Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Assertio Therapeutics, Inc | a14-3395_18k.htm |
Exhibit 99.1
|
|
A Specialty Pharmaceutical Leader Focused in Pain and Neurology January 13, 2014 |
|
|
Note on Forward-Looking Statements Statements made in this presentation that are not historical facts are forward-looking statements that involve risks and uncertainties. The inclusion of forward-looking statements, including those related to our commercialization plans for Gralise®, Zipsor®, Lazanda® and CAMBIA®, the efforts of our collaboration partners to obtain regulatory approval of product candidates and commercialize products, financial projections and expectations, and intellectual property and other litigation to which we are a party, should not be regarded as a representation that any of our plans will be achieved. Actual results may differ materially from those described in this presentation due to the risks and uncertainties inherent in our business, including, without limitation, risks and uncertainties related to: our ability to successfully commercialize our products; the success of our collaborative arrangements with development and commercialization partners; our research and development efforts, regulation by the FDA and other government agencies; the timing of regulatory approvals and product launches; and other risks detailed in our filings with the Securities and Exchange Commission, including our most recent Annual Report on Form 10-K and our most recent Quarterly Report on Form 10-Q. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. We undertake no obligation to revise or update this presentation to reflect events or circumstances that occur after the date of this presentation. 2 January 2014 |
|
|
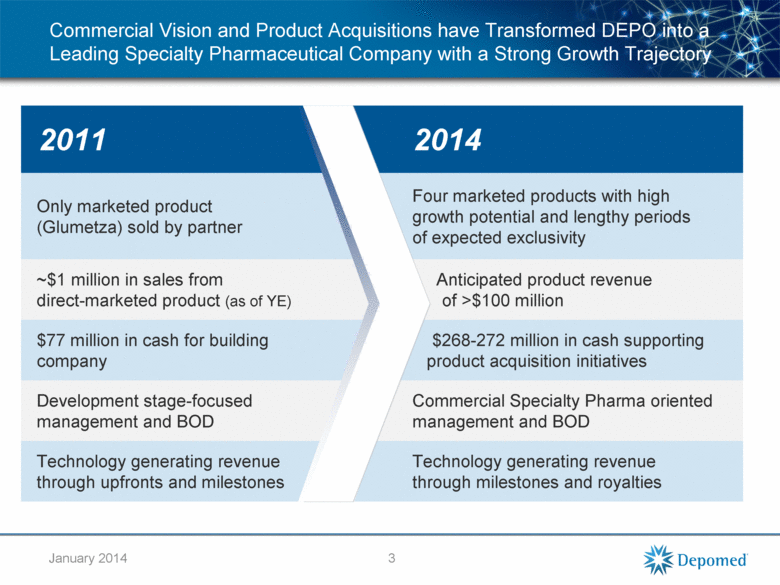
Commercial Vision and Product Acquisitions have Transformed DEPO into a Leading Specialty Pharmaceutical Company with a Strong Growth Trajectory 2011 2014 Only marketed product (Glumetza) sold by partner Four marketed products with high growth potential and lengthy periods of expected exclusivity ~$1 million in sales from direct-marketed product (as of YE) Anticipated product revenue of >$100 million $77 million in cash for building company $268-272 million in cash supporting product acquisition initiatives Development stage-focused management and BOD Commercial Specialty Pharma oriented management and BOD Technology generating revenue through upfronts and milestones Technology generating revenue through milestones and royalties 3 January 2014 |
|
|
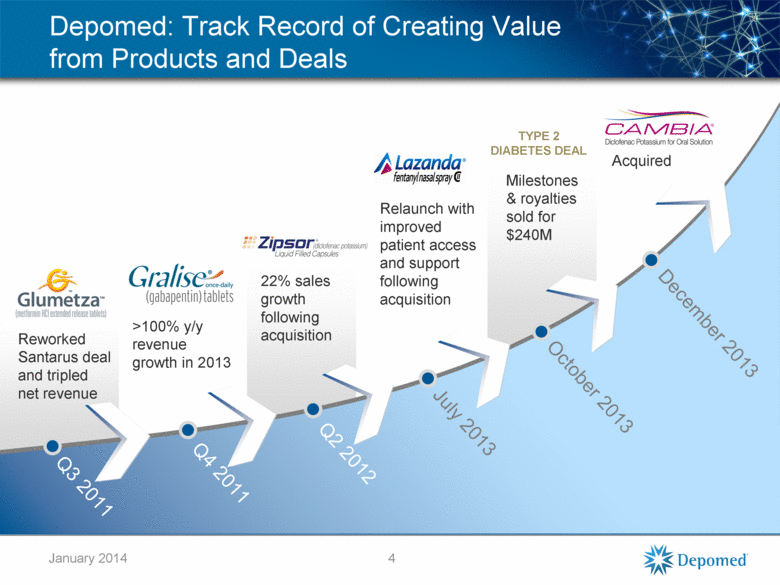
Depomed: Track Record of Creating Value from Products and Deals 4 TYPE 2 DIABETES DEAL Reworked Santarus deal and tripled net revenue >100% y/y revenue growth in 2013 22% sales growth following acquisition Relaunch with improved patient access and support following acquisition Milestones & royalties sold for $240M Acquired Q3 2011 Q4 2011 Q2 2012 July 2013 October 2013 December 2013 January 2014 |
|
|
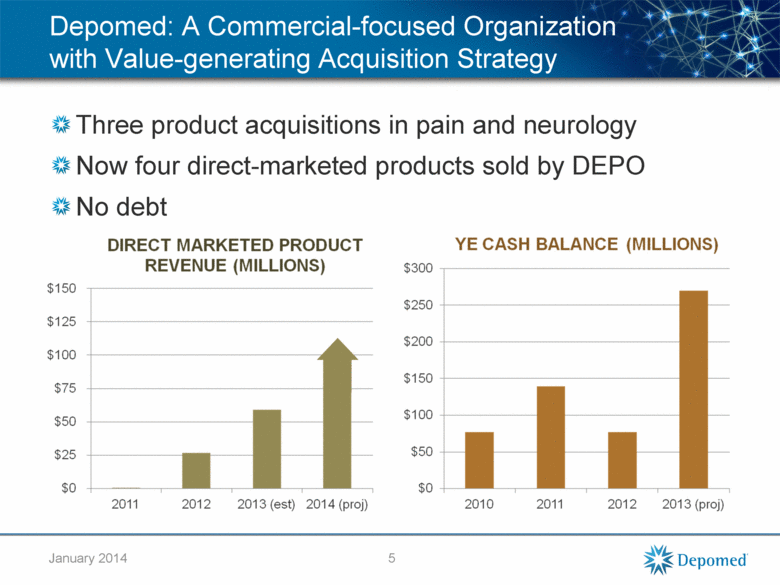
Depomed: A Commercial-focused Organization with Value-generating Acquisition Strategy Three product acquisitions in pain and neurology Now four direct-marketed products sold by DEPO No debt 5 January 2014 |
|
|
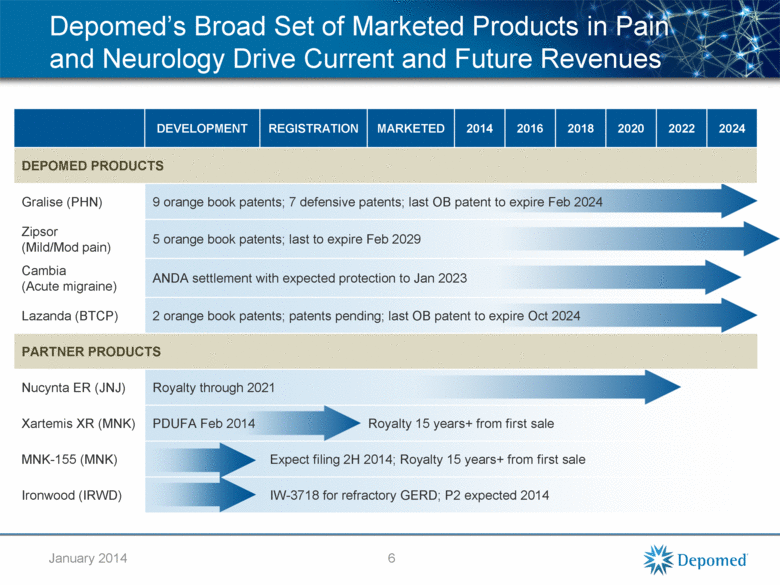
DEVELOPMENT REGISTRATION MARKETED 2014 2016 2018 2020 2022 2024 DEPOMED PRODUCTS Gralise (PHN) 9 orange book patents; 7 defensive patents; last OB patent to expire Feb 2024 Zipsor (Mild/Mod pain) 5 orange book patents; last to expire Feb 2029 Cambia (Acute migraine) ANDA settlement with expected protection to Jan 2023 Lazanda (BTCP) 2 orange book patents; patents pending; last OB patent to expire Oct 2024 PARTNER PRODUCTS Nucynta ER (JNJ) Royalty through 2021 Xartemis XR (MNK) PDUFA Feb 2014 Royalty 15 years+ from first sale MNK-155 (MNK) Expect filing 2H 2014; Royalty 15 years+ from first sale Ironwood (IRWD) IW-3718 for refractory GERD; P2 expected 2014 Depomed’s Broad Set of Marketed Products in Pain and Neurology Drive Current and Future Revenues 6 January 2014 |
|
|
Depomed’s Partnerships, Technology and IP Continue to Provide Cash, Revenue and Upside $240.5 million royalty sale to PDL (October 2013) Sale of non-core diabetes royalties and milestones Provides growth capital without dilution Final accounting and tax treatment pending Partner products provide future revenue and upside Nucynta ER (Johnson & Johnson) - royalty to 2021 Xartemis XR and MNK-155 (Mallinckrodt) – potential abuse-resistant opioids with potential near term milestones and high single digit royalties for 15+ year term IW-3718 (Ironwood) – milestones and royalty Potential cash flow from IP litigation against Purdue and Endo 7 Over past 7 years, Depomed generated >$500 million in non-dilutive capital January 2014 |
|
|
Gralise® for Postherpetic Neuralgia Indicated for the management of postherpetic neuralgia (PHN) Large and growing market opportunity >$50 million run rate (Y/E 2013) Promoted by >160 sales professionals who also sell CAMBIA and Zipsor Additional managed care contracts to improve coverage in 2014 8 January 2014 |
|
|
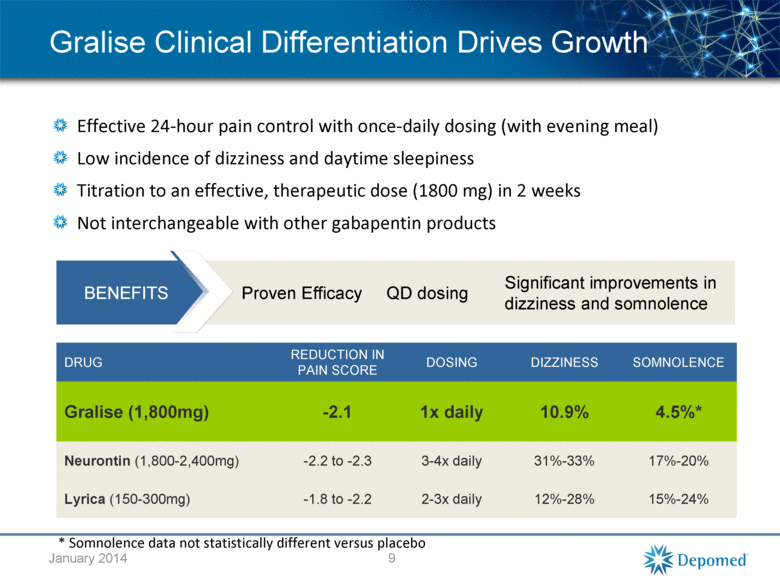
BENEFITS Proven Efficacy QD dosing Significant improvements in dizziness and somnolence Gralise Clinical Differentiation Drives Growth Effective 24-hour pain control with once-daily dosing (with evening meal) Low incidence of dizziness and daytime sleepiness Titration to an effective, therapeutic dose (1800 mg) in 2 weeks Not interchangeable with other gabapentin products DRUG REDUCTION IN PAIN SCORE DOSING DIZZINESS SOMNOLENCE Gralise (1,800mg) -2.1 1x daily 10.9% 4.5%* Neurontin (1,800-2,400mg) -2.2 to -2.3 3-4x daily 31%-33% 17%-20% Lyrica (150-300mg) -1.8 to -2.2 2-3x daily 12%-28% 15%-24% 9 * Somnolence data not statistically different versus placebo January 2014 |
|
|
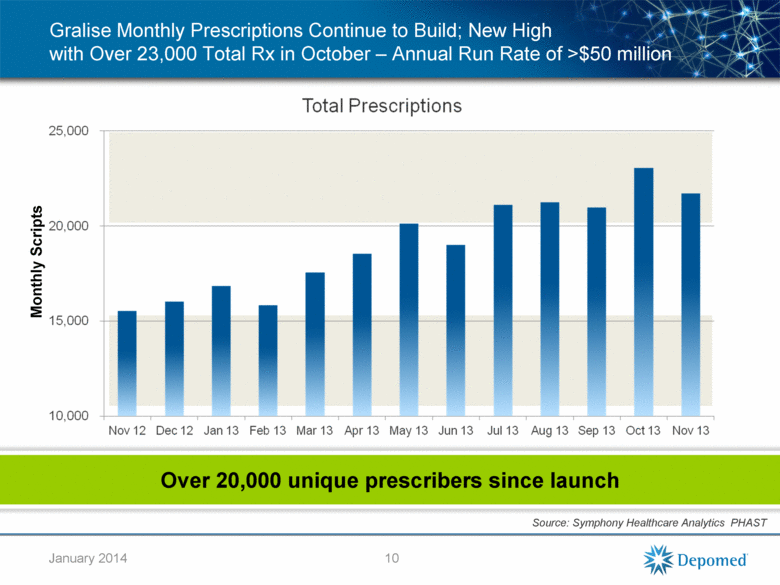
Gralise Monthly Prescriptions Continue to Build; New High with Over 23,000 Total Rx in October – Annual Run Rate of >$50 million 10 Monthly Scripts Source: Symphony Healthcare Analytics PHAST Over 20,000 unique prescribers since launch January 2014 |
|
|
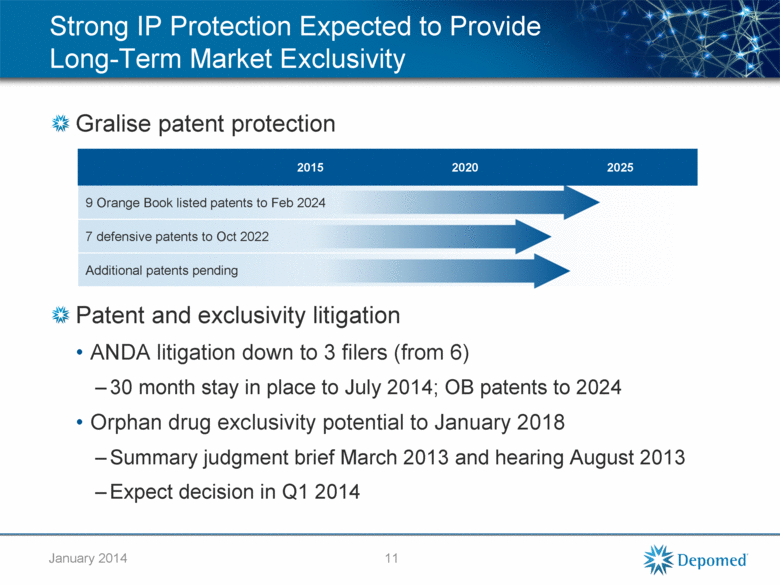
Gralise patent protection Patent and exclusivity litigation ANDA litigation down to 3 filers (from 6) 30 month stay in place to July 2014; OB patents to 2024 Orphan drug exclusivity potential to January 2018 Summary judgment brief March 2013 and hearing August 2013 Expect decision in Q1 2014 2015 2020 2025 9 Orange Book listed patents to Feb 2024 7 defensive patents to Oct 2022 Additional patents pending Strong IP Protection Expected to Provide Long-Term Market Exclusivity 11 January 2014 |
|
|
Zipsor® 12 January 2014 |
|
|
Zipsor Acquisition in 2012 Expanded Pain Franchise and Was Immediately Accretive Broad indication for mild to moderate pain Rapidly dispersed, liquid-filled capsule provides acute pain relieve in <1 hour Acquired in 2012 for ~$28 million Current annual run rate >$25 million (as of December 2013) Highly profitable with gross margin over 95% Depomed halted sales decline and resumed growth of product in 2013 13 January 2014 |
|
|
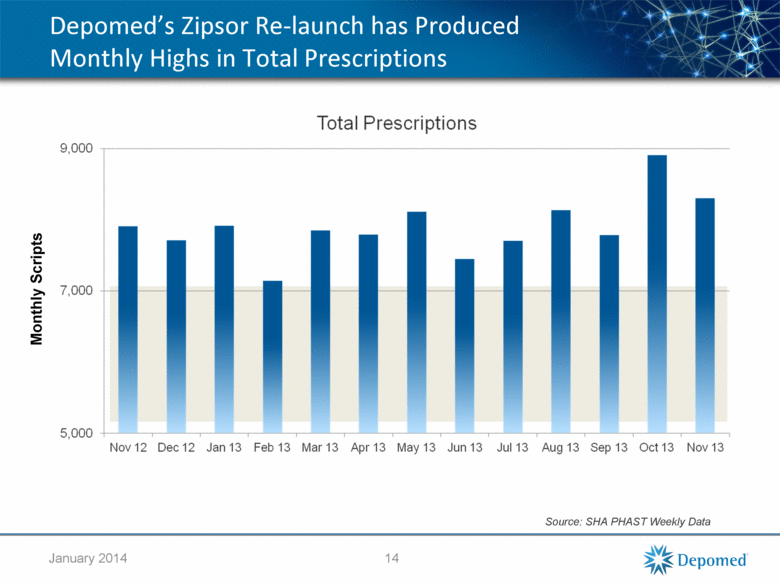
Depomed’s Zipsor Re-launch has Produced Monthly Highs in Total Prescriptions Source: SHA PHAST Weekly Data 14 Monthly Scripts January 2014 |
|
|
CAMBIA Adds Differentiated Migraine Product to Depomed in December 2013 Acquired December 2013 for $48.7 million The only single agent NSAID in the US approved for treatment of migraine attacks in adults Powdered formulation of diclofenac dissolves in liquid, provides rapid relief of pain and is easy to take Principally prescribed by neurologists 15 January 2014 |
|
|
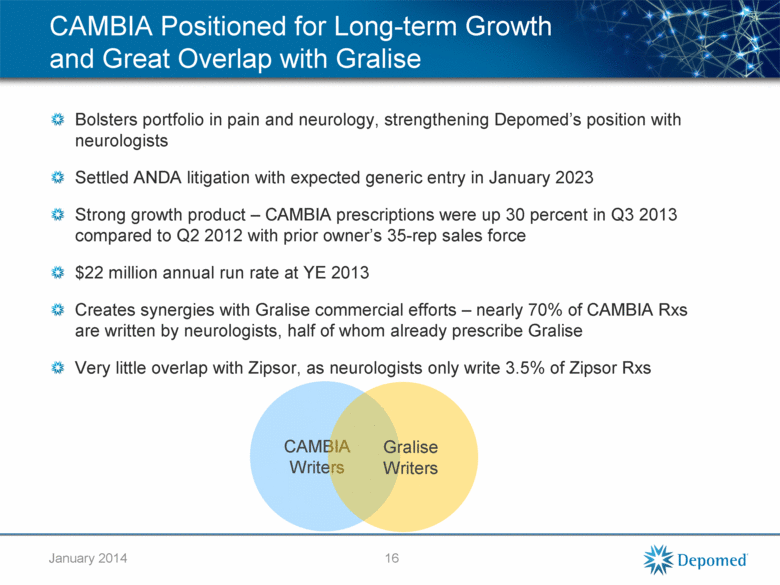
CAMBIA Positioned for Long-term Growth and Great Overlap with Gralise Bolsters portfolio in pain and neurology, strengthening Depomed’s position with neurologists Settled ANDA litigation with expected generic entry in January 2023 Strong growth product – CAMBIA prescriptions were up 30 percent in Q3 2013 compared to Q2 2012 with prior owner’s 35-rep sales force $22 million annual run rate at YE 2013 Creates synergies with Gralise commercial efforts – nearly 70% of CAMBIA Rxs are written by neurologists, half of whom already prescribe Gralise Very little overlap with Zipsor, as neurologists only write 3.5% of Zipsor Rxs 16 CAMBIA Writers Gralise Writers January 2014 |
|
|
Lazanda (fentanyl) Nasal Spray Expands Depomed’s Pain Franchise, Acquired Late July 2013 Indicated for the management of breakthrough pain in cancer patients 18 years of age and older who are already receiving and who are tolerant to opioid therapy for their underlying persistent cancer pain May only be dispensed by physicians enrolled in the TIRF REMS Access program 17 Only fentanyl product delivered nasally Rapid onset of action to handle breakthrough cancer pain May be used by patients with oral mucositis Opportunity for growth January 2014 |
|
|
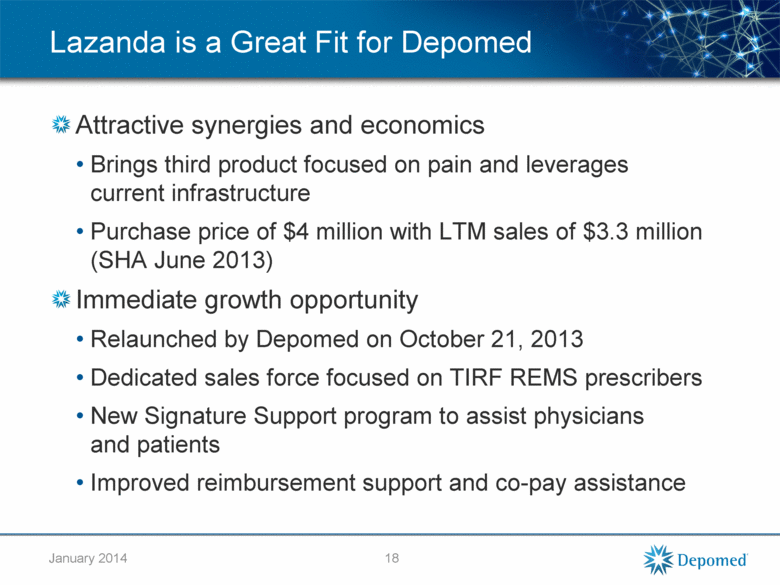
Lazanda is a Great Fit for Depomed Attractive synergies and economics Brings third product focused on pain and leverages current infrastructure Purchase price of $4 million with LTM sales of $3.3 million (SHA June 2013) Immediate growth opportunity Relaunched by Depomed on October 21, 2013 Dedicated sales force focused on TIRF REMS prescribers New Signature Support program to assist physicians and patients Improved reimbursement support and co-pay assistance 18 January 2014 |
|
|
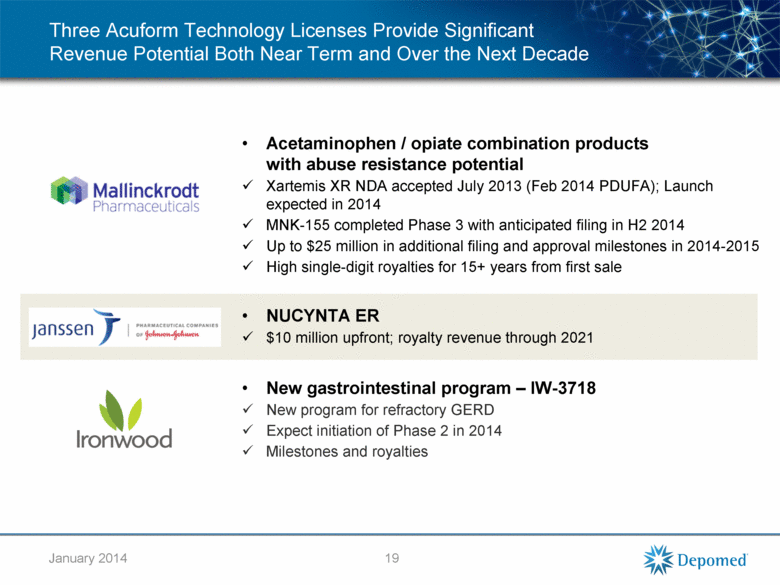
Three Acuform Technology Licenses Provide Significant Revenue Potential Both Near Term and Over the Next Decade Acetaminophen / opiate combination products with abuse resistance potential Xartemis XR NDA accepted July 2013 (Feb 2014 PDUFA); Launch expected in 2014 MNK-155 completed Phase 3 with anticipated filing in H2 2014 Up to $25 million in additional filing and approval milestones in 2014-2015 High single-digit royalties for 15+ years from first sale NUCYNTA ER $10 million upfront; royalty revenue through 2021 New gastrointestinal program – IW-3718 New program for refractory GERD Expect initiation of Phase 2 in 2014 Milestones and royalties 19 January 2014 |
|
|
Depomed (DEPO): a Specialty Pharmaceutical Leader Focused in Pain and Neurology Four proprietary, marketed products with long-term, high growth opportunity Track record of creating value from product acquisitions Significant cash to acquire additional products to fuel growth Partnerships, technology and IP continue to provide value and upside 20 January 2014 |
|
|
Thank You |