Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REPROS THERAPEUTICS INC. | v340134_8k.htm |

Developing clinical stage small molecule therapeutics to treat hormonal and reproductive system disorders

Repros Disclaimer Any statements made by the Company that are not historical facts contained in this release are forward - looking statements that involve risks and uncertainties, including the ability to raise additional needed capital on a timely basis in order for it to continue to fund development of its Androxal ® and Proellex ® programs, have success in the clinical development of its technologies, the reliability of interim results to predict final study outcomes, and such other risks which are identified in the Company's most recent Annual Report on Form 10 - K and in any subsequent quarterly reports on Form 10 - Q. These documents are available on request from Repros Therapeutics or at www.sec.gov . Repros disclaims any intention or obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise.

Investment Highlights • Focused strategy: small molecule therapeutics for reproductive disorders • Two late stage clinical programs each with +$1B sales potential • Androxal ® : PHASE 3 (SPA) oral treatment for Low Testosterone with pending patent/ patent life to the mid 2020’s(growing +$2B market) – Restoration of testicular function and testosterone levels in treatment of 2 º hypogonadism (most common cause of low T) • Proellex : PHASE 2 treatment for uterine fibroids and endometriosis with pending patent/ patent life to the mid 2020’s (+$5B market) – Chronic relief of uterine fibroid symptoms – Fibroid de - bulking – Chronic relief of the symptoms associated with endometriosis – Potential breast cancer intervention • Key late stage clinical & regulatory events driven news flow in 2013
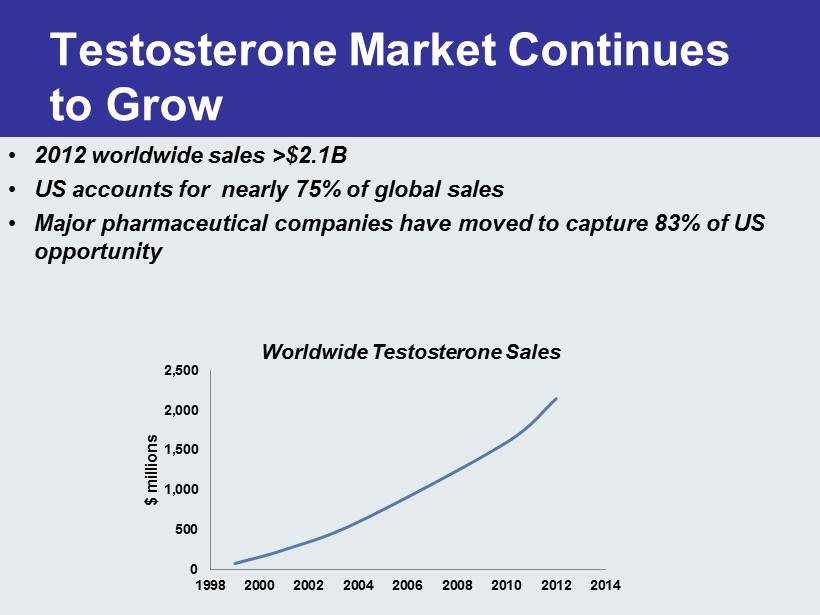
Testosterone Market Continues to Grow 0 500 1,000 1,500 2,000 2,500 1998 2000 2002 2004 2006 2008 2010 2012 2014 $ millions • 2012 worldwide sales >$2.1B • US accounts for nearly 75% of global sales • Major pharmaceutical companies have moved to capture 83% of US opportunity Worldwide Testosterone Sales

Excerpt: FDA SPA Minutes 1) Proportion of subjects with average serum concentration ( Cavg ) for T in the normal range (i.e. serum T of 300 ng / dL – 1040 ng / dL ). 2) Proportion of subjects with a 50% or greater decrease in sperm concentration from baseline to endpoint. To demonstrate efficacy with regards to the first endpoint, at least 75% of subjects in the Androxal group should achieve a Cavg for T in the normal range with the lower bound of the 95% confidence interval not below 67%. At least 100 Androxal subjects would be required to demonstrate a point estimate of 75% or better. For the second endpoint, Androxal should be non - inferior to placebo with respect to the difference in responder rates. We have found a 20% non - inferiority margin to be acceptable in prior similar trials. Values for serum T and sperm concentration at baseline and endpoint should be based on at least two assessments. Semen sampling at each time point (baseline and endpoint) should be separated by at least 48 hours. Cmax is an important safety issue. The percentage of patients with Cmax above the following three pre - determined limits (listed below) should be a secondary endpoint: • Cmax >1500 ng / dL • Cmax >1800 ng / dL and <2499 ng / dL • Cmax >2500 ng / dL

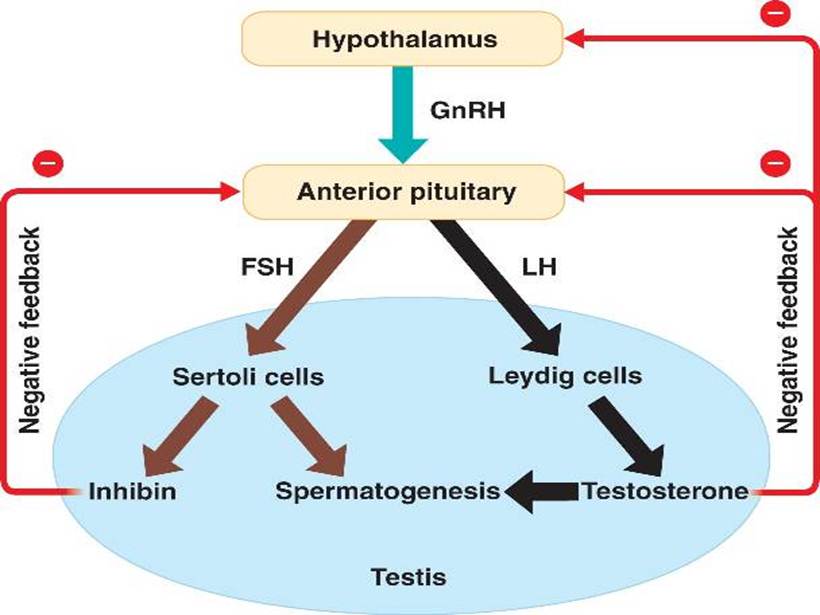
How to Impact Sperm Concentration While Treating for Hypogonadism • Suppress FSH – Administration of exogenous testosterone • Reduces sperm counts to azoospermic or oligospermic levels • Infertility or impaired fertility • Significant increase in sexual activity – In general fertility unaffected

% of Subjects with FSH Below the Lower Limit of Normal and Below the Lower Limit of Detection (0.3 mIU/ml) after 3 months ZA - 003 “Completer” Analysis 2.8 0 0 0 62.2 43.2 0 0 0 20 40 60 80 100 % with FSH < 1.4 mIU/ml % with FSH < 0.3 mIU/ml %of Subjects 12.5 mg Androxal 25 mg Androxal Androgel Placebo

FSH Effects ZA - 203 (3 month study) 0 2 4 6 8 10 12 14 12.5 mg 25 mg PL Testim FSH Treatment Effect of Treatment on Median FSH p versus Testim Before After p=0.0004 vs pbo

Sperm Concentration ZA - 203 0 10 20 30 40 50 60 70 80 90 100 12.5 mg 25 mg PL Testim [Sperm] in Millions/ml Treatment Effect of Treatment on Median Sperm Concentration p versus Testim Baseline EOS p=0.0049 vs pbo

Ejaculation Frequency Reduces Sperm Numbers (n=12 subjects (age 18 - 25) 0 10 20 30 40 50 60 70 80 90 100 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 % of Baseline Total Sperm Count Days Fertility and Sterility, Levine et al Vol.5, No. 5, May 1986
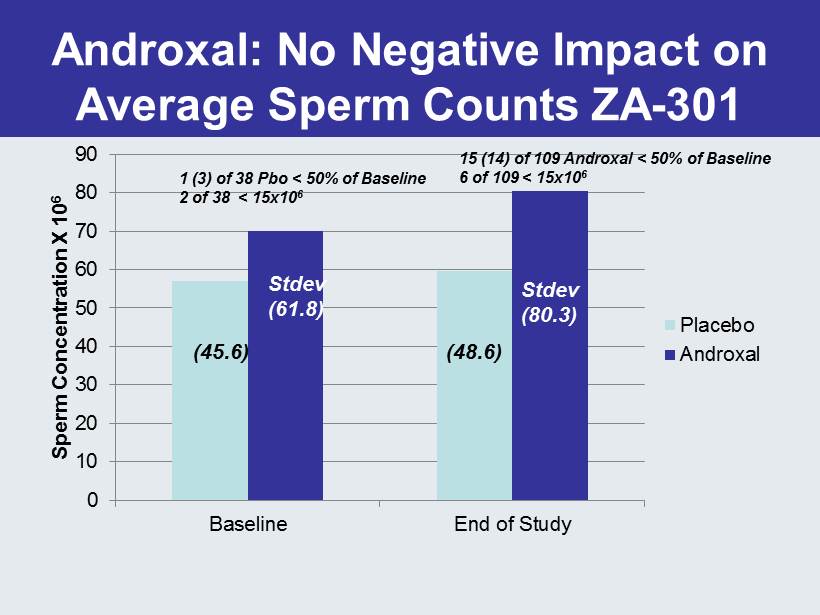
Androxal : No Negative Impact on Average Sperm Counts ZA - 301 0 10 20 30 40 50 60 70 80 90 Baseline End of Study Sperm Concentration X 10 6 Placebo Androxal (48.6) (45.6) Stdev (61.8) Stdev (80.3) 1 (3) of 38 Pbo < 50% of Baseline 2 of 38 < 15x10 6 15 (14) of 109 Androxal < 50% of Baseline 6 of 109 < 15x10 6

Breakdown of Sperm Count Drops >50% by Site ZA - 301 0 5 10 15 20 25 30 35 40 45 Site 9 Site 44 Site 6,7,10,12 Site 22,23,30,33,34 Site 41,42,43,46,49,50 Number of Subjects >50% Drop on Pbo >50% Drop on Drug <50% Drop 7.5% 25% 8% 10.7% 8.1%
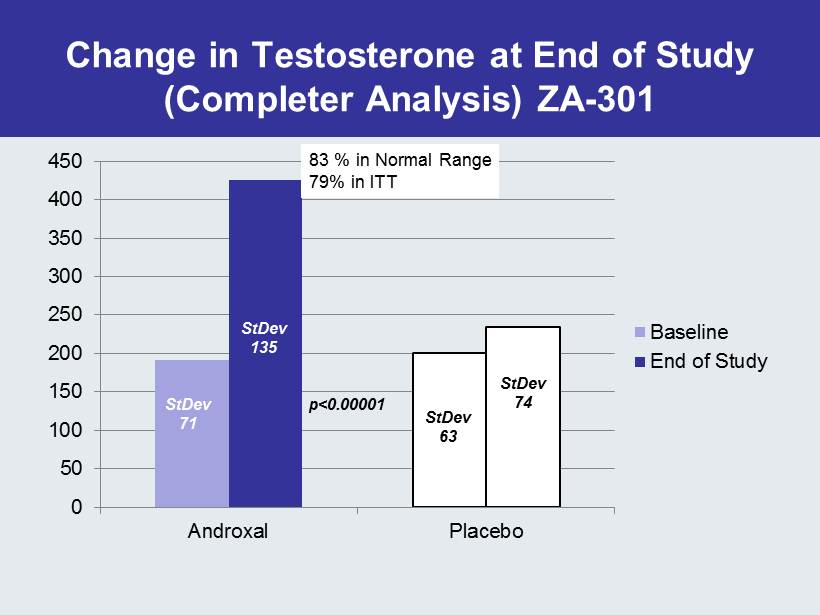
Change in Testosterone at End of Study (Completer Analysis) ZA - 301 0 50 100 150 200 250 300 350 400 450 Androxal Placebo Baseline End of Study 83 % in Normal Range 79% in ITT StDev 71 StDev 135 StDev 63 StDev 74 p<0.00001

ZA - 300: % of Men with T in the Normal Range Fully Enrolled 0 10 20 30 40 50 60 70 80 90 100 Week 6 Week 16 Week 26 % Normal n=476 n=344 N=185 Normal Range = 300 – 1040 ng / dL
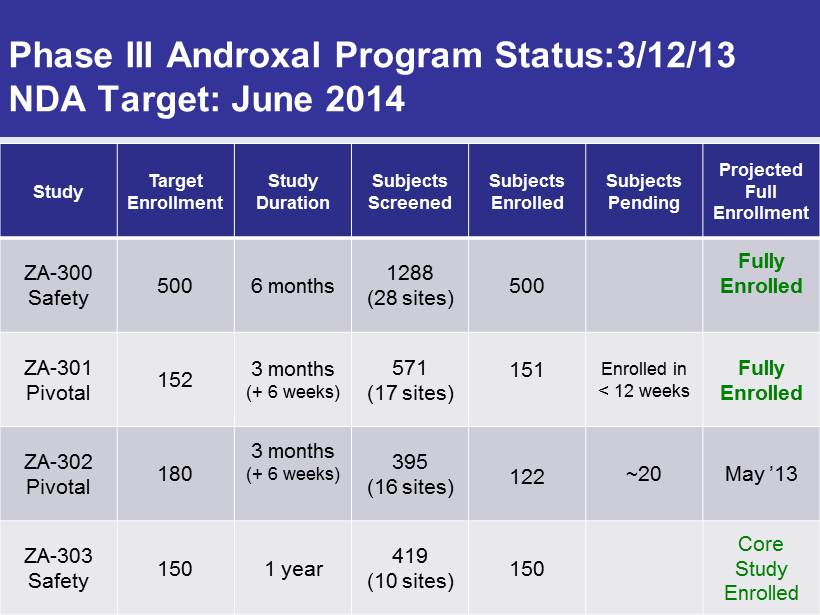
Phase III Androxal Program Status:3/12/13 NDA Target: June 2014 Study Target Enrollment Study Duration Subjects Screened Subjects Enrolled Subjects Pending Projected Full Enrollment ZA - 300 Safety 500 6 months 1288 (28 sites) 500 Fully Enrolled ZA - 301 Pivotal 152 3 months (+ 6 weeks) 571 (17 sites) 151 Enrolled in < 12 weeks Fully Enrolled ZA - 302 Pivotal 180 3 months (+ 6 weeks) 395 (16 sites) 122 ~20 May ’13 ZA - 303 Safety 150 1 year 419 (10 sites) 150 Core Study Enrolled

Androxal Profile Favorable Compared to Leading T Products T Gels/Creams Androxal Advantage Androxal Administration Applied to Skin Oral Controlled Substance Yes No Sexual Partner & Risk to Children Yes No Unpredictable Response Yes No Super High T Levels Yes No Prostate Risk Yes No Shuts Down Testes Yes No Requires Chronic Treatment Yes No

Third Party Study Suggests Favorable Reimbursement Potential for Androxal Majority of payers believe Androxal’s oral administration and non - chronic use may offer overall cost savings – Third party assessment of payers suggests vast majority (>90%) would add Androxal to formularies • Cost will be key for tier placement • 50% of plans indicated they would require a PA (Prior Authorization) to show proper diagnosis • 62 % of respondents expect Androxal to be priced at parity to Androgel • Anticipated Androxal pricing of $170 - 350/month would be competitive with Androgel

0 100 200 300 400 500 600 700 Morning TT (ng/dL) Fig. 14: Mean Morning TT Over Time (ZA - 204) 6.25mg Androxal 12.5mg Androxal 25mg Androxal Androgel Week 2 Week 4 Week 6 Last Day of Dosing One Week Follow - up One week after dosing all Androxal arms were better than baseline One week after dosing the Androgel arm was worse than baseline Men on Androxal exhibit continued improvement in T even after dosing has stopped

Who are the men using testosterone? European Male Aging Study Distribution and Selected Characteristics of Men Ages 40 - 79 ( Tajar et al) 0 10 20 30 40 50 60 70 80 90 100 Eugonadal Secondary Hypogonadal Primary Compensated Hypogonadism % of Subjects Age: 58.5 (10.7) BMI: 27.3 TT: 513.4 ng / dL LH: 5.2 U/L Age: 59.4 (10.4) BMI: 30.8 (4.8) TT: 250.9 ng / dL LH: 4.4 U/L Age: 70.0 (9.0) BMI: 29.0 (3.9) TT: 216 ng / dL LH: 18.0 U/L Age: 67.3 (9.9) BMI: 26.8 (3.6) TT: 527.8 LH: 14.1 U/L Data derived from over 3000 men Obese men (BMI>30) 8.7X more likely to have Low T than men of normal weight In 2010 there were ~90 million men in the US between the ages of 20 and 65 32% are obese Overweight BMI > 25 (6’ 190# male BMI=25.8) Obese BMI > 30 (6’ 230# male BMI =31.2)

Androxal Take Home Message • Because of Obesity, 30% of American Males are at Risk of Secondary Hypogonadism – Co - morbidities include diabetes and cardiovascular disease • Approved T Products Worsen the Underlying Condition • We believe only Androxal + Diet + Exercise can reverse this disorder

Proellex for the Treatment of Uterine Fibroids and Endometriosis Over 30 million women of reproductive age in the US afflicted with symptomatic uterine fibroids or endometriosis Over 300,000 hysterectomies performed every year in the US to treat these two disorders No acceptable chronic therapeutic options available today
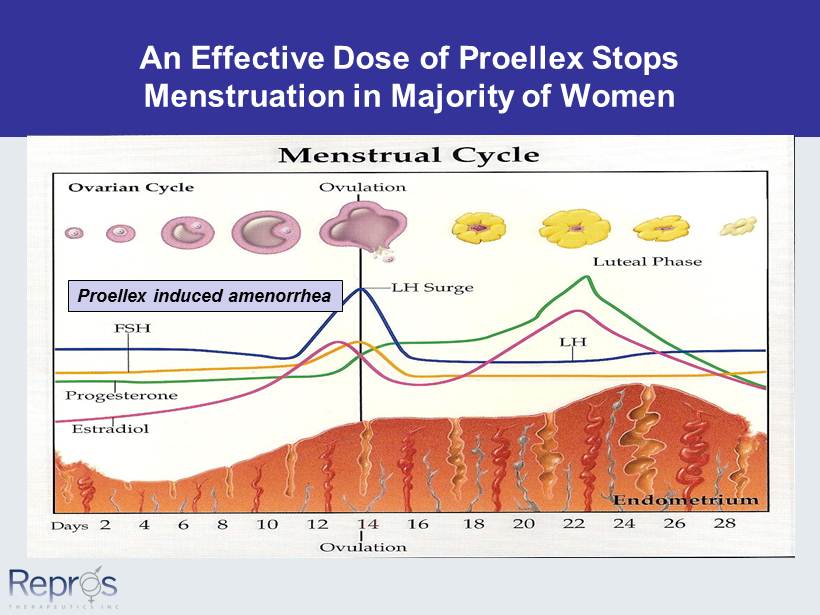
An Effective Dose of Proellex Stops Menstruation in Majority of Women Proellex induced amenorrhea

% of Women Experiencing Proellex Induced Amenorrhea in Low Dose Study 0 10 20 30 40 50 60 70 80 90 100 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg Doses for Phase 2 Endometriosis Study Doses safely tested in Phase 2 trial

ZPE - 201 Baseline vs Last 28 Days All Patient Reported Endometriosis Pain 0 10 20 30 40 50 60 70 Placebo Proellex 25mg Proellex 50 % of Baseline n=23 p - value versus placebo 25 mg = 0.001 50 mg = 0.017 n=20 n=21 Dysmenorrhea , Dyspareunia and Non Menstrual Pelvic Pain

ZPE - 201 Doses That Stop Menses Have Significant Impact on Analgesic Use in the Control of the Pain Symptoms of Endometriosis 88.9 33.4 36.4 0 10 20 30 40 50 60 70 80 90 100 Placebo 25mg Proellex 50mg Proellex % of Subjects Requiring Narcotic or Non Narcotic Analgesics at End of Study Baseline End of Study 55.5 22.2 13.6 0 10 20 30 40 50 60 70 80 90 100 Placebo 25mg Proellex 50mg Proellex % of Subjects Requiring Narcotics at End of Study Baseline End of Study FDA allows Repros to conduct Phase 2 study in women with severe endometriosis P<0.01 P<0.001

ZPE - 202 Phase 2 Endometriosis Study • 90 subject double blind placebo controlled study balanced between placebo, 6 and 12 mg oral Proellex – Subject population (confirmed endometriosis) • Severe endometriosis as determined by BBSS score • Requiring narcotics or prescription analgesics to control endometriosis related pain – Study Duration: 4 months – Study endpoints: • Reduction in need for analgesics from baseline • Change from baseline in BBSS pain scores – Status: enrolling sites and subjects

Vaginal Proellex to Eliminate the Need for Hysterectomy in Most Situations – Initial Phase 2 study to test four doses of vaginal administration in the treatment of uterine fibroids completed • Assess reduction of fibroid size and elimination of symptoms • Top line data reported – End of Phase 2 meeting request with FDA accepted by Agency for end of May 2013 – Propose 90 subject 1 st Phase 3 study and….. • 2 Phase 3 studies • 200 subjects for +1 year • 300 - 600 subjects for +6 months – Separate IND from low dose oral

Systemic Exposure to Oral Proellex Varies in a Dose Dependent Manner Significant reduction in exposure via vaginal delivery 0 200 400 600 800 1000 1200 1400 1600 1800 12 mg vaginal 1 mg 3 mg 6 mg 9 mg 12 mg 25 mg 50 mg Concentration ( ng /ml) Combined Cmax for Telapristone and Primary Metabolite Liver associated SAE’s limited to 50 mg dose 3.5% of women exposed

Vaginal Proellex Update 12 mg Dose Achieves Significant Improvement in Vaginal Bleeding 0 20 40 60 80 100 120 Baseline End of Study Mean Pictorial Blood loss Score 3 & 6 mg 12 mg 12 mg dose achieves significant reduction vs baseline p=0.0003 and lower doses p=0.045 Menorrhagia

ZPU - 003 Phase II Uterine Fibroid Study Pictoral Blood Loss 0 20 40 60 80 100 120 140 160 BL Mo 1 Mo 2 Mo 3 Mean PBAC Score mL 12.5 mg oral p < 0.0001 vs Pl n ~ 40/arm Menorrhagia

Vaginal Proellex Update 12 mg Dose Achieves Significant Improvement in Symptom Scores 0 10 20 30 40 50 60 Baseline End of Study Mean Uterine Fibroid Symptom Score 3 & 6 mg 12 mg 12 mg dose achieves significant reduction vs baseline p=0.001 and vs lower doses p=0.029 Score for normal women

12.5 mg Oral Proellex Produces Significant Clinical Benefit Resulting in Substantially Asymptomatic Uterine Fibroids in Phase 2 Study (ZPU - 003) 0 5 10 15 20 25 30 35 40 45 Symptom Severity UFSQOL Symtpm Severity Placebo 12.5 mg Oral Normal Women US Phase Iib ~ 40/arm Spies et al, Obstetrics & Gynecology, vol. 99, No. 2, February 2002 p vs placebo < 0.0001 Higher Score = Worse

Fibroid Volume Significantly Reduced at end of 4 Month Study @ 12 mg 0 20 40 60 80 100 Mean % of Initial Volume % of Initial Volume 3mg 6mg 12mg p vs 3mg = 0.002 p vs 6mg = 0.01

ZPV - 200 Ext. Proellex Open Label Update (12mg dose) 0 10 20 30 40 50 60 70 80 90 100 Baseline End of 1st Cycle End of 2nd Cycle % of Initial Volume Fibroid Volume Reduction by MRI (n=15) Paired Comparison • Baseline to Cycle 1 p=0.005 • Cycle 1 to Cycle 2 p= 0.003

Financial Summary • Cash and equivalents (as of 4/1/13, unaudited) ~ $18 M • Cash runway: Q1 2014 • Current shares outstanding: 18.6 M shares – This includes approximately 1.5 million shares resulting from the cashless exercise of 872,133 Series A Warrants and 713,741 Series B Warrants on Jan. 29, 2013. – Warrants Outstanding – Series A – 877,137 (purchased in unit deal @ $2.45); Series B – 855,680 @ $2.49 exercise price.

2013 Anticipated Milestones • Report results for Phase 2 Vaginal Proellex Study Q1 - 13 • Fully Enroll 1 year Dexa Study Q1 - 13 • Fully Enroll 500 subject 6 mos. Androxal Study Q1 - 13 • Report Results for 1 st Pivotal Androxal Study Q2 - 13 • End of Phase 2 Meeting with FDA for Vaginal Proellex Q2 - 13 • Commence Phase 3 Vaginal Proellex Study Q3 - 13 • Complete 500 subject 6 mos. Androxal Study Q3 - 13 • Report Phase 2 low dose Oral Proellex Study Q4 - 13 • Report 2 nd Pivotal Androxal Study Q4 - 13 • Request Androxal Pre - NDA Meeting with FDA for Q1’14 Q4 - 13 • Submit Androxal NDA Mid - 2014
