Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AVEO PHARMACEUTICALS, INC. | d279151d8k.htm |
 A DECADE OF
DELIVERY 2012 Marks AVEO’s Ten Year Anniversary
NASDAQ: AVEO
Exhibit 99.1 |
 This
presentation contains forward-looking statements that involve substantial risks and uncertainties, including
among other things, statements about:
-our planned
development, commercialization and manufacturing plans, timelines and strategies for tivozanib;
-the potential
therapeutic advantages and benefits of our product candidates;
-the timing and
results of our ongoing and planned preclinical studies and clinical trials;
-the potential
benefits of our strategic partnership agreements, our ability to achieve additional payments under
these arrangements and our ability to enter into additional arrangements;
-our plans to
leverage our Human Response Platform™
to inform clinical development;
-our intellectual
property position and strategies; -the expected RCC market and potential of tivozanib to enter this
market;
-our projections
with respect to our achievement of corporate milestones, including our anticipated plans for
success in the oncology markets; and
-AVEO having
sufficient capital to fund its operations beyond 2012 and AVEO's estimates for its 2011 expected
year-end cash balance.
Actual results or events could differ materially from the plans,
intentions and expectations disclosed in the
forward-looking statements we make due to a number of important factors,
including risks and uncertainties
inherent in pharmaceutical research and development, such as our ability to
successfully develop, test and gain approval of our product candidates, including
tivozanib; our ability to obtain, maintain and enforce intellectual property rights;
competition; our dependence on our alliance partners and other third parties; our ability to obtain
any necessary financing; adverse economic conditions; and those risk factors discussed in the
“Risk Factors” and
elsewhere in our most recent Form 10-K and other periodic filings made with the
Securities and Exchange Commission. You should not place undue reliance on
forward-looking statements because they involve known and unknown risks,
uncertainties and other factors that are, in some cases, beyond our control and that
could materially affect actual results, performance or achievements. The
forward-looking statements you see or hear during this presentation represent our
views as of the date of this presentation. We anticipate that subsequent events and
developments will cause our views to change. However, while we may elect to update
these forward- looking statements at some point in the future, we have no current
intention of doing so except to the extent required by applicable law. You should,
therefore, not rely on these forward-looking statements as representing our views
as of any date subsequent to the date of this presentation. Cautionary Note Regarding
Forward-Looking Statements 2 |
 Transformative
Step Towards Achieving AVEO Vision 3
Our Vision: Build a sustainable, fully integrated company to
advance oncology care with next generation products |
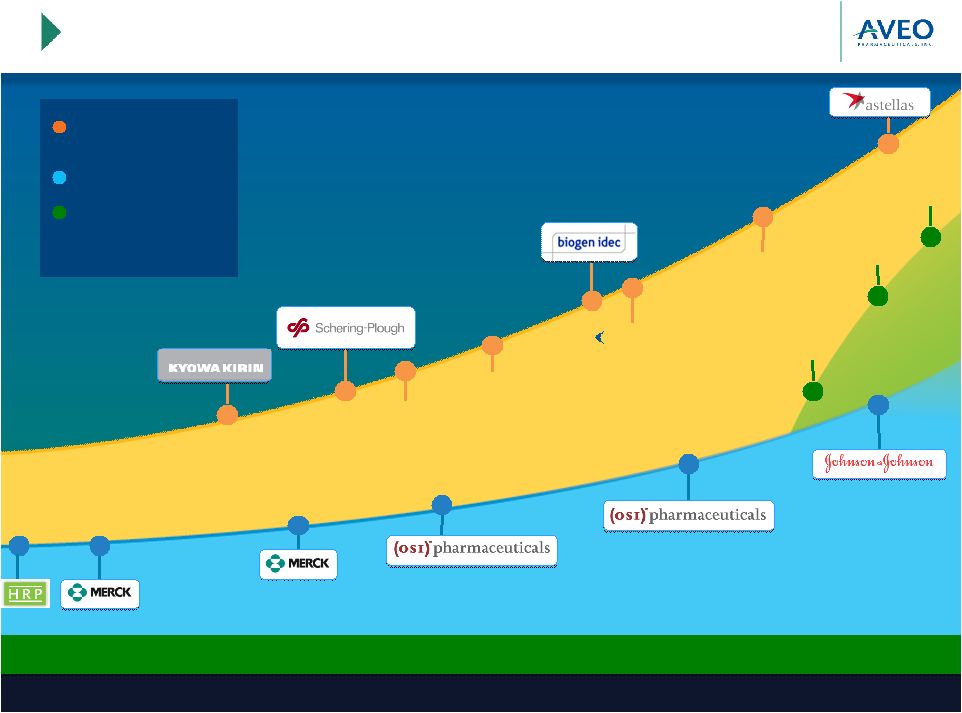 CLINICAL
ACTIVITIES AND PIPELINE DEALS
HRP AND HRP DEALS
MERGER OF HRP &
PRODUCTS/CLINICAL
PROGRAMS
SERIES A
SERIES B
SERIES C
SERIES D
SUCCESSFUL IPO
PIPE
FOLLOW-ON
TIVOZANIB PH 2 RCC
STUDY INITIATED
FICLATUZUMAB PH 1
INITIATED
AVEO REGAINS
RIGHTS TO
FICLATUZUMAB
TIVO-1
INITIATED
FICLATUZUMAB
PH 2 NSCLC INITIATED
BATON-RCC
BATON-CRC
2002-2012: A Decade of
Delivery
2002
|
|
|
|
| 2012 |
 TIVOZANIB
5
Potent, Selective and
Continuous Inhibitor of All
Three VEGF Receptors |
     TIVO-1: Pivotal
Superiority Study in 1 st
-Line RCC
6
STUDY DESIGN
Patient Eligibility
•
Advanced RCC
•
Confirmed clear-cell type
•
Prior nephrectomy
•
No prior VEGF/mTOR tx
•
ECOG PS 0-1
RANDOMIZE
TIVOZANIB
(n=260)
SORAFENIB
(n=257)
•
Well-controlled
study for registration in first-line
RCC
»
Central,
blinded,
independent
review
of
CT
scans
every
8
wks
»
Continuation of treatment until disease progression verified by independent
radiologist
•
Patients randomized to sorafenib with confirmed disease progression
eligible to join separate study to crossover to tivozanib
6 |
 TIVO-1
Top-Line: Statistically Significant Superiority 7
Efficacy
7 |
       Current Landscape:
Treatment Naïve RCC Patients 1.
Votrient PI.
2.
Sutent PI.
3.
Motzer, et al. 2011 ASCO GU Symposium, Abstract 308. Standard dose (4/2), novel dose
(continuous). 4.
AVEO and Astellas Pharma Inc., TIVO-1 top-line data announced Jan. 3, 2012.
Phase 3 Study
1
11.1 months
Phase 3 Study
2
11 months
Standard Dose
3
9.9 months
Novel Dose
3
7.1 months
TIVO-1
4
9.1 months
Pazopanib
Sunitinib
Sorafenib
8 |
 TIVO-1
Top-Line: Statistically Significant Superiority |
 Tivozanib:
Potential Standard of Care in First-Line RCC RCC market valued at $2B WW
1
Despite advances, unmet medical need persists
2
Insufficient Efficacy
•
Dose reductions or interruption
of current
therapies
due to patient
intolerability
is
major
limitation²
•
Many
patients
cannot
tolerate
current therapies
for long periods
of time, impacting
QoL
and
potential
to achieve maximal
efficacy
2
•
Low remission rates
for
patients with Stage IV
disease reflects fact
that
response rates
of
current
therapies are
insufficient
2
1. Wolters Kluwer and IMS Health
2. Campbell Alliance interviews with 7 KOLs. Aug, 8 through Aug. 12, 2011.
10 |
         Preparing for
Tivozanib Registration & Launch in RCC Prepare NDA and
MAA submissions
Investing in
North American
commercial infrastructure
•AVEO
to
lead
commercialization
in
N.A.;
will
hold
NDA
and
book
sales
•Astellas
to
provide
50%
of
sales reps and
MSLs
Launch
tivozanib
in
advanced
RCC
1
Leveraging
Astellas
EU
market
presence
•Astellas
to
lead
commercialization
in
EU;
will
hold
MAA
and
book
sales
•AVEO
to
provide
50%
of
MSLs
in
EU
11
1. Contingent upon FDA and EMA approvals |
 Our Total
Approach to Patient Access
Disease
Management
Efficacy
Tolerability
Support
Advocacy
Predictive
Biomarkers |
 Human
Response
Platform™: Insights into Cancer Biology
13
U.S. Patent No. 6,639,121 Issued in 2003
•
Discovery and
validation of
functionally
relevant targets
•
Maturing pipeline
of novel functional
antibodies
•
Biomarker-driven
identification of
responsive patient
populations
•
Genetic signatures
of tumors informs
rational choice of
drug combinations
•
Biomarker data
provides
opportunity to
optimize benefits in
selected patients
•
Supports pricing
and reimbursement |
     HRP
Biomarker
Strategy
for
Tivozanib
•
Initiated
Jan.
2011
as
exploratory
Ph
2
biomarker
study
in
pts
w/
RCC
•
Evaluate
biomarkers
in blood and archived tissue samples for
correlation
with
tivozanib
clinical
activity
and/or
drug-related
toxicity
•
Initiated
Ph
2
study
Nov.
2011
as
first-line
therapy
in
pts
w/
mCRC
•
Evaluate PFS of
tivozanib
+ mFOLFOX6 vs.
Avastin
+ mFOLFOX6
•
Assess biomarker relationships
that may predict response
14 |
 TIVOZANIB
Triple VEGF Receptor Inhibitor
TIVO-1
BATON-RCC
BATON-CRC
RCC + Torisel®
GI cancers + FOLFOX6
Breast cancer + Taxol®
Breast and colorectal cancers + Xeloda®
PRECLINICAL
PHASE
1 PHASE 2
PHASE 3
PRE-NDA Expanding Beyond RCC
*Aveo
and
Astellas
Pharma
Inc.
have
a
worldwide
agreement
to
co-develop
and
commercialize
tivozanib
outside
of
Asia
15 |
 FICLATUZUMAB
Wholly owned HGF
inhibitory antibody in
Phase 2 development
Hepatocyte growth factor (HGF) is sole ligand for c-MET receptor
16 |
 Ficlatuzumab
Targets Important HGF/c-Met Pathway •
HGF / c-Met pathway plays important role in
several cancers
–
Including lung, H&N and gastric cancers
–
Associated with aggressive growth, invasion, metastasis,
and poor prognosis
•
AVEO’s HRP provides insights into role of HGF /
c-Met pathway in many tumors
–
Proprietary models allow for exploration of activity in
different genetic backgrounds (e.g., EGFR status and c-
Met level)
17 |
     Ficlatuzumab
Phase 2 in First-Line NSCLC
18
STUDY DESIGN
RANDOMIZE
FICLATUZUMAB
+ IRESSA
IRESSA
Patient Eligibility
•
Advanced or metastatic stage
3b/4 NSCLC
•
Asian population
•
No prior chemotherapy
•
Adenocarcinoma
•
Non-
or light ex-smoker
•
ECOG PS 0-2
•
Primary
endpoints: PFS and Response
•
Enrollment completed May 2011
(n=188 patients in Asia)
•
Biomarker based outcome
»
EGFR mutation +/-
and c-Met level low/high
•
Large scale manufacturing in place with Boehringer Ingelheim
•
Data expected in 2012
18 |
 AV-203
Anti-ErbB3 Monoclonal
Antibody with Broad
Therapeutic Potential
19 |
 •
ErbB3 widely expressed in several cancers;
overexpression associated with poor prognosis
»
Including, breast, H&N, lung and prostate cancers
•
AVEO’s HRP provides insights into role of ErbB3
in many tumors
»
AV-203 inhibits
ErbB3 function and tumor growth
in
different genetic contexts in a broad
range of tumor
models
»
Identified potential biomarkers of AV-203
response; to
be
validated in
upcoming clinical trials
AV-203: First in Human Study in 2012
20 |
 = FOUNDATION
FOR BIOMARKER CLINICAL STATEGIES *Aveo and Astellas Pharma Inc. have a worldwide
agreement to co-develop and commercialize tivozanib outside of Asia **Anti-ErbB3
program is strategically partnered with Biogen Idec Building
A Sustainable Pipeline
21
Anti-ErbB3 Program
Solid Tumors
TIVOZANIB
Triple VEGF Receptor Inhibitor
TIVO-1
BATON-RCC
BATON-CRC
RCC + Torisel®
GI cancers + FOLFOX6
Breast cancer + Taxol®
Breast and colorectal cancers + Xeloda®
PRECLINICAL
PHASE
1 PHASE 2
PHASE 3
PRE-NDA FICLATUZUMAB
Anti-HGF/c-MET Pathway
NSCLC + Iressa®
Solid Tumors + Tarceva®
AV-203
PRECLINICAL
PHASE
1 PHASE 2
PHASE 3
PRE-NDA PRECLINICAL
PHASE
1 PHASE 2
PHASE 3
PRE-NDA |
 2002
|
|
|
|
| 2012
$16M
SERIES A
$44M
SERIES B
$12 M
SERIES C
$53 M
SERIES D
$90 M
IPO
$60 M
PIPE
$111 M
FOLLOW-ON
Strong Financial Resources
•
Expect to exit 2011 with ~$275M in cash
and securities
•
Sufficient capital to fund
operations beyond
2012
»
Expand
tivozanib
clinical development & increase
pre-commercial activities
»
Invest in
ficlatuzumab
development
»
Advance antibody
pipeline
•
Dual sources of capital
»
Capital markets
»
Corporate partnerships
Unaudited 2011 financial results as of 12/31/11 |
 2011 Key
Accomplishments •
Established $1.3B+ Astellas partnership*
•
Initiated BATON-RCC
•
Initiated BATON-CRC
•
Completed top-line analysis of TIVO-1
•
Completed Phase 2 enrollment
•
Presented Phase 1 data at ASCO 2011
•
Secured manufacturing agreement with Boehringer Ingelheim
•
Established $550M+ RON antibody deal with J&J*
•
Completed $111M financing
TIVOZANIB
FICLATUZUMAB
CORPORATE
23
* Deal amounts reflect upfront payments and potential milestones
|
 Key Priorities
for 2012 •
Submit NDA in 3Q in advanced RCC
•
Submit MAA following NDA
•
Submit detailed findings from TIVO-1 to ASCO
•
Expand clinical development into breast cancer
•
Advance pre-launch activities
•
Complete Phase 2 study
•
Present Phase 2 results in 2H
•
Initiate Phase 1 study
TIVOZANIB
FICLATUZUMAB
AV-203
24 |
 A Decade of
Delivery: Pieces in Place… 25 |
 …to Build
A Sustainable
Fully Integrated Oncology Company
26 |
