Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - Dex Liquidating Co. | Financial_Report.xls |
| EX-32 - EXHIBIT 32.1 - Dex Liquidating Co. | ex32-1.htm |
| EX-31 - EXHIBIT 31.1 - Dex Liquidating Co. | ex31-1.htm |
| EX-10 - EXHIBIT 10.3 - Dex Liquidating Co. | ex10-3.htm |
| EX-23 - EXHIBIT 23.2 - Dex Liquidating Co. | ex23-2.htm |
| EX-23 - EXHIBIT 23.1 - Dex Liquidating Co. | ex23-1.htm |
| EX-31 - EXHIBIT 31.2 - Dex Liquidating Co. | ex31-2.htm |
| EX-10 - EXHIBIT 10.21 - Dex Liquidating Co. | ex10-21.htm |
| EX-10 - EXHIBIT 10.14 - Dex Liquidating Co. | ex10-14.htm |
| EX-10 - EXHIBIT 10.19 - Dex Liquidating Co. | ex10-19.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
|
☑ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended June 30, 2014 | |
|
OR | |
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 000-51772
CARDICA, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
94-3287832 |
|
(State or other jurisdiction of |
(I.R.S. Employer |
|
Incorporation or Organization) |
Identification No.) |
900 Saginaw Drive
Redwood City, California 94063
(650) 364-9975
(Address, including zip code, and telephone number, including area code, of principal executive offices)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Name of each exchange on which registered |
|
Common Stock, $0.001 par value per share |
The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☑ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer ☐ |
Accelerated filer ☐ |
Non-accelerated filer ☐ |
Smaller reporting company☑ |
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
The aggregate market value of the voting stock held by non-affiliates of the registrant as of December 31, 2013, was approximately $31,888,386 (based on the closing sales price of the registrant’s common stock as reported by the NASDAQ Global Market, on December 31, 2013). For purposes of this disclosure, shares of common stock held by each officer and director (and entities affiliated therewith) have been excluded in that such persons may be deemed to be “affiliates” as that term is defined under the Rules and Regulations of the Securities Exchange Act of 1934. This determination of affiliate status is not necessarily conclusive.
The number of shares of common stock outstanding as of September 18, 2014, was 88,951,216.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement for its 2014 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission within 120 days after the registrant’s fiscal year ended June 30, 2014, are incorporated by reference in Part III, Items 10-14 of this Annual Report on Form 10-K.
Cardica, inc.
ANNUAL REPORT ON FORM 10-K
For the Year Ended June 30, 2014
TABLE OF CONTENTS
|
|
Page |
|
PART I |
|
|
Item 1. Business |
1 |
|
Item 1A. Risk Factors |
21 |
|
Item 1B. Unresolved Staff Comments |
40 |
|
Item 2. Properties |
40 |
|
Item 3. Legal Proceedings |
40 |
|
Item 4. Mine Safety Disclosures |
40 |
|
PART II |
|
|
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
40 |
|
Item 6. Selected Financial Data |
41 |
|
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations |
41 |
|
Item 7A. Quantitative and Qualitative Disclosures About Market Risk |
51 |
|
Item 8. Financial Statements and Supplementary Data |
52 |
|
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
76 |
|
Item 9A. Controls and Procedures |
76 |
|
Item 9B. Other Information |
76 |
|
PART III |
|
|
Item 10. Directors, Executive Officers and Corporate Governance |
77 |
|
Item 11. Executive Compensation |
77 |
|
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
77 |
|
Item 13. Certain Relationships and Related Transactions, and Director Independence |
78 |
|
Item 14. Principal Accountant Fees and Services |
78 |
|
PART IV |
|
|
Item 15. Exhibits, Financial Statement Schedules |
78 |
|
Signatures |
79 |
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are subject to the “safe harbor” created by those sections. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “could,” “would,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “predict,” “potential” and similar expressions intended to identify forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance, time frames or achievements to be materially different from any future results, performance, time frames or achievements expressed or implied by the forward-looking statements. We discuss many of these risks, uncertainties and other factors in this Annual Report on Form 10-K in greater detail under the heading “Risk Factors.” Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements. Also, these forward-looking statements represent our estimates and assumptions only as of the date of this filing. You should read this Annual Report on Form 10-K completely and with the understanding that our actual future results may be materially different from what we expect. We hereby qualify our forward-looking statements by our cautionary statements. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
PART I
Item 1. Business
Overview
We are commercializing and developing a microcutter product line based on our proprietary “staple-on-a-strip” technology intended for use by thoracic, bariatric, colorectal and general surgeons. Our microcutter product line consists of the currently commercially-available MicroCutter XCHANGE® 30, a cartridge based microcutter device with a 5 millimeter shaft diameter and a 30 millimeter staple line, and products in development, including the MicroCutter XCHANGE® 45, a cartridge based microcutter device with an 8 millimeter shaft and a 45 millimeter staple line, the MicroCutter FLEXCHANGE™ 30, a cartridge based microcutter device with a flexible shaft to facilitate endoscopic procedures requiring cutting and stapling, and the MicroCutter XPRESS® 45, a multi-fire endolinear microcutter device with a 45 millimeter staple line specifically designed for the bariatric and thoracic surgery markets. We estimate that the commercially-available MicroCutter XCHANGE 30, along with these planned additional products, would provide us with a commercial opportunity of approximately 1.4 million procedures annually in the United States, involving, we estimate, over four million staple cartridge deployments, three million of which we believe are deployed in laparoscopic procedures.
In March 2012, we completed the design verification for and applied Conformité Européenne, or the CE Mark, to the MicroCutter XCHANGE 30 and, in December 2012, began a controlled commercial launch of the MicroCutter XCHANGE 30 in Europe. We received from the United States Food and Drug Administration, or FDA, 510(k) clearance for the MicroCutter XCHANGE 30 and blue cartridge in January 2014, and for the white cartridge in February 2014, for use in multiple open or minimally-invasive surgical procedures for the transection, resection and/or creation of anastomoses in small and large intestine, as well as the transection of the appendix. The blue cartridge is for use in medium thickness tissue, and the white cartridge is for use in thin tissue. In March 2014, we made our first sale of the MicroCutter XCHANGE 30 in the United States. We also recently submitted our MicroCutter XCHANGE 30 blue and white cartridges application to Health Canada for regulatory approval of our MicroCutter XCHANGE 30 and, if we receive approval, anticipate launching it in Canada. In addition, in August 2013, our exclusive distributor in Japan, Century Medical, Inc., or Century, filed for regulatory approval of our MicroCutter XCHANGE 30 cartridges with the Pharmaceuticals and Medical Devices Agency in Japan and, upon approval, anticipates launching the MicroCutter XCHANGE 30 in Japan. We believe that the MicroCutter XCHANGE 30 is differentiated in the market compared to currently marketed staplers due to its significantly reduced size and ability to articulate up to 80 degrees.
Historically, our business focused on the design, manufacture and marketing of proprietary automated anastomotic systems used by cardiac surgeons to perform coronary bypass surgery. Our C-Port® Distal Anastomosis Systems, or C-Port systems, are sold in the United States and Europe. The C-Port systems are used to perform a distal anastomosis, which is the connection between a bypass graft vessel and the target coronary artery. As of June 30, 2014, more than 14,300 C- Port systems had been sold in the United States and Europe. We also currently sell our PAS-Port® Proximal Anastomosis System, or PAS-Port system, in the United States, Europe and Japan. The PAS-Port system is used to perform a proximal anastomosis, which is the connection of a bypass graft vessel to the aorta or other source of blood. As of June 30, 2014, more than 37,000 PAS-Port systems had been sold in the United States, Europe and Japan. To date, we generated revenues almost exclusively from the sale of automated anastomotic systems, and have generated minimal revenues from the commercial sales of the MicroCutter XCHANGE 30 since its introduction in Europe in December 2012, and in the United States in March 2014.
Our Strategy
Our goals are to develop and market laparoscopic microcutter products intended for use by thoracic, bariatric, colorectal and general surgeons and to increase adoption of automated anastomotic systems for coronary artery bypass grafting, or CABG, procedures and closure devices for other surgical procedures. Other existing technologies either do not enable or are less compatible with less invasive and minimally invasive surgery. Because less invasive surgery has many advantages relative to patient outcomes, our strategy involves developing and, ultimately, marketing and selling devices that enable or facilitate less invasive and minimally invasive procedures, which in turn may help expand the market for these types of surgeries. The principal elements of our strategy to achieve our vision and goals include:
|
• |
Commercializing our microcutters. We received from the FDA 510(k) clearance for the MicroCutter XCHANGE 30 and blue cartridge in January 2014, and for the white cartridge in February 2014, for use in multiple open or minimally-invasive surgical procedures for the transection, resection and/or creation of anastomoses in small and large intestine, as well as the transection of the appendix. The blue cartridge is for use in medium thickness tissue, and the white cartridge is for use in thin tissue. As part of our controlled commercial launch of the MicroCutter XCHANGE 30 in Europe and in the United States, we made our first sales in December 2012, and March 2014, respectively. We have agreements for the microcutter product line with four distributors in Europe. In addition, in August 2014, we established a subsidiary in Germany, Cardica, GmbH, to facilitate direct sales of the microcutter product. In the United States, we have secured approval from 38 hospital Value Analysis Committees (VACs) who have approved the MicroCutter XCHANGE 30 either for purchase or for an evaluation and potential subsequent purchase. We focus on developing relationships with leading clinicians who are considered to be “thought leaders” in their institutions and surgical specialties. We work closely with a limited number of targeted clinical sites to achieve routine clinical adoption of the MicroCutter XCHANGE 30 for surgical procedures in which we believe its key features are most differentiated from existing devices. We plan to leverage the lessons learned from this initial experience and the clinical experience of these key opinion leaders to bring credibility to a broader launch. We signed a distribution agreement in 2011 with Century with respect to distribution of our planned microcutter products in Japan. |
|
• |
Leveraging our proprietary “staple-on-a-strip” technology to develop a broad range of surgical stapling devices. Several of the innovative features that we are incorporating into our microcutter product line are: the ability to deploy our “staple-on-a-strip” technology for more consistent staple forms; significantly reduced tool shaft diameter; increased amount of articulation of the end-effector; and potentially larger staples. The MicroCutter XCHANGE 30 incorporates these features and is the first product we commercialized in our planned product line of microcutter devices. We believe that our technology can be adapted for a variety of surgical stapling devices, including the MicroCutter XCHANGE 45, the MicroCutter FLEXCHANGE 30, and the MicroCutter XPRESS 45. These potential products are described under “Microcutter Products and Products Under Development” below. By leveraging our technology, we believe we will expand our commercial opportunity into additional surgical markets. |
|
• |
Obtaining U.S. and international regulatory clearance of the microcutter product line. We are currently developing the MicroCutter XCHANGE 45, a cartridge based microcutter device with an eight millimeter shaft and a 45 millimeter staple line. In August 2013, our Japanese distributor, Century Medical, has filed with Pharmaceuticals and Medical Devices Agency in Japan for approval of the MicroCutter XCHANGE 30 cartridges. In addition, we are pursuing regulatory approval for the MicroCutter XCHANGE 30 in Canada. |
.
|
• |
Supporting market adoption of the C-Port and PAS-Port systems. We intend to continue to support commercial adoption of our C-Port systems and our PAS-Port system by marketing them as integrated anastomotic tools for use in both on- and off-pump CABG procedures and in robot assisted bypass surgeries, known as totally endoscopic coronary artery bypass, or TECAB, procedures. |
|
• |
Establishing a strong proprietary position. As of June 30, 2014, we had 133 issued U.S. patents, 67 additional patent applications in the United States, twelve issued foreign patents and another 25 patent applications filed in selected international markets. We plan to continue to invest in building our intellectual property portfolio. |
Microcutter Industry Background
Evolution of surgical techniques
Open surgery has been the most common form of surgery for many decades. Using open surgical techniques, a surgeon generally creates an incision large enough to allow a direct view of the operating field and inserts the instruments necessary to manipulate the patient's tissues. The large incisions and significant tissue manipulation involved in open surgery cause trauma to the patient, resulting in extended hospitalization and recovery times, increased hospital costs, and additional pain and suffering.
Over the past thirty years, technological innovations such as enhanced imaging and instrumentation have facilitated visualization and surgical access through smaller and smaller incisions. These improvements have enabled surgeons to reduce patient trauma, hospital stays and morbidity, while improving recovery times and cosmetic results. This evolution has both been made possible by, and created opportunities for, the development of new categories of surgical devices.
Minimally invasive, laparoscopic (abdominal or pelvic cavities) or thoracoscopic (chest cavity) surgery replaces the large incision typically required for open surgery with several small abdominal/thoracic openings and tubes, referred to as ports, that provide access to the organs upon which the surgeon needs to operate. The surgeon uses an endoscope to view the operating field and inserts specialized instruments through the ports to carry out the procedure. The advantages of laparoscopic/thoracoscopic surgery compared to traditional open surgical procedures include shorter post-operative recovery periods with less pain, shorter hospital stays, decreases in post-operative complications and a quicker return to routine activities.
Laparoscopic surgery was originally used by gynecologists for the diagnosis and treatment of diseases of the ovary and uterus. Removal of the gall bladder by laparoscopic techniques was introduced in the late 1980s. Since that time, many of the procedures that were performed in the past utilizing traditional open surgical techniques have transitioned to minimally invasive surgical approaches including procedures on the appendix, stomach, lungs, colon, uterus and other organs.
More recently, minimally invasive surgeons are using fewer and fewer abdominal openings and ports, such as in single incision surgery, in which the surgeon operates almost exclusively through a single entry point, typically the patient’s navel. Unlike a traditional multi-port laparoscopic approach, single port surgery leaves only a single small scar. Single incision surgery has been used to perform many types of surgery, including removal of the appendix, gall bladder and portions of the lung or colon, as well as bariatric surgeries including gastric bypass and sleeve gastrectomy.
We believe the realization of the full potential of minimally invasive surgery will depend upon the availability of surgical instruments and devices that address the unique challenges of these procedures by offering advanced capabilities, including smaller instrument shaft diameters, increased end-effector articulation, flexible shaft instruments, better ergonomics and greater ease of use than are provided by currently available devices.
Market
The use of disposable devices for closing and/or cutting in both traditional and laparoscopic/thoracoscopic surgical procedures has been broadly adopted clinically in a number of surgical specialties including colorectal, bariatric, gynecologic, urologic and thoracic surgery. Based on a 2009 Reach Consulting Services report, the world-wide laparoscopic surgery products market is estimated at $3.6 billion annually, with the cutter and stapler segment representing approximately $1.3 billion. Based on a 2010 Millennium Research Group report, 55-70% of the worldwide laparoscopic stapling-cutting closure product revenue is generated in the United States market.
We estimate there are approximately 1.4 million surgical procedures per year in the United States involving bariatric and general, thoracic, gynecologic and urologic surgery, involving, we estimate, over 4 million staple cartridge deployments, 3 million of which we believe are deployed in laparoscopic or thoracoscopic procedures.
Current Devices for Surgical Stapling
Current, conventional surgical stapling technology generally involves:
|
• |
individually placing sets of staples in reloadable cartridges, designed for single use; |
|
• |
using a deployment tool, consisting of a handle and shaft (with a minimum diameter of 12 millimeters), that is reusable within a single surgical procedure; |
|
• |
using cartridges that can be loaded, following each deployment, into a receptacle at the end of the deployment tool; |
|
• |
deploying multiple U-shaped wires against a deforming surface, called an anvil, to reshape the wires into B-shaped wires and thereby connecting or sealing tissue; and |
|
• |
deploying multiple rows of staples, usually two to three rows per side, with a tissue dividing cut between the rows. |
Unlike many other surgical instruments and devices, there have been few significant innovations in surgical stapling technology over the past ten years.
Microcutter Product Development
Based upon much of the technology we developed for our cardiac surgery anastomosis products, we are developing and have begun commercialization of our microcutter product line. We believe that our endoscopic microcutter design potentially addresses many of the limitations in currently available stapling products and provides surgeons with a smaller and more effective stapling and cutting device for more minimally invasive surgical procedures. Key features of our commercially-available MicroCutter XCHANGE 30 and our planned microcutter product line include:
|
• |
Staple Design and Formation. Our microcutter product line utilizes our innovative three dimensional, or 3D, staple design, which we engineered in connection with our vascular anastomotic products, that in vascular applications allows single rows of staples to effectively prevent blood leakage at physiological blood pressures. These 3D staples allow for a large contact surface between staple and tissue, which improves sealing while reducing the likelihood of the staple cutting through tissue. These 3D staples are guided into their final shape by the anvil rather than forced to buckle as is the case with U-shaped wire staples, which reduces the forming forces and helps to reduce the likelihood of malformed staples. The 3D design with a rectangular cross-section increases staple stiffness compared to round wire, resulting in a much stronger final form that is more resistant to opening or yielding. |
|
• |
Device Size. By changing the technology used to form the staple, we are able to design our microcutter products to have a smaller-sized end-effector and tool shaft. Depending upon the chosen staple line length and staple height, the microcutter’s outer diameter could be as small as five millimeters. Due to its smaller size, our microcutter should enable procedures requiring minimal access, such as robot-assisted surgery and the rapidly emerging area of single incision laparoscopic surgery. |
|
• |
“Staple-On-A-Strip” Technology. We have further advanced our 3D staple technology in connection with the microcutter product line by introducing an innovative design in which 3D staples are stamped from sheet metal and left connected to a metal band that is then loaded into the device. This differs from conventional technology in which individual staples are typically loaded into cartridge bays. We believe that our “staple-on-a-strip” technology will enable tighter spacing between individual staples, which improves sealing performance. |
|
• |
True Multi-Fire Capability. Our “staple-on-a-strip” technology is being designed to allow the surgeon to fire multiple deployments within a single procedure, without the need to remove the stapler from the tissue site or having to replace the staple cartridge. Conventional stapling technology requires a tedious, repetitive 10-step process after each deployment in which the stapler is first clamped and then removed from the body cavity. We believe true multi-fire capability will reduce this multi-step process to one simple step: following a deployment, the device is reset by activating a simple slider. True multi-fire capability will only be available in the MicroCutter XPRESS 45. |
|
• |
Improved Staple Formation. We have designed our microcutter products to deploy staples with significantly lower deployment forces. Reduced deployment forces potentially give the user more control during deployment. Additionally, our compact staple mechanism would allow more design space to be dedicated to the anvil, which helps to ensure favorable tissue compression. These features combine to result in staple formation. |
|
• |
Articulation, Rotation and Handling. End-effector size, articulation and rotation improve tissue access and ease of use, and we believe both are expected by surgeons in stapling devices. Our microcutter products’ designs incorporate end-effectors that articulate as much as 80 degrees, compared to the 45 degrees of maximum articulation achieved with the vast majority of currently marketed linear stapling technologies. In addition, all of our microcutter products are being designed to enable 360-degree rotation of the end-effector. Our MicroCutter XCHANGE 30 is a single-hand operated device: 360 degree rotation with up to 80 degree articulation accomplished with two articulation buttons integrated into a single knob at the end of the handle. |
Microcutter Products and Products Under Development
Subject to regulatory clearances, we intend to launch a full range of surgical stapling devices that cover the needs of thoracic, bariatric, colorectal and general surgeons as shown in the table below. These products would provide staple line lengths from 30 to 60 millimeters, come in shaft diameters ranging from five to ten millimeters, accommodate staple heights from 2.0 to 5.3 millimeters and articulate up to 80 degrees. Depending upon the specific product application, we anticipate that some of these products will have true multi-fire capability, while others will be cartridge-based. In all instances the true multi-fire or cartridge design would be combined with our unique staple design, including the “staple-on-a-strip” technology. In the true multi-fire design, we anticipate that each device will provide a number of deployments that is a function of shaft length and desired staple line length, ranging from six to twelve deployments in one device. In addition, subject to regulatory clearances, we plan to expand the microcutter product line by introducing products with flexible shafts to facilitate minimally invasive procedures. The following table summarizes our current and planned microcutter product line; the MicroCutter XCHANGE 30 is our only currently commercial product:
|
MicroCutter Product Line | ||||
|
Product Family |
Multifire |
Staple Line Length |
Shaft |
Articulation |
|
MicroCutter XCHANGE 30 |
No |
30 mm |
5 mm, Rigid |
Up to 80 degrees |
|
MicroCutter XCHANGE 45 |
No |
45 mm |
8 mm, Rigid |
Up to 80 degrees |
|
MicroCutter XCHANGE 60 |
No |
60 mm |
10 mm, Rigid |
Up to 45 degrees |
|
MicroCutter FLEXCHANGE 30 |
No |
30 mm |
5 mm, Flexible |
Up to 80 degrees |
|
MicroCutter XPRESS 45 |
Yes |
45 mm |
8 mm, Rigid |
Up to 60 degrees |
MicroCutter XCHANGE Product Family
The MicroCutter XCHANGE name refers to the current and planned group of cartridge based microcutter products with rigid shafts that include our proprietary “staple-on-a-strip” technology. The first product in this family is the MicroCutter XCHANGE 30 with a 30 mm staple line length. This 5 mm stapling device has been developed with up to 80 degrees of articulation. We also plan to develop and offer additional versions of the MicroCutter XCHANGE 30 including cartridges with a curved plastic tip at the distal end to facilitate surgeon vision and access for vascular surgical procedures and a version of the MicroCutter XCHANGE 30 with a shorter shaft to facilitate certain surgeries. Subsequently, the MicroCutter XCHANGE 45 and MicroCutter XCHANGE 60, with 45mm and 60mm staple line lengths, respectively, are planned to provide cartridge-based capability when multifire capability is not required.
We believe that the MicroCutter XCHANGE 30 is and will be differentiated in the market compared to currently marketed staplers due to its significantly reduced size and ability to articulate up to 80 degrees. We have initiated sales of the MicroCutter XCHANGE 30 in the European Union and recently in the United States, following the clearance of our 510(k) submissions to the FDA in January 2014 for use of the MicroCutter XCHANGE 30 and blue cartridge, and for the white cartridge in February 2014, for use in multiple open or minimally-invasive surgical procedures for the transection, resection and/or creation of anastomoses in small and large intestine, as well as the transection of the appendix. The blue cartridge is for use in medium thickness tissue, and the white cartridge is for use in thin tissue. We have limited the development of other products in our planned microcutter product line other than the MicroCutter XCHANGE 45 until the development and commercialization of the MicroCutter XCHANGE 45 have been completed. We also recently submitted our MicroCutter XCHANGE 30 blue and white cartridges application to Health Canada for regulatory approval of our MicroCutter XCHANGE 30 and, if we receive approval, anticipate launching it in Canada. In addition, in August 2013, our exclusive distributor in Japan, Century Medical, Inc., or Century, filed for regulatory approval of our MicroCutter XCHANGE 30 cartridges with the Pharmaceuticals and Medical Devices Agency in Japan and, upon approval, anticipates launching the MicroCutter XCHANGE 30 in Japan.
MicroCutter FLEXCHANGE Product Family
The MicroCutter FLEXCHANGE name refers to the planned group of cartridge-based microcutter products with flexible shafts that will also include our proprietary “staple-on-a-strip” technology. The first product that is planned in this family would be the Microcutter FLEXCHANGE 30 with a 30 mm staple line length. We expect this product would be the first and only 5 mm stapling device available on the market with a flexible shaft and is being developed with up to 80 degrees of articulation as currently there are no other products on the market that have these characteristics. This device is planned to facilitate endoscopic procedures requiring cutting and stapling.
MicroCutter XPRESS Product Family
The MicroCutter XPRESS name refers to the group of microcutter products that is planned to have the true multi-fire endolinear microcutter device design based on our proprietary “staple-on-a-strip” technology. Our only product planned in this group is the MicroCutter XPRESS 45 with a 45 mm staple line length, an 8mm rigid shaft and articulation up to 60 degrees.
Microcutter Technology License Agreement
On August 16, 2010, we entered into a license agreement with Intuitive Surgical Operations, Inc., or Intuitive Surgical, pursuant to which we granted to Intuitive Surgical a worldwide, sublicenseable, exclusive license to use our intellectual property in the robotics field in diagnostic or therapeutic medical procedures, excluding vascular anastomosis applications, referred to as the License Agreement. In consideration for this license, we received an up-front license fee of $9.0 million. We are also eligible to receive a contingent payment related to achieving a certain sales volume. Receipt of the contingent payment is substantively at risk given the uncertainties surrounding the development and sale of any products incorporating our patent rights. Each party has the right to terminate the License Agreement in the event of the other party’s uncured material breach or bankruptcy. Following any termination of the License Agreement, the licenses granted to Intuitive Surgical will continue, and, except in the case of termination for our or Intuitive Surgical’s uncured material breach or insolvency, Intuitive Surgical’s payment obligations will continue as well. Under the License Agreement, Intuitive Surgical has rights to improvements in our technology and intellectual property over a specified period of time.
Microcutter Product Sales and Marketing
Total product sales of our MicroCutter XCHANGE 30 were $0.5 million and $0.2 million, for fiscal years ended June 30, 2014, and 2013, respectively, representing 14 % and 5% of total revenues for fiscal years ended June 30, 2014 and 2013, respectively. There were no product sales of our MicroCutter XCHANGE 30 in fiscal year ended June 30, 2012, as it was introduced in December 2012.
United States
We have launched the MicroCutter XCHANGE 30 to a limited number of targeted clinical sites in the United States. We plan to learn from these sites the time and training required to achieve routine clinical adoption of the MicroCutter XCHANGE 30. To support this strategy, we started with a small group of direct sales representatives that have extensive backgrounds in stapling products and laparoscopic procedures and existing relationships with key surgeons and decision makers.
We will base a broader launch of the MicroCutter XCHANGE 30 on our experience from this limited product introduction. Over subsequent quarters, our plan is to add additional sales representatives in new geographic markets. As part of our controlled commercial launch, we made our first commercial sale of the MicroCutter XCHANGE 30 to a hospital in the United States in March 2014, following our FDA 510(k) clearance of the MicroCutter XCHANGE 30 blue and white cartridges in January 2014 and February 2014, respectively. Total U.S. product sales of our MicroCutter XCHANGE 30 systems since the March 2014 introduction through June 30, 2014, was $0.2 million, representing 6% of total revenue, for the fiscal year ended June 30, 2014.
International
As we are able to apply the CE Mark to additional products in our microcutter product line and are able to gain more adoption of our products, we plan to introduce these additional products to a limited number of targeted clinical sites, similar to our December 2012 introduction of our MicroCutter XCHANGE 30 in Europe. We signed a distribution agreement with Century with respect to distribution of our planned microcutter products in Japan. Century will be responsible for securing regulatory approval from the Ministry of Health in Japan. After approval for marketing in Japan, we would sell microcutter units to Century, who would then sell the microcutter devices to their customers in Japan. In August 2013, Century filed for regulatory approval of our MicroCutter XCHANGE 30 cartridges with the Pharmaceuticals and Medical Devices Agency in Japan and, upon approval, anticipates launching our MicroCutter XCHANGE 30 in Japan. We also recently submitted our MicroCutter XCHANGE 30 blue and white cartridges application to Health Canada for regulatory approval of our MicroCutter XCHANGE 30 and, if we receive approval, anticipate launching it in Canada.
Total international product sales of our MicroCutter XCHANGE 30 systems was $0.3 million, representing 8% of total revenue, for the fiscal year ended June 30, 2014.
MicroCutter Competition
The MicroCutter XCHANGE 30 competes, and the MicroCutter XCHANGE 45 and other planned products in the microcutter product line if they receive regulatory clearance and are successfully launched would compete, in the market for stapling and cutting devices against laparoscopic stapling and sealing devices currently marketed around the world. We believe the principal competitive factors in the market for laparoscopic staplers include:
|
• |
reduced product size; |
|
• |
ease of use; |
|
• |
product quality and reliability; |
|
• |
device cost-effectiveness; |
|
• |
degree of articulation; |
|
• |
surgeon relationships; |
|
• |
sales and marketing capabilities; and |
|
• |
multi-fire capability. |
Two large competitors, Ethicon Endo-Surgery, part of Johnson & Johnson, and Covidien currently control more than 80% of this market. Other large competitors in the laparoscopic device market include Stryker Endoscopy and Olympus, which acquired another competitor, Gyrus Medical. Ethicon Endo-Surgery and Covidien, which acquired a small competitor, Power Medical, each have large direct sales forces in the United States and have been the largest participants in the market for single use disposable laparoscopic stapling devices for many years. Competing against large established competitors with significant resources may make establishing a market for any products that we develop difficult and the failure to establish a market for our products would have a material adverse effect on our business. A private company, JustRight Surgical, LLC, is developing smaller surgical instruments and has announced FDA 510(k) clearance for a 5 millimeter stapler that could be considered competitive with our stapling products, but is more limited in availability of staple sizes and articulation compared to the MicroCutter XCHANGE 30. Further, we may also face additional competition from generic surgical stapling products similar to currently commercially available products following expiration of patents on our competitors’ products.
Cardiac Industry Background
Coronary Artery Disease
According to the American Heart Association, approximately 17.6 million people in the United States have coronary artery disease, and approximately 425,400 people in the United States die each year as a result of the disease. Coronary artery disease, sometimes referred to as atherosclerosis, is a degenerative disease resulting from the deposit of cholesterol and other fatty materials on the interior walls of blood vessels, forming a build-up known as plaque. The accumulation of plaque, usually over decades, causes the vessel to become inelastic and progressively narrows the interior of the artery, impairing its ability to supply blood and oxygen to the heart muscle. When there is insufficient blood flow to the heart muscle, an injury may occur, often resulting in chest pain, or angina, a heart attack or even death. Coronary artery disease is caused by aging and is exacerbated by dietary and environmental factors, as well as by genetic predisposition. As patient ages, the disease will typically advance and become more diffuse, compromising the coronary artery system more globally and occluding more small-diameter vessels.
Current Treatment Alternatives for Coronary Artery Disease
Physicians and patients may select among a variety of treatments to address coronary artery disease, with the selection often depending upon the stage and severity of the disease and the age of the patient. In addition to changes in patient lifestyle, such as smoking cessation, weight reduction, diet changes and exercise programs, the principal existing treatments for coronary artery disease include the following:
Medical Treatment with Pharmaceuticals
Before the advent of interventional cardiology or bypass surgery, medical treatment with pharmaceuticals was the only form of therapy available to patients with coronary artery disease. In patients with less severe disease, pharmaceuticals remain the primary treatment approach and include drugs such as platelet adhesion inhibitors or drugs that reduce the blood cholesterol or triglyceride levels. The objective for medical treatment with pharmaceutical agents is to reduce the incidence, progression or exacerbation of coronary artery disease and its associated symptoms. For more serious disease, however, pharmacological therapy alone is often inadequate.
Interventional Cardiology Techniques
Coronary Angioplasty. Percutaneous transluminal coronary angioplasty, commonly referred to as balloon angioplasty, is a surgical procedure that involves the dilation of the obstructed artery with a balloon catheter. To perform an angioplasty, the surgeon maneuvers a flexible balloon catheter to the site of the blockage in the coronary artery, inflates the balloon, compressing the plaque and stretching the artery wall to create a larger channel for blood flow. The balloon is then deflated and removed. Angioplasty is generally successful in increasing immediate blood flow and, relative to current surgical procedures, offers the benefits of shorter periods of hospitalization, quicker recovery times, reduced patient discomfort and lower cost. However, angioplasty does not always provide prolonged efficacy: independent studies indicate that 25% to 40% of vessels treated with balloon angioplasty return to their pre-treatment, narrowed size, a process known as restenosis, within six months following the procedure. Restenosis is primarily the result of cell proliferation in response to the “injury” caused by the angioplasty procedure.
Stents. High rates of restenosis following treatment by balloon angioplasty led to the introduction of stents, mesh-like metallic tubes that are placed within the narrowed portion of the coronary vessel to hold the vessel open after the angioplasty balloon has been removed. Although clinical outcomes for procedures using stents reflect an improvement over balloon angioplasty alone, the effectiveness of stents is still limited by restenosis, which for bare metal stents occurs in about 10% to 35% of cases within six months of the procedure.
Some manufacturers have introduced drug-eluting stents, which incorporate, on the surface of the stent, specially formulated, slow-release drugs designed to prevent restenosis. According to published studies, currently marketed drug-eluting stents have been shown in clinical trials to reduce the rate of restenosis, within the first nine months after placement, to less than 10%. Market adoption of drug-eluting stents has been rapid, and industry observers had predicted that drug-eluting stents would capture approximately 90% of the stent market within three years. However, some studies have been presented that associate drug eluting stents with late stage thrombosis, or clotting, which can be an adverse event. Drug eluting stents are still widely used, with a current market share relative to total stent usage in the range of 70-80%.
Despite the advancements and market success of drug-eluting stents and angioplasty therapies, these interventional procedures may be less effective than coronary artery bypass grafting, or CABG, procedures in addressing diffuse progressive coronary artery disease. In this advanced stage of coronary artery disease, intervention is required for multiple vessels, many of which are less than two millimeters in internal diameter, a diameter currently unsuited for angioplasty and stenting. In addition, stents have been shown to be difficult to place in patients with coronary lesions in sections with vessel branches and in patients with narrowings in the left main coronary artery.
Bypass Surgery. CABG involves the construction of an alternative path to bypass a narrowed or occluded coronary artery and restore blood flow from the aorta to an area past the occlusion. This procedure can be accomplished using either veins or arteries as bypass grafts. Veins are typically harvested from the patient’s leg (saphenous vein), while arteries are taken from either the patient’s arm (radial artery) or chest wall (mammary artery). For vein grafts and radial arteries, one end of the harvested vessel is then generally attached to the aorta for blood inflow, and the opposite end is attached to the target coronary vessel. If a mammary artery is used as the bypass graft, it must be dissected from the chest wall, leaving one end in place, while the opposite end is attached to the target vessel, providing uninterrupted blood flow from the arterial circulation. Once in place, these grafts provide sufficient blood flow to bypass the narrowed or occluded portion of the coronary artery. (See Figure Below).
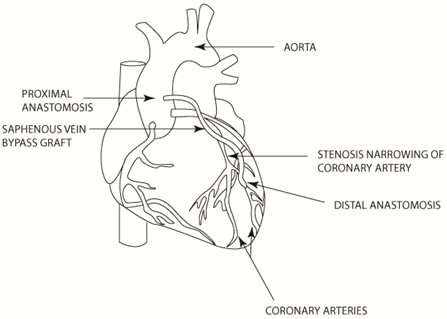
Although CABG surgery is generally a highly invasive and even traumatic procedure, an independent study comparing CABG and implantation of conventional stents has shown that CABG is the more effective treatment for coronary artery disease, achieving the best long-term patient outcomes as measured by survival rate and need for intervention. Studies have shown that following CABG, grafts can remain patent, or open, and functional for as long as 10 years in approximately 50% of venous grafts and approximately 90% of arterial grafts. In addition, CABG procedures can be used to treat diffuse, end-stage coronary artery disease states that are often not amenable to treatment by angioplasty or stents.
According to an independent analysis by Medtech Insight, a division of Windhover Information, entitled “U.S. Surgical Procedure Volumes,” dated February 2007, an estimated 257,000 CABG procedures were performed in 2007 in the United States, as compared to approximately 260,000 procedures in 2006. We believe that the decrease in CABG procedures is primarily attributable to the increase in other interventional cardiology procedures, including the increased use of drug-eluting stents. The average CABG surgery requires approximately three bypass grafts per patient, and a majority of grafts require an anastomotic connection at both ends of the graft. Assuming an average of approximately five anastomoses per CABG procedure, we estimate that approximately 1.8 million of these blood vessel connections are performed in connection with CABG procedures annually in the United States. We believe approximately two-thirds of the procedures are performed using veins as the bypass graft. A similar number of CABG procedures with similar grafting frequency are performed outside of the US.
Types of CABG Procedures
There are currently three types of CABG procedures, two of which are commonly performed:
Conventional On-Pump CABG Procedures. Conventional on-pump CABG procedures are particularly invasive and traumatic to the patient, typically requiring the surgeon to open the patient’s chest cavity by splitting the sternum and to place the patient on a pump to circulate the blood throughout the body. Redirecting the blood flow to a pump enables the surgeon to clamp the aorta and stop the heart, which results in a motionless and bloodless field in which the surgeon can perform the difficult and tedious task of manually suturing the small vessels to one another. The absence of blood flow and motion are important factors in ensuring precision and providing positive clinical outcomes; however, the use of a pump for circulation exposes the patient’s blood to foreign surfaces, which has been shown to increase the incidence of bleeding and short-term neurocognitive defects. Additionally, stopping the heart may result in impairment or damage to the heart muscle. Moreover, clamping of the aorta has been shown in clinical studies to cause the release of particles into the blood stream that may produce blockages in other parts of the body, such as the brain. Blockages in the brain can lead to neurological damage, including strokes. Clamping the aorta also carries the risk of injury to the vessel wall with later bleeding complications. Notwithstanding these potential problems, the majority of CABG procedures performed today use this on-pump technique.
Off-Pump CABG Procedures. In 1995, a method of performing CABG procedures was introduced that avoids the use of external pumps, requiring the surgeon to perform the anastomosis while the heart is beating. The clinical literature suggests that this procedure, termed off-pump coronary artery bypass, or OPCAB, offers several benefits as compared to on-pump CABG procedures, including reductions in bleeding, kidney dysfunction, short-term neurocognitive dysfunction and length of hospital stay. OPCAB procedures currently represent approximately 20% to 25% of all CABG procedures performed in the United States.
Notwithstanding these advantages, the technical challenges inherent in OPCAB have impeded its widespread adoption. Because the patient’s heart is beating during the procedure, the surgeon is required to perform the delicate anastomosis on a target vessel, which could be as small as one millimeter in internal diameter, while the vessel is moving with each heart contraction. The technical demands of the procedure, together with the longer learning curve required to achieve surgical proficiency, may also initially adversely affect long-term graft patency and completion of revascularization. In addition, surgeons will still typically be required to place a partially occluding clamp on the ascending aorta to hand suture the proximal vein graft anastomosis. As a result, even in OPCAB procedures, patients still face the risk of the serious adverse effects associated with the application of aortic clamps.
Minimally Invasive Endoscopic Procedures. Recently, a very small number of CABG procedures, referred to as totally endoscopic coronary artery bypass, or TECAB, have been performed using minimally invasive endoscopic procedures to reduce patient trauma. These TECAB procedures typically involve the use of Intuitive Surgical’s da Vinci surgical robot system. In this approach, the sternum is left intact and the surgery is performed through small access ports. The anastomoses are performed on selected, readily reachable vessels using special surgical instruments or the da Vinci robot system, and this procedure requires special surgical skills. Although endoscopic procedures offer the promise of faster post-operative patient recovery times, rapid ambulation, long-term graft patency and a low incidence of adverse outcomes, in the past there were a number of challenges to wide-scale realization of that potential, including the absence of a method to enable surgeons to perform reproducible and effective anastomoses that can be rapidly deployed through small incisions. While many patients may be eligible for minimally invasive endoscopic techniques, the TECAB procedures are currently performed in less than 1% of all CABG patients.
Surgical Techniques for Anastomoses
The current method of performing anastomoses, which surgeons generally view as the most critical aspect of CABG procedures, typically employs tedious and time-consuming hand-sewn placement of individual stitches with a continuous suture to connect the bypass graft to the aorta or coronary vessels. Conventional anastomosis can require ten to 25 minutes to suture, depending upon the size and disease state of the vessels. Proper vessel alignment and suture tension among the many individually placed fine stitches are critical for optimal bypass graft blood flow and function. Furthermore, long-term clinical outcomes may be improved if the anastomosis is “compliant,” that is, if its shape and size can adapt to changes in flow and blood pressure by placement of many single sutures rather than one continuous suture. However, most surgeons prefer the use of a continuous suture because placement of individual sutures may be more technically challenging and time-consuming. Whether the surgeon elects to operate on the patient on- or off-pump, a hand-sewn proximal anastomosis generally requires clamping of the aorta and therefore carries with it the risk of neurological damage and other serious adverse effects. Recently, new technology has been introduced that allows the surgeon to perform hand-sewn proximal anastomoses to the aorta without clamping of the aorta. These facilitating devices temporarily cover the opening in the aortic wall from the inside while the surgeon places the stitches to create the anastomosis and are removed after the anastomosis has been completed to allow blood flow into the bypass graft. We believe these systems, in their current implementations, are not suitable for endoscopic bypass surgery.
The laborious and time-consuming nature of manually applied sutures and the limitations associated with their use, together with advances occurring in coronary surgical procedures, have fueled the need for easy-to-use, fast and highly reliable automated systems to expedite and standardize the performance of anastomoses in CABG procedures. A number of companies have attempted to develop automated systems to perform anastomoses, to date, we believe Cardica is one of those companies with FDA clearance to market distal and proximal anastomosis devices in the United States, and only one other non-automated system for use in performing a proximal anastomosis is currently commercially available in the United States.
Our Cardiac Solutions
We design, manufacture and market proprietary automated anastomotic systems used by surgeons to perform anastomoses during on- or off-pump CABG procedures. We believe that by enabling consistent and reliable anastomoses of the vessels at this most critical step in CABG surgery through a fast, automated process, our products can improve the quality and consistency of these anastomoses, which we believe will ultimately contribute to improved patient outcomes. We have designed our products to meet the needs of surgeons, including:
|
• |
Physiological features. Our clips use medical grade stainless steel that is identical to that used in conventional coronary stents, which is known to be compatible with the human body (in the absence of allergies to certain components of medical grade stainless steel). Our products minimize trauma to both the graft and target vessel during loading and deployment, thereby reducing the risk of scar formation and associated narrowings or occlusions. Additionally, our PAS-Port system can be used without clamping the aorta, which has been shown to be a cause of adverse events, including neurological complications. In addition, our C-Port system creates compliant anastomoses, which potentially allow the shape and size of the anastomosis to adapt to changes in flow and blood pressure. |
|
• |
Handling features. Our anastomotic systems can create anastomoses more rapidly than hand suturing, resulting in a surgical procedure that can be performed more quickly. For example the PAS-Port system can be set-up and deployed in approximately three minutes compared with approximately ten to 25 minutes for a hand-sewn anastomosis. In addition, the system is easy to use, typically requiring only a few hours of training to become technically proficient in the technique. The C-Port system is compatible with coronary arteries as small as 1.3 millimeters in internal diameter, which is typically the lower limit of target vessels considered to be candidates for revascularization. The C-Port system can also be deployed at various angles, allowing access to all coronary targets during both on- and off-pump procedures. Both the C-Port system and the PAS-Port system are designed as integrated products, where all steps necessary to create an anastomosis are performed by a single tool, with one user interface. The need for target vessel preparation is minimal for the PAS-Port system, a feature that is especially important in patients undergoing a second or third coronary bypass procedure with the presence of significant scarring in and around the heart and aorta. |
|
• |
Standardized results. Our products enable consistent, reproducible anastomoses, largely independent of surgical technique and skill set, using a wide range in quality of graft tissues. In comparison with hand-sewn sutures, our systems offer mechanically-governed repeatability and reduced procedural complexity. |
|
• |
Reduced costs. Because our products can help to expedite the CABG procedure, we believe that they may contribute to reduced operating room time and a reduction in associated expenses, partially offset by the increased direct cost of our products compared to current alternatives, such as sutures. Additionally, our C-Port system creates anastomoses rapidly and does not require the interruption of blood flow. This may reduce some of the technical challenges inherent in performing anastomosis in off-pump procedures, which may advance adoption of the off-pump approach. By helping more surgeons perform off-pump CABG, the need for a costly pump may also be reduced or eliminated, thereby potentially reducing the total direct costs of the procedure. The C-Port Flex A allows the surgeon to perform coronary revascularization through small openings in the chest wall, thereby reducing the trauma and morbidity associated with the CABG procedure, which therefore may help reduce costs by reducing the time to patient discharge. Finally, to the extent complications such as strokes or injury to the heart muscle decrease, post-operative costs of a CABG procedure may be significantly reduced. |
Our Cardiac Products
We currently market three proprietary products to perform anastomoses, the C-Port xA system, the C-Port Flex A system and the PAS-Port system. The C-Port systems automate a distal anastomosis between the graft vessel and target artery. The C-Port xA system was developed to use veins and arteries as the bypass graft vessel and received 510(k) clearance in November 2006. A new generation of the C-Port xA system, the C-Port Flex A system, designed to further enable minimally invasive CABG surgery, received 510(k) clearance in March 2007. Each of our C-Port systems has received the CE Mark for sales in Europe. As of June 30, 2014, we had sold an aggregate of nearly 14,300 units of all the versions of our C-Port systems. The PAS-Port system automates the performance of a proximal anastomosis between a graft vessel, typically a saphenous vein, and the aorta. The PAS-Port system received 510(k) clearance in September 2008 following successful completion of a prospective, international, randomized study. Our PAS-Port system also has received the CE Mark. The PAS-Port system is marketed in the United States, Europe and Japan. As of June 30, 2014, over 37,000 PAS-Port systems had been sold, primarily in Japan and the United States.
C-Port® Distal Anastomosis Systems
C-Port® xA Anastomosis System
Our C-Port xA Distal Anastomosis System, which may be used in either on- or off-pump CABG procedures, is designed to perform an end-to-side distal anastomosis by attaching the end of a bypass graft to a coronary artery downstream of an occlusion or narrowing. The C-Port xA system is inserted in a small incision in the coronary artery with a bypass graft vessel attached to the device. The C-Port xA system is actuated by depressing a trigger which activates a manifold powered by a cylinder of compressed carbon dioxide to provide smooth actuation. Miniature stainless steel staples are deployed to securely attach the bypass graft to the coronary artery and at the same time a miniature knife completes an opening inside the coronary artery to complete the bypass. After deployment, the C-Port system is removed from the coronary artery and the entry incision is closed typically with a single stitch. Our C-Port xA system is effective in creating compliant anastomoses in vessels as small as 1.3 millimeters in internal diameter. In addition, the C-Port xA system has been designed to:
|
• |
perform an end-to-side anastomosis without interruption of native coronary blood flow, which is not possible in a conventional hand-sewn anastomosis during off-pump surgery without the use of a temporarily placed vascular shunt; |
|
• |
achieve nearly complete alignment of the natural blood lining surfaces of the coronary artery and the bypass graft to minimize scarring and potential occlusion of the anastomosis; |
|
• |
minimize the amount of foreign material in the blood stream that may cause clotting and subsequent graft failure; and |
|
• |
be suitable for all grafts typically used in CABG procedures with wall thicknesses of less than or equal to 1.4 millimeters. |
C-Port® Flex A Anastomosis System
The C-Port Flex A system includes modifications to the C-Port xA system that are designed to enable automated anastomoses to be performed as part of minimally invasive and robot-facilitated CABG procedures. The C-Port Flex A system includes all of the features and benefits of the C-Port xA system and has a flexible, rather than rigid, shaft. The flexible shaft is designed to allow the working end of the device that creates the anastomosis to be inserted through a 14-millimeter diameter port to access the chest cavity and heart. The device is designed to be loaded with the bypass graft vessel inside or outside the chest cavity and deployed to create the anastomosis to the coronary artery. This product is designed to enable technology for completion of robotically assisted, including endoscopic, CABG surgery through four or five relatively small incisions between the ribs. Avoiding both the incision through the sternum and the use of the pump should significantly reduce patient trauma and accelerate post-operative recovery.
PAS-Port® Proximal Anastomosis System
Our PAS-Port system is a fully automated device used to perform an end-to-side proximal anastomosis between a saphenous vein and the aorta. To complete a proximal anastomosis, the cardiac surgeon simply loads the bypass graft vessel into the PAS-Port system, places the end of the delivery device against the aorta and turns the knob on the opposite end of the delivery tool. The device first creates an opening in the aorta and subsequently securely attaches the bypass graft to the aortic wall, using a medical grade stainless steel implant that is formed into its final shape by the delivery tool. The innovative design of the PAS-Port system allows the surgeon to load the bypass graft and rapidly complete the anastomosis, typically in approximately three minutes, with little or no injury to the bypass graft vessel or the aorta.
An important advantage of our PAS-Port system is that, in contrast to conventional hand-sewn proximal anastomoses, the vascular connections created can be performed without clamping the aorta, potentially avoiding associated risks, such as neurological complications. Surgeons use our PAS-Port system in conventional CABG procedures and in OPCAB. Similar to hand-sewn anastomosis, anastomoses completed using our PAS-Port system occasionally require additional stitches intra-operatively to obtain hemostasis (absence of bleeding in the anastomosis site). These additional stitches may be required intra-operatively in an individual anastomosis depending on the quality of the target and graft vessels, adequacy of target site preparation and quality of the loading of the graft to the deployment cartridge.
Cardiac Product Sales and Marketing
United States
Our cardiac products focus on the needs of cardiovascular surgeons worldwide. We have a four person direct sales force, augmented by a network of independent medical device manufacturers’ representatives and distributors to sell our products domestically. We utilize manufacturers’ representatives and distributors who carry other cardiac surgery products, are clinically knowledgeable and are capable of training cardiac surgeons on the use of our products and proctoring initial cases in the operating room.
International
We currently distribute our PAS-Port system in Japan through our exclusive distributor, Century, pursuant to a distribution agreement entered into in June 2003, which has been subsequently amended. The latest amendment, effective July 1, 2014, among other things, extended the term of the distribution agreement for another five years, extending the expiration date to July 31, 2019.
For the fiscal years ended June 30, 2014, 2013 and 2012, sales to Century accounted for approximately 29%, 29% and 29%, respectively, of our total revenue and approximately 30%, 33% and 32%, respectively, of our product sales. As of June 30, 2014, Century had trained over 700 Japanese cardiac surgeons in over 350 hospitals. Century has a direct sales organization of approximately 26 representatives who are responsible for the development of the anastomotic device market and directly contact cardiac surgeons. Century provides clinical training and support for end-users in Japan. We provide Century with promotional support, ongoing clinical training, representation at trade shows and guidance in Century’s sales and marketing efforts. Our agreement with Century pertaining to the PAS-Port system, as amended, expires in July 2019, but automatically renews for an additional five-year term if Century meets certain sales milestones. Either party may terminate this agreement if the other party defaults in performance of material obligations and such default is not cured within a specified period or if the other party becomes insolvent or subject to bankruptcy proceedings. In addition, we may terminate the agreement within 90 days following a change of control by payment of a specified termination fee.
For the fiscal years ended June 30, 2014, 2013 and 2012, sales to Herz-Und Diabeteszentrum in Germany, accounted for approximately 12%, 7% and 7%, respectively, of our total revenue and approximately 12%, 8% and 8%, respectively, of our product sales.
Total product sales of our C-Port and PAS-Port systems were $3.0 million, $2.9 million and $3.3 million, for fiscal years ended June 30, 2014, 2013 and 2012, respectively. Total product sales of our C-Port and PAS-Port systems represented 83%, 83% and 89% of total revenues for fiscal years ended June 30, 2014, 2013 and 2012, respectively.
We are continuing to sell to selected international customers and will continue to evaluate further opportunities to expand our distribution network in Europe and in other parts of the world where the healthcare economics are conducive to the introduction and adoption of new medical device technologies.
Cardiac Product Competition
The market for medical devices used in the treatment of coronary artery disease is intensely competitive, subject to rapid change, and significantly affected by new product introductions and other market activities of industry participants. We believe the principal competitive factors in the market for medical devices used in the treatment of coronary artery disease include:
|
• |
improved patient outcomes; |
|
• |
access to and acceptance by leading physicians; |
|
• |
product quality and reliability; |
|
• |
ease of use; |
|
• |
device cost-effectiveness; |
|
• |
training and support; |
|
• |
novelty; |
|
• |
physician relationships; and |
|
• |
sales and marketing capabilities. |
There are numerous potential competitors in the medical device, biotechnology and pharmaceutical industries, such as Maquet Cardiovascular LLC, formerly the cardiac surgery division of Boston Scientific Corporation, Edwards Lifesciences Corporation, Johnson & Johnson, Inc., Abbott Laboratories, which acquired an additional division of Guidant Corporation, Medtronic, Inc. and St. Jude Medical, that are targeting the treatment of coronary artery disease broadly. Each of these companies has significantly greater financial, clinical, manufacturing, marketing, distribution and technical resources and experience than we have. In addition, new companies have been, and are likely to continue to be, formed to pursue opportunities in our market.
The landscape of active competitors in the market for anastomotic solutions is currently limited. Several companies market systems designed to facilitate or stabilize proximal anastomoses, such as Maquet Cardiovascular’s Heartstring Aortic Occluder and Novare Surgical Systems’ Enclose anastomotic assist device. St. Jude Medical previously had a commercially available proximal anastomotic system that was marketed both in the United States and Europe; however, St. Jude Medical voluntarily withdrew this product from the market in 2004. Johnson & Johnson obtained FDA clearance for a proximal system that was developed by Bypass Inc. but has divested the division that was originally responsible for selling this product, and this proximal anastomosis product is now not available for cardiac surgeons in the United States or abroad. Our PAS-Port system is the only commercially available automated proximal anastomosis device.
Our C-Port systems are the only automated anastomosis devices for distal anastomosis cleared for marketing in the United States. The only currently marketed facilitating device for distal anastomosis is the U-Clip, which substitutes clips for sutures, but still requires manual application of typically 12 to 14 individually placed clips per anastomosis by the surgeon.
Currently, the vast majority of anastomoses are performed with sutures and, for the foreseeable future, sutures will continue to be the principal competitor for alternative anastomotic solutions. The direct cost of sutures used for anastomoses in CABG procedures is far less expensive than the direct cost of automated anastomotic systems, and surgeons, who have been using sutures for their entire careers, have been reluctant to consider alternative technologies, despite potential advantages.
In addition, cardiovascular diseases may also be treated by other methods that do not require anastomoses, including interventional techniques such as balloon angioplasty and use of drug-eluting stents, pharmaceuticals, atherectomy catheters and lasers. Further, technological advances with other therapies for cardiovascular disease such as drugs, local gene therapy or future innovations in cardiac surgery techniques could make other methods of treating this disease safer, more effective or less expensive than CABG procedures.
Manufacturing
Our manufacturing operations, sterile products manufacturing, assembly, packaging, storage and shipping, as well as our research and development laboratories and administrative activities all take place at our headquarters facility. Our lease expires on August 31, 2015, with the option to extend for a period of two years beyond the expiration date. We believe that our current facilities will be sufficient to meet our manufacturing needs for at least the next few years.
We believe our manufacturing operations are in compliance with regulations mandated by the FDA and the European Union. Our facility is International Standards Organization, or ISO, 13485:2003 certified. In connection with our CE mark approval and compliance with European quality standards, our facility was initially certified in June 2002 and has been inspected annually thereafter.
There are a number of critical components and sub-assemblies required for manufacturing the microcutter product line and C-Port and PAS-Port systems that we purchase from third-party suppliers. The vendors for these materials are qualified through stringent evaluation and monitoring of their performance over time. We audit our critical component manufacturers on a regular basis and at varied intervals based on the nature and complexity of the components they provide and the risk associated with the components’ failure.
We use or rely upon sole source suppliers for certain components and services used in manufacturing our products, and we utilize materials and components supplied by third parties, with whom we do not have any long-term contracts. In recent years, many suppliers have ceased supplying materials for use in implantable medical devices. We cannot quickly establish additional or replacement suppliers for certain components or materials, due to both the complex nature of the manufacturing processes employed by our suppliers and the time and effort that may be required to obtain FDA clearance or other regulatory approval to use materials from alternative suppliers. Any significant supply interruption or capacity constraints affecting our facilities or those of our suppliers would affect our ability to manufacture and distribute our products.
Third-Party Reimbursement
Sales of medical products are increasingly dependent in part on the availability of reimbursement from third-party payors such as government and private insurance plans. In the United States, as well as in foreign countries, government-funded or private insurance programs, commonly known as third-party payors, pay the cost of a significant portion of a patient’s medical expenses. Successful sales of our products will depend on the availability of adequate reimbursement from third-party payors. No uniform policy of coverage or reimbursement for medical technology exists among all these payors. Therefore, coverage and reimbursement can differ significantly from payor to payor.
Hospitals and other healthcare providers that purchase medical devices, such as the ones that we manufacture, rely on third-party payors to pay for all or part of the costs and fees associated with the procedures performed with these devices. The existence of adequate reimbursement for the procedures performed with our MicroCutter and cardiac surgery products by government and private insurance plans are central to acceptance of our current and future products. We may be unable to sell our products on a profitable basis if third-party payors deny coverage or reduce their current levels of payment, or if our costs of production increase faster than increases in reimbursement levels.
Many private payors use coverage decisions and payment amounts determined by the Centers for Medicare and Medicaid Services, or CMS, which administers the Medicare program, as guidelines in setting their reimbursement policies. Future action by CMS or other government agencies may diminish payments to physicians, outpatient centers and hospitals. Those private payors that do not follow the Medicare guidelines may adopt different reimbursement policies for procedures performed with our products. For some governmental programs, such as Medicaid, reimbursement differs from state to state, and some state Medicaid programs may not pay for the procedures performed with our products in an adequate amount, if at all.
Once a device has received approval or clearance for marketing by the FDA, there is no assurance that Medicare will cover the device and related services. In some cases, CMS may place certain restrictions on the circumstances in which coverage will be available. In making such coverage determinations, CMS considers, among other things, peer-reviewed publications concerning the effectiveness of the technology, the opinions of medical specialty societies, input from the FDA, the National Institutes of Health, and other government agencies. We cannot assure you that our microcutter products and/or our cardiac surgery products will be covered by Medicare and other third-party payors. Limited coverage of our products could have a material adverse effect on our business, financial condition and results of operations.
In general, Medicare makes a predetermined, fixed payment amount for its beneficiaries receiving covered inpatient services in acute care hospitals. This payment methodology is part of the inpatient prospective payment system, or IPPS. For acute care hospitals, under IPPS, payment for an inpatient stay is based on diagnosis-related groups, or DRGs, which include reimbursement for all covered medical services and medical products that are provided during a hospital stay. Additionally, a relative weight is calculated for each individual DRG which represents the average resources required to care for cases in that particular DRG relative to the average resources required to treat cases in all DRGs. Generally, DRG relative weights are adjusted annually to reflect changes in medical practice in a budget neutral manner.
CMS has made no decisions with respect to DRG assignment when patients undergo thoracic, bariatric, colorectal, general or CABG procedures in which our microcutter or cardiac surgery products would be used, and there can be no assurance that the DRG to which such patients will be assigned will result in Medicare payment levels that are considered by hospitals to be adequate to support purchase of our products.
As is the case with other endoscopic stapling devices available in the U.S. today, we do not anticipate that our microcutter products will be reimbursed separately by third-party payors. Our cardiac surgery technologies bring added direct costs to medical providers and may not be reimbursed separately by third-party payors at rates sufficient to allow us to sell our products on a competitive and profitable basis. Many private payors look to CMS in setting their reimbursement policies and payment amounts. If CMS or other agencies limit coverage and decrease or limit reimbursement payments for hospitals and physicians, this may affect coverage and reimbursement determinations by many private payors.
Coverage and reimbursement therefore depend on our ability to demonstrate the short-term and long-term clinical and cost-effectiveness of our products from the results we obtain from clinical experience and formal clinical studies. We have not collected, and are not aware that others have collected, long-term data regarding efficacy, safety and clinical outcomes associated with the use of our microcutter products.
For classification of physician services, the American Medical Association, referred to as the AMA, has developed a coding system known as the Current Procedural Terminology, or CPT. CPT codes are established by the AMA and adopted by the Medicare program in the Healthcare Common Procedure Coding System, to describe and develop payment amounts for physician services. Physician services are reimbursed by Medicare based on a physician fee schedule whereby payment is based generally on the number of “relative value units” assigned by CMS to the service furnished by the physician. No decision has been made concerning whether existing CPT codes would be appropriate for use in coding thoracic, bariatric, colorectal, general or CABG procedures when our products are used or if new CPT codes and payment are required. We cannot assure you that codes used for submitting claims for procedures using our products will result in incremental payment to physicians. CPT codes are used by many other third-party payors in addition to Medicare. Failure by physicians to receive what they consider to be adequate reimbursement for procedures in which our products are used could have a material adverse effect on our business, financial condition and results of operations.
Our international success will depend upon the availability of reimbursement within prevailing foreign healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country and include both government-sponsored healthcare and private insurance.
All third-party reimbursement programs, whether government funded or insured commercially, whether inside the United States or outside, are developing increasingly sophisticated methods of controlling healthcare costs through prospective reimbursement and capitation programs, group purchasing, redesign of benefits, second opinions required prior to major surgery, careful review of bills, encouragement of healthier lifestyles and exploration of more cost-effective methods of delivering healthcare. These types of programs and legislative changes to reimbursement policies could potentially limit the amount which healthcare providers may be willing to pay for medical devices.
As the portion of the United States population over age 65 and eligible for Medicare continues to grow we may be more vulnerable to reimbursement limitations imposed by CMS. Furthermore, the healthcare industry in the United States has experienced a trend toward cost containment as government and private insurers seek to control healthcare costs by imposing lower payment rates and negotiating reduced contract rates with service providers. Therefore, we cannot be certain that the procedures performed with our products will be adequately reimbursed.
Research and Development
As of June 30, 2014, we had 23 employees in our research and development department. Future research and development efforts will involve development of the microcutter in a variety of formats that accommodate different staple sizes and staple line lengths and different tool form factors, such as flexible versus rigid shafts, cartridges with a curved plastic tip at the distal end to facilitate surgeon vision and access for vascular surgical procedures, and a shorter shaft to facilitate certain surgeries. We are also exploring the development of other products that can be derived from our core technology platform and intellectual property. Research and development expenses for fiscal years ended June 30, 2014, 2013 and 2012 were $6.9 million, $9.1 million and $7.2 million, respectively. We expect research and development expenses to decrease slightly in absolute dollar terms in fiscal year 2015 as we have completed the clinical trial, product testing and the tooling expenses relating to the enhancements of the MicroCutter XCHANGE 30.
Patents and Intellectual Property
We believe our competitive position will depend significantly upon our ability to protect our intellectual property. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our technology, inventions and improvements that are important to the development of our business. As of June 30, 2014, we had 133 issued U.S. patents, of which 33 are related to our microcutter products, 67 additional U.S. patent applications, of which 49 are related to our microcutter products, twelve issued foreign patents, of which three are related to our microcutter products, and another 25 patent applications filed in select international markets, of which 23 are related to our microcutter products. Our issued patents expire between 2018 and 2032, with the issued patents related to our microcutter products expiring between 2027 and 2032.
We also rely upon trade secrets, technical know-how and continuing technological innovation to develop and maintain our competitive position. We typically require our employees, consultants and advisors to execute confidentiality and assignment of inventions agreements in connection with their employment, consulting or advisory relationships with us. There can be no assurance, however, that these agreements will not be breached or that we will have adequate remedies for any breach. Furthermore, no assurance can be given that competitors will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our proprietary technology, or that we can meaningfully protect our rights in unpatented proprietary technology.
Patent applications in the United States and in foreign countries are maintained in secrecy for a period of time after filing, which results in a delay between the actual discoveries and the filing of related patent applications and the time when discoveries are published in scientific and patent literature. Patents issued and patent applications filed relating to medical devices are numerous, and there can be no assurance that current and potential competitors and other third parties have not filed or in the future will not file applications for, or have not received or in the future will not receive, patents or obtain additional proprietary rights relating to products, devices or processes used or proposed to be used by us. We are aware of patents issued to third parties that contain subject matter related to our technology. We believe that the technologies we employ in our products and systems do not infringe the valid claims of any such patents. There can be no assurance, however, that third parties will not seek to assert that our devices and systems infringe their patents or seek to expand their patent claims to cover aspects of our products and systems.
The medical device industry, in general, and the industry segment that includes products for the treatment of cardiovascular disease in particular, has been characterized by substantial litigation regarding patents and other intellectual property rights. Any such claims, regardless of their merit, could be time-consuming and expensive to respond to and could divert our technical and management personnel. We may be involved in litigation to defend against claims of infringement by other patent holders, to enforce patents issued to us, or to protect our trade secrets. If any relevant claims of third-party patents are upheld as valid and enforceable in any litigation or administrative proceeding, we could be prevented from practicing the subject matter claimed in such patents, or would be required to obtain licenses from the patent owners of each such patent, or to redesign our products, devices or processes to avoid infringement. There can be no assurance that such licenses would be available or, if available, would be available on terms acceptable to us or that we would be successful in any attempt to redesign our products or processes to avoid infringement. Accordingly, an adverse determination in a judicial or administrative proceeding or failure to obtain necessary licenses could prevent us from manufacturing and selling our products, which would have a material adverse effect on our business, financial condition and results of operations. We intend to vigorously protect and defend our intellectual property. Costly and time-consuming litigation brought by us may be necessary to enforce patents issued to us, to protect trade secrets or know-how owned by us or to determine the enforceability, scope and validity of the proprietary rights of others. See “Risk Factors.”
Government Regulation
The FDA and other regulatory bodies extensively regulate the research, development, manufacture, labeling, distribution, import/export, sales and marketing of our products. Our current products are regulated by the FDA as medical devices, and we are required to obtain review and clearance or approval from the FDA prior to commercializing our devices in the United States.
FDA regulations govern nearly all of the activities that we perform, or that are performed on our behalf, to ensure that medical products distributed domestically or exported internationally are safe and effective for their intended uses. The activities that the FDA regulates include the following:
|
• |
product design, development and manufacture; |
|
• |
product safety, testing, labeling and storage; |
|
• |
pre-clinical testing in animals and in the laboratory; |
|
• |
clinical investigations in humans; |
|
• |
marketing applications, such as 510(k) notifications and Premarket Approval, or PMA, applications; |
|
• |
record keeping and document retention procedures; |
|
• |
advertising and promotion; |
|
• |
product marketing, distribution and recalls; and |
|
• |
post-marketing surveillance and medical device reporting, including reporting of deaths, serious injuries, device malfunctions or other adverse events. |
FDA’s Premarket Clearance and Approval Requirements. Unless an exemption applies, each medical device distributed commercially in the United States will require either prior 510(k) clearance or PMA from the FDA. The FDA classifies medical devices into one of three classes. Class I devices are subject to only general controls, such as establishment registration and device listing, labeling, medical device reporting, and prohibitions against adulteration and misbranding. Class II medical devices generally require prior 510(k) clearance before they may be commercially marketed in the United States. The FDA will clear marketing of a medical device through the 510(k) process if the FDA is satisfied that the new product has been demonstrated to be substantially equivalent to another legally marketed device, or predicate, device, and otherwise meets the FDA’s requirements. Class II devices are also subject to general controls and may be subject to performance standards and other special controls. Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a predicate device, are placed in Class III, generally requiring submission of a PMA supported by clinical trial data.
510(k) Clearance Pathway. To obtain 510(k) clearance, we must submit a notification to the FDA demonstrating that our proposed device is substantially equivalent to a predicate device, i.e., a device that was in commercial distribution before May 28, 1976, a device that has been reclassified from Class III to Class I or Class II, or a 510(k)-cleared device. The FDA’s 510(k) clearance process generally takes from three to twelve months from the date the application is submitted, but can take significantly longer. If the FDA determines that the device, or its intended use, is not substantially equivalent to a previously-cleared device or use, the device is automatically placed into Class III, requiring the submission of a PMA. Any modification to a 510(k)-cleared device that would constitute a major change in its intended use, design or manufacture, requires a new 510(k) clearance and may even, in some circumstances, require a PMA, if the change raises complex or novel scientific issues. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) submission in the first instance, but the FDA may review any manufacturer’s decision. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or recall the device until 510(k) clearance or PMA is obtained. If the FDA requires us to seek 510(k) clearance or PMAs for any modifications, we may be required to cease marketing and/or recall the modified device, if already in distribution, until 510(k) clearance or PMA is obtained and we could be subject to significant regulatory fines or penalties. The FDA has undertaken a systematic review of the 510(k) clearance process that included both internal and independent recommendations for reform of the 510(k) system. The internal review has resulted in a series of recommendations that the FDA is currently acting on, and in July 2011, the Institute of Medicine, or IOM issued its independent recommendations for 510(k) reform. Ultimately and as the FDA reconciles its plan of action to respond to both the internal and IOM recommendations and public comments on both, the availability of the 510(k) pathway for our product candidates and the timing and data burden required to obtain 510(k) clearance could be adversely impacted. Furthermore, our products could be subject to voluntary recall if we or the FDA determines, for any reason, that our products pose a risk of injury or are otherwise defective. Moreover, the FDA can order a mandatory recall if there is a reasonable probability that our device would cause serious adverse health consequences or death. Delays in receipt or failure to receive clearances or approvals, the loss of previously received clearances or approvals, or the failure to comply with existing or future regulatory requirements could reduce our sales, profitability and future growth prospects.
Premarket Approval Pathway. A PMA must be submitted to the FDA if the device cannot be cleared through the 510(k) process. The PMA process is much more demanding than the 510(k) notification process. A PMA must be supported by extensive data, including but not limited to data obtained from preclinical or clinical studies or relating to manufacturing and labeling to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device.
After a PMA submission is complete, the FDA begins an in-depth review, which generally takes between one and three years, but may take significantly longer. During this review period, the FDA will typically request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will conduct a pre-approval inspection of the manufacturing facility to ensure compliance with Quality System Regulation, or QSR. New PMA applications or PMA supplements are required for significant modifications to the device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA application and may not require as extensive clinical data or the convening of an advisory panel.
Clinical Trials. Clinical trials are generally required to support a PMA application and are sometimes required for 510(k) clearance. To perform a clinical trial in the United States for a significant risk device, prior submission of an application for an Investigational Device Exemption, or IDE, to the FDA is required. An IDE amendment must also be submitted before initiating a new clinical study under an existing IDE, such as initiating a pivotal trial following the conclusion of a feasibility trial. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, and any available data on human clinical experience, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The animal and laboratory testing must meet the FDA’s good laboratory practice requirements.
The IDE and any IDE supplement for a new trial must be approved in advance by the FDA for a specific number of patients. Clinical trials conducted in the United States for significant risk devices may not begin until the IDE application or IDE supplement is approved by the FDA and the appropriate institutional review boards, or IRBs, overseeing the welfare of the research subjects and responsible for that particular clinical trial. If the product is considered a non-significant risk device under FDA regulations, only the patients’ informed consent and IRB approval are required. Under its regulations, the agency responds to an IDE or an IDE amendment for a new trial within 30 days. The FDA may approve the IDE or amendment, grant an approval with certain conditions, or identify deficiencies and request additional information. It is common for the FDA to require additional information before approving an IDE or amendment for a new trial, and thus final FDA approval on a submission may require more than the initial 30 days. The FDA may also require that a small-scale feasibility study be conducted before a pivotal trial may commence. In a feasibility trial, the FDA limits the number of patients, sites and investigators that may participate. Feasibility trials are typically structured to obtain information on safety and to help determine how large a pivotal trial should be to obtain statistically significant results.
Clinical trials are subject to extensive recordkeeping and reporting requirements. Our clinical trials must be conducted under the oversight of an IRB for the relevant clinical trial sites and must comply with FDA regulations, including but not limited to those relating to good clinical practices. We are also required to obtain the patients’ informed consent in form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. We, the FDA or the IRB may suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA approval to market the product in the United States. Similarly, in Europe the clinical study must be approved by a local ethics committee and in some cases, including studies with high-risk devices, by the ministry of health in the applicable country.
We received 510(k) clearance from the FDA in January 2014, for use of the MicroCutter XCHANGE 30 and blue cartridge, and for the white cartridge in February 2014, for use in multiple open or minimally-invasive surgical procedures for the transection, resection and/or creation of anastomoses in small and large intestine, as well as the transection of the appendix. The blue cartridge is for use in medium thickness tissue, and the white cartridge is for use in thin tissue. The MicroCutter XCHANGE 30, now that it has received 510(k) clearance, could serve as the predicate device for subsequent iterations and product line extensions.
Any products or product enhancements that we develop that require regulatory clearance, including enhancements to the MicroCutter XCHANGE 30, may not be cleared on the timelines that we currently anticipate, if cleared at all. Any new products or any product enhancements that we develop may not be subject to the shorter 510(k) clearance process, but may instead be subject to the more lengthy PMA requirements.
Pervasive and Continuing Regulation. There are numerous regulatory requirements governing the approval and marketing of a product. These include:
|
• |
product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; |
|
• |
QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
|
• |
labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication; |
|
• |
clearance or approval of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use; |
|
• |
medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to an adverse event, a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; |
|
• |
post-approval restrictions or conditions, including post-approval study commitments; |
|
• |
post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; and |
|
• |
notices of correction or removal and recall regulations. |
Advertising and promotion of medical devices are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Promotional activities for FDA-regulated products have been the subject of significant enforcement actions brought under healthcare reimbursement laws, “fraud and abuse” laws (such as those prohibiting kickbacks and false claims, discussed below), and consumer protection statutes, among other theories. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims.
We have registered with the FDA as a medical device manufacturer. The FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by the FDA to determine our compliance with the QSR, and other regulations, and these inspections may include the manufacturing facilities of our suppliers.
Failure by us or by our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or state authorities, which may include any of the following sanctions:
|
• |
warning letters, fines, injunctions, consent decrees and civil penalties; |
|
• |
customer notifications, repair, replacement, refunds, recall or seizure of our products; |
|
• |
operating restrictions, partial suspension or total shutdown of production; |
|
• |
delay in processing marketing applications for new products or modifications to existing products; |
|
• |
mandatory product recalls; |
|
• |
withdrawing approvals that have already been granted; and |
|
• |
criminal prosecution. |
Fraud and Abuse and False Claims. We are directly and indirectly subject to various federal and state laws governing our relationship with healthcare providers and pertaining to healthcare fraud and abuse, including anti-kickback laws. In particular, the federal healthcare program Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending a good or service, for which payment may be made in whole or part under federal healthcare programs, such as the Medicare and Medicaid programs. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. Where such activities involve foreign government officials, they may also potentially be subject to the Foreign Corrupt Practices Act. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. In implementing the statute, the Office of Inspector General of the U.S. Department of Health and Services, or OIG, has issued a series of regulations, known as the “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable element of a safe harbor may result in increased scrutiny by government enforcement authorities, such as the OIG.
The Federal False Claims Act imposes civil liability on any person or entity who submits, or causes the submission of a false or fraudulent claim to the United States Government. Damages under the Federal False Claims Act can be significant and consist of the imposition of fines and penalties. Under certain circumstances, the Federal False Claims Act also allows a private individual or entity with knowledge of past or present fraud on the federal government to sue on behalf of the government to recover the civil penalties and up to treble damages. The U.S. Department of Justice on behalf of the government has successfully enforced the Federal False Claims Act against medical device manufacturers. Federal suits have alleged that pharmaceutical manufacturers whose marketing and promotional practices were found to have included the off-label promotion and/or the payment of prohibited kickbacks to doctors violated the Federal False Claims Act on the grounds that these prohibited activities resulted in the submission of claims to federal and state healthcare entitlement programs such as Medicaid, resulting in the payment of claims for the off-label use that was not otherwise covered. Such manufacturers have entered into settlements with the federal government under which they paid amounts and entered into corporate integrity agreements that require, among other things, substantial reporting and remedial actions.
State authorities may likewise seek to enforce the False Claims Act (and/or the state equivalents) against medical device manufacturers.
We believe that our marketing practices are not in violation of the laws mentioned above or their state equivalents, but we cannot assure you that individuals or enforcement authorities will not attempt to take action against us and, if such action were successful, we could be required to pay significant fines and penalties and change our marketing practices. Such enforcement could have a significant adverse effect on our ability to operate.
We engage in a variety of activities that are potentially regulated under these laws, including, for example, consulting arrangements with cardiothoracic surgeons, grants for training and other education, grants for research, and other interactions with doctors. Failure to comply with applicable legal requirements could potentially result in substantial penalties to us and significant adverse effect on our ability to operate. Even if we structure our programs with the intent of compliance with such laws, there can be no certainty that we would not need to defend against enforcement or litigation, in light of the fact that there is significant enforcement interest in medical device manufacturers in the United States, and some of the applicable laws are quite broad in scope.
We may also be subject to various federal and state marketing expenditure tracking and reporting laws, such as the federal Physician Payments Sunshine Act, which generally require certain types of expenditures in the United States to be tracked and reported. Several states have enacted legislation requiring pharmaceutical and medical device companies to establish marketing compliance programs. Compliance with such requirements may require investment in infrastructure to ensure that tracking is performed properly, and some of these laws result in the public disclosure of various types of payments and relationships.
International Regulation. International sales of medical devices are subject to foreign governmental regulations, which vary substantially from country to country. The time required to obtain certification or approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ.
The primary regulatory body in Europe is the European Union, or EU, which has adopted numerous directives and has promulgated voluntary standards regulating the design, manufacture and labeling of and clinical trials and adverse event reporting for medical devices. Devices that comply with the requirements of a relevant directive will be entitled to bear CE conformity marking, indicating that the device conforms with the essential requirements of the applicable directives and, accordingly, can be commercially distributed throughout the member states of the European Union and other countries that comply with or mirror these directives. The method for assessing conformity varies depending upon the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a notified body, which is an independent and neutral institution appointed by a country to conduct the conformity assessment. This third-party assessment may consist of an audit of the manufacturer’s quality system and specific testing of the manufacturer’s device. Such an assessment is required for a manufacturer to commercially distribute the product throughout these countries. ISO 9001 and ISO 13845 certifications are voluntary standards. Compliance establishes the presumption of conformity with the essential requirements for the CE Mark. We have the authorization to affix the CE Mark to the PAS-Port and C-Port devices and to commercialize the devices in the European Union for coronary artery bypass grafting. We have received CE Mark certification for the MicroCutter XPRESS 30 and MicroCutter XCHANGE 30, in July 2011, and March 2012, respectively, and we expect to be able to apply the CE Mark to future devices within the microcutter product line that comply with the certified design and manufacturing processes in the same manner.
In Japan, medical devices must be approved prior to importation and commercial sale by the Ministry of Health, Labor and Welfare, or MHLW. Manufacturers of medical devices outside of Japan are required to utilize a contractually bound In-Country Caretaker, or ICC, to submit an application for device approval to the MHLW. The MHLW evaluates each device for safety and efficacy. As part of its approval process, the MHLW may require that the product be tested in Japanese laboratories. The approval process for products such as our existing anastomotic products is typically 13 to 14 months. Other medical devices may require a longer review period for approval. Once approved, the manufacturer may import the device into Japan for sale by the manufacturer’s contractually bound importer or distributor.
After a device is approved for importation and commercial sale in Japan, the MHLW continues to monitor sales of approved products for compliance with labeling regulations, which prohibit promotion of devices for unapproved uses and reporting regulations and reporting of product malfunctions, including serious injury or death caused by any approved device. Failure to comply with applicable regulatory requirements can result in enforcement action by the MHLW, which may include fines, injunctions, and civil penalties, recall or seizure of our products, operating restrictions, partial suspension or total shutdown of sales in Japan, or criminal prosecution.
We have received approval from the MHLW to distribute our PAS-Port system in Japan. We will be required to submit applications with respect to all new products and product enhancements for review and approval by the MHLW. Our contract with Century, our distributor in Japan, has a multi-year term and is renewable for additional multi-year terms upon mutual agreement of the parties.
In addition to MHLW oversight, the regulation of medical devices in Japan is also governed by the Japanese Pharmaceutical Affairs Law, or PAL. Under PAL, manufacturers outside of Japan must now appoint a “primary distributor” located in Japan that holds a primary distributor license for medical devices to provide primary distribution services, including conducting quality assurance and safety control tasks for each product at the time an application for the approval of each such product is submitted to the MHLW. Century serves as the “primary distributor” for Cardica. We do not anticipate that these changes will have a material impact on our existing level of third-party reimbursement for sales of our products in Japan. Century filed for regulatory approval in August 2013, of our MicroCutter XCHANGE 30 cartridges with the Pharmaceuticals and Medical Devices Agency in Japan and anticipates launching our MicroCutter XCHANGE 30 in Japan.
In Canada, medical devices are regulated by the Therapeutic Products Directorate of Health Canada (“TPD”) and are licensed for sale through submission to the TPD. The timeline for approval is similar to that of the FDA’s 510(k) process. As of January 2003, all new and existing class II, III and IV Medical Device Licenses (“MDL”) in Canada also require a valid International Organization for Standardization (ISO), 13485 or ISO 13488 Quality System Certificate from a registrar recognized by the Canadian Medical Devices Conformity Assessment System (“CMDCAS”). We are currently pursuing ISO 13485:2003 certification and had recently submitted our MicroCutter XCHANGE 30 blue and white cartridges application to Health Canada for regulatory approval of our MicroCutter XCHANGE 30, and if we receive approval, anticipate launching it in Canada.
Employees
As of June 30, 2014, we had 69 employees, including 20 employees in manufacturing, 9 employees in sales and marketing, 9 employees in clinical, regulatory and quality assurance, 8 employees in general and administrative and 23 employees in research and development. We believe that our future success will depend upon our continued ability to attract, hire and retain qualified personnel. None of our employees is represented by a labor union or party to a collective bargaining agreement, and we believe our employee relations are good.
Financial Information
Information regarding our revenues from external customers, our net loss and total assets is contained in the financial statements included in this report, which information is incorporated by reference here. For the specifics of our revenue by geographic location and long-lived assets, please see Note 1, Concentrations of Credit Risk and Certain Other Risks and Impairment of Long-Lived Assets, in our Notes to Financial Statements.
Corporate Information
We were incorporated in Delaware in October 1997 as Vascular Innovations, Inc. and changed our name to Cardica, Inc. in November 2001. Our principal executive offices are located at 900 Saginaw Drive, Redwood City, California 94063 and our telephone number is (650) 364-9975. We file annual reports, quarterly reports, current reports, proxy statements and amendments to such filings with the Securities and Exchange Commission, or SEC. We make these filings available, free of charge, on our website as soon as practicable after such material is electronically filed with the SEC. Our website address is www.cardica.com and the reports are filed under “SEC Filings”, on the Investors/Media portion of our website. You may read and copy any materials we file with the SEC the SEC's Public Reference Room at 100 F Street, NE., Washington, DC 20549, on official business days during the hours of 10 a.m. to 3 p.m. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, which is http://www.sec.gov.
Executive Officers of the Registrant
The following table sets forth certain information concerning our executive officers as of August 31, 2014:
|
Name |
Age |
Position |
|
Bernard A. Hausen, M.D., Ph.D. |
54 |
President, Chief Executive Officer, Chief Medical Officer and Director |
|
Robert Y. Newell |
66 |
Vice President, Finance and Chief Financial Officer |
|
Frederick M. Bauer |
60 |
Vice President, Operations |
|
Liam J. Burns |
48 |
Vice President, Sales and Marketing |
|
Bryan D. Knodel, Ph.D. |
54 |
Vice President, Research and Development |
Bernard A. Hausen, M.D., Ph.D. has been our President and Chief Executive Officer since December 2000. Dr. Hausen co-founded Cardica in October 1997 and has served as a director and our Chief Medical Officer since inception. Dr. Hausen received a medical degree from Hannover Medical School in Germany in 1988 and was trained there as a general and cardiothoracic surgeon. Upon completion of his training, he received a Ph.D. degree in Medical Physiology in 1999. From 1996 to 2000, he was employed as a Senior Research Scientist in the Laboratory for Transplantation Immunology of the Department of Cardiothoracic Surgery at Stanford University. Until Dr. Hausen became our full-time employee in October of 2000, he remained responsible for all surgery-related research in that laboratory.
Robert Y. Newell has been our Vice President, Finance and Chief Financial Officer since March 2003 and was Vice President, Finance and Operations, from July 2005 to July 2008. From January 2000 to February 2003 he was Vice President, Finance and Chief Financial Officer for Omnicell, Inc., a hospital supply and medication management company. Mr. Newell holds a B.A. degree in Mathematics from the College of William & Mary and an M.B.A. degree from the Harvard Business School. He currently serves as a member of the Board of Directors of ARI Network Services, Inc., a public software as a service (SaaS) company.
Frederick M. Bauer joined Cardica as our Vice President of Operations in July 2008. From August 2005 to June 2008, he was President and Owner of 3RLatex, LLC, a containment, transportation and recycling company for the construction industry and from November 2002 to November 2005, he was general manager of Amazon Environmental, a latex paint recycling company. From October 1996 to November 2001, he was Vice President Operations for the Cardiac Surgery division and Vice President Operations for the Perfusion Systems division of Medtronic, Inc., a medical device company. He also held a number of operations and engineering executive positions with Baxter Healthcare International, a healthcare company, from 1981 to 1996. He currently serves as a member of the board of the Orange County ARC, a non-profit servicing 800 developmentally disabled adults. Mr. Bauer holds a B.S. degree in Civil Engineering from the University of Detroit Mercy.
Liam J. Burns joined Cardica as our Vice President, Sales and Marketing in January 2014. Since September 2007, he has been President of EP Burns Group LLC, a healthcare and life science focused sales, marketing and leadership development consulting company that he founded, at which he was responsible for all facets of the business. From September 2006 to August 2007, he was Vice President Marketing of Power Medical Interventions, Inc. a surgical stapling company. From October 1991 to August 2006, he held various sales and marketing management positions with Ethicon, Inc., a Johnson & Johnson company. Mr. Burns holds a B.A. degree from the College of Holy Cross and an M.B.A. degree from Case Western Reserve University.
Bryan D. Knodel, Ph.D. joined Cardica as our Vice President of Research and Development in July 2005. Since January 1998, he has been president of Bryan D. Knodel, Inc., a consulting firm specializing in medical device design and product development. From April 2001 until June 2005, Dr. Knodel consulted for us in product development. From 1992 to 1997, he was a principal engineer with Ethicon Endo-Surgery, a Johnson & Johnson company developing medical devices for less invasive surgery. Dr. Knodel holds B.S., M.S. and Ph.D. degrees in Mechanical Engineering from the University of Illinois.
Item 1A. Risk Factors
We have identified the following risks and uncertainties that may have a material adverse effect on our business, financial condition or results of operations. The risks described below are not the only ones we face. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations.
Risks Related to Our Finances and Capital Requirements
We have a history of net losses, which we expect to continue for the foreseeable future, and we are unable to predict the extent of future losses or when we will become profitable, if at all.
We have incurred annual net losses since our inception in October 1997. As of June 30, 2014, our accumulated deficit was approximately $170.5 million. We expect to incur substantial additional losses until we can achieve significant commercial sales of our products, which depend upon a number of factors, including increased commercial sales of our C-Port and PAS-Port systems, as well as our commercially launched MicroCutter XCHANGE 30 in Europe and in the United States, market adoption of our planned microcutter products in Europe and receipt of regulatory clearance or approval, commercial launch and market adoption of our planned microcutter products in the United States.
Our ability to become and remain profitable depends upon our ability to generate significantly higher product sales. Our ability to generate significant and sustained revenue depends upon a number of factors, including:
|
• |
achievement of broad acceptance for our current products or future products that we may commercialize including the MicroCutter XCHANGE 30 in the United States; |
|
• |
achievement of international and U.S. regulatory clearance or approval for additional products; and |
|
• |
successful sales, manufacturing, marketing and distribution of our products. |
We have generated revenues primarily from the sale of automated anastomotic systems, and have generated minimal revenues from the commercial sales of the MicroCutter XCHANGE 30 in Europe and recently in the United States. Sales of our C-Port and PAS-Port systems have not met the levels that we had anticipated, and to date our systems have had limited commercial adoption. Sales of our products, license and development and royalties activities generated revenues of $3.6 million, $3.5 million and $3.7 million for fiscal years ended June 30, 2014, 2013 and 2012, respectively. We do not anticipate that we will generate significantly higher product sales in the next few quarters.
Our cost of product sales was 136%, 117% and 111% of our net product sales for the fiscal years ended June 30, 2014, 2013 and 2012, respectively. We expect higher cost relative to product sales for the foreseeable future due to costs associated with commercializing our microcutter product line. If, over the long term, we are unable to reduce our cost of producing goods and expenses relative to our net revenue, we will not achieve profitability even if we are able to generate significant product sales. Our failure to achieve and sustain profitability would negatively impact the market price of our common stock.
Existing lenders may have rights to our assets that are senior to our stockholders.
An existing debt arrangement with our current distributor and lender Century under which, as of June 30, 2014, $4.0 million of principal is outstanding, as well as potential future arrangements with other lenders, allow or may allow these lenders to have priority over our stockholders to our assets, including our intellectual property should we be in default of our obligations to the lenders. The proceeds of any sale or liquidation of our assets under these circumstances would be applied first to any of our debt obligations.
Our quarterly operating results and stock price may fluctuate significantly.
We expect our operating results to be subject to quarterly fluctuations. The revenue we generate, if any, and our operating results will be affected by numerous factors, many of which are beyond our control, including:
|
● |
the trading volume of our stock; |
|
● |
the extent to which we are able to raise additional capital in any equity or debt transaction; |
|
● |
market acceptance of our MicroCutter XCHANGE 30 in Europe and the United States; |
|
● |
market acceptance of our MicroCutter XCHANGE 30 cartridge in Japan if regulatory approval from the Pharmaceuticals and Medical Devices Agency of our MicroCutter XCHANGE 30 cartridge in Japan is obtained; |
|
● |
the extent of our ongoing enhancements of the MicroCutter XCHANGE 30, including alterations and post-commercialization improvements based on early adopter experience with this newly commercial product; |
|
• |
the extent of our ongoing research and development programs and related costs, including costs related to the continued development of the MicroCutter XCHANGE 45 and additional products and features in our microcutter product line; |
|
• |
our ability to enter into additional license, development and/or collaboration agreements with respect to our technology, and the terms thereof; |
|
• |
our level of revenues; |
|
• |
costs associated with our sales and marketing initiatives and manufacturing activities; |
|
• |
costs and timing of obtaining and maintaining FDA and other regulatory clearances and approvals for our products and potential additional products; |
|
• |
securing, maintaining and enforcing intellectual property rights and the costs thereof; and |
|
• |
the effects of competing technological and market developments. |
Quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially.
Risks Related to Our Business
We are dependent upon the commercial success of our MicroCutter XCHANGE 30 in Europe and in the United States which, if not successful, could prevent us from successfully commercializing our other microcutter products.
We have expended significant time, money and effort in the development of our microcutter product line and, in particular, our MicroCutter XCHANGE 30. We commercially launched the MicroCutter XCHANGE 30 in Europe in December 2012, and in the United States in March 2014. We only received U.S. regulatory approval of the MicroCutter XCHANGE 30 with blue staple cartridge in January 2014, and for the white staple cartridge in February 2014. If we are not successful in achieving market adoption of the MicroCutter XCHANGE 30 in Europe and the United States, we may never generate substantial revenue from this product line, and our business, financial condition and results of operations would be materially and adversely affected, and we may be forced to cease operations. Although we generated our first commercial revenue from the MicroCutter XCHANGE 30 in December 2012, we expect that we will need to continue to make enhancements and improvements to the MicroCutter XCHANGE 30 as early adopters in Europe continue to use the MicroCutter XCHANGE 30. We anticipate that our ability to increase our revenue significantly will depend on the continued adoption of the MicroCutter XCHANGE 30 in Europe, and adoption of the MicroCutter XCHANGE 30 in the United States.
A number of factors will influence our ability to gain clinical adoption of the MicroCutter XCHANGE 30:
|
● |
in many surgical specialties, the use of laparoscopic and open surgical stapling devices is routine in clinical practice and an accepted standard of care. Two large companies, Johnson & Johnson and Covidien, dominate the market for surgical stapling devices. For our products to be clinically adopted, they must show benefits that are significant enough for surgeons to communicate their preference and to overcome any constraints on their hospitals’ ability to purchase competing products, such as purchasing contracts, to buy one of our stapling products to replace a competing device; |
|
● |
our microcutter products must demonstrate the degree of reliability that surgeons have experienced with products that they have been using for years; |
|
● |
market acceptance of our products also depends on our ability to demonstrate consistent quality and safety of our products; |
|
● |
if physicians are not able to use our microcutter products properly, or use them on tissue thicknesses for which they are not designed, adoption of our microcutter products may be negatively impacted; |
|
● |
any recalls may impact physicians’ and hospitals’ perception of our products; |
|
● |
we will need to demonstrate the cost-effectiveness of our products, including against branded, patent protected products, as well as any generic stapling products similar to currently commercially available products following expiration of patents on our competitors’ products; |
|
● |
our ability to reduce our costs of manufacturing the MicroCutter XCHANGE 30; |
|
● |
our ability to increase our sales force; and |
|
● |
our ability to address the need for improvements in response to feedback from physicians, if any. |
We cannot predict when, if ever, we will generate significant commercial revenue from the sale of the MicroCutter XCHANGE 30 or any other potential future products or anticipated features in our microcutter product line. If we fail to achieve significant growth in market adoption of the MicroCutter XCHANGE 30, our ability to develop our other planned microcutter products, if at all, will be delayed, which would further harm our business.
We are dependent upon the success of our C-Port and PAS-Port systems to generate revenue in the near term, and sales of our C-Port and PAS-Port systems have not met the levels that we had anticipated and if we are unable to increase sales of our C-Port and PAS-Port systems, our business will be harmed.
We have expended significant time, money and effort in the development of our current commercial products used by cardiac surgeons to perform coronary bypass surgery, the C-Port and the PAS-Port systems. We commenced sales of our C-Port xA system in December 2006 (after introduction of our original C-Port system in January 2006) and our C-Port Flex A in April 2007. We commenced U.S. sales of our PAS-Port system in September 2008. To date, our anastomosis products have not gained, and we cannot assure you that our anastomosis products or any other products that we may develop will gain, any significant degree of market acceptance among physicians or patients. We believe that recommendations by physicians will be essential for market acceptance of our products; however, we cannot assure you that significant recommendations will be obtained. Physicians will not recommend our products unless they conclude, based on clinical data and other factors, that the products represent a safe and acceptable alternative to other available options. In particular, physicians may elect not to recommend using our anastomosis products in surgical procedures until such time, if ever, as we successfully demonstrate with long-term data that our products result in patency rates comparable to or better than those achieved with hand-sewn anastomoses, and we resolve any technical limitations that may arise. Further, if physicians have negative experiences with our anastomosis products in surgical procedures, whether due to the fault of our anastomosis products or the physician, the adoption of these products could be negatively impacted.
To date we have generated revenues almost exclusively from the sale of automated anastomotic systems, and have generated minimal revenues from the commercial sales of the MicroCutter XCHANGE 30 since its December 2012 introduction in Europe and recently in the United States, following our FDA clearances for the MicroCutter XCHANGE 30 with blue staple cartridge in January 2014, and for the white staple cartridge in February 2014. If we are not successful in increasing commercial adoption of our C-Port and PAS-Port systems, we may never generate substantial revenue, our business, financial condition and results of operations would be materially and adversely affected, and we may be forced to cease operations.
The limitations on the indications of use for the MicroCutter XCHANGE 30 will limit our promotional activities, which could inhibit our success in commercializing the MicroCutter XCHANGE 30 and could expose us to potential off-label risks, including fines, penalties or injunctions if we are determined to be promoting the use of our products for unapproved or “off-label” uses.
Our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition of the promotion of the off-label use of our products. Healthcare providers may use our products off-label, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. However, if the FDA determines that our promotional materials or training constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties. Although our policy is to refrain from statements that could be considered off-label promotion of our products, the FDA or another regulatory agency could disagree and conclude that we have engaged in off-label promotion. In addition, the off-label use of our products may increase the risk of product liability claims. Product liability claims are expensive to defend and could result in substantial damage awards against us and harm our reputation.
We have limited data regarding the safety and efficacy of our microcutter products. Any data that is generated in the future may not be positive or consistent with our existing data, which would affect market acceptance and the rate at which our microcutter products are adopted.
The success of our microcutter products depends on their acceptance by the surgical community as safe and effective. Even if the data collected from future clinical studies or clinical experience indicates positive results, each surgeon’s actual experience with our devices outside the clinical study setting may vary. Clinical studies conducted with our initial microcutter products may involve procedures performed by thoracic, bariatric, colorectal and general surgeons who are technically proficient, high-volume surgeons. Consequently, both short- and long-term results reported in these studies may be significantly more favorable than typical results of practicing surgeons, which could negatively impact rates of adoption of the microcutter if launched. In addition, any adverse experiences of surgeons using the microcutter products, or adverse outcomes to patients, may deter surgeons from using our products and negatively impact product adoption.
If the FDA determines that our C-Port systems or PAS-Port systems do not perform as anticipated, or if the FDA identifies new concerns related to the safety and effectiveness of these products, we may be required to withdraw these products, which could harm our business.
As a condition of its U.S market clearance, the C-Port system is subject to a mandatory Post Market Surveillance order under Section 522 of the Federal Food Drug and Cosmetic Act (which we refer to as the 522 order) to demonstrate graft patency outcomes and technical failure rate in a clinical study. Should the FDA decide that the C-Port system does not perform as anticipated, or if the FDA identifies new concerns related to the safety and effectiveness of the product, or if the FDA determines that the requirements of the 522 order are otherwise unmet, we may be required to withdraw the C-Port system from the market and may be subject to other enforcement action, which could harm our business.
Our C-Port and PAS-Port systems were designed for use with venous grafts. In addition, we have studied the use of the C-Port systems with venous grafts and arterial grafts. Using the C-Port systems with arterial grafts may not yield patency rates or material adverse cardiac event rates comparable to those found in our clinical trials using venous grafts, which could negatively affect market acceptance of our C-Port systems. In addition, the clips and staples deployed by our products are made of 316L medical-grade stainless steel, to which some patients are allergic. These allergies, especially if not previously diagnosed or unknown, may result in adverse reactions that negatively affect the patency of the anastomoses or the healing of the implants and may therefore adversely affect outcomes, particularly when compared to anastomoses performed with other materials, such as sutures. Additionally, in the event a surgeon, during the course of surgery, determines that it is necessary to convert to a hand-sewn anastomosis and to remove an anastomosis created by one of our products, the removal of the implants may result in more damage to the target vessel (such as the aorta or coronary artery) than would typically be encountered during removal of a hand-sewn anastomosis. Moreover, the removal may damage the target vessel to an extent that could further complicate construction of a replacement hand-sewn or automated anastomosis, which could be detrimental to patient outcome. These or other issues, if experienced, could limit physician adoption of our products.
Even if the data collected from future clinical studies or clinical experience indicates positive results, each physician’s actual experience with our devices outside the clinical study setting may vary. Clinical studies conducted with the C-Port and PAS-Port systems have involved procedures performed by physicians who are technically proficient, high-volume users of the C-Port and PAS-Port systems. Consequently, both short- and long-term results reported in these studies may be significantly more favorable than typical results of practicing physicians, which could negatively impact rates of adoption of the C-Port and PAS-Port systems.
If we are unable to establish sales and marketing capabilities or enter into and maintain arrangements with third parties to market and sell our products, our business may be harmed.
We have limited experience as a company in the sale, marketing and distribution of our products. To commercialize the MicroCutter XCHANGE 30 in the United States, we will have to build a sales force, which we are only beginning to do. Century is responsible for marketing and commercialization of cardiac and microcutter products in Japan. To promote our current and future products in the United States, Canada and Europe, we must develop our sales, marketing and distribution capabilities or make arrangements with third parties to perform these services. Competition for qualified sales personnel is intense. Developing a sales force is expensive and time consuming and could delay any product launch. We may be unable to establish and manage an effective sales force in a timely or cost-effective manner, if at all, and any sales force we do establish may not be capable of generating sufficient demand for our products. We have entered into arrangements with third parties to perform sales and marketing services, which may result in lower product sales than if we directly marketed and sold our products. We expect to rely on third-party distributors for substantially all of our international sales. If we are unable to establish adequate sales and marketing capabilities, independently or with others, we may not be able to generate significant revenue and may not become profitable.
Our products require training to use, and if physicians are not willing to undergo that training, or if they undergo the training but do not use our products properly, or for other reasons, our products may not gain any significant degree of market acceptance, and a lack of market acceptance would have a material adverse effect on our business.
Widespread use of our products will require the training of numerous physicians, and the time required to complete training could result in a delay or dampening of market acceptance. Even if the safety and efficacy of our products is established, physicians may use our products improperly due to unfamiliarity with the products, or may use the MicroCutter XCHANGE 30 on tissues with thicknesses greater than the specifications for the MicroCutter XCHANGE 30. If this were to happen, the MicroCutter XCHANGE 30 may not function as desired for the physicians and could be reported as a problem with the MicroCutter XCHANGE 30 rather than the physicians using it improperly, which could damage the reputation of the MicroCutter XCHANGE 30 and cause other physicians to consider the MicroCutter XCHANGE 30 to be not a safe product. Further, physicians may elect not to use our products for a number of other reasons beyond our control, including inadequate or no reimbursement from health care payors, physicians’ reluctance to use products that have not been proven through time in the market, the introduction of competing devices by our competitors and pricing for our products. Failure of our products to achieve any significant market acceptance would have a material adverse effect on our business, financial condition and results of operations.
We may not be successful in our efforts to improve and expand our product portfolio, and our failure to do so could cause our business and prospects to suffer.
While we continue to make modifications and add features to our Microcutter XCHANGE 30, we have suspended development of other potential products in our planned microcutter product line other than the MicroCutter XCHANGE 45 until the development and commercialization of the MicroCutter XCHANGE 45 have been completed. Significant additional research and development and financial resources will be required to continue the development of the MicroCutter XCHANGE 45 and other products in this planned product line into commercially viable products and to obtain necessary regulatory clearances to commercialize the devices. We cannot assure you that our development efforts will be successful or that they will be completed within our publicly stated anticipated timelines, and we may never be successful in developing a viable product for the markets intended to be addressed by the MicroCutter XCHANGE 45 or other potential microcutter products. Further, even if we do successfully develop any of these microcutter products, we may not be successful in commercializing them for any number of reasons, including failure or delays in obtaining regulatory clearances, or if surgeons do not perceive the benefits of these products to be significantly greater than current established products. We may also face additional competition from branded, patent-protected products, as well as generic stapling products similar to currently commercially available products following expiration of patents on our competitors’ products, which could create greater price competition and decrease the revenue potential of our microcutter products. Our failure to successfully develop the MicroCutter XCHANGE 45 and/or other microcutter products and improvements to our current product would have a material adverse effect on our business, growth prospects and ability to raise additional capital.
Healthcare reform measures could hinder or prevent the commercial success of our products.
The pricing and reimbursement environment may change in the future and become more challenging as a result of any of one several possible regulatory developments, including policies advanced by the United States government, new healthcare legislation or fiscal challenges faced by government health administration authorities. The U.S. government has shown significant interest in pursuing healthcare “reform” and reducing healthcare costs. For example, aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, were implemented starting in 2013. Any government-adopted reform measures that decrease the amount of reimbursement available from governmental and other third-party payers, and could potentially adversely affect our business.
Our PAS-Port and C-Port systems, our MicroCutter XCHANGE 30, and future products may face future development and regulatory difficulties and limitations on use.
Even though the current generations of the C-Port and PAS-Port systems have received U.S. regulatory clearance, the FDA may still impose significant restrictions on the indicated uses or marketing of these products or ongoing requirements for potentially costly post-clearance studies. The FDA permits commercial distribution of most new medical devices only after the device has received 510(k) clearance or is the subject of an approved PMA. Any of our future products, including planned products in our microcutter product line and any future generations of the C-Port and PAS-Port systems, may not obtain regulatory clearances required for marketing or may face these types of restrictions or requirements, particularly as the FDA is considering revising its 510(k) clearance system to, in certain cases, require human clinical data and to prohibit the combination of multiple predicate devices as the basis for a 510(k).
The process of obtaining regulatory clearances or approvals to market a medical device, particularly from the FDA, can be costly and time consuming, and there can be no assurance that such clearances or approvals will be granted on a timely basis, if at all. We rely substantially on the premarket notification process for FDA clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act. This provision allows many medical devices to avoid human clinical trials if the product is “substantially equivalent” to another device already on the market. Premarket notification requires a new device to be compared for safety, effectiveness and technological characteristics to another device (or multiple devices) already on the market. A successful 510(k) submission results in FDA clearance for commercialization. An Institute of Medicine, or IOM panel recommended that this 510(k) process be significantly revised to be more restrictive. While the IOM report is non-binding, we do not know if or when the FDA will act on this recommendation. If we can no longer use the 510(k) pathway in the future, we may be required to perform clinical trials for our new products in order to obtain clearance or approval for commercialization. If so, our development costs will increase substantially, and the likelihood of approval for some of our products may be reduced. The PMA approval process is more costly, lengthy and uncertain than the 510(k) clearance process and requires the development and submission of clinical studies supporting the safety and effectiveness of the device. Product modifications may also require the submission of a new 510(k) clearance or the approval of a PMA before the modified product can be marketed. Any products or product enhancements that we develop that require regulatory clearance or approval may not be cleared or approved on the timelines that we currently anticipate, if approved at all. Any new products or any product enhancements that we develop may not be subject to the shorter 510(k) clearance process, but may instead be subject to the more lengthy PMA requirements. Additionally, even if 510(k) or other regulatory clearance is granted for any potential product, the approved indications for use may be limited, and the FDA may require additional animal or human clinical data prior to any potential approval of additional indications.
The European Union, or EU, requires that manufacturers of medical products obtain the right to affix the CE Mark to their products before selling them in member countries of the EU. We have received CE Mark certification for the two initial microcutter surgical cutting and stapling devices that we have developed, the MicroCutter XCHANGE 30 and the MicroCutter XPRESS 30. To maintain authorization to apply the CE Mark to future devices within the microcutter product line, we are subject to annual surveillance audits and periodic re-certification audits. If we modify the intended use of new products (relative to predicate products) or change the indication for use or develop new products in the future, we may need to apply for permission to affix the CE Mark to such products. We do not know whether we will be able to obtain permission to affix the CE Mark to new or modified products or whether we will continue to meet the quality and safety standards required to maintain the authorization that we have received. If we are unable to maintain authorization to affix the CE Mark to microcutter products, we will not be able to sell these products in member countries of the EU, which would have a material adverse effect on our results of operations.
Regulatory agencies subject a product, its manufacturer and the manufacturer’s facilities to continual review, regulation and periodic inspections. If a regulatory agency discovers previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product, our collaborators or us, including requiring withdrawal of the product from the market. Our products will also be subject to ongoing FDA requirements for the labeling, packaging, storage, advertising, promotion, record-keeping and submission of safety and other post-market information on the product. If our products fail to comply with applicable regulatory requirements, a regulatory agency may impose any of the following sanctions:
|
• |
warning letters, fines, injunctions, consent decrees and civil penalties; |
|
• |
customer notifications, repair, replacement, refunds, recall or seizure of our products; |
|
• |
operating restrictions, partial suspension or total shutdown of production; |
|
• |
delay in processing marketing applications for new products or modifications to existing products; |
|
• |
withdrawing approvals that have already been granted; and |
|
• |
criminal prosecution. |
To market any products internationally, we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from that required to obtain FDA clearance or approval. The regulatory approval process in other countries may include all of the risks detailed above regarding FDA clearance or approval. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others. Failure to obtain regulatory approval in other countries or any delay or setback in obtaining such approval could have the same adverse effects detailed above regarding FDA clearance or approval, including the risk that our products may not be approved for use under all of the circumstances requested, which could limit the uses of our products and adversely impact potential product sales, and that such clearance or approval may require costly, post-marketing follow-up studies. If we fail to comply with applicable foreign regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
If we do not achieve our projected development goals in the time frames we announce and expect, the commercialization of our product candidates may be delayed and, as a result, our stock price may decline.
From time to time, we may estimate and publicly announce the timing anticipated for the accomplishment of various clinical, regulatory and other product development goals, which we sometimes refer to as milestones. These milestones may include submissions for and receipt of clearances or approvals from regulatory authorities, other clinical and regulatory events or the launch of new products. These estimates are based on a variety of assumptions. The actual timing of these milestones can vary dramatically compared to our estimates, in some cases for reasons beyond our control. If we do not meet milestones as publicly announced, the commercialization of our products may be delayed and, as a result, our stock price may decline.
Our manufacturing facilities, and those of our suppliers, must comply with applicable regulatory requirements. Failure of our manufacturing facilities to comply with quality requirements would harm our business and our results of operations.
Our manufacturing facilities and processes are subject to periodic inspections and audits by various federal, state and foreign regulatory agencies. For example, our facilities have been inspected by State of California regulatory authorities pursuant to granting a California Device Manufacturing License and by the FDA. Additionally, to market products in Europe, we are required to maintain International Standards Organization, or ISO, 13485:2003 certification and are subject to periodic surveillance audits. We are currently ISO 13485:2003 certified; however, our failure to maintain necessary regulatory compliance and permits for our manufacturing facilities could prevent us from manufacturing and selling our products.
Additionally, our manufacturing processes and, in some cases, those of our suppliers, are required to comply with the FDA’s Quality System Regulation, or QSR, which covers the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of our products, including the PAS-Port and C-Port systems and the MicroCutter XCHANGE 30. We are also subject to similar state requirements and licenses. In addition, we must engage in extensive record keeping and reporting and must make available our manufacturing facilities and records for periodic inspections by governmental agencies, including the FDA, state authorities and comparable agencies in other countries. If we are given notice of significant violations in a QSR inspection, our operations could be disrupted and our manufacturing interrupted. Failure to take adequate corrective action in response to an adverse QSR inspection could result in, among other things, a shut-down of our manufacturing operations, significant fines, suspension of product distribution or other operating restrictions, seizures or recalls of our devices and criminal prosecutions, any of which would cause our business to suffer. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with applicable regulatory requirements, which may result in manufacturing delays for our products and cause our revenue to decline.
We may also be required to recall our products due to manufacturing supply defects. If we issue recalls of our products in the future, our revenue and business could be harmed.
Lack of third-party coverage and reimbursement for our products could delay or limit their adoption.
We may experience limited sales growth resulting from limitations on reimbursements made to purchasers of our products by third-party payors, and we cannot assure you that our sales will not be impeded and our business harmed if third-party payors fail to provide reimbursement that hospitals view as adequate.
In the United States, our products are and will continue to be purchased primarily by medical institutions, which then bill various third-party payors, such as CMS which administer the Medicare program, and other government programs and private insurance plans, for the health care services provided to their patients. The process involved in applying for coverage and incremental reimbursement from CMS is lengthy and expensive. Under current CMS reimbursement policies, CMS offers a process to obtain add-on payment for a new medical technology when the existing Diagnosis-Related Group, or DRG, prospective payment rate is inadequate. To obtain add-on payment, a technology must be considered “new,” demonstrate substantial improvement in care and exceed certain payment thresholds. Add-on payments are made for no less than two years and no more than three years. We must demonstrate the safety and effectiveness of our technology to the FDA in addition to CMS requirements before add-on payments can be made. Further, Medicare coverage is based on our ability to demonstrate the treatment is “reasonable and necessary” for Medicare beneficiaries. In November 2006, CMS denied our request for an add-on payment with respect to our C-Port systems. According to CMS, we met the “new” criteria and exceeded the payment threshold but did not in their view demonstrate substantial improvement in care. Even if our products receive FDA and other regulatory clearance or approval, they may not be granted coverage and reimbursement in the foreseeable future, if at all. Moreover, many private payors look to CMS in setting their reimbursement policies and amounts. If CMS or other agencies limit coverage or decrease or limit reimbursement payments for doctors and hospitals, this may affect coverage and reimbursement determinations by many private payors.
We cannot assure you that CMS will provide coverage and reimbursement for our products. If a medical device does not receive incremental reimbursement from CMS, then a medical institution would have to absorb the cost of our products as part of the cost of the procedure in which the products are used. Acute care hospitals are now generally reimbursed by CMS for inpatient operating costs under a Medicare hospital inpatient prospective payment system. Under the Medicare hospital inpatient prospective payment system, acute care hospitals receive a fixed payment amount for each covered hospitalized patient based upon the DRG to which the inpatient stay is assigned, regardless of the actual cost of the services provided. At this time, we do not know the extent to which medical institutions would consider insurers’ payment levels adequate to cover the cost of our products. Failure by hospitals and physicians to receive an amount that they consider to be adequate reimbursement for procedures in which our products are used could deter them from purchasing our products and limit our revenue growth. In addition, pre-determined DRG payments may decline over time, which could deter medical institutions from purchasing our products. If medical institutions are unable to justify the costs of our products, they may refuse to purchase them, which would significantly harm our business.
Any clinical trials that we may conduct may not begin on time, or at all, and may not be completed on schedule, or at all.
The commencement or completion of any clinical trials that we may conduct may be delayed or halted for numerous reasons, including, but not limited to, the following:
|
• |
the FDA or other regulatory authorities suspend or place on hold a clinical trial, or do not approve a clinical trial protocol or a clinical trial; |
|
• |
the data and safety monitoring committee of a clinical trial recommends that a trial be placed on hold or suspended; |
|
• |
patients do not enroll in clinical trials at the rate we expect; |
|
• |
patients are not followed-up at the rate we expect; |
|
• |
clinical trial sites decide not to participate or cease participation in a clinical trial; |
|
• |
patients experience adverse side effects or events related to our products; |
|
• |
patients die or suffer adverse medical effects during a clinical trial for a variety of reasons, which may not be related to our product candidates, including the advanced stage of their disease and other medical problems; |
|
• |
third-party clinical investigators do not perform our clinical trials on our anticipated schedule or consistent with the clinical trial protocol and good clinical practices, or other third-party organizations do not perform data collection and analysis in a timely or accurate manner; |
|
• |
regulatory inspections of our clinical trials or manufacturing facilities may, among other things, require us to undertake corrective action or suspend or terminate our clinical trials if investigators find us not to be in compliance with regulatory requirements; |
|
• |
third-party suppliers fail to provide us with critical components that conform to design and performance specifications; |
|
• |
the failure of our manufacturing processes to produce finished products that conform to design and performance specifications; |
|
• |
changes in governmental regulations or administrative actions; |
|
• |
the interim results of the clinical trial are inconclusive or negative; |
|
• |
pre-clinical or clinical data is interpreted by third parties in different ways; or |
|
• |
our trial design, although approved, is inadequate to demonstrate safety and/or efficacy. |
Clinical trials sometimes experience delays related to outcomes experienced during the course of the trials, which may result in a material delay in the trial and could lead to more significant delays or other effects in future trials. Clinical trials may require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. Patient enrollment in clinical trials and completion of patient follow-up in clinical trials depend on many factors, including the size of the patient population, the nature of the trial protocol, the proximity of patients to clinical sites and the eligibility criteria for the study and patient compliance. For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures to assess the safety and effectiveness of our product candidates, or they may be persuaded to participate in contemporaneous trials of competitive products. Delays in patient enrollment or failure of patients to continue to participate in a study may cause an increase in costs and delays or result in the failure of the trial.
Our clinical trial costs will increase if we have material delays in our clinical trials or if we need to perform more or larger clinical trials than planned. Adverse events during a clinical trial could cause us to repeat a trial, terminate a trial or cancel an entire program.
If the third parties upon which we rely to conduct our clinical trials do not perform as contractually required or expected, we may not be able to obtain regulatory approval for or commercialize our product candidates.
We do not have the ability to independently conduct clinical trials for our product candidates, and we must rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to conduct our clinical trials. In addition, we rely on third parties to assist with our pre-clinical development of product candidates. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control, such as changes in regulations, delays in enrollment, and the like. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, any clinical trials that we may conduct may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates on a timely basis, if at all.
Because two customers account for a substantial portion of our product sales, the loss of these significant customers would cause a substantial decline in our revenue.
We derive a substantial portion of our revenue from sales to Century, our distributor in Japan, and to Herz-Und Diabeteszentrum in Germany. The loss of either of these customers would cause a decrease in revenue and, consequently, an increase in net loss. For fiscal years ended June 30, 2014 and 2013, sales to Century accounted for approximately 30% and 33%, respectively, and sales to Herz-Und Diabeteszentrum accounted for approximately 12% and 8%, respectively, of our total product sales. We expect these customers will continue to account for a substantial portion of our sales in the near term. As a result, if we lose these customers, our revenue and net loss would be adversely affected. In addition, customers that have accounted for significant revenue in the past may not generate revenue in any future period. The failure to obtain new significant customers or additional orders from existing customers will materially affect our operating results.
If our competitors for our MicroCutter XCHANGE 30 have products that are marketed more effectively or are demonstrated to be safer or more effective than ours, our commercial opportunity for our MicroCutter XCHANGE 30 and any future microcutter products will be reduced or eliminated and our business will be harmed.
Although we have commercially launched the MicroCutter XCHANGE 30 in Europe and recently in the United States, we have generated minimal revenues from this launch through June 30, 2014. We only received the FDA 510(k) clearance for the MicroCutter XCHANGE 30 and blue staple cartridge in January 2014, and for the white staple cartridge in February 2014, for use in multiple open or minimally-invasive surgical procedures for the transection, resection and/or creation of anastomoses in the small and large intestine, as well as the transection of the appendix. The MicroCutter XCHANGE 30 competes, and the MicroCutter XCHANGE 45 and other planned improvements, features and products in the microcutter product line if they receive regulatory clearance and are successfully launched, would compete in the market for stapling and cutting devices against laparoscopic stapling and sealing devices currently marketed around the world. We believe the principal competitive factors in the market for laparoscopic staplers include:
|
• |
reduced product size; |
|
• |
ease of use; |
|
• |
product quality and reliability; |
|
• |
device cost-effectiveness; |
|
• |
degree of articulation; |
|
• |
surgeon relationships; |
|
• |
sales and marketing capabilities; and |
|
• |
multi-fire capability. |
Two large competitors, Ethicon Endo-Surgery, part of Johnson & Johnson, and Covidien currently control over 80% of this market. Other large competitors in the laparoscopic device market include Stryker Endoscopy and Olympus, which acquired another competitor, Gyrus Medical. Ethicon Endo-Surgery and Covidien, which acquired a small competitor, Power Medical, each have large direct sales forces in the United States and have been the largest participants in the market for single use disposable laparoscopic stapling devices for many years. Competing against large established competitors with significant resources may make establishing a market for any products that we develop difficult which would have a material adverse effect on our business. A private company, JustRight Surgical, LLC, is developing smaller surgical instruments and has announced FDA 510(k) clearance for a 5 millimeter stapler that could be considered competitive with our stapling products, but is more limited in availability of staple sizes and articulation compared to the MicroCutter XCHANGE 30. Further, we may also face additional competition from generic surgical stapling products similar to currently commercially available products following expiration of patents on our competitors’ products.
We may require substantial additional capital to continue operations as currently conducted and as proposed to be conducted.
As of June 30, 2014, we had approximately $40.5 million of cash, cash equivalents and short-term investments, $2.3 million in long-term investments and $4.0 million of debt principal outstanding. In April 2014, we completed the public offering of our common stock and Series A Convertible Preferred Stock, resulting in net proceeds to us of approximately $44.7 million. We believe that our existing cash, cash equivalents, short-term and long-term investments will be sufficient to meet our anticipated cash needs to enable us to conduct our business substantially as currently conducted for at least the next 12 months. We may be able to extend this time period to the extent that we decrease our planned expenditures, or raise additional capital. We have based our estimate as to the sufficiency of our cash resources on assumptions that may prove to be wrong.
Because we do not anticipate that we will generate sufficient product sales to achieve profitability for the next several years, if at all, we may need to raise substantial additional capital to finance our operations in the future. To raise capital, we may seek to sell additional equity or debt securities, obtain a credit facility or enter into product development, license or distribution agreements with third parties or divest one or more of our commercialized products or products in development. However, we cannot be certain that additional funding of any kind will be available on acceptable terms, or at all. If additional funds are raised through the issuance of debt securities, these securities could have rights senior to those associated with our Series A preferred stock and common stock and could contain covenants that would restrict our operations. Any product development, licensing, distribution or sale agreements that we enter into may require us to relinquish valuable rights, including with respect to commercialized products or products in development that we would otherwise seek to commercialize or develop ourselves. We may not be able to obtain sufficient additional funding or enter into a strategic transaction in a timely manner. Our need to raise capital may require us to accept terms that may harm our business or be disadvantageous to our current stockholders. If adequate funds are not available or revenue from product sales do not increase, we would be required to further reduce our workforce, delay, reduce the scope of or eliminate our commercialization efforts with respect to one or more of our products or one or more of our research and development programs. Failure to raise additional capital may result in our ceasing to be publicly traded or ceasing operations.
If our competitors for our anastomotic solutions and cardiac bypass products have products that are approved in advance of ours, are marketed more effectively or are demonstrated to be safer or more effective than ours, our commercial opportunity for our anastomotic solutions and cardiac bypass products will be reduced or eliminated and our business will be harmed.
The market for anastomotic solutions and cardiac bypass products is competitive. Competitors include a variety of public and private companies that currently offer or are developing cardiac surgery products generally and automated anastomotic systems specifically that would compete directly with ours.
We believe that the primary competitive factors in the market for medical devices used in the treatment of coronary artery disease include:
|
• |
improved patient outcomes; |
|
• |
access to and acceptance by leading physicians; |
|
• |
product quality and reliability; |
|
• |
ease of use; |
|
• |
device cost-effectiveness; |
|
• |
training and support; |
|
• |
novelty; |
|
• |
physician relationships; and |
|
• |
sales and marketing capabilities. |
We may be unable to compete successfully on the basis of any one or more of these factors, which could have a material adverse effect on our business, financial condition and results of operations.
A number of different technologies exist or are under development for performing anastomoses, including sutures, mechanical anastomotic devices, suture-based anastomotic devices and shunting devices. Currently, substantially all anastomoses are performed with sutures and, for the foreseeable future we believe that sutures will continue to be the principal alternative to our anastomotic products. Sutures are far less expensive than our automated anastomotic products, and other anastomotic devices may be less expensive than our own. Surgeons, who have been using sutures for their entire careers, may be reluctant to consider alternative technologies, despite potential advantages. Any resistance to change among practitioners could delay or hinder market acceptance of our products, which would have a material adverse effect on our business.
Cardiovascular diseases may also be treated by other methods that do not require anastomoses, including, interventional techniques such as balloon angioplasty with or without the use of stents, pharmaceuticals, atherectomy catheters and lasers. Several of these alternative treatments are widely accepted in the medical community and have a long history of use. In addition, technological advances with other therapies for cardiovascular disease, such as drugs, or future innovations in cardiac surgery techniques could make other methods of treating these diseases more effective or lower cost than bypass procedures. For example, the number of bypass procedures in the United States and other major markets has declined in recent years and is expected to decline in the years ahead because competing treatments are, in many cases, far less invasive and provide acceptable clinical outcomes. Many companies working on treatments that do not require anastomoses may have significantly greater financial, manufacturing, marketing, distribution and technical resources and experience than we have. Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, pre-clinical testing, clinical trials, obtaining regulatory clearance or approval and marketing approved products than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Our competitors may succeed in developing technologies and therapies that are more effective, better tolerated or less costly than any that we are developing or that would render our product candidates obsolete and noncompetitive. Our competitors may succeed in obtaining clearance or approval from the FDA and foreign regulatory authorities for their products sooner than we do for ours. We will also face competition from these third parties in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient enrollment for clinical trials and in acquiring and in-licensing technologies and products complementary to our programs or advantageous to our business.
We are dependent upon a number of key suppliers, including single source suppliers, the loss of which would materially harm our business.
We use or rely upon sole source suppliers for certain components and services used in manufacturing our products, and we utilize materials and components supplied by third parties with which we do not have any long-term contracts. In recent years, many suppliers have ceased supplying materials for use in implantable medical devices. We cannot assure you that materials required by us will not be restricted or that we will be able to obtain sufficient quantities of such materials or services in the future. Moreover, the continued use by us of materials manufactured by third parties could subject us to liability exposure. Because we do not have long-term contracts, none of our suppliers is required to provide us with any guaranteed minimum production levels.
We cannot quickly replace suppliers or establish additional new suppliers for some of our components, particularly due to both the complex nature of the manufacturing process used by our suppliers and the time and effort that may be required to obtain FDA clearance or approval or other regulatory approval to use materials from alternative suppliers. Any significant supply interruption or capacity constraints affecting our facilities or those of our suppliers would have a material adverse effect on our ability to manufacture our products and, therefore, a material adverse effect on our business, financial condition and results of operations.
We have limited manufacturing experience and may encounter difficulties in increasing production to provide an adequate supply to customers.
To date, our manufacturing activities have consisted primarily of producing moderate quantities of our products for use in clinical studies and for commercial sales in Japan, Europe and the United States. Production in increased commercial quantities will require us to expand our manufacturing capabilities and to hire and train additional personnel. We may encounter difficulties in increasing our manufacturing capacity and in manufacturing larger commercial quantities, including:
|
• |
maintaining product yields; |
|
• |
maintaining quality control and assurance; |
|
• |
providing component and service availability; |
|
• |
maintaining adequate control policies and procedures; and |
|
• |
hiring and retaining qualified personnel. |
Difficulties encountered in increasing our manufacturing could have a material adverse effect on our business, financial condition and results of operations.
The manufacture of our products is a complex and costly operation involving a number of separate processes and components. Any shipment delays could harm perception of our products and have a material adverse impact on our results of operations.
The current unit costs for our products are very high, and if we are not able to bring them down we will suffer from price competition and may not become profitable.
The current unit costs for our products, based on limited manufacturing volumes, are very high, and it will be necessary to achieve economies of scale to become profitable. Certain of our manufacturing processes are labor intensive, and achieving significant cost reductions will depend in part upon reducing the time required to complete these processes. We cannot assure you that we will be able to achieve cost reductions in the manufacture of our products and, without these cost reductions, our business may never achieve profitability.
We have considered, and will continue to consider as appropriate, manufacturing in-house certain components currently provided by third parties, as well as implementing new production processes. Manufacturing yields or costs may be adversely affected by the transition to in-house production or to new production processes, when and if these efforts are undertaken, which would materially and adversely affect our business, financial condition and results of operations.
If we fail to retain key personnel, or to retain our executive management team, we may be unable to successfully develop or commercialize our products.
Our business and future operating results depend significantly on the continued contributions of our key technical personnel and senior management, including those of our co-founder, CEO and President, Bernard Hausen, M.D., Ph.D. Dr. Hausen speaks German, has many relationships valuable to our company with physicians in Europe, and is the primary contact for our company with many of the physicians using our microcutter products in Europe. These services and individuals would be difficult or impossible to replace and none of these individuals is subject to a post-employment non-competition agreement. While we are subject to certain severance obligations to Dr. Hausen, either he or we may terminate his employment at any time and for any lawful reason or for no reason. Additionally, although we have key-person life insurance in the amount of $3.0 million on the life of Dr. Hausen, we cannot assure you that this amount would fully compensate us for the loss of Dr. Hausen’s services. The loss of key employees, the failure of any key employee to perform or our inability to attract and retain skilled employees, as needed, could materially adversely affect our business, financial condition and results of operations.
As of June 30, 2014, we had 69 employees. Our business and future operating results depend significantly on our ability to attract and retain qualified management, manufacturing, technical, marketing, sales and support personnel for our operations. Competition for such personnel is intense, and there can be no assurance that we will be successful in attracting or retaining such personnel. We will need to maintain an appropriate level of managerial, operational, financial and other resources to manage and fund our operations and clinical trials, continue our research and development activities and commercialize our products, and we expect our past reductions in force will impair our ability to maintain or increase our product sales. It is possible that our management and scientific personnel, systems and facilities currently in place may not be adequate to maintain future operating activities, and we may be required to effect additional reductions in force.
We may in the future be a party to patent litigation and administrative proceedings that could be costly and could interfere with our ability to sell our products.
The medical device industry has been characterized by extensive litigation regarding patents and other intellectual property rights, and companies in the industry have used intellectual property litigation to gain a competitive advantage. We may become a party to patent infringement claims and litigation or interference proceedings declared by the U.S. Patent and Trademark Office to determine the priority of inventions. The defense and prosecution of these matters are both costly and time consuming. Additionally, we may need to commence proceedings against others to enforce our patents, to protect our trade secrets or know-how or to determine the enforceability, scope and validity of the proprietary rights of others. These proceedings would result in substantial expense to us and significant diversion of effort by our technical and management personnel.
While we are not aware of any patents issued to third parties that contain subject matter materially related to our technology, there may be patents held by third parties of which we are not aware that contain subject matter materially related to our technology. We cannot assure you that third parties will not assert that our products and systems infringe the claims in their patents or seek to expand their patent claims to cover aspects of our products and systems. An adverse determination in litigation or interference proceedings to which we may become a party could subject us to significant liabilities or require us to seek licenses. In addition, if we are found to willfully infringe third-party patents, we could be required to pay treble damages in addition to other penalties. Although patent and intellectual property disputes in the medical device area have often been settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include ongoing royalties. We may be unable to obtain necessary licenses on satisfactory terms, if at all. If we do not obtain necessary licenses, we may be required to redesign our products to avoid infringement, and it may not be possible to do so effectively. Adverse determinations in a judicial or administrative proceeding or failure to obtain necessary licenses could prevent us from manufacturing and selling the C-Port or PAS-Port systems or any other product we may develop, which would have a significant adverse impact on our business.
Intellectual property rights may not provide adequate protection, which may permit third parties to compete against us more effectively.
We rely upon patents, trade secret laws and confidentiality agreements to protect our technology and products. Our pending patent applications may not issue as patents or, if issued, may not issue in a form that will be advantageous to us. Any patents we have obtained or will obtain in the future might be invalidated or circumvented by third parties. If any challenges are successful, competitors might be able to market products and use manufacturing processes that are substantially similar to ours. We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or other trade secrets by consultants, vendors or former or current employees, despite the existence generally of confidentiality agreements and other contractual restrictions. Monitoring unauthorized use and disclosure of our intellectual property is difficult, and we do not know whether the steps we have taken to protect our intellectual property will be adequate. In addition, the laws of many foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States. To the extent that our intellectual property protection is inadequate, we are exposed to a greater risk of direct competition. In addition, competitors could purchase any of our products and attempt to replicate some or all of the competitive advantages we derive from our development efforts or design around our protected technology. If our intellectual property is not adequately protected against competitors’ products and methods, our competitive position could be adversely affected, as could our business.
We also rely upon trade secrets, technical know-how and continuing technological innovation to develop and maintain our competitive position. We require our employees, consultants and advisors to execute appropriate confidentiality and assignment-of-inventions agreements with us. These agreements typically provide that all materials and confidential information developed or made known to the individual during the course of the individual’s relationship with us be kept confidential and not disclosed to third parties except in specific circumstances and that all inventions arising out of the individual’s relationship with us shall be our exclusive property. These agreements may be breached, and in some instances, we may not have an appropriate remedy available for breach of the agreements. Furthermore, our competitors may independently develop substantially equivalent proprietary information and techniques, reverse engineer our information and techniques, or otherwise gain access to our proprietary technology.
Our products face the risk of technological obsolescence, which, if realized, could have a material adverse effect on our business.
The medical device industry is characterized by rapid and significant technological change. There can be no assurance that third parties will not succeed in developing or marketing technologies and products that are more effective than ours or that would render our technology and products obsolete or noncompetitive. Additionally, new, less invasive surgical procedures and medications could be developed that replace or reduce the importance of current procedures that use or could use our products. Accordingly, our success will depend in part upon our ability to respond quickly to medical and technological changes through the development and introduction of new products. We expect the relative speed with which we can develop products, complete clinical testing and regulatory clearance or approval processes, train physicians in the use of our products, and supply commercial quantities of products to the market to be important competitive factors. Product development involves a high degree of risk, and we cannot assure you that our new product development efforts will result in any commercially successful products. We have experienced delays in completing the development and commercialization of our planned products, and there can be no assurance that these delays will not continue or recur in the future. Any delays could result in a loss of market acceptance and market share.
We are subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, marketing expenditure tracking and disclosure (or “sunshine”) laws, health information privacy and security laws, and consumer protection laws. If we are unable to comply, or have not fully complied, with such laws, we could face criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Our operations may be directly, or indirectly, subject to various federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act. These laws may impact, among other things, our current activities with physicians, including consulting arrangements, as well as proposed sales, marketing and educational activities. In addition, we may be subject to patient privacy regulation by the federal government and by the US states and foreign jurisdictions in which we conduct our business. The laws that may affect our ability to operate include, but are not limited to:
|
● |
the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, to induce, or in return for, either the referral of an individual, or the purchase or recommendation of an item or service for which payment may be made under a federal health care program, such as the Medicare and Medicaid programs; |
|
● |
federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third party payers that are false or fraudulent; |
|
● |
federal criminal statutes created under the Health Insurance Portability and Accountability Act of 1996 (HIPAA), which prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters; |
|
● |
HIPAA, as amended by the Health Information Technology and Clinical Health Act of 2009 (HITECH), and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information; |
|
● |
state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third party payer, including commercial insurers, and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts; |
|
● |
the Foreign Corrupt Practices Act, a U.S. law which regulates certain financial relationships with foreign government officials (which could include, for example, certain medical professionals); |
|
● |
federal and state consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; and |
|
● |
state and federal marketing expenditure tracking and reporting laws, which generally require certain types of expenditures in the United States to be tracked and reported (compliance with such requirements may require investment in infrastructure to ensure that tracking is performed properly, and some of these laws result in the public disclosure of various types of payments and relationships, which could potentially have a negative effect on our business and/or increase enforcement scrutiny of our activities). |
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, possible exclusion from Medicare, Medicaid and other government healthcare programs, and curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
We could be exposed to significant product liability claims, which could be time consuming and costly to defend, divert management attention, and adversely impact our ability to obtain and maintain insurance coverage. The expense and potential unavailability of insurance coverage for our company or our customers could adversely affect our ability to sell our products, which would adversely affect our business.
The testing, manufacture, marketing, and sale of our products involve an inherent risk that product liability claims will be asserted against us. Additionally, we are currently training physicians in the United States on the use of our blue and white staple cartridges for the MicroCutter XCHANGE 30, C-Port and PAS-Port systems and in Europe for the MicroCutter XCHANGE 30. During training, patients may be harmed, which could also lead to product liability claims. Product liability claims or other claims related to our products, or their off-label use, regardless of their merits or outcomes, could harm our reputation in the industry, reduce our product sales, lead to significant legal fees, and result in the diversion of management’s attention from managing our business.
Although we maintain product liability insurance in the amount of $10.0 million, we may not have sufficient insurance coverage to fully cover the costs of any claim or any ultimate damages we might be required to pay. We may not be able to obtain insurance in amounts or scope sufficient to provide us with adequate coverage against all potential liabilities. Any product liability claims brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing continuing coverage. Product liability claims in excess of our insurance coverage would be paid out of cash reserves, harming our financial condition and adversely affecting our operating results.
Some of our customers and prospective customers may have difficulty in procuring or maintaining liability insurance to cover their operations and use of the C-Port or PAS-Port systems or the microcutter product line. Medical malpractice carriers are withdrawing coverage in certain states or substantially increasing premiums. If this trend continues or worsens, our customers may discontinue using the C-Port or PAS-Port systems and potential customers may opt against purchasing the C-Port or PAS-Port systems due to the cost or inability to procure insurance coverage.
We sell our systems internationally and are subject to various risks relating to these international activities, which could adversely affect our revenue.
To date, a substantial portion of our product sales has been attributable to sales in international markets. By doing business in international markets, we are exposed to risks separate and distinct from those we face in our domestic operations. Our international business may be adversely affected by changing economic conditions in foreign countries. Because most of our sales are currently denominated in U.S. dollars, if the value of the U.S. dollar increases relative to foreign currencies, our products could become more costly to the international customer and, therefore, less competitive in international markets, which could affect our results of operations. Engaging in international business inherently involves a number of other difficulties and risks, including:
|
|
• |
export restrictions and controls relating to technology; |
|
• |
the availability and level of reimbursement within prevailing foreign healthcare payment systems; |
|
• |
pricing pressure that we may experience internationally; |
|
• |
required compliance with existing and changing foreign regulatory requirements and laws; |
|
• |
laws and business practices favoring local companies; |
|
• |
longer payment cycles; |
|
• |
difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; |
|
• |
political and economic instability; |
|
• |
potentially adverse tax consequences, tariffs and other trade barriers; |
|
• |
international terrorism and anti-American sentiment; |
|
• |
difficulties and costs of staffing and managing any foreign operations; and |
|
• |
difficulties in enforcing intellectual property rights. |
Our exposure to each of these risks may increase our costs, impair our ability to market and sell our products and require significant management attention. We cannot assure you that one or more of these factors will not harm our business.
Our operations are currently conducted at a single location that may be at risk from earthquakes, terror attacks or other disasters.
We currently conduct all of our manufacturing, development and management activities at a single location in Redwood City, California, near known earthquake fault zones. We have taken precautions to safeguard our facilities, including insurance, health and safety protocols, and off-site storage of computer data. However, any future natural disaster, such as an earthquake, or a terrorist attack, could cause substantial delays in our operations, damage or destroy our equipment or inventory and cause us to incur additional expenses. A disaster could seriously harm our business and results of operations. Our insurance does not cover earthquakes and floods and may not be adequate to cover our losses in any particular case.
If we use hazardous materials in a manner that causes injury, we may be liable for damages.
Our research and development and manufacturing activities involve the use of hazardous materials. Although we believe that our safety procedures for handling and disposing of these materials comply with federal, state and local laws and regulations, we cannot entirely eliminate the risk of accidental injury or contamination from the use, storage, handling or disposal of these materials. We do not carry specific hazardous waste insurance coverage, and our property and casualty and general liability insurance policies specifically exclude coverage for damages and fines arising from hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury, we could be held liable for damages or penalized with fines in an amount exceeding our resources, and our clinical trials or regulatory clearances or approvals could be suspended or terminated.
Changes in tax structures may negatively impact our financial results and industry in general, which could harm our business and the value of our stock.
Effective January 1, 2013, U.S. health care law reforms under the 2010 Affordable Care Act imposed a new 2.3% excise tax on certain medical technology companies regardless of whether the companies are profitable. Industry advocates anticipate the new tax will negatively impact innovation and U.S. competitiveness. The tax may already be having an adverse impact on U.S. medical device research and development investment activity and job creation, and may force affected companies to consider cutting manufacturing operations, research and development, and employment levels. These new taxes may also adversely impact patient access to new and innovative medical technologies such as those we manufacture and develop. If any of these risks materialize, then our business may be harmed and the value of our common stock could decline. We cannot assure you that the Affordable Care Act, as currently enacted or as amended in the future, will not adversely affect our business and financial results, and we cannot predict how future federal or state legislative or administrative changes relating to healthcare reform will affect our business.
Risks Related to Our Common Stock
The conversion of shares of Series A preferred stock into common stock, or the perception that such conversions may occur, could cause the market price of our common stock to decline.
We currently have 191,474 shares of our Series A preferred stock outstanding. Each share of our Series A preferred stock is convertible into 100 shares of our common stock at any time at the option of the holder, subject to certain limitations. The conversion of substantial amounts of our Series A preferred stock would result in the issuance by us of a substantial number of additional shares of our common stock, which, subject to certain limitations, could be traded publicly. Such conversions, or the perception that such conversions may occur, could cause the market price of our common stock to decline.
The price of our common stock may continue to be volatile, and the value of an investment in our common stock may decline.
An active and liquid trading market for our common stock may not develop or be sustained. Factors that could cause volatility in the market price of our common stock include, but are not limited to:
|
• |
completion of development and commercial launch of our microcutter products, and the timing thereof; |
|
• |
perceptions that we may not be able to raise capital as needed, or that investors will be substantially diluted if we do raise capital; |
|
• |
market acceptance and adoption of our products; |
|
• |
regulatory clearance or approvals of or other regulatory developments with respect to our products; |
|
• |
volume and timing of orders for our products; |
|
• |
changes in earnings estimates, investors’ perceptions, recommendations by securities analysts or our failure to achieve analysts’ earnings estimates; |
|
• |
quarterly variations in our or our competitors’ results of operations; |
|
• |
general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors; |
|
• |
the announcement of new products or product enhancements by us or our competitors; |
|
• |
announcements related to patents issued to us or our competitors and to litigation; |
|
• |
developments in our industry; and |
|
• |
announcements regarding the proxy contest for our 2014 annual meeting of stockholders or the outcome thereof, as well other actions by stockholder activists. |
In addition, the stock prices of many companies in the medical device industry have experienced wide fluctuations that have often been unrelated to the operating performance of those companies. These factors may materially and adversely affect the market price of our common stock.
The ownership of our common stock is highly concentrated, and your interests may conflict with the interests of our existing stockholders.
Our executive officers and directors and their affiliates, together with other stockholders that own 5% or more of our outstanding common stock, beneficially owned approximately 11% of our outstanding common stock as of June 30, 2014. In addition, two stockholders collectively hold all of our Series A preferred stock and may convert those shares into 19,147,400 shares of our common stock, or another 18% of our outstanding common stock, subject to certain conditions. Accordingly, these stockholders have significant influence over the outcome of corporate actions requiring stockholder approval. The interests of these stockholders may be different than the interests of other stockholders on these matters. This concentration of ownership could also have the effect of delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could reduce the price of our common stock.
Evolving regulation of corporate governance and public disclosure will result in additional expenses and continuing uncertainty.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002, new Securities and Exchange Commission regulations and The NASDAQ Stock Market rules are creating uncertainty for public companies. We are presently evaluating and monitoring developments with respect to new and proposed rules and cannot predict or estimate the amount of the additional compliance costs we may incur or the timing of such costs. These new or changed laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity, and as a result, their application in practice may evolve over time as new guidance is provided by courts and regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. Maintaining appropriate standards of corporate governance and public disclosure will result in increased general and administrative expenses and a diversion of management time and attention from product-generating and revenue-generating activities to compliance activities. In addition, if we fail to comply with new or changed laws, regulations and standards, regulatory authorities may initiate legal proceedings against us and our business and reputation may be harmed.
Our future operating results may be below securities analysts’ or investors’ expectations, which could cause our common stock price to decline.
The revenue and income potential of our products and our business model are unproven, and we may be unable to generate significant revenue or grow at the rate expected by securities analysts or investors. In addition, our costs may be higher than we, securities analysts or investors expect. If we fail to generate sufficient revenue or our costs are higher than we expect, our results of operations will suffer, which in turn could cause our common stock price to decline. Our results of operations will depend upon numerous factors, including:
|
• |
completion of development and commercial launch of our microcutter products, and the timing thereof; |
|
• |
FDA or other regulatory clearance or approval of our products; |
|
• |
demand for our products; |
|
• |
the performance of third-party contract manufacturers and component suppliers; |
|
• |
our ability to develop sales and marketing capabilities; |
|
• |
our ability to develop, introduce and market new or enhanced versions of our products on a timely basis; and |
|
• |
our ability to obtain and protect proprietary rights. |
Our operating results in any particular period may not be a reliable indication of our future performance. In some future quarters, our operating results may be below the expectations of securities analysts or investors. If this occurs, the price of our common stock will likely decline.
Anti-takeover defenses that we have in place could prevent or frustrate attempts to change our direction or management.
Provisions of our certificate of incorporation and bylaws and applicable provisions of Delaware law may make it more difficult for or prevent a third party from acquiring control of us without the approval of our board of directors. These provisions:
|
• |
limit who may call a special meeting of stockholders; |
|
• |
establish advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted upon at stockholder meetings; |
|
• |
prohibit cumulative voting in the election of our directors, which would otherwise permit less than a majority of stockholders to elect directors; |
|
• |
prohibit stockholder action by written consent, thereby requiring all stockholder actions to be taken at a meeting of our stockholders; and |
|
• |
provide our board of directors with the ability to designate the terms of and issue a new series of preferred stock without stockholder approval. |
In addition, Section 203 of the Delaware General Corporation Law generally prohibits us from engaging in any business combination with certain persons who own 15% or more of our outstanding voting stock or any of our associates or affiliates who at any time in the past three years have owned 15% or more of our outstanding voting stock. These provisions may have the effect of entrenching our management team and may deprive stockholders of the opportunity to sell their shares to potential acquirers at a premium over prevailing prices. This potential inability to obtain a control premium could reduce the price of our common stock.
We may become involved in securities class action litigation that could divert management’s attention and harm our business.
The stock market in general, the NASDAQ Global Market and the market for medical device companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. Further, the market prices of securities of medical device companies have been particularly volatile. These broad market and industry factors may materially harm the market price of our common stock, regardless of our operating performance. In the past, following periods of volatility in the market price of a particular company’s securities, securities class action litigation has often been brought against that company. We may become involved in this type of litigation in the future. Litigation often is expensive and diverts management’s attention and resources, which could materially harm our financial condition and results of operations.
We have never paid dividends on our capital stock, and we do not anticipate paying any cash dividends in the foreseeable future.
We have paid no cash dividends on any of our classes of capital stock to date, and we currently intend to retain our future earnings to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be the sole source of gain to our stockholders for the foreseeable future.
If our stock price declines, our common stock may be subject to delisting from the NASDAQ Global Market.
In November 2012, we received notice from the NASDAQ Global Market that we did not meet the continued listing requirements for listing our common stock on the NASDAQ Global Market because for 30 consecutive business days the market value of our common stock had been below $50.0 million. Although in January 2013, our stock price increased and we received a letter from Nasdaq stating that the we had regained compliance with the listing rule and that matter was closed, we cannot guarantee that our stock price will remain sufficiently high to continue to meet the listing requirements. Further, the price of our common stock closed at below $1.00 per share on twenty consecutive trading days in May and June 2014, before trading above $1.00 again beginning on June 5, 2014. If our stock price were trade below $1.00 for a period of 30 consecutive trading days, our common stock could be subject to delisting unless we were to regain compliance within the grace periods allowed by the NASDAQ Global Market. If our stock price drops again our common stock may once again not meet the continued listing requirements for listing our common stock on the NASDAQ Global Market, and our common stock could be delisted from that market if we do not regain compliance within 180 days of any new notice received from Nasdaq.
The announcement by a dissident stockholder of an intention to conduct a proxy contest has the potential to adversely affect our business and the market price of our common stock.
Broadfin Healthcare Master Fund, LTD, or Broadfin, has announced its intention to initiate a proxy contest with respect to the election of directors at our 2014 annual meeting of stockholders, or the Annual Meeting. Broadfin is proposing to solicit proxies in opposition to us for the purpose of voting in favor of its four nominees for election to our board of directors. Further, in discussions with Kevin Kotler of Broadfin, Mr. Kotler has expressed a desire to replace our chief executive officer, Dr. Hausen. Our business, operating results or financial condition could be harmed by the potential proxy contest because, among other things:
|
• |
responding to the potential proxy contest is costly and time-consuming, is a significant distraction for our board of directors, management and employees, and diverts the attention of our board of directors and senior management from the pursuit of our business strategy, which could adversely affect our results of operations and financial condition; |
|
• |
perceived uncertainties as to our future direction, our ability to execute on our strategy, or changes to the composition of our board of directors and our chief executive officer, may lead to the perception of a change in the direction of our business, instability or lack of continuity which may be exploited by our competitors, cause concern to our current or potential future customers and suppliers, and may result in the loss of potential business opportunities and make it more difficult to attract and retain qualified personnel and business partners; |
|
• |
the expenses for legal and advisory fees and administrative and associated costs incurred in connection with responding to the potential proxy contest and any related litigation may be substantial; and |
|
• |
we may choose to initiate, or may become subject to, litigation as a result of the potential proxy contest or matters arising from the potential proxy contest, which would serve as a further distraction to our board of directors, management and employees and would require us to incur significant additional costs. |
In addition, the market price of our common stock could be subject to significant fluctuation or otherwise be adversely affected by the uncertainties described above, the outcome of the potential proxy contest, or a threat of future stockholder activism.
We may become subject to increased stockholder activism efforts that each could cause a material disruption to our business.
Certain influential institutional investors and hedge funds have taken steps to involve themselves in the governance and strategic direction of certain companies due to governance or strategic-related disagreements between such companies and such stockholders. For example, in the first quarter of fiscal 2014, we received a communication from Broadfin urging a change in our board of directors and in our chief executive officer. Broadfin has since advised us that it intends to nominate four members to our board of directors and initiate a proxy contest with respect to our 2014 annual meeting of stockholders. Increased stockholder activism efforts could result in substantial costs and a further diversion of management’s attention and resources, which could harm our business and adversely affect the market price of our common stock.
Item 1B. Unresolved Staff Comments
Not applicable.
Item 2. Properties
We currently lease approximately 30,000 square feet of office, manufacturing and laboratory space in Redwood City, California. Our monthly average rent expense is approximately $60,000 and the lease expires on August 31, 2015, with the option to extend for a period of two years beyond the expiration date. We believe that our existing facility should meet our needs for at least the next few years. Our facility is subject to periodic inspections by state and federal regulatory authorities.
Item 3. Legal Proceedings
We are not subject to any material legal proceeding.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market for Common Equity
Our common stock began trading on the NASDAQ Global Market on February 3, 2006, under the symbol “CRDC”. The table below sets forth the high and low intraday sales prices for our common stock for the periods indicated:
|
High |
Low |
|||||||
|
Fiscal Year 2014 |
||||||||
|
First Quarter ended September 30, 2013 |
$ | 1.50 | $ | 1.10 | ||||
|
Second Quarter ended December 31, 2013 |
$ | 1.35 | $ | 0.88 | ||||
|
Third Quarter ended March 31, 2014 |
$ | 1.61 | $ | 0.95 | ||||
|
Fourth Quarter ended June 30, 2014 |
$ | 1.30 | $ | 0.82 | ||||
|
Fiscal Year 2013 |
||||||||
|
First Quarter ended September 30, 2012 |
$ | 1.99 | $ | 1.25 | ||||
|
Second Quarter ended December 31, 2012 |
$ | 1.48 | $ | 0.67 | ||||
|
Third Quarter ended March 31, 2013 |
$ | 1.83 | $ | 1.12 | ||||
|
Fourth Quarter ended June 30, 2013 |
$ | 1.57 | $ | 1.10 | ||||
As of September 18, 2014, there were 103 holders of record of common stock. This number does not include the number of persons whose shares are held by a nominee or in “street name” accounts through brokers.
Dividend Policy
We have never declared or paid any dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance our operations and do not anticipate paying any cash dividends on our capital stock in the foreseeable future. Future determination as to the declaration and payment of dividends, if any, will be at the discretion of our board of directors and will depend on then existing conditions, including our operating results, financial conditions, contractual restrictions, capital requirements, business prospects and other factors our board of directors may deem relevant.
Recent Sales of Unregistered Securities
Not applicable.
Issuer Purchases of Equity Securities
During the quarter ended June 30, 2014, we did not repurchase any equity securities.
Item 6. Selected Financial Data
The following selected financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and notes to those statements included elsewhere in this report.
The following selected balance sheet data as of June 30, 2014 and 2013, and the statements of operations data for each of the three fiscal years in the period ended June 30, 2014, have been derived from our audited financial statements, which are included elsewhere in this annual report. The selected balance sheet data as of June 30, 2012, 2011 and 2010, and the selected statements of operations data for the fiscal year ended June 30, 2011 and 2010, has been derived from our audited financial statements not included in this annual report. Historical results are not necessarily indicative of the results to be expected in future periods.
|
Fiscal Year Ended June 30, |
||||||||||||||||||||
|
2014 |
2013 |
2012 |
2011 |
2010 |
||||||||||||||||
|
(in thousands, except per share data) |
||||||||||||||||||||
|
Statements of Operations Data: |
||||||||||||||||||||
|
Net revenue: |
||||||||||||||||||||
|
Product sales, net |
$ | 3,505 | $ | 3,093 | $ | 3,274 | $ | 3,889 | $ | 3,764 | ||||||||||
|
License and development revenue |
41 | 336 | 336 | 9,277 | 124 | |||||||||||||||
|
Royalty revenue |
69 | 70 | 71 | 77 | 93 | |||||||||||||||
|
Total net revenue |
3,615 | 3,499 | 3,681 | 13,243 | 3,981 | |||||||||||||||
|
Operating costs and expenses: |
||||||||||||||||||||
|
Cost of product sales |
4,770 | 3,604 | 3,638 | 3,350 | 3,687 | |||||||||||||||
|
Research and development |
6,883 | 9,145 | 7,220 | 7,495 | 5,437 | |||||||||||||||
|
Selling, general and administrative |
8,463 | 6,410 | 6,139 | 5,920 | 5,734 | |||||||||||||||
|
Total operating costs and expenses |
20,116 | 19,159 | 16,997 | 16,765 | 14,858 | |||||||||||||||
|
Loss from operations |
(16,501 | ) | (15,660 | ) | (13,316 | ) | (3,522 | ) | (10,877 | ) | ||||||||||
|
Interest income |
12 | 15 | 12 | 21 | 35 | |||||||||||||||
|
Interest expense |
(504 | ) | (457 | ) | (268 | ) | (11 | ) | (112 | ) | ||||||||||
|
Other income (expense), net |
27 | (35 | ) | (3 | ) | (5 | ) | (1 | ) | |||||||||||
|
Net loss before income tax benefit |
(16,966 | ) | (16,137 | ) | (13,575 | ) | (3,517 | ) | (10,955 | ) | ||||||||||
|
Income tax benefit |
— | — | — | — | 31 | |||||||||||||||
|
Net loss |
$ | (16,966 | ) | $ | (16,137 | ) | $ | (13,575 | ) | $ | (3,517 | ) | $ | (10,924 | ) | |||||
|
Deemed dividend related to beneficial conversion feature of convertible preferred stock |
(1,915 | ) | — | — | — | — | ||||||||||||||
|
Net loss allocable to common stockholders |
$ | (18,881 | ) | $ | (16,137 | ) | $ | (13,575 | ) | $ | (3,517 | ) | $ | (10,924 | ) | |||||
|
Basic and diluted net loss per common share |
$ | (0.32 | ) | $ | (0.40 | ) | $ | (0.44 | ) | $ | (0.14 | ) | $ | (0.50 | ) | |||||
|
Shares used in computing basic and diluted net loss per common share |
58,395 | 40,842 | 30,547 | 25,620 | 21,927 | |||||||||||||||
|
As of June 30, |
||||||||||||||||||||
|
2014 |
2013 |
2012 |
2011 |
2010 |
||||||||||||||||
|
(in thousands) |
||||||||||||||||||||
|
Balance Sheet Data: |
||||||||||||||||||||
|
Cash, cash equivalents and investments |
$ | 42,796 | $ | 12,395 | $ | 14,645 | $ | 9,325 | $ | 6,561 | ||||||||||
|
Working capital |
39,965 | 12,268 | 13,316 | 8,477 | 5,016 | |||||||||||||||
|
Total assets |
47,577 | 17,761 | 18,142 | 11,470 | 9,791 | |||||||||||||||
|
Short-term note payable |
— | — | — | — | 1,400 | |||||||||||||||
|
Long-term liabilities |
4,735 | 4,559 | 4,364 | 433 | 31 | |||||||||||||||
|
Total stockholders’ equity |
40,185 | 10,974 | 11,360 | 8,862 | 6,477 | |||||||||||||||
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and the related notes to those statements included elsewhere in this report. In addition to historical financial information, the following discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results and timing of selected events may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those discussed under “Risk Factors” and elsewhere in this Report.
Overview
We are commercializing and developing a microcutter product line based on our proprietary “staple-on-a-strip” technology intended for use by thoracic, bariatric, colorectal and general surgeons. Our microcutter product line consists of the currently commercially-available MicroCutter XCHANGE® 30, a cartridge based microcutter device with a 5 millimeter shaft diameter and a 30 millimeter staple line, and products in development, including the MicroCutter XCHANGE® 45, a cartridge based microcutter device with an 8 millimeter shaft and a 45 millimeter staple line, the MicroCutter FLEXCHANGE™ 30, a cartridge based microcutter device with a flexible shaft to facilitate endoscopic procedures requiring cutting and stapling, and the MicroCutter XPRESS® 45, a multi-fire endolinear microcutter device with a 45 millimeter staple line specifically designed for the bariatric and thoracic surgery markets. We estimate that the commercially-available MicroCutter XCHANGE 30, along with these planned additional products, would provide us with a commercial opportunity of approximately 1.4 million procedures annually in the United States, involving, we estimate, over four million staple cartridge deployments, three million of which we believe are deployed in laparoscopic procedures.
In March 2012, we completed the design verification for and applied Conformité Européenne, or the CE Mark, to the MicroCutter XCHANGE 30 and, in December 2012, began a controlled commercial launch of the MicroCutter XCHANGE 30 in Europe. We received from the United States Food and Drug Administration, or FDA, 510(k) clearance for the MicroCutter XCHANGE 30 and blue cartridge in January 2014, and for the white cartridge in February 2014, for use in multiple open or minimally-invasive surgical procedures for the transection, resection and/or creation of anastomoses in small and large intestine, as well as the transection of the appendix. The blue cartridge is for use in medium thickness tissue, and the white cartridge is for use in thin tissue. In March 2014, we made our first sale of the MicroCutter XCHANGE 30 in the United States. We also recently submitted our MicroCutter XCHANGE 30 blue and white cartridges application to Health Canada for regulatory approval of our MicroCutter XCHANGE 30 and, if we receive approval, anticipate launching it in Canada. In addition, our exclusive distributor in Japan, Century Medical, Inc., or Century, filed for regulatory approval of our MicroCutter XCHANGE 30 cartridges with the Pharmaceuticals and Medical Devices Agency in Japan and, upon approval, anticipates launching the MicroCutter XCHANGE 30 in Japan. We believe that the MicroCutter XCHANGE 30 is differentiated in the market compared to currently marketed staplers due to its significantly reduced size and ability to articulate up to 80 degrees.
Historically, our business focused on the design, manufacture and marketing of proprietary automated anastomotic systems used by cardiac surgeons to perform coronary bypass surgery. Our C-Port® Distal Anastomosis Systems, or C-Port systems, are sold in the United States and Europe. The C-Port systems are used to perform a distal anastomosis, which is the connection between a bypass graft vessel and the target coronary artery. As of June 30, 2014, more than 14,300 C- Port systems had been sold in the United States and Europe. We also currently sell our PAS-Port® Proximal Anastomosis System, or PAS-Port system, in the United States, Europe and Japan. The PAS-Port system is used to perform a proximal anastomosis, which is the connection of a bypass graft vessel to the aorta or other source of blood. As of June 30, 2014, more than 37,000 PAS-Port systems had been sold in the United States, Europe and Japan. To date, we generated revenues almost exclusively from the sale of automated anastomotic systems, and have generated minimal revenues from the commercial sales of the MicroCutter XCHANGE 30 since its introductions in Europe in December 2012, and in the United States in March 2014.
For the fiscal year ended June 30, 2014, we generated net revenue of $3.6 million, including $0.5 million from commercial sales of the MicroCutter XCHANGE 30 and $0.1 million of license and development and royalty revenues, and incurred a net loss of $17.0 million.
Since our inception, we have incurred significant net losses, and we expect to continue to incur net losses for at least the next several years. We have not generated significant revenues from the MicroCutter XCHANGE 30. To date, our C-Port and PAS-Port systems have had limited commercial adoption, and sales have not met the levels that we had anticipated. Revenues from product sales and milestone payments were not sufficient to support the operation of our business as we had planned. If we fail to obtain broader commercial adoption of our C-Port and PAS-Port systems or achieve commercial adoption of our microcutter products, we may be required to delay, further reduce the scope of or eliminate our commercialization efforts with respect to one or more of our products or one or more of our research and development programs.
As of June 30, 2014, we had approximately $40.5 million of cash, cash equivalents and short-term investments, $2.3 million in long-term investments and $4.0 million of debt principal outstanding. In April 2014, we sold 37,375,000 shares of our common stock at $0.85 per share, and 191,474 shares of Series A Convertible Preferred Stock at $85 per share. The Series A convertible preferred stock is non-voting and is convertible into shares of our common stock at a conversion rate of 100 shares of common stock for each share of Series A convertible preferred stock, provided that conversion will be prohibited if, as a result, the holder and its affiliates would own more than 9.98% of the total number of shares of the our common stock then outstanding unless the holder gives us at least 61 days prior notice of an intent to convert into shares of common stock that would cause the holder to own more than 9.98% of the total number of shares of common stock then issued and outstanding. Net proceeds from the financing to us were approximately $44.7 million. We intend to use the net proceeds from this offering for general corporate purposes, including the costs of sales and marketing activities, research and development activities, and general and administrative and manufacturing expenses.
We believe that our existing cash, cash equivalents, short-term and long-term investments will be sufficient to meet our anticipated cash needs to enable us to conduct our business substantially as currently conducted for at least the next 12 months. We may be able to extend this time period to the extent that we decrease our planned expenditures, or raise additional capital. We have based our estimate as to the sufficiency of our cash resources on assumptions that may prove to be wrong, including assumptions with respect to the level of revenue from product sales and the cost of product development, and we could exhaust our available financial resources sooner than we currently expect. The sufficiency of our current cash resources and our need for additional capital, and the timing thereof, will depend on many factors, including the extent of our ongoing research and development programs and related costs, including costs related to the development of the MicroCutter XCHANGE 45, and additional products in our planned microcutter product line, our ability to enter into additional license, development and/or collaboration agreements with respect to our technology, and the terms thereof, market acceptance and adoption of our current products or future products that we may commercialize, our level of revenues, costs associated with our sales and marketing initiatives and manufacturing activities, costs and timing of obtaining and maintaining FDA, and other regulatory clearances or approvals for our products and potential additional products, securing, maintaining and enforcing intellectual property rights and the costs thereof, and the effects of competing technological and market developments.
We may seek to sell additional equity or debt securities, obtain a credit facility, enter into product development, license or distribution agreements with third parties or divest one or more of our commercialized products or products in development. The sale of additional equity or convertible debt securities could result in significant dilution to our stockholders, particularly in light of the prices at which our common stock has been recently trading. In addition, if we raise additional funds through the sale of equity securities, new investors could have rights superior to our existing stockholders. If additional funds are raised through the issuance of debt securities, these securities could have rights senior to those associated with our common stock and could contain covenants that would restrict our operations. Any product development, licensing, distribution or sale agreements that we enter into may require us to relinquish valuable rights, including with respect to commercialized products or products in development that we would otherwise seek to commercialize or develop ourselves. We may not be able to obtain sufficient additional financing or enter into a strategic transaction in a timely manner. Our need to raise capital may require us to accept terms that may harm our business or be disadvantageous to our current stockholders.
Agreements with Century
On September 2, 2011, we signed a distribution agreement, or the Distribution Agreement, with Century Medical, Inc., or Century, with respect to distribution of our planned microcutter products in Japan. Under the terms of a secured note purchase agreement entered into at the time of the Distribution Agreement, Century agreed to loan us an aggregate of up to $4.0 million, with principal due on September 30, 2016, subject to certain conditions, which principal due date was extended to September 30, 2018, effective July 1, 2014. Under this facility, we received $2.0 million on September 30, 2011, and the remaining $2.0 million on December 27, 2011. The note bears 5% annual interest which is payable quarterly in arrears on the last business day of March, June, September and December of each year through September 30, 2018, the maturity date when the total $4.0 million of principal becomes due. In return for the loan commitment, we granted Century distribution rights to our planned microcutter product line in Japan, and a right of first negotiation for distribution rights in Japan to future products. Century is responsible for securing regulatory approval from the Ministry of Health in Japan for microcutter products. After approval for marketing in Japan, we would sell microcutter units to Century, which would then sell the microcutter devices to their customers in Japan.
Proceeds from the note and granting the distribution rights were allocated to the note based on their aggregate fair value of $2.4 million at the dates of receipt. This fair value was determined by discounting cash flows using a discount rate of 18%, which we estimated was a market rate of borrowing that could be obtained by companies with credit risk similar to ours. The remainder of the proceeds of $1.6 million was recognized as debt issuance discount and was allocated to the value of the distribution rights granted to Century under the Distribution Agreement and is included in deferred revenue. The deferred revenue will be recognized over the term of the Distribution Agreement, beginning upon the first sale by Century of microcutter products in Japan.
In addition, our distribution agreement with Century pertaining to the PAS-Port system, originally dated June 16, 2003, as amended, was last amended effective July 1, 2014. The last amendment, among other things, renewed the contract for another five years, extending the expiration date to July 31, 2019.
Agreements with Intuitive Surgical
On August 16, 2010, we entered into a license agreement, or License Agreement, with Intuitive Surgical Operations, Inc., or Intuitive Surgical, pursuant to which we granted to Intuitive Surgical a worldwide, sublicenseable, exclusive license to use our intellectual property in the robotics field in diagnostic or therapeutic medical procedures, but excluding vascular anastomosis applications, for an upfront license fee of $9.0 million. We are also eligible to receive a contingent payment if sales of any products incorporating our patent rights achieve a specified level of net sales within a specified period after the date of the License Agreement, as well as single-digit royalties on sales by Intuitive Surgical, its affiliates or its sublicensees of specified stapler and clip applier products covered by our patent rights as well as on sales of certain other products covered by our patent rights that may be developed in the future, if any. Each party has the right to terminate the License Agreement in the event of the other party’s uncured material breach or bankruptcy. Following any termination of the License Agreement, the licenses granted to Intuitive Surgical will continue, and, except in the case of termination for our uncured material breach or insolvency, Intuitive Surgical’s payment obligations will continue as well. Under the License Agreement, Intuitive Surgical has rights to improvements in our technology and intellectual property over a specified period of time.
In addition, on the same date, we entered into a stock purchase agreement with Intuitive Surgical pursuant to which Intuitive Surgical paid $3.0 million to purchase from us an aggregate of 1,249,541 shares of our common stock, or the Stock Issuance. The net proceeds recorded to stockholders’ equity based upon the fair value of our common stock on August 16, 2010, were approximately $2.0 million after offering expenses. From the premium paid of $1.0 million and the upfront license fee payment of $9.0 million, $41,000 and $0.3 million have been recorded as license and development revenue for the fiscal years ended June 30, 2014 and 2013, respectively, and there was no deferred revenue as of June 30, 2014 There were no underwriters or placement agents involved with the Stock Issuance, and no underwriting discounts or commissions or similar fees were payable in connection with the Stock Issuance.
Agreement with MLV
On August 3, 2011, we entered into the ATM Agreement with MLV, which expired on August 2, 2014, that provided for the sale of our common stock through MLV as our sales agent. As of June 30, 2014, we received net proceeds of $1.2 million from the sale of an aggregate of 884,756 shares of common stock through MLV. During the fiscal years ended June 30, 2014 and 2013, we received net proceeds of $0.4 million and $0.7 million, respectively, from the sale of an aggregate of 439,163 and 414,099 shares of common stock through MLV, respectively.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our financial statements which have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP. The preparation of our financial statements requires management to make estimates and assumptions that affect the amounts reported in our financial statements and accompanying notes. Actual results could differ materially from those estimates.
We believe that the following critical accounting policies are the most critical to an understanding of our financial statements because they require us to make significant judgments and estimates that are used in the preparation of our financial statements.
Revenue Recognition. We recognize revenue when four basic criteria are met: (1) persuasive evidence of an arrangement exists; (2) title has transferred; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. We generally use contracts and customer purchase orders to determine the existence of an arrangement. We use contractual terms, shipping documents and third-party proof of delivery to verify that title or rights have transferred. We assess whether the fee is fixed or determinable based upon the terms of the agreement associated with the transaction. To determine whether collection is probable, we assess a number of factors, including past transaction history with the customer and the creditworthiness of the customer. If we determine that collection is not reasonably assured, then the recognition of revenue is deferred until collection becomes reasonably assured, which is generally upon receipt of payment.
We record product sales net of estimated product returns and discounts from the list prices for our products. The amounts of product returns and the discount amounts have not been material to date. Our sales to distributors do not include price protection or product return rights, outside of standard warranties. We include shipping and handling costs in cost of product sales.
Payments that are contingent upon the achievement of a substantive milestone are recognized in their entirety in the period in which the milestone is achieved subject to satisfaction of all revenue recognition criteria at that time. Revenue generated from license fees and performing development services are recognized when they are earned and non-refundable upon receipt, over the period of performance, or upon incurrence of the related development expenses in accordance with contractual terms, based on the actual costs incurred to date plus overhead costs for certain project activities. Amounts paid but not yet earned on the project are recorded as deferred revenue until such time as performance is rendered or the related development expenses are incurred.
Inventory. We state our inventories at the lower of cost or market value on a first-in, first-out basis. Inventory write-downs are established when conditions indicate that the net realizable value is less than cost due to physical deterioration, usage, obsolescence, reductions in estimated future demand or reductions in selling prices. Inventory write-downs are measured as the difference between the cost of inventory and estimated net realizable value. Inventory write-downs are charged to cost of product sales and establish a lower cost basis for the inventory. We balance the need to maintain strategic inventory levels with the risk of obsolescence due to changing technology and the risk of lower customer demand levels. While we believe the current value of inventories represents all known and estimated changes in demand, we have experienced reduced demand for our C-Port systems and further unfavorable changes in market conditions may result in a need for additional inventory write-downs that could adversely impact our financial results.
Stock-Based Compensation. We account for employee and director share-based compensation plans, including stock options and restricted stock units, or RSUs, pursuant to Accounting Standards Codification, or ASC, 718 “Compensation — Stock Compensation”. Stock-based compensation cost is measured on the grant date, based on fair value-based measurement of the award, and is recognized as an expense over the requisite service period which generally equals the vesting period of each grant. We recognize compensation expense using the accelerated method and we account for the non-employee share-based grants pursuant to ASC 505-50 “Equity — Equity Based Payments to Non-Employees”.
We selected the Black-Scholes option pricing model for determining the estimated fair value-based measurements of share-based awards. The use of the Black-Scholes model requires the use of assumptions including expected term, expected volatility, risk-free interest rate and expected dividends. The expected term of options granted is determined using the standard method. Under this approach, we estimate the expected life of options granted based on historical exercise and post-vest cancellation patterns, which we believe are representative of future behavior. The risk-free interest rate for the expected term of each option is based on a risk-free zero-coupon spot interest rate at the time of grant. We have never declared or paid any cash dividends and do not plan to pay cash dividends in the foreseeable future. The expected volatility is based on our historical stock price. We estimate forfeitures in calculating the expense related to stock-based compensation. We recorded fair value-based stock-based compensation expense of $0.9 million, or $ 0.02 per share, $0.9 million, or $0.02 per share, and $0.7 million, or $0.02 per share, for the fiscal years ended June 30, 2014, 2013 and 2012, respectively.
Results of Operations
Comparison of Fiscal Years ended June 30, 2014 and 2013
Net Revenue. Net revenue increased $0.1 million, or 3%, to $3.6 million in fiscal year 2014 compared to $3.5 million in fiscal year 2013.
Net product sales increased $0.4 million, or 13%, to $3.5 million in fiscal year 2014 compared to $3.1 million in fiscal year 2013. The increase of product sales for the fiscal year ended June 30, 2014, was primarily attributable to both higher MicroCutter XCHANGE 30 and PAS-Port systems sales in the United States and international markets, offset in part by lower C-Port systems sales.
For fiscal years 2014 and 2013, sales to Century, our distributor in Japan, accounted for approximately 30% and 33%, respectively, of our total product sales. For the fiscal years ended June 30, 2014 and 2013, sales to Herz-Und Diabeteszentrum in Germany accounted for approximately 12% and 8%, respectively, of our product sales.
License and development revenue from our agreement with Intuitive Surgical and royalty revenue decreased $0.3 million, or 89%, to $0.1 million in fiscal year 2014 compared to $0.4 million in fiscal year 2013. The decrease was primarily attributable to the end of the three years amortization of the license and development agreement with Intuitive Surgical.
Cost of Product Sales. Cost of product sales consists primarily of material, labor and overhead costs. Cost of product sales increased by $1.2 million, or 32%, to $4.8 million in fiscal year 2014 compared to $3.6 million in fiscal year 2013.
The increase in cost of product sales in fiscal year 2014 compared to fiscal year 2013 is primarily driven by higher product cost related to the MicroCutter XCHANGE 30 design changes and the MicroCutter XCHANGE 30 sales in the United States and Europe in fiscal 2014.
Our cost of product sales was 136% and 117% of our net product sales in fiscal years 2014 and 2013, respectively, largely associated with our MicroCutter XCHANGE 30 product line capacity utilization. The production capacity and infrastructure has been put in place in anticipation of the foreseeable future growth of our microcutter products.
We expect higher costs relative to product sales for the next few years due to the planned commercialization of our microcutter product line.
Research and Development Expenses. Research and development expenses consist primarily of personnel costs within our product development, regulatory and clinical groups and the costs of clinical trials. Research and development expenses decreased by $2.2 million, or 25%, to $6.9 million in fiscal year 2014 compared to $9.1 million in fiscal year 2013.
The decrease in research and development expenses in fiscal year 2014 compared to fiscal year 2013 was attributable to a decrease of $1.1 million of material purchases due to the completion of the development of our MicroCutter XCHANGE 30, a decrease of $0.2 million in traveling expenses and a decrease of $0.6 million in clinical expenses, due to the completion of our microcutter clinical trial in Europe. There were also decreases in salaries and benefits expenses of $0.1 million and noncash stock compensation expenses of $0.1 million, due primarily to fewer numbers of personnel.
We anticipate that research and development expenses will decrease modestly in absolute terms in fiscal year 2015 as we have completed our clinical trial, product testing and tooling expenses related to the enhancements of the MicroCutter XCHANGE 30.
Selling, General and Administrative Expenses. Selling, general and administrative expenses consist primarily of costs for administrative and sales and marketing personnel, intellectual property and marketing expenses. Selling, general and administrative expenses increased by $2.1 million, or 32%, to $8.5 million in fiscal year 2014 compared to $6.4 million in fiscal year 2013.
The net increase in selling, general and administrative expenses in fiscal year 2014 compared to fiscal year 2013 was primarily attributable to an increase in microcutter demonstration and sample expenses of $1.1 million related to sales training, an increase in salaries and benefits expenses of $0.6 million and an increase in noncash stock compensation expenses of $0.2 million, mainly due to higher bonus percentage payout based on milestone achievements and the four additions in our sales force, and an increase in travel expenses of $0.2 million related to the recent MicroCutter XCHANGE 30 introduction in the United States.
We expect selling, general and administrative expenses to increase slightly in absolute terms in fiscal year 2015 as we continue to increase the number of individuals in sales and marketing.
Interest Income. Interest income decreased by $3,000, or 20%, to $12,000 for fiscal year 2014 from $15,000 for fiscal year 2013. The decrease in interest income in fiscal year 2014 was primarily attributable to lower cash available for investments.
Interest Expense. Interest expense increased by $47,000 to $0.5 million for fiscal year 2014 from $0.4 million in fiscal year 2013. The increase in interest expense was due to the interest, including the accretion of debt discount, on our note payable to Century, which we issued in September and December 2011. We expect interest expense to increase in future periods as the note payable is scheduled to mature on September 30, 2018, and the debt discount is accreted using the effective interest method.
Deemed dividend related to beneficial conversion feature of convertible preferred stock. We recorded a deemed dividend of $1.9 million in fiscal year ended June 30, 2014, relating to our recently issued and outstanding Series A Convertible Preferred Stock, representing a one-time beneficial conversion charge due to the difference between the common stock price and conversion price on the closing date of our recently completed public offering.
Comparison of Fiscal Years ended June 30, 2013 and 2012
Net Revenue. Net revenue decreased $0.2 million, or 5%, to $3.5 million in fiscal year 2013 compared to $3.7 million in fiscal year 2012.
Net product sales decreased $0.2 million, or 6%, to $3.1 million in fiscal year 2013 compared to $3.3 million in fiscal year 2012. The decrease of product sales for the fiscal year ended June 30, 2013, was primarily attributable to both lower PAS-Port and C-Port systems sales in the United States, offset in part by our MicroCutter XCHANGE 30 commercial sales of $0.2 million.
For fiscal years 2013 and 2012, sales to Century, our distributor in Japan, accounted for approximately 33% and 32%, respectively, of our total product sales. For each of the fiscal years ended June 30, 2013 and 2012, sales to Herz-Und Diabeteszentrum in Germany accounted for approximately 8% of our product sales.
License and development revenue was $0.3 million in both fiscal years 2013 and 2012. The license and development revenue in both years resulted from the amortization of the $1.0 million of premium from the Stock Purchase Agreement with Intuitive Surgical that we entered into in August 2010.
Cost of Product Sales. Cost of product sales consists primarily of material, labor and overhead costs. Cost of product sales decreased by $34,000, or 1%, from fiscal 2012 to fiscal 2013.
The decrease in cost of product sales in fiscal year 2013 compared to fiscal year 2012 is primarily driven by lower product revenue offset in part by higher product cost related to the introduction of the microcutter products in fiscal 2013.
Our cost of product sales was 117% and 111% of our net product sales in fiscal years 2013 and 2012, respectively, due largely to higher fixed overhead costs per unit sold in the current year due to building an infrastructure in anticipating of the foreseeable future growth and higher product cost related to the new microcutter products.
Research and Development Expenses. Research and development expenses consist primarily of personnel costs within our product development, regulatory and clinical groups and the costs of clinical trials. Research and development expenses increased by $1.9 million, or 27%, to $9.1 million in fiscal year 2013 compared to $7.2 million in fiscal year 2012.
The increase in research and development expenses in fiscal year 2013 compared to fiscal year 2012 was attributable to higher salaries and benefits of $0.4 million due primarily to an increase in the number of personnel and higher bonus percentage payout based on milestone achievements, increased prototype project materials of $0.3 million, increased facility related and depreciation expenses of $0.6 million, increased in travel expenses of $0.2 million, and increased clinical trial expenses of $0.5 million all related to microcutter activities.
Selling, General and Administrative Expenses. Selling, general and administrative expenses consist primarily of costs for administrative and sales and marketing personnel, intellectual property and marketing expenses. Selling, general and administrative expenses increased by $0.3 million, or 4%, to $6.4 million in fiscal year 2013 compared to $6.1 million in fiscal year 2012.
The net increase in selling, general and administrative expenses in fiscal year 2013 compared to fiscal year 2012 was attributable to higher salaries and benefits of $0.2 million due primarily to an increase in bonus percentage payout based on milestone achievements, an increased in professional services expenses of $0.3 million due to the MicroCutter XCHANGE 30 launch efforts in Europe, partially offset by lower facility related expenses of $0.1 million.
Interest Income. Interest income increased $3,000, or 25%, to $15,000 for fiscal year 2013 from $12,000 for fiscal year 2012. The increase in interest income in fiscal year 2013 was primarily attributable to higher overall market interest rates for the fiscal year.
Interest Expense. Interest expense increased $0.2 million to $0.5 million for fiscal year 2013 from $0.3 million in fiscal year 2012. The increase in interest expense was due to the interest, including the accretion of debt discount, on our note payable to Century, which we issued in September and December 2011.
Income Taxes
Due to uncertainty surrounding the realization of our deferred tax assets through future taxable income, we have provided a full valuation allowance, and no benefit has been recognized for our net operating losses and other deferred tax assets. Accordingly, deferred tax asset valuation allowances have been established as of June 30, 2014 and 2013, to reflect these uncertainties. At June 30, 2014, we had unrecognized tax benefits of $1.0 million, which would not currently affect our effective tax rate if recognized due to our deferred tax assets being fully offset by a valuation allowance.
As of June 30, 2014, we had net operating loss carry-forwards to reduce future taxable income, of approximately $153.2 million for federal income tax purposes and $93.2 million available to reduce future taxable income, if any, for state income taxes. The net operating loss carry-forwards begin to expire in the fiscal year 2019. We also had federal and state research and development credit carry-forwards of approximately $1.5 million and $3.5 million, respectively, at June 30, 2014. The federal credits begin to expire in fiscal year 2021 if not utilized. The California state credit carry-forwards have an unlimited carry-forward period and the State of Arizona credits begin to expire in fiscal year 2024. We have completed a study of our tax attributes under Section 382 of the Internal Revenue Code of 1986 through June 30, 2010, which resulted in significant limitations on our net operating loss and credit carry-forwards prior to utilization. The related reductions are reflected in the carry-forward amounts discussed above. The most recent analysis of our historical ownership changes was completed in 2014. Due to IRC Section 382 and 383 limitations, we only account for net operating loss and tax credit carry-forwards as deferred tax assets where we reasonably expect that these losses and carry-forwards can be utilized in future periods.
Liquidity and Capital Resources
As of June 30, 2014, our accumulated deficit was $170.5 million. As of June 30, 2014, we had cash, cash equivalents, and short-term investments of $40.5 million, and $2.3 million in long-term investments, compared to cash, cash equivalents, and short-term investments of $12.4 million and no long-term investments at June 30, 2013. We currently invest majority of our cash, cash equivalents, short-term and long-term investments in money market funds, corporate debt and commercial paper securities. As of June 30, 2014 and 2013, we had $4.0 million debt principal outstanding. Since inception, we have financed our operations primarily through private and public sales of convertible preferred stock, long-term notes payable, public and private sales of common stock, warrants to purchase common stock and license or collaboration agreements.
In April 2014, we completed the sale of 37,375,000 shares of our common stock at a price to the public of $0.85 per share and 191,474 shares of Series A Convertible Preferred Stock at a price to the public of $85 per share. Net proceeds from the financing to us were approximately $44.7 million. On March 20, 2013, we completed the sale of 14,251,368 shares of our common stock at a price to the public of $1.05 per share. Net proceeds from the financing to us were $14.0 million. In February 2012, we completed the sale of 9,091,000 shares of our common stock in an underwritten public offering at a price to the public of $1.65 per share. Net proceeds from that financing to us were $13.9 million. We have also sold stock through various agreements with other entities. As of June 30, 2014, we received net proceeds of $1.2 million from the sale of 884,756 shares of our common stock through McNicoll Lewis & Vlak LLC, or MLV, pursuant to an ATM agreement. In addition, in December 2010, we entered into a purchase agreement with Aspire Capital, which provided for the sale of our common stock subject to the conditions set forth in that agreement, which agreement terminated in February 2013. Through the termination date, we had raised $4.4 million of capital through the sale of 1,350,000 shares of our common stock under that purchase agreement; all of the capital raised under that purchase agreement had been raised prior to June 30, 2012.
On September 2, 2011, we entered into a Distribution Agreement with Century, with respect to distribution of our planned microcutter products in Japan. Additionally, under the terms of a secured note purchase agreement, Century agreed to loan us an aggregate of up to $4.0 million, with principal due five years after the first draw by us under the agreement, subject to certain conditions, which principal due date was extended by two years effective July 1, 2014. In return for the loan commitment, we granted Century distribution rights to our planned microcutter product line in Japan, and a right of first negotiation for distribution rights in Japan to future products. Century will be responsible for securing regulatory approval from the Ministry of Health in Japan for the microcutter product line. After approval for marketing in Japan, we would sell microcutter units to Century, who would then sell the microcutter devices to their customers in Japan.
Under this facility, we received $2.0 million on September 30, 2011, and the remaining $2.0 million on December 27, 2011. The note, as amended, bears 5% annual interest which is payable quarterly in arrears on the last business day of March, June, September and December of each year through September 30, 2018, the maturity date when the total $4.0 million of principal becomes due. Proceeds from the note and granting the distribution rights were allocated to the note based on its aggregate fair value of $2.4 million at the dates of receipt. This fair value was determined by discounting cash flows using a discount rate of 18%, which we estimated approximated a market rate of return on debt financing that could be obtained by companies with credit risk similar to us. The remainder of the proceeds of $1.6 million was allocated to the value of the distribution rights granted to Century under the Distribution Agreement and is included in deferred revenue. The deferred revenue will be recognized on a straight-line basis over the term of the Distribution Agreement, beginning upon the first sale by Century of the microcutter products in Japan. In August 2013, Century filed for regulatory approval of our MicroCutter XCHANGE 30 cartridge with the Pharmaceuticals and Medical Devices Agency in Japan, and upon approval, anticipates launching our MicroCutter XCHANGE 30 in Japan.
On August 3, 2011, we entered into the ATM Agreement with MLV, which expired on August 2, 2014, that provided for the sale of our common stock through MLV as our sales agent over the term of the ATM Agreement. As of June 30, 2014, we received net proceeds of $1.2 million from the sale of an aggregate of 884,756 shares of common stock through MLV. During the fiscal years ended June 30, 2014 and 2013, we received net proceeds of $0.4 million and $0.7 million, respectively, from the sale of an aggregate of 439,163 and 414,099 shares of common stock through MLV, respectively.
On November 11, 2010, we entered into an amendment, or Lease Amendment, to our facility lease. Pursuant to the Lease Amendment, the term of the lease is extended four years, through August 31, 2015, and we were granted an improvement allowance of $148,070 to be used in connection with the construction of alterations and refurbishment of improvements in the premises. In addition, under the Lease Amendment, we were granted an option to further extend the lease for a period of two years beyond August 31, 2015, or the Option Term, and the method of determination of the annual rent payable by us during the Option Term was set forth in the Lease Amendment. Under the operating lease we were required to maintain a letter of credit with a restricted cash balance at our bank. A certificate of deposit of $100,000 was recorded as restricted cash in the condensed balance sheets as of June 30, 2014, related to this letter of credit.
Summary cash flow data is as follows:
|
Fiscal Year Ended June 30, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
(In thousands) |
||||||||||||
|
Net cash used in operating activities |
$ | (13,808 | ) | $ | (14,865 | ) | $ | (9,718 | ) | |||
|
Net cash used in investing activities |
(32,332 | ) | (2,063 | ) | (7,234 | ) | ||||||
|
Net cash provided by financing activities |
45,162 | 14,829 | 17,592 | |||||||||
Our net use of cash in operating activities for fiscal year 2014 was primarily attributable to our net loss adjusted for non-cash items primarily due to the continued development and commercialization efforts related to our MicroCutter XCHANGE 30, partially offset by a decreased inventories of $0.4 million due to design changes and MicroCutter XCHANGE 30 sales which directly resulted in an increase in accounts receivable of $0.3 million. Our net use of cash for fiscal year 2013 was primarily attributable to our net loss adjusted for non-cash items, partially offset by an increase in inventories of $0.9 million due to our microcutter product builds, and a decrease in deferred revenue of $0.3 million due to the Intuitive Surgical arrangement. Our net use of cash for fiscal year 2012 was primarily attributable to our net loss adjusted for non-cash items, partially offset by an increase in accounts payable and other accrued liabilities of $0.3 million resulting from the timing of our vendor payments, a decreased inventories of $0.3 million due to lower cardiac sales, and an increase in deferred revenue of $1.3 million resulting from granting Japanese distribution rights for our microcutter product line to Century. Our net losses for the three years as adjusted for non-cash items was due to our cost of product sales exceeding our product sales and our research and development and other expenses incurred in developing our microcutter products.
Net cash used in investing activities of $32.3 million for fiscal year 2014, reflects purchases of property and equipment of $0.8 million mainly related to tools and molds modifications purchased for our microcutter development as well as a net purchases of investments of $31.5 million. Net cash used in investing activities of $2.1 million for fiscal year 2013, reflects purchases of property and equipment of $ 2.1 million mainly related to tools and molds modifications purchased for our microcutter. Net cash used in investing activities of $7.2 million for fiscal year 2012, reflects purchases of property and equipment of $ 2.5 million related to tools and molds purchased for our microcutter development as well as net purchases of short-term investments of $4.7 million.
Net cash provided by financing activities of $45.2 million for fiscal year 2014, was due primarily to the net proceeds of $44.7 million received from our public stock offering in April 2014, and an aggregate net proceeds of $0.4 million received from the sale of shares of common stock through MLV. Net cash provided by financing activities of $14.8 million for fiscal year 2013, was due primarily to the net proceeds of $14.0 million received from our common stock offering in March 2013, aggregate net proceeds of $0.8 million received from the sale of shares of common stock through MLV and proceeds from the exercise of options of $88,000. Net cash provided by financing activities of $17.6 million for fiscal year 2012, was due primarily to the net proceeds of $13.9 million received from our common stock offering in February 2012, the net proceeds from the issuance of a note payable to Century of $2.4 million and aggregate net proceeds of $1.2 million received from the sale of shares of common stock to Aspire Capital and through MLV.
We believe that our existing cash, cash equivalents, short-term and long-term investments will be sufficient to meet our anticipated cash needs to enable us to conduct our business substantially as currently conducted through at least the next 12 months. We would be able to extend this time period to the extent that we decrease our planned expenditures, or raise additional capital. We have based our estimate on assumptions that may prove to be wrong, including assumptions with respect to the level of revenue from product sales, and the cost of product development, including the process for obtaining FDA approval for the commercial use of our microcutter products in the United States and internationally, and we could exhaust our available financial resources sooner than we currently expect.
The sufficiency of our current cash resources and our need for additional capital, and the timing thereof, will depend upon numerous factors. These factors include, but are not limited to, the following:
|
● |
the extent to which we are able to raise additional capital in any equity or debt transaction; |
|
● |
market acceptance of our MicroCutter XCHANGE 30 in Europe and in the United States; |
|
● |
our success in obtaining regulatory approval from the Pharmaceuticals and Medical Devices Agency of our MicroCutter XCHANGE 30 cartridge in Japan and the timing of such approval, and market acceptance of our MicroCutter XCHANGE 30 cartridge in Japan if such approval is obtained; |
|
● |
the extent of our ongoing enhancements of the MicroCutter XCHANGE 30, including alterations and post-commercialization improvements based on early adopter experience with this newly commercial product; |
|
• |
the extent of our ongoing research and development programs and related costs, including costs related to the continued development of the MicroCutter XCHANGE 45 and additional future products and features in our microcutter product line; |
|
• |
our ability to enter into additional license, development and/or collaboration agreements with respect to our technology, and the terms thereof; |
|
• |
market acceptance and adoption of future products that we may commercialize; |
|
• |
our level of revenues; |
|
• |
costs associated with our sales and marketing initiatives and manufacturing activities; |
|
• |
costs associated with our potential proxy contest with Broadfin Capital, including costs associated with any potential litigation arising or resulting from the potential proxy contest; |
|
• |
costs and timing of obtaining and maintaining FDA and other regulatory clearances and approvals for our products and potential additional products; |
|
• |
securing, maintaining and enforcing intellectual property rights and the costs thereof; and |
|
• |
the effects of competing technological and market developments. |
As part of our controlled commercial launch of the MicroCutter XCHANGE 30 in Europe and in the United States, we made our first sales in December 2012 and March 2014, respectively. We have agreements for the microcutter product line with four distributors in Europe. In addition, in August 2014, we established a subsidiary in Germany, Cardica, GmbH, to facilitate direct sale of the microcutter product. In the United States, we have secured approval from 38 hospital Value Analysis Committees (VACs) who have approved the MicroCutter XCHANGE 30 either for purchase or for an evaluation and potential subsequent purchase. We intend to continue to make enhancements to the MicroCutter XCHANGE 30 and develop the MicroCutter XCHANGE 45 before continuing our efforts to develop other products in our planned microcutter product line. We cannot predict when, if ever, we will generate significant commercial revenue from the sale of either of these products or any other products in our planned microcutter product line. Because we do not anticipate that we will generate sufficient product sales to achieve profitability for at least the next few years, if at all, we may need to raise substantial additional capital to finance our operations in the future. Until we can generate significant continuing revenue, if ever, we expect to satisfy our future cash needs public or private equity offerings, debt financings or corporate collaboration and licensing arrangements, as well as through interest income earned on cash balances. To raise capital, we may seek to sell additional equity or debt securities, obtain a credit facility or enter into product development, license or distribution agreements with third parties or divest one or more of our commercialized products or products in development. However, we cannot be certain that additional funding of any kind will be available on acceptable terms, or at all. The sale of additional equity or convertible debt securities could result in significant dilution to our stockholders, particularly in light of the prices at which our common stock has been recently trading. If additional funds are raised through the issuance of debt securities, these securities could have rights senior to those associated with our common stock and could contain covenants that would restrict our operations. Any product development, licensing, distribution or sale agreements that we enter into may require us to relinquish valuable rights, including with respect to commercialized products or products in development that we would otherwise seek to commercialize or develop ourselves. We may not be able to obtain sufficient additional funding or enter into a strategic transaction in a timely manner. Our need to raise capital may require us to accept terms that may harm our business or be disadvantageous to our current stockholders. If adequate funds are not available or revenue from product sales do not increase, we would be required to reduce our workforce, delay, reduce the scope of or eliminate our commercialization efforts with respect to one or more of our products or one or more of our research and development programs in advance of the 12 months, to ensure that we have sufficient capital to meet our obligations and continue on a path designed to preserve stockholder value.
Broadfin Healthcare Master Fund, LTD, or Broadfin, has announced its intention to initiate a proxy contest with respect to the election of directors at our 2014 annual meeting of stockholders, or the Annual Meeting. Broadfin is proposing to solicit proxies in opposition to us for the purpose of voting in favor of its four nominees for election to our board of directors. Further, in discussions with Kevin Kotler of Broadfin, Mr. Kotler has expressed a desire to replace our chief executive officer, Dr. Hausen. Responding to the potential proxy contest is costly and time-consuming, is a significant distraction for our board of directors, management and employees, and diverts the attention of our board of directors and senior management from the pursuit of our business strategy, which could adversely affect our results of operations and financial condition. Further, we have incurred, and will continue to incur, expenses for legal and advisory fees and administrative and associated costs incurred in connection with responding to the potential proxy contest, which may include related litigation, which costs may be substantial.
Contractual Obligations
Our future contractual obligations at June 30, 2014, were as follows (in thousands):
|
Contractual Obligations: |
Total |
7/1/2014 - 6/30/2015 |
7/1/2015 - 9/30/2018 |
|||||||||
|
Operating lease obligations |
$ | 807 | $ | 689 | $ | 118 | ||||||
|
Purchase commitments |
270 | 270 | — | |||||||||
|
Note payable, including interest |
4,850 | 200 | 4,650 | |||||||||
|
Total |
$ | 5,927 | $ | 1,159 | $ | 4,768 | ||||||
This compares to future contractual obligations at June 30, 2013, of $6.3 million.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606): Revenue from Contracts with Customers, which guidance in this update will supersede the revenue recognition requirements in Topic 605, Revenue Recognition, and most industry-specific guidance when it becomes effective. ASU No. 2014-09 affects any entity that enters into contracts with customers to transfer goods or services or enters into contracts for the transfer of nonfinancial assets unless those contracts are within the scope of other standards. The core principal of ASU No. 2014-09 is that a company will recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. In doing so, companies will need to use more judgment and make more estimates than under current guidance. These may include identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation. ASU No. 2014-09 is effective for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period, which will be our fiscal year 2018 (or July 1, 2017), and entities can transition to the standard either retrospectively or as a cumulative-effect adjustment as of the date of adoption. Early adoption is prohibited. We will be evaluating the impact of the adoption of this guidance on our financial statements.
In July 2013, the FASB issued an accounting standard update which states that an unrecognized tax benefit should be presented in the financial statements as a reduction to a deferred tax asset for a net operating loss carryforward, a similar tax loss, or a tax credit carryforward if such settlement is required or expected in the event the uncertain tax position is disallowed. In situations where a net operating loss carryforward, a similar tax loss, or a tax credit carryforward is not available at the reporting date under the tax law of the applicable jurisdiction or the tax law of the jurisdiction does not require, and the entity does not intend to use the deferred tax asset for such purpose, the unrecognized tax benefit should be presented in the financial statements as a liability and should not be combined with deferred tax assets. The guidance will be effective prospectively for reporting periods beginning after December 15, 2013. We do not expect the adoption of this guidance to have a material impact on our financial statements.
In March 2013, the FASB issued an accounting standard update which requires the release of cumulative translation adjustments into net income when an entity ceases to have a controlling financial interest resulting in the complete or substantially complete liquidation of a subsidiary or group of assets within a foreign entity. The guidance will be effective prospectively for reporting periods beginning after December 15, 2013. We do not expect the adoption of this guidance to have a material impact on our financial statements.
In February 2013, the FASB issued amended standards to improve the reporting of reclassifications out of accumulated other comprehensive income by requiring an entity to report their corresponding effect(s) on net income. These amended standards are effective for annual reporting periods beginning after December 15, 2012. We adopted this guidance in July 2013, and it did not have a material impact on our financial statements for the fiscal year ended June 30, 2014.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, including structured finance, special purpose or variable interest entities.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We had cash, cash equivalents, short-term and long-term investments of $42.8 million at June 30, 2014, compared to $12.4 million at June 30, 2013. These amounts were invested primarily in money market funds and marketable securities and are held for working capital purposes. The marketable securities were invested primarily in corporate debt securities and commercial papers. We do not enter into investments for trading or speculative purposes. We do not believe that a 10% drop in interest rates would have a material effect on the fair value of our marketable securities due to the short-term nature of these instruments. Declines in interest rates, however, will reduce future investment income.
Item 8. Financial Statements and Supplementary Data
Index to Financial Statements
|
|
Page |
|
Report of Independent Registered Public Accounting Firm |
53 |
|
Report of Independent Registered Public Accounting Firm |
54 |
|
Balance Sheets |
55 |
|
Statements of Operations |
56 |
|
Statements of Comprehensive Loss |
57 |
|
Statements of Stockholders’ Equity |
58 |
|
Statements of Cash Flows |
59 |
|
Notes to Financial Statements |
60 |
|
Supplemental Data: Financial Information by Quarter |
75 |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Cardica, Inc.
We have audited the accompanying balance sheets of Cardica, Inc. as of June 30, 2014 and 2013, and the related statements of operations, comprehensive loss, stockholders’ equity and cash flows for the each of the two years in the period ended June 30, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Cardica, Inc. at June 30, 2014 and 2013, and the results of its operations and its cash flows for each of the two years in the period ended June 30, 2014, in conformity with U.S. generally accepted accounting principles.
/s/ BDO USA, LLP
San Jose, California
September 25, 2014
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Cardica, Inc.
We have audited the accompanying statements of operations, stockholders’ equity and cash flows for the year ended June 30, 2012. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the results of its operations and its cash flows for the year ended June 30, 2012, in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
Redwood City, California
September 28, 2012
Cardica, Inc.
BALANCE SHEETS
(In thousands, except share and per share data)
|
June 30, |
||||||||
|
2014 |
2013 |
|||||||
|
Assets |
||||||||
|
Current assets |
||||||||
|
Cash and cash equivalents |
$ | 5,395 | $ | 6,373 | ||||
|
Short-term investments |
35,086 | 6,022 | ||||||
|
Accounts receivable, net of allowance of $0 and $33 |
706 | 391 | ||||||
|
Inventories |
1,086 | 1,457 | ||||||
|
Prepaid expenses and other current assets |
349 | 253 | ||||||
|
Total current assets |
42,622 | 14,496 | ||||||
|
Property and equipment, net |
2,536 | 3,161 | ||||||
|
Long-term investments |
2,315 | — | ||||||
|
Restricted cash |
104 | 104 | ||||||
|
Total assets |
$ | 47,577 | $ | 17,761 | ||||
|
Liabilities and stockholders’ equity |
||||||||
|
Current liabilities |
||||||||
|
Accounts payable |
$ | 847 | $ | 764 | ||||
|
Accrued compensation |
899 | 600 | ||||||
|
Other accrued liabilities |
437 | 420 | ||||||
|
Current portion of deferred rent |
71 | — | ||||||
|
Current portion of deferred revenue |
403 | 444 | ||||||
|
Total current liabilities |
2,657 | 2,228 | ||||||
|
Deferred revenue, net of current portion |
1,610 | 1,610 | ||||||
|
Note payable |
3,092 | 2,788 | ||||||
|
Other non-current liabilities |
33 | 161 | ||||||
|
Total liabilities |
7,392 | 6,787 | ||||||
|
Commitments and contingencies (Note 5) |
||||||||
| Stockholders’ equity | ||||||||
|
Preferred stock, $0.001 par value: 5,000,000 shares authorized at June 30, 2014 and 2013, 191,474 and 0 shares issued and outstanding at June 30, 2014 and 2013, respectively |
17,214 | — | ||||||
|
Common stock, $0.001 par value: 125,000,000 and 75,000,000 shares authorized; 89,005,443 and 51,068,614 shares issued and 88,939,216 and 51,002,387 shares outstanding at June 30, 2014 and 2013, respectively |
89 | 51 | ||||||
|
Additional paid-in capital |
194,015 | 165,085 | ||||||
|
Treasury stock at cost (66,227 shares at June 30, 2014 and 2013) |
(596 | ) | (596 | ) | ||||
|
Accumulated other comprehensive loss |
(10 | ) | (5 | ) | ||||
|
Accumulated deficit |
(170,527 | ) | (153,561 | ) | ||||
|
Total stockholders’ equity |
40,185 | 10,974 | ||||||
|
Total liabilities and stockholders’ equity |
$ | 47,577 | $ | 17,761 | ||||
See accompanying notes to financial statements.
Cardica, Inc.
STATEMENTS OF OPERATIONS
(In thousands, except per share data)
|
Fiscal Year Ended June 30, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Net revenue |
||||||||||||
|
Product sales, net |
$ | 3,505 | $ | 3,093 | $ | 3,274 | ||||||
|
License and development revenue |
41 | 336 | 336 | |||||||||
|
Royalty revenue |
69 | 70 | 71 | |||||||||
|
Total net revenue |
3,615 | 3,499 | 3,681 | |||||||||
|
Operating costs and expenses |
||||||||||||
|
Cost of product sales |
4,770 | 3,604 | 3,638 | |||||||||
|
Research and development |
6,883 | 9,145 | 7,220 | |||||||||
|
Selling, general and administrative |
8,463 | 6,410 | 6,139 | |||||||||
|
Total operating costs and expenses |
20,116 | 19,159 | 16,997 | |||||||||
|
Loss from operations |
(16,501 | ) | (15,660 | ) | (13,316 | ) | ||||||
|
Interest income |
12 | 15 | 12 | |||||||||
|
Interest expense |
(504 | ) | (457 | ) | (268 | ) | ||||||
|
Other income (expense), net |
27 | (35 | ) | (3 | ) | |||||||
|
Net loss before income tax |
$ | (16,966 | ) | $ | (16,137 | ) | $ | (13,575 | ) | |||
|
Income tax benefit |
— | — | — | |||||||||
|
Net loss |
$ | (16,966 | ) | $ | (16,137 | ) | $ | (13,575 | ) | |||
|
Deemed dividend related to beneficial conversion feature of convertible preferred stock |
(1,915 | ) | — | — | ||||||||
|
Net loss allocable to common stockholders |
$ | (18,881 | ) | $ | (16,137 | ) | $ | (13,575 | ) | |||
|
Basic and diluted net loss per share allocable to common stockholders |
$ | (0.32 | ) | $ | (0.40 | ) | $ | (0.44 | ) | |||
|
Shares used in computing basic and diluted net loss per share allocable to common stockholders |
58,395 | 40,842 | 30,547 | |||||||||
See accompanying notes to financial statements.
Cardica, Inc.
STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
|
Fiscal Year Ended June 30, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Net loss |
$ | (16,966 | ) | $ | (16,137 | ) | $ | (13,575 | ) | |||
|
Other comprehensive loss: |
||||||||||||
|
Change in unrealized loss on investment |
(5 | ) | — | (4 | ) | |||||||
|
Comprehensive loss |
$ | (16,971 | ) | $ | (16,137 | ) | $ | (13,579 | ) | |||
See accompanying notes to financial statements.
Cardica, Inc.
STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands, except share data)
| Convertible Preferred Stock | Common Stock |
Additional Paid-in |
Treasury |
Accumulated other comprehensive |
Accumulated |
Total Stockholders’ |
||||||||||||||||||||||||||||||
|
Shares |
Amount |
Shares |
Amount |
Capital |
Stock |
loss |
Deficit |
Equity |
||||||||||||||||||||||||||||
|
Balance at June 30, 2011 |
— | $ | — | 26,635,115 | $ | 27 | $ | 133,281 | $ | (596 | ) | $ | (1 | ) | $ | (123,849 | ) | $ | 8,862 | |||||||||||||||||
|
Issuance of common stock upon exercise of employee stock options for cash |
— | — | 163,438 | — | 134 | — | — | — | 134 | |||||||||||||||||||||||||||
|
Issuance of common stock upon release of restricted share units |
— | — | 60,500 | — | — | — | — | — | — | |||||||||||||||||||||||||||
|
Sale of common stock, net of financing costs of $1.2 million |
— | — | 9,652,335 | 10 | 15,058 | — | — | — | 15,068 | |||||||||||||||||||||||||||
|
Stock-based compensation expense |
— | — | — | — | 875 | — | — | — | 875 | |||||||||||||||||||||||||||
|
Net loss |
— | — | — | — | — | — | — | (13,575 | ) | (13,575 | ) | |||||||||||||||||||||||||
|
Net change in unrealized loss on marketable securities |
— | — | — | — | — | — | (4 | ) | — | (4 | ) | |||||||||||||||||||||||||
|
Balance at June 30, 2012 |
— | — | 36,511,388 | 37 | 149,348 | (596 | ) | (5 | ) | (137,424 | ) | 11,360 | ||||||||||||||||||||||||
|
Issuance of common stock upon exercise of employee stock options for cash |
— | — | 10,518 | — | 88 | — | — | — | 88 | |||||||||||||||||||||||||||
|
Issuance of common stock upon release of restricted share units |
— | — | 48,000 | — | — | — | — | — | — | |||||||||||||||||||||||||||
|
Sale of common stock, Wedbush net of financing costs of $1.0 million |
— | — | 14,251,368 | 14 | 13,998 | — | — | — | 14,012 | |||||||||||||||||||||||||||
|
Sale of common stock, Aspire returned commitment shares |
— | — | (166,759 | ) | — | — | — | — | — | — | ||||||||||||||||||||||||||
|
Sale of common stock, MLV net of financing costs $25,000 |
— | — | 414,099 | — | 729 | — | — | — | 729 | |||||||||||||||||||||||||||
|
Stock-based compensation expense |
— | — | — | — | 922 | — | — | — | 922 | |||||||||||||||||||||||||||
|
Net loss |
— | — | — | — | — | — | — | (16,137 | ) | (16,137 | ) | |||||||||||||||||||||||||
|
Balance at June 30, 2013 |
— | — | 51,068,614 | 51 | 165,085 | (596 | ) | (5 | ) | (153,561 | ) | 10,974 | ||||||||||||||||||||||||
|
Issuance of common stock upon release of restricted share units |
— | — | 122,666 | — | — | — | — | — | — | |||||||||||||||||||||||||||
|
Sale of common stock, net of issuance costs of $2.3 million |
— | — | 37,375,000 | 38 | 29,375 | — | — | — | 29,413 | |||||||||||||||||||||||||||
|
Sale of preferred stock, net of issuance costs of $1.0 million |
191,474 | 15,299 | — | — | — | — | — | — | 15,299 | |||||||||||||||||||||||||||
|
Deemed dividend related to beneficial conversion feature of Series A preferred stock |
— | 1,915 | — | — | (1,915 | ) | — | — | — | — | ||||||||||||||||||||||||||
|
Sale of common stock, MLV net of issuance costs $51,000 |
— | — | 439,163 | — | 450 | — | — | — | 450 | |||||||||||||||||||||||||||
|
Stock-based compensation expense |
— | — | — | — | 1,020 | — | — | — | 1,020 | |||||||||||||||||||||||||||
|
Net loss |
— | — | — | — | — | — | — | (16,966 | ) | (16,966 | ) | |||||||||||||||||||||||||
|
Net change in unrealized loss on marketable securities |
— | — | — | — | — | — | (5 | ) | — | (5 | ) | |||||||||||||||||||||||||
|
Balance at June 30, 2014 |
191,474 | $ | 17,214 | 89,005,443 | $ | 89 | $ | 194,015 | $ | (596 | ) | $ | (10 | ) | $ | (170,527 | ) | $ | 40,185 | |||||||||||||||||
See accompanying notes to financial statements.
Cardica, Inc.
STATEMENTS OF CASH FLOWS
(In thousands)
|
Fiscal Year Ended June 30, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Operating activities |
||||||||||||
|
Net loss |
$ | (16,966 | ) | $ | (16,137 | ) | $ | (13,575 | ) | |||
|
Adjustments to reconcile net loss to net cash used in operating activities |
||||||||||||
|
Depreciation and amortization of property and equipment |
1,387 | 1,154 | 858 | |||||||||
|
Amortization of premiums on marketable securities |
126 | 135 | — | |||||||||
|
Loss on disposal or retirement of property and equipment |
60 | 68 | 102 | |||||||||
|
Stock-based compensation expense on grants of stock awards to non-employees |
107 | 65 | 134 | |||||||||
|
Stock-based compensation expense on grants of stock awards to employees |
913 | 857 | 741 | |||||||||
|
Allowance for doubtful account |
(33 | ) | 33 | — | ||||||||
|
Non cash interest expense |
304 | 256 | 142 | |||||||||
|
Changes in assets and liabilities |
||||||||||||
|
Accounts receivable |
(282 | ) | (125 | ) | 28 | |||||||
|
Prepaid expenses and other current assets |
(96 | ) | (39 | ) | (54 | ) | ||||||
|
Inventories |
371 | (881 | ) | 264 | ||||||||
|
Accounts payable and other accrued liabilities |
171 | (86 | ) | 324 | ||||||||
|
Accrued compensation |
299 | 190 | (120 | ) | ||||||||
|
Deferred revenue |
(41 | ) | (336 | ) | 1,276 | |||||||
|
Other non-current liabilities |
(128 | ) | (19 | ) | 162 | |||||||
|
Net cash used in operating activities |
(13,808 | ) | (14,865 | ) | (9,718 | ) | ||||||
|
Investing activities |
||||||||||||
|
Purchases of property and equipment |
(822 | ) | (2,079 | ) | (2,550 | ) | ||||||
|
Proceeds from maturities of investments |
8,648 | 10,082 | 6,445 | |||||||||
|
Purchases of investments |
(40,158 | ) | (10,066 | ) | (11,129 | ) | ||||||
|
Net cash used in investing activities |
(32,332 | ) | (2,063 | ) | (7,234 | ) | ||||||
|
Financing activities |
||||||||||||
|
Proceeds from sales of convertible preferred stock, net of issuance costs |
15,299 | — | — | |||||||||
|
Proceeds from sales of common stock, net of issuance costs |
29,863 | 14,741 | 15,068 | |||||||||
|
Proceeds from issuance of (repayment of) notes payable |
— | — | 2,390 | |||||||||
|
Proceeds from issuance of common stock pursuant to the exercise of stock options |
— | 88 | 134 | |||||||||
|
Net cash provided by financing activities |
45,162 | 14,829 | 17,592 | |||||||||
|
Net increase (decrease) in cash and cash equivalents |
(978 | ) | (2,099 | ) | 640 | |||||||
|
Cash and cash equivalents at beginning of period |
6,373 | 8,472 | 7,832 | |||||||||
|
Cash and cash equivalents at end of period |
$ | 5,395 | $ | 6,373 | $ | 8,472 | ||||||
|
Supplemental disclosure of cash flow information |
||||||||||||
|
Cash paid for interest |
$ | 200 | $ | 200 | $ | 77 | ||||||
|
Supplemental disclosure of non-cash investing and financing information |
||||||||||||
|
Deemed dividend related to beneficial conversion feature of convertible preferred stock |
$ | 1,915 | $ | — | $ | — | ||||||
See accompanying notes to financial statements.
Cardica, Inc.
Notes to Financial Statements
Note 1. Organization and Summary of Significant Accounting Policies
Organization
Cardica, Inc. ( the “Company”) was incorporated in the state of Delaware on October 15, 1997, as Vascular Innovations, Inc. On November 26, 2001, the Company changed its name to Cardica, Inc. The Company is commercializing and developing a microcutter product line based on its proprietary “staple-on-a-strip” technology intended for use by thoracic, bariatric, colorectal and general surgeons. The microcutter product line consists of the currently commercially-available MicroCutter XCHANGE® 30, a cartridge based microcutter device with a 5 millimeter shaft diameter and a 30 millimeter staple line, and products in development, including the MicroCutter XCHANGE® 45, a cartridge based microcutter device with an 8 millimeter shaft and a 45 millimeter staple line, the MicroCutter FLEXCHANGE™ 30, a cartridge based microcutter device with a flexible shaft to facilitate endoscopic procedures requiring cutting and stapling, and the MicroCutter XPRESS® 45, a multi-fire endolinear microcutter device with a 45 millimeter staple line specifically designed for the bariatric and thoracic surgery markets.
In March 2012, the Company completed the design verification for and applied Conformité Européenne, or the CE Mark, to the MicroCutter XCHANGE 30 and, in December 2012, began a controlled commercial launch of the MicroCutter XCHANGE 30 in Europe. The Company received from the United States Food and Drug Administration, or FDA, 510(k) clearance for the MicroCutter XCHANGE 30 and blue cartridge in January 2014, and for the white cartridge in February 2014, for use in multiple open or minimally-invasive surgical procedures for the transection, resection and/or creation of anastomoses in small and large intestine, as well as the transection of the appendix. The blue cartridge is for use in medium thickness tissue, and the white cartridge is for use in thin tissue. In March 2014, the Company made its first sale of the MicroCutter XCHANGE 30 in the United States. The Company also recently submitted the MicroCutter XCHANGE 30 blue and white cartridges application to Health Canada for regulatory approval of the MicroCutter XCHANGE 30 and, if the Company receives approval, anticipate launching it in Canada. In addition, in August 2013, the Company’s exclusive distributor in Japan, Century Medical, Inc., or Century, filed for regulatory approval of the MicroCutter XCHANGE 30 cartridges with the Pharmaceuticals and Medical Devices Agency in Japan and, upon approval, anticipates launching the MicroCutter XCHANGE 30 in Japan.
To date, the Company generated revenues almost exclusively from the sale of automated anastomotic systems, and has generated minimal revenues from the commercial sales of the MicroCutter XCHANGE 30 since its introduction in Europe in December 2012, and in the United States in March 2014.
Liquidity
The Company has incurred cumulative net losses of $170.5 million through June 30, 2014, negative cash flows from operating activities and expects to incur losses for the next several years. As of June 30, 2014, the Company had approximately $40.5 million of cash, cash equivalents and short-term investments, $2.3 million in long-term investments and $4.0 million of debt principal outstanding. The Company believes that its existing cash, cash equivalents, short-term and long-term investments, will be sufficient to meet its anticipated cash needs to enable the Company to conduct its business substantially as currently conducted for at least the next 12 months. The Company would be able to extend this time period to the extent that it decreases its planned expenditures, or raises additional capital.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles (“GAAP”) generally requires management to make estimates and assumptions that affect the amounts reported in the financial statements. Actual results could materially differ from these estimates.
Cash and Cash Equivalents
The Company’s cash and cash equivalents are maintained in checking, money market and mutual fund investment accounts. The Company considers all highly liquid investments with maturities remaining on the date of purchase of three months or less to be cash equivalents.
Accounts Receivable
Accounts receivable consists of trade receivables and other receivables. Accounts receivable are recorded at net realizable value, which approximates fair value. The Company evaluates the collectability of accounts receivable on a case-by-case basis and makes adjustments to the bad debt reserve for expected losses. The Company considers factors such as ability to pay, bankruptcy, credit ratings, payment history and past-due status of the accounts. If circumstances related to customers change, estimates of recoverability would be further adjusted. For the fiscal year ended June 30, 2014, the Company recovered $33,000 of bad debt reserve that was recorded in the fiscal year ended June 30, 2013.
Available-for-Sale Securities
Available-for-sale securities consist primarily of corporate debt securities, commercial papers, and certificates of deposits, and, by the Company's investment policy, restrict exposure to any single corporate issuer by imposing concentration limits. Although maturities may extend beyond one year, it is management's intent that these securities are available for use in current operations.
The Company held investments in marketable securities as of June 30, 2014 and 2013, with maturity dates of less than one year for short-term and greater than one year for long-term. The Company records its marketable securities at fair value and classifies them as available-for-sale. The cost of securities sold is based on the specific-identification method. Interest on securities classified as available-for-sale is included in interest income. Unrealized gains or losses on available-for-sale securities are classified as other comprehensive income or loss and reported as a separate component of stockholders’ equity until realized.
When the resulting fair value is significantly below cost basis and/or the significant decline has lasted for an extended period of time, the Company performs an evaluation to determine whether the marketable equity security is other than temporarily impaired. The evaluation that the Company uses to determine whether a marketable equity security is other than temporarily impaired is based on the specific facts and circumstances present at the time of assessment, which include significant quantitative and qualitative assessments and estimates regarding credit ratings, collateralized support, the length of time and significance of a security’s loss position and intent and ability to hold a security to maturity or forecasted recovery.
Investments are summarized as follows (in thousands):
|
As of June 30, 2014 |
||||||||||||||||
|
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value |
|||||||||||||
|
Available-for-sale securities: |
||||||||||||||||
|
Money market funds – Short-term |
$ | 17,658 | $ | — | $ | — | $ | 17,658 | ||||||||
|
Corporate debt securities – Short-term |
14,434 | — | (6 |
) |
14,428 | |||||||||||
|
Commercial paper - Short-term |
3,000 | — | — | 3,000 | ||||||||||||
|
Corporate debt securities – Long-term |
2,319 | — | (4 |
) |
2,315 | |||||||||||
|
Total |
$ | 37,411 | $ | — | $ | (10 |
) |
$ | 37,401 | |||||||
|
As of June 30, 2013 |
||||||||||||||||
|
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value |
|||||||||||||
|
Available-for-sale securities: |
||||||||||||||||
|
Corporate debt securities – Short-term |
$ | 5,527 | $ | — | $ | (5 |
) |
$ | 5,522 | |||||||
|
Commercial paper – Short-term |
500 | — | — | 500 | ||||||||||||
|
Total |
$ | 6,027 | $ | — | $ | (5 |
) |
$ | 6,022 | |||||||
Restricted Cash
Under an operating lease for its facility in Redwood City, California, the Company is required to maintain a letter of credit with a restricted cash balance at the Company’s bank. A certificate of deposit of $100,000 at June 30, 2014 and 2013, has been recorded as restricted cash in the accompanying balance sheets, related to the letter of credit (see Note 5).
A certificate of deposit of $4,000 at June 30, 2014 and 2013, has been recorded as restricted cash in the accompanying balance sheets related to the deposit on the Company’s merchant credit card.
Concentrations of Credit Risk and Certain Other Risks
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash, cash equivalents, short-term investments, long-term investments and accounts receivable. The Company places its cash, cash equivalents, short-term and long-term investments with high-credit quality financial institutions. The Company is exposed to credit risk in the event of default by the institutions holding the cash, cash equivalents, short-term and long-term investments to the extent of the amounts recorded on the balance sheet. The Company sells its products to hospitals in the U.S. and Europe and to distributors in Europe, Japan and Saudi Arabia that resell the products to hospitals. The Company does not require collateral to support credit sales. The Company has had insignificant credit losses to date.
The following table illustrates total net revenue from the geographic location in which the Company’s customers are located and sales revenue by product line.
Net revenue by geographic location:
|
Fiscal Year Ended June 30, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
United States |
45 | % | 53 | % | 59 | % | ||||||
|
Japan |
29 | % | 29 | % | 29 | % | ||||||
|
Europe |
26 | % | 17 | % | 12 | % | ||||||
|
Rest of world |
— | 1 | % | — | ||||||||
Sales revenue by product line:
|
Fiscal Year Ended June 30, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Microcutter |
$ | 488 | $ | 176 | $ | — | ||||||
|
Cardiac (automated anastomotic systems) |
3,017 | 2,917 | 3,274 | |||||||||
|
Total |
$ | 3,505 | $ | 3,093 | $ | 3,274 | ||||||
The following table illustrates concentrations of credit risk for the periods presented.
|
Percent of Total Net Revenue for Fiscal Year Ended June 30, |
Percent of Total Accounts Receivable as of June 30, |
|||||||||||||||||||
|
2014 |
2013 |
2012 |
2014 |
2013 |
||||||||||||||||
|
Century Medical |
29 | % | 29 | % | 29 | % | 35 | % | 33 | % | ||||||||||
|
Herz-Und Diabeteszentrum |
12 | % | 7 | % | 7 | % | 8 | % | — | |||||||||||
As of June 30, 2014, 2013 and 2012, and for the years then ended, no other customer accounted for equal to or greater than 10% of net revenue or account receivable balances. The Company does not believe that accounts receivable from Century Medical and Herz-Und Diabeteszentrum represent a significant credit risk based on past collection experiences and the general creditworthiness of these customers.
The Company depends upon a number of key suppliers, including single source suppliers, the loss of which would materially harm the Company’s business. Single source suppliers are relied upon for certain components and services used in manufacturing the Company’s products. The Company does not have long-term contracts with any of the suppliers; rather, purchase orders are submitted for each order. Because long-term contracts do not exist, none of the suppliers are required to provide the Company any guaranteed minimum quantities.
Inventories
Inventories are recorded at the lower of cost or market on a first-in, first-out basis. The Company periodically assesses the recoverability of all inventories, including materials, work-in-process and finished goods, to determine whether adjustments for impairment are required. Inventory that is obsolete or in excess of forecasted usage is written down to its estimated net realizable value based on assumptions about future demand and market conditions. Further reduced demand may result in the need for additional inventory write-downs in the near term. Inventory write-downs are charged to cost of product sales and establish a lower cost basis for the inventory.
Property and Equipment
Property and equipment are stated at cost and depreciated on a straight-line basis over the estimated useful lives of the related assets, which are generally three to five years. Amortization of leasehold improvements is computed using the straight-line method over the shorter of the remaining lease term or the estimated useful life of the related assets. Upon sale or retirement of assets, the costs and related accumulated depreciation and amortization are removed from the balance sheet and the resulting gain or loss is reflected in the statement of operations.
Impairment of Long-Lived Assets
The Company reviews long-lived assets, including property and equipment, for impairment whenever events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. An impairment loss is recognized when estimated undiscounted future cash flows expected to result from the use of the asset and its eventual disposition are less than its carrying amount. Impairment, if any, is assessed using discounted cash flows. All long-lived assets are in the United States, and through June 30, 2014, there have been no indications of impairment; therefore, the Company has recorded no such losses.
Revenue Recognition
The Company recognizes revenue when four basic criteria are met: (1) persuasive evidence of an arrangement exists; (2) title has transferred; (3) the fee is fixed or determinable; and (4) collectability is reasonably assured. The Company uses contracts and customer purchase orders to determine the existence of an arrangement. The Company uses shipping documents and third-party proof of delivery to verify that title has transferred. The Company assesses whether the fee is fixed or determinable based upon the terms of the agreement associated with the transaction. To determine whether collection is probable, the Company assesses a number of factors, including past transaction history with the customer and the creditworthiness of the customer. If the Company determines that collection is not reasonably assured, then the recognition of revenue is deferred until collection becomes reasonably assured, which is generally upon receipt of payment.
The Company records product sales net of estimated product returns and discounts from the list prices for its products. The amounts of product returns and the discount amounts have not been material to date. The Company’s sales to distributors do not include price protection or product return rights, outside of standard warranties. The Company includes shipping and handling costs in cost of product sales.
Payments that are contingent upon the achievement of a substantive milestone are recognized in their entirety in the period in which the milestone is achieved subject to satisfaction of all revenue recognition criteria at that time. Revenue generated from license fees and performing development services are recognized when they are earned and non-refundable upon receipt, over the period of performance, or upon incurrence of the related development expenses in accordance with contractual terms, based on the actual costs incurred to date plus overhead costs for certain project activities. Amounts paid but not yet earned on a project are recorded as deferred revenue until such time as performance is rendered or the related development expenses, plus overhead costs for certain project activities, are incurred.
Research and Development
Research and development expenses consist of costs incurred for internally sponsored research and development, direct expenses, research-related overhead expenses, and costs incurred on development contracts. Research and development costs are charged to research and development expenses as incurred.
Clinical Trials
The Company accrues and expenses costs for clinical trial activities performed by third parties based upon estimates of the percentage of work completed over the life of the individual study in accordance with agreements established with contract research organizations and clinical trial sites. The Company determines the estimates through discussion with internal clinical personnel and outside service providers as to progress or stage of completion of trials or services and the agreed upon fee to be paid for such services. Costs of setting up clinical trial sites for participation in the trials are expensed immediately as research and development expenses. Clinical trial site costs related to patient enrollment are accrued as patients are entered into the trial.
Deferred Rent
Rent expense is recognized on a straight-line basis over the non-cancelable term of the Company’s facility operating lease. The difference between the actual amounts paid and amounts recorded as rent expense is recorded to deferred rent. The current portion of deferred rent is recorded as other accrued liabilities, while the non-current portion is recorded in non-current accrued liabilities.
Income Taxes
The Company utilizes the liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between the financial reporting and tax reporting bases of assets and liabilities and are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The Company would classify interest and penalties related to uncertain tax positions in income tax expense, if applicable. There was no interest expense or penalties related to unrecognized tax benefits recorded through June 30, 2014.
Segments
The Company operates in one segment. Management uses one measurement of profitability and does not segregate its business for internal reporting purposes. All of the Company’s operations are in the United States and all of its long-lived assets are maintained in the United States.
Net Loss per Share
Basic net loss per share is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period without consideration of potential common shares. Diluted net loss per share is computed by dividing the net loss by the weighted-average number of common shares outstanding for the period less the dilutive potential common shares for the period determined using the treasury-stock method. For purposes of this calculation, options, warrants and underlying convertible preferred shares to purchase stock and unvested restricted stock awards are considered to be potential common shares and are only included in the calculation of diluted net loss per share when their effect is dilutive.
In the years the Preferred Stock was outstanding, the two-class method was used to calculate basic and diluted earnings (loss) per common share since it is a participating security under ASC 260 Earnings per Share. The two-class method is an earnings allocation formula that determines earnings per share for each class of common stock and participating security according to dividends declared (or accumulated) and participation rights in undistributed earnings. Under the two-class method, basic earnings (loss) per common share is computed by dividing net earnings (loss) attributable to common share after allocation of earnings to participating securities by the weighted-average number of common shares outstanding during the year. Diluted earnings (loss) per common share is computed using the more dilutive of the two-class method or the if-converted method. In periods of net loss, no effect is given to participating securities since they do not contractually participate in the losses of the Company.
The following table sets forth the computation of the basic and diluted net loss per share (in thousands, except per share data):
|
Fiscal Year Ended June 30, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Numerator: |
||||||||||||
|
Net loss |
$ | (16,966 | ) | $ | (16,137 | ) | $ | (13,575 | ) | |||
|
Deemed dividend related to beneficial conversion feature of convertible preferred stock |
(1,915 | ) | — | — | ||||||||
|
Net loss allocable to common stockholders |
$ | (18,881 | ) | $ | (16,137 | ) | $ | (13,575 | ) | |||
|
Denominator: |
||||||||||||
|
Weighted-average shares outstanding allocable to common stockholders |
58,395 | 40,842 | 30,547 | |||||||||
|
Denominator for basic and diluted net loss per share allocable to common stockholders |
58,395 | 40,842 | 30,547 | |||||||||
|
Basic and diluted net loss per share allocable to common stockholders |
$ | (0.32 | ) | $ | (0.40 | ) | $ | (0.44 | ) | |||
The following table sets forth the outstanding securities not included in the diluted net loss per common share calculation for the fiscal years ended June 30, 2014 and 2013, because their effect would be antidilutive (in thousands):
|
As of June 30, |
||||||||
|
2014 |
2013 |
|||||||
|
Options to purchase common stock |
5,601 | 3,936 | ||||||
|
Non-vested restricted stock units and awards |
16 | 46 | ||||||
|
Shares reserved for issuance upon conversion of Series A Preferred |
19,147 | — | ||||||
|
Warrants |
3,991 | 3,991 | ||||||
| 28,755 | 7,973 | |||||||
Stock-Based Compensation
Stock-based compensation expense related to employee and director share-based compensation plans, including stock options and restricted stock units, is measured on the grant date, based on the fair value-based measurement of the award and is recognized as an expense over the requisite service period which generally equals the vesting period of each grant. The Company recognizes compensation expense using the accelerated method and the Company accounts for the non-employee share-based grants pursuant to ASC 505-50, Equity Based Payments to Non-Employees.
The Company selected the Black-Scholes option pricing model for determining the estimated fair value-based measurements of share-based awards. The use of the Black-Scholes model requires the use of assumptions including expected term, expected volatility, risk-free interest rate and expected dividends. The Company used the following assumptions in its fair value-based measurements:
|
Fiscal Year Ended June 30, |
|||||||||||||||
|
2014 |
2013 |
2012 |
|||||||||||||
|
Risk-free interest rate |
0.91% – 1.49% |
0.44% – 0.74% |
0.58% – 0.83% |
||||||||||||
|
Dividend yield |
— | — | — | ||||||||||||
|
Weighted-average expected life (in years) |
3.8 – 4.6 |
3.8 – 4.6 |
3.8 – 4.6 |
||||||||||||
|
Volatility |
66% – 80 % |
78% – 88 % |
86% – 93 % |
||||||||||||
The Company estimates the expected life of options granted based on historical exercise and post-vest cancellation patterns, which the Company believes are representative of future behavior. The risk-free interest rate for the expected term of each option is based on a risk-free zero-coupon spot interest rate at the time of grant. The Company has never declared or paid any cash dividends and does not presently plan to pay cash dividends in the foreseeable future. The expected volatility is based on the Company’s historical stock price. The Company estimates forfeitures in calculating the expense related to stock-based compensation. The Company recorded stock-based compensation expenses under ASC 718 of $0.9 million, or $0.02 per share, $0.9 million, or $0.02 per share, and $0.7 million, or $0.02 per share for the fiscal years ended June 30, 2014, 2013 and 2012, respectively. The Company recorded stock-based compensation expenses under ASC 505-50 of $0.1 million, or $0 per share for fiscal years ended June 30, 2014, 2013 and 2012.
Total compensation expense related to unvested awards not yet recognized is approximately $0.6 million at June 30, 2014, and is expected to be recognized over a weighted average period of 3.6 years.
Included in the statement of operations is the following non-cash stock-based compensation expense for the periods reported, including non-employee stock based compensation expense and the amortization of deferred compensation (in thousands):
|
Fiscal Year Ended June 30, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Cost of product sales |
$ | 117 | $ | 88 | $ | 82 | ||||||
|
Research and development |
133 | 236 | 162 | |||||||||
|
Selling, general and administrative |
770 | 598 | 631 | |||||||||
|
Total |
$ | 1,020 | $ | 922 | $ | 875 | ||||||
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606): Revenue from Contracts with Customers, which guidance in this update will supersede the revenue recognition requirements in Topic 605, Revenue Recognition, and most industry-specific guidance when it becomes effective. ASU No. 2014-09 affects any entity that enters into contracts with customers to transfer goods or services or enters into contracts for the transfer of nonfinancial assets unless those contracts are within the scope of other standards. The core principal of ASU No. 2014-09 is that a company will recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. In doing so, companies will need to use more judgment and make more estimates than under current guidance. These may include identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation. ASU No. 2014-09 is effective for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period, which will be the Company’s fiscal year 2018 (or July 1, 2017), and entities can transition to the standard either retrospectively or as a cumulative-effect adjustment as of the date of adoption. Early adoption is prohibited. The Company will be evaluating the impact of the adoption of this guidance on the Company’s financial statements.
In July 2013, the FASB issued an accounting standard update which states that an unrecognized tax benefit should be presented in the financial statements as a reduction to a deferred tax asset for a net operating loss carryforward, a similar tax loss, or a tax credit carryforward if such settlement is required or expected in the event the uncertain tax position is disallowed. In situations where a net operating loss carryforward, a similar tax loss, or a tax credit carryforward is not available at the reporting date under the tax law of the applicable jurisdiction or the tax law of the jurisdiction does not require, and the entity does not intend to use the deferred tax asset for such purpose, the unrecognized tax benefit should be presented in the financial statements as a liability and should not be combined with deferred tax assets. The guidance will be effective prospectively for reporting periods beginning after December 15, 2013. The Company does not expect the adoption of this guidance to have a material impact on the Company’s financial statements.
In March 2013, the FASB issued an accounting standard update which requires the release of cumulative translation adjustments into net income when an entity ceases to have a controlling financial interest resulting in the complete or substantially complete liquidation of a subsidiary or group of assets within a foreign entity. The guidance will be effective prospectively for reporting periods beginning after December 15, 2013. The Company does not expect the adoption of this guidance to have a material impact on the Company’s financial statements.
In February 2013, the FASB issued amended standards to improve the reporting of reclassifications out of accumulated other comprehensive income by requiring an entity to report their corresponding effect(s) on net income. These amended standards are effective for annual reporting periods beginning after December 15, 2012. The Company adopted this guidance in July 2013, and it did not have a material impact on the Company’s financial statements for the fiscal year ended June 30, 2014.
Note 2. Fair Value Measurements
FASB Accounting Standards Codification (“ASC”) 820, “Fair Value Measurements and Disclosures,” defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. ASC 820 establishes a three-level fair value hierarchy that prioritizes the inputs used to measure fair value. The three levels of inputs used to measure fair value are as follows:
Level 1 — Quoted prices in active markets for identical assets or liabilities.
Level 2 — Observable inputs other than quoted prices included in Level 1, such as quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data.
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The Company does not have any liabilities that are measured at fair value on a recurring basis. All assets that are measured at fair value on a recurring basis have been segregated into the most appropriate level within the fair value hierarchy based on the inputs used to determine the fair value at the measurement date. These assets measured at fair value are summarized below (in thousands):
|
As of June 30, 2014 |
||||||||||||||||
|
Level 1 |
Level 2 |
Level 3 |
Total |
|||||||||||||
|
Cash equivalents: |
||||||||||||||||
|
Money market funds |
$ | 2,500 | $ | — | $ | — | $ | 2,500 | ||||||||
|
Short-term investments: |
||||||||||||||||
|
Money market funds |
17,658 | — | — | 17,658 | ||||||||||||
|
Corporate debt securities |
— | 14,428 | — | 14,428 | ||||||||||||
|
Commercial paper |
3,000 | — | 3,000 | |||||||||||||
|
Long-term investments: |
||||||||||||||||
|
Corporate debt securities |
— | 2,315 | — | 2,315 | ||||||||||||
|
Total assets at fair value |
$ | 20,158 | $ | 19,743 | $ | — | $ | 39,901 | ||||||||
|
As of June 30, 2013 |
||||||||||||||||
|
Level 1 |
Level 2 |
Level 3 |
Total |
|||||||||||||
|
Cash equivalents: |
||||||||||||||||
|
Money market funds |
$ | 5,462 | $ | — | $ | — | $ | 5,462 | ||||||||
|
Short-term investments: |
||||||||||||||||
|
Corporate debt securities |
— | 5,522 | — | 5,522 | ||||||||||||
|
Commercial paper |
— | 500 | — | 500 | ||||||||||||
|
Total assets at fair value |
$ | 5,462 | $ | 6,022 | $ | — | $ | 11,484 | ||||||||
Funds held in money market instruments, are included in Level 1 as their fair values are based on market prices/quotes for identical assets in active markets.
Corporate debt securities and commercial papers are valued primarily using market prices comparable securities, bid/ask quotes, interest rate yields, and prepayment spreads and are included in Level 2.
Cash balances of $2.9 million and $0.9 million at June 30, 2014 and 2013, respectively, are not included in the fair value hierarchy disclosure. As of June 30, 2014, the Company’s material financial assets and liabilities were reported at their current carrying values which approximate fair value given the short-term nature of less than a year, except for its note payable. As of June 30, 2014, the Company’s note payable was reported at its current carrying value which approximates fair value based on Level 3 unobservable inputs involving discounted cash flows and the estimated market rate of borrowing that could be obtained by companies with credit risk similar to the Company’s credit risk.
Note 3. Inventories
Inventories consisted of the following (in thousands):
|
June 30, 2014 |
June 30, 2013 |
|||||||
|
Raw materials |
$ | 669 | $ | 473 | ||||
|
Work in progress |
207 | 594 | ||||||
|
Finished goods |
210 | 390 | ||||||
|
Total |
$ | 1,086 | $ | 1,457 | ||||
Note 4. Property and Equipment
Property and equipment consisted of the following (in thousands):
|
June 30, |
||||||||
|
2014 |
2013 |
|||||||
|
Computer hardware and software |
$ | 70 | $ | 62 | ||||
|
Office furniture and equipment |
27 | 27 | ||||||
|
Machinery and equipment |
6,306 | 5,916 | ||||||
|
Leasehold improvements |
174 | 174 | ||||||
| 6,577 | 6,179 | |||||||
|
Less: accumulated depreciation and amortization |
(4,215 | ) | (3,079 | ) | ||||
|
Subtotal |
2,362 | 3,100 | ||||||
|
Construction in process (1) |
174 | 61 | ||||||
|
Total |
$ | 2,536 | $ | 3,161 | ||||
(1) Construction in process includes equipments paid based on installment plan, but not yet placed in service pending completion. The completion dates for these equipments range from three months to two years, and the future payments are immaterial.
Note 5. Commitments and Contingencies
On November 11, 2010, the Company entered into an amendment to its facility lease (the “Lease Amendment”). Pursuant to the Lease Amendment, the term of the lease was extended by four years, through August 31, 2015, and the Company was granted an improvement allowance of $148,070 to be used in connection with the construction of alterations and refurbishment of improvements in the premises, which was used and reimbursed in the fiscal year ended June 30, 2012. The leasehold improvement allowance will be recorded as a reduction of rent expense on a straight-line basis over the term of the lease. In addition, under the Lease Amendment, the Company was granted an option to further extend the lease for a period of two years beyond August 31, 2015 (the “Option Term”), with the annual rent payable by the Company during the Option Term to be equal to the annual rent for comparable buildings, as described in the Lease Amendment. Under the operating lease, the Company is required to maintain a letter of credit with a restricted cash balance at the Company’s bank. A certificate of deposit of $100,000 was recorded as restricted cash in the condensed balance sheets as of June 30, 2014 and 2013, related to the letter of credit.
Future minimum lease payments under the non-cancelable operating leases having initial terms of a year or more as of June 30, 2014, including the Lease Amendment, are as follows (in thousands):
|
Fiscal year ending June 30, |
Operating Leases |
|||
|
2015 |
689 | |||
|
2016 |
118 | |||
|
Total minimum lease payments |
$ | 807 | ||
Rent expense for fiscal years 2014, 2013 and 2012, was $0.6 million, $0.6 million and $0.6 million, respectively.
Note 6. Distribution, License, Development and Commercialization Agreements
Century
On September 2, 2011, the Company signed a distribution agreement (the “Distribution Agreement”) with Century Medical, Inc. (“Century”) with respect to distribution of the Company’s planned microcutter products in Japan. Under the terms of a secured note purchase agreement, Century agreed to loan the Company an aggregate of up to $4.0 million, with principal due in September 30, 2016, subject to certain conditions, which principal due date was extended by two years effective July 1, 2014. Under this facility, the Company received $2.0 million on September 30, 2011, and the remaining $2.0 million on December 27, 2011. The note bears 5% annual interest which is payable quarterly in arrears through September 30, 2018, the maturity date when the total $4.0 million of principal becomes due. In return for the loan commitment, the Company granted Century distribution rights to the Company’s planned microcutter product line in Japan, and a right of first negotiation for distribution rights in Japan to future products. Century will be responsible for securing regulatory approval from the Ministry of Health in Japan for the microcutter product line. After approval for marketing in Japan, the Company would sell microcutter units to Century, who would then sell the microcutter devices to their customers in Japan.
Proceeds from the note and granting the distribution rights were allocated to the note based on its aggregate fair value of $2.4 million at the dates of receipt. This fair value was determined by discounting cashflows using a discount rate of 18%, which the Company estimated a market rate of borrowing that could be obtained by companies with credit risk similar to the Company’s. The remainder of the proceeds of $1.6 million was recognized as debt issuance discount and was allocated to the value of the distribution rights granted to Century under the Distribution Agreement and is included in deferred revenue. The deferred revenue will be recognized over the term of the Distribution Agreement, beginning upon the first sale by Century of the microcutter products in Japan.
In addition, the distribution agreement with Century pertaining to the PAS-Port system, originally dated June 16, 2003, as amended, was last amended effective July 1, 2014. The last amendment, among other things, renewed the contract for another five years, extending the expiration date to July 31, 2019.
Cook Incorporated
In June 2007, the Company entered into, and in September 2007 and in June 2009 amended, a license, development and commercialization agreement with Cook, to develop and commercialize a specialized device, referred to as the PFO device, designed to close holes in the heart from genetic heart defects known as patent foramen ovales (“PFOs”). Under the agreement, Cook funded certain development activities and the Company and Cook jointly developed the device. The Company’s significant deliverables under the arrangement were the license rights and the associated development activities. These deliverables were determined to represent one unit of accounting as there was no standalone value to the license rights. If developed, Cook would receive an exclusive, worldwide, royalty-bearing license, with the right to grant sublicenses, to make, have made, use, sell, offer for sale and import the PFO device. Under this agreement, the Company received no payments in the fiscal years ended June 30, 2014, 2013 and 2012. Amounts paid but not yet earned on the project are recorded as deferred revenue until such time as the related development expenses for certain project activities are incurred. A total of $0.4 million under this agreement has been recorded as deferred development revenue on the balance sheet as of June 30, 2014. On January 6, 2010, the Company and Cook mutually agreed to suspend work on the PFO project and, accordingly, the Company does not anticipate receiving any additional payments or recording any additional revenue related to this agreement in the foreseeable future.
Intuitive Surgical
On August 16, 2010, the Company entered into a license agreement with Intuitive Surgical Operations, Inc., or Intuitive Surgical, (the “License Agreement”) pursuant to which the Company granted to Intuitive Surgical a worldwide, sublicenseable, exclusive license to use the Company’s intellectual property in the robotics field in diagnostic or therapeutic medical procedures, but excluding vascular anastomosis applications, for an upfront license fee of $9.0 million. The Company is also eligible to receive a contingent payment related to achieving a certain sales volume. Each party has the right to terminate the License Agreement in the event of the other party’s uncured material breach or bankruptcy. Following any termination of the License Agreement, the licenses granted to Intuitive Surgical will continue, and except in the case of termination for the Company’s uncured material breach or insolvency, Intuitive Surgical’s payment obligations will continue as well. Under the License Agreement, Intuitive Surgical has rights to improvements in the Company’s technology and intellectual property over a specified period of time.
The Company determined that there were two substantive deliverables under the License Agreement representing separate units of accounting: license rights to technology that existed as of August 16, 2010, and license rights to technology that may be developed over the following three years. The $9.0 million upfront license payment and $1.0 million premium on the stock purchase by Intuitive Surgical (see Note 8) were aggregated and allocated to the two units of accounting based upon the relative estimated selling prices of the deliverables. The relative estimated selling prices of the deliverables were determined using a probability weighted expected return model with significant inputs relating to the nature of potential future outcomes and the probability of occurrence of future outcomes. Based upon the relative estimated selling prices of the deliverables, $9.0 million of the total consideration of $10.0 million was allocated to the license rights to technology that existed as of August 16, 2010, that has been recognized as revenue in the fiscal year ended June 30, 2011, and $1.0 million was allocated to technology that may be developed over the following three years that is being recognized as revenue ratably over that three year period. In total, the revenue recognized for the fiscal years ended June 30, 2014, 2013 and 2012, related to this arrangement were $41,000, $0.3 million and $0.3 million, respectively. The Company has fully recognized such revenue, and as of June 30, 2014, no deferred revenue related to this arrangement.
Note 7. Notes Payable
In connection with the Distribution Agreement with Century (see Note 6), the Company entered into a secured note purchase agreement and a related security agreement pursuant to which Century agreed to loan to the Company up to an aggregate of $4.0 million, which amount was received in the fiscal year ended June 30, 2012, and the secured note purchase agreement was amended effective July 1, 2014, to extend the principal due date by two years. Under this facility, the Company received $2.0 million on September 30, 2011, and the remaining $2.0 million on December 27, 2011. This note bears 5% annual interest which is payable quarterly in arrears on the last business day of March, June, September and December of each year through September 30, 2018, the maturity date when the total $4.0 million of principal becomes due. The debt issuance discount of approximately $1.6 million is reflected as a reduction in long-term debt and is being amortized as interest expense over the term of the note using the effective interest method. The note is secured by substantially all of the Company's assets, including the Company’s intellectual property related to the PAS-Port® Proximal Anastomosis System, but excluding all other intellectual property, until the note is repaid. There are no covenants associated with this debt.
The Company made interest payments of $200,000, $200,000 and $77,000 in the fiscal years ended June 30, 2014, 2013 and 2012, respectively. The interest payable at June 30, 2014 and 2013, was $50,000 and $50,000, respectively, and included in other accrued liabilities in the accompanying balance sheets.
Note 8. Stockholders’ Equity
As of June 30, 2014, the total number of shares that the Company is authorized to issue is 130,000,000 shares, with 125,000,000 shares designated as common stock and 5,000,000 shares designated as preferred stock. As of June 30, 2013, the total number of shares that the Company was authorized to issue is 80,000,000 shares, with 75,000,000 shares designated as common stock and 5,000,000 shares designated as preferred stock.
Common Stock
Holders of common stock are entitled to one vote per share on all matters to be voted upon by the stockholders of the Company. Subject to the preferences that may be applicable to any outstanding shares of preferred stock, the holders of common stock are entitled to receive ratably such dividends, if any, as may be declared by the Board of Directors. No dividends have been declared to date.
In April 2014, the Company sold 37,375,000 shares of its common stock at $0.85 per share, and 191,474 shares of Series A Convertible Preferred Stock at $85 per share. The Series A convertible preferred stock is non-voting and is convertible into shares of its common stock at a conversion rate of 100 shares of common stock for each share of Series A convertible preferred stock, provided that conversion will be prohibited if, as a result, the holder and their affiliates would own more than 9.98% of the total number of shares of the Company’s common stock then outstanding unless the holder gives the Company at least 61 days prior notice of an intent to convert into shares of common stock that would cause the holder to own more than 9.98% of the total number of shares of common stock then issued and outstanding. Net proceeds from the financing to the Company were approximately $44.7 million. For fiscal year ended June 30, 2014, the Company recorded a deemed dividend of $1.9 million related to beneficial conversion feature of series A convertible preferred stock. A one-time beneficial conversion charge was due to the difference between the common stock price and conversion price on the closing date of the Company’s recently completed public offering.
On March 20, 2013, the Company completed the sale of 14,251,368 shares of its common stock at a price to the public of $1.05 per share. Net proceeds from the financing to the Company were $14.0 million.
In February 2012, the Company completed the sale of 9,091,000 shares of its common stock in an underwritten public offering at a price to the public of $1.65 per share. Net proceeds from that financing to us were $13.9 million.
On August 3, 2011, the Company entered into the At The Market Issuance Sales Agreement (the “ATM Agreement”) with McNicoll, Lewis & Vlak LLC (“MLV”), which provided for the sale of the Company’s common stock through MLV as the Company’s sales agent. The ATM Agreement expired on August 2, 2014. As of June 30, 2014, the Company had received net proceeds of $1.2 million from the sale of an aggregate of 884,756 shares of common stock through MLV. During the fiscal years ended June 30, 2014 and 2013, the Company received net proceeds of $0.4 million and $0.7 million, respectively, from the sale of an aggregate of 439,163 and 414,099 shares of common stock through MLV, respectively.
On August 16, 2010, the Company entered into a Stock Purchase Agreement with Intuitive Surgical pursuant to which Intuitive Surgical paid $3.0 million to purchase from the Company an aggregate of 1,249,541 newly-issued shares of the Company’s common stock (the “Stock Issuance”). The net proceeds recorded to stockholders’ equity based upon the fair value of the common stock on August 16, 2010, were approximately $2.0 million after offering expenses. See Note 6, Distribution, License, Development and Commercialization Agreements, for a discussion of the accounting treatment of the premium paid of $1.0 million, which is the amount Intuitive Surgical paid above the fair market value of the Company’s stock on the date of the agreement. There were no underwriters or placement agents involved with the Stock Issuance, and no underwriting discounts or commissions or similar fees were payable in connection with the Stock Issuance. Under the associated Registration Rights Agreement between the Company and Intuitive Surgical, the Company was required to meet certain obligations with respect to (1) filing a registration statement with the Securities and Exchange Commission pertaining to all common stock issued to Intuitive Surgical, and (2) using its reasonable best efforts to cause the registration statement to be declared effective within a specified number of days after filing the registration statement. The Company filed a registration statement related to the stock issued to Intuitive Surgical, and it was declared effective within the timeframes specified in the Registration Rights Agreement.
On December 14, 2010, the Company entered into a common stock purchase agreement (the “Purchase Agreement”) with Aspire Capital Fund, LLC, an Illinois limited liability company (“Aspire Capital”), which provided that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital was committed to purchase up to an aggregate of $10.0 million of shares of the Company’s common stock (the “Purchase Shares”) over the term of the Purchase Agreement at purchase prices determined in accordance with the Purchase Agreement. Pursuant to the Purchase Agreement, on any trading day on which the closing sale price of the Company’s common stock exceeded $1.00 per share, the Company had the right, in its sole discretion, to present Aspire Capital with a purchase notice, directing Aspire Capital to purchase up to (i) 100,000 shares of the Company’s common stock per trading day if the closing sale price of the Company’s common stock was above $1.00 per share, (ii) 200,000 shares of the Company’s common stock per trading day if the closing sale price of the Company’s common stock was above $2.25 per share and (iii) 300,000 shares of the Company’s common stock per trading day if the closing sale price of the Company’s common stock was above $3.50 per share. The purchase price per Purchase Share was to equal to the lesser of (i) the lowest sale price of the Company’s common stock on the purchase date or (ii) the arithmetic average of the three lowest closing sale prices for the Company’s common stock during the twelve consecutive trading days ending on the trading day immediately preceding the purchase date.
In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, the Company issued to Aspire Capital 295,567 shares of the Company’s common stock as a commitment fee (the “Commitment Shares”). The value of the Commitment Shares of $966,000 and other costs related to entering into the Purchase Agreement of $134,000 represented financing costs that were recorded to additional paid-in capital upon capital being raised under the Purchase Agreement. The Purchase Agreement provided that the Company may not issue and sell more than 4,930,747 shares of the Company’s common stock, including the Commitment Shares.
The Purchase Agreement terminated on February 10, 2013, and 166,759 shares of the Company’s common stock issued pursuant to the Purchase Agreement were returned to the Company as the maximum numbers of shares available under the Purchase Agreement were not sold to Aspire. Based on the quoted price, the shares were valued at $1.38 per share, or $230,000. The Company is no longer entitled to sell any further shares of its common stock to Aspire Capital under the Purchase Agreement. Through the termination date, a total of 1,478,808 shares of common stock (including the 128,808 Commitment Shares) had been issued to Aspire Capital pursuant to the Purchase Agreement and $4.4 million of capital had been raised through the sale of 1,350,000 shares of common stock at an average price of $3.23 per share.
Preferred Stock
The Company has 5,000,000 shares of authorized preferred stock issuable in one or more series. The Company can determine the number of shares constituting any series and the designation of such series and the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences and sinking fund terms, any or all of which may be greater than the rights of common stock. The issuance of the preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change in control of the Company or other corporate action. As of June 30, 2014, the Company had designated 250,000 shares of preferred stock as series A convertible preferred stock, and there were 191,474 shares of series A convertible preferred stock issued and outstanding. There were no shares of preferred stock designated as series A convertible preferred stock or issued and outstanding as of June 30, 2013. For the fiscal year ended June 30, 2014, the Company recorded a deemed dividend of $1.9 million related to beneficial conversion feature of series A convertible preferred stock. A one-time beneficial conversion charge was due to the difference between the common stock price and conversion price on the closing date of the Company’s recently completed public offering.
Each share of Series A preferred stock is convertible into 100 shares of the Company’s common stock at any time at the option of the holder, provided that the holder will be prohibited from converting Series A preferred stock into shares of the Company’s common stock if, as a result of such conversion, the holder, together with its affiliates, would own more than 9.98% of the total number of shares of the Company’s common stock then issued and outstanding, unless the holder gives us at least 61 days prior notice of an intent to convert into shares of common stock that would cause the holder to own more than 9.98% of the total number of shares of the Company’s common stock then issued and outstanding. In the event of the Company’s liquidation, dissolution, or winding up, holders of the Company’s Series A preferred stock will share ratably with the holders of the Company’s common stock on an as-if-converted basis. Shares of Series A preferred stock will generally have no voting rights, except as required by law and except that the consent of holders of a majority of the outstanding Series A Preferred Stock will be required to alter or change adversely the powers, preferences or rights given to the Series A preferred stock (an increase the number of authorized shares of Series A preferred stock shall not constitute an adverse change) or enter into any agreement to do so. Shares of Series A Preferred Stock will not be entitled to receive any dividends, unless a cash dividend is declared by the Company’s board of directors to be paid to the holders of common stock, in which case the holders of Series A Preferred Stock will be entitled to receive a cash dividend equal to the amount of dividends declared on the common stock on an as-if-converted basis.
Shares Reserved
Shares of common stock reserved for future issuance are as follows:
|
June 30, 2014 |
||||
|
Stock options and RSUs outstanding |
5,617,479 | |||
|
Shares available for grant under stock option plan |
716,941 | |||
|
Shares reserved for issuance upon conversion of Series A Preferred |
19,147,400 | |||
|
Warrants for common stock |
3,991,205 | |||
| 29,473,025 | ||||
Stock Options
In 1997, the Company adopted the 1997 Equity Incentive Plan (the “1997 Plan”). The 1997 Plan provides for the granting of options to purchase common stock and the issuance of shares of common stock, subject to Company repurchase rights, to directors, employees and consultants. Certain options are immediately exercisable, at the discretion of the Board of Directors. Shares issued pursuant to the exercise of an unvested option are subject to the Company’s right of repurchase which lapses over periods specified by the board of directors, generally four years from the date of grant. In February 2006, the Company terminated all remaining unissued shares under the 1997 Plan. Although the 1997 Plan terminated, all outstanding options thereunder will continue to be governed by their existing terms.
In October 2005, the Company’s Board of Directors adopted, and in December 2005 the stockholders approved, the 2005 Equity Incentive Plan, as amended (the “2005 Plan”). Pursuant to a series of amendments, a total of 6,400,000 shares of common stock have been reserved for issuance under the 2005 Plan as of June 30, 2014.
Stock awards granted under the 2005 Plan may either be incentive stock options, nonstatutory stock options, stock bonuses or rights to acquire restricted stock. Incentive stock options may be granted to employees with exercise prices of no less than the fair value of the common stock on the date of grant, as determined by the Board of Directors, and nonstatutory options may be granted to employees, directors or consultants at exercise prices of no less than the fair value. If, at the time the Company grants an option, the awardee directly or by attribution owns stock possessing more than 10% of the total combined voting power of all classes of stock of the Company, the option price shall be at least 110% of the fair value and shall not be exercisable more than five years after the date of grant. Options may be granted with vesting terms as determined by the Board of Directors. Options expire no more than 10 years after the date of grant, or earlier if employment is terminated.
Common stock options may include a provision whereby the holder, while an employee, director or consultant, may elect at any time to exercise the option as to any part or all of the shares subject to the option prior to the full vesting of the option. Any unvested shares so purchased are subject to repurchase by the Company at its option and at a price equal to the original purchase price of the stock. The Company does not consider the stock issued upon exercise of an unvested stock option substantively exercised, and the cash paid for the exercise price is considered a deposit or a prepayment of the exercise price that is recognized by the Company as a liability. As the underlying shares vest, the deposit liability is reclassified as equity. As of June 30, 2014 and 2013, no such shares are subject to the Company’s right of repurchase and excluded from stockholders’ equity.
Award activity under all Plans is as follows:
|
Outstanding Options |
||||||||||||
|
Shares Available for Grant |
Number of Shares |
Weighted- Average Exercise Price Per Share |
||||||||||
|
Balance at June 30, 2011 |
837,304 | 3,381,738 | $ | 2.59 | ||||||||
|
Shares reserved |
750,000 | — | — | |||||||||
|
Restricted stock awards granted |
(86,000 | ) | — | — | ||||||||
|
Options granted |
(899,400 | ) | 899,400 | 2.13 | ||||||||
|
Options exercised |
— | (163,438 | ) | 2.01 | ||||||||
|
Options forfeited |
263,529 | (263,529 | ) | 2.86 | ||||||||
|
Balance at June 30, 2012 |
865,433 | 3,854,171 | $ | 2.53 | ||||||||
|
Shares reserved |
750,000 | — | — | |||||||||
|
Restricted stock awards granted |
(48,000 | ) | — | — | ||||||||
|
Options granted |
(671,150 | ) | 671,150 | 1.53 | ||||||||
|
Options exercised |
— | (10,518 | ) | 1.31 | ||||||||
|
Options forfeited |
578,908 | (578,908 | ) | 2.22 | ||||||||
|
Balance at June 30, 2013 |
1,475,191 | 3,935,895 | $ | 2.43 | ||||||||
|
Shares reserved |
1,000,000 | — | — | |||||||||
|
Restricted stock awards granted |
(92,666 | ) | — | — | ||||||||
|
Options granted |
(1,944,500 | ) | 1,944,500 | 1.24 | ||||||||
|
Options forfeited |
278,916 | (278,916 | ) | 3.94 | ||||||||
|
Balance at June 30, 2014 |
716,941 | 5,601,479 | $ | 1.95 | ||||||||
The following table summarizes information about options outstanding, vested and exercisable at June 30, 2014:
|
Options Outstanding |
Options exercisable |
|||||||||||||||||||||
|
Exercise Prices |
Number of Shares |
Weighted- Average Remaining Contractual Life (years) |
Weighted Average Exercise Price per Share |
Number of Shares |
Weighted Average Exercise Price per Share |
|||||||||||||||||
| $ | $ | |||||||||||||||||||||
|
$0.99 |
– | $1.55 | 3,847,356 | 4.39 | 1.24 | 2,174,269 | 1.26 | |||||||||||||||
|
$1.56 |
– | $2.85 | 1,352,960 | 3.89 | 2.15 | 1,003,980 | 2.22 | |||||||||||||||
|
$2.86 |
– | $9.75 | 401,163 | 1.51 | 7.89 | 395,516 | 7.95 | |||||||||||||||
|
Total outstanding |
5,601,479 | 4.06 | $ | 1.95 | 3,573,765 | $ | 2.28 | |||||||||||||||
|
Options vested and expected to vest |
5,356,829 | 3.96 | $ | 1.98 | ||||||||||||||||||
The weighted average remaining contractual life for all currently exercisable options as of June 30, 2014, was 2.9 years. The aggregate intrinsic value as of June 30, 2014, of all outstanding options was $105,000, options vested and expected to vest was $93,000 and options exercisable was $22,000. The aggregate intrinsic value as of June 30, 2013, of all outstanding options was $0, options vested and expected to vest was $0 and options exercisable was $0, as a result of the fair market value at June 30, 2013, being below the grant price ranges. The aggregate intrinsic value as of June 30, 2012, of all outstanding options was $1,116,000, options vested and expected to vest was $1,093,000 and options exercisable was $769,000.
The weighted-average estimated grant date fair value of options granted to employees and directors during fiscal years 2014, 2013 and 2012 was $0.80, $1.03 and $1.45 per share, respectively. The intrinsic value of all options exercised during fiscal years 2014, 2013 and 2012 was $0, $4,000 and $122,000, respectively. The fair value of all stock options actually vesting in fiscal years 2014, 2013 and 2012 was $528,000, $746,000 and $734,000, respectively.
Restricted Stock Units and Awards
The following table summarizes information about restricted stock activity.
|
Shares |
||||
|
Non-vested restricted stock at June 30, 2011 |
20,500 | |||
|
Awarded |
86,000 | |||
|
Vested |
(60,500 | ) | ||
|
Forfeited |
— | |||
|
Non-vested restricted stock at June 30, 2012 |
46,000 | |||
|
Awarded |
48,000 | |||
|
Vested |
(48,000 | ) | ||
|
Forfeited |
— | |||
|
Non-vested restricted stock at June 30, 2013 |
46,000 | |||
|
Awarded |
92,666 | |||
|
Vested |
(122,666 | ) | ||
|
Forfeited |
— | |||
|
Non-vested restricted stock at June 30, 2014 |
16,000 | |||
The aggregate intrinsic value as of June 30, 2014, of all non-vested restricted stock awards was $18,000, and awards expected to vest was $18,000.
The estimated grant date fair value of awards granted during fiscal years 2014, 2013 and 2012, was $1.15, $1.43 and $1.82 per share, respectively. The intrinsic value of all awards granted during fiscal years 2014, 2013 and 2012 was $106,000, $66,000 and $80,000, respectively. The fair value of all stock awards actually vesting in fiscal years 2014, 2013 and 2012 was $165,000, $75,000 and $73,000, respectively.
The fair value of each restricted stock award is estimated based upon the closing price of the Company’s common stock on the grant date. Share-based compensation expense related to restricted stock units and awards is recognized over the requisite service period as adjusted for estimated forfeitures.
Warrants
The Company has outstanding warrants to purchase common stock that are all exercisable at June 30, 2014, as follows:
|
Shares |
Exercise Price Per Share |
Expiration | ||||||
| 3,991,205 | $ | 1.45 |
September 2014 | |||||
Note 9. Income Taxes
Deferred income taxes reflect the net tax effects of net operating loss and tax credit carryovers and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets are as follows (in thousands):
|
June 30, |
||||||||
|
2014 |
2013 |
|||||||
|
Net operating loss carry-forwards |
$ | 55,591 | $ | 51,005 | ||||
|
Research credits |
3,084 | 2,868 | ||||||
|
Fixed asset depreciation |
(109 | ) | (71 | ) | ||||
|
Stock compensation |
1,103 | 936 | ||||||
|
Deferred revenue |
158 | 806 | ||||||
|
Other |
764 | 617 | ||||||
|
Total deferred tax assets |
60,591 | 56,161 | ||||||
|
Valuation allowance |
(60,591 | ) | (56,161 | ) | ||||
|
Net deferred tax assets |
$ | — | $ | — | ||||
Realization of the deferred tax assets is dependent upon future taxable income, the amount and timing of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The net valuation allowance increased by approximately $4.4 million, $6.8 million and $5.6 million during fiscal years ended June 30, 2014, 2013 and 2012, respectively.
As of June 30, 2014, the Company had federal net operating loss carry-forwards and research credit carry-forwards of approximately $153.2 million and $1.5 million, respectively. The net operating loss carry-forwards begin to expire in the fiscal year 2019. The federal credits begin to expire in fiscal year 2021 if not utilized. Additionally, the Company’s state net operating loss carry-forwards of approximately $93.2 million begin to expire in the fiscal year 2015 and the Company has state research credit carry-forwards of $3.5 million. The California state credit carry-forwards have an unlimited carry-forward period and the State of Arizona credits begin to expire in fiscal year 2024.
Included in the valuation allowance balance as of June 30, 2014, is $0.3 million related to the exercise of stock options which are not reflected as an expense for financial reporting purposes. Accordingly, any future reduction in the valuation allowance relating to this amount will be credited directly to equity and not reflected as an income tax benefit in the Statement of Operations.
The reconciliation of income tax benefits attributable to the net loss computed at the U.S. federal statutory rates to the income tax benefit recorded (in thousands):
|
Fiscal Year Ended June 30, |
||||||||||||
|
2014 |
2013 |
2012 |
||||||||||
|
Tax benefit at U.S. statutory rate |
$ | (5,768 | ) | $ | (5,487 | ) | $ | (4,648 | ) | |||
|
Loss for which no tax benefit is currently recognizable |
5,584 | 5,341 | 4,502 | |||||||||
|
Stock based compensation |
164 | 128 | 131 | |||||||||
|
Other, net |
20 | 18 | 15 | |||||||||
| $ | — | $ | — | $ | — | |||||||
Utilization of the net operating loss carry-forwards and credit carry-forwards may be subject to a substantial annual limitation due to the limitations set forth in Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, and similar state provisions. In the fiscal year ended June 30, 2010, the Company completed a detailed analysis to determine whether an ownership change under Section 382 of the Internal Revenue Code had occurred. The effect of an ownership change would be the imposition of an annual limitation on the use of the net operating loss carry-forwards and credit carry-forwards attributable to periods before the change. Any subsequent ownership changes could further limit the use of net operating losses and credits. The Company concluded that approximately $4.9 million of federal net operating loss carry-forwards, $1.5 million of federal credit carry-forwards, and approximately $19.5 million of California state net operating loss carry-forwards are significantly limited to offset future income, if any. The reductions are reflected in the carry-forward amounts included above. The most recent analysis of the Company’s historical ownership changes was completed in 2014. Due to IRC Section 382 and 383 limitations, the Company only accounts for net operating loss and tax credit carryforwards as deferred tax assets where the Company reasonably expects that these losses and carryforwards can be utilized in future periods.
At June 30, 2014, the Company had unrecognized tax benefits of $1.0 million, all of which would not currently affect the Company’s effective tax rate if recognized due to the Company’s deferred tax assets being fully offset by a valuation allowance. A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
|
Amount |
||||
|
Balance at June 30, 2012 |
$ | 756 | ||
|
Additions based on tax positions related to current year |
183 | |||
|
Balance at June 30, 2013 |
939 | |||
|
Additions based on tax positions related to current year |
74 | |||
|
Balance at June 30, 2014 |
$ | 1,013 | ||
The Company would classify interest and penalties related to uncertain tax positions in income tax expense, if applicable. There was no interest expense or penalties related to unrecognized tax benefits recorded through June 30, 2014. The tax years 1998 through 2014 remain open to examination by one or more major taxing jurisdictions to which the Company is subject.
Note 10. Employee Benefit Plan
In January 2001, the Company adopted a 401(k) Profit Sharing Plan that allows voluntary contributions by eligible employees. Employees may elect to contribute up to the maximum allowed under the Internal Revenue Service regulations. The Company may make discretionary contributions as determined by the Board of Directors. No amount was contributed by the Company to the plan during fiscal years ended June 30, 2014, 2013 or 2012.
Note 11. Indemnification
From time to time, the Company enters into contracts that require the Company, upon the occurrence of certain contingencies, to indemnify parties against third-party claims. These contingent obligations primarily relate to (i) claims against the Company’s customers for violation of third-party intellectual property rights caused by the Company’s products; (ii) claims resulting from personal injury or property damage resulting from the Company’s activities or products; (iii) claims by the Company’s office lessor arising out of the Company’s use of the premises; and (iv) agreements with the Company’s officers and directors under which the Company may be required to indemnify such persons for liabilities arising out of their activities on behalf of the Company. Because the obligated amounts for these types of agreements usually are not explicitly stated, the overall maximum potential amount of these obligations cannot be reasonably estimated. No liabilities have been recorded for these obligations on the Company’s balance sheets as of June 30, 2014 or 2013, as there are no amounts currently estimable and probable of payment.
Note 12. Financial Information by Quarter
Financial Information by Quarter (unaudited)
Fiscal Year 2014:
|
1st Quarter |
2nd Quarter |
3rd Quarter |
4th Quarter |
|||||||||||||
|
(In thousands, except per share data) |
||||||||||||||||
|
Total net revenue |
$ | 805 | $ | 851 | $ | 934 | $ | 1,025 | ||||||||
|
Gross profit (loss) on product sales |
(255 | ) | (219 | ) | (452 | ) | (339 | ) | ||||||||
|
Net loss |
(3,739 | ) | (4,172 | ) | (4,379 | ) | (4,676 | ) | ||||||||
|
Net loss allocable to common stockholders |
(3,739 | ) | (4,172 | ) | (4,379 | ) | (6,591 | ) | ||||||||
|
Basic and diluted net loss per share allocable to common stockholders |
(0.07 | ) | (0.08 | ) | (0.09 | ) | (0.08 | ) | ||||||||
|
Shares used in computing basic and diluted net loss per share allocable to common stockholders |
51,089 | 51,314 | 51,587 | 79,590 | ||||||||||||
Fiscal Year 2013:
|
1st Quarter |
2nd Quarter |
3rd Quarter |
4th Quarter |
|||||||||||||
|
(In thousands, except per share data) |
||||||||||||||||
|
Total net revenue |
$ | 885 | $ | 874 | $ | 868 | $ | 872 | ||||||||
|
Gross profit (loss) on product sales |
150 | (195 | ) | (322 | ) | (144 | ) | |||||||||
|
Net loss |
(4,140 | ) | (4,192 | ) | (3,946 | ) | (3,859 | ) | ||||||||
|
Basic and diluted net loss per common share |
(0.11 | ) | (0.11 | ) | (0.10 | ) | (0.08 | ) | ||||||||
|
Shares used in computing basic and diluted net loss per common share |
36,723 | 36,951 | 38,633 | 51,060 | ||||||||||||
|
(1) |
Gross profit is computed as total net product sales less cost of product sales. |
Note 13. Subsequent Event
On June 24, 2014, the Company and Century Medical, Inc. signed a fifth amendment pursuant to the Distribution Agreement pertaining to the PAS-Port system, originally dated June 16, 2003, as amended, which amendment was effective July 1, 2014. The amendment, among other things, renewed the contract for another five years, extending the expiration date to July 31, 2019. In addition, on June 24, 2014, the Company and Century also signed an amendment pursuant to the Secured Note Purchase Agreement dated September 2, 2011, extending the maturity date of the loan under that agreement by two years from September 30, 2016, to September 30, 2018.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Effectiveness of Disclosure Controls and Procedures
Based on their evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures (as defined in Rule 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended) were effective as of June 30, 2014.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) of the Securities Exchange Act of 1934, as amended). Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of June 30, 2014, based on the criteria set forth in Internal Control — Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the criteria set forth in Internal Control — Integrated Framework (1992), our management concluded that our internal control over financial reporting was effective as of June 30, 2014.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended June 30, 2014, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Effectiveness of Controls
A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within an organization have been detected. Accordingly, our internal control over financial reporting, including our disclosure controls and procedures, are designed to provide reasonable, not absolute, assurance that the objectives of our internal control over financial reporting, including our disclosure control system, are met and, as set forth above, our principal executive officer and principal financial officer have concluded, based on their evaluation as of the end of the period covered by this report, that our internal control over financial reporting, including our disclosure controls and procedures, were effective to provide reasonable assurance that the objectives of our internal control over financial reporting, including our disclosure control system, were met.
Item 9B. Other Information
On June 24, 2014, Cardica and Century Medical, Inc., or Century, signed a fifth amendment pursuant to the Distribution Agreement pertaining to the PAS-Port system, originally dated June 16, 2003, as amended, which amendment was effective July 1, 2014. The amendment, among other things, renewed the contract for another five years, extending the expiration date to July 31, 2019. In addition, on June 24, 2014, Cardica and Century also signed an amendment to the Secured Note Purchase Agreement dated September 2, 2011, pursuant to which Century loaned us $4.0 million, extending the maturity date of the loan under this agreement by two years from September 30, 2016, to September 30, 2018. A description of the relationship between Cardica and Century is set forth in Items 1 and 7 of this Form 10-K.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Identification of Executive Officers and Directors
Reference is made to the information regarding executive officers appearing under the heading “Business — Executive Officers of the Registrant” in Part I Item 1 of this Annual Report on Form 10-K, which information is hereby incorporated by reference. Reference is made to the information regarding our directors and nominees for director appearing under the heading “Proposal 1 — Election of Directors” to be included in our proxy statement for our 2014 annual meeting of stockholders, or 2014 Proxy Statement, which information is incorporated herein by reference.
Identification of Audit Committee and Audit Committee Financial Expert
Reference is made to the information regarding directors to be included under the headings “Information Regarding the Board of Directors and Corporate Governance — Information Regarding Committees of the Board of Directors— Audit Committee” in our 2014 Proxy Statement, which information is incorporated herein by reference.
Material Changes to Procedures for Recommending Directors
Reference is made to the information regarding directors to be included under the heading “Information Regarding the Board of Directors and Corporate Governance” in our 2014 Proxy Statement, which information is incorporated herein by reference.
Compliance with Section 16(a) of the Exchange Act
Reference is made to the information to be included under the heading “Section 16(a) Beneficial Ownership Reporting Compliance” in our 2014 Proxy Statement, which information is incorporated herein by reference.
Code of Conduct
Reference is made to the information to be included under the heading “Information Regarding the Board of Directors and Corporate Governance — Code of Business Conduct and Ethics” in our 2014 Proxy Statement, which information is incorporated herein by reference. A copy of our code of business conduct and ethics can be found on our website, www.cardica.com in the “USA” section titled “About Cardica,” by clicking on “Investors/Media” and selecting the subsection titled “Corporate Governance.” The contents of our website are not a part of this Annual Report on Form 10-K.
Item 11. Executive Compensation
Reference is made to the information to be included under the heading “Executive Compensation” in our 2014 Proxy Statement, which information is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Security Ownership
Reference is made to the information to be included under the heading “Security Ownership of Certain Beneficial Owners and Management” in our 2014 Proxy Statement, which information is hereby incorporated by reference.
Equity Compensation Plan Information
Reference is made to the information to be included under the heading “Securities Authorized for Issuance under Equity Compensation Plans — Equity Compensation Plan Information” in our 2014 Proxy Statement, which information is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
Reference is made to the information to be included under the headings “Transactions with Related Persons” and “Information Regarding the Board of Directors and Corporate Governance — Independence of the Board of Directors” in our 2014 Proxy Statement, which information is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services
Reference is made to the information to be included under the heading “Principal Accountant Fees and Services” in our 2014 Proxy Statement, which information is incorporated herein by reference.
PART IV
Item 15. Exhibits, Financial Statement Schedules
(a) Documents filed as part of this report
1. Financial Statements
Reference is made to the Index to Financial Statements under Item 8, Part II hereof.
2. Financial Statement Schedules
All financial statement schedules are omitted because the information is not applicable or is presented in the Financial Statements or Notes thereto.
3. Exhibits
Reference is made to the Exhibit Index which follows the signature page of this Annual Report on Form 10-K, which is incorporated herein by reference here.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
Cardica, Inc. |
||
| Registrant | ||
|
September 25, 2014 |
/s/ ROBERT Y. NEWELL |
|
| Date | Robert Y. Newell | |
| Chief Financial Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Bernard A. Hausen and Robert Y. Newell, as his true and lawful attorney-in-fact and agent, with full power of substitution for him, and in his name in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, and any of them or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1934, this report has been signed by the following persons on behalf of the Registrant in the capacities indicated on the date set forth below:
|
Name and Signature |
Title |
Date | ||
|
/s/ BERNARD A. HAUSEN |
President, Chief Executive Officer and Director |
September 25, 2014 | ||
| Bernard A. Hausen, M.D., Ph.D. | (Principal Executive Officer) | |||
|
/s/ ROBERT Y. NEWELL |
Chief Financial Officer |
September 25, 2014 | ||
| Robert Y. Newell | (Principal Financial and Accounting Officer) | |||
|
/s/ KEVIN T. LARKIN |
Director |
September 25, 2014 | ||
| Kevin T. Larkin | ||||
|
/s/ RICHARD P. POWERS |
Director |
September 25, 2014 | ||
| Richard P. Powers | ||||
|
/s/ JEFFREY L. PURVIN |
Director |
September 25, 2014 | ||
| Jeffrey L. Purvin | ||||
|
/s/ JOHN SIMON |
Director |
September 25, 2014 | ||
| John Simon, Ph.D. | ||||
|
/s/ WILLIAM H. YOUNGER, JR. |
Director |
September 25, 2014 | ||
| William H. Younger, Jr. |
INDEX TO EXHIBITS
|
|
|
Incorporation by Reference |
| |||||||||
|
Exhibit Number |
Exhibit Description |
Form |
File Number |
Exhibit/ Appendix Reference |
Filing Date |
Filed Herewith | ||||||
|
3.1 |
Amended and Restated Certificate of Incorporation of Cardica, Inc. |
S-1 |
333-129497 |
3.2 |
01/13/2006 |
|||||||
|
3.2 |
Certificate of Amendment of Amended and Restated Certificate of Incorporation of Cardica, Inc. |
10-Q |
000-51772 |
3.3 |
11/15/2010 |
|||||||
|
3.3 |
Certificate of Correction of Certificate of Amendment of Amended and Restated Certificate of Incorporation of Cardica, Inc. |
8-K |
000-51772 |
3.2 |
11/16/2010 |
|||||||
|
3.4 |
Certificate of Amendment of Amended and Restated Certificate of Incorporation of Cardica, Inc. |
8-K |
000-51772 |
3.1 |
11/19/2012 |
|||||||
|
3.5 |
Certificate of Amendment of Amended and Restated Certificate of Incorporation of Cardica, Inc. |
8-K |
000-51772 |
3.1 |
11/15/2013 |
|||||||
|
3.6 |
Bylaws of the Registrant as currently in effect. |
8-K |
000-51772 |
3.2 |
08/19/2008 |
|||||||
|
4.1 |
Specimen Common Stock certificate of the Registrant. |
S-1 |
333-129497 |
3.5 |
02/01/2006 |
|||||||
|
10.1 |
1997 Equity Incentive Plan and forms of related agreements and documents. + |
S-1 |
333-129497 |
10.1 |
11/04/2005 |
|||||||
|
10.2 |
2005 Equity Incentive Plan. + |
10-Q |
000-51772 |
10.1 |
02/05/2014 |
|||||||
|
10.3 |
Form of Option Agreement under the Cardica, Inc. 2005 Equity Incentive Plan+ |
X | ||||||||||
|
10.4 |
Form of Restricted Stock Unit Grant Notice and Restricted Stock Unit Grant Agreement. + |
8-K |
000-51772 |
10.26 |
02/20/2009 |
|||||||
|
10.5 |
Office Lease Agreement dated April 25, 2003, and First Amendment to Office Lease Agreement dated January 21, 2004. |
S-1 |
333-129497 |
10.5 |
11/04/2005 |
|||||||
|
10.6 |
Second Amendment to Office Lease Agreement, executed and delivered in December 2007 effective November 19, 2007. |
8-K |
000-51772 |
10.1 |
12/05/2007 |
|||||||
|
10.7 |
Third Amendment to Office Lease, dated November 17, 2009, by and between Cardica, Inc., and HCP LS REDWOOD CITY, LLC (f/k/a Slough Redwood City, LLC). |
10-Q |
000-51772 |
10.29 |
11/15/2010 |
|||||||
|
10.8 |
Fourth Amendment to Lease dated November 11, 2010 |
8-K |
000-51772 |
10.30 |
11/16/2010 |
|||||||
|
10.9 |
Distribution Agreement by and between Cardica, Inc. and Century Medical, Inc. dated June 16, 2003. † |
S-1 |
333-129497 |
10.6 |
12/20/2005 |
|||||||
|
10.10 |
First Amendment to Distribution Agreement, dated March 30, 2007, by and between Cardica, Inc. and Century Medical, Inc. † |
8-K |
000-51772 |
10.6.1 |
04/05/2007 |
|||||||
|
10.11 |
Amendment No. 2 to Distribution Agreement, dated June 13, 2007, by and between Cardica, Inc. and Century Medical, Inc. † |
10-K |
000-51772 |
10.7 |
09/24/2010 |
|||||||
| Incorporation by Reference | ||||||||||||
|
Exhibit Number |
Exhibit Description | Form | File Number |
Exhibit/ Appendix Reference |
Filing Date |
Filed Herewith | ||||||
|
10.12 |
Amendment No. 3 to Distribution Agreement, dated January 24, 2008, by and between Cardica, Inc. and Century Medical, Inc. |
10-K |
000-51772 |
10.8 |
09/24/2010 |
|||||||
|
10.13 |
Amendment No. 4 to Distribution Agreement, dated April 1, 2010, by and between Cardica, Inc. and Century Medical, Inc. † |
8-K |
000-51772 |
10.8.1 |
04/07/2010 |
|||||||
|
10.14 |
Fifth Amendment to Distribution Agreement, dated as of July 1, 2014, by and between Cardica, Inc. and Century Medical, Inc. † |
X | ||||||||||
|
10.15 |
Distribution Agreement by and between Cardica, Inc. and Century Medical, Inc. dated September 2, 2011. † |
10-Q |
000-51772 |
10.36 |
11/09/2011 |
|||||||
|
10.16 |
Secured Note Purchase Agreement by and between Cardica, Inc. and Century Medical, Inc. dated September 2, 2011. † |
10-Q |
000-51772 |
10.37 |
11/09/2011 |
|||||||
|
10.17 |
Security Agreement by and between Cardica, Inc. and Century Medical, Inc. dated September 2, 2011. † |
10-Q |
000-51772 |
10.38 |
11/09/2011 |
|||||||
|
10.18 |
Form of Secured Promissory Note to Century Medical |
10-Q |
000-51772 |
10.39 |
11/09/2011 |
|||||||
|
10.19 |
Letter Agreement, dated as of July 1, 2014, extending the term of the Secured Note Purchase Agreement by and between Cardica, Inc. and Century Medical, Inc. |
X | ||||||||||
|
10.20 |
Compensation Information for named executive officers. + |
8-K |
000-51772 |
Item 5.02 |
07/18/2014 |
|||||||
|
10.21 |
Cardica, Inc. Non-Employee Director Compensation. + |
X | ||||||||||
|
10.22 |
Benefit Agreement with Bernard Hausen, M.D., Ph.D. + |
S-1 |
333-129497 |
10.4 |
02/01/2006 |
|||||||
|
10.23 |
Letter Agreement with Frederic M. Bauer+ |
10-Q |
000-51772 |
10.27 |
11/07/2008 |
|||||||
|
10.24 |
Cardica, Inc. Change in Control and Severance Benefit Plan. + |
8-K |
000-51772 |
10.25 |
02/18/2009 |
|||||||
|
10.25 |
License Agreement, dated August 16, 2010, by and between Cardica, Inc., and Intuitive Surgical Operations, Inc. † |
10-K |
000-51722 |
10.28 |
09/24/2010 |
|||||||
|
23.1 |
Consent of Independent Registered Public Accounting Firm. |
X | ||||||||||
|
23.2 |
Consent of Independent Registered Public Accounting Firm. |
X | ||||||||||
|
24.1 |
Power of Attorney (included on signature page). |
X | ||||||||||
|
31.1 |
Certification of chief executive officer. |
X | ||||||||||
|
31.2 |
Certification of chief financial officer. |
X | ||||||||||
|
32.1 |
Section 1350 Certification |
X | ||||||||||
|
101.INS |
XBRL Instance Document |
X | ||||||||||
|
101.SCH |
XBRL Taxonomy Extension Schema Document |
X | ||||||||||
| Incorporation by Reference | ||||||||||||
|
Exhibit Number |
Exhibit Description | Form | File Number |
Exhibit/ Appendix Reference |
Filing Date |
Filed Herewith | ||||||
|
101.CAL |
XBRL Taxonomy Extension Calculation Linkbase Document |
X | ||||||||||
|
101.DEF |
XBRL Taxonomy Extension Definition Linkbase |
X | ||||||||||
|
101.LAB |
XBRL Taxonomy Extension Labels Linkbase Document |
X | ||||||||||
|
101.PRE |
XBRL Taxonomy Extension Presentation Linkbase Document |
X | ||||||||||
|
† |
Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for, or grant of, confidential treatment. |
|
+ |
Indicates management contract or compensatory plan. |
82
