Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Unilife Corp | d335717d8k.htm |
| EX-99.1 - POWER POINT PRESENTATION FOR 2012 SPECIAL MEETING - Unilife Corp | d335717dex991.htm |
Exhibit 99.2

Exhibit 99.2 Australian Shareholder Briefing
April 17, 2012
NASDAQ (UNIS) and ASX (UNS)

Cautionary Note Regarding Forward-Looking Statements
This presentation contains forward looking statements under the safe harbor provisions of the US securities laws. These forward-looking statements are based on management’s beliefs and assumptions and on information currently available to our management.
Our management believes that these forward-looking statements are reasonable as and when made. However you should not place undue reliance on any such forward looking statements as these are subject to risks and uncertainties. Please refer to our press releases and our SEC filings for more information regarding the use of forward looking statements.
| 2 |
|

Everything in place, ready for execution
$40MM, 165,000sqf FDA-registered facility fully operational
World-class management team with deep industry expertise
Quality management system successfully audited on regular basis
Diversified device portfolio driven by emerging customer needs
Strong commercial pipeline, multiple partnerships accelerating
Decade of investment ready to convert to long-term agreements
| 3 |
|
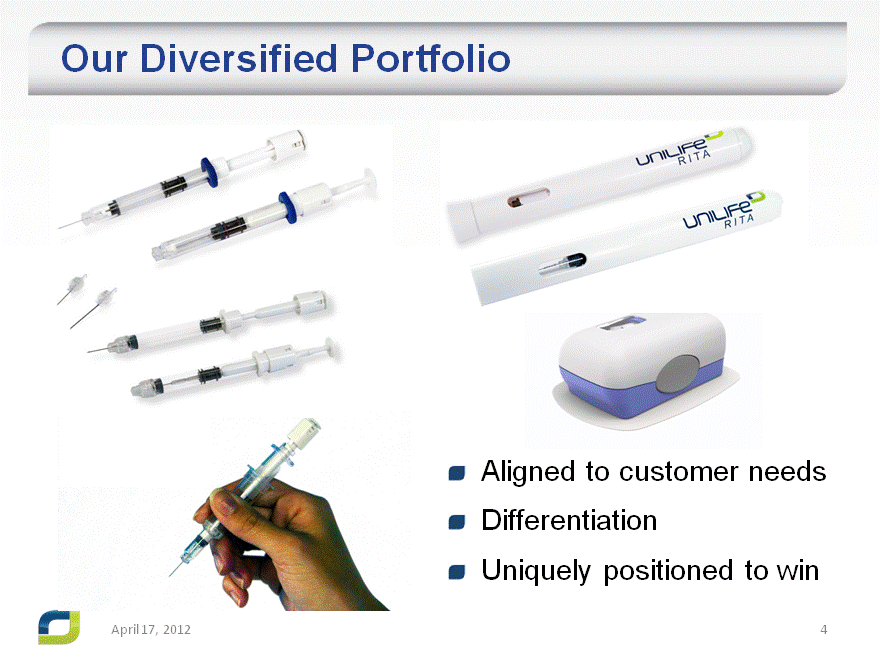
Our Diversified Portfolio
Aligned to customer needs
Differentiation
Uniquely positioned to win
April 17, 2012
| 4 |
|
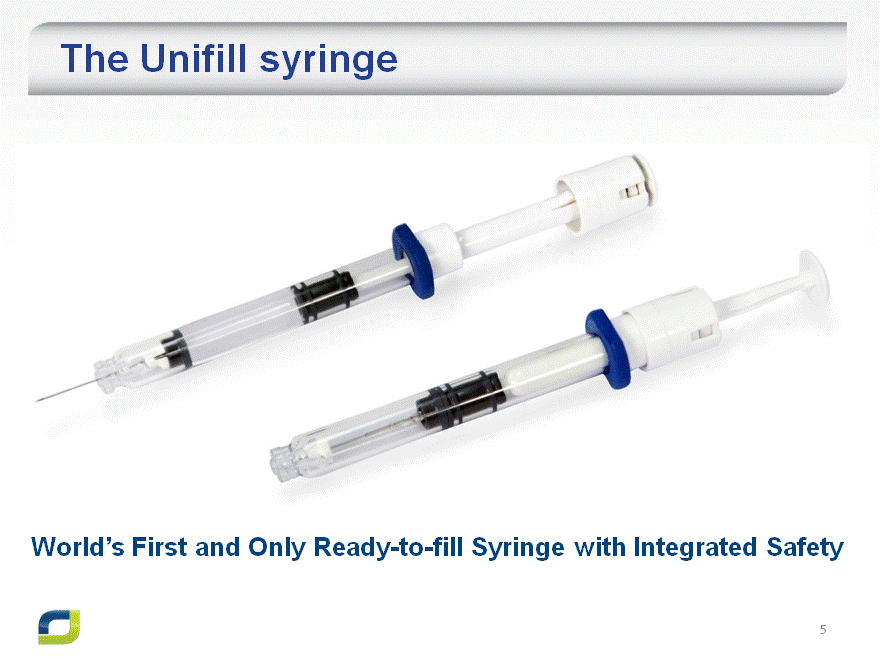
The Unifill syringe
World’s First and Only Ready-to-fill Syringe with Integrated Safety
| 5 |
|
Healthcare workers rate Unifill as Excellent
How would you rate the feedback (audible click, tactile feel) received to confirm the activation of the needle retraction & the end of dose
How would you rate the overall fit and finish, and quality of the syringe
Mean Rating
1.2
1.1
Outcome
Excellent
Excellent
– Excellent. 2. Good. 3. Neutral. 4. Poor. 5. Terrible
Self-Injecting Patients Rate Unifill as Excellent
How would you rate the feedback (audible click, tactile feel) received to confirm the activation of the needle retraction & the end of dose
How would you rate the overall fit and finish, and quality of the syringe
Mean Rating
1.3
1.3
Outcome
Excellent
Excellent
– Excellent. 2. Good. 3. Neutral. 4. Poor. 5. Terrible
| 7 |
|
Human Factor Study Remarks
“All participants found the device and associated procedure to be easy to learn and easy to use, even the first attempt.” “The most appreciated feature of the syringe was the needle retraction mechanism, which was deemed safer and easier to utilize compared to other safety syringe mechanisms.” “The Unifill syringe needle retraction technology is an innovative and advanced approach to the safety syringe and was appreciated by HCPs and patients alike.”
October, 2011
| 8 |
|
Needlestick Safety Advocacy Tour
A grassroots campaign to engage healthcare personnel to raise awareness and minimize risk of needlestick injuries Building a database of 100,000 HCW and cause advocates
50 locations and 12 conferences across 36 cities over year
9

The Pledge
I pledge to support Safe in Common in its campaign to promote and strengthen the Federal Needlestick Safety and Prevention Act, raise awareness of needlestick safety, and utilize safer engineering controls to better protect me and my fellow healthcare personnel from unnecessary needlestck injuries.
10
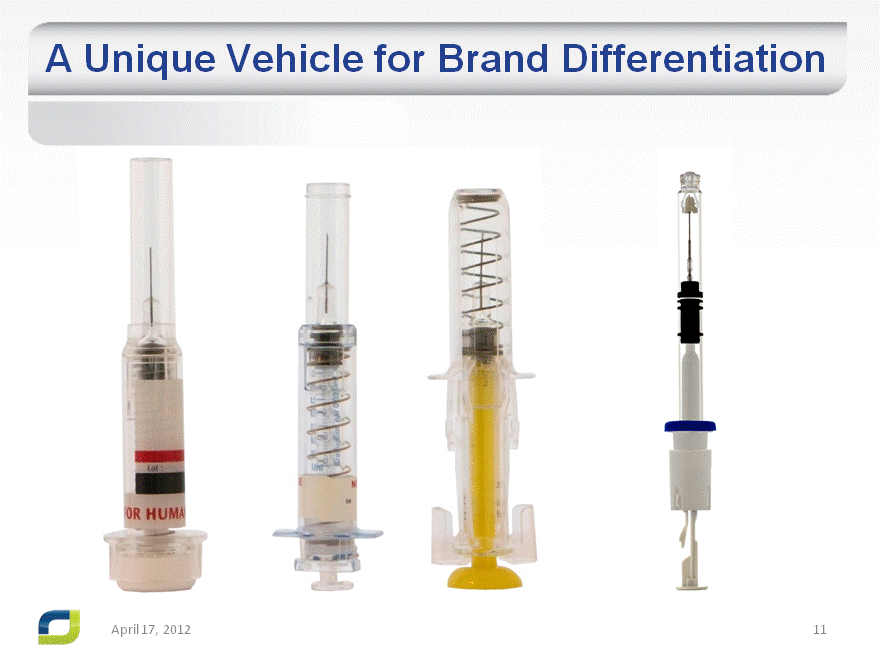
A Unique Vehicle for Brand Differentiation
April 17, 2012
11
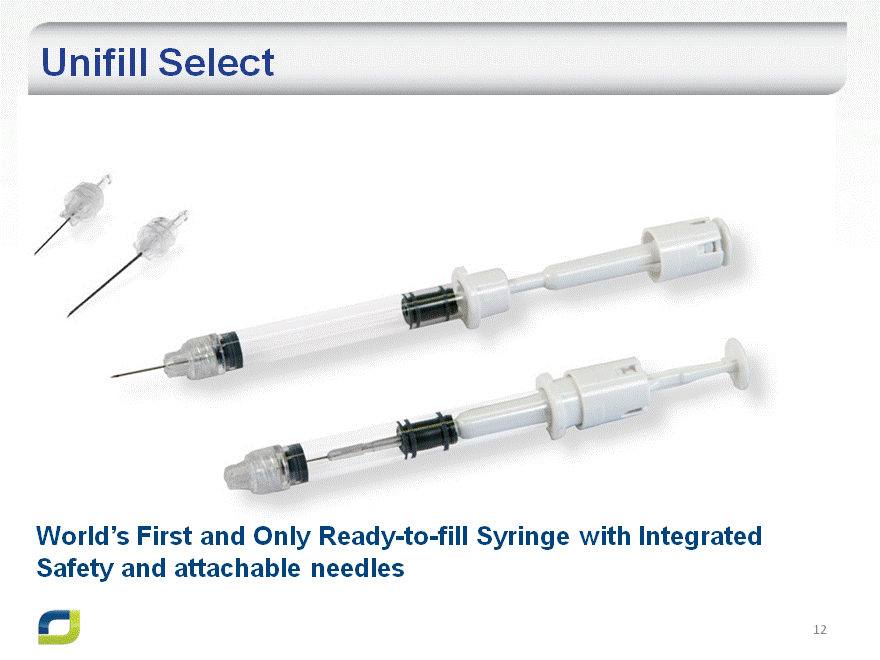
Unifill Select
World’s First and Only Ready-to-fill Syringe with Integrated Safety and attachable needles
12
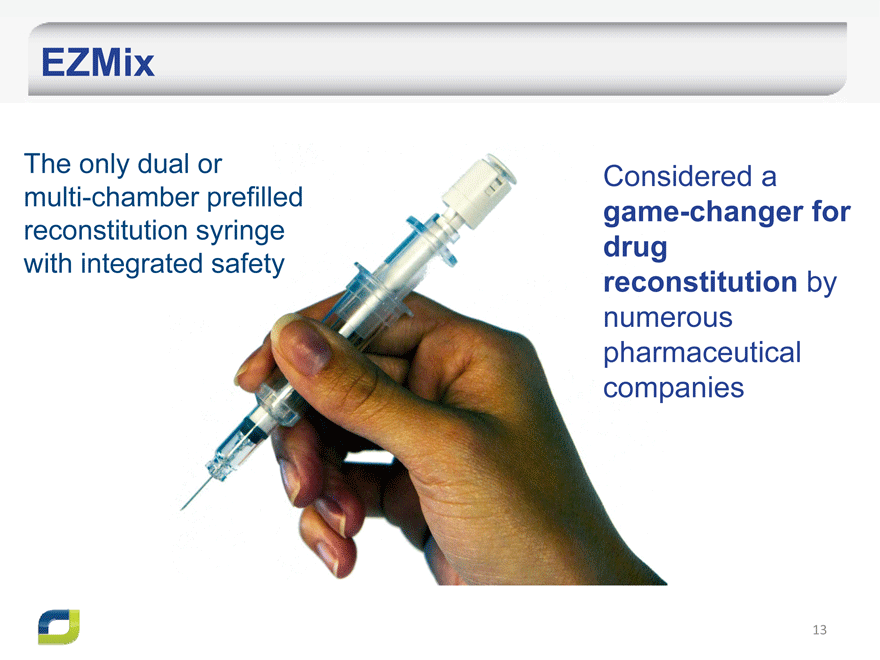
EZMix
The only dual or multi-chamber prefilled reconstitution syringe with integrated safety
Considered a game-changer for drug reconstitution by numerous pharmaceutical companies
13
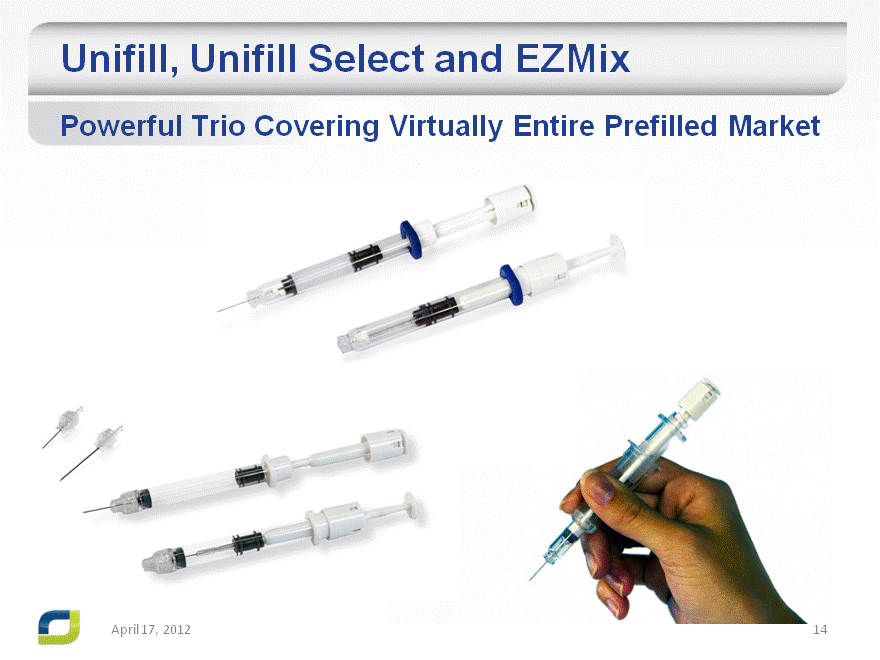
Unifill, Unifill Select and EZMix
Powerful Trio Covering Virtually Entire Prefilled Market
April 17, 2012

Unilife Rita Auto-Injector
World’s First and Only Auto-Injector with a True End-of-Dose Indicator
15

Comparison to Conventional Auto-Injector
April 17, 2012
16
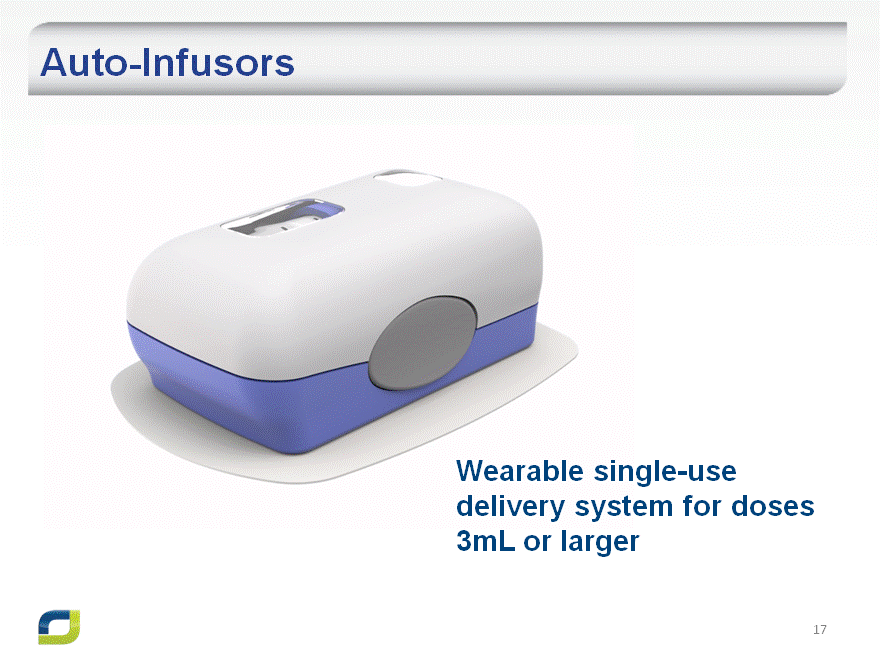
Auto-Infusors
Wearable single-use delivery system for doses 3mL or larger
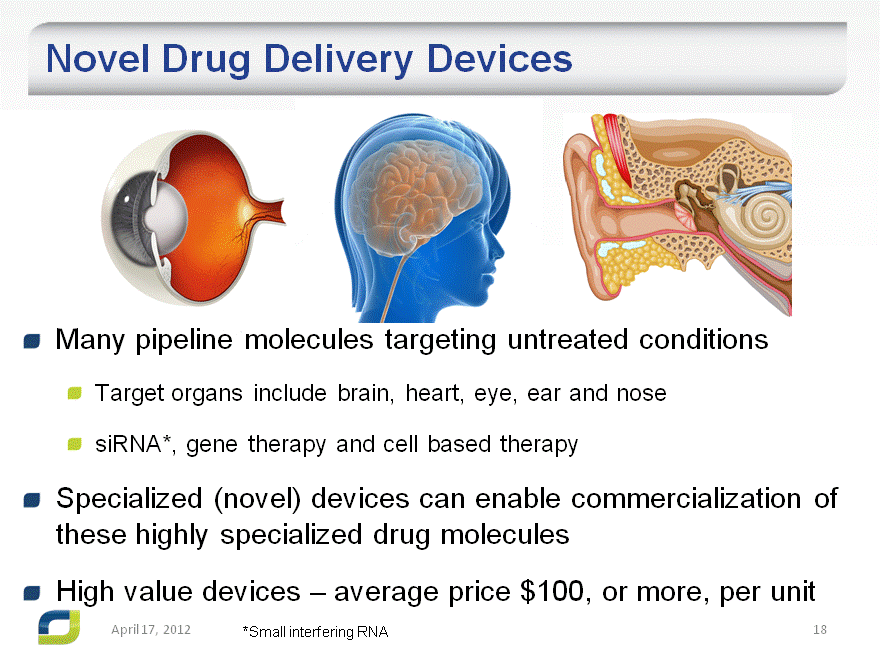
Novel Drug Delivery Devices
Many pipeline molecules targeting untreated conditions
Target organs include brain, heart, eye, ear and nose
siRNA*, gene therapy and cell based therapy
Specialized (novel) devices can enable commercialization of these highly specialized drug molecules High value devices – average price $100, or more, per unit
April 17, 2012
*Small interfering RNA
18
Building a Commercial Pipeline
Multiple pharmaceutical customers Multiple therapeutic classes targeted Multiple devices now under negotiation Multiple types of agreement anticipated Multiple deals in the commercial pipeline
April 17, 2012
19

Key Upcoming Milestones
Continued Unifill supplies to current and new customers
Unifill placement onto stability studies
Potential for additional access or royalty fees
Commercial supply agreements
Potential ordering of additional higher volume assembly lines
Agreements for additional products within portfolio
Develop or customize Unilife devices to address specific customer needs for pipeline and approved drugs and vaccines
Clinical development and supply agreements
Continuing to address unmet market needs in other areas
April 17, 2012

Questions and Answers
NASDAQ (UNIS) and ASX (UNS)
