Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Paratek Pharmaceuticals, Inc. | d327985d8k.htm |
 April 2012
April 2012
A specialty pharmaceutical company focused on the development
and commercialization of proprietary products to address important
therapeutic needs in the field of neuroscience
Exhibit 99.1 |
 Forward
looking statements Forward looking statements
This presentation contains forward-looking statements that involve substantial risks and
uncertainties. All statements, other than statements of historical facts, included in
this presentation are forward-looking statements. Examples
of
such
statements
include
our
expectation
regarding
the
potential
market
for
Intermezzo
®
and
the
potential market size for a middle of the night sleep aid; our expectations regarding an
ideal therapeutic; the current wholesale acquisition cost of Intermezzo and the
resulting revenue for each 1% of market share; Purdue plans to hire 275 sales
representatives to exclusively promote Intermezzo; Purdue plans to invest approximately
$100 million to support sales and marketing over the first 12 months of commercialization of
Intermezzo in the U.S.; the expected timing for and the success of the commercial
launch of Intermezzo by Purdue in the U.S.; the receipt of royalty and milestone
payments from Purdue pursuant to our Collaboration Agreement; anticipated
reimbursement coverage; intellectual property protection being obtained and maintained;
plans for the Phase 2 study of TO-2061, including the expected timing of clinical
trial results; and our expectations regarding the potential market size for
TO-2061 as augmentation treatment for patients with OCD. All of these forward-looking
statements are based on estimates and assumptions by our management that, although we
believe to be reasonable, are inherently uncertain. Forward-looking statements
involve risks and uncertainties, including, but not limited to, achieving acceptance
of Intermezzo by physicians, patients and third-party payors; supplying
sufficient quantities of Intermezzo from third-party manufacturers and suppliers to meet
anticipated market demand; the impact of competitive products and the market for
Intermezzo generally; our dependence on Purdue’s
commercialization
efforts,
including
our
reliance
on
Purdue
to
set
the
future
pricing
of
Intermezzo,
and
our Collaboration Agreement with Purdue; obtaining, maintaining and protecting regulatory
exclusivity and intellectual property protection for Intermezzo; competitive product
commercialization; manufacturing and supply risks for TO-2061; adverse patent
decisions at the USPTO or in court; and variability in the business of Transcept
generally.
These
and
other
risks
are
described
in
greater
detail
in
the
"Risk
Factors"
section
of
Transcept
periodic
reports filed with the Securities and Exchange Commission. Forward-looking statements do
not reflect the potential impact of any future in-licensing, collaborations,
acquisitions, mergers, dispositions, joint ventures, or investments
Transcept
may
enter
into
or
make.
Transcept
does
not
assume
any
obligation
to
update
any
forward-
looking statements, except as may be required by law.
2 |
 U.S.
primary care partnership: Purdue Pharma Option
to
co-promote
Intermezzo
to
psychiatrists
12/31/11: ~$62 M cash, equivalents & investments
$10M milestone payment from Purdue: Q4 2011
No debt
Neuroscience / psychiatry
Intermezzo:
middle of the night awakenings TO-2061
:
treatment
resistant
OCD
3
Intermezzo
product launch early April 2012
Intermezzo
prescription trends
TO-2061
Phase
2
results:
estimated
Q1
2013
Therapeutic focus
Commercial
platform
Strong balance
sheet
Large markets,
unmet needs
Near term
catalysts
Transcept: preparing for Intermezzo
®
commercialization |
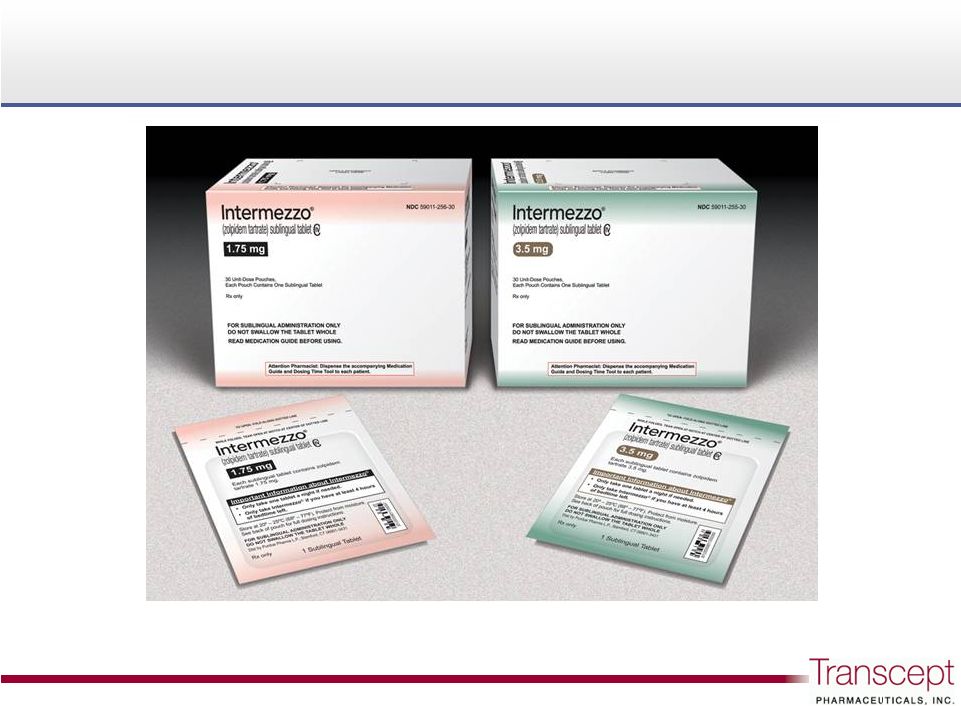
 Intermezzo
®
(zolpidem tartrate) sublingual tablet C-IV
Intermezzo
®
(zolpidem tartrate) sublingual tablet C-IV
4 |
 Intermezzo:
the first and only prescription sleep aid approved for middle-of-the-night
dosing Intermezzo: the first and only prescription sleep aid
approved for middle-of-the-night dosing
Indication statement as approved by FDA:
–
Intermezzo
is indicated for use as needed for the
treatment of insomnia when a middle-of-the-night
awakening is followed by difficulty returning to sleep.
–
Intermezzo
is not indicated for the treatment of middle-
of-the-night insomnia when the patient has fewer than 4
hours of bedtime remaining before the planned time of
waking.
5 |
 Intermezzo:
the first and only prescription sleep aid approved for middle-of-the-night
dosing Intermezzo: the first and only prescription sleep aid
approved for middle-of-the-night dosing
6 |
 Middle of the
night (MOTN) awakening: a major unmet medical need in the insomnia category
Middle of the night (MOTN) awakening:
a major unmet medical need in the insomnia category
Large U.S. insomnia market
–
79
million
new
and
refill
prescriptions
(1)
Insomnia
is
an
under-treated
condition
(2)
–
11
million
patients
receive
Rx
(3)
–
4x
to
6x
more
are
not
diagnosed
or
treated
by
a
physician
(3)
MOTN
awakening:
the
most
common
insomnia
symptom
(4)
–
35%
of
Americans
suffer
from
MOTN
awakenings
at
least
3x
/
week
(4)
–
>90% report awakenings persist more than six months;
50%
report
awakenings
persist
more
than
five
years
(5)
7
(1)
IMS
Oct
2010
to
Sep
2011;
(2)
Institute
of
Medicine
-
Sleep
disorders
and
sleep
deprivation
Apr.
2006;
(3)
BluePrint
Research
Group;
(4) Ohayon,
Nocturnal awakenings and comorbid disorders in the American general population. J of Psych
Research (2009); (5) Ohayon, Difficulty in
resuming
or
inability
to
resume
sleep
and
the
links
to
daytime
impairment,
J
of
Psych
Research
(2009). |
 Commonly
prescribed sleep aids are indicated only for bedtime use
Commonly prescribed sleep aids are indicated
only for bedtime use
MOTN awakenings typically do not occur every night
7-8 hr sleep aids (Ambien
®
, Ambien CR
®
, Lunesta
®
) require
bedtime prophylactic dosing to prevent awakenings
An ideal therapeutic would:
–
Be used only at the time patients need help returning to sleep,
not every night in advance of a problem that may not occur
–
Return patients to sleep rapidly
–
Be effective despite the low dose necessary to avoid next day
residual effects when used in the middle of the night
8 |
 Intermezzo:
the first and only sleep aid approved for middle-of-the night dosing
Intermezzo: the first and only sleep aid approved for
middle-of-the night dosing
Novel zolpidem formulation
–
Sublingual tablet
–
Bicarbonate-carbonate buffers
Approved dose
–
1.75 mg in women & patients > 65 years
–
3.5 mg in non-elderly men
Rapidly absorbed in both men and women
Effective vs. placebo in sleep laboratory & outpatient studies
Instructions to patients
–
Take Intermezzo “while in bed”
–
“When you wake up in the morning, be sure that at least 4 hours have passed
since you have taken Intermezzo
and you feel fully awake before driving. Do
not do dangerous activities until you know how
Intermezzo affects you.”
9 |
 Intermezzo:
National product launch early April 2012 Intermezzo: National product launch early
April 2012 10 |
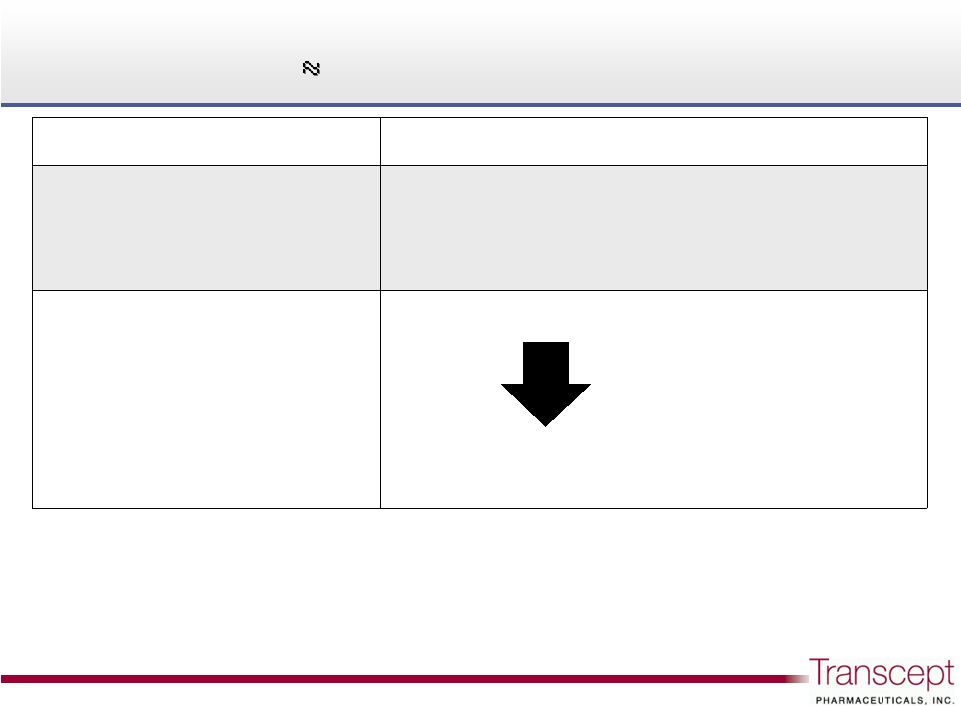 Significant
Rx insomnia market opportunity: each 1% share
$150M at gross price (WAC)
2
Significant Rx insomnia market opportunity:
each 1% share
$150M at gross price (WAC)
2
11
U.S. insomnia market
79 million TRx per year
1
Intermezzo pricing
$6.45 per tablet gross price (WAC)
2
30 tablets per Rx
3
Average Rx value: ~$190
Hypothetical market
penetration
1% Intermezzo share
790,000 TRx
approx $150M gross revenue
(1) IMS Health, National Prescription Audit, Sept 2011; (2) Published WAC, or
Wholesale Acquisition Cost, as of 4/2/12. WAC is the list price for wholesalers
and distributors before discounts, rebates, allowances, and/or other fees. (3)
Transcept estimate based on IMS Health, National Prescription Audit, Sept 2011.
|
 Promotion
to begin early April 2012 Purdue plans to invest ~$100M
during
first 12 months of Intermezzo launch
~275 field representatives in new
national
sales force devoted exclusively to the promotion of
Intermezzo to highest insomnia prescribers
Purdue began contacting drug wholesaler, retailer and
managed care decision makers in early 2012
Intermezzo launch plans
Intermezzo launch plans
12 |
 Purdue
commercialization agreement: key Transcept benefits
Purdue commercialization agreement:
key Transcept benefits
Co-promote option: foundation for a commercial future
–
Transcept
can
begin
promotion
to
psychiatrists
between
8
and
15
months after exercise of option depending on when in the calendar the
exercise is made
Royalty structure
–
Base royalty: mid-teens up to mid 20% level on net sales
–
Co-promote royalty:
•
22% to 40% on psychiatrist Rx net sales, based on timing of opt-in
•
Net revenue qualifying for this additional co-promote royalty is
capped at 15% of total Intermezzo annual net U.S. sales
Milestone payments
–
$10M
for
first
formulation
patent
listed
in
Orange
Book,
paid
in
Q4
2011
–
Up to $80M additional upon the achievement of certain patent
milestones and net sales targets
13 |
 Intermezzo:
intellectual property
Intermezzo:
intellectual property |
 Two U.S.
patents issued, additional patent applications pending
Two U.S. patents issued, additional patent
applications pending
Two issued U.S. patents -
7,682,628 and 7,658,945
Patent applications pending
15
–
Buffered formulations
–
Compositions and methods of treating insomnia
–
Claims require absorption of zolpidem across the
oral mucosa
–
Patents expire no sooner than February 2025
–
Claims are not limited to use of buffers
–
Compositions and methods of treating middle of
the night awakenings
–
Any patents issuing from these applications would
expire no sooner than February 2025 |
 TO-2061
TO-2061
16
Proposed indication: adjunctive therapy in adult patients
with obsessive compulsive disorder (OCD) not adequately
responsive to currently approved OCD medication |
 Significant
unmet medical need: OCD patients not responding adequately to approved
medication Significant unmet medical need: OCD patients not
responding adequately to approved medication
Obsessive Compulsive Disorder
–
Intrusive thoughts and repetitive actions to reduce distress
–
Affects 1% to 2% of U.S. adult population, 40% to 50% seek treatment
–
Significantly impacts everyday life activities of patients and their families
–
40% to 60% of OCD patients do not respond adequately to approved
medications,
which
include
the
SSRIs
Prozac
®
,
Luvox
®
,
Paxil
®
,
Zoloft
®
No FDA approved medication for treatment resistant OCD
–
Atypical antipsychotics are often used off-label to augment approved
medications
•
~68% of treatment resistant OCD patients do not respond
•
Frequently reported adverse events: weight gain, metabolic disorder
17 |
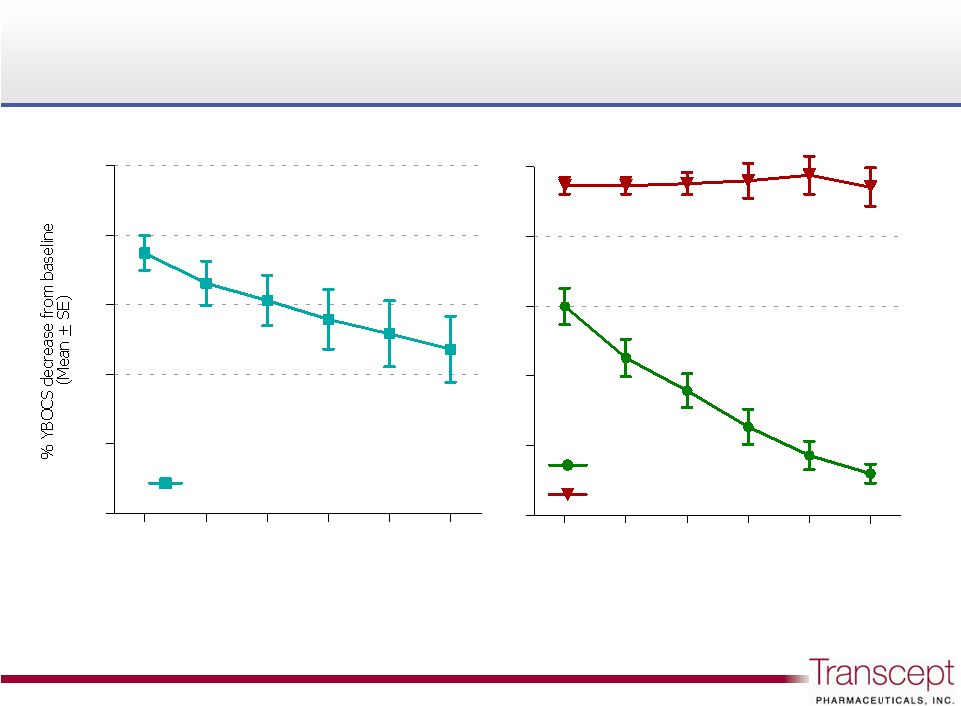 Pilot Study
B: Improvement measured as % decrease below baseline
Pilot Study B:
Improvement measured as % decrease below baseline
18
Week
2
Week
4
Week
6
Week
8
Week
10
Week
12
50%
40%
30%
20%
10%
0%
All patients
Week
2
Week
4
Week
6
Week
8
Week
10
Week
12
50%
40%
30%
20%
10%
0%
Responders (57% of patients)
Non-responders (43% of patients)
All Patients: n=21
26.3% improvement at 12 weeks
Responders: n=12 of 21 (57%)
44.3% improvement at 12 weeks
Pilot Study B, n=21 |
 TO-2061
TO-2061
505b2 NDA pathway
Phase 2 double blind, placebo controlled study, n 150,
complete enrollment est. Fall 2012, top line data est. Q1 2013
Intellectual property
–
Method of use patent application filed, priority date May 19, 2009
–
Ondansetron, up to ~1.5 mg/day
–
Pending claims for treating SSRI resistant OCD with ondansetron augmentation
Managed care survey (~108M lives): unmet medical need
acknowledged, Tier 3 formulary placement expected
Strategic fit: psychiatry
–
~87% of patient visits for OCD were to psychiatrists in 2009
*
–
Complementary to Intermezzo
psychiatry co-promote option with Purdue
19
* SDI Physician Drug and Diagnosis Audit |
 Financial
overview Financial overview |
 Cash &
investments: $62.4 M
Shares outstanding:
13.9 M
Options / warrants / other:
3.6
Total:
17.5 M
Employees:
18
Financial position
Financial position
12/31/2011
21
2/1/2012 update |
 Intermezzo
®
is a registered trademark of Transcept Pharmaceuticals, Inc.
Ambien
®
and Ambien CR
®
are registered trademarks of sanofi-aventis
Lunesta
®
is a registered trademark of Sunovion Pharmaceuticals Inc.
Zofran
®
and
Paxil
®
are registered trademarks of The GlaxoSmithKline Group of Companies
Prozac
®
is a registered trademark of Eli Lilly & Co.
Luvox
®
is a registered trademark of Solvay Pharmaceuticals, Inc.
Zoloft
®
is a registered trademark of Pfizer Inc. |
