Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REPROS THERAPEUTICS INC. | v331473_8k.htm |

Developing clinical stage small molecule therapeutics to treat hormonal and reproductive system disorders

Repros Disclaimer Any statements made by the Company that are not historical f acts contained in this release are forward-looking statements that involve risks and uncertainties, including the ability to raise addition al needed capital on a timely basis in order for it to continue to fund development of its Androxal ® and Proellex ® programs, have success in the clinical development of its technologies, the reliability of interim results to predict final study outcomes, and such other risks which are identified in the Company's most recent Annual Report on Form 10-K and in any subsequent quarterly reports on Form 10 -Q. These documents are available on request from Repros Therapeutics or at www.sec.gov. Repros disclaims any intention or obligation to update or revise any forward -looking statements, whether as a result of new information, future events or otherwise.

Investment Highlights • Focused strategy: small molecule therapeutics for reproductive disorders • Two late stage clinical programs each with +$1B sales potential • Androxal ® : PHASE 3 (SPA) oral treatment for Low Testosterone with pending patent/ patent life to the mid 2020’s(growing +$2B market) – Restoration of testicular function and testosterone levels in treatment of 2ºhypogonadism (most common cause of low T) • Proellex: PHASE 2 treatment for uterine fibroids and endometriosis with pending patent/ patent life to the mid 2020’s (+$5B market) – Chronic relief of uterine fibroid symptoms – Fibroid de-bulking – Chronic relief of the symptoms associated with endometriosis • Key late stage clinical & regulatory events expected in 2013
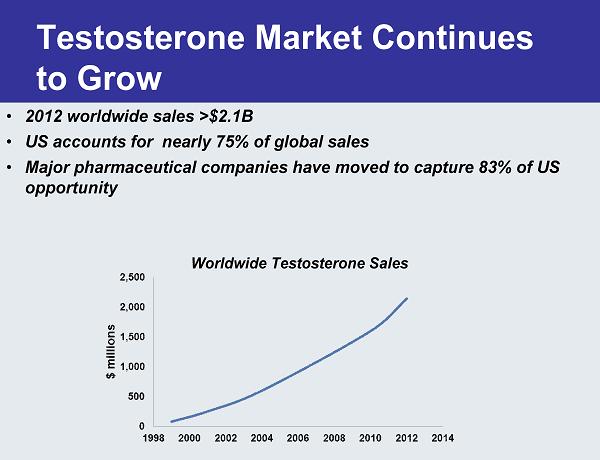
Testosterone Market Continues to Grow • 2012 worldwide sales >$2.1B • US accounts for nearly 75% of global sales • Major pharmaceutical companies have moved to capture 83% of US opportunity Worldwide Testosterone Sales
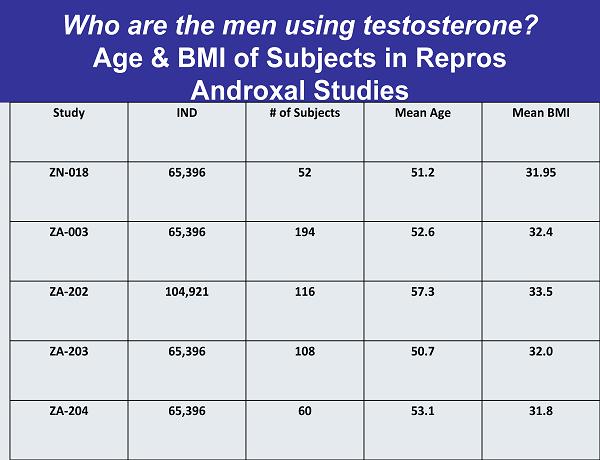
Who are the men using testosterone? Age & BMI of Subjects in Repros Androxal Studies Study IND # of Subjects Mean Age Mean BMI ZN-018 65,396 52 51.2 31.95 ZA-003 65,396 194 52.6 32.4 ZA-202 104,921 116 57.3 33.5 ZA-203 65,396 108 50.7 32.0 ZA-204 65,396 60 53.1 31.8
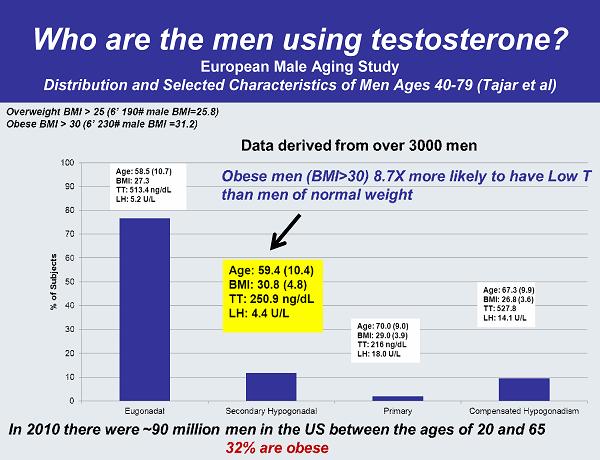
Who are the men using testosterone? European Male Aging Study Distribution and Selected Characteristics of Men Ages 40-79 (Tajar et al)
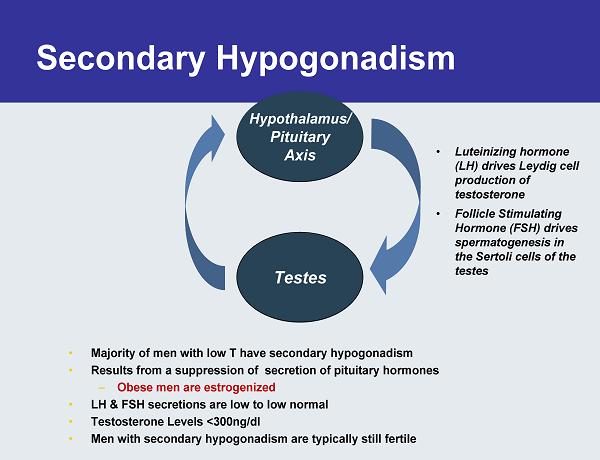
Secondary Hypogonadism • Majority of men with low T have secondary hypogonadism • Results from a suppression of secretion of pituitary hormones – Obese men are estrogenized • LH & FSH secretions are low to low normal • Testosterone Levels <300ng/dl • Men with secondary hypogonadism are typically still fertile Testes Hypothalamus/ Pituitary Axis • Luteinizing hormone (LH) drives Leydig cell production of testosterone • Follicle Stimulating Hormone (FSH) drives spermatogenesis in the Sertoli cells of the testes Testes Hypothalamus/ Pituitary Axis
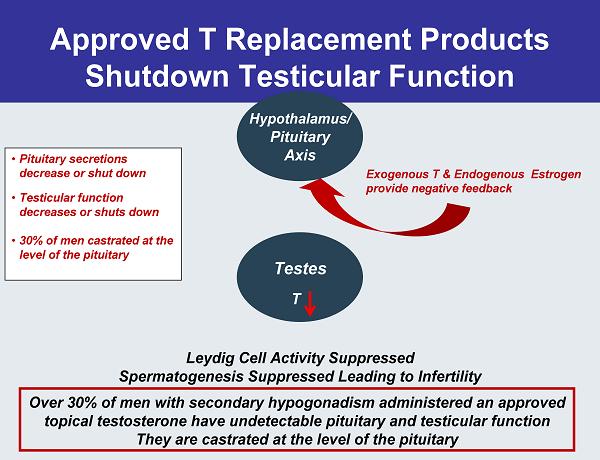
Approved T Replacement Products Shutdown Testicular Function Testes Hypothalamus/ Pituitary Axis •Pituitary secretions decrease or shut down •Testicular function decreases or shuts down •30% of men castrated at the level of the pituitary Leydig Cell Activity Suppressed Spermatogenesis Suppressed Leading to Infertility T Over 30% of men with secondary hypogonadism administered an approved topical testosterone have undetectable pituitary and testicular function They are castrated at the level of the pituitary Exogenous T & Endogenous Estrogen provide negative feedback
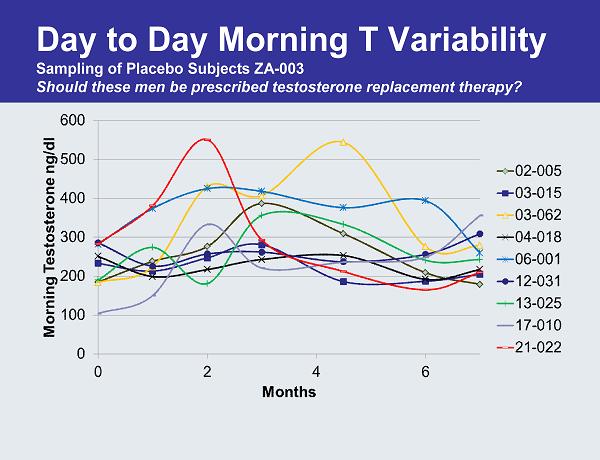
Day to Day Morning T Variability Sampling of Placebo Subjects ZA-003 Should these men be prescribed testosterone replacement therapy?
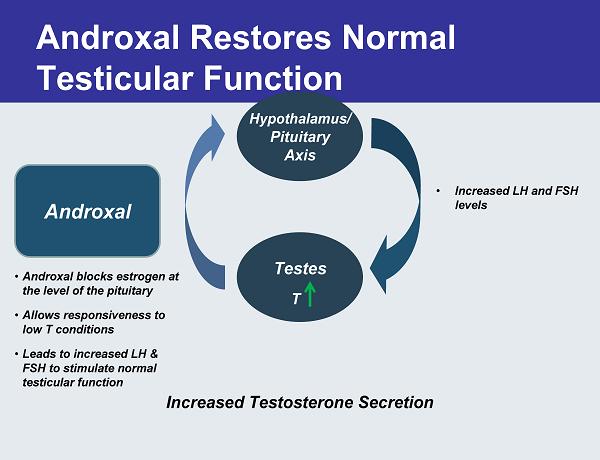
Androxal Restores Normal Testicular Function Testes Increased Testosterone Secretion Androxal •Androxal blocks estrogen at the level of the pituitary •Allows responsiveness to low T conditions •Leads to increased LH & FSH to stimulate normal testicular function • Increased LH and FSH levels T Hypothalamus/ Pituitary Axis
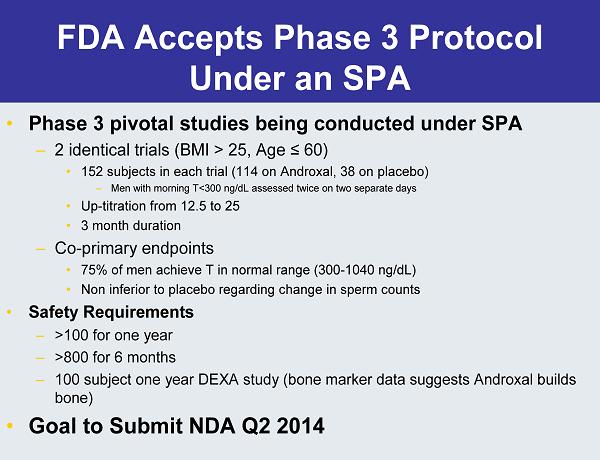
FDA Accepts Phase 3 Protocol Under an SPA • Phase 3 pivotal studies being conducted under SPA – 2 identical trials (BMI > 25, Age ≤60) • 152 subjects in each trial (114 on Androxal, 38 on placebo) – Men with morning T<300 ng/dL assessed twice on two separate days • Up-titration from 12.5 to 25 • 3 month duration – Co-primary endpoints • 75% of men achieve T in normal range (300-1040 ng/dL) • Non inferior to placebo regarding change in sperm counts • Safety Requirements – >100 for one year – >800 for 6 months – 100 subject one year DEXA study (bone marker data suggests Androxal builds bone) • Goal to Submit NDA Q2 2014
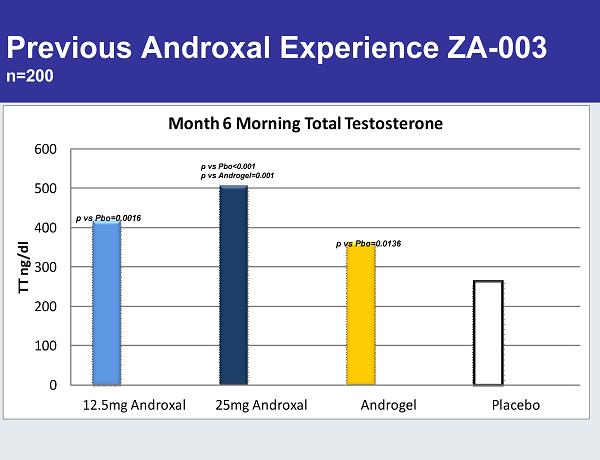
Previous Androxal Experience ZA-003 n=200 0 100 200 300 400 500 600 12.5mg Androxal 25mg Androxal Androgel Placebo TT ng/dl Month 6 Morning Total Testosterone p vs Pbo=0.0016 p vs Pbo<0.001 p vs Androgel=0.001 p vs Pbo=0.0136

Previous Studies Suggests Favorable Outcome ZA-003 Testosterone Distribution (6 Month Data) n=162 patients
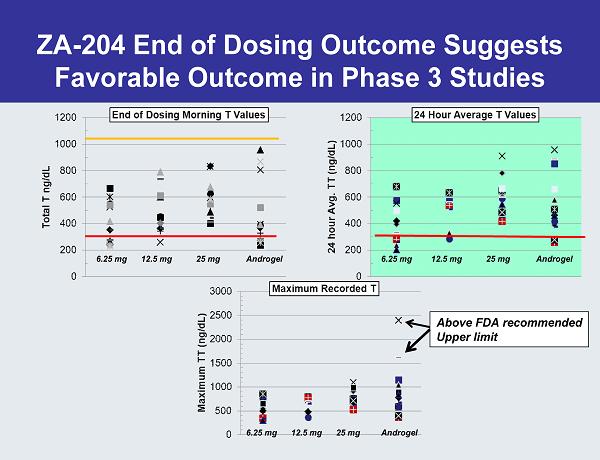
6.25 mg 12.5 mg 25 mg Androgel Androgel Androgel Above FDA recommended Upper limit ZA-204 End of Dosing Outcome Suggests Favorable Outcome in Phase 3 Studies 25 mg12.5 mg6.25 mg 6.25 mg 12.5 mg 25 mg
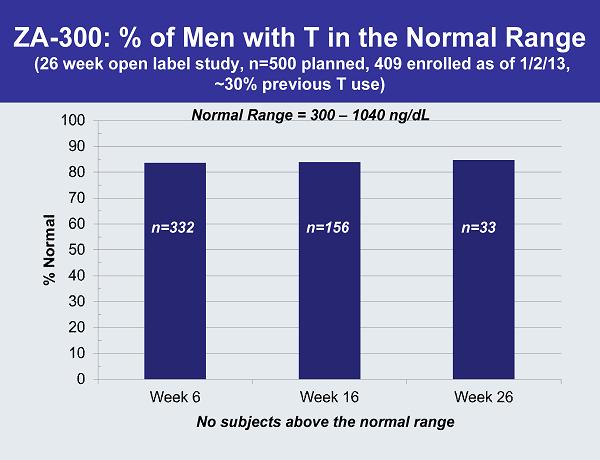
ZA-300: % of Men with T in the Normal Range (26 week open label study, n=500 planned, 409 enrolled as of 1/2/13, ~30% previous T use) n=332 n=156 n=33 Normal Range = 300 –1040 ng/dL No subjects above the normal range
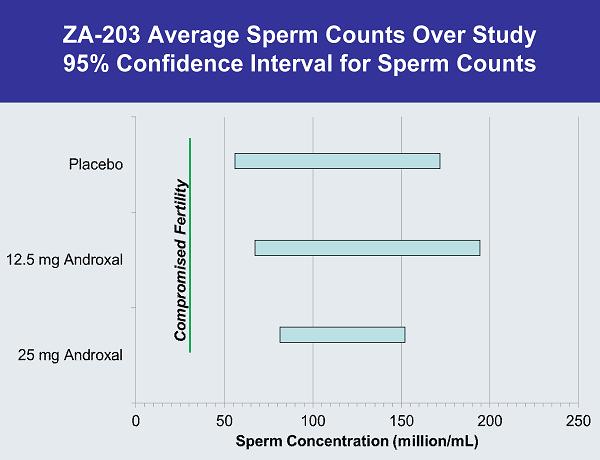
ZA-203 Average Sperm Counts Over Study 95% Confidence Interval for Sperm Counts

Androxal Preserves Testicular Function Approved Topical Products Suppress the Approved Topical Products Suppress the Hypothalamic Hypothalamic - - Pituitary Pituitary - - Testes Axis Testes Axis

24 Hour T & LH 25 mg Androxal Subject Study ZA-204 Subject 2-003 Age: 55, BMI: 32 Baseline 0 time: 8:09 AM Week 6 0 time: 7:25 AM
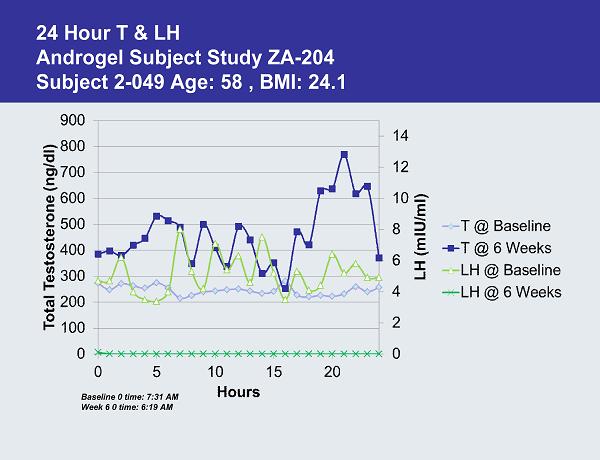
24 Hour T & LH Androgel Subject Study ZA-204 Subject 2-049 Age: 58 , BMI: 24.1 Baseline 0 time: 7:31 AM Week 6 0 time: 6:19 AM
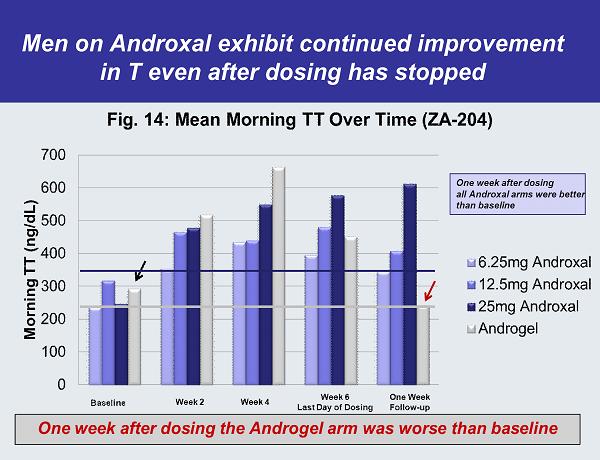
Men on Androxal exhibit continued improvement in T even after dosing has stopped
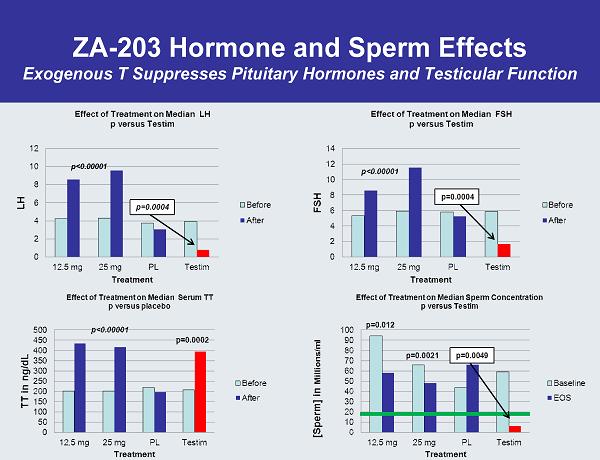
ZA-203 Hormone and Sperm Effects Exogenous T Suppresses Pituitary Hormones and Testicular Function p<0.00001

Distribution of Men Screened into ZA-300 850 subjects screened as of 11/10/12
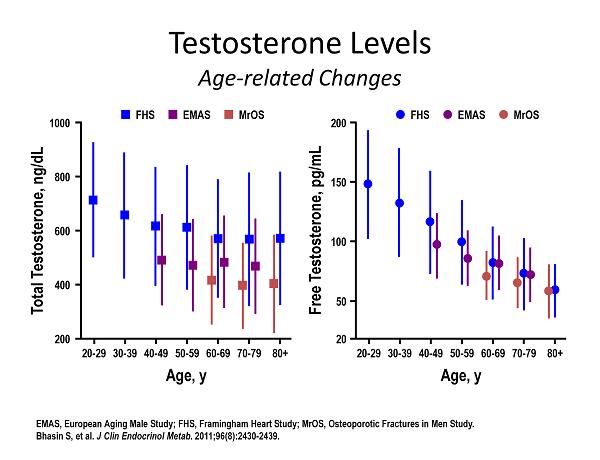
Testosterone Levels Age - related Changes EMAS, European Aging Male Study; FHS, Framingham Heart Study; MrOS, Osteoporotic Fractures in Men Study. Bhasin S, et al. J Clin Endocrinol Metab . 2011;96(8):2430 - 2439. Total Testosterone, ng/dL 20 - 29 30 - 39 40 - 49 50 - 59 60 - 69 70 - 79 1000 800 200 600 400 80+ Age, y FHS EMAS MrOS Free Testosterone, pg/mL 20 - 29 30 - 39 40 - 49 50 - 59 60 - 69 70 - 79 200 150 20 100 50 80+ Age, y FHS EMAS MrOS
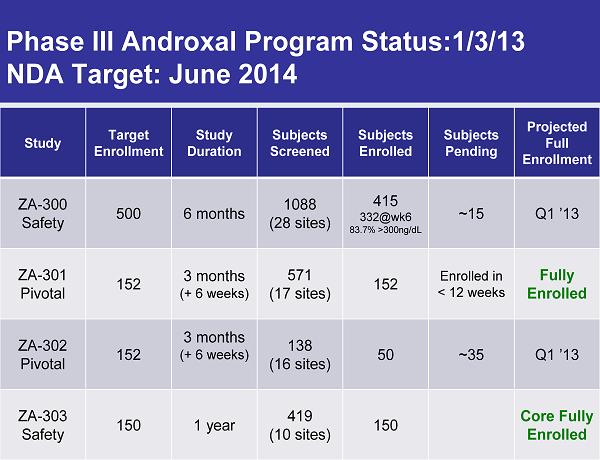
Phase III Androxal Program Status:1/3/13 NDA Target: June 2014 Study Target Enrollment Study Duration Subjects Screened Subjects Enrolled Subjects Pending Projected Full Enrollment ZA-300 Safety 500 6 months 1088 (28 sites) 415 332@wk6 83.7% >300ng/dL ~15 Q1 ’13 ZA-301 Pivotal 152 3 months (+ 6 weeks) 571 (17 sites) 152 Enrolled in < 12 weeks Fully Enrolled ZA-302 Pivotal 152 3 months (+ 6 weeks) 138 (16 sites) 50 ~35 Q1 ’13 ZA-303 Safety 150 1 year 419 (10 sites) 150 Core Fully Enrolled
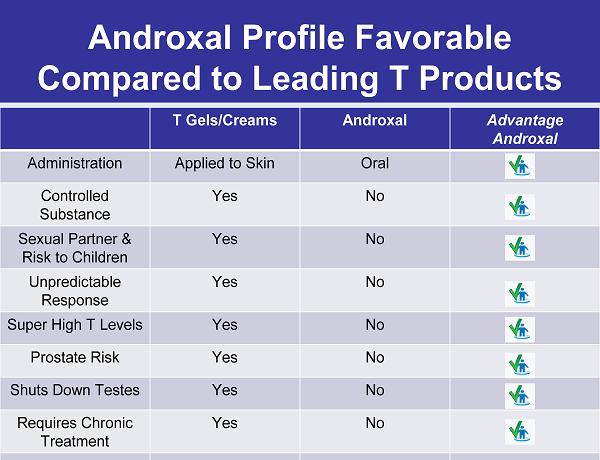
Androxal Profile Favorable Compared to Leading T Products T Gels/Creams Androxal Advantage Androxal Administration Applied to Skin Oral Controlled Substance Yes No Sexual Partner & Risk to Children Yes No Unpredictable Response Yes No Super High T Levels Yes No Prostate Risk Yes No Shuts Down Testes Yes No Requires Chronic Treatment Yes No
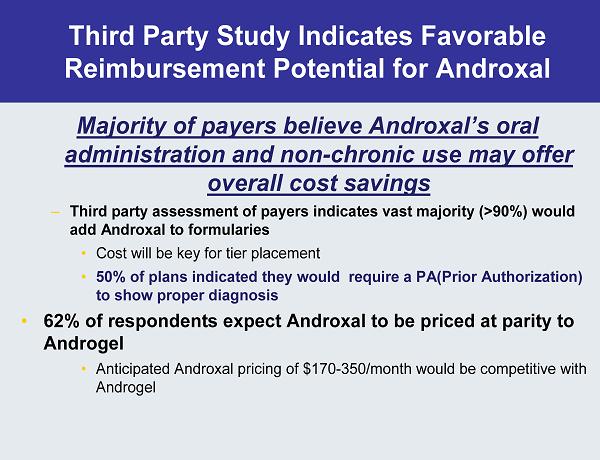
Third Party Study Indicates Favorable Reimbursement Potential for Androxal Majority of payers believe Androxal’s oral administration and non-chronic use may offer overall cost savings – Third party assessment of payers indicates vast majority (>90%) would add Androxal to formularies • Cost will be key for tier placement • 50% of plans indicated they would require a PA(Prior Authorization) to show proper diagnosis • 62% of respondents expect Androxal to be priced at parity to Androgel • Anticipated Androxal pricing of $170-350/month would be competitive with Androgel

Androxal Take Home Message • Because of Obesity, 30% of American Males are at Risk of Secondary Hypogonadism –Co-morbidities include diabetes and cardiovascular disease • Approved T Products Worsen the Underlying Condition • We believe only Androxal + Diet + Exercise can reverse this disorder

Proellex for the Treatment of Uterine Fibroids and Endometriosis Over 30 million women of reproductive age in the US afflicted with symptomatic uterine fibroids or endometriosis Over 300,000 hysterectomies performed every year in the US to treat these two disorders No acceptable chronic therapeutic options available today
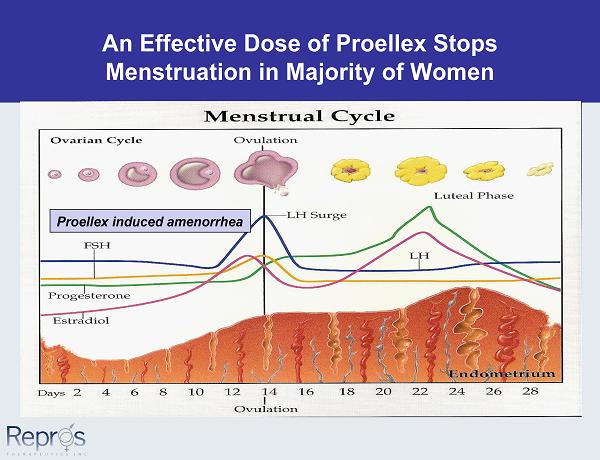
An Effective Dose of Proellex Stops Menstruation in Majority of Women Proellex induced amenorrhea
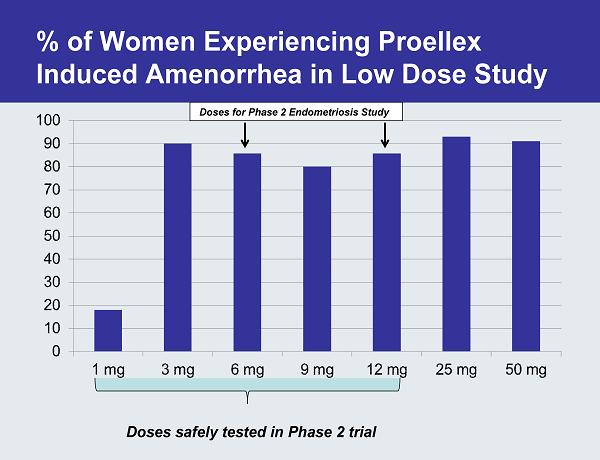
% of Women Experiencing Proellex Induced Amenorrhea in Low Dose Study Doses for Phase 2 Endometriosis Study Doses safely tested in Phase 2 trial
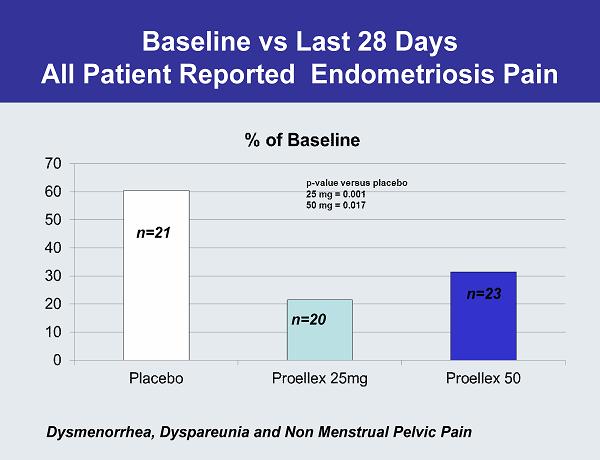
Baseline vs Last 28 Days All Patient Reported Endometriosis Pain n=20 n=21 Dysmenorrhea, Dyspareunia and Non Menstrual Pelvic Pain
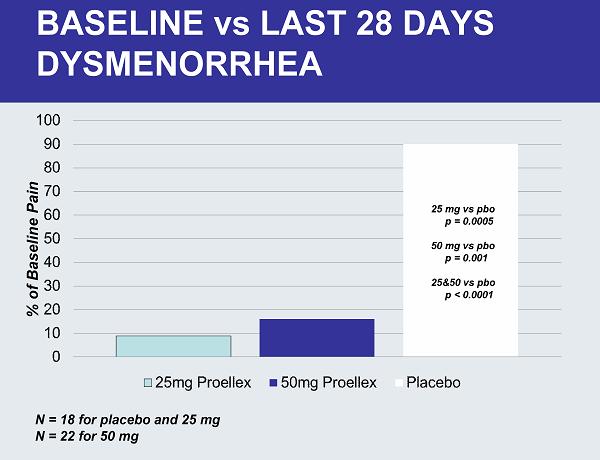
BASELINE vs LAST 28 DAYS DYSMENORRHEA N = 18 for placebo and 25 mg N = 22 for 50 mg 25 mg vs pbo p = 0.0005 50 mg vs pbo p = 0.001 25&50 vs pbo p < 0.0001 % of Baseline Pain
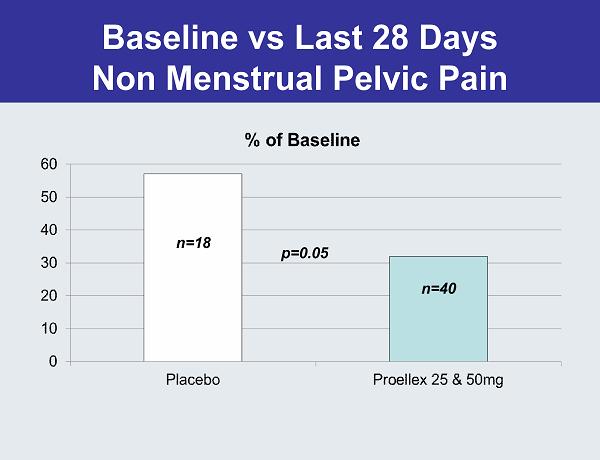
Baseline vs Last 28 Days Non Menstrual Pelvic Pain p=0.05 n=18
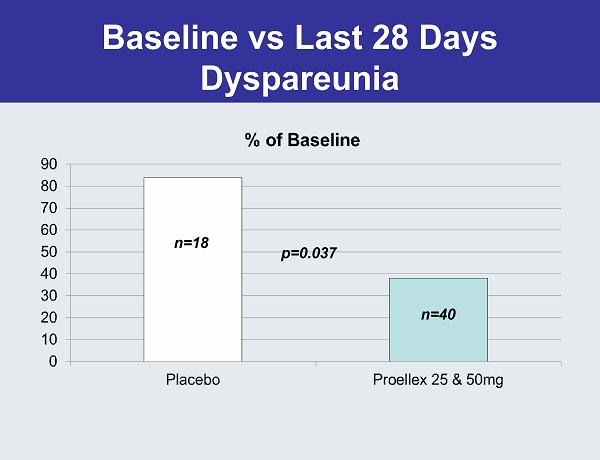
Baseline vs Last 28 Days Dyspareunia p=0.037 n=18 n=40
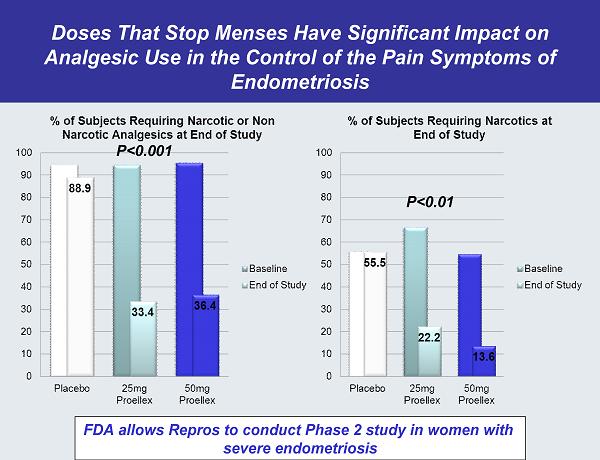
Doses That Stop Menses Have Significant Impact on Analgesic Use in the Control of the Pain Symptoms of Endometriosis FDA allows Repros to conduct Phase 2 study in women with severe endometriosis P<0.01 P<0.001

Vaginal Proellex Expected to Eliminate the Need for Hysterectomy in Most Situations –New IND effective •Unaffected by oral outcomes –Initial Phase 2 study to test four doses of vaginal administration in the treatment of uterine fibroids •Assess reduction of fibroid size and elimination of symptoms •Top line data reported
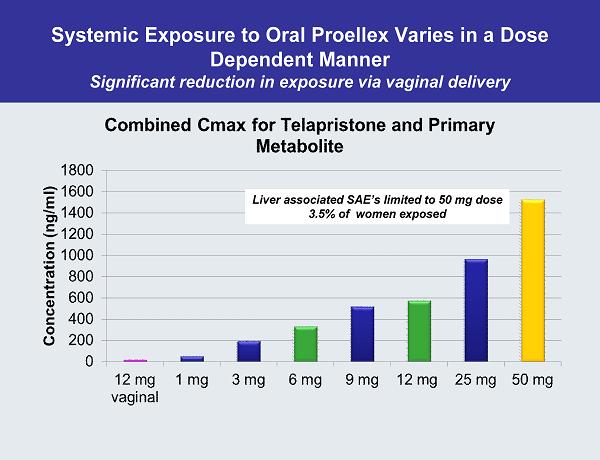
Systemic Exposure to Oral Proellex Varies in a Dose Dependent Manner Significant reduction in exposure via vaginal delivery Liver associated SAE’s limited to 50 mg dose 3.5% of women exposed
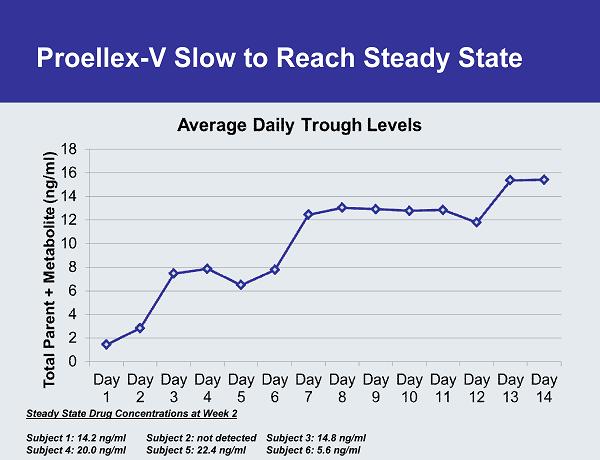
Proellex-V Slow to Reach Steady State Steady State Drug Concentrations at Week 2 Subject 1: 14.2 ng/ml Subject 2: not detected Subject 3: 14.8 ng/ml Subject 4: 20.0 ng/ml Subject 5: 22.4 ng/ml Subject 6: 5.6 ng/ml

At Steady State Proellex-V Yields Flat Exposure Profile
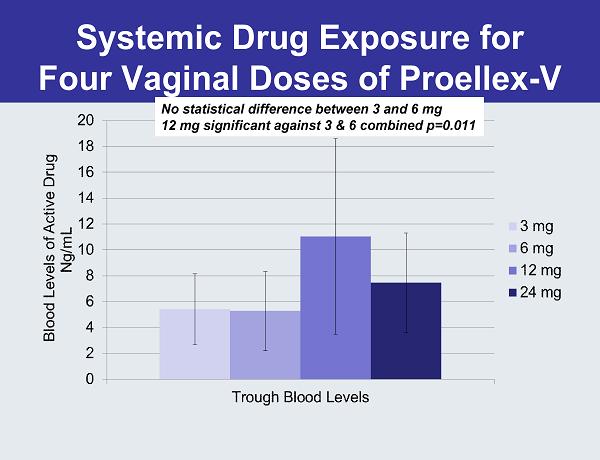
Systemic Drug Exposure for Four Vaginal Doses of Proellex-V No statistical difference between 3 and 6 mg 12 mg significant against 3 & 6 combined p=0.011
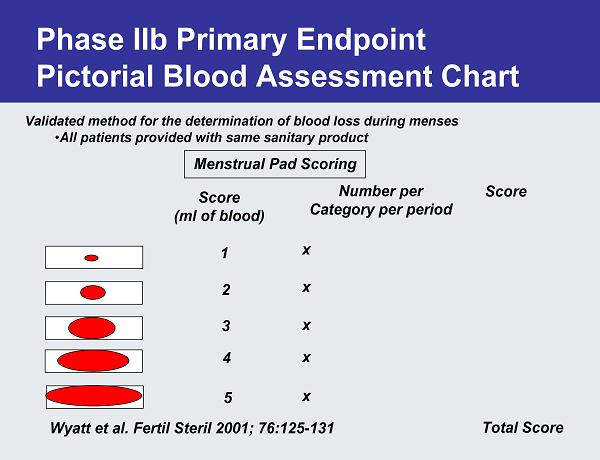
Phase IIb Primary Endpoint Pictorial Blood Assessment Chart Validated method for the determination of blood loss during menses •All patients provided with same sanitary product Menstrual Pad Scoring Score (ml of blood) 1 2 3 4 5 Number per Category per period Score Total Score x x x x x Wyatt et al. Fertil Steril 2001; 76:125-131
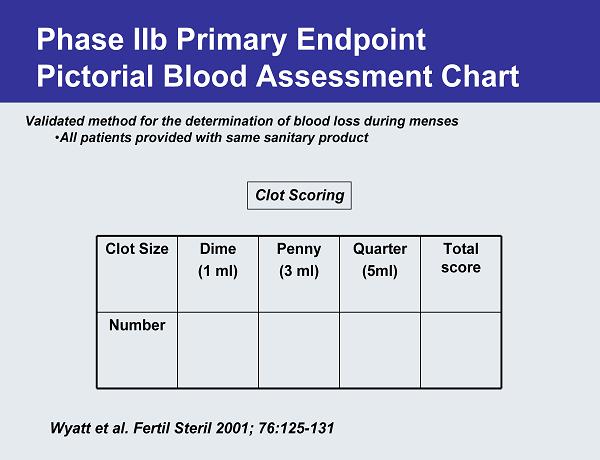
Phase IIb Primary Endpoint Pictorial Blood Assessment Chart Validated method for the determination of blood loss during menses •All patients provided with same sanitary product Clot Scoring Wyatt et al. Fertil Steril 2001; 76:125-131 Clot Size Dime (1 ml) Penny (3 ml) Quarter (5ml) Total score Number
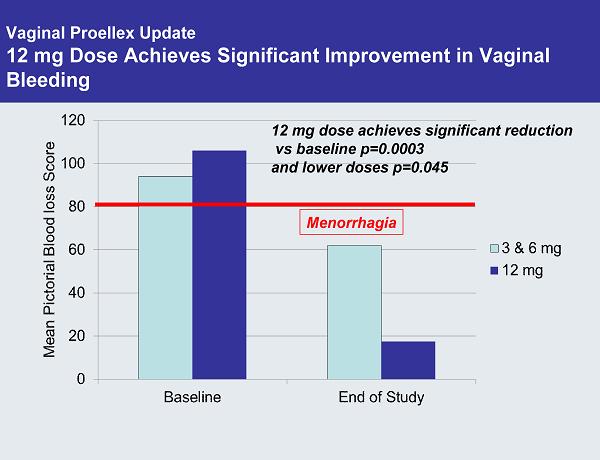
Vaginal Proellex Update 12 mg Dose Achieves Significant Improvement in Vaginal Bleeding 12 mg dose achieves significant reduction vs baseline p=0.0003 and lower doses p=0.045 Menorrhagia

UFSQOL Symptom Severity Questions 1. Heavy bleeding during your menstrual period 2. Passing blood clots during your menstrual period 3. Fluctuation in the duration of your menstrual period compared to your previous cycle 4. Fluctuation in the length of your monthly cycle compared to your previous cycles 5. Feeling tightness or pressure in your pelvic area 6. Frequent urination during the daytime hours 7. Frequent nighttime urination 8. Feeling fatigued
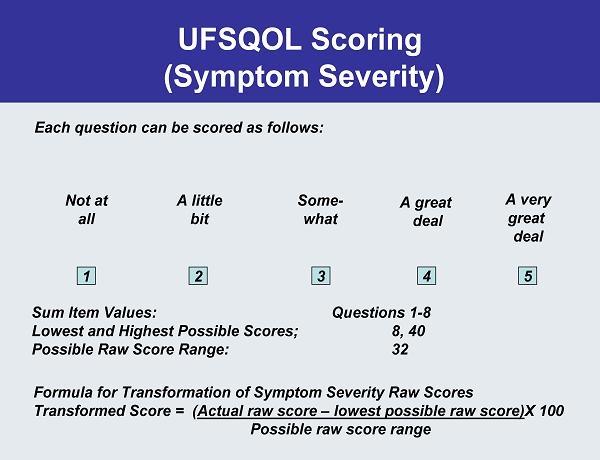
UFSQOL Scoring (Symptom Severity) Each question can be scored as follows: Not at all A little bit Some- what A great deal A very great deal 1 2 3 4 5 Sum Item Values: Questions 1-8 Lowest and Highest Possible Scores; 8, 40 Possible Raw Score Range: 32 Formula for Transformation of Symptom Severity Raw Scores Transformed Score = (Actual raw score –lowest possible raw score)X 100 Possible rawscore range
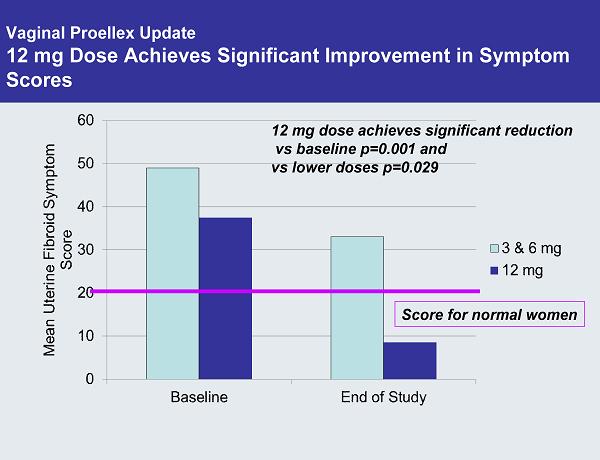
Vaginal Proellex Update 12 mg Dose Achieves Significant Improvement in Symptom Scores 12 mg dose achieves significant reduction vs baseline p=0.001 and vs lower doses p=0.029 Score for normal women
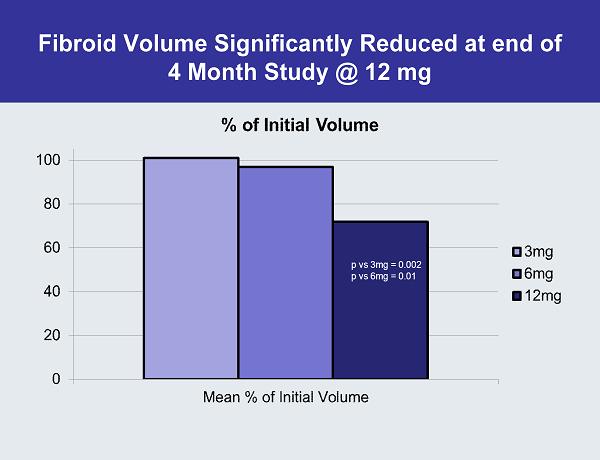
Fibroid Volume Significantly Reduced at end of 4 Month Study @ 12 mg

Financial Summary • Cash and equivalents (as of 12/31/12, unaudited) ~ $24.2M • Cash burn = 2012 = ~$14 M • Cash runway= Mid 2014 (projected) • 17.2 MM shares outstanding – Warrants outstanding = 1.75MM Series A (Purchased in unit deal @$2.46) + 1.57MM Series B (@$2.49 with cashless exercise provision) – Forced warrant strike at $8/share converts B warrants

Projected 2013 Milestones • Report results for Phase 2 Vaginal Proellex Study Q1-13 • Fully Enroll 1 year Dexa Study Q1-13 • Fully Enroll 500 subject 6 mos. Androxal Study Q1-13 • Report Results for 1 st Pivotal Androxal Study Q2-13 • End of Phase 2 Meeting with FDA for Vaginal Proellex Q2-13 • Commence Phase 3 Vaginal Proellex Study Q3-13 • Complete 500 subject 6 mos. Androxal Study Q3-13 • Report Phase 2 low dose Oral Proellex Study Q4-13 • Report 2 nd Pivotal Androxal Study Q4-13 • Request Androxal Pre-NDA Meeting with FDA for Q1’14 Q4-13
