Attached files
| file | filename |
|---|---|
| 8-K - 8-K - HERON THERAPEUTICS, INC. /DE/ | d925937d8k.htm |
| EX-99.1 - EX-99.1 - HERON THERAPEUTICS, INC. /DE/ | d925937dex991.htm |
| Exhibit 99.2
|
MAGIC: A Phase 3 Study of SUSTOL® in Patients Receiving Highly Emetogenic Chemotherapy
Positive Top-Line Results
May 28, 2015
|
|
Legal Disclaimer
This presentation contains “forward-looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve risks and uncertainties, including uncertainties associated with the development, regulatory approval, manufacture, launch and acceptance of new products, completion of clinical studies and the results thereof, the ability to establish strategic alliances and/or acquire desirable assets, progress in research and development programs and other risks and uncertainties identified in the Company’s filings with the
Securities and Exchange Commission. The Company’s lead product candidate, SUSTOL, which is discussed in this presentation, has not been approved by the FDA or any other regulatory authority. Actual results may differ materially from the results anticipated in our forward-looking statements. We caution investors that forward-looking statements reflect our analysis only on their stated date. We do not intend to update them except as required by law.
| 2 |
|
|
|
SUSTOL Background
SUSTOL® (granisetron injection, extended release) is an injectable, extended-release formulation of granisetron that utilizes Heron’s proprietary Biochronomer® drug delivery technology Granisetron is a widely used 5-HT3 antagonist approved for the prevention of chemotherapy induced nausea and vomiting (CINV)
– Long track record of safe and effective use
– Lacks cardiac toxicity (QT prolongation) observed with many other 5-HT3 antagonists
– Short half-life, so limited benefit in the delayed phase of CINV
SUSTOL is designed to deliver granisetron over 5 days to address the problem of delayed-onset CINV
Prior randomized, controlled, Phase 3 study in 1,341 patients demonstrated efficacy in acute and delayed CINV after moderately emetogenic chemotherapy (MEC), and acute-onset CINV after highly emetogenic chemotherapy (HEC)
| 3 |
|
|
|
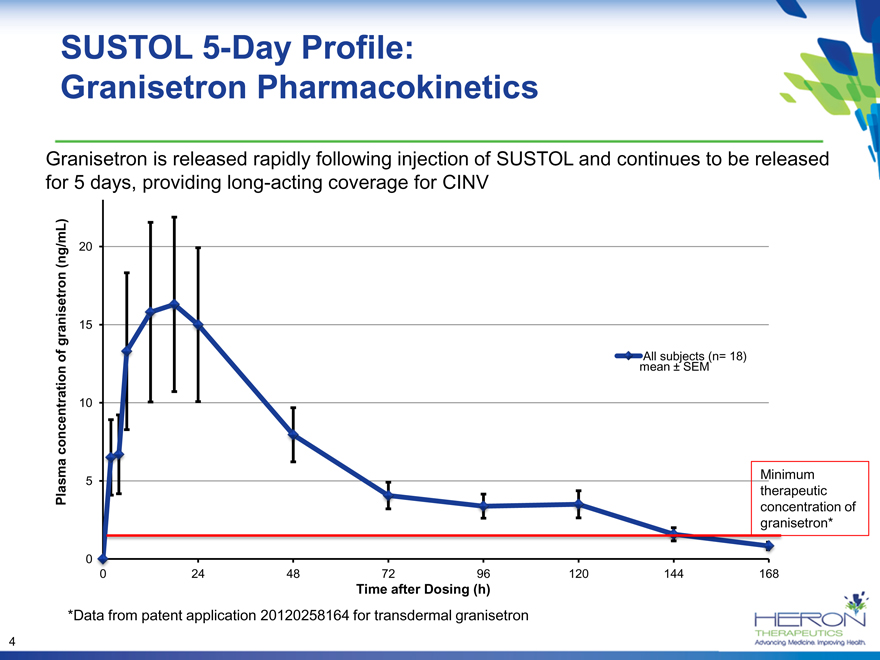
SUSTOL 5-Day Profile:
Granisetron Pharmacokinetics
Granisetron is released rapidly following injection of SUSTOL and continues to be released
for 5 days, providing long-acting coverage for CINV
(ng/mL) 20
granisetron 15
of All subjects (n= 18)
mean ± SEM
concentration 10
| 5 |
|
Minimum |
Plasma therapeutic
concentration of
granisetron*
0
0 24 48 72 96 120 144 168
Time after Dosing (h)
*Data from patent application 20120258164 for transdermal granisetron
| 4 |
|
|
|
Rationale for Pursuing HEC Study
No 5-HT3 antagonist has demonstrated efficacy in delayed nausea and vomiting in patients receiving HEC
There is a significant unmet medical need for better prophylaxis against delayed nausea and vomiting in patients receiving HEC
Reanalysis of prior Phase 3 study of SUSTOL using the 2011 ASCO definition for HEC suggested that SUSTOL may represent the best-in-class 5-HT3 antagonist
| 5 |
|
|
|
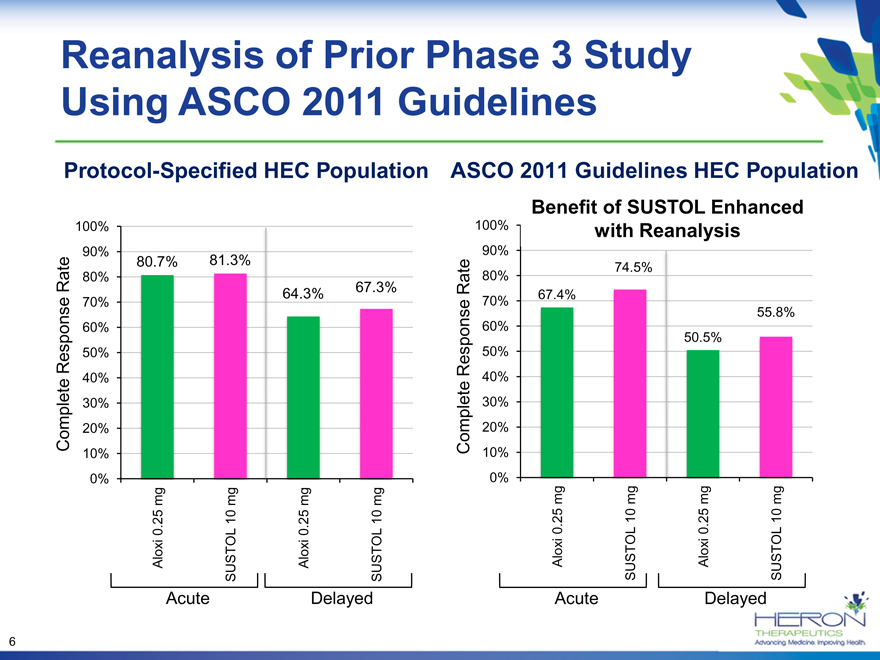
Reanalysis of Prior Phase 3 Study Using ASCO 2011 Guidelines
Protocol-Specified HEC Population
100%
90% 80.7% 81.3%
80%
Rate 64.3% 67.3%
70%
60%
Response 50%
40%
30%
Complete 20%
10%
0%
mg mg mg mg
0.25 10 0.25 10
Aloxi SUSTOL Aloxi SUSTOL
Acute Delayed
ASCO 2011 Guidelines HEC Population
Benefit of SUSTOL Enhanced
100% with Reanalysis
90%
Rate 80% 74.5%
70% 67.4%
55.8%
60%
50.5%
Response 50%
40%
30%
Complete 20%
10%
0%
mg mg mg mg
0.25 10 0.25 10
Aloxi SUSTOL Aloxi SUSTOL
Acute Delayed
| 6 |
|
|
|
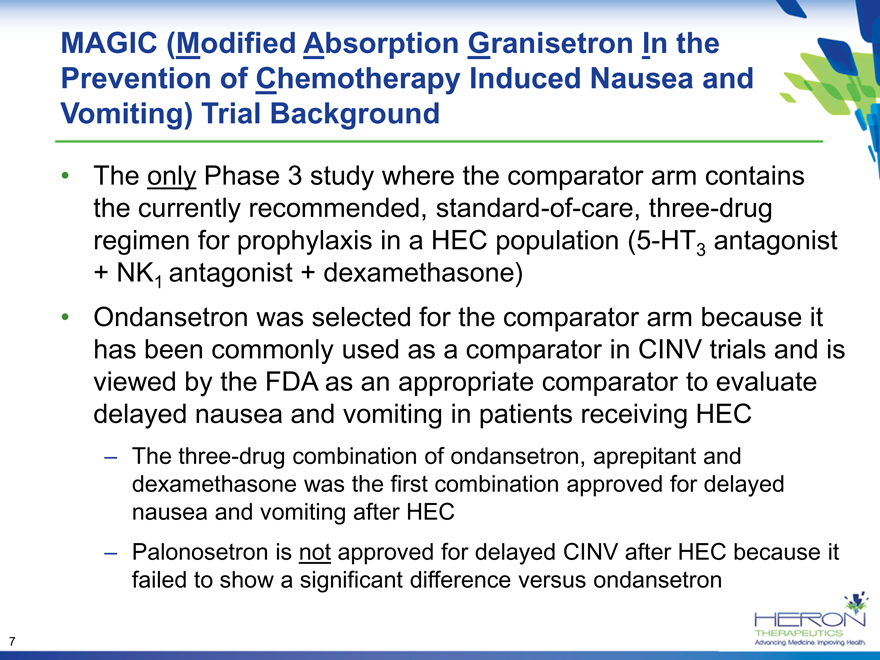
MAGIC (Modified Absorption Granisetron In the Prevention of Chemotherapy Induced Nausea and Vomiting) Trial Background
The only Phase 3 study where the comparator arm contains the currently recommended, standard-of-care, three-drug regimen for prophylaxis in a HEC population (5-HT3 antagonist
+ NK1 antagonist + dexamethasone)
Ondansetron was selected for the comparator arm because it has been commonly used as a comparator in CINV trials and is viewed by the FDA as an appropriate comparator to evaluate delayed nausea and vomiting in patients receiving HEC
– The three-drug combination of ondansetron, aprepitant and dexamethasone was the first combination approved for delayed nausea and vomiting after HEC
– Palonosetron is not approved for delayed CINV after HEC because it failed to show a significant difference versus ondansetron
| 7 |
|
|
|
MAGIC Conducted Entirely in U.S. Community Oncology Centers
83 U.S. Study Sites
| 8 |
|
|
|
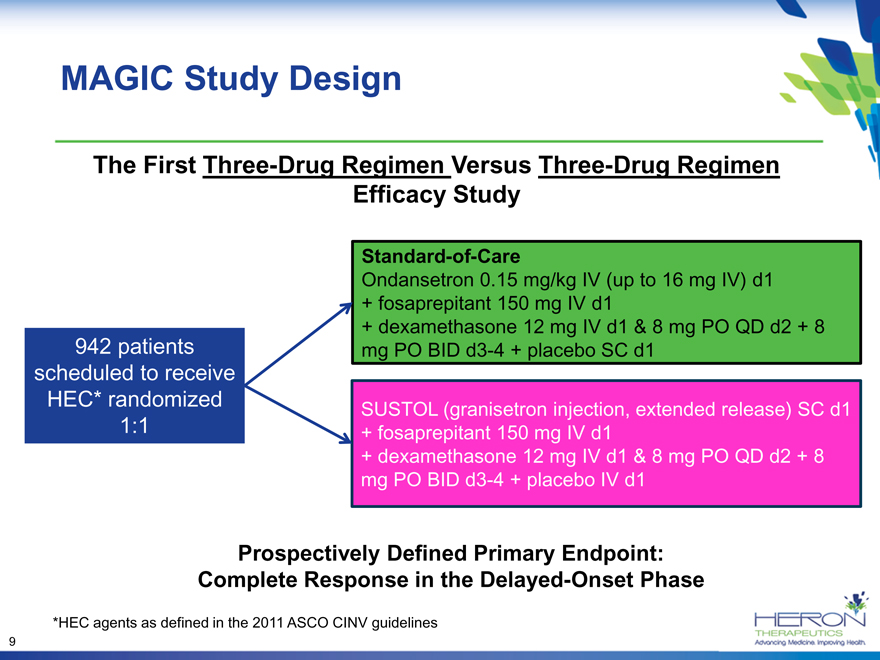
MAGIC Study Design
The First Three-Drug Regimen Versus Three-Drug Regimen
Efficacy Study
Standard-of-Care
Ondansetron 0.15 mg/kg IV (up to 16 mg IV) d1
+ fosaprepitant 150 mg IV d1
+ dexamethasone 12 mg IV d1 & 8 mg PO QD d2 + 8
942 patients mg PO BID d3-4 + placebo SC d1
scheduled to receive
HEC* randomized SUSTOL (granisetron injection, extended release) SC d1
1:1 + fosaprepitant 150 mg IV d1
+ dexamethasone 12 mg IV d1 & 8 mg PO QD d2 + 8
mg PO BID d3-4 + placebo IV d1
Prospectively Defined Primary Endpoint:
Complete Response in the Delayed-Onset Phase
*HEC agents as defined in the 2011 ASCO CINV guidelines
9
|
|
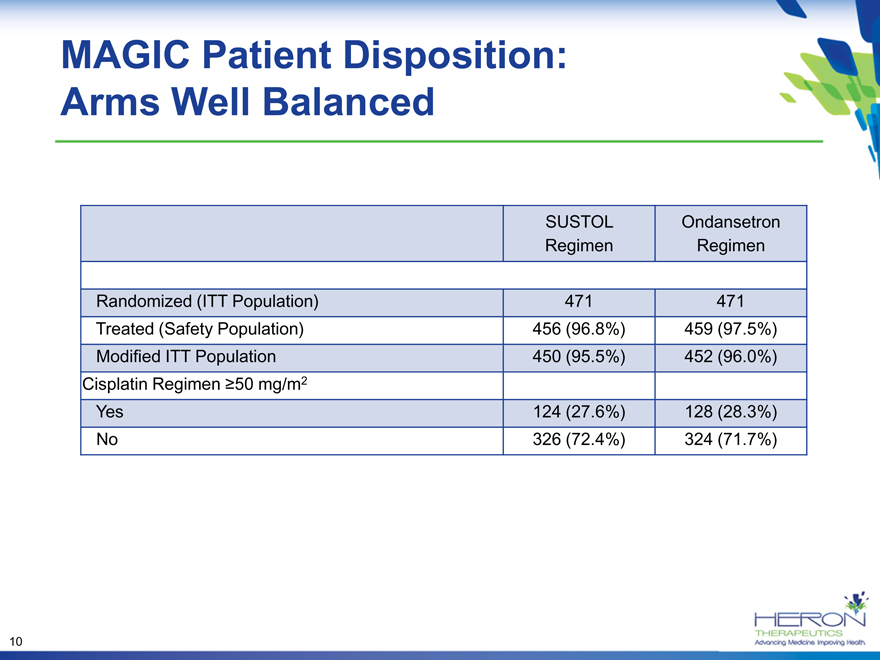
MAGIC Patient Disposition: Arms Well Balanced
SUSTOL Ondansetron
Regimen Regimen
Randomized (ITT Population) 471 471
Treated (Safety Population) 456 (96.8%) 459 (97.5%)
Modified ITT Population 450 (95.5%) 452 (96.0%)
Cisplatin Regimen ?50 mg/m2
Yes 124 (27.6%) 128 (28.3%)
No 326 (72.4%) 324 (71.7%)
10
|
|
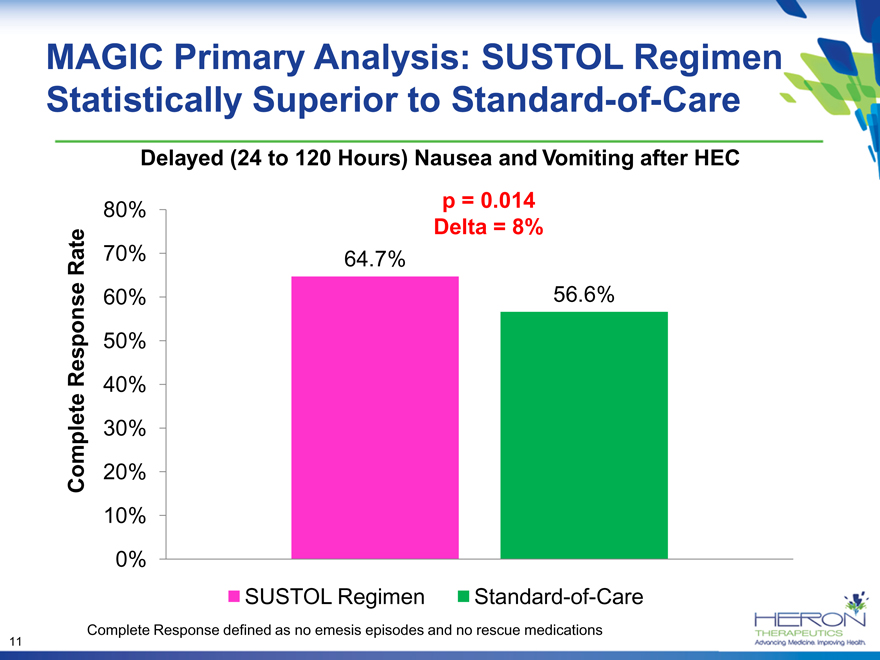
MAGIC Primary Analysis: SUSTOL Regimen Statistically Superior to Standard-of-Care
Delayed (24 to 120 Hours) Nausea and Vomiting after HEC
80% p = 0.014
Delta = 8%
Rate 70% 64.7%
60% 56.6%
Response 50%
40%
30%
Complete 20%
10%
0%
SUSTOL Regimen Standard-of-Care
Complete Response defined as no emesis episodes and no rescue medications
11
|
|
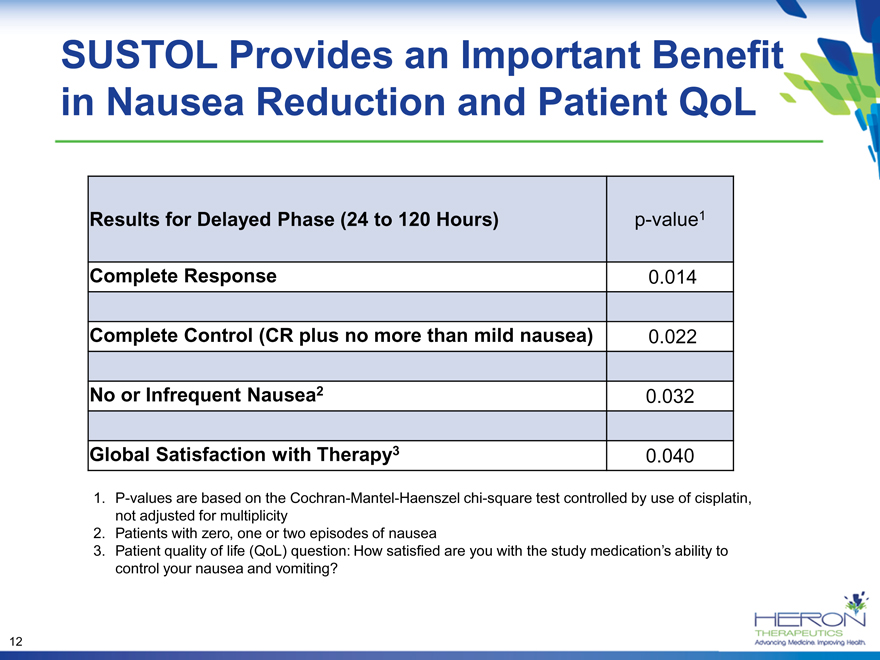
SUSTOL Provides an Important Benefit in Nausea Reduction and Patient QoL
Results for Delayed Phase (24 to 120 Hours) p-value1
Complete Response 0.014
Complete Control (CR plus no more than mild nausea) 0.022
No or Infrequent Nausea2 0.032
Global Satisfaction with Therapy3 0.040
P-values are based on the Cochran-Mantel-Haenszel chi-square test controlled by use of cisplatin, not adjusted for multiplicity
Patients with zero, one or two episodes of nausea
Patient quality of life (QoL) question: How satisfied are you with the study medication’s ability to control your nausea and vomiting?
12
|
|
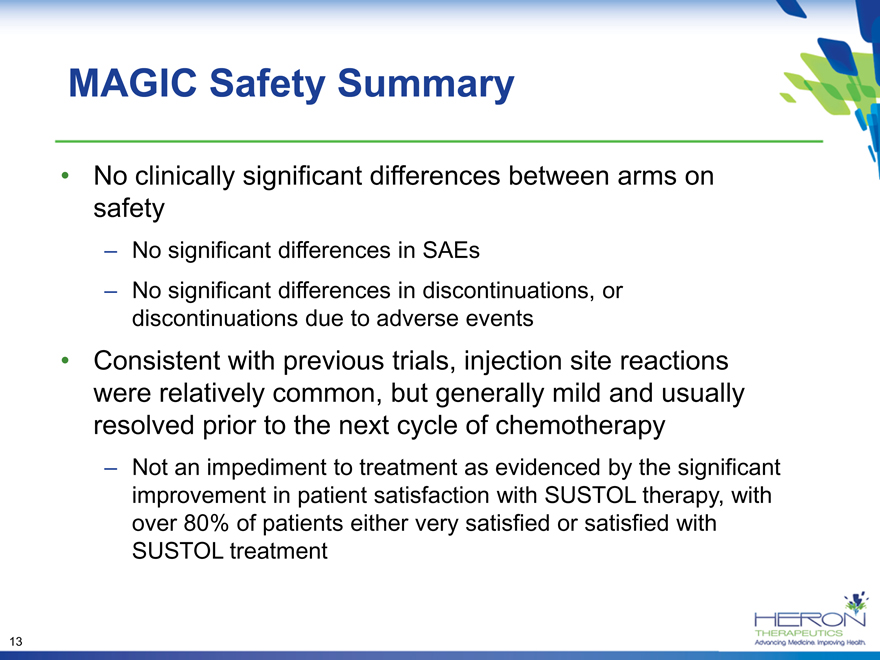
MAGIC Safety Summary
No clinically significant differences between arms on safety
– No significant differences in SAEs
– No significant differences in discontinuations, or discontinuations due to adverse events
Consistent with previous trials, injection site reactions were relatively common, but generally mild and usually resolved prior to the next cycle of chemotherapy
– Not an impediment to treatment as evidenced by the significant improvement in patient satisfaction with SUSTOL therapy, with over 80% of patients either very satisfied or satisfied with SUSTOL treatment
13
|
|
MAGIC Trial Summary
MAGIC is the first Phase 3 CINV study:
– Using a control group that receives a standard-of-care, three-drug regimen for HEC
– Conducted entirely at U.S. community oncology centers
Complete Response in delayed nausea and vomiting was significantly greater with the SUSTOL-based, three-drug regimen compared to standard-of-care
– Complete Control (which includes a measure of nausea) was significantly greater with SUSTOL
– Significantly more patients had no nausea or infrequent nausea with SUSTOL
– SUSTOL patients reported significantly greater satisfaction with therapy
Adverse events were as expected with no clinically significant differences between arms for any safety measure
14
|
|
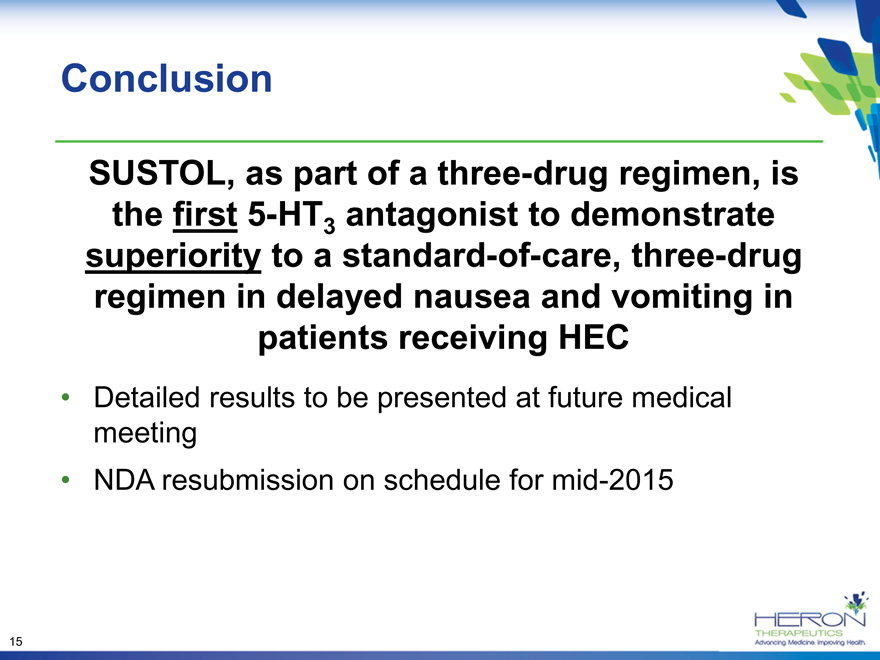
Conclusion
SUSTOL, as part of a three-drug regimen, is the first 5-HT3 antagonist to demonstrate superiority to a standard-of-care, three-drug regimen in delayed nausea and vomiting in patients receiving HEC
Detailed results to be presented at future medical meeting
NDA resubmission on schedule for mid-2015
15