed on the National Institutes of Health Human Embryonic Stem Cell Registry, or |
| · | Been deposited in the United Kingdom Stem Cell Bank, or |
| · | Been derived by, or approved for use by, a licensee of the United Kingdom Human Fertilisation and Embryology Authority, or |
| · | Been derived in accordance with the Canadian Institutes of Health Research Guidelines for Human Stem Cell Research under an application approved by the National Stem Cell Oversight Committee, or |
| · | Been derived in accordance with the Japanese Guidelines for Derivation; or |
| · | Been approved by CIRM in accordance with California Code of Regulations Title 17, Section 100081; or |
| · | Been derived under the following conditions: |
| (a) | Donors of gametes, embryos, somatic cells, or human tissue gave voluntary and informed consent; |
| (b) | Donors of gametes, embryos, somatic cells, or human tissue did not receive valuable consideration. This provision does not prohibit reimbursement for permissible expenses as determined by an IRB; |
| (c) | Donation of gametes, embryos, somatic cells, or human tissue was overseen by an IRB (or, in the case of foreign sources, an IRB-equivalent); and |
| (d) | Individuals who consented to donate stored gametes, embryos, somatic cells, or human tissue were not reimbursed for the cost of storage prior to the decision to donate. |
Other hES lines may be deemed acceptably derived if they were derived in accordance with (a), (b), and (d) above and the hES line was derived prior to the publication of the National Academy of Sciences guidelines on April 26, 2005 and a SCRO Committee has determined that the investigator has provided sufficient scientific rationale for the need for use of the line, which should include establishing that the proposed research cannot reasonably be carried out with covered lines that did have IRB approval.
California regulations also require that certain records be maintained with respect to stem cell research and the materials used, including:
| · | A registry of all human stem cell research conducted, and the source(s) of funding for this research. |
| · | A registry of human pluripotent stem cell lines derived or imported, to include, but not necessarily limited to: |
| (a) | The methods utilized to characterize and screen the materials for safety; |
| (b) | The conditions under which the materials have been maintained and stored; |
| (c) | A record of every gamete donation, somatic cell donation, embryo donation, or product of somatic cell nuclear transfer that has been donated, created, or used; |
| (d) | A record of each review and approval conducted by the SCRO Committee. |
California Proposition 71
During November 2004, California State Proposition 71 (“Prop. 71”), the California Stem Cell Research and Cures Initiative, was adopted by state-wide referendum. Prop. 71 provides for a state-sponsored program designed to encourage stem cell research in the State of California, and to finance such research with State funds totaling approximately $295,000,000 annually for 10 years beginning in 2005. This initiative created CIRM, which will provide grants, primarily but not exclusively, to academic institutions to advance both hES cell research and adult stem cell research.
Medicare, Medicaid, and Similar Reimbursement Programs
Sales of our products will depend, in part, on the extent to which the costs of our products will be covered by third-party payors, such as government health programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly challenging the prices charged for medical products and services. Additionally, the containment of healthcare costs has become a priority of federal and state governments and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. If these third-party payors do not consider our products to be cost-effective compared to other therapies, they may not cover our products after approved as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010, collectively referred to as the ACA, enacted in March 2010, is expected to have a significant impact on the health care industry. ACA is expected to expand coverage for the uninsured while at the same time containing overall healthcare costs. With regard to pharmaceutical products, among other things, ACA is expected to expand and increase industry rebates for drugs covered under Medicaid programs and make changes to the coverage requirements under the Medicare Part D program. We cannot predict the impact of ACA on pharmaceutical companies, as many of the ACA reforms require the promulgation of detailed regulations implementing the statutory provisions which has not yet occurred. In addition, although the United States Supreme Court upheld the constitutionality of most of the ACA, some states have indicated that they intend to not implement certain sections of the ACA, and some members of the U.S. Congress are still working to repeal parts of the ACA. These challenges add to the uncertainty of the legislative changes enacted as part of ACA.
In addition, in some non-U.S. jurisdictions, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the United States and generally tend to be significantly lower.
Employees
As of December 31, 2014 we employed 25 persons on a full time basis. Thirteen of our employees hold Ph.D. degrees in one or more fields of science.
Our business is subject to various risks, including those described below. You should consider the following risk factors, together with all of the other information included in this report, which could materially adversely affect our proposed operations, our business prospects, and financial condition, and the value of an investment in our business. There may be other factors that are not mentioned here or of which we are not presently aware that could also affect our business operations and prospects.
Risks Related to Our Business Operations
We have a history of operating losses and negative cash flows
Since our inception in September 2012, we have incurred operating losses and negative cash flow, and we expect to continue to incur losses and negative cash flow in the future. Our net losses for the fiscal years ended December 31, 2014, and 2013, the period from September 2012 (inception) to December 31, 2012, and the period from September 2012 (inception) to December 31, 2014 were $10,097,154, $22,379,744, $758,893 and $33,235,791, respectively, and we had an accumulated deficit of $33,235,791 and $23,138,637 as of December 31, 2014 and 2013, respectively. Our net loss for the year ended December 31, 2013 and our accumulated deficit as of that date include $17,458,766 charged as in-process research and development expenses (“IPR&D”) in accordance with Accounting Standards Codification (“ASC”) 805-50 on account of our acquisition of certain assets from Geron. See Notes 2 and 3 to Financial Statements. BioTime previously funded our formation and operating costs but we do not expect BioTime to continue to do so in the future. We have limited cash resources and will depend upon future equity financings, research grants, financings through collaborations with third parties, and sales of BioTime common shares that we as a source of funding for our operations. There is no assurance that we will be able to obtain the financing we need from any of those sources, or that any such financing that may become available will be on terms that are favorable to us and our shareholders.
Failure to attract and retain skilled personnel and key relationships could impair our research and development efforts
Our operations are still in the start-up stage and we had only 25 employees as of December 31, 2014. We will need to recruit and hire additional qualified research scientists, laboratory technicians, clinical development, and management personnel. Competition for these types of personnel is intense and we may experience delays in hiring the qualified people that we need. The inability to attract and retain sufficient qualified management, scientific, or technical personnel may significantly delay or prevent the achievement of our product development and other business objectives and could have a material adverse effect on our business, operating results and financial condition. We partially rely on BioTime to provide financial accounting management and personnel, human resources, and intellectual property management. We will also rely on consultants and advisors who are either self-employed or employed by other organizations, and they may have conflicts of interest or other commitments, such as consulting or advisory contracts with other organizations, that may affect their ability to perform services for us.
We will spend a substantial amount of our capital on research and development but we might not succeed in developing products and technologies that are useful in medicine
| · | The product development work we plan to do is costly, time consuming and uncertain as to its results. |
| · | We will attempt to develop new medical products and technologies that might not prove to be safe and efficacious in human medical applications. Many of the products and technologies that we will seek to develop have not been applied in human medicine and have only been used in laboratory studies in vitro or in animals. Only two of the product candidates that we have acquired have been used in clinical trials, and those were early stage trials involving only a small number of patients. |
| · | If we are successful in developing a new technology or product, refinement of the new technology or product and definition of the practical applications and limitations of the technology or product may take years and require the expenditure of large sums of money. |
The amount and pace of research and development work that we can do or sponsor, and our ability to commence and complete clinical trials required to obtain FDA and foreign regulatory approval of our products, depends upon the amount of money available to us
| · | We may have to limit our laboratory research and development work based on the amount of our cash resources. |
| · | We plan to seek research and development grants from government agencies and to enter into collaborative product development agreements through which third parties will provide funding or otherwise bear the cost of research and development or clinical trials of our product candidates. There is no assurance that the amount of any grants that we may receive will be adequate for our needs. The agreements we entered into to date with CIRM and CRUK are subject to termination if certain milestones are not achieved. Hence, there is no assurance that we will receive the full value of the agreement with either entity. |
| · | Unless we are able to generate sufficient revenue or raise additional funds when needed, it is likely that we will be unable to continue our planned activities, even if we make progress in our research and development projects. |
We will need to issue additional equity or debt securities in order to raise additional capital needed to pay our operating expenses
| · | We plan to incur substantial research and product development expenses, and we will need to raise additional capital to pay operating expenses until we are able to generate sufficient revenues from product sales, royalties, and license fees. |
| · | It is likely that additional sales of equity or debt securities will be required in the near future to meet our short-term capital needs, unless we receive substantial research grants and revenues from the sale of any products that receive regulatory approval or we are successful in licensing or sublicensing our technology and we receive substantial licensing fees and royalties. |
| · | Sales of additional equity securities could result in the dilution of the interests of present shareholders. |
The condition of certain cells, cell lines and other biological materials that we acquired from Geron could impact the time and cost of commencing our research and product development programs
The cells, cell lines and other biological materials that we acquired are being stored under cryopreservation protocols intended to preserve their functionality. We have successfully completed the verification of the viability of three lots of AST-OPC1 cells that we intend to use in clinical trials. However, the functional condition of the other materials cannot be certified until they are tested in an appropriate laboratory setting by qualified scientific personnel using validated equipment. We intend to perform that testing on the cells that we intend to use in our research and development programs as the need arises.
To the extent that the cells we plan to use are not sufficiently functional for our purposes, we would need to incur the time and expense of regenerating cell lines from cell banks, or regenerating cell banks from cell stocks, which could delay and increase the cost of our research and development work using those cells.
Sales of any products we may develop may be adversely impacted by the availability of competing products
| · | In order to compete with other products, particularly those that sell at lower prices, our products will have to provide medically significant advantages. |
| · | Physicians and hospitals may be reluctant to try a new product due to the high degree of risk associated with the application of new technologies and products in the field of human medicine. |
| · | There also is a risk that our competitors may succeed at developing safer or more effective products that could render our products and technologies obsolete or noncompetitive. |
Any products that receive regulatory approval may be difficult and expensive to manufacture on a commercial scale
| · | hES derived therapeutic cells have only been produced on a small scale and not in quantities and at levels of purity and viability that will be needed for wide scale commercialization. If we are successful in developing products that consist of hES cells or other cells or products derived from hES or other cells, we will need to develop, alone or in collaboration with one or more pharmaceutical companies or contract manufacturers, technology for the commercial production of those products. |
| · | Our hES cell or other cell-based products are likely to be more expensive to manufacture on a commercial scale than most other drugs on the market today. The high cost of manufacturing a product will require that we charge our customers a high price for the product in order to cover our costs and earn a profit. If the price of our products is too high, hospitals and physicians may be reluctant to purchase our products, especially if lower priced alternative products are available, and we may not be able to sell our products in sufficient volumes to recover our costs of development and manufacture or to earn a profit. |
We do not have our own marketing, distribution, and sales resources for the commercialization of any products that we might successfully develop
| · | If we are successful in developing marketable products, we will need to build our own marketing, distribution, and sales capability for our products, which would require the investment of significant financial and management resources, or we will need to find collaborative marketing partners, independent sales representatives, or wholesale distributors for the commercial sale of our products. |
| · | If we market products through arrangements with third parties, we may pay sales commissions to sales representatives or we may sell or consign products to distributors at wholesale prices. As a result, our gross profit from product sales may be lower than it would be if we were to sell our products directly to end users at retail prices through our own sales force. |
| · | There can be no assurance that we will able to negotiate distribution or sales agreements with third parties on favorable terms to justify our investment in our products or achieve sufficient revenues to support our operations. |
We do not have the ability to independently conduct clinical trials required to obtain regulatory approvals for our therapeutic product candidates
We will need to rely on third parties, such as CRUK, contract research organizations, data management companies, contract clinical research associates, medical institutions, clinical investigators and contract laboratories to conduct any clinical trials that we may undertake for our products. We may also rely on third parties to assist with our preclinical development of therapeutic product candidates. If we outsource clinical trials, we may be unable to directly control the timing, conduct and expense of our clinical trials. If we enlist third parties to conduct clinical trials and they fail to successfully carry out their contractual duties or regulatory obligations or fail to meet expected deadlines, if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to clinical protocols or regulatory requirements or for other reasons, our preclinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our therapeutic product candidates.
We will have certain obligations and may incur liabilities arising from clinical trials, and we do not yet know the scope of any resulting expenses that might arise
We face the risk of incurring liabilities to patients who participate in clinical trials of our product candidates if they incur any injuries as a result of their participation. We will also be obligated to obtain information and prepare reports about the health of the clinical trial patients. In addition, we have assumed Geron’s obligations to obtain information and prepare reports about the health of patients, and we have assumed any liabilities to those patients that might arise from any injuries they may have incurred, as a result of their participation in the clinical trials of Geron’s GRN-OPC1 cell replacement therapy for spinal cord damage and its GRN-VAC1 immunological therapy for certain cancers. We are not aware of any claims by patients alleging injuries suffered as a result of any of those clinical trials, but if any claims are made and if liability can be established, the amount of any liability that we may incur, depending upon the nature and extent of any provable injuries, could exceed our insurance coverage, and the amount of the liability could be material to our financial condition.
Our business could be adversely affected if we lose the services of the key personnel upon whom we depend
Our stem cell research program will be directed primarily by our President and Chief Executive Officer Pedro Lichtinger and by our President of Research and Development, Dr. Jane S. Lebkowski. The loss of the services of Mr. Lichtinger or Dr. Lebkowski could have a material adverse effect on us.
Our business and operations could suffer in the event of system failures
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruption of our operations. For example, the loss of data for our product candidates could result in delays in our regulatory filings and development efforts and significantly increase our costs. To the extent that any disruption or security breach was to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our product candidates could be delayed.
Failure of our internal control over financial reporting could harm our business and financial results
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting for external purposes in accordance with accounting principles generally accepted in the U.S. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our financial statements; providing reasonable assurance that receipts and expenditures of our assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected. Our growth and entry into new products, technologies and markets will place significant additional pressure on our system of internal control over financial reporting. Any failure to maintain an effective system of internal control over financial reporting could limit our ability to report our financial results accurately and timely or to detect and prevent fraud.
We will initially rely in part on financial systems maintained by BioTime and upon services provided by BioTime personnel. BioTime will allocate certain expenses among itself, us, and BioTime’s other subsidiaries, which creates a risk that the allocations may not accurately reflect the benefit of an expenditure or use of financial or other resources by us, BioTime as our parent company, and the BioTime subsidiaries among which the allocations are made.
Risks Related to Our Industry
We will face certain risks arising from regulatory, legal, and economic factors that affect our business and the business of other pharmaceutical and biological product development companies. Because we are a small company with limited revenues and limited capital resources, we may be less able to bear the financial impact of these risks than larger companies that have substantial income and available capital.
If we do not receive FDA and other regulatory approvals we will not be permitted to sell our products
The cell-based products that we are developing cannot be sold until the FDA and corresponding foreign regulatory authorities approve the products for medical use. To date, long-term safety and efficacy has not been demonstrated in clinical trials for any of our therapeutic product candidates. The need to obtain regulatory approval to market a new product means that:
| · | we will have to conduct expensive and time consuming clinical trials of new products. The full cost of conducting and completing clinical trials necessary to obtain FDA and foreign regulatory approval of a new product cannot be presently determined, but could exceed our current financial resources; |
| · | clinical trials and the regulatory approval process for a cell-based product can take several years to complete. As a result, we will incur the expense and delay inherent in seeking FDA and foreign regulatory approval of new products, even if the results of clinical trials are favorable; |
| · | data obtained from preclinical and clinical studies is susceptible to varying interpretations that could delay, limit, or prevent regulatory agency approvals. Delays in the regulatory approval process or rejections of an application for approval of a new drug or cell-based product may be encountered as a result of changes in regulatory agency policy; |
| · | because the therapeutic products we plan to develop with hES and iPS technology involve the application of new technologies and approaches to medicine, the FDA or foreign regulatory agencies may subject those products to additional or more stringent review than drugs or biologics derived from other technologies. No therapeutic product based on hES or iPS technology has been approved by the FDA to date. |
| · | a product that is approved may be subject to restrictions on use; |
| · | the FDA can limit or withdraw approval of a product if problems arise; and |
| · | we will face similar regulatory issues in foreign countries. |
Clinical trial failures can occur at any stage of the testing and we may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent commercialization of our current or future therapeutic product candidates
All of our product candidates are either at early stages of clinical development or at the preclinical or research stages of development. Clinical trial failures or delays can occur at any stage of the trials, and may be directly or indirectly caused by a variety of factors, including but not limited to:
| · | delays in securing clinical investigators or trial sites for our clinical trials; |
| · | delays in obtaining Institutional Review Board (“IRB”) and other regulatory approvals to commence a clinical trial; |
| · | slower than anticipated rates of patient recruitment and enrollment, or failing to reach the targeted number of patients due to competition for patients from other trials; |
| · | limited or no availability of coverage, reimbursement and adequate payment from health maintenance organizations and other third party payors for the use of agents used in our clinical trials; |
| · | negative or inconclusive results from clinical trials; |
| · | unforeseen side effects interrupting, delaying, or halting clinical trials of our therapeutic product candidates, and possibly resulting in the FDA or other regulatory authorities denying approval of our therapeutic product candidates; |
| · | unforeseen safety issues; |
| · | uncertain dosing issues; |
| · | approval and introduction of new therapies or changes in standards of practice or regulatory guidance that render our clinical trial endpoints or the targeting of our proposed indications obsolete; |
| · | inability to monitor patients adequately during or after treatment or problems with investigator or patient compliance with the trial protocols; |
| · | inability to replicate in large controlled studies safety and efficacy data obtained from a limited number of patients in uncontrolled trials; |
| · | inability or unwillingness of medical investigators to follow our clinical protocols; and |
| · | unavailability of clinical trial supplies. |
Government imposed bans or restrictions, and religious, moral and ethical concerns on the use of hES cells could prevent us from developing and successfully marketing stem cell products
| · | Government imposed bans or restrictions on the use of embryos or hES cells research and development in the United States and abroad could generally constrain stem cell research, thereby limiting the market and demand for any of our products that receive regulatory approval. In March 2009, President Obama lifted certain restrictions on federal funding of research involving the use of hES cells, and in accordance with President Obama’s executive order, the National Institutes of Health has adopted new guidelines for determining the eligibility of hES cell lines for use in federally funded research. The central focus of the proposed guidelines is to assure that hES cells used in federally funded research were derived from human embryos that were created for reproductive purposes, were no longer needed for this purpose, and were voluntarily donated for research purposes with the informed written consent of the donors. hES cells that were derived from embryos created for research purposes rather than reproductive purposes, and other hES cells that were not derived in compliance with the guidelines, are not eligible for use in federally funded research. |
| · | California law requires that stem cell research be conducted under the oversight of a stem cell research oversight (“SCRO”) committee. Many kinds of stem cell research, including the derivation of new hES cell lines, may only be conducted in California with the prior written approval of the SCRO. A SCRO could prohibit or impose restrictions on the research we plan to do. |
| · | The use of hES cells gives rise to religious, moral and ethical issues regarding the appropriate means of obtaining the cells and the appropriate use and disposal of the cells. These considerations could lead to more restrictive government regulations or could generally constrain stem cell research thereby limiting the market and demand for any of our products that receive regulatory approval. |
If we are unable to obtain and enforce patents and to protect our trade secrets, others could use our technology to compete with us, which could limit opportunities for us to generate revenues by licensing our technology and selling products
| · | Our success will depend in part on our ability to obtain and enforce patents and maintain trade secrets in the United States and in other countries. If we are unsuccessful in obtaining and enforcing patents, our competitors could use our technology and create products that compete with our products, without paying license fees or royalties to us. |
| · | The preparation, filing, and prosecution of patent applications can be costly and time consuming. Our limited financial resources may not permit us to pursue patent protection of all of our technology and products throughout the world. |
| · | Even if we are able to obtain issued patents covering our technology or products, we may have to incur substantial legal fees and other expenses to enforce our patent rights in order to protect our technology and products from infringing uses. We may not have the financial resources to finance the litigation required to preserve our patent and trade secret rights. |
There is no certainty that our pending or future patent applications will result in the issuance of patents
| · | We have acquired patent applications for technology that Geron developed, and we obtained licenses for a number of patent applications covering technology developed by others that we believe will be useful in producing new products, and which we believe may be of commercial interest to other companies that may be willing to sublicense the technology for fees or royalty payments. We may also file new patent applications in the future seeking patent protection for new technology or products that we develop ourselves or jointly with others. However, there is no assurance that any of the patent applications that we acquired or any licensed patent applications or any future patent applications that we may file in the United States or abroad will result in the issuance of patents. |
| · | In Europe, the European Patent Convention prohibits the granting of European patents for inventions that concern "uses of human embryos for industrial or commercial purposes." The European Patent Office is presently interpreting this prohibition broadly, and is applying it to reject patent claims that pertain to hES cells. However, this broad interpretation is being challenged through the European Patent Office appeals system. As a result, we do not yet know whether or to what extent we will be able to obtain patent protection for our hES cell technologies in Europe. |
The patent protection for our product candidates or products may expire before we are able to maximize their commercial value, which may subject us to increased competition and reduce or eliminate our opportunity to generate product revenue
The patents for our product candidates have varying expiration dates. When these patents expire, we may be subject to increased competition and our opportunity to establish or maintain product revenue could be substantially reduced or eliminated. As a result we may not be able to recover our development costs. In some of the larger economic territories, such as the United States and Europe, patent term extension/restoration may be available to compensate for time taken during aspects of the product candidate's regulatory review. We cannot, however, be certain that an extension will be granted or, if granted, what the applicable time period or the scope of patent protection afforded during any extended period will be. In addition, even though some regulatory agencies may provide some other exclusivity for a product candidate under its own laws and regulations, we may not be able to qualify the product candidate or obtain the exclusive time period.
The process of applying for and obtaining patents can be expensive and slow
| · | The preparation and filing of patent applications, and the maintenance of patents that are issued, may require substantial time and money. |
| · | A patent interference proceeding may be instituted with the USPTO when more than one person files a patent application covering the same technology, or if someone wishes to challenge the validity of an issued patent. At the completion of the interference proceeding, the USPTO will determine which competing applicant is entitled to the patent, or whether an issued patent is valid. Patent interference proceedings are complex, highly contested legal proceedings, and the USPTO’s decision is subject to appeal. This means that if an interference proceeding arises with respect to any of our patent applications, we may experience significant expenses and delay in obtaining a patent, and if the outcome of the proceeding is unfavorable to us, the patent could be issued to a competitor rather than to us. |
| · | A derivation proceeding may be instituted by the USPTO or an inventor alleging that a patent or application was derived from the work of another inventor. |
| · | Post Grant Review under the new America Invents Act will make available opposition-like proceedings in the United States. As with the USPTO interference proceedings, Post Grant Review proceedings will be very expensive to contest and can result in significant delays in obtaining patent protection or can result in a denial of a patent application. |
| · | Oppositions to the issuance of patents may be filed under European patent law and the patent laws of certain other countries. As with the USPTO interference proceedings, these foreign proceedings can be very expensive to contest and can result in significant delays in obtaining a patent or can result in a denial of a patent application. |
We may be subject to patent infringement claims that could be costly to defend, which may limit our ability to use disputed technologies, and which could prevent us from pursuing research and development or commercialization of some of our products, require us to pay licensing fees to have freedom to operate and/or result in monetary damages or other liability for us
The success of our business will depend significantly on our ability to operate without infringing patents and other proprietary rights of others. If the technology that we use infringes a patent held by others, we could be sued for monetary damages by the patent holder or its licensee, or we could be prevented from continuing research, development, and commercialization of products that rely on that technology, unless we are able to obtain a license to use the patent. The cost and availability of a license to a patent cannot be predicted, and the likelihood of obtaining a license at an acceptable cost would be lower if the patent holder or any of its licensees is using the patent to develop or market a product with which our product would compete. If we could not obtain a necessary license, we would need to develop or obtain rights to alternative technologies, which could prove costly and could cause delays in product development, or we could be forced to discontinue the development or marketing of any products that were developed using the technology covered by the patent.
Our patents may not protect any of our products from competition
We have acquired patents and patent applications filed in the United States, Canada, the European Union countries, and in other foreign countries for a variety of hES and iPS technologies.
| · | We might not be able to obtain any additional patents, and any patents that we do obtain might not be comprehensive enough to provide us with meaningful patent protection. |
| · | There will always be a risk that our competitors might be able to successfully challenge the validity or enforceability of any patent issued to us. |
| · | In addition to interference proceedings, the USPTO can reexamine issued patents at the request of a third party seeking to have the patent invalidated. This means that patents owned or licensed by us may be subject to reexamination and may be lost if the outcome of the reexamination is unfavorable to us. Our patents may be subject to inter partes review (replacing the reexamination proceeding), a proceeding in which a third party can challenge the validity of one of our patents. |
If we fail to meet our obligations under license agreements, we may lose our rights to key technologies on which our business depends
Our business will depend in part on several technologies that are based in part on technology licensed from third parties, including the University of Colorado, the University of California, and the Wisconsin Alumni Research Foundation. Those third-party license agreements impose obligations on us, including payment obligations and obligations to pursue development of commercial products under the licensed patents or technology. If a licensor believes that we have failed to meet our obligations under a license agreement, the licensor could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation our ability to carry out the development and commercialization of potential products, and our ability to raise capital, could be significantly and negatively affected. If our license rights were restricted or ultimately lost, we would not be able to continue to use the licensed technology in our business.
The price and sale of any of our products that receive regulatory approval may be limited by health insurance coverage and government regulation
Success in selling any of our products that receive regulatory approval may depend in part on the extent to which health insurance companies, HMOs, and government health administration authorities such as Medicare and Medicaid will pay for the cost of the products and related treatment. Until we actually introduce a new product into the medical market place we will not know with certainty whether adequate health insurance, HMO, and government coverage will be available to permit the product to be sold at a price high enough for us to generate a profit. In some foreign countries, pricing or profitability of health care products is subject to government control which may result in low prices for our products. In the United States, there have been a number of federal and state proposals to implement similar government controls, and new proposals are likely to be made in the future.
The implementation of the ACA in the United States may adversely affect our business.
As a result of the March 2010 adoption of the ACA in the United States, substantial changes are being made to the current system for paying for healthcare in the United States, including programs to extend medical benefits to millions of individuals who currently lack insurance coverage. The changes contemplated by the ACA are subject to rule-making and implementation timelines that extend for several years, as well as initiatives in Congress to amend or repeal the law, and this uncertainty limits our ability to forecast changes that may occur in the future. However, implementation of the ACA has already begun with respect to certain significant cost-saving measures, including changes to several government healthcare programs that may cover the cost of our future products, including Medicaid, Medicare Parts B and D, and these efforts could have a materially adverse impact on our future financial prospects and performance. For example, with respect to Medicaid, in order for a manufacturer’s products to be reimbursed by federal funding under Medicaid, the manufacturer must enter into a Medicaid rebate agreement with the Secretary of the United States Department of Health and Human Services, and must pay certain rebates to the states based on utilization data provided by each state to the manufacturer and to CMS, and based on pricing data provided by the manufacturer to the federal government. The states share this savings with the federal government, and sometimes implement their own additional supplemental rebate programs. Under the Medicaid drug rebate program, the rebate amount for most branded drug products was previously equal to a minimum of 15.1% of the Average Manufacturer Price, or AMP, or the AMP less Best Price, whichever is greater. Effective January 1, 2010, the ACA generally increases the size of the Medicaid rebates paid by manufacturers for single source and innovator multiple source (brand name) drug product from a minimum of 15.1% to a minimum of 23.1% of the AMP, subject to certain exceptions, for example, for certain clotting factors, the increase is limited to a minimum of 17.1% of the AMP. For non-innovator multiple source (generic) products, the rebate percentage is increased from a minimum of 11.0% to a minimum of 13.0% of AMP. In 2010, the ACA also newly extended this rebate obligation to prescription drugs covered by Medicaid managed care organizations. These increases in required rebates may adversely affect our future financial prospects and performance. The ACA also creates new rebate obligations for products under Medicare Part D, a partial, voluntary prescription drug benefit created by the United States federal government primarily for persons 65 years old and over. The Part D drug program is administered through private insurers that contract with CMS. Beginning in 2011, the healthcare reform law generally requires that in order for a drug manufacturer’s products to be reimbursed under Medicare Part D, the manufacturer must enter into a Medicare Coverage Gap Discount Program agreement with the Secretary of the United States Department of Health and Human Services, and reimburse each Medicare Part D plan sponsor an amount equal to 50% savings for the manufacturer’s brand name drugs and biologics which the Part D plan sponsor has provided to its Medicare Part D beneficiaries who are in the “donut hole” (or a gap in Medicare Part D coverage for beneficiaries who have expended certain amounts for drugs). The Part D plan sponsor is responsible for calculating and providing the discount directly to its beneficiaries and for reporting these amounts paid to CMS’s contractor, which notifies drug manufacturers of the rebate amounts it must pay to each Part D plan sponsor. The rebate requirement could adversely affect our future financial performance, particularly if contracts with Part D plans cannot be favorably renegotiated or the Part D plan sponsors fail to accurately calculate payments due in a manner that overstates our rebate obligation. The ACA also introduced a biosimilar pathway that will permit companies to obtain FDA approval of generic versions of existing biologics based upon reduced documentation and data requirements deemed sufficient to demonstrate safety and efficacy than are required for the pioneer biologics. The new law provides that a biosimilar application may be submitted as soon as four years after the reference product is first licensed, and that the FDA may not make approval of an application effective until 12 years after the reference product was first licensed. With the likely introduction of biosimilars in the United States, we expect in the future to face greater competition from biosimilar products, including a possible increase in patent challenges. The FDA has reported meeting with sponsors who are interested in developing biosimilar products, and is developing regulations to implement the abbreviated regulatory review pathway. Regarding access to our products, the ACA established and provided significant funding for a Patient-Centered Outcomes Research Institute to coordinate and fund Comparative Effectiveness Research, or CER. While the stated intent of CER is to develop information to guide providers to the most efficacious therapies, outcomes of CER could influence the reimbursement or coverage for therapies that are determined to be less cost-effective than others. Should any of our products be determined to be less cost effective than alternative therapies, the levels of reimbursement for these products, or the willingness to reimburse at all, could be impacted, which could materially impact our future financial prospects and results.
Risks Related to Our Relationship With BioTime
We are a subsidiary of BioTime, and accordingly our business is substantially controlled by BioTime.
As of March 9, 2015, BioTime owns approximately 67.5% of our issued and outstanding Series A Shares, and also holds warrants that, if exercised, would increase its ownership by approximately 2.9%. Because BioTime owns a majority of the outstanding Series A Shares, it has the voting power to elect our entire Board of Directors. Presently, four of the eight members of our Board of Directors are also directors or officers of BioTime, and another director is an employee of Broadwood Capital, Inc., which is the general partner of Broadwood Partners, L.P., the partnership that is the largest shareholder of BioTime. Some of our directors also serve on the Boards of Directors of one or more of BioTime’s other subsidiaries. The relationship of our directors with BioTime means that we will not have a Board of Directors making business decisions on our behalf independent from BioTime. Even those of our directors who do not serve on the BioTime Board of Directors will be elected to our Board of Directors by BioTime, and they may be removed from our Board by BioTime, as the majority shareholder.
BioTime could cause corporate actions to be taken even if the interests of BioTime conflict with the interests of our other shareholders. This concentration of voting power could have the effect of deterring or preventing a change in control that might be beneficial to our other shareholders.
As the majority shareholder, BioTime will have the voting power to approve or disapprove any matter or corporate transaction presented to our shareholders for approval, including but not limited to:
| · | any amendment of our certificate of incorporation or bylaws; |
| · | any merger or consolidation of us with another company; |
| · | any recapitalization or reorganization of our capital stock; |
| · | any sale of assets or purchase of assets; or |
| · | a corporate dissolution or a plan of liquidation of our business. |
We partially rely upon BioTime for certain services and resources
Although we have our own research facilities, scientific personnel, and some management personnel, we partially rely on BioTime to provide certain management and administrative services, including patent prosecution, certain legal services, accounting, financial management, and controls over financial accounting and reporting. We have entered into a Shared Facilities and Services Agreement”) with BioTime under which we have agreed to bear costs allocated to us by BioTime for the use of BioTime human resources and for services and materials provided for our benefit by BioTime. We pay BioTime 105% of its costs of providing personnel and services to us, and for any use of its facilities by us, including an allocation of general overhead based on that use. We may also share the services of some research personnel with BioTime.
If BioTime’s human resources and facilities are not sufficient to serve both BioTime’s needs and ours, we will have to hire additional personnel of our own, either on a full-time or part-time basis, as employees or as consultants, and the cost of doing so could be greater than the costs that would be allocated to us by BioTime. Also, any new personnel that we may need to hire may not be as familiar with our business or operations as BioTime’s personnel, which means that we would incur the expense and inefficiencies related to training new employees or consultants.
Conflicts of interest may arise from our relationship with BioTime
Our relationship with BioTime could give rise to certain conflicts of interest that could have an impact on our research and development programs, business opportunities, and operations generally.
| · | We and BioTime or any of its other subsidiaries may determine to engage in research and development of the same or similar products or technologies, or products that would otherwise compete in the market place. Even if we utilize different technologies than BioTime or its other subsidiaries, we could find ourselves in competition with them for research scientists, financing and other resources, licensing, manufacturing, and distribution arrangements, and for customers if we and BioTime or another BioTime subsidiary both bring products to market. |
| · | Because we are a subsidiary of BioTime, BioTime could prevent us from engaging in research and development programs, investments, business ventures, or agreements to develop, license, or acquire products or technologies that would or might compete with those owned, licensed, or under development by BioTime or any of its other subsidiaries. |
| · | BioTime may determine that some of our patents or technology would be useful in its business or that of another BioTime subsidiary, and BioTime or another BioTime subsidiary may hold patents or technology that we may determine would be useful in our business. In such cases we may enter into license or sublicense agreements with BioTime or another BioTime subsidiary for the use of such patents or technology. Conflicts of interest will arise in determining the scope and financial terms of any such licenses or sublicenses, including the fields of use permitted, licensing fees, and royalties, if any, and other matters. |
| · | BioTime and its other subsidiaries will engage for their own accounts in research and product development programs, investments, and business ventures, and we will not be entitled to participate or to receive an interest in those programs, investments, or business ventures. BioTime and its other subsidiaries will not be obligated to present any particular research and development, investment, or business opportunity to us, even if the opportunity would be within the scope of our research and development plans or programs, business objectives, or investment policies. These opportunities may include, for example, opportunities to acquire businesses or assets, including but not limited to patents and other intellectual property that could be used by us or by BioTime or by any of BioTime’s other subsidiaries. Our respective boards of directors will have to determine which company should pursue those opportunities, taking into account relevant facts and circumstances at the time, such as the financial and other resources of the companies available to acquire and utilize the opportunity, and the best “fit” between the opportunity and the business and research and development programs of the companies. However, since BioTime will have the ultimate power to elect the members of our Board of Directors, BioTime may have the ultimate say in decision making with respect to the allocation of opportunities. |
| · | If we enter into any patent or technology license or sublicense, or any other agreement with BioTime or with another BioTime subsidiary, the BioTime companies that are parties to the agreement may have a conflict of interest in determining how and when they should enforce their rights under the agreement if the other BioTime company that is a party were to default or otherwise fail to perform any of its obligations under the agreement. |
| · | One of our significant assets is 3,852,880 BioTime common shares that we held as of December 31, 2014. Through the Asset Contribution Agreement we acquired 8,902,077 BioTime common shares from BioTime in October 2013 and we sold a portion of those shares during June 2014. We expect to sell the remaining BioTime common shares from time to time, or to pledge those shares as collateral for loans, to raise capital to finance our operations. Because a sale of those shares could have a depressing effect on the market value of BioTime common shares, BioTime will have a continuing interest in the number of shares we sell, the prices at which we sell the shares, and time and manner in which the shares are sold. Further, we may need or find it desirable to sell BioTime common shares at the same time as BioTime, or other BioTime subsidiaries that hold BioTime common shares, also desire to sell some of their BioTime common shares. Concurrent sales of BioTime common shares by us, BioTime, or other BioTime subsidiaries could have a depressing effect on the market price of the BioTime common shares, lowering the price at which we and they are able to sell BioTime common shares and resulting in lower net proceeds from the sales. We plan to coordinate any future sales of our BioTime common shares with BioTime and its other subsidiaries in order to provide an orderly and controlled process for raising capital through the sale of BioTime shares. This will include an agreement as to the number of shares to be sold, the time period or “market window” for selling shares, the use of a common securities broker-dealer, and a fair allocation of net sales based on average sales prices during any trading day on which we and they sell BioTime shares. |
| · | Each conflict of interest will be resolved by our respective boards of directors in keeping with their fiduciary duties and such policies as they may implement from time to time. However, the terms and conditions of patent and technology licenses and other agreements between us and BioTime or other BioTime subsidiaries will not be negotiated on an arm’s-length basis due to BioTime’s ownership of a controlling interest in us and due to the commonality of directors serving on our respective boards of directors. |
Risks Related to Our Dependence on Third Parties
We could lose our CIRM grant if we fail to meet the clinical trial milestones that are a condition to CIRM’s obligation to provide funding
We are depending upon our grant from CIRM as a source of financing for the costs of conducting our Phase I/IIa clinical trial and process development of AST-OPC1. Under the terms of the CIRM grant, we must meet certain efficacy and progress milestones pertaining to the clinical trial. If we fail to meet any of the milestones within the specified time frame, CIRM may discontinue providing grant funds to us, which could force us to postpone, delay, or discontinue the clinical trial and development work for the product.
Establishing and maintaining strategic alliances is a key component of our business strategy. If we are unable to establish and maintain strategic alliances for our therapeutic product candidates, we may have to reduce or delay our product development or increase our expenditures
A key component of our current strategy for developing, manufacturing and commercializing our therapeutic product candidates will be entering into strategic alliances with pharmaceutical companies or other industry participants to advance our programs and enable us to maintain our financial and operational capacity. We will face significant competition in seeking appropriate alliances. We may not be able to negotiate alliances on acceptable terms, if at all. If our strategic alliances do not result in the successful development and commercialization of our product candidates or if one or more of our collaborators terminates its agreement with us, we may not receive any future research and development funding or milestone or royalty payments under the collaboration. If we do not receive the funding we expect under these agreements, our continued development of our product candidates could be delayed and we may need to obtain additional funding, which may be unavailable or available only on unfavorable terms.
If we are able to enter into product development and marketing arrangements with pharmaceutical companies, we may license product development, manufacturing, and marketing rights to the pharmaceutical company or to a joint venture company formed with the pharmaceutical company. Under such arrangements we might receive only a royalty on sales of the products developed or an equity interest in a joint venture company that develops the product. As a result, our revenues from the sale of those products may be substantially less than the amount of revenues and gross profits that we might receive if we were to develop, manufacture, and market the products ourselves.
We may become dependent on possible future collaborations to develop and commercialize many of our product candidates and to provide the manufacturing, regulatory compliance, sales, marketing and distribution capabilities required for the success of our business
We may enter into various kinds of collaborative research and development, manufacturing, and product marketing agreements to develop and commercialize our products. Any future milestone payments and cost reimbursements from collaboration agreements could provide an important source of financing for our research and development programs, thereby facilitating the application of our technology to the development and commercialization of our products, but there are risks associated with entering into collaboration arrangements.
There is a risk that we could become dependent upon one or more collaborative arrangements for product development or manufacturing or as a source of revenues from the sale of any products that may be developed by us alone or through one of the collaborative arrangements. A collaborative arrangement upon which we might depend might be terminated by our collaboration partner or they might determine not to actively pursue the development or commercialization of our products. A collaboration partner also may not be precluded from independently pursuing competing products and drug delivery approaches or technologies.
There is a risk that a collaboration partner might fail to perform its obligations under the collaborative arrangements or may be slow in performing its obligations. In addition, a collaboration partner may experience financial difficulties at any time that could prevent it from having available funds to contribute to the collaboration. If a collaboration partner fails to conduct its product development, manufacturing, commercialization, regulatory compliance, sales and marketing or distribution activities successfully and in a timely manner, or if it terminates or materially modifies its agreements with us, the development and commercialization of one or more product candidates could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue product development, manufacturing, and commercialization on our own.
We have no experience in manufacturing, marketing, selling or distributing products, and we may need to rely on marketing partners or contract sales companies if any of our product candidates receive regulatory approval
Even if we are able to develop our products and obtain necessary regulatory approvals, we have no experience or capabilities of our own in manufacturing, marketing, selling or distributing any of the products that we plan to develop. Accordingly, we will be dependent on our ability to build our own manufacturing, marketing, and distribution capability for our products, which would require the investment of significant financial and management resources, or we will need to find third parties to manufacture our products, and collaborative marketing partners or contract sales companies for commercial sale of those products. Even if we find one or more potential third party manufacturers and marketing partners, of which there can be no assurance, we may not be able to negotiate manufacturing, licensing, or marketing contracts on favorable terms to justify our investment or achieve adequate revenues and margins.
Industry and other market data used in this Annual Report, including those undertaken by us or our engaged consultants, may prove to be unrepresentative of current and future market conditions or future results
This Annual Report includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties, and surveys and studies we commissioned, regarding the market potential for our product candidates. Although we believe that such information has been obtained from sources believed to be reliable, neither the sources of such data, nor we, can guarantee the accuracy or completeness of such information. While we believe these industry publications and third party research, surveys and studies are reliable, we have not independently verified such data. With respect to the information from third party consultants, the results of that study represent the independent consultants’ own methodologies, assumptions, research, analysis, projections, estimations, composition of respondent pool, presentation of data, and adjustments, each of which may ultimately prove to be incorrect, and cause actual results and market viability to differ materially from those presented in such report. Readers should not place undue reliance on this information.
Risks Pertaining to Our Common Stock
Ownership of our common stock will entail certain risks associated with the volatility of prices for our shares and the fact that we do not pay dividends on our common stock.
Because we are engaged in the development of stem cell therapeutic products, the price of our common stock may rise and fall rapidly
The market price of our common stock like that of the shares of many biotechnology companies, may be highly volatile. The price of our common stock may rise or fall rapidly as a result of a number of factors, including:
| · | sales or potential sales of substantial amounts of our common stock; |
| · | results of preclinical testing or clinical trials of our product candidates or those of our competitors; |
| · | announcements about us or about our competitors, including clinical trial results, regulatory approvals, new product introductions and commercial results; |
| · | the cost of our development programs; |
| · | the success of competitive products or technologies; |
| · | litigation and other developments relating to our issued patents or patent applications or other proprietary rights or those of our competitors; |
| · | conditions in the pharmaceutical or biotechnology industries; |
| · | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
| · | variations in our financial results or those of companies that are perceived to be similar to us, including the failure of our earnings to meet analysts’ expectations; and |
| · | general economic, industry and market conditions. |
Many of these factors are beyond our control. The stock markets in general, and the market for pharmaceutical and biotechnological companies in particular, have been experiencing extreme price and volume fluctuations which have affected the market price of the equity securities without regard to the operating performance of the issuing companies. Broad market fluctuations, as well as industry factors and general economic and political conditions, may adversely affect the market price of our common stock.
The recently enacted JOBS Act allows us to postpone the date by which we must comply with certain laws and regulations intended to protect investors and to reduce the amount of information we provide in our reports filed with the Commission, which could undermine investor confidence in our company and adversely affect the market price of our securities.
The recently enacted JOBS Act is intended to reduce the regulatory burden on “emerging growth companies.” As defined in the JOBS Act, a public company whose initial public offering of common equity securities occurred after December 8, 2011 and whose annual gross revenues are less than $1.0 billion will, in general, qualify as an emerging growth company until the earliest of:
| · | the last day of its fiscal year following the fifth anniversary of the date of its initial public offering of common equity securities; |
| · | the last day of its fiscal year in which it has annual gross revenue of $1.0 billion or more; |
| · | the date on which it has, during the previous three-year period, issued more than $1.0 billion in non-convertible debt; and |
| · | the date on which it is deemed to be a “large accelerated filer,” which will occur at such time as we (a) have an aggregate worldwide market value of common equity securities held by non-affiliates of $700 million or more as of the last business day of its most recently completed second fiscal quarter, (b) have been required to file annual and quarterly reports under the Securities Exchange Act of 1934 for a period of at least 12 months, and (c) have filed at least one annual report pursuant to the Securities Exchange Act of 1934. |
Under this definition, we are an emerging growth company and could remain an emerging growth company until as late as December 31, 2019.
The JOBS Act provides that, so long as we qualify as an emerging growth company, we will, among other things:
| · | be exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting; |
| · | be exempt from the “say on pay” provisions (requiring a non-binding stockholder vote to approve compensation of certain executive officers) and the “say on golden parachute” provisions (requiring a non-binding stockholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations) of the Dodd-Frank Act and certain disclosure requirements of the Dodd-Frank Act relating to compensation of our chief executive officer; |
| · | be permitted to omit the detailed compensation discussion and analysis from proxy statements and reports filed under the Securities Exchange Act of 1934 and instead provide a reduced level of disclosure concerning executive compensation; and |
| · | be exempt from any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements. |
Although we are still evaluating the JOBS Act, we currently take advantage of the reduced regulatory and reporting requirements that are available to us so long as we qualify as an “emerging growth company,” except that we have irrevocably elected not to take advantage of the extension of time to comply with new or revised financial accounting standards available under Section 102(b) of the JOBS Act. Among other things, this means that our independent registered public accounting firm will not be required to provide an attestation report on the effectiveness of our internal control over financial reporting so long as we qualify as an emerging growth company, which may increase the risk that weaknesses or deficiencies in our internal control over financial reporting go undetected. Likewise, so long as we qualify as an emerging growth company, we may elect not to provide you with certain information, including certain financial information and certain information regarding compensation of our executive officers, that we would otherwise have been required to provide in filings we make with the Commission, which may make it more difficult for investors and securities analysts to evaluate our company. As a result, investor confidence in our company and the market price of our securities may be materially and adversely affected.
Our stock price could decline due to the large number of outstanding shares of our common stock eligible for future sale
Sales of substantial amounts of our common stock in the public market, or the perception that those sales could occur, could cause the market price of our common stock to decline. Sales of substantial amounts of common stock could also make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem appropriate.
We have not registered for sale or transfer under the Securities Act any of the 21,823,340 shares of our Series A Shares or 3,150,000 warrants that BioTime owns, or the Series A Shares that it may receive if it exercises its warrants. However, BioTime reserves the right to sell its Series A Shares and the warrants in the future or to distribute them to its shareholders.
We do not currently intend to pay dividends on any of our classes of securities and, consequently, your ability to achieve a return on your investment will depend on the appreciation in the price of our securities.
We have never declared or paid any cash dividends on any class of our securities. We currently intend to retain any future earnings to fund our future growth and do not expect to declare or pay any dividend on any class of our securities in the foreseeable future. As a result, you may only realize a gain on your investment in our securities if the market price of our securities appreciates and you sell your securities at a price above your cost after accounting for any taxes. The price of our securities may not appreciate in value or ever exceed the price that you paid for our securities.
The price of our common stock, and the value of our assets, will be affected by changes in the value of the BioTime common shares that we own
As of December 31, 2014, we held 3,852,880 of the 8,902,077 BioTime common shares we received from BioTime under the Asset Contribution Agreement. The value of our common stock will reflect, in part, the value of the BioTime common shares that we hold. The value of the BioTime common shares we hold will vary with the price at which BioTime common shares trade in the public market. The market price of BioTime common shares will be impacted by a number of factors, including the results of BioTime’s operations.
If securities analysts do not publish research or reports about our business or if they downgrade our stock, the price of our securities could decline.
The current trading market for our common stock relies in part on the research and reports that industry or financial analysts publish about us, our business, our markets and our competitors. We do not control these analysts. If securities analysts do not cover us, the lack of research coverage may adversely affect the market price of our common stock. Furthermore, if one or more of the analysts who do cover us downgrade our stock or if those analysts issue other unfavorable commentary about us or our business, our stock price would likely decline. If one or more of these analysts cease coverage of us or fails to regularly publish reports on us, we could lose visibility in the market and interest in our stock could decrease, which in turn could cause our stock price or trading volume to decline.
You may experience dilution of your ownership interests because of the future issuance of additional shares of our common stock and our preferred stock
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present shareholders. We are currently authorized to issue an aggregate of 150,000,000 shares of common stock, consisting of 75,000,000 Series A Shares and 75,000,000 of our Series B Shares. We are also authorized to issue 5,000,000 shares of “blank check” preferred stock. As of March 1, 2015, we had issued and outstanding 32,312,407 Series A Shares. We have also reserved 8,500,000 Series A Shares for issuance upon the exercise of outstanding warrants, and 4,500,000 Series A Shares for issuance under a stock option and stock purchase plan.
We may issue additional shares of common stock or other securities in order to raise additional capital, or in connection with hiring or retaining employees or consultants, or in connection with future acquisitions of licenses to technology or rights to acquire products, in connection with future business acquisitions, or for other business purposes. The future issuance of any such additional shares of common stock or other securities may create downward pressure on the trading price of our common stock.
We may also issue 5,000,000 shares of preferred stock having rights, preferences, and privileges senior to the rights of our common stock with respect to dividends, rights to share in distributions of our assets if we liquidate our company, or voting rights. Any preferred stock may also be convertible into Series A Shares on terms that would be dilutive to holders of common stock.
None
We occupy an office and research facility located at 230 Constitution Drive, Menlo Park, California under a sublease from BioTime. The building on the leased premises contains approximately 24,080 square feet of space. The sublease and underlying master lease are both for a term of three years commencing January 7, 2013. The sublease requires us to pay all rent and other amounts due, and to perform all of our other obligations as a tenant, under the master lease. We will pay base rent of $31,785.60 per month, plus real estate taxes and certain costs of maintaining the leased premises.
BioTime may assign the lease to us outright at any time after we have obtained at least $10,000,000 in equity capital through the sale of our capital stock for cash, or we have a class of capital stock registered under Section 12 of the Securities Exchange Act of 1934, as amended; provided that we must agree in writing to assume, to be bound by, and to perform the terms, covenants and conditions of the lease. We expect that the conditions to the assignment will be met.
On December 30, 2013, Asterias entered into a lease for an office and research facility located in Fremont, California, consisting of an existing building with approximately 44,000 square feet of space. The building will be used by Asterias primarily as a laboratory and production facility that can be used to produce human embryonic stem cells and related products under current good manufacturing procedures (cGMP). We plan to construct certain tenant improvements for our use, which we expect will cost approximately $5.5 million, of which a maximum $4.4 million will be paid by the landlord. The landlord’s obligation to fund the tenant improvements expires on June 30, 2015 with respect to any portion of the allowance not expended by then. Asterias expects to substantially complete construction of the built-to-suit facility during the third quarter of 2015.
The lease is for a term of 96 months commencing on October 1, 2014, with two available five-year options to extend the term, upon one year written notice by Asterias. During the first 15 months of the lease term, from October 1, 2014 through December 31, 2015, Asterias will pay monthly base rent of $50,985 representing 22,000 square feet rather than 44,000 square feet provided that Asterias is not in default in performing its obligations under the lease beyond any notice and cure periods. Beginning on January 1, 2016, base rent will increase to $105,142 per month and increase by approximately 3% annually on every October 1 thereafter.
In addition to monthly base rent, Asterias will pay all real estate taxes, insurance and the cost of maintenance, repair and replacement of the leased premises. During the first 15 months of the lease term, Asterias will pay only 50% of the real estate taxes assessed on the premises provided that Asterias is not in default in performing its obligations under the lease beyond any notice and cure periods. However, if any improvements or alterations to the premises that Asterias constructs or adds are assessed for real property tax purposes at a valuation higher than the valuation of the improvements on the premises on the date it signed the lease, Asterias will pay 100% of the taxes levied on the excess assessed valuation.
Asterias also currently pays $3,512 per month for the use of approximately 120 square feet of the office space in New York City that is used to conduct meetings and other business affairs. The lease is for a term of one year commencing July 1, 2014.
From time to time, we may be involved in routine litigation incidental to the conduct of our business. We are not presently involved in any material litigation or proceedings, and to our knowledge no such litigation or proceedings are contemplated.
Not applicable
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities |
Our Series A Shares have been traded on the NYSE MKT under the symbol AST since October 7, 2014. From July 2014 through October 7, 2014 our Series A Shares traded on the OTC Bulletin Board under the ticker symbol “ASTYV.” The following table sets forth the range of high and low closing prices for our Series A Shares during the third and fourth quarters of 2014 as report by the NYSE MKT and the OTC BB.
|
Quarter Ended
|
High
|
Low
|
|||||
|
September 30, 2014
|
$
|
7.35
|
$
|
2.07
|
|||
|
December 31, 2014
|
$
|
6.25
|
$
|
3.07
|
|||
Over-the-counter market quotations on the OTC Bulletin Board may reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
As of February 26, 2015, there were 542 holders of record of our Series A Shares.
The following table shows certain information concerning the options outstanding and available for issuance under all of our compensation plans and agreements as of December 31, 2014:
|
Plan Category
|
|
Number of Shares
to
be Issued upon
Exercise of
Outstanding
Options, Warrants,
and Rights
|
Weighted Average
Exercise Price of
the Outstanding
Options, Warrants,
and Rights
|
Number of Shares
Remaining Available
for Future Issuance
under Equity
Compensation Plans
|
|||||||
|
Asterias Equity Compensation Plans Approved by Shareholders(1)
|
3,346,666
|
$
|
2.42
|
1,150,001
|
|||||||
| (1) | Includes 200,000 shares of restricted stock granted to Pedro Lichtinger, our President and Chief Executive Officer of Asterias on June 9, 2014, which are subject to restrictions on transfer and to forfeiture until the shares vest. The restricted stock will vest at the rate of 16,667 shares per month while Mr. Lichtinger remains employed by Asterias. |
Additional information concerning our Equity Incentive Plan, the stock options and restricted stock may be found in Note 9 to the Financial Statements.
Dividend Policy
We have never paid cash dividends on our capital stock and we do not anticipate paying cash dividends in the foreseeable future, but intend to retain our capital resources for reinvestment in our business. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon our financial condition, results of operations, capital requirements and other factors as our Board of Directors deems relevant.
Performance Measurement Comparison(1)
The following graph compares total stockholder returns of Asterias Biotherapeutics, Inc.for the last six months beginning July 18, 2014 to two indices: the NYSE Amex Market Value – U.S. Companies (Amex Market Value) and the NYSE Arca Biotechnology Index (NYSE Arca Biotechnology Index). The total return for our stock and for each index assumes the reinvestment of dividends, although we have never declared dividends on Asterias Biotherapeutics, Inc., and is based on the returns of the component companies weighted according to their capitalizations as of the end of each quarterly period. The NYSE Amex Market Value tracks the aggregate price performance of equity securities of U.S. companies listed therein. The NYSE Arca Biotechnology Index represents biotechnology companies, trading on NYSE MKT under the Standard Industrial Classification (SIC) Code Nos. 283 (Drugs) and 382 (Laboratory Apparatus and Analytical, Optical) main categories (2834:Pharmaceutical Preparations; 2835: Diagnostic Substances; 2836: Biological Products; 3826: Laboratory Analytical Instruments; and 3829: Measuring & Controlling Devices). Asterias Biotherapeutics, Inc. common stock trades on the NYSE MKT and is a component of the NYSE Amex Market Value – US Companies.
Comparison of 6 Month Cumulative Total Return on Investment
|
7/18/2014
|
7/31/2014
|
8/31/2014
|
9/30/2014
|
10/31/2014
|
11/30/2014
|
12/31/2014
|
|||||||||||||||||||||||
|
Asterias Biotherapeutics, Inc.
|
Cum $
|
100.00
|
62.50
|
99.17
|
168.33
|
116.67
|
108.33
|
90.00
|
|||||||||||||||||||||
|
AMEX Market Value (US Companies)
|
Cum $
|
100.00
|
96.91
|
102.79
|
97.37
|
93.69
|
88.61
|
90.99
|
|||||||||||||||||||||
|
NYSE Arca Biotechnology Index
|
Cum $
|
100.00
|
100.47
|
115.01
|
113.60
|
123.17
|
126.27
|
126.42
|
|||||||||||||||||||||
Asterias Biotherapeutics, Inc., the Amex Market Value and Amex Biotechnology Index(2)
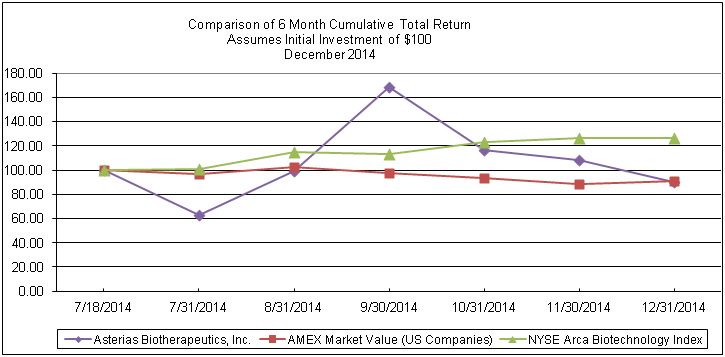
| (1) | This Section is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference in any filing of BioTime under the Securities Act of 1933, or the Securities Exchange Act of 1934, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. |
| (2) | Shows the cumulative total return on investment assuming an investment of $100 in each of BioTime, Inc., the Amex Market Value and NYSE Arca Biotechnology Index on December 31, 2009. The cumulative total return on BioTime stock has been computed based on a price of $4.23 per share, the price at which BioTime’s shares closed on December 31, 2009. |
|
Years Ended December 31,
|
Cumulative
Period from Inception
(September 24, 2012) to
|
||||||||||||||
|
2014
|
2013
|
December 31,
2012
|
December 31,
2014
|
||||||||||||
|
REVENUE
|
|||||||||||||||
|
Royalties from product sales
|
$
|
189,215
|
$
|
-
|
$
|
-
|
$
|
189,215
|
|||||||
|
Grant income
|
1,034,456
|
-
|
-
|
1,034,456
|
|||||||||||
| Total revenues | 1,233,671 | - | - | 1,233,671 | |||||||||||
|
Cost of sales
|
(94,607
|
)
|
-
|
-
|
(94,607)
|
||||||||||
|
Total gross profit
|
|
1,129,064
|
|
-
|
|
-
|
|
1,129,064
|
|||||||
|
OPERATING EXPENSES
|
|||||||||||||||
|
Research and development
|
|
(13,310,421
|
)
|
|
(4,319,494
|
)
|
|
-
|
|
(17,629,915)
|
|||||
|
Acquired in-process research and development (see Note 2)
|
-
|
(17,458,766
|
)
|
-
|
(17,458,766)
|
||||||||||
|
General and administrative
|
(5,279,859
|
)
|
(3,883,185
|
)
|
(758,563
|
)
|
(9,921,607)
|
||||||||
|
Total operating expenses
|
(18,590,280
|
)
|
(25,661,445
|
)
|
(758,563
|
)
|
(45,010,288)
|
||||||||
|
Loss from operations
|
(17,461,216
|
)
|
(25,661,445
|
)
|
(758,563
|
)
|
(43,881,224)
|
||||||||
|
OTHER INCOME/(EXPENSE)
|
|||||||||||||||
|
Interest expense, net
|
(9,991
|
)
|
(1,737
|
)
|
-
|
(11,728)
|
|||||||||
|
Other income/(expense), net
|
(1,558
|
)
|
313
|
(330
|
)
|
(1,575)
|
|||||||||
|
Gain on sale of fixed assets
|
-
|
2,430
|
-
|
2,430
|
|||||||||||
|
Total other income/(expense), net
|
(11,549
|
)
|
1,006
|
(330
|
)
|
(10,873)
|
|||||||||
|
LOSS BEFORE DEFERRED INCOME TAX BENEFIT
|
(17,472,765
|
)
|
(25,660,439
|
)
|
(758,893
|
)
|
(43,892,097)
|
||||||||
|
Deferred income tax benefit
|
7,375,611
|
3,280,695
|
-
|
10,656,306
|
|||||||||||
|
NET LOSS
|
(10,097,154
|
)
|
|
(22,379,744
|
)
|
|
(758,893
|
)
|
|
(33,235,791)
|
|||||
|
Unrealized gain/(loss) on available-for-sale securities, net
|
2,432,023
|
(2,934,686
|
)
|
-
|
(502,663)
|
||||||||||
|
COMPREHENSIVE LOSS
|
$
|
(7,665,131
|
)
|
$
|
(25,314,430
|
)
|
$
|
(758,893
|
)
|
$
|
(33,738,454)
|
||||
|
Basic and diluted net loss per common share
|
$
|
(0.33
|
)
|
$
|
(2.90
|
)
|
$
|
(14.69
|
)
|
$
|
(1.96)
|
||||
|
Weighted average number of common stock outstanding: basic and diluted
|
30,720,280
|
7,726,042
|
51,647
|
16,954,131
|
|||||||||||
| (1) | Represents the value of incomplete research and development projects acquired from Geron which Asterias intends to continue. See Notes 2 and 3 to the Financial Statements. |
|
December 31,
|
December 31,
|
||||||
|
2014
|
2013
|
||||||
|
Balance Sheet Data:
|
|||||||
|
Cash and cash equivalents
|
$
|
3,075,593
|
$
|
2,171,113
|
|||
|
Total assets
|
44,113,513
|
80,353,797
|
|||||
|
Total liabilities
|
$
|
11,482,453
|
$
|
26,573,312
|
|||
|
Accumulated deficit
|
(33,235,791
|
)
|
(23,138,637)
|
||||
|
Total stockholders' equity
|
$
|
32,631,060
|
|
$
|
53,780,485
|
||
The following Management's Discussion and Analysis of Financial Condition and Results of Operations is intended to provide information necessary to understand Asterias’ audited financial statements for the years ended December 31, 2014, 2013, the period from September 24, 2012 (Asterias’ date of inception) to December 31, 2012, and the period from September 24, 2012 to December 31, 2014, and highlight certain other information which, in the opinion of management, will enhance a reader's understanding of Asterias’ financial condition, changes in financial condition and results of operations. In particular, the discussion is intended to provide an analysis of significant trends and material changes in our financial position and the operating results of our business during the year ended December 31, 2014 as compared to 2013, and during the year ended December 31, 2013 as compared to the period from September 24, 2012 to December 31, 2012. This discussion should be read in conjunction with our consolidated financial statements for the period ended December 31, 2014 and related notes included elsewhere in this Annual Report on Form 10-K. These historical financial statements may not be indicative of our future performance. These historical financial statements may not be indicative of Asterias’ future performance. This Management's Discussion and Analysis of Financial Condition and Results of Operations contains a number of forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risks described throughout this filing, particularly in "Item 1A. Risk Factors.”
Critical Accounting Policies
Development Stage Company – We comply with the reporting requirements of ASC 915, “Development Stage Entities.”
Revenue recognition – We comply with ASC 605-10 and record revenue when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable, and collectability is reasonably assured. Grant income is recognized as revenue when earned. Royalty revenues consist of royalty payments on sales of products under a license agreement. We recognize revenue in the quarter in which the royalty reports are received rather than the quarter in which the sales took place. When we are entitled to receive up-front nonrefundable licensing or similar fees pursuant to agreements under which we have no continuing performance obligations, the fees are recognized as revenues when collection is reasonably assured. When we receive up-front nonrefundable licensing or similar fees pursuant to agreements under which we do have continuing performance obligations, the fees are deferred and amortized ratably over the performance period. If the performance period cannot be reasonably estimated, we amortize nonrefundable fees over the life of the contract until such time that the performance period can be more reasonably estimated. Milestone payments, if any, related to scientific or technical achievements are recognized in income when the milestone is accomplished if (a) substantive effort was required to achieve the milestone, (b) the amount of the milestone payment appears reasonably commensurate with the effort expended, and (c) collection of the payment is reasonably assured.
Patent costs – Costs associated with obtaining patents on products or technology developed are expensed as general and administrative expenses when incurred.
Investment in BioTime shares and other marketable equity securities investments – Marketable equity securities and debt securities not classified as held-to-maturity are classified as available-for-sale. Available-for-sale securities are carried at fair value, with the unrealized gains and losses, net of tax, reported in other comprehensive income. Realized gains and losses, and declines in value judged to be other-than-temporary related to equity securities, are included in investment income.
Equipment and furniture – Equipment and furniture are stated at cost and are being depreciated using the straight-line method over a period of 36 to 120 months.
Intangible assets – Intangible assets with finite useful lives are amortized over estimated useful lives and intangible assets with indefinite lives are not amortized but rather are tested at least annually for impairment. Acquired in-process research and development intangible assets are accounted depending on whether they were acquired as part of an acquisition of a business, or assets that do not constitute a business. When acquired in conjunction with the acquisition of a business, these assets are considered to be indefinite-lived until the completion or abandonment of the associated research and development efforts and are capitalized as an asset. If and when development is complete, the associated assets would be deemed finite-lived and would then be amortized based on their respective estimated useful lives at that point in time. However, when acquired in conjunction with an acquisition of assets that do not constitute a business (such as our acquisition of assets from Geron), in accordance with the accounting rules in ASC 805-50, such intangible assets related to in process research and development (“IPR&D”) are expensed upon acquisition.
Impairment of long-lived assets – Our long-lived assets, including tangible assets, will be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be fully recoverable. If an impairment indicator is present, we will evaluate recoverability by a comparison of the carrying amount of the assets to future undiscounted net cash flows expected to be generated by the assets. If the assets are impaired, the impairment will be recognized and measured by the amount by which the carrying amount exceeds the estimated fair value of the assets.
Warrants to purchase common stock – We generally account for warrants issued in connection with equity financings as a component of equity. None of the warrants issued by us as of December 31, 2014 include a conditional obligation to issue a variable number of shares; nor was there a deemed possibility that we may need to settle the warrants in cash. If we were to issue warrants with a conditional obligation to issue a variable number of shares or with the deemed possibility of a cash settlement, we would record the fair value of the warrants as a liability at each balance sheet date and records changes in fair value in other income and expense in our statements of operations.
Accounting for BioTime Shares – The Company accounts for the BioTime shares it holds as available-for-sale equity securities in accordance with ASC 320-10-25, Investments-Debt and Equity Securities, as the shares have a readily determinable fair value quoted on the NYSE MKT and are held principally for future working capital purposes, as necessary. These shares are measured at fair value and reported as current assets on the balance sheet based on the closing trading price of the security as of the date being presented. Unrealized holding gains and losses in changes to the fair value of these shares are excluded from the statements of operations and reported in equity as part of other comprehensive income or loss until realized. Realized gains and losses are reclassified out of other comprehensive income or loss and included in equity, as an increase or decrease in additional paid-in capital consistent with, and pursuant to, ASC 805-50, transactions between entities under common control.
Research and development – Research and development costs are expensed when incurred, and consist principally of salaries, payroll taxes, consulting fees, research and laboratory fees, and fees paid to acquire patents or licenses.
Income taxes – Asterias will file its own U.S. federal tax return for the entire 2014 calendar year. For California purposes Asterias’ activity will continue to be included in BioTime’s California combined tax return. Asterias’ operations through September 30, 2013 were included in BioTime’s consolidated U.S. federal income tax return. For the period from October 1, 2013 through December 31, 2013 Asterias filed a separate U.S federal tax return. For California, Asterias’ activity for the entire 2013 calendar year was included in BioTime’s combined tax return. The provision for income taxes was previously determined as if Asterias had filed separate tax returns for the periods presented. Accordingly, the effective tax rate of Asterias in periods subsequent to December 31, 2013 could vary from its historical effective tax rates depending on the future legal structure of Asterias and related tax elections. Asterias accounts for income taxes in accordance with the accounting principles generally accepted in the United States, which prescribe the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. Generally, Asterias is subject to income tax examinations by major taxing authorities for all years since inception. Asterias will recognize accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of December 31, 2014 and December 31, 2013. Management is currently unaware of any tax issues under review.
Stock-based compensation – Asterias adopted accounting standards governing share-based payments, which require the measurement and recognition of compensation expense for all share-based payment awards made to directors and employees, including employee stock options, based on estimated fair values. Consistent with those guidelines, Asterias utilizes the Black-Scholes-Merton option pricing model. Asterias' determination of fair value of share-based payment awards on the date of grant using that option-pricing model is affected by Asterias' stock price as well as by assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, Asterias' expected stock price volatility over the term of the awards, and actual and projected employee stock option exercise behaviors. The expected term of options granted is derived from historical data on employee exercises and post-vesting employment termination behavior. The risk-free rate is based on the U.S. Treasury rates in effect during the corresponding period of grant.
Fair value of financial instruments – ASC 820, Fair Value Measurements, clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability.
ASC 820 requires that the valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 establishes a three tier value hierarchy, which prioritizes inputs that may be used to measure fair value as follows:
| · | Level 1 – Observable inputs that reflect quoted prices for identical assets or liabilities in active market |
| · | Level 2 – Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| · | Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The carrying amounts of current assets and current liabilities approximate their fair value because of the relatively short period until they mature or are required to be settled, except for the investment in BioTime shares, which are carried at fair value based on Level 1 inputs, and BioTime Warrants and related obligation to distribute the BioTime Warrants, which are carried at fair value based on Level 2 inputs.
Results of Operations
Comparison of Years Ended December 31, 2014 and 2013
For the years ended December 31, 2014 and 2013 we recorded net losses of $10,097,154 and $22,379,744, respectively.
Revenues
The following table shows certain information about our revenues for the years ended December 31, 2014 and 2013.
|
Year Ended
December 31,
|
$
Increase/
|
%
Increase/
|
|||||||||||||
|
2014
|
2013
|
Decrease
|
Decrease
|
||||||||||||
|
License and royalties from product sales
|
$
|
189,215
|
$
|
-
|
$
|
+ 189,215
|
+ 100%
|
||||||||
|
Grant income
|
1,034,456
|
-
|
+ 1,034,456
|
+ 100%
|
|||||||||||
|
Total revenues
|
1,223,671
|
-
|
+ 1,223,671
|
+ 100%
|
|||||||||||
|
Cost of sales
|
(94,607
|
)
|
-
|
+ 94,607
|
|
+ 100% |
|
||||||||
|
Total gross profit
|
$
|
1,129,064
|
$
|
-
|
$
|
+ 1,129,064
|
+ 100% |
|
|||||||
Our license and royalty revenues from product sales is entirely from the non-exclusive license agreement with Stem Cell Technologies, Inc., Corning Life Science, Life Tech, and Millipore which Asterias assumed as part of consideration received from Geron under the Asset Contribution Agreement.
Grant revenue in 2014 is entirely from CIRM which has awarded us a $14.3 million grant for clinical development of AST-OPC1. CIRM will disburse the grant funds to us over four years in accordance with a quarterly disbursement schedule, subject to our attainment of certain progress and safety milestones. We received the first payment from CIRM in the amount of $916,554 during October 2014 and the second payment in the amount of $2,269,515 in January 2015.
Expenses
The following table shows our operating expenses for the years ended December 31, 2014 and 2013.
|
Year Ended
December 31,
|
$
Increase/
|
%
Increase/
|
|||||||||||||
|
2014
|
2013
|
Decrease
|
Decrease
|
||||||||||||
|
Research and development expenses
|
$
|
13,310,421
|
$
|
4,319,494
|
$
|
+8,990,927
|
+208% | ||||||||
|
Acquired in-process research and development expenses
|
-
|
17,458,766
|
-17,458,766
|
|
-100% | ||||||||||
|
General and administrative expenses
|
5,279,859
|
3,883,185
|
+ 1,396,674
|
+36%
|
|||||||||||
Research and development expenses – Research and development expenses increased by approximately $8,990,927 to $13,310,421 for the year ended December 31, 2014 compared to $4,319,494 for the year ended December 31, 2013. In addition, during 2013 we recognized $17,458,766 of IPR&D in connection with the consummation of our acquisition of assets from Geron. The increases in research and development expenses, excluding IPR&D, during 2014 are primarily attributed to an increase of $4,063,974 of amortization of intangible assets acquired under Asset Contribution Agreement on October 1, 2013, and following increases in expenses reflecting the ramp up of our AST-OPC1 and AST-VAC2 programs: $2,442,791 of salaries, payroll related expenses and employee stock-based compensation allocated to research and development expenses, $546,088 of license and patent related expenses, $377,215 of rent and facilities maintenance related expenses allocated to research and development expenses due to new lease in Fremont which commenced on October 1, 2014, $362,087 of laboratory expense and supplies expenses, $328,241 of outside research and services allocated to research and development expenses, $276,595 of depreciation expenses allocated to research and development expenses, $252,183 of clinical trial related expenses, $166,292 of contract manufacturing related expenses allocated to research and development expenses, $84,394 of insurance expense allocated to research and development expenses, and $54,084 of travel, lodging and meals allocated to research and development expenses.
Research and development expenses during 2013 included $17,458,766 of IPR&D expense related to the assets we acquired in 2013 under the Asset Contribution Agreement. Other research and development expenses incurred during 2014 and 2013 include laboratory study expenses, patent and technology license fees, employee compensation, rent, insurance, and science-related consultants’ fees and amortization allocated to research and development expense.
General and administrative expenses – General and administrative expenses increased by approximately $1,396,674 to $5,279,859 for the year ended December 31, 2014 compared to $3,883,185 for the year ended December 31, 2013. The increase in general and administrative expenses is primarily comprised of the following increases in expenses incurred: $1,159,296 of salaries, payroll related expenses and employee stock-based compensation allocated to general and administrative expenses, $195,784 of investor and public relations expenses, $184,277 in state corporation and franchise taxes, $114,736 of accounting, audit, and tax services, $97,734 of rent and facilities maintenance related expenses allocated to general and administrative expenses, $68,649 of overhead allocated from BioTime generally for accounting and corporate secretarial related services and general liability insurance allocated to general and administrative expenses, and $46,250 of cash compensation to directors. Those increases were in part offset by a decrease of $419,069 in legal expenses reflecting non-recurring expenses that we incurred in 2013 related to the Asset Contribution Agreement transactions, including preparing registration statements for filing with the SEC and a decrease of $101,845 in general office and computer supplies expense.
Comparison of Years Ended December 31, 2013 and 2012
The following table shows our operating expenses for the years ended December 31, 2013 and 2012.
|
Year
Ended
December 31,
2013
|
From
September
24, 2012 to
December 31,
2012
|
$
Increase/
Decrease
|
%
Increase
Decrease
|
||||||||||||
|
Research and development expenses
|
$ |
4,319,494
|
$ |
-
|
$ |
+ 4,319,494
|
+ 100%
|
||||||||
|
Acquired in-process research and development expenses
|
17,458,766
|
-
|
+ 17,458,766
|
+ 100%
|
|||||||||||
|
General and administrative expenses
|
3,883,185
|
758,563
|
+ 3,124,622
|
+ 412%
|
|||||||||||
Our activities through December 31, 2013 primarily related to our formation, the execution of the Asset Contribution Agreement, and preparation for the start of our planned research and development operations following the acquisition of assets under the Asset Contribution Agreement. Certain other expenses are primarily attributed to rent and utilities and general overhead expenses.
Research and development expenses – Research and development expenses recognized during the year ended December 31, 2013 and for the period from September 24, 2012 (inception) to December 31, 2012 amounted to $4,319,494 and $0, respectively. In addition, during 2013 we recognized $17,458,766 of IPR&D in connection with the consummation of our acquisition of assets from Geron. IPR&D represents the value allocated by management to incomplete research and development projects which we acquired from Geron and intend to continue. That value was expensed under applicable accounting rules rather than capitalized for future amortization because the acquisition was accounted for an acquisition of assets rather than an acquisition of a business. See Notes 2 and 3 to the Financial Statements. The increases in research and development expenses, other than IPR&D, during 2013 are primarily comprised of $725,425 of amortization of intangible assets acquired under the Asset Contribution Agreement on October 1, 2013, $1,416,415 of salaries, and payroll related expenses allocated to research and development expenses, $194,578 in employee stock-based compensation allocated to research and development expenses, $518,509 of rent and facilities maintenance related expenses allocated to research and development expenses, $363,964 of patent related legal fees and patent maintenance costs, $250,877 of scientific consulting expenses, $81,917 of travel, lodging and meals allocated to research and development expenses, $75,430 in outside research and services allocated to research and development expenses, $453,741 of laboratory expense and supplies expenses, and $183,525 of depreciation expenses allocated to research and development expenses. Research and development expenses, other than IPR&D, were incurred in setting up our research and product development facility and equipment, planning the initiation of our initial product development programs, licensing patents and stem cell lines from WARF, evaluating other technology that may be available for in-licensing or acquisition, preparing applications for research grants, and initiating discussions with third parties for the manufacture or co-development of product candidates.
General and administrative expenses – General and administrative expenses recognized during the year ended December 31, 2013 and for the period from September 24, 2012 (inception) to December 31, 2012 amounted to $3,883,185 and $758,563, respectively and are primarily comprised of $1,018,428 and $27,022, respectively in salaries and payroll related expenses allocated to general and administrative expenses, $527,213 and $0, respective in employee stock-based compensation allocated to general and administrative expenses, $1,221,051 and $727,123 in legal and accounting fees incurred in connection with the registration of the Series A Shares under the Securities Act of 1933, as amended, and the registration or application for exemptions from registration of those shares under the securities laws of certain states and other jurisdictions, matters related to the Asset Contribution Agreement, quarterly reviews and annual audit procedures, $299,063 and $0, respectively in rent and facilities maintenance related expenses allocated to general and administrative expenses, $238,004 and $0, respectively in director cash and stock-based compensation expense, $187,453 and $0, respectively in general office expenses, $96,276 and $120 of investor and public relations expenses, stock listing and subscription fees and securities exchange commission filing related fees, and $132,209 and $1,736, respectively of travel, lodging and meals allocated to general and administrative expenses, and $39,619 and $0, respectively of depreciation expenses allocated to general and administrative expenses.
Liquidity and Capital Resources
At December 31, 2014, we had $3,075,593 of cash and cash equivalents on hand and we held 3,852,880 BioTime common shares, with a market value of approximately $14,374,492 on that date. We may raise capital from time to time through the sale of our Series A Shares or other securities, and our BioTime common shares. We may sell our Series A Shares or other securities in public offerings registered under the Securities Act of 1933, as amended (the “Securities Act”), or in private placements to select investors. We may sell our BioTime common shares, from time to time, by any method that is deemed to be an “at-the-market” equity offering as defined in Rule 415 promulgated under the Securities Act, including sales made directly on or through the NYSE MKT or any other existing trading market for the common shares in the U.S. or to or through a market maker, at prices related to the prevailing market price, or through block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction, or through one more of the foregoing transactions. We expect to sell the BioTime common shares through Cantor Fitzgerald & Co. or such other broker-dealer as BioTime may designate. We may also sell BioTime common shares by any other method permitted by law, including in privately negotiated transactions. We will bear all broker-dealer commissions payable in connection with the sale of our Series A Shares or other securities and our BioTime common shares. Broker-dealers may receive commissions or discounts from us (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The prices at which we may issue and sell our Series A Shares or other securities and our BioTime common shares in the future are not presently determinable and will depend upon many factors, including prevailing prices for those securities in the public market.
We have outstanding warrants to purchase 3,500,000 shares of our common stock at an exercise price of $5.00 per share that will expire on September 30, 2016, and warrants to purchase 5,000,000 shares of our common stock at an exercise price of $2.34 per share that will expire on June 15, 2015. We will receive $29,200,000 if all of the warrants are exercised. There can be no assurance that the warrants will be exercised.
We plan to use the cash we have available to develop certain of our product candidates and technology, to acquire new stem cell products and technology through licenses or similar agreements from other companies, and to defray overhead expenses and to pay general and administrative expenses. We may also use available funds for any clinical trials of products that we may conduct. We expect to continue to incur operating losses and negative cash flows. BioTime contributed to the funding of our business activities from inception through December 31, 2014 but there can be no assurance they will continue to do so in the future.
We have been awarded a $14.3 million Strategic Partnership III grant by CIRM to help fund our clinical development of AST-OPC1. The grant will provide funding for us to reinitiate clinical development of AST-OPC1 in subjects with spinal cord injury, to expand clinical testing of escalating doses in the target population intended for future pivotal trials, and for product development efforts to refine and scale manufacturing methods to support eventual commercialization. CIRM will disburse the grant funds to us over four years in accordance with a quarterly disbursement schedule, subject to our attainment of certain progress and safety milestones. We received the first payment from CIRM in the amount of $916,554 during October 2014 and the second payment in the amount of $2,269,515 in January 2015. As the distributions of the CIRM grant are subject to meeting certain progress and go/no-go milestones, there can be no assurance that we will receive the entire amount granted. As the balance of the distributions of the CIRM grant are subject to meeting certain progress and go/no-go milestones, there can be no assurance that we will receive the entire amount granted.
Pursuant to the CRUK Agreement, CRUK has agreed to fund Phase I/IIa clinical development of our AST-VAC2 product candidate. We will, at our own cost, complete process development and manufacturing scale-up of the AST-VAC2 manufacturing process and will transfer the resulting cGMP-compatible process to CRUK. CRUK will, at its own cost, manufacture clinical grade AST-VAC2 and will carry out the Phase I/IIa clinical trial of AST-VAC2 in cancer patients both resected early-stage and advanced forms of lung cancer.
Since inception, Asterias has incurred net losses and has funded its operations primarily through the support from BioTime, issuance of equity securities, payments from research grants, and royalties from product sales. At December 31, 2014, we had an accumulated deficit of $33,235,791, working capital of $11,887,884 and stockholders’ equity of $32,631,060. Asterias has evaluated its projected cash flows for and believes that its cash and cash equivalents of $3,075,593 as of December 31, 2014 and financial support from BioTime as needed will be sufficient to fund its operations at least through 2015. However, clinical trials being conducted by us will be funded in part with funds from the $14,323,318 grant awarded in 2014 by the California Institute of Regenerative Medicine (“CIRM”) and not from cash on hand. If we were to lose our grant funding we may be required to delay, postpone, or cancel our clinical trials or limit the number of clinical trial sites, or otherwise reduce or curtail our operations unless we are able to obtain from another source of adequate financing that could be used for our clinical trials.
The unavailability or inadequacy of financing or revenues to meet future capital needs could force us to modify, curtail, delay, or suspend some or all aspects of our planned operations. Sales of additional equity securities could result in the dilution of the interests of our current shareholders.
Cash Flows
Cash provided used in operations
Since our inception, we have incurred losses from operations and negative cash flows from our operations. During the year ended December 31, 2014, our total research and development expenditures were $13,310,421 and our general and administrative expenditures were $5,279,859. Net loss for the year ended December 31, 2014 amounted to $10,097,154. Our sources of cash during 2014 primarily consisted of $189,215 from royalty revenues on product sales by licensees and a research grant payment of $916,554 from CIRM. As of December 31, 2014 and December 31, 2013, we had a working capital surplus of $11,887,884 and $31,835,965, respectively, and an accumulated deficit of $33,235,791 and $23,138,637, respectively, based on our operating losses and the expensed IPR&D.
Net cash used in operating activities during the year ended December 31, 2014 amounted to $11,591,510. The difference between the net loss and net cash used in operating activities during the year ended December 31, 2014 was primarily attributable to amortization of intangible assets of $4,789,399, stock-based compensation paid to employees of $1,814,120, depreciation expense of $530,391, amortization of prepaid rent of $84,586, deferred rent liabilty of $93,763, changes in accrued liabilities of $198,902, and accounts payable of $120,208. The overall difference was offset in part by $1,449,455 of our expenses paid by BioTime which is reflected in “amount due to BioTime” on our December 31, 2014 Statements of Cash Flows, grant receivable of $117,902, prepaid expenses and other current assets of $182,757, and deferred income tax benefits of $7,375,611.
Investing and financing activities
During the year ended December 31, 2014, we used $114,714 in cash to purchase equipment and furniture and $219,443 in construction in progress for our Fremont facility, and we paid $306,560 as security deposits for two of our leased facilities. During June 2014, we received $12,660,908 in proceeds from the sale of 5,049,197 of our BioTime common shares, including 49,197 shares sold in open market transactions and 5,000,000 shares sold with warrants to purchase 5,000,000 shares of Asterias common stock to two investors for $12,500,000 in cash. The exercise price of the warrants is $2.34. The exercise price and the number of shares of Asterias common stock issuable upon the exercise of the warrants are subject to adjustment in the event of a stock split, stock dividend, combination of shares, recapitalization or certain other transactions. The warrants will expire on June 15, 2015 if not exercised by that date.
During June 2014, we also received $468,000 from the sale of 200,000 shares of our common stock to our newly appointed President and Chief Executive Officer for $2.34 per share and $7,799 from the exercise of options by a former employee at an exercise price of $2.34 per share.
Off-Balance Sheet Arrangements
As of December 31, 2014, and as of December 31, 2013, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of SEC Regulation S-K.
Foreign Currency Exchange Risk
We are not presently exposed in a significant degree to foreign exchange currency risks because we are not conducting international business at this time, and we do not engage in foreign currency hedging activities. If we engage in international transactions, we will need to translate foreign currencies into U.S. dollars for reporting purposes, and currency fluctuations could have an impact on our financial results.
Credit Risk
We place some of our cash in U.S. banks and invest most of our cash in money market funds. Deposits with banks may temporarily exceed the amount of insurance provided on such deposits. We will monitor the cash balances in the accounts and adjust the cash balances as appropriate, but if the amount of a deposit at any time exceeds the federally insured amount at a bank, the uninsured portion of the deposit could be lost, in whole or in part, if the bank were to fail. Our investments in money market funds are not insured or guaranteed by the United States government or any of its agencies.
Interest Rate Risk
We invest most of our cash in money market funds. The primary objective of our investments will be to preserve principal and liquidity while earning a return on our invested capital, without incurring significant risks. Our future investment income is not guaranteed and may fall short of expectations due to changes in prevailing interest rates, or we may suffer losses in principal if the net asset value of a money market fund falls below $1 per share.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Asterias Biotherapeutics, Inc.
We have audited the accompanying balance sheet of Asterias Biotherapeutics, Inc. (a company in the development stage) as of December 31, 2014, and the related statements of operations, comprehensive loss, stockholders’ equity (deficit), and cash flows for the year then ended and for the period from September 24, 2012 (inception) to December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit. We did not audit the statements of operations, comprehensive loss, stockholders’ equity (deficit), and cash flows of the Company for the period from September 24, 2012 (inception) to December 31, 2013. Those statements were audited by other auditors whose report has been furnished to us, and our opinion, insofar as it relates to amounts for the period from inception to December 31, 2013, is based solely on the report of the other auditors.
We conducted our audit in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Asterias Biotherapeutics, Inc. (a company in the development stage) at December 31, 2014, and the results of its operations and its cash flows for the year then ended and for the period from inception to December 31, 2014, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company's internal control over financial reporting as of December 31, 2014, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 9, 2015 expressed an unqualified opinion thereon.
/s/ OUM & CO. LLP
San Francisco, California
March 9, 2015
49
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Asterias Biotherapeutics, Inc.
We have audited the internal control over financial reporting of Asterias Biotherapeutics, Inc. (a company in the development stage) as of December 31, 2014, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO criteria”). The management of the Company is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Item 9A, Management’s Report on Internal Control Over Financial Reporting”. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Asterias Biotherapeutics, Inc. (a company in the development stage) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2014, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheet of Asterias Biotherapeutics, Inc. (a company in the development stage) as of December 31, 2014, and the related statements of operations, comprehensive loss, stockholders’ equity (deficit), and cash flows for the year then ended and for the period from September 24, 2012 (inception) to December 31, 2014 and our report dated March 9, 2015 expressed an unqualified opinion thereon.
/s/ OUM & CO. LLP
San Francisco, California
March 9, 2015
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Asterias Biotherapeutics, Inc
We have audited the accompanying balance sheet of Asterias Biotherapeutics, Inc. (a company in the development stage) (the “Company”) as of December 31, 2013, and the related statements of operations, comprehensive loss, stockholders’ equity (deficit), and cash flows for the year ended December 31, 2013 and for the period from September 24, 2012 (inception) to December 31, 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinions.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Asterias Biotherapeutics, Inc. as of December 31, 2013, and the results of its operations and its cash flows for the year ended December 31, 2013 and for the period from September 24, 2012 (inception) to December 31, 2012, in conformity with accounting principles generally accepted in the United States of America.
|
/s/ Rothstein Kass
|
|
|
New York, New York
|
|
|
March 17, 2014
|
ASTERIAS BIOTHERAPEUTICS, INC.
(a company in the development stage)
BALANCE SHEETS
|
December 31,
2014
|
December 31,
2013
|
||||||
|
ASSETS
|
|||||||
|
CURRENT ASSETS
|
|||||||
|
Cash and cash equivalents
|
$
|
3,075,593
|
$
|
2,171,113
|
|||
|
Available-for-sale securities, at fair value
|
14,374,492
|
32,052,217
|
|||||
|
BioTime warrants to be distributed to holders of Series A shares (see Note 2)
|
—
|
15,568,307
|
|||||
|
Grant receivable
|
117,902
|
—
|
|||||
|
Landlord receivable
|
377,981 |
—
|
|||||
|
Prepaid expenses and other current assets
|
438,263
|
340,092
|
|||||
|
Total current assets
|
18,384,231
|
50,131,729
|
|||||
|
NONCURRENT ASSETS
|
|||||||
|
Intangible assets, net
|
23,502,185
|
28,291,584
|
|||||
|
Equipment and furniture, net
|
1,044,841
|
1,460,518
|
|||||
|
Construction in progress
|
405,730
|
—
|
|||||
|
Investment in affiliates
|
415,543
|
415,543
|
|||||
|
Other assets
|
360,983
|
54,423
|
|||||
|
TOTAL ASSETS
|
$
|
44,113,513
|
$
|
80,353,797
|
|||
|
LIABILITIES AND STOCKHOLDERS' EQUITY
|
|||||||
|
CURRENT LIABILITIES
|
|||||||
|
Obligation to distribute BioTime warrants to holders of Series A shares (see Note 2)
|
$
|
—
|
$
|
15,568,307
|
|||
|
Amount due to BioTime
|
614,977
|
2,064,432
|
|||||
|
Accounts payable
|
736,919
|
567,140
|
|||||
|
Accrued expenses and other current liabilities
|
431,503
|
95,885
|
|||||
| Deferred tax liabilities, current portion | 4,712,948 |
—
|
|||||
|
Total current liabilities
|
6,496,347
|
18,295,764
|
|||||
| LONG-TERM LIABILITIES | |||||||
|
Deferred tax liabilites, net of current portion and deferred tax assets
|
4,514,362
|
8,277,548
|
|||||
| Deferred rent liabilty, net of current portion | 93,763 |
—
|
|||||
| Lease liability | 377,981 |
—
|
|||||
|
TOTAL LIABILITIES
|
11,482,453
|
26,573,312
|
|||||
|
Commitments and contingencies (see Note 10)
|
|||||||
|
STOCKHOLDERS’ EQUITY
|
|||||||
|
Preferred Stock, $0.0001 par value, authorized 5,000,000 shares; none issued and outstanding
|
—
|
—
|
|||||
|
Common Stock, authorized 75,000,000 Series A Common Stock, $0.0001 par value, and 75,000,000 Series B Common Stock, $0.0001 par value; 30,902,152 shares Series A Common Stock and no Series B Common Stock issued and outstanding at December 31, 2014 and 6,537,779 Series A Common Stock and 23,961,040 Series B Common Stock issued and outstanding at December 31, 2013
|
3,080
|
3,050
|
|||||
|
Additional paid-in capital
|
66,366,434
|
79,850,758
|
|||||
|
Accumulated other comprehensive loss on available-for-sale investments
|
(502,663
|
)
|
(2,934,686)
|
||||
|
Deficit accumulated during the development stage
|
(33,235,791
|
)
|
(23,138,637)
|
||||
|
Total stockholders’ equity
|
32,631,060
|
53,780,485
|
|||||
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY
|
$
|
44,113,513
|
$
|
80,353,797
|
|||
The accompanying notes are an integral part of these financial statements.
ASTERIAS BIOTHERAPEUTICS, INC.
(a company in the development stage)
STATEMENTS OF OPERATIONS
|
Years Ended December 31,
|
Cumulative
Period from Inception
(September 24, 2012) to
|
||||||||||||||
|
2014
|
2013
|
December 31,
2012
|
December 31,
2014
|
||||||||||||
|
REVENUE
|
|||||||||||||||
|
Royalties from product sales
|
$
|
189,215
|
$
|
-
|
$
|
-
|
$
|
189,215
|
|||||||
|
Grant income
|
1,034,456
|
-
|
-
|
1,034,456
|
|||||||||||
| Total revenues | 1,223,671 |
-
|
-
|
1,223,671 | |||||||||||
|
Cost of sales
|
(94,607
|
)
|
-
|
-
|
(94,607)
|
||||||||||
|
Total gross profit
|
|
1,129,064
|
|
-
|
|
-
|
|
1,129,064
|
|||||||
|
OPERATING EXPENSES
|
|||||||||||||||
|
Research and development
|
|
(13,310,421
|
)
|
|
(4,319,494
|
)
|
|
-
|
|
(17,629,915)
|
|||||
|
Acquired in-process research and development (see Note 2)
|
-
|
(17,458,766
|
)
|
-
|
(17,458,766)
|
||||||||||
|
General and administrative
|
(5,279,859
|
)
|
(3,883,185
|
)
|
(758,563
|
)
|
(9,921,607
|
||||||||
|
Total operating expenses
|
(18,590,280
|
)
|
(25,661,445
|
)
|
(758,563
|
)
|
(45,010,288)
|
||||||||
|
Loss from operations
|
(17,461,216
|
)
|
(25,661,445
|
)
|
(758,563
|
)
|
(43,881,224)
|
||||||||
|
OTHER INCOME/(EXPENSE)
|
|||||||||||||||
|
Interest expense, net
|
(9,991
|
)
|
(1,737
|
)
|
-
|
(11,728)
|
|||||||||
|
Other income/(expense), net
|
(1,558
|
)
|
313
|
(330
|
)
|
(1,575)
|
|||||||||
|
Gain on sale of fixed assets
|
-
|
2,430
|
-
|
2,430
|
|||||||||||
|
Total other income/(expense), net
|
(11,549
|
)
|
1,006
|
(330
|
)
|
(10,873)
|
|||||||||
|
LOSS BEFORE DEFERRED INCOME TAX BENEFIT
|
(17,472,765
|
)
|
(25,660,439
|
)
|
(758,893
|
)
|
(43,892,097)
|
||||||||
|
Deferred income tax benefit
|
7,375,611
|
3,280,695
|
-
|
10,656,306
|
|||||||||||
|
NET LOSS
|
$
|
(10,097,154
|
)
|
$
|
(22,379,744
|
)
|
$
|
(758,893
|
)
|
$
|
(33,235,791)
|
||||
|
Basic and diluted net loss per common share
|
$
|
(0.33
|
)
|
$
|
(2.90
|
)
|
$
|
(14.69
|
)
|
$
|
(1.96)
|
||||
|
Weighted average number of common stock outstanding: basic and diluted
|
30,720,280
|
7,726,042
|
51,647
|
16,954,131
|
|||||||||||
The accompanying notes are an integral part of these financial statements.
ASTERIAS BIOTHERAPEUTICS, INC.
(a company in the development stage)
STATEMENTS OF COMPREHENSIVE LOSS
|
For the Years ended December 31,
|
Accumulated Period from Inception
(September 24, 2012) to
|
|||||||||||||||
|
2014
|
2013
|
December 31, 2012
|
December 31, 2014
|
|||||||||||||
|
NET LOSS
|
$
|
(10,097,154
|
)
|
$
|
(22,379,744
|
)
|
$
|
(758,893
|
)
|
$
|
(33,235,791
|
)
|
||||
|
Other comprehensive income/(loss), net of tax:
|
||||||||||||||||
|
Change in comprehensive income/(loss) from equity investments
|
||||||||||||||||
|
Available for sale investments:
|
||||||||||||||||
|
Change in other unrealized gain/(loss), net of taxes
|
2,432,023
|
(2,934,686
|
)
|
-
|
(502,663
|
)
|
||||||||||
|
COMPREHENSIVE LOSS
|
$
|
(7,665,131
|
)
|
$
|
(25,314,430
|
)
|
$
|
(758,893
|
)
|
$
|
(33,738,454
|
)
|
||||
The accompanying notes are an integral part of the financial statements
ASTERIAS BIOTHERAPEUTICS, INC.
(a company in the development stage)
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
|
Common Stock
|
Additional
Paid-In
|
Accumulated
Other
Comprehensive
Income
|
Accumulated
Deficit
During the
Development
|
Subscription |
Stockholders’
Equity
|
|||||||||||||||||||||||||||||||
|
Series A
|
Series B
|
Capital
|
(Loss) |
Stage
|
Receivable
|
(Deficit) | ||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
|||||||||||||||||||||||||||||||||
|
Common stock issued to BioTime on September 24, 2012 (date of inception)
|
—
|
$
|
—
|
50,000
|
$
|
5
|
$
|
49,995
|
$
|
—
|
$
|
—
|
$
|
(50,000
|
)
|
$
|
—
|
|||||||||||||||||||
|
Common stock issued to officer on September 27, 2012
|
—
|
—
|
1,700
|
—
|
1,740
|
—
|
—
|
—
|
1,740
|
|||||||||||||||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
—
|
—
|
(758,893
|
)
|
—
|
(758,893
|
)
|
|||||||||||||||||||||||||
|
Balance as of December 31, 2012
|
—
|
—
|
51,700
|
5
|
51,735
|
—
|
(758,893
|
)
|
(50,000
|
)
|
(757,153
|
)
|
||||||||||||||||||||||||
|
Common stock, at $2.40 per share, issued to Geron in connection with acquisition of various assets on October 1, 2013, net of issuance costs of $541,800
|
6,537,779
|
654
|
—
|
—
|
15,120,568
|
—
|
—
|
—
|
15,121,222
|
|||||||||||||||||||||||||||
|
Common stock, at $2.40 per share, and common stock warrants issued to BioTime in connection with transfer of various assets on October 1, 2013
|
—
|
—
|
21,773,340
|
2,177
|
58,974,935
|
—
|
—
|
—
|
58,977,112
|
|||||||||||||||||||||||||||
|
Common stock, at $2.40 per share, and common stock warrants issued to an investor for cash
|
—
|
—
|
2,136,000
|
214
|
4,999,786
|
—
|
—
|
—
|
5,000,000
|
|||||||||||||||||||||||||||
|
Reduction of subscription receivable
|
—
|
—
|
—
|
—
|
—
|
—
|
—
|
50,000
|
50,000
|
|||||||||||||||||||||||||||
|
Unrealized loss on available-for-sale securities
|
—
|
—
|
—
|
—
|
—
|
(2,934,686
|
)
|
—
|
—
|
(2,934,686
|
)
|
|||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
—
|
—
|
703,734
|
—
|
—
|
—
|
703,734
|
|||||||||||||||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
—
|
—
|
(22,379,744
|
)
|
—
|
(22,379,744
|
)
|
|||||||||||||||||||||||||
|
Balance as of December 31, 2013
|
6,537,779
|
|
654
|
23,961,040
|
|
2,396
|
|
79,850,758
|
|
(2,934,686
|
)
|
|
(23,138,637
|
)
|
|
—
|
|
53,780,485
|
||||||||||||||||||
|
Common stock issued to officer at $2.34 per share
|
—
|
—
|
200,000
|
20
|
467,980
|
—
|
—
|
—
|
468,000
|
|||||||||||||||||||||||||||
|
Sale of BioTime shares
|
—
|
—
|
—
|
—
|
(10,366,327
|
)
|
—
|
—
|
—
|
(10,366,327
|
)
|
|||||||||||||||||||||||||
|
Stock-based compensation expense
|
—
|
—
|
—
|
—
|
1,580,115
|
—
|
—
|
—
|
1,580,115
|
|||||||||||||||||||||||||||
|
Restricted stock granted for compensation at $2.34 per share
|
—
|
—
|
200,000
|
10
|
233,995
|
—
|
—
|
—
|
234,005
|
|||||||||||||||||||||||||||
|
Warrants issued to outside investors as part of the sale of 5,000,000 BioTime shares
|
—
|
—
|
—
|
—
|
3,183,891
|
—
|
—
|
—
|
3,183,891
|
|||||||||||||||||||||||||||
|
Series B converted to Series A on October 1, 2014
|
24,361,040
|
2,426
|
(24,361,040
|
)
|
(2,426
|
)
|
—
|
—
|
—
|
—
|
||||||||||||||||||||||||||
|
Exercise of options
|
3,333
|
—
|
—
|
—
|
7,799
|
—
|
—
|
—
|
7,799
|
|||||||||||||||||||||||||||
|
Deferred tax liability adjustment on BioTime shares
|
—
|
—
|
—
|
—
|
(8,591,777
|
)
|
—
|
—
|
—
|
(8,591,777
|
)
|
|||||||||||||||||||||||||
|
Unrealized gain on available-for-sale securities
|
—
|
—
|
—
|
—
|
—
|
2,432,023
|
—
|
—
|
2,432,023
|
|||||||||||||||||||||||||||
|
Net loss
|
—
|
—
|
—
|
—
|
—
|
—
|
(10,097,154
|
)
|
—
|
(10,097,154
|
)
|
|||||||||||||||||||||||||
|
Balance as of December 31, 2014
|
30,902,152
|
$
|
3,080
|
—
|
$
|
—
|
$
|
66,366,434
|
$
|
(502,663
|
)
|
$
|
(33,235,791
|
)
|
$
|
—
|
$
|
32,631,060
|
||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
ASTERIAS BIOTHERAPEUTICS, INC.
(a company in the development stage)
STATEMENTS OF CASH FLOWS
|
Years ended December 31,
|
Cumulative
Period from Inception
(September 24, 2012) to
|
||||||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
2014
|
2013
|
December 31,
2012
|
December 31,
2014
|
|||||||||||
|
Net loss
|
$
|
(10,097,154
|
)
|
$
|
(22,379,744
|
)
|
$
|
(758,893
|
)
|
$
|
(33,235,791)
|
||||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|||||||||||||||
|
Acquired in-process research and development (see Note 2)
|
—
|
17,458,766
|
—
|
17,458,766
|
|||||||||||
|
Depreciation expense
|
530,391
|
220,595
|
—
|
750,986
|
|||||||||||
|
Stock-based compensation
|
1,814,120
|
703,734
|
—
|
2,517,854
|
|||||||||||
|
Amortization of intangible assets
|
4,789,399
|
725,425
|
—
|
5,514,824
|
|||||||||||
| Amortization of prepaid rent | 84,586 | 84,586 |
—
|
169,172 | |||||||||||
|
Gain on sale of equipment, net
|
—
|
(2,430
|
)
|
—
|
(2,430)
|
||||||||||
|
Deferred income tax benefit
|
(7,375,611
|
)
|
(3,280,695
|
)
|
—
|
(10,656,306)
|
|||||||||
|
Changes in operating assets and liabilities:
|
|||||||||||||||
|
Grant receivable
|
(117,902
|
) |
—
|
—
|
(117,902)
|
||||||||||
|
Prepaid expenses and other current assets
|
(182,757
|
)
|
(422,407
|
)
|
(2,271
|
)
|
(607,435)
|
||||||||
|
Accounts payable
|
120,208
|
567,140
|
—
|
687,348
|
|||||||||||
|
Accrued expenses and other current liabilities
|
198,902
|
95,885
|
—
|
294,787
|
|||||||||||
| Deferred rent liability | 93,763 |
—
|
—
|
93,763 | |||||||||||
|
Amount due to BioTime
|
(1,449,455
|
)
|
4,902,014
|
761,164
|
4,213,723
|
||||||||||
|
Net cash used in operating activities
|
(11,591,510
|
)
|
(1,327,131
|
)
|
—
|
(12,918,641)
|
|||||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|||||||||||||||
|
Purchase of equipment and furniture
|
(114,714
|
)
|
(1,246,729
|
)
|
—
|
(1,361,443)
|
|||||||||
|
Construction in progress
|
(219,443
|
)
|
—
|
(219,443)
|
|||||||||||
|
Proceeds from sale of equipment and furniture
|
—
|
27,500
|
—
|
27,500
|
|||||||||||
|
Proceeds from the sale of available-for-sale securities
|
12,660,908
|
—
|
12,660,908
|
||||||||||||
|
Payment of security deposits
|
(306,560
|
)
|
(54,423
|
)
|
—
|
(360,983)
|
|||||||||
|
Net cash provided by (used in) investing activities
|
12,020,191
|
(1,273,652
|
)
|
—
|
10,746,539
|
||||||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|||||||||||||||
|
Proceeds from sales of common shares and warrants
|
468,000
|
5,000,000
|
—
|
5,468,000
|
|||||||||||
|
Proceeds from exercises of stock options
|
7,799
|
—
|
—
|
7,799
|
|||||||||||
|
Payment to Geron in connection with acquisition of assets on October 1, 2013
|
—
|
(228,104
|
)
|
—
|
(228,104)
|
||||||||||
|
Net cash provided by financing activities
|
475,799
|
4,771,896
|
—
|
5,247,695
|
|||||||||||
|
Net increase in cash
|
904,480
|
2,171,113
|
—
|
3,075,593
|
|||||||||||
|
Cash and cash equivalents at beginning of period
|
2,171,113
|
—
|
—
|
-
|
|||||||||||
|
Cash and cash equivalents at end of period
|
$
|
3,075,593
|
$
|
2,171,113
|
$
|
—
|
$
|
3,075,593
|
|||||||
|
SUPPLEMENTAL SCHEDULE OF NON-CASH FINANCING AND INVESTING ACTIVITIES:
|
|||||||||||||||
|
Purchase of equipment and furniture, contributed by BioTime
|
$
|
—
|
$
|
(459,454
|
)
|
$
|
—
|
$
|
(459,454)
|
||||||
|
Construction in progress in accounts payable and accrued expenses
|
$ |
186,287
|
$
|
—
|
$
|
—
|
$ |
186,287
|
|||||||
| Landlord receivable | $ | (377,981 | ) | $ |
—
|
$ |
—
|
$ |
—
|
||||||
| Lease liability | $ | 377,981 | $ |
—
|
$ |
—
|
$ |
—
|
|||||||
|
Available-for-sale BioTime securities contributed by BioTime
|
$
|
—
|
$
|
34,985,163
|
$
|
—
|
$
|
34,985,163
|
|||||||
|
Cancellation of indebtedness to BioTime
|
$
|
—
|
$
|
5,000,000
|
$
|
—
|
$
|
5,000,000
|
|||||||
|
Transaction costs paid by BioTime, on behalf of the Company
|
$
|
—
|
$
|
300,000
|
$
|
—
|
$
|
300,000
|
|||||||
|
Intangible assets acquired from Geron
|
$
|
—
|
$
|
29,017,009
|
$
|
—
|
$
|
29,017,009
|
|||||||
|
Adjustment to deferred tax liability arising from difference in book versus tax basis on Geron intangible assets acquired
|
$
|
— |
$
|
11,558,243
|
$
|
—
|
$
|
11,558,243
|
|||||||
|
Investment in affiliates, contributed by BioTime
|
$
|
—
|
$
|
415,543
|
$
|
—
|
$
|
415,543
|
|||||||
|
Common stock and common stock warrants issued to BioTime and Geron in connection with acquisition and transfer of assets
|
$
|
—
|
$
|
74,098,333
|
$
|
—
|
$
|
74,098,333
|
|||||||
|
Common stock issued upon investment by BioTime
|
$
|
—
|
$
|
—
|
$
|
50,000
|
$
|
50,000
|
|||||||
|
Reduction of subscription receivable
|
$
|
—
|
$
|
(50,000
|
)
|
$
|
—
|
$
|
(50,000)
|
||||||
|
Common stock issued in exchange for non-cash consideration in connection with investment by officer
|
$
|
—
|
$
|
—
|
$
|
1,740
|
$
|
1,740
|
|||||||
ASTERIAS BIOTHERAPEUTICS, INC.
(a company in the development stage)
NOTES TO FINANCIAL STATEMENTS
1. Organization, Basis of Presentation and Liquidity
Asterias Biotherapeutics, Inc. (“Asterias”) (a company in the development stage) was incorporated in Delaware on September 24, 2012. Asterias is a majority-owned and controlled subsidiary of BioTime, Inc. (“BioTime”).
Asterias’ primary focus is the emerging field of regenerative medicine. Asterias’ core technologies center on stem cells capable of becoming all of the cell types in the human body, a property called pluripotency. Asterias plans to develop, support and license a wide range of technologies that are based on “pluripotent” stem cells and that could be used to treat diseases or injuries in a variety of medical fields, with an initial focus on the therapeutic areas of neurology and oncology.
Through December 31, 2014, Asterias had generated little revenue and is considered to be in the development stage as defined in Statement of Financial Accounting Standards Board Accounting Standards Codification (“ASC”) Topic 915, “Development Stage Entities,” and is subject to the risks associated with activities of development stage companies.
Asterias’ activities through December 31, 2013 primarily related to Asterias’ formation, the execution of the Asset Contribution Agreement described below, preparation for the start of its planned operations following the acquisition of assets under the Asset Contribution Agreement, and acquired in-process research and development (“IPR&D”) recognized upon consummation of the asset acquisition under the Asset Contribution Agreement on October 1, 2013. Certain other expenses are primarily attributed to rent and utilities and general overhead expenses. Asterias has selected December 31 as its fiscal year end.
The financial statements presented herein, and discussed below, have been prepared on a stand-alone basis. The financial statements are presented in accordance with accounting principles generally accepted in the U.S. and with the accounting and reporting requirements of Regulation S-X of the Securities and Exchange Commission (“SEC”). BioTime has consolidated the results of Asterias into BioTime’s consolidated results based on BioTime’s ability to control Asterias’ operating and financial decisions and policies through the ownership of Asterias Series B Shares throughout the periods presented. BioTime owned 70.6% and 71.6% of the outstanding of Asterias common stock as a whole at December 31, 2014 and 2013, respectively.
BioTime allocates expenses such as salaries and payroll related expenses incurred and paid on behalf of Asterias based on the amount of time that particular employees devote to Asterias affairs. Other expenses such as legal, accounting, travel, and entertainment expenses are allocated to Asterias to the extent that those expenses are incurred by or on behalf of Asterias. BioTime also allocates certain overhead expenses such as insurance, internet, and telephone expenses based on a percentage determined by management. These allocations are made based upon activity-based allocation drivers such as time spent, percentage of square feet of office or laboratory space used, and percentage of personnel devoted to Asterias operations or management. Management evaluates the appropriateness of the percentage allocations on a quarterly basis and believes that this basis for allocation is reasonable.
Liquidity – Since inception, Asterias has incurred net losses and has funded its operations primarily through the support from BioTime, issuance of equity securities, payments from research grants, and royalties from product sales. At December 31, 2014, Asterias had an accumulated deficit of $33,235,791, working capital of $11,887,884 and stockholders’ equity of $32,631,060. Asterias has evaluated its projected cash flows for and believes that its cash and cash equivalents of $3,075,593 as of December 31, 2014 and financial support from BioTime as needed will be sufficient to fund its operations at least through 2015 (See Note 17). However, clinical trials being conducted by Asterias will be funded in part with funds from the $14,323,318 grant awarded in 2014 by the California Institute of Regenerative Medicine (“CIRM”) and not from cash on hand. If Asterias was to lose its grant funding it may be required to delay, postpone, or cancel its clinical trials or limit the number of clinical trial sites, or otherwise reduce or curtail its operations unless it is able to obtain from another source of adequate financing that could be used for its clinical trials.
2. Asset Contribution Agreement with BioTime and Geron Corporation
On January 4, 2013, Asterias entered into an Asset Contribution Agreement with BioTime and Geron Corporation (“Geron”) pursuant to which BioTime and Geron agreed to concurrently contribute certain assets to Asterias in exchange for shares of Asterias common stock and warrants to purchase common stock. The transaction closed on October 1, 2013.
Transfer of BioTime Assets
Under the Asset Contribution Agreement, BioTime contributed to Asterias 8,902,077 BioTime common shares registered for re-sale with the SEC; warrants to subscribe for and purchase 8,000,000 additional BioTime common shares (the “BioTime Warrants”) exercisable for a period of five years at a price of $5.00 per share, subject to pro rata adjustment for certain stock splits, reverse stock splits, stock dividends, recapitalizations and other transactions; a 10% common stock interest in BioTime’s subsidiary OrthoCyte Corporation; a 6% ordinary stock interest in BioTime’s subsidiary Cell Cure Neurosciences, Ltd. (“Cell Cure Neurosciences”); and a quantity of certain hES cell lines produced under “good manufacturing practices” sufficient to generate master cell banks, and non-exclusive, world-wide, royalty-free licenses to use those cell lines and certain patents pertaining to stem cell differentiation technology for any and all purposes.
In return, Asterias issued to BioTime 21,773,340 shares of its Series B Common Stock, par value $0.0001 per share (“Series B Shares”), and warrants to purchase 3,150,000 Series B Shares, exercisable for a period of three years from the date of issue at an exercise price of $5.00 per share. In addition, BioTime cancelled Asterias’ obligations under a loan of $5,000,000 from BioTime, related to cash financing provided by BioTime during 2013 prior to the Asset Contribution Agreement closing.
Because Asterias is a subsidiary of BioTime, the transfer of assets from BioTime was accounted for as a transaction under common control. Non-monetary assets received by Asterias were recorded at their historical cost basis amounts with BioTime. Monetary assets were recorded at fair value. The difference between the value of assets contributed by BioTime and the fair value of consideration issued to BioTime was recorded as an additional contribution by BioTime, in additional paid-in capital.
The assets transferred by BioTime and the related consideration were recorded as follows:
|
Consideration transferred to BioTime:
|
|||
|
Asterias Series B shares
|
$
|
52,164,568
|
|
|
Warrants to purchase Asterias Series B shares
|
2,012,481
|
||
|
Excess of contributed assets’ value over consideration
|
4,800,063
|
||
|
Total consideration issued
|
$
|
58,977,112
|
|
|
Assets transferred by BioTime:
|
|||
|
BioTime common shares, at fair value
|
$
|
34,985,163
|
|
|
BioTime Warrants, at fair value
|
18,276,406
|
||
|
Cancellation of outstanding obligation to BioTime
|
5,000,000
|
||
|
Investment in affiliates, at cost
|
415,543
|
||
|
Geron asset acquisition related transaction costs paid by BioTime
|
300,000
|
||
|
Total assets transferred
|
$
|
58,977,112
|
|
The fair value of the Asterias Series B shares issued was estimated at $2.40 based on the Asterias enterprise value as determined on January 4, 2013, at the time the Asset Contribution Agreement was negotiated and executed by its parties, and as adjusted for subsequent changes in fair values of assets the parties agreed to contribute. The fair value of the warrants to purchase Asterias Series B shares was computed using a Black Scholes Merton option pricing model, which utilized the following assumptions: expected term equal to the contractual term of three years, which is equal to the contractual life of the warrants; risk-free rate of 0.63%; 0% expected dividend yield; 69.62% expected volatility based on the average historical common stock volatility of BioTime and Geron, which were used as Asterias’ common stock does not have a trading history; a stock price of $2.40; and an exercise price of $5.00 per share.
BioTime common shares were valued using $3.93, the closing price per BioTime common shares on the NYSE MKT on October 1, 2013. The fair value of the BioTime Warrants was computed using a Black Scholes Merton option pricing model, which utilized the following assumptions: expected term equal to the contractual term of five years, which is equal to the contractual life of the warrants; risk-free rate of 1.42%; 0% expected dividend yield; 77.6% expected volatility based on historical common stock volatility of BioTime; a stock price of $3.93; and an exercise price of $5.00 per share.
The investment in affiliates represents a non-monetary asset and was recorded at BioTime’s historical cost because BioTime is a common parent to Asterias and those affiliates.
Geron Assets Acquisition
Under the Asset Contribution Agreement, Geron contributed to Asterias certain patents, patent applications, trade secrets, know-how and other intellectual property rights with respect to the technology of Geron directly related to the research, development and commercialization of certain products and know-how related to human embryonic stem (“hES”) cells; certain biological materials, reagents, laboratory equipment; as well as clinical trial documentation, files and data, primarily related to GRNAST-OPC1 clinical trials for spinal cord injury and AST-VAC1 clinical trials for acute myelogenous leukemia. Asterias assumed all obligations related to such assets that would be attributable to periods, events or circumstances after the Asset Contribution Agreement closing date, including those related certain patent interference proceedings that have since been settled.
As consideration for the acquisition of assets from Geron, Asterias issued to Geron 6,537,779 shares of Series A Common Stock, par value $0.0001 per share (“Series A Shares”), which Geron agreed to distribute to its stockholders, on a pro rata basis, subject to applicable legal requirements and certain other limitations (the “Series A Distribution”). The Series A Distribution was completed during July 2014. Asterias agreed to distribute to the holders of its Series A Shares the 8,000,000 shares of BioTime Warrants contributed to Asterias by BioTime. Geron gave notice to Asterias that the Series A Distribution was completed on August 15, 2014. On October 1, 2014 the distribution of the BioTime Warrants to the new holders of Series A Shares was completed and on October 3, 2014 Asterias’ Series B Shares were converted to Series A shares. See Note 8.
In addition, Asterias agreed to bear certain transaction costs in connection with the Asset Acquisition. Such transaction costs were allocated to acquisition of assets in the amount of $1,519,904 and issuance of equity in the amount of $541,800.
The assets contributed by Geron did not include workforce or any processes to be applied to the patents, biological materials and other assets acquired, and therefore did not constitute a business. Accordingly, the acquisition of Geron assets has been accounted for as an acquisition of assets in accordance with the relevant provisions of Accounting Standards Codification (ASC) 805-50. Total consideration payable by Asterias, including transaction costs, has been allocated to the assets acquired based on relative fair values of those assets as of the date of the transaction, October 1, 2013, in accordance with ASC 820, Fair Value Measurement.
The assets acquired from Geron and the related consideration paid were recorded as follows:
|
Consideration paid to Geron:
|
|||
|
Asterias Series A shares, net of share issuance costs of $541,800
|
$
|
15,121,222
|
|
|
Obligation to distribute BioTime Warrants
|
18,276,406
|
||
|
Transaction and other costs
|
1,519,904
|
||
|
Total consideration paid
|
$
|
34,917,532
|
|
|
Assets acquired from Geron (preliminary allocation):
|
|||
|
Patents and other intellectual property rights related to hES cells
|
$
|
29,017,009
|
|
|
Deferred tax liability arising from difference in book versus tax basis on Geron intangible assets acquired
|
(11,558,243)
|
||
|
IPR&D expensed upon acquisition
|
17,458,766
|
||
|
Total assets and in-process research and development acquired
|
$
|
34,917,532
|
|
The fair value of the Asterias Series A shares issued was estimated at $2.40 based on the estimated Asterias enterprise value as determined by parties at the time the Asset Contribution Agreement was negotiated and executed by its parties on January 4, 2013, as adjusted for subsequent changes in fair values of assets the parties agreed to contribute.
The fair value of the obligation to distribute BioTime Warrants equaled the fair value of such warrants, which was computed as noted above under “Transfer of BioTime Assets.” Because the fair value of the BioTime Warrants was expected to always be equal to the fair value of the obligation to distribute them at any date on which those values are determined, the remeasurement of those values would not result in a charge or credit on the statement of operations.
The difference between the fair value of assets contributed by Geron and the fair value of consideration issued to Geron was recorded as an additional contribution by Geron, in additional paid-in capital.
The assets acquired from Geron consist primarily of patents and other intellectual property rights related to hES cells which Asterias intends to license to various parties interested in research, development and commercialization of hES cells technologies, and in-process research and development (“IPR&D”), which includes biological materials, reagents, clinical trial documentation, files and data related primarily to certain clinical trials previously conducted by Geron, which Geron discontinued in November 2011.
Intangible assets related to IPR&D represent the value of incomplete research and development projects which the company intends to continue. In accordance with the accounting rules in ASC 805, such assets, when acquired in conjunction with acquisition of a business, are considered to be indefinite-lived until the completion or abandonment of the associated research and development efforts and are capitalized as an asset. If and when development is complete, the associated assets would be deemed finite-lived and would then be amortized based on their respective estimated useful lives at that point in time. However, when acquired in conjunction with an acquisition of assets that do not constitute a business (such as the acquisition of assets from Geron), in accordance with the accounting rules in ASC 805-50, such intangible assets related to IPR&D are expensed upon acquisition.
The values of the acquired assets were estimated as of October 1, 2013 based upon a preliminary review of those assets which took into account factors such as the condition of the cells, cell lines and other biological materials being contributed, the stage of development of particular technology and product candidates related to patents, patent applications, and know-how, the intended use of these assets and the priority assigned to the development of product candidates to which those assets relate, and the assessment of the estimated useful lives of patents. The amounts allocated to patents and other intellectual property rights that Asterias intends to license, in-process research and development with alternative future uses, and equipment were capitalized as intangible assets and are being amortized over an estimated useful life period of 10 years. The amounts allocated to IPR&D that management determined to have no alternative uses were expensed at the time of acquisition of the related assets in accordance with the requirements of ASC 805-50. The allocation was based on the relative fair value of assets eligible for capitalization and the fair value of assets representing IPR&D before assessing the deferred tax liability arising from the difference in book versus tax basis on Geron intangible assets acquired, which management estimated to be approximately equal. Accordingly, $17,458,766 was capitalized as of December 31, 2013, and $17,458,766 was expensed during the year ended December 31, 2013. The amounts recorded were preliminary as management had not yet completed a detailed assessment and valuation of the acquired assets at the time. During the year ended December 31, 2014, Asterias finalized its assessment of values pertaining to these Geron capitalized intangible assets and recorded an adjustment to reduce the gross intangible cost by $2,157,369 with a corresponding reduction to the accumulated amortization balance of $269,671, resulting in an additional amortization expense of $1,887,698 included in the statements of operations for the year ended December 31, 2014. This adjustment was wholly attributable to finalizing the California income tax rate previously used to compute both the intangible asset and the corresponding deferred income tax liability, recorded at the acquisition date. Accordingly, Asterias simultaneously recorded an adjustment to reduce the related deferred tax liability resulting in the same net amount of $1,887,698 being included in the deferred income tax benefits of $7,375,611 included in the statements of operations for the year ended December 31, 2014. See also Note 7.
Asterias is also obligated to pay Geron royalties on the sale of products, if any, that are commercialized in reliance upon patents acquired from Geron, at the rate of 4% of net sales.
Stock and Warrant Purchase Agreement with Romulus
On January 4, 2013, in connection with entering into the Asset Contribution Agreement, Asterias entered into a Stock and Warrant Purchase Agreement with Romulus Films, Ltd (“Romulus”) pursuant to which Romulus agreed to purchase 2,136,000 Series B Shares and warrants to purchase 350,000 additional Series B Shares for $5,000,000 in cash upon the consummation of asset acquisition under the Asset Contribution Agreement. On October 1, 2013, the shares and warrants were issued in exchange for $5,000,000 in cash.
3. Summary of Significant Accounting Policies
Revenue recognition – Asterias complies with ASC 605-10 and records revenue when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable, and collectability is reasonably assured. Grant income is recognized as revenue when earned. Royalty revenues consist of royalty payments on sales of products under a license agreement. Asterias recognizes revenue in the quarter in which the royalty reports are received rather than the quarter in which the sales took place. When Asterias is entitled to receive up-front nonrefundable licensing or similar fees pursuant to agreements under which Asterias has no continuing performance obligations, the fees are recognized as revenues when collection is reasonably assured. When Asterias receives up-front nonrefundable licensing or similar fees pursuant to agreements under which Asterias does have continuing performance obligations, the fees are deferred and amortized ratably over the performance period. If the performance period cannot be reasonably estimated, Asterias amortizes nonrefundable fees over the life of the contract until such time that the performance period can be more reasonably estimated. Milestone payments, if any, related to scientific or technical achievements are recognized in income when the milestone is accomplished if (a) substantive effort was required to achieve the milestone, (b) the amount of the milestone payment appears reasonably commensurate with the effort expended, and (c) collection of the payment is reasonably assured.
Development stage company – Asterias complies with the reporting requirements of ASC 915, “Development Stage Entities.”
Use of estimates – The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and cash equivalents – Asterias considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents.
Available-for-sale securities, at fair value – Marketable equity securities and debt securities not classified as held-to-maturity are classified as available-for-sale. Available-for-sale securities are carried at fair value, with the unrealized gains and losses, net of tax, reported in other comprehensive income. Realized gains and losses, and declines in value judged to be other-than-temporary related to equity securities, are included in investment income.
Equipment and furniture – Equipment and furniture are stated at cost and are being depreciated using the straight-line method over a period of 36 to 120 months.
Intangible assets – Intangible assets with finite useful lives are amortized over their estimated useful lives, and intangible assets with indefinite lives are not amortized but rather are tested at least annually for impairment. Acquired in-process research and development intangible assets are accounted depending on whether they were acquired as part of an acquisition of a business, or as assets that do not constitute a business. When acquired in conjunction with the acquisition of a business, these assets are considered to be indefinite-lived until the completion or abandonment of the associated research and development efforts and are capitalized as an asset. If and when development is complete, the associated assets would be deemed finite-lived and would then be amortized based on their respective estimated useful lives at that point in time. However, when acquired in conjunction with an acquisition of assets that do not constitute a business (such as part of the acquisition of assets from Geron), in accordance with the accounting rules in ASC 805-50, such intangible assets related to IPR&D are expensed upon acquisition.
Impairment of long-lived assets – Asterias’ long-lived assets, including intangible assets, will be reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be fully recoverable. If an impairment indicator is present, Asterias will evaluate recoverability by a comparison of the carrying amount of the assets to future undiscounted net cash flows expected to be generated by the assets. If the assets are impaired, the impairment will be recognized and measured by the amount by which the carrying amount exceeds the estimated fair value of the assets.
Warrants to purchase common stock – Asterias generally accounts for warrants issued in connection with equity financings as a component of equity. None of the warrants issued by Asterias as of December 31, 2014 and 2013 include a conditional obligation to issue a variable number of shares; nor was there a deemed possibility that Asterias may need to settle the warrants in cash.
Accounting for BioTime Shares – The Company accounts for the BioTime shares it holds as available-for-sale equity securities in accordance with ASC 320-10-25, Investments-Debt and Equity Securities, as the shares have a readily determinable fair value quoted on the NYSE MKT and are held principally for future working capital purposes, as necessary. These shares are measured at fair value and reported as current assets on the balance sheet based on the closing trading price of the security as of the date being presented. Unrealized holding gains and losses in changes to the fair value of these shares are excluded from the statements of operations and reported in equity as part of other comprehensive income or loss until realized. Realized gains and losses are reclassified out of other comprehensive income or loss and included in equity, as an increase or decrease in additional paid-in capital consistent with, and pursuant to, ASC 805-50, transactions between entities under common control.
Patent costs – Costs associated with obtaining patents on products or technology developed are expensed as general and administrative expenses when incurred.
Reclassification– Certain prior year amounts in the statement of cash flows have been reclassified to conform to the current year presentation.
Research and development – Research and development costs are expensed when incurred, and consist principally of salaries, payroll taxes, consulting fees, research and laboratory fees, and fees paid to acquire patents or licenses.
Income taxes – For 2014 and for following years, Asterias will file its own U.S. federal tax return including its activity for the entire 2014 calendar year. For California purposes their activity will continue to be included in BioTime’s California combined tax return. Prior to the year ended December 31, 2013, Asterias’ operations through September 30, 2013 were included in BioTime’s consolidated U.S. federal and certain state income tax returns. For the period from October 1, 2013 through December 31, 2013 Asterias filed a separate U.S federal tax return. For California, Asterias’ activity for the entire 2013 calendar year was included in BioTime’s combined tax return. The provision for income taxes was previously determined as if Asterias had filed separate tax returns for the periods presented. Accordingly, the effective tax rate of Asterias in periods subsequent to December 31, 2013 could vary from its historical effective tax rates depending on the future legal structure of Asterias and related tax elections. Asterias accounts for income taxes in accordance with the accounting principles generally accepted in the United States of America, which prescribe the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. Generally, Asterias is subject to income tax exemptions by major taxing authorities for all years since inception. Asterias will recognize accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of December 31, 2014 and 2013. Management is currently unaware of any tax issues.
A deferred income tax benefit of approximately $7,376,000 was recorded for the year ended December 31, 2014, of which approximately $5,155,000 was related to federal and $2,221,000 was related to state taxes. A deferred income tax benefit of approximately $3,280,000 was recorded for the year ended December 31, 2013, of which approximately $2,800,000 was related to federal and $480,000 was related to state taxes. As disclosed in Note 2, Asterias established deferred tax liabilities primarily related to its acquisition of certain intellectual property. It is more likely than not that the deferred tax assets are fully realizable since these income tax benefits are expected to be available to offset such deferred tax liabilities.
In June 2014, Asterias’ sale of BioTime shares resulted in a taxable gain of approximately $10.3 million and a tax payable of $3.6 million. Asterias received the BioTime shares from BioTime as part of their consideration under the Asset Contribution Agreement, a tax free transaction. See Note 2. This payable, however, is expected to be fully offset by available net operating losses thus, resulting in no cash income taxes due from that sale. As of December, 2014, Asterias recorded a $4.7 million deferred tax liability for the temporary taxable difference in the basis of the investment still held by Asterias in BioTime stock. Both transactions were treated as a deemed distribution by Asterias and recorded against equity.
Stock-based compensation – Asterias adopted accounting standards governing share-based payments, which require the measurement and recognition of compensation expense for all share-based payment awards made to directors and employees, including employee stock options, based on estimated fair values. Consistent with those guidelines, Asterias utilizes the Black-Scholes Merton option pricing model. Asterias' determination of fair value of share-based payment awards on the date of grant using that option-pricing model is affected by Asterias' stock price as well as by assumptions regarding a number of highly complex and subjective variables. These variables include, but are not limited to, Asterias' expected stock price volatility over the term of the awards, and actual and projected employee stock option exercise behaviors. The expected term of options granted is derived from historical data on employee exercises and post-vesting employment termination behavior. The risk-free rate is based on the U.S. Treasury rates in effect during the corresponding period of grant.
Fair value of financial instruments – ASC 820, Fair Value Measurements, clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability.
ASC 820 requires that the valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 establishes a three tier value hierarchy, which prioritizes inputs that may be used to measure fair value as follows:
| · | Level 1– Observable inputs that reflect quoted prices for identical assets or liabilities in active markets. |
| · | Level 2– Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| · | Level 3– Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The carrying amounts of current assets and current liabilities approximate their fair value because of the relatively short period until they mature or are required to be settled, except for the investment in BioTime shares, which are carried at fair value based on Level 1 inputs, and BioTime Warrants and related obligation to distribute the BioTime Warrants, which are carried at fair value based on Level 2 inputs.
Comprehensive income/loss – ASC 220, Comprehensive Income, requires that an entity’s change in equity or net assets during a period from transactions and other events from non-owner sources be reported. Asterias reports unrealized gains and losses on its available-for-sale securities as other comprehensive income (loss).
Loss per share – Basic net loss per share is computed by dividing net loss attributable to Asterias by the weighted-average number of shares of common stock outstanding for the period. Diluted net loss per share reflects the weighted-average number of shares of common stock outstanding plus the potential effect of dilutive securities or contracts which are convertible to common stock, such as options and warrants (using the treasury stock method) and shares issuable in future periods, except in cases where the effect would be anti-dilutive.
The computations of basic and diluted net loss per share are as follows:
|
|
Years ended December 31,
|
Cumulative
Period from Inception
(September 24, 2012) to
|
||||||||||||||
|
|
2014
|
2013
|
December 31,
2012
|
December 31,
2014
|
||||||||||||
|
Net loss
|
$
|
(10,097,154
|
) |
$
|
(22,379,744
|
)
|
$
|
(758,893
|
)
|
$
|
(33,235,791
|
) | ||||
|
Weighted average common shares of common stock – basic and diluted
|
30,720,280
|
7,726,042
|
51,647
|
16,954,131
|
||||||||||||
|
Net loss per share – basic and diluted
|
$
|
(0.33
|
)
|
$
|
(2.90
|
)
|
$
|
(14.69
|
)
|
$
|
(1.96
|
)
|
||||
The following common stock equivalents were excluded from the computation of diluted net loss per share of common stock for the periods presented because including them would have been antidilutive:
|
|
Years ended December 31,
|
Cumulative
Period from Inception
(September 24, 2012) to
|
||||||||||||||
|
|
2014
|
2013
|
December 31,
2012
|
December 31,
2014
|
||||||||||||
|
Stock options under Equity Incentive Plan
|
1,623,227
|
494,479
|
-
|
1,623,227
|
||||||||||||
Effect of recently issued and recently adopted accounting pronouncements – There are no recently issued accounting standards which are not yet effective which Asterias believes would materially impact the financial statements.
In May 2014, the FASB issued Accounting Standards Update (“ASU”) No. 2014-09 “Revenue from Contracts with Customers” (Topic 606). The guidance of this Update affects any entity that either issues contracts with customers or transfers goods or services or enters into contracts for the transfer of non-financial assets. The core principal of the guidance is that an entity should recognize revenue to depict the transfer of promised goods or services to customers in the amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods and services. To achieve those core principals, the ASU specifies steps that the entity should apply for revenue recognition. The guidance also specifies the accounting for some costs to obtain or fulfill the contract with customer and disclosure requirements to enable users of financial statements to understand the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers. For a public entity, ASU No. 2014-09 is effective for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period. Early application is not permitted. Asterias is currently evaluating the impact of the adoption of the ASU on its financial statements.
In May 2014, the FASB issued ASU No. 2014-10 “Development Stage Entities” (Topic 915). The objective of the ASU is to improve financial reporting by reducing the cost and complexity associated with the incremental reporting requirements for development stage entities. The ASU removes all incremental financial reporting requirements from U.S. GAAP for development stage entities, including the inception-to-date information and certain other disclosures. The ASU also eliminates an exception provided to development stage entities in Topic 810 “Consolidation” for determining whether an entity is a variable interest entity on the basis of amount of investment equity at risk. For public business entities, those amendments are effective for annual reporting periods beginning after December 15, 2014, and interim periods therein. Early application is permitted. Asterias is currently evaluating the impact of the adoption of the ASU on its financial statements.
In June 2014, the FASB issued ASU No. 2014-12 “Compensation – Stock Compensation” (Topic 718). The ASU provides guidance for accounting for share-based payments when the terms of an award provide that a performance target could be achieved after the requisite service period. That is the case when an employee is eligible to retire or otherwise terminate employment before the end of the period in which a performance target (for example, profitability target) could be achieved and still be eligible to vest in the award if and when the performance target is achieved. The ASU requires a performance target that effects vesting and that could be achieved after the requisite service period be treated as a performance condition. Compensation cost should be recognized in the period in which it becomes probable that such performance condition would be achieved and should represent the compensation cost attributable to the periods for which the requisite service has already been rendered. For public business entities, the ASU is effective for annual reporting periods beginning after December 15, 2015, and interim periods therein. Early application is permitted. Asterias is in the process of evaluating the impact of the adoption of the ASU on its financial statements.
In August 2014, the FASB issued ASU No. 2014-15 “Presentation of Financial Statements – Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern” requiring management to evaluate on a regular basis whether any conditions or events have arisen that could raise substantial doubt about the entity’s ability to continue as a going concern. The guidance 1) provides a definition for the term “substantial doubt,” 2) requires an evaluation every reporting period, interim periods included, 3) provides principles for considering the mitigating effect of management’s plans to alleviate the substantial doubt, 4) requires certain disclosures if the substantial doubt is alleviated as a result of management’s plans, 5) requires an express statement, as well as other disclosures, if the substantial doubt is not alleviated, and 6) requires an assessment period of one year from the date the financial statements are issued. The standard is effective for Asterias' reporting year ending December 31, 2016, and interim periods thereafter. Early adoption is permitted. Asterias does not expect the adoption of this guidance to have a material impact on its financial statements.
4. Balance Sheet Components
Equipment and Furniture, Net
At December 31, 2014 and 2013, equipment and furniture were comprised of the following:
|
Years Ended December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Equipment and furniture
|
$
|
1,795,827
|
$
|
1,681,113
|
|||
|
Accumulated depreciation
|
(750,986
|
)
|
(220,595)
|
||||
|
Equipment and furniture, net
|
$
|
1,044,841
|
$
|
1,460,518
|
|||
Depreciation expense amounted to $530,391 and $220,595 for the years ended December 31, 2014 and 2013, respectively.
Construction in progress
Construction in progress of $405,730 as of December 31, 2014 entirely relates to the improvements for the Fremont facility. Under the terms of the lease agreement, the landlord will provide Asterias with a tenant improvement allowance of $4,400,000, which Asterias is using to construct a laboratory and production facility that can be used to produce human embryonic stem cell and related products under current good manufacturing procedures (cGMP). See also Note 10.
5. Investment in BioTime and in BioTime Subsidiaries
Investment in BioTime
At December 31, 2014, Asterias held 3,852,880 of the 8,902,077 BioTime common shares that Asterias received under the Asset Contribution Agreement, and which are included at fair value in current assets in its balance sheet as the shares are available for use and could be sold at fair value for liquidity purposes at any time. The investment is classified as “available for sale.” Available-for-sale securities are carried at fair value, with the unrealized gains and losses, net of tax, reported in other comprehensive income. Realized gains and losses, and declines in value judged to be other-than-temporary related to equity securities, are included in investment income/loss, except as discussed below.
During June 2014, Asterias sold 5,000,000 of its BioTime common shares with warrants to purchase 5,000,000 Asterias Series B Shares to two investors for $12,500,000 in cash. Broadwood Partners, L.P., BioTime’s largest shareholder, purchased 1,000,000 of the BioTime common shares with 1,000,000 Asterias warrants. One of BioTime’s directors, Neal C. Bradsher, is President of Broadwood Capital, Inc. the investment manager of Broadwood Partners, L.P., and one of Asterias’ directors, Richard T. LeBuhn, is Senior Vice President of Broadwood Capital, Inc. The exercise price of the warrants is $2.34. Following the conversion of the Series B Shares into Series A Shares on October 3, 2014, the warrants are now exercisable into Series A Shares. The exercise price and the number of shares of Asterias common stock issuable upon the exercise of the warrants are subject to adjustment in the event of a stock split, stock dividend, combination of shares, recapitalization or certain other transactions. The warrants will expire on June 15, 2015 if not exercised by that date. During June 2014, Asterias also sold 49,197 BioTime common shares in “at-the-market” transactions for gross proceeds of $160,908 in the open market through Cantor Fitzgerald & Co., acting as Asterias’ sales agent.
The gross proceeds totaling $12,660,908 received in June 2014 from Asterias' sale of BioTime common shares with warrants to purchase Asterias common stock were allocated to the BioTime common shares and Asterias warrants based on their relative fair values resulting in $9,477,017 and $3,183,891 of the proceeds being allocated to the BioTime common shares and the Asterias warrants, respectively. This resulted in Asterias recording in a realized loss of $10,466,327 on the sale of the BioTime common shares under U.S. GAAP. The loss has been accounted for as a capital transaction and recorded as a reduction of additional paid in capital in the accompanying balance sheet at December 31, 2014. The loss was calculated using a cost basis of $19,843,344, or $3.93 per share, for the BioTime common shares reflecting the closing price of BioTime common shares as reported on the NYSE MKT on October 1, 2013, the date Asterias acquired those shares from BioTime under the Asset Contribution Agreement. See Note 2. The relative fair value of the Asterias warrants was calculated applying the Black-Scholes-Merton formula using the following assumptions: expected term of one year, the contractual life of the warrants; risk-free rate of 0. 11%; 0% expected dividend yield; 94.88% expected volatility based on the average historical common stock volatility of BioTime and Geron (which were used because Asterias’ common stock did not have a trading history); a stock price of $2.34; and an exercise price of $2.34, and may not reflect the value that the warrants would bring if a trading market for the warrants existed. Asterias also recorded an unrealized net gain of $2,432,023 in accumulated other comprehensive loss, which is primarily attributed to an unrealized gain on the remaining 3,852,880 BioTime common shares held by Asterias on December 31, 2014. At December 31, 2013, Asterias has recognized an unrealized net loss of $2,934,686 in accumulated other comprehensive loss, which is primarily attributed to an unrealized loss on the 8,902,077 BioTime common shares then held by Asterias.
Asterias reviews various factors in determining whether it should recognize an other-than-temporary impairment charge for its marketable securities, including its intent and ability to hold the investment for a period of time sufficient for any anticipated recovery in market value, and the length of time and extent to which the fair value has been less than its cost basis. Based on consideration of these factors, as of December 31, 2014, no other-than-temporary impairment was recognized.
Investments in Affiliates
Asterias’ investments in the OrthoCyte and Cell Cure Neurosciences stock received from BioTime were recorded at BioTime’s historical costs but not below zero. The investment is carried using the cost method of accounting.
6. Investment in BioTime Warrants and Related Obligation to Distribute BioTime Warrants
As part of the consideration for the issuance of Series B Shares to BioTime under the Asset Contribution Agreement, Asterias received the BioTime Warrants. Under the Asset Contribution Agreement, Asterias agreed to distribute the BioTime Warrants to holders of its Series A Shares as promptly as practicable after notice from Geron that the Series A Distribution has been completed. During August 2014, Geron gave notice to Asterias that the Series A Distribution has been completed and on October 1, 2014, Asterias completed the distribution of the BioTime Warrants to the holders of its Series A Shares.
Both the BioTime Warrants and the corresponding obligation to distribute them were measured at fair value at each relevant balance sheet date by applying a Black Scholes Merton option pricing model using assumptions deemed appropriate as of the applicable date. Because the fair value of the BioTime Warrants was expected to always be equal to the fair value of the obligation to distribute those warrants at any date on which those values are determined, remeasurement of those values did not result in a charge or credit on the statement of operations and comprehensive loss.
The estimated fair value of the BioTime Warrants and the corresponding obligation to distribute them as of December 31, 2013 was $15,568,307, based on a $5.00 per share exercise price, a $3.60 closing price on December 31, 2013, a 4.75 year term, 75.86% volatility, and a 1.75% discount rate. Because any increase or decrease in value of the BioTime Warrants accrued to the benefit of the holders of Series A Shares, and not to Asterias, the financial statements do not include and gain or loss related to any increases or decreases in value subsequent to October 1, 2013.
7. Intangible assets
As of December 31, 2013, Asterias had capitalized intangible assets acquired from Geron, primarily related to patents and other intellectual property rights related to hES cells. These assets are being amortized over the estimated economic lives of the patents on a straight-line basis, which approximates the pattern of consumption over their estimated useful lives. Asterias is currently estimating a useful life of 10 years.
Intangible assets net of accumulated amortization at December 31, 2014 and December 31, 2013 are shown in the following table:
|
Years Ended December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Intangible assets
|
$
|
26,859,640
|
$
|
29,017,009
|
|||
|
Accumulated amortization
|
(3,357,455
|
)
|
(725,425)
|
||||
|
Intangible assets, net
|
$
|
23,502,185
|
$
|
28,291,584
|
|||
Asterias amortizes its intangible assets over an estimated period of 10 years on a straight line basis. Asterias recognized $4,789,399 and $725,425 in amortization expense of intangible assets during the year ended December 31, 2014 and 2013, respectively. As further discussed in Note 2, as of December 31, 2014, Asterias recorded an adjustment to reduce the cost of the intangible asset by $2,157,369 with a corresponding reduction to the accumulated amortization balance of $269,671, resulting in an additional amortization expense of $1,887,698 included in the statements of operations for the year ended December 31, 2014.
Amortization of intangible assets for periods subsequent to December 31, 2014 is as follows:
|
Year Ended
|
Amortization
|
|||
|
December 31,
|
Expense
|
|||
|
2015
|
$
|
2,685,964
|
||
|
2016
|
2,685,964
|
|||
|
2017
|
2,685,964
|
|||
|
2018
|
2,685,964
|
|||
|
2019
|
2,685,964
|
|||
|
Thereafter
|
10,072,365
|
|||
|
Total
|
$
|
23,502,185
|
||
8. Common Stock and Warrants
At December 31, 2014, Asterias had outstanding 30,902,152 Series A Shares and no Series B Shares. At December 31, 2013, Asterias had outstanding 6,537,779 Series A Shares and 23,961,040 Series B Shares. All outstanding Series B Shares were converted into Series A Shares on October 3, 2014.
Common Stock Issuance
Significant common stock transaction during the year ended December 31, 2014 include the sale of 200,000 Series B Shares at $2.34 per share for $468,000 to Pedro Lichtinger, its President and Chief Executive Officer.
Significant common stock transactions during the year ended December 31, 2013 included the issuance of 6,537,779 Series A Shares to Geron and 21,773,340 Series B Shares to BioTime pursuant to the Asset Contribution Agreement, and the sale of 2,136,000 Series B Shares to Romulus pursuant to a Stock and Warrant Purchase Agreement, as described in Note 2.
Asterias has issued warrants to purchase its common shares. Activity related to warrants in 2014 and 2013, is presented in the table below:
|
Number of
Warrants
|
Per share
exercise
price
|
Weighted
Average
Exercise
Price
|
|||||||||
|
Outstanding, January 1, 2013
|
-
|
$
|
-
|
$
|
-
|
||||||
|
Issued in 2013
|
3,500,000
|
5.00
|
5.00
|
||||||||
|
Outstanding, January 1, 2014
|
3,500,000
|
5.00
|
5.00
|
||||||||
|
Issued in 2014
|
5,000,000
|
2.34
|
2.34
|
||||||||
|
Outstanding, December 31, 2014
|
8,500,000
|
$
|
2.34-5.00
|
$
|
3.44
|
||||||
The warrants to purchase 3,500,000 shares of its common stock will expire on September 30, 2016 and the warrants to purchase 5,000,000 shares of its common stock will expire on June 15, 2015. Asterias will receive $29,200,000 if all of the warrants are exercised.
At December 31, 2014, 8,500,000 warrants to purchase common shares with a weighted average exercise price of $3.44 and a weighted average remaining contractual life of 0.99 years were outstanding.
At December 31, 2013, 3,500,000 warrants to purchase common shares with a weighted average exercise price of $5.00 per share and a weighted average remaining contractual life of 2.75 years were outstanding.
9. Equity Incentive Plan
During March 2013, Asterias’ Board of Directors approved an Equity Incentive Plan (the “Plan”) under which Asterias has reserved 4,500,000 shares of common stock for the grant of stock options or the sale of restricted stock. Initially, Asterias issued Series B Shares under the Plan. Since the date on which all of the outstanding Series B Shares were converted into Series A Shares, Asterias has issued Series A Shares under the Plan. The Plan also permits Asterias to issue such other securities as its Board of Directors or the Compensation Committee administering the Plan may determine. Asterias’ stockholders approved the Plan in September 2013.
No options may be granted under the Plan more than ten years after the date upon which the Plan was adopted by the Board of Directors, and no options granted under the Plan may be exercised after the expiration of ten years from the date of grant. Under the Plan, options to purchase common stock may be granted to employees, directors and certain consultants at prices not less than the fair market value at date of grant, subject to certain limited exceptions for options granted in substitution of other options. Options may be fully exercisable immediately, or may be exercisable according to a schedule or conditions specified by the Board of Directors or the Compensation Committee. The Plan also permits Asterias to award restricted stock for services rendered or to sell common stock to employees subject to vesting provisions under restricted stock agreements that provide for forfeiture of unvested shares upon the occurrence of specified events under a restricted stock award agreement. Asterias may permit employees or consultants, but not officers or directors, who purchase stock under restricted stock purchase agreements, to pay for their shares by delivering a promissory note that is secured by a pledge of their shares.
Asterias may also grant stock appreciation rights (“SARs”) and hypothetical units issued with reference to Asterias common stock (“Restricted Stock Units”) under the Plan. An SAR is the right to receive, upon exercise, an amount payable in cash or shares or a combination of shares and cash, as determined by the Board of Directors or the Compensation Committee, equal to the number of shares subject to the SAR that is being exercised multiplied by the excess of (a) the fair market value of a share of Asterias common stock on the date the SAR is exercised, over (b) the exercise price specified in the SAR Award agreement.
The terms and conditions of a grant of Restricted Stock Units will be determined by the Board of Directors or Compensation Committee. No shares of stock will be issued at the time a Restricted Stock Unit is granted, and Asterias will not be required to set aside a fund for the payment of any such award. A recipient of Restricted Stock Units will have no voting rights with respect to the Restricted Stock Units. Upon the expiration of the restrictions applicable to a Restricted Stock Unit, Asterias will either issue to the recipient, without charge, one share of common stock per Restricted Stock Unit or cash in an amount equal to the fair market value of one share of common stock.
Options Granted and Restricted Stock Units Issued
As of December 31, 2014, Asterias had granted to certain officers, employees, and directors, options to purchase a total of 3,146,666 shares of common stock and 200,000 shares of restricted common stock at a weighted average exercise price of $2.42 per share.
A summary of Asterias' stock option activity and related information follows:
|
Options and
Restricted
Stock Available
for Grant
|
Number of
Options and
Restricted Stock
Outstanding
|
Weighted
Average
Exercise
Price
|
|||||||||
|
January 1, 2013
|
-
|
-
|
$
|
-
|
|||||||
|
Equity incentive plan
|
4,500,000
|
-
|
-
|
||||||||
|
Options granted in 2013
|
(2,965,000
|
)
|
2,965,000
|
2.34
|
|||||||
|
Options expired/forfeited in 2013
|
125,000
|
(125,000
|
)
|
2.34
|
|||||||
|
December 31, 2013
|
1,660,000
|
2,840,000
|
$
|
2.34
|
|||||||
|
Options granted in 2014
|
(1,590,000
|
)
|
1,590,000
|
2.50
|
|||||||
|
Restricted stock units issued in 2013
|
(200,000
|
)
|
200,000
|
2.34
|
|||||||
|
Options exercised in 2014
|
(3,333
|
)
|
2.34
|
||||||||
|
Options expired/forfeited in 2014
|
1,280,001
|
(1,280,001
|
)
|
2.34
|
|||||||
|
December 31, 2014
|
1,150,001
|
3,346,666
|
$
|
2.42
|
|||||||
Stock-Based Compensation Expense
The weighted-average estimated fair value of stock options granted during the years ended December 31, 2014 and 2013 was $1.50 and $1.39 per share respectively, using the Black-Scholes Merton model with the following weighted-average assumptions:
|
Years Ended
December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Expected life (in years)
|
3.98
|
3.99
|
||||||
|
Risk-free interest rates
|
1.31
|
%
|
0.86
|
%
|
||||
|
Volatility
|
83.49
|
%
|
73.21
|
%
|
||||
|
Dividend yield
|
0
|
%
|
0
|
%
|
||||
The risk-free rate is based on the rates in effect at the time of grant for zero coupon U.S. Treasury notes with maturities approximately equal to each grant’s expected life. A dividend yield of zero is applied since Asterias has not historically paid dividends and has no intention to pay dividends in the near future. The expected volatility is based upon the volatility of a group of publicly traded industry peer companies. The expected term of options granted is calculated using the simplified method under SEC Staff Accounting Bulletin No. 107.
Total proceeds if all options granted and outstanding as of December 31, 2014 were exercised would be $7,619,548.
Employee stock-based compensation expense is calculated and recorded based on awards ultimately expected to vest and has been reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and will be revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Cost of net revenues and operating expenses include stock-based compensation as follows:
|
Years Ended December 31,
|
|||||||
|
2014
|
2013
|
||||||
|
Research and development
|
$
|
478,049
|
$
|
194,578
|
|||
|
General and administrative
|
1,336,071
|
509,156
|
|||||
|
Total stock-based compensation expense
|
$
|
1,814,120
|
$
|
703,734
|
|||
At December 31, 2014 and 2013, Asterias had $3,311,093 and $3,237,601 of total unrecognized compensation expense, net of estimated forfeitures, related to the Plan that will be recognized over a weighted-average period of approximately 2.91 and 3.97 years, respectively.
10. Commitments and Contingencies
At December 31, 2014 Asterias had commitments consisting of an operating lab equipment lease, a sublease of its current office and research facility, a lease of its satellite office in New York, and a lease for its future office and research facility in Fremont.
Asterias subleases from BioTime, an office and research facility located in Menlo Park, California. The lease is for a term of three years commencing January 7, 2013. Base rent is $31,786 per month, plus real estate taxes and certain costs of maintaining the leased premises.
On December 30, 2013, Asterias entered into a lease for an office and research facility located in Fremont, California, consisting of an existing building with approximately 44,000 square feet of space. The building will be used by Asterias primarily as a laboratory and production facility that can be used to produce human embryonic stem cells and related products under current good manufacturing procedures (cGMP). Asterias plans to construct certain tenant improvements for its use, which it expects will cost approximately $5.5 million, of which a maximum $4.4 million will be paid by the landlord. The landlord’s obligation to fund the tenant improvements expires on June 30, 2015, 18 months from the date of the lease, with respect to any portion of the allowance not expended by then. Asterias expects to substantially complete construction of the built-to-suit facility during the third quarter of 2015.
In January 2014, Asterias paid the landlord a $300,000 security deposit and the landlord allowed access and use of the premises beginning in March 2014 to allow for the construction of the tenant improvements. The lease is for a term of 96 months, commencing on October 1, 2014, with two available five-year options to extend the term, upon one year written notice by Asterias. During the first 15 months of the lease term, from October 1, 2014 through December 31, 2015, Asterias will pay monthly base rent of $50,985 representing 22,000 square feet rather than 44,000 square feet provided that Asterias is not in default in performing its obligations under the lease beyond any notice and cure periods. Beginning on January 1, 2016, base rent will increase to $105,142 per month and increase by approximately 3% annually on every October 1 thereafter.
In addition to monthly base rent, Asterias will pay all real estate taxes, insurance and the cost of maintenance, repair and replacement of the leased premises. During the first 15 months of the lease term, Asterias will pay only 50% of the real estate taxes assessed on the premises provided that Asterias is not in default in performing its obligations under the lease beyond any notice and cure periods. However, if any improvements or alterations to the premises that Asterias constructs or adds are assessed for real property tax purposes at a valuation higher than the valuation of the improvements on the premises on the date it signed the lease, Asterias will pay 100% of the taxes levied on the excess assessed valuation.
Asterias is considered the owner of the asset under construction under ASC 840-40-55 as Asterias, among other things, has the primary obligation to pay for construction costs and Asterias will retain exclusive use of the building for its office and research facility requirements after construction is completed. In accordance with this guidance, amounts previously expended by Asterias for construction would continue to be reported as construction in progress in Asterias’ financial statements, and the proceeds received from the landlord will be reported as a liability. Once the property is placed in service, Asterias will depreciate the property and the lease payments allocated to the landlord liability will be accounted for as debt service payments on that liability. As of December 31, 2014, Asterias has incurred approximately $406,000 of construction costs included in construction in progress, of which approximately $378,000 is reimbursable by the landlord included in long term liabilities.
Asterias was provided access and rights to use the property beginning in March 2014 with “free-rent” until the lease payments commenced on October 1, 2014, as described above. Asterias commenced expensing rent beginning in March 2014 in accordance with ASC 840-20-25-10 and 11, Rent Expense During Construction. Accordingly, during the year ended December 31, 2014, Asterias has expensed approximately $247,000 included in the statements of operations and a deferred rent balance of approximately $94,000 as of December 31, 2014, included in long-term liabilities.
Asterias also currently pays $3,512 per month for the use of approximately 120 square feet of the office space in New York City that is used to conduct meetings and other business affairs. The lease is for a term of one year commencing July 1, 2014.
Remaining minimum annual lease payments under the various operating leases for the year ending after December 31, 2014 are as follows:
|
Year Ending
December 31,
|
Minimum Lease
Payments
|
||
|
2015
|
$
|
1,040,522
|
|
|
2016
|
1,271,226
|
||
|
2017
|
1,309,295
|
||
|
2018
|
1,347,364
|
||
|
2019
|
1,386,792
|
||
|
Thereafter
|
4,033,933
|
||
|
Total
|
$
|
10,389,132
|
|
11. Shared Facilities and Service Agreement
On April 1, 2013, Asterias and BioTime executed a Shared Facilities and Services Agreement (“Shared Facilities Agreement”). Under the terms of the Shared Facilities Agreement, BioTime will allow Asterias to use its premises and equipment located at Alameda, California for the sole purpose of conducting business. BioTime will provide basic accounting, billing, bookkeeping, payroll, treasury, collection of accounts receivable (excluding the institution of legal proceedings or taking of any other action to collect accounts receivable), payment of accounts payable, and other similar administrative services to Asterias. BioTime may also provide the services of attorneys, accountants, and other professionals who may also provide professional services to BioTime and its other subsidiaries. BioTime will also provide Asterias with the services of its laboratory and research personnel, including BioTime employees and contractors, for the performance of research and development work for Asterias at the premise.
BioTime will charge Asterias a fee for the services and usage of facilities, equipment, and supplies aforementioned. For each billing period, BioTime will equitably prorate and allocate its employee costs, equipment costs, insurance costs, lease costs, professional costs, software costs, supply costs, and utilities costs, between BioTime and Asterias based upon actual documented use and cost by or for Asterias or upon proportionate usage by BioTime and Asterias, as reasonably estimated by BioTime. Asterias shall pay 105% of the allocated costs (the “Use Fee”). The allocated cost of BioTime employees and contractors who provide services will be based upon records maintained of the number of hours of such personnel devoted to the performance of services.
The Use Fee will be determined and invoiced to Asterias on a quarterly basis for each calendar quarter of each calendar year. If the Shared Facilities Agreement terminates prior to the last day of a billing period, the Use Fee will be determined for the number of days in the billing period elapsed prior to the termination of the Shared Facilities Agreement. Each invoice will be payable in full by Asterias within 30 days after receipt. Any invoice or portion thereof not paid in full when due will bear interest at the rate of 15% per annum until paid, unless the failure to make a payment is due to any inaction or delay in making a payment by BioTime employees from Asterias funds available for such purpose, rather than from the unavailability of sufficient funds legally available for payment or from an act, omission, or delay by any employee or agent of Asterias.
In addition to the Use Fees, Asterias will reimburse BioTime for any out of pocket costs incurred by BioTime for the purchase of office supplies, laboratory supplies, and other goods and materials and services for the account or use of Asterias, provided that invoices documenting such costs are delivered to Asterias with each invoice for the Use Fee. Furthermore, BioTime will have no obligation to purchase or acquire any office supplies or other goods and materials or any services for Asterias, and if any such supplies, goods, materials or services are obtained for Asterias, BioTime may arrange for the suppliers thereof to invoice Asterias directly.
Asterias in turn may charge BioTime or any Other Subsidiary for similar services provided by Asterias at the same rate and terms as aforementioned. “Other Subsidiary” means a subsidiary of BioTime other than Asterias and other than a subsidiary of Asterias.
The Shared Facilities Agreement terminates on December 31, 2016, provided that, unless otherwise terminated under another provision of the Shared Facilities Agreement, the term of the Shared Facilities Agreement will automatically be renewed and the termination date will be extended for an additional year each year after December 31, 2016, unless either party gives the other party written notice stating that the Shared Facilities Agreement will terminate on December 31 of that year.
BioTime allocated $170,806, $102,156, $11,303 and $284,265 of general overhead expenses to Asterias during the years ended December 31, 2014 and 2013, and the period from inception (September 24, 2012) to December 31, 2012 and the period from inception to December 31, 2014.
12. Income Taxes
Asterias’ operations through September 30, 2013 were included in BioTime’s consolidated U.S. federal income tax returns. For California purposes Asterias’ activity will continue to be included in BioTime’s California combined tax return. The provision for income taxes has been determined as if Asterias had filed separate tax returns for the periods presented. Effective October 1, 2013, Asterias began filing separate U.S. federal income tax returns. Accordingly, the effective tax rate of Asterias in future years could vary from its historical effective tax rates depending on the future legal structure of Asterias and related tax elections.
The primary components of the net deferred tax liabilities at December 31, 2014 and 2013 were as follows:
|
Deferred tax assets:
|
2014
|
2013
|
|||||
|
Net operating loss carryforwards
|
$
|
2,242,394
|
$
|
2,198,747
|
|||
|
Research & development and other credits
|
1,368,337
|
779,568
|
|||||
|
Total deferred tax assets
|
3,610,731
|
2,978,315
|
|||||
|
Deferred tax liabilities:
|
|||||||
|
Patents and licenses
|
(8,125,093
|
)
|
(11,255,863)
|
||||
|
Securities held for sale
|
(4,712,948
|
)
|
-
|
||||
|
Total deferred tax liabilities
|
(12,838,041
|
)
|
(11,255,863)
|
||||
|
Net deferred tax liabilities
|
$
|
(9,227,310
|
)
|
$
|
(8,277,548)
|
||
Income taxes differed from the amounts computed by applying the U.S. federal income tax of 34% to pretax losses from operations as a result of the following:
| Years Ended December 31, | ||||||||
|
2014
|
2013
|
|||||||
|
Computed tax benefit at federal statutory rate
|
34
|
%
|
34
|
%
|
||||
|
Permanent differences- write off of intangibles
|
-
|
|
(23
|
%)
|
||||
|
Permanent differences- options
|
(3
|
%)
|
(1
|
%)
|
||||
|
State tax benefit, net of effect on federal income taxes
|
8
|
%
|
2
|
%
|
||||
|
Change in valuation allowance
|
-
|
1
|
%
|
|||||
|
Research & Development credits
|
2
|
%
|
-
|
|
||||
|
Other
|
1
|
%
|
-
|
|
||||
|
42
|
%
|
13
|
%
|
|||||
As of December 31, 2014, Asterias has net operating loss carryforwards of approximately $6,520,000 and $450,000 respectively for federal and California tax purposes, which expire between 2032 and 2034. In addition, Asterias has federal and California research tax credit carry forwards or $431,000 and $453,000, respectively. The federal credits expire beteween 2032 and 2034, while the state tax credits have no expiration date.
A deferred income tax benefit of approximately $7,376,000 was recorded for the year ended December 31, 2014, of which approximately $5,155,000 was related to federal taxes and $2,221,000 was related to state taxes. A deferred income tax benefit of approximately $3,280,000 was recorded for the year ended December 31, 2013, of which approximately $2,800,000 was related to federal taxes and $480,000 was related to state taxes. As disclosed in Note 2, Asterias established deferred tax liabilities primarily related to its acquisition of certain intellectual property. It is more likely than not that the deferred tax assets are fully realizable since these income tax benefits are expected to be available to offset such deferred tax liabilities.
In June 2014, Asterias’ sale of 49,197 BioTime shares in open market transactions and Asterias’ sale of 5,000,000 BioTime common shares together with Asterias warrants to two private investors resulted in a taxable gain of approximately $10.3 million and a tax payable of $3.6 million. This payable, however, is expected to be fully offset by available net operating losses, thus resulting in no cash income taxes due from that sale. As of December 31, 2014, Asterias recorded a $4.7 million deferred tax liability for the temporary taxable difference in the basis of the investment still held by Asterias in BioTime stock. Both transactions were treated as a deemed distribution by Asterias and recorded against equity.
Internal Revenue Code Section 382 places a limitation (“Section 382 Limitation”) on the amount of taxable income that can be offset by net operating loss (“NOL”) carryforwards after a change in control (generally greater than 50% change in ownership within a three-year period) of a loss corporation. California has similar rules. Generally, after a control change, a loss corporation cannot deduct NOL carryforwards in excess of the Section 382 Limitation. Due to these “change in ownership” provisions, utilization of the NOL and tax credit carryforwards may be subject to an annual limitation regarding their utilization against taxable income in future periods.
Asterias will file an income tax return in the U.S. federal jurisdiction, and may file income tax returns in various U.S. states and foreign jurisdictions.
Asterias may be subject to potential examination by U.S. federal, U.S. states or foreign jurisdiction authorities in the areas of income taxes. These potential examinations may include questioning the timing and amount of deductions, the nexus of income among various tax jurisdictions and compliance with U.S. federal, U.S. state and foreign tax laws. Asterias' management does not expect that the total amount of unrecognized tax benefits will materially change over the next twelve months.
13. Segment Information
Operating segments are defined as components of an enterprise that engage in business activities for which separate financial information is available and evaluated by the chief operating decision maker in deciding how to allocate resources and assess performance. Asterias’ executive management team represents its chief decision maker. The executive management team reviews financial information presented on a consolidated basis for purposes of allocating resources and evaluating financial performance and there are no managers who are held accountable for levels or components below the consolidated unit level. To date, management has viewed Asterias’ operations as one segment.
14. Selected Quarterly Financial Information (unaudited)
|
Year Ended December 31, 2014
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
|||||||||||
|
Revenues, net
|
$
|
61,980
|
$
|
(20,634
|
)
|
$
|
42,257
|
$
|
1,045,461
|
||||||
|
Operating expenses
|
3,693,620
|
4,287,397
|
4,036,968
|
6,572,295
|
|||||||||||
|
Loss from operations before deferred tax benefits
|
(3,635,775
|
)
|
(4,315,016
|
)
|
(3,994,976
|
)
|
(5,526,998)
|
||||||||
|
Basic and diluted net loss per share
|
(0.07
|
)
|
(0.09
|
)
|
(0.05
|
)
|
(0.11)
|
||||||||
|
Year Ended December 31, 2013
|
|||||||||||||||
|
Revenues, net
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
|||||||
|
Acquired in-process research and development(1)
|
-
|
-
|
-
|
17,458,766
|
|||||||||||
|
Operating expenses
|
826,818
|
1,319,466
|
2,672,791
|
3,383,604
|
|||||||||||
|
Loss from operations before deferred tax benefits
|
(827,158
|
)
|
(1,319,036
|
)
|
(2,668,528
|
)
|
(20,845,717)
|
||||||||
|
Basic and diluted net loss per share
|
(16.00
|
)
|
(25.51
|
)
|
(51.62
|
)
|
(0.68)
|
||||||||
| (1) | Includes IPR&D expenses related to intangible assets acquired by Asterias from Geron under the Asset Contribution Agreement on October 1, 2013. IPR&D represents the value of incomplete research and development projects which Asterias intends to continue. See Note 2. |
15. Royalty Obligations
Asterias License from WARF403
Asterias has entered into a Non-Exclusive License Agreement with WARF under which Asterias was granted a worldwide non-exclusive license under certain WARF patents and WARF-owned embryonic stem cell lines to develop and commercialize therapeutic, diagnostic and research products. The licensed patents include patents covering primate embryonic stem cells as compositions of matter, as well as methods for growth and differentiation of primate embryonic stem cells. The licensed stem cell lines include the H1, H7, H9, H13 and H14 hES cell lines.
In consideration of the rights licensed, Asterias has agreed to pay WARF an upfront license fee, payments upon the attainment of specified clinical development milestones, royalties on sales of commercialized products, and, subject to certain exclusions, a percentage of any payments that Asterias may receive from any sublicenses that it may grant to use the licensed patents or stem cell lines.
The license agreement will terminate with respect to licensed patents upon the expiration of the last licensed patent to expire. Asterias may terminate the license agreement at any time by giving WARF prior written notice. WARF may terminate the license agreement if payments of earned royalties, once begun, cease for a specified period of time or if Asterias and any third parties collaborating or cooperating with Asterias in the development of products using the licensed patents or stem cell lines fail to spend a specified minimum amount on research and development of products relating to the licensed patents or stem cell lines for a specified period of time. WARF also has the right to terminate the license agreement if Asterias breaches the license agreement or becomes bankrupt or insolvent or if any of the licensed patents or stem cell lines are offered to creditors
Asterias License from the University of California
Geron assigned to Asterias its Exclusive License Agreement with The Regents of the University of California for patents covering a method for directing the differentiation of multipotential hES cells to glial-restricted progenitor cells that generate pure populations of oligodendrocytes for remyelination and treatment of spinal cord injury. Pursuant to this agreement, Asterias has an exclusive worldwide license under such patents, including the right to grant sublicenses, to create products for biological research, drug screening, and human therapy using the licensed patents. Under the license agreement, Asterias will be obligated to pay the university a royalty of 1% from sales of products that are covered by the licensed patent rights, and a minimum annual royalty of $5,000 starting in the year in which the first sale of a product covered by any licensed patent rights occurs, and continuing for the life of the applicable patent right under the agreement. The royalty payments due are subject to reduction, but not by more than 50%, to the extent of any payments that Asterias may be obligated to pay to a third party for the use of patents or other intellectual property licensed from the third party in order to make, have made, use, sell, or import products or otherwise exercise its rights under the Exclusive License Agreement. Asterias will be obligated to pay the university 7.5% of any proceeds, excluding debt financing and equity investments, and certain reimbursements, that its receives from sublicensees, other than Asterias’ affiliates and joint ventures relating to the development, manufacture, purchase, and sale of products, processes, and services covered by the licensed patent. The license agreement will terminate on the expiration of the last-to-expire of the university's issued licensed patents. If no further patents covered by the license agreement are issued, the license agreement would terminate in 2024. The university may terminate the agreement in the event of Asterias’ breach of the agreement. Asterias can terminate the agreement upon 60 days' notice.
Asterias Sublicense from Geron
Asterias has received from Geron an exclusive sublicense under certain patents owned by the University of Colorado’s University License Equity Holdings, Inc. relating to telomerase (the “Telomerase Sublicense”). The Telomerase Sublicense entitles Asterias to use the technology covered by the patents in the development of AST-VAC1 and AST-VAC2 as immunological treatments for cancer. Under the Telomerase Sublicense, Asterias paid Geron a one-time upfront license fee of $65,000, and will pay Geron an annual license maintenance fee of $10,000 due on each anniversary of the effective date of the Telomerase Sublicense, and a 1% royalty on sales of any products that Asterias may develop and commercialize that are covered by the sublicensed patents. The Telomerase Sublicense will expire concurrently with the expiration of Geron’s license. That license will terminate during April 2017 when the licensed patents expire. The Telomerase Sublicense may also be terminated by Asterias by giving Geron 90 days written notice, by Asterias or by Geron if the other party breaches its obligations under the sublicense agreement and fails to cure their breach within the prescribed time period, or by Asterias or by Geron upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings, or upon an assignment of a substantial portion of the assets for the benefit of creditors by the other party.
16. Clinical Trial and Option Agreement and CIRM Grant Award
During September 2014, Asterias entered into a Clinical Trial and Option Agreement (the “CRUK Agreement”) with Cancer Research UK (the “Charity”) and Cancer Research Technology Limited, (“CRT”), a wholly-owned subsidiary of the Charity, pursuant to which the Charity has agreed to fund Phase I/IIa clinical development of our AST-VAC2 product candidate. Asterias will, at its own cost, complete process development and manufacturing scale-up of the AST-VAC2 manufacturing process and will transfer the resulting cGMP-compatible process to the Charity. The Charity will, at its own cost, manufacture clinical grade ASTVAC2 and will carry out the Phase I/IIa clinical trial of AST-VAC2 in cancer patients both resected early-stage and advanced forms of lung cancer. Asterias will have an exclusive first option to obtain a license to use the data from the clinical trial. If Asterias exercises that option Asterias will be obligated to make payments upon the execution of the License Agreement, upon the achievement of various milestones, and then royalties on sales of products. In connection with the CRUK Agreement, Asterias sublicensed to CRUK for use in the clinical trials and product manufacturing process certain patents that have been licensed or sublicensed to us by third parties. Asterias would also be obligated to make payments to those licensors and sublicensors upon the achievement of various milestones, and then royalties on sales of products if AST-VAC2 is successfully developed and commercialized.
On October 16, 2014 Asterias signed a Notice of Grant Award (“NGA”) with the California Institute of Regenerative Medicine (“CIRM”), effective October 1, 2014, with respect to a $14.3 million CIRM grant award for clinical development of Asterias' product, AST-OPC1. The NGA includes the terms under which CIRM will release grant funds to Asterias. CIRM will disburse the grant funds to Asterias over four years in accordance with a quarterly disbursement schedule, subject to Asterias' attainment of certain progress and safety milestones. Asterias received the first payment from CIRM in the amount of $916,554.
17. Subsequent Events
In January 2015, Asterias received the second payment from CIRM in the amount of $2,269,515.
In February 2015, Asterias raised approximately $5.5 million in aggregate gross proceeds from the sale of 1,410,255 Series A Shares at a price of $3.90 per share through an underwritten public offering and a private placement. Broadwood Partners, L.P., British & American Investment Trust PLC and Pedro Lichtinger purchased an aggregate of 1,025,640 of the shares. Broadwood Partners, L.P. is BioTime’s largest shareholder, of its directors, Neal C. Bradsher, is President of Broadwood Capital, Inc., the investment manager of Broadwood Partners, L.P., and one of Asterias’ directors, Richard T. LeBuhn, is Senior Vice President of Broadwood Capital, Inc., Pedro Lichtinger is Asterias’ Chief Executive Officer and a member of its Board of Directors. British & American Investment Trust PLC is an affiliate of a stockholder of Asterias and BioTime.
Not applicable.
Evaluation of Disclosure Controls and Procedures
It is management’s responsibility to establish and maintain adequate internal control over all financial reporting pursuant to Rule 13a-15 under the Securities Exchange Act of 1934 (“Exchange Act”). Our management, including our principal executive officer, our principal operations officer, and our principal financial officer, have reviewed and evaluated the effectiveness of our disclosure controls and procedures as of a date within ninety (90) days of the filing date of this Annual Report on Form 10-K. Following this review and evaluation , management collectively determined that our disclosure controls and procedures are effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act (i) is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms; and (ii) is accumulated and communicated to management, including our chief executive officer, our chief operations officer, and our chief financial officer, as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the period covered by this Annual Report on Form 10-K that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f), is a process designed by, or under the supervision of, our principal executive officer, our principal operations officer, and our principal financial officer, and effected by our Board of Directors, management, and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| · | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
| · | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| · | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2014, based on criteria established in the 2013 Internal Control - Integrated Framework issued by COSO. Based on this assessment, management believes that, as of that date, our internal control over financial reporting was effective.
This annual report includes an attestation report of our registered public accounting firm regarding internal control over financial reporting for the year ended December 31, 2014. The attestation is included with the accounting firm's report on our audited financial statements.
None
PART III
The name, age, and background of each of our directors are contained under the caption “Election of Directors” in our Proxy Statement for our 2015 Annual Meeting of Shareholders, and are incorporated herein by reference. Information about our executive officers, committees of the Board of Directors, and compensation of directors is reported under the caption “Corporate Governance” in our Proxy Statement for our 2015 Annual Meeting of Shareholders, and is incorporated herein by reference.
We have a written Code of Ethics that applies to our principal executive officer, our principal financial officer and accounting officer, our other executive officers, and our directors. The purpose of the Code of Ethics is to promote (i) honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; (ii) full, fair, accurate, timely, and understandable disclosure in reports and documents that we file with or submit to the Securities and Exchange Commission and in our other public communications; (iii) compliance with applicable governmental rules and regulations; (iv) prompt internal reporting of violations of the Code of Ethics to an appropriate person or persons identified in the Code; and (v) accountability for adherence to the Code. A copy of our Code of Ethics has been posted on our internet website and can be found at www.asteriasbiotherapeutics.com. If we amend or waive a provision of our Code of Ethics that applies to our chief executive officer or chief financial officer, we will post the amended Code of Ethics or information about the waiver on our internet website.
Information about our compliance with Section 16(a) of the Securities Exchange Act of 1934 is reported under the caption “Compliance with Section 16(a) of the Securities Exchange Act of 1934” in our Proxy Statement for our 2015 Annual Meeting of Shareholders, and is incorporated herein by reference.
Information on compensation of our executive officers is reported under the caption “Executive Compensation” in our Proxy Statement for our 2015 Annual Meeting of Shareholders, and is incorporated herein by reference.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management, and Related Stockholder Matters |
Information on the number of common shares of Asterias beneficially owned by each shareholder known by us to be the beneficial owner of 5% or more of our common shares, and by each director and named executive officer, and by all directors and named executive officers as a group, is contained under the caption “Principal Shareholders” in our Proxy Statement for our 2015 Annual Meeting of Shareholders, and is incorporated herein by reference.
Information about transactions with related persons; review, and approval or ratification of transactions with related persons; and director independence is reported under the caption “Election of Directors” in our Proxy Statement for our 2015 Annual Meeting of Shareholders, and is incorporated herein by reference.
Information about our Audit Committee’s pre-approval policy for audit services, and information on our principal accounting fees and services is reported under the caption “Ratification of the Selection of Our Independent Auditors” in our Proxy Statement for our 2015 Annual Meeting of Shareholders, and is incorporated herein by reference.
PART IV
(a-1) Financial Statements.
The following financial statements of Asterias Biotherapeutics, Inc. are filed in the Form 10-K:
Balance sheets
Statements of operations
Statements of stockholders' equity/(deficit)
Statements of cash flows
Notes to Financial Statements
(a-2) Financial Statement Schedules
All schedules are omitted because the required information is inapplicable or the information is presented in the financial statements or the notes thereto.
(a-3) Exhibits.
| Exhibit Numbers | Description | |
|
2.1
|
Asset Contribution Agreement, dated January 4, 2013, by and among BioTime, Inc., BioTime Acquisition Corporation, and Geron Corporation. (1) Schedules to the Asset Contribution Agreement have been omitted. Asterias agrees to furnish supplementally a copy of the omitted schedules to the Commission upon request
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation (2)
|
|
|
3.2
|
Bylaws (2)
|
|
|
4.1
|
Specimen of Series A Common Stock Certificate (3)
|
|
|
4.2
|
Warrant Agreement, dated October 1, 2013, by Asterias Biotherapeutics, Inc. for the benefit of BioTime, Inc. (4)
|
|
|
4.3
|
Form of Warrant (Included in Exhibit 4.2) (4)
|
|
|
4.4
|
Warrant Agreement, dated October 1, 2013, by Asterias Biotherapeutics, Inc. for the benefit of Romulus Films Ltd. (4)
|
|
|
4.5
|
Form of Warrant (Included in Exhibit 4.4) (4)
|
|
|
10.1
|
Stock and Warrant Purchase Agreement, dated January 4, 2013, between BioTime Acquisition Corporation and Romulus Films Ltd. (2)
|
|
|
10.2
|
Sublease dated April 1, 2013 between BioTime, Inc. and BioTime Acquisition Corporation (2)
|
|
|
10.3
|
Shared Facilities and Services Agreement, dated April 1, 2013, between Asterias Biotherapeutics, Inc. and BioTime, Inc. (2)
|
|
|
10.4
|
Promissory Note, dated April 1, 2013, payable to BioTime, Inc. (2)
|
|
|
10.5
|
2013 Equity Incentive Plan (5)
|
|
|
10.6
|
Form of Employee Incentive Stock Option Agreement (6)
|
|
|
10.7
|
Form of Non-employee Director Stock Option Agreement (6)
|
|
|
10.8
|
Employment Agreement, dated as June 24, 2013, between Thomas Okarma and Asterias Biotherapeutics, Inc. (6)
|
|
| 10.9 |
Employment Agreement, dated as of June 24, 2013, between Katharine Spink and Asterias Biotherapeutics, Inc. (6)
|
|
10.10
|
Employment Agreement, dated as of June 24, 2013, between Jane Lebkowski and Asterias Biotherapeutics, Inc. (6)
|
|
|
10.11
|
Share Exchange Agreement, dated September 25, 2012, between Thomas Okarma and BioTime Acquisition Corporation (6)
|
|
|
10.12
|
Royalty Agreement, dated October 1, 2013 between Asterias Biotherapeutics, Inc. and Geron Corporation (7)
|
|
|
10.13
|
Exclusive Sublicense Agreement between Geron Corporation and Asterias Biotherapeutics, Inc. (7)
|
|
|
10.14
|
Sublicense Agreement between BioTime, Inc. and Asterias Biotherapeutics, Inc. (7)
|
|
|
10.15
|
Exclusive License Agreement, dated February 20, 2003, and First Amendment thereto dated September 7, 2004, between The Regents of the University of California and Geron Corporation (7)
|
|
|
10.16
|
Non-exclusive License Agreement, dated October 7, 2013, between the Wisconsin Alumni Research Foundation and Asterias Biotherapeutics, Inc. (Portions of this exhibit have been omitted pursuant to a request for confidential treatment) (7)
|
|
|
10.17
|
Lease, dated December 30, 2013, by and between BMR 6300 Dumbarton Circle, LP, and Asterias Biotherapeutics, Inc. (8)
|
|
|
10.18
|
Warrant Agreement, dated June 16, 2014, by Asterias Biotherapeutics, Inc. for the benefit of certain warrant holders (9)
|
|
|
10.19
|
Form of Warrant (included in Exhibit 10.18) (9)
|
|
|
10.20
|
Employment Agreement, dated as of June 9, 2014, between Pedro Lichtinger and Asterias Biotherapeutics, Inc. (9)
|
|
|
10.21
|
Employment Agreement, dated as of June 12, 2014, between Pedro Lichtinger and Asterias Biotherapeutics, Inc. (9)
|
|
|
10.22
|
Stock Purchase Agreement, dated as of June 12, 2014, between Pedro Lichtinger and Asterias Biotherapeutics, Inc. (9)
|
|
|
10.22
|
Purchase Agreement, dated as of June 13, 2014, between Broadwood Partners, L.P. and Asterias Biotherapeutics, Inc. (9)
|
|
|
10.22
|
Purchase Agreement, dated as of June 13, 2014, between The George Karfunkel 2007 Grantor Trust #1 and Asterias Biotherapeutics, Inc. (9)
|
|
|
10.23
|
Registration Rights Agreement, dated June 16, 2014, between The George Karfunkel 2007 Grantor Trust #1, Broadwood Partners, L.P., and Asterias Biotherapeutics, Inc. (9)
|
|
|
10.24
|
Clinical Trial and Option Agreement, dated September 8, 2014, between Asterias Biotherapeutics, Inc. and Cancer Research UK and Cancer Research Technology Limited (Portions of this exhibit have been omitted pursuant to a request for confidential treatment) (10) +
|
|
|
10.25
|
Form of Subscription Agreement 2015 Private Placement
|
|
| 10.26 | Notice of Grant Award, dated as of October 14, 2014, between the California Institute for Regenerative Medicine and Asterias Biotherapeutics, Inc. ++ | |
| 10.27 | Amendment to Notice of Grant Award, dated as of November 26, 2014, between the California Institute for Regenerative Medicine and Asterias Biotherapeutics, Inc. ++ | |
|
23.1
|
Consent of OUM & Co. LLP *
|
|
|
23.2
|
Consent of Rothstein Kass*
|
|
|
31
|
Rule 13a-14(a)/15d-14(a) Certification.*
|
|
|
32
|
Section 1350 Certification.*
|
|
|
101
|
Interactive Data File
|
|
101.INS
|
XBRL Instance Document *
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema *
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase *
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase *
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase *
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Document *
|
|
+
|
Portions of this exhibit have been omitted pursuant to a confidential treatment order from the Securities and Exchange Commission.
|
|
++
|
Portions of this exhibit have been omitted pursuant to a request for confidential treatment and have been separately filed with the Securities and Exchange Commission.
|
| (1) | Incorporated by reference to Asterias’ Current Report on Form 8-K filed by BioTime, Inc. with the Securities and Exchange Commission on January 8, 2013. |
| (2) | Incorporated by reference to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on April 3, 2013. |
| (3) | Incorporated by reference to Amendment No. 3 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on September 3, 2013 |
| (4) | Incorporated by reference to Current Report on Form 8-K filed with the Securities and Exchange Commission on October 1, 2013. |
| (5) | Incorporated by reference to Amendment No. 1 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on June 26, 2013. |
| (6) | Incorporated by reference to Amendment No. 2 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on August 13, 2013. |
| (7) | Incorporated by reference to Asterias’ Quarterly Report on Form 10-Q for the quarter ended September 30, 2013. |
| (8) | Incorporated by reference to Asterias’ Annual Report on Form 10-K for the year ended December 31, 2013. |
| (9) | Incorporated by reference to Post-Effective Amendment No. 4 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on June 19, 2014. |
| (10) | Incorporated by reference to Asterias’ Quarterly Report on Form 10-Q/A-1 for the quarter ended September 30, 2014 |
* Filed herewith.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized on the 10 day of March, 2015.
|
ASTERIAS BIOTHERAPEUTICS, INC.
|
||
|
By:
|
/s/ Pedro Lichtinger
|
|
|
Pedro Lichtinger,
|
||
|
President and Chief Executive Officer
|
||
|
Signature
|
Title
|
Date
|
||
|
/s/ Pedro Lichtinger
|
Chief Executive Officer and
|
March 10, 2015
|
||
|
PEDRO LICHTINGER
|
Director (Principal Executive Officer)
|
|||
|
/s/ Robert W. Peabody
|
Chief Financial Officer (Principal
|
March 10, 2015
|
||
|
ROBERT W. PEABODY
|
Financial and Accounting Officer)
|
|||
|
/s/ Andrew Arno
|
Director
|
March 10, 2015
|
||
|
ANDREW ARNO
|
||||
|
/s/ Alfred D. Kingsley
|
Director
|
March 10, 2015
|
||
|
ALFRED D. KINGSLEY
|
||||
|
/s/ Richard LeBuhn
|
Director
|
March 10, 2015
|
||
|
RICHARD LEBUHN
|
||||
|
/s/ Natale Ricciardi
|
Director
|
March 10, 2015
|
||
|
NATALE RICCIARDI
|
||||
|
/s/ Judith Segall
|
Director
|
March 10, 2015
|
||
|
JUDITH SEGALL
|
||||
|
/s/ Michael D. West
|
Director
|
March 10, 2015
|
||
|
MICHAEL D. WEST
|
|
Exhibit Numbers
|
Description
|
|
|
2.1
|
Asset Contribution Agreement, dated January 4, 2013, by and among BioTime, Inc., BioTime Acquisition Corporation, and Geron Corporation. (1) Schedules to the Asset Contribution Agreement have been omitted. Asterias agrees to furnish supplementally a copy of the omitted schedules to the Commission upon request
|
|
|
|
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation (2)
|
|
|
|
|
|
|
3.2
|
Bylaws (2)
|
|
|
|
|
|
|
4.1
|
Specimen of Series A Common Stock Certificate (3)
|
|
|
|
|
|
|
4.2
|
Warrant Agreement, dated October 1, 2013, by Asterias Biotherapeutics, Inc. for the benefit of BioTime, Inc. (4)
|
|
|
|
|
|
|
4.3
|
Form of Warrant (Included in Exhibit 4.2) (4)
|
|
|
|
|
|
|
4.4
|
Warrant Agreement, dated October 1, 2013, by Asterias Biotherapeutics, Inc. for the benefit of Romulus Films Ltd. (4)
|
|
|
|
|
|
|
4.5
|
Form of Warrant (Included in Exhibit 4.4) (4)
|
|
|
|
|
|
|
10.1
|
Stock and Warrant Purchase Agreement, dated January 4, 2013, between BioTime Acquisition Corporation and Romulus Films Ltd. (2)
|
|
|
10.2
|
Sublease dated April 1, 2013 between BioTime, Inc. and BioTime Acquisition Corporation (2)
|
|
|
|
|
|
|
10.3
|
Shared Facilities and Services Agreement, dated April 1, 2013, between Asterias Biotherapeutics, Inc. and BioTime, Inc. (2)
|
|
|
|
|
|
|
10.4
|
Promissory Note, dated April 1, 2013, payable to BioTime, Inc. (2)
|
|
|
|
|
|
|
10.5
|
2013 Equity Incentive Plan (5)
|
|
|
|
|
|
|
10.6
|
Form of Employee Incentive Stock Option Agreement (6)
|
|
|
|
|
|
|
10.7
|
Form of Non-employee Director Stock Option Agreement (6)
|
|
|
|
|
|
|
10.8
|
Employment Agreement, dated as June 24, 2013, between Thomas Okarma and Asterias Biotherapeutics, Inc. (6)
|
|
|
|
|
|
|
10.9
|
Employment Agreement, dated as of June 24, 2013, between Katharine Spink and Asterias Biotherapeutics, Inc. (6)
|
|
|
|
|
|
|
10.10
|
Employment Agreement, dated as of June 24, 2013, between Jane Lebkowski and Asterias Biotherapeutics, Inc. (6)
|
|
|
|
|
|
|
10.11
|
Share Exchange Agreement, dated September 25, 2012, between Thomas Okarma and BioTime Acquisition Corporation (6)
|
|
|
|
|
|
|
10.12
|
Royalty Agreement, dated October 1, 2013 between Asterias Biotherapeutics, Inc. and Geron Corporation (7)
|
|
|
|
|
|
|
10.13
|
Exclusive Sublicense Agreement between Geron Corporation and Asterias Biotherapeutics, Inc. (7)
|
|
|
|
|
|
|
10.14
|
Sublicense Agreement between BioTime, Inc. and Asterias Biotherapeutics, Inc. (7)
|
|
|
|
|
|
|
10.15
|
Exclusive License Agreement, dated February 20, 2003, and First Amendment thereto dated September 7, 2004, between The Regents of the University of California and Geron Corporation (7)
|
|
|
|
|
|
|
10.16
|
Non-exclusive License Agreement, dated October 7, 2013, between the Wisconsin Alumni Research Foundation and Asterias Biotherapeutics, Inc. (Portions of this exhibit have been omitted pursuant to a request for confidential treatment) (7)
|
|
|
|
|
|
|
10.17
|
Lease, dated December 30, 2013, by and between BMR 6300 Dumbarton Circle, LP, and Asterias Biotherapeutics, Inc. (8)
|
|
|
|
|
|
|
10.18
|
Warrant Agreement, dated June 16, 2014, by Asterias Biotherapeutics, Inc. for the benefit of certain warrant holders (9)
|
|
|
|
|
|
|
10.19
|
Form of Warrant (included in Exhibit 10.18) (9)
|
|
|
|
|
|
|
10.20
|
Employment Agreement, dated as of June 9, 2014, between Pedro Lichtinger and Asterias Biotherapeutics, Inc. (9)
|
|
10.21
|
Employment Agreement, dated as of June 12, 2014, between Pedro Lichtinger and Asterias Biotherapeutics, Inc. (9)
|
|
|
|
|
|
|
10.22
|
Stock Purchase Agreement, dated as of June 12, 2014, between Pedro Lichtinger and Asterias Biotherapeutics, Inc. (9)
|
|
|
|
|
|
|
10.22
|
Purchase Agreement, dated as of June 13, 2014, between Broadwood Partners, L.P. and Asterias Biotherapeutics, Inc. (9)
|
|
|
|
|
|
|
10.22
|
Purchase Agreement, dated as of June 13, 2014, between The George Karfunkel 2007 Grantor Trust #1 and Asterias Biotherapeutics, Inc. (9)
|
|
|
|
|
|
|
10.23
|
Registration Rights Agreement, dated June 16, 2014, between The George Karfunkel 2007 Grantor Trust #1, Broadwood Partners, L.P., and Asterias Biotherapeutics, Inc. (9)
|
|
|
|
|
|
|
10.24
|
Clinical Trial and Option Agreement, dated September 8, 2014, between Asterias Biotherapeutics, Inc. and Cancer Research UK and Cancer Research Technology Limited (Portions of this exhibit have been omitted pursuant to a request for confidential treatment) (10) +
|
|
|
|
|
|
|
Form of Subscription Agreement 2015 Private Placement
|
||
| 10.26 | Notice of Grant Award, dated as of October 14, 2014, between the California Institute for Regenerative Medicine and Asterias Biotherapeutics, Inc. ++ | |
| 10.27 | Amendment to Notice of Grant Award, dated as of November 26, 2014, between the California Institute for Regenerative Medicine and Asterias Biotherapeutics, Inc. ++ | |
|
Consent of OUM & Co. LLP *
|
||
|
|
|
|
|
Consent of Rothstein Kass*
|
||
|
|
|
|
|
Rule 13a-14(a)/15d-14(a) Certification.*
|
||
|
|
|
|
|
Section 1350 Certification.*
|
||
|
|
|
|
|
101
|
Interactive Data File
|
|
|
101.INS
|
XBRL Instance Document *
|
|
|
|
|
|
|
101.SCH
|
XBRL Taxonomy Extension Schema *
|
|
|
|
|
|
|
101.CAL
|
XBRL Taxonomy Extension Calculation Linkbase *
|
|
|
|
|
|
|
101.LAB
|
XBRL Taxonomy Extension Label Linkbase *
|
|
|
|
|
|
|
101.PRE
|
XBRL Taxonomy Extension Presentation Linkbase *
|
|
|
|
|
|
|
101.DEF
|
XBRL Taxonomy Extension Definition Document *
|
|
+
|
Portions of this exhibit have been omitted pursuant to a confidential treatment order from the Securities and Exchange Commission.
|
|
++
|
Portions of this exhibit have been omitted pursuant to a request for confidential treatment and have been separately filed with the Securities and Exchange Commission.
|
| (1) | Incorporated by reference to Asterias’ Current Report on Form 8-K filed by BioTime, Inc. with the Securities and Exchange Commission on January 8, 2013. |
| (2) | Incorporated by reference to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on April 3, 2013. |
| (3) | Incorporated by reference to Amendment No. 3 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on September 3, 2013 |
| (4) | Incorporated by reference to Current Report on Form 8-K filed with the Securities and Exchange Commission on October 1, 2013. |
| (5) | Incorporated by reference to Amendment No. 1 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on June 26, 2013. |
| (6) | Incorporated by reference to Amendment No. 2 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on August 13, 2013. |
| (7) | Incorporated by reference to Asterias’ Quarterly Report on Form 10-Q for the quarter ended September 30, 2013. |
| (8) | Incorporated by reference to Asterias’ Annual Report on Form 10-K for the year ended December 31, 2013. |
| (9) | Incorporated by reference to Post-Effective Amendment No. 4 to Registration Statement on Form S-1 (333-187706) filed with the Securities and Exchange Commission on June 19, 2014. |
| (10) | Incorporated by reference to Asterias’ Quarterly Report on Form 10-Q/A-1 for the quarter ended September 30, 2014 |
82
